Abstract
Background
Community‐based primary‐level workers (PWs) are an important strategy for addressing gaps in mental health service delivery in low‐ and middle‐income countries.
Objectives
To evaluate the effectiveness of PW‐led treatments for persons with mental health symptoms in LMICs, compared to usual care.
Search methods
MEDLINE, Embase, CENTRAL, ClinicalTrials.gov, ICTRP, reference lists (to 20 June 2019).
Selection criteria
Randomised trials of PW‐led or collaborative‐care interventions treating people with mental health symptoms or their carers in LMICs.
PWs included: primary health professionals (PHPs), lay health workers (LHWs), community non‐health professionals (CPs).
Data collection and analysis
Seven conditions were identified apriori and analysed by disorder and PW examining recovery, prevalence, symptom change, quality‐of‐life (QOL), functioning, service use (SU), and adverse events (AEs).
Risk ratios (RRs) were used for dichotomous outcomes; mean difference (MDs), standardised mean differences (SMDs), or mean change differences (MCDs) for continuous outcomes.
For SMDs, 0.20 to 0.49 represented small, 0.50 to 0.79 moderate, and ≥0.80 large clinical effects.
Analysis timepoints: T1 (<1 month), T2 (1‐6 months), T3 ( >6 months) post‐intervention.
Main results
Description of studies
95 trials (72 new since 2013) from 30 LMICs (25 trials from 13 LICs).
Risk of bias
Most common: detection bias, attrition bias (efficacy), insufficient protection against contamination.
Intervention effects
*Unless indicated, comparisons were usual care at T2.
“Probably”, “may”, or “uncertain” indicates "moderate", "low," or "very low" certainty evidence.
Adults with common mental disorders (CMDs)
LHW‐led interventions
a. may increase recovery (2 trials, 308 participants; RR 1.29, 95%CI 1.06 to 1.56);
b. may reduce prevalence (2 trials, 479 participants; RR 0.42, 95%CI 0.18 to 0.96);
c. may reduce symptoms (4 trials, 798 participants; SMD ‐0.59, 95%CI ‐1.01 to ‐0.16);
d. may improve QOL (1 trial, 521 participants; SMD 0.51, 95%CI 0.34 to 0.69);
e. may slightly reduce functional impairment (3 trials, 1399 participants; SMD ‐0.47, 95%CI ‐0.8 to ‐0.15);
f. may reduce AEs (risk of suicide ideation/attempts);
g. may have uncertain effects on SU.
Collaborative‐care
a. may increase recovery (5 trials, 804 participants; RR 2.26, 95%CI 1.50 to 3.43);
b. may reduce prevalence although the actual effect range indicates it may have little‐or‐no effect (2 trials, 2820 participants; RR 0.57, 95%CI 0.32 to 1.01);
c. may slightly reduce symptoms (6 trials, 4419 participants; SMD ‐0.35, 95%CI ‐0.63 to ‐0.08);
d. may slightly improve QOL (6 trials, 2199 participants; SMD 0.34, 95%CI 0.16 to 0.53);
e. probably has little‐to‐no effect on functional impairment (5 trials, 4216 participants; SMD ‐0.13, 95%CI ‐0.28 to 0.03);
f. may reduce SU (referral to MH specialists);
g. may have uncertain effects on AEs (death).
Women with perinatal depression (PND)
LHW‐led interventions
a. may increase recovery (4 trials, 1243 participants; RR 1.29, 95%CI 1.08 to 1.54);
b. probably slightly reduce symptoms (5 trials, 1989 participants; SMD ‐0.26, 95%CI ‐0.37 to ‐0.14);
c. may slightly reduce functional impairment (4 trials, 1856 participants; SMD ‐0.23, 95%CI ‐0.41 to ‐0.04);
d. may have little‐to‐no effect on AEs (death);
e. may have uncertain effects on SU.
Collaborative‐care
a. has uncertain effects on symptoms/QOL/SU/AEs.
Adults with post‐traumatic stress (PTS) or CMDs in humanitarian settings
LHW‐led interventions
a. may slightly reduce depression symptoms (5 trials, 1986 participants; SMD ‐0.36, 95%CI ‐0.56 to ‐0.15);
b. probably slightly improve QOL (4 trials, 1918 participants; SMD ‐0.27, 95%CI ‐0.39 to ‐0.15);
c. may have uncertain effects on symptoms (PTS)/functioning/SU/AEs.
PHP‐led interventions
a. may reduce PTS symptom prevalence (1 trial, 313 participants; RR 5.50, 95%CI 2.50 to 12.10) and depression prevalence (1 trial, 313 participants; RR 4.60, 95%CI 2.10 to 10.08);
b. may have uncertain effects on symptoms/functioning/SU/AEs.
Adults with harmful/hazardous alcohol or substance use
LHW‐led interventions
a. may increase recovery from harmful/hazardous alcohol use although the actual effect range indicates it may have little‐or‐no effect (4 trials, 872 participants; RR 1.28, 95%CI 0.94 to 1.74);
b. may have little‐to‐no effect on the prevalence of methamphetamine use (1 trial, 882 participants; RR 1.01, 95%CI 0.91 to 1.13) and functional impairment (2 trials, 498 participants; SMD ‐0.14, 95%CI ‐0.32 to 0.03);
c. probably slightly reduce risk of harmful/hazardous alcohol use (3 trials, 667 participants; SMD ‐0.22, 95%CI ‐0.32 to ‐0.11);
d. may have uncertain effects on SU/AEs.
PHP/CP‐led interventions
a. probably have little‐to‐no effect on recovery from harmful/hazardous alcohol use (3 trials, 1075 participants; RR 0.93, 95%CI 0.77 to 1.12) or QOL (1 trial, 560 participants; MD 0.00, 95%CI ‐0.10 to 0.10);
b. probably slightly reduce risk of harmful/hazardous alcohol and substance use (2 trials, 705 participants; SMD ‐0.20, 95%CI ‐0.35 to ‐0.05; moderate‐certainty evidence);
c. may have uncertain effects on prevalence (cannabis use)/SU/AEs.
PW‐led interventions for alcohol/substance dependence
a. may have uncertain effects.
Adults with severe mental disorders
*Comparisons were specialist‐led care at T1.
LHW‐led interventions
a. may have little‐to‐no effect on caregiver burden (1 trial, 253 participants; MD ‐0.04, 95%CI ‐0.18 to 0.11);
b. may have uncertain effects on symptoms/functioning/SU/AEs.
PHP‐led or collaborative‐care
a. may reduce functional impairment (7 trials, 874 participants; SMD ‐1.13, 95%CI ‐1.78 to ‐0.47);
b. may have uncertain effects on recovery/relapse/symptoms/QOL/SU.
Adults with dementia and carers
PHP/LHW‐led carer interventions
a. may have little‐to‐no effect on the severity of behavioural symptoms in dementia patients (2 trials, 134 participants; SMD ‐0.26, 95%CI ‐0.60 to 0.08);
b. may reduce carers' mental distress (2 trials, 134 participants; SMD ‐0.47, 95%CI ‐0.82 to ‐0.13);
c. may have uncertain effects on QOL/functioning/SU/AEs.
Children with PTS or CMDs
LHW‐led interventions
a. may have little‐to‐no effect on PTS symptoms (3 trials, 1090 participants; MCD ‐1.34, 95%CI ‐2.83 to 0.14);
b. probably have little‐to‐no effect on depression symptoms (3 trials, 1092 participants; MCD ‐0.61, 95%CI ‐1.23 to 0.02) or on functional impairment (3 trials, 1092 participants; MCD ‐0.81, 95%CI ‐1.48 to ‐0.13);
c. may have little‐or‐no effect on AEs.
CP‐led interventions
a. may have little‐to‐no effect on depression symptoms (2 trials, 602 participants; SMD ‐0.19, 95%CI ‐0.57 to 0.19) or on AEs;
b. may have uncertain effects on recovery/symptoms(PTS)/functioning.
Authors' conclusions
PW‐led interventions show promising benefits in improving outcomes for CMDs, PND, PTS, harmful alcohol/substance use, and dementia carers in LMICs.
Plain language summary
The effects of primary‐level workers on people with mental disorders and distress in low‐ and middle‐income countries
This Cochrane Review update aims to assess the effects of engaging community‐based workers, such as primary‐care workers and teachers, to help people with mental disorders or distress. The review focused on studies from low‐ and middle‐income countries and found 95 studies for inclusion (including 23 from the previous review).
Key messages
Primary health professionals, lay health workers, teachers, and other community workers may be able to help people with mental health issues if they are trained. However, more evidence is needed.
What was studied in the review?
In low‐ and middle‐income countries, many people with mental illness do not receive the care they need because of stigma and difficulty accessing services. One solution is to offer services through ‘primary‐level workers’. These are people who are not mental health specialists but who receive some mental health training, including primary health professionals (e.g. doctors, nurses); lay health workers; community volunteers; and other community members (e.g. teachers, social workers). Primary‐level workers deliver these services alone or in collaboration with specialists.
What are the main results of the review?
95 relevant trials from 30 low‐ or middle‐income countries were found.
The review authors searched for evidence about the effects of these strategies on the number of people who had mental health problems, the number who recovered, their symptom severity, quality of life, day‐to‐day functioning, use of health services, and negative effects of treatment. All results were measured one to six months after treatment completion, except in group 5, in which results were measured immediately after treatment completion. When results are not presented, this is because there was no evidence, or because the evidence was very uncertain. Evidence of the results below is of low to moderate certainty.
1. Adults with depression and anxiety
Treatments from lay health workers compared to usual care:
a. may increase recovery;
b. may reduce the number of people with depression/anxiety;
c. may improve quality of life;
d. may slightly improve day‐to‐day functioning; and
e. may reduce risk of suicidal thoughts/attempts.
Treatments from primary‐level workers in collaboration with mental health specialists compared to usual care:
a. may increase recovery;
b. may reduce the number of people with depression/anxiety although the range for the actual effect indicates they may have little or no effect;
c. may slightly reduce symptoms;
d. may slightly improve quality of life;
e. probably have little to no effect on day‐to‐day functioning; and
f. may reduce referral to mental health specialists.
2. Women with depression related to pregnancy and childbirth
Treatments from lay health workers compared to usual care:
a. may increase recovery;
b. probably slightly reduce symptoms of depression;
c. may slightly improve day‐to‐day functioning;
d. may have little to no effect on risk of death.
3. Adults in humanitarian settings with post‐traumatic stress or depression and anxiety
Treatments from lay health workers compared to usual care:
a. may slightly reduce depression symptoms; and
b. probably slightly improve quality of life.
Treatments from primary health professionals compared to usual care:
a. may reduce the number of adults with post‐traumatic stress and depression.
4. Adults with alcohol or substance use problems
Treatments from lay health workers compared to usual care:
a. may increase recovery from harmful/hazardous alcohol use although the range for the actual effect indicates they may have little or no effect;
b. probably slightly reduce the risk of harmful/hazardous alcohol use;
c. may have little to no effect on day‐to‐day functioning; and
d. may have little to no effect on the number of people who use methamphetamine;
Treatments from primary health and community professionals compared to usual care:
a. probably have little to no effect on recovery from harmful/hazardous alcohol use;
b. probably slightly reduce risk of harmful/hazardous alcohol and substance use; and
c. probably have little to no effect on quality of life.
5. Adults with severe mental disorders (e.g. schizophrenia)
Treatments from lay health workers compared to mental specialists alone:
a. may have little to no effect on caregiver burden.
Treatments from primary health professionals alone or in collaboration with mental health specialists:
a. may improve day‐to‐day functioning.
6. Adults with dementia and their carers
Treatments from lay and professional health workers, compared to usual care:
a. may have little to no effect on the severity of behavioural symptoms in dementia patients; and
b. may reduce carers' mental distress.
7. Children in humanitarian settings with post‐traumatic stress or depression and anxiety
Treatments from lay health workers, compared to usual or no care:
a. may have little to no effect on post‐traumatic stress symptoms;
b. probably have little to no effect on depressive symptoms nor on day‐to‐day functioning; and
c. may make little or no difference in risk of adverse events.
Treatments from community professionals (teachers and social workers) compared to no care:
a. may have little to no effect on depressive symptoms; and
b. may make little or no difference in adverse events.
How up‐to‐date is this review?
Originally published in November 2013, this update includes studies published up to 20 June 2019.
Summary of findings
Background
Description of the condition
The global burden of mental disorders and distress is high. The latest global burden of disease estimates have shown that mental, behavioural, and neuropsychiatric disorders all feature in the top 30 causes of all years lived with disability; the highest contributors are major depression (ranked second), anxiety (ranked seventh), and substance use disorders (ranked 12th) (GBD 2017). The contribution of major depressive disorders to worldwide disability‐adjusted life‐years (DALYs) increased by 37% from 1990 to 2010 and is predicted to rise farther (Murray 2015a; Rehm 2019). Furthermore, self‐inflicted injuries and alcohol‐related disorders are likely to increase in the ranking of disease burden due to the decline in communicable diseases. The disease burden due to Alzheimer’s disease is also increasing, linked to the demographic transition towards an ageing population (GBD 2017; Rehm 2019).
Mental disorders and distress definitions are provided in Table 16. Mental disorder categories used and studied in LMIC align best with the World Health Organization (WHO) ICD‐11 Mental Disorders categorisation (WHO 2019). Mental illness also come with substantial economic costs. A report on the global economic burden of non‐communicable diseases (NCDs) suggests that by the early 2030s, mental disorders and distress alone will account for loss of an additional USD16.1 trillion, with dramatic impact on productivity and quality of life (Jan 2018). Data on macro‐economic costs for low‐ and middle‐income country (LMIC) settings remain poor (Trautmann 2016). However, the economic and social costs for individuals and families are substantial. High direct costs are incurred in countries where health spending is met largely through private, as opposed to public, spending, and where health insurance and employer‐met health payments are not substantial (Levin 2016). High indirect costs are also incurred due to informal caregiving and lost work opportunities, and may be related to untreated disorders and their associated disability (Razzouk 2017; Sørensen 2017).
1. Definitions.
| Adult | Patients who were ≥ 18 years old. However, if some studies had an age range from, for example, 16 years upwards, and a majority of participants are over 18 years, we included these study participants as adults |
| Children and adolescents | Children (from birth to 18 years) were considered as a separate group of participants, as they have (1) different patterns of psychopathology/mental disorders; and (2) different help‐seeking behaviours that would, therefore, require different interventions, in different settings (e.g. schools) and a different approach to care worker interventions (such as teacher‐led interventions) |
| Clinical interventions | 1. Detection (recognition and diagnosis) of illness, including screening 2. Acute interventions: drug treatment, non‐drug treatment/care (such as specific psychological therapies, or interventions with psychosocial components like counselling, psychoeducation, coping skills, etc.), referral. In this review, we refer to PW‐led psychological interventions as those where the PW delivers mainly a psychological therapy (such as cognitive‐behaviour therapy, behavioural activation, etc). We refer to PW‐led psychosocial interventions as those where the PW delivers an intervention that combines elements or adaptations of therapeutic principles or therapeutic components, may adopt a transdiagnostic approach, and may deliver in addition a social supportive component (such as debt management, family negotiation, income‐generating activity, or community/well‐being activity) 3. Follow‐up, rehabilitation, role in detecting and dealing with relapse/recurrence, compliance issues, treatment resistance, side effects of treatment, or psychosocial problems 4. Prodromal interventions: those with prodromal symptoms/distress may receive interventions such as training in self‐help, informal support, transdiagnostic psychosocial support (individualized plan addressing social and emotional functioning and problems), and high‐risk individual identification We decided not to include interventions delivered by people who were not within the medical paradigm (such as faith healers or yoga masters) |
| Community professionals (CPs) | Professional people who were involved as community‐level workers but were not within the health sector, as many people, particularly adolescents and young adults, have low contact with health workers. This category included, for example, teachers/trainers/support workers from schools and colleges, social workers, community development workers/managers, etc. These CPs have an important role, particularly in promotion of mental health and detection of mental disorders (Patel 2007; Patel 2008a; WHO 2003) Generalist social workers often linked to or overlapped with roles performed within the health sector in that they have a well‐defined and expected extended mental health support role towards patients, all social support. In certain comparisons in this review, they are therefore often combined with nurses, although occasionally they are combined with teachers We excluded certain health workers whom we classified as specialists, including those who were not traditionally thought of as specialists by the psychiatry/medical system: for example, when school counsellors were trained to exclusively do that and had a qualification, with or without extra experience, and when their sole focus was on child psychology/counselling. In this review, many of the school counsellors may not have been that highly trained and therefore were included (Lewin 2010; Patel 2007; Patel 2008a; WHO 2003) |
| First‐level care, primary care, and community | First level of contact with formal health services including community‐based interventions or primary care interventions (or both), on their own or attached to hospital settings, provided they had no specialist input apart from supervision (modified from Wiley‐Exley 2007). This would include individuals with mental illness living in the community and programmes in outpatient clinics or primary care practices. This would not include programmes in hospitals unless these programmes were providing care to outpatients (i.e. generalists in outpatient departments). Community: as mentioned above, detection of mental disorders in all age groups was often done outside the health facility, for example, through school, training, and other community settings Therefore, we considered interventions outside the health sector |
| Lay health workers (LHWs) | As per the Lewin 2010 review, lay health workers (LHWs) perform diverse functions related to healthcare delivery. Although LHWs are usually provided with job‐related training, they have no formal professional or paraprofessional tertiary education and can be involved in either paid or voluntary care. The term LHW is thus necessarily broad in scope and includes, for example, community health workers, village health workers, treatment supporters, and birth attendants. As LHWs are diverse and can be linked to a health setting or to a community organisation, we have categorised these together in comparisons as LHWs. They have broadly similar backgrounds (from local communities with little if any professional background) and minimal training We excluded from this review studies that looked at informal care provided by family members or extended members only to members of their own family (i.e. who were unavailable to other members of the community). This would have excluded certain peer‐led interventions. As previously highlighted in Lewin's Cochrane Review, "these interventions are qualitatively different from other LHW [lay health worker] interventions included in this review given that parents or spouses have an established close relationship with those receiving care which could affect the process and effects of the intervention" (Lewin 2010). We also excluded all healthcare providers within non‐biomedical systems (e.g. a yoga master), as we had not searched for these specifically and it was difficult to judge, from our perspective, what constituted for them a mental health intervention |
| Low‐ and middle‐income country (LMIC) | Any country that has ever been an LMIC, as defined by the World Bank lists of LMICs |
|
Mental condition |
Term used to encompass mental disorders, mental distress, sub‐syndromal mental illnesses, and chronic and relapsing conditions |
| Mental disorders | This review included mental disorders as defined by any criteria within included papers. For the purpose of subgroup analysis, we sub‐categorised these disorders using the International Classification of Diseases (ICD)‐10 criteria in the previous review, and have updated to the ICD‐11 criteria for this review, for mental and behavioural disorders (related ICD‐11 codes are listed in brackets). These categories are most likely to be used in LMIC mental health service delivery and are based on the World Health Organization (WHO) Mental Disorders categorisation (WHO 2019) Common mental disorders Depressive disorders (6A70‐6A7Z) Symptomatic and course presentations for mood episodes in mood disorders (including panic attacks and recurrent depressive disorders) (6A80) Anxiety‐ or fear‐related disorders (6B00 to 6B06) Perinatal mental disorders We kept perinatal depression separate, as these disorders have an impact on different services (not just primary care, but midwifery services) compared to depression. This was defined as depression starting during pregnancy and/or up to 1 year post delivery (Stuart‐Parrigon 2014 We searched for any mental or behavioural disorders associated with pregnancy, childbirth, or the puerperium (6E20‐6E2Z) Severe mental disorders Schizophrenia or other primary psychotic disorders (6A20 to 6A2Z), bipolar or related disorders (6A60‐6A6Z) Disorders specifically associated with stress Post‐traumatic stress disorder (PTSD) ‐ divided into PTSD (6B40) and complex PTSD (6B41) in ICD‐11. These are patients meeting the specific diagnostic criteria for the disorder of PTSD. Post‐traumatic stress (PTS): we have grouped all the various expressions of distress/acute or longer‐term reactions to stress caused by trauma, which would broadly equate to ICD acute reaction to severe stress (6B42, 6B4Y, 6B4Z) and adjustment disorders (6B43) Neuropsychiatric disorders Disorders with neurocognitive impairment as a major feature (including specific dementias (8A20, 8A20 to 8A2Z) and other dementias related to diseases specified elsewhere (e.g. cerebrovascular 6D85) Disorders caused by substance abuse Disorders due to substance use (4C40 to 6C4Z excluding nicotine and caffeine, as those were felt to be prevention lifestyle interventions) Substance‐induced mood disorders (6C40 to 6C4D) Substance‐induced anxiety disorders (6C40 ‐6C4G)) Mental disorders specifically related to childhood/development Common childhood /adolescent disorders were selected as per MHgap (WHO 2016) (developmental, emotional and behavioural disorders) which are represented by the following ICD‐11 categories: Mood disorders (6A60‐6A8Z) (as above) Anxiety or fear related disorders (6B00 to 6B0Z) (as above) Disorders specifically related to stress (6B40 to 6B4Z) (as above) Neurodevelopmental disorders (6A00 to 6A0Z), including autistic spectrum disorder (6A02) and Attention deficit hyperactivity disorder (ADHD) (6A05). The diagnosis could be made in clinical practice or in the context of the trial. |
| Mental distress/ prodromal stage | Term used to describe the spectrum of symptoms and states that may or may not lead to a mental disorder but are responsive to mental health interventions that are appropriate for different stages. For example, early signs of mental distress may respond to increased self‐care and informal network support, whereas sub‐threshold symptoms may require transdiagnostic psychosocial support from PHC and increased monitoring (Patel 2018). |
| Primary health professionals (PHPs) | Professionals working in the health sector who are not specialised in mental disorders or have not received in‐depth professional specialist training in this clinical area. These included clinicians such as primary care or generalist doctors, nurses, and auxiliary nurses, as well as allied health personnel such as midwives, occupational therapists, etc. (Lewin 2010) This category did not include professional specialist health workers such as psychiatrists, neurologists, psychiatric nurses, or mental health social workers. For inclusion, PHPs received some training in mental disorders (in either the control or the intervention group), but this would not constitute a professional category. Review authors made a judgement of what constitutes 'some training'. Examples of 'some training' may be an undergraduate module or a short course in mental health |
| Primary‐level workers (PWs) | Broad term to encompass PHPs, LHWs, and CPs, who are all professional or lay workers working with the health sector at the primary care level/in a generalist role, or at the community level |
| Service interventions | These include changes in staffing or changes in mechanisms of mental health service delivery (e.g. extension of mental health services through camps and such other outreach services, mobile vans) We did not include service or social interventions (initially defined as return to employment/school or general social support) if they were not part of a trial with a specific mental health intervention, as we discovered our search strategy did not address this completely and opened a whole array of studies that we had not considered at the protocol stage (such as income‐generating activities without a mental health intervention that may look at mental health outcomes) |
| Social interventions | 1. Social integration 2. Return to employment, or school 3. Helping reduce stigma and other barriers to mental health care 4. Other social or well‐being support |
More recently, mental health and ill health have been re‐framed to be seen as a continuum from health to ill health: from 'at risk' to experiencing 'mental distress' to developing 'sub‐syndromal symptoms' (some of which are suggestive of a mental disorder but are not sufficient to reach diagnostic categories) to finally developing 'mental disorders' (Patel 2018). Alongside, efforts have been made to implement interventions targeting each stage of this continuum. This reflects the growing approach towards seeing the value in treating mental ill health as a response to functional issues or common elements (transdiagnostic approach) (Dalgleish 2020). This Cochrane Review update therefore includes a broader spectrum of people with mental symptoms, ranging from distress up to more severe symptoms and diagnosed conditions.
The gap between those who could benefit from mental health interventions and those who receive such care is very large (Patel 2016; Singla 2017; WHO 2018); in LMICs, up to 90% of people needing care do not receive it (Alonso 2018; Docrat 2019; Patel 2010 CRCT India), despite the existence of a range of cost‐effective interventions in mental health care (Barbui 2020; Levin 2016; WHO 2010). Major barriers to closing the treatment gap include the huge scarcity of skilled human resources, large inequities and inefficiencies in resource distribution and utilisation (LMICs spend only USD1 per capita on mental health compared to USD80 in high‐income countries (HICs), and most of that is spent on hospital care) (Chisholm 2019; Mugashi 2017; WHO 2018), and the significant stigma associated with psychiatric illness (Semrau 2015). With increasing evidence of the economic and well‐being‐related burden of mental disorders, implementing evidence‐based mental health interventions on a large scale through task‐shifting (i.e. delegating appropriate tasks to non‐specialists) and task‐sharing (ensuring there is some collaboration and ongoing supervision with specialists) in the community should be seen as a high priority (Galvin 2020; Ola 2019; Patel 2018; Petersen 2019).
The World Health Organization (WHO) and other global organisations have long held the position that methods of providing psychological treatments that are less resource intensive, less accessible, less affordable, and non‐stigmatising to patients are of great importance for reducing the global burden of mental distress (Keynejad 2018; Lund 2016; Patel 2016; Rathod 2017; Semrau 2019; Thornicroft 2019; WHO & WONCA 2008), and one of the targets in the WHO 2013‐2020 mental health action plan is provision of comprehensive, integrated mental health and social care services in community‐based settings (https://revman.cochrane.org/#/501210041309211330/dashboard/htmlView/4.211.54?revertEnabled=false#REF‐WHO‐2013). The primary care setting is the point of entry into the health system for most people, and primary care and community‐based providers are well placed to deliver mental health interventions due to their potential for longitudinal relationships with patients and their families, their ability to respond to undifferentiated problems, their use of a bio‐psychosocial model, and their ability to integrate care of mental conditions with care of physical conditions (WHO & WONCA 2008). Over the past two decades, community‐based models of care that operationalise the principles of Wagner’s Chronic Care Model by including risk assessment and a task‐shifting or task‐sharing team‐based approach such as Katon’s Collaborative Care Model have been shown to improve access to evidence‐based mental health treatments, improve patient outcomes, enhance quality of life, reduce costs, and normalise and de‐stigmatise treatments for behavioural and psychological health disorders (Archer 2012;https://revman.cochrane.org/#/501210041309211330/dashboard/htmlView/4.211.54?revertEnabled=false#REF‐Archer‐2012Keynejad 2018; Ratzliff 2016).
Description of the intervention
Primary‐level worker (PW) interventions for the care of patients with mental disorders and distress are the focus of this review. PWs include lay health workers (LHWs), primary care health professionals (PHPs), and community professionals (CPs) (see Figure 1 for categorisation of primary‐level workers and Table 16 for definitions).
1.
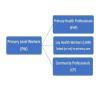
Nomenclature of primary‐level workers described in the review.
Lay health workers (LHWs). As per the Lewin 2010 review, LHWs perform diverse functions related to healthcare delivery (Lewin 2010). Although LHWs are usually provided with job‐related training, they have no formal professional or paraprofessional tertiary education and can be involved in either paid or voluntary care. The term LHW is thus necessarily broad in scope and includes, for example, community health workers, village health workers, treatment supporters, and birth attendants (Barnett 2018; Patel 2018; Shahmalak 2019). As LHWs are diverse and may be linked to a health setting or to a community organisation, we have categorised these together in comparisons as LHWs. They have broadly similar backgrounds (usually from local communities with little if any professional background) and receive minimal training.
Primary care health professionals (PHPs). These professional health workers are not specialising in mental disorders and have not received in‐depth professional specialist training in this clinical area. They may include doctors, nurses, auxiliary nurses, lay health workers, and allied health personnel such as midwives and occupational therapists. PHPs have received professional training in health care and include clinicians (e.g. physicians, nurses, physician assistants) and allied health professionals (e.g. occupational therapists, social workers). As LHWs are diverse and may be linked to a healthcare setting (lay PHW) or to a community organisation (lay CW), we have categorised these together in comparisons as LHWs. They have broadly similar backgrounds (usually from local communities with little if any professional background) and receive minimal training.
Community professionals (CPs). These non‐healthcare professionals are involved as community‐level workers but are not within the health sector. CPs are not health trained per se but play a role in promoting/monitoring mental health. As many people, particularly adolescents and young adults, have minimal contact with healthcare workers, CPs are another human resource instrumental in delivering mental health care. This category includes teachers/trainers/support workers from schools and colleges, social workers, and community development workers/managers. Generalist social workers are often linked to the health sector in that they provide well‐defined and expected extended mental health support role for patients via social support (Barnett 2018; Patel 2018; Shahmalak 2019). In the comparisons in this review, CPs are often combined with nurses, although occasionally they are combined with teachers.
Interventions may include pharmacological, psychosocial, and/or psychological treatments for the care of individuals with mental disorders and distress. They include programmes in which PWs are the main cadres delivering the intervention (e.g. primary care doctors prescribing antidepressants, LHWs delivering a psychosocial intervention) or interventions in which one or several PWs work closely in a team with mental health specialists (collaborative care). One example of a collaborative care intervention is seen when a psychiatrist may diagnose a patient with a disorder and supervise the team; the primary health professional provides follow‐up for the patient and may prescribe or adjust medication dosages or provide a psychological intervention; and an LHW provides psychosocial counselling or support. Such collaborative care teams often use a stepped‐care approach comprising different levels of care according to the patient's response to the interventions (see Table 1 for definitions). These encompass early clinical interventions and monitoring for people with mental distress or sub‐syndromal symptoms, acute interventions for people with mental disorders in the acute phase, and long‐term follow‐up and rehabilitation for people with chronic mental disorders.
Training PWs to deliver psychological or psychosocial interventions, or to participate as members of collaborative care teams, may be a way of expanding provision of services for the care of individuals with mental disorders and distress, as well as making these services more accessible and acceptable to communities. With regards to intervening at the mental distress or sub‐syndromal level, this could prevent full‐blown mental disorders from becoming established, which for many may become chronic or relapsing conditions (Patel 2018). It has been suggested that interventions that rely on PWs could deliver general health and mental health interventions that are at least as effective and acceptable as those delivered by specialist health workers (Lassi 2013; Lewin 2010; Mendenhall 2014; Padmanathan 2013). PWs have been used in various services including those delivered by governmental, private, and non‐governmental organisations (NGOs) in clinics, halfway homes, and communities. They have been involved in a variety of activities and roles, including detecting, diagnosing, treating, and preventing common and severe mental disorders, epilepsy, and learning difficulties. Their roles differ according to their level of training. For example, lay health workers (LHWs) have been involved in supporting carers, befriending, ensuring adherence, and detecting mental health problems (Chibanda 2016; Rahman 2019; Williams 2019). Nurses, social workers, and LHWs may also take on follow‐up or educational/promotional roles (Khan 2017; Patel 2017; Tol 2020). In addition, doctors with general mental health training have been involved in identification, diagnosis, treatment, and referral of complex cases (Archer 2012; Goodrich 2013; Seidman 2017). These interventions may consist of collaborative care models, whereby a PW is involved as part of a team or a step‐wise process for accessing care (Gureje 2019 (STEPCARE); Patel 2010). These models may therefore have elements of psychiatric/specialist intervention or support, and patients may even be recruited from secondary care (such as those with severe mental disorders).
Many challenges are involved in implementing such interventions in LMICs. Differences in the organisation of mental health services between LMICs and HICs, with poorer countries having few or no mental health service structures in primary care or in the community, mean that in such settings, PWs may need to work with little or no support from specialist mental health services with fewer options for referral (Chisholm 2019; Mugashi 2017). Although PW interventions often have lower up‐front costs compared with reliance on professional specialist health workers, these savings may be cancelled out by higher downstream resource use (Rocks 2020).
Other reviews have looked at the effectiveness of interventions in perinatal mental disorders (Clarke 2013; Rahman 2013), as well as in child and adolescent mental disorders (Barry 2013; Burkey 2018; Purgato 2018), or have specifically focused on psychological interventions delivered by PWs (Huntley 2012; Singla 2017) (see Table 17 for additional information). This review differs from other reviews in that it focuses particularly on whether task‐shifting and/or task‐sharing (as in the case of collaborative care) is effective broadly for all types of psychological, psychosocial, and/or pharmacological interventions in the care of mental disorders and distress. This review will also include data on the costs and cost‐effectiveness of PW interventions when available (Figure 2).
2. Agreements and disagreements with related reviews.
| Author/year | Summary of review | Agreements | Disagreements/differences |
| Barbui 2020 | An umbrella review of systematic reviews to evaluate the strength and credibility of evidence generated in low‐income and middle‐income countries (LMICs) on the efficacy of psychosocial interventions for various mental health outcomes | Review authors identified 123 studies from 8 systematic reviews (Asher 2017; Burkey 2018; Cuijpers 2018; De Silva 2013; Purgato 2018; Rahman 2013; Singla 2017; Turrini 2019), and from the previous version of this review (van Ginneken 2013). The focus was on treatment interventions only, as it was for this review. They found strongest evidence for using psychosocial intervention for adults with depression in humanitarian settings, which correlates with our findings for primary‐level workers. There was some evidence for psychosocial interventions to help functioning in schizophrenia, for adults with depression, and for adults with PTS/PTSD, and possibly for children with PTS/PTSD | Review authors cover all psychosocial interventions whether or not they are delivered by PWs. They do not include any other types of interventions such as those involving use of pharmacotherapy |
| Boer 2005 | Review on paraprofessionals delivering psychological interventions for anxiety and depression (HIC only) (Cochrane Review) | Included studies were from HICs only but support our findings that non‐professional care is generally equivalent to professional care (this review's equivalent of specialist care), and that non‐professional care is better than usual care | Some of the paraprofessionals would have been classified as specialist health workers in our review |
| Bower 2006 | Systematic review on the effects of collaborative care models on antidepressant use. All included studies were from HICs, except Araya 2003. |
Bower 2006 found improvement with antidepressant use, particularly in studies where the case manager had a mental health background, where there was adequate supervision, and where there was systematic identification of patients (rather than waiting for a referral) | We were not able to assess, as did Bower 2006, whether lengths of training, supervision, or other intervention characteristics modified these outcomes because only 5 studies were included in this comparison |
| Barry 2013 | Systematic review and narrative synthesis on interventions promoting positive mental health for young people in school and community‐based settings in LMICs | Similar to our review, Barry 2013 identified interventions that were predominantly delivered in school‐based settings across a wide range of LMIC settings, including those in areas of conflict or humanitarian need, with paucity of data in very young primary school‐aged children. Similar to ours, their findings suggest that trained teachers can effectively deliver mental health promotion interventions | Barry 2013 differed from our review, as it also included quasi‐experimental studies. Barry performed a more detailed review of psychosocial outcomes including self‐esteem, self‐efficacy, coping skills, resilience, social participation, empowerment, communication, and social support, which we did not examine. The review included only papers published in English |
| Burkey 2018 | Systematic review of randomised controlled trials examining effects of psychosocial interventions on reducing behaviour problems among children (under 18) living in LMICs | Similar to our review, this review focuses on the importance of early intervention and recognises the need for task‐shifting. This review also included RCTs | Review authors cover all psychosocial interventions whether or not they are delivered by PWs. They therefore include those delivered by specialists (the majority). They also do not include any other types of interventions such as those involving use of pharmacotherapy, and thus excluded ADHD. They included both prevention and treatment strategies but possibly with stronger emphasis on prevention |
| Clarke 2013 | Systematic review of psychological or social interventions delivered by primary health workers for prevention and care of maternal mental disorders in LMIC | This review included 2 of our treatment studies and focused (as did our review) on PHP and LHW (but not CP) delivery of interventions in LMICs. The effects of psychosocial interventions delivered by PWs for treatment and prevention (combined) compared with usual perinatal care show they may slightly improve maternal depression symptoms (SMD ‐0.34, 95% CI ‐0.53 to ‐0.16; I² = 83.9%) and may reduce the number of women with maternal depression (OR 0.59, 95% CI 0.26 to 0.92; I² = 79.3%) | All other studies (9 of their 11 included RCTs) were prevention interventions. They did not include pharmacological disorders. Clarke 2013 undertook subgroup analyses of group and individual interventions as well as of whether interventions were delivered antenatally or postnatally. However, the numbers of studies per subgroup were small. We considered similar subgroup analyses, but these were not feasible, as the subgroups included fewer than 10 studies |
| Fuhr 2014 | Systematic review of peer‐delivered interventions for severe mental illness and depression | This review is a systematic review and meta‐analysis that showed peer‐led interventions may have some effect in high‐income settings, but findings in low‐income settings were inconclusive due to insufficient evidence | Peer‐led interventions are not included in this review unless the peer has an LHW‐type role for a wider group of people ‐ not just 1‐to‐1 support or ad hoc support for peers |
| Huntley 2012 | Systematic review of the effects of CBT and group CBT | Huntley 2012 found that LHW‐led psychological interventions are effective in the short and medium term in reducing symptoms of depression | Huntley 2012 described the effects of CBT and group CBT (rather than the effects of PWs) |
| Gajaria 2018 | Systematic review of psychological interventions for perinatal depression in low‐ and middle‐income countries | “Majority of the interventions were psychosocial, and were often provided by lay health workers and in the community.” Review authors concluded that there was “evidence for the benefit of psychological interventions in perinatal depression in LMICs.” Rahman 2008 and Rojas 2007 were also included in our review |
Not all 18 studies were RCTs; several studies were preventive in nature; no meta‐analysis was performed |
| Gamieldien 2020 | Protocol paper for a scoping review to map the literature related to recovery of people living with severe mental illness in LMICs | No findings presented, as this was a study protocol | The purpose of Gamieldien 2020 is to identify where the literature has not yet been comprehensively reviewed, or where working definitions and concepts are still under development |
| Keynejad 2018 | Systematic review to identify evidence to date for mhGAP‐IG implementation in LMICs | The 2 RCTs included in this review are also included in our review: protocols of Sikander 2019 and Madhombiro 2017, which is an ongoing study. Review authors concluded that although there is substantial observational and implementation literature on mhGAP, evidence on the effectiveness of training for PWs is insufficient | The review included all grey literature and non‐RCT designs, including non‐intervention literature such as training material and reports and economic modelling |
| Klasen 2013 | Systematic review of all randomised controlled trials in child and adolescent mental health in LMICs, supplemented by evidence from HIC as well as suitable information from child programme evaluations and adult studies in LMICs | This review included 8 trials that are also included in our review. None of the other 17 RCTs met all inclusion criteria for this review (as mentioned in the next column). Given their review has many RCTs, these review authors may have more confidence than authors of our review in saying some interventions developed in HICs may be "stripped down to basic principles" (delivered by lay health workers and simplified interventions) and yet still be effective in LMICs. For example, 10 RCTs (+4 quasi RCTs) addressed trauma‐related disorders | Of the 25 RCTs included in Klasen 2013, 13 studies were prevention studies, 7 employed interventions that were not eligible for inclusion in our review, 3 reported interventions that were delivered by specialists, 1 described interventions delivered by peers, 3 provided traditional or herbal non‐evidence‐based interventions, and 1 may not be a randomised trial (we considered Thabet 2005 to be a controlled before‐and‐after study, not an RCT). In addition, this was a narrative review, so no meta‐analyses provided figures for comparison |
| Kohrt 2018 | A review‐of‐reviews to perform a narrative synthesis to map community interventions in LMIC, identify competencies for community‐based providers, and highlight research gaps | Kohrt 2018 described some of the community interventions included in our review, such as psychoeducation, case management, psychological treatments, and training and supervision of community health workers, formal and non‐formal providers outside the healthcare system, and other primary health professionals in delivering these interventions | Kohrt 2018 differed from our review, as its focus was purely on the role of community components to map community interventions, identify competencies for community‐based providers, and identify research gaps, and it was more process‐oriented than outcomes‐oriented compared to our review, and included only systematic reviews for the synthesis. Our review has included only RCTs to evaluate the effectiveness of mental health interventions delivered by non‐specialists in primary health |
| Lassi 2013 | A systematic review of studies on the role of mid‐level health workers in delivering to the general population healthcare services that are associated with achievement of Millennium Development Goals for health and nutrition or with management of non‐communicable diseases | Lassi 2013 examined the role of task‐shifting to non‐physician health providers (i.e. nurse and allied health providers) to deliver clinical care in the community or at a primary care facility or hospital. This review supported our findings that task‐sharing, especially when part of a team‐based approach with adequate training and supervision, can be useful in helping overcome issues such as poor access to care and costs; however most of the evidence of low to very low certainty | Lassi 2013 focused on non‐communicable diseases and did not examine any mental health outcomes |
| Munodawafa 2018 | Systematic review of process evaluation of task‐sharing interventions for perinatal depression in LMICs | “All three RCTs indicated that the intervention was effective.” Rahman 2008 is featured in this review as well as in ours | Of the 3 studies that were included, 2 were preventive in nature. Process evaluation data were meta‐synthesised |
| Mutamba 2013 | Systematic review of LHWs in prevention of mental, neurological, and substance use disorders in LMICs | This review was based on the same searches and principles of examining a type of health worker as the first review of PWs (van Ginneken 2013), concluding that robust evidence is insufficient for assessing effectiveness | This review will be more relevant to compare to our parallel review on prevention interventions (Purgato 2021), as it pertains to prevention of mental disorders rather than treatment. This new Cochrane prevention review will be an update and an extension of this review |
| Parker 2008 | Review on consultation liaison in primary care ‐ HICs (Cochrane protocol) | ‐ | Our review process did not find any consultation liaison in primary care in LMICs, so results cannot be compared |
| Purgato 2018 | A systematic review and meta‐analysis evaluating the effectiveness of focused psychosocial support interventions for children exposed to traumatic events in humanitarian settings in LMICs, to explore which children are likely to benefit most | Similar to our review, Purgato 2018 provided meta‐analytical evidence for the beneficial effects of focused psychosocial support interventions on PTSD/PTS symptoms (SMD ‐0.33, 95% CI ‐0.52 to ‐0.14) that were maintained at follow‐up (‐0.21, ‐0.42 to ‐0.01), benefits for functional impairment (‐0.29, ‐0.43 to ‐0.15) and for strengths: coping (‐0.22, ‐0.43 to ‐0.02), hope (‐0.29, ‐0.48 to ‐0.09), and social support (‐0.27, ‐0.52 to ‐0.02) | The focus of Purgato 2018 was to identify which population subsets benefit the most from psychosocial interventions, which was beyond the scope of our review, including analyses by age, gender, displacement status, region, and household size |
| Rahman 2013 | Systematic review on interventions for common perinatal mental disorders among women in LMICs | This was a similar but more in‐depth review of our perinatal depression pooled comparison, which also looked at LHW‐led interventions for mothers with perinatal depression. The final pooled outcome was similar in magnitude and direction to ours for our perinatal depression category (SMD ‐0.38, 95% CI ‐0.56 to ‐0.21) vs our findings (SMD ‐0.42, 95% CI ‐0.58 to ‐0.26) | This review differed from ours in that its study inclusion criteria were broader, as it included studies that measured maternal (all perinatal disorders) or child (or both) outcomes, even if the intervention was not primarily targeted at these groups. It also reported child outcomes, which ours did not |
| Shahmalak 2019 | Qualitative analysis of LHW opinions on training, barriers, and facilitators of therapy delivery, factors in successful therapy delivery, and personal impact | Many authors whose studies featured in our review conducted the studies that were featured in this review (I.e. they studied LHWs who had carried out interventions in their studies). Thus, the 2 reviews are complementary to each other | Patient outcomes were not studied |
| Singla 2017 | Systematic review of RCTs using non‐specialist providers to provide psychological treatments for depression, anxiety, and post‐traumatic stress disorder outcomes | They found a positive treatment effect favouring intervention (pooled effect size 0.49, 95% CI 0.36 to 0.62). Studies common between this review and ours include Ali 2003, Araya 2003, Bass 2013, Bolton 2014 (Iraq), Bolton 2014 (Thailand), Bolton 2003, Chibanda 2014, Dybdahl 2001, Fritsch 2007, Milani 2015, Patel 2010, Rahman 2008, Rojas 2007, Weiss 2015, and Yeomans 2010 | Search was up to 2016. Studies conducted in Taiwan and Hong Kong were included. Some included studies are not in our review but will be included in the sister prevention review ‐ Purgato 2018. Authors also studied types of psychological treatments and techniques. They did not extract dichotomous outcomes, nor did they focus on analysis by type of health worker |
| Tol 2011 | Systematic review on mental health interventions in humanitarian settings | Tol 2011 found similar results to our review for school‐based interventions for children with PTSD (i.e. no significant benefit) (an extra study was included in this comparison, which we had excluded, as it did not meet our PW definitions). This review went further and found a statistically significant benefit for improving internalising symptoms (SMD ‐0.34, 95% CI ‐0.40 to ‐0.09). For adults, potential benefit of interventions was also seen | Tol 2011 differed from our review in that it included studies of both PWs and specialists, according to our definitions |
| Woltmann 2012 | Systematic review on collaborative care/chronic care management (HICs) | These review authors also found a statistically significant effect on reduction in depression severity among the 14 HIC studies that were included in the meta‐analysis (SMD 0.31, 95% CI 0.16 to 0.47) (Araya 2003 and Patel 2010 were included in the narrative review but did not qualify for the meta‐analysis). Review authors suggested that collaborative care is of moderate benefit; however, Woltmann 2012 estimated a more conservative value of SMD > 0.5 to show moderate benefit (from the analysis of scales and how to interpret their SMDs). Our meta‐analyses of collaborative care models suggest similar improvements in symptoms and recovery from depression or from CMD (same direction of effect, similar magnitude) | In Woltmann 2012, chronic care management had a stricter definition than our collaborative care definition |
CBT: cognitive‐behavioural therapy; CI: confidence interval; CMD: common mental disorder; CP: community professional; HIC: high‐income country; LHW: lay health worker; LMIC: low‐ and medium‐income country; OR: odds ratio; PHP: primary health professional; PTS: post‐traumatic stress; PTSD: post‐traumatic stress disorder; PW: primary‐level worker; RCT: randomised controlled trial; SMD: standardised mean difference.
2.

