Abstract
The protein tyrosine kinase PYK2 has been implicated in signaling pathways activated by G-protein-coupled receptors, intracellular calcium, and stress signals. Here we describe the molecular cloning and characterization of a novel family of PYK2-binding proteins designated Nirs (PYK2 N-terminal domain-interacting receptors). The three Nir proteins (Nir1, Nir2, and Nir3) bind to the amino-terminal domain of PYK2 via a conserved sequence motif located in the carboxy terminus. The primary structures of Nirs reveal six putative transmembrane domains, a region homologous to phosphatidylinositol (PI) transfer protein, and an acidic domain. The Nir proteins are the human homologues of the Drosophila retinal degeneration B protein (rdgB), a protein implicated in the visual transduction pathway in flies. We demonstrate that Nirs are calcium-binding proteins that exhibit PI transfer activity in vivo. Activation of PYK2 by agents that elevate intracellular calcium or by phorbol ester induce tyrosine phosphorylation of Nirs. Moreover, PYK2 and Nirs exhibit similar expression patterns in several regions of the brain and retina. In addition, PYK2-Nir complexes are detected in lysates prepared from cultured cells or from brain tissues. Finally, the Nir1-encoding gene is located at human chromosome 17p13.1, in proximity to a locus responsible for several human retinal diseases. We propose that the Nir and rdgB proteins represent a new family of evolutionarily conserved PYK2-binding proteins that play a role in the control of calcium and phosphoinositide metabolism downstream of G-protein-coupled receptors.
Tyrosine phosphorylation plays an important role in the regulation of intracellular events in response to external stimuli. Activation of receptor tyrosine kinases by specific ligands leads to recruitment of signaling molecules by means of small protein modules such as the SH2, SH3, PTB, and PH domains, among others (6). Receptors that lack intrinsic protein tyrosine kinase activity access similar kinase cascades and signaling proteins through recruitment of nonreceptor protein tyrosine kinases (15, 22, 35). For example, the nonreceptor protein tyrosine kinases Lck and ZAP70 play an important role in signaling pathways which are activated upon engagement of the T-cell receptor, Src is activated in response to stimulation of receptor tyrosine kinases and different G-protein-coupled receptors, and Jak tyrosine kinases have been implicated in signaling via cytokine receptors, while the focal adhesion kinase (FAK) plays a role in integrin-mediated signal transduction cascades.
PYK2 (also known as RAFTK, CAKβ, and CADTK) (3, 19, 28, 39) and FAK belong to the same family of nonreceptor protein tyrosine kinases. In spite of their structural similarity, PYK2 and FAK exhibit different tissue expression patterns and different modes of activation. Activation of FAK is more strictly linked to integrin-mediated signaling pathways (25), whereas PYK2 is activated in response to a variety of extracellular stimuli that elevate the intracellular Ca2+ concentration (19). Calcium influx mediated by activation of the nicotinic acetylcholine receptor or by a voltage-gated calcium channel and agonists that promote calcium release from intracellular stores induce strong tyrosine phosphorylation of PYK2 in PC12 cells (12, 19, 29, 34, 39). Although the mechanism by which calcium induces PYK2 activation is not understood, PYK2 appears to function as a signaling component that, in different cell types, can mediate calcium-induced mitogen-activated protein (MAP) kinase or Jun kinase cascades in response to different external stimuli (34). PYK2 is also activated in response to agonists of G-protein-coupled receptors such as bradykinin, l-α-lysophosphatidic acid (LPA), or angiotensin II (12, 21).
Upon bradykinin or LPA stimulation, a major autophosphorylation site of PYK2, at Tyr402, functions as a docking site for the SH2 domain of Src (12). Binding to tyrosine-phosphorylated PYK2 leads to activation of Src, which, in turn, phosphorylates PYK2 on Tyr881, leading to recruitment of the Grb2-Sos complex and subsequent activation of the MAP kinase signaling pathway (12, 19). In addition, activation of PYK2 leads to tyrosine phosphorylation of the adapter protein Shc (19), focal contact proteins p130cas (2) and paxillin (20), and delayed-rectifier-type potassium channel Kv1.2 (19). Tyrosine phosphorylation of Kv1.2 suppresses outward potassium current, suggesting that PYK2 plays a role in regulation of neuronal excitability (19).
Since PYK2 is activated by a variety of extracellular stimuli in different cell types, including agonists of ion channels and G-protein-coupled receptors, and engagements of T-cell, B-cell, and integrin receptors (2, 23), as well as by stress signals (34), it was proposed that PYK2 may facilitate coupling between different intracellular signaling pathways. The identification of PYK2-binding proteins may shed light on new mechanisms for coupling between intracellular signaling pathways that are activated by different extracellular stimuli.
In this report, we describe the identification of a new family of PYK2-binding proteins designated Nirs (Nir1, Nir2, and Nir3). The three Nir proteins are the mammalian homologues of the Drosophila retinal degeneration B (rdgB) protein. We demonstrate that Nirs are calcium-binding proteins that possess phosphatidylinositol (PI) transfer activity. Nir proteins bind to PYK2 via a conserved region located in the carboxy-terminal domain. Complex formation is detected in yeast in vitro and in lysates prepared from cultured cells or from brain tissues. Moreover, Nir proteins are tyrosine phosphorylated by PYK2 in vitro and in response to agonists of PYK2 in vivo. We also show that PYK2 and Nirs exhibit similar expression patterns in several regions in the brain and in the retina. Based on these results and the genetic studies in Drosophila, we propose that Nir proteins function in concert with PYK2 downstream of G-protein-coupled receptors as components of an evolutionarily conserved calcium- and phosphoinositide-dependent signaling pathways in flies and vertebrates.
MATERIALS AND METHODS
Two-hybrid screen.
Yeast strain L40, containing the reporter genes for HIS3 and β-galactosidase (β-Gal) under the control of an upstream LexA-binding site, was used as a host for two-hybrid screening. The PYK2 N-terminal domain (PYK2-N; 2 to 425), PYKN-ΔI (2 to 377), PYK-NN (2 to 285), and the FAK N-terminal domain (2 to 412) were fused in frame to the LexA DNA-binding domain. A yeast strain expressing the LexA–PYK2-N fusion protein was transfected with a human brain cDNA library (Clontech) fused to the GAL4 transcriptional activation domain. Transformants were plated on agar selection medium lacking uracil (Ura−), tryptophan (Trp−), leucine (Leu−), and histidine (His−). The resulting colonies were isolated and retested for growth on Ura− Trp− Leu− His− plates and for β-Gal activity (38). Plasmid DNA was purified from colonies that were His+ β-Gal+ and used for retransformation of yeast strains expressing heterologous baits to determine specificity of interactions.
Isolation of Nir1, Nir2, and Nir3 cDNAs.
A human brain substania nigra cDNA library (λgt10; Clontech) was screened with a 32P-labeled probe derived from the yeast prey plasmid encoding GAL4-Nir1 (clone 20A). Four independent clones were isolated, subcloned, and analyzed by sequence determination. This analysis demonstrated that the 5′ end of the gene is missing from this clone. A human fetal brain cDNA library (λgt11; Clontech) was screened with a probe derived from the most 5′ region of the new cDNA clone. Sequence analysis of six independent clones showed that these clones belonged to the same Nir1-encoding gene, yet all were missing the 5′ end of the sequence. A specifically primed cDNA library was constructed in λZapII by utilizing human fetal brain tissue. Poly(A)+ RNA was used as the template for cDNA synthesis (Stratagene kit). Fifteen independent clones were isolated, enabling the cloning of the full-length Nir1 cDNA.
