Abstract
The long history of life on Earth has unfolded as a cause-and-effect relationship with the evolving amount of oxygen (O2) in the oceans and atmosphere. Oxygen deficiency characterized our planet's first 2 billion years, yet evidence for biological O2 production and local enrichments in the surface ocean appear long before the first accumulations of O2 in the atmosphere roughly 2.4 to 2.3 billion years ago. Much has been written about this fundamental transition and the related balance between biological O2 production and sinks coupled to deep Earth processes that could buffer against the accumulation of biogenic O2. However, the relationship between complex life (eukaryotes, including animals) and later oxygenation is less clear. Some data suggest O2 was higher but still mostly low for another billion and a half years before increasing again around 800 million years ago, potentially setting a challenging course for complex life during its initial development and ecological expansion. The apparent rise in O2 around 800 million years ago is coincident with major developments in complex life. Multiple geochemical and paleontological records point to a major biogeochemical transition at that time, but whether rising and still dynamic biospheric oxygen triggered or merely followed from innovations in eukaryotic ecology, including the emergence of animals, is still debated. This paper focuses on the geochemical records of Earth's middle history, roughly 1.8 to 0.5 billion years ago, as a backdrop for exploring possible cause-and-effect relationships with biological evolution and the primary controls that may have set its pace, including solid Earth/tectonic processes, nutrient limitation, and their possible linkages. A richer mechanistic understanding of the interplay between coevolving life and Earth surface environments can provide a template for understanding and remotely searching for sustained habitability and even life on distant exoplanets.
Key Words: Biogeochemistry, Early Earth, Coevolving life and environments, Oxygen, Planetary systems, Complex life
1. Introduction
Earth's early oxygenation history has long been expressed in terms of two fundamental steps: the Great Oxidation Event (GOE) around 2.4 to 2.3 billion years ago (Ga) and a later rise in ocean-atmosphere oxygen levels between 800 and 600 million years ago (Ma) (Canfield, 2005; Kump, 2008; Lyons et al., 2014). By some estimates, the onset of the GOE was marked by atmospheric O2 levels that were still relatively low and variable (e.g., Poulton et al., 2021). Whether this transition was a product of increasing O2 release to the biosphere or decreasing O2 sinks is debated (reviewed in Krissansen-Totton et al., 2021). It was likely followed, however, by a dramatic increase in O2 inputs to the biosphere predicted from a large and long-lived anomaly in the carbon isotope record attributed to enhanced organic carbon burial (the Lomagundi-Jatuli Event; e.g., Krissansen-Totton et al., 2015). Interpretations of this feature point to potentially high levels of O2 in the atmosphere (e.g., Bekker and Holland, 2012) and/or oxidized species (such as sulfate) in the oceans (Blättler et al., 2018). Following the Lomagundi-Jatuli Event, atmospheric O2 levels fell (e.g., Ossa Ossa et al., 2018; compare Mänd et al., 2020), ushering in the low-to-intermediate levels that would characterize the next billion years of Earth's history and creating very different environmental conditions for the beginnings of complex life.
Historically, the second rise has been linked to increasing diversity and complexity among eukaryotic organisms generally, expressed in part through increasing multicellularity and the appearance and diversification of animals specifically. In the absence of a cohesive geochemical argument for the second rise in oxygen, however, the record of the earliest animals is often treated as evidence for that oxygenation. In part because of this circular reasoning, the relationship between rising biotic complexity and the oxygenation of the ocean-atmosphere system has come under close scrutiny, producing challenges to the conventional view that increasing oxygen triggered the emergence of animals (reviewed in Cole et al., 2020). A community of researchers is now reconsidering this time-honored link, some arguing that evolutionary patterns simply reflect the prolonged time scales necessary for genetic evolution of complex eukaryotes. The assertion that oxygen was not a factor controlling the timing of eukaryotic diversification builds from the view that oxygen was sufficiently high to support animals hundreds of millions of years before they appear in the rock record (Butterfield, 2009). Contributing to this viewpoint are suggestions that the earliest animals (Mills et al., 2014) and eukaryotes more generally (Mentel and Martin, 2008) required only low levels of oxygen. Other challenges lie with the possibility that prevailingly low marine oxygen levels extended into the Paleozoic, well beyond the point where animals rose to ecological importance (Sperling et al., 2015a).
Most recently, major improvements in our ability to track small-scale changes in oxygen levels in the ocean and atmosphere through time, reinforced by new biogeochemical modeling, suggest extreme variability with low baseline surface oxygen levels throughout much of Earth's history. The foundations of this new research include evidence for very low oxygen conditions in the atmosphere and oceans during the key middle chapters of Earth's history (∼1.8 to 0.8 Ga) followed by an abrupt rise of oxygen beginning around 800 Ma (Planavsky et al., 2014). Despite the convergence of many data sets suggesting change at ∼800 Ma, an essential caveat is that correlations among environmental and evolutionary changes need not imply causation—and even if they do, challenges in distinguishing the causes from the effects can foster disagreement. Further, beyond relationships between environmental and biological coevolution, central questions remain about how our planetary system sustained clement surface conditions and modulated its atmosphere in ways that may or may not have been remotely detectable. Thus, our planet's history of accumulation of potential biosignature gases and changes to Earth's atmospheric greenhouse, viewed in the context of the collective drivers and feedbacks, informs our search for habitability and life on exoplanets (Schwieterman et al., 2018).
2. Early Records of Complex Life
We focus on complex life here because all or most of the key prokaryotic metabolisms, including oxygenic photosynthesis, methanogenesis, and oxidative and reductive cycling of nitrogen and sulfur, developed much earlier (Lyons et al., 2015). These pathways continued to influence and even dominate life in the oceans throughout Earth's middle history, and the primary controls on their impacts on the biosphere are repeated themes in this contribution. With that said, the most profound milestones specific to this interval lie with the rise and proliferation of algae, animals, and other complex life and with the cause-and-effect relationships between these evolutionary breakthroughs and environmental change. We can imagine, for example, time intervals with mostly hostile environments that stifled eukaryotic proliferation and minimized ecological impact, while at other times environmental variability and heterogeneities could have driven innovation. Landmarks in the development of complex life, for the purposes of this review, lie with several critical transitions in evolutionary history: the first physical (fossil) and geochemical (organic biomarker) records of eukaryotic life, crown group emergence and the earliest expressions of multicellularity among the eukaryotes, dramatic diversification and increasing abundances among the eukaryotes and associated ecological innovations, the first physical and geochemical signs of animal (metazoan) life, and innovation and ecological impact among animal lineages (Fig. 1).
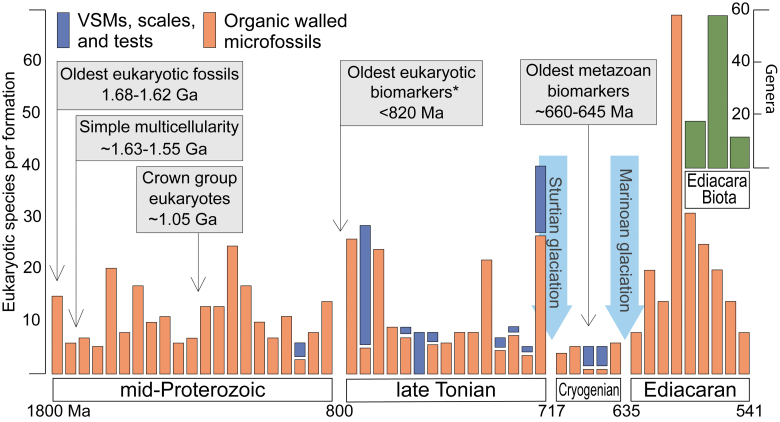
FIG. 1.
Eukaryotic microfossil diversity through time. Data include all formations of mid-Proterozoic through Ediacaran age for which greater than five eukaryotic species have been identified (updated from Cohen and Macdonald, 2015). Orange bars indicate diversity in organic-walled microfossils; blue bars indicate diversity of vase-shaped microfossils (VSMs), mineralized scales, and tests. Formations are arranged chronologically and separated into time periods of interest as discussed in the text (horizontal axis is not a linear scale). Diversity of Ediacaran animals defined in terms of global groupings based on three well-documented assemblages (Avalon, White Sea, and Nama; Erwin et al., 2011). Supporting details are provided in the supplementary material (see Supplementary Fig. S1). *Oldest passing recent scrutiny; see Brocks et al. (2017) and text.
We choose to emphasize the fossil and biomarker evidence for life transitions, rather than molecular (phylogenomic) approaches, because it is more straightforward to merge observable records of organismal presence, diversity, abundance, and ecology to coeval geochemical archives of environmental change, sometimes captured in the same samples. Such an approach sharpens our focus on the environmental significance of macroevolutionary lags—that is, the lag between the evolutionary origins of clades and later substantial, easily recognized contributions to global ecology (Erwin et al., 2011). With that said, increasingly refined efforts to integrate “omic” perspectives of biotic evolution with independent fossil records (Peterson et al., 2007) and geochemical archives of environmental change (Dupont et al., 2006; Magnabosco et al., 2018) have and will continue to yield essential fruit.
Clearly, delineating the timing of major evolutionary changes is sensitive to increasingly more sophisticated genomic approaches and dissections of the fossil record, yet the critical take-home message remains the same: key milestones in biological novelty came hundreds of millions of years prior to their ecological consequences. Some phylogenetic arguments permit extension of eukaryotic antiquity as deep into history as the beginnings of bacteria and archaea (Pace, 1997; compare Parfrey et al., 2011); however, the first observable, datable, probable eukaryotic remains occur at ∼1.7 to 1.6 Ga (Knoll and Nowak, 2017; Javaux and Lepot, 2018)—consistent with molecular clock estimates that place the last common ancestor of extant eukaryotes between ∼1.87 and 1.68 Ga (Parfrey et al., 2011; Betts et al., 2018). Until recently the earliest organic biomarker evidence for eukaryotic sterol synthesis was dated to 2.7 Ga (Brocks et al., 1999), but those records have now been convincingly tied to contamination (French et al., 2015).
Eukaryotic multicellularity at ∼1.6 Ga followed soon after those 1.7 to 1.6 Ga stem eukaryote remains (Zhu et al., 2016), and emergence of the crown group, expressed in simple multicellular red algae, is placed at ∼1.05 Ga (Butterfield, 2000; Gibson et al., 2018) and more recently with reports of macroscopic multicellular green algae at ∼1.0 Ga (Tang et al., 2020). Very recent evidence from phylogenetic analysis targeting sterol biosynthesis, an O2-requiring process, places the stem eukaryote split around the GOE (Gold et al., 2017), which may not be a coincidence, and a recent fossil study argues for crown group (red algae) at 1.6 Ga (Bengtson et al., 2017).
Perhaps of greatest relevance to defining the link between oxygen and biological evolution are the changes among eukaryotes observed in the fossil record at ∼800 to 750 Ma, including increasing diversity (Fig. 1) (reviewed in Knoll, 2014; Cohen and Macdonald, 2015; Riedman and Sadler, 2018). The mid-Proterozoic fossil record contains a total diversity of 30–40 very long-ranging (hundreds of millions of years) eukaryotic taxa, comprised exclusively of organic-walled microfossils, with individual assemblages rarely containing more than 10–15 species (reviewed in Cohen and Macdonald, 2015). While diversity among the organic-walled microfossils apparently decreased in the later Tonian (800–720 Ma), this deficit was more than made up for by the first appearances of vase-shaped microfossils (interpreted to have preyed on other eukaryotes; Porter, 2016) and scales (defensive structures), both at relatively high diversity.
If interpreted correctly, the vase-shaped microfossils, linked to testate amoebae, would be the earliest known example of eukaryophagy (protists preying on other protists). Recent work, using less aggressive separation techniques, hints at higher diversity within individual mid-Proterozoic formations (e.g., Sergeev et al., 2016; Beghin et al., 2017); however, these new findings do not greatly expand the known diversity of pre-Tonian eukaryotes. Further, the development of eukaryotic predator-prey relationships remains one of the first significant evolutionary steps after nearly a billion years of relative stasis, with relevance to drivers of diversification (reviewed in Cohen and Macdonald, 2015) and as a possible consequence of environmental change. An equally intriguing possibility lies with claims for the earliest reliable organic biomarkers for ancient eukaryotes around 800 Ma (Brocks et al., 2016, 2017; Zumberge et al., 2020; and references cited therein), despite reports of earlier occurrences that would instead reflect contamination based on newer work. These new studies have taken exceptional steps, analogous to those described in the work of French et al. (2015) for Archean samples, to screen for secondary overprints. Such old samples, which have seen long and often complicated burial, collection, and storage histories, demand particular care. This need is exacerbated by the possibility of very low levels of primary steranes in samples deriving from a prokaryote-dominated world.
Cryogenian-aged (specifically 717 to 635 Ma) organic biomarkers from sponges found in samples from Oman (Love et al., 2009; Love and Summons, 2015; Zumberge et al., 2018) and possible sponge fossils of similar age (Maloof et al., 2010) are the first traces of animal life, although a recent analysis of phylogenomic data suggests relatively rapid radiation of crown-group non-bilaterian animals in the interval leading up to the Cryogenian (Dohrmann and Wörheide, 2017). The Cryogenian is defined by the onset of snowball Earth glaciation at 717 Ma (Rooney et al., 2015). Further, possible animal (sponge) organic biomarkers have been identified in the same pre-Cryogenian time slice (Brocks et al., 2016, 2017; Zumberge et al., 2020).
We then jump to the post-glacial Ediacaran (635 to 541 Ma), with macroscopic body fossils at ∼570 Ma (Pu et al., 2016; rangeomorphs often, though not universally, assumed to be animals) followed by a great diversity of often large, dominantly soft-bodied animals, including bilaterians, and parallel advances in ecological complexity between ∼570 Ma and the Precambrian-Cambrian boundary (reviewed in Droser et al., 2017). Those ecological innovations include motility, burrowing, and heterotrophy—although pervasive deep mixing of sediments would not be seen until well into the Paleozoic, more than 100 million years after the Ediacaran (Tarhan et al., 2015). Despite the extraordinary array of early animals and lifestyles on display, the Ediacaran body fossils are limited to the interval between ∼570 Ma and the base of the Cambrian roughly 30 million years later (reviewed in Xiao and Laflamme, 2009; Droser et al., 2017; Darroch et al., 2018).
