Abstract
Background
The advent of immune checkpoint inhibitors (ICIs) therapy has resulted in significant survival benefits in patients with non-small-cell lung cancer (NSCLC) without increasing toxicity. However, the utilisation of immunotherapy for small-cell lung cancer (SCLC) remains unclear, with a scarcity of systematic comparisons of therapeutic effects and safety of immunotherapy in these two major lung cancer subtypes. Herein, we aimed to provide a comprehensive landscape of immunotherapy and systematically review its specific efficacy and safety in advanced lung cancer, accounting for histological types.
Methods
We identified studies assessing immunotherapy for lung cancer with predefined endpoints, including overall survival (OS), progression-free survival (PFS), objective response rate (ORR), and treatment-related adverse events (TRAE), from PubMed, Embase, Medline, and Cochrane library. A random-effects or fixed-effect model was adopted according to different settings.
Results
Overall, 38 trials with 20,173 patients with lung cancer were included in this study. ICI therapy resulted in a significantly prolonged survival in both patients with NSCLC and SCLC when compared with chemotherapy (hazard ratio [HR] = 0.74; 95% confidence interval [CI], 0.70–0.79] and [HR = 0.82; 95% CI, 0.75–0.90], respectively). The magnitude of disease control and survival benefits appeared superior with ICI plus standard of care (SOC) when compared with SOC alone. OS and PFS advantages were observed only when immunotherapy was employed as the first-line treatment in patients with SCLC.
Conclusion
ICI therapy is a promising therapeutic option in patients with NSCLC and SCLC. ICI plus SOC can be recommended as the optimal first-line treatment for patients with SCLC, and double-target ICIs combined with SOC are recommended in patients with NSCLC as both the first and subsequent lines of treatment. Additionally, non-first-line immunotherapy is not recommended in patients with SCLC.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-021-08662-2.
Keywords: Immune checkpoint inhibitor, Efficacy, Non-small-cell lung cancer, Small-cell lung cancer
Introduction
Lung cancer is the primary cause of cancer-related mortality and incidence, resulting in a significant economic burden [1]. Regarding histological types, lung cancer can be categorised into small-cell lung cancer (SCLC) and non-small-cell lung cancer (NSCLC). SCLC accounts for only 15% of lung cancers, with first-line treatment mainly restricted to chemotherapy or radiotherapy and presenting a worse prognosis than NSCLC [2]. In contrast, NSCLC constitutes approximately 85% of lung cancers and presents a relatively superior prognosis, given the rapid development of therapeutic techniques, including surgery, chemotherapy, radiotherapy, and targeted therapy [3, 4]; however, the actual 5-year overall survival (OS) of NSCLC remains poor. Standard of care (SOC) therapies include chemotherapy and radiotherapy for patients with lung cancer lacking specific therapeutic targets, whereas targeted therapy can be administered to those with corresponding mutated genes.
One main hypothesis for tumour invasion and metastasis is immune evasion, controlled by a combination of inhibitory or stimulatory receptors and corresponding ligands [5]. Among them, cytotoxic T-lymphocyte antigen-4 (CTLA-4) and programmed cell death 1 (PD-1) pathways are promising therapeutic targets, also known as immune checkpoints [6, 7]. Tumour cells can escape the immune system attack via forming immune checkpoints. Accordingly, blocking such immune checkpoints can activate the immune system and prevent tumour cell evasion. Currently, immune checkpoint inhibitors (ICIs) developed to treat malignant tumours, including lung cancer, can be classified into anti-PD-1, anti-PD-L1, and anti-CTLA-4 antibodies.
Accumulating evidence has reported that ICIs have higher efficacy than SOC in both NSCLC and SCLC, indicating their superior therapeutic potential. In patients with advanced NSCLC, anti-PD-1 monotherapy can achieve a median OS of 11.9 months, which was significantly superior to that with a SOC at 9.5 months (hazard ratio [HR]: 0.75; 95% confidence interval [CI]: 0.61–0.93). Furthermore, the incidence of treatment-related adverse events (TRAE) in the ICI group was reportedly lower than in the SOC group [8]. In patients with SCLC, anti-PD-L1 therapy as first-line treatment has demonstrated a better OS than platinum-etoposide treatment [9].
However, clinical trials evaluating the efficiency and safety of ICI therapy have mainly focused on NSCLC in recent years, neglecting any specific data analysis for SCLC. More importantly, systematic studies comparing ICI therapy among NSCLC patients with SCLC remain scarce.
A pooled analysis not restricted to patients with SCLC or NSCLC could provide valuable clinical information regarding anti-PD-1/PD-L1 and CTLA-4 treatments. In the present study, we aimed to validate whether immunotherapy could result in more manageable TRAEs and better efficacy than SOC in patients with advanced NSCLC or SCLC. Moreover, we compared the distinct benefits and risks of immunotherapy between patients with NSCLC and SCLC. We anticipate that our results could benefit the development of immunotherapy in lung cancer and offer practical solutions for routine clinical practice using immunotherapy in patients with NSCLC or SCLC.
Methods
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [10].
Search strategy and study selection
We performed a search for eligible randomised controlled trials (RCTs) from January 2010 to May 2021 in Medline, PubMed, Embase, and the Cochrane Central Register of Controlled Trials, using the following key words: ICIs (PD-1, PD-L1, or CTLA-4), specific ICI drug names (toripalimab, sintilimab, camrelizumab, tilelizumab, nivolumab, pembrolizumab, atezolizumab, durvalumab, avelumab, ipilimumab, and tremelimumab), and lung cancer. For further identifying unpublished studies, we retrieved abstracts from the American Society of Clinical Oncology, the European Society of Medical Oncology, the American Association for Cancer Research, and the World Conference on Lung Cancer. (Table S1).
Exclusion and inclusion criteria were predefined. Eligible RCTs were required to meet the following criteria: (a) population: diagnosed with lung cancer (NSCLC or SCLC) pathologically; (b) intervention: treatment with PD-1/PD-L1 or CTLA-4 inhibitors (toripalimab, sintilimab, camrelizumab, tislelizumab, nivolumab, pembrolizumab, atezolizumab, durvalumab, avelumab, ipilimumab and tremelimumab); (c) control: treated with chemotherapy or radiotherapy; (d) type of study: phase II and III clinical trials. Exclusion criteria were as follows: (a) the study was not a randomised controlled trial; (b) data regarding PFS/OS measured by HRs, objective response rate (ORR), or TRAEs was unavailable; (c) duplicate articles.
Data extraction and quality assessment
For all included trials, we extracted the name of the trial, year of publication, trial phase, line of treatment, age and number of patients, OS/PFS/ORR, and TRAEs of grade ≥ 3 and any grade. We adopted the Cochrane Risk of Bias Tool, consisting of allocation concealment, random sequence generation, blinding of outcome assessment, blinding protocol, selective reporting, and incomplete outcome data, to methodologically assess the quality of the enrolled RCTs [11]. The items adjudged as “low risk” were regarded as applicable. Two authors independently performed data extraction and quality assessment. Discrepancies were resolved by reaching a consensus.
Statistical analysis
Heterogeneity was identified by the Q test and quantified using the I2 and Q statistics [12]. If I2 was more than 50%, the random effect model was applied; otherwise, the fixed-effect model was selected [13]. The primary outcomes in the present study were OS and PFS, measured as HRs, 95% CIs, and p-values. ORR, grade ≥ 3 TRAEs, and Grade 1–5 TRAEs were presented as risk ratios (RRs). The Q test was used to detect heterogeneity between the subgroups and assess differences between histological types. Prespecified subgroup analyses were performed to evaluate the potential association between individual or methodological factors and immunotherapy efficacy in each histological type of lung cancer. Eggerʼs and Beggʼs tests were used to assess the publication bias for included RCTs. Stata 16.0 software (Stata Corp, College Station, TX) was used to perform all analyses. Statistical significance was set at p < 0.05.
Result
Literature search results
The initial literature search identified 30,284 related studies (Fig. 1). In total, 38 RCTs, including 41 studies with 20,173 patients with lung cancer, were included for quantitative analyses [6–8, 14–48]. Eight studies explored the efficacy of ICI versus SOC in patients with SCLC (three studies on ipilimumab, two on atezolizumab, one on nivolumab, one about durvalumab, and one assessing tremelimumab plus durvalumab). The remaining 33 studies were performed efficiency and safety comparisons between ICIs and SOC in patients with NSCLC.
Fig. 1.
