Abstract
Background
Loss of olfactory function is well recognised as a cardinal symptom of COVID‐19 infection, and the ongoing pandemic has resulted in a large number of affected individuals with abnormalities in their sense of smell. For many, the condition is temporary and resolves within two to four weeks. However, in a significant minority the symptoms persist. At present, it is not known whether early intervention with any form of treatment (such as medication or olfactory training) can promote recovery and prevent persisting olfactory disturbance.
Objectives
To assess the effects (benefits and harms) of interventions that have been used, or proposed, to prevent persisting olfactory dysfunction due to COVID‐19 infection. A secondary objective is to keep the evidence up‐to‐date, using a living systematic review approach.
Search methods
The Cochrane ENT Information Specialist searched the Cochrane COVID‐19 Study Register; Cochrane ENT Register; CENTRAL; Ovid MEDLINE; Ovid Embase; Web of Science; ClinicalTrials.gov; ICTRP and additional sources for published and unpublished studies. The date of the search was 16 December 2020.
Selection criteria
Randomised controlled trials including participants who had symptoms of olfactory disturbance following COVID‐19 infection. Individuals who had symptoms for less than four weeks were included in this review. Studies compared any intervention with no treatment or placebo.
Data collection and analysis
We used standard Cochrane methodological procedures. Our primary outcomes were the presence of normal olfactory function, serious adverse effects and change in sense of smell. Secondary outcomes were the prevalence of parosmia, change in sense of taste, disease‐related quality of life and other adverse effects (including nosebleeds/bloody discharge). We used GRADE to assess the certainty of the evidence for each outcome.
Main results
We included one study of 100 participants, which compared an intranasal steroid spray to no intervention. Participants in both groups were also advised to undertake olfactory training for the duration of the trial. Data were identified for only two of the prespecified outcomes for this review, and no data were available for the primary outcome of serious adverse effects.
Intranasal corticosteroids compared to no intervention (all using olfactory training)
Presence of normal olfactory function after three weeks of treatment was self‐assessed by the participants, using a visual analogue scale (range 0 to 10, higher scores = better). A score of 10 represented "completely normal smell sensation". The evidence is very uncertain about the effect of intranasal corticosteroids on self‐rated recovery of sense of smell (estimated absolute effect 619 per 1000 compared to 520 per 1000, risk ratio (RR) 1.19, 95% confidence interval (CI) 0.85 to 1.68; 1 study; 100 participants; very low‐certainty evidence).
Change in sense of smell was not reported, but the self‐rated score for sense of smell was reported at the endpoint of the study with the same visual analogue scale (after three weeks of treatment). The median scores at endpoint were 10 (interquartile range (IQR) 9 to 10) for the group receiving intranasal corticosteroids, and 10 (IQR 5 to 10) for the group receiving no intervention (1 study; 100 participants; very low‐certainty evidence).
Authors' conclusions
There is very limited evidence regarding the efficacy of different interventions at preventing persistent olfactory dysfunction following COVID‐19 infection. However, we have identified a small number of additional ongoing studies in this area. As this is a living systematic review, the evidence will be updated regularly to incorporate new data from these, and other relevant studies, as they become available.
For this (first) version of the living review, we identified a single study of intranasal corticosteroids to include in this review, which provided data for only two of our prespecified outcomes. The evidence was of very low certainty, therefore we were unable to determine whether intranasal corticosteroids may have a beneficial or harmful effect.
Plain language summary
Interventions for the prevention of persistent smell disorders (olfactory dysfunction) after COVID‐19 infection
Why this is important
COVID‐19 has been found to cause problems with the sense of smell. Sometimes this is a reduction in the ability to smell things, and sometimes it is a complete loss of the sense of smell. For many people this recovers in a short time, but for others it may last for weeks or months. This review considers whether there are treatments that people might take as soon as they have lost their sense of smell (within four weeks of the symptoms starting), to try and stop this becoming a long‐standing problem.
How we identified and assessed the evidence
We searched for all relevant studies in the medical literature to summarise the results. We also looked at how certain the evidence was, considering things like the size of the studies and how they were carried out. Based on this, we classed the evidence as being of very low, low, moderate or high certainty.
What we found
We only found one study that had been completed. This included 100 people, all of whom had problems with their sense of smell for a short time (less than four weeks) at the start of the study. The study compared people who were treated with a steroid spray that goes into the nose, with people who were given no treatment. All of the people in the study were also recommended to carry out 'olfactory training' – to spend a short time each day practising smelling particular scents, to try and stimulate their sense of smell to return. The researchers followed them for three weeks to see what happened. The findings from this one comparison are presented here:
Intranasal corticosteroids compared to no treatment (all using olfactory training)
We do not know whether a nasal steroid spray is better or worse than no treatment at:
‐ making people feel that their sense of smell is back to normal after three weeks; ‐ resulting in a change in the sense of smell after three weeks.
This is because the evidence was of very low certainty.
We did find a number of other studies that are being carried out, but no results from these studies were available yet to be included in this review.
What this means
We do not know whether using a nasal steroid spray has any benefit in preventing longer‐term loss of the sense of smell that is related to COVID‐19, or whether it causes any harm. We do not have any evidence about other treatments. This review is a 'living systematic review' ‐ meaning that we will keep checking for new studies that might be relevant, and the review will be continually updated when any extra results are available.
How up‐to‐date is this review?
The evidence in this Cochrane Review is current to December 2020.
Summary of findings
Summary of findings 1. Intranasal steroids compared to no intervention for the prevention of persistent post‐COVID‐19 olfactory dysfunction.
| Intranasal steroids compared to no intervention for the prevention of persistent post‐COVID‐19 olfactory dysfunction | ||||||
|
Patient or population: adults with olfactory dysfunction for < 4 weeks following COVID‐19 infection Setting: one hospital in Egypt Intervention: intranasal corticosteroid spray Comparison: no intervention | ||||||
| Outcomes | Anticipated absolute effects*(95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no treatment | Risk with intranasal steroids | |||||
| Self‐rated presence of normal olfactory function Assessed with: score of 10 on a visual analogue scale (rated 0 to 10, where 10 = "completely normal smell sensation")1 Follow‐up: ≤ 4 weeks |
Study population | RR 1.19 (0.85 to 1.68) | 100 (1 RCT) |
⊕⊝⊝⊝ very low2,3 | — | |
| 520 per 1000 | 619 per 1000 (442 to 874) | |||||
| Serious adverse effects | No studies reported on this outcome. | |||||
| Self‐rated change in sense of smell Assessed with: numeric rating scale (0 to 10, higher = better) Follow‐up: ≤ 4 weeks |
Change in sense of smell was not reported, only endpoint data. One study reported a median sense of smell score of 10 (IQR 9 to 10) in those receiving intranasal steroids and a median score of 10 (IQR 5 to 10) in those who did not receive steroids at 3 weeks' follow‐up (P = 0.16). |
— | 100 (1 RCT) | ⊕⊝⊝⊝ very low2,4 | — | |
| Prevalence of parosmia | No studies reported on this outcome. | |||||
| Change in sense of taste | No studies reported on this outcome. | |||||
| Disease‐related quality of life | No studies reported on this outcome. | |||||
| Other adverse effects | No studies reported on this outcome. | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IQR: interquartile range; RCT: randomised controlled trial; RR: risk ratio; VAS: visual analogue scale | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1We have assumed that individuals who reported a score of 10 on the VAS were included as those who reported "completely normal smell sensation".
2Serious risk of bias as this was an unblinded study, and the outcome was self‐assessed by the participants.
3Very serious imprecision due to the very small sample size, which does not reach the optimal information size (< 400 participants), and the wide confidence intervals, which range from potential harm to potential benefit.
4Very serious imprecision due to the very small sample size, which does not reach the optimal information size (< 400 participants), and because no effect size or confidence interval could be calculated.
Background
Description of the condition
Loss of olfactory function (the sense of smell) emerged as a marker of COVID‐19 infection in March 2020 (Hopkins 2020a). Since that time, it has become established that this is a cardinal symptom of COVID‐19 infection (Menni 2020), with a high predictive value (Gerkin 2020). This usually takes the form of complete or partial loss of olfactory function (anosmia and hyposmia respectively) (Lechien 2020).
Olfactory dysfunction, through loss (quantitative changes) or distortion (qualitative changes) of smell, is a debilitating condition with a variety of causes and has a major impact on quality of life (Croy 2014; Erskine 2020; Philpott 2014). It also has safety implications, through the inability to detect odours that may signal danger (such as smoke, gas or spoilt food). Through its intimate relationship with the sense of taste, the disturbance of olfactory function can also hamper the ability to enjoy food.
Post‐infectious olfactory dysfunction (PIOD) is one of the most common causes of olfactory dysfunction, representing up to 20% of all cases in specialist olfactory clinics (Cain 1988; Damm 2004; Seiden 2001). Many viruses have been implicated in PIOD, including the coronavirus family. However, the prominence of SARS‐CoV‐2 (which causes COVID‐19) as a causative agent has been notable, and can perhaps be attributed to the spotlight created by it being the cause of a pandemic.
Accurate estimates of the prevalence of olfactory dysfunction resulting from COVID‐19 are difficult to obtain, and may vary according to the clinical presentation of the disease (which ranges from mild, or relatively asymptomatic, to serious complications requiring intensive care). A recent systematic review identified an overall prevalence of smell loss of 43%, however the authors noted high variation between the estimates from different studies (von Bartheld 2020). Another systematic review showed a prevalence of 62% across the range of studies included (Rocke 2020). A large European cohort, which included hospitalised individuals with mild‐moderate symptoms, as well as individuals who did not require hospital treatment, reported the prevalence of olfactory dysfunction to be 85.6% (Lechien 2020). The majority of individuals included in this study reported anosmia, with a minority reporting hyposmia (20.4%).
The incidence of anosmia or olfactory dysfunction related to COVID‐19 appears to vary across the world, with studies from the USA and Europe typically demonstrating much higher incidence than those from Asia (Meng 2020; von Bartheld 2020). A study from Wuhan, China reported abnormalities of olfactory function in only 5.1% of their cohort (214 patients, with both severe and mild forms of the disease) (Mao 2020). It is not clear why this may be. Gender and age have also been suggested as possible effect modifiers, with some reviews suggesting preponderance in females (Meng 2020), and others suggesting an increased incidence in younger age groups (Fuccillo 2020).
The incidence of olfactory dysfunction may also vary depending on the method used to diagnose it. Studies that used self‐reported symptoms of loss of smell identified a lower prevalence than those that utilised some form of objective assessment (von Bartheld 2020). It is well recognised that, for healthy individuals, self‐rating of the sense of smell may correlate poorly with scores achieved on psychophysical testing (Landis 2003; Lötsch 2019). Correlation is better for those who report olfactory dysfunction (particularly anosmia), but on an individual level there is still considerable variation between the severity of the reported loss, and that identified with psychophysical tests (Welge‐Luessen 2005). With larger numbers reporting COVID‐19 symptoms in general, the data collected by the COVID tracker app is more likely to reflect the prevalence of olfactory dysfunction in the non‐hospitalised population (Menni 2020).
A further complication in obtaining accurate estimates of prevalence is the variety of data sources that are available. Studies conducted in a hospitalised population may present very different estimates to those where data are gathered from internet‐based surveys. This may reflect genuine differences in the presence of olfactory dysfunction in these varied populations, different methods of ascertaining olfactory function, or potentially a different preponderance to report symptoms. Internet‐based surveys may have a greater propensity for responder bias than other cross‐sectional studies ‐ those who have symptoms may be more likely to participate or complete the required data, resulting in inflated estimates of prevalence. However, some prospective series have also identified a high prevalence of olfactory dysfunction (Spinato 2020)
Other symptoms of olfactory dysfunction include phantosmia (qualitative dysfunction in the absence of an odour, or 'olfactory hallucinations') and parosmia (distorted perception of an odour stimulus) (Hummel 2016). A recent survey of individuals with COVID‐19 indicated that these symptoms occurred in fewer than 10% in the short term (Parma 2020). However, longer‐term follow‐up may demonstrate further problems at a later stage (Gerkin 2020), and reports of persisting parosmia as a consequence of COVID‐19 are increasing (Hopkins 2020b).
The exact mechanism by which the SARS‐CoV‐2 virus triggers olfactory dysfunction remains unclear (reviewed in Butowt 2020). Many viruses cause conductive olfactory impairment, with inflammation, nasal congestion and rhinorrhoea preventing detection of odours during the acute phase of the infection. These symptoms are not as common in COVID‐19 and, when present, do not correlate well with the degree of olfactory dysfunction (Parma 2020). Symptoms may also be caused by direct damage to, or death of, olfactory neurons or cells within the olfactory bulb. However, olfactory neurons lack ACE2 receptors (which facilitate viral entry to cells) and the rapid recovery for most individuals with COVID‐19 related smell loss makes this less likely. Infection of supporting cells (sustentacular cells) within the olfactory epithelium has been reported (reviewed in Bilinska 2020). These cells play a critical role in supporting the function of olfactory neurons, and their infection may consequently have an adverse effect on olfactory processing.
For many individuals with COVID‐19 related olfactory dysfunction, the condition is temporary and they recover a normal sense of smell relatively quickly (Chary 2020; Klopfenstein 2020). Complete recovery by two weeks was reported for most people (96.7%) in the study by Lechien 2020. A second case series of individuals with mild coronavirus symptoms found that 89% had complete or partial recovery of olfactory function by four weeks from the onset of the disease (Boscolo‐Rizzo 2020). However, for some individuals the problem persists. Some studies report a much higher prevalence of persisting olfactory loss, despite resolution of other COVID‐19 symptoms. Data from the Global Consortium of Chemosensory Research indicates that up to 50.7% of individuals may have persisting olfactory dysfunction at up to 40 days from the onset of COVID‐19 (Gerkin 2020). It remains unclear why some individuals experience longer‐lasting olfactory deficits. This may be due to differing extents of damage (as suggested by Butowt 2020), or different mechanisms for olfactory loss (Hopkins 2020c; Saussez 2020). Differing features of COVID‐19 related smell loss may include a potential impact on true gustatory function, as well as a greater severity of olfactory loss itself (Huart 2020); many larger studies are limited by the reliance on self‐reporting, so this is more difficult to corroborate.
This review is one of a pair that consider the effect of interventions to prevent or treat persisting olfactory dysfunction following COVID‐19. For this review, we considered interventions that may be used in the acute phase (less than four weeks since diagnosis), aiming to prevent individuals from developing persisting olfactory dysfunction. For the companion review ('Interventions for the treatment of persisting olfactory dysfunction following COVID‐19'; Webster 2021a), we considered treatment for individuals who already have persisting olfactory dysfunction at four weeks (or longer) following a diagnosis of COVID‐19.
Description of the intervention
As COVID‐19 related persisting olfactory dysfunction is a relatively new condition, there are no established interventions that are known to prevent it. However, a number of interventions have been used for other post‐viral causes of anosmia. It is possible that early intervention for those with short‐lived symptoms could help to prevent the development of persisting, long‐term olfactory dysfunction.
Steroids are commonly prescribed for olfactory dysfunction ‐ these are typically administered locally as a nasal spray, drops or rinse for conductive causes of olfactory loss ‐ where the nasal cavity is blocked, or partially blocked, by inflammation and oedema. Systemic (oral) steroids may also be used, particularly in cases where no conductive cause is identified.
Olfactory training is also frequently suggested for reduced or absent sense of smell ‐ this involves regular exposure to a number of specific odours. It can be performed in a variety of different ways, using household items or essential oils.
