Abstract
Mycoplasma genitalium infection is a sexually transmitted infection that causes urethritis, cervicitis, and pelvic inflammatory disease (PID) in men and women. The global rise in antimicrobial resistance against recommended antibiotics for the treatment of M. genitalium infection has triggered the need to explore novel drug targets against this pathogen. The application of a bioinformatics approach through subtractive genomics has proven highly instrumental in predicting novel therapeutic targets against a pathogen. This study aimed to identify essential and non-homologous proteins with unique metabolic pathways in the pathogen that could serve as novel drug targets. Based on this, a manual comparison of the metabolic pathways of M. genitalium and the human host was done, generating nine pathogen-specific metabolic pathways. Additionally, the analysis of the whole proteome of M. genitalium using different bioinformatics databases generated 21 essential, non-homologous, and cytoplasmic proteins involved in nine pathogen-specific metabolic pathways. The further screening of these 21 cytoplasmic proteins in the DrugBank database generated 13 druggable proteins, which showed similarity with FDA-approved and experimental small-molecule drugs. A total of seven proteins that are involved in seven different pathogen-specific metabolic pathways were finally selected as novel putative drug targets after further analysis. Therefore, these proposed drug targets could aid in the design of potent drugs that may inhibit the functionality of these pathogen-specific metabolic pathways and, as such, lead to the eradication of this pathogen.
Keywords: Mycoplasma genitalium, drug targets, metabolic pathways, pathogen, proteins, subtractive genomics
1. Introduction
Mycoplasma genitalium is an emerging cause of sexually transmitted infections (STIs) around the globe and has been implicated in urogenital infections of both men and women [1], which include urethritis [2], cervicitis [3], pelvic inflammatory disease (PID) [4], and preterm birth [5]. The pathogen is responsible for 30% of persistent non-gonococcal urethritis cases [6]. Coinfections of M. genitalium with other human STIs such as human immunodeficiency virus (HIV), Chlamydia trachomatis, and Neisseria gonorrhoeae have been reported in different studies [7,8,9,10]. Due to the clinical significance of M. genitalium infection, a guideline for its diagnosis, treatment, and management was included in the Sexually Transmitted Diseases Treatment Guidelines, 2015 by the Centers for Disease Control and Prevention (CDC) [11].
Despite these guidelines, the treatment of M. genitalium infection is still challenging [12]. This pathogen’s lack of cell wall has exempted the use of certain antibiotics such as the beta-lactams (penicillins and cephalosporins) that target cell wall biosynthesis [13]. The commonly used antibiotics that have proven effective in treating this infection include macrolides, tetracycline, and quinolones [14,15]. However, the rapid emergence of antimicrobial resistance against these antibiotics has become worrisome. For example, the recommended 1 g single dose of azithromycin for the treatment of non-gonococcal urethritis (GNU) has not only proven ineffective against M. genitalium but has also led to macrolide-resistant M. genitalium [16,17]. Additionally, the second-line treatment antibiotics (fluoroquinolone moxifloxacin) are effective against M. genitalium, but resistance against these antibiotics has also been reported in different countries [18,19,20,21]. The global rise in antimicrobial resistance among M. genitalium has necessitated the need to explore novel drug targets in this pathogen that can help to curtail this deadly infection.
The advances in computational biology and bioinformatics approaches through the use of omics data such as proteomics, metabolomics, and genomics have been instrumental in drug discovery as they reduce the cost and time needed for in vivo and wet-lab screening for drug design [22]. One of such bioinformatics approaches is subtractive genomics, which entails comparing pathogen and host proteomes to identify non-host proteins with unique metabolic pathways that are critical to the survival of the pathogens. These essential proteins, which must show no cross-reaction with the host, have been proposed as potential drug targets. This approach has been used in different studies to identify potential drug targets and vaccine candidates against different pathogenic bacteria [23,24,25]. A previous study by Butt et al. [26] used this same approach to predict two unique metabolic pathways (bacteria secretion system and phosphotransferase system (PTS)) of M. genitalium as potential drug targets. However, the metabolic pathways of M. genitalium in the KEGG database have been updated since then and now have 53 metabolic pathways compared to the 43 reported by Butt et al. [26]. Therefore, this demands further study to explore additional potential drug targets that could help in drug design against this pathogen.
In this study, we explored the identification of potential drug targets from the whole proteome of M. genitalium. The proteome of the pathogen was mined using a subtractive genomics approach. Cytoplasmic proteins with unique metabolic pathways that play critical roles in the pathogen’s survival with no potential of cross-reaction in the host were selected as novel drug targets. These proteins, when further experimentally confirmed, could aid in drug development against M. genitalium infection.
2. Results and Discussion
2.1. Sequence Retrieval, Filtering and Identification of Essential Proteins
The 511 complete protein sequences of M. genitalium str. G37 (Supplementary File S2) retrieved from UniProtKB were reduced to 488 sequences after removing redundant sequences by CD-HIT at a 90% threshold. The 488 sequences were further subjected to Geptop server, which predicted 381 sequences as essential proteins (Supplementary File S3). Essential proteins are mostly present in antimicrobial compounds and are promising targets for drug design [27].
2.2. Identification of Essential and Non-Homologous Proteins
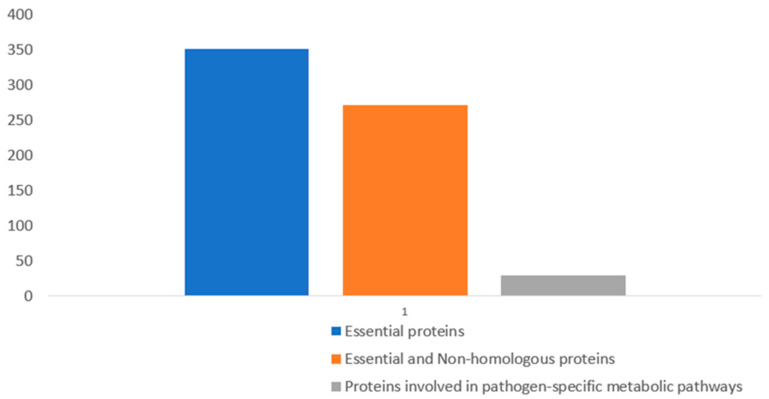
Essential proteins that contribute to the survival of a pathogen while in the host body are potential drug targets, but such proteins should be non-homologous to host (human) proteins to prevent the likelihood of adverse drug effects [28,29]. Based on this, the 381 essential proteins were subjected to BLASTp against human (Homo sapiens) proteins, and this generated 272 sequences that are regarded as essential and non-homologous proteins (Figure 1). The sequences of these proteins can be found in Supplementary File S4.
