This cross-sectional study examines self-reported nighttime sleeping patterns, performance on cognition tests, and lifestyle and demographic variables in cognitively healthy older adults.
Key Points
Question
What role does self-reported sleep duration play in brain amyloid-β accumulation, cognitive performance, and lifestyle factors in the context of healthy aging?
Findings
In this cross-sectional study of 4417 older adults with normal cognition, a higher amyloid-β burden was associated with short sleep duration. Sleeping duration of 6 hours or less or 9 hours or more was associated with distinct deficits in cognitive performance as well as greater depressive symptoms, body mass index, and daytime napping.
Meaning
Short and long sleep durations were associated with multiple adverse health outcomes, highlighting the importance of healthy sleep in aging.
Abstract
Importance
Disrupted sleep is common in aging and is associated with cognition. Age-related changes to sleep are associated with multiple causes, including early Alzheimer disease pathology (amyloid β [Aβ]), depression, and cardiovascular disease.
Objective
To investigate the associations between self-reported sleep duration and brain Aβ burden as well as the demographic, cognitive, and lifestyle variables in adults with normal cognition.
Design, Setting, and Participants
This cross-sectional study obtained data from participants in the Anti-Amyloid Treatment in Asymptomatic Alzheimer’s Disease (A4) study, which is being conducted in 67 sites in the United States, Canada, Australia, and Japan. The sample for this analysis consisted of individuals aged 65 to 85 years who underwent an Aβ positron emission tomography (PET) scan, had complete apolipoprotein E (APOE) genotype data, and were identified as clinically normal (per a Clinical Dementia Rating score of 0) and cognitively unimpaired (per a Mini-Mental State Examination score of 25 to 30 and Logical Memory Delayed Recall test score of 6 to 18). Data were analyzed from April 3, 2020, to June 20, 2021.
Main Outcomes and Measures
The outcome was self-reported nightly sleep duration (grouped by short sleep duration: ≤6 hours, normal sleep duration: 7-8 hours, and long sleep duration: ≥9 hours) compared with demographic characteristics, Aβ burden (as measured with a fluorine 18–labeled-florbetapir PET scan), objective and subjective cognitive function measures, and lifestyle variables.
Results
The 4417 participants in the study included 2618 women (59%) and had a mean (SD) age of 71.3 (4.7) years. Self-reported shorter sleep duration was linearly associated with higher Aβ burden (β [SE] = –0.01 [0.00]; P = .005), and short sleep duration was associated with reduced cognition that was mostly in memory domains. No difference in Aβ was found between long and normal sleep duration groups (β [SE] = 0.00 [0.01]; P = .99). However, compared with normal sleep duration, both short and long sleep durations were associated with higher body mass index (short vs normal sleep duration: β [SE] = 0.48 [0.17], P = .01; long vs normal sleep duration: β [SE] = 0.97 [0.31], P = .002), depressive symptoms (short vs normal sleep duration: β [SE] = 0.31 [0.05], P < .001; long vs normal sleep duration: β [SE] = 0.39 [0.09], P < .001), and daytime napping (short vs normal sleep duration: β [SE] = 2.66 [0.77], P = .001; long vs normal sleep duration: β [SE] = 3.62 [1.38], P = .01). Long sleep duration was associated with worse performance across multiple cognitive domains.
Conclusions and Relevance
In this cross-sectional study, both short and long sleep durations were associated with worse outcomes for older adults, such as greater Aβ burden, greater depressive symptoms, higher body mass index, and cognitive decline, emphasizing the importance of maintaining adequate sleep.
Introduction
Aging is associated with robust changes in sleep, resulting from shifts in lifestyle and underlying neurophysiological mechanisms.1 These changes are not uniform but instead heterogeneous, with variability in sleep architecture among older adults observed even in the absence of disease.2,3 Sleep health has important consequences for aging trajectories, and sleep disruption has been associated with increased risk of depression,4,5 cognitive decline,6,7 Alzheimer disease (AD),8,9 and cardiovascular and metabolic outcomes.10,11 Because poor sleep represents a potentially modifiable risk factor, it is critical to identify cognitive and biological correlates of sleep disturbance in the context of healthy aging.
Nocturnal sleep duration has been a focus of public health recommendations, with 7 to 8 hours of sleep typically advised for older adults.12 Focusing on cognitive decline, both short sleep (≤6 h/night) and long sleep (≥9 h/night) durations have been associated with worse outcomes.7,13,14 Although short sleep duration has been associated with greater brain amyloid-β (Aβ) burden in healthy older adults,15 which is believed to reflect a preclinical stage of AD,16,17 the modest sample size of previous Aβ positron emission tomography (PET) imaging studies and the rarity of long sleep duration have precluded a thorough investigation of the distinct factors in short and long sleep duration while modeling the outcome of elevated Aβ.
We investigated the associations between self-reported sleep duration and brain Aβ burden as well as the demographic, cognitive, and lifestyle variables in a cohort of 4417 older adults with normal cognition. This sample size allowed the opportunity to examine both the linear (ie, is self-reported sleep continuously associated with aging phenotypes?) and nonlinear (ie, how do short and long sleep duration groups compared with the normal [7-8 hours] sleep duration reference group?) associations of the outcomes in a large cohort of older individuals with Aβ status. We hypothesized that multiple independent variables would play a role in sleep duration, such that greater Aβ burden would be associated with short sleep duration, but that worse age-related cognitive and lifestyle outcomes would be associated with both short and long sleep durations.
Methods
From April 3, 2020, to June 20, 2021, we analyzed data from participants in the Anti-Amyloid Treatment in Asymptomatic Alzheimer’s Disease (A4) study, which was conducted at 67 sites across the United States, Canada, Australia, and Japan. Institutional review board approval was obtained at each site of the A4 Study. All participants provided written informed consent before participation.
A total of 6763 individuals with normal cognition between age 65 and 85 years were initially screened for the A4 Study.18,19 Participants with a Clinical Dementia Rating global score of 0, Mini-Mental State Examination (MMSE) score of 25 to 30, and Logical Memory Delayed Recall test score of 6 to 18 were eligible for fluorine 18–labeled-florbetapir PET imaging. A total of 4486 participants underwent a PET scan, and of this sample, 45 individuals were excluded from the present cross-sectional study for missing apolipoprotein E (APOE [OMIM 107741]) genotype data. An additional 24 individuals were excluded for having cognitive test scores that did not meet the screening criteria on rescreening, resulting in a final analysis sample of 4417 participants. An overview of the inclusion process in this study is presented in eFigure 1 in the Supplement.
Self-identified race/ethnicity information was collected during screening. Because both Aβ burden20 and sleep duration21,22 have been shown to differ across racial/ethnic groups, self-identified race/ethnicity was used as a covariate in the analyses. Race/ethnicity was reclassified into 4 categories: Latino or Hispanic White (n = 112), non-Hispanic Asian (n = 168), non-Hispanic Black or African American (n = 155), and non-Hispanic White (n = 3881). A fifth category, other (including Hispanic American Indian or Alaskan Native, Hispanic Asian, Hispanic Native Hawaiian or Pacific Islander, and Hispanic Black or African American; non-Hispanic American Indian or Alaskan Native and non-Hispanic Native Hawaiian or Pacific Islander; and anyone who identified as unknown, did not report race/ethnicity, or identified as more than 1 race/ethnicity19), was used for all remaining participants (n = 101).
