Abstract
Primary cardiac angiosarcoma is relatively rare, and most cases involve metastasis at the time of diagnosis. The median survival time is 14 months for patients who can be treated surgically, versus 3.8 ± 2.5 months for patients with metastasis who could not undergo surgery. Radical surgical resection, radiotherapy, chemotherapy, and targeted therapy are the main treatments, but prognosis remains poor because of rapid progression and high recurrence and metastasis rates. At present, there is no unified standard treatment, and selecting the correct treatment plan and improving patient survival and quality of life remain challenging. We have reported the case of a 45-year-old woman with a primary heart tumor that infiltrated the right atrial wall and pericardium. Angiosarcoma was verified histologically. After palliative resection of the primary tumor followed by concurrent chemoradiotherapy and targeted therapy, the patient exhibited overall survival of 23 months, highlighting the potential utility of this treatment strategy.
Keywords: Primary cardiac tumor, angiosarcoma, diagnosis, anlotinib, case report, resection, chemoradiotherapy, targeted therapy
Introduction
Primary cardiac tumors are relatively rare, and the incidence rate of malignant tumors is only 0.0017% to 0.28%.1 In total, 75% of primary cardiac tumors, including cardiac myxoma, rhabdomyoma, fibroma, hemangioma, and teratoma, are benign. Among them, myxoma is the most common, and it mostly arises in the left atrium. Twenty-five percent of primary cardiac tumors are malignant, including sarcoma, lymphoma, and mesothelioma. Among these malignancies, angiosarcoma is the most common, and it mostly arises in the right cardiac system. Radical resection is the most effective treatment for primary cardiac malignant tumor, but 89% of patients have metastasis at the time of diagnosis.2 Despite the availability of multi-disciplinary treatments such as radiotherapy, chemotherapy, and targeted therapy, patient prognosis remains poor, and the median survival of patients treated surgically is only 14 months.3 In this study, we reported a case of primary angiosarcoma and discussed the relevant literature to improve our understanding of this disease.
