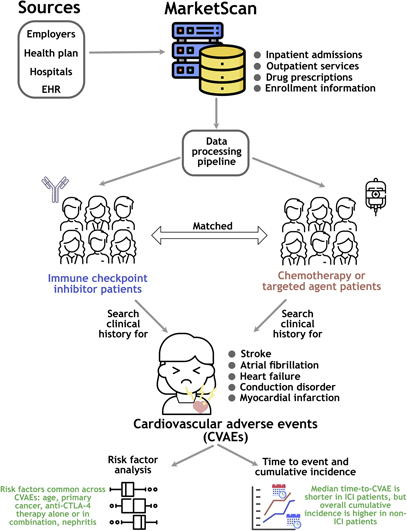
Abstract
Background
Immune checkpoint inhibitors (ICIs) can cause life-threatening cardiovascular adverse events (CVAEs) that may not be attributed to therapy. The outcomes of clinical trials may underestimate treatment-related adverse events due to restrictive eligibility, limited sample size, and failure to anticipate selected toxicities. We evaluated the incidence and clinical determinants of CVAEs in real-world population on ICI therapy.
Patients and methods
Among 2 687 301 patients diagnosed with cancer from 2011 to 2018, 16 574 received ICIs for any cancer. Patients in the ICI and non-ICI cohorts were matched in a 1 : 1 ratio according to age, sex, National Cancer Institute comorbidity score, and primary cancer. The non-ICI cohort was stratified into patients who received chemotherapy (N = 2875) or targeted agents (N = 4611). All CVAEs, non-cardiac immune-related adverse events occurring after treatment initiation, baseline comorbidities, and treatment details were identified and analyzed using diagnosis and billing codes.
Results
Median age was 61 and 65 years in the ICI and non-ICI cohorts, respectively (P < 0.001). ICI patients were predominantly male (P < 0.001). Lung cancer (43.1%), melanoma (30.4%), and renal cell carcinoma (9.9%) were the most common cancer types. CVAE diagnoses in our dataset by incidence proportion (ICI cohort) were stroke (4.6%), heart failure (3.5%), atrial fibrillation (2.1%), conduction disorders (1.5%), myocardial infarction (0.9%), myocarditis (0.05%), vasculitis (0.05%), and pericarditis (0.2%). Anti-cytotoxic T-lymphocyte-associated protein 4 increased the risk of heart failure [versus anti-programmed cell death protein 1; hazard ratio (HR), 1.9; 95% confidence interval (CI) 1.27-2.84] and stroke (HR, 1.7; 95% CI 1.3-2.22). Pneumonitis was associated with heart failure (HR, 2.61; 95% CI 1.23-5.52) and encephalitis with conduction disorders (HR, 4.35; 95% CI 1.6-11.87) in patients on ICIs. Advanced age, primary cancer, nephritis, and anti-cytotoxic T-lymphocyte-associated protein 4 therapy were commonly associated with CVAEs in the adjusted Cox proportional hazards model.
Conclusions
Our findings underscore the importance of risk stratification and cardiovascular monitoring for patients on ICI therapy.
Key words: immune checkpoint inhibitors, anti-CTLA4, anti-PD-1, anti-PD-L1, cardiovascular adverse events, real-world evidence
Graphical abstract
Highlights
-
•
Patient claims data across the United States were used to study cardiovascular adverse events (CVAEs) after ICI treatment.
-
•
Patients on ICI treatment for advanced cancer have a higher incidence of CVAEs than previously reported.
-
•
Median time to CVAE onset was significantly shorter with ICIs (~3 months) than with non-ICI systemic therapy (~8 months).
-
•
Anti-CTLA-4 monotherapy or combination had a higher risk of heart failure and stroke than anti-PD-1 therapy (1.5-2 folds).
-
•
Age, male sex, cancer type, nephritis, pneumonitis, and anti-CTLA-4 therapy were associated with a higher risk of CVAEs.
Introduction
Immune checkpoint inhibitors (ICIs) have revolutionized the cancer treatment paradigm. Immune checkpoints act as negative regulators of autoreactive T cells, resulting in immune tolerance and prevention of autoimmunity.1 Therapeutic blocking using ICIs can result in nonspecific immune activation with a unique spectrum of side-effects. These are referred to as immune-related adverse events (irAEs) and can affect any organ. Most irAEs can be managed using steroids; however, in some cases, they can lead to severe complications, if not managed promptly.2,3
At present, cardiovascular monitoring is not routinely carried out after initiating immunotherapy in clinical trials. Due to nonspecific symptoms and attribution bias, ICI-associated cardiovascular adverse events (CVAEs) are rarely reported.4 Inflammatory CVAEs with ICI treatment are well documented in both preclinical and clinical studies.5,6 Most studies describing epidemiological features and clinical characteristics, however, are single institution-based, with relatively small sample sizes. Therefore, risk factors leading to these rare but fatal CVAEs have not been well characterized and may be underestimated.
Longitudinal real-world big datasets may facilitate the identification, incidence, and trend of such rare cardiovascular side-effects. Real-world data can be processed comprehensively to generate clinically meaningful and actionable real-world evidence that better reflects the true clinical environment.7,8 Immunotherapy-related CVAEs need to be systematically evaluated using real-world big datasets. Our primary objectives were to estimate the incidence and clinical determinants of CVAEs associated with ICI treatment in advanced cancers in the real-world population. We used claims data generated from 17-53 million commercially insured individuals per year across the United States (US) and reported the clinical characteristics and factors associated with CVAEs after ICI administration in comparison with appropriately matched controls.
Methods
This is a retrospective cohort study of advanced cancer patients on ICI treatment who were not enrolled in clinical trials. ICI and chemotherapy outpatient pharmacy claims, and corresponding patient claims data were extracted from the International Business Machines (IBM) MarketScan Research Databases (Armonk, NY).
Data source
We used the IBM MarketScan data (de-identified linked inpatient, outpatient, and pharmacy claims), one of the largest proprietary US claims databases consisting of Commercial Claims and Encounter and Medicare Supplemental and Coordination of Benefits databases from 2011 to 2018. The de-identified data complied with the Health Insurance Portability and Accountability Act and the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.9 This study was exempted from review by University Hospitals' institutional review board and the need for informed consent from patients. This study adhered to the Strengthening the Reporting of Observational Studies in Epidemiology reporting guidelines for cohort studies.
