Abstract
Oxytocin receptors (OTRs) in the midbrain dorsal raphe (DR; the source of most forebrain serotonin) have recently been identified as a potential pharmacological target for treating numerous psychiatric disorders. However, almost all research on this topic has been conducted with males and the role of DR OTRs in female social and affective behaviors is mostly unknown. This may be particularly relevant during early motherhood, a time of high endogenous oxytocin signaling, but also a time of elevated risk for psychiatric dysfunction. To investigate whether OTRs in the DR are necessary for postpartum female social and affective behaviors, we constructed and then injected an adeno-associated virus permanently expressing an shRNA targeting OTR mRNA into the DR. We then observed a suite of social and affective behaviors postpartum. OTR knockdown in the maternal DR led to pup loss after parturition, decreased nursing, increased aggression, and increased behavioral despair. These effects of OTR knockdown in the DR may be due to disrupted plasticity in the primary somatosensory cortex (S1), which mediates maternal sensitivity to tactile cues from young, as we also found significantly more plasticity-restricting perineuronal nets (PNNs) in the S1 rostral barrel field and a trend for fewer PNNs in the caudal barrel field of OTR-knockdown mothers. These results demonstrate that OTRs in the midbrain DR are essential for postpartum maternal social and affective behaviors, involved in postpartum cortical plasticity, and suggest that pharmacotherapies targeting OTRs in the DR might be effective treatments for some peripartum affective disorders.
Keywords: dorsal raphe, anxiety, depression, oxytocin, perineuronal nets, postpartum
1. Introduction
The neuropeptide oxytocin (OT) is well-known to regulate social interactions (Donaldson and Young, 2008), decrease stress responsivity (Jurek and Neumann, 2018; Slattery and Neumann, 2008), and act as an anxiolytic and antidepressant (Jurek and Neumann, 2018). These effects have led investigators to suggest that OT has high therapeutic potential for treating psychiatric disorders involving social and affective dysfunction (Feifel et al., 2010; Young and Barrett, 2015). The brain sites where OT acts to produce these effects are numerous, but one currently neglected site is the midbrain dorsal raphe (DR). The DR is the source of most forebrain serotonin (Lowry et al., 2008) and serotonin-modulating drugs are still the first-line pharmacotherapies for many psychiatric disorders (Hayes et al., 2012). OT in the DR or other raphe nuclei enhances social reward (Dölen et al., 2013), decreases anxiety-like behaviors (Yoshida et al., 2009), and decreases or has no effect on aggression (Calcagnoli et al., 2015; Pagani et al., 2015) in male laboratory rodents. However, no studies have examined the influence of DR OT receptors (OTRs) on social and affective behaviors displayed during motherhood. Examining OTR influences in mothers is critical given that motherhood is a time of very high endogenous OT (Neumann et al., 1993), that 10-20% of peripartum women will develop a depressive or anxiety disorder (Pawluski et al., 2017), and that maternal mental illness is detrimental not only to mothers’ general wellbeing but also offspring development (Manassis et al., 1994; Murray et al., 1996).
We recently found that parturient laboratory rats have 250% higher OTR autoradiographic binding in their DR compared to the DR of nulliparous female rats (Grieb and Lonstein, 2021), suggesting that high OTR activity in the DR is involved in the typical expression of social and affective behaviors in new mothers. One mechanism through which OTRs in the DR could achieve this is by modulating central DR serotonin release, including that which is necessary for the neuroplasticity associated with motherhood (Hoekzema et al., 2017; Kim et al., 2010). Motherhood-induced neuroplasticity includes the primary somatosensory cortex (S1) (Rosselet et al., 2006), where the S1 representation of the ventral skin doubles during lactation in rats (Xerri et al., 1994). This is consistent with the fact that somatosensation is absolutely essential for maternal caregiving (Stern and Johnson, 1989). Most studies of serotonin’s role in S1 plasticity focus on the barrel field (BF) representing the facial vibrissae pads, though, but show that serotonin reuptake inhibition increases neuronal plasticity (Guirado et al., 2009) and that serotonin can strengthen experience-dependent synaptic plasticity in response to cross-modal sensory deprivation (Jitsuki et al., 2011).
Serotonin also influences cortical plasticity by regulating the expression of perineuronal nets (PNNs) (Guirado et al., 2014). PNNs are extracellular matrix structures surrounding the somata and proximal dendrites of parvalbumin+ GABAergic interneurons in the mature cortex (Sorg et al., 2016). PNNs are thought to be physical and chemical barriers to neuronal plasticity (Krishnan et al., 2017; Krishnan et al., 2015). Increasing extracellular serotonin with fluoxetine decreases expression of PNNs in the cerebral cortex (Ohira et al., 2013). Therefore, OTR activity in the maternal serotonergic DR may regulate postpartum social and affective behaviors by altering reproduction-related plasticity in the female S1 and, by effect, mothers’ ability to sensitively detect cues from the offspring and then respond to them (Hoekzema et al., 2017; Kim et al., 2010; Rosselet et al., 2006; Xerri et al., 1994).
To investigate whether OTRs in the DR are necessary for postpartum female social and affective behaviors, we constructed and then injected an adeno-associated virus (AAV) permanently expressing a short-hairpin RNA (shRNA) targeting OTR mRNA into the DR. This was done in early pregnancy, before most reproduction-related adaptations in females’ brain and behavior occur (Mayer and Rosenblatt, 1984) and before the rise in DR OTRs is found (Grieb and Lonstein, 2021). We then observed a suite of social and affective behaviors during the postpartum period, as well as determined the density of PNNs and the length of serotonin-immunoreactive fibers in the mothers’ S1. We hypothesized that OTRs in the DR would be critical for the typical display of postpartum social and affective behaviors, and for normal PNN expression and serotonin-immunoreactive fiber length in the S1.
2. Methods and Materials
2.1. Subjects.
Female Long-Evans rats born and raised in our breeding colony at Michigan State University (MSU) were housed with 2 or 3 same-sex littermates in cages (48 cm x 28 cm x 16 cm) containing wood chip bedding, food, and water as described previously (Grieb et al., 2020). They were maintained on a 12:12 light/dark cycle (lights on 0700 hr) and temperature 22 ± 1°C. Estrous cycles were monitored by vaginal smearing, and subjects mated on a day of proestrous. Subjects were group housed until surgery occurred on pregnancy day 8, after which they were singly housed. Litters were later culled to 4♂/4♀. Procedures were in accordance with the NIH Guide for Care and Use of Laboratory Animals and approved by the MSU IACUC.
2.2. Creation of viral construct.
Candidate shRNAs against the laboratory rat OTR mRNA (NCBI sequence: NM_012871.3) were designed using validated methods (Benskey et al., 2018) and cloned into an AAV genome under H1 promotor control. The same genome also carried a GFP reporter under the control of the chicken β-actin promotor/cytomegalovirus enhancer promotor hybrid (pCBA). shRNA candidates were evaluated in vitro using a dual-luciferase (Firefly-Renilla) assay system, and the best candidate (CGG TGA AGA TGA CCT TCA T) was found to produce ~80% knockdown of OTR mRNA. A scrambled shRNA was used as a negative control. Vector genomes were packaged into an AAV9 capsid using a triple transfection protocol followed by recovery of vector from media and cells (Benskey et al., 2016).
