Abstract
BACKGROUND:
In South Africa, tuberculosis (TB) is a leading cause of death among those <20 years of age. We describe changes in TB mortality among children and adolescents in South Africa over a 13-year period, identify risk factors for mortality, and estimate excess TB-related mortality.
METHODS:
Retrospective analysis of all patients <20 years of age routinely recorded in the national electronic drug-susceptible TB treatment register (2004–2016). We developed a multivariable Cox regression model for predictors of mortality and used estimates of mortality among the general population to calculate standardized mortality ratios (SMRs).
RESULTS:
Between 2004 and 2016, 729 463 children and adolescents were recorded on TB treatment; 84.0% had treatment outcomes and 2.5% (18 539) died during TB treatment. The case fatality ratio decreased from 3.3% in 2007 to 1.9% in 2016. In the multivariable Cox regression model, ages 0 to 4, 10 to 14, and 15 to 19 years (compared with ages 5 to 9 years) were associated with increased risk of mortality, as was HIV infection, previous TB treatment, and extrapulmonary involvement. The SMR of 15 to 19-year-old female patients was more than double that of male patients the same age (55.3 vs 26.2). Among 10 to 14-year-olds and those who were HIV-positive, SMRs increased over time.
CONCLUSIONS:
Mortality in South African children and adolescents treated for TB is declining but remains considerable, with 2% dying during 2016. Adolescents (10 to 19 years) and those people living with HIV have the highest risk of mortality and the greatest SMRs. Interventions to reduce mortality during TB treatment, specifically targeting those at highest risk, are urgently needed.
The World Health Organization (WHO) estimated that 1.1 million children (<15 years) developed tuberculosis (TB) in 2018, with 31% in Africa.1 Estimates for adolescent (10–19 years2) TB are not routinely reported by the WHO, but modeling suggests that 535 000 15- to 19-year-olds developed TB globally in 2012.3 In South Africa, children (<15 years) accounted for 7% (~16 000) of notified patients with TB in 2018; no published data are available for 15- to 19-year-olds.1
In a review of all-cause mortality for 2013, developing countries accounted for 98% of all deaths in individuals <20 years old, with HIV and TB accounting for 11% of deaths.4 Death due to TB may occur before the diagnosis of TB, before treatment initiation, during TB treatment, or after completion of TB treatment. However, TB programs routinely only report death during treatment. The WHO reports TB deaths as a proportion of all estimated incident patients with TB, combining deaths before and during TB treatment,5 and estimated that children (<15 years) accounted for 14% (208 740) of all TB deaths in 2018.1 Mortality is currently not sufficiently disaggregated by age to estimate the mortality in 15- to 19-year-olds in addition to children (<15 years). In South Africa, although general mortality rates vary by age, sex, and HIV status, TB was the leading cause of mortality among individuals 15 to 24 years old and a leading cause of mortality in children 1 to 4 years old in 2016.6
To achieve the global targets of a 95% reduction in TB deaths by 2035 compared to 20157 and prevent TB deaths, targeted strategies need to be developed and implemented. An improved understanding of the profile of patients who die and the risk factors for death during TB treatment will support this process. Given the lack of reporting of agespecific TB mortality data, we aimed to describe mortality during routine TB treatment in South Africa among all children and adolescents <20 years old, disaggregated into 4 age categories. Using the large national electronic recording system of routine individual patients with TB, we describe TB case fatality ratios (CFRs) over time; calculate standardized mortality ratios (SMRs), comparing TB mortality and population-based mortality estimates; and identify risk factors for death during TB treatment.
METHODS
Study Design
This was a retrospective cohort study of all individuals <20 years old routinely recorded in the South African drug-susceptible TB treatment register reporting cohort between 2004 and 2016. We used age categories of 0 to 4, 5 to 9, 10 to 14, and 15 to 19 years.
Electronic Tuberculosis Register
The South Africa National Department of Health’s national TB program implemented the Electronic Tuberculosis Register (http://etrnet.info/) for routine reporting of all drug-susceptible TB treatment in 2004. Drug-resistant TB is recorded in a separate Web-based register8 and is not included in this analysis. ETR. Net is an electronic system, with paper-based records as a source, that allows facility, district, provincial, and national reporting on case-finding, sputum conversion, and treatment outcome cohorts.9,10 In the 2017 evaluation of the South African TB care cascade, it was estimated that 71.1% of those estimated to have TB and 86.5% of those diagnosed with TB in South Africa were notified and treated (recorded within ETR.Net).11
Definitions
Patients with drug-susceptible TB were defined as patients who had no documented resistance to anti-TB drugs. From 2011, testing with Xpert MTB/RIF (Xpert; Cepheid, Sunnyvale, CA) for all patients with presumptive TB was introduced in South Africa with rapid detection of Mycobacterium tuberculosis and mutations conferring rifampin resistance.12 Retreatment refers to patients who had previously received <4 weeks of anti-TB treatment, regardless of the time since the previous treatment episode. Newly treated patients with TB were classified as having had no previously reported TB treatment or as having received <4 weeks of TB treatment at any stage. The site of TB disease was categorized as pulmonary TB when there was any pulmonary involvement or as extrapulmonary tuberculosis (EPTB) when patients had disease exclusively affecting any organ other than the lung parenchyma.13 International Classification of Diseases, 10th Revision (ICD-10) codes referring to central nervous system TB, including TB meningitis, or miliary TB, were combined as disseminated disease; all other ICD-10 codes were defined as not disseminated disease. HIV status was determined by using several proxies, including documentation of co-trimoxazole preventive therapy or antiretroviral therapy (ART), HIV test results, and CD4 test results. HIV status was classified as HIV-negative; HIV-positive, on ART; HIV-positive, not on ART; and HIV status unknown. The timing of ART could not be determined.
TB Treatment Outcomes
In South Africa, treatment outcomes for patients with TB are assigned by treating clinicians and captured in ETR.Net, which includes preprogrammed algorithms to verify outcomes consistent with national and WHO definitions.13,14 When a treatment outcome is not allocated or inconsistent with the definitions, a patient is reported in ETR.Net as not evaluated. For this analysis, “not evaluated” was combined with “lost to follow-up,” and 2 outcomes were used: Outcome 1 included the outcomes cured or treatment completed, died, lost to follow-up, failed, or transferred out;14 and outcome 2 defined vitality status (dead or alive; restricted to patients in whom the final vitality status was definitively recorded). Person-time was calculated as the difference between the start date of TB treatment and the treatment outcome date recorded in the register, representing person-years on TB treatment.
Mortality
Death included death due to any cause during the TB treatment episode. The CFR was calculated as the number of deaths as a proportion of the number of patients with TB for the specified group and period; 95% confidence intervals (CIs) were calculated around point estimates. To compute the CFR for each age band by sex, data for 2004–2016 were used as a single cohort. For SMRs, population estimates were used from the Thembisa model, an established publicly available mathematical model of South African HIV epidemiology and general demographic statistics.15 Thembisa uses age- and sex-specific mortality rates on the basis of an analysis of South African cause-of-death statistics and the South African National Burden of Disease study and projects mortality rates from 2016 onward.16 For HIV, estimates of mortality were available by sex but not age. The SMR was calculated as the ratio between the observed TB deaths and the expected deaths on the basis of mortality estimates of the general population. The expected deaths were the product of the mortality rates, determined from the Thembisa estimates, and the person-time from our cohort for each demographic category. Expected deaths were based on mortality due to any cause, including TB.