Study population
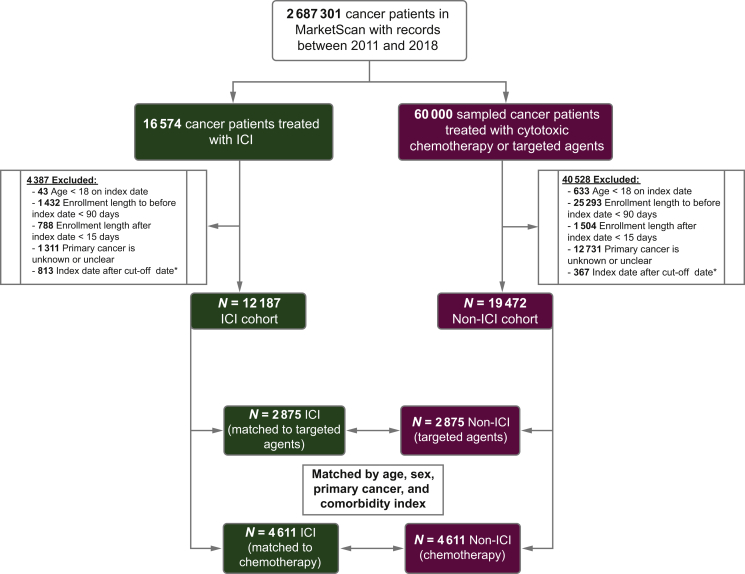
We first identified 2 687 301 patients with International Classification of Diseases (ICD 9/10) codes for any cancer in our database who were enrolled at any time between January 1 2011 and October 31 2018 (see Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2021.100252). The key inclusion criteria were patients (i) aged at least 18 years on the day of the first ICI or chemotherapy treatment and (ii) with at least one confirmed ICD-9-CM or ICD-10-CM diagnosis code for a cancer where ICI therapy was approved until 2018. To reduce the possibility of coding errors, we considered a confirmed diagnosis only if the cancer diagnosis code appeared in (at least) either (i) one inpatient claim or (ii) two outpatient claims >30 to 42 days apart to reduce misclassification10; (iii) was a discernible primary cancer as of the index date, which is defined as the date of the first administration of ICI or chemotherapy; (iv) was part of continuous health plan enrollment for at least 90 days before and 30 days after the index date. We constructed ICI and non-ICI cohorts using the aforementioned eligibility criteria (Figure 1). For the non-ICI cohort, we selected a random sample of 60 000 cancer patients who received a minimum of one dose of cytotoxic chemotherapy or targeted agents provided there was at least one inpatient claim, or two outpatient claims of administration to minimize ambiguity (list of drugs is provided in Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2021.100252). Patients in the ICI cohort were matched in a 1 : 1 ratio to those in the non-ICI cohort, either treated with chemotherapy or with targeted agents by age, sex, National Cancer Institute (NCI) comorbidity score, and primary cancer. Baseline comorbidities were identified and incorporated into the NCI comorbidity index, whereby a higher score reflects a high comorbidity burden.11
Figure 1.
Study design.
Index date is defined as the date of the first ICI treatment or the first chemotherapy or targeted agent treatment (for the non-ICI patients).
∗ Cut-off date was set to September 30 2018 (three months prior to the last recorded data in the IBM MarketScan dataset).
ICI, immune checkpoint inhibitor
Covariates
The complete list of diagnoses, procedures, and prescription codes used in the IBM MarketScan database to identify the variables of interest was compiled (Supplementary Tables S1-S7 and Figure S1, available at https://doi.org/10.1016/j.esmoop.2021.100252). The variables of interest were as follows: (i) diagnoses (ICD 9/10 CM) codes for cancer, irAEs, CVAEs, and comorbidities (according to the NCI comorbidity guideline) and (ii) procedure or drug prescription codes [Procedure Coding System (PCS); Current procedural terminology (CPT); Healthcare Common Procedure Coding System (HCPCS); National Drug Code (NDC)] for ICI therapy and non-ICI systemic treatment including cytotoxic chemotherapy or targeted agents and other cancer procedures (radiation and surgery). Claims within 90 days before drug use were used to generate the NCI comorbidity index. The NCI comorbidity index is an updated version of the Charlson comorbidity index that considers the following 15 comorbidities: acute myocardial infarction, acquired immunodeficiency syndrome, cerebrovascular disease, chronic obstructive pulmonary disease (COPD), congestive heart failure, dementia, diabetes, complications of diabetes, myocardial infarction, liver disease, paralysis, peptic ulcer disease, peripheral vascular disease, renal disease, and rheumatologic disease. The definitions of covariates and other terms used throughout the study are provided in Supplementary methods, available at https://doi.org/10.1016/j.esmoop.2021.100252.
Outcomes
The primary outcome of this study was the incidence and risk factors associated with CVAEs.
Statistical analyses
Continuous variables were presented as means (±standard deviation) or as median [with interquartile range (IQR)] for normally and non-normally distributed data, respectively. Categorical variables were presented as frequencies and percentages. Cumulative incidence rates and the median time to event were estimated using the Kaplan–Meier method and assessed using log-rank tests as implemented in the Lifelines package (version 0.24.14) for Python 3.7. All hypothesis tests were two-sided, and a P value of <0.01 was considered statistically significant.
To allow for small deviances in continuous values across cohorts, matched age with a tolerance of up to a 1-year difference and NCI comorbidity score with a tolerance of up to a 0.5-point difference were included.
We grouped ICIs into three treatment regimens: (i) cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitor (ipilimumab); (ii) anti-programmed cell death protein 1 (anti-PD-1) or anti-programmed death-ligand 1 (anti-PD-L1) (nivolumab, pembrolizumab, atezolizumab, avelumab, and durvalumab), and (iii) anti-CTLA-4 plus PD-1 combination. Data were collected on age, sex, variables in NCI comorbidity score, type of ICI, treatment year, number of ICI doses, type of CVAEs, type of non-CV irAEs, time until the first CVAE after ICI, and duration of follow-up.
We used the Kruskal–Wallis test and Bonferroni correction for P values (for five cardiovascular events). The proportional hazards assumptions were examined, and multivariate Cox proportional hazards regression was used to evaluate the risk associated with each CVAE while adjusting for age, sex, other irAEs, and comorbidities. Variable selection using random forests12 and sensitivity analyses were carried out before and after the Cox regression analysis, respectively. For details, see Supplementary methods and Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2021.100252.
