Abstract
Polycystic ovarian syndrome (PCOS), the most common endocrinopathy affecting women worldwide, is characterized by elevated luteinizing hormone (LH) pulse frequency due to the impaired suppression of gonadotrophin-releasing hormone (GnRH) release by steroid hormone negative feedback. Although neurons that co-express kisspeptin, neurokinin B, and dynorphin (KNDy cells) were recently defined as the GnRH/LH pulse generator, little is understood about their role in the pathogenesis of PCOS. We used a prenatal androgen-treated (PNA) mouse model of PCOS to determine whether changes in KNDy neurons or their afferent network underlie altered negative feedback. First, we identified elevated androgen receptor gene expression in KNDy cells of PNA mice, whereas progesterone receptor and dynorphin gene expression was significantly reduced, suggesting elevated androgens in PCOS disrupt progesterone negative feedback via direct actions upon KNDy cells. Second, we discovered GABAergic and glutamatergic synaptic input to KNDy neurons was reduced in PNA mice. Retrograde monosynaptic tract-tracing revealed a dramatic reduction in input originates from sexually dimorphic afferents in the preoptic area, anteroventral periventricular nucleus, anterior hypothalamic area and lateral hypothalamus. These results reveal 2 sites of neuronal alterations potentially responsible for defects in negative feedback in PCOS: changes in gene expression within KNDy neurons, and changes in synaptic inputs from steroid hormone-responsive hypothalamic regions. How each of these changes contribute to the neuroendocrine phenotype seen in in PCOS, and the role of specific sets of upstream KNDy afferents in the process, remains to be determined.
Keywords: PCOS, GnRH, luteinizing hormone, KNDy, prenatal androgen, mouse
Polycystic ovarian syndrome (PCOS) is estimated to affect up to 20% of women of reproductive age worldwide and is the most common cause of anovulatory infertility (1, 2). The cardinal features of PCOS include hyperandrogenemia, menstrual dysfunction, and cystic ovaries, with 2 out of 3 symptoms required for diagnosis (3, 4). Up to 75% of PCOS patients present with elevated circulating luteinizing hormone (LH) levels (5), which reflect an increase in the frequency and amplitude of pulsatile LH release from the pituitary gland (6-10). At the ovary, LH hypersecretion and a correlating reduction in follicle stimulating-hormone secretion promotes thecal cell production of androgen secretion and arrests follicle development in the preovulatory stage to create the classic cystic feature of the disease (11-14). LH secretion mirrors gonadotropin-releasing hormone (GnRH) release from GnRH neurons that reside in the hypothalamus (15, 16), suggesting that GnRH pulse frequency is increased in PCOS patients.
Compared with control women, PCOS patients have an impaired ability for estradiol and progesterone to lower LH pulse frequency, indicating central alterations which impair the ability of steroid hormones to restrain GnRH/LH pulse frequency (17, 18). Antagonism of the androgen receptor with flutamide is able to restore the sensitivity of the GnRH pulse generator to steroid hormones in PCOS patients, indicating that hyperandrogenism underlies impaired steroid hormone negative feedback (19). As GnRH neurons themselves do not express steroid hormone receptors (20-24), androgen interference with negative feedback is likely located in steroid hormone–sensitive upstream neuronal populations.
Although multiple neuronal populations have been implicated in the regulation of GnRH neurons, cells in the arcuate nucleus of the hypothalamus (ARC) that co-express the stimulatory peptides kisspeptin and neurokinin B and the inhibitory neurotransmitter dynorphin, termed KNDy cells, have recently been subject to intense interest as a central target for therapeutics to treat PCOS and other reproductive disorders (25-31). KNDy cells are highly steroid hormone–sensitive, as the vast majority express receptors required for estrogen and progesterone negative feedback (32-36). Estradiol suppresses kisspeptin expression in multiple species, which is consistent with reduced excitatory drive to GnRH neurons, and evidence from sheep strongly indicates that progesterone negative feedback is mediated by KNDy cells through dynorphin and kappa opioid receptor (KOR) signaling (37-41). Importantly, anatomical and functional studies in animal models support KNDy cells as the GnRH pulse generator. A current hypothesis states that synchronized NKB activation of KNDy cells by reciprocally connected KNDy cells drives kisspeptin activation of GnRH neurons to initiate the GnRH/LH pulse (42, 43). Subsequent release of dynorphin by KNDy neurons may stop the GnRH/LH pulse through inhibition of KNDy and/or GnRH neurons (44-46). Presynaptic KNDy populations that are sensitive to external and internal cues, including stress, steroid hormone, and metabolic signals, likely allow for the regulation of LH pulse amplitude and frequency over different physiological and pathological conditions (47-49). However, potential changes in KNDy neuron physiology that may impair restraint of the GnRH/LH pulse generator by steroid hormones in PCOS patients is largely unclear. To assess this, animal models are essential to investigate specific neuronal circuits.
Although the etiology of PCOS is likely multifactorial (50), prenatal androgen (PNA) exposure has been linked to development of the reproductive and metabolic symptoms of PCOS in women (51-53) and drives impaired steroid hormone feedback control of pulsatile LH release in the primate, sheep, rat, and mouse (54, 55). However, there are variable reports on PNA exposure impacting KNDy peptides in the brain of animal models (56-60), and the sensitivity of this population to steroid hormone feedback remains largely unassessed. In addition, anatomical assessment of KNDy neurons in the PNA sheep revealed that synaptic input from gamma-aminobutyric acid (GABA)ergic and glutamatergic neurons to KNDy cells is altered (61, 62), implicating potential changes in the regulation of KNDy neurons from afferent populations. The regions and nuclei where these inputs arise are currently unknown. However, mouse models are amenable to transgenic tools that permit identification of specific neuronal circuits controlling KNDy neurons. Therefore, the present study utilized the PNA-induced PCOS mouse model, which displays the neuroendocrine phenotype of impaired estradiol and progesterone negative feedback of pulsatile LH release (63, 64), to assess whether alterations to KNDy circuitry underlie impaired negative feedback in PCOS. First, we addressed whether PNA treatment in mice directly alters steroid hormone receptor and neurochemical gene expression in KNDy cells in a manner consistent with impaired steroid hormone negative feedback. Second, this study aimed to determine whether synaptic input to KNDy cells is altered by PNA treatment in mice, as seen in PNA sheep. Finally, we used Cre-dependent rabies-mediated viral tract-tracing tools to identify the specific brain nuclei with altered synaptic input to KNDy cells in PNA mice.
Materials and Methods
Animals
All mice were bred and housed in the Kent State University animal facility on a 12-hour light/dark cycle and given access to food and water ad libitum. Experimental procedures in mice were conducted from 50 days of age. All experimental protocols and procedures were approved by Kent State University Institutional Animal Care and Use Committee under protocol 475 LC 1819 and conform to guidelines outlined by the United States National Institutes of Health for animal research. Heterozygous Kiss1-Cre mice, in which Cre-recombinase expression is driven by Kiss1 regulatory elements (65). Breeding pairs (kindly donated by Dr. Carol Elias, JAX mice, stock #023426) were crossed with either C57Bl/6J mice, B6.129X1-Gt(ROSA)26Sortm1(EYFP)Cos/J enhanced yellow fluorescent protein (YFP) floxed-stop reporter mice (JAX mice, stock #006148) or B6.Cg-Gt-(ROSA)26Sortm9(CAG-tdTomato)Hze/J floxed-stop reporter mice (JAX mice, stock #007907) to generate Kiss1-Cre, Kiss1-Cre/YFP or Kiss1-Cre/tdTomato male and female mice, respectively.
Prenatal androgen-induced PCOS-like mice were generated as described previously (63). Adult female mice were paired with males and checked for copulatory plugs to indicate day 1 of gestation. At embryonic day 16, 17, and 18, pregnant dams received 100 µL subcutaneous injections of either sesame oil vehicle alone (prenatal vehicle [PNV] controls) or containing 250 µg of dihydrotestosterone (DHT, Sigma-Aldrich) (PNA mice). PNV male and female offspring and PNA female offspring were studied in postnatal life from 60 to 90 days of age. PNA male offspring were not included, as prior research has revealed no reproductive deficits in these animals (66). Characterization of the reproductive and neuroendocrine deficits in the PCOS mouse model have been described previously and match the PCOS phenotype of infertility and acylicity, altered ovarian morphology, increased LH pulse frequency, elevated GnRH neuron activity, and impaired progesterone and estrogen negative feedback of LH secretion (63, 64).
To ensure that PNA-induced PCOS-like mice generated here replicate previous characterization of the model, the vaginal cytology of a separate group of Kiss1-Cre± PNV (n = 6) and PNA (n = 6) mice from those used in the studies below was assessed daily for 4 weeks from 50 days of age to confirm that the phenotype of acyclicity persists in our hands. The number of days spent in proestrus, estrus, and metestrus/diestrus was calculated and expressed as a percentage of the total cycle.
Multiplex Fluorescent In Situ Hybridization
RNAscope HiPlex (ACD Bio-techne, Newark, CA, USA) fluorescent in situ hybridization, which permits detection of 12 different RNA targets in the same slide-mounted tissue, was used to assess the expression of ARC neuropeptides and receptors that mediate negative feedback control of pulsatile LH secretion in prenatal vehicle male (n = 5), PNV female (n = 4), and PNA female (n = 6) C57BL/6J mice (Fig. 1A). Adult female mice were perfused in diestrus, as determined using vaginal cytology. Mice were deeply anesthetized using intraperitoneal injection of pentobarbital (3 mg/mL) before transcardial perfusion with 4% paraformaldehyde (PFA). Brains were extracted and incubated at 4 °C for 24 hours in the same fixative. Brains were then sunk in 10%, 20%, and 30% sucrose in 0.1M phosphate-buffered saline (PBS), rapidly frozen in optimal cutting temperature (OCT) compound (FisherScientific) and stored at −80 °C until cryosectioning coronal sections onto superfrost charged slides at 12-µm thickness. Sections were stored at −80 °C until use.
