Abstract
The encoding of chemical compounds with amplifiable DNA tags facilitates the discovery of small molecule ligands for proteins. In order to investigate the impact of stereo- and regio-chemistry on ligand discovery, we synthesized a DNA-encoded library of 670,752 derivatives based on 2-azido-3-iodophenylpropionic acids. The library was selected against multiple proteins and yielded specific ligands. The selection fingerprints obtained for a set of protein targets of pharmaceutical relevance clearly showed the preferential enrichment of ortho-, meta- or para-regioisomers, which was experimentally verified by affinity measurements in the absence of DNA. The discovered ligands included novel selective enzyme inhibitors and binders to tumor-associated antigens, enabling conditional chimeric antigen receptor T (CAR-T) cell activation and tumor targeting.
Keywords: DNA-encoded chemical libraries, stereochemistry, regiochemistry, Suzuki coupling, Sonogashira coupling, Chimeric Antigen Receptor T cells (CAR-T)
Graphical abstract
There is a growing interest in the use of DNA-encoded chemical libraries (DELs) as tools for the discovery of small organic ligands against target proteins. The identification of such compounds plays an important role in Pharmaceutical research and in Chemical Biology1–9. The concept of encoding molecules with DNA barcodes, first postulated in 199210, has now found widespread applications for the discovery of ligands against targets of biochemical or pharmacological interest. By employing split-and-pool synthetic methodologies11–13, DNA-templated synthesis strategies14 or DNA self-assembling procedures15,16, libraries with large combinatorial diversity can be created, starting from a moderately-sized set of oligonucleotides and building blocks.
In full analogy to antibody phage-display libraries17,18, DELs can be screened by affinity panning on proteins immobilized on solid supports. The high-throughput sequencing of the DNA barcodes before and after selection allows the facile identification of preferentially enriched ligands, which can be subsequently resynthesized for confirmation of binding affinity3,4,6–9. While antibody phage display libraries typically yield binders against virtually all purified protein antigens used as bait19, only a few systematic studies have documented the success rate of DELs for hit discovery, so far20,21. In order to increase the probability of isolating binders from DELs, one may consider to expand library size22. This may lead to an unacceptable growth of molecular weight if three or more sets of building blocks are used, or may require substantial investments for the purchase of a large number of building blocks. Chemical strategies aimed at creating structural diversity starting from relatively compact scaffolds may help explore chemical space and facilitate hit identification, while avoiding the creation of molecules that would violate Lipinski’s rule of five23–25.
Our group23, and the group of Stuart Schreiber26, have described initial library construction activities for the incorporation of stereochemical diversity and topographic complexity into DELs, as an avenue to explore a larger chemical space and discover selective binders against proteins of biomedical interest27,28. DELs are increasingly being considered as tools for ligand discovery, but library size alone does not guarantee success in screening campaigns13,27,29. Both chemical purity (which directly depends on the robustness of the employed reactions for library synthesis) and library design contribute to the productive discovery of binders against “hard-to-drug” targets3,4,6.
Recent reports have shown how the discovery of small ligands, which selectively localize at the site of disease, may open novel biomedical applications. Small molecule-drug conjugates (SMDCs) are increasingly being considered as an alternative to antibody-drug conjugates (ADCs) for superior pharmacodelivery applications30,31. Fluorescein-labeled chemical adaptors have been used as “bridge” molecules between tumor cells and engineered T cells carrying an anti-fluorescein antibody on their surface (“Universal CAR-T cells”), thus allowing the selective killing of neoplastic cells 32–35. Small molecule adaptors diffuse into tissue more efficiently than antibodies and clear more rapidly from circulation, helping spare normal tissues30.
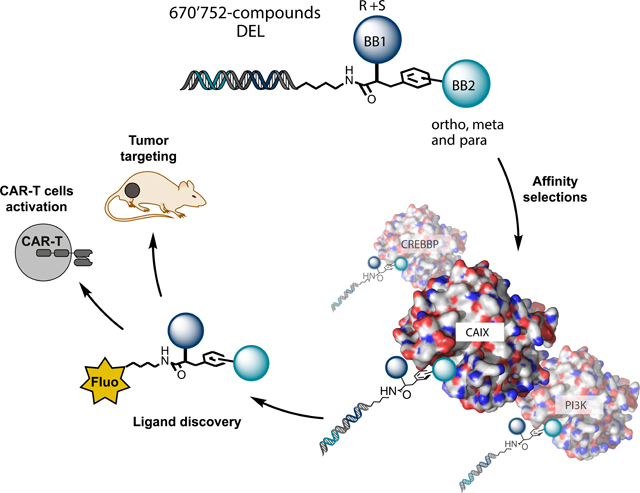
In this article, we functionalized 2-azido-3-iodophenylpropionic acid derivatives in order to construct a DEL with structural diversity both in terms of amino acid stereochemistry and of regiochemistry of phenyl ring functionalization (i.e., using substituents in ortho, meta or para position). The scaffold choice benefitted from the availability of DNA-compatible reaction conditions, that had been optimized for uniform reactivity of different regioisomers using hundreds of different building blocks23. The new library (termed NF-DEL) comprised 670,752 members, yielded binders against a large set of different protein targets and produced hits with “antibody-like” binding properties. The stereocenter within the amino acid moiety and the regiochemistry of iodophenyl ring substitution had a strong impact for certain targets, leading to structurally compact ligands for several proteins of biomedical interest.
RESULTS
Library design and construction
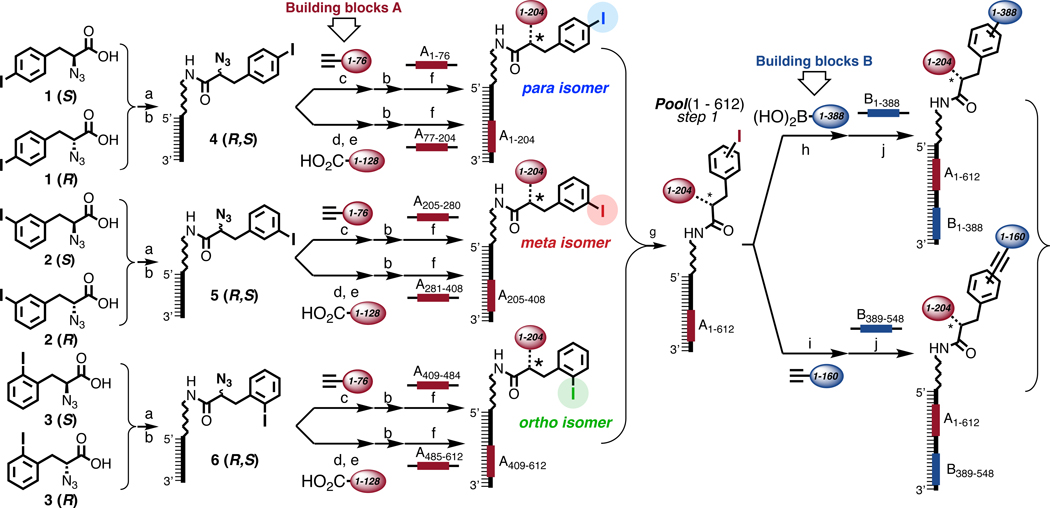
Figure 1 depicts the strategy used for the synthesis of a DEL with 670,752 members. A scaffold based on 2-azido-3-iodophenylpropionate structures, featuring an iodine in ortho, meta or para position, was used for stepwise library construction. Scaffolds were coupled to a universal amino-tagged 14-mer oligonucleotide by amide bond formation, followed by functionalization of the azido moiety by copper-catalyzed alkyne-azide cyclization with terminal alkyne derivatives or by Staudinger reduction and subsequent amide bond formation with a set of carboxylic acids. The resulting conjugates were HPLC purified prior to an encoding step, featuring a splint ligation (Building Blocks A; red code in the Figure)23,27,36. The chemical transformation in this first reaction step and, simultaneously, the regiochemistry of the iodophenyl moiety was encoded using 612 oligonucleotides [3 (regioisomers) x 204 (building blocks); Figure 1; Supplementary Figures 6 and 7].
Figure 1 |. Library design, synthesis and encoding.