Case presentation
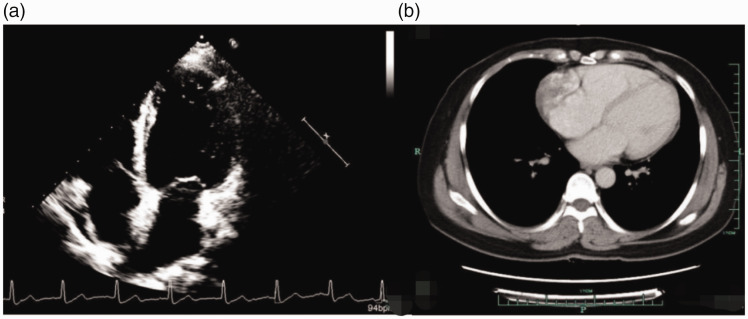
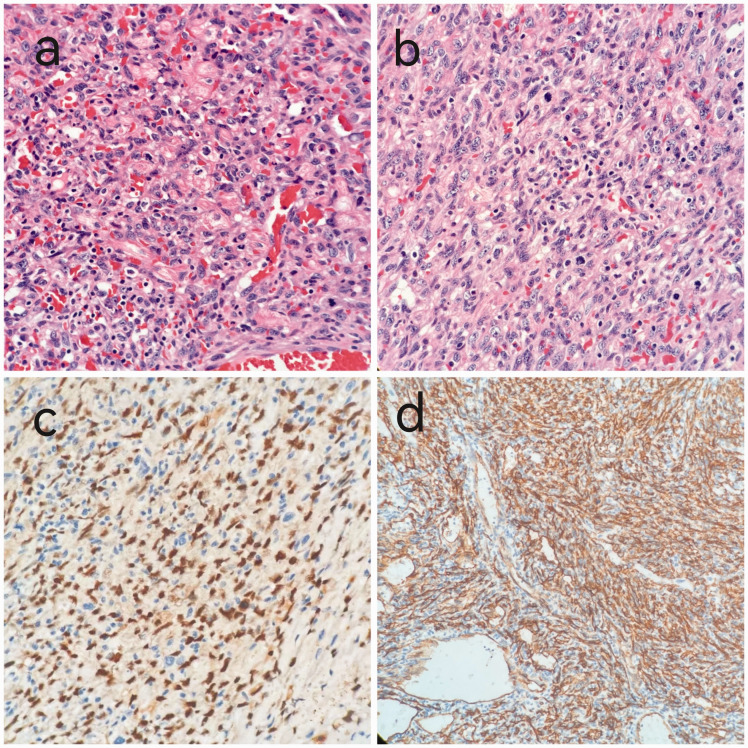
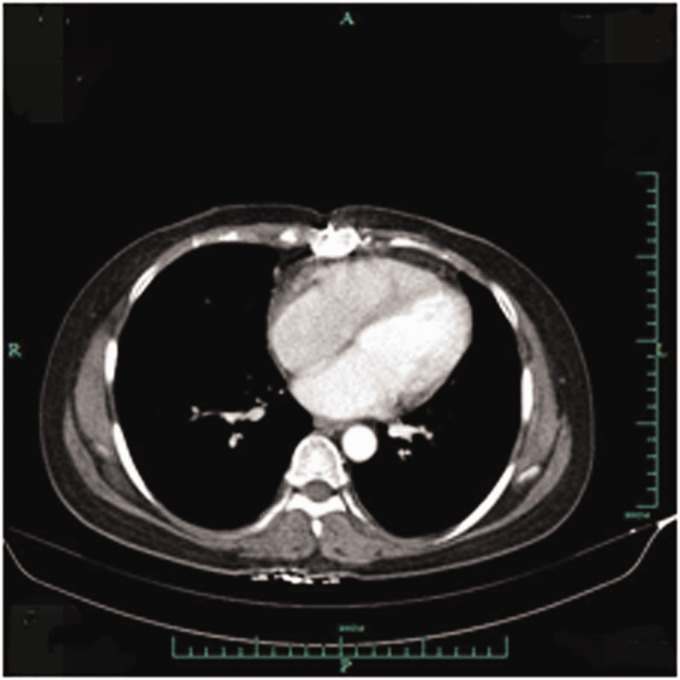
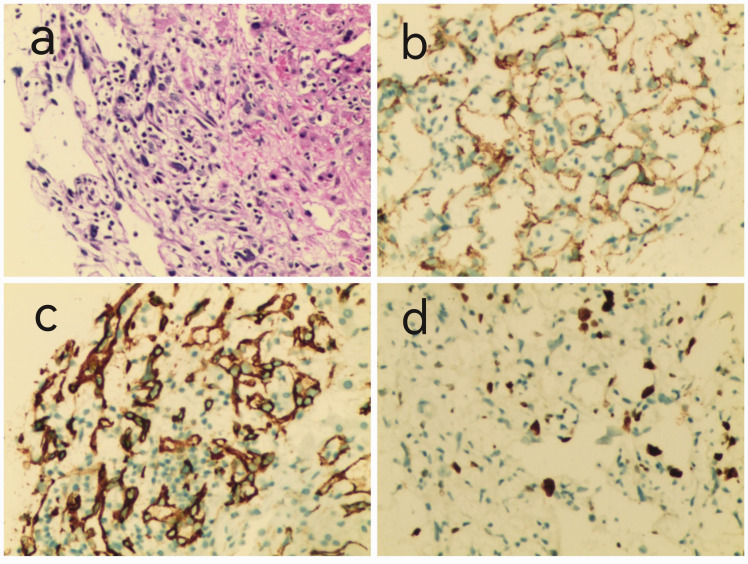
A 45-year-old woman presented with intermittent chest pain in April 2018. Echocardiography and computed tomography (CT) revealed a pericardial mass (approximately 5.5 cm × 4.2 cm × 3.1 cm; Figure 1). The patient underwent palliative resection of right atrial tumor under general anesthesia. The postoperative pathology was consistent with angiosarcoma, which was considered to have a cardiac origin based on the medical history and further immunohistochemical results. The results of immunohistochemical analysis were as follows: cytokeratin (CK) (−), vimentin (+), CD31 (+), CD34 (+), FV (+), ERG (+), D2-40 (−), SMA (−), desmin (−), S-100 (−), CK5/6 (−), calretinin (−), p53 (+), and Ki-67 (+, 10% to 15%; Figure 2). The patient was treated with adjuvant concurrent chemoradiotherapy (50 Gy/2 Gy/25 fractions) consisting of eight cycles of gemcitabine (1000 mg/m2 on days 1 and 8) + docetaxel (75 mg/m2 on day 1) intravenous chemotherapy every 3 weeks. She tolerated the treatment extremely well, and a complete response was observed (Figure 3). There was no evidence of recurrence until she presented with shortness of breath on exertion, multiple liver metastases, and pericardial effusion after 15 months. We performed ultrasound-guided puncture biopsy of the right liver mass for her, and the pathology was indicative of infiltrating or metastatic hepatic angiosarcoma. The results of immunohistochemical analysis were as follows: CK (−), EMA (−), CD34 (+), CD31 (+), SMA (+), Des (−), S-100 (−), and Ki-67 (+, 20%; Figure 4). The patient was treated with anlotinib (12 mg qd on days 1–14 q3w) for five cycles. The patient underwent ultrasound-guided radiofrequency ablation of liver metastases during anlotinib treatment. Despite treatment, she experienced dyspnea, and the amount of pleural fluid gradually increased. Then, the patient received intrapleural injections of cisplatin 60 mg, which was ineffective. Eventually, the disease was fatal after 23 months.