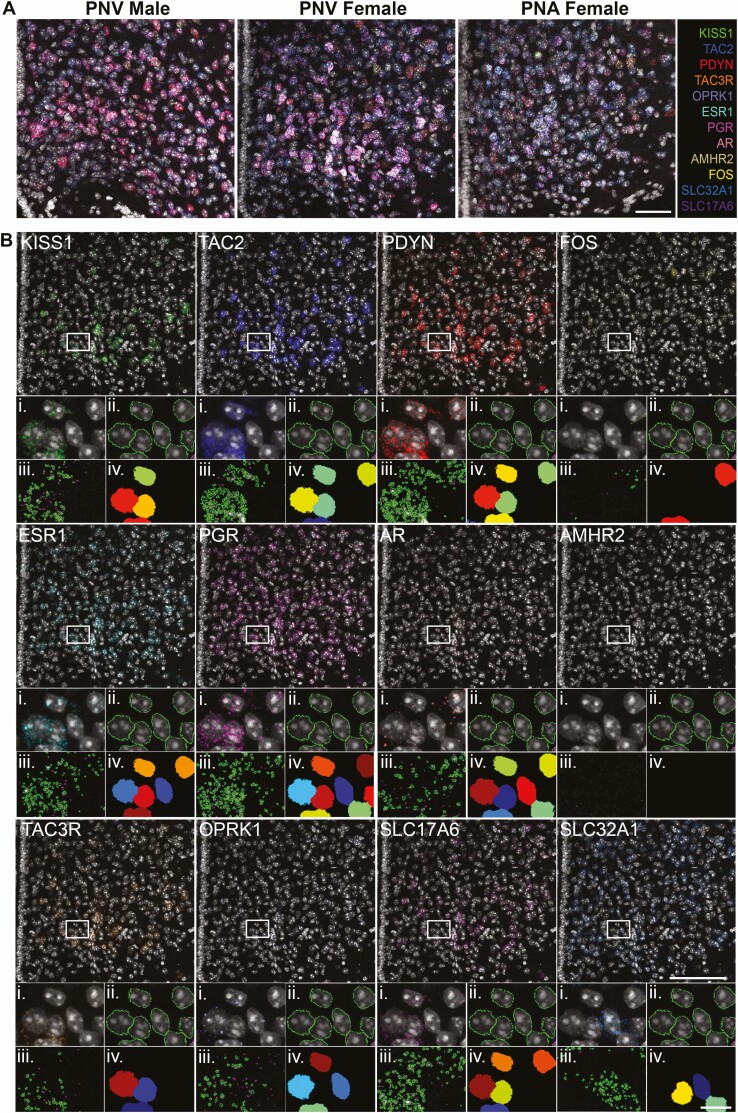
Figure 1.
Multiplex detection of RNA expression in the arcuate nucleus of control and prenatal androgen-treated mice. A, Confocal images of 12 RNA targets visualized simultaneously in representative sections from the arcuate nucleus (ARC) of PNV male, PNV female, and PNA female mice using RNAscope HiPlex fluorescent in situ hybridization. Scale bar = 100 µm. B, Representative confocal section of the ARC from a PNV female mouse containing labeling for Dapi and RNA transcripts for KISS1, TAC2, PDYN, FOS, ESR1, PGR, AR, AMRH2, TAC3R, OPRK1, SLC17A6 (vGluT2), and SLC32A1 (vGaT). Scale bar = 100 μm. (i) High magnification images of insets containing representative cells depicting automated detection of Dapi borders (ii) and RNA transcripts (iii) by Cellprofiler software. Signal that did not meet the criteria for RNA transcripts are represented by purple outlines. (iv) Dapi cells (randomly pseudocolored by CellProfiler software) containing at least 3 RNA puncta (Scale bar in inset = 10μm).
The assay was performed following manufacturer’s instructions (67). Briefly, sections were fixed in 4% PFA for 15 minutes, dehydrated with 50%, 70%, and 100% ethanol for 5 minutes each before treatment with protease reagent at 99 to 100 °C. Following tissue pretreatment, all RNA targets were simultaneously hybridized and amplified, followed by detection, imaging, and cleaving in groups of 3 targets. To achieve this, sections were incubated with the 12 pooled HiPlex probes and amplified with the solutions provided. Probes were provided by ACDBiotechne and designed to detect kisspeptin (KISS1, Cat# 500141), neurokinin B (TAC2, Cat# 446391), prodynorphin (PDYN, Cat# 318771), tachykinin receptor 3 (TAC3R, Cat# 481671), kappa opioid receptor (OPRK1, Cat# 316111), estrogen receptor (ESR1, Cat# 478201), progesterone receptor (PGR, Cat# 318921), androgen receptor (AR, Cat# 316991), anti-Müllerian hormone receptor 2 (AMHR2, Cat# 489821), vesicular glutamate transporter 2 (vGluT2, SLC17A6, Cat# 319171), vesicular GABA transporter (SLC32A1, vGaT, Cat# 319191), and a marker of neuronal activation (FOS, Cat# 316921). Cleavable versions of fluorophores AF488, Atto550, and Atto647 were applied to sections to target 3 probes at a time. Cell nuclei were counterstained using Dapi (ACDBiotechne) and slides were coverslipped using ProLong Gold Antifade Mountant (FisherScientific, Cat# P36930). After imaging each round using confocal microscopy (detailed below), samples were treated with a sodium citrate solution to remove coverslips, and fluorophores were removed using an RNAscope cleaving solution before incubation with the next round of fluorophores and reimaging using confocal microscopy in the same brain region. The process of cleaving fluorophores, applying new fluorophores to the next set of amplified probes and imaging using confocal microscopy was repeated until all 12 RNA targets had been imaged.
Stereotaxic Injection of Viral Vectors
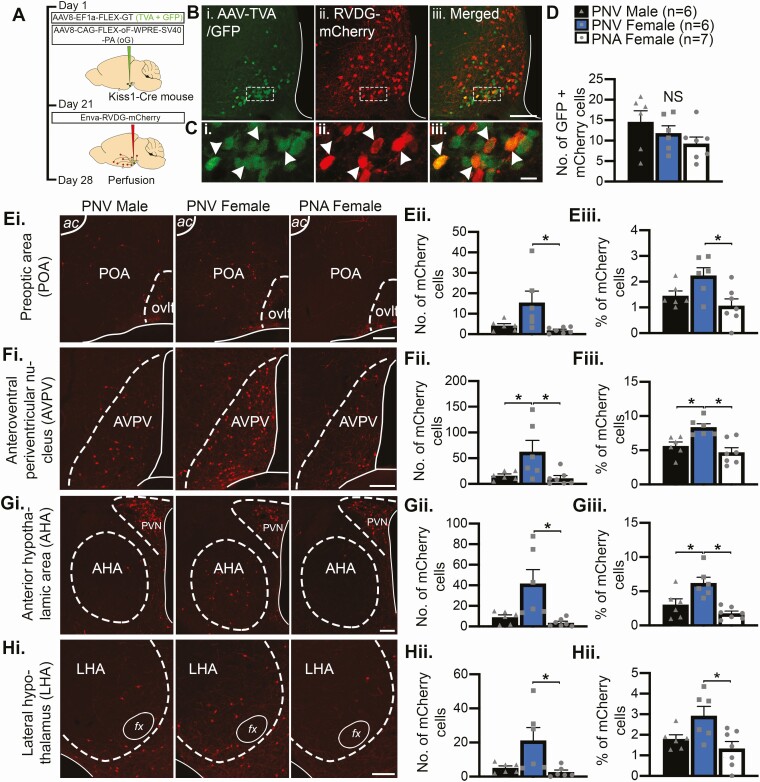
Adeno-associated viruses (AAVs) AAV8-EF1a-FLEX-GT (AAV-TVA/GFP, 1.86E+12 plaque forming units [pfu]/mL) and AAV8-CAG-FLEX-oG-WPRE-SV40-PA (AAV-oG, optimized rabies glycoprotein 8.91E+13 pfu/mL) and EnvA glycoprotein-Deleted Rabies-mCherry virus (RVDG, 3.78E+07 pfu/mL) were prepared and purified by the Gene Transfer Targeting and Therapeutics Core at the Salk Institute of Biological Studies (La Jolla, CA). Kiss1-Cre± and wild-type Kiss1-Cre-/- mice were anesthetized with isoflurane (2%) and placed in a stereotaxic frame (Stoelting Co. IL, USA). Using a Drill and Microinjection Robot (Neurostar, Tubingen, Germany), a small hole was drilled into the skull 1 mm posterior to bregma and 0.3 mm lateral to midline. A 29-gauge cannula attached to a 2.5 µL Hamilton syringe was loaded with a cocktail containing AAV-TVA/GFP:AAV-oG (25:75 µl) and slowly lowered 5.8 mm ventral to dura into the unilateral ARC. The needle was left in situ for 5 minutes before viral vectors were injected at a rate of 100 nL/minute. Following injection, syringes were left in situ for 10 minutes and the needle slowly removed. Three weeks later, mice were again anesthetized and placed in a stereotaxic frame for the injection of RVDG (400 nL) in the same coordinates. Seven days following RVDG injection, mice were given an overdose of pentobarbital (3 mg/mL, intraperitoneal), vaginal cytology was collected from females to determine estrous cycle stage, and mice perfused transcardially with 4% PFA (Fig. 4A). Kiss1-Cre±/tdTomato± mice were injected as described above with AAV-TVA/GFP and AAV-oG, but not RVDG-mCherry, and perfused transcardially with 4% PFA 3 weeks after viral injection when in diestrus (n = 5 females).
Figure 4.
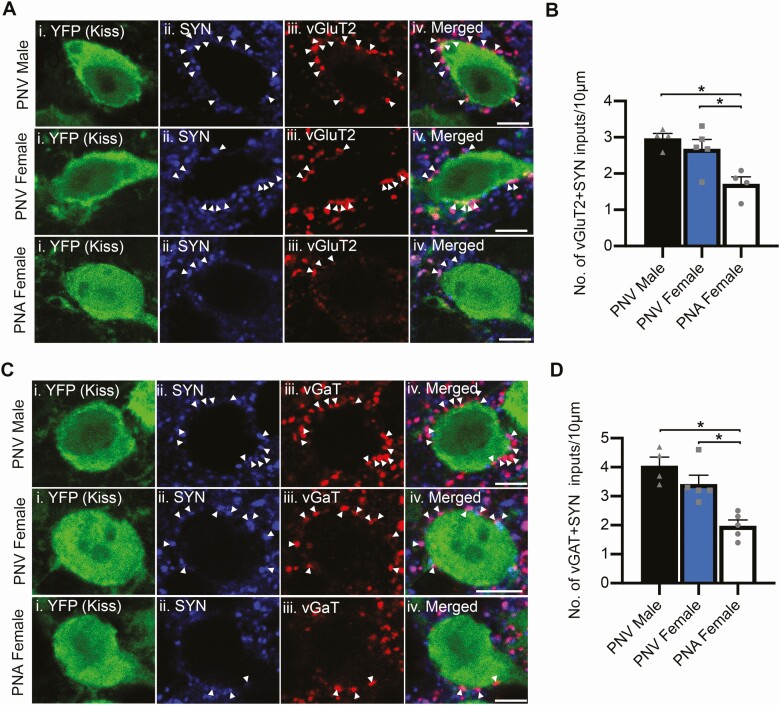
Glutamatergic and GABAergic synaptic input to KNDy neurons is reduced in PNA mice. A, C, Representative confocal images of sections triple labeled for YFP in ARC kisspeptin (Kiss) cells, (i), synaptophysin (SYN, ii) and either vGluT2 (glutamatergic inputs, Aiii) or vGaT (GABAergic inputs, Ciii) in the middle ARC of PNV male, PNV female, and PNA female mice. Arrowheads indicate synaptic terminals containing glutamate (VGLUT2 + SYN, A) or GABA (vGAT + SYN, C). B, D, Graphs depicting the mean ± SEM density of synaptic (SYP-positive) contacts immunoreactive for vGluT2 (B) or vGaT (D). *P < 0.05. Scale bars = 5 µm.