A DNA fragment derived from an expressed sequence tag (EST) fragment (T12574) was amplified from a human fetal brain cDNA by PCR. The PCR product was used as a probe for screening of a human fetal brain cDNA library (λgt11; Clontech). One positive clone exhibited sequence similarity to Nir1 and rdgB. This cDNA (1.8 kb) was used as a probe for rescreening of the same cDNA library. Seven independent clones were obtained, subcloned, and sequenced. Analysis of their sequences demonstrated that these clones belong to the gene for Nir2. However, these clones were different from the original clone that was isolated from the same cDNA library. We next used the 3′ end of the first clone (1.8 kb) as a probe for screening of a human heart cDNA library (Clontech), enabling the cloning of the two isoforms of the gene for Nir3.
Northern blot analysis.
Human multiple-tissue Northern blots (Clontech) were hybridized under high-stringency conditions by using 32P-labeled cDNA fragments of Nir1 (EcoRI-Eco47III, nucleotides 246 to 511), Nir2 (SacI-Eco47III, nucleotides 1540 to 2661), and Nir3 (BstXI, nucleotides 912 to 1472) as probe in accordance with the manufacturer’s instructions.
Plasmid constructs and expression vectors.
PCR was used to amplify different regions of PYK2 and FAK cDNAs as indicated. The amplified DNA fragments were subcloned into pBTM116 in frame to generate a fusion protein with a LexA DNA-binding domain. PCR was used to amplify different regions of Nir1, Nir2, or Nir3 cDNA as indicated. The amplified DNA fragments were subcloned into pGAD10 (Clontech) in frame to generate a fusion protein with the GAL4 activation domain. The full-length cDNAs of Nir1, Nir2, and Nir3 were subcloned into pCMP1 downstream of the cytomegalovirus promoter. A hemagglutinin (HA) epitope tag (YPYDVPDYAS) was fused in frame to their carboxy-terminal ends.
Complementation of the sec14ts growth lesion.
The PI transfer domain of Nir3 was amplified by PCR and subcloned into pAD54 (kindly provided by J. E. Gerst) downstream of the alcohol dehydrogenase promoter. Saccharomyces cerevisiae CTY483 (α ura3-52 Δhis3-200 ade2 ade3 leu2-3 sec14-1ts) (kindly provided by V. A. Bankaitis) was transformed with an expression vector containing the Nir3 PI transfer domain or with the vector alone. Yeast transformation was carried out by the standard lithium acetate method.
Antibodies, immunoprecipitation, and immunoblotting.
Antibodies against Nir1, Nir2, and Nir3 were raised in rabbits immunized with keyhole limpet hemocyanin-conjugated synthetic peptides corresponding to amino acids 965 to 974 of Nir1, 287 to 301 and 1230 to 1244 of Nir2, and 652 to 666 of Nir3. Antibodies against PYK2 were raised in a rabbit immunized with a MAP peptide corresponding to amino acids 2 to 15 of PYK2 or with a maltose-binding protein (MBP) fusion protein containing amino acids 285 to 445 of PYK2. Antibodies against FAK were purchased from Upstate Biotechnology. Immunoprecipitation and immunoblotting were performed as described previously (19). Rat brain homogenate (5%, wt/vol) was prepared by using a Teflon-glass homogenizer (five strokes) and a buffer containing 0.32 M sucrose, 20 mM HEPES (pH 7.5), 1 mM EGTA, 1.5 mM MgCl2, 1 mM phenylmethylsulfonyl fluoride, 10-μg/ml leupeptin, and 10-μg/ml aprotinin. The homogenate was centrifuged at 1,000 × g for 20 min. Triton X-100 was added to the supernatant (1%, wt/vol). Following a 30-min incubation on ice, the homogenate was centrifuged for 1 h at 100,000 × g. The supernatant was used for immunoprecipitation following addition of NaCl to 150 mM.
Calcium overlay assay.
The calcium overlay assay was performed as described previously with minor modifications (37). Briefly, MBP fusion proteins (5 μg) bound to nitrocellulose filters were incubated in washing buffer (60 mM KCl, 5 mM MgCl2, 10 mM imidazole-HCl, pH 6.8) for 20 min at room temperature. Following two additional washes, the membrane was incubated with buffer containing 45Ca2+ (1.5 μCi/ml; Du Pont NEN) for 20 min. The membrane was washed with aqueous ethanol (67%) for 10 min, dried, and exposed to X-ray film for 48 to 72 h.
Recombinant proteins.
MBP or gluthatione S-transferase (GST) fusion proteins were expressed in bacteria and purified essentially as previously described (16, 30).
Immunohistochemical staining.
Rats were perfused with 4% paraformaldehyde, and their brains were dissected, postfixed at 4°C for 5 h, and cryoprotected in 30% sucrose overnight. Sections (32 μm) were cut on a microtome and stored at 4°C as free-floating sections. Sections were blocked by incubation with TBS blocking solution, (2% bovine serum albumin, 1% glycine, 10% goat serum, 0.1% Triton X-100) for 2 h at 22°C and then incubated with affinity-purified rabbit anti-Nir1, anti-Nir2, anti-Nir3, or anti-PYK2 antibodies. Following washing with phosphate-buffered saline–0.1% Triton X-100, immunoperoxidase histochemical analysis was performed by using the ABC method (Vector). Enucleated rat eyes were quickly frozen in OCT (10.24% [wt/wt] polyvinyl alcohol, 4.26% polyethylene glycol) and stored at −80°C. The eyes were sectioned at a 20-μm thickness on a cryostat, and sections were collected on gelatin-coated slides. Following fixation with 4% paraformaldehyde for 20 min (for anti-PYK2, anti-Nir2, or anti-Nir3 antibodies) or with ethanol at −20°C for 5 min (for anti-Nir1 antibodies), the sections were washed three times with phosphate-buffered saline and then blocked as described above for brain sections.
Chromosomal localization of the gene for Nir1.
A genomic fragment of the human Nir1 gene was isolated by screening a human genomic library (Stratagene) with a Nir1 cDNA fragment corresponding to nucleotides 246 to 512. The exon-intron boundaries of this region were determined by Southern blotting and nucleotide sequencing. Eighty-three diploid radiation hybrids (Research Genetics) produced as the Stanford G3 Radiation Hybrid Panel were analyzed by PCR utilizing an oligonucleotide pair derived from the Nir1 genomic sequence (5′ CACGAGGTCATTGGAGTTCC 3′ and 5′ GCTGTTGCACGTGGAGGC 3′). The results were confirmed by PCR analysis using an additional pair of oligonucleotides derived from the same genomic fragment. The PCRs were carried out in a total volume of 10 μl under the following conditions: 2.5 min at 94°C and 40 cycles of 30 s at 94°C, 30 s at 56°C, and 45 s at 72°C. The radiation hybrid mapping package RHMAP was used to analyze the results.
RESULTS
Cloning of proteins that bind to PYK2-N.
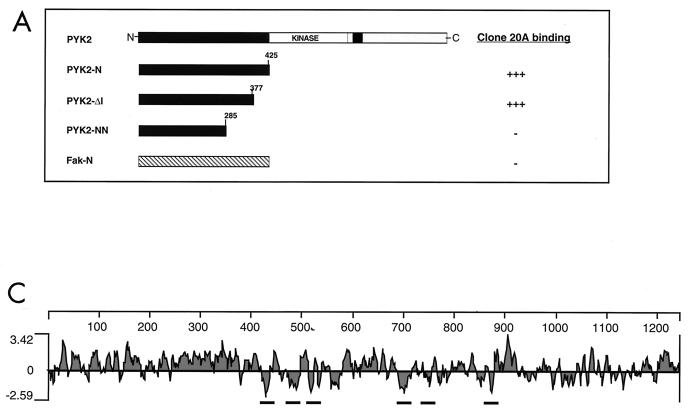
To isolate PYK2-binding proteins, a yeast two-hybrid screen of a human brain cDNA library was performed by using PYK2-N (amino acids 2 to 425) as bait. One clone which interacts specifically with PYK2-N was isolated after screening 2 × 106 transformants. The specificity of the interaction was tested by transformation of yeast strains expressing specific and heterologous baits, as shown in Fig. 1A. A similar interaction was detected in yeast strains expressing a deletion mutant protein that lacks the last 48 amino acids (PYK2-Δ1). By contrast, the interaction was eliminated by further deletions and no interaction was seen with the N-terminal domain of the related protein FAK.