2.3. Stereotaxic injections.
On pregnancy day 8, rats were anesthetized with ketamine and xylazine and a hole was drilled in the skull above the DR (A/P = 7.8 mm, M/L = 0.0 mm from bregma). One μL of OTR-shRNA (n = 16) or scrambled control vector (n = 10) was slowly injected (0.5 μL/5 min) through a Neuros Hamilton syringe −6.7 mm below the skull. The needle remained for 10 min and was then retracted slowly. Subjects received buprenorphine analgesic for two days post-operation and were then left undisturbed until parturition.
2.4. In vivo determination of OTR knockdown.
Before the behavioral studies were conducted, level of OTR knockdown was determined in vivo in a separate group of OTRKD- and Scramble-injected females that had received infusions during early pregnancy (n = 6/group). Within 3 hrs of giving birth, these dams were rendered unresponsive with CO2 and decapitated. All dams from both groups gave birth on the expected day of parturition and had normal-sized litters. Dams’ brains were removed, frozen, and stored at −80°C. Brains were cut coronally into 300-μm-thick sections to obtain three sections encompassing the DR (−7.3 to −8.3 mm from bregma). The DR was punched using a 1-mm-diameter micropuncher (Harris Micropunch) to examine OTR mRNA (Grieb et al., 2017). Because the vasopressin V1a receptor gene shares some homology with the OTR gene, PCR was also run for V1a receptor. PCR products were sequenced at the Genomics Core at MSU to confirm specificity. The ΔΔCT method calculated fold-change differences between groups, with OTR and V1a normalized to the “housekeeping” gene HPRT-1 (Schmittgen and Livak, 2008).
2.5. Undisturbed maternal behavior observations.
Dams’ home cage behavior was observed three times daily (0900, 1300, and 1500 hr) for 30 min each on postpartum days (PPD) 1-7 as previously described (Fig. 1A; Grieb et al., 2018). Maternal behaviors were recorded by spot checks every 30s. Frequencies of each behavior were totaled for each day and those totals used for data analyses. Relevant combinations of behaviors were also analyzed (i.e., erect maternal postures = kyphotic nursing + time in nest not nursing; passive maternal postures = prone + supine nursing; oral behaviors = sum of pup licking, self-grooming, and eating/drinking; non-pup directed behaviors = sum of exploring the home cage, eating/drinking, self-grooming, and resting away from the litter). Inter-rater reliability of >90% was established before beginning data collection.
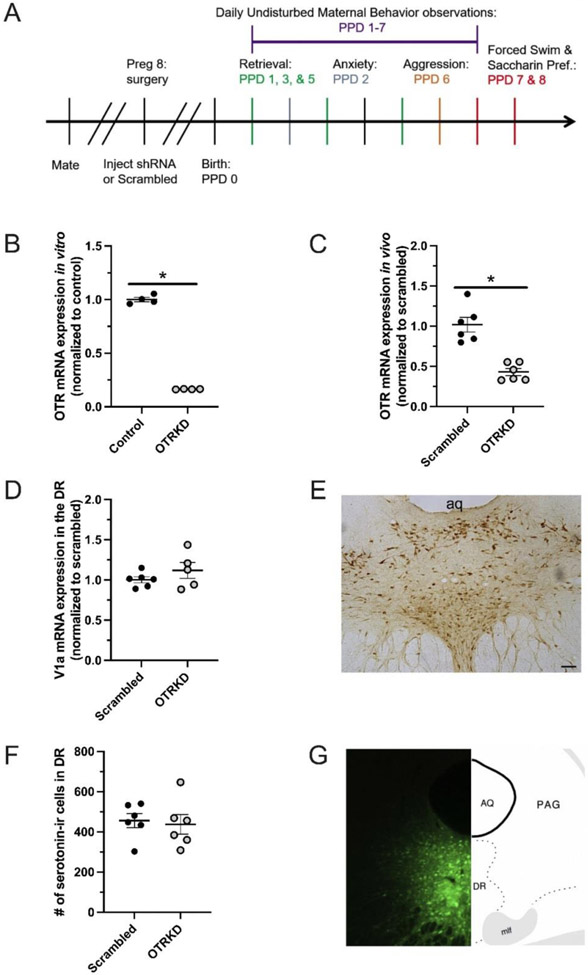
Figure 1. Validation of viral construct.
Experimental timeline (A). In vitro (B) and in vivo (C) OTR expression in the dorsal raphe (DR) of scrambled-vector treated (Scrambled) and shRNA-vector treated (OTRKD) cells and recently parturient mothers, respectively. Vasopressin V1a receptor expression in the DR of Scrambled and OTRKD dams (D). Representative photomicrograph of serotonin-immunoreactive cells in the DR (E). Number of serotonin-ir cells in the DR of Scrambled and OTRKD dams (F). Representative photomicrograph and brain atlas diagram showing distribution of viral vector in the DR (G). * indicates significant difference between groups, p < 0.05. Scale bar in D = 100 μm.
2.6. Pup Retrieval.
Litters were briefly removed to induce dams’ retrieval as an indicator of maternal motivation (Numan and Stolzenberg, 2009). On PPDs 1, 3, and 5 (Fig. 1A), immediately following undisturbed maternal behavior observations, litters were removed and placed in an incubator set to nest temperature (34 °C) for 15 min. The pups were then scattered in the home cage opposite the nest site. Latencies for the dams to retrieve each pup and hover over all of the pups in the nest were recorded. Maternal behaviors were then recorded by spot checks every 30s for an additional 10 min. Given that there were no main effects of PPD, nor any interactions between PPD and group on any behaviors, the behavioral results from the three retrieval tests were averaged together for final data analyses.
2.7. Elevated-plus maze.
On PPD 2 (Fig. 1A), following undisturbed maternal behavior observations (~1600-1700 hr), dams were brought in their home cage to a nearby room containing an elevated plus-maze (~28 lux in the opens arms, ~2 lux in the closed arms). Behavior was videorecorded and later scored with a computerized data acquisition system. An entry was coded when the dam placed her head and both front paws into an arm (Lonstein, 2005).
2.8. Maternal aggression.
On PPD 6 (Fig. 1A), following undisturbed maternal behavior observations (~1600-1700 hr), dams were brought in their home cage to a nearby behavior testing room and a smaller male intruder from our colony was placed in the dams cage with the pups present as previously described (Holschbach et al., 2018). Following the 10-min test, males were removed from the cage and sacrificed by CO2 asphyxiation. No pups were harmed during the tests. Females and litters were then returned to the colony room.
2.9. Saccharin preference.
A saccharin habituation period began in the morning of PPD 7 (Fig. 1A; ~0800 hr), when two water bottles (one containing 0.1% saccharin and another water) were weighed and placed in the home cage. Eight hr later, the bottles were weighed again, and their positions switched to avoid side preference confounds. Testing on the morning of PPD 8 (~0800 hr) involved both bottles being weighed, and then being removed from the cage along with food for 4 hr. Following deprivation, food and both water bottles were returned to the home cage and pups removed to limit potential distractions. Following a 1 hr testing period, the bottles were removed and weighed, and the regular water bottle returned. The change in bottle weights between pre- and post-1 hr testing was used for data analyses.
2.10. Forced swim.
Following undisturbed behavior observation on PPD 7 (Fig. 1A), dams were brought to a nearby testing room containing a 50 cm X 20 cm Plexiglas cylinder 40 cm full of 24 °C water for pre-exposure. Dams were placed in the cylinders and behavior recorded for 15 min. Dams were then removed from the cylinder, towel-dried, and placed into a new cage containing a prewarmed heating pad. Once dry, dams were returned to their home cages. The next day, 1 hr after saccharin preference testing the dams were brought back to the behavior testing room and placed into the water-filled cylinder for 10 min. After removal and drying, dams were placed in the home cage until sacrifice ~30 min later. Females’ behavior was scored from the digital recording with a computerized data acquisition system (Solomon Coder). The durations of time swimming/struggling and floating were coded and analyzed.
