Abstract
Osteoarthritis (OA) is the most prevalent joint degenerative disease leading to irreversible structural and functional changes in the joint and is a major cause of disability and reduced life expectancy in ageing population. Despite the high prevalence of OA, there is no disease modifying drug available for the management of OA. Oxidative stress, a result of an imbalance between the production of reactive oxygen species (ROS) and their clearance by antioxidant defense system, is high in OA cartilage and is a major cause of chronic inflammation. Inflammatory mediators, such as interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) are highly upregulated in OA joints and induce ROS production and expression of matrix degrading proteases leading to matrix degradation and joint dysfunction. ROS and inflammation are interdependent, each being the target of other and represent ideal target/s for the treatment of OA. Plant polyphenols possess potent antioxidant and anti-inflammatory properties and can inhibit ROS production and inflammation in chondrocytes, cartilage explants and in animal models of OA. The aim of this review is to discuss the chondroprotective effects of polyphenols and modulation of different molecular pathways associated with OA pathogenesis and limitations and future prospects of polyphenols in OA treatment.
Keywords: Osteoarthritis, Polyphenols, Inflammation, Redox, Nrf2, Chondrocytes
Graphical Abstract
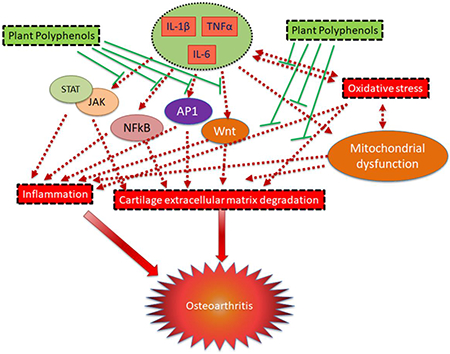
1.0. Introduction
Osteoarthritis (OA) is the most common joint disease and a major cause of disability and reduced life expectancy in the ageing population [1, 2]. OA is a multifactorial disease with etiology ranging from normal ageing process, sex, genetic background and obesity to physical factors which include trauma and injury to the joints and a strong interplay between all these factors. OA pathogenesis is modulated by genetic and environmental factors in association with the activation of molecular and cellular pathways that participate in the advancement of joint injury. Thus, OA is not a single disease, rather it is the final stage of joint failure, the initial stage of which could be triggered and propagated by injury to cartilage, ligaments or other joint tissues (post-traumatic OA) or other causal factors. The diseased joint is characterized by synovial inflammation, oxidative stress, apoptosis in chondrocytes, cartilage extracellular matrix degradation, subchondral bone sclerosis and osteophyte formation leading to stiffness of the whole joint, pain and joint failure (Figure 1). To date, there is no disease-modifying therapy available for the treatment of OA due to poor understanding of the disease pathogenesis. Further understanding of the molecular and cellular pathways and their association with joint tissues is necessary to develop new therapeutic approaches for the prevention and treatment of OA. The currently available therapies for the management of OA include non-steroidal anti-inflammatory drugs (NSAIDs) which only provide temporary relief and neither prevents cartilage degradation nor have any effect on the reversal of cartilage degeneration. In addition, these agents have adverse side effects and toxicity [3, 4]. These limitations demand the development/invention of new therapeutic approaches which have little to no side effects and in addition to anti-inflammatory and analgesic effects also improve the cartilage structure and reverse the cartilage destruction to improve overall joint health. Recent studies have shown that joint inflammation and oxidative stress is directly associated with OA progression [5, 6]. The purpose of this review is to highlight the contribution of oxidative stress and inflammation in OA pathogenesis and summarize the therapeutic potential of polyphenols for OA.
Figure 1:
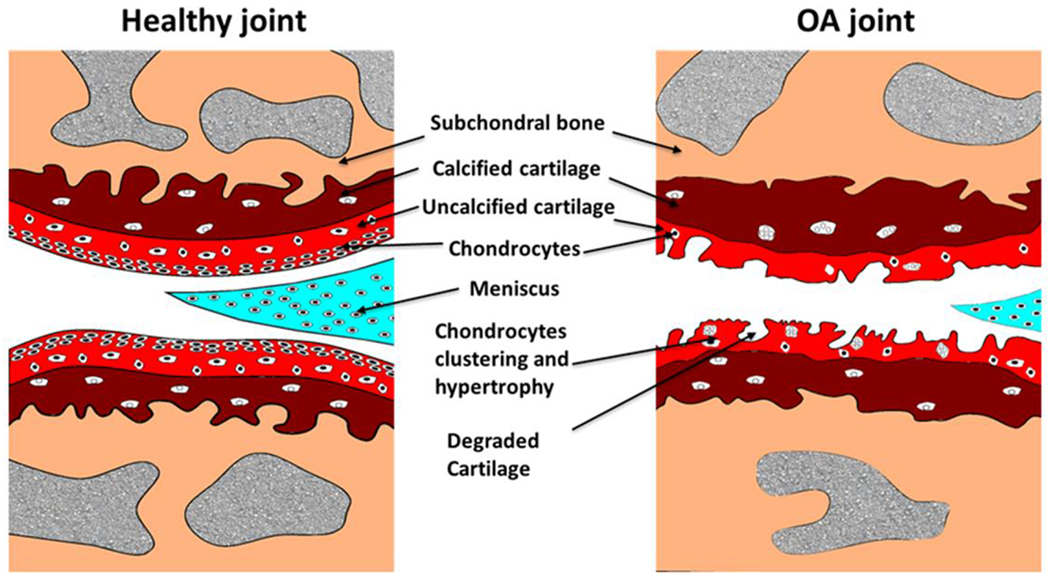
Schematic representation of normal and OA knee joint. The healthy joint (on left) has smooth cartilage surface with normal chondrocyte distribution and OA joint shows cartilage degeneration and subchondral bone changes.
2. Oxidative Stressing in Osteoarthritis
Reactive oxygen species (ROS) are oxygen containing free radicals including hydrogen peroxide (H2O2), hydroxy radical (OH−), superoxide anion (O2−) and nitric oxide (NO) and have unpaired electron which makes them unstable and highly reactive. ROS is normally produced in cells at low levels and is essential for the maintenance of cellular homeostasis and function [7]. However, the imbalance in this physiological mechanism leads to increased expression of inflammatory cytokines and chemokines, which causes oxidation of cellular macromolecules such as proteins, lipids and DNA altering their function. The major sites of ROS production include mitochondria, peroxisomes and other membranous structures containing NADPH oxidases (NOXs), Xanthine Oxidase (XO) and Nitric Oxide Synthase (NOS) [8]. It is estimated that approximately 2-3% of O2 consumed in mitochondria during oxidative phosphorylation is converted to O2− rather than to water [9]. NOX complex is consist of 3 cytosolic (p40phox, p47phox and p67phox) and 2 membrane associated (p22phox and gp91phox) protein components. Under pathological conditions, cytosolic units translocate to inner surface of plasma membrane and form fully active enzyme complex leading to increased production of ROS. NOX components are expressed in chondrocytes, the only resident cell type of the cartilage, and are the major producers of ROS [10, 11]. XO produces H2O2 during oxidation of hypoxanthine to xanthine. The evidence of high levels of ROS production in OA cartilage comes from either chondrocytes isolated from end stage diseased cartilage or from the presence of lipid peroxidation and nitrosylation products in synovial fluids and in the cartilage [12, 13]. There are three isoforms of NOS, (1) the constitutive isoform mainly expressed in neuronal cell, neuronal NOS (nNOS), (2) endothelial NOS (eNOS) and (3) inducible NOS (iNOS). The nNOS and eNOS require calcium and calmodulin and produce a very low amounts of NO. The iNOS is the inducible form of NOS induced by inflammatory cytokines and produces relatively high amounts of NO and require low concentration of calcium for its activity. Reactive nitrogen species (RNS) are molecules derived from O2− and NO and cause damage to the cell by inducing nitrosative stress. iNOS expression is highly upregulated in chondrocytes in response to inflammatory cytokines stimulation such interleukin-1β (IL-1β), tumor necrosis factor α (TNFα), interferon-γ (IFN-γ) and IL-17 etc. [14–17]. Regardless of the source of production, NO may react with cellular proteins causing their nitrosylation which alter their normal function [18, 19]. NO has been reported to increase inflammation by activating nuclear factor kappa B (NFκB) pathway causing increased production of IL-1β and TNFα [20]. Under pathological conditions, excessive amounts of ROS function as secondary messengers and promote cartilage degradation by inducing the expression of matrix degrading proteases, reducing extracellular matrix (ECM) synthesis and inducing chondrocyte apoptosis.
Under conditions of increased ROS production, the cellular defense mechanism against oxidative stress gets activated and efficiently removes the ROS molecules from the cell. The cellular antioxidant defense system includes various enzymes, such as catalases, peroxiredoxins (Prxs), glutathione peroxidase (GPx), NADPH ubiquinone oxidoreductase (NQO1) and superoxide dismutases (SODs) and nonenzymatic such as glutathione (GSH), ascorbic acid (vitamin C), α-tocopherol (vitamin E) etc. [9, 21]. SODs protect against oxidative stress by converting the O2− to H2O2, which is further eliminated by Prx, GPx and catalases. There are three isoforms of SODs, cytosolic SOD (SOD1 or Cu/Zn SOD), mitochondrial SOD (SOD2 or MnSOD) and extracellular SOD (SOD3 or EC-SOD) which is secreted outside the cell [22]. Prxs protect against H2O2 mediated oxidation of proteins by accepting the nascent oxygen at its thiol active site [23]. Catalases provide protection against oxidative stress by converting H2O2 to water and oxygen molecules. GPx protects membrane lipid oxidation by H2O2 by oxidizing GSH [24]. The expression of antioxidant defense system proteins including SOD, catalase and Gpx are downregulated in OA joints showing imbalance in redox in OA cartilage [25, 26]. Various in vitro and in vivo studies have shown that upregulation of cellular antioxidant defense system in chondrocytes suppresses the expression of catabolic genes and improves joint health. Nuclear factor (erythroid-derived 2)-like 2 (Nrf2), a master transcription factor regulator of the cellular antioxidant defense system, expression is dysregulated in OA and its deletion resulted in enhanced disease development in a mouse model of destabilization of medial meniscus (DMM) induced OA [27] which suggest a potential role of antioxidant defense system in the protection against OA. In a study, Prx3 (mitochondrial Prx) was reported to be hyperoxidized in aged and OA human cartilage indicating increased oxidative stress [28]. OA chondrocytes treated with Menadione showed high levels of oxidized Prx3 which was associated with decreased pro-survival signaling (Akt) and increased pro-death signaling (p38)[28]. Interestingly, mitochondria targeted expression of catalase (MCAT) suppressed the Menadione induced catabolic effects in chondrocytes and suppressed age-related progression of OA in a mouse model [28]. The scavenger of mitochondrial superoxide, SOD2 is downregulated in human and mouse OA cartilage [29]. Mitochondrial dysfunction or deregulation of SOD2 expression may lead to excessive ROS production which may cause irreversible damage to the chondrocytes and induce cell death by apoptosis or necrosis [9]. We have shown that autophagic clearance of dysfunctional mitochondria and suppression of ROS is essential for the survival of chondrocytes under pathological condition [30]. NO and its derivative have also been reported to increase the damage to cartilage during OA development [6, 20, 31] and targeting of NO was found to suppress the progression of OA in a mouse model of experimental OA [17]. NO is produced in chondrocytes in a two-step conversion process of L-Arginine to L-Citrulline which is catalyzed by iNOS whose expression is highly upregulated in OA cartilage and in chondrocytes under pathological conditions [32].