3. The Boring Billion
Understanding any relationships between oxygen and biological evolution requires that we explore the full time-interval over which eukaryotes first appeared, diversified, grew in numbers, and gave rise to the observed animal record. This time slice, broadly between 1.8 and 0.8 Ga, is known informally as the mid-Proterozoic. Despite its importance for the origins of eukaryotes, this middle chapter in Earth's history was once described as the “dullest time in Earth's history” owing to the observed uniformity of carbon isotope compositions for carbonate rocks of this age (Buick et al., 1995), particularly when contrasted with the striking isotopic variability that characterizes the intervals immediately before and after—arguably the most dramatic in Earth's history (Fig. 2). Because these isotope data are widely thought to be linked mechanistically to rates of organic carbon burial, the major driver of biospheric oxygenation, this billion-year period was imagined to have been equally “dull” in terms of atmospheric and oceanic oxygenation. With further work, the spatial and temporal persistence of this carbon isotope pattern has been reinforced, suggesting a “prolonged period of stability in crustal dynamics, redox state of surface environments, and planetary climate” (Brasier and Lindsay, 1998) to the degree that it is now widely referred to as the “boring billion.” We will continue to use this term because of deeply entrenched historical precedent.
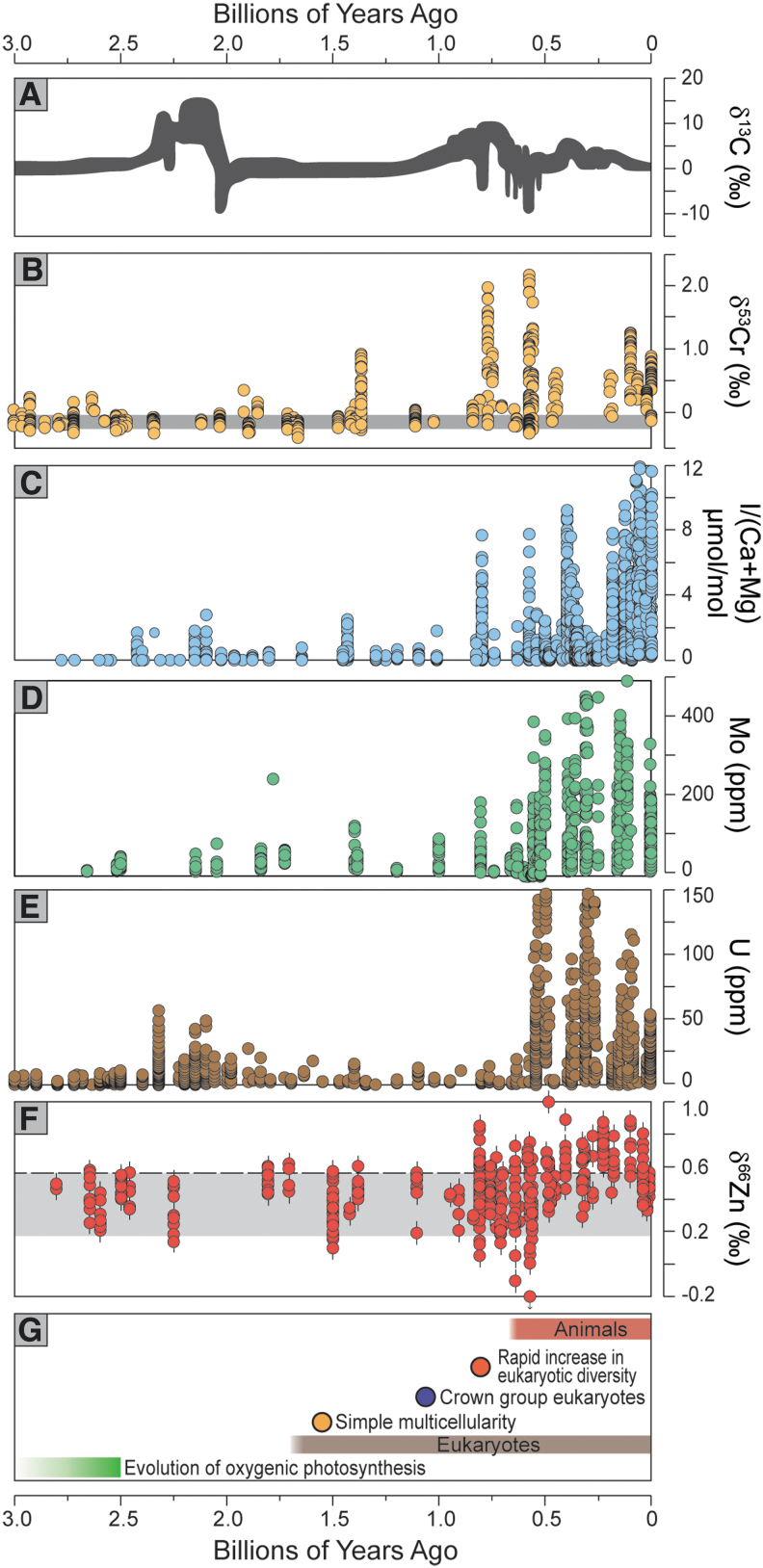
FIG. 2.
Summary of geochemical proxy records. (A) Carbon isotope data from marine carbonates (data from many sources); (B) chromium isotope data from marine ironstones and shales (Cole et al., 2016; Canfield et al., 2018); (C) iodine concentrations from shallow marine carbonates (Hardisty et al., 2017; Lu et al., 2018); (D) molybdenum (Scott et al., 2008; Reinhard et al., 2013) and (E) uranium concentrations (Partin et al., 2013) from marine shales deposited under euxinic and anoxic conditions, respectively; (F) zinc isotope data from marine sedimentary sulfides (Isson et al., 2018); and (G) timeline of evolutionary milestones. Carbon isotope data are interpreted at the first order to reflect the balance of organic-C to carbonate-C burial through time, with heavier values potentially reflecting higher organic-C burial and commensurate release of O2. Chromium isotope data are interpreted to be a direct measure of atmospheric O2 because large fractionations, like those beginning ∼800 Ma, require oxidative Cr-cycling in the presence of Mn-oxides during weathering and pO2 > 1% PAL (Planavsky et al., 2014; Cole et al., 2016). Iodine concentrations in carbonate rocks scale proportionally with concentration of iodate (the oxidized species of I) in seawater; higher concentrations beginning ∼800 Ma are interpreted to reflect increase in stability of well-oxygenated surface ocean conditions and concomitant deepening of the chemocline (Hardisty et al., 2017). The concentrations of Mo and U in marine shales scale proportionally with the concentrations of these elements in overlying seawater. The reservoir sizes of these redox-sensitive elements are modulated at first order by the spatial extent of reducing bottom-water conditions (and hence the size of the global removal flux), with expansion of oceanic euxinia and anoxia drawing down concentrations of Mo and U, respectively. The increase in Mo at ∼800 Ma is therefore interpreted to represent a decrease in the prevalence of euxinia. A corresponding increase in U concentrations has not been observed, suggesting that the event recorded in other proxies at ∼800 Ma could have been restricted to the atmosphere, shallow oceans, and intermediate depths along continental margins where euxinia prevailed—while the deep ocean remained stably anoxic until later times. A delay in the rise of phosphorus and rhenium, like that observed for uranium, could also be explained by later deep-water oxygenation (Reinhard et al., 2017a; Sheen et al., 2018). Supporting details are provided in the supplementary material.
In our discussion of oxygen's relationship with evolving life, habitats in the shallow parts of the ocean are most important, but the character and consistency of conditions in those waters is intimately related to the atmosphere above and the deep ocean below. Early estimates for stable atmospheric oxygen levels during the boring billion converged on a range from 1% to 40% of the present atmospheric level (Kump, 2008) (PAL; O2 presently comprises 21% of the atmosphere) until recent suggestions of even lower possible concentrations (addressed below).
The lower limit of 1% was based on oxidative weathering and specifically the level of O2 required to retain insoluble iron [Fe(III)] in ancient soils, which has been described from this time—in contrast to the loss of Fe in soils before the GOE (Rye and Holland, 1998). In truth, however, this approach is strongly reliant on independent estimates of atmospheric CO2 concentrations and assumptions about Fe oxidation kinetics and soil hydrology, which are equally fraught with uncertainty, and thus substantially lower or higher estimates for atmospheric O2 are possible from this method. More importantly, however, the best preserved mid-Proterozoic soils actually do bear signs of Fe loss during weathering, suggesting lower O2 (Mitchell and Sheldon, 2010).
The upper estimate of 40% PAL was grounded in something entirely different—maximum levels of O2 in the atmosphere that would have allowed the deep ocean to remain anoxic (Canfield, 1998), which is now well accepted for the boring billion (see discussions below). The estimate of 40% ultimately lies with a simple box model for O2 accumulation in the deep ocean and its relationship to O2 levels in the atmosphere and carries assumptions about patterns and efficiency of O2 mixing into and through the oceans, nutrient inventories, and O2 consumption via organic decay in the deep waters below the photic zone. Subsequently, the upper limit for the estimate was dialed down to something closer to 10% (Kump, 2008), and a recent rethink of the phosphorus assumptions employed in the landmark Canfield contribution (Derry, 2015) reduced the estimate for atmospheric pO2 to far less than 10% PAL. These and related details are discussed further below.
Nonetheless, Canfield's (1998) arguments for protracted anoxia in the deep ocean long after O2 began to accumulate in the atmosphere helped define a generation of research, demanding a search for geochemical evidence and possible biological consequences. First and foremost, protracted anoxia fundamentally challenged the previous assumption that the full ocean was O2-rich during the boring billion (Wilde, 1987; Canfield, 1998) based on the disappearance of anoxia-dependent iron formations at ∼1.8 Ga (Fe is soluble only in O2-free waters). Reconciling the disappearance of iron formations with prolonged anoxia led instead to the idea that accumulation of hydrogen sulfide (H2S) was responsible for the loss of iron formations at the start of the boring billion (Canfield, 1998). Iron is insoluble in anoxic waters when they are rich in H2S (an ocean state known as euxinia, which dominates the Black Sea but is otherwise rare in the ocean today). The pattern of stable, likely intermediate levels of atmospheric O2 during the boring billion stands in contrast to the dramatic rising and falling levels that characterized the preceding GOE and the Neoproterozoic Oxidation Event (NOE; Och and Shields-Zhou, 2012) that followed (Fig. 3; reviewed in Lyons et al., 2014). The Proterozoic spanned from 2.5 to 0.54 Ga, with the Neoproterozoic beginning at 1.0 Ga.
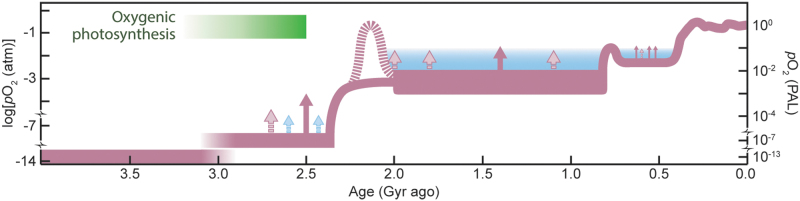
FIG. 3.
Evolution of Earth's atmospheric oxygen content through time. Red curve shows the authors' preferred model for long-term evolution (see text for details), while the blue shaded region reflects alternative views based on numerical simulations that predict O2 stability only at higher pO2 (Daines et al., 2017). Solid red arrows denote possible transient increases in pO2 for which geochemical evidence exists; pink dashed arrows indicate less certain events. Blue arrows in the late Archean indicate hypothetical “whiffs” of O2 and are included to highlight schematically the oscillatory system behavior assumed to accompany transitions from one steady state to another. Supporting details are provided in the supplementary material (see Supplementary Fig. S2). PAL = present atmospheric level.
Details from the boring billion suggest climatic stability expressed in an unusual, if not unique, lack of widespread glaciation despite the muted warming of a still faint sun and the records of the world's greatest (snowball Earth) glaciations in the intervals before and after. Plate tectonics was in full swing (Cawood, 2020), but as another testament to the unique stability of this time, the tectonic/orogenic signals are far less dramatic than those before and after (Tang et al., 2021)—marked instead by lithospheric stability and a paucity of both passive margins and geochemical records of mountain building and intense concomitant weathering (Cawood and Hawkesworth, 2014). In apparent contradiction to these observations, however, a supercontinent did form, as reviewed by Roberts (2013; see also Evans, 2013, and Cawood and Hawkesworth, 2014). The paucity of geochemical signatures may thus reflect muted intensity rather than the absence of tectonic activity. The supercontinent Nuna (also known as Columbia) was assembled by the beginning of the boring billion and remained relatively intact for hundreds of millions of years. Nascent rifting followed, particularly at ∼1.4 Ga, but the continental mass mostly retained its unified integrity rather than experiencing widespread dispersal of continental fragments. With little modification, this was followed by reassembly into Rodinia roughly a billion years ago. Again, stability and specifically subdued tectonic/orogenic drivers (Tang et al., 2021) may have been a defining quality, although details of Nuna breakup are not well known, and more dramatic dispersal is possible (as reviewed in Planavsky et al., 2015; Pehrsson et al., 2016; Salminen et al., 2020).
One could argue that the truly remarkable observation about the boring billion is that it is bracketed by some of the most striking transitions in Earth's history—expressed in oxygen, life, climate, and first-order tectonic reorganization. As such, Earth's middle chapter is both a consequence and harbinger of change, yet it reveals itself through its stability as being the opposite. It is clear to us that such a pattern of steadiness and the bookends of change on either side are not easily explained—but are anything but boring.
4. New Perspectives
It is possible that the boring billion interval offered a challenging O2 landscape for early complex life until its end, around 800 Ma. This transition is marked by strong temporal correlation among eukaryotic innovation, records of the earliest animals, and environmental change expressed in multiple proxies. It follows that ocean oxygenation may have been a critical step required for the radiation of complex, eukaryote-dominated ecosystems—O2 is certainly a prerequisite for their existence on the modern Earth. Confidence in this assertion, however, depends largely on the latest opinions about O2 during the boring billion and its immediate aftermath.