2.11. Euthanasia, perfusion, and brain extraction.
Approximately 30 min after forced swim testing, subjects were overdosed with pentobarbital and transcardially perfused with saline then 4% paraformaldehyde. Brains were extracted, postfixed overnight, and submerged in 30% sucrose for 2-3 days. Brains were cut into 40-μm sections in three series and stored in cryoprotectant at −20°C.
2.12. GFP immunohistochemistry for injection localization.
All rinses were in PBST. Sections were rinsed and blocked in 0.1% triton-PBS containing 2% NDS for 1 hr. Sections were then incubated in a rabbit anti-GFP primary antiserum (A6455, Thermo-Scientific; 1:10,000) for 24 hrs at 4°C, then donkey anti-rabbit Alexafluor 488 secondary antiserum (A21206, Fisher Scientific; 1:500) for 2 hrs at room temperature, and then mounted and coverslipped. The entirety of the midbrain was scanned for GFP-ir cells under 40X magnification. Four of the 16 shRNA-injected dams were removed from further analysis due to misplaced injections (see details in Results).
2.13. Serotonin immunohistochemistry.
To help verify that shRNA infusion did not grossly affect DR cell viability, three alternative sections per subject containing the DR (−7.5 to −8.4 mm from bregma) were analyzed for the number of serotonin-ir cells as done previously (Holschbach et al., 2018). Immunohistochemistry involved a monoclonal rabbit anti-serotonin primary antiserum (NT-102 5HTrab; Protos BiotechCorp; 1:10,000) and a biotinylated goat anti-rabbit secondary antiserum (Vector Laboratories; 1:1,000). The number of serotonin-ir cells in the DR, which was entirely captured within a standardized ROI, was counted on each section under 100X magnification. Somata with any visible serotonin immunoreactivity were included.
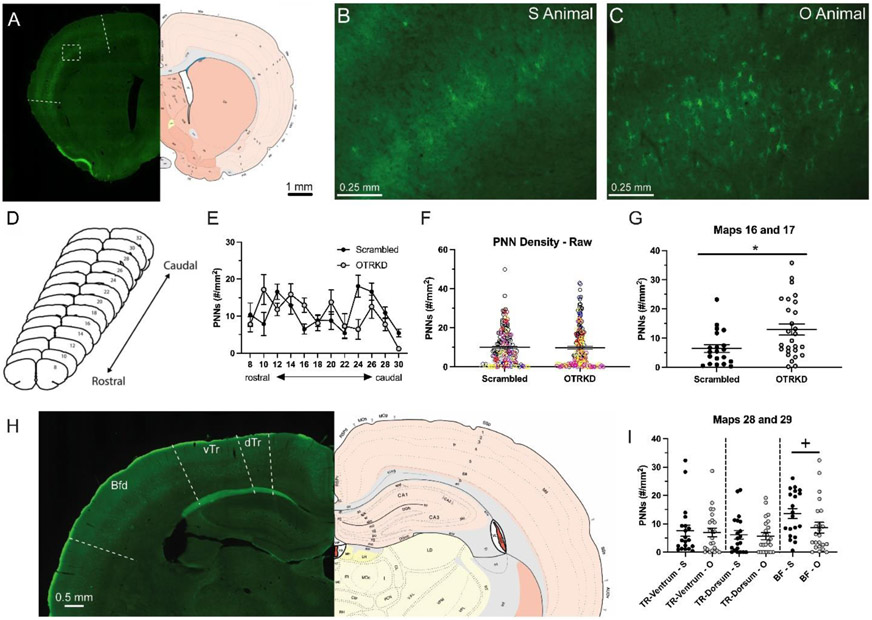
Additionally, because serotonin influences cortical plasticity by regulating the expression of PNNs (Guirado et al., 2014), we examined the effects of DR OTR knockdown on serotonin-ir fiber length (reflective of the capacity for serotonin release (Hritcu et al., 2007)) in S1 sites where significant effects on the numbers of PNNs were found (see below). This involved bilaterally analyzing one section each containing cortical maps 16-17 and maps 28-29 (n = 5/group) (Swanson, 2004). The length of serotonin-ir fibers was traced bilaterally using ImageJ software under 200X magnification. The total length of serotonin-ir fibers traced per subregion of the S1 for each subject was used for data analyses.
2.14. Perineuronal net visualization.
One series of alternate sections from each subject was rinsed 5 times in PBS, then incubated in fluorescently tagged-Wisteria floribunda lectin (FL-1351, Vector Laboratories; 1:200) for 2 hrs at room temperature in the dark. Tissue was then rinsed 5 times in PBS, mounted, and coverslipped using Fluormount-G. Analysis involved 23 images from 6 randomly selected OTRKD dams and 20 images from 6 randomly selected Scrambled controls. High magnification (10X) single-plane epifluorescent images were taken using a Keyence microscope (BZ-X710, Keyence Corp.) and stitched using BZ-X Analyzer (Keyence Corp). To identify subregions of the S1, each image was incorporated into ImageJ and overlaid with the corresponding map number based on the Swanson rat atlas (Swanson, 2004). The S1 BF (S1BF) area was identified and outlined based on the map delineations. To count high-intensity PNNs, we followed previously published protocols (Lau et al., 2020), detailed protocol available at dx.doi.org/10.17504/protocols.io.bcf8itrw. Briefly, from the ImageJ browser, in the “Brightness/Contrast” window, the rolling bar in “Contrast” was moved all the way to the right. This option eliminated all weak PNN signal from the image. In this maximized contrast view, all PNN structures were counted if they maintained 100% of their original shape (before contrast adjustment). This was the only difference compared to our previously published protocol (Lau et al., 2020). The density was calculated as the number of PNNs divided by the area analyzed.
2.15. Statistical analyses.
All data were analyzed using SPSS software. Undisturbed maternal behavior data were analyzed using repeated-measures ANOVAs (with Greenhouse-Geisser correction) involving PPD as the repeated measure and knockdown condition as the between-subject variable. In cases of statistical significance, Holm-Bonferroni post-hoc tests corrected for multiple comparisons were conducted to compare across PPD and between groups. Percentage of subjects having pup loss was analyzed with a Fisher’s exact test. Two dams lost their entire litters and the replacement litters provided, so were removed from analyses starting from the point they lost their first litters. Another three dams lost 2-3 pups, but after quickly providing replacement pups were able to maintain the litter and remained in the study. Retrieval, aggressive, forced-swim, saccharine preference, and anxiety-like behaviors, along with serotonin-immunoreactive fiber length were analyzed using two-tailed Student’s t-tests. For the PNN data and in other cases of unequal variances Welch’s t-tests were used. Partial η2 (η2p) are reported as measures of effect sizes from the ANOVAs and Welch’s t-tests, Cohen’s d are reported as measures of effect sizes for the Student’s t-tests, and φ is reported as a measure of effect size for the Fisher’s exact test. p < 0.05 was considered statistically significant.