Oxidative stress is the result of excessive production of ROS, which is beyond the capacity of the cellular antioxidant defense system to effectively remove from the cells. Overproduction of ROS and induction of oxidative stress in chondrocytes are one of the major contributors to OA pathogenesis [6, 9, 33, 34]. Many studies have shown that the ROS levels are highly upregulated in the human OA cartilage and chondrocytes [30, 34–36]. We have shown earlier in an in vitro study that stimulation of primary human OA chondrocytes and mouse chondrocytes with IL-1β increases the production of cellular and mitochondrial ROS which promotes chondrocyte apoptosis [30] mimicking the in vivo condition observed in OA cartilage [37]. Exposure of chondrocytes with H2O2, Menadione, 3-morpholinosydnonimine (SIN1), tert-butyl hydroperoxide (TBHP) or other pro-oxidants have been reported to increase inflammation and apoptosis [28, 38] showing that oxidative stress induces inflammation in chondrocytes. Increase in oxidative stress positively correlates with collagen degradation [34] suggesting a role of ROS in cartilage matrix catabolism. In addition, in different studies, NO and H2O2 have been reported to suppress proteoglycan synthesis showing the role of ROS in suppressing cartilage matrix anabolism [6]. Taken together these studies show that oxidative stress has a detrimental effect on joint health and function and targeting these pathways might be of therapeutic importance for the management of OA.
3. Inflammation in Osteoarthritis
Inflammation is a necessary cellular response in the fight against infection, however, chronic, unregulated inflammation is associated with the pathophysiology of several human diseases including neurological diseases, obesity, diabetes, autoimmune disease, cancer and rheumatoid arthritis [39, 40]. Recent studies show increased levels of proinflammatory cytokines and chemokines in the synovial fluids of end stage OA patients [41–43]. Several studies with animal models of OA also show high levels of inflammation in experimental OA joints and support the idea of anti-inflammatory approach of treatment of OA.
Chondrocytes are normally quiescent cells, however, during unfavorable conditions, get activated and produce a plethora of proinflammatory cytokines and chemokines that increase the expression of collagenases and aggrecanases leading to cartilage ECM degradation [44, 45]. Chondrocytes also express receptors for several of proinflammatory cytokines and chemokines. Thus, chondrocytes are the source as well as the target of proinflammatory cytokines in OA. TNFα, IL-1β and IL-6 represent the three highly expressed cytokines in OA joints and are actively produced by chondrocytes, synoviocytes, macrophages and osteoblast and play a critical role in the degeneration of articular cartilage matrix which makes them primary therapeutic target. Other than TNFα, IF-1β and IF-6, several other cytokines (such as IF-17, IF-18, MCP1, CXCF5, RANTES etc., see table 1), have also been reported to be associated with OA pathogenesis and may be targeted for therapeutic strategies [46–49] but the data is limited at this stage. Increase in the levels of the cytokines in joints play a central role in the pathogenesis of OA by modulating oxidative stress, cartilage ECM turnover and chondrocytes apoptosis [50].
Table 1:
Cytokines and chemokines and their role in the pathogenesis of OA
| Cytokine | Role in OA | Reference |
|---|---|---|
| IL-1β | Increased in OA joint synovial fluid, cartilage, synovial membrane and subchondral bone. It increases the production of iNOS, COX-2, IL-6, TNF-α IL-8, MCP1, RANTES and the levels of PGE2 and NO in chondrocytes and in cartilage explants. Increases the levels of matrix degrading proteases MMP-1, MMP-3, MMP-9, MMP-13, ADAMTS-4 and ADAMTS-5 and matrix degradation. Suppresses the synthesis of type II collagen and aggrecan and proteoglycan. |
[52, 74, 86] |
| TNF-α | Increased in OA joint synovial fluid, cartilage, synovial membrane and subchondral bone. It increases the production of iNOS, COX-2, IL-6, IL-8, MCP1, RANTES and the levels of PGE2 and NO in chondrocytes and in cartilage explants. Increases the levels of matrix degrading proteases MMP-1, MMP-3, MMP-9, MMP-13, ADAMTS-4 and ADAMTS-5 and matrix degradation. Suppresses the synthesis of type II collagen and aggrecan. |
[61] |
| IL-6 | Increased in OA joint synovial fluid, cartilage, synovial membrane and serum of OA patients. Upregulates MMP-13 expression in chondrocytes. Downregulates the expression of type II collagen. |
[66, 70] |
| IL-15 | Increased in the synovial fluids of OA joints. Is associated with joint pain. |
[48] |
| IL-17 | Increased in the synovial fluids of OA joints. Induces IL-1β, TNF-α and IL-6 expression and suppresses proteoglycan synthesis. |
[14, 46] |
| IL-18 | Increased in the OA joints cartilage and synovial fluid. Increases the production of MMP-1, MMP-3, MMP-13. |
[47] |
| LIF | Increased in the synovial fluids of OA joint. Enhances cartilage extracellular matrix degradation. Increases matrix degrading proteases expression and nitric oxide levels. |
[49, 139] |
| MCP1 | Increased in OA joint tissue and in chondrocytes under pathological conditions | [54] |
| RANTES | Increased in OA joint tissue and in chondrocytes under pathological conditions | [54] |
| IL-8 | Increased in OA joint tissue and in chondrocytes under pathological conditions | [54, 70] |
| IL-4 and IL-10 | Anti-inflammatory. Increase the expression of IL-IRa and TIMP and decrease IL-1β, TNF-α expression. |
[46, 54] |
Stimulation of primary chondrocytes and cartilage explants with IF-1β and TNFα mimic the in vivo pathological conditions by upregulating the expression of catabolic genes including IF-6, COX-2, iNOS, collagenases [matrix metalloprotease 13 (MMP-13)] and aggrecanases [a disintegrin and metalloproteinase with thrombospondin motif (ADAMTSs)] and by down regulating the expression of anabolic genes such as aggrecan and type II collagen [51–55]. Animal studies with ADAMTS4 [56] and ADAMTS5 [57] knockout mouse models show that ADAMTS5 is the major aggrecanase associated with OA pathogenesis and catalytically is multiple fold more active than ADAMTS4 [58]. IF-1β mediated activation of cells is through binding and activation of specific cell surface receptor, IF-1 receptor type I (IF-1RI). IF-1RI expression is highly upregulated in OA chondrocytes compared to normal chondrocytes [59]. Many cell types including chondrocytes express natural competitive inhibitor of IF-1β, IF-1 receptor antagonist (IF-IRa) which binds to IF-1RI but does not transduce a signal and has anti-inflammatory properties [60]. TNFα functions as ligand for two specific receptors [TNF receptor I (TNFRI) and TNFRII] which are expressed on cell membrane on various cell types including chondrocytes. Compared to normal chondrocytes, OA chondrocytes express high levels of TNFRI which is the dominant receptor for TNFα [61]. Intraarticular injection of either of TNFα or IL-1β into rabbit knee joints triggered the progression of OA which was augmented upon combined injection [62]. In another study, deletion of IL-1β was reported to reduce the severity of DMM induced OA in a mouse model [63]. However, deletion of IL-1β or IL-1β converting enzyme the usual suspects in OA pathogenesis, accelerated the development of experimental OA in mouse model showing that complete deletion of IL-1β or IL-1β converting enzyme augments the pathogenesis of OA [64, 65]. These results show that the proinflammatory cytokines, which appear to play pathogenic role in the development of OA, are also important for the maintenance of chondrocyte homeostasis and joint health and a fine balance of these inflammatory mediators is required for normal functioning of the joint and knowledge in this area is far from complete.
IL-6 signaling involves many components including a multimeric receptor complex consist of membrane bound IL-6 receptor (IL-6R), soluble IL-6 receptor (sIL-6R) and gpl30. Normal chondrocytes express very low levels of IL-6, whose expression is highly upregulated upon treatment with proinflammatory cytokines such as TNFα or IL-1β [66–68]. Treatment of cartilage explants with IL-6 upregulated the expression of MMP-13 [69]. Expression of levels of IL-6 and sIL-6R is increased in the synovial fluid of OA patients [70]. We found increased expression of IL-6 in chondrocytes of the damaged area of human OA cartilage [66]. Suppression of IL-6 expression in Zcchc6 knockout mice reduced the severity of experimental OA in a mouse model of post-traumatic OA[67]. Antibody mediated neutralization of systemic levels of IL-6 or small molecule inhibitor of STAT3 signaling (downstream signaling pathway of IL-6 mediated receptor activation) was reported to ameliorate cartilage degradation in a DMM induced OA mouse model [71]. Intraarticular injection of IL-6 protein promoted cartilage destruction in a mouse model [72]. However, in a study using IL-6 knockout mouse, the severity of age-related OA in male mice was significantly increased, but not in female mice [73] suggesting that low levels of IL-6 may be required to maintain chondrocyte homeostasis and play a protective role at some stage, at least in age-related OA.