Flowchart diagram of selected randomized controlled trials included
Characteristics of identified trials
The main characteristics of the 38 trials are listed in Table 1. All included trials were performed in patients with relapsed or extensive SCLC and advanced NSCLC. In total, 20,173 patients were included, of which 17,250 (85.5%) were diagnosed with NSCLC and 2923 (14.5%) with SCLC. Regarding age, most patients were ≥ 70 years old. All eligible trials were phase II or III studies, with 31 phase III trials, 6 phase II, and 1 phase II/III. Among these trials, 22 employed ICIs as first-line therapy, and the remaining trials were in a non-first-line setting. Overall, all studies, except for 17 (44.7%), confirmed improvements in OS in patients receiving immunotherapy when compared with those receiving SOC. Except for PEMBRO-RT and IFCT-1603, all trials reported total TRAEs in patients. Furthermore, several RCTs were uniquely designed, necessitating further explanation. KEYNOTE-010 evaluated the efficiency of different pembrolizumab doses (2 mg/kg and 10 mg/kg), accordingly divided into KEYNOTE-010, a and KEYNOTE-010, b. OAK established two different cohorts, ITT850 and ITT1225, both of which were treated as independent studies. Likewise, ARCTIC and CASPIAN were considered independent studies. CA184–041 was a phase II study focusing on different medication orders, which was considered four studies based on histological type and order of medication. Trials were generated through a random sequence and at low risk of selection bias, presenting good quality (Table S2). The reduced selection bias was attributed to low attrition and thorough reporting without missing cases. The funnel plot (Fig. S1), as well as Eggerʼs and Beggʼs tests, all indicated no sign of publication bias.
Table 1.
Clinical characteristics and outcomes of the included randomized controlled trials
| Trials | Trial phase | Line of treatment | Intervention (No.) | Control (No.) | Age, Median (Range) | Efficiency | TRAEs | |||
|---|---|---|---|---|---|---|---|---|---|---|
| OS (95% CI) | PFS (95% CI) | ORR | Grade 3–5 | Grade 1–5 | ||||||
| NSCLC | ||||||||||
| KEYNOTE-407, 2018 | III | 1 | PEM plus PBC (278) | PBC plus placebo (281) |
Int:65 (29–87) Con:65 (36–88) |
0.64 (0.49–0.85) | 0.56 (0.45–0.70) |
161/278 108/281 |
194/278 191/280 |
273/278 274/280 |
| KEYNOTE-021, 2016 | II | 1 | PEM plus PBC (60) | PBC (63) |
Int:62.5 (54–70) Con:63.2 (58–70) |
0.56 (0.32–0.95) | 0.53 (0.33–0.86) |
33/60 18/63 |
24/59 17/62 |
55/59 57/62 |
|
OAK ITT850 2017, 2019 |
III | > 1 | ATE (425) | DOC (425) |
Int:63.5 (33–77) Con:58.5 (34–79) |
0.75 (0.64–0.88) | 0·95 (0·82–1·10) |
58/425 57/425 |
90/609 248/578 |
390/609 496/578 |
|
CheckMate 026 2017 |
III | 1 | NIV (271) | PBC (270) |
Int:63 (32–89) Con:65 (29–87) |
1.08 (0.87–1.34) | 1.19 (0.97–1.46) |
55/211 71/212 |
47/267 133/263 |
190/267 243/263 |
|
OAK ITT1225 2018 |
III | > 1 | ATE (613) | DOC (612) |
Int:63 (25–84) Con:64 (34–85) |
0.80 (0.70–0.92) | 0.96 (0.85–1.08) |
84/613 72/612 |
243/609 322/578 |
574/609 557/578 |
| JAVELIN Lung 200, 2018 | III | > 1 | Avelumab (396) | DOC (396) |
Int:64 (58–69) Con:63 (57–69) |
0·90 (0·75–1·08) | 1·16 (0·97–1·40) |
59/396 44/396 |
39/393 180/365 |
251/393 254/365 |
| KEYNOTE-189, 2018 | III | 1 | PEM plus PBC (410) | PBC plus placebo (206) |
Int:65 (34–84) Con:63 (34–84) |
0.49 (0.38–0.64) | 0.52 (0.43–0.64) |
195/410 39/206 |
272/405 133/202 |
404/405 200/202 |
| KEYNOTE-042, 2019 | III | 1 | PEM (637) | PBC (637) |
Int:63 (57–69) Con:63 (57–69) |
0.81 (0.71–0.93) | 1.07 (0.94–1.21) |
174/637 169/637 |
113/636 252/615 |
399/636 553/615 |
| KEYNOTE-010, a, 2016 | II/III | > 1 | PEM (344) | DOC (172) |
Int:63 (56–69) Con:62 (56–69) |
0.71 (0.58–0.88) | 0.88 (0.74–1.05) |
62/344 16/172 |
43 /339 55/155 |
215/339 126/155 |
| KEYNOTE-010, b, 2016 | II/III | > 1 | PEM (346) | DOC (171) |
Int:63 (56–69) Con:62 (56–69) |
0.61 (0.49–0.75) | 0.79 (0.66–0.94) |
64/346 16/171 |
55/343 54/154 |
226/343 125/154 |
| POPLAR, 2016 | II | > 1 | ATE (144) | DOC (143) |
Int:62 (42–82) Con:62 (36–84) |
0.73 (0.53–0.99) | 0.94 (0.72–1.23) |
21/144 21/143 |
57/142 71/135 |
95/142 119/135 |
|
PACIFIC 2017, 2018 |
III | > 1 | DUR (476) | PBC plus Placebo (237) |
Int:64 (31–84) Con:64 (23–90) |
0.68 (0.47–0.997) | 0.52 (0.42–0.65) |
126/443 34/123 |
142/475 61/234 |
460/475 222/234 |
| KEYNOTE- 024, 2016, 2019 | III | 1 | PEM (154) | PBC (151) |
Int:64.5 (33–90) Con:66.0 (38–85) |
0.63 (0.47–0.86) | 0.50 (0.37–0.68) |
69/154 42/151 |
48/154 80/150 |
118/154 135/150 |
|
CheckMate 017 2015 |
III | > 1 | NIV (135) | DOC (137) |
Int:62 (39–85) Con:64 (42–84) |
0.59 (0.44–0.79) | 0.62 (0.47–0.81) |
27/135 12/137 |
9/131 71/129 |
76/131 111/129 |
|
IMpower110 2020 |
III | 1 | ATE (277) | PBC (277) |
Int:64 (30–81) Con:65 (30–87) |
0.83 (0.65–1.07) | 0.77 (0.63–0.94) |
81/277 88/277 |
97/286 149/263 |
258/286 249/263 |
|
CheckMate 057 2015 |
III | > 1 | NIV (292) | DOC (290) |
Int:61 (37–84) Con:64 (21–85) |
0.73 (0.59–0.89) | 0.92 (0.77–1.11) |
56/292 36/290 |
30/287 144/268 |
199/287 236/268 |
|
IMpower150 2018 |
III | 1 | ATE plus PBC (400) | PBC (400) |
Int:63 (31–89) Con:63 (31–90) |
0.78 (0.64–0.96) | 0.61 (0.52–0.72) |
224/353 159/331 |
230/393 197/394 |
371/393 376/394 |
|
CheckMate 078 2020 |
III | > 1 | NIV (338) | DOC (166) |
Int:60 (27 to 78) Con:60 (38 to 78) |
0.75 (0.61–0.93) | : 0.79 (0.65–0.98) |
59/338 7/166 |
41/337 74/156 |
219/337 131/156 |
|
IMpower130 2019 |
III | 1 | ATE plus PBC (483) | PBC (240) |
Int:64 (18–86) Con:65 (38–85) |
0·80 (0·65–0·99) | 0.65 (0·54–0·77) |
220/447 72/226 |
354/473 141/232 |
455/473 215/232 |
|
ARCTIC, a, 2020 |
III | > 1 | DUR (62) | Erlotinib, gemcitabine, or vinorelbine) (64) |
Int:63.5 (35–79) Con:62.0 (41–81) |
0.63 (0.42–0.93) | 0.71 (0.49–1.04) |
22/62 8/64 |
25/62 41/63 |
