Abstract
Objective: This study aimed to investigate the serum levels of inflammatory markers in adolescents with major depressive disorder (MDD) using selective serotonin reuptake inhibitors.
Methods: This was an 8-month observational study, involving 30 adolescents with and 38 without (control) MDD diagnosis. Demographic (age and gender) and anthropometric data (weight, height, and calculated body mass index [BMI] z score) were collected. Body composition was assessed with whole-body DXA scan. Depressive and anxiety symptoms were assessed using the Beck Depression and Anxiety Inventories (BDI-II and BAI), respectively. Serum levels of interleukin (IL)-6, IL-8, IL-1β, tumor necrosis factor, monocyte chemoattractant protein-1 (MCP-1), leptin, resistin, and adiponectin were measured using Bio-Plex Multiplex Immunoassays at baseline and after 8 months.
Results: At baseline, patients with MDD and controls did not differ in age, gender, BMI z score, and fat mass index (FMI) z score. At follow-up, 58.3% (21/36) of patients with MDD were in full remission. Patients with MDD had higher levels of resistin at baseline (26274.16 pg/mL [16162.68–54252.72]) than controls (21678.53 pg/mL [11221.17–37343.27]; p < 0.01). This difference remained statistically significant after adjustment for sex, age, and FMI z score. No differences in other inflammatory markers were observed between the groups. By follow up, depressive and anxiety symptom severity had decreased significantly in patients with MDD in parallel with a decrease in the serum levels of TNF (p = 0.02), IL-8 (p < 0.01) and MCP-1 (p = 0.04). Among these markers, BDI-II score was positively correlated with serum levels of MCP-1.
Conclusion: These results corroborate the view of involvement of peripheral inflammatory mechanisms in the pathophysiology of MDD in adolescents.
This trial is registered at ClinicalTrials.gov: NCT02147184.
Keywords: depression, adolescents, inflammation, cytokines, SSRI
Introduction
Major depressive disorder (MDD) is estimated to affect >264 million people worldwide (GBD 2017 Disease and Injury Incidence and Prevalence Collaborators 2018). The number of years lived with MDD increased by ∼15% between 2007 and 2017, in all age groups (GBD 2017 Disease and Injury Incidence and Prevalence Collaborators 2018). It is estimated that half of major psychiatric disorders start before 15 years of age (Kessler et al. 2005). Among adolescents, MDD is associated with increased risk for suicide and illicit drug use as well as impaired academic performance (Avenevoli et al. 2015; Mendelson and Tandon 2016).
Peripheral inflammation has been consistently associated with depressive symptoms (Moulton et al. 2015; Hickie et al. 2018; Martins et al. 2019). In adults, elevated circulating levels of inflammatory markers, such as tumor necrosis factor (TNF), interleukin (IL)-6, and C-reactive protein (CRP) are associated with MDD (Howren et al. 2009; Dowlati et al. 2010; Strawbridge et al. 2015), and antidepressant treatment decreases their levels (Strawbridge et al. 2015). Furthermore, the use of anti-inflammatory interventions for MDD has shown promising results (Kohler-Forsberg et al. 2019).
Although the evidence supporting a role for proinflammatory mechanisms in adults with MDD is robust, there is a dearth of studies in youth. A recent meta-analysis showed the potential bidirectional association between MDD and proinflammatory markers (i.e., CRP and IL-6) in children and adolescents (Colasanto et al. 2020). However, the authors highlighted the small number of studies, preventing an adequate control of confounding factors that could have influenced this association (Colasanto et al. 2020). Since immune activity, including production of cytokines, may differ by age (Mitchell and Goldstein 2014), findings in adults cannot be readily extrapolated to younger age groups. Moreover, the pathways involved in the pathogenesis of MDD in adolescents and young adults are less influenced by confounding factors, such as chronic diseases (e.g., cardiovascular and metabolic diseases), which influence immune function (Hickie et al. 2018).