To probe different cognitive domains, we examined the performance on the individual components of the Preclinical Alzheimer Cognitive Composite23,24: MMSE (score range: 0-30, with higher scores indicating better cognitive performance), Digit Symbol Substitution Test (DSST; score range: 0-91, with higher scores indicating correct responses in 90 seconds), Logical Memory Delayed Recall test (score range: 0-25, with higher scores indicating more items recalled), and Free and Cued Selective Reminding Test (FCSRT; score range: 0-48, with higher scores indicating more items recalled). We examined 2 FCSRT scores: free recall across all 3 trials (maximum score of 48) and total recall, which is a sum of both free recall and cueing of items that were not freely recalled across all 3 trials (maximum score of 48).24,25 To assess subjective reports of cognition, we used the Cognitive Function Index (CFI), a questionnaire that was administered separately to the participants and their study partner (score range: 0-15, with higher scores indicating worse reported cognitive function).26 Depressive symptoms were evaluated with the Geriatric Depression Scale (GDS), an instrument that does not include questions about sleep disturbance (score range: 0-15, with higher scores indicating higher depressive symptoms). In a lifestyle questionnaire, participants were asked to report their typical number of hours of sleep per night, minutes of daytime naps per day, and caffeinated and/or alcoholic drinks per day.
All participants received an injection of 10 mCi of fluorine 18–labeled-florbetapir and underwent a PET scan 50 to 70 minutes after injection. Mean cortical standardized uptake value ratio was calculated using a whole cerebellar reference region.19 All analyses used continuous standardized uptake value ratios.
Statistical Analysis
Demographic characteristics of the sleep duration groups (short sleep duration: ≤6 hours, normal sleep duration: 7-8 hours, and long sleep duration: ≥9 hours) were compared using χ2 tests for categorical variables and analysis of variance for continuous variables. Associations between sleep duration and outcomes were assessed using linear regression. To explore nonlinear associations with sleep duration, analyses were repeated using a 3-factor variable (short sleep duration: ≤6 hours, normal sleep duration: 7-8 hours, and long sleep duration: ≥9 hours7,27) in linear regressions, with all 3 post hoc contrasts examined (short vs normal sleep duration, long vs normal sleep duration, and short vs long sleep duration). Logistic regression was performed to identify the association between sleep duration and the maximum FCSRT total recall score (48 vs <48), given that most older individuals who are clinically normal are at the upper score limit but that slight decrements in cueing are associated with early changes related to AD.24,25,28
All analyses included the following covariates: age, sex, years of education, self-identified race/ethnicity, number of APOE ε2 alleles, and number of APOE ε4 alleles. No multiple comparisons correction was performed. All statistical analyses were performed in R, version 4.0.2 (R Foundation for Statistical Computing). We considered 2-sided P < .05 to be statistically significant.
We visualized associations by reclassifying sleep duration into 5 groups (≤5 hours [n = 288; 7%], 6 hours [n = 897; 20%], 7 hours [n = 1574; 36%], 8 hours [n = 1375; 31%], and ≥9 hours [n = 283; 6%]), using the adjusted mean and SE of each outcome by sleep duration group. This strategy was used for data visualization purposes only; group contrasts were not performed across the 5 groups.
Results
The 4417 participants included in the study consisted of 2618 women (59%) and 1799 men (40%) with a mean (SD) age of 71.3 (4.7) years. When examined individually, all demographic variables (age, sex, years of education, and race/ethnicity) were associated with sleep duration (Table 1). In a multiple regression model that examined all demographic variables simultaneously, sex, years of education, and race/ethnicity were independently associated with sleep duration. Specifically, female sex (β [SE] = 0.07 [0.03]; P = .03) and greater years of education (β [SE] = 0.02 [0.01]; P = .002) were both significantly associated with self-reported longer nightly sleep duration. Compared with non-Hispanic White participants, non-Hispanic Black or African American participants reported a mean (SD) sleep duration of 37.9 (5.0) minutes less (P < .001), non-Hispanic Asian participants reported 27.3 (5.2) minutes less (P < .001), and Latino or Hispanic White participants reported 15.0 (6.1) minutes less (P = .01).
Table 1. Demographic Characteristics of Sleep Duration Groupsa.
| Variable | No. (%) | |||
|---|---|---|---|---|
| Overall | Short sleep duration: ≤6 h | Normal sleep duration: 7-8 h | Long sleep duration: ≥9 h | |
| All participants | 4417 (100) | 1185 (27) | 2949 (67) | 283 (6) |
| Age, mean (SD), y | 71.3 (4.7) | 71.4 (4.6) | 71.2 (4.7) | 72.0 (4.8) |
| Women | 2618 (59) | 689 (58) | 1747 (59) | 182 (64) |
| Men | 1799 (41) | 496 (42) | 1202 (41) | 101 (36) |
| Years of education, mean (SD), y | 16.6 (2.8) | 16.3 (3.0) | 16.7 (2.8) | 16.7 (2.9) |
| Race/ethnicity | ||||
| Non-Hispanic Asian | 168 (4) | 81 (48) | 82 (49) | 5 (3) |
| Non-Hispanic Black or African American | 155 (4) | 74 (48) | 78 (50) | 3 (2) |
| Latino or Hispanic White | 112 (3) | 39 (35) | 69 (62) | 4 (4) |
| Non-Hispanic White | 3881 (88) | 956 (25) | 2663 (69) | 262 (7) |
| Otherb | 101 (2) | 35 (35) | 57 (56) | 9 (9) |
| APOE genotype | ||||
| ε2/ε2 | 25 (1) | 8 (32) | 16 (64) | 1 (4) |
| ε2/ε3 | 445 (10) | 131 (29) | 287 (64) | 27 (6) |
| ε2/ε4 | 115 (3) | 35 (30) | 77 (67) | 3 (3) |
| ε3/ε3 | 2405 (54) | 629 (26) | 1618 (67) | 158 (7) |
| ε3/ε4 | 1290 (29) | 341 (26) | 862 (67) | 87 (7) |
| ε4/ε4 | 137 (3) | 41 (30) | 89 (65) | 7 (5) |
Abbreviation: APOE, apolipoprotein E.
Categorical variables were compared using χ2 tests, and continuous variables were compared using analysis of variance tests. Sleep duration groups differed on the basis of age, years of education, and self-identified race/ethnicity.
Other included Hispanic American Indian or Alaskan Native, Hispanic Asian, Hispanic Native Hawaiian or Pacific Islander, and Hispanic Black or African American; non-Hispanic American Indian or Alaskan Native and non-Hispanic Native Hawaiian or Pacific Islander; and anyone who identified as unknown, did not report race/ethnicity, or identified as more than 1 race/ethnicity.
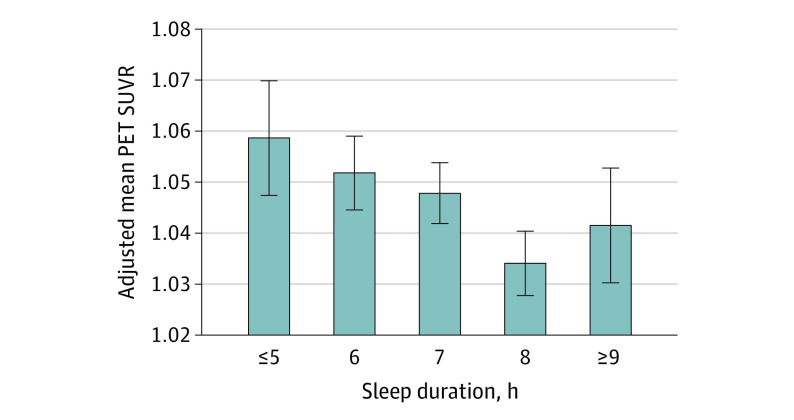
Associations between sleep duration and Aβ burden, cognitive function, and lifestyle outcomes are presented in Table 2. Higher Aβ burden was associated with fewer hours of nightly sleep (β [SE] = –0.01 [0.00]; P = .005) (Figure 1). When comparing short and long sleep durations with normal (7 to 8 hours) sleep duration, only short sleep duration was associated with elevated Aβ (short vs normal sleep duration: β [SE] = 0.01 [0.01], P = .048; long vs normal sleep duration: β [SE] = 0.00 [0.01], P = .99) (Table 2).