60/62 63/63 |
|
ARCTIC, b 2020 |
III | > 1 | DUR plus TRE (174) | Erlotinib, gemcitabine, or vinorelbine) (118) |
Int:62.5 (26–81) Con:65 (42–83) |
0.80 (0.61–1.05) | 0.77 (0.59–1.01) |
26/174 8/118 |
74/173 57/110 |
160/173 105/110 |
|
CameL 2020 |
III | 1 | CAM plus PBC (205) | PBC (207) |
Int:59 (54–64) Con:61 (53–65) |
0.73 (0.53–1.02) | 0.60 (0.45–0.79) |
124/205 80/207 |
78/205 63/207 |
146/205 132/207 |
|
CheckMate 227 2019 |
III | 1 | NIV plus IPI (583) | PBC (583) |
Int:64 (26–87) Con:64 (29–87) |
0.73 (0.64–0.84) | 0.79 (0.69–0.91) |
199/583 162/583 |
189/576 205/570 |
442/576 467/570 |
|
CheckMate 9LA 2021 |
III | 1 | NIV plus IPI plus PBC (361) | PBC (358) |
Int:65 (59–70) Con:65 (58–70) |
0·69 (0·55–0·80) | 0·68 (0·57–0·82) |
138/361 89/358 |
168/358 132/349 |
327/358 303/349 |
|
CA184–041, a 2012 |
II | 1 | Concurrent IPI plus PBC (70) | PBC (33) |
Int:59 (36–82) Con:62 (36–82) |
0.99 (0.67–1.46) | 0.88 (0.61–1.27) |
15/70 6/33 |
40/71 13/32 |
52/71 23/32 |
|
CA184–041, b 2012 |
II | 1 | Phased IPI plus PBC (68) | PBC (33) |
Int:61 (36–82) Con:62 (36–88) |
0.87 (0.59–1.28) | 0.69 (0.48–1.00) |
22/68 6/33 |
36/67 13/33 |
49/67 23/33 |
|
CA184–104 2017 |
III | 1 | IPI plus PBC (388) | PBC plus placebo (361) |
Int:64 (28–84) Con:64 (28–85) |
0.91 (0.77–1.07) | 0.87 (0.75–1.01) |
171/388 170/361 |
205/388 129/361 |
344/388 292/361 |
|
IMpower132 2020 |
III | 1 | ATE plus PBC (292) | PBC (286) |
Int:64 (31–85) Con:63 (33–83) |
0.86 (0.71–1.06) | 0.60 (0.49–0.72) |
137/292 92/286 |
208/291 166/274 |
287/291 266/274 |
|
PEMBRO-RT 2019 |
II | > 1 | PEM plus Radiotherapy (36) | Radiotherapy (40) |
Int:62 (35–78) Con:62 (38–78) |
0.66 (0.37–1.18) | 0.71 (0.42–1.18) |
13/36 7/40 |
12/35 6/37 |
NA |
|
IMpower131 2020 |
III | 1 | ATE plus PBC (343) | PBC (340) |
Int:65 (23–83) Con:65 (38–86) |
0.88 (0.73–1.05) | 0.71 (0.60–0.85) |
170/343 139/340 |
231/334 195/334 |
316/334 303/334 |
|
EMPOWER-Lung 1 2021 |
III | 1 | CEM (283) | PBC (280) |
Int:63 (58–69) Con:64 (58–70) |
0.57 (0.42–0.77) | 0.54 (0.43–0.68) |
111/283 57/280 |
50/355 134/342 |
204/355 303/342 |
|
RATIONALE 307, a 2021 |
III | 1 | TIS plus PBC (120) | PBC (61) |
Int:60 (41–74) Con:62 (34–74) |
\ | 0.52 (0.37–0.74) |
87/120 30/61 |
103/120 47/59 |
119/120 59/59 |
|
RATIONALE 307, b 2021 |
III | 1 | TIS plus PBC (119) | PBC (60) |
Int:63 (38–74) Con:62 (34–74) |
\ | 0.48 (0.34–0.68) |
89/119 30/60 |
99/118 47/58 |
117/118 58/58 |
| SCLC | ||||||||||
|
CASPIAN, a 2021 |
III | 1 | TRE plus DUR plus PBC (268) | PBC (269) |
Int:63 (58–68) Con:63 (57–68) |
0·82 (0·68–1·00) | 0·84 (0·70–1·01) |
156/267 78/134 |
196/266 86/133 |
264/266 129/133 |
|
CASPIAN, b 2021 |
III | 1 | DUR plus PBC (268) | PBC (269) |
Int:62 (58–68) Con:63 (57–68) |
0.75 (0.62–0.91) | 0·80 (0·66–0·96) |
182/268 78/135 |
171/265 87/133 |
260/265 129/133 |
|
IFCT-1603 2019 |
II | > 1 | ATE (49) | PBC (24) |
Int:65.9 (51.1–85.5) Con:63.5 (51.8–81.0) |
0.84 (0.45–1.58) | 2.26 (1.30–3.39) |
1/43 2/20 |
2/48 18/24 |
NA |
|
IMpower133 2018 |
III | 1 | ATE plus PBC (201) | PBC plus placebo (202) |
Int:64 (28–90) Con:64 (26–87) |
0.70 (0.54–0.91) | 0.77 (0.62–0.96) |
121/201 130/202 |
115/198 113/196 |
188/198 181/198 |
|
CA184–041, a 2013 |
II | 1 | Concurrent IPI plus PBC (43) | PBC plus placebo (23) |
Int:57 (44–80) Con:58 (42–82) |
0.95 (0.59–1.54) | 0.93 (0.59–1.48) |
14/43 11/23 |
19/42 10/22 |
29/42 18/22 |
|
CA184–041, b 2013 |
II | 1 | Phased IPI plus PBC (42) | Placebo plus PBC (22) |
Int:59 (43–80) Con:58 (42–82) |
0.75 (0.46–1.23) | 0.93 (0.59–1.45) |
24/42 11/22 |
22/42 9/22 |
33/42 18/22 |
|
CA184–156, 2016 |
III | 1 | IPI plus PBC (478) | Placebo plus PBC (476) |
Int:62 (39–85) Con:63 (36–81) |
0.94 (0.81–1.09) | 0.85 (0.75–0.97) |
297/478 296/476 |
231/478 214/476 |
391/478 361/478 |
|
CheckMate 331 2021 |
III | > 1 | NIV (284) | PBC (285) |
Int:62 (37–85) Con:61 (34–82) |
0.86 (0.72–1.04) | 1.41 (1.18–1.69) |
39/284 47/285 |
39/282 194/265 |
156/282 239/265 |
Abbreviations: ATE atezolizumab, AVE avelumab, DOC docetaxel, TRAE treatment-related adverse event, IPI ipilimumab, NIV nivolumab, DUR durvalumab, TRE tremelimumab, CAM camrelizumab, CEM Cemiplimab, TIS Tislelizumab, ORR objective response rate, OS overall survival, PBC platinum-based chemotherapy, PEM pembrolizumab, PFS progression-free survival
Efficiency
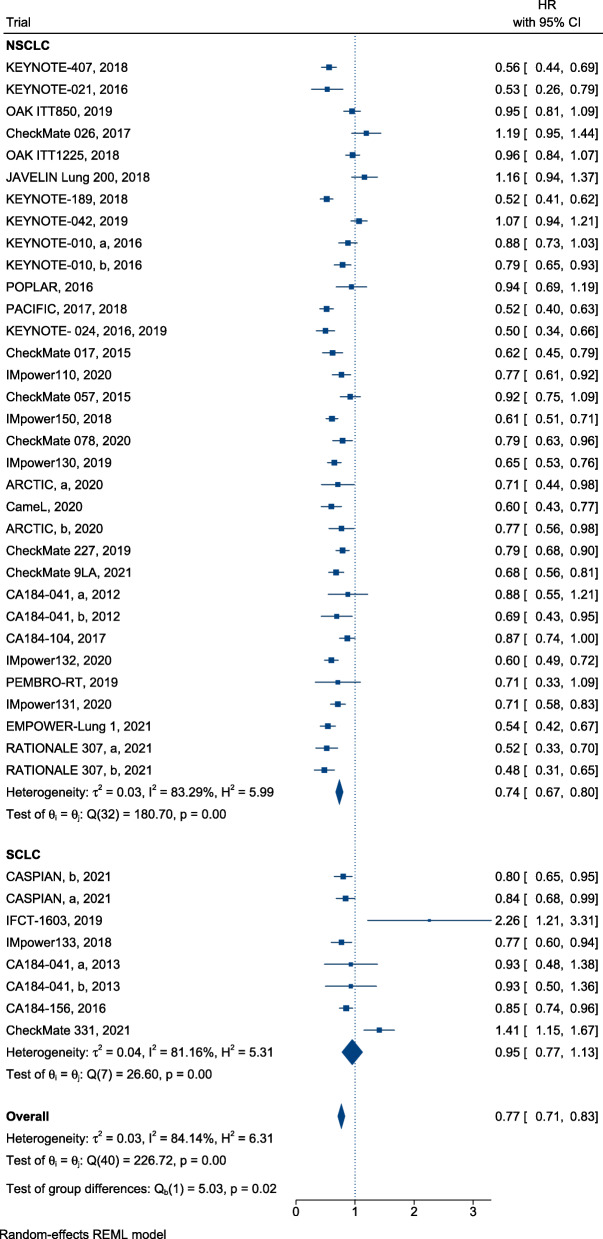
In summary, ICI treatment presented a significant advantage over SOC, with a reduction in mortality (HR, 0.76; 95% CI, 0.72–0.80) (Fig. 2) and successful control of disease progression in patients with lung cancer (HR, 0.77; 95% confidence interval [CI], 0.71–0.83) (Fig. 3). Furthermore, immunotherapy yielded superior efficacy in terms of objective response in patients with lung cancer when compared with chemotherapy or radiotherapy (RR, 1.21; 95% CI, 1.13–1.30; Fig. 4). Regarding different histological types, greater improvements in PFS following ICI therapy were observed in patients with NSCLC than in patients with SCLC ([HR = 0.74; 95% CI, 0.67–0.80] and [HR = 0.95; 95% CI, 0.77–1.13]; difference p = 0.02; Fig. 3), similar findings were documented in terms of ORR ([RR = 1.28; 95% CI, 1.18–1.39] and [RR = 1.00; 95% CI, 0.92–1.08]; difference p < 0.01; Fig. 4). In contrast, equivalent OS benefits from ICI therapy were observed in both patients with NSCLC and SCLC ([HR = 0.74; 95% CI, 0.70–0.79] and [HR = 0.82; 95% CI, 0.75–0.90]; difference p = 0.07; Fig. 2). Remarkably, disease progression was retarded in patients with NSCLC treated with ICIs when compared with patients treated with SOC ([HR = 0.74; 95% CI, 0.67–0.80], Fig. 3), risk of death ([HR = 0.74; 95% CI, 0.70–0.79], Fig. 2), and increased ORR ([RR = 1.28; 95% CI, 1.18–1.39], Fig. 4). However, the benefit of ICI therapy in patients with SCLC was only indicated by OS ([HR = 0.82; 95% CI, 0.75–0.90], Fig. 2).
