Significance
Mass coral spawning is a perplexing annual event that occurs over a few nights following a full moon. This paper demonstrates that the period of darkness between sunset and moonrise that occurs after the full moon is a trigger for spawning in coral species Dipsastraea speciosa. As this species shares similar spawning patterns with many other coral taxa, we expect this model for spawning to be applicable to other coral species. Our discovery provides insights into how coral spawning is synchronized via the lunar cycle.
Keywords: coral, moonlight, reproduction, spawning mechanism, synchronized
Abstract
Synchronized mass coral spawning typically occurs several days after a full moon once a year. It is expected that spawning day is determined by corals sensing environmental change regulated by the lunar cycle (i.e., tide or moonlight); however, the exact regulatory mechanism remains unknown. Here, we demonstrate how moonlight influences the spawning process of coral, Dipsastraea speciosa. When corals in the field were shaded 1 and 3 d before the full moon or 1 d after the full moon, spawning always occurred 5 d after shading commenced. These results suggest moonlight suppresses spawning: a hypothesis supported by laboratory experiments in which we monitored the effects of experimental moonlight (night-light) on spawning day. Different night-light treatments in the laboratory showed that the presence of a dark period between day-light and night-light conditions eliminates the suppressive effect of night-light on spawning. In nature, moonrise gets progressively later during the course of the lunar cycle, shifting to after sunset following the day of the full moon. Our results indicate that this period of darkness between sunset and moonrise triggers synchronized mass spawning of D. speciosa in nature.
Synchronized spawning is a common phenomenon that has been observed in a variety of marine organisms (1, 2). Many spawn gametes for external fertilization, which are then rapidly diffused and diluted in water (3). Synchronization is thought to have evolved in order to increase gamete density and ensure high levels of fertilization (4, 5), while predator satiation acts to increase the likelihood of survival (6, 7). Among marine invertebrates, reef-building (scleractinian) corals are the most studied in terms of lunar-synchronized spawning behavior. More than 80% of scleractinian corals are broadcasting spawners (8), many of which spawn only a few nights per year, during a specific lunar phase (6–11).
Synchronous coral spawning is regulated at three different time scales: month, day, and hour (12). Spawning month is regulated by the timing of gamete maturation, which is largely influenced by seawater temperature (13). Annual variation of seawater temperature and solar insolation may also influence spawning month (13–15). Spawning day is assumed to be determined by environmental factors that are controlled by the lunar cycle, such as moonlight and tidal fluctuations, as spawning generally occurs after the full moon (6, 11, 16–19) [some exceptions include Diploria labyrinthiformi, which spawns closer to the new moon (20)]. Corals have been separated into two groups depending on their spawning pattern (9), with one group regularly spawning several days after the full moon, while the other shows more irregularity in their spawning day, though still occurring around the full moon. The former includes at least 13 common genera (SI Appendix, Table S1), such as Favites, Platygyra, Goniastrea, and Dipsastraea. The latter includes the genus Acropora. Lastly, spawning hour is determined by sunset time (21, 22) and differs among coral species. For example, Orbicella franksi and Orbicella annularis show signs of spawning at 2 and 4 h after sunset, respectively (23).
Moonlight is considered to be the proximate factor in determining coral spawning day (5, 19). Under the current model, based on spawning behavior of Acropora millipora, moonlight is thought to positively regulate coral spawning (i.e., corals receive a moonlight signal via a light sensor [cryptochrome]), which then changes the expression of genes that induces spawning (24, 25). This model is supported by evidence that corals in genus Acropora do not spawn in the absence of moonlight (24) and that night-light pollution disturbs spawning synchronicity (26, 27). However, it remains unknown exactly how corals determine the spawning day by sensing a moonlight signal and whether this model is applicable to the majority of coral species.
In the present study, we examined the effect of light at night on the spawning day of D. speciosa after a spring full moon each year in the field and laboratory. Our results demonstrate that night-light (moonlight in field) following daylight (sunlight in field) suppresses the process of spawning and the dark interval between them eliminates this suppressive effect. Our results provide a hypothesis that the dark interval at the start of the night period, that occurs only after the night of the full moon, triggers coral spawning in D. speciosa.
Results
Blocking Moonlight Changes Spawning Day in Nature.
