Abstract
Myxobacteria are unicellular, Gram-negative, soil-dwelling, gliding bacteria that belong to class δ-proteobacteria and order Myxococcales. They grow and proliferate by transverse fission under normal conditions, but form fruiting bodies which contain myxospores during unfavorable conditions. In view of the escalating problem of antibiotic resistance among disease-causing pathogens, it becomes mandatory to search for new antibiotics effective against such pathogens from natural sources. Among the different approaches, Myxobacteria, having a rich armor of secondary metabolites, preferably derivatives of polyketide synthases (PKSs) along with non-ribosomal peptide synthases (NRPSs) and their hybrids, are currently being explored as producers of new antibiotics. The Myxobacterial species are functionally characterized to assess their ability to produce antibacterial, antifungal, anticancer, antimalarial, immunosuppressive, cytotoxic and antioxidative bioactive compounds. In our study, we have found their compounds to be effective against a wide range of pathogens associated with the concurrence of different infectious diseases.
Keywords: antibiotics, bioactive compounds, medication, Myxobacteria, human diseases
1. Introduction
Myxobacteria, bacteria belonging to family δ-proteobacteria and order Myxococcales, are unicellular, soil-dwelling, rod-shaped bacteria that display gliding motility on attachment to solid surfaces. They are omnipresent, with habitats ranging from tundra to hot deserts and from acidic soils to alkaline conditions [1,2,3]. The source for their isolation ranges from soil to decaying wood and leaves of trees up to excreta of herbivorous creatures [4,5]. Under nutrient-deficient conditions, they produce species-explicit structures (fruiting bodies) that exhibit myxospores (arisen from vegetative cells) within themselves to pass decades of unfavorable environmental conditions [6]. Withstanding regular confinement endeavors, myxospores sprout with the onset of favorable conditions into full-fledged structures, with the exception of depicted facultative anaerobic species, Anaeromyxobacter dehalogenans [7]. Recently, a large number of studies have been performed to gain a detailed account of the Myxobacterial properties along with types, dynamics and biogenesis of Myxobacteria-derived secondary metabolites [8,9,10,11,12].
The rise in resistance to armor of available antibiotic regimes represents a problem of global magnitude [13,14,15,16]. With increases in mortality and morbidity rates, it becomes imperative to have a strategic management plan to monitor the impact of resistance development and means for exploration of new molecules that can combat the emergence of different diseases among humans [12,17]. Myxobacterial species, despite exhibiting sensitiveness to tetracycline, kanamycin, erythromycin, streptomycin, neomycin and actinomycin, produce a variety of chemically different structures that in due course were found effective in combatting the growing problem of drug resistance. The present study highlights the potential of Myxobacteria as a source of new bioactive molecules, with strong emphasis on the production and screening of secondary metabolites, their effect observed in overcoming the odyssey associated with different diseases, as well as having updated information of the current development of their exploitation as a source of effective molecules with potential to compliment available drugs in the control of different diseases.
2. Distribution
Myxobacteria are largely cosmopolitan. Besides inhabiting terrestrial conditions, they mark their presence in extreme habitats, such as anaerobic/microaerophilic, freshwater, acidic soils, saline waters and others [12]. Since maximum populations of Myxobacteria predominantly inhabit terrestrial ecosystems, a large proportion of their secretions (secondary metabolites) are derived from terrestrial Myxobacterial species. On the basis of habitats, their distribution is studied under the following.
2.1. Terrestrial Habitats
Adaptation of Myxobacteria to terrestrial habitats manifests their existence in wide phenotypic characteristics, such as social swarming and gliding, resting myxospores, etc., capable of producing secondary metabolites with a wide range of antibiotic or antifungal activity as well as predation or cellulose decomposition [18]. With the help of different probes and primers, Wu et al. explored a wide range of Myxobacteria, mostly Myxococcales, from the soil samples [19]. Mohr revealed greater presentation of Myxococcus and Corallococcus genera by standardized cultivation techniques as compared to cultivation-independent clone libraries [12].
2.2. Acidic and Alkaline Habitats
Generally, Myxobacteria inhabit the soils which are neutral or slightly alkaline and show a narrow range in their pH, i.e., approximately 6.5–8.5 [12]. Myxobacteria species isolated from the alkaline bogs include Myxococcus, Archangium and Sorangium, along with others such as Melittangium [20]. Corallococcus coralloides (formerly Myxococcus coralloides) dominated in slightly acidic soils, while M. fulvus dominated in soils with a pH range in between 3.0 and 3.5 [21]. Ruckert reported that Myxobacterial diversity decreases with the decrease in the pH of the soil at alpine regions [21].
2.3. Freshwater Habitats
Freshwater-dwelling Myxobacteria share some characteristic features with soil inhabitants, which justifies that these Myxobacteria have been blown away or washed from soil into the freshwater bodies [22]. Research related to freshwater habitats of Myxobacteria reveal that in lake mud, Myxobacteria were the dominant bacterial groups [23].
2.4. Marine/Saline Environments
Though Myxobacteria are less adapted to saline environments, their existence in salty conditions was reported by Brockman in 1963, who observed Myxobacterial fruiting bodies in sand dunes from an ocean beach of South Carolina [24]. Marine Myxobacteria are represented by four different genera: Salimabromide [25], Enhygrolides [26], Haliangicin [27] and Haliamide [28]. Haliangium tepidum and H. ochraceum are the representative members of Myxobacteria from coastal salt marshes. They differ from members of the terrestrial genus with respect to the presence of anteiso-branched fatty acids, that help them to survive in greater salt concentrations (2–3% NaCl) [29]. Some genera of Myxobacteria, including Enhygromyxa [30], Plesiocystis [31] and Pseudenhygromyxa [32], are entirely detected in the saline environments. Brinkhoff et al. reported a cluster of marine Myxobacteria (MMB) from sediments of the North Sea [33,34]. Zhang et al. studied 58 species of Myxobacteria from the saline soils of Xinjiang, China [35], and Li et al. observed that species such as Sorangium, Cystobacter, Myxococcus, Polyangium, Corallococcus and Nannocystis show better survival in elevated salt conditions [36].
2.5. Facultative Anaerobic Myxobacteria
Myxobacteria are strictly aerobes, with the exception of Anaeromyxobacter dehalogenans, which is a facultative anaerobe. This strain of Myxobacteria was studied from sediments of the stream and grows with 2-chlorophenol (2-CPh) as an electron acceptor and acetate as an electron donor [7]. Later, different strains of this Myxobacteria were isolated from uranium-contaminated soils [37], flooded paddy fields [38], corrosive material of water pipelines [39] and arsenic-polluted environments [40].
2.6. Myxobacteria Inhabiting Moderate to Extreme Environments
Most of the Myxobacterial species are mesophilic, i.e., they survive in the range of 4–44 °C. However, they are also reported to survive in the extreme temperature range. Myxospores liberated by bacteria inhabiting extreme environments act as a means of sexual reproduction and can survive with temperature extremes of 58–60 °C. Production of myxospores differentiates these organisms from the rest of the faunal diversity [22]. Brockman analyzed greater diversity among Myxobacteria from regions that received greater annual rainfall (400–800 mm) as compared to the normal range of 200–400 mm [41]. Gerth and Müller [42] reported that Cystobacterineae and Sorangiineae-Myxobacterial suborders show greater morphogenesis at temperatures of 42–48 °C. Mohr et al. reported that N. konarekensis, which was studied from an Iranian desert, exhibits the best growth at 37 °C, compared with N. pusilla and N. exedens, which show optimal growth at 30 °C [43]. Though hot springs are not considered suitable for the growth of mesophilic Myxobacteria, Iizuka et al. reported four different strains of Myxobacteria that grow in geothermal conditions (optimum 45–49 °C) from Japan [44].
3. Myxobacterial Secondary Metabolites
Secondary metabolites represent incredible gathering of characteristically differing molecules blended among different creatures, such as microorganisms, plants, etc. Though they are not actively involved in development or any type of advancement, their absence prompts a long-haul disability in the survivability of living beings [45]. Production of secondary metabolites has been reported from a large number of Myxobacterial species, but a major proportion of them are reported among Myxococcus xanthus, Sorangium cellulosum and Chondromyces species [46]. In addition to ribosomally produced secondary metabolites, a major proportion of Myxobacterial metabolites were found to be derivatives of polyketide synthases (PKSs), non-ribosomal peptide synthetases (NRPSs) or hybrids of PK-NRPS systems [3,6,47]. The synthesis module in both cases proceeds through buildup of monomeric blocks: acyl CoA thioester (in case of PK metabolites) and amino acids (both proteinogenic and non-proteinogenic in case of NRPs), in a stepwise manner, followed by modification either during assembly of reaction intermediates or at the end after release from the multienzyme complex [3]. Over the past 3 decades, more than 100 secondary metabolites with over 600 analogs were reportedly isolated from more than 9000 Myxobacterial strains [48]. The production of unique metabolites among Myxobacterial strains reflects a strong correlation between genome size and the biosynthetic pathway [49,50].
Considered as a rich source of secondary metabolites, the production of a large number (>80 distinctive and 350 structural variants) of bioactive compounds by Myxobacteria puts it on par with Pseudomonas for being a rich source of antibiotics [51]. A large number of Myxobacterial secondary metabolites show similarity to those produced by Pseudomonas and Bacillus spp. Antibiotics produced as bioactive secondary metabolites have been observed for about 55% and 95% of Myxobacterial spp. that exhibit bacteriolytic and cellulolytic properties [52]. With greater potential for use in clinical settings, compounds isolated from Myxobacteria are found either as macrocyclic lactones or linear cyclic peptides [51,52]. Information on different aspects of secondary metabolites produced by different strains of Myxobacteria along with their uses is summarized in Table 1.
Table 1.
Categorization of Myxobacterial-derived secondary metabolites based on their function.
| Bioactive Compound | Chemical Structure | Classification | Myxobacterial sp. | Uses | References |
|---|---|---|---|---|---|
| Bioactive compounds exerting antimicrobial effect | |||||
| Ajudazol |
|
Depsipeptides | Chondromyces crocatus | Acts as inhibitor of mitochondrial electron transport. More effective against yeast and fungi. |
[3,47,53] |
| Althiomycin |
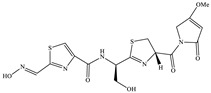
|
Polyketide, peptide | Myxococcus xanthus | Disrupts translocation of tRNA for peptide bond formation by peptidyltransferase. It is effective in treatment of injury and sepsis associated with Yersinia pestis infection. | [54] |
| Angiolactone |
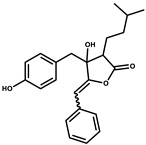
|
Furanone | Angiococcus sp. | Exhibits siderophore production which enables its antibacterial and antiproliferative activity. | [55] |
| Antalid |
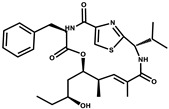
|
Depsipeptide | Polyangium sp. | NA | [56] |
| Aurachins E |
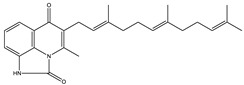
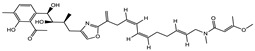
|
Quinoline alkaloids | Stigmatella aurantiaca | Exhibits antimalarial activity (effective against Plasmodium falciparum). | [57] |
| Carolacton |
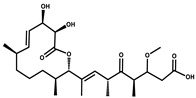
|
Macrolactone | Sorangium cellulosum | Effective in regulating the growth of biofilm-producing microbes such as Streptococcus mutans and pneumococci. | [58] |
| Chlorotonil |
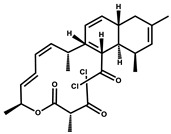
|
Macrolactone | Sorangium cellulosum | Antibacterial and antimalarial activity. | [59] |
| Corallopyronin A |
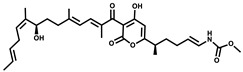
|
α-Pyrone | Corallococcus (Myxococcus) coralloides | Exhibits antibacterial action, effective in treating filariasis. | [60] |
| Corallorazine |
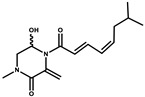
|
Piperazine | Corallococcus coralloides | Exhibits antibacterial activity. | [61] |
| Crocacin |
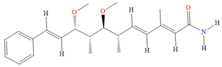
|
Depsipeptides | Chondromyces crocatus | Antibacterial. Inhibits electron transport system. | [62] |
| Cystobactamid |
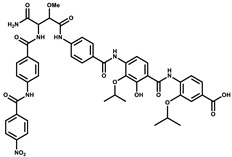
|
Peptide | Cystobacter sp. | Broad-spectrum antibacterial; topoisomerase (gyrase) inhibition. | [63] |
| Cytochromone |
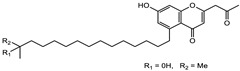
|
Polyketide, chromone | Proteus mirabilis | Essential in mitochondrial electron transport and intrinsic type II apoptosis. | [64] |
| Cystomanamide |
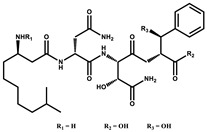
|
Lipopeptide | Cystobacter fuscus | Exhibits strong antifungal and antibacterial activity. | [65] |
| Cystothiazol |
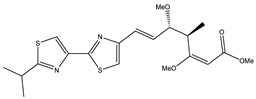
|
Heterocyclic alkaloid | Cystobacter fuscus | Antifungal/cytostatic. Inhibits sub-mitochondrial NADH oxidation. | [66] |
| Disciformycin |
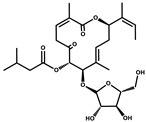
|
Macrolide | Pyxidicoccus fallax | Exhibits antibacterial activity. | [67] |
| Enhygrolide A |
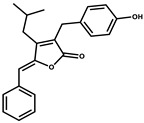
|
Furanone | Enhygromyxa salina | Effective in inhibiting the growth of Arthrobacter crystallopoietes. | [68] |
| Etnangien |
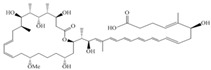
|
Polyketides | Sorangium cellulosum | Works as an inhibitor of eubacterial DNA polymerase. | [3,47,53] |
| Gulmirecin |
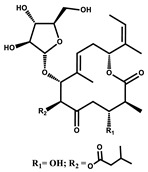
|
Macrolide | Pyxidicoccus fallax | Exhibits antibacterial activity. | [69] |
| Haliangicin |
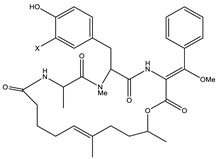
|
Polyketide | Haliangium luteum | Effective against fungi Aspergillus niger and Fusarium sp. at very low concentrations of 6–12 µg/mL. | [70] |