3. Results
3.1. OTR shRNA vector validation.
When tested in vitro, our shRNA vector produced ~80% knockdown of OTR mRNA, whereas the scrambled vector had no effect (Fig 1B). When tested in vivo, the shRNA vector injected into the DR on pregnancy day 8 resulted in ~60% knockdown of OTR mRNA when animals were euthanized postpartum (Fig 1C). Our OTR shRNA vector did not affect V1a receptor mRNA expression (Fig 1D) or the number of serotonin-ir cells in the DR (Figs 1E, F). In all cases (16 shRNA-injected dams), GFP was found in the DR (Fig 1G). However, four of the 16 shRNA-injected dams were removed from final analysis due to misplaced injections. In two cases there was minimal GFP in the DR, but GFP was present in the nearby lateral periaqueductal grey. In two other cases, GFP was found in the DR but spread rostrally to the Edinger-Westphal nucleus and ventrally to the ventral tegmental area.
3.2. OTRs in the DR regulate maternal behaviors.
Five of the twelve OTR-knockdown mothers lost >2 pups within 48 hrs after giving birth; the controls lost no pups (N = 22, p = 0.04, 95% CI [1.06,2.77], φ = 0.50; Fig 2A). During the daily undisturbed caregiving observations, OTR-knockdown mothers spent less time than the controls in any type of physical contact with pups (F(1,18) = 17.56, p < 0.01, η2p = 0.49; Fig 2B). There was also an interaction between knockdown condition and postpartum day (PPD) on time spent nursing, such that OTR-knockdown mothers spent less time than the controls nursing on most days starting on PPD 3 (F(6, 108) = 3.91, p = 0.01, η2p = 0.18; Fig 2C). This interaction was mostly driven by the reduction in kyphotic (i.e., arched-back) nursing in OTR-knockdown mothers (F(6, 108) = 3.54, p = 0.01, η2p = 0.17; Fig 2D). However, knockdown and control mothers spent the same amount of time in the nest with pups while not nursing them (F(1, 18) = 1.66; p = 0.21, η2p = 0.09; Table 1). Despite the differences in time spent nursing, there were no differences in litter weight change across the study (t(18) = 1.12, p = 0.28; d = 0.50). There was also an interaction between knockdown condition and PPD on the frequency of non-pup directed behaviors and perioral activities, such that OTR-knockdown mothers spent more time than controls displaying non-pup directed behaviors on most days starting on PPD 3 (F(6, 108) = 4.22, p < 0.01, η2p = 0.19; Fig 2E) and showed more oral activities on most days also starting on PPD 3 (F(6, 108) = 3.77, p < 0.01, η2p = 0.17; Fig 2F). This included more feeding (F(1, 18) = 19.22, p < 0.01, η2p = 0.52; Fig 2G), which is consistent with their weight gain across the study, as opposed to the small weight loss by the controls (t(18) = 2.43, p = 0.03, d = 1.06; Fig 2H). There were no effects of OTR-knockdown on the other behaviors recorded during the undisturbed observations or during the retrieval tests (Table 1).
Figure 2. Effects of oxytocin receptor knockdown in the dorsal raphe on maternal caregiving behaviors.
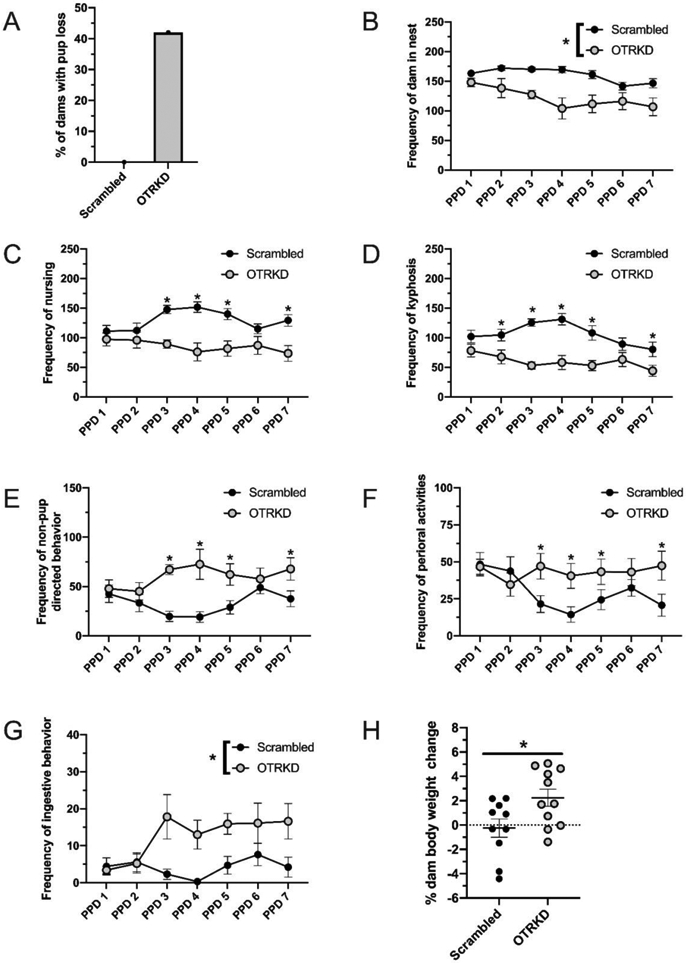
Percentage of dams with pup loss (A) and dam body weight change (G) of scrambled-vector treated (Scrambled) and shRNA-vector treated (OTRKD) mothers. Frequency of mothers in the nest (B), nursing (C), nursing in a kyphotic posture (D), non-pup directed behavior (E), perioral activity (F), and ingestive behaviors (G) by Scrambled and OTRKD dams. All data are presented as Mean ± SEM. * above bars indicates significant differences between groups, p < 0.05.
Table 1: Undisturbed maternal and retrieval behaviors.
Frequencies (Mean ± SEM) of maternal behaviors displayed by Scrambled and OTR knockdown (OTRKD) dams during three 30-min observations each day on postpartum days 2 – 8 and following retrieval testing.