Schematic representation of the strategy used for library synthesis, using regio- and stereoisomers of 2-azido-3-iodophenylpropionic acid. The chemical building blocks A and B and the corresponding encoding DNA portions are color-coded in red and blue, respectively. The first set of building blocks was conjugated to the central scaffold through a triazole ring or amide bond (*), the second set of building blocks was connected by either Suzuki- or Sonogashira coupling. a, EDC, S-NHS, DIPEA, r.t., 30’, 5’-C6-amino-GGAGCTTCTGAATT in TEA buffer (pH=10), 37 °C, 6h. b, RP-HPLC purification. c, CuAAC on-DNA reaction37 d, TCEP, TRIS buffer pH=7, 40 °C, 3h, RP-HPLC purification. e, on-DNA amide bond formation85. f, adaptor 5’-CAGCACACAGAATTCAGAAGCTCC-3’, ligase buffer, T4 DNA-ligase. g, RP-HPLC purification at 60°C. h, Pd(OAc)2, TPPTS, 200 mM Na2CO3, 60°C, 3h37. i, Pd(OAc)2, TPPTS, CuSO4, Ascorbate, 200 mM Na2CO3, 70°C, 2h37. j, adaptor 5’- CGTCGATCCGGCGCCATGG-3’, ligase buffer, T4 DNA-ligase. See Chapter 4 and 8 of the Supplementary Information for exact structures and detailed conditions.
The stereocenter within the azido-acid moiety was not encoded at this step, as the binding affinity of the different stereoisomers can be determined at the hit validation stage. The resulting conjugates were then mixed in equimolar amounts, split into 548 vials and used for a subsequent transformation step, using Suzuki coupling or Sonogashira reactions. The experimental conditions for these transformations had previously been investigated by us37 and by other groups38–43 and have further been optimized [Supplementary Figure 13]. In analogy to the first step, the chemical structure of the second set of building blocks was encoded by splint ligation (Building Blocks B; blue code in the Figure). Conjugates were then pooled, purified by RP-HPLC and used for selection in either single-stranded or double-stranded DNA format, using recently described procedures44.
Library selections and hit validation
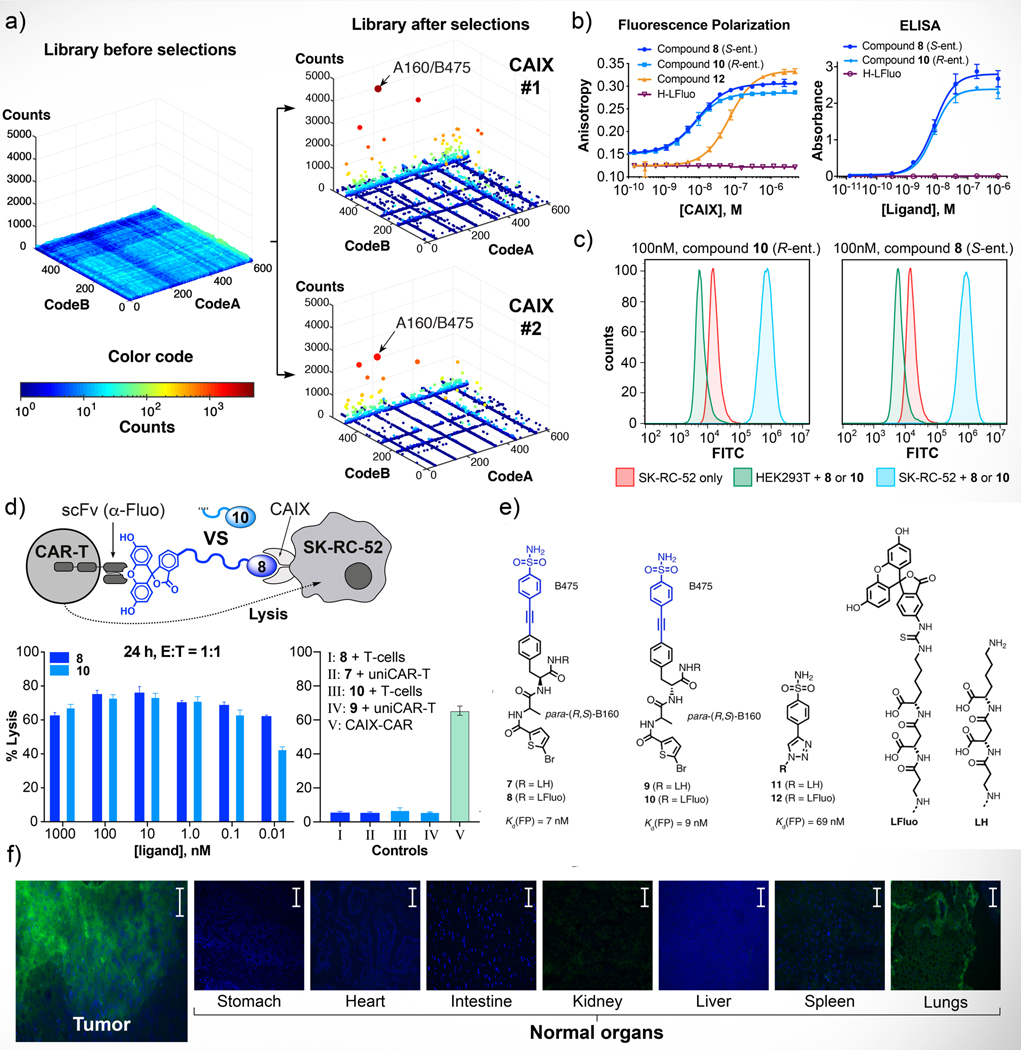
Figure 2 shows the selection results against carbonic anhydrase IX (CAIX), a tumor-associated membrane protein that can be recognized by aromatic sulfonamides16,23,27,30,45,46. The fingerprints of the library before selection or after selections (performed in duplicate) are displayed in Figure 2a, using a three-dimensional representation, in which two axes correspond to the identity of building blocks A and B, while the third axis indicates the number of sequence counts observed for individual molecules. Sequence counts are also indicated by a color code. A cut-off value (indicated in all Figures) was used in order to restrict the display of selection results only for those molecules which had reached a minimal level of sequence counts. While the distribution of sequence counts was homogeneous in the unselected library, distinctive lines of enriched compounds could be seen after CAIX selection [Figure 2a]. Fingerprints from replicate selections were remarkably reproducible and led to the identification of certain building block combinations as preferential CAIX binders.
Figure 2 |. Selection against carbonic anhydrase IX, hit validation and conversion of ligands to CAR-T cell activators.
a, NF-DEL high-throughput DNA sequencing results represented as three-dimensional fingerprints before and after selections against CAIX. The x and y axes represent the two building blocks while the color heat map and z-axis correspond to the DNA sequence counts. Selections were performed in triplicate (see Supplementary Figure 21). In order to show reproducibility of selection results, two CAIX prints are shown in this Figure. Cut-off values equal to 20 (for CAIX selection #1) and 15 (for CAIX selection #2) were applied to the fingerprints. No cut-off was applied for the naïve library fingerprint. The most enriched combination is indicated with an arrow (average EF=23±5 × 102). For the calculation of enrichment factors (EF) see Supplementary Tables 2,3 and Equation 1 (S29). b, off-DNA affinity measurements of selected compounds by FP and small-molecule ELISA, see also Supplementary Table 6. Error bars indicate standard deviation of three measurements. c, FACS with 8 and 10 on SK-RC-52 cells (renal cell carcinoma, in blue), on HEK293T (human embryonic kidney cells, control, in green), on SK-RC-52 cells alone (renal cell carcinoma, control, in red). d, Schematic representation of the immunological synapse of a fluorescein-specific universal CAR-T cell with a CAIX-presenting SK-RC-52 tumor cell, established by cross-linking the cells with the heterobifunctional crosslinker (blue). Killing was monitored after 24 h. % SK-RC-52 lysis is given as a function of ligand 8 (dark blue) and 10 (light blue) concentration. % SK-RC-52 lysis is also given for controls I-IV and the functional combination V(anti CAIX CAR-T). Error bars indicate standard deviation of three measurements e, Chemical structures of compounds 7, 8, 9, 10, 11, 12 and of tripeptide linker (LH, LFluo). f, Evaluation of the tumor-targeting performance of compound 8 after i.v. administration for human SK-RC-52 xenografted in mice, using ex vivo immunofluorescence detection of the fluorescein moiety. Images were taken 60 min after intravenous injection of 65 μg of compound 8 (green = ligand, blue = DAPI staining. Scale bar = 100 μm).
