Abstract
Accumulating evidence suggests that inflammation has a key role in the pathogenesis of Osteoarthritis (OA). Nitric oxide (NO) is established as one of the major inflammatory mediators in OA and drives many pathological changes during the development and progression of OA. Excessive production of NO in chondrocyte promotes cartilage destruction and cellular injury. Synthesis of NO in chondrocytes is catalyzed by inducible nitric oxide synthase (iNOS), which is thereby an attractive therapeutic target for the treatment of OA. A number of direct and indirect iNOS inhibitors, bioactive compounds, and plant-derived small molecules have been shown to exhibit chondroprotective effect by suppressing the expression of iNOS. Many of these iNOS inhibitors hold promise for the development of new, disease-modifying therapies for OA; however, attempts to demonstrate their success in clinical trials is not yet achieved. Many plant extracts and plant-derived small molecules have also shown promise in animal models of OA, though further studies are needed in human clinical trials to confirm their therapeutic potential. In this review, we discuss the role of iNOS in OA pathology and the effects of various iNOS inhibitors in OA.
Keywords: iNOS, nitric oxide, osteoarthritis, inflammation, iNOS inhibitor, Oxidative stress, Chondrocytes
1. Introduction
1.1. Biosynthesis of NO and Nitric oxide Synthases
NO is a free radical and endogenously it is produced by the conversion of L-arginine to L-citrulline, with NADPH acting as an electron donor. The production of NO is catalyzed by a family of enzymes called nitric oxide synthases (NOSs) that includes three different isoforms: eNOS (endothelial NOS or NOS1), iNOS (inducible NOS or NOS2) and nNOS (neuronal NOS or NOS3) (Table 1). eNOS and nNOS were first characterized in endothelial cells and neurons respectively but were later characterized in other cell types also and were then renamed to NOS1 and NOS3, respectively (Nathan & Xie, 1994). NOS1 and NOS3 are expressed constitutively, while NOS2 is only expressed in activated cells and is induced by lipopolysaccharide (LPS) and inflammatory cytokines such as IL-1β, TNF-α, or IFN-γ (Coleman, 2001). Production of NO by NOS2 is induced after several hours of stimulation, but once induced, it lasts for up to five days (Kolios, Valatas, & Ward, 2004). The physiological half-life of NO ranges from seconds to minutes, depending on its concentration (Wink & Mitchell, 1998).
Table 1.
Isoforms of nitric oxide synthases
| eNOS | iNOS | nNOS | |
|---|---|---|---|
| Alias | endothelial NOS, NOS I | inducible NOS, NOS II | neuronal NOS, NOS III |
| Function | Regulates vascular tissue, blood pressure, inhibits platelet aggregation and leukocyte adhesion | Response to stress and inflammation, involved in host defense and tissue destruction | Involved in neurotransmission, modulates response to pain and neuronal sensitization |
| Gene expression | Constitutively expressed | Induced in response to Immunostimulatory cytokines or bacterial pathogens | Constitutively expressed |
| Dependency on Calcium | Calcium- calmodulin dependent | Calcium- calmodulin independent | Calcium- calmodulin dependent |
| NO production | Small amount (pico to nanomolar) | Large sustained amount of NO (nano to micromolar) | Small amount (pico to nanomolar) |
| Signal for discontinuation of NO production | Self-limited | NOS degradation | Self-limited |
| Expression/localization | Endothelium | Chondrocyte, synoviocytes, hepatocyte, macrophages, other | Brain |
1.2. NO reactivity
The effects of NO can be divided into direct and indirect effects depending on whether NO directly interacts with the target, or first forms an effector molecule by reacting with a reactive oxygen species (ROS), which then interacts with the target (Kolios et al., 2004). The direct action of NO is exemplified by the reaction with guanylate cyclase leading to the formation of cGMP from GTP. Indirect effects of NO are characterized by the formation of a reactive nitrogen oxide species (RNOS) intermediate that is derived from the reaction of NO with either O2 or O2−. NO is quickly oxidized to RNOS under aerobic conditions; it reacts with O2− to form peroxynitrite (ONOO−), which is a potent oxidative and nitrosative agent and may lead to alteration in cellular pathways (Figure 1). Peroxynitrite is known to mediate most of the severe toxic effects of NO (Radomski & Herschorn, 1992). In biological fluids, the primary oxidative product is dinitrogen trioxide (N2O3), which is unstable and quickly nitrates to thiols or amines. NO regulates cell death and has apoptotic effects on cells as the production of RNOS like ONOO− and N2O3 can directly modify nitrogenous bases in DNA and can induce single-strand breaks in DNA (P. K. Kim, Zamora, Petrosko, & Billiar, 2001). However, regulation of cell death by NO is concentration-dependent, as at lower concentration, NO generally appears to be anti-apoptotic, but at higher concentration, it acts as a pro-apoptotic molecule (Kroncke, Fehsel, Suschek, & Kolb-Bachofen, 2001).
Figure 1.
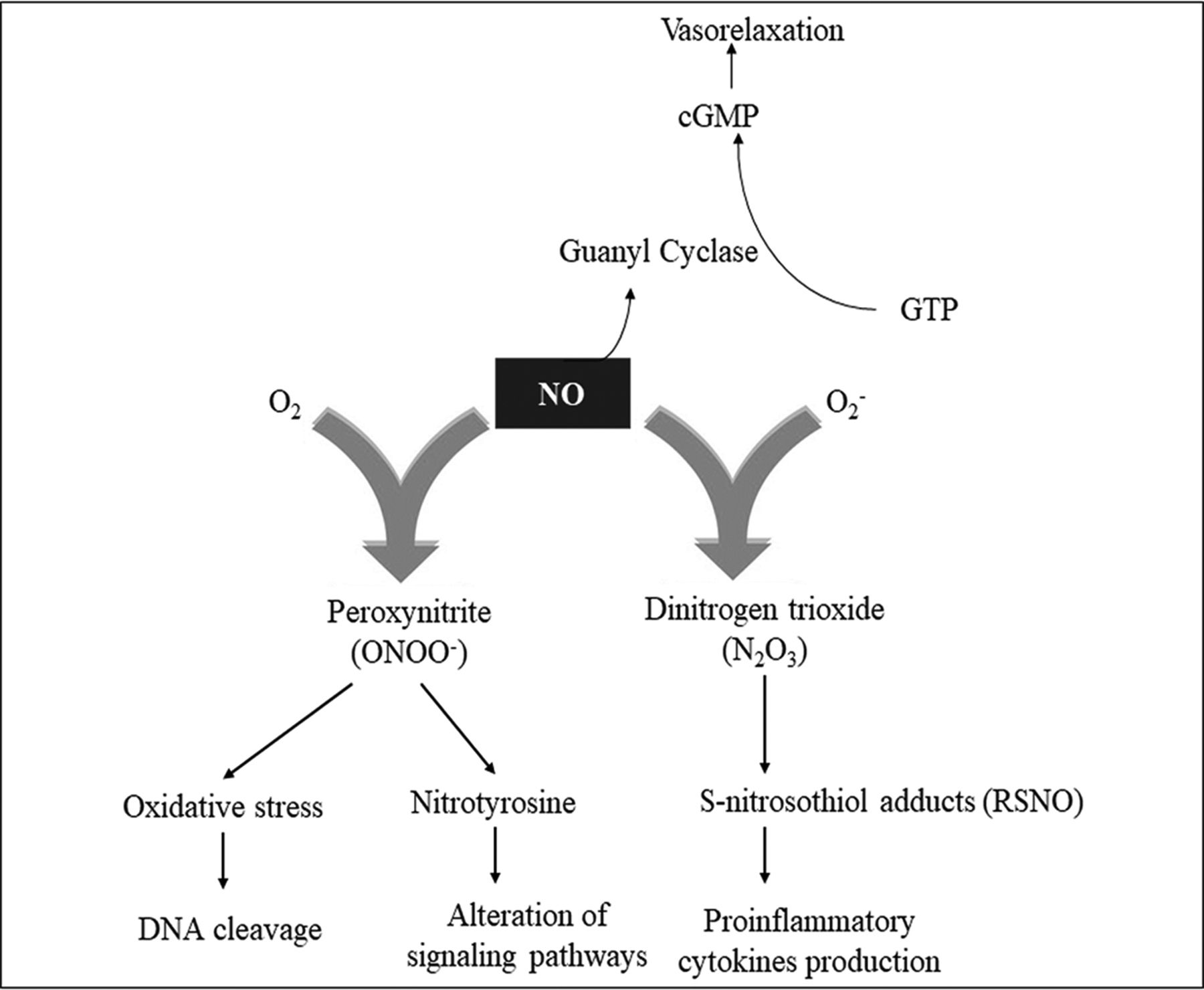
Direct and indirect effects of NO
1.3. NO and OA
Osteoarthritis (OA) is a disabling joint disease that results in substantial morbidity and reduced quality of life (Castrogiovanni et al., 2019). OA is now considered as a disease of the whole joint, as it not only affects the articular cartilage but also affects the entire joint structure and is characterized by cartilage degradation, synovitis, osteophytes formation, and thickening of subchondral bone (Loeser, Goldring, Scanzello, & Goldring, 2012). The current therapy of OA involves interventions that provide symptomatic relief only and the only definite treatment for OA is the joint arthroplasty, which is expensive and requires revision in 10–15 years (Castorina et al., 2017). Excess NO is considered a pro-inflammatory mediator that acts as a tissue-damaging molecule and is implicated in many inflammatory diseases (Chen et al., 2018). These results led to the prevailing hypothesis that NO is a pro-inflammatory factor, which is detrimental to cartilage if present in excess. Considering the pivotal role of inflammation in OA, novel anti-inflammatory therapeutics targeted against iNOS could form the basis of much-needed disease-modifying OA drug (DMOADs). Below we provide an overview of the involvement of iNOS in OA pathogenesis and the potential of various iNOS inhibitors in mitigating joint inflammation in OA. We also review here the importance of inflammation in OA and the transcriptional regulation of iNOS in chondrocytes. Additionally, we explored the chondroprotective and therapeutic potential of various plant-derived small molecules in the prevention or treatment of OA.