Given that the association between immune/inflammatory markers and MDD in adolescents requires further examination (Mills et al. 2013; Kim et al. 2014; Mitchell and Goldstein 2014), the current longitudinal study sought to investigate possible alterations in serum inflammatory markers in adolescents with MDD treated with selective serotonin reuptake inhibitors (SSRIs).
Methods
Data were obtained from participants enrolled in an observational study investigating the long-term effect of SSRIs on bone mass in adolescents (NCT02147184). The University of Iowa Institutional Review Board approved the study. Methods and main results of this study have been described elsewhere (Calarge et al. 2017).
In brief, male and female individuals, aged between 15 and 20 years, within a month of starting SSRI treatment or unmedicated were enrolled. Exclusion criteria included (1) treatment with psychotropic agents other than SSRIs during the 2 years before study entry, (2) presence of eating disorders, (3) substance use disorder, (4) severe medical conditions, and (5) pregnancy. All participants underwent an initial interview comprising the National Institute of Mental Health (NIMH) Diagnostic Interview Schedule for Children (DISC-IV) (Shaffer et al. 2000) as well as a clinical assessment by a child psychiatrist. At every visit, consensus diagnoses were generated following the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) (American Psychiatric Association 2000) based on all available information, including clinician- and self-completed symptom rating scales. Full remission of a depressive episode was defined as the resolution of all reported depressive symptoms. Participants were divided into control and MDD groups, with the baseline and 8-month assessments used.
Demographic (age and sex) and anthropometric data (weight and height) were collected. Body composition was assessed using whole-body DXA scan (Hologic QDR DELPHI-4500A unit or a Hologic Discovery A unit; Hologic, Inc., Bedford, MA). The two DXA units were cross-calibrated (Calarge et al. 2014). Age- and gender-specific z scores were generated for body mass index (BMI; calculated by the formula: weight/height2) and fat mass index (FMI; calculated by the formula: fat mass/height2) (Ogden et al. 2002; Weber et al. 2013).
Depressive and anxiety symptoms were assessed using the Beck Depression and Anxiety Inventories (BDI-II and BAI), respectively (Beck et al. 1961, 1988).
A fasting blood sample was collected. Serum levels of IL-6, IL-8, IL-1β, TNF, monocyte chemoattractant protein-1 (MCP-1), leptin, resistin, and adiponectin were measured using Bio-Plex Multiplex Immunoassays following the manufacturer's instructions (Bio-Rad Laboratories, Inc., Hercules), as routinely performed in our laboratory (Rocha et al. 2019).
Statistical analyses were performed using SPSS, version 26.0 (IBM Corp., Armonk, NY) and GraphPad Prism 5.0 (GraphPad Software, Inc., La Jolla, CA, EUA). The Kolmogorov–Smirnov test was used to test normality. The chi square test was used to compare categorical variables between those with and without MDD, and independent samples t-test and Mann–Whitney U test for continuous variables. Logistic regression was used to adjust comparison of the markers between the groups for age, sex, and FMI z score. To evaluate differences between visits, Wilcoxon test was used. MDD group was dichotomized into those who remitted from depression (full remission) and those who remained depressed (full episode) on follow-up. Pearson's and Spearman's correlation analyses were used to assess the potential association between inflammatory markers and psychiatric symptom severity of normally and non-normally distributed variables, respectively. Multivariable regression models examined the association between the inflammatory markers and the clinical parameters (BDI, BAI, FMI z score, SSRI use, and equivalent dose), using stepwise forward regression modeling. SSRI equivalent dose was defined as one SSRI unit as equivalent to 10 mg of escitalopram; 20 mg of fluoxetine, citalopram, or paroxetine; or 50 mg of sertraline (Deumic et al. 2016). Univariate analysis was performed to select clinical parameters associated with dependent variables when p < 0.20. Statistical significance was set at p < 0.05. Our sample size provides a statistical power >80% to detect an effect size of 0.70 in resistin level.