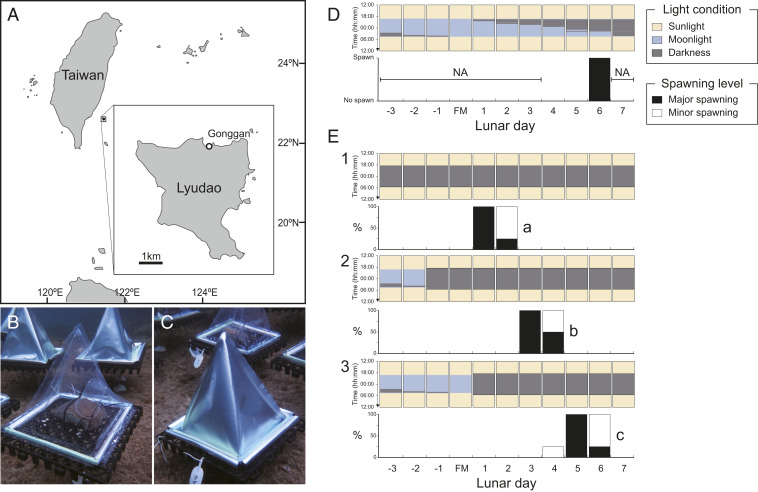
We examined the effect of moonlight on spawning day in coral D. speciosa during the month of predicted spawning in the field near Green Island in Taiwan (Fig. 1A). Coral colonies were covered with transparent plastic bags (Fig. 1B) or with aluminum foil bags that blocked moonlight (Fig. 1C). The coverings were put in place from sunset (∼18:30) to sunrise (∼05:30) 1 or 3 d before the full moon or 1 d after the full moon. In the natural populations of D. speciosa (i.e., with no coverings), spawning occurred on the sixth night after the full moon (Fig. 1D). When moonlight was blocked from 3 d before the full moon, spawning occurred on the fifth night of darkness (i.e., 1 d after the full moon, Fig. 1E, panel 1). However, when moonlight was blocked from 1 d before the full moon and 1 d after the full moon, spawning occurred on 3 d and 5 d after the full moon, respectively (Fig. 1E, panels 2 and 3). The results demonstrate that illumination by moonlight delayed spawning, with spawning occurring on the fifth night after blocking moonlight, irrespective of the day moonlight was blocked.
Fig. 1.
Spawning day of D. speciosa in the field manipulation experiment. (A) Study location at Lyudao (Green Island), Taiwan. The white dot indicates Gonggan, where D. speciosa fragments were collected and the field observation and experiment were conducted. (B) An egg trap in a transparent plastic bag and (C) an egg trap in an aluminum foil bag. (D) Spawning in natural populations of D. speciosa (>10 colonies) at the study location and (E) spawning of D. speciosa fragments under the moonlight-blocking treatment commencing at 3 d before the full moon (panel 1), 1 d before the full moon (panel 2), and 1 d after the full moon (panel 3). Black bars indicate major spawning (>hundreds of eggs), and white bars indicate minor spawning (several eggs) in four replicate fragments. Note that “NA” indicates no observation. Different letters in the panels in E indicate significant differences between the treatments (ANOVA and Tukey HSD test; P < 0.001). For detailed results of statistical analysis, refer to SI Appendix, Table S2.
Dim Light at Night Suppresses Spawning.
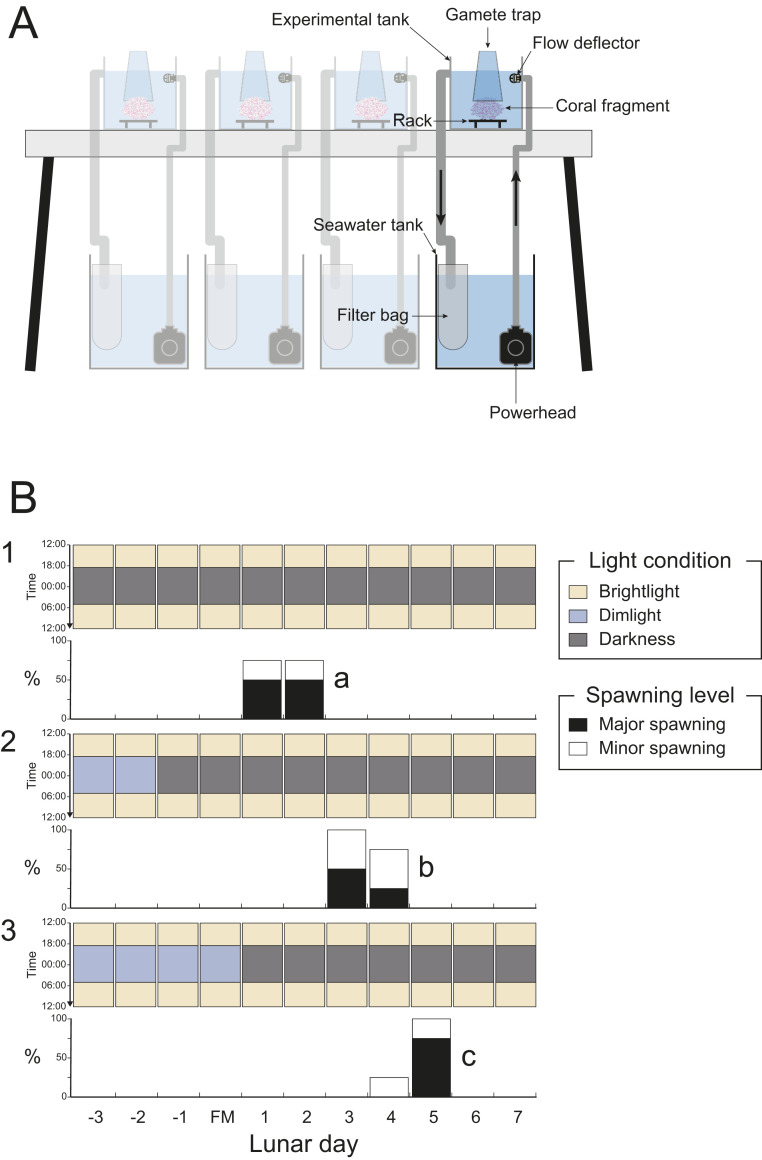
Using dim light to mimic moonlight (see SI Appendix, Fig. S1 for the spectrum), we examined the effect of light during the nighttime period (18:30 to 5:00) on the spawning day in the laboratory (Fig. 2A). Fragments of D. speciosa were transferred to the laboratory 3 d before the full moon. These fragments were then kept in total darkness overnight (Fig. 2B, panel 1) or were exposed to dim light for 2 or 4 nights (Fig. 2B, panels 2 and 3). In our experimental conditions, major spawning occurred on the fifth or sixth night of darkness, shifting consistently with the dim light treatment. There was no difference in spawning time (around 21:00 to 22:00) among the dim light treatments nor between the laboratory specimens and D. speciosa colonies in the field at the study site (9). These results demonstrate that dim light during the night suppresses spawning in coral D. speciosa.