4. Major signaling pathways in OA pathogenesis
The expression of proinflammatory cytokines, cyclooxygenase, iNOS, MMPs and other proteinases in chondrocyte is tightly regulated by inflammatory pathways, including the three (ERK, JNK and p38) mitogen activated protein kinases (MAPK), NFκB, API, JAK/STAT and Wnt pathway. The pathological effects of ROS, IL-1β, TNFα and IF-6 in chondrocytes and in cartilage are due to the activation of various proinflammatory signaling pathways (Figure 2). Stimulation of chondrocytes with IF-1β initiates a cascade of events leading to the activation of p38-, JNK- and ERK-MAPK, PKC, increase in the intracellular Ca+2 and nuclear translocation of NFκB, API, STATs and ATFs [45, 51, 54, 74–76]. Activation of NFκB signaling pathway mediates the upregulation of several inflammatory cytokine and chemokine genes, iNOS and COX-2 and increased expression of cartilage ECM degrading proteases such as MMP-1, MMP-9, MMP-13, ADAMTS4, and ADAMTS5 [77]. Recent studies have established a significant role of Wnt/β-catenin signaling in OA pathogenesis [78]. The expression of Wnt signaling mediators such as Wnt ligands and β-Catenin is upregulated in OA cartilage [79]. Activation of Wnt/β-Catenin signaling in chondrocytes augmented IF-1β induced expression of MMPs and ADAMTSs [80]. Intraarticular injection of a small molecule inhibitor of Wnt/β-Catenin signaling pathway reduced the severity of experimental OA in a mouse model [81]. ROS molecules function as intermediate signaling molecules in multiple signaling pathways. Stimulation of chondrocytes with IF-1β increases the production of ROS which induces mitochondrial dysfunction and may augment the IF-1β induced production of ROS [30, 45]. Increased ROS levels activate redox sensitive transcription factors such as AP1 and contribute to the proinflammatory phenotypic alterations in chondrocytes including the expression of IF-6, COX-2, iNOS and their products PGE2 and NO. In addition, increased prooxidant load can also suppress proteoglycan synthesis by inhibiting the PI3/Akt signaling and activating MEK/ERK signaling pathway [82]. TNFα increased the expression of cFos/AP1 via NADPH oxidase mediated production of ROS in bovine chondrocytes [83]. IF-1β induced expression of cFos and MMP-1 in chondrocytes depends on ROS production [84]. Treatment of chondrocytes with prooxidant TBHP activated the MEK/ERK pathway [82]. Activation of JNK in chondrocytes by IF-1β and TNFα is dependent on the production of ROS indicating a potential role of ROS in OA pathogenesis [85]. In a recent study we have shown that IL-1β induced the activation of cFos/AP1 and upregulated the expression of IL-6 and MMP-13 [68]. These findings suggest that ROS mediated inflammation is induced via activation cFos/AP1 pathway. In addition, direct stimulation of chondrocytes with H2O2 or NO activated JNK pathway indicating that JNK is the major target of ROS mediated inflammatory response [85]. Deletion of JNK prevented the increase in the expression of IL-1 and TNF, IL-6, IL-18 and ADAMTS4 in a DMM mouse model of OA [86].
Figure 2:
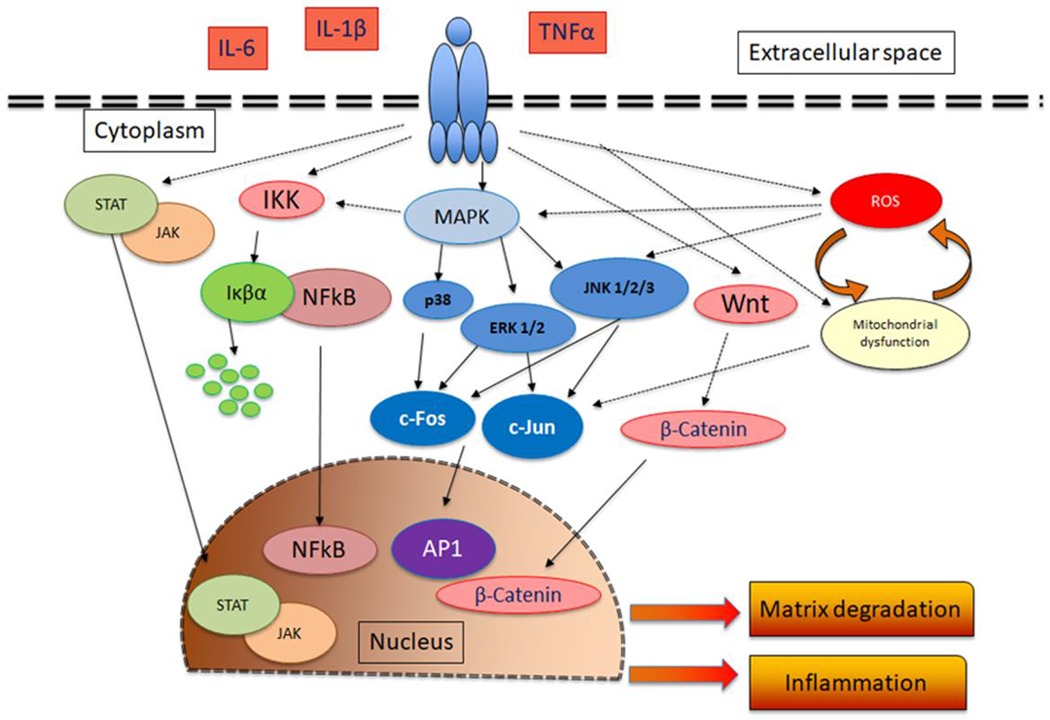
Schematic representation of the major signaling pathways activated by proinflammatory cytokines (IL-1β, TNFα and IL-6) in chondrocytes and their downstream effects. Stimulation of chondrocytes with IL-1β, TNFα and IL-6 leads to the activation of JAK/STAT, MAPK, AP1, NFκB and Wnt signaling pathways These cytokines also modulate mitochondrial function and ROS production. The activation of these pathways lead to increased expression of inflammatory molecules, matrix degrading proteases in chondrocytes.
5.0. Targeting oxidative stress and inflammation with plant polyphenols
Polyphenols are secondary metabolites produced by almost every part of plants, including fruits, flowers, leaves and bark. Many common fruits (such as grapes, cherries, apples, pomegranate, oranges), herbs and spices are very rich source of polyphenols. These compounds have potent anti-inflammatory and antioxidant properties. The antioxidants property of polyphenols depend on a polyphenol’s ability to scavenge ROS molecules, inhibit the expression of prooxidant genes and increase the expression of antioxidant genes such as SODs, catalases [87–89]. The anti-inflammatory activity of polyphenols depends on their ability to suppress pro-inflammatory signaling pathways such as MAPK, API and NFκB. Several studies have demonstrated a potential OA suppressing activity of polyphenolic compounds which depend mainly on their antioxidant and anti-inflammatory properties [87, 89]. The anti-inflammatory and antioxidant effects of many polyphenols including pomegranate extract, Butein, green tea polyphenol, EGCG, Resveratrol, Wogonin, Quercetin, Harpagoside, Curcumin, Morin and several others have been tested in in vitro and in vivo models of OA.
We have shown earlier that Butein, a chalcone, rich extract of Butea monosperma flowers and purified Butein showed potent antioxidant property and suppressed the expression of IL-6 and metalloproteases by enhancing autophagy in chondrocytes via modulation of AMPK/mTOR signaling pathway [66, 90]. Butein activates AMPKα by inducing the phosphorylation of AMPKαThr-172and suppresses mTOR activity by reducing the phosphorylation of mTORSer-2448 [66, 90]. We have also showed that an extract of Scutellaria baicalensis and purified Wogonin suppresses the IL-1β induced expression of IL-6, COX-2, iNOS and metalloproteases and reduces the production of PGE2 and NO [45, 91]. Wogonin increases the expression and activity of Nrf2, the master transcription regulator of antioxidant defense enzymes and increased the expression of HOI providing resistance against IL-1β induced oxidative stress in primary human chondrocytes [45, 91]. Harpagoside, an iridoid, suppressed IL-1β induced expression of MMP-13 and a plethora of proinflammatory cytokines and chemokines including IL-6 through the inhibition of cFos/AP-1 signaling pathway and independent of c-Jun and NFκB pathway in primary human OA chondrocytes [68]. Harpagoside when given in combination with glucosamine hydrochloride, chondroitin sulfate, methylsulfonyl methane and bromelain extract showed protective effect in formalin induced rat OA model by suppressing the expression of IL-1β and TNF-α [92].
Pomegranate (Punicagranatum) fruit extract (PFE) which is rich in gallic acid, ellagic acid and punicalagin polyphenols suppressed IL-1β induced expression of MMP-1, −3 and −13 and COX-2 expression in primary human chondrocytes through the inhibition of p38-MAPK and JNK-MAPK and their downstream transcription factors, cJun and ATF2 [93, 94]. PFE also suppressed NFκB activation by preventing the phosphorylation of IκBα [93, 94]. PFE was found to inhibit the activation of RUNX-2 transcription factor via the inhibition of MKK3/p38α-MAPK signaling pathway [75]. Delphinidin, an active constituent of pomegranate fruit, suppressed IL-1β induced expression of COX-2 and PGE2 production through the inhibition of NFκB-inducing kinase (NIK) and IL-1 receptor-associated kinase-1 (IRAKI) mediated activation of NFκB pathway in human OA chondrocytes [53].
Green tea polyphenol, Epigallocatechin 3-gallate (EGCG), a catechin, shows potent anti-inflammatory properties which depends on its ability to suppress the IL-1β induced expression of inflammatory mediators in primary human chondrocytes and cartilage explants [16, 54]. EGCG suppressed the expression of several proinflammatory cytokines and chemokines including IL-1β, IL-6, IL-7, TNFα, LIF, MCSF, Oncostatin M, MCP-1, MCP-2 and IL-8 in primary human chondrocytes through the inhibition of NFκB and MAPK signaling pathway [16, 54]. EGCG suppressed the advanced glycation end products induced expression of TNFα and MMP-13 in primary human chondrocytes via the inhibition of ρ38-, JNK- and ERK-MAPK [74, 95]. Intraarticular injection of EGCG in a collagenase-induced arthritis model in rats suppressed inflammation [96]. Intraperitoneal injection of EGCG in a DMM induced OA mouse model was shown to exert chondroprotective effects by reducing the expression levels of IL-1β, TNFα and metalloproteases [97]. In another in vitro study, EGCG was reported to suppress the expression of inflammatory mediators in human fibroblast-like synoviocytes [98].
