SUMMARY
Giardia duodenalis captured the attention of Leeuwenhoek in 1681 while he was examining his own diarrheal stool, but, ironically, it did not really gain attention as a human pathogen until the 1960s, when outbreaks were reported. Key technological advances, including in vitro cultivation, genomic and proteomic databases, and advances in microscopic and molecular approaches, have led to an understanding that this is a eukaryotic organism with a reduced genome rather than a truly premitochondriate eukaryote. This has included the discovery of mitosomes (vestiges of mitochondria), a transport system with many of the features of the Golgi apparatus, and even evidence for a sexual or parasexual cycle. Cell biology approaches have led to a better understanding of how Giardia survives with two nuclei and how it goes through its life cycle as a noninvasive organism in the hostile environment of the lumen of the host intestine. Studies of its immunology and pathogenesis have moved past the general understanding of the importance of the antibody response in controlling infection to determining the key role of the Th17 response. This work has led to understanding of the requirement for a balanced host immune response that avoids the extremes of an excessive response with collateral damage or one that is unable to clear the organism. This understanding is especially important in view of the remarkable ranges of early manifestations, which range from asymptomatic to persistent diarrhea and weight loss, and longer-term sequelae that include growth stunting in children who had no obvious symptoms and a high frequency of postinfectious irritable bowel syndrome (IBS).
KEYWORDS: Giardia, Giardia lamblia, giardiasis
INTRODUCTION
Giardia duodenalis (synonyms Giardia lamblia and Giardia intestinalis) has caught the interest of scientists and clinicians since its initial description by van Leeuwenhoek in 1681 as he described his own diarrheal stools and identified motile organisms that fit the description of the organism that was ultimately given the name of Giardia (1). G. duodenalis is the most prevalent protozoan human intestinal pathogen and is found worldwide, causing infections that ranges from asymptomatic to chronic diarrhea and malabsorption.
The life cycle of G. duodenalis consists of two stages, the trophozoite and cyst. The trophozoite is the vegetative form and replicates in the small intestine of the host. The eight flagella provide motility, and the ventral disk mediates attachment to the intestinal wall, where it gains its nutrients. More distally, in the small intestine and even extending to the large intestine, the trophozoite encysts into a cyst that is environmentally stable and can be transmitted to the next host through the fecal-oral route. The majority of outbreaks of human infection are associated with contaminated water, but food and direct human-to-human transmission via the fecal-oral route are also important.
CLASSIFICATION OF GIARDIA
Classification of Giardia as a Eukaryotic Organism
Giardia species have traditionally been categorized with other flagellated protozoa that are pathogenic to humans, but most of these other flagellates are not closely related to Giardia. Of the other human pathogens, Trichomonas vaginalis is, along with Giardia, part of the superclass Fornicata (2). Giardia species are the only human pathogens that are classified as diplomonads, while Trichomonas species are parabasalids. Diplomonad means two bodies, and the diplomonads typically have two symmetrically placed nuclei. The other diplomonads include Spironucleus and Hexamita species, which may be free-living or parasites of nonhuman animals (2).
Giardia species have an anaerobic metabolism and lack mitochondria, the Golgi apparatus, and other canonical eukaryotic organelles, and were thought at one time to be one of the earliest divergences of the eukaryotic organisms (3). As such, these species were of particular interest to evolutionary biologists. However, genome sequencing demonstrated that G. duodenalis has genes for many of the “missing” organelles and favored the alternative view that Giardia has a secondarily reduced genome (4). Although it is probably a eukaryotic organism that diverged early, it diverged after the acquisition of these “missing” organelles. Perhaps the most notable finding has been the identification of mitochondrial remnants called mitosomes (5). The identification of functional equivalents for other organelles has added to the current view that the organism has a minimized genome and cell biology. This is expanded on here in the sections on genomics and cell biology.
Classification of Giardia Species and Genotypes
Giardia species are flagellated anaerobic protozoan (or protist) organisms characterized by their dyadic symmetry and presence of two symmetrical nuclei in the trophozoites. They are intestinal parasites of animals that range phylogenetically from amphibians to mammals, depending on the Giardia species. The initial classification of Giardia species was done on the basis of host of origin and was supported by early observations that there was relatively little cross-transmission of Giardia species to different hosts (6). Subsequently, there was a move to the opposite extreme, with a designation of just three species that were based on differences that were readily observable during light microscopic evaluation of trophozoites (7). Filice (7) described the trophozoites of Giardia agilis, which is long and narrow and found primarily in amphibians, Giardia muris, which is short and wide and found in rodents, and G. duodenalis, which he obtained from rabbits but which had the same pear-shaped morphology as the human parasite.
The organism he called G. duodenalis on the basis of light microscopy is found in humans and in a broad range of other mammals. However, subsequent investigation using techniques with greater discriminatory power, primarily electron microscopy and molecular characterization, have defined differences within G. duodenalis that are frequently associated with host specificity. The first round of subdividing G. duodenalis into subgroups consisted of the formal designation of new species on the basis of morphologic differences seen by electronic microscopy (EM) (Table 1) and included Giardia psittaci, Giardia microti, and Giardia ardeae. The distinctness of these new species has subsequently been supported by DNA sequence comparisons.
TABLE 1.
Giardia species
| Species name | Hosts | Morphology determined by: |
Reference(s) for species description | Genotype(s) (reference[s]) | |
|---|---|---|---|---|---|
| Light microscopy | Electron microscopy | ||||
| G. duodenalis | Humans, numerous mammals | Pear-shaped trophozoite; claw-shaped median body | 7, 318 | AI (4) | |
| AII (17) | |||||
| B (16, 17) | |||||
| C and D (19) | |||||
| G. muris | Rodents | Short and rounded; small, rounded median body | 7; EM (319) | ||
| G. agilis | Amphibians | Long and slender; teardrop-shaped median body | 7; EM (320) | ||
| G. psittaci | Psittacine birds | Same as G. duodenalis | Incomplete ventrolateral flange, no marginal groove | 321 | |
| G. microti | Rodents | Same as G. duodenalis | Cysts contain two trophozoites with mature ventral disks | 322 | |
| G. ardeae | Herons | Same as G. duodenalis | Ventral disk and caudal flagellum similar to G. muris | 323 | |
| G. peramelis | Quenda (small marsupial) | Same as G. duodenalis | 324 | ||
| G. cricetidarum | Hamsters | Short and rounded; closer to G. muris than other species | 325 | ||
Even after excluding the organisms with morphological differences at an EM level, there is substantial variability at a DNA sequence level, and for many of them, there is a difference in host specificity (Table 2). The first recognition of differences among G. duodenalis isolates from humans was in the 1980s and consisted of different isoenzyme patterns in five axenized isolates (cell-free in vitro culture) (8, 9). In 1985, surface antigen (10) and restriction fragment length polymorphism (RFLP) patterns (11) demonstrated three groups, one of which was different enough from the other two to support the proposal that these represented different species. Subsequent refinement has been done on the basis of sequence differences in housekeeping genes (12–15). These different groups have subsequently been divided into assemblages or genotypes that now extend from A to H. Whole-genome sequences have been reported for a number of human isolates, which are found in genotypes (assemblages) A and B. The first genome was reported for the WB isolate (genotype AI), followed by those for the GS isolate (genotype B) (16, 17) and the DH isolate (genotype AII) (17). In addition, the livestock genotype (genotype E) from a pig (18) and genotypes C and D (both dog isolates) (19) have also been sequenced. Genotypes C and D were sequenced by amplifying DNA from single cysts. Phylogenetically, genotypes C and D fall into a single clade but are about as distinct from each other as genotypes A and B are from each other.
TABLE 2.
G. duodenalis genotypes
| Genotypea | Hosts | Proposed species nameb | Reference(s)c |
|---|---|---|---|
| AI | Primarily animals, but also in humans | Giardia duodenalis | 4, 11 |
| AII | Humans, numerous other mammals | Giardia duodenalis | 11, 17 |
| B | Humans, numerous other mammals | Giardia enterica | 11, 16, 17 |
| C | Dogs | Giardia canis | 326, 327 |
| D | Dogs | Giardia canis | 326, 327 |
| E | Cows, sheep, alpacas, goats, pigs | Giardia bovis | 18, 328 |
| F | Cats | Giardia cati | 327 |
| G | Rats and mice | Giardia simondi | 327 |
| H | Seals (marine vertebrates) | NA | 329 |
Other proposed subgenotypes of A are less well documented than AI and AII.
From reference 23. NA, not applicable.
Initial description and genome sequence where available.
All of the eight G. duodenalis genotypes are found in mammals (Table 2). Two of these, A and B, are routinely found in humans and occasionally in other mammals. The genotype A isolates have been divided into two groups (AI and AII) on the basis of sequence and biological differences. Genotype AI is a highly homogeneous group in which sequence differences among isolates are rare (20) and the level of allelic heterozygosity is very low (4). Recent data suggest that AI is found primarily in animals and should be considered zoonotic, while AII is seen primarily in humans (20–22). Some of the other genotypes are occasionally found in humans, but not at a frequency to implicate them as disease-causing organisms in humans. Currently, these are usually referred to as part of the same species. The name “Giardia lamblia” has typically been used in medical writing, while “Giardia intestinalis” and, later, “G. duodenalis” have been commonly used in the scientific literature. It is possible that many or all of the genotypes will ultimately be given separate species names. Interestingly, there is a greater phylogenetic difference between the human genotypes, A and B, than between these human genotypes and some of the other genotypes (C to H). Thus, it has been proposed that these two genotypes should be designated separate species (11), and subsequently, species names have been proposed; G. duodenalis for genotype A and Giardia enterica for genotype B (23). However, the argument has been made that a clonal or near-clade approach should be used for organisms such as Giardia and that assigning separate species names is premature (24). There would also be the challenge on the clinical side that molecular typing, which is not currently available, would be required for identifying these at a species level. For the purposes of this review, the term “G. duodenalis” is used for all of these genotypes, with the recognition that there remains a lack of consensus on the preferred name.
THE GIARDIA LIFE CYCLE
The Cyst
The G. duodenalis cyst is the environmentally stable stage of the parasite life cycle that facilitates the transmission of cysts passed in the feces of one host into the environment to be ingested by the subsequent host. The cyst is 5 μm by 7 to 10 μm in size with a two-layered cyst wall. The outer filamentous layer is covered by filaments 7 to 20 nm in length (25) and with N-acetylgalactosamine as the major sugar (26). The cyst has four nuclei, rather than the two found in trophozoites. The metabolic rate is only 10% to 20% that of the trophozoite (27), allowing prolonged survival in the environment, especially in cool, moist settings, perhaps explaining the higher frequency of giardiasis in the northern part of the United States compared to that in the southern areas (28).
Ingestion and Excystation
Infection of the host is initiated when cysts are ingested and pass through the acidic stomach into the duodenum where excystation occurs upon exposure to bile and a more alkaline pH. In vitro application of that sequence of pH change and exposure to bile results in a high percentage of successful excystation (29, 30). However, it is also possible to excyst Giardia at a neutral pH (31), and humans whose gastric pH is raised naturally or by medical intervention are susceptible to Giardia infection (32). In fact, they may be more susceptible to chronic giardiasis, as suggested in a report of two cases of gastric giardiasis in patients with hypochlorhydria as a result of treatment with a proton pump inhibitor, one of which resolved after discontinuing the proton pump inhibitor and without specific antimicrobial treatment (33).
Encystation
The trophozoites replicate in the small intestine, where some of the organisms will differentiate into cysts. Recent animal model data suggest that the trophozoites cluster into foci throughout the small intestine and even into the cecum and that encystation begins shortly after infection and peaks in a week. The encystation occurs in these clusters of increased organism density (34).
The development of in vitro encystation was accomplished by three different laboratories (35–37). The initial report of in vitro encystation used primary bile salts to induce encystation (37), an approach modified to a two-step process in which the second step utilized an alkaline pH of 7.8 and porcine bile (38). Alternative approaches have included high-bile medium (39) or cholesterol starvation (40). One of early events in encystation is the development of encystation-specific vesicles (ESVs) (41). These ESVs have a number of properties of the Golgi apparatus, including their involvement in protein trafficking that includes cell wall proteins 1 to 3 (CWP1 to CWP3), sensitivity to brefeldin A, and their emergence from active ER sites (42). During encystation, the trophozoites become rounded, and some of the key trophozoite cytoskeletal components are disassembled (43). There are two cycles of chromosome replication and one cycle of nuclear division, resulting in a mature cyst that has four nuclei, each of which is 4n. Then, when viable cysts are exposed to appropriate conditions, an opening at one pole of the cyst allows the emergence of the flagella and the cell body of the excyzoite (44), which undergoes two rounds of division, resulting in four trophozoites that are each 4n (two diploid nuclei).
The Giardia genome has 27 cysteine proteases that are part of the CA clan of cysteine proteases (45). Twenty-five of those cysteine proteases are expressed, with GlCP2 (CP2) being the most abundantly expressed. CP2 is a cathepsin B-like protease that is involved in excystation (46) and encystation (45, 47). It is found in the ESVs, and purified CP2 is able to cleave recombinant CWP2 into a 26-kDa fragment, which is the size found in encysting organisms (45). The roles of the other cysteine proteases remain to be confirmed.
The transcriptomics of encystation using serial analysis of gene expression (SAGE) (48) and microarray (49) identified that CWP1 to CWP3, a high-cysteine nonvariant cyst protein (HCNCp), and the transcription factor Myb are highly upregulated in the first 3 h. HCNCp was the first characterized protein from a family of high-cysteine membrane proteins (HCMPs or MCMPs) (50) (see “High-cysteine membrane proteins,” below). These and additional genes are upregulated at 7 h, including genes with Myb-binding sequences (49). A subsequent proteomic evaluation of encystation using tandem mass spectrometry found that the variety of variant-specific proteins (VSPs) had decreased 4 h after initiation of encystation in comparison to the baseline level (42). In addition, there were multiple changes in metabolic and cytoskeletal proteins. The mechanisms of initiating encystation are not well understood, but there are data suggesting the inhibition of encystation by nitric oxide (51), histone deacetylase inhibitors (52), or by the presence of lactoferrin (53). In addition, a study of the one Rho family GTPase found in Giardia (Rac) showed its localization to the ER and ESVs and demonstrated that its expression increased CWP1 expression (54). CWP1 then accumulated in the extracellular environment and was used by surrounding trophozoites to support encystation. The authors proposed that this mechanism may explain why encystation is frequently found in clusters of organisms.
GENOMICS AND PROTEOMICS OF GIARDIA
Genomics
The first Giardia genome was published (4) in 2007 and has been refined by optical mapping (55), and recently by extended reads with optical mapping (56) for a nearly complete genome of the WB isolate (genotype AI). There are five chromosomes that range in size from about 1 to 5 Mb (57) with a ploidy of four (two diploid nuclei) (44). The entire genome is about 12 Mb and is compact, with minimal noncoding regions. Introns are rare, with eight cis-spliced and five trans-spliced introns having been identified (56, 58). The genome consists of 4,863 protein-coding genes, 2,099 of which are hypothetical proteins; the others are annotated (56). In addition, there are 306 pseudogenes. The genomes of the isolates and species that have been sequenced can be found at https://Giardiadb.org/, along with multiple genome- and proteome-related resources. The database is updated frequently, with version 52 released 20 May 2021. Noncoding small RNAs (sRNAs) have also been identified in the genome and include microRNAs (miRNA), which have been associated with control of vsp gene expression (59), as well as endogenous siRNA (endo-siRNA) and tRNA-derived sRNA (60).
Giardia trophozoites are tetraploid with two diploid nuclei (see “Nuclear and chromosomal structure and replication,” below) and were assumed to be asexual in their reproduction (also discussed below). In this case, there would be an expectation of increasing allelic heterozygosity over time, as has occurred with certain bdelloid rotifers (61, 62). However, contrary to that expectation, the WB genome (genotype AI) has an extremely low level of allelic heterozygosity and was estimated at <0.01% in the initial genome assembly (4), which was subsequently revised to 0.03% (56). In comparison, the GS isolate (genotype B) has a heterozygosity level of about 0.425% (17), while the DH isolate (genotype AII) is intermediate, with a heterozygosity level of 0.037%. The level of allelic sequence heterozygosity for the dog genotypes (C and D) is estimated at 0.52 to 0.58% (19). The reasons for these differences are not known. The additional question to address is whether the heterozygosity is seen at a single-cell level. This question was addressed for genotype B by using clones that were selected by limiting dilution (63) and by sequencing single cells obtained by micromanipulation (64). These studies have confirmed heterozygosity at a single-cell level. Other studies of heterozygosity within genotype B have found that the frequency of silent substitutions in housekeeping genes make it very difficult to subtype these organisms into specific subgenotypes or subassemblages (65, 66).
In sexually reproducing organisms, the level of allelic heterozygosity is limited by the need for chromosome pairing during meiosis, and the levels found in Giardia that range from <0.01% to 0.5% are within the range expected in sexually reproducing organisms. This has raised the question of whether Giardia undergoes sexual reproduction. Support for sexual reproduction could come from examples of recombination among isolates or by documentation from cell biology of meiosis. The initial evidence for recombination among G. duodenalis isolates was derived from sequence analysis of single-copy genes of genotype AII field isolates from Peru (67, 68). Reports suggesting recombination between genotypes A and B (69) have not been confirmed by subsequent studies (19). However, recent work has suggested recombination between A and E (A is much closer phylogenetically to E than to B) (22) (see “Chromosomal organization,” below).
Proteomics and Transcriptomics
The rarity of introns in the genome, as well as the short 5′ and 3′ untranslated regions and the conserved putative polyadenylation signal, makes it relatively easy to generate the amino acid sequences directly from the genome. In addition, the transcript patterns (transcriptome) are available for a number of conditions for trophozoites or cysts. Despite that, a relatively small number of 3D protein structures have been determined experimentally, and existing sequence-based searches, including BLAST or hidden Markov model (HMM), have poor accuracy when there is a low amino acid identity to the sequences in the database. However, the alternative of structure-based searches with machine learning using the I-TASSER software suite has been used to examine the set of approximately 5,000 Giardia proteins (70). The authors generated a library of 1,095 structural models, which included 212 hypothetical proteins, at a high level of confidence and then used this library to explore the redox pathways that could contribute to resistance to nitroimidazoles.
CELL BIOLOGY
Trophozoite Cytoskeleton, Structure, Motility, and Adhesion
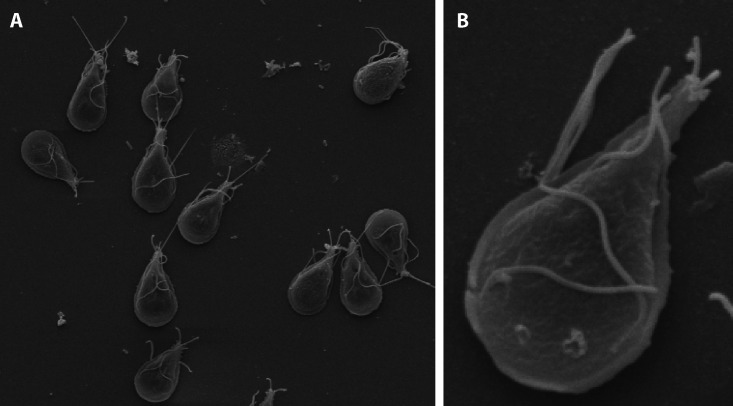
G. duodenalis trophozoites are pear-shaped, with a length of 12 to 15 μm and a width of 8 μm. They have four pairs of flagella and a concave ventral side that is surrounded by the lateral crest and a flange (Fig. 1) that allows the organism to attach to the intestinal epithelium. The dorsal surface is convex, and two symmetrically placed nuclei are in the anterior half of the organism (Fig. 2).
FIG 1.
A scanning electron micrograph shows multiple trophozoites adhering to a surface (A) and a magnified view (B). Note the flagella extending from the trophozoite.
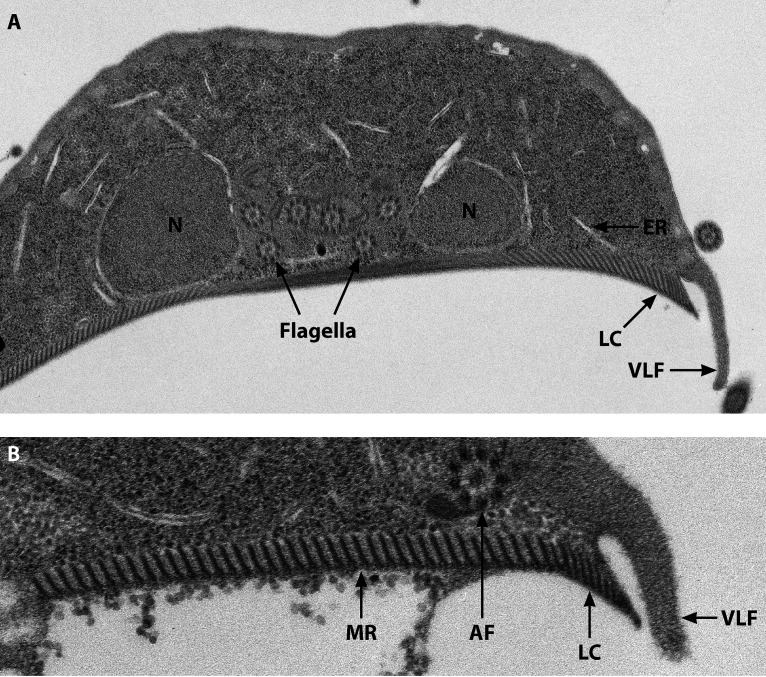
FIG 2.
(A) A transverse electron micrograph of a trophozoite through the two nuclei shows the nuclei (N), six flagellar axonemes between the nuclei with the canonical 9+2 arrangement, the ventral disk with the microribbons (MR), and the lateral crest (LC) and ventrolateral flange (VLF). (B) A higher magnification, including the anterior flagellum (AF).
The organism is remarkably adapted for survival within the small intestine, even in the absence of tissue invasion, and the cytoskeleton plays a central role in this adaptation. The cytoskeletal components include the ventral disk, the median body, and the eight flagella with their basal bodies. The core composition of the cytoskeleton consists of microtubules that are formed from a family of alpha- and beta-tubulins (71–74). The ventral disk and the median body are unique to Giardia species. The ventral disk is composed of a spiral of microtubules and associated sheets that are called microribbons (Fig. 2). There are also hundreds of disk-associated proteins, including ankyrins (initially called alpha-giardins) (75), all of which add rigid structure to the concavity of the disk (76). The disk itself has the ability to contract during attachment and utilizes the lateral crest (Fig. 2) to help generate the initial attachment (77, 78), as well as to remain attached when confronted with shear forces (79).
The flagella have the conventional eukaryotic 9 + 2 microtubule organization and are called the anterior, posterolateral, ventral, and caudal flagella according to the direction of their emergence from the basal bodies located between the nuclei (76) (Fig. 1 to 3). The flagella provide motility for the trophozoites, but their role in attachment continues to be debated. The beating of the ventral flagella occurs in conjunction with attachment to the intestinal epithelium and supports a model in which the ventral flagella would generate a hydrodynamic force resulting in suction by the ventral disk (80). However, more recent data have suggested that the ventral flagella help generate suction through a force that pushes the ventral disk against the intestinal epithelium and helps remove fluid under the disk to allow the initial attachment to occur (81, 82).