Figure 1.
Result showing the gradual reduction of essential proteins after BLASTp analysis.
2.3. Metabolic Pathways Analysis
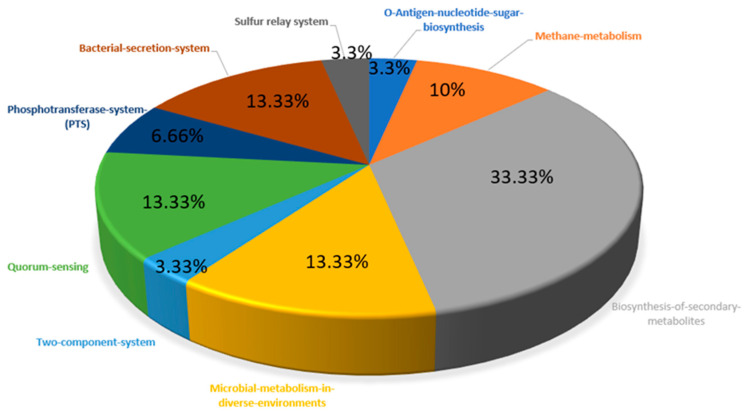
The KEGG database contains 53 and 343 metabolic pathways for M. genitalium and H. sapiens, respectively. The manual comparison of the metabolic pathways of the pathogen and host showed nine metabolic pathways to be unique to the pathogen, and as such, they were regarded as pathogen-specific metabolic pathways. The remaining 44 metabolic pathways were common to both the pathogen and the host. Out of the 272 essential and non-homologous proteins of M. genitalium, 30 were predicted via BLASTp in the KASS server to be involved in the nine pathogen-specific metabolic pathways (Table 1). The distribution of unique metabolic pathways among the essential and non-homologous proteins is also shown in Figure 2.
Table 1.
List of unique metabolic pathways in Mycoplasma genitalium with their KEGG Ortholog.
| Protein ID | Protein Name | KO Assignment | Unique Metabolic Pathways |
|---|---|---|---|
| P22746 | Bifunctional oligoribonuclease and PAP phosphatase | K06881 | mge01120-Microbial metabolism in diverse environments |
| P35888 | Chromosomal replication initiator protein | K02313 | mge02020-Two component system |
| P47259 | Bifunctional protein FolD | K01491 | mge01120-Microbial-metabolism-in-diverse-environments |
| P47269 | Fructose-bisphosphate aldolase | K01624 | mge00680-Methane-metabolism, mge01110-Biosynthesis-of-secondary-metabolites, mge01120-Microbial-metabolism-in-diverse-environments |
| P47287 | Phosphocarrier protein | K02784 | mge02060-Phosphotransferase-system-(PTS) |
| P47299 | Phosphomannomutase | K01840 | mge00541-O-Antigen-nucleotide-sugar-biosynthesis, mge01110-Biosynthesis-of-secondary-metabolites |
| P47301 | Uncharacterised protein | K03073 | mge02024-Quorum-sensing, mge03070-Bacterial-secretion-system |
| P47315 | PTS system | K20118 | mge02060-Phosphotransferase-system-(PTS) |
| P47318 | Protein translocase subunit | K03070 | mge02024-Quorum-sensing, mge03070-Bacterial-secretion-system |
| P47323 | Oligopeptide transport system permease protein | K15581 | mge02024-Quorum-sensing |
| P47324 | Oligopeptide transport system permease protein | K15582 | mge02024-Quorum-sensing |
| P47357 | Glucose-6-phosphate isomerase | K01810 | mge01110-Biosynthesis-of-secondary-metabolites, mge01120-Microbial-metabolism-in-diverse-environments |
| P47391 | Bifunctional riboflavin kinase | K11753 | mge01110-Biosynthesis-of-secondary-metabolites |
| P47416 | Protein translocase subunit | K03076 | mge02024-Quorum-sensing, mge03070-Bacterial-secretion-system |
| P47489 | Glycerol-3-phosphate acyltransferase | K08591 | mge01110-Biosynthesis-of-secondary-metabolites |
| P47514 | Dihydrolipoyllysine-residue acetyltransferase | K00627 | mge01110-Biosynthesis-of-secondary-metabolites, mge01120-Microbial-metabolism-in-diverse-environments |
| P47515 | Pyruvate dehydrogenase E1 component subunit beta | K00162 | mge01110-Biosynthesis-of-secondary-metabolites, mge01120-Microbial-metabolism-in-diverse-environments |
| P47516 | Pyruvate dehydrogenase E1 component subunit alpha | K00161 | mge01110-Biosynthesis-of-secondary-metabolites, mge01120-Microbial-metabolism-in-diverse-environments |
| P47529 | Acyl carrier protein homolog | K02078 | mge01110-Biosynthesis-of-secondary-metabolites |
| P47541 | Phosphate acetyltransferase | K00625 | mge00680-Methane-metabolism mge01120-Microbial-metabolism-in-diverse-environments |
| P47599 | Acetate kinase | K00925 | mge00680-Methane-metabolism, mge01120-Microbial-metabolism-in-diverse-environments |
| P47612 | Probable tRNA sulfurtransferase | K03151 | mge04122-Sulfur relay system |
| P47636 | Probable ribose-5-phosphate isomerase B | K01808 | mge01110-Biosynthesis-of-secondary-metabolites, mge01120-Microbial-metabolism-in-diverse-environments |
| P47668 | Phosphoenolpyruvate-protein phosphotransferase | K08483 | mge02060-Phosphotransferase-system-(PTS) |
| P47669 | 2,3-bisphosphoglycerate-independent phosphoglycerate mutase | K15633 | mge00680-Methane-metabolism, mge01110-Biosynthesis-of-secondary-metabolites, mge01120-Microbial-metabolism-in-diverse-environments |
| P47696 | Hypoxanthine-guanine phosphoribosyltransferase | K00760 | mge01110-Biosynthesis-of-secondary-metabolites |
| P47702 | Membrane protein insertase | K03217 | mge02024-Quorum-sensing, mge03070-Bacterial-secretion-system |
| P58061 | Probable protein-export membrane protein | K03075 | mge02024-Quorum-sensing, mge03070-Bacterial-secretion-system |
| Q49409 | Uncharacterised protein | K12257 | mge02024-Quorum-sensing, mge03070-Bacterial-secretion-system |
| Q49427 | Phosphate acyltransferase | K03621 | mge01110-Biosynthesis-of-secondary-metabolites |
Figure 2.
