Abstract
Protein tyrosine kinase Pyk2 is activated by a variety of G-protein-coupled receptors and by extracellular signals that elevate intracellular Ca2+ concentration. We have identified a new Pyk2 binding protein designated Pap. Pap is a multidomain protein composed of an N-terminal α-helical region with a coiled-coil motif, followed by a pleckstrin homology domain, an Arf-GAP domain, an ankyrin homology region, a proline-rich region, and a C-terminal SH3 domain. We demonstrate that Pap forms a stable complex with Pyk2 and that activation of Pyk2 leads to tyrosine phosphorylation of Pap in living cells. Immunofluorescence experiments demonstrate that Pap is localized in the Golgi apparatus and at the plasma membrane, where it is colocalized with Pyk2. In addition, in vitro recombinant Pap exhibits strong GTPase-activating protein (GAP) activity towards the small GTPases Arf1 and Arf5 and weak activity towards Arf6. Addition of recombinant Pap protein to Golgi preparations prevented Arf-dependent generation of post-Golgi vesicles in vitro. Moreover, overexpression of Pap in cultured cells reduced the constitutive secretion of a marker protein. We propose that Pap functions as a GAP for Arf and that Pyk2 may be involved in regulation of vesicular transport through its interaction with Pap.
Protein tyrosine kinases can be divided into receptor and nonreceptor classes by virtue of whether they possess or lack extracellular ligand-binding and transmembrane domains (57). On the basis of sequence similarity in the catalytic kinase domain and the presence of common structural motifs, numerous families of nonreceptor tyrosine kinases have been defined (45). Nonreceptor tyrosine kinases may be recruited to the plasma membrane, where they mediate cellular signaling by cell surface receptors lacking intrinsic protein tyrosine kinase activities. For instance, members of the Src family of protein tyrosine kinases are activated in response to stimulation of growth factor receptors, different G-protein-coupled receptors, and many other extracellular stimuli (63). Focal adhesion kinase (FAK) on the other hand plays a central role in integrin-mediated signaling (56).
FAK and Pyk2 (proline-rich tyrosine kinase 2 [also known as RAFTK, CAKβ, and CADTK]) comprise one family of PTKs (56). Pyk2 and FAK exhibit approximately 45% amino acid identity and similar domain structure: a unique N terminus, a centrally located protein tyrosine kinase domain, and two proline-rich regions at the C terminus. Pyk2 can be activated by a variety of extracellular signals that elevate intracellular calcium concentration (37). In addition, treatments with phorbol esters or agonists of G-protein-coupled receptors lead to Pyk2 tyrosine phosphorylation (17, 37, 40). Moreover, Pyk2, like FAK, can be regulated by the activation of integrin receptors (4, 38). However, Pyk2 is not localized in focal contacts but rather concentrated in the perinuclear region of cells. The major autophosphorylation site of Pyk2 at tyrosine 402 functions as a docking site for the SH2 domain of Src. It has been shown that, together with Src, Pyk2 functions as a link between heterotrimeric G-protein-coupled receptors and the mitogen-activated protein (MAP) kinase signaling pathway (17).
There is evidence indicating that nonreceptor protein tyrosine kinases may be involved in the regulation of some aspects of membrane trafficking, such as secretory vesicle formation from the trans-Golgi network in endocrine cells (5). Src family kinases have been found associated with coated-membrane regions in platelets (62), while Src itself copurified with synaptic vesicles in PC12 cells (41). It was shown that Src associates with and phosphorylates several proteins involved in membrane trafficking, such as the neuronal synaptic vesicle-associated proteins synapsin I, synaptophysin, and synaptogyrin (6, 22, 32).
Vesicular traffic is controlled by different regulatory proteins, including Ras-like GTPases (54), heterotrimeric G proteins (8, 18), phosphatidyl inositol (PI) transfer proteins (46), PI-3 kinases (16), phospholipase D (PLD) (19), Ca2+ influx (26), as well as by protein kinase C (10, 59). Substantial evidence indicates that heterotrimeric G proteins may control vesicular transport through the Golgi apparatus by regulating the activity of the small GTP-binding protein Arf (ADP-ribosylation factor) that controls vesicle formation (8).
Arfs were first isolated as cytosolic cofactors required for cholera toxin-dependent ADP ribosylation of the Gsα in an in vitro assay (33). This property, and its ability to rescue the lethal double arf1-arf2 deletion in the yeast Saccharomyces cerevisiae, distinguish Arfs from structurally similar Arf-like proteins (34). Six Arf family members have been so far identified. Arf1, the best characterized of the mammalian Arfs, functions in the recruitment of coat proteins to membranes of the Golgi apparatus either directly or through the activation of PLD (18, 55). It was shown that disruption of the Golgi complex by the fungal metabolite brefeldin A is due to the inhibition of GDP-to-GTP exchange on Arf1 (29). Arf1 has been also implicated in endoplasmic reticulum (ER)-to-Golgi transport, endosome function, and synaptic-vesicle formation (18, 20). Recent experiments provide evidence that Arf1 may physically associate with G-protein-coupled receptors at the plasma membrane and enhance activation of PLD (44). Arf6 is localized to the plasma membrane (11), where it appears to modulate both assembly of actin cytoskeleton and endocytosis (15, 48). The sites of action of other Arf family members are currently unknown.
Arfs are distinct from other small GTPases in their GTP-dependent binding to membranes and in the absence of intrinsic GTPase activity. The completion of the GTPase cycle, therefore, requires interaction with guanine-nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs). Three Arf GEFs have been characterized, (12, 36, 43), but their substrate specificity in vivo remains to be determined (23). Several Arf GAP activities from yeast cells (47), bovine brain (51), and rat liver (42, 50) have been detected. Acidic lipids and PtdIns(4,5)P2 were found to be necessary cofactors for stimulation of GTP hydrolysis by certain Arf GAPs (35, 49). Recently, a GAP protein for Arf1 was identified (14). Arf1-GAP protein contains a zinc finger sequence of approximately 120 amino acids termed Arf GAP domain. This part of Arf1 GAP exhibits a high degree of similarity to the S. cerevisiae family of “zinc finger” proteins. One of them, GCS1, mediates the transition from stationary phase to cell proliferation (30) and has been shown to function as yeast Arf GAP in vitro and in vivo (47). It remains uncertain whether GAPs drive the GTPase cycle of Arfs autonomously or whether their GAP activity can be regulated by other proteins as well as by extracellular signals.
In this report we describe the cloning and characterization of a novel protein that specifically binds to Pyk2 and is designated Pap (Pyk2 C terminus-associated protein). Analysis of the primary structure of Pap revealed an N-terminal α-helical region with coiled-coil motif, a pleckstrin homology (PH) domain, a zinc finger containing Arf GAP domain, an ankyrin homology region, a proline-rich region, and an SH3 domain. We demonstrate an association between Pap and Pyk2, both in vitro and in vivo, and show that endogenous Pap is phosphorylated on tyrosine residues in response to phorbol ester stimulation. Activation of Pyk2 leads to tyrosine phosphorylation of Pap but the closely related kinase FAK does not phosphorylate Pap. Immunofluorescence analysis of Pap reveals localization in the plasma membrane, in the cytoplasm, and in the Golgi compartment. The addition of recombinant Pap strongly activates GTP hydrolysis on Arf in vitro and inhibits Arf-dependent generation of post-Golgi vesicles. Moreover, while overexpressed in 293 cells, Pap downregulates constitutive secretion of a marker protein (SEAP). We propose that Pap functions as an Arf GAP protein in vivo and that, when recruited to Golgi membranes, it can control Arf-mediated vesicle budding. In addition, Pap functions as a substrate and downstream target for the protein tyrosine kinases Pyk2 and Src. We propose that through their interaction with Pap, these two protein tyrosine kinases may be involved in the regulation of some aspects of vesicular transport.
MATERIALS AND METHODS
SRS.
The Sos recruitment system (SRS) for detecting protein-protein interactions has been described elsewhere (2, 3). Temperature-sensitive S. cerevisiae cdc25-2, pADNS-h5′Sos constructs and a galactose-inducible expression library of rat pituitary cDNA fused to the Src myristylation signal were obtained from A. Aronheim, Haifa, Israel. Full-length Pyk2 was subcloned into pADNS expression vector in frame with h5′Sos. Lysates of S. cerevisiae cdc25-2 transfected with pADNS-h5′SOS-Pyk2 were subjected to immunoprecipitation with anti-Pyk2 antibodies followed by blotting with anti-Pyk2 or anti-Sos antibodies to verify the expression of h5′Sos-Pyk2 fusion protein.
Approximately 4 × 105 cdc25-2 transformants containing library plasmids and the h5′Sos-Pyk2 “bait” were grown at room temperature, replica plated onto galactose plates, and incubated at 37°C. Library plasmids (pYES2 expression vector) were isolated from clones that exhibited galactose-dependent growth at 37°C and retransformed into strain cdc25-2 cells with pADNS vector expressing either h5′Sos-Pyk2, nonrelevant bait (h5′SOS-FAK), or h5′Sos alone. Clones which suppressed the cdc25-2 phenotype only in the presence of h5′Sos-Pyk2 were considered positive and further analyzed. Conventional yeast manipulation protocols were used. Yeast transfection was done as described previously (25). Media composition and replica plating were as previously described (3).
Northern blot analysis.
Human multiple tissues for Northern blotting (Clontech) were hybridized under high-stringency conditions with a 32P-labeled cDNA fragment of Papα corresponding to amino acids 281 to 691 as a probe according to the manufacturer’s instructions; this was followed by autoradiography.
