Abstract
South Africa (SA) is facing a rising prevalence of obesity and diet-related chronic diseases. The government is seeking to develop effective, evidence-based policy measures to address this. A well-designed, fit-for-purpose nutrient profiling model (NPM) can aid policy development. The aim of this study was to develop a fit-for-purpose NPM in SA. Steps included: (1) determining the purpose and target population; (2) selecting appropriate nutrients and other food components to include; (3) selecting a suitable NPM type, criteria and base; and (4) selecting appropriate numbers and thresholds. As part of the evaluation, the nutritional composition of packaged foods containing nutritional information (n = 6747) in the SA food supply chain was analyzed, a literature review was undertaken and various NPMs were evaluated. Our findings indicated that it is most appropriate to adapt an NPM and underpin regulation with a restrictive NPM that limits unhealthy food components. The Chile 2019 NPM was identified as suitable to adapt, and total sugar, saturated fat, sodium and non-sugar sweetener were identified as appropriate to restrict. This NPM has the potential to underpin restrictive policies, such as front-of-package labelling and child-directed marketing regulations in SA. These policies will support the fight against obesity and NCDs in the country.
Keywords: nutrient profiling, South Africa, LMIC, food policy
1. Introduction
There is global consensus that better policies are needed to address the obesity pandemic [1,2]. Comprehensive, operationalizable and solidly designed food policies have the capacity to substantially improve diets at a local, national and international level. These benefits are not only for a select few, but also reach disadvantaged, lower socioeconomic groups [3]. Underpinning various country-level food policies with one well-designed nutrient profiling model (NPM) can support good regulatory practice and a consistent regulatory approach through providing a transparent basis on which to distinguish healthier and less healthy foods for policy application [4]. NPMs developed together with industry are more lenient and permit more foods than those grounded with scientific evidence [5], resulting in little progress, if any, in addressing rising rates of malnutrition in all its forms and NCDs. As such, the development of an NPM should be independent of industry involvement.
There are a range of policy measures that can benefit from an NPM as their foundation. These include policies and regulations on front-of-package labelling, marketing to children, school food guidelines, health and nutrition claims on foodstuff, food taxation, food fortification, food procurement in hospitals, prisons and old age homes and the informing of welfare support schemes [6]. Several countries have introduced mandatory food policies that make use of NPMs. For instance, Chile has used the same NPM to effectively restrict foods that have front-of-pack (FOP) warning labels from being sold or eaten on or near school grounds, and to prohibit marketing of foods carrying an FOP label [7].
1.1. The Need for Evidence-Based Restrictive Food Policies in South Africa
SA has undergone a transition from infective to non-communicable diseases [8] and South Africans are consuming more and more harmful, ultra-processed foods high in fats, sugar and salt [9]. Given this, the SA government has identified the need to implement policies and practices to prevent and control obesity [10]. Indeed, the current COVID-19 pandemic, with poorer prognosis and higher mortality rates linked to obesity and NCDs [11] has highlighted the strain that obesity places on the healthcare system [12].
The active SA food labelling regulation, R146, was implemented in 2010 [13]. Following this, in 2014, a draft regulation, R429, was published [14] that makes recommendations for an NPM for health and nutrition claims (hereafter referred to as the SA HNC NPM). In June 2016, SA implemented mandatory upper sodium limits in various processed food categories [15]. Regulation 127 of 2011, for trans-fats, prohibits foods that contain more than 2 g of trans-fat per 100 g of oil or fat [16]. These actions suggest that there is recognition of the potential harms associated with certain ingredients or amounts of nutrients in SA and a need to restrict them.
There is a need for strong policies to promote health and prevent non-communicable diseases (NCDs) in low- and middle-income countries (LMICs) [17]. A transparent, evidence-based approach without industry interference should be followed in their development [17,18]. In SA, powerful commercial actors have been shown to influence health policy formulation processes to favor their interests over those of the public’s health and pockets [19,20,21,22]. As such, an independently developed NPM may support the government to develop a strong policy.
1.2. Considerations for a Fit-for-Purpose NPM to Underpin Restrictive Food Policy in SA
Different NPMs vary significantly in purpose and complexity, so it is of utmost importance that policymakers consider the purpose and operationalizability of the model they intend to implement, in order to select a model that will achieve the intended purpose [6,23]. Implementation of policy related to NPMs has been slow in LMICs, possibly due to limited resources and a lack of population level nutritional data that are required to inform the development of NPMs within these countries [17,24,25]. Additionally, the LMIC setting faces a number of challenges in the food policy arena, including struggles with multi-sectoral collaboration, implementation of proposed policies and the ability to follow through with long-term commitment to policy goals [26].
According to a 2018 systematic review of all NPMs used in government regulation globally (78 models worldwide), only one NPM has been developed for Africa [6]. This is the SA HNC NPM, included in the draft R429 of 2014 for the purpose of regulating health and nutrition claims. It has also been validated for use in child-directed marketing restrictions [27,28]. This model is based on the Food Standards Australia/New Zealand’s (FSANZ) NPM, which in turn was adapted from the UK Ofcom NPM [29], and thus originally designed for high-income countries. Since then, the World Health Organization (WHO) African Region has proposed an NPM [30], although it has yet to be implemented by any country.
A single fit-for-purpose NPM that can be used to ground various restrictive food policies in SA is an ideal starting point for regulating the processed food environment and will ensure a consistent message for the public and the food industry. Using one NPM in various policies can also reduce administrative burden [4]. If regulations are to be put in place, they need to be easy to implement, require limited resources to enforce and they must not be costly. The objective of this paper is to identify a suitable, context-specific NPM for food policy in SA, using an established step-wise approach.
The LMIC setting of SA offers a diverse and challenging context. It is important that an independent, robust approach following accepted scientific process is used to develop a feasible, context-specific NPM [17,31,32,33]. This paper contributes to existing scientific research on NPMs by investigating the various aspects to consider when developing a fit-for-purpose NPM for restrictive food policies in SA, which has the potential to influence food policy in South Africa, and more broadly, other LMICs in Africa.
2. Materials and Methods
For this paper, the NPM development process was informed by the internationally accepted seven-step approach developed by Scarborough, Rayner and Stockley [32] and the six steps recommended for NPM development or adaptation in the WHO draft guidelines on front-of-pack labelling (FOPL) [33]. Although this is presented as a step-wise process, these steps are practically interrelated and interdependent and decisions made in one step affect the decisions in other steps [34]. Steps will be discussed in combination under these broad headings:
Determining the purpose, and target population of the NPM;
Selecting appropriate nutrients and other food components to include;
Selecting a suitable NPM type, criteria and base;
Selecting appropriate numbers and thresholds.
The decisions are informed by a combination of location-specific primary research, as well as lessons learnt through existing literature. Where literature was reviewed, the authors used narrative literature review methodology to identify appropriate, targeted literature. Taking the resource limitations of the country into account, provisions for straightforward classification, implementation and evaluation were considered at each step.
2.1. Data
Primary data collection was necessary to evaluate the composition of packaged foods in SA. Nutritional information on packaged foods and beverages (n = 6747) with nutrition information panels (NIP) was collected photographically by trained fieldworkers from large supermarkets in SA (Pick ‘n Pay, Woolworths, Checkers, Spar and Shoprite) in 2018. Products that did not contain an NIP were excluded from analysis (Supplementary Tables S1 and S2 and Supplementary Figure S1 contain detailed methodological information). These data were used to calculate the mean nutritional content of various nutrients of concern. For non-sugar sweetener (NSS) specifically, where the mean content could not be calculated (as data are not provided), the number of different NSS’s was assessed by identifying NSS in the ingredient list using a standardized list of search terms (Supplementary Table S3).
2.2. Steps in NPM Development
2.2.1. Step 1: Determining the Purpose and Target Population of the NPM
To determine the purpose and target population, we reviewed relevant population level data [35,36,37,38,39,40,41] and the policy context to identify the key nutritional problems faced by the SA population. We researched existing literature to identify the target population, their disease burden and dietary intake patterns to select the most appropriate NPM. We also reviewed strategic plans and dietary guidelines developed by the SA Department of Health to gain insights into the nutrition problems identified by the government for the population as a whole.
To assess the level of processing in South African packaged foods, collected data were evaluated by making use of the NOVA classification system as described elsewhere [42,43].
2.2.2. Step 2: Deciding Which Nutrients and Other Food Components to Include
Making use of literature review, whilst considering the purpose of the NPM and risk profile of the target population, international dietary guidelines and nutrients and food components considered for inclusion in the NPM were reviewed to identify those most appropriate to include.
As current draft regulations [14] in SA make use of an NPM that includes nutrients of concern to both encourage and limit, in this study we compared differences between an NPM that only considers unhealthy nutrients of concern to restrict, and one that includes both healthy and unhealthy components by applying their criteria to the SA packaged food supply (n = 6747). The Chile Warning Octagon (CWO) 2019 was selected as it assesses nutrients of concern linked only to poor health outcomes (sugar, sodium, saturated fat and energy) that need to be restricted through food policy. A number of studies indicate its success in Chile [44,45,46,47,48], and other countries have used Chile’s NPM [49,50]. The SA HNC NPM assessed health and nutrition claims in draft SA regulation [14] and was adopted from Food Standards Australia and New Zealand’s (FSANZ) NPM (which relied on the UK Ofcom NPM in its development [29]). To determine the difference between the SA HNC NPM baseline score (which is the score solely for ‘nutrients to limit’—sugar, sodium, saturated fat and energy) and the final SA HNC NPM score (which includes points for fiber, protein, fruits, vegetables, nuts and legumes as ‘nutrients to encourage’), we evaluated the baseline and final SA HNC NPM separately, alongside the CWO 2019 NPM.
Algorithms were generated in STATA (version 15, StataCorp, College Station, TX, USA) to evaluate the packaged food supply against these NPMs. We classified foods and beverages as either compliant or non-compliant, depending on the nutritional criteria of the NPM. For the SA HNC NPM, a product was considered compliant when it met the criterion of being allowed to carry a health claim (for beverages a score of less than 1, for processed cheese and fats a score of less than 28 and for other foods a score of less than 4). Similarly, for the SA baseline HNC NPM, a product was considered compliant when it met the criterion of being allowed to carry a health claim—but for this model, points were not awarded for ‘nutrients to encourage’. For the CWO 2019 NPM, a product was considered compliant when it was excluded from carrying a warning label according to the 2019 criteria (it did not exceed any of the model’s limits for energy, sugar, sodium or saturated fat).
A fruits, vegetables, nuts and legumes (FVNL) score needed to be calculated for the SA HNC NPM. As the percentage of fruits and vegetables is not routinely included on the NIP in SA, we manually estimated scores. A similar methodology, as described by Bernstein et al. [51], was followed for the calculations. An FVNL score of 0 was assigned to sub-categories without FVNLs (e.g., fats and oils). For groups which may contain FVNLs, products were individually reviewed within the context of its group. We considered the order of ingredients, the form of the FVNL ingredients (concentrated or non-concentrated), number of FVNL ingredients compared to the number of non-FVNL ingredients and type of product in order to determine the score. A dietitian performed all classifications.