Fig. 2.
Spawning day of D. speciosa under different moonlight exposure days. (A) The design of one experimental unit, including four replicate tank systems for each experimental treatment. (B) D. speciosa fragments were exposed to three experimental moonlight (dim light [∼0.3 lx]) conditions with different exposure days at nighttime (18:30 to 05:00): no light treatment (panel 1), 2-d exposure treatment (panel 2), and 4-d exposure treatment (panel 3). Black bars indicate major spawning (>hundreds of eggs), and white bars indicate minor spawning (several eggs) in four replicate fragments. Different letters in the panels indicate significant differences between the treatments (ANOVA and Tukey HSD test; P ≤ 0.001). For detailed results of statistical analysis, refer to SI Appendix, Table S2.
Dark Interval between Sunset and Moonrise Triggers Spawning.
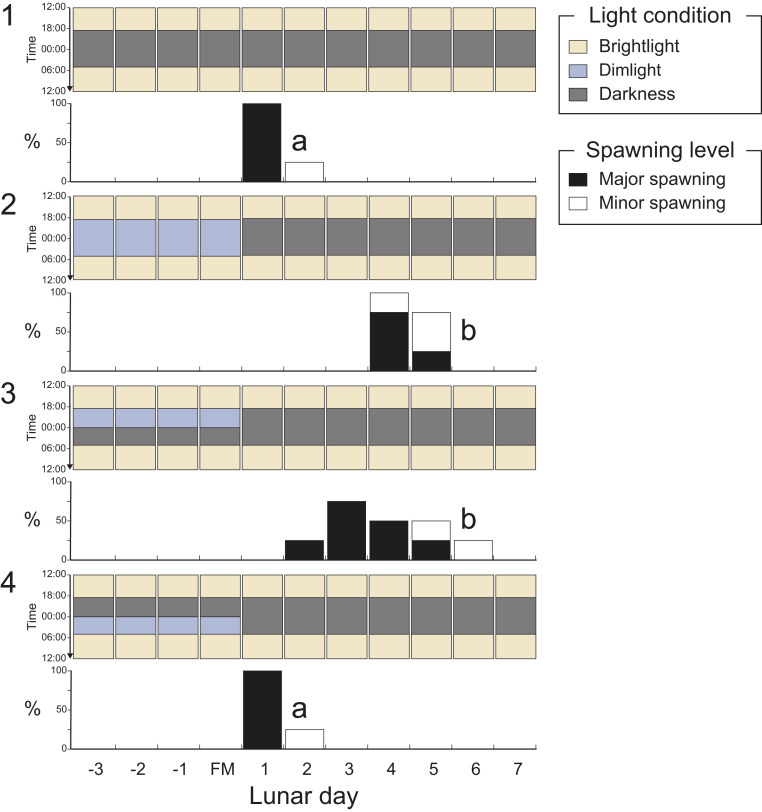
To further elucidate the suppressive effect of moonlight on spawning, coral fragments were exposed to a range of dim light treatments, including darkness (Fig. 3, panel 1), dim light (Fig. 3, panel 2), dim light for the first half of the night (Fig. 3, panel 3), and dim light for the second half of the night (Fig. 3, panel 4). For the coral fragments kept in total darkness overnight, spawning occurred on the fifth night of darkness (Fig. 3, panel 1). By comparison, major spawning was delayed for 3 to 4 nights in the coral exposed to 4 nights of dim light (Fig. 3, panel 2). This delay in spawning was also observed in the coral exposed to dim light at the start of the night period and also resulted in less synchronized spawning (Fig. 3, panel 3). However, coral fragments exposed to dim light only in the second half of the night showed no delay in spawning (Fig. 3, panel 4), with major spawning occurring on the fifth night, as was observed in the coral kept in total darkness for four nights (Fig. 3, panel 1). Our results demonstrate that continuous light during the transition from day to night is key for suppressing spawning, whereas darkness in the first half of the night can be a trigger. In fact, subsequent dim light treatments demonstrated that the absence of light for just 1 h at the start of the night period was sufficient to trigger spawning, though less efficiently compared with total darkness for the entire night period (SI Appendix, Fig. S2, panel 3). When colonies were exposed to two nights of total darkness followed by two nights of dim light (SI Appendix, Fig. S2, panel 4), major spawning day clearly spread into two timings, indicating that two consecutive nights of darkness are necessary to ensure spawning synchronicity.