FIG. 1.
Cloning and amino acid sequences of Nir1, Nir2, and Nir3. (A) Identification of PYK2-binding proteins using the yeast two-hybrid system. PYK2-N is shown along with heterologous baits that were used to determine binding specificity toward clone 20A. Yeast two-hybrid interactions were determined by induction of the reporter genes for HIS3 and β-Gal. (B) Comparison of amino acid sequences of human Nir1, Nir2, and Nir3; Drosophila rdgB; and human PI transfer protein α. The amino acid sequences are shown in single-letter code, and the numbers represent the positions of amino acid residues. Amino acids present in two or more sequences are boxed. Boundaries of the PI transfer domain (first 270 amino acids at the amino terminus) and the PYK2-binding domain (last 350 amino acids at the carboxy terminus) are marked by arrows and vertical lines to the right. The six putative transmembrane domains are overlined. The hydrophobicity of transmembrane domains 1 to 4 is stronger (solid lines) than the hydrophobicity of transmembrane domains 5 and 6 (dashed lines). The putative calcium-binding site is marked with a dotted line. Three Xs were incorporated into the Drosophila rdgB sequence; two represent amino acid deletions, whereas the third X located the position of a predicted frame shift in the published sequence that extends the rdgB-Nir homology. (C) Hydropathy profile of Nir2. The profile was calculated with a window size of 15 amino acids (18).
A full-length cDNA was obtained by screening several human brain cDNA libraries as described in Materials and Methods. The full-length cDNA, designated Nir1 (PYK2-N-interacting receptor), encodes a protein of 974 amino acids with a predicted molecular mass of 108 kDa (Fig. 1B). A search of the Nir1 sequence in the GenBank database revealed a strong similarity to the Drosophila retinal degeneration B (rdgB) protein (36). Furthermore, two additional fragments representing two distinct human homologues for Nir1 were identified in the EST database. A probe derived from the T12574 EST fragment was used to screen a human brain cDNA library from which the full-length cDNA of Nir2 was isolated. The full-length Nir2 cDNA (4,186 bp) encodes a protein of 1,244 amino acids with a predicted molecular mass of 140 kDa (Fig. 1B). A full-length cDNA for Nir3 was obtained by screening human brain and heart cDNA libraries as described in Materials and Methods. Two isoforms that are probably generated by alternative RNA splicing were identified: a long Nir3 isoform that encodes a protein of 1,349 amino acids with a predicted molecular mass of 150 kDa and a shorter isoform lacking amino acids 50 to 328 with a predicted molecular mass of 125 kDa. The gene for Nir2 was previously cloned by Chang et al. (9) and, because of its similarity to the Drosophila homologue, designated human rdgB. According to this nomenclature, Nir1 and Nir3 may be designated human rdgB1 and rdgB3, respectively.
Primary structures, domain organization, tissue distribution, and chromosomal localization of Nirs.
Multiple sequence alignments of Nir1, Nir2, and Nir3 with the Drosophila homologue revealed high similarity throughout the entire sequences of these proteins (Fig. 1B). The N-terminal domains of Nir2 and Nir3 exhibit 45 and 47% amino acid sequence identity, respectively, with human PI transfer proteins (PI-TPα and PI-TPβ) (11, 33). By contrast, Nir1 does not contain a PI transfer domain. The PI transfer domains of Nir2 and Nir3 exhibit 72% sequence identity with each other and 65% sequence identity with the Drosophila homologue (Fig. 1B).
In the center of their sequences (Fig. 1B), Nir1, Nir2, and Nir3 contain an acidic region, followed by several conserved regions including six hydrophobic stretches that probably function as transmembrane domains (Fig. 1B and C and 2A) and three additional highly conserved regions. These regions (Fig. 2A) exhibit 66 to 88% amino acid sequence identity with human Nirs, 41 to 75% sequence identity with the Drosophila homologue (Fig. 2A), 39 to 61% sequence identity with the Caenorhabditis elegans homologue (the Genbank accession numbers of the two overlapping cosmids are Z77131 and Z46381), and 27 to 66% sequence identity with three additional ESTs (Z49956, Z74930, and Z95334).
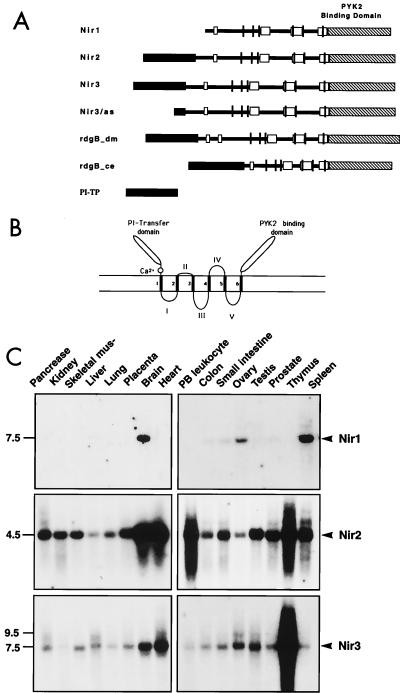
FIG. 2.
Structure of Nir proteins and mRNA distribution. (A) Schematic diagram of the primary structures of Nir1, Nir2, and Nir3. The amino-terminal PI transfer domain (black), the acidic domain (white), the three conserved subdomains (gray), and the carboxy-terminal PYK2-binding domain (striped) are represented. The six putative transmembrane domains are shown as vertical lines. (B) Model of the structure of Nir proteins. The amino-terminal PI transfer domain, the acidic calcium-binding domain, and the carboxy-terminal PYK2-binding domain are located on one face of the membrane. The six transmembrane domains are numbered 1 to 6. The loops between the transmembrane domains are designated I, II, III, and IV. (C) Northern blot analysis of Nir mRNA expression in various human tissues. Human multiple-tissue Northern blots were hybridized with radiolabeled probes for Nir1 (top), Nir2 (middle), and Nir3 (bottom). Specific probes are described in Materials and Methods. Marker sizes are shown in kilobases at the left.
The carboxy-terminal portions of Nir1, Nir2, and Nir3 (Fig. 1B) exhibit 60 to 63% amino acid sequence identity with human Nirs and approximately 40 to 60% sequence identity with Drosophila rdgB. Because of strong conservation of the 3′ sequences of Drosophila rdgB and human Nir1, Nir2, and Nir3, we propose a correction in the 3′ sequence of Drosophila rdgB. Addition of a single base to the published sequence (36) will extend the open reading frame of Drosophila rdgB from 1,054 to 1,250 amino acids (Fig. 1B). This correction will eliminate the discrepancy between the amino acid sequences of the human protein and the Drosophila homologue and the difference between the previously reported apparent and calculated molecular weights of the Drosophila rdgB protein (37).
The tissue expression patterns of Nir1, Nir2, and Nir3 were revealed by Northern blot analysis of various human tissues with specific probes as shown in Fig. 2C. While Nir1 is expressed exclusively in the brain, spleen, and ovary as a transcript of approximately 7.5 kb, Nir2 is ubiquitously expressed, with the highest expression levels in the brain, heart, thymus, and peripheral blood leukocytes (4.5 kb). By contrast, Nir3 is expressed mainly in the thymus, heart, brain, ovary, and testis as two transcripts of 7.5 and 9.5 kb.