To consider nutrients of concern within packaged foods in SA, we divided the packaged food supply into sub-categories and the mean content of nutrients of concern was calculated. Most of this information was available from the NIP, however, free sugar content of products was estimated according to the method proposed by the Pan American Health Organization (PAHO) [52] when not available on the NIP. To evaluate the suitability of the inclusion of energy in the NPM products excessive in energy only (and not sugar, saturated fat or sodium), the CWO 2019 NPM was utilized.
2.2.3. Step 3: Selection of a Suitable NPM Type, Criteria and Base
To inform the selection of the NPM type, we used the approach described by Rayner, Scarborough and Stockley [31,32] to decide on the base unit of measure, whether to make use of a categorical or continuous model, as well as whether to select across-the-board thresholds or category-specific criteria.
The criteria underpinning an NPM can be applied across all foods, (‘across-the-board’) or alternatively, it can differentiate between food categories, allowing either comparison across different food categories or comparison within food categories (‘category-specific’) [53]. Models can be either threshold-based or continuous [32]. Threshold-based NPMs identify predetermined cut-points that will place food into different categories in a binary manner. Different cut-points can be used for different nutrients in one food (e.g., a different cut-point each for sugar, sodium and saturated fat). With a continuous model, foods are classified on a continuous scale, ranking nutritionally better, or worse, than another product. Points are awarded based on different criteria for various nutrients and a summative healthfulness score is calculated from this, depending on the NPM’s criteria [31].
To inform the decision, we conducted a review of the literature, examining the pros and cons of various approaches, whilst taking the LMIC context of SA into consideration.
2.2.4. Step 4: Choosing the Thresholds to Use
In order to decide whether to use the same cut-points as an existing NPM, or to adapt them for SA, we followed the same methodological approach as that described in the Chilean paper [54] for the development of cut-points. For the SA list of foods, two dietitians analyzed the SA Medical Research Council Food Composition Table (FCT) [55] independently of each other. Any discrepancies in classification were resolved in a group discussion with a third dietitian. All three dietitians were in agreement about the final list of foods included. As the SA FCT is not as comprehensive as the USDA FCT, the final list of foods was shorter, at 183 foods.
3. Results
3.1. The Purpose and Target Population of the NPM
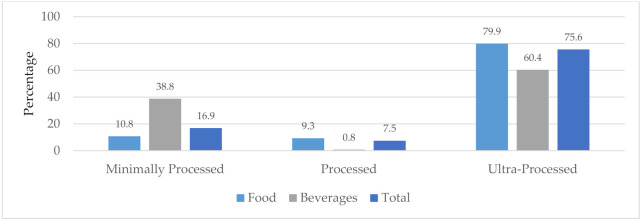
South Africans are consuming more and more harmful, ultra-processed foods [9,56] that are linked to NCDs [57]. This is clear from the analysis of the packaged food supply in SA (Figure 1 and Supplementary Table S4). In 2018, 83% of packaged products evaluated in SA supermarkets (89% of foods and 61% of beverages) were either processed or ultra-processed, according to the NOVA classification [42,43].
Figure 1.
Packaged foods and beverages in the SA marketplace (2018) classified as minimally processed, processed and ultra-processed, according to the NOVA classification system. Note: the NOVA category, ‘culinary ingredients’ has been omitted from analysis.
The NCD mortality rate has steadily increased and NCDs are the main cause of death in SA [58]. The 2016 SA Demographic and Health Survey (SADHS) reported that overweight, obesity and hypertension prevalence have been on the rise since 1998, with 31% of men and 68% of women overweight or obese [35]. A recent report by the World Obesity Federation anticipates that SA is likely to have the 10th highest level of childhood obesity in the world by 2030, with approximately 28% of children aged 5 to 19 obese in 2030 [36].
Additionally, a review of all adult dietary studies done in SA between 2000 and 2015 found that there is still a high prevalence of micronutrient deficiencies. High food prices and limited availability of nutrient-dense foods in townships and poorer urban centers are thought to be contributing factors [37]. A predominantly carbohydrate-based diet with low nutrient density is common in SA [38,39,40,41].
The nutrition policy priorities in SA are clearly articulated in the National Department of Health’s Strategy for the Prevention and Control of Obesity in SA 2015–2020: ‘create an enabling environment that supports the availability and accessibility of healthy food choices in various settings’. It highlights the need for the development of norms and standards on sugar and fat content of ultra-processed foods and also notes the importance of FOPL and consideration of ethical marketing of food to children [10]. Currently, draft regulation [14] in SA includes an NPM to assess health and nutrition claims, but no NPM has been developed for SA to use in restrictive food policies, such as FOPL and marketing to children.
Although those at risk for developing NCDs and children (with rapidly increasing obesity prevalence) will benefit most from the potential restrictive food policies that the NPM can underpin, these food policies are broadly applicable across different population groups and age groups. Due to heterogeneity in nutritional requirements across different life stages, it is recommended that adult dietary guidelines be used to guide NPM criteria [33]. The only age group excluded from NPMs is children below the age of six months, where exclusive breastfeeding is recommended and protected by existing regulation in SA [59].
Recommendation
The purpose of the NPM should be to identify unhealthy, processed packaged foods that result in poor health outcomes. The appropriate target population for the NPM is all adults and children above the age of six months.
3.2. Nutrients and Other Food Components to Include
Literature review indicated that countries implementing regulations (with mandatory NPM applications) have predominately focused on negative nutrients of concern linked to poor health outcomes, which they restrict through policies such as FOPL, marketing restrictions and regulations in the school environment [18]. This is because generally, using an NPM that includes both ‘nutrients to limit’ (such as salt, sugar and saturated fat which are harmful to health) and ‘nutrients to encourage’ (such as fiber, fruit and vegetables, nuts and legumes which are beneficial to health), in a restrictive regulatory environment is limited and becomes complicated to implement. To date, only Israel has added another, positive FOPL in the form of a green logo for healthy, minimally processed foods [60] that do not contradict their warning labels.
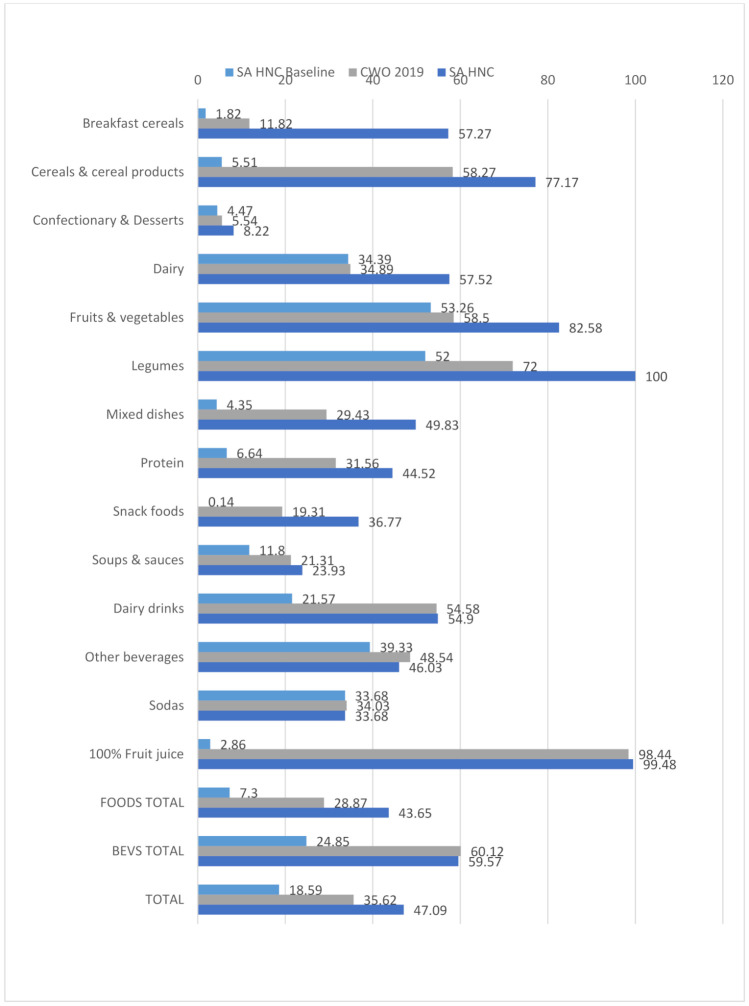
From the results in Figure 2 and Table 1, it is apparent that the additional points awarded for ‘nutrients to encourage’ in the final SA HNC NPM give rise to a more lenient score than the initial SA HNC NPM baseline score, which only includes ‘nutrients to limit’, resulting in more compliant products in all categories, except for sodas, where it does not change. These results highlight the leniency that ‘nutrients to encourage’ introduces into the final score of an NPM. Of the models included in this study, the most lenient model was the SA HNC NPM, with 47% of foodstuffs compliant, and the SA HCN Baseline model was the strictest, with 19% compliance.
Figure 2.
Proportion of SA packaged food and beverage products compliant with the SA HNC, SA HNC baseline and CWO 2019 NPM criteria by groups.
Table 1.
Qualitative table reflecting how the addition of ‘nutrients to encourage’ affects NPM leniency, in terms of the proportion of products compliant (i.e., that fall beneath the thresholds and are not deemed ‘unhealthy’).
| Highlighted Food Categories | SA HNC Baseline (Only ‘Nutrients to Limit’ | CWO 2019 (Only ‘Nutrients to Limit’) | SA HNC (Nutrients to Limit and Encourage) | |
|---|---|---|---|---|
| Categories where the addition of ‘nutrients to encourage’ may potentially be beneficial | Dairy Drinks | 21.57% compliant with criteria | 54.6% compliant with criteria | 54.9% compliant with criteria |
| In this category one would want to mitigate the effects of lactose, a carbohydrate naturally present in milk, which contributes to the total sugar score. Here the addition of ‘nutrients to encourage’ potentially assists; although given the differences in algorithms, the CWO criteria (which only includes ‘nutrients to limit’) has a similar compliance level. | ||||
| Categories where the addition of ‘nutrients to encourage’ allows for a more lenient score | Breakfast cereals | 1.8% compliant with criteria | 11.8% compliant with criteria | 57.3% compliant with criteria |
| Dairy (food) | 34.39% | 34.89% | 57.52% | |
| Fruits and vegetables | 53.26 | 58.5% | 82.58% | |
| Legumes | 52% | 72% | 100% | |
| These categories highlight where the more lenient score of the SA HNC NPM causes contestation due to the addition of protein, fiber, FVNL (fruit, vegetables, nuts and legumes) points. In all of these categories the SA HNC NPM scored at least 23% higher than for the CWO 2019. Although these foods may include healthy components, the impact of undesirable ingredients cannot be negated by ‘nutrients to encourage’. Most of the fruits, vegetables and legumes that are restricted by the CWO 2019 contain high levels of sugar or sodium. The significant change in compliance due to the addition of ‘nutrients to encourage’ is seen in the difference in scores between the baseline and final SA HNC NPM. | ||||
| Categories that are strict regardless of nutrients to encourage or limit | Confectionery & Dessert | 4.47% | 5.54% | 8.22% |
| Soda | 33.68% | 34.03% | 33.68% | |
| These categories contain a large number of unhealthy products that are linked to poor health outcomes. Even when ‘nutrients to encourage’ are added, they score poorly across different NPMs. The addition of ‘nutrients to encourage’ does not result in a more lenient score. | ||||
A review of the literature indicates that trans-fat, saturated fat, added or free sugar and sodium are harmful to health, and contribute to the obesity and NCD epidemic [61,62,63,64,65,66]. According to a review of NPMs used in government-led nutrition policies, all NPMs (n = 78) included ‘nutrients to limit’. The most common nutrients of concern identified in these NPMs were sodium, saturated fat and total sugar [6]. Table 2 provides the mean nutrient content of unhealthy nutrients of concern in packaged foods in SA.
