Abstract
Background
High‐resistance inspiratory muscle strength training (IMST) is a novel, time‐efficient physical training modality.
Methods and Results
We performed a double‐blind, randomized, sham‐controlled trial to investigate whether 6 weeks of IMST (30 breaths/day, 6 days/week) improves blood pressure, endothelial function, and arterial stiffness in midlife/older adults (aged 50–79 years) with systolic blood pressure ≥120 mm Hg, while also investigating potential mechanisms and long‐lasting effects. Thirty‐six participants completed high‐resistance IMST (75% maximal inspiratory pressure, n=18) or low‐resistance sham training (15% maximal inspiratory pressure, n=18). IMST was safe, well tolerated, and had excellent adherence (≈95% of training sessions completed). Casual systolic blood pressure decreased from 135±2 mm Hg to 126±3 mm Hg (P<0.01) with IMST, which was ≈75% sustained 6 weeks after IMST (P<0.01), whereas IMST modestly decreased casual diastolic blood pressure (79±2 mm Hg to 77±2 mm Hg, P=0.03); blood pressure was unaffected by sham training (all P>0.05). Twenty‐four hour systolic blood pressure was lower after IMST versus sham training (P=0.01). Brachial artery flow‐mediated dilation improved ≈45% with IMST (P<0.01) but was unchanged with sham training (P=0.73). Human umbilical vein endothelial cells cultured with subject serum sampled after versus before IMST exhibited increased NO bioavailability, greater endothelial NO synthase activation, and lower reactive oxygen species bioactivity (P<0.05). IMST decreased C‐reactive protein (P=0.05) and altered select circulating metabolites (targeted plasma metabolomics) associated with cardiovascular function. Neither IMST nor sham training influenced arterial stiffness (P>0.05).
Conclusions
High‐resistance IMST is a safe, highly adherable lifestyle intervention for improving blood pressure and endothelial function in midlife/older adults with above‐normal initial systolic blood pressure.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT03266510.
Keywords: exercise training, flow‐mediated dilation, hypertension, NO, oxidative stress, reactive oxygen species
Subject Categories: High Blood Pressure, Exercise, Aging, Mechanisms
Nonstandard Abbreviations and Acronyms
- AU
arbitrary units
- CFPWV
carotid‐femoral pulse wave velocity
- CV
coefficient of variation
- DBP
diastolic blood pressure
- eNOS
endothelial nitric oxide synthase
- FMDBA
brachial artery flow‐mediated dilation
- HUVEC
human umbilical vein endothelial cell
- IMST
inspiratory muscle strength training
- IMT
intima‐media thickness
- PIMAX
maximal inspiratory pressure
- PME‐
estrogen‐deficient postmenopausal
- ROS
reactive oxygen species
- SBP
systolic blood pressure
Clinical Perspective
What Is New?
This is the first trial in healthy midlife and older adults to show that high‐resistance inspiratory muscle strength training lowers blood pressure and improves endothelial function.
We provide the first evidence that inspiratory muscle strength training increases NO bioavailability by increasing endothelial NO synthase activation and decreasing oxidative stress.
We provide initial evidence that inspiratory muscle strength training decreases systemic inflammation, induces potentially beneficial changes to the plasma metabolome, promotes impressively high adherence, and is both safe and tolerable.
What Are the Clinical Implications?
Older individuals have a greater risk for cardiovascular diseases, partly because of increases in blood pressure and endothelial dysfunction.
Healthy lifestyle practices can decrease cardiovascular disease risk, but adherence is low.
This study demonstrates that high‐resistance inspiratory muscle strength training, a novel time‐ and effort‐efficient lifestyle intervention, lowers blood pressure, improves endothelial function, and promotes adherence in midlife and older adults with above‐normal systolic blood pressure at baseline. Improving blood pressure control and vascular endothelial function through inspiratory muscle strength training could decrease the risk of cardiovascular diseases and other clinical disorders.
Aging increases the risk of developing cardiovascular diseases (CVD),1, 2 the leading cause of death in developed societies.3 Much of this risk is attributable to increased blood pressure (BP), particularly systolic BP (SBP), which is a highly prevalent and clinically important modifiable CVD risk factor.4, 5, 6 Over 65% of midlife/older (aged ≥50 years) adults in the United States have above‐normal SBP (ie, ≥120 mm Hg).3 In addition to BP, endothelial dysfunction and increased arterial stiffness also are key features of cardiovascular aging that contribute to CVD risk.7, 8
The above changes in cardiovascular function with aging are driven, in part, by decreased NO bioavailability.8, 9 The reduction in NO bioavailability is, in turn, mediated by increases in reactive oxygen species (ROS; oxidative stress) that react with NO and possibly by reduced activation of the NO‐producing enzyme, endothelial NO synthase (eNOS).7, 9, 10 Chronic low‐grade inflammation also is an important mechanism that promotes cardiovascular aging.7, 11, 12
Aerobic exercise is one of the most evidence‐based interventions to promote healthy cardiovascular aging.7, 13, 14 However, adherence to aerobic exercise guidelines (150 min/wk moderate‐intensity aerobic exercise or 75 min/wk vigorous aerobic exercise15) is poor, with <40% of midlife/older adults meeting the guidelines.16, 17 This low rate of adherence is because of barriers such as lack of time, facility access, transportation, mobility issues, and financial costs of membership to exercise facilities.18, 19, 20, 21, 22, 23 Therefore, there is a need to establish novel lifestyle strategies that overcome these barriers to achieve high adherence while also showing efficacy for reducing SBP and improving vascular health in midlife/older adults.24
Inspiratory muscle strength training (IMST) is an alternative form of physical training that uses the diaphragm and accessory respiratory muscles to repeatedly inhale against resistance.24 Historically, clinical research studies assessing potential health benefits of IMST have used a training intensity of low/moderate resistance to inspiration (eg, ≈30% of maximal inspiratory pressure [PIMAX]) performed for a sustained duration (≥30 min/session), with multiple sessions per week, resulting in an overall weekly time commitment similar to moderate‐intensity aerobic exercise training guidelines.25, 26, 27, 28, 29 Moreover, many such studies performed IMST in a clinical research laboratory setting, requiring frequent travel to conduct the training sessions.30, 31, 32
More recently, Bailey and colleagues developed a novel high‐resistance paradigm of IMST that requires only 30 breaths (≈5 minutes) per session performed with an affordable and portable handheld device at home.33, 34 When performed 5 to 7 days (25–35 total minutes) per week for 6 weeks, reductions in casual (resting) BP, particularly SBP, were observed in young healthy adults,35, 36 as well as patients with obstructive sleep apnea.33, 34 The reductions in BP with IMST were associated with reductions in systemic vascular resistance in the absence of changes in heart rate,36 suggesting adaptations in vascular function.
To establish the safety, adherence, and efficacy of high‐resistance IMST for lowering SBP and improving vascular function in midlife/older adults with above‐normal SBP, we conducted a randomized, double‐blind, sham‐controlled trial of high‐resistance IMST for 6 weeks in adults aged 50 to 79 years with baseline SBP ≥120 mm Hg. Casual SBP was the primary outcome. To determine if at least part of the BP‐lowering effects of IMST are sustained, measurements were repeated 6 weeks after cessation of training. Twenty‐four hour (ambulatory) BP, endothelial function, and arterial stiffness were measured, and safety, tolerability, and adherence to IMST were documented. Blood biomarkers were assessed to determine the influence of IMST on systemic oxidative stress, inflammation, and sympathoadrenal activity. To determine if improvements in endothelial function with IMST were mediated by changes in circulating factors that increase endothelial NO bioavailability, cultured human umbilical vein endothelial cells (HUVECs) were incubated with serum obtained from subjects before and after IMST and sham training, and NO production, ROS bioactivity, and eNOS activation were assessed. Finally, to identify examples of circulating metabolites that may have been altered by IMST, we performed a targeted plasma metabolomics analysis of blood sampled from our subjects before and after IMST and sham training.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request. All procedures were reviewed and approved by the institutional review board at the University of Colorado, Boulder. The nature, benefits, and risks of all study procedures were explained to volunteers, and their written informed consent was obtained before participation in the study. The study was registered on CinicalTrials.gov (NCT03266510).
Participants
Men and postmenopausal women from Boulder County, Colorado and the surrounding areas were studied. All postmenopausal women were amenorrheic ≥1 year, and postmenopausal women aged ≤56 years had a follicle stimulating hormone concentration ≥40 IU/L. All participants demonstrated above‐normal SBP, defined as an average SBP ≥120 mm Hg taken on 2 occasions >24 hours apart. Potential participants were excluded if they had fasting plasma glucose ≥126 mg/dL, total cholesterol ≥240 mg/dL, uncontrolled thyroid disease, severe obesity (body mass index >40 kg/m2), were not weight stable (>3 kg change in body mass) for at least 3 months before enrolling in the study, or had alcohol dependence determined through a medical history and physical exam performed by the University of Colorado Boulder Clinical Translational Research Center study physician. Participants on antihypertensive or other prescription medications (eg, statins) were enrolled if their treatment regimen had been stable for >3 months and remained stable throughout the study.
Study Design and Randomization
The study consisted of a 6‐week randomized, double‐blind, sham‐controlled, parallel‐design clinical trial. Additionally, all subjects were invited to return 6 weeks after completing the intervention for an optional follow‐up assessment to gain initial insight into the long‐lasting effects of IMST on the primary outcome, casual SBP, and other functional measures. Subjects were randomized to either the high‐resistance IMST or the low‐resistance sham training control group by a study team member not involved in the assessment of study outcomes. Subjects were randomly assigned to IMST or sham training using a block randomization scheme. Subjects were stratified into a block based on age (midlife: aged 50–64 years versus older: aged 65–79 years), sex (men versus women), and SBP (elevated SBP/stage 1 hypertension: 120–139 mm Hg versus stage 2 hypertension: ≥140 mm Hg). Subjects within each block were randomly assigned to a group in a 1:1 ratio using a computer‐generated allocation sequence.
Intervention
Maximal Inspiratory Pressure
PIMAX was assessed by having each subject perform a series of maximal inspiratory efforts against a near‐infinite resistance using a custom‐built pressure transducer (Omega‐Dyne). These maneuvers were performed until the 3 largest pressures were within 5% of each other; PIMAX was defined as the average of these 3 measures.35, 37
IMST and Sham Training
Subjects in both groups used the POWERbreathe K3 inspiratory muscle training device. All subjects performed 30 inspiratory maneuvers (5 sets of 6, 1‐minute rest between sets), 6 days per week, for 6 weeks. Subjects in the IMST group trained at 55% PIMAX during week 1, 65% PIMAX during week 2, and 75% PIMAX during weeks 3 to 6. The sham groups trained at 15% PIMAX throughout the intervention. One training session per week was observed by an unblinded research assistant. PIMAX was assessed before each supervised training session and the training device was set to the appropriate resistance. The remaining 5 training sessions each week were performed unsupervised at home.