We have determined the chromosomal localization of the gene for Nir1 by using the Stanford G3 Radiation Hybrid Panel (7, 10). By using this approach, we demonstrated that the human Nir1 gene is closely linked to the D175938 marker on chromosome 17p13.1. Interestingly, mutations in the same locus were shown to be responsible for three retinal disorders: Leber’s congenital amaurosis (LCA), an autosomal recessive disease responsible for congenital blindness (8); cone-rod dystrophy (CORD5), an inherited retinal dystrophy that causes visual impairment (4); and autosomal dominant retinitis pigmentosa. Moreover, the Nir2 gene (also known as H-rdgB) was also mapped to a locus at chromosome 11q13.1 that is known to be responsible for several retinal diseases (5, 9, 14).
Functional domains in Nir1, Nir2, and Nir3.
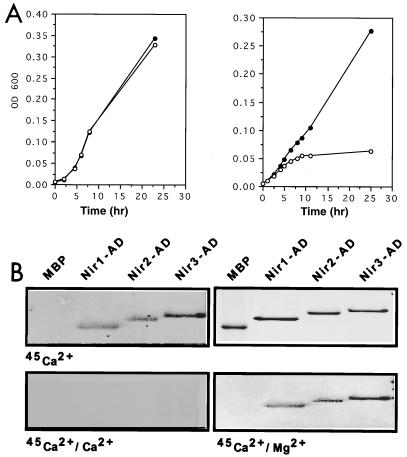
The N-terminal parts of Nir2 and Nir3 contain a typical PI transfer domain, suggesting that these proteins may possess PI transfer activity (Fig. 1B and 2A). It was previously shown that a recombinant protein containing the first 296 amino acids of rdgB exhibits PI transfer activity in vitro (37). To determine whether the PI transfer domain of Nirs possesses functional PI transfer activity in vivo, we used an experimental approach that was previously applied for cloning of rat PI-TPβ (33). This approach is based on the ability of PI transfer protein to rescue the growth defect of the S. cerevisiae sec14ts mutant, a yeast strain carrying a lesion in the structural gene for yeast PI-TP (SEC14). Inactivation of the sec14 gene product in a temperature-sensitive (sec14ts) mutant leads to arrest of a secretory pathway at the late Golgi compartment, leading to growth arrest. The experimental results presented in Fig. 3A show that expression of the Nir3 PI transfer domain suppressed the growth lesion of sec14ts at 35°C, whereas the vector alone did not have any effect, thus demonstrating that the Nir3 PI transfer domain can function as a PI transfer protein in vivo.
FIG. 3.
Functional analysis of the PI transfer and acidic domains of Nirs. (A) Rescue of the growth lesion of a sec14ts mutant by a Nir PI transfer domain. Growth curves of a sec14ts mutant transformed either with a yeast expression vector (○) or with an expression vector containing the PI transfer domain of Nir3 (●) at 25 and 35°C. Growth was determined by measuring the optical density at 600 nm (OD 600) of a liquid culture at different time points as indicated. An identical growth curve was observed at 25°C, while Nir3 rescued the growth lesion of the sec14ts mutant at 39°C. (B) The acidic domain (AD) of Nir proteins binds calcium. The acidic domains of Nir1, Nir2, and Nir3 were expressed in Escherichia coli in the form of MBP fusion proteins. Following affinity purification, the recombinant acidic domains and MBP alone were resolved on a sodium dodecyl sulfate–12% polyacrylamide gel and either stained with Coomassie brilliant blue (upper right) or transferred to a nitrocellulose filter and incubated with 45Ca2+ as described in Materials and Methods in the absence (upper left) or presence of 10 mM CaCl2 (lower left) or MgCl2 (lower right).
The acidic domain of Nir binds calcium.
All three Nirs contain an acidic region that may function as a calcium-binding domain. Moreover, it has been demonstrated that the Drosophila rdgB protein binds calcium (37). To examine the possibility that the acidic regions of Nirs are able to bind calcium, the appropriate regions in Nir1 (amino acids 2 to 76), Nir2 (amino acids 278 to 370), and Nir3 (amino acids 278 to 386) were expressed in the form of MBP fusion proteins and tested for the ability to bind 45Ca2+ in an overlay assay. The experiment presented in Fig. 3B shows specific binding of calcium to the acidic domains of Nir1, Nir2, and Nir3 but not to MBP alone. The binding specificity was confirmed by inhibition of 45Ca2+ binding in the overlay assay in the presence of unlabeled CaCl2 but not in the presence of MgCl2. We therefore conclude that Nir1, Nir2, and Nir3 bind calcium by means of their conserved acidic domains.
The C-terminal domains of Nir proteins bind the N-terminal domain of PYK2.
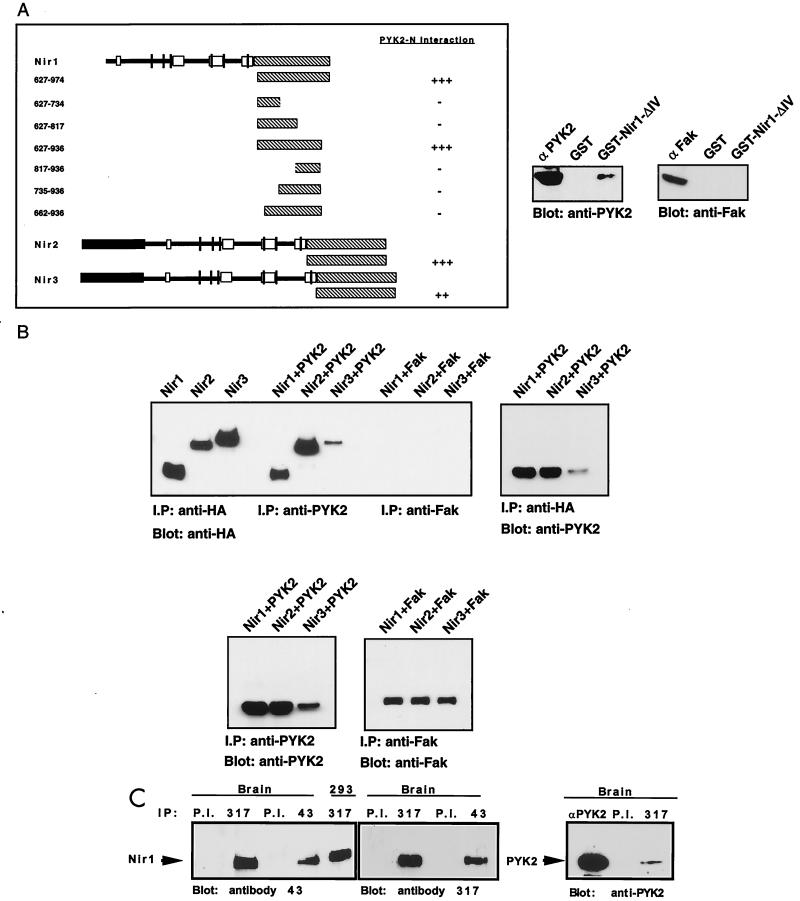
To define the minimal PYK2-binding region within Nirs, we prepared a series of deletion mutant Nir1 proteins attached to the GAL4 transcription activation domain (Fig. 4A). The ability of these deletion mutant proteins to interact with PYK2-N was determined by using the yeast two-hybrid system. These experiments demonstrated that a region composed of almost the entire C-terminal part of Nir1 is essential for PYK2-N binding (Fig. 4A). A similar strategy was used to demonstrate that corresponding regions in Nir2 and Nir3 function as binding sites for PYK2 (Fig. 4A). We have also performed direct binding experiments between a GST fusion protein containing the binding region of Nir1 (GST-ΔIV) and PYK2. This experiment demonstrates that PYK2 binds to immobilized GST-ΔIV but not to GST alone (Fig. 4A). Furthermore, immobilized GST-ΔIV did not bind to the closely related kinase FAK, thus providing further evidence of specificity of binding. Taken together, these experiments demonstrate that the C-terminal domains of Nir1, Nir2, and Nir3 function as binding sites for PYK2-N.
FIG. 4.
