Abstract
Background:
There is evidence that the risk of mortality is increased in patients with essential tremor (ET), however, there are few longitudinal, prospective data on the predictors of mortality in ET. There is also evidence that ET is associated with cognitive impairment; yet, it is unknown whether this is associated with elevated risk of mortality.
Methods:
In a longitudinal, prospective study of 194 elders with ET, an extensive neuropsychological test battery was performed at three time points: baseline, 18 months, and 36 months, and cognitive diagnoses (normal, mild cognitive impairment [MCI], and dementia) were assigned during consensus conferences. We used Cox proportional hazards models to estimate hazard ratios (HR) for death.
Results:
The mean baseline age was 79.1 ± 9.7 years. During follow-up, 52 (26.8%) died. In initial univariate models, a variety of baseline factors were associated with increased risk of mortality, including demographic variables (i.e., older age), cognitive variables and gait and balance variables. In the final multivariate Cox model, baseline dementia (HR = 2.66, p = 0.006), older baseline age (HR = 1.18, p < 0.001), and more reported falls at baseline (HR = 1.10, p < 0.001) were independently associated with increased risk of mortality. Amnestic MCI was marginally associated with increased risk of mortality (HR = 1.93, p = 0.08) in primary analyses and significantly (p < 0.05) in several sensitivity analyses.
Conclusions:
In this longitudinal, prospective study, baseline dementia resulted in a 2- to 3-times increase in risk of mortality in ET, further highlighting the clinical significance of cognitive impairment, specifically dementia, in this population.
Keywords: essential tremor, mortality, dementia, cognitive aging, epidemiology, longitudinal
Introduction
Essential tremor (ET) is one of the most common movement disorders among adults, with a prevalence of ~5% in individuals age 40 and older, and over 20% in individuals ≥ 95 years [1–2]. While kinetic tremor is the primary feature of ET, additional clinical characteristics have been associated with the disorder, including motor problems (i.e., ataxic gait) and non-motor problems (i.e., anxiety symptoms, depressive symptoms, mild cognitive impairment, and dementia) [3–5], some of which may be due to underlying cerebellar dysfunction [6–8]. Due to its progressive and multifaceted nature, ET can manifest with functional declines and disability.
There are few longitudinal studies of mortality in ET. Of the two longitudinal studies that included controls [9–10], only one enrolled ET cases and controls at the same time, and that demonstrated an increased risk of mortality in ET [10]. Studies of the predictors of mortality in ET are even more rare. A preliminary study using a portion of the current cohort of elderly ET cases followed these cases from baseline to their first 18-month follow-up evaluation [11]. The study found that older age, a higher clinical dementia rating (CDR) score, and a higher Geriatric Depression Scale (GDS) score were independently associated with increased risk of mortality in ET. That study did not attempt to disentangle the effects of mild cognitive impairment (MCI) or dementia on risk of mortality in ET.
A growing number of studies demonstrate that, on average, individuals with ET have poorer cognitive performance compared to age-matched controls, supporting an association between ET and cognitive dysfunction [12–15]. One study has shown that ET patients have a higher prevalence of MCI than controls [16]. Two studies have demonstrated an increased risk of dementia in ET compared to controls [17–18] and a third study, although not reporting an overall risk increase, reported that the hazard ratio for incident dementia in the comparison between ET onset age ≥65 vs. ET onset < age 65 was 2.1, suggesting a higher risk of incident dementia in older onset ET cases than controls, although those analyses were not performed [19–20]. Two studies found that amnestic MCI is more typical than non-amnestic MCI in ET cases [21–22] whereas another found the opposite pattern [23]. Guehne et al. [24] determined that individuals without ET or other known neurological illness diagnosed with MCI had an increased risk of mortality, though no studies have explored MCI as a predictor of mortality specifically in an ET cohort. Additionally, dementia is known to be a significant risk factor of mortality in the elderly, but has not been examined as an independent predictor of mortality in ET [25–27].
Thus, dementia and possibly MCI are likely predictors of mortality in ET, although this has not been formally studied or quantified. We now have longer follow-up data on our complete cohort and are able to quantify the risk of mortality associated with both MCI and dementia in ET cases. Identifying the risk factors of mortality and incorporating an assessment of these factors may enhance the quality of care provided to ET patients, as well as increase their longevity. These results will provide greater insight on the risk factors of mortality in ET patients and further assist clinicians in managing ET patients from a comprehensive viewpoint.
Methods
Study Design
ET cases were enrolled in a prospective, longitudinal study examining cognition in ET (Clinical Pathological Study of Cognitive Impairment in Essential Tremor, COGNET, NINDS R01 NS086736) beginning in July 2014. The purpose of the study is to characterize the neuropathological basis of cognitive deficits in ET and to examine the course of cognition in ET. The eligibility criteria are: (1) diagnosis of ET; (2) minimum age of 55 years old; (3) no history of brain surgery for the treatment of ET; (4) willingness to partake in a cognitive test battery; and (5) willingness to enroll in the Essential Tremor Centralized Brain Repository. We recruited cases through advertisements on the study website, along with other websites (i.e., International Essential Tremor Foundation).
Three assessments were scheduled for each participant: baseline (T1), 18 months after baseline (T2), and 36 months after baseline (T3). Trained research assistants conducted in-person evaluations in the homes of ET cases at each study visit, which consisted of an extensive clinical evaluation, cognitive test battery, and a videotaped neurological examination. These visits typically took place over two consecutive days to minimize participant fatigue.
All study procedures were approved by the UT Southwestern Medical Center, Yale University, and Columbia University Internal Review Boards. All cases signed written informed consents.
Clinical Battery
The clinical evaluation included questions regarding demographic (e.g., age, gender, ethnicity, education) and clinical (e.g., age of tremor onset, medication usage, current cigarette smoker) features. Researchers also asked about the number of falls the patient had experienced in the past year. Additional questionnaires were also implemented: Geriatric Depression Scale (GDS) [28], Pittsburgh Sleep Quality Index (PSQI) [29], and Physical Activity Scale for the Elderly (PASE) [30]. The GDS [28] is a valid and reliable 30-item self-report measure of depressive symptoms, specifically designed for the elderly, with a higher score indicating more depressive symptoms (range = 0–30). The PSQI [29] is a self-rated questionnaire assessing sleep quality and disturbances (e.g., average hours of sleep, sleep quality). The PASE [30] is a self-rated assessment of physical activity (e.g., leisure, household activity, occupational activity) for older adults, with higher scores indicating greater physical activity (range = 0–400 or more).
Cognitive Evaluation
A neuropsychologist (SC) developed the cognitive test battery specifically for the COGNET study [11]. The battery included two global cognitive screening measures, the Montreal Cognitive Assessment (MoCA) [31] and the Mini Mental State Exam (MMSE) [32], with higher scores indicating normal cognition (range = 0–30). The battery included assessments of five cognitive domains: attention, visuospatial function, memory, language, and executive function.