Plasmids, subcloning, isolation of Pap cDNA, and sequence analysis.
The clone obtained in the screen of rat pituitary cDNA library contained the C-terminal region of Pap protein and the 3′ region of the Pap gene. To identify the 5′ end of the gene, mouse brain lambda cDNA library (Stratagene) was screened with 32P-labeled probe corresponding to the Pap C terminus (639 bp) according to standard procedures. Positive clones were plaque purified, and excised cDNAs were sequenced in both directions from internal and external primers by using an automated sequencer (Applied Biosystems). Clone KIAA0400 containing Papα was kindly provided by P. A. Nagaso, Kisarazu, Japan. Genetics Computer Group sequence analysis software (University of Wisconsin, Madison, Wis.) was used to analyze DNA and amino acid sequences. The PH domain was identified by comparison with the PH domain database (31).
For mammalian expression, cDNAs encoding Papα and Papβ were subcloned into pRK5 expression vector (39). A myc tag was fused in frame to the C-terminal end of Papα. pRK5 vectors containing FAK, hemagglutinin (HA)-tagged Pyk2, and HA-tagged PKM were as previously described (37). To generate PC12 cells stably expressing Papβ, cDNA of Papβ was subcloned into pLXSN retrovirus by using PCR.
For glutathione S-transferase (GST)-PAP fusion protein, the C-terminal part of Pap encoding the proline-rich region and SH3 domain was amplified by PCR, subcloned into pGEX-2T vector (Pharmacia Biotech, Inc.) in frame with GST, expressed in Escherichia coli, and purified by affinity chromatography on glutathione-Sepharose beads as previously described (61).
For Arf GAP assay, the region of Papβ containing the PH domain, the Arf GAP domain, and ankyrin homology region (amino acids 111 to 522) was amplified by PCR and subcloned into bacterial expression vector pET22b(+) (Novagen) in frame with a His tag sequence. The protein product was expressed in bacteria and purified by sequential chromatography on Hiload Q and nickel-chelating columns (Pharmacia Biotech, Inc.).
Cell lines and transient and stable transfections.
Human kidney (293), monkey kidney (COS-7), and human epithelial (HeLa) cells were grown in Dulbecco modified Eagle medium (DMEM; Cellgro) supplemented with 10% fetal bovine serum (Life Technologies, Inc.). Rat pheochromocytoma (PC12) cells were grown in DMEM with 10% fetal bovine serum and 10% horse serum. PC12 cells were starved in growth medium without serum for 24 h before stimulation with phorbol myristate acetate (PMA; 2 μM) for 10 min or with the mixture of H2O2 and NaVO3 (1 mM) for 20 min. Nearly confluent 293 cells were transfected by using the calcium precipitation method (13) with 1 μg of DNA per well of six-well plates, or as indicated. Stably transfected PC12 cells expressing Papβ were generated by using the PLXSN retroviral expression vector essentially as described previously (28). The expression level of Papβ was determined by immunoblotting the PC12 cell lysates after selection.
Antibodies, immunoprecipitation, and immunoblotting.
Antibodies against Pap were raised in rabbits immunized with keyhole limpet hemocyanin-conjugated synthetic peptide corresponding to amino acids 612 to 623 (Papβ) or with GST-PAP fusion protein (see above). Antibodies against FAK were obtained from Transduction Laboratories. Antibodies against Pyk2 were as previously described (17). Anti-Pyk2 antibodies recognized Pyk2 and PKM but did not recognize FAK or Pap. Polyclonal anti-phosphotyrosine antibodies were as previously described (7). The anti-mannosidase II and anti-β-Cop polyclonal antibodies were a kind gift from Marilyn Farquhar (San Diego, Calif.) and Kelley Moremen (Atlanta, Ga.). Rabbit polyclonal anti-Sos and anti-Src antibodies, anti-HA tag, anti-myc tag, and anti-GST monoclonal antibodies were from Santa Cruz Biotechnology, Inc.
For immunoprecipitation and immunoblotting analysis, the cells were washed with ice-cold phosphate-buffered saline (PBS) and lysed in 50 mM HEPES (pH 7.2), 150 mM NaCl, 1 mM EDTA, 10% glycerol, 1% Triton X-100, 1 mM sodium orthovanadate, 40 mM β-gycerophosphate, 10 mM sodium pyrophosphate, 1 mM phenylmethyl sulfonyl fluoride (PMSF), 10 μg of leupeptin per ml, and 10 μg of aprotinin per ml (lysis buffer). Cell extracts were precleared by centrifugation and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (total lysate) or incubated with antibodies cross-linked to protein A-Sepharose beads in a nutator at 4°C for 3 h or overnight. Immunocomplexes were washed in lysis buffer and analyzed as described earlier (24).
For Far Western blot, total cell lysates and immunoprecipitates from transfected 293 cells were separated by SDS-PAGE and transferred to nitrocellulose by conventional techniques. Filters were blocked overnight with TBS buffer containing 5% bovine serum albumin at 4°C and incubated with the mixture of GST-PAP fusion protein (3 μg/ml) and anti-GST monoclonal antibodies (1:1,000) overnight at 4°C. Filters were then processed as regular immunoblots.
Mouse brain homogenate (20% [wt/vol]) was prepared by rapidly excising the brain from the sacrificed mice. The tissue was soaked briefly in ice-cold PBS and then homogenized in a Teflon-glass homogenizer (10 strokes) in an ice-cold lysis buffer. The extract was centrifuged at 4°C in table-top centrifuge for 10 min at maximum speed and then recentrifuged at 4°C for 1 h (100,000 × g). The supernatant was used for immunoprecipitation and immunoblotting.
Arf GAP assay.
Arf GAP activity was determined by an in vitro assay that measures a single round of GTP hydrolysis on recombinant Arf (51). Crude phosphoinositides, phosphatidylinositol (PI) from bovine liver, phosphatidylinositol-4,5-bisphosphate (PIP2), phosphatidylcholine (PC), phosphatidylserine (PS) from bovine brain, and phosphatidic acid (PA) prepared from lecithin were obtained from Sigma. Phospholipids were solubilized in 0.1% Triton X-100 and added to the assay as mixed micelles. Myristoylated Arf1, nonmyristoylated Arf1, Arl2, and myristoylated Arf5 were prepared as described earlier (50, 52). While comparing the Arf specificities of Pap, all Arfs were used in myristoylated form. The cDNA for Arf6 was expressed in E. coli BL21(DE3) and purified as described previously (9). To determine Arf GAP activity in cell lysates, 293 cell extracts were prepared as described above.
Immunofluorescence analysis and subcellular fractionation.
HeLa or COS-7 cells were grown on uncoated coverslips, transfected by the calcium precipitation method, washed twice in PBS (37°C), fixed in 2% formaldehyde for 20 min at room temperature, permeabilized in PBS containing Triton X-100 (0.2%) for 20 min, and washed with PBS. Coverslips were incubated with primary antibodies or preimmune serum diluted in TBS buffer containing bovine serum albumin (5%) for 1 h, washed in PBS, incubated with secondary antibodies for 1 h, washed again in PBS, mounted in Fluorostab (ICN Pharmaceuticals, Inc.), and inspected with a confocal microscope (Sarastro-2000). For double-label immunofluorescence experiments, the incubation with primary (rabbit) and secondary (anti-rabbit) antibodies was followed by incubation with the second set of primary (mouse) and secondary (anti-mouse) antibodies. In control experiments primary antibodies from the second set were not used.
For subcellular fractionation, 293 cells overexpressing Papα were lysed in lysis buffer without detergent by repeatedly freezing and thawing three times. Total lysate was separated into soluble and particulate fractions by a 30-min centrifugation at 16,000 × g at 4°C.
In vitro generation of post-Golgi vesicles.
Golgi fractions were isolated from vesicular stomatitis virus (VSV)-infected MDCK cells and the cytosolic proteins fractions from rat liver cytosol (60). The Golgi fractions contained 35S-labeled sialylated VSV-G protein that had been allowed to accumulate in the trans-Golgi Network (TGN) during incubation of the cells at 20°C for 2 h prior to lysis. Vesicle generation proceeded during the incubation at 37°C for 60 min and was supported by either ATP (1 mM) or the poorly hydrolyzable GTP analog guanylyl-imidodiphosphate (GMP-PNP) (100 μM). The reactions were terminated by cooling on ice, Golgi membranes were removed by sedimentation at 10,000 × g for 10 min, and the release of labeled viral glycoprotein was measured as the percentage of total labeled protein initially present in the Golgi fraction that appeared in the supernatant. In some instances cooled reactions were analyzed by sucrose density gradient centrifugation, which allowed separation of the released vesicles (60).
Secreted alkaline phosphatase (SEAP) assay.
Human 293 cells grown in six-well plates were cotransfected with pCDNA3.SEAP and pRK5-based constructs (1:100) by the calcium precipitation method. At 36 h after transfection the medium was changed. Then, 4 h later the culture supernatant probes were taken, and the cells were lysed in ice-cold growth medium supplemented with 1% Triton X-100 and 1 mM PMSF, 10 μg of leupeptin per ml, and 10 μg of aprotinin per ml. SEAP activity in culture supernatants and in cell lysates was determined by using the SEAP reporter gene assay (Boehringer Mannheim). The presence of 1% Triton X-100 did not affect the SEAP activity in cell lysates (not shown). Secretion was expressed as the ratio of SEAP activity in culture supernatants to the sum of SEAP activity in culture supernatants and cell lysates. All experiments were performed three times in triplicate. The expression of pRK5-based constructs was verified by immunoblotting.