Results
Between 2011 and 2018, a total of 2 687 301 patients were diagnosed with cancer in the IBM MarketScan database. A total of 16 574 patients received at least one dose of ICI for any advanced cancer. Of these, 12 187 met the study eligibility criteria, contributing to 9985 person-years of follow-up (Figure 1). The non-ICI cohort included 60 000 patients (~5 times the number of patients in the ICI cohort) with advanced active cancer who received either cytotoxic chemotherapy or targeted agents. Of these, 19 472 met the eligibility criteria, contributing to 10 534.6 person-years of follow-up (Figure 1). Based on the underlying pathophysiology, we classified CVAEs into myocardial infarction, heart failure, conduction disorder, atrial fibrillation, stroke, myocarditis, and others (pericarditis and vasculitis) and identified five major CVAE groups most commonly reported in our dataset. The spectrum and percentage of CVAEs reported for all immunotherapy regimens are shown in Supplementary Figure S3A, available at https://doi.org/10.1016/j.esmoop.2021.100252. We observed minimal overlap or co-occurrence of CVAEs with each other (Supplementary Figure S3B, available at https://doi.org/10.1016/j.esmoop.2021.100252).
Patient characteristics
In the ICI cohort (N = 12 187), the majority of patients (72.8%) received PD-1/PD-L1 inhibitor monotherapy, followed by anti-CTLA-4 monotherapy (16.4%), a combination of anti-CTLA-4 and anti-PD-1 (6.1%), and anti-PD-L1 monotherapy (4.6%). Patient demographics, comorbidities, and cancer data are shown in Supplementary Table S8, available at https://doi.org/10.1016/j.esmoop.2021.100252. In the ICI cohort, the median age of patients was 61 years (55-69) versus 65 years (58-74) in the control cohort (P < 0.001). ICI patients were predominantly male (58.4% versus 47.0%, P < 0.001). Most patients receiving ICI had lung cancer (43.1%), followed by malignant melanoma (30.4%) and renal cell carcinoma (9.9%). Other cancers comprised <10% of the overall population (Supplementary Table S8 and Figure S4, available at https://doi.org/10.1016/j.esmoop.2021.100252). COPD was the most common comorbid condition identified in patients, with and without ICI usage (43.8% versus 44.6%, P = 0.163). The non-ICI cohort had higher rates of diabetes mellitus (37.8% versus 25.4%, P < 0.001), history of congestive heart failure (13.2% versus 11.0%, P < 0.001), cerebrovascular disease (3.1% versus 2.4%, P < 0.001), and dementia (2.3% versus 1.5%, P < 0.001).
CVAEs
ICI cohort
The incidence rates of stroke, heart failure, atrial fibrillation, conduction disorders, myocardial infarction, myocarditis, vasculitis, and pericarditis were 4.6%, 3.5%, 2.1%, 1.5%, 0.9%, 0.05%, 0.05%, and 0.2%, respectively. The clinical characteristics of the prespecified CVAE groups are shown in Table 1. Males were more affected than females. Stroke events were more commonly reported in patients with malignant melanoma [N = 302 (53.6%) of 564 patients], whereas atrial fibrillation [N = 132 (52.8%) of 250 patients], myocardial infarction [N = 58 (51.8%) of 112 patients], heart failure [N = 205 (48.2%) of 425 patients], and conduction disorders [N = 66 (35.9%) of 184 patients] were more commonly reported in patients with lung cancer. The clinical characteristics of the prespecified CVAE groups in the non-ICI cohort are shown in Supplementary Table S9, available at https://doi.org/10.1016/j.esmoop.2021.100252.
Table 1.
Baseline characteristics of ICI patients overall and by CVAE type (MarketScan database, 2011-2018)
| Baseline characteristic at or before the index date | ICI cohort (N = 12,187) | Stroke (N = 564) | Atrial fibrillation (N = 250) | Heart failure (N = 425) | Conduction disorder (N = 184) | Myocardial Infarction (N = 112) | Myocarditis (N = 6) |
|---|---|---|---|---|---|---|---|
| Age, median (IQR) | 61 (55-69) | 60 (52-66.25) | 64 (58.25-76) | 64 (58-75) | 62 (56-72) | 64 (60-74) | 50.5 (37.75-61.0) |
| Males, no. (%) | 7113 (58.37) | 357 (63.29) | 162 (64.8) | 249 (58.58) | 115 (62.5) | 80 (71.42) | 1 (16.66) |
| Females, no. (%) | 5074 (41.63) | 207 (36.70) | 88 (35.2) | 176 (41.42) | 69 (37.5) | 32 (28.57) | 5 (83.33) |
| NCI comorbidity score, median (IQR) | 2.09 (1.3-4.37) | 2.09 (0.0-3.62) | 2.73 (1.34-4.80) | 3.34 (1.69-5.38) | 3.24 (1.60-5.15) | 3.75 (1.69-6.02) | 1.89 (1.69-2.09) |
| Days to CVAE, median (IQR) | N/A | 135.5 (52.5-285.5) | 101 (42.0-229.5) | 123 (50.0-266.0) | 145 (48.75-340.75) | 124 (36.0-252.0) | 116.5 (52.0-136.0) |
| CVAE hospitalization rate, no. (%) | N/A | 474 (84.04) | 199 (79.6) | 363 (85.41) | 159 (86.41) | 102 (91.07) | 5 (83.33) |
| ICI doses before onset of CVAE, median (IQR) | N/A | 4 (2-6) | 3 (1.25-5) | 4 (2-6) | 4 (2-8) | 3 (2-6) | 3.5 (1.5-5.5) |
| Comorbidities, no. (%) | |||||||
| History of acute myocardial infarction | 664 (5.45) | 22 (3.9) | 12 (4.8) | 41 (9.65) | 12 (6.52) | 9 (8.04) | 0 (0) |