Adherence, Safety, and Tolerability
Adherence, safety, and tolerability were monitored throughout the intervention. Adherence, defined as the number and quality of completed versus prescribed training sessions, was monitored using the internal data storage of the POWERbreathe K3. Training session data were downloaded at the end of each week during supervised training sessions to determine adherence. The safety of the intervention was assessed by recording adverse events reported by participants, whereas tolerability was assessed by the rate at which enrolled subjects dropped out because of adverse events.
Measurements
All measurements were performed at the University of Colorado Boulder Clinical Translational Research Center or Integrative Physiology of Aging Laboratory. All outcome measures were obtained after a >12‐hour fast from food and caffeine (water allowed), and >24‐hour abstention from alcohol, physical activity, dietary supplements, and over‐the‐counter medications. Subjects continued to take antihypertensive medications as normal but refrained from taking all other prescription medications in the morning until after completing their experimental visit. Supervised training sessions were conducted without restrictions.
Participant Characteristics and Clinical Blood Assays
Body mass index was determined by anthropometry. Percent body fat was measured using dual‐energy x‐ray absorptiometry. Blood samples were drawn either via venipuncture (screening, follow‐up testing) or from an intravenous catheter (baseline, end‐intervention) placed in an antecubital vein. The Boulder Community Hospital Clinical Laboratory measured fasting serum lipids and plasma glucose at screening, baseline, end‐intervention, and follow‐up.
Blood markers of systemic oxidative stress, antioxidant defenses, inflammation, and sympathoadrenal activity were measured by the Colorado Clinical and Translational Sciences Institute Clinical Translational Research Center Core Laboratory at baseline and end‐intervention. Plasma interleukin (IL)‐6, IL‐10, and tumor necrosis factor α were measured by multiplex ELISA (R&D). Plasma oxidized low‐density lipoprotein was measured by ELISA (Mercordia). High‐sensitivity plasma C‐reactive protein (CRP) was measured by immunoturbidimetry (Beckman Coulter). Plasma total antioxidant status was determined by the colorimetric method (Randox Laboratories). Plasma norepinephrine and epinephrine were measured as markers of sympathoadrenal activity by high‐performance liquid chromatography (BioRad). Values below assay detection level were imputed as the lower limit of the detection level (epinephrine: 20 pg/mL, IL‐6: 1.9 pg/mL, IL‐10: 0.92 pg/mL; samples in all other assays were above detection limits). The day‐to‐day coefficient of variation (CV) for these assays is between 1.8% and 8.2%.
Blood Pressure
Casual SBP and diastolic BP (DBP) were measured according to American College of Cardiology/American Heart Association guidelines6 by a single trained investigator via brachial auscultation on the subject's nondominant arm after ≥5 minutes of quiet rest. Subjects were seated with their back supported, feet flat on the floor, and arm at heart level. BP was measured in triplicate, with 1 minute between each measurement. Casual BP was measured on 2 separate days at each time point (screening, baseline, end‐intervention, follow‐up), and the average value from the 6 BP measurements (3 per day) at each time point was reported. Our laboratory's day‐to‐day CVs for casual SBP and DBP are 3.4% and 3.7%, respectively.
Ambulatory BP was measured every 20 minutes during the day and every 60 minutes at night over 24 hours (Oscar 2; SunTech Medical). Ambulatory BP was measured at baseline and after 6 weeks of IMST or sham training. Daytime (16 hours) and nighttime (8 hours) were determined individually on the basis of personal sleep patterns, and the same day–night cycle was used pre‐ and end‐intervention. Ambulatory recordings were analyzed for mean 24‐hour, daytime, and nighttime SBP and DBP. Our laboratory's day‐to‐day CVs for 24‐hour SBP and DBP are 2.5% and 3.3%, respectively. We also calculated SBP and DBP dipping, defined as the percent difference between mean daytime and nighttime pressures.6
Vascular Endothelial Function
Endothelium‐dependent dilation was measured using brachial artery flow‐mediated dilation (FMDBA) following a 5‐minute forearm cuff occlusion with high‐resolution ultrasonography, as described previously.38, 39 FMDBA was measured on 2 separate days at each time point (baseline, end‐intervention, follow‐up), and the average percentage and absolute change from baseline diameter at each time point was reported.38 FMDBA peak shear rate was calculated as (8×mean velocity [m/s])/occlusion diameter (m).38 Brachial artery diameters at end diastole and blood velocities were captured and analyzed using Vascular Research Tools 5.10.9 (Medical Imaging Applications). Our day‐to‐day CVs for baseline brachial artery diameter and peak brachial diameter are 4.1% and 3.9%, respectively. Our day‐to‐day CV for FMDBA is 36%, which is on the lower end of the typical range of 13% to 84% reported in the literature.40, 41, 42 To explore any potential effects of changes in shear rate or resting brachial artery diameter on changes in endothelial function, we also analyzed FMDBA data normalized to peak shear and through allometric scaling for baseline diameter.43
To determine if changes in the circulating milieu following IMST may contribute to improving endothelial function by decreasing oxidative stress and subsequently increasing NO bioavailability, we performed innovative ex vivo experiments where HUVECs were treated with serum obtained from subjects before and after IMST or sham training. HUVECs (PromoCell; used after 2–4 passages) were plated in 96‐well culture plates and incubated under standard conditions (37°C, 5% CO2) for 24 hours in basal media supplemented with 10% subject serum. Following incubation, cells were coincubated with the fluorescent probes CellROX Deep Red (Thermo Fisher) to detect ROS production, and DAF‐FM diacetate (Cayman), to detect NO production. Cells stained with DAF‐FM diacetate were imaged 5 minutes after addition of 200 µmol/L acetylcholine (Sigma) to the cell culture media to stimulate NO production. Analysis was done with ImageJ (National Institutes of Health).
To assess the influence of circulating factors on eNOS activation for increasing NO bioavailability, HUVECs treated with 10% subject serum were washed in ice‐cold PBS and incubated with ice‐cold RIPA buffer containing protease and phosphatase inhibitors (Thermo Fisher) for 10 minutes. Cell lysates were then sonicated for 20 seconds and incubated on ice for 10 minutes. Thereafter, cell lysates were clarified by centrifugation at 13 000g at 4°C for 10 minutes. The supernatant was collected, and protein concentrations of phosphorylated (p)‐eNOSser1177, a primary activation site of eNOS,44 and p‐eNOSthr495, a key site of eNOS inactivation,44 were determined by capillary electrophoresis immunoassay (Wes; ProteinSimple) using rabbit antibodies (Thermo Fisher). Protein expression was normalized to total protein in the sample and presented as arbitrary units (AU).
Plasma Metabolomics
To further interrogate the circulating factors that may be mediating the response to IMST, targeted plasma metabolomics was performed. Plasma samples were thawed on ice, and a 20‐µL aliquot was diluted with 480‐µL of ice‐cold methanol/acetonitrile/water (5/3/2). Extractions were performed as previously described45 and resulting supernatants analyzed using a 5‐minute C18 gradient on a Thermo Fisher Vanquish ultra‐high‐performance liquid chromatograph coupled to a Thermo Fisher Q Exactive mass spectrometer exactly as previously described.46, 47 Raw files were converted to mzXML format using RawConverter (Yates Laboratory, The Scripps Research Institute, La Jolla, CA). Signals in the mass spectra were annotated and integrated using Maven (Princeton University, Princeton, NJ) in conjunction with the Kyoto Encyclopedia of Genes and Genomes database and an in‐house standard library. Partial least squares–discriminant analysis and heat maps with hierarchical clustering were prepared using MetaboAnalyst (Wishart Research Group, University of Alberta) version 4.0 following data normalization to median with autoscaling.
Arterial Stiffness
Aortic stiffness was measured using carotid–femoral pulse wave velocity (CFPWV), the gold‐standard measure of arterial stiffness in humans.48 Pressure waveforms were recorded simultaneously from the carotid and femoral arteries using applanation tonometry (Millar). The transit time of the aortic pulse wave was determined by measuring the time delay between the foot of the carotid and femoral pressure waves using LabChart (ADInstruments) analysis software. CFPWV was calculated by subtracting the distance from the suprasternal notch and the carotid measurement site from the distance between the suprasternal notch and the femoral measurement site, and dividing the resultant distance by the aortic transit time.49 Our laboratory's day‐to‐day CV for CFPWV is 5.4%.
Carotid artery compliance was assessed using ultrasonography to measure arterial diameter from end‐systole to end‐diastole and applanation tonometry to measure carotid arterial pressure changes and calculated as described previously.50 Intima‐media thickness (IMT) was measured at end‐diastole. Carotid artery diameters and IMT were captured and analyzed by Vascular Research Tools 5.10.9 (Medical Imaging Applications). Our day‐to‐day CV for carotid artery compliance is 12.0%, in line with reported reproducibility of 14%.51 Our day‐to‐day CV for IMT is 6.4%.
Statistical Analysis
Data from DeLucia et al36 indicated an effect size of high‐resistance IMST versus low‐resistance sham training on casual SBP, our primary outcome, of 1.28. Based on this, 18 subjects per group (36 total) were determined sufficient to observe a between‐group difference in the change in SBP from baseline to end‐intervention at 95% power and an α level of 0.05. Statistical analyses were performed with SPSS version 27 (IBM, Armonk, NY). PIMAX, casual BP, ambulatory BP, FMDBA, measures of arterial stiffness, anthropometrics, blood glucose and lipids, ex vivo endothelial cell function, plasma metabolomics, and plasma markers of inflammation, oxidative stress, and sympathoadrenal activity were measured at baseline and end‐intervention. Only casual BP, FMDBA, arterial stiffness, anthropometrics, blood glucose, and lipids were measured at the 6‐week follow‐up.
Continuous variables were assessed using a repeated measure analysis implemented with a mixed‐effects model. The outcome of interest (eg, casual SBP, FMDBA) was entered into the model as the dependent variable, whereas time point, group, and the interaction of time point and group were entered as dependent variables. For follow‐up testing analysis, Sidak's post hoc test was used for all within‐group comparisons to correct for multiple comparisons. The χ2 test was used to assess between‐group differences in categorical variables. The plasma metabolome of each group was assessed with partial least squares‐discriminant analysis, and heat maps with hierarchical clustering were prepared using MetabolAnalyst version 4.0 following data normalization to median with autoscaling. Between‐group differences in individual plasma metabolites were assessed with unpaired t tests. To gain initial insight into any potential trends for sex‐specific effects of the intervention on the main functional outcomes, changes in BP, endothelial function, and arterial stiffness pre–post IMST were assessed separately in women and men, though between‐group comparisons were not conducted because of the small number of subjects in those subgroups. Data are expressed as mean±SEM for continuous variables and as frequency for categorical variables. Statistical significance was set a priori at α=0.05.
Results
Participants
Sixty‐four participants consented for the study. Twenty‐six participants were either excluded or withdrew before study randomization. Thirty‐eight participants were equally randomized to each group, with 36 subjects (n=18 [9 men/9 women] IMST, n=18 [10 men/8 women] sham) included in the final analysis. Twenty‐nine participants (n=15 [7 men/8 women] IMST, n=14 [9 men/5 women] sham) completed optional follow‐up testing for casual BP. Reasons for exclusion and withdrawal are presented in Figure 1.