| Hyalachelin |
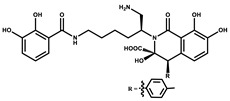
|
Catechol | Hyalangium minutum | Shows sidrophore, i.e., iron-chelating activity, and cytotoxic activity is minor. | [71] |
| Hyaladione |
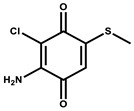
|
Quinone | Hyalangium minutum | Exhibits antimicrobial and cytotoxic activity. | [72] |
| Hyapyrroline |
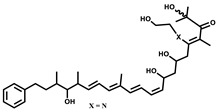
|
Polyketide, pyrrole | Hyalangium minutum | NA | [73] |
| Hyapyrone |
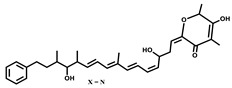
|
Polyketide, pyrone | Hyalangium minutum | Exhibits weak antibacterial and antifungal activity. | [73] |
| p-Hydroxyacetophenone amide |
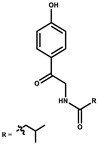
|
Amide | Cystobacter ferrugineus | Shows marginal activity against microalgae (P. simplex). | [74] |
| 1-Hydroxyphenazin-6-yl-a-Darabinofuranoside |
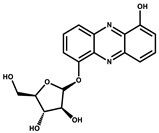
|
Glycoside | Nannocystis pusilla | Exhibits weak antimicrobial activity. | [75] |
| Icumazol |
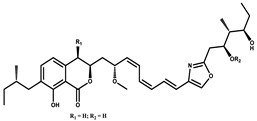
|
Polyketide | Sorangium cellulosum | Effective antifungal. Inhibition of NADH oxidation. | [76] |
| Indiacen |
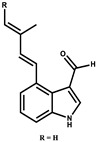
|
Indole | Sandaracinus amylolyticus | Exhibits antibacterial and antifungal activity. | [77,78] |
| Indothiazinone |
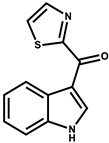
|
Indole | Ohtaekwangia kribbensis | Weak antimicrobial and cytotoxic activity. | [75] |
| Kulkenon |
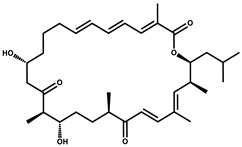
|
Macrolactone | Sorangium cellulosum | Exhibits antibacterial activity. | [79] |
| Leupyrrins |
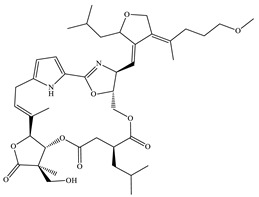
|
Macrolides | Sorangium cellulosum | Exhibits antibacterial activity. | [80] |
| Macyranone |
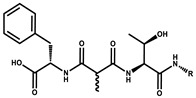
|
Peptide | Cystobacter fuscus | Shows moderate cytotoxic activity; antiparasitic (L. donovani); proteasome inhibitor (CT-L activity). | [81] |
| Maltepolid |
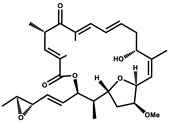
|
Macrolactone | Exhibits moderate cytotoxic activity. | [82] | |
| Methyl indole-3-carboxylate |
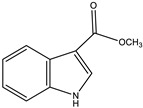
|
Indole | Sorangium cellulosum | NA | [75] |
| Melithiazols |
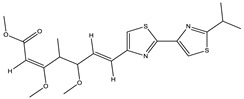
|
Heterocyclic alkaloid | Archangium gephyra | Antibacterial. Inhibits NADH oxidation. | [83] |
| Microsclerodermin |
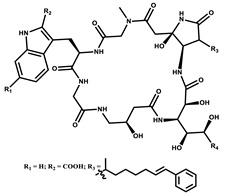
|
Cyclic peptide | Microscleroderma, theonella | Exhibits antifungal activity, NF-kB inhibition and induction of apoptosis. | [84,85] |
| Myxalamids |
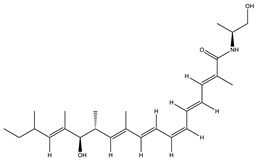
|
Amide | Myxococcus xanthus | Exhibits antibacterial and antifungal activity; inhibits electron transport system. | [3,47,53] |
| Myxochelin |
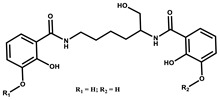
|
Peptide | Angiococcus disciformis | Shows siderophore production. Exhibits antibacterial, antitumor and antiproliferative activities: inhibits 5-lipoxygenase. | [86,87] |
| Myxocoumarin |
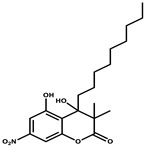
|
Coumarin | Stigmatella aurantiaca | Exhibits antifungal activity. | [88] |
| Myxoprincomide |
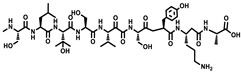
|
Peptide | Myxococcus xanthus | NA | [89,90,91] |
| Myxopyronin B |
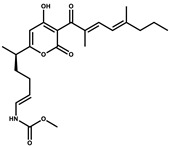
|
Peptide | Myxococcus fulvus | Effective in combating diseases caused by Staphylococcus aureus. | [92] |
| Myxothiazol |
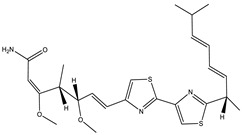
|
Macrocyclic | Myxococcus fulvus | Inhibits mitochondrial cytochrome c reductase. | [3,47,53] |
| Myxovalargin |
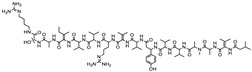
|
Lipopeptide | Myxococcus fulvus | Exhibits antibacterial activity against Micrococcus luteus and Corynebacterium Mediolanum. Disrupts memebrane integrity and aminoacyl-tRNA binding to site A during translation. | [93] |
| Myxovirescin |
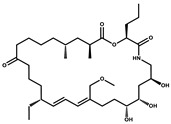
|
Macrocyclic | Myxococcus xanthus | Exhibits antibacterial activity. Blocks bacterial cell wall synthesis via interference in lipid-disaccharide pentapeptide polymerization, as well as targeting type II signal peptidase LspA. | [94,95] |
| Nannozinone |
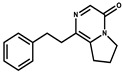
|
Pyrrolopyrazinoe | Nannocystis pusilla | Exhibits weak antimicrobial and cytotoxic activity. | [75] |
| Noricumazol |
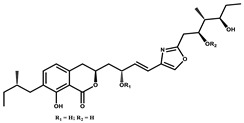
|
Polyketide | Sorangium cellulosum | Inhibits conductance of potassium channel KscA. Exhibits antiviral (EBOV, HCV) activity. | [76,96,97] |
| Phenoxan |
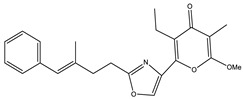
|
Lipopeptide | Polyganium sp. | Effective as an inhibitor of eukaryotic respiratory chain (blocks Complex I). Exhibits antifungal activity. | [3,47,53] |
| Phoxalone |
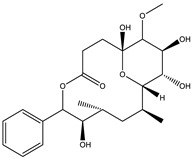
|
Macrolides | Sorangium cellulosum | Exhibits antimicrobial activity. | [98] |
| Pyrronazol |
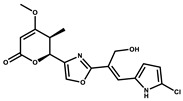
|
Pyrrole | Nannocystis pusilla | Shows weak antifungal activity. | [75] |
| Ripostatin B |
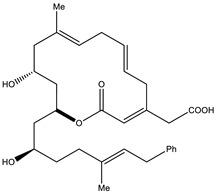
|
Polyketide | Sorangium cellulosum | Effective in treating tuberculosis. | [99] |
| Roimatacene |
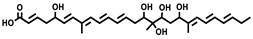
|
Cyclic peptide | Cystobacter ferrugineus | Exhibits antibacterial activity. | [74] |
| Saframycin Mx1 |
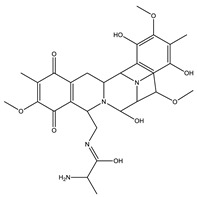
|
α-cyanoamine | Myxococcus xanthus | Acts as a broad-spectrum inhibitor for a wide range of Gram-positive and halobacteria. Shows week activity against Gram-negative bacteria. | [3,47,53] |
| Salimyxin A and Salimabromide |
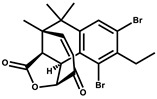
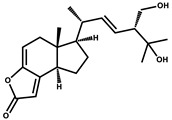
|
Sterol, Furano lactone |
Enhygromyxa salina | Effective against Arthrobacter cristallopoietes. | [100] |
| Sesqiterpene |
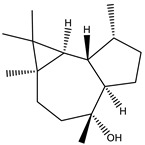
|
Terpenes | Sorangium cellulosum | Exhibits antimicrobial activity. | [101,102] |
| Sorangicin |
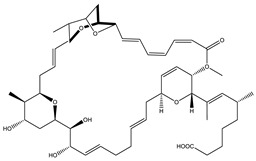
|
Macrolides | Sorangium cellulosum | Exhibits antimicrobial activity. | [82] |
| Sorangiadenosine |
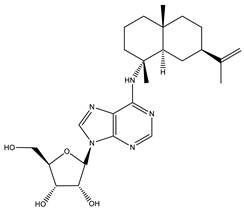
|
Macrolides | Sorangium cellulosum | Exhibits antimicrobial activity. | [101] |
| Soraphinol |
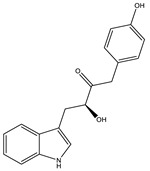
|
Macrolides | Sorangium cellulosum | Exhibits antimicrobial activity. | [103] |
| Sorazinnone |
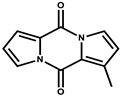
|
Pyrazinone | Pyxidicoccus fallax | Siderophore production. Exhibits antibacterial activity. | [75] |
| Sorazolon |
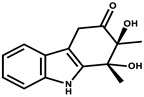
|
Indole | Sorangium cellulosum | Weak activity against Gram-positive bacteria. | [104] |
| Stigmatellin |
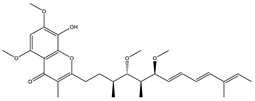
|
Macrolactone | Stigmatella aurantica | Exhibits strong antifungal activity. Inhibits quinol oxidation of mitochondrial cytochrome bc1 complex. | [3,47,53] |
| Sulfangolid |
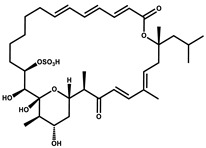
|
Macrolactone | Sorangium cellulosum | Exhibits antiviral (HIV-1) activity. | [105,106] |
| Thuggacin |
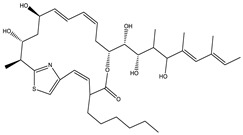
|
Macrolactone | Sorangium cellulosum | Effective against Mycobacterium tuberculosis. | [107,108] |
| Bioactive compounds exerting cytotoxic effects | |||||
| Aetheramide |
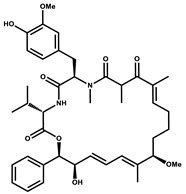
|
Cyclic peptide | Atherobacter rufus | Shows cytotoxic and moderate antifungal activity. | [109,110,111] |
| Archazolid |
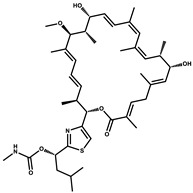
|
Macrolactone |
Archangium gephyra, Cystobacter violaceus |
Exhibits cytotoxic and antitumor activity. Inhibits V-ATPase. | [112] |
| Argyrin |
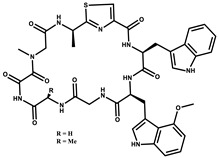
|
Peptolide | Archangium gephyra | Acts as a potential inhibitor of antibody formation by murine B-cells. Exhibits antibacterial and cytotoxic activity. | [113] |
| Bengamide |
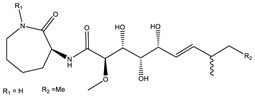
|
Caprolactam | Myxococcus virescens | Shows cytotoxic, antitumor, antibacterial and anthelmintic activity. Inhibits MetAP. Acts as an anti-inflammatory. | [114] |
| Chivosazol |
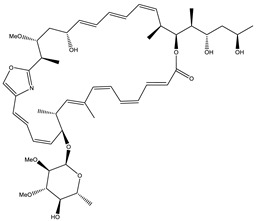
|
Peptide | Sorangium cellulosum | Effective antifungal activity at higher concentration. Exhibits strong cytotoxic activity. Destroys cyto-skeleton. | [3,47,53] |
| Chrondramide |
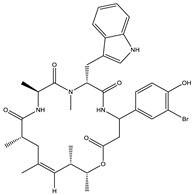
|
Depsipeptide | Chondromyces crocatus | Exhibits strong cytotoxic activity; effective against breast cancer metastasis. | [3,47,53] |
| Cystodienoic acid |
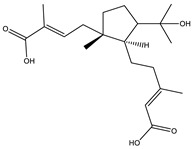
|
Terpene | Cystobacter ferrugineus | Exhibits cytotoxic activity. | [115] |
| Disorazol |
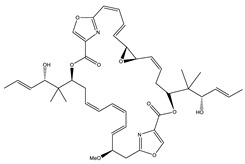
|
Peptide | Sorangium cellulosum | Exhibits strong antifungal activity; inhibits proliferation of different cancer cell lines. | [116] |
| Eliamid |
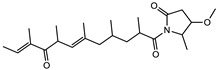
|
polyketide | Sorangium cellulosum | Exhibits cytotoxic activity; shows moderate anthelmintic and antifungal activity; acts as a respiratory chain inhibitor. | [117] |
| Epothilone |
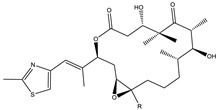
|
Peptide | Sorangium cellulosum | Acts as an inhibitor of microtubule function concerning cell division. | [118] |
| Haliamide |
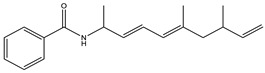
|
Polyene | Haliangium ochraceum | Exhibits moderate cytotoxic activity. | [28] |
| Hyafurone |
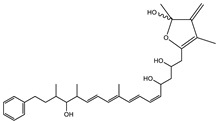
|
Polyketide, furanone | Hyalangium minutum | Exhibits moderate cytotoxic activity, as well as showing marginal antiparasitic activity. | [73] |
| Miuraenamide |
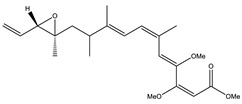
|
cyclic depsipeptides | Paraliomyxa miuraensis | Exhibits antibacterial and cytotoxic activity. | [119] |
| Nannocystin |
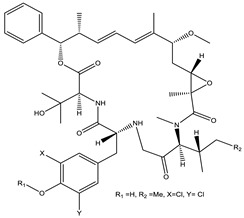
|
Macrocyclic epoxyamide | Nannocystis sp. | Exhibits strong antifungal and cytotoxic activity; inhibits eukaryotic translation elongation factor 1α. | [120,121] |
| Pellasoren |
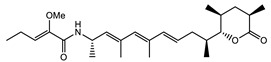
|
Polyketide | Sorangium cellulosum | Exhibits cytotoxic activity. | [51,122] |
| Ratjadone A |
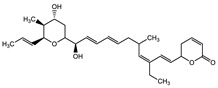
|
α-pyrone | Sorangium cellulosum | Acts as an antiviral drug. Inhibits HIV infection by ceasing the Rev/CRM1-mediated nuclear export. | [106] |
| Rhizopodin |
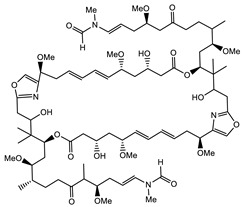
|
Amide | Myxococcus stipitatus | Effective against cancer cell lines. Interferes with cytoskeleton assembly. Acts as a strong antiviral. | [3,123] |
| Spirangien |
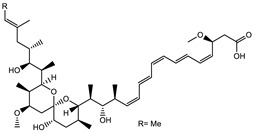
|
Polyketide | Sorangium cellulosum | Exhibits antifungal, cytotoxic, antiviral (HIV) and anti-inflamatory activity. | [124] |
| Tubulysin |
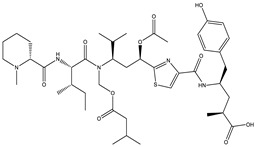
|
Peptide |
Archangium gephyra and Angiococcus disciformis |
It has been found to be effective in treating the cancer associated with Luteinizing Hormone-releasing hormone receptor. Effective in cell cycle arrest at G2/M phase. | [125] |
| Bioactive compounds exerting beneficial effects in agriculture | |||||
| Ambruticin |
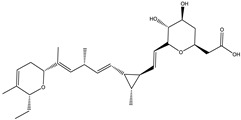
|
Polyketide | Sorangium cellulosum | Acts as a fungicide, effective against Hansenula anomala and other plant pathogens such as Botrytis cinerea, via interference in osmoregulation system. | [126] |
| Pyrrolnitrin |
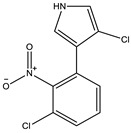
|
Pyrrole | Myxococcus fulvus, Carallococcus exiguous, Cystobacter ferrugineus | Exhibits strong antifungal activity. | [3,47,53] |
| Tartrolon |
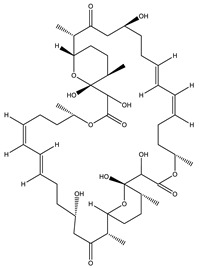
|
Pilyketide | Sorangium cellulosum | Effective against Gram-positive bacteria and mammalian cells. | [127] |
| Thiangazol |
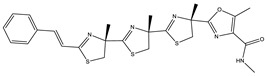
|
Amide | Polyangium sp. | Exhibits strong antifungal, acaricidal and insecticidal activities, as well as having anti-HIV activity. | [128] |
| Soraphen A |
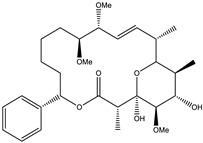
|
Polyketide | Sorangium cellulosum | Effective as a plant disease control agent. Possesses strong antifungal activity. Acts as a broad-spectrum antiviral (effective against HIV and Hepatitis C Virus). | [129,130] |
4. Pharmacological Effects of Myxobacteria-Derived Bioactive Compounds
Myxobacteria, an adaptable cosmopolitan, produces a wide range of bioactive molecules. About 40% of Myxobacteria-derived compounds represent novel (mostly non-glycosylated) chemical structures that act against targets often not covered by compounds derived from Actinomycetes, Bacillus and Pseudomonas. A variety of bioactive compounds produced by Myxobacterial spp. play a vital role in biological activities, and mostly, their activities are antifungal, antibacterial, anti-cancerous, antiparasitic and immunomodulatory.
4.1. Myxobacteria and Infectious Diseases
Before the advent of an era of widely accessible anti-infectious agents, mankind was considered vulnerable to infections such as cholera, which reached the extent of epidemics that caused a huge loss of human lives [131]. With the passage of time, the period of anti-infectious agents moved along from quinine (utilized against fever), to Salvarsan (arsenic compound used against syphilis) and Sulpha drugs such as Protonsil (utilized against diseases caused by Gram-positive cocci). The circumstances profoundly improved with the discovery of the β-lactam drug Penicillin, from Penicillium spp. [132]. The era of antibiotics moved on to aminoglycosides [133], macrolides [134] and so on to treat ailments that were considered untreatable. Inaccurate recommendation and wrong use of antibiotics in human medication, veterinary and horticulture expanded portability, and as such, quick spread of microbes, that raised alarm regarding the use of multi-tranquilize safe microbes. Many pharmaceutical companies withdrew from manufacturing new drugs due to high-cost screening systems developed for nosocomial infections caused by ESKAPE (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacter spp.) pathogens [135]. With less new medications, the dying antimicrobial pipeline caused by an absence in development and inefficient ways of screening bioactive substances presented a dreadful situation that led to obstruction in the production of drugs [136,137]. The bottlenecks that choked the production of anti-infective agents prompted qualified countermeasures to be implemented regarding improvements in the production of engineered medications, proper screening of the metabolite markers, followed by assessment of the rediscovered drugs. At this instance, exploration of new genera and species are of extraordinary intrigue [138] as it may involve the creation of auxiliary metabolites in scaleup forms or fitting hardware for maturation and release of substances from fermenter stock for resolving biotic and abiotic conditions of the maker strain.