| History of cerebrovascular disease | 287 (2.35) | 9 (1.6) | 6 (2.4) | 13 (3.06) | 5 (2.72) | 6 (5.36) | 0 (0) |
| History of chronic obstructive pulmonary disease | 5337 (43.79) | 195 (34.57) | 120 (48.0) | 218 (51.29) | 78 (42.39) | 64 (57.14) | 3 (50.0) |
| History of congestive heart failure | 1340 (11.0) | 42 (7.45) | 31 (12.4) | 26 (6.12) | 33 (17.93) | 28 (25) | 0 (0) |
| History of dementia | 185 (1.52) | 11 (1.95) | 1 (0.4) | 6 (1.41) | 4 (2.17) | 2 (1.79) | 0 (0) |
| History of diabetes | 3099 (25.42) | 128 (22.70) | 76 (30.4) | 163 (38.35) | 55 (29.89) | 47 (41.96) | 1 (16.67) |
| ICI type, no. (%) | |||||||
| Anti-CTLA-4 + anti-PD-1 combo as first ICI | 747 (6.13) | 46 (8.16) | 8 (3.2) | 29 (6.82) | 14 (7.61) | 3 (2.68) | 2 (33.33) |
| Anti-CTLA-4 monotherapy as first ICI | 2001 (16.42) | 201 (35.46) | 62 (24.8) | 77 (18.12) | 41 (22.28) | 24 (21.43) | 1 (16.67) |
| Anti-PD-1 monotherapy as first ICI | 8875 (72.82) | 298 (52.84) | 163 (65.2) | 304 (71.53) | 120 (65.22) | 81 (75.32) | 3 (50) |
| Anti-PD-L1 monotherapy as first ICI | 564 (4.63) | 19 (3.37) | 17 (6.8) | 15 (3.53) | 9 (4.89) | 4 (3.57) | 0 (0) |
| Received radiation before onset of CVAE, no. (%) | 4626 (37.96) | 271 (48.04) | 82 (32.8) | 143 (33.65) | 69 (37.5) | 34 (30.35) | 1 (16.66) |
| Received chemotherapy before first ICI, no. (%) | 5333 (43.76) | 184 (32.62) | 110 (44.0) | 190 (44.70) | 72 (39.13) | 55 (49.10) | 1 (16.66) |
| Received targeted agent therapy before first ICI, no. (%) | 2666 (21.87) | 108 (19.14) | 41 (16.4) | 100 (23.53) | 41 (22.28) | 25 (22.32) | 0 (0) |
| Received chemotherapy after ICI, no. (%) | 2316 (19.0) | 89 (15.78) | 54 (21.6) | 68 (16.0) | 18 (9.78) | 17 (15.18) | 0 (0) |
| Received targeted agent therapy after first ICI, no. (%) | 1430 (11.73) | 80 (14.18) | 18 (7.20) | 59 (13.88) | 21 (11.41) | 17 (15.18) | 2 (33.33) |
| Malignant melanoma, no. (%) | 3708 (30.43) | 302 (53.55) | 79 (31.6) | 117 (27.53) | 65 (35.33) | 35 (31.25) | 2 (33.33) |
| Lung cancer, no. (%) | 5255 (43.12) | 184 (32.62) | 132 (52.8) | 205 (48.24) | 66 (35.87) | 58 (51.79) | 3 (50.0) |
| Renal cell carcinoma, no. (%) | 1211 (9.94) | 44 (7.8) | 19 (7.6) | 61 (14.35) | 25 (13.59) | 8 (7.14) | 0 (0) |
| Other cancers,a no. (%) | 2013 (16.51) | 34 (6.02) | 20 (8.00) | 42 (9.88) | 28 (15.22) | 11 (9.82) | 1 (16.67) |
CTLA-4, cytotoxic T-lymphocyte-associated protein 4; CVAE, cardiovascular adverse events; ICI, immune checkpoint inhibitor; IQR, interquartile range; PD-1, programmed cell death protein 1.
Other cancers include Hodgkin's lymphoma, bladder cancer, head and neck cancer, Merkel cell carcinoma, colorectal cancer, hepatocellular carcinoma, gastric cancer, esophageal cancer, cervical cancer, and primary mediastinal B-cell lymphoma.
After ICI initiation, the median time to onset for stroke was 4.5 months ( IQR 1.75-9.52 months). Atrial fibrillation, heart failure, myocardial infarction, and conduction disorders had median time to onset of 3.37 months (IQR 1.4-7.65 months), 4.1 months (IQR 1.66-8.87 months), 4.13 months (IQR 1.2-8.4 months), and 4.83 months (IQR 1.62-11.36 months), respectively. In most cases, patients with cardiovascular AEs required hospitalization with hospitalization rates of 91.1% for myocardial infarction, 86.4% for conduction disorders, 85.5% for heart failure, 84.0% for stroke, and 79.6% for atrial fibrillation. The ICI discontinuation rates were 17.55% for stroke, 10.59% for heart failure, 10.8% for atrial fibrillation, 16.30% for conduction disorders, and 8.93% for myocardial infarction (Table 1). The most common irAEs that co-occurred with CVAEs were nephritis (N = 266, 19.54%), thyroiditis (N = 130, 9.55%), and colitis (N = 72, 5.29%). The co-occurrence of each CVAE and non-cardiovascular irAEs is detailed in Supplementary Table S10, available at https://doi.org/10.1016/j.esmoop.2021.100252.
Clinical determinants of increased risk of CVAEs
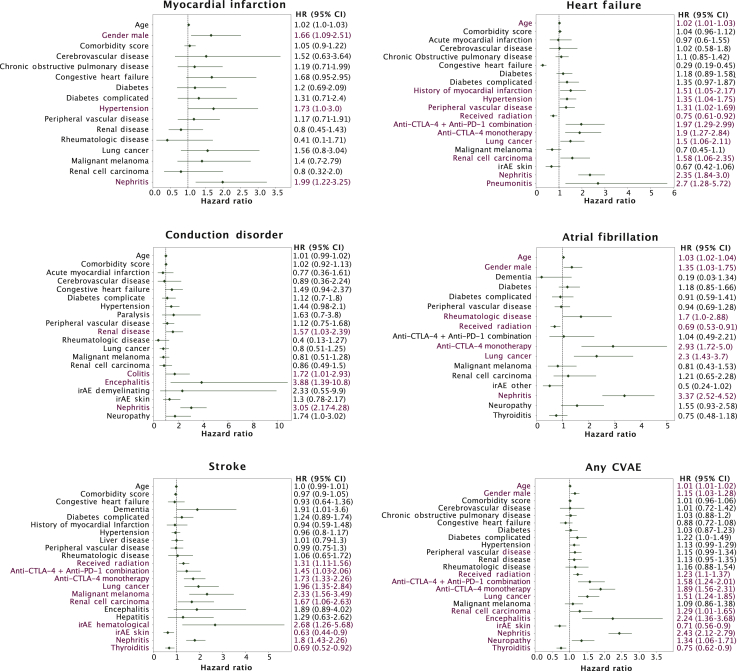
The use of ICIs was associated with a higher risk of developing myocardial infarction in men [hazard ratio (HR), 1.7; 95% confidence interval (CI) 1.12-2.58; P = 0.012] (Figure 2). Among patients who developed nephritis, use of ICIs was associated with a twofold to threefold higher risk of myocardial infarction (HR, 2.03; 95% CI 1.25-3.31; P = 0.004), heart failure (HR, 2.37; 95% CI 1.86-3.03; P < 0.001), conduction disorders (HR, 3.06; 95% CI 2.17-4.3; P = 0.001), atrial fibrillation (HR, 3.29; 95% CI 2.46-4.4; P < 0.001), and stroke (HR, 1.75; 95% CI 1.39-2.2; P < 0.001). Patients who developed pneumonitis while on ICIs were found to be associated with heart failure (HR, 2.61; 95% CI 1.23-5.52) and patients who developed encephalitis for conduction disorders (HR, 4.35; 95% CI 1.6-11.87). Age, type of primary cancer, and nephritis were associated with increased risk of CVAEs in patients on ICI treatment.