Figure 1. Participant progress through the study.
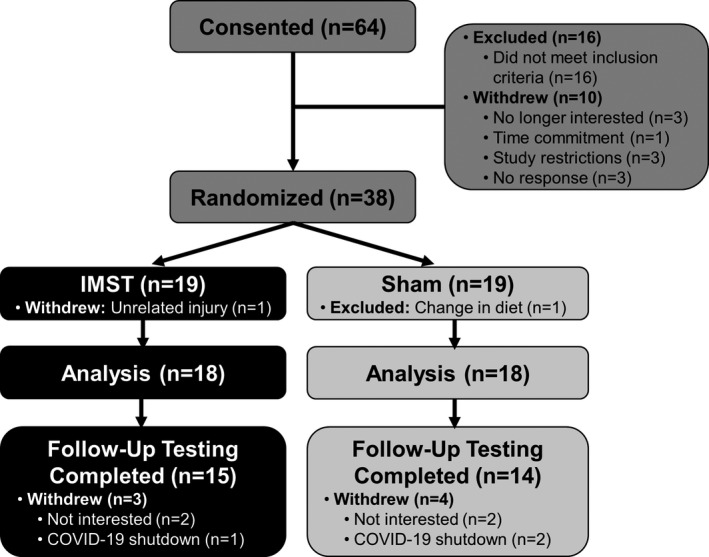
IMST indicates inspiratory muscle strength training.
Participant characteristics and clinical blood markers of the 36 participants who completed the 6‐week IMST/sham intervention trial were well matched because there were no differences between groups or changes across the 6‐week intervention (Table; all P>0.05). Subject characteristics for the 29 subjects who completed follow‐up testing were similarly well‐matched, with no differences between groups or changes across the intervention (Table S1; all P>0.05).
Table .
Subject Characteristics
| Sham | IMST | |||
|---|---|---|---|---|
| Baseline | End‐Intervention | Baseline | End‐Intervention | |
| No. of men/women | 10/8 | … | 9/9 | … |
| Age, y | 67±2 | … | 67±2 | … |
| Body mass, kg | 80±4 | 81±4 | 73±3 | 73±3 |
| Body mass index, kg/m2 | 27.2±0.5 | 27.5±0.5 | 25.7±1.0 | 25.7±1.0 |
| Body fat, % | 33±3 | 33±3 | 34±2 | 33±2 |
| Resting heart rate, beats/min | 63±2 | 63±3 | 65±3 | 64±3 |
| Total cholesterol, mg/dL | 169±7 | 172±7 | 169±5 | 169±5 |
| HDL‐cholesterol, mg/dL | 51±4 | 51±4 | 48±3 | 49±4 |
| LDL‐cholesterol, mg/dL | 99±5 | 101±5 | 100±4 | 100±4 |
| Triglycerides, mg/dL | 96±9 | 99±10 | 109±11 | 107±12 |
| Glucose, mg/dL | 91±2 | 89±1 | 90±1 | 90±2 |
Data are mean±SEM. HDL indicates high‐density lipoprotein; IMST, inspiratory muscle strength training; and LDL low‐density lipoprotein.
Adherence, Safety, and Tolerability
The average number of training sessions completed by the IMST group was 39.7±5.2 and by the sham group was 37.4±4.3, with no difference in the number of training sessions between groups (P=0.17). The IMST group completed 94.4% of prescribed training sessions, whereas the sham group completed 90.0% of prescribed training sessions. There was no difference in adherence between groups (P=0.17).
Two treatment‐related adverse events were reported by 2 subjects in the IMST group (neck muscle soreness, n=1; lightheadedness, n=1), both minor in severity. Neither subject dropped out because of treatment‐related adverse events. No adverse events occurred in the sham group.
Maximal Inspiratory Pressure
PIMAX increased from 64±5 mm Hg at baseline to 74±4 mm Hg end‐intervention in the IMST group (P<0.01), whereas PIMAX was unchanged in the sham group (baseline: 69±5 mm Hg, end‐intervention: 72±4 mm Hg; P=0.33). The group×time point interaction for PIMAX was P=0.06.
Blood Pressure
Casual BP
SBP decreased from 135±2 mm Hg at baseline to 126±3 mm Hg after 6 weeks of IMST (P<0.01) but was unchanged with sham training (baseline: 134±2 mm Hg, end‐intervention: 131±3 mm Hg; P=0.07). There was a significant interaction effect for casual SBP (P=0.02). When stratified by sex, IMST reduced casual SBP both in men (baseline: 136±3 mm Hg, end‐intervention: 125±3 mm Hg; P<0.01) and women (baseline: 134±3 mm Hg, end‐intervention: 127±4 mm Hg; P=0.02). Casual DBP decreased from 79±2 mm Hg at baseline to 77±2 mm Hg after 6 weeks of IMST (P=0.03) but was unchanged with sham training (baseline: 81±1 mm Hg, end‐intervention: 81±1 mm Hg; P=0.75) (Figure 2). The interaction effect for casual DBP was P=0.17. Casual DBP was 82±2 mm Hg at baseline versus 81±3 mm Hg after 6 weeks of IMST in men (P=0.16) and 76±3 mm Hg at baseline versus 74±3 mm Hg after IMST in women (P=0.01).
Figure 2. Casual systolic blood pressure (SBP) (A) and diastolic BP (DBP) (B) at baseline and after 6 weeks of inspiratory muscle strength training (IMST) or sham training.
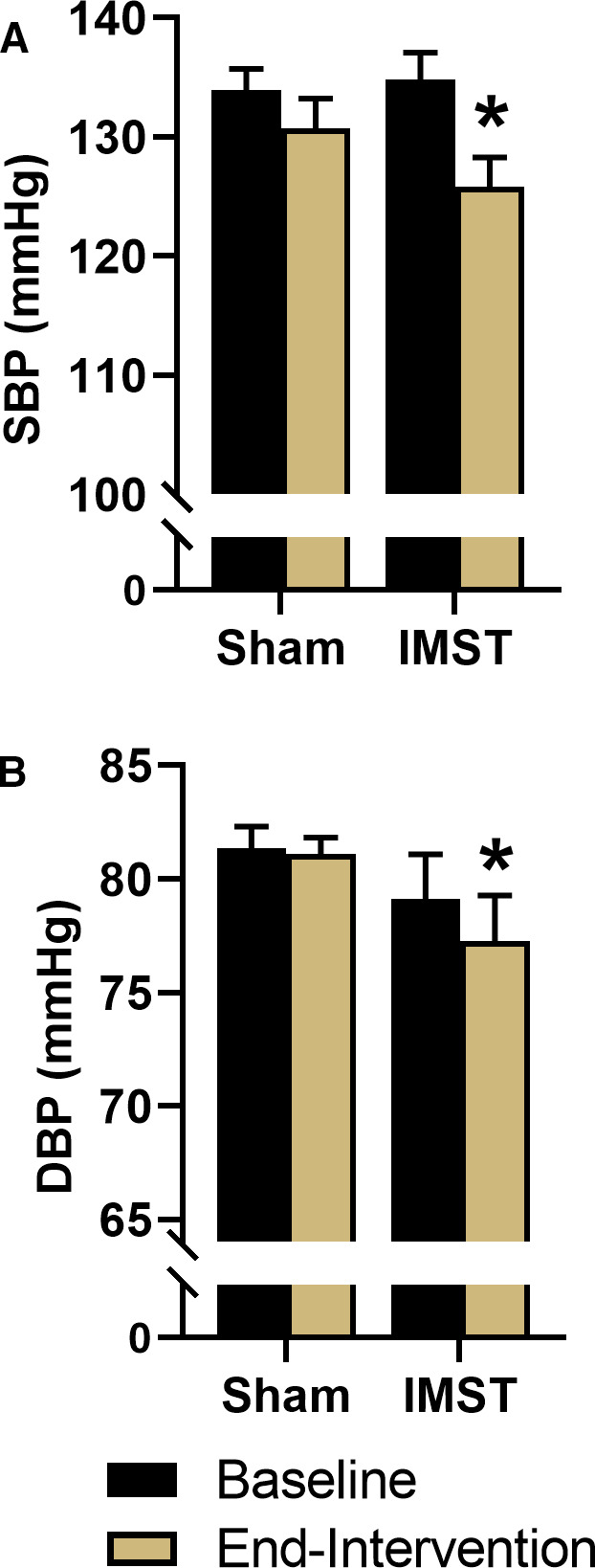
Data are mean±SEM. *P<0.05 vs baseline.
In the IMST subjects who completed follow‐up testing (n=15), the decrease in casual SBP from baseline to end‐intervention (135±2 mm Hg versus 126±3 mm Hg, P<0.01) was identical to the whole group (n=18). Six weeks after cessation of IMST, casual SBP in the subgroup remained significantly lower (128±4 mm Hg, P<0.01) than baseline and not different from end‐intervention (P=0.71). Casual SBP did not change at any time point in the subgroup of sham subjects (all P>0.05) (Figure 3A). There was a significant interaction effect for casual SBP for those who completed follow‐up testing (P=0.01). Casual DBP did not change at any time point or differ between groups in the subjects who completed follow‐up testing (Figure 3B; interaction effect P=0.31).
Figure 3. Casual systolic blood pressure (SBP) (A) and diastolic BP (DBP) (B) at baseline, after 6 weeks of inspiratory muscle strength training (IMST) or sham training and after 6 weeks of abstaining from training (follow‐up).