Results
Sixty-eight individuals were included in this study, of which 38 were diagnosed with MDD. Data on two participants with MDD were available at baseline but not at the 8-month follow-up. Patients and controls did not differ in age, gender, BMI z score, or FMI z score. As expected, the MDD group had higher baseline BDI-II and BAI scores (Table 1).
Table 1.
Demographic and Clinical Parameters of Depressive Patients and Controls at the Baseline
| Control (n = 30) | MDD (n = 38) | p-Value | |
|---|---|---|---|
| Age | 19 (15–20) | 19 (15–20) | 0.42a |
| Sex, n (%) | |||
| Female | 17 (56.7) | 29 (76.3) | |
| Male | 13 (43.3) | 9 (23.7) | 0.12b |
| BMI z score | 0.48 ± 0.92 | 0.14 ± 0.77 | 0.17c |
| FMI z score | 0.29 ± 0.79 | −0.03 ± 0.59 | 0.17c |
| BAI | 1 (0–12) | 12 (0–29) | <0.01a |
| BDI-II | 2 (0–7) | 20 (3–46) | <0.01a |
Mann–Whitney.
Chi-square.
Independent samples t-test.
BMI, body mass index; BAI, Beck Anxiety Inventory; BDI-II, Beck Depression Inventory-II; FMI, fat mass index; MDD, major depressive disorder.
At baseline, 28 participants with MDD were taking SSRI (fluoxetine, n = 10; sertraline, n = 7; citalopram, n = 8, escitalopram, n = 2, and paroxetine, n = 1). Five patients (13.2%) were using medications other than SSRIs (omeprazole, n = 3; propranolol, n = 1, and cetirizine, n = 1). At the 8-month follow-up, 58.3% (21/36) of patients with MDD had fully remitted and the rate of SSRI use dropped from 73.7% (28/38) to 41.7% (15/36) (Table 2).
Table 2.
Major Depressive Disorders Status and Selective Serotonin Reuptake Inhibitors Use at the Baseline and 8-Month Follow-Up Visit
| Baseline (n = 38) | Month 8 (n = 36) | |
|---|---|---|
| MDD status, n (%) | ||
| Full episode | 38 (100.0) | 15 (41.7) |