Table 2.
Mean content of nutrients of concern in packaged foods in SA per 100 g (2018).
| Energy (kJ) | Total Sugar (g) | Free Sugar (g) | Total Fat (g) | Saturated Fat (g) | Trans Fat (g) | Sodium (mg) | Contains NSS n (%) | |
|---|---|---|---|---|---|---|---|---|
| Breakfast cereals n = 110 |
1588.2 | 17.2 | 16.3 | 8.9 | 2.9 | 0.03 | 210.6 | 0 (0) |
| Cereals & cereal products n = 254 |
989.9 | 3.1 | 3.2 | 6.1 | 2.4 | 0.11 | 338.5 | 8 (3.2) |
| Confectionary & dessert n = 1119 |
1559.8 | 38.4 | 35.1 | 14.0 | 7.7 | 0.12 | 142.5 | 143 (12.8) |
| Dairy n = 791 |
766.5 | 6.4 | 3.5 | 12.6 | 8.8 | 0.33 | 322.1 | 70 (8.9) |
| Fruits & vegetables n = 196 |
677.4 | 29.8 | 15.2 | 2.1 | 1.2 | 0.02 | 41.9 | 0 (0) |
| Vegetables n = 510 |
315.9 | 3.3 | 3.3 | 3.6 | 0.7 | 0.03 | 392.6 | 5 (1.0) |
| Legumes n = 100 |
342.7 | 2.1 | 2.1 | 0.8 | 0.2 | 0.03 | 290.3 | 0 (0) |
| Mixed dishes n = 299 |
813.0 | 3.3 | 3.2 | 9.3 | 4.0 | 0.17 | 429.2 | 10 (3.3) |
| Protein n = 602 |
787.4 | 1.4 | 1.4 | 9.9 | 3.5 | 0.13 | 826.0 | 18 (3.0) |
| Snack foods n = 699 |
2059.4 | 6.8 | 6.1 | 27.9 | 7.7 | 0.06 | 476.8 | 58 (8.3) |
| Soups & sauces n = 610 |
676.1 | 9.7 | 9.6 | 11.2 | 2.2 | 0.07 | 746.3 | 35 (5.9) |
| Food total n = 5290 |
1072.8 | 13.4 | 11.6 | 12.4 | 5.1 | 0.12 | 411.2 | 347 (6.6) |
| Dairy drinks n = 306 |
255.1 | 6.0 | 4.9 | 1.8 | 1.1 | 0.07 | 43.3 | 58 (19.0) |
| Other beverages n = 478 |
116.7 | 5.8 | 4.0 | 0.1 | 0.08 | 0.004 | 13.4 | 213 (44.6) |
| Sodas n = 288 |
125.1 | 6.9 | 6.9 | 0.04 | 0.02 | 0.01 | 18.6 | 160 (55.6) |
| 100% fruit juice n = 385 |
190.0 | 10.4 | 6.0 | 0.05 | 0.02 | 0.0 | 9.5 | 1 (0.3) |
| Beverage total n = 1457 |
160.7 | 7.2 | 5.1 | 0.45 | 0.3 | 0.01 | 19.7 | 432 (29.6) |
| Food & beverage total n = 6747 |
875.7 | 12.1 | 10.7 | 9.8 | 4.1 | 0.09 | 326.6 | 779 (11.5) |
3.2.1. Sugar
International guidelines recommend the restriction of free or added sugar (rather than total sugar) as this is the sugar that is harmful to health [67]. Please refer to Supplementary Table S5 for the definitions of sugar used in this paper. Intrinsic sugar is not believed to be as harmful as free sugar [67]. These differences can be seen in Table 2 for the dairy and fruit categories, where the total and free sugar content differ significantly due to the high intrinsic sugar levels.
WHO dietary guidelines recommend that a maximum of 10% of daily energy should be derived from total sugar and 5% from free sugar [67]. Based on a reference energy intake of 8400 kJ (or 2000 kCal) per day, this equates to 1 g of total sugar per 168 kJ and 1 g of free sugar per 336 kJ consumed in a day. As the average energy content per 100 g of foods in this sample is 1072.8 kJ (see Table 2 above), in order to align with WHO dietary guidelines, total sugar should ideally be below 6.4 g/100 g and free sugar should be below 3.2 g/100 g. Similarly, for beverages (with an average energy content of 160.7 kJ/100 mL), total sugar should be below 1 g/100 mL and free sugar below 0.5 g/100 mL. However, the sugar content of foods in this sample far exceeds the sugar recommendations in the WHO dietary guidelines, as 21.2% of energy (13.4 g/100 g) is attributed to total sugar and 18.4% (11.6 g/100 g) to free sugar. Although beverages contain less sugar per 100 mL than foods, sugar contributes most of the energy in beverages, at 76.2% (7.2 g/100 mL) and 54.0% (5.1 g/100 mL), respectively, for total and free sugar.
As the chemical structure of free (or added) sugar is the same as total sugar they cannot be differentiated objectively through laboratory tests [68]. One could require manufacturers to report the added sugar on the NIP (as in the USA), although this is reliant on the manufacturer being trustworthy about the recipe composition. Alternatively, the approximate amount of free or added sugars in foods can be calculated [52,69]. However, this approach requires assumptions to be made, is time-consuming and open to misinterpretation. In the resource-limited LMIC setting, this is not a suitable method to use. The total and added sugar challenge is not new. When the UK Office of Communications (Ofcom) NPM was being developed, added sugar was proposed, but due to the technical difficulties involved in analyzing added sugars, total sugar was selected instead [70]. Numerous other countries, including Chile [7], Israel [50] and Peru [49], have opted to use total sugar in their regulations.
In order to work around this, Chile recommends applying the total sugar cut-point of the NPM only to those foods that have added sugar, salt or saturated fat [54]. This is to prevent foods such as fresh fruit from receiving a classification of ‘high in sugar’ (as one would not want to accidentally restrict a healthy product, such as fresh fruit, through the application of an NPM). However, this allows 100% fruit juice to ‘pass’ the CWO 2019 NPM criteria as it is considered free, but not added, sugar (see Table 3 below in which 99 percent of 100% fruit juices are compliant with the CWO 2019 sugar criteria).
Table 3.
Number of packaged SA products that would be regulated by the CWO 2019 NPM criteria (overall, for sugar, sodium, saturated fat and energy).
| Number Regulated (Overall) |
Number Regulated for Sugar |
Number Regulated for Sodium |
Number Regulated for Saturated Fat |
Number Regulated for Energy |
Number Regulated for Only Energy |
|
|---|---|---|---|---|---|---|
| Breakfast cereals n = 110 |
97 | 74 | 16 | 31 | 94 | 11 |
| Cereals & cereal products n = 254 |
106 | 1 | 71 | 34 | 47 | 10 |
| Confectionary & dessert n = 1119 |
1057 | 997 | 83 | 600 | 912 | 12 |
| Dairy n = 791 |
515 | 262 | 246 | 56 | 74 | 3 |
| Fruits & vegetables n = 706 |
293 | 129 | 163 | 13 | 29 | 8 |
| Legumes n = 100 |
28 | 0 | 28 | 0 | 3 | 0 |
| Mixed dishes n = 299 |
211 | 14 | 177 | 113 | 36 | 3 |
| Protein n = 602 |
412 | 6 | 390 | 88 | 88 | 3 |
| Snack foods n = 699 |
564 | 95 | 388 | 394 | 552 | 35 |
| Soups & sauces n = 610 |
480 | 206 | 416 | 106 | 244 | 12 |
| Food total n = 5290 |
3763 | 1784 | 1978 | 1435 | 2079 | 97 |
| Dairy drinks n = 306 |
139 | 135 | 4 | 0 | 39 | 1 |
| Other beverages n = 478 |
246 | 243 | 1 | 0 | 3 | 2 |
| Sodas n = 288 |
190 | 190 | 0 | 0 | 2 | 0 |
| 100% juice n = 385 |
6 | 3 | 3 | 0 | 0 | 0 |
| Beverage total n = 1457 |
581 | 571 | 8 | 0 | 44 | 3 |
| Food & beverage total n = 6747 |
4344 | 2355 | 1986 | 1435 | 2123 | 100 |
This is of some concern, as long-term overconsumption of fructose (the sugar found in fruit juice) may result in cardiovascular and metabolic diseases [71] as well as increased all-cause mortality risk [72]. It has been argued that efforts to reduce sugar consumption need to be extended to 100% fruit juice [72]. In 2019, the Indian Academy of Pediatrics recommended fruit juice should not be given to infants and young children below the age of two years, and that very limited amounts should be given to older children [73].
Given the difficulties of measuring added or free sugar, it is recommended that SA make use of total sugar in the NPM. However, unlike Chile, where added sugar is used as a qualifying criterion, it is recommended that the SA NPM use free sugar—including any form of fruit juice concentrate (e.g., pulp)—rather than added sugar in the qualifying criteria.
3.2.2. Fat
Consideration should be given to total fat, saturated fat and trans-fat.
Total Fat: The WHO recommends an intake of between 15 and 30% of total energy from fat [74]. Recently, the US dietary guidelines removed total fat as a nutrient of concern to focus instead on unhealthy saturated fats [75].
Total fat has not been identified as appropriate to include in this NPM because fat is not harmful to health per se. Indeed, certain components, such as mono- and polyunsaturated fatty acids are beneficial to health and provide protection against certain NCDs, such as cardiovascular disease [76,77,78,79].
Saturated fat: The WHO recommends that less than 10% of the total daily energy intake should be from saturated fats [80]. The Codex Alimentarius non-communicable disease guidelines for saturated fat (NRV-NCD) recommend that saturated fatty acid intake should not exceed 20 g/day, based on a reference energy intake of 8400 kJ (or 2000 kCal) [81].
The Heart and Stroke Foundation SA, as well as international organizations such as the American Heart Association and Heart UK, recommend limiting saturated fat intake due to the risk of elevated cholesterol levels and the increased risk of heart disease. As saturated fat is known to be harmful to health [76,77,78], it is recommended that it should be included in the NPM.
Trans-fat: In line with WHO recommendations, SA implemented a regulation on trans-fat, R127, in 2011 [16]. The WHO guideline recommends that less than 1% of the total energy intake be derived from trans-fat [80]. The R127 effectively deals with trans-fats and prohibits foods sold in SA to have more than 2 g of trans-fat per 100 g of fats and oils. The analysis of the mean trans-fat content of packaged foods in SA (Table 2) indicates that the mean trans-fat level is 0.09 g, which is below 1 g per 100 g. Thus, it is unnecessary for the NPM in SA to include trans-fat as the country has already effectively dealt with this harmful nutrient through regulation R127 of 2011.