Statistical Analysis
Descriptive statistics of demographic and clinical variables were completed for the overall cohort, for patients with TB with a known vitality status, and for patients with TB who were documented to have died. HIV status was evaluated for completeness; analysis for predictors of mortality was restricted to the period of 2013–2016, the years during which <80% of patients with TB had a known HIV status in each age category. Missing data were excluded from analysis, except for HIV status, for which unknown HIV status was included as a predictor for mortality. A Cox proportional hazards regression model for hazard ratios (HRs) predicting death was developed. Univariate analyses were conducted, and collinearity in the final model was avoided. We added predictors incrementally, observing the change in significance of the likelihood ratio test of each model, to produce a final adjusted model. Survival analysis was completed by using Kaplan-Meier curves. SAS software (version 9.4; SAS Institute, Inc, Cary, NC) was used for the data analysis.
Ethical Considerations
Approval was received from the Stellenbosch University Health Research Ethics Committee (N16/07/088), and permission was obtained from the South African National Department of Health for the use of the national ETR.Net data set.
RESULTS
Between January 1, 2004, and December 31, 2016, the ETR.Net reporting cohort included 729 463 patients with TB <20 years of age who were treated for drug-susceptible TB. The vital status was recorded in 612 655 (84.0%) patients with TB, and of these, 18 539 (3.0%) died during TB treatment. Unknown treatment outcomes were more common among patients who were previously treated, those with EPTB, and those with disseminated TB, but they decreased over time, with 89.7% of patients having a known treatment outcome in 2016 (Supplemental Table 3).
There were 339 719 (46.6%) patients <5 years of age; 37 628 (5.2%) were previously treated for TB; 65 418 (9.0%) had only EPTB; and 12 245 (1.9%) had disseminated TB (Table 1). HIV testing changed over time, from 0.4% of children and adolescents with TB having a known HIV status in 2004 to 94.3% in 2016.
TABLE 1.
Demographic and Clinical Characteristics of Children and Adolescents Treated for Drug-Susceptible TB in the South African Reporting Cohort, by Vitality Status and Mortality, 2004–2016
| Variable | All Patients With TB in Reporting Cohort (N = 729 463), n | Patients With TB With Known Vitality Status (n = 612 655 [84.0%]), n (%) | Patients With TB Who Died (n = 18 539 [3.0%]), n (%) | CFRa (Patients With TB Who Died: 2.5%), % |
|---|---|---|---|---|
| Age, y | ||||
| 0–4 | 339 719 | 287 085 (84.51) | 7709 (2.69) | 2.26 |
| 5–9 | 134 616 | 115 156 (85.54) | 2776 (2.41) | 2.06 |
| 10–14 | 74 674 | 63 615 (85.19) | 2417 (3.80) | 3.23 |
| 15–19 | 180 454 | 146 799 (81.35) | 5637 (3.84) | 3.12 |
| Sex | ||||
| Male | 355 560 | 298 020 (83.82) | 8614 (2.89) | 2.42 |
| Female | 373 897 | 314 634 (84.15) | 9925 (3.15) | 2.65 |
| HIV status | ||||
| HIV-negative | 245 787 | 216 753 (88.19) | 2387 (1.10) | 0.97 |
| HIV-positive, on ART | 56 068 | 48 493 (86.49) | 2680 (5.53) | 4.77 |
| HIV-positive, no ART | 46 575 | 37 418 (80.34) | 2917 (7.80) | 6.26 |
| HIV status unknown | 381 033 | 309 991 (81.36) | 10 555 (3.40) | 2.77 |
| Previous TB history | ||||
| New | 691 834 | 584 350 (84.46) | 16 610 (2.84) | 2.40 |
| Retreatment | 37 628 | 28 305 (75.22) | 1929 (6.82) | 5.13 |
| Site of TB diseaseb | ||||
| Pulmonary TB | 664 041 | 561 255 (84.52) | 15 760 (2.81) | 2.37 |
| EPTB | 65 418 | 51 400 (78.57) | 2779 (5.41) | 4.25 |
| Disseminated diseasec | ||||
| Not disseminated | 640 136 | 541 006 (84.51) | 15 189 (2.81) | 2.37 |
| Disseminated | 12 245 | 9389 (76.68) | 882 (9.39) | 7.20 |
| Year | ||||
| 2004 | 53 081 | 40 389 (76.09) | 1505 (3.73) | 2.83 |
| 2005 | 55 426 | 44 096 (79.56) | 1670 (3.79) | 3.01 |
| 2006 | 60 482 | 49 638 (82.07) | 1842 (3.71) | 3.04 |
| 2007 | 62 981 | 52 069 (82.67) | 2101 (4.04) | 3.34 |
| 2008 | 68 106 | 56 206 (82.53) | 2072 (3.69) | 3.04 |
| 2009 | 69 559 | 58 800 (84.53) | 1930 (3.28) | 2.77 |
| 2010 | 64 003 | 53 342 (83.34) | 1674 (3.14) | 2.62 |
| 2011 | 63 887 | 55 268 (86.51) | 1364 (2.47) | 2.14 |
| 2012 | 55 002 | 48 037 (87.34) | 1127 (2.35) | 2.05 |
| 2013 | 51 572 | 45 454 (88.14) | 942 (2.07) | 1.82 |
| 2014 | 47 904 | 39 784 (83.05) | 878 (2.21) | 1.83 |
| 2015 | 42 986 | 38 635 (89.88) | 769 (1.99) | 1.79 |
| 2016 | 34 474 | 30 937 (89.74) | 665 (2.15) | 1.93 |
| Outcome 1 | ||||
| Cured or completed | 591 640 | 591 640 (100.00) | — | — |
| Died | 18 539 | 18 539 (100.00) | 18 539 (100.00) | — |
| Loss to follow-up | 116 808 | 0 (0.00) | — | — |
| Failed or drug resistant | 2476 | 2476 (100.00) | — | — |
—, not applicable.
CFR was calculated as a percentage by using the number of deaths over the total number of patients with TB.
The binary classification of site of disease included pulmonary TB, which was based on the presence of any pulmonary TB, and EPTB, which was restricted to exclusive EPTB.
Disseminated disease was based on ICD-10 coding, with neurologic TB and miliary TB recorded as disseminated disease and all other ICD-10 codes recorded as not disseminated.
The overall CFR was 2.5%, which increased from 2004 to a peak in 2007, gradually declining thereafter (Fig 1). CFRs differed by age category and were higher among 10- to 19-year-olds, with no decline over time (Fig 1). When applied to the whole cohort, the CFR was highest in the first year of life and then declined steeply over the next 2 years, with no difference by sex. CFRs increased in later childhood and peaked for boys at 12 years of age (CFR = 4.3%) before declining through adolescence. Girls had a lower but earlier peak (CFR = 3.2% at 11 years) and a plateau during early adolescence, followed by a steep increase from 16 years of age to a CFR of 4.2% at the age of 19 years (Fig 2).
FIGURE 1.