Logic model for prevention ‐ Purgato 2021 ‐ and treatment reviews ‐ van Ginneken 2019.
How the intervention might work
Studies have shown that mental health care can be delivered effectively in primary healthcare and community‐based settings through task‐shifting, task‐sharing, or collaborative care approaches that engage and support skilled non‐specialist health professionals, lay workers, affected individuals, and caregivers in mental health service delivery (Kakuma 2011). Implementation studies of these complex interventions have indicated that although the composition of the mental health workforce may vary, a key contributor to its effectiveness lies in the quality and intensity of training, supervision, and subsequent mentoring of non‐specialist workers (Kakuma 2011). Due to the spectrum of illness encompassed in common mental disorders, most are non‐pharmacological interventions and tend to be abbreviated, time‐limited versions of well‐established, evidence‐based transdiagnostic psychological therapies such as cognitive‐behavioural therapy and problem‐solving therapy (Dalgleish 2020). To ensure that interventions are appropriately applied, many involve a stepped approach whereby patients with more severe or complex illnesses, or who do not respond adequately to treatment, are 'stepped up' to more intensive treatments. This may entail having more treatment sessions with the PW, being 'stepped‐up' to receive more intensive pharmacological or psychological treatments provided by health workers with more training or expertise, or, in some cases, being referred to specialist care (Rathod 2017).
Why it is important to do this review
The growing burden of mental disorders coupled with treatment gaps and shortages of specialist human resources in LMICs has made the need to involve non‐specialists in mental healthcare provision more urgent (Patel 2016). People in LMICs need care for mental disorders and distress as much as people in HICs do, yet many LMICs have poorer mental health resources and organisation, fewer psychiatrists, and fewer psychiatric nurses than HICs. The median number of psychiatrists is 172 times lower in low‐income countries (LICs) than in high‐income countries (HICs) (Kakuma 2011; WHO 2018). Training and retaining sufficient numbers of mental health specialists in LMICs is not feasible in the near future. It is therefore important in these settings to consider options for expanding access to mental health services. The use of PWs, who are more numerous, affordable, and accessible than specialists, is one such option. This task‐shifting/sharing model, in which tasks traditionally allocated to a mental health professional are done by non‐specialists, requires far fewer specialist resources and makes available a larger taskforce. Primary‐level providers are less stigmatising, more accessible, and more acceptable to people needing mental health care, who may be reluctant or unaware of how to access mental health services (Rathod 2017). Reliable evidence is needed on the effectiveness of PWs in scaling up mental health interventions, including for detection, treatment, and rehabilitation of mental disorders. This systematic review will provide the evidence needed to inform policy development for sustainable scaling up of mental health services in LMICs.
The aim of this first update of the original 2013 Cochrane Review ‐ van Ginneken 2013 ‐ is to evaluate the effectiveness of mental health treatments delivered by PWs to people with mental distress/disorders living in LMICs. To expand the applicability of findings, this review update builds on the first review by including people with a broader spectrum of illness and symptom severity than was addressed in the 2013 review, to include people experiencing mental distress, sub‐syndromal symptoms, and diagnosable disorders. Following consultation with a panel of stakeholders (clinicians delivering care in LMICs, project implementers, academics, and policymakers), the review team decided to conduct an additional parallel review on the role of PWs in delivering interventions focused on prevention of mental ill health and promotion of mental well‐being (Purgato 2021).
Objectives
To evaluate the effectiveness of mental health treatments delivered by trained PWs in LMICs to persons with mental distress/disorders, compared to usual care or care delivered by untrained PWs or specialists.
Methods
Criteria for considering studies for this review
Types of studies
We included only randomised trials including cluster‐randomised trials in this review update. In the previous version of this review (van Ginneken 2013), we included a range of non‐randomised studies (NRSs). However these other study designs (non‐randomised trials, controlled before‐and‐after studies, and interrupted time series studies) contributed little to the results due to serious risk of bias. Furthermore, these NRSs were often used as precursor studies to randomised trials.
We included only studies conducted in LMICs, as defined by the World Bank on the year(s) that each study was conducted, and we excluded all studies conducted in HICs. Similar to the 2013 review, this update remained focused on LMICs as a response to the call for more community‐based models of care to address the challenges encountered in delivering mental health care in these settings.
When data were available, we included economic studies if they were conducted as part of an effectiveness trial. These included full economic evaluations (cost‐effectiveness analyses, cost‐utility analyses, and cost‐benefit analyses), cost analyses, and comparative resource utilisation studies. 'Cost‐effectiveness' refers to analyses that examine the ratio of the cost of a particular intervention to a chosen unit of effectiveness, which is different from 'cost‐saving', which refers only to the monetary value of an intervention without consideration of its effects. We described whether the analysis took a 'societal perspective' (which takes into account costs incurred to all, such as to the health service, the patient, etc.), or whether it took a 'health system perspective' (which takes into account only costs to the health service and system) (Zilberberg 2010). We extracted and reported only cost and resource usage outcomes from these studies.
Types of participants
We included children (aged < 18 years) and adults with mental disorders or distress seeking first‐level care/primary care or detected in the community in LMICs. We also included participants who had a diagnosis established in secondary care or by specialists in community care and for whom the intervention was then performed in primary or community settings (e.g. follow‐up monitoring to improve/maintain mental health after discharge, collaborative care/shared care between primary and secondary care). Additionally, we included carers of people with mental disorders or distress (i.e. any relatives or friends of any age who defined themselves as key supporters to a person with a mental condition), as some interventions may be directed at the carers rather than at patients themselves (e.g. interventions to alleviate carer burden).
Mental disorders included are in accordance with the ICD‐11 classification of mental disorders (WHO 2019), and include common mental disorders, severe mental disorders, perinatal mental disorders, disorders specifically associated with stress, disorders associated with substance abuse, neurocognitive disorders such as dementia, as well as all mental developmental (e.g. autism), emotional (e.g. mood disorders) and behavioural (e.g. ADHD) disorders associated with childhood (based on those included in the Mental Health Gap Action Programme (mhGAP) guide (WHO 2016).
(See Table 16 for further definitions of participants, 'LMIC', and 'primary care', and for the list of included mental disorders, as well as a definition of mental distress.)
Types of interventions
Cadres of interventionists
Primary‐level workers (PWs) include primary healthcare professionals (PHPs), such as doctors, nurses, and pharmacists; lay health workers (LHWs) (i.e. people living at the community level with no prior health professional training); and community professionals (CPs), such as social workers, teachers, and development workers. We combined PHPs and/or CPs and LHWs (1) when interventions and roles were similar but due to different contexts, different cadres were used (comparisons 6, 8, 10, and 13), or (2) because multi‐disciplinary teams were involved, such as within collaborative care (comparison 2). For example, social workers were combined with PHPs (doctors, pharmacists, nurses) in comparison 6, as psychosocial interventions and roles were similar in helping adults in humanitarian settings with mental distress and post‐traumatic stress with similar regular extended mental health supportive roles (also as described in the study descriptions) within their work for these populations, who often needed more intensive community support. Community‐based lay providers could be linked to the health sector or attached to community networks or organisations. Because they were very similar in terms of function, trainability, and educational background, and were embedded in communities, we opted to categorise all lay workers together within comparisons as 'lay health workers' (LHWs). See Figure 1 for the PW nomenclature hierarchy, and see Table 16 for full definitions of primary‐level workers and types of interventions.
Description of interventions
We included clinical (medical and psychological) and PW educational interventions that were intended to improve the mental health of patients in LMICs. Clinical interventions are mental health therapies that aim to alleviate acute mental health symptoms, promote recovery from mental disorders or distress, or monitor and manage chronic mental illness. In addition, we included a broader suite of interventions that may be delivered by PWs to those with mental distress/prodromal symptoms, such as training people in self‐help interventions, providing informal support or transdiagnostic psychosocial support (such as developing individualised plans to address social and emotional functioning and problems), and providing interventions directed at high‐risk individual identification. We did not include service or social interventions (such as income generation or general social support) if the trial did not also include a specific mental health intervention.
Acute clinical interventions
Acute interventions delivered by PWs include various forms of psychotherapeutic or pharmacological treatments. In this review, we refer to PW‐led 'psychological interventions' as those in which the PW delivers mainly a psychological therapy (such as cognitive‐behavioural therapy, behavioural activation, etc.). We refer to PW‐led 'psychosocial interventions' as those in which the PW delivers an intervention that combines elements or adaptations of therapeutic principles or therapeutic components, adopts a more transdiagnostic approach, and may deliver a social supportive component (such as debt management, family negotiation, income‐generating activity, or community/well‐being activity).
Long‐term clinical interventions
Long‐term interventions delivered by primary and community workers could include roles in follow‐up or rehabilitation of people with severe mental disorders, as well as roles in detecting and dealing with relapse/recurrence, compliance issues, treatment resistance, side effects of treatment, or psychosocial problems. Modifications to the interventions included are consistent with recent recommendations of The Lancet Commission on Global Mental Health and Sustainable Development staging approach to classification of mental disorders (Patel 2018).
Case‐finding interventions
We included studies that considered effects of detection, screening, or case‐finding of mental distress and disorders by primary‐level workers on subsequent patient and health provider outcomes versus primary‐level workers not actively detecting cases, or when specialists performed the detection.
Identification methods used by primary‐level workers could include 'naturalistic’ detection (i.e. detection in the course of a routine clinical consultation) or detection using a validated screening/detection tool (e.g. in the context of a trial). We did not compare diagnostic accuracy between these primary‐level workers and specialists, as this variable was likely to be confounded by the screening/detection tools used. Therefore, it would be difficult to differentiate between effects of the screening tool and effects of the skills of health workers (specialist or non‐specialist).
Training/professional development interventions
We also included studies in which the intervention purely consisted of a training course provided to PWs, usually conducted by mental health specialists or by the research team, on the topic of psychiatric illness and/or its management. We excluded such trials if no patient outcomes were measured (e.g. when they assessed only knowledge or attitude changes, as in pre‐post training evaluations).
Studies with a prevention component
From our previous 2013 review (van Ginneken 2013), 12 out of 38 included studies had interventions that combined both treatment and prevention. For trials that included subgroup analyses that split out these different populations, we retained treatment outcomes in this review and we will include prevention outcomes in a parallel prevention review (Purgato 2021; Figure 2).
We decided whether the aim of each study was prevention or treatment, and we looked at the inclusion criteria for participants (studies had to have as an inclusion criterion the presence of mental distress/prodromal symptoms or a diagnosable disorder). When there was no clear distinction between prevention and treatment groups, we made a pragmatic decision about whether these trials were primarily about well‐being/prevention or were primarily about treatment, and we then allocated trials to the appropriate review, or we included them in both reviews and performed sensitivity analyses while excluding them.
Comparators
For all study populations aside from adults with severe mental disorders, PW interventions were compared to ‘usual care’. Usual care could consist of functional usual care (e.g. provided by a non‐trained existing PW), non‐functioning theoretical usual care, or essentially no care (i.e. care that is nominally there but is poorly accessed or is not always available/accessible). In addition, due to the heterogeneity of provision, we included 'enhanced usual care' (defined as minimal additional mental healthcare intervention such as a one‐day training workshop or provision of a leaflet or manual) or external support or some other minimal follow‐up arrangement (such as the option of referral to a specialist), or any other non‐mental health intervention (such as a lecture on physical disease prevention).
For adults with severe mental disorders (comparisons 11 and 12), primary‐level worker treatments were compared to treatments provided by mental health specialists in primary care and in the community.
Types of outcome measures
We grouped outcomes into three sets of time points.
T1: short term/immediate post intervention (defined as 0 to 1 month post intervention) to detect illness recovery/symptom reduction of the intervention.
T2: intermediate term (defined as 1 to 6 months post intervention) to detect sustained illness recovery/symptom reduction.
T3: longer term (defined as 7 to 24 months post intervention) as a measure of medium‐ to long‐term avoidance of recurrence and chronicity. Subgroup analyses were performed for 1‐ to 2‐year outcomes if available.
If an outcome was reported more than once during any of the above time points, we used the latest time point within that category (e.g. if there was a measure at 3 months and at 6 months, we used the results at 6 months for T2) or the time point that correlated best with other studies compared within each outcome.
We organised relevant outcomes into categories by drawing on the Cochrane Consumers and Communication Review Group's outcome taxonomy (La Trobe 2008), by consulting with co‐reviewers and service users from the Movement for Global Mental Health discussion board, and by having recent consultations with current implementers and policymakers in LMICs (see below). As in the previous review, when studies reported more than one measure for each relevant outcome, we abstracted the primary or main measure (as defined by study authors). We separately documented the other measures used, as necessary.
The time points in this review update differ slightly from those in the 2013 review (van Ginneken 2013), where T1 was 0 to 2 months, T2 4 to 6 months, and T3 8 to 12 months post intervention, as we wanted to better capture the difference between post‐intervention recovery and remission, and we wanted to include a measure of long‐term outcomes. A 2019 review concluded that the duration criteria for declaring remission versus recovery may be unnecessary and not meaningful (de Zwart 2019), even though these had previously been thought to be important (Spijker 2002). Depressive remission can be defined as the asymptomatic state after a depressive episode, without application of any duration criterion. Stability of remission is relatively low on the first day but increases gradually with its duration. The term 'recovery' is then used as a concept that would involve more than absence of symptoms and would also include better social functioning or subjective well‐being, and may include the absence of significant treatment, as this would better fit the concept of recovery from a patient’s perspective (de Zwart 2019).
This review does not attempt to present the illness recovery outcome as a single outcome, although individual studies may provide some of the information pertaining to illness recovery (such as social functioning).
Primary outcomes
1. Recovery and prevalence
1.1. Clinical illness recovery: number of people who recover from mental distress or mental disorder (defined by study authors as number of people reaching minimal or no symptom category on a validated symptom scale, such as the Hamilton Depression Rating Scale (HDRS)). If the time point was < 1 month post intervention (T1), we called this 'remission'
1.2. Disease prevalence: number of people with the illness at a point in time. Some trial authors separated recovery and prevalence outcomes, as prevalence did not equate to 'one minus recovery'. Disease prevalence involves a person having an illness based on diagnostic criteria (e.g. for the Patient Health Questionnaire‐9 (PHQ‐9) for depression, this would include those scoring > 10). Recovery applies to those who were well, so below a certain threshold on a scale (e.g. with PHQ‐9, this would be < 5). However those who scored as having mild or sub‐threshold symptoms would not feature in either (in this example, PHQ‐9 between 5 and 9). We were therefore not able to combine or transform figures to create just a single outcome
2. Clinical symptom severity: average clinical symptom scores for a study population at a point in time, or change in average clinical scores from baseline (i.e. average improvement or change in symptom scale across the study population), such as scores or change in scores from baseline on the Harvard Trauma Questionnaire (HTQ), the PHQ‐9, and the Edinburgh Postnatal Depression Scale (EPDS)
3. Quality of life (QOL): meaningful functioning and human development (e.g. WHO Quality of Life Assessment (WHOQOL), 36‐Item Short Form Survey (SF‐36), EuroQoL Group Quality of Life Questionnaire based on 5 dimensions (EQ‐5D)). QOL outcomes were deemed different from outcomes related to psychosocial functioning, as the former encompass a summary of many other aspects of life in addition to psychosocial functioning
4. Functional impairment and/or disability: as measured by levels of dependency (e.g. WHO Disability Assessment Schedule (WHO‐DAS))
5. Service utilisation (demand) and coverage (supply): including admission/re‐admission rates to hospital whether related to mental disorder or not; attendance rates with regards to utilisation of primary or community services; or increased demand and/or referral rates from primary/community care setting to mental health specialists
6. Adverse events: number of people who sustained harm during the intervention, measured by rates of adverse effects of interventions, which could be clinical indicators (e.g. suicide/deliberate self‐harm rates, needing referral to psychiatric care), social indicators (social exclusion), or service delivery indicators (i.e. service utilisation; hospital admission/re‐admission rates), regardless of whether the study team attributed these events to the intervention. These were systematically extracted.
We did not base inclusion decisions on whether a reference or a validated standard measure (either a screening instrument or a psychiatric assessment) had been used in studies to differentiate between those correctly and incorrectly diagnosed by PWs, but this featured as part of the assessment of quality of evidence (within‐study limitations).
We included service delivery and utilisation outcomes as primary rather than as secondary outcomes in this updated review, as this was of great interest to stakeholders (decision‐makers and providers).
Secondary outcomes
Direct cost and cost‐effectiveness of the intervention
-
Resource use and societal costs
for health services (e.g. health service personnel's time allocated, cost of extra consultations or referrals, other opportunity costs of the intervention for other aspects of the health service)
for patients (e.g. opportunity costs to patients such as extra costs of travel, time, or medication; lost productivity; employment status; income; work absenteeism; retention; educational attainment)
The service utilisation figures in primary outcomes may relate to some health service additional costs (e.g. inpatient admissions, referrals). This is highlighted when both the number of attendance rates and the cost of these are reported in studies.
The economic outcome measures considered were informed by the training material of, and in discussion with, the Campbell and Cochrane Economics Methods Group (CCEMG 2010). We included in this review only measures related to resource use and costs. We recognise that costs and resource use are intertwined, but we divided the outcomes in this way to make it clear which outcomes we had assessed.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases for primary studies on 20 June 2019.
Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 6), in the Cochrane Library.
MEDLINE Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, MEDLINE Daily and MEDLINE 1946 to present, Ovid.
Embase 1974 to present, Ovid.
CINAHL (Cumulative Index to Nursing and Allied Health Literature), 1980 to present, EBSCO.
PsycINFO 1806 to present, Ovid.
World Health Organization (WHO) Global Health Library (Including: World Health Organization Library Information System (WHOLIS), AIM (AFRRO), IMEMR (EMRO), IMSEAR (SEARO, WPRIM, WPRO), which includes the Latin American Caribbean Health Sciences Literature (LILACS).
The EPOC Information Specialist revised the search strategy in collaboration with the review team. The search strategy was peer reviewed by two Cochrane Information Specialists using the PRESS checklist 2016. Search strategy amendments included new search terms (such as mental distress, sub‐syndromal syndromes) and removal of some exclusions (such as epilepsy). Search strategies comprised natural language and controlled vocabulary terms related to PWs and mental health. We did not apply any language or date limits.
We used two search filters ‐ one to restrict search retrieval to randomised trials (Cochrane RCT sorter) and one for LMICs.
We employed strategies to search for and include relevant unpublished studies. These strategies included searching the grey literature and prospective trial registration databases to overcome time‐lag bias.
This search method was used for both this treatment review and the parallel prevention review, so screening was done simultaneously for both.
We repeated the search on 24 August 2020 (Figure 3).
3.
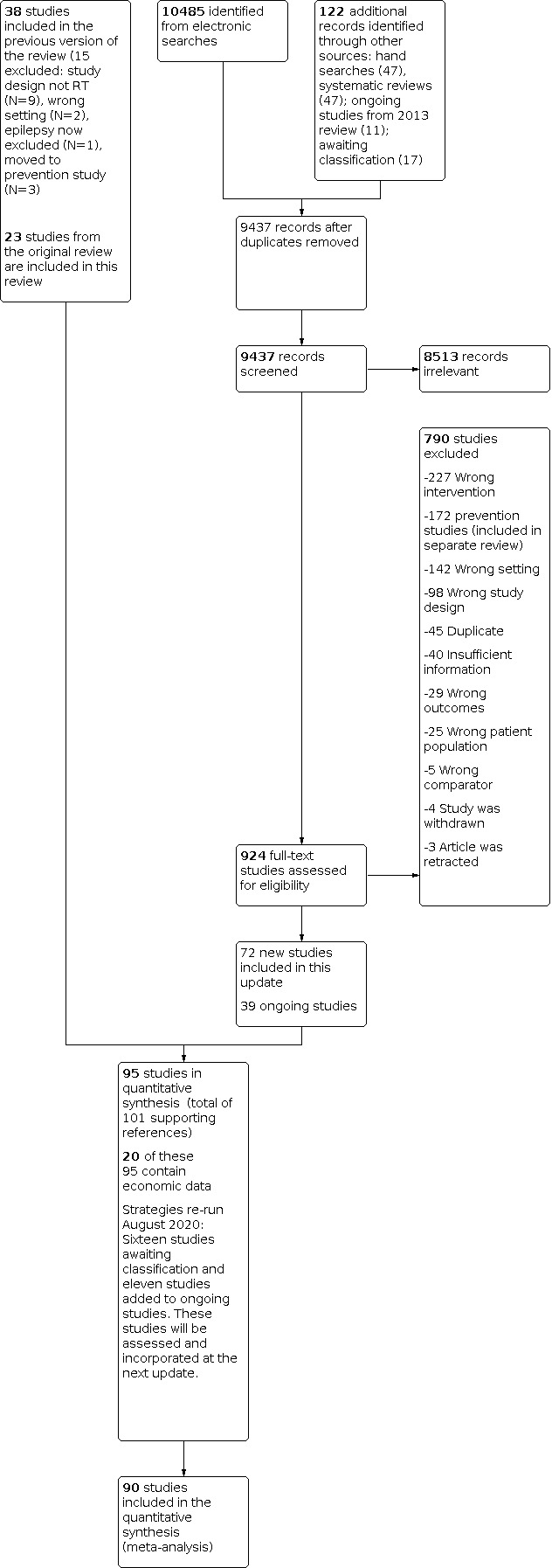
Study PRISMA flow diagram.
See Appendix 1 for all search strategies used.
Searching other resources
We searched the following trial registries on 20 June 2019 for ongoing studies.
Clinical Trials Register, US National Library of Medicine (clinicaltrials.gov).
WHO International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/en/).
We also searched the reference lists of existing reviews (De Vet 2008).
We did not search for economic analyses. We retrieved potentially eligible economic analyses when screening records generated by the various searches reported above, but we selected only those performed alongside identified effectiveness studies. We contacted the authors of all included effectiveness studies to ask for information on any published or unpublished economic studies related to their trials. We also scanned the reference lists of eligible trials and economic analyses (where these were reported separately for eligible trials) and other related reviews and papers for additional eligible studies.
Data collection and analysis
Selection of studies
Review authors independently screened all records obtained through the searches. For studies retrieved in languages in which the review authors were not competent (Portuguese and Farsi), we found reviewers who were native speakers of the language to screen and review these papers. We retrieved full‐text copies of all articles identified as potentially relevant by at least one review author. Two review authors checked each full paper for inclusion criteria. We resolved disagreements on inclusion by discussion. If no agreement was reached, we asked a third review author to make an independent assessment (NvG or SL). When appropriate, we contacted study authors by email to ask for further information.
Data extraction and management
Two review authors independently extracted descriptive and outcome data for each paper using an adapted version of the EPOC data collection checklist into a Microsoft Excel spreadsheet. The two extractions were cross‐checked by the two review authors, and a third review author (NvG, WC, YCL, NH, LYC) resolved discrepancies and re‐checked all descriptive and outcome data during analysis. Chinese and Iranian papers relied on data extracted by one bilingual researcher and translation of key parts of the paper to allow double assessment of risk of bias and outcome extraction by a second review author. Review authors contacted trial authors to ask for missing statistical data and additional descriptive data and most often received responses particularly to statistical queries. Review authors entered the final agreed descriptive extracted data into relevant tables of characteristics in Review Manager 5 (RevMan 2012). Two review authors (NH and LYC) entered the checked outcome data into Review Manager 5 for meta‐analysis and cross checked each other's entries (RevMan 2012).
We extracted the following information for all included studies.
Details of the intervention: type and length of each of the clinical, psychosocial, and service interventions; a full description of cadre(s) of PWs consulting with the patient, including details of their training and supervision/support; length, frequency, and type of intervention delivered by each PW; a description of the specialist providing care (including type, experience, training in using reference standard).
Participants: a full description of participants (including sex, age, socioeconomic status, ethnicity) and details of the mental condition being treated.
Setting: country, type of health service (e.g. government funded, NGO), rural versus urban, organisation of primary care and specialist services, specialist outreach or generalist. We used the World Bank classification of countries by gross national income per capita in all calendar year(s); the study was conducted to assign studies to the appropriate low‐income and middle‐income country category.
Data from included studies were not transformed before presentation in the review. When 2 × 2 data or means and standard deviations were not available for each group in the included studies, relative measures of effect were extracted and meta‐analysed by the generic inverse variance method.
Assessment of risk of bias in included studies
Two review authors independently assessed each study for risk of bias while extracting data. The lead authors (NvG, WYC, YCL) independently checked assessments for all studies. We followed the Cochrane Effectiveness of Practice and Organisation of Care (EPOC) group format (Ballini 2010, EPOC 2017a; EPOC 2017b), which follows the Cochrane approach (Higgins 2009; Higgins 2011), to assess risk of bias. We assessed attrition bias for two types of outcomes: efficacy outcomes and safety outcomes (e.g. adverse events, unintended consequences). If no mention was made of safety outcomes being assessed, the study was rated as having ‘unsure’ risk of bias for safety outcomes. We divided the blinding domain into blinding of participants and personnel and blinding of outcome assessment. We considered incomplete outcome data separately for efficacy and for adverse outcomes.
For economic studies, we adapted the Consensus on Health Economic Criteria (CHEC) list (see Appendix 2) to include an extra question on the sources of data used, and we re‐formulated other CHEC criteria headings into discrete sub‐questions. We had a total of 24 questions.
Measures of treatment effect
For dichotomous outcomes, we calculated risk ratios (RRs) with 95% confidence intervals (CIs).
For continuous outcomes, we calculated mean differences (MDs), standardised mean differences (SMDs), or mean change differences (MCDs) with 95% CIs. When studies reported only relative measures of effect (and not raw data per group), we used the generic inverse variance method to combine trials.
For SMDs, we used the Cochrane Handbook for Systematic Reviews of Interventions to interpret their clinical relevance using the following cut‐offs: 0.2 represented a small clinically appreciable effect, 0.5 a moderate effect, and 0.8 a large effect (Cohen 1988). We attempted to establish minimally important differences for each outcome (as suggested in Guyatt 2013), but this was not possible due to the wide variety of instruments used.
Unit of analysis issues
When possible, we re‐analysed studies that randomised or allocated clusters (health professionals, healthcare settings, or geographical areas) but did not account for clustering in the analysis (Ukoumunne 1999). We adjusted the results for clustering by multiplying the standard errors of estimates by the square root of the design effect when the design effect was calculated as DEff = 1 + (M ‐ 1) ICC, where M is mean cluster size and ICC is the intracluster correlation coefficient (ICC). When included studies did not report ICCs for the respective outcome measures, we derived ICCs from a different outcome from the same study, or from a different study included in the same meta‐analysis. Details on the ICC used and the sources are provided in the footnotes of each analysis under the forest plots.
We combined adjusted measures of effects of cluster‐randomised trials with the results of non‐cluster trials, when it was possible to adjust adequately the results of cluster trials. There were too few studies per meta‐analysis to perform sensitivity analyses comparing effects estimates with and without inclusion of cluster trials.
If a single study compared two or more primary‐level worker (PW) interventions (with or without a control arm), we labelled the arms separately in analyses. Each PW‐led intervention within a trial was analysed separately, and if necessary, the control group was split to perform independent comparisons.
Dealing with missing data
For missing or unclear information, we contacted study investigators for clarification or additional information. We were able to access all required authors for the purpose of requesting statistical information. Some remaining missing information on the qualitative description of interventions that we did not get despite several attempts at following up with study authors is highlighted in the Characteristics of included studies tables. To reduce the risk of overly positive answers, we used open‐ended questions when contacting investigators (as recommended in the Cochrane Handbook for Systematic Reviews of Interventions;Higgins 2009).
When possible, we extracted data to allow an intention‐to‐treat (ITT) analysis in which all randomised participants were analysed in the groups to which they were originally assigned. For studies that reported continuous data but did not report standard deviations, we calculated these from other available data such as standard errors or confidence intervals, or we imputed these using the methods suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2009). We did not make any assumptions about loss to follow‐up for continuous data, and we analysed results for those who completed the trial.
Assessment of heterogeneity
We first made a qualitative assessment of the extent to which studies assessing a particular comparison were similar to one another. This included assessment of settings, interventions, participants, and outcomes to determine whether a meta‐analysis was appropriate. We obtained an initial visual overview of statistical heterogeneity by scrutinising the forest plots and examining the overlap between confidence intervals around the estimate for each included study. To quantify inconsistency across studies, and thus the impact of heterogeneity on the meta‐analysis, we used the I² statistic, and we defined I² > 50% as indicative of substantial heterogeneity. We then considered these assessments when interpreting the results of a pooled analysis.
For analyses where the 12 statistic was 95‐97%, we opted to present the results and then to grade down the evidence on the basis of high levels of statistical heterogeneity. We took this approach as the interventions cover a wide range of settings and disorders and so we anticipated higher levels of statistical heterogeneity. We do not report pooled results for analyses where the I2 was found to be 98% or higher. However, these pooled analyses are visible in the Analyses section as it is not possible to selectively de‐activate pooling for some sub‐analyses.
Assessment of reporting biases
To assess publication bias, funnel plots were to be produced for outcomes with more than four studies to visualise whether there was asymmetry. None of the plots showed asymmetry. We did not perform any statistical testing for funnel plot asymmetry, as none of the meta‐analyses included more than 10 studies.
Data synthesis
We grouped the studies into 15 comparisons by:
Type of disorder: there were six disorder categories: common mental disorders (including depression and anxiety), perinatal depression, post‐traumatic stress (some distress following trauma)/post‐traumatic stress disorder (the disorder meeting diagnostic criteria), dementia, severe mental disorders, and substance use disorders (see Table 16 for details of categories of disorders included);
Age of participant: child interventions (< 18 years old) were separated from interventions for adults;
Type of provider: we separated interventions by types of health workers based on whether they were lay or professional, and whether they worked in the health sector or in the community (such as teachers, NGOs). All lay workers, whether they were linked to the health sector or to the community, were grouped together as LHWs for analyses. Hence, primary‐level workers (PWs) were classified as primary health professionals (PHPs), as community professionals (CPs), or as LHWs (see Table 16 for definitions). These providers worked in a collaborative capacity (as part of a larger complex intervention) or as a sole component of a psychological intervention. Because their roles sometimes overlapped, the interventions were similar, and/or they worked collaboratively, these cadres were combined together for some comparisons; and
Comparator group: comparators were usual care, enhanced usual care (EUC), or specialist care. On occasion, usual care was essentially no care, as access to usual care sometimes was not feasible for participants.
We did this as these groupings and these categories fit with current models of service delivery in LMICs. This grouping was chosen after consultation with stakeholders in the first review, and was checked before the start of this update with a focus group of stakeholders who agreed that it was important to divide the information by types of disorders because of relevance to different professionals and patients of classifying and treating disorders (Patel 2018). It was also important to divide these studies by types of PW and whether PWs were delivering an add‐on intervention or were working as part of a collaborative team, as policy and practice stakeholders need to know for the purpose of allocating their workforce (Kakuma 2011).
For the common mental disorders comparisons, where trials reported only depression scores, these were combined within the common mental disorder analysis (which included both anxiety and depression).
For each comparison, we created tables of summary statistics including baseline and follow‐up summary statistics, effect estimates, and their precision. We used forest plots to display the data graphically. When pooling was considered appropriate, we employed a random‐effects meta‐analysis using the DerSimonian and Laird method (DerSimonian 1986), as it was assumed that effect size might vary across studies and settings, and that the included results are estimates of different but related quantities.
When outcomes assessed and settings and interventions were very diverse (as agreed by at least two review authors), we did not consider it appropriate to combine the results quantitatively. For these results, we presented a descriptive summary of the data (Reeves 2009).
Economic data
We conducted all elements of the economics component of this review according to current guidance on the use of economics methods in preparation and maintenance of Cochrane Reviews (Aluko 2021). We classified the included economic evaluations based on an established system (Drummond 2005; Trautmann 2016). We summarised the characteristics and results of included economic evaluations using additional tables, supplemented by a narrative summary that compared and evaluated methods used and principal results between studies.
We displayed resource use and cost data in a table, along with unit cost data (when available). A unit cost was defined as the cost of each specific resource input calculated by multiplying the measured number of units (quantities) of an item of resource use (e.g. the number of hours of time provided by a senior teacher) by an applicable unit cost (e.g. the salary cost of one hour of senior teacher time). We reported the currency and price year applicable to measures of costs and unit costs in each original study. Measures of costs are highly likely to vary across and within study settings, and over time as the result of variations in underlying quantities of resource use and variations in underlying unit costs .
Because the data on resource use and costs were very heterogeneous, meta‐analysis was not appropriate, and we presented the findings narratively. We discuss the limitations of this approach below.
Subgroup analysis and investigation of heterogeneity
Within each comparison, we planned the following subgroups.
Categories of health workers: primary health professionals (PHPs) (e.g. doctors, nurses); community professionals (CPs) (e.g. teachers); and non‐professional lay workers (LHWs).
Types of interventions (e.g. collaborative versus psychological interventions).
Settings (e.g. government versus non‐government).
After considering the studies included in this review, we revised the comparisons, so that separate analyses would be performed for categories of health workers and types of community interventions as the main analyses ‐ not as subgroups.
We were not able to perform other subgroup analyses to check if the intervention effect varied with different population characteristics, as the number of included studies for each comparison was insufficient (RevMan 2012).
Sensitivity analysis
When data were sufficient, we planned to perform sensitivity analyses by removing from the analysis studies that were at high risk of bias. However, the number of included studies for each comparisons was insufficient to allow sensitivity analyses to be conducted.
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach to assess the level of certainty of evidence related to each of the primary outcomes (Guyatt 2008; Schünemann 2009). We used the GRADEpro profiler to import data from Review Manager 5 (GRADEpro GDT; RevMan 2012), and we created 'Summary of findings' tables for each comparison. Only the 1 to 6 month (T2) time point is presented for each outcome in the 'Summary of findings' tables, as this approach best shows whether participants have gone into remission (see explanation of time points under Types of outcome measures).
For assessments of the overall certainty of evidence for each outcome that included pooled data, we downgraded the evidence from 'high certainty' by one level for serious (or by two levels for very serious) study limitations (risk of bias), indirectness of evidence (applicability), inconsistency of results (heterogeneity), imprecision of effect estimates (few events or wide confidence intervals), and publication bias.
We used these assessments, along with evidence for absolute benefit or harm of the intervention and the sum of available data on all outcomes from each study included for each comparison, to draw conclusions about the effectiveness of primary‐level workers in providing mental health care in LMICs.
Results
Description of studies
Detailed descriptions of all studies are found in Included studies; Appendix 3; Appendix 4; Appendix 5; Appendix 6; Appendix 7; Appendix 8; Appendix 9; Appendix 10; Appendix 11; Appendix 12; Appendix 13; Appendix 14; Appendix 15; and Appendix 16; these sections contain detailed descriptions of 93 studies, and two studies are described in detail under Effects of interventions.
Results of the search
We included 95 randomised trials in the quantitative synthesis of this treatment review, 23 of which were included in the original review, and we identified 39 ongoing trials (i.e. a total of 134 trials relevant to the treatment review). From our search of all above databases and trial registries on 20 June 2019, we screened 9437 titles and abstracts, of which we sourced 924 full texts to check their eligibility (Figure 3). We performed a second search on 20 August 2020 and identified 16 studies that are awaiting classification. We updated the number of ongoing studies to 50.
Included studies
Study design
Of the 95 included studies, 67 were randomised trials and 28 were cluster‐randomised trials.
Setting
Of the 95 included studies, 25 were conducted in 13 low‐income countries: Afghanistan (one study), Bosnia (one study), Burundi (one study), China (one study), Democratic Republic of Congo (four studies), India (one study), Kenya (two studies), Nepal (two studies), Pakistan (two studies), Rwanda (one study), Sierra Leone (one study), Uganda (five studies), and Zimbabwe (three studies); and 71 included studies were conducted in 21 middle‐income countries: Brazil (three studies), Chile (three studies), China (nine studies), Colombia (one study), Egypt (one study), India (ten studies), Indonesia (two studies), Iran (four studies), Iraq (three studies), Kenya (three studies), Kosovo (one study), Malaysia (one study), Nigeria (four studies), Pakistan (five studies), Palestinian Territories (two studies), Russia (one study), South Africa (nine studies), Sri Lanka (two studies), Thailand (three studies), Vietnam (three studies), and Zambia (one study). All studies except one were based in just one country. The exception was Humeniuk 2012, which was a multi‐site study across different countries including Brazil and India that was performed at a time when India was a low‐income country.
In this section, as well as in the following sections (participants, interventions, etc.), the number of studies when added up may exceed 95, as some studies belonged to more than one category. There were 41 studies from rural, 65 from urban, and five from refugee camp settings. Most interventions were delivered at primary healthcare (PHC) centres (39 studies). Others were delivered at community areas/centres (21 studies), at home (21 studies), in schools or universities (14 studies), at other health clinics (five studies), or online (two studies).
Participants
There were 80 studies that included adults and 17 that included children. Of the 17 studies that included children, four included children up to the age of 12 years, five focused on adolescents aged 12 to 17 years, one included young adults up to age 24 years, five included pre‐teens and/or younger adolescents aged 8 to 15 years, and two included children over a wider age range of 5 to 18 years.
Conditions
Most studies covered post‐traumatic stress or common mental disorders in humanitarian settings (34 studies) and common mental disorders (23 included depression and anxiety). Seventeen studies covered alcohol and substance use disorders, nine covered severe mental disorders (schizophrenia, bipolar disorder, and schizoaffective disorder), nine covered perinatal depression, two covered dementia, and two covered other childhood diagnoses (autism spectrum disorder and attention‐deficit hyperactivity disorder). See Effects of interventions and Appendix 3 through Appendix 16 for details of these by analysis groups.
Interventionists
Various cadres of primary‐level workers were used: lay health workers (68 studies), doctors (24 studies), nurses (22 studies), teachers (six studies), social workers (five studies), and other primary health professionals such as midwives, physician assistants, and pharmacists (eight studies). The educational level of LHWs was documented in 34 studies: 15 selected LHWs with a minimum of high school education, 12 selected LHWs with a minimum of secondary school education, six included LHWs who had college degrees or diplomas, and one included LHWs who had primary school education. Remuneration was generally poorly described. The training and supervision of these providers are described in detail under Included studies and in Appendix 3 through Appendix 16.
Interventions
In 45 studies, combinations of different types of interventions were used. In 22 studies, pharmacotherapy was provided as well as follow‐up to check adherence, the effects of medication, and side effects, and was provided by a doctor (15 studies), a nurse/clinical officer (four studies), or an LHW (three studies). The drugs used were typically amitriptyline or fluoxetine according to WHO guidelines for depression, although two studies used sertraline at a starting dose of 50 mg or 25 mg instead (Chen 2015; Indu 2018); fluoxetine 20 to 40mg per day or sertraline 50 to 100 mg per day for perinatal depression (Rojas 2007); and antipsychotics for severe mental disorders, such as depot antipsychotics (Malakouti 2015), equivalents of chlorpromazine up to 238 mg ± 9.67 mg per day (Tan 2005), or equivalents of perphenazine up to 29.05 ± 9.83 mg per day (Li 2002), with one study adding on sulpiride up to 200 mg per day for depressed mood, apathy, or withdrawal and alprazolam for poor sleep (Li 2002). In 63 studies, some form of psychosocial intervention was provided such as psychoeducation, various support and general counselling/coping skills interventions, and stimulation programmes for children. One study utilised a day rehabilitation set‐up, and one utilised a clubhouse model set‐up to deliver psychosocial interventions (Shen 2016). In 66 studies, specific psychological interventions were used on their own or as part of a collaborative care model: nine used motivational interviewing, five used problem‐solving therapy, 13 used cognitive‐behavioural therapy (CBT), six used interpersonal therapy (IPT), six used behavioural activation, nine used other methods, and 20 used combinations of methods; one studied cognitive processing therapy versus a transdiagnostic intervention versus control (Weiss 2015), and one studied motivational interviewing with or without problem‐solving therapy versus control (Sorsdahl 2015). One study used alternative therapies (yoga) in combination with psychosocial interventions (Niemi 2016). No studies examined detection by PWs, and none reported relevant health worker outcomes. More details on these are provided under Effects of interventions and in Appendix 3 through Appendix 16.
Economic studies
Of the 20 studies that included economic evaluations, 13 were cost‐effectiveness studies (Araya 2006, reference in Araya 2003; Barfar 2017; Burtoff 2013, reference in Patel 2010; Fuhr 2019; Gureje 2019 (EXPONATE); Gureje 2019 (STEPCARE); Lund 2020; Malakouti 2015; Nadkarni 2017; Nadkarni 2019; Patel 2017; Sikander 2019; Sorsdahl 2015; Tan 2005), one combined cost‐effectiveness and cost‐utility analysis (Chatterjee 2014), two were cost‐benefit analyses (Barron 2013; Galarraga 2017, reference in Papas 2020), and three were cost analyses (Li 2002; Tol 2008; Tol 2012).
Excluded studies
We excluded 790 studies: 695 were of interest but were excluded due to specific participants, interventions, comparators, and outcomes (PICOS) not met (documented in Characteristics of excluded studies and in Figure 3 in the PRISMA flow chart), including 12 studies from the previous review, the most common reason being wrong intervention (e.g. interventions not performed by or not involving PWs), followed by wrong patient population (prevention trials or non‐primary care populations), followed by wrong setting (conducted in HICs) and wrong study design (not randomised trials or cluster‐randomised trials). Three other studies that were included in the 2013 review were moved to the prevention review, as the more stringent criteria of participants for this review (given splitting of the review into separate prevention and treatment reviews) were not met. Of the remaining studies, 45 were excluded because they were duplicate records of a study that had already been excluded or included, 40 were excluded because we had insufficient information despite our best efforts to classify them (e.g. trial registry entries with incomplete information and with no published findings, 2 to 3 attempts to contact study authors for clarification failed), four were withdrawn, and three were retracted.
We identified 13 studies in the 2013 review that included economic data on mental disorders and distress but were not linked to studies included in this review. These are summarised in Appendix 17.
Risk of bias in included studies
The most often identified biases across studies were lack of blinding of outcome assessment, incomplete outcome (efficacy) data, and insufficient protection against contamination (Figure 4; Figure 5).
4.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
5.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Six studies had high risk of bias with regards to allocation sequence generation. Although they were randomised, they sometimes used alternate allocation of randomised people or other potentially quasi‐randomised methods. However due to some randomisation featuring in the allocation sequence generation, it was agreed to include these studies and downgrade them accordingly. Four utilised an alternating or planned sequence to allocate participants (Ayoughi 2012; Connolly 2011; Jiang 2017; Neuner 2008); one randomised interventionists who then selected a pool of potential participants on whom screening for eligibility was performed (Barron 2013); and one used a combination of random and non‐random sequence generation (Sherman 2009). For 14 studies, risk of bias was unclear because the method of random sequence generation was not described. A total of 74 studies had low risk of bias.
For allocation concealment, 57 studies had low risk of bias. Eight studies had high risk of bias, as they explicitly did not conceal allocation. For 30 studies, risk of bias was unclear due to poor reporting.
We judged four studies to be at high risk of bias due to unequal baseline characteristics between groups (Bolton 2007; Neuner 2008; Peltzer 2019; Sherman 2009). For three studies, risk of bias was high due to unequal baseline measurements of outcomes (Barron 2013; Pradeep 2014; Sherman 2009). These possibly reflected lack of adequate randomisation. Risk of bias due to unequal baseline characteristics was low in 77 studies and was unclear in 14 studies. Risk of bias due to unequal baseline measurements of outcomes was low in 82 studies and was unclear in 10.
Blinding
When assessing risk of performance bias, we recognised the difficulty of blinding participants and personnel in studies employing psychosocial or psychological interventions. For two studies, risk of bias was high, as lack of blinding of participants and personnel was likely to have influenced the results (Neuner 2008; Xie 2019). For 32 studies, although there was lack of blinding of participants and personnel, it is unclear how this could have influenced results. In the remaining 61 studies, risk of bias was low because there was blinding of participants and personnel, and lack of blinding was unlikely to have influenced the outcome. Almost two‐thirds of studies blinded outcome assessors to participants' allocated intervention group. For 30 studies, risk of bias was high, as there was no blinding of outcome assessments. Of the remaining studies, risk of bias was low in 58 studies and was unclear in seven studies because blinding of assessors was not described or was incomplete, or because unmasking was thought to have occurred.
Incomplete outcome data
We considered incomplete outcome data separately for efficacy and for adverse outcomes. Studies were deemed at high risk of attrition bias if they had greater than 20% attrition in any arm, or, in the case of Dybdahl 2001, an unreported number of participants participated in outcome measures at each time point. For 59 studies, efficacy data were complete; however for 20 studies, this was unclear, and 16 had high risk of bias due to high rates of attrition (Adewuya 2019; Ali 2003; Arjadi 2018; Bonilla‐Escobar 2018; Bryant 2017; Chen 2015; Connolly 2011; Dawson 2016; Dybdahl 2001; Indu 2018; Malakouti 2015; Neuner 2008; Noknoy 2010; Peltzer 2019; Petersen 2014; Pradeep 2014). For 35 studies, safety data were complete. Three studies had high risk of incompleteness of safety data due to high attrition rates (Chen 2015; Indu 2018; Malakouti 2015); for eight studies, it is unclear whether attrition resulted in incomplete safety data because of incomplete adverse event reporting or loss of records (Araya 2003; Bass 2013; Chibanda 2014; Dias 2008; Gureje 2019 (EXPONATE); Li 2018; Oladeji 2015; Weiss 2015); in about half of studies (49), adverse events were not monitored, rendering this bias category "not applicable" (Figure 4; Figure 5). This made analysis of adverse outcomes difficult for most comparisons.
Selective reporting
For 43 studies, there appeared to be no selective reporting based on outcomes listed in the associated published protocols and on contact with authors where there was doubt. Nine studies were at high risk of bias due to selective reporting when outcomes that were planned in published trial protocols were not reported in the final publication (Arjadi 2018; Bryant 2017; Chatterjee 2014; Dawson 2016; Dias 2008; Indu 2018; Lund 2020; Peltzer 2019; Zhong 2015). For 43 studies, the risk of selective reporting was not clear (see Characteristics of included studies tables).
Other potential sources of bias
Risk of contamination was quite common among studies. We assessed 31 studies as having unclear risk because insufficient information was available regarding whether contamination across groups was likely, and conclusive information on this could not be obtained from study authors. We assessed an additional ten studies as being at high risk of contamination (Abas 2018; Araya 2003; Berger 2009; Bolton 2007; Bolton 2014 (Iraq); Bolton 2014 (Thailand); Khan 2017; Neuner 2008; Sherman 2009; Weiss 2015). Contamination was documented in Abas 2018 (a number of control participants received the intervention due to concerns about their mental health amongst the staff) or was thought to be likely, as in Berger 2009, in which all teachers had received training and students assigned to the control group may have inadvertently received elements of the intervention; or was thought to be highly possible, as in Bolton 2007, in which recipients in both groups lived close to one another and may have shared information and skills acquired during the intervention.
Other sources of bias that were detected included (1) control and intervention arms potentially delivered interventions that were too similar, as mentioned by the study authors (Sherman 2009); (2) sources of bias related to statistical analysis due to lack of correction for clustering (Berger 2009); and (3) conduct of multiple analyses that were not pre‐specified (Dybdahl 2001).
Possible conflicts of interest were declared in a small number of studies. These were related to receiving royalties for books on treatment of psychological difficulties (Abas 2018); receiving payments for conducting workshops (Araya 2003), lectures (Arjadi 2018; Nadkarni 2017 ‐ unrelated to study; Patel 2017 ‐ unrelated to study), or training sessions (Arjadi 2018); travel expenses (Arjadi 2018; Bass 2013); and receiving expenses, payments, research fellowships, or research grants from the funding source (Araya 2003; Nadkarni 2017; Patel 2010; Patel 2017). As each of these studies had been assessed for risk of bias by the same process that was used in the other studies, these possible conflicts of interest were deemed to have no significant impact on the results of this review.
Economic studies ‐ risk of bias assessment with adapted CHEC list criteria
All studies had some significant risks of bias (Table 18), although we considered no study to be at high risk of bias on more than seven of the 24 adapted CHEC list criteria. The risk of identified biases was potentially important for interpretation of costing, such as not discounting costs in all but one study (Galarraga 2017, reference in Papas 2020), not conducting sensitivity analysis in all except Barfar 2017, not including appropriate costs or outcomes, and not valuing some outcomes appropriately. In one study (Galarraga 2017, reference in Papas 2020), this occurred because study authors presented modelled costs rather than actual costs.
3. Risk of bias economic studies ‐ CHEC list criteria.
| Study | Risk of bias issues |
| PW‐led care for adults with common mental disorders | |
|
Gureje 2019 (STEPCARE) |
No discounting Time horizon = 1 year Not explicitly stated perspective/viewpoint |
| Araya 2003/Araya 2006 | No discounting Time horizon < 1 year A societal perspective would have been more appropriate Not all relevant costs reported Not all relevant outcomes included (only ambulatory, not hospital) |
| Patel 2017 | No discounting No sensitivity analysis |
| Patel 2010/Buttorff 2012 | No discounting Time horizon < 1 year |
| PW‐led care for women with maternal depression | |
|
Lund 2020 |
No discounting Time horizon = 1 year |
|
Gureje 2019 (EXPONATE) |
No discounting Not all outcomes are appropriately valued Time horizon = 1 year |
|
Fuhr 2019 |
No discounting Not all outcomes are appropriately valued No source of data mentioned Time horizon = 1 year |
|
Sikander 2019 |
No discounting Time horizon < 1 year |
| PW‐led care for adults with alcohol or substance use disorders | |
|
Nadkarni 2019 |
No discounting Time horizon = 1 year |
|
Sorsdahl 2015 /Dwommoh 2018 |
No discounting Time horizon < 1 year |
|
Papas 2020 /Galarraga 2017 |
No discussion of ethical and distribution issues |
|
Nadkarni 2017 |
No discounting Time horizon = 1 year |
| PW‐led care for adults with severe mental disorders | |
| Tan 2005 | No discounting No sensitivity analysis No incremental analysis of costs performed No discussion of conflicts of interests Not all outcomes are appropriately valued No source of data mentioned |
|
Barfar 2017 |
No discounting No discussion of conflicts of interests Not all outcomes are appropriately valued |
|
Li 2002 |
No discounting No sensitivity analysis No incremental analysis of costs performed No discussion of conflicts of interests Not all outcomes are appropriately valued No source of data mentioned Time horizon < 1 year |
|
Malakouti 2015 |
No discounting No sensitivity analysis Time horizon = 1 year |
|
Chatterjee 2014 |
No discounting No sensitivity analysis No source of data mentioned |
| PW‐led care for children and adolescents with PTS and CMDs | |
|
Barron 2016 |
No discounting No sensitivity analysis No incremental analysis of costs performed No discussion of conflicts of interests No source of data mentioned |
|
Tol 2008 /Jordans 2011 |
No discounting No sensitivity analysis No incremental analysis of costs performed No perspective/viewpoint explicitly stated |
| Tol 2012 /Jordans 2011 | No discounting No sensitivity analysis No incremental analysis of costs performed No perspective/viewpoint explicitly stated |
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9; Table 10; Table 11; Table 12; Table 13; Table 14; Table 15
Summary of findings 1. Lay health worker‐led psychological interventions compared to usual care in treating common mental disorders in adults in low‐ and middle‐income countries.
| What are the effects of lay health worker‐led psychological interventions vs usual care in treating common mental disorders (CMDs) in low‐ and middle‐income countries? | ||||||
| Patient or population: adults with common mental disorders (CMDs) Setting: low‐ and middle‐income countries (Brazil (1 study), India (1 study), Vietnam (1 study), Zimbabwe (2 studies)) Intervention: lay health worker (LHW)‐led psychological interventions Comparison: usual care (including 1 of the following: routine primary care, HIV care, nurse‐led psychoeducation, antidepressants as needed, and referral to mental health specialists) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | №. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with usual care in CMD | LHW‐led psychological interventions | |||||
|
Recovery from CMDs ‐ intermediate term (1 to 6 months post intervention) Recovery defined by HDRS < 8a; SRQ‐20 ≤ 7b (RR > 1 denotes greater likelihood of recovery) |
456 per 1000 participants | 593 per 1000 participants (488 to 721) | RR 1.29 (1.06 to 1.56) | 308 (2 RCTs) | ⊕⊕⊝⊝ LOWc | LHW‐led psychological interventions may increase intermediate‐term recovery from CMDs (1 to 6 months post intervention) compared to usual care |
|
Prevalence of CMDs ‐ intermediate term (1 to 6 months post intervention) Diagnosis defined by SRQ‐20 > 7b; PHQ‐9 ≥ 11d (RR < 1 denotes lower prevalence compared to control) |
463 per 1000 participants | 195 per 1000 participants (83 to 445) | RR 0.42 (0.18 to 0.96) | 479 (2 RCTs) | ⊕⊕⊝⊝ LOWe | LHW‐led psychological interventions may reduce the prevalence of CMDs (at 1 to 6 months post intervention) compared to usual care |
|
Severity of CMD symptoms (including anxiety and depression) ‐ intermediate term (1 to 6 months post intervention) Measured by PHQ‐9a,d,f; SRQ‐20b (higher score, higher severity) |
Mean SRQ‐20 score with usual care was 7.5b | Mean SRQ‐20 score in the intervention group was 2.66 (0.72 to 4.55) lower | 798 (4 RCTs) | ⊕⊕⊝⊝ LOWg | Scores estimated based on an SMD of ‐0.59 (95% CI ‐1.01 to ‐0.16). LHW‐led psychological interventions may reduce symptom severity of CMDs (at 1 to 6 months post intervention) compared to usual care | |
|
Quality of life ‐ intermediate term (1 to 6 months post intervention) Measured by EQ‐5D (higher score = better quality of life) |
Mean EQ‐5D score with usual care was 0.72d | Mean EQ‐5D score in the intervention group was 0.17 (0.11 to 0.23) lower | 521 (1 RCT)d | ⊕⊕⊝⊝ LOWh | Scores estimated based on an SMD of 0.51 (95% CI 0.34 to 0.69). LHW‐led psychological interventions may improve the quality of life of people with CMDs (at 1 to 6 months post intervention) compared to usual care | |
|
Functional impairment/disability ‐ intermediate term (1 to 6 months post intervention) Measured by WHODAS 2.0 (higher score = higher disability) |
Mean WHODAS 2.0 score with usual care was 21.0b | Mean WHODAS 2.0 score in the intervention group was 3.9 (6.6 to 1.25) lower | SMD ‐0.47 (‐0.8 to ‐0.15) | 1399 (3 RCTs)b,d,i | ⊕⊕⊝⊝ LOWj | Scores estimated based on an SMD of ‐0.47 (95% CI ‐0.8 to ‐0.15). LHW‐led psychological interventions may slightly reduce functional impairment in people with CMDs (at 1 to 6 months post intervention) compared to usual care |
| Service utilisation (1 to 6 months post intervention) | In Patel 2017 RCT India, there were 7/248 (3%) unplanned hospitalisations | In Patel 2017 RCT India, there were 3/245 (1%) unplanned hospitalisations | RR 0.43 (0.11 to 1.66) | 493 (1 RCT) | ⊕⊝⊝⊝ VERY LOWk | It is uncertain whether LHW‐led psychological interventions have any effect on unplanned hospitalisations in people with CMDs (at 1 to 6 months post intervention) compared to usual care |
|
Adverse events RR < 1 indicates lower risk of adverse events |
In Chibanda 2016, N = 32 (12.3%) were identified as having suicidal ideation In Patel 2017, there were no deaths (0%) and 3 (1%) were identified as having a suicide attempt For Murphy 2020, the committee met 3 times during the trial and identified no concerns regarding safety or adverse events |
In Chibanda 2016, N = 6 (2.3%) were identified as having suicidal ideation In Patel 2017, 2 (1%) deaths occurred, and 4 (2%) were identified as having a suicide attempt |
RR (suicide attempt/ideation) 0.29 (0.14 to 0.58) | 1014 (2 RCTs d,i) | ⊕⊕⊝⊝ LOWl | LHW‐led psychological interventions may reduce suicide ideation or attempts in people with CMDs (at 1 to 6 months post intervention) compared to usual care |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CMD: common mental disorder; EQ‐5D: EuroQol‐5D; HDRS: Hamilton Depression Rating Scale; LHW: lay health worker; PHQ‐9: Patient Health Questionnaire‐9; RCT: randomised controlled trial; RR: risk ratio; SMD: standardised mean difference; SRQ‐20: Self‐Reporting Questionnaire 20‐Item; WHODAS 2.0: World Health Organization Disability Assessment Score 2.0. | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aMatsuzaka 2017. Trained community health workers delivered interpersonal counselling to intervention participants. Control participants were referred to specialised public mental health care, facilitated by research psychologists.
bMurphy 2020. Trained lay health workers delivered cognitive‐behavioural therapy to intervention participants. Primary care providers delivered regular medical care to control participants.
cDowngraded by one level for indirectness: the estimate is determined by only one study (Murphy 2020) due to the very small sample size of the other study. Downgraded by one level for imprecision due to low event number and small total number of participants.
dChibanda 2016. Trained lay health workers delivered problem‐solving therapy to intervention participants. Control participants received nurse‐led care including psychoeducation and antidepressants when necessary.
eDowngraded by one level for imprecision due to low event number and small total number of participants. Downgraded by one level for unexplained inconsistency (I² = 87%): a variety of comparison group interventions were evaluated amongst a relatively small number of trials, limiting our ability to draw conclusions.
fAbas 2018. Trained antiretroviral therapy adherence counsellors delivered problem‐solving therapy. Control participants received routine HIV care.
gDowngraded by one level for unexplained inconsistency (I² = 83%): a variety of comparison group interventions were evaluated amongst a relatively small number of trials, limiting our ability to draw conclusions. Downgraded by one level for imprecision: the estimated effect size ranges from showing important benefits of LHW‐led interventions to LHW‐led interventions having no clinical effect compared to usual care. Note that a small clinically appreciable benefit was set at SMD 0.2 to 0.5, a moderate benefit at SMD 0.5 to 0.8, and a large benefit at > 0.8 (Cohen 1988).
hDowngraded by one level for indirectness: evidence was derived from one trial only. Downgraded by one level for imprecision: the estimated effect size ranges from showing a small clinical effect to a moderate clinical effect.
iPatel 2017. Trained lay counsellors delivered a manualised psychological treatment based on behavioural activation. Primary care providers delivered regular medical care to control participants.
jDowngraded by one level for imprecision: the estimated effect size ranges from showing important benefits of LHW‐led interventions to LHW‐led interventions having no clinical effect compared to usual care. Downgraded by one level for unexplained inconsistency (I² = 89%): a variety of comparison group interventions were evaluated amongst a relatively small number of trials, limiting our ability to draw conclusions. Downgraded by one level for indirectness (extensive training of LHW in Patel 2017).
kDowngraded by one level for indirectness: evidence was derived from one trial only. Downgraded by two levels for imprecision: event numbers are very low and the relative risk ratio ranges from favouring LHW‐led interventions to favouring usual care.
lDowngraded by one level for indirectness (extensive training of LHW in Patel 2017). Downgraded by one level for inconsistency (one study showed benefit of LHW‐led interventions, and the other showed no difference).
Summary of findings 2. Primary‐level worker‐led collaborative care compared to usual care in treating common mental disorders (CMDs) in adults in low‐ and middle‐income countries.
| What are the effects of primary‐level worker‐led collaborative care vs usual care in treating common mental disorders in adults in low‐ and middle‐income countries? | ||||||
| Patient or population: adults with common mental disorders Setting: low‐ and middle‐income countries (China (2 studies), Chile (2 studies), Kenya (1 study), India (3 studies), Nepal (1 study), Nigeria (3 studies)) Intervention: primary‐level worker‐led collaborative care Comparison: usual care (1 of the following: encouragement to attend primary health care, continuation of primary health care, primary health care aided by depression guidelines such as mhGAP, monthly symptom review, physical examination and general health education, or referral to mental health specialist) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | №. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with usual care in CMD | PW‐led collaborative care | |||||
|
Recovery from CMD ‐ intermediate term (1 to 6 months post intervention) Recovery defined by GDS ≤ 11a; HDRS < 7b; HDRS < 8c; PHQ‐9 < 6d; PHQ‐9 < 5 or 50% reduction from baselinee (RR > 1 denotes greater likelihood of recovery) |
227 per 1000 participants | 513 per 1000 participants (340 to 778) | RR 2.26 (1.50 to 3.43) | 804 (5 RCTs) | ⊕⊕⊝⊝ LOWf | PW‐led collaborative care may increase intermediate‐term recovery from CMDs (1 to 6 months post intervention) compared to usual care |
|
Prevalence of CMD ‐ intermediate term (1 to 6 months post intervention) Diagnosis defined by presence of ICD‐10 diagnosis on CIS‐Rg; prevalence defined by [Total number ‐ number recovered (recovery defined by HDRS < 8)]c (RR < 1 denotes lower prevalence compared to control) |
426 per 1000 participants |
243 per 1000 participants (136 to 431) |
RR 0.57 (0.32 to 1.01) | 2820 (2 RCTs) | ⊕⊕⊝⊝ LOWh | PW‐led collaborative care may reduce the prevalence of CMDs (at 1 to 6 months post intervention) compared to usual care, although the range where the actual effect may be indicates that it may have little to no effect |
|
CMD symptoms ‐ intermediate term (1 to 6 months post intervention) Measured by change in CIS‐Rg; GHQ‐12i; HDRSc; PHQ‐9e,j,k scores from baseline (greater decline = greater improvement) |
Mean CIS‐R score with usual care was 22.6g | Mean CIS‐R score in the intervention group was 2.5 (4.5 to 0.6) lower | 4419 (6 RCTs) | ⊕⊕⊝⊝ LOWl | Scores estimated based on an SMD of ‐0.35 (95% CI ‐0.63 to ‐0.08). PW‐led collaborative care may slightly reduce the symptoms of CMDs (at 1 to 6 months post intervention) compared to usual care | |
|
Quality of life ‐ intermediate term (1 to 6 months post intervention) EQ‐5Di; SF‐36c,m; WHOQOL‐BREFe,j,n (higher score = better quality of life) |
Mean WHOQOL‐BREF score with usual care was 78.2e | Mean WHOQOL‐BREF score in the intervention group was 3.9 (1.8 to 6.1) higher | 2199 (6 RCTs) | ⊕⊕⊝⊝ LOWo | Scores estimated based on an SMD of 0.34 (95% CI 0.16 to 0.53). PW‐led collaborative care may slightly improve quality of life in people with CMDs (at 1 to 6 months post intervention) compared to usual care | |
|
Functional impairment ‐ intermediate term (1 to 6 months post intervention) WHODAS 2.0e,g,i,j,k (higher score = higher disability) |
Mean WHODAS score with usual care was 19.5j | Mean WHODAS score in the intervention group was 0.8 (1.8 to 0.2) lower | 4216 (5 RCTs) | ⊕⊕⊕⊝ MODERATEp | Scores estimated based on an SMD of ‐0.13 (95% CI ‐0.28 to 0.03). PW‐led collaborative care probably has little to no effect on functional impairment in people with common mental disorders (at 1 to 6 months post intervention) compared to usual care | |
| Service utilisation ‐ referral to mental health team ‐ long term (7 to 12 months post intervention) | In Adewuya 2019, 95/451 participants (21.1%) were referred to the mental health team at 7 to 12 months post intervention | In Adewuya 2019, 44/456 participants (9.6%) were referred to the mental health team at 7 to 12 months post intervention In Oladeji 2015, at 1 to 6 months post intervention, 48/165 (29%) participants were discussed with the primary care physician by telephone, and of these, 17 participants (10.3%) required an in‐person consultation with the primary care physician and 3 (2%) were referred to a psychiatrist |
RR 0.46 (0.33 to 0.64) | 907 (1 RCT) | ⊕⊕⊝⊝ LOWq | PW‐led stepped care interventions may reduce referral to mental health specialists in people with CMDs (at 7 to 12 months post intervention) compared to usual care |
| Adverse events ‐ death | In Adewuya 2019, at 7 to 12 months post intervention, there were 15/451 (3.3%) deaths In Gureje 2019 (STEPCARE), there were 16/456 deaths at 12 months In Jenkins 2013, at 1 to 6 months post intervention, there were 0/475 deaths In Indu 2018r, at less than 1 month post intervention, there were 0/16 serious adverse events In Patel 2010, at 1 to 6 months post intervention, there were 6/1269 deaths. No deaths were from suicide |
In Adewuya 2019, at 7 to 12 months post intervention, there were 3/456 (0.6%) deaths In Gureje 2019 (STEPCARE), there were 17/542 deaths at 12 months In Jenkins 2013, at 1 to 6 months post intervention, there were 0/453 deaths In Indu 2018, at less than 1 month post intervention, there were 0/22 serious adverse events In Patel 2010, at 1 to 6 months post intervention, there were 3/1160 deaths. No deaths were from suicide |
RR 0.63 (0.38 to 1.06) | 5300 (5 RCTs) | ⊕⊝⊝⊝ VERY LOWs | It is uncertain whether PW‐led collaborative care has any effect on deaths in people with CMDs (up to 7 to 12 months post intervention) compared to usual care |
| *The risk in the intervention group (and its 95% confidence interval) is based on the observed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval;CIS‐R: revised Clinical Interview Schedule; CMD: common mental disorder;EQ‐5D: EuroQol‐5D; GDS: Geriatric Depression Scale; GHQ‐12: 12‐Item General Health Questionnaire; HDRS: Hamilton Depression Rating Scale; ICD‐10: International Classification of Diseases, Tenth Edition; PHQ‐9: Patient Health Questionnaire‐9; PW: primary‐level worker; RCT: randomised controlled trial; RR: risk ratio; SF‐36: 36‐Item Short Form Survey; SMD: standardised mean difference; WHODAS 2.0: World Health Organisation Disability Assessment Score 2.0; WHOQOL‐BREF: World Health Organization Quality of Life‐BREF. | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aXie 2019. PW‐led collaborative care including behavioural activation and activity scheduling vs usual care involving symptom review, physical examination, and general health education.
bChen 2015. PW‐led stepped care including psychoeducation and pharmacotherapy vs usual care aided by depression guidelines.
cAraya 2003. PW‐led stepped care including psychological intervention with problem‐solving components and pharmacotherapy vs usual care aided by depression guidelines.
dAdewuya 2019. PW‐led stepped care including problem‐solving therapy and pharmacotherapy vs usual care aided by depression guidelines.
eOladeji 2015. PW‐led stepped care including problem‐solving therapy and pharmacotherapy vs usual care aided by depression guidelines.
fDowngraded by one level due to high risk of bias. There were limitations in study design and execution (Adewuya 2019 ‐ attrition bias, Araya 2003 ‐ contamination bias, Chen 2015 – allocation concealment, outcome assessment bias, and attrition bias, Xie 2019 – performance bias, outcome assessment bias). Downgraded by one level for inconsistency (I² = 67%): a variety of comparison group interventions were evaluated amongst a relatively small number of trials, limiting our ability to draw conclusions.
gPatel 2010. PW‐led stepped care including interpersonal therapy and pharmacotherapy vs usual care aided by depression guidelines.
hAlthough there were study limitations in Araya 2003, study results did not affect the estimate of the effect size. Downgraded by one level for inconsistency (I² = 86%): a variety of comparison group interventions were evaluated amongst a relatively small number of trials, limiting our ability to draw conclusions.
iJenkins 2013. PW‐led collaborative care with psychoeducation and pharmacotherapy vs usual primary health care.
jGureje 2019 (STEPCARE). PW‐led stepped care with problem‐solving therapy and pharmacotherapy vs usual care aided by depression guidelines.
kJordans 2019. PW‐led collaborative care with psychological intervention with problem‐solving therapy and behavioural activation components vs usual care aided by depression guidelines.
lDowngraded by one level for inconsistency (I² = 89%): a variety of comparison group interventions were evaluated amongst a relatively small number of trials, limiting our ability to draw conclusions. Downgraded by one level for imprecision: the estimated effect size ranged from moderate to no clinical effect. Note that a small clinically appreciable benefit was set at SMD 0.2 to 0.5, a moderate benefit at SMD 0.5 to 0.8, and a large benefit at > 0.8 (Cohen 1988).
mFritsch 2007. PW‐led collaborative care with psychoeducation and pharmacotherapy vs usual care aided by depression guidelines.
nPradeep 2014. PW‐led collaborative care with psychoeducation and pharmacotherapy vs usual care (encouraged to attend primary health centre).
oAlthough there were study limitations in Araya 2003 and Pradeep 2014, sensitivity analyses showed that results did not affect the estimate of the effect size. Downgraded by one level for inconsistency (I² = 75%): a variety of comparison group interventions were evaluated amongst a relatively small number of trials, limiting our ability to draw conclusions. Downgraded for imprecision: the estimated effect size ranged from no clinical effect to moderate clinical effect.
pDowngraded by one level for imprecision: the estimated effect size ranged from small to no clinical effect.
qDowngraded by two levels for indirectness, as the outcome was derived from only one study population (Nigerian primary care patients) that may not be sufficiently representative of all LMIC settings, and a specific intervention (stepped care for depression) was used that may not be implement‐able in all LMIC settings.
rIndu 2018. PW‐led collaborative care with cognitive‐behavioural therapy and pharmacotherapy vs usual care (referral to mental health specialists).
sDowngraded by one level for indirectness. In Adewuya 2019, study authors reported that the deaths were not due to study procedures. In Gureje 2019 (STEPCARE), it is unclear whether any of the deaths were related to CMD, although none were attributed to study procedures. In Patel 2010, the causes of deaths were not reported, apart from stating they were not due to suicide. It is not clear whether the deaths were related to CMD. Downgraded by two levels for imprecision, as event numbers were small and the confidence interval of the risk ratio ranged from indicating possible benefit to indicating no effect by LHW‐led collaborative care compared to usual care.
Summary of findings 3. Lay health worker‐led psychosocial interventions compared to enhanced usual care for treating perinatal depression in low‐ and middle‐income countries.
| What are the effects of lay health worker‐led psychosocial interventions vs enhanced usual care for treating perinatal depression in low‐ and middle‐income countries? | ||||||
|
Patient or population: women with perinatal depression Setting: low‐ and middle‐income countries (India (2 studies), Pakistan (2 studies), South Africa (2 studies), Zimbabwe (1 study)) Intervention: lay health worker‐led psychosocial interventions Comparison: enhanced usual care (including routine antenatal care and 1 of the following: increased gynaecologist visits, prevention of mother‐to‐child transmission, health promotion and disease prevention, visits by lay health worker without mental health training, monthly phone calls, pharmacotherapy) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | №. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with enhanced usual care | Risk with LHW‐led psychosocial interventions | |||||
|
Recovery from depression ‐ intermediate term (1 to 6 months post intervention) Recovery defined by Edinburgh Postnatal Depression Scale ≤ 5a; PHQ‐9 < 5b,c; absence of major depression based on DSM‐IV criteriad (RR > 1 denotes greater likelihood of recovery) |
501 per 1000 participants** |
646 per 1000 participants (541 to 772) |
RR 1.29 (1.08 to 1.54) | 1243 (4 RCTs) | ⨁⨁⊝⊝ LOWe | LHW‐led interventions for women with perinatal depression may increase recovery from depression compared to usual care at 1 to 6 months post intervention (**Absolute effects = means of Fuhr 2019 and Sikander 2019, the two largest studies) |
| Prevalence of perinatal depression (1 to 6 months post intervention) | No studies that measured this outcome were identified | |||||
|
Severity of depression symptoms ‐ intermediate term (1 to 6 months post intervention) Edinburgh Postnatal Depression Scalea; Hamilton Depression Rating Scaled,f; PHQ‐9b,c (higher score = higher severity) |
Mean Hamilton Depression Rating Scale score with usual care was 8.7f | Mean Hamilton Depression Rating Scale score in the intervention group was 1.9 (2.7 to 1.0) lower | 1989 (5 RCTs) | ⨁⨁⨁⊝ MODERATEg | Scores estimated based on an SMD of ‐0.26 (95% CI ‐0.37 to ‐0.14). LHW‐led interventions for women with perinatal depression probably slightly reduce perinatal depressive symptoms compared to enhanced usual care at 1 to 6 months post intervention | |
| Quality of life (1 to 6 months post intervention) | No studies that measured this outcome were identified | |||||
|
Functional impairment ‐ intermediate term (1 to 6 months post intervention) Global assessment of functioning scale, WHODAS (higher score = higher functional impairment) |
Mean Global Assessment of Functioning Scale score with usual care was 72f | Mean Global Assessment of Functioning Scale score in the intervention group was 2.7 (4.9 to 0.5) lower | SMD ‐0.23 (‐0.41 to ‐0.04) | 1856 (4 RCTs)b,c,d,f | ⨁⨁⊝⊝ LOWh | Scores estimated based on an SMD of ‐0.23 (95% CI ‐0.41 to ‐0.04). LHW‐led interventions for women with perinatal depression may slightly reduce functional impairment compared with enhanced usual care at 1 to 6 months post intervention |
| Service utilisation ‐ maternal or child hospitalisations ‐ intermediate term (1 to 6 months post intervention) |
Fuhr 2019: 7/140 (5%) including mother and child Sikander 2019: 11/287 (4%) (1 mother, 10 children) |
Fuhr 2019: 7/140 (5%) including mother and child Sikander 2019: 9/283 (3%) (all children) |
RR 1.12 (0.60 to 2.06) | 850 (2 RCTs) |
⨁⊝⊝⊝ VERY LOWi | It is uncertain whether LHW‐led interventions for women with perinatal depression have any effect on hospitalisations compared with enhanced usual care at 1 to 6 months post intervention |
| Adverse events (1 to 6 months post intervention) | No significant harms (Lund 2020). 3 patients discontinued pharmacotherapy due to adverse effects (Chibanda 2014j). Serious adverse events: 24 (19%) (Fuhr 2019) and 47 (16%) (Sikander 2019). No deaths |
Lund 2020 had no adverse events in intervention groups. Chibanda 2014 had no adverse events in intervention groups. Serious adverse events: 27 (17%) (Fuhr 2019); 43 (15%) (Sikander 2019). No deaths. |
No difference in deaths | 1205 (4 RCTs) | Deaths: ⨁⨁⊝⊝ LOWk | LHW‐led interventions for women with perinatal depression may have little or no effect on the risk of deaths compared with enhanced usual care at 1 to 6 months post intervention |
| The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders Fourth Edition; LHW: lay health worker; PHQ‐9: Patient Health Questionnaire‐9; RCT: randomised controlled trial; RR: risk ratio; SD: standard deviation; SMD: standardised mean difference; WHODAS: World Health Organization Disability Assessment Score. | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aPeltzer 2019. LHW‐led structured behavioural prevention of mother‐to‐child transmission (PMTCT) and anxiety‐reduction intervention vs enhanced usual care (PMTCT counselling and physical health promotion/disease prevention).
bFuhr 2019. LHW‐led Thinking Healthy Programme (THP) with cognitive‐behavioural therapy components vs enhanced usual care (seeing a gynaecologist more often).
cSikander 2019. LHW‐led Thinking Health Programme with cognitive‐behavioural therapy components vs enhanced usual care (visits by LHWs without mental health training).
dRahman 2008. LHW‐led Thinking Health Programme with cognitive‐behavioural therapy components vs enhanced usual care (visits by LHWs without mental health training).
eDowngraded by one level for inconsistency (I² = 72%): unexplained statistical heterogeneity. Downgraded by one level for imprecision: effect ranges from no clinical effect to moderate clinical effect. Note that a small clinically appreciable benefit was set at SMD 0.2 to 0.5, a moderate benefit at SMD 0.5 to 0.8, and a large benefit at > 0.8 (Cohen 1988).
fLund 2020. LHW‐led problem‐solving therapy vs enhanced usual care (routine antenatal care + monthly phone calls).
gNot downgraded for limitations in design: Peltzer 2019 had high risk of bias in several domains but contributed little weight in this analysis. Downgraded by one level for imprecision: SMD confidence interval ranges from no clinical effect to small clinical effect.
hDowngraded by one level for inconsistency (I² = 73%): unexplained statistical heterogeneity. Downgraded by one level for imprecision: SMD confidence interval ranges from no clinical effect to small clinical effect.
iDowngraded by one level for inconsistency: one study shows no difference and the other shows more hospitalisations in LHW‐led interventions compared to enhanced usual care. Downgraded by two levels for imprecision: few total events. Risk ratio ranges from clinical effect favouring LHW‐led interventions to favouring enhanced usual care.
jChibanda 2014. LHW‐led problem‐solving therapy vs enhanced usual care (pharmacotherapy). Both groups received PMTCT counselling.
kDowngraded by two levels for imprecision: very few total events.
Summary of findings 4. Primary health professional‐led collaborative care interventions compared to usual or enhanced care in treating perinatal depression in low‐ and middle‐income countries.
| What are the effects of primary health professional‐led collaborative care interventions vs usual or enhanced care in treating perinatal depression in low‐ and middle‐income countries? | ||||||
|
Patient or population: women with perinatal depression Setting: low‐ and middle‐income countries (Chile (1 study), Nigeria(1 study)) Intervention: primary health professional‐led collaborative care interventions Comparison: enhanced usual care (usual care with pharmacological treatment or aided by mental health guidelines) | ||||||
| Outcomes | Anticipated absolute effects*(95% CI) | Relative effect (95% CI) | №. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with enhanced usual care | Risk with primary health professional (PHP)‐led collaborative care interventions | |||||
| Depression recovery ‐ intermediate term (1 to 6 months post intervention) | No studies that reported this outcome were identified | |||||
| Disease prevalence (1 to 6 months post intervention) | No studies that reported this outcome were identified | |||||
|
Depression symptoms ‐ intermediate term (1 to 6 months post intervention) Edinburgh Postnatal Depression Scale score (higher score = higher severity) |
Mean severity of symptoms in the PHP intervention group was 1.6 points lower (3.49 lower to 0.29 higher) compared with enhanced usual care |
MD ‐1.6 (‐3.49 to 0.29) |
230 (1 RCTa) | ⨁⊝⊝⊝ VERY LOWb | It is uncertain whether PHP‐led collaborative care interventions have any effect on symptoms of depression compared with enhanced usual care at 1 to 6 months | |
|
Quality of life ‐ intermediate term (1 to 6 months post intervention) SF‐36 social functioning (higher score = higher quality of life) |
Mean quality of life in the PHP intervention group was 3.5 points higher (4.55 lower to 11.55 higher) compared to enhanced usual care |
MD 3.5 (‐4.55 to 11.55) |
230 (1 RCTa) | ⨁⊝⊝⊝ VERY LOWb | It is uncertain whether PHP‐led collaborative care interventions have any effect on quality of life among women with perinatal depression compared with usual enhanced care at 1 to 6 months post intervention | |
| Functional impairment ‐ intermediate term (1 to 6 months post intervention) | No studies that reported this outcome were identified | |||||
|
Service utilisation ‐ intermediate term (1 to 6 months post intervention) Mean number of medical consultations |
0.4 per person (SD 1.0) | 0.2 per person (SD 0.6) | 0.2 fewer per person (from 0.4 fewer to 0.0) | 230 (1 RCTa) |
⨁⊝⊝⊝ VERY LOWc | It is uncertain whether PHP‐led collaborative care interventions make any difference in the mean number of medical consultations for women with perinatal depression compared with usual enhanced care at 1 to 6 months post intervention |
| Adverse events (7 to 24 months post intervention) | Maternal deaths 0/234 Stillbirths 11/234 (5%) No deaths were judged to be related to the study |
Maternal deaths 3/452 (none were due to suicide) Stillbirths 25/452 (6%) No deaths were judged to be related to the study |
Maternal deaths: 7 more per 1000 participants (from 1 less to 14 more) | 686 (1 RCTd) |
⨁⊝⊝⊝ VERY LOWe | It is uncertain whether PHP‐led collaborative care interventions have any effect on deaths in women with perinatal depression compared with usual enhanced care at 7 to 24 months post intervention |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference;PHP: primary health professional; RCT: randomised controlled trial; SD: standard deviation. | ||||||
|
GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aRojas 2007. PHP‐led pharmacological therapy and group psychosocial therapy vs usual care including pharmacological treatment.
bDowngraded by one level for indirectness: although study population was a very generic intervention in a representative LMIC, this is evidence from just one country. Downgraded by two levels for imprecision: low total numbers and MD confidence interval ranges from significant clinical effect favouring PHP‐led collaborative care to favouring enhanced usual care.
cDowngraded by one level for indirectness: although study population was a very generic intervention in a representative LMIC, this is evidence from just one country. Downgraded by two levels for imprecision: low total numbers.
dGureje 2019 (EXPONATE). PHP‐led stepped care including problem‐solving therapy and pharmacological therapy vs usual care aided by guidelines.
eDowngraded by one level for indirectness. Single study in a single setting. Deaths may be unrelated to perinatal depression. Downgraded by two levels for imprecision: low number of events. Absolute risk reduction confidence interval ranges from favouring low‐intensity intervention (control) to no difference.
Summary of findings 5. Lay health worker‐led psychological interventions compared to usual care in treating adults with post‐traumatic stress or common mental disorders in humanitarian settings in low‐ and middle‐income countries.
| What are the effects of lay health worker‐led psychological interventions vs usual care in treating adults with post‐traumatic stress or common mental disorders in humanitarian settings in low‐ and middle‐income countries? | ||||||
|
Patient or population: adults with post‐traumatic stress and CMDs Setting: in humanitarian settings in low‐ and middle‐income countries (Egypt (1 study), Kenya (2 studies), Pakistan (3 studies), Uganda (2 studies)) Intervention: lay health worker‐led psychological interventions Comparison: usual (including routine antenatal visits or wait‐list control) or enhanced usual care (including 1 or more of the following: single psychoeducation session, care by briefly trained primary healthcare providers without supervision, information on seeking care with primary or tertiary care provider) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | №. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with usual care | Risk with LHW‐led psychological interventions | |||||
| Recovery from PTSD (1 to 6 months post intervention) | No studies that reported on this outcome were identified |
|||||
|
Prevalence of PTSD ‐ intermediate term (1 to 6 months) Diagnosis defined by fulfilment of PTSD diagnostic criteria using the PCL‐5a; fulfilment of DSM‐IV PTSD diagnostic criteria using the CIDIb (RR < 1 denotes lower prevalence compared to control) |
198 per 1000 participants | 152 per 1000 participants (59 to 396) | RR 0.77 (0.30 to 2.00) | 380 (2 RCTs) | ⨁⊝⊝⊝ VERY LOWc | It is uncertain whether LHW‐delivered interventions have any effect on the number of people with PTSD compared to usual care 1 to 6 months post intervention |
|
PTS symptoms ‐ intermediate term (1 to 6 months post intervention) PCL‐5a,d,e; PCL‐6f; Post‐traumatic Stress Diagnostic Scaleb (higher score = higher severity) |
Mean PCL‐6 score with usual care was 17.7f | Mean PCL‐6 score in the intervention group was 1.6 (2.4 to 0.8) lower | 2045 (5 RCTs) | ⨁⊝⊝⊝ VERY LOWg | Scores estimated based on an SMD of ‐0.27 (95% CI ‐0.41 to ‐0.13). It is uncertain whether LHW‐delivered interventions have any effect on PTS symptoms compared to usual care 1 to 6 months post intervention | |
|
Depression symptoms ‐ intermediate term (1 to 6 months post intervention) GHQ‐12a; PHQ‐9d,e,f; self‐reporting questionnaireh (higher score = higher severity) |
Mean PHQ‐9 score with usual care was 10.8f | Mean PHQ‐9 score in the intervention group was 1.8 (2.9 to 0.8) lower | 1986 (5 RCTs) | ⨁⨁⊝⊝ LOWi | Scores estimated based on an SMD of ‐0.36 (95% CI ‐0.56 to ‐0.15). LHW‐delivered interventions may slightly reduce depression symptoms compared to usual care 1 to 6 months post intervention | |
|
Quality of life ‐ intermediate term (1 to 6 months post intervention) PSYCHLOPS (higher score = poorer outcome) |
Mean PSYCHLOPS score with usual care was 13.1f | Mean PSYCHLOPS score in the intervention group was 1.3 (1.9 to 0.7) lower | 1918 (4 RCTa,d,e,f) | ⨁⨁⨁⊝ MODERATEj | Scores estimated based on an SMD of ‐0.27 (95% CI ‐0.39 to ‐0.15). LHW‐delivered interventions probably slightly improve quality of life compared to usual care 1 to 6 months post intervention | |
|
Functional impairment ‐ intermediate term (1 to 6 months post intervention) WHODAS (higher score = more severity impairment) |
Mean WHODAS score with usual care was 17.3f | Mean WHODAS score in the intervention group was 2.3 (3.8 to 0.1) lower | 1914 (4 RCTsa,d,e,f) | ⨁⊝⊝⊝ VERY LOWk | Scores estimated based on an SMD of ‐0.26 (95% CI ‐0.42 to ‐0.1). It is uncertain whether LHW‐delivered interventions have any effect on functional impairment compared to usual care 1 to 6 months post intervention | |
|
Service utilisation ‐ hospital admissions (1 to 6 months post intervention) |
3.07% of people in control group were admitted to hospital | 2.56% of people in the intervention group were admitted to hospital | RR 1.13 (0.53 to 2.34) | 319 (1 RCTa) | ⨁⊝⊝⊝ VERY LOWl | It is uncertain whether LHW‐delivered interventions have any effect on hospital admissions compared to usual care 1 to 6 months post intervention |
| Adverse events | Unknown | No adverse events reported in 4 studies, except 6 (of unknown nature except not to be related to the intervention and not known in which group) inTol 2020 | ‐ | 1701 (5 RCTsa,e,f,m,n) | ⨁⊝⊝⊝ VERY LOWo | It is uncertain whether LHW‐delivered interventions have any effect on adverse events compared to usual care up to 6 months post intervention |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CIDI: Composite International Diagnostic Interview; CMD: common mental disorder; DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders Fourth Edition; GHQ‐12: general health questionnaire 12; LHW: lay health worker; PCL‐5, PCL‐6: Post‐traumatic Stress Diagnostic Scale 5 and 6; PHQ‐9: Patient Health Questionnaire‐9; PSYCHLOPS: psychological outcomes profile; PTS: post‐traumatic stress; PTSD: post‐traumatic stress disorder; RCT: randomised controlled trial; RR: risk ratio; SMD: standardised mean difference; WHODAS: WHO disability assessment scale. | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aBryant 2017. Problem Management Plus vs non‐specific counselling without supervision.
bNeuner 2008. Narrative exposure therapy or trauma counselling vs wait‐list control.
cDowngraded by one level for limitations in design: Bryant 2017 had high risk of attrition and reporting bias. Neuner 2008 had high risk of selection bias (random sequence generation and allocation concealment), unequal baseline characteristics, high risk of attrition bias, and high risk of contamination. Downgraded by one level for imprecision: small sample size and event number; downgraded by one level for inconsistency: unexplained statistical heterogeneity (I² = 84%).
dRahman 2016. Individual Problem Management Plus vs visit and psychoeducation with primary care physician trained for 6 days in community mental health.
eRahman 2019. Group Problem Management Plus vs option for psychoeducation, care by LHWs or by primary healthcare providers briefly (0.5 days) trained in detection and treatment of mental health problems, or care at tertiary centre.
fTol 2020. Self‐Help Plus vs 30‐minute psychoeducation by trained LHW followed by information on accessing mental health specialists or basic psychosocial support by trained LHW.
gDowngraded by one level for inconsistency: mild unexplained statistical heterogeneity (I² = 50%). Downgraded by one level for indirectness: two studies included female and male participants (Neuner 2008Rahman 2016), which if removed, change the estimate to no clinical effect (all others were female only). Downgraded by one level for imprecision: confidence interval of SMD ranges from no clinical effect to small clinical effect favouring LHW‐led interventions. Note that a small clinically appreciable benefit was set at SMD 0.2 to 0.5, a moderate benefit at SMD 0.5 to 0.8, and a large benefit at > 0.8 (Cohen 1988).
hKhan 2017. Psychoeducation vs routine antenatal LHW visits.
iDowngraded by one level for inconsistency: moderate unexplained statistical heterogeneity (I² = 78%). Not downgraded for indirectness: although only Rahman 2016 had both male and female participants (other studies had only female participants), when this study is removed, the overall estimate remains clinically significant. Downgraded by one level for imprecision: confidence interval of SMD ranges from no clinical effect to moderate clinical effect favouring intervention.
jDowngraded by one level for imprecision: confidence interval of SMD ranges from no clinical effect to small clinical effect favouring intervention. Not downgraded for indirectness: although only Rahman 2016 RCT Pakistan had both male and female participants (other studies had only female participants), when this study is removed, the effect estimate still shows clinically important benefit.
kDowngraded by one level for inconsistency: moderate unexplained statistical heterogeneity (I² = 67%). Downgraded by one level for imprecision: confidence interval of SMD ranges from no clinical effect to moderate clinical effect favouring LHW‐led interventions. Downgraded by one level for indirectness: as Rahman 2016 RCT Pakistan had both male and female participants (other studies had only female participants). when this study is removed, SMD changes from slight clinical effect to no or little effect.
lDowngraded by one level for limitations in design: Bryant 2017 had high risk of attrition and reporting bias. Downgraded by one level for indirectness: participants were females who had experienced gender‐based violence. Population may not be generalisable to other populations in LMICs with post‐traumatic stress symptoms or disorder. Downgraded by two levels for imprecision: Low total numbers. Confidence interval of risk ratio ranged from effect favouring usual care to effect favouring LHW‐led interventions.
mDawson 2016. Problem Management Plus vs primary health care by nurses briefly trained in supportive counselling (1 day) without supervision.
nMeffert 2014. Interpersonal therapy vs wait‐list control.
oDowngraded by one level for risk of bias: Bryant 2017 ‐ high risk of reporting bias; Dawson 2016 ‐ high risk of reporting bias; Meffert 2014 ‐ high risk of detection bias. Downgraded by one level for indirectness: Bryant 2017 RCT Kenya; Dawson 2016 RCT Kenya; Rahman 2019 CRCT Pakistan; Tol 2020 CRCT Uganda had only female participants. Downgraded by two levels for imprecision: very low event number.
Summary of findings 6. Primary health professional‐led psychological interventions compared to usual or no care for treating adults with post‐traumatic stress or common mental disorders in humanitarian settings in low‐ and middle‐income countries.
| What are the effects of primary health professional‐led psychological interventions vs usual or no care for treating adults with post‐traumatic stress or common mental disorders in humanitarian settings in low‐ and middle‐income countries? | ||||||
|
Patient or population: adults with post‐traumatic stress or common mental disorders Setting: humanitarian settings in low‐ and middle‐income countries (Democratic Republic of Congo (1 study), Iraq (3 studies), Thailand (1 study)) Intervention: primary health professional‐led psychological interventions Comparison: usual (including 1 of the following: psychosocial support, identification and referral to mental health specialist, monthly follow‐up, poorly accessed counselling service) or no care (wait list) | ||||||
| Outcomes | Anticipated absolute effects*(95% CI) | Relative effect (95% CI) | №. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with usual care | Risk with primary health professional‐led psychological intervention | |||||
| Recovery from PTSD | No studies that reported on this outcome were identified | |||||
|
Prevalence of adults with probable PTSD (1 to 6 months post intervention) Diagnosis defined by HTQ ≥ 1.75 (RR > 1 denotes lower prevalence compared to control) |
417 per 1000 participants |
87 per 1000 participants (15 to 152) |
RR 5.50 (2.50 to 12.10) | 313 (1 RCTa) | ⨁⨁⊝⊝ LOWb | PHPs delivering psychological interventions may reduce the number of people with probable PTSD compared to usual or no care |
|
Prevalence of adults with probable depression or anxiety (1 to 6 months post intervention) Diagnosis defined by HSCL‐25 ≥ 1.75 (RR > 1 denotes lower prevalence compared to control) |
417 per 1000 participants |
87 per 1000 participants (15 to 152) |
RR 4.60 (2.10 to 10.08) | 313 (1 RCTa) | ⨁⨁⊝⊝ LOWb | PHPs delivering psychological interventions may reduce the number of people with depression compared to usual or no care |
|
PTS symptoms (1 to 6 months post intervention) Harvard Trauma Questionnaire (higher score = higher severity) |
Mean HTQ score with usual care was 1.5a | Mean HTQ score in the intervention group was 0.5 (1.0 to 0.1) lower | SMD ‐0.78 (‐1.43 to ‐0.13) | 680 (2 RCTsa,c) | ⨁⊝⊝⊝ VERY LOWd | Scores estimated based on an SMD of ‐0.78 (95% CI ‐1.43 to ‐0.13). It is uncertain whether PHPs delivering interventions have any effect on PTS symptoms compared to usual or no care 1 to 6 months post intervention |
|
Depression symptoms (1 to 6 months post intervention) Hopkins Symptom Checklist ‐ depression (higher score = higher severity) |
Mean HSCL score with usual care was 1.5a | Mean HSCL score in the intervention group was 0.5 (1.0 to 0.1) lower | SMD ‐0.91 (‐1.73 to ‐0.1) | 680 (2 RCTsa,c) | ⨁⊝⊝⊝ VERY LOWe | Scores estimated based on an SMD of ‐0.91 (95% CI ‐1.73 to ‐0.1). It is uncertain whether PHPs delivering interventions have any effect on depression symptoms compared with usual or no care 1 to 6 months post intervention |
| Quality of life | No studies that reported on this outcome were identified | |||||
|
Functional impairment (1 to 6 months post intervention) Locally developed functional impairment scale (higher score = higher functional impairment) |
Mean functional impairment score with usual care was 1.8a | Mean functional impairment score in the intervention group was 0.6 (1.2 lower to 0.04 higher) lower | SMD ‐0.64 (‐1.31 to 0.04) | 680 (2 RCTsa,c) | ⨁⊝⊝⊝ VERY LOWf | Scores estimated based on an SMD of ‐0.64 (95% CI ‐1.31 to 0.04). It is uncertain whether PHPs delivering psychological interventions have any effect on functional impairment 1 to 6 months post intervention compared to usual or no care |
| Service utilisation | 1/66 participants referred to a psychiatrist for worsening symptoms (Bolton 2014 (Iraq)) | 1/223 hospitalised for severe depression and 1/223 self‐referred to a psychiatrist (Weiss 2015) | Similar in both arms | 1572 (5 RCTsa,c,g,h,i) |
⨁⊝⊝⊝ VERY LOWj | It is uncertain whether primary health professionals delivering psychological interventions have any effect on service utilisation up to 6 months post intervention compared to usual or no care |
|
Adverse events (RR > 1 denotes greater risk of harm) |
1/50 deaths (Bolton 2014 (Iraq)) 1 participant died of a heart attack (not stated in which arm) (Weiss 2015, deemed unrelated to study) No adverse events detected (Bass 2013; Bass 2016; Bolton 2014 (Thailand)) |
1/215 participants died; 1/215 participants reported being verbally abused by her husband for getting treatment (Bolton 2014 (Iraq)) 1/223 attempted suicide (Weiss 2015), 1 had a heart attack (not mentioned in which arm; deemed unrelated to study) (Weiss 2015) Bolton 2014 (Thailand); Bass 2013; Bass 2016 1/157 participants died (Bass 2013) 1/182 participants died (Bolton 2014 (Thailand) (deemed unrelated to study)) No adverse events detected (Bass 2016) |
Deaths: RR 2.22 (0.23 to 21.34) | 1242 (4 RCTsa,g,h,i) | ⨁⊝⊝⊝ VERY LOWk | It is uncertain whether PHPs delivering psychological interventions have any effect on adverse events up to 6 months post intervention compared to usual or no care |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HSCL‐25: Hopkins Symptom Checklist ‐ depression; HTQ: Harvard Trauma Questionnaire; PHP: primary health professional; PTS: post‐traumatic stress; PTSD: post‐traumatic stress disorder; RCT: randomised controlled trial; RR: risk ratio; SMD: standardised mean difference. | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aBass 2013. Cognitive processing therapy vs psychosocial support.
bDowngraded by one level for indirectness: results are from a single study done in a low‐income country in which participants were female survivors of sexual violence. Study population may not be generalisable to other adults with PTSD in LMICs. Downgraded by one level for imprecision: low total number.
cWeiss 2015. Cognitive processing therapy vs transdiagnostic Common Elements Treatment Approach (CETA) vs identification and referral.
dDowngraded by one level for limitations in design: high risk of detection bias ‐ Bass 2013 ‐ and contamination ‐ Weiss 2015. Downgraded by one level for inconsistency: large unexplained statistical heterogeneity (I² = 93%). Downgraded by one level for imprecision: confidence interval of SMD ranges from large clinical effect favouring intervention to no effect. Note that a small clinically appreciable benefit was set at SMD 0.2 to 0.5, a moderate benefit at SMD of 0.5 to 0.8, and a large benefit at > 0.8 (Cohen 1988).
eDowngraded by one level for limitations in design: high risk of detection bias ‐ Bass 2013 ‐ and contamination ‐ Weiss 2015. Downgraded by one level for imprecision: confidence interval of SMD ranges from large clinical effect favouring intervention to no effect. Downgraded by one level for inconsistency: large unexplained statistical heterogeneity (I² = 96%).
fDowngraded by one level for limitations in design: high risk of detection bias ‐ Bass 2013 ‐ and contamination ‐ Weiss 2015. Downgraded by one level for imprecision: confidence interval of SMD ranges from large clinical effect favouring intervention to no effect. Downgraded by one level for inconsistency: large unexplained statistical heterogeneity (I² = 94%).
gBass 2016. Locally designed psychological intervention vs wait‐list control.
hBolton 2014 CRCT Iraq. Cognitive processing therapy vs behavioural activation vs monthly follow‐up.
iBolton 2014 RCT Thailand. CETA vs usual care (poorly accessed counselling service).
jDowngraded by one level for risk of bias: high risk of detection bias in Bass 2013 and Bass 2016; high risk of contamination bias in Bolton 2014 (Iraq); Bolton 2014 (Thailand); and Weiss 2015. Downgraded by two levels for imprecision: very few events.
kDowngraded by one level for risk of bias: high risk of detection bias in Bass 2013 and Bass 2016; high risk of contamination bias in Bolton 2014 (Iraq) and Bolton 2014 (Thailand). Downgraded by one level for indirectness: it is unclear if any of the deaths were related to post‐traumatic stress symptoms or study procedures. Downgraded by two levels for imprecision: very few events. The confidence interval of the risk ratio ranged from indicating harm by PHP‐delivered interventions to indicating benefit.
Summary of findings 7. Lay health worker‐led interventions for adult patients with harmful or hazardous alcohol or substance use compared to enhanced usual care in low‐ and middle‐income countries.
| What are the effects of lay health worker‐led interventions vs enhanced usual care for adult patients with harmful or hazardous alcohol or substance use? | ||||||
|
Patient or population: adult patients with harmful or hazardous alcohol or substance use Setting: low‐ and middle‐income countries (Brazil (1 study), Kenya (2 studies), India (1 study), Nepal (1 study), South Africa (2 studies), Thailand (1 study)) Intervention: lay health worker‐delivered psychological interventions Comparison: enhanced usual care (including 1 or more of the following: routine medical care, feedback on score, information leaflet, primary health care aided by mental health guidelines, healthy lifestyle intervention, life skills‐building intervention) | ||||||
| Outcomes | Anticipated absolute effects*(95% CI) | Relative effect (95% CI) | №. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with enhanced usual care | Risk with lay health worker‐led interventions | |||||
|
Clinical recovery ‐ harmful or hazardous alcohol use (1 to 6 months post intervention) Assessed with AUDIT score < 7a/8b/9c; abstinent from alcohold (RR > 1 denotes higher likelihood of recovery) |
540 per 1000 participants | 692 per 1000 participants (508 to 940) | RR 1.28 (0.94 to 1.74) | 872 (4 RCTs) | ⨁⨁⊝⊝ LOWe | LHW‐delivered interventions may increase clinical recovery from harmful or hazardous alcohol use 1 to 6 months post intervention compared with enhanced usual care, although the range where the actual effect may be indicates that lay health workers may have little or no effect |
|
Prevalence of methamphetamine use (1 to 6 months post intervention) (RR < 1 denotes lower prevalence compared to control) |
607 per 1000 participants | 613 per 1000 participants (552 to 686) | RR 1.01 (0.91 to 1.13) | 882 (1 RCT)f | ⨁⨁⊝⊝ LOWg | LHW‐delivered interventions may have little to no effect on prevalence of methamphetamine use at 1 to 6 months post intervention compared to enhanced usual care |
|
Clinical symptoms ‐ alcohol use (1 to 6 months post intervention) ‐ risk of hazardous or harmful alcohol use Assessed with ASSIST scoreh; AUDIT scorea,c (lower score = lower risk) |
Mean AUDIT score with usual care was 3.6c | Mean AUDIT score in the intervention group was 1.4 (2.0 to 0.7) lower | 667 (3 RCTs) | ⨁⨁⨁⊝ MODERATEi | Scores estimated based on an SMD of ‐0.22 (95% CI ‐0.32 to ‐0.11). LHW‐delivered interventions probably slightly reduce risk of harmful or hazardous drinking 1 to 6 months post intervention compared with enhanced usual care | |
|
Clinical symptoms ‐ alcohol and substance use (1 to 6 months post intervention) Assessed with ASSIST score (lower score = lower risk) |
Mean ASSIST score with usual care was 22.4h | Mean ASSIST score in the intervention group was 0.2 (2.6 lower to 2.3 higher) lower | 540 (2 RCTs)h,j | ⨁⨁⨁⊝ MODERATEk | Scores estimated based on an SMD of ‐0.01 (95% CI ‐0.15 to 0.13). LHW‐delivered interventions probably have little to no effect on drug and alcohol use 1 to 6 months post intervention compared with enhanced usual care | |
| Quality of life | No studies that reported on this outcome were identified | |||||
|
Functional impairment (1 to 6 months post intervention) Assessed with WHODAS II score (lower score = lower functional impairment) |
Mean WHODAS score with usual care was 3.5b | Mean WHODAS score in the intervention group was 0.7 (1.7 lower to 0.2 higher) lower | 498 (2 RCTs)b,c | ⨁⨁⊝⊝ LOWl | Scores estimated based on an SMD of ‐0.14 (95% CI ‐0.32 to 0.03). LHW‐delivered interventions may have little to no effect on functional impairment 1 to 6 months post intervention compared to enhanced usual care | |
|
Service utilisation ‐ unplanned hospitalisations (1 to 6 months post intervention) (RR < 1 denotes lower risk) |
41 per 1000 participants | 37 per 1000 participants (13 to 107) | RR 0.90 (0.29 to 2.72) | 336 (1 RCT)b | ⨁⊝⊝⊝ VERY LOWm | It is uncertain whether LHW‐delivered interventions have any effect on unplanned hospitalisations 1 to 6 months post intervention compared with enhanced usual care |
|
Adverse events ‐ deaths (RR < 1 denotes lower risk) |
20 per 1000 participants | 7 per 1000 (2 to 23) | RR 0.34 (0.10 to 1.18) | 1025 (3 RCTs)b,d,n | ⨁⊝⊝⊝ VERY LOWo | It is uncertain whether LHW‐delivered interventions have any effect on deaths up to 12 months post intervention compared to enhanced usual care |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ASSIST: Alcohol, Smoking and Substance Involvement Screening Test;AUDIT: Alcohol Use Disorders Identification Test; CI: confidence interval; LHW: lay health worker; LMICs: low‐ and middle‐income countries; MD: mean difference; RCT: randomised controlled trials; RR: risk ratio; SMD: standardised mean difference; WHODAS: World Health Organization Disability Assessment Scale; WHODAS II: World Health Organization Disability Assessment Schedule 2.0. | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
a Peltzer 2013 CRCT South Africa. Brief intervention vs an information leaflet.
b Nadkarni 2017 RCT India. A manualised psychological intervention (“Counselling for Alcohol Problems”) vs WHO mhGAP enhanced usual care.
c Jordans 2019 RCT Nepal. A manualised psychological intervention (“Counselling for Alcohol Problems”) vs WHO mhGAP enhanced usual care.
d Papas 2011 RCT Kenya. Cognitive‐behavioural therapy vs routine medical care.
e Downgraded by one level for inconsistency: large unexplained statistical heterogeneity (I² = 81%). Downgraded by one level for imprecision: confidence interval of the risk ratio ranges from no clinical effect to favouring intervention.
fSherman 2009 RCT Thailand. Peer education with a social network vs life skills‐building intervention.
g Downgraded by one level for indirectness: one study in a single setting. Downgraded by one level for risk of bias: high risk of selection (random sequence generation) and detection bias, unequal baseline characteristics and outcome measures, and high risk of contamination.
hChristoff 2015 RCT Brazil. Brief intervention vs feedback on ASSIST score.
i Downgrade by one level for imprecision: confidence interval of SMD ranges from no clinical effect to small clinical effect favouring intervention. Note that a small clinically appreciable benefit was set at SMD 0.2 to 0.5, a moderate benefit at SMD 0.5 to 0.8, and a large benefit at > 0.8 (Cohen 1988).
jSorsdahl 2015 RCT South Africa. Brief intervention vs brief intervention blended with problem‐solving therapy vs an information brochure.
k Downgraded by one level for indirectness: Christoff 2015 studied university students, and Sorsdahl 2015 studied patients attending primary healthcare or emergency departments; both were performed in middle‐income countries.
l Downgraded by one level for indirectness. In Jordans 2019 and Nadkarni 2017, interventionists received intense training, which may not be scalable to other settings in LMICs. Downgraded by one level for imprecision: Confidence interval of SMD ranges from no clinical effect to small clinical effect favouring intervention.
m Downgraded by one level for indirectness: interventionists received intensive training. Downgraded by two levels for imprecision: very few event numbers.
nPapas 2020 in press RCT Kenya. Cognitive‐behavioural therapy vs healthy lifestyle education intervention.
o Downgraded by one level for indirectness: Papas 2011 and Papas 2020 studied HIV patients; Nadkarni 2017 studied patients in primary health care. All three delivered intensive interventions by interventionists who had received intensive training. Downgraded by two levels for imprecision: very low event numbers.
Summary of findings 8. Primary health professional‐ and community professional‐led interventions compared to enhanced usual care for adult patients with harmful or hazardous alcohol or substance use in low‐ and middle‐income countries.
| What are the effects of primary health professional‐ and community professional‐led interventions vs enhanced usual care for adult patients with harmful or hazardous alcohol or substance use? | ||||||
|
Patient or population: adult patients with harmful or hazardous alcohol or substance use Setting: low‐ and middle‐income countries (Brazil (1 study), India (1 study), South Africa (3 studies), Thailand (1 study)) Intervention: primary health professionals (PHPs) (5 studies) and community professionals (CPs) (1 study) delivering psychological interventions Comparison: enhanced usual care (including 1 or more of the following: questionnaire, feedback on score, information leaflet, resource list) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | №. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with enhanced usual care | Risk with PHP‐ or CP‐led interventions | |||||
|
Clinical recovery ‐ harmful or hazardous alcohol use (1 to 6 months post intervention) Assessed with change to low risk score on AUDITa,b/ASSISTc (RR > 1 denotes higher likelihood of recovery) |
412 per 1000 participants | 383 per 1000 participants (317 to 461) | RR 0.93 (0.77 to 1.12) | 1075 (3 RCTs) | ⨁⨁⨁⊝ MODERATEd | PHP‐ or CP‐delivered interventions probably have little to no effect on the likelihood of recovery from harmful or hazardous alcohol use 1 to 6 months post intervention compared to enhanced usual care |
|
Prevalence of cannabis use (1 to 6 months post intervention) (RR < 1 denotes lower prevalence compared to control) |
282 per 1000 participants | 310 per 1000 participants (189 to 507) | RR 1.10 (0.67 to 1.80) | 152 (1 RCT)b | ⨁⊝⊝⊝ VERY LOWe | It is uncertain whether PHP‐ or CP‐delivered interventions have any effect on the prevalence of cannabis use 1 to 6 months post intervention compared to enhanced usual care |
|
Clinical symptoms ‐ risk of harmful or hazardous drinking (1 to 6 months post intervention) Assessed with AUDIT scorea,b; ASSIST scorec (lower score = lower risk) |
Mean ASSIST score with usual care was 9.1c | Mean ASSIST score in the intervention group was 1.5 (2.8 to 0.3) lower | 1075 (3 RCTs) | ⨁⨁⊝⊝ LOWf | Scores estimated based on an SMD of ‐0.15 (95% CI ‐0.27 to ‐0.03). PHP‐ or CP‐delivered interventions may slightly reduce risk of harmful or hazardous drinking at 1 to 6 months post intervention compared to enhanced usual care | |
|
Clinical symptoms ‐ overall risk of harmful or hazardous alcohol and substance use (1 to 6 months post intervention) Assessed with ASSIST score (lower score = lower risk) |
Mean ASSIST score with usual care was 15.1c | Mean ASSIST score in the intervention group was 3.2 (5.6 to 0.8) lower | 705 (2 RCTs)c,g | ⨁⨁⨁⊝ MODERATEh | Scores estimated based on an SMD of ‐0.20 (95% CI ‐0.35 to ‐0.05).PHP‐ or CP‐delivered interventions probably slightly reduce overall risk of harmful or hazardous alcohol and substance use 1 to 6 months post intervention compared to enhanced usual care | |
|
Quality of life (1 to 6 months post intervention) Assessed with WHOQOL‐HIV BREF (higher score = higher quality of life) |
Mean quality of life score in this PHP intervention was 0 points (i.e. no different) (0.1 lower to 0.1 higher) compared to enhanced usual care | MD 0.00 (‐0.10 to 0.10) | 560 (1 RCT)a | ⨁⨁⨁⊝ MODERATEi | PHP‐ or CP‐delivered interventions probably have little to no effect on quality of life 1 to 6 months post intervention compared to enhanced usual care | |
| Functional impairment | No studies that reported on this outcome were identified | |||||
|
Service utilisation (1 to 6 months post intervention) ‐ incidence of visits to primary care centres due to alcohol consumption
(RR > 1 denotes greater risk) |
3 out of 51 | 0 out of 56 | RR 0 (0.01 to 2.5) | 107 (1 RCT)j |
⨁⊝⊝⊝ VERY LOWk | It is uncertain whether PHP‐delivered interventions have any effect on primary health centre visits due to alcohol consumption 1 to 6 months post intervention compared to enhanced usual care |
|
Adverse events (1 to 6 months post intervention) ‐ incidence of alcohol‐related consequences ‐ accidents
(RR > 1 denotes greater risk) |
4 out of 51 | 1 out of 56 | RR 0.23 (0.03 to 1.97) | 107 (1 RCT)j |
⨁⊝⊝⊝ VERY LOWk | It is uncertain whether PHP‐delivered interventions have any effect on accidents due to alcohol consumption 1 to 6 months post intervention compared to enhanced usual care |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ASSIST: Alcohol, Smoking and Substance Involvement Screening Test; AUDIT: Alcohol Use Disorder Identification Test;CI: confidence interval; CP: community professional; LMIC: low‐ to middle‐income country; MD: mean difference; PHP: primary health professional; RCT: randomised clinical trial; RR: risk ratio; SMD: standardised mean difference; WHOQOL‐HIV BREF: World Health Organization Quality of Life Assessment for people living with HIV, abbreviated. | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aHuisIntVeld 2019. Brief intervention vs information leaflet.
bPengpid 2013. Brief intervention vs feedback on scores and information leaflet.
cMertens 2014. Brief intervention vs resource list.
dDowngraded by one level for indirectness: HuisIntVeld 2019 was performed on patients with HIV attending primary care clinics in South Africa. Mertens 2014 was performed on young adults age 18 to 24 who attended primary care clinics in South Africa. Pengpid 2013 was performed on university students in South Africa. As only one country was represented and each study studied a specific sub‐population, overall population characteristics were not easily generalisable to LMIC populations.
eDowngraded by one level for indirectness: single study in a single setting. Downgraded by two levels for imprecision: small total event numbers. Confidence interval of risk ratio ranged from favouring LHW‐led interventions to favouring usual care.
fDowngraded by one level for indirectness: HuisIntVeld 2019 was performed on patients with HIV attending primary care clinics in South Africa. Mertens 2014 was performed on young adults age 18 to 24 who attended primary care clinics in South Africa. Pengpid 2013 was performed on university students in South Africa. As only one country was represented and each study studied a specific sub‐population, overall population characteristics were not easily generalisable to LMIC populations. Downgraded by one level for imprecision: confidence interval of SMD ranged from small clinical effect to no effect. Note that a small clinically appreciable benefit was set at SMD 0.2 to 0.5, a moderate benefit at SMD 0.5 to 0.8, and a large benefit at > 0.8 (Cohen 1988).
gHumeniuk 2012. Brief intervention vs ASSIST questionnaire only.
hNot downgraded for indirectness: taken together, study populations and interventions were representative of alcohol and substance use populations and interventions in LMICs. Downgraded by one level for imprecision: confidence interval of SMD ranged from small clinical effect to no clinical effect.
iDowngraded by one level for indirectness: performed on patients with HIV attending primary care clinics in South Africa. As only one country was represented and the study studied a specific sub‐population, the overall population characteristics were not easily generalisable to LMIC populations.
jNoknoy 2010. Motivational enhancement therapy vs AUDIT questionnaire only.
kDowngraded by one level for indirectness: the outcome was derived from only one study population (Thai primary care patients) that may not be sufficiently representative of all LMIC settings. Downgraded by two levels for imprecision: very low event and total numbers. Confidence interval of risk ratio ranged from favouring PHP‐delivered interventions to favouring usual care.
Summary of findings 9. Lay health worker‐led interventions compared to enhanced usual care for adult patients with alcohol dependence in low‐ and middle‐income countries.
| What are the effects of lay health worker‐led interventions vs enhanced usual care for adult patients with alcohol dependence in low‐ and middle‐income countries? | ||||||
|
Patient or population: adult patients with alcohol dependence Setting: low‐ and middle‐income countries (India (1 study)) Intervention: lay health worker‐led psychological interventions Comparison: enhanced usual care (screening and referral) | ||||||
| Outcomes | Anticipated absolute effects*(95% CI) | Relative effect (95% CI) | №. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with enhanced usual care | Risk with lay health worker‐led interventions | |||||
|
Clinical recovery ‐ harmful or dependent alcohol use (1 to 6 months post intervention) Defined by AUDIT score < 8 (RR > 1 denotes higher likelihood of recovery) |
145 per 1000 participants | 271 per 1000 participants (131 to 566) | RR 1.87 (0.90 to 3.90) | 121 (1 RCT)a | ⨁⊝⊝⊝ VERY LOWb | It is uncertain whether LHW‐delivered interventions for adult patients with dependent use of alcohol have any effect on recovery from dependent alcohol use 1 to 6 months post intervention compared to enhanced usual care |
| Prevalence of alcohol dependence (1 to 6 months post intervention) | No studies that reported on this outcome were identified | |||||
|
Clinical symptoms ‐ alcohol use (1 to 6 months post intervention) Assessed with grams of ethanol consumed (lower number = lower amount consumed) |
Mean alcohol use in this LHW intervention is 0.3 grams of ethanol lower (21.6 lower to 21.0 higher) compared to enhanced usual care | MD ‐0.3 (‐21.6 to 21.0) | 121 (1 RCT)a | ⨁⊝⊝⊝ VERY LOWc | It is uncertain whether LHW‐delivered interventions for adult patients with dependent use of alcohol have any effect on alcohol use 1 to 6 months post intervention compared to enhanced usual care | |
|
Clinical symptoms ‐ depression (1 to 6 months post intervention) Assessed with PHQ‐9 (higher score = higher depression symptom severity) |
Mean depression score in this LHW intervention is 0.5 points lower (2.68 lower to 1.68 higher) compared to enhanced usual care | MD ‐0.5 (‐2.68 to 1.68) | 121 (1 RCT)a | ⨁⊝⊝⊝ VERY LOWc | It is uncertain whether LHW‐delivered interventions for adult patients with dependent use of alcohol have any effect on depression symptoms 1 to 6 months post intervention compared to enhanced usual care | |
| Quality of life | No studies that reported on this outcome were identified | |||||
|
Functional impairment (1 to 6 months post intervention) Assessed with WHODAS II (lower score = less functional impairment) |
Mean functional impairment score in this LHW intervention is 0.9 points lower (3.43 lower to 1.63 higher) compared to enhanced usual care | MD ‐0.9 (‐3.43 to 1.63) | 121 (1 RCT)a | ⨁⊝⊝⊝ VERY LOWc | It is uncertain whether LHW‐delivered interventions for adult patients with dependent use of alcohol have any effect on functional impairment 1 to 6 months post intervention compared to enhanced usual care | |
|
Service utilisation ‐ Unplanned hospitalisation in past 12 months (RR > 1 denotes higher risk of hospitalisation) |
148 per 1000 participants | 104 per 1000 participants (39 to 279) | RR 0.70 (0.26 to 1.88) | 112 (1 RCT)a | ⨁⊝⊝⊝ VERY LOWd | It is uncertain whether LHW‐delivered interventions for adult patients with dependent use of alcohol have any effect on unplanned hospitalisations more than 6 months post intervention compared to enhanced usual care |
|
Adverse events ‐ death in past 12 months (RR > 1 denotes higher risk of death) |
15 per 1000 participants | 5 per 1000 participants (0 to 117) | RR 0.32 (0.01 to 7.70) | 135 (1 RCT)a | ⨁⊝⊝⊝ VERY LOWd | It is uncertain if LHW‐delivered interventions for adult patients with dependent use have any effect on death more than 6 months post intervention compared to enhanced usual care |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AUDIT: Alcohol Use Disorder Identification Test; CI: confidence interval; LMIC: low‐ to middle‐income country; LHW: lay health worker; MD: mean difference; PHQ‐9: Patient Health Questionnaire‐9; RCT: randomised controlled trial; RR: risk ratio; WHODAS II: World Health Organization DIsability Assessment Schedule 2.0. | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aNadkarni 2019. Manualised psychological intervention (“Counselling for Alcohol Problems”) vs enhanced usual care (screening and referral).
bDowngraded by one level for indirectness: Nadkarni 2019 was performed in a lower‐middle‐income country, and interventionists underwent 2 weeks of classroom training followed by 6 months of internship. Patients were males only. Training may not be scalable to other populations in LMICs. Single trial in a single setting. Downgraded by two levels for serious imprecision: few events. Confidence interval ranges from no clinical effect to favouring LHW‐led intervention.
cDowngraded by one level for indirectness: Nadkarni 2019 was performed in a lower‐middle‐income country, and interventionists underwent 2 weeks of classroom training followed by 6 months of internship. Patients were males only. Training may not be scalable to other populations in LMICs. Single trial in a single setting. Downgraded by two levels for serious imprecision: Low total numbers. Confidence interval ranges from favouring LHW‐led interventions to enhanced usual care.
dDowngraded by one level for indirectness: Nadkarni 2019 was performed in a lower‐middle‐income country, and interventionists underwent 2 weeks of classroom training followed by 6 months of internship. Patients were males only. Training may not be scalable to other populations in LMICs. Single trial in a single setting. Downgraded by two levels for serious imprecision: Very few events. Confidence interval ranges from favouring LHW‐led intervention to favouring enhanced usual care.
Summary of findings 10. Primary health professional‐ and community professional‐led interventions compared to enhanced usual care for adult patients with substance dependence in low‐ and middle‐income countries.
| What are the effects of primary health professional‐ and community professional‐ led interventions on adult patients with substance dependence vs. enhanced usual care in low‐ and middle‐income countries? | ||||||
|
Patient or population: adult patients with substance dependence Setting: middle‐income country (China (1 study)) Intervention: community professional (1 study)‐led psychosocial interventions (N.B.: the study reporting the primary health professional is not included in this SOF) Comparison: enhanced usual care (monthly visits) | ||||||
| Outcomes | Anticipated absolute effects*(95% CI) | Relative effect (95% CI) | №. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with enhanced usual care | Risk with primary health professional‐led interventions | |||||
| Clinical recovery | No studies that reported on this outcome were identified | |||||
|
Prevalence of morphine use Assessed with urine tests for morphine (RR < 1 denotes lower prevalence compared to control) |
235 per 1000 participants | 249 per 1000 participants (148 to 424) | RR 1.06 (0.63 to 1.80) | 173 (1 RCT)a | ⨁⊝⊝⊝ VERY LOWb | It is uncertain whether CP‐led interventions for adult patients with substance dependence have any effect on prevalence of positive urine morphine tests less than 1 month post intervention compared to enhanced usual care |
|
Clinical symptoms ‐ alcohol use (< 1 month post intervention) Assessed with average number of months of alcohol use in the last 12 months (higher number = higher usage) |
Mean alcohol use in the PHP intervention was 0.1 months higher (0.91 lower to 1.11 higher) compared to enhanced usual care | MD 0.1 (‐0.91 to 1.11) | 155 (1 RCT)a | ⨁⊝⊝⊝ VERY LOWc | It is uncertain whether CP‐led interventions for adults with drug dependency have any effect on amount of alcohol use less than 1 month post intervention compared to enhanced usual care | |
|
Clinical symptoms ‐ drug use (< 1 month post intervention) ‐ heroin Assessed with average number of months of heroin use in the last 12 months (higher number = higher usage) |
Mean heroin use in the PHP intervention was 0.03 months lower (0.22 lower to 0.16 higher) compared to enhanced usual care | MD ‐0.03 (‐0.22 to 0.16) | 155 (1 RCT)a | ⨁⊝⊝⊝ VERY LOWc | It is uncertain whether CP‐led interventions for adults with drug dependency have any effect on heroin use less than 1 month post intervention compared to enhanced usual care | |
|
Quality of life (< 1 month post intervention) ‐ social functioning Assessed with social functioning sub‐scale of SF‐36 (higher score = higher quality of life) |
Mean quality of life social functioning score in the PHP intervention was 48.36 higher (41.8 higher to 54.92 higher) compared to enhanced usual care | MD48.36 (41.8 to 54.92) | 155 (1 RCT)a | ⨁⊝⊝⊝ VERY LOWd | It is uncertain whether CP‐led psychological interventions for adults with drug dependency have any effect on quality of life (social functioning) less than 1 month post intervention compared to enhanced usual care | |
| Functional impairment | No studies that reported on this outcome were identified | |||||
| Service utilisation | No studies that reported on this outcome were identified | |||||
| Adverse events | No studies that reported on this outcome were identified | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CP: community professional; LMIC: low‐ to middle‐income country; MD: mean difference; PHP: primary health professional; RR: risk ratio; RCT: randomised controlled trial; SF‐36: 36‐Item Short Form Health Survey. | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aZhong 2015. Psychosocial rehabilitation programme vs monthly visits.
bDowngraded by one level for risk of bias: high risk of detection and reporting bias. Downgraded by one level for indirectness: population characteristics not easily generalisable to LMIC populations. Only one study in one country where patients had been through 2 years of mandatory drug rehabilitation before entering study. Downgraded by one level for imprecision: low total event numbers.
cDowngraded by one level for risk of bias: high risk of detection and reporting bias. Downgraded by one level for indirectness: population characteristics not easily generalisable to LMIC populations. Only one study in one country where patients had been through 2 years of mandatory drug rehabilitation before entering study. Downgraded by one level for imprecision: low total number.
dDowngraded by one level for risk of bias: high risk of detection and reporting bias. Downgraded by one level for indirectness: population characteristics not easily generalisable to LMIC populations. Only one study in one country where patients had been through 2 years of mandatory drug rehabilitation before entering study. Downgraded by one level for imprecision: low total number. Note that the two groups were unequal at baseline for this outcome (almost 2× difference).
Summary of findings 11. Lay health worker‐ compared to specialist‐led care for people with severe mental disorder in low‐ and middle‐income countries.
| What are the effects of lay health worker (LHW)‐ vs specialist‐led care for people with severe mental disorder in low‐ and middle‐income countries? | |||||||
| Patient or population: people with severe mental disorder Setting: low‐ and middle‐income countries (China (1 study), India (1 study)) Intervention: lay health worker‐led care Comparison: specialist‐led care | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | №. of participants (studies) | Certainty of the evidence (GRADE) | Comments | ||
| Risk with specialist‐led care for people with severe mental disorder | Risk with LHW | ||||||
| Recovery from severe mental disorders | No studies that reported on this outcome were identified | ||||||
| Prevalence of severe mental disorders | No studies that reported on this outcome were identified | ||||||
|
Schizophrenia symptoms severity ‐ immediately post intervention Assessed by BPRSa; PANSSb (Chatterjee 2014) (higher scores = greater symptom severity) |
‐ | ‐ | 364 (2 RCTs) | ⊕⊝⊝⊝ VERY LOWc | It is uncertain whether LHW‐led interventions for people with severe mental disorder have any effect on schizophrenia symptom severity compared to specialist‐led care. In view of important differences between studies, the results of each study are described narratively in the text |
||
|
Caregiver burden symptom severity ‐ immediately post intervention Assessed by Burden Assessment Schedule (higher scores = higher burden) |
‐ | Mean caregiver burden score with LHW‐led interventions was 0.04 points lower (0.18 lower to 0.11 higher) compared to specialist‐led care | Adjusted MD ‐0.04 (‐0.18 to 0.11) | 253 (1 RCT)b | ⊕⊕⊝⊝ LOWd | LHW‐led interventions for people with severe mental disorder may have little to no effect on caregiver burden compared to specialist‐led care | |
| Quality of life | No studies that reported on this outcome were identified | ||||||
|
Functional impairment ‐ immediately post intervention Assessed by IDEASb; SDSSa (lower scores = less functional impairment) |
‐ | 364 (2 RCTs) | ⊕⊝⊝⊝ VERY LOWe | It is uncertain whether LHW‐led interventions for people with severe mental disorder have any effect on functional impairment compared to specialist‐led care. In view of important differences between studies, the results of each study are described narratively in the text |
|||
|
Service utilisation ‐ hospitalisation during intervention (RR > 1 denotes greater risk) |
1 out of 95 | 17 out of 187 | RR 8.64 (1.17 to 63.92) | 282 (1RCT)b | ⊕⊝⊝⊝ VERY LOWf | It is uncertain whether LHW‐led interventions for people with severe mental disorder have any effect on hospitalisations compared to specialist‐led care | |
|
Adverse events ‐ death from suicide during intervention (RR > 1 denotes greater risk) |
1 out of 95 | 1 out of 187 | RR 0.51 (0.03 to 8.03) | 282 (1RCT)b | ⊕⊝⊝⊝ VERY LOWg | It is uncertain whether LHW‐led interventions for people with severe mental disorder have any effect on deaths from suicide compared to specialist‐led care | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BPRS: Brief Psychiatric Rating Scale; CI: confidence interval; IDEAS: Indian Disability and Assessment Evaluation Scale; LHW: lay health worker; MD: mean difference; PANSS: Positive and Negative Syndrome Scale; RCT: randomised controlled trial; RR: risk ratio; SDSS: Social Disability Screening Schedule; SMD: standardised mean difference. | |||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||||
aChatterjee 2014 and Shen 2016. Clubhouse rehabilitation vs routine community psychiatric care.
bChatterjee 2014. Community case management vs facility‐based care.
cDowngraded by one level for inconsistency: large statistical heterogeneity (I² = 98%). There were important differences between studies. Study population: newly diagnosed schizophrenia in recovery phase ‐ Shen 2016 ‐ vs chronic schizophrenia of moderate severity ‐ Chatterjee 2014. Intervention: community case management ‐ Chatterjee 2014 ‐ vs clubhouse model ‐ Shen 2016. Downgraded by two levels for imprecision: low total number. Confidence interval of SMD ranges from large (< 0.80) effect favouring intervention to moderate (0.5 to 0.8) effect favouring control. Note that a small clinically appreciable benefit was set at SMD 0.2 to 0.5, a moderate benefit at SMD 0.5 to 0.8, and a large benefit at > 0.8 (Cohen 1988).
dDowngraded by one level for indirectness: single trial in one setting. Downgraded by one level for imprecision: low total number.
eChatterjee 2014. Downgraded by one level for inconsistency: large statistical heterogeneity (I² = 99%). There were important differences between studies. Study population: newly diagnosed schizophrenia in recovery phase ‐ Shen 2016 ‐ vs chronic schizophrenia of moderate severity ‐ Chatterjee 2014. Intervention: community case management ‐ Chatterjee 2014 ‐ vs clubhouse model ‐ Shen 2016. Downgraded by two levels for imprecision: low total number. Confidence interval of SMD ranges from large (< 0.80) effect favouring intervention to moderate (0.5 to 0.8) effect favouring control.
fDowngraded by one level for indirectness: single trial in one setting. Seven of the 18 admissions were related to physical health problems. Downgraded by two levels for imprecision: low event and total numbers.
gDowngraded by one level for indirectness: single trial in one setting. Downgraded by two levels for imprecision: low event and total numbers.
Summary of findings 12. Primary health professional‐led or collaborative care compared to specialist‐led care for people with severe mental disorder in low‐ and middle‐income countries.
| What are the effects of primary health professional‐led or collaborative care vs specialist‐led care for people with severe mental disorders? | ||||||
|
Patient or population: people with severe mental disorder Setting: low‐ and middle‐income countries (China (5 studies), Iran (2 studies)) Intervention: primary health professional‐led or collaborative care Comparison: specialist‐led care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | №. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with specialist‐led care | Risk with primary health professional‐led or collaborative care | |||||
|
Clinical recovery from severe mental disorder (immediately post intervention) Defined by BPRS decreased by ≥ 80% (RR > 1 denotes greater likelihood of recovery) |
684 per 1000 participants | 739 per 1000 participants (554 to 985) | RR 1.08 (0.81 to 1.44) | 76 (1 RCT)a | ⨁⊝⊝⊝ VERY LOWb | It is uncertain whether PHP‐led collaborative care compared to specialist‐led care for people with severe mental disorder has any effect on clinical recovery immediately post intervention |
|
Relapse of severe mental disorder (immediately post intervention) Defined by re‐appearance of symptoms or worsening of symptoms necessitating adjustment of medicationa; Li 2002; based on 1 item scoring ≥ 5 or 2 items scoring ≥ 4 in items 4, 7, 11, 12, and 15 of the BPRSc; determined clinicallyd,e (RR > 1 denotes higher risk of relapse) |
211 per 1000 participants | 63 per 1000 participants (34 to 116) | RR 0.30 (0.16 to 0.55) | 492 (4 RCTs) | ⨁⊝⊝⊝ VERY LOWf | It is uncertain whether PHP‐led or collaborative care compared to specialist‐led care for people with severe mental disorder have any effect on relapse |
| Prevalence of severe mental disorder | No studies that reported on this outcome were identified | |||||
|
Schizophrenia symptoms severity (immediately post intervention) Assessed with BPRSa,c,d,g; PANSSh,i (higher score = greater severity) |
Mean PANSS score with usual care was 80.6i | 836 (6 RCTs) | ⨁⊝⊝⊝ VERY LOWj | Scores estimated based on an SMD ‐0.30 (95% CI ‐0.71 to 0.11). It is uncertain whether PHP‐led or collaborative care compared to specialist‐led care for people with severe mental disorder has any effect on schizophrenia symptom severity immediately post intervention | ||
|
Depression symptom severity (immediately post intervention) Assessed with HDRSb; SCL‐90d (higher score = higher symptom severity) |
Mean Hamilton Rating Scale for Depression score with usual care was 11.9b | Mean Hamilton Rating Scale for Depression score in the intervention group was 3 (8.2 lower to 2.3 higher) lower | 270 (2 RCTs) | ⨁⊝⊝⊝ VERY LOWk | Scores estimated based on an SMD of ‐0.41 (95% CI ‐1.13 to 0.32). It is uncertain whether PHP‐led or collaborative care compared to specialist‐led care for people with severe mental disorders has any effect on severity of depression symptoms immediately post intervention | |
|
Quality of life (immediately post intervention) Assessed with SF‐36i; WHOQOL BREFe,h (higher score = higher quality of life) |
Mean WHOBREF score with usual care was 84.4 | Mean WHOBREF score in the intervention group was 9.04 (8.36 lower to 26.4 higher) higher | 536 (3 RCTs) | ⨁⊝⊝⊝ VERY LOWl | Scores estimated based on an SMD of 0.40 (95% CI ‐0.37 to 1.17). It is uncertain whether PHP‐led or collaborative care compared to specialist‐led care for people with severe mental disorders has any effect on quality of life immediately post intervention | |
|
Functional impairment ‐ immediately post intervention Assessed with GAF (results were multiplied by ‐1)h; KELSi; SDSSa,c,d,g; self care ADL and Instrumental ADLe (lower score = less functional impairment) |
Mean function score with usual care was 9.58i | Mean function score in the intervention group was 5.0 (7.8 to 2.1) lower | 874 (7 RCTs) | ⨁⨁⊝⊝ LOWm | Scores estimated based on an SMD of ‐1.13 (95% CI ‐1.78 to ‐0.47). PHP‐led or collaborative care for people with severe mental disorders may reduce functional impairment immediately post intervention compared to specialist‐led care | |
|
Service utilisation ‐ hospital re‐admission (during intervention) (RR > 1 denotes greater risk) |
360 per 1000 | 216 per 1000 (101 to 461) | RR 0.60 (0.28 to 1.28) | 441 (3 RCTs)e,h,i | ⨁⊝⊝⊝ VERY LOWn | It is uncertain whether PHP‐led or collaborative care compared to specialist‐led care for people with severe mental disorders has any effect on hospital re‐admission immediately post intervention |
| Adverse events | No studies that reported on this outcome were identified | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ADL: activities of daily living; BPRS: Brief Psychiatric Rating Scale; CI: confidence interval; GAF: Global Assessment of Functioning Scale; GP: general practitioner; HDRS: Hamilton Depression Rating Scale; KELS: Kohlman Evaluation of Living Skills; LMIC: low‐ to middle‐income country; PANSS: Positive and Negative Syndrome Scale; PHP: primary health professional; RCT: randomised clinical trial; RR: risk ratio; SCL‐90: Symptom Checklist‐90; SDSS: Social Disability Screening Schedule; SF‐36: 36‐Item Short‐Form Health Survey; SMD: standardised mean difference; WHOQOL BREF: World Health Organization Quality of Life assessment abbreviated. | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aLi 2002. Community rehabilitation (medications, counselling, requested work or social activities) vs inpatient care for patients with first episode of late‐onset schizophrenia.
bDowngraded by one level for risk of bias: high risk of selection (lack of allocation concealment) and detection (lack of blinding of outcome assessors) bias. Downgraded by one level for indirectness: the only study in this analysis was conducted in a lower‐middle‐income country, on people age 50 and older experiencing their first episode of illness (schizophrenia); intervention comprised antipsychotics and weekly home visits. Study population, setting, and intervention are not generalisable to all patients with serious mental disorders in LMICs. Downgraded by one level for imprecision: small event numbers.
cTan 2005. Community observation (medications, symptom monitoring, psychoeducation, rehabilitation) vs hospitalisation as needed for patients with schizophrenia.
dLing 1999. Family intervention (education on medication side effects and adherence, symptom monitoring, psychoeducation, counselling, family communication training) vs community psychiatric nurse‐led care for patients with schizophrenia.
eWu 2016. Self‐care model combined with collaborative care (medications, counselling, family communication training, requested work or social activities, self‐care training) vs community psychiatric nurse‐led care for patients with chronic stable schizophrenia.
fDowngraded by one level for risk of bias: high risk of detection bias (lack of blinding in outcome assessments) in all four studies, and selection bias (lack of allocation concealment) in one study (Li 2002). Downgraded by one level for indirectness: only one country represented among these studies. Downgraded by one level for imprecision: Small total event numbers.
gYao 2014. Community day rehabilitation (medications, symptom monitoring, psychoeducation, counselling, social skills training, rehabilitation) vs community psychiatric nurse‐led care for patients with chronic stable schizophrenia.
hBarfar 2017. Aftercare service (medications, education on medication side effects and adherence, symptom monitoring, psychoeducation, telephone reminders to attend outpatient clinics, social skills training) vs usual specialist care in outpatient clinics or inpatient services for patients with schizophrenia, bipolar disorder, or schizoaffective disorder.
iMalakouti 2015. Home visits by nurse or GP (medications, education on medication side effects and adherence, symptom monitoring, psychoeducation) vs usual specialist‐led outpatient clinic or hospitalisation during exacerbation for patients with schizophrenia or bipolar disorder with difficult‐to‐treat disease.
jDowngraded by one level for risk of bias: high risk of detection bias (lack of blinding in outcome assessments) in four studies (Barfar 2017; Li 2002; Ling 1999; Tan 2005), selection bias (lack of allocation concealment) in one study (Li 2002), and attrition bias in one study (Malakouti 2015). Downgraded by one level for inconsistency: large unexplained statistical heterogeneity (I² = 87%). Downgraded by one level for imprecision: confidence level of SMD ranges from moderate clinical effect favouring PHP‐led or collaborative care to no clinical effect. Note that a small clinically appreciable benefit was set at SMD 0.2 to 0.5, a moderate benefit at SMD 0.5 to 0.8, and a large benefit at > 0.8 (Cohen 1988).
kDowngraded by one level for risk of bias: high risk of detection bias in Barfar 2017 and Ling 1999. Downgraded by one level for inconsistency: large unexplained statistical heterogeneity (I² = 89%). Downgraded by one level for imprecision: low total number. Confidence interval of SMD ranged from favouring PHP‐led or collaborative care to favouring specialist‐led care.
lDowngraded by one level for risk of bias: high risk of detection bias in Barfar 2017 and Wu 2016, and attrition bias in Malakouti 2015. Downgraded by one level for inconsistency: large unexplained statistical heterogeneity (I² = 94%). Downgraded by one level for imprecision: confidence interval of effect size ranges from large clinical effect favouring PHP‐led or collaborative care to favouring specialist‐led care.
mDowngraded by one level for risk of bias: high risk of detection bias (lack of blinding in outcome assessments) in five studies (Barfar 2017; Li 2002; Ling 1999; Tan 2005; Wu 2016), selection bias (lack of allocation concealment) in one study (Li 2002), and attrition bias in one study (Malakouti 2015). Downgraded by one level for inconsistency: large unexplained statistical heterogeneity (I² = 95%).
nDowngraded by one level for risk of bias: high risk of detection bias in Barfar 2017 and Wu 2016, and attrition bias in Malakouti 2015. Downgraded by one level for inconsistency: moderate unexplained statistical heterogeneity (I² = 74%). Downgraded by one level for imprecision: small event numbers. Confidence interval of risk ratio ranged from favouring PHP‐led or collaborative care to favouring specialist‐led care.
Summary of findings 13. Primary health professionals and lay health workers compared with usual care in improving dementia patients' and carers' outcomes in low‐ and middle‐income countries.
| What are the effects of primary health professional‐ and lay health worker‐led care vs usual care in improving dementia patients' and carers' outcomes for mental health care in low‐ and middle‐income countries? | ||||||
|
Patient or population: people with dementia and their carers
Settings: middle‐income countries (India (1 study), Russia (1 study)) Intervention: PHP‐ and primary care‐based LHW‐led intervention Comparison: usual care (limited dementia education or wait‐list control) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Estimate effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
|
Risk with usual care |
Risk with PHP/LHW‐led care | |||||
| Clinical illness recovery | No studies that reported on this outcome were identified | |||||
| Disease prevalence | No studies that reported on this outcome were identified | |||||
|
Severity of behavioural symptoms (patient) (1 to 6 months post intervention)
Measured using the behavioural symptom scale (NPI‐S) (high score = greater severity) |
Mean NPI‐S score with usual care was 8.4a | Mean NPI‐S score in the intervention group was 1.3 (3.1 lower to 0.4 higher) lower | 134 (2 RCTsa,b) | ⨁⨁⊝⊝
LOWc |
Scores estimated based on an SMD of ‐0.26 (95% CI ‐0.6 to 0.08)d. PHP‐ and LHW‐led carer interventions for dementia compared to usual care may have little to no effect on severity of behavioural symptoms 1 to 6 months post intervention | |
|
Severity of mental distress (carer) (1 to 6 months post intervention) Neuropsychiatric Interview for dementia (higher score = better quality of life) |
Mean NPI‐D score with usual care was 7.1a |
Mean NPI‐D score in the intervention group was 3 (5.2 to 0.8) lower |
134 (2 RCTsa,b) | ⨁⨁⊝⊝ LOWe |
Scores estimated based on an SMD of ‐0.47 (95% CI ‐0.82 to ‐0.13)d. PHP‐ and LHW‐led carer interventions for dementia compared to usual care may reduce carers' mental distress 1 to 6 months post intervention |
|
|
Quality of life (patient) (1 to 6 months post intervention) DEMQOL (higher score = better quality of life) |
Mean quality of life with this brief carer intervention was 0.43 points lower (0.98 lower to 0.12 higher) compared to usual care |
MD ‐0.43 (‐0.98 to 0.12) | 53 (1 RCTb) | ⨁⊝⊝⊝ VERY LOWf |
It is uncertain whether PHPs delivering care to dementia patients compared with usual care have any effect on dementia sufferers' quality of life at 1 to 6 months post intervention | |
|
Quality of life (carer) (1 to 6 months post intervention) WHOQOL‐BREF (higher score = better quality of life) |
Mean improvement in quality of life with this brief carer intervention was 0.37 standard deviations lower (0.93 lower to 0.17 higher) compared to usual care |
MD ‐0.37 (95% CI ‐0.92 to 0.17) | 53 (1 RCTb) | ⨁⊝⊝⊝ VERY LOWf |
It is uncertain whether PHPs delivering care to dementia patients compared with usual care have any effect on carers' quality of life at 1 to 6 months post intervention | |
|
Functional impairment (patient) (1 to 6 months post intervention) EASI (higher score = higher impairment in activities of daily living) |
Mean functional impairment with this brief carer intervention was 0.24 points lower (0.67 lower to 0.20 higher) compared to usual care |
MD ‐0.24 (‐0.67 to 0.20) | 81 (1 RCTa) |
⨁⊝⊝⊝ VERY LOWf |
It is uncertain whether LHWs delivering care to dementia patients compared to usual care have any effect on functional impairment at 1 to 6 months post intervention | |
| Service utilisation ‐ home visits by LHW and psychiatrist (during intervention) | No visits | Excess of home visits by specialist supervisor (21 home visits) and by LHW (mean visits 12.3, SD 3.1) | More visits | 81 (1 RCTa) |
⨁⊝⊝⊝ VERY LOWg |
It is uncertain whether LHWs delivering care to dementia patients compared with usual care have any effect on home visits during intervention |
|
Adverse events ‐ number of deaths (during intervention) |
There were 14 deaths out of 70 participants (20%) | There were 11 deaths out of 71 participants in the intervention arm (15%). Note: causes of death in both groups were stroke, pneumonia, myocardial infarction, septicaemia, pulmonary embolism, intestinal obstruction, diabetic coma |
RR 0.77 (from 0.38 to 1.59) | 141 (2 RCTsa,b) | ⨁⊝⊝⊝ VERY LOWh |
It is uncertain whether PHPs and LHWs delivering care to dementia patients compared with usual care have any effect on deaths during intervention |
| *The basis for the assumed risk is the mean control group risk across studies for pooled results and the control group risk for single studies. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DEMQOL: Quality of Life Assessment in Dementia; EASI: Everyday Abilities Scale for India; LHW: lay health worker; MD: mean difference; NPI‐S: Neuropsychiatric Inventory ‐ Severity; PHP: primary health professional; RCT: randomised controlled trial; RR: risk ratio; SMD: standardised mean difference; WHOQOL‐BREF: abbreviated World Health Organisation Quality of Life Assessment. | ||||||
| GRADE Working Group grades of evidence. High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDias 2008. Collaborative care package delivered by LHWs based on 10/66 dementia initiative vs limited dementia education.
bGavrilova 2009. Carer training delivered by PHPs based on 10/66 dementia initiative vs wait‐list control.
cNot downgraded as no serious study limitations: Gavrilova 2009 was unclear whether allocation concealed. Dias 2008 was at low risk of bias and contributed > 60% of the weight to pooled estimates. Removal of the former study did not alter the results. Downgraded by two levels for imprecision: 95% CI for pooled estimates indicates appreciable benefit for PHP/LHW care and non‐appreciable benefit for usual care, and the total number of participants is small.
dNote that a small clinically appreciable benefit was set at SMD 0.2 to 0.5, and a moderate benefit at SMD 0.5 to 0.8 (Cohen 1988).
eNot downgraded as no serious study limitations: Gavrilova 2009 was unclear whether allocation concealed. Dias 2008 was at low risk of bias and contributed > 60% of the weight to pooled estimates. Removal of the former study did not alter the results. Downgraded by two levels for imprecision: small total number of participants and effect confidence intervals range from favouring intervention to no effect.
fDowngraded by one level for indirectness: only one study (in Russia for quality of life, in India for functional impairment) (i.e. may not be representative of all LMICs). Downgraded by two levels for imprecision: low total number, and effect confidence intervals ranged from favouring intervention to no effect.
gDowngraded by one level for indirectness. Single trial in a single setting. Downgraded by two levels for imprecision: very low total number.
hDowngraded by one level for inconsistency: Dias 2008 RCT India showed fewer deaths in intervention arm than in control arm (not statistically significant). Gavrilova 2009 RCT Russia showed more deaths in intervention arm than in control arm (not statistically significant). Downgraded by two levels for imprecision: low total and event numbers.
Summary of findings 14. Lay health worker‐led psychosocial interventions vs usual or no care in treating children with post‐traumatic stress and common mental disorders in humanitarian settings in low‐ and middle‐income countries.
| What are the effects of lay health workers conducting interventions for children with post‐traumatic stress or common mental disorders in humanitarian settings in low‐ and middle‐income countries? | ||||||
|
Patient or population: children/adolescents with post‐traumatic stress and related depressive/anxiety symptoms Settings: humanitarian settings in low‐ and middle‐income countries (Indonesia (1 study), Nepal (1 study), Sri Lanka (1 study), Uganda (1 study)) Intervention: lay health workers delivering psychosocial interventions Comparison: usual or no care (wait‐list control or intervention for suicidal ideation) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with usual or no care | Risk with community professionals | |||||
| Depression or PTSD recovery | No studies that reported on these outcomes were identified | |||||
| Disease prevalence | No studies that reported on these outcomes were identified. | |||||
|
Depression symptoms (1 to 6 months post intervention) Depression self‐rating scale (higher score = higher severity) |
Mean change in depression severity score with LHW‐led interventions was 0.61 lower (1.23 lower to 0.02 higher) compared to usual or no care | MCD ‐0.61 (‐1.23 to 0.02) | 1092 (3 RCTsa,b,c) | ⨁⨁⨁⊝ MODERATEd | LHW‐delivered psychosocial interventions for children with post‐traumatic stress or CMD compared to usual or no care probably have little to no effect on depression symptoms 1 to 6 months post intervention | |
|
PTS symptoms (1 to 6 months post intervention) Child post‐traumatic stress scale (higher score = higher severity) |
Mean change in PTS symptom severity score with LHW‐led interventions was 1.34 lower (2.83 lower to 0.14 higher) compared to usual or no care | MCD ‐1.34 (‐2.83 to 0.14) | 1090 (3 RCTsa,b,c) | ⨁⨁⊝⊝ LOWe | LHW‐delivered psychosocial interventions for children with post‐traumatic stress or CMD compared to usual or no care may have little to no effect on PTS symptoms 1 to 6 months post intervention | |
| Quality of life | No studies that reported on this outcome were identified | |||||
|
Functional impairment (1 to 6 months post intervention) Locally developed functional scale (higher score = higher impairment) |
Mean change in functional impairment score with LHW‐led interventions was 0.81 lower (1.48 lower to 0.13 lower) compared to usual or no care | MCD ‐0.81 (‐1.48 to ‐0.13) | 1092 (3 RCTsa,b,c) | ⨁⨁⨁⊝ MODERATEd | Lay health workers delivering psychosocial interventions for children with post‐traumatic stress or CMD compared to usual or no care probably have little to no effect on functional impairment at 1 to 6 months post intervention | |
| Service utilisation | No studies that reported on this outcome were identified | |||||
| Adverse events (during intervention) | There were no adverse events in control groups (Ertl 2011; Jordans 2010; Tol 2012) | There were no adverse events in intervention groups (Ertl 2011; Jordans 2010; Tol 2012) | No differences | 809 (3 RCTsa,c,f) |
⨁⨁⊝⊝ LOWg |
LHW‐delivered psychosocial interventions for children with post‐traumatic stress or CMD compared to usual or no care may result in little to no difference in adverse events |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval;CMD: common mental disorder; LHW: lay health worker; MCD: mean change difference; PTS: post‐traumatic stress; PTSD: post‐traumatic stress disorder; RCT: randomised controlled trial. | ||||||
|
GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aJordans 2010. Classroom‐based intervention with creative‐expressive therapy and co‐operative play components vs wait‐list control.
bTol 2008. Classroom‐based intervention with creative‐expressive therapy and co‐operative play components vs wait‐list control.
cTol 2012. Classroom‐based intervention with creative‐expressive therapy and co‐operative play components vs wait‐list control.
dNot downgraded, as study populations were in different conflict areas and received scalable generic interventions. They covered children 8 to 18 years of age, so not generalisable to younger children. Downgraded by one level for imprecision: confidence interval of mean change difference ranged from favouring LHW‐delivered psychosocial interventions to no clinical effect.
eNot downgraded, as study populations were in different conflict areas and received scalable generic interventions. They covered children 8 to 18 years of age, so not generalisable to younger children. Downgraded by one level for imprecision: confidence interval of mean change difference ranged from favouring LHW‐delivered psychosocial interventions to no clinical effect. Downgraded by one level for inconsistency: unexplained statistical heterogeneity (I² = 57%).
fErtl 2011. Psychoeducation and academic catch‐up vs narrative exposure therapy vs no care except for intervention for those with suicidal ideation.
gDowngraded by two levels for serious imprecision: very small event number.
Summary of findings 15. Community professional‐led interventions vs no care in treating children with post‐traumatic stress and common mental disorders in humanitarian settings in low‐ and middle‐income countries.
| What are the effects of community professionals conducting interventions for children with post‐traumatic stress or common mental disorders in humanitarian settings in low‐ and middle‐income countries? | ||||||
|
Patient or population: children/adolescents with post‐traumatic stress and related depressive/anxiety symptoms
Settings: humanitarian settings in low‐ and middle‐income countries (DR Congo (2 studies), Kosovo (1 study), Palestine (1 study), Sierra Leone (1 study), Sri Lanka (1 study))
Intervention: community professionals delivering psychological and psychosocial interventions Comparison: no care (wait‐list control) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with usual or no care | Risk with community professionals | |||||
|
Recovery from probable PTSD in children (< 1 month post intervention) Defined by number of participants whose CRIES‐13 score was above cutoff for diagnosis of PTSD at baseline ‐ number of participants whose CRIES‐13 score was above cutoff for the same < 1 month post intervention (RR > 1 denotes higher likelihood of recovery) |
120 per 1000 participants | 472 per 1000 participants (157 to 1000) | RR 3.93 (1.31 to 11.80) | 78 (1 RCTa) | ⨁⊝⊝⊝ VERY LOWb |
It is uncertain whether CP‐led interventions have any effect on the number of children with probable PTSD compared to no care |
| Prevalence of PTSD | No studies that reported on this outcome were identified | |||||
|
PTS symptoms (1 to 6 months post intervention) Harvard Trauma Questionnairec; PTSD‐RId; UCLA PTSD indexe (higher score = higher severity) |
Mean UCLA PTSD index score with usual care was 45.71e | Mean UCLA PTSD index score in the intervention group was 2.8 (9 lower to 3.5 higher) lower | 679 (3 RCTs) | ⨁⊝⊝⊝ VERY LOWf | Scores estimated based on an SMD of ‐0.37 (95% CI ‐1.2 to 0.47). It is uncertain whether CP‐led interventions have any effect on PTS symptoms in children with post‐traumatic stress compared to no care 1 to 6 months post intervention | |
|
Depression symptoms (1 to 6 months post intervention) Beck Depression Inventorye; Oxford Measure of Psychosocial Adjustmentd (higher score = higher severity) |
Mean Beck Depression Inventory score with usual care was 3.7e |
Mean Beck Depression Inventory score in the intervention group was 0.6 (1.7 lower to 0.57 higher) lower | 602 (2 RCTs) | ⨁⨁⊝⊝ LOWg | Scores estimated based on an SMD of ‐0.19 (95% CI ‐0.57 to 0.19). CP‐led interventions may have little to no effect on depression symptoms in children with post‐traumatic stress compared to no care 1 to 6 months post intervention | |
| Quality of life | No studies that reported on this outcome were identified | |||||
|
Functional impairment (1 to 6 months post intervention) Child diagnostic interview schedulee; WHODAS IId (higher score = higher functional impairment) |
Mean function score with usual care was 11.79e | Mean function score in the intervention group was 1.56 (3.96 lower to 0.84 higher) lower | 602 (2 RCTs) | ⨁⊝⊝⊝ VERY LOWh | Scores estimated based on an SMD of ‐0.39 (95% CI ‐0.99 to 0.21). It is uncertain whether CP‐led interventions have any effect on functional impairment in children with post‐traumatic stress compared to no care 1 to 6 months post intervention | |
| Service utilisation | No studies that reported on this outcome were identified | |||||
| Adverse events (during intervention) | There were no adverse events in the control group (Gordon 2008; O'Callaghan 2013i; O'Callaghan 2015j) | There were no adverse events in the intervention group (Gordon 2008; O'Callaghan 2013; O'Callaghan 2015). | No differences | 180 (3 RCTs) |
⨁⨁⊝⊝ LOWk |
CP‐led interventions may not result in more adverse events compared to no care during intervention |
| *The basis for the risk with usual or no care is the mean control group risk across studies for pooled results and the control group risk for single studies. The risk with community professionals (and its 95% CI) is based on the risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CP: community professional; CRIES‐13: Child Revised Impact Event Scale; PTS: post‐traumatic stress; PTSD: post‐traumatic stress disorder; PTSD‐RI: Post‐traumatic Stress Disorder Reaction index; RCT: randomised controlled trial; RR: risk ratio; SMD: standardised mean difference; UCLA: University of California at Los Angeles; WHODAS II: World Health Organization Disability Adjustment Scale. | ||||||
| GRADE Working Group grades of evidence. High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aBarron 2013. Teaching Recovery Techniques trauma recovery programme vs wait‐list control.
bDowngraded by one level for limitations in design: high risk of bias in random sequence generation, allocation concealment, and similarity of baseline outcome measures in the two groups. Downgraded by one level for indirectness: population (Palestine, area with high level of violence and poverty, age 11 to 13) may not be generalisable to other children with post‐traumatic stress with or without PTSD in low‐ and middle‐income countries.
cGordon 2008. Mind‐body techniques vs wait‐list control.
dBetancourt 2014. Psychoeducation, psychological, and social interventions vs wait‐list control.
eBerger 2009. Psychoeducation, psychological, and social interventions vs wait‐list control.
fDowngraded by one level for limitations in design: Berger 2009 had high risk of selection bias (poor allocation concealment) and contamination bias. Gordon 2008 had high risk of selection bias due to poor allocation concealment. Downgraded by one level for inconsistency: large unexplained statistical heterogeneity (I² = 94%). Downgraded by two levels for serious imprecision: confidence interval crosses from favouring CP‐led intervention to favouring no care. Note that a small clinically appreciable benefit was set at SMD 0.2 to 0.5, moderate benefit at SMD 0.5 to 0.8, and large benefit a > 0.8 (Cohen 1988).
gNot downgraded for risk of bias: although Berger 2009 had design limitations, removal of this study from the analysis still yielded an SMD showing no clinical effect. Downgraded for inconsistency: unexplained statistical heterogeneity (I² = 69%). Downgraded by one level for imprecision: confidence interval crosses from moderate effect favouring CP‐led intervention to no clinical effect.
hDowngraded by one level for limitations in design: Berger 2009 had high risk of selection (poor allocation concealment) and contamination bias. Downgraded by one level for inconsistency: Large unexplained statistical heterogeneity (I² = 91%). Downgraded by two levels for imprecision: confidence interval ranged from large clinical effect favouring CP‐led interventions to small clinical effect favouring no care.
iO'Callaghan 2013. Psychoeducation, psychological, and social interventions vs wait‐list control.
jO'Callaghan 2015. Psychoeducation, psychological, and social interventions vs wait‐list control.
kDowngraded by two levels for imprecision: very low number of participants and very few events.
Results of meta‐analyses of outcomes for the following comparisons were described (except comparison 9, for which results from the one study in this category were described).
Lay health worker‐led psychological interventions versus usual care in treating common mental disorders (N = 10 studies).
Primary‐level worker‐led collaborative care versus usual care in treating common mental disorders in adults (N = 13).
Lay health worker‐led psychosocial interventions versus usual care in treating perinatal depression (N = 7 studies).
Primary health professional‐led collaborative interventions versus enhanced usual care in treating perinatal depression (N = 2 studies).
Lay health worker‐led psychological interventions versus usual care in adults with post‐traumatic stress or common mental disorders in humanitarian settings (N = 15 studies).
Primary health professional‐led and community professional‐led psychological interventions versus usual or no care in adults with post‐traumatic stress or common mental disorders in humanitarian settings (N = 5 studies).
Lay health worker‐led interventions versus enhanced usual care in adult patients with harmful or hazardous alcohol or substance use (N = 8 studies).
Primary health professional‐led and community professional‐led interventions versus enhanced usual care in adult patients with harmful or hazardous alcohol or substance use (N = 6 studies).
Lay health worker‐led interventions versus enhanced usual care in adult patients with alcohol dependence (N = 1 study).
Primary health professional‐led and community professional‐led interventions versus enhanced usual care in adult patients with substance dependence (N = 2 studies).
Lay health worker‐led interventions versus specialist‐led care for adults with severe mental disorders (N = 2 studies).
Primary health professional‐led or collaborative care versus specialist‐led care for people with severe mental disorders (N = 7 studies).
Primary health professional‐led and lay health worker‐led psychosocial interventions versus usual care in improving dementia patients' and carers' outcomes (N = 2 studies).
Lay health worker‐led psychosocial interventions versus usual or no care in child post‐traumatic stress or common mental disorders in humanitarian settings (N = 7 studies).
Community professional‐led psychosocial interventions versus no care in child post‐traumatic stress or common mental disorders in humanitarian settings (N = 8 studies).
Two studies pertaining to children were not pooled, as they were individual studies of different disorders that could not be meta‐analysed (autism and attention‐deficit hyperactivity disorder); results of these studies were reported narratively.
The full GRADE evidence profiles for this review are available on Zenodo (van Ginneken 2021).
Comparison 1. Lay health worker‐led psychological interventions versus usual care in treating common mental disorders (CMDs)
We identified ten studies from nine countries across Africa (Abas 2018; Bolton 2003; Chibanda 2016; Petersen 2014), Asia (Ali 2003; Arjadi 2018; Jiang 2017; Murphy 2020; Patel 2017), and South America (Matsuzaka 2017). Two studies included only elderly adult males and females aged > 60 years (Chibanda 2016; Jiang 2017). Two studies included patients receiving HIV antiretroviral therapy (Abas 2018; Petersen 2014). In three studies, participants were from very low‐income households with high rates of unemployment (Bolton 2003; Patel 2017; Petersen 2014).
Details of study settings, participants, interventions, and comparisons are described in Appendix 3.
A summary of key findings has been tabled in Table 1.
Primary outcomes
1. Recovery from common mental disorders (CMDs)
1.1. Analysis.

Comparison 1: Lay health worker‐led psychological interventions versus usual care in treating common mental disorders (CMDs), Outcome 1: Recovery from common mental disorders
Three studies reported recovery from CMDs, defined as scoring < 8 on the Hamilton Depression Rating Scale (HDRS) in Matsuzaka 2017, scoring ≤ 7 on the 20‐Item Self‐Reporting Questionnaire (SRQ‐20) in Murphy 2020, and scoring < 5 on the Patient Health Questionnaire‐9 (PHQ‐9) in Patel 2017. Lay health worker (LHW)‐led psychological interventions may increase the likelihood of remission from CMDs (short‐term recovery) up to 1 month post intervention compared to usual care (risk ratio (RR) 1.50, 95% confidence interval (CI) 1.04 to 2.16; 1 study, 222 participants; P = 0.03; low certainty due to serious indirectness and imprecision) (Murphy 2020). LHW‐led psychological interventions may increase the likelihood of medium‐term recovery for people with CMDs 1 to 6 months post intervention compared to usual care (RR 1.29, 95% CI 1.06 to 1.56; 2 studies, 308 participants; I² = 0%; P = 0.010; low certainty due to serious indirectness and imprecision) (Matsuzaka 2017; Murphy 2020). LHW‐led psychological interventions may increase the likelihood of long‐term recovery from CMDs > 6 months post intervention compared to usual care (RR 1.96, 95% CI 1.34 to 2.87; 1 study, 493 participants; P = 0.0005; low certainty due to serious indirectness and imprecision) (Patel 2017).
2. Prevalence of common mental disorders (CMDs)
1.2. Analysis.

Comparison 1: Lay health worker‐led psychological interventions versus usual care in treating common mental disorders (CMDs), Outcome 2: Prevalence of common mental disorders
Three studies reported change in prevalence of CMDs defined as Diagnostic and Statistical Manual of Mental Disorders Fourth Edition (DSM‐IV) diagnosable major depression in Bolton 2003, scoring ≥ 9 on the PHQ‐9 in Chibanda 2016, and scoring > 7 on the SRQ‐20 in Murphy 2020. It is uncertain whether LHW‐led psychological interventions reduce the prevalence of common mental disorders up to 1 month post intervention compared to usual care (RR 0.51, 95% CI 0.15 to 1.77; 2 studies, 336 participants; I² = 92%; P = 0.29; very low certainty due to serious study limitations, inconsistency, indirectness, and imprecision) (Bolton 2003; Murphy 2020). LHW‐led psychological interventions may reduce the prevalence of CMDs at 1 to 6 months post intervention compared to usual care (RR 0.42, 95% CI 0.18 to 0.96; 2 studies, 479 participants; I² = 87%; P = 0.04; low certainty due to serious inconsistency and imprecision) (Chibanda 2016; Murphy 2020).
3. Severity of common mental disorder (CMD) symptoms
1.3. Analysis.

Comparison 1: Lay health worker‐led psychological interventions versus usual care in treating common mental disorders (CMDs), Outcome 3: Common mental disorder symptoms
Eight studies reported change in symptom severity of CMDs (Abas 2018; Ali 2003; Bolton 2003; Chibanda 2016; Matsuzaka 2017; Murphy 2020; Patel 2017; Petersen 2014). In these trials, symptom severity was measured using depression symptom inventories (e.g. PHQ‐9) or more generic measures of psychological distress containing items related to both anxiety and depression symptoms (e.g. Hopkins Symptom Checklist, SRQ‐20). For the purpose of this meta‐analysis, we reported pooled depression and CMD scores, as the interventions used were transdiagnostic. Outcomes of analyses when only depression inventory scores were used were almost identical to results based on CMD scores.
LHW‐led psychological interventions may reduce the symptom severity of CMDs up to 1 month post intervention compared to usual care (Ali 2003; Bolton 2003; Murphy 2020; Petersen 2014); however the range at which the actual effect may be noted (the "margin of error") indicates that the intervention may make little or no difference (standardised mean difference (SMD) ‐0.93, 95% CI ‐1.68 to ‐0.18; 4 studies, 717 participants; I² = 94%; P = 0.01; low certainty due to serious inconsistency and serious imprecision). LHW‐led psychological interventions may reduce the symptom severity of CMDs at 1 to 6 months post intervention compared to usual care (SMD ‐0.59, 95% CI ‐1.01 to ‐0.16; 4 studies, 798 participants; I² = 83%; P = 0.007; low certainty due to serious inconsistency and imprecision) (Abas 2018; Chibanda 2016; Matsuzaka 2017; Murphy 2020). LHW‐led psychological interventions may slightly reduce the symptom severity of CMDs at > 6 months post intervention compared to usual care (SMD ‐0.32, 95% CI ‐0.49 to ‐0.14; 1 study, 493 participants; P = 0.0005; low certainty due to serious study indirectness and imprecision) (Patel 2017).
4. Quality of life (QOL)
1.4. Analysis.

Comparison 1: Lay health worker‐led psychological interventions versus usual care in treating common mental disorders (CMDs), Outcome 4: Quality of life
Only one study reported change in quality of life (QOL) as an outcome (Chibanda 2016). LHW‐led psychological interventions may improve the QOL of people with CMDs at 1 to 6 months post intervention compared to usual care (SMD 0.51, 95% CI 0.34 to 0.69; 1 study, 521 participants; low certainty due to serious imprecision and indirectness, as the evidence was derived from one trial in one setting only).
5. Functional impairment and disability
1.5. Analysis.

Comparison 1: Lay health worker‐led psychological interventions versus usual care in treating common mental disorders (CMDs), Outcome 5: Functional impairment/disability
LHW‐led psychological interventions may have little to no effect on functioning in people with CMDs up to 1 month post intervention compared to usual care (SMD ‐0.09, 95% CI ‐0.24 to 0.06; 2 studies, 659 participants; I² = 0%; P = 0.25; low certainty due to serious study limitations and imprecision) (Bolton 2003; Murphy 2020). LHW‐led psychological interventions may slightly reduce functional impairment in people with CMDs at 1 to 6 months post intervention compared to usual care (SMD ‐047, 95% CI ‐0.80 to ‐0.15; 3 studies, 1399 participants; I² = 89%; P = 0.005; low certainty due to serious inconsistency and imprecision) (Chibanda 2016; Murphy 2020; Patel 2017). LHW‐led psychological interventions may have little to no effect on functioning in people with CMDs at > 6 months post intervention compared to usual care (SMD ‐0.16, 95% CI ‐0.34 to 0.02; 1 study, 493 participants; P = 0.08; low certainty due to serious imprecision and indirectness) (Patel 2017).
6. Service utilisation
One study reported missed outpatient appointments (study on persons living with HIV with depression; Abas 2018). At baseline, a similar proportion of participants in the enhanced usual care (EUC) arm (22%) and in the problem‐solving therapy‐addiction (PST‐AD) arm (29%) had missed appointments in the previous three months. However, at follow‐up, a greater proportion in the EUC arm (44%) than in the PST‐AD arm (21%) had missed appointments in the prior 3 months. Given that this was a pilot study with a small number of participants, this finding was not statistically significant (P = 0.266). The intervention used a stepped care approach, and 36% (5/14) of participants were ‘stepped up’ to step two (to be seen by the psychologist at session four). Two of these 14 patients (14%) were then further referred to see a doctor for step three (to be commenced on antidepressant therapy). Patel 2017 reported unplanned hospitalisations in 3/245 and 7/248 participants in the usual care and LHW‐led interventions care arms, respectively (P = 0.34; RR 0.43, 95% CI 0.11 to 1.66; 1 study, 493 participants; very low certainty due to serious indirectness and very serious imprecision).
7. Adverse events
Three trials reported a monitoring process for adverse events (Chibanda 2016; Murphy 2020; Patel 2017). In Chibanda 2016, no evidence of harm was associated with the intervention. At follow‐up, 32 participants (12.3%) in the control group and 6 (2.3%) in the intervention group were identified as having suicidal ideation. In Murphy 2020, the monitoring committee met three times during the trial and identified no concerns regarding safety or adverse events. In Patel 2017, deaths and suicide attempts were infrequent and were similar between groups (6 (2%) in the EUC plus Health Activity Programme (HAP) group versus 3 (1%) in the EUC alone group). LHW‐led interventions may reduce the likelihood of suicidal ideation or attempts compared to usual care (RR 0.29, 95% CI 0.14 to 0.58; 2 studies, 1014 participants; low certainty due to indirectness and inconsistency). The evidence is uncertain regarding whether LHW‐led interventions have any effect on deaths compared to usual care.
| Study | Adverse events | |||
| Chibanda 2016 | No evidence of harm was associated with the intervention. At follow‐up, 32 participants (12.3%) in the control group and 6 (2.3%) in the intervention group were identified as having suicidal ideation | |||
| Murphy 2020 | The committee met 3 times during the trial and identified no concerns regarding safety or adverse events | |||
| Patel 2017 | At 6 months, in the HAP plus EUC group vs the EUC alone group, there were 2 deaths (1%) vs none (P = 0.24) and 4 suicide attempts (2%) vs 3 (1%) (P = 0.72) At 12 months, serious adverse events were infrequent and prevalence was similar by arm Prevalence of serious adverse events (HAP plus EUC arm, 23; EUC arm, 23) and proportion of participants prescribed antidepressant medications (HAP plus EUC arm, 7; EUC arm, 11) did not differ between intervention arms |
|||
Secondary outcomes
1. Cost analyses and resource use
Economic data related to Patel 2017 are reported below under economic data analysis.
2. Online psychological interventions facilitated by LHW
Two trials involving LHWs facilitating the delivery of online psychological interventions were not included in the meta‐analysis because interventions were very different from one other and from other interventions in the above comparisons (Arjadi 2018; Jiang 2017).
Arjadi 2018 evaluated an 8‐week online psychoeducational and behavioural activation intervention supported by lay counsellors for adults with PHQ‐9 scores ≥ 10 in urban Indonesia. The control group received online psychoeducation only. A total of 159 and 154 subjects were randomised to the intervention arm and the comparison arm, respectively. At 10 weeks (2 weeks post intervention), participants in the intervention group had a 50% higher chance of remission at 10 weeks (RR 1.5, 95% CI 1.19 to 1.88; P < 0.0001). PHQ‐9 scores were significantly lower in the intervention group than in the comparison group (mean difference (MD) –1.26 points, 95% CI –2.29 to –0.23; P = 0.017). The effect was sustained over time up to 6 months (effect size = 0.24 at 10 weeks, 0.24 at 3 months, and 0.27 at 6 months). No adverse events were reported in either group.
Jiang 2017 evaluated a 6‐month online intervention in Hunan Province for Chinese ‘empty nesters’ aged ≥ 60 years who scored 11 to 25 on the Geriatric Depression Scale (GDS). Nursing school students and community volunteers trained participants to use Chinese social media platforms, online shopping, online health services, and other cloud‐based services. Services included a 24‐hour health and psychology help line run by doctors and nurses to help with physical and mental health issues. The comparison group had access to the usual community health care but did not receive any additional interventions. Of the 80 participants, 40 were randomised to the intervention and 40 to the control. At baseline, mean GDS scores were 17.72 (standard deviation (SD) ± 4.78) in the intervention group and 16.65 (SD ± 4.84) in the control group (P = 0.945). At the end of the 6‐month intervention, mean GDS scores were 11.82 (SD ± 3.86) in the intervention group and 15.02 (SD ± 4.26) in the control group (P = 0.001), indicating that GDS scores were significantly lower in the intervention group.
Comparison 2. Primary‐level worker‐led collaborative care versus usual care in treating common mental disorders (CMDs) in adults
We identified 13 studies from 7 countries across Africa (Adewuya 2019; Gureje 2019 (STEPCARE); Jenkins 2013; Oladeji 2015), South America (Araya 2003; Fritsch 2007), and Asia (Chen 2015; Indu 2018; Jordans 2019; Niemi 2016; Patel 2010; Pradeep 2014; Xie 2019).
Details of study settings, participants, interventions, and comparisons are described in Appendix 4.
A summary of key findings has been tabled in Table 2.
Primary outcomes
1. Recovery from common mental disorders
2.1. Analysis.