Interaction between PYK2 and Nirs in yeast, in cultured cells, and in brain tissue. (A) Determination of the PYK2-binding domain of Nir proteins. The binding region responsible for binding of Nir1 to PYK2-N was determined by assaying growth on selection medium lacking histidine by a β-Gal assay (left) and by an in vitro binding assay (right). The diagram shows the sequences of Nir1 that were fused to the GAL-4 activation domain. The numbers indicate the amino acid residues of Nir1 that were included in each fusion protein. The interaction of the C-terminal domains of Nir2 and Nir3 with PYK2-N is shown at the bottom. An in vitro assay of PYK2 binding to an immobilized GST fusion protein expressing the Nir1-ΔIV cDNA (amino acids 627 to 936) is shown at the right. Lysates of cells expressing either PYK2 or FAK were incubated with immobilized GST or GST–Nir1-ΔIV for 2 h at 4°C. Following extensive washing, binding of PYK2 or FAK to the recombinant proteins was detected by immunoblotting with anti-PYK2 or anti-FAK antibodies as indicated. (B) Association between PYK2 and Nir proteins in vivo. Lysates from 293T cells transfected with Nirs-HA alone, Nirs-HA cotransfected with PYK2, or Nirs-HA cotransfected with FAK were immunoprecipitated with anti-HA, anti-PYK2, or anti-FAK antibodies as indicated. Immunoprecipitates (I.P) were subjected to immunoblotting with anti-HA, anti-PYK2, or anti-FAK antibodies as indicated. PYK2 was detected in Nir immunoprecipitates (left), and Nir proteins were detected in PYK2 immunoprecipitates (right). Expression levels of PYK2 and FAK in cells coexpressing Nir proteins are shown at the bottom. (C) Association of PYK2 with Nir1 in brain tissue. Adult rat brain was homogenized as described in Materials and Methods. The homogenate was subjected to immunoprecipitation with different antibodies as indicated: P.I. (preimmune serum) anti-Nir1 antibodies, antibody 317 raised against a synthetic peptide corresponding to the last 10 amino acids, antibody 43 raised against a GST fusion protein containing amino acids 260 to 380 of Nir1, or anti-PYK2 antibodies. The Nir1 protein was also immunoprecipitated from 293 cells transfected with a Nir1 expression vector as indicated. Antibodies against Nir1 recognized a single protein that migrates in sodium dodecyl sulfate gels with an apparent molecular mass of 120 kDa (two left panels). This protein was coimmunoprecipitated with anti-PYK2 antibodies (right panel).
In vivo association between the PYK2 and Nir proteins.
To analyze the association between PYK2 and Nirs in vivo, each of the three Nirs, alone or together with PYK2 or FAK, was expressed in 293T cells. The Nirs were tagged with the HA epitope to allow identification of each protein with the same antibodies. Nir, PYK2, or FAK protein expression was determined by immunoprecipitation and immunoblotting with anti-HA, anti-PYK2, or anti-FAK antibodies, respectively (Fig. 4B). Associations between Nirs and PYK2 or between Nirs and FAK were determined by immunoprecipitation with anti-PYK2 or anti-FAK antibodies, respectively, followed by immunoblotting with anti-HA antibodies. The experiment presented in Fig. 4B demonstrates that Nirs associate specifically with PYK2 lysates prepared from cotransfected cells. However, association was not detected between Nirs and FAK in the same experiment (Fig. 4B). Similar results were obtained when these cell lysates were subjected to immunoprecipitation with anti-Nir antibodies followed by immunoblotting with anti-PYK2 or anti-FAK antibodies.
Association between PYK2 and Nir1 was also detected in lysates prepared from rat brain. A crude solubilized brain extract was incubated with anti-Nir1 antibodies or preimmune serum followed by immunoblotting with anti-PYK2 antibodies. The experiment presented in Fig. 4C shows that anti-Nir1 antibodies recognize a polypeptide with an apparent molecular mass of 120 kDa. The same polypeptide coimmunoprecipitated with PYK2 (Fig. 4C), thus revealing an association between these two proteins in brain tissue.
Localization of PYK2 and Nir proteins in the brain and retina.
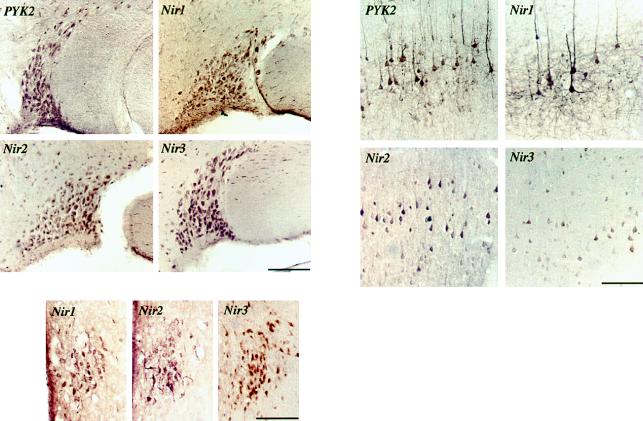
To determine more precisely the distribution patterns of PYK2 and Nirs in the brain, we performed an immunohistochemical analysis of adult rat brain. A similar localization of PYK2 and Nirs was observed in different regions of the brain, including the supraoptic nucleus, the cortex, and the middle of the preoptic region. External pyramidal layer V of the cortex was strongly labeled by anti-PYK2 or anti-Nir1 antibodies (Fig. 5). Strong labeling was detected in the pyramidal cell bodies and their processes. Moderate labeling was seen in other cortical layers. Identical results were obtained when the expression patterns of PYK2 and Nir1 were analyzed by confocal microscopy using fluorescently labeled antibodies (data not shown). Immunohistochemical analysis with anti-Nir2 and anti-Nir3 antibodies demonstrated that these proteins are prominently expressed in the pyramidal cell bodies. In addition, PYK2 and all three Nirs are highly expressed in the supraoptic nucleus. The three Nir proteins were also detected in the middle preoptic area (Fig. 5). However, Nir1 and Nir2 were not detected in the cerebellum, while both Nir3 and PYK2 were detected in Purkinje cells (data not shown).
FIG. 5.
Immunohistochemical analysis of PYK2, Nir1, Nir2, or Nir3 distribution in the rat brain. The left panel shows the localization of PYK2, Nir1, Nir2, or Nir3 in the supraoptic nucleus (scale bar, 130 μm). The right panel shows staining in pyramidal layer V (scale bar, 95 μm), and the bottom panel depicts staining of the three Nir proteins in the middle preoptic area (scale bar, 100 μM).
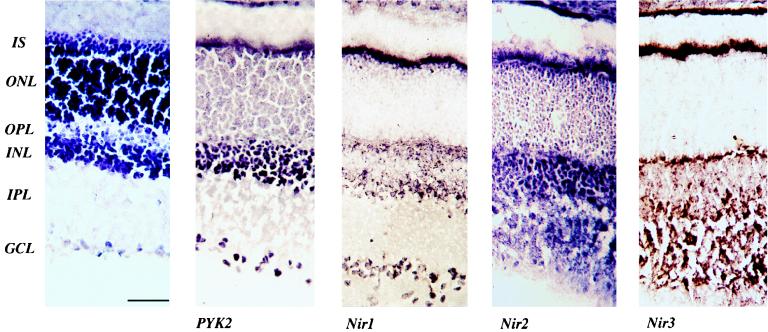
Since the genes for both Nir1 and Nir2 were mapped to chromosomal regions known to be involved in retinal diseases, we also compared the distribution of the PYK2 and Nir proteins in the rat retina. Immunoblot experiments confirmed the expression of PYK2 and Nirs in the rat retina (data not shown). Rat retinal sections were stained with antibodies specific for PYK2, Nir1, Nir2, and Nir3. The experiment presented in Fig. 6 shows that PYK2 is highly expressed in the inner nuclear layer and in the ganglion cell layers. Moderate labeling was detected in photoreceptors, while PYK2 staining was not observed in the inner and outer plexiform layers (Fig. 6). Similar to PYK2, Nir1 is strongly expressed in the ganglion cell layer. Nir1 is expressed in the inner segment and in the outer plexiform layer containing the photoreceptor terminals. Nir1 is also expressed in the inner nuclear layer; most of the staining was detected at the bottom of the inner nuclear layer, probably in amacrine cells. Nir1 staining was not detected in the outer nuclear layer and in the inner plexiform layer. Nir2 immunoreactivity was observed throughout the retina, particularly in the outer nuclear layer and in the inner plexiform layer. Nir3 immunoreactivity was detected in the inner segment and the inner and outer plexiform layers. However, staining was not observed in the outer nuclear layer and in the ganglion cell layer. The specificity of staining with anti-Nir1, anti-Nir2, anti-Nir3, and anti-PYK2 antibodies was confirmed by control staining experiments in the presence of competitive antigens. These experiments demonstrate that the PYK2 and Nir proteins are specifically expressed in different cell types of the retina. The differential distribution of Nir proteins in cells of the retina may represent specific cellular functions.