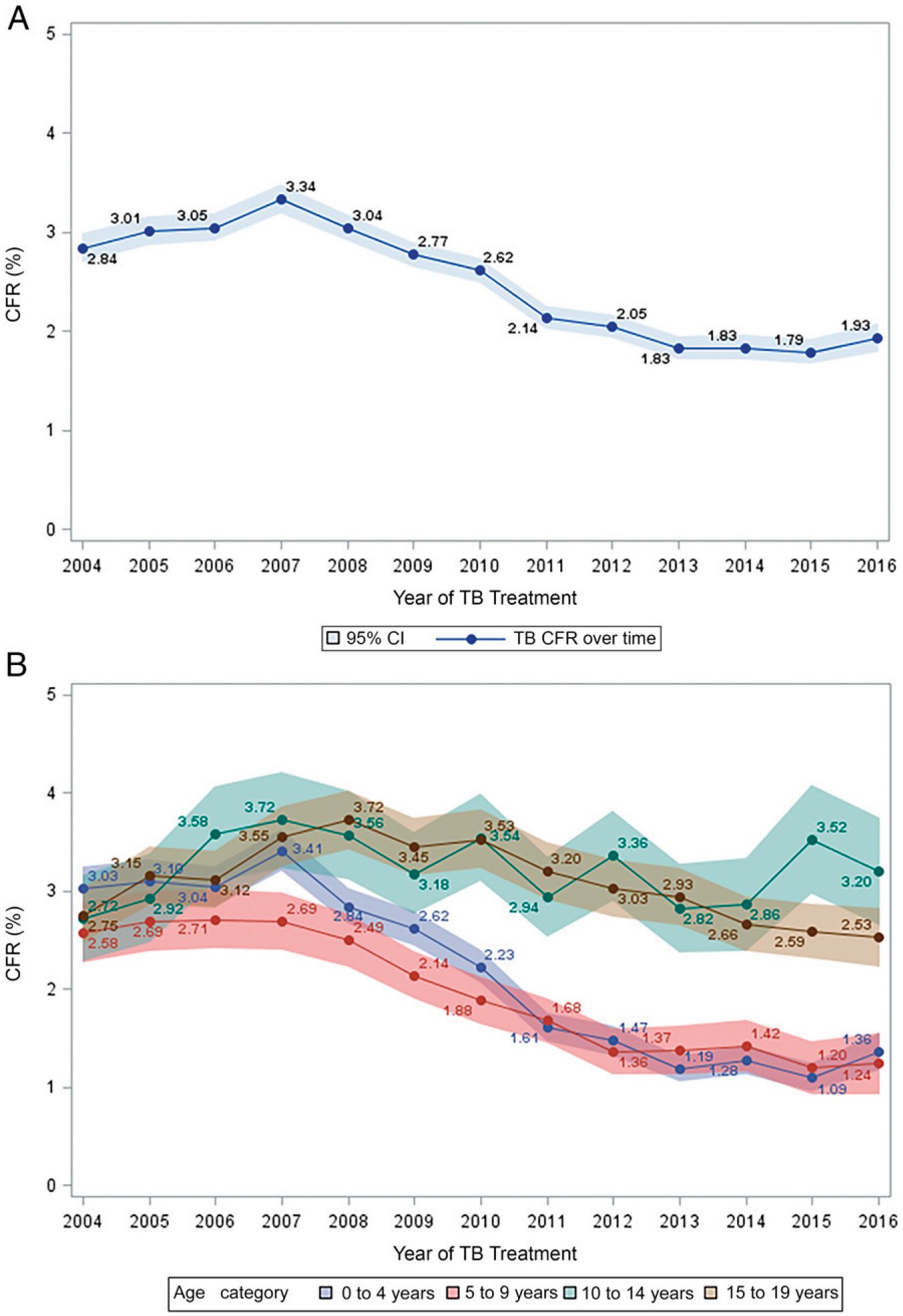
Overall CFR and stratification by age category for children and adolescents treated for drug-susceptible TB, South Africa, 2004–2016. A, Overall CFR. B, Stratification by age category.
FIGURE 2.
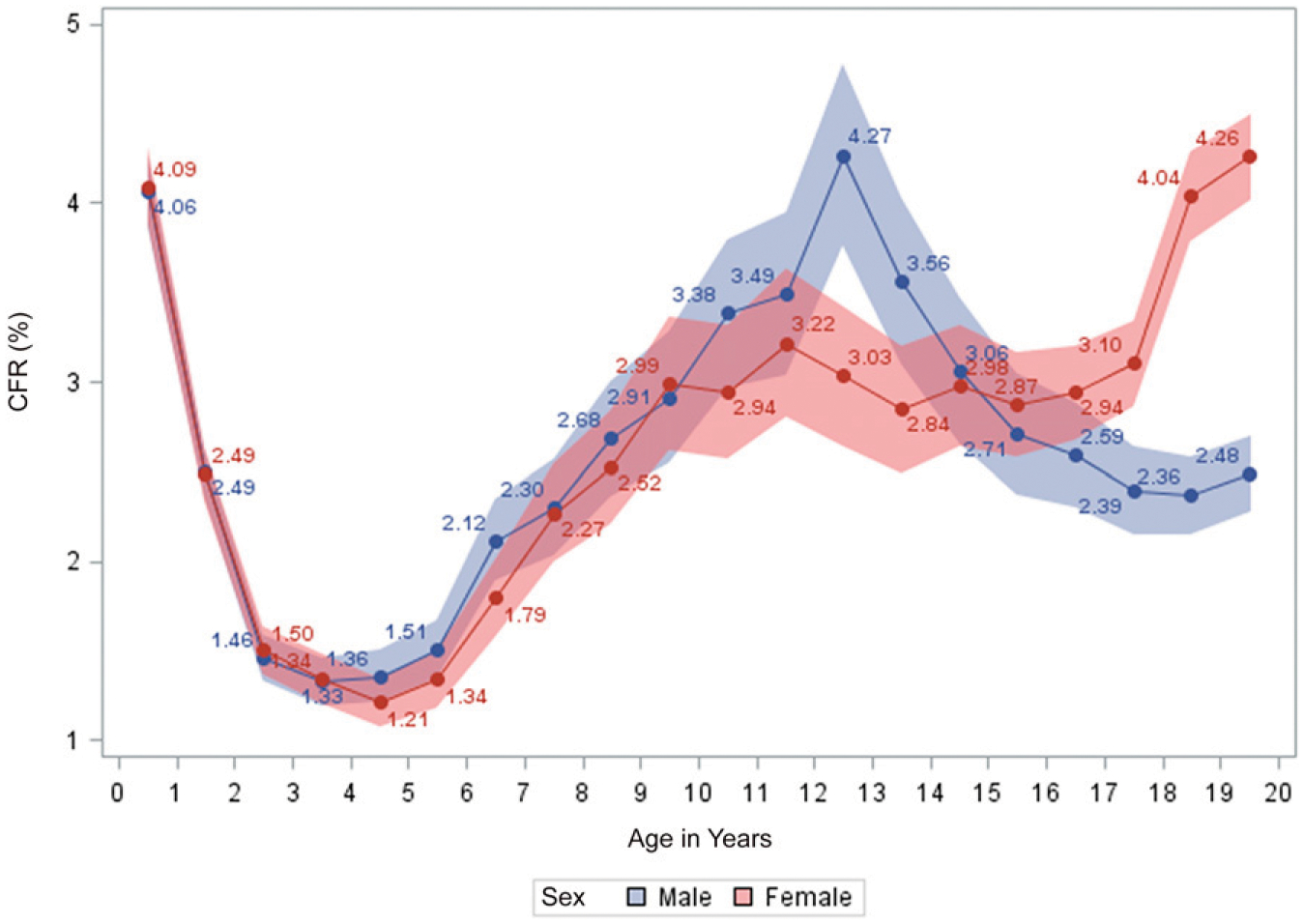
CFR of all children and adolescents treated for drug-susceptible TB in South Africa between 2004 and 2016, stratified by age and sex.
