Abstract
An extract from activated Xenopus eggs joins both matching and nonmatching ends of exogenous linear DNA substrates with high efficiency and fidelity (P. Pfeiffer and W. Vielmetter, Nucleic Acids Res. 16:907–924, 1988). In mammalian cells, such nonhomologous end joining (NHEJ) is known to require the Ku heterodimer, a component of DNA-dependent protein kinase. Here I investigated whether Ku is also required for the in vitro reaction in the egg extract. Immunological assays indicate that Ku is very abundant in the extract. I found that all NHEJ was inhibited by autoantibodies against Ku and that NHEJ between certain combinations of DNA ends was also decreased after immunodepletion of Ku from the extract. The formation of a joint between a DNA end with a 5′-protruding single strand (PSS) and an end with a 3′-PSS, between two ends with 3′-PSS, and between two blunt ends was most Ku dependent. On the other hand, NHEJ between two DNA ends bearing 5′-PSS was Ku independent. These results show that the Xenopus cell-free system will be useful to biochemically dissect the role of Ku in eukaryotic NHEJ.
Xenopus laevis has proved to be a useful system for studying both homologous recombination and nonhomologous DNA end joining (NHEJ) of exogenous DNA molecules. Both processes were studied in vivo by microinjection of DNA as well as in vitro in extracts derived from various stages of oogenesis and early embryogenesis (12, 18, 26). In oocytes, homologous recombination is the prevalent mechanism for the joining of two linear DNA molecules and NHEJ is virtually undetectable. Upon oocyte maturation and in early embryos, NHEJ becomes the prominent mechanism, even though absolute levels of homologous recombination remain little changed. An extract from fertilized or activated Xenopus eggs has been an invaluable tool for the detailed characterization of the NHEJ products generated from defined substrates (32). These experiments have shown that the egg extract has the capability to join pairs of DNA ends bearing various combinations of 5′-protruding single strands (PSS), 3′-PSS, and blunt ends, as well as chemically modified ends (15), with high efficiency and precision. Thus, DNA ends are typically joined without nucleotide loss by end-to-end alignment and filling-in of any gaps (“fill-in” mode). Somewhat more heterogeneous and less-predictable products are formed with pairs of nonmatching 5′- or 3′-PSS, in which case the antiparallel PSS align by forming overlaps whose extent is influenced by the sequence in the PSS (“overlap” mode) (31). This largely error-free NHEJ appears to be a characteristic of the Xenopus egg extract and sets it apart from similar cell-free systems derived from mammalian cells where, possibly because of higher levels of exonucleases, deletions during NHEJ are more frequent (9, 10, 29).
Based on the findings with the Xenopus egg extract it was postulated that there must be an “alignment factor” that holds the two DNA ends in place for the nucleotide fill-in and strand ligation reaction. The existence of such a factor was particularly suggested by the finding that fill-in of 3′-PSS termini can precede ligation, which implies that fill-in DNA synthesis of one strand can proceed past a nick in the opposite strand (39). Such a process is difficult to envision without an apparatus that holds the two DNA ends together.
Independent of this work in X. laevis, genetic studies with mammalian cells established that the three protein components of DNA-dependent protein kinase (DNA-PK), the 470-kDa catalytic subunit (DNA-PKcs), and the Ku heterodimer (Ku70 and Ku80) are all required for double-strand break repair upon damage by ionizing radiation and also for the related process of V(D)J recombination during lymphoid development (4, 21, 37, 38; reviewed in references 2, 7, 11, and 20). Some of these genetic findings have been confirmed with biochemical experiments in cell-free systems (8, 40). DNA-PKcs is a member of the phosphatidylinositol kinase-related kinases, is activated by double-stranded DNA ends, and preferentially phosphorylates proteins that are bound to the same DNA molecule. Even though the Ku proteins are thought to target DNA-PK to DNA and thus cause the activation of the catalytic subunit, there is evidence that DNA-PKcs and Ku do not always act as a complex. Genetic studies show that only the Ku heterodimer, but not DNA-PKcs, is required for the formation of the signal joints during V(D)J recombination (4). Furthermore, Ku appears to have a separate function in maintaining telomere length and end structure in yeast cells (5, 14). Finally, atomic force microscopy studies provide evidence that both the catalytic subunit and Ku by themselves can hold together two linear DNA molecules (30, 41). Both components of DNA-PK could thus fulfill a structural alignment function during double-strand break repair.
Despite immense progress in this field in recent years, the precise role of DNA-PK during NHEJ remains unknown. However, the data summarized above suggest that NHEJ in the Xenopus egg extract is also a DNA-PK-dependent reaction and that this system thus might be useful to further elucidate the role of DNA-PK during NHEJ. In this study I have used antibody inhibition and immunodepletion experiments to show that the DNA-PK component Ku is indeed required for NHEJ in this cell-free system. I discuss the possibility that Ku is the postulated alignment factor present in the egg extract.
MATERIALS AND METHODS
Reagents.
Purified HeLa Ku was generously provided by W. S. Dynan and S. Yoo (Augusta, Ga.). Ku protein was stored in 0.1 M KCl–50 mM Tris-HCl (pH 7.9)–1 mM EDTA–0.02% Tween 20–20% glycerol–1 mM dithiothreitol (DTT) (“Ku buffer”). Purified monoclonal antibody (MAb) N3H10 was obtained from Kamiya Biomedical Company (Seattle, Wash.). Human autoimmune sera were received from J. A. Hardin (Augusta, Ga.). The identifying initials of sera αKu-3 and αKu-4 were HT and TT, respectively, while the origin of sera αKu-1 and αKu-2 could no longer be established. Ascites fluid containing MAbs 18-2 and 42-26 was provided by W. S. Dynan. Purified immunoglobulin G2b (IgG2b) were from Pharmingen (San Diego, Calif.), and purified human DNA-PK was purchased from Promega (Madison, Wis.).
Extract preparation.
The extract from Ca2+-ionophore-activated Xenopus eggs was prepared as described by Schaal et al. (36), except that in the extraction buffer HEPES was used instead of Tris (90 mM KCl, 30 mM HEPES [pH 7.9], 2 mM EGTA, 10 mM β-glycerophosphate, 1 mM DTT) and only 1/3 volume instead of 1 volume of extraction buffer was added to the packed eggs before centrifugation. The protein concentration of the extract was about 16 mg/ml.
DNA repair reactions and analysis of repair products.
DNA repair substrates were prepared by digestion of pBluescript SK (pBSK; Stratagene) with a single or two different restriction enzymes, followed by gel purification of the ∼2.9-kb linear DNA. A typical in vitro reaction consisted of 8 μl of undiluted extract, 1 μl of DNA (10 ng/μl), and 1 μl of experimental addition. Human serum was added in a volume of 1 μl after dilution in 0.1 M KCl–20 mM HEPES (pH 7.9)–20% glycerol–0.2 mM EDTA–0.5 mM DTT–0.5 mM Phenylmethylsulfonyl fluoride–50 μg of bovine serum albumin (BSA) per ml (DB/BSA buffer). Reactions were incubated at 15°C for 2 to 3 h and stopped by the addition of 90 μl of 0.3 M sodium acetate, 10 mM EDTA, 0.5% sodium dodecyl sulfate (SDS), 50 mM Tris (pH 7.6), 0.5 mg of proteinase K per ml, and 10 μg of carrier Escherichia coli RNA and incubation at 37°C for 30 min. DNA was recovered by organic extraction and ethanol precipitation and then electrophoresed in 1% agarose gels in 50 mM Tris (pH 7.8)–20 mM sodium acetate–2 mM EDTA (TAE buffer) containing 1 μg of ethidium bromide per ml. Southern blot hybridization was performed according to standard procedures by using labeled pBSK sequences as a probe. For the experiment shown in Fig. 3, lanes 14 to 22, DNA was electrophoresed in 1% agarose in 50 mM Tris–50 mM boric acid (pH 8.3)–2 mM EDTA (TBE buffer) containing 1 μg of ethidium bromide per ml.