A large number of other interventions have been used for PIOD, and may therefore be of use for post‐COVID‐19 olfactory dysfunction. A variety of vitamins, minerals and nutritional supplements have been proposed to be of benefit ‐ either taken as an oral supplement, or in some instances used intranasally (such as intranasal vitamin A drops). Glutamate antagonists and xanthine derivatives are used occasionally in the treatment of post‐viral olfactory dysfunction and may therefore be assessed in relation to COVID‐19. Trials of acupuncture have also taken place.
Clinical trials are ongoing to assess a variety of interventions for the treatment of COVID‐19. These include antivirals, such as remdesivir, and monoclonal antibodies. It is possible that these interventions may also benefit individuals with olfactory dysfunction, if these symptoms are assessed.
For many individuals, smell loss is anticipated to improve with time. There is no intervention that could currently be regarded as standard care for individuals with post‐COVID‐19 related anosmia. Interventions are therefore likely to be compared to no treatment, or to placebo (dummy) treatment. However, olfactory training is often suggested as an intervention with few, if any, adverse effects, and may be used alongside other treatments, therefore we anticipate that this may be advised to be undertaken concurrently in some studies.
How the intervention might work
Steroids are frequently prescribed to ensure that any intranasal inflammatory component that is exacerbating the PIOD is adequately treated. Whether steroids have a persisting effect after discontinuation is unclear. Intranasal steroids are used for a number of other conditions, and serious side effects are rare, but they may cause nasal irritation, nosebleeds or other localised complications. Steroids may also be administered systemically ‐ typically as oral tablets, or sometimes parenterally.
Olfactory training aims to stimulate the olfactory neurons with a variety of odours in order to enhance smell detection. It is unclear whether any changes occur within the olfactory epithelium itself, in the olfactory bulb, or involve reorganisation of neural olfactory pathways. Although olfactory training may not restore olfactory function, it may improve the performance of the olfactory system. Two recent systematic reviews suggest that olfactory training may give some benefit to those with olfactory disorders (Pekala 2016; Sorokowska 2017). However, the majority of included studies were prospective cohorts, with only one RCT included.
A number of vitamins and minerals have been suggested to have a beneficial effect on the olfactory epithelium, including vitamins A, B12 and D, and zinc. It is thought that metabolites of vitamin A may play a role in regeneration of tissue in the olfactory epithelium or olfactory bulb, and this has been used intranasally to treat individuals with post‐viral olfactory loss (Hummel 2017). Vitamin B12 is known to be important in the maintenance of central and peripheral nervous function, and deficiency of vitamin B12 has been associated with olfactory impairment (Derin 2016). Vitamin D deficiency has also been linked to olfactory impairment (Bigman 2020), and there is ongoing interest in the potential use of vitamin D to prevent or treat other symptoms of COVID‐19 infection (Martineau 2020). Zinc deficiency has also been shown to have an association with olfactory dysfunction and zinc was historically used intranasally as a potential treatment for anosmia, although there are concerns over toxicity (Alexander 2006).
Antioxidants, such as alpha lipoic acid and omega 3 fatty acids, have also been suggested as possible interventions to treat anosmia (Hummel 2002). They are thought to have neuroprotective properties that may help restore function within olfactory neurons or the olfactory bulb. Minocycline has also been trialled in post‐viral olfactory loss ‐ due to its neuroprotective properties, rather than its traditional role as an antibiotic (Reden 2011).
It is possible that antiviral agents, some of which have already been shown to impact on the severity of COVID‐19, may also affect the olfactory dysfunction. Reducing viral replication (and consequently lowering the viral load in an individual) may result in reduced severity of olfactory loss, or hasten the recovery. Monoclonal antibodies have also been used to treat COVID‐19, and could also have an impact on the severity and persistence of olfactory impairment.
There have also been small studies to assess the possible benefit of acupuncture in olfactory loss (Dai 2016; Vent 2010).
Glutamate plays an important role in neurotransmission for olfactory neurons and within the olfactory bulb. Glutamate antagonists, such as caroverine, have been proposed to help protect against neurotoxicity, and consequently improve olfactory function (Quint 2002). Finally, xanthine derivatives such as theophylline (sometimes given intranasally) and pentoxifylline have been proposed to stimulate olfactory neuron activity, and may therefore have an effect on olfactory function.
It is possible that individuals with a longer duration of anosmia have a different underlying disease process than those with temporary olfactory dysfunction related to COVID‐19. Consequently the efficacy of different interventions may vary between these groups.
Why it is important to do this review
The COVID‐19 pandemic has resulted in an enormous number of individuals becoming infected with SARS‐CoV‐2. Fortunately, many individuals recover completely. However, the long‐term consequences of infection are only just becoming apparent. Although the prevalence of persisting olfactory dysfunction may be small, with huge numbers of global infections, the actual number of individuals suffering from post‐COVID‐19 related anosmia is large. We can assume an estimated 60% suffer olfactory dysfunction at the onset of the infection and that at least 10% of these go on to experience PIOD. Given the number of infections (> 125 million infections worldwide, as of March 2021), we estimate that up to 7.5 million people may have been affected to date. The burden of this disorder is also considerable, with significant effects on quality of life, as well as safety implications (due to the inability to detect harmful or dangerous smells). Therefore, identification of potential treatments that may improve the outcome for sufferers is timely and important.
Many interventions carry a risk of adverse effects. If the beneficial effect of an intervention is small or negligible, then side effects may be such that individuals do not consider it worthwhile. With this review we aimed to comprehensively assess the benefits and harms of interventions to prevent persisting olfactory dysfunction related to COVID‐19, to ensure that patients can make an informed choice regarding the management of their condition.
Given the recent emergence of COVID‐19, there is currently a great deal of uncertainty about how best to manage the olfactory dysfunction that occurs as a result of the virus. The sheer numbers of infected individuals worldwide also means that evidence that supports decision‐making for management of COVID‐19 is a priority for decision‐makers globally. There is also a strong emphasis on COVID‐19 research at present, and we anticipate that there is likely to be new evidence available over the coming months and years. Therefore, this review is a living systematic review, which will be continually updated to incorporate any important new evidence as it becomes available.
Objectives
To assess the effects (benefits and harms) of interventions that have been used, or proposed, to prevent persisting olfactory dysfunction due to COVID‐19 infection.
A secondary objective is to keep the evidence up‐to‐date, using a living systematic review approach.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials and quasi‐randomised trials (where trials were designed as RCTs, but the sequence generation for allocation of treatment used methods such as alternative allocation, birth dates, alphabetical order etc.).
We considered that olfactory dysfunction is unlikely to be stable over long periods of time, and individuals may experience considerable fluctuation of symptoms over a given time period. Therefore, cross‐over trials were unlikely to be identified. If we identified any cross‐over studies, we planned to only include data from the first phase of these studies in the review.
We included studies where the main purpose of the trial was to assess the effect of treatment on olfactory function. Many interventions are used in the treatment of COVID‐19 (such as steroids, antivirals) ‐ these may have beneficial effects on olfactory function, but the primary aim of most trials will be to assess their impact on other features of the disease (such as need for ventilation, mortality etc.). Therefore, we only included studies where olfactory function had been assessed at the trial baseline, and the main aim of the study was to determine the effect of an intervention on olfaction.
We only included studies where patients were followed up for at least one week. The aim of this review was to synthesise evidence for treatments that may have a lasting effect on olfactory function, rather than those that may have a very brief or temporary impact.
We included studies in any language. We planned to include outcome data reported on a trial registry, even if no published results were available. However, we did not identify any studies where this was applicable. If we identified material from a pre‐print server then we planned to note this in the 'What's New' section of the review, pending identification of fully published data. If no published data were identified within four months of the pre‐print article being made available then we planned to incorporate the data in the review. However, we did not identify any pre‐print articles during the searches.
Types of participants
We included studies of adult participants (aged 18 years or older) with a diagnosis of COVID‐19 and olfactory dysfunction that had lasted less than four weeks. We anticipated that some studies would report this as less than four weeks of olfactory dysfunction, rather than less than four weeks since a positive test for COVID‐19 ‐ either of these measures were included in the review.
We included individuals with anosmia (absent sense of smell) or hyposmia (reduced sense of smell). We anticipated that some trials may also include a small number of individuals with symptoms of pure parosmia or phantosmia. We planned to include data from these trials, providing the majority (≥ 80% of participants) report anosmia or hyposmia.
We included studies where olfactory dysfunction was identified with either psychophysical (objective) testing, or through self‐report of symptoms. We planned to investigate whether this had any impact on the effect estimates using subgroup analysis (see Subgroup analysis and investigation of heterogeneity).
We included studies where COVID‐19 has been diagnosed through either objective testing (e.g. viral polymerase chain reaction (PCR) from nasopharyngeal swabs) or through a clinical diagnosis (for example, sudden onset of olfactory dysfunction with other symptoms of COVID‐19, or in the context of contact with an infected individual).
For inclusion in this review, all participants in the trial must have had abnormalities of their sense of smell. We did not include studies where only some participants are eligible (i.e. not all participants had olfactory dysfunction at the start of the trial).
Types of interventions
Interventions
We included any intervention proposed to specifically prevent persisting olfactory dysfunction. We anticipated that this may include the following interventions:
Intranasal steroid drops/rinses
Intranasal steroid sprays
Systemic steroids
Olfactory training
Vitamin A
Zinc
Antioxidants (e.g. omega 3 fatty acids, alpha lipoic acid, minocycline)
Antiviral agents (e.g. remdesivir)
Other vitamins and nutritional supplements (to be analysed according to the type of vitamin/supplement, rather than as a pooled comparison)
Acupuncture
Monoclonal antibodies
Glutamate antagonists (e.g. caroverine)
Xanthine derivatives (e.g. theophylline, pentoxifylline)
Saline irrigation
If we had identified studies of additional interventions then these would also have been included.
All routes of administration, doses and duration of treatment were included.
Olfactory training was considered to be a complex intervention, as the method of delivery varies considerably in different studies. We planned to assess this using subgroup analyses, if we identified any trials of this intervention (see below).
Comparator(s)
The main comparison is:
placebo or no treatment.
Concurrent treatments
We anticipated that some trials may include olfactory training (or other interventions) as concurrent therapy for both arms. We placed no limits on the type of concurrent treatments used. We planned to pool these trials with those where no concurrent treatment was used and use sensitivity analyses to determine whether the effect estimates are changed because of this.
Types of outcome measures
We analysed the following outcomes in the review, but we did not use them as a basis for including or excluding studies. All outcomes were assessed at three possible time points:
≤ 4 weeks;
> 4 weeks to 3 months (this was the main time frame of interest);
> 3 months to 6 months.
These time points relate to the time since treatment was started.
Outcomes at less than four weeks following COVID‐19 were considered too short to comprehensively assess whether individuals have persisting olfactory problems. However, in the absence of other evidence they may provide some indication about the likely efficacy of treatments to prevent later problems.
As most individuals with temporary problems should have complete resolution of their olfactory symptoms by four weeks (Boscolo‐Rizzo 2020), we considered this time frame (> 4 weeks) to be of importance to identify those who truly have persisting problems. However, we recognised that some individuals may experience fluctuations in their symptoms, and develop recurrent olfactory problems at a later stage. We therefore included outcomes that were measured at a later point to identify whether early intervention could help to prevent these problems from developing.
Primary outcomes
-
Presence of normal olfactory function:
as assessed by the participants (e.g. self‐rated complete recovery);
as assessed using psychophysical testing, using Sniffin' Sticks, University of Pennsylvania Smell Identification Test (UPSIT) or another validated test.
Serious adverse effects (as defined by the trialists).
-
Change in sense of smell:
as assessed by the participants (e.g. using a visual analogue score);
as assessed using psychophysical testing, using Sniffin' Sticks, UPSIT or another validated test.
It is well recognised that self‐rated sense of smell correlates poorly with the results of psychophysical testing of olfactory function. Therefore we have included both types of outcome measurements separately for the outcome domains that relate to sense of smell. If data had been obtained for both of these measures we would not have combined them, but would have reported them as two separate analyses. However, at present the only included study includes data using self‐reported olfactory function only.
Secondary outcomes
Prevalence of parosmia, as assessed by the participants.
Change in sense of taste, as assessed by psychophysical gustatory tests, such as the sip and spit method or other validated tests.
Disease‐related quality of life, as assessed by the Olfactory Disorders Questionnaire, or another validated questionnaire (which specifically relates to olfactory dysfunction).
Other adverse effects (including nosebleeds/bloody discharge).
We recognise that parosmia is a challenging symptom to define and assess. If we had identified data for this outcome then we would have included any results reported by the study authors, and described the definitions used in the study. However, this outcome was not assessed by the study included in the review.
Where possible, we planned to compare the threshold for appreciable change in these outcomes to published minimally important differences. These have been reported for psychophysical olfactory testing using Sniffin' Sticks (MID 5.5 points, Gudziol 2006) and the Olfactory Disorders Questionnaire (MID 5.2 points, Mattos 2018). However, we did not identify any data for these outcomes in the review.
Search methods for identification of studies
The Cochrane ENT Information Specialist conducted systematic searches for randomised controlled trials and controlled clinical trials. There were no language or publication status restrictions. Some of the search terms were limited by publication year, due to the novel nature of post‐COVID‐19 olfactory dysfunction. We contacted original authors for clarification and further data if trial reports were unclear and arranged translations of papers where necessary.
Electronic searches
The Information Specialist searched:
the Cochrane ENT Trials Register (searched via the Cochrane Register of Studies to 16 December 2020);
the Cochrane Central Register of Controlled Trials (CENTRAL) (searched via the Cochrane Register of Studies to 16 December 2020);
Ovid MEDLINE(R) Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) (1946 to 16 December 2020);
Ovid Embase (1974 to 16 December 2020);
Web of Knowledge, Web of Science (1945 to 16 December 2020);
-
ClinicalTrials.gov, www.clinicaltrials.gov:
searched via the Cochrane Register of Studies to 16 December 2020;
searched via www.clinicaltrials.gov to 16 December 2020;
-
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP):
searched via the Cochrane Register of Studies to 16 December 2020;
searched via https://apps.who.int/trialsearch/ to 16 December 2020;
Cochrane COVID‐19 Study Register, https://covid-19.cochrane.org/ (searched via the Cochrane Register of Studies to 16 December 2020);
World Health Organization (WHO) COVID‐19 'Global literature on coronavirus disease', https://search.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov (searched to 16 December 2020).
The Information Specialist modelled subject strategies for databases on the search strategy designed for CENTRAL. Where appropriate, they were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials (as described in the Technical Supplement to Chapter 4 of the Cochrane Handbook for Systematic Reviews of Interventions version 6.1) (Lefebvre 2020). Search strategies for major databases including CENTRAL are provided in Appendix 1.
Clinical trials are ongoing to assess a variety of interventions for the treatment of COVID‐19. As few studies have currently been published, the search strategy developed is highly sensitive in order to try to capture all interventions as they are introduced. The Information Specialist will review the search methods (the sources and search frequency) and the search terms (index terms and free text terms) on an annual basis. The search strategy may evolve over time, as a greater body of literature is published and a more focused list of interventions are identified.
Living systematic review considerations
As a living systematic review, the Information Specialist conducts monthly searches of the sources listed above, except the following which are searched less frequently, and as a minimum on a:
-
quarterly basis:
World Health Organization (WHO) COVID‐19 'Global literature on coronavirus disease' https://search.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov (search to date);
COAP COVID‐19 Living Evidence, Institute of Social and Preventive Medicine (ISPM), University of Bern https://zika.ispm.unibe.ch/assets/data/pub/search_beta/ (search to date); or
-
an annual basis:
ClinicalTrials.gov (search via www.clinicaltrials.gov to date);
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (search via https://apps.who.int/trialsearch/ to date).