Quercetin (3,3’,4’,5,7-pentahydroxy-flavone), a flavonoid found in many common fruits and vegetables such as onion, has strong antioxidant activity and reduced the levels of ROS by increasing the expression of glutathione and glutathione peroxidase in a rat model of OA [99]. An extract of Ginkgo biloba leaves enriched in Quercetin has anti-inflammatory activity and suppressed IL-1β and LPS induced expression of iNOS, COX-2 and NO and PGE2 production in human OA chondrocytes and in a rat model of OA [100]. Intraperitoneal injection of Quercetin suppressed the oxidative stress and reduced the severity of OA in a rat model through the activation of SIRT1/AMPK pathway [101]. Intraarticular injection of Quercetin mixed with thermosensitive hydrogel suppressed the cartilage degradation and slowed the progression of OA in a rat model [102]. In another study, intraarticular injection of Quercetin was shown to suppress inflammatory response in a rat model of OA by inhibiting Akt/NFκB signaling pathway [103].
Morin, a flavanol, found in members of Moraceae family, increased the expression of HO1 and suppressed the IL-1β induced oxidative stress in chondrocytes via activation of the transcription factor Nrf2 [104]. Morin also suppressed the IL-1β induced expression of MMP-13, iNOS and COX-2 and their product NO and PGE2 in chondrocytes through the inhibition of NFκB signaling pathway [104, 105]. In another study, Morin suppressed the IL-1β induced expression of MMP-3 and MMP-13 and upregulated the expression of TIMP-1 through the suppression of JNK-, p38- and ERK-MAPK signaling pathway [106]. Oral administration of Morin slowed the progression of ACLT induced OA in a rat model [106].
Curcumin, a phenylpropanoids and the major constituent of turmeric, is a common spice and has been widely shown to have potent anti-inflammatory properties. The chondroprotective effect of curcumin has been shown in several in vitro and in vivo studies using chondrocytes, cartilage explants and various animal models [107–109]. Oral administration of Curcumin and tetrahydrocurcumin suppressed the expression of IL-1β, IL-6 and metalloproteases and alleviated the pain and cartilage degeneration in a rat [107] and mouse [108] model of experimental OA. Another study showed that chemically modified curcumin suppressed inflammation and slowed the progression of OA in an ACLT induced OA model in rabbit [110]. Ferulic acid, a derivative of curcumin and a component of the cell walls of various plants including oats, rice and the seeds of orange and apples, possess strong anti-inflammatory and antioxidant properties and was reported to suppress H202 induced expression of TNFα and IL-1β [111].
Resveratrol (trans-3,4’,5-trihydroxystilbene), a phytoalexin, found in the skin of red grapes has been shown to have potent antioxidant and anti-inflammatory activity [112]. Intra-articular injection of Resveratrol in ACLT induced OA in rabbit suppressed the expression of iNOS and production of NO [113]. Resveratrol also suppressed the IL-1β, TNF-α and IL-6 expression levels in rats with experimental OA [114]. In another study, Resveratrol reduced AGEs induced expression of iNOS, COX-2 and MMP-13 in chondrocytes via the inhibition of NFκB and API signaling pathways [115]. Resveratrol was found to activate SIRT1 in chondrocytes and blocked NFκB activation and suppressed IL-1β induced expression of iNOS in human chondrocytes [116]. Resveratrol activated SIRT1 and suppressed IL-1β induced expression of HIF-2α in human chondrocytes and intraarticular injection of resveratrol slowed the progression of experimental OA in a mouse model of OA [117]. Resveratrol also protected rabbit chondrocytes against sodium nitroprusside induced apoptosis by scavenging the SNP induced ROS and NO [118]. Resveratrol induced the expression ofHO-1 via the activation ofNrf2 and suppressed oxidative stress in rat with OA [114]. Oral administration of Resveratrol suppressed inflammation via the inhibition of TLR4 signaling and ameliorated high fat diet induced OA [119].
Olive oil is a rich source of polyphenols and is extensively used in Mediterranean diet [120]. Several in vitro and in vivo studies using olive oil have been reported to improve joint health and function [121, 122]. Hydroxytyrosol, a polyphenol found in olive oil, activates autophagy and prevents chondrocyte’s death [123]. Oral uptake of extra virgin olive oil rich diet has anti-inflammatory effects and suppressed IL-6 expression and upregulated the expression of lubricin in a rat model of ACLT induced OA [124, 125]. These and other studies provide support to the use of Olive oil containing diets as a possible approach to maintain healthy joint function.
In addition to the above mentioned plant-derived compounds, several other polyphenols were shown to suppress oxidative stress and inflammation in chondrocytes and alleviated OA pathogenesis. We showed recently that Imperatorin, a secondary metabolite found in the plants of Apiaceae and Rutaceae family members suppressed the expression of iNOS and NO production by suppressing ERKl/2-AP1(cFos/cJun) pathway [32]. We also showed that Imperatorin can bind to iNOS and suppress its enzymatic activity. In an in vitro study, Genistein, an isoflavone, suppressed the LPS and IL-1β induced expression of COX-2, iNOS and NO production in chondrocytes [126]. An aqueous extract of Java tea (Orthsiphonstaminens) suppressed inflammation in cartilage explant and reduced the severity of OA in monosodium iodoacetate (MIA) induced OA in rat [127]. Olive and grape seed extracts enriched in hydroxytyrosol and procyanidins (HT/PCy) suppressed the expression of iNOS, COX-2 and metalloproteases in chondrocytes stimulated with IL-1β and showed chondroprotective effects in post-traumatic models of OA in mice and rabbits [128]. In an in vivo study using guinea pig model of spontaneous OA, Oleuropein enriched diet significantly suppressed the synovial inflammation and serum levels of PGE2 [129]. Chlorogenic acid treatment inhibited IL-1β induced expression of iNOS and COX-2 and suppressed the production of PGE2 and NO in human chondrocytes [130]. Chlorogenic acid enriched butanol extract of WIN-34B inhibited the expression and production of inflammatory mediators TNFα, IL-1β, PGE2 and NO in human cartilage explant and chondrocytes through the inhibition of IL-1β induced JNK-, p38-MAPK signaling pathway [131]. Chlorogenic acid enriched aqueous extract of Anthriscnssylvestris leaves were shown to suppress the expression of inflammatory mediators, such as, iNOS and COX-2 and production of NO and PGE2 in rat primary chondrocytes via the inhibition of MAPK and NFKB pathway [132]. Oral uptake of Pycnogenol was reported to reduce joint pain and other symptoms of OA [133, 134]. Stachydrine prevented IL-1β induced expression of IL-6, COX-2 and iNOS in chondrocytes through the inhibition of NFκB pathway [135]. We have shown earlier that an extract from cat’s claw (Uncaria guianensis) suppressed IL-1β induced production of NO through the upregulation of insulin like growth factor-1 [136]. Apigenin was reported to block the IL-1β induced NFκB and Smad2/3 pathway in SW1353 chondrosarcoma cells [137]. Ginger extract suppressed IL-1β induced expression of TNFa, IL-6 and IL-8 through the inhibition of p38-MAPK, JNK-MAPK pathway using cartilage explants and primary human chondrocytes [138].
6.0. Conclusions and Future Studies
Oxidative stress and inflammation in chondrocytes and other joint tissues are associated with the progression and severity of the disease making them ideal targets for its management. Recent studies have provided new insights and have increased our understanding of the molecular pathways involved in the pathogenesis of OA. The studies discussed in this review show that polyphenolic compounds, such as EGCG, Butein, Wogonin, Resveratrol, Curcumin have strong anti-inflammatory and antioxidant properties and exert chondroprotective effects in chondrocytes and cartilage explants cultures and animal models of OA. These polyphenols have been shown to scavenge ROS and activate the antioxidant defense system in chondrocytes and suppressed inflammation by inhibiting pro-inflammatory signaling pathways. Future studies focused on the delivery of therapeutic amounts of polyphenolic compounds to the affected joints, which is a major limitation associated with OA treatment, are required to increase the treatment efficacy and improve joint health and function. In summary, the recent findings have shown that plant polyphenols have the potential to be developed as an effective therapy for the management of OA. Randomized clinical trial studies with polyphenols and with large number of volunteers are required to fully establish the chondroprotective role of polyphenols.
Highlights.
Here, we discussed the role of oxidative stress and inflammation in OA pathogenesis.
Here, we discussed the antioxidant and anti-inflammatory activities of polyphenols.
Polyphenolic compounds suppress oxidative stress and inflammation in OA joints.
Polyphenols inhibit the activation of key signaling pathways in OA pathogenesis.
We discuss here the possibility of development of polyphenol(s) for OA management.
Acknowledgments and Funding:
This work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) and the National Center for Complementary and Integrative Health (NCCIH) of the National Institutes of Health (NIH) under award numbers R01-AR067056, and R01-AT007373 respectively and funds from NEOMED to TMH.
Abbreviations
- ADAMTS
A Disintegrin and Metalloproteinase with Thrombospondin Motifs
- COX-2
Cyclooxygenase-2
- DMM
Destabilization of Medial Meniscus
- ECM
Extracellular Matrix
- GPx
Glutathione peroxidase
- GSH
Glutathione
- H2O2
Hydrogen peroxide
- IFN-γ
Interferon-γ
- IL-1RI
IL-1 receptor type I
- IL-IRa
IL-1 receptor antagonist
- IL-1β
Interleukin-1β
- IL-6
Interleukin 6
- IL-6R
IL-6 receptor
- MAPK
mitogen activated protein kinases
- MMP
Matrix Metalloproteinase
- NFκB
Nuclear Factor Kappa B
- eNOS
endothelial Nitric Oxide Synthase
- iNOS
inducible Nitric Oxide Synthase
- nNOS
neuronal Nitric Oxide Synthase
- NO
Nitric Oxide
- NOS
Nitric Oxide Synthase
- NOXs
NADPH oxidases
- NQO1
NADPH ubiquinone oxidoreductase
- Nrf2
Nuclear factor (erythroid-derived 2)-like 2
- NSAIDs
Non-Steroidal Anti-inflammatory Drugs
- OA
Osteoarthritis
- PGE2
Prostaglandin E2
- Prxs
Peroxiredoxins
- RNS
Reactive Nitrogen Species
- ROS
Reactive Oxygen Species
- SODs
superoxide dismutases
- TNF-α
Tumor Necrosis Factor-α
- XO
Xanthine Oxidase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors have no conflict of interest.