RESULTS
Isolation of a Pyk2-associated protein by using the SRS.
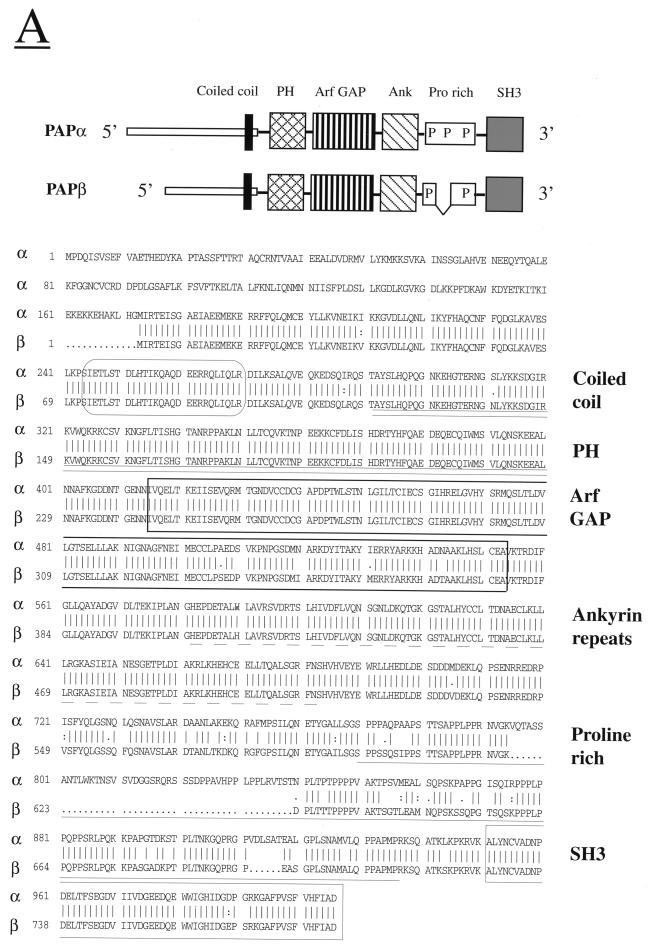
The SRS (also known as the cytoplasmic two-hybrid screen) is a genetic method that enables the detection of protein-protein interactions in yeast cells (3). To identify Pyk2 binding proteins, we used SRS for screening of a rat pituitary cDNA library with Pyk2 as a bait. One clone interacting specifically with Pyk2 but not with a heterologous bait (FAK) was isolated after screening 4 × 105 transformants. This clone (639 bases) was used as a probe for screening a mouse brain cDNA library to identify two new clones. Sequence analysis of one of the clones revealed an open reading frame of 783 amino acids with a predicted molecular size of 88 kDa. A human homolog (KIAA0400) containing 5,711 bases was identified by searching the database with the murine sequence. The human homolog exhibits 95% sequence identity with the murine sequence and contains 1,006 amino acids, with a predicted molecular mass of 112 kDa. The human homologue is larger than murine Pyk2-associated protein (Pap) since it contains additional sequences that are probably generated by alternative splicing. The shorter form lacks 45 amino acids from the proline-rich domain and 172 amino acids from the N terminus. The long form is designated Papα and the short form is designated Papβ (Fig. 1A). Papα and Papβ cDNAs were transiently expressed in 293 cells, and proteins with appropriate molecular sizes were detected by immunoprecipitation with polyclonal rabbit anti-Pap antibodies followed by SDS-PAGE and autoradiography.
FIG. 1.
Primary structure of Pap isoforms and tissue expression pattern. (A) Schematic diagram and amino acid sequences of Papα (human) and Papβ (murine). The amino acid sequences are shown in single-letter code. The numbers represent positions of the amino acid residues. Predicted coiled-coil region (CoilScan program, GCG package) is enclosed in a rectangle with rounded corners. The PH domain is underlined with a double line. The Arf GAP domain is boxed. The ankyrin homology region is underlined with a dashed line. The proline-rich region is underlined with a solid line. The SH3 domain is boxed. (B) Comparison of Arf GAP domains of murine Pap, Arf1 GAP, and GCS1. Multiple sequence alignment of the regions containing the zinc finger motif (CXXCX16CXXC, where X is any amino acid) of Arf1 GAP (residues 1 to 119), GCS1 (residues 5 to 122), and murine Pap (residues 243 to 381). Identical residues are framed and shadowed. The positions of four conserved cysteins of zinc finger motif are marked by dots. (C) Northern blot analysis of Pap mRNA expression in various human tissues. Human multiple tissues for Northern blotting were hybridized with radiolabeled probe for Pap as described in Materials and Methods. Size markers in kilobases are shown on the right of the figure. Abbreviations: H, heart; B, brain; Pl, placenta; Lu, lungs; Li, liver; S, spleen; K, kidney; Pa, pancreas.
Analysis of the primary structures of murine and human sequences shows that Pap is a multidomain protein composed of several previously described sequence motifs. The N terminus of Pap contains a unique amino acid sequence that is followed by a PH domain, an Arf GAP domain, three ankyrin repeats, a proline-rich region, and an SH3 domain (Fig. 1A). The Arf GAP domain of Pap contains a typical CXXCX16CXXC zinc finger sequence with high sequence identity to the zinc finger-containing domains of Arf1 GAP and GCS1 proteins (Fig. 1B). It was demonstrated that Arf1 GAP and GCS1 function as GTPase-activating proteins for Arf1 in vitro and in yeast cells, respectively (14, 47) and that the zinc finger domain is essential for GAP activity (14). The proline-rich sequence of Pap contains several consensus binding sites for SH3 domains (PXXP), including four binding sites for type II SH3 domains (PXXPXR) (21). Splicing out 45 amino acids from this region distinguishes Papα from Papβ.
The chromosomal localization of Papα was determined by using the Genebridge 4 Radiation Hybrid Panel. Using this approach we demonstrated that the Papα gene is closely linked to the D2S359 marker on chromosome 2p24. The tissue expression pattern of Pap was determined by Northern blot analysis of various human tissues with a specific Pap probe (Fig. 1C). Pap transcript of approximately 5.7 kb was detected predominantly in brain, kidney, and heart tissues, as well as in the placenta, lungs, and pancreas.
A closely related protein termed ASAP1 composed of similar sequence motifs was recently identified (9). Both Pap and ASAP1 contain a unique amino-terminal sequence followed by a PH domain, Arf GAP domain, three ankyrin repeats, a proline-rich sequence, and an SH3 domain. Pap and ASAP1 exhibit overall 68% identity; the PH and Arf GAP domains are 69% identical, while the SH3 domains are 75% identical.
In vitro and in vivo association between Pyk2 and Pap.
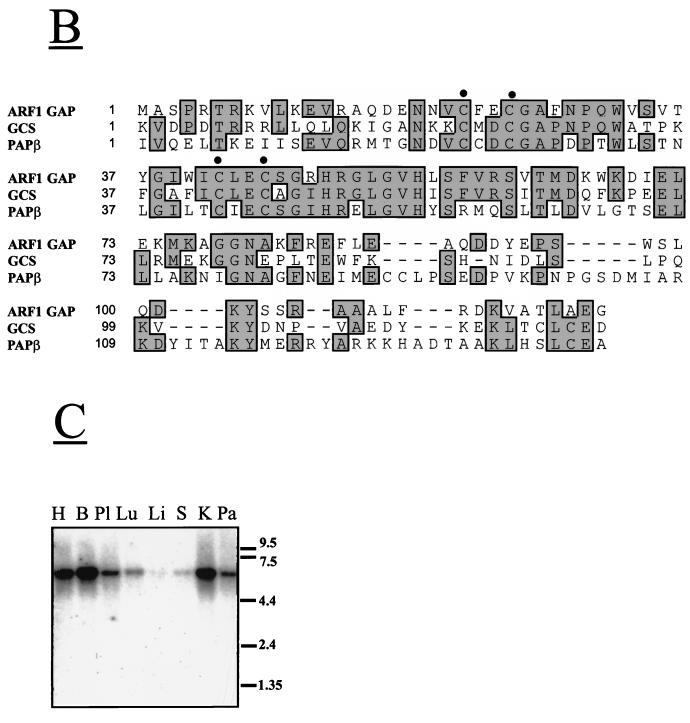
The association between Pap and Pyk2 was confirmed by Far Western blot analysis by using a GST fusion protein containing the proline-rich region and the SH3 domain of Pap as a probe for examining direct binding to Pyk2. The experiment presented in Fig. 2A demonstrates that this region of Pap binds specifically to Pyk2 and to the kinase-negative Pyk2 mutant PKM.
FIG. 2.