Distribution of essential and non-homologous proteins involved in the unique metabolic pathways of M. genitalium.
2.4. Prediction of Subcellular Localization
Subcellular localization provides information and insight about the location and function of an essential protein. Cytoplasmic proteins are more suitable as drug targets [29]. Out of the 30 essential and non-homologous proteins involved in unique metabolic pathways, 21 proteins were predicted by CELLO and PSLpred as cytoplasmic proteins, as shown in Supplementary Table S1.
2.5. Screening for Druggable Proteins and Detection of Novel Drug Targets
Druggability shows the likelihood of a small-molecule drug to modulate the activity of a therapeutic target [30]. Subjecting the 21 cytoplasmic proteins to BLASTp against druggable proteins sequences in DrugBank Database generated 13 druggable M. genitalium proteins that shared similarity with FDA-approved and experimental small-molecule drugs (Table 2). All these 13 druggable proteins can serve as drug targets in the design of antibiotics drug against this pathogen. Moreover, the remaining eight proteins (seven characterised proteins and one uncharacterised protein) that showed no similarity with any known drug/drug targets in the DrugBank database were termed novel drug targets (Table 3), and the characterised proteins were selected for further analysis. A similar approach has been used by different authors to detect novel drug targets against different pathogens [23,24,31]. The unique metabolic pathways of these proposed novel drug targets include the following: microbial metabolism in diverse environments, two-component system, biosynthesis of secondary metabolites, O-antigen nucleotide sugar biosynthesis, quorum sensing, bacterial secretion, sulphur relay system, and methane metabolism (Table 3). Brief information on some of the unique metabolic pathways as proposed novel drug targets in bacteria is summarised below.
Table 2.
List of 13 druggable proteins of Mycoplasma genitalium.
| Protein ID | Drug Name | DrugBank ID | Drug Group | Subcellular Localisation |
|---|---|---|---|---|
| P47259 | Tetrahydrofolic acid | DB00116 | Nutraceutical | Cytoplasmic |
| NADH | DB00157 | Approved | ||
| LY374571 | DB02358 | Experimental | ||
| Nicotinamide adenine dinucleotide phosphate | DB03461 | Experimental | ||
| LY249543 | DB04322 | Experimental | ||
| P47269 | Phosphoglycolohydroxamic acid | DB03026 | Experimental | Cytoplasmic |
| P47287 | Dexfosfoserine | DB04522 | Experimental | Cytoplasmic |
| P47357 | N-Bromoacetyl-aminoethyl Phosphate | DB02257 | Experimental | Cytoplasmic |
| 5-Phosphoarabinonic acid | DB03042 | Experimental | ||
| P47514 | NADH | DB00157 | Approved | Cytoplasmic |
| Radicicol | DB03758 | Experimental | ||
| Dihydrolipoic acid | DB03760 | Experimental | ||
| P47515 | Pyruvic acid | DB00119 | Approved | Cytoplasmic |
| NADH | DB00157 | Approved | ||
| P47516 | NADH | DB00157 | Approved | Cytoplasmic |
| P47541 | Acetylphosphate | DB02897 | Experimental | Cytoplasmic |
| P47599 | Formic acid | DB01942 | Experimental | Cytoplasmic |
| Adenosine-5′-[Beta, Gamma-Methylene]Triphosphate | DB03909 | Experimental | ||
| P47636 | L-cysteic acid | DB03661 | Experimental | Cytoplasmic |
| 4-phospho-D-erythronic acid | DB03108 | Experimental | ||
| 4-Phospho-D-erythronohydroxamic acid | DB04496 | Experimental | ||
| P47668 | Diethylene glycol diethyl ether | DB08357 | Experimental | Cytoplasmic |
| P47669 | 2-phospho-D-glyceric acid | DB01709 | Experimental | Cytoplasmic |
| 3-phospho-D-glyceric acid | DB04510 | Experimental | ||
| P47696 | Inosinic acid | DB04566 | Experimental | Cytoplasmic |
| 5-O-phosphono-alpha-D-ribofuranosyl diphosphate | DB01632 | Approved | ||
| Formycin B | DB04198 | Experimental | ||
| Mercaptopurine | DB01033 | Approved | ||
| Tioguanine | DB00352 | Approved | ||
| Azathioprine | DB00993 | Approved | ||
| Artenimol | DB11638 | Approved |
Table 3.
List of novel drug targets in Mycoplasma genitalium showing no similarity with human anti-target proteins.
| Protein ID | Protein Name | Subcellular Localisation | Unique Metabolic Pathways | Anti-Target Similarity |
|---|---|---|---|---|
| P22746 | Bifunctional oligoribonuclease and PAP phosphatase | Cytoplasmic | mge01120-Microbial metabolism in diverse environments | No |
| P35888 | Chromosomal replication initiator protein | Cytoplasmic | mge02020-Two component system | No |
| P47299 | Phosphomannomutase | Cytoplasmic | mge01110-Biosynthesis of secondary metabolites | No |
| mge00541-O-Antigen nucleotide sugar biosynthesis | ||||
| P47318 | Protein translocase subunit | Cytoplasmic | mge02024-Quorum-sensing | No |
| mge03070-Bacterial secretion system | ||||
| P47529 | Acyl carrier protein homolog | Cytoplasmic | mge01110-Biosynthesis of secondary metabolites | No |
| P47612 | Probable tRNA sulfurtransferase | Cytoplasmic | mge04122-Sulfur relay system | No |
| Q49427 | Phosphate acyltransferase | Cytoplasmic | mge01110-Biosynthesis of secondary metabolites | No |
2.5.1. Methane Metabolism
Energy production in the form of ATP is essential for maintaining cellular activities in all living organisms. Methane metabolism, which entails the breaking down of one-carbon compound (methane) to obtain energy, is one of the unique metabolic pathways in bacteria. One of the proteins predicted in this study participates in the methane metabolism pathway. This unique pathway is crucial to the survival of bacteria and has been suggested as a potential drug target in different bacteria such as Mycobacterium tuberculosis, Mycobacterium avium, and Enterococcus faecium [32,33,34].