| Full remission | 0 (0.0) | 21 (58.3) |
| SSRIs use, n (%) | 28 (73.7) | 15 (41.7) |
MDDs, major depressive disorders; SSRIs, selective serotonin reuptake inhibitors.
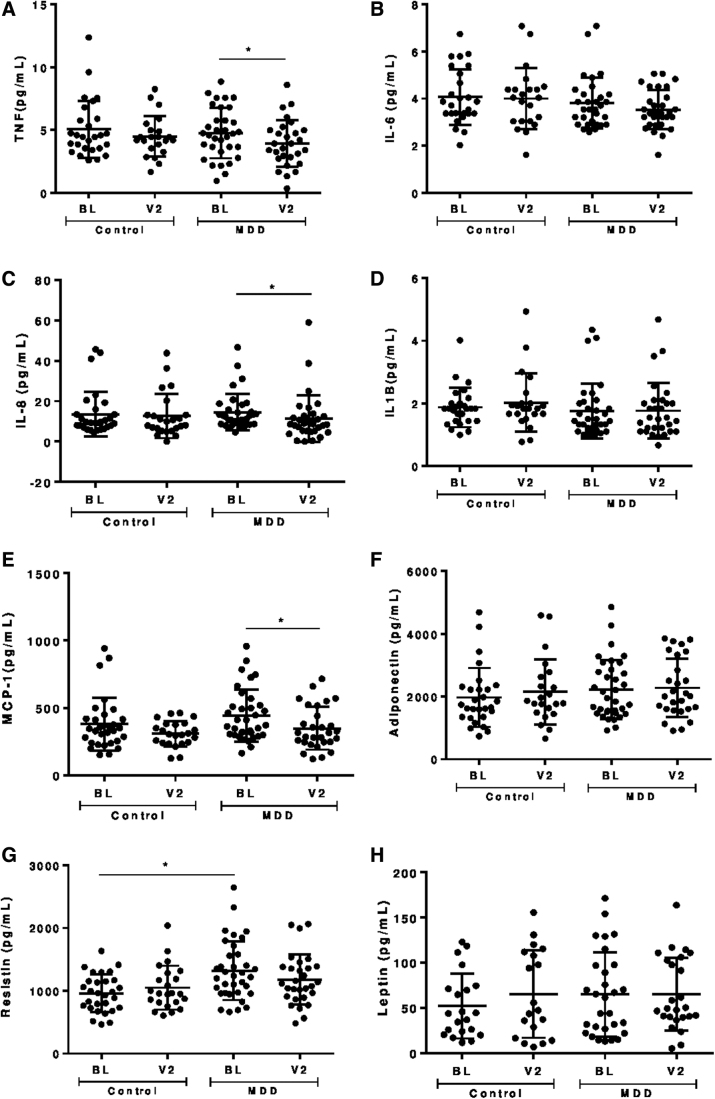
Figure 1 shows serum levels of inflammatory markers in both groups, at baseline and follow-up. Patients with MDD had higher levels of resistin at baseline (Fig. 1G). This difference remained statistically significant after adjustment for potential confounders (sex, age, and FMI z score). No significant differences in the other inflammatory markers were observed.
FIG. 1.
Serum levels of (A) TNF; (B) IL-6; (C) IL-8; (D) IL-1β; (E) MCP-1; (F) adiponectin; (G) resistin; (H) leptin in patients with MDD and controls at BL and after 8 months of follow-up (V2). BL, baseline; IL, interleukin; MCP-1, monocyte chemoattractant protein-1; MDD, major depression disorder; TNF, tumor necrosis factor.
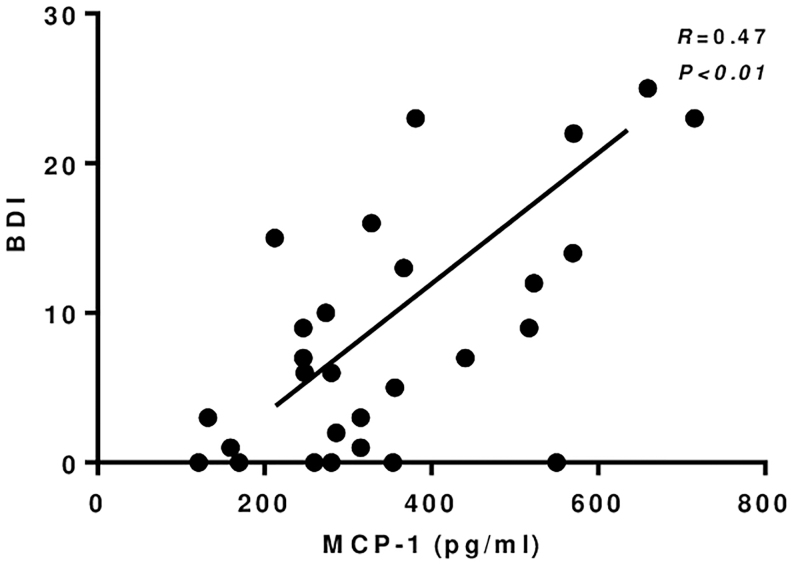
Depressive and anxiety symptom severity decreased significantly in patients with MDD on follow-up (from 20.8 [3–46] to 8.7 [0–29] in the BDI-II and from 12.7 [0–31] to 6.1 [0–22] in the BAI score; p < 0.01), in parallel with a decrease in the serum levels of TNF (p = 0.02), IL-8 (p < 0.01) and MCP-1 (p = 0.04) (Fig. 1A, C, E). Among these markers, BDI-II score positively correlated with serum levels of MCP-1 only at follow-up (Fig. 2). In a multivariate analysis, serum MCP-1 levels (adjusted R square = 0.328) were associated with BDI-II scores (β = 11.58, p < 0.01) and SSRI use (β = −144.2, p = 0.01) in participants with MDD. No other association between any of the other inflammatory markers, on the one hand, and the BDI-II and BAI scores or the use of SSRI, on the other, was observed.