Figure 1.
(a, b) Echocardiography and computed tomography revealed a pericardial mass (approximately 5.5 cm × 4.2 cm × 3.1 cm).
Figure 2.
(a, b) Hematoxylin and eosin staining revealed atypical spindle shaped and epithelioid tumor cells (×400). (c, d) On immunohistochemistry, the tumor cells were positive for CD34 and ERG (×400).
Figure 3.
Computed tomography revealed pericardial thickening but not tumor recurrence.
Figure 4.
(a) Hematoxylin and eosin staining disclosed atypical spindle-shaped and epithelioid tumor cells (×200). (b–d) On immunohistochemistry, the tumor cells were positive for CD34, CD31, and Ki-67 (×200).
Discussion
Angiosarcoma is a highly malignant endothelial cell tumor that accounts for 1% to 2% of soft tissue tumors. It is highly aggressive malignancy with a poor prognosis. It can occur in any part of the body. Approximately 60% of lesions arise in the skin, soft tissue, liver, spleen, bones, and breasts, whereas the heart and kidneys are less frequently affected.4 Primary cardiac angiosarcoma (PCAS) accounts for 33% of all primary malignant cardiac neoplasms,5 and the disease has a male predominance (incidence ratio of 2–3:1),6,7 and the onset age is 30 to 50 years old. The lesion mostly originates in the right atrium and atrial septum, whereas right ventricle and left cardiac system involvement is relatively rare. PCAS is a pedicled tumor that often grows into the right atrial cavity. Its appearance is lobulated, with bleeding and necrotic tissue observed in the center. Advanced angiosarcoma can completely invade the atrial wall, fill the entire heart cavity, and often invade adjacent structures, most often involving the epicardium and pericardium. The clinical manifestations mainly depend on the size and location of the tumor and the degree of invasion. Dyspnea, chest pain, and heart failure are common symptoms, whereas pericardial effusion, vena cava obstruction, pulmonary embolism, and hemoptysis are relatively rare. Because patients have no hemodynamic disturbance in the early stage of the disease, they generally have no obvious clinical symptoms and signs, allowing the malignancy to be easily ignored or missed. Congestive heart failure, pericardial effusion, tamponade, arrhythmia, cardiac rupture, embolism, and acute respiratory distress syndrome occur only when tumor infiltrates the pericardium and myocardium or affects blood flow,8–12 and recurrent pericardial effusion is the most common clinical feature.13 Because of the high degree of malignancy and rapid progression of PCAS, the local recurrence and metastasis rates are extremely high. Approximately 66% to 89% of patients have already had metastasis at the time of diagnosis,2 and the most common sites of metastasis are the lungs, liver, bone, lymph nodes, and brain.14,15
X-ray, echocardiography, CT, magnetic resonance imaging (MRI), and positron emission tomography (PET)-CT can all be used as imaging methods for primary cardiac tumors in the clinic, with CT and MRI serving as the main diagnostic methods.16 Echocardiography can be used to evaluate the shape and function of all four cardiac chambers, and it can accurately determine the location, size, shape, and activity of cardiac tumors and clarify hemodynamic changes for preliminary qualitative diagnosis. CT and MRI can be used to observe the size of cardiac tumors and the relationship of the tumors and the surrounding blood vessels, which can provide the basis for the selection of surgical methods. PET-CT can both differentiate benign and malignant tumors and indicate whether local and systemic tumors are invasive and metastatic. Typical immunohistochemical markers of PCAS confirmed that tumor cells were derived from endothelial cells, as indicated by positivity for CD31, CD34, ERG, and factor VIII. However, because of the cellular heterogeneity of angiosarcoma, immunohistochemistry can only be used as an auxiliary diagnostic tool.3,17.