Interaction between Pyk2 and Pap in vitro, in cultured cells, and in brain tissue. (A) Lysates from 293 cells transfected with expression vectors for Pyk2 or PKM or with vector alone were subjected to SDS-PAGE immediately (total lysate) or after immunoprecipitation (IP) with anti-Pyk2 antibodies, then transferred to nitrocellulose filters, and blotted with GST fusion protein containing the proline-rich region and the SH3 domain of Pap (3 μg/ml) and anti-GST monoclonal antibodies (1:1,000) (upper panel). The same filter was reprobed with anti-Pyk2 antibodies (lower panel). About 0.5 mg of total protein was used per immunoprecipitation experiment. Endogenous Pyk2 protein could not be detected with anti-Pyk2 antibodies under these conditions. Arrows mark the Pyk2 and PKM. Positions of standard protein markers (in kilodaltons) are indicated on the right. (B) Lysates from 293 cells transfected with expression vectors for Pyk2-HA and Papβ, with expression vectors for PKM-HA and Papβ, or with Papβ and Pyk2-HA alone were immunoprecipitated (IP) with anti-HA, anti-Pap, or anti-Pyk2 antibodies. Immunoprecipitates were subjected to immunoblotting (IB) with anti-HA, anti-Pyk2, or anti-Pap antibodies. About 5 mg of total protein was used per immunoprecipitation experiment. Arrows mark the Pyk2/PKM or Papβ. Positions of standard protein markers (in kilodaltons) are indicated on the right. (C) Lysates of PC12 cells infected with Papβ virus were immunoprecipitated with anti-Pyk2 antibodies and preimmune serum followed by immunoblotting (IB) with anti-Pap (left upper panels) or anti-Pyk2 (left lower panels) antibodies. Neither Pyk2 nor Pap were detected in this experiment with a preimmune serum (PI). Arrows mark the Papα or Papβ. Adult mouse brain homogenate was subjected to immunoprecipitation (IP) with anti-Pyk2 antibodies and immunoblotting (IB) with anti-Pap antibodies (right upper panel) or anti-Pyk2 antibodies (right lower panel). (D) Lysates from 293 cells transfected with expression vector for Src or with expression vectors for Papα and Src were immunoprecipitated (IP) with either preimmune (PI) or anti-Pap antibodies (PAP). Immunoprecipitations were performed either in lysis buffer or in lysis buffer supplemented with 1% Nonidet P-40, 0.5% deoxycholate, and 0.1% SDS instead of 1% Triton X-100 (SDS). Immunoprecipitates were subjected to immunoblotting (IB) with anti-Src antibodies. The arrows mark Src or the immunoglobulin G (IgG) heavy chain. Positions of standard protein markers (in kilodaltons) are indicated on the right.
We next cotransfected human 293 cells with expression vectors for Pyk2-HA and Papβ or expression vectors for PKM-HA and Papβ or each expression vector alone. The transfected cells were lysed and subjected to immunoprecipitation and immunoblotting with anti-Pap and anti-HA antibodies. The experiment presented in Fig. 2B shows that Papβ forms a complex with Pyk2 and with PKM, indicating that the association between these two proteins is independent of the kinase activity of Pyk2.
Association between Pyk2 and Pap was also detected in lysates prepared from mouse brain and from PC12 cells infected with Papβ virus (Fig. 2C). In brain lysates, anti-Pyk2 antibodies immunoprecipitated a protein that migrates in the SDS gel with an apparent molecular size of 112 kDa that was specifically recognized by anti-Pap antibodies (Fig. 2C, upper right panel). Similarly, in PC12 cells infected with Papβ virus, anti-Pyk2 antibodies immunoprecipitated both the exogenously expressed murine 90-kDa form of Papβ and the endogenous 112-kDa form of rat Papα (Fig. 2C, upper left panel).
We have noticed that the proline-rich region of Papα or Papβ contains a PPLPPRNVGK sequence that closely resembles the consensus binding site for the SH3 domain of Src (53). To test the possibility of whether Src can bind to Pap, human 293 cells were transfected with expression vectors for Src and Papα, and lysates from transfected cells were subjected to immunoprecipitation with anti-Pap antibodies followed by immunoblotting with anti-Src antibodies. The experiment presented in Fig. 2D demonstrates stable complex formation between Papα and Src in lysates from these cells.
Tyrosine phosphorylation of Pap by Pyk2 or Src kinases.
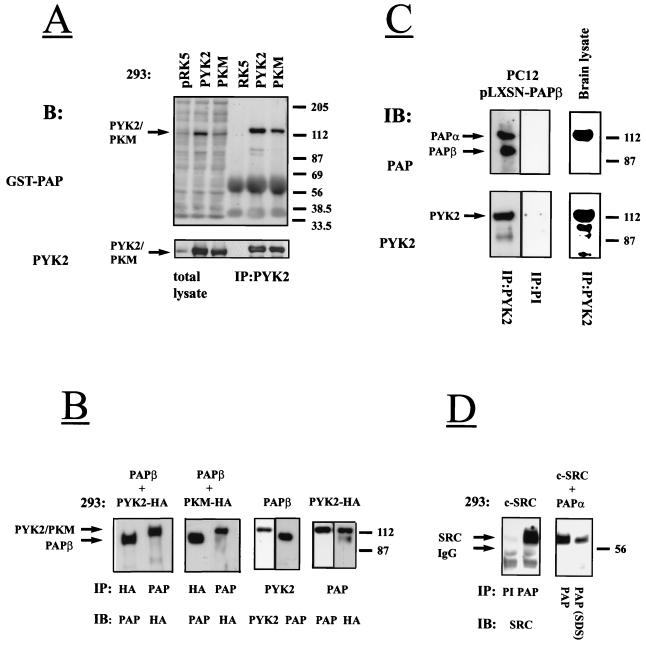
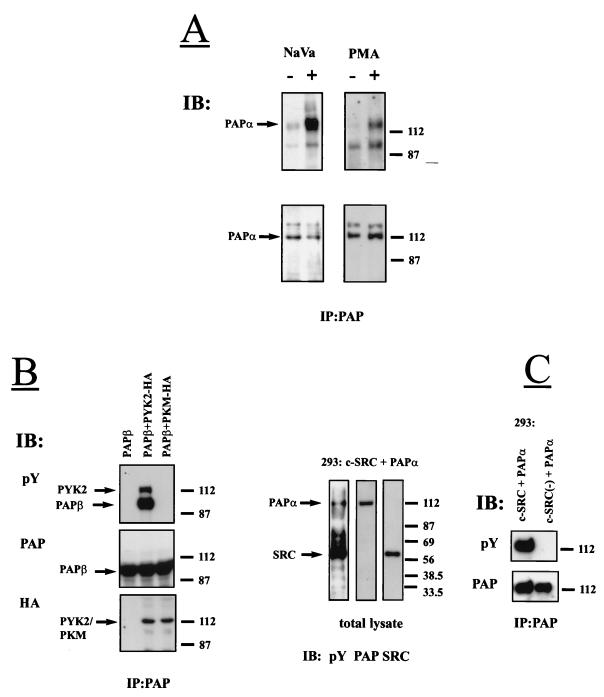
We have previously demonstrated that the phorbol ester PMA or pervanadate (NaVO3) stimulate tyrosine phosphorylation of Pyk2 in PC12 and other cell types (17, 37). We therefore examined the status of Pap phosphorylation in response to pervanadate or PMA stimulation of PC12 cells. Stimulated or unstimulated cells were lysed, subjected to immunoprecipitation with anti-Pap antibodies, and immunoblotted with antibodies against phosphotyrosine. The result of this experiment (Fig. 3A) shows that both PMA and vanadate treatment induce tyrosine phosphorylation of Papα. To further examine the possibility of whether Pap is phosphorylated by Pyk2, 293 cells were cotransfected with expression vectors for Pyk2-HA and Papβ or expression vectors for PKM-HA and Papβ. Cell lysates were subjected to immunoprecipitation with anti-Pap antibodies, and phosphorylation of Papβ on tyrosine residues was determined by immunoblotting with anti-phosphotyrosine antibodies (Fig. 3B). Analysis of Papβ immunoprecipitates of lysates prepared from cells coexpressing Papβ and Pyk2 demonstrated that the two proteins form a complex and are tyrosine phosphorylated. By contrast, Papβ was not tyrosine phosphorylated in cells expressing Papβ alone or in cells coexpressing Papβ and the kinase negative mutant of Pyk2-PKM (Fig. 3B).
FIG. 3.
Tyrosine phosphorylation of Pap by Pyk2 and by Src kinases. (A) PC12 cells were stimulated with the mixture of H2O2 and Na3VO4 (1 mM) for 20 min or with PMA (2 μM) for 10 min at 37°C. Pap was immunoprecipitated from unstimulated (−) or stimulated (+) cells, immunoblotted (IB) with anti-pY antibodies (upper panels), and reprobed with anti-Pap antibodies (lower panels). The arrows mark Papα. Positions of standard protein markers (in kilodaltons) are indicated on the right. Apart from endogenous Papα (112 kDa), anti-Pap antibodies precipitated a band with an apparent molecular size of 140 kDa (lower panels), which may represent an additional uncharacterized PAP isoform expressed in PC12 cells. (B) Lysates from 293 cells transfected with expression vectors for Papβ and Pyk2-HA, expression vectors for Papβ and PKM-HA, or expression vectors for Papβ alone were subjected to immunoprecipitation with anti-Pap antibodies and immunoblotting with anti-pY antibodies. The same filters were reprobed with anti-Pap and anti-HA antibodies. The arrows mark the Pyk2/PKM or Papβ. Positions of standard protein markers (in kilodaltons) are indicated on the right. (C) Lysates from 293 cells transfected with expression vectors for Papα and Src or expression vectors for Papα and Src(−) (kinase-negative mutant of Src) were either separated by SDS-PAGE, immunoblotted with anti-pY antibodies, and reprobed with anti-Pap or anti-Src antibodies or else subjected to immunoprecipitation with anti-Pap antibodies and immunoblotting with anti-pY and anti-Pap antibodies. The arrows mark the Papα or Src. Positions of standard protein markers (in kilodaltons) are indicated on the right.