FIG. 2.
Correlation between MCP-1 and BDI-II at follow-up. BDI-II, Beck Depression Inventory Score-II; MCP-1, monocyte chemoattractant protein-1.
Discussion
The number and quality of studies examining inflammatory markers in adolescents with MDD are still limited, with less robust evidence implicating immune/inflammatory mechanisms as compared with the adult population (Mills et al. 2013; Kim et al. 2014). The main findings of this study include: (1) adolescents with MDD have higher serum levels of resistin than controls; (2) despite the improvement of depressive and anxiety symptoms occurring in parallel with the decrease of the serum levels of inflammatory markers (TNF, IL-8, and MCP-1), there was no clear association between them.
Resistin is an adipokine associated with the regulation of both central and peripheral insulin sensitivity (Benomar et al. 2013). The circulating level of resistin has been associated with insulin resistance in metabolic diseases, such as obesity and diabetes mellitus type 2 (DM2) (Su et al. 2019). Given that individuals with obesity and DM2 are more likely to develop MDD, this suggests that these conditions may share common pathophysiological mechanisms, such as inflammation, oxidative stress, and dysregulation of the hypothalamic–pituitary–adrenal axis (Moulton et al. 2015; Martins et al. 2019). In addition, recent studies have shown that childhood trauma might increase the circulating levels of resistin (Veru-Lesmes et al. 2021). The association between childhood trauma and depression in adolescents is well established (Gardner et al. 2019). Although history of trauma was not evaluated in this analysis, it is tempting to hypothesize that it might have contributed to the higher levels of resistin in depressed subjects. Only a few studies have evaluated circulating levels of resistin in MDD, with conflicting findings. Aliyazicioglu et al. (2011) observed lower serum resistin levels in adults with MDD compared with controls, whereas Papakostas et al. (2013) did not find any difference. Similarly, Pan et al. (2008) found no association between depressive symptom severity and plasma resistin level in middle-aged and older adults. In contrast, Lehto et al. (2010) found a positive correlation between serum resistin levels and depressive symptoms in adults. With regard to antidepressant treatment, Weber-Hamann et al. (2007) showed that plasma levels of resistin decreased in adult patients with MDD who remitted from depression after treatment with amitriptyline or paroxetine. To our knowledge, no study has examined circulating resistin concentration in adolescents with MDD. We found that resistin levels are increased in adolescents with MDD, but they were neither correlated with symptom severity nor with treatment response. Further studies are needed to fully characterize the association between resistin and MDD, especially in younger age groups.
Over the 8-month follow-up period, depressive and anxiety symptoms improved in parallel with changes in serum levels of inflammatory markers. The severity of depressive symptoms and use of SSRI were correlated with MCP-1 serum levels at follow-up. MCP-1 (also called chemokine (C-C motif) ligand 2; CCL2) is involved in immune responses through regulation of monocytes/macrophages migration to and infiltration of various organs, including the brain (Deshmane et al. 2009; Barnes et al. 2017). Recent meta-analyses have shown that circulating levels of MCP-1 are higher in adult patients with MDD (Eyre et al. 2016; Leighton et al. 2018) and bipolar disorder during depressive episodes (Misiak et al. 2020) compared with controls, suggesting that MCP-1 is likely to play a pathophysiological role in depression. In this study, correlation between MCP-1 and depressive symptoms occurred only at follow-up, highlighting the need for additional investigations.