Figure 2.
Multivariate Cox regression analyses of cardiovascular adverse event onset in the immune checkpoint inhibitor cohort, by type of cardiovascular adverse event and overall, showing hazard ratios with 95% confidence intervals.
For the bottom right panel (any CVAE analysis), only the first of any five CVAEs to occur was considered an event. Significant risk factors are highlighted in red (P < 0.05).
CI, confidence interval; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; CVAE, cardiovascular adverse event; HR, hazard ratio; irAE, immune-related adverse event; PD-1, programmed cell death protein 1.
Anti-CTLA-4 used as monotherapy or in combination with anti-PD-1 increased the risk of heart failure and stroke by ~1.5-2-fold with the following values: heart failure for combination therapy (HR, 2.0; 95% CI 1.31-3.04) and heart failure for monotherapy (HR, 1.9; 95% CI 1.27-2.84) and stroke for combination therapy (HR 1.43; 95% CI 1.01-2.03) and stroke for monotherapy (HR 1.7; 95% CI 1.3-2.22). Similarly, anti-CTLA-4 monotherapy also increased the risk of developing atrial fibrillation in patients >2.5-fold, compared with those on anti-PD-1 monotherapy (HR, 2.88; 95% CI 1.69-4.91) (Figure 2, Supplementary Table S11, available at https://doi.org/10.1016/j.esmoop.2021.100252).
The use of ICIs was associated with an increased risk of heart failure in patients with a history of comorbid conditions such as hypertension (1.35; 95% CI 1.04-1.75; P = 0.022), myocardial infarction (HR, 1.55; 95% CI 1.08-2.21; P = 0.016), diabetes (HR, 1.38; 95% CI 0.99-1.92; P = 0.058), and peripheral vascular diseases (HR, 1.38; 95% CI 1.06-1.78; P = 0.015). The type of primary cancer was also found to have a significant correlation with individual CVAE types. Patients with lung cancer, renal cell carcinoma, and malignant melanoma were at a higher risk of developing heart failure [lung cancer (HR, 1.49; 95% CI 1.05-2.1), renal cell carcinoma (HR, 1.59; 95% CI 1.07-2.39), atrial fibrillation (lung cancer; HR, 2.28; 95% CI 1.42-3.67)] and stroke [lung cancer (HR, 1.93; 95% CI 1.33-2.79), renal cell carcinoma (HR, 1.64; 95% CI 1.05-2.58), malignant melanoma (HR, 2.35; 95% CI 1.57-3.51)] compared with patients with other cancers (Figure 2). According to the sensitivity analysis, the use of cytotoxic chemotherapy or targeted agents before initiation of ICIs subsequently did not influence CVAE outcomes (see Supplementary Results and Supplementary Table S12, available at https://doi.org/10.1016/j.esmoop.2021.100252).
Several reports in the literature highlight that irAEs, including CV side-effects, may occur even after a single dose of ICI.3,6,13 In our analysis, the number of ICI doses administered before onset of a CVAE varied slightly between the reported CVAEs, ranging from a median of three (atrial fibrillation) to four (conduction disorders) doses (Table 1).
The median (IQR) times to onset (months) of reported CVAEs in patients on combination therapy (anti-CTLA-4 plus anti-PD-1) were 4.98 (1.34-11.25), 3.26 (2.29-6.34), 3.43 (2.0-6.87), 1.28 (0.675-3.26), and 0.77 (0.52-0.97) for stroke, atrial fibrillation, heart failure, conduction disorder, and myocardial infarction, respectively. In general, the time to onset of CVAEs was earlier in patients receiving combination ICI therapy than in patients receiving monotherapy (anti-CTLA-4 or anti-PD-1/anti-PD-L1) (Supplementary Figure S5, available at https://doi.org/10.1016/j.esmoop.2021.100252). Furthermore, patients on anti-PD-1 or anti-PD-L1 monotherapy showed earlier onset of CVAEs compared with patients on anti-CTLA-4 monotherapy as evidenced by the following (Supplementary Figure S5, available at https://doi.org/10.1016/j.esmoop.2021.100252): stroke, 3.8 (1.5-7.87) versus 5.83 (2.67-13.13); atrial fibrillation, 3.23 (1.28-7.27) versus 4.13 (1.98-11.6); heart failure, 3.5 (1.53-8.13) versus 5.8 (3.23-14.9); conduction disorder, 4.9 (1.63-10.13) versus 7.73 (2.26-18.53); and myocardial infarction, 4.36 (1.2-8.5) versus 3.88 (1.62-7.44). The median time to onset of myocardial infarction (3.88 months) was earlier with anti-CTLA-4 compared with that with anti-PD-1 monotherapy, which had a median time to onset of 4.46 months (Supplementary Figure S5, available at https://doi.org/10.1016/j.esmoop.2021.100252).