Participants also identified an informant (i.e., someone close to the participant who knew them well) to participate in a short phone interview in which they answered questions about the participant’s behavior and everyday functioning. The informant interviews were instrumental in determining the participants’ Clinical Dementia Rating (CDR) scores (0 = no dementia, 0.5 = questionable dementia, 1 = mild dementia, 2 = moderate dementia, and 3 = severe dementia) [33]. This semi-structured and rated interview was used to determine changes across six dimensions (memory, orientation, judgment and problem solving, community affairs, home and hobbies, and personal care). If an informant was not available, CDR was established through participant self-report and examiner impression. CDR was confirmed and a cognitive diagnosis was decided during diagnostic case conferences, thereby providing two cognitive classifications.
Test scores, informant interviews, and CDR scores were presented at consensus conferences, and diagnoses were assigned by a neuropsychologist (SC) and geriatric psychiatrist (EDH). The three main cognitive diagnoses assigned were: (1) normal cognition; (2) MCI (CDR = 0.5 and impairment on two MCI-designated tests); and (3) dementia (CDR > 1 and impairment in multiple cognitive domains). We defined impairment on a single test as a z-score ≤ −1.5. To determine cognitive domain z-scores, we created domain aggregates (mean z-scores for each domain) based on published normative data, which were adjusted by age, gender, and/or education. MCI was further stratified into subtypes based on guidelines by Peterson [34]: amnestic (single or multi-domain) and non-amnestic (single or multi-domain).
Videotaped Neurological Examination
A videotaped neurological examination included one test for postural tremor and five tests for kinetic tremor [11]. A senior movement disorders neurologist (EDL) used the Washington Heights-Inwood Genetic Study of ET (WHIGET) rating scale and diagnostic criteria [1], which are reliable [35] and valid [36], to provide a tremor rating score for each task (0–3) and to assign a diagnosis in each case. The ratings added up to a total tremor score (TTS; range = 0–36) and were assigned at each interval (T1, T2, and T3). EDL also assessed tandem gait (when the toes of the back foot touch the heel of the front foot at each step) and the number of missteps during a 10-step walk.
Statistical Analyses
We used the Statistical Package for Social Sciences (SPSS) version 26. We tested the normality of test variables using a Kolmogorov Smirnov test, and used non-parametric tests as needed. We compared baseline demographic and clinical characteristics of participants who died vs. participants who remained alive during follow-up (Table 1). Additionally, we compared demographic and clinical characteristics of cases with dementia to cases who were not demented at baseline (Table 2). We imputed 8 missing values in the number of falls variable to account for possible biased estimates. For these analyses, we used student’s t-tests, chi-square tests, Fisher’s exact tests, and Mann-Whitney U tests. Cox proportional hazards models were used to estimate hazard ratios (HRs) for death with 95% confidence intervals (CIs). A person-years variable was calculated using the time between T1 and most recent contact for living cases, while a person-years variable was calculated using the time between T1 and death for the deceased cases.
Table 1.
Baseline demographic and clinical characteristics of deceased vs. living ET cases.
| All ET Cases N = 194 | Deceased N = 52 | Living N = 142 | Significance (Deceased vs. Living) | |
|---|---|---|---|---|
| Age (years) | 79.1 ± 9.7 | 88.0 ± 5.1 | 75.9 ± 8.9 | p < 0.001 a |
| Female | 118 (60.8) | 32 (61.5) | 86 (60.6) | p = 0.90b |
| White race | 189 (97.4) | 52 (100.0) | 137 (96.5) | p = 1.00c |
| Education (years) | 15.9 ± 2.5 | 15.3 ± 2.8 | 16.1 ± 2.4 | p = 0.07a |
| Number of prescription medications | 5.7 ± 4.0 [5.0] | 7.3 ± 4.7 [6.0] | 5.1 ± 3.6 [5.0] | p = 0.002 d |
| Current cigarette smoker | 8 (4.1) | 1 (1.9) | 7 (4.9) | p = 0.68c |
| Age of tremor onset (years) | 39.7 ± 22.4 [40.0] | 44.8 ± 24.6 [50.0] | 37.8 ± 21.4 [40.0] | p = 0.06d |
| Tremor duration (years) | 39.3 ± 22.1 [34.0] | 43.2 ± 24.4 [34.0] | 37.8 ± 21.1 [33.0] | p = 0.20d |
| Total tremor score | 20.1 ± 5.0 | 21.6 ± 5.4 | 19.6 ± 4.8 | p = 0.02 a |
| MoCA score | 24.6 ± 3.6 | 22.0 ± 4.5 | 25.5 ± 2.8 | p < 0.001 a |
| MMSE score | 28.2 ± 2.7 | 26.1 ± 4.1 | 29.0 ± 1.4 | p < 0.001 a |
| Dementia | 15 (7.7) | 13 (25.0) | 2 (1.4) | |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Language | 0.03 ± 0.62 [0.09] | −0.14 ± 0.86 [−0.04] | 0.09 ± 0.50 [0.11] | p = 0.12d |
| GDS | 6.4 ± 5.2 [5.0] | 7.0 ± 5.1 [7.0] | 6.2 ± 5.2 [5.0] | p = 0.21d |
| Number of falls reported in the past year | 1.4 ± 3.3 [0.0] | 2.1 ± 4.6 [1.0] | 1.1 ± 2.7 [0.0] | p = 0.005 d |
| Number of tandem missteps | 5.3 ± 4.1 [5.0] | 8.7 ± 2.8 [10.0] | 4.1 ± 3.8 [3.0] | p < 0.001 d |
| PASE score | 96.8 ± 71.3 [85.0] | 52.3 ± 40.0 [36.8] | 108.9 ± 73.2 [97.4] | p < 0.001 d |
| PSQI item: average hours of sleep | 7.2 ± 1.4 | 7.2 ± 1.8 | 7.2 ± 1.2 | p = 0.81a |
| PSQI item: Sleep quality | 0.90 ± 0.77 [1.0] | 0.88 ± 0.74 [1.0] | 0.90 ± 0.78 [1.0] | p = 0.98d |
Values are number (percentage) or mean ± standard deviation [median]. Percentages are column percentages.
2 individuals had cognitive impairment that was not classified as MCI or dementia. Bolded values are statistically significant (p < 0.05).
CDR, Clinical Dementia Rating; ET, essential tremor; GDS, Geriatric Depression Scale; MCI, mild cognitive impairment; MMSE; Mini-Mental State Exam; MoCA, Montreal Cognitive Assessment; PASE, Physical Activity Scale for the Elderly; PSQI, Pittsburgh Sleep Quality Index.