Table 2. Summary of Sleep Duration Associations With Amyloid-β Positron Emission Tomography (PET), Cognitive Performance, and Lifestyle Outcomesa.
| Variable | Overall | Short sleep duration: ≤6 h | Normal sleep duration: 7-8 h | Long sleep duration: ≥9 h | Linear regression | Short vs normal sleep duration: ≤6 h vs 7-8 h | Long vs normal sleep duration: ≥9 h vs 7-8 h | Short vs long sleep duration: ≤6 h vs ≥9 h | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β (SE) | P value | β (SE) | P value | β (SE) | P value | β (SE) | P value | |||||
| No. of participants (%) | 4417 | 1185 (27) | 2949 (67) | 283 (6) | NA | NA | NA | NA | NA | NA | NA | NA |
| Sleep, h/night | 7.1 (0.5) | 5.7 (0.5) | 7.4 (0.5) | 9.2 (0.5) | NA | NA | NA | NA | NA | NA | NA | NA |
| Daytime nap, min/d | 12.5 (22.6) | 14.6 (26.2) | 11.5 (20.3) | 15.2 (27.1) | −0.86 (0.32) | .01 | 2.66 (0.77) | .001 | 3.62 (1.38) | .01 | −0.96 (1.48) | .52 |
| Caffeine intake, drinks/d | 2.2 (2.1) | 2.2 (2.2) | 2.2 (2.1) | 2.1 (1.7) | −0.02 (0.03) | .53 | 0.04 (0.07) | .59 | −0.02 (0.13) | .88 | 0.06 (0.14) | .67 |
| Alcohol intake, drinks/d | 0.8 (1.2) | 0.7 (1.1) | 0.8 (1.0) | 1.1 (2.5) | 0.07 (0.02) | <.001 | −0.03 (0.04) | .51 | 0.38 (0.07) | <.001 | −0.41 (0.08) | <.001 |
| PET SUVR | 1.09 (0.2) | 1.10 (0.2) | 1.09 (0.2) | 1.10 (0.2) | −0.01 (0.00) | .005 | 0.01 (0.01) | .048 | 0.00 (0.01) | .99 | 0.01 (0.01) | .30 |
| GDS total scoreb | 1.0 (1.5) | 1.3 (1.7) | 0.9 (1.3) | 1.3 (1.6) | −0.06 (0.02) | .005 | 0.31 (0.05) | <.001 | 0.39 (0.09) | <.001 | −0.08 (0.10) | .38 |
| BMI | 27.5 (5.1) | 27.8 (5.3) | 27.3 (5.0) | 28.2 (5.2) | −0.06 (0.07) | .43 | 0.48 (0.17) | .01 | 0.97 (0.31) | .002 | −0.49 (0.33) | .13 |
| MMSE scorec | 28.8 (1.2) | 28.7 (1.2) | 28.8 (1.2) | 28.8 (1.3) | 0.02 (0.02) | .15 | −0.08 (0.04) | .04 | −0.09 (0.07) | .25 | 0.00 (0.08) | .97 |
| FCSRT free recall scored | 29.0 (5.6) | 29.1 (5.4) | 29.0 (6.5) | 28.4 (6.0) | −0.14 (0.07) | .052 | 0.23 (0.18) | .21 | −0.50 (0.33) | .12 | 0.73 (0.35) | .04 |
| FCSRT total recall scoree | 47.4 (0.9) | 47.3 (0.9) | 47.4 (0.9) | 47.4 (1.0) | 0.03 (0.03) | .26 | −0.18 (0.07) | .01 | −0.20 (0.13) | .12 | 0.02 (0.14) | .90 |
| Logical Memory Delayed Recall test scoref | 11.7 (3.2) | 11.4 (3.1) | 11.8 (3.2) | 11.8 (3.1) | 0.06 (0.04) | .15 | −0.23 (0.11) | .03 | −0.04 (0.19) | .83 | −0.19 (0.21) | .35 |
| DSST scoreg | 43.8 (9.0) | 43.4 (9.1) | 44.0 (8.9) | 42.6 (9.3) | −0.16 (0.12) | .17 | −0.14 (0.29) | .63 | −1.17 (0.52) | .02 | 1.04 (0.55) | .06 |
| Participant-assessed CFIh | 3.0 (2.0) | 3.3 (2.2) | 2.8 (1.9) | 3.4 (2.5) | −0.06 (0.03) | .03 | 0.34 (0.07) | <.001 | 0.57 (0.12) | <.001 | −0.22 (0.13) | .09 |
| Study partner–assessed CFIi | 2.2 (1.8) | 2.4 (2.0) | 2.1 (1.7) | 2.5 (2.0) | −0.05 (0.03) | .06 | 0.22 (0.06) | <.001 | 0.33 (0.11) | .003 | −0.11 (0.12) | .34 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CFI, Cognitive Function Index; DSST, Digit Symbol Substitution Test; FCSRT, Free and Cued Selective Reminding Test; GDS, Geriatric Depression Scale; MMSE, Mini-Mental State Examination; NA, not applicable; SUVR, standardized uptake value ratio.
Mean (SD) values for each sleep duration group are not adjusted. Linear regressions included age, sex, self-identified race/ethnicity, years of education, number of APOE ε2 alleles, and number of APOE ε4 alleles as covariates.
GDS score range: 0-15, with higher scores indicating higher depressive symptoms.
MMSE score range: 0-30, with higher scores indicating better cognitive performance.
FCSRT free recall score range: 0-48, with higher scores indicating more items recalled.
FCSRT total recall score range: 0-48, with higher scores indicating more items recalled.
Logical Memory Delayed Recall score range: 0-25, with higher scores indicating more items recalled.
DSST score range: 0-91, with higher scores indicating correct responses in 90 seconds.
Participant-assessed CFI score range: 0-15, with higher scores indicating worse reported cognitive function.
Study partner–assessed CFI score range: 0-15, with higher scores indicating worse reported cognitive function.
Figure 1. Association of Sleep Duration With Amyloid-β Positron Emission Tomography (PET) Standardized Uptake Value Ratio (SUVR).
Adjusted mean (SE) within each sleep duration group (≤5, 6, 7, 8, and ≥9 hours) was calculated for a mean age (71.3 [4.7] years), mean years of education (16.6 [2.8] years), and non-Hispanic White male participants with an APOE ε33 allele genotype.
No significant linear associations were found between sleep duration and objective cognitive test performance. However, when categorized by short, normal, or long sleep duration, the differences in cognitive performance were observed across groups, suggesting nonlinear associations. Participants with short sleep duration performed significantly worse on the MMSE than those who reported normal sleep duration (β [SE] = –0.08 [0.04]; P = .04) (Figure 2A; Table 2) and Logical Memory Delayed Recall test (β [SE] = –0.23 [0.11]; P = .03) (Figure 2B; Table 2), and they were less likely to obtain the maximum FCSRT total recall score (β [SE] = –0.18 [0.07]; P = .01) (Table 2). Conversely, participants with long sleep duration performed significantly worse on the DSST than those who reported normal sleep duration (β [SE] = –1.17 [0.52]; P = .02) (Figure 2C; Table 2). In addition, those who had short sleep duration performed better on the FCSRT free recall than those with long sleep duration (β [SE] = 0.73 [0.35]; P = .04) (Table 2). No other differences in cognitive test scores were found between the short and long sleep duration groups.
Figure 2. Association of Sleep Duration With Cognitive Function.