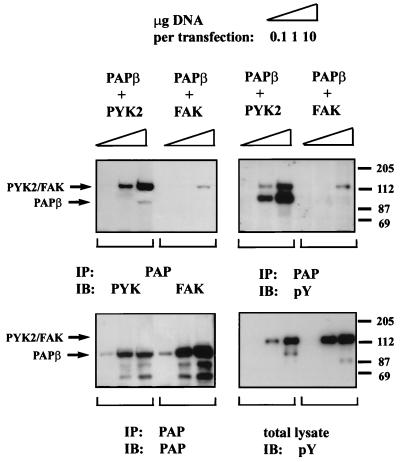
To examine the specificity of tyrosine phosphorylation by and the association between Pyk2 and Pap, 293 cells were cotransfected with Papβ and Pyk2 expression vectors or with expression vectors for Papβ and FAK. The experiment presented in Fig. 4 shows that Papβ is tyrosine phosphorylated in Pyk2-expressing cells but not in FAK-expressing cells. Upon vast overexpression, trace amounts of FAK were found in Papβ immunoprecipitates; however, no tyrosine phosphorylation of Papβ was detected. Taken together, these experiments demonstrate that Papβ is tyrosine phosphorylated by Pyk2 but not by the closely related kinase FAK.
FIG. 4.
Pap is tyrosine phosphorylated by Pyk2 but not by FAK. Human 293 cells were transfected with different amounts of expression vectors for Papβ and Pyk2 or expression vectors for Papβ and FAK. Lysates from these cells were subjected to SDS-PAGE immediately (total lysate) or after immunoprecipitation (IP) with anti-Pap antibodies and then processed for immunoblotting with anti-pY antibodies (right lower and upper panels). The right upper filter was reprobed with anti-Pyk2 or anti-FAK antibodies (left upper panel) and anti-Pap antibodies (left lower panel). The tyrosine phosphorylated 112-kDa proteins detected in the total cell lysate (right lower panel) correspond to Pyk2 and FAK, as determined by reprobing the same filter with anti-Pyk2 or anti-FAK antibodies (data not shown). The arrows mark the Pyk2, FAK, or Papβ. Positions of standard protein markers (in kilodaltons) are indicated on the right.
To examine the possibility of whether Pap is the substrate of Src, lysates from 293 cells cotransfected with Papα and Src were separated by SDS-PAGE and immunoblotted with anti-phosphotyrosine antibodies. The same filter was reprobed with anti-Src and anti-Pap antibodies. Fig. 3C shows that Src and Papα are tyrosine phosphorylated when coexpressed in 293 cells. However, tyrosine phosphorylation of Papα was not detected in lysates from 293 cells cotransfected with expression vectors for Papα and for a kinase-negative mutant of Src (Fig. 3C, right panel).
Pap protein exhibits Arf GAP activity in vitro.
The presence of an Arf GAP domain in Pap amino acid sequence (Fig. 1B) implied that Pap may activate GTP hydrolysis by Arf. The part of Pap containing the PH domain, the Arf GAP domain, and the ankyrin homology region (amino acids 111 to 522) was expressed in bacteria and tested in vitro for Arf GAP activity (51) by using Arf1, Arf5, Arf6, and Arl2 as substrates. In the presence of crude phosphoinositides containing mainly PtdIns(4,5)P2, recombinant Pap exhibited GAP activity towards Arf1 and Arf5, weaker activity towards Arf6, and no activity towards Arl2 (Fig. 5A). We also determined the effect of phospholipids upon Arf GAP activity exhibited by recombinant Pap. As shown in Fig. 5B, GAP activity was detected only in the presence of PtdIns(4,5)P2 but not in the presence of PA, PI, PS, or PC. Finally, lysates prepared from 293 cells transfected with myc-tagged Papα had approximately 100-fold-greater Arf GAP activity towards Arf1 compared to lysates prepared from cells transfected with vector alone (data not shown).
FIG. 5.
Recombinant Pap exhibits Arf GAP activity in vitro. (A) A single round of GTP hydrolysis on myristoylated Arf1 (open circles), myristoylated Arf5 (solid circles), myristoylated Arf6 (squares), and unmodified Arl2 (triangles) was measured in the presence of crude phosphoinositides (1 mg/ml) as a source of PtdIns(4,5)P2 and the indicated concentrations of recombinant Pap. GAP activity is expressed as the percentage of initially bound GTP hydrolyzed in 4 min. Myristoylated Arf1 was indistinguishable from nonmyristoylated Arf1 (data not shown). (B) A single round of GTP hydrolysis on nonmyristoylated Arf1 was measured in the presence of 1.5 nM recombinant Pap, and the indicated phospholipids are as described in Materials and Methods. None, no added phospholipid; PIP2, 90 μM phosphatidylinositol 4,5-bisphosphate; PA, 750 μM phosphatidic acid; PI, 720 μM phosphatidylinositol; PS, 720 μM phosphatidylserine; PC, 720 μM phosphatidylcholine. Error bars indicate the standard deviation.
Intracellular localization of Pap.
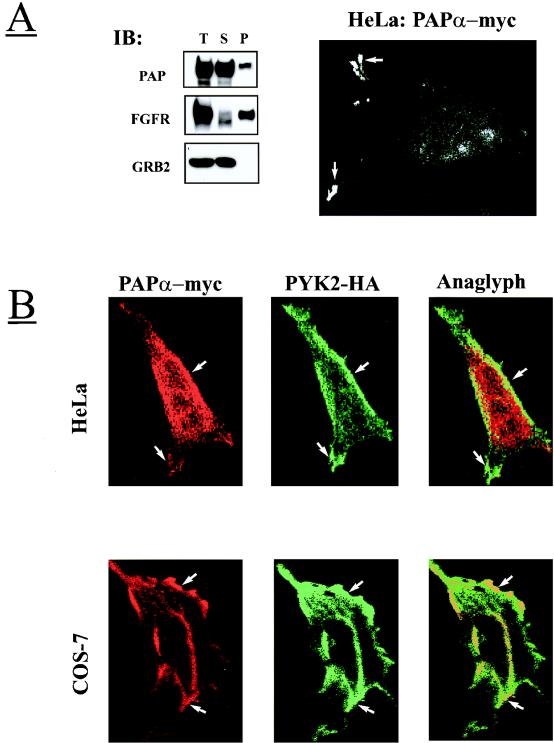
We have determined the intracellular localization of Pap by using immunofluorescence microscopy to visualize permeabilized HeLa cells overexpressing myc-tagged Papα. We were not able to determine the cellular localization of endogenous Pap by using our currently available antibodies; the experiments presented below reveal the cellular localization of exogenous Pap expressed alone or coexpressed with Pyk2 in transfected cells. Inspection of the fluorescently labeled HeLa cells showed that Papα is located in the cytoplasm and at the edge of the cells in membrane protrusions (Fig. 6A). Plasma membrane localization of Papα was further confirmed by inspecting permeabilized HeLa or COS-7 cells overexpressing Papα and Pyk2-HA by double-label immunofluorescence microscopy with anti-Pap and anti-HA antibodies, respectively. This experiment demonstrates (Fig. 6B) that in both cell lines Pyk2 and Papα are localized in the plasma membrane and in membrane protrusions. These results are consistent with a subcellular fractionation experiment demonstrating that a certain amount of overexpressed Papα protein is constantly associated with the particulate fraction (Fig. 6A).
FIG. 6.
Subcellular localization of Pap at the cell surface and in the Golgi complex. (A) 293 cells overexpressing Papα, fibroblast growth factor receptor (FGFR1, transmembrane protein), or Grb2 (cytosolic protein) were subjected to subcellular fractionation as described in Materials and Methods. Total (T), soluble (S), and particulate (P) fractions were separated by SDS-PAGE and immunoblotted (IB) with anti-Pap, anti-FGFR1, or anti-Grb2 antibodies. HeLa cells were transiently transfected with expression vector for Papα-myc. After 48 h, the cells were fixed, permeabilized, labeled with anti-myc antibodies, stained with fluorescein-conjugated anti-myc antibodies, and then examined with a confocal microscope. The arrows mark the Papα localization in the plasma membrane protrusions. (B) HeLa and COS-7 cells were transiently transfected with Papα-myc and Pyk2-HA expression vectors. After 48 h, the cells were fixed, permeabilized, and double labeled with anti-HA monoclonal antibodies and anti-Pap polyclonal antibodies, followed by staining with fluorescein-conjugated anti-mouse IgG antibodies and rhodamine-conjugated anti-rabbit IgG antibodies, and then examined by confocal fluorescence microscopy. The images were superimposed (anaglyph) to detect the areas of overlapping localization. The arrows mark the regions of Pap and Pyk2 colocalization at the plasma membrane. (C) COS-7 cells were transiently transfected with expression vectors for Papα-myc. After 48 h, cells were fixed, permeabilized, labeled with anti-myc, anti-mannosidase II, or anti-β-Cop antibodies. The cells were then stained with fluorescein-conjugated anti-mouse IgG antibodies and with rhodamine-conjugated anti-rabbit IgG antibodies and examined by confocal fluorescence microscopy. The images were superimposed (anaglyph) to detect the areas of overlapping localization. The arrows indicate the regions of Pap and mannosidase II or β-Cop colocalization in the perinuclear area.
The cytoplasmic location of Pap was further analyzed by performing double-label immunofluorescence microscopy analysis with antibodies that recognize known marker proteins. In these experiments COS-7 cells overexpressing Papα-myc were permeabilized, labeled with anti-myc antibodies, and with antibodies that recognize specific intracellular compartments. These experiments demonstrated that a population of Papα molecules is colocalized with β-Cop and mannosidase II, two specific markers of the Golgi compartment (Fig. 6C). However, while coexpressed with Pyk2 in COS-7 or HeLa cells, Pap did not colocalize with Golgi complex markers but was found at the plasma membrane. It is noteworthy that overexpression of Papα did not influence the integrity of the Golgi compartment in contrast to Arf1-GAP which, upon overexpression, causes fusion of the Golgi complex with the ER (1).