Myxobacteria, together with actinomycetes [139] and Bacillus spp., are considered as the best producers of bioactive compounds [140]. A large proportion of Myxobacteria-derived bioactive compounds (29%) displaying antibacterial properties reflect their competitiveness for existence in their natural habitats. These characteristic products demonstrate a more extensive scope of biological activities which are regularly less direct to rationalize, as the production of regular objects from different Myxobacterial spp. requires regular screening and enormous scaleup development [6].
4.2. Myxobacteria and Viral Diseases
4.2.1. Human Immunodeficiency Virus (HIV)
Human Immunodeficiency Virus is a single-strand RNA (ssRNA) lentivirus which targets human immune cells, and integrates into host DNA by reverse transcription. Secondary metabolites extracted from different Myxobacterial strains are reported to play crucial roles against HIV. The Sulfangolids are an important class of antiviral secondary metabolites secreted by different strains of Sorangium cellulosum [105]. Myxobacterial extracts such as spirangien B, sulfangolid C, soraphen F and epothilon D at different concentrations showed impressive activity against HIV [124]. Soraphens exert antiviral activity by inhibiting acetyl-CoA carboxylate transferase [141], while epothilones stabilize the activity of macrophage microtubuli in a parallel way to Taxol® [142,143]. Ixabepilone®, an FDA-registered anticancer drug, is derived from epothilone B [144]. Epothilon D and spirangien B are believed to decrease the phosphorylation, and as such degradation of inhibitor of kappa B (IkBS) [143,145]. Rhizopodin, a well-known actin inhibitor, extracted from Myxococcus stipitatus [124], interferes in virus synapses and hence blocks the virological synapse arrangement. Stigmatellin extracted from Stiginatella aurantiaca Sga15, disorazol extracted from Sorangium cellulosum Soce 56 and tubulysin extracted from Archangium gephyrs strain Ar315 shows mild anti-HIV activity [124], while Phenalamide A1, phenoxan and thiangazole separated from Polyangium sp. and Myxococcus stipitatus strain Mxs40 suppress HIV-1-mediated cell death in the MT-4 cell assay, thereby exhibiting high anti-HIV activity [146]. Aetheramide A and B isolated from the genus Aetherobacter, that inhibits HIV-1 infection, show IC50 values of 0.015 and 0.018 M, respectively [109,147,148]. Similarly, Ratjadon A (a compound isolated from Sorangium cellulosum Soce 360), capable of blocking the Rev/CRM1-mediated nuclear export, inhibits HIV infectivity; however, its toxicity and low SI value becomes a limiting factor for its exploitation as a potential therapeutic molecule [106,149].
4.2.2. Human Cytomegalovirus (HCMV)
Infections of Human Cytomegalovirus are associated with diseases such as glandular fever and pneumonia. Myxochelin, a secondary metabolite obtained from different Myxobacterial strains, responsible for iron uptake during iron-limiting circumstances, was found to be a potent antitumor agent [87,150,151]. The ability of nannochelins and hylachelins (siderophores of Myxobacterial source) in inhibiting the human 5-lipoxygenase (5-LO, a gene associated with the proliferation of cancerous cells) were found exerting antitumor activity [87,142,143,144,145,146,147,148,149,150,151,152,153,154]. It is believed that a similar pathway of inhibiting 5-LO is associated with the strong anticancer activity of myxochelin [153,155]. Of the different Myxochelins, which are either isolated from Angiococcus disciformis (strain And30) or synthesized [155,156], Myxochelin C is capable of inhibiting HCMV (IC50 value of 0.7 g/mL) [150,157]. It opens avenues for testing other known siderophores, such as nannochelins, hylachelins and myxochelin analogues, in the future for their possible role in inhibiting HCMV [158]. Additionally, structure–activity relationships of the siderophores need to be studied for possible discovery of more potent antivirals [123].
4.2.3. Ebola Virus Disease (EVD)
Ebola virus (EBOV) is a single-stranded RNA virus which causes hemorrhagic fever. Different metabolites extracted from Myxobacteria were analyzed for their possible activity in inhibiting the Ebola virus using GP-pseudo-typed lentiviral vectors expressing Ebola envelope glycoprotein [97]. Chondramides extracted from the genus Chondromyces [159] of Myxobacteria were found capable of inhibiting the EBOV-GP-mediated transduction [123]. Noricumazole, a polyketide extracted from Sorangium cellulosum, exerts an EBOV-GP inhibitory effect with an IC50 value of 0.33 M. [97]. The secondary metabolite is believed to lower the virulence of EBOV via blocking of the potassium channels [76,97].
4.2.4. Hepatitis C Virus (HCV)
Hepatitis C virus, a single-stranded RNA virus, undergoes transmission through blood transfusions. Heterocyclic metabolites such as labindoles A and B [160], 3-chloro-9H-carbazole and 4-hydroxymethyl-quinoline extracted from Myxobacterial strain Labilithrix luteola, exert potent antiviral activity, and thereby help to overcome the effects of HCV [160]. Of the different macrolactones, Soraphens A obtained from Myxobacterial species was found to inhibit HCV replication in in vitro HCV culture models (cells in sub-genomic and full-length replicons) and in cell culture-adapted virus with an IC50 value of 5 nM [96,161,162,163]. Lanyamycin, a macrolide obtained from Sorangium cellulosum (strain Soce 481) that exhibits similarity to bafilomycins of actinobacteria effective against influenza A virus (IC50 value of 0.1 nM), was found to moderately inhibit HCV [96,160,164].
4.3. Myxobacterial Metabolites as Anti-Neurodegenerative Diseases
Inside the cell, the endoplasmic reticulum (ER) helps in the processing of proteins before their transport to the target sites. However, any kind of ER dysfunction due to protein misfolding may lead to neurodegenerative disorder or cell death [165,166,167]. Myxobacterial secondary metabolites act on protein GRP78/Bip, which helps to release any kind of stress created in the ER [168]. It also decreases the release of apoptosis-inducing factor (AIF) and cytochrome C (an apoptosis-related marker proteins). Therefore, Myxobacterial secondary metabolites help in combating the Parkinson’s disease (PD) pathology via decreasing the ER stress, which contributes to inhibition of cell apoptosis [169]. Microtubules play a major role in the axoplasmic transport of different constituents of the cell (mitochondria, synaptic vesicles, lipids, proteins) [170]. Neurodegenerative diseases such as Alzheimer’s disease (AD), Amyotrophic lateral Sclerosis (ALS) and PD arise by distraction in the axoplasmic transport due to microtubules linked to tau proteins—the phenomenon known as tauopathy [171,172,173,174,175]. Epothilones (A–F) are a particular class of secondary metabolites produced by Sorangium cellulosum strain So ce90 that exhibit antifungal and anti-cancerous potential [176]. These compounds bind to microtubules and help them in stabilization, hence resulting in the elevation of axoplasmic transport in neurodegenerative disorders [177]. Of the different Epothilones, Epothilone D plays an important role in improving the axonal transport, as well as protecting cognitive deficits in a mouse tauopathy model having overexpression of P301S (a mutant tau), thereby contributing to inhibition of tau pathology [178]. Epothilone D also plays an active role in alleviating the microtubule defects in a C57Bl model of PD [179].
Neurodegenerative diseases such as PD, AD and Huntington’s Disease (HD) are the outcomes of different mitochondrial dysfunctions [180]. Earlier studies predicted that certain prokaryotes have the ability to synthesize PUFAs, however, these predictions failed as some extremophilic bacteria which inhabit extreme environments of seas and oceans invalidated this hypothesis [181,182]. Among different terrestrial prokaryotes, Myxobacteria are considered as a major contributor of PUFAs [183]. In the studies employing the genome mining approach, two Myxobacterial species, Sorangium and Aetherobacter, were found, having different organization of gene clusters associated with biosynthetic PUFA compared with their marine counterparts [184]. Myxobacterial omega 3 PUFAs play an antagonistic role against prenatal stress, which arises from mitochondrial abnormalities such as changes in mitochondrial complexes, DNA damage and memory deficiency [185,186]. Having a remarkable effect regarding the phospholipid profile, and as such fluidity of the mitochondrial membrane, DHA was observed to play a critical role in maintaining stability of the structure, and as such functions of the mitochondrial membrane, and thereby in non-amyloidogenic processing of APP in the HEK-APP cell line [187].
Immune Modulating Myxobacterial Compounds
Employment of Myxobacterial secondary metabolites such as Soraphen A, bengamide A and B and Spirangiens as immune-enhancing compounds has attracted the attention of different researchers throughout the world [188]. Castro et al. worked out the immune-enhancing responses of Soraphen A [189]. Acting on the biotin carboxylase (BC) domain, Soraphen A extracted from Sorangium cellulosum So ce26 was found to exert an inhibitory effect on acetyl-CoA carboxylase (ACC) [141]. Bengamides, an important class of secondary metabolites produced by Myxococcus virescens, exert both anti-inflammatory as well as immune-boosting effects via regulation of the nuclear factor-KB (NF-KB) and pro-inflammatory cytokines (IL-6, TNFα and MCP-1) [190]. Spirangien A produced by Sorangium cellulosum strain So ce90 shows antifungal activity, as well as suppressing transcription of IL-8 in response to IL-1 (cytotoxic activity). The compound along with its derivative, spirangien M522, were found effective in inhibiting IL-8 gene expression in the HeLa cell line [145].
4.4. Myxobacterial Compounds Attributing Cytotoxic Effects
Myxobacterial secondary metabolites display unique structural properties and exhibit novel modes of action. These metabolites mainly target the cellular structures that are rarely hit by metabolites from other sources.
4.4.1. Compounds Targeting Electron Transport
Myxobacterial compounds such as crocacins [191] and aurachin C [192,193], along with a group of closely related bithiazole derivatives, particularly myxothiazol, cystothiazol and melithiazole [66,194,195,196], were found effective in inhibiting mitochondrial respiration through interference in the functioning of complex-I (NADH-Ubiquinone oxidoreductase) and complex-III (Cyt b–C1 complex). Stigmatellin was found to exert its inhibitory effect at complex III of the mitochondria [6] and Cyt b6/f of the photosynthetic apparatus in plants [197,198,199].
4.4.2. Compounds Targeting RNA and Protein Synthesis
With enormous potential to lead as building blocks for drug development, compounds of Myxobacteria origin such as saframycin tie to DNA [200], ambruticin helps in osmoregulation of fungi [126] and gephyronic acid [201] and myxovalargin [93,202] repress eukaryotic and prokaryotic protein synthesis, respectively [83]. Etnangien is a metabolite that targets protein synthetic machinery via inhibition of the eubacterial RNA polymerases. In addition to rifampicins utilized maximally in clinics, other inhibitors of RNA polymerase of Myxobacterial origin include thiolutin [203,204], streptolydigin [205] and holomycin [206]. These molecules (ripostatin and corallopyronin) show no cross-resistance with rifamycin, and likewise concentrate on the commencement of RNA synthesis [207]. Acting in an alternate way to rifamycin, it is believed that these metabolites can potentially be used to overcome rifamycin resistance in bacteria [208,209]. Inhibition of the protein synthetic machinery is mediated by both naturally occurring compounds such as sorangicins and ripostatins that exert their effect during initiation (sorangicins) [210,211] and chain elongation (ripostatins) [212,213], as well as by chemically related myxopyronins [93] and corallopyronins [214].
Compounds of Myxobacterial origin (10% of Myxobacterial compounds), that interfere with the microtubule assembly (cytoskeleton) and thereby hinder cell proliferation and promote apoptosis, are currently being used in cancer chemotherapies. Similar to notorious fungal toxins obtained from mushrooms (preferably green and white cap mushrooms), Myxobacterial compounds such as rhizopodin [215,216] and chondramides [159,217,218] are reported to work explicitly on the actin [214]. Though all chondramide variants exert similar effects, chondramide C was found to be most effective in its action on actin [217]. Of the different compounds, a few compounds, such as epothilones [219,220], play important roles in retaining tubular polymerization under in vitro conditions, while others, such as tubulysins [221,222], favor depolymerization events of the tubulin. Epothilones and their analogs have shown antitumor activity towards multidrug-resistant and paclitaxel-safe tumor cell lines [223]. In 2007, the FDA recommended Ixabepilone (IxempraTM)—a derivative of epothilone—for the treatment of metastatic breast cancer, while epothilones B and D are currently undergoing clinical trials [224]. From the tubulysins class, tubulysin D displays action that surpasses other tubulin modifiers, such as taxol, epothilones and vinblastine, by 20–100-fold [225,226]. Additionally, tubulysin A is currently explored for its pharmacological properties related to its use as an antiangiogenic and antiproliferative agent [227].
4.4.3. Other Activities
Soraphen A from Sorangium cellulosum was found to hinder normal functioning of acetyl-CoA carboxylase through interference with its biotin carboxylase (BC) domain. With its novel modus operandi, Soraphen A has explicit utility as a promising therapeutic (novel inhibitor of ACCs) in the treatment of cancers [3,228]. Its utility as a potent inhibitor in cancers hindered by its poor water solubility and less bioavailability is overruled through generation of either structural variants of this metabolite or through the genetic engineering approach, upholding its bioactivity.
4.5. Myxobacteria and Plant Diseases of Bacterial and Fungal Origin
Although the contribution of Myxobacteria to plant health remains largely unexplored, studies have assessed the role of Myxobacterial secondary metabolites in the predation of microorganisms and other plant pathogens. Based on their ability to degrade biomolecules, two groups of Myxobacterial spp., i.e., bacteriolytic and cellulolytic, have been formed [229]. The Myxobacteria of the bacteriolytic category produce a large number of agriculturally important compounds such as pyrrolnitrin, a thiangazoletic that acts as an antagonistic in the control of phytopathogens that destroy crops [230]. Pyrrolnitrin produced by Myxobacterial spp. (Myxococcus fulvus, Cystobacter ferrugineus and Corallococcus exiguous) was found effective in controlling the damping-off of diseases of cotton caused by Rhizoctonia solani [229,230]. The ability of Myxobacteria to utilize cellulose categorizes them into two groups: Group I, capable of utilizing inorganic nitrogen compounds during their growth on cellulose and glucose sources (members of the Sorangineae suborder), and Group II, unable to make direct use of cellulose (majority of Myxobacterial spp.) and as such, dependent on enzymatic degradative products of proteins (peptides and amino acids) as their source of nitrogen [230]. Under natural conditions, Group II Myxobacterial spp. causes lysis of other organisms, such as eubacteria, via secretion of exoenzymes (proteases, lipases, xylanases, etc.). The lysate generated thereof is used as a nutrient by these Myxobacterial spp., and tags them with the name “micro-predators” [231], Myxobacterial proteolytic enzymes exhibiting both cellulolytic (genus Sorangium) and predatory roles (genus Myxococcus). These proteases are believed to perform lysis of prey, cellular membrane disruption for cytoplasmic content release and protein hydrolysis for supplying amino acids to the Myxobacteria-like functions [232]. Lipids containing fatty acids c16:1ω5c, utilized along with proteins as an energy and carbon source during the growth of myxobacteria, play pivotal roles in the predation by acting as chemo-attractants for the prey. In Myxococcus xanthus, lipolytic enzymes belonging to three families—α/β hydrolases, patatin and GDSL lipases—disintegrate the membrane barrier, thereby releasing fatty acids and cytoplasmic contents of the prey. Genus Polyangium was found perforating, and as such lysing, the conidia of Cochliobolus miyabeanus and hyphae of R. solani. Genus Sorangium reduces damping-off of conifers in addition to lysis of microorganisms under culture conditions [231,232]. Additionally, the production of unsaturated fats by Myxoxoccus xanthus was found to exert an inhibitory effect on the growth of Fusarium roseum [233]. Taken together, the production of agriculturally important compounds along with a series of lytic enzymes show that Myxobacteria have potential for use as biocontrol agents.
5. Techniques for Exploring Myxobacterial Metabolites
As emerging endeavors of whole-genome sequencing together with metabolic profiling of Myxobacterial species revealed high profundity of secondary metabolites, it becomes necessary to have information on mining genomes of both terrestrial and marine Myxobacteria for novel metabolites [234]. It becomes obligatory to have a strategic plan regarding the methodology (in terms of media composition, temperature, pH, along with others) adopted for identification of secondary metabolites from cultivated strains under standard research laboratory conditions. One such strategy is OSMAC (one strain many compounds), initially introduced in Actinomycetes and fungi during isolation of new secondary metabolites [235]. Traditional but untested strategies for isolation of secondary metabolites include inoculation of microorganisms into the culture, much like induction of cytotoxic compounds [236].
Optimization of the growth conditions along with addition of the explicit precursors would be a way to support generation and expansion of the metabolite yield [234]. The adoption of the genetic engineering techniques for producing a strain with desired characters can be achieved. For instance, overexpression of a particular gene activator regulating biosynthesis of a cryptic gene cluster might be activated, as recently illustrated for the fungus Aspergillus nidulans [237]. The irregular transposon mutagenesis approach was adopted to obtain genetic information regarding gene clusters of metabolites produced from a prepared cosmid library of the strain [238]. The methodology helped in obtaining information of the gene clusters for ambruticin/jerangolid [239,240], aurachin [240,241], disorazol [242] and tubulysin [243] metabolites. In Cystobacter fuscus Cb f17, irregular transposon mutation helped in the recognition of a particular regulatory element for a metabolite [244]. The adopted methodology helped in unravelling information of the biosynthetic gene cluster with two components (StiR) associated with the synthesis of the polyketide stigmatellin. Recognition of ChiR protein following detachment of the promoter binding protein by the biomagnetic bead assay revealed its role in the biosynthesis of the metabolite chivosazol in Sorangium cellulosum So ce56, as its overexpression led to a 5-fold increase in the production of chivosazol [245]. Alternatively, intentional inactivation of the gene cluster followed by screening of mutants for non-production of the explicit metabolite compared with the wild phenotypes helped in the recognition of myxochelins, myxochromides and aurafurones [246,247]. Additionally, shot-gun genome sequencing can be adopted to obtain information of the gene clusters for the identification of different metabolites, as observed for phosphoglycolipid moenomycin A [248,249].