Fig. 3.
Spawning of D. speciosa under different moonlight exposure hours. D. speciosa fragments were exposed to four experimental moonlight conditions (dim light [∼0.3 lx]) with different exposure hours at nighttime (18:30 to 05:00) for the first 4 d of the experiment: no light (panel 1), dim light in 18:30 to 05:00 (panel 2), dim light in 18:30 to 00:00 (panel 3), and dim light in 00:00 to 05:00 (panel 4). Black bars indicate major spawning (>hundreds of eggs), and white bars indicate minor spawning (several eggs) in four replicate fragments. Different letters in the panels indicate significant differences between the treatments (ANOVA and Tukey HSD test; P < 0.001). For detailed results of statistical analysis, refer to SI Appendix, Table S2.
Suppression by Moonlight Is Not Blue Light Specific.
Previous studies have demonstrated that a blue light sensor, cryptochrome, senses moonlight and controls spawning day (25). Therefore, we examined the effect of dim colored light at night on spawning day in D. speciosa. Corals were exposed to two nights (18:30 to 05:00) of darkness or dim light (∼0.3 lx) with three different light spectra from 1 d before the full moon (SI Appendix, Fig. S3). As shown, dim light at night delayed spawning relative to coral fragments kept in darkness overnight; however, there was no observed difference in spawning day between the three different light spectra tested. Our results demonstrate that the suppressive effect of moonlight on spawning day seen in our study is not blue light specific, suggesting that regulation of this mechanism may be independent of cryptochrome.
Discussion
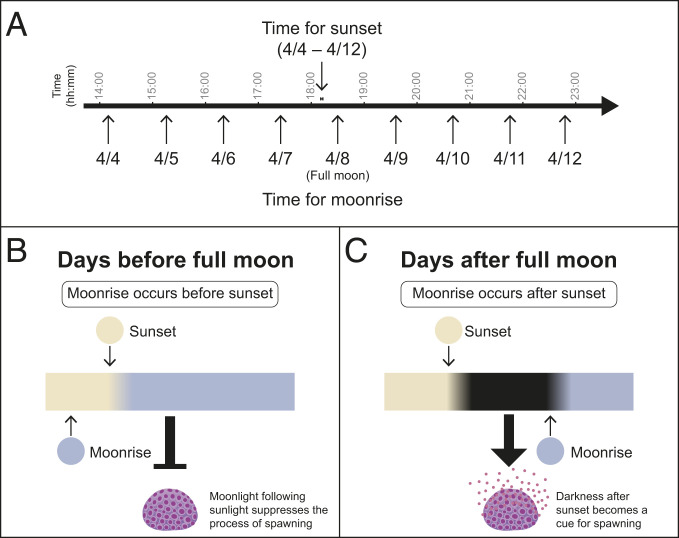
Many coral species synchronize spawning to occur over only a few nights per year during a specific lunar phase. Previous studies have suggested that moonlight is a key factor for this synchronization (24, 28, 29); however, the regulatory mechanism is unknown. In the field, sunset timing differs by just a few minutes each week; however, moonrise timing shifts an average of 48 min each day (Fig. 4A). This means that before the night of the full moon, corals are exposed to continuous light in the evening since moon rise occurs before sunset, whereas after the full moon, there is a period of darkness between sunset and before moonrise. In this study, we have shown that continuous light during the transition from day to night has a suppressive effect on spawning and that an interval of darkness eliminates this suppression (Fig. 3). These findings illustrate that the dark interval at the start of the night period, that occurs only after the night of the full moon, triggers coral spawning in D. speciosa (Fig. 4 B and C).
Fig. 4.
Schematic relationship of sunset and moonrise time regulate the spawning behavior in D. speciosa. (A) Sunset and moonrise time in Lyudao during the spawning season, periods from April 4th to April 12th (4 d before full moon to 4 d after full moon), 2020, were shown as an example. Moonrise occurs prior to sunset before the full moon day (April 8th), whereas moonrise occurs following sunset after the full moon day. Note that sunset time changes from 18:11 on April 4th to 18:14 on April 12th, and moonrise time changes from 14:11 on April 4th to 22:48 on April 12th. (B) Before the full moon day, the presence of moonlight after sunset suppresses the process of spawning. (C) After the full moon day, the presence of darkness after sunset triggers the process of spawning.