Student’s t-test
Chi-square test
Fisher’s exact test
Mann-Whitney U test
Table 2.
Demographic and clinical characteristics of ET cases with vs. without baseline dementia.
| All ET Cases N = 192 | Baseline Dementia N = 15 | Baseline Not Demented N = 177 | Significance (Dementia vs. Non-Demented) | |
|---|---|---|---|---|
| Age (years) | 79.1 ± 9.7 | 88.2 ± 5.0 | 78.4 ± 9.4 | p < 0.001 a |
| Female | 118 (60.8) | 8 (53.5) | 110 (62.1) | p = 0.50b |
| White race | 189 (97.4) | 15 (100.0) | 172 (97.2) | p = 1.00c |
| Education (years) | 15.9 ± 2.5 | 15.7 ± 2.7 | 15.9 ± 2.6 | p = 0.81a |
| Number of prescription medications | 5.7 ± 4.0 | 8.4 ± 4.2 | 5.5 ± 4.0 | p = 0.007 a |
| Current cigarette smoker | 8 (4.1) | 0 (0.0) | 8 (4.5) | p = 1.00c |
| Age of tremor onset (years) | 39.7 ± 22.4 [40.0] | 44.0 ± 24.2 [52.5] | 39.4 ± 22.4 [40.0] | p = 0.43d |
| Tremor duration (years) | 39.3 ± 22.1 [34.0] | 44.1 ± 23.8 [36.5] | 38.9 ± 22.0 [33.0] | p = 0.39d |
| Total tremor score | 20.1 ± 5.0 [20.5] | 22.3 ± 6.6 [22.0] | 19.9 ± 4.9 [20.5] | p = 0.03 d |
| MoCA score | 24.6 ± 3.6 | 16.9 ± 3.6 | 25.1 ± 3.0 | p < 0.001 a |
| MMSE score | 28.2 ± 2.7 | 22.0 ± 4.2 | 28.7 ± 1.8 | p < 0.001 a |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Language | 0.03 ± 0.62 [0.09] | −1.05 ± 0.95 [1.04] | 0.11 ± 0.50 [0.11] | p < 0.001 d |
| GDS | 6.4 ± 5.2 [5.0] | 5.9 ± 6.2 [3.0] | 6.5 ± 5.2 [5.0] | p = 0.33d |
| Number of falls reported in the past year | 1.4 ± 3.3 [0.0] | 2.4 ± 2.9 [2.0] | 1.3 ± 3.3 [0.0] | p = 0.004 d |
| Number of tandem missteps | 5.3 ± 4.1 [5.0] | 9.4 ± 2.1 [10.0] | 5.1 ± 4.1 [4.0] | p = 0.001 d |
| PASE score | 96.8 ± 71.3 | 53.4 ± 59.1 | 97.7 ± 71.7 | p = 0.17a |
| PSQI item: average hours of sleep | 7.2 ± 1.4 | 8.5 ± 1.3 | 7.1 ± 1.3 | p = 0.002 a |
| PSQI item: sleep quality | 0.90 ± 0.77 [1.0] | 0.78 ± 0.83 [1.0] | 0.90 ± 0.77 [1.0] | p = 0.64d |
Values are number (percentage) or mean ± standard deviation [median]. Percentages are column percentages.
Bolded values are statistically significant (p < 0.05).
CDR, Clinical Dementia Rating; ET, essential tremor; GDS, Geriatric Depression Scale; MCI, mild cognitive impairment; MMSE; Mini-Mental State Exam; MoCA, Montreal Cognitive Assessment; PASE, Physical Activity Scale for the Elderly; PSQI, Pittsburgh Sleep Quality Index.
Student’s t-test
Chi-square test
Fisher’s exact test
Mann-Whitney U test
For the Cox proportional hazards models, we first performed univariate models (i.e., entered independent variables one at a time in a series of regression models). In the univariate models, our cognitive diagnosis groups were normal cognition (reference group), MCI, and dementia. Based on initial modeling, we identified variables as predictors of mortality when p < 0.05, and incorporated each into subsequent multivariate Cox models. As such, along with our primary variable of interest (cognitive diagnosis) we added the identified variables successively, one at a time instead of en bloc, to account for any potential collinearity between independent variables (e.g., between the numerous gait and balance variables or between the numerous cognitive variables). For the multivariate models, we further subdivided MCI and our groups were normal cognition (reference group), non-amnestic MCI, amnestic MCI, and dementia. We set the significant p-value at < 0.05 for these analyses. Furthermore, we conducted two sensitivity analyses using Cox proportional hazards models to examine the predictors of mortality. The first excluded 15 cases with dementia diagnoses in order to provide a cleaner dataset for the direct comparison between MCI and normal cognition; these analyses used a cohort of 179 cases with normal cognition or MCI. The second analysis excluded 8 cases with imputed falls to examine a cohort of 186 cases to assess the performance and robustness of our imputation method.
We checked the proportional hazards assumption and time dependence for each model. Multicollinearity was evaluated through variance inflation factors (VIF) of the variables. We also computed dfbetas and standardized residual charts to detect any influential observations. The proportional hazards assumption was satisfied in every model when we included time dependent covariates in the regression (p > 0.05) and plotted the log(-log(survival)) versus log of survival time curves. We found no collinearity between the variables when using the variance inflation factor test (vif < 1.27, r2 < 0.45 in each model). At the same time, no high leverage observations were observed (dfbeta < 0.22, studentized residuals within the t distribution).
Results
There were 194 ET cases with a baseline age of 79.1 ± 9.7 years (Table 1). By the end of the study, 52 (26.8%) ET cases had died. Of the 52 deceased participants, 22 (42.3%) died after T1, 9 (17.3%) died after T2, and 21 (40.4%) died after T3. Among living participants, the mean time from T1 to most recent contact was 5.1 ± 0.5 years. Among deceased participants, the mean time from T1 to death was 2.0 ± 1.7 years. At baseline, there were 21 amnestic and 8 non-amnestic MCI participants and 15 demented participants included in the below analyses.
We compared baseline demographic and clinical characteristics of 52 participants who died vs. 142 participants who remained alive during follow-up (Table 1). At baseline, those who died were older, took more prescription medications, had a higher TTS, more reported falls, more tandem missteps, and lower physical activity scores. Furthermore, they had lower scores on global cognitive screens (MoCA and MMSE) and composite measures of memory, executive function, and attention; higher CDR scores; and fewer diagnoses of normal cognition. The two groups did not differ significantly in terms of gender, race, years of education, cigarette smoking, age of tremor onset, tremor duration, depressive symptoms, average hours of sleep, or sleep quality, although some of these were of marginal significance (Table 1).