FIG. 6.
Distribution of the PYK2 and Nir proteins in the rat retina. (A) Hematoxylin staining of a vertical cryostat section of a rat retina showing different layers of the retina. (B) PYK2 immunoreactivity in the retina. Strong staining for PYK2 is detected throughout the inner nuclear layer (INL) and in the ganglion cell layer (GCL); specific staining is also observed in the outer nuclear layer (ONL). (C) Nir1 immunoreactivity in the retina. Strong staining is detected in the inner segment (IS), in the outer plexiform layer (OPL), in specific cells within the inner nuclear layer, and in the ganglion cell layer. (D) Nir2 immunostaining is seen in the inner segment, outer nuclear layer, and inner plexiform layer (IPL), and a moderate expression level is seen in the inner nuclear layer and the outer plexiform layer. (E) Strong immunostaining of Nir3 is detected in the inner segment and the inner and outer plexiform layers (scale bar, 50 μM).
Tyrosine phosphorylation of Nir proteins by PYK2.
The strong and specific association of Nirs with PYK2 both in vitro and in vivo raised the possibility that these proteins are substrates of PYK2. To examine this possibility, a Nir2 expression vector, alone or together with a PYK2 or PKM (a kinase-negative mutant PYK2 protein) (19) expression vector, was transfected into 293T cells. The phosphorylation of Nir2 on tyrosine residues was determined by immunoblotting with antiphosphotyrosine antibodies (Fig. 7A). Analysis of Nir2 or PYK2 immunoprecipitates from cells coexpressing Nir2 and PYK2 demonstrated that both Nir2 and PYK2 are tyrosine phosphorylated and are present in the same immunocomplex. By contrast, Nir2 was not tyrosine phosphorylated in cells expressing the kinase-negative mutant protein PKM. However, Nir2 was found to be associated with PKM in a complex, thus demonstrating that tyrosine kinase activity is not essential for complex formation between these two proteins (Fig. 7A). Similar results were obtained with Nir1- and Nir3-expressing cells, indicating that all three proteins are likely substrates of PYK2 (data not shown).
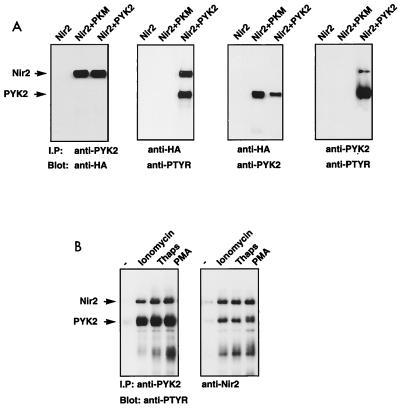
FIG. 7.
Tyrosine phosphorylation of Nir2 in response to PYK2 activation. (A) Human 293T cells were transfected with expression vectors for Nir2-HA, PKM, or PYK2. Lysates from transfected cells were subjected to immunoprecipitation (I.P) with anti-HA or anti-PYK2 antibodies as indicated. Tyrosine phosphorylation was determined by immunoblotting with antiphosphotyrosine antibodies (anti-PTYR). (B) Quiescent HL60 cells were stimulated with ionomycin (6 μM), thapsigargin (Thaps; 2 μM), or phorbol myristate acetate (PMA; 1.6 μM) for 10 min at 37°C. PYK2 and Nir2 were immunoprecipitated from lysates of unstimulated (−) and stimulated cells. The samples were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to a nitrocellulose filter, and immunoblotted with anti-P-Tyr antibodies.
To further evaluate the status of Nir2 in vivo, we examined the effect of external stimuli on the tyrosine phosphorylation of endogenous PYK2 and Nir2. Quiescent HL60 cells, a human promyelocytic leukemia cell line that expresses both PYK2 and Nir2, were stimulated with agonists known to induce tyrosine phosphorylation of PYK2 such as calcium ionophore, thapsigargin, or phorbol myristate acetate. Following stimulation, the cells were lysed, subjected to immunoprecipitation with anti-Nir2 antibodies, and then immunoblotted with antiphosphotyrosine antibodies. The experimental results presented in Fig. 7B show that these three agonists induce strong phosphorylation of Nir2 on tyrosine residues. Moreover, upon immunoprecipitation with anti-Nir2 or anti-PYK2 antibodies, both endogenous PYK2 and Nir2 were found to be associated in a stable complex (Fig. 7B). The tight association with PYK2 and the tyrosine phosphorylation of Nir2 in response to known PYK2 agonists indicate that Nir2 is most likely a substrate of PYK2. However, it is possible that tyrosine phosphorylation of Nir proteins is induced by Src or by another protein tyrosine kinase which can be activated by PYK2.
DISCUSSION
By using PYK2-N as bait in a yeast two-hybrid screen, we have isolated a new family of PYK2-binding proteins designated Nir1, Nir2, and Nir3. The Nir family of proteins shows sequence similarity to the Drosophila rdgB protein, a protein implicated in the phototransduction signaling pathway (24). It has been shown that rdgB mutant flies exhibit light-enhanced retinal degeneration and electroretinogram defects. It was proposed that inactivation of rdgB causes abnormalities in phototransduction in Drosophila because of aberrations in Ca2+ signaling and/or phosphoinositide metabolism (26, 27, 37).
Nirs are multidomain proteins containing six putative transmembrane domains, a calcium-binding region, and a carboxy-terminal domain that functions as a PYK2-binding site. The amino-terminal parts of Nir2 and Nir3 contain an additional PI transfer domain, a similar domain was not found in Nir1. In lysates prepared from brain tissue or cultured cells, Nir proteins form a complex with PYK2 and are tyrosine phosphorylated in response to PYK2 activation. It is now well established that PYK2 is activated by a variety of G-protein agonists such as bradykinin, LPA, and angiotensin II, among many other stimuli (12, 19, 21, 34). In addition, activation of protein kinase C (PKC) and an increase in the cytosolic calcium concentration lead to the activation of PYK2 (19). Here we demonstrate that PYK2 forms a complex with Nir proteins leading to their tyrosine phosphorylation both in vitro and in living cells. It is therefore likely that these proteins function as downstream targets of PYK2 in different tissues and cell types. However, other possibilities are that Nir proteins act upstream of PYK2 and that activation of PYK2 mediated by Nirs induces tyrosine phosphorylation of Nir proteins.
Although Nir proteins exhibit different tissue expression patterns (Fig. 2C), all three proteins are expressed in the brain. We demonstrated that the PYK2 and Nir proteins exhibit similar expression patterns in some, but not all, neuronal cell populations in the rat brain. Expression of PYK2 and Nir proteins was detected in the supraoptic nucleus, in the cortex, and in the middle preoptic area, and both proteins are found to be associated in a complex in lysates prepared from these tissues.