When corals in the field were shaded from moonlight for 1 or 3 d before the full moon or 1 d after the full moon, major spawning occurred on the fifth night after the initiation of shading, irrespective of the lunar phase (Fig. 1E). Similar trends were seen in the laboratory experiments using experimental moonlight, with major spawning occurring on the fifth or sixth day after initiating shading (Fig. 2B). From these results, it is conceivable that the final gametogenesis processes, including gamete maturation [i.e., germinal vesicle migration and breakdown in eggs (30) and formation of egg–sperm bundles inside polyps (31)], may require at least 4 d for completion after receiving the cue for spawning (i.e., the presence of a dark period before moonlight exposure). At the study site tested in the field experiments, major spawning occurred 5 to 8 d after the full moon in D. speciosa (9). This suggests the length of the dark period between sunset and moonrise, for the first few days after the full moon, is important in triggering spawning in the field. The duration and time window of the dark period may be the reason we observe less synchronicity in spawning (Fig. 3, panel 3), as a nonnatural dark period was used. Indeed, a 1-h dark period was sufficient to trigger spawning but with less efficiency compared to colonies kept in total darkness for the entire night period (SI Appendix, Fig. S2, panel 3). Finally, colonies exposed to two nights of total darkness followed by two nights of dim light showed that more than two consecutive nights of darkness at sunset are necessary to ensure spawning synchronicity (SI Appendix, Fig. S2, panel 4).
Our proposed mechanism, negative regulation of coral spawning by moonlight, contrasts with the current proposed mechanism (24). Indeed, it was shown that A. millepora did not spawn when moonlight was blocked (24). Both mechanisms agree however, that distinct spawning patterns are observed between the two dominant coral taxa: Merulinid corals, which includes D. speciosa, and Acropora corals (9). Merulinid corals spawn over a couple of nights around a specific lunar phase, the last quarter moon (9), whereas Acropora corals have a high spawning variability during the lunar phase from the full moon until the last quarter moon, with similar variability occurring in Taiwan, Japan, the Red Sea (9), and the Caribbean (32). The two proposed spawning mechanisms may explain variability in spawning day between the two, as the latter (moonlight dependent) is influenced by the disturbance of moonlight by clouds, whereas the former (dark dependent) is not. Additionally, the gametogenesis and spawning time in Acropora corals can be influenced by artificial light at night (24, 26, 27). Therefore, as the dark period plays a vital role in triggering spawning in our proposed mechanism, light pollution may also have the potential to influence spawning in D. speciosa.
The observed spawning mechanism in D. speciosa allows for stable, synchronous spawning around the last quarter moon. This trait is not unique to D. speciosa, as many other coral species spawn together around the last quarter moon, including 34 species from 13 genera in 5 families (SI Appendix, Table S1). This suggests that these coral species use a similar spawning mechanism and/or the last quarter moon is an optimal spawning period for these species. The last quarter moon does coincide with the neap tide with minimum tidal change. This may help to minimize sperm dilution and increase fertilization success of spawned gametes (6, 33–35). In addition, as the moon rises at midnight around the last quarter moon, corals spawn in near complete darkness, which may reduce the risk of visual predatory fish preying on spawned gametes (6, 35, 36).
Here we present a synchronized spawning mechanism that the appearance of a dark period between sunset and moonrise is the cue to trigger spawning in coral D. speciosa. In other words, moonrise timing is the key factor to synchronize spawning. As D. speciosa spawns at a similar time to many coral species (9), we suspect that this mechanism can be applicable to a variety of corals and could also explain synchronized behavior, occurring a few days after the full moon, in other marine organisms [e.g., palolo worm (37) and sponges (38)]. This study elucidates the regulatory mechanisms of synchronous spawning in corals. Thus, these findings directly address a gap in knowledge concerning spawning mechanisms, which has been present for decades (5). These results will significantly advance our understanding of synchronous coral spawning as well as the maintenance and recovery of reef-building corals that are facing unprecedented pressures worldwide.
Materials and Methods
Materials.
One field and three tank experiments were conducted in Lyudao (Green Island), Taiwan (22°40' N, 121°30' E) in April–May of 2018 to 2020. D. speciosa, used in this study, is a hermaphroditic, broadcast spawning scleractinian coral that is common across the Indo-Pacific (8, 39). In each experiment, we collected colonies of D. speciosa with mature gametes (purple eggs) 2 to 5 d before the full moon using a hammer and a chisel at a depth of 3 to 8 m in Gonggan, Lyudao (Fig. 1A). After collection, each colony was separated into 3 to 5 large fragments (ca. 12 cm in diameter), and fragments were left to recover in a small fishing harbor in Gonggan for 1 to 2 d before use.
Field Experiment.