2.5.2. Biosynthesis of Secondary Metabolites
Secondary metabolites are small bioactive molecules produced in bacteria during their stationary phase of growth [35]. They are not essentially important for the growth and survival of bacteria, but they provide competitive advantages [36]. Three proteins from this study predicted to be involved in the biosynthesis of secondary metabolites are unique to M. genitalium. This is similar to reports from other studies where the proteins associated with this pathway have been described to be essential, non-homologous, and unique to different bacteria pathogens such as Vibrio parahaemolyticus and Staphylococcus saprophyticus [23,37]. Different studies have reported the manipulation of bacterial secondary metabolites pathways and their use as antibiotics, antiviral drugs, and other pharmaceutical drugs [38,39,40,41].
2.5.3. Quorum Sensing
Bacteria use quorum-sensing cell communication to regulate several cellular processes such as virulence, biofilm formation, pathogenicity, and drug resistance [42,43,44]. Other activities controlled by quorum sensing include antibiotic production and sporulation [45]. This present study showed that the protein translocase subunit that was predicted as a novel drug target is involved in the quorum-sensing metabolic pathway in M. genitalium. A recent study by Daubenspeck et al. [46] indicated that biofilm formation contributes to antibiotics resistance in M. genitalium. Due to the significant role of quorum sensing in biofilm formation in most bacteria, proteins from this metabolic pathway have been proposed as potential therapeutic targets [47,48].
2.5.4. Two-Component System (TCS)
Bacteria widely use two-component system (TCS) to respond to environmental signals [49]. TCS consists of histidine kinase and a sensor regulator. It is involved in the expression of virulence and antibiotic-resistance responses in pathogenic bacteria [49]. For example, VicRK (a TCS) has been reported to regulate biofilm formation, virulence, and lipid metabolism [50,51]. Similarly, the VanS/VanR two-component system controls the resistance of enterococci to vancomycin [52]. Though one of the seven proteins predicted as novel drug targets in this study is involved in the TCS metabolic pathway, no TCS has been reported so far in M. genitalium in the literature [53]. However, serine/threonine kinases (STK) and phosphatases (STP), which are signal transduction systems, have been indicated to perform similar functions as a TCS [54]. A report by Martinez et al. [53] also showed that STP is involved in the virulence of M. genitalium. Other TCSs such as DegS-DegU, Pho regulon (such as PhoR-PhoP, PhoR–PhoS, and PhoP-PhoQ), and QseC-QsecB—which are present in Bacillus subtilis [55,56], Corynebacterium glutamicum [57], Shigella species [58], and enterohaemorrhagic E. coli [59]—are involved in the pathogenesis and biofilm formation of bacteria.
As such, a TCS serves as a potential antibiotic target [60].
2.5.5. Bacterial Secretion System
The bacterial secretion system facilitates the transport of proteins from the cytoplasm of most bacteria either to their host environment or directly into their host cells. They aid in bacteria’s virulence and pathogenicity, leading to severe damage to the host cell [61]. In this study, the protein translocase subunit was found to be involved in the bacterial secretion system metabolic pathway, which was similar to a previous study by Butt et al. [26] that also predicted the bacteria secretion system as a novel drug target to be considered in the design of a potent antimicrobial agent against M. genitalium. Different classes of this secretion system in bacteria (type I–VII) have been regarded as potential drug targets [62,63,64].
2.6. Anti-Target Analysis of Novel Drug Targets and 3D Structure Analysis
Toxicity and other adverse drug reactions could cause drug withdrawal from public use [65,66]. To ensure the effectiveness and safety of potential drugs, it is essential to screen the proposed drug targets against possible anti-target proteins. The shortlisted eight novel drug target proteins showed no similarity with the 210 human anti-target proteins using NCBI BLASTp (Table 3). The novelty of these proposed novel drug targets was also confirmed by their low query coverage and/or percentage identity with known 3D protein structures in the PDB (Supplementary File S5). In summary, seven essential, non-homologous, and cytoplasmic proteins that are involved in seven unique pathways (Table 3) were proposed as potential drug targets from this study. Our study correlates with previous studies where some of these proposed unique metabolic pathways have been regarded as drug targets in different bacteria [67,68,69].
3. Materials and Methods
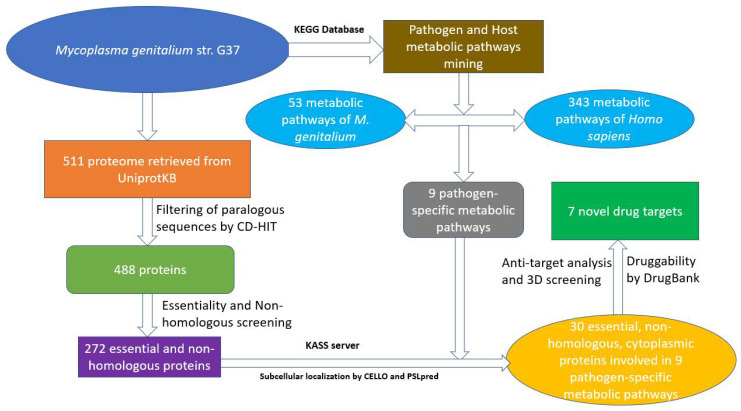
The subtractive genomics approach is a reliable method of prioritising potential drug targets from the whole proteome of a pathogen. A flow-chart showing all the steps carried out in this work is shown in Figure 3.
Figure 3.
The workflow showing the prediction of novel drug targets.
3.1. Sequence Retrieval and Removal of Paralogous Sequences
The whole proteome of Mycoplasma genitalium str. G37 consisting of 511 protein sequences was downloaded from the UniProtKB database (https://www.uniprot.org/, accessed on 15 April 2021). Out of these, paralogous sequences with a cut-off score of 0.9 (90% identity) were excluded from the protein sequences using the CD-HIT server. CD-HIT is a server that clusters biological sequences, thereby reducing the redundancy of protein sequences [70].
3.2. Prediction of Essential Proteins
After excluding paralogous sequences, the remaining protein sequences were subjected to Geptop 2.0 server to screen for essential proteins, which play a critical role in the survival of a pathogen. With an essentiality cut-off score of 0.24, the Geptop server identifies essential genes in bacteria by comparing query protein ortholog and phylogeny with experimentally determined essential gene datasets present in the Database of Essential Genes (DEG) [71].
3.3. Screening for Non-Homologous Protein
Using the default parameters of NCBI BLASTp [72], the predicted essential proteins were screened against the human protein (Homo sapiens). Essential proteins showing no hits and those with an identity of <20% but a query coverage of >70% were selected as non-homologous and used for further analysis.