| Undisturbed Maternal Behavior Tests |
Scrambled (M±SEM) |
OTRKD (M±SEM) |
Group (F(1, 18); p; η2p) | Day (F(6); p; η2p) | Interaction (F6,108); p; η2p) |
|---|---|---|---|---|---|
| Dam in nest | 1124 ± 24 | 916 ± 44 | 17.56; <0.01; 0.49 | 4.86; <0.01*; 0.21 | 2.57; >0.05; 0.13 |
| All Nursing | 918 ± 39 | 652 ± 56 | 15.16; <0.01; 0.46 | 0.82; 0.52; 0.04 | 3.91; 0.01; 0.18 |
| Kyphosis | 741 ± 37 | 455 ± 48 | 22.14; <0.01; 0.55 | 3.40; 0.01*; 0.16 | 3.54; 0.01; 0.17 |
| Supine nursing | 79 ± 20 | 76 ± 30 | 0.01; 0.92; <0.01 | 5.81; <0.01^;0.24 | 0.90; 0.45; 0.05 |
| Prone nursing | 97 ± 17 | 122 ± 12 | 1.33; 0.26; 0.07 | 1.72; 0.15; 0.09 | 1.36; 0.25; 0.07 |
| Hovering over the litter | 206 ± 31 | 264 ± 31 | 1.66; 0.21; 0.09 | 5.12; <0.01*; 0.16 | 1.01; 0.41; 0.05 |
| Erect postures (hovering over + kyphosis) | 947 ± 29 | 718 ± 40 | 21.19; <0.01; 0.54 | 8.68; <0.01*; 0.33 | 2.22 ; 0.07; 0.11 |
| Passive postures (supine + prone nursing | 177 ± 23 | 198 ± 35 | 0.25 ; 0.62; 0.01 | 3.42; 0.01^; 0.16 | 1.44; 0.23; 0.07 |
| Licking pups | 105 ± 19 | 140 ± 25 | 1.32; 0.27; 0.07 | 3.79; 0.01^; 0.17 | 0.81; 0.52; 0.04 |
| Retrieval | 0.9 ± 0.8 | 1.9 ± 1.1 | 0.52 ; 0.48; 0.03 | - | - |
| Non-pup directed behavior | 230 ± 26 | 442 ± 41 | 18.87; <0.01; 0.51 | 1.44; 0.22; 0.07 | 4.22; <0.01; 0.19 |
| Nesting | 13 ± 5 | 31 ± 8 | 3.29; 0.09; 0.16 | 1.66; 0.18; 0.09 | 1.54; 0.21; 0.08 |
| Nest quality | 38 ± 3 | 27 ± 5 | 4.67; 0.04; 0.21 | 4.55; <0.01*; 0.20 | 2.00; 0.10; 0.10 |
| Self-grooming | 72 ± 10 | 115 ± 12 | 7.80; 0.01; 0.30 | 0.84; 0.51; 0.05 | 4.04; <0.01; 0.18 |
| Eating/drinking | 29 ± 6 | 94 ± 14 | 19.22; <0.01; 0.52 | 2.02; 0.10; 0.10 | 2.27; 0.07; 0.11 |
| Perioral activity (self-grooming, eating/drinking, licking pups) | 206 ± 30 | 350 ± 30 | 11.74; <0.01; 0.40 | 0.96; 0.43; 0.05 | 3.77; <0.01; 0.17 |
| Exploring | 63 ± 10 | 136 ± 16 | 15.00; <0.01; 0.46 | 1.10; 0.36; 0.06 | 2.77; 0.04; 0.13 |
| Sleeping away from pups | 53 ± 12 | 56 ± 17 | 0.35; 0.56; 0.02 | 3.47; 0.01^; 0.16 | 0.56; 0.69; 0.03 |
| Pup Retrieval Tests | |||||
| Latency to retrieve first pup (s) | 19 ± 5 | 27 ± 9 | 0.23; 0.83; 0.10 | - | - |
| Latency to group all pups (s) | 173 ± 23 | 186 ± 23 | 0.45; 0.66; 0.20 | - | - |
| Kyphosis | 0.6 ± 0.4 | 0.5 ± 0.3 | 0.31; 0.76; 0.14 | - | - |
| Hovering over litter | 16 ± 1 | 15 ± 1 | 1.14; 0.27; 0.53 | - | - |
| Licking pups | 9 ± 1 | 8 ± 1 | 1.52; 0.15; 0.67 | - | - |
| Non-pup directed behavior | 10 ± 1 | 11 ± 2 | 0.09; 0.93; 0.55 | - | - |
| Self-grooming | 3 ± 1 | 3 ± 0.4 | 0.94; 0.36; 0.40 | - | - |
| Exploring | 7 ± 1 | 8 ± 1 | 1.26; 0.22; 0.57 | - | - |
indicates increasing frequency of behavior across postpartum day.
indicates decreasing frequency of behavior across postpartum day.
3.3. OTRs in the DR regulate aggressive and affective behaviors.
OTR knockdown in the DR increased the number of attacks that mothers made against male intruders and the time spent attacking them (t(16) = 3.96, p < 0.01, d = 1.95 and t(16) = 3.52, p < 0.01, d = 1.58, respectively; Figs 3A,B). OTR-knockdown mothers were also faster than control mothers to begin attacking (t(16) = 5.47, p < 0.01, d = 2.98; Fig 3C).
Figure 3. Effects of oxytocin receptor knockdown in the dorsal raphe on maternal affective behaviors.
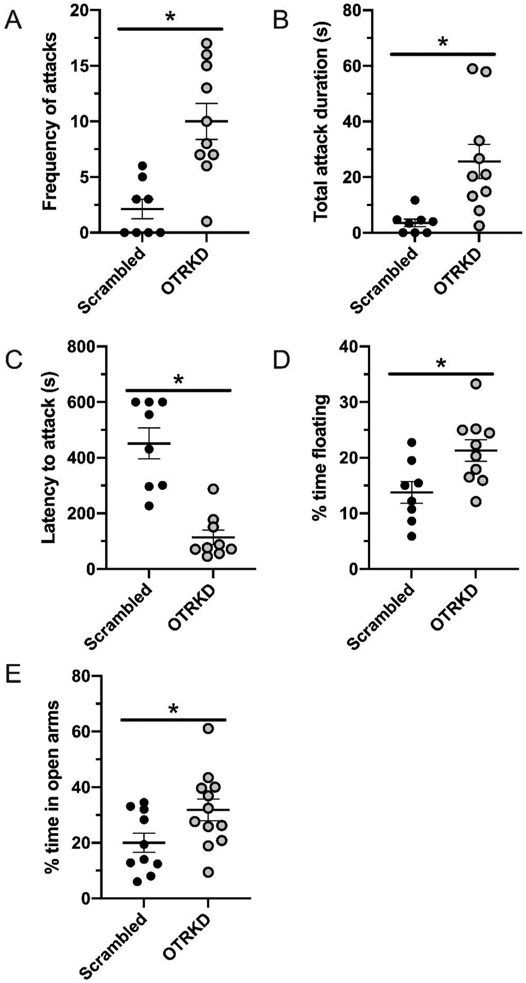
Frequency of attacks (A) by scrambled-vector treated (Scrambled) and oxytocin receptor knockdown (OTRKD) dams. Total duration of attacks (B) and latency to attack (C) by Scrambled and OTRKD dams. Percentage of time floating in the forced swim test (D) and time in the open arm of an elevated plus maze (E) by Scrambled and OTRKD dams. All data are presented as Mean ± SEM. * above bars indicates significant differences between groups, p < 0.05.
With regards to affective behaviors, OTR-knockdown mothers spent more time floating in the forced-swim test (t(16) = 2.70, p = 0.02, d = 1.29; Fig 3D), but there were no differences between groups in saccharine intake (t(16) = 1.03, p = 0.32; d = 0.48). OTR knockdown unexpectedly decreased anxiety-like behavior in the elevated plus maze, as indicated by a significantly greater percentage time spent in the open arms (t(20) = 2.20, p = 0.04, d = 0.95; Fig 3E). There were no differences between groups in locomotor activity as indicated by the number of closed-arm entries (t(20) = 1.25, p = 0.23; d = 0.55).
3.4. Primary somatosensory cortex perineuronal nets and serotonin-immunoreactive fiber length.
PNNs were visible throughout the rostrocaudal axis of the S1 (Figs 4A-E). When examining the entire S1 there was no effect of OTR knockdown on PNN density (t(379) = 0.13, p = 0.90, η2p < 0.01; Fig 4F). There were, however, significant differences between groups in some rostrocaudal levels and subregions of the S1. Specifically, at levels 16 and 17, which contain the microvibrissae areas of the rostral BF cortex (Brecht et al., 1997), PNN density with significantly higher in OTR-knockdown mothers than in the controls (t(43) = 2.77, p < 0.01, η2p = 0.15; Fig 4G). Conversely, more caudally at maps 28 and 29, which contains both the trunk and macrovibrissae BF subregions (Fig 4H), we found that neither the ventral (t(37) = 0.23, p = 0.82, η2p < 0.01; Fig 4I) nor dorsal trunk (t(38) = 0.26, p = 0.80, η2p < 0.01; Fig 4I) representations in the S1 were affected by OTR knockdown, although OTR-knockdown dams showed a trend for lower PNN density in the BF compared to the controls (t(41) = 1.93, p = 0.06, η2p = 0.08; Fig 4I).