We resynthesized molecules based on the A160/B475 combination on solid phase, using a three amino acid linker [HO-βAla-Asp-Lys-NH2; LH in Figure 2] at the position which was originally occupied by the DNA tag within the library. The charged peptide linker was chosen in order to contribute to an increased compound solubility in water. The resulting amine derivatives (e.g. compounds 7, 9 and 11) were reacted with fluorescein isothiocyanate (FITC), giving rise to thiourea products that could be purified by HPLC and characterized. Fluorescence polarization measurements performed at increasing concentrations of CAIX, revealed a dissociation constant of 8.8±0.3 nM and 7.2±0.3 nM for compounds 8 [(S)-Phe/(R,S)A160/B475] and 10 [(R)-Phe/(R,S)A160/B475] [Figure 2b], respectively. In comparison to compound 12 (B475, a derivative of triazol-benzenesulfonamide) and 4-sulfamoylbenzoic acid (p-SABA)47, the dissociation constants were improved up to thirty-fold [Supplementary Table 6]. Protein binding was also confirmed using a modified enzyme-linked immunosorbent assay (ELISA) procedure, featuring the binding of the fluorescein-conjugated molecules to immobilized protein on a solid support, followed by washing steps and incubation with anti-fluorescein antibody reagents48. ELISA tests are normally performed using monoclonal antibodies as primary reagents, but fluorescein-conjugated small organic molecules exhibited an “antibody-like” behavior in this assay [Figure 2b]. The binding of compound 8 [(R)-Phe/(R,S)A160/B475] and compound 10 [(S)-Phe/(R,S)A160/B475] to CAIX on CAIX-positive tumor cell lines (SK-RC-52) was also confirmed by fluorescence-activated cell sorting [Figure 2c].
Encouraged by the selection of high-affinity CAIX ligands, we studied whether fluorescein-conjugated derivatives of compounds 8 and 10 could be used as small molecule “adaptors” for conditional tumor cell killing by universal chimeric antigen receptor T (UniCAR-T) cells35, and for tumor targeting applications. Human CAR-T cells, engineered to display a scFv antibody fragment specific to fluorescein, efficiently killed SK-RC-52 renal cell carcinomas only in the presence of the “bridge” molecules 8 and 10 [Figure 2d and 2e]. In a separate experiment, compound 8 was injected into tumor-bearing mice, which were sacrificed 60 minutes later and whose tissues were analyzed using fluorescence microscopy techniques30. A preferential uptake of compound 8 in tumor sections, compared to normal organs, was clearly visible [Figure 2f].
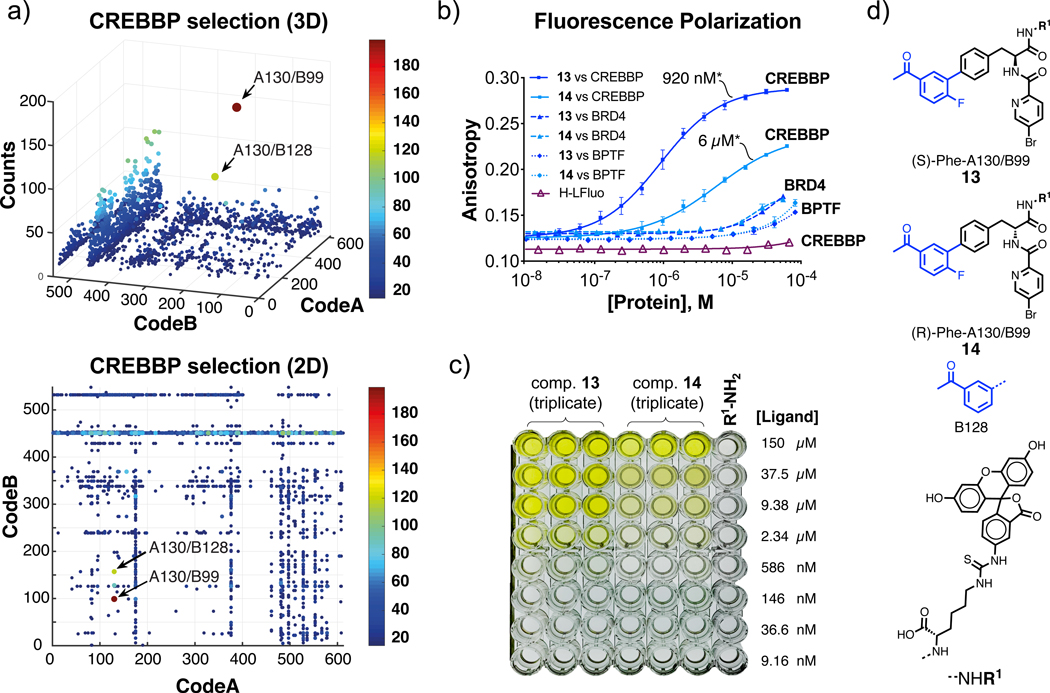
Figure 3 shows the results of DEL selections against cyclic-AMP response element binding protein (CREB) binding protein (CREBBP) bromodomain, a transcriptional co-regulator that is involved in many key intracellular processes49–52.
Figure 3 |. Selection against CREBBP bromodomain, hit validation and selectivity determination.
a, Fingerprint of a CREBBP bromodomain selection plotted in 3D and 2D representation, respectively. The x and y axes represent the two building blocks while the color heat map and z-axis correspond to the DNA sequence counts. A cut-off value equal to 15 was applied to fingerprints. Combinations A130/B99 and A130/B128 were preferentially enriched (EFA130/B99=149±16 and EF A130/B128=76±3. For the calculation of EFs see Supplementary Tables 2,3 and Equation 1 (S29). Selections were performed in triplicate (see Supplementary Figure 21). b, Fluorescence polarization of FITC-labelled compounds 13 and 14 with CREBBP bromodomain and control bromodomains BRD4(1) and BPTF. Error bars indicate standard deviation of three measurements.
The asterisk (*) indicates the dissociation constant value (Kd). c, Snapshot of maxisorp plate carrying small-molecule ELISA of compounds 13 and 14 after addition of TMB and 1M H2SO4. The assay was performed in triplicate against immobilized CREBBP bromodomain. d, Chemical structures of selected compounds and building blocks.
Selection fingerprints (represented in Figure 3a both in 3D and 2D rendering) led to the identification of A130/B99 as preferential binders. Resynthesis and characterization of these molecules (13 and 14) as FITC derivatives revealed that the (S) enantiomer bound to the cognate tag (compound 13) with a Kd = 0.91±0.06 μM, while the corresponding (R) stereoisomer (compound 14) exhibited a substantially reduced binding affinity (Kd = 6.0±0.7 μM) [Figure 3b]. These findings were confirmed by small molecule ELISA [Figure 3c]. Moreover, compounds 13 and 14 exhibited a substantially reduced interaction with the bromodomain-containing proteins BRD4(1) and BPTF, suggesting a preferential interaction with the CREBBP bromodomain [Figure 3b].
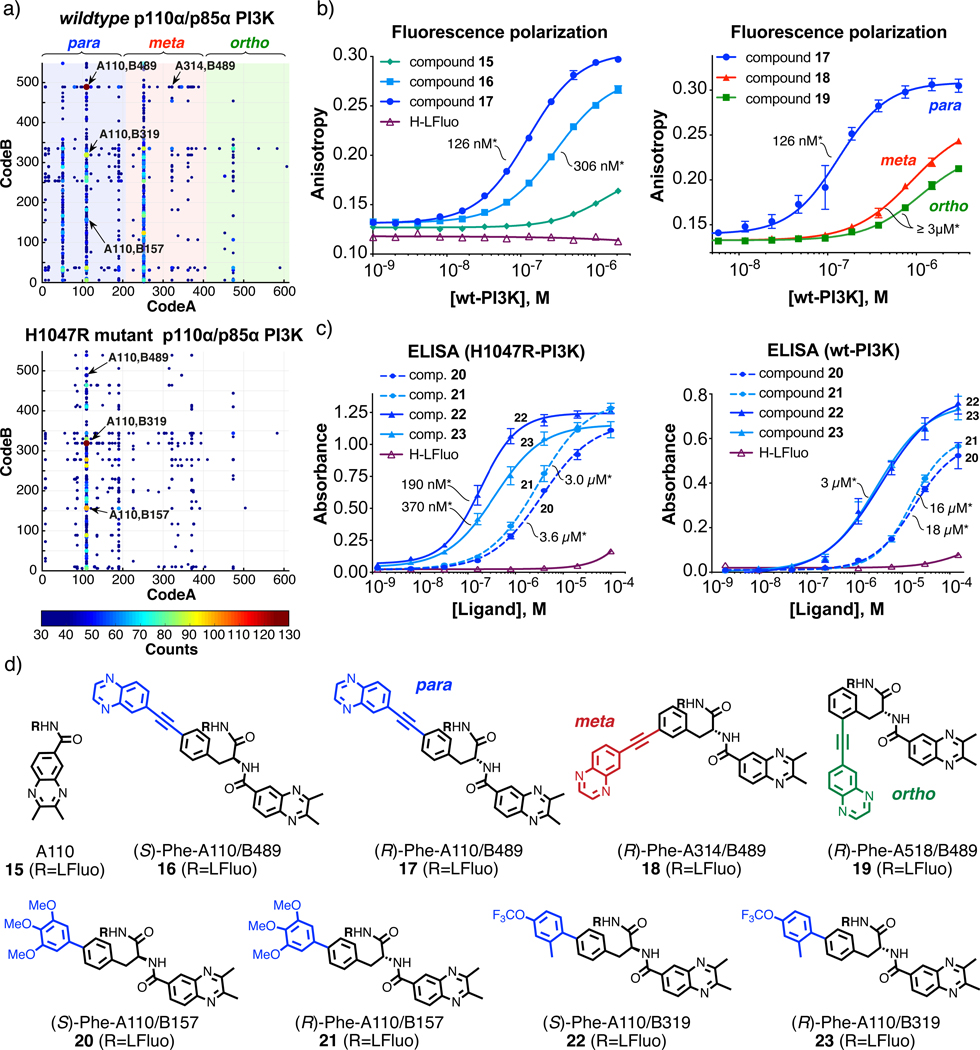
We also performed selections against variants of phosphoinositide 3-kinase (p110α/p85α PI3K)53–56, in order to learn whether binders could be found that discriminate between the wildtype enzyme and a mutant form (e.g., His1047→Arg) frequently found in tumor cells56,57. A comparative inspection of selection fingerprints revealed a preferential enrichment of A110/B489 (para regioisomer) against the wildtype protein and of A110/B319 against the mutant [Figure 4a].