Matched analysis of ICI and non-ICI cohorts
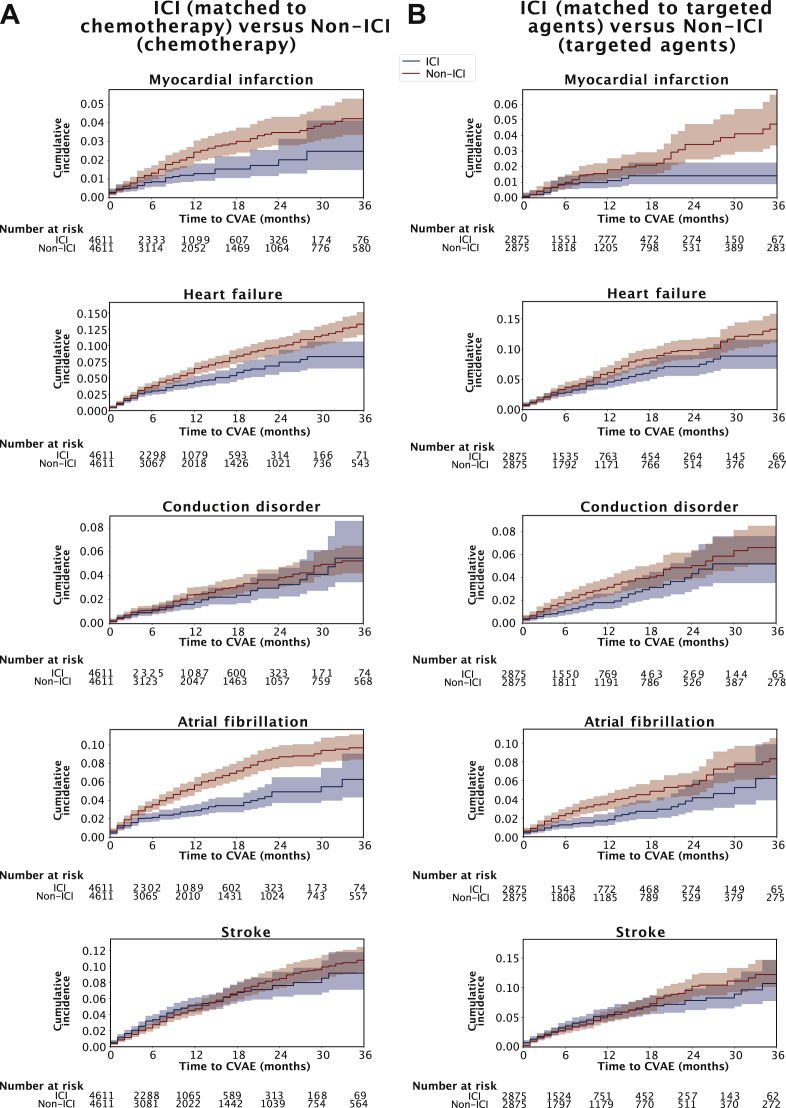
Kaplan–Meier curves of cumulative incidence (including cytotoxic chemotherapy and molecular targeted agents) in the ICI cohort and matched non-ICI cohort and their event rates at 3 years are shown in Figure 3. The two groups were matched for age, sex, cancer type, and NCI comorbidity index. The complete baseline characteristics of these matched cohorts are shown in Supplementary Table S13, available at https://doi.org/10.1016/j.esmoop.2021.100252
Figure 3.
Cumulative incidence of cardiovascular adverse events across matched cohorts by type of treatment over 3 years.
Immune checkpoint inhibitor (ICI; blue lines) and non-ICI (red lines) patients were matched by age, sex, comorbidity index, and primary cancer. (A) ICI patients compared with non-ICI patients treated with cytotoxic chemotherapy. (B) ICI patients matched with non-ICI patients treated with targeted agents. Shaded regions indicate 95% confidence intervals, and the P values correspond to log-rank tests.
CVAE, cardiovascular adverse event; ICI, immune checkpoint inhibitor.
The 3-, 6-, and 1-year cumulative incidence of CVAEs in patients who received ICIs were generally lower than those who received cytotoxic chemotherapy or targeted agents (Supplementary Table S14, available at https://doi.org/10.1016/j.esmoop.2021.100252). The cumulative incidence rates of myocardial infarction, heart failure, and atrial fibrillation were lower in patients who received ICIs than in those who received chemotherapy (P < 0.005). Similarly, the cumulative incidence rate was lower in the ICI cohort than in those who received targeted agents for all CVAEs; the difference was significant for atrial fibrillation (P < 0.005) and tended to be significant for heart failure and conduction disorders (Figure 3).
In general, time to event was shorter in the ICI cohort than in the control cohort, although the overall cumulative incidence was lower in the former than that in the latter (Figure 3). The median time to onset for CVAEs was earlier in patients who received ICIs compared with patients who received cytotoxic chemotherapy, which may represent different causal mechanism between the types of cancer therapy in Supplementary Figure S6A, available at https://doi.org/10.1016/j.esmoop.2021.100252 [myocardial infarction, median of 2.78 months (IQR, 0.74-8.4 months) versus 7.2 months (3.0-13.33 months), P < 0.001; heart failure, 3.7 months (1.58-8.18 months) versus 7.66 months (3.03-17.33 months), P < 0.001; conduction disorders, 4.22 months (1.45-10.19 months) versus 9.46 months (2.73-16.53 months), P < 0.01; atrial fibrillation, 2.83 months (1.28-6.35 months) versus 5.88 months (2.33-13.125 months), P = 0.004; stroke, 4.13 months (1.55-7.88 months) versus 8.23 months (3.81-17.47 months), P < 0.001] and targeted agents in Supplementary Figure S6B, available at https://doi.org/10.1016/j.esmoop.2021.100252 [myocardial infarction, 4.36 months (2.28-5.23 months) versus 8.38 months (3.36-21.44 months), P = 0.002; heart failure, 3.7 months (1.25-9.63 months) versus 8.62 months (3.35-15.94 months), P < 0.001; conduction disorders, 6.06 months (1.85-14.09 months) versus 6.77 months (2.48-15.69 months), P = 0.36; atrial fibrillation, 4.3 months (1.23-13.18 months) versus 5.83 months (2.26-15.66 months), P = 0.17; stroke, 4.46 months (1.39-8.80 months) versus 8.76 months (3.33-18.5 months), P < 0.001].
Discussion
To the best of our knowledge, this is the largest longitudinal retrospective pharmacovigilance study of CVAEs associated with immune checkpoint blockade in the US population. Using IBM MarketScan research databases from 2011 to 2018, cancer patients undergoing ICI therapy were evaluated to identify the incidence, clinical determinants, and time to onset of CVAEs. Our findings underscore the importance of increased awareness of cardiovascular risk and monitoring during ICI treatment.