Clinical trials are ongoing to assess a variety of interventions for the treatment of COVID‐19. We plan to conduct surveillance activity and will commence monthly searches when we anticipate that the first trials will have data available.
The Information Specialist will apply appropriate date restrictions and auto‐alerts as available and appropriate, and will provide details in an appendix to the published review.
Searching other resources
We scanned the reference lists of identified publications for additional trials and contacted trial authors if necessary. The Information Specialist also ran non‐systematic searches of Google Scholar to retrieve grey literature and other sources of potential trials.
We did not perform a separate search for adverse effects. We considered adverse effects described in included studies only.
We planned to make efforts to identify full‐text papers regardless of language of publication and to endeavour to seek help with translation; however, we intended that this should not hold up the rapid review process. Any papers that we were unable to source in time for the scheduled living review update, or were unable to get translated, would be listed as awaiting assessment. Fortunately, we were able to identify and locate all papers of relevance for this review, and we did not require any translation.
Living systematic review considerations
As a living systematic review, we scanned the reference lists of identified publications for additional trials and contacted trial authors if necessary. In addition, the Information Specialist searched on an annual basis Ovid MEDLINE to retrieve existing systematic reviews relevant to this systematic review, so that we could scan their reference lists for additional trials. The Information Specialist conducted annual searches of the Web Knowledge Science Citation Index for articles referencing the published review and its included studies and non‐systematic searches of Google Scholar to retrieve grey literature and other sources of potential trials.
Data collection and analysis
Selection of studies
The Cochrane ENT Information Specialist used the first two components of Cochrane's Screen4Me workflow to help assess the search results. Screen4Me comprises three components:
Known assessments – a service that matches records in the search results to records that have already been screened in Cochrane Crowd and been labelled as 'a RCT' or as 'not a RCT'.
The machine learning classifier (RCT model) (Wallace 2017), available in the Cochrane Register of Studies (CRS‐Web), which assigns a probability of being a true RCT (from 0 to 100) to each citation. For citations that are assigned a probability score below the cut‐point at a recall of 99% we will assume these to be non‐RCTs. For those that score on or above the cut‐point we will either manually dual screen these results or send them to Cochrane Crowd for screening.
Cochrane Crowd is Cochrane's citizen science platform where the Crowd help to identify and describe health evidence. For more information about Screen4Me and the evaluations that have been done, please go to the Screen4Me website on the Cochrane Information Specialist's portal and see Marshall 2018; McDonald 2017; Noel‐Storr 2018 and Thomas 2017.
We did not use the third component because of the relatively small number of results retrieved by the search.
Two review authors (LOB, KW) independently screened the remaining titles and abstracts retrieved by the search to identify potentially relevant studies. The same authors independently evaluated the full text of each potentially relevant study to determine whether it met the inclusion/exclusion criteria for this review. We resolved any differences by discussion and consensus. We planned to involve a third author where necessary, but this was not required.
Living systematic review considerations
We will immediately screen any new citations retrieved by the monthly searches using the approach outlined above.
Data extraction and management
Two review authors (LOB, KW) independently extracted outcome data from each study using a standardised data collection form. Where a study had more than one publication, we retrieved all publications to ensure complete extraction of data (for example, published articles and details from trial registries). Any discrepancies in the data extracted by the two authors were checked against the original reports, and differences were resolved through discussion and consensus. We planned to consult a third author where necessary, but this was not required. If required, we contacted the study authors for clarification.
We collected information on study design and setting, participant characteristics (including disease severity and age), study eligibility criteria, details of the intervention(s) given, the outcomes assessed, the source of study funding and any conflicts of interest stated by the investigators. We also included details of the baseline characteristics of trial participants, with particular regard to prognostic features such as age, gender, severity of infection and duration of time since COVID‐19 infection.
The primary effect of interest for this review was the effect of treatment assignment (which reflects the outcomes of treatment for people who were assigned to the intervention) rather than a per protocol analysis (the outcomes of treatment only for those who completed the full course of treatment as planned). For the outcomes of interest in this review, we extracted the findings from the studies on an available case basis, i.e. all available data from all participants at each time point, based on the treatment to which they were randomised. This was irrespective of compliance, or whether participants had received the intervention as planned.
In addition to extracting prespecified information about study characteristics and aspects of methodology relevant to risk of bias, we extracted the following summary statistics for each trial and outcome:
For continuous data: the mean values, standard deviation and number of patients for each treatment group at the different time points for outcome measurement. Where endpoint data were not available, we extracted the values for change‐from‐baseline data instead. If values for the individual treatment groups were not reported, we planned to extract summary statistics (e.g. mean difference) from the studies.
For binary data: we extracted information on the number of participants experiencing an event, and the number of participants assessed at that time point. If values for the individual treatment groups were not reported, we planned to extract summary statistics (e.g. risk ratio) from the studies.
For ordinal scale data: if we identified data reported on an ordinal scale and if the data appeared to be normally distributed, or if the analysis performed by the investigators indicated that parametric tests were appropriate, then we planned to treat the outcome measure as continuous data. Alternatively, if data were available, we planned to convert these to binary data. However, we were not able to confirm that the ordinal data we obtained (from a visual analogue scale of sense of smell) was normally distributed, therefore this was not possible.
For time‐to‐event data: if we identified data reported as time‐to‐event, we planned to extract data on hazard ratios from individual studies. If these data were not reported then we planned to extract alternative measures of treatment effect, such as the observed and expected number of events in each group, a P value and the number of events in each arm, or data in a Kaplan Meier curve. However, we did not identify any time‐to‐event data.
We prespecified time points of interest for the outcomes in this review. Where studies reported data at multiple time points, we planned to take the longest available follow‐up point within each of the specific time frames. For example, if a study reported an outcome at 6 weeks, 8 weeks and 12 weeks of follow‐up then the 12‐week data would have been included for the time point > 4 weeks to 3 months.
Assessment of risk of bias in included studies
Two authors undertook assessment of the risk of bias of the included trials independently, with the following taken into consideration, as guided by the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011):
sequence generation;
allocation concealment;
blinding;
incomplete outcome data;
selective outcome reporting; and
other sources of bias.
We used the Cochrane risk of bias tool in RevMan 5.4 (RevMan 2020), which involves describing each of these domains as reported in the trial and then assigning a judgement about the adequacy of each entry: 'low', 'high' or 'unclear' risk of bias.
Measures of treatment effect
We summarised the effects of dichotomous outcomes (e.g. prevalence of olfactory dysfunction) as risk ratios (RR) with 95% confidence intervals (CIs). For the key outcomes that we presented in the summary of findings tables, we also expressed the results as absolute numbers based on the pooled results and compared to the assumed risk. For future iterations of this living review, we may also calculate the number needed to treat to benefit (NNTB) using the pooled results to aid understanding. The assumed baseline risk is typically either (a) the median of the risks of the control groups in the included studies, this being used to represent a 'medium‐risk population' or, alternatively, (b) the average risk of the control groups in the included studies is used as the 'study population' (Handbook 2020). As a single study was included, we used the baseline risk from this study for all calculations. If a large number of studies are available in future, and where appropriate, we may also present additional data based on the assumed baseline risk in (c) a low‐risk population and (d) a high‐risk population.
For continuous outcomes, we planned to express treatment effects as a mean difference (MD) with standard deviation (SD) or as a standardised mean difference (SMD) if different scales have been used to measure the same outcome. We planned to provide a clinical interpretation of the SMD values using either Cohen's d or by conversion to a recognised scale if possible.
For time‐to‐event outcomes we planned to summarise the effects as a hazard ratio (HR) with 95% CI. If necessary, and where possible (if sufficient alternative data were provided), we planned to estimate the HR from individual studies according to the methods outlined in Tierney 2007. However, no time‐to‐event data were identified for the review.
Unit of analysis issues
Cross‐over trials and cluster‐randomised trials were not anticipated for this review topic, and none were identified. Post‐COVID‐19 related anosmia is unlikely to be a stable condition, and interventions may not have a temporary effect. If cross‐over trials were identified then we planned to use only the data from the first phase of the study. If cluster‐randomised trials were identified then we would have ensured that analysis methods were used to account for clustering in the data (Handbook 2020).
Dealing with missing data
We planned to contact study authors via email whenever an outcome of interest was not reported, if the methods of the study suggested that the outcome had been measured. We planned to do the same if not all data required for meta‐analysis had been reported, unless the missing data were standard deviations. If standard deviation data were not available, we would have approximated these using the standard estimation methods from P values, standard errors or 95% CIs if these were reported, as detailed in the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2020). If it was impossible to estimate these, we would have contacted the study authors.
Apart from imputations for missing standard deviations, we planned to conduct no other imputations. We extracted and analysed all data using the available case analysis method.
Assessment of heterogeneity
We planned to assess clinical heterogeneity (which may be present even in the absence of statistical heterogeneity) by examining the included trials for potential differences between studies in the types of participants recruited, interventions or controls used and the outcomes measured. However, this was not possible due to the inclusion of a single study.
We planned to assess statistical heterogeneity by visually inspecting the forest plots and by considering the Chi² test (with a significance level set at P value < 0.10) and the I² statistic, which calculates the percentage of variability that is due to heterogeneity rather than chance (Handbook 2020). Again, this was not necessary due to the inclusion of a single study.
Assessment of reporting biases
We assessed reporting bias as within‐study outcome reporting bias and between‐study publication bias.
Outcome reporting bias (within‐study reporting bias)
We assessed within‐study reporting bias by comparing the outcomes reported in the published report against the study protocol or trial registry, whenever this could be obtained. If the protocol or trial registry entry was not available, we compared the outcomes reported to those listed in the methods section. If results are mentioned but not reported adequately in a way that allows analysis (e.g. the report only mentions whether the results were statistically significant or not), bias in a meta‐analysis is likely to occur. We planned to seek further information from the study authors. If no further information was found, we noted this as being a 'high' risk of bias when the risk of bias tool is used. If there was insufficient information to judge the risk of bias we noted this as an 'unclear' risk of bias (Handbook 2011).
Publication bias (between‐study reporting bias)
We planned to assess funnel plots if sufficient studies (more than 10) were available for an outcome. If we observed asymmetry of the funnel plot, we planned to conduct more formal investigation using the methods proposed by Egger 1997. We planned to also report on whether there were any studies identified through trial registries and other sources (Searching other resources), with unpublished reports.
Data synthesis
Where possible and appropriate (if participants, interventions, comparisons and outcomes were sufficiently similar in the trials identified), we planned to conduct a quantitative synthesis of results. We planned to conduct all meta‐analyses using a fixed‐effect model in RevMan 5.4. However, at present a single study is included in this review, precluding meta‐analysis.
We planned to include all studies in the meta‐analyses, regardless of their risk of bias. However, we intended to incorporate a summary assessment of risk of bias in the measure of certainty of the evidence for each outcome, using the GRADE system.
For dichotomous data, we analysed treatment differences as a risk ratio (RR) calculated using the fixed‐effect Mantel‐Haenszel methods.
For continuous outcomes, we planned to use the inverse variance, fixed‐effect method of meta‐analysis. If all data were from the same scale, we planned to pool mean follow‐up values with change‐from‐baseline data and report this as a mean difference. If there was a need to report standardised mean differences then we would not pool endpoint and change‐from‐baseline data.
For time‐to‐event data we planned to use a generic inverse variance, fixed‐effect method of meta‐analysis.
Sense of smell may be tested using a variety of methods, which consider different aspects of the sense of smell. These are:
identification ‐ the ability to identify and name a specific odour;
threshold ‐ the concentration of an odour that can be detected;
discrimination ‐ the ability to discriminate between odours.
We included methods that consider any or all of the above aspects of sense of smell. If meta‐analysis is appropriate in future iterations of this review, we will only pool results that look at the same individual aspect (or aspects) of sense of smell.
If meta‐analysis was not possible (for example, due to incompletely reported outcomes/effect estimates or different effect measures that cannot be combined) then we considered presenting alternative synthesis methods. This would have included summarising the effect estimates from individual studies, combining P values or vote counting based on the direction of effect, depending on the data available.
Living systematic review considerations
Whenever new evidence relevant to the review is identified in our monthly searches, we will extract the data, assess risk of bias and incorporate it into the synthesis every four months, as appropriate. Formal sequential meta‐analysis approaches will not be used for updated meta‐analyses.
Subgroup analysis and investigation of heterogeneity
A number of factors are likely to impact on the outcomes included in this review. Where possible (if appropriate data are reported), we planned to assess these with subgroup analysis, regardless of whether statistical heterogeneity is identified. These are the following:
-
Age of participants in the trial (under 60 years versus those aged 60 or over):
age is well recognised to impact on olfactory function, with sense of smell worsening with time. The ability to detect smells may therefore differ considerably between younger and older adults.
-
Gender of participants in the trial (female versus male):
gender has an influence on olfactory function, and may also impact recovery rates.
-
Method used to determine olfactory dysfunction at trial baseline (self‐reported versus psychophysical testing):
rates of olfactory dysfunction vary depending on whether self‐report or psychophysical testing is used to identify olfactory loss. Effect estimates in these two groups may therefore differ.
-
Time elapsed between diagnosis and treatment (< 2 weeks compared to 2 to 4 weeks before commencing treatment):
currently, patients are likely to be required to self‐isolate for two weeks once diagnosed with COVID‐19. Therefore, it would be informative to know whether a delay of two weeks in initiating treatment has an impact on outcomes.
If trials did not report data for particular subgroups of participants, we planned to synthesise data at the level of the individual trial, where appropriate. We would have identified studies as belonging to a particular subgroup if more than 2/3 participants (66%) belong to that category.
If trials had presented data for subgroups of individuals within the trial, we would have used this for subgroup analysis, where applicable, regardless of whether trials had stratified their randomisation according to those subgroups.
We anticipate that the varying methods used for olfactory training may be a source of heterogeneity in effects. If we had identified heterogeneity in the comparison of olfactory training then we would have explored this considering the following factors:
classical versus modified olfactory training (using the same scents throughout, compared to changing the scents);
the duration of the intervention.
Sensitivity analysis
We planned to carry out sensitivity analyses to determine whether the findings are robust to the decisions made in the course of identifying, screening and analysing the trials. We would have conducted sensitivity analysis for the following factors, whenever possible:
impact of model chosen: fixed‐effect versus random‐effects model;
inclusion of studies with concurrent treatments: including and excluding these studies from the pooled estimates of effect for any intervention;
method of COVID‐19 diagnosis: to exclude studies where only a clinical method of COVID‐19 diagnosis was used (rather than laboratory confirmed).
Summary of findings and assessment of the certainty of the evidence
Two independent authors (LOB/KW) used the GRADE approach to rate the overall certainty of evidence using GRADEpro GDT (https://gradepro.org/). The certainty of evidence reflects the extent to which we are confident that an estimate of effect is correct and we will apply this in the interpretation of results. There are four possible ratings: high, moderate, low and very low. A rating of high certainty of evidence implies that we are confident in our estimate of effect and that further research is very unlikely to change our confidence in the estimate of effect. A rating of very low certainty implies that any estimate of effect obtained is very uncertain.
The GRADE approach rates evidence from RCTs that do not have serious limitations as high certainty. However, several factors can lead to the downgrading of the evidence to moderate, low or very low. The degree of downgrading is determined by the seriousness of these factors:
study limitations (risk of bias);
inconsistency;
indirectness of evidence;
imprecision; and
publication bias.
We included a summary of findings table, constructed according to the recommendations described in Chapter 14 of the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2020), for the following comparison(s):
intranasal steroid drops/rinses versus no treatment/placebo;
intranasal steroid sprays versus no treatment/placebo;
olfactory training versus no treatment/placebo;
intranasal vitamin A versus no treatment/placebo.