Figure 4 |. Selections against PI3KCA variants.
a, Two-dimensional fingerprints of NF-DEL selections against the PI3Ks wildtype p110α/p85α PI3K (upper panel) and H1047R-p110α mutant of p110α/p85α PI3K (lower panel). The x and y axes represent the two building blocks while the color heat map shows the HTDS sequence counts. Building blocks A, corresponding to para- (1–204), meta- (205–408) and ortho- (409–612) regio-isomers, are highlighted in blue, red and green, respectively. Cut-off values equal to 25 (for wt-PI3K variant selection) and 30 (for H1047R mutant of PI3K variant selection) were applied to the fingerprints. The most enriched building block combinations are indicated with arrows. For the calculation of EFs see Supplementary Tables 2,3 and Equation 1 (S29). b, The binding of the most enriched combination (A110/B489, EF=65±7) against the wildtype protein corresponding to stereoisomers 15 and 16 was validated by fluorescence polarization and compared with a derivative of building block A110 alone (15). The meta- and ortho-isomers of 17 corresponding to combinations A314/B489 (18) and A518/B489 (19) were also measured for comparison. Error bars indicate standard deviation of three measurements. The asterisk (*) indicates the dissociation constant value (Kd). c, Enriched compounds corresponding to combinations A110/B157 (20 and 21) and A110/B319 (22 and 23) were validated by small-molecule ELISA against both H1047R mutant (left panel) and wildtype PI3K (right panel). Error bars indicate standard deviation of three measurements. The asterisk (*) indicates the dissociation constant value (Kd). d, Chemical structures of selected compounds.
Affinity measurements showed a Kd = 126±2 nM for the (R) stereoisomer (compound 17) and Kd = 306±8 nM for the (S) enantiomer (compound 16) of A110/B489 towards the wildtype protein [Figure 4b]. Importantly, the A110/B319 combination (compound 22) exhibited a Kd = 185±20 nM for H1047R-PI3K, with a 20-fold selectivity compared to the wildtype protein [Figure 4c,d]. Additional combinations of building blocks (e.g., A110/B157) were enriched against the mutant protein and displayed an increased potency in ELISA [Figure 4c,d]. These results were reproducible in triplicate selection experiments [Supplementary Figure 21].
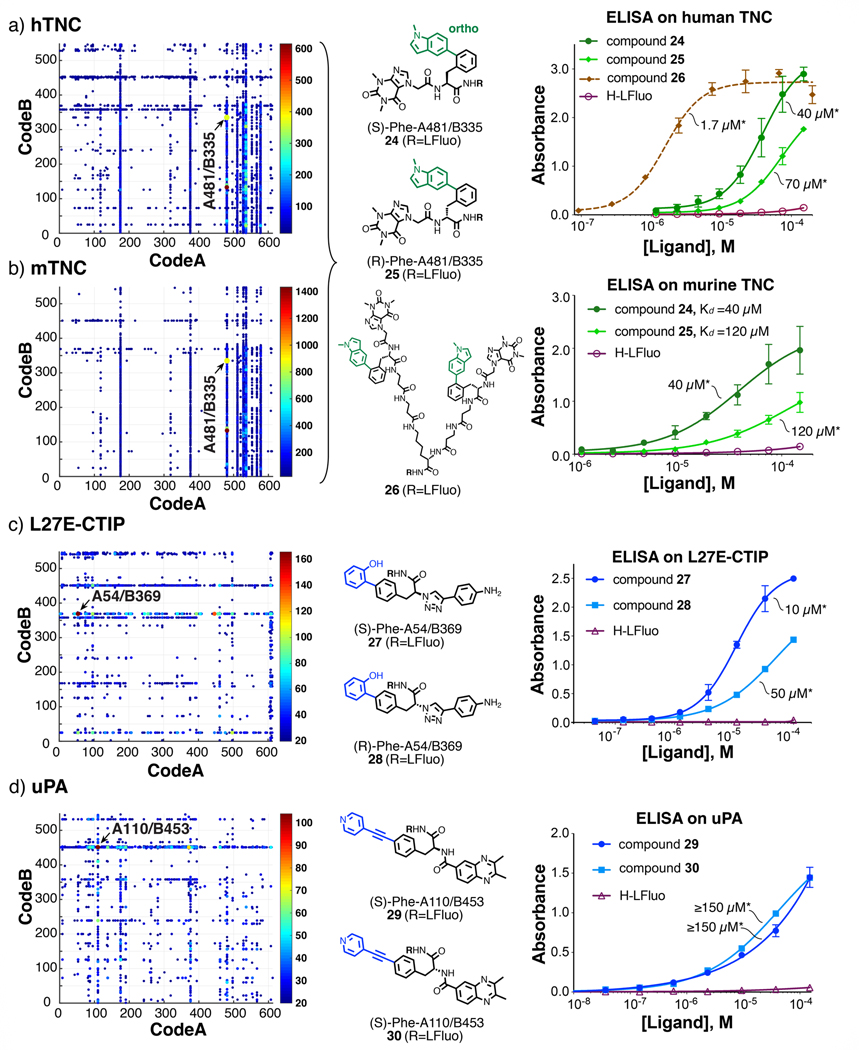
Selections were also performed against cancer targets, including murine and human extradomain D of tenascin-C (a tumor-associated antigen which is abundantly found in the neoplastic extracellular matrix58) [Figure 5a,b], the Leu27→Glu mutant of human CtIP (a multifunctional protein involved in the repair of DNA double-strand breaks59) [Figure 5c] and urokinase-type plasminogen activator (uPA, a strong prognostic marker of poor outcome in metastatic breast cancer60) [Figure 5d]. The Leu27→Glu mutation abrogates CtIP tetramer assembly, leading to strong defects in DNA end resection and homologous recombination61. Selection fingerprints were displayed in two-dimensional format, featuring the building blocks in the two dimensions and sequence counts as color code44. A preferential enrichment of A481/B335 was observed in the tenascin-C selections, both for the murine and the human antigen, which share a 90% sequence identity [Figure 5a,b]. Compounds with apparent Kd values in the 10–30 μM range were identified [Figure 5a,b, Supplementary Figure 22 and Supplementary table 6]. Connecting two moieties of (S)-Phe/A481/B335 through a β-alanine/β-alanine/lysine linker led to the dimeric compound 26, which bound to human tenascin C, displaying an apparent Kd value of 1.7 ± 0.1 μM. Interesting binders directed against L27E-CtIP and uPA could further be identified and confirmed by ELISA after resynthesis [Figure 5c,d].
Figure 5 |. Selections against tumor-associated antigens and human CtIP.
Two-dimensional fingerprints of selections against: a, human tenascin-C (hTNC); b, murine tenascin-C (mTNC); c, the L27E mutant of N-terminal CtIP d, urokinase-type plasminogen activator (uPA). A cut-off value equal to 20 was applied to all the fingerprints. The most enriched combinations (A481/B335 for hTNC and mTNC, A110/B453 for uPA and A54/B369 for L27E-CtIP) were re-synthesized off-DNA and validated by small-molecule ELISA. Error bars indicate standard deviation of three measurements. Details on DNA counts for individual library members, enrichment factors and dissociation constants are reported in Supplementary Tables 2,3 and 6. The asterisk (*) indicates the dissociation constant value (Kd).