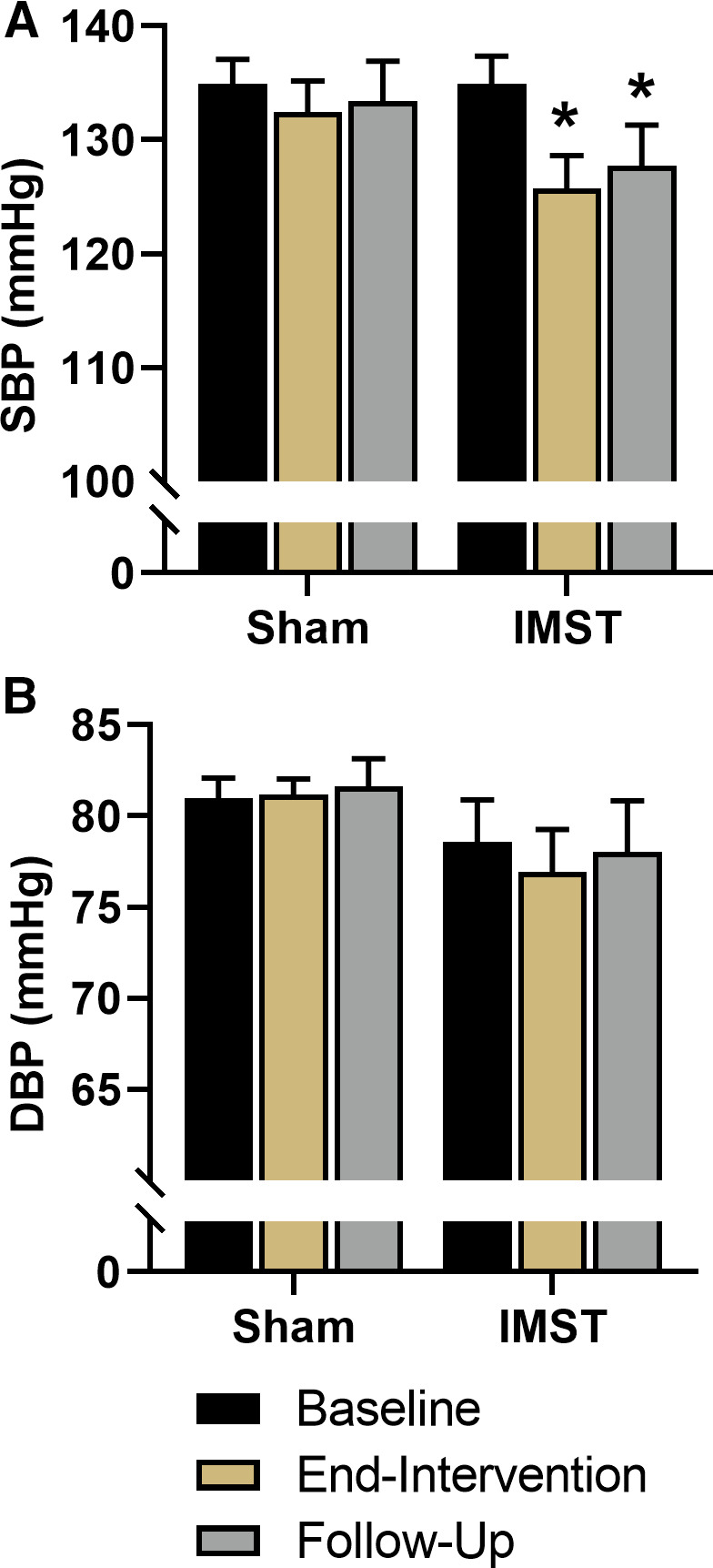
n=15 IMST, n=14 sham. Data are mean±SEM. *P<0.05 vs baseline.
Twenty‐Four‐Hour BP
Mean 24‐hour SBP did not change following 6 weeks of IMST (baseline: 131±3 mm Hg, end‐intervention: 127±3 mm Hg; P=0.52) but increased with sham training (baseline: 135±3 mm Hg, end‐intervention: 141±4 mm Hg; P=0.04) such that 24‐hour SBP was significantly lower in the IMST group versus sham end‐intervention (P=0.02). The interaction effect for 24‐hour SBP was P=0.07. When stratified by sex, 24‐hour SBP at baseline was 128±3 mm Hg versus 125±4 mm Hg after 6 weeks of IMST in men (P=0.90) and 133±4 mm Hg at baseline versus 128±4 mm Hg after IMST in women (P=0.58). Mean 24‐hour DBP was unchanged in the IMST group (baseline: 75±2 mm Hg, end‐intervention: 74±2 mm Hg; P=0.63) and increased modestly in the sham group (baseline: 74±2 mm Hg, end‐intervention: 78±2 mm Hg; P=0.04). However, 24‐hour DBP was not different between groups at either time point (P>0.05). The interaction effect for 24‐hour DBP was P=0.08. There was no influence of sex on 24‐hour DBP (data not shown).
Similar trends for daytime and nighttime SBP and DBP were observed compared with 24‐hour values (Table S2). Specifically, daytime and nighttime SBP and DBP did not change significantly across the intervention in the IMST group (all P>0.05), whereas nighttime SBP (P=0.05) and daytime DBP (P=0.02) increased modestly in the sham training group. At end‐intervention, daytime SBP (P=0.01), nighttime SBP (P=0.05), and nighttime DBP (P=0.05) were all lower in the IMST group versus sham. The interaction effects for daytime and nighttime SBP were P=0.07 and P=0.12, respectively, whereas the interaction effects for daytime and nighttime DBP were P=0.09 and P=0.16. There was no effect on BP dipping, defined as the percent change in BP values between daytime and nighttime measurement periods, for either SBP (interaction effect P=0.63) or DBP (interaction effect P=0.72) dipping (Table S2). There were no sex‐specific effects on any of these outcomes (data not shown).
Vascular Endothelial Function
In Vivo Endothelial Function (FMDBA)
When expressed as a percent change in brachial artery diameter, FMDBA was ≈45% higher after IMST versus baseline (P<0.01) and was unchanged with sham training (P=0.73), such that FMDBA was higher in the IMST versus sham group at end‐intervention (P=0.01) (Figure 4A and 4B); the same within‐ and between‐group differences were observed when expressed as an absolute (millimeters) change in brachial artery diameter (Table S3). The interaction effects for FMDBA expressed as either a relative or absolute change in brachial artery diameter were significant (all P<0.01). Brachial artery baseline diameter (interaction effect P=0.10), peak shear rate (interaction effect P=0.61), and time to peak dilation (interaction effect P=0.60) were unaffected (Table S3). Shear‐normalized FMDBA increased from baseline to end‐intervention with IMST (P=0.02) but not sham training (P=0.82), such that shear‐normalized FMDBA was significantly greater in the IMST versus sham training group at end‐intervention (P=0.03). The interaction effect for FMDBA normalized to peak shear rate was P=0.07. Allometric scaling was used to control for baseline diameter. Scaled FMDBA increased from baseline to end‐intervention with IMST (P<0.01) but not sham training (P=0.86), such that scaled FMDBA was significantly greater in the IMST versus sham training group at end‐intervention (P=0.01). The interaction effect for baseline scaled FMDBA was P=0.01 (Table S3).
Figure 4. Brachial artery flow‐mediated dilation (FMDBA) expressed as percent dilation in all subjects as average (A) and individual data (B), midlife/older men (n=9 inspiratory muscle strength training [IMST], n=10 sham) (C), and estrogen‐deficient postmenopausal (PME‐) women (n=7 IMST, n=8 sham) (D) at baseline and after 6 weeks of IMST or sham training.
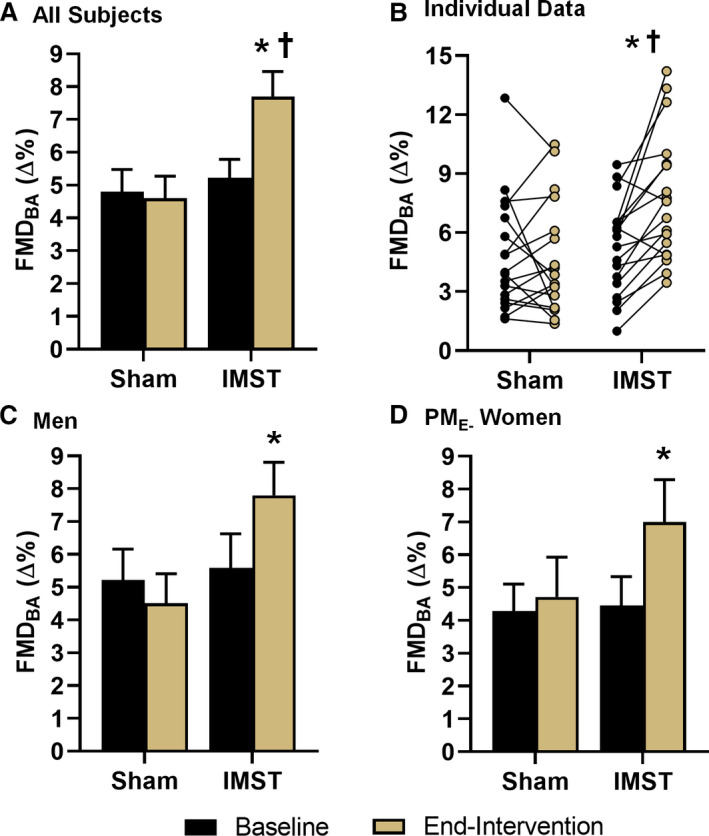
Data are mean±SEM. *P<0.05 vs baseline. † P<0.05 vs sham.
Our laboratory has found that aerobic exercise consistently improves vascular endothelial function in midlife/older men and postmenopausal women prescribed hormone replacement therapy, but not in estrogen‐deficient postmenopausal (PME‐) women.52, 53, 54, 55 In the present study, in men, FMDBA increased ≈40% with IMST (P<0.01) but was unchanged with sham training (P=0.20) (Figure 4C). The IMST group included 7 PME‐ women and 2 women who were taking hormone replacement therapy. In the 7 PME‐ women, FMDBA increased ≈57% with IMST (P=0.04) but was unchanged with sham training (P=0.68) (Figure 4D). FMDBA was increased +45% (P=0.05) with IMST when including the 2 women on hormone replacement therapy in the analysis.
Follow‐Up Period
In the subjects that completed follow‐up testing, FMDBA returned to baseline levels after 6 weeks of cessation from IMST; other brachial artery characteristics were unchanged at the follow‐up time point. No interaction effects were observed for any measure related to FMDBA at the follow‐up time point (all P>0.05) (Figure S1 and Table S4).
Ex Vivo Endothelial Function: Role of Circulating Factors on NO Bioavailability, eNOS Activation, and ROS Bioactivity
We have recently shown that in mice, interventions that increase endothelial function in intact arteries induce changes in the circulation that directly increase NO bioavailability in cultured endothelial cells.56 To assess this potential mechanism in the improvement in FMDBA in the present pilot clinical trial in humans, we treated HUVECs with subject serum obtained at baseline and end‐intervention for 24 hours. NO bioavailability in HUVECs treated with subject serum was slightly higher (+7%) after versus before sham training (P<0.01) but was more greatly increased (+17%) after versus before IMST (P<0.01). NO bioavailability was significantly greater after treatment in the IMST versus sham group (P=0.01) (Figure 5A). There was a significant interaction for NO bioavailability (P<0.01). The results were similar when examining only the men or PME‐ women (data not shown).
Figure 5. Human umbilical vein endothelial cell NO production (A), phosphorylated endothelial NO synthase (p‐eNOSser1177) abundance (B), and reactive oxygen species (ROS) activity (C), following a 24‐hour incubation with serum from subjects, with example fluorescent images below ROS activity and NO production.
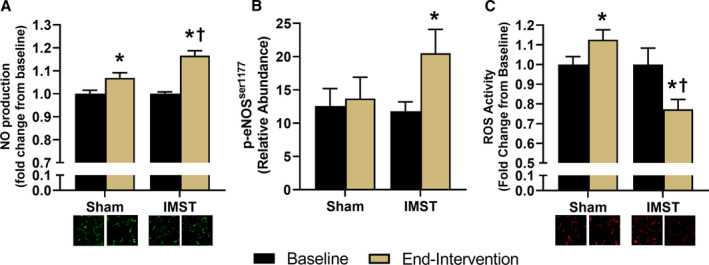
Data are mean±SEM. *P<0.05 vs baseline. † P<0.05 vs sham. IMST indicates inspiratory muscle strength training.