Comparison 2: Primary‐level worker‐led collaborative care versus usual care in treating common mental disorders in adults, Outcome 1: Recovery from common mental disorders
Seven studies reported recovery from CMDs, defined as scoring < 6 on the PHQ‐9 in Adewuya 2019 and Gureje 2019 (STEPCARE), scoring ≤ 4 on the PHQ‐9 in Niemi 2016, scoring < 5 or having a 50% reduction from baseline on the PHQ‐9 in Oladeji 2015, scoring < 8 on the HDRS in Araya 2003, scoring < 7 on the HDRS in Chen 2015, and scoring ≤ 11 on the GDS in Xie 2019. Collaborative care may increase the likelihood of short‐term remission/recovery from CMDs up to 1 month post intervention compared to usual care (RR 3.75, 95% CI 1.68 to 8.34; 5 studies, 781 participants; I² = 70%; P = 0.001; low certainty due to serious study limitations and inconsistency) (Adewuya 2019; Araya 2003; Chen 2015; Niemi 2016; Xie 2019). Collaborative care may increase the likelihood of intermediate‐term recovery from CMDs at 1 to 6 months post intervention compared to usual care (RR 2.26, 95%CI 1.50 to 3.43; 5 studies, 804 participants; I² = 67%; P = 0.0001; low certainty due to serious study limitations and inconsistency) (Adewuya 2019; Araya 2003; Chen 2015; Oladeji 2015; Xie 2019). For recovery from CMD > 6 months post intervention, pooled results for this outcome are not reported due to very high statistical heterogeneity (98% to 99%). Rather, the individual results of contributing trials are reported. It is uncertain whether collaborative care has any effect on long‐term recovery from CMDs at > 6 months post intervention compared to usual care. In Adewuya 2019, recovery was observed in 60.3% of participants in the intervention group compared to 18.2% in the control group (absolute risk reduction 3.10, 95% CI 2.15 to 3.87). In Chen 2015, recovery was observed in 57% of participants in the intervention group compared to 9% in the control group (odds ratio (OR) 12.7%, 95% CI 9.5 to 17). In Gureje 2019 (STEPCARE), however, recovery was similar in the intervention and control groups, at 76% and 77%, respectively (OR 1.0, 95% CI 0.7 to 1.4). The certainty of evidence was very low due to serious study limitations, serious inconsistency, and serious imprecision (3 studies, 922 participants; Adewuya 2019; Chen 2015; Gureje 2019 (STEPCARE).
2. Prevalence of common mental disorders (CMDs)
2.2. Analysis.

Comparison 2: Primary‐level worker‐led collaborative care versus usual care in treating common mental disorders in adults, Outcome 2: Prevalence of common mental disorders
Two studies reported change in prevalence of CMDs, defined as one minus the percentage recovered in Araya 2003, and International Classification of Diseases Tenth Revision (ICD‐10) diagnosis of CMD on the Clinical Interview Schedule ‐ Revised (CIS‐R) in Patel 2010. Collaborative care may reduce the intermediate‐term prevalence of CMDs at 1 to 6 months post intervention compared to usual care, although the range at which the actual effect may be noted indicates that collaborative care may have little or no effect (RR 0.57, 95% CI 0.32 to 1.01; 2 studies, 781 participants; I² = 86%; P = 0.05; low certainty due to serious inconsistency and imprecision) (Araya 2003; Patel 2010). Collaborative care may have little or no effect on reducing the longer‐term prevalence of CMDs at 1 year post intervention compared to usual care (RR 0.95, 95% CI 0.68 to 1.33; 1 study, 2009 participants; P = 0.77; low certainty due to serious indirectness and imprecision) (Patel 2010).
3. Severity of depression/CMD symptoms
2.3. Analysis.

Comparison 2: Primary‐level worker‐led collaborative care versus usual care in treating common mental disorders in adults, Outcome 3: Common mental disorder symptoms
Severity of depression/CMD symptoms was measured in 12 studies (CMD scores: Jenkins 2013; Patel 2010; depression scores: Araya 2003; Chen 2015; Gureje 2019 (STEPCARE); Fritsch 2007; Indu 2018; Jenkins 2013; Jordans 2019; Niemi 2016; Oladeji 2015; Xie 2019).
It is uncertain whether collaborative care has any effect on short‐term symptom severity of depression/CMDs up to 1 month post intervention compared to usual care because the certainty of the evidence is very low (SMD ‐1.23, 95% CI ‐1.68 to ‐0.79; 2 studies, 118 participants; I² = 0%; P < 0.00001; very low certainty due to serious indirectness, serious imprecision, and serious study limitations) (Niemi 2016; Xie 2019).
Collaborative care interventions may slightly reduce intermediate‐term symptom severity of depression/CMDs in the intermediate term at 1 to 6 months post intervention when compared to usual care in studies with change from baseline data (SMD ‐0.35, 95% CI ‐0.63 to ‐0.08; 6 studies, 4419 participants; I² = 89%; P = 0.01; low certainty due to serious inconsistency and serious imprecision) (Araya 2003; Gureje 2019 (STEPCARE); Jenkins 2013; Jordans 2019; Oladeji 2015; Patel 2010).
For longer‐term symptom severity of CMDs at > 6 months post intervention, pooled results for this outcome are not reported due to very high statistical heterogeneity (98% to 99%). Rather, the individual results of contributing trials are reported. It is uncertain whether collaborative care has any effect on longer‐term symptom severity of CMDs at > 6 months post intervention compared to usual care. Chen 2015 reported a between‐group difference of ‐6.5 points on the HDRS favouring the intervention group (95% CI ‐7.1 to ‐5.9). In Gureje 2019 (STEPCARE), the adjusted mean difference in PHQ‐9 scores between intervention and control groups was only ‐0.3 (95% CI ‐0.7 to 0.1). In Jordans 2019, the adjusted mean difference in PHQ‐9 scores differed by ‐3.7, favouring the intervention group (95% CI ‐5.7 to ‐1.7). In Patel 2010, amongst participants who were diagnosed with depression based on the ICD‐10, the mean difference in CIS‐R scores between intervention and control groups was ‐2.14, favouring the intervention group (95% CI ‐4.32 to 0.04). The certainty of evidence is very low due to serious study limitations, serious inconsistency, and serious imprecision (4 studies, 3274 participants; Chen 2015; Gureje 2019 (STEPCARE); Jordans 2019; Patel 2010).
It is uncertain whether collaborative care has any effect on the short‐term symptom severity of depression up to 1 month post intervention compared to usual care in studies with endpoint data (SMD ‐0.87, 95% CI ‐1.55 to ‐0.19; 1 study, 38 participants; P = 0.01; very low certainty due to serious study limitations, serious indirectness, serious imprecision, and sparse data) (Indu 2018).
Collaborative care may slightly reduce intermediate‐term symptom severity of depression/CMDs at 1 to 6 months in studies with endpoint data (SMD ‐0.30, 95% CI ‐0.5 to ‐0.09; 3 studies, 548 participants; I² = 0%; P = 0.004; low certainty due to serious indirectness and serious imprecision) (Fritsch 2007; Pradeep 2014; Xie 2019).
We could not examine differences in the severity of CMDs between outcomes for government and private facilities due to limited data.
4. Quality of life in adults with common mental disorders
2.4. Analysis.

Comparison 2: Primary‐level worker‐led collaborative care versus usual care in treating common mental disorders in adults, Outcome 4: Quality of life
Eight studies reported QOL in adults with CMD (Araya 2003; Chen 2015; Fritsch 2007; Gureje 2019 (STEPCARE); Indu 2018; Jenkins 2013; Oladeji 2015; Pradeep 2014). It is uncertain whether collaborative care improved short‐term QOL up to 1 month post intervention in people with CMDs compared to usual care because the certainty of evidence was very low (SMD 0.73, 95% CI 0.47 to 0.99; 2 studies, 249 participants; I² = 0%; P < 0.00001; very low certainty due to serious study limitations, indirectness, and sparse data) (Araya 2003; Indu 2018). Collaborative care may slightly improve intermediate‐term QOL (1 to 6 months post intervention) in people with CMDs compared to usual care (SMD 0.34, 95% CI 0.16 to 0.53; 6 studies, 2199 participants; I² = 75%; P = 0.0003; low certainty due to serious inconsistency and imprecision) (Araya 2003; Fritsch 2007; Gureje 2019 (STEPCARE); Jenkins 2013; Oladeji 2015; Pradeep 2014). For long‐term QOL, pooled results for this outcome are not reported due to very high statistical heterogeneity (98% to 99%). Rather, the individual results of contributing trials are reported. It is uncertain whether collaborative care has any effect on long‐term QOL at > 6 months post intervention for people with CMDs compared to usual care. In Chen 2015, the between‐group difference in 12‐Item Short Form Survey (SF‐12) scores was 9.6, favouring the intervention group (95% CI 8.1 to 11.1), but in Gureje 2019 (STEPCARE), intervention and control groups scored similarly on the World Health Organization Quality of Life Instrument, Short Form (WHOQOL‐BREF) (SMD 0.08, 95% CI ‐0.08 to 0.25). The certainty of evidence was very low due to serious study limitations, serious inconsistency, and serious imprecision (2 studies, 711 participants; Chen 2015; Gureje 2019 (STEPCARE)).
5. Functional impairment and disability in adults with common mental disorders (CMDs)
2.5. Analysis.

Comparison 2: Primary‐level worker‐led collaborative care versus usual care in treating common mental disorders in adults, Outcome 5: Functional impairment
Seven studies reported functional impairment and disability in adults with CMDs (Araya 2003; Fritsch 2007; Gureje 2019 (STEPCARE); Jenkins 2013; Jordans 2019; Oladeji 2015; Patel 2010). Collaborative care interventions probably have little to no effect on intermediate‐term functional impairment at 1 to 6 months post intervention in people with CMDs compared to usual care (SMD ‐0.13, 95% CI ‐0.28 to 0.03; 7 studies, 4701 participants; I² = 48%; P = 0.11; moderate certainty due to serious imprecision) (Araya 2003; Fritsch 2007; Gureje 2019 (STEPCARE); Jenkins 2013; Jordans 2019; Oladeji 2015; Patel 2010). It is uncertain whether collaborative care has any effect on functioning in people with CMDs at > 6 months post intervention compared to usual care because the certainty of evidence was very low (SMD ‐0.22, 95% CI ‐0.56 to 0.13; 3 studies, 2591 participants; I² = 83%; P = 0.22; very low certainty due to serious inconsistency and very serious imprecision) (Gureje 2019 (STEPCARE); Jordans 2019; Patel 2010).
6. Service utilisation
Two trials reported service utilisation outcomes (Adewuya 2019; Oladeji 2015). In Adewuya 2019 at 12 months, compared with the intervention group, the EUC group reported more referrals to the mental health team (21.1% versus 9.6%) and more losses to follow‐up (21.3% versus 14.3%). In Oladeji 2015, all 165 participants received the first intervention session, 123 (74.5%) received at least two sessions, and 34.6% completed at least six sessions of counselling. Of the 165 participants, 42 (25.5.%) were prescribed antidepressants, and 25 (60%) of these participants completed at least 3 months of treatment. A total of 48 (29%) participants were discussed with the primary care physician by telephone; of these, 17 participants (10.3%) required an in‐person consultation with the primary care physician, and 3 (2%) were referred to a psychiatrist. Based on results from Adewuya 2019, stepped care interventions led by primary health workers may reduce referral to mental health specialists for people with CMDs (at 7 to 12 months post intervention) compared to usual care (RR 0.46, 95% CI 0.33 to 0.64; 1 study, 907 participants; low certainty due to very serious indirectness).
7. Adverse events
Only five trials reported adverse events (Adewuya 2019; Gureje 2019 (STEPCARE); Indu 2018; Jenkins 2013; Patel 2010). In Adewuya 2019 at the 12th month, fewer deaths (adjusted RR 0.20, 95% CI 0.07 to 0.65) and fewer cases of deliberate self‐harm (adjusted RR 0.28, 95% CI 0.15 to 0.55) were reported with the stepped care intervention compared to usual care. The 18 deaths that occurred within the 12‐month follow‐up were investigated and were deemed to be unrelated to study procedures. Gureje 2019 (STEPCARE) reported 17 deaths in the intervention group (n = 562) and 16 in the control group (n = 473); however all adverse events were deemed not related to the study. In Indu 2018, adverse events were monitored but none occurred. In Jenkins 2013, no adverse events were reported. In Patel 2010, there was no difference in suicide attempts among those diagnosed with CMDs at 1 year (RR 0.56, 95% CI 0.24 to 1.32; 1905 participants) and within 2 to 6 months. For deaths, the certainty of evidence was very low due to very serious imprecision and serious indirectness. For attempted suicide or deliberate self‐harm, PW‐led collaborative care may reduce risk compared to usual care (RR 0.36, 95% CI 0.26 to 0.66; 2 studies, 3336 participants; low certainty due to very serious imprecision).
| Study | Adverse events |
| Adewuya 2019 |
At 12 months, compared with the stepped care intervention group, the enhanced usual care group reported significantly more deaths (15/451; 3.3% vs 3/456; 0.6%) and more cases of deliberate self‐harm (35/451; 7.8% vs 10/456; 2.2%) |
| Gureje 2019 (STEPCARE) | At 12 months, 17 deaths had occurred in the intervention group (total 562) and 16 in the control group (total 473). Deaths were due to hypertension or heart disease in 8 patients, tuberculosis in 6 patients, diabetes in 2 patients, liver failure in 3 patients, typhoid fever in 2 patients, asthma in 2 patients, cancer in 1 patient, and old age or unknown causes in 9 patients. One patient developed psychosis and 1 developed bipolar disorder in the intervention group, and 1 developed bipolar disorder in the control group. Suicidal ideation was reported in 57 participants in the intervention group (10%) and in 66 participants in the control group (14%). All adverse events were deemed not related to study procedures |
| Jenkins 2013 | No deaths in either arm No reported mention of suicide attempt |
| Indu 2018 | No serious adverse events including new onset of suicidality or bipolar disorder or psychotic features |
| Patel 2010 | There were 7 serious adverse events (3 deaths and 4 suicide attempts) in the collaborative stepped care group and 12 in the enhanced usual care group (6 deaths and 6 suicide attempts). None of the deaths were the result of suicide |
Secondary outcomes
1. Cost analyses and resource use
Three trials reported cost‐effectiveness analyses (Gureje 2019 (STEPCARE); Patel 2010; Araya 2003). These results are reported below under economic analysis.
Comparison 3. Lay health worker‐led psychosocial interventions versus usual care in treating perinatal depression
We identified seven studies in five countries across Africa (Chibanda 2014; Lund 2020; Peltzer 2019), the Middle East (Milani 2015), and South Asia (Fuhr 2019; Rahman 2008; Sikander 2019). These studies were conducted in urban settings (Chibanda 2014; Lund 2020; Milani 2015), in rural settings (Peltzer 2019; Rahman 2008; Sikander 2019), and in both urban and rural settings (Fuhr 2019). An eighth study ‐ Khan 2017, which has been included in the post‐traumatic stress disorder (PTSD) adult comparison but is only narratively described alongside findings here, was performed in Pakistan. One study ‐ Peltzer 2019 ‐ recruited women who had HIV.
Details of study settings, participants, interventions, and comparisons are described in Appendix 5.
A summary of key findings have been tabled in Table 3.
Primary outcomes
1. Recovery from perinatal depression
(Analysis 3.1).
3.1. Analysis.

Comparison 3: Lay health worker‐led psychosocial interventions versus usual care in treating perinatal depression, Outcome 1: Recovery from depression
Immediately post intervention (up to 1 month), LHW‐led psychosocial interventions compared to usual care probably increase the likelihood of women going into remission (short‐term recovery up to 1 month post intervention) from depression (RR 1.19, 95%CI 1.08 to 1.31; 679 participants, 2 studies; I² = 0%; P = 0.0005; moderate certainty due to serious imprecision), defined as scoring < 5 on the PHQ‐9 in Fuhr 2019 and Sikander 2019.
Similarly, at 1 to 6 months post intervention, LHW interventions compared to usual care may increase the likelihood of recovery from perinatal depression (RR 1.29, 95%CI 1.08 to 1.54; 1243 participants, 4 studies; I² = 72%; P = 0.005; low certainty due to serious inconsistency and imprecision) (Peltzer 2019, where recovery was defined as scoring ≤ 12 on the Edinburgh Postnatal Depression Scale (EPDS); Fuhr 2019 and Rahman 2008, where recovery was determined by structured interview for DSM‐IV diagnosis; Sikander 2019). A sensitivity analysis was performed without Peltzer 2019, as it is also included in the prevention review (Purgato 2021), and had only 50% of women at baseline with depression with similar results (RR 1.30, 95% CI 1.04 to 1.63; 3 studies; I² = 81; P = 0.02).
However, in the long term (> 6 months post intervention), it is uncertain whether LHW interventions have any effect on recovery from perinatal depression (RR 1.39, 95% CI 0.94 to 2.06; 919 participants, 3 studies; I² = 85%; P = 0.18; very low certainty due to serious study limitations, inconsistency, and imprecision) (Lund 2020; Peltzer 2019; Rahman 2008). A sensitivity analysis performed without Peltzer 2019 changed the effect estimate and the confidence interval, making us more uncertain about the finding (RR 1.73, 95% CI 1.46 to 2.03; 761 participants, 2 studies; I² = 0%; P < 0.00001).
2. Prevalence of perinatal depression
No studies reported on this outcome.
3. Severity of perinatal depressive symptoms
3.2. Analysis.

Comparison 3: Lay health worker‐led psychosocial interventions versus usual care in treating perinatal depression, Outcome 2: Depression symptoms
It is uncertain whether LHW interventions have any effect on severity of perinatal depressive symptoms post intervention (SMD ‐0.31, 95% CI ‐0.51 to ‐0.11; 5 studies, 1062 participants; I² = 52%; very low certainty due to serious study limitations, inconsistency, and imprecision), but moderate‐certainty evidence indicates that LHW interventions probably slightly reduce perinatal depressive symptoms at 1 to 6 months (SMD ‐0.26, 95% CI ‐0.37 to ‐0.14; 1989 participants, 5 studies; I² = 36%; moderate certainty due to serious imprecision). It is uncertain whether LHW interventions have any effect on perinatal depressive symptoms at 12 months (SMD ‐0.35, 95% CI ‐0.53 to ‐0.16; 1274 participants, 3 studies; I² = 55%; very low certainty due to serious study limitations, inconsistency, and imprecision).
Removing Peltzer 2019 (also in prevention review, as only 50% of participants were depressed at baseline) did not affect effect estimates or confidence intervals for medium‐ or long‐term outcomes.
Khan 2019, which is included in the adult PTSD comparison, reported similar findings for the immediate post intervention outcome, as it suggests a slight reduction (improvement) in SRQ‐20 (depression) score (MD ‐1.08, 95% CI ‐2.75 to 0.59; 71 participants; P = 0.2) versus usual care.
4. Quality of life
No studies reported on this outcome.
5. Functional impairment and disability
3.3. Analysis.

Comparison 3: Lay health worker‐led psychosocial interventions versus usual care in treating perinatal depression, Outcome 3: Functional impairment
Immediately (up to 1 month) post intervention, moderate‐certainty evidence shows that LHWs probably slightly reduce functional impairment (SMD ‐0.22, 95% CI ‐0.35 to ‐0.10; 3 studies, 966 participants; I² = 0%; moderate certainty due to serious imprecision). The effects of LHWs may be sustained, as they may also slightly reduce functional impairment in the medium term (1 to 6 months) (SMD ‐0.23, 95% CI ‐0.41 to ‐0.04; 4 studies, 1856 participants; I² = 73%; P = 0.02; low certainty due to serious inconsistency and imprecision). In the long term (> 6 months post intervention), the effect of LHW‐led interventions is uncertain (SMD ‐0.26, 95% CI ‐0.86 to 0.34; 2 studies, 1116 participants; I² = 95%; P = 0.40; very low certainty due to serious inconsistency and very serious imprecision).
6. Service utilisation
Fuhr 2019, Lund 2020, and Sikander 2019 reported service utilisation outcomes for maternal services and admissions (child and maternal admissions were recorded together as admissions in Fuhr 2019). In Lund 2020, LHWs may not have any effect on the likelihood of postnatal visits (RR 0.97, 95% CI 0.84 to 1.12; P = 0.690) or completed immunisations (RR 1.02, 95% CI 0.94 to 1.10; P = 0.664; low‐certainty evidence) versus enhanced usual care, although they may reduce the likelihood of admission to hospital for children (RR 0.64, 95% CI 0.29 to 1.43; P = 0.276; low‐certainty evidence). In Sikander 2019, LHWs seemed to have no effect on the mean number of visits to ante/postnatal clinics (intervention n = 104, mean 3.81, 95% CI 3.26 to 4.35 versus control n = 94, mean 3.58, 95% CI 3.06 to 4.11) nor on admissions as inpatients (intervention n = 79, mean 1.05, 95% CI 1.00 to 1.10 versus control n = 72, mean 1.11, 95% CI 1.00 to 1.22) (intervention: 9 out of 283 participants had at least one hospitalisation versus control: 11 out of 283 participants had at least one hospitalisation; all except one in the control arm were child admissions) over the period of the study (1 to 6 months post intervention). Fuhr 2019 recorded hospitalisations as serious adverse events: 11 out of 140 in the intervention group compared to 7 out of 140 in the control group were hospitalised; 9 were child admissions and 9 were maternal admissions (not stated how many child and maternal admissions occurred in each arm). It is uncertain whether LHW‐led interventions have any effect on hospitalisations compared to enhanced usual care (RR 1.12, 95% CI 0.60 to 2.09; 2 studies, 850 participants; very low certainty due to serious inconsistency and very serious imprecision).
7. Adverse events
3.4. Analysis.
Comparison 3: Lay health worker‐led psychosocial interventions versus usual care in treating perinatal depression, Outcome 4: Adverse events
| Adverse events | |
| Study | Heading 1 |
| Chibanda 2014 | 3 participants in pharmacological arm discontinued study drug due to adverse effects. |
| Fuhr 2019 | Overall, 24 (17%) participants in the intervention group and 27 (19%) participants in the control group had at least one serious adverse event; the most common of these events were hospital admissions, experiences of physical violence, and stigmatisation. However, there was no evidence of any differences between the groups. |
| Lund 2020 | There were no significant harms associated with the intervention, and no notable differences between the two arms in the number of adverse events. |
| Sikander 2019 | Overall, 43 (15%) women in the intervention group and 47 (16%) women in the control group had at least one serious adverse event, which were evenly distributed between the groups (p=0·72). The most common serious adverse events were death of the child, hospital admissions (mainly of the child), and experience of physical violence; however, there was no evidence of any difference between the groups. |
LHW‐led interventions for women with perinatal depression may have little or no effect on maternal deaths compared with enhanced usual care at 1 to 6 months post intervention (no deaths in either arm; RR not calculable; 4 studies, 1205 participants; low‐certainty evidence due to very serious imprecision).
| Study | Adverse events |
| Chibanda 2014 | 3 participants in the control arm (pharmacological arm) discontinued the study drug due to adverse effects |
| Fuhr 2019 | Overall, 24 (17%) participants in the intervention group and 27 (19%) participants in the control group had at least 1 serious adverse event; the most common of these events were hospital admissions, experiences of physical violence, and stigmatisation. However, there was no evidence of any differences between groups |
| Lund 2020 | No significant harms were associated with the intervention, and no notable differences in the number of adverse events were observed between the 2 arms |
| Sikander 2019 | Overall, 43 (15%) women in the intervention group and 47 (16%) women in the control group had at least 1 serious adverse event; adverse events were evenly distributed between groups (P = 0·72). The most common serious adverse events were death of the child (24 (8%) in the intervention group vs 25 (9%) in the control group; P = 0.92), hospital admissions (mainly of the child – intervention 9 (3%) vs control 11 (4%); P = 0.66), and experiences of physical violence (intervention 7 (2%), control 9 (2%); P = 0.63), with no evidence of any differences between groups. No deaths occurred in either group, although 2 (1%) suicide attempts were reported in the intervention group |
Secondary outcomes
1. Cost analyses and resource use
Three studies assessed cost‐effectiveness of lay health worker interventions versus enhanced usual care in pregnant women 18 years of age or older (Fuhr 2019; Lund 2020; Sikander 2019). See below for economic analysis.
Comparison 4. Primary health professional‐led collaborative interventions versus enhanced usual care in treating perinatal depression
We identified two studies from two countries in South America and Africa (Gureje 2019 (EXPONATE); Rojas 2007), which were conducted in urban settings (in Rojas 2007), and in both urban and rural settings (in Gureje 2019 (EXPONATE)). These trials recruited mothers from primary healthcare (PHC) clinics in the second trimester of pregnancy (Gureje 2019 (EXPONATE)), or in the first postnatal year (Rojas 2007). Participants had a lower socioeconomic background.
Other details of study settings, participants, interventions, and comparisons are described in Appendix 6.
A summary of key findings has been tabled in Table 4.
Primary outcomes
1. Recovery from perinatal depression
4.1. Analysis.

Comparison 4: Primary health professional‐led collaborative interventions versus enhanced usual care in treating perinatal depression, Outcome 1: Recovery from depression
Primary health professional (PHP)‐led collaborative interventions may have little to no effect on the likelihood of remission from perinatal depression (defined as scoring < 6 on the EPDS) compared with enhanced usual care immediately post intervention (RR 0.90, 95% CI 0.63 to 1.07 (ORs reported in analysis: OR 0.77, 95% CI 0.47 to 1.25); 1 study ‐ Gureje 2019 (EXPONATE), 576 participants; low certainty due to serious indirectness and imprecision). No data were provided for the time points of 1 to 6 months (medium term) or > 6 months post intervention (long term).
2. Prevalence of perinatal depression
No studies reported on this outcome.
3. Severity of perinatal depressive symptoms
4.2. Analysis.

Comparison 4: Primary health professional‐led collaborative interventions versus enhanced usual care in treating perinatal depression, Outcome 2: Depression symptoms
Primary health professional‐led collaborative interventions may have little to no effect on perinatal depressive symptoms immediately post intervention (MD ‐2.44, 95% CI ‐5.86 to 0.99; 2 studies, 806 participants; I² = 90%; P = 0.16; low certainty due to serious inconsistency and imprecision). Due to very low‐certainty evidence, it is uncertain whether there was any effect at 1 to 6 months post intervention (MD ‐1.60, 95% CI ‐3.49 to 0.29; 1 study, 230 participants; very low certainty due to serious indirectness, and very serious imprecision) (Rojas 2007). Primary health professionals probably have little to no clinically relevant effect on perinatal depressive symptoms in the long term at > 6 months post intervention (MD ‐0.9, 95% CI ‐1.7 to ‐0.1; 1 study, 686 participants; moderate certainty due to serious indirectness) (Gureje 2019 (EXPONATE)).
4. Quality of life
4.3. Analysis.

Comparison 4: Primary health professional‐led collaborative interventions versus enhanced usual care in treating perinatal depression, Outcome 3: Quality of life
Primary health professional‐led collaborative interventions may improve mothers’ QOL (MD 18.30, 95% CI 10.42 to 26.18; 1 study, 230 participants; low certainty due to serious indirectness and imprecision) on the 36‐Item Short Form Survey (SF‐36) social functioning scale – an 18‐point change on the score shows about 20% improved QOL ‐ immediately post intervention. Due to very low‐certainty evidence, it is uncertain whether PHPs have any effect on mothers' QOL 1 to 6 months post intervention (MD 3.50, 95% CI ‐4.55 to 11.55; 1 study, 230 participants; very low certainty due to serious indirectness and very serious imprecision).
5. Functional impairment or disability
4.4. Analysis.

Comparison 4: Primary health professional‐led collaborative interventions versus enhanced usual care in treating perinatal depression, Outcome 4: Functional impairment
Primary health professional‐led collaborative interventions may have little to no effect on reducing functional impairment of women with perinatal depression immediately post intervention (MD ‐0.6, 95% CI ‐1.10 to ‐0.10; 1 study, 686 participants; low certainty due to serious indirectness and imprecision) or in the long term at > 6 months post intervention (MD ‐0.2, 95% CI ‐0.7 to 0.3; 1 study, 686 participants; low certainty due to serious indirectness and imprecision). No outcomes were reported at 1 to 6 months post interventions.
6. Service utilisation
In Rojas 2007, the number of medical consultations was higher post intervention than in usual care (intervention: mean 1.2, SD 2.1; control: mean 0.5, SD 1.0); however at 3 months, it was no longer certain whether this was the case (intervention: mean 0.2, SD 0.6; control: mean 0.4, SD 1.0; mean difference ‐0.2, 95% CI ‐0.4 to 0.0). The evidence is of very low certainty due to the small number of participants (230 in total) and data derived from just one country.
7. Adverse events
4.5. Analysis.
Comparison 4: Primary health professional‐led collaborative interventions versus enhanced usual care in treating perinatal depression, Outcome 5: Adverse events
| Adverse events | |
| Study | Heading 1 |
| Gureje 2019 (EXPONATE) | There were three maternal deaths, all in the HIT group, none of which was from suicide. Eight miscarriages were recorded: five in the LIT arm and three in the HIT arm. There were 36 stillbirths, 25 (6%) in the HIT group and 11 (5%) in the LIT group. None of the adverse events were judged by the independent Trial Steering Committee to be related to the study procedures. |
It is uncertain whether PHP‐led care increases risk of maternal death compared to enhanced usual care (absolute risk of death 7 per 1000 participants higher, 95% CI 1 per 1000 participants fewer to 14 per 1000 participants higher; 1 study, 686 participants; very low certainty due to serious indirectness and very serious imprecision).
| Study | Adverse events |
| Gureje 2019 (EXPONATE) | Three maternal deaths occurred, all in the high intensity (HIT) group, none of which was the result of suicide. Eight miscarriages were recorded: 5 in the low intensity (LIT) arm and 3 in the HIT arm. A total of 36 stillbirths were reported ‐ 25 (6%) in the HIT group and 11 (5%) in the LIT group. No adverse events were judged by the independent trial steering committee to be related to study procedures |
| Rojas 2007 | Adverse events were not mentioned |
Secondary outcomes
1. Cost analyses and resource use
One study assessed cost‐effectiveness of professional (nurse/midwife)‐led and LHW‐led interventions in pregnant women aged 16 or older versus a low‐intensity intervention (World Health Organization mental health gap programme (mhGAP)) (Gureje 2019 (EXPONATE)). See below for an economic data summary.
Comparison 5. Lay health worker‐led psychological interventions versus usual care in adults with post‐traumatic stress or common mental disorders in humanitarian settings
We identified 15 studies in which participants were refugees (S. Sudanese refugees in Uganda ‐ Tol 2020; Afghan refugees in Malaysia ‐ Shaw 2018; Sudanese refugees in Cairo ‐ Meffert 2014; refugee settlements ‐ Neuner 2008; internally displaced populations ‐ Dybdahl 2001, Yeomans 2010; survivors of conflict in their area ‐ Ayoughi 2012, Khan 2017, Khan 2019, Rahman 2016, Rahman 2019; genocide survivors in Rwanda ‐ Connolly 2011; others who experienced violence ‐ Bonilla‐Escobar 2018; and survivors of gender‐based violence ‐ Bryant 2017, Dawson 2016). Six studies recruited only women (Dybdahl 2001; Khan 2019; Khan 2019; Rahman 2019; Shaw 2018; Tol 2020); one of these recruited only pregnant women (Khan 2017). Participants had a post‐traumatic stress disorder (PTSD) diagnosis (i.e. meeting the diagnostic criteria for PTSD; Meffert 2014 ‐ Harvard Trauma Questionnaire (HTQ); Connolly 2011 and Neuner 2008 ‐ DSM‐IV); had post‐traumatic stress (PTS) (i.e. those with some trauma symptoms who had not been formally diagnosed and those expressing stress/reactionary symptoms not meeting the diagnostic criteria of PTSD; see definition in Table 16) (Dybdahl 2001; Rahman 2016; Rahman 2019 Dybdahl 2001); or had psychological distress/CMDs (Ayoughi 2012; Bonilla‐Escobar 2018; Bryant 2017; Dawson 2016; Khan 2017; Khan 2019; Rahman 2016; Rahman 2019; Shaw 2018; Tol 2020).
The meta‐analysis for PTS symptoms combined change in PTS symptoms for both those with PTS and those with PTSD.
Other details of study settings, participants, interventions, and comparisons are described in Appendix 7.
A summary of key findings has been tabled in Table 5.
Primary outcomes
1. Recovery from post‐traumatic stress disorder
No studies reported this outcome.
2. Prevalence of post‐traumatic stress disorder and depression
5.1. Analysis.

Comparison 5: Lay health worker‐led psychological interventions versus usual care in adults with post‐traumatic stress or common mental disorders in humanitarian settings, Outcome 1: Prevalence of PTSD
It is uncertain whether LHW‐led interventions reduce the number of people with PTSD compared with usual care at 1 to 6 months post intervention (in Bryant 2017 and in narrative exposure therapy arm of Neuner 2008) (RR 0.77, 95% CI 0.30 to 2.00; 2 studies; I² = 84%; 445 participants; very low‐certainty evidence due to serious study limitations, inconsistency, and imprecision). PTSD was diagnosed based on the PTSD Checklist for Diagnostic and Statistical Manual of Mental Disorders (DSM‐5) (PCL) in Bryant 2017 and on DSM‐IV criteria for the Composite International Diagnostic Interview (CIDI) in Neuner 2008.
No data were provided for PTSD recovery in the short or long term.
Rahman 2019 also reported depression prevalence (i.e. people with a score > 10 on the PHQ‐9). This showed a lower likelihood of depression diagnosis 3 months post intervention in the group that had received LHW‐led interventions than in the group that had received enhanced usual care (15% vs 30%; RR 0.49, 95% CI 0.35 to 0.68; OR reported in the study).
3. Severity of post‐traumatic stress (PTS) symptoms
(Analysis 5.2).
5.2. Analysis.

Comparison 5: Lay health worker‐led psychological interventions versus usual care in adults with post‐traumatic stress or common mental disorders in humanitarian settings, Outcome 2: Post‐traumatic stress symptoms
Immediately post‐intervention data from 10 studies suggest that LHW‐led interventions may slightly reduce PTS symptoms (SMD ‐0.38, 95% CI ‐0.49 to ‐0.28; 10 studies, 2536 participants; I² = 34%; P < 0.00001; use of endpoint scores; low certainty due to serious study limitations and imprecision) (Bonilla‐Escobar 2018; Bryant 2017; Dawson 2016; Dybdahl 2001; Khan 2019; Meffert 2014; Rahman 2016; Rahman 2019; Tol 2020; Yeomans 2010). Two studies reporting symptom change scores showed an uncertain effect of LHW‐led psychological interventions on PTS symptoms post intervention (SMD ‐2.52, 95% CI ‐7.08 to 2.04; 2 studies, 174 participants; I² = 97%; use of change scores; very low certainty due to serious study limitations and very serious imprecision) (Connolly 2011; Shaw 2018), as this result was skewed by a very large effect in Shaw 2018 and less so in Connolly 2011.
We pooled five studies, which showed at 1 to 6 months post intervention that it is uncertain whether LHWs improved PTS symptoms (SMD ‐0.27, 95% CI ‐0.41 to ‐0.13; 5 studies, 2045 participants; I² = 50%; P = 0.08; very low certainty due to serious inconsistency, indirectness, and imprecision) (Bryant 2017; Neuner 2008; Rahman 2016; Rahman 2019; Tol 2020).
A sensitivity analysis excluding Neuner 2008 (as it uses quasi‐randomisation) showed a lower effect size and imprecision in the first comparison (SMD ‐0.22, 95% CI ‐0.54 to 0.10; 2 studies, 151 participants; I² = 0%; P = 0.03), with similar results for the other comparisons using the other intervention arms. A subgroup analysis excluding Dybdahl 2001, which was teacher‐led, and therefore retaining only LHWs suggested a slightly higher magnitude of effect (SMD ‐0.47, 95% CI ‐0.90 to ‐0.05; 2 studies, 148 participants; I² = 34%; P = 0.03).
In addition, Connolly 2011 reported that LHW‐led interventions did not reduce PTS symptom severity or frequency at 2 years post intervention compared to immediately post intervention.
4. Severity of depressive symptoms
5.3. Analysis.

Comparison 5: Lay health worker‐led psychological interventions versus usual care in adults with post‐traumatic stress or common mental disorders in humanitarian settings, Outcome 3: Depression symptoms
Immediately post intervention, LHWs may reduce depression severity in studies reporting endpoint scores (SMD ‐0.75, 95% CI ‐1.04 to ‐0.46; 10 studies, 2510 participants; I² = 90%; low certainty due to serious study limitations and inconsistency) (Ayoughi 2012; Bonilla‐Escobar 2018; Bryant 2017; Dawson 2016; Khan 2019; Meffert 2014; Rahman 2016; Rahman 2019; Tol 2020; Yeomans 2010), although evidence from two further studies reporting change scores was uncertain was uncertain (SMD ‐3.51, 95% CI ‐8.90 to 1.87; 2 studies, 174 participants; I² = 97%; very low certainty due to serious study limitations, inconsistency, and very serious imprecision) (Connolly 2011; Shaw 2018). As above, this latter result was skewed due to a very large effect in Shaw 2018 and a much smaller effect in Connolly 2011.
LHW‐led psychological interventions may also slightly reduce depression symptom severity at 1 to 6 months post intervention (SMD ‐0.36, 95% CI ‐0.56 to ‐0.15; 5 studies, 1986 participants; I² = 78%; low‐certainty due to serious inconsistency and imprecision) (Bryant 2017; Khan 2017; Rahman 2016; Rahman 2019; Tol 2020).
However, Connolly 2011 reported that LHW‐led interventions did not reduce symptom severity of depression or anxiety at 2 years post intervention compared to immediately post intervention.
Further analyses of studies that combined emotional distress scores showed that the effect of LHWs was similar/went in the same direction. At all time points, they probably slightly reduce emotional distress post intervention (SMD ‐0.45, 95% CI ‐0.57 to ‐0.33; 3 studies, 1104 participants; I² = 0%; P < 0.00001; moderate certainty due to serious indirectness) (Bryant 2017; Dawson 2016; Tol 2020), and they may continue to do so in the medium term (SMD ‐0.22, 95% CI ‐0.34 to ‐0.10; 2 studies, 1032 participants; I² = 2%; P = 0.0005; low certainty due to serious indirectness and imprecision) (Bryant 2017; Tol 2020). When change scores were measured, the effects of LHW‐led interventions on emotional distress post intervention were uncertain (SMD ‐6.86, 95% CI ‐8.92 to ‐4.80; 1 study, 29 participants; P < 0.00001; very low certainty due to serious study limitations, indirectness, and very serious imprecision) (Analysis 5.5; Shaw 2018).
5.5. Analysis.

Comparison 5: Lay health worker‐led psychological interventions versus usual care in adults with post‐traumatic stress or common mental disorders in humanitarian settings, Outcome 5: Emotional distress symptoms
5. Severity of anxiety symptoms
5.4. Analysis.

Comparison 5: Lay health worker‐led psychological interventions versus usual care in adults with post‐traumatic stress or common mental disorders in humanitarian settings, Outcome 4: Anxiety symptoms
LHWs may reduce anxiety severity post intervention in studies reporting endpoint scores (Ayoughi 2012; Bonilla‐Escobar 2018; Khan 2019; Rahman 2016; Rahman 2019) (SMD ‐0.95, 95% CI ‐1.44 to ‐0.46; 5 studies, 1326 participants; I² = 94%; P = 0.0001; low certainty due to serious study limitations and inconsistency), although the evidence from two further studies reporting change scores is uncertain (SMD ‐3.34, 95% CI ‐8.47 to 1.78; 2 studies, 128 participants; I² = 97%; P = 0.20; very low certainty due to serious study limitations, inconsistency, and very serious imprecision) (Connolly 2011; Shaw 2018).
This effect is carried forward, as in the medium term (1 to 6 months), LHW‐led psychological interventions also may reduce anxiety severity (SMD ‐0.52, 95% CI ‐1.02 to ‐0.02; 2 studies, 883 participants; I² = 92%; low certainty due to serious inconsistency and imprecision) (Rahman 2016; Rahman 2019).
6. Quality of life
5.6. Analysis.

Comparison 5: Lay health worker‐led psychological interventions versus usual care in adults with post‐traumatic stress or common mental disorders in humanitarian settings, Outcome 6: Quality of life
Immediately post intervention, LHWs may slightly improve QOL compared to usual care (SMD ‐0.47, 95% CI ‐0.63 to ‐0.31; 4 studies, 1565 participants; I² = 61%; P < 0.00001; low certainty due to serious inconsistency and indirectness) (Bryant 2017; Khan 2019; Rahman 2019; Tol 2020).
Similarly, at 1 to 6 months post intervention, LHWs probably slightly improve QOL compared to usual care (SMD ‐0.27, 95% CI ‐0.39 to ‐0.15; 4 studies, 1918 participants; I² = 40%; P < 0.00001; moderate certainty due to serious imprecision) (Bryant 2017; Rahman 2016; Rahman 2019; Tol 2020).
No longer‐term time point data (> 6 months) were provided.
7. Functional impairment and disability
5.7. Analysis.

Comparison 5: Lay health worker‐led psychological interventions versus usual care in adults with post‐traumatic stress or common mental disorders in humanitarian settings, Outcome 7: Functional impairment
Post intervention, LHWs may slightly reduce functional impairment (SMD ‐0.40, 95% CI ‐0.56 to ‐0.25; 7 studies, 2357 participants; I² = 67%; P < 0.00001; low certainty due to serious inconsistency and indirectness) (Bonilla‐Escobar 2018; Bryant 2017; Dawson 2016; Khan 2019; Rahman 2016; Rahman 2019; Tol 2020).
We pooled four studies (Bryant 2017; Rahman 2016; Rahman 2019; Tol 2020), which showed that at 1 to 6 months post intervention, it is uncertain whether LHWs have any effect on functional impairment (SMD ‐0.26, 95% CI ‐0.42 to ‐0.10; 4 studies, 1914 participants; I² = 67%; P = 0.001; very low certainty due to serious inconsistency, indirectness, and imprecision).
8. Service utilisation
5.8. Analysis.

Comparison 5: Lay health worker‐led psychological interventions versus usual care in adults with post‐traumatic stress or common mental disorders in humanitarian settings, Outcome 8: Service utilisation (dichotomous outcomes)
Two studies reported dichotomous outcomes for service utilisation; Bryant 2017 suggested that LHW‐led interventions did not result in any difference in the likelihood of hospital admissions compared to usual/no care (RR 1.13, 95% CI 0.53 to 2.34; OR reported in the paper), although the evidence was uncertain due to limitations in study design, indirectness, and a small number of events. Bryant 2017 reported that there was no difference between groups in the number of outpatient consultations (MD 0.03, 95% CI ‐0.59 to 0.53), in instances of medication use (MD 0.29, 95% CI ‐0.54 to 1.12), or in traditional healer engagement (MD 0.12, 95% CI ‐0.03 to 0.27) (all low certainty due to serious study limitations and indirectness). Khan 2017 suggested that a larger number of people receiving LHW‐led care were seeking help compared to those receiving usual care (27 (71%) vs 14 (46%); P = 0.036) (RR 0.35, 95% CI 0.13 to 0.94; 1 study, 71 participants, very low certainty due to serious study limitations, indirectness, and imprecision).
9. Adverse events
Five studies (1702 participants) reported that interventions by LHWs had little to no increased risk of adverse events compared to usual care (no events in each arm; RR incalculable) (Table 5). However, the evidence is uncertain due to serious limitations in design, indirectness, and very serious imprecision.
| Study | Adverse Events |
| Bryant 2017 | No reported adverse effects occurred during treatment in the intervention arm |
| Dawson 2016 | No serious adverse events were reported during the course of treatment or at post treatment assessment in the intervention arm |
| Meffert 2014 | No adverse events occurred. One participant withdrew because her husband forbade her to continue. One dropped out secondary to time constraints |
| Rahman 2019 | No adverse events were recorded |
| Tol 2020 | With regard to safety considerations, the independent data safety management board responded to 6 adverse events (although it is unclear what adverse events occurred), and none were evaluated to be concerns in response to the intervention |
Secondary outcomes
1. Cost analyses and resource use
No economic data were linked to these studies.
Comparison 6. Primary health professional‐led and community professional‐led psychological interventions versus usual or no care in adults with post‐traumatic stress or common mental disorders in humanitarian settings
We identified five studies that addressed survivors of conflict in their area, including survivors of torture (Bass 2016; Bolton 2014 (Iraq); Weiss 2015), Burmese in Thailand (Bolton 2014 (Thailand)), and survivors of gender‐based violence (Bass 2013). Of note, Bass 2013 recruited only women. Recruited adults had trauma exposure and distress (post‐traumatic stress) with significant depressive symptoms (Bolton 2014 (Iraq) Bolton 2014 (Thailand)), met PTSD diagnostic criteria (Bass 2013; Weiss 2015), or had both PTS and PTSD (Bass 2016).
Interventions were delivered by PHPs in Bass 2013, Bass 2016, Bolton 2014 (Iraq), and Weiss 2015, and by community professionals (CPs) in Bolton 2014 (Thailand).
Details of study settings, participants, interventions, and comparisons are described in Appendix 8.
A summary of key findings has been tabled in Table 6.
Primary outcomes
1. Recovery from post‐traumatic stress disorder
No studies reported this outcome.
2. Prevalence of post‐traumatic stress disorder
6.1. Analysis.

Comparison 6: Primary health professional‐ and community professional‐led psychological interventions versus usual or no care in adults with post‐traumatic stress or common mental disorders in humanitarian settings, Outcome 1: Prevalence of PTSD
In Bass 2013, psychosocial assistant/counsellor‐led psychological intervention may result in a large reduction in prevalence of PTSD defined as scoring ≥ 1.75 on the Harvard Trauma Questionnaire (HTQ) immediately post intervention (RR 12.30, 95% CI 5.20 to 29.10; 1 study, 270 participants; P < 0.00001; low certainty due to serious indirectness and imprecision) and at 1 to 6 months post intervention (RR 5.50, 95% CI 2.50 to 12.10; 1 study, 313 participants; P < 0.0001; low certainty due to serious indirectness and imprecision).
There were no longer‐term outcomes (> 7 months).
3. Prevalence of depression or anxiety
6.2. Analysis.

Comparison 6: Primary health professional‐ and community professional‐led psychological interventions versus usual or no care in adults with post‐traumatic stress or common mental disorders in humanitarian settings, Outcome 2: Prevalence of probable depression or anxiety
Primary health professional‐led psychological interventions may greatly reduce the number of people with depression or anxiety defined as scoring ≥ 1.75 on the Hopkins Symptom Checklist‐25 (HSCL‐25) immediately post intervention compared to usual care (RR 7.30, 95% CI 3.40 to 15.68; 1 study, 270 participants; P < 0.00001) and by a factor of five at 1 to 6 months post intervention (RR 4.60, 95% CI 2.10 to 10.08; 1 study, 313 participants; P = 0.0001) (both low certainty due to serious indirectness and imprecision) (Bass 2013).
There were no long‐term outcomes to report.
4. Severity of post‐traumatic stress symptoms
6.3. Analysis.

Comparison 6: Primary health professional‐ and community professional‐led psychological interventions versus usual or no care in adults with post‐traumatic stress or common mental disorders in humanitarian settings, Outcome 3: Post‐traumatic stress symptoms
Immediately post intervention, primary health professional (PHP)‐led interventions may reduce PTS symptom severity compared to usual care (SMD ‐0.71, 95% CI ‐1.11 to ‐0.30; 4 studies (2 arms of Bolton 2014 (Iraq)), 1107 participants; I² = 89%; P = 0.0006; low certainty due to serious study limitations and inconsistency).
At 1 to 6 months post intervention, it is uncertain whether PHP‐led interventions have any effect on PTS symptoms (SMD ‐0.78, 95% CI ‐1.43 to ‐0.13; 2 studies (Weiss 2015 has 2 arms), 680 participants; I² = 93%; P = 0.02; very low certainty due to serious study limitations, inconsistency, indirectness, and imprecision).
There were no long‐term outcome data to report.
5. Severity of depressive symptoms
6.4. Analysis.

Comparison 6: Primary health professional‐ and community professional‐led psychological interventions versus usual or no care in adults with post‐traumatic stress or common mental disorders in humanitarian settings, Outcome 4: Depression symptoms
Immediately post intervention, PHP‐led interventions may reduce depression severity (SMD ‐0.81, 95% CI ‐1.36 to ‐0.26; 4 studies (Bolton 2014 (Iraq) has 2 arms), 1107 participants; I² = 94%; P = 0.004; low certainty due to serious study limitations and inconsistency).
However, at 1 to 6 months post intervention, it is uncertain whether PHP‐led interventions have any effect on depression symptoms (SMD ‐0.91, 95% CI ‐1.73 to ‐0.10; 2 studies (Weiss 2015 has 2 arms included), 680 participants; I² = 96%; P < 0.03; very low certainty due to serious study limitations, inconsistency, indirectness, and imprecision).
6. Severity of anxiety symptoms
6.5. Analysis.

Comparison 6: Primary health professional‐ and community professional‐led psychological interventions versus usual or no care in adults with post‐traumatic stress or common mental disorders in humanitarian settings, Outcome 5: Anxiety symptoms
Immediately post intervention, PHP‐led interventions probably reduce anxiety symptom severity (SMD ‐0.39, 95% CI ‐0.54 to ‐0.24; 3 studies (Bolton 2014 (Iraq) has 2 arms), 837 participants; I² = 1%; P < 0.00001; moderate certainty due to serious study limitations).
However at 1 to 6 months post intervention, the effect of PHP‐led interventions is uncertain (SMD ‐0.67, 95% CI ‐1.37 to 0.03; 1 study (Weiss 2015 2 arms), 367 participants; I² = 89%; P = 0.06; very low certainty due to serious study limitations, inconsistency, indirectness, and imprecision).
7. Quality of life
No data were available for this outcome.
8. Functional impairment and disability
6.6. Analysis.

Comparison 6: Primary health professional‐ and community professional‐led psychological interventions versus usual or no care in adults with post‐traumatic stress or common mental disorders in humanitarian settings, Outcome 6: Functional impairment
Post intervention, PHP‐led interventions may reduce functional impairment (SMD ‐0.65, 95% CI ‐1.01 to ‐0.30; 4 studies (Bolton 2014 (Iraq) has 2 arms); I² = 86%; P = 0.0003; low certainty due to serious study limitations and inconsistency).
However, at 1 to 6 months post intervention, it is uncertain whether PHP‐led interventions reduce functional impairment (SMD ‐0.64, 95% CI ‐1.31 to 0.04; 2 studies (Weiss 2015 has 2 arms included), 680 participants; I² = 94%; P = 0.06; very low certainty due to serious study limitations, inconsistency, indirectness, and imprecision).
9. Service utilisation
In Bolton 2014 (Iraq), three participants in the intervention arms sought psychiatric help and one participant from the control arm was referred to a psychiatrist for worsening symptoms. In Weiss 2015, one participant from the intervention arm was hospitalised for depression. The evidence was of very low certainty due to serious study limitations and very serious imprecision.
10. Adverse events
It is uncertain whether PHP‐led interventions increase or reduce risk of death in the intervention group compared with usual care (RR 2.22, 95% CI 0.23 to 21.34; 5 studies, 1242 participants; very low certainty due to serious study limitations and very small numbers of events).
None of the study investigators believed that the adverse events that occurred were related to the trial.
Two studies reported adverse events. In Bolton 2014 (Iraq), one participant in the intervention group developed psychosis and one control group participant was referred to a psychiatrist. In Weiss 2015, one participant was hospitalised for severe depression, one attempted suicide, and one death due to heart attack (assumed unrelated) occurred.
| Study | Adverse events | |
| Bolton 2014 (Iraq) | One CPT participant was referred for psychosis, and one left the intervention arm after being verbally abused by her husband for getting treatment. One death (out of 50 participants) was reported in the control arm, and one death (out of 215 participants) in the intervention arm, but reasons for these deaths were not given | |
| Weiss 2015 | From the intervention groups, 1 out of 223 participants (although not specified from which ‐ CPT or CETA (common elements treatment approach)), attempted suicide after completing intake and the first therapy session, and 1 died from a heart attack (not clear whether from intervention or control arm). Five (out of 114) participants in the control group were lost to follow‐up with no reason identified | |
|
Bolton 2014 (Thailand); Bass 2013; Bass 2016 |
No adverse events were reported. In Bass 2013, 1 (out of 157 participants) in the intervention arm died (with no reason identified). In Bolton 2014 (Thailand), 1 (out of 182 participants) in the intervention arm died, and this death was deemed unrelated to the study |
Secondary outcomes
1. Cost analyses and resource use
No economic data are linked to these studies.
Comparison 7. Lay health worker‐led interventions versus enhanced usual care in adult patients with harmful or hazardous alcohol or substance use
We found eight studies from Brazil (Christoff 2015), Kenya (Papas 2011; Papas 2020), India (Nadkarni 2017), Nepal (Jordans 2019), South Africa (Peltzer 2013; Sorsdahl 2015), and Thailand (Sherman 2009). They were conducted in both urban ‐ Christoff 2015; Nadkarni 2017; Papas 2011; Papas 2020; Peltzer 2013; Sherman 2009; Sorsdahl 2015 ‐ and rural ‐ Jordans 2019; Peltzer 2013 ‐ settings. Of note, participants in Peltzer 2013 were receiving active tuberculosis (TB) treatment, and patients in Papas 2011 and Papas 2020 were eligible for or had been initiated on antiretroviral therapy for HIV. Participants in Sherman 2009 were recruited first from index patients and then from network contacts. Participants in Christoff 2015 and Sherman 2009 were young adults, and those in Nadkarni 2017 were male only.
Details of study settings, participants, interventions, and comparisons are described in Appendix 9.
A summary of key findings has been tabled in Table 7.
Primary outcomes
1. Recovery from harmful or hazardous alcohol use
7.1. Analysis.

Comparison 7: Lay health worker‐led interventions versus enhanced usual care in adult patients with harmful or hazardous alcohol or substance use, Outcome 1: Clinical recovery ‐ harmful or hazardous alcohol use
Recovery from harmful or hazardous alcohol use was reported at < 1 month post intervention in Papas 2011 (defined as abstinence), at 1 to 6 months post intervention in Jordans 2019 (defined as scoring < 9 on the Alcohol Use Disorders Identification Test (AUDIT)), Nadkarni 2017 (defined as scoring < 8 on the AUDIT), Papas 2011 and Peltzer 2013 (defined as scoring < 7 on the AUDIT), and at < 6 months post intervention in Jordans 2019 and Nadkarni 2017.
At < 1 month post intervention, lay health worker (LHW)‐led interventions may increase the likelihood of recovery from harmful or hazardous alcohol use compared to enhanced usual care (RR 2.55, 95% CI 1.34 to 4.88; 1 study, 75 participants; P = 0.005; low‐certainty due to serious indirectness and imprecision).
At 1 to 6 months post intervention, LWH‐led interventions may increase the likelihood of recovery from harmful or hazardous alcohol use compared with enhanced usual care, although the range at which the actual effect may occur indicates that LHW‐led interventions may have little or no effect (RR 1.28, 95% CI 0.94 to 1.74; 4 studies, 872 participants; I² = 81%; P = 0.11; low certainty due to serious inconsistency and imprecision).
At > 6 months post intervention, LHW‐led interventions may increase the likelihood of recovery from harmful or hazardous alcohol compared to enhanced usual care (RR 1.48, 95% CI 1.05 to 2.10; 2 studies, 477 participants; I² = 47%; P = 0.03; low‐certainty due to serious indirectness and imprecision).
2. Prevalence of harmful or hazardous alcohol or substance use
7.2. Analysis.

Comparison 7: Lay health worker‐led interventions versus enhanced usual care in adult patients with harmful or hazardous alcohol or substance use, Outcome 2: Prevalence ‐ drug use
Prevalence of harmful or hazardous drinking or alcohol dependence was reported in Peltzer 2013. Among the 853 participants who completed follow‐up assessments at 5 months post intervention, 21.2% in the control group and 16.8% in the intervention group reported AUDIT scores in the harmful/hazardous or dependent range (adjusted OR 0.70, 95% CI 0.41 to 1.19; P = 0.186). Prevalence of methamphetamine use was reported in Sherman 2009. At 1 to 6 months post intervention, lay health worker‐led interventions may have little to no effect on prevalence of methamphetamine use compared to enhanced usual care (RR 1.01, 95% CI 0.91 to 1.13; 1 study, 882 participants; P = 0.79; low certainty due to serious study limitations and indirectness).
3. Severity of harmful or hazardous alcohol use ‐ risk of harmful or hazardous alcohol use based on Alcohol, Smoking, and Substance Involvement Screening Test (ASSIST) or Alcohol Use Disorders Identification Test (AUDIT) scores
7.4. Analysis.

Comparison 7: Lay health worker‐led interventions versus enhanced usual care in adult patients with harmful or hazardous alcohol or substance use, Outcome 4: Clinical symptoms ‐ alcohol use (1 to 6 months post intervention)
7.5. Analysis.

Comparison 7: Lay health worker‐led interventions versus enhanced usual care in adult patients with harmful or hazardous alcohol or substance use, Outcome 5: Clinical symptoms ‐ alcohol use (> 6 months post intervention)
Risk of harmful or hazardous alcohol use was reported at 1 to 6 months post intervention in Christoff 2015, Jordans 2019, and Peltzer 2013 and at the more than 6 months time point in Jordans 2019.
At 1 to 6 months post intervention, LHW‐led interventions probably slightly reduce risk of harmful or hazardous alcohol use compared with enhanced usual care (SMD ‐0.22, 95% CI ‐0.32 to ‐0.11; 3 studies, 667 participants; I² = 0%; P < 0.0001; moderate certainty due to serious imprecision) (Analysis 7.4).
At > 6 months post intervention, LHW‐led interventions may have little to no effect on risk of harmful or hazardous alcohol use compared to enhanced usual care (SMD ‐0.13, 95% CI ‐0.44 to 0.18; 1 study, 162 participants; P = 0.41; low certainty due to serious indirectness and imprecision) (Analysis 7.5).
4. Severity of overall harmful or hazardous alcohol and substance use ‐ risk of harmful or hazardous alcohol and substance use based on total ASSIST scores
7.6. Analysis.

Comparison 7: Lay health worker‐led interventions versus enhanced usual care in adult patients with harmful or hazardous alcohol or substance use, Outcome 6: Clinical symptoms ‐ drug and alcohol use
Overall risk of harmful or hazardous alcohol and substance use was reported at 1 to 6 months post intervention in Christoff 2015 and Sorsdahl 2015.
At 1 to 6 months post intervention, LHW‐led interventions probably have little to no effect on risk of harmful or hazardous drug and alcohol use (based on total ASSIST scores) compared with enhanced usual care (SMD ‐0.01, 95% CI ‐0.15 to 0.13; 2 studies, 540 participants; I² = 13%; P = 0.87; moderate certainty due to serious indirectness) (Analysis 7.6).
5. Severity of harmful or hazardous alcohol use ‐ amount of alcohol consumed
(Analysis 7.3 Analysis 7.4 Analysis 7.5)
7.3. Analysis.

Comparison 7: Lay health worker‐led interventions versus enhanced usual care in adult patients with harmful or hazardous alcohol or substance use, Outcome 3: Clinical symptoms ‐ alcohol use (up to 1 month post intervention)
The amount of alcohol consumed was reported at the less than 1 month post intervention time point in Papas 2011 and Papas 2020 at the 1 to 6 month time point in Nadkarni 2017, Papas 2011, and Papas 2020; and at the more than 6 month time point in Nadkarni 2017 and Papas 2020.
At < 1 month post intervention, LHW‐led interventions probably reduce the amount of alcohol consumed compared to enhanced usual care (SMD ‐0.37, 95% CI ‐0.52 to ‐0.22; 2 studies, 684 participants; I² = 0%; P < 0.00001; moderate certainty due to serious indirectness) (Analysis 7.3).
It is however uncertain at 1 to 6 months post intervention whether LHW‐led interventions have any effect on the amount of alcohol consumed compared to enhanced usual care because the certainty of evidence is very low (SMD ‐0.23, 95% CI ‐0.56 to 0.09; 3 studies, 781 participants; I² = 61%; P = 0.15; very low certainty due to serious inconsistency, indirectness, and imprecision) (Analysis 7.4).
At > 6 months post intervention, LHW‐led interventions probably have little to no effect on the amount of alcohol consumed compared to enhanced usual care (SMD ‐0.11, 95% CI ‐0.29 to 0.06; 2 studies, 930 participants; I² = 42%; P = 0.21; moderate certainty due to serious imprecision) (Analysis 7.5).
Some other outcomes pertaining to alcohol consumption were measured in some of the studies and are described below.
In Nadkarni 2017, study authors reported that LHW‐led interventions increased the percent of days abstinent compared to enhanced usual care (adjusted MD 16.0, 95% CI 8.1 to 24.1; 336 participants; P < 0.0001).
At < 1 month post intervention, Papas 2011 reported that LHW‐led interventions decreased the percentage of drinking days compared to enhanced usual care (MCD 24.93, 95% CI 12.43 to 37.43; 70 participants; P = 0.0002). Papas 2020 reported that LHW‐led interventions reduced the percentage of drinking days compared to enhanced usual care (MD 2.76, 95% CI 0.65 to 4.86; 573 participants; P = 0.0102). At 1 to 6 months post intervention, Papas 2011 reported that LHW‐led interventions decreased the percentage of drinking days compared to enhanced usual care (MCD 16.93, 95% CI 3.17 to 30.68; 68 participants). At > 6 months post intervention, Papas 2020 reported that LHW‐led interventions decreased the percentage of drinking days compared to enhanced usual care (MD 2.08, 95% CI 0.13 to 4.04; 520 participants; P = 0.037).
At < 1 month post intervention, Papas 2011 reported that LHW‐led interventions decreased the number of drinks per drinking day compared to enhanced usual care (MCD 2.88, 95% CI 1.05 to 4.70; 70 participants; P = 0.002). Papas 2020 reported similar results with a greater number of participants (MD 0.67, 95% CI 0.38 to 0.96; 573 participants; P < 0.0001). At 1 to 6 months post intervention, Papas 2011 reported that LHW‐led interventions decreased the number of drinks per drinking day compared to enhanced usual care (MCD 2.51, 95% CI 0.35 to 4.68; 68 participants). At > 6 months post intervention, Papas 2020 reported that LHW‐led interventions decreased the number of drinks per drinking day (MD 0.31, 95% CI 0.05 to 0.58; 520 participants; P = 0.0218).
Nadkarni 2017 reported that LHW‐led interventions increased the odds of abstinence in the past 14 days compared to enhanced usual care at 1 to 6 months (adjusted odds ratio 3.00, 95% CI 1.76 to 5.13; 336 participants; P < 0.0001) and at more than 6 months post intervention (adjusted odds ratio 1.92, 95% CI 1.19 to 3.1; 316 participants; P = 0.008).
Nadkarni 2017 reported that LHW‐led interventions had no effect on percentage of days with heavy drinking at 1 to 6 months post intervention (adjusted MD –0.40, 95% CI –5.7 to 4.9; 336 participants; P = 0.88) as well as at > 6 months post intervention (adjusted MD 1.5, 95% CI –4.9 to 7.9; 316 participants; P = 0.65).
Peltzer 2013 reported that at 1 to 6 months post intervention LHW‐led interventions had no effect on heavy episodic drinking compared to enhanced usual care (RR 0.97, 95% CI 0.55 to 1.56 [reported in paper as adjusted OR 0.96, 95% CI 0.46 to 2.02); 853 participants; P = 0.921).
6. Severity of harmful or hazardous substance use ‐ risk of substance use based on ASSIST score
Christoff 2015 reported that at 1 to 6 months post intervention, LHW‐led interventions had no effect on risk of tobacco use (126 participants; P = n.s.), had no effect on risk of marijuana use (70 participants; P = n.s.), and had no effect on risk of other substance use (not tobacco, alcohol, or marijuana) (65 participants; P = n.s.) compared to enhanced usual care.
7. Severity of depression symptoms
7.7. Analysis.

Comparison 7: Lay health worker‐led interventions versus enhanced usual care in adult patients with harmful or hazardous alcohol or substance use, Outcome 7: Depression symptoms
Depression symptom severity was reported at 1 to 6 months post intervention in Sorsdahl 2015 and at > 6 months post intervention in Sherman 2009.
At 1 to 6 months post intervention, it is uncertain whether LHW‐led interventions had any effect on depression symptoms compared to enhanced usual care (MD ‐2.12, 95% CI ‐6.42 to 2.18; 1 study, 335 participants; I² = 63%; P = 0.33; very low certainty due to serious inconsistency, indirectness, and imprecision).
At > 6 months post intervention, LHW‐led interventions may reduce depression symptoms compared to enhanced usual care (MD ‐2.20, 95% CI ‐4.03 to ‐0.37; 1 study, 415 participants; P = 0.02; low certainty due to serious study limitations and indirectness).
8. Quality of life
No data were available for this outcome.
9. Functional impairment and disability
7.8. Analysis.

Comparison 7: Lay health worker‐led interventions versus enhanced usual care in adult patients with harmful or hazardous alcohol or substance use, Outcome 8: Functional impairment
Functional impairment was reported for the 1 to 6 months and the more than 6 months time points in Jordans 2019 and Nadkarni 2017.
At 1 to 6 months post intervention, LHW‐led interventions may have little to no effect on functional impairment compared to enhanced usual care (SMD ‐0.14, 95% CI ‐0.32 to 0.03; 2 studies, 498 participants; I² = 0%; P = 0.11; low certainty due to serious indirectness and imprecision).
Similarly, at > 6 months post intervention, LHW‐led interventions may have little to no effect on functional impairment compared to enhanced usual care (SMD ‐0.1, 95% CI ‐0.28 to 0.08; 2 studies, 478 participants; I² = 1%; P = 0.28; low certainty due to serious indirectness and imprecision).
10. Service utilisation: unplanned hospital admissions
7.9. Analysis.

Comparison 7: Lay health worker‐led interventions versus enhanced usual care in adult patients with harmful or hazardous alcohol or substance use, Outcome 9: Service utilisation ‐ unplanned admissions to hospital
Unplanned hospital admissions were reported in Nadkarni 2017. It is uncertain whether at 1 to 6 months post intervention, LHW‐led interventions had any effect on the likelihood of unplanned admissions to hospital compared with enhanced usual care (RR 0.90, 95% CI 0.29 to 2.72; 1 study, 336 participants; P = 0.85; very low certainty due to serious indirectness and very serious imprecision).
11. Service utilisation: healthcare visits related to alcohol use
7.10. Analysis.

Comparison 7: Lay health worker‐led interventions versus enhanced usual care in adult patients with harmful or hazardous alcohol or substance use, Outcome 10: Service utilisation ‐ healthcare visits
Healthcare visits related to alcohol use were reported in Sorsdahl 2015. At 1 to 6 months post intervention, it is uncertain whether LHW‐led interventions had any effect on the number of healthcare visits compared with enhanced care (MD 0.12, 95% CI ‐0.05 to 0.29; 1 study, 335 participants; P = 0.16; very low certainty due to serious indirectness and very serious imprecision).
12. Adverse events
7.11. Analysis.

Comparison 7: Lay health worker‐led interventions versus enhanced usual care in adult patients with harmful or hazardous alcohol or substance use, Outcome 11: Adverse events
Deaths were reported in Nadkarni 2017, Papas 2011, and Papas 2020. It is uncertain whether LHW‐led interventions had any effect on risk of death compared to enhanced usual care (RR 0.34, 95% CI 0.10 to 1.18; 3 studies, 1025 participants; I² = 0%; P = 0.71; very low certainty due to serious indirectness and very serious imprecision) (Table 7).
Other adverse events are summarised below.
| Study | Adverse events |
| Nadkarni 2017 | Lay health workers delivering interventions had little to no effect on perpetration of intimate partner violence (RR 0.83, 95% CI 0.41 to 1.68; P = 0.60), suicide attempts (RR 0.15, 95% CI 0.01 to 2.88; P = 0.21), or overall serious adverse events (RR 0.73, 95% CI 0.45 to 1.19; P = 0.21) (1 study, 336 participants) |
| Sorsdahl 2015 | Lay health workers delivering interventions had little or no effect on verbal arguments (MD 0.24, 95% CI ‐0.05 to 0.54), physical fights (MD 0.06, 95% CI 0.12 to 0.25), police interactions (MD ‐0.05, 95% CI ‐0.19 to 0.09), or injuries (MD ‐0.01, 95% CI ‐0.16 to 0.14) (1 study, 223 participants) |
Secondary outcomes
1. Cost analyses and resource use
Two trials reported cost‐effectiveness analyses (Nadkarni 2017; Sorsdahl 2015), and one trial reported cost‐benefit analysis (Papas 2020). These results are reported below under economic analysis.
Comparison 8. Primary health professional‐led and community professional‐led interventions versus enhanced usual care in adult patients with harmful or hazardous alcohol or substance use
We identified six studies ‐ four conducted in South Africa (HuisIntVeld 2019; Marais 2011; Mertens 2014; Pengpid 2013), one in Thailand (Noknoy 2010), and one in Brazil and India (Humeniuk 2012). These took place in rural ‐ Marais 2011; Noknoy 2010 ‐ and urban settings ‐ HuisIntVeld 2019; Humeniuk 2012; Mertens 2014; Pengpid 2013. Of note, in HuisIntVeld 2019, Pengpid 2013, and Marais 2011, patients were recruited from HIV clinics, a university, and antenatal clinics, respectively.
Interventions were delivered by PHPs ‐ HuisIntVeld 2019; Humeniuk 2012; Mertens 2014; Noknoy 2010; Pengpid 2013 ‐ and by CPs ‐ Marais 2011.
Details of study settings, participants, interventions, and comparisons are described in Appendix 10.
A summary of key findings has been tabled in Table 8.
Primary outcomes
1. Recovery from harmful or hazardous alcohol use
8.1. Analysis.

Comparison 8: Primary health professional‐ and community professional‐led interventions versus enhanced usual care in adult patients with harmful or hazardous alcohol or substance use, Outcome 1: Clinical recovery ‐ harmful or hazardous alcohol or drug use
Recovery from harmful or hazardous alcohol use was reported in HuisIntVeld 2019, Mertens 2014, and Pengpid 2013 at 1 to 6 months post intervention, and in HuisIntVeld 2019 and Pengpid 2013 at > 6 months post intervention. Recovery was defined as attaining an AUDIT score indicating low risk or abstinence in HuisIntVeld 2019 and Pengpid 2013, and an ASSIST score indicating low risk in Mertens 2014.
PHP‐led interventions probably had little to no effect on the likelihood of recovery from harmful or hazardous alcohol use at 1 to 6 months post intervention compared to enhanced usual care (RR 0.93, 95% CI 0.77 to 1.12; 3 studies, 1075 participants; I² = 28%; P = 0.43; moderate certainty due to serious indirectness).
PHP‐led psychological interventions may have little to no effect on the likelihood of recovery from harmful or hazardous alcohol use at > 6 months post intervention compared to enhanced usual care (RR 0.88, 95% CI 0.73 to 1.06; 2 studies, 712 participants; I² = 4%; P = 0.18; low certainty due to serious indirectness and imprecision).
2. Prevalence of harmful or hazardous alcohol or substance use
8.2. Analysis.

Comparison 8: Primary health professional‐ and community professional‐led interventions versus enhanced usual care in adult patients with harmful or hazardous alcohol or substance use, Outcome 2: Prevalence ‐ cannabis drug use (dichotomous data)
Prevalence of cannabis use was reported in Pengpid 2013. It is uncertain whether PHP‐led interventions increase or decrease the prevalence of use of cannabis at 1 to 6 months post intervention compared to enhanced usual care (RR 1.10, 95% CI 0.67 to 1.80; 1 study, 152 participants; P = 0.72; very low certainty due to serious indirectness and very serious imprecision) (Analysis 8.2).
Mertens 2014 reported that PHP‐led interventions had little to no effect on the prevalence of at‐risk alcohol use (P = 0.96), at‐risk cannabis use (P = 0.62), or at‐risk methamphetamine use (P = 0.75) compared to enhanced usual care at 1 to 6 months post intervention (363 participants).
Noknoy 2010 reported that PHP‐led interventions decreased the prevalence of hazardous drinking per drinking day during the previous week (P = 0.04) and of hazardous drinking per week during the previous week (P = 0.005) compared to enhanced usual care at 1 to 6 months post intervention (92 participants).
Mertens 2014 reported that PHP‐led interventions had little to no effect on the prevalence of heavy drinking compared to enhanced usual care at 1 to 6 months post intervention (RR 0.84, 95% CI 0.64 to 1.05 (reported as OR in the paper: OR 0.71, 95% CI 0.44 to 1.12); 363 participants).
3. Severity of harmful or hazardous alcohol use ‐ risk of harmful or hazardous drinking based on AUDIT or Alcohol ASSIST score
(Analysis 8.3 Analysis 8.4 Analysis 8.5)
8.3. Analysis.

Comparison 8: Primary health professional‐ and community professional‐led interventions versus enhanced usual care in adult patients with harmful or hazardous alcohol or substance use, Outcome 3: Clinical symptoms ‐ alcohol use (up to 1 month post intervention)
8.4. Analysis.

Comparison 8: Primary health professional‐ and community professional‐led interventions versus enhanced usual care in adult patients with harmful or hazardous alcohol or substance use, Outcome 4: Clinical symptoms ‐ alcohol use (1‐6 months post intervention)
8.5. Analysis.

Comparison 8: Primary health professional‐ and community professional‐led interventions versus enhanced usual care in adult patients with harmful or hazardous alcohol or substance use, Outcome 5: Clinical symptoms ‐ alcohol use (> 6 months post intervention)
Risk of harmful or hazardous drinking based on the AUDIT or Alcohol ASSIST score was reported at < 1 month post CP intervention for Marais 2011; at 1 to 6 months post PHP intervention for HuisIntVeld 2019, Mertens 2014, and Pengpid 2013; and at 6 months post PHP intervention for HuisIntVeld 2019 and Pengpid 2013.
CP‐led interventions may reduce the risk of harmful or hazardous drinking at < 1 month post intervention compared to enhanced usual care (SMD ‐0.46, 95% CI ‐0.76 to ‐0.16; 1 study, 179 participants; P = 0.002; low certainty due to serious indirectness and imprecision) (Analysis 8.3).
PHP‐led psychological interventions may slightly reduce risk of harmful or hazardous drinking at 1 to 6 months post intervention compared to enhanced usual care (SMD ‐0.15, 95% CI ‐0.27 to ‐0.03; 3 studies, 1075 participants; I² = 0%; P = 0.01; low certainty due to serious indirectness and imprecision) (Analysis 8.4).
It is uncertain whether PHP‐led psychological interventions increase or decrease the risk of harmful or hazardous drinking at > 6 months post intervention compared to enhanced usual care (SMD 0.12, 95% CI ‐0.32 to 0.55; 2 studies, 712 participants; I² = 83%; P = 0.60; very low certainty due to serious inconsistency, indirectness, and very serious imprecision) (Analysis 8.5).
4. Severity of overall harmful or hazardous alcohol and substance use ‐ overall risk of harmful or hazardous alcohol and substance use based on total ASSIST scores
8.6. Analysis.

Comparison 8: Primary health professional‐ and community professional‐led interventions versus enhanced usual care in adult patients with harmful or hazardous alcohol or substance use, Outcome 6: Clinical symptoms ‐ drug and alcohol use (1 to 6 months post intervention)
Overall risk of harmful or hazardous alcohol and substance use based on total ASSIST scores was reported in Humeniuk 2012 and Mertens 2014.
PHP‐led psychological interventions probably slightly reduce the overall risk of harmful and hazardous drug and alcohol use at 1 to 6 months post intervention compared to enhanced usual care, although the range where the actual effect may occur indicated that health professionals may have little to no effect (SMD ‐0.20, 95% CI ‐0.35 to ‐0.05; 2 studies, 705 participants; I² = 0%; P = 0.009; moderate certainty due to serious imprecision) (Analysis 8.6).
5. Severity of harmful or hazardous alcohol use ‐ amount of alcohol consumed
The amount of alcohol consumed was reported in Noknoy 2010.
It is uncertain whether PHP‐led psychological interventions have any effect on the amount of alcohol consumed at 1 to 6 months post intervention compared to enhanced usual care (SMD ‐0.52, 95% CI ‐0.94 to ‐0.10; 1 study, 92 participants; P = 0.01; very low certainty due to study limitations, indirectness and imprecision).
Noknoy 2010 reported that PHP‐led interventions had no effect on the frequency of binge drinking (P = 0.121) or of being drunk (P = 0.139) compared to enhanced usual care at 1 to 6 months post intervention (92 participants). Pengpid 2013 reported that PHP‐led interventions decreased heavy episodic drinking scores compared to enhanced usual care at > 6 months post intervention (P = 0.007; 52 participants).
6. Severity of harmful or hazardous substance use ‐ cannabis ASSIST score
8.7. Analysis.

Comparison 8: Primary health professional‐ and community professional‐led interventions versus enhanced usual care in adult patients with harmful or hazardous alcohol or substance use, Outcome 7: Clinical symptoms ‐ cannabis drug use (continuous data)
Risk of harmful or hazardous cannabis use based on cannabis ASSIST score was reported in Mertens 2014.
It is uncertain whether PHP‐led psychological interventions increase or decrease the risk of harmful or hazardous use of cannabis at 1 to 6 months post intervention compared to enhanced usual care (MD ‐0.60, 95% CI ‐1.34 to 0.14; 1 study, 363 participants; P = 0.11; very low certainty due to serious indirectness and very serious imprecision).
7. Severity of harmful or hazardous substance use ‐ stimulant ASSIST score
Humeniuk 2012 reported that PHPs delivering interventions reduced the risk of harmful or hazardous use of stimulants (including amphetamine‐type stimulants and cocaine) based on ASSIST score (1 study, 53 participants; P < 0.01) and use of opioids (1 study, 71 participants; P < 0.001) at 1 to 6 months post intervention compared to enhanced usual care.
Mertens 2014 reported that PHP‐led interventions had no effect on the risk of harmful or hazardous use of methamphetamine based on ASSIST score (1 study, 363 participants; P = 0.23) at 1 to 6 months post intervention compared to enhanced usual care.
8. Severity of depression symptoms
8.8. Analysis.

Comparison 8: Primary health professional‐ and community professional‐led interventions versus enhanced usual care in adult patients with harmful or hazardous alcohol or substance use, Outcome 8: Depression symptoms
Depression symptom severity was reported in HuisIntVeld 2019 and Pengpid 2013.
PHP‐led interventions probably have little to no effect on depression symptoms at > 6 months post intervention compared to enhanced usual care (MD ‐0.14, 95% CI ‐0.70 to 0.42; 2 studies, 712 participants; I² = 0%; P = 0.48; moderate certainty due to serious indirectness).
9. Severity of PTS symptoms
Pengpid 2013 reported that PHP‐led interventions had no effect on PTS symptom severity compared to enhanced usual care at > 6 months post intervention (147 participants; P = 0.221).
10. Quality of life
8.9. Analysis.

Comparison 8: Primary health professional‐ and community professional‐led interventions versus enhanced usual care in adult patients with harmful or hazardous alcohol or substance use, Outcome 9: Quality of life
Quality of life was reported in HuisIntVeld 2019.
PHP‐led interventions probably have little to no effect on quality of life at 1 to 6 months post intervention compared to enhanced usual care (MD 0.00, 95% CI ‐0.10 to 0.10; 1 study, 560 participants; moderate certainty due to serious indirectness).
In keeping with these findings, Pengpid 2013 reported that PHP‐led interventions had no effect on self‐rated health compared to enhanced usual care at > 6 months post intervention (147 participants; P = 0.501).
11. Functional impairment and disability
No data were available for this outcome.
12. Service utilisation
This was reported in Noknoy 2010. It is uncertain whether PHP‐led interventions result in lower likelihood of visits to primary care centres due to alcohol consumption at 1 to 6 months post intervention compared to enhanced usual care (0/56 and 3/51 in intervention and control groups, respectively; RR 0, 95% CI 0.01 to 2.5; 1 study, 107 participants; very low certainty due to serious indirectness and very serious imprecision).
13. Adverse events
| Study | Adverse events |
| Noknoy 2010 | It is uncertain whether PHP‐led interventions have any effect on risk of alcohol‐related consequences compared to enhanced usual care at 1 to 6 months post intervention, including accidents (1/56 and 4/51 in intervention group and control groups, respectively; RR 0.23, 95% CI 0.03 to 1.97; 107 participants) and traffic accidents (3/56 and 5/51 in intervention and control groups, respectively; RR 0.55, 95% CI 0.14 to 2.17; 107 participants). The certainty of evidence was very low due to serious indirectness and very serious imprecision |
Secondary outcomes
1. Cost analyses and resource use
None of these trials reported any economic analysis.
Comparisons 9 and 10. Primary‐level worker‐led interventions versus enhanced usual care in adults with alcohol and substance dependence
We identified three studies, all of which were conducted in lower‐middle income countries ‐ two in urban settings (Nadkarni 2019; Zhong 2015), and one in urban and rural settings (Li 2018). Li 2018 and Nadkarni 2019 were conducted in primary care settings, and Zhong 2015 was conducted at a community centre (Appendix 11). Of note, in Zhong 2015, people who met DSM‐IV criteria for heroin dependence and had just been released from a mandatory two‐year rehabilitation programme were recruited. Interventions were delivered by LHWs (Nadkarni 2019), PHPs (Li 2018), and CPs (Zhong 2015).
As Nadkarni 2019 was the only study performed on adults with alcohol dependence, it was not meta‐analysed. Li 2018 and Zhong 2015 provided no combinable data.
Details of study settings, participants, interventions, and comparisons are described in Appendix 11.
A summary of key findings has been tabled in Table 9 and Table 10.
Primary outcomes
1. Recovery from alcohol or substance dependence
9.1. Analysis.

Comparison 9: Lay health worker‐ and primary health professional‐led interventions versus enhanced usual care in adult patients with alcohol dependence, Outcome 1: Clinical recovery ‐ dependent alcohol use
It is uncertain whether LHW‐led interventions compared to enhanced usual care have any effect on recovery (defined as scoring < 8 on AUDIT) from alcohol dependence at 1 to 6 months post intervention (RR 1.87, 95% CI 0.90 to 3.90; 1 study, 121 participants; P = 0.10) or at > 6 months post intervention (RR 1.68, 95% CI 0.85 to 3.30; 1 study, 112 participants; P = 0.14) (both very low certainty due to serious indirectness and very serious imprecision) (Nadkarni 2019.
In Zhong 2015, the authors reported that CP‐led interventions for adults with substance dependence had no effect on relapse rate at < 1 month post intervention compared to enhanced usual care (25.9% and 22.7% in intervention and control groups, respectively; 155 participants; P = not significant).
2. Prevalence of substance dependence
10.1. Analysis.

Comparison 10: Primary health professional‐ and community professional‐led interventions versus enhanced usual care in adult patients with substance dependence, Outcome 1: Prevalence ‐ drug use (up to 1 month post intervention)
It is uncertain whether CP‐led interventions for adults with substance dependence have any effect on the prevalence of positive urine morphine tests at < 1 month post intervention compared to enhanced usual care (RR 1.06, 95% CI 0.63 to 1.80; 1 study, 173 participants; P = 0.82; very low certainty due to serious study limitations, indirectness and imprecision) (Zhong 2015). Li 2018 did not find a difference between its PHP‐led intervention and control for this outcome (decrease in prevalence was 10% in both arms; 900 participants; P = n.s.) (insufficient data for meta‐analysis).
It is also uncertain whether PHP‐led interventions for adults with substance dependence have any effect on the prevalence of positive urine methamphetamine tests at < 1 month post intervention compared to enhanced usual care (RR 1.10, 95% CI 0.42 to 2.91; 1 study, 173 participants; P = 0.84; very low certainty due to serious study limitations, indirectness, and imprecision).
3. Severity of alcohol and substance dependence symptoms ‐ amount of alcohol consumed
9.2. Analysis.

Comparison 9: Lay health worker‐ and primary health professional‐led interventions versus enhanced usual care in adult patients with alcohol dependence, Outcome 2: Clinical symptoms ‐ alcohol use
10.2. Analysis.

Comparison 10: Primary health professional‐ and community professional‐led interventions versus enhanced usual care in adult patients with substance dependence, Outcome 2: Clinical symptoms ‐ alcohol use
It is uncertain whether LHW‐led interventions for adult patients with alcohol dependency compared to enhanced usual care have any effect on the amount of alcohol consumed at 1 to 6 months post intervention (MD –0.30, 95% CI –21.60 to 21.00; 1 study, 121 participants; P = 0.98; very low certainty) or at > 6 months post intervention (MD –15.20, 95% CI –30.51 to 0.11; 1 study, 112 participants; P = 0.05; very low certainty due to serious indirectness and very serious imprecision) (Analysis 9.2) (Nadkarni 2019).
It is uncertain whether CP‐led interventions for adult patients with drug dependence have any effect on the amount of alcohol consumed at < 1 month post intervention compared to enhanced usual care (MD 0.10, 95% CI –0.91 to 1.11; 1 study, 155 participants; P = 0.85; very low certainty due to serious study limitations, indirectness, and imprecision) (Analysis 10.2) (Zhong 2015).
Other outcomes pertaining to the amount of alcohol consumed were reported in Nadkarni 2019. Study authors reported that LHW‐led interventions made no difference in the percent of days abstinent at 1 to 6 months post intervention compared to enhanced usual care (adjusted MD 9.4, 95% CI –6.5 to 25.2; 121 participants; P = 0.24). At > 6 months post intervention, there was even less difference (adjusted MD 0.9, 95% CI –15.9 to 17.6; 112 participants; P = 0.92). They also reported that LHW‐led interventions made no difference in the percentage of days of heavy drinking (adjusted MD –2.2, 95% CI –15.8 to 11.4; 121 participants; P = 0.75) compared to enhanced usual care at 1 to 6 months post intervention. However, at > 6 months post intervention, LHW‐led interventions may reduce the percentage of days of heavy drinking (adjusted MD –9.9, 95% CI –20.9 to 1.1; 112 participants; P = 0.08), although the range at which the effect may occur indicates that there still may be no difference.
4. Severity of substance dependence symptoms ‐ self‐reported heroin use
10.3. Analysis.

Comparison 10: Primary health professional‐ and community professional‐led interventions versus enhanced usual care in adult patients with substance dependence, Outcome 3: Clinical symptoms ‐ drug use (up to 1 month post intervention)
It is uncertain whether CP‐led interventions for adults with drug dependence have any effect on heroin use by self‐report at < 1 month post intervention compared to enhanced usual care (MD –0.03, 95% CI ‐0.22 to 0.16; 1 study, 155 participants; P = 0.76; very low certainty due to serious study limitations, indirectness, and imprecision) (Zhong 2015).
5. Severity of substance dependence symptoms ‐ self‐reported amphetamine use
It is uncertain whether CP‐led interventions for adults with drug dependence have any effect on amphetamine use by self‐report at < 1 month post intervention compared to enhanced usual care (MD 0.00, 95% CI –0.04 to 0.04; 1 study, 155 participants; P = 1.00; very low certainty due to serious study limitations, indirectness, and imprecision) (Zhong 2015).
6. Severity of substance dependence symptoms ‐ drug avoidance self‐efficacy
10.4. Analysis.

Comparison 10: Primary health professional‐ and community professional‐led interventions versus enhanced usual care in adult patients with substance dependence, Outcome 4: Clinical symptoms ‐ drug avoidance self‐efficacy
This was reported in Li 2018.
PHP‐led interventions for adults with drug dependence probably increase drug avoidance self‐efficacy compared to enhanced usual care at 1 to 6 months post intervention (MD 1.21, 95% CI 0.54 to 1.88; 1 study, 900 participants; P = 0.0004) and at > 6 months post intervention (MD 1.38, 95% CI 0.71 to 2.05; 1 study, 900 participants; P < 0.0001) (both moderate‐certainty evidence due to serious indirectness).
7. Severity of depressive symptoms
9.3. Analysis.

Comparison 9: Lay health worker‐ and primary health professional‐led interventions versus enhanced usual care in adult patients with alcohol dependence, Outcome 3: Depression symptoms
10.5. Analysis.

Comparison 10: Primary health professional‐ and community professional‐led interventions versus enhanced usual care in adult patients with substance dependence, Outcome 5: Clinical symptoms ‐ mental health status
Depression symptom severity based on PHQ‐9 scores was reported in Nadkarni 2019.
It is uncertain whether LHW‐led interventions for adults with alcohol dependence have any effect on depression symptoms compared to enhanced usual care at 1 to 6 months post intervention (MD –0.50, 95% CI –2.68 to 1.68; 1 study, 121 participants; P = 0.65) and at > 6 months post intervention (MD –1.80, 95% CI –4.21 to 0.61; 1 study, 112 participants; P = 0.14) (both very low certainty due to serious indirectness and very serious imprecision).
Mental health based on Hopkins Symptom Checklist‐90 (HSCL‐90) score was reported in Zhong 2015.
It is uncertain whether PHP‐led interventions for adults with drug dependence have any effect on mental health status based on HSCL‐90 score compared to enhanced usual care at < 1 month post intervention (MD –4.23, 95% CI –13.66 to 5.20; 1 study, 155 participants; P = 0.38; very low certainty due to serious study limitations, indirectness, and very serious imprecision) (Analysis 10.5).
8. Quality of life
10.6. Analysis.

Comparison 10: Primary health professional‐ and community professional‐led interventions versus enhanced usual care in adult patients with substance dependence, Outcome 6: Quality of life (up to 1 month post intervention)
These were reported in Zhong 2015.
It is uncertain whether CP‐led interventions for adults with substance dependence have any effect on social functioning based on the 36‐Item Short Form Survey (SF‐36) at < 1 month post intervention compared to enhanced usual care (MD 48.36, 95% CI 41.80 to 54.92; 1 study, 155 participants; P < 0.00001). It is similarly uncertain whether there are any effects on physical functioning (MD –6.78, 95% CI –12.69 to –0.87; 1 study, 155 participants; P = 0.02) or on emotional role functioning (MD –1.75, 95% CI –12.53 to 9.03; 1 study, 155 participants; P = 0.75) based on SF‐36 (all very low certainty due to serious study limitations, indirectness, and imprecision). The authors of this study reported that participants in the CP‐led intervention group had a reduction in physical limitations (P = 0.03) and in emotional limitations (P = 0.02) and no differences in pain, vitality, general health perceptions, and mental health perceptions based on SF‐36 scores compared to enhanced usual care at < 1 month post intervention.
9. Functional impairment
9.4. Analysis.

Comparison 9: Lay health worker‐ and primary health professional‐led interventions versus enhanced usual care in adult patients with alcohol dependence, Outcome 4: Functional impairment
These were reported in Nadkarni 2019.
It is uncertain whether LHW‐led interventions for adults with alcohol dependence have any effect on functional impairment 1 to 6 months post intervention compared to enhanced usual care (MD –0.90, 95% CI –3.43 to 1.63; 1 study, 121 participants; P = 0.48; very low certainty due to serious indirectness and very serious imprecision). However, at > 6 months post intervention, LHW‐led interventions may decrease functional impairment compared to enhanced usual care (MD –3.30, 95% CI –6.22 to –0.38; 1 study, 112 participants; P = 0.03; low certainty due to serious indirectness and imprecision).
10. Service utilisation
9.5. Analysis.

Comparison 9: Lay health worker‐ and primary health professional‐led interventions versus enhanced usual care in adult patients with alcohol dependence, Outcome 5: Service utilisation
It is uncertain whether LHW‐led interventions for adults with alcohol dependence have any effect on unplanned hospitalizations > 6 months post intervention (RR 0.70, 95% CI 0.26 to 1.88; 1 study, 112 participants; P = 0.48; very low certainty due to serious indirectness and very serious imprecision).
11. Adverse events
It is uncertain whether LHW‐led interventions for adults with alcohol dependence have any effect on death, suicide behaviour, or intimate partner violence (very low certainty due to serious indirectness and very serious imprecision).
| Study | Adverse events |
| Nadkarni 2019 | At 1 to 6 months post intervention, there appeared to be no difference in suicidal behaviour (RR 1.18, 95% CI 0.49 to 2.86; P = 0.71; reported as adjusted OR in paper; Analysis 9.6) nor in perpetration of intimate partner violence (RR 1.07, 95% CI 0.36 to 2.46; reported as adjusted OR in paper) between LHW‐led intervention and enhanced usual care (121 participants). At > 6 months post intervention, there was no difference in deaths (RR 0.32, 95% CI 0.01 to 7.70; P = 0.48; 135 participants), suicidal behaviour (RR 0.76, 95% CI 0.34 to 1.69; P = 0.50; 112 participants; reported as adjusted OR in paper; Analysis 9.6), or perpetration of intimate partner violence (RR 3.47, 95% CI 0.74 to 6.44; reported as adjusted OR in paper; 112 participants) between LHW‐led intervention and enhanced usual care |
Secondary outcomes
1. Cost analyses and resource use
One trial reported cost‐effectiveness analyses (Nadkarni 2019). These results are reported below under economic analysis.
Comparison 11. Lay health worker‐led interventions versus specialist‐led care for adults with severe mental disorders
We identified two studies, which were conducted in China ‐ Shen 2016 ‐ and India ‐ Chatterjee 2014. Study settings were urban ‐ Shen 2016; Chatterjee 2014 ‐ and rural ‐ Chatterjee 2014. Participants were adults with schizophrenia who were recovering after their first episode of illness (Shen 2016), or who had had disease for at least moderately severe disease for at least 12 months (Chatterjee 2014).
Other details of study settings, participants, interventions, and comparisons are described in Appendix 12.
A summary of key findings has been tabled in Table 11.
Primary outcomes
1. Recovery from severe mental disorders
This was not measured in either of the two studies.
2. Prevalence of severe mental disorders
This was not measured in either of the two studies.
3. Severity of schizophrenia symptoms
11.1. Analysis.

Comparison 11: Lay health worker‐led interventions versus specialist‐led care for adults with severe mental disorders, Outcome 1: Schizophrenia symptoms
Due to very high statistical heterogeneity (98% to 99%), pooled results for this outcome are not reported. Rather, the individual results of contributing trials are reported. It is uncertain whether LHW‐led interventions for persons with schizophrenia have any effect on the severity of schizophrenia symptoms immediately post intervention compared to specialist care. In Chatterjee 2014, LHWs delivering collaborative community‐based care had little to no effects on severity of schizophrenia symptoms immediately post intervention compared to specialist‐led care (SMD ‐0.22, 95% CI ‐0.48 to 0.04). In Shen 2016, LHWs delivering clubhouse‐model interventions reduced the severity of schizophrenia symptoms immediately post intervention compared to specialist‐led care (SMD ‐2.11, 95% CI ‐2.62 to ‐1.61). The certainty is very low due to serious inconsistency and very serious imprecision (2 studies, 364 participants; Chatterjee 2014; Shen 2016).
4. Severity of depressive symptoms
11.2. Analysis.

Comparison 11: Lay health worker‐led interventions versus specialist‐led care for adults with severe mental disorders, Outcome 2: Depression symptoms
This was reported in Shen 2016. LHW‐led interventions for persons with schizophrenia may reduce depression symptoms immediately post intervention compared to enhanced usual care (MD –12.69, 95% CI –15.12 to –10.26; 1 study, 111 participants; P < 0.00001; low certainty due to serious indirectness and imprecision).
5. Severity of anxiety symptoms
11.4. Analysis.

Comparison 11: Lay health worker‐led interventions versus specialist‐led care for adults with severe mental disorders, Outcome 4: Anxiety
This was reported in Shen 2016. LHW‐led interventions for persons with schizophrenia may reduce anxiety symptoms immediately post intervention compared to enhanced usual care (MD –12.23, 95% CI –14.54 to –9.92; 1 study, 111 participants; P < 0.00001; low certainty due to serious indirectness and imprecision)
6. Severity of caregiver burden symptoms
This was reported in Chatterjee 2014. LHW‐led interventions for persons with schizophrenia may have little to no effect on caregiver burden immediately post intervention compared with specialist care (adjusted mean difference –0.04, 95% CI –0.18 to 0.11; 246 participants; low certainty due to serious indirectness and imprecision).
7. Quality of life
This was not measured in either of the two studies.
8. Functional impairment and disability
11.3. Analysis.

Comparison 11: Lay health worker‐led interventions versus specialist‐led care for adults with severe mental disorders, Outcome 3: Functional impairment
Due to very high statistical heterogeneity (98% to 99%), pooled results for this outcome are not reported. Rather, the individual results of contributing trials are reported. It is uncertain whether LHW‐led interventions for persons with schizophrenia have any effect on functional impairment and disability immediately post intervention compared to specialist care. In Chatterjee 2014, LHWs delivering collaborative community‐based care had little to no effect on functional impairment immediately post intervention compared to specialist‐led care (SMD ‐0.20, 95% CI ‐0.46 to 0.06). In Shen 2016, LHWs delivering clubhouse‐model interventions reduced functional impairment immediately post intervention compared to specialist‐led care (SMD ‐2.89, 95% CI ‐3.46 to ‐2.32). The certainty of evidence is very low due to serious inconsistency and very serious imprecision (2 studies, 364 participants; Chatterjee 2014; Shen 2016).
9. Service utilisation
The incidence of hospital admissions was reported in Chatterjee 2014. Seventeen of 187 participants in the intervention group and 1 of 95 participants in the control group were hospitalised during the course of the year‐long intervention (RR 8.64, 95% CI 1.17 to 63.92; 282 participants; very low certainty due to serious indirectness and very serious imprecision).
10. Adverse events
| Study | Adverse events |
| Chatterjee 2014 | There were 4 deaths in total ‐ 1 out of 187 participants in the intervention group, 1 out of 95 participants in the control group (from suicide), and the other 2 from complications from a road traffic accident and pre‐existing cardiac disease (assigned groups not reported). It is uncertain whether lay heath workers delivering collaborative community‐based care have any effect on risk of death from suicide during intervention (RR 0.51, 95% CI 0.03 to 8.03; 282 participants; very low certainty due to serious indirectness and very serious imprecision) |
Secondary outcomes
1. Cost analyses and resource use
One trial reported cost‐effectiveness analyses (Chatterjee 2014). These results are reported below under economic analysis.
Comparison 12. Primary health professional‐led or collaborative care versus specialist‐led care for people with severe mental disorders
We identified seven studies, which were conducted in China ‐ Li 2002; Ling 1999; Tan 2005; Wu 2016; Yao 2014 ‐ and Iran ‐ Barfar 2017; Malakouti 2015. Study settings were rural ‐ Li 2002; Tan 2005 ‐ or urban ‐ Barfar 2017; Ling 1999; Malakouti 2015; Wu 2016; Yao 2014. Participants were adults with schizophrenia (all seven studies), bipolar disorder (Barfar 2017; Malakouti 2015), and schizoaffective disorder (Appendix 13) (Barfar 2017). Of note, Li 2002 recruited participants having their first episode of late‐onset schizophrenia, and Malakouti 2015 recruited patients with difficult‐to‐treat disease defined by having had two or more hospitalisations in the past two years and poor compliance with medications.
Details of study settings, participants, interventions, and comparisons are described in Appendix 13.
A summary of key findings has been tabled in Table 12.
Primary outcomes
1. Recovery from severe mental disorders
(Analysis 12.1; Analysis 12.2)
12.1. Analysis.

Comparison 12: Primary health professional‐led or collaborative care versus specialist‐led care for people with severe mental disorders, Outcome 1: Clinical recovery from severe mental disorders
12.2. Analysis.

Comparison 12: Primary health professional‐led or collaborative care versus specialist‐led care for people with severe mental disorders, Outcome 2: Relapse of severe mental disorders
It is uncertain whether interventions delivered by PHPs or collaborative care have any effect on remission defined as an 80% or higher decrease in brief Psychiatric Rating Scale (BPRS) score immediately post intervention compared with specialist‐led care because the certainty of evidence is very low (RR 1.08, 95% CI 0.81 to 1.44; 1 study, 76 participants; very low certainty due to serious study limitations, indirectness, and imprecision) (Li 2002).
Relapse was reported in Li 2002, Ling 1999, Tan 2005, and Wu 2016. Despite a risk ratio showing apparent benefit, it is uncertain whether interventions delivered by PHPs or collaborative care have any effect on relapse immediately post intervention compared to specialist‐led care because the certainty of evidence is very low (RR 0.30, 95% CI 0.16 to 0.55; 4 studies, 492 participants; I² = 13%; P = 0.0001; very low certainty due to serious study limitations, indirectness, and imprecision).
2. Prevalence of severe mental disorders
No studies reported this outcome.
3. Severity of schizophrenia symptoms
12.3. Analysis.

Comparison 12: Primary health professional‐led or collaborative care versus specialist‐led care for people with severe mental disorders, Outcome 3: Schizophrenia symptoms
Schizophrenia symptom severity was reported in Barfar 2017, Li 2002, Ling 1999, Malakouti 2015, Tan 2005, and Yao 2014. It is uncertain whether interventions delivered by PHPs or collaborative care have any effect on the severity of schizophrenia symptoms immediately post intervention compared to specialist‐led care because the evidence is of very low certainty (SMD ‐0.30, 95% CI ‐0.71 to 0.11; 6 studies, 489 participants; I² = 87%; P = 0.15; very low certainty due to serious study limitations, inconsistency, and imprecision).
4. Severity of depressive symptoms
12.4. Analysis.

Comparison 12: Primary health professional‐led or collaborative care versus specialist‐led care for people with severe mental disorders, Outcome 4: Depression symptoms
Depression symptom severity was reported in Barfar 2017 and Ling 1999. It is uncertain whether interventions delivered by PHPs or collaborative care have any effect on severity of depression symptoms immediately post intervention compared to specialist‐led care because the certainty of evidence is very low (SMD ‐0.41, 95% CI ‐1.13 to 0.32; 2 studies, 270 participants; I² = 89%; P = 0.27; very low certainty due to serious study limitations, inconsistency, and imprecision).
5. Severity of caregiver general health or burden symptoms
Caregiver outcomes were reported in Malakouti 2015. Interventions delivered by PHPs or collaborative care did not have any effect on caregiver general health or burden compared to specialist‐led care (1 study, 152 participants; P = 0.08 for caregiver burden; P = 0.20 for caregiver general health). (Issues with caregiver burden data preclude further analysis.)
6. Quality of life
12.5. Analysis.

Comparison 12: Primary health professional‐led or collaborative care versus specialist‐led care for people with severe mental disorders, Outcome 5: Quality of life
Quality of life measures were reported in Barfar 2017, Malakouti 2015, and Wu 2016.
It is uncertain whether interventions delivered by PHPs or collaborative care have any effect on quality of life immediately post intervention compared to specialist‐led care because the certainty of evidence is very low (SMD 0.40, 95% CI ‐0.37 to 1.17; 3 studies, 536 participants; I² = 94%; P = 0.31; very low certainty due to serious study limitations, inconsistency, and imprecision).
7. Functional impairment and disability
12.6. Analysis.

Comparison 12: Primary health professional‐led or collaborative care versus specialist‐led care for people with severe mental disorders, Outcome 6: Functional impairment
Functional impairment was reported in all seven studies (Barfar 2017; Li 2002; Ling 1999; Malakouti 2015; Tan 2005; Wu 2016; Yao 2014). Interventions delivered by PHPs or collaborative care may reduce functional impairment immediately post intervention compared to specialist‐led care (SMD ‐1.13, 95% CI ‐1.78 to ‐0.47; 7 studies, 874 participants; I² = 95%; P = 0.0007; low certainty due to serious study limitations and inconsistency).
8. Service utilisation
12.7. Analysis.

Comparison 12: Primary health professional‐led or collaborative care versus specialist‐led care for people with severe mental disorders, Outcome 7: Service utilisation ‐ hospital re‐admission (1 year from randomisation)
Incidence of hospital re‐admissions was reported in Barfar 2017, Malakouti 2015 and Wu 2016. It is uncertain whether interventions delivered by PHPs or collaborative care have any effect on the likelihood of hospital re‐admissions immediately post intervention compared to specialist‐led care because the certainty of evidence is very low (RR 0.60, 95% CI 0.28 to 1.28; 3 studies, 441 participants; I² = 74%; P = 0.18; very low certainty due to serious study limitations, inconsistency, and imprecision).
9. Adverse events
No data were available.
Secondary outcomes
1. Cost analyses and resource use
Two trials reported cost‐effectiveness analyses (Barfar 2017; Malakouti 2015), and two trials reported cost analyses (Li 2002; Tan 2005). These results are reported below under economic analysis.
Comparison 13. Primary health professional‐led and lay health worker‐led psychosocial interventions versus usual care in improving dementia patients' and carers' outcomes
We found two studies, which were conducted in urban areas in India by LHWs and in Russia by doctors (Dias 2008; Gavrilova 2009, respectively). Compared to the previous review (van Ginneken 2013), analysis results are unchanged, although GRADE findings may have been altered for consistency with the current review.
Details of study settings, participants, interventions, and comparisons are described in Appendix 14.
A summary of key findings has been tabled in Table 13.
Primary outcomes
1. Recovery from dementia
No studies reported this outcome.
2. Prevalence of dementia
No studies reported this outcome.
3. Severity of patients' behavioural symptoms
13.1. Analysis.