The aforementioned claim of persistent deep-ocean anoxia during the boring billion (Canfield, 1998) has gained a strong footing through detailed analysis of fine-grained sedimentary rocks and the use of well-established geochemical tracers. Specifically, the Fe chemistry of shales can delineate local anoxic conditions and even the presence of hydrogen sulfide in the water column as witnessed by the extent of associated conversion to pyrite (FeS2). Distributions of trace metals sensitive to the redox conditions of their surroundings, such as molybdenum and uranium, and their isotopic signatures can bring complementary value by giving us a global view. Temporal patterns among these trace metals in marine shales are controlled by the sizes of their reservoirs in global seawater, which are in turn modulated by the overall redox landscape of the oceans and lower solubilities of the metals under anoxic and euxinic conditions. Based on the collective data for the boring billion (Arnold et al., 2004; Scott et al., 2008; Planavsky et al., 2011; Poulton and Canfield, 2011; Partin et al., 2013; Reinhard et al., 2013; as reviewed in Lyons et al., 2014), Fe-rich (ferruginous) waters must have dominated the deeper ocean. Areas of the seafloor bathed in bottom waters containing hydrogen sulfide were likely restricted to productive portions of ocean margins. Although limited in extent, euxinia then was likely two orders of magnitude more extensive than in today's oceans where it blankets much less than 1% of the seafloor (Reinhard et al., 2013).
Some recent approximations of atmospheric O2 during the boring billion fall far below conventional estimates (Fig. 3). In particular, chromium (Cr) isotope data for the boring billion suggest pO2 levels of <1% PAL and perhaps as low as 0.1% PAL or less (Crowe et al., 2013; Planavsky et al., 2014), thus challenging the traditional view of 1% to 10% PAL (and as high as 40%). Details of the Cr isotope method are provided elsewhere (Frei et al., 2009; Planavsky et al., 2014), but there are three essential take-home messages as follows: (1) Cr isotope fractionation is typically attributed to coupled manganese (Mn) oxidation in soil profiles, a process that requires a critical threshold of surface oxygen; (2) Fe must be quantitatively oxidized within the soil profile such that isotopically fractionated Cr can escape the soil; and (3) these are difficult estimates to make because of the challenges intrinsic to using soil redox proxies, or the siliciclastic archives that were sourced by those soils and accumulated downstream, as quantitative windows to the overlying atmosphere (Daines et al., 2017). Nevertheless, the signatures of these soil processes can potentially be captured in marine sediments, and corroborating evidence for these relationships was recently reported from ancient soil horizons spanning the same time interval (Colwyn et al., 2019). Further research will determine the veracity of the recent pO2 estimates, bolstered by continued use of well-established models for Fe oxidation in ancient soils. Regardless of any remaining uncertainties surrounding the pO2 estimates, many of the Cr isotope data are strikingly unfractionated during the boring billion, suggesting fundamentally different biospheric redox relative to the interval that followed (Fig. 2). These hypothesized low O2 levels are consistent with a fresh view of the paleosol data (Planavsky et al., 2018), including mid-Proterozoic soils that lost Fe during weathering—the oft-cited paleobarometer of atmospheric pO2.
Likely of greatest importance in our discussion were the redox conditions in the shallowest oceans during the boring billion when the earliest eukaryotes were finding their first footholds. Ironically, these biologically critical settings have been assessed only rarely because standard geochemical methods are aimed instead at deeper marine waters and the atmosphere. One remedy may lie with the iodine content of carbonate rocks. Iodide (I-) in seawater oxidizes to iodate (IO3-) under low (micromolar) levels of dissolved oxygen similar to those that define the low end of eukaryotic tolerance. Importantly, iodate but not iodide readily substitutes into carbonate rocks and can fingerprint the presence of O2 in ancient marine waters (Hardisty et al., 2017). The record for the boring billion reveals a persistence of low iodate levels in shallow water settings, suggesting the presence of oxygen (Fig. 2; Hardisty et al., 2017). However, the low values are most reminiscent of those seen today on the margins of oxygen minimum zones—reflecting the mixing between proximal oxic and anoxic waters and the slow reaction kinetics of iodide-to-iodate oxidation. Cerium (Ce) records from the mid-Proterozoic point similarly to low oxygen conditions even in shallow waters where carbonates were forming (Tang et al., 2016; Liu et al., 2021).
We can reasonably interpret the record of the boring billion as reflecting a shallow chemocline at the upper boundary of deep anoxic waters with frequent upward mixing and consequent low and unstable O2 in the surface oceans. If correct, episodic upward intrusion of toxic H2S may have swamped ocean margins. Such conditions would have permitted, yet at the same time frequently challenged, O2-dependent life in the shallow ocean.
Recent three-dimensional modeling of redox landscapes in the ancient oceans provides additional perspective on the patterns and mechanisms of oxygenation and habitability during the boring billion (Reinhard et al., 2016). Even at levels as high as a few percent of present-day atmospheric O2, we can expect disequilibrium between the atmosphere and the local, photosynthetically sourced O2 of the shallow ocean, resulting in spatial concentration heterogeneities (O2 oases in the upper ocean) in regions of intense upwelling and thus highest biological production. The models further predict a general absence of O2 in bottom waters except perhaps in the shallowest regions. This constraint matters most to the earliest animals, which are known to have been benthic. Simpler calculations, as informed in a general sense by processes occurring in the modern shallow oceans, also predict pronounced temporal (seasonal) variability in O2 production and accumulation. These model results from the work of Reinhard et al. (2016), together with the iodine and cerium data, thus suggest an O2 landscape that may have been challenging for early eukaryotic life—and one that may have delayed the first animals (see Cole et al., 2020, for contrasting views).
The possibility of a world with atmospheric pO2 <0.1–1% PAL demands thorough consideration of the patterns and controls on primary production, nutrient availability, carbon cycling, and related stabilizing forcings and feedbacks. These deliberations distil down to the critical O2 sources and sinks, and in particular whether and how they respond to ambient ocean-atmosphere O2 levels in ways that provide negative feedback or promote instability. For example, it is widely accepted that a decrease in atmospheric O2 levels should, in general, lead to an increase in organic matter burial (and thus O2 release), while also leading to a decrease in organic matter weathering and thus reduced O2 consumption (Laakso and Schrag, 2014). As a result, there is widespread agreement that the most effective mechanism for stabilizing low atmospheric O2 is reducing global rates of biospheric productivity (Laakso and Schrag, 2014; Derry, 2015; Reinhard et al., 2017a). Indeed, the stability of the carbon isotope curve—hovering around zero per mil (Fig. 2)—contributed to the initial definition of the boring billion (Buick et al., 1995; Brasier and Lindsay, 1998) and attests to a relatively low steady-state background of organic burial and thus O2 release during this interval.
Nearly two decades ago the idea of limited organic production and burial during the boring billion gained momentum with the publication of Anbar and Knoll (2002). The authors developed the concept of nutrient throttling, specifically through low availability of trace elements critical to major microbial enzymatic pathways—most notably molybdenum's role as a cofactor in nitrogenase during nitrogen (N) fixation (Anbar and Knoll, 2002). Muted metal enrichments in euxinic shales support the possibility of low marine molybdenum (Mo) inventories during the boring billion due to high rates of sediment uptake within the anoxic and euxinic oceans (Scott et al., 2008), but sensitivity of the N cycle and specifically N2-fixing microbes to Mo availability remains a hot topic of research (Glass et al., 2012; Zerkle and Mikhail, 2017). Extensive microbial denitrification in the broadly anoxic waters of the ocean would have contributed to fixed-N limitation during the boring billion (Reinhard et al., 2017a), although this effect might have been muted under the Fe-rich conditions of those waters (Michiels et al., 2017). It remains unknown whether, and to what degree, scarcity of Mo and other trace metals limited N bioavailability and primary production more generally during the boring billion (Reinhard et al., 2013; Large et al., 2018).
A recurring theme in these discussions is the relationship between the likely persistence of anoxia in the deep oceans during the boring billion and impacts on nutrient cycling. Recent efforts have shifted much of the conversation to phosphorus(P)-based controls on primary production and associated oxygenation during this time window (Ozaki et al., 2019; Canfield et al., 2020). Central to these arguments is the coupling between P and Fe cycling in widely ferruginous oceans—as specifically related to Fe-P mineral formation and the strong capacity for P scavenging by Fe oxides (Laakso and Schrag, 2014; Derry, 2015; Reinhard et al., 2017a; Guilbaud et al., 2020; compare Johnson et al., 2020). Other models note important contributions to P limitation from muted recycling of P under anoxic marine conditions (Kipp and Stüeken, 2017; Laakso and Schrag, 2018, 2019). The extent of P limitation remains a critical question. Intense throttling of biospheric productivity by P deficiencies can strongly reduce biospheric N demand and thus commensurately decrease the importance of N fixation. Regardless, it appears that primary production during the boring billion was limited—as supported by recent novel triple oxygen isotope measurements (Crockford et al., 2018; Hodgskiss et al., 2019). These low levels of production were likely stabilized by P limitation but with potential reinforcement by transient N limitation during attempted excursions to higher pO2 (Reinhard et al., 2017a). Implicit to a world dominated by P limitation is the importance of tectonic and volcanic activity that can enhance P delivery to the oceans via weathering. In sum, a number of avenues seem to be converging on a generally low-P, high-Fe, and low-O2 Earth system state for much of Proterozoic time with limited biospheric productivity (Planavsky et al., 2018).
Other recent research highlights the importance of concomitant O2 sinks linked to oxidation of reduced species on land and in the oceans—compounds bearing reduced C, sulfur (S), and Fe in particular, including organic matter, pyrite (FeS2), and dissolved S and Fe in seawater (Laakso and Schrag, 2014; Daines et al., 2017). Relevant in this discussion is the degree to which the proxies and models are (Planavsky et al., 2014) or are not (Daines et al., 2017) consistent with stabilization of very low (∼0.1% PAL) O2 in the atmosphere. Specifically, recent model-based arguments assert that loss of stabilizing feedbacks at low atmospheric O2 challenges the possibility of a stable mid-Proterozoic atmosphere with O2 levels lower than 1% PAL. Levels higher than 1% PAL have also been suggested based on trace element contents of pyrite (Large et al., 2019; Steadman et al., 2020; Yierpan et al., 2020). Uncertainties in model-based estimates lie with insufficiently known parameters such as sulfide oxidation rates and strong sensitivity to other poorly known environmental factors. At the same time, geochemical proxies, including the pyrite data, rely on similarly uncertain calibrations dependent on under-constrained model parameters. Further still, very low atmospheric O2 levels can be difficult to reconcile with expected photolysis of O2 in the lower atmosphere in the absence of sufficient ozone shielding (Goldblatt et al., 2006). The good news is that all approaches point consistently to intermediate atmospheric pO2 during the boring billion, but the atmospheric pO2 estimates via competing methods can differ by as much as 2 orders of magnitude. No doubt these differences contribute to contrasting views of O2's role in modulating the pace and pattern of evolution during the boring billion.
Another consequence of low and thus more easily perturbed levels of biospheric O2 is the possibility of oscillation around a quasi-stable baseline (Fig. 3), with dynamic pO2 favored during the boring billion by still relatively low pO2 in the atmosphere controlled by a complex, feedback-rich interplay between nutrients, tectonics, erosion, and the related weathering sinks. Despite the suggestions of overall low atmospheric and marine O2 during the boring billion, a growing body of research points to at least episodes of higher O2 (e.g., Sperling et al., 2014; Gilleaudeau et al., 2016; Mukherjee and Large, 2016; Tang et al., 2016; Hardisty et al., 2017; Yang et al., 2017; Planavsky et al., 2018; Sheen et al., 2018; Zhang et al., 2018; Mänd et al., 2020; Kendall, 2021; see also Zhang et al., 2016, but compare Diamond et al., 2018). It remains to be seen whether each is truly a manifestation of higher O2 in the atmosphere and global ocean and, if true, whether drivers such as solid-Earth processes and emplacement of large igneous provinces (LIPs) specifically (Diamond et al., 2021) may have played a role. If oxygenation events were present and even prevalent during the boring billion, the question then becomes whether there were biological consequences, such as early multicellularity and development of many of the basic characteristics of eukaryotic life, as observed for example in the Roper Group of Australia at 1.4 Ga (Javaux and Knoll, 2017). The data are presently too sparse to illuminate the full scope of such records, and biases reflecting sample availability are a real concern. Nevertheless, a particularly rich archive of potentially more oxygenated conditions has emerged at roughly 1.4 Ga, including records from the Roper Group (Yang et al., 2017)—right in the middle of the boring billion.
Dynamic redox at that time is certainly consistent with the notion that O2 levels were generally much lower than today's and were set and pulsed by the balance between production and consumption. Temporal variability may well help us reconcile seemingly conflicting reports of low (even very low) and higher pO2 over the same billion years of Earth history. Tectonic models during this interval as specifically related to rifting during breakup of the Nuna supercontinent at ∼1.4 Ga could provide a mechanism for periods of enhanced organic burial and related transient oxygenation (Zhang et al., 2012; Canfield et al., 2018; Diamond and Lyons, 2018; Large et al., 2019; Steadman et al., 2020). This and other possible oxygenation events could be more specifically related to activity in LIPs and concomitant enhanced delivery of nutrients to the oceans (P and trace metals in particular; Diamond et al., 2021). Others have speculated on additional oxygenation events during the boring billion and possible relationships to tectonics/LIPs activity and life (e.g., Campbell and Allen, 2008; Diamond and Lyons, 2018; Zhang et al., 2018; Diamond et al., 2021). Finally, the likelihood of redox heterogeneities in the oceans (Slack et al., 2007; Sperling et al., 2014; Reinhard et al., 2016) tells us that coeval regions of more and less oxygenated waters may also have been a common feature during the boring billion.