2. Inflammation and OA
Traditionally, OA was considered a degenerative disease resulting from the daily wear and tear in the joints, predominantly due to mechanical factors. This concept was based on the observation that chondrocytes, the only cell type present in cartilage are avascular, non-innervated, and have no ability to regenerate (Sophia Fox, Bedi, & Rodeo, 2009). However, with the advancement of molecular biology in the past century, there came a paradigm shift in our understanding of the pathological mechanisms underlying OA. Now, there is strong evidence that OA is a multifactorial disease, and the structural changes observed in OA are due to a combination of factors among which inflammation has a key role (Robinson et al., 2016). By the early twenty-first century, synovitis, occurring due to interactions between damaged tissue and the immune system, was established as a critical feature of OA and was accepted as a driver of the pathogenesis of OA (Zhuo, Yang, Chen, & Wang, 2012).
Inflammation in OA is characterized by the involvement of innate immune system mainly and to some extent, an adaptive immune system (Haseeb & Haqqi, 2013). Joint inflammation is clearly reflected in many of the clinical symptoms of OA, such as joint swelling, warmth, and pain (Sellam & Berenbaum, 2010). We have shown previously that ZCCHC6 knockout mice develop less severe OA due to reduced expression of IL-6 (Ansari et al., 2019). Inflammation of the synovium (synovitis) is a common finding in OA and is characterized by synovial hypertrophy and infiltration of the sub lining tissue with inflammatory cells (Scanzello & Goldring, 2012). The inflammation in OA is modest and less pronounced as compared to RA and also differs in terms of the cellular and molecular players involved (de Lange-Brokaar et al., 2012).
The most accepted hypothesis to explain the inflammation in OA is that once the degraded cartilage fragments come into contact with the synovium, they are considered foreign particles, and there is a protective inflammatory response by the synoviocytes (Berenbaum, 2013). The products of cartilage damage trigger the activation of inflammatory signaling pathways including nuclear factor-kappa b (NF-κB), that plays a central role in the inflammatory response (Scanzello, 2017). Inflammation in the joint can also be triggered by stress response, and obesity-related systemic inflammation might add to the local inflammation (Scanzello, 2017). Cytokines including IL-1β, TNF-α, IL-6, IL-17, IL-18, and IL-21 have been implicated in the pathogenesis of OA and are among the most widely studied mediators of inflammation (Kapoor, Martel-Pelletier, Lajeunesse, Pelletier, & Fahmi, 2011). These inflammatory mediators are deleterious for the joint and initiate or perpetuate the cartilage damage and amplify the low-grade inflammation, which may also induce other inflammatory diseases that are affected by systemic low-grade chronic inflammation (Berenbaum, 2013).
3. Role of iNOS in OA pathogenesis
Production of NO is abnormally high in OA cartilage as high levels of NO are produced by OA chondrocytes (Scher, Pillinger, & Abramson, 2007). Moreover, elevated concentrations of nitrotyrosine, a marker of NO-dependent oxidative damage has been found in synovial fluid from OA patients, and excess NO production in OA cartilage has been illustrated by immunostaining with anti-nitrotyrosine antibodies (Loeser, Carlson, Del Carlo, & Cole, 2002). In tandem, expression of iNOS has been demonstrated by Western blot and immunohistochemical staining in OA cartilage (Vuolteenaho, Moilanen, Al-Saffar, Knowles, & Moilanen, 2001). Elevated levels of nitrite and iNOS mRNA and protein and have been found in the synovium of patients with OA (Ersoy et al., 2002; McInnes et al., 1996). However, cartilage is the major source of NO in OA joints as more enhanced expression of iNOS is found in chondrocytes as compared to the synovial cells in OA patients (Melchiorri et al., 1998). Chondrocytes isolated from OA cartilage show overexpression of iNOS mainly in the superficial zone, whereas chondrocytes isolated from patients without OA do not express iNOS (Amin et al., 1995). Aberrant expression of iNOS plays a crucial role in many inflammatory diseases like OA, colitis, asthma, multiple sclerosis, and psoriasis (Kroncke, Fehsel, & Kolb-Bachofen, 1998). iNOS generated NO seems to be crucially involved in the pathomechanisms of OA, and it contributes to the OA pathogenesis by modulating ECM homeostasis and cytokines expression, causing oxidative damage and chondrocyte apoptosis (Figure 2). Excess production of NO by iNOS leads to cartilage damage by enhancing MMP activity and downregulating biosynthesis of aggrecan and collagen (Lepetsos & Papavassiliou, 2016).
Figure 2.