Comparison 13: Primary health professional‐ and lay health worker‐led psychosocial interventions versus usual care in improving dementia patients' and carers' outcomes, Outcome 1: Behavioural problem symptoms
At 1 to 6 months post intervention, PHP‐ and LHW‐led carer interventions for dementia may have little to no effect on the severity of behavioural symptoms in patients with dementia (SMD ‐0.26, 95% CI ‐0.60 to 0.08; 2 studies, 134 participants; I² = 0%; P = 0.13; low certainty due to very serious imprecision) (Analysis 13.1).
4. Severity of carer outcome symptoms
(Analysis 13.4 Analysis 13.5 Analysis 13.6)
13.4. Analysis.

Comparison 13: Primary health professional‐ and lay health worker‐led psychosocial interventions versus usual care in improving dementia patients' and carers' outcomes, Outcome 4: Carer distress
13.5. Analysis.

Comparison 13: Primary health professional‐ and lay health worker‐led psychosocial interventions versus usual care in improving dementia patients' and carers' outcomes, Outcome 5: Carer mental health status
13.6. Analysis.

Comparison 13: Primary health professional‐ and lay health worker‐led psychosocial interventions versus usual care in improving dementia patients' and carers' outcomes, Outcome 6: Carer burden
At 1 to 6 months post intervention, PHP‐ and LHW‐led carer interventions for dementia may improve/slightly improve carer outcomes, including reducing severity of burden (SMD ‐0.50, 95% CI ‐0.84 to ‐0.15; 2 studies, 134 participants; I² = 0%; P = 0.005) (Analysis 13.6), mental health status symptoms (SMD ‐0.42, 95% CI ‐0.76 to ‐0.08; 2 studies, 134 participants; I² = 0%; P = 0.02) (Analysis 13.5), and mental distress (SMD ‐0.47, 95% CI ‐0.82 to ‐0.13; 2 studies, 134 participants; I² = 0%; P = 0.007) (all low certainty due to very serious imprecision) (Analysis 13.4).
5. Quality of life
13.2. Analysis.

Comparison 13: Primary health professional‐ and lay health worker‐led psychosocial interventions versus usual care in improving dementia patients' and carers' outcomes, Outcome 2: Quality of life (patient)
13.7. Analysis.

Comparison 13: Primary health professional‐ and lay health worker‐led psychosocial interventions versus usual care in improving dementia patients' and carers' outcomes, Outcome 7: Carer quality of life
This was reported in Gavrilova 2009.
It is uncertain whether PHP‐led carer interventions lead to any difference in quality of life of patients with dementia at 1 to 6 months post intervention (MD ‐0.43, 95% CI ‐0.98 to 0.12; 1 study, 53 participants; P = 0.12; very low certainty due to serious indirectness and very serious imprecision) (Analysis 13.2).
It is uncertain whether PHP‐led carer interventions lead to any difference in quality of life of carers of patients with dementia at 1 to 6 months post intervention (MD ‐0.37, 95% CI ‐0.92 to 0.17; 1 study, 53 participants; P = 0.18; very low certainty due to serious indirectness and very serious imprecision) (Analysis 13.7). Study authors suggested that this result, which was out of keeping with the other carer outcomes, may be due to a type 2 error because the study was not statistically powered to detect differences of this size in the quality of life outcome.
6. Functional impairment and disability
13.3. Analysis.

Comparison 13: Primary health professional‐ and lay health worker‐led psychosocial interventions versus usual care in improving dementia patients' and carers' outcomes, Outcome 3: Functional impairment (patient)
This was reported in Dias 2008.
It is uncertain whether interventions delivered by LHWs at 1 to 6 months post intervention lessen functional impairment in patients with dementia (MD ‐0.24, 95% CI ‐0.67 to 0.20; 1 study, 81 participants; P = 0.29; very low certainty due to serious indirectness and very serious imprecision).
7. Service utilisation
It is uncertain whether LHW‐led carer interventions for dementia compared to usual care have any effect on the number of home visits at 1 to 6 months post intervention. Dias 2008 reported a large number of LHW home visits (mean number of visits 12.3; SD 3.1), which lasted 30 minutes to 1 hour, as well as phone calls (mean 1.3; SD 2.1). Although psychiatrists planned to see patients at least once in clinic, patients often were unable to come, and they needed to carry out 21 home visits. The evidence is of very low certainty due to serious indirectness and very serious imprecision.
8. Adverse events
It is uncertain whether PHPs or LHWs delivering care to dementia patients compared with usual care reduce death at 1 to 6 months post intervention (RR 0.77, 95% CI 0.38 to 1.59; 2 studies, 141 participants; very low certainty due to serious inconsistency and very serious imprecision).
| Study | Adverse events |
| Dias 2008 | The intervention (LHW‐led carer intervention) led to a reduction in the number of deaths in patients with dementia compared to usual care (RR 0.40, 95% CI 0.01 to 1.02 (reported in paper as OR 0.34, 95% CI 0.01 to 1.03)), although mortality overall was high (18%), of which 6/41 deaths occurred in the intervention arm and 12/40 deaths in the control arm. Causes of death were stroke (n = 4), pneumonia (n = 4), myocardial infarction (n = 3), and septicaemia (n = 2) |
| Gavrilova 2009 | In all, 5/30 deaths occurred in the intervention arm (stroke in 2 participants; pneumonia, pulmonary embolism, and intestinal obstruction) and 2/30 in the control arm (pneumonia and diabetic coma) |
Secondary outcomes
1. Cost analyses and resource use
No economic data are linked to these studies.
Comparison 14. Lay health worker‐led psychosocial interventions versus usual or no care in child post‐traumatic stress or common mental disorders in humanitarian settings
We identified seven studies from 6 countries across Asia ‐ Jordans 2010, Tol 2008, Tol 2012 ‐ and Africa ‐ Bolton 2007, Ertl 2011, Murray 2015, O'Callaghan 2014 ‐ that were conducted in post‐conflict or peri‐conflict settings (Bolton 2007; Ertl 2011; Jordans 2010; O'Callaghan 2014; Tol 2008; Tol 2012). One study was conducted in a non‐war setting, with inclusion of children exposed to abuse/neglect/HIV in Zambia (Murray 2015). Settings were rural/semi‐rural (Bolton 2007; Jordans 2010; O'Callaghan 2014; Tol 2008), or they were urban and rural (Ertl 2011; Murray 2015; Tol 2012). One study included child soldiers aged 12 to 25 years (Ertl 2011). Most children came from low‐resource backgrounds. Children with post‐traumatic stress disorder (PTSD) diagnoses ‐ Dawson 2016; Ertl 2011; Jordans 2010; Murray 2015; Tol 2008; Tol 2012; those with post‐traumatic stress symptoms (PTS) ‐ Bolton 2007; and those with both ‐ O'Callaghan 2014 ‐ were included.
Details of study settings, participants, interventions, and comparisons are described in Appendix 15.
A summary of key findings has been tabled in Table 14.
Primary outcomes
1. Recovery from PTSD
No data were provided for this outcome.
2. Prevalence of PTSD
No data were provided for this outcome.
3. Severity of post‐traumatic stress (PTS) symptoms
(Analysis 14.1; Analysis 14.2; Analysis 14.3)
14.1. Analysis.

Comparison 14: Lay health worker‐led psychosocial interventions versus usual or no care in child post‐traumatic stress or common mental disorders in humanitarian settings, Outcome 1: Post‐traumatic stress symptoms
14.2. Analysis.

Comparison 14: Lay health worker‐led psychosocial interventions versus usual or no care in child post‐traumatic stress or common mental disorders in humanitarian settings, Outcome 2: Post‐traumatic stress symptoms (change scores)
14.3. Analysis.

Comparison 14: Lay health worker‐led psychosocial interventions versus usual or no care in child post‐traumatic stress or common mental disorders in humanitarian settings, Outcome 3: Post‐traumatic stress symptoms
Because of differences in outcome measures for short‐term outcomes (mean change differences could not be combined with standardised mean differences), we present these outcomes separately. We followed this approach for all outcomes in this comparison.
Severity of PTSD symptoms immediately post intervention was reported as mean score in Murray 2015 and O'Callaghan 2014, and as mean change score in Tol 2008. Post intervention (0 to 1 month), LHW‐led interventions may slightly improve PTS symptoms in children with mean scores (SMD ‐0.77, 95% CI ‐1.48 to ‐0.06; 2 studies, 416 participants; I² = 92%; P = 0.03, low certainty due to serious inconsistency and imprecision) but have an uncertain effect on mean change scores (SMD ‐0.45, 95% CI ‐0.78 to ‐0.12; 1 study, 145 participants; P = 0.007; very low certainty due to serious study limitations, indirectness, and imprecision) (overall low certainty due to serious inconsistency and imprecision).
At 1 to 6 months post intervention, mean scores were reported in Ertl 2011 and mean change scores were reported in Jordans 2010, Tol 2008, and Tol 2012. At 1 to 6 months post intervention, LHW‐led psychosocial interventions may have little to no effect on PTS symptoms in children with mean scores (SMD ‐0.18, 95% CI ‐0.65 to 0.28; 1 study (2 arms), 77 participants; I² = 0%; P = 0.30; very low certainty due to serious indirectness and very serious imprecision) and on mean change scores (MCD ‐1.34, 95% CI ‐2.83 to 0.14; 1090 participants, 3 studies; I² = 57%; P = 0.08) (overall low certainty due to serious inconsistency and imprecision) (Analysis 14.3). In Tol 2012, PTS symptoms improved among girls in the control group (not in the intervention group), but no difference was noted among boys.
At 11 months post intervention, mean scores were reported in Ertl 2011. It is uncertain whether at 11 months, LHWs reduce PTS symptom severity (SMD ‐0.25, 95% CI ‐0.72 to 0.22; 1 study, 76 participants; I² = 0%; P = 0.30; very low certainty due to serious indirectness and very serious imprecision).
4. Severity of depressive symptoms
(Analysis 14.4, Analysis 14.5)
14.4. Analysis.

Comparison 14: Lay health worker‐led psychosocial interventions versus usual or no care in child post‐traumatic stress or common mental disorders in humanitarian settings, Outcome 4: Depression symptoms
14.5. Analysis.

Comparison 14: Lay health worker‐led psychosocial interventions versus usual or no care in child post‐traumatic stress or common mental disorders in humanitarian settings, Outcome 5: Depression symptoms
Severity of depressive symptoms immediately post intervention was reported as mean change scores in Bolton 2007 and Tol 2008, and as mean scores in O'Callaghan 2014. Post intervention (0 to 1 month), it is uncertain whether LHW‐led interventions reduce symptom severity in studies reporting mean change scores (MCD ‐4.55, 95% CI ‐12.64 to 3.54; 421 participants, 2 studies; I² = 76%; P = 0.27; very low certainty due to serious study limitations, inconsistency, and very serious imprecision) (Analysis 14.5). Among those reporting mean scores, LHW‐led interventions probably had little to no effect (SMD ‐0.01, 95% CI ‐0.12 to 0.11; 1162 participants, 1 study; P = 0.91; moderate certainty due to serious indirectness).
At 1 to 6 months post intervention, mean change scores were reported in Jordans 2010, Tol 2008, and Tol 2012, and mean scores were reported in Ertl 2011. In the medium term (1 to 6 months post intervention), interventions delivered by LHWs probably result in little to no difference in depressive symptoms compared with usual care in studies measuring mean change scores (MCD ‐0.61, 95% CI ‐1.23 to 0.02; 3 studies, 1092 participants; I² = 15%; P = 0.06) (Analysis 14.5) and in one study (two arms) with a small sample size measuring mean scores (SMD ‐0.01, 95% CI ‐0.47 to 0.46; 1 study, 77 participants; I² = 0%; P = 0.98; very low certainty due to serious indirectness and very serious imprecision) (overall moderate certainty due to serious imprecision).
At 11 months post intervention, mean scores were reported in Ertl 2011. It is uncertain whether at 11 months post intervention, LHW‐led interventions reduce depression severity (SMD 0.25, 95% CI –0.22 to 0.72; 1 study, 76 participants; I² = 0%; P = 0.29; very low certainty due to serious indirectness and very serious imprecision).
5. Severity of anxiety symptoms
14.6. Analysis.

Comparison 14: Lay health worker‐led psychosocial interventions versus usual or no care in child post‐traumatic stress or common mental disorders in humanitarian settings, Outcome 6: Anxiety symptoms
This was reported immediately post intervention in Bolton 2007 and Tol 2008. It is uncertain whether LHWs reduce anxiety severity immediately post intervention (MCD ‐1.14, 95% CI ‐2.94 to 0.65; 2 studies, 425 participants; I² = 84%; P = 0.21; very low certainty due to serious study limitations, inconsistency, and very serious imprecision).
At 1 to 6 months post intervention, severity of anxiety symptoms was reported in Jordans 2010, Tol 2008, and Tol 2012. In the medium term (1 to 6 months post intervention), LHW‐led interventions probably have little to no effect on reducing anxiety severity in children compared with usual care (MCD ‐0.34, 95% CI ‐0.75 to 0.07; 3 studies; I² = 18%; P = 0.10; moderate certainty due to serious imprecision). Tol 2012 undertook a subgroup analysis by sex that showed there may be little to no difference for boys (MCD ‐0.63, 95% CI ‐1.23 to ‐0.03; 245 participants, 1 study; low certainty due to serious indirectness and imprecision).
No long‐term outcomes were reported.
6. Quality of life
No data were available.
7. Functional impairment and disability
14.7. Analysis.

Comparison 14: Lay health worker‐led psychosocial interventions versus usual or no care in child post‐traumatic stress or common mental disorders in humanitarian settings, Outcome 7: Functional impairment
This was reported immediately post intervention as mean scores in Bolton 2007, Murray 2015, and O'Callaghan 2014, and as mean change scores in Tol 2008. Immediately post intervention, LHWs may have little to no effect on reducing functional impairment in studies reporting mean scores (SMD ‐0.48, 95% CI ‐1.08 to 0.12; 3 studies, 625 participants; I² = 92%; P = 0.12). In studies reporting mean change scores, the clinical effect on reducing functional impairment is uncertain (MCD ‐2.19, 95% CI ‐4.22 to ‐0.16; 1 study, 104 participants; P = 0.03; very low certainty due to serious study limitations, indirectness, and very serious imprecision) (Analysis 14.8). The overall evidence was of low certainty due to serious inconsistency and imprecision.
14.8. Analysis.

Comparison 14: Lay health worker‐led psychosocial interventions versus usual or no care in child post‐traumatic stress or common mental disorders in humanitarian settings, Outcome 8: Functional impairment
In the medium term (1 to 6 months post intervention), mean change scores were reported in Jordans 2010, Tol 2008, and Tol 2012, and mean scores were reported in Ertl 2011. LHW‐led interventions probably also have little to no effect on reducing functional impairment in studies reporting mean change scores (MCD ‐0.81, 95% CI ‐1.48 to ‐0.13; 3 studies, 1092 participants; I² = 7%; P = 0.02) (Analysis 14.8), and mean scores in a small study showed no difference (SMD ‐0.08, 95% CI ‐0.55 to 0.38; 1 study (2 arms), 77 participants; I² = 0%; P = 0.72; very low certainty due to serious indirectness and very serious imprecision). The overall evidence is of moderate certainty due to serious imprecision.
At 11 months, it is uncertain whether two LHW‐led arms in Ertl 2011 reduced functional impairment (SMD ‐0.36, 95% CI ‐0.86 to 0.13; 1 study, 76 participants; I² = 9%; P = 0.15; very low certainty due to serious indirectness and very serious imprecision).
8. Service utilization
There were no service utilisation outcomes as defined in the protocol (i.e. attendance rates in primary/community care, referral rates from primary care and hospital admission rates).
9. Adverse events
LHWs delivery of psychosocial interventions to children with post‐traumatic stress or CMDs compared to usual or no care resulted in increased risk of adverse events (no adverse events in either arm, relative risk not calculable; 3 studies, 809 participants; low certainty due to very serious imprecision).
| Study | Adverse events |
| Ertl 2011 | No negative events of narrative exposure therapy (NET) were observed in this trial. Clinically reliable aggravation of symptoms was not present in the NET group but was present in 4.4% of the academic catch‐up group and in 10.7% of waiting‐list participants |
| Jordans 2010 | No adverse outcomes were detected |
| Tol 2012 | No adverse outcomes/events were detected. An unintended effect was that girls in the wait‐list control group were found to have greater improvement in symptoms related to their PTSD compared to girls in the intervention group |
| Bolton 2007; Murray 2015; O'Callaghan 2014; Tol 2008 | These 4 studies do not provide information about adverse events |
Secondary outcomes
1. Cost analyses and resource use
Two studies reported cost analysis data (Tol 2008; Tol 2012). See below for an economic data summary.
Comparison 15. Community professional‐led psychosocial interventions versus no care in child post‐traumatic stress or common mental disorders in humanitarian settings
We identified eight studies from seven countries within Europe (Dybdahl 2001; Gordon 2008), the Middle East (Barron 2013; Barron 2016), Africa (Betancourt 2014; O'Callaghan 2013; O'Callaghan 2015), and Asia (Berger 2009). Seven studies were undertaken in post‐conflict or peri‐conflict settings, including among internally displaced populations (Dybdahl 2001), although Berger 2009 was conducted following a natural disaster. Settings were rural/semi‐rural ‐ Barron 2016; Bolton 2007; Gordon 2008; O'Callaghan 2015 ‐ and urban ‐ Berger 2009; Dybdahl 2001. Of note, most participants in Betancourt 2014 had been child soldiers. Studies included children who were exposed to trauma and were distressed (Berger 2009; Betancourt 2014 ‐ specifically screened to have psychological distress or functional impairment; Dybdahl 2001; O'Callaghan 2013; O'Callaghan 2015); met PTSD diagnostic criteria (Barron 2013; Barron 2016; Gordon 2008); or included children with either PTS or PTSD (Berger 2009).
Severity of PTS symptoms was assessed in studies that looked at improvement in symptoms of patients with PTSD and of patients with PTS.
Details of study settings, participants, interventions, and comparisons are described in Appendix 16.
A summary of key findings has been tabled in Table 15.
Primary outcomes
1. Recovery from PTSD
15.1. Analysis.

Comparison 15: Community professional‐led psychosocial interventions versus no care in child post‐traumatic stress or common mental disorders in humanitarian settings, Outcome 1: Recovery from PTSD
This was reported in Barron 2013, where recovery was derived from taking the difference between the total number scoring above the cutoff for PTSD on the Child Revised Impact of Events Scale‐13 (CRIES‐13) at baseline and the number scoring above the cutoff for PTSD on CRIES‐13 on follow‐up divided by the total number scoring above the cutoff for PTSD on CRIES‐13 at baseline. It is uncertain whether psychosocial interventions led by community professionals (new school counsellors) improve recovery from PTSD in children at 2 weeks post intervention, as despite an apparently important effect, this study was conducted on a very small number of people (RR 3.93, 95% CI 1.31 to 11.80; 78 participants, 1 study; P = 0.01; very low certainty due to serious study limitations, indirectness, and very serious imprecision).
No data for 1 to 6 month or > 6 month post‐intervention time points were provided.
2. Prevalence of PTSD
No data for this outcome were provided.
3. Severity of PTS symptoms
15.2. Analysis.

Comparison 15: Community professional‐led psychosocial interventions versus no care in child post‐traumatic stress or common mental disorders in humanitarian settings, Outcome 2: Post‐traumatic stress symptoms
This was reported immediately post intervention in Barron 2016, Betancourt 2014, Gordon 2008, O'Callaghan 2013, and O'Callaghan 2015. Community professional (CP)‐led psychosocial interventions probably reduce PTS symptom severity versus no care immediately post intervention (0 to 1 month) (SMD ‐1.10, 95% CI ‐1.83 to ‐0.38; 753 participants, 5 studies; I² = 94%; P = 0.003; moderate certainty due to serious inconsistency). Barron 2013 could not be combined in the meta‐analysis, as it did not provide standard deviations for effect estimates and provided mean scores only on a graph. Study authors reported a large effect on reduction in PTS symptoms in the intervention group compared to the control group post intervention (d = 0.76; P < 0.05), which correlated with the moderate clinical effect noted above in the meta‐analysis.
At 1‐6 months post intervention, severity of PTS symptoms was reported in Berger 2009, Betancourt 2014, and Gordon 2008. Due to large variation between studies in reported effects and serious risk of bias, it is uncertain whether CP‐led interventions reduce PTS symptoms among children versus existing care at 1 to 6 months post intervention (SMD ‐0.37, 95% CI ‐1.20 to 0.47; 3 studies, 679 participants; I² = 94%; P = 0.39; very low certainty due to serious study limitations, inconsistency, and very serious imprecision).
No data for long‐term outcomes (> 6 months) were reported.
4. Severity of depressive symptoms
15.3. Analysis.

Comparison 15: Community professional‐led psychosocial interventions versus no care in child post‐traumatic stress or common mental disorders in humanitarian settings, Outcome 3: Depression symptoms
This was reported immediately post intervention in Barron 2016, Betancourt 2014, Dybdahl 2001, O'Callaghan 2013, and O'Callaghan 2015. CP‐led psychosocial interventions may reduce depression symptom severity versus no care immediately post intervention (0 to 1 month) (SMD ‐0.57, 95% CI ‐1.13 to 0.00; 750 participants, 5 studies; I² = 91%; P = 0.05; low certainty due to serious inconsistency and imprecision). Barron 2013 could not be combined in the meta‐analysis, as it did not provide standard deviations for effect estimates and provided mean scores only on a graph. Study authors reported a large effect on reduction in depression symptoms in the intervention group compared to the control group post intervention (d = 1.24; P < 0.05), which correlated with the moderate clinical effect noted in the point effect within the above meta‐analysis.
This was reported at 1 to 6 months post intervention in Berger 2009 and Betancourt 2014. At 1 to 6 months post intervention, CP‐led psychosocial interventions may have little to no effect on depressive symptoms compared with no care (SMD ‐0.19, 95% CI ‐0.57 to 0.19; 2 studies, 602 participants; I² = 69%; P = 0.33; low certainty due to serious inconsistency and imprecision).
No data for long‐term outcomes (> 6 months) were provided.
5. Quality of life
No data for quality of life were provided.
6. Functional impairment and disability
15.4. Analysis.