To overcome the problem of recalcitrance of a strain for manipulation, heterologous expression of gene clusters (both orphan and known) in a suitable host that offers advantages for genetic manipulation seems a suitable alternative for exploring the function of genes [247]. Using specific hosts such as Myxococcus xanthus and a few other bacterial strains such as Pseudomonas, it is possible to arrange different gene sets in a codon-optimized manner for heterologous expression that abolishes the requirement for genetic engineering of the host [250]. Though Myxococcus xanthus shares codon usage and other physiological parameters with a majority of the Myxobacterial species, Pseudomonads offers the advantage of a growth rate on par with E. coli, with plasmids harboring inducible promoters. Examples of heterologous expression of gene clusters for metabolites, such as epothilone in M. Xanthus [251], Streptomyces coelicolor [252] and E. coli [253], myxochromide S in Pseudomonas putida [247,254], soraphen in Streptomyces lividans [255], myxothiazol in both M. Xanthus [256] and P. putida [257] and flaviolin in three Pseudomonas strains [257], are available. Employment of Red/ET recombination technology has overcome the limitation of cluster reconstruction associated with the heterologous gene expression by enabling reconstruction of gene clusters onto a suitable vector [258]. Recently, an approach of combining Myxobacterial biosynthetic machineries has been explored for production of novel metabolites in a so-called combinatorial biosynthesis approach [259].
6. Conclusions and Future Perspectives
The escalating problem of resistance against the current regime of antibiotics has increased concern, particularly related to treatment of human diseases. It has resulted in a community crisis, necessitating the requirement to undertake studies towards development of effective alternatives that could replace or supplement the antibiotics in counteracting occurrence at a global scale. Based on this scenario, studies were undertaken to explore natural resources towards the development of potent products that offer promise for treatment of different diseases. Exhibiting potent antimicrobial activity, secondary metabolites of microbial origin (in particular Myxobacteria) were investigated for possible use in the prevention and treatment of diseases. Myxobacteria, a highly adaptable and cosmopolitan group of microorganisms, were screened at genome and metabolome levels for identification and characterization of metabolites that can serve as potent lead structures for drug development. Evaluation of the rich repertoire of Myxobacterial metabolites for safety, specificity, distribution, immune modulation and anti-infectivity potential revealed information of novel antimicrobials that offer great potential to be utilized in the manufacturing of drugs. Despite the fact that Myxobacterials exhibit survival under different habitats and extreme climatic conditions, secondary metabolites of Myxobacterial origin were found effective in the treatment of a wide range of diseases. Studies need to be undertaken to gain insight into the production mechanism that holds promise in elucidating the regulatory circuit of different secondary metabolites towards optimal design of a strategic plan for enhancing their production. Alongside strategic approaches for elucidating the potency of the secondary metabolites using recently developed techniques that offer flexibility to approval strategies, consistency in safety, efficacy and delivery methods need to be adapted to broaden exploration, and as such adoption of the secondary metabolites of Myxobacterial origin.
Acknowledgments
The authors extend their appreciation to their fellow colleagues, whose helped in improving the contents of the manuscript.
Author Contributions
Conceptualization, A.A.S. and A.T.J.; writing—original draft preparation, M.A.B. (Mudasir Ahmad Bhat), M.A.B. (Mujtaba Aamir Bhat) and A.K.M.; writing—editing, M.I.B., S.R., I.A.R. and A.T.J.; structures, O.B.; supervision, A.A.S. and A.T.J.; funding acquisition, A.K.M., I.A.R. and A.T.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Department of Science and Technology, India, under the Science and Engineering Research Board (DST-SERB), grant no. CRG/2019/004106.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gerth K., Pradella S., Perlova O., Beyer S., Müller R. Myxobacteria: Proficient producers of novel natural products with various biological activities-Past and future biotechnological aspects with the focus on the genus Sorangium. J. Biotechnol. 2003;106:233–253. doi: 10.1016/j.jbiotec.2003.07.015. [DOI] [PubMed] [Google Scholar]
- 2.Kim Y.S., Bae W.C., Back S.J. Bioactive substances from myxobacteria. Korean J. Microbiol. Biotechnol. 2003;31:1–12. [Google Scholar]
- 3.Weissman K.J., Muller R. Myxobacterial secondary metabolites: Bioactivities and modes-of-action. Nat. Prod. Rep. 2010;27:1276–1295. doi: 10.1039/c001260m. [DOI] [PubMed] [Google Scholar]
- 4.Dawid W. Biology and global distribution of myxobacteria in soils. FEMS Microbiol. Rev. 2000;24:403–427. doi: 10.1111/j.1574-6976.2000.tb00548.x. [DOI] [PubMed] [Google Scholar]
- 5.Shimkets L.J., Dworkin M., Reichenbach H. The Prokaryotes. Springer; New York, NY, USA: 2006. The myxobacteria; pp. 31–115. [Google Scholar]
- 6.Reichenbach H. Myxobacteria, producers of novel bioactive substances. J. Ind. Microbiol. Biotech. 2001;27:149–156. doi: 10.1038/sj.jim.7000025. [DOI] [PubMed] [Google Scholar]
- 7.Sanford R.A., Cole J.R., Tiedje J.M. Characterization and Description of Anaeromyxobacter dehalogenans gen. nov., sp. nov., an Aryl-Halorespiring Facultative Anaerobic Myxobacterium. Appl. Environ. Microbiol. 2002;68:893–900. doi: 10.1128/AEM.68.2.893-900.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma G., Yao A.I., Smaldone G.T., Liang J., Long M., Facciotti M.T., Singer M. Global gene expression analysis of the Myxococcus xanthus developmental time course. Genomics. 2021;113:120–134. doi: 10.1016/j.ygeno.2020.11.030. [DOI] [PubMed] [Google Scholar]
- 9.Wrótniak-Drzewiecka W., Brzezińska A.J., Dahm H., Ingle A.P., Rai M. Current trends in myxobacteria research. Ann. Microbiol. 2016;66:17–33. doi: 10.1007/s13213-015-1104-3. [DOI] [Google Scholar]
- 10.Livingstone P.G., Morphew R.M., Whitworth D.E. Genome Sequencing and Pan-Genome Analysis of 23 Corallococcus spp. Strains Reveal Unexpected Diversity, With Particular Plasticity of Predatory Gene Sets. Front. Microbiol. 2018;9:3187. doi: 10.3389/fmicb.2018.03187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sajedi H., Mohammadipanah F., Pashaei A. Automated identification of Myxobacterial genera using Convolutional Neural Network. Sci. Rep. 2019;9:18238. doi: 10.1038/s41598-019-54341-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohr K.I. Diversity of Myxobacteria-We Only See the Tip of the Iceberg. Microorganisms. 2018;6:84. doi: 10.3390/microorganisms6030084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Azam M., Jan A.T., Haq Q.M.R. blaCTX-M-152, a Novel Variant of CTX-M-group-25, Identified in a Study Performed on the Prevalence of Multidrug Resistance among Natural Inhabitants of River Yamuna, India. Front. Microbiol. 2016;7:176. doi: 10.3389/fmicb.2016.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hemlata, Jan A.T., Tiwari A. The Ever-Changing Face of Antibiotic Resistance: Prevailing Problems and Preventive Measures. Curr. Drug Metab. 2017;18:69–77. doi: 10.2174/1389200217666161014163324. [DOI] [PubMed] [Google Scholar]
- 15.Hemlata, Bhat M.A., Kumar V., Ahmed M.Z., Alqahtani A.S., Alqahtani M.S., Jan A.T., Rahman S., Tiwari A. Screening of natural compounds for identification of novel inhibitors against β-lactamase CTX-M-152 reported among Kluyvera georgiana isolates: An in vitro and in silico study. Microb. Pathog. 2021;150:104688. doi: 10.1016/j.micpath.2020.104688. [DOI] [PubMed] [Google Scholar]
- 16.Sultan I., Rahman S., Jan A.T., Siddiqui M.T., Mondal A.H., Haq Q.M.R. Antibiotics, Resistome and Resistance Mechanisms: A Bacterial Perspective. Front. Microbiol. 2018;9:2066. doi: 10.3389/fmicb.2018.02066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.AlSheikh H.M.A., Sultan I., Kumar V., Rather I.A., Al-Sheikh H., Tasleem Jan A., Haq Q.M.R. Plant-Based Phytochemicals as Possible Alternative to Antibiotics in Combating Bacterial Drug Resistance. Antibiotics. 2020;9:480. doi: 10.3390/antibiotics9080480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ringel S.M., Greenough R.C., Roemer S., Connor D., Gutt A.L., Blair B., Kanter G., von Strandtmann M. Ambruticin (W7783), a new antifungal antibiotic. J. Antibiot. 1977;30:371–375. doi: 10.7164/antibiotics.30.371. [DOI] [PubMed] [Google Scholar]
- 19.Wu Z.H., Jiang D.M., Li P., Li Y.Z. Exploring the diversity of myxobacteria in a soil niche by myxobacteria-specific primers and probes. Environ. Microbiol. 2005;7:1602–1610. doi: 10.1111/j.1462-2920.2005.00852.x. [DOI] [PubMed] [Google Scholar]
- 20.Hook L.A. Distribution of Myxobacters in Aquatic Habitats of an Alkaline Bog. Appl. Environ. Microbiol. 1977;34:333–335. doi: 10.1128/aem.34.3.333-335.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rückert G. Myxobakterien-Artenspektren von Boden in Abhängigkeit von bodenbildenden Faktoren unterbesonderer Berücksichtigung der Bodenreaktion. Z. Pflanzenernaehr. Bodenkd. 1979;142:330–343. doi: 10.1002/jpln.19791420307. [DOI] [Google Scholar]
- 22.Reichenbach H. The ecology of the myxobacteria. Environ. Microbiol. 1999;1:15–21. doi: 10.1046/j.1462-2920.1999.00016.x. [DOI] [PubMed] [Google Scholar]
- 23.Li S.G., Zhou X.W., Li P.F., Han K., Li W., Li Z.F., Wu Z.H., Li Y.Z. The existence and diversity of myxobacteria in lake mud-A previously unexplored myxobacteria habitat. Environ. Microbiol. Rep. 2012;4:587–595. doi: 10.1111/j.1758-2229.2012.00373.x. [DOI] [PubMed] [Google Scholar]
- 24.Brockman E.R. Fruiting myxobacteria from the South Carolina coast. J. Bacteriol. 1963;94:1253–1254. doi: 10.1128/jb.94.4.1253-1254.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Felder S., Dreisigacker S., Kehraus S., Neu E., Bierbaum G., Wright P.R., Menche D., Schäberle T.F., König G.M. Salimabromide: Unexpected chemistry from the obligate marine myxobacterium Enhygromxya salina. Chemistry. 2013;19:9319–9324. doi: 10.1002/chem.201301379. [DOI] [PubMed] [Google Scholar]
- 26.Felder S., Kehraus S., Neu E., Bierbaum G., Schäberle T.F., König G.M. Salimyxins and enhygrolides: Antibiotic, sponge-related metabolites from the obligate marine myxobacterium Enhygromyxa salina. Chem. Bio. Chem. 2013;14:1363–1371. doi: 10.1002/cbic.201300268. [DOI] [PubMed] [Google Scholar]
- 27.Fudou R., Iizuka T., Sato S., Ando T., Shimba N., Yamanaka S. Haliangicin, a novel antifungal metabolite produced by a marine myxobacterium. 2. Isolation and structural elucidation. J. Antibiot. 2001;54:153–156. doi: 10.7164/antibiotics.54.153. [DOI] [PubMed] [Google Scholar]
- 28.Sun Y., Tomura T., Sato J., Iizuka T., Fudou R., Ojika M. Isolation and Biosynthetic Analysis of Haliamide, a New PKS-NRPS Hybrid Metabolite from the Marine Myxobacterium Haliangium ochraceum. Molecules. 2016;21:59. doi: 10.3390/molecules21010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fudou R., Jojima Y., Iizuka T., Yamanaka S. Haliangium ochraceum gen. nov., sp. nov. and Haliangium tepidum sp. nov.: Novel moderately halophilic myxobacteria isolated from coastal saline environments. J. Gen. Appl. Microbiol. 2002;48:109–116. doi: 10.2323/jgam.48.109. [DOI] [PubMed] [Google Scholar]
- 30.Iizuka T., Jojima Y., Fudou R., Tokura M., Hiraishi A., Yamanaka S. Enhygromyxa salina gen. nov.; sp. nov., a slightly halophilic myxobacterium isolated from the coastal areas of Japan. Syst. Appl. Microbiol. 2003;26:189–196. doi: 10.1078/072320203322346038. [DOI] [PubMed] [Google Scholar]
- 31.Iizuka T., Jojima Y., Fudou R., Hiraishi A., Ahn J.W., Yamanaka S. Plesiocystis pacifica gen. nov.; sp. nov.; a marine myxobacterium that contains dihydrogenated menaquinone, isolated from the Pacific coasts of Japan. Int. J. Syst. Evol. Microbiol. 2003;53:189–195. doi: 10.1099/ijs.0.02418-0. [DOI] [PubMed] [Google Scholar]
- 32.Iizuka T., Jojima Y., Hayakawa A., Fujii T., Yamanaka S., Fudou R. Pseudenhygromyxa salsuginis gen. nov., sp. nov., a myxobacterium isolated from an estuarine marsh. Int. J. Syst. Evol. Microbiol. 2013;63:1360–1369. doi: 10.1099/ijs.0.040501-0. [DOI] [PubMed] [Google Scholar]
- 33.Brinkhoff T., Fischer D., Vollmers J., Voget S., Beardsley C., Thole S., Mussmann M., Kunze B., Wagner-Döbler I., Daniel R., et al. Biogeography and phylogenetic diversity of a cluster of exclusively marine myxobacteria. Int. J. Syst. Evol. Microbiol. 2012;6:1260–1272. doi: 10.1038/ismej.2011.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tian F., Yong Y., Chen B., Li H., Yao Y.F., Guo X.K. Bacterial, archaeal and eukaryotic diversity in Arctic sediment as revealed by 16S rRNA and 18S rRNA gene clone libraries analysis. Polar Biol. 2009;32:93–103. doi: 10.1007/s00300-008-0509-x. [DOI] [Google Scholar]
- 35.Zhang X., Yao Q., Cai Z., Xie X., Zhu H. Isolation and Identification of Myxobacteria from Saline-Alkaline Soils in Xinjiang, China. PLoS ONE. 2013;8:e70466. doi: 10.1371/journal.pone.0070466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li B., Yao Q., Zhu H. Approach to analyze the diversity of myxobacteria in soil by semi-nested PCR-denaturing gradient gel electrophoresis (DGGE) based on taxon-specific gene. PLoS ONE. 2014;9:e108877. doi: 10.1371/journal.pone.0108877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomas S.H., Padilla-Crespo E., Jardine P.M., Sanford R.A., Löffler F.E. Diversity and distribution of anaeromyxobacter strains in a uranium-contaminated subsurface environment with a nonuniform groundwater flow. Appl. Environ. Microbiol. 2009;75:3679–3687. doi: 10.1128/AEM.02473-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Treude N., Rosencrantz D., Liesack W., Schnell S. Strain FAc12, a dissimilatory iron-reducing member of the Anaeromyxobacter subgroup of Myxococcales. FEMS Microbiol. Ecol. 2003;44:261–269. doi: 10.1016/S0168-6496(03)00048-5. [DOI] [PubMed] [Google Scholar]
- 39.Lin J., Ratering S., Schnell S. Microbial iron cylce in corrosion material of drinking water pipelines. Ann. Agrar. Sci. 2011;9:18–25. [Google Scholar]
- 40.Kudo K., Yamaguchi N., Makino T., Ohtsuka T., Kimura K., Dong D.T., Amachi S. Release of arsenic from soil by a novel dissimilatory arsenate reducing bacterium, Anaeromyxobacter sp. strain PSR-1. Appl. Environ. Microbiol. 2013;79:4635–4642. doi: 10.1128/AEM.00693-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brockman E.R. Myxobacters from Arid Mexican Soil. Appl. Environ. Microbiol. 1976;32:642–644. doi: 10.1128/aem.32.4.642-644.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gerth K., Müller R. Moderately thermophilic Myxobacteria: Novel potential for the production of natural products isolation and characterization. Environ. Microbiol. 2005;7:874–880. doi: 10.1111/j.1462-2920.2005.00761.x. [DOI] [PubMed] [Google Scholar]