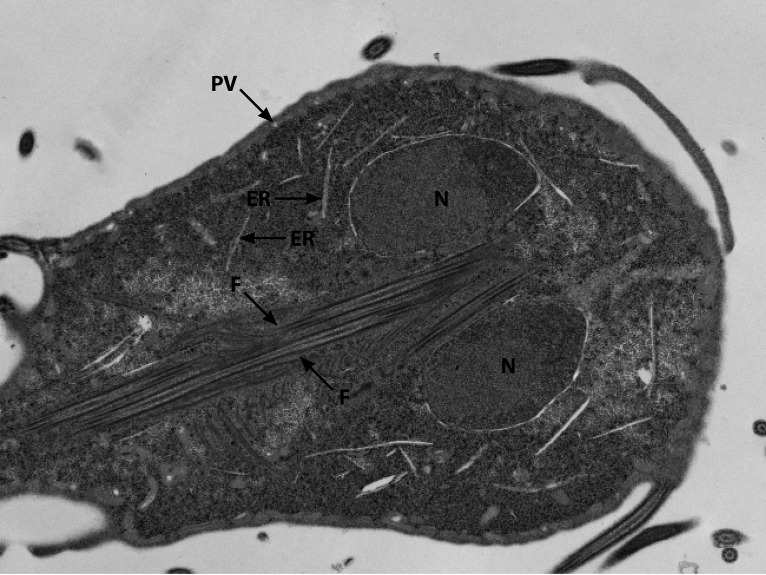
FIG 3.
A coronal view through the nuclei (N) also demonstrate the multiple flagellar axonemes (F) traversing through posteriorly. The endoplasmic reticulum (ER) and peripheral vesicles (vacuoles) can also be seen.
The median body is not only unique to Giardia species but is one of the identifying features for the various species. The median body of G. duodenalis has been called a “crooked smile” (72, 83). Its function remains unknown, but hypotheses include a role as a reservoir of tubulin subunits during cytokinesis (84) or that it may play a role in detachment (85). A protein called the median body protein (MBP) was initially found in the median body, but a subsequent study found that it is also in the ventral disk and is required for a proper dome shape of the ventral disk and thus for attachment (79).
A single highly divergent actin has been identified in the Giardia genome (4), but canonical eukaryotic actin-binding proteins have not been identified. However, recent studies have identified an actin cytoskeleton (86, 87). In addition, the eukaryotic actin-associated protein phosphoserine phosphothreonine 14-3-3 has been shown to interact with actin in G. duodenalis (88). However, the role of the actin cytoskeleton has not yet been determined.
Cytokinesis
Cell division is a complex process during which each of the trophozoite’s two mastigonts must replicate and segregate to the two daughter trophozoites, all while the organism remains attached to the intestinal wall. Trophozoites replicate by binary fission in a longitudinal plane (89). The ventral disk replicates with the daughter disks being formed at the anterior dorsal side of the trophozoite, followed by division along the longitudinal plane (90). The four pairs of flagella demonstrate semiconservative replication in which each daughter cell receives one parental flagellum in addition to a newly synthesized flagellum. During this process, the newly synthesized flagellum requires three replication cycles to mature, and during this time, the location of the flagella changes from posterolateral and ventral to anterolateral and ultimately to caudal, which is the most mature location and is the organizing center for the ventral disk microtubules (91). Actin appears to play a minimal and as yet not understood role in Giardia, and myosin has not been identified in Giardia, so the mechanism of cytokinesis must be different from that of canonical eukaryotes, in which cytokinesis depends on actin and myosin. A myosin-independent model has been suggested in which actin positions the microtubule cytoskeleton and works in cooperation with Rab11 to allow the division furrow to progress, with flagellar propulsion finally helping to complete the process (84).
Nuclear and Chromosomal Structure and Replication
Chromosomal organization.
Many of the Giardia organelles contrast significantly with those of canonical eukaryotes (Table 3), and of course the two transcriptionally active nuclei in dyadic symmetry provide one example. Initial data based on pulsed-field gel electrophoresis revealed the presence of five distinct chromosomes, but with up to four size variants for individual chromosomes (57). The size variants were subsequently shown to result from variation in the size of repeat regions in the subtelomeric regions; some of these repeats consisted of ribosomal DNA (rDNA) (92, 93). The five chromosomes have subsequently been confirmed by optical mapping (55) and by cytogenetic approaches.
TABLE 3.
Organelles of Giardia
| Conventional eukaryotic organelle | Equivalent Giardia organelle | Reference(s) |
|---|---|---|
| Nucleus | Two nearly identical nuclei | |
| Nucleolus | rDNA not organized in nucleolar pattern, but genes for nucleolus-localized proteins in genome and candidate nucleolus demonstrated by EM and confocal microscopy | 110, 330, 331 |
| Mitochondrion | Mitosome | 5 |
| Golgi | Numerous features of Golgi transport done by ER | 142 |
| Endosome/lysosome | Peripheral vacuoles or vesicles | 145, 332–334 |
| Peroxisome | Peroxisome-like proteins in cytoplasmic vesicles | 131 |
| Exosome | Exosome-like vesicles with minimal ESCRT | 150 |
Tetraploidy was confirmed by flow cytometry, with the interesting finding that trophozoites spend the majority of time in the G2 phase (after DNA replication) during in vitro cultivation (44). Thus, some of the earlier quantitative DNA analysis suggesting a ploidy of eight (57) resulted from analyzing organisms that were primarily in the G2 phase. This means that Giardia trophozoites in the G1 phase normally have a ploidy of four, with two diploid nuclei that are identical in most ways that have been tested (94). However, a small degree of aneuploidy has been documented by cytogenetic methods (95, 96). An interesting example has been described in the WB isolate and in a cloned line of WB, called WBC6. (WBC6 was cloned from the WB isolate shortly after its initial description in 1982 [97]). The WBC6 line was found to be aneuploid for chromosome 5, with three copies in one nucleus and one in the other (98). Chromosome 5 in the nucleus haploid for that chromosome had a deletion of a subtelomeric region, and five genes from that region were expressed only from one nucleus.
The chromosomes are highly compacted in comparison to many other eukaryotic chromosomes and possess 10-nm fibrils, 30-nm fibrils, and chromomeres (99). During replication, mitosis is semiopen. Prophase chromatin condensation, chromosome alignment, and movement to the spindle poles is similar to that in other eukaryotes, but the nuclear movement to the center of the cell and the telophase spindle structure differ from those of other eukaryotes (89). The centromere can be identified by the presence of a cenH3 histone variant (100). The eukaryotic spindle assembly checkpoint, which monitors attachment of the chromosome to the spindle during metaphase, has not been identified in Giardia (101). The telomeres at the end of the chromosomes consist of a 5-nucleotide repeat of TAGGG (92) and, in contrast to the 6-nucleotide human telomere of TTAGGG, the Giardia telomere forms a tetramolecular antiparallel structure (102), although the significance of this is unknown.
The above-described findings raise an interesting question regarding the replication of the nuclei of Giardia. On one hand, the two nuclei have essentially the same complement of DNA, and both are transcriptionally active (103). On the other hand, there are differences between the nuclei that are maintained through replication, providing evidence for a semiconservative distribution of the nuclei (94, 98). Initial data suggested that the same right-left asymmetry was maintained (94), but subsequent data have supported a model with mirror image replication of the nuclei (89). The semiconservative replication of the nuclei implies that there must be a mechanism of DNA exchange between nuclei at some point, since heterozygosity would continue to accumulate if there were no exchange. Direct evidence for exchange of nuclear material was supported by the EM observation of frequent fused nuclei in cysts, as well as by DNA fluorescent in situ hybridization (FISH) of plasmids transfected into the nuclei (104). This led to the conclusion that during encystation, the two nuclear pairs migrate from polar ends, fuse, and separate. Investigators suggested a model in which homologous recombination occurs after nuclear envelope fusion via a parasexual process called diplomixis (104). Indeed, recombination in eukaryotes can occur by canonical meiotic sexual reproduction or by a parasexual process, which is found in the common yeast pathogen, Candida albicans (105, 106). Meiotic genes are involved in parasexual processes as well, so the observations of the study support a role for the previously documented meiotic genes in the genome (4, 107).
Nucleolus.
The eukaryotic nucleolus is a small structure in the nucleus in which rRNA biogenesis occurs (108). The structure can be seen by electron or light microscopy and contains separate regions called fibrillar centers, dense fibrillar components, and granular components. Nucleoli were long thought to be absent in Giardia nuclei, and fibrillarin, which is involved in ribosome assembly, was found diffusely in the nuclei rather than in discrete nucleoli (109). More recently, structures consistent with nucleoli have been identified by light and electron microscopy (110, 111), but there is no evidence of a conventional eukaryotic nucleolar organizing region (112).
DNA repair.
Giardia has a DNA repair system in which the recombinase DMC1B functions as Rad51 (113), and Rad52 also plays a role in DNA repair (114). The dramatic differences among the genotypes in the level of allelic sequence heterozygosity, especially between genotypes AI and B, raise the question of whether these organisms differ significantly in the efficiency of their DNA repair systems. In addition, the ability of plasmid DNA to integrate into the genotype B genome but not the AI genome supports the possibility of differences in the repair mechanisms (115).
Transcription and translation.
The genes are present for eukaryotic RNA polymerases I, II, and III. For RNA polymerase II, the subunits rpb4 and rpb9 have not been identified, and in contrast to RNA polymerase II from other eukaryotes, transcription is resistant to amanitin (116). Although RNA splicing is relatively rare in Giardia, a spliceosome, along with core spliceosome proteins and small nuclear RNA candidates, has been identified (117). An earlier report suggested the absence of a 5-methyl-guanosine cap on mature mRNA (118), but subsequent studies confirmed conventional capping (119, 120). In view of the very short 5′ untranslated regions (UTRs), the first AUG is the one that is utilized for translation (119). Eukaryotic translation initiation normally involves the eIF4F trimer complex and the preinitiation complex. The eIF4F complex comprises eIF4A and eIF4E held together by eIF4G. However, the latter has not been identified in the genome, and there is evidence that the two remaining components can interact directly with the preinitiation complex (121), providing yet another example of the minimalization of the genome.
Mitosomes
In aerobic eukaryotic organisms, ATP is generated by oxidative phosphorylation in double-membraned organelles called mitochondria. In certain anaerobic protozoa, such as Tritrichomonas foetus (122) and Tritrichomonas vaginalis (123), there is an organelle called a hydrogenosome in which ATP is generated by substrate-level phosphorylation with the production of hydrogen. In contrast, Giardia trophozoites have an even more rudimentary organelle called a mitosome (5) or mitochondrion-like organelle (MLO) (124, 125). The mitosome is a double-membraned organelle that contains iron-sulfur (Fe-S) clusters, which play an essential role in assembling proteins with Fe-S, but there is no ATP production. The Giardia mitosome has the Fe-S cluster proteins and proteins related to biogenesis of the mitosome, but no other known functions (126–128). Unlike conventional mitochondria, the replication of the mitosomes is linked to mitosis (129).
Peroxisomes
Peroxisomes are named after their ability to reduce O2 to H2O2 and then to reduce the H2O2. They are nearly universal in eukaryotes but differ greatly in function, with the most common functions being detoxification and fatty acid synthesis (130). Giardia has long been believed to have no peroxisomes, but recent evidence suggests that a rudimentary form of this organelle may be present. Candidates for the peroxisome proteins, acyl-coenzyme A (CoA) synthetase long-chain family member 4 (ACSL-4) and peroxin-4 (PEX-4), are present in the Giardia proteome (131). Acosta-Virgen et al. used antibodies against these proteins, as well as cytochemical approaches, to localize their localization to vesicles that were distinct from encystation-specific vesicles.
Biosynthesis and Energy Metabolism
In keeping with its status as an obligate parasitic organism and its genomic minimalization, G. duodenalis also has very limited biosynthetic and energy production pathways (132). The energy metabolism is anaerobic, lacking cytochrome-mediated oxidative phosphorylation. Glucose is the major carbohydrate source of energy and is metabolized into various concentrations of acetate, ethanol, alanine, and CO2. The relative concentrations depend on the oxygen concentration, with alanine as the major product under strict anaerobic conditions. Figures 1 to 3 in my prior review (1) give the detailed pathways. At oxygen concentrations greater than 0.25 μM, the production of alanine is inhibited and ethanol is stimulated, but both are produced. At an oxygen concentration of >46 μM, both are replaced by acetate and CO2 (133). This range of oxygen concentration likely represents the range of oxygen exposure seen by the trophozoites in the small intestinal environment.
Giardia trophozoites have very little endogenous amino acid synthesis (reviewed in reference 1) but do use amino acids acquired from the host as an important source of energy. The most studied pathway is the arginine deiminase pathway, in which arginine is metabolized to produce ornithine with the generation of ATP from ADP (1, 134). This pathway is present in other diplomonads, as well in the parabasalids to which T. vaginalis belongs (135). In addition to being an important source of energy (134), it may play a role in pathogenesis (see “Proteases and Secreted Substances,” below). Aspartate may be an additional energy source through conversion to pyruvate through a malate intermediate.
Many pathogenic protozoa depend on purine salvage, as does Giardia. In addition, Giardia also depends on pyrimidine salvage, as do the trichomonads (1).
Fatty acids play key roles in the life cycle and metabolism of Giardia, including in encystation. Cholesterol and bovine bile from growth medium are able to induce encystation, and genes linked to cholesterol biosynthesis are upregulated during encystation (136). In addition, there is evidence for synthesis of phosphatidylglycerol and phosphatidylethanolamine in vegetative and encysting trophozoites (137), which led to a proposed model of lipid metabolic pathways in Giardia (see Fig. 4 in the review of Yichoy et al. [138]).
The Endomembrane Transport System
The eukaryotic Golgi apparatus is a distinct-appearing membrane-bound organelle consisting of membrane stacks and vesicles. It accepts molecules from the ER, modifies them as required, and delivers them to their required location on the basis of appropriate signal sequences. Thus, it has always been surprising that Golgi apparatus are not found in vegetatively growing Giardia trophozoites. For many years, it was assumed that Giardia had no Golgi apparatus and perhaps had emerged from the eukaryotic lineage before the development of the Golgi apparatus (139, 140). However, the discovery of structures consistent with the Golgi apparatus in encysting trophozoites (41) and the identification of protein transport that was inhibited by brefeldin A, which is used as a tool for studying Golgi transport (141), gave an indication that at least some of the functions of Golgi transport were present. Subsequent studies have suggested that the endoplasmic reticulum (ER) fills many of the Golgi functions in Giardia (142). In a canonical eukaryotic transport system, a COPII coat assembly mediates the transfer of its cargo from the ER to the Golgi apparatus, and transfer from the Golgi apparatus to the ER is mediated by COPI. In Giardia, members of the COPII complex are located at specific sites in the ER where cyst wall protein 1 (CWP1) is transported and transferred to ESVs (143). This occurs in vegetative and encysting trophozoites. Members of the COPII complex interact physically with CWPI at these sites (142). The eukaryotic KDEL receptors function in protein transport at the Golgi apparatus-ER junction, and the single Giardia KDEL receptor is also found at the same specific ER site. These findings all suggest that the ER has taken over the functions of the Golgi apparatus in Giardia (140).
Endocytosis is performed by the peripheral vesicles (PV), which are acidified for importing materials required by the trophozoite (144) and function as endosome-lysosomes (145). Invaginations of the dorsal side of the trophozoite that are associated with the presence of PVs on the inside suggest that import of these materials occurs by means of membrane fusion (146). The PVs also interact with the Giardia clathrin heavy chain, which is involved in eukaryotic endocytosis, and with associated proteins (146).
Exocytosis involves the VSPs in particular, which are secreted in high quantities (147) and form a protein coat over each trophozoite (148, 149). The secretion system is not well characterized, but a recent study identified exosome-like vesicles in in vitro cultures and demonstrated the role of ESCRT-associated proteins and ceramide in forming intraluminal vesicles inside the PVs (150). This supports the idea that the secretory process of Giardia also depends on the ER and the PVs.
Membrane and Surface Proteins
The VSPs and vsp genes.
The variant-specific surface proteins (VSPs) were initially called cysteine-rich protein (CRP) (151), trophozoite surface antigen (TSA) (152), and trophozoite surface protein (TSP) (153). They are currently called VSPs (154) because of their ability to undergo antigenic variation at a rate of up to 10−3 per cell division (155). These proteins are cysteine rich (about 12% cysteine), with frequent CXXC motifs (151). The vsp genes that were initially described all encoded a C-terminal CRGKA (154). They are heterogeneous in size, ranging from about 2 to 6 kb, corresponding to about 60 to 180 kDa for the translated protein. The size of the vsp gene repertoire for WB has been estimated at 270 to 300, making up 4% of the genome. It includes 30 with tandem repeats (as does vspA6, the first vsp described), and 14 in pairs with head-to-head or tail-to-tail arrangements (156). How many of these are functional vsp genes cannot be determined by a genomic approach. Thus, a study to determine the sequence motifs that are required to result in expression on the surface of the organism determined that the CRGKA motif was not required but that two other motifs (called motifs 1 and 2) are required for localization to the cell membrane (157). Motif 1 consists of 45 AA with CXXC flanking 12 to 15 AA near the C terminus, and motif 2 consists of the 38 hydrophobic AA at the C terminus. Li et al. identified 73 genes meeting these criteria and proposed them as true vsp genes. However, five were missing the CRGKA, and the CRGKA was modified in another three. Since all experimentally described vsp genes include the CRGKA, it may still be that this motif plays another essential role. This also opens the question of whether the additional 200 putative vsp genes are expressed and, if so, what their function is.
The VSPs appear to play a role in immune evasion (see “Immune response,” below). Some VSPs protect the trophozoites from intestinal proteases (158) and may play other roles in adaptation to different hosts (159, 160). The biological role of the VSP is not known, but based on the amount of the genome dedicated to the encoding genes, it most likely plays an important role.
A number of studies on the mechanism of antigenic variation of the vsp genes have been performed, but the mechanism of change is only partially understood. The rapid change in expression, estimated at every 6 to 13 generations (155) suggests an epigenetic mechanism. There is no current evidence that DNA sequence alteration or recombination plays a role in antigenic variation. This is in contrast to the antigenic variation that occurs in African trypanosomes, in which sequence alterations and rearrangements are commonly associated with antigenic variation (161).
A study of antigenic variation of the vspA6 (CRP170) gene indicates the loss of the expressed version in variants, which likely means the loss of the expressed allele (162). It is possible that the loss of the expressed version represents loss of the chromosome containing the expressed vspA6 (see evidence for aneuploidy in a paper by Tůmová et al. [98]).
Control of antigenic variation was studied for the vspH7 gene from the GS isolate (genotype B) by using stable transfection with homologous integration of a hemagglutinin (HA)-tagged vspH7 gene into the vspH7 genome location (163). The authors showed that expression of the native vspH7 and the HA-tagged vspH7 were independent of each other, implying that expression was allele specific. In addition, lysine acetylation occurred immediately upstream of the integration in association with expression, suggesting a potential epigenetic mechanism for allele-specific expression.
There is also evidence for the role of RNA interference (RNAi) in the control of vsp expression. Organisms expressing a single vsp have one stable transcript, but other vsp genes are transcribed but do not result in stable transcripts. These silenced genes correlate with small RNAs consistent with an RNAi process preventing their expression (164). The Giardia genome encodes an RNA-dependent RNA polymerase (RdRp), Argonaute, and Dicer as components of an RNAi system (164, 165). When the RNAi pathway was disrupted by constitutively expressing antisense transcripts for RdRp, Argonaute, and Dicer, multiple vsp genes were expressed simultaneously (164). Giardia has 25 small nucleolar RNAs (snoRNAs) (166), and two snoRNAs were identified that were precursors of the miRNAs miR6 and miR10, which are processed by Dicer into a mature miRNA duplex (166). Forty-four of the vsp genes have potential binding sites for miR6 in the 3′ UTR, and 159 have potential binding sites for miR10 at the 3′ end of the open reading frame (ORF); 33 have binding sites for both. Gerbils infected with G. duodenalis could be protected from subsequent infection by exposure to RNAi-disrupted organisms expressing multiple VSPs, but not by those expressing a single VSP, providing evidence that antigenic variation is important for survival of the organism in the host intestine (167).
In summary, there is evidence supporting at least the following two mechanisms for control of vsp expression: (i) lysine acetylation, which can limit expression to even one allele of four, and (ii) an RNAi system that limits expression to one vsp at a posttranscriptional level. However, the actual mechanisms for switching from expression of one vsp gene to another remain to be determined.
High-cysteine membrane proteins.
In addition to the vsp genes, there is a family of cysteine-rich HCMPs that do not undergo antigenic variation, but some of these are upregulated during encystation (50). The estimated number of HCMP genes is now 116 on the basis of a more complete genome assembly of the WB isolate (56). The HCMPs localize to the nuclei of trophozoites. During encystation, they are transported by the encystation-specific vesicles (ESV) and can be found in the cell body as well as in the wall of mature cysts. The role of these proteins remains to be determined.
Other membrane-associated proteins have also been described but are not yet characterized (168).
Giardiavirus (Giardia lamblia Virus)
Giardiavirus (Giardia lamblia virus [GLV]) (169) is a double-stranded RNA virus found in Giardia trophozoites from genotypes A and B. It is a member of the Totiviridae family, which also has members that infect fungi, Trichomonas, and Leishmania (170). After internalization by what may be receptor-mediated endocytosis, it is concentrated in the peripheral vacuoles (171). Although many of the isolates are not infected, the majority are susceptible to infection when exposed to the virus. The RNA is 6.2 kb in length and encodes a virus composed of a 100-kDa viral capsid protein and an RNA-dependent RNA polymerase (RdRp). Giardiavirus-infected trophozoites do not appear to differ from uninfected organisms in their fitness or virulence (172, 173). An miRNA encoded by the RdRp gene has been identified that may limit the viral copy number (174).
EPIDEMIOLOGY
G. duodenalis is distributed worldwide, but there is important variation in geographic distribution and epidemiology. Cysts survive in the environment longer in cool, moist areas. Thus, in temperate climates, which tend to be low-prevalence areas, giardiasis is often seen in the form of symptomatic waterborne outbreaks (28, 175). In fact, it was in the 1960s and 1970s with reports of outbreaks among Finnish travelers (from a low-prevalence region) to St. Petersburg (Leningrad), which had inadequately treated water at that time, that Giardia became generally accepted as a human pathogen. Some of the reported series had attack rates of over 50% (176–178). Similarly, important waterborne outbreaks have been documented in northern regions, such as Bergen, Norway, in 2004 (179, 180) and British Columbia (181). In the United States, it was found that in outbreaks of giardiasis at ski resorts, visitors were at higher risk than local residents, suggesting partial acquired immunity in the residents (182). The partial protection of the local residents suggests that the outbreak-associated pattern of high attack rates is due to infection in hosts without prior immunity. In contrast, giardiasis regions where it is highly endemic, particularly in low-income areas in tropical and subtropical areas (e.g., shantytowns of Lima, Peru) (183), is typically subclinical.
Within the United States, giardiasis may be outbreak related and is usually associated with drinking water or recreational water exposure. Food-borne outbreaks also occur but are less frequently documented. For sporadic cases, the risk factors are numerous and include direct and indirect fecal contact, as well as host factors (175, 184). Major risk factors found in a study of reported cases from Colorado and Minnesota, the major risk factors are shown in Table 2 of Reses et al. (184).
Some risk factors, such as male-male sexual contact and international travel, have very high odds ratios, but on a population basis, additional risk factors with lower odds ratios are still important because of their high prevalence; these include daycare exposure, swimming in or drinking from natural water bodies, and even chronic gastrointestinal conditions or the use of antibiotics. Animal exposure has not been a risk factor in recent studies (175, 184), and the role of animals remains controversial. There are human cases with reasonably well-documented animal sources, but quantitatively, animals play a small role (see for example, references 67 and 185). In fact, data from Brazil suggest that transmission from humans to animals is more common than that from animals to humans (186). Food-borne outbreaks are occasionally reported but are likely underrecognized, and sporadic cases due to food are very difficult to identify (187), so the true importance of foodborne transmission remains unknown.