Comparison 15: Community professional‐led psychosocial interventions versus no care in child post‐traumatic stress or common mental disorders in humanitarian settings, Outcome 4: Functional impairment
This was reported immediately post intervention in Betancourt 2014, Dybdahl 2001, O'Callaghan 2013, and O'Callaghan 2015, and at 1 to 6 months post intervention in Berger 2009 and Betancourt 2014.
Immediately post intervention (0 to 1 month), CP‐led interventions probably slightly reduce functional impairment (SMD ‐0.29, 95% CI ‐0.55 to ‐0.03; 4 studies, 623 participants; I² = 43%; P = 0.03; moderate certainty due to serious imprecision). Barron 2013 could not be combined in the meta‐analysis, as it did not provide standard deviations for effect estimates and provided mean scores only on a graph. Study authors reported a small effect on improvement of school performance in the intervention group compared to the control group post intervention (d = 0.35; P < 0.05), which correlated with the small clinical effect noted in the point effect within the above meta‐analysis.
However, it is uncertain whether CP‐led interventions reduce functional impairment at 1 to 6 months post intervention (SMD ‐0.39, 95% CI ‐0.99 to 0.21; 2 studies, 602 participants; I² = 91%; P = 0.20; very low certainty due to serious study limitations, inconsistency, and very serious imprecision).
No data for long‐term outcomes (> 6 months) were provided.
7. Service utilisation
There were no service utilisation data as per protocol suggestions.
8. Adverse events
Three out of the eight studies reported adverse outcomes (Gordon 2008; O'Callaghan 2013; O'Callaghan 2015). They reported no adverse events. CP‐led interventions for children with PTS or CMD may lead to no increase in risk of adverse events compared to no care (no adverse events recorded in either arm, relative risk not calculable; 3 studies, 180 participants; low certainty due to very serious imprecision) (Table 15).
Secondary outcomes
1. Cost analyses and resource use
One study presented cost‐effectiveness data (Barron 2016). See below for an economic data summary.
Studies not assigned to the above comparisons
Two studies performed on children did not fall into the above categories.
These studies included the following comparisons.
1. Community workers delivering interventions (group drawing) to children with attention‐deficit/hyperactivity disorder who are hyper‐aggressive versus usual care
In Momeni 2016, in a school in an urban area, children aged 8 to 12 with diagnosed attention‐deficit/hyperactivity disorder with hyperactive or aggressive symptoms participated in group drawing facilitated by teachers. Training of teachers to carry out the intervention was not described. Children underwent nine sessions held twice a week, each lasting 45 to 60 minutes. Each session consisted of a drawing activity, which was done as a pair or as a group. Children in the control group received usual care. At < 1 month post intervention, children who received teacher‐facilitated group drawing had reduced physical aggression (P < 0.05), relational aggression (P < 0.001), and reactive verbal‐hyperactive aggression/impulsivity (P < 0.05) (1 study, 24 participants).
2. Lay health workers delivering interventions to children with autism spectrum disorder and their families versus usual care
In Divan 2019, in a rural area in patients’ homes, children aged 2 to 9 with diagnosed autism spectrum disorder and their parents (parent‐child dyads) participated in a parent‐mediated intervention for autism spectrum disorder plus co‐morbidity module ("PASS plus"). Lay health workers delivered the intervention after undergoing 10 days of classroom training on the PASS module, one month of internship, and an additional two days of training on the Plus modules. They were supervised weekly to fortnightly by senior clinicians and peers. The intervention consisted of 12 fortnightly sessions conducted over six months, each lasting 60 to 90 minutes. The PASS module involved video‐feedback of a 10‐minute play session to support parents in recognising their child’s verbal and non‐verbal communication signals and which of their own actions had a positive effect on the interactions. Parents were then guided to choose intervention strategies to try out, and the effects of these strategies were reviewed during the next session. The Plus modules involved giving advice and strategies using a decision algorithm to manage a co‐morbidity that parents identified as most disruptive to their family. Participants in the control group received usual care. At < 1 month post intervention, children in the LHW‐facilitated intervention group showed reduction in autism symptom severity as measured by the Brief Observation of Social Communication Change (BOSCC), although the range at which the actual effect occurred may indicate little or no difference (adjusted mean difference (MD) ‐2.42, 95% CI –7.75 to 2.92) and an increase in the proportion of child‐initiated communication (adjusted MD 0.17, 95% CI 0.03 to 0.32) but little to no difference in co‐morbid learning disability (adjusted MD –9.00, 95% CI –24.26 to 6.26) or adaptive behaviour (adjusted MD 0.67, 95% CI –3.8 to 5.15) compared to usual care. Parents in the LHW‐facilitated intervention group showed an increased proportion of parent synchronous responses (adjusted MD 0.35, 95% CI 0.18 to 0.52) and improved mental health as measured by PHQ‐9 (adjusted MD –4.55, 95% CI –8.52 to –0.58) but little to no difference in the proportion of time in shared attention (adjusted MD 0.1, 95% CI –0.07 to 0.27) compared to usual care (1 study, 35 participants).
Economic studies related to included studies
Although literature is emerging on the effectiveness of primary‐level workers in delivering mental health services, very limited data on unit costs and resource requirements are available. This is mainly due to the difficulties associated with conducting economic analyses, time lags from inputs to outcomes, and many confounding variables.
Table 19 shows the data from the 20 included studies that reported cost‐effectiveness or costs in relation to the care of adults and children with mental health disorders. These studies underline the feasibility and potential cost‐effectiveness of PWs in providing mental health care, and report costs related to absenteeism and healthcare utilisation. However, all of the studies had significant risks of bias that cast doubt on the accuracy and reliability of these data. Not all relevant alternatives and costs (such as productivity loss) were considered or reported, some costs relied on estimates, future costs were not discounted properly, and chosen time horizons were less than one year in most cases.
4. Summary of costs and resource use from included studies.
|
Author Year Currency/Price per year Intervention dates |
Type of economic analysis | Study population | Intervention(s) | Intervention‐specific costs and cost‐effectiveness |
Resource use (i.e. costs to health services other than intervention costs; patient/society costs and productivity) |
| PW‐led care for adults with common mental disorders | |||||
|
Gureje 2019 (STEPCARE) (US$, 2016. 1 US$150 Nigerian Naira) September 2013 to July 2016 |
Cost‐effectiveness analysis Instrument: service utilisation questionnaire |
Both men and women with major depressive disorder | Stepped‐care vs usual care Delivered by PWs: doctor + nurse + LHW (multi‐disciplinary) in Nigeria |
Costs: over whole 12‐month period: cost per patient stepped care: US$187.50; usual care: US$183.40 Cost‐effectiveness: STEPCARE intervention showed a 55% to 60% probability for the intervention being a cost‐effective approach compared with usual care once a willingness‐to‐pay level of US$333 was reached. Reduction in cost per 1‐point improvement, stepped care vs control: PHQ‐9: US$30 at 6 months; US$272 at 12 months; per 1‐point improvement, WHODAS: US$26 at 6 months; US$136 at 12 months |
Health service costs: STEPCARE intervention showed considerably greater reductions in service costs compared to usual care Patient costs: time and travel costs (out‐of‐pocket expenditure) mentioned in methods but no results given |
|
Araya 2003/Araya 2006 (US$, 2004. 1 US$288.7 Chilean pesos) March 2000 to March 2002 |
Cost‐effectiveness analysis Data from the Chilean Ministry of Health price lists in Chilean pesos |
Women with depression | Stepped‐care (multi‐component programme) vs usual care Delivered by PHP: doctors, nurses, other; 12 hours' training in Chile |
Costs: stepped‐care programme was found to be marginally more expensive than usual care (an extra US$0.75 per depression‐free day; total mean cost of intervention: US$87.85 (95% CI 78.94 to 103.41) vs control: US$51.46 (95% CI 43.02 to 60.51) Cost‐effectiveness: high probability (90%) that the incremental cost of obtaining an extra depression‐free day with the intervention would not exceed US$1.04 |
Health service costs: costs for the intervention were similar in both arms. No additional data on impact on the rest of health services Patient costs: mean medication costs included in intervention costs, but unknown how much this is per patient and if cost is borne by patient. No other out‐of‐pocket payments presented |
|
Patel 2017/Weobong 2017 International dollars, 2015 (no Indian Rupees (INR) reported) 2015 PPP 19.2336 October 2013 to August 2016 |
Cost‐effectiveness analysis Instrument: client service receipt inventory |
Both men and women with severe depression | EUC with Healthy Activity Program (HAP) vs EUC only Delivered by LHW in India |
Costs: higher costs of HAP + EUC than EUC at 3 months, but by 12 months, costs offset by reductions in health service use Cost‐effectiveness: economic analysis per QALY gained at 3 months indicates that HAP is cost‐effective vs EUC, at only US$287.06 (95% CI ‐1843.21 to 4326.75); at 12 months it was ‐US$4332.75 (95% CI ‐24415.31 to 4191.12). Both use a societal perspective, which considers effects on productivity and other patient costs. The economic case is further strengthened with a high probability that the intervention could be cost‐saving at 3 months (7%, willingness‐to‐pay US$4819.50) |
Health service costs: hospital admissions at 3 months: HAP + EUC: US$2.40 (SD 18.60) vs EUC: US$6.60 (SD 30, P = 0.08); total public healthcare costs: HAP + EUC: US$14.10 (35.09) vs EUC: US$20.10 (39.59) (P = 0.07) Patient costs: at 3 months of time, cost to patients and family was HAP + EUC: US$15.60 (SD 18.60) vs EUC: US$12.00 (SD 16.51); MD: US$3.60 (95% CI 0.30 to 6.60, P = 0.03); productivity losses were HAP + EUC: US$26.10 (SD 34.79) vs EUC: US$41.69 (SD42.29); MD US$‐15.60 (95% CI ‐22.49 to ‐8.70, P < 0.0001). At 12 months, there seemed to be no difference in mean days unable to work between arms: HAP + EUC US$8.24 vs EUC US$1.82 (SD 8.81, P = 0.12) |
|
Patel 2010 /Buttorff 2012 (US$, 2009. 1 US$46.5 INR) April 2007 to September 2009 |
Cost–utility and cost‐effectiveness Instrument: no specific instrument used |
People with depression and/or anxiety in primary care | Task‐sharing psychological intervention vs EUC Delivered by LHW in Goa, India |
Costs: despite additional resources required for the intervention led by lay health workers, health system costs incurred over 12 months of follow‐up were similar across the 2 arms (Intervention: US$89 (246) vs control: US$88 (140)). In the public (but not the private) facilities investigated, time costs were lower and health outcomes significantly better in the intervention arm than in the control arm: US$229 (SD 274) vs 177 (SD 342) Cost‐effectiveness: health system costs are similar between intervention and control when data from both public and private settings were analysed. However in public primary care facilities, the intervention appeared to be not only cost–effective but also cost‐saving (if taking into account a societal perspective); participants in the intervention arm used and/or lost less cash and showed greater improvement in their mental state than control participants |
Health service costs: health system costs for outpatients were: intervention: US$32 (SD 68) vs control: US$42 (SD 61); and for inpatients: intervention: US$27.00 (SD 195) vs control: US$24.00 (SD 102) Patient costs: mean total costs for patients (including time costs for participants and families, i.e. opportunity costs of time spent travelling to, waiting for, or receiving care, as well as wages from any days of work lost, re‐treat costs were intervention: US$88.00 (153) vs control: US$141.00 (198). There was also greater productivity in intervention arm compared to control arm (complete or partial days worked in intervention arm vs control arm: MD 62 days extra worked (95% CI 49.6 to 75) |
| PW‐led care for women with maternal depression | |||||
|
Lund 2020 (1 US$12.77 ZAR, South Africa Rand), 2014 to 2016 |
Cost‐effectiveness analysis Instrument: client service receipt inventory (CSRI) + client sociodemographic and service receipt inventory – European version |
Adult pregnant women aged 18 years or older | Structured psychological intervention vs EUC Delivered by LHWs in South Africa |
Costs: no significant differences in participants’ unit costs. GP per visit cost for EUC was US$4.70 (SD 11.17) vs psychological treatment US$4.10 (SD 11.13); specialist per visit cost was EUC US$2.74 (SD 2.74) vs psychological treatment US$0 (no visits). Psychological treatment was more costly per participant per year in the intervention group (US$117.16, 95% CI 94.05 to 140.26) compared to EUC (US$85.30, 95% CI 55.98 to 114.62; P = 0.04), in part due to counselling being face to face rather than over the phone Cost‐effectiveness: no notable differences were found in mean HDRS scores at 3 months postpartum between the 2 arms; this resulted in negative incremental cost‐effectiveness ratios for the intervention arm in relation to patient, health system, and total costs |
Health service costs: no significant differences in participant unit cost and service utilisation patterns between the 2 arms (e.g. patient time costs, average visit to GPs) Patient and caregiver costs: intervention took more patient time than control; time cost MD: US$31.41 (95% CI 5.29 to 57.52) |
|
Gureje 2019 (EXPONATE) (US$, 2016. 1 US$150 Nigerian Naira) June 2013 to December 2015 |
Cost‐effectiveness analysis Instrument: client service receipt inventory – post natal depression version |
Antenatal women aged 16 to 45 years | High‐intensity treatment (HIT; stepped psychological intervention) vs low‐Intensity treatment (LIT, which is mhGAP with minimal training) in Nigeria Both arms delivered by primary maternal care provider (PMCP)‐community midwives (primary health professional). LHWs in HIT group too |
Costs: total estimated costs over full 1‐year period following baseline were approximately double in HIT group (US$46.85 per participant) compared with LIT group (US$24.00 per participant) Cost effectiveness: cost‐effectiveness was also assessed with respect to disability; for this outcome, cost per 1‐unit improvement on WHODAS was US$1.24 (95% CI ‐7.03 to 9.39) at 6 months; US$0.39 (95% CI ‐3.47 to 3.79) at 12 months. HIT represents a cost‐effective alternative to LIT, but because LIT was associated with similar changes in health, functioning, and cost, there is no significant advantage of the more intensive strategy |
Health service costs: overall higher service costs in intervention compared to control group, although service costs per participant per month were reduced similarly in both groups, HIT: US$6.54 (baseline) to US$5.25 (at 6 months); US$2.53 (at 12 months); LIT: US$5.39 (baseline) to US$3.23 (at 6 months); US$0.95(at 12 months) Patient costs: no data found |
|
Fuhr 2019 US$, 2015 October 2014 to June 2017 |
Cost‐effectiveness analysis Instrument: adapted CSRI |
Pregnant women aged 18 years or older | Thinking Healthy Programme (THP) + EUC vs EUC Delivered by LHWs (peers/Sakhis) in India |
Costs: mean cost of providing THPP was US$1.36 per beneficiary (95% CI 1.32 to 1.39); 12% of this cost was attributed to incentives alone. When other healthcare costs (health system perspective) and time and productivity costs (societal perspective) were added, costs did not differ significantly between groups at 3 and 6 months after childbirth. Adjusted MD in societal costs between intervention and control groups was US$–53·98 (95% CI ‐109.64 to 1.68) at 3 months after childbirth and US$–17·69 (‐45.94 to 10.54) at 3 to 6 months after childbirth Cost‐effectiveness: ICERs for remission and recovery also showed the THPP + EUC intervention was cost‐saving compared to EUC only; for example, incremental cost per additional recovered case is US$–151·07 (health system perspective) and US$–736 (societal perspective). THPP is relatively cheap to deliver and is cost‐saving through reduced healthcare, time, and productivity costs |
Health service costs: total costs to health system (including costs of the intervention) during third trimester and 6 months after childbirth did not differ (MD –US$14.86, 95% CI ‐46.91 to 17.19) Patient costs: total MD of societal costs (health system and productivity costs) during third trimester and 6 months after childbirth were potentially lower in intervention compared to control (MD –US$72.41, 95% CI ‐141·84 to –2·98) |
|
Sikander 2019 US$, 2015 (1 US$105 PKR (Pakistani rupee)) October 2014 to February 2016 |
Cost‐effectiveness analysis Instrument: CSRI |
Pregnant women aged 18 or older | Thinking healthy Programme (THPP) & EUC vs EUC only Delivered by LHWs (local volunteer peers) in Pakistan |
Costs: THPP intervention cost US$133.00 per participant to deliver. THPP offered appreciable improvement in health at low marginal cost. Over a willingness‐to‐pay threshold of US$60.00 per unit of improvement on PHQ‐9 score Cost‐effectiveness: they found that with a willingness‐to‐pay threshold of $60 per unit of improvement on PHQ‐9 score, intervention had a 98% probability of being cost‐effective with control group. THPP offered appreciable improvement in health at low marginal cost; costs per unit improvement in PHQ‐9 score of $15.50 (9.59 to 21.61) from 0 to 6 months |
Health service costs: overall health system costs, including costs of intervention, did not differ significantly between groups at 3 and 6 months after childbirth, nor did time and productivity costs. Travel costs of LHWs to attend training and supervisions included in intervention costs Patient costs: no difference between intervention and control with regards to numbers of days unable to work. During third trimester and the 3 months after, productivity costs did not differ between intervention and control groups (MD US$‐3.39, 95% CI ‐20.67 to 13.87) |
| PW‐led care for adults with alcohol or substance use | |||||
|
Nadkarni 2019 presented in paper as International dollars ((I$, 2015; 64.13 INR = 1 US$ ‐ PPP in 2015 = 19.236) October 2013 to August 2016 |
Cost‐effectiveness analysis Instrument: client service receipt inventory (CSRI) |
Men with alcohol dependence in primary care | Counselling for alcohol problem (CAP) plus enhanced usual care (EUC) vs EUC Delivered by LHW in India |
Costs: Total health system costs were greater in the CAP plus EUC arm (MD US$34.28 per person, 95% CI ‐3.47 to 72.15) Cost‐effectiveness: 20% chance of CAP being cost‐effective at the willingness‐to‐pay threshold of US$124.48, which is equivalent to 1 month’s wages for an unskilled manual worker in Goa Societal perspective: 53% chance of CAP being cost‐effective |
Health service costs: MD in total health service utilisation costs (including intervention costs) was US$22.30 (95% CI ‐15.70 to 60.31). There were no control costs presented Patient costs: time costs to service users and families and productivity losses in intervention group were very imprecise (wide confidence intervals, so it is not possible to draw conclusions from the figure (MD US$13.71, 95% CI ‐36.39 to 63.83; MD US$‐36.49, 95% CI ‐81.20 to 8.23, respectively) |
|
Sorsdahl 2015/Dwommoh 2018 US$, 2012 to 2013 (US$1 = ZAR 10.3952) March 2012 to March 2013 |
Cost‐effectiveness analysis Cost data described in detail in the paper |
Both men and women with substance use problem | Motivational interviewing (MI) vs MI + problem solving therapy (MI‐PST) interventions vs control group Delivered by LHWs (peer counsellors) in South Africa |
Costs: cost per patient is low in all 3 intervention groups (US$11, US$16, and US$33 for control, MI, and MI‐PST, respectively) Cost effectiveness: MI or MI‐PST interventions delivered by lay peer counsellors have the potential to be cost‐effective strategies for reduction of substance abuse disorder vs control group. MI vs control costs an additional US$119 per unit reduction in ASSIST score (US$20 for CES‐D); MI‐PST vs MI costs US$131 or US$33 per unit reduction in ASSIST or CES‐D scores, respectively |
Health service costs: included in cost of intervention under cost/cost‐effectiveness (screening and intervention costs) Patient costs: transport costs: none for MI intervention; MI‐PST: US$3 (cost per visit = US$0.96) Total patient costs were higher the more intensive the arm due to travel, counselling sessions (MI‐PST), and time off work (control: US$95; MI: US$129; MI‐PST: US$282) |
|
Papas 2020/Galarraga 2017 (US$, 2013; Kenyan Shilling) July 2012 to August 2016 (5.5 years' study) |
Cost‐benefit analysis (modelling exercise before start of intervention) Cost data from cross‐sectional survey (Larson et al 2013) |
Both men and women with alcohol problem among HIV+ persons | Cognitive‐behavioural therapy (CBT) vs healthy lifestyles (HL) Offered by paraprofessionals (LHW) in Kenya |
Costs: training costs in the first year were US$158,000, which was higher than subsequent years, primarily due to personnel costs. However, costs declined across the final 4 years to about US$94,000 in year 5. Total discounted cost over 5‐year roll‐out was approximately US$554,000. Over 5‐year period, average cost‐per‐participant was US$44. (Cost breakdowns are presented in Tables 2 and 3.) Benefits totalled US$49,000; US$118,000; US$137,000; US$133,000; US$129,000; and US$62,000 in years 1, 2, 3, 4, 5, and 6, respectively Cost‐effectiveness: overall benefit‐cost ratio was 1.13. Shifting CBT to paraprofessionals is cost‐effective compared to HL. However in the first year, costs were higher than benefits, but this reversed from year 2 to year 5 of the modelling exercise. Intervention can generate economic savings for the health system in the medium and long term |
Health service costs: no additional health service costs predicted other than those borne by the intervention Patient costs: none |
|
Nadkarni 2017 presented in paper as International dollars (I$, 2015; 64.13 INR = 1 US$ ‐ PPP in 2015 = 19.236) October 2013 and July 2015 |
Cost‐effectiveness analysis Instrument: CSRI |
Among male primary care attendees with harmful drinking | Counselling for alcohol problem (CAP) plus enhanced usual care (EUC) vs EUC Delivered by lay counsellors (LHW) in India |
Costs: from health system perspective, by 12 months, mean estimated costs to the health system of providing the intervention were no longer significantly different from the costs of EUC, being slightly lower (although not statistically significantly so) than those for EUC, at US$53.87 compared to US$62.08 (MD US$ ‐8.23, 95% CI ‐31.76 to 15.33; P = 0.49) CAP provides better outcomes at lower costs from a societal perspective vs EUC Cost‐effectiveness: health system perspective: from health system perspective, total healthcare cost per person, including intervention cost, was significantly higher in the EUC + CAP group than in the EUC alone group, with no significant difference in QALY scores. Societal perspective: CAP is said to have a more than 99% chance of being considered cost‐effective |
Health service costs: higher costs for hospital doctor consultations per person in CAP compared to control (MD US$7.47, 95% CI 10.23 to 39.59) Patient/family costs: MD in time costs to service users and families and productivity losses show no significant differences between them (MD US$1.20, 95% CI –1.8 to 2.70; MD US$ ‐3.30, 95% CI –11.10 to 2.70, respectively) |
| PW‐led care for adults with severe mental disorders | |||||
|
Barfar 2017 (US$, 2010) 12 months. 2010 to 2011 |
Cost‐effectiveness analysis Cost data: minimum hourly wage in Iran to calculate indirect costs |
Patients (both men and women) with severe mental disorders | Aftercare service vs treatment‐as‐usual (TAU) Delivered by PWs: doctor (general practitioner (PHP) + social worker (CP)) in Iran vs TAU (specialist care) |
Costs: average total cost of the intervention for personnel, medical supplies, and travelling is US$28,926 Cost‐effectiveness: no significant differences in effectiveness measures between the 2 groups were found. Aftercare service was about US$66,000 cheaper than treatment as usual |
Health service costs: no anticipated additional costs to health service other than those borne by the intervention Patient costs: mean total cost per patient for patient and family ‐ which include direct costs (out‐of‐pocket payments to health service, travel, costs at home) and indirect costs (time cost, lost productivity) ‐ was more expensive in the TAU group: US$4651 (SD 1381) compared to US$3823 (SD 837) in the aftercare service (AS) group. However, costs were overall much higher in AS telephone follow‐up group compared to AS home visit group |
|
Chatterjee 2014 (I$ 1 = Indian Rupee (INR) 19.13, 2009; 46.37 INR = 1 US$) January 2009 to December 2010 |
Cost‐effectiveness + cost utility analysis Instrument: cost of illness schedule (CIS) + EQ‐5D for QALY |
People (both men & women) with schizophrenia | Collaborative community‐based care (CCBC) vs standard facility‐based care (FBC) Delivered by LHW at least 10 years of education + weeks of training in India |
Costs: health economic findings showed that costs of the intervention group (CCBC + FBC) were greater than those of the control group (FBC), that is, MD is US$203.30 (95% CI 141.99 to 263.62). Up to a third of these extra costs were attributable to supervision Cost‐effectiveness: this ICER shows a cost of US$51.93 is needed to achieve a 1‐point reduction in PANSS. ICER based on IDEAS is US$201.96 divided by 0.95, i.e. US$214.00 to achieve a 1‐point reduction in IDEAS |
Health service costs: total service costs (including intervention costs) were higher in intervention compared to control group and varied among sites, with highest costs for participants in intervention group in Goa (i.e. Tamil Nadu: control: US$104.90 vs intervention: US$264.01; Goa: control: US$127.06 vs intervention: US$488.16; Satara: control: US$139.12 vs intervention: US$245.70). No additional health service costs apart from those included in the intervention Patient costs: cost of travel in intervention group was higher for Tamil Nadu compared to Goa or Satara because of more travel time (US$68.34 vs US$39.66 and/or US$25.40) |
|
Li 2002 (8.28 Yuan = 1 US$) June 1999 to March 2001 |
Cost analysis Cost data: collected by researchers |
Both men & women with late‐onset schizophrenia | Hospital treatment group vs community rehabilitation group Delivered by PW in China |
Costs: direct costs incurred by hospital treatment were 3.19 times those of community rehabilitation (i.e. US$29.10 and US$128.62). Cost of each home visit for GPs and nurses was US$13.67 and US$11.48, respectively. Community rehabilitation can avoid unnecessary costs such as hospital bed costs and hospital treatment fees. Going to work while supervised by family members can bring economic benefits Cost‐effectiveness: cost analyses represent basic calculations. Presented data did not allow for more sophisticated analysis |
Health service costs: no reported other costs for health service other than those reported related to intervention costs Patient costs: no patient costs done or reported |
|
Malakouti 2015 (1 US$ = 9143 IRR Iranian Rial) December 2007 to December 2008 |
Cost‐effectiveness analysis QALY ‐ HRQoL score from SF‐36 |
Patients (both men & women) with severe mental illness | Home visit clinical case management aftercare service by general physicians or nurses vs usual psychiatric care Delivered by PHP: doctor or nurse in Iran |
Costs: total costs for study participants were highest in the nurses' group US$34,828.23; corresponding costs in the GP group were US$28,843.86 and in the control group US$29,636.00. The major difference in costs among different groups was the cost of hospitalisations. The cost of each home visit for GPs and nurses was US$13.67 and US$11.48, respectively Cost‐effectiveness: incremental cost‐effectiveness ratio was US$627.89 and US$552.17 per QALY gained in general practitioner and nurse groups, respectively Nurse group was found to be most effective intervention due to lower incremental cost‐effectiveness ratio |
Health service costs: no reported other costs for health service other than those reported related to intervention costs Patient costs: indirect costs included in this study were patient transportation, case managers’ transportation, and loss of income for caregivers; no figures given |
|
Tan 2005 (Chinese Yuan, December 2003; 827.70 CNY = US$100) May 2003 to June 2004 |
Cost‐analysis Cost data: collected by researchers |
Patients (both men & women) with schizophrenia | Community observation group (collaboration between hospital doctor and community doctor) vs control Delivered by PHP: doctor in China |
Costs: costs of treatment were significantly less in the intervention group than in the control group, which is US$27.91 per month less. Hospitalisation costs for control amounted to US$6919.37 compared to US$0 (no hospitalisations) for community observation Cost‐effectiveness: cost analyses represent basic calculations. Presented data did not allow for more sophisticated analysis |
Health service costs: hospitalisation costs: control group, 15 participants hospitalised 23 times, resulting in total hospitalisation costs of US$6919.37. No hospitalisations in community observation group Patient costs: productivity loss: this PHP‐led intervention resulted on average in 7.7 days/month fewer missed workdays compared to specialists, which is equivalent to US$27.91/month Patients’ family members’ missed workdays were also reduced by 2.3 days/month, equivalent to US$7.13/month |
| PW‐led care for children and adolescents with PTS and CMD | |||||
|
Barron 2016 (US$, 2015 ) Study performed in 2015 |
Cost‐benefit analysis Calculated by field researchers |
Adolescents with high level of PTS | Teaching recovery techniques vs wait‐list (no care) Delivered by community professional: school counsellor (but received only 3 days of training) in Palestine |
Costs: total cost for intervention group was US$3867.52; cost per adolescent was US$38.68. No costs were reported for the control group Cost‐effectiveness: given that costs were relatively low and results clinically significant, study authors conclude that the intervention may be cost beneficial, and they make a case for TRT to be delivered throughout the West Bank |
Health service costs: no reported costs for health service other than those reported related to intervention costs Patient costs: participant travel cost was US$465.60 in intervention group. No costs for control group were reported. No productivity costs were reported |
|
Tol 2008/Jordans 2011 (US$; 1 EUR = US$1.33) (2005 to 2008) |
Costs analysis Cost data: calculated |
Children (both male and female, 7 to 15 years of age) Psychosocial care package | Classroom‐based intervention (CBI) vs wait‐list Delivered by LHWs (paraprofessional interventionists) in Indonesia |
Costs: cost analyses for intervention group demonstrated mean cost per service user was US$21.77 (59% of which is human resources cost) Cost‐effectiveness: cost analyses represent basic calculations. Presented data did not allow for more sophisticated analyses |
Health service costs: costs mentioned under 'costs' also include development and implementation of entire care package (combination of 3‐tiered intervention structure, i.e. psychoeducation, non‐therapeutic resilience groups, CBI and counselling, including development, supervision, and ongoing capacity building) Patient cost: none reported |
|
Tol 2012/Jordans 2011 (US$;1 EUR = US$1.33) (2005 to 2008) |
Cost analysis Cost data: calculated |
Children (both male and female, 9 to 12 years or age) Psychosocial care package | Classroom‐based intervention (CBI) vs wait‐list Delivered by LHWs (paraprofessional interventionists) Training: 2 weeks in Sri Lanka |
Costs: cost analyses for intervention group demonstrated mean cost per service user was US$8.85 (56% of which is human resources cost) Cost‐effectiveness: cost analyses represent basic calculations. Presented data did not allow for more sophisticated analyses |
Health service costs: 'costs' include broader package as mentioned above under Tol 2008 Patient cost: none reported |
CSRI: client service receipt inventory; EUC: enhanced usual care; I$: international dollar; LHW: lay health worker; MD: mean difference; PKR: Pakistani rupee; US: US dollar.
1. PW‐led interventions for adults with common mental disorders compared to usual care (Comparisons 1 and 2)
Four of the 12 included randomised trials in the CMD comparison groups reported some form of economic evaluation: all four are cost‐effectiveness studies of PWs delivering psychological interventions for depressive disorders. Two studies were multi‐disciplinary (i.e. were delivered by doctors, nurses, and LHWs) (Gureje 2019 (STEPCARE); Araya 2006, reference in Araya 2003), and one was non‐professional LHW delivered (Patel 2017). A combined cost‐effectiveness and cost‐utility analysis was conducted (Buttorff 2013, reference in Patel 2010), which was linked to a randomised trial on people with depression and/or anxiety by Patel 2010.
Findings in two studies suggest that PW‐delivered psychological interventions for depressive disorders may be cost‐effective (Gureje 2019 (STEPCARE); Patel 2017), whilst Araya 2006 (reference in Araya 2003) suggests that the stepped‐care programme was marginally expensive when compared to usual care. Patel 2017's assessment of cost‐effectiveness (incremental cost‐effectiveness ratio (ICER)) showed that the incremental cost per quality‐adjusted life‐year (QALY) gained was −$1,721. This indicates that Health Activity Programme (HAP) plus enhanced usual care (EUC) was associated with both lower costs and better outcomes than EUC alone. These researchers concluded that the HAP was cost‐effective when wide societal effects on productivity were considered. Gureje 2019 (STEPCARE) showed considerable reductions in service costs. The reduction in cost per 1‐point improvement on the PHQ‐9 with the stepped‐care intervention compared with the control was USD 29.69 (95% CI USD ‐21.56 to 89.45). Study authors concluded that their stepped‐care intervention combined with enhanced usual care lowered costs more than enhanced usual care alone, with some evidence for a more favourable cost‐effectiveness profile for treating depression in this setting. Araya 2006 (reference in Araya 2003) estimated that the stepped‐care programme cost an extra USD0.37 per depression‐free day compared to the usual care programme. The results in Buttorff 2012 (reference in Patel 2010) showed that health costs of the task‐sharing psychological intervention delivered by LHWs were similar to those of the EUC. However, time costs were lower and health outcomes significantly better in the intervention arm than in the control arm. In addition, the intervention appeared to be both cost–effective and cost‐saving for the public primary‐care facilities. Therefore, participants in the intervention arm used and/or lost less cash and showed greater improvement in mental state than control participants. Evidence for cost‐effectiveness of LHW‐led interventions is very uncertain due to serious study limitations, indirectness, and imprecision. PHP‐led collaborative care interventions are probably cost‐effective compared to usual care (moderate certainty due to serious study limitations).
There seemed to be no change in either hospital outpatient or inpatient costs between arms in Patel 2017. Patient costs seemed to be overall less in the intervention arms compared to the control arms, and their productivity was increased for two of the four studies in which this was reported (Patel 2010; Patel 2017). Evidence for health system costs of LHW‐led interventions is very uncertain due to serious study limitations, indirectness, and imprecision. PHP‐led collaborative care is probaby marginally more expensive compared to usual care (moderate certainty due to serious study limitations).
2. PW‐led interventions for women with maternal depression compared to usual or enhanced usual care (Comparisons 3 and 4)
Four of the nine included studies in the perinatal comparison group had economic evaluations: three studies assessed cost‐effectiveness of LHW interventions versus EUC in pregnant women 18 years of age or older (Fuhr 2019; Lund 2020; Sikander 2019), whilst one study assessed cost‐effectiveness of professional (nurse/midwife)‐led interventions and LHWs for pregnant women aged 16 years or older (Gureje 2019 (EXPONATE)) versus a low‐intensity intervention (mhGAP).
The only study to say that a PW intervention was not cost‐effective was Lund 2020. This group found no significant differences in participant unit costs nor in mean costs per visit to a healthcare provider but learned that psychological treatment was more costly per participant per year (USD 117.16) versus EUC (USD 85.30). However, in Fuhr 2019, ICER estimates showed that the Thinking Healthy Programme peer‐delivered (THPP) intervention was cost‐saving through reduced health care and time and productivity costs and was relatively cheap compared to the EUC only. In addition, the THPP delivered in Sikander 2019 offered an appreciable improvement in health at a low marginal cost over a willingness‐to‐pay threshold of USD 60 per unit of improvement in PHQ‐9 score. The high‐intensity stepped‐psychological intervention in Gureje 2019 (EXPONATE) was cost‐effective, but because the mhGAP (low‐intensity treatment) in the control group was associated with similar changes in health, functioning, and cost, no significant difference was found for the more intensive strategy. We found that three of the studies in this group showed that interventions were cost‐effective (Fuhr 2019; Gureje 2019 (EXPONATE); Sikander 2019) ‐ although high‐intensity treatment in Gureje 2019 (EXPONATE) was not more cost‐effective than its low‐intensity control ‐ while Lund 2020 found that the psychological treatment was more costly per participant per year. LHW‐led interventions may or may not be cost‐effective compared to EUC (low‐certainty evidence due to serious study limitations and inconsistency). PHP‐led collaborative care interventions may be slightly or no more cost‐effective than EUC (low certainty due to serious study limitations and indirectness).
We found few data on resource use, and of those reported, health service costs were high and patient costs were lower in intervention versus control groups. LHW‐led interventions may or may not incur higher health service costs than EUC (low certainty due to serious study limitations and inconsistency). PHP‐led collaborative care interventions may incur higher health service costs compared to EUC (low certainty due to serious study limitations and indirectness).
3. LHW‐led interventions for adults with alcohol and substance use compared to enhanced usual care (Comparison groups 7 to 10)
Four of the 17 included studies in the alcohol and substance use comparison reported some form of economic evaluation: three cost‐effectiveness studies (Nadkarni 2019; Dwommoh 2018. see Sorsdahl 2015; Nadkarni 2017), and one cost‐benefit study (Galarraga 2017, see Papas 2020), which examined LHWs delivering psychological interventions for alcohol dependence ‐ Nadkarni 2017 ‐ and harmful or hazardous drug use ‐ Dwommoh 2018, see Sorsdahl 2015. Galarraga 2017 (see under Papas 2020) used model‐estimated costs rather than real cost data in its economic evaluation. None of the trials that reported economic data evaluated interventions delivered by health professionals.
Findings of all four studies in this comparison group suggest that LHW‐delivered interventions may be cost‐effective (see Table 19). All three studies that assessed cost‐effectiveness from a societal perspective found that these task‐shifting interventions had potential to be effective and to incur low costs (Nadkarni 2019; Dwommoh 2018, see Nadkarni 2017; Sorsdahl 2015). The study that undertook a cost‐benefit analysis of shifting interventions for alcohol dependence among HIV‐positive persons from professionals to paraprofessional providers showed that this could generate economic benefits in the medium and long term (Galarraga 2017, see Papas 2020). LHW‐led interventions for patients with harmful and hazardous alcohol use may be cost‐effective compared to EUC (low certainty due to serious study limitations and indirectness), but the evidence for LHW‐led interventions for patients with alcohol dependence is very uncertain (due to serious indirectness and very serious imprecision).
We found few data on resource use, and among those reported, there seemed to be little to no difference between intervention and control groups, although Sorsdahl 2015 suggested total patient costs were higher the more intensive the arm control due to travel, counselling sessions (motivational interviewing/problem‐solving therapy (MI‐PST)), and time off work. Costs to patients were lower or uncertain. Evidence for health service costs for LHW‐led interventions for harmful or hazardous alcohol use or dependent alcohol use compared to EUC is very uncertain (due to serious study limitations, indirectness, and imprecision, and to serious indirectness and very serious imprecision, respectively).
4. PW‐led interventions for adults with severe mental disorders compared to specialist‐led care (Comparisons 11 and 12)
Four out of the seven studies in the comparison of primary health professional‐led or collaborative care versus specialist‐led care for people with severe mental disorders performed economic evaluations. A cost‐effectiveness analysis was performed in three of them (Barfar 2017; Malakouti 2015; Tan 2005), and a cost analysis was performed in one (Li 2002). In all four studies, interventions delivered by primary health professionals or collaborative care resulted in cost savings compared to specialist‐led care. In Barfar 2017, cost‐effectiveness analysis showed that primary health professional‐led or collaborative care interventions were USD 66,000 cheaper than specialist‐led care, and the average total cost per patient in the specialist‐led group was USD 4651 compared to USD 3823 in the primary health professional‐led group (equivalent to a cost reduction of about USD 800 per person). In Malakouti 2015, interventions delivered by general physicians and nurses yielded ICERs of USD 583.20 and USD 512.86 per QALY gained, respectively, leading study authors to conclude that nurse‐led care was most cost‐effective. In Tan 2005, study authors reported that collaborative care involving primary health professionals resulted in lower direct costs (USD 17.02 ± 18.34 versus 82.08 ± 53.96) (P = 0.002) and indirect costs (USD 0.38 ± 1.63 versus 14.97 ± 29.67) (P = 0.0022) compared to specialist‐led care. In Li 2002, trial authors found that specialist‐led care cost 3.19 times more in direct costs compared to primary health professional‐led care. The cost‐effectiveness of PHP‐led collaborative care compared to specialist‐led care is very uncertain due to serious study limitations, indirectness, and imprecision.
In Chatterjee 2014 ‐ one of the two studies that compared LHW‐led interventions for people with severe mental disorders versus specialist‐led care, a cost‐effectiveness and cost‐utility analysis was performed, and findings were that costs in the intervention group were on average greater than those in the control group, and that about a third of these additional costs were attributable to supervision. After adjustments for baseline, the mean difference between the two groups was USD 194.73 (95% CI USD 136.00 to USD 252.51). The ICER based on the Positive and Negative Syndrome Scale (PANSS) shows that a cost of USD 51.93 was needed to achieve a 1‐point reduction on the PANSS and cost of USD 204.98 on the IDEAS. The cost‐effectiveness and health service costs of LHW‐led interventions compared to specialist‐led care are very uncertain due to serious study limitations, indirectness, and imprecision.
We found few data on resource use and patient costs. Overall health service costs were higher, although costs of hospitalisations were reduced (Tan 2005). Patient costs were variable, depending on the type of intervention. Tan 2005 also reported that patients receiving collaborative care involving primary health professionals had improved productivity; they missed on average 7.7 fewer days of work per month for health reasons, saving USD 27.90/month as a result, compared to patients receiving specialist‐led care, and their family members missed 2.3 fewer days of work per month, saving USD 7.13/month as a result. Evidence for health system costs of PHP‐led collaborative care is very uncertain due to serious study limitations, inconsistency, and imprecision.
5. PW‐led interventions for children and adolescents with post‐traumatic stress (PTS) or common mental disorders (CMDs) compared to usual or no care (Comparisons 14 and 15)
Three of the 15 included studies in this comparison group reported some economic evaluation: one cost‐effectiveness study ‐ Barron 2016 ‐ and two cost analysis studies ‐ Jordans 2011, see Tol 2008; Tol 2012 ‐ of LHWs delivering psychological interventions for PTS and CMD.
Findings of both studies showed economic gains and recommended delivery of these interventions to other populations throughout the West Bank or to populations of distressed children. Given the high returns and low costs of the Teaching Recovery Techniques (TRT) intervention versus the wait‐list (cost‐effectiveness), Barron 2016 concluded that the TRT could be cost‐effective and could be delivered throughout the West Bank. However, evidence for cost‐effectiveness of CP‐led interventions was very uncertain due to serious study limitations, indirectness, and imprecision. Cost analysis of a school‐based psychosocial intervention versus wait‐list control for children aged 7 to 15 years in Indonesia ‐ Jordans 2011 (reference in Tol 2008) ‐ estimated that the mean cost per service user lies between USD 4.60 and 23.04. The same cost analysis conducted in Sri Lanka of school‐based psychosocial intervention versus wait‐list control for children aged 9 to 12 years ‐ Jordans 2011 (reference in Tol 2012) ‐ showed that mean cost per user was USD 8.85. This study suggests that a multi‐layer psychosocial package appeared feasible and satisfactory for reaching out to populations of distressed children through different levels of care. LHW‐led interventions probably can be delivered at low cost compared to no care (moderate certainty due to serious study limitations).
We found no data on resource use nor on patient costs or productivity, apart from Barron 2016, which acknowledged some transport costs that may be an important out‐of‐pocket cost for patients. Evidence for health service costs of CP‐led interventions is very uncertain due to serious study limitations, indirectness and imprecision.
Discussion
Summary of main results
This review identified 95 randomised trials evaluating the effectiveness of primary‐level worker (PW) interventions for treatment of mental disorders and distress in 30 low‐ and middle‐income countries (LMICs). In general, PWs seem to have a small to moderate effect on reducing mental health symptoms and improving quality of life and functioning. Details of this summary are described in the abstract and in the plain language summary.
Overall completeness and applicability of evidence
This review aimed to assess the effectiveness of PWs including primary health professionals (PHPs), lay health workers (LHWs), and community professionals (CPs) such as teachers in delivering care for people with mental disorders, to provide guidance to health policy makers in LMICs. Several issues need to be considered when judgements are made about the applicability of these findings to large‐scale programmes.
Factors related to the type and role of primary‐level workers
The included studies used many different types of PWs, some of whom were existing cadres within the health service sector, while others were additionally trained resources, particularly for common mental disorders (CMDs) and post‐traumatic stress disorder (PTSD). However, we identified only a few studies for most comparisons and details of intervention and training often were inadequately reported. We found sufficient studies to divide the analyses by professional versus non‐professional cadres/lay workers, which we hope is useful for policy makers in seeing which cadres may best fit with their current workforce, so that they can prioritise specific interventions accordingly. However, we were not able to explore the effects of interventions according to different PW characteristics (such as selection, training, support, incentives, or remuneration). Although mentioned in many studies, the training and support of PWs were not described in detail in more than half the studies. Incentives and remuneration were mentioned in only three studies. We also were not able to explore the independent effects of PWs when they were part of complex interventions (such as collaborative care) nor the effects of the intensity of PW‐led interventions, as this was also inadequately described. More complete and uniform reporting of this information would help guide policy makers in tailoring the types of PWs and their roles within scaled‐up programmes appropriately and may be featured in a separate publication.
Furthermore, many studies considered the role of PWs as an 'add‐on' to usual care. Only the nine studies within the two severe mental disorders comparisons compared these cadres versus specialists, but these studies had serious risk of bias issues and some outcomes that could not be pooled. Therefore, we cannot be certain whether task‐shifting (with appropriate supervision) to non‐specialists (i.e. actually substituting specialists with non‐specialists) leads to equivalent quality of care or equivalent results in terms of appropriate care. Very few studies measured adverse events or unintended consequences of PW‐led care, and such effects could impact the appropriateness and quality of care, potentially leading to patient harm.
Interventions
Comparisons of studies were performed by mental disorder, by broad types of interventions (such as collaborative care and psychological interventions), and by professional or lay cadre of workers delivering the intervention(s).
With regards to cadres delivering the intervention, it was possible, given the low to moderate certainty of evidence, to conclude that both LHWs and PHPs may be slightly or moderately effective for most mental disorders, delivering a variety of psychological or psychosocial/social interventions and collaborative care interventions (sometimes with pharmacological interventions prescribed by primary health professionals).
None of the included studies addressed the indirect impact of delivering mental health care on other elements of health care delivered by PWs or on other roles (e.g. the impact of a mental health intervention on primary care doctors' other tasks such as treating patients with diabetes, the impact on their working pattern such as consultation times). However, several studies within CMD, perinatal depression, and severe mental disorder comparisons did provide information on hospital admissions and outpatient consultation attendances. Similarly, more recent studies published since the previous version of this review were more likely to report adverse events. Even so, less than 20% of studies reported service utilisation and adverse events.
Service or social interventions that measure mental health outcomes were not included ‐ only specific mental health interventions. Mental health outcomes are closely related to social determinants of health such as poverty. This will have excluded interventions (probably most often delivered by lay workers or social workers) that primarily affect mental health outcomes but are not mental health interventions. We recommend this to be the focus of subsequent reviews. Some of those identified are listed in our Excluded studies.
We categorised mental disorders in this review similarly to our 2013 review, but we took a more nuanced approach to the grouping of post‐traumatic stress syndromes. It was important to differentiate PTSD (meeting specific diagnostic criteria) from PTS symptoms (acute reactions to stress), notably to understand the progression of symptoms along the mental health spectrum. This was important with regards to differentiating recovery from progression of symptoms in this review but will also be important for differentiating primary prevention from trauma exposure to PTS symptoms, and secondary prevention from PTS symptoms to PTSD, in our sister review on the use of PWs in prevention of mental illness and promotion of mental health (Purgato 2021).
Programme delivery
Several issues need to be considered in applying these findings to healthcare delivery systems.
First, these are interventions delivered in research settings where PWs are more likely to have been carefully selected; project leaders are more motivated; remuneration may be more available because of research funding; and training, supervision, and monitoring are generally much more intensive. These conditions may not be replicable at scale or may not be as effective at scale.
Second, the types of study design chosen here were not appropriate or sufficient to inform judgements regarding the sustainability of programmes; alternative study designs, such as longitudinal studies, economic evaluations, and qualitative studies are needed for this.
Third, the applicability of review findings needs to be considered in each setting where decisions on task‐sharing or task‐shifting for mental health treatment are made (Lavis 2009). Factors that decision‐makers may wish to consider include the extent to which their settings resemble those of the included studies, such as on‐the‐ground constraints, health service and system arrangements, differences in baseline conditions, presence of specific groups that might benefit from the intervention, sociocultural factors in those populations such as cultural perceptions of mental health and stigma, and availability of routine data on mental health issues and treatment. Although the review covered 30 LMICs (13 of which were conducted in low‐income settings), data were insufficient for any subgroup analyses by country or income setting within the overarching classification of mental disorders and types of health workers.
Fourth, it is important to know the financial burden of such interventions. Only 20 studies reported cost data, and these were all from different settings, with no more than five studies pertaining to one comparison. This suggests that interventions may be cost‐effective and of low cost for some studies, but due to localised costs and data, the results cannot be generalised to all LMICs. Also no studies included health service or societal perspective costs for training specialists (for delivery or for supervision of the community intervention). This may therefore lead to underestimation of the costs of these interventions when specialists are involved in collaborative care or shared care. Given that only cost and cost‐effectiveness data from included studies feature in the cost and cost‐effectiveness analysis, this analysis may be incomplete. A systematic review of the literature to look for stand‐alone cost or cost‐effectiveness studies would complete these findings.
Outcomes
This review attempted to be transdiagnostic by looking at mental distress and mental illness as a spectrum. However, we were limited by what the studies reported. It was not possible to combine concepts of recovery, prevalence, and severity due to the very heterogeneous definitions of these, even within each of these concepts. We therefore resorted to report as best we could on mental disorders. Furthermore for two comparisons (Summary of findings table 1; Summary of findings table 3), the same tool (and thus data) was used with different thresholds for recovery and change in symptoms (e.g. Patient Health Questionnaire‐9 (PHQ‐9) was used to look at continuous outcomes of symptom change with the view that if the score is < 5, this indicates recovery). This may have led to overestimation of the effect.
We reviewed the inclusion criteria for studies that looked at mental distress or prodromal disorders and decided to include some of these in the prevention review instead as secondary or tertiary prevention interventions (Purgato 2021). Studies that were transdiagnostic and had as their inclusion criteria people who met the diagnostic criteria for disorders and those who did not are included in both reviews.
Studies awaiting classification
An update in 2020 of the searches for this review identified 16 further studies that are awaiting classification. Due to resource limitations, it was not possible to incorporate them into this review update. These additional studies may lead to some changes in review findings at a future update, but current findings represent a substantial step forward in understanding the effectiveness of mental health treatments delivered by primary‐level workers.
Quality of the evidence
This review included 95 randomised trials covering a wide range of interventions and settings. For studies included in the meta‐analyses, evidence for most outcomes was of low to moderate certainty. Risk of bias assessments highlighted concerns regarding insufficient information on sequence generation and allocation concealment; differences in baseline outcome measurements; detection bias due to lack of blinding of outcome assessors; and failure to address incomplete outcome data adequately, particularly safety data and data on contamination between intervention and control arms. With regards to safety data, most studies did not report adverse events, and among those that did, event numbers were very low. This may impact confidence intervals (CIs) around effect estimates, and this would be taken into account in the GRADE assessment.
When meta‐analysis was possible, the results were fairly consistent in showing improvements in favour of PW interventions, although for some interventions and outcomes, there were important variations in reported effects that could not be explained. For example, it is not clear in the harmful alcohol and substance use comparison why LHWs may be more effective than health professionals, and why for some studies results favoured the control group.
Some studies assessed large numbers of outcomes, increasing the probability of finding statistically significant differences for some outcomes by chance. Furthermore, the diversity of psychometric and other outcome measures used made interpretation of statistically pooled outcome data difficult. The validity and psychometric properties of the same measure across different settings may also vary, which may further limit the reliability of the information.
Our primary search date occurred in June 2019, and a repeat search was performed in August 2020. Due to the complexity and size of the review and the multiple iterations we had been through, we put the 16 new studies identified into 'awaiting classification', and we plan to update the review with these findings soon. This may limit the up‐to‐date completeness of findings presented in the meta‐analyses.
This update of the review has highlighted a wealth of information on several different disorders, and we note that publications in this field are likely to continue to increase, particularly in light of the response to the COVID‐19 pandemic and its effects. Further updates will need to consider subdividing this area by different disorders rather than including all disorders in one review. This would allow more in‐depth analysis into other variations and factors and may help provide more nuanced answers about which types of interventions and which types of professionals/lay workers (and training and other perspectives) may be more specifically effective.
Potential biases in the review process
PWs, particularly LHWs, remain poorly indexed in the literature. Although we tried to cover a broad range of synonyms for these health workers, it is possible that some studies have been missed. In addition, PWs and LHWs do not have standard widely accepted definitions, so some readers may disagree with these definitions or with how this review has aggregated different health worker cadres.
We found too few studies within each comparison to assess publication bias through assessment of asymmetry. However, we assessed publication bias by checking on ongoing studies/those awaiting classification from the 2013 review and did not notice any publication bias (all had been published; those that had not been published had been aborted for non‐clinical reasons).
Many meta‐analyses were performed; therefore, some of the findings may be due to chance. Many pooled results were statistically and clinically heterogeneous, mainly because of the small number of included studies and the breadth of geographical, health worker, intervention, and patient characteristics. Therefore the results need to be interpreted with caution.
A further limitation was that 33 of the 89 trials meta‐analysed that did not conduct an intention‐to‐treat analysis generally were not re‐analysed or their missing data were not imputed (except for one analysis for which we were able to source data: PW‐led psychological interventions for depression ‐ prevalence of depression). This may have an impact on the estimates of effect.
Agreements and disagreements with other studies or reviews
We identified 23 reviews related to this current review, which examined various aspects of primary or community mental health care delivered by a non‐specialist workforce (Table 17). None of these reviews presented a comprehensive summary of all PWs across all mental disorders/distress in LMICs. Also, a number of these reviews are based on searches that are now very out‐of‐date, which may limit the usefulness of their findings. Several systematic reviews reviewed models of delivery (but not specifically types of health workers) such as collaborative care (Bower 2006; Woltmann 2012), psychological or psychosocial interventions delivered (Barbui 2020; Huntley 2012; Shahmalak 2019), mental health interventions provided in humanitarian settings (Purgato 2018; Tol 2011), interventions provided for common perinatal mental disorders among women in LMICs (Clarke 2013; Gajaria 2018; Munodawafa 2018; Rahman 2013), school‐ and community‐based mental health interventions for young people in settings in LMICs (Barry 2013; Burkey 2018; Klasen 2013), and recovery of people living with severe mental illness in LMICs (Gamieldien 2020).
Certain reviews focused on health workforce competencies for community‐based providers (Kohrt 2018), as well as roles of mid‐level health workers in delivering healthcare services to the general population (Lassi 2013). Only four reviews specifically examined the effectiveness of non‐specialist providers (1) in providing psychological treatments for depression, anxiety, and PTSD outcomes (randomised controlled trials (RCTs)) (Singla 2017); (2) in implementing mhGAP (a World Health Organization mental health gap programme) in LMICs (Keynejad 2018); (3) in using LHWs for prevention of mental disorders (Mutamba 2013); and (4) in using peers to deliver mental health interventions (Fuhr 2014). Similar to our findings, the Singla 2017 meta‐analysis showed pooled results for anxiety, depression, and PTSD outcomes demonstrating benefit (standardised mean difference (SMD) 0.49, 95% CI 0.36 to 0.62). However, this review did not divide findings by type of health worker, nor did review authors extract dichotomous outcomes. Both Fuhr 2014 and Mutamba 2013 suggested that peers or LHWs may improve psychosocial and mental outcomes, although these findings are out‐of‐date (Mutamba 2013 is being updated by Purgato 2021). Furthermore, this review did not include peers unless recruited as LHWs to perform a wider community role. Peers often have a different informal role just within their own family or environment, and these roles are qualitatively different, as they rely on a close relationship (see definitions in Table 16). Keynejad 2018 could not perform a meta‐analysis, as only two randomised trials were identified. Most identified literature on the mhGAP was implementation and observational literature.
An additional 14 reviews incorporated aspects of interventions delivered by non‐specialists/paraprofessionals/primary workers that were included in this current review (Barry 2013; Boer 2005; Bower 2006; Clarke 2013; Gajaria 2018; Huntley 2012; Keynejad 2018; Munodawafa 2018; Purgato 2018; Rahman 2013; Shahmalak 2019; Singla 2017; Tol 2011; Woltmann 2012). All of these review authors agree with our review findings of mild to moderate benefit of PWs, also among those that reviewed studies in high‐income countries (Boer 2005; Bower 2006; Woltmann 2012). Most of these were reviews without meta‐analyses, although the four studies that did include meta‐analyses reported findings and magnitude of effects similar to ours (Purgato 2018; Rahman 2013; Tol 2011; Woltmann 2012). For example, Rahman 2013, a systematic review on interventions for women with perinatal disorders in LMICs, included LHW‐led interventions, and the final pooled estimate was very similar to ours, showing that these interventions may lead to benefit (SMD ‐0.38, 95% CI ‐0.56 to ‐0.21).
Details of these reviews and on how they agree or differ from this review are presented in Table 17.
Authors' conclusions
Implications for practice.
Most results from the 95 randomised trials suggest that primary‐level workers (PWs) delivering interventions for the care of individuals with mental disorders and distress have some impact on patient outcomes, although most evidence is of low certainty. Given the multitude of settings, disorders, interventions, and health worker expertise covered in this review, studies within each category are still too few to allow conclusions on specific intervention characteristics (such as type of health worker, duration of intervention, levels of training and supervision, etc.) that may impact effectiveness.
Evidence does show important results across studies, in particular, the impact of lay health workers (LHWs) and primary health professionals (PHPs) on clinical symptoms and improvement in functioning and quality of life for patients, as mentioned above.
1. Adults with depression and anxiety
Treatments from LHWs compared to usual care may increase recovery, may reduce the number of people with depression/anxiety, may improve quality of life, may slightly improve day‐to‐day functioning, and may reduce risk of suicidal thoughts or attempts.
Treatments from primary‐level workers in collaboration with mental health specialists compared to usual care may increase recovery; may reduce the number of people with depression/anxiety, although the range for the actual effect indicates they may have little or no effect; may slightly reduce symptoms; may slightly improve quality of life; probably have little to no effect on day‐to‐day functioning; and may reduce referral to mental health specialists.
2. Women with depression related to pregnancy and childbirth
Treatments from LHWs compared to usual care may increase recovery; probably slightly reduce symptoms of depression; may slightly improve day‐to‐day functioning; and may have little to no effect on risk of death.
3. Adults in humanitarian settings with post‐traumatic stress or depression and anxiety
Treatments from LHWs compared to usual care may slightly reduce symptoms of depression; probably slightly improving quality of life.
Treatments from primary health professionals compared to usual care may reduce the numbers of adults with post‐traumatic stress and depression.
4. Adults with alcohol or substance use problems
Treatments from LHWs compared to usual care may increase recovery from harmful/hazardous alcohol use, although the range for the actual effect indicates they may have little to no effect; probably slightly reduce the risk of harmful or hazardous drinking; may have little to no effect on day‐to‐day functioning; and may have little to no effect on the number of people who use methamphetamine.
Treatments from primary health and community professionals compared to usual care probably have little to no effect on recovery from harmful/hazardous alcohol use; probably slightly reduce risk of harmful/hazardous alcohol and substance use; and probably have little to no effect on quality of life.
5. Adults with severe mental disorders such as schizophrenia
Treatments from primary health professionals alone or in collaboration with mental health specialists compared to mental health specialists alone may improve day‐to‐day functioning.
6. Adults with dementia and their carers
Treatments from lay and professional health workers may have little to no effect on the severity of behavioural symptoms in dementia patients and may reduce carers' mental distress.
7. Children in humanitarian settings with post‐traumatic stress or depression and anxiety
Treatments from LHWs may have little to no effect on post‐traumatic stress symptoms; probably have little to no effect on depressive symptoms or on day‐to‐day functioning; and make little to no difference in adverse events.
Treatments from community professionals (teachers and social workers) may have little to no effect on depressive symptoms; and make little to no difference in adverse events.
Very few studies measured unintended consequences of PW‐led care. We divided adverse events (as per description in the methods) into clinical indicators, service delivery indicators, and social indicators. How we defined and categorised adverse events was arbitrary, reflecting lack of reporting for this important outcome. Health service utilisation was thus included within adverse events to reflect that increased utilisation is linked to worsening symptoms. Few studies reported adverse effects (although these studies seemed to describe a few of these). Of the adverse events reported, most were clinical indicators (such as suicide rates and worsening of mental health) and health service delivery indicators (hospital re‐admissions, increased use of outpatient or alternative mental healthcare services). There were no social indicators (such as measuring impact on social exclusion/integration), nor were there indirect effects on other parts of the primary health service delivery (e.g. diversion of resources leading to neglect of other aspects of care) or on carers. Such effects could impact the appropriateness and quality of care.
Economic evaluation techniques are useful for conducting cost‐effectiveness analyses of different interventions to inform policy. Results from this review show that, in general, task‐shifting for the care of mental disorders and distress in LMICs could be cost‐effective, particularly for child and adolescent PTSD, perinatal depression, and alcohol and drug use, for which findings were consistent. This approach may be cost‐effective or cost‐saving for common mental disorders and severe mental disorders, though these findings were less consistent (see details in Table 19). We found no data on adult PTSD or dementia. Although several studies that showed cost‐effectiveness in their settings have recommended implementing the intervention in LMIC settings, the numbers of studies identified were small. However, these results could start to inform and influence policy making regarding allocation of resources at a national level.
Implications for research.
Although this review has identified a large number of studies conducted in low‐ and middle‐income countries (LMICs), a number of important research questions remain. Research recommendations have been subdivided into those for trialists, those for systematic reviewers, and those for other researchers.
Trialists
Trialists need to:
describe trial interventions better, for example, in terms of training, supervision, and incentives for primary‐level workers (PWs). This will allow systematic reviewers to identify and compare characteristics that may help to better explain the effects of PW interventions;
conduct trials comparing interventions with different characteristics/types of PWs, modes of delivery, and types of training and supervision or intensity of intervention, to enhance understanding of the effects of these variations. This is particularly applicable to collaborative care and other complex interventions for which there may be several types of specialists and PWs and several types of interventions on offer (such as stepped care);
conduct trials of different high‐risk populations (people living with HIV, people who are victims of domestic violence, veterans, etc.), as different populations may respond differently to these therapies and may have different outcomes of interest;
compare PWs versus specialists to assess the potential for task‐shifting/(substitution of roles);
consistently consider whether and how to include adverse effects or unintended consequences of PWs related to safety. We noticed about half of trials did not report safety data. This could have affected confidence intervals around point estimates. Not all trials of health systems interventions (such as task‐shifting) will explicitly collect safety data, as it is often the case that main concerns do not reflect safety in the clinical sense but unintended adverse effects (e.g. negative changes in quality of care; fewer appropriate referrals). This points to the need for more trials to assess the most relevant adverse outcomes, whether for the patient or the service, and other adverse impact;
include better data on service utilisation, which are important for understanding costs and cost‐effectiveness, but also for understanding (as mentioned above) the indirect consequences of an intervention for broader services provided and utilised;
improve the conduct of trials including more rigorous allocation concealment, randomisation, outcome assessment, and reporting; local validation of instruments; and agreement on standard instruments for specific outcomes and disorders to facilitate pooling and comparing of data;
use core outcome sets when available. This would be useful for developing core outcome sets when these are not yet available;
focus on clinical issues that have been poorly addressed to date, including severe mental disorders, alcohol/substance dependence, and child mental disorders;
include process evaluations alongside trials, to (1) better understand intervention fidelity and the pathways through which interventions impact outcomes; (2) assess the indirect impact of delivering mental health care (when this is added to existing roles and tasks) on other elements of PW health care or other roles (e.g. is it taking time away from other job roles); and (3) assess the impact on well‐being of PWs (stress/burnout); and
consistently include economic data in their trials, as costs and cost‐effectiveness are important for health planning across all mental disorders and distress;
Low‐ to moderate‐certainty clinical evidence is available for common mental disorders (CMDs), adult post‐traumatic stress (PTS), and perinatal depression, but additional studies are needed to examine severe mental disorders, alcohol and drug use, dementia, child PTS, and other child mental disorders, which were not meta‐analysed.
Systematic reviewers
Further systematic reviews drawing on a range of study designs (such as reviews on processes of care, but also economic evaluations and qualitative work) are needed, particularly to evaluate these trials through the implementation science lens and to provide broader certainty about whether they are feasible, acceptable, effective, and sustainable in their various settings. In particular, mixed qualitative and quantitative reviews should focus on:
factors affecting the sustainability of PW interventions when scaled up;
effectiveness of different approaches to ensure programme sustainability, including use of different types of incentives and payment systems for PWs;
mechanisms for integrating LHW programmes into the formal health system;
equity impact of these programmes and factors of accessibility and acceptability;
fidelity and quality of these programmes; and
cost‐effectiveness, coverage, and scalability of these programmes.
Other researchers
Given the very broad range of PWs with considerable variation in their characteristics (e.g. training, supervision), settings, interventions, and delivery mechanisms in mental health care, there is a need to develop a comprehensive typology for PWs (and how they are selected, trained, and supported/supervised), as well as for the interventions they provide, which would help health planners and future researchers to develop more standardised and comparable interventions and situations.
What's new
| Date | Event | Description |
|---|---|---|
| 18 August 2020 | New search has been performed | This is the first update of the Cochrane Review published in 2013. A new search was conducted and other content updated. |
| 18 August 2020 | New citation required and conclusions have changed | 72 new studies were included in this update. The total number of included studies in the review is now 95. Some non‐randomised trials from the previous version of the review were removed, as the inclusion criteria for study design have changed to include only randomised trials. Review authors have changed as well due to change in availability. |
History
Protocol first published: Issue 5, 2011 Review first published: Issue 11, 2013
| Date | Event | Description |
|---|---|---|
| 30 October 2013 | Amended | Addressed all copy editors' issues |
Acknowledgements
We are very grateful to Paul Garner (PG), from the Department of Clinical Sciences, Liverpool School of Tropical Medicine, for invaluable direction and help during the stakeholder analysis, for thinking about how to focus this review and how to build treatment and prevention reviews in parallel, and for helping with their protocols. We would also like to thank all stakeholders whom we consulted to ensure this review update was relevant to current policy and practice. These include LMIC health workers at the Liverpool School of Tropical Medicine, and academics and professionals working in LMICs (Crick Lund, Atif Rahman, Cindy Lam, Thelma Narayan, Dickens Akena, Dr Elie Karam from IDRAAC (Institute for Development, Research, Advocacy and Applied Care, Lebanon)) and Ms Bou Sleimam, Co‐ordinator, and Dr Kranti Raymanee, Director, Community Health & Research, Aga Khan Health Services (AKHS).
We thank the following people, who helped with aspects of the analysis: Chris Rose from Cochrane Effective Practice and Organisation of Care (EPOC) in Norway, for statistical guidance and support; Espen Movik from the Campbell & Cochrane Economics Methods Group (CCEMG), for reviewing and providing guidance on development of the economics component of this review; Laura Amato (Co‐ordinating Editor Cochrane Drugs and Alcohol) and Stefan Leucht (Editor Cochrane Schizophrenia Group), for advice on combining different outcomes and tools in the respective comparisons ‐ Alcohol and Substance Use and Severe Mental Disorders. Lee‐Yee Chong from the Cochrane Public Health and Health Systems Network provided methodological support for meta‐analysis and other analysis/grading.
We thank the following people from EPOC: Marit Johansen and Paul Miller for help in compiling and running the search strategy; Elizabeth Paulsen for guidance on editorial matters. We also thank Elizabeth Royle and the Copy Edit Support team for copy‐editing the review.
Thank you to the following people for their help with papers that were not published in the English language: Daniela Gonçalves‐Bradley (Spanish and Portuguese), and Laleh Rashidian (Farsi).
We appreciate the financial support provided by the World Health Organisation for performing the review, as well as the human resources support from the Cochrane Public Health and Health Systems Network https://publichealth.cochrane.org.
In addition, we would like to thank the authors of the previous review for their input into the prior version of the protocol and results: Prathap Tharyan, Vikram Patel, Meera S. Maheedariah, Jessica Pian, and Girish Rao.
We would like to thank Jibril Abdul Malik, Meera S.Maheedariah, and Jessica Pian for helping with screening of articles for this review.
This Cochrane Review is associated with the Research, Evidence and Development Initiative (READ‐It). READ‐It (project number 300342‐104) is funded by UK aid from the UK government; however, the views expressed do not necessarily reflect the UK government’s official policies.
Appendices
Appendix 1. Search strategies
CENTRAL, Cochrane Library
| #1 | [mh “mental disorders”] | 64650 |
| #2 | [mh “mental health”] | 1282 |
| #3 | [mh “depression”] | 10255 |
| #4 | [mh "child development"] | 2352 |
| #5 | [mh “mentally disabled persons”] | 58 |
| #6 | [mh "self‐injurious behavior"] | 1190 |
| #7 | (mental next health* or mental* next ill* or mental* next disorder* or mental* next well*):ti,ab | 19469 |
| #8 | ((substance or alcohol or opioid or morphine or marijuana or heroin or cocaine) near/2 (disorder? or illness* or dependence or abuse or misuse or use)):ti,ab | 17249 |
| #9 | (depressi* near/2 (sign* or symptom* or disorder*)):ti,ab | 21100 |
| #10 | (depress* near/3 (acute or clinical* or diagnos* or disorder* or major or unipolar or illness or scale* or score* or adult* or child* or adolesc* or teen* or youth? or elder* or late* life* or patient* or participant* or people or inpatient* or (in next patient*) or outpatient* or (out next patient*))):ti,ab | 36894 |
| #11 | ((depress* or distress*) near/3 (postnatal* or post natal* or maternal*)):ti,ab | 2551 |
| #12 | (depression or anxiety or alzheimer? or schizoaffective or mania or manic or "borderline personality" or (stress near/2 disorder*) or (adjustment next disorder?) or (psychological next/1 trauma*) or schizophrenia or psychoses or psychosis or (stress next syndrome?) or (distress next syndrome?) or (combat next disorder?) or (war next disorder?) or ptsd or dementia):ti,ab | 111604 |
| #13 | (((post next trauma*) or posttrauma*) near/3 (stress* or disorder?)):ti,ab | 3765 |
| #14 | ((psychological next trauma) or psychotrauma*):ti,ab | 119 |
| #15 | (alcoholism or alcoholic? or (drug next addict*) or (drug next abus*) or (drug next misuse) or (drug next user?)):ti,ab | 9301 |
| #16 | ((learning or mental* or intellectual) next (disabled or disabilit* or disorder? or difficult*)):ti,ab | 5833 |
| #17 | ((dissociative near/3 (disorder* or reaction*)) or dissociation):ti,ab | 1181 |
| #18 | ((bipolar or behavioral or beahavioural or obsessive or panic or mood or delusional) near/2 (disorder? or illness* or disease?)):ti,ab | 11291 |
| #19 | (trichotillomani* or OCD or (obsess* next compulsi*) or GAD or (stress next reaction?) or "acute stress" or neurosis or neuroses or neurotic):ti,ab | 6283 |
| #20 | (affective* next (disorder? or disease? or illness* or symptom?)):ti,ab | 1862 |
| #21 | ((mental or psychological or emotional or (psycho next social) or psychosocial) next (stress* or distress*)):ti,ab | 5759 |
| #22 | ((sub‐syndrom* or sub‐threshold or sub‐clinical or subsyndrom* or subthreshold or subclinical or minor or brief) next (symptom* or disorder* or condition* or depress* or anxiety)):ti,ab | 1193 |
| #23 | ("mental relapse" or fatigue or (somatic next symptom?) or worry or worries or panic or (low next mood?) or (mood next problem?)):ti,ab | 25986 |
| #24 | ((anxiety next disorder?) or agoraphobi* or (general* next anxi*) or "separation anxiety" or "neurocirculatory asthenia" or (neurotic next disorder?) or (social next phobi*) or (self next harm*) or (self next injur*) or suicid*):ti,ab | 11556 |
| #25 | (slow* next (thought? or think*)):ti,ab | 17 |
| #26 | (mental* next develop*):ti,ab | 429 |
| #27 | {or #1‐#26} | 200145 |
| #28 | [mh “primary health care”] | 6586 |
| #29 | [mh “physicians, family”] | 443 |
| #30 | [mh “physicians, primary care”] | 143 |
| #31 | [mh “general practitioners”] | 236 |
| #32 | [mh “general practice”] | 2398 |
| #33 | [mh “family practice”] | 1965 |
| #34 | [mh “social support”] | 3147 |
| #35 | [mh “community health workers”] | 416 |
| #36 | [mh "allied health personnel"] | 1065 |
| #37 | [mh “community health services”] | 12672 |
| #38 | [mh “schools”] | 2702 |
| #39 | [mh “school health services”] | 1431 |
| #40 | [mh “rural health”] | 522 |
| #41 | [mh “rural population”] | 1529 |
| #42 | [mh “nurses, community health”] | 15 |
| #43 | [mh “nurses, public health”] | 2 |
| #44 | [mh “family nursing”] | 36 |
| #45 | [mh “primary care nursing”] | 27 |
| #46 | [mh “rural nursing”] | 1 |
| #47 | [mh “community health nursing”] | 341 |
| #48 | [mh “school nursing”] | 79 |
| #49 | (primary near/5 (care or health*)):ti,ab | 28306 |
| #50 | ((family next practi*) or (family next doctor*) or (family next physician*) or gp* or (general next practi*)):ti,ab | 18585 |
| #51 | (school* or teacher* or rural* or community):ti,ab | 61995 |
| #52 | ((non next specialist*) or nonspecialist* or (social next worker*) or trainer?):ti,ab | 2884 |
| #53 | (psycho next social or psychosocial):ti,ab | 11755 |
| #54 | (caregiver* or (care next giver?) or layperson*):ti,ab | 10219 |
| #55 | (paraprofessional* or (para next professional*) or (non next physician?) or (non next clinician?)):ti,ab | 335 |
| #56 | (allied near/2 (professional? or person* or staff or worker?)):ti,ab | 168 |
| #57 | (lay near/2 (heal* or person* or counsellor? or counselor? or worker? or therapist?)):ti,ab | 482 |
| #58 | (midwife or midwive* or pharmacist* or pharmacy or pharmacies or (practice next nurs*) or (district next nurs*) or (health next visitor?)):ti,ab | 8837 |
| #59 | {or #28‐#58} | 132822 |
| #60 | #27 and #59 | 38692 |
| #61 | [mh “developing countries”] | 812 |
| #62 | (africa or asia or caribbean or “west indies” or “south America” or “latin America” or “central America”):ti,ab,kw | 10965 |
| #63 | (afghanistan or albania or algeria or angola or antigua or barbuda or argentina or armenia or armenian or aruba or azerbaijan or bahrain or bangladesh or barbados or benin or byelarus or byelorussian or belarus or belorussian or belorussia or belize or bhutan or bolivia or bosnia or herzegovina or hercegovina or botswana or brasil or brazil or bulgaria or “burkina faso” or “burkina fasso” or “upper volta” or burundi or urundi or cambodia or “khmer republic” or kampuchea or cameroon or cameroons or cameron or camerons or “cape verde” or “central african republic” or chad or chile or china or colombia or comoros or “comoro islands” or comores or mayotte or congo or zaire or “costa rica” or “cote d'ivoire” or “ivory coast” or croatia or cuba or cyprus or czechoslovakia or “czech republic” or slovakia or “slovak republic” or djibouti or “french Somaliland” or dominica or “dominican republic” or “east timor” or “east timur” or “timor leste” or ecuador or egypt or “united arab republic” or “el Salvador” or eritrea or estonia or ethiopia or fiji or gabon or “gabonese republic” or gambia or gaza or “georgia republic” or “georgian republic” or ghana or “gold coast” or greece or grenada or guatemala or guinea or guam or guiana or guyana or haiti or honduras or hungary or india or maldives or indonesia or iran or iraq or “isle of man” or jamaica or jordan or kazakhstan or kazakh or kenya or kiribati or korea or kosovo or kyrgyzstan or kirghizia or “kyrgyz republic” or kirghiz or kirgizstan or “lao pdr” or laos or latvia or lebanon or lesotho or basutoland or liberia or libya or lithuania or macedonia or madagascar or “malagasy republic” or malaysia or malaya or malay or sabah or sarawak or malawi or nyasaland or mali or malta or “marshall islands” or mauritania or mauritius or “agalega islands” or mexico or micronesia or “middle east” or moldova or moldovia or moldovian or mongolia or montenegro or morocco or ifni or mozambique or myanmar or myanma or burma or namibia or nepal or “netherlands Antilles” or “new caledonia” or nicaragua or niger or nigeria or “northern mariana islands” or oman or muscat or pakistan or palau or palestine or panama or paraguay or peru or philippines or philipines or phillipines or phillippines or poland or portugal or “puerto rico” or romania or rumania or roumania or russia or russian or rwanda or ruanda or “saint kitts” or “st kitts” or nevis or “saint lucia” or “st lucia” or “saint vincent” or “st vincent” or grenadines or samoa or “samoan islands” or “navigator island” or “navigator islands” or “sao tome” or “saudi arabia” or senegal or serbia or montenegro or seychelles or “sierra leone” or slovenia or “sri lanka” or ceylon or “solomon islands” or somalia or “south Africa” or sudan or suriname or surinam or swaziland or syria or tajikistan or tadzhikistan or tadzhik or tanzania or thailand or togo or “togolese republic” or tonga or trinidad or tobago or tunisia or turkey or turkmenistan or turkmen or uganda or ukraine or uruguay or ussr or “soviet union” or “union of soviet socialist republics” or uzbekistan or uzbek or vanuatu or “new Hebrides” or venezuela or vietnam or “viet nam” or “west bank” or yemen or yugoslavia or zambia or zimbabwe or rhodesia):ti,ab,kw | 74075 |
| #64 | ((developing or less* next developed or under next developed or underdeveloped or middle next income or low* next income or underserved or under next served or deprived or poor*) next (countr* or nation? or population? or world)):ti,ab,kw | 6194 |
| #65 | ((developing or less* next developed or under next developed or underdeveloped or middle next income or low* next income) next (economy or economies)):ti,ab,kw | 15 |
| #66 | (low* next (gdp or gnp or “gross domestic” or “gross national”)):ti,ab,kw | 49 |
| #67 | (low near/3 middle near/3 countr*):ti,ab,kw | 1102 |
| #68 | (lmic or lmics or “third world” or lami next countr*):ti,ab,kw | 333 |
| #69 | (transitional next countr*):ti,ab,kw | 6 |
| #70 | {or #61‐#69} | 81091 |
| #71 | #60 and #70 | 3087 |
| #72 | #59 and #70 with 'Dementia and Cognitive Improvement', 'Schizophrenia', 'Common Mental Disorders', 'Drugs and Alcohol', 'Developmental, Psychosocial and Learning Problems' in Cochrane Groups | 583 |
| #73 | #71 or #72 | 3142 |
MEDLINE, Ovid
| 1 | exp mental disorders/ | 1178064 |
| 2 | mental health/ | 34125 |
| 3 | depression/ | 109655 |
| 4 | child development/ | 43848 |
| 5 | mentally disabled persons/ | 3510 |
| 6 | exp self‐injurious behavior/ | 66942 |
| 7 | (mental health* or mental* ill* or mental* disorder* or mental* well*).ti,ab,kf. | 193920 |
| 8 | ((substance or alcohol or opioid or morphine or marijuana or heroin or cocaine) adj2 (disorder? or illness* or dependence or abuse or misuse or "use")).ti,ab,kf. | 130723 |
| 9 | (depressi* adj2 (sign* or symptom* or disorder?)).ti,ab,kf. | 102869 |
| 10 | (depress* adj3 (acute or clinical* or diagnos* or disorder* or major or unipolar or illness or scale* or score* or adult* or child* or adolesc* or teen* or youth? or elder* or late* life* or patient* or participant* or people or inpatient* or in‐patient* or outpatient* or out‐patient*)).ti,ab,kf. | 159137 |
| 11 | ((depress* or distress*) adj3 (postnatal* or post natal* or maternal*)).ti,ab,kf. | 7599 |
| 12 | (depression or anxiety or alzheimer? or schizoaffective or mania or manic or borderline personality or (stress adj2 disorder*) or adjustment disorder? or (psychological adj1 trauma*) or schizophrenia or psychoses or psychosis or stress syndrome? or distress syndrome? or combat disorder? or war disorder? or ptsd or dementia).ti,ab,kf. | 777081 |
| 13 | ((post‐trauma* or posttrauma*) adj3 (stress* or disorder?)).ti,ab,kf. | 31212 |
| 14 | (psychological trauma or psychotrauma*).ti,ab,kf. | 1616 |
| 15 | (alcoholism or alcoholic? or drug addict* or drug abus* or drug misuse or drug user?).ti,ab,kf. | 128352 |
| 16 | ((learning or mental* or intellectual) adj (disabled or disabilit* or disorder? or difficult*)).ti,ab,kf. | 71915 |
| 17 | ((dissociative adj3 (disorder* or reaction*)) or dissociation).ti,ab,kf. | 106783 |
| 18 | ((bipolar or behavio?ral or obsessive or panic or mood or delusional) adj2 (disorder? or illness* or disease?)).ti,ab,kf. | 71595 |
| 19 | (trichotillomani* or OCD or obsess*‐compulsi* or GAD or stress reaction? or acute stress or neuros#s or neurotic).ti,ab,kf. | 52159 |
| 20 | (affective* adj (disorder? or disease? or illness* or symptom?)).ti,ab,kf. | 18722 |
| 21 | ((mental or psychological or emotional or psycho‐social or psychosocial) adj (stress* or distress*)).ti,ab,kf. | 45389 |
| 22 | ((sub‐syndrom* or sub‐threshold or sub‐clinical or subsyndrom* or subthreshold or subclinical or minor or brief) adj (symptom* or disorder* or condition* or depress* or anxiety)).ti,ab,kf. | 6405 |
| 23 | (mental relapse or fatigue or somatic symptom? or worry or worries or panic or low mood? or mood problem?).ti,ab,kf. | 118612 |
| 24 | (anxiety disorder? or agoraphobi* or general* anxi* or separation anxiety or neurocirculatory asthenia or neurotic disorder? or social phobi* or self‐harm* or self‐injur* or suicid*).ti,ab,kf. | 111671 |
| 25 | (slow* adj (thought? or think*)).ti,ab,kf. | 65 |
| 26 | (mental* adj develop*).ti,ab,kf. | 3107 |
| 27 | or/1‐26 | 2066598 |
| 28 | primary health care/ | 72211 |
| 29 | physicians, family/ | 16035 |
| 30 | physicians, primary care/ | 2983 |
| 31 | general practitioners/ | 6962 |
| 32 | general practice/ | 12278 |
| 33 | family practice/ | 64522 |
| 34 | exp social support/ | 67224 |
| 35 | community health workers/ | 4840 |
| 36 | allied health personnel/ | 11402 |
| 37 | exp community health services/ | 290117 |
| 38 | schools/ | 35190 |
| 39 | school health services/ | 16553 |
| 40 | rural health/ | 23042 |
| 41 | rural population/ | 55375 |
| 42 | nurses, community health/ | 749 |
| 43 | nurses, public health/ | 378 |
| 44 | family nursing/ | 1397 |
| 45 | primary care nursing/ | 424 |
| 46 | rural nursing/ | 97 |
| 47 | community health nursing/ | 19250 |
| 48 | school nursing/ | 5189 |
| 49 | (primary adj5 (care or health*)).ti,ab,kf. | 151793 |
| 50 | (family practi* or family doctor* or family physician* or gp* or general practi*).ti,ab,kf. | 255078 |
| 51 | (school* or teacher* or rural* or community).ti,ab,kf. | 806828 |
| 52 | (non‐specialist* or nonspecialist* or social worker* or trainer?).ti,ab,kf. | 20475 |
| 53 | (psycho‐social or psychosocial).ti,ab,kf. | 92424 |
| 54 | (caregiver* or care giver? or layperson*).ti,ab,kf. | 60937 |
| 55 | (lay adj2 (heal* or person* or counsellor? or counselor? or worker? or therapist?)).ti,ab,kf. | 2000 |
| 56 | (paraprofessional? or para‐professional? or (allied health* adj (professional? or person* or staff or worker?)) or non‐physician? or non‐clinician?).ti,ab,kf. | 4648 |
| 57 | (midwife or midwive* or pharmacist* or pharmacy or pharmacies or practice nurs* or district nurs* or health visitor?).ti,ab,kf. | 91175 |
| 58 | or/28‐57 | 1645330 |
| 59 | 27 and 58 | 308752 |
| 60 | Developing Countries.sh,kf. | 83497 |
| 61 | (Africa or Asia or Caribbean or West Indies or South America or Latin America or Central America).hw,kf,ti,ab,cp. | 259315 |
| 62 | (Afghanistan or Albania or Algeria or Angola or Antigua or Barbuda or Argentina or Armenia or Armenian or Aruba or Azerbaijan or Bahrain or Bangladesh or Barbados or Benin or Byelarus or Byelorussian or Belarus or Belorussian or Belorussia or Belize or Bhutan or Bolivia or Bosnia or Herzegovina or Hercegovina or Botswana or Brasil or Brazil or Bulgaria or Burkina Faso or Burkina Fasso or Upper Volta or Burundi or Urundi or Cambodia or Khmer Republic or Kampuchea or Cameroon or Cameroons or Cameron or Camerons or Cape Verde or Central African Republic or Chad or Chile or China or Colombia or Comoros or Comoro Islands or Comores or Mayotte or Congo or Zaire or Costa Rica or Cote d'Ivoire or Ivory Coast or Croatia or Cuba or Cyprus or Czechoslovakia or Czech Republic or Slovakia or Slovak Republic or Djibouti or French Somaliland or Dominica or Dominican Republic or East Timor or East Timur or Timor Leste or Ecuador or Egypt or United Arab Republic or El Salvador or Eritrea or Estonia or Ethiopia or Fiji or Gabon or Gabonese Republic or Gambia or Gaza or Georgia Republic or Georgian Republic or Ghana or Gold Coast or Greece or Grenada or Guatemala or Guinea or Guam or Guiana or Guyana or Haiti or Honduras or Hungary or India or Maldives or Indonesia or Iran or Iraq or Isle of Man or Jamaica or Jordan or Kazakhstan or Kazakh or Kenya or Kiribati or Korea or Kosovo or Kyrgyzstan or Kirghizia or Kyrgyz Republic or Kirghiz or Kirgizstan or Lao PDR or Laos or Latvia or Lebanon or Lesotho or Basutoland or Liberia or Libya or Lithuania or Macedonia or Madagascar or Malagasy Republic or Malaysia or Malaya or Malay or Sabah or Sarawak or Malawi or Nyasaland or Mali or Malta or Marshall Islands or Mauritania or Mauritius or Agalega Islands or Mexico or Micronesia or Middle East or Moldova or Moldovia or Moldovian or Mongolia or Montenegro or Morocco or Ifni or Mozambique or Myanmar or Myanma or Burma or Namibia or Nepal or Netherlands Antilles or New Caledonia or Nicaragua or Niger or Nigeria or Northern Mariana Islands or Oman or Muscat or Pakistan or Palau or Palestine or Panama or Paraguay or Peru or Philippines or Philipines or Phillipines or Phillippines or Poland or Portugal or Puerto Rico or Romania or Rumania or Roumania or Russia or Russian or Rwanda or Ruanda or Saint Kitts or St Kitts or Nevis or Saint Lucia or St Lucia or Saint Vincent or St Vincent or Grenadines or Samoa or Samoan Islands or Navigator Island or Navigator Islands or Sao Tome or Saudi Arabia or Senegal or Serbia or Montenegro or Seychelles or Sierra Leone or Slovenia or Sri Lanka or Ceylon or Solomon Islands or Somalia or South Africa or Sudan or Suriname or Surinam or Swaziland or Syria or Tajikistan or Tadzhikistan or Tadjikistan or Tadzhik or Tanzania or Thailand or Togo or Togolese Republic or Tonga or Trinidad or Tobago or Tunisia or Turkey or Turkmenistan or Turkmen or Uganda or Ukraine or Uruguay or USSR or Soviet Union or Union of Soviet Socialist Republics or Uzbekistan or Uzbek or Vanuatu or New Hebrides or Venezuela or Vietnam or Viet Nam or West Bank or Yemen or Yugoslavia or Zambia or Zimbabwe or Rhodesia).hw,kf,ti,ab,cp. | 3522182 |
| 63 | ((developing or less* developed or under developed or underdeveloped or middle income or low* income or underserved or under served or deprived or poor*) adj (countr* or nation? or population? or world)).ti,ab,kf. | 120543 |
| 64 | ((developing or less* developed or under developed or underdeveloped or middle income or low* income) adj (economy or economies)).ti,ab,kf. | 501 |
| 65 | (low* adj (gdp or gnp or gross domestic or gross national)).ti,ab,kf. | 230 |
| 66 | (low adj3 middle adj3 countr*).ti,ab,kf. | 13666 |
| 67 | (lmic or lmics or third world or lami countr*).ti,ab,kf. | 6689 |
| 68 | transitional countr*.ti,ab,kf. | 156 |
| 69 | or/60‐68 | 3668723 |
| 70 | exp randomized controlled trial/ | 484601 |
| 71 | controlled clinical trial.pt. | 93116 |
| 72 | randomi#ed.ti,ab. | 575067 |
| 73 | placebo.ab. | 198687 |
| 74 | randomly.ti,ab. | 314043 |
| 75 | Clinical Trials as topic.sh. | 187357 |
| 76 | trial.ti. | 200592 |
| 77 | or/70‐76 | 1263534 |
| 78 | exp animals/ not humans/ | 4590542 |
| 79 | 77 not 78 | 1164068 |
| 80 | 59 and 69 and 79 | 3931 |
Embase, Ovid
| 1 | exp *mental disease/ | 1279242 |
| 2 | exp *mental health/ | 40054 |
| 3 | *mentally disabled person/ | 135 |
| 4 | *child development/ | 19386 |
| 5 | *automutilation/ | 7273 |
| 6 | (mental health* or mental* ill* or mental* disorder* or mental* well*).ti,ab,kw. | 232825 |
| 7 | ((substance or alcohol or opioid or morphine or marijuana or heroin or cocaine) adj2 (disorder? or illness* or dependence or abuse or misuse or "use")).ti,ab,kw. | 182023 |
| 8 | (depressi* adj2 (sign* or symptom* or disorder?)).ti,ab,kw. | 141085 |
| 9 | (depress* adj3 (acute or clinical* or diagnos* or disorder* or major or unipolar or illness or scale* or score* or adult* or child* or adolesc* or teen* or youth? or elder* or late* life* or patient* or participant* or people or inpatient* or in‐patient* or outpatient* or out‐patient*)).ti,ab,kw. | 225351 |
| 10 | ((depress* or distress*) adj3 (postnatal* or post natal* or maternal*)).ti,ab,kw. | 9993 |
| 11 | (depression or anxiety or alzheimer? or schizoaffective or mania or manic or borderline personality or (stress adj2 disorder*) or adjustment disorder? or (psychological adj1 trauma*) or schizophrenia or psychoses or psychosis or stress syndrome? or distress syndrome? or combat disorder? or war disorder? or ptsd or dementia).ti,ab,kw. | 1060133 |
| 12 | ((post‐trauma* or posttrauma*) adj3 (stress* or disorder?)).ti,ab,kw. | 38871 |
| 13 | (psychological trauma or psychotrauma*).ti,ab,kw. | 2365 |
| 14 | (alcoholism or alcoholic? or drug addict* or drug abus* or drug misuse or drug user?).ti,ab,kw. | 173313 |
| 15 | ((learning or mental* or intellectual) adj (disabled or disabilit* or disorder? or difficult*)).ti,ab,kw. | 85774 |
| 16 | ((dissociative adj3 (disorder* or reaction*)) or dissociation).ti,ab,kw. | 109038 |
| 17 | ((bipolar or behavio?ral or obsessive or panic or mood or delusional) adj2 (disorder? or illness* or disease?)).ti,ab,kw. | 107332 |
| 18 | (trichotillomani* or OCD or obsess*‐compulsi* or GAD or stress reaction? or acute stress or neuros#s or neurotic).ti,ab,kw. | 64971 |
| 19 | (affective* adj (disorder? or disease? or illness* or symptom?)).ti,ab,kw. | 26269 |
| 20 | ((mental or psychological or emotional or psycho‐social or psychosocial) adj (stress* or distress*)).ti,ab,kw. | 61738 |
| 21 | ((sub‐syndrom* or sub‐threshold or sub‐clinical or subsyndrom* or subthreshold or subclinical or minor or brief) adj (symptom* or disorder* or condition* or depress* or anxiety)).ti,ab,kw. | 8753 |
| 22 | (mental relapse or fatigue or somatic symptom? or worry or worries or panic or low mood? or mood problem?).ti,ab,kw. | 182245 |
| 23 | (anxiety disorder? or agoraphobi* or general* anxi* or separation anxiety or neurocirculatory asthenia or neurotic disorder? or social phobi* or self‐harm* or self‐injur* or suicid*).ti,ab,kw. | 145418 |
| 24 | (slow* adj (thought? or think*)).ti,ab,kw. | 104 |
| 25 | (mental* adj develop*).ti,ab,kw. | 4058 |
| 26 | or/1‐25 | 2422199 |
| 27 | exp *primary health care/ | 56644 |
| 28 | *general practitioner/ | 21882 |
| 29 | *general practice/ | 36570 |
| 30 | exp *social support/ | 20847 |
| 31 | exp *health auxiliary/ | 2626 |
| 32 | exp *community care/ | 53629 |
| 33 | exp *paramedical personnel/ | 211362 |
| 34 | *family nursing/ | 821 |
| 35 | *rural health nursing/ | 59 |
| 36 | exp *school/ | 142054 |
| 37 | exp *school health service/ | 10563 |
| 38 | *rural health care/ | 7500 |
| 39 | *rural health/ | 281 |
| 40 | *rural population/ | 11554 |
| 41 | (primary adj5 (care or health*)).ti,ab,kw. | 200393 |
| 42 | (family practi* or family doctor* or family physician* or gp* or general practi*).ti,ab,kw. | 326828 |
| 43 | (school* or teacher* or rural* or community).ti,ab,kw. | 979096 |
| 44 | (non‐specialist* or nonspecialist* or social worker* or trainer?).ti,ab,kw. | 30591 |
| 45 | (psycho‐social or psychosocial).ti,ab,kw. | 128077 |
| 46 | (caregiver* or care giver? or layperson*).ti,ab,kw. | 85553 |
| 47 | (paraprofessional? or para‐professional? or (allied health* adj (professional? or person* or staff or worker?)) or non‐physician? or non‐clinician?).ti,ab,kw. | 6724 |
| 48 | (lay adj2 (heal* or person* or counsellor? or counselor? or worker? or therapist?)).ti,ab,kw. | 2522 |
| 49 | (midwife or midwive* or pharmacist* or pharmacy or pharmacies or practice nurs* or district nurs* or health visitor?).ti,ab,kw. | 158047 |
| 50 | or/27‐49 | 2020917 |
| 51 | 26 and 50 | 361756 |
| 52 | random*.ti,ab. | 1421261 |
| 53 | factorial*.ti,ab. | 35432 |
| 54 | (crossover* or cross over*).ti,ab. | 100176 |
| 55 | ((doubl* or singl*) adj blind*).ti,ab. | 220035 |
| 56 | (assign* or allocat* or volunteer* or placebo*).ti,ab. | 972238 |
| 57 | crossover procedure/ | 59516 |
| 58 | single blind procedure/ | 35469 |
| 59 | randomized controlled trial/ | 554495 |
| 60 | double blind procedure/ | 161641 |
| 61 | or/52‐60 | 2168841 |
| 62 | exp animal/ not human/ | 4600643 |
| 63 | 61 not 62 | 1951147 |
| 64 | developing country.sh. | 90981 |
| 65 | (africa or asia or caribbean or west indies or south america or latin america or central america).hw,ti,ab,cp. | 320253 |
| 66 | (afghanistan or albania or algeria or angola or antigua or barbuda or argentina or armenia or armenian or aruba or azerbaijan or bahrain or bangladesh or barbados or benin or byelarus or byelorussian or belarus or belorussian or belorussia or belize or bhutan or bolivia or bosnia or herzegovina or hercegovina or botswana or brasil or brazil or bulgaria or burkina faso or burkina fasso or upper volta or burundi or urundi or cambodia or khmer republic or kampuchea or cameroon or cameroons or cameron or camerons or cape verde or central african republic or chad or chile or china or colombia or comoros or comoro islands or comores or mayotte or congo or zaire or costa rica or cote d'ivoire or ivory coast or croatia or cuba or cyprus or czechoslovakia or czech republic or slovakia or slovak republic or djibouti or french somaliland or dominica or dominican republic or east timor or east timur or timor leste or ecuador or egypt or united arab republic or el salvador or eritrea or estonia or ethiopia or fiji or gabon or gabonese republic or gambia or gaza or georgia republic or georgian republic or ghana or gold coast or greece or grenada or guatemala or guinea or guam or guiana or guyana or haiti or honduras or hungary or india or maldives or indonesia or iran or iraq or isle of man or jamaica or jordan or kazakhstan or kazakh or kenya or kiribati or korea or kosovo or kyrgyzstan or kirghizia or kyrgyz republic or kirghiz or kirgizstan or lao pdr or laos or latvia or lebanon or lesotho or basutoland or liberia or libya or lithuania or macedonia or madagascar or malagasy republic or malaysia or malaya or malay or sabah or sarawak or malawi or nyasaland or mali or malta or marshall islands or mauritania or mauritius or agalega islands or mexico or micronesia or middle east or moldova or moldovia or moldovian or mongolia or montenegro or morocco or ifni or mozambique or myanmar or myanma or burma or namibia or nepal or netherlands antilles or new caledonia or nicaragua or niger or nigeria or northern mariana islands or oman or muscat or pakistan or palau or palestine or panama or paraguay or peru or philippines or philipines or phillipines or phillippines or poland or portugal or puerto rico or romania or rumania or roumania or russia or russian or rwanda or ruanda or saint kitts or st kitts or nevis or saint lucia or st lucia or saint vincent or st vincent or grenadines or samoa or samoan islands or navigator island or navigator islands or sao tome or saudi arabia or senegal or serbia or montenegro or seychelles or sierra leone or slovenia or sri lanka or ceylon or solomon islands or somalia or south africa or sudan or suriname or surinam or swaziland or syria or tajikistan or tadzhikistan or tadjikistan or tadzhik or tanzania or thailand or togo or togolese republic or tonga or trinidad or tobago or tunisia or turkey or turkmenistan or turkmen or uganda or ukraine or uruguay or ussr or soviet union or union of soviet socialist republics or uzbekistan or uzbek or vanuatu or new hebrides or venezuela or vietnam or viet nam or west bank or yemen or yugoslavia or zambia or zimbabwe or rhodesia).hw,ti,ab,cp. | 3819559 |
| 67 | ((developing or less* developed or under developed or underdeveloped or middle income or low* income or underserved or under served or deprived or poor*) adj (countr* or nation? or population? or world)).ti,ab. | 114831 |
| 68 | ((developing or less* developed or under developed or underdeveloped or middle income or low* income) adj (economy or economies)).ti,ab. | 638 |
| 69 | (low* adj (gdp or gnp or gross domestic or gross national)).ti,ab. | 336 |
| 70 | (low adj3 middle adj3 countr*).ti,ab. | 15324 |
| 71 | (lmic or lmics or third world or lami countr*).ti,ab. | 7812 |
| 72 | transitional countr*.ti,ab. | 223 |
| 73 | or/64‐72 | 4024805 |
| 74 | 51 and 63 and 73 | 7134 |
| 75 | limit 74 to embase | 4038 |
CINAHL, EBSCO
| S1 | (MH "Mental Disorders+") | 491 562 |
| S2 | (MH "Mental Health") | 29 733 |
| S3 | (MH "Mentally Disabled Persons") | 4 278 |
| S4 | (MH "Child Development") | 19 880 |
| S5 | (MH "Injuries, Self‐Inflicted") OR (MH "Self‐Injurious Behavior") OR (MH "Suicide+") | 30 860 |
| S6 | TI (mental health* or mental* ill* or mental* disorder* or mental* well*) OR AB (mental health* or mental illness* or mental disorder* or mental* well*) | 110 455 |
| S7 | TI ((substance or alcohol or opioid or morphine or marijuana or heroin or cocaine) N2 (disorder? or illness* or dependence or abuse or misuse or use)) OR AB ((substance or alcohol or opioid or morphine or marijuana or heroin or cocaine) N2 (disorder? or illness* or dependence or abuse or misuse or "use")) | 64 854 |
| S8 | TI (depressi* N2 (sign* or symptom* or disorder?)) OR AB (depressi* N2 (sign* or symptom* or disorder?)) | 44 456 |
| S9 | TI (depress* N3 (acute or clinical* or diagnos* or disorder* or major or unipolar or illness or scale* or score* or adult* or child* or adolesc* or teen* or youth? or elder* or late* life* or patient* or participant* or people or inpatient* or in‐patient* or outpatient* or out‐patient*)) OR AB (depress* N3 (acute or clinical* or diagnos* or disorder* or major or unipolar or illness or scale* or score* or adult* or child* or adolesc* or teen* or youth? or elder* or late* life* or patient* or participant* or people or inpatient* or in‐patient* or outpatient* or out‐patient*)) | 59 922 |
| S10 | TI ((depress* or distress*) N3 (postnatal* or post natal* or maternal*)) OR AB ((depress* or distress*) N3 (postnatal* or post natal* or maternal*)) | 4 636 |
| S11 | TI (depression or anxiety or alzheimer? or schizoaffective or mania or manic or borderline personality or (stress N2 disorder*) or adjustment disorder? or (psychological N1 trauma*) or schizophrenia or psychoses or psychosis or stress syndrome? or distress syndrome? or combat disorder? or war disorder? or ptsd or dementia) OR AB (depression or anxiety or alzheimer? or schizoaffective or mania or manic or borderline personality or (stress N2 disorder*) or adjustment disorder? or (psychological N1 trauma*) or schizophrenia or psychoses or psychosis or stress syndrome? or distress syndrome? or combat disorder? or war disorder? or ptsd or dementia) | 231 266 |
| S12 | TI ((post‐trauma* or posttrauma*) N3 (stress* or disorder?)) OR AB ((post‐trauma* or posttrauma*) N3 (stress* or disorder?)). | 13 352 |
| S13 | TI (psychological trauma or psychotrauma*) OR AB (psychological trauma or psychotrauma*) | 1 092 |
| S14 | TI (alcoholism or alcoholic? or drug addict* or drug abus* or drug misuse or drug user?) OR AB (alcoholism or alcoholic? or drug addict* or drug abus* or drug misuse or drug user?) | 25 418 |
| S15 | TI ((learning or mental* or intellectual) N0 (disabled or disabilit* or disorder? or difficult*)) OR AB ((learning or mental* or intellectual) N0 (disabled or disabilit* or disorder? or difficult*)) | 28 260 |
| S16 | TI ((dissociative N3 (disorder* or reaction*)) or dissociation) OR AB ((dissociative N3 (disorder* or reaction*)) or dissociation) | 3 997 |
| S17 | TI ((bipolar or behavio?ral or obsessive or panic or mood or delusional) N2 (disorder? or illness* or disease?)) OR AB ((bipolar or behavio?ral or obsessive or panic or mood or delusional) N2 (disorder? or illness* or disease?)) | 17 676 |
| S18 | TI (trichotillomani* or OCD or obsess*‐compulsi* or GAD or stress reaction? or acute stress or neuros#s or neurotic) OR AB (trichotillomani* or OCD or obsess*‐compulsi* or GAD or stress reaction? or acute stress or neuros#s or neurotic) | 10 045 |
| S19 | TI (affective* N0 (disorder? or disease? or illness* or symptom?)) OR AB (affective* N0 (disorder? or disease? or illness* or symptom?)) | 2 762 |
| S20 | TI ((mental or psychological or emotional or psycho‐social or psychosocial) N0 (stress* or distress*)) OR AB ((mental or psychological or emotional or psycho‐social or psychosocial) N0 (stress* or distress*)) | 18 466 |
| S21 | TI ((sub‐syndrom* or sub‐threshold or sub‐clinical or subsyndrom* or subthreshold or subclinical or minor or brief) N0 (symptom* or disorder* or condition* or depress* or anxiety)) OR AB ((sub‐syndrom* or sub‐threshold or sub‐clinical or subsyndrom* or subthreshold or subclinical or minor or brief) N0 (symptom* or disorder* or condition* or depress* or anxiety)) | 2 315 |
| S22 | TI (mental relapse or fatigue or somatic symptom? or worry or worries or panic or low mood? or mood problem?) OR AB (mental relapse or fatigue or somatic symptom? or worry or worries or panic or low mood? or mood problem?) | 43 895 |
| S23 | TI (anxiety disorder? or agoraphobi* or general* anxi* or separation anxiety or neurocirculatory asthenia or neurotic disorder? or social phobi* or self‐harm* or self‐injur* or suicid*) OR AB (anxiety disorder? or agoraphobi* or general* anxi* or separation anxiety or neurocirculatory asthenia or neurotic disorder? or social phobi* or self‐harm* or self‐injur* or suicid*) | 43 084 |
| S24 | TI (slow* N0 (thought? or think*)) OR AB (slow* N0 (thought? or think*)) | 34 |
| S25 | TI (mental* N0 develop*) OR AB (mental* N0 develop*) | 1 026 |
| S26 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 | 741 526 |
| S27 | (MH "Primary Health Care") | 54 978 |
| S28 | (MH "Physicians, Family") | 16 868 |
| S29 | (MH "Family Practice") | 22 238 |
| S30 | (MH "Support, Psychosocial+") | 69 168 |
| S31 | (MH "Community Health Workers") | 2 787 |
| S32 | (MH "Community Health Services+") | 371 940 |
| S33 | (MH "Schools+") | 63 490 |
| S34 | (MH "School Health Services+") | 19 364 |
| S35 | (MH "Rural Health") | 5 820 |
| S36 | (MH "Rural Population") | 8 799 |
| S37 | (MH "Community Health Nursing+") OR (MH "Family Nursing") OR (MH "School Health Nursing") OR (MH "Rural Health Nursing") | 36 909 |
| S38 | TI (primary N5 (care or health*)) OR AB (primary N5 (care or health*)) | 79 822 |
| S39 | TI (family practi* or family doctor* or family physician* or gp* or general practi*) OR AB (family practi* or family doctor* or family physician* or gp* or general practi*) | 60 786 |
| S40 | TI (school* or teacher* or rural* or community) OR AB (school* or teacher* or rural* or community) | 350 063 |
| S41 | TI (non‐specialist* or nonspecialist* or social worker* or trainer?) OR AB (non‐specialist* or nonspecialist* or social worker* or trainer?) | 16 018 |
| S42 | TI (psycho‐social or psychosocial) OR AB (psycho‐social or psychosocial) | 43 614 |
| S43 | TI (caregiver* or care giver? or layperson*) OR AB (caregiver* or care giver? or layperson*) | 41 568 |
| S44 | TI (lay N2 (heal* or person* or counsellor? or counselor? or worker? or therapist?)) OR AB (lay N2 (heal* or person* or counsellor? or counselor? or worker? or therapist?)) | 1 159 |
| S45 | TI (paraprofessional? or para‐professional? or (allied health* N0 (professional? or person* or staff or worker?)) or non‐physician? or non‐clinician?) OR AB (paraprofessional? or para‐professional? or (allied health* N0 (professional? or person* or staff or worker?)) or non‐physician? or non‐clinician?) | 2519 |
| S46 | TI (midwife or midwive* or pharmacist* or pharmacy or pharmacies or practice nurs* or district nurs* or health visitor?) OR AB (midwife or midwive* or pharmacist* or pharmacy or pharmacies or practice nurs* or district nurs* or health visitor?) | 105388 |
| S47 | S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 OR S46 | 974832 |
| S48 | S26 AND S47 | 200752 |
| S49 | PT randomized controlled trial | 87050 |
| S50 | PT clinical trial | 86256 |
| S51 | TI ( randomis* or randomiz* or randomly) OR AB ( randomis* or randomiz* or randomly) | 255358 |
| S52 | (MH "Clinical Trials+") | 261313 |
| S53 | (MH "Random Assignment") | 55112 |
| S54 | S49 OR S50 OR S51 OR S52 OR S53 | 406456 |
| S55 | S48 AND S54 | 19680 |
| S56 | (MH "Developing Countries") OR (MH "Low and Middle Income Countries") | 17592 |
| S57 | TX (africa or asia or caribbean or west indies or south america or latin america or central america) | 264810 |
| S58 | TX (afghanistan or albania or algeria or angola or antigua or barbuda or argentina or armenia or armenian or aruba or azerbaijan or bahrain or bangladesh or barbados or benin or byelarus or byelorussian or belarus or belorussian or belorussia or belize or bhutan or bolivia or bosnia or herzegovina or hercegovina or botswana or brasil or brazil or bulgaria or burkina faso or burkina fasso or upper volta or burundi or urundi or cambodia or khmer republic or kampuchea or cameroon or cameroons or cameron or camerons or cape verde or central african republic or chad or chile or china or colombia or comoros or comoro islands or comores or mayotte or congo or zaire or costa rica or cote d'ivoire or ivory coast or croatia or cuba or cyprus or czechoslovakia or czech republic or slovakia or slovak republic or djibouti or french somaliland or dominica or dominican republic or east timor or east timur or timor leste or ecuador or egypt or united arab republic or el salvador or eritrea or estonia or ethiopia or fiji or gabon or gabonese republic or gambia or gaza or georgia republic or georgian republic or ghana or gold coast or greece or grenada or guatemala or guinea or guam or guiana or guyana or haiti or honduras or hungary or india or maldives or indonesia or iran or iraq or isle of man or jamaica or jordan or kazakhstan or kazakh or kenya or kiribati or korea or kosovo or kyrgyzstan or kirghizia or kyrgyz republic or kirghiz or kirgizstan or lao pdr or laos or latvia or lebanon or lesotho or basutoland or liberia or libya or lithuania or macedonia or madagascar or malagasy republic or malaysia or malaya or malay or sabah or sarawak or malawi or nyasaland or mali or malta or marshall islands or mauritania or mauritius or agalega islands or mexico or micronesia or middle east or moldova or moldovia or moldovian or mongolia or montenegro or morocco or ifni or mozambique or myanmar or myanma or burma or namibia or nepal or netherlands antilles or new caledonia or nicaragua or niger or nigeria or northern mariana islands or oman or muscat or pakistan or palau or palestine or panama or paraguay or peru or philippines or philipines or phillipines or phillippines or poland or portugal or puerto rico or romania or rumania or roumania or russia or russian or rwanda or ruanda or saint kitts or st kitts or nevis or saint lucia or st lucia or saint vincent or st vincent or grenadines or samoa or samoan islands or navigator island or navigator islands or sao tome or saudi arabia or senegal or serbia or montenegro or seychelles or sierra leone or slovenia or sri lanka or ceylon or solomon islands or somalia or south africa or sudan or suriname or surinam or swaziland or syria or tajikistan or tadzhikistan or tNikistan or tadzhik or tanzania or thailand or togo or togolese republic or tonga or trinidad or tobago or tunisia or turkey or turkmenistan or turkmen or uganda or ukraine or uruguay or ussr or soviet union or union of soviet socialist republics or uzbekistan or uzbek or vanuatu or new hebrides or venezuela or vietnam or viet nam or west bank or yemen or yugoslavia or zambia or zimbabwe or rhodesia) | 960450 |
| S59 | TI ((developing or less* developed or under developed or underdeveloped or middle income or low* income or underserved or under served or deprived or poor*) N0 (countr* or nation? or population? or world)) OR AB ((developing or less* developed or under developed or underdeveloped or middle income or low* income or underserved or under served or deprived or poor*) N0 (countr* or nation? or population? or world)) | 26025 |
| S60 | TI ((developing or less* developed or under developed or underdeveloped or middle income or low* income) N0 (economy or economies)) OR AB ((developing or less* developed or under developed or underdeveloped or middle income or low* income) N0 (economy or economies)) | 112 |
| S61 | TI (low* N0 (gdp or gnp or gross domestic or gross national)) OR AB (low* N0 (gdp or gnp or gross domestic or gross national)) | 53 |
| S62 | TI (low N3 middle N3 countr*) OR AB (low N3 middle N3 countr*) | 5991 |
| S63 | TI (lmic or lmics or third world or lami countr*) OR AB (lmic or lmics or third world or lami countr*) | 2175 |
| S64 | TI transitional countr* OR AB transitional countr* | 92 |
| S65 | S56 OR S57 OR S58 OR S59 OR S60 OR S61 OR S62 OR S63 OR S64 | 1044607 |
| S66 | S55 AND S65 | 3378 |
| S67 | S66 Limiters ‐ Exclude MEDLINE records | 1328 |
PsycINFO, Ovid
| 1 | exp mental disorders/ | 817205 |
| 2 | exp mental health/ | 60973 |
| 3 | "depression (emotion)"/ | 24950 |
| 4 | childhood development/ | 66242 |
| 5 | exp intellectual development disorder/ | 43653 |
| 6 | exp learning disorders/ | 33332 |
| 7 | exp learning disabilities/ | 26955 |
| 8 | exp self‐injurious behavior/ | 5553 |
| 9 | exp suicide/ | 32825 |
| 10 | (mental health* or mental* ill* or mental* disorder* or mental* well*).ti,ab. | 233142 |
| 11 | ((substance or alcohol or opioid or morphine or marijuana or heroin or cocaine) adj2 (disorder? or illness* or dependence or abuse or misuse or "use")).ti,ab. | 112765 |
| 12 | (depressi* adj2 (sign* or symptom* or disorder?)).ti,ab. | 91413 |
| 13 | (depress* adj3 (acute or clinical* or diagnos* or disorder* or major or unipolar or illness or scale* or score* or adult* or child* or adolesc* or teen* or youth? or elder* or late* life* or patient* or participant* or people or inpatient* or in‐patient* or outpatient* or out‐patient*)).ti,ab. | 139008 |
| 14 | ((depress* or distress*) adj3 (postnatal* or post natal* or maternal*)).ti,ab. | 6846 |
| 15 | (depression or anxiety or alzheimer? or schizoaffective or mania or manic or borderline personality or (stress adj2 disorder*) or adjustment disorder? or (psychological adj1 trauma*) or schizophrenia or psychoses or psychosis or stress syndrome? or distress syndrome? or combat disorder? or war disorder? or ptsd or dementia).ti,ab. | 569468 |
| 16 | ((post‐trauma* or posttrauma*) adj3 (stress* or disorder?)).ti,ab. | 36579 |
| 17 | (psychological trauma or psychotrauma*).ti,ab. | 2105 |
| 18 | (alcoholism or alcoholic? or drug addict* or drug abus* or drug misuse or drug user?).ti,ab. | 60437 |
| 19 | ((learning or mental* or intellectual) adj (disabled or disabilit* or disorder? or difficult*)).ti,ab. | 88069 |
| 20 | ((dissociative adj3 (disorder* or reaction*)) or dissociation).ti,ab. | 18641 |
| 21 | ((bipolar or behavio?ral or obsessive or panic or mood or delusional) adj2 (disorder? or illness* or disease?)).ti,ab. | 70644 |
| 22 | (trichotillomani* or OCD or obsess*‐compulsi* or GAD or stress reaction? or acute stress or neuros#s or neurotic).ti,ab. | 54598 |
| 23 | (affective* adj (disorder? or disease? or illness* or symptom?)).ti,ab. | 18692 |
| 24 | ((mental or psychological or emotional or psycho‐social or psychosocial) adj (stress* or distress*)).ti,ab. | 35967 |
| 25 | ((sub‐syndrom* or sub‐threshold or sub‐clinical or subsyndrom* or subthreshold or subclinical or minor or brief) adj (symptom* or disorder* or condition* or depress* or anxiety)).ti,ab. | 5345 |
| 26 | (mental relapse or fatigue or somatic symptom? or worry or worries or panic or low mood? or mood problem?).ti,ab. | 54715 |
| 27 | (anxiety disorder? or agoraphobi* or general* anxi* or separation anxiety or neurocirculatory asthenia or neurotic disorder? or social phobi* or self‐harm* or self‐injur* or suicid*).ti,ab. | 104780 |
| 28 | (slow* adj (thought? or think*)).ti,ab. | 48 |
| 29 | (mental* adj develop*).ti,ab. | 3071 |
| 30 | or/1‐29 | 1330283 |
| 31 | primary health care/ | 17324 |
| 32 | family physicians/ | 1526 |
| 33 | general practitioners/ | 5725 |
| 34 | exp allied health personnel/ | 5188 |
| 35 | social support/ | 34353 |
| 36 | exp community health/ | 4947 |
| 37 | exp community services/ | 45076 |
| 38 | exp school based intervention/ | 17304 |
| 39 | exp schools/ | 66343 |
| 40 | school nurses/ | 822 |
| 41 | rural environments/ | 16557 |
| 42 | (primary adj5 (care or health*)).ti,ab. | 37043 |
| 43 | (family practi* or family doctor* or family physician* or gp* or general practi*).ti,ab. | 30315 |
| 44 | (school* or teacher* or rural* or community).ti,ab. | 674768 |
| 45 | (non‐specialist* or nonspecialist* or social worker* or trainer?).ti,ab. | 29801 |
| 46 | (psycho‐social or psychosocial).ti,ab. | 79092 |
| 47 | (caregiver* or care giver? or layperson*).ti,ab. | 46053 |
| 48 | paraprofessional*.ti,ab. | 2170 |
| 49 | (lay adj2 (heal* or person* or counsellor? or counselor? or worker? or therapist?)).ti,ab. | 1401 |
| 50 | (paraprofessional? or para‐professional? or (allied health* adj (professional? or person* or staff or worker?)) or non‐physician? or non‐clinician?).ti,ab. | 3323 |
| 51 | (midwife or midwive* or pharmacist* or pharmacy or pharmacies or practice nurs* or district nurs* or health visitor?).ti,ab. | 10800 |
| 52 | or/31‐51 | 904056 |
| 53 | 30 and 52 | 293055 |
| 54 | exp clinical trial/ | 11422 |
| 55 | random*.ti,ab. | 187891 |
| 56 | ((clinical or control*) adj3 trial*).ti,ab. | 68780 |
| 57 | ((singl* or doubl* or trebl* or tripl*) adj5 (blind* or mask*)).ti,ab. | 25354 |
| 58 | (volunteer* or control group or controls).ti,ab. | 235982 |
| 59 | placebo/ or placebo*.ti,ab. | 38969 |
| 60 | or/54‐59 | 436702 |
| 61 | 53 and 60 | 35477 |
| 62 | developing countries.sh. | 5252 |
| 63 | (africa or asia or caribbean or west indies or south america or latin america or central america).hw,ti,ab. | 32659 |
| 64 | (afghanistan or albania or algeria or angola or antigua or barbuda or argentina or armenia or armenian or aruba or azerbaijan or bahrain or bangladesh or barbados or benin or byelarus or byelorussian or belarus or belorussian or belorussia or belize or bhutan or bolivia or bosnia or herzegovina or hercegovina or botswana or brasil or brazil or bulgaria or burkina faso or burkina fasso or upper volta or burundi or urundi or cambodia or khmer republic or kampuchea or cameroon or cameroons or cameron or camerons or cape verde or central african republic or chad or chile or china or colombia or comoros or comoro islands or comores or mayotte or congo or zaire or costa rica or cote d'ivoire or ivory coast or croatia or cuba or cyprus or czechoslovakia or czech republic or slovakia or slovak republic or djibouti or french somaliland or dominica or dominican republic or east timor or east timur or timor leste or ecuador or egypt or united arab republic or el salvador or eritrea or estonia or ethiopia or fiji or gabon or gabonese republic or gambia or gaza or georgia republic or georgian republic or ghana or gold coast or greece or grenada or guatemala or guinea or guam or guiana or guyana or haiti or honduras or hungary or india or maldives or indonesia or iran or iraq or isle of man or jamaica or jordan or kazakhstan or kazakh or kenya or kiribati or korea or kosovo or kyrgyzstan or kirghizia or kyrgyz republic or kirghiz or kirgizstan or lao pdr or laos or latvia or lebanon or lesotho or basutoland or liberia or libya or lithuania or macedonia or madagascar or malagasy republic or malaysia or malaya or malay or sabah or sarawak or malawi or nyasaland or mali or malta or marshall islands or mauritania or mauritius or agalega islands or mexico or micronesia or middle east or moldova or moldovia or moldovian or mongolia or montenegro or morocco or ifni or mozambique or myanmar or myanma or burma or namibia or nepal or netherlands antilles or new caledonia or nicaragua or niger or nigeria or northern mariana islands or oman or muscat or pakistan or palau or palestine or panama or paraguay or peru or philippines or philipines or phillipines or phillippines or poland or portugal or puerto rico or romania or rumania or roumania or russia or russian or rwanda or ruanda or saint kitts or st kitts or nevis or saint lucia or st lucia or saint vincent or st vincent or grenadines or samoa or samoan islands or navigator island or navigator islands or sao tome or saudi arabia or senegal or serbia or montenegro or seychelles or sierra leone or slovenia or sri lanka or ceylon or solomon islands or somalia or south africa or sudan or suriname or surinam or swaziland or syria or tajikistan or tadzhikistan or tadjikistan or tadzhik or tanzania or thailand or togo or togolese republic or tonga or trinidad or tobago or tunisia or turkey or turkmenistan or turkmen or uganda or ukraine or uruguay or ussr or soviet union or union of soviet socialist republics or uzbekistan or uzbek or vanuatu or new hebrides or venezuela or vietnam or viet nam or west bank or yemen or yugoslavia or zambia or zimbabwe or rhodesia).hw,ti,ab. | 199260 |
| 65 | ((developing or less* developed or under developed or underdeveloped or middle income or low* income or underserved or under served or deprived or poor*) adj (countr* or nation? or population? or world)).ti,ab. | 16521 |
| 66 | ((developing or less* developed or under developed or underdeveloped or middle income or low* income) adj (economy or economies)).ti,ab. | 346 |
| 67 | (low* adj (gdp or gnp or gross domestic or gross national)).ti,ab. | 43 |
| 68 | (low adj3 middle adj3 countr*).ti,ab. | 2713 |
| 69 | (lmic or lmics or third world or lami countr*).ti,ab. | 1649 |
| 70 | transitional countr*.ti,ab. | 62 |
| 71 | or/62‐70 | 221990 |
| 72 | 61 and 71 | 2786 |
WHO Global Health Library
| substance related disorder OR "substance related disorders" OR "substance abuse" OR "alcohol abuse" OR "alcohol dependence" OR "alchohol related" OR depressi* OR anxiety OR schizophrenia OR psychoses OR psychosis OR "stress syndrome" OR "distress syndrome" OR "combat disorder" OR "war disorder" OR "posttrauma stress" OR "post‐trauma stress" OR "post‐traumatic stress" OR ptsd OR dementia OR alcoholism OR alcoholic OR "drug addict" OR "drug abuse" OR "drug abuser" OR "drug misuse" OR "drug user" OR "drug users" OR "learning disabled" OR "learning disability" OR "learning disabilities" OR "learning disorder" OR "learning disorders" OR "learning difficulty" OR "learning difficulties" OR "mental disabled" OR "mental disability" OR "mental disabilities" OR "mental disorder" OR "mental disorders" OR "mental difficulty" OR "mental difficulties" OR "mentally disabled" OR "intellectual disability" OR "intellectual disabilities" OR "intellectual disorder" OR "intellectual disorders" OR "intellectual difficulty" OR "intellectual difficulties" OR "inellectually disabled" OR "mental health" | ||
| AND | "primary health" OR "primary care" OR "primary healthcare" OR "community" OR school* OR teacher* OR rural OR "psycho‐social" OR psychosocial OR caregiver* OR paraprofessional* OR "lay counsellor" OR "lay counselor" OR "lay worker" OR "lay therapist" OR "lay counsellors" OR "lay counselors" OR "lay workers" OR "lay therapists" OR "general practice" OR " family practice" OR "midwife" OR "midwives" OR "health visitor" OR "social worker" | |
| Type of study = controlled clinical trial | 61 |
WHO International Clinical Trials Registry Platform (ICTRP)
| Condition | mental health OR mental illness OR mental disorder OR depression | |
| AND | ||
| Intervention | school OR psychosocial OR lay OR non‐specialist OR teacher OR paraprofessional OR community‐based OR community mental health OR community worker OR primary care OR general practice OR family practice | |
| 198 |
ClinicalTrials.gov
| Condition | mental health OR mental illness OR mental disorder OR depression | |
| AND | ||
| Intervention | school OR psychosocial OR lay OR non‐specialist OR teacher OR paraprofessional OR community‐based OR community mental health OR community worker OR primary care OR general practice OR family practice | |
| Limits | Interventional Studies | First posted from 01/01/2011 to 06/20/2019 | 1347 |
Appendix 2. Adapted CHEC criteria list
| yes | no | Not applicable | Details | ||
| 1 | Are competing alternatives clearly described? | ||||
| 2 | Is a well‐defined economic question posed in an answerable form? | ||||
| 3 | Is the economic study design appropriate to the stated objective? | ||||
| 4 | Was there a comparison between 2 more groups receiving different interventions? | ||||
| 5 | Is the chosen time horizon appropriate to include relevant costs and consequences? | ||||
| 6 | Is the perspective/viewpoint** of the analysis explicitly stated? If yes, give details | ||||
| 7 | Have they mentioned the sources of data? If so, give details | ||||
| 8 | Is the actual perspective chosen appropriate? | ||||
| 9 | Are all important and relevant costs for each alternative identified? | ||||
| 10 | Are costs measured? If yes, give details of costs measured | ||||
| 11 | Are all costs measured appropriately in physical units? | ||||
| 12 | Are costs valued appropriately? | ||||
| 13 | Are all important and relevant outcomes for each alternative identified? | ||||
| 14 | Were outcomes measured? If yes, give details of outcomes measured | ||||
| 15 | Are all outcomes measured appropriately? | ||||
| 16 | Are outcomes valued appropriately? | ||||
| 17 | Is an incremental analysis of costs and outcomes of alternatives performed? | ||||
| 18 | Are all future costs and outcomes discounted appropriately? *(where appropriate) | ||||
| 19 | Were sensitivity analyses undertaken? If yes, give details of forms of sensitivity analyses | ||||
| 20 | Are all important variables, whose values are uncertain, appropriately subjected to sensitivity analysis? | ||||
| 21 | Do the conclusions follow from the data reported? | ||||
| 22 | Does the study discuss the generalisability of the results to other settings and patient/client groups? | ||||
| 23 | Does the article indicate that there is no potential conflict of interest of study researcher(s) and funder(s)? | ||||
| 24 | Are ethical and distributional issues discussed appropriately? |
Appendix 3. Study descriptions (Comparison 1). Lay health worker‐led psychological interventions versus usual care in treating common mental disorders
Settings
Ten studies were identified from nine countries across Africa (Abas 2018; Bolton 2003; Chibanda 2016; Petersen 2014), Asia (Ali 2003; Arjadi 2018; Jiang 2017; Murphy 2020; Patel 2017), and South America (Matsuzaka 2017).
Interventions were delivered in urban and periurban settings (Abas 2018Ali 2003Arjadi 2018 Chibanda 2016; Jiang 2017Matsuzaka 2017Patel 2017Petersen 2014), rural settings (Bolton 2003), and both (Murphy 2020).
Participants
Six trials included adult males and females aged ≥ 18 years (Abas 2018; Bolton 2003; Matsuzaka 2017; Murphy 2020; Patel 2017; Petersen 2014), one trial included only females aged 18 to 50 years (Ali 2003), and two trials included elderly adult males and females aged > 60 years (Chibanda 2016; Jiang 2017). Two studies were conducted on patients receiving HIV antiretroviral therapy (Abas 2018; Petersen 2014). Six trials provided descriptions of participants' socioeconomic status. In three studies, participants were from very low‐income households with high rates of unemployment (Bolton 2003; Patel 2017; Petersen 2014), one study took place in a lower‐middle class setting (Ali 2003), and in one study the average household income was US$690/month (Matsuzaka 2017).
Participants were recruited from primary care facilities in six trials (Abas 2018; Chibanda 2016; Matsuzaka 2017; Murphy 2020; Patel 2017; Petersen 2014), one trial recruited using random household screening (Ali 2003), and one trial used local village leaders to help identify potentially at‐risk individuals for screening (Bolton 2003).
Eight trials included participants with at least mild CMD symptoms using locally developed or locally adapted screening instruments including the PHQ‐9 (Abas 2018), the Shona Symptom Questionnaire (Abas 2018; Chibanda 2016), the Aga Khan Anxiety and Depression Scale (Ali 2003), the WHO Self‐Reporting Questionnaire (Murphy 2020; Petersen 2014), the Zung Scale (Matsuzaka 2017), and Hopkins Symptom Checklist (Bolton 2003). Of these, two trials further evaluated participants using a Structured Clinical Interview for DSM‐IV disorders (Petersen 2014), or the Mini International Neuropsychiatric Interview (Matsuzaka 2017), and included only those with a confirmed major depressive disorder. One trial included only participants with severe depressive symptoms identified by PHQ‐9 scores ≥ 15 (Patel 2017).
Two studies used online interventions with no face‐to‐face delivery of psychological interventions (Arjadi 2018; Jiang 2017). Outcomes for these studies have not been pooled with those of other studies for meta‐analysis and are reported narratively only.
Interventions
Training and supervision of LHWs
A large variety of backgrounds and training were provided for lay health workers delivering the intervention. Two trials used existing HIV antiretroviral therapy (ART) adherence counsellors from local primary health clinics to deliver the intervention. In Abas 2018, ART adherence counsellors (primary care counsellors or nurse aides with at least secondary school education) were trained over 2.5 days by the research team to deliver the intervention; thereafter LHWs met weekly with a local psychologist to discuss their caseload. In Petersen 2014, lay HIV counsellors were provided with 4 days of training by a clinical psychologist, and psychology trainees and lay counsellors had weekly then monthly supervision meetings with psychology trainees.
Four other trials used existing lay workers from local primary health centres to deliver psychological interventions. In Chibanda 2016, non‐health professional employees of the local primary health centre with a minimum of 10 years of schooling were provided with nine days of training on intervention delivery by the research team, supported by an intervention manual. Subsequent supervision and support were provided by a clinical psychologist and other research team members, who could be contacted as needed by mobile phone. In Matsuzaka 2017, community health workers working in the local primary health centre were provided with three days of group‐based training by the research team, and subsequently attended group‐based monthly supervision meetings. Support was available as needed by telephone, text, or email. In Murphy 2020, lay social workers with pre‐existing community involvement (e.g. as village care workers, as NGO volunteers) were provided with three days of training in study logistics and intervention delivery. Each lay worker received two visits from the research team to observe delivery of the intervention, to provide support and assess fidelity.
One trial collaborated with an NGO to identify and train lay health workers. In Bolton 2003, local gender‐matched non‐clinician World Vision employees were fluent in English and Luganda and had completed high school level education. LHWs received two weeks of intensive treatment by faculty members of the New York Psychiatric Institute assisted by a trained psychologist and an experienced group therapist employed by World Vision. Subsequent supervision was provided by World Vision mental health professionals.
Three trials recruited and trained community volunteers to deliver psychological interventions. In Ali 2003, female volunteers belonging to the local community could read and write Urdu. Each LHW received 11 three‐hour training sessions held over four weeks led by a family practitioner, a sociologist, a psychiatrist, or a clinical psychologist. The lay counsellors had access to the training team for support when needed. In Patel 2017, lay counsellors were recruited through advertisements in newspapers and a local television channel. They needed to have a minimum of 10 years of schooling and no prior mental health training. Each underwent a three‐week training workshop focused on general counselling skills and manualised treatments followed by a six‐month internship. Knowledge was assessed via an exam and performance on role‐plays using vignettes. Support was provided through weekly peer‐led small group meetings.
Description of interventions
There was wide variation in the range and intensity of psychological interventions delivered by LHWs. Three trials used interpersonal counselling (Bolton 2003; Matsuzaka 2017; Petersen 2014), and three trials used problem‐solving therapy (Abas 2018; Ali 2003; Chibanda 2016). One trial used a manualised psychological treatment based on behavioural activation (Patel 2017), and one trial used bibliotherapy to deliver CBT with support by the LHW (Murphy 2020).
The number of intervention sessions ranged from three to four sessions in Matsuzaka 2017 to 16 sessions in Bolton 2003, with most ranging between six and eight counselling therapy sessions (Abas 2018; Ali 2003; Chibanda 2016; Murphy 2020; Patel 2017; Petersen 2014). Two interventions were group‐based (Bolton 2003; Petersen 2014), and one intervention consisted of six individual counselling sessions, followed by six group‐based peer support sessions (Chibanda 2016). In one trial, the intervention was delivered at the participant’s home (Murphy 2020). In the others, the intervention was delivered at a community or primary health centre.
Comparators
In seven trials, the comparison group was provided with ‘usual care’ relevant to the setting in which participants were recruited. This included routine HIV care and ART adherence counselling (Abas 2018; Petersen 2014), treatment by local traditional healers (Bolton 2003), nurse‐led evaluation with psychoeducation and antidepressants if necessary (Chibanda 2016), and regular medical care delivered by primary care providers (Murphy 2020; Patel 2017). In one trial, ‘usual care’ was essentially no or minimal care due to poor access to health care (Ali 2003). In another trial, the comparison group received case management by research psychologists funded through the study but who had not been trained to deliver interpersonal counselling (Matsuzaka 2017).
Appendix 4. Study descriptions (Comparison 2). Primary‐level worker‐led collaborative care versus usual care in treating common mental disorders in adults
Settings
We identified 13 studies from seven countries in Africa (Adewuya 2019; Gureje 2019 (STEPCARE); Jenkins 2013; Oladeji 2015), South America (Araya 2003; Fritsch 2007), and Asia (Chen 2015; Indu 2018; Jordans 2019; Niemi 2016; Patel 2010; Pradeep 2014; Xie 2019).
Interventions were delivered in urban settings (Araya 2003; Chen 2015; Fritsch 2007; Gureje 2019 (STEPCARE); Indu 2018), in rural settings (Jordans 2019; Niemi 2016; Pradeep 2014; Xie 2019), and in both (Adewuya 2019; Jenkins 2013; Oladeji 2015; Patel 2010).
Participants
11 trials included adults aged ≥ 16 or ≥ 18 years (Adewuya 2019; Araya 2003 RCT Chile; Fritsch 2007; Gureje 2019 (STEPCARE); Indu 2018; Jenkins 2013; Jordans 2019; Niemi 2016; Oladeji 2015; Patel 2010; Pradeep 2014). Two studies examined only elderly depression in participants aged ≥ 60 to 65 years (Chen 2015; Xie 2019). Three studies were conducted in very low socioeconomic status settings including economically deprived women from urban Santiago (Araya 2003), Kenyan primary care patients with high levels of food insecurity (Jenkins 2013), and Indian women from Bangalore villages (Pradeep 2014). Four studies included only females with depression (Araya 2003; Fritsch 2007; Indu 2018; Pradeep 2014). All studies included patients with depressive symptoms identified using screening instruments including PHQ‐9 (Adewuya 2019; Chen 2015; Gureje 2019 (STEPCARE); Jordans 2019; Oladeji 2015; Niemi 2016), GHQ‐12 (Patel 2010; Fritsch 2007; Jenkins 2013; Pradeep 2014), the Hamilton Depression Rating Scale (Indu 2018), and the Geriatric Depression Scale (Xie 2019). Seven trials further evaluated participants to confirm the presence a major depressive disorder using a Structured Clinical Interview for DSM‐IV disorders (Adewuya 2019; Chen 2015), the Composite International Diagnostic Interview (Gureje 2019 (STEPCARE); Oladeji 2015), the Mini International Neuropsychiatric Interview (Indu 2018, Pradeep 2014), or the Clinical Interview Schedule Revised ICD‐10 (Patel 2010). All studies included patients with depression. Two studies also included patients with other common mental disorders (Jenkins 2013; Patel 2010).
Interventions
Types of PWs
These collaborative care models involved multi‐disciplinary teams consisting of existing PHC staff, including private and government PHC doctors (Adewuya 2019; Araya 2003; Chen 2015; Fritsch 2007; Gureje 2019 (STEPCARE); Indu 2018; Jenkins 2013; Jordans 2010; Niemi 2016; Oladeji 2015; Patel 2010; Pradeep 2014), non‐medical professional primary care or community staff (nurses, social workers, other professional community health workers) (Adewuya 2019; Araya 2003; Chen 2015; Gureje 2019 (STEPCARE); Indu 2018; Jenkins 2013; Jordans 2019; Oladeji 2015; Niemi 2016; Pradeep 2014; Xie 2019), and LHWs (Fritsch 2007; Jordans 2019; Patel 2010) with access to referral to specialist care for complex cases.
Training and supervision of PWs
Training of PWs was described in 12 studies. Training duration and intensity varied from 3 to 12 hours (Chen 2015; Fritsch 2007; Araya 2003; Indu 2018), to several days (Adewuya 2019; Gureje 2019 (STEPCARE); Jenkins 2013; Niemi 2016; Oladeji 2015; Pradeep 2014), to two months (Patel 2010), to six months (Jordans 2019). Training for doctors was typically a half‐ or full‐day session focused primarily on diagnosis and guideline‐driven use of antidepressants, often supplemented with a manual such as the mhGAP‐IG training manual (Adewuya 2019; Gureje 2019 (STEPCARE); Oladeji 2015). Most studies that reported the content of the training had a mixture of didactic and practical training. In Niemi 2016, in addition to being trained to assess and manage depression, doctors, nurses, and assistant doctors were trained to teach a slow movement and breathing yoga course. Training for nurses, social workers, and lay health workers was directed primarily at psychoeducation and structured psychological interventions.
Ongoing supervision of PWs was described in 11 trials (Adewuya 2019; Araya 2003; Chen 2015; Fritsch 2007; Gureje 2019 (STEPCARE); Indu 2018; Jordans 2019; Jenkins 2013; Indu 2018; Oladeji 2015; Patel 2010). Supervision ranged from minimal or ad hoc supervision in Gureje 2019 (STEPCARE); Indu 2018; and Jenkins 2013 to regular monthly visits in Adewuya 2019; Chen 2015; Patel 2010; and Fritsch 2007 and intensive mentoring in Jordans 2019. Supervision was provided by specialists (psychiatrist/psychologist) or by the research team (Adewuya 2019; Chen 2015; Fritsch 2007; Jordans 2019; Niemi 2016; Patel 2010; Pradeep 2014), or by a local senior non‐mental health specialist physician (Gureje 2019 (STEPCARE); Indu 2018; Oladeji 2015; Patel 2010). In three trials, access to supervision was attained primarily by mobile phone (Adewuya 2019; Gureje 2019 (STEPCARE); Oladeji 2015).
Description of interventions
All collaborative care interventions involved multi‐disciplinary teams with complex multi‐component interventions.
Six trials used a stepped care approach (Araya 2003; Adewuya 2019; Chen 2015; Gureje 2019 (STEPCARE); Oladeji 2015; Patel 2010). A four‐step process was used with regular monitoring and increasing intensity of interventions provided for participants with more severe symptoms or for those who did not respond adequately to initial treatment. Stepped care interventions included an intake assessment to assess symptom severity. All patients received psychoeducation (step 1). Participants with mild to moderate symptoms were offered a limited series (four to six sessions) of structured psychological counselling (step 2). For participants with moderate to moderately severe symptoms, or who did not respond to initial psychological interventions, treatments were intensified with the addition of an antidepressant by the doctor or with additional sessions of counselling, or with both (step 3). Participants with very severe symptoms or who were at risk of suicide were referred for specialist care (step 4).
Seven trials used an integrated collaborative care approach (Fritsch 2007; Indu 2018; Jenkins 2013; Jordans 2019; Niemi 2016; Pradeep 2014; Xie 2019), whereby doctors diagnosed patients, provided medical treatment, and provided follow‐up/referral as per the existing government health delivery model, and non‐medical clinic staff (nurses, social workers, community health workers, or lay workers) provided adjunctive psychological treatment or other supportive services such as adherence counselling or monitoring.
Seven trials involved a combination of structured psychological interventions and pharmacotherapy (Adewuya 2019; Araya 2003; Gureje 2019 (STEPCARE); Indu 2018; Oladeji 2015; Patel 2010; Pradeep 2014). Three trials did not include any pharmacotherapy component (Jordans 2019; Niemi 2016; Xie 2019). Three trials did not include any structured psychological interventions (Chen 2015; Fritsch 2007; Jenkins 2013). All trials included psychoeducation. Other non‐pharmacological interventions offered included cognitive‐behavioural therapy (Indu 2018), problem‐solving therapy (Adewuya 2019; Gureje 2019 (STEPCARE); Jordans 2019; Oladeji 2015), behavioural activation/activity scheduling (Jordans 2019; Patel 2010; Xie 2019), interpersonal therapy (Patel 2010), telephone monitoring and adherence counselling (Adewuya 2019; Chen 2015), home visits (Adewuya 2019; Indu 2018), and yoga (Niemi 2016; Patel 2010). The intensity of structured psychological interventions ranged from 6 to 12 weekly sessions (Adewuya 2019; Araya 2003; Gureje 2019 (STEPCARE); Indu 2018; Jordans 2019; Niemi 2016; Oladeji 2015; Patel 2010), with more sessions offered to those with more severe symptoms. Antidepressant regimens (when used) were dictated by local availability of selective serotonin reuptake inhibitors or tricyclic antidepressants.
Comparators
Comparison groups were in the same PHC settings in which PWs did not receive additional training (‘usual care’) (Fritsch 2007; Gureje 2019 (STEPCARE); Indu 2018; Jenkins 2013; Jordans 2019; Niemi 2016; Pradeep 2014; Xie 2019), or where PWs were provided with a manual or guidelines on diagnosis and treatment of depression (‘enhance usual care’) (Adewuya 2019; Araya 2003; Chen 2015; Oladeji 2015; Patel 2010). In one trial, the comparison group also received psychoeducation provided by a lay health worker (Adewuya 2019).
Appendix 5. Study descriptions (Comparison 3). Lay health worker‐led psychosocial interventions versus usual care in treating perinatal depression
Settings
We identified seven studies in five countries across Africa (Chibanda 2014; Lund 2020; Peltzer 2019), the Middle East (Milani 2015), and South Asia (Fuhr 2019; Rahman 2008; Sikander 2019). These studies were conducted in urban settings ((Chibanda 2014; Lund 2020; Milani 2015), in rural settings in Pakistan (Peltzer 2019; Rahman 2008Sikander 2019), and in urban and rural settings (Fuhr 2019). An eighth study, Khan 2017 (which has been included in the PTSD adult comparison but narratively described along side findings here) was performed in Pakistan.
Participants
Women over the age of 16 were recruited antenatally (Fuhr 2019; Lund 2020Peltzer 2019; Rahman 2008Sikander 2019), or postpartum (Chibanda 2014; Milani 2015), mainly from primary health clinics, except for Rahman 2008, Sikander 2019, and Milani 2015, which were recruited from the community. Peltzer 2019 recruited women who had HIV. Participants were generally from lower socioeconomic backgrounds.
Interventions
LHWs
Lay health workers were selected from peers/similar communities as participants (Fuhr 2019Khan 2017; Milani 2015; Sikander 2019), existing grassroot/NGO workers (Chibanda 2014; Lund 2020 (also peers)), or existing government aides/LHWs (Peltzer 2019; Rahman 2008).
LHW training varied from very intensive in Fuhr 2019 (25‐ to 40‐hour classroom teaching + 2‐month clinical internship + assessment) to quite minimal 2‐day training (Chibanda 2014). Other studies had intermediate length of training (Lund 2020, Peltzer 2019 ‐ 5 days; Rahman 2008, Sikander 2019 ‐ 2 days initial training + 1 or more days refresher training). Supervision varied from weekly supervision (individual ‐ Chibanda 2014; group ‐ Lund 2020), to fortnightly group supervision (Fuhr 2019), to regular supervision (Rahman 2008; Sikander 2019). Milani 2015 did not specify the duration of training nor supervision, although both were present.
Description of interventions
Interventions were delivered at home (using telephone in Milani 2015, or face‐to‐face in Sikander 2019 and Rahman 2008), at home or in the antenatal clinic (according to participant choice) (Lund 2020), at community centres (Fuhr 2019Peltzer 2019), or in primary care clinics (Chibanda 2014).
All interventions had a psychoeducation component. In addition, they offered different psychological interventions: problem‐solving techniques (Chibanda 2014; Lund 2020), telephone general support (Milani 2015), group problem‐solving therapy (Chibanda 2014), Thinking Healthy Programme (THP) ‐ CBT‐like (Fuhr 2019; Rahman 2008Sikander 2019), and structured behavioural prevention of mother‐to‐child transmission (PMTCT) and anxiety‐reduction intervention (Peltzer 2019).
Interventions were delivered for varying lengths of time from six weeks to several months, at different perinatal stages: all interventions spanned the antenatal and postnatal periods except for Milani 2015 and Chibanda 2014, in which interventions were delivered postnatally only.
Comparators
The Milani 2015 control group was given usual antenatal care (also received by intervention group). All other studies offered enhanced care. This was most extensive in Chibanda 2014 and involved pharmacotherapy (not received by the intervention group) and PMTCT counselling (also received by the intervention group). One study had a less intensive intervention not received by the intervention arm: seeing a gynaecologist more often (Fuhr 2019). The other control groups were described as enhanced care (i.e. control group was receiving an extra small intervention). This enhanced care was also a co‐intervention received by the intervention arm(s) in the following studies: routine antenatal care + monthly phone calls (Lund 2020), or visits by LHWs without mental health training (Rahman 2008, Sikander 2019), or having PMTCT counselling and physical health promotion/disease prevention (Peltzer 2019).
Appendix 6. Study descriptions (Comparison 4). Primary health professional‐led collaborative interventions versus enhanced usual care in treating perinatal depression
Settings
We identified two studies from two countries in South America ‐ Rojas 2007 ‐ and in Africa Gureje 2019 (EXPONATE), which were conducted in urban settings (Rojas 2007), and in both urban and rural settings (Gureje 2019 (EXPONATE)).
Participants
Trials recruited mothers from PHC clinics in the second trimester of pregnancy (Gureje 2019 (EXPONATE)), or in the first postnatal year (Rojas 2007). Participants were from lower socioeconomic backgrounds.
Interventions
PHPs(primary health professionals)
These interventions were delivered by several existing government cadres, who held different roles: the main intervention deliverers were nurses and midwives (Gureje 2019 (EXPONATE); Rojas 2007), with additional support provided by doctors (Rojas 2007), and by LHWs (community extension workers) (Gureje 2019 (EXPONATE)). Training was similar in both studies (two to three days), and supervision was cascaded from specialists down the hierarchy of primary care cadres (doctor > nurse/midwife > LHW).
Description of interventions
Interventions were delivered in primary care clinics and included collaborative care as multiple components offering all participants pharmacological (fortnightly, then monthly visits for six months) and group psychosocial treatment (eight weekly sessions; Rojas 2007), along with a stepped care approach with problem‐solving treatment offered initially (weekly eight individual sessions antenatally, four to eight sessions at six weeks postpartum), and combined pharmacological and psychological treatments as second line if psychological intervention steps were ineffective (Gureje 2019 (EXPONATE)).
Comparators
Comparison groups were quite different, as Gureje 2019 (EXPONATE) had ‘low‐intensity’ treatment (1.5 days of training in mhGAP), whereas Rojas 2007 had usual primary care (mainly pharmacological treatment offered).
Appendix 7. Study descriptions (Comparison 5). Lay health worker‐led psychological interventions vs usual care in adults with post‐traumatic stress or common mental disorders in humanitarian settings
Settings
We identified 15 studies, where participants were refugees (S. Sudanese refugees in Uganda ‐ Tol 2020 Afghan refugees in Malaysia ‐ Shaw 2018; Sudanese refugees living in Cairo ‐ Meffert 2014; refugee settlements ‐ Neuner 2008 internally displaced populations ‐ Dybdahl 2001 Yeomans 2010 survivors of conflict in their area ‐ Ayoughi 2012, Khan 2019, Khan 2017, Rahman 2016, Rahman 2019; genocide survivors in Rwanda ‐ Connolly 2011 victims of other violence ‐ Bonilla‐Escobar 2018; and survivors of gender‐based violence ‐ Bryant 2017, Dawson 2016).
Participants
Adults > 18 years (except Ayoughi 2012, which included 14 to 60‐year‐olds) of both sexes or just women (Dybdahl 2001; Khan 2019; Khan 2017; Rahman 2019; Shaw 2018; Tol 2020), as well as pregnant women (Khan 2017), women with PTSD diagnosis (Meffert 2014 ‐ HTQ; Connolly 2011 and Neuner 2008 ‐ DSM IV PTSD), women with PTSD symptoms (Dybdahl 2001; Rahman 2016; Rahman 2019), or women with psychological distress/CMDs ‐ in Ayoughi 2012; Bonilla‐Escobar 2018; Bryant 2017; Dawson 2016; Khan 2017; Khan 2019; Rahman 2016; Rahman 2019; Shaw 2018; Tol 2020 + functional impairment in Bryant 2017; Dawson 2016; Khan 2019; Rahman 2019.
Interventions
LHWs
Lay people delivering interventions had no previous mental health training. Training varied from 1 to 5 days (Connolly 2011; Dybdahl 2001; Yeomans 2010), to 6 to 15 days (Bonilla‐Escobar 2018; Bryant 2017; Dawson 2016; Khan 2019; Rahman 2016; Rahman 2019; Tol 2020), to 1.5 to 3.5 months (Ayoughi 2012; Neuner 2008), to ‘intensive’ (Shaw 2018) (Meffert 2014 ‐ length unknown). All trainings tried to include knowledge transfer (e.g. on intervention components) and a practical session. Some included competency assessments (Bryant 2017; Dawson 2016; Rahman 2019. These people were locally employed at clinics (Ayoughi 2012), as community‐based LHWs (Bryant 2017; Dawson 2016; Khan 2017; Rahman 2016; Rahman 2019), or as other lay people (Connolly 2011) (women including survivors of violence/displacement ‐ Bonilla‐Escobar 2018 or preschool teachers ‐ Dybdahl 2001). Training and supervision were delivered by trained trainers (Bryant 2017; Khan 2017; Khan 2019), by research team/specialists/experts (Bonilla‐Escobar 2018; Connolly 2011 Dybdahl 2001; Meffert 2014; Neuner 2008; Shaw 2018), or by both (Ayoughi 2012; Dawson 2016; Rahman 2016; Rahman 2019; Tol 2020) (Yeomans 2010 ‐ unknown).
Description of interventions
Duration: Connolly 2011 had one and Khan 2017 two brief intervention sessions. A great majority of studies had four to six sessions (Ayoughi 2012; Bryant 2017; Dawson 2016; Khan 2019; Meffert 2014; Neuner 2008; Rahman 2016; Rahman 2019; Tol 2020; Yeomans 2010) (weekly intervals except Neuner 2008 and Meffert 2014 ‐ twice a week, Yeomans 2010 ‐ 3‐day workshop + 1 session 1 month later; Ayoughi 2012 ‐ additional 8 sessions according to need), 8 to 12 weekly sessions (Bonilla‐Escobar 2018; Shaw 2018), or many more intensive sessions (Dybdahl 2001 ‐ weekly sessions for five months ‐ 20 sessions).
Location: these were delivered as individual sessions in the home (Bryant 2017; Dawson 2016; Khan 2017; Neuner 2008), or in the PC facility (Ayoughi 2012 ‐ individual, Shaw 2018 ‐ group), or at community centres/locations as individual sessions (Bonilla‐Escobar 2018 (also in homes); Connolly 2011; Khan 2017 – whole family; Meffert 2014; Rahman 2016), or as group sessions (Dybdahl 2001; Khan 2019; Rahman 2019; Tol 2020; Yeomans 2010).
Content: the primary aim of many psychological interventions was symptom improvement: PM+ (Bryant 2017; Dawson 2016; Khan 2019; Rahman 2016; Rahman 2019); CETA (Bonilla‐Escobar 2018); IPT (Meffert 2014); cognitive‐behaviour therapy (Ayoughi 2012); locally designed psychosocial therapy (Dybdahl 2001); somatic‐focused CBT (Shaw 2018); narrative exposure therapy (Neuner 2008); trauma counselling (Neuner 2008); and workshop with and without psychoeducation (Yeomans 2010). The primary aim of other interventions was to promote self‐care (although several other interventions had it as a component) or to provide self‐help plus (Tol 2020), PsychoEducation (Khan 2017), Thought Field therapy (Connolly 2011), or a general physical and mental well‐being family intervention (Khan 2017).
Comparators
Control groups varied quite widely from (a) enhanced usual care (small amount of training) to LWH (Bryant 2017; Dawson 2016; Khan 2019; Rahman 2016; Rahman 2019; Tol 2020), previously trained PHC nurses (Bryant 2017), wait‐list control with psychologist providing follow‐up safety + identification and referral (Bonilla‐Escobar 2018), primary care doctors providing weekly consultations and medication as necessary (antidepressants, pain‐killers, and sleeping tablets) (Ayoughi 2012); to (b) usual care (Dybdahl 2001; Khan 2017); and to (c) no care (wait‐list control for Bonilla‐Escobar 2018; Connolly 2011; Meffert 2014; Neuner 2008; Shaw 2018; Yeomans 2010).
Appendix 8. Study descriptions (Comparison 6). Primary health professional‐ and community professional‐led psychological interventions versus usual or no care in adults with post‐traumatic stress or common mental disorders in humanitarian settings
Settings
We identified five studies that addressed survivors of conflict in their area, including survivors of torture (Bass 2016 Bolton 2014 (Iraq); Weiss 2015), Burmese in Thailand (Bolton 2014 (Thailand)), and survivors of gender‐based violence (Bass 2013).
Participants
Adults with trauma exposure and distress (post‐traumatic stress; Bass 2013), with significant depressive symptoms (Bolton 2014 (Iraq)Bolton 2014 (Thailand)), meeting PTSD diagnostic criteria (Bass 2013; Weiss 2015), or with both PTS and PTSD (Bass 2016), and women only (Bass 2013).
Interventions
PHPs and CPs
Interventions were delivered often by several professional health worker cadres: doctors and nurses (Weiss 2015), psychosocial assistants (Bass 2013), physician assistant, nurse, pharmacist (Bass 2016; Bolton 2014 (Iraq)), and teachers, health workers, and counsellors (Bolton 2014 (Thailand)). In most of these studies, health workers had pre‐existing training and/or experience in mental health care (some up to 9 years of experience in Bolton 2014 (Iraq)), apart from Bolton 2014 (Thailand), where teachers and health workers (in addition to counsellors) were trained from minimal experience. There was also intensive supervision: all had weekly contact at least by phone with formal monthly supervisions and possibly extra ongoing training.
PHPs and CPs delivered 8 to 12 weekly sessions of different forms of psychological or psychosocial interventions across these studies. Interventions were locally designed (Bass 2016). Others were evidence‐based interventions adapted to their settings, such as cognitive processing therapy (Bass 2013; Bolton 2014 (Iraq); Weiss 2015), CETA (Bolton 2014 (Thailand); Weiss 2015), behavioural activation (second arm of Bolton 2014 (Iraq)). These were delivered at community centres (Bass 2013; Bass 2016), to community groups (Bolton 2014 (Thailand)), or in primary care facilities (Weiss 2015; Bolton 2014 (Iraq)), but all provide individual sessions.
Comparators
These were all wait‐list controls. However in some studies, the control group received during their wait enhanced care: psychosocial assistants offering psychosocial support without CPT (Bass 2013), monthly follow‐up by health workers (Bolton 2014 (Iraq)), identification/referral (Weiss 2015), or available but poorly accessed (for fear of deportation) counselling services (Bolton 2014 (Thailand)).
Appendix 9. Study descriptions (Comparison 7). Lay health worker‐led interventions versus enhanced usual care in adult patients with harmful or hazardous alcohol or substance use
Settings
We found eight studies from Brazil (Christoff 2015), Kenya (Papas 2011; Papas 2020), India (Nadkarni 2017), Nepal (Jordans 2019), South Africa (Peltzer 2013; Sorsdahl 2015), and Thailand (Sherman 2009). They were conducted in both urban ‐ Christoff 2015; Nadkarni 2017; Papas 2011; Papas 2020; Peltzer 2013; Sherman 2009; Sorsdahl 2015 ‐ and rural ‐ Jordans 2019; Peltzer 2013 ‐ settings.
Participants
Adults with harmful or hazardous alcohol use (Christoff 2015; Jordans 2019; Nadkarni 2017; Papas 2011; Papas 2020; Peltzer 2013; Sorsdahl 2015), and/or those with substance use (Christoff 2015; Sherman 2009; Sorsdahl 2015), were recruited from primary care facilities (Jordans 2019; Nadkarni 2017; Peltzer 2013; Sorsdahl 2015), emergency departments (Sorsdahl 2015), HIV clinics (Papas 2011; Papas 2020), and universities (Christoff 2015). Participants in Peltzer 2013 were receiving active TB treatment, and patients in Papas 2011 and Papas 2020 were eligible for or had been initiated on antiretroviral therapy for HIV. Participants in Sherman 2009 were recruited first from index patients and then through their network contacts. Participants in Christoff 2015 and Sherman 2009 were young adults, and those in Nadkarni 2017 were male only. Participants were recruited based on positive screening with the AUDIT test (Jordans 2019; Nadkarni 2017; Peltzer 2013), the ASSIST test (Christoff 2015; Sorsdahl 2015), the AUDIT‐C test (Papas 2011; Papas 2020), recent alcohol use and/or binge‐drinking (Papas 2011; Papas 2020), and recent methamphetamine use and being sexually active (Sherman 2009).
Interventions
LHWs
Lay health workers who had received training delivered interventions. Training intensity was variable and was about 24 hours in Peltzer 2013, 40 hours in Sherman 2009 and Sorsdahl 2015, 175 to 300 hours in Papas 2020, more than 400 hours in Papas 2011, more than 900 hours in Jordans 2019 and Nadkarni 2017, and unspecified in Christoff 2015. Interventionists were supervised weekly in Nadkarni 2017Papas 2011, and Papas 2020, or bi‐weekly in Jordans 2019Peltzer 2013, and Sorsdahl 2015; supervision details were not provided in Christoff 2015 and Sherman 2009.
Description of interventions
Interventions consisted of a single ASSIST‐linked motivational interviewing session (Christoff 2015; one arm of Sorsdahl 2015), two brief intervention sessions one month apart (Peltzer 2013), four weekly or fortnightly sessions of a manualised motivation intervention that involved assessment, feedback, cognitive and behavioural skills, and management of actual and potential relapses ("Counselling for Alcohol Problems"; Jordans 2019; Nadkarni 2017), six weekly cognitive‐behaviour therapy sessions (Papas 2011; Papas 2020), blended treatment with one motivational interviewing session and four problem‐solving therapy sessions held weekly (one arm of Sorsdahl 2015), and peer education with a social network (Sherman 2009).
Comparators
The comparison group was given enhanced usual care. Participants received feedback on their ASSIST score in Christoff 2015, a leaflet on responsible drinking in Peltzer 2013, a brochure on the effect of substance use in Sorsdahl 2015, the WHO‐mhGAP programme in Jordans 2019 and Nadkarni 2017, routine medical care in Papas 2011, a time and attention controlled healthy lifestyle education intervention in Papas 2020, and a seven‐session life skills‐building intervention in Sherman 2009.
Appendix 10. Study descriptions (Comparison 8). Primary health professional‐ and community professional‐led interventions versus enhanced usual care in adult patients with harmful or hazardous alcohol or substance use
Settings
We identified six studies ‐ four conducted in South Africa (HuisIntVeld 2019; Marais 2011; Mertens 2014; Pengpid 2013), one in Thailand (Noknoy 2010), and one in Brazil and India (Humeniuk 2012). These took place in rural ‐ Marais 2011; Noknoy 2010 ‐ and urban settings ‐ HuisIntVeld 2019; Humeniuk 2012; Mertens 2014; Pengpid 2013.
Participants
All participants recruited adults (age 15 and above ‐ Marais 2011, 16 to 62 ‐ Humeniuk 2012, 18 to 24 ‐ Mertens 2014, 18 to 65 ‐ HuisIntVeld 2019; Noknoy 2010, 18 and above ‐ Pengpid 2013) in attending primary care clinics (Humeniuk 2012; Mertens 2014; Noknoy 2010), HIV clinics (HuisIntVeld 2019), a university (Pengpid 2013), and antenatal clinics (Marais 2011) who screened positive for harmful or hazardous alcohol or substance use on the AUDIT (HuisIntVeld 2019; Noknoy 2010; Pengpid 2013), ASSIST (Humeniuk 2012), or a screening question (Mertens 2014), except for Marais 2011, which included all patients less than 20 weeks' pregnant attending clinics allocated to the intervention for antenatal care who consented to the study regardless of baseline alcohol use.
Interventions
PHPs and CPs
These were nurses (HuisIntVeld 2019; Noknoy 2010; Pengpid 2013), nurse practitioners (Mertens 2014), clinicians, clinical research interviewers, and researchers (Humeniuk 2012), and a social scientist and a social worker (Marais 2011), all of whom were trained for six hours (Noknoy 2010), three days (Mertens 2014), five days (Pengpid 2013), or for an unspecified duration (HuisIntVeld 2019; Humeniuk 2012; Marais 2011). Most were supervised twice weekly by study authors (HuisIntVeld 2019), bi‐weekly by the project manager (Pengpid 2013), weekly for the first six weeks then monthly by the study trainer (Mertens 2014), and by a psychologist experienced in addiction (frequency unspecified ‐ Humeniuk 2012).
Description of interventions
These centred around motivational interviewing techniques, including a single session of Brief Intervention based on the AUDIT (HuisIntVeld 2019; Pengpid 2013), a single ASSIST‐linked Brief intervention (Humeniuk 2012), a single session of Brief Motivational Interviewing (Mertens 2014), four sessions of Brief Intervention based on AUDIT (Marais 2011), and three sessions of Motivational Enhancement Therapy (Noknoy 2010).
Comparators
The comparison group was given enhanced usual care. Participants received the AUDIT (HuisIntVeld 2019; Marais 2011; Noknoy 2010; Pengpid 2013), or they completed the ASSIST questionnaire (Humeniuk 2012), and they received feedback about their scores (Pengpid 2013), a leaflet on responsible drinking (HuisIntVeld 2019; Pengpid 2013), a take‐home alcohol booklet (Marais 2011), a or a referral resource list for drinking and drug use (Mertens 2014).
Appendix 11. Study descriptions (Comparisons 9 and 10). Primary‐level worker‐led interventions versus enhanced usual care in adult patients with alcohol and substance drug dependence
Settings
We identified three studies, all of which were conducted in lower‐middle‐income countries ‐ two in urban settings (Nadkarni 2019; Zhong 2015), and one in urban and rural settings (Li 2018). Li 2018 and Nadkarni 2019 were conducted in primary care settings, and Zhong 2015 was conducted at a community centre.
Participants
Adults 18 years of age and older were recruited in Li 2018, 18 to 65 years in Nadkarni 2019, and 18 to 60 years in Zhong 2015. In Li 2018, commune health workers, who were doctors, assistant doctors, nurses, pharmacists, midwives, laboratory technicians, and public health workers who had contact with persons who injected drugs (PWID), as well as PWID, and with people who had a history of injecting drugs, were recruited. In Zhong 2015, persons who met DSM‐IV criteria for heroin dependence and who had just been released from a mandatory two‐year rehabilitation programme were recruited. In Nadkarni 2019, males who scored 20 or more on the AUDIT test, indicating alcohol dependence, were recruited. Most participants were male (100% in Nadkarni 2019, 97.8% amongst PWIDs in Li 2018, and 78.3% in Zhong 2015).
Interventions
PWs
Primary‐level workers were lay health workers (LHWs) in Nadkarni 2019, primary health professionals (PHPs) in Li 2018 (doctors and assistant doctors, i.e. local health educators with some medical training), and community professionals (CPs) in Zhong 2015 (experienced social workers). All were trained, to a varying extent, by two‐week classroom training followed by six‐month internship in Nadkarni 2019, two‐month‐long training in Zhong 2015, and a one‐week‐long session in Li 2018. All were supervised, at all sessions in Zhong 2015 by certified counsellors or psychologists and in Li 2018, and weekly by peers and twice monthly by peers and local experts in Nadkarni 2019. In addition, local supervisors in Nadkarni 2019 received supervision by international experts monthly via Skype.
Description of interventions
Nadkarni 2019 employed a manualised intervention, "Counselling for Alcohol Problems", which was based on individualised assessment, feedback, cognitive‐behaviour skills and techniques, and management of potential or actual relapse using these skills. Participants received four sessions at weekly or fortnightly intervals, which lasted 30 to 45 minutes each. Zhong 2015 carried out a one‐year psychosocial rehabilitation programme, comprising weekly individual sessions lasting 60 minutes each and two‐monthly group sessions lasting 100 minutes each, which engaged participants' intrinsic motivations, and identified risk factors and triggers for relapse; including psychoeducation, HIV, HCV, and high‐risk sexual behaviour education and techniques to prevent relapse; identification and coping with negative emotions; interpersonal skills training; time management; healthy alternative habits; and usage of community resources. Li 2018 utilised a communication training approach, in which commune health workers received three weekly sessions and two‐monthly booster sessions, each lasting 90 minutes, for 12 months, on roles and responsibilities of health workers in HIV and drug control; challenges in and solutions to working with PWID; stages of behavioural change; client‐centred goal‐setting; and motivational communication tools and skills. Commune health workers then delivered three individual sessions to PWID, each lasting one hour, focusing on improving physical and mental health, engaging them in harm reduction and HIV services, and enhancing family and social support in positive behaviour change.
Comparators
The comparison was enhanced usual care in all three studies. In Nadkarni 2019, participants in the control arm received a consultation with a physician, where they were provided with screening results and were referred to a de‐addiction centre. Participants in the intervention arm received a similar consultation. In Zhong 2015, participants in the control arm received monthly visits from social workers, urine tests, and simple advice regarding life events. In Li 2018, commune health workers in the control arm received a group lecture on topics related to drug use.
Appendix 12. Study descriptions (Comparison 11). Lay health worker‐led intervention versus specialist‐led care delivering interventions to adults with severe mental disorders
Settings
We identified two studies that were conducted in China ‐ Shen 2016 ‐ and in India ‐ Chatterjee 2014. Settings were urban in Shen 2016 and Chatterjee 2014 and rural in Chatterjee 2014.
Participants
Adults with schizophrenia, recovering after their first episode of illness (Shen 2016), or having had disease for at least 12 months, with at least moderately severe disease (Chatterjee 2014). Mean age was 36 years (SD 10 years) in Chatterjee 2014 and 45 years in Shen 2016 (SD 8 years). Both genders were represented.
Interventions
LHWs
In Chatterjee 2014, lay workers were trained over six weeks to deliver the intervention under the supervision of psychiatric social workers and psychiatrists. In Shen 2016, lay workers worked as employees in a clubhouse, which participants attended.
Description of interventions
In Chatterjee 2014, lay health workers performed six to eight home visits in the first three months, visits every 15 days for the next four months, and monthly visits for the next five months. Content included individualised psychoeducation, health promotion, rehabilitation, and linkage to self‐help groups and community agencies to address social, legal, and employment issues. In Shen 2016, participants attended a "clubhouse" eight hours a day, five days a week for a year, where they worked alongside employees at tasks such as manning the reception and news‐stand, meal preparation, cleaning, publishing the newsletter, and managing inventory and storage; participated in classes and clubs based on their interests as well as on weekly entertainment/cultural activities including trips; and volunteered at libraries and elderly homes.
Comparators
In Chatterjee 2014, the control group received facility‐based care from mental health practitioners. In Shen 2016, the control group received routine community psychiatric care from psychiatrists and psychiatric nurses, comprising monthly two‐hour treatment sessions that included psychoeducation.
Appendix 13. Study descriptions (Comparison 12). Primary health professional‐led or collaborative care versus specialists delivering interventions to adults with severe mental disorders
Setting
We identified seven studies that were conducted in China (Li 2002; Ling 1999; Tan 2005; Wu 2016; Yao 2014), and in Iran (Barfar 2017; Malakouti 2015). Settings were rural in Li 2002 and Tan 2005 or urban in Barfar 2017Ling 1999Malakouti 2015Wu 2016 and Yao 2014.
Participants
Adults with schizophrenia (all seven studies), bipolar disorder (Barfar 2017; Malakouti 2015), and schizoaffective disorder were included (Barfar 2017). Two studies excluded older patients (60 and older: Wu 2016; 66 and older: Barfar 2017), and one included only patients who were 50 years of age or older (Li 2002). Both genders were represented. Disease severity and subtypes ranged from chronic stable schizophrenia (Yao 2014; Wu 2016), to first episode of late‐onset schizophrenia (Li 2002), to difficult‐to‐treat disease, defined by two or more hospitalisations in the past two years and poor compliance with medications (Malakouti 2015).
Interventions
PWs
These included health professionals such as doctors (Barfar 2017; Li 2002; Malakouti 2015; Tan 2005; Wu 2016; Yao 2014), nurses (Malakouti 2015; Wu 2016), and allied professional workers such as social workers (Barfar 2017), rehabilitation therapists (Wu 2016), case managers (Barfar 2017), and disability agency workers (Yao 2014). In Ling 1999, the cadre of primary health professional who performed the psychological intervention was not specified. Primary health professionals were trained by hospital community medicine specialists in Li 2002, by psychiatrists in Ling 1999, and by unspecified personnel in Barfar 2017, Malakouti 2015, and Wu 2016. They were supervised by psychiatrists in Barfar 2017 and Malakouti 2015, by hospital community medicine specialists in Li 2002, by a manager in Wu 2016, and by a nurse in Yao 2014. They worked individually (case managers in Barfar 2017, primary care doctors in Li 2002 and Tan 2005, unspecified primary health professionals in Ling 1999, primary care doctors or nurses in Malakouti 2015) or as a multi‐disciplinary team (primary care doctors and social workers in Barfar 2017 doctors, therapists, and nurses in Wu 2016; and nurses, psychiatrists, disability agency workers, and primary care doctors in Yao 2014). Psychiatrists provided the overall care plan for each participant in Barfar 2017 and Tan 2005. They also gave regular outpatient care in Barfar 2017, were informed when issues arose in Malakouti 2015, and delivered psychological intervention and/or medical therapy, with the primary health professional playing a supportive role, in Tan 2005 and Yao 2014.
Description of interventions
These included prescribing, administering, and adjusting medications (Barfar 2017; Li 2002; Malakouti 2015; Tan 2005; Wu 2016; Yao 2014); educating on medication side effects and the importance of compliance (Barfar 2017; Ling 1999; Malakouti 2015); monitoring symptoms (Barfar 2017; Ling 1999; Malakouti 2015; Tan 2005; Yao 2014), providing psychoeducation (Barfar 2017; Ling 1999; Malakouti 2015; Tan 2005; Yao 2014), counselling (Li 2002; Ling 1999; Wu 2016; Yao 2014), and telephone reminders to attend outpatient clinics (Barfar 2017); as well as family interventions including communication training (Ling 1999; Wu 2016), social skills training (Barfar 2017; Yao 2014), requested work or social activities (Li 2002; Wu 2016), rehabilitation (Tan 2005; Yao 2014), and self‐care training (Wu 2016). Duration of the intervention was about two months in Li 2002, six months in Ling 1999, and 12 months in the rest (Barfar 2017; Malakouti 2015; Tan 2005; Wu 2016; Yao 2014).
Comparators
In the control group, patients were cared for by mental health specialists, including those providing inpatient care (Li 2002), those in any outpatient or inpatient services contacted by the patient (Barfar 2017), those in an outpatient clinic to which they were referred or in a hospital during an exacerbation (Malakouti 2015), those in a hospital when their disease became active (Tan 2005), and community psychiatric nurse‐led teams (Ling 1999; Wu 2016; Yao 2014).
Appendix 14. Study descriptions (Comparison 13). Primary health professional‐ and lay health worker‐led psychosocial interventions versus usual care in improving dementia patients' and carers' outcomes
Setting
We found two studies that were conducted in urban areas in India ‐ Dias 2008 ‐ and in Russia ‐ Gavrilova 2009.
Participants
Interventions were directed at carers of people with dementia. Carers were generally aged between 50 and 60 years and had varying economic backgrounds.
Interventions
PHPs and LHWs
Dias 2008 used two types of LHWs (home care advisors and lay counsellors) trained intensively for one week, whereas Gavrilova 2009 used newly qualified doctors trained for two days, to deliver the intervention. LHWs were supervised every two weeks by a specialist. The supervision provided to doctors was not described.
Description of interventions
In both studies, brief carer interventions were conducted, based on a larger 10/66 dementia initiative (Prince 2004). However, Gavrilova 2009 organised a short training package for carers only, and supervision/psychiatric involvement was not specified, whereas Dias 2008 implemented a collaborative care package (LHWs undertook psychoeducation and counselling, facilitated group support meetings, and followed up on treatment effects during home visits, supervised by a psychiatrist who saw them at least once and who may prescribe anticholinesterases).
Comparators
Both were wait‐list controls with usual (but not very available) physical care, although Dias 2008 had a small enhanced component of providing a small amount of education and information regarding dementia.
Appendix 15. Study descriptions (Comparison 14). Lay health worker‐led psychosocial interventions versus usual or no care in child post‐traumatic stress or common mental disorders in humanitarian settings
Setting
We identified seven studies from 6 countries in Asia ‐ Tol 2008; Jordans 2010; Tol 2012 ‐ and in Africa Bolton 2007; Ertl 2011; O'Callaghan 2014; Murray 2015 ‐ that were conducted in post‐conflict or peri‐conflict setting camps (Bolton 2007; Ertl 2011; Jordans 2010; O'Callaghan 2014; Tol 2008; Tol 2012). One study was conducted in a non‐war setting, with inclusion of children exposed to abuse/neglect/HIV in Zambia (Murray 2015). Settings were rural/semi‐rural (Bolton 2007; Jordans 2010; O'Callaghan 2014; Tol 2008), or they were urban and rural (Ertl 2011; Murray 2015; Tol 2012).
Participants
Children with PTSD diagnoses ‐ Dawson 2016; Ertl 2011; Jordans 2010; Murray 2015; Tol 2008; Tol 2012 ‐ with PTS ‐ Bolton 2007 ‐ or with both ‐ O'Callaghan 2014 ‐ were included. Other than Ertl 2011 and Murray 2015, children were also screened for depressive and anxiety symptoms, or for behavioural problems. The ages of children varied from broader age categories: from 5 (or 7) to 18 years (Murray 2015; O'Callaghan 2014), to narrower primary school or secondary school age (Bolton 2007 (14 to 18 years); Jordans 2010 (11 to 14 years); Tol 2008; Tol 2012 (8 to 13 years)). One study included child soldiers aged 12 to 25 years (Ertl 2011). Most children came from low‐resource backgrounds.
Interventions
PWs
LHWs (of both sexes) were people selected by the community who received manual‐based training for their respective interventions (Bolton 2007; Ertl 2011; Jordans 2010; Tol 2008; Tol 2012), or via apprenticeship on‐site training (Murray 2015), with training duration of about 2 weeks (Bolton 2007; Jordans 2010; Murray 2015; Tol 2008; Tol 2012). Supervision varied from regular weekly in Bolton 2007; Jordans 2010; Tol 2008; Tol 2012; and Murray 2015 to intensive (e.g. case discussions of treatment sessions and of notes) in Ertl 2011.
Description of interventions
Most interventions were delivered to groups in schools, except one in community groups (Bolton 2007), and two at home (Ertl 2011, Murray 2015). All interventions were psychological or psychosocial interventions targeted at children. They had different focuses of therapy: psychoeducational (Ertl 2011, in most; O'Callaghan 2014); social/creative (Ertl 2011); academic catch‐up (Bolton 2007, arm 2 creative play; O'Callaghan 2014); therapy‐based such as trauma‐focused CBT in Murray 2015 and narrative exposure therapy in Ertl 2011 (arm 1); group interpersonal therapy in Bolton 2007 (arm 1); or a combination. Jordans 2010, Tol 2008, and Tol 2012 had the same manual‐based, classroom‐based intervention (CBI) (creative‐expressive therapy, co‐operative play, and CBT). Interventions were mainly delivered in groups except for Ertl 2011 (individual therapy), and in child‐carer dyads for Murray 2015, over 8 to 15 sessions, weekly or thrice weekly (Ertl 2011Jordans 2010; O'Callaghan 2014Tol 2008Tol 2012), spread over three weeks in O'Callaghan 2014 to four months in Bolton 2007.
Comparators
This was overall eclectic, although most studies had no care (or existing care was often poorly available) and wait‐list control (Bolton 2007Jordans 2010O'Callaghan 2014 Tol 2008Tol 2012). Two studies enhanced their control groups with existing community outreach psychosocial counselling and support groups (Murray 2015), or with a suicidal intervention for those with suicidal ideation (Ertl 2011).
Appendix 16. Study descriptions (Comparison 15). Community professional‐led psychosocial interventions versus no care in child post‐traumatic stress or common mental disorders in humanitarian settings
Setting
We identified eight studies from seven countries within Europe (Dybdahl 2001; Gordon 2008), the Middle East (Barron 2016; Barron 2013), Africa (Betancourt 2014; O'Callaghan 2013; O'Callaghan 2015), and Asia (Berger 2009). Seven studies were undertaken in post‐conflict or peri‐conflict settings, including in internally displaced populations (Dybdahl 2001), although Berger 2009 was conducted following a natural disaster. Settings were rural/semi‐rural (Barron 2016; Bolton 2007; Gordon 2008; O'Callaghan 2015), or they were urban (Barron 2013; Berger 2009; Betancourt 2014; Dybdahl 2001; O'Callaghan 2013).
Participants
Children included were exposed to trauma and were distressed (Berger 2009; Betancourt 2014 ‐ specifically screened to have psychological distress or functional impairment; Dybdahl 2001; O'Callaghan 2013; O'Callaghan 2015), they met PTSD diagnostic criteria (Barron 2013; Barron 2016; Gordon 2008), or they had either PTS or PTSD (Berger 2009). Most children were from the general population, although in Betancourt 2014, most children had been child soldiers. The ages of children included pre‐school children (5 to 6‐year‐olds and their mothers ‐ Dybdahl 2001); those under 14 years old (6 to 14 years; Berger 2009; Barron 2013; O'Callaghan 2013); and those 12 to 17 years and over 24 years old (14 to 17 ‐ O'Callaghan 2015; 14 to 18 ‐ Gordon 2008; 15 to 24 ‐ Betancourt 2014).
Interventions
CPs
Four studies trained teachers (Berger 2009; Dybdahl 2001 (pre‐school teacher); Gordon 2008; O'Callaghan 2015); three studies trained school counsellors (newly trained with a bachelor's in social work or related field; Barron 2013; Barron 2016; Betancourt 2014 ‐ manualised); and two studies trained social workers (O'Callaghan 2013; O'Callaghan 2015). They were given 3 days (Berger 2009; Barron 2013; Barron 2016), 5 to 6 days (Dybdahl 2001; O'Callaghan 2015), or 2 weeks of training by researchers (Betancourt 2014; Gordon 2008). Supervision was weekly (Berger 2009; Dybdahl 2001, Betancourt 2014), or it was provided regularly (Gordon 2008), by mental health professionals. Less intensive supervision was delivered in Barron 2016 (in pairs or groups monthly).
Description of interventions
All interventions were targeted at children, except in Dybdahl 2001, where the target group was mothers, and in O'Callaghan 2013, which had an additional three sessions aimed at carers. Interventions were delivered to groups in schools except in two for community groups (Betancourt 2014; Dybdahl 2001), one for individuals (Barron 2013), and two that combined individual and group sessions (O'Callaghan 2013; O'Callaghan 2015). Interventions were psychological therapeutic interventions (Teaching Recovery Techniques/trauma recovery + CBT ‐ Barron 2013; Barron 2016), mind‐body techniques (Gordon 2008), therapeutic group discussions/coping skills (Dybdahl 2001), or a combination of psychoeducation and psychological and social interventions (Berger 2009; Betancourt 2014; O'Callaghan 2013; O'Callaghan 2015).
Group interventions varied from five sessions (Dybdahl 2001; Barron 2013; Barron 2016), to nine sessions (O'Callaghan 2013; O'Callaghan 2015), to 12 sessions (Berger 2009; Betancourt 2014; Gordon 2008), to 20 sessions delivered weekly for most studies (except for Gordon 2008 ‐ twice weekly for 6 weeks; and a 5‐day workshop ‐ Dybdahl 2001). Most interventions were manualised.
Comparators
No accessible care was described for most studies, and most included wait‐list controls (Barron 2013; Barron 2016; Berger 2009; Betancourt 2014; O'Callaghan 2013; O'Callaghan 2015), although the latter mentions that control group teachers received training for the intervention at baseline but were asked to implement it only one year later (risk of spillover effect). Only Dybdahl 2001 mentions available usual care (i.e. medical physical care provided by local physicians).
Appendix 17. Other economic studies of relevance but not included (from 2013 review and not updated)
Thirteen economic studies did not meet our inclusion criteria, as they did not relate to one of the included studies. Their findings are presented and compared with those included in this review to enhance the usefulness and applicability of the Cochrane Review for healthcare decision‐making. The economic questions addressed in excluded studies mainly fall into three broad categories in terms of cost analysis of specific disease conditions, carer and family burden, and comparison of improved or integrated mental health care with primary care with usual or no care.
Studies that looked at healthcare costs cannot be compared with included studies, as they were from different settings, conditions, and outcomes.
Health services costs
Chisolm 2000 dealt with integration of mental health services into primary health care in India and Pakistan and found that a significant category of healthcare costs consisted of consultations with GPs. In Luengo‐Fernandez 2011, primary care was costed in European middle‐income countries as constituting 36% (Portugal) and 9% (Greece) of total healthcare costs. There is no costing specific to NSHWs. One review showed that collaborative care costs are no greater than costs of usual care (Woltmann 2012). A community outreach intervention in rural India for untreated schizophrenia study found that the costs of informal care sector visits and family caregiving costs were considerably reduced during the follow‐up period from US$10 to about US$2 (Murthy 2005). This study gives detailed costs of outreach clinic setup, unit costs per person accessing services, and outcome data at intervention baseline and follow‐up to 18 months. It shows that costs of services increase over time (the increase in costs is for specialist outreach services ‐ not for PHC services), and that overall costs remain stable (around US$34). This study also emphasises the need for early diagnosis, and availability of services close to affected populations helps in increased uptake of services and reduces associated costs. The most promising study on service changes and costs is from South Africa, where Petersen 2012 estimated that the costs of a primary healthcare staffing package (one post for a mental health counsellor or equivalent, and 7.2 community mental health worker posts) would be offset by a reduction in the number of other specialist and non‐specialist health personnel required to close service gaps at a primary care level. The cost of these personnel amounts to GBP28,457 per 100,000 population.
Costs of specific interventions
Suh 2006 in a study on economic costs of dementia in Korea found that costs of care for dementia patients needing full‐time care in community (US$44,121) were about 10 times higher than for those who did not need long‐term care (US$3986), and that costs of informal care were very high, but it is unclear what costs related to NSHWs were. Another study dealt with societal costs of dementia (mainly informal costs) in both developed countries and LMICs but does not explicitly state the costs of an NSHW‐delivered service (Wimo 2007). The costs of providing epilepsy care through primary care in Zambia is estimated at under US$25 a day (Birbeck 2012).
Informal care costs
The high level of burden among family carers was also highlighted in other studies (Chisolm 2000; Murthy 2005; Papastavrou 2010; van Steenbergen‐Weijenburg 2010; Woltmann 2012), and this was significantly related to the severity and frequency of patient symptoms, patient gender, and educational level of the carer. Resource requirement analysis and resource use
Some studies have described the status of resource use. Chisolm 2000 showed a low level of service utilisation in government centres. Others attempt to calculate resource requirements. Scaling‐up specific interventions such as child and adolescent mental health services in their country context was done by modelling (Lund 2009), for different levels of coverage in South Africa. The model suggests most costs should be spent at a primary care level, with a range of NSHWs (occupational therapists, social workers, general nurses) and specialists (psychiatric nurses). However, this forecasted ideal situation is currently unrealistic due to budgetary constraints. Siskind 2010 estimated the cost‐effectiveness of usual care compared with improved primary care for depression in Chile using a computer‐based Markov cohort model. They found the incremental cost‐effectiveness ratio (ICER) of usual care CLP113 per quality‐adjusted life‐year (QALY) gained versus no treatment, whereas stepped care had an ICER of CLP468 per QALY versus usual care. A sensitivity analysis was performed, and the results were sensitive to assumptions made about recurrent episode coverage and cost of treatment, and were insensitive to changes in health state utility of depression and rate of recurrence.
We found one cost‐effectiveness study on a mental health intervention package in Nigeria (Gureje 2007), which estimated costs per DALYs averted for schizophrenia, depression, epilepsy, and alcohol use. The most cost‐effective intervention for schizophrenia was 70% coverage of antipsychotic drugs with psychosocial treatment or case management, with cost per DALY US$642 and US$680, respectively. Cost per DALY averted for depression was lowest for older antidepressant drugs, with psychotherapy at US$767. Similarly, for epilepsy, older antiepileptic drugs in primary care implemented at 80% coverage offered the best cost per DALY at US$100 per DALY averted. Random roadside breath‐testing for alcohol had a cost per DALY averted at US$85 (Gureje 2007). A systematic review that included two cost‐effectiveness studies in LMICs for costs of collaborative care showed these to be cost‐effective (van Steenbergen‐Weijenburg 2010).
Data and analyses
Comparison 1. Lay health worker‐led psychological interventions versus usual care in treating common mental disorders (CMDs).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Recovery from common mental disorders | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1.1 Short term (up to 1 month post intervention) | 1 | 222 | Risk Ratio (M‐H, Random, 95% CI) | 1.50 [1.04, 2.16] |
| 1.1.2 Intermediate (1 to 6 months post intervention) | 2 | 308 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [1.06, 1.56] |
| 1.1.3 Long term (> 6 months post intervention) | 1 | 493 | Risk Ratio (M‐H, Random, 95% CI) | 1.96 [1.34, 2.87] |
| 1.2 Prevalence of common mental disorders | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.2.1 Short term (up to 1 month post intervention) | 2 | 336 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.15, 1.77] |
| 1.2.2 Intermediate term (1 to 6 months post intervention) | 2 | 479 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.18, 0.96] |
| 1.3 Common mental disorder symptoms | 8 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.3.1 Short term (up to 1 month post intervention) | 4 | 717 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.93 [‐1.68, ‐0.18] |
| 1.3.2 Intermediate term (1 to 6 months post intervention) | 4 | 798 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐1.01, ‐0.16] |
| 1.3.3 Long term (> 6 months post intervention) | 1 | 493 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.49, ‐0.14] |
| 1.4 Quality of life | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.4.1 Intermediate term (1 to 6 months post intervention) | 1 | 521 | Std. Mean Difference (IV, Random, 95% CI) | 0.51 [0.34, 0.69] |
| 1.5 Functional impairment/disability | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.5.1 Short term (up to 1 month post intervention) | 2 | 659 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.24, 0.06] |
| 1.5.2 Intermediate term (1 to 6 months post intervention) | 3 | 1399 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐0.80, ‐0.15] |
| 1.5.3 Long term (> 6 months post intervention) | 1 | 493 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.34, 0.02] |
Comparison 2. Primary‐level worker‐led collaborative care versus usual care in treating common mental disorders in adults.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Recovery from common mental disorders | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1.1 Short term (up to 1 month post intervention) | 5 | 781 | Risk Ratio (M‐H, Random, 95% CI) | 3.75 [1.68, 8.34] |
| 2.1.2 Intermediate term (1 to 6 months post intervention) | 5 | 804 | Risk Ratio (M‐H, Random, 95% CI) | 2.26 [1.50, 3.43] |
| 2.1.3 Long term (> 6 months post intervention) | 3 | 922 | Risk Ratio (M‐H, Random, 95% CI) | 2.65 [0.70, 9.98] |
| 2.2 Prevalence of common mental disorders | 2 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 2.2.1 Intermediate term (1 to 6 months post intervention) | 2 | 2820 | Risk Ratio (IV, Random, 95% CI) | 0.57 [0.32, 1.01] |
| 2.2.2 All facilities medium term (at 1 year post intervention) | 1 | 2009 | Risk Ratio (IV, Random, 95% CI) | 0.95 [0.68, 1.33] |
| 2.3 Common mental disorder symptoms | 12 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.3.1 Short term (up to 1 month post intervention) ‐ change scores | 2 | 118 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.23 [‐1.68, ‐0.79] |
| 2.3.2 Intermediate term (1 to 6 months post intervention) ‐ change scores | 6 | 4419 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐0.63, ‐0.08] |
| 2.3.3 Long term (> 6 months post intervention) ‐ change scores | 4 | 3386 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.76 [‐1.50, ‐0.03] |
| 2.3.4 Short term (up to 1 month post intervention) ‐ endpoint data | 1 | 38 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.87 [‐1.55, ‐0.19] |
| 2.3.5 Intermediate term (1 to 6 months post intervention) ‐ endpoint data | 3 | 548 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.50, ‐0.09] |
| 2.4 Quality of life | 8 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.4.1 Short term (up to 1 month post intervention) | 2 | 249 | Std. Mean Difference (IV, Random, 95% CI) | 0.73 [0.47, 0.99] |
| 2.4.2 Intermediate term (1 to 6 months post intervention) | 6 | 2199 | Std. Mean Difference (IV, Random, 95% CI) | 0.34 [0.16, 0.53] |
| 2.4.3 Long term (> 6 months post intervention) | 2 | 711 | Std. Mean Difference (IV, Random, 95% CI) | 0.96 [‐0.78, 2.69] |
| 2.5 Functional impairment | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.5.1 Intermediate term (1 to 6 months post intervention) ‐ endpoint data | 5 | 4216 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.28, 0.03] |
| 2.5.2 Long term (> 6 months post intervention) ‐ endpoint data | 3 | 2591 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.56, 0.13] |
Comparison 3. Lay health worker‐led psychosocial interventions versus usual care in treating perinatal depression.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Recovery from depression | 5 | 2841 | Risk Ratio (IV, Random, 95% CI) | 1.29 [1.14, 1.46] |
| 3.1.1 Short term (up to 1 month post intervention) | 2 | 679 | Risk Ratio (IV, Random, 95% CI) | 1.19 [1.08, 1.31] |
| 3.1.2 Intermediate term (1 to 6 months post intervention) | 4 | 1243 | Risk Ratio (IV, Random, 95% CI) | 1.29 [1.08, 1.54] |
| 3.1.3 Long term (> 6 months post intervention) | 3 | 919 | Risk Ratio (IV, Random, 95% CI) | 1.39 [0.94, 2.06] |
| 3.2 Depression symptoms | 7 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.2.1 Short term (up to 1 month post intervention) | 5 | 1062 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.51, ‐0.11] |
| 3.2.2 Intermediate term (1 to 6 months post intervention) | 5 | 1989 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.37, ‐0.14] |
| 3.2.3 Long term (> 6 months post intervention) | 3 | 1274 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐0.53, ‐0.16] |
| 3.3 Functional impairment | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.3.1 Short term (up to 1 month post intervention) | 3 | 966 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.35, ‐0.10] |
| 3.3.2 Intermediate term (1 to 6 months post intervention) | 4 | 1856 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.41, ‐0.04] |
| 3.3.3 Long term (> 6 months post intervention) | 2 | 1116 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.86, 0.34] |
| 3.4 Adverse events | 4 | Other data | No numeric data |
Comparison 4. Primary health professional‐led collaborative interventions versus enhanced usual care in treating perinatal depression.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Recovery from depression | 1 | Odds Ratio (IV, Random, 95% CI) | Subtotals only | |
| 4.1.1 Short term (up to 1 month post intervention) | 1 | 576 | Odds Ratio (IV, Random, 95% CI) | 0.77 [0.47, 1.25] |
| 4.2 Depression symptoms | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.2.1 Short term (up to 1 month post intervention) | 2 | 806 | Mean Difference (IV, Random, 95% CI) | ‐2.44 [‐5.86, 0.99] |
| 4.2.2 Intermediate term (1 to 6 months post intervention) | 1 | 230 | Mean Difference (IV, Random, 95% CI) | ‐1.60 [‐3.49, 0.29] |
| 4.2.3 Long term (> 6 months post intervention) | 1 | 686 | Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐1.70, ‐0.10] |
| 4.3 Quality of life | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.3.1 Short term (up to 1 month post intervention) | 1 | 230 | Mean Difference (IV, Random, 95% CI) | 18.30 [10.42, 26.18] |
| 4.3.2 Intermediate term (1 to 6 months post intervention) | 1 | 230 | Mean Difference (IV, Random, 95% CI) | 3.50 [‐4.55, 11.55] |
| 4.4 Functional impairment | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.4.1 Short term (up to 1 month post intervention) | 1 | 686 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐1.10, ‐0.10] |
| 4.4.2 Long term (> 6 months post intervention) | 1 | 686 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.70, 0.30] |
| 4.5 Adverse events | 1 | Other data | No numeric data |
Comparison 5. Lay health worker‐led psychological interventions versus usual care in adults with post‐traumatic stress or common mental disorders in humanitarian settings.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Prevalence of PTSD | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1.1 Intermediate term (1 to 6 months post intervention) | 2 | 380 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.30, 2.00] |
| 5.1.2 Neuner second arm (1 to 6 months post intervention) | 1 | 65 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.33, 0.93] |
| 5.2 Post‐traumatic stress symptoms | 13 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.2.1 Short term (up to 1 month post intervention) | 10 | 2536 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.49, ‐0.28] |
| 5.2.2 Intermediate term (1 to 6 months post intervention) | 5 | 2045 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.41, ‐0.13] |
| 5.2.3 Short term (< 1 month post intervention) ‐ change scores | 2 | 174 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.52 [‐7.08, 2.04] |
| 5.3 Depression symptoms | 13 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.3.1 Short term (up to 1 month post intervention) | 10 | 2510 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐1.04, ‐0.46] |
| 5.3.2 Intermediate term (1 to 6 months post intervention) | 5 | 1986 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐0.56, ‐0.15] |
| 5.3.3 Short term (up to 1 month post intervention) ‐ change scores | 2 | 174 | Std. Mean Difference (IV, Random, 95% CI) | ‐3.51 [‐8.90, 1.87] |
| 5.4 Anxiety symptoms | 7 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.4.1 Short term (up to 1 month post intervention) | 5 | 1326 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.95 [‐1.44, ‐0.46] |
| 5.4.2 Intermediate term (1 to 6 months post intervention) | 2 | 883 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐1.02, ‐0.02] |
| 5.4.3 Short term (up to 1 month post intervention) ‐ change scores | 2 | 128 | Std. Mean Difference (IV, Random, 95% CI) | ‐3.34 [‐8.47, 1.78] |
| 5.5 Emotional distress symptoms | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.5.1 Short term (up to 1 month post intervention) | 3 | 1104 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.57, ‐0.33] |
| 5.5.2 Intermediate term (1 to 6 months post intervention) | 2 | 1034 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.34, ‐0.10] |
| 5.5.3 Short term (up to 1 month post intervention) ‐ change scores | 1 | 29 | Std. Mean Difference (IV, Random, 95% CI) | ‐6.86 [‐8.92, ‐4.80] |
| 5.6 Quality of life | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.6.1 Short term (up to 1 month post intervention) | 4 | 1745 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐0.63, ‐0.31] |
| 5.6.2 Intermediate term (1 to 6 months post intervention) | 4 | 1918 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.39, ‐0.15] |
| 5.7 Functional impairment | 7 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.7.1 Short term (up to 1 month post intervention) | 7 | 2357 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.56, ‐0.25] |
| 5.7.2 Intermediate term (1 to 6 months post intervention) | 4 | 1914 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.42, ‐0.10] |
| 5.8 Service utilisation (dichotomous outcomes) | 2 | Odds Ratio (IV, Random, 95% CI) | Subtotals only | |
| 5.8.1 Hospital admissions | 1 | 319 | Odds Ratio (IV, Random, 95% CI) | 1.13 [0.52, 2.46] |
| 5.8.2 Seeking assistance for mental health | 1 | 71 | Odds Ratio (IV, Random, 95% CI) | 0.35 [0.13, 0.94] |
| 5.9 Service utilisation (continuous outcomes) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.9.1 Outpatient consultations | 1 | 421 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.59, 0.53] |
| 5.9.2 Medication use | 1 | 421 | Mean Difference (IV, Random, 95% CI) | 0.29 [‐0.54, 1.12] |
| 5.9.3 Traditional healer engagement | 1 | 421 | Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.03, 0.27] |
| 5.10 Adverse events | 5 | Other data | No numeric data |
5.9. Analysis.