3.2.3. Sodium
Salt should be restricted to less than 5 g per day, and sodium to less than 2 g per day according to WHO Guidelines [82]. Codex Alimentarius recommends that sodium intake should not exceed 2000 mg per day, based on a reference energy intake of 8400 kJ (or 2000 kCal) to prevent NCDs. This translates to 1 mg per 4.18 kJ [81]. SA introduced sodium regulations (R214/2013 [15]) in two phases, from 2016 to 2019. The intended purpose of this regulation is to reduce sodium levels in foods with the aim to reduce hypertension, and is category specific and does not target all foods [83].
As the mean energy content in SA packaged foods (Table 2) is 1072.8 kJ/100 g, one would expect the sodium content to be below 258 mg/100 g to align with recommended dietary intake [81,82]. However, the mean sodium content is high, at 411 mg/100 g. There are four food categories: mixed dishes; protein; snack foods; and soups and sauces, which remain particularly high in sodium. Unlike the trans-fat regulations, which adequately address trans-fat by virtually removing it from the SA market, sodium needs to be included in the NPM as certain products have excessive quantities of sodium.
3.2.4. Energy
Although Chile [7] and the WHO African Region [30] have opted to include energy as a criterion in their NPMs, it is postulated that packaged and processed foods high in energy are also high in sugar, saturated fat and/or sodium. Further, there is an expectation that by including criteria for sugar, saturated fat and sodium, most foods high in energy will be addressed.
This was found to be true when applying the thresholds for the CWO 2019 NPM to the SA packaged food supply (Table 3). Very few products were excessive only in energy. Of the 4344 products regulated, only 100 products (or 2.3%) were above the cut-off for energy, but no other nutrient. The other 97.7% of products were regulated for sugar, sodium and/or saturated fat. This is in line with Camacho and Ruppel [84] who argue that by focusing on the calorie balance (total energy) in policies, one gives the food industry a convenient exit strategy so that they can avoid engaging with the obesity crisis. Diet composition, particularly in the case of processed foods, is potentially more harmful to health than the overall calorie balance. In countries facing high levels of stunting, wasting and micronutrient deficiencies, NPMs that focus on energy may be problematic [85].
3.2.5. Non-Sugar Sweetener
For this paper, following the NPM for the WHO Africa Region [30], the term non-sugar sweeteners (NSS) will be used (definition in Supplementary Table S5). The use of NSS, such as artificial sweeteners and polyols, in the food supply is becoming more commonplace and has become central to sugar substitution [86]. They are consumed not only through foods, but also in medicines, food supplements and other products such as toothpaste [87]. NSSs are among the most widely used food additives globally [88]. This is partly because consumers are interested in reducing sugar intake [89] and also because the introduction of food policies such as a sugary beverage tax or front-of-pack labelling has incentivized the food industry to reformulate and replace sugar with NSS [90,91] instead of reformulating into products that are less sweet. Table 2 indicates that 55.6% of sodas (and 29.6% of all packaged beverages) and 12.8% of confectionery (and 6.6% of all packaged foods) in SA already contained NSS in 2018 (before the implementation of South Africa’s sugar-sweetened beverage tax known as the Health Promotion Levy).
Because of the increased use of NSS, it is important that a thorough evaluation be conducted of its risks and benefits before advocating for, or discouraging, its use [92]. Although numerous studies and systematic reviews of these studies have been conducted on the topic of safety in the use of NSS, there is no consensus among researchers. One of the challenges is that many different NSSs exist and new NSSs are constantly becoming available [89].
The sweetness level of different NSSs also differs [93,94]. They do not all have the same physiological effect [95], and quantities of NSS intake are largely unknown. Worldwide, only two countries (Chile [7] and very recently Saudi Arabia [96]) include quantities of each type of NSS on the NIP. This makes it nearly impossible to accurately investigate consumption volumes across the world as the data are simply not available.
In Chile, food companies are reformulating products to replace sugar (which is regulated) [91] with NSS (which is not regulated). More than half (55.5%) of all packaged products in Chile now contain at least one NSS, making it difficult to select NSS-free options [97]. In SA, since the introduction of the HPL, there has been growing evidence of product reformulation [98], and although NSSs have yet to be investigated, many brands have reformulated sugar down from above 10 g/100 mL to less than 5 g/100 mL [99]. It is likely that much of this sugar has been replaced with NSS.
It is impossible to set a cut-point for NSS, unlike for other nutrients of concern such as sodium, saturated fat or sugar which have evidence-based cut-points, as there is currently inadequate evidence to identify an NSS cut-point. However, there is growing concern around children’s exposure to NSS and its effects on their sweetness preferences later in life [100,101] and gut health [102]. The impact of prolonged use remains unclear [102], and recently, NPMs targeting children have recommended that children’s exposure to sweeteners be restricted [30,103].
As food policies should be proactive in protecting the health of the population, the evidence currently available suggests that it is wise to regulate the use of NSS or at the very least, require clear information about its presence and amounts in food products to monitor its presence better. After all, the purpose of food policies is to encourage a shift towards the consumption of more whole, unprocessed foods rather than alternative, ultra-processed foods.
3.2.6. Recommendation
An easy to operationalize NPM aimed at reducing the demand for processed foods linked with NCDs and obesity should include ‘nutrients to limit’, and exclude ‘nutrients to encourage’. Saturated fat, sodium, non-sugar sweetener and total sugar (with free sugar, not added sugar, used as the qualifying criteria when assessing the inclusion criteria of the NPM) have been identified as appropriate to include in the NPM.
3.3. Selecting the NPM Type, Criteria and Base
Base unit of measure: WHO and Codex Alimentarius dietary guidelines provide guidance with regard to nutrients of concern in reference to their contribution to the percentage of total energy [67,80] or as a nutrient reference value that should not be exceeded per day [81,82]. However, these refer to the total daily intake per person, and it is not easy to practically implement because different people have different energy requirements, and packaged foods represent only a portion of overall daily intake. The draft WHO FOPL guidelines recommend NPMs be developed using a per 100 g approach [53]. The portion size or per serving approach results in several challenges as different age groups should have different portion sizes, and consumption patterns differ among individuals [70]. Portions are easier for the food industry to manipulate, and often represent portion sizes that are ‘healthier’ but not realistic in relation to the package size or the amount that people eat. Consistent with Codex Alimentarius guidelines [81], nutritional information in SA is displayed in a per 100 g/100 mL format for foods and liquids. Considering the pros and cons of various options available (Table 4), and given the current 100 g/100 mL format used in SA, continuing in the same manner would be practical.
Table 4.
Pros and cons of different base approaches.
| Pros | Cons | |
|---|---|---|
| Per 100 g/100 mL | Simple to conceptualize and easy to compare foods Used on nutrition information panels on SA packaged foods |
Certain foods are eaten in very small quantities (e.g., oil) while others are consumed in large quantities (e.g., beverages) |
| Per 100 kJ/% total energy | Allows for food consumed in smaller quantities to be put into context | Difficult to make sense of individual food items that do not represent total energy intake for the day |
| Per serving | Recognizes that portion sizes of different food types vary significantly, and if eaten in large quantities, will contribute more to nutritional intake than smaller amounts | Serving sizes are determined by the food producer and as a result vary significantly, even within a food category Easy to manipulate serving sizes to appear ‘healthier’, but these are not representative of the amount usually consumed |
Across the board vs. food category specific: An across-the-board approach establishes consistent criteria which limit the risk of misinterpretation or incorrect classification [6]. It is not resource-intensive and is straightforward to implement. However, as all foodstuffs are treated in the same manner, regardless of their inherent nutrient composition, it could suppress reformulation within a category if changes are needed for most of the foods in that category. A category-specific approach, such as is used for marketing restrictions by the WHO Africa Region NPM [30], allows for criteria that are specific to the nutritional composition of different types (or categories) of food; and the criteria can be informed by the nutritional content of existing foods in the category [6]. However, the numerous categories with different thresholds make it difficult for regulators to implement and it potentially allows leeway for the food industry to manipulate within category thresholds.
It is important to consider the context, and to weigh up robustness with the ability to apply it appropriately when selecting the most appropriate NPM [6]. To date, all countries that have adopted a mandatory warning label model have focused on only two categories (food and non-alcoholic beverages). This includes Chile [104], Israel [50], Peru [49] Uruguay [105], Mexico [106] and most recently, Brazil [107]. To ensure a simple, easy-to-implement approach, a category-specific approach is not appropriate for the SA setting as it is more resource-intensive (both for implementation and evaluation). An across-the-board approach is recommended for the NPM.
Threshold vs. continuous: A threshold-based approach has been successfully implemented in the mandatory, restrictive food policies of a number of countries, including Israel [50], Chile [104] and Peru [49]. It is administratively simple, as no calculation or comparisons need to be made before classifying a food (it either meets the cut-point or it does not). Previously, when the generally encouraged model was one that included both nutrients to encourage and limit, a continuous or scoring system was well justified. The rationale was that foods were composed of many nutrients and a single cut-off would result in the loss of valuable information [70]. However, this argument only holds true when both nutrients to encourage and restrict are considered or when the NPM is being used to underpin a positive logo that focuses only on whole, minimally processed foods, as in Israel [60].
Continuous models require a number of different calculations to be performed and can be human resource-heavy. Ultimately, when used together with an FOPL or health claim system, a threshold is still used to determine whether a product can carry a claim or not, or whether it is red, yellow or green. In this sense, a scoring approach is always used in conjunction with a threshold.
Recommendation
The SA NPM should use a straightforward approach and an across-the-board, threshold-based approach, that is applied to all packaged foodstuffs and uses a ‘per 100 g’ base for solids and the ‘per 100 mL’ base for liquids.
3.4. Thresholds to Use
The most appropriate and relevant nutrient cut-points are selected based on the recommendations made in steps one through three.
Where possible, it is recommended to adapt existing NPMs to make them context-specific, rather than inventing them from scratch due to the immense time and resources required to develop an NPM [33]. Based on the above considerations, the CWO 2019 NPM [7] appears to be the most suitable NPM to adapt. For the purpose of this paper, other NPMs considered include Mexico [106], Peru [49], Israel [50], PAHO [52], WHO Africa Region [30] and the FSANZ (SA HNC) [14] models (Supplementary Table S6). The PAHO NPM considers the percentage-of-energy approach, rather than a per 100 mL or per 100 g approach, and it is therefore not appropriate. The SA HNC NPM considers both nutrients to encourage and limit, which is difficult to implement and does not meet the purpose as discussed earlier. The WHO African Region NPM uses a category-specific approach rather than an across-the-board approach, which is why it has not been selected. Compared to other NPMs considered, the CWO 2019 NPM most appropriately meets requirements based on recommended components for the SA FOPL NPM. It is a mandatory, threshold-based, across-the-board model using a ‘per 100 g’ or ‘per 100 mL; approach and it focuses on negative ‘nutrients to limit’. A number of other countries have already adopted this NPM into their regulation, including Peru [49] and Israel [50]. Furthermore, the Chilean NPM has shown some promising results in the food policies it is underpinning in Chile [44,45,46,47,48].