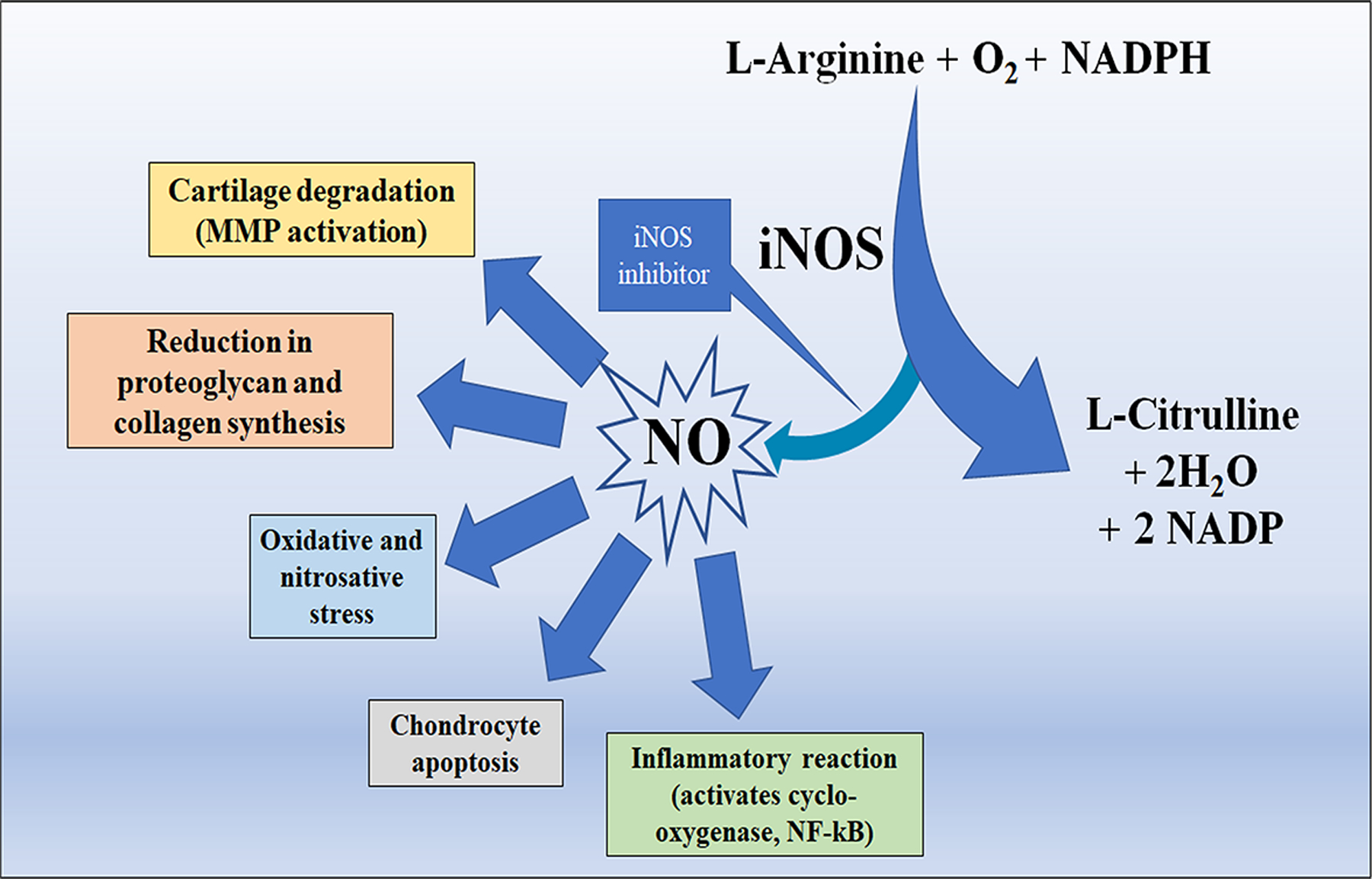
Synthesis of NO and its deleterious effects on cartilage.
3.1. Cartilage destruction
iNOS is preferentially expressed in the superficial zone of OA cartilage, which is the surface zone that defines the site of initial damage, indicating that the damaged OA cartilage actively produces NO (Melchiorri et al., 1998). Well documented actions of NO in cartilage include acceleration of chondrocyte mediated matrix degradation and inhibition of cartilage matrix synthesis, and together these effects lead to the loss of cartilage matrix (Scher et al., 2007). The destructive action of NO is mediated by inhibiting the formation of ECM components such as type II collagen at a post-translational level by inhibiting prolyl hydroxylase, which is involved in hydroxylation of proline and collagen crosslinking (Hauselmann, Stefanovic-Racic, Michel, & Evans, 1998). Furthermore, NO is implicated in the inhibition of synthesis of proteoglycan such as aggrecan (Johnson et al., 2000). Earlier, matrix degradation in OA was considered as a consequence of mainly mechanical factors, but later it was established that active resorption of matrix components is also involved in matrix degradation. NO promotes degradation of ECM by enhancing the activity of matrix metalloproteinases (MMPs) that subsequently leads to joint destruction (Davies et al., 2013). Several molecules, such as endothelin 1 that regulate MMP expression in cartilage, are themselves controlled by NO (Manacu et al., 2005). Inhibition of iNOS decreases the loss of glycosaminoglycan content in the cartilage in an OA model (Balaganur et al., 2014). Besides, NO plays a regulatory role in the activation of MMP-9 and inhibits the activity of TIMP-1 (Ridnour et al., 2007).
3.2. Pro-inflammatory reaction
NO acts as a proinflammatory and destructive mediator in OA and increases the production of PGE2 and inflammatory cytokines (Amin et al., 1997). Additionally, NO has been shown to disrupt the cytokine balance leading to a pro-inflammatory and destructive response by reducing the synthesis of endogenous interleukin-1 receptor antagonist (IL-1Ra) and anabolic mediator TGF-β (Vuolteenaho, Moilanen, Hamalainen, & Moilanen, 2003). It has been reported that NO also increases the susceptibility to injury by other oxidants like H2O2 and by upregulating IL-18 and TNF-α (Pelletier, Mineau, Ranger, Tardif, & Martel-Pelletier, 1996). IL-1β and TNF-α induce the production of NO as well as ROS, which can react with NO to form peroxynitrite and augment NF-κB activation (Scher et al., 2007), thereby providing persistent signaling for NF-κB dependent gene transcription (Clancy, Gomez, & Abramson, 2004).
3.3. Cytotoxicity
Inappropriate chondrocyte apoptosis is one of the central features of OA pathogenesis, and NO is a principal inducer of chondrocyte apoptosis, via the action of caspase-3 and tyrosine kinases (Maneiro et al., 2005), however, the exact mechanism of NO-induced apoptosis in OA has not been elucidated yet (Scher et al., 2007). Also, whether NO can independently trigger apoptosis is not established, and it is argued that ROS are the actual mediators of chondrocyte apoptosis (Del Carlo & Loeser, 2002).Whiteman et al. proposed that peroxynitrite (ONOO−) rather than NO per se, triggers apoptosis in chondrocytes (Whiteman et al., 2004). Since the effect of NO in cartilage is dependent on concentration and duration of action, and the local concentration of NO is an important determinant of cytotoxicity, so the small amounts (picomolar concentration) of NO produced for a limited duration by eNOS and nNOS are sufficient for physiological signaling and homeostasis and is known to be protective and essential for chondrocyte maturation , but large amounts (micromolar concentration) produced by iNOS for a sustained period results in inflammation, cytotoxicity and chondrocyte catabolism causing damage to chondrocytes and surrounding tissues (Abramson, 2008).
3.4. Oxidative/Nitrosative stress
In addition to inflammation, catabolic cytokines, and other factors, recent studies have supported the role of oxidative/nitrosative stress in OA pathology (Alcaraz, Megias, Garcia-Arnandis, Clerigues, & Guillen, 2010). Free radicals are eliminated by antioxidants, such as superoxide dismutase and non-enzymatic agents, such as tocopherol that protect from oxidation. An imbalance created between the production of ROS/RNS and the cellular scavenging antioxidants, is defined as oxidative/nitrosative stress, which contributes to OA pathogenesis (Ziskoven et al., 2011). Excess of NO derived RNS and ROS can be detrimental in chondrocytes by causing mitochondrial dysfunction (Maneiro et al., 2005). RNS are able to react directly with ROS by producing highly reactive molecules that can induce DNA fragmentation (Beckman & Koppenol, 1996). Additionally, both ROS and RNS are able to reduce the synthesis capacity of proteoglycans and collagen by chondrocytes (Franz et al., 2018).
4. Transcriptional regulation of iNOS expression in chondrocytes
Regulation of NO produced by iNOS is regulated by several mechanisms and varies according to species, cell type, and the inducer and is regulated both at the transcriptional and post-transcriptional level but is largely controlled by transcriptional mechanisms (Frasca et al., 2010). Induction of iNOS expression in most human cells requires the synergistic action of a complex mixture of cytokines (Kleinert, Pautz, Linker, & Schwarz, 2004). Interestingly, the production of NO in primary human chondrocytes can be induced by a single cytokine such as IL-1β, TNF-α, IL-17, or IFN- γ (Henrotin et al., 2000). Bacterial LPS, fibronectin fragment (FN-f) and shear stress have also been reported to induce NO production in human chondrocytes (Vuolteenaho, Moilanen, Knowles, & Moilanen, 2007). IL-1β induced NO synthesis in primary human chondrocytes is time and concentration-dependent and starts after a lag period of 6 hours (Palmer, Hickery, Charles, Moncada, & Bayliss, 1993). On the other hand, induction of iNOS in C-28/I2 chondrocytes depends on triple combination of cytokines (Kleinert et al., 2004). In human chondrocytes, iNOS is mainly regulated by MAPK (ERK, p38, JNK), NF-κB and JAK2-STAT1α pathway. In C-28/I2 chondrocytes also, iNOS induction is regulated by p38 MAPK, JAK2-STAT-1α and NF-κB pathways (Schmidt et al., 2010). The promoter region of the human iNOS gene contains binding sites for several TFs like NF-κB, AP-1, STAT1α, IL-6, and Interferon regulating factor (Kleinert et al., 2004). Binding sites for C/EBP, CREB, GATA, HF, p53, Sp1, and KLF-6 have also been validated (Pautz et al., 2010).