5. A World of Change 800 Million Years Ago?
The conditions, causes, and consequences of the boring billion must rank among the most important and elusive in studies of early Earth and its life, but the more intriguing stories may lie at its end. It has long been assumed that the second major step in Earth's oxygenation history (the Neoproterozoic Oxidation Event, NOE) occurred late in the Proterozoic, in the window from roughly 800 to 600 Ma (Fig. 3). The vagueness of this age constraint, however, has left open the possibility that this NOE was either the cause or a consequence of the snowball Earth-scale glaciations that started around 717 Ma or, through various feedbacks, that both are true. It is not our goal to resolve or even fully articulate that debate, but we will outline evidence for important changes in both the environment and life at roughly 800 Ma and argue that these environmental changes could reflect extrinsic drivers—particularly the role of plate tectonics.
The list of geochemical and paleontological markers of change at roughly 800 Ma is long and growing—providing an easy target for defining the end of the boring billion period (Fig. 2). Foremost, the long run of stable carbon isotope (δ13C) values shows a marked shift to positive values (Fig. 2), suggesting a fundamental transition in C cycling likely related to increased organic carbon burial and associated O2 release to the biosphere (Krissansen-Totton et al., 2015). The precise timing of this jump is not well known because of poor age control (Halverson and Shields-Zhou, 2011), but recent data place it in the interval immediately before the Bitter Springs δ13C anomaly (Macdonald et al., 2010; Kuznetsov et al., 2017). The Bitter Springs event, the onset of which has been well dated at ∼810 Ma (Macdonald et al., 2010), is the first in a series of late Proterozoic negative isotope excursions, but it is preceded by obvious positive δ13C values that seem to define the end of the boring billion. Clearly, the “boring” isotope record of the preceding billion years had given way to dramatic variability, both positive and negative, intimately tied to C cycling and likely coupled to release and consumption of O2. Yet the mechanisms and implications of those carbon-oxygen relationships are beyond simple burial and oxidation of marine organic matter and remain topics of discussion.
Many other indicators suggest a fundamental shift in redox structuring around 800 Ma, including fingerprints of higher trace metal concentrations in seawater and elevated dissolved sulfate levels expressed by the first appearance of massive sulfate evaporite deposits worldwide (Thomson et al., 2015; Turner and Bekker, 2016), which both likely record increased O2 in the oceans. Widespread anoxic conditions in the oceans such as during the boring billion, in contrast, favor burial of redox-sensitive metals and pyrite derived from microbial sulfate reduction. Similarly, Cr isotope data from shales show their earliest indications of increased atmospheric oxygenation (Planavsky et al., 2014; Cole et al., 2016). Selenium (Se) isotope data insinuate increasingly oxidizing conditions in the ocean starting around 770 Ma (Von Strandmann et al., 2015), and iodine results in carbonate rocks suggest, for the first time, appreciable and sustained O2 at least locally in the surface ocean coincident with the Bitter Springs C isotope anomaly (Lu et al., 2017). Consistent with these observations, more comprehensive Fe oxidation in the oceans in the ca. 800 Ma window is suggested in the Fe isotope record of ironstones (Wang et al., unpublished data).
Oxygen likely took a step up in the ocean-atmosphere system, but it is hard to know how big that step was. Such constraints will be a popular area of future research, and new models will continue to emerge (e.g., Kanzaki and Kump, 2017). Moreover, recent data for gas inclusions in halite dated at 815 Ma, interpreted to reflect trapped ancient atmosphere, put the rise to 50% PAL (Blamey et al., 2016; also Steadman et al., 2020). That number was brought down to less than 10% PAL in a reassessment of those data (Yeung, 2017), which might still have been a large increase depending on levels present during the boring billion. We should continue to refine the precision of the age relationships and the strength of our paleoredox proxies while developing coherent mechanistic models. Many of the observations are closer to suggestions than statistically robust trends (Cole et al., 2020), and a lack of sufficient age controls challenges our ability to feel comfortable about the specific timing relationships among the proxies, their synchroneity, or possible cause-and-effect relationships. Nevertheless, wide-ranging signs seem to be pointing in the same direction.
There is a remarkable overarching temporal coincidence among the archives of biotic innovation and proliferation and the aforementioned geochemical expressions of environmental change, such as the striking transformation in C isotope behavior. Eukaryotic fossils show considerable taxonomic and ecological diversification at ∼800 to 750 Ma (reviewed in Knoll, 2014; Cohen and Macdonald, 2015; Riedman and Sadler, 2018), almost a billion years after their first known appearance. And, in what may be the most important tie point in this discussion, organic biomarker evidence for eukaryotic life (steranes), when vetted for miscues linked to contamination, does not appear until ∼820 Ma (Brocks et al., 2017; Brocks, 2018; Zumberge et al., 2020)—despite many searches in diverse paleoenvironmental settings for earlier occurrences (e.g., Nguyen et al., 2019). Consistent with these organic trends, zinc (Zn) isotope data, an emerging geochemical tracer, offer tantalizing hints of increasing eukaryotic biomass at ∼800 Ma (Isson et al., 2018) supported by greater eukaryotic microfossil abundances in the same time window (Cohen and Macdonald, 2015). The long gap between the first appearance of fossils at about 1.7 to 1.6 Ga and reliable biomarkers almost a billion years later can reasonably be viewed as an expression of generally low eukaryote abundances (ecological significance) through most of the boring billion (Gold, 2018), assuming early synthesis of sterols (the precursors of the fossil steranes) by eukaryotes (Gold et al., 2017, compare Porter, 2020). For example, early eukaryotes living under low O2 conditions may not have produced sterols (Takishita et al., 2017). This is also consistent with models of relatively low nutrient P in the ocean interior, which may have limited the overall scope of Earth's eukaryotic biosphere despite the presence of eukaryotic life in the surface ocean (Reinhard et al., 2020).
It has long been tempting to call upon the largest-scale tectonic processes to explain the NOE. Such propositions can be straightforward (compare Alcott et al., 2019), and the breakup of Rodinia falls in just the right temporal window. Also, Rodinia breakup was far more decisive than the possibly half-hearted efforts of Nuna, and the relative consequences for ocean chemistry, nutrient cycling, and inevitably oxygenation may have differed commensurately. Li et al. (2008) noted widespread continental rifting between ∼825 and 740 Ma and episodic plume events at ∼825, ∼780, and ∼750 Ma. Rifted basins are efficient sediment traps and favor large-scale organic burial along continental margins. The plume events and associated LIPs (concentrated between 850 and 720 Ma) may have pumped out copious amounts of P via basalt weathering—stimulating primary production, organic burial, and gains in biospheric O2 (Horton, 2015, but compare Planavsky, 2018).
The possibility of increasing oxygenation prior to snowball Earth glaciation has invited some researchers to blame the dramatic cooling and onset of global-scale ice cover on the rise in O2—specifically through decreasing methane stability in the atmosphere (Pavlov et al., 2003). Alternatively, methane may have mostly been much lower than previously imagined during the boring billion due to muted production and preservation in the oceans and photochemical instability in the atmosphere (Olson et al., 2016). The balance of greenhouse gases that maintained a mostly warm, ice-free boring billion in the face of a still faint sun is a work in progress (Fiorella and Sheldon, 2017; Zhao et al., 2018).
Still other studies highlight the importance of snowball Earth glaciation, through the sheer magnitude of related weathering processes (Hoffman et al., 1998, 2017), as a trigger for high nutrient fluxes to the oceans, associated primary production, O2 release (Planavsky et al., 2010; Sahoo et al., 2012), and eukaryotic diversification (Brocks et al., 2017). This is a classic chicken-or-the-egg argument, and many other controls are possible, but it is also reasonable to imagine both scenarios at play—that is, a one-two punch, with glaciation and its impact on O2 as a positive feedback set in motion by previous tectonically driven oxygenation. In other words, imagine that rising oxygen both triggered and benefited from large-scale glaciation, that O2 may have risen and fallen more than once during this interval, and that complex life would have been affected at each step along the way.
6. Evolutionary Lags, Oxygen Triggers, and the Emergence of Complex Life—A Synthesis
Eukaryotic life took big steps forward during the boring billion, but those advances have not been correlated with clear signatures of environmental change. Further, if animals were around during the boring billion, they left no fossils, biomarkers, or traces of their ecological impacts, and recent molecular clock studies agree on a Tonian origin (Erwin et al., 2011; Dos Reis et al., 2015; Dohrmann and Wörheide, 2017). Overall, early eukaryotes seemed content to continue their development in the absence of great diversity, abundance, and ecological consequence. Life, then, was in many senses experiencing its own kind of stasis through the boring billion, and the evolutionary novelties that did occur may or may not have derived from environmental drivers.
Some milestones, including the first expressions of eukaryotic life, multicellularity, and even the earliest animals, may instead owe their timing to the evolutionary pacing intrinsic to developmental-genetic toolkits rather than something extrinsic (Butterfield, 2009), as in oxygen or other major environmental factors. Alternatively, the external drivers may have been more subtly expressed (perhaps acting transiently) or less clearly related to oxygen specifically. Again, we are making the critical distinction between the beginnings of eukaryotic life as recorded in the geologic record and their later proliferation and innovation to the point of profoundly impacting ecosystems.
The earliest animals have captured much of the attention. Recent estimates for their physiological oxygen requirements are very low (Sperling et al., 2013a; Mills et al., 2014)—mostly well below the traditional 1% to 10% PAL atmospheric O2 hypothesized for the hundreds of millions of years leading up to the arrival of animals. This possibility has contributed to the idea that rising O2 played little, if any, role in triggering the beginnings of animal life (Mills and Canfield, 2014; Mills et al., 2014; Butterfield, 2018; Hammarlund et al., 2018; Mills et al., 2018, reviewed in Cole et al., 2020). Much of this discussion has centered on animal physiology—that is, the often very low thresholds of O2 tolerance for animals in modern natural and experimental settings (Sperling et al., 2013a; Mills et al., 2014)—and whether or not the boring billion interval could have supported animal life. If one argues that there was ample O2, then their later appearance, roughly a billion years after the first eukaryotes, could well have little or nothing to do with changes in environmental oxygen levels.
Alternatively, we suggest that those early redox conditions, because of their dynamics; heterogeneities; and the potential, on average, for low levels of O2 in the surface ocean could have combined to form a challenging ecological and evolutionary landscape for the earliest animal life. It remains possible that animals could have emerged and persisted under such conditions, but in the absence of a record it is hard to know. Rather than focusing on the origin of animals, we instead have directed our attention to times when the environment and life show dramatic, easily recognized in-phase changes—and whether the former drove the latter. The coincidences are strong, and it follows reasonably that ramped-up eukaryotic life and the molecular clock and geochemical suggestions for animals at roughly 800 to 700 Ma could reflect increasing and more stable O2 in the surface oceans. Comparative studies of development, however, indicate that the earliest animals likely possessed relatively few cell types and lacked complex developmental patterning (Davidson and Erwin, 2006, 2009; Erwin, 2020), with more complex development and morphology arising convergently in different lineages during the Ediacaran. Such low complexity for early metazoans relative to those that appeared later is consistent with still challenging environmental conditions in the late Tonian and Cryogenian and the possibility of higher (perhaps episodically) O2 during the Ediacaran.
Given the temporal coincidence for records of biotic and environmental change, the dissenting opinion to oxygen's chokehold on evolutionary development is that eukaryotic expansion and the appearance of animals must have driven oxygenation—giving us another chicken-or-the-egg argument. The notion that the rise of complex life and its profound ecological impacts was a cause rather than consequence of oxygenation has been a popular view (Lenton et al., 2014, reviewed in Erwin et al., 2011). We acknowledge complex life's potential role in facilitating further oxygenation via a set of positive feedbacks, but perhaps less convincing is the idea that early algae and animals were responsible for the NOE, including the signals centered around 800 Ma (compare Lenton et al., 2014). For example, any assertion that planktonic grazing by large eukaryotes might have sufficiently enhanced the biological pump by increasing particle size and thus settling rates, with consequent increases in organic burial efficiency, must acknowledge that the advent of such contributions is not well dated and that grazing also disaggregates particles. Any contribution from fecal pellet production (Logan et al., 1995) certainly came much later and may have been triggered by increasing O2. Lastly, the possibility that early, minute sponges, through filtering, stripped large amounts of reduced C and thus O2 sinks from the water column demands operation at a massive scale. Currently, there is no evidence in the record for the predicted voluminous burial of sponge biomass.
Rather than stimulating oxygenation, animals can also trigger negative feedbacks through bioturbation, which can decrease the O2 content of the atmosphere through enhanced sulfide and organic matter oxidation and phosphorus retention in the sediments (Canfield and Farquhar, 2009; Boyle et al., 2014; Lenton et al., 2014). In any case, this hypothesis is largely irrelevant to our discussion, as biological mixing of sediments on marine shelves at the levels required to impact global-scale biogeochemical cycles was delayed well into the Paleozoic (likely at least the late Silurian)—hundreds of millions of years after our ∼800 Ma window (Tarhan et al., 2015). There is no doubt that these and perhaps many other processes related to complex life contributed to the pattern of oxygenation, particularly as positive and perhaps negative feedbacks, but it is far less clear to us that these influences were more important than extrinsic factors, such as tectonics.
We imagine a boring billion that was permissive of eukaryotic development, which emerged under challenging conditions with or without subtle environmental drivers. Certainly O2 oases as early footholds in a world rife with O2 heterogeneity during the Archean (Olson et al., 2013) and the Proterozoic (Reinhard et al., 2016) could have played a role, perhaps allowing emergence without major spikes in diversification and proliferation for aerobic prokaryotes and eukaryotes. Only later, however, did vast radiation of eukaryotes and complex eukaryote-dominated ecosystems follow—coincident with, and perhaps in response to, major environmental change. Pitched this way, the end-member proponents for and against the need for rising O2 as a prerequisite for evolution of complex life find a point of compromise. Further, molecular and geochemical evidence point to initial animal divergence perhaps one to two hundred million years before the rich body fossil record, suggesting a macroevolutionary lag that separated the initial developmental toolkits and much later ecological success (Erwin et al., 2011) perhaps also triggered by environmental O2 and/or nutrient abundance (Evans et al., 2018). Our goal here has been only to touch upon highlights of the oxygen-animal debate with the hope that the reader will leave feeling curious and wanting more information on this lively and important conversation. For additional reading, Cole et al. (2020) provided a thorough discussion addressing all sides of the story in rigorous detail.