Numerous studies of the relative importance of genotypes A (usually AII) and B have been reported, but the results of these studies do not clearly identify a difference in epidemiology. In contrast, there is accumulating evidence that genotype AI is primarily a zoonotic infection (21, 22).
PATHOGENESIS
G. duodenalis is a noninvasive pathogen of the small intestine and produces a wide range of clinical presentations, including chronic diarrhea with weight loss, postinfectious complications of irritable bowel and chronic fatigue, growth stunting, and asymptomatic infections. These varied manifestations may result from host, parasite, or microbiota differences and make it particularly difficult to elucidate the mechanisms resulting in this range of illness. Nevertheless, there has been substantial progress over the past 2 decades in elucidating some of the mechanisms. Recent in-depth reviews of these advances are also available (188–191).
The trophozoites adhere tightly to the small intestinal mucosa, leaving a detectable imprint when they detach from the intestinal epithelium (192), so the possibility of direct pathogenesis from the mechanical attachment has been raised. However, there is currently no evidence to support this possibility. Rather, current data suggest that a combination of secreted proteases and other Giardia factors, the host immune response, and the interaction of these factors with the intestinal microbiota contribute to the various manifestations (191, 193). Through the entire immune response, it is actually remarkable that in patients who have biopsies for symptomatic giardiasis, the findings consist of flattening of the villi but no obvious inflammatory changes. However, an inflammatory picture can be seen and may actually be separate from the location of the trophozoites. A series of cases was reported in which trophozoites were seen in ileal biopsy specimens from ileocolonoscopy of symptomatic patients but inflammatory changes were found in the duodenal biopsy specimens of these patients (194). All patients had ileal blunting or atrophy, 6 of 11 had neutrophilic infiltration, and one had findings consistent with celiac disease. The finding of trophozoites in the ileum is consistent with the animal model in which trophozoites were concentrated in the proximal small intestine but were sometimes found in the ileum or even the cecum (34). These findings raise interesting new questions about the pathogenesis of giardiasis that are being pursued by ongoing studies.
Proteases and Secreted Substances
The role of excretory secretory products (ESPs), most notably the cysteine proteases, has received substantial attention over the last decade (193). Although serine proteases are also present in the ESP, the major portion of the protease activity is from the cysteine proteases (195). It may be that the primary role of these proteases is to control encystation and excystation, but they also play roles in the interaction between the trophozoite and the host intestinal epithelium.
Trophozoites interfere with the intestinal tight junction by number of mechanisms that include the cysteine proteases (193, 196, 197). A study of the effect of CP2 (called giardipain 1 in this study) on an intestinal epithelial cell line (IEC-6) monolayer demonstrated damage to the cell junctions that was prevented by the protease inhibitor E-64 (198). In addition, CP2 induces apoptosis and damages the villi by its interaction with villin (199). The intestinal mucus layer is a key component of innate defense against pathogens and, as such, it is notable that the cysteine proteases have a complex effect on the mucus. MUC2 (human mucus protein) is degraded in vitro, and MUC2 gene expression in goblet-like cells is increased, leading to the hypothesis that the Giardia cysteine proteases may deplete the normal intestinal mucus reserve (200). In the same study, the mucus was thinned in infected wild-type mice, and Muc2-deficient mice developed weight loss when infected. Bacterial translocation was also increased in the wild-type mice (200).
It is likely that many additional ESPs play a role in pathogenesis, so a systematic approach was used to identify the secretome of Giardia and intestinal epithelial cells (IECs) differentiated from Caco-2 cells using two different isolates (WB from genotype AI and GS from genotype B) (201). Proteins related to metabolism were among the dominant Giardia ESPs and included those related to glycolysis, arginine metabolism, phospholipid remodeling, and nucleotide salvage. In addition, proteins involved in encystation, VSPs, HCMPs, alpha-giardins (annexins), and tenascins were increased. When the GS and WB isolates were compared, the classes of proteins were similar, although the number of proteins differed between the isolates. In addition to the effect of IECs on Giardia, the response of IECs to Giardia was also studied. The response included chemokine production for recruitment of immune cells, impact on glucose and lipid metabolism, and induction of apoptosis.
A transcriptomic study of the interaction of WB (genotype AI) trophozoites with IECs was done to explore which genes were upregulated (202). Overall, the HCMPs showed the greatest increase in expression, but were varied in their responses. Another study of the secretome of these two Giardia isolates, focused particularly on the tenascins, found similar results (203). The mammalian tenascins are extracellular matrix proteins, but tenascins are not well characterized in unicellular eukaryotes. The Giardia tenascins have been proposed as virulence factors, but specific studies have not yet been reported.
Arginine consumption by Giardia reduces nitric oxide production (51, 204) and proliferation of IECs (205). The Giardia arginine deiminase is a cytoplasmic enzyme that is secreted upon interaction with IECs in abundant quantities (206) and is responsible for arginine depletion. Arginine depletion also impacts the immune response to Giardia by increasing tumor necrosis factor alpha (TNF-α) and decreasing interleukin 10 (IL-10) in dendritic cells (207) and by reducing T-cell proliferation in intestinal epithelial cells (204). Thus, arginine deiminase can be considered a virulence factor, in addition to its role in energy production for trophozoites.
Immune Response
Early assumptions and even dogma about the central role of the antibody response for immunity to Giardia infections was supported by observations of prolonged giardiasis with total immunoglobulin or IgA deficiency. Those observations were never well documented, and the story has subsequently become much more complicated and interesting. There is evidence for a significant role of the IgA response in view of the observation of a brisk IgA response in infected humans that is directed particularly against semiconserved portions of the VSP (208).
Since the VSPs undergo antigenic variation, this raises the question of whether the antigenic variation is to avoid the host immune system. Earlier data in gerbils (160) showed that after the change in VSP expression that occurred within the first 7 days, there were no further changes by 28 days, suggesting that antigenic variation might be unrelated to avoiding the host immune response. However, in human volunteers, the data on the change in VSP expression were more consistent with a role for antigenic variation in survival in the host (159). Subsequent data have accumulated that strongly support a role for antigenic variation in surviving the host immune response. A vaccination study was done using a WB isolate that had been transfected to silence Dicer and the RNA-dependent RNA polymerase, with the effect of suppressing RNAi and allowing multiple VSPs to be expressed simultaneously. This organism was used to infect gerbils and induced effective immunity against reinfection (167). Gerbils were also protected by immunization with a vaccine composed of the multiple VSPs. The early immune response was primarily Th1, including gamma interferon (IFN-γ), TNF-α, and IL-17, and it was followed by a Th2 response. A similar vaccine was given to domestic animals and provided protection from infection (209). The ability to develop a protective immune response after exposure to multiple VSPs suggests their important role in evoking an immune response, as well as the role that antigenic variation plays in surviving the host immune response.
TH17 cells produce IL-17A as well as other cytokines and, in addition to protective immunity, play a role in autoimmune diseases, including inflammatory bowel disease, meaning that precise regulation is required (210, 211). IL-17A has been recognized as a key component of the immune response to Giardia infection and has been shown in mice infected with G. duodenalis (212) and with Giardia muris (213) and facilitates the IgA response (214). Studies have also shown that the IL-17 receptor A (IL-17RA) is a regulator of the immune response to Giardia infection and that upregulation of the receptor is required for a local IgA response (214, 215). When IL-17RA knockout mice were infected with G. muris, expression of many of the genes that are normally upregulated by Giardia infection was inhibited (215). These animal model studies suggest a key role of IL-17 A and IL-17RA in controlling Giardia infection. A Th1 (CD4) response with IL-17A-producing CD4 cells has also been documented in infected human travelers (216), as well as a trend toward increased Il-17 levels in children in Brazil (217), showing that the mouse model correlates with human infections. A study of the Th17 response to G. muris was done in BALB/c mice compared to C57BL/6 mice. The BABL/c mice had an impaired Th17 response that correlated with impaired clearance of Giardia, leading to the conclusion that a correctly balanced Th17 response appears to be required for effective G. muris infection in mice (218). An ineffective response results in prolonged infection, while an excessively exuberant response may result in a host inflammatory response that is damaging to the host. Perhaps inadequately regulated immunity can explain the great variability in symptoms, as well as that in clearing the organism.
Mast cells are recruited to the intestine following infection, play a role in controlling infection (189), and may also contribute to the abdominal cramping that is common in symptomatic giardiasis (219). A recent study has suggested that mast cells may be activated by arginine deiminase or by its product, citrulline (220). There is also an accumulation of macrophages that produce arginase 1 and nitric oxide synthase 2 in mouse lamina propria following G. duodenalis infection (221), but macrophage depletion does not impair clearance of the trophozoites (222), so the significance of the macrophages is not yet clear.
In addition to the inflammatory response induced by Giardia infection, there are also examples where Giardia actually attenuates the inflammatory response. G. duodenalis extracts increase IL-10 production by dendritic cells (223, 224) and decrease IL-12 (223) through the phosphoinositide-3-kinase (PI3K) pathway (223). The importance of this attenuation is emphasized by a study in which G. muris infection of IL-10-deficient mice caused colitis (225). The colitis was Myd88 dependent and was facilitated by the intestinal microbiota. G. muris also reduces the severity of intestinal disease in mice caused by the attaching and effacing pathogen Citrobacter rodentium (226). This was dependent on the NLRP3 inflammasome and was associated with increased antimicrobial peptide production by the host (149). G. duodenalis also reduced neutrophil infiltration in biopsy specimens from humans with active Crohn’s disease (227). G. duodenalis cathepsin B cysteine proteases degraded the neutrophil chemoattractant IL-8 in biopsy specimens infected with nontyphoidal Salmonella (228).
Malnutrition and Long-Term Sequelae
Over the last decade, there has been an increasing appreciation of the pathological and physiological roles of the intestinal microbiota. In fact, for about 50 years, there has been a recognition that Clostridioides difficile (formerly Clostridium difficile) colitis is facilitated by the predominance of bacterial flora that allows the colitis to develop, confirmed by evidence that repopulating the colonic flora using bacteria from healthy hosts substantially reduces the risk of C. difficile colitis (229). More recently, the use of next-generation sequencing has allowed systematic evaluation of the microbial makeup of the intestine and its association with health and disease. A landmark study on the role of the microbiota in malnutrition was done in twins in Malawi who were discordant for severe malnutrition (230). Colonic flora from the malnourished children, but not that from their healthy twins, reproduced malnutrition in a mouse model. Subsequently, alterations in intestinal flora have been associated with disorders that range from inflammatory gastroenterological diseases (210) to metabolic diseases, including diabetes (231).
Early data specific to Giardia regarding the importance of bacterial flora came from evidence that germfree animals would not develop disease from Giardia despite being susceptible to infection (232, 233). Mice also differ in susceptibility to Giardia infection with differences in microbial flora (234). These observations emphasize the importance of understanding the interplay between Giardia, the intestinal microbiota, and the host immune response (191). The impact of Giardia infection was examined in a mouse model of G. duodenalis infection using the GS isolate (genotype B), which establishes infection more easily than genotype A organisms and requires less use of antibiotics to establish infection (235). The investigators infected animals with Giardia (with and without antibiotic supplementation) and found that the number of Giardia organisms in the proximal small intestine was much greater in the animals who received antibiotics. The alpha diversity, a measure of diversity within the microbiota, was not changed. However, there was a shift in the microbiota (dysbiosis) induced by Giardia in animals, whether those animals were treated with antibiotics or not, particularly a shift to Proteobacteria and away from Firmicutes. This provides an interesting contrast with a study in which a human microbiota was exposed to Giardia trophozoites (genotype not stated) and then infused into germfree mice, resulting in a microbiota in these mice that was enriched in Clostridiales species from the Firmicutes group. These effects on microbiota substantiate the complex interaction between the bacterial flora and Giardia during Giardia infection. Other investigators using the GS isolate in a mouse model found that Giardia infection resulted in decreased sucrase activity, an effect that was attenuated by an antibiotic combination with a broad spectrum of Gram-positive and Gram-negative activity, but without activity against Giardia (236). They also found that pathological T-cell CD8 activation was prevented by antibiotics, suggesting a role of the microbiota in altering the immune response to giardiasis. Others have used an ex vivo approach to study the interaction of Giardia trophozoites with colonic biopsy material with a microbial biofilm. They found that Giardia, by its excretory secretory proteases, altered the biofilm. The microbiota enhanced lymphocyte production of proinflammatory cytokines and, in epithelial cells, decreased tight junction ZO-1 protein expression and increased proinflammatory CXCL-8 production and Toll-like receptor 4 (TLR4) expression. This led to their hypothesis that the pathogenesis of giardiasis is mediated through a TLR4 response and could explain its association with irritable bowel syndrome (IBS) (237). The complex interaction between Giardia and the intestinal bacteria was also demonstrated by the disruption of the tight junction after a resolved Giardia infection in a mouse model using GS (genotype B) Giardia. The disruption of the tight junction persisted after resolution of the Giardia and led to an influx of bacteria along with a neutrophilic inflammatory reaction (238). These findings from IECs contrast with a mouse model in which TLR2-deficient mice displayed enhanced clearance of Giardia along with increased proinflammatory cytokine production (239). In the latter study, there was no modification of the microbiota.
Several observational studies of humans have now been reported. A microbiota study of fecal samples of 20 people from the Ivory Coast, some with abdominal pain and some with no obvious symptoms, found that the 10 infected with Giardia had a dysbiotic pattern, as measured by a decreased Faecalibacterium prausnitzii:Escherichia coli ratio (240). A study from Colombia evaluated 23 fecal samples from 290 initial samples, each containing only one species of parasite, using 16S sequence analysis. Nine had Giardia with or without other parasites. They found that children with giardiasis had a distinct microbiota type that was characterized by an increased prevalence of Prevotella spp. (241). A study of 37 asymptomatic children in Argentina using whole-genome sequencing found that the 13 with >1 fg/μl of Giardia DNA in stool had decreased alpha diversity, along with increased abundance of Prevotella spp. (242). A study was reported using 1,004 participants from the Global Enteric Multicenter Study (GEMS) who had microbiome data, 215 of whom had Giardia identified. The GEMS study included children from low-resource countries who had moderate to severe diarrhea (243). In addition, there were about 913 fecal samples from Peruvian children under 2 years of age who were enrolled in the Malnutrition and the Consequences for Child Health (MAL-ED) study (244) and had 16S sequencing done. Those from the GEMS study (with acute symptoms) had increased abundance of Prevotella spp. and decreased abundance of Gammaproteobacteria, and those from the MAL-ED study (without acute symptoms) also had increased Prevotella and decreased E. coli (the most abundant member of the Gammaproteobacteria family) abundance (245). These observational studies, including one with a large sample size, showed consistent findings of increased Prevotella and decreased Gammaproteobacteria abundance in association with Giardia infection and represent the beginning of research that is likely to move from investigating associations to determining causality.
A neonatal rat model of postgiardiasis irritable bowel syndrome (IBS) has been developed, recreating some of the features of human IBS, including visceral hypersensitivity (246). These rats had persistent visceral hypersensitivity after Giardia infection, in addition to an impaired tight junction and increased translocation of commensal intestinal bacteria. C-fos has been a marker for the painful stimulus of IBS, and its expression was increased in this model, supporting the validity of the model.
A study done to understand the mechanism of malnutrition caused by Giardia used a weaned mouse model infected with Giardia cysts to explore the impact of Giardia on malnutrition (247). Mice were given normal or protein-deficient diets and infected with cysts from the GS isolate. Giardia caused epithelial hyperplasia and villous blunting through a Th2 immune response, along with worsened malnutrition, primarily in the protein-deficient mice. In addition, the malnutrition prevented an effective immune response. Thus, there was a synergistic effect of malnutrition and giardiasis in this model. These investigators also demonstrated a synergistic pathogenesis between Giardia and enteroaggregative E. coli, in which Giardia prevented a microbiota-dependent clearance of the E. coli bacteria (248). These animal model studies provide an important insight into potential mechanisms of Giardia persistence, as well as associated malnutrition.
CLINICAL MANIFESTATIONS
Giardia infection is best known for a subacute onset of diarrhea beginning 1 to 2 weeks after exposure, which consists of loose, greasy stools and flatulence and is frequently accompanied by weight loss (Table 4). Fever and other systemic symptoms are uncommon, and patients typically present for medical evaluation days to weeks after the onset of symptoms. However, other clinical manifestations frequently receive less attention, and the majority of infections are subclinical, especially in regions where giardiasis is endemic.
TABLE 4.
Signs and symptoms of giardiasis
| Symptom | % Reported in reference: |
|||||||
|---|---|---|---|---|---|---|---|---|
| Brodsky et al. (335) (N = 324) | Moore et al. (336) (N = 56) | Walzer et al. (176) (N = 32) | Kent et al. (337) (N = 240) | Shaw et al. (338) (N = 183) | Osterholm et al. (339) (N = 29) | Steen and Damsgaard (340) (N = 200) | Median % | |
| Diarrhea or loose stools | 96 | 93 | 72 | 98 | 92 | 100 | 95 | 95 |
| Malaise or weakness | 72 | 80 | 88 | 86 | 20 | 97 | 83 | |
| Foul-smelling stools | 57 | 75 | 79 | 75 | ||||
| Abdominal cramps | 61 | 77 | 59 | 85 | 70 | 83 | 53 | 70 |
| Wt loss | 62 | 73 | 69 | 13 | 59 | 6 | 60 | |
| Nausea | 60 | 59 | 59 | 74 | 58 | 59 | 59 | |
| Decreased appetite | 60 | 56 | 2 | 56 | ||||
| Greasy stools | 55 | 52 | 54 | |||||
| Bloating or distension | 42 | 62 | 34 | 75 | 9 | 79 | 34 | 42 |
| Flatulence | 35 | 30 | 89 | 6 | 32 | |||
| Vomiting | 29 | 34 | 36 | 23 | 17 | 29 | ||
| Belching | 26 | 30 | 28 | |||||
| Fever | 17 | 17 | 15 | 28 | 21 | 17 | ||
| Constipation | 9 | 1 | 5 | |||||
In these settings where giardiasis is highly endemic, children are infected early in life and usually do not have diarrhea. A study in a high-prevalence area of Peru reported in 1988 (183) that within 6 months after treatment with a nitroimidazole, 98% again had Giardia detected in stool specimens. However, these children had no symptoms and no excess fat excretion in association with their giardiasis. A study from the same area in Peru showed a lack of correlation of Giardia infection with diarrhea or growth stunting (249). Subsequent studies in other areas where Giardia is highly endemic have confirmed the lack of acute symptoms (250), with some even finding that Giardia was associated with reduced diarrhea (183, 251), resulting in the suggestion that Giardia might be protective. Despite the general absence of diarrhea, some studies of giardiasis in children have found evidence of malnutrition and/or growth stunting (252–255), while others have not (249, 256). The multicenter MAL-ED study prospectively followed 2,089 children to determine the impact of giardiasis (257), and in this study, only one site in Pakistan showed a negative association of giardiasis and diarrhea. In that one site, the apparent association mostly resolved when controlling for the use of metronidazole shortly before the diagnosis of giardiasis. In contrast, there was evidence across the sites for malnutrition, including growth stunting and an increased lactulose:mannose fecal excretion ratio suggestive of malabsorption. Higher zinc and vitamin A levels were associated with reduced incidence of subsequent giardiasis, indicating that reduced levels of these micronutrients were a risk factor for subsequent giardiasis. Whether the levels were also reduced as a result of giardiasis was not answered. In the same study, Giardia infection was not associated with elevated fecal inflammatory markers, in contrast to increased levels with other pathogens, including Campylobacter and enteroaggregative E. coli (258). Quantitative PCR was done in 1,469 of the children during a 2-year follow-up period, and Giardia, along with Shigella, E. coli, and Campylobacter, was associated with a decreased growth rate (259). Giardia infection was specifically associated with malnutrition in a cohort of children between 6 months and 2 years of age from Bangladesh who were selected based on impaired growth (260). In summary, the cumulative evidence suggests that Giardia infection is associated with growth retardation and other measures of malnutrition, even in children with no overt symptoms.
Postinfectious IBS is common after infection with a variety of gastrointestinal pathogens (261), but until recently, little attention was given to IBS following giardiasis. The occurrence of a large outbreak of symptomatic giardiasis in Bergen, Norway, provided an opportunity to prospectively follow these patients to determine long-term sequelae. In the fall and winter of 2004, an outbreak of giardiasis included over 1,500 patients with laboratory-diagnosed giardiasis (179, 180) and a total of 2,500 who were treated for giardiasis. These patients were followed long term for sequelae, including IBS and chronic fatigue syndrome (CFS). A case-control study of 817 of these patients found that 3 years later, 46% had IBS by Rome III criteria compared to 14% of controls (262), and 46% had CFS compared to 12% of controls. Even 6 years after the exposure, 39% still had IBS and 31% had CFS. In comparison, a U.S. study based on insurance information found an IBS rate of 3.8% after giardiasis compared to 0.4% for controls for an adjusted hazard ratio of 3.9 (263), and a study of U.S. military personnel showed a 2.1-fold increased risk (264). Thus, symptomatic giardiasis connotes a risk for IBS and CFS as sequelae, which may be higher than that reported for other gastrointestinal pathogens (265).
A risk for postinfectious joint pain has also been suggested on the basis of U.S. insurance data (odds ratio, 1.5) (266). Other sequelae, such as urticaria (267), have been reported rarely, primarily at a case report level or on the basis of finding an increased frequency of Giardia organisms in the stool, so causality and level of risk are more difficult to determine.
DIAGNOSIS
The diagnosis of giardiasis should be considered in a patient who presents with subacute or chronic diarrhea, especially when accompanied by the typical symptoms (Table 4). The “gold standard” for clinical diagnosis consists of microscopy of a fecal sample. Ideally, this should consist of a wet preparation of a fresh specimen for detection of trophozoites and a fixed specimen for detection of cysts or trophozoites. However, one limitation of microscopic identification is the dependence on expertise of the microscopist, since there may be artifacts in fecal specimens that can be confused with Giardia. Immunologic detection of stool antigen has a sensitivity similar to that of microscopy, as well as good specificity, and it is less labor-intensive, so in some settings, it is a good substitute for microscopy. There are also numerous nucleic acid detection tests that are now on the market, including many that are multiplex (Table 5). Some of the multiplex tests are specific to parasitic agents, while others cover the entire range of fecal pathogens (268–270). These tests are highly specific and are more sensitive than microscopy, but they are expensive, and availability is limited. In addition, the greater sensitivity of the test may result in diagnosing giardiasis in a greater number of asymptomatic people; whether to treat asymptomatic patients is not always clear, especially in settings where giardiasis is highly endemic. In these patients, submitting more than one stool for ova and parasite microscopy is discouraged (270). However, there are some patients with giardiasis who present with prolonged diarrhea and malabsorption for whom microscopy or other tests are negative. For these patients, the traditional gold standard has been performance of microscopy on three fecal specimens collected on different days, since cysts are intermittently detected in fecal specimens (271). However, the data suggest that PCR on a single fecal specimen is more sensitive than microscopy performed on three consecutive specimens, so in settings where PCR is available, it should be considered for these patients (272).
TABLE 5.