Enhancing Pap levels inhibits the generation of post-Golgi vesicles and reduces constitutive secretion.
The role of Arf1-GTP in the facilitation of vesicles budding from the TGN is well established (18, 60). The immunofluorescence localization experiments described here suggest that one of the potential sites of PAP action is in the Golgi compartment. We postulated that the recruitment of Pap to the Golgi compartment may inhibit vesicle budding by reducing the pool of Arf1-GTP associated with the TGN. To examine the possibility that Pap may function in the Golgi complex, we have utilized an in vitro system for the generation of post-Golgi vesicles from an isolated Golgi fraction prepared from VSV-infected MDCK cells (60). In this system, vesicle generation is cytosol and temperature dependent and requires a source of nucleotide triphosphates. When vesicle generation is supported by ATP, the released vesicles are 50 to 80 nm in diameter and lack coat structure (60). In this case ATP serves to generate GTP molecules required for Arf-dependent coat assembly (59). When ATP is replaced by the poorly hydrolyzable GTP analog GMP-PNP, the released post-Golgi vesicles remained coated with a non-clathrin COP-1 coat due to the fact that uncoating requires the hydrolysis of GTP bound to Arf (60).
The experiments presented in Fig. 7A and B show that in the presence of ATP, recombinant Pap inhibited vesicle generation in a concentration-dependent manner. When nucleotides were excluded from the reaction mixture no vesicle production occurred (Fig. 7C), but when GMP-PNP was used instead of ATP, Pap did not have any effect on coated vesicle production (Fig. 7D). The fact that PAP could only inhibit vesicle release when it was supported by hydrolyzable nucleotides and had no effect when the nonhydrolyzable GTP analog was used indicates that it serves as an Arf GAP that prevents the stable association of Arf with the TGN membranes.
FIG. 7.
Inhibition of generation of post-Golgi vesicles by Pap. (A) Assay mixtures containing Golgi fractions with 35S-labeled sialylated VSV-G proteins, liver cytosol proteins, and ATP were incubated at 37°C for 1 h with the indicated concentrations of recombinant Pap, and the reactions were stopped by chilling on ice. The radioactivity recovered in the supernatant after removal of the residual Golgi membranes is expressed as a percentage of the initial radioactivity in the Golgi (release of VSV-G [%]). Assay mixtures were incubated at 37°C for 1 h with (solid circles) or without (open circles) recombinant Pap (0.25 mg/ml), with liver cytosol, with (B) or without (C) ATP (1 mM), or with GMP-PNP (100 μM) (D). After incubation, the mixtures were chilled on ice, and the released vesicles were separated in a sucrose density gradient as described in Materials and Methods. The radioactivity distribution in the gradient fractions, loading zone (S), and resuspended pellet (P) is expressed as a percentage of the total VSV-G radioactivity recovered in the gradient. Uncoated vesicles (fractions 2 to 5) sedimented more slowly than coated ones (fractions 5 to 11). Points represent the averages from three independent experiments with two different Golgi and cytosolic protein preparations. Error bars represent the standard-deviation values.
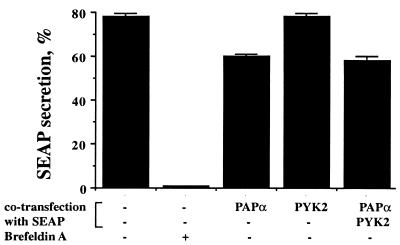
We next determined whether overexpression of Pap has an inhibitory effect on secretion in vivo, as would be expected from its effect on post-Golgi vesicle production in an in vitro assay. When expressed in transfected cells, the truncated form of placental alkaline phosphatase (SEAP) serves as a marker to assess constitutive secretion (27). This protein undergoes N-glycosylation in the Golgi apparatus, from which it is transported to the plasma membrane for release into the culture medium. The experiment presented in Fig. 8 shows that approximately 80% of the total SEAP synthesized in transiently transfected 293 cells was released into the medium after 4 h. As expected, brefeldin A treatment blocked secretion completely, indicating that this process requires Arf-GTP. However, when Pap was cotransfected with SEAP into 293 cells, a small but reproducible decrease in SEAP secretion was detected (Fig. 8). Cotransfection with Pyk2 did not affect either SEAP secretion or Pap-mediated inhibition of SEAP secretion.
FIG. 8.
Inhibition of SEAP secretion in 293 cells by overexpression of Pap. 293 cells were cotransfected with expression vectors for SEAP or with expression vectors for SEAP together with expression vector(s) for Pap or Pyk2 or both. Brefeldin A (5 μg/ml) was added to the medium for the entire duration of the assay (see Materials and Methods). The graphs depict the amount of SEAP released into the medium as a percentage of the total SEAP expressed. All experiments were done three times in triplicate. Error bars represent standard deviation values.
DISCUSSION
By using the full-length Pyk2 as a bait in a yeast two-hybrid screen, we have isolated a new Pyk2 binding protein designated Pap. Northern blot analysis with a specific probe demonstrated that Pap mRNA is most abundant in brain, kidney, and heart tissues; lower expression is detected in the placenta, lungs, and liver. Pap is a multidomain protein composed of a unique N-terminal domain, a PH domain, a zinc finger containing the Arf GAP domain, three ankyrin repeats, a proline-rich region containing potential binding sites for SH3 domains, and an SH3 domain in the carboxy terminus of the protein. The amino terminus of Pap exhibits weak homology to the α-helical sequences of myosin and kinesin. It contains predicted coiled-coil structure followed by a typical PH domain. It is now well established that the PH domains function as membrane-targeting signals and that many PH domains bind specifically to phosphoinositides. For example, the PH domain of PLCδ1 binds specifically to PtdIns(4,5)P2, and the PH domains of PKB or Grp1 bind to PtdIns(3,4,5)P3 (31). Immunofluorescence experiments demonstrated that a population of Pap molecules is localized at the plasma membrane (Fig. 6). Moreover, when the PH domain of Pap was subcloned in frame with h5′Sos construct and expressed in temperature-sensitive S. cerevisiae cdc25-2 (2, 3, 31), the yeast cells grew at nonpermissive temperatures, demonstrating that the PH domain of Pap is targeted to the cell membrane (data not shown). Although the exact ligand of the PH domain of Pap is not yet known, the PH domain is probably responsible for targeting the protein to cell membranes.
We have cloned and characterized two Papα and Papβ isoforms that differ by deletion of 45 amino acids from the proline-rich region and by 172 amino acids from the N terminus of the protein. Using Pap-specific antibodies, several immunoreactive species were identified, suggesting that additional isoforms of Pap may be generated by alternative splicing. A protein closely related to Pap termed ASAP1 was recently cloned by using Src as a bait in a yeast two-hybrid screen (9). Pap and ASAP1 contain identical structural elements and similar Arf GAP activities. However, Pap and ASAP1 exhibit different tissue expression patterns, and the two proteins contain distinct proline-rich sequences, suggesting interaction with divergent SH3 or WW domains containing signaling molecules.
We have demonstrated that a GST fusion protein containing the SH3 domain of Pap binds to Pyk2 in vitro. It appears, therefore, that the interaction between Pyk2 and Pap is mediated by binding of the SH3 domain of Pap to the proline-rich region in the C terminus of Pyk2. The proline-rich region of Papα contains four putative binding sites for SH3 domains; one of them is spliced out to generate the Papβ isoform, and one site is nearly identical to the canonical binding site for the SH3 domain of Src. Therefore, complex formation with Src is probably mediated by binding of the SH3 domain of Src to the proline-rich sequence of Pap.
We have shown that Pyk2 forms a stable complex with Pap both in vitro and in vivo and that these two proteins are localized in plasma membrane (Fig. 6). Moreover, endogenous Pap is tyrosine phosphorylated in vivo in response to stimulation with PMA, a well-known activator of Pyk2 in different cell types. In 293 cells Pap is tyrosine phosphorylated by Src or Pyk2 but not by the closely related protein tyrosine kinase FAK. Tyrosine phosphorylation of Pap by Pyk2 or by the other protein tyrosine kinases may generate binding sites for SH2 domains of signaling proteins (58); Tyr-470, for example, resides within a consensus binding site for the SH2 domain p85, the regulatory subunit of the PI-3 kinase (PXXM).
The presence of the Arf GAP domain implied that Pap may contain an intrinsic GAP activity for Arf proteins. Indeed, a recombinant protein composed of the PH domain, the Arf GAP domain, and the ankyrin homology region exhibited strong GAP activity towards Arf1 and Arf5 and weaker activity towards Arf6 (Fig. 5). The GAP activity was strictly dependent on the presence of PtdIns(4,5)P2 in the assay mixture, and a truncated protein lacking the PH domain did not have detectable GAP activity (data not shown). The PH domain, therefore, may mediate the membrane association of Pap, allowing PtdIns(4,5)P2-dependent stimulation of Arf GAP activity.