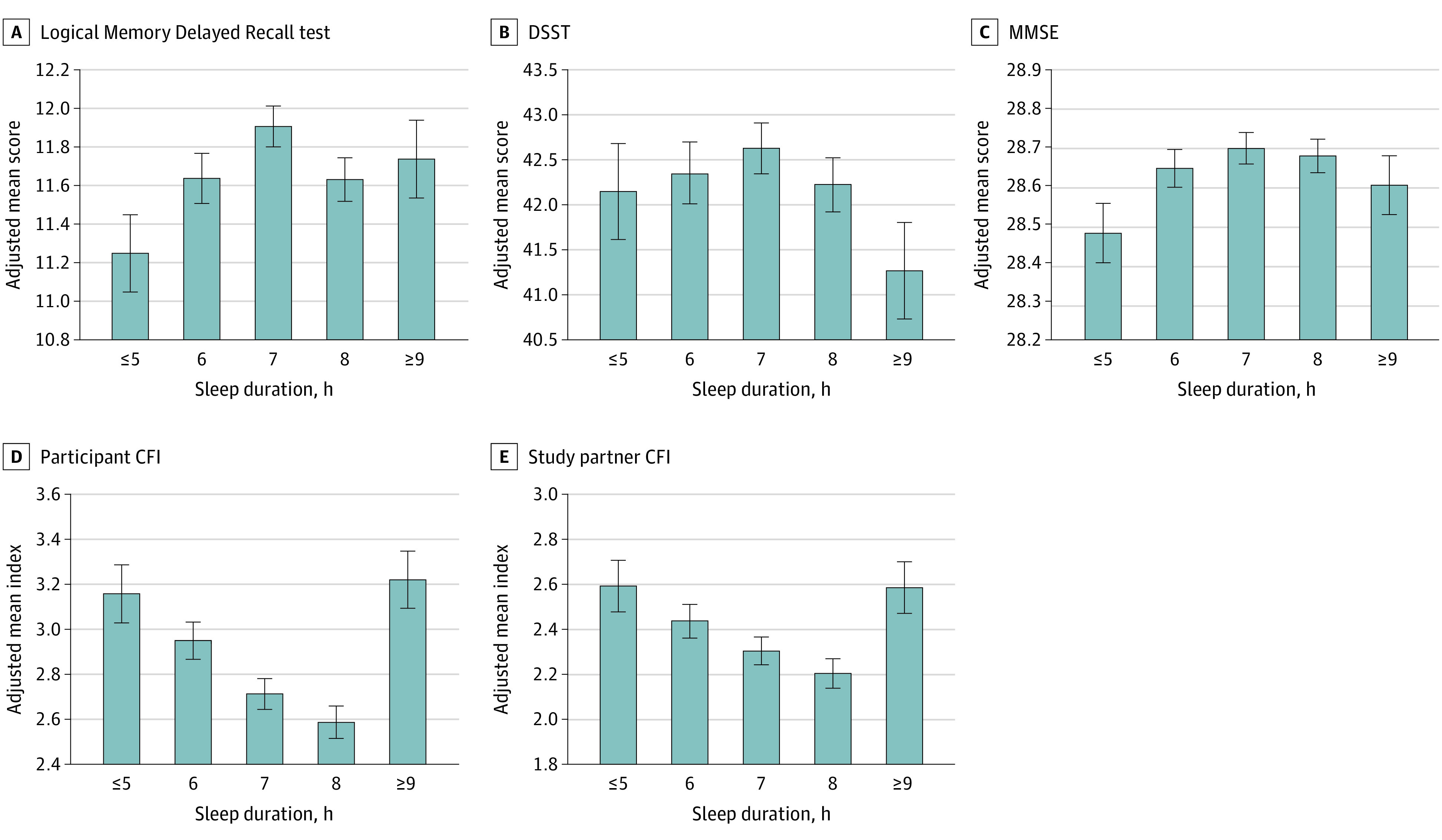
Adjusted mean (SE) within each sleep duration group (≤5, 6, 7, 8, and ≥9 hours) was calculated for a mean age (71.3 [4.7] years), mean years of education (16.6 [2.8] years), and non-Hispanic White male participants with an APOE ε33 allele genotype. CFI indicates Cognitive Function Index (score range: 0-15, with higher scores indicating worse reported cognitive function); DSST, Digit Symbol Substitution Test (score range: 0-91, with higher scores indicating correct responses in 90 seconds); and MMSE, Mini-Mental State Examination (score range: 0-30, with higher scores indicating better cognitive performance).
Subjective report of cognitive ability, measured by the CFI, also differed on the basis of sleep duration. Better self-reported cognitive function (lower score on the participant-assessed CFI) had a linear association with longer sleep duration (β [SE] = –0.06 [0.03]; P = .03) (Table 2). Short and long sleep durations also were both associated with worse self-reported cognitive function compared with normal sleep duration on the participant-assessed CFI (short vs normal sleep duration: β [SE] = 0.34 [0.07], P < .001; long vs normal sleep duration: β [SE] = 0.57 [0.12], P < .001]) (Figure 2D; Table 2). The study partner–assessed CFI was also worse for the short and long sleep duration groups vs the normal sleep duration group (short vs normal sleep duration: β [SE] = 0.22 [0.06], P < .001; long vs normal sleep duration: β [SE] = 0.33 [0.11], P = .003) (Figure 2E; Table 2). No differences in study partner–assessed CFI scores were found between the short and long sleep duration groups (β [SE] = –0.11 [0.12]; P = .34).
Greater endorsement of depressive symptoms on the GDS had a linear association with shorter sleep duration (β [SE] = –0.06 [0.02]; P = .005) (Figure 3A; Table 2), and both short and long sleep duration groups reported more depressive symptoms than the normal sleep duration group (short vs normal sleep duration: β [SE] = 0.31 [0.05], P < .001; long vs normal sleep duration: β [SE] = 0.39 [0.09], P < .001) (Table 2). No linear association was observed between body mass index (BMI) and sleep duration, but BMI was elevated in both short and long sleep duration groups (short vs normal sleep duration: β [SE] = 0.48 [0.17], P = .01; long vs normal sleep duration: β [SE] = 0.97 [0.31], P = .002) (Figure 3B; Table 2). Self-reported caffeine consumption was not associated with sleep duration. Self-reported alcohol consumption had a linear association with nighttime sleep duration (β [SE] = 0.07 [0.02]; P < .001) (Table 2), and those in the long sleep duration group consumed more alcoholic drinks compared with those in both normal and short sleep duration groups (long vs normal sleep duration: β [SE] = 0.38 [0.07], P < .001; long vs short sleep duration: β [SE] = –0.41 [0.08], P < .001) (Table 2). Longer daytime nap was associated with less continuous nighttime sleep duration (β [SE] = –0.86 [0.32]; P = .01) (Table 2) and was significantly elevated in participants in both short and long sleep duration groups vs normal sleep duration group (short vs normal sleep duration: β [SE] = 2.66 [0.77], P = .001; long vs normal sleep duration: β [SE] = 3.62 [1.38], P = .01; short vs long duration: β [SE] = –0.96 [1.48], P = .52) (Figure 3C; Table 2).
Figure 3. Association of Sleep Duration With Lifestyle Outcomes.