Commercially available tests for fecal diagnosis of giardiasisa
| Manufacturer | Test name | Antigen detected | Method |
|---|---|---|---|
| Meridian | ImmunoCard Stat Giardia/Cryptosporidium | Not stated | Immunochromatographic assay |
| Meridian | Merifluor Giardia/Cryptosporidium | Not stated | Direct fluorescent antibody |
| TechLab | Pt5012-Giardia II | CWP1 | ELISAb |
| TechLab | Tri-Combo parasite screen | Enzyme immunoassay | |
| Biosite | Triage parasite panel for Giardia, Entamoeba histolytica, and Cryptosporidium | Enzyme immunoassay | |
| Becton Dickinson | ColorPAC Giardia/Crypto | CWP1 | Immunochromatographic assay |
| Remel | X/pect Giardia or Giardia/Crypto | Not stated | Rapid immunoassay |
| Luminex | xTAG GPP | Multiplex PCR | |
| Becton Dickinson | BD Max | Multiplex PCR | |
| bioMérieux BioFire | FilmArray gastroenteritis panel | Multiplex PCR | |
| Savyon | NanoCHIP GIP | Multiplex PCR | |
| Genetic Signatures | EasyScreen parasite detection | Multiplex PCR | |
| Roche | LightMix modular assay gastro parasites | Multiplex PCR |
Reproduced from reference 347 with permission of Elsevier. Numerous antigen-based and nucleic acid-based tests are on the market. The list in this table is a partial list. The major focus in nucleic acid testing has been on multiplex tests, some of which are limited to parasites and others of which include parasites, bacteria, and viruses (341–344).
ELISA, enzyme-limited immunosorbent assay.
Endoscopic biopsy and examination of duodenal contents can be considered with prompt microscopic examination of the intestinal contents for trophozoites. An advantage of this approach is the potential of identifying other conditions leading to diarrhea and malabsorption, such as gluten enteropathy. An alternative method is the Entero-Test (string test), in which the patient swallows a capsule on the end of a string (273, 274). The string is left in place for 4 h to overnight and is then removed for microscopic examination for trophozoites or other parasitic organisms.
Serum antibody testing has been used in research settings to study the epidemiology of giardiasis, but has no role in clinical diagnosis because of limited sensitivity and specificity. Finally, G. duodenalis can be cultured in vitro, but the process of establishing an in vitro culture is laborious and unpredictable and is not used in the clinical laboratory setting. The reader is referred to an extensive review of the diagnosis of gastrointestinal parasites for greater detail on these tests (270).
TREATMENT
In view of the range of clinical manifestations of Giardia infection from asymptomatic to prolonged diarrhea with malnutrition, the clinical challenge is to determine who should be treated for giardiasis. In settings where it is highly endemic, there is probably no value in treating giardiasis in people with no symptoms. Even though Giardia infection is associated with growth stunting, reinfection rates may be so high that the effectiveness of treatment in eradicating the infection is minimal (183). In addition, the long-term effect of nitroimidazoles and other antibiotics on antimicrobial resistance and alteration of the microbiota of the person being treated are not known well enough to advise treatment in this group. A stronger case for treatment may be made for asymptomatic people in a low-prevalence setting, in view of the potential of reducing transmission to others, but no data exist for this situation. In contrast, symptomatic giardiasis should normally be treated, since the duration is frequently long in the absence of treatment. In fact, a duration of diarrhea of at least 5 or 7 days is sometimes used as a cutoff for epidemiological studies of giardiasis.
The first drug that came into common use for treatment of giardiasis was quinacrine, which was considered the drug of choice for many years. It has a long half-life (5 to 14 days) and is well absorbed from the gastrointestinal tract. Clinical studies have resulted in cure rates of 77% to 100%, and it may even decrease cyst viability (27). However, there are some important disadvantages of the drug as well. It leaves a bitter taste and frequently causes vomiting and can cause toxic psychosis. In addition, it must be administered 3× daily for five to 7 days, making it difficult for some patients to complete a treatment course. Its potential role is primarily as a single agent or as part of combination therapy for treatment-resistant giardiasis, but it is no longer available in the United States.
Metronidazole was the first 5-nitroimidazole to be developed and came into widespread use in the 1960s for giardiasis and a number of other infections caused by anaerobic protozoa or bacteria (275). It is reduced to toxic intermediates in the anerobic cell and has a variety of effects, including DNA reactivity. Pyruvate-ferredoxin oxidoreductase (PFOR) (276) and thioredoxin reductase (TrxR) (277–279) have been proposed as contributing to its activity, but there are likely to be other contributing pathways as well (275). Because of the DNA reactivity, metronidazole has been considered to possibly having carcinogenic activity. However, clinical studies have found little or no correlation (280–283). The drug is not teratogenic (284) and has been used widely in pregnancy after the first trimester. Metronidazole, is now, along with other nitroimidazoles, the drug of choice for giardiasis, although, ironically, it has never been given FDA approval for that indication. Metronidazole is 100% absorbed orally and is even absorbed fairly well from mucosal surfaces, including oral, vaginal, and rectal mucosa. A small study demonstrated the efficacy of rectally administered metronidazole for treating giardiasis in children (285). Because of its efficient absorption, levels in the intestinal lumen are relatively lower than those for drugs that are less efficiently absorbed, but whether that affects clinical activity is not known.
Metronidazole is given for a period of 5 to 10 days, and numerous studies have demonstrated high efficacy for treatment of giardiasis (see File S1 in the supplemental material) (286, 287). However, the required duration of treatment sometimes limits compliance and tolerability, so single-dose treatment has also been evaluated, but with poor results. Newer 5-nitroimidazoles have been developed, including tinidazole, which is a derivative of metronidazole and has been used in Europe since 1969, but it was not approved by the FDA until 2004 (288). Secnidazole was approved in 2017, but not for treatment of giardiasis, and ornidazole has not yet been approved. These drugs have longer half-lives than metronidazole and have all been evaluated for single dose use (File S1) (286). Tinidazole has been studied the most extensively, and a review of the literature that included 60 randomized controlled trials (RCTs) concluded that tinidazole is more effective than metronidazole or albendazole (286), so it may be considered the drug of choice.
The benzimidazoles are broad-spectrum antihelminthics that bind to beta-tubulin and cause irreversible damage to the parasite (289). Mebendazole has been studied, but with generally disappointing results. However, albendazole has demonstrated excellent efficacy when given as a 5-day course. Oral absorption is 1% to 5%, and it is metabolized to the active drug albendazole sulfoxide (290). Some studies, including a review with meta-analyses (291), have suggested that it is better tolerated than the nitroimidazoles (Table 6). However, albendazole is known to be embryotoxic in animals and must be avoided during pregnancy.
TABLE 6.
Drugs for treatment of giardiasisa
| Class | Drug | Oral bioavail ability (% if known) | Half lifec | Dose (adult and max pediatric dose) and duration | Availability in United Statesb | FDA approved for giardiasisb | Common side effects | Concerns |
|---|---|---|---|---|---|---|---|---|
| Nitroimidazole | Metronidazole | 100 | 6–10 h | 5 mg/kg t.i.d. (250 mg), 5–10 days | Y | N | Metallic taste, nausea, anorexia | DNA mutagenesis and possibility of carcinogenesis |
| Tinidazole | 100 | 12 h | 50 mg/kg (2 g), single dose | Y | Y | Metallic taste, nausea, anorexia | DNA mutagenesis and possibility of carcinogenesis | |
| Ornidazole | 100 | 11–14 h | 40 mg/kg (2 g), single dose | N | N | Metallic taste, nausea, anorexia | DNA mutagenesis and possibility of carcinogenesis | |
| Secnidazole | 100 | 14–20 h | 30 mg/kg (1.5 g), single dose | Y | N | Metallic taste, nausea, anorexia | DNA mutagenesis and possibility of carcinogenesis | |
| Benzimidazole | Albendazole | 1–5 | 8–12 h | 15 mg/kg q.i.d. (400 mg), 5 days | Y | N | Embryotoxicity | |
| Aminoglycoside | Paromomycin | 0 | NA | 10 mg/kg t.i.d. (500 mg), 5–10 days | Y | N | ||
| Nitrofuran | Furazolidone | 10 min | 2 mg/kg q.i.d. (100 mg), 7–10 days | Nd | Y | Adverse effect on reproduction, mutagenesis | ||
| Others | Nitazoxanide | Parent drug not detected | 1.3–1.8 h | 500 mg b.i.d. (adult), 100 mg b.i.d. (ages 1–3), 200 mg b.i.d. (ages 4–11), 3 days | Y | Y | ||
| Quinacrine | Good | 5–14 days | 2 mg/kg t.i.d. (100 mg), 5–7 days | Nd | Y | Nausea and vomiting | Toxic psychosis, common nausea and vomiting |
Reproduced from reference 345.
Y, yes; N, no.
NA (not applicable).
No longer generally available in the United States.
t.i.d., three times a day; q.i.d., four times a day; b.i.d., twice a day.
Nitazoxanide is a nitroheterocyclic compound that is notable as the only effective drug for treatment of Cryptosporidium spp., and it is also effective for giardiasis (292). After absorption from the gastrointestinal tract, it is rapidly converted to the active metabolite tizoxanide meaning that nitazoxanide is not detected in the serum. It has been proposed to work as a noncompetitive of PFOR (293, 294). When given as a 3-day course, it is well tolerated, but the average efficacy of 71% is significantly lower than those for the nitroimidazoles or albendazole (286).
Paromomycin is a nonabsorbed aminoglycoside that is moderately effective and is sometimes used in the first trimester of pregnancy because of lack of adequate data for the safety of nitroimidazoles in the first trimester (295). However, paromomycin is less effective, and in someone with malnutrition/weight loss, the more effective treatment should be given even during the first trimester. The antimalarial chloroquine has also been studied in children, and its efficacy is comparable to that of the nitroimidazoles (296, 297).
Many of these drugs have been compared in various combinations in children and/or adults and in symptomatic or asymptomatic individuals (File S1). A number of meta-analyses or other reviews have included many of these studies (286, 287, 298–300).
Clinical treatment failure accompanied by microscopic evidence for persistence of Giardia is occasionally seen following treatment of symptomatic giardiasis (301). These observations have raised the question of drug resistance, particularly as it related to the nitroimidazoles. Indeed, a number of studies have demonstrated trophozoites with in vitro resistance to metronidazole (302). However, documentation of microbiologically resistant isolates requires isolation from the host with a clinically resistant infection, followed by demonstration of in vitro resistance, which is difficult to do because of the challenges of adapting the organisms to in vitro culture. In addition, no genetic markers of resistance have been identified. Thus, it is more accurate to refer to treatment-refractory cases than to resistance. However, there are a few clinical correlation studies to suggest that true resistance is found for the nitroimidazoles (298). The increase in treatment failure from 15% in 2008 to 40% in 2013 for travelers returning to England suggests a true development of resistance (303), as does the higher frequency in travelers to Asia than to other areas (298). It is not yet known how many of these treatment failures are due to true resistance. Combination therapy with a nitroimidazole plus quinacrine (304) or a nitroimidazole plus albendazole (305) results in a high success rate in patients with treatment failure. In addition, quinacrine alone may be effective (298, 306).
There are also other potential reasons for the persistence or recurrence of clinical symptoms in the absence of resistance. This may be due to postinfectious IBS or to the existence of a second diagnosis, especially when the symptoms are not typical of giardiasis. Thus, when a patient has suspected treatment failure, it is critical that the presence of parasites be documented. If parasites are absent, these other possibilities must be considered. Retreatment with antimicrobial therapy is not indicated when organisms are not found on the follow-up fecal examinations (307).
TOOLS FOR STUDYING GIARDIA
A number of tools are now available for studying Giardia, many of which are summarized by Jex et al. (308). The advance that has made the single biggest impact on the study of G. duodenalis has been the development of an axenic (without organisms other than Giardia) in vitro culture system (309) (Table 7). The medium described in that report is still used today, frequently with minor modifications, such as the use of fetal calf serum in place of bovine serum if the study requires minimalization of potentially confounding antibodies.
TABLE 7.
Axenic growth medium for G. duodenalisa
| Component | Quantity per liter | Final concn |
|---|---|---|
| K2HPO4-H2O | 1 g | 4.4 mM |
| KH2PO4 | 600 mg | 4.4 mM |
| Trypticase (casein peptone) | 20 g | |
| Yeast extract | 10 g | |
| d-Glucose | 10 g | 56 mM |
| NaCl | 2 g | 34 mM |
| Cysteine-HCl | 2 g | 16.5 mM |
| Ascorbic acid | 200 mg | 1.1 mM |
| Ferric NH4 citrate (Sigma F-5879) | 22.8 mg | |
| Bovine bile (Sigma B-8381)-NaOH | 40 ml of 6.5% solution to bring pH to 7.0–7.2 (1.65 ml of 10 M solution) | |
| Serum (bovine or fetal calf) | 100 ml | 10% |
Reproduced from reference 1. Ingredients in the formula of TYI-S-33 (309), the most commonly used medium for growth of G. duodenalis trophozoites. The ferric NH4 citrate and bovine bile are generally kept as solutions at 4°C. All ingredients except the serum are dissolved in water, brought to 200 ml, sterilized with a 0.22- or 0.45-μm filter, and added to 700 ml sterilized water. Heat-inactivated (56°C for 20 min) serum is then added. During in vitro growth, the organisms are grown in sealed glass containers filled nearly full with medium. When this is impossible, such as during cloning by limiting dilution in 96-well plates (346), a sealed bag containing an anaerobic generator is used. At 4°C, the shelf life is limited to 5 to 7 days, primarily because of the degradation of the cysteine.
The development of in vitro excystation (30, 310) and encystation (35–37) have made it possible to study the life cycle of Giardia.
The development of genomics and proteomics databases began with the initial Giardia genome project of the WB isolate (genotype A), reported in 2007 (4), and was followed by reports of the GS isolate (genotype B) (16, 17). These are found at the GiardiaDB web site (https://Giardiadb.org/) and have provided the foundation for proteome analyses and databases as well (70, 311, 312).
A variety of tools for alteration of gene expression have been developed, beginning with transient transfection using a luciferase reporter (313). This was followed by stable transfection of a plasmid containing a gene for puromycin acetyltransferase (115) and using a bacterial neomycin phosphotransferase gene (neo) gene (314). Interestingly, the plasmids were maintained episomally in the WB isolate (genotype A), but in the GS isolate (genotype B), linear or circular DNA was integrated by recombination. Subsequent studies have used a variety of modifications to this initial approach. One of the limitations of transfection systems is that knockouts cannot easily be obtained, since the trophozoites are tetraploid. Thus, RNAi has also been used to study antigenic variation (164) and has been used for gene silencing to search for new drug targets (315). CRISPR interference (CRISPRi) (316) and CRISPR/Cas9 (317) have also been developed for stable repression of gene expression.
Advances in microscopic techniques that range from electron microscopy to fluorescence/confocal microscopy have included functional components, as well as improved preparation techniques, and are contributing greatly to improved understanding of the cytogenetic and cytoskeletal aspects of the organism. Numerous animal models have been developed, primarily with mice or other rodents. Some have used G. duodenalis, while others have used G. muris. Many of these are discussed in the sections on immune response and malnutrition and long-term sequelae.
These new tools for investigation of the biology of Giardia and pathogenesis of infections caused by this organism will contribute to ever accelerating advances in understanding of this organism and its impact on its host.
CONCLUSIONS
G. duodenalis as an organism caught immense interest when it was considered to be one of the earliest-diverging eukaryotes. With the finding that it actually has a minimalized genome with minimization of many canonical eukaryote functions, the emphasis has switched to understanding how that adaptation works for an organism that is an obligate intestinal parasite. As such, the organism has the ability to persist in the host intestine in the absence of tissue invasion and despite the mounting of a host immune response. This is especially remarkable in view of the requirement for trophozoites to replicate while their ventral disk adheres to the intestinal wall. The spectrum of illness caused during human infection with G. duodenalis ranges from asymptomatic to prolonged diarrhea and weight loss. Ongoing studies of pathogenesis and the immune response are beginning to shed light on the causes of these differences and will ultimately translate into more accurate guidance regarding appropriate therapy in various settings.
ACKNOWLEDGMENTS
I declare no conflict of interest.
No funding was provided for this work.
Biography

Rodney D. Adam, M.D., is currently a Professor of Pathology and Medicine at Aga Khan University in Nairobi, Kenya, and is working in the areas of molecular diagnostics and clinical infectious diseases. He received a Doctor of Medicine degree from the University of Illinois, followed by training in internal medicine and infectious diseases at the University of Arizona and molecular parasitology training at the National Institutes of Health. He joined the faculty of the University of Arizona College of Medicine, where his laboratory worked on antigenic variation and the genome of Giardia. He was a Fulbright scholar at the University of Nairobi in 2011 and then joined the Aga Khan University faculty, Nairobi, Kenya, in clinical microbiology and infectious diseases, where he is currently leading molecular diagnosis and is chair of the infection control committee.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Adam RD. 2001. Biology of Giardia lamblia. Clin Microbiol Rev 14:447–475. doi: 10.1128/CMR.14.3.447-475.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adam RD. 2017. Diplomonadida. In Archibald JM, Simpson AGB, Slamovits CH (ed), Handbook of the protists. Springer, Cham, Switzerland. [Google Scholar]
- 3.Sogin ML, Gunderson JH, Elwood HJ, Alonso RA, Peattie DA. 1989. Phylogenetic meaning of the kingdom concept: an unusual ribosomal RNA from Giardia lamblia. Science 243:75–77. doi: 10.1126/science.2911720. [DOI] [PubMed] [Google Scholar]
- 4.Morrison HG, McArthur AG, Gillin FD, Aley SB, Adam RD, Olsen GJ, Best AA, Cande WZ, Chen F, Cipriano MJ, Davids BJ, Dawson SC, Elmendorf HG, Hehl AB, Holder ME, Huse SM, Kim UU, Lasek-Nesselquist E, Manning G, Nigam A, Nixon JE, Palm D, Passamaneck NE, Prabhu A, Reich CI, Reiner DS, Samuelson J, Svard SG, Sogin ML. 2007. Genomic minimalism in the early diverging intestinal parasite Giardia lamblia. Science 317:1921–1926. doi: 10.1126/science.1143837. [DOI] [PubMed] [Google Scholar]
- 5.Tovar J, Leon-Avila G, Sanchez LB, Sutak R, Tachezy J, van der Giezen M, Hernandez M, Muller M, Lucocq JM. 2003. Mitochondrial remnant organelles of Giardia function in iron-sulphur protein maturation. Nature 426:172–176. doi: 10.1038/nature01945. [DOI] [PubMed] [Google Scholar]
- 6.Hegner RW. 1926. The biology of host-parasite relationships among protozoa living in man. Q Rev Biol 1:393–418. doi: 10.1086/394252. [DOI] [Google Scholar]
- 7.Filice FP. 1952. Studies on the cytology and life history of a Giardia from the laboratory rat. University of California Press, Berkeley, CA. [Google Scholar]
- 8.Bertram MA, Meyer EA, Lile JD, Morse SA. 1983. A comparison of isozymes of five axenic Giardia isolates. J Parasitol 69:793–801. doi: 10.2307/3281031. [DOI] [PubMed] [Google Scholar]
- 9.Korman SH, Le Blancq SM, Spira DT, el On J, Reifen RM, Deckelbaum RJ. 1986. Giardia lamblia: identification of different strains from man. Z Parasitenkd 72:173–180. doi: 10.1007/BF00931144. [DOI] [PubMed] [Google Scholar]
- 10.Nash TE, Keister DB. 1985. Differences in excretory-secretory products and surface antigens among 19 isolates of Giardia. J Infect Dis 152:1166–1171. doi: 10.1093/infdis/152.6.1166. [DOI] [PubMed] [Google Scholar]
- 11.Nash TE, McCutchan T, Keister D, Dame JB, Conrad JD, Gillin FD. 1985. Restriction-endonuclease analysis of DNA from 15 Giardia isolates obtained from humans and animals. J Infect Dis 152:64–73. doi: 10.1093/infdis/152.1.64. [DOI] [PubMed] [Google Scholar]
- 12.Baruch AC, Isaac-Renton J, Adam RD. 1996. The molecular epidemiology of Giardia lamblia: a sequence-based approach. J Infect Dis 174:233–236. doi: 10.1093/infdis/174.1.233. [DOI] [PubMed] [Google Scholar]
- 13.Read CM, Monis PT, Thompson RC. 2004. Discrimination of all genotypes of Giardia duodenalis at the glutamate dehydrogenase locus using PCR-RFLP. Infect Genet Evol 4:125–130. doi: 10.1016/j.meegid.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Wielinga CM, Thompson RC. 2007. Comparative evaluation of Giardia duodenalis sequence data. Parasitology 134:1795–1821. doi: 10.1017/S0031182007003071. [DOI] [PubMed] [Google Scholar]