Studies have reported a median survival of 6 to 11 months for primary cardiac malignancies.18 The median survival was 14 months in patients who could be treated surgically compared with 3.8 ± 2.5 months for patients with metastasis who could not undergo surgery.19 Surgical resection reduces the risks of sudden death and embolism.20 Surgical resection combined with radiotherapy and chemotherapy can significantly improve the short-term prognosis of patients, but there is no obvious advantage concerning long-term prognosis. Patients who cannot undergo tumor resection despite having no metastasis of other organs can undergo heart transplantation, but the long-term use of immunosuppressant after surgery is likely to induce tumor recurrence and metastasis. In recent years, taxanes have displayed good efficacy as adjuvant chemotherapy for cardiac angiosarcoma.21 Molecular targeted drugs, such as anlotinib, imatinib, sorafenib, and bevacizumab, also exerted certain therapeutic effects.22–25 However, a clinical trial identified no significant difference in outcomes between docetaxel combined with bevacizumab and docetaxel monotherapy.26 Postoperative adjuvant radiotherapy can improve the local control rate, reduce the recurrence rate, and prolong patient survival. Because the heart and cardiomyocytes have poor tolerance to radiotherapy, high-dose irradiation may cause damage to the heart itself and surrounding tissues. In currently reported studies, the appropriate irradiation range is 10 to 64 Gy, and the median radiotherapy dose is 50 Gy.27 We treated the patient with eight cycles of gemcitabine combined with docetaxel chemotherapy and concurrent radiotherapy. There was no obvious adverse reaction during radiotherapy, and the local lesion was stable after radiotherapy. The progression-free survival of the patient was 15 months. After progression, the patient received five cycles of oral anlotinib, and her overall survival was 23 months.
As a targeted drug independently developed in China, anlotinib is a multi-target small-molecule tyrosine kinase inhibitor. During the course of tumor development, blood vessel formation continues uncontrollably. Anlotinib mainly blocks tumor angiogenesis and inhibits tumor growth and metastasis by targeting multiple targets such as vascular endothelial growth factor receptor (VEGFR)-1, VEGFR-2, VEGFR-3, platelet-derived growth factor receptor, fibroblast growth factor receptor, c-Kit, and Ret.28,29 Recent clinical trials30–34 revealed that anlotinib has good safety and efficacy in the treatment of a variety of tumors, such as lung cancer, soft tissue sarcoma, medullary thyroid cancer, renal cell carcinoma, and esophageal squamous cell carcinoma. Anlotinib became the first approved targeted drug for soft tissue sarcoma in China on June 24, 2019, and its efficacy and safety have been confirmed in many clinical studies. Chinese Society of Clinical Oncology guidelines recommend anlotinib as the second-line treatment for soft tissue sarcoma. Clinical studies confirmed the safety and efficacy of anlotinib in patients with soft tissue sarcoma that did not respond to chemotherapy,22,35 in line with the results of this study.
In conclusion, although the incidence rate of primary cardiac tumors is relatively low in the clinic, there is no unified standard treatment or effective preventive measures. Although surgery, radiotherapy, chemotherapy, and targeted therapy represent treatment options in the clinic, their therapeutic efficacy varies greatly, and patient prognosis is poor. Therefore, selecting the optimal treatment plan and effectively improving patient survival and quality of life remain difficult problems worthy of further exploration.
Footnotes
Ethics statement: Informed written consent was obtained from the patient’s husband for publication of this case report and the accompanying images. The reporting of this study conforms to the CARE guidelines. The study was approved by the Hangzhou Cancer Hospital Ethics Committee (approval number: HZCH-2021-054; approval date: June 16, 2021).