To speculate more broadly, questions about the importance of oxygen in eukaryotic evolution remain central, fostered by debates specific to animals but extending to early eukaryotes more generally, including suggestions that the earliest eukaryotes might have required very low O2 or been anaerobic (Mentel and Martin, 2008; Porter et al., 2018). The argument for oxygen pacing the rise of eukaryotes to ecological dominance starts from a single assumption—namely, that it is energetically favorable, in a net sense, for a eukaryote to live in a persistently oxic environment. This argument makes sense intuitively, as the energy yield from aerobic respiration is significantly greater than that of any anaerobic pathway. However, living in an oxic environment also comes at great expense. An enormous amount of energy production at the cellular level is devoted to maintenance, as cells continuously battle against damage of biomolecules from a variety of processes (racemization, cross-linking, depurination, oxidation, etc.). Many of these destructive processes occur more rapidly in oxic environments, and the initial cost of monomer synthesis is also higher (reviewed in Lever et al., 2015). Altogether, this relationship amounts to an energy requirement that is over an order of magnitude greater for a cell to survive over time in an oxic environment—a number that approaches or exceeds the increased ATP yield from aerobic oxidation of glucose.
If the initial energetic cost/benefit were not positive, evolution would not necessarily have exploited oxygenated environments in the mid-Proterozoic for that reason, particularly if anoxic habitat space were abundant in the photic zone. As the surface oceans became more pervasively oxygenated, however, anoxic habitats would have correspondingly declined, potentially providing the evolutionary pressure to develop adaptations that allowed for life under the extreme conditions of persistently oxygenated waters—leading ultimately to innovation and correspondingly greater diversity among eukaryotes, including the very energetic processes of subsequent animal life.
This alternative argument, based more on shifting habit availability than energy yield, provides a possible explanation for why eukaryotes including animals responded to rising oxygen in the late Neoproterozoic rather than the weakly oxygenated mid-Proterozoic surface oceans, even if the O2 concentrations were sufficient to support basal animal life. In other words, the initial steps toward well-oxygenated settings were motivated less by cellular oxygen need and associated energy benefits and more a response to shallow anaerobic and aerobic environments that were declining and expanding, respectively.
7. What Came Next?—The Fabric of Latest Proterozoic Oxygenation
One model is that oxygen rose in the atmosphere about 800 Ma to a new baseline—perhaps well above the average levels of the boring billion—but that it stayed still mostly lower than today through the remainder of the Proterozoic and into the Paleozoic, which started at 541 Ma. An issue of particular interest is whether O2 in the atmosphere and oceans continued to be dynamic, rising and falling above the new baseline that loosely defined the NOE (Fig. 3). In addition to the indicators for significant oxygenation centered around 800 Ma, some evidence suggests increasing O2 in the immediate wake of Marinoan glaciation about 635–630 Ma (Sahoo et al., 2012, 2016; Kunzmann et al., 2017, compare Miller et al., 2017). One implication is that O2 in the atmosphere and oceans may have dipped following the rise at roughly 800 Ma, perhaps before the onset of glaciation, and stayed relatively low or variable during the Cryogenian and then rose again (Cheng et al., 2018).
Some recent organic geochemical data suggest that bacteria continued to dominate primary production until the interglacial between the Sturtian and Marinoan glaciations (Brocks et al., 2017) starting at roughly 660 Ma, while other data indicate a proliferation of eukaryotes starting closer to 800–750 Ma (Brocks et al., 2017; Brocks, 2018; Isson et al., 2018). The interglacial is the same interval that produced the earliest records of animals (sponges; Love et al., 2009; Love and Summons, 2015), as well as diversification among eukaryotes more generally, including emergence of the earliest predatory rhizarians (Brocks et al., 2017)—a group that includes radiolaria and foraminifera.
The microfossil data point to a Tonian rise in eukaryotic diversity, followed by a decline before and extending through the Cryogenian, and a large jump in diversity in the early Ediacaran (Cohen and Macdonald, 2015; Riedman and Sadler, 2018; Fig. 1). The microfossils could thus be taken to track a rise in O2 in the atmosphere and oceans around 800 Ma followed by a pre-Sturtian drop and post-Marinoan bounce back up (Fig. 1)—again, the latter possibly initiated at, and triggered by, the end of glaciation (Planavsky et al., 2010; Sahoo et al., 2012).
It now seems clear from the records of environmental and biological change that the period of particular interest spans from just before 800 Ma though the Cryogenian (720–635 Ma), defined by snowball Earth glaciations, and into the Ediacaran. This timeline raises questions about the timing, structure, and duration of the conjectured oxygenation event (Fig. 3), including the possibility of rising and falling biospheric O2 in this window—leading to both opportunities and challenges for innovation among complex organisms. It also leaves open the possibility of cause-and-effect relationships between oxygenation and global-scale glaciation.
Other data suggest a persistence of mostly low O2 at least in the deep oceans throughout the Ediacaran (Canfield et al., 2008; Sperling et al., 2015a)—perhaps in combination with transient increases in the atmosphere and oceans (Kendall et al., 2015; Sahoo et al., 2016; see also Shields et al., 2019; compare Ostrander et al., 2020, and Jin et al., 2021). This fabric of latest Proterozoic O2 and specifically ephemeral oceanic oxygenation may have played a role in early animal evolution and Ediacaran carbon cycling, and the interval from 580 to 550 Ma containing the famous Shuram carbon isotope anomaly (Grotzinger et al., 2011) has emerged as a time of particular interest (e.g., Fike et al., 2006; Canfield et al., 2007; McFadden et al., 2008; Hardisty et al., 2017; Li et al., 2017a; Evans et al., 2018; Shi et al., 2018; Rooney et al., 2020). Perhaps both innovation and extinction among the early animals were driven by ephemeral environmental change (Evans et al., 2018; Muscente et al., 2019).
The natural corollary in this discussion is that biospheric O2 must have risen to something like modern levels much later (Dahl et al., 2010; Lu et al., 2018), particularly in the deep ocean (Stolper and Keller, 2018), and that pulsed oxygenation in an ocean that was still mostly anoxic may have continued even into the Paleozoic. From this vantage, the NOE becomes a protracted and dynamic event, much like the GOE (as reviewed in Lyons et al., 2014). It follows that the ensuing “Cambrian explosion” of animal life and its possible relationship to environmental change, including dramatic and persistent shifts in atmospheric and oceanic O2 and stably oxic conditions in the surface-most waters, remains unresolved (Sperling et al., 2013b, 2016; Chen et al., 2015; Jin et al., 2016; Li et al., 2017b, 2018; Lu et al., 2018). Nonetheless, we suggest that the oceans overall remained dynamic, heterogeneous, broadly reducing, and transitional—much like the Proterozoic—well beyond the end of the Ediacaran.
The latter part of the Ediacaran witnessed impressive diversification among the animals and corresponding ecosystem complexity, including the emergence of bilaterians, large mostly soft body plans, the earliest infaunal activity, mobility, advances in heterotrophy, possible stem-group molluscs, and even the first skeletons (reviewed in Droser et al., 2017). These steps can be viewed as energy-demanding innovations favored by higher O2 availability. The tremendous advantages of O2-dependent metabolisms are reviewed in the work of Taverne et al. (2018). On the other hand, some researchers have argued that because O2 requirements for animals can be very low (Sperling et al., 2013a, 2015b), oxygenation events may not have been necessary to explain the beginnings of animals or even bilaterians.
Questions remain as to whether often-cited modern systems and organisms, capturing animal life at its lowest levels of tolerance, are meaningful analogs for the ecological complexity and possible O2-demanding taxa of the late Ediacaran, including, for example, the mobility of Kimberella (a putative stem group mollusc) as motivated by grazing (Sperling et al., 2015b). We acknowledge that many of the Ediacaran animals may have been happy even at very low O2 (Sperling et al., 2015b, compare Evans et al., 2018), but absent major increases in oxygenation, benthic conditions—even during the Ediacaran—could have been frequently inhospitable. Further, as complexity of both body plans and behaviors increased, so too would have O2 demand. If it turns out that major events in eukaryote diversification, the rise of animals, and even the patterns of Ediacaran innovation do indeed line up with independent indicators of rising and perhaps pulsed oxygenation (e.g., Sahoo et al., 2016), future research will continue to explore possible cause-and-effect relationships. At this point, however, it is critical to remember that animals need oxygen. While the earliest animals may have had very low oxygen requirements and perhaps even evolved in a low O2 environment, it seems equally important to acknowledge the trajectory of their subsequent development and evolution—which was toward more energetically demanding body plans and behaviors, requiring higher and more stable environmental O2. Even if sufficient O2 existed for some sponge species in the mid-Proterozoic, it does not mean that all animals would have been happy living at that time. At some point, by any estimation, environmental O2 began to matter. The affinity animals have for oxygenated waters is apparent today and played out countless times in the geological record (e.g., Hammarlund et al., 2017).
8. Summary and Implications beyond Earth
We can think of Precambrian oxygenation as occurring in steps with continued variability around increasing baseline pO2, including the possibility of rising and falling oxygen through the boring billion, the Ediacaran, and into the Paleozoic (Fig. 3; Li et al., 2017b)—this variability may have driven extinction and innovation in first-order ways. This conceptual model, of course, must be weighed first and foremost against the data, along with a better marriage of those results with numerical models and large-scale geological observations. The grand challenge is to link the environmental and tectonic patterns extracted from the rock record for the oceans, atmosphere, and continents to the parallel patterns in the evolution of Earth's biosphere. Such an effort will ultimately need to focus on the least constrained portion of the ocean—the surface waters particularly along ocean margins, where all or most of the action was for complex life. The most important message to carry forward from the fossil record is that the numbers game can be misleading, given the myriad intrinsic biases and gaps in the data available; we need better community agreement on how to assess patterns of diversity and ecological innovation. This fabric is something we need to test and refine, including the timing of the “terminal oxygenation”—that is, the jump to dominantly strongly oxygenated conditions perhaps long after the end of the Proterozoic (e.g., Bergman et al., 2004; Dahl et al., 2010).
While details remain poorly known, there is general agreement that O2 levels in the atmosphere and oceans during Earth's middle history were likely low compared to the intervals before and after. This possibility carries important implications for life, but it also impacts our search for life on distant exoplanets. Specifically, the atmospheric estimates on the low end (perhaps much less than 1% of PAL) suggest that O2 in the atmosphere may have been below detection were distant observers scanning our atmosphere for signs of life during the boring billion (Reinhard et al., 2017b)—despite oceans with long-established prokaryotic life and emerging eukaryotic diversity. Such conditions might require observing indirect “proxies” for O2, such as O3 (Reinhard et al., 2019). As discussed, these muted levels of O2 might have reflected correspondingly low levels of primary production in the oceans.
There is another noteworthy consequence of such low O2 levels. We can imagine oceans during the boring billion with appreciable levels of dissolved sulfate (e.g., Kah et al., 2004; Fakhraee et al., 2019; Blättler et al., 2020). In contrast to the preceding Archean eon, when marine sulfate concentrations were vanishingly small (Habicht et al., 2002; Crowe et al., 2014) and methane (CH4) undoubtedly contributed significantly to greenhouse warming, both methane production and preservation may have been challenged during the boring billion. Appreciable sulfate can limit microbiological methanogenesis because of the competition with sulfate-reducing microbes for organic substrates. Sulfate can also favor significant loss of methane in marine sediments and seawater via anaerobic oxidation of methane. The low levels of methane sourced to the atmosphere could then have faced considerable photochemical destruction if O2 and thus ozone (O3) were very low. It is therefore possible that both O2 and CH4—both promising biosignatures in exoplanet research—would have been below remote detection limits. Moreover, the detection of both, a classic disequilibrium biosignature (e.g., Sagan et al., 1993), would have been lost to observers. Such co-occurrences, because of the incompatibilities of reduced and oxidized gases, are commonly attributed to appreciable biological production of both. The possibility of Earth's middle history failing to reveal life to remote observers through the presence of O2 and CH4 alone or in combination has helped fuel the concept of a false negative—that is, “cryptic biospheres that are widespread and active on a planet's surface but are ultimately undetectable or difficult to detect in the composition of a planet's atmosphere” (Reinhard et al., 2017b). Importantly, as part of the false-negative concept, we can also imagine oases of high O2 in the surface oceans that supported aerobic life beneath an O2-poor atmosphere.
Earth's history, therefore, has taught us that missed signatures of life may be just as important to consider as false positives in searches beyond our solar system. This example is only one highlight on the long list of many reasons to include studies of Earth's past when developing a toolbox for exploring distant habitability and life. More generally, this discussion of ancient Earth is filled with many other examples of how a complex, evolving planetary system can sustain habitability and life, and those lessons must be considered when assessing the habitability and prospects for life detection on distant worlds.
Supplementary Material
Acknowledgments
The authors acknowledge financial support from the NSF FESD Program (TWL), as well as the NASA Astrobiology Institute under Cooperative Agreement No. NNA15BB03A, the NASA Interdisciplinary Consortia for Astrobiology Research (ICAR) Program, and the NSF Earth-Life Transitions Program (TWL, NJP, and CTR). CL acknowledges support from the National Natural Science Foundation of China (grant # 41825019, 41821001) and the 111 Project of China (grant # BP0820004). Much of the paper was written while Lyons was a Leverhulme Fellow at the University of St Andrews.