At baseline, 15 (7.8%) of 192 ET cases had a clinical diagnosis of dementia. We compared demographic and clinical characteristics of these 15 demented ET cases to the 177 ET cases who were not demented at baseline (Table 2). Cases with baseline dementia were older, took more prescription medications, had a higher TTS, more reported falls, more tandem missteps, and reported on average more hours of sleep (Table 2). Moreover, as expected, cases with baseline dementia had lower scores on global cognitive screens and in all cognitive domains, as well as higher CDR scores.
We entered variables one at a time for the univariate Cox regression models (Table 3). Older age (HR = 1.18, p < 0.001), fewer years of education (HR = 0.89, p = 0.04), more prescription medications (HR = 1.09, p = 0.001), and less physical activity (HR = 0.99 p < 0.001) were associated with increased risk of mortality. Furthermore, more falls in the past year (HR = 1.08, p = 0.02), more tandem missteps (HR = 1.36, p < 0.001), and a higher TTS (HR = 1.07, p = 0.02) were associated with increased risk of mortality.
Table 3.
Predictors of mortality in ET cases (Univariate Cox proportional hazards models)
| Variable | Beta | HR | 95% CI | Significance |
|---|---|---|---|---|
| Age (years) | 0.16 | 1.18 | 1.12–1.23 | p < 0.001 |
| Female | 0.003 | 1.00 | 0.57–1.76 | p = 0.99 |
| White race | −3.03 | 0.05 | 0.00–570.47 | p = 0.53 |
| Education (years) | −0.11 | 0.89 | 0.80–1.00 | p = 0.04 |
| Number of prescription medications | 0.09 | 1.09 | 1.04–1.15 | p = 0.001 |
| Current cigarette smoker | −0.79 | 0.46 | 0.06–3.30 | p = 0.46 |
| Age of tremor onset (years) | 0.01 | 1.01 | 1.00–1.03 | p = 0.06 |
| Tremor duration (years) | 0.01 | 1.01 | 0.98–1.02 | p = 0.15 |
| Total tremor score | 0.07 | 1.07 | 1.01–1.13 | p = 0.02 |
| MoCA score | −0.20 | 0.82 | 0.76–0.87 | p < 0.001 |
| MMSE score | −0.28 | 0.75 | 0.70–0.81 | p < 0.001 |
| Dementia | 2.03 | 7.61 | 3.89–14.89 | p < 0.001 |
| Overall | 1.80 | 6.02 | 3.55–10.22 | p < 0.001 |
| Language | −0.51 | 0.60 | 0.39–0.92 | p = 0.02 |
| GDS | 0.03 | 1.03 | 0.98–1.08 | p = 0.26 |
| Number of falls reported in the past year | 0.08 | 1.08 | 1.02–1.15 | p = 0.02 |
| Number of tandem missteps | 0.31 | 1.36 | 1.22–1.52 | p < 0.001 |
| PASE score | −0.02 | 0.99 | 0.98–0.99 | p < 0.001 |
| PSQI item: average hours of sleep | 0.002 | 1.00 | 0.77–1.31 | p = 0.99 |
| PSQI item: sleep quality | −0.03 | 0.97 | 0.62–1.51 | p = 0.89 |
Bolded values are statistically significant (p < 0.05).
CDR, Clinical Dementia Rating; CI, confidence interval; ET, essential tremor; GDS, Geriatric Depression Scale; HR, hazard ratio; MCI, mild cognitive impairment; MoCA, Montreal Cognitive Assessment; MMSE; Mini-Mental State Exam; ESS, Epworth Sleepiness Scale; PASE, Physical Activity Scale for the Elderly.
In terms of cognition, the models showed that lower MoCA scores (HR = 0.82, p < 0.001), lower MMSE scores (HR = 0.75, p < 0.001), higher CDR scores (HR = 6.02, p < 0.001; CDR score of 0.5 [HR = 2.00, p = 0.04); CDR score of 1 [(HR = 5.59, p < 0.001); CDR score of 2 [HR = 40.77, p < 0.001]), amnestic MCI diagnoses (HR = 3.71, p < 0.001), and dementia diagnoses (HR = 7.61, p < 0.001) were related to increased risk of mortality. Lower z-scores in the cognitive domains of memory (HR = 0.46, p < 0.001), executive function (HR = 0.41, p < 0.001), attention (HR = 0.57, p = 0.006), and language (HR = 0.60, p = 0.02) were also associated with mortality.
In the final multivariate Cox model, dementia (HR = 2.66, p = 0.006), older age (HR = 1.18, p < 0.001) and more falls (HR = 1.10, p < 0.001) were independently associated with increased risk of mortality (Table 4); numerous other factors identified in univariate models (e.g., total tremor score) dropped out of this model. As age was included as a covariate in this model, the HRs for dementia and falls adjusted for age. Survival curves are shown for cognitive diagnoses (Figure 1) where cases were stratified into normal cognition, non-amnestic MCI, amnestic MCI, and dementia. Hazard ratios (95% confidence interval) in the Cox proportional hazards model were as follows: non-amnestic MCI = 0.67 (0.16 – 2.83), p = 0.58; amnestic MCI = 1.93 (0.93 – 3.98,) p = 0.08; dementia = 2.66 (1.32 – 5.34), p = 0.006.
Table 4.
Predictors of mortality in ET cases (multivariate Cox proportional hazards models)
| Variable | Beta | HR | 95% CI | Significance |
|---|---|---|---|---|
| Dementia | 0.98 | 2.66 | 1.32–5.34 | p = 0.006 |
| Age (years) | 0.16 | 1.18 | 1.12–1.23 | p < 0.001 |
| Number of falls reported in the past year | 0.10 | 1.10 | 1.01–1.20 | p < 0.001 |
Bolded values are statistically significant (p < 0.05).
CI, confidence interval; HR, hazard ratios; MCI, mild cognitive impairment.
Figure 1. Survival Curve by Clinical Diagnosis Category.