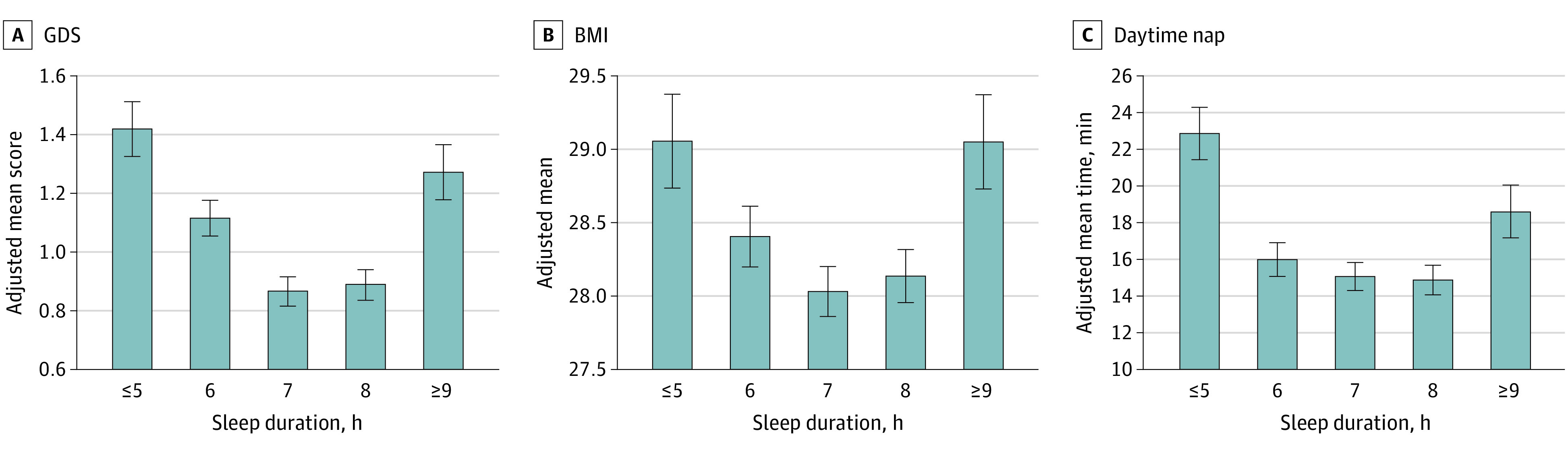
Adjusted mean (SE) within each sleep duration group (≤5, 6, 7, 8, and ≥9 hours) was calculated for a mean age (71.3 [4.7] years), mean years of education (16.6 [2.8] years), and non-Hispanic White male participants with an apolipoprotein E ε33 allele genotype. BMI indicates body mass index (calculated as weight in kilograms divided by height in meters squared); GDS, Geriatric Depression Scale (score range: 0-15, with higher scores indicating higher depressive symptoms).
Two additional sets of analyses were performed to ascertain whether associations between nighttime sleep duration and Aβ burden as well as nighttime sleep duration and cognition remained significant after controlling for lifestyle factors. First, we repeated the analyses with Aβ PET and cognitive outcomes as the dependent variables and included lifestyle variables as the covariates (summarized in eTable 1 in the Supplement). Adding these covariates did not change the association between nighttime sleep duration and Aβ or cognitive outcomes, with the exception of DSST score, which was associated with GDS score, BMI, and daytime napping duration but was no longer significantly associated with long sleep duration. Napping was a significant independent factor in FCSRT free recall (β [SE] = –0.01 [0.00]; P < .001) and participant-assessed CFI (β [SE] = 0.00 [0.00]; P < .001) scores in addition to nighttime sleep duration (eTable 1 in the Supplement).
Second, given the nonlinear association between nighttime sleep and daytime nap duration (Figure 3C), we tested the interaction between nighttime sleep and daytime nap to ascertain whether the associations with napping were dependent on short vs long sleep duration. However, we found no significant interaction between nocturnal sleeping and daytime napping, with the exception of FCSRT total recall performance (β [SE] = –0.01 [0.01]; P = .03) (eTable 2 and eFigure 2 in the Supplement).
Discussion
We found that self-reported short and long sleep durations were associated with worse outcomes in cognitively healthy older adults. Sleep duration of 6 hours or less was associated with higher Aβ burden and lower memory performance. In contrast, sleep duration of 9 hours or more was associated with worse executive function test performance. Both short and long sleep durations were associated with worse self-reported cognitive function and multiple lifestyle outcomes, including higher BMI, greater depressive symptoms, and more time spent napping during the day, suggesting a U-shaped association between sleep duration and these variables. Overall, this pattern highlights that multiple independent factors are associated with sleep duration in aging.
We identified an association between self-reported shorter sleep duration and greater Aβ burden. This finding is consistent with results of a previous study of 70 older adults, which showed that self-reported short sleep duration was associated with elevated Aβ burden,15 and studies that found that sleep restriction accelerated Aβ deposition in rodent models of AD.29,30 The association between sleep loss and Aβ accumulation has been attributed to increased production of Aβ at synapses during wake vs sleep29,31 combined with a reduction in clearance of Aβ through the glymphatic system, which is preferentially active during non–rapid eye movement sleep.32,33,34 In the present cross-sectional study, the subjective measure of short sleep duration may serve as a proxy measure for other indices of poor sleep quality. This suggestion is supported by previous work that reported an association between greater Aβ burden and several metrics of decreased sleep quality, including subjective sleep quality,35,36 objective sleep efficiency and fragmentation,37,38 and disruptions of non–rapid eye movement slow wave activity.39,40,41 The association between short sleep duration and Aβ is also consistent with our finding that short sleep duration was associated with subtle deficits in memory (per Logical Memory Delayed Recall test and FCSRT total recall scores). Although the direction of these associations is unclear, the findings in this study provide further support to the theory that short sleep in aging is associated with early AD processes.42,43 The causality of these associations cannot be established with these cross-sectional data.
We found no difference in association with Aβ burden between long and normal sleep durations. To our knowledge, this study was the first to examine whether long sleep duration, which is believed to be a marker of poor health in older adults,44,45 is associated with Aβ levels. The finding suggests that early AD processes do not mediate the established association between long sleep duration and cognitive decline.46 Long sleep duration was associated with a number of other phenotypes related to aging (eg, worse executive function, worse subjective cognition, higher GDS score, higher BMI), highlighting that the adverse impact of longer sleep is generally associated with aging processes that are independent of early Aβ. Reported long sleep duration reflected a small proportion of the population in the Netherlands, United Kingdom, and United States,3 drawing attention to a unique feature of the present study: the ability to analyze this group in a large sample of older individuals with normal cognition characterized through Aβ PET. This group has largely been ignored in biomarker cohorts that typically comprised fewer than 200 individuals. This study found that, although this group had no evidence of elevated Aβ levels, it demonstrated subtle cognitive decline, especially in executive function (per DSST and FCSRT free recall scores). We also found some evidence of a dissociation within the FCSRT scores, with subtle deficits in the free recall portion (which is believed to capture frontal retrieval and attention processes) associated with long sleep duration and the cueing component (which is more specific to AD and may be mediated by the medial temporal lobe47) associated with short sleep. Overall, the association between executive function and long sleep duration highlights the role of additional age-related processes. Future work is needed to explore the neural correlates of cognitive aging in individuals with long sleep duration.
The findings on cognition build on previous descriptions of inverted U-shaped associations between self-reported sleep duration and cognition in aging, in which extreme sleep durations were associated with worse cognitive performance.6,7,13,14,46 A meta-analysis of sleep duration and cognition in aging reported that this U-shaped association is generally consistent across the literature, but the associations with specific cognitive domains are inconsistent.13 It remains controversial whether some cognitive domains are more affected by extreme sleep duration than other domains and whether short and long sleep durations may be associated with distinct patterns of cognitive dysfunction. In a large, well-characterized cohort of healthy older adults who met the screening criteria for the A4 Study, we found a distinction between sleep duration phenotypes in which those with short sleep duration performed worse on tests of memory and those with long sleep duration performed worse in an executive function task.