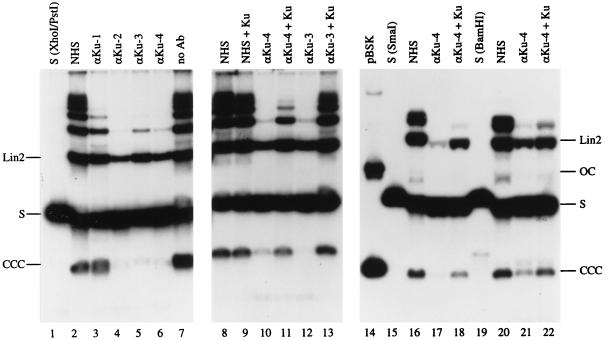
FIG. 3.
Inhibition of NHEJ by anti-Ku autoantibodies. Lanes 1 to 7: Southern blot analysis of NHEJ reactions in the presence of 1 μl of NHS (lane 2), 1 μl of αKu-1 serum (lane 3), 0.25 μl of αKu-2 serum (lane 4), 1 μl of αKu-3 serum (lane 5), 0.25 μl of αKu-4 serum (lane 6), or without addition (lane 7). Lane 1 shows 10 ng of input XhoI/PstI substrate. The bands corresponding to the substrate (S) and the main NHEJ products CCC and linear dimer (Lin2) are indicated. Lanes 8 to 13 are similar to the reactions shown in lanes 2, 5, and 6, except that the sera were preincubated with 210 ng of purified Ku (lanes 9, 11, and 13) or with the same volume (3 μl) of Ku buffer (lanes 8, 10, and 12). Note that preincubation of anti-Ku antibodies with Ku relieves the inhibition of NHEJ. Lanes 15 to 22 are similar to the reactions shown in lanes 8 to 11, except that the substrate was pBSK linearized with SmaI (lanes 16 to 18; input substrate is shown in lane 15) or pBSK linearized with BamHI (lanes 20 to 22; input substrate is shown in lane 19). Lane 14, circular pBSK. The DNA analyzed in lanes 14 to 22 was electrophoresed in TBE buffer to separate the linear substrate (S) from the open circles (OC). The slightly different migration of purified linear substrate DNA (S; lanes 1, 15, and 19) and unreacted substrate in NHEJ reactions (other lanes) is due to the presence in the latter samples of a large amount of RNA and possibly other impurities originating from the egg extract.
DNA binding assay for Ku.
pBSK(+) was biotinylated at the HindIII site as described earlier (23). After a second cut with PstI, the linear plasmid DNA was gel purified. Then, 50 ng of DNA was bound to 5 μl of streptavidin-coated paramagnetic particles (10 mg/ml; M-280 Dynabeads; Dynal) as described previously (23) and incubated with 8 μl of egg extract at 15°C for 1 h. After the addition of 10 μl of NTN (0.15 M NaCl, 50 mM Tris [pH 8], 0.1% Nonidet P-40), the beads were separated from the non-DNA binding fraction (the “supernatant”) by magnetic separation. The beads were washed twice with 150 μl of NTN, and the proteins were eluted from the DNA beads by heating in SDS loading buffer (62.5 mM Tris-HCl [pH 6.8], 2% [wt/vol] SDS, 10% glycerol, 40 mM DTT, 0.01% [wt/vol] bromophenol blue).
Preparation of antibodies against Xenopus DNA-PKcs.
To generate antibodies against Xenopus DNA-PK, the kinase domain of Xenopus DNA-PK (amino acid residues 264 to 640; numbering as in reference 24) was expressed as a hexahistidine-tagged recombinant protein in E. coli BL21(DE3) by using the vector pET-28a(+) (Novagen). The 46.9-kDa protein (his-DNPK) was purified on nickel-nitrilotriacetic acid-agarose under denaturing conditions and further purified by preparative SDS–12% polyacrylamide gel electrophoresis for the production of polyclonal antibodies in rabbits (BioWorld, Dublin, Ohio). DNA-PK-specific antibodies (anti-xDNPKcs) were purified from the serum by affinity chromatography on CNBr-activated Sepharose 4B (Pharmacia) containing the covalently bound recombinant antigen.
Western blot analysis.
Proteins were electrophoresed on 5% (for assay of DNA-PKcs) or 7.5% (for assay of Ku) discontinuous SDS-polyacrylamide gels and electrotransferred to nitrocellulose filters in 25 mM Tris (pH 8.3), 192 mM glycine, 20% (vol/vol) methanol, and 0.02% SDS. Filters were probed with the primary antibody in 0.5 M NaCl, 20 mM Tris (pH 7.5), 0.1% Tween 20, and 1% nonfat dry milk (Bio-Rad). Reactive proteins were detected with peroxidase-conjugated secondary antibody and enhanced chemiluminescence by using the SuperSignal Substrate (Pierce).
Immunoprecipitation.
To label extract proteins with 32P, egg extract was incubated in the presence of [γ-32P]ATP, 6 mM MgCl2, and 50 ng of linear DNA. Labeled Ku proteins were immunoprecipitated by using protein A-Sepharose (Pharmacia) in a buffer containing 500 mM NaCl, 50 mM NaF, 20 mM sodium phosphate (pH 8.0), and 0.2% Nonidet P-40 according to standard protocols.
Immunodepletion.
A 10-μl portion bead volume of protein G-Sepharose (Sigma) was incubated with 2 μl of human serum or 7.5 to 15 μg of immunoglobulins (purified MAb) in DB/BSA buffer in a volume of 250 μl overnight at 6°C. Beads were washed once in DB/BSA and twice in egg extraction buffer containing 0.5 mM phenylmethylsulfonyl fluoride (250 μl each). After the washes, extra liquid was removed from the beads, and 12 μl of egg extract was added. After end-over-end rotation at 6°C for 90 min, the samples were centrifuged and 9 μl of depleted extract (supernatant) was removed and used in NHEJ assays. For “double depletion”, the first supernatant was added to another 10 μl of IgG2b- or N3H10-coated protein G-Sepharose, and the incubation was continued for 60 min before the supernatant was used in NHEJ assays. To analyze the proteins bound to protein G-Sepharose, beads were washed two times with 500 μl of NTN and resuspended in SDS-gel loading buffer.
RESULTS
Molecular and immunological characterization of Xenopus DNA-PK.
Before investigating whether DNA-PK was required during NHEJ in the Xenopus egg extract, it was important to demonstrate the presence of DNA-PK in the extract. Xenopus DNA-PK had not been characterized very well, and only a partial cDNA encoding a C-terminal segment of DNA-PKcs had been isolated (24). Western blot analysis showed that antibodies directed against this recombinant C-terminal kinase domain of Xenopus DNA-PKcs (anti-xDNPKcs) reacted with a protein of very high molecular weight in the egg extract (Fig. 1A, lanes 5 to 8). This same band was also detected with MAbs against human DNA-PKcs (clones 18-2 and 42-26 [6]), and it comigrated with human DNA-PKcs, indicating that Xenopus DNA-PKcs is similar in size to its human counterpart (Fig. 1B, lanes 7 to 9, lower panel). The band reactive with MAb 42-26 could also be immunoprecipitated from Xenopus extract with anti-xDNPKcs (not shown), thus confirming that this band is the Xenopus homologue of DNA-PKcs.