References:
- [1].Hunter DJ, Bierma-Zeinstra S, Osteoarthritis, Lancet 393(10182) (2019) 1745–1759. [DOI] [PubMed] [Google Scholar]
- [2].Geyer M, Schonfeld C, Novel Insights into the Pathogenesis of Osteoarthritis, Curr Rheumatol Rev 14(2) (2018) 98–107. [DOI] [PubMed] [Google Scholar]
- [3].Ghanem CI, Perez MJ, Manautou JE, Mottino AD, Acetaminophen from liver to brain: New insights into drug pharmacological action and toxicity, Pharmacological research 109 (2016) 119–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Horl WH, Nonsteroidal Anti-Inflammatory Drugs and the Kidney, Pharmaceuticals 3(7) (2010) 2291–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Xie J, Lin J, Wei M, Teng Y, He Q, Yang G, Yang X, Sustained Akt signaling in articular chondrocytes causes osteoarthritis via oxidative stress-induced senescence in mice, Bone Res 7 (2019) 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bolduc JA, Collins JA, Loeser RF, Reactive oxygen species, aging and articular cartilage homeostasis, Free radical biology & medicine 132 (2019) 73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Trachootham D, Lu W, Ogasawara MA, Nilsa RD, Huang P, Redox regulation of cell survival, Antioxidants & redox signaling 10(8) (2008) 1343–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lismont C, Nordgren M, Van Veldhoven PP, Fransen M, Redox interplay between mitochondria and peroxisomes, Frontiers in cell and developmental biology 3 (2015) 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lepetsos P, Papavassiliou AG, ROS/oxidative stress signaling in osteoarthritis, Biochimica et biophysica acta 1862(4) (2016) 576–591. [DOI] [PubMed] [Google Scholar]
- [10].Grange L, Nguyen MV, Lardy B, Derouazi M, Campion Y, Trocme C, Paclet MH, Gaudin P, Morel F, NAD(P)H oxidase activity of Nox4 in chondrocytes is both inducible and involved in collagenase expression, Antioxidants & redox signaling 8(9–10) (2006) 1485–96. [DOI] [PubMed] [Google Scholar]
- [11].van Lent PL, Nabbe KC, Blom AB, Sloetjes A, Holthuysen AE, Kolls J, Van De Loo FA, Holland SM, Van Den Berg WB, NADPH-oxidase-driven oxygen radical production determines chondrocyte death and partly regulates metalloproteinase-mediated cartilage matrix degradation during interferon-gamma-stimulated immune complex arthritis, Arthritis research & therapy 7(4) (2005) R885–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Nemirovskiy OV, Radabaugh MR, Aggarwal P, Funckes-Shippy CL, Mnich SJ, Meyer DM, Sunyer T, Rodney Mathews W, Misko TP, Plasma 3-nitrotyrosine is a biomarker in animal models of arthritis: Pharmacological dissection of iNOS’ role in disease, Nitric oxide : biology and chemistry 20(3) (2009) 150–6. [DOI] [PubMed] [Google Scholar]
- [13].Altay MA, Erturk C, Bilge A, Yapti M, Levent A, Aksoy N, Evaluation of prolidase activity and oxidative status in patients with knee osteoarthritis: relationships with radiographic severity and clinical parameters, Rheumatology international 35(10) (2015) 1725–31. [DOI] [PubMed] [Google Scholar]
- [14].Chen B, Deng Y, Tan Y, Qin J, Chen LB, Association between severity of knee osteoarthritis and serum and synovial fluid interleukin 17 concentrations, J Int Med Res 42(1) (2014) 138–44. [DOI] [PubMed] [Google Scholar]
- [15].Ahmed S, Rahman A, Hasnain A, Lalonde M, Goldberg VM, Haqqi TM, Green tea polyphenol epigallocatechin-3-gallate inhibits the IL-1 beta-induced activity and expression of R01-AT007373 respectively and funds from NEOMED to TMH. cyclooxygenase-2 and nitric oxide synthase-2 in human chondrocytes, Free radical biology & medicine 33(8) (2002) 1097–105. [DOI] [PubMed] [Google Scholar]
- [16].Singh R, Ahmed S, Islam N, Goldberg VM, Haqqi TM, Epigallocatechin-3-gallate inhibits interleukin-1beta-induced expression of nitric oxide synthase and production of nitric oxide in human chondrocytes: suppression of nuclear factor kappaB activation by degradation of the inhibitor of nuclear factor kappaB, Arthritis and rheumatism 46(8) (2002) 2079–86. [DOI] [PubMed] [Google Scholar]
- [17].Pelletier JP, Jovanovic DV, Lascau-Coman V, Fernandes JC, Manning PT, Connor JR, Currie MG, Martel-Pelletier J, Selective inhibition of inducible nitric oxide synthase reduces progression of experimental osteoarthritis in vivo: possible link with the reduction in chondrocyte apoptosis and caspase 3 level, Arthritis and rheumatism 43(6) (2000) 1290–9. [DOI] [PubMed] [Google Scholar]
- [18].Oh CK, Sultan A, Platzer J, Dolatabadi N, Soldner F, McClatchy DB, Diedrich JK, Yates JR 3rd, Ambasudhan R, Nakamura T, Jaenisch R, Lipton SA, S-Nitrosylation of PINK1 Attenuates PINK1/Parkin-Dependent Mitophagy in hiPSC-Based Parkinson’s Disease Models, Cell reports 21(8) (2017) 2171–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Radi R, Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine, Proceedings of the National Academy of Sciences of the United States of America 115(23) (2018) 5839–5848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ahmad N, Ansari MY, Haqqi TM, Role of iNOS in osteoarthritis: Pathological and therapeutic aspects, Journal of cellular physiology (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Luo J, Mills K, le Cessie S, Noordam R, van Heemst D, Ageing, age-related diseases and oxidative stress: What to do next?, Ageing research reviews 57 (2020) 100982. [DOI] [PubMed] [Google Scholar]
- [22].Fukui M, Zhu BT, Mitochondrial superoxide dismutase SOD2, but not cytosolic SOD1, plays a critical role in protection against glutamate-induced oxidative stress and cell death in HT22 neuronal cells, Free radical biology & medicine 48(6) (2010) 821–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Rhee SG, Woo HA, Kil IS, Bae SH, Peroxiredoxin functions as a peroxidase and a regulator and sensor of local peroxides, The Journal of biological chemistry 287(7) (2012) 4403–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lubos E, Loscalzo J, Handy DE, Glutathione peroxidase-1 in health and disease: from molecular mechanisms to therapeutic opportunities, Antioxidants & redox signaling 15(7) (2011) 1957–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Regan EA, Bowler RP, Crapo JD, Joint fluid antioxidants are decreased in osteoarthritic joints compared to joints with macroscopically intact cartilage and subacute injury, Osteoarthritis and cartilage 16(4) (2008) 515–21. [DOI] [PubMed] [Google Scholar]
- [26].Ostalowska A, Birkner E, Wiecha M, Kasperczyk S, Kasperczyk A, Kapolka D, Zon-Giebel A, Lipid peroxidation and antioxidant enzymes in synovial fluid of patients with primary and secondary osteoarthritis of the knee joint, Osteoarthritis and cartilage 14(2) (2006) 139–45. [DOI] [PubMed] [Google Scholar]
- [27].Cai D, Yin S, Yang J, Jiang Q, Cao W, Histone deacetylase inhibition activates Nrf2 and protects against osteoarthritis, Arthritis research & therapy 17 (2015) 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Collins JA, Wood ST, Nelson KJ, Rowe MA, Carlson CS, Chubinskaya S, Poole LB, Furdui CM, Loeser RF, Oxidative Stress Promotes Peroxiredoxin Hyperoxidation and Attenuates Pro-survival Signaling in Aging Chondrocytes, The Journal of biological chemistry 291(13) (2016) 6641–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Regan E, Flannelly J, Bowler R, Tran K, Nicks M, Carbone BD, Glueck D, Heijnen H, Mason R, Crapo J, Extracellular superoxide dismutase and oxidant damage in osteoarthritis, Arthritis and rheumatism 52(11) (2005) 3479–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ansari MY, Khan NM, Ahmad I, Haqqi TM, Parkin clearance of dysfunctional mitochondria regulates ROS levels and increases survival of human chondrocytes, Osteoarthritis and cartilage 26(8) (2018) 1087–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Abramson SB, Osteoarthritis and nitric oxide, Osteoarthritis and cartilage 16Suppl 2 (2008) S15–20. [DOI] [PubMed] [Google Scholar]
- [32].Ahmad N, Ansari MY, Bano S, Haqqi TM, Imperatorin suppresses IL-1beta-induced iNOS expression via inhibiting ERK-MAPK/AP1 signaling in primary human OA chondrocytes, International immunopharmacology 85 (2020) 106612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Loeser RF, The Role of Aging in the Development of Osteoarthritis, Transactions of the American Clinical and Climatological Association 128 (2017) 44–54. [PMC free article] [PubMed] [Google Scholar]
- [34].Altindag O, Erel O, Aksoy N, Selek S, Celik H, Karaoglanoglu M, Increased oxidative stress and its relation with collagen metabolism in knee osteoarthritis, Rheumatology international 27(4) (2007) 339–44. [DOI] [PubMed] [Google Scholar]
- [35].Loeser RF, Collins JA, Diekman BO, Ageing and the pathogenesis of osteoarthritis, Nature reviews. Rheumatology 12(7) (2016) 412–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Afonso V, Champy R, Mitrovic D, Collin P, Lomri A, Reactive oxygen species and superoxide dismutases: role in joint diseases, Joint, bone, spine : revue du rhumatisme 74(4) (2007) 324–9. [DOI] [PubMed] [Google Scholar]
- [37].Hwang HS, Kim HA, Chondrocyte Apoptosis in the Pathogenesis of Osteoarthritis, International journal of molecular sciences 16(11) (2015) 26035–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Carlo MD Jr., Loeser RF, Increased oxidative stress with aging reduces chondrocyte survival: correlation with intracellular glutathione levels, Arthritis and rheumatism 48(12) (2003) 3419–30. [DOI] [PubMed] [Google Scholar]