The cut-points developed for the CWO 2019 NPM were based on nutrient composition analysis of 358 whole, unprocessed foods, using the USDA nutrient database [54]. No similarly comprehensive database is available for SA. The SA FCT includes 183 whole, unprocessed foods (110 foods with nutrients analyzed in SA, 65 based on the USDA FCT and eight on FCTs from other countries), resulting in an SA-specific sample size 69.3% smaller than that used for Chile. Given this, it was deemed appropriate to adopt the Chilean cut-points as-is for sodium, saturated fat and total sugar. This approach was also used by Israel and Peru [49,50].
Recommendation
This analysis indicates that the following cut-points (Table 5) be used for the proposed NPM, that have been adapted from the Chilean approach.
Table 5.
Final proposed cut-points for an NPM suitable to be used in restrictive food policies in SA.
| Solid Food (g) Cut-Points | Liquids (mL) Cut-Points | ||
|---|---|---|---|
| Sodium mg/100 g | 400 mg | Sodium mg/100 mL | 100 mg |
| Total sugar g/100 g | 10 g | Total sugar g/100 mL | 5 g |
| Saturated fat g/100 g | 4 g | Saturated fat g/100 mL | 3 g |
| Non-sugar sweetener | Contains any | Non-sugar sweetener | Contains any |
It is recommended that this NPM, if used, should be applied to all packaged foods and non-alcoholic beverages in SA containing any of the following:
Free sugar;
Added sodium;
Added saturated fat;
Non-sugar sweetener.
4. Discussion
Given the proposed NPM’s ability to identify unhealthy products, it is appropriate for use to underpin restrictive food policy in South Africa. This could include FOPL regulations (which indicate packaged products high in unhealthy nutrients of concern), marketing restrictions, taxation policies and policies that restrict unhealthy foods in schools, hospitals and other government institutions. Elsewhere in the world these restrictive policies have been successful initiatives towards promoting a healthier food environment by supporting a move away from unhealthier food choices. [108].
The use of an evidence-based NPM built on a scientific basis that supports non-discriminatory policy measures is necessary in the international trade context [109,110], where limitations imposed by international trade and investment agreements have been found to impede public health policies [111]. A scientific basis of measure in policy development ensures trade and investment agreements are respected and do not place undue limitations on public health priorities [109,112]. Without adequately researched, evidence-based regulations, governments run the risk of being forced to retract regulations due to trade and investment agreements, as was seen with the turkey tail ban in Samoa [113].
Taking the resource limitations of SA into account, an important consideration in the development of this NPM was straightforward classification, implementation and evaluation for any NPM that is used in national regulations. For example, in our evaluation of the SA HNC NPM, the FVNL score had to be calculated manually for each product as it is not routinely reported on NIPs in South Africa. This was a time-consuming task, performed by nutrition experts. From a monitoring and evaluation standpoint, it is not a feasible assessment approach (due to the time and skills required) and it is not advisable to include an FVNL score in an NPM used in regulation in South Africa without mandating an FVNL nutritional declaration on the product packaging. This principle applies to all food components included in an NPM. It would be prudent, if including NSS in the NPM, to follow Chile [7] and Saudi Arabia [96], and strengthen current sweetener regulations in South Africa [114] by requiring mandatory reporting of NSS quantities on nutritional packaging. One of the newer approaches to NSS policy can be found in Mexico [106]. Their new law on front-of-package profiling includes an information box that states: ‘Contains non-sugar sweeteners, not recommended for children’. This is placed in a large black box with white characters. They originally intended having a warning label for NSSs but, based on industry objections and World Trade Organization concerns, they shifted to an information box which has passed legal scrutiny.
As with most NPMs, the proposed NPM cut-points are applicable to foods and beverages in an as-consumed form. Should an NPM be used in regulations in SA, food labels should include the nutritional composition per 100 g ‘as-consumed’ alongside the ‘as-packaged’ composition if reconstitution through home preparation is required (e.g., concentrated fruit drinks). Manual calculations are time-intensive and may result in errors. Future regulations should stipulate that, should the ‘as-consumed’ information be missing, the NPM criteria will be applied to the ‘as-packaged’ information to encourage the ‘as-consumed’ information to be included. In addition, should nutritional information be unavailable on the product packaging (or missing for certain nutrients of concern), then by default the product should be assessed as ‘excessive’ in the nutrient of concern for which there is no information.
Distinguishing between healthy and unhealthy foods to regulate through policy is challenging for policy makers as the food industry contests definitions and argues that they are vilifying foods by differentiating them. Because of this, it is important that the purpose of the NPM is clearly understood. Recently, the argument has been made that NPMs in countries with high levels of malnutrition and stunting should include ‘nutrients to encourage’ [85]; however, this was not identified as suitable for the purpose of our proposed NPM. Positive components do not neutralize the negative health consequences of consuming the unhealthy components in the same product. The SA HNC NPM included in this analysis was developed to allow health and nutrition claims, which focus on ‘nutrients to encourage’. However, research by authors involved in the development of the SA HNC NPM concluded that the NPM was more lenient than other NPMs when marketing restrictions were applied to foods high in fat, sugar and salt [27]. This supports concerns that the addition of (healthy) ‘nutrients to encourage’ can confuse the matter when trying to identify unhealthy foods [23]. The approach of classifying foods as ‘healthy’ or ‘healthier’ has allowed the industry to add nutrients or additives (e.g., isolated or synthetic non-digestible carbohydrates that count towards fiber) to otherwise unhealthy products [115]. ‘Healthier does not necessarily mean healthy per se, and the notions of ‘better than’ may mislead consumers away from what is best’ [70]. An NPM with the purpose of identifying unhealthy products to restrict should thus only include ‘nutrients to limit’.
However, there is still space to consider ‘nutrients to encourage’. Work has already been done in SA in the development of a positive FOP logo [116] and an approach similar to Israel could be considered, where additional criteria are used to identify healthy, minimally processed foods that may carry a positive FOP logo [60]. Although we have not identified the current SA HNC NPM proposed in draft R429/2014 as appropriate for restrictive food policy, it could still be useful for its intended purpose—to regulate health and nutrition claims on packaged foods. It is possible that the two NPMs could work in tandem; permitting foods that are not identified as unhealthy via the SA FOPL NPM to be assessed for eligibility of health and/or nutrition claims via the SA HNC NPM. If this approach is considered, we recommend that it be designed carefully to complement the restrictive NPM, only permitting positive messaging on products not carrying an FOP warning label. Having both a warning label and positive message could provide a mixed message and confuse consumers.
Limitations and Strengths of the Study
No NPM is without limitations. Consumption frequency, as part of normal daily dietary intake was not considered, and instead we focused on the nutrient profile of the foods themselves, rather than their role in the diet. Nationally representative dietary intake data in SA are sparse, as nutrition surveys are not regularly done, nor are they representative of the population [37]. This makes assessing dietary intake in the country challenging.
Similarly, certain assumptions, which may not have always been accurate, had to be made as free sugar and FVNL values were not available. Data were collected in the Western Cape Province of SA, at big retail outlets, so it is possible that products that only appear in certain locations or shops were excluded.
A large number of products were excluded from analysis as the nutritional content of packaged foods could not be assessed for compliance if they did not have an NIP, which is not a legal requirement in SA. To overcome this, the SA government could regulate NIPs on all packaged foods as mandatory. The information this panel provides can be used to assess compliance with various food regulations. In accordance with Codex guidelines [81], the panel should be clear and easy to understand and presented in a standardized manner.
After an NPM has been proposed, it is important that it be validated and tested for appropriateness by applying it to the local food supply chain [117,118]. This was outside the scope of this paper, although it is an essential step before an NPM can be accepted as appropriate for a certain setting. This has been mitigated to some extent by consulting with dietitians on the nutrient thresholds in South Africa, following the approach used in Chile. The validation study has been submitted elsewhere (currently under consideration for publication).
This study makes use of a recently collected dataset, which, to our knowledge, is the most comprehensive dataset of nutritional information on packaged foods in South Africa. A systematic process was followed to assess various NPM options using available data and literature, as well as existing regulations. Cultural dimensions are often overlooked in policy making processes. Adapting an NPM for a specific context, as has been done for South Africa throughout this NPM development process, is critical to ensure context-specific solutions.
5. Conclusions
This article proposes a fit-for-purpose NPM that is suitable to use in restrictive food policies in SA. It is adapted from the Chilean NPM and includes criteria for sodium, total sugar, saturated fat and non-sugar sweetener. It has the potential to be the foundation for FOPL and child-directed marketing regulations, broader taxation policies and to act as a guide for products to prohibit them from being sold or served in schools, hospitals or other government facilities. Although implementing these policies will not resolve the obesity and NCD crisis in the country, they will be an additional step in the fight. It has the potential to inspire other LMIC in Africa and can be scaled up for use elsewhere.
Acknowledgments
We also wish to thank Barry Popkin for reviewing early versions of the manuscript, Donna Miles for exceptional assistance with data management, Bridget Hollingsworth for research assistance and Alice Khan for superb data quality control. We also want to thank Lucrechia McAllen, Vashika Chibba, Thandokazi Mahuzi and Kholiswa Manisi, who conducted nutrition label data collection in 2018, and Stephanie Rohrs and Erica Yeh who assisted with the SA FCT classification of foods. For the software used to collect and enter the nutrition label data, we thank the National Center for Advancing Translational Sciences (NCATS), NIH UL1TR001111. Funders and individuals mentioned in this acknowledgement had no role in the data analyses, interpretation and writing of this paper. The findings and recommendations reflected this paper are those of the authors only.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/nu13082584/s1, Figure S1: Flow diagram representing initial and final datasets, and reasons for exclusion, Table S1: Primary data collection methodology, Table S2: Number and proportion of products included in analysis (N = 6747), overall and by food category, Table S3: Non-sugar sweetener (NSS) search terms used to identify NSS ingredients in products, Table S4: Number and proportion of food and beverages (N = 6747) in the SA marketplace (2018), overall and by food category classified as ultra-processed according to the NOVA classification system, Table S5: Definitions of sugar and non-sugar sweetener used in this paper, Table S6: Characteristics of nutrient profiling models considered for inclusion.
Author Contributions
T.F., A.-M.T., S.W.N. and E.C.S. conceptualized and planned the methodology for the manuscript; validation was performed by T.F., J.O., S.W.N. and E.C.S.; T.F. and J.O. took care of data curation; M.B. assisted with initial investigation; formal analysis, investigation and original draft preparation were carried out by T.F.; review and editing were performed by T.F., A.-M.T., S.W.N., J.O., M.B. and E.C.S.; visualization was performed by T.F.; project administration was performed by J.O.; funding acquisition and resources were handled by S.W.N. and E.C.S.; and E.C.S. supervised the project. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Bloomberg Philanthropies, with additional support from the US NIH grant number CPC P2C HD050924. Student scholarship was funded by International Research Development Canada (IDRC) project number 108425-001.