Abbreviations Used
- Ga
billion years ago
- GOE
Great Oxidation Event
- LIPs
large igneous provinces
- Ma
million years ago
- NOE
Neoproterozoic Oxidation Event
- PAL
present atmospheric level
Supplementary Material
Associate Editor: Christopher McKay
References
- Alcott LJ, Mills BJ, and Poulton SW (2019) Stepwise Earth oxygenation is an inherent property of global biogeochemical cycling. Science 366:1333–1337 [DOI] [PubMed] [Google Scholar]
- Anbar AD and Knoll AH (2002) Proterozoic ocean chemistry and evolution: a bioinorganic bridge? Science 297:1137–1142 [DOI] [PubMed] [Google Scholar]
- Arnold GL, Anbar AD, Barling J, et al. (2004) Molybdenum isotope evidence for widespread anoxia in mid-Proterozoic oceans. Science 304:87–90 [DOI] [PubMed] [Google Scholar]
- Beghin J, Storme JY, Blanpied C, et al. (2017) Microfossils from the late Mesoproterozoic–early Neoproterozoic Atar/El Mreïti group, Taoudeni basin, Mauritania, northwestern Africa. Precambrian Res 291:63–82 [Google Scholar]
- Bekker A and Holland HD (2012) Oxygen overshoot and recovery during the early Paleoproterozoic. Earth Planet Sci Lett 317:295–304 [Google Scholar]
- Bengtson S, Sallstedt T, Belivanova V, et al. (2017) Three-dimensional preservation of cellular and subcellular structures suggests 1.6 billion-year-old crown-group red algae. PLoS Biol 15, doi: 10.1371/journal.pbio.2000735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman NM, Lenton TM, and Watson AJ (2004) COPSE: a new model of biogeochemical cycling over Phanerozoic time. Am J Sci 304:397–437 [Google Scholar]
- Betts HC, Puttick MN, Clark JW, et al. (2018) Integrated genomic and fossil evidence illuminates life's early evolution and eukaryote origin. Nature Ecol Evol 2:1556–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blamey NJ, Brand U, Parnell J, et al. (2016) Paradigm shift in determining Neoproterozoic atmospheric oxygen. Geology 44:651–654 [Google Scholar]
- Blättler CL, Claire MW, Prave AR, et al. (2018) Two-billion-year-old evaporites capture Earth's great oxidation. Science 360:320–323 [DOI] [PubMed] [Google Scholar]
- Blättler CL, Bergmann KD, Kah LC, et al. (2020) Constraints on Meso- to Neoproterozoic seawater from ancient evaporite deposits. Earth Planet Sci Lett 532, doi: 10.1016/j.epsl.2019.115951 [DOI] [Google Scholar]
- Boyle RA, Dahl TW, Dale AW, et al. (2014) Stabilization of the coupled oxygen and phosphorus cycles by the evolution of bioturbation. Nat Geosci 7:671–676 [Google Scholar]
- Brasier MD and Lindsay JF (1998) A billion years of environmental stability and the emergence of eukaryotes: new data from northern Australia. Geology 26:555–558 [DOI] [PubMed] [Google Scholar]
- Brocks JJ (2018) The transition from a cyanobacterial to algal world and the emergence of animals. Emerg Top Life Sci 2:181–190 [DOI] [PubMed] [Google Scholar]
- Brocks JJ, Logan GA, Buick R, et al. (1999) Archean molecular fossils and the early rise of eukaryotes. Science 285:1033–1036 [DOI] [PubMed] [Google Scholar]
- Brocks JJ, Jarrett AJM, Sirantoine E, et al. (2016) Early sponges and toxic protists: possible sources of cryostane, an age diagnostic biomarker antedating Sturtian Snowball Earth. Geobiology 14:129–149 [DOI] [PubMed] [Google Scholar]
- Brocks JJ, Jarrett AJ, Sirantoine E, et al. (2017) The rise of algae in Cryogenian oceans and the emergence of animals. Nature 548:578–581 [DOI] [PubMed] [Google Scholar]
- Buick R, Des Marais DJ, and Knoll AH (1995) Stable isotopic compositions of carbonates from the Mesoproterozoic Bangemall Group, northwestern Australia. Chem Geol 123:153–171 [DOI] [PubMed] [Google Scholar]
- Butterfield NJ (2000) Bangiomorpha pubescens n. gen., n. sp.: implications for the evolution of sex, multicellularity, and the Mesoproterozoic/Neoproterozoic radiation of eukaryotes. Paleobiology 26:386–404 [Google Scholar]
- Butterfield NJ (2009) Oxygen, animals and oceanic ventilation: an alternative view. Geobiology 7:1–7 [DOI] [PubMed] [Google Scholar]
- Butterfield NJ (2018) Oxygen, animals and aquatic bioturbation: an updated account. Geobiology 16:3–16 [DOI] [PubMed] [Google Scholar]
- Campbell IH and Allen CM (2008) Formation of supercontinents linked to increases in atmospheric oxygen. Nat Geosci 1:554–558 [Google Scholar]
- Canfield DE (1998) A new model for Proterozoic ocean chemistry. Nature 396:450–453 [Google Scholar]
- Canfield DE (2005) The early history of atmospheric oxygen: homage to Robert M. Garrels. Annu Rev Earth Planet Sci 33:1–36 [Google Scholar]
- Canfield DE and Farquhar J (2009) Animal evolution, bioturbation, and the sulfate concentration of the oceans. Proc Natl Acad Sci USA 106:8123–8127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canfield DE, Poulton SW, and Narbonne GM (2007) Late-Neoproterozoic deep-ocean oxygenation and the rise of animal life. Science 315:92–95 [DOI] [PubMed] [Google Scholar]
- Canfield DE, Poulton SW, Knoll AH, et al. (2008) Ferruginous conditions dominated later Neoproterozoic deep-water chemistry. Science 321:949–952 [DOI] [PubMed] [Google Scholar]
- Canfield DE, Zhang S, Frank AB, et al. (2018) Highly fractionated chromium isotopes in Mesoproterozoic-aged shales and atmospheric oxygen. Nat Commun 9, doi: 10.1038/s41467-018-05263-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canfield DE, Bjerrum CJ, Zhang S, et al. (2020) The modern phosphorus cycle informs interpretations of Mesoproterozoic Era phosphorus dynamics. Earth Sci Rev 208, doi: 10.1016/j.earscirev.2020.103267 [DOI] [Google Scholar]
- Cawood PA (2020) Earth Matters: a tempo to our planet's evolution. Geology 48:525–526 [Google Scholar]
- Cawood PA and Hawkesworth CJ (2014) Earth's middle age. Geology 42:503–506 [Google Scholar]
- Chen X, Ling HF, Vance D, et al. (2015) Rise to modern levels of ocean oxygenation coincided with the Cambrian radiation of animals. Nat Commun 6, doi: 10.1038/ncomms8142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng M, Li C, Chen X, et al. (2018) Delayed Neoproterozoic oceanic oxygenation: evidence from Mo isotopes of the Cryogenian Datangpo Formation. Precambrian Res 319:187–197 [Google Scholar]
- Cohen PA and Macdonald FA (2015) The Proterozoic record of eukaryotes. Paleobiology 41:610–632 [Google Scholar]
- Cole DB, Reinhard CT, Wang X, et al. (2016) A shale-hosted Cr isotope record of low atmospheric oxygen during the Proterozoic. Geology 44:555–558 [Google Scholar]
- Cole DB, Mills DB, Erwin DH, et al. (2020) On the co-evolution of surface oxygen levels and animals. Geobiology 18:260–281 [DOI] [PubMed] [Google Scholar]
- Colwyn DA, Sheldon ND, Maynard JB, et al. (2019) A paleosol record of the evolution of Cr redox cycling and evidence for an increase in atmospheric oxygen during the Neoproterozoic. Geobiology 17:579–593 [DOI] [PubMed] [Google Scholar]
- Crockford PW, Hayles JA, Bao H, et al. (2018) Triple oxygen isotope evidence for limited mid-Proterozoic primary productivity. Nature 559:613–616 [DOI] [PubMed] [Google Scholar]
- Crowe SA, Døssing LN, Beukes NJ, et al. (2013) Atmospheric oxygenation three billion years ago. Nature 501:535–538 [DOI] [PubMed] [Google Scholar]
- Crowe SA, Paris G, Katsev S, et al. (2014) Sulfate was a trace constituent of Archean seawater. Science 346:735–739 [DOI] [PubMed] [Google Scholar]
- Dahl TW, Hammarlund EU, Anbar AD, et al. (2010) Devonian rise in atmospheric oxygen correlated to the radiations of terrestrial plants and large predatory fish. Proc Natl Acad Sci USA 107:17911–17915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daines SJ, Mills BJ, and Lenton TM (2017) Atmospheric oxygen regulation at low Proterozoic levels by incomplete oxidative weathering of sedimentary organic carbon. Nat Commun 8, doi: 10.1038/ncomms14379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darroch SA, Smith EF, Laflamme M, et al. (2018) Ediacaran extinction and Cambrian explosion. Trends Ecol Evol 33:653–663 [DOI] [PubMed] [Google Scholar]
- Davidson EH and Erwin DH (2006) Gene regulatory networks and the evolution of animal body plans. Science 311:796–800 [DOI] [PubMed] [Google Scholar]
- Davidson EH and Erwin DH (2009) January. An integrated view of Precambrian eumetazoan evolution. Cold Spring Harb Symp Quant Biol 74:65–80 [DOI] [PubMed] [Google Scholar]
- Derry LA (2015) Causes and consequences of mid-Proterozoic anoxia. Geophys Res Lett 42:8538–8546 [Google Scholar]
- Diamond CW and Lyons TW (2018) Mid-Proterozoic redox evolution and the possibility of transient oxygenation events. Emerg Top Life Sci 2:235–245 [DOI] [PubMed] [Google Scholar]
- Diamond CW, Planavsky NJ, Wang C, et al. (2018) What the ∼1.4 Ga Xiamaling Formation can and cannot tell us about the mid-Proterozoic ocean. Geobiology 16:219–236 [DOI] [PubMed] [Google Scholar]
- Diamond CW, Ernst RE, Zhang SH, et al. (2021) Breaking the Boring Billion: a case for solid-Earth processes as drivers of system-scale environmental variability during the mid-Proterozoic. In Large Igneous Provinces: A Driver of Global Environmental and Biotic Changes, AGU Geophysical Monograph 255, American Geophysical Union, Washington, DC, doi: 10.1002/9781119507444.ch21 [DOI] [Google Scholar]
- Dohrmann M and Wörheide G (2017) Dating early animal evolution using phylogenomic data. Sci Rep 7, doi: 10.1038/s41598-017-03791-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Reis M, Thawornwattana Y, Angelis K, et al. (2015) Uncertainty in the timing of origin of animals and the limits of precision in molecular timescales. Curr Biol 25:2939–2950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droser ML, Tarhan LG, and Gehling JG (2017) The rise of animals in a changing environment: global ecological innovation in the late Ediacaran. Annu Rev Earth Planet Sci 45:593–617 [Google Scholar]
- Dupont CL, Yang S, Palenik B, et al. (2006) Modern proteomes contain putative imprints of ancient shifts in trace metal geochemistry. Proc Natl Acad Sci USA 103:17822–17827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwin DH (2020) The origin of animal body plans: a view from fossil evidence and the regulatory genome. Development 147, doi: 10.1242/dev.182899 [DOI] [PubMed] [Google Scholar]
- Erwin DH, Laflamme M, Tweedt SM, et al. (2011) The Cambrian conundrum: early divergence and later ecological success in the early history of animals. Science 334:1091–1097 [DOI] [PubMed] [Google Scholar]
- Evans DA (2013) Reconstructing pre-Pangean supercontinents. Geol Soc Am Bull 125:1735–1751 [Google Scholar]
- Evans SD, Diamond CW, Droser ML, et al. (2018) Dynamic oxygen and coupled biological and ecological innovation during the second wave of the Ediacara Biota. Emerg Top Life Sci 2:223–233 [DOI] [PubMed] [Google Scholar]
- Fakhraee M, Hancisse O, Canfield DE, et al. (2019) Proterozoic seawater sulfate scarcity and the evolution of ocean–atmosphere chemistry. Nat Geosci 12:375–380 [Google Scholar]
- Fike DA, Grotzinger JP, Pratt LM, et al. (2006) Oxidation of the Ediacaran ocean. Nature 444:744–747 [DOI] [PubMed] [Google Scholar]
- Fiorella RP and Sheldon ND (2017) Equable end Mesoproterozoic climate in the absence of high CO2. Geology 45:231–234 [Google Scholar]
- Frei R, Gaucher C, Poulton SW, et al. (2009) Fluctuations in Precambrian atmospheric oxygenation recorded by chromium isotopes. Nature 461:250–253 [DOI] [PubMed] [Google Scholar]
- French KL, Hallmann C, Hope JM, et al. (2015) Reappraisal of hydrocarbon biomarkers in Archean rocks. Proc Natl Acad Sci USA 112:5915–5920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson TM, Shih PM, Cumming VM, et al. (2018) Precise age of Bangiomorpha pubescens dates the origin of eukaryotic photosynthesis. Geology 46:135–138 [Google Scholar]
- Gilleaudeau GJ, Frei R, Kaufman AJ, et al. (2016) Oxygenation of the mid-Proterozoic atmosphere: clues from chromium isotopes in carbonates. Geochem Perspect Lett 2:178–187 [Google Scholar]
- Glass JB, Axler RP, Chandra S, et al. (2012) Molybdenum limitation of microbial nitrogen assimilation in aquatic ecosystems and pure cultures. Front Microbiol 3, doi: 10.3389/fmicb.2012.00331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold DA (2018) The slow rise of complex life as revealed through biomarker genetics. Emerg Top Life Sci 2:191–199 [DOI] [PubMed] [Google Scholar]
- Gold DA, Caron A, Fournier GP, et al. (2017) Paleoproterozoic sterol biosynthesis and the rise of oxygen. Nature 543:420–423 [DOI] [PubMed] [Google Scholar]
- Goldblatt C, Lenton TM, and Watson AJ (2006) Bistability of atmospheric oxygen and the Great Oxidation. Nature 443:683–686 [DOI] [PubMed] [Google Scholar]
- Grotzinger JP, Fike DA, and Fischer WW (2011) Enigmatic origin of the largest-known carbon isotope excursion in Earth's history. Nat Geosci 4:285–292 [Google Scholar]