5. iNOS inhibition as a therapeutic strategy for OA
Currently, there is no approved DMOAD that could inhibit the structural progression of OA; however many potential agents are under investigation (Davies et al., 2013). Since NO is established as a major catabolic factor in OA pathogenesis, iNOS inhibitors are considered as a class of prospective DMOADs and are currently under investigation (Watt & Gulati, 2017). In animal models, iNOS inhibitors were shown to reduce NO levels as well as the levels of prostaglandins at the inflammation site (Clancy, Amin, & Abramson, 1998), indicating that inhibiting iNOS could be an attractive therapeutic target for OA (Chevalier, Eymard, & Richette, 2013). iNOS knockout mice have been used to study the importance of iNOS in OA pathology and development. Preclinical studies have demonstrated that iNOS KO mice are resistant to developing OA (Salerno, Sorrenti, Di Giacomo, Romeo, & Siracusa, 2002). In a classic study, OA was induced in iNOS deficient mice and cartilage damage, cartilage proteoglycan depletion, and osteophytes formation were evaluated (van den Berg, van de Loo, Joosten, & Arntz, 1999). They found that cartilage damage, osteophyte formation, and proteoglycan depletion were significantly reduced in the iNOS-deficient mice, suggesting that iNOS and NO play a significant role in the induction and progression of OA in this model. On the other hand, in another study it was found that iNOS deficient mice showed accelerated development of OA (Clements et al., 2003). The authors suggested that this observation could be because of overexpression of eNOS or nNOS (Clements et al., 2003).
Expression of iNOS and NO production can be inhibited by a wide variety of compounds, which are either direct or indirect inhibitors of iNOS. Direct inhibitors bind directly to the iNOS enzyme and inhibit its activity, whereas indirect inhibitors mainly target NF-κB and STAT-1 signaling pathways thereby downregulating iNOS expression. Based on the site of binding, iNOS inhibitors are classified into four categories. The first category of iNOS inhibitors bind to the arginine-binding site, and examples include L-NMMA, L-NIL, 1400W, GW274150, and L-NAME. The second category includes iNOS inhibitors like Cindunistat that mimic the tetrahydrobiopterin cofactor. The third class includes a set of compounds that interact directly with the heme, and the fourth class includes compounds that interact with the calmodulin or flavine cofactors (Alderton, Cooper, & Knowles, 2001). Studies published with various iNOS inhibitors and OA are presented analytically in Table 2.
Table. 2.
iNOS inhibitors and OA
| iNOS inhibitor | Specificity | Model | Main finding | References | |
|---|---|---|---|---|---|
| 1 | L-NMMA | Not selective for different isoforms of NOS | Human chondrocytes | Expression of iNOS was inhibited by L-NMMA | (Mathy-Hartert et al., 2002) |
| 2 | 1400W | Selective iNOS inhibitor | Human OA cartilage | Treatment with 1400W reduced MMP-10 in IL-1β treated OA cartilage | (Jarvinen et al., 2008) |
| 3 | L-NIL (n-iminoethyl-L-lysine) | Selective iNOS inhibitor | ACLT induced canine model | L-NIL treatment showed a reduction in the size of osteophytes and decrease in the severity of cartilage lesions and the progression of experimental OA | (Pelletier et al., 2000) |
| 4 | GW 274150 | selective | Collagen-induced arthritis in mice | GW 274150 attenuated inflammation and tissue damage associated with collagen-induced arthritis in mice | (Cuzzocrea et al., 2002) |
| 5 | L-NAME | selective | Adjuvant induced arthritis in rats | L-NAME suppressed arthritis progression and increase weight gain | (Ialenti, Moncada, & Di Rosa, 1993) |
| 6 | SMT (S-methyl isothiourea) | Preferential iNOS inhibitor | MIA-induced arthritis in rats | SMT reduced NO production in synovial fluid and reduced | (More et al., 2013) |
| 7 | Cindunistat | Selective iNOS inhibitor | Double-blinded, placebo-controlled Randomized controlled trial | Cindunistat slowed joint space narrowing in patients with mild OA but had no effect on patients with severe OA. | (Hellio le Graverand et al., 2013) |
In animal models, the use of iNOS inhibitors significantly reduced cartilage degeneration and osteophyte formation (Pelletier et al., 1998). In a dog model of OA, inhibition of iNOS by L-NIL decreased general MMP activity, incidence and size of osteophytes and the size of cartilage lesions (Martel-Pelletier, Wildi, & Pelletier, 2012). Methylisothiourea attenuated monosodium acetate induced histopathological changes in knee joint (More et al., 2013). Conversely, in a recent 2-year randomized, double-blind, placebo-controlled clinical trial of another iNOS inhibitor, cindunistat, there was no significant reduction in joint space narrowing (JSN) and progression of OA in patients with severe OA (Hellio le Graverand et al., 2013). Many pharmaceutical drugs act as indirect iNOS inhibitors and have been shown to exert their chondroprotective effects by inhibiting iNOS expression at the transcriptional level. For example, in a double-blinded, placebo-controlled RCT, it was found that doxycycline decreased Joint space narrowing (Brandt et al., 2005). Another indirect iNOS inhibitor, Ursodeoxycholic acid (UDCA) reduced the expressions of MMP-3, MMP-13, and ADAMTS-5 in IL-1β-stimulated OA chondrocytes (Moon et al., 2014).
Plant-derived polyphenolic compounds are well known for their anti-inflammatory and antioxidant properties. Previous studies from our laboratory have shown that plant polyphenols like Epigallocatechin-3-gallate (EGCG) and Wogonin inhibit NO production in IL-1β-stimulated human OA chondrocytes by inhibiting the expression of iNOS mRNA (Ahmed et al., 2002; Akhtar & Haqqi, 2012; Khan et al., 2017). We showed that EGCG inhibited the IL-1β-induced inflammation in primary human OA chondrocytes (Akhtar & Haqqi, 2011). Many dietary polyphenols like Gallic acid, Silibinin, Carvacrol, and Sinapic acid have also been shown to inhibit iNOS and have anti-inflammatory effects in chondrocytes as shown in Table 3 (Huang et al., 2018; Wen et al., 2015; Xiao, Li, Liu, & Ma, 2018; Zheng et al., 2017). Additionally, chondroprotective and OA suppressive effects of Pomegranate extract in a rabbit model of OA have also been shown (Akhtar, Khan, Ashruf, & Haqqi, 2017). We have demonstrated the chondroprotective effects of pomegranate extract in IL-1β-stimulated primary human chondrocytes (Haseeb, Khan, Ashruf, & Haqqi, 2017). We also found that the mechanism behind the chondroprotective activity of Pomegranate extract was via suppression of the IL-1β-induced activation of p38α-MAPK (Rasheed, Akhtar, & Haqqi, 2010). Studies reporting the chondroprotective effects of selected plant extracts via inhibition of iNOS are presented in Table-4.