Institutional Review Board Statement
The study was approved by the Humanities and Social Science Research Ethics Committee of University of the Western Cape (ethics reference number HS19/6/3 29 July 2019).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data analyzed for this paper form part of a primary project which is currently being written up in other publications. Data used for this paper will therefore be available upon request and granted for replication purposes. Data are available from the UNC Carolina Digital Repository (https://cdr.lib.unc.edu/). For data inquiries, please contact Donna Miles (drmiles@email.unc.edu) and Jessica Ostrowski (jessica.ostrowski@unc.edu).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wells J.C., Sawaya A.L., Wibaek R., Mwangome M., Poullas M.S., Yajnik C.S., Demaio A. The double burden of malnutrition: Aetiological pathways and consequences for health. Lancet. 2020;395:75–88. doi: 10.1016/S0140-6736(19)32472-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Food and Agriculture Organization. World Health Organization . The Nutrition Challenge: Food System Solutions. FAO; Rome, Italy: 2018. [Google Scholar]
- 3.Hawkes C., Smith T.G., Jewell J., Wardle J., Hammond R.A., Friel S., Thow A.M., Kain J. Smart food policies for obesity prevention. Lancet. 2015;385:2410–2421. doi: 10.1016/S0140-6736(14)61745-1. [DOI] [PubMed] [Google Scholar]
- 4.Sacks G., Rayner M., Stockley L., Scarborough P., Snowdon W., Swinburn B. Applications of nutrient profiling: Potential role in diet-related chronic disease prevention and the feasibility of a core nutrient-profiling system. Eur. J. Clin. Nutr. 2011;65:298–306. doi: 10.1038/ejcn.2010.269. [DOI] [PubMed] [Google Scholar]
- 5.Contreras-Manzano A., Jáuregui A., Velasco-Bernal A., Vargas-Meza J., Rivera J., Tolentino-Mayo L., Barquera S. Comparative Analysis of the Classification of Food Products in the Mexican Market According to Seven Different Nutrient Profiling Systems. Nutrients. 2018;10:737. doi: 10.3390/nu10060737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Labonté M.-È.E., Gladanac B., Ahmed M., Rayner M., Poon T., Franco-Arellano B., L’Abbé M.R., Gladanac B., Ahmed M., Franco-Arellano B., et al. Nutrient Profile Models with Applications in Government-Led Nutrition Policies Aimed at Health Promotion and Noncommunicable Disease Prevention: A Systematic Review. Adv. Nutr. 2018;9:741–788. doi: 10.1093/advances/nmy045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Food and Agriculture Organization. Pan American Health Organization . Approval of a New Food Act in Chile: Process Summary. FAO; Santiago, Chile: 2017. [Google Scholar]
- 8.Shisana O., Labadarios D., Rehle T., Simbayi L., Zuma K., Dhansay A., Reddy P., Parker W., Hooisan E., Naidoo P., et al. South African National Health and Nutrition Examination Survey (SANHANES-1) HSRC Press; Cape Town, South Africa: 2013. [Google Scholar]
- 9.Ronquest-Ross L.-C., Vink N., Sigge G. Food consumption changes in South Africa since 1994. S. Afr. J. Sci. 2015;111:1–12. doi: 10.17159/sajs.2015/20140354. [DOI] [Google Scholar]
- 10.National Department of Health . Strategy for the Prevention and Control of Obesity in South Africa 2015–2020. National Department of Health; Pretoria, South Africa: 2015. [Google Scholar]
- 11.Popkin B.M., Corvalan C., Grummer-Strawn L.M. Dynamics of the double burden of malnutrition and the changing nutrition reality. Lancet. 2020;395:65–74. doi: 10.1016/S0140-6736(19)32497-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erzse A., Fraser H., Levitt N., Hofman K. Prioritising action on diabetes during COVID-19. South African Med. J. 2020;110:719. doi: 10.7196/SAMJ.2020.v110i8.14961. [DOI] [PubMed] [Google Scholar]
- 13.National Department of Health . Regulations Relating to the Labelling and Advertsing of Foodstuffs (R146/2010) National Department of Health; Pretoria, South Africa: 2010. pp. 3–53. [Google Scholar]
- 14.National Department of Health . Draft Regulations Relating to Labelling and Advertising of Foods (R429/2014) National Department of Health; Pretoria, South Africa: 2014. [Google Scholar]
- 15.National Department of Health . Regulations Relating to the Reduction of Sodium in Certain Foodstuffs and Related Matters (R214/2013) National Department of Health; Pretoria, South Africa: 2013. pp. 3–7. [Google Scholar]
- 16.National Department of Health . Regulations Relating to Trans-Fat in Foodstuffs (R127/2011) National Department of Health; Pretoria, South Africa: 2011. pp. 3–6. [Google Scholar]
- 17.Reeve E., Naseri T., Martyn T., Bollars C., Thow A.-M. Developing a context-specific nutrient profiling system for food policy in Samoa. Health Promot. Int. 2018;34:e94–e105. doi: 10.1093/heapro/day089. [DOI] [PubMed] [Google Scholar]
- 18.Jones A., Neal B., Reeve B., Ni Mhurchu C., Thow A.M. Front-of-pack nutrition labelling to promote healthier diets: Current practice and opportunities to strengthen regulation worldwide. BMJ Glob. Health. 2019;4:e001882. doi: 10.1136/bmjgh-2019-001882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bertscher A., London L., Orgill M. Unpacking policy formulation and industry influence: The case of the draft control of marketing of alcoholic beverages bill in South Africa. Health Policy Plan. 2018;33:786–800. doi: 10.1093/heapol/czy049. [DOI] [PubMed] [Google Scholar]
- 20.Fooks G.J., Williams S., Box G., Sacks G. Corporations’ use and misuse of evidence to influence health policy: A case study of sugar-sweetened beverage taxation. Global Health. 2019;15:56. doi: 10.1186/s12992-019-0495-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abdool Karim S., Kruger P., Hofman K. Industry strategies in the parliamentary process of adopting a sugar-sweetened beverage tax in South Africa: A systematic mapping. Global Health. 2020;16:116. doi: 10.1186/s12992-020-00647-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mialon M., Crosbie E., Sacks G. Mapping of food industry strategies to influence public health policy, research and practice in South Africa. Int. J. Public Health. 2020;1 doi: 10.1007/s00038-020-01407-1. [DOI] [PubMed] [Google Scholar]
- 23.Labonté M.-È., Poon T., Mulligan C., Bernstein J.T., Franco-Arellano B., L’Abbé M.R. Comparison of global nutrient profiling systems for restricting the commercial marketing of foods and beverages of low nutritional quality to children in Canada. Am. J. Clin. Nutr. 2017;106:1471–1481. doi: 10.3945/ajcn.117.161356. [DOI] [PubMed] [Google Scholar]
- 24.Bell W., Colaiezzi B.A., Prata C.S., Coates J.C. Scaling up Dietary Data for Decision-Making in Low-Income Countries: New Technological Frontiers. Adv. Nutr. 2017;8:916–932. doi: 10.3945/an.116.014308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pitt C., Vassall A., Teerawattananon Y., Griffiths U.K., Guinness L., Walker D., Foster N., Hanson K. Foreword: Health Economic Evaluations in Low- and Middle-income Countries: Methodological Issues and Challenges for Priority Setting. Health Econ. 2016;25:1–5. doi: 10.1002/hec.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dodd R., Reeve E., Sparks E., George A., Vivili P., Win Tin S.T., Buresova D., Webster J., Thow A.-M. The politics of food in the Pacific: Coherence and tension in regional policies on nutrition, the food environment and non-communicable diseases. Publ. Health Nutr. 2020;23:168–180. doi: 10.1017/S1368980019002118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wicks M., Wright H., Wentzel-Viljoen E. Restricting the marketing of foods and non-alcoholic beverages to children in South Africa: Are all nutrient profiling models the same? Br. J. Nutr. 2017;116:2150–2159. doi: 10.1017/S0007114516004244. [DOI] [PubMed] [Google Scholar]
- 28.Wicks M., Wright H., Wentzel-Viljoen E. Assessing the construct validity of nutrient profiling models for restricting the marketing of foods to children in South Africa. Eur. J. Clin. Nutr. 2020;74:1065–1072. doi: 10.1038/s41430-020-0566-y. [DOI] [PubMed] [Google Scholar]
- 29.Dunford E., Huang L., Peters S., Crino M., Neal B., Ni Mhurchu C. Evaluation of Alignment between the Health Claims Nutrient Profiling Scoring Criterion (NPSC) and the Health Star Rating (HSR) Nutrient Profiling Models. Nutrients. 2018;10:1065. doi: 10.3390/nu10081065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Health Organization Regional Office for Africa . Nutrient Profile Model for the WHO African Region: A Tool for Implementing WHO Recommendations on the Marketing of Foods and Non-Alcoholic Beverages to Childre. World Health Organization Regional Office for Africa; Brazzaville, Congo: 2019. [Google Scholar]
- 31.Rayner M., Scarborough P., Stockley L. Nutrient Profiles: Options for Definitions for Use in Relation to Food Promotion and Children’s Diets: Final Report; British Heart Foundation Health Promotions Research Group. University of Oxford; Oxford, UK: 2004. [Google Scholar]
- 32.Scarborough P., Rayner M., Stockley L. Developing nutrient profile models: A systematic approach. Publ. Health Nutr. 2007;10:330–336. doi: 10.1017/S1368980007223870. [DOI] [PubMed] [Google Scholar]
- 33.Joint FAO/WHO Food Standards Programme Codex Committee on Food Labelling . Proposed Draft Guidelines on Front-of-Pack Nutrition Labelling. Joint FAO/WHO Food Standards Programme Codex Committee on Food Labelling; Ottawa, Canada: 2019. [Google Scholar]
- 34.Verhagen H., van den Berg H. A simple visual model to compare existing nutrient profiling schemes. Food Nutr. Res. 2008;52:1649. doi: 10.3402/fnr.v52i0.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Statistics South Africa . South African Demographic and Health Survey (SADHS) Statistics South Africa; Pretoria, South Africa: 2017. [Google Scholar]
- 36.Lobstein T., Brinsden H. Atlas of Childhood Obesity. World Obesity Federation; London, UK: 2019. [Google Scholar]
- 37.Mchiza Z., Steyn N., Hill J., Kruger A., Schönfeldt H., Nel J., Wentzel-Viljoen E. A Review of Dietary Surveys in the Adult South African Population from 2000 to 2015. Nutrients. 2015;7:8227–8250. doi: 10.3390/nu7095389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Armstrong M.E.G., Lambert M.I., Lambert E. V Secular trends in the prevalence of stunting, overweight and obesity among South African children (1994–2004) Eur. J. Clin. Nutr. 2011;65:835–840. doi: 10.1038/ejcn.2011.46. [DOI] [PubMed] [Google Scholar]
- 39.Oldewage-Theron W.H., Slabbert T.J.C. Impact of food and nutrition interventions on poverty in an informal settlement in the Vaal Region of South Africa. Proc. Nutr. Soc. 2008;67:91–97. doi: 10.1017/S002966510800606X. [DOI] [PubMed] [Google Scholar]