Although we found a decrease in the serum levels of other markers at follow-up, they did not associate with change in depressive and anxiety symptom severity or with SSRI use. Previous studies have also failed to find a consistent longitudinal association between inflammatory markers and depressive symptoms. For instance, in a 6-year study, no association was observed between depressive symptoms as assessed by the BDI-II and serum IL-6 or CRP concentrations (Stewart et al. 2009). Similarly, Duivis et al. (2011) found that the association between depressive symptoms, as evaluated using the 9-item Patient Health Questionnaire, and inflammatory markers (IL-6, CRP, and fibrinogen) in patients with established coronary artery disease followed for 5 years was no longer significant after accounting for health behaviors (i.e., physical activity, current smoking, and BMI) (Duivis et al. 2011). Morris et al. (2011) also observed that the association between CRP and depressive symptom severity in women was no longer significant after controlling for metabolic risk factors (smoking, waist circumference, and systolic blood pressure by waist circumference) (Morris et al. 2011).
The limitations of this study include its open-label (or naturalistic) approach and the relatively small sample size. In addition, we did not control for some potential confounding factors (e.g., physical activity). It is worth mentioning that other potential confounders (medical comorbidities, polypharmacy, etc.) are much less prevalent in adolescents than in adults.
Conclusion
In sum, this was the first study to show alteration in serum resistin levels in adolescent patients with MDD and the putative association between MCP-1 and depressive symptom and SSRI use in these patients. These results corroborate the view of involvement of peripheral inflammatory mechanisms in the pathophysiology of MDD in adolescents. Future longitudinal studies involving larger samples are necessary to confirm these findings.
Clinical Significance
Although the evidence supporting a role for proinflammatory mechanisms in adults with MDD is robust, there is a dearth of studies in youth. A better understanding of the mechanisms involved in the pathophysiology of MDD in adolescents can contribute to the development of more efficient treatment strategies.
Acknowledgment
The Neuropsychiatry Program/Immuno-Psychiatry Lab is supported by the UT Health Department of Psychiatry and Behavioral Sciences.
Disclosures
No competing financial interests exist.
References
- Aliyazicioglu R, Değer O, Vanizor Kural B, Hocaoğlu Ç, Çolak M, Balaban Yücesan F: The relationship between the peroxisome proliferator-activated receptor gamma 2 gene polymorphism, lipids and adipokines in patients with major depression. Turkiye Klinikleri J Med Sci 31:1065–1072, 2011 [Google Scholar]
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. Washington, DC: American Psychiatric Association; 2000 [Google Scholar]
- Avenevoli S, Swendsen J, He JP, Burstein M, Merikangas KR: Major depression in the national comorbidity survey-adolescent supplement: Prevalence, correlates, and treatment. J Am Acad Child Adolesc Psychiatry 54:37–44.e2, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes J, Mondelli V, Pariante CM: Genetic contributions of inflammation to depression. Neuropsychopharmacology 42:81–98, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA: An inventory for measuring clinical anxiety: Psychometric properties. J Consult Clin Psychol 56:893–897, 1988 [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J: An inventory for measuring depression. Arch Gen Psychiatry 4:561–571, 1961 [DOI] [PubMed] [Google Scholar]