Figure 4. Effects of oxytocin receptor knockdown in the dorsal raphe on perineuronal nets in the primary somatosensory cortex.
Representative photomicrographs (A, B, C, H) and brain atlas plates (A, H) showing perineuronal net (PNN) expression in the primary somatosensory cortex (S1) of scrambled-vector treated (Scrambled) and shRNA-vector treated (OTRKD) mothers. Rostrocaudal extent of the S1 (D) and PNN density by map (E) in Scrambled and OTRKD mothers. (F) Statistical analysis of averaged PNN densities of combined rostral-caudal data revealed no significant difference between scrambled-vector treated and shRNA-vector treated mothers. Note: Each dot represents PNN density of one brain hemisphere, different colors represent different animals. PNN density from maps #16 and 17 (G) of the S1 in Scrambled and OTRKD mothers. PNN density in the ventrum (TR-Ventrum), dorsum (TR-Dorsum), and barrel field (BF) from maps #28 and 29 (H) of the S1 in Scrambled (S) and OTRKD (O) mothers. All data are presented as Mean ± SEM. * indicates significant difference between groups, p < 0.05. + indicates a trend, p < 0.01.
Serotonin-ir fiber length in the BF on maps 16 and 17 (t(8) = 1.17, p = 0.28, d = 0.74), or on maps 28 and 29 (t(8) = 0.14, p = 0.89, d = 0.09), was unaffected by OTR knockdown. Similarly, there were no effects of OTR knockdown on the length of serotonin-ir fibers in the dorsal (t(8) = 1.18, p = 0.27, d = 0.75) or ventral (t(8) = 1.37, p = 0.21, d = 0.87) trunk regions of the S1 on maps 28 and 29.
4. Discussion
Inhibiting OTR activity in the midbrain raphe nuclei of male rodents decreases social reward (Dölen et al., 2013), increases anxiety-like behaviors (Yoshida et al., 2009), and decreases or does not affect aggression (Calcagnoli et al., 2015; Pagani et al., 2015). However, the influence of DR OTRs on social and affective behaviors in naturally postpartum females has not previously been investigated. Given the high endogenous OT in mothers (Neumann et al., 1993), and our recent finding that new mother rats have over twice as much OTR autoradiographic binding in the DR compared to non-mothers (Grieb and Lonstein, 2021), we hypothesized that OTRs in the DR would be critical for the normal display of social and affective behaviors in postpartum rats. Supporting our hypothesis, we found that OTR-knockdown in the maternal DR led to pup loss soon after parturition, decreased nursing, increased aggression, increased behavioral despair, and decreased anxiety-like behavior.
OTR-knockdown mothers spent ~20% less time than controls in physical contact with their litters during the daily undisturbed observations. This does not appear to be driven by a lack of maternal motivation, given that the OTR knockdown did not affect dams’ latency or duration to retrieve scattered pups (which reflects maternal motivation (Numan and Stolzenberg, 2009)). This reduced pup contact by OTR-knockdown mothers could have led to their high pup loss (which is extremely rare in untreated postpartum female laboratory rats) because harming the young is usually inhibited by the almost constant physical contact between mothers and pups during the very early postpartum period (Jakubowski and Terkel, 1985). Importantly, the nature of this reduction in mother-pup contact in our OTR-knockdown group was largely due to nursing, with OTR-knockdown mothers spending only ~70% as much time nursing as did the controls. OTR-knockdown mothers did spend a normal amount of time in the nest not nursing, though, again suggesting that there was no general aversion to the litter. This deficit in nursing after DR OTR knockdown may instead have resulted from their inability to sensitively detect tactile inputs from the young, specifically those directed to the mother’s rostral snout. Mother rats require snout inputs from young pups to not only successfully retrieve and lick them, but also for the normal transition from active caregiving to quiescent nursing (Stern and Johnson, 1989). Our finding that OTR-knockdown dams had denser expression of plasticity-restricting PNNs in the rostral, microvibrissae-representing BF of the S1 suggests inhibited cortical plasticity and perhaps impaired ability to use tactile perception via the snout to detect the pups’ features. In support, the same region of the BF is known to be necessary for object recognition in male and female rats (Brecht et al., 1997). A lack of microvibrissae perception could also explain why OTR-knockdown mothers spent more time than controls performing perioral activities including self-grooming, licking, and ingestive behaviors. This decrease in nursing and persistence in oral behaviors is very similar to what occurs when mother rats cannot process pup tactile inputs due to trigeminal nerve lesions or mystacial pads anesthetization (Stern and Johnson, 1989).
In addition to DR OTR-knockdown mothers having denser expression of plasticity-restricting PNNs in the rostral BF, we found a trend for less dense expression of PNNs in their caudal, macrovibrissae representing BF cortex. This region is involved in distance decoding (Brecht et al., 1997), which could be useful for navigational tasks associated with mothering. Less dense expression of restrictive PNNs could have promoted plasticity in this caudal, macrovibrissae region, which could explain why OTR-knockdown mothers did not show deficits in highly spatial-dependent pup retrieval. The more general finding of sub-regional differences in PNN density in the maternal S1 are novel findings in laboratory rats, but were recently reported in maternal mice (Lau et al., 2020). The potential impact of these sub-regional differences in PNN density on different aspects of maternal care in either species remains to be elucidated. Given that serotonin influences cortical plasticity by regulating the expression of PNNs (Guirado et al., 2014), we were surprised to find that there were no effects of OTR knockdown on serotonin-ir fiber length in the BF regions representing the micro- and macrovibrissae regions. However, more direct measures of serotonin release or turnover into those regions using HPLC or in vivo microdialysis could reveal effects of DR OTR manipulations. It is also possible that DR OTR knockdown affected serotonin function in the S1 earlier than when we examined the brains our subjects (i.e., PPD8), such that it DR OTR effects are more involved in establishing PNNs and other aspects of S1 plasticity in the first few days postpartum when mothers first begin to interact with pups, rather than for maintaining it later in the postpartum period.
OTR knockdown not only decreased aspects of maternal caregiving, but also dramatically increased aggressive behavior and behavioral despair. Specifically, we found that the OTR knockdown mothers showed >3-fold more attacks and >6-fold more time attacking male intruders to the home cage. Given that many serotonin neurons in the DR express OTRs (Grieb and Lonstein, 2021), this elevated aggression by OTR-knockdown dams is consistent with our group’s prior work demonstrating that serotonin-selective lesions of the DR decrease postpartum maternal aggression (Holschbach et al., 2018). Our results also demonstrate that the normally elevated OTRs in the maternal DR are involved in postpartum affective behaviors, as OTR knockdown in the DR increased the time that mothers spent floating in the forced-swim test, indicative of less active coping and greater behavioral despair (Slattery and Cryan, 2012). Knockdown did not increase all stress-related behaviors, though, as seen by the similar saccharin preference (a reflection of hedonic pleasure (Liu et al., 2018)) between groups. Thus, OTRs in the DR could be a valuable target to particularly improve coping style in depressed individuals. This could be especially important during motherhood, given that SSRIs are the first-line pharmacotherapies during pregnancy and postpartum (Hayes et al., 2012), but do not improve active coping in stressed maternal rats (Pawluski et al., 2012).