Risk Factors for Mortality on TB Treatment, Restricted to 2013–2016
Ages 0 to 4, 10 to 14, and 15 to 19 years (compared with ages 5–9 years); previous TB treatment; only having EPTB; having disseminated disease; and HIV infection (with and without current ART use) were all associated with an increased hazard of death (Table 2). The cumulative mortality at 2 and 6 months of anti-TB treatment, respectively, was 4.8% and 7.5% if the patient was HIV-positive and not on ART, 2.4% and 4.9% if the patient was HIV-positive and on ART, and 0.5% and 0.9% if the patient was HIV-negative (Fig 3).
TABLE 2.
Crude and Adjusted Cox Proportional Regression Model Predicting HRs of Death for Children and Adolescents Treated for Drug-Susceptible TB in South Africa Between 2013 and 2016 (Data Set = 175 530 and 154 135 Included in Final Model, Respectively)
| Variable | HR (95% CI) | P | aHR (95% CI) | P |
|---|---|---|---|---|
| Age, y | ||||
| 0–4 | 0.94 (0.83–1.06) | .29 | 1.33 (1.18–1.51) | <.001 |
| 5–9 | Reference | — | Reference | — |
| 10–14 | 2.27 (1.99–2.60) | <.001 | 1.75 (1.53–2.00) | <.001 |
| 15–19 | 2.09 (1.86–2.35) | <.001 | 2.12 (1.89–2.39) | <.001 |
| Sex | ||||
| Male | Reference | — | Reference | — |
| Female | 1.08 (1.01–1.16) | .03 | 0.96 (0.90–1.04) | .32 |
| HIV status | ||||
| HIV-negative | Reference | — | Reference | — |
| HIV status unknown | 2.01 (1.74–2.31) | <.001 | 2.1 1 (1.83–2.43) | <.001 |
| HIV-positive, no ART | 8.48 (7.47–9.61) | <.001 | 7.99 (7.02–9.09) | <.001 |
| HIV-positive, on ART | 5.66 (5.22–6.12) | <.001 | 5.11 (4.71–5.55) | <.001 |
| Previous TB history | ||||
| New | Reference | — | Reference | — |
| Retreatment | 2.11 (1.83–2.44) | <.001 | 1.37 (1.18–1.58) | <.001 |
| Site of TB diseasea | ||||
| Pulmonary TB | Reference | — | Reference | — |
| EPTB | 2.17 (1.98–2.39) | <.001 | 1.68 (1.53–1.85) | <.001 |
| Disseminated diseaseb | ||||
| Not disseminated | Reference | — | — | — |
| Disseminated | 3.23 (2.75–3.78) | <.001 | — | — |
| Year | ||||
| Continuous, for 1-y increase | 1.00 (0.97–1.04) | .82 | 0.99 (0.96–1.03) | .70 |
HIV status was evaluated for completeness, and analysis for predictors of mortality was restricted to the period of 2013–2016, the years during which >80% of patients with TB had a known HIV status in each age category. aHR, adjusted hazard ratio; —, not applicable.
The binary classification of site of disease included pulmonary TB, which was based on the presence of any pulmonary TB, and EPTB, which was restricted to exclusive EPTB.
Disseminated disease was based on ICD-10 coding, with neurologic TB and miliary TB recorded as disseminated disease and all other ICD-10 codes recorded as not disseminated. Because of collinearity with site of disease, disseminated disease was not included in the final model.
FIGURE 3.
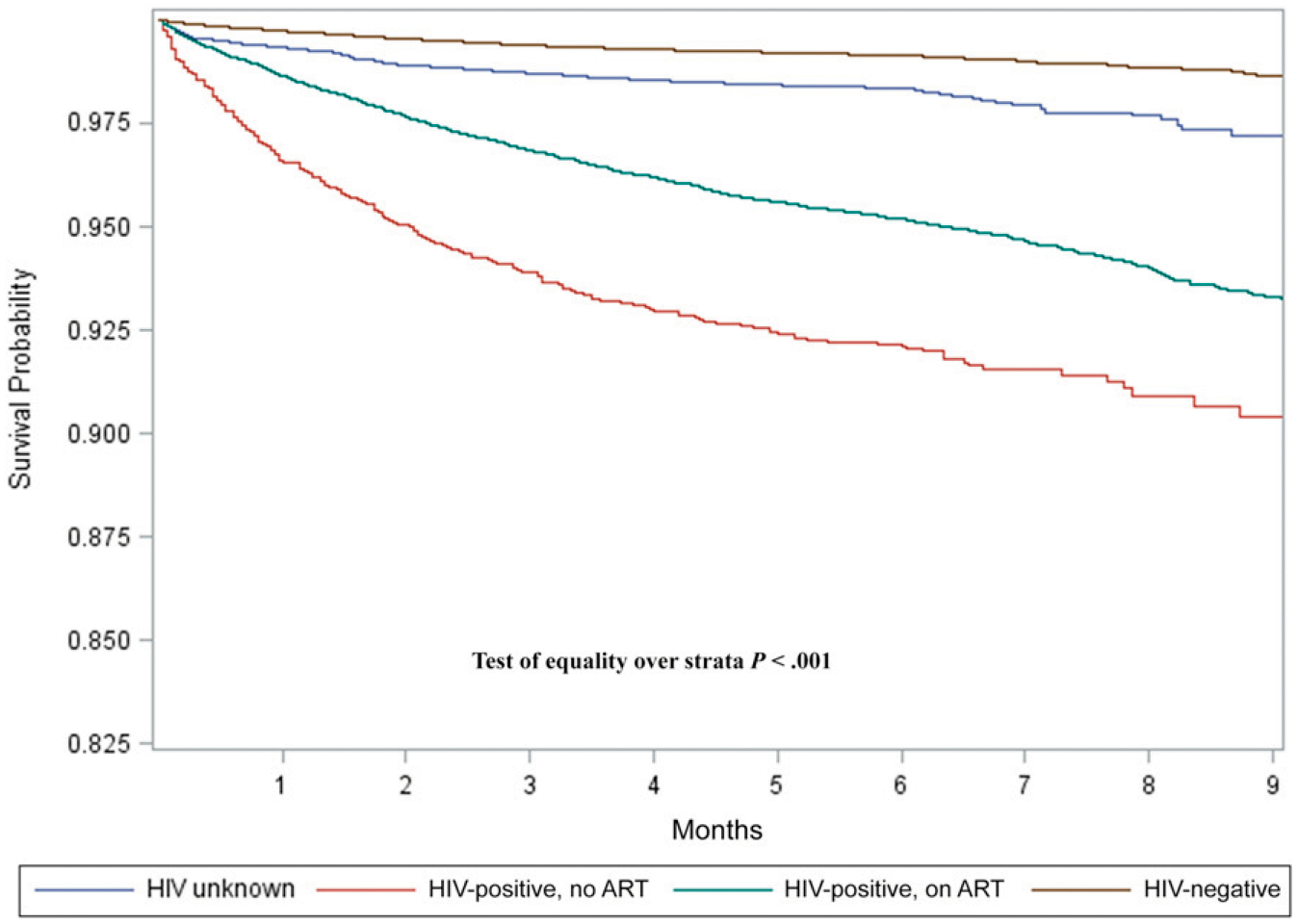
Kaplan-Meier survival curve stratified by HIV and ART status of children and adolescents on drug-susceptible TB treatment between 2013 and 2016 in South Africa.