- Benomar Y, Gertler A, De Lacy P, Crepin D, Ould Hamouda H, Riffault L, Taouis M: Central resistin overexposure induces insulin resistance through Toll-like receptor 4. Diabetes 62:102–114, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calarge CA, Butcher BD, Burns TL, Coryell WH, Schlechte JA, Zemel BS: Major depressive disorder and bone mass in adolescents and young adults. J Bone Miner Res 29:2230–2237, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calarge CA, Mills JA, Janz KF, Burns TL, Coryell WH, Zemel BS: Body composition in adolescents during treatment with selective serotonin reuptake inhibitors. Pediatrics 140:e20163943, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colasanto M, Madigan S, Korczak DJ: Depression and inflammation among children and adolescents: A meta-analysis. J Affect Disord 277:940–948, 2020 [DOI] [PubMed] [Google Scholar]
- Coryell W, Mills J, Dindo L, Calarge CA: Predictors of depressive symptom trajectories in a prospective follow-up of late adolescents. Psychol Med 50:2283–2288, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmane SL, Kremlev S, Amini S, Sawaya BE: Monocyte chemoattractant protein-1 (MCP-1): An overview. J Interferon Cytokine Res 29:313–326, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deumic E, Butcher BD, Clayton AD, Dindo LN, Burns TL, Calarge CA: Sexual functioning in adolescents with major depressive disorder. J Clin Psychiatry 77:957–962, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctot KL: A meta-analysis of cytokines in major depression. Biol Psychiatry 67:446–457, 2010 [DOI] [PubMed] [Google Scholar]
- Duivis HE, de Jonge P, Penninx BW, Na BY, Cohen BE, Whooley MA: Depressive symptoms, health behaviors, and subsequent inflammation in patients with coronary heart disease: Prospective findings from the heart and soul study. Am J Psychiatry 168:913–920, 2011 [DOI] [PubMed] [Google Scholar]
- Eyre HA, Air T, Pradhan A, Johnston J, Lavretsky H, Stuart MJ, Baune BT: A meta-analysis of chemokines in major depression. Prog Neuropsychopharmacol Biol Psychiatry 68:1–8, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner MJ, Thomas HJ, Erskine HE: The association between five forms of child maltreatment and depressive and anxiety disorders: A systematic review and meta-analysis. Child Abuse Negl 96:104082, 2019 [DOI] [PubMed] [Google Scholar]
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators: Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 392:1789–1858, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickie I, Carpenter JS, Scott EM: Adolescent-onset depressive disorders and inflammation. In: Inflammation and Immunity in Depression. Edited by Baune BT. Cambridge, Massachusetts, Academic Press, 2018, pp. 427–443 [Google Scholar]
- Howren MB, Lamkin DM, Suls J: Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosom Med 71:171–186, 2009 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE: Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 62:593–602, 2005 [DOI] [PubMed] [Google Scholar]
- Kim JW, Szigethy EM, Melhem NM, Saghafi EM, Brent DA: Inflammatory markers and the pathogenesis of pediatric depression and suicide: A systematic review of the literature. J Clin Psychiatry 75:1242–1253, 2014 [DOI] [PubMed] [Google Scholar]
- Kohler-Forsberg O, Lydholm CN, Hjorthoj C, Nordentoft M, Mors O, Benros ME: Efficacy of anti-inflammatory treatment on major depressive disorder or depressive symptoms: Meta-analysis of clinical trials. Acta Psychiatr Scand 139:404–419, 2019 [DOI] [PubMed] [Google Scholar]
- Lehto SM, Huotari A, Niskanen L, Tolmunen T, Koivumaa-Honkanen H, Honkalampi K, Ruotsalainen H, Herzig KH, Viinamaki H, Hintikka J: Serum adiponectin and resistin levels in major depressive disorder. Acta Psychiatr Scand 121:209–215, 2010 [DOI] [PubMed] [Google Scholar]
- Leighton SP, Nerurkar L, Krishnadas R, Johnman C, Graham GJ, Cavanagh J: Chemokines in depression in health and in inflammatory illness: A systematic review and meta-analysis. Mol Psychiatry 23:48–58, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins LB, Monteze NM, Calarge C, Ferreira AVM, Teixeira AL: Pathways linking obesity to neuropsychiatric disorders. Nutrition 66:16–21, 2019 [DOI] [PubMed] [Google Scholar]
- Mendelson T, Tandon SD: Prevention of depression in childhood and adolescence. Child Adolesc Psychiatr Clin N Am 25:201–218, 2016 [DOI] [PubMed] [Google Scholar]