- 40.Steyn N.P., Nel J.H., Casey A. Secondary data analyses of dietary surveys undertaken in South Africa to determine usual food consumption of the population. Publ. Health Nutr. 2003;6:631–644. doi: 10.1079/PHN2003482. [DOI] [PubMed] [Google Scholar]
- 41.Temple N.J., Steyn N.P. The cost of a healthy diet: A South African perspective. Nutrition. 2011;27:505–508. doi: 10.1016/j.nut.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 42.Monteiro C.A., Cannon G., Levy R., Moubarac J.-C., Jaime P., Martins A.P., Canella D., Louzada M., Parra D. NOVA. The star shines bright. World Nutr. 2016;7:28–38. [Google Scholar]
- 43.Monteiro C.A., Cannon G., Levy R.B., Moubarac J.-C., Louzada M.L.C., Rauber F., Khandpur N., Cediel G., Neri D., Martinez-Steele E., et al. Ultra-processed foods: What they are and how to identify them. Publ. Health Nutr. 2019;22:936–941. doi: 10.1017/S1368980018003762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Correa T., Fierro C., Reyes M., Dillman Carpentier F.R., Taillie L.S., Corvalan C., Smith Taillie L., Corvalan C. Responses to the Chilean law of food labeling and advertising: Exploring knowledge, perceptions and behaviors of mothers of young children. Int. J. Behav. Nutr. Phys. Act. 2019;16:1–10. doi: 10.1186/s12966-019-0781-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mediano Stoltze F., Reyes M., Smith T.L., Correa T., Corvalán C., Carpentier F.R.D. Prevalence of Child-Directed Marketing on Breakfast Cereal Packages before and after Chile’s Food Marketing Law: A Pre- and Post-Quantitative Content Analysis. Int. J. Environ. Res. Publ. Health. 2019;16:4501. doi: 10.3390/ijerph16224501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caro J.C., Valizadeh P., Correa A., Silva A., Ng S.W. Combined fiscal policies to promote healthier diets: Effects on purchases and consumer welfare. PLoS ONE. 2020;15:e0226731. doi: 10.1371/journal.pone.0226731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dillman Carpentier F.R., Correa T., Reyes M., Taillie L.S. Evaluating the impact of Chile’s marketing regulation of unhealthy foods and beverages: Pre-school and adolescent children’s changes in exposure to food advertising on television. Publ. Health Nutr. 2020;23:747–755. doi: 10.1017/S1368980019003355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taillie L.S., Reyes M., Colchero M.A., Popkin B., Corvalán C. An evaluation of Chile’s Law of Food Labeling and Advertising on sugar-sweetened beverage purchases from 2015 to 2017: A before-and-after study. PLoS Med. 2020;17:e1003015. doi: 10.1371/journal.pmed.1003015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ministry of Health; Lima, Peru: 2018. [(accessed on 14 July 2021)]. Ministerio de Salud del Perú Aprueban Manual de Advertencias; pp. 58–63. Available online: https://busquedas.elperuano.pe/download/url/aprueban-manual-de-advertencias-publicitarias-en-el-marco-de-decreto-supremo-n-012-2018-sa-1660606-1. [Google Scholar]
- 50.Ministry of Agriculture . Nutrition Labelling Regulation Israel: 7904. Ministry of Agriculture; Tel Aviv, Israel: 2017. [(accessed on 14 July 2021)]. Available online: https://www.health.gov.il/Subjects/FoodAndNutrition/Nutrition/Adequate_nutrition/Documents/THE%20PROTECTION%20OF%20PUBLIC%20HEALTH%20%28FOOD%29%20%28NUTRITIONAL%20LABELING%29%20REGULATIONS%2C%205778%20-%202017.pdf. [Google Scholar]
- 51.Bernstein J.T., Franco-Arellano B., Schermel A., Labonté M.-È., L’Abbé M.R. Healthfulness and nutritional composition of Canadian prepackaged foods with and without sugar claims. Appl. Physiol. Nutr. Metab. 2017;42:1217–1224. doi: 10.1139/apnm-2017-0169. [DOI] [PubMed] [Google Scholar]
- 52.Pan American Health Organization. WHO . Pan American Health Organisation Nutrient Profile Model. Pan American Health Organization; Washington, DC, USA: 2016. [Google Scholar]
- 53.World Health Organization . Draft Guiding Principles and Framework Manual for Front-of-Pack Labelling for Promoting Healthy Diet. WHO; Geneva, Switzerland: 2019. [Google Scholar]
- 54.Corvalán C., Reyes M., Garmendia M.L., Uauy R. Structural responses to the obesity and non-communicable diseases epidemic: Update on the Chilean law of food labelling and advertising. Obes. Rev. 2019;20:367–374. doi: 10.1111/obr.12802. [DOI] [PubMed] [Google Scholar]
- 55.Medical Research Council . Food Composition Tables for South Africa. 5th ed. Medical Research Council; Cape Town, South Africa: 2017. [Google Scholar]
- 56.Baker P., Machado P., Santos T., Sievert K., Backholer K., Hadjikakou M., Russell C., Huse O., Bell C., Scrinis G., et al. Ultra-processed foods and the nutrition transition: Global, regional and national trends, food systems transformations and political economy drivers. Obes. Rev. 2020;21:1–22. doi: 10.1111/obr.13126. [DOI] [PubMed] [Google Scholar]
- 57.Adams J., Hofman K., Moubarac J.-C., Thow A.M. Public health response to ultra-processed food and drinks. BMJ. 2020;369:m2391. doi: 10.1136/bmj.m2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pillay-van Wyk V., Msemburi W., Laubscher R., Dorrington R.E., Groenewald P., Glass T., Nojilana B., Joubert J.D., Matzopoulos R., Prinsloo M., et al. Mortality trends and differentials in South Africa from 1997 to 2012: Second National Burden of Disease Study. Lancet Glob. Heal. 2016;4:e642–e653. doi: 10.1016/S2214-109X(16)30113-9. [DOI] [PubMed] [Google Scholar]
- 59.National Department of Health . Regulations Relating to Foodstuffs for Infants and Young Children (R991/2012) National Department of Health; Pretoria, South Africa: 2012. pp. 3–32. [Google Scholar]
- 60.Gillon-Keren M., Kaufman-Shriqui V., Goldsmith R., Safra C., Shai I., Fayman G., Berry E., Tirosh A., Dicker D., Froy O., et al. Development of Criteria for a Positive Front-of-Package Food Labeling: The Israeli Case. Nutrients. 2020;12:1875. doi: 10.3390/nu12061875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cordain L., Eaton S.B., Sebastian A., Mann N., Lindeberg S., Watkins B.A., O’Keefe J.H., Brand-Miller J. Origins and evolution of the Western diet: Health implications for the 21st century. Am. J. Clin. Nutr. 2005;81:341–354. doi: 10.1093/ajcn.81.2.341. [DOI] [PubMed] [Google Scholar]
- 62.Lustig R.H., Schmidt L.A., Brindis C.D. The toxic truth about sugar. Nature. 2012;482:27–29. doi: 10.1038/482027a. [DOI] [PubMed] [Google Scholar]
- 63.Norat T., Scoccianti C., Boutron-Ruault M.-C., Anderson A., Berrino F., Cecchini M., Espina C., Key T., Leitzmann M., Powers H., et al. European Code against Cancer 4th Edition: Diet and cancer. Cancer Epidemiol. 2015;39:S56–S66. doi: 10.1016/j.canep.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 64.Johnson R.J., Segal M.S., Sautin Y., Nakagawa T., Feig D.I., Kang D., Gersch M.S., Benner S., Sanchez-Lozada L.G. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am. J. Clin. Nutr. 2007;86:899–906. doi: 10.1093/ajcn/86.4.899. [DOI] [PubMed] [Google Scholar]
- 65.Yeh C.-J., Chang H.-Y., Pan W.-H. Time trend of obesity, the metabolic syndrome and related dietary pattern in Taiwan: From NAHSIT 1993-1996 to NAHSIT 2005-2008. Asia Pac. J. Clin. Nutr. 2011;20:292–300. [PubMed] [Google Scholar]
- 66.World Health Organization . Diet, Nutrition and the Prevention of Chronic Diseases. WHO; Geneva, Switzerland: 2003. [PubMed] [Google Scholar]
- 67.World Health Organization . Guideline: Sugars Intake for Adults and Children. WHO; Geneva, Switzerland: 2015. [PubMed] [Google Scholar]
- 68.Louie J.C.Y., Moshtaghian H., Boylan S., Flood V.M., Rangan A.M., Barclay A.W., Brand-Miller J.C., Gill T.P. A systematic methodology to estimate added sugar content of foods. Eur. J. Clin. Nutr. 2015;69:154–161. doi: 10.1038/ejcn.2014.256. [DOI] [PubMed] [Google Scholar]
- 69.Sánchez-Pimienta T.G., Batis C., Lutter C.K., Rivera J.A. Sugar-Sweetened Beverages Are the Main Sources of Added Sugar Intake in the Mexican Population. J. Nutr. 2016;146:1888S–1896S. doi: 10.3945/jn.115.220301. [DOI] [PubMed] [Google Scholar]
- 70.Lobstein T., Davies S. Defining and labelling “healthy” and “unhealthy” food. Publ. Health Nutr. 2009;12:331–340. doi: 10.1017/S1368980008002541. [DOI] [PubMed] [Google Scholar]
- 71.Tappy L. Fructose-containing caloric sweeteners as a cause of obesity and metabolic disorders. J. Exp. Biol. 2018;221:jeb164202. doi: 10.1242/jeb.164202. [DOI] [PubMed] [Google Scholar]
- 72.Collin L.J., Judd S., Safford M., Vaccarino V., Welsh J.A. Association of Sugary Beverage Consumption with Mortality Risk in US Adults. JAMA Netw. Open. 2019;2:e193121. doi: 10.1001/jamanetworkopen.2019.3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gupta P., Shah D., Kumar P., Bedi N., Mittal H.G., Mishra K., Khalil S., Elizabeth K.E., Dalal R., Harish R., et al. Indian Academy of Pediatrics Guidelines on the Fast and Junk Foods, Sugar Sweetened Beverages, and Energy Drinks. Ind. Pediatr. 2019:849–850. doi: 10.1007/s13312-019-1612-5. [DOI] [PubMed] [Google Scholar]
- 74.World Health Organization . Global Strategy on Diet, Physical Activity and Health. WHO; Geneva, Switzerland: 2004. [Google Scholar]
- 75.Dietary Guidelines Advisory Committee . Scientific Report of the 2020 Dietary Guidelines Advisory Committee: Advisory Report to the Secretary of Agriculture and the Secretary of Health and Human Services. Dietary Guidelines Advisory Committee; Washington, DC, USA: 2020. [Google Scholar]
- 76.Kris-Etherton P.M., Krauss R.M. Public health guidelines should recommend reducing saturated fat consumption as much as possible: YES. Am. J. Clin. Nutr. 2020;112:13–18. doi: 10.1093/ajcn/nqaa110. [DOI] [PubMed] [Google Scholar]
- 77.Kris-Etherton P.M., Petersen K., Van Horn L. Convincing evidence supports reducing saturated fat to decrease cardiovascular disease risk. BMJ Nutr. Prev. Heal. 2018;1:23–26. doi: 10.1136/bmjnph-2018-000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Clifton P.M., Keogh J.B. A systematic review of the effect of dietary saturated and polyunsaturated fat on heart disease. Nutr. Metab. Cardiovasc. Dis. 2017;27:1060–1080. doi: 10.1016/j.numecd.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 79.Forouhi N.G., Krauss R.M., Taubes G., Willett W. Dietary fat and cardiometabolic health: Evidence, controversies, and consensus for guidance. BMJ. 2018;2139:k2139. doi: 10.1136/bmj.k2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.World Health Organization . Draft Guideline: Saturated Fatty Acid and Trans-Fatty Acid Intake for Adults and Children. World Health Organization; Geneva, Switzrland: 2018. [Google Scholar]