SMRs
The SMRs for 0- to 4- and 5- to 9-year-olds did not differ by sex and remained between 3 and 5 for 0- to 4-year-olds and between 30 and 45 for 5- to 9-year-olds over time. For 10- to 14-year-olds, the SMR increased differentially by sex, from <60 in both boys and girls in 2004 to 77 in boys and 92 in girls in 2016. For 15- to 19-year-olds, the SMRs in male patients increased from 20 in 2004 to a peak of 35 in 2010 and decreased to 26 in 2016. In female patients, the SMR increased from 60 in 2004 to a peak of 76 in 2008 and decreased to 55 in 2016 (Fig 4, Supplemental Table 4). The SMRs for HIV-negative individuals remained constant, across sex, between 2013 and 2016. For HIV-positive individuals, SMRs increased from 9 to 12 in female patients and from 4 to 6.5 in male patients (Fig 5, Supplemental Table 5).
FIGURE 4.
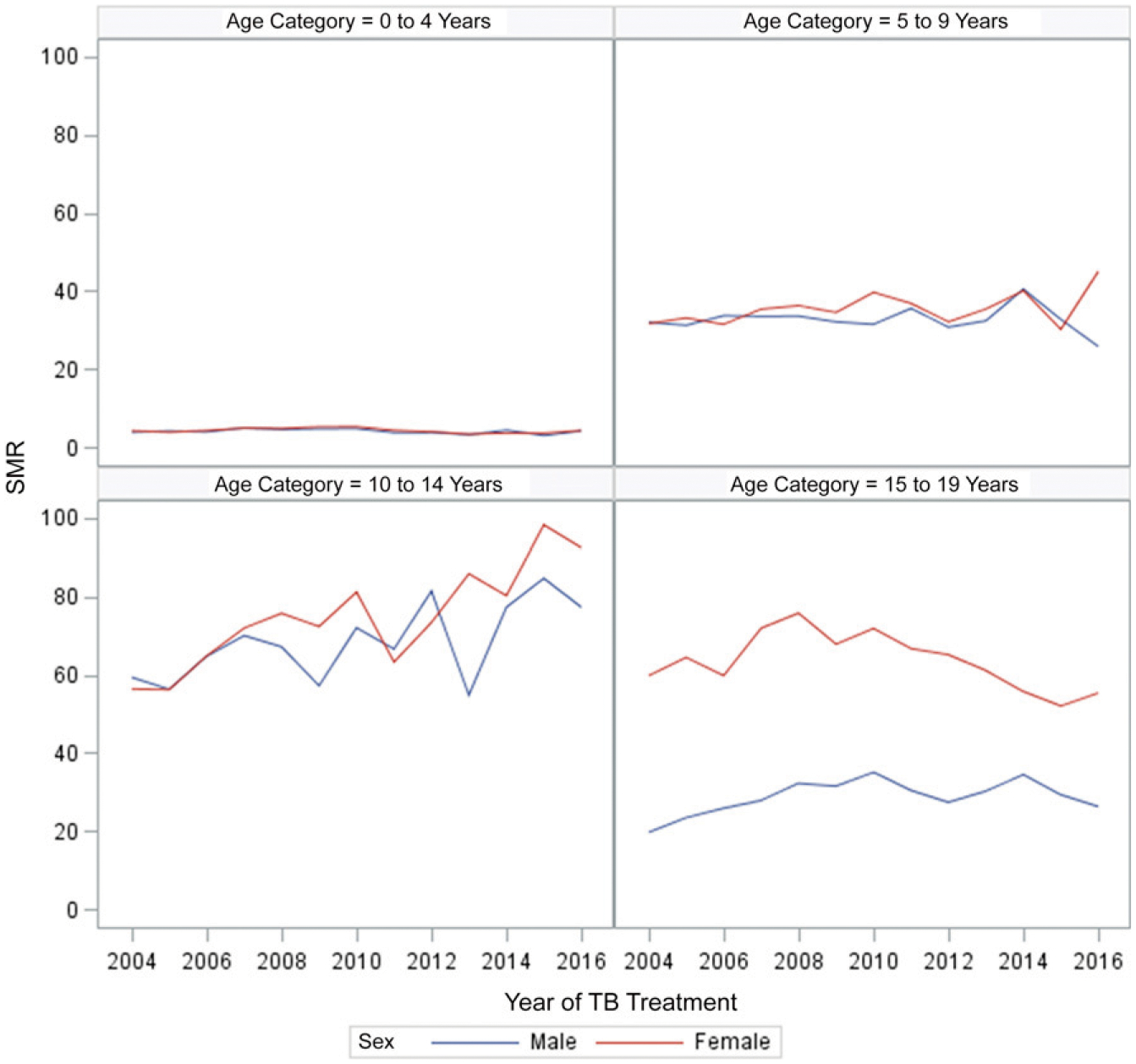
SMRs of children and adolescents on drug-susceptible TB treatment in South Africa, 2004–2016. SMR is the ratio of observed TB deaths to the expected deaths and is based on the Thembisa estimates of mortality rates for the general population. Expected mortality is based on the product of the age- and sex-specific population estimates of mortality rates from Thembisa and the person-time in the TB cohort.
FIGURE 5.
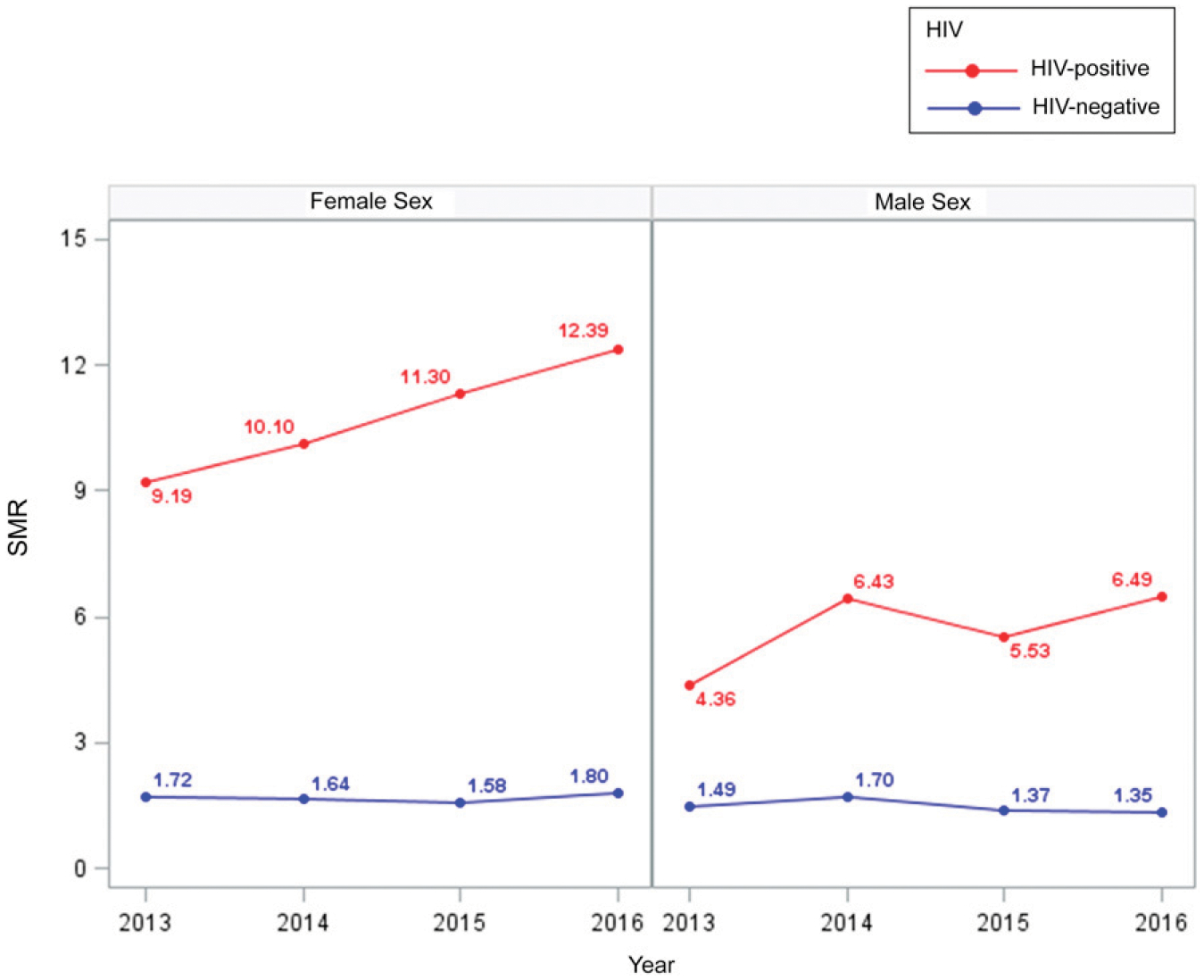
SMRs by HIV status of children and adolescents on drug-susceptible TB treatment in South Africa, 2013–2016. SMR is the ratio of observed TB deaths to the expected deaths and is based on the Thembisa estimates of mortality rates for the general population. Expected mortality is based on products of the HIV-positive and HIV-negative population estimates of mortality rates (regardless of age) from the Thembisa model and the person-time in the TB cohort.