- Mills NT, Scott JG, Wray NR, Cohen-Woods S, Baune BT: Research review: The role of cytokines in depression in adolescents: A systematic review. J Child Psychol Psychiatry 54:816–835, 2013 [DOI] [PubMed] [Google Scholar]
- Misiak B, Bartoli F, Carra G, Malecka M, Samochowiec J, Jarosz K, Banik A, Stanczykiewicz B: Chemokine alterations in bipolar disorder: A systematic review and meta-analysis. Brain Behav Immun 88:870–877, 2020 [DOI] [PubMed] [Google Scholar]
- Mitchell RH, Goldstein BI: Inflammation in children and adolescents with neuropsychiatric disorders: A systematic review. J Am Acad Child Adolesc Psychiatry 53:274–296, 2014 [DOI] [PubMed] [Google Scholar]
- Morris AA, Zhao L, Ahmed Y, Stoyanova N, De Staercke C, Hooper WC, Gibbons G, Din-Dzietham R, Quyyumi A, Vaccarino V: Association between depression and inflammation—Differences by race and sex: The META-Health study. Psychosom Med 73:462–468, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulton CD, Pickup JC, Ismail K: The link between depression and diabetes: The search for shared mechanisms. Lancet Diabetes Endocrinol 3:461–471, 2015 [DOI] [PubMed] [Google Scholar]
- Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, Grummer-Strawn LM, Curtin LR, Roche AF, Johnson CL: Centers for Disease Control and Prevention 2000 growth charts for the United States: Improvements to the 1977 National Center for Health Statistics version. Pediatrics 109:45–60, 2002 [DOI] [PubMed] [Google Scholar]
- Pan A, Ye X, Franco OH, Li H, Yu Z, Wang J, Qi Q, Gu W, Pang X, Liu H, Lin X: The association of depressive symptoms with inflammatory factors and adipokines in middle-aged and older Chinese. PLoS One 3:e1392, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papakostas GI, Shelton RC, Kinrys G, Henry ME, Bakow BR, Lipkin SH, Pi B, Thurmond L, Bilello JA: Assessment of a multi-assay, serum-based biological diagnostic test for major depressive disorder: A pilot and replication study. Mol Psychiatry 18:332–339, 2013 [DOI] [PubMed] [Google Scholar]
- Rocha NP, Colpo GD, Bravo-Alegria J, Lincoln JA, Wolinsky JS, Lindsey JW, Teixeira AL, Freeman L: Exploring the relationship between Endothelin-1 and peripheral inflammation in multiple sclerosis. J Neuroimmunol 326:45–48, 2019 [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME: NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry 391:28–38, 2000 [DOI] [PubMed] [Google Scholar]
- Stewart JC, Rand KL, Muldoon MF, Kamarck TW: A prospective evaluation of the directionality of the depression-inflammation relationship. Brain Behav Immun 23:936–944, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawbridge R, Arnone D, Danese A, Papadopoulos A, Herane Vives A, Cleare AJ: Inflammation and clinical response to treatment in depression: A meta-analysis. Eur Neuropsychopharmacol 25:1532–1543, 2015 [DOI] [PubMed] [Google Scholar]
- Su KZ, Li YR, Zhang D, Yuan JH, Zhang CS, Liu Y, Song LM, Lin Q, Li MW, Dong J: Relation of circulating resistin to insulin resistance in type 2 diabetes and obesity: A systematic review and meta-analysis. Front Physiol 10:1399, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veru-Lesmes F, Guay S, Shah JL, Schmitz N, Giguere CE, Joober R, Iyer SN, Malla AK: Adipose tissue dysregulation at the onset of psychosis: Adipokines and social determinants of health. Psychoneuroendocrinology 123:104915, 2021 [DOI] [PubMed] [Google Scholar]
- Weber DR, Moore RH, Leonard MB, Zemel BS: Fat and lean BMI reference curves in children and adolescents and their utility in identifying excess adiposity compared with BMI and percentage body fat. Am J Clin Nutr 98:49–56, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber-Hamann B, Kratzsch J, Kopf D, Lederbogen F, Gilles M, Heuser I, Deuschle M: Resistin and adiponectin in major depression: the association with free cortisol and effects of antidepressant treatment. J Psychiatr Res 41:344–350, 2007 [DOI] [PubMed] [Google Scholar]