Experiments were conducted in April 2019. The experimental area (50 m from the nearest reef) was located in a small fishing harbor away from the local residence of Gonggan, ∼100 m from the closest street light. Three moonlight conditions were used in the experiment: no moonlight, no moonlight from the third night of the experiment, and no moonlight from the fifth night of the experiment. Three fragments (ca. 12 cm in diameter) were collected from each of the four donor colonies and used for the three moonlight conditions, respectively (i.e., each experimental condition had one fragment from each of the four donor colonies). The three fragments from the same donor colony were grouped, and four groups were deployed in an area ∼3 × 3 m at a depth of 2 to 3 m in the harbor. Each fragment was attached to a plastic grid base (30 × 30 cm) using cable ties, which was fixed on the reef substrate using iron pegs (Fig. 1). The field experiment was conducted from 3 d before the full moon until 7 d after the full moon. During the experiment, each fragment was covered by either a triangular aluminum foil bag to block moonlight or a transparent plastic bag of the same shape to allow moonlight to pass through (Fig. 1 B and C). The covers were attached to the plastic grid base using magnets from sunset to sunrise during nighttime (18:30 to 05:30) and detached during the daytime (05:30 to 18:30). The grid base allowed seawater exchange inside the plastic bag.
Tank Experiment.
Each tank system consisted of an experimental tank (40 × 30 × 22 cm) and a seawater tank (40 × 30 × 35 cm) placed below the experimental tank (Fig. 2A). In the system, seawater was constantly pumped up from the seawater tank by a powerhead, introduced to the experimental tank through a rotating water deflector to create a gentle flow and acute waves, and overflowed back into the seawater tank through a 100-μm filter bag to skim organic waste detached from coral fragments. The temperature for the experiment was controlled at 27 to 28 °C by air conditioning in the laboratory. Seawater used in the experiments was collected and stored in a 1,500-L tank at the beginning of the experiments. During the experiments, a quarter of seawater and the filter bag in the seawater tank was changed every 3 d.
Three tank experiments were conducted using the same tank system (four replicate tank systems for each experimental condition), and the same rearing methods were used with different experimental moonlight (dim light) conditions. Four donor colonies were used in each experiment. Four to five fragments (ca. 12 cm in diameter) were collected from the same donor colony and used in different experimental conditions. As a result, each experimental condition had one fragment from each of the four donor colonies (i.e., four replications in each condition). In the laboratory, coral fragments were kept in independent tanks under fluorescent lamps (MASTER TL5 HO 54W/865 SLV/40, Philips) (SI Appendix, Fig. S1) during the daytime (05:00 to 18:30) (6,000 lx) and exposed to experimental moonlight conditions during the nighttime (18:30 to 05:00) (∼0.3 lx).
Fluorescent lamps were used to provide the white light (MASTER TL5 HO 54W/865 SLV/40, Philips), and LED panels were used for blue (5464SUBD/MS, Everlight), green (5464SUGD/MS, Everlight), and red lights (5463SURD/S400-A8/MS, Everlight). Light intensity was adjusted to the local moonlight intensity during the full moon (∼0.3 lx) using neutral density filters for fluorescent lamps or dimmers for LED panels. The intensity of dim light was measured by the light meter (TR-74Ui, T&D). The spectrums of blue, green, and red lights used in the experiments are shown in Fig. 3B. The light spectra were measured by the spectrometer (LA-105, NK system).
The first experiment was conducted from 3 d before full moon to 7 d after full moon in April to May 2018, with three light conditions using the white dim light at nighttime (18:30 to 05:00): no light, dim light for the first 2 d and no light afterward, and dim light for the first 4 d and no light afterward (Fig. 2B). The second experiment was conducted in April 2019 from 3 d before full moon to 7 d after full moon. In the experiments, four light conditions using the white dim light were examined at nighttime (18:30 to 05:00): no light, dim light, dim light between 18:30 to 0:00 and dim light between 0:00 to 05:00, and no light between 18:30 to 0:00 and dim light between 0:00 to 05:00 (Fig. 3). The third experiment was conducted in April 2020, from 1 d before the full moon to 6 d after full moon; four light conditions using different spectra were examined at nighttime (18:30 to 05:00): blue (476 nm), green (530 nm), red (622 nm), dim light, and no light (SI Appendix, Fig. S3).
Monitoring of Spawning.
Spawning was determined using egg traps (cylindrical transparent plastic containers; 7 to 9 cm in diameter and 8 to 15 cm in height) placed on each coral fragment. Egg traps were inspected every morning for the presence or absence of spawned eggs in the trap. If any, spawning was recorded at two levels of magnitude: minor (several eggs) or major spawning (>hundreds of eggs). During the experimental periods, spawning of conspecific populations was also monitored in the field (Gonggan). In situ spawning observation was performed by scuba at nighttime (21:00 to 22:30) 4 to 6 d after the full moon. Moonlight intensity (lux) was recorded every minute during nighttime (18:30 to 05:00) using a light meter (TR-74Ui, T&D) on the roof of the Lyudao Marine Station, located at Gonggan.
Statistical Analysis.