DISCUSSION
Between 2004 and 2016, 2.5% (18 539) of all children and adolescents in the routine national TB treatment register died, but with a decrease in mortality over time. CFRs and SMRs changed over time and differed by age, sex, HIV status, and ART use.
Most previous research on TB mortality in children and adolescents has been restricted to age <15 or ≥15 years old. Therefore, there are limited data across the age continuum. In a retrospective study from Kenya, 4.4% of children (<15 years) died during drug-susceptible TB treatment,17 whereas in a systematic review, the pooled TB case fatality estimate for children (<15 years) in low–HIV prevalence settings was 0.9%.18 We have shown an initial peak in the CFR in the first year of life, followed by a second peak in early adolescence. By age band, 10-to 14-year-olds had the highest CFR (3.2%); even when analyzed by continuous age and disaggregated by sex, the highest CFR was reported in 12-year-olds. In a systematic review, CFRs in 0- to 4 year-olds (pooled estimate 2.0%; 95% CI: 0.5–7.4) were consistently higher than those in 5- to 14-year-olds (pooled estimate 0.8%; 95% CI: 0.3–2.1).18 The use of broad age bands for children between 5 and 14 years of age may have obscured a peak in early adolescent TB CFR. A limited case-series from South Africa has been used to describe adult-type pulmonary TB in 10- to 14-year-olds,19 and challenges with adherence to TB medication and ART have been described in this group.20,21 The higher CFR noted in this group may reflect a combination of poor adherence related to health system engagement and the type and severity of disease in this age group. We showed that both younger age and relative older age were associated with increased mortality, consistent with studies that have confirmed the increased risk of TB and death in infants,22 children <218,23 or 5 years of age,17,24,25 and 15- to 19-year- olds.26 It is important that routine TB programs collect sufficient detail for routine monitoring and evaluation in more nuanced age categories.
Although children 0 to 4 years of age were at a higher risk of death, the SMRs revealed that in 2016, excess TB mortality among 0- to 4-year-olds was fourfold, whereas excess TB mortality among 5- to 9-year-olds, 10- to 14-year-olds, and 15 to 19-year-olds was 25 to 45 times, 77 to 90 times, and 26 to 55 times higher, respectively. In South Africa, TB is the leading cause of natural death among men, but it ranks fifth among women. When disaggregated by age, TB is not among the 10 leading causes of death for infants, but it is ranked fourth in children aged 1 to 14 years and first in those aged 15 to 64 years.6 Under-5 mortality in South Africa has mostly been attributed to neonatal causes, associated with prematurity, diarrhea, and pneumonia, whereas the devastating effect of HIV had been largely reversed by 2011,27 attributed to the successful implementation of the prevention of mother-to-child transmission (PMTCT) program and to the scale-up of ART access. We note that excess mortality in 0- to 4-year-olds is much lower than that in other pediatric groups because there are additional reasons for the youngest children to die. In South Africa, only 1.3% of all deaths are reported among 5- to 14-year-olds, and 10- to 14-year-olds had the lowest absolute numbers of death between 2010 and 2015.6 This lower expected mortality, combined with the high CFR, in 10- to 14-year-olds may explain the highest SMRs being recorded in 10- to 14-year-olds, who have limited other reasons for death but a high TB CFR. Earlier work has revealed how age-standardized death rates for HIV and AIDS and TB increased rapidly from 1997 to 2006 and then declined to 2012, whereas deaths due to other causes increased.28 The difference in the SMR in 10- to 19-year-old male and female patients is notable. In 2016, 15- to 19-year-old female patients had an SMR more than double that of male patients because of the higher CFR and the lower expected mortality in female patients. During early adolescence and puberty, there may be more TB in girls compared with boys,29 and differential access to health services by sex30,31 makes girls more likely to access the health care system. This results in a higher chance of diagnosis and subsequent recording of death while on treatment, compared with boys who may die before diagnosis. In addition, the significant burden of TB among pregnant women32 and the threefold increased risk of maternal mortality with TB in HIV-positive pregnant women33 further contribute to the greater CFR. Among adolescents and young adults in South Africa, almost 50% of deaths are due to unnatural causes, and 84.6% of these unnatural deaths occur in male adolescents and young adults.6 Specifically, the greater expected mortality among young boys in South Africa due to disproportionate exposure to interpersonal violence has been shown among 10- to 17-year-olds, with homicide as a leading cause of death, mainly affecting young boys.34 Female patients between 15 and 19 years of age attending reproductive health services could be identified for TB education and opportunities for TB and HIV prevention.
The risk of mortality associated with severe forms of TB is well documented. The authors of a systematic review reported the risk of death for children treated for TB meningitis to be 19.3% (95% CI: 14.0–26.1).35 We documented a CFR of 7.4% among children and adolescents with neurologic and miliary TB (which is lower than published estimates) for several potential reasons. First, we combined all neurologic TB and miliary TB as a single category of disseminated TB. Second, we did not restrict this analysis to children but included older adolescents. Third, we only included children recorded in the routine TB treatment register. In South Africa, it is estimated that at least 14.4% of all patients diagnosed with TB are not notified and treated,11 and in a hospital-based study of 0- to 12-year-olds, in-hospital death and a diagnosis of TB meningitis were associated with lack of registration.36 Future work combining reported TB deaths, vital registration data, and autopsy data will likely provide better estimates of TB mortality, including disseminated forms.
Consistent with earlier work,18,37,38 we have shown that CFRs in HIV-positive children were higher than those in HIV-negative children, and the difference was reduced but persisted despite ART. This is similar to work from Kenya in which ART reduced the adjusted hazard ratio for death from 4.8 among HIV-positive children not on ART to 3.7 among children (≤15 years) on ART with TB.17 Cumulative mortality in HIV-positive individuals halved at 2 and 6 months when we compared those on ART with those not on ART. The SMR among HIV-negative individuals has remained constant, whereas the SMR among HIV-positive individuals has increased. The SMR remains higher in HIV-positive female patients compared with male patients, likely reflecting the earlier and greater access to ART among female patients and the lower overall HIV mortality compared with TB mortality among female patients in ART programs.39
During the study period, South Africa scaled up PMTCT and HIV testing and expanded access to universal ART.40,41 The reductions in vertical HIV transmission through PMTCT may contribute to the reduction in TB CFRs in the youngest age bands over time. The HIV profile of older children and adolescents includes vertical transmission among those born before the scale-up of PMTCT, children infected despite PMTCT, and horizontal transmission. Well-functioning PMTCT programs will reduce vertical transmission of HIV, but it remains critical that all HIV-positive children and adolescents have access to immediate ART. Regular screening for TB and TB preventive therapy will reduce TB incidence and may improve TB outcomes through early diagnosis.