Arf1 was implicated in the control of vesicle transport in different intracellular compartments, including the Golgi complex. It has been shown that the integrity of the Golgi complex and the recruitment of coat proteins are dependent upon Arf1 activation. Overexpression of Arf1 GAP in cells caused disintegration of Golgi complex due to depletion of Arf-GTP associated with Golgi membranes (1). The immunofluorescence localization experiments presented here demonstrate that Pap is indeed localized in the Golgi compartment. Nevertheless, overexpression of Pap did not influence the integrity of the Golgi complex (Fig. 6C). To determine whether Pap possesses Arf GAP activity when recruited to Golgi membranes, we took advantage of an in vitro experimental system that allows generation of post-Golgi vesicles from an isolated Golgi fraction (60). Experiments are presented demonstrating that post-Golgi vesicle release is inhibited in the presence of recombinant Pap protein. The inhibition took place only when a hydrolyzable nucleotide was used and therefore was due to enhancement of the GTPase activity of an endogenous Arf protein associated with Golgi membranes (Fig. 7). We therefore conclude that endogeneous Arf1 proteins in Golgi membranes respond to the Arf GAP activity of Pap. Moreover, overexpression of Pap protein in 293 cells caused partial inhibition of the constitutive secretion of SEAP, indicating that Pap may also exert similar Arf GAP activity in these cells. However, Pap-mediated reduction in SEAP secretion was not influenced by the expression of Pyk2. This result is consistent with the cellular distribution of Pyk2 and Pap analyzed in cells transfected with Pap or Pyk2 expression vectors. Pap and Pyk2 are colocalized in the plasma membrane but, unlike Pap, Pyk2 is not found in the Golgi compartment. It appears therefore that the interaction between Pyk2 and Pap is restricted to the plasma membrane; Pap may interact with other tyrosine kinases in the Golgi complex.
Pyk2 was shown to be activated by various G-protein-coupled receptors, by phorbol ester, and by extracellular stimuli that elevate the intracellular Ca2+ concentration (17, 37, 40), signals that also play a role in the control of vesicular transport and Arf activity. It is not yet clear how the interaction between the protein tyrosine kinases Pyk2 and Src with Arf can regulate these processes. Nevertheless, Pap represents a new target of Pyk2 and Src, and future studies will reveal the biological role of Pap and its mode of regulation by Pyk2, Src and other proteins involved in the control of intracellular signaling processes.
ACKNOWLEDGMENT
We thank Y. Hadari for helpful discussions.
REFERENCES
- 1.Aoe T, Cukierman E, Lee A, Cassel D, Peters P J, Hsu V W. The KDEL receptor, ERD2, regulates intracellular traffic by recruiting a GTPase-activating protein for Arf1. EMBO J. 1997;16:7305–7316. doi: 10.1093/emboj/16.24.7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aronheim A. Improved efficiency Sos recruitment system: expression of the mammalian GAP reduces isolation of Ras GTPase false positives. Nucleic Acids Res. 1997;25:3373–3374. doi: 10.1093/nar/25.16.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aronheim A, Zandi E, Hennemann H, Elledge S J, Karin M. Isolation of an AP-1 repressor by a novel method for detecting protein-protein interactions. Mol Cell Biol. 1997;17:3094–3102. doi: 10.1128/mcb.17.6.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Astier A, Manie S N, Avraham H, Hirai H, Law S F, Zhang Y, Golemis E A, Fu Y, Druker B J, Haghayeghi N, Freedman A S, Avraham S. The related focal adhesion tyrosine kinase differentially phosphorylates p130Cas and the Cas-like protein, p105HEF1. J Biol Chem. 1997;272:19719–19724. doi: 10.1074/jbc.272.32.19719. [DOI] [PubMed] [Google Scholar]
- 5.Austin C D, Shields D. Formation of nascent secretory vesicles from the trans-Golgi network of endocrine cells is inhibited by tyrosine kinase and phosphatase inhibitors. J Cell Biol. 1996;135:1471–1483. doi: 10.1083/jcb.135.6.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnekow A, Jahn R, Schartl M. Synaptophysin: a substrate for the protein tyrosine kinase pp60c-src in intact synaptic vesicles. Oncogene. 1990;5:1019–1024. [PubMed] [Google Scholar]
- 7.Batzer A G, Rotin D, Urena J M, Skolnik E Y, Schlessinger J. Hierarchy of binding sites for Grb2 and Shc on the epidermal growth factor receptor. Mol Cell Biol. 1994;14:5192–5201. doi: 10.1128/mcb.14.8.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bomsel M, Mostov K. Role of heterotrimeric G proteins in membrane traffic. Mol Biol Cell. 1992;3:1317–1328. doi: 10.1091/mbc.3.12.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown M, Andrade J, Radhakrishna H, Donaldson J, Cooper J, Randazzo P. ASAP1, a phospholipid-dependent Arf GTPase-activating protein that associates with and is phosphorylated by Src. Mol Cell Biol. 1998;18:7038–7051. doi: 10.1128/mcb.18.12.7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buccione R, Bannykh S, Santone I, Baldassarre M, Facchiano F, Bozzi Y, Di Tullio G, Mironov A, Luini A, De Matteis M A. Regulation of constitutive exocytic transport by membrane receptors. A biochemical and morphometric study. J Biol Chem. 1996;271:3523–3533. doi: 10.1074/jbc.271.7.3523. [DOI] [PubMed] [Google Scholar]
- 11.Cavenagh M M, Whitney J A, Carroll K, Zhang C, Boman A L, Rosenwald A G, Mellman I, Kahn R A. Intracellular distribution of Arf proteins in mammalian cells. Arf6 is uniquely localized to the plasma membrane. J Biol Chem. 1996;271:21767–21774. doi: 10.1074/jbc.271.36.21767. [DOI] [PubMed] [Google Scholar]
- 12.Chardin P, Paris S, Antonny B, Robineau S, Beraud-Dufour S, Jackson C L, Chabre M. A human exchange factor for Arf contains Sec7-and pleckstrin-homology domains. Nature. 1996;384:481–484. doi: 10.1038/384481a0. [DOI] [PubMed] [Google Scholar]
- 13.Chen C, Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cukierman E, Huber I, Rotman M, Cassel D. The Arf1 GTPase-activating protein: zinc finger motif and Golgi complex localization. Science. 1995;270:1999–2002. doi: 10.1126/science.270.5244.1999. [DOI] [PubMed] [Google Scholar]
- 15.D’Souza-Schorey C, Li G, Colombo M I, Stahl P D. A regulatory role for Arf6 in receptor-mediated endocytosis. Science. 1995;267:1175–1178. doi: 10.1126/science.7855600. [DOI] [PubMed] [Google Scholar]
- 16.De Camilli P, Emr S D, McPherson P S, Novick P. Phosphoinositides as regulators in membrane traffic. Science. 1996;271:1533–1539. doi: 10.1126/science.271.5255.1533. [DOI] [PubMed] [Google Scholar]
- 17.Dikic I, Tokiwa G, Lev S, Courtneidge S A, Schlessinger J. A role for Pyk2 and Src in linking G-protein-coupled receptors with MAP kinase activation. Nature. 1996;383:547–550. doi: 10.1038/383547a0. [DOI] [PubMed] [Google Scholar]
- 18.Donaldson J G, Klausner R D. Arf: a key regulatory switch in membrane traffic and organelle structure. Curr Opin Cell Biol. 1994;6:527–532. doi: 10.1016/0955-0674(94)90072-8. [DOI] [PubMed] [Google Scholar]
- 19.Exton J H. New developments in phospholipase D. J Biol Chem. 1997;272:15579–15582. doi: 10.1074/jbc.272.25.15579. [DOI] [PubMed] [Google Scholar]
- 20.Faundez V, Horng J T, Kelly R B. A function for the AP3 coat complex in synaptic vesicle formation from endosomes. Cell. 1998;93:423–432. doi: 10.1016/s0092-8674(00)81170-8. [DOI] [PubMed] [Google Scholar]
- 21.Feng S, Chen J K, Yu H, Simon J A, Schreiber S L. Two binding orientations for peptides to the Src SH3 domain: development of a general model for SH3-ligand interactions. Science. 1994;266:1241–1247. doi: 10.1126/science.7526465. [DOI] [PubMed] [Google Scholar]
- 22.Foster-Barber A, Bishop J M. Src interacts with dynamin and synapsin in neuronal cells. Proc Natl Acad Sci USA. 1998;95:4673–4677. doi: 10.1073/pnas.95.8.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frank S, Upender S, Hansen S H, Casanova J E. ARNO is a guanine nucleotide exchange factor for ADP-ribosylation factor 6. J Biol Chem. 1998;273:23–27. doi: 10.1074/jbc.273.1.23. [DOI] [PubMed] [Google Scholar]
- 24.Galisteo M L, Chernoff J, Su Y C, Skolnik E Y, Schlessinger J. The adaptor protein Nck links receptor tyrosine kinases with the serine-threonine kinase Pak1. J Biol Chem. 1996;271:20997–21000. doi: 10.1074/jbc.271.35.20997. [DOI] [PubMed] [Google Scholar]
- 25.Gietz R D, Schiestl R H, Willems A R, Woods R A. Studies on the transformation of intact yeast cells by the LiAc/SS-DNA/PEG procedure. Yeast. 1995;11:355–360. doi: 10.1002/yea.320110408. [DOI] [PubMed] [Google Scholar]