We included the following outcomes in the summary of findings tables:
presence of normal olfactory function (as reported by the participants);
serious adverse effects;
change in sense of smell (as reported by the participants);
prevalence of parosmia;
change in sense of taste;
disease‐related quality of life;
other adverse effects (including nosebleeds/bloody discharge).
Methods for future updates
Living systematic review considerations
We will review the scope and methods of this review approximately yearly (or more frequently if appropriate) in the light of potential changes in the topic area, or the evidence being included in the review (for example, additional comparisons, interventions or outcomes, or new review methods available).
Conditions under which the review will no longer be maintained as a living systematic review
The review will no longer be maintained as a living systematic review once there is high‐certainty evidence obtained for the primary effectiveness outcomes of the review; once new studies are not expected to be conducted regularly for the interventions included in this review; or once the review topic is no longer a priority for health care decision‐making
Results
Description of studies
Results of the search
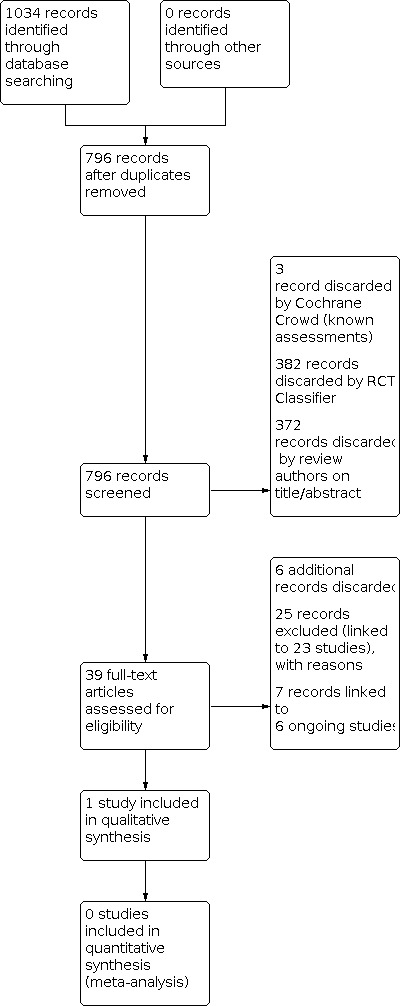
The searches (December 2020) retrieved a total of 1034 records. This reduced to 796 after the removal of duplicates. The Cochrane ENT Information Specialist sent all 796 records to the Screen4Me workflow. The Screen4Me workflow identified four records as having previously been assessed: three had been rejected as not RCTs and one had been assessed as a possible RCT. The RCT classifier rejected an additional 382 records as not RCTs (with 99% sensitivity). We did not send any records to the Cochrane Crowd for assessment. Following this process, the Screen4Me workflow had rejected 385 records and identified 411 possible RCTs for title and abstract screening.
| Possible RCTs | Rejected | |
| Known assessments | 1 | 3 |
| RCT classifier | 410 | 382 |
| Total | 411 | 385 |
We screened the titles and abstracts of the remaining 411 records. We discarded 372 records and assessed 39 full‐text records. We discarded six additional records at the full‐text screening stage.
We excluded 25 records (linked to 23 studies) with reasons recorded in the review (see Excluded studies).
We included one completed study (one record) where results were available (Abdelalim 2021). We identified one additional reference to a published paper linked to the study identified by the search. The paper was published after the search was run.
We identified six ongoing studies (seven records). We identified one additional reference to a published paper linked to an ongoing study identified by the search. The paper was published after the search was run. See Characteristics of ongoing studies for further details of all six ongoing studies.
A flow chart of study retrieval and selection is provided in Figure 1.
1.
Included studies
One study was included in the review. Abdelalim 2021 was an open‐label randomised controlled trial of intranasal corticosteroids (100 µg mometasone furoate in each nostril, once daily) compared to no treatment. The study was conducted in Egypt. Participants in both arms of the study were also recommended to use olfactory training with rose, lemon and cloves for 20 seconds each, twice per day. The authors of the study confirmed that all participants had symptoms of olfactory disturbance, which had lasted between 10 and 28 days at baseline. The study included 100 participants, all of whom self‐reported olfactory disturbance at the start of the study (no psychophysical testing was used). Treatment was given for three weeks, and outcomes were assessed at the end of the treatment period.
Excluded studies
We excluded 23 studies from the review. We present the main reasons for the exclusion of the studies below, although some studies had multiple reasons for exclusion:
Fifteen studies assessed the wrong population:
10 of these studies included all individuals with a diagnosis of COVID‐19, not just those with olfactory dysfunction (ACTION (NCT04332107); COPPS (NCT04662060); COVIDAtoZ (NCT04342728); CTRI/2020/08/027477; NCT04414124; NCT04458519; NCT04474483; NCT04513184; NCT04622891; NCT04662086);
a further four studies included participants with more than four weeks of olfactory dysfunction prior to enrolment (COVIDORL (NCT04361474)); IRCT20200522047542N1; Odorat‐Covid (NCT04598763); Vaira 2020); and
one further study included participants with any post‐viral olfactory disturbance, not specifically COVID‐19 (NCT04406584).
Two studies were not randomised controlled trials (NCT04382547; NCT04427332).
Two articles were narrative reviews, without any primary data (Begam 2020; Vroegop 2020).
Two articles were letters to a journal editor, without any primary data (Patel 2021; Pinna 2020).
Finally, two studies would have been relevant for this review, but the studies were withdrawn prior to any participant enrolment (Co‐STAR (NCT04422275); NCT04374474).
Risk of bias in included studies
A single study was included in this review (Abdelalim 2021). Overall, we considered there to be a high risk of performance and detection bias due to the lack of blinding in the study. We considered other domains to be at low or unclear risk of bias. See Figure 2.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Abdelalim 2021 described using random allocation, but did not provide further details of the method used, or details regarding concealment of the allocation sequence. Correspondence with the study authors confirmed that an adequate method was used for randomisation (simple randomisation, using drawing of lots).
Blinding
Abdelalim 2021 was an open‐label study with no placebo group. The only included outcome was self‐reported olfactory function (as rated by the participants themselves) therefore we judged this to be at high risk of performance and detection bias.
Incomplete outcome data
Few dropouts were reported for the study (7.4% in total) and these were balanced across the two groups, therefore we judged Abdelalim 2021 to be at low risk of attrition bias. We noted that a per protocol analysis appeared to have been conducted, with the exclusion of two participants from each group due to non‐compliance (use of additional medication, or no use/irregular use of the study medication). However, due to the low numbers involved, we considered that this was unlikely to introduce a high risk of bias in the reported results.
Selective reporting
We were able to identify a registered protocol for Abdelalim 2021, and the outcomes were reported according to the trial registration, therefore we judged this at low risk of reporting bias.
Other potential sources of bias
We did not detect any additional potential sources of bias for Abdelalim 2021.
Effects of interventions
See: Table 1
Comparison 1: Intranasal steroids compared to no intervention
One study (100 participants) investigated intranasal steroids compared to no intervention. See Table 1.
Presence of normal olfactory function
At ≤ 4 weeks
Recovery of sense of smell was assessed using a visual analogue scale (VAS) of 0 to 10, where 0 represented total loss of smell and 10 represented completely normal smell sensation. At three weeks follow‐up, 31 out of 50 participants in the intranasal steroid group reported completely normal smell sensation, compared to 26 out of 50 in the control group (we assume that this equates to a score of 10 on the VAS). The evidence is very uncertain as to whether intranasal steroids affect the number of people who report completely normal smell sensation at up to four weeks, given the small number of participants included and the wide confidence intervals around the effect (risk ratio (RR) 1.19, 95% confidence interval (CI) 0.85 to 1.68; 1 study; 100 participants; very low‐certainty evidence; Analysis 1.1).
1.1. Analysis.

Comparison 1: Intranasal steroids compared to no intervention, Outcome 1: Presence of normal olfactory function at ≤ 4 weeks (as assessed by participants)
No data were reported for later time points of interest in this review, and no data were reported using psychophysical tests of olfactory function.
Serious adverse effects
These were not assessed or reported.
Change in sense of smell
At ≤ 4 weeks
Change in sense of smell was also reported according to the VAS of 0 to 10, with higher scores representing better olfactory function. An estimate of the change in sense of smell was not available ‐ the only data reported were endpoint data, comparing the median sense of smell in the two groups after the treatment period (three weeks). As the data were reported as median values no effect estimate could be calculated. The evidence is very uncertain about the effect of intranasal steroids on change in sense of smell at up to four weeks. Those receiving steroids had a median sense of smell score of 10 (interquartile range (IQR) 9 to 10) and those not receiving steroids had a median score of 10 (IQR 5 to 10) (P = 0.16; 1 study; 100 participants; very low‐certainty evidence).
No data were reported for later time points of interest in this review, and no data were reported using psychophysical tests of olfactory function.
Prevalence of parosmia
This was not assessed or reported.
Change in sense of taste
This was not assessed or reported.
Disease‐related quality of life
This was not assessed or reported.
Other adverse effects
These were not assessed or reported.
Discussion
Summary of main results
This review includes a single study that assessed the effect of intranasal steroids compared to no intervention for the prevention of persisting olfactory dysfunction related to COVID‐19. All participants were encouraged to use olfactory training methods whilst participating in the study, and all had symptoms of olfactory dysfunction, which had lasted less than four weeks. At three weeks follow‐up, the effect of intranasal steroids on the number of participants with self‐reported recovery of their sense of smell, or change in their sense of smell, is very uncertain.
Overall completeness and applicability of evidence
A major limitation to the findings of this review is the identification of a single study, with a very small sample size, which addressed the efficacy of just one intervention. This study provides a very small amount of evidence regarding the effect of intranasal steroids on self‐rated recovery of normal olfactory function and change of sense of smell. However, we did not identify any evidence for other outcomes, including serious adverse effects, presence of parosmia, change in sense of taste, disease‐related quality of life and other (non‐serious) adverse effects. The duration of follow‐up for this study was also limited, with outcomes being reported at three weeks (immediately after treatment was stopped). Therefore, we are unable to assess whether the intervention may have persisting effects beyond this time, or whether the discontinuation of treatment may affect the outcomes.
Adverse effects were not reported by the single study included in this review. However, if the adverse effect profile is similar to that seen when intranasal steroids are used for other sinonasal disease, then the adverse events are likely to be modest. They may include epistaxis, gastrointestinal disturbance and headache (Demoly 2008). Systemic effects are thought to be rare (Allen 2000).
The sense of smell is also important to distinguish flavour ‐ whilst the true tastes of sweet, sour, salty, bitter and umami can be sensed with the tongue, awareness of different flavours requires a functioning olfactory system. Consequently, changes in olfactory function are typically accompanied by altered flavour perception. Assessment of taste using self‐reporting is challenging (due to the need to distinguish between true taste and retronasal olfaction) and there is a lack of widespread use of psychophysical testing methods, which are needed to determine the accurate picture of olfactory and gustatory performance. Therefore, we have focused predominantly on the sense of smell for this review, but we acknowledge that an impaired sense of taste may be a real or perceived issue for many individuals who are recovering from COVID‐19.
Quality of the evidence
We judged the certainty of the evidence to be very low for both outcomes assessed. This was predominantly due to the small sample size, leading to a lack of precision in the effect estimates. The study design was also a factor, as the unblinded nature of the study may have led to bias in the effect estimates.
Potential biases in the review process
This review is one of a pair that address the prevention and treatment of olfactory dysfunction related to COVID‐19. Therefore, we excluded studies from this review if participants had more than four weeks of olfactory disturbance at baseline ‐ these studies are included in the companion review on treatment of olfactory dysfunction (Webster 2021a). We considered that this was an appropriate distinction, due to the high rate of resolution of olfactory dysfunction in the first four weeks after COVID‐19 infection.
A limitation of this review is the focus on studies where all participants had olfactory dysfunction at baseline. Whilst these are the population of interest, additional evidence for the prevention of persisting olfactory dysfunction may be available from studies of other interventions for COVID‐19. We are aware that many studies will have enrolled participants with COVID‐19 (regardless of the presence of olfactory dysfunction at baseline) and may have reported on olfactory outcomes. These studies are excluded from this review, as the overall population do not adhere to the inclusion criteria. However, they may provide additional evidence of the benefits and harms of interventions for preventing persisting olfactory dysfunction.
Agreements and disagreements with other studies or reviews
We are not aware of any other systematic reviews that consider the prevention of persisting olfactory dysfunction related to COVID‐19, therefore there are no relevant reviews to compare our findings to.
Authors' conclusions
Implications for practice.
At present there are very few data to assess the effects of interventions on preventing persistent olfactory dysfunction following COVID‐19. The only evidence available is for intranasal steroids, and this is of very low certainty, based on one study. Therefore, we are unable to draw any conclusions regarding the efficacy ‐ or potential adverse effects ‐ of intranasal steroids at preventing persistent olfactory dysfunction following COVID‐19 infection. As this is a living systematic review, the data will be updated regularly as new evidence becomes available.
Implications for research.
We are aware of a number of ongoing studies that may be relevant for this review on publication. As a living systematic review, we will update this review as new data become available.
Although olfactory disturbance is a common symptom of COVID‐19, the natural course of the disease does have a relatively high spontaneous resolution rate. Therefore, we believe that it is essential for researchers to clearly define the affected population included in their trial, according to the duration of their symptoms. The risks and benefits of treatment in the early stages of olfactory disturbance may be very different to those for individuals with a longer duration of symptoms.
We recognise that there may be poor agreement between self‐rated olfactory function and that assessed using psychophysical testing. A recent study demonstrated that only 18% of patients with COVID‐19 self‐reported ongoing olfactory dysfunction at six months' follow‐up (Boscolo‐Rizzo 2021). However, 60% of participants were shown to have some olfactory impairment on psychophysical testing. Given this discrepancy, we consider it important to assess recovery of the sense of smell using both patient‐reported and psychophysical testing, to better understand the impact of any interventions.
What's new
| Date | Event | Description |
|---|---|---|
| 27 August 2021 | Amended | This is a living systematic review: searches are run and screened monthly. The last search date was 17 August 2021. We are in the process of incorporating data from the following newly published studies: Abdelmaksoud 2021; Kasiri 2021; Mohamad 2021 (previously NCT04657809); Rashid 2021 (previously NCT04569825) and Yildiz 2021. We have also identified 10 new ongoing studies: IRCT20210205050247N1; IRCT20210311050671N1; NCT04764981; NCT04797936; NCT04900415; NCT04951362; NCT04952389; NCT04959747; NCT04964414; UMIN000043537. |
History
Protocol first published: Issue 3, 2021 Review first published: Issue 7, 2021
| Date | Event | Description |
|---|---|---|
| 17 August 2021 | Amended | This is a living systematic review: searches are run and screened monthly. The last search date was 22 July 2021. We are in the process of incorporating data from the following newly published studies: Abdelmaksoud 2021; Kasiri 2021; Mohamad 2021 (previously NCT04657809); Rashid 2021 (previously NCT04569825) and Yildiz 2021. We also identified 10 new ongoing studies: IRCT20210205050247N1; IRCT20210311050671N1; NCT04764981; NCT04797936; NCT04900415; NCT04951362; NCT04952389; NCT04959747; NCT04964414; UMIN000043537. |
Acknowledgements
This review forms part of NIHR132103 ‐ The prevention and treatment of persisting olfactory dysfunction following COVID‐19 infection; a suite of Cochrane living systematic reviews. This study is one of a number of COVID‐19 studies that have been funded by the National Institute for Health Research (NIHR) as part of its Recovery and Learning call, totalling £5.5m in funding, to help better manage current and future waves of the COVID‐19 pandemic and investigate its long‐term impact on the health and care system beyond the acute phase.