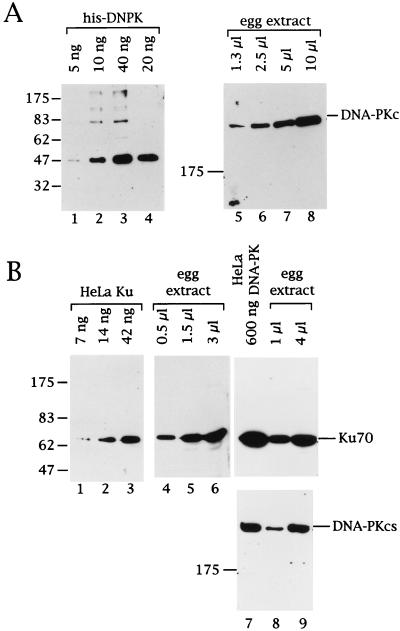
FIG. 1.
Immunological characterization of DNA-PKcs and Ku in the Xenopus egg extract. (A) Various amounts (as indicated above the lanes) of recombinant C-terminal domain of Xenopus DNA-PKcs (lanes 1 to 4, his-DNPK) or egg extract (lanes 5 to 8) were analyzed by Western blotting. Filters were probed with polyclonal rabbit antibodies against his-DNPK. (B) Various amounts of purified human Ku (lanes 1 to 3), purified human DNA-PK (lane 7), and egg extract (lanes 4 to 6, 8 and 9) were analyzed by Western blotting. Filters were probed with anti-Ku70 MAb N3H10 (lanes 1 to 9, upper panel) or anti-DNA-PKcs MAb 42-26 (lanes 7 to 9, lower panel). The migration of prestained marker proteins and their approximate molecular weight in kilodaltons is indicated to the left. The signals corresponding to Ku70 and DNA-PKcs are labeled to the right.
An MAb against human Ku70 (clone N3H10 [22]) reacted very strongly with a Xenopus protein comigrating with purified human Ku (Fig. 1B, lanes 1 to 6), indicating that this band represents the Xenopus homologue of Ku70. This interpretation was supported by the finding that the same N3H10-reactive protein was immunoprecipitated from egg extracts with anti-Ku autoimmune sera but not with normal human serum (see Fig. 4, bottom, lanes 1 to 3). When anti-Ku autoantibodies were used in immunoprecipitation experiments with 32P-labeled extract proteins, two proteins of the predicted sizes for Ku70 and Ku80 were immunoprecipitated, suggesting that Xenopus Ku is also a heterodimer (Fig. 2A). Finally, the N3H10-reactive protein binds to streptavidin beads containing biotinylated (linear) DNA but not to control beads without DNA, a finding consistent with the DNA end-binding properties of Ku protein (Fig. 2C, lanes 1 and 2).
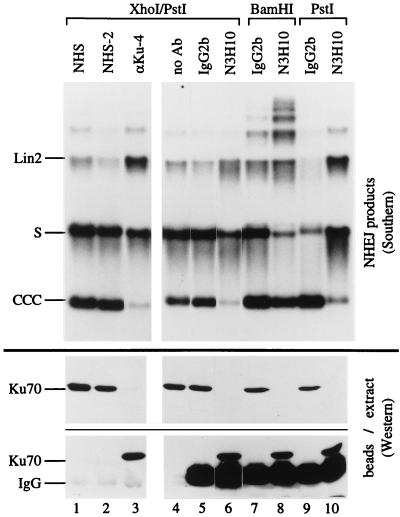
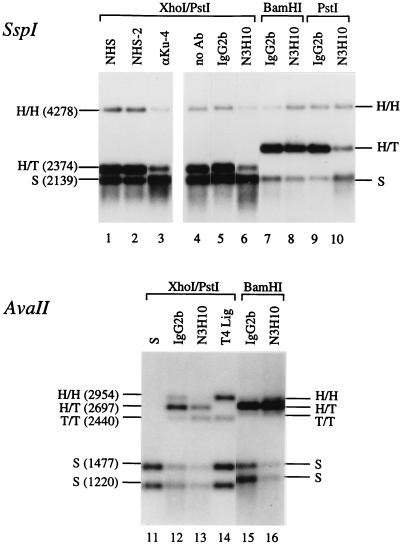
FIG. 4.
NHEJ in Ku-immunodepleted extracts. Egg extract was treated with protein G-Sepharose containing the antibodies indicated above the lanes. NHS and NHS-2 are two different control sera. After removal of the Sepharose beads, proteins in the extract and bound to the beads were analyzed by Western blots probed with N3H10 (lower panels); the extract was tested for NHEJ activity, and the products were analyzed by Southern blotting (upper panel). The linear DNA substrates used were as follows (indicated above the brackets on top): lanes 1 to 6, XhoI/PstI substrate; lanes 7 and 8, BamHI substrate; and lanes 9 and 10, PstI substrate. The migration of the DNA substrate (S), major NHEJ products (Lin2 and CCC), Ku70, and immunoglobulins (IgG) is indicated to the left. Note that Ku depletion strongly affects CCC formation with the XhoI/PstI substrate (lanes 3 and 6) and the PstI substrate (lane 10) but much less so with the BamHI substrate (lane 8).
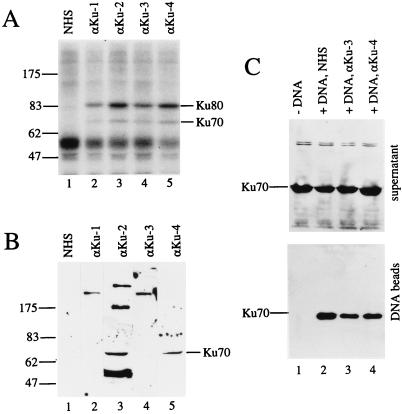
FIG. 2.
Characterization of Xenopus Ku with human anti-Ku autoantibodies. (A) Immunoprecipitation of 32P-labeled Xenopus Ku from egg extracts. Three microliters of autoimmune sera αKu-1 (lane 2) and αKu-3 (lane 4), 1.5 μl of αKu-2 (lane 3), or 2 μl of αKu-4 (lane 5) and NHS (lane 1) was used. Note that two proteins of the expected sizes for Xenopus Ku70 and Ku80 are precipitated by the autoimmune sera but not by the NHS. (B) Western blot analysis of egg extract proteins. Filters were probed with the sera indicated above the lanes. Sera were used at the following dilutions: NHS, αKu-1, and αKu-3 at 1:1,000; αKu-4 at 1:2,000; and αKu-2 at 1:4,000. The band recognized by αKu-2 and αKu-4 and corresponding to Xenopus Ku70 is indicated. Note that αKu-1 and αKu-3 recognize a protein of about 200 kDa. (C) Western blot analysis of unbound (supernatant, upper panel) and DNA-bound Ku70 (DNA beads, lower panel) after incubation of immobilized DNA in the egg extract. Lane 1 shows a control with paramagnetic particles without DNA. For lanes 3 and 4, the extract was preincubated with 1 μl of autoimmune serum αKu-3 or 0.25 μl of serum αKu-4, respectively, amounts that strongly inhibit NHEJ (see Fig. 3, lanes 5 and 6), while the control reactions shown in lanes 1 and 2 contain NHS.
DNA-PK is very abundant in the egg extract.