- 26.Goda Y, Sudhof T C. Calcium regulation of neurotransmitter release: reliably unreliable? Curr Opin Cell Biol. 1997;9:513–518. doi: 10.1016/s0955-0674(97)80027-0. [DOI] [PubMed] [Google Scholar]
- 27.Gorr S. Differential storage of prolactin, granins (chromogranin B and secretogranin II), and constitutive secretory markers in rat pituitary GH4C1 cells. J Biol Chem. 1996;271:3575–3580. doi: 10.1074/jbc.271.7.3575. [DOI] [PubMed] [Google Scholar]
- 28.Hadari Y R, Kouhara H, Lax I, Schlessinger J. Binding of Shp2 tyrosine phosphatase to FRS2 is essential for FGF-induced PC12 cell differentiation. Mol Cell Biol. 1998;18:3966–3973. doi: 10.1128/mcb.18.7.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Helms J B, Rothman J E. Inhibition by brefeldin A of a Golgi membrane enzyme that catalyses exchange of guanine nucleotide bound to Arf. Nature. 1992;360:352–354. doi: 10.1038/360352a0. [DOI] [PubMed] [Google Scholar]
- 30.Ireland L S, Johnston G C, Drebot M A, Dhillon N, DeMaggio J A, Hoekstra M F, Singer R A. A member of a novel family of “Zn-finger” proteins mediates the transition from stationary phase to cell proliferation. EMBO J. 1994;13:3812–3821. doi: 10.1002/j.1460-2075.1994.tb06692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Isakoff S J, Cardozo T, Andreev J, Aronheim A, Lemmon M, Skolnik E Y. Identification and analysis of PH domain containing 3-phosphoinositide binding proteins using a novel in vivo assay in yeast. EMBO J. 1998;17:5374–5387. doi: 10.1093/emboj/17.18.5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janz R, Sudhof T C. Cellugyrin, a novel ubiquitous form of synaptogyrin that is phosphorylated by pp60c-src. J Biol Chem. 1998;273:2851–2857. doi: 10.1074/jbc.273.5.2851. [DOI] [PubMed] [Google Scholar]
- 33.Kahn R A, Gilman A G. The protein cofactor necessary for ADP ribosylation of Gs by cholera toxin is itself a GTP binding protein. J Biol Chem. 1986;261:7906–7911. [PubMed] [Google Scholar]
- 34.Kahn R A, Kern F G, Clark J, Gelmann E P, Rulka C. Human ADP-ribosylation factors. A functionally conserved family of GTP binding proteins. J Biol Chem. 1991;266:2606–2614. [PubMed] [Google Scholar]
- 35.Kahn R A, Terui T, Randazzo P A. Effects of acid phospholipids on Arf activities: potential roles in membrane traffic. J Lipid Mediat Cell Signal. 1996;14:209–214. doi: 10.1016/0929-7855(96)00527-5. [DOI] [PubMed] [Google Scholar]
- 36.Klarlund J K, Rameh L E, Cantley L C, Buxton J M, Holik J J, Sakelis C, Patki V, Corvera S, Czech M P. Regulation of GRP1-catalyzed ADP ribosylation factor guanine nucleotide exchange by phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:1859–1862. doi: 10.1074/jbc.273.4.1859. [DOI] [PubMed] [Google Scholar]
- 37.Lev S, Moreno H, Martinez R, Canoll P, Peles E, Musacchio J M, Plowman G D, Rudy B, Schlessinger J. Protein tyrosine kinase Pyk2 involved in Ca2+-induced regulation of ion channel and MAP kinase functions. Nature. 1995;376:737–745. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- 38.Li J, Avraham H, Rogers R A, Raja S, Avraham S. Characterization of RAFTK, a novel focal adhesion kinase, and its integrin-dependent phosphorylation and activation in megakaryocytes. Blood. 1996;88:417–428. [PubMed] [Google Scholar]
- 39.Li W, Hu P, Skolnik E Y, Ullrich A, Schlessinger J. The SH2 and SH3 domain-containing Nck protein is oncogenic and a common target for phosphorylation by different surface receptors. Mol Cell Biol. 1992;12:5824–5833. doi: 10.1128/mcb.12.12.5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li X, Earp H S. Paxillin is tyrosine-phosphorylated by and preferentially associates with the calcium-dependent tyrosine kinase in rat liver epithelial cells. J Biol Chem. 1997;272:14341–14348. doi: 10.1074/jbc.272.22.14341. [DOI] [PubMed] [Google Scholar]
- 41.Linstedt A D, Vetter M L, Bishop J M, Kelly R B. Specific association of the proto-oncogene product pp60c-src with an intracellular organelle, the PC12 synaptic vesicle. J Cell Biol. 1992;117:1077–1084. doi: 10.1083/jcb.117.5.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Makler V, Cukierman E, Rotman M, Admon A, Cassel D. ADP-ribosylation factor-directed GTPase-activating protein. Purification and partial characterization. J Biol Chem. 1995;270:5232–5237. doi: 10.1074/jbc.270.10.5232. [DOI] [PubMed] [Google Scholar]
- 43.Meacci E, Tsai S C, Adamik R, Moss J, Vaughan M. Cytohesin-1, a cytosolic guanine nucleotide-exchange protein for ADP-ribosylation factor. Proc Natl Acad Sci USA. 1997;94:1745–1748. doi: 10.1073/pnas.94.5.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mitchell R, McCulloch D, Lutz E, Johnson M, MacKenzie C, Fennell M, Fink G, Zhou W, Sealfon S C. Rhodopsin-family receptors associate with small G proteins to activate phospholipase D. Nature. 1998;392:411–414. doi: 10.1038/32937. [DOI] [PubMed] [Google Scholar]
- 45.Neet K, Hunter T. Vertebrate nonreceptor protein-tyrosine kinase families. Genes Cells. 1996;1:147–169. doi: 10.1046/j.1365-2443.1996.d01-234.x. [DOI] [PubMed] [Google Scholar]
- 46.Ohashi M K, Jan deVries R, Frank R, Snoek G, Bankaitis V, Wirtz K, Huttner W B. A role for phosphatidylinositol transfer protein in secretory vesicle formation. Nature. 1995;377:544–547. doi: 10.1038/377544a0. [DOI] [PubMed] [Google Scholar]
- 47.Poon P P, Wang X, Rotman M, Huber I, Cukierman E, Cassel D, Singer R A, Johnston G C. Saccharomyces cerevisiae Gcs1 is an ADP ribosylation factor GTPase activating protein. Proc Natl Acad Sci USA. 1996;93:10074–10077. doi: 10.1073/pnas.93.19.10074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Radhakrishna H, Donaldson J G. ADP-ribosylation factor 6 regulates a novel plasma membrane recycling pathway. J Cell Biol. 1997;139:49–61. doi: 10.1083/jcb.139.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Randazzo P A. Functional interaction of ADP-ribosylation factor 1 with phosphatidylinositol 4,5-bisphosphate. J Biol Chem. 1997;272:7688–7692. [PubMed] [Google Scholar]
- 50.Randazzo P A. Resolution of two ADP-ribosylation factor 1 GTPase-activating proteins from rat liver. Biochem J. 1997;324:413–419. doi: 10.1042/bj3240413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Randazzo P A, Kahn R A. GTP hydrolysis by ADP-ribosylation factor is dependent on both an ADP-ribosylation factor GTPase-activating protein and acid phospholipids. J Biol Chem. 1994;269:10758–10763. [PubMed] [Google Scholar]
- 52.Randazzo P A, Weiss O, Kahn R A. Preparation of recombinant ADP-ribosylation factor. Methods Enzymol. 1992;219:362–369. doi: 10.1016/0076-6879(92)19036-6. [DOI] [PubMed] [Google Scholar]
- 53.Rickles R J, Botfield M C, Zhou X M, Henry P A, Brugge J S, Zoller M J. Phage display selection of ligand residues important for Src homology 3 domain binding specificity. Proc Natl Acad Sci USA. 1995;92:10909–10913. doi: 10.1073/pnas.92.24.10909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rothman J E, Orci L. Molecular dissection of the secretory pathway. Nature. 1992;355:409–415. doi: 10.1038/355409a0. [DOI] [PubMed] [Google Scholar]
- 55.Sabatini D D, Adesnik M, Ivanov I E, Simon J P. Mechanism of formation of post-Golgi vesicles from TGN membranes: Arf-dependent coat assembly and PKC-regulated vesicle scission. Biocell. 1996;20:287–300. [PubMed] [Google Scholar]
- 56.Schlaepfer D D, Hunter T. Integrin signaling and tyrosine phosphorylation: just the FAKs? Trends Cell Biol. 1998;8:151–157. doi: 10.1016/s0962-8924(97)01172-0. [DOI] [PubMed] [Google Scholar]
- 57.Schlessinger J. Cellular signaling by receptor tyrosine kinases. Harvey Lectures Ser. 1995;89:105–123. [PubMed] [Google Scholar]
- 58.Schlessinger J. SH2/SH3 signaling proteins. Curr Opin Gen Dev Biol. 1994;4:25–30. doi: 10.1016/0959-437x(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 59.Simon J P, Ivanov I E, Adesnik M, Sabatini D D. The production of post-Golgi vesicles requires a protein kinase C-like molecule, but not its phosphorylating activity. J Cell Biol. 1996;135:355–70. doi: 10.1083/jcb.135.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Simon J P, Ivanov I E, Shopsin B, Hersh D, Adesnik M, Sabatini D D. The in vitro generation of post-Golgi vesicles carrying viral envelope glycoproteins requires an Arf-like GTP-binding protein and a protein kinase C associated with the Golgi apparatus. J Biol Chem. 1996;271:16952–16961. doi: 10.1074/jbc.271.28.16952. [DOI] [PubMed] [Google Scholar]
- 61.Smith D B, Johnson K S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 62.Stenberg P E, Pestina T I, Barrie R J, Jackson C W. The Src family kinases, Fgr, Fyn, Lck, and Lyn, colocalize with coated membranes in platelets. Blood. 1997;89:2384–2393. [PubMed] [Google Scholar]
- 63.Thomas S M, Brugge J S. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]