One-way blocked ANOVA was used to examine the effect of the moonlight treatment on spawning day of D. speciosa in each experiment. In the ANOVA, spawning day (days after full moon) was used as a dependent factor, moonlight treatment as an independent factor, and fragments from the same donor colonies as a blocking factor. For spawning day, we used the major spawning day in each fragment for each experiment. For fragments that did not have major spawning, minor spawning day was used. When multiple major or minor spawning days were observed in a fragment, we used the average of major or minor spawning days. Tukey’s honestly significant difference (HSD) post hoc test was used for pairwise comparison among experimental treatments. Software R (version 3.6.3) with package car (version 3.0-10), package multcompView (version 0.1-8), and package lsmeans (version 2.3) was used for the analysis.
Supplementary Material
Acknowledgments
We thank C.-L. Fong, J.-H. Shiu, T.-Y. Lai, and V.D.H. Dang for assistance in the laboratory and Chiu-Fu Diving Shop and Green Island Marine Station for field support. Comments from Eldon Ball, Sara Milward, and two anonymous reviewers improved the manuscript. This study was funded by an internal research grant from the Biodiversity Research Center, Academia Sinica, to Y.N. This work was also supported by Japan Society for the Promotion of Science (JSPS) Grants-in Aid for Scientific Research (KAKENHI) 20H03330 and 18K19240 (both to S.T.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2101985118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix.
References
- 1.Tessmar-Raible K., Raible F., Arboleda E., Another place, another timer: Marine species and the rhythms of life. BioEssays 33, 165–172 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naylor E., Chronobiology of Marine Organisms (Cambridge University Press, 2010). [Google Scholar]
- 3.Levitan D. R., “Sperm limitation, sperm competition and sexual selection in external fertilizers” in Sperm Competition and Sexual Selection, Birkhead T., Moller A., Eds. (Academic Press, San Diego, 1998), pp. 173–215. [Google Scholar]
- 4.Levitan D. R., Fogarty N. D., Jara J., Lotterhos K. E., Knowlton N., Genetic, spatial, and temporal components of precise spawning synchrony in reef building corals of the Montastraea annularis species complex. Evolution 65, 1254–1270 (2011). [DOI] [PubMed] [Google Scholar]
- 5.Harrison P. L., et al., Mass spawning in tropical reef corals. Science 223, 1186–1189 (1984). [DOI] [PubMed] [Google Scholar]
- 6.Babcock R. C., et al., Synchronous spawnings of 105 scleractinian coral species on the great barrier reef. Mar. Biol. 90, 379–394 (1986). [Google Scholar]
- 7.Harrison P. L., “Sexual reproduction of scleractinian corals” in Coral Reefs: An Ecosystem in Transition, Dubinsky Z., Stambler N., Eds. (Springer Netherlands, 2011), pp. 59–85. [Google Scholar]
- 8.Baird A. H., Guest J. R., Willis B. L., Systematic and biogeographical patterns in the reproductive biology of scleractinian corals. Annu. Rev. Ecol. Evol. Syst. 40, 551–571 (2009). [Google Scholar]
- 9.Lin C. H., Nozawa Y., Variability of spawning time (lunar day) in Acropora versus merulinid corals: A 7-yr record of in situ coral spawning in Taiwan. Coral Reefs 36, 1269–1278 (2017). [Google Scholar]
- 10.Bouwmeester J., et al., Multi-species spawning synchrony within scleractinian coral assemblages in the Red Sea. Coral Reefs 34, 65–77 (2015). [Google Scholar]
- 11.Mendes J. M., Woodley J. D., Timing of reproduction in Montastraea annularis: Relationship to environmental variables. Mar. Ecol. Prog. Ser. 227, 241–251 (2002). [Google Scholar]
- 12.Fogarty N. D., Marhaver K. L., Coral spawning, unsynchronized. Science 365, 987–988 (2019). [DOI] [PubMed] [Google Scholar]
- 13.Keith S. A., et al., Coral mass spawning predicted by rapid seasonal rise in ocean temperature. Proc. Biol. Sci. 283, 20160011 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Woesik R., Lacharmoise F., Köksal S., Annual cycles of solar insolation predict spawning times of Caribbean corals. Ecol. Lett. 9, 390–398 (2006). [DOI] [PubMed] [Google Scholar]
- 15.Penland L., Kloulechad J., Idip D., van Woesik R., Coral spawning in the western Pacific Ocean is related to solar insolation: Evidence of multiple spawning events in Palau. Coral Reefs 23, 133–140 (2004). [Google Scholar]
- 16.Kojis B. L., Quinn N. J., Aspects of sexual reproduction and larval development in the shallow water hermatypic coral, Goniastrea australensis (Edwards and Haime, 1857). Bull. Mar. Sci. 31, 558–573 (1981). [Google Scholar]
- 17.Simpson C. J., Mass spawning of scleractinian corals in the Dampier Archipelago and the implications of management of coral reefs in Western Australia. Aust. Dep. Conserv. Environ. Bull. 244, 1–35 (1985). [Google Scholar]