Comparison 5: Lay health worker‐led psychological interventions versus usual care in adults with post‐traumatic stress or common mental disorders in humanitarian settings, Outcome 9: Service utilisation (continuous outcomes)
5.10. Analysis.
Comparison 5: Lay health worker‐led psychological interventions versus usual care in adults with post‐traumatic stress or common mental disorders in humanitarian settings, Outcome 10: Adverse events
| Adverse events | |
| Study | Heading 1 |
| Bryant 2017 | No reported adverse effects occurred during treatment. |
| Dawson 2016 | No serious adverse events were reported during the course of treatment or at posttreatment assessment. |
| Meffert 2014 | There were no adverse events. One participant withdrew because her husband forbade her to continue. One dropped out secondary to time constraints. |
| Rahman 2019 | No adverse events were recorded |
| Tol 2020 | With regard to safety considerations, the independent data safety management board responded to six adverse events, and none were evaluated to be concerns in response to the intervention. |
Comparison 6. Primary health professional‐ and community professional‐led psychological interventions versus usual or no care in adults with post‐traumatic stress or common mental disorders in humanitarian settings.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Prevalence of PTSD | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 6.1.1 Short term (up to 1 month post intervention) | 1 | 270 | Risk Ratio (IV, Random, 95% CI) | 12.30 [5.20, 29.10] |
| 6.1.2 Intermediate term (1 to 6 months post intervention) | 1 | 313 | Risk Ratio (IV, Random, 95% CI) | 5.50 [2.50, 12.10] |
| 6.2 Prevalence of probable depression or anxiety | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 6.2.1 Short term (up to 1 month post intervention) | 1 | 270 | Risk Ratio (IV, Random, 95% CI) | 7.30 [3.40, 15.68] |
| 6.2.2 Intermediate term (1 to 6 months post intervention) | 1 | 313 | Risk Ratio (IV, Random, 95% CI) | 4.60 [2.10, 10.08] |
| 6.3 Post‐traumatic stress symptoms | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.3.1 Short term (up to 1 month post intervention) | 4 | 1107 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.71 [‐1.11, ‐0.30] |
| 6.3.2 Intermediate term (1 to 6 months post intervention) | 2 | 680 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐1.43, ‐0.13] |
| 6.4 Depression symptoms | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.4.1 Short term (up to 1 month post intervention) | 4 | 1107 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.81 [‐1.36, ‐0.26] |
| 6.4.2 Intermediate term (1 to 6 months post intervention) | 2 | 680 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.91 [‐1.73, ‐0.10] |
| 6.5 Anxiety symptoms | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.5.1 Short term (up to 1 month post intervention) | 3 | 837 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.54, ‐0.24] |
| 6.5.2 Intermediate term (1 to 6 months post intervention) | 1 | 367 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐1.37, 0.03] |
| 6.6 Functional impairment | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.6.1 Short term (up to 1 month post intervention) | 4 | 1107 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐1.01, ‐0.30] |
| 6.6.2 Intermediate term (1 to 6 months post intervention) | 2 | 680 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐1.31, 0.04] |
| 6.7 Adverse events | 2 | Other data | No numeric data |
6.7. Analysis.
Comparison 6: Primary health professional‐ and community professional‐led psychological interventions versus usual or no care in adults with post‐traumatic stress or common mental disorders in humanitarian settings, Outcome 7: Adverse events
| Adverse events | |
| Study | Heading 1 |
| Bolton 2014 (Iraq) | One CPT and 2 BATD participants left to seek psychiatric help. One CPT participant moved away, one was referred for psychosis, and one left after being verbally abused by her husband for getting treatment. One control was referred to a psychiatrist for worsening symptoms. |
| Weiss 2015 | One client attempted suicide after doing the intake and the first therapy session. Another client was hospitalised with severe depression, one patient died from a heart attack. |
Comparison 7. Lay health worker‐led interventions versus enhanced usual care in adult patients with harmful or hazardous alcohol or substance use.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Clinical recovery ‐ harmful or hazardous alcohol use | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1.1 Short term (up to 1 month post intervention) | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 2.55 [1.34, 4.88] |
| 7.1.2 Intermediate term (1 to 6 months post intervention) | 4 | 872 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.94, 1.74] |
| 7.1.3 Long term (> 6 months post intervention) | 2 | 477 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [1.05, 2.10] |
| 7.2 Prevalence ‐ drug use | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.2.1 Intermediate term (1 to 6 months post intervention) | 1 | 882 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.91, 1.13] |
| 7.3 Clinical symptoms ‐ alcohol use (up to 1 month post intervention) | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.3.1 Amount of alcohol consumed | 2 | 684 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.52, ‐0.22] |
| 7.4 Clinical symptoms ‐ alcohol use (1 to 6 months post intervention) | 6 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.4.1 Amount of alcohol consumed | 3 | 781 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.56, 0.09] |
| 7.4.2 Risk of hazardous or harmful drinking | 3 | 667 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.32, ‐0.11] |
| 7.5 Clinical symptoms ‐ alcohol use (> 6 months post intervention) | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.5.1 Amount of alcohol consumed | 2 | 930 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.29, 0.06] |
| 7.5.2 Risk of hazardous or harmful drinking | 1 | 162 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.44, 0.18] |
| 7.6 Clinical symptoms ‐ drug and alcohol use | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.6.1 Intermediate term (1 to 6 months post intervention) | 2 | 540 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.15, 0.13] |
| 7.7 Depression symptoms | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.7.1 Intermediate term (1 to 6 months post intervention) | 1 | 335 | Mean Difference (IV, Random, 95% CI) | ‐2.12 [‐6.42, 2.18] |
| 7.7.2 Long term (> 6 months post intervention) | 1 | 415 | Mean Difference (IV, Random, 95% CI) | ‐2.20 [‐4.03, ‐0.37] |
| 7.8 Functional impairment | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.8.1 Intermediate term (1 to 6 months post intervention) | 2 | 498 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.32, 0.03] |
| 7.8.2 Long term (> 6 months post intervention) | 2 | 478 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.28, 0.08] |
| 7.9 Service utilisation ‐ unplanned admissions to hospital | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.10 Service utilisation ‐ healthcare visits | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.11 Adverse events | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.11.1 Serious adverse events | 1 | 336 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.45, 1.19] |
| 7.11.2 Deaths | 3 | 1025 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.10, 1.18] |
| 7.11.3 Suicide attempts | 1 | 336 | Risk Ratio (M‐H, Random, 95% CI) | 0.15 [0.01, 2.88] |
| 7.11.4 Perpetration of intimate partner violence | 1 | 267 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.41, 1.68] |
Comparison 8. Primary health professional‐ and community professional‐led interventions versus enhanced usual care in adult patients with harmful or hazardous alcohol or substance use.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Clinical recovery ‐ harmful or hazardous alcohol or drug use | 3 | 2150 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.80, 1.02] |
| 8.1.1 Recovery from harmful or hazardous alcohol use ‐ Intermediate term (1 to 6 months post intervention) | 3 | 1075 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.77, 1.12] |
| 8.1.2 Recovery from harmful or hazardous alcohol use ‐ Long term (> 6 months post intervention) | 2 | 712 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.73, 1.06] |
| 8.1.3 Recovery from at‐risk cannabis use ‐ Intermediate term (1 to 6 months post intervention) | 1 | 363 | Risk Ratio (M‐H, Random, 95% CI) | 1.82 [0.63, 5.22] |
| 8.2 Prevalence ‐ cannabis drug use (dichotomous data) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.2.1 Intermediate term (1 to 6 months post intervention) | 1 | 152 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.67, 1.80] |
| 8.3 Clinical symptoms ‐ alcohol use (up to 1 month post intervention) | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.3.1 Risk of harmful or hazardous drinking | 1 | 179 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐0.76, ‐0.16] |
| 8.4 Clinical symptoms ‐ alcohol use (1‐6 months post intervention) | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.4.1 Risk of harmful or hazardous drinking | 3 | 1075 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.27, ‐0.03] |
| 8.4.2 Amount of alcohol consumed | 1 | 92 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐0.94, ‐0.10] |
| 8.5 Clinical symptoms ‐ alcohol use (> 6 months post intervention) | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.5.1 Risk of harmful or hazardous drinking | 2 | 712 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.32, 0.55] |
| 8.6 Clinical symptoms ‐ drug and alcohol use (1 to 6 months post intervention) | 2 | 705 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.35, ‐0.05] |
| 8.6.1 Intermediate term (1 to 6 months post intervention) | 2 | 705 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.35, ‐0.05] |
| 8.7 Clinical symptoms ‐ cannabis drug use (continuous data) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.7.1 Intermediate term (1 to 6 months post intervention) | 1 | 363 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐1.34, 0.14] |
| 8.8 Depression symptoms | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.8.1 Long term (> 6 months post intervention) | 2 | 712 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.70, 0.42] |
| 8.9 Quality of life | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.9.1 Intermediate term (1 to 6 months post intervention) | 1 | 560 | Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.10, 0.10] |
Comparison 9. Lay health worker‐ and primary health professional‐led interventions versus enhanced usual care in adult patients with alcohol dependence.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Clinical recovery ‐ dependent alcohol use | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1.1 Intermediate term (1 to 6 months post intervention) | 1 | 121 | Risk Ratio (M‐H, Random, 95% CI) | 1.87 [0.90, 3.90] |
| 9.1.2 Long term (> 6 months post intervention) | 1 | 112 | Risk Ratio (M‐H, Random, 95% CI) | 1.68 [0.85, 3.30] |
| 9.2 Clinical symptoms ‐ alcohol use | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.2.1 Intermediate term (1 to 6 months post intervention) | 1 | 121 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐21.60, 21.00] |
| 9.2.2 Long term (> 6 months post intervention) | 1 | 112 | Mean Difference (IV, Random, 95% CI) | ‐15.20 [‐30.51, 0.11] |
| 9.3 Depression symptoms | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.3.1 Intermediate term (1 to 6 months post intervention) | 1 | 121 | Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐2.68, 1.68] |
| 9.3.2 Long term (> 6 months post intervention) | 1 | 112 | Mean Difference (IV, Random, 95% CI) | ‐1.80 [‐4.21, 0.61] |
| 9.4 Functional impairment | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.4.1 Intermediate term (1 to 6 months post intervention) | 1 | 121 | Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐3.43, 1.63] |
| 9.4.2 Long term (> 6 months post intervention) | 1 | 112 | Mean Difference (IV, Random, 95% CI) | ‐3.30 [‐6.22, ‐0.38] |
| 9.5 Service utilisation | 1 | 112 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.26, 1.88] |
| 9.5.1 Unplanned hospitalisation in past 12 months | 1 | 112 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.26, 1.88] |
| 9.6 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.6.1 Death in past 12 months | 1 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.01, 7.70] |
| 9.6.2 Suicidal behaviour at 3 months | 1 | 121 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.49, 2.86] |
| 9.6.3 Suicidal behaviour at 12 months | 1 | 112 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.34, 1.69] |
9.6. Analysis.