- 15.Caccio SM, Beck R, Lalle M, Marinculic A, Pozio E. 2008. Multilocus genotyping of Giardia duodenalis reveals striking differences between assemblages A and B. Int J Parasitol 38:1523–1531. doi: 10.1016/j.ijpara.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Franzen O, Jerlstrom-Hultqvist J, Castro E, Sherwood E, Ankarklev J, Reiner DS, Palm D, Andersson JO, Andersson B, Svard SG. 2009. Draft genome sequencing of Giardia intestinalis assemblage B isolate GS: is human giardiasis caused by two different species? PLoS Pathog 5:e1000560. doi: 10.1371/journal.ppat.1000560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adam RD, Dahlstrom EW, Martens CA, Bruno DP, Barbian KD, Ricklefs SM, Hernandez MM, Narla NP, Patel RB, Porcella SF, Nash TE. 2013. Genome sequencing of Giardia lamblia genotypes A2 and B isolates (DH and GS) and comparative analysis with the genomes of genotypes A1 and E (WB and Pig). Genome Biol Evol 5:2498–2511. doi: 10.1093/gbe/evt197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jerlstrom-Hultqvist J, Franzen O, Ankarklev J, Xu F, Nohynkova E, Andersson JO, Svard SG, Andersson B. 2010. Genome analysis and comparative genomics of a Giardia intestinalis assemblage E isolate. BMC Genomics 11:543. doi: 10.1186/1471-2164-11-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kooyman FNJ, Wagenaar JA, Zomer A. 2019. Whole-genome sequencing of dog-specific assemblages C and D of Giardia duodenalis from single and pooled cysts indicates host-associated genes. Microb Genom 5:12. doi: 10.1099/mgen.0.000302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsui CK, Miller R, Uyaguari-Diaz M, Tang P, Chauve C, Hsiao W, Isaac-Renton J, Prystajecky N. 2018. Beaver fever: whole-genome characterization of waterborne outbreak and sporadic isolates to study the zoonotic transmission of giardiasis. mSphere 3:e00090-18. doi: 10.1128/mSphere.00090-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sprong H, Caccio SM, van der Giessen JW, ZOOPNET network and partners. 2009. Identification of zoonotic genotypes of Giardia duodenalis. PLoS Negl Trop Dis 3:e558. doi: 10.1371/journal.pntd.0000558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ankarklev J, Lebbad M, Einarsson E, Franzen O, Ahola H, Troell K, Svard SG. 2018. A novel high-resolution multilocus sequence typing of Giardia intestinalis assemblage A isolates reveals zoonotic transmission, clonal outbreaks and recombination. Infect Genet Evol 60:7–16. doi: 10.1016/j.meegid.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 23.Monis PT, Caccio SM, Thompson RC. 2009. Variation in Giardia: towards a taxonomic revision of the genus. Trends Parasitol 25:93–100. doi: 10.1016/j.pt.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 24.Tibayrenc M, Ayala FJ. 2014. Cryptosporidium, Giardia, Cryptococcus, Pneumocystis genetic variability: cryptic biological species or clonal near-clades? PLoS Pathog 10:e1003908. doi: 10.1371/journal.ppat.1003908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erlandsen SL, Bemrick WJ, Pawley J. 1989. High-resolution electron microscopic evidence for the filamentous structure of the cyst wall in Giardia muris and Giardia duodenalis. J Parasitol 75:787–797. doi: 10.2307/3283065. [DOI] [PubMed] [Google Scholar]
- 26.Jarroll EL, Manning P, Lindmark DG, Coggins JR, Erlandsen SL. 1989. Giardia cyst wall-specific carbohydrate: evidence for the presence of galactosamine. Mol Biochem Parasitol 32:121–131. doi: 10.1016/0166-6851(89)90063-7. [DOI] [PubMed] [Google Scholar]
- 27.Paget TA, Jarroll EL, Manning P, Lindmark DG, Lloyd D. 1989. Respiration in the cysts and trophozoites of Giardia muris. J Gen Microbiol 135:145–154. doi: 10.1099/00221287-135-1-145. [DOI] [PubMed] [Google Scholar]
- 28.Coffey CM, Collier SA, Gleason ME, Yoder JS, Kirk MD, Richardson AM, Fullerton KE, Benedict KM. 2021. Evolving epidemiology of reported giardiasis cases in the United States, 1995–2016. Clin Infect Dis 72:764–770. doi: 10.1093/cid/ciaa128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bingham AK, Meyer EA. 1979. Giardia excystation can be induced in vitro in acidic solutions. Nature 277:301–302. doi: 10.1038/277301a0. [DOI] [PubMed] [Google Scholar]
- 30.Rice EW, Schaefer FW. 1981. Improved in vitro excystation procedure for Giardia lamblia cysts. J Clin Microbiol 14:709–710. doi: 10.1128/jcm.14.6.709-710.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feely DE, Gardner MD, Hardin EL. 1991. Excystation of Giardia muris induced by a phosphate-bicarbonate medium: localization of acid phosphatase. J Parasitol 77:441–448. doi: 10.2307/3283133. [DOI] [PubMed] [Google Scholar]
- 32.Cook GC. 1985. Infective gastroenteritis and its relationship to reduced gastric acidity. Scand J Gastroenterol 111:17–23. doi: 10.3109/00365528509093751. [DOI] [PubMed] [Google Scholar]
- 33.Reynaert H, Fernandes E, Bourgain C, Smekens L, Devis G. 1995. Proton-pump inhibition and gastric giardiasis: a causal or casual association? J Gastroenterol 30:775–778. doi: 10.1007/BF02349646. [DOI] [PubMed] [Google Scholar]
- 34.Barash NR, Nosala C, Pham JK, McInally SG, Gourguechon S, McCarthy-Sinclair B, Dawson SC. 2017. Giardia colonizes and encysts in high-density foci in the murine small intestine. mSphere 2:e00343-16. doi: 10.1128/mSphere.00343-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schupp DG, Januschka MM, Sherlock LA, Stibbs HH, Meyer EA, Bemrick WJ, Erlandsen SL. 1988. Production of viable Giardia cysts in vitro: determination by fluorogenic dye staining, excystation, and animal infectivity in the mouse and Mongolian gerbil. Gastroenterology 95:1–10. doi: 10.1016/0016-5085(88)90283-1. [DOI] [PubMed] [Google Scholar]
- 36.Sterling CR, Kutob RM, Gizinski MJ, Verastegui M, Stetzenbach L. 1988. Giardia detection using monoclonal antibodies recognizing determinants of in vitro derived cysts, p 219–222. In Wallis PM, Hammond BR (ed), Advances in Giardia research. University of Calgary Press, Calgary, Canada. [Google Scholar]
- 37.Gillin FD, Reiner DS, Gault MJ, Douglas H, Das S, Wunderlich A, Sauch JF. 1987. Encystation and expression of cyst antigens by Giardia lamblia in vitro. Science 235:1040–1043. doi: 10.1126/science.3547646. [DOI] [PubMed] [Google Scholar]
- 38.Boucher SE, Gillin FD. 1990. Excystation of in vitro-derived Giardia lamblia cysts. Infect Immun 58:3516–3522. doi: 10.1128/iai.58.11.3516-3522.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kane AV, Ward HD, Keusch GT, Pereira ME. 1991. In vitro encystation of Giardia lamblia: large-scale production of in vitro cysts and strain and clone differences in encystation efficiency. J Parasitol 77:974–981. doi: 10.2307/3282752. [DOI] [PubMed] [Google Scholar]
- 40.Lujan HD, Mowatt MR, Nash TE. 1997. Mechanisms of Giardia lamblia differentiation into cysts. Microbiol Mol Biol Rev 61:294–304. doi: 10.1128/mmbr.61.3.294-304.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reiner DS, McCaffery M, Gillin FD. 1990. Sorting of cyst wall proteins to a regulated secretory pathway during differentiation of the primitive eukaryote, Giardia lamblia. Eur J Cell Biol 53:142–153. [PubMed] [Google Scholar]
- 42.Faso C, Bischof S, Hehl AB. 2013. The proteome landscape of Giardia lamblia encystation. PLoS One 8:e83207. doi: 10.1371/journal.pone.0083207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palm D, Weiland M, McArthur AG, Winiecka-Krusnell J, Cipriano MJ, Birkeland SR, Pacocha SE, Davids B, Gillin F, Linder E, Svard S. 2005. Developmental changes in the adhesive disk during Giardia differentiation. Mol Biochem Parasitol 141:199–207. doi: 10.1016/j.molbiopara.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 44.Bernander R, Palm JE, Svard SG. 2001. Genome ploidy in different stages of the Giardia lamblia life cycle. Cell Microbiol 3:55–62. doi: 10.1046/j.1462-5822.2001.00094.x. [DOI] [PubMed] [Google Scholar]
- 45.DuBois KN, Abodeely M, Sakanari J, Craik CS, Lee M, McKerrow JH, Sajid M. 2008. Identification of the major cysteine protease of Giardia and its role in encystation. J Biol Chem 283:18024–18031. doi: 10.1074/jbc.M802133200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ward W, Alvarado L, Rawlings ND, Engel JC, Franklin C, McKerrow JH. 1997. A primitive enzyme for a primitive cell: the protease required for excystation of Giardia. Cell 89:437–444. doi: 10.1016/S0092-8674(00)80224-X. [DOI] [PubMed] [Google Scholar]
- 47.Touz MC, Nores MJ, Slavin I, Carmona C, Conrad JT, Mowatt MR, Nash TE, Coronel CE, Lujan HD. 2002. The activity of a developmentally regulated cysteine proteinase is required for cyst wall formation in the primitive eukaryote Giardia lamblia. J Biol Chem 277:8474–8481. doi: 10.1074/jbc.M110250200. [DOI] [PubMed] [Google Scholar]
- 48.Birkeland SR, Preheim SP, Davids BJ, Cipriano MJ, Palm D, Reiner DS, Svard SG, Gillin FD, McArthur AG. 2010. Transcriptome analyses of the Giardia lamblia life cycle. Mol Biochem Parasitol 174:62–65. doi: 10.1016/j.molbiopara.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morf L, Spycher C, Rehrauer H, Fournier CA, Morrison HG, Hehl AB. 2010. The transcriptional response to encystation stimuli in Giardia lamblia is restricted to a small set of genes. Eukaryot Cell 9:1566–1576. doi: 10.1128/EC.00100-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davids BJ, Reiner DS, Birkeland SR, Preheim SP, Cipriano MJ, McArthur AG, Gillin FD. 2006. A new family of giardial cysteine-rich non-VSP protein genes and a novel cyst protein. PLoS One 1:e44. doi: 10.1371/journal.pone.0000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eckmann L, Laurent F, Langford TD, Hetsko ML, Smith JR, Kagnoff MF, Gillin FD. 2000. Nitric oxide production by human intestinal epithelial cells and competition for arginine as potential determinants of host defense against the lumen-dwelling pathogen Giardia lamblia. J Immunol 164:1478–1487. doi: 10.4049/jimmunol.164.3.1478. [DOI] [PubMed] [Google Scholar]
- 52.Carranza PG, Gargantini PR, Prucca CG, Torri A, Saura A, Svard S, Lujan HD. 2016. Specific histone modifications play critical roles in the control of encystation and antigenic variation in the early-branching eukaryote Giardia lamblia. Int J Biochem Cell Biol 81:32–43. doi: 10.1016/j.biocel.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 53.Frontera LS, Moyano S, Quassollo G, Lanfredi-Rangel A, Ropolo AS, Touz MC. 2018. Lactoferrin and lactoferricin endocytosis halt Giardia cell growth and prevent infective cyst production. Sci Rep 8:18020. doi: 10.1038/s41598-018-36563-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krtkova J, Thomas EB, Alas GC, Schraner EM, Behjatnia HR, Hehl AB, Paredez AR. 2016. Rac regulates Giardia lamblia encystation by coordinating cyst wall protein trafficking and secretion. mBio 7:e01003-16. doi: 10.1128/mBio.01003-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perry DA, Morrison HG, Adam RD. 2011. Optical map of the genotype A1 WB C6 Giardia lamblia genome isolate. Mol Biochem Parasitol 180:112–114. doi: 10.1016/j.molbiopara.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu F, Jex A, Svard SG. 2020. A chromosome-scale reference genome for Giardia intestinalis WB. Sci Data 7:38. doi: 10.1038/s41597-020-0377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Adam RD, Nash TE, Wellems TE. 1988. The Giardia lamblia trophozoite contains sets of closely related chromosomes. Nucleic Acids Res 16:4555–4567. doi: 10.1093/nar/16.10.4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xue M, Chen B, Ye Q, Shao J, Lyu Z, Wen J. 2018. Sense-antisense gene overlap is probably a cause for retaining the few introns in Giardia genome and the implications. Biol Direct 13:23. doi: 10.1186/s13062-018-0226-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saraiya AA, Li W, Wu J, Chang CH, Wang CC. 2014. The microRNAs in an ancient protist repress the variant-specific surface protein expression by targeting the entire coding sequence. PLoS Pathog 10:e1003791. doi: 10.1371/journal.ppat.1003791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liao JY, Guo YH, Zheng LL, Li Y, Xu WL, Zhang YC, Zhou H, Lun ZR, Ayala FJ, Qu LH. 2014. Both endo-siRNAs and tRNA-derived small RNAs are involved in the differentiation of primitive eukaryote Giardia lamblia. Proc Natl Acad Sci U S A 111:14159–14164. doi: 10.1073/pnas.1414394111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Welch DM, Meselson M. 2000. Evidence for the evolution of bdelloid rotifers without sexual reproduction or genetic exchange. Science 288:1211–1215. doi: 10.1126/science.288.5469.1211. [DOI] [PubMed] [Google Scholar]
- 62.Welch DB, Meselson MS. 2001. Rates of nucleotide substitution in sexual and anciently asexual rotifers. Proc Natl Acad Sci U S A 98:6720–6724. doi: 10.1073/pnas.111144598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wielinga C, Ryan U, Andrew Thompson RC, Monis P. 2011. Multi-locus analysis of Giardia duodenalis intra-assemblage B substitution patterns in cloned culture isolates suggests sub-assemblage B analyses will require multi-locus genotyping with conserved and variable genes. Int J Parasitol 41:495–503. doi: 10.1016/j.ijpara.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 64.Ankarklev J, Svard SG, Lebbad M. 2012. Allelic sequence heterozygosity in single Giardia parasites. BMC Microbiol 12:65. doi: 10.1186/1471-2180-12-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lecová L, Weisz F, Tůmová P, Tolarová V, Nohýnková E. 2018. The first multilocus genotype analysis of Giardia intestinalis in humans in the Czech Republic. Parasitology 145:1577–1587. doi: 10.1017/S0031182018000409. [DOI] [PubMed] [Google Scholar]
- 66.Lecova L, Tumova P, Nohynkova E. 2019. Clone-based haplotyping of Giardia intestinalis assemblage B human isolates. Parasitol Res 118:355–361. doi: 10.1007/s00436-018-6161-7. [DOI] [PubMed] [Google Scholar]
- 67.Cooper MA, Adam RD, Worobey M, Sterling CR. 2007. Population genetics provides evidence for recombination in Giardia. Curr Biol 17:1984–1988. doi: 10.1016/j.cub.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 68.Cooper MA, Sterling CR, Gilman RH, Cama V, Ortega Y, Adam RD. 2010. Molecular analysis of household transmission of Giardia lamblia in a region of high endemicity in Peru. J Infect Dis 202:1713–1721. doi: 10.1086/657142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lasek-Nesselquist E, Welch DM, Thompson RC, Steuart RF, Sogin ML. 2009. Genetic exchange within and between assemblages of Giardia duodenalis. J Eukaryot Microbiol 56:504–518. doi: 10.1111/j.1550-7408.2009.00443.x. [DOI] [PubMed] [Google Scholar]
- 70.Ansell BRE, Pope BJ, Georgeson P, Emery-Corbin SJ, Jex AR. 2019. Annotation of the Giardia proteome through structure-based homology and machine learning. GigaScience 8:giy150. doi: 10.1093/gigascience/giy150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Elmendorf HG, Dawson SC, McCaffery JM. 2003. The cytoskeleton of Giardia lamblia. Int J Parasitol 33:3–28. doi: 10.1016/S0020-7519(02)00228-X. [DOI] [PubMed] [Google Scholar]
- 72.Dawson SC. 2010. An insider’s guide to the microtubule cytoskeleton of Giardia. Cell Microbiol 12:588–598. doi: 10.1111/j.1462-5822.2010.01458.x. [DOI] [PubMed] [Google Scholar]
- 73.Hagen KD, McInally SG, Hilton ND, Dawson SC. 2020. Microtubule organelles in Giardia, p 25–96. In Ortega-Pierres MG (ed), Advances in parasitology, vol 107. Academic Press, Cambridge, MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gadelha APR, Benchimol M, de Souza W. 2020. The structural organization of Giardia intestinalis cytoskeleton, p 1–23. In Ortega-Pierres MG (ed), Advances in parasitology, vol 107. Academic Press, Cambridge, MA. [DOI] [PubMed] [Google Scholar]
- 75.Peattie DA, Alonso RA, Hein A, Caulfield JP. 1989. Ultrastructural localization of giardins to the edges of disk microribbons of Giardia lamblia and the nucleotide and deduced protein sequence of alpha giardin. J Cell Biol 109:2323–2335. doi: 10.1083/jcb.109.5.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brown JR, Schwartz CL, Heumann JM, Dawson SC, Hoenger A. 2016. A detailed look at the cytoskeletal architecture of the Giardia lamblia ventral disc. J Struct Biol 194:38–48. doi: 10.1016/j.jsb.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Feely DE, Schollmeyer JV, Erlandsen SL. 1982. Giardia spp.: distribution of contractile proteins in the attachment organelle. Exp Parasitol 53:145–154. doi: 10.1016/0014-4894(82)90100-x. [DOI] [PubMed] [Google Scholar]
- 78.House SA, Richter DJ, Pham JK, Dawson SC. 2011. Giardia flagellar motility is not directly required to maintain attachment to surfaces. PLoS Pathog 7:e1002167. doi: 10.1371/journal.ppat.1002167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Woessner DJ, Dawson SC. 2012. The Giardia median body protein is a ventral disc protein that is critical for maintaining a domed disc conformation during attachment. Eukaryot Cell 11:292–301. doi: 10.1128/EC.05262-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Holberton DV. 1974. Attachment of Giardia—a hydrodynamic model based on flagellar activity. J Exp Biol 60:207–221. doi: 10.1242/jeb.60.1.207. [DOI] [PubMed] [Google Scholar]
- 81.Lenaghan SC, Davis CA, Henson WR, Zhang Z, Zhang M. 2011. High-speed microscopic imaging of flagella motility and swimming in Giardia lamblia trophozoites. Proc Natl Acad Sci U S A 108:E550–E558. doi: 10.1073/pnas.1106904108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lenaghan SC, Chen J, Zhang M. 2013. Modeling and analysis of propulsion in the multiflagellated micoorganism Giardia lamblia. Phys Rev E 88:e012726. doi: 10.1103/PhysRevE.88.012726. [DOI] [PubMed] [Google Scholar]
- 83.Ankarklev J, Jerlstrom-Hultqvist J, Ringqvist E, Troell K, Svard SG. 2010. Behind the smile: cell biology and disease mechanisms of Giardia species. Nat Rev Microbiol 8:413–422. doi: 10.1038/nrmicro2317. [DOI] [PubMed] [Google Scholar]
- 84.Hardin WR, Li R, Xu J, Shelton AM, Alas GCM, Minin VN, Paredez AR. 2017. Myosin-independent cytokinesis in Giardia utilizes flagella to coordinate force generation and direct membrane trafficking. Proc Natl Acad Sci U S A 114:E5854–E5863. doi: 10.1073/pnas.1705096114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Piva B, Benchimol M. 2004. The median body of Giardia lamblia: an ultrastructural study. Biol Cell 96:735–746. doi: 10.1016/j.biolcel.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 86.Paredez AR, Assaf ZJ, Sept D, Timofejeva L, Dawson SC, Wang CJ, Cande WZ. 2011. An actin cytoskeleton with evolutionarily conserved functions in the absence of canonical actin-binding proteins. Proc Natl Acad Sci U S A 108:6151–6156. doi: 10.1073/pnas.1018593108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Paredez AR, Nayeri A, Xu JW, Krtkova J, Cande WZ. 2014. Identification of obscure yet conserved actin-associated proteins in Giardia lamblia. Eukaryot Cell 13:776–784. doi: 10.1128/EC.00041-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Krtkova J, Xu J, Lalle M, Steele-Ogus M, Alas GCM, Sept D, Paredez AR. 2017. 14-3-3 Regulates actin filament formation in the deep-branching eukaryote Giardia lamblia. mSphere 2:e00248-17. doi: 10.1128/mSphere.00248-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sagolla MS, Dawson SC, Mancuso JJ, Cande WZ. 2006. Three-dimensional analysis of mitosis and cytokinesis in the binucleate parasite Giardia intestinalis. J Cell Sci 119:4889–4900. doi: 10.1242/jcs.03276. [DOI] [PubMed] [Google Scholar]
- 90.Tůmová P, Kulda J, Nohýnková E. 2007. Cell division of Giardia intestinalis: assembly and disassembly of the adhesive disc, and the cytokinesis. Cell Motil Cytoskeleton 64:288–298. doi: 10.1002/cm.20183. [DOI] [PubMed] [Google Scholar]
- 91.Nohynkova E, Tumova P, Kulda J. 2006. Cell division of Giardia intestinalis: flagellar developmental cycle involves transformation and exchange of flagella between mastigonts of a diplomonad cell. Eukaryot Cell 5:753–761. doi: 10.1128/EC.5.4.753-761.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Adam RD, Nash TE, Wellems TE. 1991. Telomeric location of Giardia rDNA genes. Mol Cell Biol 11:3326–3330. doi: 10.1128/mcb.11.6.3326-3330.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Adam RD. 1992. Chromosome-size variation in Giardia lamblia: the role of rDNA repeats. Nucleic Acids Res 20:3057–3061. doi: 10.1093/nar/20.12.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yu LZ, Birky CW, Jr, Adam RD. 2002. The two nuclei of Giardia each have complete copies of the genome and are partitioned equationally at cytokinesis. Eukaryot Cell 1:191–199. doi: 10.1128/EC.1.2.191-199.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tůmová P, Hofstetrová K, Nohýnková E, Hovorka O, Král J. 2007. Cytogenetic evidence for diversity of two nuclei within a single diplomonad cell of Giardia. Chromosoma 116:65–78. doi: 10.1007/s00412-006-0082-4. [DOI] [PubMed] [Google Scholar]
- 96.Tůmová P, Uzlíková M, Jurczyk T, Nohýnková E. 2016. Constitutive aneuploidy and genomic instability in the single-celled eukaryote Giardia intestinalis. MicrobiologyOpen 5:560–574. doi: 10.1002/mbo3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Smith PD, Gillin FD, Spira WM, Nash TE. 1982. Chronic giardiasis: studies on drug sensitivity, toxin production, and host immune response. Gastroenterology 83:797–803. doi: 10.1016/S0016-5085(82)80008-5. [DOI] [PubMed] [Google Scholar]
- 98.Tůmová P, Dluhošová J, Weisz F, Nohýnková E. 2019. Unequal distribution of genes and chromosomes refers to nuclear diversification in the binucleated Giardia intestinalis. Int J Parasitol 49:463–470. doi: 10.1016/j.ijpara.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 99.Tůmová P, Uzlíková M, Wanner G, Nohýnková E. 2015. Structural organization of very small chromosomes: study on a single-celled evolutionary distant eukaryote Giardia intestinalis. Chromosoma 124:81–94. doi: 10.1007/s00412-014-0486-5. [DOI] [PubMed] [Google Scholar]