In addition to affecting active coping/behavioral despair, OTR knockdown in the maternal DR reduced anxiety-related behaviors. Because central OT is anxiolytic in non-parous rodents (Jurek and Neumann, 2018), as well as after infusion into the ventral periaqueductal gray and mPFC of postpartum females (Figueira et al., 2008; Sabihi et al., 2014), this reduction of anxiety was unexpected. Consistent with our results, though, increased OTR expression in the DR of female hamsters following hormone-simulated pseuodopregnancy was very recently shown to be anxiogenic (Hedges et al., 2021). These results collectively suggest that oxytocin’s effects on anxiety are brain-site specific, with OTR activity in the DR having anxiogenic effects. This may be due to elevated serotonin release particularly in the bed nucleus of the stria terminalis, which increases anxiety-like behaviors (at least in males; Hammack et al., 2009).
While we were careful to design the order of behavior testing from the least likely stressful to the most likely stressful (Fig. 1A), it could be possible that the timeline of testing might have affected our behavioral results. However, OTR knockdown does not seem to have caused any generalized vulnerability to stress, as it led to less anxiety-like behavior, more passive coping, and no difference in saccharine preference. Taken together, this suggests a more specific and unique role of OTRs in the DR in regulating specific socioemotional behaviors.
In sum, the present study expands our understanding of the neuropeptidergic control of postpartum behaviors by demonstrating that high OTR expression in the midbrain DR is essential for the normal display of postpartum social and affective behaviors. The influence of DR OTRs on PNN expression is likely critical for S1 plasticity underlying maternal perception of pups, and how tactile cues from pups regulate their mother’s social and affective behaviors. Our results also suggest an intriguing sex difference in the role of OTRs in the DR. Previous studies have found that OTRs in the DR enhance social reward, decrease anxiety-like behaviors, and increase aggression in male rodents. In contrast, we found that OTRs in the DR do not normally increase social motivation (at least for pup retrieval), are anxiogenic, and decrease aggression in postpartum females. Thus, these data extend previous research suggesting fundamental sex differences in the neurochemical regulation of social and affective behaviors (Caldwell, 2018), with implications for how to best consider oxytocin as a treatment for psychiatric disorders in males and in females.
Highlights:
Oxytocin receptor knockdown (OTRKD) in the dorsal raphe affected mothering
OTRKD in the dorsal raphe increased maternal aggression
OTRKD in the dorsal raphe increased behavioral despair
OTRKD in the dorsal raphe decreased anxiety-like behavior
OTRKD in the dorsal raphe disrupted cortical plasticity
Acknowledgements
These studies were funded by NICHD HD097085 awarded to Joseph S. Lonstein. The authors would also like to thank Dr. Erika Vitale, Maggie Ahearn, and Katrina Linning for their assistance with the undisturbed maternal behavior observations.
Footnotes
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data availability statement
Data will be made available with reasonable request.
References
- Benskey MJ, Sandoval IM, & Manfredsson FP (2016). Continuous collection of adeno-associated virus from producer cell medium significantly increases total viral yield. Human gene therapy methods, 27(1), 32–45. [DOI] [PubMed] [Google Scholar]
- Benskey MJ, Sellnow RC, Sandoval IM, Sortwell CE, Lipton JW, & Manfredsson FP (2018). Silencing alpha synuclein in mature nigral neurons results in rapid neuroinflammation and subsequent toxicity. Frontiers in molecular neuroscience, 11, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecht M, Preilowski B, & Merzenich MM (1997). Functional architecture of the mystacial vibrissae. Behavioural brain research, 84(1-2), 81–97. [DOI] [PubMed] [Google Scholar]
- Calcagnoli F, Stubbendorff C, Meyer N, de Boer SF, Althaus M, & Koolhaas JM (2015). Oxytocin microinjected into the central amygdaloid nuclei exerts anti-aggressive effects in male rats. Neuropharmacology, 90, 74–81. [DOI] [PubMed] [Google Scholar]
- Caldwell HK (2018). Oxytocin and sex differences in behavior. Current opinion in behavioral sciences, 23, 13–20. [Google Scholar]
- Dölen G, Darvishzadeh A, Huang KW, & Malenka RC (2013). Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature, 501(7466), 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson ZR, & Young LJ (2008). Oxytocin, vasopressin, and the neurogenetics of sociality. Science, 322(5903), 900–904. [DOI] [PubMed] [Google Scholar]
- Feifel D, Macdonald K, Nguyen A, Cobb P, Warlan H, Galangue B, Perry W (2010). Adjunctive intranasal oxytocin reduces symptoms in schizophrenia patients. Biological psychiatry, 68(7), 678–680. [DOI] [PubMed] [Google Scholar]
- Figueira RJ, Peabody MF, & Lonstein JS (2008). Oxytocin receptor activity in the ventrocaudal periaqueductal gray modulates anxiety-related behavior in postpartum rats. Behavioral neuroscience, 122(3), 618. [DOI] [PubMed] [Google Scholar]
- Grieb Z, Holschbach M, Lonstein JS (2018). Interaction between postpartum stage and litter age on maternal caregiving and medial preoptic area orexin. Physiology and behavior, 194, 430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieb Z, Tierney S, Lonstein J (2017). Postpartum inhibition of ovarian steroid action increases aspects of maternal caregiving and reduces medial preoptic area progesterone receptor expression in female rats. Hormones and behavior, 96, 31–41. [DOI] [PubMed] [Google Scholar]
- Grieb ZA, & Lonstein JS (2021). Oxytocin receptor expression in the midbrain dorsal raphe is dynamic across female reproduction in rats. Journal of neuroendocrinology, 33(2), e12926. [DOI] [PubMed] [Google Scholar]
- Grieb ZA, Vitale EM, Morrell JI, Lonstein JS, & Pereira M (2020). Decreased mesolimbic dopaminergic signaling underlies the waning of maternal caregiving across the postpartum period in rats. Psychopharmacology, 1–13. [DOI] [PubMed] [Google Scholar]
- Guirado R, Perez-Rando M, Sanchez-Matarredona D, Castrén E, & Nacher JJ (2014). Chronic fluoxetine treatment alters the structure, connectivity and plasticity of cortical interneurons. Neuropsychopharmacology, 17(10), 1635–1646. [DOI] [PubMed] [Google Scholar]
- Guirado R, Varea E, Castillo-Gómez E, Gómez-Climent M, Rovira-Esteban L, Blasco-Ibáñez J, Nacher J (2009). Effects of chronic fluoxetine treatment on the rat somatosensory cortex: activation and induction of neuronal structural plasticity. Neuroscience letters, 457(1), 12–15. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Guo J-D, Hazra R, Dabrowska J, Myers KM, & Rainnie DG (2009). The response of neurons in the bed nucleus of the stria terminalis to serotonin: implications for anxiety. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 33(8), 1309–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes RM, Wu P, Shelton RC, Cooper WO, Dupont WD, Mitchel E (2012). Maternal antidepressant use and adverse outcomes: a cohort study of 228,876 pregnancies. American journal od obstetrics and gynecology, 207(1), 49. e41–49. e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges VL, Heaton EC, Amaral C, Benedetto LE, Bodie CL, D’Antonio BI, O’Sullivan EC (2020). Estrogen withdrawal increases postpartum anxiety via oxytocin plasticity in the paraventricular hypothalamus and dorsal raphe nucleus. Biological psychiatry, 89(9), 929–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekzema E, Barba-Müller E, Pozzobon C, Picado M, Lucco F, García-García D, Crone EA (2017). Pregnancy leads to long-lasting changes in human brain structure. Nature neuroscience, 20(2), 287. [DOI] [PubMed] [Google Scholar]