- Guilbaud R, Poulton SW, Thompson J, et al. (2020) Phosphorus-limited conditions in the early Neoproterozoic ocean maintained low levels of atmospheric oxygen. Nat Geosci 13:296–301 [Google Scholar]
- Habicht KS, Gade M, Thamdrup B, et al. (2002) Calibration of sulfate levels in the Archean ocean. Science 298:2372–2374 [DOI] [PubMed] [Google Scholar]
- Halverson GP and Shields-Zhou G (2011) Chemostratigraphy and the Neoproterozoic glaciations. Geological Society, London, Memoirs 36:51–66 [Google Scholar]
- Hammarlund EU, Gaines RR, Prokopenko MG, et al. (2017) Early Cambrian oxygen minimum zone-like conditions at Chengjiang. Earth Planet Sci Lett 475:160–168 [Google Scholar]
- Hammarlund EU, Von Stedingk K, and Påhlman S (2018) Refined control of cell stemness allowed animal evolution in the oxic realm. Nat Ecol Evol 2:220–228 [DOI] [PubMed] [Google Scholar]
- Hardisty DS, Lu Z, Bekker A, et al. (2017) Perspectives on Proterozoic surface ocean redox from iodine contents in ancient and recent carbonate. Earth Planet Sci Lett 463:159–170 [Google Scholar]
- Hodgskiss MS, Crockford PW, Peng Y, et al. (2019) A productivity collapse to end Earth's Great Oxidation. Proc Natl Acad Sci USA 116:17207–17212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman PF, Kaufman AJ, Halverson GP, et al. (1998) A Neoproterozoic snowball Earth. Science 281:1342–1346 [DOI] [PubMed] [Google Scholar]
- Hoffman PF, Abbot DS, Ashkenazy Y, et al. (2017) Snowball Earth climate dynamics and Cryogenian geology-geobiology. Sci Adv 3, doi: 10.1126/sciadv.1600983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton F (2015) Did phosphorus derived from the weathering of large igneous provinces fertilize the Neoproterozoic ocean? Geochem Geophys Geosys 16:1723–1738 [Google Scholar]
- Isson TT, Love GD, Dupont CL, et al. (2018) Tracking the rise of eukaryotes to ecological dominance with zinc isotopes. Geobiology 16:341–352 [DOI] [PubMed] [Google Scholar]
- Javaux EJ and Knoll AH (2017) Micropaleontology of the lower Mesoproterozoic Roper Group, Australia, and implications for early eukaryotic evolution. J Paleontol 91:199–229 [Google Scholar]
- Javaux EJ and Lepot K (2018) The Paleoproterozoic fossil record: implications for the evolution of the biosphere during Earth's middle-age. Earth-Science Reviews 176:68–86 [Google Scholar]
- Jin C, Li C, Algeo TJ, et al. (2016) A highly redox-heterogeneous ocean in South China during the early Cambrian (∼529–514 Ma): implications for biota-environment co-evolution. Earth Planet Sci Lett 441:38–51 [Google Scholar]
- Jin C, Li C, Algeo TJ, et al. (2021) Spatial heterogeneity of redox-sensitive trace-metal enrichments in upper Ediacaran anoxic black shales. Journal of the Geological Society, in press, doi: 10.1144/jgs2020-234 [DOI] [Google Scholar]
- Johnson BR, Tostevin R, Gopon P, et al. (2020) Phosphorus burial in ferruginous SiO2-rich Mesoproterozoic sediments. Geology 48:92–96 [Google Scholar]
- Kah LC, Lyons TW, and Frank TD (2004) Low marine sulphate and protracted oxygenation of the Proterozoic biosphere. Nature 431:834–838 [DOI] [PubMed] [Google Scholar]
- Kanzaki Y and Kump LR (2017) Biotic effects on oxygen consumption during weathering: implications for the second rise of oxygen. Geology 45:611–614 [Google Scholar]
- Kendall B (2021) Recent advances in geochemical paleo-oxybarometers. Annu Rev Earth Planet Sci 49:399–433 [Google Scholar]
- Kendall B, Komiya T, Lyons TW, et al. (2015) Uranium and molybdenum isotope evidence for an episode of widespread ocean oxygenation during the late Ediacaran Period. Geochim Cosmochim Acta 156:173–193 [Google Scholar]
- Kipp MA and Stüeken EE (2017) Biomass recycling and Earth's early phosphorus cycle. Sci Adv 3, doi: 10.1126/sciadv.aao4795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll AH (2014) Paleobiological perspectives on early eukaryotic evolution. Cold Spring Harb Perspect Biol 6, doi: 10.1101/cshperspect.a016121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll AH and Nowak MA (2017) The timetable of evolution. Sci Adv 3, doi: 10.1126/sciadv.1603076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krissansen-Totton J, Buick R, and Catling DC (2015) A statistical analysis of the carbon isotope record from the Archean to Phanerozoic and implications for the rise of oxygen. Am J Sci 315:275–316 [Google Scholar]
- Krissansen-Totton J, Kipp MA, and Catling DC (2021) Carbon cycle inverse modeling suggests large changes in fractional organic burial are consistent with the carbon isotope record and may have contributed to the rise of oxygen. Geobiology 19:342–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kump LR (2008) The rise of atmospheric oxygen. Nature 451:277–278 [DOI] [PubMed] [Google Scholar]
- Kunzmann M, Bui TH, Crockford PW, et al. (2017) Bacterial sulfur disproportionation constrains timing of Neoproterozoic oxygenation. Geology 45:207–210 [Google Scholar]
- Kuznetsov AB, Bekker A, Ovchinnikova GV, et al. (2017) Unradiogenic strontium and moderate-amplitude carbon isotope variations in early Tonian seawater after the assembly of Rodinia and before the Bitter Springs Excursion. Precambrian Res 298:157–173 [Google Scholar]
- Laakso TA and Schrag DP (2014) Regulation of atmospheric oxygen during the Proterozoic. Earth Planet Sci Lett 388:81–91 [Google Scholar]
- Laakso TA and Schrag DP (2018) Limitations on limitation. Global Biogeochem Cycles 32:486–496 [Google Scholar]
- Laakso TA and Schrag DP (2019) A small marine biosphere in the Proterozoic. Geobiology 17:161–171 [DOI] [PubMed] [Google Scholar]
- Large RR, Mukherjee I, Zhukova I, et al. (2018) Role of upper-most crustal composition in the evolution of the Precambrian ocean–atmosphere system. Earth Planet Sci Lett 487:44–53 [Google Scholar]
- Large RR, Mukherjee I, Gregory D, et al. (2019) Atmosphere oxygen cycling through the Proterozoic and Phanerozoic. Mineralium Deposita 54:485–506 [Google Scholar]
- Lenton TM, Boyle RA, Poulton SW, et al. (2014) Co-evolution of eukaryotes and ocean oxygenation in the Neoproterozoic era. Nat Geosci 7:257–265 [Google Scholar]
- Lever MA, Rogers KL, Lloyd KG, et al. (2015) Life under extreme energy limitation: a synthesis of laboratory-and field-based investigations. FEMS Microbiol Rev 39:688–728 [DOI] [PubMed] [Google Scholar]
- Li C, Hardisty DS, Luo G, et al. (2017a) Uncovering the spatial heterogeneity of Ediacaran carbon cycling. Geobiology 15:211–224 [DOI] [PubMed] [Google Scholar]
- Li C, Jin C, Planavsky NJ, et al. (2017b) Coupled oceanic oxygenation and metazoan diversification during the early–middle Cambrian? Geology 45:743–746 [Google Scholar]
- Li C, Cheng M, Zhu M, et al. (2018) Heterogeneous and dynamic marine shelf oxygenation and coupled early animal evolution. Emerg Top Life Sci 2:279–288 [DOI] [PubMed] [Google Scholar]
- Li ZX, Bogdanova SV, Collins AS, et al. (2008) Assembly, configuration, and break-up history of Rodinia: a synthesis. Precambrian Res 160:179–210 [Google Scholar]
- Liu XM, Kah LC, Knoll AH, et al. (2021) A persistently low level of atmospheric oxygen in Earth's middle age. Nat Commun 12, doi: 10.1038/s41467-020-20484-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GA, Hayes JM, Hieshima GB, et al. (1995) Terminal Proterozoic reorganization of biogeochemical cycles. Nature 376:53–56 [DOI] [PubMed] [Google Scholar]
- Love GD and Summons RE (2015) The molecular record of Cryogenian sponges—a response to Antcliffe (2013). Palaeontology 58:1131–1136 [Google Scholar]
- Love GD, Grosjean E, Stalvies C, et al. (2009) Fossil steroids record the appearance of Demospongiae during the Cryogenian period. Nature 457:718–721 [DOI] [PubMed] [Google Scholar]
- Lu W, Wörndle S, Halverson GP, et al. (2017) Iodine proxy evidence for increased ocean oxygenation during the Bitter Springs Anomaly. Geochem Perspect Lett 5:53–57 [Google Scholar]
- Lu W, Ridgwell A, Thomas E, et al. (2018) Late inception of a resiliently oxygenated upper ocean. Science 361:174–177 [DOI] [PubMed] [Google Scholar]
- Lyons TW, Reinhard CT, and Planavsky NJ (2014) The rise of oxygen in Earth's early ocean and atmosphere. Nature 506:307–315 [DOI] [PubMed] [Google Scholar]
- Lyons TW, Fike DA, and Zerkle A (2015) Emerging biogeochemical views of Earth's ancient microbial worlds. Elements 11:415–421 [Google Scholar]
- Macdonald FA, Schmitz MD, Crowley JL, et al. (2010) Calibrating the Cryogenian. Science 327:1241–1243 [DOI] [PubMed] [Google Scholar]
- Magnabosco C, Moore KR, Wolfe JM, et al. (2018) Dating phototrophic microbial lineages with reticulate gene histories. Geobiology 16:179–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloof AC, Rose CV, Beach R, et al. (2010) Possible animal-body fossils in pre-Marinoan limestones from South Australia. Nat Geosci 3:653–659 [Google Scholar]
- Mänd K, Lalonde SV, Robbins LJ, et al. (2020) Palaeoproterozoic oxygenated oceans following the Lomagundi–Jatuli Event. Nat Geosci 13:302–306 [Google Scholar]
- McFadden KA, Huang J, Chu X, et al. (2008) Pulsed oxidation and biological evolution in the Ediacaran Doushantuo Formation. Proc Natl Acad Sci USA 105:3197–3202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentel M and Martin W (2008) Energy metabolism among eukaryotic anaerobes in light of Proterozoic ocean chemistry. Philos Trans R Soc Lond B Biol Sci 363:2717–2729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michiels CC, Darchambeau F, Roland FA, et al. (2017) Iron-dependent nitrogen cycling in a ferruginous lake and the nutrient status of Proterozoic oceans. Nat Geosci 10:217–221 [Google Scholar]
- Miller AJ, Strauss JV, Halverson GP, et al. (2017) Tracking the onset of Phanerozoic-style redox-sensitive trace metal enrichments: new results from basal Ediacaran post-glacial strata in NW Canada. Chem Geol 457:24–37 [Google Scholar]
- Mills DB and Canfield DE (2014) Oxygen and animal evolution: did a rise of atmospheric oxygen “trigger” the origin of animals? BioEssays 36:1145–1155 [DOI] [PubMed] [Google Scholar]
- Mills DB, Ward LM, Jones C, et al. (2014) Oxygen requirements of the earliest animals. Proc Natl Acad Sci USA 111:4168–4172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills DB, Francis WR, Vargas S, et al. (2018) The last common ancestor of animals lacked the HIF pathway and respired in low-oxygen environments. Elife 7, doi: 10.7554/eLife.31176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell RL and Sheldon ND (2010) The ∼1100 Ma Sturgeon Falls paleosol revisited: implications for Mesoproterozoic weathering environments and atmospheric CO2 levels. Precambrian Res 183:738–748 [Google Scholar]
- Mukherjee I and Large RR (2016) Pyrite trace element chemistry of the Velkerri Formation, Roper Group, McArthur Basin: evidence for atmospheric oxygenation during the Boring Billion. Precambrian Res 281:13–26 [Google Scholar]
- Muscente AD, Bykova N, Boag TH, et al. (2019) Ediacaran biozones identified with network analysis provide evidence for pulsed extinctions of early complex life. Nat Commun 10, doi: 10.1038/s41467-019-08837-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen K, Love GD, Zumberge JA, et al. (2019) Absence of biomarker evidence for early eukaryotic life from the Mesoproterozoic Roper Group: searching across a marine redox gradient in mid-Proterozoic habitability. Geobiology 17:247–260 [DOI] [PubMed] [Google Scholar]
- Och LM and Shields-Zhou GA (2012) The Neoproterozoic oxygenation event: environmental perturbations and biogeochemical cycling. Earth-Science Reviews 110:26–57 [Google Scholar]
- Olson SL, Kump LR, and Kasting JF (2013) Quantifying the areal extent and dissolved oxygen concentrations of Archean oxygen oases. Chem Geol 362:35–43 [Google Scholar]
- Olson SL, Reinhard CT, and Lyons TW (2016) Limited role for methane in the mid-Proterozoic greenhouse. Proc Natl Acad Sci USA 113:11447–11452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossa Ossa F, Eickmann B, Hofmann A, et al. (2018) Two-step deoxygenation at the end of the Paleoproterozoic Lomagundi Event. Earth Planet Sci Lett 486:70–83 [Google Scholar]
- Ostrander CM, Owens JD, Nielsen SG, et al. (2020) Thallium isotope ratios in shales from South China and northwestern Canada suggest widespread O2 accumulation in marine bottom waters was an uncommon occurrence during the Ediacaran Period. Chem Geol 557, doi: 10.1016/j.chemgeo.2020.119856 [DOI] [Google Scholar]
- Ozaki K, Reinhard CT, and Tajika E (2019) A sluggish mid-Proterozoic biosphere and its effect on Earth's redox balance. Geobiology 17:3–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace NR (1997) A molecular view of microbial diversity and the biosphere. Science 276:734–740 [DOI] [PubMed] [Google Scholar]