- 100.Dawson SC, Sagolla MS, Cande WZ. 2007. The cenH3 histone variant defines centromeres in Giardia intestinalis. Chromosoma 116:175–184. doi: 10.1007/s00412-006-0091-3. [DOI] [PubMed] [Google Scholar]
- 101.Markova K, Uzlikova M, Tumova P, Jirakova K, Hagen G, Kulda J, Nohynkova E. 2016. Absence of a conventional spindle mitotic checkpoint in the binucleated single-celled parasite Giardia intestinalis. Eur J Cell Biol 95:355–367. doi: 10.1016/j.ejcb.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 102.Bansal A, Kukreti S. 2020. The four repeat Giardia lamblia telomere forms tetramolecular G-quadruplex with antiparallel topology. J Biomol Struct Dyn 38:1975–1983. doi: 10.1080/07391102.2019.1623074. [DOI] [PubMed] [Google Scholar]
- 103.Kabnick KS, Peattie DA. 1990. In situ analyses reveal that the two nuclei of Giardia lamblia are equivalent. J Cell Sci 95:353–360. doi: 10.1242/jcs.95.3.353. [DOI] [PubMed] [Google Scholar]
- 104.Poxleitner MK, Carpenter ML, Mancuso JJ, Wang CJ, Dawson SC, Cande WZ. 2008. Evidence for karyogamy and exchange of genetic material in the binucleate intestinal parasite Giardia intestinalis. Science 319:1530–1533. doi: 10.1126/science.1153752. [DOI] [PubMed] [Google Scholar]
- 105.Forche A, Alby K, Schaefer D, Johnson AD, Berman J, Bennett RJ. 2008. The parasexual cycle in Candida albicans provides an alternative pathway to meiosis for the formation of recombinant strains. PLoS Biol 6:e110. doi: 10.1371/journal.pbio.0060110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Birky CW, Jr. 2010. Giardia sex? Yes, but how and how much? Trends Parasitol 26:70–74. doi: 10.1016/j.pt.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 107.Ramesh MA, Malik SB, Logsdon JM, Jr.. 2005. A phylogenomic inventory of meiotic genes; evidence for sex in Giardia and an early eukaryotic origin of meiosis. Curr Biol 15:185–191. doi: 10.1016/S0960-9822(05)00028-X. [DOI] [PubMed] [Google Scholar]
- 108.Boisvert F-M, van Koningsbruggen S, Navascués J, Lamond AI. 2007. The multifunctional nucleolus. Nat Rev Mol Cell Biol 8:574–585. doi: 10.1038/nrm2184. [DOI] [PubMed] [Google Scholar]
- 109.Narcisi EM, Glover CV, Fechheimer M. 1998. Fibrillarin, a conserved pre-ribosomal RNA processing protein of Giardia. J Eukaryot Microbiol 45:105–111. doi: 10.1111/j.1550-7408.1998.tb05077.x. [DOI] [PubMed] [Google Scholar]
- 110.Tian XF, Yang ZH, Shen H, Adam RD, Lu SQ. 2010. Identification of the nucleoli of Giardia lamblia with TEM and CFM. Parasitol Res 106:789–793. doi: 10.1007/s00436-009-1715-3. [DOI] [PubMed] [Google Scholar]
- 111.Jimenez-Garcia LF, Zavala G, Chavez-Munguia B, Ramos-Godinez MP, Lopez-Velazquez G, Segura-Valdez ML, Montanez C, Hehl AB, Arguello-Garcia R, Ortega-Pierres G. 2008. Identification of nucleoli in the early branching protist Giardia duodenalis. Int J Parasitol 38:1297–1304. doi: 10.1016/j.ijpara.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 112.Lara-Martinez R, De Lourdes Segura-Valdez M, De La Mora-De La Mora I, Lopez-Velazquez G, Jimenez-Garcia LF. 2016. Morphological studies of nucleologenesis in Giardia lamblia. Anat Rec 299:549–556. doi: 10.1002/ar.23323. [DOI] [PubMed] [Google Scholar]
- 113.Torres-Huerta AL, Martinez-Miguel RM, Bazan-Tejeda ML, Bermudez-Cruz RM. 2016. Characterization of recombinase DMC1B and its functional role as Rad51 in DNA damage repair in Giardia duodenalis trophozoites. Biochimie 127:173–186. doi: 10.1016/j.biochi.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 114.Martinez-Miguel RM, Sandoval-Cabrera A, Bazan-Tejeda ML, Torres-Huerta AL, Martinez-Reyes DA, Bermudez-Cruz RM. 2017. Giardia duodenalis Rad52 protein: biochemical characterization and response upon DNA damage. J Biochem 162:123–135. doi: 10.1093/jb/mvx009. [DOI] [PubMed] [Google Scholar]
- 115.Singer SM, Yee J, Nash TE. 1998. Episomal and integrated maintenance of foreign DNA in Giardia lamblia. Mol Biochem Parasitol 92:59–69. doi: 10.1016/s0166-6851(97)00225-9. [DOI] [PubMed] [Google Scholar]
- 116.Seshadri V, McArthur AG, Sogin ML, Adam RD. 2003. Giardia lamblia RNA polymerase II: amanitin-resistant transcription. J Biol Chem 278:27804–27810. doi: 10.1074/jbc.M303316200. [DOI] [PubMed] [Google Scholar]
- 117.Gomez V, Wasserman M. 2017. Interactions between Giardia duodenalis Sm proteins and their association with spliceosomal snRNAs. Parasitol Res 116:617–626. doi: 10.1007/s00436-016-5326-5. [DOI] [PubMed] [Google Scholar]
- 118.Yu DC, Wang AL, Botka CW, Wang CC. 1998. Protein synthesis in Giardia lamblia may involve interaction between a downstream box (DB) in mRNA and an anti-DB in the 16S-like ribosomal RNA. Mol Biochem Parasitol 96:151–165. doi: 10.1016/S0166-6851(98)00126-1. [DOI] [PubMed] [Google Scholar]
- 119.Li L, Wang CC. 2004. Capped mRNA with a single nucleotide leader is optimally translated in a primitive eukaryote, Giardia lamblia. J Biol Chem 279:14656–14664. doi: 10.1074/jbc.M309879200. [DOI] [PubMed] [Google Scholar]
- 120.Hausmann S, Altura MA, Witmer M, Singer SM, Elmendorf HG, Shuman S. 2005. Yeast-like mRNA capping apparatus in Giardia lamblia. J Biol Chem 280:12077–12086. doi: 10.1074/jbc.M412063200. [DOI] [PubMed] [Google Scholar]
- 121.Adedoja AN, McMahan T, Neal JP, Hamal Dhakal S, Jois S, Romo D, Hull K, Garlapati S. 2020. Translation initiation factors GleIF4E2 and GleIF4A can interact directly with the components of the pre-initiation complex to facilitate translation initiation in Giardia lamblia. Mol Biochem Parasitol 236:111258. doi: 10.1016/j.molbiopara.2020.111258. [DOI] [PubMed] [Google Scholar]
- 122.Lindmark DG, Muller M. 1973. Hydrogenosome, a cytoplasmic organelle of the anaerobic flagellate Tritrichomonas foetus, and its role in pyruvate metabolism. J Biol Chem 248:7724–7728. doi: 10.1016/S0021-9258(19)43249-3. [DOI] [PubMed] [Google Scholar]
- 123.Johnson PJ, d’Oliveira CE, Gorrell TE, Müller M. 1990. Molecular analysis of the hydrogenosomal ferredoxin of the anaerobic protist Trichomonas vaginalis. Proc Natl Acad Sci U S A 87:6097–6101. doi: 10.1073/pnas.87.16.6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shiflett AM, Johnson PJ. 2010. Mitochondrion-related organelles in eukaryotic protists. Annu Rev Microbiol 64:409–429. doi: 10.1146/annurev.micro.62.081307.162826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Voleman L, Dolezal P. 2019. Mitochondrial dynamics in parasitic protists. PLoS Pathog 15:e1008008. doi: 10.1371/journal.ppat.1008008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Regoes A, Zourmpanou D, Leon-Avila G, van der GM, Tovar J, Hehl AB. 2005. Protein import, replication, and inheritance of a vestigial mitochondrion. J Biol Chem 280:30557–30563. doi: 10.1074/jbc.M500787200. [DOI] [PubMed] [Google Scholar]
- 127.Jedelsky PL, Dolezal P, Rada P, Pyrih J, Smid O, Hrdy I, Sedinova M, Marcincikova M, Voleman L, Perry AJ, Beltran NC, Lithgow T, Tachezy J. 2011. The minimal proteome in the reduced mitochondrion of the parasitic protist Giardia intestinalis. PLoS One 6:e17285. doi: 10.1371/journal.pone.0017285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pyrihova E, Motyckova A, Voleman L, Wandyszewska N, Fiser R, Seydlova G, Roger A, Kolisko M, Dolezal P. 2018. A single Tim translocase in the mitosomes of Giardia intestinalis illustrates convergence of protein import machines in anaerobic eukaryotes. Genome Biol Evol 10:2813–2822. doi: 10.1093/gbe/evy215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Voleman L, Najdrová V, Ástvaldsson Á, Tůmová P, Einarsson E, Švindrych Z, Hagen GM, Tachezy J, Svärd SG, Doležal P. 2017. Giardia intestinalis mitosomes undergo synchronized fission but not fusion and are constitutively associated with the endoplasmic reticulum. BMC Biol 15:27. doi: 10.1186/s12915-017-0361-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gabaldon T, Ginger ML, Michels PA. 2016. Peroxisomes in parasitic protists. Mol Biochem Parasitol 209:35–45. doi: 10.1016/j.molbiopara.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 131.Acosta-Virgen K, Chavez-Munguia B, Talamas-Lara D, Lagunes-Guillen A, Martinez-Higuera A, Lazcano A, Martinez-Palomo A, Espinosa-Cantellano M. 2018. Giardia lamblia: identification of peroxisomal-like proteins. Exp Parasitol 191:36–43. doi: 10.1016/j.exppara.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 132.Lloyd D, Williams CF. 2014. Comparative biochemistry of Giardia, Hexamita and Spironucleus: enigmatic diplomonads. Mol Biochem Parasitol 197:43–49. doi: 10.1016/j.molbiopara.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 133.Paget TA, Raynor MH, Shipp DW, Lloyd D. 1990. Giardia lamblia produces alanine anaerobically but not in the presence of oxygen. Mol Biochem Parasitol 42:63–67. doi: 10.1016/0166-6851(90)90113-Z. [DOI] [PubMed] [Google Scholar]
- 134.Schofield PJ, Edwards MR, Matthews J, Wilson JR. 1992. The pathway of arginine catabolism in Giardia intestinalis. Mol Biochem Parasitol 51:29–36. doi: 10.1016/0166-6851(92)90197-R. [DOI] [PubMed] [Google Scholar]
- 135.Novák L, Zubáčová Z, Karnkowska A, Kolisko M, Hroudová M, Stairs CW, Simpson AGB, Keeling PJ, Roger AJ, Čepička I, Hampl V. 2016. Arginine deiminase pathway enzymes: evolutionary history in metamonads and other eukaryotes. BMC Evol Biol 16:197. doi: 10.1186/s12862-016-0771-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hernandez PC, Wasserman M. 2006. Do genes from the cholesterol synthesis pathway exist and express in Giardia intestinalis? Parasitol Res 98:194–199. doi: 10.1007/s00436-005-0039-1. [DOI] [PubMed] [Google Scholar]
- 137.Yichoy M, Nakayasu ES, Shpak M, Aguilar C, Aley SB, Almeida IC, Das S. 2009. Lipidomic analysis reveals that phosphatidylglycerol and phosphatidylethanolamine are newly generated phospholipids in an early-divergent protozoan, Giardia lamblia. Mol Biochem Parasitol 165:67–78. doi: 10.1016/j.molbiopara.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yichoy M, Duarte TT, De Chatterjee A, Mendez TL, Aguilera KY, Roy D, Roychowdhury S, Aley SB, Das S. 2011. Lipid metabolism in Giardia: a post-genomic perspective. Parasitology 138:267–278. doi: 10.1017/S0031182010001277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Faso C, Hehl AB. 2019. A cytonaut’s guide to protein trafficking in Giardia lamblia, p 105–127. In Ortega-Pierres MG (ed), Advances in parasitology, vol 106. Academic Press, Cambridge, MA. [DOI] [PubMed] [Google Scholar]
- 140.Touz MC, Zamponi N. 2017. Sorting without a Golgi complex. Traffic 18:637–645. doi: 10.1111/tra.12500. [DOI] [PubMed] [Google Scholar]
- 141.Lujan HD, Marotta A, Mowatt MR, Sciaky N, Lippincott-Schwartz J, Nash TE. 1995. Developmental induction of Golgi structure and function in the primitive eukaryote Giardia lamblia. J Biol Chem 270:4612–4618. doi: 10.1074/jbc.270.9.4612. [DOI] [PubMed] [Google Scholar]
- 142.Zamponi N, Zamponi E, Mayol GF, Lanfredi-Rangel A, Svard SG, Touz MC. 2017. Endoplasmic reticulum is the sorting core facility in the Golgi-lacking protozoan Giardia lamblia. Traffic 18:604–621. doi: 10.1111/tra.12501. [DOI] [PubMed] [Google Scholar]
- 143.Faso C, Konrad C, Schraner EM, Hehl AB. 2013. Export of cyst wall material and Golgi organelle neogenesis in Giardia lamblia depend on endoplasmic reticulum exit sites. Cell Microbiol 15:537–553. doi: 10.1111/cmi.12054. [DOI] [PubMed] [Google Scholar]
- 144.Abodeely M, DuBois KN, Hehl A, Stefanic S, Sajid M, Desouza W, Attias M, Engel JC, Hsieh I, Fetter RD, McKerrow JH. 2009. A contiguous compartment functions as endoplasmic reticulum and endosome/lysosome in Giardia lamblia. Eukaryot Cell 8:1665–1676. doi: 10.1128/EC.00123-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rivero MR, Jausoro I, Bisbal M, Feliziani C, Lanfredi-Rangel A, Touz MC. 2013. Receptor-mediated endocytosis and trafficking between endosomal-lysosomal vacuoles in Giardia lamblia. Parasitol Res 112:1813–1818. doi: 10.1007/s00436-012-3253-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zumthor JP, Cernikova L, Rout S, Kaech A, Faso C, Hehl AB. 2016. Static clathrin assemblies at the peripheral vacuole-plasma membrane interface of the parasitic protozoan Giardia lamblia. PLoS Pathog 12:e1005756. doi: 10.1371/journal.ppat.1005756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nash TE, Gillin FD, Smith PD. 1983. Excretory-secretory products of Giardia lamblia. J Immunol 131:2004–2010. [PubMed] [Google Scholar]
- 148.Pimenta PF, da Silva PP, Nash T. 1991. Variant surface antigens of Giardia lamblia are associated with the presence of a thick cell coat: thin section and label fracture immunocytochemistry survey. Infect Immun 59:3989–3996. doi: 10.1128/iai.59.11.3989-3996.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Manko-Prykhoda A, Allain T, Motta JP, Cotton JA, Feener T, Oyeyemi A, Bindra S, Vallance BA, Wallace JL, Beck P, Buret AG. 2020. Giardia spp. promote the production of antimicrobial peptides and attenuate disease severity induced by attaching and effacing enteropathogens via the induction of the NLRP3 inflammasome. Int J Parasitol 50:263–275. doi: 10.1016/j.ijpara.2019.12.011. [DOI] [PubMed] [Google Scholar]
- 150.Moyano S, Musso J, Feliziani C, Zamponi N, Frontera LS, Ropolo AS, Lanfredi-Rangel A, Lalle M, Touz M. 2019. Exosome biogenesis in the protozoa parasite Giardia lamblia: a model of reduced interorganellar crosstalk. Cells 8:1600. doi: 10.3390/cells8121600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Adam RD, Aggarwal A, Lal AA, de la Cruz V, McCutchan T, Nash TE. 1988. Antigenic variation of a cysteine-rich protein in Giardia lamblia. J Exp Med 167:109–118. doi: 10.1084/jem.167.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gillin FD, Hagblom P, Harwood J, Aley SB, Reiner DS, McCaffery M, So M, Guiney DG. 1990. Isolation and expression of the gene for a major surface protein of Giardia lamblia. Proc Natl Acad Sci U S A 87:4463–4467. doi: 10.1073/pnas.87.12.4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ey PL, Khanna K, Andrews RH, Manning PA, Mayrhofer G. 1992. Distinct genetic groups of Giardia intestinalis distinguished by restriction fragment length polymorphisms. J Gen Microbiol 138:2629–2637. doi: 10.1099/00221287-138-12-2629. [DOI] [PubMed] [Google Scholar]
- 154.Mowatt MR, Aggarwal A, Nash TE. 1991. Carboxy-terminal sequence conservation among variant-specific surface proteins of Giardia lamblia. Mol Biochem Parasitol 49:215–227. doi: 10.1016/0166-6851(91)90065-e. [DOI] [PubMed] [Google Scholar]
- 155.Nash TE, Banks SM, Alling DW, Merritt JW, Jr, Conrad JT. 1990. Frequency of variant antigens in Giardia lamblia. Exp Parasitol 71:415–421. doi: 10.1016/0014-4894(90)90067-M. [DOI] [PubMed] [Google Scholar]
- 156.Adam RD, Nigam A, Seshadri V, Martens CA, Farneth GA, Morrison HG, Nash TE, Porcella SF, Patel R. 2010. The Giardia lamblia vsp gene repertoire: characteristics, genomic organization, and evolution. BMC Genomics 11:424. doi: 10.1186/1471-2164-11-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Li W, Saraiya AA, Wang CC. 2013. Experimental verification of the identity of variant-specific surface proteins in Giardia lamblia trophozoites. mBio 4:e00321-13–e00313. doi: 10.1128/mBio.00321-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nash TE, Merritt JW, Jr, Conrad JT. 1991. Isolate and epitope variability in susceptibility of Giardia lamblia to intestinal proteases. Infect Immun 59:1334–1340. doi: 10.1128/iai.59.4.1334-1340.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Nash TE, Herrington DA, Levine MM, Conrad JT, Merritt JW, Jr.. 1990. Antigenic variation of Giardia lamblia in experimental human infections. J Immunol 144:4362–4369. [PubMed] [Google Scholar]
- 160.Aggarwal A, Nash TE. 1988. Antigenic variation of Giardia lamblia in vivo. Infect Immun 56:1420–1423. doi: 10.1128/iai.56.6.1420-1423.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Horn D. 2014. Antigenic variation in African trypanosomes. Mol Biochem Parasitol 195:123–129. doi: 10.1016/j.molbiopara.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Adam RD, Yang YM, Nash TE. 1992. The cysteine-rich protein gene family of Giardia lamblia: loss of the CRP170 gene in an antigenic variant. Mol Cell Biol 12:1194–1201. doi: 10.1128/mcb.12.3.1194-1201.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kulakova L, Singer SM, Conrad J, Nash TE. 2006. Epigenetic mechanisms are involved in the control of Giardia lamblia antigenic variation. Mol Microbiol 61:1533–1542. doi: 10.1111/j.1365-2958.2006.05345.x. [DOI] [PubMed] [Google Scholar]
- 164.Prucca CG, Slavin I, Quiroga R, Elias EV, Rivero FD, Saura A, Carranza PG, Lujan HD. 2008. Antigenic variation in Giardia lamblia is regulated by RNA interference. Nature 456:750–754. doi: 10.1038/nature07585. [DOI] [PubMed] [Google Scholar]
- 165.Saraiya AA, Wang CC. 2008. snoRNA, a novel precursor of microRNA in Giardia lamblia. PLoS Pathog 4:e1000224. doi: 10.1371/journal.ppat.1000224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Li W, Saraiya AA, Wang CC. 2012. The profile of snoRNA-derived microRNAs that regulate expression of variant surface proteins in Giardia lamblia. Cell Microbiol 14:1455–1473. doi: 10.1111/j.1462-5822.2012.01811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rivero FD, Saura A, Prucca CG, Carranza PG, Torri A, Lujan HD. 2010. Disruption of antigenic variation is crucial for effective parasite vaccine. Nat Med 16:551–557. doi: 10.1038/nm.2141. [DOI] [PubMed] [Google Scholar]
- 168.Touz MC, Feliziani C, Ropolo AS. 2018. Membrane-associated proteins in Giardia lamblia. Genes 9:404. doi: 10.3390/genes9080404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wang AL, Wang CC. 1986. Discovery of a specific double-stranded RNA virus in Giardia lamblia. Mol Biochem Parasitol 21:269–276. doi: 10.1016/0166-6851(86)90132-5. [DOI] [PubMed] [Google Scholar]
- 170.Wang AL, Wang CC. 1991. Viruses of parasitic protozoa. Parasitol Today 7:76–80. doi: 10.1016/0169-4758(91)90198-w. [DOI] [PubMed] [Google Scholar]
- 171.Tai JH, Ong SJ, Chang SC, Su HM. 1993. Giardiavirus enters Giardia lamblia WB trophozoite via endocytosis. Exp Parasitol 76:165–174. doi: 10.1006/expr.1993.1019. [DOI] [PubMed] [Google Scholar]
- 172.Furfine ES, White TC, Wang AL, Wang CC. 1989. A single-stranded RNA copy of the Giardia lamblia virus double- stranded RNA genome is present in the infected Giardia lamblia. Nucleic Acids Res 17:7453–7467. doi: 10.1093/nar/17.18.7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lagunas-Rangel FA, Kameyama-Kawabe LY, Bermúdez-Cruz RM. 2021. Giardiavirus: an update. Parasitol Res 120:1943–1948. doi: 10.1007/s00436-021-07167-y. [DOI] [PubMed] [Google Scholar]
- 174.Gong P, Li X, Wu W, Cao L, Zhao P, Li X, Ren B, Li J, Zhang X. 2020. A novel MicroRNA from the translated region of the Giardiavirus rdrp gene governs virus copy number in Giardia duodenalis. Front Microbiol 11:569412. doi: 10.3389/fmicb.2020.569412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Benedict KM, Collier SA, Marder EP, Hlavsa MC, Fullerton KE, Yoder JS. 2019. Case-case analyses of cryptosporidiosis and giardiasis using routine national surveillance data in the United States—2005–2015. Epidemiol Infect 147:e178. doi: 10.1017/S0950268819000645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Walzer PD, Wolfe MS, Schultz MG. 1971. Giardiasis in travelers. J Infect Dis 124:235–237. doi: 10.1093/infdis/124.2.235. [DOI] [PubMed] [Google Scholar]
- 177.Kettis AA, Magnius L. 1973. Giardia lamblia infection in a group of students after a visit to Leningrad in March 1970. Scand J Infect Dis 5:289–292. doi: 10.3109/inf.1973.5.issue-4.10. [DOI] [PubMed] [Google Scholar]
- 178.Jokipii L, Jokipii AM. 1974. Giardiasis in travelers: a prospective study. J Infect Dis 130:295–299. doi: 10.1093/infdis/130.3.295. [DOI] [PubMed] [Google Scholar]
- 179.Robertson LJ, Hermansen L, Gjerde BK, Strand E, Alvsvag JO, Langeland N. 2006. Application of genotyping during an extensive outbreak of waterborne giardiasis in Bergen, Norway, during autumn and winter 2004. Appl Environ Microbiol 72:2212–2217. doi: 10.1128/AEM.72.3.2212-2217.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Nygard K, Schimmer B, Sobstad O, Walde A, Tveit I, Langeland N, Hausken T, Aavitsland P. 2006. A large community outbreak of waterborne giardiasis-delayed detection in a non-endemic urban area. BMC Public Health 6:141. doi: 10.1186/1471-2458-6-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Isaac-Renton JL, Lewis LF, Ong CS, Nulsen MF. 1994. A second community outbreak of waterborne giardiasis in Canada and serological investigation of patients. Trans R Soc Trop Med Hyg 88:395–399. doi: 10.1016/0035-9203(94)90397-2. [DOI] [PubMed] [Google Scholar]
- 182.Istre GR, Dunlop TS, Gaspard GB, Hopkins RS. 1984. Waterborne giardiasis at a mountain resort: evidence for acquired immunity. Am J Public Health 74:602–604. doi: 10.2105/ajph.74.6.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Gilman RH, Marquis GS, Miranda E, Vestegui M, Martinez H. 1988. Rapid reinfection by Giardia lamblia after treatment in a hyperendemic third world community. Lancet 331:343–345. doi: 10.1016/S0140-6736(88)91131-2. [DOI] [PubMed] [Google Scholar]
- 184.Reses HE, Gargano JW, Liang JL, Cronquist A, Smith K, Collier SA, Roy SL, Vanden Eng J, Bogard A, Lee B, Hlavsa MC, Rosenberg ES, Fullerton KE, Beach MJ, Yoder JS. 2018. Risk factors for sporadic Giardia infection in the USA: a case-control study in Colorado and Minnesota. Epidemiol Infect 146:1071–1078. doi: 10.1017/S0950268818001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.de Lucio A, Bailo B, Aguilera M, Cardona GA, Fernandez-Crespo JC, Carmena D. 2017. No molecular epidemiological evidence supporting household transmission of zoonotic Giardia duodenalis and Cryptosporidium spp. from pet dogs and cats in the province of Alava, Northern Spain. Acta Trop 170:48–56. doi: 10.1016/j.actatropica.2017.02.024. [DOI] [PubMed] [Google Scholar]