To determine if the greater enhancement of endothelial NO production with serum after IMST versus sham training was linked to increased eNOS activation, key eNOS activation (p‐eNOSser1177) and inhibition (p‐eNOSthr495) sites were measured in HUVECs treated with subject serum sampled at baseline or end‐intervention. Cell expression of p‐eNOSser1177 in serum‐treated HUVECs increased with IMST (P=0.003) but not sham training (P=0.68) (Figure 5B). The interaction effect for HUVEC expression of eNOS1177 was P=0.06. Treatment with subject serum did not change HUVEC expression of p‐eNOSthr495 in either the IMST (baseline: 24.5±2.7 AU, end‐intervention: 22.2±4.1 AU) or sham (baseline: 16.6±2.9 AU, end‐intervention: 17.0±3.1 AU; interaction effect P=0.62) groups. These patterns were similar when examining the men and PME‐ women subgroups (data not shown).
ROS, such as superoxide, readily react with NO, consequently reducing NO bioavailability. To determine if reduced ROS bioactivity may contribute to the greater NO bioavailability in HUVECs treated with serum after IMST versus sham training, we also assessed ROS in our HUVEC culture experiments. ROS bioactivity was decreased relative to baseline in the IMST group (P=0.01) but increased in the sham group (P=0.05) such that end‐intervention ROS activity was 35% lower in IMST versus sham serum‐treated HUVECs (Figure 5C) (P<0.01). There was a significant interaction effect for ROS bioactivity (P<0.01). The results were similar in the men and PME‐ and all women subgroups (data not shown).
Systemic Circulating Markers of Oxidative Stress, Inflammation, and Sympathoadrenal Activity
Plasma concentrations of CRP, a circulating marker of systemic inflammation, were 30% lower after IMST versus baseline (baseline: 1.37±0.23 mg/L, end‐intervention: 0.96±0.17 mg/L; P=0.05) but were unchanged with sham training (baseline: 1.19±0.35 mg/L, end‐intervention: 1.23±0.31 mg/L; P=0.85). The interaction effect for plasma CRP concentrations was P=0.12. In contrast, there were no significant between‐ or within‐group (across time points) differences in other circulating markers of inflammation (IL‐6 and tumor necrosis factor α), the anti‐inflammatory cytokine IL‐10, plasma oxidized low‐density lipoprotein (marker of oxidative stress), plasma total antioxidant capacity, or plasma norepinephrine or epinephrine (markers of sympathoadrenal activity) (Table S5) (all interaction effects P>0.05).
Plasma Metabolomics
Targeted plasma metabolomics was performed to gain initial insight into possible circulating factors and pathways involved in the changes in cardiovascular function associated with IMST. Variation of the plasma metabolomic profile between IMST and sham participants was assessed using partial least squares‐discriminant analysis, which revealed a small but consistent difference in the shift of the plasma metabolome between IMST and sham (Figure S2), with principal component 1 explaining 7.3% of the variance. From the metabolites with the highest partial least squares‐discriminant analysis loadings we identified potential markers of cardiovascular‐acting effects, which were observed to increase with IMST but not sham training. Among the metabolites with the highest loading, we identified 3 potential markers of cardiovascular‐acting effects, which were observed to increase with IMST but not sham training: L‐ornithine, an amino acid and precursor for the NO substrate, L‐arginine57 (IMST: 1.33±0.09‐fold change, sham: 1.03±0.06‐fold change; P=0.01); indole, a microbial metabolite reported to decrease BP through central nervous system mechanisms58 (IMST: 1.31±0.17‐fold change, sham: 0.89±0.08‐fold change; P=0.03); and hexanoic acid, a short‐chain fatty acid that may mediate anti‐inflammatory responses59 (IMST: 1.36±0.16‐fold change, sham: 0.96±0.09‐fold change; P=0.04).
Arterial Stiffness
There were no baseline differences between groups in any measure of arterial stiffness, including CFPWV, carotid artery compliance, and beta stiffness index, or for carotid IMT (all P>0.05), and none of these outcomes changed following 6 weeks of IMST or sham training (all P>0.05). Similarly, there were no between‐ or within‐group differences in carotid IMT (all P>0.05) (Table S6). Markers of arterial stiffness and carotid IMT remained unchanged following 6 weeks of cessation from IMST or sham training (all P>0.05) (Table S7). There were no significant interaction effects for any measure of arterial stiffness or carotid IMT in the whole group or the subgroup that completed follow‐up testing (all interaction effects P>0.05). No sex‐specific effects were observed (data not shown).
Discussion
In the current study, we performed a rigorous randomized, double‐blind, sham‐controlled, parallel‐design trial to evaluate the efficacy of high‐resistance IMST for improving cardiovascular function in midlife/older adults with above‐normal SBP. We show that 6 weeks of IMST reduced casual SBP, our primary outcome, and largely maintained this reduction following cessation from training. We also found that IMST increased FMDBA, a well‐established functional bioassay of NO‐mediated endothelium‐dependent dilation and vascular endothelial function. Endothelial cell culture experiments showed that exposure to serum from subjects who performed IMST increased NO production, and this improvement was associated with increased eNOS activation and reduced endothelial cell ROS bioactivity. In addition, we found that IMST reduced plasma CRP, the most well‐established clinical marker of systemic inflammation. IMST also changed concentrations of select plasma metabolites related to BP regulation, NO bioavailability, and inflammation. Lastly, 6 weeks of IMST was safe, tolerable, and promoted excellent adherence in our midlife/older subjects. Collectively, these findings provide support for high‐resistance IMST as a novel lifestyle intervention for lowering SBP and improving vascular endothelial function in both midlife/older men and postmenopausal women, with favorable adherence compared with other healthy lifestyle interventions.
Blood Pressure
We found that 6 weeks of high‐resistance IMST lowered casual SBP and DBP. This is the first study to demonstrate efficacy of high‐resistance IMST for lowering casual BP in otherwise healthy midlife/older adults with above‐normal SBP. Importantly, the observed 9‐mm Hg reduction in casual SBP is greater than or equal to reductions observed with other healthy lifestyle strategies,6 including aerobic exercise,6, 60 while requiring only ≈30 minutes per week. This improvement in SBP is clinically meaningful because it is associated with a 30% to 40% lower risk of death from CVD.61 The reduction in DBP with IMST was smaller, but our subjects had clinically normal DBP at baseline and aging is not associated with increases in DBP.8
The reduction in casual SBP was largely sustained after 6 weeks of abstaining from IMST, with about 75% of the initial reduction preserved. Our results suggest endothelial function and arterial stiffness are unlikely to be involved in the sustained reductions in SBP, because there were no differences in these measures of vascular function between the baseline versus follow‐up time points. At present, the mechanisms underlying the sustained reductions in casual SBP after cessation of IMST are unknown and require further investigation.
Casual SBP tends to rise quickly following cessation of aerobic exercise62, 63 or antihypertensive medication treatment,64, 65, 66 suggesting IMST may produce more long‐lasting reductions in SBP compared with other forms of physical training or pharmacotherapy. Deep breathing with low resistance is reported to have sustained SBP‐lowering effects as well, though with a much greater time requirement (≈30 min/d) than high‐resistance IMST.67 Moreover, our sham group controlled for the independent effects of deep breathing in our intervention. That SBP was unchanged in our sham subjects suggests the high‐intensity resistance of IMST is required for the initial and sustained reductions in SBP. This is supported by findings in young adults in which high‐resistance IMST performed with or without changes in lung volume elicit equal reductions in casual SBP, whereas inspiring with a large lung volume against a negligible resistance does not influence SBP.35
Twenty‐four‐hour daytime and nighttime SBP were lower in the IMST group compared with sham group at end‐intervention attributable to a trend for reductions in SBP with IMST concomitant with modest increases in the sham control group. Of note, the 4‐mm Hg reduction in 24‐hour SBP we observed following IMST is similar to the effectiveness of aerobic exercise for lowering 24‐hour SBP as described in a recent meta‐analysis.68 This observation suggests a comparable effect of IMST to aerobic exercise, but, again, with much less total weekly time commitment. BP dipping remained unchanged, because IMST equally affected daytime and nighttime pressures, though larger trials will be important to compare the effectiveness of IMST in groups with differing circadian BP profiles. Overall, these results are promising and suggest high‐resistance IMST may lower ambulatory measures of SBP.
Finally, our results indicate similar effects of high‐resistance IMST on BP in midlife/older men and postmenopausal women. This observation is consistent with reports that conventional aerobic exercise training also appears to have a similar influence on BP in men and women.69, 70 However, in contrast to aerobic exercise training, in which chronic reductions in BP are associated with a period of relative hypotension after each exercise training session,71 recent findings in young adults suggest that BP remains slightly elevated above baseline resting levels following one training bout of high‐resistance IMST, even as heart rate returns to resting levels.72 These findings further support the idea that high‐resistance IMST and aerobic exercise exert different acute influences on BP regulation immediately following a bout of training.
Endothelial Function
Here we show for the first time that IMST of any intensity and specifically high‐resistance IMST robustly improves vascular endothelial function, as indicated by a 45% mean increase in FMDBA in the overall group. The average improvement in FMDBA measurement units in response to IMST was ≈2.5∆%, which represents a potentially clinically meaningful effect because each 1∆%‐unit increase in FMDBA is associated with an 8% to 13% lower risk for incident CVD.73, 74, 75, 76, 77
The magnitude of improvement in FMDBA with IMST in the present study compares favorably with the responses to much more time‐ and effort‐consuming lifestyle therapies that we have studied in the past in this population, including conventional aerobic exercise,53, 55, 78 dietary sodium restriction,79 and energy restriction‐based weight loss.80 Importantly, we have shown that although time‐intensive aerobic exercise training increases endothelial function in previously inactive midlife/older men,53, 55 this commonly recommended, first‐line healthy lifestyle strategy does not consistently improve endothelial function in PME‐ women,52, 53, 54, 81 a group representing >90% of women aged 50 years and older. Our PME‐ women demonstrated similar or slightly greater improvements in FMDBA (≈2.5∆%) as their male peers (≈2.2∆%) when expressed in FMDBA measurement units. This suggests that IMST may work through somewhat different physiological signaling mechanisms than aerobic exercise in PME‐ women, and that IMST represents a highly promising lifestyle practice for improving vascular endothelial function in that large, ever‐growing population at increased CVD risk.3, 24, 81
FMDBA is an in vivo functional bioassay of endothelium‐dependent dilation largely mediated by NO. That FMDBA was improved following IMST suggests that there was an increase in NO bioavailability. We asked if changes in circulating factors induced by IMST were involved in the presumed improvement in NO bioavailability and, if so, through what mechanisms. Using an innovative ex vivo model to determine the effects of circulating factors in HUVEC culture, we found that serum from subjects who completed IMST induced an increase in NO bioavailability that was greater than that observed with exposure to sham training. The greater improvement in NO bioavailability in response to serum from IMST was associated with increased phosphorylation of the ser1177 activation site, but no change in the thr495 inhibitory site, of the NO‐producing enzyme, eNOS, suggestive of increased eNOS activation and NO production. In addition, serum from subjects who completed IMST reduced the production of potentially NO‐scavenging ROS by endothelial cells. Similar effects of IMST serum on HUVEC NO bioavailability, eNOS activation, and ROS bioactivity were observed in men and in PME‐ women as found in the overall cohort. In contrast to IMST, exposure to serum from sham‐trained subjects did not induce eNOS activation nor suppress ROS bioactivity. Taken together, the results of these novel translational assays suggest the improvements in endothelial function with IMST are driven, in part, by increased NO bioavailability secondary to increased eNOS activation and suppression of endothelial ROS bioactivity.