- [39].Federico A, Morgillo F, Tuccillo C, Ciardiello F, Loguercio C, Chronic inflammation and oxidative stress in human carcinogenesis, International journal of cancer 121(11) (2007) 2381–6. [DOI] [PubMed] [Google Scholar]
- [40].Straub RH, Schradin C, Chronic inflammatory systemic diseases: An evolutionary trade off between acutely beneficial but chronically harmful programs, Evolution, medicine, and public health 2016(1) (2016) 37–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Shen J, Abu-Amer Y, O’Keefe RJ, McAlinden A, Inflammation and epigenetic regulation in osteoarthritis, Connective tissue research 58(1) (2017) 49–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Greene MA, Loeser RF, Aging-related inflammation in osteoarthritis, Osteoarthritis and cartilage 23(11) (2015) 1966–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Griffin TM, Scanzello CR, Innate inflammation and synovial macrophages in osteoarthritis pathophysiology, Clin Exp Rheumatol 37 Suppl 120(5) (2019) 57–63. [PMC free article] [PubMed] [Google Scholar]
- [44].Goldring MB, Otero M, Inflammation in osteoarthritis, Current opinion in rheumatology 23(5) (2011) 471–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Khan NM, Haseeb A, Ansari MY, Devarapalli P, Haynie S, Haqqi TM, Wogonin, a plant derived small molecule, exerts potent anti-inflammatory and chondroprotective effects through the activation of ROS/ERK/Nrf2 signaling pathways in human Osteoarthritis chondrocytes, Free radical biology & medicine 106 (2017) 288–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Lubberts E, Joosten LA, van de Loo FA, van den Gersselaar LA, van den Berg WB, Reduction of interleukin-17-induced inhibition of chondrocyte proteoglycan synthesis in intact murine articular cartilage by interleukin-4, Arthritis and rheumatism 43(6) (2000) 1300–6. [DOI] [PubMed] [Google Scholar]
- [47].Dai SM, Shan ZZ, Nishioka K, Yudoh K, Implication of interleukin 18 in production of matrix metalloproteinases in articular chondrocytes in arthritis: direct effect on chondrocytes may not be pivotal, Annals of the rheumatic diseases 64(5) (2005) 735–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Sun JM, Sun LZ, Liu J, Su BH, Shi L, Serum interleukin-15 levels are associated with severity of pain in patients with knee osteoarthritis, Dis Markers 35(3) (2013) 203–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Jiang Y, Xiao Q, Hu Z, Pu B, Shu J, Yang Q, Lao H, Hao J, Tissue levels of leukemia inhibitory factor vary by osteoarthritis grade, Orthopedics 37(5) (2014) e460–4. [DOI] [PubMed] [Google Scholar]
- [50].Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H, Role of proinflammatory cytokines in the pathophysiology of osteoarthritis, Nature reviews. Rheumatology 7(1) (2011) 33–42. [DOI] [PubMed] [Google Scholar]
- [51].Daheshia M, Yao JQ, The interleukin 1beta pathway in the pathogenesis of osteoarthritis, The Journal of rheumatology 35(12) (2008) 2306–12. [DOI] [PubMed] [Google Scholar]
- [52].Ansari MY, Haqqi TM, Interleukin-1beta induced Stress Granules Sequester COX-2 mRNA and Regulates its Stability and Translation in Human OA Chondrocytes, Scientific reports 6 (2016) 27611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Haseeb A, Chen D, Haqqi TM, Delphinidin inhibits IL-1beta-induced activation of NF-kappaB by modulating the phosphorylation of IRAK-1(Ser376) in human articular chondrocytes, Rheumatology 52(6) (2013) 998–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Akhtar N, Haqqi TM, Epigallocatechin-3-gallate suppresses the global interleukin-1beta-induced inflammatory response in human chondrocytes, Arthritis research & therapy 13(3) (2011) R93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Khan NM, Ansari MY, Haqqi TM, Sucrose, But Not Glucose, Blocks IL1-beta-Induced Inflammatory Response in Human Chondrocytes by Inducing Autophagy via AKT/mTOR Pathway, Journal of cellular biochemistry 118(3) (2017) 629–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Glasson SS, Askew R, Sheppard B, Carito BA, Blanchet T, Ma HL, Flannery CR, Kanki K, Wang E, Peluso D, Yang Z, Majumdar MK, Morris EA, Characterization of and osteoarthritis susceptibility in ADAMTS-4-knockout mice, Arthritis and rheumatism 50(8) (2004) 2547–58. [DOI] [PubMed] [Google Scholar]
- [57].Glasson SS, Askew R, Sheppard B, Carito B, Blanchet T, Ma HL, Flannery CR, Peluso D, Kanki K, Yang Z, Majumdar MK, Morris EA, Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis, Nature 434(7033) (2005) 644–8. [DOI] [PubMed] [Google Scholar]
- [58].Fushimi K, Troeberg L, Nakamura H, Lim NH, Nagase H, Functional differences of the catalytic and non-catalytic domains in human ADAMTS-4 and ADAMTS-5 in aggrecanolytic activity, The Journal of biological chemistry 283(11) (2008) 6706–16. [DOI] [PubMed] [Google Scholar]
- [59].Tabeian H, Betti BF, Dos Santos Cirqueira C, de Vries TJ, Lobbezoo F, Ter Linde AV, Zandieh-Doulabi B, Koenders MI, Everts V, Bakker AD, IL-1beta Damages Fibrocartilage and Upregulates MMP-13 Expression in Fibrochondrocytes in the Condyle of the Temporomandibular Joint, International journal of molecular sciences 20(9) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Palmer G, Guerne PA, Mezin F, Maret M, Guicheux J, Goldring MB, Gabay C, Production of interleukin-1 receptor antagonist by human articular chondrocytes, Arthritis research 4(3) (2002) 226–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Wojdasiewicz P, Poniatowski LA, Szukiewicz D, The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis, Mediators of inflammation 2014 (2014)561459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Page Thomas DP, King B, Stephens T, Dingle JT, In vivo studies of cartilage regeneration after damage induced by catabolin/interleukin-1, Annals of the rheumatic diseases 50(2) (1991) 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Glasson SS, In vivo osteoarthritis target validation utilizing genetically-modified mice, Current drug targets 8(2) (2007) 367–76. [DOI] [PubMed] [Google Scholar]
- [64].Clements KM, Price JS, Chambers MG, Visco DM, Poole AR, Mason RM, Gene deletion of either interleukin-1beta, interleukin-1beta-converting enzyme, inducible nitric oxide synthase, or stromelysin 1 accelerates the development of knee osteoarthritis in mice after surgical transection of the medial collateral ligament and partial medial meniscectomy, Arthritis and rheumatism 48(12) (2003) 3452–63. [DOI] [PubMed] [Google Scholar]
- [65].Nasi S, Ea HK, So A, Busso N, Revisiting the Role of Interleukin-1 Pathway in Osteoarthritis: Interleukin-1alpha and −1beta, and NLRP3 Inflammasome Are Not Involved in the Pathological Features of the Murine Menisectomy Model of Osteoarthritis, Frontiers in pharmacology 8 (2017) 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Ansari MY, Ahmad N, Haqqi TM, Butein Activates Autophagy Through AMPK/TSC2/ULK1/mTOR Pathway to Inhibit IL-6 Expression in IL-1beta Stimulated Human Chondrocytes, Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology 49(3) (2018) 932–946. [DOI] [PubMed] [Google Scholar]
- [67].Ansari MY, Khan NM, Ahmad N, Green J, Novak K, Haqqi TM, Genetic Inactivation of ZCCHC6 Suppresses Interleukin-6 Expression and Reduces the Severity of Experimental Osteoarthritis in Mice, Arthritis & rheumatology 71(4) (2019) 583–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Haseeb A, Ansari MY, Haqqi TM, Harpagoside suppresses IL-6 expression in primary human osteoarthritis chondrocytes, Journal of orthopaedic research : official publication of the Orthopaedic Research Society 35(2) (2017) 311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Rowan AD, Koshy PJ, Shingleton WD, Degnan BA, Heath JK, Vernallis AB, Spaull JR, Life PF, Hudson K, Cawston TE, Synergistic effects of glycoprotein 130 binding cytokines in combination with interleukin-1 on cartilage collagen breakdown, Arthritis and rheumatism 44(7) (2001) 1620–32. [DOI] [PubMed] [Google Scholar]
- [70].Kaneko S, Satoh T, Chiba J, Ju C, Inoue K, Kagawa J, Interleukin-6 and interleukin-8 levels in serum and synovial fluid of patients with osteoarthritis, Cytokines, cellular & molecular therapy 6(2) (2000) 71–9. [DOI] [PubMed] [Google Scholar]
- [71].Latourte A, Cherifi C, Maillet J, Ea HK, Bouaziz W, Funck-Brentano T, Cohen-Solal M, Hay E, Richette P, Systemic inhibition of IL-6/Stat3 signalling protects against experimental osteoarthritis, Annals of the rheumatic diseases 76(4) (2017) 748–755. [DOI] [PubMed] [Google Scholar]
- [72].Ryu JH, Yang S, Shin Y, Rhee J, Chun CH, Chun JS, Interleukin-6 plays an essential role in hypoxia-inducible factor 2alpha-induced experimental osteoarthritic cartilage destruction in mice, Arthritis and rheumatism 63(9) (2011) 2732–43. [DOI] [PubMed] [Google Scholar]
- [73].de Hooge AS, van de Loo FA, Bennink MB, Arntz OJ, de Hooge P, van den Berg WB, Male IL-6 gene knock out mice developed more advanced osteoarthritis upon aging, Osteoarthritis and cartilage 13(1) (2005) 66–73. [DOI] [PubMed] [Google Scholar]
- [74].Ahmed S, Wang N, Lalonde M, Goldberg VM, Haqqi TM, Green tea polyphenol epigallocatechin-3-gallate (EGCG) differentially inhibits interleukin-1 beta-induced expression of matrix metalloproteinase-1 and −13 in human chondrocytes, The Journal of pharmacology and experimental therapeutics 308(2) (2004) 767–73. [DOI] [PubMed] [Google Scholar]