Subjective cognitive function, as assessed with the CFI,26 also showed a U-shaped association with sleep duration, with both short and long duration phenotypes endorsing worse cognitive function compared with normal sleep duration. The CFI focuses on ability to perform activities of daily living but excludes sleep. This pattern held for both participant-assessed and study partner–assessed CFI, which meant that older individuals who were sleeping outside the recommended 7 to 8 hours (and their study partners) reported worse cognitive function, even when adjusted for other known factors in late-life cognition, such as demographic characteristics and APOE ε2 and ε4 alleles. This association supports a previous finding that CFI scores were associated with worse subjective sleep quality,48 and this finding is important because CFI performance is a sign of subsequent clinical progression.26
Sleep duration was shorter in male participants; individuals with fewer years of education; and individuals who identified as non-Hispanic Black or African American, non-Hispanic Asian, or Latino or Hispanic White, replicating previous reports that sociodemographic variables were associated with sleep duration in late life.21,22,49,50 The substantive differences in sleep duration across racial/ethnic groups provide further evidence that disparities in sleep may be associated with disparities in other areas, such as cardiovascular and metabolic health.21,51 Although socioeconomic factors likely play a role,52,53 previous examinations of the link between racial/ethnic identity and sleep duration have suggested that racial discrimination and perceived racism are associated with less sleep.54,55,56 More data are needed from racial/ethnic minority populations to assess whether the sleep duration associations reported in studies involving mostly non-Hispanic White participants (such as the present cohort) are generalizable to the population at large.
Sleep duration was associated with several lifestyle variables of health outcomes in aging. First, those with short and long sleep durations reported more depressive symptoms than individuals who slept 7 to 8 hours, which is consistent with previous reports that linked subjective sleep disruption to mood in older adults.4,5 Second, BMI was elevated in participants with short and long sleep durations. Insufficient sleep has been associated with higher BMI in several studies of older adults, and both short and long sleep durations have been found to increase the risk for cardiovascular disease.10,57,58 The association between BMI and AD risk is complex, with higher BMI in midlife associated with late-life dementia risk but lower BMI in late life associated with elevated Aβ.59 Thus, further work is needed to evaluate the associations among BMI, early-onset AD, and sleep pattern across the life span.
Third, participants in both short and long sleep duration groups reported napping longer during the day. Napping behaviors in aging are complex but have been established as a risk factor in future decline, independent of nighttime sleep,60 which is consistent with a finding in the present study that nighttime and daytime sleep independently relate to some measures of cognition. In this study, the U-shaped association between nocturnal sleep duration and daytime napping suggests that individuals with short sleep duration may compensate with napping, whereas those with long sleep duration may experience excessive sleepiness throughout the day. Analyses that included an interaction term between sleep duration and daytime nap duration found that daytime napping was an independent factor in several models (ie, FCSRT free recall, DSST, and CFI), suggesting that daytime napping is independently associated with these outcomes in addition to nocturnal sleep duration. In models that included multiple lifestyle factors (ie, BMI, GDS, and daytime napping) as covariates, these variables were generally not associated with Aβ or cognition. The exception was the DSST, with which nocturnal sleep was no longer significantly associated (although a pattern of continuous sleep duration was found); instead, all lifestyle variables showed significant associations with this cognitive measure. This finding implies that multiple factors (eg, depression, BMI, and daytime napping) may be independently associated with worse executive function and may have an upstream physiological basis, such as cardiovascular health, that was not measured in the current study.
Limitations
This study has some limitations. First, the study lacked data on sleep-disordered breathing, which is a risk factor for cognitive decline61,62 and elevated Aβ63,64,65,66 but is typically not associated with self-reported sleep duration.67 We also did not have data on use of medications that might affect sleep duration and could be associated with worse cognitive function.7 Second, we used self-reported sleep duration information rather than sleep data from objective measures, such as actigraphy or polysomnography. Subjective (self-reported) and objective sleep times differ and may represent distinct signals of an individual’s sleep health.3,68 Comparisons of these objective and subjective measures of sleep in older adults have shown that these measures are typically not correlated,69,70,71 suggesting that self-reported measures may carry information that is different from that in objective measures.72
Third, the cross-sectional design of this study did not allow us to establish the direction of associations between sleep and the outcome variables, which is likely bidirectional. Fourth, the modest effect sizes of some of the associations with self-reported sleep duration limited the clinical implications of these findings.
Conclusions
Findings from this cross-sectional study show that short and long sleep duration were both associated with worse outcomes in older adults with normal cognition; the specific differences between sleep duration phenotypes suggest their distinct physiological bases. The association between sleep duration and multiple intersecting health outcomes, such as greater Aβ burden, greater depressive symptoms, higher BMI, and cognitive decline, highlight the importance of maintaining adequate sleep in late life.
eFigure 1. Flow Chart of Participant Inclusion Process
eFigure 2. Nocturnal Sleep Duration and Daytime Nap Duration Associations With Amyloid PET and Cognitive Function
eTable 1. Summary of Sleep Duration Associations With Amyloid PET and Cognitive Performance With Lifestyle Outcomes Included as Covariates
eTable 2. Summary of Associations Between the Interaction Term of Sleep Duration * Daytime Napping With Amyloid PET and Cognitive Performance
References
- 1.Mander BA, Winer JR, Walker MP. Sleep and human aging. Neuron. 2017;94(1):19-36. doi: 10.1016/j.neuron.2017.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27(7):1255-1273. doi: 10.1093/sleep/27.7.1255 [DOI] [PubMed] [Google Scholar]
- 3.Kocevska D, Lysen TS, Dotinga A, et al. Sleep characteristics across the lifespan in 1.1 million people from the Netherlands, United Kingdom and United States: a systematic review and meta-analysis. Nat Hum Behav. 2021;5(1):113-122. doi: 10.1038/s41562-020-00965-x [DOI] [PubMed] [Google Scholar]
- 4.Franzen PL, Buysse DJ. Sleep disturbances and depression: risk relationships for subsequent depression and therapeutic implications. Dialogues Clin Neurosci. 2008;10(4):473-481. doi: 10.31887/DCNS.2008.10.4/plfranzen [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Potvin O, Lorrain D, Belleville G, Grenier S, Préville M. Subjective sleep characteristics associated with anxiety and depression in older adults: a population-based study. Int J Geriatr Psychiatry. 2014;29(12):1262-1270. doi: 10.1002/gps.4106 [DOI] [PubMed] [Google Scholar]