Two independent approaches were taken to estimate the amount of DNA-PK in the egg extract. By using the recombinant kinase domain of Xenopus DNA-PKcs as a standard in a Western blot probed with anti-xDNPKcs, I found that the 470-kDa DNA-PKcs present in 10 μl of extract gave a signal similar to that of 40 ng of the recombinant 47-kDa protein (Fig. 1A, compare lanes 1 to 4 to lanes 5 to 8), indicating that 1 μl of extract contains 40 ng of DNA-PKcs. In the second approach, preparations of purified human Ku and DNA-PK were used as standards in Western blots probed with MAbs against the human proteins. This approach is based on the assumption that these MAbs bind to the Xenopus proteins with equal affinity as to their human counterparts, an assumption that, if incorrect, more likely would lead to an underestimate of the amount of Xenopus DNA-PK in the extract. These experiments showed that the Xenopus Ku70 signal obtained with 1 μl of egg extract was about as intense as the signal obtained with 30 to 40 ng of purified human Ku heterodimer (Fig. 1B, lanes 1 to 6) or with 150 ng of purified human DNA-PK holoenzyme (lanes 7 to 9; upper panel). Furthermore, 1 μl of egg extract gave a DNA-PKcs signal of about the same intensity as 150 ng of human DNA-PK (Fig. 1B, lanes 7 to 9; lower panel). These data also indicated that DNA-PKcs and Ku were present in about equimolar amounts in both the preparation of purified, active human DNA-PK and the Xenopus egg extract. Based on these two estimates and by using a dilution factor of the protein concentration from the egg to the extract of 1.33, I conclude that one Xenopus egg (∼1 μl) contains 70 to 200 ng of DNA-PK or 0.7 × 1011 to 2 × 1011 molecules.
Assay for NHEJ in the Xenopus egg extract.
The standard NHEJ substrate used in the present study was a linear plasmid DNA molecule bearing nonmatching XhoI and PstI ends (Fig. 3, lane 1; see diagram in Fig. 5). Upon incubation in the extract, this substrate underwent both intramolecular NHEJ to give rise to monomeric covalently closed circles (CCC), as well as intermolecular NHEJ to form multimeric forms (Fig. 3, lane 2). Whereas the monomeric circular products can only be formed by joining an XhoI end to a PstI end in a head-to-tail configuration (H/T), the multimers generated from the XhoI/PstI substrate can be formed both by joining of mismatched ends (H/T), as well as by ligation of pairs of cohesive ends as head-to-head (H/H) or tail-to-tail joints (T/T) (see Fig. 5). Sequence analysis of cloned circularized products showed that the majority of XhoI and PstI ends were joined without nucleotide loss, i.e., by end-to-end joining of the 5′- and 3′-PSS and filling in of the 8-nucleotide (nt) gap (25), which is in agreement with previous studies (32, 39). Likewise, digestion of the repair products with XhoI and PstI indicated that H/H and T/T joints were generated in an error-free manner (data not shown).
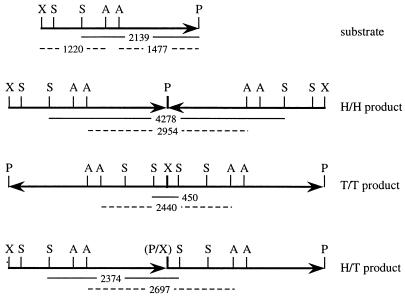
FIG. 5.
Diagram of XhoI/PstI substrate and its NHEJ dimer products. The arrows arbitrarily define the PstI (P) end of the 2,919-bp segment of the pBSK(−) plasmid as the head (H) and the XhoI (X) end as the tail (T). The three possible dimer combinations H/H, T/T, and H/T are shown. Also indicated are the sites for SspI (S) and AvaII (A) and the corresponding restriction fragments (with lengths in base pairs) generated from the three types of NHEJ products. The joint between the PstI and XhoI sites, which destroys both recognition sequences, is indicated by (P/X). The SspI fragments are shown as solid lines, and the AvaII fragments are shown as dashed lines. Note that monomeric circles (CCC) are also a H/T product.
Autoantibodies against Ku inhibit NHEJ.
To determine whether DNA-PK was required for NHEJ in the egg extract, I attempted to inhibit the reaction by the addition of antibodies against DNA-PK components. Whereas even high amounts of various antibodies against DNA-PKcs did not affect the NHEJ reaction (data not shown; see Discussion), inhibitory effects were readily detected with antibodies against the Ku component of DNA-PK. The present study therefore focuses entirely on the role of Ku during NHEJ in the Xenopus egg extract.
Four different anti-Ku autoimmune sera were used. As shown above in Fig. 2A, all four sera immunoprecipitated the Ku heterodimer. However, to precipitate similar amounts of Ku, two to four times more αKu-1 and αKu-3 serum than αKu-2 and αKu-4 serum was necessary, indicating that αKu-2 and αKu-4 had a higher titer. To further characterize these four sera, different dilutions were used to probe Western blots of Xenopus egg extract proteins. Sera αKu-1 and αKu-3 appeared to be very similar; they both did not react with the denatured Ku proteins but at a dilution of 1:1,000 recognized an unidentified protein of about 200 kDa (Fig. 2B, lanes 2 and 4). This band was detected with dilutions of these sera of up to 1:4,000 (not shown). Sera αKu-2 and αKu-4, on the other hand, both recognized a band comigrating with Ku70 (lanes 3 and 5). While this band was the only reactive band when serum αKu-4 was used at a 1:2,000 dilution, αKu-2 also reacted with three major additional proteins, up to a dilution of 1:8,000. These Western blot analyses thus indicate that αKu-1 and αKu-3 on the one hand, and αKu-2 and αKu-4 on the other hand, belong to two different subclasses of anti-Ku autoimmune sera (1, 34, 35).
When directly added to NHEJ reactions, all four anti-Ku autoimmune sera were found to inhibit end-joining reactions with the XhoI/PstI substrate (Fig. 3, lanes 3 to 6). Consistent with their higher titer, αKu-2 and αKu-4 strongly inhibited NHEJ even when only 0.25 μl of serum was added to the reaction (lanes 4 and 6), while 1 μl of αKu-1 and αKu-3 had to be added to see inhibition (lanes 3 and 5). The addition of 1 μl of normal human serum (NHS) did not inhibit the reaction (lane 2). To test whether the inhibition was due to anti-Ku antibodies or to antibodies with other specificities, which are often present in autoimmune sera (33), I preincubated the autoantibodies with purified human Ku protein. Figure 3, lanes 8 to 13, shows that the inhibition of NHEJ by sera αKu-3 and αKu-4 was indeed neutralized by this preincubation (lanes 11 and 13), while preincubation of NHS with Ku protein had no effect (lane 9). This result thus demonstrates that anti-Ku antibodies specifically inhibit NHEJ by binding to Ku. NHEJ reactions with immobilized DNA showed that inhibitory amounts of αKu-3 and αKu-4 serum did not significantly affect the amount of Ku binding to DNA (Fig. 2C, lanes 3 and 4). Thus, inhibition of NHEJ by anti-Ku antibodies appears not to be due to interference with the DNA-binding site of Ku.
Linear repair substrates bearing blunt ends (SmaI) or cohesive 4-nt 5′-PSS (BamHI) were circularized and multimerized in the extract with similar efficiency as the XhoI/PstI substrate (Fig. 3, lanes 15, 16, 19, and 20). Additional experiments showed that these NHEJ products could be completely digested with SmaI or BamHI, respectively, indicating that the joining was error-free (data not shown). NHEJ of these substrates was also inhibited by the αKu-4 serum (lanes 17 and 21), and the inhibition was relieved by preincubation of the αKu-4 serum with Ku protein (lanes 18 and 22). In the experiment with the SmaI and BamHI substrates, the NHEJ products were analyzed under electrophoretic conditions that separated the “open” (nicked or gapped) circles from the input linear substrates (Fig. 3, lanes 14 to 22). The results showed that the formation of these incompletely repaired intermediates was also inhibited by the αKu-4 serum, indicating that the inhibition by anti-Ku antibodies occurs at an early step of the NHEJ reaction.
NHEJ in Ku-depleted extracts.