- 18.Babcock R. C., Wills B. L., Simpson C. J., Mass spawning of corals on a high latitude coral reef. Coral Reefs 13, 161–169 (1994). [Google Scholar]
- 19.Hunter C., “Environmental cues controlling spawning in two Hawaiian corals, Montipora verrucosa and M. dilatata” in Proceedings of the 6th International Coral Reef Symposium (Australian Institute of Marine Science, Townsville, Australia, 1988), pp. 727–732. [Google Scholar]
- 20.Chamberland V. F., Snowden S., Marhaver K. L., Petersen D., Vermeij M. J. A., The reproductive biology and early life ecology of a common Caribbean brain coral, Diploria labyrinthiformis (Scleractinia: Faviinae). Coral Reefs 36, 83–94 (2016). [Google Scholar]
- 21.Brady A. K., Hilton J. D., Vize P. D., Coral spawn timing is a direct response to solar light cycles and is not an entrained circadian response. Coral Reefs 28, 677–680 (2009). [Google Scholar]
- 22.Knowlton N., Maté J. L., Guzmán H. M., Rowan R., Jara J., Direct evidence for reproductive isolation among the three species of the Montastraea annularis complex in Central America (Panamá and Honduras). Mar. Biol. 127, 705–711 (1997). [Google Scholar]
- 23.Levitan D. R., et al., Mechanisms of reproductive isolation among sympatric broadcast-spawning corals of the Montastraea annularis species complex. Evolution 58, 308–323 (2004). [PubMed] [Google Scholar]
- 24.Kaniewska P., Alon S., Karako-Lampert S., Hoegh-Guldberg O., Levy O., Signaling cascades and the importance of moonlight in coral broadcast mass spawning. eLife 4, e09991 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levy O., et al., Light-responsive cryptochromes from a simple multicellular animal, the coral Acropora millepora. Science 318, 467–470 (2007). [DOI] [PubMed] [Google Scholar]
- 26.Rosenberg Y., Doniger T., Harii S., Sinniger F., Levy O., Demystifying circalunar and diel rhythmicity in Acropora digitifera under constant dim light. iScience 22, 477–488 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ayalon I., et al., Coral gametogenesis collapse under artificial light pollution. Curr. Biol., 31, 413–419.e3 (2020). [DOI] [PubMed] [Google Scholar]
- 28.Oldach M. J., Workentine M., Matz M. V., Fan T. Y., Vize P. D., Transcriptome dynamics over a lunar month in a broadcast spawning acroporid coral. Mol. Ecol. 26, 2514–2526 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Wuitchik D. M., et al., Seasonal temperature, the lunar cycle and diurnal rhythms interact in a combinatorial manner to modulate genomic responses to the environment in a reef-building coral. Mol. Ecol. 28, 3629–3641 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suwa R., Nakamura M., A precise comparison of developmental series of oocyte growth and oocyte maturation between real-oocytes and pseudo-oocytes in the coral Galaxea fascicularis. Galaxea J. Coral Reef Stud. 20, 1–7 (2018). [Google Scholar]
- 31.Padilla-Gamiño J. L., Weatherby T. M., Waller R. G., Gates R. D., Formation and structural organization of the egg-sperm bundle of the scleractinian coral Montipora capitata. Coral Reefs 30, 371–380 (2011). [Google Scholar]
- 32.Fogarty N. D., Vollmer S. V., Levitan D. R., Weak prezygotic isolating mechanisms in threatened Caribbean Acropora corals. PLoS One 7, e30486 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oliver J., Babcock R., Aspects of the fertilization ecology of broadcast spawning corals: Sperm dilution effects and in situ measurements of fertilization. Biol. Bull. 183, 409–417 (1992). [DOI] [PubMed] [Google Scholar]
- 34.Pennington J. T., The ecology of fertilization of echinoid eggs: The consequences of sperm dilution, adult aggregation, and synchronous spawning. Biol. Bull. 169, 417–430 (1985). [DOI] [PubMed] [Google Scholar]
- 35.Nanami A., Sato T., Ohta I., Akita Y., Suzuki N., Preliminary observations of spawning behavior of white-streaked grouper (Epinephelus ongus) in an Okinawan coral reef. Ichthyol. Res. 60, 380–385 (2013). [Google Scholar]
- 36.Babcock R., Mundy C., Keesing J., Oliver J., Predictable and unpredictable spawning events:in situbehavioural data from free-spawning coral reef invertebrates. Invertebr. Reprod. Dev. 22, 213–227 (1992). [Google Scholar]
- 37.Caspers H., Spawning periodicity and habitat of the palolo worm Eunice viridis (Polychaeta: Eunicidae) in the Samoan Islands. Mar. Biol. 79, 229–236 (1984). [Google Scholar]
- 38.Hoppe W. F., Reichert M. J. M., Predictable annual mass release of gametes by the coral reef sponge Neofibularia nolitangere (Porifera: Demospongiae). Mar. Biol. 94, 277–285 (1987). [Google Scholar]
- 39.Veron J. E., Corals of the World (Australian Institute of Marine Science, Townsville, 2000), vol. 1–3. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.