This project was also supported by the National Institute for Health Research, via Cochrane Infrastructure, Cochrane Programme Grant or Cochrane Incentive funding to Cochrane ENT. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Evidence Synthesis Programme, NIHR, NHS or the Department of Health.
Lisa O' Byrne, Evidence Synthesis Ireland Fellow, was part‐supported by the Health Research Board (Ireland) and the HSC Public Health Agency (Grant number CBES‐2018‐001) through Evidence Synthesis Ireland/Cochrane Ireland.
The authors would like to extend their sincere thanks to the board members of AbScent and Fifth Sense for their insights and significant contributions to the development of this protocol. We would also like to thank the many individuals affected by smell loss who took the time to complete our surveys, which were invaluable when prioritising outcomes for this review.
The authors would like to acknowledge Lee Yee Chong for her work on generic methods content, some of which has been adapted for use in this review (with permission).
The authors are grateful to Joanne Abbott, Information Specialist with Cochrane Pain, Palliative and Supportive Care, for providing peer review comments on the draft search methods, and Iris Gordon, Information Specialist with Cochrane Eyes & Vision, for providing peer review comments on the updated search methods introduced in July 2021.
Lastly, we would like to thank Professor Peter Tugwell, Senior Editor Cochrane MOSS Network, for acting as sign‐off editor for this review.
Appendices
Appendix 1. Search strategies
CENTRAL (CRS)
1 (("2019 nCoV" or 2019nCoV or "COVID 19" or COVID19 or "new coronavirus" or "novel coronavirus" or "novel corona virus" or "SARS CoV‐2" or SARS‐CoV2 or SARSCoV2 or "2019‐novel CoV" or ncov19 or ncov‐19 or nCov 2019 or COVID‐19 or SARSCoV‐2 or SARS‐CoV‐2)):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 2844
2 ((Wuhan and (coronavirus or "corona virus"))):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 124
3 (((coronavirus or "corona virus" or COVID) adj3 "2019")):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 582
4 ((wuhan adj2 (disease or virus))):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 4
5 ((coronavirus or "corona virus" or COVID) ):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 2919
6 (2020 or 2021):YR AND CENTRAL:TARGET 63399
7 #5 AND #6 AND CENTRAL:TARGET 2801
8 #1 OR #2 OR #3 OR #4 OR #7 AND CENTRAL:TARGET 2877
9 MESH DESCRIPTOR Olfaction Disorders EXPLODE ALL AND CENTRAL:TARGET 118
10 (Olfaction or olfactory or Dysosmia* or Paraosmia* or Anosmia* or hyposmia* or phantosmia* or Cacosmia* or microsmia*):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 1201
11 (smell* adj6 (disorder* or loss or distort* or alter* or dsyfunction or impair* or abscen* or reduce* or different* or sensation* or abnormal* or perception* or change* or expected or decreas* or deficit*)):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 424
12 (smell* adj6 (prevent* or rehab* or recover* or therap* or train* or retrain*)):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 151
13 #9 OR #10 OR #11 OR #12 AND CENTRAL:TARGET 1474
14 #13 AND #8 AND CENTRAL:TARGET 34
MEDLINE (Ovid)
1 ("2019 nCoV" or 2019nCoV or "COVID 19" or COVID19 or "new coronavirus" or "novel coronavirus" or "novel corona virus" or "SARS CoV‐2" or SARS‐CoV2 or SARSCoV2 or "2019‐novel CoV" or ncov19 or ncov‐19 or nCov 2019 or COVID‐19 or SARSCoV‐2 or SARS‐CoV‐2).ab,ti. 64357
2 (Wuhan and (coronavirus or "corona virus")).ab,ti. 2686
3 (coronavirus or "corona virus" or COVID) adj3 "2019").ab,ti. 14622
4 (wuhan adj2 (disease or virus)).ab,ti. 69
5 ("LAMP assay" or "COVID‐19" or "COVID‐19 drug treatment" or "COVID‐19 diagnostic testing" or "COVID‐19 serotherapy" or "COVID‐19 vaccine" or "severe acute respiratory syndrome coronavirus 2" or "spike protein, SARS‐CoV‐2").os. 27706
6 (coronavirus or "corona virus" or COVID).ab,ti. 73711
7 limit 6 to yr="2020 ‐Current" 64172
8 1 or 2 or 3 or 4 or 5 or 7 68776
9 exp olfaction disorders/ 4393
10 (Olfaction or olfactory or Dysosmia* or Paraosmia* or Anosmia* or hyposmia* or phantosmia* or Cacosmia* or microsmia*).ab,ti. 49706
11 (smell* adj6 (disorder* or loss or distort* or alter* or dsyfunction or impair* or abscen* or reduce* or different* or sensation* or abnormal* or perception* or change* or expected or decreas* or deficit*)).ab,ti. 2401
12 (smell* adj6 (prevent* or rehab* or recover* or therap* or train* or retrain*)).ab,ti. 180
13 9 or 10 or 11 or 12 51681
14 8 and 13 768
15 randomized controlled trial.pt. 516072
16 controlled clinical trial.pt. 93905
17 randomized.ab. 496684
18 placebo.ab. 212020
19 drug therapy.fs. 2246822
20 randomly.ab. 343595
21 trial.ab. 525027
22 groups.ab. 2108399
23 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 4824610
24 exp animals/ not humans.sh. 4750713
25 23 not 24 4189853
26 14 and 25 72
Cochrane COVID‐19 Study Register (CRS)
1 MESH DESCRIPTOR Olfaction Disorders EXPLODE ALL AND COVID19:INREGISTER 5
2 (Olfaction or olfactory or Dysosmia* or Paraosmia* or Anosmia* or hyposmia* or phantosmia* or Cacosmia* or microsmia*) AND COVID19:INREGISTER 392
3 (smell* adj6 (disorder* or loss or distort* or alter* or dsyfunction or impair* or abscen* or reduce* or different* or sensation* or abnormal* or perception* or change* or expected or decreas* or deficit*)) AND COVID19:INREGISTER 180
4 (smell* adj6 (prevent* or rehab* or recover* or therap* or train* or retrain*)) AND COVID19:INREGISTER 17
5 #1 OR #2 OR #3 OR #4 483
6 (interventional):SY AND COVID19:INREGISTER 3460
7 #6 AND #5 37
Cochrane ENT Register (CRS)
1 (("2019 nCoV" or 2019nCoV or "COVID 19" or COVID19 or "new coronavirus" or "novel coronavirus" or "novel corona virus" or "SARS CoV‐2" or SARS‐CoV2 or SARSCoV2 or "2019‐novel CoV" or ncov19 or ncov‐19 or nCov 2019 or COVID‐19 or SARSCoV‐2 or SARS‐CoV‐2)):AB,EH,KW,KY,MC,MH,TI,TO AND INREGISTER
2 ((Wuhan and (coronavirus or "corona virus"))):AB,EH,KW,KY,MC,MH,TI,TO AND INREGISTER
3 (((coronavirus or "corona virus" or COVID) adj3 "2019")):AB,EH,KW,KY,MC,MH,TI,TO AND INREGISTER
4 ((wuhan adj2 (disease or virus))):AB,EH,KW,KY,MC,MH,TI,TO AND INREGISTER
5 ((coronavirus or "corona virus" or COVID) ):AB,EH,KW,KY,MC,MH,TI,TO AND INREGISTER
6 (2020 or 2021):YR AND INREGISTER
7 #5 AND #6 AND INREGISTER
8 #1 OR #2 OR #3 OR #4 OR #7 AND INREGISTER
9 MESH DESCRIPTOR Olfaction Disorders EXPLODE ALL AND INREGISTER
10 (Olfaction or olfactory or Dysosmia* or Paraosmia* or Anosmia* or hyposmia* or phantosmia* or Cacosmia* or microsmia*):AB,EH,KW,KY,MC,MH,TI,TO AND INREGISTER
11 (smell* adj6 (disorder* or loss or distort* or alter* or dsyfunction or impair* or abscen* or reduce* or different* or sensation* or abnormal* or perception* or change* or expected or decreas* or deficit*)):AB,EH,KW,KY,MC,MH,TI,TO AND INREGISTER
12 (smell* adj6 (prevent* or rehab* or recover* or therap* or train* or retrain*)):AB,EH,KW,KY,MC,MH,TI,TO AND INREGISTER
13 #9 OR #10 OR #11 OR #12 AND INREGISTER
14 #13 AND #8 AND INREGISTER
Embase (Ovid)
1 ("2019 nCoV" or 2019nCoV or "COVID 19" or COVID19 or "new coronavirus" or "novel coronavirus" or "novel corona virus" or "SARS CoV‐2" or SARS‐CoV2 or SARSCoV2 or "2019‐novel CoV" or ncov19 or ncov‐19 or nCov 2019 or COVID‐19 or SARSCoV‐2 or SARS‐CoV‐2).ab,ti.
2 (Wuhan and (coronavirus or "corona virus")).ab,ti.
3 ((coronavirus or "corona virus" or COVID) adj3 "2019").ab,ti.
4 (wuhan adj2 (disease or virus)).ab,ti.
5 (coronavirus or "corona virus" or COVID).ab,ti.
6 limit 5 to yr="2020 ‐Current"
7 1 or 2 or 3 or 4 or 6
8 exp smelling disorder/
9 (Olfaction or olfactory or Dysosmia* or Paraosmia* or Anosmia* or hyposmia* or phantosmia* or Cacosmia* or microsmia*).ab,ti.
10 (smell* adj6 (disorder* or loss or distort* or alter* or dsyfunction or impair* or abscen* or reduce* or different* or sensation* or abnormal* or perception* or change* or expected or decreas* or deficit*)).ab,ti.
11 (smell* adj6 (prevent* or rehab* or recover* or therap* or train* or retrain*)).ab,ti.
12 8 or 9 or 10 or 11
13 7 and 12
14 Randomized controlled trial/
15 Controlled clinical study/
16 Random$.ti,ab.
17 randomization/
18 intermethod comparison/
19 placebo.ti,ab.
20 (compare or compared or comparison).ti.
21 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab.
22 (open adj label).ti,ab.
23 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab.
24 double blind procedure/
25 parallel group$1.ti,ab.
26 (crossover or cross over).ti,ab.
27 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab.
28 (assigned or allocated).ti,ab.
29 (controlled adj7 (study or design or trial)).ti,ab.
30 (volunteer or volunteers).ti,ab.
31 human experiment/
32 trial.ti.
33 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32
34 (random$ adj sampl$ adj7 ("cross section$" or questionnaire$1 or survey$ or database$1)).ti,ab.
35 comparative study/ or controlled study/
36 randomi?ed controlled.ti,ab.
37 randomly assigned.ti,ab.
38 35 or 36 or 37
39 34 not 38
40 Cross‐sectional study/
41 randomized controlled trial/ or controlled clinical study/ or controlled study/
42 (randomi?ed controlled or control group$1).ti,ab.
43 41 or 42
44 40 not 43
45 (((case adj control$) and random$) not randomi?ed controlled).ti,ab.
46 (Systematic review not (trial or study)).ti.
47 (nonrandom$ not random$).ti,ab.
48 "Random field$".ti,ab.
49 (random cluster adj3 sampl$).ti,ab.
50 (review.ab. and review.pt.) not trial.ti.
51 "we searched".ab.
52 review.ti. or review.pt.
53 51 and 52
54 "update review".ab.
55 (databases adj4 searched).ab.
56 (rat or rats or mouse or mice or swine or porcine or murine or sheep or lambs or pigs or piglets or rabbit or rabbits or cat or cats or dog or dogs or cattle or bovine or monkey or monkeys or trout or marmoset$1).ti. and animal experiment/
57 39 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 53 or 54 or 55
58 33 not 57
59 13 and 58
Web of Science (Web of Knowledge)
#1 TS=("2019 nCoV" or 2019nCoV or "COVID 19" or COVID19 or "new coronavirus" or "novel coronavirus" or "novel corona virus" or "SARS CoV‐2" or SARS‐CoV2 or SARSCoV2 or "2019‐novel CoV" or ncov19 or ncov‐19 or nCov 2019 or COVID‐19 or SARSCoV‐2 or SARS‐CoV‐2)
#2 TS=(Wuhan and (coronavirus or "corona virus") )
#3 TS=(((coronavirus or "corona virus" or COVID) NEAR/3 "2019"))
#4 TOPIC: (wuhan NEAR/2 (disease or virus) )
#5 TS=(coronavirus or "corona virus" or COVID)
#6 #5 OR #4 OR #3 OR #2 OR #1
#7 TS=(Olfaction or olfactory or Dysosmia* or Paraosmia* or Anosmia* or hyposmia* or phantosmia* or Cacosmia* or microsmia*)
#8 TS=(smell* NEAR/6 (disorder* or loss or distort* or alter* or dsyfunction or impair* or abscen* or reduce* or different* or sensation* or abnormal* or perception* or change* or expected or decreas* or deficit*) )
#9 TS=(smell* NEAR/6 (prevent* or rehab* or recover* or therap* or train* or retrain*) )
#10 #9 OR #8 OR #7
#11 #10 AND #6
#12 TS=((randomised OR randomized OR randomisation OR randomisation OR placebo* OR (random* AND (allocat* OR assign*) ) OR (blind* AND (single OR double OR treble OR triple) )))
#13 #12 AND #11
World Health Organization (WHO) COVID‐19 'Global literature on coronavirus disease'
(ti:(olfaction OR olfactory OR dysosmia* OR paraosmia* OR anosmia* OR hyposmia* OR phantosmia* OR cacosmia* OR microsmia* OR smell*)) OR (mh:(olfato OR l'olfaction OR cacosmia OR paraosmia OR anosmia))
Trial Registry Records (CENTRAL via CRS)
1 (("2019 nCoV" or 2019nCoV or "COVID 19" or COVID19 or "new coronavirus" or "novel coronavirus" or "novel corona virus" or "SARS CoV‐2" or SARS‐CoV2 or SARSCoV2 or "2019‐novel CoV" or ncov19 or ncov‐19 or nCov 2019 or COVID‐19 or SARSCoV‐2 or SARS‐CoV‐2)) AND CENTRAL:TARGET
2 ((Wuhan and (coronavirus or "corona virus"))) AND CENTRAL:TARGET
3 (((coronavirus or "corona virus" or COVID) adj3 "2019")) AND CENTRAL:TARGET
4 ((wuhan adj2 (disease or virus))) AND CENTRAL:TARGET
5 (coronavirus or "corona virus" or COVID) AND CENTRAL:TARGET
6 (2020 or 2021):YR AND CENTRAL:TARGET
7 #5 AND #6
8 #1 OR #2 OR #3 OR #4 OR #7
9 (Olfaction or olfactory or Dysosmia* or Paraosmia* or Anosmia* or hyposmia* or phantosmia* or Cacosmia* or microsmia* or smell*) AND CENTRAL:TARGET
10 #8 AND #9
11 http*:SO AND CENTRAL:TARGET
12 (NCT0* or ACTRN* or ChiCTR* or DRKS* or EUCTR* or eudract* or IRCT* or ISRCTN* or JapicCTI* or JPRN* or NTR0* or NTR1* or NTR2* or NTR3* or NTR4* or NTR5* or NTR6* or NTR7* or NTR8* or NTR9* or SRCTN* or UMIN0*):AU AND CENTRAL:TARGET
13 #11 OR #12
14 #10 AND #13
ICTRP (WHO Portal)
covid* AND anomsia OR covid* AND smell OR covid* AND olfact* OR coronavirus AND anomsia OR coronavirus AND smell OR coronavirus AND olfact* OR SARS‐CoV* AND anomsia OR SARS‐CoV* AND smell OR SARS‐CoV* AND olfact*
ClinicalTrials.gov
(COVID‐19 OR 2019‐nCoV OR SARS‐CoV‐2 OR 2019 novel coronavirus OR severe acute respiratory syndrome coronavirus 2 OR Wuhan coronavirus OR coronavirus) AND (anosmia OR smell OR Olfaction or olfactory)
AND Interventiona
Data and analyses
Comparison 1. Intranasal steroids compared to no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Presence of normal olfactory function at ≤ 4 weeks (as assessed by participants) | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.85, 1.68] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abdelalim 2021.