Fig. 2.
Forest plots of HRs comparing overall survival of immunotherapy between NSCLC and SCLC
Fig. 3.
Forest plots of HRs comparing progression-free survival of immunotherapy between NSCLC and SCLC
Fig. 4.
Forest plots of RRs comparing overall response rate of immunotherapy between NSCLC and SCLC
Safety
Compared with SOC alone, immunotherapy for patients with lung cancer reduced the risk of Grade 3–5 TRAEs (RR, 0.76; 95% CI, 0.64–0.89, Fig. 5) and Grade 1–5 TRAEs (RR, 0.95; 95% CI, 0.92–0.98, Fig. 6). In terms of Grade 3–5 TRAEs, no significant difference in risk reduction was observed among patients with different subtypes of lung cancer receiving ICI treatment when compared with SOC ([RR = 0.75; 95%, CI, 0.63–0.90] and [RR = 0.76; 95% CI, 0.48–1.18], respectively; difference p = 0.98; Fig. 5). The risk of Grade 1–5 TRAEs was equivalent among patients with different subtypes of lung cancer treated with ICIs and SOC ([RR = 0.95; 95% CI, 0.92–0.98] and [RR = 0.96; 95% CI, 0.87–1.07], respectively; p = 0.78; Fig. 6).
Fig. 5.
Forest plots of RRs comparing Grade 3–5 TRAEs of immunotherapy between NSCLC and SCLC
Fig. 6.
Forest plots of RRs comparing Grade 1–5 TRAEs of immunotherapy between NSCLC and SCLC
Subgroup analysis
Table 2 and Table S3 display differences in the efficiency of ICI therapy between patients with NSCLC and SCLC. Importantly, as indicated by PFS, patients with NSCLC presented greater benefits following ICI therapy plus SOC than those with SCLC, when corresponding ICI-treated patients were used as a standard for comparison ([HR, 0.63; 95% CI, 0.57–0.69] and [HR, 0.83; 95% CI, 0.76–0.90], respectively, difference p < 0.01); similar results were observed following ICI monotherapy ([HR, 0.82; 95% CI, 0.73–0.91] and [HR, 1.68; 95% CI, 0.90–2.45], respectively; p = 0.03). Moreover, we further assessed differences on efficiency between patients with NSCLC and SCLC when immunotherapy was employed as the first or subsequent line of treatment. We detected an advantage in terms of PFS in patients with NSCLC when compared with patients with SCLC in both the first ([HR, 0.68; 95% CI, 0.60–0.76] and [HR, 0.83; 95% CI, 0.76–0.90], respectively, difference p = 0.01) and subsequent line of therapy ([HR, 0.83; 95% CI, 0.73–0.92] and [HR, 1.68; 95% CI, 0.90–2.45], respectively, p = 0.03). However, further subgroup analyses of sex, age, drug target, and Eastern Cooperative Oncology Group Performance Status (ECOG PS) score showed no statistically significant differences on PFS between patients with NSCLC and SCLC. In addition, we conducted subgroup analyses, including sex, age, smoking status, line of therapy, research methodology, drug target, and ECOG PS score, for OS and found no statistically significant differences in OS among patients with NSCLC and SCLC in all subgroups (Table S3).
Table 2.
Differences in PFS benefits of Immunotherapy in NSCLC and SCLC by subgroups
| Variable | Study | Test for Difference | |||
|---|---|---|---|---|---|
| NSCLC | SCLC | χ2 | P Value | ||
| Overall | 41 | 0.74 [0.67; 0.80] | 0.95 [0.77; 1.13] | 5.03 | 0.02 |
| Sex | |||||
| Male | 18 | 0.63 [0.56; 0.69] | 0.87 [0.64; 1.10] | 3.16 | 0.08 |
| Female | 18 | 0.69 [0.57; 0.82] | 0.59 [0.37; 0.81] | 0.67 | 0.41 |
| Age | |||||
| < 65 yr | 18 | 0.62 [0.54; 0.70] | 0.76 [0.54; 0.98] | 1.40 | 0.24 |
| ≥ 65 yr | 14 | 0.67 [0.58; 0.77] | 0.76 [0.53; 0.99] | 0.44 | 0.51 |
| Line of therapy | |||||
| First | 26 | 0.68 [0.60; 0.76] | 0.83 [0.76; 0.90] | 7.68 | 0.01 |
| Subsequent | 15 | 0.83 [0.73; 0.92] | 1.68 [0.90; 2.45] | 4.59 | 0.03 |
| Research methodology | |||||
| ICI vs non-ICI | 20 | 0.82 [0.73; 0.91] | 1.68 [0.90; 2.45] | 4.66 | 0.03 |
| ICI + non-ICI vs non-ICI | 21 | 0.63 [0.57; 0.69] | 0.83 [0.76; 0.90] | 15.28 | < 0.01 |
| Drug target | |||||
| Anti-PD-1/PD-L1 | 31 | 0.73 [0.56; 0.81] | 1.16 [0.64; 1.68] | 2.61 | 0.11 |
| Anti-CTLA-4 | 6 | 0.84 [0.73; 0.95] | 0.86 [0.76; 0.96] | 0.07 | 0.80 |
| Anti-PD-1/PD-L1 + CTLA-4 | 4 | 0.75 [0.66; 0.83] | 0.84 [0.68; 0.99] | 1.12 | 0.29 |
| ECOG PS | |||||
| 0 | 17 | 0.64 [0.54; 0.75] | 0.84 [0.53; 1.15] | 1.42 | 0.23 |
| 1 | 17 | 0.64 [0.57; 0.71] | 0.72 [0.53; 0.92] | 0.54 | 0.46 |
| Trial phase | |||||
| II | 8 | 0.75 [0.59; 0.91] | 1.23 [0.54; 1.91] | 1.17 | 0.18 |
| III | 33 | 0.73 [0.66; 0.81] | 0.92 [0.71; 1.13] | 2.65 | 0.10 |
Discussion
The present study is the first systematic review and meta-analysis to evaluate the association between ICIs and long-term outcomes in patients with NSCLC and SCLC. We used published data from 38 RCTs of high quality, including more than 20,000 patients with lung cancer, revealing that ICIs were associated with a better therapeutic effect on reducing the risk of death in patients with NSCLC and SCLC without increasing TRAEs when compared with SOC. However, in terms of ORR and control of disease progression, benefits were primarily observed in patients with NSCLC, who showed significant improvements when compared with patients with SCLC. Compared with SOC, immunotherapy resulted in significantly prolonged PFS in patients with NSCLC than in patients with SCLC, with a significant difference noted between the two subgroups. Furthermore, among the treatment strategies, ICIs plus SOC led to a better improvement in PFS than ICI monotherapy in both patients with NSCLC and SCLC patients; accordingly, it is recommended for patients with advanced lung cancer as a preferential option. However, I2 > 50% in PFS analyses of NSCLC and SCLC indicated heterogeneity. In terms of NSCLC, we conducted subgroup analysis for drug targets, revealing that I2 of CTLA-4 and PD-1/PD-L1 plus CTLA-4 groups was 0 and 10.17% after grouping; however, the heterogeneity for the PD-1/PD-L1 group persisted (Fig. S2). On carefully comparing therapeutic regimens, we observed that the CTLA-4 and PD-1/PD-L1 plus CTLA-4 groups adopted similar ICI regimens among different trials. Nevertheless, the number of ICIs in the PD-1/PD-L1 group reached eight, with some employed in only one trial. Therefore, we believe that variations in ICIs possibly accounted for the heterogeneity. For SCLC, we found only two trials that assessed non-first-line treatment. Accordingly, we conducted a subgroup analysis for the line of therapy and observed that the I2 for first-line treatment became 0; this suggested that the different non-first-line treatments were sources of heterogeneity (Fig. S3).