Comparison 9: Lay health worker‐ and primary health professional‐led interventions versus enhanced usual care in adult patients with alcohol dependence, Outcome 6: Adverse events
Comparison 10. Primary health professional‐ and community professional‐led interventions versus enhanced usual care in adult patients with substance dependence.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 Prevalence ‐ drug use (up to 1 month post intervention) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1.1 Morphine | 1 | 173 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.63, 1.80] |
| 10.1.2 Methamphetamine | 1 | 173 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.42, 2.91] |
| 10.2 Clinical symptoms ‐ alcohol use | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.2.1 Short term (up to 1 month post intervention) | 1 | 155 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.91, 1.11] |
| 10.3 Clinical symptoms ‐ drug use (up to 1 month post intervention) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.3.1 Heroin | 1 | 155 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.22, 0.16] |
| 10.3.2 Amphetamine | 1 | 155 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.04, 0.04] |
| 10.4 Clinical symptoms ‐ drug avoidance self‐efficacy | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.4.1 Intermediate term (1 to 6 months post intervention) | 1 | 900 | Mean Difference (IV, Random, 95% CI) | 1.21 [0.54, 1.88] |
| 10.4.2 Long term (> 6 months post intervention) | 1 | 900 | Mean Difference (IV, Random, 95% CI) | 1.38 [0.71, 2.05] |
| 10.5 Clinical symptoms ‐ mental health status | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.5.1 Short term (up to 1 month post intervention) | 1 | 155 | Mean Difference (IV, Random, 95% CI) | ‐4.23 [‐13.66, 5.20] |
| 10.6 Quality of life (up to 1 month post intervention) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.6.1 Physical functioning | 1 | 155 | Mean Difference (IV, Random, 95% CI) | ‐6.78 [‐12.69, ‐0.87] |
| 10.6.2 Social functioning | 1 | 155 | Mean Difference (IV, Random, 95% CI) | 48.36 [41.80, 54.92] |
| 10.6.3 Emotional role functioning | 1 | 155 | Mean Difference (IV, Random, 95% CI) | ‐1.75 [‐12.53, 9.03] |
Comparison 11. Lay health worker‐led interventions versus specialist‐led care for adults with severe mental disorders.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 11.1 Schizophrenia symptoms | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 11.1.1 Short term (up to 1 month post intervention) | 2 | 364 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.15 [‐3.01, 0.70] |
| 11.2 Depression symptoms | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 11.2.1 Short term (up to 1 month post intervention) | 1 | 121 | Mean Difference (IV, Random, 95% CI) | ‐12.69 [‐15.12, ‐10.26] |
| 11.3 Functional impairment | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 11.3.1 Short term (up to 1 month post intervention) | 2 | 364 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.53 [‐4.17, 1.11] |
| 11.4 Anxiety | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 11.4.1 Short term (up to 1 month post intervention) | 1 | 111 | Mean Difference (IV, Random, 95% CI) | ‐12.23 [‐14.54, ‐9.92] |
Comparison 12. Primary health professional‐led or collaborative care versus specialist‐led care for people with severe mental disorders.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 12.1 Clinical recovery from severe mental disorders | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.81, 1.44] |
| 12.1.1 Short term (up to 1 month post intervention) | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.81, 1.44] |
| 12.2 Relapse of severe mental disorders | 4 | 492 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.16, 0.55] |
| 12.2.1 Short term (up to 1 month post intervention) | 4 | 492 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.16, 0.55] |
| 12.3 Schizophrenia symptoms | 6 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 12.3.1 Short term (up to 1 month post intervention) | 6 | 836 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.71, 0.11] |
| 12.4 Depression symptoms | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 12.4.1 Short term (up to 1 month post intervention) | 2 | 270 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐1.13, 0.32] |
| 12.5 Quality of life | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 12.5.1 Short term (up to 1 month post intervention) | 3 | 536 | Std. Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.37, 1.17] |
| 12.6 Functional impairment | 7 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 12.6.1 Short term (up to 1 month post intervention) | 7 | 874 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.13 [‐1.78, ‐0.47] |
| 12.7 Service utilisation ‐ hospital re‐admission (1 year from randomisation) | 3 | 441 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.28, 1.28] |
Comparison 13. Primary health professional‐ and lay health worker‐led psychosocial interventions versus usual care in improving dementia patients' and carers' outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 13.1 Behavioural problem symptoms | 2 | 134 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.60, 0.08] |
| 13.1.1 Intermediate term (1 to 6 months post intervention) | 2 | 134 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.60, 0.08] |
| 13.2 Quality of life (patient) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 13.2.1 Intermediate term (1 to 6 months post intervention) | 1 | 53 | Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.98, 0.12] |
| 13.3 Functional impairment (patient) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 13.3.1 Intermediate term (1 to 6 months post intervention) | 1 | 81 | Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.67, 0.20] |
| 13.4 Carer distress | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 13.4.1 Intermediate term (1 to 6 months post intervention) | 2 | 134 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐0.82, ‐0.13] |
| 13.5 Carer mental health status | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 13.5.1 Intermediate term (1 to 6 months post intervention) | 2 | 134 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐0.76, ‐0.08] |
| 13.6 Carer burden | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 13.6.1 Intermediate term (1 to 6 months post intervention) | 2 | 134 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐0.84, ‐0.15] |
| 13.7 Carer quality of life | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 13.7.1 Intermediate term (1 to 6 months post intervention) | 1 | 53 | Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.92, 0.17] |
Comparison 14. Lay health worker‐led psychosocial interventions versus usual or no care in child post‐traumatic stress or common mental disorders in humanitarian settings.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 14.1 Post‐traumatic stress symptoms | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 14.1.1 Short term (up to 1 month post intervention) | 2 | 416 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.77 [‐1.48, ‐0.06] |
| 14.1.2 Intermediate term (1 to 6 months post intervention) | 1 | 77 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.65, 0.28] |
| 14.1.3 Long term (> 6 months post intervention) | 1 | 76 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.72, 0.22] |
| 14.2 Post‐traumatic stress symptoms (change scores) | 1 | 145 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.78, ‐0.12] |
| 14.2.1 Short term (up to 1 month post intervention) ‐ change scores | 1 | 145 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.78, ‐0.12] |
| 14.3 Post‐traumatic stress symptoms | 3 | Mean Change Difference (IV, Random, 95% CI) | Subtotals only | |
| 14.3.1 Intermediate term (1 to 6 months post intervention) | 3 | 1090 | Mean Change Difference (IV, Random, 95% CI) | ‐1.34 [‐2.83, 0.14] |
| 14.4 Depression symptoms | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 14.4.1 Short term (up to 1 month post intervention) | 1 | 1162 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.12, 0.11] |
| 14.4.2 Intermediate term (1 to 6 months post intervention) | 1 | 77 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.47, 0.46] |
| 14.4.3 Long term (> 6 months post intervention) | 1 | 76 | Std. Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.22, 0.72] |
| 14.5 Depression symptoms | 4 | Mean Change Difference (IV, Random, 95% CI) | Subtotals only | |
| 14.5.1 Short term (up to 1 month post intervention) | 2 | 421 | Mean Change Difference (IV, Random, 95% CI) | ‐4.55 [‐12.64, 3.54] |
| 14.5.2 Bolton second arm (up to 1 month post intervention) | 1 | 209 | Mean Change Difference (IV, Random, 95% CI) | ‐2.51 [‐11.42, 6.40] |
| 14.5.3 Intermediate term (1 to 6 months post intervention) | 3 | 1092 | Mean Change Difference (IV, Random, 95% CI) | ‐0.61 [‐1.23, 0.02] |
| 14.6 Anxiety symptoms | 4 | Mean Change Difference (IV, Random, 95% CI) | Subtotals only | |
| 14.6.1 Short term (up to 1 month post intervention) | 2 | 425 | Mean Change Difference (IV, Random, 95% CI) | ‐1.14 [‐2.94, 0.65] |
| 14.6.2 Intermediate term (1 to 6 months post intervention) | 3 | 1092 | Mean Change Difference (IV, Random, 95% CI) | ‐0.34 [‐0.75, 0.07] |
| 14.7 Functional impairment | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 14.7.1 Short term (up to 1 month post intervention) | 3 | 625 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐1.08, 0.12] |
| 14.7.2 Intermediate term (1 to 6 months post intervention) | 1 | 77 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.55, 0.38] |
| 14.7.3 Long term (> 6 months post intervention) | 1 | 76 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐0.86, 0.13] |
| 14.8 Functional impairment | 3 | Mean Change Difference (IV, Random, 95% CI) | Subtotals only | |
| 14.8.1 Short term (up to 1 month post intervention) | 1 | 104 | Mean Change Difference (IV, Random, 95% CI) | ‐2.19 [‐4.22, ‐0.16] |
| 14.8.2 Intermediate term (1 to 6 months post intervention) | 3 | 1092 | Mean Change Difference (IV, Random, 95% CI) | ‐0.81 [‐1.48, ‐0.13] |
Comparison 15. Community professional‐led psychosocial interventions versus no care in child post‐traumatic stress or common mental disorders in humanitarian settings.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 15.1 Recovery from PTSD | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 15.1.1 Short term (up to 1 month post intervention) | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 3.93 [1.31, 11.80] |
| 15.2 Post‐traumatic stress symptoms | 6 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 15.2.1 Short term (up to 1 month post intervention) | 5 | 753 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.10 [‐1.83, ‐0.38] |
| 15.2.2 Intermediate term (1 to 6 months post intervention) | 3 | 679 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐1.20, 0.47] |
| 15.3 Depression symptoms | 6 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 15.3.1 Short term (up to 1 month post intervention) | 5 | 750 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.57 [‐1.13, 0.00] |
| 15.3.2 Intermediate term (1 to 6 months post intervention) | 2 | 602 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.57, 0.19] |
| 15.4 Functional impairment | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 15.4.1 Short term (up to 1 month post intervention) | 4 | 623 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.55, ‐0.03] |
| 15.4.2 Intermediate term (1 to 6 months post intervention) | 2 | 602 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.99, 0.21] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abas 2018.
| Study characteristics | ||
| Methods |
Study design: RCT Duration of study: recruitment of subjects September to November 2014, January 2015. Intervention for 6 weeks. Follow‐up at 6 months |
|
| Participants |
Country: Zimbabwe Income classification: low‐income country in 2014‐2015 Geographical scope: Parirenyatwa Hospital Family Care Centre (PHFCC), a government clinic affiliated with the University of Zimbabwe College of Health Sciences (UZCHS); urban (Harare) Healthcare setting: PC facility Mental health condition: depression Population 1. Age ≥ 18 years 2. Gender: both 3. Socioeconomic background: not mentioned 4. Inclusion criteria a. Aged 18 or over b. On ART for HIV for at least 4 months; risk of poor adherence as indicated by any one of the following (1) Missed at least 1 clinic appointment in the last 3 months (2) Falling CD4 count or detectable viral load in previous 6 months (3) Self‐report of poor adherence (admitting to having missed 1 or more doses, taking treatment late, or being forgetful with treatment) c. Screen positive for at least mild depression, as defined by scoring at least 5 on the Patient Health Questionnaire and/or at least 9 on the Shona Symptom Questionnaire 5. Exclusion criteria: a. Score < 6 on International HIV Dementia Scale (IHDS) b. Score ≥ 3 for women and ≥ 4 for men on short AUDIT‐C screen for alcohol dependence c. On medication for tuberculosis d. Suicidal intent as determined by P4 Suicidality Screener e. Interviewer assessment of person too unwell/agitated to take part |
|
| Interventions |
Stated purpose: aims of this feasibility study were to measure acceptability of the PST‐AD intervention for participants and for clinic staff; to test methods that would inform a future randomised trial; and to gather data to inform a sample size calculation for a future trial INTERVENTION (n = 18) Name: Problem‐Solving Therapy for Depression and HIV Medication Adherence (PST‐AD), locally named TENDAI and Stepped Care Delivered by LHW Title/name of PW and number: adherence counsellor (ADC) (n = 1) 1. Selection: counselling is provided by ADCs. This cadre usually consists of primary care HIV counsellors or nurse aides 2. Educational background: secondary school education and 6 months of training in HIV/AIDS basic counselling 3. Training: PI (psychiatrist) trained the first local psychologist (A.C.), who has been working in HIV clinical research since 1998. PI and psychologist (A.C.) introduced LifeSteps to the second psychologist (T.B.), who, together with C.O., S.A.S., and J.F.M., worked on preparations for local training. C.O. and J.F.M. carried out the first training over 2.5 days in English to the second psychologist and to adherence counsellors. This comprised approximately 30% didactic and 70% skill‐based work. The 2 bilingual local psychologists (A.C. and T.B.) who had been involved in the formative work conducted a further 2 days' training in Shona comprising 20% didactic and 80% skill‐based work. From then on, the local psychologist (T.B.) led supervision of adherence counsellors, with mainly Skype supervision from experts based in Boston 4. Supervision: counsellor met weekly with psychologist to discuss her caseload 5. Incentives/remuneration: employed at clinic Intervention details 1. Duration/frequency: 6 weekly sessions 2. Content of intervention: session 1: motivational and informational and behavioural steps; sessions 2 to 6: problem‐solving therapy; if SSQ‐14 > 8 by session 4, stepped up to psychologist‐led counselling (Session 5) ± referral to psychiatrist for antidepressants (Session 6) CONTROL: enhanced usual care (EUC) (n = 14) Four ADCs who had not been trained in the PST‐AD intervention delivered the EUC. We enhanced usual care by (1) increasing usual care from 1 session only, to 1 session a month for 3 months, and (2) ensuring continuity of the same counsellor for all sessions. ADCs providing EUC were asked to repeat the information they normally provide. They were not trained in the local version of LifeSteps CO‐INTERVENTIONS: nil |
|
| Outcomes |
Patients 1. Viral suppression 2. Missed clinic appointments 3. Depression Shona Symptom Questionnaire Score 4. PHQ‐9 5. Electronically measured ART adherence 6. Self‐reported missed dose in the last month Carers Nil Process/health workers 1. Switched to second‐ or third‐line regimen 2. Missed at least 1 HIV care appointment in last 3 months 3. Number of missed HIV care appointments in last 3 months Economic outcomes Nil Time points: baseline, 6 months (post intervention) |
|
| Notes |
Source of funding: NIMH R21 Grant Notes on validation of instruments (screening and outcomes): all validated Additional information: declarations of interest ‐ Dr. Steven Safren received royalties from Oxford University Press and Guilford Publications for books on treatments for psychological difficulties. All other study authors reported no conflicts of interest Handling the data: as per footnotes in data and analysis Prospective trial registration number: PACTR20151100115030 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomisation was conducted by participants selecting one numbered card at random from a bag; each number had been pre‐allocated to either the intervention or EUC arm" Judgement comment: adequate random sequence generation |
| Allocation concealment (selection bias) | Low risk | Quote: "after giving written informed consent, patients were randomised in a 1:1 ratio to intervention or EUC arms" Judgement comment: allocation by patient |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Participants were at least partly blinded to their group allocation as both arms were offered extra sessions of counselling, which is different from standard care" |
| Blinding of outcome assessment (detection bias) all outcomes | Unclear risk | "We piloted independent assessment of follow‐up data on self‐report outcomes of depression and adherence in 25% of participants, but resources precluded doing this for all participants"; "quantitative analysis was performed by two independent researchers blinded to the content of the sessions" |
| Baseline outcome measurements similar | Low risk | From Table 3, baseline outcomes similar for depression and viral suppression |
| Baseline characteristics similar? | Unclear risk | Many more unemployed in the intervention arm (57%) than in the usual care arm (28%). This may be significant; unclear risk |
| Incomplete outcome data (attrition bias) Efficacy data | Unclear risk | Low dropout (Fig. 2: 13/14 followed up in intervention group; 15/18 in control group) |
| Protection against contamination | High risk | Quote: "further contamination was revealed through interviewing the ADC who delivered the PST‐AD intervention. It emerged she had given two sessions of PST‐AD to one EUC arm participant and one session each to 6 EUC arm participants because EUC counsellors referred these clients to her after their EUC sessions due to concerns about the participants’ psychosocial issues" Judgement comment: visible contamination happened as control participants referred to take part in the intervention group |
| Selective reporting (reporting bias) | Low risk | Same outcomes as protocol |
| Other bias | Low risk | No other risks of bias were found |
Adewuya 2019.
| Study characteristics | ||
| Methods |
Study design: cluster‐RCT Unit of randomisation: comprehensive primary health care centres (CPHCs) Duration of study: February 2013 to December 2016. Recruitment from October 2014 to April 2015. Intervention lasted 14 weeks. Trial ended at 12‐month follow‐up |
|
| Participants |
Country: Nigeria Income classification: low‐middle income from 2014 to 2016 Geographical scope: Lagos; 10 CPHCs (6 from rural and 4 from urban settings) Healthcare setting: primary health centre Mental health condition: depression Population: adults with diagnosed depression attending 10 primary health centres (PHCs) in Lagos, Nigeria 1. Age 18 to 60 years 2. Gender: both 3. Socioeconomic background: urban and rural mixed 4. Inclusion criteria a. Adults aged 18 to 60 years b. Scoring > 10 on PHQ‐9 or persistent score > 5 but < 10 on PHQ‐9 for 2 weeks c. Intent to stay in the project area for at least 18 months d. Literate enough to read English, pidgin English, or any of the 3 local languages (Yoruba, Hausa, and Igbo) e. Completed written informed consent form 5. Exclusion criteria a. Children (below 18 years of age) and the elderly (above 60 years of age) b. Serious medical condition or disability necessitating specialist care c. Presently having any form of psychosis or under psychiatric care or showing suicidal ideation or attempt |
|
| Interventions |
Stated purpose: to evaluate whether collaborative stepped care intervention for depression in primary care is more effective in reducing symptoms of depression compared with treatment as usual INTERVENTION (n = 456) Name: Stepped Care Intervention (SCI) Delivered by: PHPs and LHWs Title/name of PW and number 1. Selection: CPHCs qualified for selection if they have at least 2 medical doctors, 10 nurses/midwives, 5 community health officers (CHOs), 5 community health extension workers (CHEWs), and 2 pharmacy technicians 2. Educational background: medical doctors, nurses/midwives, CHOs, CHEWs ‐ LHWs, pharmacy technicians 3. Training: health staff of CPHCs offering SCI were trained in delivering the full intervention using the mhGAP‐IG training manual. The structured training comprised initial 5‐day workshop and 2‐day refresher course 4 weeks later. Training covered general introduction to depression, identification, methods of providing care for clients with depression, overall structure of the intervention, and specific intervention components. It also covered evaluation for improvement in symptoms, assessment for symptoms of psychosis and suicidality, and referral pathway to the mental health specialist for those having psychotic or suicidal symptoms. Training methods included lectures, role‐plays, and discussions groups and was standardised with the use of video/audiotapes 4. Supervision: mental health team provided clinical support and supervision to CPHC teams via mobile telephone and made site visits to each of the CPHCs once a month. 5. Incentives/remuneration: not mentioned Intervention details 1. Duration/frequency: psychoeducation ?single session, followed by antidepressants for 6 weeks or weekly individual Problem‐Solving Therapy in Primary Care (PST‐PC) sessions for 3 to 6 sessions. For those without at least 2‐point reduction on the PHQ‐9 after 3 sessions of PST‐PC or at least 4‐point reduction on the PHQ‐9 after 6 sessions of PST‐PC or 6 weeks of antidepressants, combination therapy of antidepressants (6 months in total) and PST‐PC (a total of 10 sessions, with the first 6 delivered weekly and the next 4 delivered fortnightly) was offered 2. Content: step 1 ‐ psychoeducation ‐ delivered by trained CHEWs focusing on educating patients about symptoms, the association between depression and interpersonal difficulties, the need to share emotional symptoms with the carer and to share personal difficulties with family members caring for them or other key people in their social network. In addition, they received a 4‐paged information leaflet about depression, its causes, symptoms, and ways of preventing and managing it; step 2 ‐ problem‐solving therapy in primary care (PST‐PC) consisting of 6 sessions is offered to patients with moderate depression (PHQ 10‐14), pregnant women, breastfeeding mothers, those with comorbid medical conditions, and any patients with severe depression not wanting medications. Antidepressants were offered for patients with severe depression (PHQ > 14) and for those not wanting PST‐PC. Amitriptyline or fluoxetine was the medication of choice and was given by the primary care doctor; step 3 ‐ combination therapy. For those without at least 2‐point reduction on the PHQ‐9 after 3 sessions of PST‐PC or at least 4‐point reduction on the PHQ‐9 after 6 sessions of PST‐PC or 6 weeks of antidepressants, combination therapy of antidepressants (6 months in total) and PST‐PC (a total of 10 sessions, with the first 6 delivered weekly and the next 4 delivered fortnightly) was offered; step 4 ‐ support and supervision from the mental health team. The mental health team provided clinical support and supervision to the CPHC teams via mobile telephone and made site visits to each of the CPHCs once a month CONTROL (n = 451): enhanced usual care (eUCA) – psychoeducation provided by a CHEW plus information leaflets about depression, its causes, symptoms, and ways of preventing it. Health staff at CPHCs offering eUCA were given 1‐day training on providing psychoeducation; and assessment of patients for symptoms of depression, symptoms of psychosis and suicidality, and referral to the mental health specialist if there were signs of psychosis or suicidal behaviour CO‐INTERVENTIONS: both groups received psychoeducation and information leaflets |
|
| Outcomes |
Patients Primary outcome Recovery (PHQ‐9 score < 6) at 12 months Secondary outcomes at 4 and 6 months 1. Recovery (PHQ‐9 score < 6) at 4 months 2. Recovery (PHQ‐9 score < 6) at 6 months Secondary outcomes at 12 months 1. Reduced in disability (WHODAS score > 12) 2. Good QOL (WHOQOL‐BREF overall score > 3) Carers None Process/health workers Secondary outcomes at 12 months 1. Number of deaths 2. Number with reported deliberate self‐harm 3. Good adherence (> 2 on adherence scale) 4. Switch to combination therapy (step 3) 4. Referral to mental health specialist 5. Loss to follow up Economic outcomes Health economic cost is calculated using the Client Service Receipt Inventory at 6 and 12 months (from protocol but not reported in this paper) Time points: baseline; 4, 6, 12 months (post intervention) |
|
| Notes |
Source of funding: Grand Challenges Canada (GCC) Notes on validation of instruments (screening and outcomes): all instruments validated Additional information: declarations of interest ‐ none Handling the data: as per footnotes in data and analysis Prospective trial registration number: ISRCTN66243738 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization was done by an independent centre"; "computer was used to generate the clusters using the random allocation rule" |
| Allocation concealment (selection bias) | Low risk | Quote: "allocation was based on clusters. The CHPCs were stratified into urban and rural to ensure even distribution. Computer was used to generate the clusters using the random allocation rule and each cluster was then assigned a study number and the research coordinator was informed of treatment allocation, for onward information to the health workers. To minimise the possibility of selection bias we identified clusters and recruited them before randomisation and we included all patients within a cluster meeting the eligibility criteria in the study. Also, the assessment of outcome measures was done by an independent group blinded to the allocation of clusters" Judgement comment: adequate allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and interventionists were not blinded to allocation, but this was unlikely to have affected results |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | "the assessment of outcome measures was done by an independent group blinded to the allocation of clusters" |
| Baseline outcome measurements similar | Low risk | See table 1: similar |
| Baseline characteristics similar? | Low risk | They are similar for age, sex, marital status, religion, education, and presence of chronic illness |
| Incomplete outcome data (attrition bias) Efficacy data | High risk | There is 20% loss to f/u in intervention group and 14.3% loss to f/u in control group at 12 months; unequal loss to f/u at all times. Also overall 18 deaths/907 participants (approx. 2%), which is significant, although none were judged to be related to study procedures. However they were unequal between groups (15 in control and 3 in treatment) |
| Incomplete outcome data (attrition bias) Safety data (e.g. adverse events) | Low risk | "We investigated the 18 deaths within 12 months' follow‐up and none was adjudged by the Trial Steering Committee to be related to the study procedures" |
| Protection against contamination | Low risk | Judgement comment: contamination unlikely as people get intervention from specific health workers, so cannot necessarily benefit from the intervention directly, unless people participating in it share their knowledge with others in the control group |
| Selective reporting (reporting bias) | Low risk | Similar in protocol |
| Other bias | Low risk | No other likely risks of bias |
Ali 2003.
| Study characteristics | ||
| Methods |
Study design: RCT Duration of study: baseline survey January to April 2001 and considering the 8‐week intervention must be provided between May and June‐July 2001 |
|
| Participants |
Country: Pakistan Income classification: low income Geographical scope: semi‐urban: in Qayoomabad, lower middle class semi‐urban community with a population of 80,000 in Karachi Healthcare setting: home Mental health condition: common mental disorders Population: adults 1. Age: 18 to 50 years 2. Gender: female 3. Socioeconomic background: lower‐middle class. Women predominantly aged 26 to 40 years, half with no formal education, not involved in revenue generation, two‐thirds with household income > 3000 PKR, nearly 60% residing longer than 10 years 4. Inclusion criteria a. Women 18 to 50 years old b. Able to communicate in Urdu c. Planning to live in the study area longer than 1 year d. No bereavement in past 6 weeks e. Identified as anxious and/or depressed based on screening with Aga Khan University Anxiety and Depression Scale 5. Exclusion criteria a. Participant women b. Those actively suicidal |
|
| Interventions |
Stated purpose: to assess effects on levels of anxiety or depression (or both), among women who had attended counselling sessions, provided by briefly trained counsellors in their own community INTERVENTION (n = 70) Name: counselling Delivered by: LHW Title/name of PW and number: minimally trained counsellors ‐ 21 1. Selection: "women were informed by word of mouth and by leaflets; out of 73 women who came for interview, 21 selected based on communications skills, motivation, attitude, ability to read and write Urdu and freedom to move in the community" 2. Educational background: "ability to read and write Urdu" and belonging to local community 3. Training: 11 training sessions held over 4 weeks. Each lesson lasted 3 hours and was led by family practitioner, sociologist, psychiatrist, or 3 clinical psychologists a. Contents: basic information regarding anxiety, depression, stress/anger management, and communication/counselling skills. Communication covered active listening, probing, and feedback, whereas counselling dealt with supportive problem‐solving and cognitive‐behavioural techniques. "Manual incorporating the training material is being published and is planned to train master training who could replicate the study in several urban and rural centres" b. Supervision: "women had ready access to members of the training team throughout the study period" c. Incentives/remuneration: not specified Intervention details 1. Duration/frequency: 8 sessions (?possibly weekly). Supportive, cognitive, and problem‐solving counselling was provided at day and time convenient for the woman 2. Content of intervention: trained counsellors provided supportive, cognitive, and problem‐solving counselling at client residence at convenient time CONTROL (n = 91): usual care, no intervention, just had AKUADS administered at baseline and end of study; however, "as the effectiveness of counselling was proved, for ethical reasons the control group was also counselled" possibly at the end of the study CO‐INTERVENTIONS: nil |
|
| Outcomes |
Patients Reduction in Aga Khan University Anxiety and Depression Scale scores Carers Not applicable Process/health workers Not specified Economic outcomes None Time points: baseline, end of 8 weeks |
|
| Notes |
Source of funding: academic body; Aga Khan University Research Council Notes on validation of instruments (screening and outcomes): AKUADS (indigenous screening scale, developed from complaints of patients with anxiety/depression, recorded verbatim in Urdu) previously validated against psychiatrist evaluation as gold standard and compared with SRQ Additional information: declarations of interest ‐ none Handling the data: as per footnotes in data and analysis Prospective trial registration number: not specified |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "every third household was systematically sampled in all of Qayoomabad. [...] One woman was randomly chosen from each selected household and screened for anxiety and/or depression. [...] Using computer‐generated random numbers, 216 [of 1218 women] cases were randomised to the intervention and 150 to the control group". The initial selection was quasi‐random, but then allocation to control or intervention was random |
| Allocation concealment (selection bias) | Unclear risk | Quote: "computer‐generated random numbers" Comment: even though sequence generation was centrally done, it was unclear how allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: not able to blind participants or personnel. Unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | No selective reporting. Independent data collectors blind to allocation and to previous scores |
| Baseline outcome measurements similar | Low risk | Comment: yes, similar, both across intervention and control, and between dropouts and non‐dropouts |
| Baseline characteristics similar? | Low risk | Comment: yes, similar. All P values over 0.2 comparing dropouts vs non‐dropouts and intervention vs control groups |
| Incomplete outcome data (attrition bias) Efficacy data | High risk | Comment:intervention: 68% dropout between baseline and those completing the intervention; control: 33% dropout. Although characteristics are similar between dropouts and non‐dropouts (including baseline scores), scores may have been different at follow‐up |
| Protection against contamination | Low risk | Quote: "the spontaneous decrease in the score [in the control group] could be attributed to the natural history of depression, which waxes and wanes, but a contaminant effect of counselling cannot be ruled out"; "the effect of summer holidays occurring during the study period was also considered as possibly causing contamination" |
| Selective reporting (reporting bias) | Unclear risk | Comment: no selective reporting. but no protocol to assess if this is the case |
| Other bias | Low risk | Comment: none detected |
Araya 2003.
| Study characteristics | ||
| Methods |
Study design: RCT Duration of study: March 2000 to March 2002 |
|
| Participants |
Country: Chile Income classification: upper‐middle income Geographical scope: deprived urban areas in Santiago Healthcare setting: PC facilities that were underfunded and insufficiently resourced Mental health condition: women with persistent depression Population: women 1. Age: 18 to 70 years 2. Gender: female 3. Socioeconomic background: most were housewives from deprived areas 4. Inclusion criteria a. Age 18 to 70 years b. Current major depression illness (2 screenings of GHQ‐12 with score > 5 at 2‐week interval) c. Female PHC patients 5. Exclusion criteria a. Women who had psychiatric consultation or admission to hospital in the 3 months before baseline interview b. Current psychotic symptoms c. Serious suicide risks d. History of mania e. Alcohol abuse |
|
| Interventions |
Stated purpose: to compare the effectiveness of a stepped‐care programme vs usual care in primary care management of depression in low‐income women in Santiago, Chile INTERVENTION (n = 120) Name: Stepped Care Delivered by: professionals (PHPs and CPs) Title/name of PW and number: PC physician and group leaders (non‐medical workers) 1. Selection: Group leaders and doctors ‐ both were employed at local PHC units selected for the study 2. Educational background: group leaders included a nurse or a social worker. Doctors had a medical degree 3. Training a. Group leaders ‐ 12‐hour training by principal investigator psychiatrist b. Content ‐ not specified c. Doctors ‐ 4‐hour training by psychiatrist to understand the brief pharmacotherapy protocol (medical algorithm of fluoxetine, amitriptyline, imipramine) and initial and follow‐up assessments 4. Supervision a. Group leader ‐ 8 hours of supervision by principal investigator over the course of the intervention 6. Incentives/remuneration: none, as they are employees Intervention details 1. Duration/frequency: psychoeducation: 7 weekly sessions and 2 booster sessions (weeks 9 and 12), each lasting 75 minutes; groups of 20 participants 2. Content of intervention: group leaders provide psychoeducation, which consists of information on symptoms, causes of depressions, treatments available, positive activities, problem‐solving techniques, basic cognitive and relapse‐prevention techniques; patients are given a manual on session contents and examples/exercises; follow‐up by group leaders: monitoring medication adherence, attendance at follow‐up visits for patients receiving pharmacotherapy. They also refer to the doctor if HDRS score > 12 at 6 weeks with psychoeducation; doctors: detect and diagnose using their brief pharmacotherapy protocol, then prescribe according to the medical algorithm (which includes use of fluoxetine, amitriptyline, or imipramine), then follow‐up with patients CONTROL (n = 120): usual care: normally available services in PC clinic: included antidepressant medication, referral to specialist (usually takes 2 months to be seen by psychiatrist); given guidelines on treating depression in PC before initiation of study. No services restricted/withheld CO‐INTERVENTIONS: none |
|
| Outcomes |
Patients 1. GHQ‐12 screening 2. MINI 3. Diagnosis for DSM‐IV 4. HDRS* ‐ Severity of depression, SF‐36 ‐ scores for mental health, emotional role, social functioning, vitality. Carers None Process/health workers None Economic outcomes None (*study's primary outcomes) Time points: baseline, 3 months, 6 months |
|
| Notes |
Source of funding: US National Institute of Mental Health Notes on validation of instruments (screening and outcomes): all instruments validated Additional information: information from authors: no study protocol, so unable to check primary and secondary outcomes. Declarations of interest ‐ Araya R received payment from Wyeth for conducting a workshop. Rojas G received payment from Wyeth and Servier, and Fritsch R received payment from Wyeth for participation in clinical trials. Simon G received research grants from Eli Lilly and Solvay Pharmaceuticals Handling the data: as per footnotes in data and analysis Prospective trial registration number: none (only National Institute of Mental Health proposal) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomisation of patients was stratified by clinic, done in blocks of 20 using computer‐generated random number" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "individuals who recruited patients were neither involved in nor aware of the procedure used to generate allocations. Allocations in numbered sealed envelopes in each clinic and opened by an individual who had not recruited patients" Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Individuals who recruited patients were neither involved in nor aware of the procedure used to generate allocations. Allocations in numbered sealed envelopes in each clinic and opened by an individual who had not recruited patients" |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | Quotes: "at baseline, a clinician administered the three assessments"; "follow‐up interviews were done by an independent clinician blinded to treatment assignment"; "rates of participation in the intervention programme were high, and participation in blinded outcome assessments exceeded 85% in both groups" |
| Baseline outcome measurements similar | Low risk | Comment: similar baseline outcome measurements. No adjustment therefore needed |
| Baseline characteristics similar? | Low risk | Comment: similar baseline characteristics |
| Incomplete outcome data (attrition bias) Efficacy data | Low risk | Comment: 3 months: 18 (stepped care) vs 11 (usual care); 6 months: 16 vs 13 out of 120 patients in each group. This represents more than 80% follow‐up rate |
| Incomplete outcome data (attrition bias) Safety data (e.g. adverse events) | Unclear risk | Author information: "there was a record kept [of adverse outcomes] but do not know where it is" |
| Protection against contamination | High risk | Comment: 3 clinics that can have both intervention and control people and the same GP could be delivering both interventions, so theoretical risk of contamination |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol. Study author suggests all outcomes reported |
| Other bias | Low risk | None |
Arjadi 2018.
| Study characteristics | ||
| Methods |
Study design: community‐based country‐wide 2‐group randomised controlled trial Duration of study: recruitment from September 2016 to May 2017. Intervention for 8 weeks. Follow‐up at 10 weeks |
|
| Participants |
Country: Indonesia Income classification: low‐middle income from 2016 to 2017 Geographical scope: predominantly urban/suburban from Java and Sumatra Healthcare setting: general community Mental health condition: depression Population 1. Age: ≥ 16 years 2. Gender: both 3. Socioeconomic background: 31.1% employed in the past year 4. Inclusion criteria a. Meet cutoff score ≥ 10 on PHQ‐9 (Kroenke, Spitzer, & Williams, 2001) b. Meet criteria for diagnosis of major depressive disorder or persistent depressive disorder on SCID‐5 (First, Williams, Karg, & Spitzer, 2015) c. Age ≥ 16 years d. Proficient in Indonesian language e. Have fluency to use the Internet 5. Exclusion criteria a. Current or previous mania or hypomania episode b. Current or previous psychotic disorder c. Current substance use disorder d. Current acute suicidality e. Currently following weekly or more intensive psychological intervention (non‐medication) for mental health complaints |
|
| Interventions |
Stated purpose: to examine the effectiveness of Internet‐based intervention guided by lay counsellors for depression INTERVENTION (n = 159) Name: Guided Act and Feel Indonesia (GAF‐ID) Delivered by: LHW Title/name of PW and number: lay counsellors 1. Selection a. Age between 20 and 40 years with no restriction on gender b. Minimum senior high school education c. Willing to participate fully during trial process d. No professional background as mental health specialist e. Willingness to participate in training for lay counsellor in this study 2. Educational background: minimum senior high school education 3. Training: all lay counsellors receive 2 days of intensive training, during which all features of Internet‐based BA are discussed and role‐plays are conducted. Other technical issues addressed during training include how to handle technical problems that may arise, how to handle participants with low motivation, and how to monitor suicidality or other serious deteriorations during the intervention. Printed training modules are provided to help with tasks during the trial 4. Supervision: lay counsellors were supervised by a licensed clinical psychologist during their work. 5. Incentives/remuneration: not mentioned Intervention details 1. Duration/frequency: GAF‐ID is offered in a secure online environment and consists of a series of 8 structured modules that can be completed over 8 weeks 2. Content of intervention: content of GAF‐ID programme is based on face‐to‐face BA intervention [7] and on Dutch online BA intervention, called “Act and Feel”. Programme consists of a series of 8 weekly structured modules that can be completed in 30 to 45 minutes per module, including psychoeducation about depression and basic background of behavioural activation, monitoring mood and behaviour or activities, expansion of potential mood‐independent pleasurable activities, overcoming difficulties during the process, gaining insight into the effect of avoidance behaviour, and building a strategy for relapse prevention CONTROL: ‘online minimal psychoeducation’ (n = 154) Minimal PE is presented as a short, online leaflet consisting of basic information about depression and basic tips on how it can be addressed, representing information that can be easily and freely accessed online outside of this programme |
|
| Outcomes |
Patients Primary measure Self‐reported symptoms of depression at post treatment, 10 weeks from baseline, measured on Patient Health Questionnaire‐9 (PHQ‐9) Secondary measures 1. Rate of remission/recovery of depression (major depressive disorder or persistent depressive disorder) using Structured Clinical Interview for DSM‐5 (SCID‐5; First et al., 2015) 2. Inventory of Depressive Symptomatology Self‐Report (IDS‐SR; Rush, Gullion, Basco, Jarrett, & Trivedi, 1996; Rush et al., 1986) 3. Fear Questionnaire (FQ; Marks & Mathews, 1979) 4. Multi‐dimensional Scale of Perceived Social Support (MSPSS; Zimet, Dahlem, Zimet, & Farley, 1988) 5. Brief version of WHO Quality of Life (WHOQOL‐BREF; The WHOQOL Group, 1998) Potential mediators and moderators 1. Visual analogue scale (VAS) of mood (1‐item mood scale; van Rijsbergen, Bockting, Berking, Koeter, & Schene, 2012) 2. Positive and Negative Affect Scale (PANAS; Watson, Clark, & Tellegen, 1998) 3. Behavioral Activation for Depression Scale Short Form (BADS‐SF; Manos, Kanter, & Luo, 2011) 4. Life‐events Scale (Garnefski, Kraaij, & Spinhoven, 2001) 5. History of depressive disorders (major depressive disorder and persistent depressive disorder) and trauma‐ and stressor‐related disorders as assessed using SCID‐5 interview (First et al., 2015) 6. Childhood Trauma (self‐developed) Carers None Process/health workers None Economic outcomes None Time points: baseline, post intervention (10 weeks from baseline), follow‐up (3 months and 6 months from baseline) 1. Bi‐weekly/once every 2 weeks (weeks 2, 4, 6, 8): primary outcome, selection of potential mediators and moderators (VAS, PANAS, BADS‐SF) 2. Post intervention (t0 + 10 weeks): primary outcome, all secondary outcomes, selection of potential mediators and moderators (VAS, PANAS, BADS‐SF) 3. Follow‐up (t0 + 3 months): primary outcome, all secondary outcomes (except SCID‐5 interview), selection of potential mediators and moderators (VAS, PANAS, BADS‐SF) 4. Follow‐up (t0 + 6 months): primary outcome, all secondary outcomes (except SCID‐5 interview), selection of potential mediators and moderators (VAS, PANAS, BADS‐SF) |
|
| Notes |
Source of funding: Indonesia Endowment Fund for Education (Lembaga Pengelola Dana Pendidikan), Ministry of Finance, Republic of Indonesia Additional information: www.actandfeelindonesia.com; www.actandfeel.com. Declarations of interest ‐ MHN reports travel expenses and speaker honoraria for lectures or clinical training workshops paid for by mental health centres. CLHB is a member of the Dutch multi‐disciplinary guideline for anxiety and depression, a co‐editor of PLoS One and European Psychology, a member of the scientific board in the Dutch national statutory insured package, for which she receives an honorarium, and has received honoraria for keynote addresses at the European Association for Behavioural and Cognitive Therapies, the European Psychiatry Association, and the European Conference Association, and for clinical training workshops (paid by mental health centres), and receives book royalties Handling the data: as per footnotes in data and analysis Prospective trial registration number: Nederlands Trial Register (www.trialregister.nl): NTR5920 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization will be performed within in a permuted block design. The size of the blocks and the exact strata are not revealed in this design paper, so that the underlying algorithm remains unpredictable for the research assistants, but it is stated on the trial registration" |
| Allocation concealment (selection bias) | Low risk | Randomisation performed by research assistant at the start of the study Quote: "as participants are screened into the study, they will be randomized using a web‐based program that was built for this trial. Randomization will be performed within in a permuted block design. The size of the blocks and the exact strata are not revealed in this design paper, so that the underlying algorithm remains unpredictable for the research assistants, but it is stated on the trial registration" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were not informed whether their assigned treatment was intervention or control |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | Research assistants performing SCID 5 were not aware of participants' assignments and participants were asked not to inform them Quote: "this study is single‐blind: the research assistants, who will be involved in conducting the clinical interviews after randomization, will be blind to the treatment condition and the participants will be asked not to reveal their treatment condition during the interview. Research assistants who perform the assessments are not involved in the intervention process and they will be asked to guess the treatment allocation per participant" |
| Baseline outcome measurements similar | Unclear risk | UNCLEAR: only baseline PHQ‐9 (primary outcome) results were reported. SIMILAR: baseline results of secondary outcomes: IDS‐SR, FQ, MSPSS and WHO‐QOL‐BREF were not reported |
| Baseline characteristics similar? | Low risk | Baseline age, gender, ethnicity, place of residence, area, marital status, educational level, socioeconomic status, occupation, comorbidity with PTSD and anxiety were similar across both groups |
| Incomplete outcome data (attrition bias) Efficacy data | High risk | More dropouts in intervention group than in control group; a number of dropouts in both groups due to lack of improvement, several dropouts had no quoted reason ‐ participants who dropped out may have had an impact on study outcomes if they had stayed on. Higher dropout in intervention group likely to change results Quote: "the drop‐out rate was higher in the intervention group than in the control group, a pattern that is frequently reported in studies of Internet‐based interventions. The increased frequency of dropout in the intervention group in our study was probably because of the greater demands put on participants in the GAF‐ID group compared with those in the psychoeducation group." |
| Incomplete outcome data (attrition bias) Safety data (e.g. adverse events) | Low risk | Low: "no adverse events were reported in either group" No adverse events happened |
| Protection against contamination | Low risk | GAF‐ID intervention available only on a secure online platform. Lay support not provided to participants in the control group. Contamination unlikely |
| Selective reporting (reporting bias) | High risk | Not all relevant outcomes from protocol reported: PANAS (Positive and Negative Affects) |
| Other bias | Low risk | No other sources of bias found |
Ayoughi 2012.
| Study characteristics | ||
| Methods |
Study design: RCT Duration of study: recruitment September to October 2009, intervention for 5 to 13 weeks, follow‐up at 3 months; study concluded in March 2010 |
|
| Participants |
Country: Afghanistan Income classification: low income from 2009 to 2010 Geographical scope: in Balkh Province of Afghanistan, in its capital Mazar‐e‐Sharif, 320 km northwest of Kabul, urban Healthcare setting: PC facility Mental health condition: depression Population 1. Age: ≥14 years 2. Gender: both (but all men dropped out, so only females in study sample) 3. Socioeconomic background: more than 30 years of war has disrupted the lives of 2 generations of people; most were unemployed ‐ intervention group 83.9%, control group 96.7%; most have no education 4. Inclusion criteria a. Adults, aged 18 years or over with score ≥ 11 on Patient Health Questionnaire (PHQ‐9) b. Confirmed 5th edition of Diagnostic and Statistical Manual of Mental Disorders (DSM‐V) diagnosis of depression using the Composite International Diagnostic Interview (CIDI) c. Informed consent provided 5. Exclusion criteria a. Schizophrenia b. Mental retardation c. Neurological disorder |
|
| Interventions |
Stated purpose: to assess common treatment for mental health patients in Afghanistan INTERVENTION (n = 31) Name: Psychosocial Counselling Delivered by: lay PHWs Title/name of PW and number: psychosocial counsellors (N = 30 trained and deployed at local health care centres in North Afghanistan, with N = 3 at a counselling centre at Mazar‐e‐Sharif) 1. Selection: not mentioned 2. Educational background: not mentioned 3. Training: 3.5 months of intensive training ending with a final examination, ensuring the required quality standard of counsellors set by the Ministry of Public Health. Training provided by experienced local physicians who had been trained as psychosocial counsellors in an extensive, 2‐year training programme for psychosocial counselling in 2005/2006 and gathering considerable experience in counselling thereafter 4. Supervision: research team based in Kabul 5. Incentives/remuneration: employed at clinic Intervention details 1. Duration/frequency: first 5 held over 5 weeks. Each session 45 to 60 minutes long; in case of clinical necessity, up to 8 additional counselling sessions could be added, following selected intervention modules of cognitive‐behaviour therapy. Supervising team needed to agree on whether patient required additional sessions 2. Content of intervention: approach has been developed and adapted to the sociocultural background of Afghanistan. Watzlawick's short‐term therapy and Antonowsky's salutogenetic approach lie at the core; selected intervention modules of cognitive‐behaviour therapy have additionally been included. Resource‐ and problem‐solving approach that aims at restoring self‐efficacy and developing resources, enabling patients to re‐participate in their daily life in a satisfying and responsible way. Approach is geared towards improving patients' general mental, physical, social, and spiritual health. Emphasis on a sense of coherence, covering comprehensibility, manageability, and meaningfulness; followed treatment guidelines in the manual "Professional Package for Psychosocial Counsellors working in the BPHS in Afghanistan"; no medication prescribed CONTROL: routine medical treatment (n = 30) Usual medical treatment was carried out by 4 local physicians, who regularly examined patients in the control group and prescribed medication. We agreed with them on a weekly appointment and precise documentation on prescribed medication. This intervention can be described as usual treatment within the Basic Public Health Care System for patients reporting mental suffering and psychosocial problems. Routine medical treatment by local physicians started immediately after initial expert interview. Patients receiving medical care were treated at local health care centre for the following 3 months CO‐INTERVENTIONS: nil |
|
| Outcomes |
Patients 1. HSCL Depression Score 2. HSCL Anxiety Score 3. MINI diagnosis of major depression 4. Screening for depression change between pretest and follow‐up Carers Nil Process/health workers Nil Economic outcomes None Time points: baseline, 3 months post baseline (*intervention length for intervention /counselling group = 5 to 8 weeks, intervention length for control/medication group = 3 months) |
|
| Notes |
Source of funding: EU Delegation in Kabul Notes on validation of instruments (screening and outcomes): all validated Additional information: declarations of interest ‐ none Handling the data: as per footnotes in data and analysis Prospective trial registration number: ClinicalTrials.gov Identifier: NCT01155687 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Judgement comment: not completely random, as every other day "Our team allocated the participants to one of the treatment conditions based on a daily alternation routine, meaning that alternately, one day patients were allocated to the medication group, and the next day to the counselling group" |
| Allocation concealment (selection bias) | High risk | Judgement comment: randomisation was not concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Patients and assessors were all aware of treatment allocation, but this may not have influenced results |
| Blinding of outcome assessment (detection bias) all outcomes | High risk | "The interviewers who carried out the follow‐up test were not fully blind to the treatment condition as the two types of intervention (psycho‐ vs pharmacotherapy) were very different and thus sometimes revealed through unsolicited information given by the patient. Moreover, although the knowledge about the treatment condition was not updated before follow‐up, we cannot rule out that the expert‐interviewer still remembered the treatment condition of some patients" |
| Baseline outcome measurements similar | Unclear risk | Yen ‐ LOW: similar baseline HSCL depression, HSCL anxiety, MINI, screening for depression, psychosocial stressors, and coping mechanisms results in both groups Nadja ‐ HIGH: OK apart from how many people were on medication (none in psychosocial counselling and 29 in control (medication only)) |
| Baseline characteristics similar? | Low risk | Baseline gender, ethnicity, religion, marital status, education, employment, number of people living in the household, medication, and age all showed no statistically significant difference |
| Incomplete outcome data (attrition bias) Efficacy data | Unclear risk | Yen ‐ LOW: efficacy analysis; analysis was carried out with and without participants who dropped out; there was no difference in treatment effect Nadja ‐ UNCLEAR: no access to protocol to check |
| Protection against contamination | Unclear risk | Judgement comment: Yen ‐ LOW: psychosocial counselling (intervention) and usual medical care (control) delivered by different providers, and psychosocial counselling programme highly specialised, making contamination between groups unlikely, even though they may have lived in the same area and received treatment at centres close to one another. Nadja ‐ UNCLEAR: although in the same clinic, it would not be possible for control group to be contaminated by psychosocial counselling, unless hearing indirectly about it from friends who may be included in the intervention arm. Given the 2 arms are in the same clinic, contamination is possible |
| Selective reporting (reporting bias) | Low risk | All outcomes planned in clinical trial protocol were reported in this article |
| Other bias | Low risk | No other sources of bias were found |
Barfar 2017.
| Study characteristics | ||
| Methods |
Study design: RCT Duration of study: 12 months; 2010 to 2011 |
|
| Participants |
Country: Iran Income classification: upper‐middle income in 2010 to 2011 Geographical scope: urban Healthcare setting: 3 university‐affiliated hospitals and participants’ homes Mental health condition: serious mental disorders (schizophrenia, schizoaffective disorder, bipolar I disorder) Population (mention whether patient, carer, or dyad) 1. Age: 15 to 65 years 2. Gender: both 3. Socioeconomic background: more than 60% of participants were unemployed 4. Inclusion criteria a. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM‐IV), diagnosis of schizophrenia, schizoaffective disorder, or bipolar I disorder b. Age 15 to 65 c. Previous history of psychiatric hospitalisation(s) d. Residing in catchment area of the hospital 5. Exclusion criteria 1. Mental retardation 2. Severe organic condition 3. Non‐Farsi‐speaking |
|
| Interventions |
Interventions Stated purpose: to determine whether providing services for patients with severe mental disorders (schizophrenia, schizoaffective, and bipolar I disorder) through an Aftercare Service programme improves quality of life and global functioning, increases patient satisfaction, and is cost‐effective when compared with routine conventional care INTERVENTION 1 (n = 80) Name: Aftercare Service (2 groups, analysed together) Delivered by: professionals (PHPs and CPs) Title/name of PW and number: general practitioner (GP), social worker, case manager; number not specified 1. Selection: GP and social worker with experience in psychiatric home health care 2. Educational background: not specified 3. Training: specially trained; no details given 4. Supervision: for home visiting team, care plan was established and reviewed by faculty member psychiatrist, and weekly meetings were held with psychiatrists to discuss problems and to establish decisions. Intervention details: home visits by general practitioner and social worker for 25 patients who were non‐compliant, were difficult to engage, and/or had high service use (home visiting care group). Telephone follow‐up by case manager for 55 patients who did not meet above requirement for home visits (telephone follow‐up group) 1. Duration/frequency: monthly home visit (home visit team), phone calls before monthly outpatient follow‐up (telephone follow‐up group), psychoeducation sessions (frequency not specified), intervention duration 1 year 2. Content of intervention: home visiting care included assessing patients, co‐ordinating care, managing symptoms/medication, prescribing and adjusting medications, ensuring compliance, educating patient and family about the illness and about medications and warning signs of a relapse, recognising early phases of a relapse, managing a relapse (which may involve raising medication dosage or referring for hospital admission), and guiding the family on how to access supportive and community resources. Telephone follow‐up involved telephone calls by a case manager to encourage patients to attend outpatient clinic at the hospital for monthly follow‐up visits by a resident of psychiatry or a general practitioner, where medication management and patient education were provided. Both groups received family psychoeducation and social skills training in individual (in‐home visiting care) or group format (telephone follow‐up, 6 sessions) CONTROL (type and descriptions) (n = 80) Existing services provided by outpatient or inpatient services inside or outside hospitals that might be contacted by patients. Outpatient services included visits by a psychiatric resident at the hospital who prescribed medications for the patient. No active follow‐up or any form of rehabilitation services CO‐INTERVENTIONS: nil |
|
| Outcomes |
Patients 1. 1‐Year rehospitalization rate* 2. Clinical Severity Index (CGI) 3. Global Functioning (GAF) 4. General symptoms of psychosis (PANSS) 5. Positive symptoms of psychosis (PANSS) 6. Negative symptoms of psychosis (PANSS) 7. Total symptoms of psychosis (PANSS) 8. Depression (HDRS) 9. Mania (YMRS) 10. Quality of life (WHOQOL‐BREF) Carers Nnil Process/health workers CSQ‐8: Satisfaction Economic outcomes 1. Intervention costs 2. Patient and family costs 3. Total costs 4. Tables 3, 4, and 5 (asterisk for study's primary outcomes; star: outcomes that we have not reported in this review) Time point: 0 months |
|
| Notes |
Source of funding: Mental Health Office at Ministry of Health and Tehran University of Medical Sciences Notes on validation of instruments (screening and outcomes): validated Additional information: Hajebi et al. A multicenter randomized controlled trial of aftercare services for severe mental illness: study protocol. BMC Psychiatry 2013;13:17. Declarations of interest ‐ none Handling the data: nil Prospective trial registration number: IRCT201009052557N2 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A total of 160 post‐discharge eligible patients were randomized into two equal patient groups using stratified balanced block randomization method. A psychiatrist at the center stratified patients by sex and severity of illness in an effort to insure that equal numbers of similar patients were allocated to each group" |
| Allocation concealment (selection bias) | Unclear risk | Allocation of participants between arms of the study was equal (allocation ratio 1:1). Randomisation was provided by an independent statistician at the medical university. Eligible patients were assigned to intervention or control (TAU) groups by stratified balanced block randomisation method with allocation concealment. A psychiatrist at each centre was responsible for concealment procedures. It is unclear how allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The nature of such services prevents adequate blinding of participants. It was not possible to blind raters who will perform follow‐up evaluations on cases and controls because of direct contact with patients when rating them. It was not possible to conceal knowledge of allocation to groups. Lack of blinding of personnel and participants may not have influenced study results |
| Blinding of outcome assessment (detection bias) all outcomes | High risk | Blinding of outcome assessors was not performed |
| Baseline outcome measurements similar | Unclear risk | Baseline outcomes have been clearly reported in the table. Although similar between groups, it is unclear whether any differences were adjusted for during analyses |
| Baseline characteristics similar? | Low risk | Patients in experimental and control groups were comparable at baseline with regard to demographic and clinical data, with no significant differences (Table 1) |
| Incomplete outcome data (attrition bias) Efficacy data | Low risk | The proportion of missing data was similar in intervention and control groups and therefore was unlikely to bias the results |
| Incomplete outcome data (attrition bias) Safety data (e.g. adverse events) | Low risk | The proportion of missing data was similar in intervention and control groups and was not linked to adverse events |
| Protection against contamination | Unclear risk | As control group received treatment as usual with visits from psychiatrists, it is unclear whether communication between intervention and control could have occurred |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in trial protocol and registry have been reported |
| Other bias | Low risk | No other biases were present |
Barron 2013.
| Study characteristics | ||
| Methods |
Study design: RCT Unit of allocation: individual Duration of study: not given |
|
| Participants |
Country: Palestine Income classification: lower‐middle income Geographical scope: urban Healthcare setting: school Mental health condition: PTSD Population (mention whether patient, carer, or dyad) 1. Age: 11 to 13 years 2. Gender: female and male 3. Socioeconomic background: participants are from Nablus, an area with high levels of ongoing violence and high levels of poverty 4. Inclusion criteria (including threshold cut off score of measurement tool) a. 10 students with highest CRIES‐13 scores in each class were selected for participation in the study 5. Exclusion criteria: not reported |
|
| Interventions |
Stated purpose: to assess the Teaching Recovery Techniques (TRT) trauma recovery programme within the context of ongoing violence INTERVENTION (n = 83) Name: TRT Delivered by: community professional (CP) Title/name of PW and number: school counsellors ‐ 14 1. Selection: not described 2. Educational background: not described 3. Training (contents, duration, by whom): school counsellors received 3 days of training in programme delivery by 2 expert trainers from the Children and War Foundation covering programme values, content, and processes. Training method: information giving, modelling, experiential learning, reflection, feedback 4. Supervision: 2 counsellors present during programme delivery ‐ 1 to present and the other to observe Intervention details (according to PHW/CWs and whether aimed at carers and/or patients) 1. Duration/frequency: sessions were delivered over 5 consecutive weeks. Each session lasted 1 hour and 30 minutes 2. Content of intervention (by type of health worker and per patient/carer): this cognitive‐behavioural programme includes 5 sessions that focus on normalising trauma and strategies for intrusive memories, hyperarousal, and avoidance symptoms of PTSD. The fifth session focuses on children’s response to loss CONTROL (n = 50) Students in wait‐list. Participants in wait‐list received their usual social education curriculum involving art, civic education, geography, history, and national education; intervention group experienced TRT CO‐INTERVENTIONS: none |
|
| Outcomes |
Patients 1. (CRIES‐13)* 2. Depression (Depression Self‐rating Scale for Children (DSRS*)) 3. Traumatic grief (Traumatic Grief Inventory for Children (TGIC*), Impact on School Performance Scale (ISPS), Strength and Difficulties Questionnaire (SDQ), Exposure to War Stressors Questionnaire (EWSQ)) 4. Students’ subjective experience of programme delivery was assessed through a random sample focus group of 10 students Carers None Process/health workers 1. Programme fidelity was assessed by counsellors and observers (n = 18) completing a fidelity questionnaire following programme delivery 2. Counsellors’ subjective experience was assessed through a focus group of the 9 intervention group counsellors Economic outcomes (and where these can be found, e.g. ref or table number) None (asterisk for study's primary outcomes; star: outcomes that we have not reported in this review) Time points: baseline, 2 weeks post intervention |
|
| Notes |
Source of funding: The Children and War Foundation Notes on validation of instruments (screening and outcomes): all validated Additional information (e.g. provided by authors, existence of a published study protocol): pilot to Barron 2016. Declaration of interests ‐ none Handling the data (e.g. imputed values/other calculations we have made): all measures and subscales were analysed using paired t‐tests (pre/post‐test) and analysis of covariance comparing intervention and wait‐list groups. The Tukey HSD (honestly significance difference) test was used in conjunction with ANOVA to check for differences between classes. In the meta‐analysis, only students with results above the cutoff before intervention in both arms were considered (53 in the intervention group, 25 in the control group) Prospective trial registration number: not given |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "twenty school counselors were trained in TRT and then randomly assigned by the principal researcher (names on cards and blindly selected from a container) to intervention and wait‐list control groups" Judgement comment: "name on cards and blindly selected from a container"; "simple method but acceptable during the complex environment where the study took place. The problem is that they didn't randomize students in different classes" (page 309); "the remaining 14 counsellors each identified a school class of at least 40 11–13‐year‐old students" |
| Allocation concealment (selection bias) | High risk | Judgement comment: principal investigator was involved in allocation of counsellors; schools were not randomised |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information given |
| Blinding of outcome assessment (detection bias) all outcomes | Unclear risk | No mention of blinding in this report |
| Baseline outcome measurements similar | High risk | Page 312‐313: "a comparison between the intervention and wait‐list groups at pre‐test showed significantly higher levels of posttraumatic stress, negative school impact, and mental health difficulties in the intervention group (P < 0.05), regardless of inclusion or exclusion of the high exposure class in the analysis. Levels of traumatic grief and depression were matched across both groups"; and further, page 313: "significant variance between the intervention and wait‐list groups at pre‐test" |
| Baseline characteristics similar? | Unclear risk | Limited information provided. No differences in age |
| Incomplete outcome data (attrition bias) Efficacy data | Unclear risk | Page 311: "seven intervention students were omitted due to incomplete pre‐test data (see Figure 1)"; instead, no dropouts in the control group; not clear why data were missing or how this would impact analyses |
| Protection against contamination | Low risk | Judgement comment: allocation by school |
| Selective reporting (reporting bias) | Unclear risk | No reference to protocol or to online trial registration. Results are not reported numerically in a clear way in a table |
| Other bias | Low risk | No further bias identified |
Barron 2016.
| Study characteristics | ||
| Methods |
Study design: RCT Unit of allocation: individual Duration of study: started at beginning of 2015. End not clear |
|
| Participants |
Country: Palestine Income classification: lower‐middle income Geographical scope: rural. 10 villages (Jabaa, Hezma, Anata, Bo Dees, Bethany, Bir Nabala, Qatana, Shuafat, Alram, Biet Anan) near East Jerusalem along the separation wall Healthcare setting: school Mental health condition: PTS Population (mention whether patient, carer, dyad): adolescents 1. Age: 11 to 15 years 2. Gender: both 3. Socioeconomic background: lived in area with high military presence 4. Inclusion criteria (including threshold cutoff score of measurement tool) a. Participants were randomly selected from 10 randomly selected high schools (70 in the geographical area) situated in 10 villages b. Students fulfilling criteria indicative of PTSD on the CRIES–8, that is, score ≥ 17 on intrusion and avoidance subscales 5. Exclusion criteria a. Not specified |
|
| Interventions |
Stated purpose: to assess effects of a cognitive‐behavioural group intervention, Teaching Recovery Techniques (TRT), for adolescents with high levels of post‐traumatic stress (n = 154) from villages in occupied Palestine INTERVENTION: Teaching Recovery Technique (n = 75) Delivered by: community professional (CP) Title/name of PW and number: school counsellors ‐ 1 1. Selection: not specified 2. Educational background: not specified 3. Training (contents, duration, by whom): school counsellors received 3 days of training in programme delivery by 2 expert trainers from the Children and War Foundation covering programme values, content, and processes. Training methods included information giving, modelling, experiential learning, reflection, and feedback 4. Supervision: counsellors met monthly in pairs and in small groups for supervision to prepare and reflect on lesson delivery Intervention details: TRT programme was developed by the Children and War Foundation, Bergen 1. Duration/frequency: 5 sessions, duration not stated 2. Content of intervention (by type of health worker and per patient/carer): Teaching Recovery Technique. This cognitive‐behavioural programme includes 5 sessions that focus on normalising trauma and strategies for intrusive memories, hyperarousal, and avoidance symptoms of PTS. The group‐delivered programme, based on CBT, focuses specifically on children’s symptoms of PTSD. The 5 sessions help students to understand the causes of trauma and to recognise signs and symptoms. Adolescents are taught a range of coping skills to stop flashbacks and other intrusive images, sounds, or smells. Student hyperarousal is addressed through stabilisation and relaxation techniques and phobic avoidance behaviour is gradually desensitised through use of relaxation with anxiety and anger hierarchies CONTROL (n = 64) No care. Wait‐list includes students who were not in the intervention. Participants in wait‐list received their usual social education curriculum involving art, civic education, geography, history, and national education; intervention group experienced TRT CO‐INTERVENTIONS: none |
|
| Outcomes |
Patients 1. Exposure to War Stressors Questionnaire (EWSQ) 2. CRIES–13* 3. Depression Self‐Rating Scale (DSRS)* 4. Adolescent Dissociative Experiences Scale (ADES)* Carers None Process/health workers Programme fidelity measured through presenter self‐report and observer report of programme delivery (page 968) Economic outcomes (and where these can be found, e.g. reference or table number) Assessment of future TRT delivery and evaluation; analysis of costs was calculated for 10 counsellors and 2 local rather than international trainers delivering TRT within their own geographical location (can be found within the paper page 968) (asterisk for study's primary outcomes; star: outcomes that we have not reported in this review) Time points: baseline, 2 weeks post intervention |
|
| Notes |
Source of funding: Children and War Foundation Notes on validation of instruments (screening and outcomes): none Additional information(e.g. provided by authors, existence of a published study protocol): declaration of interests ‐ none Handling the data (e.g. imputed values/other calculations we have made): omnibus multi‐variate analyses were conducted on all standardised measures and subscales. Following analysis of intervention and wait‐list data from participants who completed the TRT and from pretest and post‐test measures (n = 139), an intention‐to‐treat analysis (ITT) was conducted on all participants in both conditions, when at least pretest data were available. A conservative estimate of treatment was used when participant pretest scores were also used as post‐test scores. An ITT effect size analysis was then conducted on PTSD, depression, and dissociation between intervention and wait‐list participants Prospective trial registration number: not provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "school counselors randomly allocated adolescents to TRT and wait list groups by tossing a coin for each participant" Judgement comment: tossing a coin is considered random allocation |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: allocation was randomised, but if school counsellors performed randomisation, this is not concealed. Unclear whether efforts were made to conceal allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and personnel were not blinded, but this was unlikely to affect the outcome |
| Blinding of outcome assessment (detection bias) all outcomes | High risk | No information regarding blinding of outcome assessors was given |
| Baseline outcome measurements similar | Low risk | Similar scores for PTSD symptoms, depression, and dissociation at baseline |
| Baseline characteristics similar? | Unclear risk | No table. Data not clearly reported. Intervention group was more likely to be in public female schools and more likely to be female. Depression and exposure to stressors seemed to be higher among women |
| Incomplete outcome data (attrition bias) Efficacy data | Unclear risk | Intention‐to‐treat analysis. More dropout in waiting‐list compared to intervention group due to military violence (~ 10% across groups). Not clear whether this would have biased results |
| Protection against contamination | Low risk | Judgement comment: students within schools were allocated to 1 of 2 interventions. Students receiving the intervention may have influenced students on the wait‐list, although this is unlikely to have a big impact on results |
| Selective reporting (reporting bias) | Unclear risk | No reference to protocol or to online trial registration. All items in the methods are also reported in the results |
| Other bias | Low risk | No other sources of bias were found |
Bass 2013.
| Study characteristics | ||
| Methods |
Study design: cluster‐RCT (8 villages in control arm, 7 villages in therapy arm) Duration of study: intervention period lasted from April through July 2011. Follow‐up data were collected within 1 month after treatment ended and 6 months later |
|
| Participants |
Country: Democratic Republic of Congo Income classification: low income from 2011 to 2012 Geographical scope: 14 villages in South Kivu Province and 2 villages on the border in North Kivu Province Healthcare setting: community groups (?NGO office) Mental health condition: PTSD Population 1. Age: 18 to 90 years 2. Gender: female 3. Socioeconomic background: despite regional instability, 80% of women were living in their territory of origin. As compared with participants in the therapy group, those in the individual‐support group were younger and were less likely to be married, and they lived with fewer people. Marital status is noted with numbers 4. Inclusion criteria a. Survivor of sexual violence mental health symptom severity cutoff b. Functional impairment cutoff 5. Exclusion criteria a. Active suicidality b. Not living at study site c. 1 village was excluded after training because of concerns regarding competency of interventionist |
|
| Interventions |
Stated purpose: to evaluate an adaptation of group cognitive processing therapy provided by community‐based paraprofessionals (psychosocial assistants), supervised by psychosocial staff at a non‐governmental organisation (NGO) and by clinical experts based in the United States. To evaluate the benefits of adding this therapy to services offered by workers trained only in case management and individual supportive counselling INTERVENTION (n = 157) Name: Cognitive Processing Therapy Delivered by: PHPs Title/name of PW and number: psychosocial assistants ‐ 15 1. Selection: not specified 2. Educational background: all psychosocial assistants had 1 to 9 years of experience providing case management and individual supportive counselling to survivors of sexual violence and at least 4 years of post–primary school education 3. Training: all underwent a 5‐ to 6‐day training session conducted in case management and specific topics, including counselling, family mediation, stress management, clinical care of survivors, and prevention of human immunodeficiency virus infection and other sexually transmitted diseases. Training provided by the International Rescue Committee (IRC) 4. Supervision: monitoring of services provided by means of monthly visits and reviews of interim monitoring forms. Congolese psychosocial supervisors who were employees of the IRC provided direct supervision to psychosocial assistants through weekly telephone or in‐person meetings; a bilingual clinical social worker trained in the United States provided in‐country supervision and communicated with US trainers through weekly calls for supervision and quality assurance 5. Incentives/remuneration: not specified Intervention details 1. Duration/frequency: cognitive processing treatment included 1 individual session (1 hour) and 11 sessions with six to eight women per group (2 hours each). Each psychosocial assistant con‐\currently led three groups. Participants in the therapy group had access to the psychosocial assistants as desired outside the therapy. 2. Content of intervention: cognitive processing therapy is a protocol‐based therapy for treating depression, anxiety, and PTSD in sexual‐violence survivors. The group format was chosen to reach large numbers of women. We used the cognitive‐only model (i.e., without a trauma narrative) because its efficacy is similar to that of the full version of the therapy, providing greater ease of administration in groups and greater retention by participants CONTROL: treatment as usual (n = 248) Support provided by Psychosocial Assistants without training in CPT. When women were informed of their eligibility, psychosocial assistants invited them to receive individual support services as desired, including psychosocial support and economic, medical, and legal referrals CO‐INTERVENTIONS: nil |
|
| Outcomes |
Patients 1. Assessments of combined depression and anxiety symptoms (average score on Hopkins Symptom Checklist (range, 0 to 3, with higher scores indicating worse symptoms)) 2. PTSD symptoms (average score on Harvard Trauma Questionnaire (range, 0 to 3, with higher scores indicating worse symptoms)) 3. Functional impairment (average score across 20 tasks (range, 0 to 4, with higher scores indicating greater impairment)) Carers Nil Process/health workers Nil Economic outcomes Nil Time points: baseline, end of treatment, 6 months after treatment ended |
|
| Notes |
Source of funding: US Agency for International Development Victims of Torture Fund and the World Bank Additional information: declarations of interest ‐ Bass J has received travel expenses from the American Red Cross for attending a meeting with the scientific advisory board, Cetinoglu T received travel expenses for a meeting as advisor to the WHO, was employed by the International Rescue Committee from Feb 2010 to Sept 2012, and was a board member of the Medecins Sans Frontieres (MSF) Greek Section between 2008 (May‐June) and 2011 (June‐July). No other authors reported any potential conflicts of interest Handling the data: as per footnotes in data and analysis Prospective trial registration number: NCT01385163 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: allocation described as "randomized", but not specified how in protocol or paper |
| Allocation concealment (selection bias) | Low risk | Judgement comment: randomisation done at interventionist level. From protocol: "randomization will be done at the level of Psychosocial Assistant (PSA). The 19 PSA will be randomly assigned to either receive training in CPT at the beginning of the study, and subsequently provide CPT to their study participants, or to wait until year 2 to receive the CPT training, and therefore continue to provide the treatment as usual" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "there will be no blinding in this study" Judgement: participants and personnel were not blinded; however, cluster‐randomisation would ensure minimal risk of performance bias because villages where intervention was given were separate from villages randomised to usual care |
| Blinding of outcome assessment (detection bias) all outcomes | High risk | Interviewers were not blinded. They were included as part of the screening interviews and helped with selection; the same interviewers then collected data in villages |
| Baseline outcome measurements similar | Low risk | Baseline outcomes differed, but effects were adjusted "To assess whether higher baseline scores in the individual‐support group biased the results, we performed sensitivity analyses restrict‐ ed to women with baseline HSCL‐25 scores higher than 2.0 (84 women in the therapy group and 171 in the individual‐support group) and found that effect sizes remained greater than 1.0" |
| Baseline characteristics similar? | Low risk | Small differences in baseline characteristics |
| Incomplete outcome data (attrition bias) Efficacy data | Low risk | Around 30% completed baseline assessment and 1 follow‐up assessment in both groups, but only 52% completed baseline and both follow‐up assessments in the individual support group compared to 65% in the therapy group. Difference is unlikely to have changed the outcome significantly |
| Incomplete outcome data (attrition bias) Safety data (e.g. adverse events) | Unclear risk | Safety data not mentioned in the paper. However, it is mentioned in the protocol that "any significant worsening of symptoms among any of the participants will be reported to the IRB [institutional review board], along with the measures taken and the results". This features as incomplete reporting, and for the purpose of this heading, it is unclear, as they may have been measured or not in the actual study |
| Protection against contamination | Unclear risk | Judgement comment: professionals were allocated within a clinic or practice, and it is possible that communication between intervention and control professionals could have occurred |
| Selective reporting (reporting bias) | Unclear risk | All relevant outcomes in the methods section are reported in the results section. Published trial protocol planned for severity of mental health symptoms as primary outcomes, which were reported, and economic indicators (non‐clinical outcome) as secondary outcomes, which were not reported. Instead, functional impairment was reported. Also safety data are not mentioned in the paper. However, it is mentioned in the protocol that "any significant worsening of symptoms among any of the participants will be reported to the IRB [institutional review board], along with the measures taken and the results". We are unsure how much these unreported data would affect the results |
| Other bias | Low risk | No other sources of bias were found |
Bass 2016.
| Study characteristics | ||
| Methods |
Study design: RCT Duration of study: recruitment June 2009 to June 2010. Intervention 3 to 5 months. Follow‐up was performed between 5 and 7 months post baseline |
|
| Participants |
Country: Iraq Income classification: lower‐middle income from 2009 to 2011 Geographical scope: Northern Iraq, Dohuk region Healthcare setting: this randomised controlled trial was conducted through primary health clinics staffed by study CMHWs Mental health condition: CMDs with post‐traumatic stress Population: Survivors of Torture and Related Trauma, in Kurdistan, Northern Iraq 1. Age: ≥ 18 years 2. Gender: both 3. Socioeconomic background: most of the sample was married; approximately half reported they were unemployed 4. Inclusion criteria a. ≥ 18 years of age b. Residing in the Dohuk governorate c. Reporting experiences of torture (defined as personally experiencing or witnessing physical torture, imprisonment, and/or military attacks) d. *Presenting with significant depressive symptoms e. Not currently psychotic or actively suicidal f. Mentally competent to give consent g. Significant depression was defined as reporting a total score ≥ 20 on the 20‐symptom, adapted Hopkins Symptom Checklist (HSCL) depression scale and meeting both of the following specific criteria necessary for a DSM‐IV (Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition) diagnosis of a major depressive episode: crying or feeling depressed most or all of the time in the last 2 weeks, and loss of interest in sex or loss of interest in things generally (as evidenced by being unable to enjoy festivals and celebrations most or all of the time) in the last 2 weeks 5. Exclusion criteria a. Nil mentioned |
|
| Interventions |
Stated purpose: to evaluate the impact of a trauma‐informed support, skills, and psychoeducation intervention provided by community mental health workers (CMHWs) on depressive symptoms and dysfunction (primary outcomes), as well as on post‐traumatic stress, traumatic grief, and anxiety symptoms (secondary outcomes) INTERVENTION (n = 159) Name: counselling intervention Delivered by: PHPs Title/name of PW and number: community mental health worker (CMHWs) ‐ 11 1. Selection: CMHWs were recruited through a joint selection process by the Department of Health in the Dohuk governorate, the Health Staff Association of Kurdistan, and staff of Heartland Alliance International (US based NGO). The main selection criteria were clinical staff from local primary clinics who had time and expressed an interest in gaining skills in mental health and psychosocial support and had experience working in rural areas with people who had experienced torture and trauma. 11 CMHWs were given refresher training on a much‐shortened version of the original HAI program that was specific for survivors of torture and imprisonment. They were presented with 9 counselling techniques and 4 to 6 activities per technique. Training emphasised core clinical skills of empathic reflection, trust building, emotional expression and regulation, and message of hope and meaning 2. Educational background: These staff, who would become CMHWs, included pharmacists, nurses, and physician assistants, and were permanent employees of the Ministry of Health. None of the CMHWs had any formal mental health training prior to the HAI project 3. Training: the project used an iterative, participatory action model for curriculum development, which took several months to complete and included (1) identifying learning needs in collaboration with Iraqi staff and CMHWs; (2) gathering information via interviews with Iraqi staff and CMHWs to map curriculum content; (3) drafting the curriculum; (4) testing the curriculum during pilot train‐the‐trainer sessions; (5) gathering post‐pilot evaluative information to revise training materials; (6) implementing revised training with CMHWs; and (7) providing ongoing evaluation and further refinement. The curriculum development team consisted of US‐based adult learning experts and mental health technical staff, as well as Iraqi programme staff with diverse expertise in curriculum development, trauma‐focused mental health practice, and Iraqi culture and society. US‐educated, licensed clinical social workers facilitated the train‐the‐trainer programme, and HAI programme staff in Iraq, mainly physicians, facilitated CMHW training 4. Supervision: monthly on‐site group supervision by a psychiatrist (TM) and weekly check‐in via mobile phone. TM available on phone anytime for questions. TM also reviewed clinical notes 5. Incentives/remuneration: not mentioned Intervention details 1. Duration/frequency: 6 to 12 sessions depending on presenting problems and client progress 2. Content of intervention: time‐limited trauma‐informed support, skills, and psychoeducation intervention. CMHWs were trained to organise interactions with clients into (1) a preparatory first session that set the stage for the development of a trusting relationship and engaged the client in the work; (2) a series of 4 to 10 ‘‘response’’ sessions in which difficulties related to the principal concerns of PTSD, depression, anxiety, traumatic grief, and impaired functioning were assessed and strategies were taught to address them; and (3) a concluding session that focused on exploring progress made in treatment, consolidation of work and skills learned, and planning for the future. The counselling process was expected to require 6 to 12 sessions depending on presenting problems and client progress CONTROL: wait‐list control (n = 50) Brief monthly check by telephone with instructions to contact CMHWs if symptoms worsened, with referral if necessary (including transport to psychiatrist or rehabilitation and training centre) CO‐INTERVENTIONS: nil |
|
| Outcomes |
Patients Adapted and translated versions of 1. Hopkins Symptom Checklist‐25 (HSCL‐25) (a 25‐item version of the HSCL) for symptoms of depression and anxiety 2. Harvard Trauma Questionnaire (HTQ) for symptoms of post‐traumatic stress 3. Inventory of Traumatic Grief for symptoms of traumatic grief 4. Functional impairment questionnaire (self‐developed) Carers Nil Process/health workers Nil Economic outcomes Nil Time points: baseline, 0 to 1 month post intervention, 3 to 5 months after baseline (control group) |
|
| Notes |
Source of funding: USAID Victims of Torture Fund (VOT) Notes on validation of instruments (screening and outcomes): instruments adapted, translated, and validated for local use. Functionality was defined based on a series of tasks and activities, identified during a prior qualitative study, regularly done by adults in Dohuk to take care of themselves and their families, and to participate in the community. Separate measures were developed for men and women Additional information: declarations of interest ‐ none Handling the data: as per footnotes in data and analysis Prospective trial registration number: not available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: ID numbers were randomly allocated to study condition by study author using Stata's randomisation function |
| Allocation concealment (selection bias) | Low risk | Judgement comment: study CMHWs were given a set of pre‐numbered consent forms with the designation of intervention or wait‐list on a piece of paper that was folded and stapled to the back |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding of participants or personnel, but this does not affect measurement and blinding was not possible in this trial |
| Blinding of outcome assessment (detection bias) all outcomes | High risk | Quote: "the majority (82%, n=154) of the follow‐up interviews were implemented by CMHWs who were blinded to the participant’s treatment status, whereas 18% (n = 34) were implemented by CMHWs or study supervisors who were unblinded" Judgement: analysis conducted by removing the 34 participants who were assessed unblinded to their treatment resulted in smaller effect sizes for depression, dysfunction, and anxiety, and larger effect sizes for trauma and traumatic grief |
| Baseline outcome measurements similar | Low risk | No important differences in baseline outcomes were noted across study groups |
| Baseline characteristics similar? | Low risk | "Demographic characteristics of the participants across the 2 arms were comparable, with no differences reaching statistical significance" |
| Incomplete outcome data (attrition bias) Efficacy data | Unclear risk | Hakan ‐ LOW: proportion of missing data was similar in intervention and control groups Yen ‐ UNCLEAR: 10% attrition rate in total. Individuals lost to follow‐up were significantly more likely to be female, self‐employed, and unmarried. If they had not dropped out of the study, they might have made a difference in the results |
| Protection against contamination | Unclear risk | Judgement comment Hakan ‐ HIGH: randomisation at participant level Yen ‐ LOW: although wait‐list participants lived in the same community, the intervention was a one‐to‐one intervention provided by CMHWs designated to each intervention participant following a set plan; therefore contamination between groups is unlikely |
| Selective reporting (reporting bias) | Unclear risk | No published clinical trial is available. All outcomes from methods section were reported on |
| Other bias | Low risk | No other risk of biases found. "This study was solely funded by the USAID Victims of Torture Fund (VOT) under grant #101978. USAID/VOT was not involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript" |
Berger 2009.
| Study characteristics | ||
| Methods |
Study design: cluster‐RCT (unit of allocation: class; unit of analysis: individual patient. 6 classes in each arm) Duration of study: February to May 2006 |
|
| Participants |
Country: Sri Lanka Income classification: lower‐middle income Geographical scope: southern coast of Sri Lanka, small town of Welligama Healthcare setting: schools Mental health condition: PTSD Population: children/adolescents 1. Age: 9 to 14 years old 2. Gender: both male and female 3. Socioeconomic background: almost all children in this school lost their homes; many lost family members or relatives in the tsunami 4. Inclusion criteria a. Aged 9 to 14 years b. All exposed to tsunami 5. Exclusion criteria a. Not specified |
|
| Interventions | INTERVENTION Name: ES‐Sl (ERASE Stress Srilanka) (n = 84) Delivered by: CP Title/name of PW and number: homeroom teachers ‐ 12 (12 trained, but 6 took part in intervention and 6 in wait‐list control) 1. Selection: teachers at the chosen school 2. Educational background: primary and secondary school teachers 3. Training: ES‐Sl course 3 days of 8‐hour training (24 hours in total). Trainers were study researchers too 4. Supervision: throughout the application of the programme, teachers were supervised on a weekly basis by 2 local mental health professionals previously trained by researchers, to ensure programme fidelity (monitoring of protocol adherence by trainers). During the first 2 sessions of the intervention, all teachers in the active group participated in two 3‐hour supervisory sessions delivered by trainers and assisted by 2 local mental health professionals, to ensure reliability of application of the protocol and to overcome potential problems. Adherence to protocol was monitored during these sessions, which included a point‐by‐point discussion of the training procedure by the trainers. Because the trainers could not remain in Sri Lanka for the entire intervention period, further fidelity was monitored by local professionals and by periodic phone and Internet supervision by the first author (R.B.) 5. Incentives/remuneration: not specified Intervention details a. Duration/frequency: twelve 90‐minute sessions (18 hours) delivered on a weekly basis b. Content of intervention: each teacher in charge of 1 class only (12 to 16 students). The 12 sessions included homework review, warm‐up exercises, experiential group activity, psychoeducational presentations, practical coping skills training, and a closure exercise, followed by a new home assignment. Each teacher was given a manual CONTROL (n = 82) Wait‐list religious class control but teachers had received training for the intervention at baseline (risk of spillover effect). Due to perform intervention on other 6 classes the following year CO‐INTERVENTIONS As above (usual care) |
|
| Outcomes |
Patients (children) 1. Two objective exposure‐related questions analysed as 2 Guttman scales § 2. Subjective exposure: Pat ‐ Horencyck questionnaire § 3. Significant distress, helplessness, and horror: 3 questions querying whether participants experienced any of those emotions as related to the tsunami, using a 5‐point scale from 1 (did not experience this emotion at all) to 5 (experienced this emotion often). So as to avoid over‐inclusion, 1 score of at least 4 was necessary to fulfil criterion A2 of PTSD § 4. Major trauma life questionnaire § 5. UCLA PTSD index 6. Subjective functional impairment: 7 items derived from the Child DIS 5‐point scale ranging from 1 (not at all impaired) to 5 (very much impaired) 7. Somatic complaints related to terrorism: 5 yes/no categorical items from the Diagnostic Predictive Scales § 8. Hope: 6‐item self‐report questionnaire § 9. Depression: 7‐ item brief BDI Carers None Process/health workers None Economic outcomes None (*: primary outcomes; §: outcomes that we have not reported in this review) Time points: none |
|
| Notes |
Source of funding: not specified Notes on validation of instruments: instruments 1, 2, and 4 are not validated. Instrument 3 is validated only in Israeli settings. 5 ‐ UCLA PTSD index is validated in Sri Lankan population. Instruments 6 to 9: internal reliability only for current setting; validated elsewhere in other settings (Beck 1974; Lucas 2001; Snyder 1997) Additional information: declarations of interest ‐ none Handling the data: as per footnotes in data and analysis Prospective trial registration number: not specified |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the randomisation procedure was done by coin tossing and choosing 1 class for each age group" |
| Allocation concealment (selection bias) | High risk | Comment: there was no allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: there was no blinding of teachers or students, but this is unlikely to affect outcomes |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | Quote: "local trained volunteers blinded to the experimental conditions administered questionnaires" |
| Baseline outcome measurements similar | Low risk | Quote: "further analyses show no difference in outcome measures at the first assessment between the ES‐SL and WL [wait‐list] groups (table 1)" |
| Baseline characteristics similar? | Low risk | Quote: "no differences between the ES‐SL experimental group and the WL [wish‐list] control group were found for gender, grade level and personal or important other exposure to tsunami" |
| Incomplete outcome data (attrition bias) Efficacy data | Low risk | Quote: "there were no missing data" |
| Protection against contamination | High risk | Quote: "there may have been a spillover effect since all the homeroom teachers participated in the training" |
| Selective reporting (reporting bias) | Unclear risk | Comment: not mentioned and not able to find protocol |
| Other bias | High risk | Comment: high risk, as clustering error not adjusted for |
Betancourt 2014.
| Study characteristics | ||
| Methods |
Study design: RCT Unit of allocation: individual Duration of study: April 2012 to December 2014 |
|
| Participants |
Country: Sierra Leone Income classification: low income Geographical scope: urban Healthcare setting: community group Mental health condition: PTSD Population: war‐affected youth ‐ patients 1. Age: 15 to 24 years 2. Gender: male and female 3. Socioeconomic background: not specified 4. Inclusion criteria (including threshold cutoff score of measurement tool) a. Youth in war‐affected regions, many of whom were child soldiers (SRQ‐20 ≥ 8) b. Age 15 to 24 years (consistent with UN definition of “youth”) c. Indication of interest to continue education per a series of survey screening questions d. Psychological distress as indicated by a total score 0.5 standard deviations above total psychological distress levels (combined internalising and externalising problem scores) e. Self‐reported impairment in daily functioning 5. Exclusion criteria a. Individuals were excluded and referred for mental health services for active suicidality or psychosis |
|
| Interventions |
Stated purpose: to test the effectiveness of a 10‐session cognitive‐behavioural therapy (CBT)–based group mental health intervention for multi‐symptomatic war‐affected youth (aged 15 to 24 years) in Sierra Leone INTERVENTION (n = 222) Name: Youth Readiness Intervention (YRI) Delivered by: PHP Title/name of PW and number: 4 male and 4 female local mental health workers were trained as counsellors 1. Selection: not specified 2. Educational background: all counsellors had a bachelor’s degree or a diploma in social work or a related field 3. Training (contents, duration, by whom): 2‐week training conducted by members of the authorship team. Four counsellors who completed an intensive 2‐week training conducted by members of the authorship team led training workshops for other potential counsellors. Those who completed training and achieved a high level of competency in the manualised treatment were employed by the study (n = 8) 4. Supervision: a senior local mental health worker provided weekly supervision to all counsellors in‐country; study leaders, including 2 clinical psychologists, provided additional weekly group clinical supervision by telephone Intervention details: Youth Readiness Intervention (YRI), which integrates evidence‐based common practice elements from cognitive‐behavioural therapy (CBT) and group interpersonal therapy (IPT) to address co‐occurring mental health symptoms and functional problems that may impede life success and functioning in war‐affected youth. Delivered by CHW for patients 1. Duration/frequency: delivered over 10 to 12 sessions per week for 90 minutes 2. Content of intervention (by types of health workers and per patients/carers): core components include the following: psychoeducation about trauma and its impact on interpersonal relationships; self‐regulation and relaxation skills (e.g. deep abdominal breathing); cognitive restructuring (i.e. addressing negative self‐perceptions due to trauma); behavioural activation; communication and interpersonal skills; and sequential problem‐solving. The YRI incorporates trauma psychoeducation and discussion of the impact of trauma on interpersonal relationships and self‐concept as a core guiding framework. The trauma‐informed focus on comorbid anger, emotion dysregulation, and overall distress (internalising/externalising problems and interpersonal and functional impairments, including school functioning) was identified via intervention development research on the mental health of war‐affected youth in the region CONTROL (n = 214) Wait‐list control group: received the intervention 1 year after the intervention group CO‐INTERVENTIONS: after the YRI intervention period (during which half of all YRI and control participants were randomly assigned to receive the YRI), youth were randomly assigned to receive access to a free educational opportunity, EducAid, in Fall 2012 or 2013, stratifying by condition (YRI or control). EducAid is a programme run by a British charity that uses an alternative educational style in which students study in small groups and work at their own pace to achieve competency per each grade of the national curriculum. Students then sit for a grade completion examination per the national standard. Although subsidies were offered only at EducAid, we followed up on all participants in any educational opportunities that they pursued |
|
| Outcomes |
Patients 1. Oxford Measure of Psychosocial Adjustment (OMPA) 2. Difficulties in Emotion Regulation Scale 3. Post‐traumatic Stress Disorder Reaction Index (PTSD‐RI) 4. Classroom Performance Scale ‐ 20‐item teacher survey Carers None Process/health workers None Economic outcomes (and where these can be found, e.g. ref or table number) None (asterisk for study's primary outcomes; star: outcomes that we have not reported in this review) Time points: baseline, 10 weeks, 6 months |
|
| Notes |
Source of funding: this study was supported by the United States Institute of Peace (USIP‐008‐10F), the UBS Optimus Foundation (UBS‐5253), the National Institute for Mental Health (5K01MH077246‐05; 1F31MH097333‐01A1), the National Institute of Aging (5P30AG024409‐08), Harvard Catalyst, the Julie Henry Junior Faculty Development Fund, the Australian Psychological Society, and the Australian National Health and Medical Research Council Notes on validation of instruments (screening and outcomes): validated instruments Additional information: none. Declarations of interest ‐ none Handling the data(e.g. imputed values/other calculations we have made): we used linear mixed‐effects regression models to assess effects of the YRI on mental health and functional outcomes over time, as well as the effect of the education subsidy. The primary mode of analysis was intention‐to‐treat, with 20 multiply imputed data sets incorporated to account for missing values for all individuals, including those lost to follow‐up (10% post intervention, 15% at 6‐month follow‐up). Multiple imputation was used to impute values for scales when item‐level missingness was greater than 25% for a given scale. Otherwise, missing items within scales were imputed using Markov Chain Monte Carlo (MCMC) methods with an added error term Prospective trial registration number: NCT01684488 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: "a randomization sequence generated in STATA 12.0 SE 23 was used to assign participants to condition, stratified by sex and age (younger: 15–17 years old; older: 18–24 years old)" |
| Allocation concealment (selection bias) | Low risk | Judgement comment: "randomization occurred after baseline assessment; assessors were blinded to participants’ condition (Figure 1 provides a Consolidated Standards of Reporting [CONSORT] diagram)" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and personnel were not blinded to treatment allocation, but this unlikely had any influence on the outcome |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | "A team of trained local research assistants conducted blinded interviews (approximately 90 minutes) in Krio at baseline, postintervention assessment, and 6‐month follow‐up" |
| Baseline outcome measurements similar | Low risk | Not commented on, but baseline outcomes similar |
| Baseline characteristics similar? | Low risk | Not commented on, but baseline characteristics similar (Table 1) |
| Incomplete outcome data (attrition bias) Efficacy data | Low risk | Loss to follow‐up similar in different groups (Figure 1) |
| Protection against contamination | Unclear risk | Judgement comment: allocation by patient; risk of contamination |
| Selective reporting (reporting bias) | Unclear risk | Symptom severity at post‐intervention assessment; 6‐month follow‐up Showed coefficients post intervention but not at 6‐month follow‐up |
| Other bias | Low risk | No other sources of bias were found |
Bolton 2003.
| Study characteristics | ||
| Methods |
Study design: cluster‐randomised parallel‐group gender‐stratified controlled clinical trial (unit of randomisation: village; unit of analysis: individual. 15 villages in each arm) Duration of study: February 2002 to July 2002; 6‐month follow‐up completed in January 2003 |
|
| Participants |
Country: Uganda Income classification: low income Geographical scope: 30 villages in Rakkai Province and contiguous half of Masaka Province in South West Uganda; rural Healthcare setting: community (community centres, churches, open spaces) Mental health condition: depression (DSM‐IV depression and sub‐syndromal depression) Population: patients 1. Age: adults (> 18 years); mean age ranged from 27 years (SD 13.5) to 66 years (SD 10.5) 2. Gender: both (stratified for gender) 3. Socioeconomic background: not stated except for education (mean 4.7 years (SD 2.8) Intervention; 3.9 years (SD 3.3) control) 4. Inclusion criteria: 3‐stage screening: Stage 1 (by trained local World Vision staff) identified 20 people from the selected 15 villages (8 for males and 5 for females) with depressive symptoms in local idiom; Stage 2: same interviews visited identified people, and if they admitted to having 1 of 2 locally approximate depressive conditions, informed consent was sought; Stage 3: eligibility expanded to include sub‐syndromal depression by DSM‐IV criteria (less 1 DSM criterion); screening for depression was done by 10 trained and experienced local World Vision staff using a composite instrument (Bolton 2004) consisting of HSCL (to assess depressive symptoms and to diagnose DSM‐IV major depression (excluding criteria related to exclusion of medical causes and drug effects), a previously validated algorithm), a locally developed culturally appropriate instrument to assess functional impairment (separately for women and for men), and ethnographically validated questions that assessed significant distress and duration of depression 5. Exclusion criteria a. Absence of symptoms of depression b. Age < 18 years c. Unwillingness to meet weekly (additional criteria revised after screening commenced) d. People very different in age from the rest included in a village e. Those appearing currently suicidal |
|
| Interventions |
Stated purpose: to test the efficacy of a manual‐based, time‐limited group psychotherapeutic approach in relieving depressive symptoms and improving functioning; and to demonstrate that psychotherapy trials are feasible in sub‐Saharan Africa INTERVENTION (n = 107) Name: Group Interpersonal Therapy for Uganda (IPT‐G‐U), 116 people (of 163 in 15 villages originally randomised, and 139 invited to participate; 107 completed intervention and follow‐up) Delivered by: LHWs Title/name of PW and number: group leaders ‐ 9/10 who completed training 1. Selection: local person of the same sex as the sex‐segregated group; non‐clinicians fluent in English and Luganda employed by World Vision 2. Educational background: completed high school (college‐level) 3. Training: duration: 2 weeks intensive training. Trained by 2 faculty members of the New York State Psychiatric Institute (members of the team led by Myrna Weissman that developed IPT and the group adaptation of IPT) assisted by a trained psychologist and an experienced group therapist employed by World Vision. Content of training: participating in local adaptations of the IPT manual; explanations of treatment process and contract; explanations of the role of group leaders in helping members to identify problem areas and in discussing locally acceptable variations to absolute confidentiality; identification of, and agreement about, interpersonal problem areas likely to be encountered in group work according to the 4 domains in IPT; using the principles of IPT to identify personal problems and to support one other to find options and to facilitate implementation. Format: didactic teaching and experiential group processes with role‐plays and group exercises 4. Supervision: by local World Vision mental health professionals involved in training; format and duration not described 5. Incentives/remuneration: weekly payment for 16 weeks (amount not stated) Intervention details 1. Duration/frequency: 16 weekly 90‐minute sessions 2. Content of intervention: group work led by group leader who first diagnoses depression; works with group member to identify problem areas associated with current symptoms and to identify the 4 areas of interpersonal difficulties that served as triggers for depression; conducts weekly review of mood and encouragement of participant's description of events that could link to the mood; and facilitates support and solutions from group members CONTROL (n = 117) Treatment as usual (treatment by local traditional healers, no treatment, or, in rare cases, hospitalisation), 138 people (of 178 randomised in 15 villages and 145 invited to participate; 117 completed intervention and follow‐up) CO‐INTERVENTIONS: no restrictions on additional interventions (utilisation and nature of any not described) |
|
| Outcomes |
Patients 1. Screening: HSCL and local functional impairment scale 2. Prevalence of DSM‐IV major depression (excluding criteria related to exclusion of medical causes and drug effects ‐ using Mollica DSM‐IV algorithm for A, C, and E criteria)* 3. HSCL mean scores 4. Functional Impairment scores (sex‐specific 9‐item questionnaire) 5. Depression in subgroups continuing informal group meetings between 2 weeks and 6 months vs subgroup not meeting after group intervention Carers Not applicable Process/health workers No direct outcomes reported: indirect outcomes are the results of the trial Economic outcomes Not reported Time points: initial assessment 2 weeks after intervention, follow‐up at 6 months (*: primary outcomes) |
|
| Notes |
Source of funding: supported by World Vision, Washington, DC; Psychotherapy Core of the Child Intervention Research Center Columbia University (NIMH grant #5P30 MH60570); Center for International Emergency Disaster and Refugee Studies; Johns Hopkins Bloomberg School of Public Health; Mellon Foundation Notes on validation of instruments (screening and outcomes): all screening instruments and outcome measures locally adapted and validated in previous exercises and published; the HSCL scale consisted of 14 items, with 4 responses for each item related to the degree of distress due to a particular symptom (range 0 to 42 points); higher scores indicate more severe depression; function scale consisted of 9 items, with 5 responses for each item, indicating degree of difficulty in completing the activity (range 0 to 36 points); higher scores indicate more dysfunction Additional information: IPT attendance was high: 54% attended at least 14/16 sessions; 4% attended ≤ 10 sessions. Declarations of interest ‐ none Handling the data: as per footnotes in data and analysis Prospective trial registration number: not prospectively registered |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from report: "random assignment was performed by enumerating the villages and using a random number table to determine study allocation" Comment: cluster‐randomisation of 30 villages to 15 in each arm was done using a random numbers table; the 30 villages of 154 eligible villages were chosen for a previous prevalence study ‐ Bolton 2002, unpublished ‐ that used weighted random sampling based on government census data |
| Allocation concealment (selection bias) | Unclear risk | Quote from report: "each list began with those who met the original diagnostic criteria, followed by those who fell short by a single criterion, in order of decreasing depression score. Interviewers visited all persons in the order they appeared on the list. The interviewer re‐read the consent form, advised persons about the study group to which their village had been allocated, and asked them to confirm their willingness to continue in the study. Interviewers continued down the list until they had at least 8 participants (at which point they did not contact the remainder of the list) or until they reached the end of the list" Comment: allocation of participants was not concealed although cluster‐randomisation of villages was the unit of randomisation; eligibility criteria were modified to exclude people whose age varied widely from the rest of those selected in each village, to ensure better outcomes with group IPT (based on previous experiences) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: participants and personnel were not blinded; however, cluster‐randomisation would ensure minimal risk of performance bias because villages where intervention was given were separate from villages randomised to usual care |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | Quote from report: "the baseline assessments were conducted in the villages, with the randomisation of village groups to intervention or control or control status done afterwards to ensure that interviewers were not aware of participant trial status at baseline. In an effort to keep interviewers unaware of the participants’ intervention status, the post‐intervention and 6‐month follow‐up assessments were conducted at a centrally located community centre. At these assessments, trial participants were transferred from their villages and were asked not to divulge either their village of origin or their treatment assignment status. To reduce measurement error that might have arisen from different interviewing styles, study participants were interviewed by the same interviewer at each stage of the study" Comment: the period from recruitment to first assessment was 18 weeks and to second assessment was a further 6 months. There is a possibility that the recruiter (who did not administer the intervention) may have guessed allocation for the first assessment in a few instances, but this is unlikely to have altered results significantly, given the magnitude of the differences in results between groups |
| Baseline outcome measurements similar | Low risk | Quote from 6‐month follow‐up report: "at baseline 86% of participants in the intervention group met the modified diagnostic criteria for major depressive disorder and 94% of those in the control group met these criteria (prevalence difference was not significant)" Quote from primary report: "however, there was a significant difference in the proportions who met the original depression diagnostic criteria, both among those who completed the study and all those on the original lists of eligible participants (TABLE 1). (Tests for differences in baseline characteristics were performed using standard significance tests and were not adjusted for cluster effects. However, because we found a positive correlation between clusters, adjusting for cluster effects would tend to reduce variance and cause group differences to be even less significant than the values reported herein)" Comment: discrepancy in interpretation of baseline differences in the 2 reports; results adjusted for clustering and for baseline outcome differences |
| Baseline characteristics similar? | Low risk | Comment: no differences in age or education nor in symptom duration |
| Incomplete outcome data (attrition bias) Efficacy data | Low risk | Quote from 6‐month follow‐up report: "six months after the post‐intervention 103 (96%) of the 107 participants in the intervention group who completed the trial and 113 (97%) of the 117 completed the trial and 113 (97%) of the 117 controls were reassessed" Comment: attrition was high in both groups due to the 3‐stage screening process; however, 116/163 eligible and randomised to IPT consented to participate; 132/178 randomised to control consented to participate; results did not differ in completer analyses and in 2 sets of ITT analyses |
| Protection against contamination | Low risk | Comment: cluster‐randomisation of intervention arms precluded contamination |
| Selective reporting (reporting bias) | Unclear risk | Comment: trial was not prospectively registered, but all pre‐stated outcomes were reported |
| Other bias | Low risk | Comment: no other biases were detected |
Bolton 2007.
| Study characteristics | ||
| Methods |
Study design: randomised parallel‐group assessor‐blinded 3‐arm clinical trial Duration of study: May 2005 to December 2005 |
|
| Participants |
Country: Uganda Income classification: low income Geographical scope: 2 camps (Awer and Unyama) for internally displaced people near Gulu town in northern Uganda; semi‐rural; > 20,000 inhabitants each; minimal socioeconomic facilities Healthcare setting: group meetings Mental health condition: anxiety, depression, conduct problems, some PTSD symptoms Population: adolescents 1. Age: 14 to 17 years 2. Gender: both 3. Socioeconomic background: Acholi youth from socioeconomically deprived backgrounds living in camps for displaced youths 4. Inclusion criteria a. Age 14 to 17 years b. Scored > 32 on depression scale; > 0 on function scale c. Symptoms > 1 month d. Camp resident for previous month 5. Exclusion criteria a. Inability to be interviewed due to physical or cognitive difficulties b. Severe suicidal ideation or behaviour |
|
| Interventions |
Stated purpose: to assess effects of locally feasible interventions on depression, anxiety, and conduct problem symptoms among adolescent survivors of war and displacement in northern Uganda INTERVENTION 1 Name: G‐IPT (psychotherapy‐based intervention); 105 people randomised (103 enrolled) Delivered by: LHWs Title/name of PW and number: G‐IPT facilitators ‐ 12 1. Selection: same gender as groups; local Acholi, spoke both English and the local language Luo, and had minimal previous mental health intervention experience 2. Educational background: not stated 3. Training: 2 weeks of intensive training by Columbia University faculty using a locally adapted G‐IPT treatment manual (unpublished) 4. Supervision: weekly direct supervision by World Vision Uganda staff and weekly phone supervision of written case notes for adherence to study protocol with study personnel in the USA; supervisors had previous IPT experience and received weekly telephone supervision with US trainer 5. Incentives/remuneration: Not stated Intervention details 1. Duration/frequency: 16 weekly group meetings lasting 90 to 180 minutes (preceded by 1 to 2 individual meetings to explain treatment and to draw up a treatment plan) 2. Content of intervention: 6 to 8 same sex groups of adolescent Acholi youths per facilitator; manualised G‐IPT based on the concept that depressive episodes are related to difficulties in 1 or more of 4 interpersonal areas: grief, interpersonal disputes, role transitions, and interpersonal deficits. The focus is on improving depressive symptoms and functioning by identifying interpersonal problems most relevant to the current depression and by assisting the individual in building skills to manage those problems. "The flow and organization of the IPT‐G sessions was organized in three phases: The initial phase (corresponding roughly to sessions 1‐4) focused on building rapport, setting personal treatment goals and learning to identify mood states. The middle or working phase (corresponding roughly to sessions 5‐12) involved exploring major issues related to grief, transitions, disputes and building interpersonal skills and connections among group members. The final, closure phase (corresponding roughly to sessions 13‐16) was dedicated to preparing for the end of the IPT‐G intervention and the close of formal group meetings. During this final phase, participants in the IPT‐G intervention groups were encouraged to discuss how they might continue to provide support and connection to one another after the formal ending of the group (if this topic arose naturally)" INTERVENTION 2 Name: creative play (activity‐based intervention); 105 people randomised (99 enrolled) Delivered by: LHWs Title/name of PW and number: creative play facilitators ‐ 2 people 1. Selection: War Child Holland staff (selection not described) 2. Educational background: not stated 3. Training (contents, duration, by whom): not stated 4. Supervision: weekly or bi‐monthly supervision by War Child Holland psychosocial specialist, who reported bimonthly by telephone with US study personnel 5. Incentives/remuneration: not stated Intervention details 1. Duration/frequency: 16 weekly group meetings lasting 90 to 180 minutes (preceded by 1 to 2 individual sessions where treatment was explained) 2. Content of intervention: 4 groups (2 per camp) of 25 to 30 adolescents of both genders per group; based on War Child Holland manual adapted for adolescents with depression; for war‐affected youth, based on the premise that a youth’s resilience is strengthened by verbal and non‐verbal expression of thoughts and feelings through age‐appropriate creative activities such as songs, art, role‐plays, music, sports, games, and debates. Each activity served specific psychosocial goals, and after the activities, facilitators led discussions on what participants and facilitators thought about the activity as a means of drawing real‐life lessons. "Sessions 1‐4 focused on getting to know one another and setting the group rules. Sessions 5‐12 were more in‐depth and focused on issues in the group, in particular the interrelationships between the adolescents in the group and developing opportunities for self‐expression. Sessions 13‐15 were dedicated to closure and preparing for a closing inter‐generational event. The final CP [creative play] session (session 16) was an inter‐generational event where caregivers were invited to attend along with the young people. This final session at each camp was hosted by one of the young people serving as Master of Ceremonies and facilitated by the participating young people themselves. The youth facilitated some of their CP activities for the family members who attended" CONTROL: wait‐list controls, 104 people (102 enrolled); received no specific intervention but were free to access any services or programmes that they would have received in the absence of the study CO‐INTERVENTIONS: not stated |
|
| Outcomes |
Patients Locally developed 1. Acholi Psychosocial Assessment Instrument depression symptom scale scores* 2. Improvements in anxiety symptoms, conduct problems, and functioning on the APAI (minimum score for clinically significant symptoms on the APAI = 32; maximum score 105; higher scores = more symptoms) 3. Functional impairment scores (range 0 to 36 for girls (9 items) and 0 to 20 for boys (5 items), with higher scores representing a greater degree of impairment) 4. Qualitative interviews § Carers Not applicable Process/health workers Not assessed Economic outcomes Not reported (*: primary outcomes; §: outcomes that we have not reported in this review) Time points: baseline, 2 weeks to 1 month of completing interventions |
|
| Notes |
Source of funding: World Vision and War Child Holland; Ruth and David Levine Foundation Notes on validation of instruments (screening and outcomes): APAI locally developed: scale reliability and validity were evaluated for a sub‐sample (178 people) of adolescents interviewed for trial eligibility (667 people). Cronbach alpha (a measure of internal reliability) was 0.92. Concurrent validity established by comparing depression symptoms scale scores between cases and non‐cases were identified by carer‐youth pairs and threshold scale score of 32 identified (1 SD below mean score for cases); test‐re‐test reliability for depression symptom scale was 0.84 (in 30 convenience sub‐samples re‐administered the APAI after 5 days) Additional information (e.g. provided by authors; existence of a published study protocol): declarations of interest ‐ none Handling the data: as per footnotes in data and analysis Prospective trial registration number: not prospectively registered |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "eligible youths were then randomly assigned to a study group. Random allocation was done by computerized generation of a random number between 1 and 400 for each eligible participant, ordering them by number and assigning the first third to IPT‐G, the second third to CP and the final third to the wait‐control group"; "of the total sample screened (N = 667), 300 individuals met original inclusion criteria, were stratified by camp and sex, and randomised to a study group. Of these 300, 290 were enrolled in the study. Of the remaining 10 individuals, 1 was already involved in the CP program in a neighbouring camp, 4 could not be located, and 5 refused. To meet our original sample size (300), we randomised an additional 38 individuals whose depression symptom scores were between 28 and 31 points. This relaxation of a trial eligibility criterion is acceptable when study design consequences are minimal.19 The first 14 individuals all consented and therefore, the remainder were not approached" Comment: very few people are in this non‐randomised group; unlikely to make any difference in outcomes |
| Allocation concealment (selection bias) | Unclear risk | Quote: "eligible youths were then randomly assigned to a study group. Random allocation was done by computerized generation of a random number between 1 and 400 for each eligible participant, ordering them by number and assigning the first third to IPT‐G, the second third to CP and the final third to the wait‐control group" Comment: not specified who allocated them and whether allocation was concealed from them; however, there were no major differences in baseline prognostic variables or outcome measures |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: no blinding of participants or personnel but unlikely to affect outcomes |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | Quote from report: "interviewers were blinded to interviewees' intervention status" Comment: outcome assessors were blinded to allocation |
| Baseline outcome measurements similar | Low risk | Baseline depression and function scores were similar in intervention and control arms |
| Baseline characteristics similar? | High risk | Comment: "except for a slightly older age among wait‐list controls, the 3 study groups did not vary significantly", but age was not adjusted for in statistical analysis |
| Incomplete outcome data (attrition bias) Efficacy data | Low risk | Quote: "the study instrument was re‐administered to 282 (90%) of the original 314 participants within 1 month of completing both interventions" Comment: 304/314 enrolled and 261 (82 + 89 + 90; i.e. 83%) completed analysis |
| Protection against contamination | High risk | Comment: only 2 camps chosen with refugee settings. Likely to be contamination (no clustering) |
| Selective reporting (reporting bias) | Unclear risk | Comment: all outcomes found in report and in paper, although conduct not reported in published article. These were available when study author was asked |
| Other bias | Low risk | Comment: none detected |
Bolton 2014 (Iraq).
| Study characteristics | ||
| Methods |
Study design: 3‐arm cluster‐RCT (health workers were trained in either of 2 interventions: 11 BATD, 9 CPT); unit of allocation: individual patients (treatment or wait‐list control). Blinding of assessors was intended but was violated in 15% of interviews Duration of study: 16 months. Recruitment May 2009 to June 2010. Intervention June 2009 to August 2010. Assessments concluded in January 2011 |
|
| Participants |
Country: Iraq Income classification: lower‐middle income from 2009 to 2011 Geographical scope: rural; Kurdistan, Northern Iraq Healthcare setting: 14 government primary healthcare clinics and 1 outpatient clinic Mental health condition: survivors of systematic violence Population: survivors of systematic violence 1. Age: 18 and older 2. Gender: both 3. Socioeconomic background: respondents in a qualitative study conducted during the year before trial commencement reported that their current problems included poverty and reduced economic function. Between 48% and 61% of participants in this trial were unemployed 4. Inclusion criteria a. Survivors of systematic violence (experiencing and/or witnessing physical torture, imprisonment, and/or military attacks) b. Living in the governorates of Erbilor or Sulaimaniyah c. Age 18 or over d. Fluent in Sorani Kurdish e. Reported significant depression symptoms using an adaptation of the HSCL‐25 that included the 15 standard depression items plus 5 local depression‐like symptoms (a score of 2 or 3 on ≥ 1 of the DSM‐IV A criteria related to presence of depressive symptoms or anhedonia and total symptoms score ≥ 20) f. Mentally competent to consent 5. Exclusion criteria a. Current psychotic symptoms or active suicidality |
|
| Interventions |
Stated purpose: to assess the effectiveness of 2 psychotherapeutic interventions: Behavioural Activation Treatment for Depression (BATD) and Cognitive Processing Therapy (CPT) in reducing depression symptoms INTERVENTION 1 (n = 114) Name: Behavioural Activation Treatment for Depression (BATD) Delivered by: PHPs Title/name of PW and number: community mental health workers ‐ 11 1. Selection: nurses, pharmacist assistants, or physician assistants employed by clinics. Worked in government clinics in rural areas; previously trained by Heartland Alliance International in basic supportive counselling; seeing clients before the study; randomised to receive training in Behavioural Activation Treatment for Depression 2. Educational background: completed high school; previously trained in supportive counselling 3. Training: by US trainers for 2 weeks, then ongoing training by supervisors 4. Supervision: local supervisors who were trained by US trainers first in‐person for 2 weeks, then via Skype weekly Intervention details: BATD (Behavioural Activation) 1. Duration/frequency: 12 sessions, meant to be completed within 3 to 5 months but took up to 15.5 months during implementation 2. Content of intervention: psychotherapy based on helping individuals plan for and engage in positive activities daily based on values and goals of the individual in multiple life areas. Adapted culturally and for limited language/writing proficiency and extreme poverty INTERVENTION 2 (n = 101) Name: Cognitive Processing Therapy for Depression Delivered by: health professionals Title/name of PW and number: community mental health workers ‐ 9 1. Selection: nurses, pharmacist assistants, or physician assistants employed by clinics. Wrked in government clinics in rural areas; previously trained by Heartland Alliance International in basic supportive counselling; seeing clients before the study; randomised to receive training in Cognitive Processing Therapy for Depression 2. Educational background: completed high school; previously trained in supportive counselling 3. Training: by US trainers for 2 weeks, then ongoing training by supervisors 4. Supervision: local supervisors who were trained by US trainers first in‐person for 2 weeks, then via Skype weekly Intervention details: CPT 1. Duration/frequency: 12 sessions, meant to be completed within 3 to 5 months but took up to 15.5 weeks during implementation 2. Content of intervention: included cognitive re‐structuring (identifying, challenging, and modifying maladaptive beliefs) and emotional processing of traumatic events, with ultimate goals for clients to be able to approach (vs avoid) their feelings about the trauma and to modify rigid, inaccurate, or over‐generalised trauma‐related beliefs to be more flexible, accurate, and adaptive. Adapted culturally and for limited language/writing proficiency CONTROL (n = 66) Enhanced usual care. Wait‐list control with monthly follow‐up by BATD or CPT CMHWs and specialist referral if needed for worsening symptoms CO‐INTERVENTIONS: nil |
|
| Outcomes |
Patients 1. HSCL‐25: Depression* 2. Function scale: dysfunction* 3. HTQ: post‐traumatic stress 4. Inventory of Traumatic Grief: traumatic grief 5. HSCL‐25: Anxiety Carers Nil Process/health workers Nil Economic outcomes Nil (asterisk for study's primary outcomes; star: outcomes that we have not reported in this review) Time points: post end of intervention: 1 month |
|
| Notes |
Source of funding: USAID Victims of Torture Fund (VOT) Notes on validation of instruments (screening and outcomes): validated Additional information (e.g. provided by authors, existence of a published study protocol): protocol available. Declarations of interest ‐ none Handling the data: nil Prospective trial registration number: ClinicalTrials.Gov NCT00925262 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization of CMHWs and participant IDs was done by JB using Stata’s randomization function. Investigators kept a master list of each study ID’s assignment for checking randomization fidelity" Judgement comment: low risk; Stata was used to randomise |
| Allocation concealment (selection bias) | Low risk | Quote: "if a person consented the CMHW opened a sealed envelope attached to the consent form containing the participant’s assignment" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and personnel were not blinded; however, cluster‐randomisation would ensure minimal risk of performance bias because villages where intervention was given were separate from villages randomised to usual care |
| Blinding of outcome assessment (detection bias) all outcomes | Unclear risk | 85% of interviews were done by CMHWs or supervisors blinded to allocation Judgement: as not all are blinded, unclear what the risk of bias would be for the 15% of unblinded assessments |
| Baseline outcome measurements similar | Low risk | Mean dysfunction and grief scores higher among CPT‐site participants. Otherwise, similar outcome measurements. Mean dysfunction and grief scores higher among CPT‐site participants. Otherwise, similar outcome measurements |
| Baseline characteristics similar? | Low risk | Baseline characteristics pretty similar. However, "[a] review of demographic characteristics (Table 3) identifies that the proportion of females is smaller among BATD‐site controls than the BATD group; the opposite is true for CPT and CPT‐site controls. There were also differences in marital status across the groups with the pro‐ portion of widows smallest in the BATD group and the proportion of single/divorced the smallest in the CPT control group. Employment status also varied by group, with the CPT controls having the highest proportion of not working participants and the BATD participants having the highest proportion of self‐employed or irregular workers" |
| Incomplete outcome data (attrition bias) Efficacy data | Low risk | Seven BATD participants (6%) never began treatment, and 25 (28%) dropped out before completion (nine sessions). Those who did not begin or dropped out of BATD were more likely to be from the Sulaimaniyah governorate, have no education and be self‐employed, or have irregular work compared with those who completed treatment. Six CPT participants (6%) did not start treatment, and 15 (21%) dropped out before completion (also 9 sessions). Those who did not begin or dropped out of CPT were more likely to be male and married compared with those who completed treatment. Ten (15%) controls dropped out and 2 (3%) could not be located at follow‐up. Those controls who were not followed up were more likely to be male, living in Sulaimaniyah governorate, and to have at least some education compared with controls who were followed up. Participants who dropped out of the trial after having started treatment rarely gave reasons beyond not wanting to continue |
| Incomplete outcome data (attrition bias) Safety data (e.g. adverse events) | Low risk | Safety data were not reported as a specific outcome, but it is mentioned that only 1 unintended effect was reported in the study. "Participants who dropped out of the trial after having started the treatment rarely gave reasons beyond not wanting to continue. One CPT and 2 BATD participants left to seek psychiatric help. One CPT participant moved away, one was referred for psychosis, and one left after being verbally abused by her husband for getting treatment. This was the only significant harm or unintended effect reported in the study. One control was referred to a psychiatrist for worsening symptoms" |
| Protection against contamination | High risk | Judgement comment: both patients and CMHWs were randomised, but it is likely that patients could have received some of the intervention from other patients |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in paper and trial registry were reported |
| Other bias | Low risk | No other sources of bias were found |
Bolton 2014 (Thailand).
| Study characteristics | ||
| Methods |
Study design: RCT single‐blinded wait‐list randomised controlled trial Duration of study: August 2011 to November 2012 |
|
| Participants |
Country: Thailand Income classification: upper‐middle income Geographical scope: urban; Mae Sot is in northwest Thailand, 5 km from Myanmar Healthcare setting: community groups Mental health condition: post‐traumatic stress and moderate/severe depression Population (mention whether patient, carer, or dyad) 1. Age > 18 2. Gender: any 3. Socioeconomic background: Burmese survivors of imprisonment, torture, and related traumas, with flexibility based on client presentation. Displaced to Thailand. Over 50% unemployed; over 50% educated high school or higher 4. Inclusion criteria (including threshold cutoff score of measurement tool) a. Reported trauma exposure b. Met severity criteria for moderate/severe depression (HSCL‐25: DSM‐IV criteria ‐ criterion A or B + 3 or 4 symptoms from categories within criterion C; and/or post‐traumatic stress: HTQ: DSM‐IV criteria: any 2 or 4 criteria) 5. Exclusion criteria a. Active psychosis |
|
| Interventions | INTERVENTION (n = 182) Name: CETA (Common Elements Treatment Approach) Delivered by: PHPs and CPs Title/name of PW and number: counsellors 1. Selection: teachers, health workers, and counsellors from the community 2. Educational background: as above 3. Training (contents, duration, by whom): 10 full days; role‐play; small groups; didactic; by US‐based clinical psychologist 4. Supervision: weekly meetings between counsellor and clinical supervisor and in the form of weekly phone conversations between clinical supervisors and JHU trainers/clinical psychologists. Clinical supervisors had at least a high school education, were bilingual in English and Burmese, and preferably had counselling experience 5. Incentives/remuneration: not specified Intervention details (according to PHW/CWs and whether aimed at carers and/or patients) 1. Duration/frequency: approximately 8 to 12 weekly individual sessions of 50 to 60 minutes in length; total length of intervention 3 to 5 months 2. Content of intervention (by types of health workers and per patients/carers): designed to treat symptoms of common mental health disorders. Consisted of 9 elements that focused on a torture and violence‐exposed population (engagement, psychoeducation, anxiety management, behavioural activation, cognitive coping/re‐structuring thinking, imaginal gradual exposure, in vivo exposure, safety, screening, and brief intervention for alcohol) CONTROL (n = 165) No care: wait‐list. During this study, few mental health services were available to Burmese refugees, except counselling at a Burmese‐run clinic. Many Burmese reported reluctance to go there (or to other public places) for fear of apprehension and deportation by Thai authorities CO‐INTERVENTIONS: nil |
|
| Outcomes |
Patients 1. Hamilton Symptom Check List (HSCL‐25) ‐ depression subscale* 2. Harvard Trauma Questionnaire (HTQ) (PTS symptoms)* 3. Local functional impairment scale* 4. HSCL‐25 ‐ Anxiety subscale* 5. Aggression questionnaire 6. Alcohol Use Disorders Identification Test (AUDIT) 7. Numbers and types of traumatic events either witnessed or experienced 8. Current problems (6 items: food insecurity, negative workplace experiences, fear of police harassment, fear of detention, financial difficulties, social relationship problems) Carers None Process/health workers None Economic outcomes (and where these can be found, e.g. ref or table number) None (asterisk for study's primary outcomes; star: outcomes that we have not reported in this review) Time points: 14 months post end of intervention |
|
| Notes |
Source of funding: funding was provided by USAID Victims of Torture Fund Notes on validation of instruments (screening and outcomes): validated instruments; partially translated Additional information: declarations of interest ‐ study authors declared no competing interests Handling the data: as per footnotes in data and analysis Prospective trial registration number: ClinicalTrials.gov NCT01459068 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: "the project site director generated these random numbers using STATA" |
| Allocation concealment (selection bias) | Low risk | Judgement comment: "each counselor then assigned participants the next available ID number from a block of 20 sequential participant ID numbers per counselor randomly allocated to intervention or wait‐list control (WLC) status. The project site director generated these random numbers using STATA. Counselors opened a pre‐sealed envelope (corresponding to the ID number) containing assignment to immediate treatment or wait‐list" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding of participants or personnel but unlikely to affect outcomes |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | "It was single‐blinded in that interviewers at baseline and follow‐up did not know to which study arm the interviewees belonged" |
| Baseline outcome measurements similar | Low risk | Baseline outcomes were similar. |
| Baseline characteristics similar? | Low risk | Baselines characteristics were similar (Table 2) |
| Incomplete outcome data (attrition bias) Efficacy data | Unclear risk | CETA arm: 34/182 lost to follow‐up. Control arm: 39/165 lost to follow‐up. Significant loss to follow‐up ‐ missing data may have affected outcome estimates |
| Incomplete outcome data (attrition bias) Safety data (e.g. adverse events) | Low risk | Adverse events reported as "there were no adverse events" |
| Protection against contamination | High risk | Judgement comment: allocated by patient; high risk of contamination |
| Selective reporting (reporting bias) | Low risk | All outcomes from NCT and methods section included |
| Other bias | Low risk | No other sources of bias found |
Bonilla‐Escobar 2018.
| Study characteristics | ||
| Methods |
Study design: RCT: initially clustered in 2 areas, but both clusters analysed as separate trials; block randomisation Duration of study: June 2012 to June 2014 |
|
| Participants |
Country: Colombia Income classification: upper‐middle income Geographical scope: urban. The 2 biggest cities of the Colombian Pacific region, a historically impoverished area, are Buenaventura, a harbour city in Valle del Cauca Province (department), and Quibdó, the capital of Chocó Province Healthcare setting: homes, community centres including schools and churches, ACOPLE (“Community‐Based Treatment Services for Afro‐Colombian Victims of Conflict and Torture”) centres Mental health condition: had at least 1 violent traumatic experience and have any reduced functionality in routine activities Population 1. Age > 18 2. Gender: both 3. Socioeconomic background: about half were unemployed; about a quarter lived in houses with tamped ground flooring 4. Inclusion criteria (including threshold cutoff score of measurement tool) a. Identified as Afro‐Colombian b. Had had at least 1 violent traumatic experience c. Displayed suffering, sadness, or symptoms of depression d. TMHS score ≥ 0.77 e. Reduced functionality in routine activities 5. Exclusion criteria a. All those endorsing or having suicidal thoughts and requiring psychological or medical care, as determined by psychologists (1) Psychological treatment was provided or individuals were referred to local health services |
|
| Interventions |
Stated purpose: to evaluate an individualised Common Elements Treatment Approach (CETA), a transdiagnostic psychotherapy model based on cognitive‐behavioural therapy for adult trauma survivors INTERVENTION Name: Common Elements Treatment Approach (CETA) Delivered by: LHWs Title/name of PW and number: lay psychosocial community workers (LPCWs) ‐ 20 (10 in each city) 1. Selection: Afro‐Colombian survivors of violence and displacement; recognised leaders and/or caregivers in their receiving communities 2. Educational background: 5 years post primary education 3. Training (contents, duration, by whom): 10 days followed by weekly practice groups and personalised supervision meeting with psychologist and local supervisor after each session. Assessment: LPCW had to treat 2 non‐study (pilot) patients satisfactorily before they were allowed to provide CETA to participants 4. Supervision: CETA experts ‐ 2 of the trial authors who developed CETA (Laura Murray, Shannon Dorsey) and 2 others 5. Incentives/remuneration: not specified Intervention details (according to PWs and whether aimed at carers and/or patients) 1. Duration/frequency: treatment duration is 8 to 12 sessions, 45 minutes to 1 hour each time 2. Content of intervention (by types of health workers and per patients/carers): individually administered transdiagnostic psychotherapy model based on cognitive‐behavioural therapy, covering elements of psychoeducation, cognitive coping, gradual exposure to traumatic memories, cognitive re‐processing, safety skills, relaxation, behavioural activation, live gradual exposure. Lay psychosocial community workers performed the intervention. They were allowed to make decisions regarding selecting elements, sequencing elements, and dosing elements of the intervention programme based on participants' presentation and response to treatment INTERVENTION 2 ‐ 2 identical interventions: 1 in Quibdo (n = 83) and 1 in Buenaventura (n = 92) CONTROL (Quibdo, n = 83; Buenaventura, n = 88) Wait‐list control. No intervention. Monthly monitoring via phone calls to screen for acute serious mental problems. Assessed by psychologist. If necessary, excluded from study and provided with appropriate care or referral to psychiatrist. Psychological evaluation offered and provided after completion of follow‐up, and, if necessary, psychological care provided CO‐INTERVENTIONS: narrative community‐based group therapy (described in a different paper) |
|
| Outcomes |
Patient 1. TMHS (Total Mental Health Score) (locally relevant symptoms and sub‐scales of depression (n = 15 symptoms), anxiety (n = 10 symptoms) and post‐ traumatic stress symptoms (PTSS) (n = 16 symptoms))* 2. Validated instrument built based on the Hopkins Symptom Checklist (HSCL‐25) 3. Harvard Trauma Questionnaire (HTQ) 4. PTSD CheckList–Civilian Version (PCL‐C) 5. Local dysfunction scale (gender specific)* Carer None Process/health workers None Economic outcomes (and where these can be found, e.g. ref or table number) None (asterisk for study's primary outcomes; star: outcomes that we have not reported in this review) Time points: 2 weeks post intervention (3 to 4 months post baseline) |
|
| Notes |
Source of funding: US Agency for International Development (USAID) Victims of Torture Fund Notes on validation of instruments (screening and outcomes): validated instruments in Spanish Additional information (e.g. provided by authors, existence of a published study protocol): there is a protocol and trial registration. Further information from the study author too. Declarations of interests ‐ study authors declared no competing interests Handling the data: as per footnotes in data and analysis Prospective trial registration number: ClinicalTrials.gov NCT01856673 (https://clinicaltrials.gov/ct2/show/NCT01856673) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: both reviewers rated "LOW" |
| Allocation concealment (selection bias) | Low risk | Judgement comment: both reviewers rated "LOW" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Once study began, participants were aware of their allocation; unlikely to have affected outcomes |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | Participants and interventionists were not blinded, but this was unlikely to have affected the results |
| Baseline outcome measurements similar | Low risk | Interviewers who conducted baseline and follow‐up assessments were masked |
| Baseline characteristics similar? | Low risk | Baseline characteristics (gender, age, marital status, education, displaced, employment, main material in house flooring, healthcare regimen, number of traumatic experiences) were similar between control and intervention groups |
| Incomplete outcome data (attrition bias) Efficacy data | High risk | High attrition rate. Large number lost to follow‐up in Buenaventura control group (23/88). Large number lost to follow‐up in Buenaventura intervention group (21/92). Large number lost to follow‐up in Quibdo control group (15/83). Large number lost to follow‐up in Quibdo intervention group (27/83). Participants who dropped out could have influenced results had they stayed in the study |
| Protection against contamination | Unclear risk | Judgement comment: Yen ‐ "LOW" ‐ control group had no access to psychotherapy. Lay psychosocial community workers' relatives were excluded from the study to prevent contamination. Hakan ‐ "HIGH" ‐ allocation by participant |
| Selective reporting (reporting bias) | Low risk | No, all planned outcomes in methods section and in clinical trial protocol were reported in results section |
| Other bias | Low risk | No other sources of bias were found |
Bryant 2017.
| Study characteristics | ||
| Methods |
Study design: single‐blind parallel randomised controlled trial Duration of study: between 15 April 2015 and 20 August 2015 (with final follow‐up assessments completed on 16 January 2016) |
|
| Participants |
Country: Kenya Income classification: lower‐middle income from 2015 to 2016 Geographical scope: peri‐urban; Nairobi, Kenya Healthcare setting: home Mental health condition: common mental disorders Population: women exposed to gender‐based violence and indicating significant distress as reflected in scores on GHQ > 2 and impaired functioning reflected in scores on WHODAS > 16 1. Age ≥ 18 years 2. Gender: female 3. Socioeconomic background: intervention 49.8% working; control 50.9% working 4. Inclusion criteria a. History of GBV ‐ any (prior or current) experience of interpersonal violence on the Life Events Checklist (LEC) or the WHO Violence Against Women Instrument (WHO‐VAW) b. Distress as reflected in scores on GHQ > 2 c. Impaired functioning as reflected in scores on WHODAS > 16 5. Exclusion criteria a. Imminent suicidal intent b. Severe mental disorder c. Severe cognitive impairment d. Acute protection risks e. Exposure to trauma in last month f. Male gender |
|
| Interventions |
Stated purpose: to assess the effectiveness of Problem Management Plus (PM+) for alleviating distress in women who had experienced gender‐based violence (GBV) in peri‐urban slums in Nairobi, Kenya, compared to enhanced usual care (EUC) delivered by qualified community nurses INTERVENTION (n = 209) Name: Problem Management Plus (PM+) Delivered by: LHWs Title/name of PW and number: community health workers ‐ 23 1. Selection: no prior training or experience in mental health care. Social collaborators may be recruited to their role due to existing community involvement and leadership (e.g. as village care workers, Red Cross volunteers, Women’s Union staff). Passed competency assessments conducted in the form of mock interviews after training 2. Educational background: 10 years' school education 3. Training: 64‐hour training programme over 8 days. covered knowledge of common mental health conditions, basic counselling delivery, PM+, and self‐care strategies. CHWs also received a 1‐day training in psychological first aid (PFA) to prepare them for managing people in crisis (e.g. ongoing violence) who required immediate attention and possible referral. Training also addressed issues related to GBV, as well as ethical and confidentiality matters. Provided by the Research Team 4. Supervision: 2 hours of weekly supervision by the local supervisor, who provided supervision in 4 separate groups to CHWs (5 CHWs per group). Local supervisors received 1.5 hours of weekly training and mentoring in supervision by the research team via Skype 5. Incentives/remuneration: not mentioned Intervention details 1. Duration/frequency: 5 weekly 90‐minute individual sessions 2. Content of intervention: PM+ commenced with an introduction to the programme, motivational interviewing, psychoeducation, and stress management (Session 1); problem‐solving strategies focused on specific problems nominated by the participant and review of stress management strategies (Session 2); behavioural activation and review of problem‐solving and stress management (Session 3); strengthening of social supports and review of stress management, problem‐solving, behavioural activation, and social supports (Session 4); and reinforcement of all strategies and relapse prevention education (Session 5) CONTROL: enhanced usual care (n = 212) EUC was provided by 6 community nurses at primary healthcare centres in the area, where nurses provided non‐specific counselling, using strategies and numbers of sessions they deemed appropriate |
|
| Outcomes |
Patients 1. GHQ‐12 2. WHO‐DAS 2.0 3. PCL‐5 4. Psychological outcomes profile (PSYCHLOPS) Carers None Process/health workers None Economic outcomes None Time points: baseline, post‐treatment, 3 months post‐intervention |
|
| Notes |
Source of funding: Grand Challenges Canada #0368‐04 (www. grandchallenges.ca/), World Vision Canada (http://www.worldvision.ca/), and World Vision Australia (www.worldvision.com.au/ home‐east‐africa) Notes on validation of instruments (screening and outcomes): all validated Additional information: trial protocol; declaration of interests ‐ study authors declared no competing interests Handling the data: as per footnotes in data and analysis Prospective trial registration number: Australian New Zealand Clinical Trials Registry ACTRN12614001291673 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization was performed using computerized software by an independent colleague (i.e. off‐site in Sydney and not involved in the trial)" |
| Allocation concealment (selection bias) | Low risk | Judgement comment: participants randomly allocated to intervention or control group using computer programme conducted by an independent researcher; therefore allocation concealment could be ensured |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and interventionists were not blinded, but this is unlikely to have affected outcomes |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | Post‐treatment assessments were completed by independent assessors who were unaware of the treatment condition of participants. Blindness was maintained; therefore considered low risk |
| Baseline outcome measurements similar | Low risk | "There were no differences detected between participants in the PM+ and ETAU conditions on any of the pre‐treatment outcome measures, demographics, or trauma exposure"; therefore, considered low risk |
| Baseline characteristics similar? | Low risk | Table 1: baseline characteristics are reported and are similar |
| Incomplete outcome data (attrition bias) Efficacy data | High risk | Higher level of dropout in control group (10 vs 5); likely to impact results as the groups are small |
| Incomplete outcome data (attrition bias) Safety data (e.g. adverse events) | Low risk | No adverse or serious adverse events were reported by women receiving the intervention, suggesting that PM+ did not cause harm nor exacerbate distress beyond one's capacity to cope with it. PM+ did not appear to result in increased risk to safety, and no instances of stigma associated with receiving the intervention were reported |
| Protection against contamination | Unclear risk | Quote: "careful attention was paid to ensure assessors had no contact with CHWs or ETAU nurses by having them work in different locations. Any adverse reactions reported spontaneously" Judgement comment: allocated by patient; unclear risk of contamination |
| Selective reporting (reporting bias) | High risk | Three outcomes from trial registry not reported. Personalised outcomes as measured by the Psychological Outcomes Profile (PSYCHLOPS) scale; health service use as measured by reported access of Nairobi Health Services; stressful life events as measured by the Life Events Checklist. Also, follow‐up after post‐treatment assessment is not included in this feasibility trial, but all this is included in the full trial |
| Other bias | Low risk | No other sources of bias were found |
Chatterjee 2014.
| Study characteristics | ||
| Methods |
Study design: multi‐centre parallel‐group RCT; single‐blind (outcome assessors were masked to participant allocation) Duration of study: Jan 2009 to Dec 2011; follow up assessments were at 12 months post baseline. |
|
| Participants |
Country: India Income classification: lower‐middle income from 2009 to 2010 Geographical scope: rural (Tamil Nadu), rural and urban (Goa and Satara district of Maharashtra) Healthcare setting: participants’ homes Mental health condition: schizophrenia Population 1. Age: 16 to 60 2. Gender: both 3. Socioeconomic background: around 70% of participants were not income‐generating 4. Inclusion criteria a. Primary diagnosis of schizophrenia based on ICD‐10 b. Illness duration of at least 12 months c. Illness severity at least moderate on the Clinical Global Impression‐Schizophrenia scale d. Intended to reside in the study region for 12 months 5. Exclusion criteria a. Nil |
|
| Interventions |
Stated purpose: to compare effectiveness of a combination of facility‐based care and collaborative community‐based care and facility‐based care alone for people with moderate to severe schizophrenia. INTERVENTION (n = 167) Name: COPSI: community‐based intervention for people with schizophrenia and their caregivers in India Delivered by: LHW Title/name of PW and number: community health workers ‐ number not specified 1. Selection: good interpersonal skills 2. Educational background: at least 10 years of schooling 3. Training (contents, duration, by whom): systematically trained over 6 weeks, based on intervention manual, covered aspects of the illness, specific components of the intervention, trial‐related documents, and supervision 4. Supervision: psychiatric social workers – on‐site; psychiatrists – on‐site and during quarterly review Intervention details 1. Duration/frequency: 1 year – 6 to 8 visits in the first 3 months; sessions every 15 days in the next 4 months; monthly sessions during the last 5 months 2. Content of intervention: individualised, flexible intervention to improve collaboration between patient, caregiver, and treatment team; psychoeducation regarding illness and its management; identifying and addressing stigma and discrimination; adherence, health promotion, and rehabilitation strategies; linkage to support groups and community agencies; addressing social problems in the family; facilitating employment and access to social and legal benefits CONTROL (n = 86) Facility‐based care by specialist mental health practitioners. Each consultation lasted 10 to 15 minutes; participants were prescribed antipsychotic drugs, were given information about the illness, were encouraged to adhere to drugs, and discussed specific concerns with their psychiatrists CO‐INTERVENTIONS: nil |
|
| Outcomes |
Patients 1. PANSS (all and for each area)* 2. Reduction > 20% in total symptoms, IDEAS score* 3. Improvement ≥ 20% on IDEAS total score, sub‐categories of IDEAS: self‐care, interpersonal activities, communication and understanding, work 4. PANSS subscales: positive, negative, and general, death# 5. Hospital admission 6. Adherence to antipsychotic treatment# 7. Alienation (=stigma)# 8. Willingness to disclose illness# 9. Negative discrimination# 10. Anticipated discrimination# Carers 1. Change in knowledge and attitude about the illness# 2. Change in perceived caregiver burden# 3. Reported stigma# 4. Willingness to disclose family member’s illness# 5. Experiences of stigma and discrimination# Process/health workers 1. Number of sessions received 2. Number of contacts with treating psychiatrist Economic outcomes 1. Cost of sessions 2. Travel and supervision 3. Total intervention cost 4. Other service costs of whole sample 5. Tamil Nadu, Goa and Satara, ICER# (asterisk for study's primary outcomes; star: outcomes that we have not reported in this review) Time point: 0 months post intervention |
|
| Notes |
Source of funding: Wellcome Trust Notes on validation of instruments (screening and outcomes): validated (IDEAS has been validated in India) Additional information: trial protocol ‐ Chatterjee S, Leese M, Koschorke M, McCrone P, Naik S, John S, Dabholkar H, Goldsmith K, Balaji M, Varghese M, Thara R, Patel V, Thornicroft G. The COmmunity care for People with Schizophrenia in India (COPSI) group. Collaborative community based care for people and their families living with schizophrenia in India: protocol for a randomised controlled trial. Trials 2011;12:12. Declarations of interest ‐ study authors declared no competing interests Handling the data: nil Patient outcomes presented in this treatment review. Carer outcomes summarised but also to be included in prevention review, as for them the intervention is preventing mental distress/disorders Prospective trial registration number: ISRCTN 56877013. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomly assigned in a 2:1 ratio, via computer‐generated randomisation list with block sizes of three, six, or nine, to receive either collaborative community‐based care plus facility‐based care or facility‐based care alone" Judgement comment: randomisation clearly described and allocated via a computer programme |
| Allocation concealment (selection bias) | Low risk | Quote: "for each site, the randomisation list was generated independently by the trial statistician and transferred to the site data manager before recruitment. The data manager had no role in the recruiting of participants and held the passwords for the randomisation lists; individuals recruiting participants did not have access to the randomisation lists or the passwords" Judgement comment: allocation was conducted by a member of the team who did not have a role in recruitment. Allocation was concealed from individuals recruiting. The unit of allocation was clearly defined |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and interventionists were not blinded to treatment allocation, but this is unlikely to have influenced outcomes |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | "Outcome assessors were masked to group allocation"; "if unmasking happened at the time of the 6 month assessment, a separate researcher undertook the 12 month assessments" Precautions were taken to ensure masking of the interventions |
| Baseline outcome measurements similar | Low risk | Patient outcomes were measured before the intervention, and no important differences were noted across study groups "Clinical characteristics of participants were similar between the treatment groups"; "small differences in baseline outcome scores; mean difference adjusted for baseline scores" |
| Baseline characteristics similar? | Low risk | Sociodemographic and clinical characteristics of participants were similar between treatment groups Comments: baseline characteristics mostly similar. More married participants and more rural participants in intervention arm compared to control arm |
| Incomplete outcome data (attrition bias) Efficacy data | Low risk | No imputation was made because missing data were fewer than the predefined threshold specified in the trial protocol Comments: missing data were fewer than in the predefined threshold of 95% power; around 9% to 11% dropout in both groups; reasons for dropout reported |
| Incomplete outcome data (attrition bias) Safety data (e.g. adverse events) | Low risk | Four patients died during the study. Two (50%) of these deaths were the result of suicide (1 in each treatment group), whereas the other 2 (50%) were due to complications of a road traffic accident and pre‐existing cardiac disease. 18 (73%) patients were admitted to hospital during the course of the trial; of these, 17 were in the intervention group. Seven (39%) of these admissions were related to physical health problems, such as acute gastritis and vomiting, road accident, high fever, or cardiovascular disease. Comments: missing data were fewer than in the specified threshold. Potential adverse events were adequately explained |
| Protection against contamination | Low risk | Quote: "to minimise this possibility, we kept the research and intervention teams physically separate during the trial, asked participants and caregivers first at the time of assessments to not disclose whether they had received home visits from the community health worker, and the primary outcome measures (positive and negative syndrome scale [PANSS] and the Indian disability evaluation assessment scale [IDEAS]) were completed first" Judgement comment: minimal risk of contamination because participants in intervention group received additional treatment from community health workers, whereas those in the control group did not |
| Selective reporting (reporting bias) | High risk | Quality of life mentioned in protocol but not reported |
| Other bias | Low risk | No other sources of bias found |
Chen 2015.
| Study characteristics | ||
| Methods |
Study design: cluster‐RCT (unit of allocation: primary care clinic; 8 in each arm) Duration of study: 17 January 2011 to 30 November 2013 |
|
| Participants |
Country: China Income classification: upper‐middle income from 2011 to 2013 Geographical scope: Shangcheng districts, Hangzhou City, eastern China Healthcare setting: PC facility (free standing) Mental health condition: depression Population: Chinese primary care patients with late‐life depression 1. Age: 60 to 90 years 2. Gender: both 3. Socioeconomic background: not mentioned 4. Inclusion criteria a. PHQ‐9 ≥ 10 b. Diagnosis of MDD on SCID c. Age ≥ 60 years d. Community‐dwelling residences e. Capable of independent communication 5. Exclusion criteria a. Mini‐Mental State Examination (MMSE) score < 18 b. Incapable of giving written informed consent for this study c. Acute high suicide risk at baseline assessment d. Psychosis (as assessed by a psychiatrist) |
|
| Interventions |
Stated purpose: to examine whether collaborative care depression care management (DCM) is an effective treatment for patients with late‐life depression in urban China Our specific aims are 1. To determine whether DCM intervention results in improved outcomes compared with CAU at both provider (e.g. greater adherence to quality indicators) and patient levels (e.g. greater reduction in depressive symptoms) 2. To compare DCM with CAU with regards to a range of outcomes in other pertinent domains, at both provider (e.g. improvements in knowledge/attitudes) and patient (e.g. functioning, satisfaction) levels 3. The study will take place at 16 primary care clinics (PCCs) randomly assigned to deliver either DCM or CAU (8 clinics each) to 320 patients (aged ≥ 60 years) with major depression (20/clinic; n = 160 in each treatment condition). In the DCM arm, PCPs will prescribe 16 weeks of antidepressant medication according to the TG protocol. CMs monitor the progress of treatment and side effects, educate patients/family, and facilitate communication between providers; psychiatrists will provide weekly group psychiatric consultation and CM supervision INTERVENTION (n = 164) Name: Depression Care Management (DCM) Delivered by: PHPs Title/name of PW and number: primary care physician (PCP) and depression care manager (CM) 1. Selection: PCPs and nurses working in the intervention cluster 2. Educational background: medical degree (doctor)/at least 3 years of post‐secondary school education (nurse) 3. Training: trained and supervised (once per month) by the psychiatrist consultant. Trained to use antidepressant drugs and mental health referral. 3 hours of group learning; 1 hour of supervision by psychiatrist every month for the duration of the study 4. Supervision: as above 5. Incentives/remuneration: not mentioned Intervention details 1. Duration/frequency: 8 weeks of treatment with Sertraline, another 8 weeks of treatment augmentation with bupropion if patients fail to respond in the initial trial. For more complicated cases, transfer to psychiatrists is indicated 2. Content of intervention: DCM intervention was based on the 3‐component model for late‐life depression and combined provision of antidepressant treatment guidelines. Antidepressant medication treatment guidelines included 2 stages: (1) 8 weeks of treatment with sertraline (starting dose of 50 mg per day; option of weekly increases by 50‐mg increments to a maximum dose of 200 mg per day); (2) augmentation with bupropion extended‐release 200 to 400 mg per day for patients whose PHQ‐9 scores had decreased by less than 50% from baseline after 8 weeks of sertraline treatment; 1 nurse from each clinic was designated as the clinic's depression care manager. Responsibilities included education of patients and their families about their illnesses, assistance with communication between patients and their providers, and support of patients' adherence to treatment. They telephoned patients every 2 weeks and encouraged them to keep their appointments. Patients attended the clinic every alternate week. PHQs were administered every week in person or via telephone CONTROL: enhanced care as usual (n = 162) Physicians were provided with a copy of written guidelines on depression treatment and were informed of each patient's PHQ‐9 score and diagnosis of major depression. Patients were referred to the Hangzhou Mental Health Centre if the primary care doctor recognised a problem they were not comfortable with managing CO‐INTERVENTIONS: nil |
|
| Outcomes |
Patients 1. Depressive symptoms ‐ Hamilton Rating Scale for Depression (HAM‐D) score 2. Response rates (defined as proportion of patients with ≥ 50% reduction in HAM‐D score) 3. Remission rates (defined as proportion of patients with HAM‐D score < 7) 4. SF‐12 5. Treatment stigma 6. Client Satisfaction Questionnaire (CSQ‐8) 7. Safety outcomes Carers Nil Process/health workers 1. Antidepressant prescription 2. Mental health referral 3. Dropouts Economic outcomes Nil Time points: baseline; 3, 6, 12 months after the start of treatment |
|
| Notes |
Source of funding: this study was funded in part by the Fogarty International Center of the National Institutes of Health, MD, USA (R01TW008699), and by the Program for New Century Excellent Talents in Universities of China Notes on validation of instruments (screening and outcomes): all culturally validated Additional information: declarations of interest ‐ study authors declared no competing interests Handling the data: as per footnotes in data and analysis Prospective trial registration number: NCT01287494 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using a computer algorithm, 16/34 clinics in the Shangcheng district were allocated to participate in the study. With computer‐generated number sequences, 8 of these 16 were assigned to intervention and 8 were allocated to control |
| Allocation concealment (selection bias) | High risk | Quote: "after assignment, patients were invited to participate (with written informed consent) from each clinic knowing the treatment assignment of their treating clinic. Primary care centre staff and research personnel were also not masked to treatment assignment" Patients knew the treatment assignment at their treating clinic |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Lack of blinding of participants and interventionists |
| Blinding of outcome assessment (detection bias) all outcomes | High risk | Staff at the clinic and research staff who carried out the intervention and outcome assessments were aware of their clinic assignments |
| Baseline outcome measurements similar | Low risk | Baseline HAM‐D results were similar in the 2 groups |
| Baseline characteristics similar? | Low risk | The 2 groups did not differ significantly in baseline characteristics (age, gender, marital status, educational level, physical illness, and living situation) |
| Incomplete outcome data (attrition bias) Efficacy data | High risk | Dropout rates were balanced in the 2 groups (33% in intervention, 36% in control) but were high in each and could have made a difference in the results had these participants stayed on in the study |
| Incomplete outcome data (attrition bias) Safety data (e.g. adverse events) | High risk | SUPPORTING ANNOTATIONS Over the 12 months, 19 patients in clusters assigned to DCM and 26 patients from clusters assigned to enhanced care as usual had at least 1 hospital admission for physical illness. No patients were admitted into hospital for psychiatric illness. One patient for each assigned intervention died from heart disease; no other deaths occurred COMMENTS Adverse events reported in the study were hospitalisation from physical illness (19/164 in intervention group, 26/162 in control group), hospitalisation from psychiatric illness (0 in each group), and death from heart disease (1 in each group). Dropout rates were similar in each group (33% in intervention, 36% in control). Even if study authors reported, as above, a low incidence of adverse effects, lots of dropouts occurred during the study in both groups (> 30%) |
| Protection against contamination | Low risk | Judgement comment: cluster‐randomised trial |
| Selective reporting (reporting bias) | Unclear risk | Some outcomes planned in the published protocol were not reported in the results of the study, but these may or may not have been relevant. SSI (replaced by PHQ‐9 and HAM‐D). CAS (replaced by HAM‐D) |
| Other bias | Low risk | Funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all data in the study and had final responsibility for the decision to submit for publication. Study authors declared no competing interests (page 338) |
Chibanda 2014.
| Study characteristics | ||
| Methods |
Study design: RCT Duration of study: 6 weeks. According to trial registry, first patient was enrolled in August 2006 |
|
| Participants |
Country: Zimbabwe Income classification: low income from 2006 to 2014 Geographical scope: this study was done at 2 urban primary care clinics in Chitungwiza, a peri‐urban community with a population of 1.5 million, located on the outskirts of the city of Harare, Zimbabwe Healthcare setting: PC clinics Mental health condition: perinatal MD Population 1. Age 18+ years 2. Gender: female 3. Socioeconomic background: mean education in years: problem‐solving group 10.6; pharmacotherapy 11.2 4. Inclusion criteria (including threshold cutoff score of measurement tool) a. EPDS cutoff ≥ 11 b. All study participants were subsequently subjected to mental status examination by 2 psychiatrists who were blinded to participants’ EDPS test results c. Diagnosis of postpartum depression was confirmed by Diagnostic and Statistical Manual of Mental Disorders Fourth Edition (DSM‐IV) criteria for major depression 5. Exclusion criteria a. Did not reside in the local area b. Unable to give informed consent c. Had psychosis, severe depression, or suicidal ideation |
|
| Interventions |
Stated purpose: to determine the efficacy of group problem‐solving therapy (PST) delivered by peer counsellors vs pharmacotherapy for PND in a cohort of postpartum HIV‐infected and uninfected women attending primary care postnatal clinics in urban Zimbabwe INTERVENTION (n = 27) Name: Group Problem Solving Therapy Delivered by: LHW Title/name of PW and number: trained peer counsellors in a private setting at the antenatal clinic ‐ 6 1. Selection: HIV‐infected women who previously participated in a "Prevent mother‐to‐child transmission of HIV (PMTCT)" programme, were currently enrolled in support groups, and had disclosed their positive HIV status to partner or family member 2. Educational background: not specified 3. Training (contents, duration, by whom): 2‐day training on identification of kufungisisa 4. Supervision: weekly supervision by psychiatrist 5. Incentives/remuneration: not stated Intervention details (according to PHW/CWs and whether aimed at carers and/or patients) 1. Duration/frequency: each group met twice weekly for 6 weeks, with every session lasting for 60 minutes 2. Content of intervention (by types of health workers and per patients/carers): problem identification, exploration of solutions, practice, discussion, reinforcement, support, identification of achievements and obstacles. Patients with psychosis, severe depression, and suicidal ideation were referred to a specialised psychiatric unit located at Harare Central Hospital for treatment CONTROL (n = 22) Name: Intervention With Pharmacotherapy Delivered by: PHW, PC nurse Intervention details 1. Duration/frequency: each session lasted 20 to 30 minutes, and participants were then given their medication 2. Content of intervention (by types of health workers and per patients/carers: amitriptyline was prescribed by the primary care nurse as part of her routine clinic work. All women underwent a physical examination including measurement of blood pressure and examination of the cardiovascular system. Women were then informed of the effects of amitriptyline and how it would help in the treatment of kufungisisa (depression). An initial dose of 50 mg to be taken at night was provided. Amitriptyline was increased by 25 mg after every 3 days depending on the symptoms. Potential adverse effects of the drug were explained to study participants. They were advised to visit the clinic nurse every week for evaluation of their progress. The group under the supervision of a peer counsellor talked about education on PMTCT, including safe breast‐feeding practices and the importance of exclusive breast‐feeding CO‐INTERVENTIONS: none |
|
| Outcomes |
Patients EPDS Carers None Process/health workers None Economic outcomes (and where these can be found, e.g. ref or table number) (asterisk for study's primary outcomes; star: outcomes that we have not reported in this review) Time points post intervention: post intervention (6 weeks post baseline) |
|
| Notes |
Source of funding: study author(s) received no financial support for research, authorship, and/or publication of this article Notes on validation of instruments (screening and outcomes): all scales are validated Additional information: information on trial registry at https://pactr.samrc.ac.za/Search.aspx; declarations of interest ‐ study authors declared no competing interests Handling the data: as per footnotes in data and analysis Prospective trial registration number: this trial is registered at the Pan African Clinical Trial Registry; trial number: PACTR201303000485383 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "postpartum mothers meeting criteria for major depression according to DSM‐IV were randomly assigned to group PST (delivered by trained primary care counsellors) or pharmacotherapy with amitriptyline using computer‐generated random numbers" Judgement comment: randomisation was performed using computer‐generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not clear whether and how a computer‐generated list was concealed from researchers and handled by an independent researcher |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and peer counsellors was not performed. Peer counsellors involved in delivering the intervention also screened for depression symptoms |
| Blinding of outcome assessment (detection bias) all outcomes | High risk | No description of blinding of outcome assessors was provided in this article |
| Baseline outcome measurements similar | Low risk | Similar baseline EPDS scores and no statistically significant differences |
| Baseline characteristics similar? | Low risk | Participants in intervention and control groups had similar baseline characteristics (age, parity, education level, HIV status, marital status, employment status, negative life event) |