Because of the structural similarities between human Nir proteins and the Drosophila rdgB homologue and because of the extensive genetic and electrophysiological information concerning the role of rdgB in phototransduction, it is possible that Nirs play a similar biological role in the phototransduction pathway in vertebrates. Indeed, it was recently reported that the murine Nir2 gene rescues the phenotype of rdgB mutant flies (9), thus demonstrating the Nir and rdgB proteins are functional homologues in vertebrates and Drosophila, respectively. We therefore compared the cellular distribution of Nirs in the rat retina. The results presented in Fig. 6 demonstrate that Nir proteins and PYK2 exhibit similar expression patterns in different cell types of the retina (13). PYK2 is highly expressed in the inner nuclear layer and in the ganglion cell layer, and Nir2 immunoreactivity is detected throughout the retina. The immunoreactivity of Nir1 is mostly confined to the outer plexiform layer, to the ganglion cell layer, and to a population of cells in the inner nuclear layer. Nir3 is highly expressed in the inner segment and in the inner and outer plexiform layers. The differences in the expression patterns of the three Nir proteins may reflect differences in their cellular functions. It is not surprising that proteins involved in PI transfer, such as Nir2 and Nir3, are expressed in the inner segment of the retina, potentially participating in the control of membrane turnover, vesicle trafficking, or vesicle fusion. Nir1 does not have a PI transfer domain and therefore may have another regulatory function. An additional possibility is that Nirs play a role in calcium homeostasis, as was previously proposed for the Drosophila rdgB protein (32, 36).
On the basis of genetic, electrophysiological, and pharmacological studies with Drosophila, it was proposed that rdgB is involved in the pumping of calcium into intracellular stores and perhaps also out of the cell (32, 36). In the fly retina, light-induced activation of rhodopsin stimulates a G-protein cascade, leading to the activation of phospholipase Cβ. Upon activation, phospholipase Cβ (NorpA) hydrolyzes PI biphosphate to generate diacylglycerol and Ins(1,4,5)P3. Diacylglycerol then activates PKC (inaC), and Ins(1,4,5)P3 releases calcium from intracellular stores (40). Genetic and electrophysiological studies with Drosophila (31, 32, 40) placed rdgB downstream of NorpA and inaC, suggesting that rdgB uses its PI transfer domain for restoration of Ptd Ins(4,5)P2, which was utilized during the activation phase of this G-protein-coupled cascade. The experiments described here suggest that PYK2 functions as an additional regulatory component in this process. PYK2 was shown to be activated by a variety of G-protein agonists, such as bradykinin, LPA, and angiotensin II, as well as by many other extracellular stimuli (12, 19, 21, 34). In addition, phorbol ester-induced activation of PKC and an increase in the cytosolic calcium concentration lead to the activation of PYK2 (19). Here we demonstrate that PYK2 forms a complex with Nir proteins, leading to tyrosine phosphorylation of these proteins both in vitro and in living cells. A reasonable possibility is that PYK2 and its Drosophila homologue function as upstream regulators of Nir and rdgB proteins in response to extracellular signals that stimulate G-protein-coupled receptors.
Finally, the finding that the gene for Nir1 is localized to human chromosome 17p13.1 near the marker D17S938 suggests that Nir1 is a candidate gene for inherited retinal diseases. Indeed, the short arm of chromosome 17 has emerged as a hot spot responsible for distinct retinal disorders (17); LCA, an autosomal recessive disease responsible for congenital blindness (8); and CORD5, an inherited retinal dystrophy that causes visual impairment and autosomal dominant retinitis pigmentosa. While retinal guanylate cyclase has been shown to be involved in LCA, the gene responsible for CORD5 has not been identified (4). In addition, the human Nir2-encoding gene was mapped to chromosome 11q13.1 at a locus where genes for several retinal diseases have also been mapped (1, 5, 9, 14). It remains to be determined whether Nir proteins play a role in these retinal disorders.
ACKNOWLEDGMENTS
We thank V. A. Bunkaitis for providing S. cerevisiae CTY483. We are grateful to H. Schulman for help with the calcium overlay assay. We also thank M. Gishizky, T. Hunter, D. Stokes, and A. Weiss for critical reading of the manuscript.
REFERENCES
- 1.Aikawa Y, Hara H, Watanabe T. Molecular cloning and characterization of mammalian homologues of the Drosophila retinal degeneration B gene. Biochem Biophys Res Commun. 1997;236:559–564. doi: 10.1006/bbrc.1997.7009. [DOI] [PubMed] [Google Scholar]
- 2.Astier A, Avraham H, Manie S N, Groopman J, Canty T, Avraham S, Freedman A S. The related adhesion focal tyrosine kinase is tyrosine-phosphorylated after beta1-integrin stimulation in B cells and binds to p130cas. J Biol Chem. 1997;272:228–232. doi: 10.1074/jbc.272.1.228. [DOI] [PubMed] [Google Scholar]
- 3.Avraham S, London R, Fu Y, Ota S, Hiregowdara D, Li J, Jiang S, Pasztor L M, White R A, Groopman J E, et al. Identification and characterization of a novel related adhesion focal tyrosine kinase (RAFTK) from megakaryocytes and brain. J Biol Chem. 1995;270:27742–27751. doi: 10.1074/jbc.270.46.27742. [DOI] [PubMed] [Google Scholar]
- 4.Balciuniene J, Johansson K, Sandgren O, Wachtmeister L, Holmgren G, Forsman K. A gene for autosomal dominant progressive cone dystrophy (CORD5) maps to chromosome 17p12-p13. Genomics. 1995;30:281–286. doi: 10.1006/geno.1995.9876. [DOI] [PubMed] [Google Scholar]
- 5.Banfi S, Borsani G, Rossi E, Bernard L, Guffanti A, Rubboli F, Marchitiello A, Giglio S, Coluccia E, Zollo M, Zuffardi O, Ballabio A. Identification and mapping of human cDNAs homologous to Drosophila mutant genes through EST database searching. Nat Genet. 1996;13:167–174. doi: 10.1038/ng0696-167. [DOI] [PubMed] [Google Scholar]
- 6.Birge R B, Knudsen B S, Besser D, Hanafusa H. SH2 and SH3-containing adaptor proteins: redundant or independent mediators of intracellular signal transduction. Genes Cells. 1996;1:595–613. doi: 10.1046/j.1365-2443.1996.00258.x. [DOI] [PubMed] [Google Scholar]
- 7.Boehnke M, Lange K, Cox D R. Statistical methods for multipoint radiation hybrid mapping. Am J Hum Genet. 1991;49:1174–1188. [PMC free article] [PubMed] [Google Scholar]
- 8.Camuzat A, Dollfus H, Rozet J M, Gerber S, Bonneau D, Bonnemaison M, Briard M L, Dufier J L, Ghazi I, Leowski C. A gene for Leber’s congenital amaurosis maps to chromosome 17p. Hum Mol Genet. 1995;4:1447–1452. doi: 10.1093/hmg/4.8.1447. [DOI] [PubMed] [Google Scholar]
- 9.Chang J T, Milligan S, Li Y, Chew C E, Wiggs J, Copeland N G, Jenkins N A, Campochiaro P A, Hyde D R, Zack D J. Mammalian homolog of drosophila retinal degeneration B rescues the mutant fly phenotype. J Neurosci. 1997;17:5881–5890. doi: 10.1523/JNEUROSCI.17-15-05881.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cox D R, O’Connor K, Hebert S, Harris M, Lee R, Stewart B, DiSibio G, Boehnke M, Lange K, Goold R, Myers R M. Construction and analysis of panel of “whole genome” radiation hybrid. Am J Hum Genet. 1994;55:A23. [Google Scholar]