- 43.Mohr K.I., Moradi A., Glaeser S.P., Kämpfer P., Gemperlein K., Nübel U., Schumann P., Müller R., Wink J. Nannocystis konarekensis sp. nov.; a novel myxobacterium from an Iranian desert. Int. J. Syst. Evol. Microbiol. 2018;68:721–729. doi: 10.1099/ijsem.0.002569. [DOI] [PubMed] [Google Scholar]
- 44.Reichenbach H. The Myxococcales. In: Garrity G.M., editor. Bergey’s Manual of Systematic Bacteriology. 2nd ed. Springer; New York, NY, USA: 2005. [Google Scholar]
- 45.Mohiuddin A.K. Chemistry of Secondary Metabolites. Ann. Clin. Toxicol. 2019;2:1014. [Google Scholar]
- 46.Xiao Y., Wei X., Ebright R., Wall D. Antibiotic production by myxobacteria plays a role in predation. J. Bacteriol. 2011;193:4626–4633. doi: 10.1128/JB.05052-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wenzel S.C., Muller R. Myxobacteria-Microbial factories for the production of bioactive secondary metabolites. Mol. BioSyst. 2009;5:567–574. doi: 10.1039/b901287g. [DOI] [PubMed] [Google Scholar]
- 48.Herrmann J., Fayad A.A., Müller R. Natural products from myxobacteria: Novel metabolites and bioactivities. Nat. Prod. Rep. 2017;34:135–160. doi: 10.1039/C6NP00106H. [DOI] [PubMed] [Google Scholar]
- 49.Korp J., Gurovic M.S.V., Nett M. Antibiotics from predatory bacteria. Beilstein J. Org. Chem. 2016;12:594–607. doi: 10.3762/bjoc.12.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Albataineh H., Stevens D.C. Marine Myxobacteria: A Few Good Halophiles. Mar. Drugs. 2018;16:209. doi: 10.3390/md16060209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reichenbach H., Höfle G. Myxobacteria as Producers of Secondary Metabolites. In: Grabley S., Thiericke R., editors. Drug Discovery from Nature. Springer; Berlin, Germany: 1999. pp. 149–179. [Google Scholar]
- 52.Reichenbach H., Höfle G. Biologically active secondary metabolites from myxobacteria. Biotechnol. Adv. 1993;11:219–277. doi: 10.1016/0734-9750(93)90042-L. [DOI] [PubMed] [Google Scholar]
- 53.Kaur R., Singh S.K., Kaur R., Kumari A., Kaur R. Myxococcus xanthus: A source of antimicrobials and natural bio-control agent. Pharm. Innov. 2017;6:260–262. [Google Scholar]
- 54.Wilson C.N. Endacea Inc. Methods for Preventing and Treating Tissue Injury and Sepsis Associated with Yersinia pestis Infection. 12/220,377. U.S. Patent. 2009 Feb 19;
- 55.Raju R., Garcia R., Müller R. Angiolactone, a new Butyrolactone isolated from the terrestrial myxobacterium, Angiococcus sp. J. Antibiot. 2014;67:725–726. doi: 10.1038/ja.2014.55. [DOI] [PubMed] [Google Scholar]
- 56.Tautz T., Hoffmann J., Hoffmann T., Steinmetz H., Washausen P., Kunze B., Huch V., Kitsche A., Reichenbach H., Muller R., et al. Isolation, structure elucidation, biosynthesis, and synthesis of Antalid, a secondary metabolite from Polyangium species. Org. Lett. 2016;18:2560–2563. doi: 10.1021/acs.orglett.6b00810. [DOI] [PubMed] [Google Scholar]
- 57.Hofle G., Böhlendorf B., Fecker T., Sasse F., Kunze B. Semisynthesis and antiplasmodial activity of the quinoline alkaloid aurachin E. J. Nat. Prod. 2008;71:1967–1969. doi: 10.1021/np8004612. [DOI] [PubMed] [Google Scholar]
- 58.Kunze B., Reck M., Dötsch A., Lemme A., Schummer D., Irschik H., Steinmetz H., Wagner-Döbler I. Damage of Streptococcus mutans biofilms by carolacton, a secondary metabolite from the myxobacterium Sorangium cellulosum. BMC Microbiol. 2010;10:199. doi: 10.1186/1471-2180-10-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jungmann K., Jansen R., Gerth K., Huch V., Krug D., Fenical W., Müller R. Two of a Kind-The Biosynthetic Pathways of Chlorotonil and Anthracimycin. ACS Chem. Biol. 2015;10:2480–2490. doi: 10.1021/acschembio.5b00523. [DOI] [PubMed] [Google Scholar]
- 60.Schiefer A., Schmitz A., Schäberle T.F., Specht S., Lämmer C., Johnston K.L., Vassylyev D.G., König G.M., Hoerauf A., Pfarr K. Corallopyronin A specifically targets and depletes essential obligate Wolbachia endobacteria from filarial nematodes in vivo. J. Infect. Dis. 2012;206:249–257. doi: 10.1093/infdis/jis341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schmitz A., Kehraus S., Schaberle T.F., Neu E., Almeida C., Roth M., König G.M. Corallorazines from the Myxobacterium Corallococcus coralloides. J. Nat. Prod. 2014;77:159–163. doi: 10.1021/np400740u. [DOI] [PubMed] [Google Scholar]
- 62.Kunze B., Jansen R., Höfle G., Reichenbach H. Crocacin, a new electron transport inhibitor from Chondromyces crocatus (myxobacteria). Production, isolation, physico-chemical and biological properties. J. Antibiot. 1994;47:881–886. doi: 10.7164/antibiotics.47.881. [DOI] [PubMed] [Google Scholar]
- 63.Baumann S., Herrmann J., Raju R., Steinmetz S., Mohr K.I., Huttel S., Harmrolfs K., Stadler M., Muller R. Cystobactamids: Myxobacterial topoisomerase inhibitors exhibiting potent antibacterial activity. Angew. Chem. Int. Ed. 2014;53:14605–14609. doi: 10.1002/anie.201409964. [DOI] [PubMed] [Google Scholar]
- 64.Nadmid S., Plaza A., Garcia R., Müller R. Cystochromones, Unusual Chromone-Containing Polyketides from the Myxobacterium Cystobacter sp. MCy9104. J. Nat. Prod. 2015;78:2023–2028. doi: 10.1021/acs.jnatprod.5b00343. [DOI] [PubMed] [Google Scholar]
- 65.Etzbach L., Plaza A., Garcia R., Baumann S., Müller R. Cystomanamides: Structure and biosynthetic pathway of a family of glycosylated lipopeptides from myxobacteria. Org. Lett. 2014;16:2414–2417. doi: 10.1021/ol500779s. [DOI] [PubMed] [Google Scholar]
- 66.Ojika M., Suzuki Y., Tsukamoto A., Sakagami Y., Fudou R., Yoshimura T., Yamanaka S. Cystothiazoles A and B, new bithiazole-type antibiotics from the myxobacterium Cystobacter fuscus. J. Antibiot. 1998;51:275–281. doi: 10.7164/antibiotics.51.275. [DOI] [PubMed] [Google Scholar]
- 67.Surup F., Viehrig K., Mohr K.I., Herrmann J., Jansen R., Müller R. Disciformycins A and B: 12-membered macrolide glycoside antibiotics from the myxobacterium Pyxidicoccus fallax active against multiresistant staphylococci. Angewandte Chemie (International ed. in English) Angew. Chem. Int. Ed. Engl. 2014;53:13588–13591. doi: 10.1002/anie.201406973. [DOI] [PubMed] [Google Scholar]
- 68.Muddala R., Acosta J.A., Barbosa L.C., Boukouvalas J. Synthesis of the Marine Myxobacterial Antibiotic Enhygrolide A. J. Nat. Prod. 2017;80:2166–2169. doi: 10.1021/acs.jnatprod.7b00405. [DOI] [PubMed] [Google Scholar]
- 69.Schieferdecker S., König S., Weigel C., Dahse H.M., Werz O., Nett M. Structure and biosynthetic assembly of gulmirecins, macrolide antibiotics from the predatory bacterium Pyxidicoccus fallax. Chemistry. 2014;20:15933–15940. doi: 10.1002/chem.201404291. [DOI] [PubMed] [Google Scholar]
- 70.Dávila-Céspedes A., Hufendiek P., Crüsemann M., Schäberle T.F., König G.M. Marine-derived myxobacteria of the suborder Nannocystineae: An underexplored source of structurally intriguing and biologically active metabolites. Beilstein J. Org. Chem. 2016;12:969. doi: 10.3762/bjoc.12.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nadmid S., Plaza A., Lauro G., Garcia R., Bifulco G., Müller R. Hyalachelins A-C, unusual siderophores isolated from the terrestrial myxobacterium Hyalangium minutum. Org. Lett. 2014;16:4130–4133. doi: 10.1021/ol501826a. [DOI] [PubMed] [Google Scholar]
- 72.Okanya P.W., Mohr K.I., Gerth K., Steinmetz H., Huch V., Jansen R., Müller R. Hyaladione, an S-methyl cyclohexadiene-dione from Hyalangium minutum. J. Nat. Prod. 2012;75:768–770. doi: 10.1021/np200776v. [DOI] [PubMed] [Google Scholar]
- 73.Okanya P., Mohr K., Gerth K., Kessler W., Jansen R., Stadler M., Müller R. Hyafurones, hyapyrrolines and hyapyrones: Polyketides from Hyalangium minutum. J. Nat. Prod. 2014;77:1420–1429. doi: 10.1021/np500145f. [DOI] [PubMed] [Google Scholar]
- 74.Zander W., Mohr K.I., Gerth K., Jansen R., Müller R. P-hydroxyacetophenone amides from cystobacter ferrugineus, strain Cb G35. J. Nat. Prod. 2011;74:1358–1363. doi: 10.1021/np1006789. [DOI] [PubMed] [Google Scholar]
- 75.Jansen R., Sood S., Huch V., Kunze B., Stadler M., Müller R. Pyrronazols, metabolites from the mxobacteria Nannocystis pusilla and N. exedens are unusual chlorinated pyrone-oxazole-pyrroles. J. Nat. Prod. 2014;77:320–326. doi: 10.1021/np400877r. [DOI] [PubMed] [Google Scholar]
- 76.Barbier J., Jansen R., Irschik H., Benson S., Gerth K., Böhlendorf B., Höfle G., Reichenbach H., Wegner J., Zeilinger C., et al. Isolation and total synthesis of icumazoles and noricumazoles-Antifungal antibiotics and cation-channel blockers from Sorangium cellulosum. Angew. Chem. Int. Ed. 2012;51:1256–1260. doi: 10.1002/anie.201106435. [DOI] [PubMed] [Google Scholar]
- 77.Steinmetz H., Mohr K., Zander W., Jansen R., Müller R. Indiacens A and B: Prenyl indoles from the myxobacterium Sandaracinus amylolyticus. J. Nat. Prod. 2012;75:1803–1805. doi: 10.1021/np300288b. [DOI] [PubMed] [Google Scholar]
- 78.Marsch N., Jones P.G., Lindel T. SmI2-mediated dimerization of indolylbutenones and synthesis of the myxobacterial natural product indiacen B. Beilstein J. Org. Chem. 2015;11:1700–1706. doi: 10.3762/bjoc.11.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Symkenberg G., Kalesse M. Structure elucidation and total synthesis of kulkenon. Angew. Chem. Int. Ed. 2014;53:1795–1798. doi: 10.1002/anie.201309386. [DOI] [PubMed] [Google Scholar]
- 80.Kopp M., Irschik H., Gemperlein K., Buntin K., Meiser P., Weissman K.J., Bode H.B., Müller R. Insights into the complex biosynthesis of the leupyrrins in Sorangium cellulosum So ce690. Mol. Biosyst. 2011;7:1549–1563. doi: 10.1039/c0mb00240b. [DOI] [PubMed] [Google Scholar]
- 81.Keller L., Plaza A., Dubiella C., Groll M., Kaiser M., Müller R. Macyranones: Structure, Biosynthesis, and Binding Mode of an Unprecedented Epoxyketone that Targets the 20S Proteasome. J. Am. Chem. Soc. 2015;137:8121–8130. doi: 10.1021/jacs.5b03833. [DOI] [PubMed] [Google Scholar]
- 82.Irschik H., Washausen P., Sasse F., Fohrer J., Huch V., Müller R., Prusov E.V. Isolation, structure elucidation, and biological activity of maltepolides: Remarkable macrolides from myxobacteria. Angew. Chem. Int. Ed. 2013;52:5402–5405. doi: 10.1002/anie.201210113. [DOI] [PubMed] [Google Scholar]
- 83.Irschik H., Schummer D., Höfle G., Reichenbach H., Steinmetz H., Jansen R. Etnangien, a macrolide-polyene antibiotic from Sorangium cellulosum that inhibits nucleic acid polymerases. J. Nat. Prod. 2007;70:1060–1063. doi: 10.1021/np070115h. [DOI] [PubMed] [Google Scholar]
- 84.Hoffmann T., Müller S., Nadmid S., Garcia R., Müller R. Microsclerodermins from terrestrial myxobacteria: An intriguing biosynthesis likely connected to a sponge symbiont. J. Am. Chem. Soc. 2013;135:16904–16911. doi: 10.1021/ja4054509. [DOI] [PubMed] [Google Scholar]
- 85.Guzman E.A., Maers K., Roberts J., Kemami-Wangun H.V., Harmody D., Wright A.E. The marine natural product microsclerodermin A is a novel inhibitor of the nuclear factor kappa B and induces apoptosis in pancreatic cancer cells. Invest. New Drugs. 2015;33:86–94. doi: 10.1007/s10637-014-0185-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kunze B., Bedorf N., Kohl W., Höfle G., Reichenbach H. Myxochelin A, a new iron-chelating compound from Angiococcus disciformis (Myxobacterales). Production, isolation, physico-chemical and biological properties. J. Antibiot. 1989;42:14–17. doi: 10.7164/antibiotics.42.14. [DOI] [PubMed] [Google Scholar]
- 87.Schieferdecker S., König S., Koeberle A., Dahse H.M., Werz O., Nett M. Myxochelins target human 5-lipoxygenase. J. Nat. Prod. 2015;78:335–338. doi: 10.1021/np500909b. [DOI] [PubMed] [Google Scholar]
- 88.Gulder T.A., Neff S., Schüz T., Winkler T., Gees R., Böhlendorf B. The myxocoumarins A and B from Stigmatella aurantiaca strain MYX-030. Beilstein J. Org. Chem. 2013;9:2579–2585. doi: 10.3762/bjoc.9.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cortina N.S., Krug D., Plaza A., Revermann O., Müller R. Myxoprincomide: A natural product from Myxococcus xanthus discovered by comprehensive analysis of the secondary metabolome. Angew. Chem. Int. Ed. 2012;51:811–816. doi: 10.1002/anie.201106305. [DOI] [PubMed] [Google Scholar]
- 90.Goldman B.S., Nierman W.C., Kaiser D., Slater S.C., Durkin A.S., Eisen J.A., Ronning C.M., Barbazuk W.B., Blanchard M., Field C., et al. Evolution of sensory complexity recorded in a myxobacterial genome. Proc. Natl. Acad. Sci. USA. 2006;103:15200–15205. doi: 10.1073/pnas.0607335103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schley C., Altmeyer M.O., Swart R., Müller R., Huber C.G. Proteome analysis of Myxococcus xanthus by off-line two-dimensional chromatographic separation using monolithic poly-(styrene-divinylbenzene) columns combined with ion-trap tandem mass spectrometry. J. Proteome Res. 2006;5:2760–2768. doi: 10.1021/pr0602489. [DOI] [PubMed] [Google Scholar]
- 92.Moy T.I., Daniel A., Hardy C., Jackson A., Rehrauer O., Hwang Y.S., Zou D., Nguyen K., Silverman J.A., Li Q., et al. Evaluating the activity of the RNA polymerase inhibitor myxopyronin B against Staphylococcus aureus. FEMS Microbiol. Lett. 2011;319:176–179. doi: 10.1111/j.1574-6968.2011.02282.x. [DOI] [PubMed] [Google Scholar]
- 93.Irschik H., Gerth K., Kemmer T., Steinmetz H., Reichenbach H. The myxovalargins, new peptide antibiotics from Myxococcus fulvus (myxobacterales). I. cultivation, isolation, and some chemical and biological properties. J. Antibiot. 1983;36:6–12. doi: 10.7164/antibiotics.36.6. [DOI] [PubMed] [Google Scholar]
- 94.Gerth K., Irschik H., Reichenbach H., Trowitzsch W. The myxovirescins, a family of antibiotics from Myxococcus virescens (Myxobacterales) J. Antibiot. 1982;35:1454–1459. doi: 10.7164/antibiotics.35.1454. [DOI] [PubMed] [Google Scholar]
- 95.Vogeley L., El-Arnaout T., Bailey J. Structural basis of lipoprotein signal peptidase II action and inhibition by the antibiotic globomycin. Science. 2016;351:876–880. doi: 10.1126/science.aad3747. [DOI] [PubMed] [Google Scholar]
- 96.Gentzsch J., Hinkelmann B., Kaderali L., Irschik H., Jansen R., Sasse F., Frank R., Pietschmann T. Hepatitis C virus complete life cycle screen for identification of small molecules with pro- or antiviral activity. Antivir. Res. 2011;89:136–148. doi: 10.1016/j.antiviral.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 97.Beck S., Henß L., Weidner T., Herrmann J., Müller R., Chao Y., Weber C., Sliva K., Schnierle S. Identification of inhibitors of Ebola virus pseudotyped vectors from a myxobacterial compound library. Antivir. Res. 2016;132:85–91. doi: 10.1016/j.antiviral.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 98.Guo W.J., Tao W.Y. Phoxalone, a novel macrolide from Sorangium cellulosum: Structure identification and its anti-tumor bioactivity in vitro. Biotechnol. Lett. 2008;30:349–356. doi: 10.1007/s10529-007-9550-z. [DOI] [PubMed] [Google Scholar]