3.4. Analysis of Metabolic Pathways
The Kyoto Encyclopedia of Genes and Genome (KEGG) database [73], which contains the complete metabolic pathways of most living organisms, was used to retrieve the metabolic pathways of M. genitalium str. G37 and human host (Homo sapiens) using the KEGG three letter organism codes ‘mge’ and ‘hsa,’ respectively. The metabolic pathways of the pathogen and host were compared, and those pathways found in the pathogen but absent in the human host were regarded as unique pathways. Common pathways are those found in both a pathogen and its human host. The essential and non-homologous proteins of M. genitalium were subjected to BLASTp present in KEGG Automatic Annotation Server (KASS), an automatic genome annotation and pathway reconstruction too [74]. KASS provides KEGG Orthology identifiers (KO) and information about the metabolic pathways. The essential and non-homologous proteins of M. genitalium with unique metabolic pathways were selected for subsequent analysis.
3.5. Prediction of Subcellular Localization
Sub-cellular localisation prediction classifies proteins into different positions, including cytoplasm, inner membrane, periplasmic, and outer membrane. Proteins located in the cytoplasm and outer membrane are potential drug and vaccine targets, respectively. CELLO and PSLpred servers that employ support vector machine (SVM) and hybrid SVM-based approaches with prediction accuracies of 89% and 91%, respectively, were used to predict subcellular localisation [75,76].
3.6. Druggability Analysis and Detection of Novel Drug Targets
To identify druggable proteins, the essential and non-homologous proteins of M. genitalium with unique metabolic pathways were screened against the DrugBank database using the default parameters. The DrugBank database consists of 14,542 drug entries, and these include FDA-approved drugs, nutraceuticals, and experimental drugs [77]. Novel putative drug targets that showed no hits with known drug/drug targets in the DrugBank were selected for further analysis.
3.7. Anti-Target Analysis of Novel Drug Targets and 3D Structure Analysis
Anti-target proteins are important proteins in the host cell that could dock with potential therapeutic compounds designed against a pathogen. In humans, 210 of such anti-target proteins (Supplementary File S1) have been reported in the literature, including P-glycoprotein (P-gly), adrenergic receptor, dopaminergic receptor, and ether-a-go-go-related protein. To avoid the cross-reaction of these anti-target proteins with the proposed drug targets, which could lead to toxic effects, the novel drug targets were subjected to NCBI BLASTp against these 210 anti-targets proteins with the following parameters: an E-value of <0.005, a query coverage of >30%, and an identity of <25%. To further verify the novelty of these novel drug targets, their sequences were screened against known 3D structures in the Protein Data Bank (PDB) using PSI-BLAST in NCBI.
4. Conclusions
The rapid emergence of antimicrobial resistance among Gram-positive bacteria has triggered the need to explore novel drug targets that could assist in designing new antimicrobial agents. This present study provided seven cytoplasmic proteins of Mycoplasma genitalium as novel putative drug targets. These proteins are involved in pathogen-specific metabolic pathways, some of which have been reported as therapeutic targets in different microorganisms. The lack of similarities of these proposed novel putative drug targets with human proteome and anti-targets has further reduced the chances of interaction between drugs and human proteins. Therefore, the design of new antimicrobial agents that would inhibit these novel putative drug targets could provide effective control against M. genitalium infection, though these putative drug targets still need to be subjected to further analysis and be experimentally validated.
Acknowledgments
The postdoctoral fellowship awarded to the first author by the College of Agriculture, Engineering and Science, University of KwaZulu-Natal is gratefully appreciated.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/pathogens10080921/s1, Table S1: List of consensus cytoplasmic proteins generated by CELLO and PSLpred servers; File S1: List of anti-target protein sequences in human, File S2: The complete protein sequences of M. genitalium str. G37, File S3: List of essential proteins predicted by Geptop server, File S4: List of essential and non-homologous protein, File S5: List of known 3D protein structure generated by PSI-BLAST.
Author Contributions
Conceptualization, A.J.F., M.O. and M.A.A.; methodology, A.J.F.; validation, A.J.F., M.O. and M.A.A.; formal analysis, A.J.F.; investigation, A.J.F.; resources, M.A.A.; writing—original draft preparation, A.J.F.; writing—review and editing, A.J.F., M.O. and M.A.A.; supervision, M.A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sethi S., Singh G., Samanta P., Sharma M. Mycoplasma genitalium: An emerging sexually transmitted pathogen. Indian J. Med. Res. 2012;136:942–955. [PMC free article] [PubMed] [Google Scholar]
- 2.Jensen J.S. Mycoplasma genitalium: The aetiological agent of urethritis and other sexually transmitted diseases. J. Eur. Acad. Dermatol. Venereol. 2004;18 doi: 10.1111/j.1468-3083.2004.00923.x. [DOI] [PubMed] [Google Scholar]
- 3.Lis R., Rowhani-Rahbar A., Manhart L.E. Mycoplasma genitalium infection and female Reproductive Tract Disease: A Meta-analysis. Clin. Infect. Dis. 2015;61:418–426. doi: 10.1093/cid/civ312. [DOI] [PubMed] [Google Scholar]