Our study was associated with several strengths and limitations. We used a large individual-level national data set spanning 13 years to identify patient factors associated with mortality and the timing of death, but we were limited to those who started TB treatment. We quantified unknown vitality status and, for the purpose of estimating CFRs, assumed all those lost to follow-up to be alive. Because we were restricted to those patients registered and on treatment, we probably underreport on pediatric TB because children and adolescents with TB may be undiagnosed, untreated, or unreported. Additional work is required to estimate the losses of children and adolescents with TB across the care cascade. Although mortality during treatment occurs as a discrete event and is probably accurately noted, our CFRs are likely an underestimate of mortality, with additional unreported mortality expected among those undiagnosed or lost to follow-up. In addition, we do not have data on mortality in those who did not initiate TB treatment or who were not in the TB treatment register. Future work to reduce loss to follow-up and ascertain definitive outcomes among those lost to follow-up is required. Because of the reliance on treatment register data, we were not able to evaluate pretreatment mortality and the role of additional risk factors for mortality, including nutritional status, BCG vaccination status, pregnancy status, the degree of HIV-related immune suppression, HIV viral load, and the precise timing and duration of ART. More work is needed to explore the relationship between pregnancy and death during TB treatment, especially considering the high TB CFR in female individuals of reproductive age.
We report on TB mortality over a 13-year period, which overlaps with significant progress made in the management of HIV in South Africa and reveals that overall mortality during treatment has declined in children and adolescents. This reduction in the hazard of death is consistent with earlier work from South Africa.23,42 We highlighted the high risk of TB mortality in the youngest age group, during early adolescence, and among female patients in later adolescence. The modulating effect of HIV and ART on TB mortality continues to be highly relevant. Early detection and treatment of HIV with TB remains essential, and tailored approaches to treatment support are required in infants and during adolescence.
Supplementary Material
WHAT’S KNOWN ON THIS SUBJECT:
Modeling studies have highlighted the significant burden of tuberculosis (TB) mortality in children and adolescents, but adolescent reporting has been limited. The effect of HIV on TB mortality and the reversal with antiretroviral therapy has been well described.
WHAT THIS STUDY ADDS:
In this study, we provide child and adolescent TB mortality estimates in the context of the world’s largest antiretroviral program. We provide the first quantification of standardized mortality ratios for children and adolescents with TB.
ACKNOWLEDGMENTS
We acknowledge the South African Department of Health, the National Tuberculosis Program, and the Research, Information, Monitoring, Evaluation, and Surveillance unit for the support and extraction of the ETR.Net data set.
FUNDING:
Funded by the South African Medical Research Council through its Division of Research Capacity Development under the Bongani Mayosi National Health Scholars Program from funding received from the Public Health Enhancement Fund, South African National Department of Health (to Dr Osman). The contents of any publications from any studies during this degree are solely the responsibility of the authors and do not necessarily represent the official views of the South African Medical Research Council or the funders. Dr du Preez is supported by the Fogarty International Center of the National Institutes of Health under award K43TW011006. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr Hesseling is financially supported by the South African National Research Foundation through a South African Research Chairs Initiative, and Dr du Preez received grant holder–linked student support. Dr Welte is supported financially by a grant from the South African National Research Foundation through its funding of the Centre of Excellence in Epidemiological Modelling and Analysis. The financial assistance of the National Research Foundation toward this research is hereby acknowledged. Opinions expressed, and conclusions arrived at, are those of the authors and are not necessarily to be attributed to the National Research Foundation. Dr Seddon is supported by a Clinician Scientist Fellowship jointly funded by the UK Medical Research Council and the UK Department for International Development under the UK Medical Research Council–Department for International Development concordat agreement (MR/R007942/1). Initial data extraction and cleaning was funded by URC through the Tuberculosis Care II Project (US Agency for International Development cooperative agreement AID-OAA-A-10-00021).
ABBREVIATIONS
- ART
antiretroviral therapy
- CFR
case fatality ratio
- CI
confidence interval
- EPTB
extrapulmonary tuberculosis
- ETR.Net
Electronic Tuberculosis Register
- HR
hazard ratio
- ICD-10
International Classification of Diseases, 10th Revision
- PMTCT
prevention of mother-to-child transmission
- SMR
standardized mortality ratio
- TB
tuberculosis
- WHO
World Health Organization
Footnotes
Deidentified individual participant data will not be made available. Deidentified individual participant data are available from the South African National Department of Health on request with an approved protocol.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
COMPANION PAPER: A companion to this article can be found online at www.pediatrics.org/cgi/doi/10.1542/peds.2020-039404.
REFERENCES
- 1.World Health Organization. Global Tuberculosis Report 2019. Geneva, Switzerland: World Health Organization; 2019 [Google Scholar]
- 2.World Health Organization. Health for the World’s Adolescents: A Second Chance in the Second Decade. Geneva, Switzerland: World Health Organization; 2014 [Google Scholar]
- 3.Snow KJ, Sismanidis C, Denholm J, Sawyer SM, Graham SM. The incidence of tuberculosis among adolescents and young adults: a global estimate. Eur Respir J. 2018;51(2):1702352. [DOI] [PubMed] [Google Scholar]
- 4.Kyu HH, Pinho C, Wagner JA, et al. ; Global Burden of Disease Pediatrics Collaboration. Global and national burden of diseases and injuries among children and adolescents between 1990 and 2013: findings from the global burden of disease 2013 study. JAMA Pediatr. 2016;170(3):267–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glaziou P, Dodd P, Dean A, Floyd K. Methods Used by WHO to Estimate the Global Burden of TB Disease. Geneva, Switzerland: World Health Organization; 2019 [Google Scholar]
- 6.Statistics South Africa. Mortality and Causes of Death in South Africa, 2016: Findings From Death Notification. Pretoria, South Africa: Statistics South Africa; 2018 [Google Scholar]
- 7.World Health Organization. The End TB Strategy. Geneva, Switzerland: World Health Organization; 2015 [Google Scholar]
- 8.National Department of Health. Management of Drug-Resistant Tuberculosis: Policy Guidelines. Pretoria, South Africa: National Department of Health; 2013 [Google Scholar]
- 9.Nadol P, Stinson KW, Coggin W, et al. Electronic tuberculosis surveillance systems: a tool for managing today’s TB programs. Int J Tuberc Lung Dis. 2008; 12(3, suppl 1):8–16 [PubMed] [Google Scholar]
- 10.Coggin W. Overview of the electronic TB register ETR.Net. Available at: https://www.who.int/tb/err/coggin.pdf.AccessedNovember 4, 2019