- 99.Glaus F., Dedić D., Tare P., Nagaraja V., Rodrigues L., Aínsa J.A., Kunze J., Schneider G., Hartkoorn R.C., Cole S.T., et al. Total Synthesis of Ripostatin B and Structure–Activity Relationship Studies on Ripostatin Analogs. J. Org. Chem. 2018;83:7150–7172. doi: 10.1021/acs.joc.8b00193. [DOI] [PubMed] [Google Scholar]
- 100.Blunt J.W., Copp B.R., Keyzers R.A., Munro M.H., Prinsep M.R. Marine natural products. Nat. Prod. Rep. 2015;32:116–211. doi: 10.1039/C4NP00144C. [DOI] [PubMed] [Google Scholar]
- 101.Ahn J.W., Jang K.H., Chung S.C., Oh K.B., Shin J. Sorangiadenosine, a new sesquiterpene adenoside from the myxobacterium Sorangium cellulosum. Org. Lett. 2008;10:1167–1169. doi: 10.1021/ol800061h. [DOI] [PubMed] [Google Scholar]
- 102.Okoth D.A., Hug J.J., Garcia R., Spröer C., Overmann J., Müller R. 2-Hydroxysorangiadenosine: Structure and Biosynthesis of a Myxobacterial Sesquiterpene-Nucleoside. Molecules. 2020;25:2676. doi: 10.3390/molecules25112676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li Y., Weissman K.J., Müller R. Myxochelin biosynthesis: Direct evidence for two- and four-electron reduction of a carrier protein-bound thioester. J. Am. Chem Soc. 2008;130:7554–7555. doi: 10.1021/ja8025278. [DOI] [PubMed] [Google Scholar]
- 104.Karwehl S., Jansen R., Huch V., Stadler M. Sorazolons, Carbazole Alkaloids from Sorangium cellulosum Strain Soce375. J. Nat. Prod. 2016;79:369–375. doi: 10.1021/acs.jnatprod.5b00997. [DOI] [PubMed] [Google Scholar]
- 105.Zander W., Irschik H., Augustiniak H., Herrmann M., Jansen R., Steinmetz H., Gerth K., Kessler W., Kalesse M., Hofle G., et al. Sulfangolids, macrolide sulfate esters from Sorangium cellulosum. Chemistry. 2012;18:6264–6271. doi: 10.1002/chem.201100851. [DOI] [PubMed] [Google Scholar]
- 106.Fleta-Soriano E., Martinez J.P., Hinkelmann B., Gerth K., Washausen P., Diez J., Frank R., Sasse F., Meyerhans A. The myxobacterial metabolite ratjadone A inhibits HIV infection by blocking the Rev/CRM1-mediated nuclear export pathway. Microb. Cell Factories. 2014;13:17. doi: 10.1186/1475-2859-13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Steinmetz H., Irschik H., Kunze B., Reichenbach H., Hoefle G., Jansen R. Thuggacins, macrolide antibiotics active against Mycobacterium tuberculosis: Isolation from myxobacteria, structure elucidation, conformation analysis and biosynthesis. Chemistry. 2007;13:5822–5832. doi: 10.1002/chem.200700269. [DOI] [PubMed] [Google Scholar]
- 108.Mdluli K., Kaneko T., Upton A. The tuberculosis drug discovery and development pipeline and emerging drug targets. Cold Spring Harb. Perspect. Med. 2015;5:a021154. doi: 10.1101/cshperspect.a021154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Plaza A., Garcia R., Bifulco G., Martinez J.P., Hüttel S., Sasse F., Meyerhans A., Stadler M., Müller R. Aetheramides A and B, potent HIV-inhibitory depsipeptides from a myxobacterium of the new genus “Aetherobacter”. Org. Lett. 2012;14:2854–2857. doi: 10.1021/ol3011002. [DOI] [PubMed] [Google Scholar]
- 110.Ghosh A.K., Rao K.V., Akasapu S. An Enantioselective Synthesis of a MEM-Protected Aetheramide a Derivative. Tetrahedron Lett. 2014;55:5191–5194. doi: 10.1016/j.tetlet.2014.07.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gerstmann L., Kalesse M. Total Synthesis of Aetheramide A. Chemistry. 2016;22:11210–11212. doi: 10.1002/chem.201602682. [DOI] [PubMed] [Google Scholar]
- 112.Zhang S., Schneider L.S., Vick B., Grunert M., Jeremias I., Menche D., Müller R., Vollmar A.M., Liebl J. Anti-leukemic effects of the V-ATPase inhibitor Archazolid A. Oncotarget. 2015;6:43508–43528. doi: 10.18632/oncotarget.6180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sasse F., Steinmetz H., Schupp T., Petersen F., Memmert K., Hofmann H., Heusser C., Brinkmann V., Von-matt P., Hofle G., et al. Argyrins, immunosuppressive cyclic peptides from myxobacteria. I. Production, isolation, physico-chemical and biological properties. J. Antibiot. 2002;55:543–551. doi: 10.7164/antibiotics.55.543. [DOI] [PubMed] [Google Scholar]
- 114.Wenzel S.C., Hoffmann H., Zhang J., Debussche L., Haag-Richter S., Kurz M., Nardi F., Lukat P., Kochems I., Tietgen H., et al. Production of the Bengamide Class of Marine Natural Products in Myxobacteria: Biosynthesis and Structure-Activity Relationships. Angew. Chem. Int. Ed. Engl. 2015;54:15560–15564. doi: 10.1002/anie.201508277. [DOI] [PubMed] [Google Scholar]
- 115.Raju R., Mohr K., Bernecker S., Herrmann J., Müller R. Cystodienoic acid: A new diterpene isolated from the myxobacterium Cystobacter sp. J. Antibiot. 2015;68:473–475. doi: 10.1038/ja.2015.8. [DOI] [PubMed] [Google Scholar]
- 116.Elnakady Y., Sasse F., Lünsdorf H., Reichenbach H. Disorazol A(1), a highly effective antimitotic agent acting on tubulin polymerization and inducing apoptosis in mammalian cells. Biochem. Pharmacol. 2004;67:927–935. doi: 10.1016/j.bcp.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 117.Höfle G., Gerth K., Reichenbach H., Kunze B., Sasse F., Forche E., Prusov E.V. Isolation, biological activity evaluation, structure elucidation, and total synthesis of eliamid: A novel complex I inhibitor. Chemistry. 2012;18:11362–11370. doi: 10.1002/chem.201201879. [DOI] [PubMed] [Google Scholar]
- 118.Altmann K.H., Pfeiffer B., Arseniyadis S., Pratt B., Nicolaou K. The Chemistry and Biology of Epothilones—The Wheel Keeps Turning. Chem. Med. Chem. 2007;2:396–423. doi: 10.1002/cmdc.200600206. [DOI] [PubMed] [Google Scholar]
- 119.Ojima D., Yasui A., Tohyama K., Tokuzumi K., Toriihara E., Ito K., Iwasaki A., Tomura T., Ojika M., Suenaga K. Total Synthesis of Miuraenamides A and D. J. Org. Chem. 2016;81:9886–9894. doi: 10.1021/acs.joc.6b02061. [DOI] [PubMed] [Google Scholar]
- 120.Hoffmann H., Kogler H., Heyse W., Matter H., Caspers M., Schummer D., Klemke-Jahn C., Bauer A., Penarier G., Debussche L., et al. Discovery, Structure Elucidation, and Biological Characterization of Nannocystin A, a Macrocyclic Myxobacterial Metabolite with Potent Antiproliferative Properties. Angew. Chem. Int. Ed. Engl. 2015;54:10145–10148. doi: 10.1002/anie.201411377. [DOI] [PubMed] [Google Scholar]
- 121.Krastel P., Roggo S., Schirle M., Ross N., Perruccio F., Aspesi P., Aust T., Buntin K., Estoppey D., Liechty B., et al. Nannocystin A: An Elongation Factor 1 Inhibitor from Myxobacteria with Differential Anti-Cancer Properties. Angew. Chem. Int. Ed. Engl. 2015;54:10149–10154. doi: 10.1002/anie.201505069. [DOI] [PubMed] [Google Scholar]
- 122.Jahns C., Hoffmann T., Müller S., Gerth K., Washausen P., Höfle G., Reichenbach H., Kalesse M., Müller R. Pellasoren: Structure elucidation, biosynthesis, and total synthesis of a cytotoxic secondary metabolite from Sorangium cellulosum. Angew. Chem. Int. Ed. Engl. 2012;51:5239–5243. doi: 10.1002/anie.201200327. [DOI] [PubMed] [Google Scholar]
- 123.Mulwa L.S., Stadler M. Antiviral compounds from Myxobacteria. Microorganisms. 2018;6:73. doi: 10.3390/microorganisms6030073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Martinez J.P., Hinkelmann B., Fleta-Soriano E., Steinmetz H., Jansen R., Diez J., Frank R., Sasse F., Meyerhans A. Identification of myxobacteria-derived HIV inhibitors by a high-throughput two-step infectivity assay. Microb. Cell Factories. 2013;12:85. doi: 10.1186/1475-2859-12-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Roy J., Kaake M., Srinivasarao M., Low P.S. Targeted Tubulysin B Hydrazide Conjugate for the Treatment of Luteinizing Hormone-Releasing Hormone Receptor-Positive Cancers. Bioconjug. Chem. 2018;29:2208–2214. doi: 10.1021/acs.bioconjchem.8b00164. [DOI] [PubMed] [Google Scholar]
- 126.Knauth P., Reichenbach H. On the mechanism of action of the myxobacterial fungicide ambruticin. J. Antibiot. 2000;53:1182–1190. doi: 10.7164/antibiotics.53.1182. [DOI] [PubMed] [Google Scholar]
- 127.Irschik H., Schummer D., Gerth K., Höfle G., Reichenbach H. ChemInform Abstract: The Tartrolons, New Boron-Containing Antibiotics from a Myxobacterium, Sorangium cellulosum. J. Antibiot. 2010;48:26–30. doi: 10.7164/antibiotics.48.26. [DOI] [PubMed] [Google Scholar]
- 128.Kunze B., Jansen R., Pridzun L., Jurkiewicz E., Hunsmann G., Höfle G., Reichenbach H. Thiangazole, a new thiazoline antibiotic from Polyangium sp. (myxobacteria): Production, antimicrobial activity and mechanism of action. J. Antibiot. 1993;46:1752–1755. doi: 10.7164/antibiotics.46.1752. [DOI] [PubMed] [Google Scholar]
- 129.Gerth K., Bedorf N., Irschik H., Höfle G., Reichenbach H. The soraphens: A family of novel antifungal compounds from Sorangium cellulosum (Myxobacteria) J. Antibiot. 1994;47:23–31. doi: 10.7164/antibiotics.47.23. [DOI] [PubMed] [Google Scholar]
- 130.Fleta-Soriano E., Smutná K., Martinez J.P., Oró C.L., Sadiq S.K., Mirambeau G., Lopez-Iglesias C., Bosch M., Pol A., Brönstrup M., et al. The myxobacterial metabolite soraphen A inhibits HIV-1 by reducing virus production and altering virion composition. Antimicrob. Agents Chemother. 2017;61:e00739-17. doi: 10.1128/AAC.00739-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mohr K.I., Zindler T., Wink J., Wilharm E., Stadler M. Myxobacteria in high moor and fen: An astonishing diversity in a neglected extreme habitat. MicrobiologyOpen. 2017;6:e00464. doi: 10.1002/mbo3.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Houbraken J., Frisvad J.C., Samson R.A. Fleming’s penicillin producing strain is not Penicillium chrysogenum but P. rubens. IMA Fungus. 2011;1:87–95. doi: 10.5598/imafungus.2011.02.01.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Schatz A., Bugie E., Waksman S. Streptomycin: A substance exhibiting antibiotic activity against gram positive and gram-negative bacteria. Proc. Exp. Biol. Med. 1944;55:66–69. doi: 10.3181/00379727-55-14461. [DOI] [PubMed] [Google Scholar]
- 134.McGuire J.M., Bunch R.L., Anderson R.C., Boaz H.E., Flynn E.H., Powell H.M., Smith J.W. Ilotycin a new antibiotic. Antibiot. Chemother. 1952;2:281–283. [PubMed] [Google Scholar]
- 135.Bartlett J.G., Gilbert D.N., Spellberg B. Seven ways to preserve the miracle of antibiotics. Clin. Infect. Dis. 2013;56:1445–1450. doi: 10.1093/cid/cit070. [DOI] [PubMed] [Google Scholar]
- 136.Ventola C.L. The Antibiotic Resistance Crisis: Part 1: Causes and Threats. Pharm. Ther. 2015;40:277–283. [PMC free article] [PubMed] [Google Scholar]
- 137.Hesterkamp T. Antibiotics Clinical Development and Pipeline. In: Stadler M., Dersch P., editors. How to Overcome the Antibiotic Crisis-Facts, Challenges, Technologies and Future Perspective. Springer; Berlin, Germany: 2016. pp. 447–474. [DOI] [PubMed] [Google Scholar]
- 138.Hoffmann T., Krug D., Bozkurt N., Duddela S., Jansen R., Garcia R., Gerth K., Steinmetz H., Müller R. Correlating chemical diversity with taxonomic distance for discovery of natural products in myxobacteria. Nat. Commun. 2018;9:803. doi: 10.1038/s41467-018-03184-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Landwehr W., Wolf C., Wink J. Actinobacteria and Myxobacteria-Two of the Most Important Bacterial Resources for Novel Antibiotics. In: Stadler M., Dersch P., editors. How to Overcome the Antibiotic Crisis-Facts, Challenges, Technologies & Future Perspective. Springer; Berlin, Germany: 2016. [DOI] [PubMed] [Google Scholar]
- 140.Sansinenea E., Ortiz A. Secondary metabolites of soil Bacillus spp. Biotechnol. Lett. 2011;33:1523–1538. doi: 10.1007/s10529-011-0617-5. [DOI] [PubMed] [Google Scholar]
- 141.Shen Y., Volrath S.L., Weatherly S.C., Elich T.D., Tong L. A mechanism for the potent inhibition of eukaryotic acetyl-coenzyme A carboxylase by soraphen A, a macrocyclic polyketide natural product. Mol. Cell. 2004;16:881–891. doi: 10.1016/j.molcel.2004.11.034. [DOI] [PubMed] [Google Scholar]
- 142.Mühlradt P.F., Sasse F. Epothilone B stabilizes microtubuli of macrophages like taxol without showing taxol-like endotoxin activity. Cancer Res. 1997;57:3344–3346. [PubMed] [Google Scholar]
- 143.Goodin S., Kane M.P., Rubin E.H. Epothilones: Mechanism of action and biologic activity. J. Clin. Oncol. 2004;22:2015–2025. doi: 10.1200/JCO.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 144.Peterson J.K., Tucker C., Favours E., Cheshire P.J., Creech J., Billups C.A., Smykla R., Lee F.Y.F., Houghton P.J. In vivo evaluation of Ixabepilone (BMS247550), A novel epothilone B derivative, against pediatric cancer models. Clin. Cancer Res. 2005;11:6950–6958. doi: 10.1158/1078-0432.CCR-05-0740. [DOI] [PubMed] [Google Scholar]
- 145.Reboll M.R., Ritter B., Sasse F., Niggemann J., Frank R., Nourbakhsh M. The myxobacterial compounds spirangien A and spirangien M522 are potent inhibitors of IL-8 expression. Chem. Bio. Chem. 2012;13:409–415. doi: 10.1002/cbic.201100635. [DOI] [PubMed] [Google Scholar]
- 146.Jurkliewicz E., Jansen R., Kunze B., Trowitzsch-Klenast W., Porche E., Reichenbach H., Höfle G., Hunsmann G. Three new potent HIV-1 inhibitors from myxobacteria. Antivir. Chem. Chemother. 1992;3:189–193. doi: 10.1177/095632029200300401. [DOI] [Google Scholar]
- 147.Trowitzsch-Kienast W., Forche E., Wray V., Reichenbach H., Jurkiewicz E., Hunsmann G., Höfle G. Phenalamide, neue HIV-1-Inhibitoren aus Myxococcus stipitatus Mx s40. Liebigs Ann. Chem. 1992;1992:659–664. doi: 10.1002/jlac.1992199201112. [DOI] [Google Scholar]
- 148.Garcia R., Stadler M., Gemperlein K., Müller R. Aetherobacter fasciculatus gen. nov.; sp. nov. and Aetherobacter rufus sp. nov.; novel myxobacteria with promising biotechnological applications. Int. J. Syst. Evol. Microbiol. 2016;66:928–938. doi: 10.1099/ijsem.0.000813. [DOI] [PubMed] [Google Scholar]
- 149.Gerth K., Schummer D., Höfle G., Irschik H., Reichenbach H. Ratjadon: A new antifungal compound from Sorangium cellulosum (Myxobacteria) Production, physico-chemical and biological properties. J. Antibiot. 1995;48:787–792. doi: 10.7164/antibiotics.48.973. [DOI] [PubMed] [Google Scholar]
- 150.Kaul D.R., Stoelben S., Cober E., Ojo T., Sandusky E., Lischka P., Zimmermann H., Rübsamen-Schaeff H. First report of successful treatment of multidrug-resistant cytomegalovirus disease with the novel anti-CMV compound AIC246. Am. J. Transpl. 2011;11:1079–1084. doi: 10.1111/j.1600-6143.2011.03530.x. [DOI] [PubMed] [Google Scholar]
- 151.Nagoba B., Vedpathak D. Medical applications of siderophores. Eur. J. Gen. Med. 2011;8:229–235. doi: 10.29333/ejgm/82743. [DOI] [Google Scholar]
- 152.Miyanaga S., Obata T., Onaka H., Fujita T., Saito N., Sakurai H., Saiki I., Furumai T., Igarashi Y. Absolute configuration and antitumor activity of myxochelin a produced by Nonomuraea pusilla TP-A0861. J. Antibiot. 2006;59:698–703. doi: 10.1038/ja.2006.93. [DOI] [PubMed] [Google Scholar]