Finally, unlike the sustained effects observed with casual SBP, improvements in endothelial function did not persist 6 weeks after cessation of IMST. This suggests that, unlike SBP, IMST may need to be performed regularly to maintain benefits on endothelial function. The mechanisms by which IMST induces sustained improvements in SBP, but not endothelial function, 6 weeks after cessation of training are not clear, because we only assessed these functional outcomes and not our mechanistic probes at the follow‐up time point to decrease subject burden and maximize participant retention in this initial trial. However, it should be noted that our findings are consistent with previous observations that FMDBA rapidly returns to baseline levels after terminating aerobic exercise training.82, 83 Further investigation will be needed to gain insight into the mechanisms underlying the differential SBP and FMDBA responses to cessation of IMST.
Circulating Markers
We observed a reduction in CRP, the gold‐standard clinical marker of systemic inflammation and an evolving CVD risk factor,84 with IMST. This suggests that along with inhibiting ROS production and oxidative stress, IMST may lower BP and/or improve endothelial function by suppressing proinflammatory signaling. Plasma catecholamines, circulating markers of sympathoadrenal activity, were unchanged in our study. It has been reported that high‐resistance IMST reduces sympathetic activity in patients with obstructive sleep apnea, either measured directly with microneurography34 or using plasma norepinephrine concentrations.33 Our results do not show an obvious role for reductions in sympathetic activity contributing to improvements in cardiovascular function in our otherwise healthy midlife/older adults with above‐normal SBP. These differences may be due in part to the fact that obstructive sleep apnea is independently associated with increases in basal sympathetic activity.85 Baseline plasma norepinephrine concentrations in the obstructive sleep apnea patients who undertook high‐resistance IMST were nearly double those observed in our midlife/older cohort.33
To further identify the nature of the circulating factors that may have contributed to the effects of IMST on BP and vascular endothelial function, we performed a targeted plasma metabolomics analysis focusing on central energy and redox metabolism. We observed a modest but clear difference in the pre–post intervention changes in the overall plasma metabolite profiles of the IMST and sham groups. However, metabolomics analysis identified 3 metabolites, ornithine, indole, and hexanoic acid, that changed most with IMST but were unaffected by sham training. L‐ornithine is a byproduct of the urea cycle and a precursor of L‐arginine,57 the substrate for NO production by eNOS, whereas greater circulating L‐ornithine could reflect increased substrate bioavailability and NO production with IMST. Indole is an aromatic heterocyclic organic compound downstream of tryptophan that reportedly acts on the central nervous system to decrease BP,58 so it might be involved in the BP‐lowering effects of IMST. Finally, hexanoic acid is a short chain fatty acid. Short chain fatty acids are known to mediate anti‐inflammatory responses,59 so it is possible that the increase in hexanoic acid with IMST may play some role in the observed reduction in inflammation, shown by decreased plasma levels of CRP.
Arterial Stiffness
High‐resistance IMST did not improve measures of arterial stiffness, including CFPWV and carotid artery compliance and beta stiffness index. Unlike changes in endothelial function, which are mediated by rapid alterations in cellular signaling and occur quickly in response to lifestyle interventions,86, 87 changes in arterial stiffness typically involve remodeling of the extracellular matrix of the arterial wall and therefore generally require a longer duration, particularly in humans. For example, it often takes 3 months or more for aerobic exercise or other healthy lifestyle interventions to improve arterial stiffness.88 Thus, our 6‐week intervention may have been too short to observe benefits. Longer IMST interventions should be performed to see whether arterial stiffness improves.
Adherence, Safety, and Tolerability
Healthy lifestyle interventions, such as aerobic exercise, are first‐line strategies to lower BP and improve arterial function.6, 14 However, there are many deterrents to achieving healthy lifestyle guidelines, including time availability, cost, transportation, and mobility issues among others.18, 19, 20, 21, 22, 23 Here, we found that 30 minutes per week of IMST performed with a handheld device promotes excellent rates of adherence, because subjects in the IMST group completed ≈95% of prescribed training sessions at the appropriate intensity. In comparison, <40% of all midlife/older adults in the United States meet current recommendations for 150 minutes per week of moderate‐intensity aerobic exercise or 75 minutes per week of vigorous aerobic exercise.16, 17 It should be noted, however, that our subjects were predominantly non‐Hispanic White adults with a college degree or higher, a group with self‐reported rates of adherence to aerobic exercise of ≈65% to 70%.89 Although adherence to aerobic exercise in this demographic tends to be higher than the general population, it is still less than the 95% rate of adherence to IMST observed in this study. Thus, the rate of adherence to IMST observed here is promising compared with corresponding values for conventional forms of aerobic exercise. Additional studies that include more diverse populations and translation outside of the clinic are needed to fully determine adherence to high‐resistance IMST among a variety of demographic groups differing in race, ethnicity, and socioeconomic status.
In the present study, IMST also was shown to be safe, with only 2 minor intervention‐related adverse events reported, which involved neck muscle soreness and transient lightheadedness. Both events occurred early in the 6‐week intervention and dissipated with continued training. Neither participant experiencing an event dropped out of the study, suggesting high tolerability to the minor side effects of IMST.
Experimental Considerations
The results from this pilot study will need to be confirmed in a larger trial, perhaps with a longer treatment duration. It is possible that a longer‐term training stimulus would produce even greater improvements in cardiovascular function. These initial results suggest IMST is equally effective at improving endothelial function in midlife/older men and women, though larger studies are needed to confirm this finding. Comparative effectiveness trials of IMST versus aerobic exercise in midlife/older healthy and at‐risk adults, particularly PME‐ women, a group that does not consistently exhibit improvements in endothelial function following aerobic exercise training, would be highly informative from a medical guidelines and public health perspective. At present, the mechanism(s) through which IMST improves SBP and endothelial function is unknown. Repeated shear stress because of the large changes in lung volume may be an important stimulus, but the acute effects of high‐resistance IMST on arterial blood flow and shear rate have yet to be determined.
Conclusions
Here, we demonstrate that in both midlife/older men and postmenopausal women with above‐normal SBP, high‐resistance IMST lowers casual SBP and DBP, and reductions in the former are largely maintained at least 6 weeks after cessation of training. In addition, IMST lowers 24‐hour SBP compared with sham training and improves vascular endothelial function, in part by increasing NO bioavailability via increased eNOS activation and reduced ROS production and oxidative stress. Finally, IMST was found to be safe, tolerable, and to promote excellent adherence. Our results provide support for high‐resistance IMST as a promising lifestyle intervention for improving cardiovascular function and possibly decreasing the risk of CVD and other clinical disorders, such as cognitive dysfunction and chronic kidney disease.
Sources of Funding
This work was supported by National Institutes of Health awards R21AG061677, T32DK007135, UL1TR002535, P30CA046934 and American Heart Association Award 18POST33990034.
Disclosures
None.
Supporting information
Tables S1–S7
Figures S1–S2
Acknowledgments
The authors thank the staff of the University of Colorado Boulder Clinical and Translational Research Center for their technical assistance. The authors acknowledge Dr DeLucia for her helpful comments on the article.
(J Am Heart Assoc. 2021;10:e020980. DOI: 10.1161/JAHA.121.020980.)
See Editorial by Joyner and Baker
For Sources of Funding and Disclosures, see page 14.