Table 3.
iNOS inhibition by plant-derived molecules in chondrocytes and animal OA models.
| Molecule | Source | Outcome | Model | Reference | |
|---|---|---|---|---|---|
| 1 | EGCG | Green tea | EGCG inhibited the IL-1 beta-induced activity and expression of iNOS via blocking the NF-κB pathway activation | Human chondrocytes | (Ahmed et al., 2002; Singh, Ahmed, Islam, Goldberg, & Haqqi, 2002) |
| 2 | Wogonin | Scutellaria baicalensis | Wogonin suppressed the IL1-β- induced NO production and iNOS expression via activation of the ROS/ERK/Nrf2 signaling pathway | Human chondrocytes | (Khan et al., 2017) |
| 3 | Gallic acid (GA) | Gallnuts | GA suppressed the AGE-induced expression of iNOS in articular chondrocytes, playing a chondroprotective role against OA progression | Rabbit model of knee OA | (Wen et al., 2015) |
| 4 | Silibinin | Siltbum Marianum |
Silibinin inhibited the IL1-β-induced NO production and iNOS expression via inhibiting the NF-κB pathway | Human chondrocytes | (Zheng et al., 2017) |
| 5 | Carvacrol | Oregano and thyme | Carvacrol inhibited the IL1-β-induced NO production and iNOS expression via inhibiting the NF-κB pathway | Human chondrocytes | (Xiao et al., 2018) |
| 6 | Sinapic acid | Vinegar and wine | Sinapic acid inhibited the IL1-β-induced NO production and iNOS expression via inhibiting the MAPK pathway | Rat chondrocytes | (Huang et al., 2018) |
| 7 | Sulforaphane | Broccoli, cabbage | Sulforaphane treatment inhibited NO synthesis and cartilage matrix degradation | Human chondrocytes and DMM model of osteoarthritis in mouse | (Davidson et al., 2013) |
6. Concluding remarks
It is now well established that inflammation plays a central role in the pathogenesis of OA, and NO acts as a major pro-inflammatory mediator during joint inflammation. NO plays a destructive role during the pathomechanism of OA and leads to cartilage damage and matrix degradation. A number of studies suggest that inhibition of NO has chondroprotective effects in OA. Hence, inhibitors of iNOS are attractive molecules for the development of DMOADs. iNOS inhibitors like L-NMMA, L-NIL and 1400W have shown chondroprotective effects, and they need to be assessed further in human phase I trials. Despite the promising results obtained with most of the iNOS inhibitors in vitro and in vivo, no successful outcome has been observed in clinical trials yet. Studies from our group and others have demonstrated that plant-derived compounds and herbal medicines have positive effects on OA in animal models, but further human studies need to be conducted to confirm the beneficial effects of dietary and bioactive compounds in OA.
Table 4.
Use of plant extracts and herbal medicine to inhibit iNOS in OA.
| Plant extract/ herbal medicine | Plant source | Outcome | Model | Reference | |
|---|---|---|---|---|---|
| 1 | Ginkgo biloba extract (EGb) | Ginkgo biloba | EGb inhibited NO production, iNOS expression, chemokine production, and the JNK-AP1 signaling pathway. | Human OA chondrocytes | (Ho et al., 2013) |
| 2 | Cherry seed extract (SCE) | Prunus avium | Patients who were treated with SCE topically showed significant decreased inflammatory cytokines levels, decreased pain and decreased serum CRP levels. | Double-blind, placebo-controlled RCT | (Mahmoud, Al-Awadhi, & Haines, 2015) |
| 3 | Herbal-Leucine mixture (HLM) | Uncaria tomentosa, Boswellia spp., Lepidium meyenii and L-Leucine | HLM showed chondroprotective and anti-inflammatory activity in OA. | Primary OA chondrocytes or OA cartilage explants | (Akhtar, Miller, & Haqqi, 2011) |
| 4 | Ginsenoside Rb1 (G-Rb1) | Panax ginseng | G-Rb1 reduced the levels of IL-1β, TNF-α, NO, MMP-1 and MMP-13, by suppressing iNOS expression and reducing ROS production. | Rat articular chondrocytes | (S. Kim et al., 2012) |
| 5 | Duhuo Jisheng decoction (DHJSD) | Radix angelicae pubescentis and Herba taxilli | DHJSD inhibited NO-induced apoptosis in rat articular chondrocytes. DHJSD combined with Western medicine or sodium hyaluronate injection appeared to have benefits for knee OA. | Rat articular Chondrocytes, Randomized clinical trials | (Zhang et al., 2016) |
8. Funding source:
This work was supported in part by the NIH/National Institute of Arthritis and Musculoskeletal and Skin Diseases and the NIH/National Center for Complementary and Integrative Health of the National Institutes of Health under Award Numbers R01AR067056 and RO1AT007373 and funds from the Northeast Ohio Medical University to TMH.
Footnotes
Conflict of Interest.
Authors declare no conflict of interest.
Data Availability.
Data sharing not applicable to this article as no datasets were generated during the current study.
All cited references are available in Pubmed.gov
11. References
- Abramson SB (2008). Osteoarthritis and nitric oxide. Osteoarthritis Cartilage, 16 Suppl 2, S15–20. doi: 10.1016/S1063-4584(08)60008-4 [DOI] [PubMed] [Google Scholar]
- Ahmed S, Rahman A, Hasnain A, Lalonde M, Goldberg VM, & Haqqi TM (2002). Green tea polyphenol epigallocatechin-3-gallate inhibits the IL-1 beta-induced activity and expression of cyclooxygenase-2 and nitric oxide synthase-2 in human chondrocytes. Free Radic Biol Med, 33(8), 1097–1105. doi: 10.1016/s0891-5849(02)01004-3 [DOI] [PubMed] [Google Scholar]
- Akhtar N, & Haqqi TM (2011). Epigallocatechin-3-gallate suppresses the global interleukin-1beta-induced inflammatory response in human chondrocytes. Arthritis Res Ther, 13(3), R93. doi: 10.1186/ar3368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar N, & Haqqi TM (2012). Current nutraceuticals in the management of osteoarthritis: a review. Ther Adv Musculoskelet Dis, 4(3), 181–207. doi: 10.1177/1759720X11436238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar N, Khan NM, Ashruf OS, & Haqqi TM (2017). Inhibition of cartilage degradation and suppression of PGE2 and MMPs expression by pomegranate fruit extract in a model of posttraumatic osteoarthritis. Nutrition, 33, 1–13. doi: 10.1016/j.nut.2016.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar N, Miller MJ, & Haqqi TM (2011). Effect of a Herbal-Leucine mix on the IL-1beta-induced cartilage degradation and inflammatory gene expression in human chondrocytes. BMC Complement Altern Med, 11, 66. doi: 10.1186/1472-6882-11-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcaraz MJ, Megias J, Garcia-Arnandis I, Clerigues V, & Guillen MI (2010). New molecular targets for the treatment of osteoarthritis. Biochem Pharmacol, 80(1), 13–21. doi: 10.1016/j.bcp.2010.02.017 [DOI] [PubMed] [Google Scholar]
- Alderton WK, Cooper CE, & Knowles RG (2001). Nitric oxide synthases: structure, function and inhibition. Biochem J, 357(Pt 3), 593–615. doi: 10.1042/0264-6021:3570593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin AR, Attur M, Patel RN, Thakker GD, Marshall PJ, Rediske J, … Abramson SB (1997). Superinduction of cyclooxygenase-2 activity in human osteoarthritis-affected cartilage. Influence of nitric oxide. J Clin Invest, 99(6), 1231–1237. doi: 10.1172/JCI119280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin AR, Di Cesare PE, Vyas P, Attur M, Tzeng E, Billiar TR, … Abramson SB (1995). The expression and regulation of nitric oxide synthase in human osteoarthritis-affected chondrocytes: evidence for up-regulated neuronal nitric oxide synthase. J Exp Med, 182(6), 2097–2102. doi: 10.1084/jem.182.6.2097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari MY, Khan NM, Ahmad N, Green J, Novak K, & Haqqi TM (2019). Genetic Inactivation of ZCCHC6 Suppresses Interleukin-6 Expression and Reduces the Severity of Experimental Osteoarthritis in Mice. Arthritis Rheumatol, 71(4), 583–593. doi: 10.1002/art.40751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaganur V, Pathak NN, Lingaraju MC, More AS, Latief N, Kumari RR, … Tandan SK (2014). Effect of S-methylisothiourea, an inducible nitric oxide synthase inhibitor, in joint pain and pathology in surgically induced model of osteoarthritis. Connect Tissue Res, 55(5–6), 367–377. doi: 10.3109/03008207.2014.953629 [DOI] [PubMed] [Google Scholar]