- 186.Coelho CH, Durigan M, Leal DAG, Schneider ADB, Franco RMB, Singer SM. 2017. Giardiasis as a neglected disease in Brazil: systematic review of 20 years of publications. PLoS Negl Trop Dis 11:e0006005. doi: 10.1371/journal.pntd.0006005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ryan U, Hijjawi N, Feng Y, Xiao L. 2019. Giardia: an under-reported foodborne parasite. Int J Parasitol 49:1–11. doi: 10.1016/j.ijpara.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 188.Certad G, Viscogliosi E, Chabe M, Caccio SM. 2017. Pathogenic mechanisms of Cryptosporidium and Giardia. Trends Parasitol 33:561–576. doi: 10.1016/j.pt.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 189.Singer SM, Fink MY, Angelova VV. 2019. Recent insights into innate and adaptive immune responses to Giardia. Adv Parasitol 106:171–208. doi: 10.1016/bs.apar.2019.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Buret AG. 2019. Acceptance of the 2019 Stoll-Stunkard Memorial Lectureship Award: the study of host-parasite interactions to better understand fundamental host physiology: the model of giardiasis. J Parasitol 105:955–960. [PubMed] [Google Scholar]
- 191.Fink MY, Singer SM. 2017. The intersection of immune responses, microbiota, and pathogenesis in giardiasis. Trends Parasitol 33:901–913. doi: 10.1016/j.pt.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Erlandsen SL, Chase DG. 1974. Morphological alterations in the microvillous border of villous epithelial cells produced by intestinal microorganisms. Am J Clin Nutr 27:1277–1286. doi: 10.1093/ajcn/27.11.1277. [DOI] [PubMed] [Google Scholar]
- 193.Allain T, Fekete E, Buret AG. 2019. Giardia cysteine proteases: the teeth behind the smile. Trends Parasitol 35:636–648. doi: 10.1016/j.pt.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 194.Oberhuber G, Mesteri I, Kopf W, Muller H. 2016. Demonstration of trophozoites of G. Lamblia in ileal mucosal biopsy specimens may reveal giardiasis in patients with significantly inflamed parasite-free duodenal mucosa. Am J Surg Pathol 40:1280–1285. doi: 10.1097/PAS.0000000000000665. [DOI] [PubMed] [Google Scholar]
- 195.de Carvalho TB, David EB, Coradi ST, Guimaraes S. 2008. Protease activity in extracellular products secreted in vitro by trophozoites of Giardia duodenalis. Parasitol Res 104:185–190. doi: 10.1007/s00436-008-1185-z. [DOI] [PubMed] [Google Scholar]
- 196.Allain T, Amat CB, Motta JP, Manko A, Buret AG. 2017. Interactions of Giardia sp. with the intestinal barrier: epithelium, mucus, and microbiota. Tissue Barriers 5:e1274354. doi: 10.1080/21688370.2016.1274354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Liu J, Ma'ayeh S, Peirasmaki D, Lundstrom-Stadelmann B, Hellman L, Svard SG. 2018. Secreted Giardia intestinalis cysteine proteases disrupt intestinal epithelial cell junctional complexes and degrade chemokines. Virulence 9:879–894. doi: 10.1080/21505594.2018.1451284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ortega-Pierres G, Arguello-Garcia R, Laredo-Cisneros MS, Fonseca-Linan R, Gomez-Mondragon M, Inzunza-Arroyo R, Flores-Benitez D, Raya-Sandino A, Chavez-Munguia B, Ventura-Gallegos JL, Zentella-Dehesa A, Bermudez-Cruz RM, Gonzalez-Mariscal L. 2018. Giardipain-1, a protease secreted by Giardia duodenalis trophozoites, causes junctional, barrier and apoptotic damage in epithelial cell monolayers. Int J Parasitol 48:621–639. doi: 10.1016/j.ijpara.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 199.Bhargava A, Cotton JA, Dixon BR, Gedamu L, Yates RM, Buret AG. 2015. Giardia duodenalis surface cysteine proteases induce cleavage of the intestinal epithelial cytoskeletal protein villin via myosin light chain kinase. PLoS One 10:e0136102. doi: 10.1371/journal.pone.0136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Amat CB, Motta JP, Fekete E, Moreau F, Chadee K, Buret AG. 2017. Cysteine protease-dependent mucous disruptions and differential mucin gene expression in Giardia duodenalis infection. Am J Pathol 187:2486–2498. doi: 10.1016/j.ajpath.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 201.Ma’ayeh SY, Liu J, Peirasmaki D, Hornaeus K, Bergstrom Lind S, Grabherr M, Bergquist J, Svard SG. 2017. Characterization of the Giardia intestinalis secretome during interaction with human intestinal epithelial cells: the impact on host cells. PLoS Negl Trop Dis 11:e0006120. doi: 10.1371/journal.pntd.0006120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Peirasmaki D, Ma’ayeh SY, Xu F, Ferella M, Campos S, Liu J, Svard SG. 2020. High cysteine membrane proteins (HCMPs) are up-regulated during Giardia-host cell interactions. Front Genet 11:913. doi: 10.3389/fgene.2020.00913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Dubourg A, Xia D, Winpenny JP, Al Naimi S, Bouzid M, Sexton DW, Wastling JM, Hunter PR, Tyler KM. 2018. Giardia secretome highlights secreted tenascins as a key component of pathogenesis. GigaScience 7:1–13. doi: 10.1093/gigascience/giy003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Stadelmann B, Hanevik K, Andersson MK, Bruserud O, Svard SG. 2013. The role of arginine and arginine-metabolizing enzymes during Giardia-host cell interactions in vitro. BMC Microbiol 13:256. doi: 10.1186/1471-2180-13-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Stadelmann B, Merino MC, Persson L, Svard SG. 2012. Arginine consumption by the intestinal parasite Giardia intestinalis reduces proliferation of intestinal epithelial cells. PLoS One 7:e45325. doi: 10.1371/journal.pone.0045325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ringqvist E, Palm JE, Skarin H, Hehl AB, Weiland M, Davids BJ, Reiner DS, Griffiths WJ, Eckmann L, Gillin FD, Svard SG. 2008. Release of metabolic enzymes by Giardia in response to interaction with intestinal epithelial cells. Mol Biochem Parasitol 159:85–91. doi: 10.1016/j.molbiopara.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Banik S, Renner Viveros P, Seeber F, Klotz C, Ignatius R, Aebischer T. 2013. Giardia duodenalis arginine deiminase modulates the phenotype and cytokine secretion of human dendritic cells by depletion of arginine and formation of ammonia. Infect Immun 81:2309–2317. doi: 10.1128/IAI.00004-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hjollo T, Bratland E, Steinsland H, Radunovic M, Langeland N, Hanevik K. 2018. Longitudinal cohort study of serum antibody responses towards Giardia lamblia variant-specific surface proteins in a non-endemic area. Exp Parasitol 191:66–72. doi: 10.1016/j.exppara.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 209.Serradell MC, Saura A, Rupil LL, Gargantini PR, Faya MI, Furlan PJ, Lujan HD. 2016. Vaccination of domestic animals with a novel oral vaccine prevents Giardia infections, alleviates signs of giardiasis and reduces transmission to humans. NPJ Vaccines 1:16018. doi: 10.1038/npjvaccines.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Chang JT. 2020. Pathophysiology of inflammatory bowel diseases. N Engl J Med 383:2652–2664. doi: 10.1056/NEJMra2002697. [DOI] [PubMed] [Google Scholar]
- 211.Plichta DR, Graham DB, Subramanian S, Xavier RJ. 2019. Therapeutic opportunities in inflammatory bowel disease: mechanistic dissection of host-microbiome relationships. Cell 178:1041–1056. doi: 10.1016/j.cell.2019.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Solaymani-Mohammadi S, Singer SM. 2011. Host immunity and pathogen strain contribute to intestinal disaccharidase impairment following gut infection. J Immunol 187:3769–3775. doi: 10.4049/jimmunol.1100606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Dreesen L, De Bosscher K, Grit G, Staels B, Lubberts E, Bauge E, Geldhof P. 2014. Giardia muris infection in mice is associated with a protective interleukin 17A response and induction of peroxisome proliferator-activated receptor alpha. Infect Immun 82:3333–3340. doi: 10.1128/IAI.01536-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Dann SM, Manthey CF, Le C, Miyamoto Y, Gima L, Abrahim A, Cao AT, Hanson EM, Kolls JK, Raz E, Cong Y, Eckmann L. 2015. IL-17A promotes protective IgA responses and expression of other potential effectors against the lumen-dwelling enteric parasite Giardia. Exp Parasitol 156:68–78. doi: 10.1016/j.exppara.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Paerewijck O, Maertens B, Dreesen L, Van Meulder F, Peelaers I, Ratman D, Li RW, Lubberts E, De Bosscher K, Geldhof P. 2017. Interleukin-17 receptor A (IL-17RA) as a central regulator of the protective immune response against Giardia. Sci Rep 7:8520. doi: 10.1038/s41598-017-08590-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Saghaug CS, Sørnes S, Peirasmaki D, Svärd S, Langeland N, Hanevik K. 2016. Human memory CD4+ T cell immune responses against Giardia lamblia. Clin Vaccine Immunol 23:11–18. doi: 10.1128/CVI.00419-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Cascais-Figueiredo T, Austriaco-Teixeira P, Fantinatti M, Silva-Freitas ML, Santos-Oliveira JR, Coelho CH, Singer SM, Da-Cruz AM. 2019. Giardiasis alters intestinal fatty acid binding protein (I-FABP) and plasma cytokines levels in children in brazil. Pathogens 9:7. doi: 10.3390/pathogens9010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Yordanova IA, Cortes A, Klotz C, Kuhl AA, Heimesaat MM, Cantacessi C, Hartmann S, Rausch S. 2019. ROR-γt+ Treg to Th17 ratios correlate with susceptibility to Giardia infection. Sci Rep 9:20328. doi: 10.1038/s41598-019-56416-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Solaymani-Mohammadi S, Singer SM. 2010. Giardia duodenalis: the double-edged sword of immune responses in giardiasis. Exp Parasitol 126:292–297. doi: 10.1016/j.exppara.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Munoz-Cruz S, Gomez-Garcia A, Matadamas-Martinez F, Alvarado-Torres JA, Meza-Cervantez P, Arriaga-Pizano L, Yepez-Mulia L. 2018. Giardia lamblia: identification of molecules that contribute to direct mast cell activation. Parasitol Res 117:2555–2567. doi: 10.1007/s00436-018-5944-1. [DOI] [PubMed] [Google Scholar]
- 221.Maloney J, Keselman A, Li E, Singer SM. 2015. Macrophages expressing arginase 1 and nitric oxide synthase 2 accumulate in the small intestine during Giardia lamblia infection. Microbes Infect 17:462–467. doi: 10.1016/j.micinf.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Fink MY, Maloney J, Keselman A, Li E, Menegas S, Staniorski C, Singer SM. 2019. Proliferation of resident macrophages is dispensable for protection during Giardia duodenalis infections. ImmunoHorizons 3:412–421. doi: 10.4049/immunohorizons.1900041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Kamda JD, Singer SM. 2009. Phosphoinositide 3-kinase-dependent inhibition of dendritic cell interleukin-12 production by Giardia lamblia. Infect Immun 77:685–693. doi: 10.1128/IAI.00718-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Summan A, Nejsum P, Williams AR. 2018. Modulation of human dendritic cell activity by Giardia and helminth antigens. Parasite Immunol 40:e12525. doi: 10.1111/pim.12525. [DOI] [PubMed] [Google Scholar]
- 225.Dann SM, Le CHY, Hanson EM, Ross MC, Eckmann L. 2018. Giardia infection of the small intestine induces chronic colitis in genetically susceptible hosts. J Immunol 201:548–559. doi: 10.4049/jimmunol.1700824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Manko A, Motta JP, Cotton JA, Feener T, Oyeyemi A, Vallance BA, Wallace JL, Buret AG. 2017. Giardia co-infection promotes the secretion of antimicrobial peptides beta-defensin 2 and trefoil factor 3 and attenuates attaching and effacing bacteria-induced intestinal disease. PLoS One 12:e0178647. doi: 10.1371/journal.pone.0178647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Cotton JA, Motta JP, Schenck LP, Hirota SA, Beck PL, Buret AG. 2014. Giardia duodenalis infection reduces granulocyte infiltration in an in vivo model of bacterial toxin-induced colitis and attenuates inflammation in human intestinal tissue. PLoS One 9:e109087. doi: 10.1371/journal.pone.0109087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Cotton JA, Bhargava A, Ferraz JG, Yates RM, Beck PL, Buret AG. 2014. Giardia duodenalis cathepsin B proteases degrade intestinal epithelial interleukin-8 and attenuate interleukin-8-induced neutrophil chemotaxis. Infect Immun 82:2772–2787. doi: 10.1128/IAI.01771-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, Visser CE, Kuijper EJ, Bartelsman JFWM, Tijssen JGP, Speelman P, Dijkgraaf MGW, Keller JJ. 2013. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med 368:407–415. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 230.Smith MI, Yatsunenko T, Manary MJ, Trehan I, Mkakosya R, Cheng J, Kau AL, Rich SS, Concannon P, Mychaleckyj JC, Liu J, Houpt E, Li JV, Holmes E, Nicholson J, Knights D, Ursell LK, Knight R, Gordon JI. 2013. Gut microbiomes of Malawian twin pairs discordant for Kwashiorkor. Science 339:548–554. doi: 10.1126/science.1229000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Fan Y, Pedersen O. 2021. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol 19:55–71. doi: 10.1038/s41579-020-0433-9. [DOI] [PubMed] [Google Scholar]
- 232.Torres MR, Silva ME, Vieira EC, Bambirra EA, Sogayar MI, Pena FJ, Nicoli JR. 1992. Intragastric infection of conventional and germfree mice with Giardia lamblia. Braz J Med Biol Res 25:349–352. [PubMed] [Google Scholar]
- 233.Torres MF, Uetanabaro AP, Costa AF, Alves CA, Farias LM, Bambirra EA, Penna FJ, Vieira EC, Nicoli JR. 2000. Influence of bacteria from the duodenal microbiota of patients with symptomatic giardiasis on the pathogenicity of Giardia duodenalis in gnotoxenic mice. J Med Microbiol 49:209–215. doi: 10.1099/0022-1317-49-3-209. [DOI] [PubMed] [Google Scholar]
- 234.Singer SM, Nash TE. 2000. The role of normal flora in Giardia lamblia infections in mice. J Infect Dis 181:1510–1512. doi: 10.1086/315409. [DOI] [PubMed] [Google Scholar]
- 235.Barash NR, Maloney JG, Singer SM, Dawson SC. 2017. Giardia alters commensal microbial diversity throughout the murine gut. Infect Immun 85:e00948-16. doi: 10.1128/IAI.00948-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Keselman A, Li E, Maloney J, Singer SM. 2016. The microbiota contributes to CD8+ T cell activation and nutrient malabsorption following intestinal infection with Giardia duodenalis. Infect Immun 84:2853–2860. doi: 10.1128/IAI.00348-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Beatty JK, Akierman SV, Motta JP, Muise S, Workentine ML, Harrison JJ, Bhargava A, Beck PL, Rioux KP, McKnight GW, Wallace JL, Buret AG. 2017. Giardia duodenalis induces pathogenic dysbiosis of human intestinal microbiota biofilms. Int J Parasitol 47:311–326. doi: 10.1016/j.ijpara.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 238.Chen TL, Chen S, Wu HW, Lee TC, Lu YZ, Wu LL, Ni YH, Sun CH, Yu WH, Buret AG, Yu LC. 2013. Persistent gut barrier damage and commensal bacterial influx following eradication of Giardia infection in mice. Gut Pathog 5:26. doi: 10.1186/1757-4749-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Li X, Zhang X, Gong P, Xia F, Li L, Yang Z, Li J. 2017. TLR2−/− mice display decreased severity of giardiasis via enhanced proinflammatory cytokines production dependent on AKT signal pathway. Front Immunol 8:1186. doi: 10.3389/fimmu.2017.01186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Iebba V, Santangelo F, Totino V, Pantanella F, Monsia A, Di Cristanziano V, Di Cave D, Schippa S, Berrilli F, D’Alfonso R. 2016. Gut microbiota related to Giardia duodenalis, Entamoeba spp. and Blastocystis hominis infections in humans from Cote d’Ivoire. J Infect Dev Ctries 10:1035–1041. doi: 10.3855/jidc.8179. [DOI] [PubMed] [Google Scholar]
- 241.Toro-Londono MA, Bedoya-Urrego K, Garcia-Montoya GM, Galvan-Diaz AL, Alzate JF. 2019. Intestinal parasitic infection alters bacterial gut microbiota in children. PeerJ 7:e6200. doi: 10.7717/peerj.6200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Mejia R, Damania A, Jeun R, Bryan PE, Vargas P, Juarez M, Cajal PS, Nasser J, Krolewiecki A, Lefoulon E, Long C, Drake E, Cimino RO, Slatko B. 2020. Impact of intestinal parasites on microbiota and cobalamin gene sequences: a pilot study. Parasit Vectors 13:200. doi: 10.1186/s13071-020-04073-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque AS, Zaidi AK, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acacio S, Biswas K, O'Reilly CE, Mintz ED, Berkeley LY, Muhsen K, Sommerfelt H, Robins-Browne RM, Levine MM. 2013. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 244.Yori PP, Lee G, Olortegui MP, Chavez CB, Flores JT, Vasquez AO, Burga R, Pinedo SR, Asayag CR, Black RE, Caulfield LE, Kosek M. 2014. Santa Clara de Nanay: the MAL-ED cohort in Peru. Clin Infect Dis 59(Suppl 4):S310–S316. doi: 10.1093/cid/ciu460. [DOI] [PubMed] [Google Scholar]
- 245.Berry ASF, Johnson K, Martins R, Sullivan MC, Farias Amorim C, Putre A, Scott A, Wang S, Lindsay B, Baldassano RN, Nolan TJ, Beiting DP. 2020. Natural infection with Giardia is associated with altered community structure of the human and canine gut microbiome. mSphere 5:e00670-20. doi: 10.1128/mSphere.00670-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Halliez MC, Motta JP, Feener TD, Guerin G, LeGoff L, Francois A, Colasse E, Favennec L, Gargala G, Lapointe TK, Altier C, Buret AG. 2016. Giardia duodenalis induces paracellular bacterial translocation and causes postinfectious visceral hypersensitivity. Am J Physiol Gastrointest Liver Physiol 310:G574–85. doi: 10.1152/ajpgi.00144.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Bartelt LA, Roche J, Kolling G, Bolick D, Noronha F, Naylor C, Hoffman P, Warren C, Singer S, Guerrant R. 2013. Persistent G. lamblia impairs growth in a murine malnutrition model. J Clin Invest 123:2672–2684. doi: 10.1172/JCI67294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Bartelt LA, Bolick DT, Mayneris-Perxachs J, Kolling GL, Medlock GL, Zaenker EI, Donowitz J, Thomas-Beckett RV, Rogala A, Carroll IM, Singer SM, Papin J, Swann JR, Guerrant RL. 2017. Cross-modulation of pathogen-specific pathways enhances malnutrition during enteric co-infection with Giardia lamblia and enteroaggregative Escherichia coli. PLoS Pathog 13:e1006471. doi: 10.1371/journal.ppat.1006471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Hollm-Delgado MG, Gilman RH, Bern C, Cabrera L, Sterling CR, Black RE, Checkley W. 2008. Lack of an adverse effect of Giardia intestinalis infection on the health of Peruvian children. Am J Epidemiol 168:647–655. doi: 10.1093/aje/kwn177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Muhsen K, Levine MM. 2012. A systematic review and meta-analysis of the association between Giardia lamblia and endemic pediatric diarrhea in developing countries. Clin Infect Dis 55:S271–S293. doi: 10.1093/cid/cis762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Muhsen K, Cohen D, Levine MM. 2014. Can Giardia lamblia infection lower the risk of acute diarrhea among preschool children? J Trop Pediatr 60:99–103. doi: 10.1093/tropej/fmt085. [DOI] [PubMed] [Google Scholar]
- 252.Donowitz JR, Alam M, Kabir M, Ma JZ, Nazib F, Platts-Mills JA, Bartelt LA, Haque R, Petri WA, Jr.. 2016. A prospective longitudinal cohort to investigate the effects of early life giardiasis on growth and all cause diarrhea. Clin Infect Dis 63:792–797. doi: 10.1093/cid/ciw391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Prado MS, Cairncross S, Strina A, Barreto ML, Oliveira-Assis AM, Rego S. 2005. Asymptomatic giardiasis and growth in young children; a longitudinal study in Salvador, Brazil. Parasitology 131:51–56. doi: 10.1017/s0031182005007353. [DOI] [PubMed] [Google Scholar]
- 254.Boeke CE, Mora-Plazas M, Forero Y, Villamor E. 2010. Intestinal protozoan infections in relation to nutritional status and gastrointestinal morbidity in Colombian school children. J Trop Pediatr 56:299–306. doi: 10.1093/tropej/fmp136. [DOI] [PubMed] [Google Scholar]
- 255.Sackey ME, Weigel MM, Armijos RX. 2003. Predictors and nutritional consequences of intestinal parasitic infections in rural Ecuadorian children. J Trop Pediatr 49:17–23. doi: 10.1093/tropej/49.1.17. [DOI] [PubMed] [Google Scholar]
- 256.Centeno-Lima S, Rosado-Marques V, Ferreira F, Rodrigues R, Indeque B, Camara I, De Sousa B, Aguiar P, Nunes B, Ferrinho P. 2013. Giardia duodenalis and chronic malnutrition in children under five from a rural area of Guinea-Bissau. Acta Medica Portuguesa 26:721–724. [PubMed] [Google Scholar]
- 257.Rogawski ET, Bartelt LA, Platts-Mills JA, Seidman JC, Samie A, Havt A, Babji S, Trigoso DR, Qureshi S, Shakoor S, Haque R, Mduma E, Bajracharya S, Gaffar SMA, Lima AAM, Kang G, Kosek MN, Ahmed T, Svensen E, Mason C, Bhutta ZA, Lang DR, Gottlieb M, Guerrant RL, Houpt ER, Bessong PO, Investigators M-EN, the MAL-ED Network Investigators. 2017. Determinants and impact of Giardia infection in the first 2 years of life in the MAL-ED birth cohort. J Pediatric Infect Dis Soc 6:153–160. doi: 10.1093/jpids/piw082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Kosek MN, Investigators M-EN, MAL-ED Network Investigators. 2017. Causal pathways from enteropathogens to environmental enteropathy: findings from the MAL-ED birth cohort study. EBioMedicine 18:109–117. doi: 10.1016/j.ebiom.2017.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Rogawski ET, Liu J, Platts-Mills JA, Kabir F, Lertsethtakarn P, Siguas M, Khan SS, Praharaj I, Murei A, Nshama R, Mujaga B, Havt A, Maciel IA, Operario DJ, Taniuchi M, Gratz J, Stroup SE, Roberts JH, Kalam A, Aziz F, Qureshi S, Islam MO, Sakpaisal P, Silapong S, Yori PP, Rajendiran R, Benny B, McGrath M, Seidman JC, Lang D, Gottlieb M, Guerrant RL, Lima AAM, Leite JP, Samie A, Bessong PO, Page N, Bodhidatta L, Mason C, Shrestha S, Kiwelu I, Mduma ER, Iqbal NT, Bhutta ZA, Ahmed T, Haque R, Kang G, Kosek MN, Houpt ER, MAL-ED Network Investigators. 2018. Use of quantitative molecular diagnostic methods to investigate the effect of enteropathogen infections on linear growth in children in low-resource settings: longitudinal analysis of results from the MAL-ED cohort study. Lancet Glob Health 6:e1319–e1328. doi: 10.1016/S2214-109X(18)30351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Platts-Mills JA, Taniuchi M, Uddin MJ, Sobuz SU, Mahfuz M, Gaffar SA, Mondal D, Hossain MI, Islam MM, Ahmed AS, Petri WA, Haque R, Houpt ER, Ahmed T. 2017. Association between enteropathogens and malnutrition in children aged 6–23 mo in Bangladesh: a case-control study. Am J Clin Nutr 105:1132–1138. doi: 10.3945/ajcn.116.138800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Futagami S, Itoh T, Sakamoto C. 2015. Systematic review with meta-analysis: post-infectious functional dyspepsia. Aliment Pharmacol Ther 41:177–188. doi: 10.1111/apt.13006. [DOI] [PubMed] [Google Scholar]