We report a wide spectrum of CVAEs after ICI treatment with absolute incidence rates of stroke (5%), heart failure (3.5%), atrial fibrillation (2%), and conduction disorders (1.5%) higher than those reported in clinical trials, meta-analyses, and other large retrospective studies.14, 15, 16 This is suggestive of the fact that ICI-associated CVAEs may be underestimated in the literature. One possible reason could be the inadequate screening of patients and lack of awareness regarding ICI-associated cardiovascular toxicities in the clinical setting. In our analysis, the incidence of myocarditis was very low (0.05%), as has been reported in previous studies.15,17 The low prevalence can possibly be attributed to its heterogeneous clinical presentation and difficulty associated with diagnosis.
The increased incidence of heart failure, atrial fibrillation, and conduction disorders suggests that these CVAEs may have an underlying pathophysiology unrelated to ICI-associated myocarditis.5,18 The higher incidence of CVAEs in the US claims database is not completely clear; one possibility is that the real-world population of cancer patients on ICIs have higher underlying comorbidities and/or susceptibility to increased risk factors leading to cardiovascular issues compared with those who received ICIs in clinical trials.
There is preclinical and clinical evidence of accelerated atherosclerosis associated with ICIs.19, 20, 21, 22 In a recent single-center study by Drobni et al.,23 patients on ICIs were at a threefold higher risk of myocardial infarction and stroke due to increased aortic atherosclerotic plaque burden, as compared with non-ICI patients. Although we did not analyze this specifically in our study, accelerated atherosclerosis may explain the higher incidence of some CVAEs.
In our analysis, anti-CTLA-4 alone or in combination with anti-PD-L1/anti-PD-1 monotherapy was associated with increased risk of heart failure, atrial fibrillation, and stroke. Our findings are consistent with other studies reporting higher incidence of CVAEs with use of anti-CTLA4 agents.4,17,18 Advanced age, sex, cancer type, underlying comorbidities, history of cardiovascular disease, and irAEs affecting other organs were found to have a significant association with CVAEs. Consistent with our findings, a few small studies have reported association of CVAEs with advanced age and sex, with males being more susceptible.6,13,15,24 Specifically, patients with lung cancer, melanoma, and renal cell carcinoma on ICIs were at a higher risk for developing CVAEs than patients diagnosed with other cancers. There could be a possible bias in our analysis due to overrepresentation of these cancer types in our dataset. Lung cancer and cardiovascular disease also have certain common risk factors.25 The multivariable Cox proportional hazards model allowed us to adjust for multiple variables (confounders) in estimating the risk by each variable. Further stratified Cox model analysis for each pre-existing comorbidity could not be carried out due to low sample size resulting from low event rate of each CVAE.
Kidney irAE or nephritis was found to have a significant association with the development of cardiotoxicity. In addition, pneumonitis was associated with heart failure and encephalitis with conduction disorders. The association between irAEs and CVAEs, other than myocarditis, has not been assessed in previous studies. The mechanism of this association is not entirely clear and may be explained by off-target immune effects within the heart due to overactivation of the immune system.
The risk factor analysis we carried out is difficult in a single-center study. Our dataset's advantage is that it combines health outcome information from multiple academic and community practices, providing a better representation of the real-world population.
Our study also offers a perspective of CVAEs in patients treated with ICIs matched with non-ICI controls. In a risk matched analysis, the cumulative incidence of CVAEs was lower in the ICI cohort than the non-ICI cohort, indicating an overall better toxicity profile of ICIs, consistent with clinical experience. We acknowledge the recent independent studies by Drobni et al.23 and D'Souza et al.,26 which showed a higher incidence of CVAEs associated with ICIs in comparison to non-ICI therapy. It is important to note that the non-ICI comparison arm in these studies was a relatively heterogeneous population and may be representative of patients with early-stage cancers. Since ICI agents are approved only in advanced or metastatic cancers, in our study, we constructed the control arm of non-ICI patients by selecting only those who received either targeted agents or cytotoxic chemotherapy and excluded patients who did not receive systemic treatment. Thus, we indirectly controlled for the cancer stage as a possible confounder for the overestimation of the risk of CVAEs due to ICIs.
An awareness of incidence and factors that can significantly influence the development of CV side-effects in patients on ICIs will be beneficial for the clinician in formulating an individualized monitoring strategy for CVAEs. Moreover, this will aid the clinician in optimization of modifiable risk factors and timely involvement of cardio-oncology.
Our study has several possible limitations. In addition to the fact that this is a retrospective analysis, the claims dataset is non-standardized, incomplete, lacking laboratory, image, and pathology reports leading to challenges in identifying causality. We are not able to account for the intrinsic selection bias when comparing patient cohorts based on their treatment, ICI versus non-ICI, particularly, with respect to targeted agents, since the claims dataset lacks patient stratification based on actionable genomic alterations and information on accurate cancer stage. Our control group was randomly selected from the database, and the findings were not verified with an independent set of patients. However, the comparison between the ICI and non-ICI cohorts was carried out after a 1 : 1 match for age, sex, NCI comorbidity score, and primary cancer. Competing risks or cause-specific analysis for developing CVAE could not be considered, as it was not possible to identify specific causes or contributing factors. We included, however, the NCI comorbidity index and other irAEs in the multivariate regression models to account for other factors that might contribute to the occurrence of CVAEs. We did not consider a separate analysis of the multiplicative effect on incidence. When we plotted the overlap of the type of CVAE, however, we observed that the majority were isolated events with minimal overlap. Additional investigation examining one CVAE as a risk factor for another is needed. Finally, there is a likelihood that some myocarditis events may have been coded as having heart failure, which cannot be deciphered further because of the administrative nature of the dataset.
Conclusions
Patients undergoing ICI treatment of advanced cancer have a higher incidence of CVAEs in a large contemporary real-world cohort than previously reported. Onset of heart failure, stroke, and myocardial infarction was earlier than in risk matched cancer patients who received non-ICI treatment. The cumulative incidence of CVAEs treated with ICIs was lower than those treated with traditional and other targeted therapies, suggesting that ICIs have a relatively better safety profile. The association between CVAEs and specific types of cancer and other noncardiac irAEs requires further evaluation. A personalized risk-based strategy is important to maximize the clinical benefit from ICIs at minimal cardiovascular toxicity, enhancing precision cardio-oncology and immunotherapy.
Acknowledgements
We thank Dr. Balazs Halmos for his review and suggestions to improve the manuscript draft.
Funding
This work was supported by the University Hospitals Research and Education (UHR&E) Big Data/Health Informatics Pilot Grant (P0466) awarded to Dr Prantesh Jain.