- Holschbach MA, Vitale EM, & Lonstein JS (2018). Serotonin-specific lesions of the dorsal raphe disrupt maternal aggression and caregiving in postpartum rats. Behavioural brain research, 348, 53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hritcu L, Clicinschi M, Nabeshima TJP, & behavior. (2007). Brain serotonin depletion impairs short-term memory, but not long-term memory in rats. Physiology and behavior, 91(5), 652–657. [DOI] [PubMed] [Google Scholar]
- Jakubowski M, & Terkel JJ (1985). Incidence of pup killing and parental behavior in virgin female and male rats (Rattus norvegicus): Differences between Wistar and Sprague-Dawley stocks. Journal of comparative psychology, 99(1), 93. [PubMed] [Google Scholar]
- Jitsuki S, Takemoto K, Kawasaki T, Tada H, Takahashi A, Becamel C, Ziff EB (2011). Serotonin mediates cross-modal reorganization of cortical circuits. Neuron, 69(4), 780–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurek B, & Neumann ID(2018). The oxytocin receptor: from intracellular signaling to behavior. Physiological reviews, 98(3), 1805–1908. [DOI] [PubMed] [Google Scholar]
- Kim P, Leckman JF, Mayes LC, Feldman R, Wang X, & Swain JE (2010). The plasticity of human maternal brain: longitudinal changes in brain anatomy during the early postpartum period. Behavioral neuroscience, 124(5), 695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan K, Lau BY, Ewall G, Huang ZJ, & Shea SD(2017). MECP2 regulates cortical plasticity underlying a learned behaviour in adult female mice. Nature communications, 8, 14077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan K, Wang B-S, Lu J, Wang L, Maffei A, Cang J, & Huang ZJ (2015). MeCP2 regulates the timing of critical period plasticity that shapes functional connectivity in primary visual cortex. Proceedings of the National Academy of Sciences, 112(34), E4782–E4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau BY, Krishnan K, Huang ZJ, & Shea SD (2020). Maternal experience-dependent cortical plasticity in mice is circuit-and stimulus-specific and requires MECP2. Journal of Neuroscience, 40(7), 1514–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M-Y, Yin C-Y, Zhu L-J, Zhu X-H, Xu C, Luo C-X, Zhou Q-G (2018). Sucrose preference test for measurement of stress-induced anhedonia in mice. Nature protocols, 13(7), 1686–1698. [DOI] [PubMed] [Google Scholar]
- Lonstein JS (2005). Reduced anxiety in postpartum rats requires recent physical interactions with pups, but is independent of suckling and peripheral sources of hormones. Hormones and behavior, 47(3), 241–255. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Evans AK, Gasser PJ, Hale MW, Staub DR, & Shekhar A (2008). Topographic organization and chemoarchitecture of the dorsal raphe nucleus and the median raphe nucleus Serotonin and sleep: molecular, functional and clinical aspects (pp. 25–67): Springer. [Google Scholar]
- Manassis K, Bradley S, Goldberg S, Hood J, & Swinson RP (1994). Attachment in mothers with anxiety disorders and their children. Journal of the American Academy of Child & Adolescent Psychiatry, 33(8), 1106–1113. [DOI] [PubMed] [Google Scholar]
- Mayer AD, & Rosenblatt JS (1984). Prepartum changes in maternal responsiveness and nest defense in Rattus norvegicus. Journal of comparative psychology, 98(2), 177. [PubMed] [Google Scholar]
- Murray L, Fiori-Cowley A, Hooper R, & Cooper P (1996). The impact of postnatal depression and associated adversity on early mother-infant interactions and later infant outcome. Child development, 67(5), 2512–2526. [PubMed] [Google Scholar]
- Neumann I, Russell J, & Landgraf R (1993). Oxytocin and vasopressin release within the supraoptic and paraventricular nuclei of pregnant, parturient and lactating rats: a microdialysis study. Neuroscience, 53(1), 65–75. [DOI] [PubMed] [Google Scholar]
- Numan M, & Stolzenberg DS (2009). Medial preoptic area interactions with dopamine neural systems in the control of the onset and maintenance of maternal behavior in rats. Frontiers in neuroendocrinology, 30(1), 46–64. [DOI] [PubMed] [Google Scholar]
- Ohira K, Takeuchi R, Iwanaga T, & Miyakawa TJ (2013). Chronic fluoxetine treatment reduces parvalbumin expression and perineuronal netsin gamma-aminobutyric acidergic interneurons of the frontal cortex in adultmice. Molecular brain, 6(1), 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani JH, Williams Avram S, Cui Z, Song J, Mezey É, Senerth JM, Young WS (2015). Raphe serotonin neuron-specific oxytocin receptor knockout reduces aggression without affecting anxiety-like behavior in male mice only. Genes, Brain and Behavior, 14(2), 167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawluski JL, Charlier TD, Fillet M, Houbart V, Crispin HT, Steinbusch HW, & van den Hove DL (2012). Chronic fluoxetine treatment and maternal adversity differentially alter neurobehavioral outcomes in the rat dam. Behavioural brain research, 228(1), 159–168. [DOI] [PubMed] [Google Scholar]
- Pawluski JL, Lonstein JS, & Fleming AS (2017). The neurobiology of postpartum anxiety and depression. Trends in Neurosciences, 40(2), 106–120. [DOI] [PubMed] [Google Scholar]
- Rosselet C, Zennou-Azogui Y. i., & Xerri CJ (2006). Nursing-induced somatosensory cortex plasticity: temporally decoupled changes in neuronal receptive field properties are accompanied by modifications in activity-dependent protein expression. Journal of neuroscience, 26(42), 10667–10676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabihi S, Dong SM, Durosko NE, & Leuner BJ (2014). Oxytocin in the medial prefrontal cortex regulates maternal care, maternal aggression and anxiety during the postpartum period. Frontiers in behavioral neuroscience, 8, 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD, & Livak KJ (2008). Analyzing real-time PCR data by the comparative C T method. Nature protocols, 3(6), 1101. [DOI] [PubMed] [Google Scholar]
- Slattery DA, & Cryan JF (2012). Using the rat forced swim test to assess antidepressant-like activity in rodents. Nature protocols, 7(6), 1009–1014. [DOI] [PubMed] [Google Scholar]
- Slattery DA, & Neumann ID (2008). No stress please! Mechanisms of stress hyporesponsiveness of the maternal brain. The Journal of physiology, 586(2), 377–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorg BA, Berretta S, Blacktop JM, Fawcett JW, Kitagawa H, Kwok JC, & Miquel MJ (2016). Casting a wide net: role of perineuronal nets in neural plasticity. Journal of neuroscience, 36(45), 11459–11468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern JM, & Johnson SK (1989). Perioral somatosensory determinants of nursing behavior in Norway rats (Rattus norvegicus). Journal of Comparative Psychology, 103(3), 269. [DOI] [PubMed] [Google Scholar]
- Swanson L (2004). Brain maps: structure of the rat brain: Gulf Professional Publishing. [Google Scholar]
- Xerri C, Stern JM, & Merzenich M (1994). Alterations of the cortical representation of the rat ventrum induced by nursing behavior. Journal of neuroscience, 14(3), 1710–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Takayanagi Y, Inoue K, Kimura T, Young LJ, Onaka T, & Nishimori K (2009). Evidence that oxytocin exerts anxiolytic effects via oxytocin receptor expressed in serotonergic neurons in mice. Journal of Neuroscience, 29(7), 2259–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, & Barrett CE (2015). Can oxytocin treat autism? Science, 347(6224), 825–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available with reasonable request.