Furthermore, the current study indicated that the magnitude of immunotherapy treatment effects was related to the ICI drug targets. Based on the checkpoints, ICIs are roughly classified as anti-PD-1/ PD-L1 and anti-CTLA-4 drugs. Some researchers have highlighted that combining anti-PD-1/ PD-L1 with anti-CTLA-4 might lead to additive antitumour effects [16]. Herein, we demonstrated that, among different drug targets, the combination of anti-PD-1/PD-L1 and anti-CTLA-4 decreased the risk of death by 28% in patients with NSCLC, which was only 26% in the anti-PD-1/PD-L1 group and 9% in the anti-CTLA-4 group, consistent with the former hypothesis. Similarly, the magnitude of PFS benefits seemed to favour anti-PD-1/PD-L1 plus anti-CTLA-4 treatment in both patients with NSCLC and SCLC. Nevertheless, the magnitude of OS benefits favoured the anti-PD-1/PD-L1 group most in patients with SCLC, revealing that the combination of anti-PD-1/PD-L1 and anti-CTLA-4 treatment has better therapeutic effects in patients with NSCLC. Given the limited number of clinical trials, additional research is needed to comprehensively evaluate the efficiency of drug combinations. Nevertheless, there is a potential explanation for the promising effects of combined anti-PD-1/PD-L1 with anti-CTLA-4 treatment. Although the anti-PD-1/PD-L1 and anti-CTLA-4 antibodies are distinct ICIs, they may play a synergistic role. More precisely, anti-PD-1/PD-L1 antibodies restore the antitumour function of T cells, whereas anti-CTLA-4 antibodies activate antitumour T-cell responses and induce the proliferation of T-cells involving memory T cells [49].
In addition, we observed that therapy with ICIs plus SOC conferred greater treatment benefits than ICI monotherapy. This finding was in line with findings reported by Wang and colleagues, which revealed that ICI plus SOC results in significantly prolonged PFS when compared with monotherapy with immunotherapy [50]. However, we compared both NSCLC and SCLC rather than just NSCLC. In theory, chemotherapy or radiotherapy can induce the expression of immune checkpoints on infiltrating immune cells and tumour cells, which might enhance the curative effects of ICI therapy [50]. Thus, a combination of ICIs and SOCs should be adopted as the optimal treatment for SCLC and NSCLC. For NSCLC, we recommended a combination of SOC and anti-PD-1/PD-L1 plus anti-CTLA-4 antibodies. Furthermore, although men and women exhibited distinct immunological responses to antigens, no significant association of sex in terms of survival and disease control advantages was detected in patients with NSCLC and SCLC, in agreement with a previous study by Wallis et al. [51].
Currently, no RCTs comparing the therapeutic effects of ICIs in patients with NSCLC and SCLC patients have been reported. In the past decade, most drugs were found to be ineffective in SCLC management, in contrast to the success in the NSCLC field. In 2018, the IMpower133 trial revealed that the combination of atezolizumab and chemotherapy significantly prolonged OS and PFS when compared with chemotherapy alone for patients with advanced-stage SCLC [19]; this challenged the traditional chemotherapy-based treatment strategies for patients with SCLC. Subsequently, atezolizumab was adopted as the first-line treatment for SCLC. To date, only one study has compared first-line treatment strategies for SCLC, which only included two studies of ICI therapy, while most other trials in the SCLC field were limited in chemotherapy subtypes [52]. Another novelty of our study lies in the subgroup analyses according to individual conditions and treatment methods. Herein, we demonstrated that for patients with SCLC, ICI plus SOC therapy confers superior advantages over SOC, as indicated by OS and PFS. Furthermore, our study revealed that patients with NSCLC presented greater PFS benefits than SCLC patients receiving ICI monotherapy and ICI plus SOC therapy regarding different lung cancer subtypes. In terms of the line of therapy, patients with NSCLC benefited more from ICI treatment than patients with SCLC in both the first and subsequent lines of therapy, with significant differences between groups. These findings indicate that NSCLC might benefit more from ICI treatment than SCLC, regardless of the methodology of drug administration.
Implications of the study
Providing optimal treatment strategies for patients with lung cancer
Our study had several clinical implications. We recommend treatment strategies for patients with lung cancer based on sufficient evidence. With the development and gradual maturity of ICI treatment, it is necessary for oncologists, respiratory physicians, and thoracic surgeons to navigate multiple treatment strategies, including various ICI therapies, and to determine the optimal treatment for patients with lung cancer. Therefore, we recommend that patients with SCLC undergo ICI plus SOC therapy based on findings in the present study. For patients with NSCLC, a combination of anti-PD-1/PD-L1 and anti-CTLA-4 antibodies and SOC could serve as the optimal treatment strategy.
Discovering novel therapeutic regimen for SCLC
In addition, our research provides a new approach for SCLC therapy. The median OS for SCLC, especially for extensive-stage SCLC, is less than 10 months, emphasising the need for novel promising treatments [2]. However, several clinical trials, including targeted drugs, have declared treatment failure for SCLC in the past few decades. In 2013 and 2016, CA184–041 and CA184–156 were conducted by Reck et al. to evaluate the therapeutic effect of ipilimumab in patients with SCLC patients. The authors reported that ipilimumab had no significant efficacy when compared with traditional chemotherapy [20, 21]. Recently, IMpower133 and CASPIAN assessed anti-PD-1/PD-L1 antibodies with or without anti-CTLA-4 antibodies as the first-line of therapy for patients with SCLC, revealing better therapeutic effects in prolonging OS and PFS in patients with SCLC than chemotherapy [16, 19], which indicated a major development in SCLC therapy. However, IFCT-1603 and CheckMate 331 used anti-PD-1/PD-L1 antibodies as first-line therapy when compared with traditional chemotherapy and observed no significant difference in prolonging OS. In terms of PFS, immunotherapy led to worse results than chemotherapy [17, 18]. In the current study, we systematically analysed data from these RCTs and validated that ICI therapy could prolong OS in patients with SCLC. Considering these discrepancies, we conducted subgroup analyses in line with therapy and drug targets, which recommended ICI treatment as the first-line therapy for SCLC, affording better OS and PFS than with the subsequent line of therapy. Among different drug targets, anti-PD-1/PD-L1 antibodies with or without anti-CTLA-4 antibodies presented a superior advantage in reducing the risk of death; this indicated that anti-PD-1/PD-L1 antibodies with or without anti-CTLA-4 antibodies should be adopted as the first-line therapy for patients with SCLC. Moreover, additional trials should be conducted to further validate the treatment effects of anti-PD-1/PD-L1 antibodies with or without anti-CTLA-4 antibodies as the first-line therapy for SCLC.
Landscape of ICI treatment efficacy among lung cancer
Another clinical implication of our study is that NSCLC might benefit more from ICI therapy than SCLC among different histological subtypes. Currently, available studies are insufficient to compare the treatment effects of ICIs in patients with NSCLC and SCLC. However, we conducted the first analysis to evaluate differences in ICI treatment between patients with NSCLC and SCLC. The results revealed that patients with NSCLC benefited more from immunotherapy than patients with SCLC in almost all subgroups, regardless of treatment methodology and individual patient conditions. Notably, ICI treatment presented a statistically significant advantage in terms of therapeutic efficiency in patients with NSCLC when compared with patients with SCLC, irrespective of first or subsequent line of therapy and treatment methodology (ICIs alone or ICIs plus SOC). In terms of PFS and ORR, patients with SCLC receiving immunotherapy showed no difference from those on SOC regimens, both of which were significantly lower than in patients with NSCLC. Thus, the above results demonstrated that although the OS of patients with SCLC could benefit from immunotherapy, PFS and ORR fail to demonstrate promising effects equivalent to those in patients with NSCLC.
Strengthens and weaknesses of this study
First, this is the first study to comprehensively review the relative benefits and risks of ICI treatment between patients with NSCLC and SCLC and indirectly compare the efficiency of treatment methodology in each histological lung cancer subtype, including the largest number of trials and patients. As few studies have analysed the efficiency and safety of ICI treatment in patients with SCLC, and no comparison directly included patients with SCLC versus those with NSCLC, to a certain extent, we bridged the gap in efficiency and safety data for ICI therapy among patients with NSCLC and SCLC. Previously, Maung et al. have shown that ICIs conferred better survival benefits than chemotherapy in both NSCLC and SCLC [53]. However, their conclusions were mainly based on qualitative analysis, without data analysis of clinical trials. In contrast, the quantitative analysis in our study could lead to more accurate and convincing results. Furthermore, our findings confirmed that immunotherapy could better benefit patients with NSCLC in prolonging PFS and increasing ORR than patients with SCLC. Given that the therapeutic effects of ICI treatment for SCLC remain controversial, we conducted a comprehensive assessment to compare its efficacy with chemotherapy. We observed that ICIs could undoubtedly reduce the risk of death in patients with SCLC, with a statistically significant difference, which has compensated for the lack of assessments of immunotherapy in the SCLC field. Second, one of the distinct strengths of our study is the data quality involved in our analyses. We employed 38 well-designed RCTs, gathered data from more than 20,000 patients with lung cancer, and carried out analyses according to predefined primary endpoints of OS and PFS and second endpoints of TRAEs with different grades. Our study was the largest scale of ICI analyses in patients with lung cancer. Under most circumstances, one essential factor in reducing statistical errors in a meta-analysis involves a large-scale quantity of subjects with high quality. Third, this study recommends optimal ICI treatment strategies in patients with NSCLC and SCLC. For NSCLC, the combination of anti-PD-1/PD-L1 and anti-CTLA-4 antibodies plus SOC is recommended for both first and subsequent lines of immunotherapy. In patients with SCLC, we only recommend the first-line treatment as anti-PD-1/PD-L1 plus SOC with or without anti-CTLA-4 antibodies.