Immunofluorescence
Brains were fixed for a further hour in 4% PFA following perfusion fixation before being sunk in 20% sucrose. Brains were then cut into 3 parallel series of coronal sections at 30-µm thickness using a freezing microtome. For free-floating immunofluorescent histochemistry, all tissue was initially washed in 0.1M PBS for a minimum of 4 hours. To assess glutamatergic and GABAergic input to KNDy cells in Kiss1-Cre/eYFP mice, tissue underwent 10-minute washes for 3 hours in 0.1M PBS containing 0.4% Triton-X-100 to increase permeability of the tissue to synaptic markers. The sections were next incubated in an antibody incubation solution (0.1% bovine serum albumin [Thermo Fisher Scientific] and 0.4% Triton-X 100 in 0.1M PBS) for 1 hour before incubation for 17 hours in antibody incubation solution containing mouse anti-synaptophysin (1:200, Sigma, Catalog no. S5768 (68)), chicken anti-green fluorescent protein (GFP) to enhance YFP in cells (1:2000, Aves Laboratories, Catalog no. GFP-1020 (69)) and either rabbit anti-vesicular glutamate transporter 2 (vGluT2, 1:750, Synaptic Systems, Catalog no. 135402 (70)) or rabbit anti-GABA (vGaT, 1:750, Synaptic Systems, Catalog no. 131-003 (71)). Sections were washed in PBS and incubated in antibody solution containing Dylight donkey anti-chicken 488 (1:200), Dylight donkey anti-rabbit 550 (1:100), and Dylight goat anti-mouse 647 (1:100) secondary antibodies for 30 minutes before final washes in 0.1M phosphate-buffer (PB).
To enhance endogenous GFP and mCherry in sections transfected with the trio of AAV and RVDG vectors, sections were incubated in antibody solution for 1 hour before a 17-hour incubation in antibody solution containing rabbit anti-mCherry (1:4000, Abcam, Cat AB167453, (72)) and chicken anti-GFP (1:2000, Aves Laboratories). Sections were washed in PBS and incubated for 30 minutes in Dylight donkey anti-chicken 488 (1:200) and Dylight donkey anti-rabbit 550 (1:100) before final washes in 0.1M PB. All tissue was mounted onto superfrost charged slices, air dried, and coverslipped using an aqueous mounting medium (Gelvatol (73); containing the antifade agent 1,4-diazabicyclo(2,2)octane (Sigma-Aldrich; 50 mg/mL).
Image Acquisition and Analysis
RNA transcript imaging and analysis following HiPlex fluorescent in situ hybridization
Upon incubation with each round of fluorophores, 2 sections containing the middle arcuate nucleus were imaged per animal using an Olympus FV3000 confocal microscope. A 20× objective was used to enable imaging of the entire ARC area. Optical sections with a 2-µm step size were acquired using Dapi, 488 nm, 550 nm, and 647 nm channels. Confocal files were converted to TIFFS and images from all rounds of staining were registered to each other using HiPlex image registration software to assess up to 12 gene targets per cell. For unbiased, automated quantification of 12 genes in the same tissue, we modified a previously published pipeline (74) in Cellprofiler software (75) that was originally developed to quantify RNAscope transcripts for 3 gene targets (Fig. 1B). Cellprofiler software identified cells via Dapi (Fig. 1Bii), and RNA transcripts were identified when above background intensity, as defined by negative control sections, and a dot diameter equal or greater than 3 pixels and less than 30 pixels (Fig. 1Biii). Within the entire image, Cellprofiler software quantified the number of overlying RNA transcripts for each gene within the border of Dapi labeling. A cell was deemed to express a target gene when 3 or more transcripts overlayed Dapi (Fig. 1Biv). Using these rules, the pipeline was configured to automatically quantify the number of cells expressing each gene in the ARC (Table 1), the percentage of Kiss1, non-kisspeptinergic vGaT and non-kisspeptinergic vGluT2 cells colocalized with the other genes (Table 2), and the average number of RNA transcripts overlying Kiss1, non-kisspeptinergic vGaT and non-kisspeptinergic vGluT2 cells (Table 3).
Table 1.
Mean ± SEM number of cells containing RNA transcripts in the ARC of PNV male (n = 5), PNV female (n = 4), and PNA female (n = 6) mice
| AMHR2 | AR | ESR1 | FOS | KISS1 | OPRK1 | PDYN | PGR | VGLUT2 | VGAT | TAC2 | TAC3R | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PNV Male | 1.9 ± 0.2 | 247.9 ± 25.3 | 185.4 ± 40.7 | 79.0 ± 12.8 | 39.0 ± 7.7 | 141.3 ± 15.4 | 184.4 ± 36.2 | 334.3 ± 42.9 | 209.9 ± 23.4 | 318.0 ± 36.2 | 43.1 ± 8.4 | 47.1 ± 5.5 |
| PNV Female | 2.1 ± 0.7 | 222.6 ± 10.7 | 246.5 ± 20.8 | 80.9 ± 22.6 | 63.5 ± 10.1 | 112.4 ± 6.3 | 185.7 ± 16.8 | 362.0 ± 27.7 | 182.2 ± 19.6 | 338.7 ± 16.2 | 72.4 ± 9.5 a | 70.4 ± 10.7 |
| PNA Female | 1.8 ± 0.3 | 308.7 ± 19.4 b | 249.9 ± 14.1 | 61.9 ± 9.2 | 56.9 ± 3.4 | 214.9 ± 26.5 b | 198.1 ± 14.9 | 365.4 ± 26.0 | 213.0 ± 16.1 | 353.1 ± 16.9 | 72.7 ± 4.2 a | 80.4 ± 9.1 |
Abbreviations: ARC, arcuate nucleus; PNA, prenatal androgen-treated; PNV, prenatal vehicle; SEM, standard error of the mean.
a significantly different vs PNV male;
b significantly different vs PNV female
Table 2.
Mean ± SEM percentage of kisspeptinergic, non-kisspeptinergic VGLUT2 cells and non-kisspeptinergic VGAT cells containing RNA transcripts in the ARC of PNV male (n = 5), PNV female (n = 4), and PNA female (n = 6) mice
| AMHR2 | AR | ESR1 | FOS | OPRK1 | PDYN | PGR | VGLUT2 | VGAT | TAC2 | TAC3R | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| KISS1 cells | PNV Male | 0.5 ± 0.2 | 88.5 ± 1.9 b | 92.5 ± 2.3 | 15.4 ± 5.1 | 64.4 ± 3.2 | 93.1 ± 2.1 | 97.4 ± 0.8 | 95.7 ± 1.4 | 48.0 ± 4.8 | 80.0 ± 1.8 b | 72.3 ± 5.0 |
| PNV Female | 0.5 ± 0.3 | 58.2 ± 4.4 | 95.8 ± 1.0 | 15.9 ± 3.2 | 58.5 ± 5.4 | 93.7 ± 2.1 | 97.2 ± 0.9 | 90.5 ± 4.5 | 53.4 ± 4.4 | 90.1 ± 1.7 | 81.5 ± 1.4 | |
| PNA Female | 0.8 ± 0.7 | 83.2 ± 2.3 b | 95.7 ± 1.2 | 15.0 ± 1.6 | 79.9 ± 2.7 a,b | 91.0 ± 1.4 | 91.7 ± 1.5 a,b | 88.2 ± 2.7 | 51.7 ± 4.7 | 91.3 ± 1.7 a | 86.0 ± 2.6 a | |
| VGLUT2 cells | PNV Male | 0.6 ± 0.2 | 59.5 ± 3.4 | 41.3 ± 5.4 | 17.5 ± 2.5 | 35.0 ± 5.1 | 49.6 ± 7.1 | 78.4 ± 3.1 | N/A | 44.5 ± 4.7 | 6.8 ± 1.4 | 7.9 ± 1.4 |
| PNV Female | 1.1 ± 0.3 | 59.8 ± 1.7 | 53.9 ± 5.4 | 19.0 ± 4.1 | 24.2 ± 3.3 | 51.7 ± 3.5 | 76.1 ± 3.8 | N/A | 43.3 ± 4.3 | 7.2 ± 0.9 | 7.6 ± 1.4 | |
| PNA Female | 0.5 ± 0.1 | 66.8 ± 2.6 | 44.2 ± 3.2 | 16.5 ± 1.6 | 44.4 ± 5.8 b | 48.4 ± 3.0 | 72.9 ± 3.4 | N/A | 41.8 ± 5.5 | 7.6 ± 1.0 | 12.8 ± 3.5 | |
| VGAT cells | PNV Male | 0.2 ± 0.1 | 46.8 ± 3.5 | 38.7 ± 6.0 | 18.9 ± 2.7 | 28.1 ± 5.1 | 30.7 ± 3.6 | 74.3 ± 3.1 | 26.7 ± 44 | N/A | 2.4 ± 0.1 | 3.1 ± 0.2 |
| PNV Female | 0.3 ± 0.03 | 44.3 ± 3.0 | 49.3 ± 3.1 | 18.1 ± 4.9 | 19.9 ± 1.5 | 26.7 ± 1.9 | 72.0 ± 4.2 | 18.0 ± 3.5 | N/A | 3.8 ± 0.6 | 4.6 ± 1.5 | |
| PNA Female | 0.3 ± 0.1 | 55.9 ± 2.7 | 48.8 ± 2.7 | 14.8 ± 1.2 | 36.3 ± 6.1 | 28.1 ± 1.2 | 71.9 ± 3.5 | 20.6 ± 2.6 | N/A | 4.0 ± 0.4 | 5.6 ± 1.1 |
Abbreviations: ARC, arcuate nucleus; N/A, not assessable; PNA, prenatal androgen-treated; PNV, prenatal vehicle; SEM, standard error of the mean.
a significantly different vs PNV male,
b significantly different vs PNV female.
Table 3.