- Parfrey LW, Lahr DJ, Knoll AH, et al. (2011) Estimating the timing of early eukaryotic diversification with multigene molecular clocks. Proc Natl Acad Sci USA 108:13624–13629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partin CA, Bekker A, Planavsky NJ, et al. (2013) Large-scale fluctuations in Precambrian atmospheric and oceanic oxygen levels from the record of U in shales. Earth Planet Sci Lett 369:284–293 [Google Scholar]
- Pavlov AA, Hurtgen MT, Kasting JF, et al. (2003) Methane-rich Proterozoic atmosphere? Geology 31:87–90 [Google Scholar]
- Pehrsson SJ, Eglington BM, Evans DA, et al. (2016) Metallogeny and its link to orogenic style during the Nuna supercontinent cycle. Geological Society of London Special Publications 424:83–94 [Google Scholar]
- Peterson KJ, Summons RE, and Donoghue PC (2007) Molecular palaeobiology. Palaeontology 50:775–809 [Google Scholar]
- Planavsky N (2018) From orogenies to oxygen. Nat Geosci 11, doi: 10.1038/s41561-017-0040-1 [DOI] [Google Scholar]
- Planavsky NJ, Rouxel OJ, Bekker A, et al. (2010) The evolution of the marine phosphate reservoir. Nature 467:1088–1090 [DOI] [PubMed] [Google Scholar]
- Planavsky NJ, McGoldrick P, Scott CT, et al. (2011) Widespread iron-rich conditions in the mid-Proterozoic ocean. Nature 477:448–451 [DOI] [PubMed] [Google Scholar]
- Planavsky NJ, Reinhard CT, Wang X, et al. (2014) Low Mid-Proterozoic atmospheric oxygen levels and the delayed rise of animals. Science 346:635–638 [DOI] [PubMed] [Google Scholar]
- Planavsky NJ, Tarhan LG, Bellefroid EJ, et al. (2015). Late Proterozoic transitions in climate, oxygen, and tectonics, and the rise of complex life. In Earth-Life Transitions: Paleobiology in the Context of Earth System Evolution, The Paleontological Society Papers, Vol. 21, The Paleontological Society, Boulder, CO, pp 47–82 [Google Scholar]
- Planavsky NJ, Cole DB, Isson TT, et al. (2018) A case for low atmospheric oxygen levels during Earth's middle history. Emerg Top Life Sci 2:149–159 [DOI] [PubMed] [Google Scholar]
- Porter SM (2016) Tiny vampires in ancient seas: evidence for predation via perforation in fossils from the 780–740 million-year-old Chuar Group, Grand Canyon, USA. Proc R Soc Lond B Biol Sci 283, doi: 10.1098/rspb.2016.0221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter SM (2020) Insights into eukaryogenesis from the fossil record. Interface Focus 10, doi: 10.1098/rsfs.2019.0105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter SM, Agić H, and Riedman LA, 2018. Anoxic ecosystems and early eukaryotes. Emerg Top Life Sci 2:299–309 [DOI] [PubMed] [Google Scholar]
- Poulton SW and Canfield DE (2011) Ferruginous conditions: a dominant feature of the ocean through Earth's history. Elements 7:107–112 [Google Scholar]
- Poulton SW, Bekker A, Cumming VM, et al. (2021) A 200-million-year delay in permanent atmospheric oxygenation. Nature 592:232–236 [DOI] [PubMed] [Google Scholar]
- Pu JP, Bowring SA, Ramezani J, et al. (2016) Dodging snowballs: geochronology of the Gaskiers glaciation and the first appearance of the Ediacaran biota. Geology 44:955–958 [Google Scholar]
- Reinhard CT, Planavsky NJ, Robbins LJ, et al. (2013) Proterozoic ocean redox and biogeochemical stasis. Proc Natl Acad Sci USA 110:5357–5362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhard CT, Planavsky NJ, Olson SL, et al. (2016) Earth's oxygen cycle and the evolution of animal life. Proc Natl Acad Sci USA 113:8933–8938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhard CT, Planavsky NJ, Gill BC, et al. (2017a) Evolution of the global phosphorus cycle. Nature 541:386–389 [DOI] [PubMed] [Google Scholar]
- Reinhard CT, Olson SL, Schwieterman EW, et al. (2017b) False negatives for remote life detection on ocean-bearing planets: lessons from the early Earth. Astrobiology 17:287–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhard CT, Schwieterman EW, Olson SL, et al. (2019) The remote detectability of Earth's biosphere through time and the importance of UV capability for characterizing habitable exoplanets. arXiv:1903.05611 [Google Scholar]
- Reinhard CT, Planavsky NJ, Ward BA, et al. (2020) The impact of marine nutrient abundance on early eukaryotic ecosystems. Geobiology 18:139–151 [DOI] [PubMed] [Google Scholar]
- Riedman LA and Sadler PM (2018) Global species richness record and biostratigraphic potential of early to middle Neoproterozoic eukaryote fossils. Precambrian Res 319:6–18 [Google Scholar]
- Roberts NM (2013) The boring billion? Lid tectonics, continental growth and environmental change associated with the Columbia supercontinent. Geoscience Frontiers 4:681–691 [Google Scholar]
- Rooney AD, Strauss JV, Brandon AD, et al. (2015) A Cryogenian chronology: two long-lasting synchronous Neoproterozoic glaciations. Geology 43:459–462 [Google Scholar]
- Rooney AD, Cantine MD, Bergmann KD, et al. (2020) Calibrating the coevolution of Ediacaran life and environment. Proc Natl Acad Sci USA 117:16824–16830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rye R and Holland HD (1998) Paleosols and the evolution of atmospheric oxygen: a critical review. Am J Sci 298:621–672 [DOI] [PubMed] [Google Scholar]
- Sagan C, Thompson WR, Carlson R, et al. (1993) A search for life on Earth from the Galileo spacecraft. Nature 365:715–721 [DOI] [PubMed] [Google Scholar]
- Sahoo SK, Planavsky NJ, Kendall B, et al. (2012) Ocean oxygenation in the wake of the Marinoan glaciation. Nature 489:546–549 [DOI] [PubMed] [Google Scholar]
- Sahoo SK, Planavsky NJ, Jiang G, et al. (2016) Oceanic oxygenation events in the anoxic Ediacaran ocean. Geobiology 14:457–468 [DOI] [PubMed] [Google Scholar]
- Salminen J, Pehrsson S, Evans D, et al. (2020) Chapter 15: Neoarchean-Paleoproterozoic supercycle. In Ancient Supercontinents and the Paleogeography of the Earth. Elsevier, Amsterdam [Google Scholar]
- Schwieterman EW, Kiang NY, Parenteau MN, et al. (2018) Exoplanet biosignatures: a review of remotely detectable signs of life. Astrobiology 18:663–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott C, Lyons TW, Bekker A, et al. (2008) Tracing the stepwise oxygenation of the Proterozoic ocean. Nature 452:456–459 [DOI] [PubMed] [Google Scholar]
- Sergeev VN, Knoll AH, Vorob'eva NG, et al. (2016) Microfossils from the lower Mesoproterozoic Kaltasy formation, east European platform. Precambrian Res 278:87–107 [Google Scholar]
- Sheen AI, Kendall B, Reinhard CT, et al. (2018) A model for the oceanic mass balance of rhenium and implications for the extent of Proterozoic ocean anoxia. Geochim Cosmochim Acta 227:75–95 [Google Scholar]
- Shi W, Li C, Luo G, et al. (2018) Sulfur isotope evidence for transient marine-shelf oxidation during the Ediacaran Shuram Excursion. Geology 46:267–270 [Google Scholar]
- Shields GA, Mills BJ, Zhu M, et al. (2019) Unique Neoproterozoic carbon isotope excursions sustained by coupled evaporite dissolution and pyrite burial. Nature Geosci 12:823–827 [Google Scholar]
- Slack JF, Grenne T, Bekker A, et al. (2007) Suboxic deep seawater in the late Paleoproterozoic: evidence from hematitic chert and iron formation related to seafloor-hydrothermal sulfide deposits, central Arizona, USA. Earth Planet Sci Lett 255:243–256 [Google Scholar]
- Sperling EA, Frieder CA, Raman AV, et al. (2013a) Oxygen, ecology, and the Cambrian radiation of animals. Proc Natl Acad Sci USA 110:13446–13451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling EA, Halverson GP, Knoll AH, et al. (2013b) A basin redox transect at the dawn of animal life. Earth Planet Sci Lett 371:143–155 [Google Scholar]
- Sperling EA, Rooney AD, Hays L, et al. (2014) Redox heterogeneity of subsurface waters in the Mesoproterozoic ocean. Geobiology 12:373–386 [DOI] [PubMed] [Google Scholar]
- Sperling EA, Wolock CJ, Morgan AS, et al. (2015a) Statistical analysis of iron geochemical data suggests limited late Proterozoic oxygenation. Nature 523:451–454 [DOI] [PubMed] [Google Scholar]
- Sperling EA, Knoll AH, and Girguis PR (2015b) The ecological physiology of Earth's second oxygen revolution. Annu Rev Ecol Evol Syst 46:215–235 [Google Scholar]
- Sperling EA, Carbone C, Strauss JV, et al. (2016) Oxygen, facies, and secular controls on the appearance of Cryogenian and Ediacaran body and trace fossils in the Mackenzie Mountains of northwestern Canada. Geol Soc Am Bull 128:558–575 [Google Scholar]
- Steadman JA, Large RR, Blamey NJ, et al. (2020) Evidence for elevated and variable atmospheric oxygen in the Precambrian. Precambrian Res 343, doi: 10.1016/j.precamres.2020.105722 [DOI] [Google Scholar]
- Stolper DA and Keller CB (2018) A record of deep-ocean dissolved O2 from the oxidation state of iron in submarine basalts. Nature 553:323–327 [DOI] [PubMed] [Google Scholar]
- Takishita K, Chikaraishi Y, Tanifuji G, et al. (2017) Microbial eukaryotes that lack sterols. J Eukaryot Microbiol 64:897–900 [DOI] [PubMed] [Google Scholar]
- Tang D, Shi X, Wang X, et al. (2016) Extremely low oxygen concentration in mid-Proterozoic shallow seawaters. Precambrian Res 276:145–157 [Google Scholar]
- Tang M, Chu X, Hao J, et al. (2021) Orogenic quiescence in Earth's middle age. Science 371:728–731 [DOI] [PubMed] [Google Scholar]
- Tang Q, Pang K, Yuan X, et al. (2020) A one-billion-year-old multicellular chlorophyte. Nat Ecol Evol 4:543–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarhan LG, Droser ML, Planavsky NJ, et al. (2015) Protracted development of bioturbation through the early Palaeozoic Era. Nat Geosci 8:865–869 [Google Scholar]
- Taverne YJ, Merkus D, Bogers AJ, et al. (2018) Reactive oxygen species: radical factors in the evolution of animal life: a molecular timescale from Earth's earliest history to the rise of complex life. Bioessays 40, doi: 10.1002/bies.201700158 [DOI] [PubMed] [Google Scholar]
- Thomson D, Rainbird RH, Planavsky N, et al. (2015) Chemostratigraphy of the Shaler Supergroup Victoria Island NW Canada: a record of ocean composition prior to the Cryogenian glaciations. Precambrian Res 263:232–245 [Google Scholar]
- Turner EC and Bekker A (2016) Thick sulfate evaporite accumulations marking a mid-Neoproterozoic oxygenation event (Ten Stone Formation, Northwest Territories, Canada). Geol Soc Am Bull 128:203–222 [Google Scholar]
- Von Strandmann PAP, Stüeken EE, Elliott T, et al. (2015) Selenium isotope evidence for progressive oxidation of the Neoproterozoic biosphere. Nat Commun 6, doi: 10.1038/ncomms10157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde P (1987) Model of progressive ventilation of the late Precambrian–early Paleozoic ocean. Am J Sci 287:442–459 [Google Scholar]
- Xiao S and Laflamme M (2009) On the eve of animal radiation: phylogeny ecology and evolution of the Ediacara biota. Trends Ecol Evol 24:31–40 [DOI] [PubMed] [Google Scholar]
- Yang S, Kendall B, Lu X, et al. (2017) Uranium isotope compositions of mid-Proterozoic black shales: evidence for an episode of increased ocean oxygenation at 1.36 Ga and evaluation of the effect of post-depositional hydrothermal fluid flow. Precambrian Res 298:187–201 [Google Scholar]
- Yeung LY (2017) Low oxygen and argon in the Neoproterozoic atmosphere at 815 Ma. Earth Planet Sci Lett 480:66–74 [Google Scholar]
- Yierpan A, König S, Labidi J, et al. (2020) Recycled selenium in hot spot–influenced lavas records ocean-atmosphere oxygenation. Science Adv 6, doi: 10.1126/sciadv.abb6179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerkle AL and Mikhail S (2017) The geobiological nitrogen cycle: from microbes to the mantle. Geobiology 15:343–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Zhu X, Wood RA, et al. (2018) Oxygenation of the Mesoproterozoic ocean and the evolution of complex eukaryotes. Nat Geosci 11:345–350 [Google Scholar]
- Zhang S, Li ZX, Evans DA, et al. (2012) Pre-Rodinia supercontinent Nuna shaping up: a global synthesis with new paleomagnetic results from North China. Earth Planet Sci Lett 353:145–155 [Google Scholar]
- Zhang S, Wang X, Wang H, et al. (2016) Sufficient oxygen for animal respiration 1,400 million years ago. Proc Natl Acad Sci USA 113:1731–1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Reinhard CT, and Planavsky N (2018) Terrestrial methane fluxes and Proterozoic climate. Geology 46:139–142 [Google Scholar]
- Zhu S, Zhu M, Knoll AH, et al. (2016) Decimetre-scale multicellular eukaryotes from the 1.56-billion-year-old Gaoyuzhuang Formation in North China. Nat Commun 7, doi: 10.1038/ncomms11500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumberge JA, Love GD, Cárdenas P, et al. (2018) Demosponge steroid biomarker 26-methylstigmastane provides evidence for Neoproterozoic animals. Nat Ecol Evol 2:1709–1714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumberge JA, Rocher D, and Love GD (2020) Free and kerogen-bound biomarkers from late Tonian sedimentary rocks record abundant eukaryotes in mid-Neoproterozoic marine communities. Geobiology 18:326–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.