| Study characteristics | ||
| Methods | Two‐arm, non‐blinded, parallel‐group randomised controlled trial with 3 weeks' duration of treatment and follow‐up | |
| Participants |
Location: Egypt, single‐centre study Setting of recruitment and treatment: recruited at Benha University Hospital Sample size: 108
Participants:
Baseline characteristics:
Inclusion criteria for the study:
Exclusion criteria for the study:
|
|
| Interventions |
Intervention group: Topical corticosteroid spray (mometasone furoate nasal spray), 2 puffs (100 µg) once daily in each nostril for 3 weeks Comparator group: no intervention Use of additional interventions in both groups: none reported |
|
| Outcomes |
Outcomes of interest in the review: Primary outcomes: Presence of normal olfactory function
Serious adverse effects
Change in sense of smell
Secondary outcomes: Prevalence of parosmia
Change in sense of taste
Disease‐related quality of life
Other adverse effects (including nosebleeds/bloody discharge)
Other outcomes reported by the study: None reported |
|
| Funding sources | No financial support was reported for the study | |
| Declarations of interest | No conflict of interest was declared | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The participants in this study were randomly assigned to two groups (simple 1:1 randomisation)". Comment: correspondence from the author confirmed that adequate methods were used (random selection of groups from an envelope). |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information was provided regarding concealment of allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: open‐label study, with no placebo group. Participants were aware of their allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: open‐label study, with no placebo group. The only outcome assessed was self‐reported by the (unblinded) participants. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: few dropouts (7.4%) and balanced across the groups. Two participants in each group may have been excluded due to non‐adherence to the trial protocol (intervention group: 1 discontinued treatment, 1 used treatment inconsistently, control group: 2 received other medications). However, the impact of this on the results was not felt to be sufficient to result in high risk of bias. |
| Selective reporting (reporting bias) | Low risk | Comment: trial protocol accessed on clinical trial registry, and no further outcomes were planned. |
| Other bias | Low risk | Comment: no other source of bias detected. |
IQR interquartile range; PCR polymerase chain reaction; VAS visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ACTION (NCT04332107) | Wrong population: study does not specifically include participants with olfactory dysfunction |
| Begam 2020 | Narrative review article, no primary data |
| COPPS (NCT04662060) | Wrong population: study does not specifically include participants with olfactory dysfunction |
| Co‐STAR (NCT04422275) | Although this study fits the inclusion criteria for the review, it was withdrawn prior to any participant enrolment |
| COVIDAtoZ (NCT04342728) | Wrong population: study does not specifically include participants with olfactory dysfunction |
| COVIDORL (NCT04361474) | Wrong population: all participants in the study have had symptoms of olfactory disturbance for at least 4 weeks. This study is relevant for the companion review "Interventions for the treatment of persistent post‐COVID‐19 olfactory dysfunction" (Webster 2021a). |
| CTRI/2020/08/027477 | Wrong population: study does not specifically include participants with olfactory dysfunction |
| IRCT20200522047542N1 | Wrong population: all participants in the study have had symptoms of olfactory disturbance for at least 4 weeks. This study is relevant for the companion review "Interventions for the treatment of persistent post‐COVID‐19 olfactory dysfunction" (Webster 2021a). |
| NCT04374474 | Although this study fits the inclusion criteria for the review, it was withdrawn prior to any participant enrolment |
| NCT04382547 | Wrong study design: not a randomised controlled trial |
| NCT04406584 | Wrong population: includes participants with any post‐viral olfactory disturbance (not specifically COVID‐19) |
| NCT04414124 | Wrong population: study does not specifically include participants with olfactory dysfunction |
| NCT04427332 | Wrong study design: observational study, not a randomised controlled trial |
| NCT04458519 | Wrong population: study does not specifically include participants with olfactory dysfunction |
| NCT04474483 | Wrong population: study does not specifically include participants with olfactory dysfunction |
| NCT04513184 | Wrong population and wrong comparator: study does not specifically include participants with olfactory dysfunction; intervention is compared to intravenous dexamethasone |
| NCT04622891 | Wrong population: study does not specifically include participants with olfactory dysfunction |
| NCT04662086 | Wrong population: study does not specifically include participants with olfactory dysfunction |
| Odorat‐Covid (NCT04598763) | Wrong population: all participants in the study have had symptoms of olfactory disturbance for at least 4 weeks. This study is relevant for the companion review "Interventions for the treatment of persistent post‐COVID‐19 olfactory dysfunction" (Webster 2021a). |
| Patel 2021 | Letter to the editor: no primary data included |
| Pinna 2020 | Letter to the editor: no primary data included |
| Vaira 2020 | Wrong population: all participants in the study have had symptoms of olfactory disturbance for at least 4 weeks. This study is relevant for the companion review "Interventions for the treatment of persistent post‐COVID‐19 olfactory dysfunction" (Webster 2021a). |
| Vroegop 2020 | Narrative review article: no primary data |
Characteristics of ongoing studies [ordered by study ID]
NCT04495816.
| Study name | Randomised control trial of omega‐3 fatty acid supplementation for the treatment of COVID‐19 related olfactory dysfunction |
| Methods | Parallel‐group randomised controlled trial |
| Participants | Adults with self‐reported new onset olfactory dysfunction and COVID‐19 infection Inclusion criteria:
Exclusion criteria:
Planned sample size: estimated enrolment 126 participants (from clinical trial register). Additional publication states estimated sample size of 176 (88 per group). |
| Interventions |
Intervention: omega‐3 fatty acid, 1000 mg (administered as 2 soft gels, containing 683 mg eicosapentaenoic acid and 252 mg docosahexaenoic acid) twice daily for 6 weeks Comparator: placebo (administered as 2 placebo soft gels) twice daily for 6 weeks |
| Outcomes |
Outcomes of interest in the review: Primary outcomes: Presence of normal olfactory function
Serious adverse effects
Change in sense of smell
Secondary outcomes: Prevalence of parosmia
Change in sense of taste
Disease‐related quality of life
Other adverse effects (including nosebleeds/bloody discharge)
Other outcomes reported by the study:
|
| Starting date | 15 July 2020 |
| Contact information | Alfred‐Marc Iloreta Email: alfred-marc.iloreta@mountsinai.org |
| Notes | Estimated study completion date: June 2021 It is unclear from the description of this trial whether participants will have symptoms of olfactory disturbance for fewer than 4 weeks from the onset of COVID‐19. Correspondence with the study team has confirmed that they will recruit a mixed population, comprising individuals with fewer than and longer than 4 weeks of symptoms. |
NCT04528329.
| Study name | Anosmia and/or ageusia and early corticosteroid use |
| Methods | Randomised controlled trial |
| Participants | Adult participants with mild to moderate severity COVID‐19 Inclusion
Exclusion
Planned sample size: 300 participants |
| Interventions |
Intervention: "Early dexamethasone use as early as confirmation of inflammation" Comparator: "Late dexamethasone use as soon as deterioration" |
| Outcomes |
Outcomes of interest in the review: Primary outcomes: Presence of normal olfactory function
Serious adverse effects
Change in sense of smell
Secondary outcomes: Prevalence of parosmia
Change in sense of taste
Disease‐related quality of life
Other adverse effects (including nosebleeds/bloody discharge)
Other outcomes reported by the study:
|
| Starting date | 30 August 2020 |
| Contact information | Emad R Issak Email: dr.emad.r.h.issak@gmail.com |
| Notes | Estimated study completion date: 15 December 2020 Trial registered in Egypt Uncertainty over future inclusion in the review: It is not clear from the description provided whether participants will all have olfactory dysfunction at baseline and, if so, whether they will have ≤ 4 weeks of olfactory dysfunction. We are awaiting confirmation from the study authors. |
NCT04530409.
| Study name | Timing of corticosteroids in COVID‐19 |
| Methods | Randomised controlled trial |
| Participants | Adult participants with mild or moderate COVID‐19 Included
Excluded
Planned sample size: 450 patients |
| Interventions |
Intervention: "Early dexamethasone use as early as confirmation of inflammation" Comparator: "Late dexamethasone use as soon as deterioration" |
| Outcomes |
Outcomes of interest in the review: Primary outcomes: Presence of normal olfactory function
Serious adverse effects
Change in sense of smell
Secondary outcomes: Prevalence of parosmia
Change in sense of taste
Disease‐related quality of life
Other adverse effects (including nosebleeds/bloody discharge)
Other outcomes reported by the study: Primary:
Secondary:
|
| Starting date | 26 August 2020 |
| Contact information | Emad R Issak Email: dr.emad.r.h.issak@gmail.com |
| Notes | Estimated study completion date: 1 December 2020 Trial registered in Egypt Uncertainty over future inclusion in the review: It is not clear from the description provided whether participants will all have olfactory dysfunction at baseline and, if so, whether they will have ≤ 4 weeks of olfactory dysfunction. |
NCT04569825.
| Study name | Effect of nasal steroid in the treatment of anosmia due to COVID‐19 disease |
| Methods | Parallel‐group randomised controlled trial |
| Participants | Individuals with a confirmed diagnosis of COVID‐19 (real‐time PCR) with recent onset of anosmia and or ageusia Inclusion criteria:
Exclusion criteria:
Planned sample size: estimated enrolment 250 participants |
| Interventions |
Intervention: intranasal opthamesone (steroid) drops (frequency and duration not stated) Comparator: intranasal normal saline drops (frequency and duration not stated) |
| Outcomes |
Outcomes of interest in the review: Primary outcomes: Presence of normal olfactory function
Serious adverse effects
Change in sense of smell
Secondary outcomes: Prevalence of parosmia
Change in sense of taste
Disease‐related quality of life
Other adverse effects (including nosebleeds/bloody discharge)
Other outcomes reported by the study:
|
| Starting date | 1 August 2020 |
| Contact information | Raid M Al‐Ani Email: med.raed.alani2003@uoanbar.edu.iq |
| Notes | Estimated study completion date: 15 October 2020 Trial registered in Iraq Correspondence from the trial investigator confirms that all participants in this trial will have had recently diagnosed COVID‐19, and that symptoms of anosmia will have lasted for less than 2 weeks at the start of the trial. |
NCT04638673.
| Study name | NeuroCovid rehab and recovery related to COVID‐19 diagnosis |
| Methods | To evaluate a transcutaneous auricular vagus nerve stimulation (taVNS) in the treatment of the neurological symptoms of COVID‐19 termed NEUROCOVID |
| Participants | Patients with COVID‐19 suffering from the neurological symptoms associated with infection Inclusion criteria:
Exclusion criteria:
Planned sample size: estimated enrolment 30 participants |
| Interventions |
Intervention group: active‐active stimulation group; participants will receive active taVNS stimulation for weeks 1 to 4 of the stimulation portion of this study using Soterix taVNS model 0125‐LTE Comparator group: sham‐active stimulation group; participants will receive sham taVNS stimulation for weeks 1 and 2 and active stimulation for weeks 3 and 4 of the stimulation portion of this study |
| Outcomes |
Outcomes of interest in the review: Primary outcomes: Presence of normal olfactory function
Serious adverse effects
Change in sense of smell
Secondary outcomes: Prevalence of parosmia
Change in sense of taste
Disease‐related quality of life
Other adverse effects (including nosebleeds/bloody discharge)
Other outcomes reported by the study:
|
| Starting date | 19 November 2020 |
| Contact information | Sarah Huffman
Email: huffmans@musc.edu Morgan Dancy Email: maddoxm@musc.edu |
| Notes | Estimated study completion date: June 2021 Trial registered in USA Uncertainty over future inclusion in the review: It is not clear from the description provided whether participants will all have olfactory dysfunction at baseline and, if so, whether they will have had ≤ 4 weeks of olfactory dysfunction. We are awaiting confirmation from the study authors. |
NCT04657809.
| Study name | Clinical assessment of insulin fast dissolving film in treatment of post infection anosmia |
| Methods | Parallel‐group randomised controlled trial |
| Participants | Adult participants with loss of sense of smell after COVID‐19 infection Inclusion criteria
Exclusion criteria
Planned sample size: estimated enrolment 40 participants |
| Interventions |
Intervention: insulin fast‐dissolving film containing 100 IU of insulin applied intranasally 3 times a week for 4 weeks Comparator: formulated bio‐adhesive fast‐dissolving film containing no drugs applied intranasally 3 times a week for 4 weeks |
| Outcomes |
Outcomes of interest in the review: Primary outcomes: Presence of normal olfactory function
Serious adverse effects
Change in sense of smell
Secondary outcomes: Prevalence of parosmia
Change in sense of taste
Disease‐related quality of life
Other adverse effects (including nosebleeds/bloody discharge)
Other outcomes reported by the study:
|
| Starting date | 1 December 2020 |
| Contact information | Soad Ali, no contact details provided |
| Notes | Estimated study completion date: 15 January 2021 Trial registered in Egypt It is unclear from the description of this trial whether participants will have symptoms of olfactory disturbance for fewer than 4 weeks from the onset of COVID‐19 |
ALT: alanine aminotransferase; BiPAP bilevel positive airway pressure; BSIT Brief Smell Identification Test; CPAP continuous positive airway pressure; CRP: c‐reactive protein; LDH: lactate dehydrogenase; mQOD‐NS Modified Brief Questionnaire of Olfactory Dysfunction; SNOT‐22 Sinonasal Outcomes Test
Differences between protocol and review
When preparing the protocol for this review we intended to present the following outcomes in the summary of findings table:
presence of normal olfactory function (as reported by the participants);
serious adverse effects;
change in sense of smell (as identified by psychophysical testing);
prevalence of parosmia;
change in sense of taste;
disease‐related quality of life;
other adverse effects (including nosebleeds/bloody discharge).
No data were available from psychophysical testing for the outcome "Change in sense of smell". However, we did identify data for this outcome that had been self‐reported by the participants, therefore these data were included in the summary of findings table.
Contributions of authors
Katie Webster: scoped, designed and drafted the protocol with the help of the other authors. Sifted studies, carried out data extraction, risk of bias assessment, performed analyses and GRADE assessment for included studies, drafted and revised the review.
Lisa O'Byrne: sifted studies, carried out data extraction, risk of bias assessment and GRADE assessment for included studies, drafted and revised the review.
Samuel MacKeith: clinical guidance at all stages of project scoping and protocol development; commented on and edited the draft review, and agreed the final version.
Carl Philpott: clinical guidance at all stages of project scoping and protocol development; commented on and edited the draft review, and agreed the final version.
Claire Hopkins: clinical guidance at all stages of project scoping and protocol development; commented on and edited the draft review, and agreed the final version.
Martin Burton: clinical guidance at all stages of project scoping and protocol development; commented on and edited the draft review, and agreed the final version.
Sources of support
Internal sources
No sources of support provided
External sources
-
National Institute for Health Research, UK
Infrastructure funding for Cochrane ENT
NIHR COVID‐19 Recovery and Learning programme, UK
Declarations of interest
Katie Webster: none known.
Lisa O' Byrne: none known.