Since autoantibodies were found to immunoprecipitate Ku from the extract (see Fig. 2A), I also used these antibodies to prepare Ku-depleted extracts. Western blot analysis showed that Ku protein could effectively be removed from the extract with protein G-Sepharose containing αKu-4 immunoglobulins (Fig. 4, bottom panel, lane 3). Ku remained in the extract when protein G-Sepharose containing NHS immunoglobulins were used (lanes 1 and 2). The same extracts were then tested for NHEJ activity (Fig. 4, top panel). Unlike NHEJ reactions with crude, untreated extract (as in Fig. 3), mock-depleted extracts reproducibly showed very efficient circularization but less multimerization (Fig. 4, lanes 1 and 2). This phenomenon was not further examined, but it is likely a consequence of the depletion protocol. In Ku-depleted extracts the circularization of the XhoI/PstI substrate was reduced to 15% of the level observed in control extracts treated with NHS (lane 3 [band labeled CCC]). Interestingly, however, there was an increase in linear dimer products, and the low level of higher multimers formed was barely affected (see below).
To confirm the observations with the αKu-4 serum, immunodepletion experiments were also carried out with MAb N3H10. Western blot assays of aliquots of the same depleted extracts used for the NHEJ reactions showed that N3H10 was as effective in removing Ku from the extract as was αKu-4 (Fig. 4, bottom panel, lanes 5 to 10). Again, control reactions with mock-depleted extracts resulted in a high level of circularization and a lower level of multimerization of the XhoI/PstI substrate (Fig. 4, top panel, lanes 4 and 5). Upon the depletion of Ku from the extract with protein G-Sepharose-bound N3H10, circularization of the XhoI/PstI substrate was reduced and dimer formation was slightly increased (lane 6). Thus, the Ku immunodepletion experiments with autoantibodies and with MAb N3H10 gave comparable results.
In the experiments shown in Fig. 4, lanes 1 to 6, the decrease in NHEJ (CCC formation) upon Ku depletion was measured to be about sixfold with both types of antibodies. However, in other experiments the decrease was as small as twofold (see, e.g., Fig. 7), even though Ku was not detectable in the immunodepleted extracts by Western blotting. Nevertheless, a small residual amount of Ku is likely to be present and could be responsible for the remaining NHEJ activity. Indeed, based on the present estimates for the amount of Ku in a standard NHEJ reaction, even the removal of 99% of Ku would still leave an amount of Ku that is approximately equimolar to the number of DNA ends (10 fmol). In an attempt to further reduce NHEJ after Ku depletion, extracts were subjected to two consecutive rounds of immunodepletion with either IgG2b- or N3H10-coated protein G-Sepharose beads. Circularization of the XhoI/PstI substrate in such double-Ku-depleted extracts was reduced to 5% of the circularization in double-mock-depleted extracts (data not shown, but see Fig. 7, panels 1 and 1a), thus demonstrating a correlation between NHEJ activity and the extent of Ku depletion.
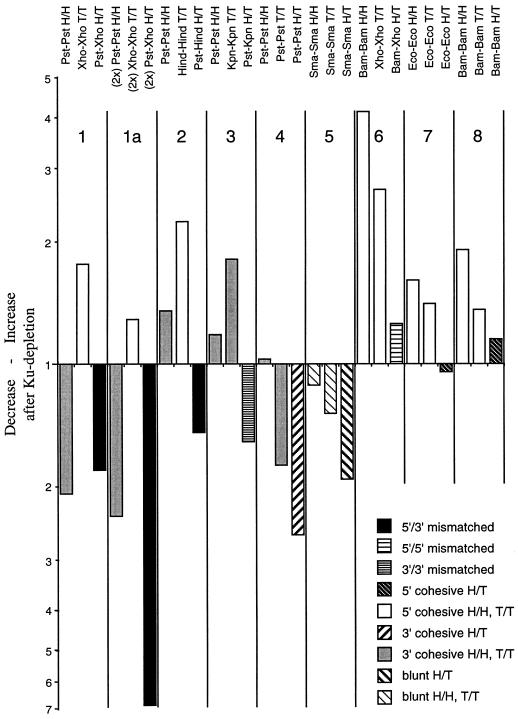
FIG. 7.
Effect of Ku depletion on NHEJ with eight different DNA substrates. Phosphorimager analysis of Southern blots of AvaII-digested repair products (as in Fig. 6, lanes 11 to 16). Increases after Ku depletion are indicated by upward bars, while decreases are shown by downward bars. The type of joints (DNA ends and configuration) are indicated at the top, and bars representing the same class of joint are patterned in the same way (see legend in lower right corner of bar graph). The DNA repair substrates were pBSK linearized with the following restriction enzymes: panels 1 and 1a, XhoI/PstI; panel 2, HindIII/PstI; panel 3, KpnI/PstI; panel 4, PstI; panel 5, SmaI; panel 6, BamHI/XhoI; panel 7, EcoRI; and panel 8, BamHI. The data presented in panel 1a were from NHEJ in “double-depleted” extracts, as described in Materials and Methods. Note that joint formation between nonmatching 5′- and 3′-PSS is always decreased upon Ku depletion (black bars), while joint formation between cohesive 5′-PSS is always increased (open bars).
Effect of DNA end structure on Ku dependence of NHEJ.
To examine how Ku depletion affects the joining of DNA ends with cohesive 4-nt 5′- or 3′-PSS, I used BamHI- and PstI-linearized plasmids as repair substrates. As shown in Fig. 4, lanes 7 and 8, Ku depletion led only to a slight decrease in circularization of the BamHI substrate. Significantly, the formation of all types of multimers was increased upon Ku depletion. On the other hand, the PstI substrate gave a result that was very similar to the result obtained with the XhoI/PstI substrate (Fig. 4, lanes 9 and 10): Ku depletion reduced circularization to 20% of control levels, and dimer products were increased. Thus, when focusing on the circularization of the linear substrate as a measure for NHEJ, Ku removal inhibited the joining of mismatched ends and cohesive ends with 4-nt 3′-PSS, while the joining of cohesive ends with 4-nt 5′-PSS was little changed.
To determine the relative abundance of H/T, H/H, and T/T joints formed in the NHEJ reactions, the purified DNA was digested with either SspI or AvaII. The lengths of the restriction fragments generated from the different NHEJ products are indicated in the diagrams presented in Fig. 5 (for the XhoI/PstI substrate only). Figure 6, lanes 1 to 10, shows SspI digests of aliquots of the same NHEJ reactions shown in Fig. 4. The data show that H/T joint formation was the most prominent type of joint in the reaction (80 to 90% of all joints in control reactions) and that circularization accounted for most, if not all, of the H/T joints. Accordingly, Ku-dependent changes of the H/T bands in Fig. 6 paralleled the changes observed with the undigested CCC products, i.e., H/T joining was decreased upon Ku depletion in reactions with the XhoI/PstI substrate (lanes 1 to 6, 12, and 13) and the PstI substrate (lanes 9 and 10) but was essentially unchanged in reactions with the BamHI substrate (lanes 7, 8, 15, and 16).
FIG. 6.