Mean ± SEM number of RNA transcripts in kisspeptinergic, non-kisspeptinergic VGLUT2 and non-kisspeptinergic VGAT cells in the ARC of PNV male (n = 5), PNV female (n = 4), and PNA female (n = 6) mice
| AMHR2 | AR | ESR1 | FOS | KISS1 | OPRK1 | PDYN | PGR | VGLUT2 | VGAT | TAC2 | TAC3R | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| KISS1 cells | PNV male | 8.0 ± 1.2 | 9.8 ± 0.8 b | 13.6 ± 0.6 b | 6.4 ± 0.7 | 9.4 ± 0.5 | 8.2 ± 0.2 | 23.3 ± 0.6 | 15.0 ± 0.5 | 19.5 ± 0.9 | 12.8 ± 0.1 | 15.2 ± 0.6 | 10.4 ± 0.6 |
| PNV female | 4.0 ± 0.8 | 6.0 ± 0.1 | 17.0 ± 0.8 | 7.1 ± 0.3 | 11.9 ± 0.9 | 7.1 ± 0.3 | 20.1 ± 1.2 | 18.0 ± 1.2 | 17.0 ± 1.3 | 12.6 ± 0.8 | 18.2 ± 0.8 | 11.3 ± 0.9 | |
| PNA female | 4.4 ± 0.4 | 8.4 ± 0.3 b | 16.5 ± 0.6a | 6.1 ± 1.0 | 11.7 ± 0.6 | 11.6 ± 1.0 a,b | 15.4 ± 0.6 a,b | 12.9 ± 0.8 a,b | 14.2 ± 0.4 | 12.9 ± 0.6 | 18.3 ± 0.6 | 13.0 ± 0.8 | |
| VGLUT2 cells | PNV Male | 4.2 ± 0.3 | 7.3 ± 0.7 | 8.1 ± 0.5 | 10.3 ± 1.0 | N/A | 6.5 ± 0.2 | 12.9 ± 1.4 | 10.3 ± 0.6 | 13.0 ± 0.9 | 14.8 ± 0.6 | 7.2 ± 1.0 | 7.4 ± 0.9 |
| PNV Female | 3.3 ± 0.1 | 6.9 ± 0.3 | 10.1 ± 0.7 | 9.4 ± 1.2 | N/A | 7.1 ± 0.5 | 11.6 ± 0.7 | 11.2 ± 0.7 | 11.5 ± 0.6 | 14.5 ± 0.3 | 7.3 ± 0.7 | 6.9 ± 0.8 | |
| PNA Female | 3.3 ± 0.2 | 7.7 ± 0.4 | 9.9 ± 0.3 | 7.8 ± 0.4 | N/A | 7.1 ± 0.3 | 11.0 ± 0.5 | 8.7 ± 0.4 b | 12.1 ± 0.7 | 15.5 ± 0.2 | 8.7 ± 1.0 | 7.2 ± 1.0 | |
| VGAT cells | PNV Male | 4.2 ± 0.9 | 6.1 ± 0.3 | 7.5 ± 0.6 | 10.0 ± 0.6 | N/A | 6.3 ± 0.2 | 9.9 ± 0.8 | 8.9 ± 0.4 | 10.6 ± 0.2 | 14.8 ± 0.6 | 6.9 ± 0.7 | 6.1 ± 0.8 |
| PNV Female | 3.3 ± 0.1 | 5.6 ± 0.2 | 8.9 ± 0.6 | 9.2 ± 0.6 | N/A | 6.6 ± 0.3 | 8.3 ± 0.2 | 9.8 ± 0.3 | 9.3 ± 0.7 | 14.5 ± 0.3 | 6.9 ± 0.5 | 5.5 ± 0.2 | |
| PNA Female | 3.4 ± 0.2 | 6.4 ± 0.2 | 8.9 ± 0.3 | 7.1 ± 0.4 a | N/A | 6.7 ± 0.3 | 8.1 ± 0.1 a | 8.0 ± 0.5 b | 9.8 ± 0.8 | 15.5 ± 0.2 | 7.2 ± 0.5 | 6.1 ± 0.7 |
Abbreviations: ARC, arcuate nucleus; N/A, not assessable; PNA, prenatal androgen-treated; PNV, prenatal vehicle; SEM, standard error of the mean.
a significantly different vs PNV male,
b significantly different vs PNV female. N/A = not assessable.
vGluT2 and vGAT synaptic appositions with KNDy cells
Light microscopy image acquisition of glutamatergic and GABAergic synapses to KNDy cells was performed using an Olympus FV3000 confocal microscope. In 2 sections from each of the rostral, middle, and caudal regions of the ARC, 1-µm optical sections of YFP-expressing Kiss1 cells were collected using a 60× objective. Thirty cells were analyzed per animal (10 cells/arcuate region). The perimeter of the soma was measured across optical sections and expressed as an average per cell, per animal, and per group. Synaptic terminals apposing KNDy cells containing synaptophysin (SYP) and SYP with glutamate (vGluT2 + SYN) or GABA (vGaT + SYN) were counted when no black pixels existed between YFP and the synaptic marker. The density of synaptic contacts was expressed as the number of contacts per 10 µm of soma perimeter.
Rabies-mediated tract-tracing
Brain-wide mapping of RVDG-mCherry cells was conducted using epifluorescent microscopy (DM500B, Lecia Microsystems) and a digital camera (Microfire A/R; Optronics) paired with MicroBrightField Neurolucida Software (Williston, Vermont USA) to permit rapid imaging of brain series. For whole-brain mapping of afferents to KNDy neurons, cells positive for mCherry were quantified in 2 representative brain sections per nuclei per animal using Image J software. In the ARC, mCherry-labeled cells were separated into mCherry only and mCherry + GFP neurons. To normalize for differences in viral transfection, the number of mCherry-positive cells in each brain nuclei were expressed as a percentage of the total mCherry-positive cells quantified across the brain. In all analyses, brain regions were determined by anatomical landmarks and brain nuclei defined using a region-of-interest tool in ImageJ. Brain nuclei were based on the Mouse Brain Atlas in Stereotaxic Coordinates by Franklin and Paxinos, second edition (76).
To analyze co-expression of AAV-TVA/GFP with either Kiss1-Cre/tdTomato or RVDG-mCherry, confocal z-stacks of 1-µm thick optical sections were imaged using a 20× objective with 1.5× zoom in 2 sections per rostral, middle, and caudal ARC regions. Confocal Z-stacks of 1-µm thick optical sections were captured through the ARC. The number of GFP-positive cells, tdTomato or mCherry-positive cells, and GFP cells and colocalized with either tdTomato or mCherry were counted. The percentage of tdTomato-positive cells colocalized with GFP and the percentage of GFP-positive cells colocalized with tdTomato or mCherry was calculated.
Statistical Analysis
One-way ANOVAs with Tukey post hoc tests were used to analyze statistical significance between PNV male, PNV female, and PNA female mice. In the RVDG-tract-tracing study, the number of mCherry cells counted in PNV male, PNV female, and PNA female mice were compared using the Kruskal-Wallis nonparametric test due to variability in the number of mCherry transfected cells in PNV female mice. In all analyses, experimenters were blinded to experimental group and statistical comparisons were made using Prism, Graphpad. All data is reported as the mean ± standard error of the mean (SEM) per group.
Results
PNA Treatment Alters Steroid Hormone Receptor and Peptide Expression in KNDy Neurons
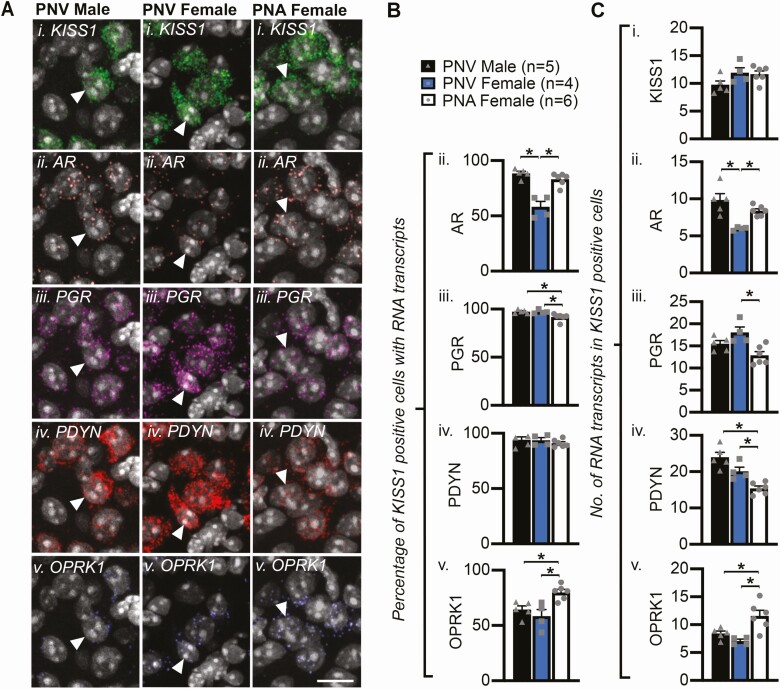
Assessment of vaginal cytology daily for 4 weeks confirmed that PNA mice exhibited a significant increase in the percentage of the cycle spent in diestrus compared with PNV female controls (PNA = 76.9 ± 6.8%, PNV = 40.9 ± 3.1%, P < 0.05), a significant reduction in the percentage of the cycle spent in estrus compared with female controls (PNA = 24.7 ± 4.4% vs PNV = 42.5 ± 2.7%, P < 0.05), and the complete abolishment of proestrus (PNA = 0 ± 0%, PNV = 16.7 ± 1.0%, P < 0.05). These results mirror previous characterization of acyclicity induced by PNA treatment (63). RNAscope HiPlex fluorescent in situ hybridization revealed that the number of AR-expressing cells within the ARC was significantly elevated in PNA mice compared with control PNV females (Table 1). Increased androgen receptor (AR) was apparent within ARC KISS1 neurons, as a significant male-dominant sexual dimorphism (P < 0.05) in the percentage of KISS1 cells expressing AR was lost following PNA treatment in female mice (Fig. 2Bii, P < 0.05). Similarly, PNA treatment significantly elevated the average number of AR RNA transcripts within KISS1 cells in female mice to levels equivalent to control males (Fig. 2Cii, P < 0.05). Conversely, a small but significant reduction in the percentage of KISS1 cells expressing PGR was detected in PNA mice compared with control groups (Fig. 2Biii, P < 0.05), and a significant reduction in the average number of PGR RNA transcripts in KISS1 cells was detected in PNA mice when compared with female controls (Fig. 2Ciii, P < 0.05). In KISS1 cells from PNA mice that display elevated AR and reduced PGR gene expression, the average number of PDYN RNA transcripts was significantly lower compared with both male (P < 0.05) and female (P < 0.05) control groups (Fig. 2Civ). Finally, PNA treatment significantly elevated the number of ARC cells containing the kappa opioid receptor (OPRK1), which has high affinity for dynorphin, compared with PNV females (Table 1, P < 0.05). The percentage of KISS1 cells containing OPRK1 (Fig. 2B v), as well as the number of OPRK1 transcripts in KISS1 cells, was significantly elevated in PNA mice when compared with control groups (Fig. 2Cv, P < 0.05). Surprisingly, despite support for the role of anti-Müllerian hormone (AMH) in the prenatal origins of PCOS (77), very little RNA for the AMH receptor (AMHR2) was detected in KNDy neurons or other cells in the ARC (Fig. 1, Tables 1-3).
Figure 2.
Androgen receptor RNA is increased whereas progesterone and dynorphin RNA is reduced in ARC KISS1 (KNDy) cells in PNA mice. A, Confocal images of representative cells in the ARC (identified by DAPI, white) containing RNA transcripts for KISS1 (i), AR (ii), PDYN (iii), PGR (iv), and OPRK1 (v). Arrows depict examples of KISS1-positive cells co-expressing AR, PGR, DYN, and OPRK1 transcripts. Scale bars = 10 µm. B, Mean percentage ± SEM of KISS1 neurons expressing AR (ii), PGR (iii), PDYN (iv), and OPRK1 (v) transcripts. C, Mean number ± SEM of KISS1 (i), AR (ii), PGR (iii), PDYN (iv), and OPRK1 (v) RNA transcripts in KISS1 cells. *P < 0.05.