Samuel MacKeith: Sam MacKeith is Assistant Co‐ordinating Editor of Cochrane ENT, but had no role in the editorial process for this review.
Carl Philpott: Professor Carl Philpott sees and treats patients with COVID‐19 related smell loss. He has written various online publications on the topic and conducted interviews and webinars internationally. He is a Trustee for the charity Fifth Sense. He is the senior author on the Clinical Olfactory Working Group consensus document on the management of post‐infectious olfactory dysfunction and the consensus document on the use of systemic corticosteroids in COVID‐19 related olfactory dysfunction.
Claire Hopkins: Professor Claire Hopkins sees and treats patients with COVID‐19 related smell loss. She has spoken on the association between COVID and smell loss in multiple media outlets. She is senior author of the British Rhinological Society position paper on management of COVID‐19 related smell loss.
Martin Burton: Professor Martin Burton is Co‐ordinating Editor of Cochrane ENT, but had no role in the editorial process for this review.
Edited (no change to conclusions)
References
References to studies included in this review
Abdelalim 2021 {published data only}
- Abdelalim AA, Mohamady AA, Elsayed RA, Elawady MA, Ghallab AF. Corticosteroid nasal spray for recovery of smell sensation in COVID-19 patients: a randomized controlled trial. American Journal of Otolaryngology 2021;42(2):102884. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT04484493. Corticosteroid nasal spray in COVID-19 anosmia [Role of corticosteroid nasal spray in recovery of smell sensation in COVID-19 patients]. https://clinicaltrials.gov/show/NCT04484493 (first received 23 July 2020). [CENTRAL: CN-02145535]
References to studies excluded from this review
ACTION (NCT04332107) {published data only}
- NCT04332107. Azithromycin for COVID-19 treatment in outpatients nationwide [Azithromycin for prevention of disease progression in patients with mild or moderate COVID-19]. https://clinicaltrials.gov/show/NCT04332107 (first received 2 April 2020).
Begam 2020 {published data only}
- Begam N, Bashar MA. Olfactory and taste disorders in patients with SARS-CoV-2 infection. International Archives of Otorhinolaryngology 2020;24(3):e391-2. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
COPPS (NCT04662060) {published data only}
- NCT04662060. COVID-19 Outpatient Pragmatic Platform Study (COPPS) - Acebilustat Sub-Protocol [COVID-19 Outpatient Pragmatic Platform Study (COPPS): a pragmatic multi-arm, adaptive, phase 2, blinded, randomized placebo-controlled platform trial to assess the efficacy of different investigational therapeutics in reducing time to disease resolution or viral load cessation, as compared to standard supportive care in outpatients with COVID-19]. https://clinicaltrials.gov/show/NCT04662060 (first received 10 December 2020).
Co‐STAR (NCT04422275) {published data only}
- NCT04422275. Coronavirus smell therapy for anosmia recovery. https://clinicaltrials.gov/show/NCT04422275 (first received 9 June 2020). [CENTRAL: CN-02118715]
COVIDAtoZ (NCT04342728) {published data only}
- NCT04342728. Coronavirus 2019 (COVID-19)- using ascorbic acid and zinc supplementation [Coronavirus disease 2019- using ascorbic acid and zinc supplementation (COVIDAtoZ) research study a randomized, open label single center study]. https://clinicaltrials.gov/show/NCT04342728 (first received 13 April 2020).
COVIDORL (NCT04361474) {published data only}
- Daval M, Corre A, Palpacuer C, Housset J, Poillon G, Eliezer M, et al. Efficacy of local budesonide therapy in the management of persistent hyposmia in COVID-19 patients without signs of severity: a structured summary of a study protocol for a randomised controlled trial. Trials 2020;21(1):666. [CENTRAL: CN-02177639] [EMBASE: 632388256] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT04361474. Trial evaluating the efficacy of local budesonide therapy in the management of hyposmia in COVID-19 patients without signs of severity [A randomized controlled trial evaluating the efficacy of local budesonide therapy in the management of hyposmia in COVID-19 patients without signs of severity]. https://clinicaltrials.gov/show/NCT04361474 (first received 24 April 2020). [CENTRAL: CN-02180471]
CTRI/2020/08/027477 {published data only}
- CTRI/2020/08/027477. Evaluation of safety and efficacy of T-AYU-HM Premium and Onion steam vaporization/nebulization in Covid-19 patients. http://www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2020/08/027477 (first received 2020).
IRCT20200522047542N1 {published data only}
- IRCT20200522047542N1. Effect of Corton on olfactory dysfunction in COVID-19 patients [Effect of inhaled corticosteroids in the treatment of anosmia in patients with COVID-19]. http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20200522047542N1 (first received 2020). [CENTRAL: CN-02187839]
NCT04374474 {published data only}
- NCT04374474. Anosmia rehabilitation in patients post coronavirus disease (COVID 19) [Olfactory retraining therapy and budesonide nasal rinse for anosmia treatment in patients post-COVID 19. A randomized controlled trial]. https://clinicaltrials.gov/show/NCT04374474 (first received 5 May 2020). [CENTRAL: CN-02093904]
NCT04382547 {published data only}
- NCT04382547. Treatment of Covid-19 associated pneumonia with allogenic pooled olfactory mucosa-derived mesenchymal stem cells. https://clinicaltrials.gov/show/NCT04382547 (first received 11 May 2020).
NCT04406584 {published data only}
- NCT04406584. Intranasal Injection of PRP versus saline for treatment of olfactory dysfunction [Intranasal injection of platelet-rich plasma versus saline for treatment of olfactory dysfunction]. https://clinicaltrials.gov/show/NCT04406584 (first received 28 May 2020). [CENTRAL: CN-02124993]
NCT04414124 {published data only}
- NCT04414124. A clinical study to assess the natural history of COVID-19 and effects of KB109 and supportive self-care in outpatients with mild-to-moderate COVID-19 [A randomized, open label, prospective, parallel group study to assess the natural history of COVID-19 and effects of KB109 in addition to supportive self care (SSC) compared to SSC alone on measures of health in non-hospitalized patients with mild-moderate COVID-19]. https://clinicaltrials.gov/show/NCT04414124 (first received 4 June 2020).
NCT04427332 {published data only}
- NCT04427332. Smell and taste disorders in COVID-19 patients [Smell and taste disorders in COVID-19 patients: prospective observational study]. ClinicalTrials.gov (first received 2020).
NCT04458519 {published data only}
- NCT04458519. Efficacy of intranasal probiotic treatment to reduce severity of symptoms in COVID19 infection [Randomised single blinded clinical study of efficacy of intranasal probiotic treatment to reduce severity of symptoms in COVID19 infection]. https://clinicaltrials.gov/show/NCT04458519 (first received 7 July 2020).
NCT04474483 {published data only}
- NCT04474483. Safety and efficacy of melatonin in outpatients infected with COVID-19 [A pilot placebo-controlled randomized double-blind trial of melatonin in outpatients with COVID-19 infection]. https://clinicaltrials.gov/show/NCT04474483 (first received 16 July 2020).
NCT04513184 {published data only}
- NCT04513184. Randomized clinical trial of intranasal dexamethasone as an adjuvant in patients with COVID-19. https://clinicaltrials.gov/show/NCT04513184 (first received 14 August 2020). [CENTRAL: CN-02146132]
NCT04622891 {published data only}
- NCT04622891. Clarithromycin versus azithromycin in treatment of mild COVID-19 infection [Efficacy of clarithromycin in comparison to azithromycin in treatment of mild COVID-19 infection, randomized controlled trial]. https://clinicaltrials.gov/show/NCT04622891 (first received 10 November 2020). [CENTRAL: CN-02184257]
NCT04662086 {published data only}
- NCT04662086. COVID-19 Outpatient Pragmatic Platform Study (COPPS) - master protocol [COVID-19 Outpatient Pragmatic Platform Study (COPPS): a pragmatic multi-arm, adaptive, phase 2, blinded, randomized placebo-controlled platform trial to assess the efficacy of different investigational therapeutics in reducing time to disease resolution or viral load cessation, as compared to standard supportive care in outpatients with COVID-19]. https://clinicaltrials.gov/show/NCT04662086 (first received 10 December 2020).
Odorat‐Covid (NCT04598763) {published data only}
- NCT04598763. Evaluation of two methods of olfactory rehabilitation in post-viral loss of smell: classic and intensive. https://clinicaltrials.gov/show/NCT04598763 (first received 22 October 2020).
Patel 2021 {published data only}
- Levy JM. Regarding use of topical steroids in patients with COVID-19-associated olfactory loss - Reply. JAMA Otolaryngology--Head & Neck Surgery 2021;147(1):110-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel ZM. Regarding use of topical steroids in patients with COVID-19-associated olfactory loss. JAMA Otolaryngology - Head and Neck Surgery 2021;147(1):110. [DOI] [PubMed] [Google Scholar]
Pinna 2020 {published data only}
- Pinna FR, Brandao Neto D, Fornazieri MA, Voegels RL. Olfaction and COVID: the little we know and what else we need to know. International Archives of Otorhinolaryngology 2020;24(3):e386-7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vaira 2020 {published data only}
- Vaira LA, Hopkins C, Petrocelli M, Lechien JR, Cutrupi S, Salzano G, et al. Efficacy of corticosteroid therapy in the treatment of long- lasting olfactory disorders in COVID-19 patients. Rhinology 2020;59(1):21-5. [CENTRAL: CN-02210463] [EMBASE: 633663621] [PMID: ] [DOI] [PubMed] [Google Scholar]
Vroegop 2020 {published data only}
- Vroegop AV, Eeckels AS, Van Rompaey V, Vanden Abeele D, Schiappoli M, Alobid I, et al. COVID-19 and olfactory dysfunction - an ENT perspective to the current COVID-19 pandemic. B-ENT 2020;16(1):81-5. [Google Scholar]
References to ongoing studies
NCT04495816 {published data only}
- Lerner D, Garvey K, Arrighi-Allisan A, Filimonov A, Filip P, Liu K, et al. Letter to the editor: study summary - randomized control trial of omega-3 fatty acid supplementation for the treatment of COVID-19 related olfactory dysfunction. Trials 2020;21(1):942. [CENTRAL: CN-02200204] [EMBASE: 2007355105] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT04495816. COVID-19 anosmia study. https://clinicaltrials.gov/show/NCT04495816 (first received 3 August 2020). [CENTRAL: CN-02145765]
NCT04528329 {published data only}
- NCT04528329. Anosmia and / or ageusia and early corticosteroid use [Time to recover of anosmia and / or ageusia and early corticosteroid use]. ClinicalTrials.gov (first received 2020).
NCT04530409 {published data only}
- NCT04530409. Timing of corticosteroids in COVID-19. https://clinicaltrials.gov/show/NCT04530409 (first received 28 August 2020).
NCT04569825 {published data only}
- NCT04569825. Effect of nasal steroid in the treatment of anosmia due to COVID-19 disease. https://clinicaltrials.gov/show/NCT04569825 (first received 30 September 2020). [CENTRAL: CN-02181592]
NCT04638673 {published data only}
- NCT04638673. NeuroCovid Rehab and Recovery Related to COVID-19 Diagnosis [Testing a wearable telemedicine-controllable taVNS device for NeuroCovid recovery and rehab]. https://clinicaltrials.gov/show/NCT04638673 (first received 20 November 2020).
NCT04657809 {published data only}
- NCT04657809. Clinical assessment of insulin fast dissolving film in treatment of post infection anosmia [Insulin fast dissolving film for intranasal delivery via olfactory region, a promising approach for the treatment of anosmia in COVID 19 patients: design, in-vitro characterization and clinical evaluation]. https://clinicaltrials.gov/show/NCT04657809 (first received 8 December 2020). [DOI] [PubMed]
Additional references
Abdelmaksoud 2021
- Abdelmaksoud AA, Ghweil AA, Hassan MH, Rashad A, Khodeary A, Aref ZF, et al. Olfactory disturbances as presenting manifestation among Egyptian patients with COVID-19: possible role of zinc. Biological Trace Element Research 2021 Jan 7 [Epub ahead of print]. [DOI: 10.1007/s12011-020-02546-5] [DOI] [PMC free article] [PubMed]
Alexander 2006
- Alexander TH, Davidson TM. Intranasal zinc and anosmia: the zinc-induced anosmia syndrome. Laryngoscope 2006;116(2):217-20. [DOI: 10.1097/01.mlg.0000191549.17796.13] [PMID: ] [DOI] [PubMed] [Google Scholar]
Allen 2000
- Allen DB. Systemic effects of intranasal steroids: an endocrinologist’s perspective . Journal of Allergy and Clinical Immunology 2000;106(4):S179-90. [DOI] [PubMed] [Google Scholar]
Bigman 2020
- Bigman G. Age-related smell and taste impairments and vitamin D associations in the U.S. adults national health and nutrition examination survey. Nutrients 2020;12(4):984. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bilinska 2020
- Bilinska K, Butowt R. Anosmia in COVID-19: a bumpy road to establishing a cellular mechanism. ACS Chemical Neuroscience 2020;11(15):2152-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boscolo‐Rizzo 2020
- Boscolo-Rizzo P, Borsetto D, Fabbris C, Spinato G, Frezza D, Menegaldo A, et al. Evolution of altered sense of smell or taste in patients with mildly symptomatic COVID-19. JAMA Otolaryngology - Head & Neck Surgery 2020;146(8):729-32. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boscolo‐Rizzo 2021
- Boscolo-Rizzo P, Menegaldo A, Fabbris C, Spinato G, Borsetto D, Vaira LA, et al. Six-month psychophysical evaluation of olfactory dysfunction in patients with COVID-19. Chemical Senses 2021 Feb 12 [Epub ahead of print]. [DOI: 10.1093/chemse/bjab006] [DOI] [PMC free article] [PubMed]
Butowt 2020
- Butowt R, Bartheld CS. Anosmia in COVID-19: underlying mechanisms and assessment of an olfactory route to brain infection. Neuroscientist 2020 Sep 11 [Epub ahead of print]. [DOI: 10.1177/1073858420956905] [PMID: ] [DOI] [PMC free article] [PubMed]
Cain 1988
- Cain WS, Gent JF, Goodspeed RB, Leonard G. Evaluation of olfactory dysfunction in the Connecticut Chemosensory Clinical Research Center. Laryngoscope 1988;98(1):83-8. [DOI] [PubMed] [Google Scholar]
Chary 2020
- Chary E, Carsuzaa F, Trijolet J-P, Capitaine A-L, Roncato-Saberan M, Fouet K, et al. Prevalence and recovery from olfactory and gustatory dysfunctions in COVID-19 infection: a prospective multicenter study. American Journal of Rhinology & Allergy 2020;34(5):686-93. [DOI: 10.1177/1945892420930954] [DOI] [PMC free article] [PubMed] [Google Scholar]
Croy 2014
- Croy I, Nordin S, Hummel T. Olfactory disorders and quality of life--an updated review. Chemical Senses 2014;39(3):185-94. [DOI: 10.1093/chemse/bjt072] [DOI] [PubMed] [Google Scholar]
Dai 2016
- Dai Q, Pang Z, Yu H. Recovery of olfactory function in postviral olfactory dysfunction patients after acupuncture treatment. Evidence-based Complementary and Alternative Medicine: ECAM 2016;2016:4986034. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Damm 2004
- Damm M, Temmel A, Welge-Lüssen A, Eckel HE, Kreft MP, Klussmann JP, et al. Olfactory dysfunctions. Epidemiology and therapy in Germany, Austria and Switzerland [Riechstörungen. Epidemiologie und Therapie in Deutschland, Osterreich und der Schweiz]. HNO 2004;52(2):112-20. [DOI] [PubMed] [Google Scholar]
Demoly 2008
- Demoly P. Safety of intranasal corticosteroids in acute rhinosinusitis. American Journal of Otolaryngology 2008;29(6):403-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Derin 2016
- Derin S, Koseoglu S, Sahin C, Sahan M. Effect of vitamin B12 deficiency on olfactory function. International Forum of Allergy & Rhinology 2016;6(10):1051-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629. [DOI] [PMC free article] [PubMed] [Google Scholar]
Erskine 2020
- Erskine SE, Philpott CM. An unmet need: patients with smell and taste disorders. Clinical Otolaryngology 2020;45(2):197-203. [DOI] [PubMed] [Google Scholar]
Fuccillo 2020
- Fuccillo E, Saibene AM, Canevini MP, Felisati G. Olfactory disorders in coronavirus disease 2019 patients: a systematic literature review. Journal of Laryngology & Otology 2020 Sep 15 [Epub ahead of print]. [DOI: 10.1017/S0022215120002005] [PMID: ] [DOI] [PMC free article] [PubMed]
Gerkin 2020
- Gerkin RC, Ohla K, Veldhuizen MG, Joseph PV, Kelly CE, Bakke AJ, et al. Recent smell loss is the best predictor of COVID-19 among individuals with recent respiratory symptoms. Chemical Senses 2020 Dec 25 [Epub ahead of print]. [DOI: 10.1093/chemse/bjaa081] [PMID: ] [DOI] [PMC free article] [PubMed]
Gudziol 2006
- Gudziol V, Lötsch J, Hähner A, Zahnert T, Hummel T. Clinical significance of results from olfactory testing. Laryngoscope 2006;116(10):1858-63. [PMID: ] [DOI] [PubMed] [Google Scholar]
Handbook 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Handbook 2020
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Hopkins 2020a
- Hopkins C, Kumar N. Loss of sense of smell as a marker of COVID-19 infection. https://www.sforl.org/wp-content/uploads/2020/03/Loss-of-sense-of-smell-as-marker-of-COVID-21-Mar-2020.pdf. [DOI] [PMC free article] [PubMed]
Hopkins 2020b
- Hopkins C, Burges Watson DL, Kelly C, Deary V, Smith BC. Managing long COVID: don't overlook olfactory dysfunction. BMJ 2020;370:m3736. [DOI] [PubMed] [Google Scholar]
Hopkins 2020c
- Hopkins C, Lechien JR, Saussez S. More that ACE2? NRP1 may play a central role in the underlying pathophysiological mechanism of olfactory dysfunction in COVID-19 and its association with enhanced survival. Medical Hypotheses 2021;146:110406. [DOI: 10.1016/j.mehy.2020.110406.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Huart 2020
- Huart C, Philpott C, Konstantinidis I, Altundag A, Whitcroft KL, Trecca EMC, et al. Comparison of COVID-19 and common cold chemosensory dysfunction. Rhinology 2020;58(6):623-5. [DOI: 10.4193/Rhin20.251] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hummel 2002
- Hummel T, Heilmann S, Hüttenbriuk KB. Lipoic acid in the treatment of smell dysfunction following viral infection of the upper respiratory tract. Laryngoscope 2002;112(11):2076-80. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hummel 2016
- Hummel T, Whitcroft KL, Andrews P, Altundag A, Cinghi C, Costanzo RM, et al. Position paper on olfactory dysfunction. Rhinology 2016;56(1):1-30. [DOI] [PubMed] [Google Scholar]
Hummel 2017
- Hummel T, Whitcroft KL, Rueter G, Haehner A. Intranasal vitamin A is beneficial in post-infectious olfactory loss. European Archives of Oto-rhino-laryngology 2017;274(7):2819-25. [PMID: ] [DOI] [PubMed] [Google Scholar]
IRCT20210205050247N1
- IRCT20210205050247N1. The effect of olfactory training and vitamin A in the olfactory loss of patients with covid-19 [A comparative study of the effect of olfactory training and vitamin A in the olfactory loss of patients with covid-19]. http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20210205050247N1 (first received 2021).