Despite these strengths, several limitations exist in the present study. First, differences in risks and benefits between patients with NSCLC and SCLC were determined and compared through indirect analyses. To date, no RCTs have directly compared the efficiency and safety of immunotherapy between patients with SCLC and patients with NSCLC. Therefore, our results remain suggestive but not conclusive. Second, although our study is based on the largest scale of ICI analysis for lung cancer, more research is needed to comprehensively investigate the efficiency of immunotherapy in SCLC. Third, in selecting immunotherapy, the risk of toxicity is as important as the therapeutic effect, which should be thoroughly investigated. However, we only considered TRAEs of grade ≥ 3 and any grade, as information regarding TRAEs stratified by predefined subgroups was unavailable. Furthermore, additional factors should be used to evaluate toxicity.
Conclusion
In conclusion, for patients with NSCLC and SCLC, ICI therapies are promising therapeutic options with advantages in terms of survival and toxicity over SOC. Furthermore, ICIs plus SOC are recommended as the optimal first-line therapy for patients with SCLC. Anti-PD-1/PD-L1 plus SOC with anti-CTLA-4 antibodies is recommended for patients with NSCLC without mutated gene targets in both the first and subsequent lines of therapy. In addition, immunotherapy as a subsequent line is not recommended as a standard strategy for patients with SCLC.
Supplementary Information
Additional file 1: Fig. S1 Funnel plot of the effect size for each trial. Fig. S2 Drug targets analysis for NSCLC. Fig. S3 Therapeutic scheme analysis for SCLC. Table S1 Search strategies. Table S2 The methodological quality of included RCTs. Table S3 Differences in OS benefits of Immunotherapy in NSCLC and SCLC by subgroups
Acknowledgments
Not applicable.
Abbreviations
- ATE
Atezolizumab
- CI
Confidence interval
- CTLA-4
Cytotoxic T lymphocyte-associated antigen 4
- DOC
Docetaxel
- ECOG PS
Eastern Cooperative Oncology Group Performance Status
- HR
Hazard ratio
- ICI
Immune checkpoint inhibitor
- IPI
Ipilimumab
- NIV
Nivolumab
- DUR
Durvalumab
- TRE
Tremelimumab
- CAM
Camrelizumab
- CEM
Cemiplimab
- TIS
Tislelizumab
- SCLC
Small-cell lung cancer
- NSCLC
Non-small-cell lung cancer
- ORR
Objective response rate
- OS
Overall survival
- PBC
Platinum-based chemotherapy
- PD-1
Programmed cell death 1
- PD-L1
Programmed cell death 1 ligand 1
- PEM
Pembrolizumab
- PFS
Progression-free survival
- RCT
Randomised controlled trial
- RR
Risk ratio
- TRAEs
Treatment-related adverse events
- SOC
Standard of care
Authors’ contributions
Y. L. and H. G. designed the study. C. W. and J. L. collected the data and analyzed the data. C. W. and J. L. wrote the initial manuscript. C. W., J. W., Q. Z., Y. X., and L. S. participated in the manuscript correcting and data analyses. All authors participated in the manuscript writing and approved the final manuscript.
Funding
This work was supported by Sichuan Science and Technology Program (No. 2020YFS0572); National Guided Science and Technology Development Project of Sichuan Province (No: 2020ZYD009); the Science and Technology Project of Chengdu (No: 2017-CY02–00030-GX); Postdoctoral Program of West China Hospital, Sichuan University (No: 2020HXBH084); Postdoctoral Program of Sichuan University (2021SCU12018); Grant of Innovative Research Project for College Students, Sichuan University, Ministry of Education (No: C2021116604). The funding bodies played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
All data generated or analyzed during this study are included in this published article [and its supplementary information files]. All the data were available from the corresponding authors for reasonable request.
Declarations
Ethics approval and consent to participate
All analyses were based on previously published studies, thus no ethical approval and patient consent are required.
Consent for publication
Not applicable.
Competing interests
All authors declared that there was no conflict of interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hanlin Gong, Email: 9463382@qq.com.
Yalun Li, Email: liyalun@wchscu.cn.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Sabari JK, Lok BH, Laird JH, Poirier JT, Rudin CM. Unravelling the biology of SCLC: implications for therapy. Nat Rev Clin Oncol. 2017;14(9):549–561. doi: 10.1038/nrclinonc.2017.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Niksic M, Bonaventure A, Valkov M, Johnson CJ, Esteve J, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet (London, England) 2018;391(10125):1023–1075. doi: 10.1016/S0140-6736(17)33326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y, Zhou C, Reungwetwattana T, Cheng Y, Chewaskulyong B, Shah R, Cobo M, Lee KH, Cheema P, Tiseo M, John T, Lin MC, Imamura F, Kurata T, Todd A, Hodge R, Saggese M, Rukazenkov Y, Soria JC. Overall survival with Osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2020;382(1):41–50. doi: 10.1056/NEJMoa1913662. [DOI] [PubMed] [Google Scholar]
- 5.Liu M, Guo F. Recent updates on cancer immunotherapy. Precis Clin Med. 2018;1(2):65-74. [DOI] [PMC free article] [PubMed]
- 6.Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, Hermes B, Çay Şenler F, Csőszi T, Fülöp A, Rodríguez-Cid J, Wilson J, Sugawara S, Kato T, Lee KH, Cheng Y, Novello S, Halmos B, Li X, Lubiniecki GM, Piperdi B, Kowalski DM. Pembrolizumab plus chemotherapy for squamous non-small-cell lung Cancer. N Engl J Med. 2018;379(21):2040–2051. doi: 10.1056/NEJMoa1810865. [DOI] [PubMed] [Google Scholar]
- 7.Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim SW, Carcereny Costa E, Park K, Alexandru A, Lupinacci L, de la Mora JE, et al. Nivolumab plus Ipilimumab in advanced non-small-cell lung Cancer. N Engl J Med. 2019;381(21):2020–2031. doi: 10.1056/NEJMoa1910231. [DOI] [PubMed] [Google Scholar]
- 8.Lu S, Wang J, Cheng Y, Mok T, Chang J, Zhang L, Feng J, Tu HY, Wu L, Zhang Y, et al. Nivolumab versus docetaxel in a predominantly Chinese patient population with previously treated advanced non-small cell lung cancer: 2-year follow-up from a randomized, open-label, phase 3 study (CheckMate 078) Lung Cancer (Amsterdam, Netherlands) 2020;152:7–14. doi: 10.1016/j.lungcan.2020.11.013. [DOI] [PubMed] [Google Scholar]
- 9.Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, Statsenko G, Hochmair MJ, Özgüroğlu M, Ji JH, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet (London, England) 2019;394(10212):1929–1939. doi: 10.1016/S0140-6736(19)32222-6. [DOI] [PubMed] [Google Scholar]
- 10.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 11.Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ (Clinical research ed) 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical research ed) 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins J, Thompson S, Deeks J, Altman D. Statistical heterogeneity in systematic reviews of clinical trials: a critical appraisal of guidelines and practice. J Health Serv Res Policy. 2002;7(1):51–61. doi: 10.1258/1355819021927674. [DOI] [PubMed] [Google Scholar]
- 14.Lynch TJ, Bondarenko I, Luft A, Serwatowski P, Barlesi F, Chacko R, Sebastian M, Neal J, Lu H, Cuillerot JM, Reck M. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol. 2012;30(17):2046–2054. doi: 10.1200/JCO.2011.38.4032. [DOI] [PubMed] [Google Scholar]
- 15.Govindan R, Szczesna A, Ahn MJ, Schneider CP, Gonzalez Mella PF, Barlesi F, Han B, Ganea DE, Von Pawel J, Vladimirov V, et al. Phase III trial of Ipilimumab combined with paclitaxel and carboplatin in advanced squamous non-small-cell lung Cancer. J Clin Oncol. 2017;35(30):3449–3457. doi: 10.1200/JCO.2016.71.7629. [DOI] [PubMed] [Google Scholar]