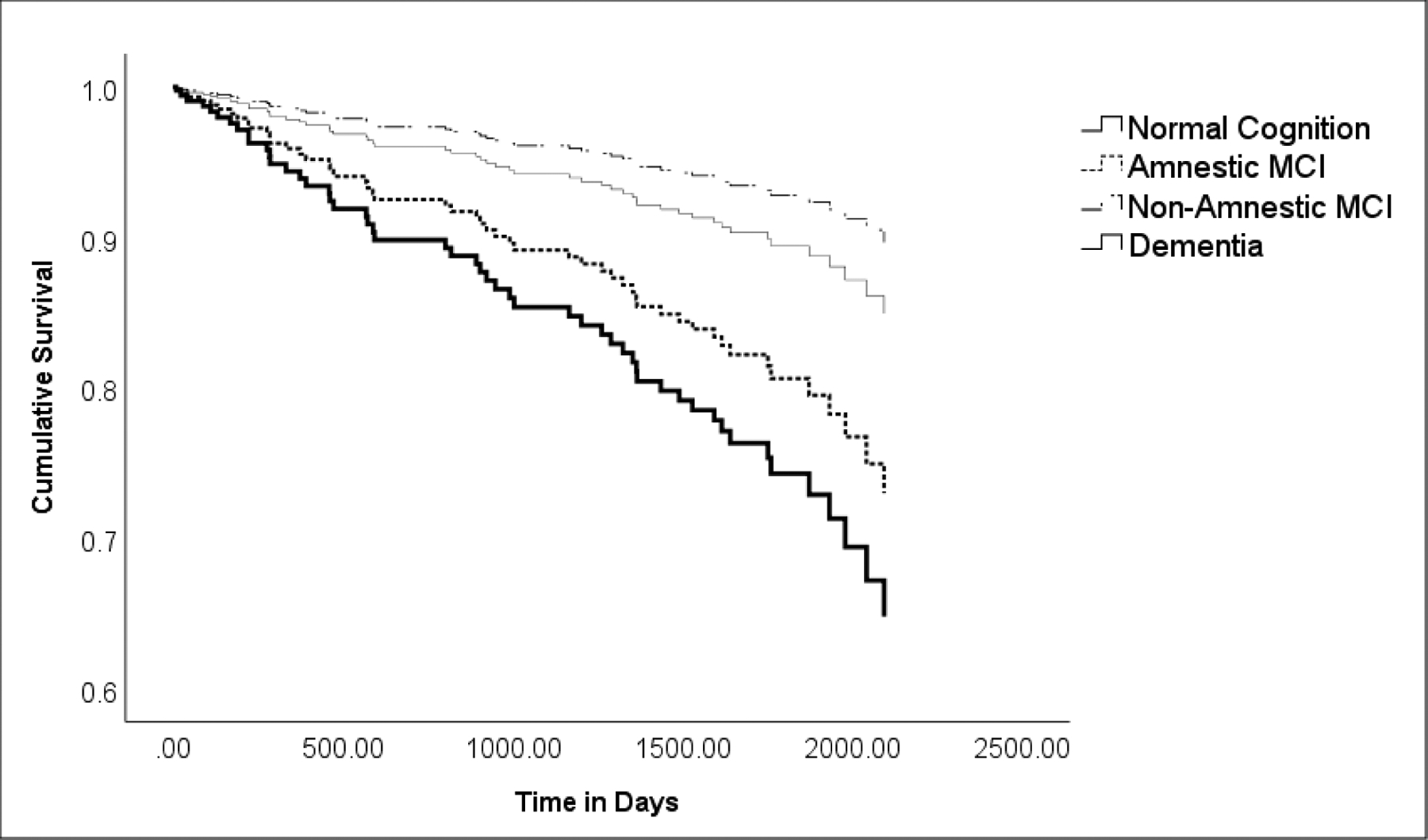
ET cases were stratified into four cognitive diagnoses: normal cognition, amnestic MCI, non-amnestic MCI, and dementia. Hazards ratio (95% Confidence Interval) in Cox proportional hazards model for non-amnestic MCI = 0.67 (0.16 – 2.83); amnestic MCI = 1.93 (0.93 – 3.98,) p = 0.08; dementia = 2.66 (1.32 – 5.34), p = 0.006. While the curves for normal cognition and amnestic MCI diverge, the difference was not significant (p = 0.58).
We performed a sensitivity analysis excluding 15 dementia cases, which yielded several significant predictors of mortality. In the initial unadjusted Cox models, older age (HR = 1.17, p < 0.001), longer tremor duration (HR = 1.01, p = 0.04), more prescription medications (HR = 1.01, p = 0.01), and less physical activity (HR = 0.99, p = 0.002) were independent predictors of mortality. Furthermore, more tandem missteps (HR = 1.32, p < 0.001) and a higher TTS (HR = 1.07, p = 0.05) were also predictors. In regards to cognition, lower MOCA scores (HR = 0.84, p < 0.001), lower MMSE scores (HR = 0.71, p < 0.001), higher CDR scores (HR = 4.11, p = 0.03), amnestic MCI diagnosis (HR = 3.79, p < 0.001), and lower z -scores in the memory cognitive domain (HR = 0.54, p = 0.001) were each predictors. In the multivariate Cox model, amnestic MCI (HR = 2.21, p = 0.03) and older age (HR = 1.19, p < 0.001) remained significant predictors of mortality in the ET cohort.
Lastly, we performed a sensitivity analysis excluding the imputed number of falls to account for potential changes in hazard ratios. The results did not vary significantly for the univariate models. However, in the multivariate model, we identified amnestic MCI (HR = 2.16, p = 0.04) as a predictor of mortality; furthermore, dementia (HR = 2.33, p = 0.03), older age (HR = 1.18, p < 0.001), and more falls in the past year (HR= 1.19, p < 0.001) were significant.
Discussion
In this study, we examined predictors of mortality in a group of 194 cases among whom 52 (26.8%) died during prospective, longitudinal follow-up. In the final multivariate model, a clinical diagnosis of dementia, older age, and more falls in the past year were independent predictors of mortality in ET cases.
Although dementia is a likely predictor of mortality in ET, prior to the current analysis, this had not been formally studied or quantified. The most prominent finding in the current study is that dementia more than doubles the risk of mortality in ET, with the increased risk being 2 to 3 times greater than that seen in non-demented ET cases [18]. Past studies have also shown that there is an association between ET and both prevalent [11] and incident dementia [21]. With growing evidence of this association, it seems that dementia is a feature that selectively affects ET patients above and beyond age-matched controls [18]. Dementia can be challenging for ET patients and their families, especially caregivers [37], and increased preparedness and knowledge about dementia and its downstream effects is important.
Once cognitive impairment sets in, the clinical outlook and prognosis are important to establish. A recent paper identified certain critical gaps in knowledge regarding the presence and correlates of cognitive impairment in ET [4]. ET patients with dementia may reach a variety of critical endpoints, including admission to a hospital, institutionalization, or death, and they may also encounter specific medical events (e.g., falls) [38]. The extent to which cognitive impairment in ET increases risk for these endpoints is unknown and in this paper, we tackle a specific endpoint – death. We address the increased risk of mortality that accompanies dementia in ET.
Ours is a study of ET patients. For context, one may ask how the presence of dementia increases the risk of mortality in an ET-free group. Prior studies suggest that the risk of mortality in tremor-free demented persons aged 65 years and older is 1.7 – 3.8 times higher than it is for their counterparts without dementia [39]. In this sense, our ET patients are comparable to similarly-aged controls.