DISCUSSION
A regio- and stereo-defined DEL has been synthesized, which has been screened against multiple protein targets and has produced specific hits, often with dissociation constants in the submicromolar range. Ligands exhibited antibody-like binding properties, as target recognition could be confirmed in all cases using an adapted ELISA methodology62 with human monoclonal IgG1 antibody bearing the anti-fluorescein variable heavy and light chains previously described48.
We tested our library by performing selections against biomedically relevant proteins, some of which belonged to classes which are considered to be “difficult to drug” using small molecule ligands (e.g., transcription factors, protein-protein interactions). We found ligands that discriminated between closely-related protein targets. The discovery of selective binders 22 and 23, with a 20-fold higher affinity towards the H1047R mutant of p110α in the p110α/p85α PI3K complex, may be of practical relevance since the mutation is frequently found in cancer and is associated with PI3K/AKT/mTOR signaling activation63,64. Similarly, 13 and 14 recognized CREBBP bromodomain but not the closely-related bromodomains of BRD4(1) and BPTF. CREBBP malfunctioning may cause mental diseases and growth deficiencies such as Rubinstein-Taybi Syndrome 1 (RSTS1)65 and Menke-Hennekam Syndrome 1 (MKHK1)66 as well as the growth of some types of cancer49,67–69. For therapeutic applications, a high CREBBP selectivity over other bromodomain-containing proteins is desirable52,70.
For certain targets, the regiochemistry of iodophenylalanine modification and the chiral center of this amino acid contributed to the experimentally observed enrichment factors and affinity constants. For example, PI3K binders based on the A110-B489 combination revealed that the para regioisomers had >20-fold improved affinity, while a 2.5-fold difference in Kd values between the R and S stereoisomers of the para derivative was observed [Figure 4].
Small-molecules used for in vivo tumor-targeting applications have often been derived from naturally-occurring compounds. For example, folate derivatives have been used to target folate receptors on ovarian cancer and other types of tumor cells33, while somatostatin analogues have been used to target somatostatin receptors overexpressed in neuroendocrine tumors71,72. “Drugs of the future” for targeted cancer therapy should not be limited to those cancer entities for which natural ligands exist. Both the discovery of accessible tumor markers73–75 and the facile identification of small organic ligands76 will be crucially important.
Small organic ligands for tumor-associated antigens can serve as versatile building blocks for the development of innovative biomedical strategies. This concept is well exemplified by the use of fluorescein conjugates in UniCAR-T cell technology. Conventional chimeric antigen receptor (CAR) T cells have been used to treat certain hematological malignancies, leading to durable complete responses77. However, CAR-T cells (which display anti-tumor antibodies on their surface) exhibit a constitutive biocidal activity, that cannot easily be switched off and which may lead to in vivo toxicity (e.g., B cell aplasia in anti-CD19 CAR-T therapeutics)77. UniCAR-T cells kill tumor cells only in the presence of suitable fluorescein-conjugates, which act as a “bridge” between the engineered T cell and the cancer cell. When the fluorescein-conjugated adaptor has cleared from the body (e.g., by renal clearance), the UniCAR-T cell loses its biocidal activity, which can be reactivated at a later time point by a subsequent administration of the adaptor molecule78. Compared to antibodies small organic ligands can reach the target rapidly in vivo, and also clear from circulation within minutes after intravenous injection30,79–82. Small organic ligand derivatives (e.g., fluorescein conjugates) might outperform antibodies for UniCAR-T cell therapy33,34,78. CAIX ligands are ideally suited for the in vivo targeting [Figure 2f] of renal cell carcinomas30,31, but other targets expressed on a broader set of cancer entities may be preferable for practical applications. For example, tenascin-C splice isoforms [Figure 5a,b] are found in the majority of solid tumors, lymphomas and in the bone marrow of acute leukemias, while being virtually undetectable in normal adult tissues83,84.
In our laboratory, we have built DELs for the last 17 years, and the NF-DEL has proven to be one of the most productive sources of protein binding specificities, despite containing only 670,752 compounds. The library had a high purity, as reactions with excellent conversion yields were used. Moreover, the compact structures generated by Suzuki or Sonogashira coupling enabled the regio- and stereo-defined projection of functional groups towards the cognate target protein. Collectively, this work provides additional evidence for the potential of DEL technology as a versatile and convenient source of small molecule ligands for important therapeutic targets. Compared to conventional collections of chemical compounds for high-throughput screening, which may contain up to one million molecules and can cost over a billion dollars2,3, a library such as the one described contains a similar number of compounds, does not require expensive logistics, and can be productively screened against multiple protein targets in a relatively short timeframe.
METHODS
Detailed methods, synthetic procedures and characterization of compounds are described in the Supplementary Information.
Library synthesis.
Equimolar mixtures of scaffolds 1(S) and 1(R), 2(S) and 2(R), 3(S) and 3(R) (Supplementary Figure 1) were coupled to a universal 5’ amino-modified oligonucleotide (5 μmol, 5’-C6-amino-GGAGCTTCTGAATT-3’) using the 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) / N-hydroxysulfosuccinimide (S-NHS) method1 (Supplementary Figure 2). The HPLC-purified derivatives 4 (R,S), 5 (R,S) and 6 (R,S) either were conjugated with 76 alkynes with a reported “on-DNA” CuAAC method2 or reduced with tris(2-carboxyethyl)phosphine (TCEP, in TRIS pH=8 buffer, 40°C, 3h) and conjugated to additional 128 carboxylic acids1. The obtained 612 derivatives were individually purified by RP-HPLC. To each oligonucleotide-conjugates (5 nmol), 5′-phosphorylated-oligonucleotides (code A: 5’-CTGTGTGCTGXXXXXXCGAGTCCCATGGCGC-3’ where X correspond to a variable region, 7.5 nmol), a ‘splint’ oligonucleotide (s1: 5’- CAGCACACAGAATTCAGAAGCTCC-3’, 8.5 nmol) and NEB T4-ligase buffer were added. The reactions were heated for 5 minutes at 70 °C, cooled to room temperature followed by addition of T4 DNA ligase3–5. The ligation was kept at room temperature for 2 hours. The enzyme was deactivated by adding acetate buffer pH=4.7 and by heating at 70 °C for 5 minutes. The encoded derivatives were pooled, precipitated by ethanol and purified by RP-HPLC at 60°C (Supplementary Figures 6, 7 and 9). The step 1 of Library was split in 548 reaction vessels (1 nmol each) and coupled with 388 boronates and 160 alkynes through Suzuki and Sonogashira “on-DNA” cross-coupling2 respectively (Supplementary Figures 12 and 13). The obtained derivatives (2 nmol) were precipitated and encoded by enzymatic ligation3–5 using a ‘splint’ oligonucleotide (s2: 5’- CGTCGATCCGGCGCCATGG-3’, 3.5 nmol) and an additional phosphorylated code (code B: 5’-GGATCGACGYYYYYYYGCGTCAGGCAGC-3’ where Y correspond to a variable region, 3 nmol). The ligation products were pooled in equimolar amount and finally precipitated and purified (RP-HPLC, 60°C, Supplementary Figure 14) obtaining a final library containing 670’752 different combinations.
Affinity Selections.
The purified NF-DEL was screened in duplicate or in triplicate against the target proteins of interest as previously reported6,7. Additional details are reported in Chapter 5 of the Supplementary Information.
Hit re-synthesis.
The CREBBP binders were synthesized starting from (S)-2-amino-3-(4-iodophenyl)propanoic acid, (R)-2-amino-3-(4-iodophenyl)propanoic acid and (5-acetyl-2-fluorophenyl)boronic acid. The Suzuki products were coupled with 4-bromopyridine-2-carboxylic acid and derivatized as α- L-lysine amide (Supplementary Information, Chapter 6.1). CAIX, wt-PI3K, H1047R-PI3K, uPA, hTNC, mTNC, CtIP binders and the negative control (FluoL-NH2) have been synthesized on solid phase starting from Fmoc- L-Lys(Boc)-Wang resin in which L-Aspartic acid α-tert-butyl ester and β-alanine have been added as linker to the iodo-phenylalanine scaffolds. The building blocks A were added by amide coupling or CuAAC and the building blocks B by palladium-catalyzed Suzuki or Sonogashira cross-couplings. The final compounds were cleaved from the resin in acidic conditions, obtaining the fully-deprotected peptides (Supplementary Information, Chapter 6.2). The obtained lysine derivatives were further conjugated with fluorescein-5-isothiocyanate to obtain the desired FITC-labelled compounds (Supplementary Table 5).