IRCT20210311050671N1
- IRCT20210311050671N1. Evaluation of the effect of laser acupuncture on improving the sense of smell in patients with COVID-19 [Effect of auricular acupuncture with the laser in post-viral anosmia during the COVID-19 pandemic]. http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20210311050671N1 (first received 2021).
Kasiri 2021
- Kasiri H, Rouhani N, Salehifar E, Ghazaeian M, Fallah S. Mometasone furoate nasal spray in the treatment of patients with COVID-19 olfactory dysfunction: a randomized, double blind clinical trial. International Immunopharmacology 2021;98:107871. [DOI] [PMC free article] [PubMed] [Google Scholar]
Klopfenstein 2020
- Klopfenstein T, Kadiane-Oussou NJ, Toko L, Royer PY, Lepiller Q, Gendrin V, et al. Features of anosmia in COVID-19. Medecine et Maladies Infectieuses 2020;50(5):436-9. [DOI: 10.1016/j.medmal.2020.04.006] [DOI] [PMC free article] [PubMed] [Google Scholar]
Landis 2003
- Landis BN, Hummel T, Hugentobler M, Giger R, Lacroix JS. Ratings of overall olfactory function. Chemical Senses 2003;28(8):691-4. [DOI] [PubMed] [Google Scholar]
Lechien 2020
- Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. European Archives of Oto-rhino-laryngology 2020;277(8):2251-61. [DOI: 10.1007/s00405-020-05965-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2020
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Technical Supplement to Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook.
Lötsch 2019
- Lötsch J, Hummel T. Clinical usefulness of self-rated olfactory performance-a data science-based assessment of 6000 patients. Chemical Senses 2019;44:357-64. [DOI: 10.1093/chemse/bjz029] [PMID: ] [DOI] [PubMed] [Google Scholar]
Mao 2020
- Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurology 2020;77(6):683-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marshall 2018
- Marshall J, Noel-Storr AH, Kuiper J, Thomas J, Wallace BC. Machine learning for identifying randomized controlled trials: an evaluation and practitioner's guide. Research Synthesis Methods 2018;9(4):602-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martineau 2020
- Martineau AR, Forouhi NG. Vitamin D for COVID-19: a case to answer? Lancet. Diabetes & Endocrinology 2020;8(9):735-6. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mattos 2018
- Mattos JL, Schlosser RJ, Mace JC, Smith TL, Soler ZM. Establishing the minimal clinically important difference for the Questionnaire of Olfactory Disorders. International Forum of Allergy & Rhinology 2018;8(9):1041-6. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
McDonald 2017
- McDonald S, Noel-Storr AH, Thomas J. Harnessing the efficiencies of machine learning and Cochrane Crowd to identify randomised trials for individual Cochrane reviews. In: Global Evidence Summit; Sep 13-17, Cape Town, South Africa. 2017.
Meng 2020
- Meng X, Deng Y, Dai Z, Meng Z. COVID-19 and anosmia: a review based on up-to-date knowledge. American Journal of Otolaryngology 2020;41(5):102581. [DOI] [PMC free article] [PubMed] [Google Scholar]
Menni 2020
- Menni C, Valdes AM, Freidin MB, Sudre CH, Nguyen LH, Drew DA, et al. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nature Medicine 2020;26(7):1037-40. [DOI: 10.1038/s41591-020-0916-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mohamad 2021
- Mohamad SA, Badawi AM, Mansour HF. Insulin fast-dissolving film for intranasal delivery via olfactory region, a promising approach for the treatment of anosmia in COVID-19 patients: design, in-vitro characterization and clinical evaluation. International Journal of Pharmaceutics 2021;601:120600. [DOI] [PubMed] [Google Scholar]
NCT04764981
- NCT04764981. Olfactory training for olfactory dysfunction after coronavirus disease - 19 (COVID-19) [Clinical outcomes of olfactory training for treatment of olfactory dysfunction after COVID-19]. https://clinicaltrials.gov/show/NCT04764981 (first received 21 February 2021).
NCT04797936
- NCT04797936. BNO 1030 extract (Imupret) in the treatment of mild forms of COVID-19 [A randomized, open-label, multicentre, comparative study of therapeutic efficacy, safety, and tolerability of BNO 1030 extract, in the treatment of mild forms of COVID-19]. https://clinicaltrials.gov/show/NCT04797936 (first received 15 March 2021).
NCT04900415
- NCT04900415. Olfactory and neurosensory rehabilitation in COVID-19-related olfactory dysfunction [Olfactory and neurosensory rehabilitation in coronavirus 2019 (COVID-19)-related olfactory dysfunction (OD)]. https://clinicaltrials.gov/show/NCT04900415 (first received 25 May 2021).
NCT04951362
- NCT04951362. Role of Ivermectin nanosuspension as nasal spray in treatment of persistant post COVID19 anosmia [Studying the expected effect of Ivermectin nanosuspension as nasal spray upon post COVID19 persistant anosmia]. https://clinicaltrials.gov/show/NCT04951362 (first received 6 July 2021).
NCT04952389
- NCT04952389. Acupuncture therapy for COVID-related olfactory loss. https://clinicaltrials.gov/show/NCT04952389 (first received 7 July 2021).
NCT04959747
- NCT04959747. Acupuncture for olfactory dysfunction in infected COVID-19 patients [Acupuncture for olfactory dysfunction in infected COVID-19 patients: a randomized, sham-controlled clinical trial]. https://clinicaltrials.gov/show/NCT04959747 (first received 13 July 2021).
NCT04964414
- NCT04964414. Treatment of pediatric patients that lost sense of smell due to COVID-19 [Prospective randomized study of the treatment of pediatric patients that lost sense of smell due to COVID-19]. https://clinicaltrials.gov/show/NCT04964414 (first received 16 July 2021).
Noel‐Storr 2018
- Noel-Storr AH. The Project Transform Team. Cochrane Crowd: new ways of working together to produce health evidence. In: Evidence Live; 2018 June 18-20; Oxford, UK. 2018.
Parma 2020
- Parma V, Ohla K, Veldhuizen MG, Niv MY, Kelly CE, Bakke AJ, et al. More than smell - COVID-19 is associated with severe impairment of smell, taste and chemesthesis. Chemical Senses 2020;45(7):609–22. [DOI: 10.1093/chemse/bjaa041] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pekala 2016
- Pekala K, Chandra RK, Turner JH. Efficacy of olfactory training in patients with olfactory loss: a systematic review and meta-analysis. International Forum of Allergy & Rhinology 2016;6(3):299-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Philpott 2014
- Philpott CM, Boak D. The impact of olfactory disorders in the United Kingdom. Chemical Senses 2014;39(8):711-8. [DOI: 10.1093/chemse/bju043] [DOI] [PubMed] [Google Scholar]
Quint 2002
- Quint C, Temmel AF, Hummel T, Ehrenberger K. The quinoxaline derivative caroverine in the treatment of sensorineural smell disorders: a proof-of-concept study. Acta Oto-laryngologica 2002;122(8):877-81. [PMID: ] [PubMed] [Google Scholar]
Rashid 2021
- Rashid RA, Zgair A, Al-Ani RM. Effect of nasal corticosteroid in the treatment of anosmia due to COVID-19: a randomised double-blind placebo-controlled study. American Journal of Otolaryngology 2021;42(5):103033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reden 2011
- Reden J, Herting B, Lill K, Kern R, Hummel T. Treatment of postinfectious olfactory disorders with minocycline: a double-blind, placebo-controlled study. Laryngoscope 2011;121(3):679-82. [PMID: ] [DOI] [PubMed] [Google Scholar]
RevMan 2020 [Computer program]
- The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Rocke 2020
- Rocke J, Hopkins C, Philpott C, Kumar N. Is loss of sense of smell a diagnostic marker in COVID-19: a systematic review and meta-analysis. Clinical Otolaryngology 2020;45(6):914-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saussez 2020
- Saussez S, Lechien JR, Hopkins C. Anosmia: an evolution of our understanding of its importance in COVID-19 and what questions remain to be answered. European Archives of Oto-rhino-laryngology 2020 Sep 9 [Epub ahead of print]. [DOI: 10.1007/s00405-020-06285-0] [PMID: ] [DOI] [PMC free article] [PubMed]
Seiden 2001
- Seiden AM, Duncan HJ. The diagnosis of a conductive olfactory loss. Laryngoscope 2001;111(1):9-14. [DOI] [PubMed] [Google Scholar]
Sorokowska 2017
- Sorokowska A, Drechsler E, Karwowski M, Hummel T. Effects of olfactory training: a meta-analysis. Rhinology 2017;55(1):17-26. [DOI] [PubMed] [Google Scholar]
Spinato 2020
- Spinato G, Fabbris C, Polesel J, Cazzador D, Borsetto D, Hopkins C, et al. Alterations in smell or taste in mildly symptomatic outpatients with SARS-CoV-2 infection. JAMA 2020;323(20):2089-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thomas 2017
- Thomas J, Noel-Storr AH, Marshall I, Wallace B, McDonald S, Mavergames C, et al, Living Systematic Review Network. Living systematic reviews 2: combining human and machine effort. Journal of Clinical Epidemiology 2017;91:31-7. [DOI] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI: 10.1186/1745-6215-8-16.] [DOI] [PMC free article] [PubMed] [Google Scholar]
UMIN000043537
- UMIN000043537. Post COVID-19 anosmia [Post COVID-19 anosmia treatments - anosmia treatments]. http://www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000043537 (first received 2021).
Vent 2010
- Vent J, Wang DW, Damm M. Effects of traditional Chinese acupuncture in post-viral olfactory dysfunction. Otolaryngology - Head and Neck Surgery 2010;142(4):505-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
von Bartheld 2020
- Bartheld CS, Hagen MM, Butowt R. Prevalence of chemosensory dysfunction in COVID-19 patients: a systematic review and meta-analysis reveals significant ethnic differences. ACS Chemical Neuroscience 2020;11(19):2944-61. [DOI: 10.1021/acschemneuro.0c00460] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wallace 2017
- Wallace BC, Noel-Storr AH, Marshall IJ, Cohen AM, Smalheiser NR, et al. Identifying reports of randomized controlled trials (RCTs) via a hybrid machine learning and crowdsourcing approach. Journal of the American Medical Informatics Association 2017;24(6):1165-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Webster 2021a
- Webster KE, MacKeith S, Philpott C, Hopkins C, Burton MJ. Interventions for the treatment of persistent post-COVID-19 olfactory dysfunction. Cochrane Database of Systematic Reviews 2021, Issue 7. Art. No: CD013876. [DOI: 10.1002/14651858.CD013876.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Welge‐Luessen 2005
- Welge-Luessen A, Hummel T, Stojan T, Wolfensberger M. What is the correlation between ratings and measures of olfactory function in patients with olfactory loss? American Journal of Rhinology 2005;19(6):567-71. [PubMed] [Google Scholar]
Yildiz 2021
- Yildiz E, Koca Yildiz S, Kuzu S, Günebakan Ç, Bucak A, Kahveci OK. Comparison of the healing effect of nasal saline irrigation with triamcinolone acetonide versus nasal saline irrigation alone in COVID-19 related olfactory dysfunction: a randomized controlled study. Indian Journal of Otolaryngology and Head and Neck Surgery 2021 Jul 10 [Epub ahead of print]. [DOI: 10.1007/s12070-021-02749-9] [DOI] [PMC free article] [PubMed]
References to other published versions of this review
Webster 2021b
- Webster KE, MacKeith S, Philpott C, Hopkins C, Burton MJ. Interventions for the prevention of persistent post‐COVID‐19 olfactory dysfunction. Cochrane Database of Systematic Reviews 2021, Issue 3. Art. No: CD013877. [DOI: 10.1002/14651858.CD013877] [DOI] [PMC free article] [PubMed] [Google Scholar]