- 4.Lewis J., Horner P.J., White P.J. Incidence of pelvic inflammatory disease associated with Mycoplasma genitalium infection: Evidence Synthesis of Cohort Study Data. Clin. Infect. Dis. 2020;71:2719–2722. doi: 10.1093/cid/ciaa419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Averbach S.H., Hacker M.R., Yiu T., Modest A.M., Dimitrakoff J., Ricciotti H.A. Mycoplasma genitalium and preterm delivery at an urban community health center. Int. J. Gynaecol. Obstet. 2013;123:54–57. doi: 10.1016/j.ijgo.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manhart L.E., Gillespie C.W., Lowens M.S., Khosropour C.M., Colombara D.V., Golden M.R., Hakhu N.R., Thomas K.K., Hughes J.P., Jensen N.L., et al. Standard treatment regimens for nongonococcal urethritis have similar but declining cure rates: A randomized controlled trial. Clin. Infect. Dis. 2013;56:934–942. doi: 10.1093/cid/cis1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahlangu M.P., Müller E.E., Venter J.M.E., Maseko D.V., Kularatne R.S. The prevalence of Mycoplasma genitalium and association with Human Immunodeficiency Virus infection in symptomatic patients, Johannesburg, South Africa, 2007–2014. Sex. Transm. Dis. 2019;46:395–399. doi: 10.1097/OLQ.0000000000000984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Napierala Mavedzenge S., Müller E.E., Lewis D.A., Chipato T., Morrison C.S., Weiss H.A. Mycoplasma genitalium is associated with increased genital HIV Type 1 RNA in Zimbabwean Women. J. Infect. Dis. 2015;211:1388–1398. doi: 10.1093/infdis/jiu644. [DOI] [PubMed] [Google Scholar]
- 9.Harrison S.A., Olson K.M., Ratliff A.E., Xiao L., Van Der Pol B., Waites K.B., Geisler W.M. Mycoplasma genitalium coinfection in women with Chlamydia trachomatis infection. Sex. Transm. Dis. 2019;46:e101–e104. doi: 10.1097/OLQ.0000000000001028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernández-Huerta M., Espasa M. Mycoplasma genitalium co-infection with Chlamydia trachomatis and Neisseria gonorrhoeae among asymptomatic patients: The silent wick for macrolide resistance spread. Sex. Transm. Infect. 2019;95 doi: 10.1136/sextrans-2018-053848. [DOI] [PubMed] [Google Scholar]
- 11.Workowski K.A., Bolan G.A. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm. Rep. 2015;64:1–137. [PMC free article] [PubMed] [Google Scholar]
- 12.Horner P.J., Martin D.H. Mycoplasma genitalium Infection in Men. J. Infect. Dis. 2017;216:S396–S405. doi: 10.1093/infdis/jix145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jensen J.S., Bradshaw C. Management of Mycoplasma genitalium infections—Can we hit a moving target? BMC Infect. Dis. 2015;15:343. doi: 10.1186/s12879-015-1041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bradshaw C.S., Jensen J.S., Waites K.B. New Horizons in Mycoplasma genitalium Treatment. J. Infect. Dis. 2017;216:S412–S419. doi: 10.1093/infdis/jix132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horner P.J., Blee K., Falk L., Van Der Meijden W., Moi H. 2016 European guideline on the management of non-gonococcal urethritis. Int. J. STD AIDS. 2016:1–10. doi: 10.1177/0956462416648585. [DOI] [PubMed] [Google Scholar]
- 16.Taylor-robinson D. Diagnosis and antimicrobial treatment of Mycoplasma genitalium infection: Sobering thoughts. Expert Rev. Anti-Infect. Infect. Ther. 2014:715–722. doi: 10.1586/14787210.2014.919220. [DOI] [PubMed] [Google Scholar]
- 17.Jensen J.S., Cusini M., Gomberg M., Moi H. 2016 European guideline on Mycoplasma genitalium infections. J. Eur. Acad. Dermatol. Venereol. 2016;30:1650–1656. doi: 10.1111/jdv.13849. [DOI] [PubMed] [Google Scholar]
- 18.Manhart L.E., Jensen J.S., Bradshaw C.S., Golden M.R., Martin D.H. Efficacy of Antimicrobial Therapy for Mycoplasma genitalium Infections. Clin. Infect. Dis. 2015;61:S802–S817. doi: 10.1093/cid/civ785. [DOI] [PubMed] [Google Scholar]
- 19.Ke W., Li D., Tso L.S., Wei R., Lan Y., Chen Z., Zhang X., Wang L., Liang C., Liao Y., et al. Macrolide and fluoroquinolone associated mutations in Mycoplasma genitalium in a retrospective study of male and female patients seeking care at a STI Clinic in Guangzhou, China, 2016–2018. BMC Infect. Dis. 2020;20:950. doi: 10.1186/s12879-020-05659-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muller E.E., Mahlangu M.P., Lewis D.A., Kularatne R.S. Macrolide and fluoroquinolone resistance-associated mutations in Mycoplasma genitalium in Johannesburg, South Africa, 2007–2014. BMC Infect. Dis. 2019;19:148. doi: 10.1186/s12879-019-3797-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murray G.L., Bradshaw C.S., Bissessor M., Danielewski J., Garland S.M., Jensen J.S., Fairley C.K., Tabrizi S.N. Increasing Macrolide and Fluoroquinolone Resistance in Mycoplasma genitalium. Emerg. Infect. Dis. 2017;23:809–812. doi: 10.3201/eid2305.161745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin X., Li X., Lin X. A Review on Applications of Computational Methods in Drug Screening and Design. Molecules. 2020;25:1375. doi: 10.3390/molecules25061375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hasan M., Azim K.F., Imran M.A.S., Chowdhury I.M., Urme S.R.A., Parvez M.S.A., Uddin M.B., Ahmed S.S.U. Comprehensive genome based analysis of Vibrio parahaemolyticus for identifying novel drug and vaccine molecules: Subtractive proteomics and vaccinomics approach. PLoS ONE. 2020;15:e0237181. doi: 10.1371/journal.pone.0237181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan M.T., Mahmud A., Iqbal A., Hoque S.F., Hasan M. Subtractive genomics approach towards the identification of novel therapeutic targets against human Bartonella bacilliformis. Inform. Med. Unlocked. 2020;20:100385. doi: 10.1016/j.imu.2020.100385. [DOI] [Google Scholar]
- 25.Solanki V., Tiwari V. Subtractive proteomics to identify novel drug targets and reverse vaccinology for the development of chimeric vaccine against Acinetobacter baumannii. Sci. Rep. 2018;8:9044. doi: 10.1038/s41598-018-26689-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Butt A.M., Tahir S., Nasrullah I., Idrees M., Lu J., Tong Y. Mycoplasma genitalium: A comparative genomics study of metabolic pathways for the identification of drug and vaccine targets. Infect. Genet. Evol. 2012;12:53–62. doi: 10.1016/j.meegid.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 27.Jackson N., Czaplewski L., Piddock L.J. V Discovery and development of new antibacterial drugs: Learning from experience? J. Antimicrob. Chemother. 2018;73:1452–1459. doi: 10.1093/jac/dky019. [DOI] [PubMed] [Google Scholar]