Restriction analysis of NHEJ products. Lanes 1 to 10 show SspI digests of aliquots of the same NHEJ products as shown in Fig. 4. The sizes of the restriction fragments (for the XhoI/PstI substrate only) and the type of joint that they represent are indicated (see Fig. 5). The BamHI and PstI substrates were made from pBSK(+), giving rise to a larger fragment for H/T joints (2,828 bp) than the XhoI/PstI substrate [made from pBSK(−)]. To make the changes in the signals between the control lanes (IgG2b) and minus-Ku lanes (N3H10) more visible, the exposure shown for lanes 7 to 10 is twice as short as that shown for lanes 4 to 6. Lanes 11 to 16, AvaII digests of independent NHEJ reactions in mock- and Ku-depleted extracts with the XhoI/PstI (lanes 12 and 13) or the BamHI substrate (lanes 15 and 16). Lane 11, AvaII digest of input substrate (S); lane 14, AvaII digest of XhoI/PstI substrate treated with T4 DNA ligase. The AvaII fragments generated from the reactions with the BamHI substrate (lanes 15 and 16) are slightly different from the sizes indicated to the left for the XhoI/PstI substrate. (The sizes are as follows: H/H, 2,930 bp; H/T, 2,736 bp; T/T, 2,542 bp; and S, 1,465 and 1,271 bp.) Note that with the XhoI/PstI substrate the H/H band (PstI-PstI joints) decreases, whereas the T/T band (XhoI-XhoI joints) increases upon Ku depletion (lanes 12 and 13).
On the other hand, H/H and T/T joint formations were much less frequent events (10 to 20% of all joints). Interestingly, their formation showed a Ku and substrate dependence that was similar, but clearly not identical, to the formation of the H/T joints described above. With the XhoI/PstI substrate, Ku depletion led to a decrease in the PstI-PstI joints (H/H), while in the same reaction the XhoI-XhoI joints (T/T) were increased (Fig. 6, lanes 12 and 13; for H/H, see also lanes 1 to 6). In reactions with the BamHI substrate, both H/H and T/T BamHI-BamHI joints were increased upon Ku depletion (Fig. 6, lanes 7 and 8 and lanes 15 and 16). However, in reactions with the PstI substrate, the formation of PstI-PstI joints in H/H configuration was unchanged (Fig. 6, lanes 9 and 10), while their formation was decreased in T/T configuration (see Fig. 7, panel 4), indicating that not all types of joints involving cohesive 4-nt 3′-PSS show the same Ku dependence. These results also provide an explanation for the increased dimer formation seen with the XhoI/PstI and PstI substrates (Fig. 4): mathematical models (25; not shown) predict that decreased probability for H/H joining at one end of a linear repair substrate and increased or unchanged probability for T/T joining at the other end would indeed lead to increased probability for dimer formation.
To further test the validity and generality of these findings, an independent experiment with the XhoI/PstI, BamHI, and PstI substrates, as well as five additional NHEJ substrates, was carried out and its quantitative analysis is shown in Fig. 7. The Ku-dependent changes in H/T, H/H, and T/T joint formation were determined by AvaII digestion of the repair products and phosphorimager analysis of the Southern blots. The results can be summarized as follows: a decrease in NHEJ after Ku depletion was always observed with a nonmatching pair having a 5′-PSS and a 3′-PSS (XhoI-PstI and HindIII-PstI; Fig. 7, black bars) or two 3′-PSS (KpnI-PstI) and with pairs of blunt ends (SmaI-SmaI) or cohesive ends with 3′-PSS (PstI-PstI) in H/T configuration (third bar in panels 1 to 5). On the other hand, an increase in NHEJ after Ku depletion was consistently seen with pairs of cohesive ends bearing 5′-PSS in H/H or T/T configuration (BamHI-BamHI, EcoRI-EcoRI, XhoI-XhoI, and HindIII-HindIII; open bars), while Ku depletion had little effect on joint formation between pairs of ends with 5′-PSS in H/T configuration (BamHI-BamHI, EcoRI-EcoRI, and BamHI-XhoI; third bar in panels 6 to 8; it should be noted that the nonmatching XhoI and BamHI ends can anneal by forming a 2-bp overlap). Mixed results were again obtained with pairs of cohesive ends with 3′-PSS in H/H or T/T configuration (dark gray bars in panels 1 to 4): while the formation of PstI-PstI joints was reproducibly decreased in reactions with the XhoI/PstI substrate (see also Fig. 6), it showed minor increases with other substrates, and the formation of the single KpnI-KpnI joint tested in these experiments was increased after Ku depletion. From the combined data it can be concluded that Ku dependence of NHEJ is primarily a function of the structure of the two DNA ends, but that NHEJ between two ends in H/T configuration is generally more Ku dependent than NHEJ between the same two ends in H/H or T/T configuration, suggesting that the presence of extended inverted repeats flanking the joining site can obviate the need for Ku.
DISCUSSION
NHEJ in an extract from activated Xenopus eggs had been characterized in detail with respect to the repair products formed from defined exogenous linear DNA substrates (31, 32). However, little is known about the protein factors in the extract that are involved in this process. Here I identify the DNA-PK component Ku as a protein factor that is required for the joining of various types of DNA ends in this system.
Both DNA-PKcs and Ku are very abundant in the egg extract. They are present in about equimolar amounts, and my results indicate that one egg contains ca. 70 to 200 ng or 0.7 × 1011 to 2 × 1011 molecules of the DNA-PK complex. This is in the same range as the chromatin components histones (140 ng/oocyte) or nucleoplasmin (250 ng/oocyte) (see reference 17). DNA-PK has long been recognized to be abundant, and it has been reported that a single somatic cell (HeLa) contains 400,000 molecules of Ku and up to 100,000 molecules of DNA-PKcs (2, 3). Since oocytes contain about 100,000 somatic cell equivalents of many proteins involved in DNA metabolism (17), the relative abundance of DNA-PK in Xenopus oocytes and eggs appears to be similar to that in HeLa cells. One can speculate that the high concentration of both Ku and DNA-PKcs is necessary to ensure that wherever DNA damage would occur, the generated DNA ends would immediately be protected from degradation and be prevented from diffusing apart.
In the present study I used two independent approaches to demonstrate a requirement for Ku in NHEJ: addition of anti-Ku antibodies to the NHEJ reaction and immunodepletion of Ku from the NHEJ reaction. Whereas the results obtained with both methods indicate a requirement for Ku during NHEJ, only the immunodepletion experiments revealed clear effects of different DNA ends on the Ku dependence of the reaction. Based on the properties of the Ku heterodimer, it can be expected to bind equally to all the different DNA ends tested in the present experiments (28). It is therefore possible that the presence of Ku-IgG complexes at the DNA ends sterically inhibits NHEJ, even in a reaction where the joining of the two DNA ends (e.g., the BamHI ends) would not require Ku. Nevertheless, quantitative analysis of the data shown in Fig. 3 revealed that even in these antibody addition experiments the joining of blunt SmaI ends was more sensitive toward anti-Ku autoantibodies (both circularization and dimerization were reduced to 10%) than the joining of the cohesive BamHI ends (circularization and dimerization reduced to 35%), and that with the XhoI/PstI substrate circularization was consistently at least twofold more inhibited by anti-Ku antibodies than was dimer formation, probably reflecting the presence of XhoI-XhoI (T/T) products among the dimers.
These differences in the Ku dependence between NHEJ reactions with different types of linear DNA substrates were much more evident in immunodepletion experiments, where the inhibition of NHEJ cannot be due to steric hindrance but must reflect the lack of an essential component of the NHEJ machinery. While more work is needed to understand why the joining of certain pairs of DNA ends requires Ku and others do not, it is intriguing that Ku is required for NHEJ between a 5′-PSS and a 3′-PSS, as well as for NHEJ between two blunt ends. In both of these cases, the alignment of the two DNA ends cannot be achieved by base pairing, and Ku might be necessary to hold the two ends together. On the other hand, the joining of two ends bearing the same type of PSS is clearly Ku-dependent for 3′-PSS but shows little Ku dependence for 5′-PSS. Thus, at least in the case of 5′-PSS, the ability of two ends to align by base pairing seems to obviate the need for Ku.