Although very few studies have looked at predictors of mortality in ET, by contrast, many studies have investigated predictors of mortality in Parkinson’s disease (PD). Studies have shown that cognitive impairment [40–41] and older age [41–42] are independent predictors of mortality in PD. Dementia was particularly important since it increased the risk of mortality by more than double (HR = 2.62, p < 0.001) [40].
While dementia was the strongest predictor of mortality, in several analyses, amnestic MCI was also either a marginally significant or significant independent predictor of mortality in ET. MCI has not been extensively examined as a predictor of mortality in ET, but has been found to increase risk of mortality in otherwise healthy controls [24,44]. However, the risk of mortality based on MCI subtypes is still controversial. Some population-based studies found an increase in mortality for non-amnestic MCI [45–47], while others have found amnestic MCI cohorts to have increased risk of mortality [48–49]. Differences in study methodology and samples could account for these differences. In PD cohorts, cognitive impairment is associated with higher mortality [50] and MCI is associated with a faster rate of progression to dementia [51]. To our knowledge, no studies in PD cohorts have made the distinction between non-amnestic and amnestic MCI as potential predictors of mortality. Predictors of mortality only partially overall overlapped in ET and PD, suggesting that predictors of ET cannot be assumed from PD, so further investigation of ET’s unique features is warranted.
The association between age and risk of mortality is consistent with previous studies, thus age remains a predictor of mortality in ET [18]. In our results, we found an 18% increased risk of mortality with each additional year of age. This finding is congruent with other neurodegenerative diseases (i.e., PD, amyotrophic lateral sclerosis) [52].
A greater number of falls also increased the risk of mortality, and each fall was associated with a further 10% increment. In older cohorts with no cognitive impairment, the risk of falls increased with age; one study found that old age and frequent falls were risk factors of mortality after adjusting for relevant covariates [53]. Surprisingly, recurrent falling was not identified as a predictor in PD cohorts [54], even if falling is a disabling feature of this disease [55–56]. Falls have important consequences for older adults, leading to serious injuries, disabilities, and loss of independence, hence further interventions to control this risk factor might improve the quality of life of patients affected by it.
There are several limitations inherent in this study, the most prominent being the lack of a control group. Despite this, we are able to say that within the context of ET that the presence of dementia, and possibly certain forms of MCI, increase risk of mortality, and in the case of dementia, by 2 – 3 fold. Both of these findings are novel – they are ones for which there has not been any published data to date. These data are conceptually important because they serve to further reinforce the notion that within the context of ET, cognitive impairment can have significant downstream implications. Without a control group, we cannot compare the increased risk associated with dementia in an ET cohort to that of a non-ET cohort. However, the goal of this study was to understand among those diagnosed with ET, which demographic and clinical features inform mortality risk, and not to determine the extent to which these predictors are specific to ET. Future studies should include control groups to address that separate question.
Another limitation is that the majority of the cohort had normal cognition at baseline. Only 44 (22.7%) out of 194 cases had a clinical diagnosis of MCI or dementia at baseline. Despite this, we identified a significant association between baseline dementia and mortality during follow-up. In addition, the longitudinal data used in the analyses is limited to three time intervals. A longer time duration with more deaths would further enhance the power of this study to detect additional associations. ET cases enrolled in the COGNET study were self-referred and 189 (97.4%) participants identified as white. This group is not necessarily representative of ET in the community. Despite the fact that we had a limited number of cases with baseline MCI (n = 29), we were able in several analyses to detect significant associations between MCI and mortality. However, future studies with larger sample sizes would allow for narrower confidence intervals around our point estimates and would contribute to greater overall power to detect small associations. Finally, our analyses did not include neuroimaging and genetic data, which could be useful in determining the biological predictors of mortality in ET and the underlying structural correlates of our results.
While there were limitations, the study also had various strengths. To our knowledge, COGNET is the only ongoing longitudinal, prospective study that tracks a population-based group of ET cases at several time points. We examined a broad variety of possible predictors of mortality, including demographic and clinical variables, which yielded many significant associations. Additionally, phenotyping of the cohort in terms of tremor features and cognition was carefully assigned at each time point by a senior movement disorders specialist, neuropsychologist, and geriatric psychiatrist.
Given that ET is a prevalent movement disorder, the influence of clinical features of ET on a patient’s life span has valuable clinical implications. These findings present a useful tool for clinicians, patients, and their families to understand and plan for disease-related features. With these identified predictors, future studies can assess ways to mitigate risks in ET patients since identifying risk factors is important for diagnosis, prognosis, and treatment. While age and clinical diagnoses may not be modifiable risk factors, there have been multiple studies looking at falls prevention in older adults, including exercise programs [57] and vitamin D supplementation [58]. Given the association of even single falls with increased mortality, clinicians could institute fall precautions even prior to the occurrence of falls. Further prospective longitudinal studies are necessary to characterize predictors, outcomes, and the underlying pathological mechanisms of ET. Overall, this study highlights several independent predictors of mortality among older ET patients. This study contributes to growing evidence that dementia is of importance in ET cohorts as it leads to an increased risk of mortality.
Dementia leads to an increased risk of mortality in people with essential tremor
More falls and older age lead to an increased risk of mortality
Amnestic mild cognitive impairment is margixy2nally significant in risk of mortality
Funding:
This work was supported by the National Institutes of Health R01NS086736. This funding body played no role in the design of the study, the collection, analysis, and interpretation of data, or the writing of the manuscript.