- 262.Wensaas KA, Langeland N, Hanevik K, Morch K, Eide GE, Rortveit G. 2012. Irritable bowel syndrome and chronic fatigue 3 years after acute giardiasis: historic cohort study. Gut 61:214–219. doi: 10.1136/gutjnl-2011-300220. [DOI] [PubMed] [Google Scholar]
- 263.Nakao JH, Collier SA, Gargano JW. 2017. Giardiasis and subsequent irritable bowel syndrome: a longitudinal cohort study using health insurance data. J Infect Dis 215:798–805. doi: 10.1093/infdis/jiw621. [DOI] [PubMed] [Google Scholar]
- 264.Dormond M, Gutierrez RL, Porter CK. 2016. Giardia lamblia infection increases risk of chronic gastrointestinal disorders. Trop Dis Travel Med Vaccines 2:17. doi: 10.1186/s40794-016-0030-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Svendsen AT, Bytzer P, Engsbro AL. 2019. Systematic review with meta-analyses: does the pathogen matter in post-infectious irritable bowel syndrome? Scand J Gastroenterol 54:546–562. doi: 10.1080/00365521.2019.1607897. [DOI] [PubMed] [Google Scholar]
- 266.Painter JE, Collier SA, Gargano JW. 2017. Association between Giardia and arthritis or joint pain in a large health insurance cohort: could it be reactive arthritis? Epidemiol Infect 145:471–477. doi: 10.1017/S0950268816002120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Giacometti A, Cirioni O, Antonicelli L, D’Amato G, Silvestri C, Del Prete MS, Scalise G. 2003. Prevalence of intestinal parasites among individuals with allergic skin diseases. J Parasitol 89:490–492. doi: 10.1645/0022-3395(2003)089[0490:POIPAI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 268.Paulos S, Saugar JM, de Lucio A, Fuentes I, Mateo M, Carmena D. 2019. Comparative performance evaluation of four commercial multiplex real-time PCR assays for the detection of the diarrhoea-causing protozoa Cryptosporidium hominis/parvum, Giardia duodenalis and Entamoeba histolytica. PLoS One 14:e0215068. doi: 10.1371/journal.pone.0215068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Hitchcock MM, Hogan CA, Budvytiene I, Banaei N. 2019. Reproducibility of positive results for rare pathogens on the FilmArray GI panel. Diagn Microbiol Infect Dis 95:10–14. doi: 10.1016/j.diagmicrobio.2019.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Garcia LS, Arrowood M, Kokoskin E, Paltridge GP, Pillai DR, Procop GW, Ryan N, Shimizu RY, Visvesvara G. 2018. Laboratory diagnosis of parasites from the gastrointestinal tract. Clin Microbiol Rev 31:e00025-17. doi: 10.1128/CMR.00025-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Cartwright CP. 1999. Utility of multiple-stool-specimen ova and parasite examinations in a high-prevalence setting. J Clin Microbiol 37:2408–2411. doi: 10.1128/JCM.37.8.2408-2411.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Jangra M, Dutta U, Shah J, Thapa BR, Nada R, Gupta N, Sehgal R, Sharma V, Khurana S. 2020. Role of polymerase chain reaction in stool and duodenal biopsy for diagnosis of giardiasis in patients with persistent/chronic diarrhea. Dig Dis Sci 65:2345–2353. doi: 10.1007/s10620-019-06042-2. [DOI] [PubMed] [Google Scholar]
- 273.Beal CB, Viens P, Grant RGL, Hughes JM. 1970. A new technique for sampling duodenal contents-demonstration of upper small-bowel pathogens. Am J Trop Med Hyg 19:349–352. doi: 10.4269/ajtmh.1970.19.349. [DOI] [PubMed] [Google Scholar]
- 274.Rosenthal P, Liebman WM. 1980. Comparative study of stool examinations, duodenal aspiration, and pediatric Entero-Test for giardiasis in children. J Pediatr 96:278–279. doi: 10.1016/S0022-3476(80)80826-2. [DOI] [PubMed] [Google Scholar]
- 275.Leitsch D. 2019. A review on metronidazole: an old warhorse in antimicrobial chemotherapy. Parasitology 146:1167–1178. doi: 10.1017/S0031182017002025. [DOI] [PubMed] [Google Scholar]
- 276.Lindmark DG, Muller M. 1976. Antitrichomonad action, mutagenicity, and reduction of metronidazole and other nitroimidazoles. Antimicrob Agents Chemother 10:476–482. doi: 10.1128/AAC.10.3.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Leitsch D, Burgess AG, Dunn LA, Krauer KG, Tan K, Duchene M, Upcroft P, Eckmann L, Upcroft JA. 2011. Pyruvate:ferredoxin oxidoreductase and thioredoxin reductase are involved in 5-nitroimidazole activation while flavin metabolism is linked to 5-nitroimidazole resistance in Giardia lamblia. J Antimicrob Chemother 66:1756–1765. doi: 10.1093/jac/dkr192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Leitsch D, Drinic M, Kolarich D, Duchene M. 2012. Down-regulation of flavin reductase and alcohol dehydrogenase-1 (ADH1) in metronidazole-resistant isolates of Trichomonas vaginalis. Mol Biochem Parasitol 183:177–183. doi: 10.1016/j.molbiopara.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Leitsch D, Schlosser S, Burgess A, Duchene M. 2012. Nitroimidazole drugs vary in their mode of action in the human parasite Giardia lamblia. Int J Parasitol Drugs Drug Resist 2:166–170. doi: 10.1016/j.ijpddr.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Beard CM, Noller KL, O’Fallon WM, Kurland LT, Dahlin DC. 1988. Cancer after exposure to metronidazole. Mayo Clin Proc 63:147–153. doi: 10.1016/S0025-6196(12)64947-7. [DOI] [PubMed] [Google Scholar]
- 281.Dobias L, Cerna M, Rossner P, Sram R. 1994. Genotoxicity and carcinogenicity of metronidazole. Mutation Res 317:177–194. doi: 10.1016/0165-1110(94)90001-9. [DOI] [PubMed] [Google Scholar]
- 282.Friedman GD, Jiang SF, Udaltsova N, Quesenberry CP, Jr, Chan J, Habel LA. 2009. Epidemiologic evaluation of pharmaceuticals with limited evidence of carcinogenicity. Int J Cancer 125:2173–2178. doi: 10.1002/ijc.24545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Falagas ME, Walker AM, Jick H, Ruthazer R, Griffith J, Snydman DR. 1998. Late incidence of cancer after metronidazole use: a matched metronidazole user/nonuser study. Clin Infect Dis 26:384–388. doi: 10.1086/516306. [DOI] [PubMed] [Google Scholar]
- 284.Koss CA, Baras DC, Lane SD, Aubry R, Marcus M, Markowitz LE, Koumans EH. 2012. Investigation of metronidazole use during pregnancy and adverse birth outcomes. Antimicrob Agents Chemother 56:4800–4805. doi: 10.1128/AAC.06477-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Vakkilainen S, Nieminen T, Bjorkbacka S, Saavalainen-Hakala T, Salo E. 2020. Treatment of giardiasis in children: randomized trial of rectal metronidazole versus oral tinidazole. J Infect 81:816–846. doi: 10.1016/j.jinf.2020.08.050. [DOI] [PubMed] [Google Scholar]
- 286.Ordonez-Mena JM, McCarthy ND, Fanshawe TR. 2018. Comparative efficacy of drugs for treating giardiasis: a systematic update of the literature and network meta-analysis of randomized clinical trials. J Antimicrob Chemother 73:596–606. doi: 10.1093/jac/dkx430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Pasupuleti V, Escobedo AA, Deshpande A, Thota P, Roman Y, Hernandez AV. 2014. Efficacy of 5-nitroimidazoles for the treatment of giardiasis: a systematic review of randomized controlled trials. PLoS Negl Trop Dis 8:e2733. doi: 10.1371/journal.pntd.0002733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Fung HB, Doan TL. 2005. Tinidazole: a nitroimidazole antiprotozoal agent. Clin Ther 27:1859–1884. doi: 10.1016/j.clinthera.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 289.MacDonald LM, Armson A, Thompson AR, Reynoldson JA. 2004. Characterisation of benzimidazole binding with recombinant tubulin from Giardia duodenalis, Encephalitozoon intestinalis, and Cryptosporidium parvum. Mol Biochem Parasitol 138:89–96. doi: 10.1016/j.molbiopara.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 290.Dayan AD. 2003. Albendazole, mebendazole and praziquantel. Review of non-clinical toxicity and pharmacokinetics. Acta Trop 86:141–159. doi: 10.1016/s0001-706x(03)00031-7. [DOI] [PubMed] [Google Scholar]
- 291.Escobedo AA, Almirall P, Gonzalez-Fraile E, Ballesteros J. 2019. Efficacy of 5-nitroimidazole compounds for giardiasis in Cuban children: systematic review and meta-analysis. Infezioni in Medicina 27:58–67. [PubMed] [Google Scholar]
- 292.Raether W, Hanel H. 2003. Nitroheterocyclic drugs with broad spectrum activity. Parasitol Res 90:S19–S39. doi: 10.1007/s00436-002-0754-9. [DOI] [PubMed] [Google Scholar]
- 293.Hoffman PS, Sisson G, Croxen MA, Welch K, Harman WD, Cremades N, Morash MG. 2007. Antiparasitic drug nitazoxanide inhibits the pyruvate oxidoreductases of Helicobacter pylori, selected anaerobic bacteria and parasites, and Campylobacter jejuni. Antimicrob Agents Chemother 51:868–876. doi: 10.1128/AAC.01159-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Anderson VR, Curran MP. 2007. Nitazoxanide: a review of its use in the treatment of gastrointestinal infections. Drugs 67:1947–1967. doi: 10.2165/00003495-200767130-00015. [DOI] [PubMed] [Google Scholar]
- 295.Gardner TB, Hill DR. 2001. Treatment of giardiasis. Clin Microbiol Rev 14:114–128. doi: 10.1128/CMR.14.1.114-128.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Escobedo AA, Nunez FA, Moreira I, Vega E, Pareja A, Almirall P. 2003. Comparison of chloroquine, albendazole and tinidazole in the treatment of children with giardiasis. Ann Trop Med Parasitoly 97:367–371. doi: 10.1179/000349803235002290. [DOI] [PubMed] [Google Scholar]
- 297.Canete R, Rivas DE, Escobedo AA, Gonzalez ME, Almirall P, Brito K. 2010. A randomized, controlled, open-label trial evaluating the efficacy and safety of chloroquine in the treatment of giardiasis in children. West Indian Med J 59:607–611. [PubMed] [Google Scholar]
- 298.Morch K, Hanevik K. 2020. Giardiasis treatment: an update with a focus on refractory disease. Curr Opin Infect Dis 33:355–364. doi: 10.1097/QCO.0000000000000668. [DOI] [PubMed] [Google Scholar]
- 299.Escobedo AA, Ballesteros J, Gonzalez-Fraile E, Almirall P. 2016. A meta-analysis of the efficacy of albendazole compared with tinidazole as treatments for Giardia infections in children. Acta Tropica 153:120–127. doi: 10.1016/j.actatropica.2015.09.023. [DOI] [PubMed] [Google Scholar]
- 300.Solaymani-Mohammadi S, Genkinger JM, Loffredo CA, Singer SM. 2010. A meta-analysis of the effectiveness of albendazole compared with metronidazole as treatments for infections with Giardia duodenalis. PLoS Negl Trop Dis 4:e682. doi: 10.1371/journal.pntd.0000682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Lalle M, Hanevik K. 2018. Treatment-refractory giardiasis: challenges and solutions. Infect Drug Resist 11:1921–1933. doi: 10.2147/IDR.S141468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Emery SJ, Baker L, Ansell BRE, Mirzaei M, Haynes PA, McConville MJ, Svard SG, Jex AR. 2018. Differential protein expression and post-translational modifications in metronidazole-resistant Giardia duodenalis. GigaScience 7:giy024. doi: 10.1093/gigascience/giy024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Nabarro LE, Lever RA, Armstrong M, Chiodini PL. 2015. Increased incidence of nitroimidazole-refractory giardiasis at the Hospital for Tropical Diseases, London: 2008–2013. Clin Microbiol Infect 21:791–796. doi: 10.1016/j.cmi.2015.04.019. [DOI] [PubMed] [Google Scholar]
- 304.Nash TE, Ohl CA, Thomas E, Subramanian G, Keiser P, Moore TA. 2001. Treatment of patients with refractory giardiasis. Clin Infect Dis 33:22–28. doi: 10.1086/320886. [DOI] [PubMed] [Google Scholar]
- 305.Escobedo AA, Almirall P, Chirino E, Pacheco F, Duque A, Avila I. 2018. Treatment of refractory paediatric giardiasis using secnidazole plus albendazole: a case series. Infezioni in Medicina 26:379–384. [PubMed] [Google Scholar]
- 306.Requena-Mendez A, Goni P, Rubio E, Pou D, Fumado V, Lobez S, Aldasoro E, Cabezos J, Valls ME, Trevino B, Martinez Montseny AF, Clavel A, Gascon J, Munoz J. 2017. The use of quinacrine in nitroimidazole-resistant Giardia duodenalis: an old drug for an emerging problem. J Infect Dis 215:946–953. doi: 10.1093/infdis/jix066. [DOI] [PubMed] [Google Scholar]
- 307.Hanevik K, Morch K, Eide GE, Langeland N, Hausken T. 2008. Effects of albendazole/metronidazole or tetracycline/folate treatments on persisting symptoms after Giardia infection: a randomized open clinical trial. Scand J Infect Dis 40:517–522. doi: 10.1080/00365540701827481. [DOI] [PubMed] [Google Scholar]
- 308.Jex AR, Svärd S, Hagen KD, Starcevich H, Emery-Corbin SJ, Balan B, Nosala C, Dawson SC. 2020. Recent advances in functional research in Giardia intestinalis, p 97–137. In Ortega-Pierres MG (ed), Advances in parasitology, vol 107. Academic Press, Cambridge, MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Keister DB. 1983. Axenic culture of Giardia lamblia in TYI-S-33 medium supplemented with bile. Trans R Soc Trop Med Hyg 77:487–488. doi: 10.1016/0035-9203(83)90120-7. [DOI] [PubMed] [Google Scholar]
- 310.Bingham AK, Jarroll EL, Jr, Meyer EA, Radulescu S. 1979. Giardia sp.: physical factors of excystation in vitro, and excystation vs eosin exclusion as determinants of viability. Exp Parasitol 47:284–291. doi: 10.1016/0014-4894(79)90080-8. [DOI] [PubMed] [Google Scholar]
- 311.Emery-Corbin SJ, Vuong D, Lacey E, Svard SG, Ansell BRE, Jex AR. 2018. Proteomic diversity in a prevalent human-infective Giardia duodenalis sub-species. Int J Parasitol 48:817–823. doi: 10.1016/j.ijpara.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 312.Emery SJ, Lacey E, Haynes PA. 2016. Quantitative proteomics in Giardia duodenalis—achievements and challenges. Mol Biochem Parasitol 208:96–112. doi: 10.1016/j.molbiopara.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 313.Yee J, Nash TE. 1995. Transient transfection and expression of firefly luciferase in Giardia lamblia. Proc Natl Acad Sci U S A 92:5615–5619. doi: 10.1073/pnas.92.12.5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Sun CH, Chou CF, Tai JH. 1998. Stable DNA transfection of the primitive protozoan pathogen Giardia lamblia. Mol Biochem Parasitol 92:123–132. doi: 10.1016/S0166-6851(97)00239-9. [DOI] [PubMed] [Google Scholar]
- 315.Marcial-Quino J, Gomez-Manzo S, Fierro F, Rufino-Gonzalez Y, Ortega-Cuellar D, Sierra-Palacios E, Vanoye-Carlo A, Gonzalez-Valdez A, Torres-Arroyo A, Oria-Hernandez J, Reyes-Vivas H. 2017. RNAi-mediated specific gene silencing as a tool for the discovery of new drug targets in Giardia lamblia: evaluation using the NADH oxidase gene Genes 8:303. doi: 10.3390/genes8110303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.McInally SG, Hagen KD, Nosala C, Williams J, Nguyen K, Booker J, Jones K, Dawson SC. 2019. Robust and stable transcriptional repression in Giardia using CRISPRi. Mol Biol Cell 30:119–130. doi: 10.1091/mbc.E18-09-0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Lin ZQ, Gan SW, Tung SY, Ho CC, Su LH, Sun CH. 2019. Development of CRISPR/Cas9-mediated gene disruption systems in Giardia lamblia. PLoS One 14:e0213594. doi: 10.1371/journal.pone.0213594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Cheissin EM. 1964. Ultrastructure of Lamblia duodenalis. I. Body surface, sucking disc and median bodies. J Protozool 11:91–98. doi: 10.1111/j.1550-7408.1964.tb01725.x. [DOI] [PubMed] [Google Scholar]
- 319.Friend DS. 1966. The fine structure of Giardia muris. J Cell Biol 29:317–332. doi: 10.1083/jcb.29.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Feely DE, Erlandsen SL. 1985. Morphology of Giardia agilis: observation by scanning electron microscopy and interference reflexion microscopy. J Protozool 32:691–693. doi: 10.1111/j.1550-7408.1985.tb03103.x. [DOI] [PubMed] [Google Scholar]
- 321.Erlandsen SL, Bemrick WJ. 1987. SEM evidence for a new species, Giardia psittaci. J Parasitol 73:623–629. doi: 10.2307/3282146. [DOI] [PubMed] [Google Scholar]
- 322.Feely DE. 1988. Morphology of the cyst of Giardia microti by light and electron microscopy. J Protozool 35:52–54. doi: 10.1111/j.1550-7408.1988.tb04075.x. [DOI] [PubMed] [Google Scholar]
- 323.Erlandsen SL, Bemrick WJ, Wells CL, Feely DE, Knudson L, Campbell SR, van Keulen H, Jarroll EL. 1990. Axenic culture and characterization of Giardia ardeae from the great blue heron (Ardea herodias). J Parasitol 76:717–724. doi: 10.2307/3282988. [DOI] [PubMed] [Google Scholar]
- 324.Hillman A, Ash A, Elliot A, Lymbery A, Perez C, Thompson RCA. 2016. Confirmation of a unique species of Giardia, parasitic in the quenda (Isoodon obesulus). Int J Parasitol Parasites Wildl 5:110–115. doi: 10.1016/j.ijppaw.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Lyu Z, Shao J, Xue M, Ye Q, Chen B, Qin Y, Wen J. 2018. A new species of Giardia Kunstler, 1882 (Sarcomastigophora: Hexamitidae) in hamsters. Parasit Vectors 11:202. doi: 10.1186/s13071-018-2786-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Monis PT, Andrews RH. 1998. Molecular epidemiology: assumptions and limitations of commonly applied methods. Int J Parasitol 28:981–987. doi: 10.1016/s0020-7519(98)00042-3. [DOI] [PubMed] [Google Scholar]
- 327.Monis PT, Andrews RH, Mayrhofer G, Ey PL. 1999. Molecular systematics of the parasitic protozoan Giardia intestinalis. Mol Biol Evol 16:1135–1144. doi: 10.1093/oxfordjournals.molbev.a026204. [DOI] [PubMed] [Google Scholar]
- 328.Ey PL, Mansouri M, Kulda J, Nohynkova E, Monis PT, Andrews RH, Mayrhofer G. 1997. Genetic analysis of Giardia from hoofed farm animals reveals artiodactyl-specific and potentially zoonotic genotypes. J Eukaryot Microbiol 44:626–635. doi: 10.1111/j.1550-7408.1997.tb05970.x. [DOI] [PubMed] [Google Scholar]
- 329.Lasek-Nesselquist E, Welch DM, Sogin ML. 2010. The identification of a new Giardia duodenalis assemblage in marine vertebrates and a preliminary analysis of G. duodenalis population biology in marine systems. Int J Parasitol 40:1063–1074. doi: 10.1016/j.ijpara.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Guo J, Chen Y, Zhou K, Li J. 2005. Distribution of rDNA in the nucleus of Giardia lamblia: detection by Ag-I silver stain. Anal Quant Cytol Histol 27:79–82. [PubMed] [Google Scholar]
- 331.Xin DD, Wen JF, He D, Lu SQ. 2005. Identification of a Giardia krr1 homolog gene and the secondarily anucleolate condition of Giaridia lamblia. Mol Biol Evol 22:391–394. doi: 10.1093/molbev/msi052. [DOI] [PubMed] [Google Scholar]
- 332.Feely DE, Dyer JK. 1987. Localization of acid phosphatase activity in Giardia lamblia and Giardia muris trophozoites. J Protozool 34:80–83. doi: 10.1111/j.1550-7408.1987.tb03137.x. [DOI] [PubMed] [Google Scholar]
- 333.Touz MC, Lujan HD, Hayes SF, Nash TE. 2003. Sorting of encystation-specific cysteine protease to lysosome-like peripheral vacuoles in Giardia lamblia requires a conserved tyrosine-based motif. J Biol Chem 278:6420–6426. doi: 10.1074/jbc.M208354200. [DOI] [PubMed] [Google Scholar]
- 334.Thirion J, Wattiaux R, Jadot M. 2003. The acid phosphatase positive organelles of the Giardia lamblia trophozoite contain a membrane bound cathepsin C activity. Biol Cell 95:99–105. doi: 10.1016/s0248-4900(03)00006-6. [DOI] [PubMed] [Google Scholar]
- 335.Brodsky RE, Spencer HC, Jr, Schultz MG. 1974. Giardiasis in American travelers to the Soviet Union. J Infect Dis 130:319–323. doi: 10.1093/infdis/130.3.319. [DOI] [PubMed] [Google Scholar]
- 336.Moore GT, Cross WM, McGuire D, Mollohan CS, Gleason NN, Healy GR, Newton LH. 1969. Epidemic giardiasis at a ski resort. N Engl J Med 281:402–407. doi: 10.1056/NEJM196908212810802. [DOI] [PubMed] [Google Scholar]
- 337.Kent GP, Greenspan JR, Herndon JL, Mofenson LM, Harris JA, Eng TR, Waskin HA. 1988. Epidemic giardiasis caused by a contaminated public water supply. Am J Public Health 78:139–143. doi: 10.2105/ajph.78.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Shaw PK, Brodsky RE, Lyman DO, Wood BT, Hibler CP, Healy GR, Macleod KI, Stahl W, Schultz MG. 1977. A communitywide outbreak of giardiasis with evidence of transmission by a municipal water supply. Ann Intern Med 87:426–432. doi: 10.7326/0003-4819-87-4-426. [DOI] [PubMed] [Google Scholar]
- 339.Osterholm MT, Forfang JC, Ristinen TL, Dean AG, Washburn JW, Godes JR, Rude RA, McCullough JG. 1981. An outbreak of foodborne giardiasis. N Engl J Med 304:24–28. doi: 10.1056/NEJM198101013040106. [DOI] [PubMed] [Google Scholar]
- 340.Steen K, Damsgaard E. 2007. The Giardia epidemic in 2004 and out-of-hours service in Bergen. Tidsskr nor Laegeforen 127:187–189. [PubMed] [Google Scholar]
- 341.Cama VA, Mathison BA. 2015. Infections by intestinal coccidia and Giardia duodenalis. Clin Lab Med 35:423–444. doi: 10.1016/j.cll.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Zhang H, Morrison S, Tang Y-W. 2015. Multiplex PCR tests for detection of pathogens associated with gastroenteritis. Clin Lab Med 35:461–486. doi: 10.1016/j.cll.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Ryan U, Paparini A, Oskam C. 2017. New technologies for detection of enteric parasites. Trends Parasitol 33:532–546. doi: 10.1016/j.pt.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 344.Hanson KE, Couturier MR. 2016. Multiplexed molecular diagnostics for respiratory, gastrointestinal, and central nervous system infections. Clin Infect Dis 63:1361–1367. doi: 10.1093/cid/ciw494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Adam RD, Nellen JFJB, Zaat JOM, Speelman P. 2010. Giardia lamblia (giardiasis). In Yu VLW, Raoult D (ed), Antimicrobial therapy and vaccines, 3rd ed, vol 1. Infectious Disease and Antimicrobial Agents, Antimicrobe, Pittsburgh, PA. [Google Scholar]
- 346.Nash TE, Aggarwal A, Adam RD, Conrad JT, Merritt JW, Jr.. 1988. Antigenic variation in Giardia lamblia. J Immunol 141:636–641. [PubMed] [Google Scholar]
- 347.Adam RA. 2020. Giardiasis, p 707–711. In Ryan ET, Hill DR, Solomon T, Aronson NE, Endy TP (ed), Hunter’s tropical medicine and emerging infectious diseases, 10th ed. Elsevier, New York, NY. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Download CMR.00024-19-s0001.pdf, PDF file, 0.3 MB (320.4KB, pdf)