References
- 1.Sistino JJ, Fitzgerald DC. Epidemiology of cardiovascular disease in the United States: implications for the perfusion profession. A 2017 update. Perfusion. 2017;32:501–506. DOI: 10.1177/0267659117696140. [DOI] [PubMed] [Google Scholar]
- 2.Joseph P, Leong D, McKee M, Anand SS, Schwalm J‐D, Teo K, Mente A, Yusuf S. Reducing the global burden of cardiovascular disease, part 1: the epidemiology and risk factors. Circ Res. 2017;121:677–694. DOI: 10.1161/CIRCRESAHA.117.308903. [DOI] [PubMed] [Google Scholar]
- 3.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, et al. Heart disease and stroke statistics‐2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–e596. DOI: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 4.Huang Y, Wang S, Cai X, Mai W, Hu Y, Tang H, Xu D. Prehypertension and incidence of cardiovascular disease: a meta‐analysis. BMC Med. 2013;11:177. DOI: 10.1186/1741-7015-11-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Danaei G, Ding EL, Mozaffarian D, Taylor B, Rehm J, Murray CJL, Ezzati M. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med. 2009;6:e1000058. DOI: 10.1371/journal.pmed.1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13–e115. DOI: 10.1161/HYP.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 7.Seals DR. Edward F. Adolph Distinguished Lecture: the remarkable anti‐aging effects of aerobic exercise on systemic arteries. J Appl Physiol. 2014;117:425–439. DOI: 10.1152/japplphysiol.00362.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107:139–146. DOI: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 9.Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clin Sci. 2011;120:357–375. DOI: 10.1042/CS20100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Durrant JR, Seals DR, Connell ML, Russell MJ, Lawson BR, Folian BJ, Donato AJ, Lesniewski LA. Voluntary wheel running restores endothelial function in conduit arteries of old mice: direct evidence for reduced oxidative stress, increased superoxide dismutase activity and down‐regulation of NADPH oxidase. J Physiol. 2009;587:3271–3285. DOI: 10.1113/jphysiol.2009.169771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guzik TJ, Touyz RM. Oxidative stress, inflammation, and vascular aging in hypertension. Hypertension. 2017;70:660–667. DOI: 10.1161/HYPERTENSIONAHA.117.07802. [DOI] [PubMed] [Google Scholar]
- 12.Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part III: cellular and molecular clues to heart and arterial aging. Circulation. 2003;107:490–497. DOI: 10.1161/01.cir.0000048894.99865.02. [DOI] [PubMed] [Google Scholar]
- 13.Craighead DH, Freeberg KA, Seals DR. The protective role of regular aerobic exercise on vascular function with aging. Curr Opin Physiol. 2019;10:55–63. DOI: 10.1016/j.cophys.2019.04.005. [DOI] [Google Scholar]
- 14.Seals DR, Brunt VE, Rossman MJ. Keynote lecture: strategies for optimal cardiovascular aging. Am J Physiol Heart Circ Physiol. 2018;315:H183–H188. DOI: 10.1152/ajpheart.00734.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, George SM, Olson RD. The physical activity guidelines for Americans. JAMA. 2018;320:2020–2028. DOI: 10.1001/jama.2018.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schoenborn CA, Stommel M. Adherence to the 2008 adult physical activity guidelines and mortality risk. Am J Prev Med. 2011;40:514–521. DOI: 10.1016/j.amepre.2010.12.029. [DOI] [PubMed] [Google Scholar]
- 17.Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40:181–188. DOI: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 18.Kelly S, Martin S, Kuhn I, Cowan A, Brayne C, Lafortune L. Barriers and facilitators to the uptake and maintenance of healthy behaviours by people at mid‐life: a rapid systematic review. PLoS One. 2016;11:e0145074. DOI: 10.1371/journal.pone.0145074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siddiqi Z, Tiro JA, Shuval K. Understanding impediments and enablers to physical activity among African American adults: a systematic review of qualitative studies. Health Educ Res. 2011;26:1010–1024. DOI: 10.1093/her/cyr068. [DOI] [PubMed] [Google Scholar]
- 20.Yarwood J, Carryer J, Gagan MJ. Women maintaining physical activity at midlife: contextual complexities. Nurs Prax N Z. 2005;21:24–37. [PubMed] [Google Scholar]
- 21.Babakus WS, Thompson JL. Physical activity among South Asian women: a systematic, mixed‐methods review. Int J Behav Nutr Phys Act. 2012;9:150. DOI: 10.1186/1479-5868-9-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stutts WC. Physical activity determinants in adults. Perceived benefits, barriers, and self efficacy. AAOHN J. 2002;50:499–507. DOI: 10.1177/216507990205001106. [DOI] [PubMed] [Google Scholar]
- 23.El Ansari W, Lovell G. Barriers to exercise in younger and older non‐exercising adult women: a cross sectional study in London, United Kingdom. Int J Environ Res Public Health. 2009;6:1443–1455. DOI: 10.3390/ijerph6041443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Craighead DH, Heinbockel TC, Hamilton MN, Bailey EF, MacDonald MJ, Gibala MJ, Seals DR. Time‐efficient physical training for enhancing cardiovascular function in midlife and older adults: promise and current research gaps. J Appl Physiol. 2019;127:1427–1440. DOI: 10.1152/japplphysiol.00381.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saglam M, Arikan H, Vardar‐Yagli N, Calik‐Kutukcu E, Inal‐Ince D, Savci S, Akdogan A, Yokusoglu M, Kaya EB, Tokgozoglu L. Inspiratory muscle training in pulmonary arterial hypertension. J Cardiopulm Rehabil Prev. 2015;35:198–206. DOI: 10.1097/HCR.0000000000000117. [DOI] [PubMed] [Google Scholar]
- 26.Ferreira JB, Plentz RDM, Stein C, Casali KR, Arena R, Lago PD. Inspiratory muscle training reduces blood pressure and sympathetic activity in hypertensive patients: a randomized controlled trial. Int J Cardiol. 2013;166:61–67. DOI: 10.1016/j.ijcard.2011.09.069. [DOI] [PubMed] [Google Scholar]
- 27.Kaminski DM, Schaan BD, da Silva AMV, Soares PP, Lago PD. Inspiratory muscle training in patients with diabetic autonomic neuropathy: a randomized clinical trial. Clin Auton Res. 2015;25:263–266. DOI: 10.1007/s10286-015-0291-0. [DOI] [PubMed] [Google Scholar]
- 28.Dall’Ago P, Chiappa GRS, Guths H, Stein R, Ribeiro JP. Inspiratory muscle training in patients with heart failure and inspiratory muscle weakness: a randomized trial. J Am Coll Cardiol. 2006;47:757–763. DOI: 10.1016/j.jacc.2005.09.052. [DOI] [PubMed] [Google Scholar]
- 29.Corrêa APS, Ribeiro JP, Balzan FM, Mundstock L, Ferlin EL, Moraes RS. Inspiratory muscle training in type 2 diabetes with inspiratory muscle weakness. Med Sci Sports Exerc. 2011;43:1135–1141. DOI: 10.1249/MSS.0b013e31820a7c12. [DOI] [PubMed] [Google Scholar]
- 30.Weiner P, Waizman J, Magadle R, Berar‐Yanay N, Pelled B. The effect of specific inspiratory muscle training on the sensation of dyspnea and exercise tolerance in patients with congestive heart failure. Clin Cardiol. 1999;22:727–732. DOI: 10.1002/clc.4960221110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adamopoulos S, Schmid J‐P, Dendale P, Poerschke D, Hansen D, Dritsas A, Kouloubinis A, Alders T, Gkouziouta A, Reyckers I, et al. Combined aerobic/inspiratory muscle training vs. aerobic training in patients with chronic heart failure: the Vent‐HeFT trial: a European prospective multicentre randomized trial. Eur J Heart Fail. 2014;16:574–582. DOI: 10.1002/ejhf.70. [DOI] [PubMed] [Google Scholar]
- 32.Mancini DM, Henson D, La Manca J, Donchez L, Levine S. Benefit of selective respiratory muscle training on exercise capacity in patients with chronic congestive heart failure. Circulation. 1995;91:320–329. DOI: 10.1161/01.cir.91.2.320. [DOI] [PubMed] [Google Scholar]
- 33.Vranish JR, Bailey EF. Inspiratory muscle training improves sleep and mitigates cardiovascular dysfunction in obstructive sleep apnea. Sleep. 2016;39:1179–1185. DOI: 10.5665/sleep.5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramos‐Barrera GE, DeLucia CM, Bailey EF. Inspiratory muscle strength training lowers blood pressure and sympathetic activity in older adults with OSA: a randomized controlled pilot trial. J Appl Physiol. 2020;129:449–458. DOI: 10.1152/japplphysiol.00024.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vranish JR, Bailey EF. Daily respiratory training with large intrathoracic pressures, but not large lung volumes, lowers blood pressure in normotensive adults. Respir Physiol Neurobiol. 2015;216:63–69. DOI: 10.1016/j.resp.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 36.DeLucia CM, De Asis RM, Bailey EF. Daily inspiratory muscle training lowers blood pressure and vascular resistance in healthy men and women. Exp Physiol. 2018;103:201–211. DOI: 10.1113/EP086641. [DOI] [PubMed] [Google Scholar]
- 37.Kellerman BA, Martin AD, Davenport PW. Inspiratory strengthening effect on resistive load detection and magnitude estimation. Med Sci Sports Exerc. 2000;32:1859–1867. DOI: 10.1097/00005768-200011000-00007. [DOI] [PubMed] [Google Scholar]
- 38.Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow‐mediated dilation. Hypertension. 2010;55:1075–1085. DOI: 10.1161/HYPERTENSIONAHA.110.150821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow‐mediated dilatation with sedentary and physically active human ageing. J Physiol. 2004;556:315–324. DOI: 10.1113/jphysiol.2003.057042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.West SG, Wagner P, Schoemer SL, Hecker KD, Hurston KL, Likos Krick A, Boseska L, Ulbrecht J, Hinderliter AL. Biological correlates of day‐to‐day variation in flow‐mediated dilation in individuals with type 2 diabetes: a study of test‐retest reliability. Diabetologia. 2004;47:1625–1631. DOI: 10.1007/s00125-004-1502-8. [DOI] [PubMed] [Google Scholar]
- 41.Kanani PM, Sinkey CA, Browning RL, Allaman M, Knapp HR, Haynes WG. Role of oxidant stress in endothelial dysfunction produced by experimental hyperhomocyst(e)inemia in humans. Circulation. 1999;100:1161–1168. DOI: 10.1161/01.cir.100.11.1161. [DOI] [PubMed] [Google Scholar]
- 42.De Roos NM, Bots ML, Schouten EG, Katan MB. Within‐subject variability of flow‐mediated vasodilation of the brachial artery in healthy men and women: implications for experimental studies. Ultrasound Med Biol. 2003;29:401–406. DOI: 10.1016/s0301-5629(02)00709-3. [DOI] [PubMed] [Google Scholar]
- 43.Thijssen DHJ, Bruno RM, van Mil ACCM, Holder SM, Faita F, Greyling A, Zock PL, Taddei S, Deanfield JE, Luscher T, et al. Expert consensus and evidence‐based recommendations for the assessment of flow‐mediated dilation in humans. Eur Heart J. 2019;40:2534–2547. DOI: 10.1093/eurheartj/ehz350. [DOI] [PubMed] [Google Scholar]
- 44.Bauer PM, Fulton D, Boo YC, Sorescu GP, Kemp BE, Jo H, Sessa WC. Compensatory phosphorylation and protein‐protein interactions revealed by loss of function and gain of function mutants of multiple serine phosphorylation sites in endothelial nitric‐oxide synthase. J Biol Chem. 2003;278:14841–14849. DOI: 10.1074/jbc.M211926200. [DOI] [PubMed] [Google Scholar]
- 45.Nemkov T, D’Alessandro A, Hansen KC. Three‐minute method for amino acid analysis by UHPLC and high‐resolution quadrupole orbitrap mass spectrometry. Amino Acids. 2015;47:2345–2357. DOI: 10.1007/s00726-015-2019-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nemkov T, Reisz JA, Gehrke S, Hansen KC, D’Alessandro A. High‐throughput metabolomics: isocratic and gradient mass spectrometry‐based methods. Methods Mol Biol. 2019;1978:13–26. DOI: 10.1007/978-1-4939-9236-2_2. [DOI] [PubMed] [Google Scholar]