Abbreviations:
- CDR
Clinical Dementia Rating
- COGNET
Clinical-Pathological Study of Cognitive Impairment in Essential Tremor
- ET
essential tremor
- GDS
Geriatric Depression Scale
- MCI
mild cognitive impairment
- MMSE
Mini Mental State Exam
- MoCA
Montreal Cognitive Assessment
- PASE
Physical Activity Scale for the Elderly
- PD
Parkinson’s disease
- PSQI
Pittsburgh Sleep Quality Index
- WHIGET
Washington Heights-Inwood Genetic Study of Essential Tremor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Louis ED, Ottman R, Ford B, Pullman S, Martinez M, Fahn S, Hauser WA. The Washington heights-inwood genetic study of essential tremor: methodologic issues in essential-tremor research. Neuroepidemiology 1997April;16(3):124–33. 10.1159/000109681. [DOI] [PubMed] [Google Scholar]
- 2.Louis ED, Ferreira JJ. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov Disord. 2010April;25(5):534–41. 10.1002/mds.22838. [DOI] [PubMed] [Google Scholar]
- 3.Louis ED, Rao AK, Gerbin M. Functional correlates of gait and balance difficulty in essential tremor: balance confidence, near misses and falls. Gait Posture. 2012January1;35(1):43–7. 10.1016/j.gaitpost.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Louis ED, Joyce JL, Cosentino S. Mind the gaps: what we don’t know about cognitive impairment in essential tremor. Parkinsonism Relat Disord. 2019June;63(1):10–9. 10.1016/j.parkreldis.2019.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sengul Y, Sengul HS, Yucekaya SK, Yucel S, Bakim B, Pazarci NK, et al. Cognitive functions, fatigue, depression, anxiety, and sleep disturbances: assessment of nonmotor features in young patients with essential tremor. Acta Neurol Belg. 2015September;115(3):281–7. 10.1007/s13760-014-0396-6. [DOI] [PubMed] [Google Scholar]
- 6.Bareš M, Husárová I, Lungu OV. Essential tremor, the cerebellum, and motor timing: towards integrating them into one complex entity. Tremor Other Hyperkinet Mov. 2012September12; 2. [PMC free article] [PubMed] [Google Scholar]
- 7.Adamaszek M, D’Agata F, Ferrucci R, Habas C, Keulen S, Kirkby KC, et al. Consensus paper: cerebellum and emotion. Cerebellum. 2017April1;16(2):552–76. 10.1007/s12311-016-0815-8. [DOI] [PubMed] [Google Scholar]
- 8.Koziol LF, Budding D, Andreasen N, D’Arrigo S, Bulgheroni S, Imamizu H, et al. Consensus paper: the cerebellum’s role in movement and cognition. Cerebellum. 2014February1;13(1):151–77. 10.1007/s12311-013-0511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rajput AH, Offord KP, Beard CM, Kurland LT. Essential tremor in Rochester, Minnesota: a 45-year study. J Neurol Neurosurg Psychiatry. 1984May1;47(5):466–70. 10.1136/jnnp.47.5.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Louis ED, Benito-León J, Ottman R, Bermejo-Pareja F. A population-based study of mortality in essential tremor. Neurology. 2007November20;69(21):1982–9. 10.1212/01.wnl.0000279339.87987.d7 [DOI] [PubMed] [Google Scholar]
- 11.Zubair A, Cersonsky TE, Kellner S, Huey ED, Cosentino S, Louis ED. What predicts mortality in essential tremor? A prospective, longitudinal study of elders. Front Neur. 2018December;9:1077. 10.3389/fneur.2018.01077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lombardi WJ, Woolston DJ, Roberts JW, Gross RE. Cognitive deficits in patients with essential tremor. Neurology. 2001September;57(5):785–90. 10.1212/WNL.57.5.785. [DOI] [PubMed] [Google Scholar]
- 13.Chandran V, Pal PK. Essential tremor: beyond the motor features. Parkinsonism Relat Disord. 2012June1;18(5):407–13. 10.1016/j.parkreldis.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Janicki SC, Cosentino S, Louis ED. The cognitive side of essential tremor: what are the therapeutic implications? Ther Adv Neurol Disord. 2013November;6(6):353–68. 10.1177/1756285613489591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Louis ED. Non-motor symptoms in essential tremor: a review of the current data and state of the field. Parkinsonism Relat Disord. 2016January1;22:S115–8. 10.1016/j.parkreldis.2015.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benito-León J, Louis ED, Mitchell AJ, Bermejo-Pareja F. Elderly-onset essential tremor and mild cognitive impairment: a population-based study (NEDICES). J Alzheimer’s Dis. 2011January1;23(4):727–35. 10.3233/JAD-2011-101572. [DOI] [PubMed] [Google Scholar]
- 17.Bermejo‐Pareja F, Louis ED, Benito‐León J. Risk of incident dementia in essential tremor: a population‐based study. Mov Disord. 2007August15;22(11):1573–80. 10.1002/mds.21553. [DOI] [PubMed] [Google Scholar]
- 18.Thawani SP, Schupf N, Louis ED. Essential tremor is associated with dementia: prospective population-based study in New York. Neurology. 2009August25;73(8):621–5. 10.1212/WNL.0b013e3181b389f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shill HA, Hentz JG, Jacobson SA, Belden C, Sabbagh MN, Beach TG, Driver-Dunckley E, Adler CH. Essential tremor in the elderly and risk for dementia. J Neurodegener Dis. 2014April9:2014: 328765. 10.1155/2014/328765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Louis ED, Radler KH, Huey ED, Cosentino S. Progression to dementia in patients with essential tremor. Parkinsonism Relat Disord. 2020December30:S1353–8020. 10.1016/j.parkreldis.2020.12.011. [DOI] [PubMed] [Google Scholar]
- 21.Bologna M, Berardelli I, Paparella G, Ferrazzano G, Angelini L, Giustini P, et al. Tremor distribution and the variable clinical presentation of essential tremor. Cerebellum. 2019October;18(5):866–72 10.1007/s12311-019-01070-0. [DOI] [PubMed] [Google Scholar]
- 22.Collins K, Rohl B, Morgan S, Huey ED, Louis ED, Cosentino S. Mild cognitive impairment subtypes in a cohort of elderly essential tremor cases. J Int Neuropsychol Soc. 2017May;23(5):390. 10.1017/S1355617717000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park IS, Oh YS, Lee KS, Yang DW, Song IU, Park JW, et al. Subtype of mild cognitive impairment in elderly patients with essential tremor. Alzheimer Dis Assoc Disord. 2015April;29(2):141–5. 10.1097/WAD.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 24.Guehne U, Luck T, Busse A, Angermeyer MC, Riedel-Heller SG. Mortality in individuals with mild cognitive impairment. Neuroepidemiology. 2007;29(3–4):226–34. 10.1159/000112479. [DOI] [PubMed] [Google Scholar]
- 25.Agüero-Torres H, Fratiglioni L, Guo Z, Viitanen M, Winblad B. Mortality from dementia in advanced age: a 5-year follow-up study of incident dementia cases. J Clin Epidemiol. 1999August;52(8):737–43. 10.1016/S0895-4356(99)00067-0. [DOI] [PubMed] [Google Scholar]
- 26.Connors MH, Ames D, Boundy K, Clarnette R, Kurrle S, Mander A, et al. Predictors of mortality in dementia: the PRIME study. J Alzheimer’s Dis. 2016January1;52(3):967–74. 10.3233/JAD-150946. [DOI] [PubMed] [Google Scholar]