- 153.Korp J., König S., Schieferdecker S., Dahse H.M., König G.M., Werz O., Nett M. Harnessing enzymatic promiscuity in myxochelins biosynthesis for the production of 5-lipoxygenase inhibitors. Chem. Bio. Chem. 2015;16:2445–2450. doi: 10.1002/cbic.201500446. [DOI] [PubMed] [Google Scholar]
- 154.Schieferdecker S., König S., Simona Pace S., Werz O., Nett M. Myxochelin-inspired 5-lipoxygenase inhibitors: Synthesis and biological evaluation. Chem. Med. Chem. 2017;12:23–27. doi: 10.1002/cmdc.201600536. [DOI] [PubMed] [Google Scholar]
- 155.Miyanga S., Sakurai H., Saiki I., Onaka H., Igarashi Y. Synthesis and evaluation of myxochelins analogues as antimetastatic agents. Biol. Med. Chem. 2009;17:2724–2732. doi: 10.1016/j.bmc.2009.02.040. [DOI] [PubMed] [Google Scholar]
- 156.Ambrosi H.D., Hartmann V., Pistorius D., Reissbrodt R., Trowitzsch-Kienast W. Myxochelins B, C, D, E and F: A new structural principle for powerful siderophores imitating Nature. Eur. J. Org. Chem. 1998;1998:541–551. doi: 10.1002/(SICI)1099-0690(199803)1998:3<541::AID-EJOC541>3.0.CO;2-A. [DOI] [Google Scholar]
- 157.Britt W.J., Vugler L., Butfiloski E.D., Stevens E.B. Cell surface expression of Human Cytomegalovirus (HCMV): Use of HCMV-recombinant vaccinia virus-infected cells in analysis of the human neutralizing antibody response. J. Virol. 1990;64:1079–1085. doi: 10.1128/jvi.64.3.1079-1085.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Saha M., Sarkar S., Sarkar B., Sharma B.K., Bhattacharjee S., Prosun T. Microbial siderophores and their potential applications: A review. Environ. Sci. Pollut. Res. 2016;23:3984–3999. doi: 10.1007/s11356-015-4294-0. [DOI] [PubMed] [Google Scholar]
- 159.Kunze B., Jansen R., Sasse F., Höfle G., Reichenbach H. Chondramides A-D, new antifungal and cytostatic depsipeptides from Chondromyces crocatus (Myxobacteria). Production, physicochemical and biological properties. J. Antibiot. 1995;48:1262–1266. doi: 10.7164/antibiotics.48.1262. [DOI] [PubMed] [Google Scholar]
- 160.Mulwa L., Jansen R., Praditya D., Mohr K., Wink J., Steinmann E., Stadler M. Six heterocyclic metabolites from the Myxobacterium Labilithrix luteola. Molecules. 2018;23:542. doi: 10.3390/molecules23030542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bedorf N., Schomburg D., Gerth K., Reichenbach H., Höfle G., Bedorf N., Schomburg D., Gerth K., Reichenbach H., Höfle G. Isolation and structure elucidation of soraphen A1, a novel antifungal macrolide from Sorangium cellulosum. Liebigs Ann. Chem. 1993;1993:1017–1021. doi: 10.1002/jlac.1993199301161. [DOI] [Google Scholar]
- 162.Singaravelu R., Desrochers G.F., Srinivasan P., O’Hara S., Lyn R., Müller R., Jones D.M., Russell R., Pezacki J.P. Soraphen A: A probe for investigating the role of de novo lipogenesis during viral infection. ACS Infect. Dis. 2015;1:130–134. doi: 10.1021/acsinfecdis.5b00019. [DOI] [PubMed] [Google Scholar]
- 163.Koutsoudakis G., Romero-Brey I., Berger C., Pérez-Vilaró G., Monteiro P.P., Vondran F.W.R., Kalesse M., Harmrolfs K., Müller R., Martinez J.P., et al. Soraphen A: A broad-spectrum antiviral natural product with potent anti-hepatitis C virus activity. J. Hepatol. 2015;63:813–821. doi: 10.1016/j.jhep.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 164.Yeganeh B., Ghavami S., Kroeker A.L., Mahood T.H., Stelmack G.L., Klonisch T., Coombs K.M., Halayko A.J. Suppression of influenza A virus replication in human lung epithelial cells by noncytotoxic concentrations bafilomycin A1. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015;308:270–286. doi: 10.1152/ajplung.00011.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Oslowski C.M., Urano F. Measuring ER stress and the unfolded protein response using mammalian tissue culture system. Methods Enzymol. 2011;490:71. doi: 10.1016/B978-0-12-385114-7.00004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rahman S., Jan A.T., Ayyagari A., Kim J., Kim J., Minakshi R. Entanglement of UPRER in Aging Driven Neurodegenerative Diseases. Front. Aging Neurosci. 2017;9:341. doi: 10.3389/fnagi.2017.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rahman S., Archana A., Jan A.T., Minakshi R. Dissecting Endoplasmic Reticulum Unfolded Protein Response (UPRER) in Managing Clandestine Modus Operandi of Alzheimer’s Disease. Front. Aging Neurosci. 2018;10:30. doi: 10.3389/fnagi.2018.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Dehhaghi M., Mohammadipanah F., Guillemin G.J. Myxobacterial natural products: An under-valued source of products for drug discovery for neurological disorders. NeuroToxicology. 2018;66:195–203. doi: 10.1016/j.neuro.2018.02.017. [DOI] [PubMed] [Google Scholar]
- 169.Kim S.J., Lee Y.-J., Kim J.-B. Myxobacterial metabolites enhance cell proliferation and reduce intracellular stress in cells from a Parkinson’s disease mouse model. Gene. 2013;514:36–40. doi: 10.1016/j.gene.2012.10.088. [DOI] [PubMed] [Google Scholar]
- 170.Sabry J., O’Connor T.P., Kirschner M.W. Axonal transport of tubulin in tit pioneer neurons in situ. Neuron. 1995;14:1247–1256. doi: 10.1016/0896-6273(95)90271-6. [DOI] [PubMed] [Google Scholar]
- 171.Chu Y., Morfini G.A., Langhamer L.B., He Y., Brady S.T., Kordower J.H. Alterations in axonal transport motor proteins in sporadic and experimental Parkinson’s disease. Brain. 2012;135:2058–2073. doi: 10.1093/brain/aws133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ikenaka K., Katsuno M., Kawai K., Ishigaki S., Tanaka F., Sobue G. Disruption of axonal transport in motor neuron diseases. Int. J. Mol. Sci. 2012;13:1225–1238. doi: 10.3390/ijms13011225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ye X., Tai W., Zhang D. The early events of Alzheimer’s disease pathology: From mitochondrial dysfunction to BDNF axonal transport deficits. Neurobiol. Aging. 2012;33:1122.e1-10. doi: 10.1016/j.neurobiolaging.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 174.Magen I., Gozes I. Microtubule-stabilizing peptides and small molecules protecting axonal transport and brain function: Focus on davunetide (NAP) Neuropeptides. 2013;47:489–495. doi: 10.1016/j.npep.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 175.Saman S., Kim W., Raya M., Visnick Y., Miro S., Saman S., Jackson B., McKee A.C., Alvarez V.E., Lee N.C. Exosome-associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early Alzheimer disease. J. Biol. Chem. 2012;287:3842–3849. doi: 10.1074/jbc.M111.277061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Gerth K., Bedorf N., Höfle G., Irschik H., Reichenbach H. Epothilons A and B: Antifungal and cytotoxic compounds from Sorangium cellulosum (Myxobacteria) J. Antibiot. 1996;49:560–563. doi: 10.7164/antibiotics.49.560. [DOI] [PubMed] [Google Scholar]
- 177.De Vos K.J., Grierson A., Ackerley S., Miller C. Role of axonal transport in neurodegenerative diseases. Annu. Rev. Neurosci. 2008;31:151–173. doi: 10.1146/annurev.neuro.31.061307.090711. [DOI] [PubMed] [Google Scholar]
- 178.Zhang B., Carroll J., Trojanowski J.Q., Yao Y., Iba M., Potuzak J.S., Hogan A.-M.L., Xie S.X., Ballatore C., Smith A.B., et al. The microtubule-stabilizing agent, epothilone D, reduces axonal dysfunction, neurotoxicity, cognitive deficits, and Alzheimer-like pathology in an interventional study with aged tau transgenic mice. J. Neurosci. 2012;32:3601–3611. doi: 10.1523/JNEUROSCI.4922-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Cartelli D., Casagrande F., Busceti C.L., Bucci D., Molinaro G., Traficante A., Passarella D., Giavini E., Pezzoli G., Battaglia G., et al. Microtubule alterations occur early in experimental parkinsonism and the microtubule stabilizer epothilone D is neuroprotective. Sci. Rep. 2013;3:1837. doi: 10.1038/srep01837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Eckert G.P., Lipka U., Muller W.E. Omega-3 fatty acids in neurodegenerative diseases: Focus on mitochondria. Prostaglandins Leukotrienes Essent. Fat. Acids (PLEFA) 2013;88:105–114. doi: 10.1016/j.plefa.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 181.Nichols D.S., McMeekin T.A. Biomarker techniques to screen for bacteria that produce polyunsaturated fatty acids. J. Microbiol. Methods. 2002;48:161–170. doi: 10.1016/S0167-7012(01)00320-7. [DOI] [PubMed] [Google Scholar]
- 182.Yano Y., Nakayama A., Yoshida K. Distribution of polyunsaturated fatty acids in bacteria present in intestines of deep-sea fish and shallow-sea poikilothermic animals. Appl. Environ. Microbiol. 1997;63:2572–2577. doi: 10.1128/aem.63.7.2572-2577.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hayashi S., Satoh Y., Ujihara T., Takata Y., Dairi T. Enhanced production of polyunsaturated fatty acids by enzyme engineering of tandem acyl carrier proteins. Sci. Rep. 2016;6:35441. doi: 10.1038/srep35441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Gemperlein K., Rachid S., Garcia R.O., Wenzel S.C., Müller R. Polyunsaturated fatty acid biosynthesis in myxobacteria: Different PUFA synthases and their product diversity. Chem. Sci. 2014;5:1733–1741. doi: 10.1039/c3sc53163e. [DOI] [Google Scholar]
- 185.Feng Z., Zou X., Jia H., Li X., Zhu Z., Liu X., Bucheli P., Ballevre O., Hou Y., Zhang W., et al. Maternal docosahexaenoic acid feeding protects against impairment of learning and memory and oxidative stress in prenatally stressed rats: Possible role of neuronal mitochondria metabolism. Antioxid. Redox. Signal. 2012;16:275–289. doi: 10.1089/ars.2010.3750. [DOI] [PubMed] [Google Scholar]
- 186.Song L., Zheng J., Li H., Jia N., Suo Z., Cai Q., Bai Z., Cheng D., Zhu Z. Prenatal stress causes oxidative damage to mitochondrial DNA in hippocampus of offspring rats. Neurochem. Res. 2009;34:739–745. doi: 10.1007/s11064-008-9838-y. [DOI] [PubMed] [Google Scholar]
- 187.Eckert G.P., Zheng J., Li H., Jia N., Suo Z., Cai Q., Bai Z., Cheng D., Zhu Z. Liposome-incorporated DHA increases neuronal survival by enhancing non-amyloidogenic APP processing. Biochim. Biophys. Acta BBA Biomembr. 2011;1808:236–243. doi: 10.1016/j.bbamem.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 188.White K.N., Tenney K., Crews P. The Bengamides: A Mini-Review of Natural Sources, Analogues, Biological Properties, Biosynthetic Origins, and Future Prospects. J. Nat. Prod. 2017;80:740–755. doi: 10.1021/acs.jnatprod.6b00970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Castro C., Freitag J., Berod L., Lochner M., Sparwasser T. Microbe-associated immunomodulatory metabolites: Influence on T cell fate and function. Mol. Immunol. 2015;68:575–584. doi: 10.1016/j.molimm.2015.07.025. [DOI] [PubMed] [Google Scholar]
- 190.Johnson T.A., Sohn J., Vaske Y.M., White K.N., Cohen T.L., Vervoort H.C., Tenney K., Valeriote F.A., Bjeldanes L.F., Crews P. Myxobacteria versus sponge-derived alkaloids: The bengamide family identified as potent immune modulating agents by scrutiny of LC–MS/ ELSD libraries. Bioorg. Med. Chem. 2012;20:4348–4355. doi: 10.1016/j.bmc.2012.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Jansen R., Washausen P., Kunze B., Reichenbach H., Höfle G. ChemInform Abstract: Antibiotics from Gliding Bacteria. Part 83. The Crocacins, Novel Antifungal and Cytotoxic Antibiotics from Chondromyces crocatus and Chondromyces pediculatus (Myxobacteria): Isolation and Structure Elucidation. Cheminform. 2010;30 doi: 10.1002/chin.199930200. [DOI] [Google Scholar]
- 192.Kunze B., Hofle G., Reichenbach H. The aurachins, new quinoline antibiotics from myxobacteria: Production, physico-chemical and biological properties. J. Antibiot. 1987;40:258–265. doi: 10.7164/antibiotics.40.258. [DOI] [PubMed] [Google Scholar]
- 193.Miyoshi H., Takegami K., Sakamoto K., Mogi T., Iwamura H. Characterization of the ubiquinol oxidation sites in cytochromes bo and bd from Escherichia coli using aurachin C analogues. J. Biochem. 1999;125:138–142. doi: 10.1093/oxfordjournals.jbchem.a022250. [DOI] [PubMed] [Google Scholar]
- 194.Gerth K., Irschik H., Reichenbach H., Trowitzsch W. Myxothiazol, an antibiotic from Myxococcus fulvus (Myxobacterales) J. Antibiot. 1980;33:1474–1479. doi: 10.7164/antibiotics.33.1474. [DOI] [PubMed] [Google Scholar]
- 195.Thierbach G., Reichenbach H. Myxothiazol, a new antibiotic interfering with respiration. Antimicrob. Agents Chemother. 1981;19:504–507. doi: 10.1128/AAC.19.4.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Sasse F., Böhlendorf B., Herrmann M., Kunze B., Forche E., Steinmetz H., Höfle G., Reichenbach H. Melithiazols, new beta-methoxyacrylate inhibitors of the respiratory chain isolated from myxobacteria. Production, isolation, physico-chemical and biological properties. J. Antibiot. 1999;52:721–729. doi: 10.7164/antibiotics.52.721. [DOI] [PubMed] [Google Scholar]
- 197.Oettmeier W., Godde D., Höfle G. Stigmatellin. A dual type inhibitor of photosynthetic electron transport. Biochim. Biophys. Acta. 1985;807:216–219. doi: 10.1016/0005-2728(85)90125-2. [DOI] [Google Scholar]
- 198.Yamashita E., Zhang H., Cramer W.A. Structure of the cytochrome b6f complex: Quinone analogue inhibitors as ligands of heme cn. J. Mol. Biol. 2007;370:39–52. doi: 10.1016/j.jmb.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Dejon L., Speicher A. Synthesis of aurachin D and isoprenoid analogues from the myxobacterium Stigmatella aurantiaca. Tetrahedron Lett. 2013;54:6700–6702. doi: 10.1016/j.tetlet.2013.09.085. [DOI] [Google Scholar]
- 200.Irschik H., Trowitzsch-Kienast W., Gerth K., Hofle G., Reichenbach H. Saframycin Mx1, a new natural saframycin isolated from a myxobacterium. J. Antibiot. 1988;41:993–998. doi: 10.7164/antibiotics.41.993. [DOI] [PubMed] [Google Scholar]
- 201.Sasse F., Steinmetz H., Höfle G., Reichenbach H. Gephyronic acid, a novel inhibitor of eukaryotic protein synthesis from Archangium gephyra (myxobacteria). Production, isolation, physico-chemical and biological properties, and mechanism of action. J. Antibiot. 1995;48:21–25. doi: 10.7164/antibiotics.48.21. [DOI] [PubMed] [Google Scholar]
- 202.Irschik H., Reichenbach H. The mechanism of action of myxovalargin A, a peptide antibiotic from Myxococcus fulvus. J. Antibiot. 1985;38:1237–1245. doi: 10.7164/antibiotics.38.1237. [DOI] [PubMed] [Google Scholar]