- [75].Rasheed Z, Akhtar N, Haqqi TM, Pomegranate extract inhibits the interleukin-1beta-induced activation of MKK-3, p38alpha-MAPK and transcription factor RUNX-2 in human osteoarthritis chondrocytes, Arthritis research & therapy 12(5) (2010) R195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Saklatvala J, Inflammatory signaling in cartilage: MAPK and NF-kappaB pathways in chondrocytes and the use of inhibitors for research into pathogenesis and therapy of osteoarthritis, Current drug targets 8(2) (2007) 305–13. [DOI] [PubMed] [Google Scholar]
- [77].Rigoglou S, Papavassiliou AG, The NF-kappaB signalling pathway in osteoarthritis, The international journal of biochemistry & cell biology 45(11) (2013) 2580–4. [DOI] [PubMed] [Google Scholar]
- [78].Zhou Y, Wang T, Hamilton JL, Chen D, Wnt/beta-catenin Signaling in Osteoarthritis and in Other Forms of Arthritis, Current rheumatology reports 19(9) (2017) 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Corr M, Wnt-beta-catenin signaling in the pathogenesis of osteoarthritis, Nature clinical practice. Rheumatology 4(10) (2008) 550–6. [DOI] [PubMed] [Google Scholar]
- [80].Yuasa T, Otani T, Koike T, Iwamoto M, Enomoto-Iwamoto M, Wnt/beta-catenin signaling stimulates matrix catabolic genes and activity in articular chondrocytes: its possible role in joint degeneration, Laboratory investigation; a journal of technical methods and pathology 88(3) (2008) 264–74. [DOI] [PubMed] [Google Scholar]
- [81].Lietman C, Wu B, Lechner S, Shinar A, Sehgal M, Rossomacha E, Datta P, Sharma A, Gandhi R, Kapoor M, Young PP, Inhibition of Wnt/beta-catenin signaling ameliorates osteoarthritis in a murine model of experimental osteoarthritis, JCI insight 3(3) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Yin W, Park JI, Loeser RF, Oxidative stress inhibits insulin-like growth factor-I induction of chondrocyte proteoglycan synthesis through differential regulation of phosphatidylinositol 3-Kinase-Akt and MEK-ERK MAPK signaling pathways, The Journal of biological chemistry 284(46) (2009) 31972–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Lo YY, Cruz TF, Involvement of reactive oxygen species in cytokine and growth factor induction of c-fos expression in chondrocytes, The Journal of biological chemistry 270(20) (1995) 11727–30. [DOI] [PubMed] [Google Scholar]
- [84].Lo YY, Conquer JA, Grinstein S, Cruz TF, Interleukin-1 beta induction of c-fos and collagenase expression in articular chondrocytes: involvement of reactive oxygen species, Journal of cellular biochemistry 69(1) (1998) 19–29. [DOI] [PubMed] [Google Scholar]
- [85].Lo YY, Wong JM, Cruz TF, Reactive oxygen species mediate cytokine activation of c-Jun NH2-terminal kinases, The Journal of biological chemistry 271(26) (1996) 15703–7. [DOI] [PubMed] [Google Scholar]
- [86].Ismail HM, Miotla-Zarebska J, Troeberg L, Tang X, Stott B, Yamamoto K, Nagase H, Fosang AJ, Vincent TL, Saklatvala J, Brief Report: JNK-2 Controls Aggrecan Degradation in Murine Articular Cartilage and the Development of Experimental Osteoarthritis, Arthritis & rheumatology 68(5) (2016) 1165–71. [DOI] [PubMed] [Google Scholar]
- [87].Akhtar N, Haqqi TM, Current nutraceuticals in the management of osteoarthritis: a review, Therapeutic advances in musculoskeletal disease 4(3) (2012) 181–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Oliviero F, Scanu A, Zamudio-Cuevas Y, Punzi L, Spinella P, Anti-inflammatory effects of polyphenols in arthritis, Journal of the science of food and agriculture 98(5) (2018) 1653–1659. [DOI] [PubMed] [Google Scholar]
- [89].Henrotin Y, Mobasheri A, Natural Products for Promoting Joint Health and Managing Osteoarthritis, Current rheumatology reports 20(11) (2018) 72. [DOI] [PubMed] [Google Scholar]
- [90].Ansari MY, Khan NM, Haqqi TM, A standardized extract of Butea monosperma (Lam.) flowers suppresses the IL-1beta-induced expression of IL-6 and matrix-metalloproteases by activating autophagy in human osteoarthritis chondrocytes, Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 96 (2017) 198–207. [DOI] [PubMed] [Google Scholar]
- [91].Khan NM, Haseeb A, Ansari MY, Haqqi TM, A wogonin-rich-fraction of Scutellaria baicalensis root extract exerts chondroprotective effects by suppressing IL-1beta-induced activation of AP-1 in human OA chondrocytes, Scientific reports 7 (2017) 43789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Ucuncu Y, Celik N, Ozturk C, Turkoglu M, Cetin N, Kockara N, Sener E, Dundar C, Arslan A, Dogan H, Kurt N, Suleyman H, Chondroprotective effects of a new glucosamine combination in rats: Gene expression, biochemical and histopathological evaluation, Life sciences 130 (2015) 31–7. [DOI] [PubMed] [Google Scholar]
- [93].Shukla M, Gupta K, Rasheed Z, Khan KA, Haqqi TM, Bioavailable constituents/metabolites of pomegranate (Punica granatum L) preferentially inhibit COX2 activity ex vivo and IL-1beta-induced PGE2 production in human chondrocytes in vitro, Journal of inflammation 5 (2008) 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Ahmed S, Wang N, Hafeez BB, Cheruvu VK, Haqqi TM, Punica granatum L extract inhibits IL-1beta-induced expression of matrix metalloproteinases by inhibiting the activation of MAP kinases and NF-kappaB in human chondrocytes in vitro, The Journal of nutrition 135(9) (2005)2096–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Rasheed Z, Anbazhagan AN, Akhtar N, Ramamurthy S, Voss FR, Haqqi TM, Green tea polyphenol epigallocatechin-3-gallate inhibits advanced glycation end product-induced expression of tumor necrosis factor-alpha and matrix metalloproteinase-13 in human chondrocytes, Arthritis research & therapy 11(3) (2009) R71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Natarajan V, Madhan B, Tiku ML, Intra-Articular Injections of Polyphenols Protect Articular Cartilage from Inflammation-Induced Degradation: Suggesting a Potential Role in Cartilage Therapeutics, PloS one 10(6) (2015) e0127165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Leong DJ, Choudhury M, Hanstein R, Hirsh DM, Kim SJ, Majeska RJ, Schaffler MB, Hardin JA, Spray DC, Goldring MB, Cobelli NJ, Sun HB, Green tea polyphenol treatment is chondroprotective, anti-inflammatory and palliative in a mouse post-traumatic osteoarthritis model, Arthritis research & therapy 16(6) (2014) 508. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- [98].Oliviero F, Sfriso P, Scanu A, Fiocco U, Spinella P, Punzi L, Epigallocatechin-3-gallate reduces inflammation induced by calcium pyrophosphate crystals in vitro, Frontiers in pharmacology 4 (2013) 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Qiu L, Luo Y, Chen X, Quercetin attenuates mitochondrial dysfunction and biogenesis via upregulated AMPK/SIRT1 signaling pathway in OA rats, Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 103 (2018) 1585–1591. [DOI] [PubMed] [Google Scholar]
- [100].Chen YJ, Tsai KS, Chiu CY, Yang TH, Lin TH, Fu WM, Chen CF, Yang RS, Liu SH, EGb761 inhibits inflammatory responses in human chondrocytes and shows chondroprotection in osteoarthritic rat knee, Journal of orthopaedic research : official publication of the Orthopaedic Research Society 31(7) (2013) 1032–8. [DOI] [PubMed] [Google Scholar]
- [101].Feng K, Chen Z, Pengcheng L, Zhang S, Wang X, Quercetin attenuates oxidative stress-induced apoptosis via SIRT1/AMPK-mediated inhibition of ER stress in rat chondrocytes and prevents the progression of osteoarthritis in a rat model, Journal of cellular physiology 234(10) (2019) 18192–18205. [DOI] [PubMed] [Google Scholar]
- [102].Mok SW, Fu SC, Cheuk YC, Chu IM, Chan KM, Qin L, Yung SH, Kevin Ho KW, Intra-Articular Delivery of Quercetin Using Thermosensitive Hydrogel Attenuate Cartilage Degradation in an Osteoarthritis Rat Model, Cartilage (2018) 1947603518796550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Hu Y, Gui Z, Zhou Y, Xia L, Lin K, Xu Y, Quercetin alleviates rat osteoarthritis by inhibiting inflammation and apoptosis of chondrocytes, modulating synovial macrophages polarization to M2 macrophages, Free radical biology & medicine 145 (2019) 146–160. [DOI] [PubMed] [Google Scholar]
- [104].Qu Y, Wang C, Liu N, Gao C, Liu F, Morin Exhibits Anti-Inflammatory Effects on IL-1beta-Stimulated Human Osteoarthritis Chondrocytes by Activating the Nrf2 Signaling Pathway, Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology 51(4) (2018) 1830–1838. [DOI] [PubMed] [Google Scholar]
- [105].Chen WP, Wang YL, Tang JL, Hu PF, Bao JP, Wu LD, Morin inhibits interleukin-1beta-induced nitric oxide and prostaglandin E2 production in human chondrocytes, International immunopharmacology 12(2) (2012) 447–52. [DOI] [PubMed] [Google Scholar]
- [106].Chen WP, Hu PF, Bao JP, Wu LD, Morin exerts antiosteoarthritic properties: an in vitro and in vivo study, Experimental biology and medicine 237(4) (2012) 380–6. [DOI] [PubMed] [Google Scholar]