The present results suggest the existence of an alternative end-joining pathway, which becomes more dominant in the absence of Ku. Thus “simple ligation” of ends with 4-nt 5′-PSS in H/H or T/T configuration is stimulated upon Ku removal, leading to increased multimer formation with the BamHI substrate and to increased dimer formation with the XhoI/PstI substrate. And even for DNA substrates with other end structures, the formation of the H/H and T/T products is less Ku dependent than the corresponding H/T products (Fig. 7). The preference of this Ku-independent mechanism for H/H and T/T is reminiscent of an NHEJ activity described by Derbyshire et al. (10). This activity in nuclear extracts preferentially forms H/H and T/T joints after 3′-exonucleolytic modification of the DNA ends. However, BamHI digestion of the products formed in Ku-depleted Xenopus egg extracts indicates that the majority of ends are still joined with high precision (data not shown). The present results thus suggest a mechanism during which two ends in H/H or T/T configuration can anneal by melting of the ends and the formation of a cruciform-like structure between the extended homologous single strands, which then would promote the ligation of the two strands without nucleotide loss. Such a hypothetical mechanism again would be consistent with the notion that in the egg extract Ku as an alignment factor is less important for the joining of DNA ends that can interact by base pairing.
Genetic approaches have shown that in mammalian cells the catalytic subunit of DNA-PK is required in addition to the Ku heterodimer for the repair of double-stranded DNA breaks (4, 21). Furthermore, coding joint formation during V(D)J recombination was found to be inhibited by anti-DNA-PKcs antibodies (MAb 18-2) in a cell-free system (40). On the other hand, signal joint formation does not require DNA-PKcs (4, 27), and no DNA-PKcs homologue has been identified in yeast, suggesting that certain types of DNA end-joining reactions do not require DNA-PKcs. Attempts to inhibit NHEJ in the Xenopus egg extract with antibodies against DNA-PKcs, including MAb 18-2 and the X. laevis-specific anti-xDNPKcs (used in Fig. 1A), were unsuccessful. While there are several possible explanations for these negative results, one could speculate that the error-free NHEJ in the Xenopus egg extract is mechanistically related to signal joint formation and does not require DNA-PKcs. However, it has been reported that NHEJ in the Xenopus egg extract is sensitive toward wortmannin (15, 16), an inhibitor of DNA-PK and phosphatidylinositol kinase-related kinases (19). When increasing concentrations of wortmannin have been tested, there was a good correlation between the inhibition of NHEJ and the inhibition of DNA-PK activity, suggesting that the wortmannin-sensitive kinase that is required at an early step during NHEJ is indeed DNA-PK. I could confirm these findings by using the present assay system for NHEJ (25). However, my results show that, unlike Ku depletion, wortmannin inhibits NHEJ between pairs of cohesive 4-nt 5′-PSS and 3′-PSS to a similar degree. These observations thus suggest that the kinase activity of DNA-PK and the Ku heterodimer are required at different steps during NHEJ.
Addition of purified human Ku to Ku-depleted Xenopus egg extracts did not restore NHEJ activity but instead further reduced the residual NHEJ activity (25). Additional data show that up to 50% of DNA-PKcs in the extract coimmunoprecipitates with Ku during the present Ku depletion protocol (25). However, purified DNA-PK was also unable to rescue NHEJ in Ku-depleted extracts. These findings could indicate that Ku function is species specific or that additional factors required for NHEJ are bound to Ku or DNA-PKcs and have been removed from the extract during immunodepletion. To address these questions, to learn more about the mechanism and the biochemistry of the NHEJ reaction, and to ultimately identify all of the protein factors required for NHEJ in this system, it will be necessary to fractionate the egg extract and to reconstitute NHEJ from purified components.
ACKNOWLEDGMENTS
This work was supported by grant MCB-9630773 from the National Science Foundation.
I thank W. S. Dynan, S. Yoo, and J. A. Hardin (Augusta, Ga.) for generously providing me with antibody and protein reagents.
REFERENCES
- 1.Abu-Elheiga L, Yaneva M. Antigenic determinants of the 70-kDa subunit of the Ku autoantigen. Clin Immunol Immunopathol. 1992;64:145–152. doi: 10.1016/0090-1229(92)90192-q. [DOI] [PubMed] [Google Scholar]
- 2.Anderson C W, Carter T H. The DNA-activated protein kinase: DNA-PK. Curr Top Microbiol Immunol. 1996;217:91–111. doi: 10.1007/978-3-642-50140-1_7. [DOI] [PubMed] [Google Scholar]
- 3.Anderson C W, Lees-Miller S P. The nuclear serine/threonine protein kinase DNA-PK. Crit Rev Eukaryotic Gene Expr. 1992;2:283–314. [PubMed] [Google Scholar]
- 4.Blunt T, Finnie N J, Taccioli G E, Smith G C M, Demengeot J, Gottlieb T M, Mizuta R, Varghese A J, Alt F W, Jeggo P A, Jackson S P. Defective DNA-dependent protein kinase activity is linked to V(D)J recombination and DNA repair defects associated with the murine scid mutation. Cell. 1995;80:813–823. doi: 10.1016/0092-8674(95)90360-7. [DOI] [PubMed] [Google Scholar]
- 5.Boulton S J, Jackson S P. Identification of a Saccharomyces cerevisiae Ku80 homologue: roles in DNA double strand break rejoining and in telomeric maintenance. Nucleic Acids Res. 1996;24:4639–4648. doi: 10.1093/nar/24.23.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carter T, Vancurova I, Sun I, Lou W, DeLeon S. A DNA-activated protein kinase from HeLa cell nuclei. Mol Cell Biol. 1990;10:6460–6471. doi: 10.1128/mcb.10.12.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chu G. Double strand break repair. J Biol Chem. 1997;272:24097–24100. doi: 10.1074/jbc.272.39.24097. [DOI] [PubMed] [Google Scholar]
- 8.Cortes P, Weis-Garcia F, Misulovin Z, Nussenzweig A, Lai J-S, Li G, Nussenzweig M C, Baltimore D. In vitro V(D)J recombination: signal joint formation. Proc Natl Acad Sci USA. 1996;93:14008–14013. doi: 10.1073/pnas.93.24.14008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daza P, Reichenberger S, Göttlich B, Hagmann M, Feldmann E, Pfeiffer P. Mechanisms of nonhomologous DNA end joining in frogs, mice and men. Biol Chem Hoppe-Seyler. 1996;377:775–786. doi: 10.1515/bchm3.1996.377.12.775. [DOI] [PubMed] [Google Scholar]
- 10.Derbyshire M K, Epstein L H, Young C S H, Munz P L, Fishel R. Nonhomologous recombination in human cells. Mol Cell Biol. 1994;14:156–169. doi: 10.1128/mcb.14.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dynan W S, Yoo S. Interaction of Ku protein and DNA-dependent protein kinase catalytic subunit with nucleic acids. Nucleic Acids Res. 1998;26:1551–1559. doi: 10.1093/nar/26.7.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goedecke W, Vielmetter W, Pfeiffer P. Activation of a system for the joining of nonhomologous DNA ends during Xenopus egg maturation. Mol Cell Biol. 1992;12:811–816. doi: 10.1128/mcb.12.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gottlieb T M, Jackson S P. The DNA-dependent protein kinase: requirement for DNA ends and association with Ku antigen. Cell. 1993;72:131–142. doi: 10.1016/0092-8674(93)90057-w. [DOI] [PubMed] [Google Scholar]