- 11.Naidoo P, Theron G, Rangaka MX, et al. The South African tuberculosis care cascade: estimated losses and methodological challenges. J Infect Dis. 2017;216(suppl 7):S702–S713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nicol MP, Whitelaw A, Wendy S. Using Xpert MTB/RIF. Curr Respir Med Rev. 2013;9:187–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Department of Health. National Tuberculosis Management Guidelines 2014. Pretoria, South Africa: National Department of Health; 2014 [Google Scholar]
- 14.World Health Organization. Definitions and Reporting Framework for Tuberculosis – 2013 Revision. Geneva, Switzerland: World Health Organization; 2013 [Google Scholar]
- 15.Johnson L, Dorrington R. Thembisa Version 4.1: A Model for Evaluating the Impact of HIV/AIDS in South Africa. Cape Town, South Africa: University of Cape Town; 2018 [Google Scholar]
- 16.Johnson LF, May MT, Dorrington RE,et al. Estimating the impact of antiretroviral treatment on adult mortality trends in South Africa:a mathematical modelling study. PLoS Med. 2017;14(12):e1002468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Onyango DO, Yuen CM, Masini E, Borgdorff MW. Epidemiology of pediatric tuberculosis in Kenya and risk factors for mortality during treatment: a national retrospective cohort study. J Pediatr. 2018;201:115–121 [DOI] [PubMed] [Google Scholar]
- 18.Jenkins HE, Yuen CM, Rodriguez CA,et al. Mortality in children diagnosed with tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2017;17(3):285–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marais BJ, Gie RP, Hesseling AH, Beyers N. Adult-type pulmonary tuberculosis in children 10–14 years of age. Pediatr Infect Dis J. 2005;24(8):743–744 [DOI] [PubMed] [Google Scholar]
- 20.Adejumo OA, Malee KM, Ryscavage P, Hunter SJ, Taiwo BO. Contemporary issues on the epidemiology and antiretroviral adherence of HIV-infected adolescents in sub-Saharan Africa: a narrative review. J Int AIDS Soc. 2015; 18(1):20049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munro SA, Lewin SA, Smith HJ, Engel ME, Fretheim A, Volmink J. Patient adherence to tuberculosis treatment: a systematic review of qualitative research. PLoS Med. 2007;4(7):e238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atalell KA, Birhan Tebeje N, Ekubagewargies DT. Survival and predictors of mortality among children co-infected with tuberculosis and human immunodeficiency virus at University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia. A retrospective follow-up study. PLoS One. 2018;13(5):e0197145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Osman M, Lee K, Du Preez K, Dunbar R, Hesseling AC, Seddon JA. Excellent treatment outcomes in children treated for tuberculosis under routine operational conditions in Cape Town, South Africa. Clin Infect Dis. 2017;65(9): 1444–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wood R, Liang H, Wu H, et al. Changing prevalence of tuberculosis infection with increasing age in high-burden townships in South Africa. Int J Tuberc Lung Dis. 2010;14(4):406–412 [PMC free article] [PubMed] [Google Scholar]
- 25.Drobac PC, Shin SS, Huamani P, et al. Risk factors for in-hospital mortality among children with tuberculosis: the 25-year experience in Peru. Pediatrics. 2012;130(2). Available at: https://pediatrics.aappublications.org/content/130/2/e373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Snow K, Hesseling AC, Naidoo P, Graham SM, Denholm J, du Preez K. Tuberculosis in adolescents and young adults: epidemiology and treatment outcomes in the Western Cape. Int J Tuberc Lung Dis. 2017;21(6):651–657 [DOI] [PubMed] [Google Scholar]
- 27.Nannan NN, Groenewald P, Pillay-van Wyk V, et al. Child mortality trends and causes of death in South Africa, 1997–2012, and the importance of a national burden of disease study. S Afr Med J. 2019;109(7):480–485 [DOI] [PubMed] [Google Scholar]
- 28.Pillay-van Wyk V, Msemburi W, Laubscher R, et al. Mortality trends and differentials in South Africa from 1997 to 2012: second National Burden of Disease Study. [published correction appears in Lancet Glob Health. 2017; 5(3):e27].Lancet Glob Health. 2016;4(9): e642–e653 [DOI] [PubMed] [Google Scholar]
- 29.Seddon JA, Chiang SS, Esmail H, Coussens AK. The wonder years: what can primary school children teach us about immunity to Mycobacterium tuberculosis? Front Immunol. 2018;9: 2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horton KC, MacPherson P, Houben RMGJ, White RG, Corbett EL. Sex differences in tuberculosis burden and notifications in low- and middle-income countries: a systematic review and meta-analysis. PLoS Med. 2016;13(9): e1002119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Osler M, Cornell M, Ford N, Hilderbrand K, Goemaere E, Boulle A. Population-wide differentials in HIV service access and outcomes in the Western Cape for men as compared to women, South Africa: 2008 to 2018:a cohort analysis. J Int AIDS Soc. 2020; 23(suppl 2):e25530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sugarman J, Colvin C, Moran AC, Oxlade O. Tuberculosis in pregnancy: an estimate of the global burden of disease. Lancet Glob Health. 2014;2(12): e710–e716 [DOI] [PubMed] [Google Scholar]
- 33.Zumla A, Bates M, Mwaba P. The neglected global burden of tuberculosis in pregnancy. Lancet Glob Health. 2014; 2(12):e675–e676 [DOI] [PubMed] [Google Scholar]
- 34.Mathews S, Martin LJ, Coetzee D, et al. The South African child death review pilot: a multiagency approach to strengthen healthcare and protection for children. S Afr Med J. 2016;106(9): 895–899 [DOI] [PubMed] [Google Scholar]
- 35.Chiang SS, Khan FA, Milstein MB, et al. Treatment outcomes of childhood tuberculous meningitis: a systematic review and meta-analysis. Lancet Infect Dis. 2014;14(10):947–957 [DOI] [PubMed] [Google Scholar]
- 36.du Preez K, Schaaf HS, Dunbar R, et al. Incomplete registration and reporting of culture-confirmed childhood tuberculosis diagnosed in hospital. Public Health Action. 2011;1(1):19–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soeters M, de Vries AM, Kimpen JL, Donald PR, Schaaf HS. Clinical features and outcome in children admitted to a TB hospital in the Western Cape–the influence of HIV infection and drug resistance. S Afr Med J. 2005;95(8): 602–606 [PubMed] [Google Scholar]
- 38.Mukadi YD, Wiktor SZ, Coulibaly IM,et al. Impact of HIV infection on the development, clinical presentation, and outcome of tuberculosis among children in Abidjan, Côte d’Ivoire. AIDS. 1997;11(9):1151–1158 [DOI] [PubMed] [Google Scholar]
- 39.Druyts E, Dybul M, Kanters S, et al. Male sex and the risk of mortality among individuals enrolled in antiretroviral therapy programs in Africa: a systematic review and meta-analysis. AIDS. 2013;27(3):417–425 [DOI] [PubMed] [Google Scholar]
- 40.Goga A, Dinh TH, Jackson D; SAPMTCTE Study Group. Evaluation of the Effectiveness of the National Prevention of Mother-to-Child Transmission (PMTCT) Programme Measured at Six Weeks Postpartum in South Africa, 2010. Cape Town, South Africa: South African Medical Research Council, National Department of Health of South Africa, and PEPFAR/US Centers for Disease Control and Prevention; 2012 [Google Scholar]
- 41.Simelela NP, Venter WDF. A brief history of South Africa’s response to AIDS. S Afr Med J. 2014;104(3, suppl 1):249–251 [DOI] [PubMed] [Google Scholar]
- 42.Heunis JC, Kigozi NG, Chikobvu P, Botha S, van Rensburg HD. Risk factors for mortality in TB patients: a 10-year electronic record review in a South African province. BMC Public Health. 2017;17(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