- 28.Sakharkar K.R., Sakharkar M.K., Chow V.T.K. A novel genomics approach for the identification of drug targets in pathogens, with special reference to Pseudomonas aeruginosa. In Silico Biol. 2004;4:355–360. [PubMed] [Google Scholar]
- 29.Uddin R., Siddiqui Q.N., Sufian M., Azam S.S., Wadood A. Proteome-wide subtractive approach to prioritize a hypothetical protein of XDR-Mycobacterium tuberculosis as potential drug target. Genes Genom. 2019;41:1281–1292. doi: 10.1007/s13258-019-00857-z. [DOI] [PubMed] [Google Scholar]
- 30.Owens J. Determining druggability. Nat. Rev. Drug Discov. 2007;6:187. doi: 10.1038/nrd2275. [DOI] [Google Scholar]
- 31.Mahmud A., Khan M.T., Iqbal A. Identification of novel drug targets for humans and potential vaccine targets for cattle by subtractive genomic analysis of Brucella abortus strain 2308. Microb. Pathog. 2019;137:103731. doi: 10.1016/j.micpath.2019.103731. [DOI] [PubMed] [Google Scholar]
- 32.Amir A., Rana K., Arya A., Kapoor N., Kumar H., Siddiqui M.A. Mycobacterium tuberculosis H37Rv: In Silico Drug Targets Identification by Metabolic Pathways Analysis. Int. J. Evol. Biol. 2014;2014:284170. doi: 10.1155/2014/284170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uddin R., Siraj B., Rashid M., Khan A., Halim S.A., Al-Harrasi A. Genome subtraction and comparison for the identification of novel drug targets against mycobacterium avium subsp. Hominissuis. Pathogens. 2020;9:368. doi: 10.3390/pathogens9050368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karim M., Islam M.D.N., Azad Jewel G.M.N. In Silico identification of potential drug targets by subtractive genome analysis of Enterococcus faecium. bioRxiv. 2020 doi: 10.1101/2020.02.14.948232. [DOI] [Google Scholar]
- 35.Ruiz B., Chávez A., Forero A., García-Huante Y., Romero A., Sánchez M., Rocha D., Sánchez B., Rodríguez-Sanoja R., Sánchez S., et al. Production of microbial secondary metabolites: Regulation by the carbon source. Crit. Rev. Microbiol. 2010;36:146–167. doi: 10.3109/10408410903489576. [DOI] [PubMed] [Google Scholar]
- 36.Craney A., Ahmed S., Nodwell J. Towards a new science of secondary metabolism. J. Antibiot. 2013;66:387–400. doi: 10.1038/ja.2013.25. [DOI] [PubMed] [Google Scholar]
- 37.Shahid F., Ashfaq U.A., Saeed S., Munir S., Almatroudi A., Khurshid M. In silico subtractive proteomics approach for identification of potential drug targets in staphylococcus saprophyticus. Int. J. Environ. Res. Public Health. 2020;17:3644. doi: 10.3390/ijerph17103644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weber T., Welzel K., Pelzer S., Vente A., Wohlleben W. Exploiting the genetic potential of polyketide producing streptomycetes. J. Biotechnol. 2003;106:221–232. doi: 10.1016/j.jbiotec.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 39.Zhan J. Biosynthesis of Bacterial Aromatic Polyketides. Curr. Top. Med. Chem. 2009:1598–1610. doi: 10.2174/156802609789941906. [DOI] [PubMed] [Google Scholar]
- 40.Ozcengiz G., Demain A.L. Recent advances in the biosynthesis of penicillins, cephalosporins and clavams and its regulation. Biotechnol. Adv. 2013;31:287–311. doi: 10.1016/j.biotechadv.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 41.Onaka H. Novel antibiotic screening methods to awaken silent or cryptic secondary metabolic pathways in actinomycetes. J. Antibiot. 2017;70:865–870. doi: 10.1038/ja.2017.51. [DOI] [PubMed] [Google Scholar]
- 42.Pena R.T., Blasco L., Ambroa A., González-Pedrajo B., Fernández-García L., López M., Bleriot I., Bou G., García-Contreras R., Wood T.K., et al. Relationship Between Quorum Sensing and Secretion Systems. Front. Microbiol. 2019;10:1100. doi: 10.3389/fmicb.2019.01100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hawver L.A., Jung S.A., Ng W.-L. Specificity and complexity in bacterial quorum-sensing systems. FEMS Microbiol. Rev. 2016;40:738–752. doi: 10.1093/femsre/fuw014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao X., Yu Z., Ding T. Quorum-Sensing Regulation of Antimicrobial Resistance in Bacteria. Microorganisms. 2020;8:425. doi: 10.3390/microorganisms8030425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abisado R.G., Benomar S., Klaus J.R., Dandekar A.A., Chandler J.R. Bacterial Quorum Sensing and Microbial Community Interactions. MBio. 2018;9:e02331-17. doi: 10.1128/mBio.02331-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Daubenspeck J.M., Totten A.H., Needham J., Feng M., Balish M.F., Atkinson T.P., Dybvig K. Mycoplasma genitalium Biofilms Contain Poly-GlcNAc and Contribute to Antibiotic Resistance. Front. Microbiol. 2020;11 doi: 10.3389/fmicb.2020.585524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang Q., Chen J., Yang C., Yin Y., Yao K. Quorum Sensing: A Prospective Therapeutic Target for Bacterial Diseases. BioMed Res. Int. 2019;2019:2015978. doi: 10.1155/2019/2015978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haque S., Ahmad F., Dar S.A., Jawed A., Mandal R.K., Wahid M., Lohani M., Khan S., Singh V., Akhter N. Developments in strategies for Quorum Sensing virulence factor inhibition to combat bacterial drug resistance. Microb. Pathog. 2018;121:293–302. doi: 10.1016/j.micpath.2018.05.046. [DOI] [PubMed] [Google Scholar]
- 49.Lingzhi L., Haojie G., Dan G., Hongmei M., Yang L., Mengdie J., Chengkun Z., Xiaohui Z. The role of two-component regulatory system in β-lactam antibiotics resistance. Microbiol. Res. 2018;215:126–129. doi: 10.1016/j.micres.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 50.Ahn S.-J., Burne R.A. Effects of oxygen on biofilm formation and the AtlA autolysin of Streptococcus mutans. J. Bacteriol. 2007;189:6293–6302. doi: 10.1128/JB.00546-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ng W.-L., Tsui H.-C.T., Winkler M.E. Regulation of the pspA virulence factor and essential pcsB murein biosynthetic genes by the phosphorylated VicR (YycF) response regulator in Streptococcus pneumoniae. J. Bacteriol. 2005;187:7444–7459. doi: 10.1128/JB.187.21.7444-7459.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gagnon S., Lévesque S., Lefebvre B., Bourgault A.-M., Labbé A.-C., Roger M. vanA-containing Enterococcus faecium susceptible to vancomycin and teicoplanin because of major nucleotide deletions in Tn1546. J. Antimicrob. Chemother. 2011;66:2758–2762. doi: 10.1093/jac/dkr379. [DOI] [PubMed] [Google Scholar]