- Beckman JS, & Koppenol WH (1996). Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol, 271(5 Pt 1), C1424–1437. doi: 10.1152/ajpcell.1996.271.5.C1424 [DOI] [PubMed] [Google Scholar]
- Berenbaum F (2013). Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthritis Cartilage, 21(1), 16–21. doi: 10.1016/j.joca.2012.11.012 [DOI] [PubMed] [Google Scholar]
- Brandt KD, Mazzuca SA, Katz BP, Lane KA, Buckwalter KA, Yocum DE, … Heck LW (2005). Effects of doxycycline on progression of osteoarthritis: results of a randomized, placebo-controlled, double-blind trial. Arthritis Rheum, 52(7), 2015–2025. doi: 10.1002/art.21122 [DOI] [PubMed] [Google Scholar]
- Castorina S, Guglielmino C, Castrogiovanni P, Szychlinska MA, Ioppolo F, Massimino P, … Musumeci G (2017). Clinical evidence of traditional vs fast track recovery methodologies after total arthroplasty for osteoarthritic knee treatment. A retrospective observational study. Muscles Ligaments Tendons J, 7(3), 504–513. doi: 10.11138/mltj/2017.7.3.504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrogiovanni P, Di Rosa M, Ravalli S, Castorina A, Guglielmino C, Imbesi R, … Musumeci G (2019). Moderate Physical Activity as a Prevention Method for Knee Osteoarthritis and the Role of Synoviocytes as Biological Key. Int J Mol Sci, 20(3). doi: 10.3390/ijms20030511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, … Zhao L (2018). Inflammatory responses and inflammation-associated diseases in organs. Oncotarget, 9(6), 7204–7218. doi: 10.18632/oncotarget.23208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier X, Eymard F, & Richette P (2013). Biologic agents in osteoarthritis: hopes and disappointments. Nat Rev Rheumatol, 9(7), 400–410. doi: 10.1038/nrrheum.2013.44 [DOI] [PubMed] [Google Scholar]
- Clancy RM, Amin AR, & Abramson SB (1998). The role of nitric oxide in inflammation and immunity. Arthritis Rheum, 41(7), 1141–1151. doi: [DOI] [PubMed] [Google Scholar]
- Clancy RM, Gomez PF, & Abramson SB (2004). Nitric oxide sustains nuclear factor kappaB activation in cytokine-stimulated chondrocytes. Osteoarthritis Cartilage, 12(7), 552–558. doi: 10.1016/j.joca.2004.04.003 [DOI] [PubMed] [Google Scholar]
- Clements KM, Price JS, Chambers MG, Visco DM, Poole AR, & Mason RM (2003). Gene deletion of either interleukin-1beta, interleukin-1beta-converting enzyme, inducible nitric oxide synthase, or stromelysin 1 accelerates the development of knee osteoarthritis in mice after surgical transection of the medial collateral ligament and partial medial meniscectomy. Arthritis Rheum, 48(12), 3452–3463. doi: 10.1002/art.11355 [DOI] [PubMed] [Google Scholar]
- Coleman JW (2001). Nitric oxide in immunity and inflammation. Int Immunopharmacol, 1(8), 1397–1406. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, Chatterjee PK, Mazzon E, McDonald MC, Dugo L, Di Paola R, … Thiemermann C (2002). Beneficial effects of GW274150, a novel, potent and selective inhibitor of iNOS activity, in a rodent model of collagen-induced arthritis. Eur J Pharmacol, 453(1), 119–129. doi: 10.1016/s0014-2999(02)02338-5 [DOI] [PubMed] [Google Scholar]
- Davidson RK, Jupp O, de Ferrars R, Kay CD, Culley KL, Norton R, … Clark IM (2013). Sulforaphane represses matrix-degrading proteases and protects cartilage from destruction in vitro and in vivo. Arthritis Rheum, 65(12), 3130–3140. doi: 10.1002/art.38133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PS, Graham SM, MacFarlane RJ, Leonidou A, Mantalaris A, & Tsiridis E (2013). Disease-modifying osteoarthritis drugs: in vitro and in vivo data on the development of DMOADs under investigation. Expert Opin Investig Drugs, 22(4), 423–441. doi: 10.1517/13543784.2013.770837 [DOI] [PubMed] [Google Scholar]
- de Lange-Brokaar BJ, Ioan-Facsinay A, van Osch GJ, Zuurmond AM, Schoones J, Toes RE, … Kloppenburg M (2012). Synovial inflammation, immune cells and their cytokines in osteoarthritis: a review. Osteoarthritis Cartilage, 20(12), 1484–1499. doi: 10.1016/j.joca.2012.08.027 [DOI] [PubMed] [Google Scholar]
- Del Carlo M Jr., & Loeser RF (2002). Nitric oxide-mediated chondrocyte cell death requires the generation of additional reactive oxygen species. Arthritis Rheum, 46(2), 394–403. doi: 10.1002/art.10056 [DOI] [PubMed] [Google Scholar]
- Ersoy Y, Ozerol E, Baysal O, Temel I, MacWalter RS, Meral U, & Altay ZE (2002). Serum nitrate and nitrite levels in patients with rheumatoid arthritis, ankylosing spondylitis, and osteoarthritis. Ann Rheum Dis, 61(1), 76–78. doi: 10.1136/ard.61.1.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz A, Joseph L, Mayer C, Harmsen JF, Schrumpf H, Frobel J, … Zilkens C (2018). The role of oxidative and nitrosative stress in the pathology of osteoarthritis: Novel candidate biomarkers for quantification of degenerative changes in the knee joint. Orthop Rev (Pavia), 10(2), 7460. doi: 10.4081/or.2018.7460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasca G, Panico AM, Bonina F, Messina R, Rizza L, Musumeci G, … Cardile V (2010). Involvement of inducible nitric oxide synthase and cyclooxygenase-2 in the anti-inflammatory effects of a red orange extract in human chondrocytes. Nat Prod Res, 24(15), 1469–1480. doi: 10.1080/14786410903169987 [DOI] [PubMed] [Google Scholar]
- Haseeb A, & Haqqi TM (2013). Immunopathogenesis of osteoarthritis. Clin Immunol, 146(3), 185–196. doi: 10.1016/j.clim.2012.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseeb A, Khan NM, Ashruf OS, & Haqqi TM (2017). A Polyphenol-rich Pomegranate Fruit Extract Suppresses NF-kappaB and IL-6 Expression by Blocking the Activation of IKKbeta and NIK in Primary Human Chondrocytes. Phytother Res, 31(5), 778–782. doi: 10.1002/ptr.5799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauselmann HJ, Stefanovic-Racic M, Michel BA, & Evans CH (1998). Differences in nitric oxide production by superficial and deep human articular chondrocytes: implications for proteoglycan turnover in inflammatory joint diseases. J Immunol, 160(3), 1444–1448. [PubMed] [Google Scholar]
- Hellio le Graverand MP, Clemmer RS, Redifer P, Brunell RM, Hayes CW, Brandt KD, … Vignon E (2013). A 2-year randomised, double-blind, placebo-controlled, multicentre study of oral selective iNOS inhibitor, cindunistat (SD-6010), in patients with symptomatic osteoarthritis of the knee. Ann Rheum Dis, 72(2), 187–195. doi: 10.1136/annrheumdis-2012-202239 [DOI] [PubMed] [Google Scholar]
- Henrotin YE, Zheng SX, Labasse AH, Deby GP, Crielaard JM, & Reginster JY (2000). Modulation of human chondrocyte metabolism by recombinant human interferon. Osteoarthritis Cartilage, 8(6), 474–482. doi: 10.1053/joca.1999.0323 [DOI] [PubMed] [Google Scholar]