FP measurements with FITC-labelled compounds.
The FITC-labelled compounds were diluted to a final concentration between 10 and 50 nM (5 μL) and incubated for 15 minutes in a black 384-well plate (Greiner small-volume, non-binding) with serial dilutions of protein (5 μL). The fluorophore was excited at 485 nm and the emission was measured at 535 nM on a Spectra Max Paradigm multimode plate reader (Molecular Devices). The experiments were performed in triplicate and the resulting data was fitted by Prism 7 ([Inhibitor] vs. response, Variable slope four parameters).
ELISA measurements with FITC-labelled compounds.
The protein (200 nM) was coated on F8 maxisorp (Thermo Scientific) plate overnight at 4 °C. The immobilized protein was incubated for 30 minutes in the dark with serial dilutions of FITC-labelled compound and an additional 30 minutes with human monoclonal IgG1 anti-fluorescein antibody8. After several washing steps, protein A-HRP (1 μg/mL) was added to each well and the non-bound protein was washed away. The substrate 3,3′,5,5′-tetramethylbenzidine (TMB) was added and developed in the dark for 1–5 minutes. The reaction was quenched by adding 50 μL of 1 M sulfuric acid. The absorbance was measured on a Spectra Max Paradigm multimode plate reader (Molecular Devices) at 620–650 nm and 450 nm. Additional details are reported in the Supplementary Information (Chapter 7.2).
UniCAR-T production and Killing assay.
The production of anti-fluorescein uniCAR-T and the killing assay against SK-RC-52 in presence of FITC-labelled small CAIX-binders were performed essentially as previously described9,10, but using the vectors of Myburgh and colleagues for viral particle production and for cell transduction11.
Ex-Vivo.
BALB/c nu/nu mice bearing established subcutaneous SK-RC-52 tumors (∼400 mm3) were injected intravenously with fluorescein-labelled compound 8 (50 nmol) dissolved in sterile PBS buffer pH 7.4 (100 μL). After 1 hour, the animals were sacrificed by CO2 asphyxiation. The organs and tumors were extracted and flash-frozen in Neg-50 cryo medium (Thermo Fisher Scientific) with dry ice. The samples were cut into sections of 10 μm width and nuclear staining was performed with Fluorescence Mounting Medium containing DAPI (Dako Omnis). All pictures were acquired on an Axioskop 2 fluorescence microscope (Zeiss).
Supplementary Material
Acknowledgments
Financial support by the ETH Zürich, the Swiss National Science Foundation (grant number 310030_182003/1), the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (grant agreement 670603), the Federal Commission for Technology and Innovation (KTI, grant number 12803.1 VOUCH-LS), the Clinical Research Priority Program “ImmunoCure” of the University of Zurich to M.G.M and D.N, a University Research Priority Project Translational Cancer Research grant to R.M.; a Swiss Cancer Research grant to M.G.M. and D.N. (KFS-3846-02-2016) is gratefully acknowledged; the National Cancer Institute (R35 CA197582 to PKV) is gratefully acknowledged (TSRI ms. #29970). Work in the Sartori laboratory is supported by the Swiss National Science Foundation (grant number: 31003A_176161) and the Swiss Cancer Research Foundation (grant number: KFS-4702-02-2019). M.M. thanks the EPSRC Centre for Doctoral Training in Synthesis for Biology and Medicine (EP/L015838/1) for studentship support, supported by AstraZeneca, Diamond Light Source, Defence Science and Technology Laboratory, Evotec, GlaxoSmithKline, Janssen, Novartis, Pfizer, Syngenta, Takeda, UCB, and Vertex. S.J.C. thanks St Hugh’s College, Oxford, for research funding. We further thank Davide Bianchi and Cressida Harvey for the synthesis of some of the molecules described in this manuscript and Marco Catalano for providing the anti-FITC antibody used in ELISA procedures. We thank Adriano Martinelli for his help with data evaluation and we are most grateful to Bernhard Pfeiffer for help with NMR measurements. We are also grateful to the Functional Genomics Center Zurich for help with high-throughput DNA sequencing. Instant JChem (ChemAxon) was used for structure and data management (http://www.chemaxon.com).
Footnotes
Competing Interests Statement
D.N. is co-founder and shareholder of Philochem AG (http://www.philochem.com), a company active in the field of DNA-encoded chemical libraries.
Data availability.
The main data supporting the findings of this study are available within this Article and its Supplementary Information. Extra data and materials are available from the corresponding authors upon reasonable request. Software for the evaluation of high-throughput DNA sequencing has been previously reported87.
REFERENCES
- 1.Lerner RA & Brenner S. DNA-Encoded Compound Libraries as Open Source: A Powerful Pathway to New Drugs. Angew Chem Int Ed Engl 56, 1164–1165, doi: 10.1002/anie.201612143 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Neri D. & Lerner R. DNA-Encoded Chemical Libraries: A Selection System Based On Endowing Organic Compounds with Amplifiable Information. Annual Review of Biochemistry 87, 24 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goodnow RA Jr., Dumelin CE & Keefe AD DNA-encoded chemistry: enabling the deeper sampling of chemical space. Nat Rev Drug Discov 16, 131–147, doi: 10.1038/nrd.2016.213 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Favalli N, Bassi G, Scheuermann J. & Neri D. DNA-encoded chemical libraries - achievements and remaining challenges. FEBS Lett 592, 2168–2180, doi: 10.1002/1873-3468.13068 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dumelin CE et al. A portable albumin binder from a DNA-encoded chemical library. Angew Chem Int Ed Engl 47, 3196–3201, doi: 10.1002/anie.200704936 (2008). [DOI] [PubMed] [Google Scholar]
- 6.Franzini RM, Neri D. & Scheuermann J. DNA-encoded chemical libraries: advancing beyond conventional small-molecule libraries. Acc Chem Res 47, 1247–1255, doi: 10.1021/ar400284t (2014). [DOI] [PubMed] [Google Scholar]
- 7.Liu R, Li X. & Lam KS Combinatorial chemistry in drug discovery. Curr Opin Chem Biol 38, 117–126, doi: 10.1016/j.cbpa.2017.03.017 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu Z. & Cuozzo J. High-throughput affinity-based technologies for small-molecule drug discovery. J Biomol Screen 14, 1157–1164, doi: 10.1177/1087057109350114 (2009). [DOI] [PubMed] [Google Scholar]
- 9.Zhao G, Huang Y, Zhou Y, Li Y. & Li X. Future challenges with DNA-encoded chemical libraries in the drug discovery domain. Expert Opinion on Drug Discovery 14, 19 (2019). [DOI] [PubMed] [Google Scholar]
- 10.Brenner S. & Lerner RA Encoded combinatorial chemistry. Proceedings of the National Academy of Sciences of the United States of America 89, 5381–5383 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mannocci L. et al. High-throughput sequencing allows the identification of binding molecules isolated from DNA-encoded chemical libraries. Proceedings of the National Academy of Sciences 105, 17670–17675 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buller F. et al. High-throughput sequencing for the identification of binding molecules from DNA-encoded chemical libraries. Bioorg Med Chem Lett 20, 4188–4192, doi: 10.1016/j.bmcl.2010.05.053 (2010). [DOI] [PubMed] [Google Scholar]
- 13.Clark MA et al. Design, synthesis and selection of DNA-encoded small-molecule libraries. Nat Chem Biol 5, 647–654, doi: 10.1038/nchembio.211 (2009). [DOI] [PubMed] [Google Scholar]
- 14.Gartner ZJ et al. DNA-Templated Organic Synthesis and Selection of a Library of Macrocycles. Science 305, 1601 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melkko S, Scheuermann J, Dumelin CE & Neri D. Encoded self-assembling chemical libraries. Nat Biotechnol 22, 568–574, doi: 10.1038/nbt961 (2004). [DOI] [PubMed] [Google Scholar]
- 16.Wichert M. et al. Dual-display of small molecules enables the discovery of ligand pairs and facilitates affinity maturation. Nat Chem 7, 241–249, doi: 10.1038/nchem.2158 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Winter G, Griffiths AD, Hawkins RE & Hoogenboom HR MAKING ANTIBODIES BY PHAGE DISPLAY TECHNOLOGY. Annual Review of Immunology 12, 23 (1994). [DOI] [PubMed] [Google Scholar]