- 16.Goldman JW, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, Statsenko G, Hochmair MJ, Özgüroğlu M, Ji JH, Garassino MC, Voitko O, Poltoratskiy A, Ponce S, Verderame F, Havel L, Bondarenko I, Każarnowicz A, Losonczy G, Conev NV, Armstrong J, Byrne N, Thiyagarajah P, Jiang H, Paz-Ares L, Dvorkin M, Trukhin D, Statsenko G, Voitko N, Poltoratskiy A, Bondarenko I, Chen Y, Kazarnowicz A, Paz-Ares L, Özgüroglu M, Conev N, Hochmair M, Burghuber O, Havel L, Çiçin I, Losonczy G, Moiseenko V, Erman M, Kowalski D, Wojtukiewicz M, Adamchuk H, Vasilyev A, Shevnia S, Valev S, Reinmuth N, Ji JH, Insa Molla MA, Ursol G, Chiang A, Hartl S, Horváth Z, Pajkos G, Verderame F, Hotta K, Kim SW, Smolin A, Göksel T, Dakhil S, Roubec J, Bogos K, Garassino MC, Cornelissen R, Lee JS, Garcia Campelo MR, Lopez Brea M, Alacacioglu A, Casarini I, Ilieva R, Tonev I, Somfay A, Bar J, Zer Kuch A, Minelli M, Bartolucci R, Roila F, Saito H, Azuma K, Lee GW, Luft A, Urda M, Delgado Mingorance JI, Majem Tarruella M, Spigel D, Koynov K, Zemanova M, Panse J, Schulz C, Pápai Székely Z, Sárosi V, Delmonte A, Bettini AC, Nishio M, Okamoto I, Hendriks L, Mandziuk S, Lee YG, Vladimirova L, Isla Casado D, Domine Gomez M, Navarro Mendivil A, Morán Bueno T, Wu SY, Knoble J, Skrickova J, Venkova V, Hilgers W, Laack E, Bischoff H, Fülöp A, Laczó I, Kósa J, Telekes A, Yoshida T, Kanda S, Hida T, Hayashi H, Maeda T, Kawamura T, Nakahara Y, Claessens N, Lee KH, Chiu CH, Lin SH, Li CT, Demirkazik A, Schaefer E, Nikolinakos P, Schneider J, Babu S, Lamprecht B, Studnicka M, Fausto Nino Gorini C, Kultan J, Kolek V, Souquet PJ, Moro-Sibilot D, Gottfried M, Smit E, Lee KH, Kasan P, Chovanec J, Goloborodko O, Kolesnik O, Ostapenko Y, Lakhanpal S, Haque B, Chua W, Stilwill J, Sena SN, Girotto GC, de Marchi PRM, Martinelli de Oliveira FA, Dos Reis P, Krasteva R, Zhao Y, Chen C, Koubkova L, Robinet G, Chouaid C, Grohe C, Alt J, Csánky E, Somogyiné Ezer É, Heching NI, Kim YH, Aatagi S, Kuyama S, Harada D, Nogami N, Nokihara H, Goto H, Staal van den Brekel A, Cho EK, Kim JH, Ganea D, Ciuleanu T, Popova E, Sakaeva D, Stresko M, Demo P, Godal R, Wei YF, Chen YH, Hsia TC, Lee KY, Chang HC, Wang CC, Dowlati A, Sumey C, Powell S, Goldman J, Zarba JJ, Batagelj E, Pastor AV, Zukin M, Baldotto CSR, Schlittler LA, Calabrich A, Sette C, Dudov A, Zhou C, Lena H, Lang S, Pápai Z, Goto K, Umemura S, Kanazawa K, Hara Y, Shinoda M, Morise M, Hiltermann J, Mróz R, Ungureanu A, Andrasina I, Chang GC, Vynnychenko I, Shparyk Y, Kryzhanivska A, Ross H, Mi K, Jamil R, Williamson M, Spahr J, Han Z, Wang M, Yang Z, Hu J, Li W, Zhao J, Feng J, Ma S, Zhou X, Liang Z, Hu Y, Chen Y, Bi M, Shu Y, Nan K, Zhou J, Zhang W, Ma R, Yang N, Lin Z, Wu G, Fang J, Zhang H, Wang K, Chen Z. Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2021;22(1):51–65. doi: 10.1016/S1470-2045(20)30539-8. [DOI] [PubMed] [Google Scholar]
- 17.Pujol JL, Greillier L, Audigier-Valette C, Moro-Sibilot D, Uwer L, Hureaux J, Guisier F, Carmier D, Madelaine J, Otto J, Gounant V, Merle P, Mourlanette P, Molinier O, Renault A, Rabeau A, Antoine M, Denis MG, Bommart S, Langlais A, Morin F, Souquet PJ. A randomized non-comparative phase II study of anti-programmed cell death-ligand 1 Atezolizumab or chemotherapy as second-line therapy in patients with small cell lung Cancer: results from the IFCT-1603 trial. J Thorac Oncol. 2019;14(5):903–913. doi: 10.1016/j.jtho.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Spigel DR, Vicente D, Ciuleanu TE, Gettinger S, Peters S, Horn L, Audigier-Valette C, Aranda NP, Juan-Vidal O, Cheng Y, et al. Second-line Nivolumab in relapsed small-cell lung Cancer: CheckMate 331. Ann Oncol. 2021;32(5):631–641. doi: 10.1016/j.annonc.2021.01.071. [DOI] [PubMed] [Google Scholar]
- 19.Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, Huemer F, Losonczy G, Johnson ML, Nishio M, Reck M, Mok T, Lam S, Shames DS, Liu J, Ding B, Lopez-Chavez A, Kabbinavar F, Lin W, Sandler A, Liu SV. First-line Atezolizumab plus chemotherapy in extensive-stage small-cell lung Cancer. N Engl J Med. 2018;379(23):2220–2229. doi: 10.1056/NEJMoa1809064. [DOI] [PubMed] [Google Scholar]
- 20.Reck M, Luft A, Szczesna A, Havel L, Kim SW, Akerley W, Pietanza MC, Wu YL, Zielinski C, Thomas M, Felip E, Gold K, Horn L, Aerts J, Nakagawa K, Lorigan P, Pieters A, Kong Sanchez T, Fairchild J, Spigel D. Phase III randomized trial of Ipilimumab plus etoposide and platinum versus placebo plus etoposide and platinum in extensive-stage small-cell lung Cancer. J Clin Oncol. 2016;34(31):3740–3748. doi: 10.1200/JCO.2016.67.6601. [DOI] [PubMed] [Google Scholar]
- 21.Reck M, Bondarenko I, Luft A, Serwatowski P, Barlesi F, Chacko R, Sebastian M, Lu H, Cuillerot JM, Lynch TJ. Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trial. Ann Oncol. 2013;24(1):75–83. doi: 10.1093/annonc/mds213. [DOI] [PubMed] [Google Scholar]
- 22.Langer CJ, Gadgeel SM, Borghaei H, Papadimitrakopoulou VA, Patnaik A, Powell SF, Gentzler RD, Martins RG, Stevenson JP, Jalal SI, Panwalkar A, Yang JC, Gubens M, Sequist LV, Awad MM, Fiore J, Ge Y, Raftopoulos H, Gandhi L, KEYNOTE-021 investigators Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016;17(11):1497–1508. doi: 10.1016/S1470-2045(16)30498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barlesi F, Vansteenkiste J, Spigel D, Ishii H, Garassino M, de Marinis F, Özgüroğlu M, Szczesna A, Polychronis A, Uslu R, Krzakowski M, Lee JS, Calabrò L, Arén Frontera O, Ellers-Lenz B, Bajars M, Ruisi M, Park K. Avelumab versus docetaxel in patients with platinum-treated advanced non-small-cell lung cancer (JAVELIN lung 200): an open-label, randomised, phase 3 study. Lancet Oncol. 2018;19(11):1468–1479. doi: 10.1016/S1470-2045(18)30673-9. [DOI] [PubMed] [Google Scholar]
- 24.Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ, Powell SF, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung Cancer. N Engl J Med. 2018;378(22):2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 25.Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, Castro G, Jr, Srimuninnimit V, Laktionov KK, Bondarenko I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet (London, England) 2019;393(10183):1819–1830. doi: 10.1016/S0140-6736(18)32409-7. [DOI] [PubMed] [Google Scholar]
- 26.Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet (London, England) 2016;387(10027):1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 27.Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, Park K, Smith D, Artal-Cortes A, Lewanski C, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet (London, England) 2016;387(10030):1837–1846. doi: 10.1016/S0140-6736(16)00587-0. [DOI] [PubMed] [Google Scholar]
- 28.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Kurata T, Chiappori A, Lee KH, de Wit M, Cho BC, Bourhaba M, Quantin X, Tokito T, Mekhail T, Planchard D, Kim YC, Karapetis CS, Hiret S, Ostoros G, Kubota K, Gray JE, Paz-Ares L, de Castro Carpeño J, Faivre-Finn C, Reck M, Vansteenkiste J, Spigel DR, Wadsworth C, Melillo G, Taboada M, Dennis PA, Özgüroğlu M. Overall survival with Durvalumab after Chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379(24):2342–2350. doi: 10.1056/NEJMoa1809697. [DOI] [PubMed] [Google Scholar]
- 29.Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, et al. Updated analysis of KEYNOTE-024: Pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung Cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol. 2019;37(7):537–546. doi: 10.1200/JCO.18.00149. [DOI] [PubMed] [Google Scholar]
- 30.Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung Cancer. N Engl J Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O’Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung Cancer. N Engl J Med. 2016;375(19):1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 32.Herbst RS, Giaccone G, de Marinis F, Reinmuth N, Vergnenegre A, Barrios CH, Morise M, Felip E, Andric Z, Geater S, Özgüroğlu M, Zou W, Sandler A, Enquist I, Komatsubara K, Deng Y, Kuriki H, Wen X, McCleland M, Mocci S, Jassem J, Spigel DR. Atezolizumab for first-line treatment of PD-L1-selected patients with NSCLC. N Engl J Med. 2020;383(14):1328–1339. doi: 10.1056/NEJMoa1917346. [DOI] [PubMed] [Google Scholar]