- [107].Park S, Lee LR, Seo JH, Kang S, Curcumin and tetrahydrocurcumin both prevent osteoarthritis symptoms and decrease the expressions of pro-inflammatory cytokines in estrogen-deficient rats, Genes & nutrition 11 (2016) 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Zhang Z, Leong DJ, Xu L, He Z, Wang A, Navati M, Kim SJ, Hirsh DM, Hardin JA, Cobelli NJ, Friedman JM, Sun HB, Curcumin slows osteoarthritis progression and relieves osteoarthritis-associated pain symptoms in a post-traumatic osteoarthritis mouse model, Arthritis research & therapy 18(1) (2016) 128. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- [109].Bannuru RR, Osani MC, Al-Eid F, Wang C, Efficacy of curcumin and Boswellia for knee osteoarthritis: Systematic review and meta-analysis, Seminars in arthritis and rheumatism (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Coury JR, Nixon R, Collins M, Schwartz J, Chahine NO, Grande DA, Oral Administration of a Chemically Modified Curcumin, TRB-N0224, Reduced Inflammatory Cytokines and Cartilage Erosion in a Rabbit ACL Transection Injury Model, Cartilage (2018) 1947603518815263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Chen MP, Yang SH, Chou CH, Yang KC, Wu CC, Cheng YH, Lin FH, The chondroprotective effects of ferulic acid on hydrogen peroxide-stimulated chondrocytes: inhibition of hydrogen peroxide-induced pro-inflammatory cytokines and metalloproteinase gene expression at the mRNA level, Inflammation research : official journal of the European Histamine Research Society … [et al.] 59(8) (2010) 587–95. [DOI] [PubMed] [Google Scholar]
- [112].Frischholz S, Berberich O, Bock T, Meffert RH, Blunk T, Resveratrol counteracts IL-1beta-mediated impairment of extracellular matrix deposition in 3D articular chondrocyte constructs, J Tissue Eng Regen Med (2020). [DOI] [PubMed] [Google Scholar]
- [113].Wang J, Gao JS, Chen JW, Li F, Tian J, Effect of resveratrol on cartilage protection and apoptosis inhibition in experimental osteoarthritis of rabbit, Rheumatology international 32(6) (2012) 1541–8. [DOI] [PubMed] [Google Scholar]
- [114].Wei Y, Jia J, Jin X, Tong W, Tian H, Resveratrol ameliorates inflammatory damage and protects against osteoarthritis in a rat model of osteoarthritis, Molecular medicine reports 17(1) (2018) 1493–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Liu FC, Hung LF, Wu WL, Chang DM, Huang CY, Lai JH, Ho LJ, Chondroprotective effects and mechanisms of resveratrol in advanced glycation end products-stimulated chondrocytes, Arthritis research & therapy 12(5) (2010) R167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Lei M, Wang JG, Xiao DM, Fan M, Wang DP, Xiong JY, Chen Y, Ding Y, Liu SL, Resveratrol inhibits interleukin 1beta-mediated inducible nitric oxide synthase expression in articular chondrocytes by activating SIRT1 and thereby suppressing nuclear factor-kappaB activity, European journal of pharmacology 674(2–3) (2012) 73–9. [DOI] [PubMed] [Google Scholar]
- [117].Li W, Cai L, Zhang Y, Cui L, Shen G, Intra-articular resveratrol injection prevents osteoarthritis progression in a mouse model by activating SIRT1 and thereby silencing HIF-2alpha, Journal of orthopaedic research : official publication of the Orthopaedic Research Society 33(7) (2015) 1061–70. [DOI] [PubMed] [Google Scholar]
- [118].Liang Q, Wang XP, Chen TS, Resveratrol protects rabbit articular chondrocyte against sodium nitroprusside-induced apoptosis via scavenging ROS, Apoptosis : an international journal on programmed cell death 19(9) (2014) 1354–63. [DOI] [PubMed] [Google Scholar]
- [119].Jiang M, Li X, Yu X, Liu X, Xu X, He J, Gu H, Liu L, Oral Administration of Resveratrol Alleviates Osteoarthritis Pathology in C57BL/6J Mice Model Induced by a High-Fat Diet, Mediators of inflammation 2017 (2017) 7659023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Gorzynik-Debicka M, Przychodzen P, Cappello F, Kuban-Jankowska A, Marino Gammazza A, Knap N, Wozniak M, Gorska-Ponikowska M, Potential Health Benefits of Olive Oil and Plant Polyphenols, International journal of molecular sciences 19(3) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Ravalli S, Szychlinska MA, Leonardi RM, Musumeci G, Recently highlighted nutraceuticals for preventive management of osteoarthritis, World J Orthop 9(11) (2018) 255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Castrogiovanni P, Trovato FM, Loreto C, Nsir H, Szychlinska MA, Musumeci G, Nutraceutical Supplements in the Management and Prevention of Osteoarthritis, International journal of molecular sciences 17(12) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Cetrullo S, D’Adamo S, Guidotti S, Borzi RM, Flamigni F, Hydroxytyrosol prevents chondrocyte death under oxidative stress by inducing autophagy through sirtuin 1-dependent and -independent mechanisms, Biochimica et biophysica acta 1860(6) (2016) 1181–91. [DOI] [PubMed] [Google Scholar]
- [124].Szychlinska MA, Castrogiovanni P, Trovato FM, Nsir H, Zarrouk M, Lo Furno D, Di Rosa M, Imbesi R, Musumeci G, Physical activity and Mediterranean diet based on olive tree phenolic compounds from two different geographical areas have protective effects on early osteoarthritis, muscle atrophy and hepatic steatosis, Eur J Nutr 58(2) (2019) 565–581. [DOI] [PubMed] [Google Scholar]
- [125].Musumeci G, Trovato FM, Pichler K, Weinberg AM, Loreto C, Castrogiovanni P, Extra-virgin olive oil diet and mild physical activity prevent cartilage degeneration in an osteoarthritis model: an in vivo and in vitro study on lubricin expression, The Journal of nutritional biochemistry 24(12) (2013) 2064–75. [DOI] [PubMed] [Google Scholar]
- [126].Hooshmand S, Soung do Y, Lucas EA, Madihally SV, Levenson CW, Arjmandi BH, Genistein reduces the production of proinflammatory molecules in human chondrocytes, The Journal of nutritional biochemistry 18(9) (2007) 609–14. [DOI] [PubMed] [Google Scholar]
- [127].Bokhari RA, Tantowi N, Lau SF, Mohamed S, Java Tea (Orthosiphon stamineus) protected against osteoarthritis by mitigating inflammation and cartilage degradation: a preclinical study, Inflammopharmacology 26(4) (2018) 939–949. [DOI] [PubMed] [Google Scholar]
- [128].Mevel E, Merceron C, Vinatier C, Krisa S, Richard T, Masson M, Lesoeur J, Hivernaud V, Gauthier O, Abadie J, Nourissat G, Houard X, Wittrant Y, Urban N, Beck L, Guicheux J, Olive and grape seed extract prevents post-traumatic osteoarthritis damages and exhibits in vitro anti IL-1beta activities before and after oral consumption, Scientific reports 6 (2016) 33527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Horcajada MN, Sanchez C, Membrez Scalfo F, Drion P, Comblain F, Taralla S, Donneau AF, Offord EA, Henrotin Y, Oleuropein or rutin consumption decreases the spontaneous development of osteoarthritis in the Hartley guinea pig, Osteoarthritis and cartilage 23(1) (2015) 94–102. [DOI] [PubMed] [Google Scholar]
- [130].Chen WP, Wu LD, Chlorogenic acid suppresses interleukin-1beta-induced inflammatory mediators in human chondrocytes, International journal of clinical and experimental pathology 7(12) (2014) 8797–801. [PMC free article] [PubMed] [Google Scholar]
- [131].Huh JE, Seo BK, Baek YH, Lee S, Lee JD, Choi DY, Park DS, Standardized butanol fraction of WIN-34B suppresses cartilage destruction via inhibited production of matrix metalloproteinase and inflammatory mediator in osteoarthritis human cartilage explants culture and chondrocytes, BMC complementary and alternative medicine 12 (2012) 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Lee SA, Moon SM, Han SH, Hwang EJ, Park BR, Kim JS, Kim DK, Kim CS, Chondroprotective effects of aqueous extract of Anthriscus sylvestris leaves on osteoarthritis in vitro and in vivo through MAPKs and NF-kappaB signaling inhibition, Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 103 (2018) 1202–1211. [DOI] [PubMed] [Google Scholar]
- [133].Rohdewald PJ, Review on Sustained Relief of Osteoarthritis Symptoms with a Proprietary Extract from Pine Bark, Pycnogenol, Journal of medicinal food 21(1) (2018) 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Cisar P, Jany R, Waczulikova I, Sumegova K, Muchova J, Vojtassak J, Durackova Z, Lisy M, Rohdewald P, Effect of pine bark extract (Pycnogenol) on symptoms of knee osteoarthritis, Phytotherapy research : PTR 22(8) (2008) 1087–92. [DOI] [PubMed] [Google Scholar]
- [135].Wu H, Zhang M, Li W, Zhu S, Zhang D, Stachydrine attenuates IL-1beta-induced inflammatory response in osteoarthritis chondrocytes through the NF-kappaB signaling pathway, Chemico-biological interactions 326 (2020) 109136. [DOI] [PubMed] [Google Scholar]
- [136].Miller MJ, Ahmed S, Bobrowski P, Haqqi TM, The chrondoprotective actions of a natural product are associated with the activation of IGF-1 production by human chondrocytes despite the presence of IL-1beta, BMC complementary and alternative medicine 6 (2006) 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Davidson RK, Green J, Gardner S, Bao Y, Cassidy A, Clark IM, Identifying chondroprotective diet-derived bioactives and investigating their synergism, Scientific reports 8(1) (2018) 17173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Ruangsuriya J, Budprom P, Viriyakhasem N, Kongdang P, Chokchaitaweesuk C, Sirikaew N, Chomdej S, Nganvongpanit K, Ongchai S, Suppression of Cartilage Degradation by Zingerone Involving the p38 and JNK MAPK Signaling Pathway, Planta medica 83(3–04) (2017) 268–276. [DOI] [PubMed] [Google Scholar]
- [139].Alaaeddine N, Di Battista JA, Pelletier JP, Kiansa K, Cloutier JM, Martel-Pelletier J, Differential effects of IL-8, LIF (pro-inflammatory) and IL-11 (anti-inflammatory) on TNF-alpha-induced PGE(2)release and on signalling pathways in human OA synovial fibroblasts, Cytokine 11(12) (1999) 1020–30. [DOI] [PubMed] [Google Scholar]