- 47.Gehrke S, Rice S, Stefanoni D, Wilkerson RB, Nemkov T, Reisz JA, Hansen KC, Lucas A, Cabrales P, Drew K, et al. Red blood cell metabolic responses to torpor and arousal in the hibernator arctic ground squirrel. J Proteome Res. 2019;18:1827–1841. DOI: 10.1021/acs.jproteome.9b00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker‐Boudier H; European Network for Non‐invasive Investigation of Large Arteries . Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. DOI: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 49.Martens CR, Denman BA, Mazzo MR, Armstrong ML, Reisdorph N, McQueen MB, Chonchol M, Seals DR. Chronic nicotinamide riboside supplementation is well‐tolerated and elevates NAD+ in healthy middle‐aged and older adults. Nat Commun. 2018;9:1286. DOI: 10.1038/s41467-018-03421-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Santos‐Parker JR, Strahler TR, Bassett CJ, Bispham NZ, Chonchol MB, Seals DR. Curcumin supplementation improves vascular endothelial function in healthy middle‐aged and older adults by increasing nitric oxide bioavailability and reducing oxidative stress. Aging. 2017;9:187–208. DOI: 10.18632/aging.101149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gamble G, Zorn J, Sanders G, MacMahon S, Sharpe N. Estimation of arterial stiffness, compliance, and distensibility from M‐mode ultrasound measurements of the common carotid artery. Stroke. 1994;25:11–16. DOI: 10.1161/01.str.25.1.11. [DOI] [PubMed] [Google Scholar]
- 52.Santos‐Parker JR, Strahler TR, Vorwald VM, Pierce GL, Seals DR. Habitual aerobic exercise does not protect against micro‐ or macrovascular endothelial dysfunction in healthy estrogen‐deficient postmenopausal women. J Appl Physiol. 2017;122:11–19. DOI: 10.1152/japplphysiol.00732.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pierce GL, Eskurza I, Walker AE, Fay TN, Seals DR. Sex‐specific effects of habitual aerobic exercise on brachial artery flow‐mediated dilation in middle‐aged and older adults. Clin Sci. 2011;120:13–23. DOI: 10.1042/CS20100174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moreau KL, Stauffer BL, Kohrt WM, Seals DR. Essential role of estrogen for improvements in vascular endothelial function with endurance exercise in postmenopausal women. J Clin Endocrinol Metab. 2013;98:4507–4515. DOI: 10.1210/jc.2013-2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age‐related declines in endothelium‐dependent vasodilation in healthy men. Circulation. 2000;102:1351–1357. DOI: 10.1161/01.CIR.102.12.1351. [DOI] [PubMed] [Google Scholar]
- 56.Ballak DB, Brunt VE, Sapinsley ZJ, Ziemba BP, Richey JJ, Zigler MC, Johnson LC, Gioscia‐Ryan RA, Culp‐Hill R, Eisenmesser EZ, et al. Short‐term interleukin‐37 treatment improves vascular endothelial function, endurance exercise capacity, and whole‐body glucose metabolism in old mice. Aging Cell. 2020;19:e13074. DOI: 10.1111/acel.13074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morris SM. Regulation of enzymes of the urea cycle and arginine metabolism. Annu Rev Nutr. 2002;22:87–105. DOI: 10.1146/annurev.nutr.22.110801.140547. [DOI] [PubMed] [Google Scholar]
- 58.Huć T, Nowinski A, Drapala A, Konopelski P, Ufnal M. Indole and indoxyl sulfate, gut bacteria metabolites of tryptophan, change arterial blood pressure via peripheral and central mechanisms in rats. Pharmacol Res. 2018;130:172–179. DOI: 10.1016/j.phrs.2017.12.025. [DOI] [PubMed] [Google Scholar]
- 59.Nishitsuji K, Xiao J, Nagatomo R, Umemoto H, Morimoto Y, Akatsu H, Inoue K, Tsuneyama K. Analysis of the gut microbiome and plasma short‐chain fatty acid profiles in a spontaneous mouse model of metabolic syndrome. Sci Rep. 2017;7:15876. DOI: 10.1038/s41598-017-16189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Whelton SP, Chin A, Xin X, He J. Effect of aerobic exercise on blood pressure: a meta‐analysis of randomized, controlled trials. Ann Intern Med. 2002;136:493–503. DOI: 10.7326/0003-4819-136-7-200204020-00006. [DOI] [PubMed] [Google Scholar]
- 61.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration . Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. DOI: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 62.Nolan PB, Keeling SM, Robitaille CA, Buchanan CA, Dalleck LC. The effect of detraining after a period of training on cardiometabolic health in previously sedentary individuals. Int J Environ Res Public Health. 2018;15:2303. DOI: 10.3390/ijerph15102303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mora‐Rodriguez R, Ortega JF, Hamouti N, Fernandez‐Elias VE, Cañete Garcia‐Prieto J, Guadalupe‐Grau A, Saborido A, Martin‐Garcia M, Guio de Prada V, Ara I, et al. Time‐course effects of aerobic interval training and detraining in patients with metabolic syndrome. Nutr Metab Cardiovasc Dis. 2014;24:792–798. DOI: 10.1016/j.numecd.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 64.Nelson MR, Reid CM, Krum H, Muir T, Ryan P, McNeil JJ. Predictors of normotension on withdrawal of antihypertensive drugs in elderly patients: prospective study in second Australian national blood pressure study cohort. BMJ. 2002;325:815. DOI: 10.1136/bmj.325.7368.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sasamura H, Nakaya H, Julius S, Tomotsugu N, Sato Y, Takahashi F, Takeuchi M, Murakami M, Ryuzaki M, Itoh H, et al. Feasibility of regression of hypertension using contemporary antihypertensive agents. Am J Hypertens. 2013;26:1381–1388. DOI: 10.1093/ajh/hpt105. [DOI] [PubMed] [Google Scholar]
- 66.van der Wardt V, Harrison JK, Welsh T, Conroy S, Gladman J. Withdrawal of antihypertensive medication: a systematic review. J Hypertens. 2017;35:1742–1749. DOI: 10.1097/HJH.0000000000001405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sangthong B, Ubolsakka‐Jones C, Pachirat O, Jones DA. Breathing training for older patients with controlled isolated systolic hypertension. Med Sci Sports Exerc. 2016;48:1641–1647. DOI: 10.1249/MSS.0000000000000967. [DOI] [PubMed] [Google Scholar]
- 68.Saco‐Ledo G, Valenzuela PL, Ruiz‐Hurtado G, Ruilope LM, Lucia A. Exercise reduces ambulatory blood pressure in patients with hypertension: a systematic review and meta‐analysis of randomized controlled trials. J Am Heart Assoc. 2020;9:e018487. DOI: 10.1161/JAHA.120.018487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fagard RH, Cornelissen VA. Effect of exercise on blood pressure control in hypertensive patients. Eur J Cardiovasc Prev Rehabil. 2007;14:12–17. DOI: 10.1097/HJR.0b013e3280128bbb. [DOI] [PubMed] [Google Scholar]
- 70.Cornelissen VA, Buys R, Smart NA. Endurance exercise beneficially affects ambulatory blood pressure: a systematic review and meta‐analysis. J Hypertens. 2013;31:639–648. DOI: 10.1097/HJH.0b013e32835ca964. [DOI] [PubMed] [Google Scholar]
- 71.Halliwill JR. Mechanisms and clinical implications of post‐exercise hypotension in humans. Exerc Sport Sci Rev. 2001;29:65–70. DOI: 10.1097/00003677-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 72.DeLucia CM, DeBonis DR, Schwyhart SM, Bailey EF. Acute cardiovascular responses to a single bout of high intensity inspiratory muscle strength training in healthy young adults. J Appl Physiol. 2021;130:114–1121. DOI: 10.1152/japplphysiol.01015.2020. [DOI] [PubMed] [Google Scholar]
- 73.Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow‐mediated vasodilatation of brachial artery: a meta‐analysis. Int J Cardiovasc Imaging. 2010;26:631–640. DOI: 10.1007/s10554-010-9616-1. [DOI] [PubMed] [Google Scholar]
- 74.Green DJ, Jones H, Thijssen D, Cable NT, Atkinson G. Flow‐mediated dilation and cardiovascular event prediction: does nitric oxide matter? Hypertension. 2011;57:363–369. DOI: 10.1161/HYPERTENSIONAHA.110.167015. [DOI] [PubMed] [Google Scholar]
- 75.Ras RT, Streppel MT, Draijer R, Zock PL. Flow‐mediated dilation and cardiovascular risk prediction: a systematic review with meta‐analysis. Int J Cardiol. 2013;168:344–351. DOI: 10.1016/j.ijcard.2012.09.047. [DOI] [PubMed] [Google Scholar]
- 76.Xu Y, Arora RC, Hiebert BM, Lerner B, Szwajcer A, McDonald K, Rigatto C, Komenda P, Sood MM, Tangri N. Non‐invasive endothelial function testing and the risk of adverse outcomes: a systematic review and meta‐analysis. Eur Heart J Cardiovasc Imaging. 2014;15:736–746. DOI: 10.1093/ehjci/jet256. [DOI] [PubMed] [Google Scholar]
- 77.Matsuzawa Y, Kwon T‐G, Lennon RJ, Lerman LO, Lerman A. Prognostic value of flow‐mediated vasodilation in brachial artery and fingertip artery for cardiovascular events: a systematic review and meta‐analysis. J Am Heart Assoc. 2015;4:e002270. DOI: 10.1161/JAHA.115.002270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pierce GL, Donato AJ, LaRocca TJ, Eskurza I, Silver AE, Seals DR. Habitually exercising older men do not demonstrate age‐associated vascular endothelial oxidative stress. Aging Cell. 2011;10:1032–1037. DOI: 10.1111/j.1474-9726.2011.00748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jablonski KL, Racine ML, Geolfos CJ, Gates PE, Chonchol M, McQueen MB, Seals DR. Dietary sodium restriction reverses vascular endothelial dysfunction in middle‐aged/older adults with moderately elevated systolic blood pressure. J Am Coll Cardiol. 2013;61:335–343. DOI: 10.1016/j.jacc.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pierce GL, Beske SD, Lawson BR, Southall KL, Benay FJ, Donato AJ, Seals DR. Weight loss alone improves conduit and resistance artery endothelial function in young and older overweight/obese adults. Hypertension. 2008;52:72–79. DOI: 10.1161/HYPERTENSIONAHA.108.111427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Seals DR, Nagy EE, Moreau KL. Aerobic exercise training and vascular function with ageing in healthy men and women. J Physiol. 2019;597:4901–4914. DOI: 10.1113/JP277764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vona M, Codeluppi GM, Iannino T, Ferrari E, Bogousslavsky J, von Segesser LK. Effects of different types of exercise training followed by detraining on endothelium‐dependent dilation in patients with recent myocardial infarction. Circulation. 2009;119:1601–1608. DOI: 10.1161/CIRCULATIONAHA.108.821736. [DOI] [PubMed] [Google Scholar]
- 83.Vona M, Rossi A, Capodaglio P, Rizzo S, Servi P, De Marchi M, Cobelli F. Impact of physical training and detraining on endothelium‐dependent vasodilation in patients with recent acute myocardial infarction. Am Heart J. 2004;147:1039–1046. DOI: 10.1016/j.ahj.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 84.Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, Lowe GDO, Pepys MB, Gudnason V. C‐reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–1397. DOI: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 85.Narkiewicz K, Somers VK. Sympathetic nerve activity in obstructive sleep apnoea. Acta Physiol Scand. 2003;177:385–390. DOI: 10.1046/j.1365-201X.2003.01091.x. [DOI] [PubMed] [Google Scholar]
- 86.Tinken TM, Thijssen DHJ, Black MA, Cable NT, Green DJ. Time course of change in vasodilator function and capacity in response to exercise training in humans. J Physiol. 2008;586:5003–5012. DOI: 10.1113/jphysiol.2008.158014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brunt VE, Howard MJ, Francisco MA, Ely BR, Minson CT. Passive heat therapy improves endothelial function, arterial stiffness and blood pressure in sedentary humans. J Physiol. 2016;594:5329–5342. DOI: 10.1113/JP272453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pierce GL. Aortic stiffness in aging and hypertension: prevention and treatment with habitual aerobic exercise. Curr Hypertens Rep. 2017;19:90. DOI: 10.1007/s11906-017-0788-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Du Y, Liu B, Sun Y, Snetselaar LG, Wallace RB, Bao W. Trends in adherence to the physical activity guidelines for Americans for aerobic activity and time spent on sedentary behavior among US adults, 2007 to 2016. JAMA Netw Open. 2019;2:e197597. DOI: 10.1001/jamanetworkopen.2019.7597. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S7
Figures S1–S2