- 6.Devore EE, Grodstein F, Duffy JF, Stampfer MJ, Czeisler CA, Schernhammer ES. Sleep duration in midlife and later life in relation to cognition. J Am Geriatr Soc. 2014;62(6):1073-1081. doi: 10.1111/jgs.12790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Virta JJ, Heikkilä K, Perola M, et al. Midlife sleep characteristics associated with late life cognitive function. Sleep. 2013;36(10):1533-1541, 1541A. doi: 10.5665/sleep.3052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lim ASP, Yu L, Kowgier M, Schneider JA, Buchman AS, Bennett DA. Modification of the relationship of the apolipoprotein E ε4 allele to the risk of Alzheimer disease and neurofibrillary tangle density by sleep. JAMA Neurol. 2013;70(12):1544-1551. doi: 10.1001/jamaneurol.2013.4215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lysen TS, Luik AI, Ikram MK, Tiemeier H, Ikram MA. Actigraphy-estimated sleep and 24-hour activity rhythms and the risk of dementia. Alzheimers Dement. 2020;16(9):1259-1267. doi: 10.1002/alz.12122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cappuccio FP, Cooper D, D’Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J. 2011;32(12):1484-1492. doi: 10.1093/eurheartj/ehr007 [DOI] [PubMed] [Google Scholar]
- 11.Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2010;33(2):414-420. doi: 10.2337/dc09-1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirshkowitz M, Whiton K, Albert SM, et al. National Sleep Foundation’s updated sleep duration recommendations: final report. Sleep Health. 2015;1(4):233-243. doi: 10.1016/j.sleh.2015.10.004 [DOI] [PubMed] [Google Scholar]
- 13.Lo JC, Groeger JA, Cheng GH, Dijk D-J, Chee MWL. Self-reported sleep duration and cognitive performance in older adults: a systematic review and meta-analysis. Sleep Med. 2016;17:87-98. doi: 10.1016/j.sleep.2015.08.021 [DOI] [PubMed] [Google Scholar]
- 14.Ma Y, Liang L, Zheng F, Shi L, Zhong B, Xie W. Association between sleep duration and cognitive decline. JAMA Netw Open. 2020;3(9):e2013573. doi: 10.1001/jamanetworkopen.2020.13573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spira AP, Gamaldo AA, An Y, et al. Self-reported sleep and β-amyloid deposition in community-dwelling older adults. JAMA Neurol. 2013;70(12):1537-1543. doi: 10.1001/jamaneurol.2013.4258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jack CR Jr, Bennett DA, Blennow K, et al. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14(4):535-562. doi: 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jagust W. Imaging the evolution and pathophysiology of Alzheimer disease. Nat Rev Neurosci. 2018;19(11):687-700. doi: 10.1038/s41583-018-0067-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sperling RA, Rentz DM, Johnson KA, et al. The A4 study: stopping AD before symptoms begin? Sci Transl Med. 2014;6(228):228fs13. doi: 10.1126/scitranslmed.3007941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sperling RA, Donohue MC, Raman R, et al. ; A4 Study Team . Association of factors with elevated amyloid burden in clinically normal older individuals. JAMA Neurol. 2020;77(6):735-745. doi: 10.1001/jamaneurol.2020.0387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deters KD, Napolioni V, Sperling RA, et al. Amyloid PET imaging in self-identified non-Hispanic Black participants of the anti-amyloid in asymptomatic Alzheimer’s disease (A4) study. Neurology. 2021;96(11):e1491-e1500. doi: 10.1212/WNL.0000000000011599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kingsbury JH, Buxton OM, Emmons KM. Sleep and its relationship to racial and ethnic disparities in cardiovascular disease. Curr Cardiovasc Risk Rep. 2013;7(5). doi: 10.1007/s12170-013-0330-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carnethon MR, De Chavez PJ, Zee PC, et al. Disparities in sleep characteristics by race/ethnicity in a population-based sample: Chicago Area Sleep Study. Sleep Med. 2016;18:50-55. doi: 10.1016/j.sleep.2015.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donohue MC, Sperling RA, Salmon DP, et al. ; Australian Imaging, Biomarkers, and Lifestyle Flagship Study of Ageing; Alzheimer’s Disease Neuroimaging Initiative; Alzheimer’s Disease Cooperative Study . The preclinical Alzheimer cognitive composite: measuring amyloid-related decline. JAMA Neurol. 2014;71(8):961-970. doi: 10.1001/jamaneurol.2014.803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mormino EC, Papp KV, Rentz DM, et al. Early and late change on the preclinical Alzheimer’s cognitive composite in clinically normal older individuals with elevated amyloid β. Alzheimers Dement. 2017;13(9):1004-1012. doi: 10.1016/j.jalz.2017.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grober E, Sanders AE, Hall C, Lipton RB. Free and cued selective reminding identifies very mild dementia in primary care. Alzheimer Dis Assoc Disord. 2010;24(3):284-290. doi: 10.1097/WAD.0b013e3181cfc78b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amariglio RE, Donohue MC, Marshall GA, et al. ; Alzheimer’s Disease Cooperative Study . Tracking early decline in cognitive function in older individuals at risk for Alzheimer disease dementia: the Alzheimer’s Disease Cooperative Study Cognitive Function Instrument. JAMA Neurol. 2015;72(4):446-454. doi: 10.1001/jamaneurol.2014.3375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kronholm E, Sallinen M, Suutama T, Sulkava R, Era P, Partonen T. Self-reported sleep duration and cognitive functioning in the general population. J Sleep Res. 2009;18(4):436-446. doi: 10.1111/j.1365-2869.2009.00765.x [DOI] [PubMed] [Google Scholar]
- 28.Papp KV, Rentz DM, Mormino EC, et al. Cued memory decline in biomarker-defined preclinical Alzheimer disease. Neurology. 2017;88(15):1431-1438. doi: 10.1212/WNL.0000000000003812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang J-E, Lim MM, Bateman RJ, et al. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326(5955):1005-1007. doi: 10.1126/science.1180962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roh JH, Huang Y, Bero AW, et al. Disruption of the sleep-wake cycle and diurnal fluctuation of β-amyloid in mice with Alzheimer’s disease pathology. Sci Transl Med. 2012;4(150):150ra122. doi: 10.1126/scitranslmed.3004291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lucey BP, Hicks TJ, McLeland JS, et al. Effect of sleep on overnight cerebrospinal fluid amyloid β kinetics. Ann Neurol. 2018;83(1):197-204. doi: 10.1002/ana.25117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342(6156):373-377. doi: 10.1126/science.1241224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hablitz LM, Plá V, Giannetto M, et al. Circadian control of brain glymphatic and lymphatic fluid flow. Nat Commun. 2020;11(1):4411. doi: 10.1038/s41467-020-18115-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fultz NE, Bonmassar G, Setsompop K, et al. Coupled electrophysiological, hemodynamic, and cerebrospinal fluid oscillations in human sleep. Science. 2019;366(6465):628-631. doi: 10.1126/science.aax5440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Branger P, Arenaza-Urquijo EM, Tomadesso C, et al. Relationships between sleep quality and brain volume, metabolism, and amyloid deposition in late adulthood. Neurobiol Aging. 2016;41:107-114. doi: 10.1016/j.neurobiolaging.2016.02.009 [DOI] [PubMed] [Google Scholar]
- 36.Sprecher KE, Koscik RL, Carlsson CM, et al. Poor sleep is associated with CSF biomarkers of amyloid pathology in cognitively normal adults. Neurology. 2017;89(5):445-453. doi: 10.1212/WNL.0000000000004171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ju YE-S, McLeland JS, Toedebusch CD, et al. Sleep quality and preclinical Alzheimer disease. JAMA Neurol. 2013;70(5):587-593. doi: 10.1001/jamaneurol.2013.2334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ettore E, Bakardjian H, Solé M, et al. Relationships between objectives sleep parameters and brain amyloid load in subjects at risk for Alzheimer’s disease: the INSIGHT-preAD Study. Sleep. 2019;42(9):zsz137. doi: 10.1093/sleep/zsz137 [DOI] [PubMed] [Google Scholar]