- 18.Lerner RA et al. Antibodies from combinatorial libraries use functional receptor pleiotropism to regulate cell fates. Quarterly Reviews of Biophysics 48, 389–394, doi: 10.1017/s0033583515000049 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Griffiths D, et al A. Isolation of high affinity human antibodies directly from large synthetic repertoires. EMBO J 13, 16 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Machutta CA et al. Prioritizing multiple therapeutic targets in parallel using automated DNA-encoded library screening. Nat Commun 8, 16081, doi: 10.1038/ncomms16081 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eidam O. & Satz AL Analysis of the productivity of DNA encoded libraries. MedChemComm 7, 1323–1331, doi: 10.1039/c6md00221h (2016). [DOI] [Google Scholar]
- 22.Perelson AS & Oster GF Theoretical Studies of Clonal Selection: Minimal Antibody Repertoire Size and Reliability of Self-Non-self Discriminationt. J. theor. Biol. 81, 26 ( 1979). [DOI] [PubMed] [Google Scholar]
- 23.Favalli N. et al. A DNA-encoded library of chemical compounds based on common scaffolding structures reveals the impact of ligand geometry on protein recognition. ChemMedChem 13, 5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuen LH et al. A Focused DNA-Encoded Chemical Library for the Discovery of Inhibitors of NAD(+)-Dependent Enzymes. J Am Chem Soc 141, 5169–5181, doi: 10.1021/jacs.8b08039 (2019). [DOI] [PubMed] [Google Scholar]
- 25.Lipinski CA Lead- and drug-like compounds: the rule-of-five revolution. Drug Discov Today Technol 1, 337–341, doi: 10.1016/j.ddtec.2004.11.007 (2004). [DOI] [PubMed] [Google Scholar]
- 26.Gerry CJ, Wawer MJ, Clemons PA & Schreiber SL DNA Barcoding a Complete Matrix of Stereoisomeric Small Molecules. J Am Chem Soc 141, 10225–10235, doi: 10.1021/jacs.9b01203 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y. et al. Versatile protein recognition by the encoded display of multiple chemical elements on a constant macrocyclic scaffold. Nature Chemistry 10, 441–448, doi: 10.1038/s41557-018-0017-8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buller F. et al. Design and synthesis of a novel DNA-encoded chemical library using Diels-Alder cycloadditions. Bioorg Med Chem Lett 18, 5926–5931, doi: 10.1016/j.bmcl.2008.07.038 (2008). [DOI] [PubMed] [Google Scholar]
- 29.Franzini RM & Randolph C. Chemical Space of DNA-Encoded Libraries. J Med Chem 59, 6629–6644, doi: 10.1021/acs.jmedchem.5b01874 (2016). [DOI] [PubMed] [Google Scholar]
- 30.Cazzamalli S, Dal Corso A, Widmayer F. & Neri D. Chemically Defined Antibody- and Small Molecule-Drug Conjugates for in Vivo Tumor Targeting Applications: A Comparative Analysis. J Am Chem Soc 140, 1617–1621, doi: 10.1021/jacs.7b13361 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cazzamalli S, Corso AD & Neri D. Targeted Delivery of Cytotoxic Drugs: Challenges, Opportunities and New Developments. Chimia (Aarau) 71, 712–715, doi: 10.2533/chimia.2017.712 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Urbanska K. et al. A universal strategy for adoptive immunotherapy of cancer through use of a novel T-cell antigen receptor. Cancer Res 72, 1844–1852, doi: 10.1158/0008-5472.CAN-11-3890 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee YG et al. Use of a Single CAR T Cell and Several Bispecific Adapters Facilitates Eradication of Multiple Antigenically Different Solid Tumors. Cancer Res 79, 387–396, doi: 10.1158/0008-5472.CAN-18-1834 (2019). [DOI] [PubMed] [Google Scholar]
- 34.Pellegrino Christian et al. Impact of ligand size and conjugation chemistry on the performance of universal chimeric antigen receptor T cells for tumor killing. Bioconjugate Chem. 31, 9 (2020). [DOI] [PubMed] [Google Scholar]
- 35.Ma JS et al. Versatile strategy for controlling the specificity and activity of engineered T cells. Proc Natl Acad Sci U S A 113, E450–458, doi: 10.1073/pnas.1524193113 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Litovchick A. et al. Encoded Library Synthesis Using Chemical Ligation and the Discovery of sEH Inhibitors from a 334-Million Member Library. Sci Rep 5, 10916, doi: 10.1038/srep10916 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Favalli N, Bassi G, Zanetti T, Scheuermann J. & Neri D. Screening of Three Transition Metal Mediated Reactions Compatible with DNA Encoded Chemical Libraries. Helvetica Chimica Acta 102, doi: 10.1002/hlca.201900033 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Satz AL et al. DNA Compatible Multistep Synthesis and Applications to DNA Encoded Libraries. Bioconjug Chem 26, 1623–1632, doi: 10.1021/acs.bioconjchem.5b00239 (2015). [DOI] [PubMed] [Google Scholar]
- 39.de Pedro Beato E. et al. Mild and Efficient Palladium-mediated C-N Cross-Coupling Reaction between DNA-conjugated Aryl Bromides and Aromatic Amines. ACS Comb Sci, doi: 10.1021/acscombsci.8b00142 (2019). [DOI] [PubMed] [Google Scholar]
- 40.Li JY & Huang H. Development of DNA-Compatible Suzuki-Miyaura Reaction in Aqueous Media. Bioconjug Chem 29, 3841–3846, doi: 10.1021/acs.bioconjchem.8b00676 (2018). [DOI] [PubMed] [Google Scholar]
- 41.Hervé G. & Len C. Heck and Sonogashira couplings in aqueous media – application to unprotected nucleosides and nucleotides. Sustainable Chemical Processes 3, doi: 10.1186/s40508-015-0029-2 (2015). [DOI] [Google Scholar]
- 42.Ding Y. & Clark MA Robust Suzuki–Miyaura Cross-Coupling on DNA-Linked Substrates. ACS Combinatorial Science 17, 1–4, doi: 10.1021/co5001037 (2014). [DOI] [PubMed] [Google Scholar]
- 43.Ding Y, DeLorey JL & Clark MA Novel Catalyst System for Suzuki-Miyaura Coupling of Challenging DNA-Linked Aryl Chlorides. Bioconjug Chem 27, 2597–2600, doi: 10.1021/acs.bioconjchem.6b00541 (2016). [DOI] [PubMed] [Google Scholar]
- 44.Bassi G. et al. Comparative evaluation of DNA-encoded chemical selections performed using DNA in single-stranded or double-stranded format. Biochem Biophys Res Commun 533, 223–229, doi: 10.1016/j.bbrc.2020.04.035 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Supuran CT Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 7, 168–181 (2008). [DOI] [PubMed] [Google Scholar]
- 46.Alterio V, Esposito D, Monti SM, Supuran CT & De Simone G. Crystal structure of the human carbonic anhydrase II adduct with 1-(4-sulfamoylphenyl-ethyl)-2,4,6-triphenylpyridinium perchlorate, a membrane-impermeant, isoform selective inhibitor. J Enzyme Inhib Med Chem 33, 151–157, doi: 10.1080/14756366.2017.1405263 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sannino A. et al. Quantitative Assessment of Affinity Selection Performance by Using DNA-Encoded Chemical Libraries. ChemBioChem 20, 7, doi:10.1002/ (2019). [DOI] [PubMed] [Google Scholar]
- 48.Midelfort KS et al. Substantial energetic improvement with minimal structural perturbation in a high affinity mutant antibody. J Mol Biol 343, 685–701, doi: 10.1016/j.jmb.2004.08.019 (2004). [DOI] [PubMed] [Google Scholar]
- 49.Breen ME & Mapp AK Modulating the masters: chemical tools to dissect CBP and p300 function. Curr Opin Chem Biol 45, 195–203, doi: 10.1016/j.cbpa.2018.06.005 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rooney TP et al. A series of potent CREBBP bromodomain ligands reveals an induced-fit pocket stabilized by a cation-pi interaction. Angew Chem Int Ed Engl 53, 6126–6130, doi: 10.1002/anie.201402750 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schiedel M. & Conway SJ Small molecules as tools to study the chemical epigenetics of lysine acetylation. Curr Opin Chem Biol 45, 166–178, doi: 10.1016/j.cbpa.2018.06.015 (2018). [DOI] [PubMed] [Google Scholar]