PNA Treatment Reduces Progesterone Receptor Expression in Non-KNDy ARC Glutamate and GABA Neurons
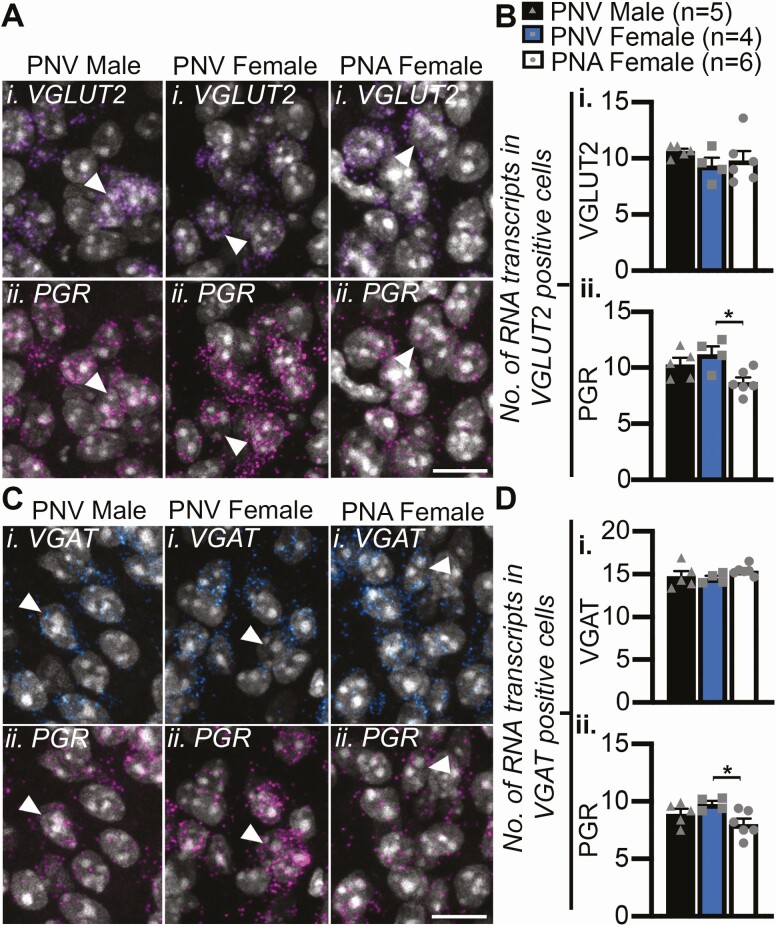
In addition to the observed changes in gene expression within ARC KISS1 neurons, changes in steroid hormone receptor gene expression were recorded in non-KISS1 cells in the ARC following PNA treatment. The average number of PGR RNA transcripts was significantly reduced in PNA female mice compared with male (P < 0.05) and female (P < 0.05) PNV control groups in both VGLUT2- (Fig. 3A-3B) and VGAT- (Fig. 3C-3D) expressing cells that were not colocalized with KISS1. Furthermore, the percentage of vGluT2 cells containing OPRK1 was significantly higher in PNA females compared with PNV controls (Table 2, P < 0.05). However, an increase in AR expression within these populations did not reach significance (Table 2, 3).
Figure 3.
PNA treatment reduces PGR expression in non-KNDy ARC glutamatergic and GABAergic cells following PNA treatment. A, C, Confocal images of representative cells in the ARC (identified by DAPI, white) containing RNA transcripts for VGLUT2 (Ai) or vGAT (Ci) and PGR (Aii, Cii). Arrows depict examples of VGLUT2-positive cells co-expressing PGR transcripts. B, Mean number ± SEM of VGLUT2 (i) and PGR (ii) transcripts in glutamatergic cells. D, Mean number ± SEM of VGAT (i) and PGR (ii) transcripts in GABAergic cells. *P < 0.05. Scale bars = 10 μm.
Glutamatergic and GABAergic Synaptic Input to KNDy Neurons Is Reduced in PNA Mice
Somal circumference of KNDy neurons, as visualized using EYFP reporter expression in KISS1-positive cells in the ARC, was not significantly different between PNV male (95.9 ± 9.4 µm), PNV female (101.6 ± 7.3 µm) and PNA female (102.4 ± 6.8 µm) mice. The density of synaptic input to KNDy cells, as identified through closely apposed synaptophysin (SYP) puncta to KNDy soma, was nearly halved in PNA mice (3.8 ± 0.36 inputs/10 µm of somal circumference) in comparison with PNV male (6.7 ± 0.45 SYP inputs/10 µm of somal circumference, P < 0.05) and PNV female (7.0 ± 0.64 inputs/10 µm of somal circumference, P < 0.05) controls. Reduced synaptic input originated from both glutamatergic and GABAergic cells, as the density of SYP puncta closely apposed to KNDy soma that were colocalized with either vGluT2 (Fig. 4A-4B) or vGaT (Fig. 4C-4D) was significantly lower in PNA mice compared with male and female PNV control groups (P < 0.05, both measurements). No significant differences in glutamatergic or GABAergic synaptic input to KNDy cells was observed between male and female control mice.
Retrograde Monosynaptic Tract-Tracing Reveals Reduced Synaptic Input Originates From Preoptic and Hypothalamic Nuclei in PNA Mice
To identify the location of altered synaptic input to KNDy cells in PNA mice, rabies-mediated retrograde monosynaptic tract-tracing was performed as previously described (47). The specificity of the Cre-dependent AAV-TVA/GFP vector, which infers infection of the glycoprotein-deleted rabies virus (RVDG-mCherry) to Cre-positive cells, was characterized through transfection of AAV vectors into the ARC of female Kiss1-Cre mice crossed onto floxed tdTomato reporter mice (Kiss1-Cre/tdTomato). In doing so, 45.8% ± 9.6% of Kiss1-Cre/tdTomato cells were transfected by AAV-TVA/GFP and 92.6% ± 2.3% of AAV-TVA/GFP neurons were colocalized with tdTomato, indicating up to half of the kisspeptin population was targeted by AAVs and the vast majority of AAV transfection was specific to Cre-positive kisspeptin cells, respectively. In wild-type female mice injected with all viral vectors (n = 4), no GFP-positive cells were observed and only 5 mCherry-positive cells were detected in total across the brain of all animals, supporting the concept that Cre is required for mCherry expression.
Stereotaxic injection of AAV and RVDG-mCherry viral vectors into the ARC of PNV- and PNA-treated Kiss1-Cre mice was performed to quantify presynaptic KNDy cells (Fig. 5A). In the ARC, the total number of Kiss1-Cre (presumptive KNDy) cells transfected with both AAV and RVDG viral vectors (GFP + mCherry) was not significantly different between groups (Fig. 5B-5D). Although this indicates that the number of KNDy cells initially transfected by the rabies virus (starter cells) was also not significantly different between groups, it is possible that GFP + mCherry ARC cells also represent reciprocally connected KNDy cells transfected with AAV-TVA/GFP. Therefore, to normalize potential differences in the number of starter cells between animals, mCherry transfections in each brain nuclei were reported as both the average number of mCherry cells and as a percentage of the total number of mCherry cells in the brain.
Figure 5.
Reduced synaptic input to KNDy neurons in PNA mice originates from preoptic and hypothalamic nuclei. A, Illustration of the rabies virus tract-tracing workflow. B, Representative confocal images of Kiss1-Cre cells in the ARC transfected with AAV-TVA/GFP (i), RVDG-mCherry (ii), and the merged images (iii). Scale bar = 100 µm. C, High magnification insets from Bi-Biii with examples of cells that co-express AAV-TVA/GFP and RVDG-mCherry (arrowheads), which represent either starter cells or presynaptic KNDy inputs to KNDy neurons. Scale bar = 10 µm. D, Graph illustrating no significant difference (NS) in the mean ± SEM number of GFP + mCherry cells transfected in the ARC of PNV male, PNV female, and PNA female mice. Scale bars = 100 µm. E-I, (i) Representative images of mCherry-positive cells (presynaptic neurons to KNDy cells) in the preoptic area (POA, E), anteroventral periventricular nucleus (AVPV, F), anterior hypothalamic area (AHA, G) and lateral hypothalamus (LHA, H). E-H, Graphs depicting the mean ± SEM number of mCherry-positive cells in afferent areas (ii), and the distribution of mCherry cells as shown by the mean ± SEM percentage of total mCherry cells in each brain nuclei (iii). *P < 0.05.
For all experimental groups, mCherry cells were observed in the same brain regions reported previously in nontreated male and female mice (47) and are reported in Table 4. PNV female mice showed a greater number of mCherry cells (1478 ± 428 cells) across the whole brain compared with PNV males (631 ± 108 cells) and PNA females (464 ± 170 cells), although this did not reach significance. Quantification of mCherry labeling in afferent nuclei revealed the average number of mCherry cells in the preoptic area (POA, Fig. 5Eii), anteroventral periventricular nucleus (AVPV, Fig. 5Fii), anterior hypothalamic area (AHA, Fig. 5Gii) and lateral hypothalamus (LHA, Fig. 5Hii) was significantly higher in PNV females compared with PNA females (P < 0.05). The percentage of total mCherry cells located in the POA (Fig. 5Eiii), AVPV (Fig. 5Fiii), AHA (Fig. 5Giii) and LHA (Fig. 5Hiii) was also significantly higher in PNV female compared with PNA female mice (P < 0.05), indicating that it is unlikely that the difference between groups was due to an overall higher number of mCherry cells in the brain of PNV female mice. The percentage of mCherry cells in the AVPV (Fig. 5Fiii) and AHA (Fig. 5Giii) of PNV females was also significantly higher compared with PNV males (P < 0.05). In the ARC, quantification of non-GFP expressing mCherry cells revealed that the percentage of total afferent input to KNDy neurons originating from local afferent inputs was significantly higher in PNA females compared with both PNV male and female control mice (Table 4, P < 0.05). However, no differences in mCherry cell numbers were recorded in the ARC between PNV male (221.8 ± 41.7), PNV female (342.7 ± 70.8), and PNA female (213.6 ± 65.1) mice. Therefore, this elevated percentage likely reflects the reduction in input occurring from non-ARC preoptic and hypothalamic nuclei in PNA female mice.
Table 4.