- 39.Mander BA, Marks SM, Vogel JW, et al. β-amyloid disrupts human NREM slow waves and related hippocampus-dependent memory consolidation. Nat Neurosci. 2015;18(7):1051-1057. doi: 10.1038/nn.4035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Varga AW, Wohlleber ME, Giménez S, et al. Reduced slow-wave sleep is associated with high cerebrospinal fluid Aβ42 levels in cognitively normal elderly. Sleep. 2016;39(11):2041-2048. doi: 10.5665/sleep.6240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Winer JR, Mander BA, Kumar S, et al. Sleep disturbance forecasts β-amyloid accumulation across subsequent years. Curr Biol. 2020;30(21):4291-4298.e3. doi: 10.1016/j.cub.2020.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mander BA, Winer JR, Jagust WJ, Walker MP. Sleep: a novel mechanistic pathway, biomarker, and treatment target in the pathology of Alzheimer’s disease? Trends Neurosci. 2016;39(8):552-566. doi: 10.1016/j.tins.2016.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang C, Holtzman DM. Bidirectional relationship between sleep and Alzheimer’s disease: role of amyloid, tau, and other factors. Neuropsychopharmacology. 2020;45(1):104-120. doi: 10.1038/s41386-019-0478-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grandner MA, Drummond SPA. Who are the long sleepers? Towards an understanding of the mortality relationship. Sleep Med Rev. 2007;11(5):341-360. doi: 10.1016/j.smrv.2007.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Benito-León J, Louis ED, Bermejo-Pareja F. Cognitive decline in short and long sleepers: a prospective population-based study (NEDICES). J Psychiatr Res. 2013;47(12):1998-2003. doi: 10.1016/j.jpsychires.2013.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yaffe K, Falvey CM, Hoang T. Connections between sleep and cognition in older adults. Lancet Neurol. 2014;13(10):1017-1028. doi: 10.1016/S1474-4422(14)70172-3 [DOI] [PubMed] [Google Scholar]
- 47.Lemos R, Duro D, Simões MR, Santana I. The free and cued selective reminding test distinguishes frontotemporal dementia from Alzheimer’s disease. Arch Clin Neuropsychol. 2014;29(7):670-679. doi: 10.1093/arclin/acu031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nicolazzo J, Xu K, Lavale A, et al. Sleep symptomatology is associated with greater subjective cognitive concerns: findings from the community-based Healthy Brain Project. Sleep. 2021;zsab097. doi: 10.1093/sleep/zsab097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leng Y, Wainwright NWJ, Cappuccio FP, et al. Self-reported sleep patterns in a British population cohort. Sleep Med. 2014;15(3):295-302. doi: 10.1016/j.sleep.2013.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.George KM, Peterson RL, Gilsanz P, et al. Racial/ethnic differences in sleep quality among older adults: Kaiser Healthy Aging and Diverse Life Experiences (KHANDLE) study. Ethn Dis. 2020;30(3):469-478. doi: 10.18865/ed.30.3.469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grandner MA, Williams NJ, Knutson KL, Roberts D, Jean-Louis G. Sleep disparity, race/ethnicity, and socioeconomic position. Sleep Med. 2016;18:7-18. doi: 10.1016/j.sleep.2015.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stamatakis KA, Kaplan GA, Roberts RE. Short sleep duration across income, education, and race/ethnic groups: population prevalence and growing disparities during 34 years of follow-up. Ann Epidemiol. 2007;17(12):948-955. doi: 10.1016/j.annepidem.2007.07.096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Whinnery J, Jackson N, Rattanaumpawan P, Grandner MA. Short and long sleep duration associated with race/ethnicity, sociodemographics, and socioeconomic position. Sleep. 2014;37(3):601-611. doi: 10.5665/sleep.3508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Slopen N, Williams DR. Discrimination, other psychosocial stressors, and self-reported sleep duration and difficulties. Sleep. 2014;37(1):147-156. doi: 10.5665/sleep.3326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thomas KS, Bardwell WA, Ancoli-Israel S, Dimsdale JE. The toll of ethnic discrimination on sleep architecture and fatigue. Health Psychol. 2006;25(5):635-642. doi: 10.1037/0278-6133.25.5.635 [DOI] [PubMed] [Google Scholar]
- 56.Beatty DL, Hall MH, Kamarck TA, et al. Unfair treatment is associated with poor sleep in African American and Caucasian adults: Pittsburgh SleepSCORE project. Health Psychol. 2011;30(3):351-359. doi: 10.1037/a0022976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Altman NG, Izci-Balserak B, Schopfer E, et al. Sleep duration versus sleep insufficiency as predictors of cardiometabolic health outcomes. Sleep Med. 2012;13(10):1261-1270. doi: 10.1016/j.sleep.2012.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Knutson KL. Sleep duration and cardiometabolic risk: a review of the epidemiologic evidence. Best Pract Res Clin Endocrinol Metab. 2010;24(5):731-743. doi: 10.1016/j.beem.2010.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hsu DC, Mormino EC, Schultz AP, et al. ; Harvard Aging Brain Study . Lower late-life body-mass index is associated with higher cortical amyloid burden in clinically normal elderly. J Alzheimers Dis. 2016;53(3):1097-1105. doi: 10.3233/JAD-150987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leng Y, Redline S, Stone KL, Ancoli-Israel S, Yaffe K. Objective napping, cognitive decline, and risk of cognitive impairment in older men. Alzheimers Dement. 2019;15(8):1039-1047. doi: 10.1016/j.jalz.2019.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yaffe K, Laffan AM, Harrison SL, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306(6):613-619. doi: 10.1001/jama.2011.1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Osorio RS, Gumb T, Pirraglia E, et al. ; Alzheimer’s Disease Neuroimaging Initiative . Sleep-disordered breathing advances cognitive decline in the elderly. Neurology. 2015;84(19):1964-1971. doi: 10.1212/WNL.0000000000001566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ju Y-ES, Finn MB, Sutphen CL, et al. Obstructive sleep apnea decreases central nervous system-derived proteins in the cerebrospinal fluid. Ann Neurol. 2016;80(1):154-159. doi: 10.1002/ana.24672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sharma RA, Varga AW, Bubu OM, et al. Obstructive sleep apnea severity affects amyloid burden in cognitively normal elderly. A longitudinal study. Am J Respir Crit Care Med. 2018;197(7):933-943. doi: 10.1164/rccm.201704-0704OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.André C, Rehel S, Kuhn E, et al. ; Medit-Ageing Research Group . Association of sleep-disordered breathing with Alzheimer disease biomarkers in community-dwelling older adults: a secondary analysis of a randomized clinical trial. JAMA Neurol. 2020;77(6):716-724. doi: 10.1001/jamaneurol.2020.0311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bubu OM, Pirraglia E, Andrade AG, et al. ; Alzheimer’s Disease Neuroimaging Initiative . Obstructive sleep apnea and longitudinal Alzheimer’s disease biomarker changes. Sleep. 2019;42(6):zsz048. doi: 10.1093/sleep/zsz048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Duarte RLM, Mendes BA, Oliveira-E-Sá TS, Magalhães-da-Silveira FJ, Gozal D. Perception of sleep duration in adult patients with suspected obstructive sleep apnea. PLoS One. 2020;15(8):e0238083. doi: 10.1371/journal.pone.0238083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Self-reported and measured sleep duration: how similar are they? Epidemiology. 2008;19(6):838-845. doi: 10.1097/EDE.0b013e318187a7b0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Landry GJ, Best JR, Liu-Ambrose T. Measuring sleep quality in older adults: a comparison using subjective and objective methods. Front Aging Neurosci. 2015;7:166. doi: 10.3389/fnagi.2015.00166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Matthews KA, Patel SR, Pantesco EJ, et al. Similarities and differences in estimates of sleep duration by polysomnography, actigraphy, diary, and self-reported habitual sleep in a community sample. Sleep Health. 2018;4(1):96-103. doi: 10.1016/j.sleh.2017.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kaplan KA, Hardas PP, Redline S, Zeitzer JM; Sleep Heart Health Study Research Group . Correlates of sleep quality in midlife and beyond: a machine learning analysis. Sleep Med. 2017;34:162-167. doi: 10.1016/j.sleep.2017.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Buysse DJ. Sleep health: can we define it? Does it matter? Sleep. 2014;37(1):9-17. doi: 10.5665/sleep.3298 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Flow Chart of Participant Inclusion Process
eFigure 2. Nocturnal Sleep Duration and Daytime Nap Duration Associations With Amyloid PET and Cognitive Function
eTable 1. Summary of Sleep Duration Associations With Amyloid PET and Cognitive Performance With Lifestyle Outcomes Included as Covariates
eTable 2. Summary of Associations Between the Interaction Term of Sleep Duration * Daytime Napping With Amyloid PET and Cognitive Performance