- 27.Todd S, Barr S, Roberts M, Passmore AP. Survival in dementia and predictors of mortality: a review. Int J Geriatr Psychiatry. 2013November;28(11):1109–24. 10.1002/gps.3946. [DOI] [PubMed] [Google Scholar]
- 28.Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: a preliminary report. Journal Psychiatr Res. 1982January;17(1):37–49. 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 29.Buysse DJ, Reynolds III CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989May1;28(2):193–213. 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 30.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993February1;46(2):153–62. 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 31.Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005April;53(4):695–9. 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 32.Folstein MF, Robins LN, Helzer JE. The mini-mental state examination. Arch Gen Psychiatry. 1983July1;40(7):812. 10.1001/archpsyc.1983.01790060110016. [DOI] [PubMed] [Google Scholar]
- 33.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412–4. 10.1212/WNL.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 34.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004September;256(3):183–94. 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 35.Louis ED, Ford B, Bismuth B. Reliability between two observers using a protocol for diagnosing essential tremor. Mov Disord. 1998March; 13(2):287–93. 10.1002/mds.870130215. [DOI] [PubMed] [Google Scholar]
- 36.Louis ED, Pullman SL. Comparison of clinical vs. electrophysiological methods of diagnosing of essential tremor. Mov Disord. 2001) July;16(4):668–73. 10.1002/mds.1144. [DOI] [PubMed] [Google Scholar]
- 37.Morgan S, Kellner S, Gutierrez J, Collins K, Rohl B, Migliore F, et al. The experience of essential tremor caregivers: burden and its correlates. Front Neur. 2017August14;8:396. 10.3389/fneur.2017.00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alamgir H, Muazzam S, Nasrullah M. Unintentional falls mortality among elderly in the United States: time for action. Injury. 2012December1;43(12):2065–71. 10.1016/j.injury.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 39.Guehne U, Riedel-Heller S, Angermeyer MC. Mortality in dementia. Neuroepidemiology. 2005September;25(3):153–62. 10.1159/000086680. [DOI] [PubMed] [Google Scholar]
- 40.Posada IJ, Benito‐León J, Louis ED, Trincado R, Villarejo A, Medrano MJ, et al. Mortality from Parkinson’s disease: a population‐based prospective study (NEDICES). Mov Disord Clin Pract. 2011December;26(14):2522–9. 10.1002/mds.23921. [DOI] [PubMed] [Google Scholar]
- 41.Marder K, Leung D, Tang M, Bell K, Dooneief G, Cote L, et al. Are demented patients with Parkinson’s disease accurately reflected in prevalence surveys? A survival analysis. Neurology. 1991August1;41(8):1240. 10.1212/WNL.41.8.1240. [DOI] [PubMed] [Google Scholar]
- 42.Piccirilli M, D’Alessandro P, Finali G, Piccinin GL. Neuropsychological follow-up of parkinsonian patients with and without cognitive impairment. Dement Geriatr Cogn Disord. 1994;5(1):17–22. 10.1159/000106689. [DOI] [PubMed] [Google Scholar]
- 43.Hughes TA, Ross HF, Mindham RH, Spokes EG. Mortality in Parkinson’s disease and its association with dementia and depression. Acta Neurol Scand. 2004August;110(2):118–23. 10.1111/j.1600-0404.2004.00292.x. [DOI] [PubMed] [Google Scholar]
- 44.Wilson RS, Aggarwal NT, Barnes LL, Bienias JL, de Leon CF, et al. Biracial population study of mortality in mild cognitive impairment and Alzheimer disease. Arch Neurol. 2009June1;66(6):767–72. 10.1001/archneurol.2009.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bae JB, Han JW, Kwak KP, Kim BJ, Kim SG, Kim JL, et al. Impact of mild cognitive impairment on mortality and cause of death in the elderly. J Alzheimer’s Dis. 2018January1;64(2):607–16. 10.3233/JAD-171182. [DOI] [PubMed] [Google Scholar]
- 46.Vassilaki M, Cha RH, Aakre JA, Therneau TM, Geda YE, Mielke MM, et al. Mortality in mild cognitive impairment varies by subtype, sex, and lifestyle factors: the Mayo Clinic Study of Aging. J Alzheimer’s Dis. 2015January1;45(4):1237–45. 10.3233/JAD-143078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yaffe K, Petersen RC, Lindquist K, Kramer J, Miller B. Subtype of mild cognitive impairment and progression to dementia and death. Dement Geriatr Cogn Disord. 2006;22(4):312–9. 10.1159/000095427. [DOI] [PubMed] [Google Scholar]
- 48.Bermejo-Pareja F, Contador I, Trincado R, Lora D, Sánchez-Ferro Á, Mitchell AJ, et al. Prognostic significance of mild cognitive impairment subtypes for dementia and mortality: data from the NEDICES cohort. J Alzheimer’s Dis. 2016January1;50(3):719–31. 10.3233/JAD-150625. [DOI] [PubMed] [Google Scholar]
- 49.Contador I, Bermejo‐Pareja F, Mitchell AJ, Trincado R, Villarejo A, Sánchez‐Ferro Á et al. Cause of death in mild cognitive impairment: a prospective study (NEDICES). Eur J Neurol. 2014February;21(2):253–e9. 10.1111/ene.12278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oosterveld LP, Allen JC Jr, Reinoso G, Seah SH, Tay KY, Au WL, et al. Prognostic factors for early mortality in Parkinson’s disease. Parkinsonism Relat Disord. 2015March1;21(3):226–30. 10.1016/j.parkreldis.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 51.Aarsland D, Kurz MW. The epidemiology of dementia associated with Parkinson’s disease. Brain Pathol. 2010May;20(3):633–9. 10.1111/j.1750-3639.2009.00369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Steenland K, MacNeil J, Seals R, Levey A. Factors affecting survival of patients with neurodegenerative disease. Neuroepidemiology. 2010;35(1):28–35. 10.1159/000306055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gillespie LD, Robertson MC, Gillespie WJ, Sherrington C, Gates S, Clemson L, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012September12;9. 10.1002/14651858.CD007146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matinolli M, Korpelainen JT, Sotaniemi KA, Myllylä VV, Korpelainen R. Recurrent falls and mortality in Parkinson’s disease: a prospective two‐year follow‐up study. Acta Neurol Scand. 2011March;123(3):193–200. 10.1111/j.1600-0404.2010.01386.x. [DOI] [PubMed] [Google Scholar]
- 55.Coughlin L, Templeton J. Hip fractures in patients with Parkinson’s disease. Clin Orthop Relat Res. 1980May1(148):192–5. [PubMed] [Google Scholar]
- 56.Okuma Y Freezing of gait and falls in Parkinson’s disease. J Parkinsons Dis. 2014January1;4(2):255–60. 10.3233/JPD-130282. [DOI] [PubMed] [Google Scholar]
- 57.Spaniolas K, Cheng JD, Gestring ML, Sangosanya A, Stassen NA, Bankey PE. Ground level falls are associated with significant mortality in elderly patients. J Trauma Acute Care Surg. 2010October1;69(4):821–5. 10.1097/TA.0b013e3181efc6c6. [DOI] [PubMed] [Google Scholar]
- 58.Waldron N, Hill AM, Barker A. Falls prevention in older adults: assessment and management. Aust Fam Physician. 2012December;41(12):930–5. [PubMed] [Google Scholar]