- 33.von Pawel J, Bordoni R, Satouchi M, Fehrenbacher L, Cobo M, Han JY, Hida T, Moro-Sibilot D, Conkling P, Gandara DR, et al. Long-term survival in patients with advanced non-small-cell lung cancer treated with atezolizumab versus docetaxel: Results from the randomised phase III OAK study. Eur J Cancer (Oxford, England : 1990) 2019;107:124–132. doi: 10.1016/j.ejca.2018.11.020. [DOI] [PubMed] [Google Scholar]
- 34.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhäufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crinò L, Blumenschein GR, Jr, Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, Brahmer JR. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung Cancer. N Engl J Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, de Wit M, Cho BC, Bourhaba M, Quantin X, Tokito T, Mekhail T, Planchard D, Kim YC, Karapetis CS, Hiret S, Ostoros G, Kubota K, Gray JE, Paz-Ares L, de Castro Carpeño J, Wadsworth C, Melillo G, Jiang H, Huang Y, Dennis PA, Özgüroğlu M. Durvalumab after Chemoradiotherapy in stage III non-small-cell lung Cancer. N Engl J Med. 2017;377(20):1919–1929. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 36.Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, Rodríguez-Abreu D, Moro-Sibilot D, Thomas CA, Barlesi F, Finley G, Kelsch C, Lee A, Coleman S, Deng Y, Shen Y, Kowanetz M, Lopez-Chavez A, Sandler A, Reck M. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288–2301. doi: 10.1056/NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- 37.Planchard D, Reinmuth N, Orlov S, Fischer JR, Sugawara S, Mandziuk S, Marquez-Medina D, Novello S, Takeda Y, Soo R, Park K, McCleod M, Geater SL, Powell M, May R, Scheuring U, Stockman P, Kowalski D. ARCTIC: durvalumab with or without tremelimumab as third-line or later treatment of metastatic non-small-cell lung cancer. Ann Oncol. 2020;31(5):609–618. doi: 10.1016/j.annonc.2020.02.006. [DOI] [PubMed] [Google Scholar]
- 38.Fehrenbacher L, von Pawel J, Park K, Rittmeyer A, Gandara DR, Ponce Aix S, Han JY, Gadgeel SM, Hida T, Cortinovis DL, Cobo M, Kowalski DM, de Marinis F, Gandhi M, Danner B, Matheny C, Kowanetz M, He P, Felizzi F, Patel H, Sandler A, Ballinger M, Barlesi F. Updated efficacy analysis including secondary population results for OAK: a randomized phase III study of Atezolizumab versus docetaxel in patients with previously treated advanced non-small cell lung Cancer. J Thorac Oncol. 2018;13(8):1156–1170. doi: 10.1016/j.jtho.2018.04.039. [DOI] [PubMed] [Google Scholar]
- 39.Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M, Felip E, van den Heuvel MM, Ciuleanu TE, Badin F, Ready N, Hiltermann TJN, Nair S, Juergens R, Peters S, Minenza E, Wrangle JM, Rodriguez-Abreu D, Borghaei H, Blumenschein GR Jr, Villaruz LC, Havel L, Krejci J, Corral Jaime J, Chang H, Geese WJ, Bhagavatheeswaran P, Chen AC, Socinski MA, CheckMate 026 Investigators First-line Nivolumab in stage IV or recurrent non-small-cell lung Cancer. N Engl J Med. 2017;376(25):2415–2426. doi: 10.1056/NEJMoa1613493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols MC, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet (London, England) 2017;389(10066):255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, Kopp HG, Daniel D, McCune S, Mekhail T, Zer A, Reinmuth N, Sadiq A, Sandler A, Lin W, Ochi Lohmann T, Archer V, Wang L, Kowanetz M, Cappuzzo F. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(7):924–937. doi: 10.1016/S1470-2045(19)30167-6. [DOI] [PubMed] [Google Scholar]
- 42.Theelen W, Peulen HMU, Lalezari F, van der Noort V, de Vries JF, Aerts J, Dumoulin DW, Bahce I, Niemeijer AN, de Langen AJ, et al. Effect of Pembrolizumab after stereotactic body radiotherapy vs Pembrolizumab alone on tumor response in patients with advanced non-small cell lung Cancer: results of the PEMBRO-RT phase 2 randomized clinical trial. JAMA Oncol. 2019;5(9):1276–1282. doi: 10.1001/jamaoncol.2019.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jotte R, Cappuzzo F, Vynnychenko I, Stroyakovskiy D, Rodriguez-Abreu D, Hussein M, Soo R, Conter HJ, Kozuki T, Huang KC, et al. Atezolizumab in combination with carboplatin and nab-paclitaxel in advanced squamous NSCLC (IMpower131): results from a randomized phase III trial. J Thorac Oncol. 2020;15(8):1351–1360. doi: 10.1016/j.jtho.2020.03.028. [DOI] [PubMed] [Google Scholar]
- 44.Nishio M, Barlesi F, West H, Ball S, Bordoni R, Cobo M, Longeras PD, Goldschmidt J Jr., Novello S, Orlandi F, et al. Atezolizumab plus chemotherapy for first-line treatment of nonsquamous NSCLC: results from the randomized phase 3 IMpower132 trial. J Thorac Oncol. 2021;16(4):653-64. [DOI] [PubMed]
- 45.Paz-Ares L, Ciuleanu TE, Cobo M, Schenker M, Zurawski B, Menezes J, Richardet E, Bennouna J, Felip E, Juan-Vidal O, Alexandru A, Sakai H, Lingua A, Salman P, Souquet PJ, de Marchi P, Martin C, Pérol M, Scherpereel A, Lu S, John T, Carbone DP, Meadows-Shropshire S, Agrawal S, Oukessou A, Yan J, Reck M. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(2):198–211. doi: 10.1016/S1470-2045(20)30641-0. [DOI] [PubMed] [Google Scholar]
- 46.Zhou C, Chen G, Huang Y, Zhou J, Lin L, Feng J, Wang Z, Shu Y, Shi J, Hu Y, et al. Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy-naive patients with advanced non-squamous non-small-cell lung cancer (CameL): a randomised, open-label, multicentre, phase 3 trial. Lancet Respir Med. 2021;9(3):305-14. [DOI] [PubMed]
- 47.Sezer A, Kilickap S, Gumus M, Bondarenko I, Ozguroglu M, Gogishvili M, Turk HM, Cicin I, Bentsion D, Gladkov O, et al. Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: a multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet (London, England) 2021;397(10274):592–604. doi: 10.1016/S0140-6736(21)00228-2. [DOI] [PubMed] [Google Scholar]
- 48.Wang J, Lu S, Yu X, Hu Y, Sun Y, Wang Z, Zhao J, Yu Y, Hu C, Yang K, Feng G, Ying K, Zhuang W, Zhou J, Wu J, Leaw SJ, Zhang J, Lin X, Liang L, Yang N. Tislelizumab plus chemotherapy vs chemotherapy alone as first-line treatment for advanced squamous non-small-cell lung Cancer: a phase 3 randomized clinical trial. JAMA Oncol. 2021;7(5):709–717. doi: 10.1001/jamaoncol.2021.0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sharma P, Allison JP. Dissecting the mechanisms of immune checkpoint therapy. Nat Rev Immunol. 2020;20(2):75–76. doi: 10.1038/s41577-020-0275-8. [DOI] [PubMed] [Google Scholar]
- 50.Wang C, Qiao W, Jiang Y, Zhu M, Shao J, Wang T, Liu D, Li W. The landscape of immune checkpoint inhibitor plus chemotherapy versus immunotherapy for advanced non-small-cell lung cancer: a systematic review and meta-analysis. J Cell Physiol. 2020;235(5):4913–4927. doi: 10.1002/jcp.29371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wallis C, Butaney M, Satkunasivam R, Freedland S, Patel S, Hamid O, et al. Association of patient sex with efficacy of immune checkpoint inhibitors and overall survival in advanced cancers: a systematic review and meta-analysis. JAMA Oncol. 2019;5(4):529–36. [DOI] [PMC free article] [PubMed]
- 52.Zhou T, Zhang Z, Luo F, Zhao Y, Hou X, Liu T, Wang K, Zhao H, Huang Y, Zhang L. Comparison of first-line treatments for patients with extensive-stage small cell lung Cancer: a systematic review and network Meta-analysis. JAMA Netw Open. 2020;3(10):e2015748. doi: 10.1001/jamanetworkopen.2020.15748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maung TZ, Ergin HE, Javed M, Inga EE, Khan S. Immune checkpoint inhibitors in lung Cancer: role of biomarkers and combination therapies. Cureus. 2020;12(5):e8095. doi: 10.7759/cureus.8095. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Fig. S1 Funnel plot of the effect size for each trial. Fig. S2 Drug targets analysis for NSCLC. Fig. S3 Therapeutic scheme analysis for SCLC. Table S1 Search strategies. Table S2 The methodological quality of included RCTs. Table S3 Differences in OS benefits of Immunotherapy in NSCLC and SCLC by subgroups
Data Availability Statement
All data generated or analyzed during this study are included in this published article [and its supplementary information files]. All the data were available from the corresponding authors for reasonable request.