- 203.Jimenez A., Tipper D.J., Davies J. Mode of action of thiolutin, an inhibitor of macromolecular synthesis in Saccharomyces cerevisiae. Antimicrob. Agents Chemother. 1973;3:729–738. doi: 10.1128/AAC.3.6.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Tipper D.J. Inhibition of yeast ribonucleic acid polymerases by thiolutin. J. Bacteriol. 1973;116:245–256. doi: 10.1128/jb.116.1.245-256.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Tuske S., Sarafianos S.G., Wang X., Hudson B., Sineva E., Mukhopadhyay J., Birktoft J.J., Leroy O., Ismail S., Clark A.D., Jr., et al. Inhibition of bacterial RNA polymerase by streptolydigin: Stabilization of a straight-bridge-helix active-center conformation. Cell. 2005;122:541–552. doi: 10.1016/j.cell.2005.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Oliva B., O’Neill A., Wilson J.M., O’Hanlon P.J., Chopra I. Antimicrobial properties and mode of action of the pyrrothine holomycin. Antimicrob. Agents Chemother. 2001;45:532–539. doi: 10.1128/AAC.45.2.532-539.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Artsimovitch I., Vassylyeva M.N., Svetlov D., Svetlov V., Perederina A., Igarashi N., Matsugaki N., Wakatsuki S., Tahirov T.H., Vassylyev D.G. Allosteric modulation of the RNA polymerase catalytic reaction is an essential component of transcription control by rifamycins. Cell. 2005;122:351–363. doi: 10.1016/j.cell.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 208.O’Neill A.J., Huovinen T., Fishwick C.W., Chopra I. Molecular genetics and structural modeling studies of Staphylococcus aureus RNA polymerase and the fitness of rifampin resistance genotypes in relation to clinical prevalence. Antimicrob. Agents Chemother. 2006;50:298–309. doi: 10.1128/AAC.50.1.298-309.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.O’Neill A., Oliva B., Storey C., Hoyle A., Fishwick C., Chopra I. RNA polymerase inhibitors with activity against rifampin-resistant mutants of Staphylococcus aureus. Antimicrob. Agents Chemother. 2000;44:3163–3166. doi: 10.1128/AAC.44.11.3163-3166.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Campbell E.A., Pavlova O., Zenkin N., Leon F., Irschik H., Jansen R., Severinov K., Darst S.A. Structural, functional, and genetic analysis of sorangicin inhibition of bacterial RNA polymerase. EMBO J. 2005;24:674–682. doi: 10.1038/sj.emboj.7600499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Irschik H., Jansen R., Gerth K., Höfle G., Reichenbach H. The sorangicins, novel and powerful inhibitors of eubacterial RNA polymerase isolated from myxobacteria. J. Antibiot. 1987;40:7–13. doi: 10.7164/antibiotics.40.7. [DOI] [PubMed] [Google Scholar]
- 212.Augustiniak H., Höfle G., Irschik H., Reichenbach H. Antibiotics from gliding bacteria, LXXVIII. Ripostatin A, B, and C: Isolation and structure and structure elucidation of novel metabolites from Sorangium cellulosum. Liebigs Ann. 1996;1996:1657–1663. doi: 10.1002/jlac.199619961026. [DOI] [Google Scholar]
- 213.Irschik H., Augustiniak H., Gerth K., HöFle G., Reichenbach H. Antibiotics from gliding bacteria. No. 68. The Ripostatins, Novel Inhibitors of Eubacterial RNA Polymerase Isolated from myxobacteria. J. Antibiot. 1995;48:787–792. doi: 10.7164/antibiotics.48.787. [DOI] [PubMed] [Google Scholar]
- 214.Irschik H., Jansen R., Höfle G., Gerth K., Reichenbach H. The corallopyronins, new inhibitors of bacterial RNA synthesis from Myxobacteria. J. Antibiot. 1985;38:145–152. doi: 10.7164/antibiotics.38.145. [DOI] [PubMed] [Google Scholar]
- 215.Gronewold T., Sasse F., Lünsdorf H., Reichenbach H. Effects of rhizopodin and latrunculin B on the morphology and on the actin cytoskeleton of mammalian cells. Cell Tissue Res. 1999;295:121–129. doi: 10.1007/s004410051218. [DOI] [PubMed] [Google Scholar]
- 216.Sasse F., Steinmetz H., Höfle G., Reichenbach H. Rhizopodin, a new compound from Myxococcus stipitatus (Myxobacteria) causes formation of rhizopodia-like structures in animal cell cultures. Production, isolation, physico-chemical and biological properties. J. Antibiot. 1993;46:741–748. doi: 10.7164/antibiotics.46.741. [DOI] [PubMed] [Google Scholar]
- 217.Holzinger A., Lütz-Meindl U. Chondramides, novel cyclodepsipeptides from myxobacteria, influence cell development and induce actin filament polymerization in the green alga Micrasterias. Cell Motil. Cytoskelet. 2001;48:87–95. doi: 10.1002/1097-0169(200102)48:2<87::AID-CM1000>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 218.Sasse F., Kunze B., Gronewold T., Reichenbach H. The chondramides: Cytostatic agents from myxobacteria acting on the actin cytoskeleton. J. Natl Cancer Inst. 1998;90:48–52. doi: 10.1093/jnci/90.20.1559. [DOI] [PubMed] [Google Scholar]
- 219.Bollag D.M., McQueney P.A., Zhu J., Hensens O., Koupal L., Liesch J., Goetz M., Lazarides E., Woods C.M. Epothilones, a new class of microtubule-stabilizing agents with a taxol-like mechanism of action. Cancer Res. 1995;55:2325–2333. [PubMed] [Google Scholar]
- 220.Höfle G., Bedorf N., Steinmetz H., Schomburg D., Gerth K., Reichenbach H. Epothilone A and B-Novel 16-membered macrolides with cytotoxic activity: Isolation, crystal structure, and conformation in solution. Angew. Chem. Int. Ed. Engl. 1996;35:1567–1569. doi: 10.1002/anie.199615671. [DOI] [Google Scholar]
- 221.Khalil M.W., Sasse F., Lünsdorf H., Elnakady Y.A., Reichenbach H. Mechanism of action of tubulysin, an antimitotic peptide from myxobacteria. ChemBioChem. 2006;7:678–683. doi: 10.1002/cbic.200500421. [DOI] [PubMed] [Google Scholar]
- 222.Sasse F., Steinmetz H., Heil J., Höfle G., Reichenbach H. Tubulysins, New Cytostatic Peptides from Myxobacteria Acting on Microtubuli. Production, Isolation, Physico-chemical and Biological Properties. J. Antibiot. 2000;53:879–885. doi: 10.7164/antibiotics.53.879. [DOI] [PubMed] [Google Scholar]
- 223.Cheng K.L., Bradley T., Budman D.R. Novel microtubule-targeting agents—The epothilones. Biol. Targets Ther. 2008;2:789–811. doi: 10.2147/btt.s3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Fumoleau P., Coudert B., Isambert N., Ferrant E. Novel tubulin-targeting agents:anticancer activity and pharmacologic profile of epothilones and related analogues. Ann. Oncol. 2007;18:9–15. doi: 10.1093/annonc/mdm173. [DOI] [PubMed] [Google Scholar]
- 225.Steinmetz H., Glaser N., Herdtweck E., Sasse F., Reichenbach H., Höfle G. Isolation, crystal and solution structure determination, and biosynthesis of tubulysins-powerful inhibitors of tubulin polymerization from myxobacteria. Angew. Chem. Int. Ed. Engl. 2004;43:4888–4892. doi: 10.1002/anie.200460147. [DOI] [PubMed] [Google Scholar]
- 226.Patterson A.W., Peltier H.M., Sasse F., Ellman J.A. Design, synthesis, and biological properties of highly potent tubulysin D analogues. Chemistry. 2007;13:9534–9541. doi: 10.1002/chem.200701057. [DOI] [PubMed] [Google Scholar]
- 227.Kaur G., Hollingshead M., Holbeck S., Schauer-Vukasinovic V., Camalier R.F., Domling A., Agarwal S. Biological evaluation of tubulysin A: A potential anticancer and antiangiogenic natural product. Biochem. J. 2006;396:235–242. doi: 10.1042/BJ20051735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Beckers A., Organe S., Timmermans L., Scheys K., Peeters A., Brusselmans K., Verhoeven G., Swinnen J.V. Chemical inhibition of acetyl-CoA carboxylase induces growth arrest and cytotoxicity selectively in cancer cells. Cancer Res. 2007;67:8180–8187. doi: 10.1158/0008-5472.CAN-07-0389. [DOI] [PubMed] [Google Scholar]
- 229.Rosenberg E., Varon M. Antibiotics and Lytic Enzymes. In: Rosenberg E., editor. Myxobacteria. Springer; New York, NY, USA: 1984. (Springer Series in Molecular Biology). [Google Scholar]
- 230.Hill D.S., Stein J.I., Torkewitz N.R., Morse A.M., Howell C.R., Pachlatko J.P., Becker J.O., Ligon J.M. Cloning of genes involved in the synthesis of pyrrolnitrin from Pseudomonas fluorescens and role of pyrrolnitrin synthesis in biological control of plant disease Appl. Environ. Microb. 1994;60:78–85. doi: 10.1128/aem.60.1.78-85.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Hocking D., Cook F.D. Myxobacteria exert partial control of damping-off and root disease in container-grown tree seedlings. Can. J. Microbiol. 1972;18:1557–1560. doi: 10.1139/m72-237. [DOI] [PubMed] [Google Scholar]
- 232.Homma Y. Perforation and lysis of hyphae of Rhizoctonia solani and conidia of Cochliobolus miyabeanus by soil myxobacteria. Agris. 1984;74:1234–1239. [Google Scholar]
- 233.Norén B., Odham G. Antagonistic effects of Myxococcus xanthus on fungi: II. Isolation and characterization of inhibitory lipid factors. Lipids. 1973;8:573–583. doi: 10.1007/BF02532714. [DOI] [PubMed] [Google Scholar]
- 234.Lautru S., Deeth R.J., Bailey L.M., Challis G.L. Discovery of a new peptide natural product by Streptomyces coelicolor genome mining. Nature Chem. Biol. 2005;1:265–269. doi: 10.1038/nchembio731. [DOI] [PubMed] [Google Scholar]
- 235.Bode H.B., Bethe B., Höfs R., Zeeck A. Big effects from small changes: Possible ways to explore nature’s chemical diversity. ChemBioChem. 2002;3:619–627. doi: 10.1002/1439-7633(20020703)3:7<619::AID-CBIC619>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 236.Christian O.E., Compton J., Christian K.R., Mooberry S.L., Valeriote F.A., Crews P. Using jasplakinolide to turn on pathways that enable the isolation of new chaetoglobosins from Phomopsis asparagi. J. Nat. Prod. 2005;68:1592–1597. doi: 10.1021/np050293f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Bergmann S., Schümann J., Scherlach K., Lange C., Brakhage A., Hertweck C. Genomics-driven discovery of PKS-NRPS hybrid metabolites from Aspergillus nidulans. Nat. Chem. Biol. 2007;3:213–217. doi: 10.1038/nchembio869. [DOI] [PubMed] [Google Scholar]
- 238.Sandmann A., Frank B., Müller R. A transposon-based strategy to scale up myxothiazol production in myxcobacterial cell factories. J. Biotechnol. 2008;135:255–261. doi: 10.1016/j.jbiotec.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 239.Julien B., Tian Z.Q., Reid R., Reeves C.D. Analysis of the ambruticin and jerangolid gene clusters of Sorangium cellulosum reveals unusual mechanisms of polyketide biosynthesis. Chem. Biol. 2006;13:1277–1286. doi: 10.1016/j.chembiol.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 240.Weissman K.J., Müller R. A brief tour of myxobacterial secondary metabolism. Bioorg. Med. Chem. 2009;17:2121–2136. doi: 10.1016/j.bmc.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 241.Sandmann A., Dickschat J., Jenke-Kodama H., Kunze B., Dittmann E., Müller R. A Type II Polyketide Synthase from the Gram-Negative Bacterium Stigmatella aurantiaca Is Involved in Aurachin Alkaloid Biosynthesis. Angew. Chem. Int. Ed. 2007;46:2712–2716. doi: 10.1002/anie.200603513. [DOI] [PubMed] [Google Scholar]
- 242.Carvalho R., Reid R., Viswanathan N., Gramajo H., Julien B. The biosynthetic genes for disorazoles, potent cytotoxic compounds that disrupt microtubule formation. Gene. 2005;359:91–98. doi: 10.1016/j.gene.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 243.Sandmann A., Sasse F., Müller R. Identification and analysis of the core biosynthetic machinery of tubulysin, a potent cytotoxin with potential anticancer activity. Chem. Biol. 2004;11:1071–1079. doi: 10.1016/j.chembiol.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 244.Rachid S., Sasse F., Beyer S., Müller R. Identification of StiR, the first regulator of secondary metabolite formation in the myxobacterium Cystobacter fuscus Cb f17.1. J. Biotechnol. 2006;121:429–441. doi: 10.1016/j.jbiotec.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 245.Rachid S., Gerth K., Kochems I., Müller R. Deciphering regulatory mechanisms for secondary metabolite production in the myxobacterium Sorangium cellulosum So ce56. Molecul. Microbiol. 2007;63:1783–1796. doi: 10.1111/j.1365-2958.2007.05627.x. [DOI] [PubMed] [Google Scholar]
- 246.Silakowski B., Kunze B., Nordsiek G., Blöcker H., Höfle G., Müller R. The myxochelin iron transport regulon of the myxobacterium Stigmatella aurantiaca Sg a15. Eur. J. Biochem. 2000;267:6476–6485. doi: 10.1046/j.1432-1327.2000.01740.x. [DOI] [PubMed] [Google Scholar]
- 247.Wenzel S., Kunze B., Höfle G., Silakowski B., Scharfe M., Blöcker H., Müller R. Structure and Biosynthesis of Myxochromides S1-3 in Stigmatella aurantiaca: Evidence for an Iterative Bacterial Type I Polyketide Synthase and for Module Skipping in Nonribosomal Peptide Biosynthesis. ChemBioChem. 2005;6:375–385. doi: 10.1002/cbic.200400282. [DOI] [PubMed] [Google Scholar]
- 248.Ostash B., Saghatelian A., Walker S. A streamlined metabolic pathway for the biosynthesis of moenomycin A. Chem. Biol. 2007;14:257–267. doi: 10.1016/j.chembiol.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Ostash B., Campbell J., Luzhetskyy A., Walker S. MoeH5: A natural glycorandomizer from the moenomycin biosynthetic pathway. Mol. Microbiol. 2013;90:1324–1338. doi: 10.1111/mmi.12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Kodumal S., Patel K., Reid R., Menzella H., Welch M., Santi D. Total synthesis of long DNA sequences: Synthesis of a contiguous 32-kb polyketide synthase gene cluster. Proc. Nat. Acad. Sci. USA. 2004;101:15573–15578. doi: 10.1073/pnas.0406911101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Julien B., Shah S. Heterologous expression of epothilone biosynthetic genes in Myxococcus xanthus. Antimicrob. Agents Chemother. 2002;46:2772–2778. doi: 10.1128/AAC.46.9.2772-2778.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Tang L., Shah S., Chung L., Carney J., Katz L., Khosla C., Julien B. Cloning and heterologous expression of the epothilone gene cluster. Science. 2000;287:640–642. doi: 10.1126/science.287.5453.640. [DOI] [PubMed] [Google Scholar]
- 253.Mutka S.C., Carney J.R., Liu Y., Kennedy J. Heterologous production of epothilone C and D in Escherichia coli. Biochemistry. 2006;45:1321–1330. doi: 10.1021/bi052075r. [DOI] [PubMed] [Google Scholar]
- 254.Fu J., Wenzel S.C., Perlova O., Wang J., Gross F., Tang Z., Yin Y., Stewart A.F., Müller R., Zhang Y. Efficient transfer of two large secondary metabolite pathway gene clusters into heterologous hosts by transposition. Nucleic Acids Res. 2008;36:e113. doi: 10.1093/nar/gkn499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Zirkle R., Ligon J.M., Molnár I. Heterologous production of the antifungal polyketide antibiotic soraphen A of Sorangium cellulosum So ce26 in Streptomyces lividans. Microbiology. 2004;150:2761–2774. doi: 10.1099/mic.0.27138-0. [DOI] [PubMed] [Google Scholar]
- 256.Perlova O., Fu J., Kuhlmann S., Krug D., Stewart A., Zhang Y., Müller R. Reconstitution of the Myxothiazol Biosynthetic Gene Cluster by Red/ET Recombination and Heterologous Expression in Myxococcus xanthus. Appl. Environ. Microbiol. 2007;72:7485–7494. doi: 10.1128/AEM.01503-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Gross F., Luniak N., Perlova O., Gaitatzis N., Jenke-Kodama H., Gerth K., Gottschalk D., Dittmann E., Müller R. Bacterial type III polyketide synthases: Phylogenetic analysis and potential for the production of novel secondary metabolites by heterologous expression in pseudomonads. Arch. Microbiol. 2006;185:28–38. doi: 10.1007/s00203-005-0059-3. [DOI] [PubMed] [Google Scholar]
- 258.Zhang Y., Buchholz F., Muyrers J.P., Stewart A.F. A new logic for DNA engineering using recombination in Escherichia coli. Nat. Genet. 1998;20:123–128. doi: 10.1038/2417. [DOI] [PubMed] [Google Scholar]
- 259.Weissman K.J., Leadlay P.F. Combinatorial biosynthesis of reduced polyketides. Nat. Rev. Microbiol. 2005;3:925–936. doi: 10.1038/nrmicro1287. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