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Xin Fang https://orcid.org/0000-0003-4151-692X
References
- 1.Look Hong NJ, Pandalai PK, Hornick JL, et al. Cardiac angiosarcoma management and outcomes: 20- year single-institution experience. Ann Surg Oncol 2012; 19: 2707–2715. [DOI] [PubMed] [Google Scholar]
- 2.Bouma W, Lexis CP, Willems TP, et al. Successful surgical excision of primary right atrial angiosarcoma. J Cardiothorac Surg 2011; 6: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel SD, Peterson A, Bartczak A, et al. Primary cardiac angiosarcoma – a review. Med Sci Monit 2014; 20: 103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boni A, Cochetti G, Sidoni A, et al. Primary angiosarcoma of the kidney: case report and comprehensive literature review. Open Med (Wars) 2019; 14: 443–455. doi: 10.1515/med-2019-0048. eCollection 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu C, Zhao Y, Yin Z, et al. Right atrial epithelioid angiosarcoma with multiple pulmonary metastasis confirmed by multimodality imaging- guided pulmonary biopsy: A case report and literature review. Medicine (Baltimore) 2018; 97: e11588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang R, Li L, Li X, et al. Primary cardiac angiosarcoma: A case report. Medicine (Baltimore) 2017; 96: e7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reardon MJ, Walkes JC, Benjamin R.Therapy insight: malignant primary cardiac tumors. Nat Clin Pract Cardiovasc Med 2006; 3: 548–553. [DOI] [PubMed] [Google Scholar]
- 8.Palkar AV, Gupta A, Greenstein Y, et al. Primary cardiac angiosarcoma: a rare cause of diffuse alveolar haemorrhage. BMJ Case Rep 2018; 2018: bcr-2018-225365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jain G, Mukhopadhyay S, Kurien S, et al. Ruptured cardiac angiosarcoma with pulmonary metastases: A rare disease with a common(mis)diagnosis. Indian Heart J 2012; 64: 603–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang M, Fu G, Jiang H, et al. Multimodality treatment for cardiac angiosarcoma. Intern Med 2014; 53: 1949–1953. [DOI] [PubMed] [Google Scholar]
- 11.Wang H, Shi J, Liu H, et al. Clinical and diagnostic features of angiosarcoma with pulmonary metastases: A retrospective observational study. Medicine (Baltimore) 2017; 96: e8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaballah AH, Jensen CT, Palmquist S, et al. Angiosarcoma: clinical and imaging features from head to toe. Br J Radiol 2017; 90: 20170039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong Y, Zhu D, Dong L, et al. Primary cardiac angiosarcoma with pulmonary and lumbar metastases presenting as unexplained pericardial effusion in a 41 year-old man. Heart Lung Circ 2014; 23: e145–e146. [DOI] [PubMed] [Google Scholar]
- 14.Jain A, Simon S, Elangovan I.F-fluoro-deoxyglucose positron emission tomography -computed tomography in initial assessment and diagnosis of right atrial angiosarcoma with widespread visceral metastases: A rare case report and review of the literature. Indian J Nucl Med 2015; 30: 51–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waness A, Batoon AA, Mirza I, et al. Elusive cardiac angiosarcoma in a young pregnant female: Rare presentation with fatal outcome. Cardiol Res 2015; 6: 292–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elsayad K, Scobioala S, Kriz J, et al. Advances in Image-Guided Radiation Therapy for Primary Cardiac Angiosarcoma: The Role of PET-CT and MRI. Oncol Res Treat 2016; 39(5): 290–294. [DOI] [PubMed] [Google Scholar]
- 17.Ge Y, Ro JK, Kim D, et al. Clinicopathologic and immunohistochemical characteristics of adult primary cardiac angiosarcomas: analysis of 10 cases. Ann Diagn Pathol 2011; 15(4): 262–267. [DOI] [PubMed] [Google Scholar]