Distribution of RVDG-mCherry afferents to KNDy cells, as expressed by the mean ± SEM percentage of total mCherry cells, in PNV male (n = 6), PNV female (n = 6), and PNA female (n = 7) mice in the brain
| Brain region | PNV male | PNV female | PNA female |
|---|---|---|---|
| Septal nucleus | 1.5 ± 0.3 | 1.6 ± 0.3 | 1.3 ± 0.5 |
| Bed nucleus of the stria terminalis | 1.5 ± 0.2 | 1.7 ± 0.3 | 1.0 ± 0.1 |
| Preoptic area | 1.5 ± 0.2 | 2.2 ± 0.3 | 1.1 ± 0.3 b |
| Ventromedial preoptic nucleus | 1.9 ± 0.3 | 1.7 ± 0.3 | 0.8 ± 0.2 a |
| Organum vasculosum of the stria terminalis | 1.3 ± 0.3 | 0.7 ± 0.2 | 0.7 ± 0.3 |
| Medial preoptic nucleus | 6.9 ± 1.1 | 5.4 ± 0.7 | 4.2 ± 0.8 |
| Anteroventral periventricular nucleus | 5.6 ± 0.6 | 8.4 ± 0.5a | 4.7 ± 0.7 b |
| Periventricular nucleus | 1.5 ± 0.2 | 2.9 ± 0.4 | 2.2 ± 0.6 |
| Paraventricular nucleus | 6.3 ± 1.1 | 4.1 ± 0.4 | 4.2 ± 1.2 |
| Lateroanterior hypothalamic nucleus | 1.3 ± 0.2 | 1.7 ± 0.2 | 1.1 ± 0.3 |
| Anterior hypothalamic area | 3.1 ± 0.8 | 6.2 ± 0.9 a | 1.8 ± 0.3 b |
| Ventromedial hypothalamus | 3.1 ± 0.5 | 3.7 ± 0.6 | 3.1 ± 0.3 |
| Supraoptic nucleus | 4.9 ± 1.1 | 2.7 ± 0.4 | 2.6 ± 0.9 |
| Accessory groups of the supraoptic nucleus | 13.0 ± 2.8 | 8.6 ± 1.2 | 7.0 ± 2.6 |
| Arcuate nucleus | 26.1 ± 2.1 | 20.2 ± 3.0 | 43.5 ± 3.3 a,b |
| Dorsomedial hypothalamic nucleus | 3.3 ± 0.9 | 4.5 ± 0.6 | 3.7 ± 2.0 |
| Lateral hypothalamic area | 1.8 ± 0.2 | 2.9 ± 0.5 | 1.3 ± 0.3 b |
| Posterior hypothalamic area | 1.7 ± 0.5 | 4.1 ± 1.6 | 2.4 ± 1.6 |
| Premammillary nucleus | 6.6 ± 1.0 | 5.9 ± 0.6 | 6.8 ± 1.9 |
| Tuberal nucleus | 3.2 ± 0.7 | 4.0 ± 0.6 | 2.6 ± 0.7 |
| Supramammilary nucleus | 0.8 ± 0.2 | 2.4 ± 0.9 | 1.1 ± 0.4 |
| Zona incerta | 0.6 ± 0.2 | 1.8 ± 0.4 | 1.7 ± 1.1 |
| Paraventricular thalamic nucleus | 0.7 ± 0.1 | 1.1 ± 0.3 | 0.2 ± 0.1 |
| Medial amygdala | 0.7 ± 0.2 | 1.0 ± 0.1 | 0.5 ± 0.2 |
| Amygdalohippocampal area | 0.9 ± 0.3 | 0.4 ± 0.1 | 0.4 ± 0.2 |
Abbreviations: KNDy, kisspeptin/neurokinin B/dynorphin; PNA, prenatal androgen-treated; PNV, prenatal vehicle; RVDG, glycoprotein-deleted rabies virus; SEM, standard error of the mean.
a significantly different to PNV male,
b significantly different to PNV female.
Discussion
These findings demonstrate that the PCOS neuroendocrine phenotype of impaired progesterone negative feedback may be a consequence of changes in steroid hormone sensitivity and synaptic regulation of KNDy neurons. Using a well-characterized PNA-induced mouse model of PCOS, we find that AR RNA expression is increased while PGR and dynorphin RNA expression is reduced in KNDy cells. This may indicate a potential mechanism through which elevated testosterone in PCOS inhibits PGR-mediated negative feedback regulation of GnRH neurons by dynorphin. In addition to direct changes in steroid hormone receptor gene expression at KNDy cells, ARC glutamate and GABA cells had reduced PGR RNA expression, and KNDy cells exhibited a dramatic reduction in synaptic input from both neurotransmitters. Retrograde monosynaptic tract-tracing supported that PNA treatment leads to significantly less synaptic input to KNDy cells and demonstrated this reduction originates from multiple sexually dimorphic hypothalamic nuclei in steroid hormone-responsive regions. Together, these data indicate that testosterone actions, either directly at KNDy neurons or in presynaptic steroid hormone-responsive populations, may result in impaired steroid hormone negative feedback in PCOS.
The resistance to progesterone negative feedback in PCOS patients is, at least partially, driven by testosterone action through AR, as treatment with the AR antagonist flutamide restores estrogen and progesterone suppression of LH pulse frequency in PCOS patients (19) and reproductive cycles in preclinical PCOS models (78, 79). In line with this, androgen excess in animal models during either prenatal or adult life reduces PGR expression in the hypothalamus, supporting impaired central sensitivity to progesterone (60, 64, 80, 81). Using multiplex in situ hybridization, our data revealed a parallel increase in AR RNA with reduced PGR RNA in ARC KNDy cells of PCOS-like PNA mice. The percentage of KNDy cells expressing PGR RNA also had a significant, but small, decrease in PNA-treated mice. However, the small effect size of this reduction may be due to the high sensitivity of the RNAscope assay to detect single RNAs, combined with our criteria in which only 3 RNA transcripts were required for a cell to be counted as positive for the gene. Although the significance of progesterone negative feedback has been questioned in rodents due to the lack of a true luteal phase, the knockout of PGR in mice leads to anovulation and increased basal LH levels (82, 83) and episodic KNDy population activity in mice recorded with fiber photometry found that progesterone reduces the frequency of correlated KNDy episodic activity and pulsatile LH release (84). As such, progesterone may play a more significant role in regulating LH pulse frequency in rodents than previously anticipated.
Importantly, KNDy cells with elevated AR and reduced PR RNA also exhibited reduced dynorphin RNA in PNA mice. There is significant evidence from sheep models that progesterone negative feedback of GnRH/LH pulse frequency occurs predominantly through dynorphin and OPKR1 actions in the ARC (40, 85), and progesterone upregulates ARC dynorphin expression (41). Therefore, the results here suggest a mechanism through which high circulating testosterone in PCOS may act at KNDy cells to reduce PGR expression which may, in turn, reduce the production of dynorphin and the inhibition of GnRH neurons and/or KNDy cells. Somewhat surprisingly, ESR1, Kiss1, and Tac2 RNA expression remain unchanged in PNA-treated mice, indicating that estradiol regulation of excitatory KNDy neuropeptides is potentially unimpaired in this model. As such, the cellular mechanism underlying the previously reported phenomena of impaired estradiol negative feedback in PNA mice remains to be identified (63). This is consistent with reports of unchanged kisspeptin and neurokinin B cell numbers in prenatal testosterone-treated sheep (60). Of note, this data stands in contrast to a recent report of increased Kiss1 and Tac2 arcuate gene expression in a PCOS mouse model induced using peripubertal treatment with letrozole, an aromatase inhibitor that reduces testosterone conversion to estrogen (86). The underlying mechanisms that result in KNDy gene expression levels that differ between models needs further investigation. Nonetheless, this comparison may help elucidate how manipulations to steroid hormone levels at different developmental timepoints differentially impact KNDy neurons to elevate LH pulse frequency, a potentially important consideration when examining a heterogenous syndrome such as PCOS.
Similar to KNDy neurons, ARC GABA and glutamate cells exhibited reduced PGR levels following PNA treatment, although this was not concurrent with elevated AR. Reduced PGR has been detected in non-KNDy ARC cells in PNA sheep and in an ARC GABA population with enhanced synaptic input directly to GnRH neurons in PNA mice (64, 78, 79, 87), but changes in synaptic input from progesterone-sensitive populations to KNDy cells in this model was unexplored. Here, we show that PNA treatment in mice reduces synaptic input from vGluT2-positive glutamatergic and vGaT-positive GABAergic neurons to KNDy neurons. As stimulation of glutamate and GABA is reported to depolarize the membrane potential of KNDy neurons (88-91), these results may indicate that synaptic input from 2 excitatory resources is removed by PNA treatment. However, the neuropeptidergic phenotype of these inputs are unknown and may supersede the effects of neurotransmitters on KNDy neuron firing activity (90, 92). Further, we postulate that reduced synaptic input from glutamatergic and GABAergic cells in PNA mice may impair modulation of the pulse generator by afferent steroid hormone–sensitive neurons. In line with this hypothesis, rabies-mediated monosynaptic retrograde tract-tracing identified reduced synaptic input to KNDy neurons in PNA mice originates from hypothalamic areas that are highly steroid hormone-dependent. Synaptic input to KNDy cells in PNA mice was reduced to levels seen in control males, indicating that elevated testosterone in PCOS may rewire synaptic structure through either organizational and/or activational changes in female-dominant inputs to KNDy cells. Unfortunately, toxicity induced by the rabies virus prevents quantification of steroid hormone receptor expression in transfected cells. However, reduced input was observed from the AVPV, which is highly steroid hormone–sensitive in control animals and exhibits increased AR- and reduced PR-positive cells in PNA mice (64). This may indicate that high testosterone action in the AVPV of PNA mice reduces progesterone sensitivity and synaptic input to KNDy neurons. In addition, input from the lateral LHA and AHA were reduced in PNA mice when compared with control females. The LHA contains diverse neuronal populations and outputs; however, a role for linking energy balance and reproductive regulation has been reported, such as by orexin- and melanin-concentrating hormone synthesizing neurons that have direct input to GnRH neurons (93, 94). The exact role of changes in synaptic input from the AHA and LHA to KNDy cells is so far unknown.
Together, our observations suggest a potential mechanism through which high testosterone in PCOS acts directly at KNDy cells to impair progesterone negative feedback and may modulate synaptic plasticity between KNDy cells and upstream steroid hormone–sensitive hypothalamic populations to perturb negative feedback suppression of KNDy neuron activity in PCOS. It remains to be assessed whether changes to KNDy cells and associated networks are permanently programmed by prenatal AR activation or driven by high circulating testosterone in PCOS-like mice. Additionally, functional assessment of the detected changes in KNDy steroid hormone receptor expression and presynaptic input will be required to elucidate the significance of the abnormalities identified here in the generation of impaired steroid hormone feedback. Nevertheless, this work supports abnormalities to KNDy neurons and upstream regulators may contribute to neuroendocrine dysfunction in PCOS. Although these observations have been made in a preclinical model, elevated LH pulsatile secretion is temporally linked to kisspeptin pulsatile secretion in PCOS women (95) and antagonism of the neurokinin B receptor, Tac3R, is able to lower LH pulse frequency and subsequent hyperandrogenism (96). Therefore, both clinical studies and the basic research presented here support continued efforts for the development of therapeutic targets against KNDy cells as a treatment for LH hypersecretion in PCOS.
Acknowledgments
We would like to thank Dr. Richard Piet for his helpful review and comments on this manuscript.
Financial Support: Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Numbers K99HD096120 to A.M.M. and R01HD039916 to M.N.L.
Author Contributions: A.M.M., L.M.C., and M.N.L. designed the research; A.M.M. and D.M. performed the research; and A.M.M. wrote the initial draft of the paper. All authors reviewed and edited the manuscript.