- 81.Codex Alimentarius Guidelines on Nutrition Labelling. Cac/Gl 2-1985. 2013;53:1689–1699. doi: 10.1017/CBO9781107415324.004. [DOI] [Google Scholar]
- 82.World Health Organization . Guideline: Sodium Intake for Adults and Children. WHO; Geneva, Switzerland: 2012. [PubMed] [Google Scholar]
- 83.Peters S., Dunford E., Ware L., Harris T., Walker A., Wicks M., van Zyl T., Swanepoel B., Charlton K., Woodward M., et al. The Sodium Content of Processed Foods in South Africa during the Introduction of Mandatory Sodium Limits. Nutrients. 2017;9:404. doi: 10.3390/nu9040404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Camacho S., Ruppel A. Is the calorie concept a real solution to the obesity epidemic? Glob. Health Action. 2017;10:1289650. doi: 10.1080/16549716.2017.1289650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Drewnowski A., Amanquah D., Gavin-Smith B. Perspective: How to Develop Nutrient Profiling Models Intended for Global Use: A Manual. Adv. Nutr. 2021:1–12. doi: 10.1093/advances/nmab018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Samaniego-Vaesken M.d.L., Partearroyo T., Cano A., Urrialde R., Varela-Moreiras G. Novel database of declared low- and no-calorie sweeteners from foods and beverages available in Spain. J. Food Compos. Anal. 2019;82:103234. doi: 10.1016/j.jfca.2019.103234. [DOI] [Google Scholar]
- 87.Serra-Majem L., Raposo A., Aranceta-Bartrina J., Varela-Moreiras G., Logue C., Laviada H., Socolovsky S., Pérez-Rodrigo C., Aldrete-Velasco J., Meneses Sierra E., et al. Ibero–American Consensus on Low- and No-Calorie Sweeteners: Safety, Nutritional Aspects and Benefits in Food and Beverages. Nutrients. 2018;10:818. doi: 10.3390/nu10070818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Suez J., Korem T., Zeevi D., Zilberman-Schapira G., Thaiss C.A., Maza O., Israeli D., Zmora N., Gilad S., Weinberger A., et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 2014;514:181–186. doi: 10.1038/nature13793. [DOI] [PubMed] [Google Scholar]
- 89.Dunford E.K., Taillie L.S., Miles D.R., Eyles H., Tolentino-Mayo L., Ng S.W. Non-nutritive sweeteners in the packaged food supply—an assessment across 4 countries. Nutrients. 2018;10:257. doi: 10.3390/nu10020257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vyth E.L., Steenhuis I.H.M., Roodenburg A.J.C., Brug J., Seidell J.C. Front-of-pack nutrition label stimulates healthier product development: A quantitative analysis. Int. J. Behav. Nutr. Phys. Act. 2010;7:65. doi: 10.1186/1479-5868-7-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Reyes M., Smith Taillie L., Popkin B., Kanter R., Vandevijvere S., Corvalán C. Changes in the amount of nutrient of packaged foods and beverages after the initial implementation of the Chilean Law of Food Labelling and Advertising: A nonexperimental prospective study. PLoS Med. 2020;17:e1003220. doi: 10.1371/journal.pmed.1003220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Durán Agüero S., Angarita Dávila L., Escobar Contreras M.C., Rojas Gómez D., de Assis Costa J. Noncaloric Sweeteners in Children: A Controversial Theme. Biomed Res. Int. 2018;2018:1–7. doi: 10.1155/2018/4806534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brown R.J., de Banate M.A., Rother K.I. Artificial Sweeteners: A systematic review of metabolic effects in youth. Int. J. Pediatr. Obes. 2010;5:305–312. doi: 10.3109/17477160903497027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Toews I., Lohner S., Küllenberg de Gaudry D., Sommer H., Meerpohl J.J. Association between intake of non-sugar sweeteners and health outcomes: Systematic review and meta-analyses of randomised and non-randomised controlled trials and observational studies. BMJ. 2019;364:k4718. doi: 10.1136/bmj.k4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sardarodiyan M., Hakimzadeh V. Artificial Sweeteners. Int. J. Pharm. Tech. Res. 2016;9:357–363. [Google Scholar]
- 96.United States Department of Agriculture. Global Agricultural Information Network . Food and Agricultural Import Regulations and Standards: Saudi Arabia. Office of Agricultural Affairs of the USDA/Foreign Agricultural Service; Riyadh, Saudi Arabia: 2020. [Google Scholar]
- 97.Sambra V., López-Arana S., Cáceres P., Abrigo K., Collinao J., Espinoza A., Valenzuela S., Carvajal B., Prado G., Peralta R., et al. Overuse of Non-caloric Sweeteners in Foods and Beverages in Chile: A Threat to Consumers’ Free Choice? Front. Nutr. 2020;7:1–8. doi: 10.3389/fnut.2020.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Essman M., Taillie L.S., Frank T., Ng S.W., Popkin B.M., Swart E.C. Taxed and untaxed beverage intake by South African young adults after a national sugar-sweetened beverage tax: A before-and-after study. PLoS Med. 2021;18:e1003574. doi: 10.1371/journal.pmed.1003574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stacey N., Mudara C., Ng S.W., van Walbeek C., Hofman K., Edoka I. Sugar-based beverage taxes and beverage prices: Evidence from South Africa’s Health Promotion Levy. Soc. Sci. Med. 2019;238:112465. doi: 10.1016/j.socscimed.2019.112465. [DOI] [PubMed] [Google Scholar]
- 100.Appleton K.M., Tuorila H., Bertenshaw E.J., de Graaf C., Mela D.J. Sweet taste exposure and the subsequent acceptance and preference for sweet taste in the diet: Systematic review of the published literature. Am. J. Clin. Nutr. 2018;107:405–419. doi: 10.1093/ajcn/nqx031. [DOI] [PubMed] [Google Scholar]
- 101.Swithers S.E. Artificial sweeteners are not the answer to childhood obesity. Appetite. 2015;93:85–90. doi: 10.1016/j.appet.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 102.Archibald A., Dolinsky V., Azad M. Early-Life Exposure to Non-Nutritive Sweeteners and the Developmental Origins of Childhood Obesity: Global Evidence from Human and Rodent Studies. Nutrients. 2018;10:194. doi: 10.3390/nu10020194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.World Health Organization Regional Office for Europe . WHO Regional Office for Europe Nutrient Profile Model. WHO; Copenhagen, Denmark: 2015. [Google Scholar]
- 104.Reyes M., Garmendia M.L., Olivares S., Aqueveque C., Zacarías I., Corvalán C. Development of the Chilean front-of-package food warning label. BMC Publ. Health. 2019;19:906. doi: 10.1186/s12889-019-7118-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ministerio de Salud de Uruguay Manual . Ministry of Health; Montevideo, Uruguay: 2018. [(accessed on 14 July 2021)]. Para la Aplicación del Decreto No 272/018 Sobre Rotulado Frontal de Alimentos. Available online: https://www.gub.uy/ministerio-salud-publica/sites/ministerio-salud-publica/files/documentos/publicaciones/MSP_MANUAL_APLICACION_ROTULADO_FRONTAL_ALIMENTOS_0.pdf. [Google Scholar]
- 106.Secretaria de Economia . Mexico Regulation NOM-051. Volume 1. Ministry of Economy; Mexico City, Mexico: 2020. [(accessed on 14 July 2021)]. pp. 4–40. Available online: https://www.dof.gob.mx/2020/SEECO/NOM_051.pdf. [Google Scholar]
- 107.National Agency of Sanitary Surveillance (ANVISA) Ministério da Saúde . Resolution of the Collegiate Board 429 and Normative Instruction 75. ANVISA; Sau Paulo, Brazil: 2020. [(accessed on 14 July 2021)]. Available online: https://www.in.gov.br/en/web/dou/-/resolucao-de-diretoria-colegiada-rdc-n-429-de-8-de-outubro-de-2020-282070599. [Google Scholar]
- 108.Popkin B.M., Barquera S., Corvalan C., Hofman K.J., Monteiro C., Ng S.W., Swart E.C., Taillie L.S. Towards unified and impactful policies to reduce ultra-processed food consumption and promote healthier eating. Lancet Diabetes Endocrinol. 2021;8587:1–10. doi: 10.1016/S2213-8587(21)00078-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dorlach T., Mertenskötter P. Interpreters of International Economic Law: Corporations and Bureaucrats in Contest over Chile’s Nutrition Label. Law Soc. Rev. 2020;54:571–606. doi: 10.1111/lasr.12495. [DOI] [Google Scholar]
- 110.Thow A.M., Jones A., Hawkes C., Ali I., Labonté R. Nutrition labelling is a trade policy issue: Lessons from an analysis of specific trade concerns at the World Trade Organization. Health Promot. Int. 2017;33:daw109. doi: 10.1093/heapro/daw109. [DOI] [PubMed] [Google Scholar]
- 111.Garton K., Thow A.M., Swinburn B. International Trade and Investment Agreements as Barriers to Food Environment Regulation for Public Health Nutrition: A Realist Review. Int. J. Heal. Policy Manag. 2020:1–21. doi: 10.34172/ijhpm.2020.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Thow A.M., Gleeson D. Advancing Public Health on the Changing Global Trade and Investment Agenda Comment on “The Trans-Pacific Partnership: Is It Everything We Feared for Health?”. Int. J. Health Policy Manag. 2017;6:295–298. doi: 10.15171/ijhpm.2016.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Thow A.M., Reeve E., Naseri T., Martyn T., Bollars C. Food supply, nutrition and trade policy: Reversal of an import ban on turkey tails. Bull. World Health Organ. 2017;95:723–725. doi: 10.2471/BLT.17.192468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.National Department of Health . Regulations Relating to the use of Sweeteners in Foodstufss (R733/2012) National Department of Health; Pretoria, South Africa: 2012. pp. 3–6. [Google Scholar]
- 115.Tong T., Rangan A., Gemming L. Evaluating the Nutritional Content of Children’s Breakfast Cereals in Australia. Children. 2018;5:84. doi: 10.3390/children5070084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Koen N., Wentzel-Viljoen E., Blaauw R. The development of a single health-endorsement logo for South Africa. Publ. Health Nutr. 2018;21:1444–1454. doi: 10.1017/S1368980018000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Townsend M.S. Where is the science? What will it take to show that nutrient profiling systems work? Am. J. Clin. Nutr. 2010;91:1109S–1115S. doi: 10.3945/ajcn.2010.28450F. [DOI] [PubMed] [Google Scholar]
- 118.Cooper S.L., Pelly F.E., Lowe J.B. Construct and criterion-related validation of nutrient profiling models: A systematic review of the literature. Appetite. 2016;100:26–40. doi: 10.1016/j.appet.2016.02.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data analyzed for this paper form part of a primary project which is currently being written up in other publications. Data used for this paper will therefore be available upon request and granted for replication purposes. Data are available from the UNC Carolina Digital Repository (https://cdr.lib.unc.edu/). For data inquiries, please contact Donna Miles (drmiles@email.unc.edu) and Jessica Ostrowski (jessica.ostrowski@unc.edu).