- Ho LJ, Hung LF, Liu FC, Hou TY, Lin LC, Huang CY, & Lai JH (2013). Ginkgo biloba extract individually inhibits JNK activation and induces c-Jun degradation in human chondrocytes: potential therapeutics for osteoarthritis. PLoS One, 8(12), e82033. doi: 10.1371/journal.pone.0082033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Pan Q, Mao Z, Zhang R, Ma X, Xi Y, & You H (2018). Sinapic Acid Inhibits the IL-1beta-Induced Inflammation via MAPK Downregulation in Rat Chondrocytes. Inflammation, 41(2), 562–568. doi: 10.1007/s10753-017-0712-4 [DOI] [PubMed] [Google Scholar]
- Ialenti A, Moncada S, & Di Rosa M (1993). Modulation of adjuvant arthritis by endogenous nitric oxide. Br J Pharmacol, 110(2), 701–706. doi: 10.1111/j.1476-5381.1993.tb13868.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvinen K, Vuolteenaho K, Nieminen R, Moilanen T, Knowles RG, & Moilanen E (2008). Selective iNOS inhibitor 1400W enhances anti-catabolic IL-10 and reduces destructive MMP-10 in OA cartilage. Survey of the effects of 1400W on inflammatory mediators produced by OA cartilage as detected by protein antibody array. Clin Exp Rheumatol, 26(2), 275–282. [PubMed] [Google Scholar]
- Johnson K, Jung A, Murphy A, Andreyev A, Dykens J, & Terkeltaub R (2000). Mitochondrial oxidative phosphorylation is a downstream regulator of nitric oxide effects on chondrocyte matrix synthesis and mineralization. Arthritis Rheum, 43(7), 1560–1570. doi: [DOI] [PubMed] [Google Scholar]
- Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, & Fahmi H (2011). Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol, 7(1), 33–42. doi: 10.1038/nrrheum.2010.196 [DOI] [PubMed] [Google Scholar]
- Khan NM, Haseeb A, Ansari MY, Devarapalli P, Haynie S, & Haqqi TM (2017). Wogonin, a plant derived small molecule, exerts potent anti-inflammatory and chondroprotective effects through the activation of ROS/ERK/Nrf2 signaling pathways in human Osteoarthritis chondrocytes. Free Radic Biol Med, 106, 288–301. doi: 10.1016/j.freeradbiomed.2017.02.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim PK, Zamora R, Petrosko P, & Billiar TR (2001). The regulatory role of nitric oxide in apoptosis. Int Immunopharmacol, 1(8), 1421–1441. [DOI] [PubMed] [Google Scholar]
- Kim S, Na JY, Song KB, Choi DS, Kim JH, Kwon YB, & Kwon J (2012). Protective Effect of Ginsenoside Rb1 on Hydrogen Peroxide-induced Oxidative Stress in Rat Articular Chondrocytes. J Ginseng Res, 36(2), 161–168. doi: 10.5142/jgr.2012.36.2.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinert H, Pautz A, Linker K, & Schwarz PM (2004). Regulation of the expression of inducible nitric oxide synthase. Eur J Pharmacol, 500(1–3), 255–266. doi: 10.1016/j.ejphar.2004.07.030 [DOI] [PubMed] [Google Scholar]
- Kolios G, Valatas V, & Ward SG (2004). Nitric oxide in inflammatory bowel disease: a universal messenger in an unsolved puzzle. Immunology, 113(4), 427–437. doi: 10.1111/j.1365-2567.2004.01984.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroncke KD, Fehsel K, & Kolb-Bachofen V (1998). Inducible nitric oxide synthase in human diseases. Clin Exp Immunol, 113(2), 147–156. doi: 10.1046/j.1365-2249.1998.00648.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroncke KD, Fehsel K, Suschek C, & Kolb-Bachofen V (2001). Inducible nitric oxide synthase-derived nitric oxide in gene regulation, cell death and cell survival. Int Immunopharmacol, 1(8), 1407–1420. [DOI] [PubMed] [Google Scholar]
- Lepetsos P, & Papavassiliou AG (2016). ROS/oxidative stress signaling in osteoarthritis. Biochim Biophys Acta, 1862(4), 576–591. doi: 10.1016/j.bbadis.2016.01.003 [DOI] [PubMed] [Google Scholar]
- Loeser RF, Carlson CS, Del Carlo M, & Cole A (2002). Detection of nitrotyrosine in aging and osteoarthritic cartilage: Correlation of oxidative damage with the presence of interleukin-1beta and with chondrocyte resistance to insulin-like growth factor 1. Arthritis Rheum, 46(9), 2349–2357. doi: 10.1002/art.10496 [DOI] [PubMed] [Google Scholar]
- Loeser RF, Goldring SR, Scanzello CR, & Goldring MB (2012). Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum, 64(6), 1697–1707. doi: 10.1002/art.34453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud FF, Al-Awadhi AM, & Haines DD (2015). Amelioration of human osteoarthritis symptoms with topical ‘biotherapeutics’: a phase I human trial. Cell Stress Chaperones, 20(2), 267–276. doi: 10.1007/s12192-014-0553-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manacu CA, Martel-Pelletier J, Roy-Beaudry M, Pelletier JP, Fernandes JC, Shipkolye FS, … Moldovan F (2005). Endothelin-1 in osteoarthritic chondrocytes triggers nitric oxide production and upregulates collagenase production. Arthritis Res Ther, 7(2), R324–332. doi: 10.1186/ar1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maneiro E, Lopez-Armada MJ, de Andres MC, Carames B, Martin MA, Bonilla A, … Blanco FJ (2005). Effect of nitric oxide on mitochondrial respiratory activity of human articular chondrocytes. Ann Rheum Dis, 64(3), 388–395. doi: 10.1136/ard.2004.022152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel-Pelletier J, Wildi LM, & Pelletier JP (2012). Future therapeutics for osteoarthritis. Bone, 51(2), 297–311. doi: 10.1016/j.bone.2011.10.008 [DOI] [PubMed] [Google Scholar]
- Mathy-Hartert M, Deby-Dupont GP, Reginster JY, Ayache N, Pujol JP, & Henrotin YE (2002). Regulation by reactive oxygen species of interleukin-1beta, nitric oxide and prostaglandin E(2) production by human chondrocytes. Osteoarthritis Cartilage, 10(7), 547–555. [DOI] [PubMed] [Google Scholar]
- McInnes IB, Leung BP, Field M, Wei XQ, Huang FP, Sturrock RD, … Liew FY (1996). Production of nitric oxide in the synovial membrane of rheumatoid and osteoarthritis patients. J Exp Med, 184(4), 1519–1524. doi: 10.1084/jem.184.4.1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchiorri C, Meliconi R, Frizziero L, Silvestri T, Pulsatelli L, Mazzetti I, … Facchini A (1998). Enhanced and coordinated in vivo expression of inflammatory cytokines and nitric oxide synthase by chondrocytes from patients with osteoarthritis. Arthritis Rheum, 41(12), 2165–2174. doi: [DOI] [PubMed] [Google Scholar]
- Moon SJ, Jeong JH, Jhun JY, Yang EJ, Min JK, Choi JY, & Cho ML (2014). Ursodeoxycholic Acid ameliorates pain severity and cartilage degeneration in monosodium iodoacetate-induced osteoarthritis in rats. Immune Netw, 14(1), 45–53. doi: 10.4110/in.2014.14.1.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- More AS, Kumari RR, Gupta G, Lingaraju MC, Balaganur V, Pathak NN, … Tandan SK (2013). Effect of iNOS inhibitor S-methylisothiourea in monosodium iodoacetate-induced osteoathritic pain: implication for osteoarthritis therapy. Pharmacol Biochem Behav, 103(4), 764–772. doi: 10.1016/j.pbb.2012.12.013 [DOI] [PubMed] [Google Scholar]
- Nathan C, & Xie QW (1994). Nitric oxide synthases: roles, tolls, and controls. Cell, 78(6), 915–918. doi: 10.1016/0092-8674(94)90266-6 [DOI] [PubMed] [Google Scholar]
- Palmer RM, Hickery MS, Charles IG, Moncada S, & Bayliss MT (1993). Induction of nitric oxide synthase in human chondrocytes. Biochem Biophys Res Commun, 193(1), 398–405. doi: 10.1006/bbrc.1993.1637 [DOI] [PubMed] [Google Scholar]