Glossary
Abbreviations
- AAV
adeno-associated virus
- AHA
anterior hypothalamic area
- AMH
anti-Müllerian hormone
- AR
androgen receptor
- ARC
arcuate nucleus
- AVPV
anteroventral periventricular nucleus
- ESR1
estrogen receptor
- GABA
gamma-aminobutyric acid
- GFP
green fluorescent protein
- GnRH
gonadotropin-releasing hormone
- KISS1
kisspeptin
- KNDy
kisspeptin/neurokinin B/dynorphin
- LH
luteinizing hormone
- LHA
lateral hypothalamus
- OPRK1
kappa opioid receptor
- PBS
phosphate-buffered saline
- PCOS
polycystic ovary syndrome
- PDYN
prodynorphin
- PFA
paraformaldehyde
- pfu
plaque forming units
- PGR
progesterone receptor
- PNA
prenatal androgen-treated
- PNV
prenatal vehicle
- POA
preoptic area
- RVDG
glycoprotein-deleted rabies virus
- SEM
standard error of the mean
- SYP
synaptophysin
- TAC2
neurokinin B
- YFP
yellow fluorescent protein
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Dumesic DA, Oberfield SE, Stener-Victorin E, Marshall JC, Laven JS, Legro RS. Scientific statement on the diagnostic criteria, epidemiology, pathophysiology, and molecular genetics of polycystic ovary syndrome. Endocr Rev. 2015;36(5):487-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lizneva D, Suturina L, Walker W, Brakta S, Gavrilova-Jordan L, Azziz R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil Steril. 2016;106(1):6-15. [DOI] [PubMed] [Google Scholar]
- 3. Teede HJ, Misso ML, Costello MF, et al. ; International PCOS Network . Erratum. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod. 2019;34(2):388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rotterdam EA-SPCWG. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19(1):41-47. [DOI] [PubMed] [Google Scholar]
- 5. Taylor AE, McCourt B, Martin KA, et al. . Determinants of abnormal gonadotropin secretion in clinically defined women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1997;82(7):2248-2256. [DOI] [PubMed] [Google Scholar]
- 6. Yoo RY, Dewan A, Basu R, Newfield R, Gottschalk M, Chang RJ. Increased luteinizing hormone pulse frequency in obese oligomenorrheic girls with no evidence of hyperandrogenism. Fertil Steril. 2006;85(4):1049-1056. [DOI] [PubMed] [Google Scholar]
- 7. Arroyo A, Laughlin GA, Morales AJ, Yen SS. Inappropriate gonadotropin secretion in polycystic ovary syndrome: influence of adiposity. J Clin Endocrinol Metab. 1997;82(11):3728-3733. [DOI] [PubMed] [Google Scholar]
- 8. Daniels TL, Berga SL. Resistance of gonadotropin releasing hormone drive to sex steroid-induced suppression in hyperandrogenic anovulation. J Clin Endocrinol Metab. 1997;82(12):4179-4183. [DOI] [PubMed] [Google Scholar]
- 9. Rebar R, Judd HL, Yen SSC, Rakoff J, Vandenberg G, Naftolin F. Characterization of inappropriate gonadotropin-secretion in polycystic ovary syndrome. J Clin Investig. 1976;57(5):1320-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Waldstreicher J, Santoro NF, Hall JE, Filicori M, Crowley WF. Hyperfunction of the hypothalamic-pituitary axis in women with polycystic ovarian disease - indirect evidence for partial gonadotroph desensitization. J Clin Endocrinol Metab. 1988;66(1):165-172. [DOI] [PubMed] [Google Scholar]
- 11. Gilling-Smith C, Willis DS, Beard RW, Franks S. Hypersecretion of androstenedione by isolated thecal cells from polycystic ovaries. J Clin Endocrinol Metab. 1994;79(4):1158-1165. [DOI] [PubMed] [Google Scholar]
- 12. Eisner JR, Barnett MA, Dumesic DA, Abbott DH. Ovarian hyperandrogenism in adult female rhesus monkeys exposed to prenatal androgen excess. Fertil Steril. 2002;77(1):167-172. [DOI] [PubMed] [Google Scholar]
- 13. Jonard S, Dewailly D. The follicular excess in polycystic ovaries, due to intra-ovarian hyperandrogenism, may be the main culprit for the follicular arrest. Hum Reprod Update. 2004;10(2):107-117. [DOI] [PubMed] [Google Scholar]
- 14. Schoemaker J, van Weissenbruch MM, Scheele F, van der Meer M. The FSH threshold concept in clinical ovulation induction. Baillieres Clin Obstet Gynaecol. 1993;7(2):297-308. [DOI] [PubMed] [Google Scholar]
- 15. Clarke IJ, Cummins JT. The temporal relationship between gonadotropin releasing hormone (GnRH) and luteinizing hormone (LH) secretion in ovariectomized ewes. Endocrinology. 1982;111(5):1737-1739. [DOI] [PubMed] [Google Scholar]
- 16. Wildt L, Häusler A, Marshall G, et al. . Frequency and amplitude of gonadotropin-releasing hormone stimulation and gonadotropin secretion in the rhesus monkey. Endocrinology. 1981;109(2):376-385. [DOI] [PubMed] [Google Scholar]
- 17. Pastor CL, Griffin-Korf ML, Aloi JA, Evans WS, Marshall JC. Polycystic ovary syndrome: evidence for reduced sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab. 1998;83(2):582-590. [DOI] [PubMed] [Google Scholar]
- 18. Chhabra S, McCartney CR, Yoo RY, Eagleson CA, Chang RJ, Marshall JC. Progesterone inhibition of the hypothalamic gonadotropin-releasing hormone pulse generator: evidence for varied effects in hyperandrogenemic adolescent girls. J Clin Endocrinol Metab. 2005;90(5):2810-2815. [DOI] [PubMed] [Google Scholar]
- 19. Eagleson CA, Gingrich MB, Pastor CL, et al. . Polycystic ovarian syndrome: evidence that flutamide restores sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab. 2000;85(11):4047-4052. [DOI] [PubMed] [Google Scholar]
- 20. Simonian SX, Spratt DP, Herbison AE. Identification and characterization of estrogen receptor alpha-containing neurons projecting to the vicinity of the gonadotropin-releasing hormone perikarya in the rostral preoptic area of the rat. J Comp Neurol. 1999;411(2):346-358. [DOI] [PubMed] [Google Scholar]
- 21. Skinner DC, Caraty A, Allingham R. Unmasking the progesterone receptor in the preoptic area and hypothalamus of the ewe: no colocalization with gonadotropin-releasing neurons. Endocrinology. 2001;142(2):573-579. [DOI] [PubMed] [Google Scholar]
- 22. Herbison AE, Skinner DC, Robinson JE, King IS. Androgen receptor-immunoreactive cells in ram hypothalamus: distribution and co-localization patterns with gonadotropin-releasing hormone, somatostatin and tyrosine hydroxylase. Neuroendocrinology. 1996;63(2):120-131. [DOI] [PubMed] [Google Scholar]
- 23. Huang X, Harlan RE. Absence of androgen receptors in LHRH immunoreactive neurons. Brain Res. 1993;624(1-2):309-311. [DOI] [PubMed] [Google Scholar]
- 24. Lehman MN, Karsch FJ. Do gonadotropin-releasing hormone, tyrosine hydroxylase-, and beta-endorphin-immunoreactive neurons contain estrogen receptors? a double-label immunocytochemical study in the Suffolk ewe. Endocrinology. 1993;133(2):887-895. [DOI] [PubMed] [Google Scholar]
- 25. Coyle C, Campbell RE. Pathological pulses in PCOS. Mol Cell Endocrinol. 2019;498:110561. [DOI] [PubMed] [Google Scholar]
- 26. Araújo BS, Baracat MCP, Dos Santos Simões R, et al. . Kisspeptin influence on polycystic ovary syndrome-a mini review. Reprod Sci. 2020;27(2):455-460. [DOI] [PubMed] [Google Scholar]
- 27. Szeliga A, Podfigurna A, Bala G, Meczekalski B. Kisspeptin and neurokinin B analogs use in gynecological endocrinology: where do we stand? J Endocrinol Invest. 2020;43(5):555-561. [DOI] [PubMed] [Google Scholar]
- 28. Abbara A, Eng PC, Phylactou M, et al. . Kisspeptin receptor agonist has therapeutic potential for female reproductive disorders. J Clin Invest. 2020;130(12):6739-6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Skorupskaite K, George JT, Veldhuis JD, Millar RP, Anderson RA. Kisspeptin and neurokinin B interactions in modulating gonadotropin secretion in women with polycystic ovary syndrome. Hum Reprod. 2020;35(6):1421-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Romero-Ruiz A, Skorupskaite K, Gaytan F, et al. . Kisspeptin treatment induces gonadotropic responses and rescues ovulation in a subset of preclinical models and women with polycystic ovary syndrome. Hum Reprod. 2019;34(12):2495-2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hunjan T, Abbara A. Clinical Translational Studies of Kisspeptin and Neurokinin B. Semin Reprod Med. 2019;37(3):119-124. [DOI] [PubMed] [Google Scholar]
- 32. Smith JT, Dungan HM, Stoll EA, et al. . Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology. 2005;146(7):2976-2984. [DOI] [PubMed] [Google Scholar]
- 33. Burke MC, Letts PA, Krajewski SJ, Rance NE. Coexpression of dynorphin and neurokinin B immunoreactivity in the rat hypothalamus: Morphologic evidence of interrelated function within the arcuate nucleus. J Comp Neurol. 2006;498(5):712-726. [DOI] [PubMed] [Google Scholar]
- 34. Franceschini I, Lomet D, Cateau M, Delsol G, Tillet Y, Caraty A. Kisspeptin immunoreactive cells of the ovine preoptic area and arcuate nucleus co-express estrogen receptor alpha. Neurosci Lett. 2006;401(3):225-230. [DOI] [PubMed] [Google Scholar]
- 35. Goubillon ML, Forsdike RA, Robinson JE, Ciofi P, Caraty A, Herbison AE. Identification of neurokinin B-expressing neurons as an highly estrogen-receptive, sexually dimorphic cell group in the ovine arcuate nucleus. Endocrinology. 2000;141(11):4218-4225. [DOI] [PubMed] [Google Scholar]
- 36. Foradori CD, Coolen LM, Fitzgerald ME, Skinner DC, Goodman RL, Lehman MN. Colocalization of progesterone receptors in parvicellular dynorphin neurons of the ovine preoptic area and hypothalamus. Endocrinology. 2002;143(11):4366-4374. [DOI] [PubMed] [Google Scholar]
- 37. Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146(9):3686-3692. [DOI] [PubMed] [Google Scholar]
- 38. Smith JT, Clay CM, Caraty A, Clarke IJ. KiSS-1 messenger ribonucleic acid expression in the hypothalamus of the ewe is regulated by sex steroids and season. Endocrinology. 2007;148(3):1150-1157. [DOI] [PubMed] [Google Scholar]
- 39. Rometo AM, Rance NE. Changes in prodynorphin gene expression and neuronal morphology in the hypothalamus of postmenopausal women. J Neuroendocrinol. 2008;20(12):1376-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Goodman RL, Coolen LM, Anderson GM, et al. . Evidence that dynorphin plays a major role in mediating progesterone negative feedback on gonadotropin-releasing hormone neurons in sheep. Endocrinology. 2004;145(6):2959-2967. [DOI] [PubMed] [Google Scholar]
- 41. Foradori CD, Goodman RL, Adams VL, Valent M, Lehman MN. Progesterone increases dynorphin a concentrations in cerebrospinal fluid and preprodynorphin messenger ribonucleic Acid levels in a subset of dynorphin neurons in the sheep. Endocrinology. 2005;146(4):1835-1842. [DOI] [PubMed] [Google Scholar]