- 52.Brand M. et al. Controlling Intramolecular Interactions in the Design of Selective, High-Affinity, Ligands for the CREBBP Bromodomain. ChemRxiv. Preprint 10.26434/chemrxiv.12081999.v1 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang CH The structure of a human p110alpha/p85alpha complex elucidates the effects of oncogenic PI3Kalpha mutations. Science 318, 1744–1748, doi: 10.1126/science.1150799 (2007). [DOI] [PubMed] [Google Scholar]
- 54.Katso R. et al. CELLULAR FUNCTION OF PHOSPHOINOSITIDE 3-KINASES: Implications for Development, Immunity, Homeostasis, and Cancer. Annu. Rev. Cell Dev. Biol. 17, 61 (2001). [DOI] [PubMed] [Google Scholar]
- 55.Zhao L. & Vogt PK Class I PI3K in oncogenic cellular transformation. Oncogene 27, 5486–5496, doi: 10.1038/onc.2008.244 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Samuels Y. et al. High Frequency of Mutations of the PIK3CA Gene in Human Cancers. SCIENCE 304, 1 (2004). [DOI] [PubMed] [Google Scholar]
- 57.Miller MS et al. Identification of allosteric binding sites for PI3Kalpha oncogenic mutant specific inhibitor design. Bioorg Med Chem 25, 1481–1486, doi: 10.1016/j.bmc.2017.01.012 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brack SS, Silacci M, Birchler M. & Neri D. Tumor-targeting properties of novel antibodies specific to the large isoform of tenascin-C. Clin Cancer Res 12, 3200–3208, doi: 10.1158/1078-0432.CCR-05-2804 (2006). [DOI] [PubMed] [Google Scholar]
- 59.Sartori AA et al. Human CtIP promotes DNA end resection. Nature 450, 509–514, doi: 10.1038/nature06337 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kubala MH & DeClerck YA The plasminogen activator inhibitor-1 paradox in cancer: a mechanistic understanding. Cancer Metastasis Rev 38, 483–492, doi: 10.1007/s10555-019-09806-4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Davies OR et al. CtIP tetramer assembly is required for DNA-end resection and repair. Nat Struct Mol Biol 22, 150–157, doi: 10.1038/nsmb.2937 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Prati L, Bigatti M, Donckele EJ, Neri D. & Samain F. On-DNA hit validation methodologies for ligands identified from DNA-encoded chemical libraries. Biochem Biophys Res Commun 533, 235–240, doi: 10.1016/j.bbrc.2020.04.030 (2020). [DOI] [PubMed] [Google Scholar]
- 63.Yang H. et al. Discovery of a Potent Class of PI3Kalpha Inhibitors with Unique Binding Mode via Encoded Library Technology (ELT). ACS Med Chem Lett 6, 531–536, doi: 10.1021/acsmedchemlett.5b00025 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Janku F. et al. PIK3CA mutation H1047R is associated with response to PI3K/AKT/mTOR signaling pathway inhibitors in early-phase clinical trials. Cancer Res 73, 276–284, doi: 10.1158/0008-5472.CAN-12-1726 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alari V. et al. Generation of three iPSC lines (IAIi002, IAIi004, IAIi003) from Rubinstein-Taybi syndrome 1 patients carrying CREBBP non sense c.4435G>T, p.(Gly1479*) and c.3474G>A, p.(Trp1158*) and missense c.4627G>T, p.(Asp1543Tyr) mutations. Stem Cell Research 40, doi: 10.1016/j.scr.2019.101553 (2019). [DOI] [PubMed] [Google Scholar]
- 66.Banka S. et al. Genotype-phenotype specificity in Menke-Hennekam syndrome caused by missense variants in exon 30 or 31 of CREBBP. Am J Med Genet A 179, 1058–1062, doi: 10.1002/ajmg.a.61131 (2019). [DOI] [PubMed] [Google Scholar]
- 67.Steven A. & Seliger B. Control of CREB expression in tumors: from molecular mechanisms and signal transduction pathways to therapeutic target. Oncotarget 7, 12 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang H, Xu J, Lazarovici P, Quirion R. & Zheng W. cAMP Response Element-Binding Protein (CREB): A Possible Signaling Molecule Link in the Pathophysiology of Schizophrenia. Front Mol Neurosci 11, 255, doi: 10.3389/fnmol.2018.00255 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Belmaker RH & Agam G. Major Depressive Disorder. The New England Journal of Medicine 358, 14 (2008). [DOI] [PubMed] [Google Scholar]
- 70.Schiedel M. Chemical Epigenetics: The Impact of Chemical and Chemical Biology Techniques on Bromodomain Target Validation. Angew Chem Int Ed Engl 58, 17930–17952, doi: 10.1002/anie.201812164 (2019). [DOI] [PubMed] [Google Scholar]
- 71.Reubi JC, Mäcke HR & Krenning EP Candidates for Peptide Receptor Radiotherapy Today and in the Future. J Nucl Med 46, 9 (2005). [PubMed] [Google Scholar]
- 72.Reubi JC et al. SST3-selective potent peptidic somatostatin receptor antagonists. Proc Natl Acad Sci U S A 97, 13973–13978, doi: 10.1073/pnas.250483897 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rybak JN et al. In vivo protein biotinylation for identification of organ-specific antigens accessible from the vasculature. Nat Methods 2, 291–298, doi: 10.1038/nmeth745 (2005). [DOI] [PubMed] [Google Scholar]
- 74.Bodenmiller B. et al. Multiplexed mass cytometry profiling of cellular states perturbed by small-molecule regulators. Nat Biotechnol 30, 858–867, doi: 10.1038/nbt.2317 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Parker CG et al. Ligand and Target Discovery by Fragment-Based Screening in Human Cells. Cell 168, 527–541 e529, doi: 10.1016/j.cell.2016.12.029 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Krall N, Scheuermann J. & Neri D. Small targeted cytotoxics: current state and promises from DNA-encoded chemical libraries. Angew Chem Int Ed Engl 52, 1384–1402, doi: 10.1002/anie.201204631 (2013). [DOI] [PubMed] [Google Scholar]
- 77.Maude SL et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med 378, 439–448, doi: 10.1056/NEJMoa1709866 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee YG et al. Regulation of CAR T cell-mediated cytokine release syndrome-like toxicity using low molecular weight adapters. Nat Commun 10, 2681, doi: 10.1038/s41467-019-10565-7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Krall N. et al. A small-molecule drug conjugate for the treatment of carbonic anhydrase IX expressing tumors. Angew Chem Int Ed Engl 53, 4231–4235, doi: 10.1002/anie.201310709 (2014). [DOI] [PubMed] [Google Scholar]
- 80.Lindner T. et al. Development of Quinoline-Based Theranostic Ligands for the Targeting of Fibroblast Activation Protein. J Nucl Med 59, 1415–1422, doi: 10.2967/jnumed.118.210443 (2018). [DOI] [PubMed] [Google Scholar]
- 81.Loktev A. et al. Development of Fibroblast Activation Protein-Targeted Radiotracers with Improved Tumor Retention. J Nucl Med 60, 1421–1429, doi: 10.2967/jnumed.118.224469 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Giesel FL et al. 68Ga-FAPI PET/CT: Biodistribution and Preliminary Dosimetry Estimate of 2 DOTA-Containing FAP-Targeting Agents in Patients with Various Cancers. Journal of Nuclear Medicine 60, 386–392, doi: 10.2967/jnumed.118.215913 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Silacci M. et al. Human monoclonal antibodies to domain C of tenascin-C selectively target solid tumors in vivo. Protein Eng Des Sel 19, 471–478, doi: 10.1093/protein/gzl033 (2006). [DOI] [PubMed] [Google Scholar]
- 84.Schliemann C. et al. Three clinical-stage tumor targeting antibodies reveal differential expression of oncofetal fibronectin and tenascin-C isoforms in human lymphoma. Leuk Res 33, 1718–1722, doi: 10.1016/j.leukres.2009.06.025 (2009). [DOI] [PubMed] [Google Scholar]
- 85.Li Y. et al. Optimized Reaction Conditions for Amide Bond Formation in DNA-Encoded Combinatorial Libraries. ACS Comb Sci 18, 438–443, doi: 10.1021/acscombsci.6b00058 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Franzini RM et al. Identification of structure-activity relationships from screening a structurally compact DNA-encoded chemical library. Angew Chem Int Ed Engl 54, 3927–3931, doi: 10.1002/anie.201410736 (2015). [DOI] [PubMed] [Google Scholar]
- 87.Decurtins W. Automated screening for small organic ligands using DNA-encoded chemical libraries. Nat Protoc 11, 764–780, doi: 10.1038/nprot.2016.039 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Myburgh R. et al. Anti-human CD117 CAR T-cells efficiently eliminate healthy and malignant CD117-expressing hematopoietic cells. Leukemia 34, 2688–2703, doi: 10.1038/s41375-020-0818-9 (2020). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.