- Pautz A, Art J, Hahn S, Nowag S, Voss C, & Kleinert H (2010). Regulation of the expression of inducible nitric oxide synthase. Nitric Oxide, 23(2), 75–93. doi: 10.1016/j.niox.2010.04.007 [DOI] [PubMed] [Google Scholar]
- Pelletier JP, Jovanovic D, Fernandes JC, Manning P, Connor JR, Currie MG, … Martel-Pelletier J (1998). Reduced progression of experimental osteoarthritis in vivo by selective inhibition of inducible nitric oxide synthase. Arthritis Rheum, 41(7), 1275–1286. doi: [DOI] [PubMed] [Google Scholar]
- Pelletier JP, Jovanovic DV, Lascau-Coman V, Fernandes JC, Manning PT, Connor JR, … Martel-Pelletier J (2000). Selective inhibition of inducible nitric oxide synthase reduces progression of experimental osteoarthritis in vivo: possible link with the reduction in chondrocyte apoptosis and caspase 3 level. Arthritis Rheum, 43(6), 1290–1299. doi: [DOI] [PubMed] [Google Scholar]
- Pelletier JP, Mineau F, Ranger P, Tardif G, & Martel-Pelletier J (1996). The increased synthesis of inducible nitric oxide inhibits IL-1ra synthesis by human articular chondrocytes: possible role in osteoarthritic cartilage degradation. Osteoarthritis Cartilage, 4(1), 77–84. [DOI] [PubMed] [Google Scholar]
- Radomski SB, & Herschorn S (1992). Risk factors associated with penile prosthesis infection. J Urol, 147(2), 383–385. doi: 10.1016/s0022-5347(17)37243-9 [DOI] [PubMed] [Google Scholar]
- Rasheed Z, Akhtar N, & Haqqi TM (2010). Pomegranate extract inhibits the interleukin-1beta-induced activation of MKK-3, p38alpha-MAPK and transcription factor RUNX-2 in human osteoarthritis chondrocytes. Arthritis Res Ther, 12(5), R195. doi: 10.1186/ar3166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridnour LA, Windhausen AN, Isenberg JS, Yeung N, Thomas DD, Vitek MP, … Wink DA (2007). Nitric oxide regulates matrix metalloproteinase-9 activity by guanylyl-cyclase-dependent and -independent pathways. Proc Natl Acad Sci U S A, 104(43), 16898–16903. doi: 10.1073/pnas.0702761104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson WH, Lepus CM, Wang Q, Raghu H, Mao R, Lindstrom TM, & Sokolove J (2016). Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat Rev Rheumatol, 12(10), 580–592. doi: 10.1038/nrrheum.2016.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salerno L, Sorrenti V, Di Giacomo C, Romeo G, & Siracusa MA (2002). Progress in the development of selective nitric oxide synthase (NOS) inhibitors. Curr Pharm Des, 8(3), 177–200. [DOI] [PubMed] [Google Scholar]
- Scanzello CR (2017). Role of low-grade inflammation in osteoarthritis. Curr Opin Rheumatol, 29(1), 79–85. doi: 10.1097/BOR.0000000000000353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanzello CR, & Goldring SR (2012). The role of synovitis in osteoarthritis pathogenesis. Bone, 51(2), 249–257. doi: 10.1016/j.bone.2012.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher JU, Pillinger MH, & Abramson SB (2007). Nitric oxide synthases and osteoarthritis. Curr Rheumatol Rep, 9(1), 9–15. [DOI] [PubMed] [Google Scholar]
- Schmidt N, Pautz A, Art J, Rauschkolb P, Jung M, Erkel G, … Kleinert H (2010). Transcriptional and post-transcriptional regulation of iNOS expression in human chondrocytes. Biochem Pharmacol, 79(5), 722–732. doi: 10.1016/j.bcp.2009.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellam J, & Berenbaum F (2010). The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol, 6(11), 625–635. doi: 10.1038/nrrheum.2010.159 [DOI] [PubMed] [Google Scholar]
- Singh R, Ahmed S, Islam N, Goldberg VM, & Haqqi TM (2002). Epigallocatechin-3-gallate inhibits interleukin-1beta-induced expression of nitric oxide synthase and production of nitric oxide in human chondrocytes: suppression of nuclear factor kappaB activation by degradation of the inhibitor of nuclear factor kappaB. Arthritis Rheum, 46(8), 2079–2086. doi: 10.1002/art.10443 [DOI] [PubMed] [Google Scholar]
- Sophia Fox AJ, Bedi A, & Rodeo SA (2009). The basic science of articular cartilage: structure, composition, and function. Sports Health, 1(6), 461–468. doi: 10.1177/1941738109350438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg WB, van de Loo F, Joosten LA, & Arntz OJ (1999). Animal models of arthritis in NOS2-deficient mice. Osteoarthritis Cartilage, 7(4), 413–415. doi: 10.1053/joca.1999.0228 [DOI] [PubMed] [Google Scholar]
- Vuolteenaho K, Moilanen T, Al-Saffar N, Knowles RG, & Moilanen E (2001). Regulation of the nitric oxide production resulting from the glucocorticoid-insensitive expression of iNOS in human osteoarthritic cartilage. Osteoarthritis Cartilage, 9(7), 597–605. doi: 10.1053/joca.2001.0431 [DOI] [PubMed] [Google Scholar]
- Vuolteenaho K, Moilanen T, Hamalainen M, & Moilanen E (2003). Regulation of nitric oxide production in osteoarthritic and rheumatoid cartilage. Role of endogenous IL-1 inhibitors. Scand J Rheumatol, 32(1), 19–24. doi: 10.1080/03009740310000355 [DOI] [PubMed] [Google Scholar]
- Vuolteenaho K, Moilanen T, Knowles RG, & Moilanen E (2007). The role of nitric oxide in osteoarthritis. Scand J Rheumatol, 36(4), 247–258. doi: 10.1080/03009740701483014 [DOI] [PubMed] [Google Scholar]
- Watt FE, & Gulati M (2017). New Drug Treatments for Osteoarthritis: What is on the Horizon? Eur Med J Rheumatol, 2(1), 50–58. [PMC free article] [PubMed] [Google Scholar]
- Wen L, Qu TB, Zhai K, Ding J, Hai Y, & Zhou JL (2015). Gallic acid can play a chondroprotective role against AGE-induced osteoarthritis progression. J Orthop Sci, 20(4), 734–741. doi: 10.1007/s00776-015-0718-4 [DOI] [PubMed] [Google Scholar]
- Whiteman M, Armstrong JS, Cheung NS, Siau JL, Rose P, Schantz JT, … Halliwell B (2004). Peroxynitrite mediates calcium-dependent mitochondrial dysfunction and cell death via activation of calpains. FASEB J, 18(12), 1395–1397. doi: 10.1096/fj.03-1096fje [DOI] [PubMed] [Google Scholar]
- Wink DA, & Mitchell JB (1998). Chemical biology of nitric oxide: Insights into regulatory, cytotoxic, and cytoprotective mechanisms of nitric oxide. Free Radic Biol Med, 25(4–5), 434–456. doi: 10.1016/s0891-5849(98)00092-6 [DOI] [PubMed] [Google Scholar]
- Xiao Y, Li B, Liu J, & Ma X (2018). Carvacrol ameliorates inflammatory response in interleukin 1beta-stimulated human chondrocytes. Mol Med Rep, 17(3), 3987–3992. doi: 10.3892/mmr.2017.8308 [DOI] [PubMed] [Google Scholar]
- Zhang W, Wang S, Zhang R, Zhang Y, Li X, Lin Y, & Wei X (2016). Evidence of Chinese herbal medicine Duhuo Jisheng decoction for knee osteoarthritis: a systematic review of randomised clinical trials. BMJ Open, 6(1), e008973. doi: 10.1136/bmjopen-2015-008973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Feng Z, Lou Y, Chen C, Zhang C, Tao Z, … Ying X (2017). Silibinin protects against osteoarthritis through inhibiting the inflammatory response and cartilage matrix degradation in vitro and in vivo. Oncotarget, 8(59), 99649–99665. doi: 10.18632/oncotarget.20587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo Q, Yang W, Chen J, & Wang Y (2012). Metabolic syndrome meets osteoarthritis. Nat Rev Rheumatol, 8(12), 729–737. doi: 10.1038/nrrheum.2012.135 [DOI] [PubMed] [Google Scholar]
- Ziskoven C, Jager M, Kircher J, Patzer T, Bloch W, Brixius K, & Krauspe R (2011). Physiology and pathophysiology of nitrosative and oxidative stress in osteoarthritic joint destruction. Can J Physiol Pharmacol, 89(7), 455–466. doi: 10.1139/y11-055 [DOI] [PubMed] [Google Scholar]