- 14.Gravel S, Larrivée M, Labrecque P, Wellinger R J. Yeast Ku as a regulator of chromosomal DNA end structure. Science. 1998;280:741–744. doi: 10.1126/science.280.5364.741. [DOI] [PubMed] [Google Scholar]
- 15.Gu X-Y, Bennett R A O, Povirk L F. End-joining of free radical-mediated DNA double-strand breaks in vitro is blocked by the kinase inhibitor wortmannin at a step preceding removal of damaged 3′ termini. J Biol Chem. 1996;271:19660–19663. doi: 10.1074/jbc.271.33.19660. [DOI] [PubMed] [Google Scholar]
- 16.Gu X-Y, Weinfeld M A, Povirk L F. Implication of DNA-dependent protein kinase in an early, essential, local phosphorylation event during end-joining of DNA double-strand breaks in vitro. Biochemistry. 1998;37:9827–9835. doi: 10.1021/bi980198o. [DOI] [PubMed] [Google Scholar]
- 17.Gurdon J B, Wickens M P. The use of Xenopus oocytes for the expression of cloned genes. Methods Enzymol. 1983;101:370–386. doi: 10.1016/0076-6879(83)01028-9. [DOI] [PubMed] [Google Scholar]
- 18.Hagmann M, Adlkofer K, Pfeiffer P, Bruggmann R, Georgiev O, Rungger D, Schaffner W. Dramatic changes in the ratio of homologous recombination to nonhomologous DNA-end joining in oocytes and early embryos of Xenopus laevis. Biol Chem Hoppe-Seyler. 1996;377:239–250. doi: 10.1515/bchm3.1996.377.4.239. [DOI] [PubMed] [Google Scholar]
- 19.Hartley K O, Gell D, Smith G C M, Zhang H, Divecha N, Connelly M A, Admon A, Lees-Miller S P, Anderson C W, Jackson S P. DNA-dependent protein kinase catalytic subunit: a relative of phosphatidylinositol 3-kinase and the ataxia telangiectasia gene product. Cell. 1995;82:849–856. doi: 10.1016/0092-8674(95)90482-4. [DOI] [PubMed] [Google Scholar]
- 20.Jackson S P, Jeggo P A. DNA double-strand break repair and V(D)J recombination: involvement of DNA-PK. Trends Biol Sci. 1995;20:412–415. doi: 10.1016/s0968-0004(00)89090-8. [DOI] [PubMed] [Google Scholar]
- 21.Kirchgessner C U, Patil C K, Evans J W, Cuomo C A, Fried L M, Carter T, Oettinger M A, Brown J M. DNA-dependent kinase (p350) as a candidate gene for the murine SCID defect. Science. 1995;267:1178–1183. doi: 10.1126/science.7855601. [DOI] [PubMed] [Google Scholar]
- 22.Knuth M W, Gunderson S I, Thompson N E, Strasheim L A, Burgess R R. Purification and characterization of proximal sequence element-binding protein 1, a transcription activating protein related to Ku and TREF that binds the proximal sequence element of the human U1 promoter. J Biol Chem. 1990;265:17911–17920. [PubMed] [Google Scholar]
- 23.Labhart P. The Xenopus 9-bp ribosomal terminator (T3 box) is a pause signal for the RNA polymerase I elongation complex. Nucleic Acids Res. 1995;23:2252–2258. doi: 10.1093/nar/23.12.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Labhart P. mRNA encoding the catalytic subunit of DNA-dependent protein kinase is widely expressed in Xenopus cells. Gene. 1997;203:235–240. doi: 10.1016/s0378-1119(97)00498-8. [DOI] [PubMed] [Google Scholar]
- 25.Labhart, P. Unpublished observations.
- 26.Lehman C W, Clemens M, Worthylake D K, Trautman J K, Carroll D. Homologous and illegitimate recombination in developing Xenopus oocytes and eggs. Mol Cell Biol. 1993;13:6897–6906. doi: 10.1128/mcb.13.11.6897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lieber M R, Hesse J E, Lewis S, Bosma G C, Rosenberg N, Mizuuchi K, Bosma M J, Gellert M. The defect in murine severe combined immune deficiency: joining of signal sequences but not coding segments in V(D)J recombination. Cell. 1988;55:7–16. doi: 10.1016/0092-8674(88)90004-9. [DOI] [PubMed] [Google Scholar]
- 28.Mimori T, Hardin J A. Mechanism of interaction between Ku protein and DNA. J Biol Chem. 1986;261:10375–10379. [PubMed] [Google Scholar]
- 29.Nicolas A L, Young C S H. Characterization of DNA end joining in a mammalian cell nuclear extract: junction formation is accompanied by nucleotide loss, which is limited and uniform but not site specific. Mol Cell Biol. 1994;14:170–180. doi: 10.1128/mcb.14.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pang D, Yoo S, Dynan W S, Jung M, Dritschilo A. Ku proteins join DNA fragments as shown by atomic force microscopy. Cancer Res. 1997;57:1412–1415. [PubMed] [Google Scholar]
- 31.Pfeiffer P, Thode S, Hancke J, Vielmetter W. Mechanisms of overlap formation in nonhomologous DNA end joining. Mol Cell Biol. 1994;14:888–895. doi: 10.1128/mcb.14.2.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pfeiffer P, Vielmetter W. Joining of nonhomologous DNA double strand breaks in vitro. Nucleic Acids Res. 1988;16:907–924. doi: 10.1093/nar/16.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reeves W H. Use of monoclonal antibodies for the characterization of novel DNA-binding proteins recognized by human autoimmune sera. J Exp Med. 1985;161:18–39. doi: 10.1084/jem.161.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reeves W H. Antibodies to the p70/p80 (Ku) antigens in systemic lupus erythematosus. Rheum Dis Clin N Am. 1992;18:391–414. [PubMed] [Google Scholar]
- 35.Reeves W H, Pierani A, Chou C H, Ng T, Nicastri C, Roeder R G, Sthoeger Z M. Epitopes of the p70 and p80 (Ku) lupus autoantigens. J Immunol. 1991;146:2678–2686. [PubMed] [Google Scholar]
- 36.Schaal H, Pfeiffer P, Klein M, Gehrmann P, Scheid A. Use of DNA end joining activity of a Xenopus laevis egg extract for construction of deletions and expression vectors for HIV-1 Tat and Rev proteins. Gene. 1993;124:275–280. doi: 10.1016/0378-1119(93)90405-r. [DOI] [PubMed] [Google Scholar]
- 37.Smider V, Rathmell W K, Lieber M R, Chu G. Restoration of x-ray resistance and V(D)J recombination in mutant cells by Ku cDNA. Science. 1994;266:288–291. doi: 10.1126/science.7939667. [DOI] [PubMed] [Google Scholar]
- 38.Taccioli G E, Gottlieb T M, Blunt T, Priestley A, Demengeot J, Mizuta R, Lehmann A R, Alt F W, Jackson S P, Jeggo P A. Ku80: product of the XRCC5 gene and its role in DNA repair and V(D)J recombination. Science. 1994;265:1442–1445. doi: 10.1126/science.8073286. [DOI] [PubMed] [Google Scholar]
- 39.Thode S, Schäfer A, Pfeiffer P, Vielmetter W. A novel pathway of DNA end-to-end joining. Cell. 1990;60:921–928. doi: 10.1016/0092-8674(90)90340-k. [DOI] [PubMed] [Google Scholar]
- 40.Weis-Garcia F, Besmer E, Sawchuk D J, Yu W, Hu Y, Cassard S, Nussenzweig M C, Cortes P. V(D)J recombination: in vitro coding joint formation. Mol Cell Biol. 1997;17:6379–6385. doi: 10.1128/mcb.17.11.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yaneva M, Kowalewski T, Lieber M R. Interaction of DNA-dependent protein kinase with DNA and with Ku: biochemical and atomic-force microscopy studies. EMBO J. 1997;16:5098–5112. doi: 10.1093/emboj/16.16.5098. [DOI] [PMC free article] [PubMed] [Google Scholar]