- 53.Martinez M.A., Das K., Saikolappan S., Materon L.A., Dhandayuthapani S. A serine/threonine phosphatase encoded by MG_207 of Mycoplasma genitalium is critical for its virulence. BMC Microbiol. 2013;13:44. doi: 10.1186/1471-2180-13-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hoch J.A. Two-component and phosphorelay signal transduction. Curr. Opin. Microbiol. 2000;3:165–170. doi: 10.1016/S1369-5274(00)00070-9. [DOI] [PubMed] [Google Scholar]
- 55.Hulett F.M., Lee J., Shi L., Sun G., Chesnut R., Sharkova E., Duggan M.F., Kapp N. Sequential action of two-component genetic switches regulates the PHO regulon in Bacillus subtilis. J. Bacteriol. 1994;176:1348–1358. doi: 10.1128/jb.176.5.1348-1358.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fabret C., Feher V.A., Hoch J.A. Two-component signal transduction in Bacillus subtilis: How one organism sees its world. J. Bacteriol. 1999;181:1975–1983. doi: 10.1128/JB.181.7.1975-1983.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wösten M.M.S.M., Parker C.T., Van Mourik A., Guilhabert M.R., Van Dijk L., Van Putten J.P.M. The Campylobacter jejuni PhosS/PhosR operon represents a non-classical phosphate-sensitive two-component system. Mol. Microbiol. 2006;62:278–291. doi: 10.1111/j.1365-2958.2006.05372.x. [DOI] [PubMed] [Google Scholar]
- 58.Moss J.E., Fisher P.E., Vick B., Groisman E.A., Zychlinsky A. The regulatory protein PhoP controls susceptibility to the host inflammatory response in Shigella flexneri. Cell. Microbiol. 2000;2:443–452. doi: 10.1046/j.1462-5822.2000.00065.x. [DOI] [PubMed] [Google Scholar]
- 59.Breland E.J., Eberly A.R., Hadjifrangiskou M. An Overview of Two-Component Signal Transduction Systems Implicated in Extra-Intestinal Pathogenic E. coli Infections. Front. Cell. Infect. Microbiol. 2017;7:162. doi: 10.3389/fcimb.2017.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hirakawa H., Kurushima J., Hashimoto Y., Tomita H. Progress Overview of Bacterial Two-Component Regulatory Systems as Potential Targets for Antimicrobial Chemotherapy. Antibiotics. 2020;9:635. doi: 10.3390/antibiotics9100635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Waksman G. Bacterial secretion comes of age. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012;367:1014–1015. doi: 10.1098/rstb.2011.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Green E.R., Mecsas J. Bacterial Secretion Systems: An Overview. Microbiol. Spectr. 2016;4 doi: 10.1128/microbiolspec.VMBF-0012-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McShan A.C., De Guzman R.N. The bacterial type III secretion system as a target for developing new antibiotics. Chem. Biol. Drug Des. 2015;85:30–42. doi: 10.1111/cbdd.12422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cegelski L., Marshall G.R., Eldridge G.R., Hultgren S.J. The biology and future prospects of antivirulence therapies. Nat. Rev. Microbiol. 2008;6:17–27. doi: 10.1038/nrmicro1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lasser K.E., Allen P.D., Woolhandler S.J., Himmelstein D.U., Wolfe S.M., Bor D.H. Timing of New Black Box Warnings and Withdrawals for Prescription Medications. JAMA. 2002;287:2215–2220. doi: 10.1001/jama.287.17.2215. [DOI] [PubMed] [Google Scholar]
- 66.Lerner A., Klein M. Dependence, withdrawal and rebound of CNS drugs: An update and regulatory considerations for new drugs development. Brain Commun. 2019;1 doi: 10.1093/braincomms/fcz025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morya V.K., Dewaker V., Mecarty S.D., Singh R. In silico Analysis Metabolic Pathways for Identi fi cation of Putative Drug Targets for Staphylococcus aureus. J. Vet. Sci. 2010;3:62–69. doi: 10.4172/jcsb.1000058. [DOI] [Google Scholar]
- 68.Chhabra G., Sharma P., Anant A., Deshmukh S., Kaushik H., Gopal K., Srivastava N., Sharma N., Garg L.C. Identification and modeling of a drug target for Clostridium perfringens SM101. Bioinformation. 2010;4:278–289. doi: 10.6026/97320630004278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barh D., Kumar A. In silico Identification of Candidate Drug and Vaccine Targets from Various Pathways in Neisseria gonorrhoeae. In Silico Biol. 2009;9:225–231. doi: 10.3233/ISB-2009-0399. [DOI] [PubMed] [Google Scholar]
- 70.Huang Y., Niu B., Gao Y., Fu L., Li W. CD-HIT Suite: A web server for clustering and comparing biological sequences. Bioinformatics. 2010;26:680–682. doi: 10.1093/bioinformatics/btq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wen Q.F., Liu S., Dong C., Guo H.X., Gao Y.Z., Guo F.B. Geptop 2.0: An updated, more precise, and faster Geptop server for identification of prokaryotic essential genes. Front. Microbiol. 2019;10 doi: 10.3389/fmicb.2019.01236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 73.Kanehisa M., Goto S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moriya Y., Itoh M., Okuda S., Yoshizawa A.C., Kanehisa M. KAAS: An automatic genome annotation and pathway reconstruction server. Nucleic Acids Res. 2007;35:W182–W185. doi: 10.1093/nar/gkm321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yu C.-S., Cheng C.-W., Su W.-C., Chang K.-C., Huang S.-W., Hwang J.-K., Lu C.-H. CELLO2GO: A Web Server for Protein subCELlular LOcalization Prediction with Functional Gene Ontology Annotation. PLoS ONE. 2014;9:e99368. doi: 10.1371/journal.pone.0099368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bhasin M., Garg A., Raghava G.P.S. PSLpred: Prediction of subcellular localization of bacterial proteins. Bioinformatics. 2005;21:2522–2524. doi: 10.1093/bioinformatics/bti309. [DOI] [PubMed] [Google Scholar]
- 77.Wishart D.S., Knox C., Guo A.C., Shrivastava S., Hassanali M., Stothard P., Chang Z., Woolsey J. DrugBank: A comprehensive resource for in silico drug discovery and exploration. Nucleic Acids Res. 2006;34:D668–D672. doi: 10.1093/nar/gkj067. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.