- 42. Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. 2010;151(8):3479-3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009;29(38):11859-11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Weems PW, Witty CF, Amstalden M, Coolen LM, Goodman RL, Lehman MN. κ-opioid receptor is colocalized in GnRH and KNDy cells in the female ovine and rat brain. Endocrinology. 2016;157(6):2367-2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Clarkson J, Han SY, Piet R, et al. . Definition of the hypothalamic GnRH pulse generator in mice. Proc Natl Acad Sci U S A. 2017;114(47):E10216-E10223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Han SY, McLennan T, Czieselsky K, Herbison AE. Selective optogenetic activation of arcuate kisspeptin neurons generates pulsatile luteinizing hormone secretion. Proc Natl Acad Sci U S A. 2015;112(42):13109-13114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Moore AM, Coolen LM, Lehman MN. Kisspeptin/Neurokinin B/Dynorphin (KNDy) cells as integrators of diverse internal and external cues: evidence from viral-based monosynaptic tract-tracing in mice. Sci Rep. 2019;9(1):14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yeo SH, Kyle V, Blouet C, Jones S, Colledge WH. Mapping neuronal inputs to Kiss1 neurons in the arcuate nucleus of the mouse. Plos One. 2019;14(3):e0213927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Xu Y, Nedungadi TP, Zhu L, et al. . Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. 2011;14(4):453-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Azziz R. Polycystic ovary syndrome. Obstet Gynecol. 2018;132(2):321-336. [DOI] [PubMed] [Google Scholar]
- 51. Sir-Petermann T, Codner E, Pérez V, et al. . Metabolic and reproductive features before and during puberty in daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2009;94(6):1923-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Maliqueo M, Galgani JE, Pérez-Bravo F, et al. . Relationship of serum adipocyte-derived proteins with insulin sensitivity and reproductive features in pre-pubertal and pubertal daughters of polycystic ovary syndrome women. Eur J Obstet Gynecol Reprod Biol. 2012;161(1):56-61. [DOI] [PubMed] [Google Scholar]
- 53. Risal S, Pei Y, Lu H, et al. . Prenatal androgen exposure and transgenerational susceptibility to polycystic ovary syndrome. Nat Med. 2019;25(12):1894-1904. [DOI] [PubMed] [Google Scholar]
- 54. Walters KA, Bertoldo MJ, Handelsman DJ. Evidence from animal models on the pathogenesis of PCOS. Best Pract Res Clin Endocrinol Metab. 2018;32(3):271-281. [DOI] [PubMed] [Google Scholar]
- 55. Stener-Victorin E, Padmanabhan V, Walters KA, et al. . Animal models to understand the etiology and pathophysiology of polycystic ovary syndrome. Endocr Rev. 2020;41(4):538-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Caldwell AS, Eid S, Kay CR, et al. . Haplosufficient genomic androgen receptor signaling is adequate to protect female mice from induction of polycystic ovary syndrome features by prenatal hyperandrogenization. Endocrinology. 2015;156(4):1441-1452. [DOI] [PubMed] [Google Scholar]
- 57. Hu Q, Jin J, Zhou H, et al. . Crocetin attenuates DHT-induced polycystic ovary syndrome in mice via revising kisspeptin neurons. Biomed Pharmacother. 2018;107:1363-1369. [DOI] [PubMed] [Google Scholar]
- 58. Osuka S, Iwase A, Nakahara T, et al. . Kisspeptin in the hypothalamus of 2 rat models of polycystic ovary syndrome. Endocrinology. 2017;158(2):367-377. [DOI] [PubMed] [Google Scholar]
- 59. Yan X, Yuan C, Zhao N, Cui Y, Liu J. Prenatal androgen excess enhances stimulation of the GNRH pulse in pubertal female rats. J Endocrinol. 2014;222(1):73-85. [DOI] [PubMed] [Google Scholar]
- 60. Cheng G, Coolen LM, Padmanabhan V, Goodman RL, Lehman MN. The kisspeptin/neurokinin B/dynorphin (KNDy) cell population of the arcuate nucleus: sex differences and effects of prenatal testosterone in sheep. Endocrinology. 2010;151(1):301-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Porter DT, Moore AM, Cobern JA, et al. . Prenatal testosterone exposure alters GABAergic synaptic inputs to GnRH and KNDy neurons in a sheep model of polycystic ovarian syndrome. Endocrinology. 2019;160(11):2529-2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cernea M, Padmanabhan V, Goodman RL, Coolen LM, Lehman MN. Prenatal testosterone treatment leads to changes in the morphology of KNDy neurons, their inputs, and projections to GnRH cells in female sheep. Endocrinology. 2015;156(9):3277-3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Moore AM, Prescott M, Campbell RE. Estradiol negative and positive feedback in a prenatal androgen-induced mouse model of polycystic ovarian syndrome. Endocrinology. 2013;154(2):796-806. [DOI] [PubMed] [Google Scholar]
- 64. Moore AM, Prescott M, Marshall CJ, Yip SH, Campbell RE. Enhancement of a robust arcuate GABAergic input to gonadotropin-releasing hormone neurons in a model of polycystic ovarian syndrome. Proc Natl Acad Sci U S A. 2015;112(2):596-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Cravo RM, Margatho LO, Osborne-Lawrence S, et al. . Characterization of Kiss1 neurons using transgenic mouse models. Neuroscience. 2011;173:37-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Holland S, Prescott M, Pankhurst M, Campbell RE. The influence of maternal androgen excess on the male reproductive axis. Sci Rep. 2019;9(1):18908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wang F, Flanagan J, Su N, et al. . RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn. 2012;14(1):22-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. RRID:AB_477523. [Google Scholar]
- 69. RRID:AB_10000240. [Google Scholar]
- 70. RRID:AB_2187539. [Google Scholar]
- 71. RRID:AB_887869. [Google Scholar]
- 72. RRID:AB_2571870. [Google Scholar]
- 73. Kuiper LB, Beloate LN, Dupuy BM, Coolen LM. Drug-taking in a socio-sexual context enhances vulnerability for addiction in male rats. Neuropsychopharmacology. 2019;44(3):503-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Erben L, He MX, Laeremans A, Park E, Buonanno A. A novel ultrasensitive in situ hybridization approach to detect short sequences and splice variants with cellular resolution. Mol Neurobiol. 2018;55(7):6169-6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Carpenter AE, Jones TR, Lamprecht MR, et al. . CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 2006;7(10):R100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Franklin KB, Paxinos G.. Mouse brain in stereotaxic coordinates. 2nd ed. San Diego: Academic Press; 2001. [Google Scholar]
- 77. Tata B, Mimouni NEH, Barbotin AL, et al. . Elevated prenatal anti-Müllerian hormone reprograms the fetus and induces polycystic ovary syndrome in adulthood. Nat Med. 2018;24(6):834-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sullivan SD, Moenter SM. Prenatal androgens alter GABAergic drive to gonadotropin-releasing hormone neurons: implications for a common fertility disorder. Proc Natl Acad Sci U S A. 2004;101(18):7129-7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Silva MS, Prescott M, Campbell RE. Ontogeny and reversal of brain circuit abnormalities in a preclinical model of PCOS. JCI insight. 2018;3(7):e99405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Foecking EM, Szabo M, Schwartz NB, Levine JE. Neuroendocrine consequences of prenatal androgen exposure in the female rat: absence of luteinizing hormone surges, suppression of progesterone receptor gene expression, and acceleration of the gonadotropin-releasing hormone pulse generator. Biol Reprod. 2005;72(6):1475-1483. [DOI] [PubMed] [Google Scholar]
- 81. Foecking EM, Levine JE. Effects of experimental hyperandrogenemia on the female rat reproductive axis: suppression of progesterone-receptor messenger RNA expression in the brain and blockade of luteinizing hormone surges. Gend Med. 2005;2(3):155-165. [DOI] [PubMed] [Google Scholar]
- 82. Lydon JP, DeMayo FJ, Conneely OM, O’Malley BW. Reproductive phenotpes of the progesterone receptor null mutant mouse. J Steroid Biochem Mol Biol. 1996;56(1-6 Spec No):67-77. [DOI] [PubMed] [Google Scholar]
- 83. Chappell PE, Lydon JP, Conneely OM, O’Malley BW, Levine JE. Endocrine defects in mice carrying a null mutation for the progesterone receptor gene. Endocrinology. 1997;138(10):4147-4152. [DOI] [PubMed] [Google Scholar]
- 84. McQuillan HJ, Han SY, Cheong I, Herbison AE. GnRH pulse generator activity across the estrous cycle of female mice. Endocrinology. 2019;160(6):1480-1491. [DOI] [PubMed] [Google Scholar]
- 85. Goodman RL, Holaskova I, Nestor CC, et al. . Evidence that the arcuate nucleus is an important site of progesterone negative feedback in the ewe. Endocrinology. 2011;152(9):3451-3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Esparza LA, Schafer D, Ho BS, Thackray VG, Kauffman AS. Hyperactive LH pulses and elevated kisspeptin and NKB gene expression in the arcuate nucleus of a PCOS mouse model. Endocrinology. 2020;161(4):bqaa018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Berg T, Silveira MA, Moenter SM. Prepubertal development of GABAergic transmission to Gonadotropin-Releasing Hormone (GnRH) neurons and postsynaptic response are altered by prenatal androgenization. J Neurosci. 2018;38(9):2283-2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. DeFazio RA, Elias CF, Moenter SM. GABAergic transmission to kisspeptin neurons is differentially regulated by time of day and estradiol in female mice. J Neurosci. 2014;34(49):16296-16308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. DeFazio RA, Navarro MA, Adams CE, Milescu LS, Moenter SM. Estradiol enhances the depolarizing response to GABA and AMPA synaptic conductances in arcuate kisspeptin neurons by diminishing voltage-gated potassium currents. J Neurosci. 2019;39(48):9532-9545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Qiu J, Nestor CC, Zhang C, et al. . High-frequency stimulation-induced peptide release synchronizes arcuate kisspeptin neurons and excites GnRH neurons. eLife. 2016;5:e16246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Wang L, Burger LL, Greenwald-Yarnell ML, MyersMG, Jr., Moenter SM. Glutamatergic transmission to hypothalamic kisspeptin neurons is differentially regulated by estradiol through estrogen receptor alpha in adult female mice. J Neurosci. 2018;38(5):1061-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Piet R, Kalil B, McLennan T, Porteous R, Czieselsky K, Herbison AE. Dominant neuropeptide cotransmission in Kisspeptin-GABA regulation of GNRH neuron firing driving ovulation. J Neurosci. 2018;38(28):6310-6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Skrapits K, Kanti V, Savanyú Z, et al. . Lateral hypothalamic orexin and melanin-concentrating hormone neurons provide direct input to gonadotropin-releasing hormone neurons in the human. Front Cell Neurosci. 2015;9:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Wu M, Dumalska I, Morozova E, van den Pol A, Alreja M. Melanin-concentrating hormone directly inhibits GnRH neurons and blocks kisspeptin activation, linking energy balance to reproduction. Proc Natl Acad Sci U S A. 2009;106(40):17217-17222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Katulski K, Podfigurna A, Czyzyk A, Meczekalski B, Genazzani AD. Kisspeptin and LH pulsatile temporal coupling in PCOS patients. Endocrine. 2018;61(1):149-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. George JT, Kakkar R, Marshall J, et al. . Neurokinin B receptor antagonism in women with polycystic ovary syndrome: a randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2016;101(11):4313-4321. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.