Disclosure
AD has received consultancy fees for advisory committees from Bristol Myers Squibb (BMS), AstraZeneca, Bayer, Jazz Pharmaceuticals. VV, COI: Consultant/Advisor: AstraZeneca, BMS, Merck, EMD Serono, Novartis, Eli Lily, Foundation Medicine, Alkermes, Reddy Labs, Novocure, Boston Scientific. The remaining authors have declared no conflicts of interest.
Supplementary data
References
- 1.Bour-Jordan H., Bluestone J.A. Regulating the regulators: costimulatory signals control the homeostasis and function of regulatory T cells. Immunol Rev. 2009;229:41–66. doi: 10.1111/j.1600-065X.2009.00775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Puzanov I., Diab A., Abdallah K. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5:95. doi: 10.1186/s40425-017-0300-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martins F., Sofiya L., Sykiotis G.P. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019;16:563–580. doi: 10.1038/s41571-019-0218-0. [DOI] [PubMed] [Google Scholar]
- 4.Mahmood S.S., Fradley M.G., Cohen J.V. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71:1755–1764. doi: 10.1016/j.jacc.2018.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lyon A.R., Yousaf N., Battisti N.M.L., Moslehi J., Larkin J. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol. 2018;19:e447–e458. doi: 10.1016/S1470-2045(18)30457-1. [DOI] [PubMed] [Google Scholar]
- 6.Moslehi J.J., Salem J.E., Sosman J.A., Lebrun-Vignes B., Johnson D.B. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet. 2018;391:933. doi: 10.1016/S0140-6736(18)30533-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corrigan-Curay J., Sacks L., Woodcock J. Real-world evidence and real-world data for evaluating drug safety and effectiveness. JAMA. 2018;320:867–868. doi: 10.1001/jama.2018.10136. [DOI] [PubMed] [Google Scholar]
- 8.Miksad R.A., Abernethy A.P. Harnessing the power of real-world evidence (RWE): a checklist to ensure regulatory-grade data quality. Clin Pharmacol Ther. 2018;103:202–205. doi: 10.1002/cpt.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.IBM Watson Health . IBM Corporation; United States of America: 2018. IBM MarketScan Research Databases for Health Services Researchers (White Paper) [Google Scholar]
- 10.Schulman K.L., Berenson K., Tina Shih Y.C. A checklist for ascertaining study cohorts in oncology health services research using secondary data: report of the ISPOR oncology good outcomes research practices working group. Value Health. 2013;16:655–669. doi: 10.1016/j.jval.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Stedman MR, P Doria-Rose P, Warren JL, Klabunde CN, Mariotto A. The impact of different SEER-medicare claims-based comorbidity indexes on predicting non-cancer mortality for cancer patients. Comorbidity Technical Report; 2019. Available at https://healthcaredelivery.cancer.gov/seermedicare/considerations/comorbidity-report.html. Accessed January 1, 2020.
- 12.Wang H., Li G. A selective review on random survival forests for high dimensional data. Quant Biosci. 2017;36:85–96. doi: 10.22283/qbs.2017.36.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Escudier M., Cautela J., Malissen N. Clinical features, management, and outcomes of immune checkpoint inhibitor-related cardiotoxicity. Circulation. 2017;136:2085–2087. doi: 10.1161/CIRCULATIONAHA.117.030571. [DOI] [PubMed] [Google Scholar]
- 14.Hu Y.B., Zhang Q., Li H.J. Evaluation of rare but severe immune related adverse effects in PD-1 and PD-L1 inhibitors in non-small cell lung cancer: a meta-analysis. Transl Lung Cancer Res. 2017;6:S8–S20. doi: 10.21037/tlcr.2017.12.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salem J.E., Manouchehri A., Moey M. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018;19:1579–1589. doi: 10.1016/S1470-2045(18)30608-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson D.B., Manouchehri A., Haugh A.M. Neurologic toxicity associated with immune checkpoint inhibitors: a pharmacovigilance study. J Immunother Cancer. 2019;7:134. doi: 10.1186/s40425-019-0617-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson D.B., Balko J.M., Compton M.L. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375:1749–1755. doi: 10.1056/NEJMoa1609214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stein-Merlob A.F., Rothberg M.V., Holman P., Yang E.H. Immunotherapy-associated cardiotoxicity of immune checkpoint inhibitors and chimeric antigen receptor T cell therapy: diagnostic and management challenges and strategies. Curr Cardiol Rep. 2021;23:11. doi: 10.1007/s11886-021-01440-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gotsman I., Grabie N., Dacosta R. Proatherogenic immune responses are regulated by the PD-1/PD-L pathway in mice. J Clin Invest. 2007;117:2974–2982. doi: 10.1172/JCI31344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bu D.X., Tarrio M., Maganto-Garcia E. Impairment of the programmed cell death-1 pathway increases atherosclerotic lesion development and inflammation. Arterioscler Thromb Vasc Biol. 2011;31:1100–1107. doi: 10.1161/ATVBAHA.111.224709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsumoto T., Sasaki N., Yamashita T. Overexpression of cytotoxic T-lymphocyte-associated antigen-4 prevents atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2016;36:1141–1151. doi: 10.1161/ATVBAHA.115.306848. [DOI] [PubMed] [Google Scholar]
- 22.Fernandez D.M., Rahman A.H., Fernandez N.F. Single-cell immune landscape of human atherosclerotic plaques. Nat Med. 2019;25:1576–1588. doi: 10.1038/s41591-019-0590-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drobni Z.D., Alvi R.M., Taron J. Association between immune checkpoint inhibitors with cardiovascular events and atherosclerotic plaque. Circulation. 2020;142:2299–2311. doi: 10.1161/CIRCULATIONAHA.120.049981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oren O., Yang E.H., Molina J.R., Bailey K.R., Blumenthal R.S., Kopecky S.L. Cardiovascular health and outcomes in cancer patients receiving immune checkpoint inhibitors. Am J Cardiol. 2020;125:1920–1926. doi: 10.1016/j.amjcard.2020.02.016. [DOI] [PubMed] [Google Scholar]
- 25.Meijers W.C., de Boer R.A. Common risk factors for heart failure and cancer. Cardiovasc Res. 2019;115:844–853. doi: 10.1093/cvr/cvz035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D'Souza M., Nielsen D., Svane I.M. The risk of cardiac events in patients receiving immune checkpoint inhibitors: a nationwide Danish study. Eur Heart J. 2020;42:1621–1631. doi: 10.1093/eurheartj/ehaa884. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.