- 18.Antonuzzo L, Rotella V, Mazzoni F, et al. Primary cardiac angiosarcoma: a fatal disease. Case Rep Med 2009; 2009: 591512. doi: 10.1155/2009/591512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jang Y, Kim J, Shim JW, et al. Primary cardiac angiosarcoma: a prolonged response to surgical resection followed by concurrent chemoradiotherapy with docetaxel. Springerplus 2016; 5: 648. doi: 10.1186/s40064-016-2248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J, Wang B, Hu Y, et al. Clinicopathologic features and outcomes of primary cardiac tumors: a 16-year-experience with 212 patients at a Chinese medical center. Cardiovasc Pathol 2018; 33: 45–54. [DOI] [PubMed] [Google Scholar]
- 21.Kajihara I, Kanemaru H, Miyake T, et al. Combination chemotherapy with S-1 and docetaxel for cutaneous angiosarcoma resistant to paclitaxel. Drug Discov Ther 2015; 9: 75–77. DOI: 10. 5582/ddt. 2015. 01005. [DOI] [PubMed] [Google Scholar]
- 22.Chi Y, Fang Z, Hong X, et al. Safety and Efficacy of Anlotinib, a Multikinase Angiogenesis Inhibitor, in Patients with Refractory Metastatic Soft-Tissue Sarcoma. Clin Cancer Res2018; 11: 5233–5238. [DOI] [PubMed] [Google Scholar]
- 23.Maki RG, D'Adamo DR, Keohan ML, et al. Phase II study of sorafenib in patients with metastatic or recurrent sarcomas. J Clin Oncol 2009; 27: 3133–3140. DOI: 10. 1200/JCO. 2008. 20. 4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chugh R, Wathen JK, Maki RG, et al. Phase II multicenter trial of imatinib in 10 histologic subtypes of sarcoma using a bayesian hierarchical statistical model. J Clin Oncol 2009; 27: 3148–3153. DOI: 10. 1200/JCO. 2008. 20. 5054. [DOI] [PubMed] [Google Scholar]
- 25.Agulnik M, Yarber JL, Okuno SH, et al. An open-label, multicenter, phase II study of bevacizumab for the treatment of angiosarcoma and epithelioid hemangioendotheliomas. Ann Oncol 2012; 24: 257–263. DOI: 10. 1093/annonc/mds237. [DOI] [PubMed] [Google Scholar]
- 26.Ray-Coquard IL, Domont J, Tresch-Bruneel E, et al. Paclitaxel given once per week with or without bevacizumab in patients with advanced angiosarcoma: a randomized phase II trial. J Clin Oncol 2015; 33: 2797–2802. DOI: 10. 1200/JCO. 2015. 60. 8505. [DOI] [PubMed] [Google Scholar]
- 27.Takenaka S, Hashimoto N, Araki N, et al. Eleven cases of cardiac metastases from soft-tissue sarcomas. Jpn J Clin Oncol 2011; 41: 514–518. DOI: 10. 1093/jjco/hyq246. [DOI] [PubMed] [Google Scholar]
- 28.Bussolino F, Mantovani A, Persico G.Molecular mechanisms of blood vessel formation. Trends Biochem Sci 1997; 22: 251–256. [DOI] [PubMed] [Google Scholar]
- 29.Carmeliet P.Angiogenesis in health and disease. Nat Med 2003; 9: 653–660. [DOI] [PubMed] [Google Scholar]
- 30.Han B, Li K, Zhao Y, et al. Anlotinib as a third-line therapy in patients with refractory advanced non-small-cell lung cancer: a multicentre, randomised phase II trial (ALTER0302). Br J Cancer 2018; 118: 654–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chi Y, Fang Z, Hong X, et al. Safety and efficacy of anlotinib, a multikinase angiogenesis inhibitor, in patients with refractory metastatic soft tissue sarcoma. Clin Cancer Res 2018; 24: 5233–5238. [DOI] [PubMed] [Google Scholar]
- 32.Li D, Tang P, Chen X, et al. Anlotinib treatment in locally advanced or metastatic medullary thyroid carcinoma: a multicentre, randomised, double-blind, placebo-controlled phase IIB trail. J Clin Oncol 2019; 37: 6019. [Google Scholar]
- 33.Zhou AP, Bai Y, Song Y, et al. Anlotinib in metastatic renal cell carcinoma (mRCC) with a previous anti-VEGFR TKI: preliminary results from a multicenter, phase II trial. J Clin Oncol 2016; 34: e16082. [Google Scholar]
- 34.Sun Y, Du F, Gao M, et al. Anlotinib for the treatment of patients with locally advanced or metastatic medullary thyroid cancer. Thyroid 2018; 28: 1455–1461. [DOI] [PubMed] [Google Scholar]
- 35.Chen H, Shen J, Choy E, et al. Targeting protein kinases to reverse multidrug resistance in sarcoma [J]. Cancer Treat Rev, 2016, 43: 8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]