- 11.Dickeson S K, Helmkamp G M, Yarbrough L R. Sequence of a human cDNA encoding phosphatidylinositol transfer protein and occurrence of a related sequence in widely divergent eukaryotes. Gene. 1994;142:301–305. doi: 10.1016/0378-1119(94)90279-8. [DOI] [PubMed] [Google Scholar]
- 12.Dikic I, Tokiwa G, Lev S, Courtneidge S A, Schlessinger J. A role for Pyk2 and Src in linking G-protein-coupled receptors with MAP kinase activation. Nature. 1996;383:547–550. doi: 10.1038/383547a0. [DOI] [PubMed] [Google Scholar]
- 13.Dowling J E. The retina: an approachable part of the brain. Cambridge, Mass: The Belknap Press of Harvard University; 1987. [Google Scholar]
- 14.Guo J, Yu F X. Cloning and characterization of human homologue of Drosophila retinal degeneration B: a candidate gene for degenerative retinal diseases. Dev Genet. 1997;20:235–245. doi: 10.1002/(SICI)1520-6408(1997)20:3<235::AID-DVG6>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 15.Hanks S K, Polte T R. Signaling through focal adhesion kinase. Bioessays. 1997;19:137–145. doi: 10.1002/bies.950190208. [DOI] [PubMed] [Google Scholar]
- 16.Jayakumar A, Huang W Y, Raetz B, Chirala S S, Wakil S J. Cloning and expression of the multifunctional human fatty acid synthase and its subdomains in Escherichia coli. Proc Natl Acad Sci USA. 1996;93:14509–14514. doi: 10.1073/pnas.93.25.14509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joshi A R, Mullen L, Small K W. The retina: genetic studies of several retinopathies located on the short arm of chromosome 17. Curr Opin Neurol. 1997;10:31–35. [PubMed] [Google Scholar]
- 18.Kyte J, Doolittle R F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 19.Lev S, Moreno H, Plowman G D, Martinez R, Peles E, Canoll P, Musacchio J M, Rudy B, Schlessinger J. Protein tyrosine kinase PYK2 involved in Ca+2 induced regulation of ion channel and MAP kinase functions. Nature. 1995;376:737–745. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- 20.Li X, Earp H S. Paxillin is tyrosine-phosphorylated by and preferentially associates with the calcium-dependent tyrosine kinase in rat liver epithelial cells. J Biol Chem. 1997;272:14341–14348. doi: 10.1074/jbc.272.22.14341. [DOI] [PubMed] [Google Scholar]
- 21.Li X, Yu H, Graves L M, Earp H S. Protein kinase c and protein kinase A inhibit calcium-dependent but not stress-dependent c-Jun N-terminal kinase activation in rat liver epithelial cells. J Biol Chem. 1997;272:14996–15002. doi: 10.1074/jbc.272.23.14996. [DOI] [PubMed] [Google Scholar]
- 22.Neet K, Hunter T. Vertebrate non-receptor protein-tyrosine kinase families. Genes Cells. 1996;1:147–169. doi: 10.1046/j.1365-2443.1996.d01-234.x. [DOI] [PubMed] [Google Scholar]
- 23.Qian D, Lev S, van Oers N S, Dikic I, Schlessinger J, Weiss A. Tyrosine phosphorylation of Pyk2 is selectively regulated by Fyn during TCR signaling. J Exp Med. 1997;185:1253–1259. doi: 10.1084/jem.185.7.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ranganathan R, Malicki D M, Zuker C S. Signal transduction in Drosophila photoreceptors. Annu Rev Neurosci. 1995;18:283–317. doi: 10.1146/annurev.ne.18.030195.001435. [DOI] [PubMed] [Google Scholar]
- 25.Richardson A, Parsons J T. Signal transduction through integrins: a central role for focal adhesion kinase? Bioessays. 1995;17:229–236. doi: 10.1002/bies.950170309. [DOI] [PubMed] [Google Scholar]
- 26.Sahly I, Bar Nachum S, Suss-Toby E, Rom A, Peretz A, Kleiman J, Byk T, Selinger Z, Minke B. Calcium channel blockers inhibit retinal degeneration in the retinal-degeneration-B mutant of Drosophila. Proc Natl Acad Sci USA. 1992;89:435–439. doi: 10.1073/pnas.89.1.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sahly I, Schroder W H, Zierold K, Minke B. Accumulation of calcium in degenerating photoreceptors of several Drosophila mutants. Vis Neurosci. 1994;11:763–772. doi: 10.1017/s0952523800003060. [DOI] [PubMed] [Google Scholar]
- 28.Sasaki H, Nagura K, Ishino M, Tobioka H, Kotani K, Sasaki T. Cloning and characterization of cell adhesion kinase beta, a novel protein-tyrosine kinase of the focal adhesion kinase subfamily. J Biol Chem. 1995;270:21206–21219. doi: 10.1074/jbc.270.36.21206. [DOI] [PubMed] [Google Scholar]
- 29.Siciliano J C, Toutant M, Derkinderen P, Sasaki T, Girault J A. Differential regulation of proline-rich tyrosine kinase 2/cell adhesion kinase β (PYK2/CAKβ) and pp125(FAK) by glutamate and depolarization in rat hippocampus. J Biol Chem. 1996;271:28942–28946. doi: 10.1074/jbc.271.46.28942. [DOI] [PubMed] [Google Scholar]
- 30.Smith D B, Johnson K S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 31.Smith D P, Ranganathan R, Hardy R W, Marx J, Tsuchida T, Zuker C S. Photoreceptor deactivation and retinal degneration mediated by a photoreceptor-specific protein kinase C. Science. 1991;254:1478–1484. doi: 10.1126/science.1962207. [DOI] [PubMed] [Google Scholar]
- 32.Smith D P, Stamnes M A, Zuker C S. Signal transduction in the visual system of Drosophila. Annu Rev Cell Biol. 1991;7:161–190. doi: 10.1146/annurev.cb.07.110191.001113. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka S, Hosaka K. Cloning of cDNA encoding a second phosphatidylinositol transfer protein of rat brain by complementation of yeast sec14 mutation. J Biochem. 1994;115:981–984. doi: 10.1093/oxfordjournals.jbchem.a124448. [DOI] [PubMed] [Google Scholar]
- 34.Tokiwa G, Dikic I, Lev S, Schlessinger J. Activation of Pyk2 by stress signals and coupling with JNK signaling pathway. Science. 1996;273:792–794. doi: 10.1126/science.273.5276.792. [DOI] [PubMed] [Google Scholar]
- 35.Van Oers N S, Weiss A. The Syk/ZAP-70 protein tyrosine kinase connection to antigen receptor signalling processes. Semin Immunol. 1995;7:227–236. doi: 10.1006/smim.1995.0027. [DOI] [PubMed] [Google Scholar]
- 36.Vihtelic T S, Hyde D R, O’Tousa J E. Isolation and characterization of the Drosophila retinal degeneration B (rdgB) gene. Genetics. 1991;127:761–768. doi: 10.1093/genetics/127.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vihtelic T S, Goebl M, Milligan S, O’Tousa J E, Hyde D R. Localization of drosophila retinal degeneration B, a membrane-associated phosphatidylinositol transfer protein. J Cell Biol. 1993;122:1013–1022. doi: 10.1083/jcb.122.5.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vojtek A B, Hollenberg S, Cooper J A. Mammalian Ras interacts directly with the serine/threonine kinase Raf. Cell. 1993;74:205–214. doi: 10.1016/0092-8674(93)90307-c. [DOI] [PubMed] [Google Scholar]
- 39.Yu H, Li X, Marchetto G S, Dy R, Hunter D, Calvo B, Dawson T L, Wilm M, Anderegg R J, Graves L M, Earp H S. Activation of a novel calcium-dependent protein-tyrosine kinase. Correlation with c-Jun N-terminal kinase but not mitogen-activated protein kinase activation. J Biol Chem. 1996;271:29993–29998. doi: 10.1074/jbc.271.47.29993. [DOI] [PubMed] [Google Scholar]
- 40.Zuker C S. The biology of vision of Drosophila. Proc Natl Acad Sci USA. 1996;93:571–576. doi: 10.1073/pnas.93.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]