| Incomplete outcome data (attrition bias) Efficacy data | Unclear risk | Higher dropout in pharmacotherapy arm than in psychotherapy arm, probably due to medication, but not all reasons for loss to follow‐up were reported Attrition: 3 in intervention group (all lost to follow‐up), 6 in control group (3 due to adverse events, 2 lost to follow‐up, 1 declined). Participants whose outcome data are unknown may have impacted the results if their outcomes were known |
| Incomplete outcome data (attrition bias) Safety data (e.g. adverse events) | Unclear risk | Not all reasons for dropout have been reported Study numbers are small; hence if an adverse event such as suicide had occurred among any of the participants who dropped out of the intervention or control group, there would be an impact on the results of the study. Not all reasons for dropout have been reported |
| Protection against contamination | Unclear risk | Judgement comment: it seems like the same peer counsellors who delivered the psychotherapy intervention supervised a peer support group for the pharmacotherapy arm, so it is possible that elements from the psychotherapy intervention would have been included in the pharmacotherapy arm |
| Selective reporting (reporting bias) | Unclear risk | All outcome measures planned in methods were reported in results. Trial registration (PACTR201303000485383) refers to study to validate the EPDS scale, not to evaluate the intervention. Trial registration was edited after completion of the study. Unclear whether EPDS was the only measure used |
| Other bias | Low risk | No other sources of bias were found |
Chibanda 2016.
| Study characteristics | ||
| Methods |
Study design: cluster‐RCT (unit of allocation: primary care clinics. 12 clinics in each arm, 24 in total) Duration of study: 1 September 2014 to 25 May 2015 |
|
| Participants |
Country: Zimbabwe Income classification: low income Geographical scope: Harare, Zimbabwe Healthcare setting: PC facility Mental health condition: common mental disorders Population 1. Age: 18 and above 2. Gender: both 3. Socioeconomic background: not mentioned 4. Inclusion criteria a. All persons residing in the area and attending local clinics who are aged 18 and above and are able to give written informed consent will be eligible for enrolment b. Individuals scoring at or above a cutoff point of 9 on the SSQ‐14 will be invited to participate 5. Exclusion criteria a. Persons who are unable to comprehend the nature of the study in either English or Shona (local language) b. Those with suicidal intent, end‐stage AIDS, currently in psychiatric care, or presenting with current psychosis, intoxication, and/or dementia c. Those excluded for medical reasons will be referred for appropriate care to 1 of 2 tertiary facilities in Harare d. Those reported to be physically unwell by the clinic Nurse‐in‐Charge e. Pregnant women in the third trimester and women within the 3 months post delivery period f. Those not residing in the geographical locality or whose address cannot be verified through clinic registries |
|
| Interventions |
Stated purpose: to evaluate effectiveness of this culturally adapted intervention for common mental disorders delivered by existing LHWs in primary care in Harare, Zimbabwe INTERVENTION (n = 260) Name: Friendship Bench Intervention Delivered by: LHWs Title/name of PW and number: community workers (lay health workers) 1. Selection: LHW was attached to the clinic and employed by the local health authority; all were female 2. Educational background: mean of 10 years education 3. Training: 9 days' training. Topics included common mental disorders, counselling skills, problem‐solving supported with a manual developed by Friendship Bench team 4. Supervision: LHWs will receive supervision and support from the clinical team at the site level or through mobile phones using voice calls and, when necessary, SMS messaging. The SMS messaging/voice call will be sent by the LHW, and when challenges such as being unable to contact a participant are encountered, project co‐ordinators will follow up with a voice call; if this yields no results, a physical home visit will be carried out. The support structure is based on a predetermined algorithm developed during formative research. This consists of study screen tool cutoff scores, criteria for referral including assessment for “red flags”, clients who are suicidal, and immediate referral to a tertiary facility 5. Incentives/remuneration: not mentioned Intervention details 1. Duration/frequency: 6 weekly sessions of 30 to 45 minutes delivered through the Friendship Bench over 6 weeks, including home visits when deemed necessary 2. Content of intervention: Friendship Bench intervention is problem‐solving therapy, in which the patient identifies a problem (e.g. unemployment) rather than a diagnosis or symptom; this has been shown to be feasible and acceptable in this resource‐poor setting. The psychological approach of problem‐solving therapy works by enabling a more positive orientation towards resolving problems and empowering people to have a sense of greater coping and control over their lives 3. Lay health workers followed a detailed script contained in a manual to conduct 6 sessions on a bench located in a discreet area outside the clinic 4. The care model was driven by a trained and supervised LHW attached to the clinic and employed by the local health authority. After 6 sessions of individual therapy, the LHW referred those not improving or with suicidal ideation to a supervisor trained in mental health to re‐assess and manage the case if needed 5. Participants in the intervention group received up to 6 text messages, phone calls, or both during the intervention, which reinforced the problem‐solving therapy approach and encouraged participants, particularly those attending fewer than 3 sessions during the first 4 weeks, to follow their action plan. As part of the improved management programme, participants were re‐assessed by the LHW after the third session using the SSQ‐14, and those whose score had worsened by 1 or more scale points or who had suicidal ideation were assessed by a psychiatrist. These results were not used for research purposes. If participants missed a session, the LHWs followed up with a phone call, a home visit, or both if there was no response 6. After 4 individual sessions, all intervention group participants were invited to join a peer‐led group called Circle Kubatana Tose, or “holding hands together”. These weekly meetings consisted of sharing personal experiences while crocheting a bag from recycled plastic materials. The latter activity was a skill for generating income by making and selling the bags. Participants in the intervention group were also offered enhanced usual care (EUC) CONTROL (n = 261) The control group received the standard usual care consisting of a nurse‐led evaluation, brief support counselling, and an option for medication, as well as information, education, and support for common mental disorders including assessment for antidepressant medication prescribed by the clinic nurse, referral to a psychiatric facility, or both if needed. Participants also received 2 to 3 supportive Short Message Service messages or calls, with the last message a reminder to attend the 6‐month assessment CO‐INTERVENTIONS: nil |
|
| Outcomes |
Patients 1. Continuous: SSQ‐14; PHQ‐9; GAD‐7; WHODAS.2.0; EQ‐5D 2. Binary: PHQ‐9 ≥ 11, PHQ‐9 diagnostic algorithm, GAD ≥ 10, SSQ‐14 ≥ 9, WHODAS2.0 ≥ 20 Carers Nil Process/health workers Nil Economic outcomes Nil Time points: baseline, 6 months |
|
| Notes |
Source of funding: Grand Challenges Canada (grant KCU‐0087‐042) Notes on validation of instruments (screening and outcomes): all validated Additional information: declarations of interest ‐ study authors reported no conflict of interest Handling the data: as per footnotes in data and analysis Prospective trial registration number: PACTR201410000876178 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "on each day of screening, computer‐generated preprinted random numbers were used to select clinic attenders based on their queue position number" Judgement comment: random allocation of patients with computer‐generated lists. Also randomisation of clinics (not specified how) |
| Allocation concealment (selection bias) | Low risk | Quote: "the research assistants responsible for outcome assessment were masked to the allocation" Judgement comment: allocation was conducted by city health staff not involved in the study and was blinded to the study team. Allocation unit was clearly specified Therefore, low risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Participants in both groups were not aware which group was the intervention" Interventionists were unlikely to be blinded, but this was unlikely to have influenced outcomes |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | "Research assistants conducting follow‐up interviews in the clinics could have ascertained allocation by the presence of the bench, but we attempted to minimise bias by keeping research assistants independent of intervention delivery and implementation" Therefore, low risk |
| Baseline outcome measurements similar | Low risk | Mean SSQ‐14 score at baseline similar between groups. Other measures mostly similar; PHQ showed participants with high scores in the intervention group |
| Baseline characteristics similar? | Low risk | Participants in the intervention group were more likely to be women, younger, and better educated, and were less likely to be HIV positive COMMENTS Some differences in baseline characteristics but not very large |
| Incomplete outcome data (attrition bias) Efficacy data | Low risk | "Because of a high 6 month follow up rate (91%), complete case analysis was used, and missing data were not imputed at the individual level" Missing data unlikely to bias results; therefore low risk "Data were not imputed for 9% of participants lost to follow‐up and with missing data. However, missing outcome was associated with baseline SSQ‐14, PHQ‐9, and WHODAS2.0 scores, and the complete‐case analysis should therefore be unbiased" 26 dropouts in each group; all clinics included in analyses |
| Incomplete outcome data (attrition bias) Safety data (e.g. adverse events) | Low risk | Reasons for dropout were reported. Study authors report no evidence of harm. Proportions of missing data were similar in intervention and control groups |
| Protection against contamination | Low risk | Judgement comment: allocation by primary care clinic |
| Selective reporting (reporting bias) | Unclear risk | Protocol is published; primary outcome is the same but not all secondary outcomes are specified in the protocol. All outcomes described in methods are reported |
| Other bias | Low risk | No other sources of bias were found |
Christoff 2015.
| Study characteristics | ||
| Methods |
Study design: 3‐arm parallel‐group RCT Duration of study: 3 months |
|
| Participants |
Country: Brazil Income classification: upper‐middle income between 2006 and 2015 Geographical scope: urban Healthcare setting: university Mental health condition: Alcohol Use Disorder Population (mention whether patient, carer, or dyad) 1. Age: 18 years and older 2. Gender: both 3. Socioeconomic background: 58% of participants were in socioeconomic class B 4. Inclusion criteria (including threshold cutoff score of measurement tool) a. College students from 2 universities with alcohol or substance abuse b. ASSIST score 4 to 26 (moderate) and above (dependent) for substance use and/or 11 to 26 (moderate) and above (dependent) for alcohol use c. Ability and consent to participate in 2 sessions that lasted from 5 minutes (control group) to 40 minutes (other 2 groups) with no compensation or payment for participation d. Declaration that they would not engage in other substance treatments/programmes before or during the study 5. Exclusion criteria a. None |
|
| Interventions |
Stated purpose: to evaluate the efficacy of a computer‐based intervention programme, called ASSIST/Motivational Brief Intervention (ASSIST/MBIc), for substance involvement compared with only feedback about ASSIST scores (control group) and feedback plus MBI in an interview (ASSIST/MBIi) INTERVENTION 1 (n = 128) Name: computer‐based ASSIST/Motivational Brief Intervention (ASSIST/MBIc) Delivered by: computer programme Title/name of PW and number: none 1. Selection: none 2. Educational background: none 3. Training (contents, duration, by whom): none 4. Supervision: none Intervention details: NA (will not analyse) 1. Duration/frequency: NA (will not analyse) INTERVENTION 2 (n = 106) Name: feedback plus MBI in an interview (ASSIST/MBIi) Delivered by: CP (community professional) Title/name of PW and number: interviewers ‐ 6 1. Selection: not specified 2. Educational background: not specified 3. Training: in ASSIST and motivational interviewing by principal investigator using the WHO manual 4. Supervision: none specified Intervention details 1. Duration/frequency: a single‐session brief intervention following the ASSIST interview lasting 5 to 20 minutes. Contents: a personal face‐to‐face intervention that provides feedback on scores, gives advice about identifying potential problems, encourages behavioural change, allows participants to report substance‐related problems, lists the advantages and disadvantages of using the substance(s), lists skills to cope with risky behaviours related to the substance(s), and provides goals to change behaviours in the short, medium, and long term. ASSISTi on follow‐up at 3 months CONTROL (n = 99) Randomly screened by ASSISTi or ASSISTc and received feedback about the score. Wait‐list – received MBIi after the study ended CO‐INTERVENTIONS: none |
|
| Outcomes |
Patients 1. ASSIST Total* (Men, Women) 2. ASSIST Alcohol (Men, Women) 3. ASSIST Marijuana (Men, Women) 4. ASSIST Other Drugs (Men, Women) 5. ASSIST Tobacco (Men, Women)# Carers Nil Process/health workers Nil Economic outcomes (and where these can be found, e.g. ref or table number) Nil (asterisk for study's primary outcomes; star: outcomes that we have not reported in this review) Time points: 3 months |
|
| Notes |
Source of funding: institutional (Universidade Federal do Paraná) support only Notes on validation of instruments (screening and outcomes): validated Additional information (e.g. provided by authors, existence of a published study protocol): declaration of interests ‐ all study authors reported no conflicts of interest Prospective trial registration number: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: Yen rated "unclear" and stated "random sequence generation is not described, apart from mentioning that each student received a personal code at time of enrolment"; Antonio rated "low" without comment. Final decision by Yen ‐ "unclear" |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: Yen rated "unclear" and stated "allocation concealment is not described"; Antonio rated "low" without comment. Final decision by Yen "unclear" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Yen rated "high"; "subjects received the intervention and were thus not blinded to their intervention. Assessments were performed by an interviewer or were self‐assessed using computer software. Blinding was not performed" Antonio rated "unclear"; final decision by Yen ‐ "unclear" because although study design made it difficult to blind subjects to their intervention; this may have influenced the outcome as it is self‐assessed for some participants |
| Blinding of outcome assessment (detection bias) all outcomes | High risk | Assessments were performed by an interviewer or were self‐assessed using computer software. Blinding was not performed |
| Baseline outcome measurements similar | Low risk | Baseline total involvement, tobacco, alcohol, marijuana, and other drug ASSIST scores were statistically similar in all 3 groups |
| Baseline characteristics similar? | Low risk | Randomisation successfully balanced intervention assignments and ensured equal characteristics in the groups (no signiciant differences; chi² test) |
| Incomplete outcome data (attrition bias) Efficacy data | Unclear risk | Students: 458 students scored on ASSIST; 4 did not agree to participate in the RCT (1 in the ASSIST/MBIi group, 3 in the control group). Over the course of the trial, 121 students were lost (14%; i.e. not found after 3 attempts by phone or personal contact within 1 month after scheduled follow‐up) or dropped out (11%; i.e. when they personally or by phone/email gave up the study), for an overall response rate of 75%. Twenty‐three per cent of ASSIST/MBIc, 25% of ASSIST/MBIi, and 28% of control did not complete the 2 sessions, with no significant differences among groups Significant dropout rate (25%) in total ‐ this may have influenced results had these participants not dropped out |
| Protection against contamination | Low risk | Judgement comment: both reviewers rated "low" |
| Selective reporting (reporting bias) | Unclear risk | No published clinical trial protocol available; outcomes planned in methods section were reported in results section |
| Other bias | Low risk | No other sources of bias were found |
Connolly 2011.
| Study characteristics | ||
| Methods |
Study design: RCT; randomised wait‐list control study Duration of study: 2 years |
|
| Participants |
Country: Rwanda Income classification: low income Geographical scope: urban Healthcare setting: community group setting. The study took place in a vacant rental home and backyard with plastic chairs and tables inside and outside Mental health condition: post‐traumatic stress symptoms and disorder Population 1. Age > 18 2. Gender: both 3. Socioeconomic background: Rwandan genocide survivors 4. Inclusion criteria (including threshold cutoff score of measurement tool) a. 18 years or older b. All but a few were able to read Kinyarwanda, the language into which consent forms and testing instruments were translated c. met DSM‐IV criterion A1 for post‐traumatic stress disorder symptoms (American Psychiatric Association (DSM‐IV‐TR), 2000): “exposure to an extreme traumatic stressor involving direct personal experience of an event that involves actual or threatened death or serious injury, or other threat to one’s physical integrity; or witnessing an event that involves death, injury, or a threat to the physical integrity of another person; or learning about unexpected or violent death, serious harm, or threat of death or injury experienced by a family member or other close associate” (page 463) by having been in Rwanda and having survived the genocide of 1994 5. Exclusion criteria a. Not specified |
|
| Interventions |
Stated purpose: to examine the efficacy of Thought Field Therapy (TFT) in reducing post‐traumatic stress disorder symptoms among survivors of the 1994 genocide in Rwanda INTERVENTION (n = 71) Name: Thought Field Therapy Delivered by: LHWs Title/name of PW and number: Rwandan therapists ‐ 28 1. Selection: female members of Women’s Foundation Ministry community. The exception was a Rwandan male orphanage director who had missed a previous training in Kigali and had asked to attend this training. All therapists and participants were native Rwandans who spoke Kinyarwanda 2. Educational background: none of the Rwandan therapists in this study were mental health professionals 3. Training (contents, duration, by whom): therapists received 2 days of training in TFT at the algorithm level provided by PI, including hands‐on practice. Standardised TFT manual in French or English included 4. Supervision: conducted by trial authors, who used translators 5. Incentives/remuneration: not specified Intervention details (according to PHW/CWs and whether aimed at carers and/or patients) 1. Duration/frequency: 1 session, mean 41 minutes (SD 29 minutes), median 30 minutes 2. Content of intervention (by types of health workers and per patients/carers): psychoeducation and therapy. Thought Field Therapy is a brief treatment, often used as a self‐help treatment. Participants identified 1 or more unwanted emotions relative to their past experiences, such as anxiety, fear, anger, guilt, or depression, that they wished to address. Once a participant has identified a specific problem, a typical TFT session begins with exposure to the problem, usually by the therapist asking the participant to think about the problem. While the participant is thinking about the problem and identifying feelings elicited by thinking about the problem, the participant is asked to simultaneously stimulate selected acupoints on the surface of the skin by tapping with the fingers, in a sequence that is specific to the identified emotion(s). Each TFT tapping protocol or algorithm designates the specific acupoints to be tapped, as well as the order in which they are to be tapped. These algorithms address a range of emotions such as anxiety, fear, anger, guilt, shame, depression, embarrassment, and addictive urges. Elements of PTSD, such as hyperarousal, dissociation, and defensive avoidance, are targeted by a trauma treatment protocol. A participant first rates the emotional intensity he or she feels when thinking about the problem, usually by giving it a 0 to 10 subjective units of distress (SUD) rating (Wolpe, 1958). The practitioner then selects the most appropriate tapping protocols for the participant's identified emotions and models the tapping sequence. The participant simultaneously taps his or her body, tapping on the points modelled by the practitioner, while keeping the memory or trigger mentally activated. Then an SUD is taken and, if symptoms are lessening, another round of tapping is done after some auxiliary activities (including eye movements, bilateral stimulation, and counting). A subsequent SUD is assessed. The process is repeated following additional auxiliary activities involving other acupoints, until the rating is down to 0 or the lowest it can go for the participant in the time available. Then, whatever other traumatic memories or triggers have arisen or remain may be addressed. Optimally, in treating a trauma survivor, each major traumatic memory that triggers the individual is addressed. Although there are more advanced levels of TFT that require more extensive training, only the TFT algorithms described above were taught and applied in this study CONTROL (n = 74) No care. Wait‐list control: the treatment group and the wait‐list group were asked to return 7 days following their treatment to complete the post‐tests. The wait‐list group received treatment with TFT 2 days following the post‐test and returned 7 days after treatment to take a second post‐test |
|
| Outcomes |
Patients 1. Modified PTSD Symptom Scale (MPSS)* 2. Trauma Symptom Inventory (TSI)* various sub‐scales: Anxious, Arousal, Depression, Anger/Irritability, Intrusive Experience, Defensive Avoidance, Dissociation, Sexual Concern, Dysfunctional Sexual Behavior, Impaired Self‐Reference, and Tension Reduction Behaviour Carers None Process/health workers None Economic outcomes (and where these can be found, e.g. ref or table number) None (asterisk for study's primary outcomes; star: outcomes that we have not reported in this review) Time points post intervention: 0, 7 days; 2 years |
|
| Notes |
Source of funding: Association of Thought Field Therapy Foundation through contributions from the Ruth Lane Charitable Foundation, the Linden Root Dickinson Foundation, the PepsiCo Foundation, and individual donors Notes on validation of instruments (screening and outcomes): instruments were translated and back‐translated. Not validated in Kinyarwanda but validated internationally in other languages Additional information: declarations of interest ‐ none Handling the data: as per footnotes in data and analysis Prospective trial registration number: nil |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "randomized waitlist control group design was used. If, after reading the consent letter, the participants gave verbal consent, they were randomly assigned to an immediate treatment group or the waitlist control group. Blank surveys were in file folders delineated as treatment (blue folders) or waitlist group (red folders) and were stacked alternately. The intake person removed the top file from the stack and assigned the participant to that group, continuing with alternating group assignments" Judgement comment: alternate stacking is supposedly random, but given it relies on the person definitely taking the top folder, randomisation could be broken. Not fully clear how randomisation was conducted and who conducted it |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: it is unclear whether allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and interventionists were not blinded to treatment allocation. As outcomes were self‐assessed, this may have influenced outcomes |
| Blinding of outcome assessment (detection bias) all outcomes | High risk | Outcomes were self‐administered; therefore could not be conducted blindly |
| Baseline outcome measurements similar | Low risk | Baseline outcome measurements represented in Table 2 are similar |
| Baseline characteristics similar? | Low risk | Demographic differences between participants in the treatment group and those in the control group were examined using Chi² analyses and t‐tests. No significant differences were found Comments: although it has been reported that no significant differences were found between baseline characteristics, characteristics of participants have not been presented in a table. We think there are unlikely to be significant differences if these were not reported |
| Incomplete outcome data (attrition bias) Efficacy data | High risk | "The treatment group was 80.3% female (n = 57), and the control group was 83.8% female (n = 62)"; "pretests on the TSI required the exclusion of inconsistent data, identified by the creator, Briere (1995), as a score of 75 or over on the Inconsistent Data subscale. Forty‐six total cases were removed per Briere’s criteria (21 from the treatment group and 25 from the control group). The resulting group sizes for TSI analysis were treatment group (n = 50) and control group (n = 49)" Over 20% dropout; this is significant and may affect the effect size |
| Protection against contamination | Low risk | Judgement comment: control group did not have communication with therapists prior to receiving intervention themselves; therefore unlikely to have been contaminated |
| Selective reporting (reporting bias) | Unclear risk | No protocol, so not clear |
| Other bias | Low risk | No other sources of bias found |
Dawson 2016.
| Study characteristics | ||
| Methods |
Study design: RCT Duration of study: recruitment March to September 2014. Intervention for 5 weeks. Assessments conducted 2 weeks after last session |
|
| Participants |
Country: Kenya Income classification: lower‐middle income in 2014 Geographical scope: 3 peri‐urban villages that are part of the primary healthcare system of Dagoretti sub‐county in Nairobi, Kenya Healthcare setting: after‐school programme Mental health condition: PTSD Population: women exposed to adversity including possible gender‐based violence 1. Age > 18 years 2. Gender: female 3. Socioeconomic background: low‐income areas 4. Inclusion criteria a. Female b. Over 18 years of age c. Score ≥ 3 on General Health Questionnaire (GHQ‐12; a measure of general anxiety and depression) and ≥ 17 on WHO Disability Assessment Schedule version 2.0 (WHO‐DAS2.0; a measure of functional disability). These cutoffs were used to ensure that only women with both marked distress and impairment were recruited 5. Exclusion criteria a. Considered to be at risk of ending their life or displaying severe mental disorder (i.e. psychotic disorders and substance dependence) or severe cognitive impairment (i.e. severe intellectual disability or dementia) |
|
| Interventions |
Stated purpose: feasibility trial for a brief psychological intervention to alleviate symptoms of common mental disorders among women exposed to adversity INTERVENTION (n = 30) Name: Problem Management Plus Delivered by: LHWs Title/name of PW and number: community health workers ‐ 23 1. Selection: intervention providers were women engaged in community health work with the government 2. Educational background: CHWs have varied levels of education and do not receive any training or experience in mental health care (encompassing counselling, psychology, or psychiatry) 3. Training: an 8‐day training programme was delivered by the master trainer (KSD), directly to the CHWs (n = 23) and to 3 Kenyan psychologists who would provide supervision for the CHWs. Training included the provision of basic theoretical knowledge of common mental disorders, basic counselling skills, delivery of the PM+ intervention, and self‐care practices. Classroom training was followed by 4 weeks of practice cases (approximately 3 clients per CHW) under close supervision. CHWs were then required to pass competency assessments before PM+ was offered to participants involved in the feasibility study. CHWs also received training in psychological first aid to know how to react in case people were exposed to new traumatic events during the study 4. Supervision: CHWs were supervised on a weekly basis by 1 of 3 local supervisors who were clinical psychologists with previous experience in providing clinical supervision. Local supervisors were supervised weekly to fortnightly for 1 to 2 hours by the master trainer and a fourth local supervisor (LN). Supervision comprised building skills in the PM+ intervention as well as in training and supervision of CHWs with emphasis on research principles, such as standardisation and fidelity of treatment. Thus, supervision was cascaded from a foreign intervention specialist to local experts, and onwards to CHWs 5. Incentives/remuneration: not mentioned Intervention details 1. Duration/frequency: 5 x 90‐minute sessions 2. Content of intervention: PM+ is an innovative, evidence‐informed, and scalable intervention that aims to provide psychological support to adults exposed to adversity. Specifically, it aims to address common symptoms of mental disorders such as depression, anxiety, and stress, as well as client self‐identified practical problems, such as interpersonal conflict and financial problems CONTROL: enhanced treatment as usual (ETAU) (n = 25) ETAU consisted of receiving care from primary care clinicians (nurses) at 1 of 3 local primary healthcare clinics (PHCs). For the purposes of this study, and given that treatment as usual for mental disorders in this setting often equates to no care, primary care nurses – who already had training and experience in counselling people with HIV/AIDS – received 1‐day training in psychological first aid and an additional day of training in supportive counselling, based on the International Federation of Red Cross manual, without follow‐up supervision CO‐INTERVENTIONS: nil |
|
| Outcomes |
Patients 1. GHQ 2. WHO‐DAS2.0 3. PCL‐5 Carers Nil Process/health workers Nil Economic outcomes Nil Time points: baseline, immediately post intervention (1 to 2 weeks after last session (or approximately 6 weeks later for control group)) |
|
| Notes |
Source of funding: Grand Challenges Canada, World Vision Canada, and World Vision Australia; with additional support from the World Health Organization and the University of New South Wales Notes on validation of instruments (screening and outcomes): validated Additional information: declaration of interests ‐ study authors report no competing interests Handling the data: as per footnotes in data and analysis Prospective trial registration number: ACTRN12614001291673 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed by an independent colleague using computerised software (i.e. off‐site in Sydney and not involved in the trial) |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed by an independent colleague using computerised software (i.e. off‐site in Sydney and not involved in the trial) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and interventionists were not blinded; however this was unlikely to have influenced outcomes |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | Post‐treatment assessments were completed by independent assessors who were unaware of the treatment conditions of participants. Blindness was maintained by ensuring that assessors who conducted assessments did not have access to (1) condition allocation of participants or (2) participant notes. In addition, careful attention was paid to ensure assessors had no contact with CHWs or ETAU nurses by having them work in different locations |
| Baseline outcome measurements similar | Low risk | No differences were detected between participants in the PM+ and ETAU conditions on any of the pretreatment outcome measures, demographics, or trauma exposure |
| Baseline characteristics similar? | Low risk | No differences were detected between participants in the PM+ and ETAU conditions on any of the pretreatment outcome measures, demographics, or trauma exposure |
| Incomplete outcome data (attrition bias) Efficacy data | High risk | Rates of dropout at post assessment also provide some support for acceptability of PM+. Eighty‐six per cent of women who received the intervention were willing to participate in the post assessment, compared to 71% of ETAU women Altogether, 15 of 70 participants dropped out of the study. This could have impacted the efficacy results. The study was a pilot study and thus was not powered to determine efficacy of the intervention |
| Incomplete outcome data (attrition bias) Safety data (e.g. adverse events) | Low risk | Any adverse reactions reported spontaneously by participants or observed by the research team were reported to a local independent advisory board that comprised an independent medical officer, an independent counselling psychologist, the site principal investigator, and a clinical supervisor. An adverse reaction was defined as any undesirable experience occurring to a participant during the study, whether or not it was considered related to the research procedure No adverse or serious adverse events were reported by women receiving the intervention, suggesting that PM+ did not cause harm or exacerbate distress beyond one’s capacity to cope with it |
| Protection against contamination | Low risk | Project staff met periodically with nurses providing the ETAU to establish the types of support and counselling they offered to participants in this condition and to check that there was no contamination of PM+ strategies used in the ETAU condition |
| Selective reporting (reporting bias) | High risk | PSYCHLOPS, health service use, and life events checklist were listed in the trial protocol, but results were not reported in this paper |
| Other bias | Unclear risk | it was revealed through supervision processes that the in vivo exposure strategy was rarely implemented, and when it was, it was done so incorrectly. For instance, in vivo exposure was applied to women reporting symptoms of cognitive worry as opposed to anxious avoidance. In vivo exposure was not used in an exploratory trial of PM+ in Peshawar, Pakistan [33]. In vivo exposure is arguably a more complex strategy to train non‐specialist providers to deliver effectively, although other research groups have managed this [34]. In this study, in vivo exposure required the CHW to identify the source of anxious avoidance, to develop a gradual exposure plan, and to accompany the client during initial steps of this plan. It is possible the specific source of avoidance was difficult for CHWs to define, and that accompanying the client was logistically prohibitive; therefore in vivo exposure was readily abandoned as a strategy to implement with clients. Consequently, the revised version of PM+ omits in vivo exposure [35] |
Dias 2008.
| Study characteristics | ||
| Methods |
Study design: RCT Duration of study: unknown |
|
| Participants |
Country: India Income classification: lower‐middle Geographical scope: Taluka; semi‐urban Healthcare setting: home‐based care Mental health condition: dementia Population (patient and carer dyads) 1. Age: carers around 53 years; patients with dementia around 78 years 2. Gender: both 3. Socioeconomic background: 40% of patients with dementia and 20% of carers had below primary education. Most (90%) were unable to afford paid help 4. Inclusion criteria a. Clinical Dementia Rating scale: mild to moderate dementia b. Carers c. Person identified by the family 5. Exclusion criteria a. Clinical Dementia Rating scale: severe dementia or severe co‐morbid physical health condition |
|
| Interventions |
Stated purpose: to test effectiveness of the 10/66 intervention in reducing carer burden, promoting carer mental health, and reducing behaviour problems in elderly people with dementia INTERVENTION (n = 41) Name: 10/66 Flexible Stepped‐Care Brief Carer Intervention Delivered by: LHW Title/name of PHW and number: 4 healthcare assistants (HCAs) (2 in each taluk); 1 lay health counsellor (LHC) (shared by both taluks) 1. Selection: HCA: knowledge of local language, literate, motivated to involve in community care of older people; LC: part of the intervention team/authors; member of the Dementia Society in Goa 2. Educational background: HCA: passed higher secondary school; LC: not specified 3. Training: HCA: intensive training module over 1 week developed/adapted to local settings. Trained in key skills including listening and counselling skills, bereavement counselling, stress management, and health advice for common health problems. Trained by study author (geriatrician/epidemiologist) and LHC; LHC: not specified 4. Supervision: for HCA: meetings every 2 weeks with psychiatrist and LC. HCA would meet the psychiatrist twice a month to give update on person with dementia, especially if person was taking medication. In addition, met with LC every 2 weeks to share experiences, support one another, and problem‐solve difficult situations; LC: supervised by psychiatrists 5. Incentives/remuneration: LC: Rs 5000/month; HCA: not specified; psychiatrist remunerated Rs 3000/month for monitoring/supervising LCs Intervention details 1. Duration/frequency: home visits at least every 2 weeks for 6 months 2. Content of intervention (by types of health workers and per patients/carers): HCAs: intervention for carers: psychoeducation plus follow‐up and some counselling skills. Patients or carers (or both) had follow‐up with psychiatrist and patients may have been prescribed medication CONTROL (n = 40) Control arm dyads received only education and information regarding dementia and then were placed on a waiting list to receive the intervention after 6 months CO‐INTERVENTIONS: both intervention and control groups were free to utilise existing health services during this time |
|
| Outcomes |
Patients 1. Severity of behavioural problems (NPI‐S) 2. Functional ability of participant (Everyday Abilities Scales for India) Carers 1. Carer mental health (GHQ score)* 2. Carer perceived burden (ZBS) 3. Carer distress due to problem behaviours (NPI‐D) Process/health workers 1. Process indicators: mean number of visits by HCA, visits by psychiatrists 2. Use of medication not reported Economic outcomes 1. Protocol mentions primary outcome: cost of illness, but not reported (*: primary outcomes of study) Time points: 3 months and 6 months after baseline |
|
| Notes |
Source of funding: WHO Notes on validation of instruments (screening and outcomes): all were validated (Dias 2004) Additional information: study authors provided supplementary information on supervision, remuneration, and other elements. We had access to the study protocol Patient outcomes are presented in this treatment review. Carer outcomes are summarised in this review, but will also be included in prevention review, as for them, the intervention is preventing mental distress/disorders. Declaration of interests ‐ study authors declared no competing interests Handling the data: as per footnotes in data and analysis Prospective trial registration number: NCT00479271 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization of dyads comprising the person with dementia and their principal caregiver was carried out by an independent person, based on simple random number tables, either to the intervention or waiting list group" Comment: this was carried out by using simple random numbers tables |
| Allocation concealment (selection bias) | Low risk | Comment: allocation was done by an 'independent person' |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Patients and carers recommended by the family and by personnel knew who was allocated to the intervention. Personnel did not take part in measuring the outcome, so this does not affect the outcome |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | Quote: "outcome evaluations were carried out by researchers who were masked to the allocation status until the end of the project. We attempted to blind outcome evaluations by ensuring that allocation status was kept in a separate office from the outcome evaluation teams. We had also instructed the families not to divulge information on the visits by the Home Care Advisor. However, we anticipated that some unmasking would occur because both the intervention and outcome evaluations were home‐based. In order to evaluate the masking process, researchers were asked to guess the intervention status. Another limitation in trials of this nature is that the researchers did, during the course of their outcome evaluation, correctly guess the allocation status in nearly two‐thirds of individuals because of the information on health care use which typically led some care‐givers to share contacts with the intervention team" Comment: study authors have mentioned the possibility of unmasking and measures they took to minimise this. Mortality is an objective outcome and was reported completely. Agree with low risk assessment |
| Baseline outcome measurements similar | Low risk | Comment: There were differences in outcome measures at baseline: mean GHQ score was different ‐ higher in the intervention group (Table 2). This difference was adjusted for in subsequent analyses |
| Baseline characteristics similar? | Low risk | Comment: there were no baseline differences in SES nor in psychiatric comorbidity. Outcome measures at baseline were also similar |
| Incomplete outcome data (attrition bias) Efficacy data | Low risk | Comment: there was a > 20% dropout rate (only 59 remain at follow‐up compared with 81 randomised), but this was a small sample size. The most common causes of death were stroke (4 people), pneumonia (4 people), myocardial infarction (3 people), and septicaemia (2 people). 2 families moved out of the study area, and 2 refused to continue with the trial. However, there was no significant difference in baseline characteristics among those who died or were alive to the end of the trial (P = 0.05 for GHQ, NPI‐S, NPI‐D, Everyday Abilities Scales for India, and ZBS scores) |
| Incomplete outcome data (attrition bias) Safety data (e.g. adverse events) | Unclear risk | Comment: deaths were reported, but adverse effects of interventions on carers were not specified |
| Protection against contamination | Unclear risk | Comment: insufficient information was provided on how close together intervention and control groups were placed (e.g. were they in same village, mean details of dementia care in Goa) (cultural view) ‐ contacted study author for this |
| Selective reporting (reporting bias) | High risk | Comment: study authors have not reported the cost of illness nor process indicators: mean number of visits by home care advisor, visits by psychiatrists, use of medication. The protocol mentions primary outcomes as (1) carer mental health, (2) carer burden, (3) behaviour problems and activities of daily living in elderly people with dementia, and (4) costs of illness, but in the results section, the last point is not reported |
| Other bias | Low risk | Comment: none detected |
Divan 2019.
| Study characteristics | ||
| Methods |
Study design: RCT; 2‐arm single (assessor)‐blinded Duration of study: January to December 2016 |
|
| Participants |
Country: India Income classification: lower‐middle income in 2016 Geographical scope: rural; Kolhapur, Maharashtra, India Healthcare setting: participants' homes Mental health condition: autism spectrum disorder Population: children with autism and their parents (parent‐child dyads) 1. Age: children 2 to 9 years old 2. Gender: children – both 3. Socioeconomic background: more than half of participants' mothers had received undergraduate education or higher 4. Inclusion criteria (including threshold cutoff score of measurement tool) a. Development age 12 months or older b. Diagnosis of autism spectrum disorder using the INCLEN Diagnostic Tool for Autism Spectrum Disorder (Juneja et al, 2014) 5. Exclusion criteria a. Children with uncontrolled epileptic seizures, severe hearing, or visual impairment b. Residence outside trial area c. Parents with severe hearing or visual impairment, severe psychiatric disorder, residence outside the trial area |
|
| Interventions | Stated purpose: to evaluate the feasibility and effectiveness of the PASS Plus intervention for Autism Spectrum Disorders compared to usual care INTERVENTION 1 (n = 19) Name: parent‐mediated intervention for autism spectrum disorder plus (PASS Plus) Delivered by: LHW Title/name of PW and number: facilitators (lay health workers) ‐ 4 1. Selection: a first‐stage competency assessment evaluated knowledge and skills of selected candidates, who were then allowed to co‐deliver the intervention to non‐trial practice dyads under supervision. During 1‐month internship period, each trainee delivered a minimum of 3 sessions independently, after which a second‐level objective competency assessment was administered on PASS specific knowledge and skills. Those who achieved a pre‐determined competency score then engaged with trial dyads. Two months into the case practice sessions, lay health workers received an additional 2‐day training on Plus modules 2. Educational background: college graduates 3. Training (contents, duration, by whom): 10‐day training by senior clinicians and mid‐level supervisor, which included classroom‐based instruction on child development and autism, observations in special education settings of children with social communication impairments, and practice‐based learning of the modular intervention PASS 4. Supervision: first session on engagement was conducted with a supervisor present. All sessions were videotaped, and these were used initially for one‐on‐one supervision by PASS Plus trainers. Over the trial period, supervision evolved from initial high‐intensity group supervision conducted once a week by a senior clinician to peer‐led supervision. These were then supervised every fortnight by senior clinicians. The implementation team was rated on fidelity measures conducted by a therapy expert based in the UK on 10% of randomly selected treatment sessions Intervention details (according to PWs and whether aimed at carers and/or patients) 1. Duration/frequency: twelve 60‐ to 90‐minute fortnightly sessions over a 6‐month period 2. Content of intervention (by types of health workers and per patients/carers): 2 distinct manualised components: PASS social communication modules and “Plus” comorbidity modules. During the core social communication intervention (PASS), facilitators used video feedback on play sessions recorded during a 10‐minute period of play between parent and child to support the parent to recognise the child’s non‐verbal and verbal signals, which reflect the child’s communication intentions, and to recognise which of their own actions have a positive effect on the interaction. The parent is then guided to choose intervention strategies, which include simple but effective strategies such as paying attention to parent positioning, watching and waiting, and reducing the use of questions and directives to try out and the effects of these on their dyadic interaction, which is reviewed at the start of the next session. Plus modules address common comorbidities via a psychosocial approach and are introduced in the fourth session. After supporting the parent to identify the comorbidity most disruptive for the family, the decision algorithm enables the facilitator to identify the most relevant advice and strategies for the family. Parents are requested to practise the communication strategies for 30 minutes every day in the intervening fortnight CONTROL: usual care. (n = 21) Participants were able to access regular treatment: children visited allopathic private doctors and Ayurvedic/homeopathic doctors CO‐INTERVENTIONS: participants in both arms were able to access regular treatment. About 15% of the children in both groups attended a specialist school, and about half attended mainstream schools. Schools offered largely respite care with some remedial education, with no specific intervention for autism |
|
| Outcomes |
Patients 1. Autism symptom severity – Brief Observation of Social Communication Change (BOSCC)* 2. Dyadic Communication Measure for Autism (DCMA)* ‐ proportion of parent synchronous responses, proportion of child acts of communication initiation, proportion of time in shared attention 3. Comorbidity severity ‐ Developmental Behaviour Checklist (DBC) 4. Adaptive child behaviours ‐ standard Vineland Adaptive Behaviour Scale (VABS) total score and communication, receptive, expressive, written, and socialisation sub‐scores Carers 1. Parental mental well‐being ‐ PHQ‐9 2. Parent self‐perception of knowledge, skills, acceptance, empowerment, and advocacy, using a measure adapted from the Research on Autism and Families in India (RAFIN) study (Daley 2013) (the RAFIN tool) Process/health workers Nil Economic outcomes Nil (asterisk for study's primary outcomes; star: outcomes that we have not reported in this review) Time points post end of intervention: 0 months |
|
| Notes |
Source of funding: Grand Challenges Canada Global Mental Health Stream Notes on validation of instruments (screening and outcomes): validated Additional information: study protocol available. Declaration of interests ‐ study authors declared no competing interests Handling the data: nil Prospective trial registration number: ISRCTN10260663. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Each child participant was assigned a sequential identification number that was sent to an independent randomisation centre. Randomisation lists were stratified by age and by functional impairment |
| Allocation concealment (selection bias) | Low risk | Allocation was conveyed by telephone and by email to the site co‐ordinator, who communicated with the intervention team. Research members of staff were masked to treatment allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and interventionists were not blinded, but this was unlikely to have influenced outcomes |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | Assessors were masked to participants' allocation and to the time point at which participants were videotaped |
| Baseline outcome measurements similar | Low risk | Baseline outcome measurements were similar in both groups |
| Baseline characteristics similar? | Low risk | Baseline characteristics of the 2 groups (age, gender, mother's educational level) were similar |
| Incomplete outcome data (attrition bias) Efficacy data | Unclear risk | Small sample size: attrition rate of 12.5% (5/40); participants who had dropped out may have influenced results had they stayed |
| Incomplete outcome data (attrition bias) Safety data (e.g. adverse events) | Low risk | Low attrition and no safety issues; 2 participants dropped out because of intervention (1 withdrew because parent did not understand intervention; 1 family withdrew because they felt the intervention was not relevant to the child's difficulties). No safety issues were reported |
| Protection against contamination | Low risk | No participants received speech, language, or occupational therapy nor physiotherapy during the trial period. Although participants attended specialist or mainstream schools, these had no notable specific intervention for autism. Control group participants were not exposed to the intervention, as the intervention was home‐based and was delivered by trained workers |
| Selective reporting (reporting bias) | Unclear risk | All outcomes planned in the methods section were reported in the results section. As this was a pilot trial, trial protocol was not published online |
| Other bias | Low risk | Nil |
Dybdahl 2001.
| Study characteristics | ||
| Methods |
Study design: randomised 2‐sided parallel‐group open‐label assessor‐blinded controlled trial (unit of randomisation: mother‐child dyads; unit of analysis: individuals) Duration of study: 1995 to 1996 |
|
| Participants |
Country: Bosnia Income classification: low income Geographical scope: urban (town of Tuzla, a multi‐ethnic industrial town in northeastern Bosnia) Healthcare setting: home (1 refugee settlement; private accommodation for refugees) Mental health condition: child mental health (PTSD, mental health, behavioural problems, scholastic difficulties) Population: mother‐child dyads (internally displaced refugees) 1. Age: mothers: mean 30.7 years (SD 4.9), range 20 to 44 years; children: mean 5.5 years (SD 0.7) 2. Gender: both (children: 48 girls, 39 boys) 3. Socioeconomic background: mothers: 85% urban origin, education 14% illiterate (mean 5.3 years, SD 2.8; range 0 to 14 years), married 63%, widowed 36%, divorced 1%, living in private accommodation 60%, living in refugee camp 40% 4. Inclusion criteria: internally displaced Bosnian mothers with a child aged 5 to 6 years 5. Exclusion criteria a. Not participating in any other intervention programme b. Unlikely to move out of the area before November 1996 |
|
| Interventions |
Stated purpose: to provide early childhood care and education as well as psychosocial support to traumatised children by working with their mothers to help them resolve grief and improve parenting and by providing a well‐functioning family environment utilising non‐medical professionals in a post‐conflict situation INTERVENTION Name: Psychosocial Intervention (+ basic medical care) ‐ 42 people Delivered by: CP Title/name of PW and number: group leaders ‐ preschool teachers trained for the study ‐ 5 1. Selection: not specified in this report 2. Educational background: as above 3. Training (contents, duration, by whom): to a group of 3 to 8 group leaders, provided by mental health professional a. Duration: 5‐day workshop. Before arrival, participants received basic information about the programme and its background and aims b. Content: participants were introduced to one another and received written material and introductory training on some of the key issues such as trauma, child development, and the importance of interaction and communication (mother‐child) in two 3‐hour seminars. Then 3 days of more detailed description of the programme and reinforcement through group work, demonstrations, role‐plays, and discussion of the above topics (roles of caretaker, trauma and its effects on adults and children, groups and group dynamics, supervision, logbook) 4. Supervision: weekly group meetings (with 6 to 8 group leaders along with a supervisor (a mental health professional) (later twice a month) 5. Incentives/remuneration: as above Intervention details 1. Duration/frequency: group leader met weekly with 2 groups of mothers (5 per group) for 5 months; 1 additional visit to each mother at her home at start of programme 2. Content of intervention: group work using a manual‐based approach derived from therapeutic discussions with war‐traumatised women at the Psychological Centre in Tuzla (1993‐1996) and the ICDP; semi‐structured group discussions introduced by group leaders dedicated to providing information about trauma and trauma reactions in adults and children, as well as suggestions for how to meet common post‐traumatic needs and problems, with emphasis on strengthening participants' own coping strategies and reinforcing existing normal basic communication and interaction skills. Direct attention was given to mothers and their mental health, to their beliefs and knowledge about children, and to the reactions and needs of adults and children following traumatic events. Mothers were also visited once at home to establish rapport and to express support CONTROL: non‐intervention group; participated in evaluations and received free basic medical care (45 people) CO‐INTERVENTIONS: free basic medical care by local physicians provided for both groups; vitamins or iron was given to 52 children (66% in intervention group; 81% in control group) |
|
| Outcomes |
Children 1. Description of child (rated by mothers; 11 characteristics; 7‐point differential) 2. Mothers' ratings of children's problems § (10 problems; 4‐point scale; total 30 points) 3. Mothers' ratings of concentration problems § (yes/no) 4. Raven's Coloured Progressive Matrices § 5. Children's interview (modified Birleson Depressive Inventory; modified by removing 2 of 13 items; scored 0 to 32; 11 used as cutoff for depression) 6. Well‐being § 7. Psychologists' observations § (video‐rated; 14 items; 4‐point scale; scored on 2 factors ‐ problems 0 to 32; resources 0 to 16) 8. Anthropometrics: haemoglobin § Mothers 1. Perceived Social Support 2. IES (reported in adult PTSD) 3. Well‐being § Process/health workers Not reported Economic outcomes Not reported (*: primary outcomes of the study; §: outcomes that we have not reported in this review) Outcomes not used in quantitative synthesis War Trauma Questionnaire (given at baseline) Time points: baseline, 5 to 6 months after recruitment (0 to 1 month post intervention) |
|
| Notes |
Source of funding: UNICEF; University of Tromso Notes on validation of instruments (screening and outcomes): mothers' ratings of child's concentration and concentration problems; perceived social support: not validated separately; IES scores: not diagnostic of PTSD but some literature suggests IES score above 33 suggestive of PTSD Additional information: group work is described in Dybdahl 1996 and Dybdahl 1999. Declaration of interests ‐ none Also included in prevention review, as some mothers seem to have a mental disorder, and others minimal to moderate psychological distress Handling the data: as per footnotes in data and analysis Prospective trial registration number: not registered Also included in prevention review, as unsure about the population (roughly half the intervention group has mental distress or a mental disorder at baseline; thus the intervention may be a treatment, whereas for the other half, it could be a prevention strategy) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from report: "the assignment was random. All the names of the mother–child dyads were written on pieces of paper, which were folded, mixed together, and then separated into two piles at random so that one pile formed the intervention group and the other pile formed the control group" |
| Allocation concealment (selection bias) | Unclear risk | Comment: not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: participants and intervention personnel were not blinded to allocation, but no evidence of impact on outcome is provided |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | Comment objective outcomes: physical and psychosocial outcomes were measured by teams of physicians and experienced health worker assistants not involved in delivering interventions and blind to interventions Comment subjective outcomes: physical and psychosocial outcomes were conducted by teams of physicians and experienced health worker assistants not involved in delivering interventions and blind to interventions |
| Baseline outcome measurements similar | Low risk | Comment: baseline imbalances in prognostic variables noted for psychosocial support for mothers and for well‐being (but not statistically significant) and for children's haemoglobin (P = 0.3); however, analyses included differences between groups in changes from baseline |
| Baseline characteristics similar? | Unclear risk | Comment: mothers in refugee camps reported more war trauma and were more likely to be widowed during the conflict |
| Incomplete outcome data (attrition bias) Efficacy data | High risk | Quote: "twelve of the families dropped out of the study and did not participate in scheduled interventions: 7 from the intervention group, and 5 from the control group. Several of the mothers and children did not complete all tests at both test periods for a variety of reasons; thus the number of participants varied from test to test" Comment: denominators for each of the tests are not provided by intervention or control |
| Protection against contamination | Unclear risk | Comment: mothers in refugee camps could have discussed contents of the intervention while supporting mothers in control group |
| Selective reporting (reporting bias) | Unclear risk | Comment: protocol is not available, but all measures stated in methods are reported |
| Other bias | High risk | Comment: multiple statistical analyses were used without pre‐specified primary or secondary outcomes; analyses corrected for multiple comparisons yielded non‐significant results |
Ertl 2011.
| Study characteristics | ||
| Methods |
Study design: RCT Duration of study: trial conducted between November 2007 and October 2009 (last follow‐up). Preceded/overlapped by an epidemiological survey July 2007 to April 2008 |
|
| Participants |
Country: Uganda Income classification: low income Geographical scope: rural and urban; takes place in IDP camps and new settlement areas in 3 regions of Northern Uganda: Anaka: rural area with the most documented rebel activity; Awer: urban relatively safe area close to large town called Gulu, Padibe; rural (long distance from Gulu and more affected by the war) Healthcare setting: home Mental health condition: child mental disorder ‐ PTSD Population: patients; children/adolescents (child soldiers) 1. Age: 12 to 25 years; mean age 18.66 years (SD 3.77) 2. Gender: both 3. Socioeconomic background: former child soldiers; mean economic status in Euros (as measured by household possessions weighted by current local market prices divided by household size): EUR44‐55 4. Inclusion criteria a. Clinical diagnosis of PTSD derived from expert interviews b. Member of the group of formerly abducted people or former child soldiers c. Note: to keep the trial naturalistic, we did not exclude patients with suicidal ideation, substance abuse, or depression 5. Exclusion criteria a. Current substance dependence b. Mental retardation c. Psychotic disorder |
|
| Interventions |
Stated purpose: to examine whether individual‐based, trauma‐focused NET is feasible and effective in reducing PTSD symptoms among traumatised former child soldiers living in the IDP camps of Northern Uganda when carried out by trained local lay therapists directly in communities INTERVENTION 1 (n = 29) Name: NET Delivered by: LHWs Title/name of PW and number: local lay counsellors ‐ 14 (7 women, 7 men) 1. Selection: not specified 2. Educational background: not specified 3. Training: training in and performance of NET were as outlined by an adapted field version of manual, duration, and trainers: unspecified 4. Supervision: "treatment fidelity and therapeutic competence were monitored by case discussions in supervision meetings, observation and evaluation of treatment sessions via video recordings, and review of the obligatory treatment process notes for each session. In the case of NET, testimonies were additionally reviewed to check for trauma focus and richness of detail" ‐ not specified by whom 5. Incentives/remuneration: not specified Intervention details 1. Duration/frequency: 8 sessions of individual therapy; "sessions lasted between 90 and 120 minutes and were scheduled 3 times a week" 2. Content of intervention: "we chose an individual‐based over a group‐based treatment, because we expected this approach to better meet the requirements of former child soldiers, who present with high levels of PTSD as well as mistrust"; "narrative exposure therapy is a short‐term, trauma‐focused treatment developed for use in low‐resource countries affected by crises and conflict. Intended for survivors of multiple trauma, this therapy results in the detailed documentation of the patients' lives as part of the therapy process"; "irrespective of treatment condition, the first session included psychoeducation on PTSD, its symptoms and consequences for the individual, and explanation of the rationale for narrative exposure therapy or academic catch‐up". Participant constructs chronological account of self biography with therapist and reconstructs fragmented memories of traumatic events and habituation INTERVENTION 2 (n = 28) Name: Academic Catch‐up Training Delivered by: lay PHWs Title/name of PHW/CW and number: local lay counsellors ‐ 14 (7 women, 7 men) 1. Selection: not specified by whom 2. Educational background: not specified by whom 3. Training: written guidelines that summarised basic counselling skills and session outlines for academic catch‐up training; duration and trainers unspecified 4. Supervision: "treatment fidelity and therapeutic competence were monitored by case discussions in supervision meetings, observation and evaluation of treatment sessions via video recordings, and review of the obligatory treatment process notes for each session". Not specified by whom 5. Incentives/remuneration: not specified by whom Intervention details 1. Duration/frequency: 8 sessions of individual therapy; "sessions lasted between 90 and 120 minutes and were scheduled 3 times a week" 2. Content of intervention: "carried out according to written guidelines that summarized basic counselling skills and session outlines for the academic catch‐up training"; "irrespective of treatment condition, the first session included psychoeducation on PTSD, its symptoms and consequences for the individual, and explanation of the rationale for narrative exposure therapy or academic catch‐up"; "an intensive English catch‐up course using the official Ugandan schoolbooks for different skill levels was developed. Evaluation of process notes revealed that counsellors spent 55% of total time allocated for academic catch‐up doing academic training. The rest of the time was equally dedicated to providing psychoeducation, conducting discussions on coping with symptoms, and dealing with current problems. None of the counsellors deviated from the restriction that they should not focus on traumatic experiences in this condition. In the last session, participants received the English textbooks and exercise books they had been working on with their counsellors" CONTROL (n = 28) Wait‐list control; 10 received suicide intervention due to suicidal ideation. "After the 12‐month follow‐up, each waiting‐list and academic catch‐up participant still presenting with PTSD was offered narrative exposure therapy" CO‐INTERVENTIONS: wait list with suicide intervention for those who exhibited high levels of suicide ideation (10 people) |
|
| Outcomes |
Patients 1. PTSD symptom load* (Clinician‐Administered PTSD Scale ‐ CAPS) 2. Functional impairment* (CAPS) 3. Guilt § (CAPS) 4. Symptoms of depression (MINI Neuropsychiatric Interview for depression module A ‐ MINI) 5. Suicidal ideation (MINI) 6. Stigmatisation § (Perceived Stigmatization Questionnaire ‐ PSQ) Carers N/a Process/health workers 1. "Treatment fidelity and therapeutic competence were monitored by case discussions in supervision meetings, observation and evaluation of treatment sessions via video recordings, and review of the obligatory treatment process notes for each session. In the case of narrative exposure therapy, testimonies were additionally reviewed to check for trauma focus and richness of detail. No deviations from the study protocol were noted" 2. None reported in the study § Economic outcomes None (*: primary outcomes of the study; §: outcomes that we have not reported in this review) Time points: baseline; 3‐, 6‐, and 12‐month follow‐up |
|
| Notes |
Source of funding: this study was supported by the NGO vivo and by funding from the DFG (Deutsche Forschungsgemeinschaft) and the Ein Herz für Kinder Foundation Notes on validation of instruments (screening and outcomes): CAPS and MINI validated; PSQ not validated Additional information:clinicaltrialsgov/show/NCT00552006. Declaration of interests ‐ study authors reported no competing interests Handling the data: as per footnotes in data and analysis Prospective trial registration number: NCT00552006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: randomly selected, but study does not specify how |
| Allocation concealment (selection bias) | Unclear risk | Comment: unspecified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: unspecified |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | Quote: "pretreatment assessments as well as follow‐up assessments at 3 months, 6 months, and 12 months after treatment were conducted by 13 clinical psychologists blinded to treatment conditions" Comment: appropriate blinding; no objective outcomes |
| Baseline outcome measurements similar | Low risk | Comment: no statistical differences Quote: "there were no systematic pretreatment differences in sociodemographic data, traumatic load, and psychological impairment between the 3 groups" |
| Baseline characteristics similar? | Low risk | Comment: no statistical differences Quote: "there were no systematic pretreatment differences in sociodemographic data, traumatic load, and psychological impairment between the 3 groups" |
| Incomplete outcome data (attrition bias) Efficacy data | Low risk | Comment: 3‐month follow‐up: 26 included and 2 discontinued in NET; 24 included, 2 discontinued, and 1 died in academic catch‐up (ACU) at 6 months' follow‐up; 26 included in NET, 23 included, 1 not found in ACU at 12 months' follow‐up; 25 included, 1 loss to follow‐up, 23 in NET; all 28 wait‐list participants remained throughout treatment Quote: "apart from providing participants with the written documentation of their lives or with the English textbooks and exercise books, no incentives were offered. During follow‐up periods, individuals who had relocated far from the former IDP camps were refunded travel expenses" Comment: this would have reduced attrition; unlikely to have affected outcomes |
| Incomplete outcome data (attrition bias) Safety data (e.g. adverse events) | Low risk | Comment: no negative effects of NET were observed in this trial. Clinically reliable aggravation of symptoms was not present in the NET group but was present in 4.4% of academic catch‐up and in 10.7% of waiting list participants |
| Protection against contamination | Low risk | Comment: lay counsellors were instructed not to integrate treatment material from NET to ACU |
| Selective reporting (reporting bias) | Low risk | Comment: all pre‐specified outcomes in protocol were reported |
| Other bias | Low risk | Comment: none were detected |
Fritsch 2007.
| Study characteristics | ||
| Methods |
Study design: RCT Duration of study: 6 months |
|
| Participants |
Country: Chile Income classification: upper‐middle income Geographical scope: urban (Santiago) Healthcare setting: 5 PHC clinics Mental health condition: major depression Population 1. Age: 18 to 70 years 2. Gender: female 3. Socioeconomic background: about 30% employed, 8% unemployed, 5% student 4. Inclusion criteria a. As above, with depression for 3 months (screening with GHQ‐12 (≥ 5) twice, 2 weeks apart) b. At least 1 child aged 6 to 16 living with her Exclusion criteria a. Abuse/dependence on alcohol or drugs b. Bipolar disorder c. Psychotic symptoms (present or past) d. Suicidal ideation e. Pregnancy f. Physical or mental disabilities that would hamper participation in the study |
|
| Interventions |
Stated purpose: to compare monitored pharmacotherapy intervention with current treatment in PC INTERVENTION (n = 143) Name: Monitored Pharmacotherapy Delivered by: PHP and LHWs Title/name of PW and number: 5 generalist doctors/GPs (1 per practice) and non‐professional trained staff from 5 clinics 1. Selection: based on practice selection 2. Educational background: qualified doctors 3. Training: for doctors: 6 hours of training by principal investigators; for non‐professional trained staff: 2 hours 4. Supervision: doctors had permanent monitoring by principal investigators. In addition, doctors participated in monthly meetings with a psychiatrist to discuss cases 5. Incentives/remuneration: not specified Intervention details 1. Duration/frequency: regular visits to GP by patients 2. Content of intervention: regular visits to GP with pharmacotherapy structured using clinical algorithms (use of available antidepressants: fluoxetine, amitriptyline, imipramine). Regular telephone contact by non‐professional, but trained personnel provided education, monitoring of drug intake and side effects, and reminders/reinforcement of the need for regular follow‐up with the doctor CONTROL: usual care (n = 131) Based on Ministry of Health programme for treatment of depression in PC: consultations with GPs, pharmacotherapy, individual or group psychotherapy with psychologists, and referral to psychiatrists CO‐INTERVENTIONS: none |
|
| Outcomes |
Patients 1. Diagnosis of depression (MINI) 2. Severity of symptoms (HDRS) 3. QoL (SF‐36) Carers None Process/health workers None Economic outcomes None Time points: 3 months and 6 months |
|
| Notes |
Source of funding: Fondecyt, Chile Notes on validation of instruments: all instruments were validated internationally and in Chilean setting Additional information: no protocol was provided. Declaration of interests ‐ none Handling the data: as per footnotes in data and analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: patients were assigned randomly; this took place at the individual level, using computer systems managed at a central level |
| Allocation concealment (selection bias) | Low risk | Comment: patients were assigned randomly; this took place at the individual level, using computer systems managed at a central level |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: due to the nature of the intervention, participants could not be blinded to the intervention; this is unlikely to create any bias to the results |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | Comment: assessors were not involved in the design of the study, did not know the study hypotheses, and were blinded to group assignment. No objective outcomes |
| Baseline outcome measurements similar | Low risk | Comment: the 2 study groups did not vary significantly |
| Baseline characteristics similar? | Low risk | Comment: the 2 study groups did not vary significantly |
| Incomplete outcome data (attrition bias) Efficacy data | Unclear risk | Comment: MINI scores are not reported at follow‐up. In addition, study author does not show comparative tables of results at 3 and 6 months (only individual figures per allocated group; no summary statistics) |
| Protection against contamination | Unclear risk | Comment: we have incomplete information; we are not sure if GPs in this setting may be providing both intervention and control interventions |
| Selective reporting (reporting bias) | Unclear risk | Comment: no published clinical trial available; no selective reporting based on paper alone |
| Other bias | Low risk | Comment: no other sources of bias |
Fuhr 2019.
| Study characteristics | ||
| Methods |
Study design: RCT Duration of study: 24 October 2014 to 9 June 2017 |
|
| Participants |
Country: India Income classification: lower‐middle income Geographical scope: urban and rural areas of Goa Healthcare setting: women were recruited from PC clinics and antenatal clinics; home delivered intervention Mental health condition: perinatal mental disorder Population (mention whether patient, carer, or dyad) 1. Age: 18+ 2. Gender: women 3. Socioeconomic background: high literacy rates: males 94.7% and females 89% 4. Inclusion criteria (including threshold cutoff score of measurement tool) a. Women who were potentially eligible were invited to be screened for depression with a locally validated version of the Patient Health Questionnaire 9 (PHQ‐9) after providing written informed consent for screening (or witnessed informed consent or audio recordings by illiterate participants) b. Women who screened positive for depression (defined as PHQ‐9 score ≥ 10) were eligible for enrolment 5. Exclusion criteria a. Participants who did not speak Konkani, Hindi, or Marathi b. Those who needed immediate medical or psychiatric inpatient care |
|
| Interventions |
Stated purpose: to determine whether use of Sakhis, representing a wider range of non‐specialist providers, to deliver the Thinking Healthy Programme (THP) intervention would be feasible and effective on a greater scale than is possible with community health workers INTERVENTION (n = 122) Name: Thinking Healthy Programme (THP) Delivered by: LHWs Title/name of PW and number: Sakhis (non‐specialist workers ‐ lay women) ‐ 26 1. Selection: THPP peers were middle‐aged with children, had a similar sociodemographic background as participants, and were selected for their good communication skills; they were referred to as Sakhi, which translates to "friend" in Hindi. Additional criteria required to become Sakhis have previously been published. Sakhis were recruited from the local community through word of mouth, particularly through key informants in women’s self‐help groups and community health workers who were responsible for the well‐being and nutrition of mothers and their newborns 2. Educational background: not specified 3. Training (contents, duration, by whom): 26 Sakhis delivered trial interventions, and each received 25 to 40 hours of classroom‐based training that focused on intervention content and relationship‐building skills. Training included sessions on dealing with difficult situations, recognition of symptom worsening, and serious adverse events. Training was primarily interactive and comprised discussion and role‐plays. A clinical internship period of 2 months followed training, during which Sakhis delivered 2 to 4 sessions of THPP to at least 2 mothers. At the end of their training and internship period, Sakhis were assessed on their competence via standardised role‐plays. Only Sakhis who passed pre‐defined competence assessments were selected for THPP delivery 4. Supervision: during the trial, Sakhis continued to receive fortnightly group supervision sessions with 4 to 5 Sakhis per group, once a month with a supervisor present and once a month without a supervisor present. A peer group leader was chosen on a rotational basis at each session to lead the discussion; when the supervisor was present, the peer group leader and the supervisor co‐facilitated the session. An audio‐recorded THPP session delivered by a Sakhi was played at each session, and successes and difficulties were discussed. Audio recordings were rated on the Therapy Quality Scale, and feedback was exchanged within the group 5. Incentives/ remuneration: notably, it costs only a little over $1 per beneficiary mother to provide THPP (12% of which is attributed to the cost of incentives) Intervention details (according to PWs and whether aimed at carers and/or patients) 1. Duration/frequency: THPP was delivered over 6 to 14 individual sessions in 4 phases over 7 to 12 months, depending on the eligible trimester of recruitment, with each session lasting between 30 and 45 minutes 2. Content of intervention (by types of health workers and per patients/carers): participants in the intervention group received THPP in addition to EUC. THPP was developed during a 2‐year formative research phase. Two major adaptations, related to content and delivery mechanism, were made to the original THP intervention to make it deliverable by peers. First, we narrowed the focus from CBT (the theoretical basis of the original THP intervention) to behavioural activation because formative research indicated that CBT is more difficult for lay providers, such as peers, to learn CONTROL (n = 129) Treatment for control group (i.e. receiving EUC) was referred to as enhanced because, in India, perinatal depression is not treated. Participants in EUC only group received standard care from the gynaecologist and enhanced treatment CO‐INTERVENTIONS: none |
|
| Outcomes |
Patients 1. Symptom severity (PHQ‐9)* 2. Remission (PHQ‐9 < 5 at 6 months post partum)* 3. Remission at 3 months 4. Recovery (PHQ‐9 < 5 at 3 and 6 months postpartum) 5. WHODAS 6. Days unable to work in the past month 7. Maternal support required# 8. Exclusive breastfeeding# 9. Infant weight‐for‐age and height‐for‐age# 10. Minimal clinically important difference at 6 months after birth# (asking participants how much of their tension had changed since entry into the study) 11. Serious adverse events Carers None Process/health workers Number of sessions attended# Economic outcomes 1. Health system costs 2. Societal costs (asterisk for study's primary outcomes; star: outcomes that we have not reported in this review) Time points post intervention: baseline, post intervention (3 months postnatal), at 3 months (6 months postnatal) |
|
| Notes |
Source of funding: National Institute of Mental Health (USA) Notes on validation of instruments (screening and outcomes): PHQ‐9 was validated Additional information: declaration of interests ‐ study authors declared no competing interests Handling the data: as per footnotes in data and analysis Prospective trial registration number: trial is registered with ClinicalTrials.gov, number NCT02104232 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation list consisted of randomly sized blocks of 4 or 6 that were stratified by area of residence (urban or rural) Blocked randomisation was used to generate list by area; therefore considered low risk |
| Allocation concealment (selection bias) | Low risk | Residence (urban or rural) was generated by an independent statistician who had no subsequent involvement in the trial. Randomisation code was concealed from participants and researchers before allocation by use of sequentially numbered opaque sealed envelopes that were administered after consent was provided, to inform participants in the group; this allocation concealment scheme has been used successfully in previous trials in this setting. Research assistants opened the envelopes immediately after consent for enrolment and the baseline questionnaire had been completed; participants were assigned to the indicated group. Data manager did daily cross‐checks to confirm that allocations were consistent with the allocation code. Outcome assessors were independent. Allocation was conducted by an independent researcher and was concealed from participants and researchers using numbered opaque envelopes; therefore low risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Independent outcome assessors and the gynecologists providing care to participants in both groups were masked to the treatment allocation. Outcome assessors had no interaction with the study team"; therefore, low risk |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | "Independent outcome assessors and the gynecologists providing care to participants in both groups were masked to the treatment allocation. Outcome assessors had no interaction with the study team" |
| Baseline outcome measurements similar | Unclear risk | It is unclear whether baseline outcomes were similar between groups. However, study authors do state, "There was no evidence of a difference in baseline characteristics in women for whom we had 6‐month outcome data and for whom we did not" |
| Baseline characteristics similar? | Low risk | Baseline characteristics were broadly similar by treatment group (table 1) Baseline characteristics were similar and reported; therefore low risk |
| Incomplete outcome data (attrition bias) Efficacy data | Low risk | "We adjusted for pre‐specified baseline variables that are associated with the outcome or missing data"; missing outcome data were adjusted for in analyses; therefore considered low risk |
| Incomplete outcome data (attrition bias) Safety data (e.g. adverse events) | Low risk | There was no evidence of significant differences in serious adverse events between groups. Therefore we consider low risk |
| Protection against contamination | Low risk | We assumed a more conservative effect size to allow for the possibility of contamination between groups and a diluted effect due to delivery of the intervention by Sakhis Possible contamination between groups was adjusted for during analyses; therefore considered low risk |
| Selective reporting (reporting bias) | Low risk | All outcomes planned in clinical trial protocol NCT02104232 were reported in the results section |
| Other bias | Low risk | No other sources of bias were found |
Gavrilova 2009.
| Study characteristics | ||
| Methods |
Study design: randomised parallel‐group single‐blind controlled clinical trial Duration of study: 2000 to 2004 |
|
| Participants |
Country: Russia Income classification: middle Geographical scope: urban (Moscow ‐ South administrative district; patients registered at 3 general practices) Healthcare setting: group community training Mental health condition: dementia Population: patient‐carer dyad 1. Age: patients: > 65 years; carers' mean age: 61.5 years (SD 17.6) 2. Gender: both 3. Socioeconomic background: not specified 4. Inclusion criteria a. Patients > 65 years b. Met DSM‐IV criteria for dementia 5. Exclusion criteria a. Serious current physical illness b. No family carer c. > 1 person with dementia in same household |
|
| Interventions |
Stated purpose: to test the effectiveness of the 10/66 Dementia Research Group brief carer intervention among people with dementia and their carers INTERVENTION (n = 25) Name: 10/66 Brief Carer Intervention Delivered by: PHP Title/name of PW: newly qualified doctors (number not specified) 1. Selection: not specified 2. Educational background: medical degree 3. Training (contents, duration, by whom): 2‐day training, using the 10/66 intervention manual (includes vignettes, role‐plays, live interviews) 4. Supervision: not specified. 5. Incentives/remuneration: not specified Intervention details 1. Duration/frequency: 5 weekly 30‐minute sessions 2. Content of intervention: intervention for carers: content (manualised approach): 3 modules: assessment of cognitive and functional impairment, carers' knowledge and understanding, care arrangements (1 session), basic education about dementia illness, what to expect in future, local available resources (2 sessions), training regarding dealing with specific problem behaviours (2 sessions) CONTROL (n = 28) Usual medical care (on a wait‐list for the intervention) CO‐INTERVENTIONS: medical care for both intervention and control |
|
| Outcomes |
Patients 1. Behavioural and psychological symptoms of dementia (NPI‐Q) 2. DEMQOL Carers 1. ZBI 2. SRQ‐20 ‐ carer mental health 3. Caregiver QoL (WHOQOL‐BREF) Process/health workers Not assessed Economic outcomes Not reported Time points: baseline, 6 months No mention of study's primary or secondary outcomes |
|
| Notes |
Source of funding: WHO Notes on validation of instruments (screening and outcomes): validated Additional information (e.g. provided by study authors, existence of a published study protocol): none Patient outcomes presented in this treatment review. Carer outcomes summarised in this review but will also be included in prevention review, as for them the intervention is preventing mental distress/disorders. Declarations of interests ‐ study authors reported no competing interests Handling the data: as per footnotes in data and analysis Prospective trial registration number: ISRCTN41039907 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from report: "randomisation was carried out in London, with the codes transmitted immediately back to the Moscow centre by e‐mail. We used a stratified permuted block method to ensure as fare as possible an even distribution of baseline caregiver strain assessed using the Zarit Burden Interview" Comment: central randomisation apparently computer generated |
| Allocation concealment (selection bias) | Unclear risk | Comment: not reported Comment: even though sequence generation was centrally done, it is unclear how allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: the control group was a wait‐list, so differential interventions were unlikely |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | Comment: this was an open‐label trial; however, assessors were blind to treatment allocation |
| Baseline outcome measurements similar | Low risk | Comment: all similar |
| Baseline characteristics similar? | Low risk | Comment: all similar; there were baseline imbalances in the degree of care needed by patients in the control group. However, this was adjusted for in statistical analysis |
| Incomplete outcome data (attrition bias) Efficacy data | Low risk | Comment:attrition was low in both groups (only deaths), and this was adjusted for in statistical analysis |
| Protection against contamination | Low risk | Comment: wait‐list control, so unlikely to be contamination |
| Selective reporting (reporting bias) | Low risk | Comment: trial prospectively registered; all pre‐stated outcomes reported |
| Other bias | Low risk | Comment: none detected |
Gordon 2008.
| Study characteristics | ||
| Methods |
Study design: RCT Duration of study: September 2004 to May 2005 |
|
| Participants |
Country: Kosovo Income classification: lower‐middle income Geographical scope: rural; Suhareka region, a fertile agricultural area in the southern part if of Kosovo Healthcare setting: small group school setting ‐ high school Mental health condition: PTSD Population: patients (adolescents only) 1. Age: 14 to 18 years; mean age 16.3 years 2. Gender: both; significantly more girls than boys 3. Socioeconomic background: war‐traumatised area with students who had lost both or 1 parent and 90% of homes in that area were destroyed 4. Inclusion criteria a. Students having PTSD as defined according to a scoring algorithm of the HTQ previously described by the Harvard Refugee Trauma group and used in a Kosovar Albanian population (1) This definition of PTSD requires a score of 3 or 4 on a Likert scale of 1 to 4, on at least 1 of 4 re‐experiencing symptoms (Criterion B), on at least 3 of 7 avoidance and numbing symptoms (Criterion C), and on at least 2 of 5 arousal symptoms (Criterion D), in addition to exposure to a traumatic event (Criterion A) 5. Exclusion criteria a. No specific exclusion criteria b. Students with PTSD symptoms as defined above may participate in the study |
|
| Interventions |
Stated purpose: to determine whether participation in a mind‐body skills group programme based on psychological self‐care, mind‐body techniques, and self‐expression decreases symptoms of PTSD INTERVENTION (n = 38) Name: mind‐body school‐based skill group Delivered by: CP Title/name of PW and number: high school teachers ‐ 4 1. Selection: information from study author: "the teachers were self‐selected" 2. Educational background: information from study author: "all graduated from the university but did not have advanced degrees. They would have whatever certification is required to teach high school in Kosovo" 3. Training: 2‐part, 10‐day intensive training undertaken in 1999 to 2000; Washington DC‐based faculty of the Centre for Mind‐Body Medicine (CMBM). Info from study author: "when we went to Kosovo after the war to train health professionals, the teachers from this village came to our training and brought the mind‐body techniques back to their school in the rural village and began using them with their students. We did one pilot study before we did the RCT" 4. Supervision: CMBM's Kosovo faculty of psychiatrist and psychologist 5. Incentives/remuneration: information from study author: "they were paid a small stipend" Intervention details 1. Duration/frequency: 12 sessions for 2 hours twice a week for 6 weeks 2. Content of intervention: self‐expression and personal sharing with instruction in and use of meditative and imaginative mind‐body techniques; given in small group sessions (about 10 students per group). Format is now manualised. The aim is not to discuss traumatic events but to create a supportive environment in which self‐awareness, sharing, and listening are encouraged, to teach them self‐care techniques, and to give them skills to deal with traumatic events in their daily life and to understand the trauma they suffered CONTROL (n = 40) Wait‐list control group, who received the 12‐session mind‐body skill training after the first intervention group finished their 12 sessions CO‐INTERVENTIONS: none |
|
| Outcomes |
Patients HTQ Carers NA Process/health workers None Economic outcomes None reported Time points: baseline (pre‐intervention), immediately post intervention (i.e. after 6 weeks), 3‐month follow‐up after the intervention |
|
| Notes |
Source of funding: Oswald Family Foundation, Minnesota, USA; Oak Foundation, Geneva, Switzerland; deLaski Family Foundation, Virginia, USA; Ms Lyn Rales, Potomac, Maryland, USA; Ms Judith Loeb Chiara, New York, New York, USA; Helen Clay Frick Foundation, New York, USA Notes on validation of instruments (screening and outcomes): used previously in Kosovo, as described in Cardozo 2000 Additional information:clinicaltrials.gov/ct2/show/NCT00136357?term=NCT00136357. Declaration of interests ‐ study authors report no competing interests Handling the data (e.g. imputed values, other calculations we have made): none Prospective trial registration number: NCT00136357 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "students were stratified according to gender and randomly assigned by the research director using random numbers generated by Microsoft Excel 2003" |
| Allocation concealment (selection bias) | High risk | Quote: "the list of assigned groups was given to the teachers, who then notified the students of their group assignment" Comment: no allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: not blinded but unlikely to affect outcomes |
| Blinding of outcome assessment (detection bias) all outcomes | Unclear risk | Quote: "while it is possible that students wanted to please the teachers by reporting a decrease in symptomatology after the groups, the teachers' experience, and that of the observers was that greater familiarity with the teachers, on the contrary, facilitated more frank discussions and sharing of problems and symptoms after as well as before and during the intervention" Comment: teachers both performed intervention and delivered the instruments but given explanation above, may be classified as unclear risk; no objective outcomes |
| Baseline outcome measurements similar | Low risk | Comment: all similar |
| Baseline characteristics similar? | Low risk | Information from study author: age, sex, and baseline PTSD were all similar |
| Incomplete outcome data (attrition bias) Efficacy data | Low risk | Comment: low dropout rate |
| Incomplete outcome data (attrition bias) Safety data (e.g. adverse events) | Low risk | Information from study author: "we did not look at any adverse outcomes. The teachers received ongoing supervision. The supervisors and teachers would have notified us if any adverse events occurred as required by the IRB, but there were none. We did not formally record this" Comment: low risk |
| Protection against contamination | Unclear risk | Comment: just 1 school in the study, so may have been contamination. However, control group received intervention as soon as intervention group had finished. Some of the results (e.g. arousal) suggest improvement in control group before intervention was received, which may suggest contamination |
| Selective reporting (reporting bias) | Low risk | Comment: only 1 outcome on the protocol ‐ HTQ |
| Other bias | Low risk | Comment: none detected |
Gureje 2019 (EXPONATE).
| Study characteristics | ||
| Methods |
Study design: CRCT. Unit of allocation: maternal care clinics Duration of study: 18 June 2013 and 11 December 2015 |
|
| Participants |
Country: Nigeria Income classification: lower‐middle income Geographical scope: study was conducted in Oyo State in southwestern Nigeria. Nine local government areas (4 urban and 4 rural) were randomly selected for the study Healthcare setting: PC facility: maternal care clinics Mental health condition: perinatal MD Population (mention whether patient, carer, or dyad) 1. Age: 16 to 45 years 2. Gender: female 3. Socioeconomic background: mean education 10.6 years (3.1 SD) 4. Inclusion criteria a. Spoke Yoruba b. Scored ≥ 12 on the Edinburgh Postnatal Depression Scale (EPDS) c. Confirmed presence of major depression according to DSM‐IV (1994) criteria d. Signed informed consent e. Were going to be available in the study area up to 12 months after childbirth 5. Exclusion criteria a. Immediate need for medical attention b. Actively suicidal c. Presence of bipolar or psychotic disorder d. Unlikely to be in the neighbourhood in the following 12 months |
|
| Interventions |
Stated purpose: to determine whether an intervention package consisting of primary care worker‐administered problem‐solving treatment delivered within a stepped‐care approach would be more effective than enhanced care as usual for alleviating perinatal depression 6 months after childbirth INTERVENTION: high‐intensity treatment (HIT) (n = 379) Delivered by: PHP and LHW Title/name of PW and number: PHWs: primary maternal care providers (non‐physician primary care providers and midwives who have been trained as nurses, community health officers, and community health extension workers) 1. Selection: clinics offered full maternal and child health services and provided explicit consent to participate. One clinic was excluded because the staffing profile was too thin to permit effective participation 2. Educational background: minimum of 2 to 3 years post secondary education and certified by respective boards 3. Training: initial 3‐day training and 2‐day top‐up training (1 month later) 4. Supervision: ongoing structured support and supervision from primary care physicians who, in turn, could consult with a psychiatrist when needed 5. Incentives/ remuneration: not specified Intervention details (according to PHW/CWs and whether aimed at carers and/or patients) 1. Duration/frequency: step 1 comprised 8 sessions of psychological interventions, delivered weekly in the antenatal period. Step 2 commenced 6 weeks after delivery, during the mother’s routine postnatal visit. Depending on participants’ EPDS scores (< 12 or ≥ 12), providers delivered 4 fortnightly top‐up sessions of the PST or 8 weekly intervention sessions. At completion of step 2, participants who still had EPDS scores ≥ 12 proceeded to step 3, in which they were re‐assessed by the community physician with a view to initiate pharmacotherapy, in addition to continuing with the psychological intervention or referral to a specialist service. Each session of the psychological intervention lasted approximately 30 to 45 minutes 2. Content of intervention (by types of health workers and per patients/carers): in addition to enhanced usual care (see below), providers offered stepped‐care treatment, using a manualised psychological intervention package, the core component of which was a locally adapted form of problem‐solving treatment (PST) for primary care. With this intervention, the patient is guided through a step‐by‐step process of breaking down current psychosocial stressors and then exploring and trying out options for their resolution, which includes using personal resources and available social support. Mothers in the HIT arm of the study also received parenting skills training CONTROL: enhanced care as usual (low‐intensity treatment) (n = 197) PMCP in the low‐intensity treatment (LIT) arm received a 1.5‐day training on use of the mhGAP – Intervention Guide (mhGAP‐IG) ‐ and were given copies of the mhGAP‐IG, as well as a manual describing the nature and standard treatment approaches for perinatal depression. Providers delivered the intervention to participants by using the basic specifications of mhGAP‐IG ‐ psychoeducation, addressing current psychosocial stressors and reactivation of social network. No structured sessions were stipulated, and no stepped‐care procedure was specified; number/frequency of visits and content of psychosocial interventions were determined at the discretion of the PMCP CO‐INTERVENTIONS: none |
|
| Outcomes |
Patients 1. EDPS 2. Remission from depression at 6 months post partum, defined as EPDS score < 6* 3. WHODAS 2.0 Carers None Process/health workers None Economic outcomes None (asterisk for study's primary outcomes; star: outcomes that we have not reported in this review) Time points post intervention: baseline, 0 to 2 months (6 months postpartum), 6 to 8.5 months (12 months postpartum) |
|
| Notes |
Source of funding: Grand Challenges Canada (0082‐04) Notes on validation of instruments (screening and outcomes): all instruments were validated Additional information: Gureje O, Oladeji BD, Araya R, Montgomery AA, Kola L, Kirmayer L, Zelkowitz P, Groleau D. Expanding care for perinatal women with depression (EXPONATE): study protocol for a randomized controlled trial of an intervention package for perinatal depression in primary care. BMC Psychiatry 2015;15:136. Declaration of interests ‐ none Handling the data: as per footnotes in data and analysis Prospective trial registration number: trial is registered with the International Standard Randomised Controlled Trials Number Registry (http://www.isrctn.com/isrctn) under trial number ISRCTN60041127 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation was conducted by one of the authors (A.A.M.), using anonymous codes for clinics and local government areas provided by other members of the research team Randomisation was conducted using codes; therefore sufficient for random sequence generation and considered low risk |
| Allocation concealment (selection bias) | Low risk | Considering that allocation concealment and selection bias could be a problem in cluster‐randomised trials, where all participants are not consented and recruited prior to allocation of clusters, consecutive attendees at selected MCCs were invited to participate by trained research staff while waiting to see the midwife. They were briefly educated about perinatal depression and were invited to take the screening interview. Those who agreed were screened with the Edinburgh Postnatal Depression Scale (EPDS) Allocation was conducted by one of the authors (A.A.M.), using anonymous codes for clinics and local government areas provided by other members of the research team Allocation was concealed and was adjusted for clustering |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and interventionists were not blinded; however, this is unlikely to have influenced outcomes |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | "All outcome assessments were conducted ... by experience research interviewers... were not involved in participant's recruitment and who were blind to the participant's treatment". |
| Baseline outcome measurements similar | Unclear risk | No baseline measure for EPDS; it is unclear whether these were different at the start; baseline measures for WHODAS are similar |
| Baseline characteristics similar? | Unclear risk | Variation in cluster size was not ignorable when design effect was estimated Imbalance in the ratio of women recruited at around 1.9 in favour of the HIT arm Significant differences in baseline characteristics (different cluster size and numbers recruited between HIT and LIT arms; these were not adjusted for; however baseline characteristics were similar. unclear effect) |
| Incomplete outcome data (attrition bias) Efficacy data | Low risk | In view of the high follow‐up rate, the main approach to analysis was modified intention‐to‐treat at the individual level, that is, analysis according to randomised group regardless of adherence to allocation and without imputation of missing outcome data High follow‐up rate Any missing outcome data were adjusted for in analyses |
| Incomplete outcome data (attrition bias) Safety data (e.g. adverse events) | Unclear risk | Only 3 of 6 deaths explained; not sure about the 3 unaccounted for |
| Protection against contamination | Unclear risk | Although interventions were conducted in different clinics by own primary care provider, it is unclear whether there was contamination between groups |
| Selective reporting (reporting bias) | Low risk | All outcomes planned in the protocol were reported in the results section |
| Other bias | Low risk | None were detected |
Gureje 2019 (STEPCARE).
| Study characteristics | ||
| Methods |
Study design: cluster‐RCT. Unit of allocation: primary care clinics Duration of study: November 2013 to October 2016 |
|
| Participants |
Country: Nigeria Income classification: lower‐middle income Geographical scope: city of Ibadan, a large metropolis in the southwest of Nigeria Healthcare setting: PC facility Mental health condition: depression Population: Nigerian primary care patients 1. Age: ≥ 18 years 2. Gender: both 3. Socioeconomic background: not mentioned 4. Inclusion criteria a. Adults ≥ 18 years b. Patient Health Questionnaire (PHQ‐9) ≥ 11 c. MDD diagnosed on Composite International Diagnostic Interview (CIDI) d. Provides informed consent 5. Exclusion criteria a. Immediate need for medical attention b. Pregnant c. Actively suicidal d. Presence of bipolar or psychotic disorder or severe substance dependence e. Unlikely to be in the neighbourhood in the following 12 months |
|
| Interventions |
Stated purpose: to evaluate the effectiveness and cost‐effectiveness, over a 12‐month period, of structured problem‐solving therapy delivered within a stepped‐care approach by non‐physician, lay health workers for moderate to severe depression INTERVENTION (n = 631) Name: MhGAP‐IG Stepped Care Delivered by: PHP and LHW Title/name of PW and number: primary health care workers ‐ 36 1. Selection: at each participating clinic, 2 front‐line primary care providers, of any cadre (i.e. nurse, community health officer, or community health extension worker), were selected and trained to provide treatment appropriate to the study group 2. Educational background: no specific info; each has 2 to 3 years of post‐secondary school professional training (i.e. average of 14 years’ education) 3. Training: primary healthcare workers in the intervention group were given the 2‐day top‐up MHgap training + were trained to deliver a structured psychological intervention consisting of behavioural activation (activity scheduling) and problem‐solving therapy, previously culturally adapted and pilot tested by study authors. These providers received 6 days of training on problem‐solving therapy and on use of the mhGAP‐IG to identify and treat depression, which included didactic lectures, clinical demonstrations, role‐plays on delivery of manualised intervention, procedures for support and supervision by general practitioner through mobile phones, and how to monitor patients on antidepressant medication 4. Supervision: supervision and support to all primary healthcare centres in each local government area (typically 8 to 10 centres) are provided by a general practitioner, acting as primary healthcare co‐ordinator, who runs outpatient clinics, provides clinical supervision on a scheduled regular basis across clinics, responds to clinical emergency calls, and has administrative management duties 5. Incentives/remuneration: employed at clinic Intervention details 1. Duration/frequency: eight 30‐ to 40‐minute sessions of individual problem‐solving therapy delivered by primary healthcare workers, with an extra 2 to 4 sessions if needed 2. Content of intervention: structured problem‐solving therapy delivered within a stepped‐care approach combined with usual care (enhanced with use of the WHO mhGAP‐IG) in which specifications for treatment of depression consist of simple psychosocial approaches, including psychoeducation and counselling to address stressors and activate social networks, and pharmacotherapy when necessary CONTROL: enhanced usual care (n = 547) Participants in control group received enhanced usual care alone (2 providers received day's training). Choice of intervention (unstructured psychological treatment or medications as stipulated in the mhGAP‐IG) was made at the discretion of the primary healthcare worker, and no specification as to the number of sessions was provided CO‐INTERVENTIONS: nil |
|
| Outcomes |
Patients 1. PHQ‐9 2. WHODAS 3. WHOQOL Carers Nil Process/health workers Nil Economic outcomes Cost effectiveness based on a service utilisation questionnaire Time points: baseline; 3, 6, and 12 months (post baseline). Intervention is 2 to 3 months long. so equivalent to 0 to 1 month, 3 to 4 months, and 9 to 10 months |
|
| Notes |
Source of funding: UK Medical Research Council. Notes on validation of instruments (screening and outcomes): all validated Additional information: Gureje O, Oladeji BD, Araya R, Montgomery AA. A cluster randomized clinical trial of a stepped care intervention for depression in primary care (STEPCARE) ‐ study protocol. BMC Psychiatry 2015;15:148. Declaration of interests ‐ study authors declared no competing interests Handling the data: as per footnotes in data and analysis Prospective trial registration number: ISRCTN46754188 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: "anonymised codes for each clinic were provided by the research team in Ibadan to the study statistician (AAM), who generated the allocation sequence and carried out the random allocation. For each local government area, a single balanced block equal to stratum size was generated with use of computer‐generated random numbers to ensure balanced allocation to treatment groups" Allocation of groups clearly described |
| Allocation concealment (selection bias) | Low risk | Judgement comment: unclear whether study statistician was independent of study team. However, "datasets did not contain the allocation status of the participants which was kept as a separate file and was available only to the trial statistician" Therefore, considered low risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and interventionists were not blinded, but this unlikely influenced the outcome |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | "Outcome assessors were blinded to the patients’ group allocations, were not involved with screening or recruitment of trial participants in the clinic, and were randomly assigned to participants from any clinic in either group of the study" |
| Baseline outcome measurements similar | Low risk | From Table 3, baseline outcomes similar for depression and viral suppression |
| Baseline characteristics similar? | Unclear risk | Many more were unemployed in the intervention arm (57%) than in the usual care arm (28%). This may be significant. Unclear risk |
| Incomplete outcome data (attrition bias) Efficacy data | Low risk | Low dropout (Fig 2: 13/14 followed up in intervention group, 15/18 in control group) |
| Protection against contamination | Low risk | Judgement comment: as intervention and control clinics were separate, there is unlikely to be contamination between groups |
| Selective reporting (reporting bias) | Low risk | Same outcomes as protocol |
| Other bias | Low risk | None detected |
HuisIntVeld 2019.
| Study characteristics | ||
| Methods |
Study design: randomised controlled clinical trial Duration of study: January 2012 to December 2013. Recruitment between January and June 2012 |
|
| Participants |
Country: South Africa Income classification: upper‐middle income Geographical scope: urban (townships surrounding Pretoria) Healthcare setting: 3 HIV clinics based within primary healthcare clinics Mental health condition: alcohol use disorder Population 1. Age: 18 to 65 2. Gender: both 3. Socioeconomic background: not specified 4. Inclusion criteria (including threshold cutoff score of measurement tool) a. Adults b. HIV‐1 infection c. Visited clinic for HIV care d. AUDIT scores meeting criteria for hazardous or harmful alcohol use (8 to 19 men, 7 to 19 women) 5. Exclusion criteria a. Mental impairment b. Unable to provide informed consent c. Receiving treatment for alcohol disorder d. Pregnant |
|
| Interventions |
Stated purpose: to reduce alcohol use from harmful/hazardous level to abstinence or low‐risk level INTERVENTION (n = 267) Name: brief intervention Delivered by: PHP Title/name of PW and number: research assistants ‐ 4 1. Selection: not specified 2. Educational background: nurses 3. Training (contents, duration, by whom): role‐play, general skills, training on alcohol‐, HIV‐ and sexual‐related issues. Details on duration and trainers not specified 4. Supervision: twice weekly by investigators Intervention details (according to PHW/CWs and whether aimed at carers and/or patients) 1. Duration/frequency: single session (median time 40 minutes) 2. Content of intervention (by types of health workers and per patients/carers): research assistant gave personalised feedback on AUDIT results, including specific harms and emphasising seriousness of the condition. A health education leaflet on responsible drinking was given and discussed. Brief counselling based on Information‐Motivation‐Behavioural Skills model on reducing excessive alcohol use was then given CONTROL (n = 293) Enhanced usual care. After AUDIT assessment, participants were given a health education leaflet on responsible drinking CO‐INTERVENTIONS: usual HIV care |
|
| Outcomes |
Patients 1. Reduction of alcohol use from harmful/hazardous level to abstinence or low‐risk level* 2. Reduction in absolute AUDIT score 3. Percentage decrease in AUDIT score 4. Depression score (CESD‐10) 5. WHOQoL‐HIVBREF (health‐related quality of life) score 6. Mean last measured CD4 count# 7. Last viral load# 8. Therapy adherence to antiviral therapy# 9. Internalised AIDS‐related stigma scale score# 10. Tobacco use# Carers None Process/health workers None Economic outcomes (and where these can be found, e.g. ref or table number) None (asterisk for study's primary outcomes; star: outcomes that we have not reported in this review) Time points: 5 months, 12 months |
|
| Notes |
Source of funding: BMRF, Foundation for Alcohol Research (https://www.abmrf.org/), Directorate General for Development Cooperation through the Flemish Interuniversity Council (VLIR‐UOS) (https://www.vliruos.be/en/home/1) Notes on validation of instruments (screening and outcomes): validated Additional information (e.g. provided by study authors, existence of a published study protocol): protocol published in 2012: Huis In 't Veld D et al. The efficacy of a brief intervention to reduce alcohol misuse in patients with HIV in South Africa: study protocol for a randomized controlled trial.” Trials 2012;13:190; doi:10.1186/1745‐6215‐13‐190. Declaration of interests ‐ study authors declared no competing interests Handling the data (e.g. imputed values/other calculations we have made): none Prospective trial registration number: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: both reviewers rated LOW |
| Allocation concealment (selection bias) | Low risk | Judgement comment: Ujala ‐ HIGH; unit of allocation was by individual. Allocation was not blinded Yen ‐ LOW; randomisation was done by trial co‐ordinator using concealed, centrally allocated computer‐generated random numbers and made available for research assistants in envelopes Email sent to discuss |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding for nurses who delivered the intervention, who also conducted baseline and follow‐up assessments |
| Blinding of outcome assessment (detection bias) all outcomes | High risk | No blinding for nurses who delivered the intervention nor for the statistician. Nurses conducted baseline and follow‐up assessments and carried out intervention; therefore high risk of bias |
| Baseline outcome measurements similar | Unclear risk | Difference in AUDIT scores between intervention and control groups at baseline, with higher mean score in the intervention compared to the control arm. However, because randomisation was done, any observed differences at baseline are attributed purely to chance, and no conclusion from this can be derived. Unclear whether differences were adjusted for during analysis |
| Baseline characteristics similar? | Low risk | Baseline characteristics have been reported in table 1 |
| Incomplete outcome data (attrition bias) Efficacy data | Unclear risk | It is unclear whether missing data were adjusted for during analysis |
| Incomplete outcome data (attrition bias) Safety data (e.g. adverse events) | Low risk | No adverse events were reported |
| Protection against contamination | Unclear risk | Judgement comment: Ujala rated UNCLEAR and commented, "Control group received leaflet; therefore unlikely to have come in contact with intervention participants or nurses. However, this is not clear" Yen rated LOW and commented, "Although they visited the same clinic, the intervention group received face to face intervention whereas the control group received only a leaflet; therefore contamination between groups is unlikely" Final decision by Yen ‐ UNCLEAR. In view of participants in both groups treated in the same location and could have shared information amongst themselves |
| Selective reporting (reporting bias) | Low risk | All outcomes described in trial registry ‐ PACTR201202000355384 ‐ have been reported |
| Other bias | Low risk | None detected |
Humeniuk 2012.
| Study characteristics | ||
| Methods |
Study design: RCT Duration of study: recruitment and follow‐up period varied from country to country and generally occurred between September 2003 and December 2006 |
|
| Participants |
Country: Brazil and India (Australia and USA were also sites for this study) Income classification: Brazil lower‐middle income from 2003 to 2005 and upper‐middle income in 2006; India low income between 2003 and 2006. Australia and USA high‐income countries ‐ results from these 2 sites are excluded from our review Geographical scope: urban Healthcare setting: Brazil ‐ 30 primary healthcare (PHC) units, 2 health centres specialising in sexually transmitted diseases, and 1 outpatient setting linked to a general hospital; India ‐ community health centres Mental health condition: substance (drug) abuse Population 1. Age: 16 to 62 2. Gender: both 3. Socioeconomic background: Brazil: 60% employed; India: 94% employed. Both: majority lived in their own homes or in rented accommodations 4. Inclusion criteria a. Age 16 to 62 years b. Able to participate in 3‐month follow‐up c. Able to give contact details for at least 2 to 3 other people d. Having a fixed address e. Scoring moderate risk (4 to 26) for cannabis, cocaine, amphetamine‐type stimulants (ATS) or opioids on the screening ASSIST test 5. Exclusion criteria a. Pending incarceration within the next 3 months b. Cognitive impairment or severe behaviour problems c. Intoxicated or going through withdrawal from alcohol or other drugs d. Currently in drug or alcohol treatment (apart from treatment for nicotine dependence) |
|
| Interventions |
Stated purpose: to evaluate the effectiveness of a brief intervention (BI) for illicit drugs (cannabis, cocaine, amphetamine‐type stimulants (ATS) and opioids) in PHC patients INTERVENTION (Brazil, n = 94) (India, n = 89) Name: ASSIST‐linked Brief Intervention Delivered by: LHW (India); PHP (Brazil) Title/name of PW and number: India ‐ clinical research interviewers ‐ number not specified; Brazil ‐ clinicians and researchers ‐ number not specified 1. Selection: not specified 2. Educational background: Brazil ‐ medical degree (clinicians), not specified (researchers); India ‐ some level of tertiary education in health 3. Training: India ‐ trained by study co‐ordinator at each site to administer the ASSIST and brief intervention; Brazil ‐ trained by local study co‐ordinators to administer the test battery 4. Supervision: by mental health‐trained persons. Person overseeing the trainers was a psychologist with 16 years of addiction experience Intervention details: 1 ASSIST‐linked Brief Intervention 1. Duration/frequency: 1 session. Brazil ‐ 8 to 60 minutes, mean 23.3 minutes, median 20 minutes; India ‐ 7 to 15 minutes, mean 10.9 minutes, median 11 minutes 2. Content of intervention: brief intervention for the drug receiving the highest moderate‐risk specific substance involvement score on ASSIST or the substance that was of greatest concern to the participant if there was more than 1 substance for which he or she was at moderate risk. The aim was to move participants through the stages of change using the technique of FRAMES (Feedback, Responsibility, Advice, Menu, Empathy, Self‐Efficacy) and motivational interviewing. Specific content was culturally adapted in each country according to these principles CONTROL (Brazil, n = 71) (India, n = 88) Usual primary care CO‐INTERVENTIONS: all participants were administered the ASSIST questionnaire. Time taken at baseline and follow up: Brazil ‐ 3 to 25 minutes (mean 7.2 ± 3.7 minutes), India ‐ 4 to 12 minutes (mean 6.6 ± 1.9 minutes) at baseline and 1 to 40 minutes (mean 11.3 ± 6.9 minutes) at follow‐up for all 4 countries |
|
| Outcomes |
Patients 1. Total illicit substance involvement scores 2. Cannabis‐specific substance involvement scores 3. Stimulant‐specific substance involvement scores 4. Opioid‐specific substance involvement scores Carer Nil Process/health workers Nil Economic outcomes Nil (asterisk for study's primary outcomes; star: outcomes that we have not reported in this review) Time point: 3 months |
|
| Notes |
Source of funding: WHO, the Australian Commonwealth Department of Health and Ageing, grants and in‐kind contributions from individual sites. Funding for participation of sites in USA was provided by the US National Institute on Drug Abuse Notes on validation of instruments (screening and outcomes): validated Additional information: socioeconomic background of participants; duration of ASSIST questionnaires and BI sessions: Humeniuk R, Dennington V, Ali R. The effectiveness of a brief intervention for illicit drugs linked to the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) in primary health care settings: a technical report of phase III findings of the WHO ASSIST randomized controlled trial. Geneva: World Health Organization, 2008. www.who.int/substance_abuse/activities/assist_technicalreport_ phase3_final.pdf. Information regarding supervision of interventionists: personal communication. Declaration of interests ‐ none Handling the data: nil Prospective trial registration number: nil |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation lists for each drug category and country were prepared by the co‐ordinating centre in Australia using a web‐based randomisation programme Computer programme used for randomisation; therefore low risk |
| Allocation concealment (selection bias) | Unclear risk | Randomisation was stratified by gender, substance, and level of use (high/low). Participants who were within the moderate‐risk range on ASSIST for cannabis, cocaine, ATS, or opioids were classified as "high‐use" if they scored between 16 and 26 or "low‐use" if they scored between 4 and 15. Randomisation lists for each drug category and country were prepared by the co‐ordinating centre in Australia using a web‐based randomisation programme Unit of allocation was clearly defined. Randomisation was performed by co‐ordinating centre using computer programme; however, unclear how allocation sequence was concealed from researchers |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Clinical research staff were not blind to the intervention allocation, as they were responsible for administering the intervention at baseline.
In the majority of cases, the same clinical researcher performed both the baseline and follow‐up interviews" It is unclear how lack of blinding of participants and interventionists may have influenced study results |
| Blinding of outcome assessment (detection bias) all outcomes | High risk | "Clinical research staff were not blind to the intervention allocation, as they were responsible for administering the intervention at baseline.
In the majority of cases, the same clinical researcher performed both the baseline and follow‐up interviews" Interventionists performed outcome assessments ‐ high likelihood of bias. ASSIST questionnaire is of a self‐report nature ‐ under‐reporting may have occurred |
| Baseline outcome measurements similar | Low risk | Similar baseline ASSIST |
| Baseline characteristics similar? | Unclear risk | Characteristics of intervention and control described together. No table of separate control and intervention group characteristics given |
| Incomplete outcome data (attrition bias) Efficacy data | Low risk | Similar numbers lost to follow‐up for both intervention and control. All participants in control and intervention groups were included in analyses |
| Protection against contamination | Unclear risk | "Control participants were invited to contact the clinical interviewer if they had concerns about their substance abuse during this time" Control participants may have had some level of communication with intervention provider; therefore unclear |
| Selective reporting (reporting bias) | Low risk | This is a WHO study on the use of ASSIST and ASSIST‐linked BI for people using illicit drugs. ASSIST scores were the only outcomes planned for in the methods section and reported in the results section. Although no published protocol is available, no evidence of selective reporting can be found in this paper |
| Other bias | Low risk | No other sources of bias were found |
Indu 2018.
| Study characteristics | ||
| Methods |
Study design: RCT Duration of study: recruitment began in 2012 and ended in 2014 |
|
| Participants |
Country: India Income classification: lower‐middle income between 2012 and 2015 Geographical scope: trial was conducted in Medical College Health Unit (MCH Unit), Pangappara. It is a primary health centre under the administrative control of Department of Community Medicine, Government Medical College, Trivandrum. This unit provides primary care to around 120,000 people who live in the area of 11 family welfare sub‐centres, which are functionally attached to it. Trivandrum is the urban capital of the southern Indian state of Kerala Healthcare setting: primary health centre Mental health condition: depression Population 1. Age: adults 18 to 60 years of age 2. Gender: women 3. Socioeconomic background: middle and lower socioeconomic strata 4. Inclusion criteria a. Age group 18 to 60 years b. Permanent residents living in the area of MCH unit Pangappara for at least past 6 months c. Diagnosis of moderate to severe depression d. Informed consent to participate in the trial, to record examination findings, and to use the data in research and future publications e. Literacy to read and write in Malayalam f. Having an active phone number 5. Exclusion criteria: a. Past or current diagnosis or history of treatment for bipolar disorder, dementia, psychosis b. Currently managed by a psychiatrist or psychologist c. Taking any psychotropic drug d. High risk of suicidality as measured by a suicidality score > 17 e. Pregnant, breast‐feeding, or planning to become pregnant in next 6 months f. Seriously ill or bedridden patient g. Not able to communicate with the health worker |
|
| Interventions |
Stated purpose: to evaluate the effectiveness of community‐based depression intervention programme (ComDIP) compared to treatment as usual (TAU) in managing women with moderate to severe depression in primary care INTERVENTION (n = 22) Name: community‐based depression intervention programme (ComDIP) Delivered by: PHP Title/name of PW and number: medical officers (primary care physicians) and junior public health nurses (JPHNs) 1. Selection: recruited from the primary health centre 2. Educational background: nurse/doctor 3. Training: 2 initial trainings and 1 booster session were conducted based on a module in the local language Malayalam. Teaching/learning media and methods included in the psychiatrist‐led training included PowerPoint presentations, videos, and case demonstration with hands on 4. Supervision: medical officers supervised the JPHN 5. Incentives/remuneration: not mentioned Intervention details 1. Duration/frequency: implemented by health workers in 6 sessions spread over 8 weeks. Each session will last for 30 minutes and will be delivered during house visit of the health worker 2. Content of intervention: sertraline 50 mg once daily as night dose prescribed by Medical Officer at primary health centre; home‐based psychosocial (CBT) intervention provided by JPHN. Core contents included psychoeducation of patients and caregivers about nature and symptoms of depression, validation and monitoring of symptoms, and behavioural activation by facilitating getting back to activities performed earlier. Relation between negative thoughts and behaviours was discussed during the visit. JPHNs during their interaction emphasised and monitored compliance to medication and encouraged reporting to Medical Officer for follow‐up visits CONTROL: treatment as Usual (TAU) (n = 16) Patients will be referred to a psychiatrist at Medical College hospital, mental health centre, or general hospital, or in the private sector |
|
| Outcomes |
Patients Primary outcome measure Reduction in severity of depression at 8 weeks measured by HAM‐D Secondary outcome measures 1. Montgomery‐Asberg Depression Rating Scale (MADRS) 2. Health‐related quality of life by Short Form 8 (SF‐8) at 8 weeks Carers None Process/health workers None Economic outcomes None Time points: baseline, 8 weeks |
|
| Notes |
Source of funding: unfunded Notes on validation of instruments (screening and outcomes): validated Additional information: declaration of interests ‐ study authors reported no conflicts of interest Handling the data: as per footnotes in data and analysis Prospective trial registration number: CTRI/2011/08/001978 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: random allocation sequence with variable block size was used |
| Allocation concealment (selection bias) | Low risk | Quote: "they were kept in the safe custody of the staff nurse, in sequentially numbered sealed opaque envelopes. On completion of baseline assessment, staff nurse was contacted for allo‐ cation. She opened the numbered envelope and allotted the patient to the concerned arm. Those who were responsible for establishing randomisation procedures were not involved in the day to day conduct of the trial" Judgement comment: random allocation sequence was kept by a staff nurse in sequentially numbered sealed opaque envelopes, which she opened and allotted to patients after baseline assessment was completed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not blinded; however unlikely to have influenced outcomes |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | Outcomes were assessed by a psychiatrist who was blinded to participant allocation |
| Baseline outcome measurements similar | Low risk | Patients and healthcare workers were aware of their allocation, but the assessor who rated outcomes was blinded to their allocation |
| Baseline characteristics similar? | Low risk | Baseline characteristics of 2 groups were similar (age, type of family, marital status, occupation, education, serious physical illness, high blood pressure, severity of depression, past history of suicide attempt) except for presence of diabetes mellitus (P = 0.04) |
| Incomplete outcome data (attrition bias) Efficacy data | High risk | High rate of attrition, especially in control arm, can influence results. A higher proportion of control arm participants who did not drop out of study may not be getting psychiatric care compared to control participants who dropped out of study; this can show the effect of the intervention as falsely high |
| Incomplete outcome data (attrition bias) Safety data (e.g. adverse events) | High risk | High dropout rate in both arms may have led to loss of important data regarding adverse events |
| Protection against contamination | Unclear risk | Judgement comment: Hakan ‐ HIGH: individual randomisation at 1 site Yen ‐ LOW: patients in intervention arm received sertraline and psychotherapy at 1 primary health centre; patients in control arm were referred to locally available mental health services. Contamination is unlikely, as patients in control arm who visited the primary health centre for other reasons were unlikely to receive sertraline and psychotherapy at the primary health centre |
| Selective reporting (reporting bias) | High risk | Remission assessment by Mini Neuropsychiatric Interview for Depression, improvement in suicidality score, Clinical Global Impression of Severity score, Clinical Global Impression of Change or Improvement score, modified global assessment of functioning score, treatment adherence, and acceptability was planned in the published clinical trial protocol but was not reported in the results section |
| Other bias | Low risk | None detected |
Jenkins 2013.
| Study characteristics | ||
| Methods |
Study design: cluster‐RCT, allocated by clinic, analysed at individual level for patient outcomes, analysed at clinic level for GHQ cases. 49 clinics in intervention group, 50 clinics in control group Duration of study: conducted in 2010 |
|
| Participants |
Country: Kenya Income classification: low income Geographical scope: urban and rural; Nyanza Province, Kenya, as this was the region where the national training programme 2005/2010 had hitherto trained fewest staff, and thus most clinics were eligible for th study. Districts of Siaya, Bondo, and Rachuonya were selected, allocated around Kisumu, near Lake Victoria Healthcare setting: PC facilities (dispensaries and PHC centres) Mental health condition: all mental disorders Population: patients attending level 2 or 3 public health facilities in Nyanza Province 1. Age: > 18 years 2. Gender: both 3. Socioeconomic background: livelihoods were based on subsistence farming, an extensive fishing industry along the lake, and some commercial business. The majority tribe is Luo. The area was the site of significant election violence in January 2007 4. Inclusion criteria a. Level 2 and 3 publicly funded primary care facilities in the Ministry of Health list of PHCs in Siaya, Bondo, and Rachuonya districts in Nyanza Province b. Two staff from each clinic were invited to attend the mental health training course c. All attenders from each clinic were recruited for the primary outcome d. Criteria for entry for patients were that they were over 18 years of age and were able to speak the language spoken by the researchers e. First 12 consecutive GHQ‐positive (GHQ score ≥ 3) clients (patients) from each clinic were recruited for secondary outcomes 5. Exclusion criteria a. Centres where staff had previously received training from the KMTC mental health training programme b. Those with dementia or learning disability of such severity as to be unable to complete the questionnaires c. Life‐threatening illness d. Did not speak the language spoken by researchers e. Refused to co‐operate |
|
| Interventions |
Stated purpose: to test effects of a low‐cost training intervention, integrated with national health sector reforms, on (1) competencies of primary care staff to recognise mental disorders, treat, and make appropriate referrals to scarce specialist services, and (2) recovery (improved health and social outcomes and quality of life) of patients INTERVENTION (n = 468) Name: impact of short structured general mental health in service training programme Delivered by: PHP Title/name of PW and number: staff (all nurses and clinical officers (doctors) eligible for training) from level 2 and 3 publicly funded primary care facilities in Nyanza Province, Kenya ‐ 2 from each PHC 1. Selection: self‐selection: 2 invited from each centre 2. Educational background: nurses and clinical officers at PHC 3. Training: RJ (psychiatrist) trained local trainers (3 courses) to deliver the course to front‐line workers in 2005 and gave them a refresher course in 2009 (40 hours in total). Trainers had done the Kenya medical college (KMTC) mental health training and had been delivering training since then. Trainers included 20 senior staff from KMTC (i.e. from Nairobi, provincial medical training colleges and the Ministry of Health rural health training centres). They were supplied with good practice guidelines and handouts for those who attended the training course, and the project provided a training course on mental health for local district public health nurses. Local trainers trained staff from PHCs randomised to intervention. 98 staff from the 49 PHCs randomised to the intervention group were trained in 5 courses. Each course, a comprehensive structured interactive mental health training programme, was held over 5 days and lasted 40 hours. Curriculum and teaching materials were developed by the WHO Collaborating Centre in dialogue with Kenya partners, based on the Kenya adaptation of the WHO primary care guidelines. Content: 5 modules: (1) core concepts of mental health, mental disorders, their contributions to physical health economic and social outcomes; (2) core skills (examination, communication, assessment, managing difficult cases/violence/bad news); (3) neurological disorders (epilepsy, Parkinson's disease, headache, dementia, toxic confusional states); (4) psychiatric disorders (content based on WHO primary care PC guidelines for mental health, Kenya adaptation); (5) system issues of policy; legislation; links between mental health and child health, reproductive health, HIV and malaria, and roles and responsibilities; health management information systems; working with community health worker (CHWs) and with traditional healers; and integration of mental health into annual operational plans. Use of role‐plays (25 each), theory, discussion, videos; emphasis on acquisition of practical skills and competencies for assessment, diagnosis, and management. 98 staff from PHCs were trained in 5 courses 4. Supervision: local district public health nurses whose role is to provide support and supervision to primary care were provided a training course on mental health 5. Incentives/remuneration: "each health facility is staffed by one or more nurses and clinical officers on Ministry of Health salaries, and around 15‐20 community health workers are not remunerated by the Ministry by the Ministry of Health but are now expected to receive small remuneration from the community" Intervention details 1. Duration/frequency: varying, depending on patient 2. Content of intervention: diagnosis and treatment with medicines according to WHO primary care guidelines and follow‐up CONTROL (n = 478) Usual care, PHCs that had not received prior KMTC training; neither were given training during this intervention CO‐INTERVENTIONS: patients in both intervention and control groups were treated as health workers routinely decided, based on their knowledge, experience, and training |
|
| Outcomes |
Patients 1. GHQ change in patients (neurotic symptoms, including morbid rating) 2. EQ5D § (health outcomes for wide range of health conditions and treatments) 3. WHODAS II (disability according to ICF) Carers NA Process/health worker outcomes GHQ identification index of clinics: detection rate of mental disorder (agreement/disagreement of staff diagnosis with patient‐rated GHQ score cutoff (GHQ score ≥ 3 being positive))* Economic outcomes None reported Time points: baseline (3 months post training), 3 months (6 months post training) (*: primary outcomes of the study; §: outcomes that we have not reported in this review) |
|
| Notes |
Source of funding: Nuffield Foundation and Department for International Development (UK) Notes on validation of instruments: all instruments available in English and Kiswahili; all validated in local setting. GHQ: widely validated in Africa; WHODAS II: validated (Ref from WHO; www.who.int/classifications/icf/en/); EUROQOL 5D: "the special validated calculator used in this project is derived from normative data from Zimbabwe for the EQ" Global Forum for Health Research (2002), the 10/9 Report on Health Research, 2001‐2002, Geneva, Switzerland Additional information: (www.controlled-trials.com/ISRCTN53515024). Declaration of interests ‐ RJ has received previous grants from DFID and Nuffield. SO, FK, HO, JA, PB, and CO have no competing interests Handling the data: as per footnotes in data and analysis Prospective trial registration number: ISRCTN53515024 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote 1: "all public level 2 and 3 health facilities were eligible for randomisation, which was done by DK and the Great Lakes University Knowledge Management and Research Department, using a table of random numbers drawn from JT McLure and F Dietrich 1994, Statistics, Macmillan College Publishing Co. pp 909‐911" Quote 2: "a random sample of 99 centres were selected stratified by health facility level, which were then randomly allocated to intervention and control groups, resulting in 33 dispensaries and 16 health centres in the intervention group and 37 dispensaries and 13 health centres in the control group" |
| Allocation concealment (selection bias) | Low risk | Information from study author: allocation to intervention and control was concealed from research assistants |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "the clinic staff were not blind as to whether they had received the training. We did not run a quantitative check on whether recruited clinic clients were aware of the trained status of their health workers" Comment: unlikely to affect outcomes; this was performed in real conditions in Kenya |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | Quote: "the research assistants were blind to whether the clinic staff had received the mental health training course, and to whether clients were attending clinics with trained or untrained staff. JA, who organised the research assistants in the field, was not blind to the clinic status" |
| Baseline outcome measurements similar | Low risk | Comment: all similar |
| Baseline characteristics similar? | Low risk | Quote: "the groups were generally similar on these parameters except that intervention clinics had more availability of benzodiazepines, and more clients who were unmarried" Comment: these were adjusted for in the analysis |
| Incomplete outcome data (attrition bias) Efficacy data | Low risk | Quote: "to reduce the possibility of attrition bias [31], we paid the 12 participants per cluster £2 per day to complete their initial assessment day (3 months after training of the health workers) and follow up day 12 weeks later, as compensation for their transport costs and time" Comment: in addition, dropout rate was very small (> 90% retention rate) |
| Incomplete outcome data (attrition bias) Safety data (e.g. adverse events) | Low risk | Information from study author: no major adverse events were noted (e.g. suicides). NB: trial was of training, not of a specific medicine or a specific intervention |
| Protection against contamination | Low risk | Quote: "tandomisation was conducted at the cluster level, namely PHC level rather than individual health worker level. If randomisation had taken place at individual health worker level, the risk of contamination between the practice of trained and untrained staff would be high, since they work closely in small teams" Comment: in addition, mentioned that other training is happening simultaneously (HIV, malaria, nutrition, paediatrics), but none of the training covered mental health issues (HIV training was only about pre‐post‐test counselling for HIV) |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes in protocol and in paper are the same |
| Other bias | Low risk | Comment: first study author confirmed that all results were "adjusted for clustering" |
Jiang 2017.
| Study characteristics | ||
| Methods |
Study design: RCT Duration of study: 9 months. Recruitment June 2015. Intervention for 9 months. Follow‐up assessments performed at 12 months post baseline |
|
| Participants |
Country: China Income classification: upper‐middle income between 2015 and 2016 Geographical scope: 4 communities in Yueyang City, Yueyanglou District, Hunan Province Healthcare setting: home‐based Mental health condition: depression Population 1. Age: ≥ 60 years 2. Gender: both 3. Socioeconomic background: not specified, but education is middle school or above 4. Inclusion criteria a. Age 60 or older b. Children living in another city c. Living alone or with spouse d. GDS score 11 to 25 e. Education middle school and above f. Willing to participate g. Possesses smartphone and computer or economic ability to purchase 5. Exclusion criteria: nil |
|
| Interventions |
Stated purpose: to evaluate the effectiveness of a cloud‐based spiritual support platform on mild to moderate depression among elderly “empty‐nesters” living in the community INTERVENTION (n = 40) Name: cloud‐based spiritual support platform model Delivered by: LH Title/name of PW and number: community workers ‐ number not specified 1. Selection: nursing student volunteers ‐ enrolled in 3‐year higher nursing college 2. Educational background: nursing school student volunteers, computer teachers, and elderly care workers with at least high school education 3. Training: 4 weeks' training on Internet platform 4. Supervision: not mentioned 5. Incentives/remuneration: not mentioned Intervention details 1. Duration/frequency: duration ‐ training 3 months, usage of cloud‐based platform 6 months. Frequency – training period: twice a week for 1 hour each time; actual intervention period: health and psychology help line available 24 hours a day, online health lectures every 2 weeks, health information disseminated once every 2 days via Weixingqun; other activities were ad hoc 2. Content of intervention: participants were trained for 3 months to use the Internet and the computer, specifically, Baidu, Weixing, QQ (social media platforms), online shopping, online health services, and Gerenkongjian (Personal Space). Training included demonstrations by research team and computer teachers, as well as practice by community workers and student volunteers. Subsequently, for 6 months, teams composed of hospital staff, elderly care workers, schoolteachers, and students ran an online and offline co‐ordinated spiritual support service. Services included 24‐hour health and psychology help line run by doctors and nurses to help with physical and mental health issues as they arose; online health lectures that participants could access and participate in using their smartphone or computer; creation and maintenance of personal webspace by each participant showing health, activities, hobbies, enquiries, and feelings, which their family members and medical staff can access and know about and help with any issues that they may have; use of Weixingqun to disseminate information such as food safety, weather, help line, and other services; holding themed discussions such as "My childhood", "My marriage", "My family" to encourage reminiscence and regular chats on Weixing and QQ to satisfy participants' need for family and social interaction; formation of interest‐based groups such as those based on hobbies, diet, exercise, art, etc. CONTROL: usual care: traditional community home‐based services (n = 40) Community health centres: doctors and nurses provided regular care based on health needs, including help with daily needs, physical examination, chronic disease treatment, occupational training guidance, safety advice, health education, etc., for 6 months CO‐INTERVENTIONS: nil |
|
| Outcomes |
Patients Geriatric depression scale Carers NA Process/health workers 1. Self‐designed evaluation questionnaire for intervention participants only, with 8 items that participants answered as Helpful/As usual/Not helpful 2. Enhanced connection with children and reduced sense of loneliness 3. Making new friends and increasing social interaction 4. Broadened perspective and increased self‐worth 5. Keeping up with times and maintaining a young mentality 6. Understanding of national affairs and following issues of societal importance 7. Attaining effective health education and improved self‐management of chronic diseases 8. Experiencing new things and greater convenience 9. Feeling respected by society as an elderly person# (did not analyse) Economic outcomes nil Time points: baseline, immediately after intervention |
|
| Notes | Article in Chinese Source of funding: Hunan Province 2014 4th Province‐level Scientific Project, 2015 Hunan Province Education Subject Notes on validation of instruments (screening and outcomes): Chinese version of GDS has been validated; questionnaire has not been validated Additional information: declarations of interest ‐ none Handling the data: nil Prospective trial registration number: nil |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Judgement comment: a convenience sample was taken from 4 communities in Yueyanglou District, Yueyang City. Elderly empty nesters were screened with Geriatric Depression Scale (GDS), and 80 subjects who fulfilled the criteria were selected for the study. Based on the serial numbers on their baseline surveys, participants were divided into intervention and control groups, with odd numbers in 1 group and even numbers in the other |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: randomisation was performed after baseline assessment and before intervention |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding was performed. Participants in intervention and control groups received different treatment approaches and hence were aware of their allocation |
| Blinding of outcome assessment (detection bias) all outcomes | High risk | No details were given about how post‐intervention GDS was administered |
| Baseline outcome measurements similar | Low risk | Similar baseline outcome results (GDS score) in both groups |
| Baseline characteristics similar? | Low risk | Both groups were similar in age, gender, educational level, economic status, living arrangement, religion, use of media, etc (P ≥ 0.05) |
| Incomplete outcome data (attrition bias) Efficacy data | Low risk | No missing data were reported |
| Protection against contamination | Unclear risk | Although only intervention subjects were on the cloud‐based platform, it is possible for them to share information such as health lectures and health news with their friends in the control group, as they live in the same community. Use of social media to communicate with family was an important aspect of the intervention, but social media was also available widely in the general population, including among subjects in the control group |
| Selective reporting (reporting bias) | Unclear risk | According to the paper, GDS was the only outcome measure planned and measured. No published clinical trial protocol is available |
| Other bias | Low risk | None were detected |
Jordans 2010.
| Study characteristics | ||
| Methods |
Study design: cluster‐RCT. Unit of allocation: district; unit of analysis: individual. 2 districts in each arm Duration of study: December 2006 to March 2007 |
|
| Participants |
Country: Nepal Income classification: low income Geographical scope: 4 districts of rural southwestern Nepal (Banke, Dang, Bardia, Kailali) Healthcare setting: school Mental health condition: psychosocial distress (including PTSD symptoms) Population: patients (children/adolescents) 1. Age: 11 to 14 years 2. Gender: both; more girls in treatment group 3. Socioeconomic background: significant differences in groups despite randomisation: more Brahmins in treatment group, Terai caste in wait‐list (none in intervention group). Higher education among treatment group. Religion and place of residence statistically different but of minimal importance: majority Hindu in both groups and living in a village other than their original village 4. Inclusion criteria a. School‐aged children b. Positive Child Psychosocial Distress Screener score (cutoff score unspecified) 5. Exclusion criteria a. Psychiatric problems (mutism, mental retardation, dissociative disorders, epilepsy without medication, panic or phobic disorders, child psychosis) b. Schools excluded if they were in Village Development Committees (VDCs) where the intervention was already implemented c. Schools in adjoining VDCs excluded to avoid contamination |
|
| Interventions |
Stated purpose: to assess the efficacy of CBIs among school‐going children in rural Nepal as a psychosocial intervention to assist children affected by armed conflict in LAMIC INTERVENTION (n = 164) Name: CBI Delivered by: LHW Title/name of PW and number: 16 paraprofessional interventionists/facilitators 1. Selection: gender‐balanced group, from targeted communities 2. Educational background: based on previous experience and affinity to work with children 3. Training: 15‐day skills‐oriented course (duration and trainers not specified) 4. Supervision: regular supervision by experienced counsellor 5. Incentives/remuneration: information from study author: facilitators received a monthly remuneration of 4000 NPR for running the CBI sessions Intervention details 1. Duration/frequency: 5 weeks, 15 sessions (about 60‐minute sessions) 2. Content of intervention: protocolised group intervention; eclectic intervention based on concepts from creative‐expressive and experiential therapy, co‐operative play, and CBT. Use of the same manual as for Tol 2008 (Center for Trauma Psychology in Boston) CONTROL: usual care (wait‐list control) (n = 161) CO‐INTERVENTIONS: CBI was offered as part of a multi‐layered care system that included activities geared towards strengthening community resilience through parental support groups, recreational activities, community sensitisation, and psychoeducation (tier 1); the CBI to target children with elevated psychosocial distress upon primary screening (tier 2); and individual supportive and problem‐solving counselling and referral to psychiatric care (if available) for children, mainly referred on from the group intervention, in need of more individualised or specialised care (tier 3) |
|
| Outcomes |
Patients Primary outcomes 1. SCARED (anxiety)* 2. Children's Aggression Scale for Parents* § (physical aggression) 3. CPSS (Child PTSD)* 4. DSRS* 5. SDQ* § Secondary outcomes 1. Concern for others scale § (prosocial behaviour) 2. Children's Functional Impairment (protocol mentioned secondary outcomes would also be daily functioning and self efficacy ‐ these are not reported here) Carers N/A Process/health workers Not assessed Economic outcomes None Time points: baseline, 3‐month follow‐up (*: primary outcomes of the study; §: outcomes that we have not reported in this review) |
|
| Notes |
Source of funding: Save the Children USA (Nepal Office) Notes on validation of instruments: translated and validated; "test–retest reliability of the instruments was determined among 20 participants"; 1 screening measure, the CPDS, was developed for Nepali context specifically and was described in Bolton 2002 Additional information: (www.controlled-trials.com/ISRCTN48004304/ISRCTN48004304) declarations of interest ‐ no conflicts declared Handling the data: as per footnotes in data and analysis Prospective trial registration number: SRCTN48004304 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "allocation to study conditions followed a three‐step procedure. First, districts were randomly allocated to either CBI or control condition (2 CBI districts, 2 wait‐ list districts). Second, two schools per district were randomly selected from a list of all eligible schools. Exclusion criteria for schools were (a) schools in Village Development Committees (VDC; the smallest administrative unit in Nepal) where CBI had already been implemented and schools in adjoining VDCs to avoid contamination; (b) schools in parts of the district with large geographic or ethnic differences compared to the majority of the district to increase group homogeneity within districts. Third, children were randomly selected from a list of all children aged 11‐14 years in the school. The randomisation was done, without imposing a randomisation constraint, by use of computer‐generated random numbers (in SPSS) by the research team in Amsterdam. Out of 53 eligible schools, 8 were randomly selected with a total of 1367 eligible children of whom 149 were absent and 30 refused" |
| Allocation concealment (selection bias) | High risk | Quote: "randomisation was done, without imposing a randomisation constraint, by use of computer‐generated random numbers (in SPSS) by the research team in Amsterdam" Comment: schools, districts, and students randomised through computer‐generated random numbers by research team in Amsterdam but still not clear whether at the point of allocation whether allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: schoolchildren and teachers could not be blinded due to nature of intervention, but outcomes are unlikely to be affected by blinding |
| Blinding of outcome assessment (detection bias) all outcomes | High risk | Research assistants not blinded to treatment condition; interviewed children's self‐report (children not blinded to treatment condition) |
| Baseline outcome measurements similar | Unclear risk | Comment: report that no significant baseline differences between boys and girls on outcomes but data not presented between control and intervention groups. Baseline outcome measures seem similar between groups (table 2), except perhaps SCARED, physical aggression, and prosocial behaviour. In addition, noted in limitations that SCARED reliability between assessors was poor, so may not be reliable |
| Baseline characteristics similar? | Low risk | Comment: baseline differences in gender, ethnicity, religion, place of residence, and level of education, which were adjusted for |
| Incomplete outcome data (attrition bias) Efficacy data | Low risk | Comment: lost to follow‐up at T2, 2 in treatment group, 0 in wait‐list control group |
| Incomplete outcome data (attrition bias) Safety data (e.g. adverse events) | Low risk | Information from trial author: "there were no adverse outcomes" |
| Protection against contamination | Low risk | Comment: cluster design, which is unlikely to lead to contamination and wait‐list control |
| Selective reporting (reporting bias) | Low risk | Comment: 2 secondary outcomes reported in protocol are not reported in results (self‐efficacy and daily functioning) Study author response: "with regards to the secondary outcomes; (a) 'daily functioning' has been included in the paper but has been renamed as 'functional impairment' (following the paper that was written on the development and validation of that scale); (b) 'self‐efficacy' was included in the protocol, but no instrument was found with sufficient cross‐cultural validity. As a result we have opted to include a 'coping scale (KID‐COPE)', which was not included in the reporting because of unforeseen problems with the analyses (i.e. we were not able to adequately analyse the combined response format of dichotomous and ordinal scales per respondents of the KID‐COPE)" Comment: good explanation |
| Other bias | Low risk | Comment: ICC done with adjustment for clustering |
Jordans 2019.
| Study characteristics | ||
| Methods |
Study design: nested RCT (in alcohol use disorder and depression cohort studies) Duration of study: August 2014 to 2016 (recruitment and baseline interviews August 2014 to 2015; F/U interviews December 2014 to August 2016) |
|
| Participants |
Country: Nepal Income classification: low income Geographical scope: Chitwan, a predominantly rural district in Southern Nepal (total population 579 984) Healthcare setting: 10 primary healthcare facilities in Chitwan, Nepal Mental health condition: adults with depression and/or alcohol use disorder (AUD) Population 1: alcohol use disorder 1. Age: 16 and older 2. Gender: both 3. Socioeconomic background: about three‐quarters of participants were employed, but more than 95% had some form of food insecurity 4. Inclusion criteria (including threshold cutoff score of measurement tool) a. Adults with AUDIT score ≥ 9 (1) AUD took precedence over depression. This meant that some of the AUD participants had also been diagnosed with depression as a secondary diagnosis (n = 3) 5. Exclusion criteria a. Pregnancy, psychosis, or epilepsy b. Unable to communicate clearly c. Needing urgent medical attention Population 2: depression 1. Age: ≥16 years 2. Gender: male and female 3. Socioeconomic background: 70% unemployed 4. Inclusion criteria a. Adults aged ≥ 16 screening positive with depression (PHQ‐9 ≥ 10) 5. Exclusion criteria a. Pregnancy b. Needing urgent medical treatment c. Diagnosis with psychosis or epilepsy d. Unable to communicate clearly |
|
| Interventions |
Stated purpose: to evaluate the effectiveness of adding community‐based, counsellor‐delivered psychological interventions for adults who initiated Mental Health Gap‐Action Programme (mhGAP)‐based primary care services for depression and AUD in Nepal INTERVENTION 1 (n = 80) Name: mhGAP plus Counselling for Alcohol Problems (CAP) Delivered by: LHW Title/name of PW and number: community‐based counsellors ‐ 6 1. Selection: members of local community 2. Educational background: completed at least a high school education 3. Training: base training of 400 hours of classroom learning, 150 hours of clinical supervision, 350 hours of practice, and 10 hours of personal therapy spread out over 6 months. For this study, counsellors had 10 additional days of combined training for HAP (intervention for depression arm) and CAP 4. Supervision: bi‐weekly, by psychologist Intervention details: delivered to persons with AUD 1. Duration/frequency: 4 individual sessions delivered weekly 2. Content of intervention: manualised motivational intervention consisting of assessment, cognitive and behavioural skills pertaining to drink refusal, peer pressure, problem‐solving and difficult emotions, and management of potential relapses INTERVENTION 2 (n = 60) Name: mhGAP plus Healthy Activity Program (HAP) for depression Delivered by: lay PHW Title/name of PHW/CW and number: 6 community‐based counsellors ‐ 4 female, 2 male 1. Selection: members of local community 2. Educational background: completed at least a high school education 3. Training: counsellors received a base training, which included 400 hours of classroom learning, 150 hours of clinical supervision, 350 hours of practice, and 10 hours of personal therapy, spread out over 6 months. In addition, for this study, counsellors followed a 10‐day combined training for HAP for depression and CAP for AUD 4. Supervision: CAP and HAP manuals were translated from their original into Nepali by a bilingual Nepali psychologist, who had also received a training of trainers from the original developers. Bi‐weekly supervision by the same trainer was delivered for counsellors during trials 5. Incentives/remuneration: none, as they are employees Intervention details: delivered to persons with depression 1. Duration/frequency: 6 to 8 individual sessions delivered weekly 2. Content of intervention: manualised intervention that consists of behavioural activation as the core therapeutic framework, which includes psychoeducation, behavioural assessment, activity monitoring, activity structuring, problem‐solving, and activation of social networks CONTROL (CAP trial, n = 82) (HAP trial, n = 60) mhGAP‐based services: pharmacological treatment (fluoxetine or amitriptyline), basic psychosocial support by trained non‐prescribing health workers, referral to formal mental health services when indicated, and community mental health sensitization programmes CO‐INTERVENTIONS: all 3 groups received mhGAP |
|
| Outcomes |
Patients with AUD 1. AUDIT score (symptom severity)* 2. WHODAS score (disability)* 3. Reduction to low‐risk drinking levels: defined as sub‐threshold AUDIT score (< 9) at follow‐up Patients with depression 1. PHQ‐9 (symptom severity)* 2. WHODAS score (disability)* 3. Response rate for depression: defined as ≥ 50% reduction in PHQ‐9 score compared with baseline Carers None Process/health workers Numbers of CAP and HAP sessions# Economic outcomes None (* study's primary outcomes, #not included in this review) Time points post intervention: 1 to 3 months, 10 to 12 months |
|
| Notes |
Source of funding: UK Department of International Development Notes on validation of instruments (screening and outcomes): validated Additional information: ISRCTN Registry (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5840717/). Declarations of interest ‐ study authors reported no conflicts of interest Handling the data: none Prospective trial registration number: ISRCTN72875710 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Via computer‐generated random numbers (in SPSS Version 22 for Windows), a list of numbers (1 to 400) was randomised, so that each number corresponded to either the treatment group or the control group Sequence generation was conducted with computer software |
| Allocation concealment (selection bias) | Low risk | Randomisation was done by the research co‐ordinator in Kathmandu (N.P.L.) via computer‐generated random numbers (in SPSS Version 22 for Windows). A list of numbers (1 to 400) was randomised, so that each number corresponded to either the treatment group or the control group. The ID code of each new eligible participant was sent to the research co‐ordinator, who then matched it to the next number on the list Allocation was concealed by researchers, and each participant was given an ID code |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Participants could not be blinded", but this probably did not influence outcomes |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | Research assessors were blinded to allocation |
| Baseline outcome measurements similar | Low risk | Any differences in baseline characteristics between control and treatment were accounted for during analyses |
| Baseline characteristics similar? | Low risk | Some differences in baseline characteristics were found; however these were adjusted for during analyses |
| Incomplete outcome data (attrition bias) Efficacy data | Low risk | All outcome analyses were on an intention‐to‐treat basis with use of multiple imputation for those with missing outcome data All incomplete date were adjusted for during analyses |
| Protection against contamination | Unclear risk | It is unclear whether there was communication between primary health workers and community‐based counsellors who delivered the intervention |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the trial (ISRCTN72875710) were reported |
| Other bias | Low risk | No concerns |
Khan 2017.
| Study characteristics | ||
| Methods |
Study design: RCT Duration of study: data collection took place between July 2016 and November 2017 |
|
| Participants |
Country: Pakistan Income classification: lower‐middle income Geographical scope: Swat Valley, Pakistan (rural); armed conflict area Healthcare setting: primary care Mental health condition: common mental disorders Population 1. Age: 15 to 45 years 2. Gender: pregnant women 3. Socioeconomic background: middle and lower socioeconomic strata 4. Inclusion criteria a. SRQ score > 9 at any stage of pregnancy 5. Exclusion criteria a. Patients with suicidal intent b. Severe mental or medical illness c. Recently given birth d. Living with another woman with an SRQ score ≥ 9 |
|
| Interventions |
Stated purpose: to evaluate the feasibility and acceptability of a psychoeducational intervention for pregnant women with common mental disorders in rural Pakistan INTERVENTION (n = 34) Name: "Happy Mother, Healthy Child in Ten Steps" Delivered by: LHW Title/name of PW and number: lady health workers (LHWs) 1. Selection: worked as LHW in catchment area 2. Educational background: LHWs are community health workers trained to provide maternal and child health care and education to a catchment area of approximately 1000 people or 150 homes (LHWs = local community health workers with no mental health experience) 3. Training: intervention was fully manualised. Alongside the manual, counselling cards were developed as job aids for LHWs to use while delivering each session 4. Supervision: by the Lady Health Supervisor 5. Incentives/remuneration: not mentioned Intervention details 1. Duration/frequency: 2 sessions 2. Content of intervention: session 1 (20 minutes): to build rapport and for family buy‐in and participation in the next visit; session 2: delivered to the whole family in their home by the local community health worker. Intervention focuses attention on maternal psychosocial well‐being, associating it with optimal growth and development of the unborn child. Main areas of the intervention were empathetic listening, availability of social support, ensuring the pregnant woman regarding the circle of support that is available, domestic peace, balanced diet, rest, engagement of the pregnant woman in pleasurable activities, routine check‐up during pregnancy, consulting doctor in case the distress is not relieved, and maintenance of household peace and harmony throughout. These were described as steps for the health and well‐being of the pregnant woman and her unborn child. Each of these steps has a maternal well‐being message for the whole family. Intervention uses a simple pictorial approach of paired illustrations with 1 showing unwanted behaviours and the other positive actions to achieve the desired outcome of support for the mother; they were designed specifically for this intervention CONTROL (n = 37) Routine care was conducted by local community health workers (LHWs) to control arm participants, as is their routine official duty. During routine visits, LHWs conduct educational sessions (awareness for prevention of common diseases) and provide the household with routine over‐the‐counter medicine for common ailments, along with iron and folic acids |
|
| Outcomes |
Patients Primary outcome measure Help‐seeking for psychological distress by pregnant women (semi‐structured interview) Secondary outcome measures 1. Psychological distress as measured by the Self‐Reporting Questionnaire (SRQ) 2. Social support as assessed through the Multidimensional Scale of Perceived Social Support (MSPSS) Carers None Process/health workers 1. Response rate 2. Retention rates 3. Complaints/objections 4. Untoward incidents Economic outcomes None Time points: baseline, 2 months post intervention |
|
| Notes |
Source of funding: study author(s) received no financial support for research, authorship, and/or publication of this article Notes on validation of instruments (screening and outcomes): all validated Additional information: declarations of interest ‐ study authors declare that they have no competing interests. K.B. is funded by NIHR CLAHRC NWC Handling the data: as per footnotes in data and analysis Prospective trial registration number: ACTRN12616001630404 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "of the 133 pregnant women with SRQ score of ≥9, 81 pregnant women (within the catchment area of 27 LHWs) consented to participate in the trial and were randomized on 1:1 allocation ratio by an independent researcher" |
| Allocation concealment (selection bias) | Low risk | Judgement comment: from registry: "randomization was done by a researcher at the independent trial center at the Human Development Research Foundation who was not involved in intervention delivery, clinical supervision, independent assessment or other aspects of the day‐to‐day running of the study" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding; unclear how this may have influenced outcomes |
| Blinding of outcome assessment (detection bias) all outcomes | Unclear risk | RAs gathering outcome data were masked to intervention allocation; however, participants were aware of their own study status and could have divulged this or could have been biased based on expectations |
| Baseline outcome measurements similar | Low risk | "Table 2 depicts the baseline psychological distress and perceived social support using SRQ and MSPSS, respectively. There were no differences between the SRQ and MSPSS scores in two arms. In Table 3, the stressful life events in the post‐conflict year, measured through the life events checklist, and the self‐recognition of their psychological distress at baseline are compared and showed no differences" |
| Baseline characteristics similar? | Low risk | Table 1 compares sociodemographic characteristics of intervention and control arms at baseline. There were more uneducated pregnant women (65% vs 41%) and less primigravida (6% vs 27%) in the intervention compared to the control arm. There were no differences between arms in terms of age, duration of pregnancy, pregnancy loss, or infant death. Factors pertaining to family and socioeconomic status were evenly distributed between intervention and control arms |
| Incomplete outcome data (attrition bias) Efficacy data | Unclear risk | There was a high response rate, and trial attrition rates were low, as more than 95% participants completed follow‐up assessments Comments: 8/42 allocated to intervention were excluded, as they did not fulfil inclusion criteria vs 0/39 in the control group. This could affect the results as sample sizes are so small |
| Protection against contamination | High risk | Judgement comment: individual randomisation: "contamination of the intervention across trial arms is a particular problem in trials evaluating educational interventions because of their easy spillage (Sullivan, 2011). In the current trial, such contamination cannot be ruled out because women live in close proximity and interact regularly. Some steps taken in this study, however, may have minimized this: (a) LHWs delivered the sessions only to those families they were assigned by the research team, (b) participants living in the same household were excluded and (c) active supervision and fidelity checks were conducted to ensure the sessions were conducted as planned" |
| Selective reporting (reporting bias) | Low risk | All outcomes in registry and in methods section are reported |
| Other bias | Low risk | None |
Khan 2019.
| Study characteristics | ||
| Methods |
Study design: 2‐arm cluster‐randomised controlled feasibility trial. Unit of randomisation: Lady Health Worker's catchment area. 10 LHW catchment areas in each arm (20 in total) Duration of study: 21 March until 12 August 2015 |
|
| Participants |
Country: Pakistan Income classification: lower‐middle income Geographical scope: Qambar Union Council (UC), a rural conflict‐affected area in Swat, Khyber Pakhtunkhwa Province, Pakistan Healthcare setting: primary care facility (Basic Health Unit (BHU) staffed by a physician, a Lady Health Visitor (primary healthcare worker stationed at a health unit and providing maternal and child health services), a vaccinator, a midwife and a Lady Health Worker) Mental health condition: PTSD Population: psychologically distressed women in a conflict‐affected setting 1. Age: ≥ 18 years 2. Gender: female 3. Socioeconomic background: mainly housewives; 43% to 46% financially empowered 4. Inclusion criteria a. Females ≥ 18 years of age b. Referred for screening based on judgement of their LHW that they were psychologically distressed c. Score > 2 on General Health Questionnaire (GHQ) d. Score > 16 on WHO Disability Assessment Schedule (WHO‐DAS) 5. Exclusion criteria a. Imminent suicide risk b. Cognitive impairment (e.g. severe intellectual disability, dementia) c. Mental disorder (psychotic disorder or substance dependence) |
|
| Interventions |
Stated purpose: to evaluate the feasibility and acceptability of locally adapted Group Problem Management Plus (PM+) intervention for women in conflict‐affected settings in Swat, Pakistan INTERVENTION (n = 54) Name: Group Problem Management Plus (Group PM+) Delivered by: LHW Title/name of PW and number: lay helpers ‐ 2; lady health workers ‐ 10 1. Selection: of the 24 lady health workers (in Qambar UC), 20 were eligible for randomisation (reasons for exclusion: 2 for personal reasons, 2 not fully inducted) 2. Educational background a. Lay helpers: 16 years of education (graduates) with no formal training of or prior experience in mental health b. Lady health workers (LHWs): trained to provide mother and child health care and education to a catchment area of approximately 1000 people or 150 homes, conducting monthly routine health visits 3. Training: lay helpers received 6 days of training by one of the study authors ("Master Trainer"), together with 3 non‐specialist supervisors. Training covered common mental disorders, basic counselling and group management skills, the Group PM+ intervention, and self‐care strategies. This was followed by 4 weeks of practice cases with weekly group supervision through Skype (2 to 3 hours' duration) by the 3 supervisors. Lady health workers received a half‐day training session by lay helpers regarding their roles and responsibilities in the trial 4. Supervision: group supervision was continued throughout the trial, providing opportunities for peer learning and collective problem‐solving 5. Incentives/remuneration: not mentioned Intervention details 1. Duration/frequency: 5 weekly group sessions, each lasting 2 hours 2. Content of intervention: PM+ is a WHO psychological intervention developed on evidence of established problem‐solving, counselling, and behavioural techniques. The multi‐component nature of PM+ allows many symptoms of common mental health disorders to be addressed with 1 intervention (i.e. transdiagnostic). Group PM+ is an adaptation of the individual intervention. The manual has been translated into Urdu and Pashtu, and has been culturally adapted to the Swat setting. Group PM+ was delivered by lay helpers. LHWs served to facilitate introduction of lay helpers to the community, encourage participant attendance, and provide a space in which sessions could be conducted. LHWs were present when Group PM+ sessions were conducted but were not active facilitators of intervention content CONTROL: enhanced usual care (EUC) (n = 58) The 10 LHWs randomised to control intervention received training in primary care referral pathways for treatment of common mental disorders, and primary care physicians in the corresponding Basic Health Unit received training in assessment and treatment of common mental disorders by the Swat District psychiatrist in separate half‐day sessions. Participants with severe psychiatric disorders (e.g. psychosis) or problems (e.g. suicidality) that required immediate specialist treatment and follow‐up were referred to the physician at the Basic Health Unit or District Headquarter Hospital for specialised psychiatric care, depending upon their needs CO‐INTERVENTIONS: nil |
|
| Outcomes |
Patients 1. Hospital Anxiety and Depression Scale 2. GHQ 3. WHO‐DAS 4. PHQ‐9 5. Psychological outcomes profile (PSYCHLOPS) 6. PCL‐5 for PTSD symptoms Carers Nil Process/health workers Nil Economic outcomes Nil Time points: baseline, 1 week post intervention |
|
| Notes |
Source of funding: Office of Foreign Disaster Assistance (OFDA) Notes on validation of instruments (screening and outcomes): all locally validated Additional information: declarations of interest ‐ study authors declared no conflicts of interest Handling the data: as per footnotes in data and analysis Prospective trial registration number: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: LHW clusters were randomised on a 1:1 allocation ratio using computer‐based software through a simple randomisation method Randomised via a computer programme, therefore low risk |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: allocation was carried out by an independent researcher. However, it is unclear if allocation was concealed from members of the research team |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not blinded, although unlikely to have influenced outcomes |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | Blinding was ensured during assessments through LHW instructing participants not to disclose their allocation status. In case of disclosure, assessments were rescheduled through the other assessor Blinding was ensured, therefore considered low risk |
| Baseline outcome measurements similar | Low risk | Participant outcomes were measured before the intervention, and no important differences were present across study groups |
| Baseline characteristics similar? | Low risk | Baseline characteristics are reported in the table and are similar |
| Incomplete outcome data (attrition bias) Efficacy data | Low risk | Missing outcome measures were similar; this is unlikely to bias the results |
| Incomplete outcome data (attrition bias) Safety data (e.g. adverse events) | Low risk | No adverse events happened. Missing data were similar for intervention and control; this is unlikely to bias the results |
| Protection against contamination | Low risk | Judgement comment: allocation was by geographical area of the LHWs. Control group received enhanced usual care; therefore unlikely that there was contamination by primary care staff with lay helpers |
| Selective reporting (reporting bias) | Low risk | All outcomes described in trial registry (ACTRN12615000210572) were reported in the paper |
| Other bias | Low risk | No other risk of bias present |
Li 2002.
| Study characteristics | ||
| Methods |
Study design: RCT Duration of study; 9 months. Recruitment between June 1999 and March 2001. Intervention lasted 50 to 51 days. Follow‐up assessments 6 months post intervention |
|
| Participants |
Country: China Income classification: lower‐middle income between 1999 and 2002 Geographical scope: rural Healthcare setting: home and community Mental health condition: schizophrenia (specifically late‐onset) Population (mention if patient, carer, or dyad) 1. Age: 50 years and older 2. Gender: both 3. Socioeconomic background: over 80% were farmers 4. Inclusion criteria a. Met Chinese Classification of Mental Disorder (CCMD)‐2‐R criteria for schizophrenia b. Age 50 and above c. First episode of illness 5. Exclusion criteria: nil |
|
| Interventions |
Stated purpose: to determine the efficacy of community rehabilitation for patients with late‐onset schizophrenia INTERVENTION (n = 38) Name: community rehabilitation Delivered by: PHP Title/name of PW and number: village and street‐level doctors ‐ number not specified 1. Selection: existing infrastructure 2. Educational background: medical degree 3. Training (contents, duration, by whom): briefed by a hospital community medicine doctor regarding general mental health prevention and treatment and specific issues to take note of 4. Supervision: hospital community medicine doctor in charge of patient Intervention details: home visits and telephone enquiries 1. Duration/frequency: duration 50.12 ± 28.19 days, frequency of home visits at least weekly, telephone enquiries as needed 2. Content of intervention (by types of health workers and per patients/carers): medication following specific guidelines (see below), counselling, requested work, social activities CONTROL (n = 38) Specialist care ‐ inpatient care; doctors in charge were psychiatrists, treated mainly with medication according to guidelines (same as intervention group), supplemented by occupational therapy and psychotherapy. Duration 51.03 ± 29.17 days CO‐INTERVENTIONS: medication guidelines were to begin with a low dose of a typical antipsychotic such as perphenazine, gradually increased as needed up to 29.05 ± 9.83 mg/d, to add on sulpiride up to 200mg/d for depressed mood, apathy, or withdrawal, and to add on alprazolam if there was obvious insomnia |
|
| Outcomes |
Patients 1. Recovery (BPRS reduced by ≥ 80%) 2. BPRS 3. SDSS 4. Relapse (reappearance of symptoms or worsening of original symptoms requiring medication modification) 5. Disease much improved (BPRS reduced by ≥ 60%)# 6.Disease improved (BPRS reduced by ≥ 30%)# 7. Disease showing no improvement (BPRS reduction <3 0%)# Carers Nil Process/health workers Nil Economic outcomes Direct costs of treatment (page 2 of article (page 712 of journal), right column, paragraph numbered “4”) (asterisk for study's primary outcomes; star: outcomes that we have not reported in this review) Time points: 0 months, 6 months |
|
| Notes | Article in Chinese Source of funding: none Notes on validation of instruments (screening and outcomes): validated Additional information: declarations of interest ‐ none Handling the data: none Prospective trial registration number: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: randomised based on sequence of admission to hospital |
| Allocation concealment (selection bias) | High risk | Judgement comment: no description of any efforts to conceal allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No effort to blind participants was mentioned in the paper |
| Blinding of outcome assessment (detection bias) all outcomes | High risk | No attempt at blinding researchers was mentioned in the paper |
| Baseline outcome measurements similar | Low risk | Baseline outcome measures (table 2 ‐ BPRS, table 3 ‐ SDSS) were similar in both groups |
| Baseline characteristics similar? | Low risk | Baseline characteristics (age, gender, occupation, education, marital status, family history) were similar in both arms |
| Incomplete outcome data (attrition bias) Efficacy data | Low risk | There is no attrition in this study |
| Protection against contamination | Low risk | Judgement comment: groups were treated in different settings (hospital, community); therefore unlikely to contaminate each other |
| Selective reporting (reporting bias) | Unclear risk | All outcomes planned in materials and methods section were reported in results section. No clinical trial protocol is available |
| Other bias | Low risk | No other sources of bias have been found |
Li 2018.
| Study characteristics | ||
| Methods |
Study design: RCT, cluster. Unit of allocation: commune health centres; 30 commune health centres in each arm (60 total) Duration of study: 2 years; October 2014 to October 2016 |
|
| Participants |
Country: Vietnam Income classification: lower‐middle income between 2014 and 2016 Geographical scope: rural and urban (Vınh Phúc and Phú Thọ Provinces of Vietnam) Healthcare setting: commune health centres Mental health condition: substance abuse (heroin) Population 1: commune health workers 1. Age: 18 and older 2. Gender: both 3. Socioeconomic background: 17% were doctors; 39% were assistant doctors 4. Inclusion criteria a. Aged 18 years or older b. Doctor, assistant doctor, nurse, pharmacist, midwife, lab technician, or public health worker who had contact with persons who inject drugs (PWID) in the study communes 5. Exclusion criteria: nil Population 2: persons who inject drugs (PWID) 1. Age: 18 and older 2. Gender: 97.8% men 3. Socioeconomic background: 73% had annual family income < ₫750 000 (US $3290). Approximately 5% of PWID came from poor households, based on the country’s standard 4. Inclusion criteria a. 18 years of age or older b. History of injecting drug use c. Resident of selected communes 5. Exclusion criteria: nil |
|
| Interventions |
Stated purpose: to evaluate the efficacy of an intervention targeted to commune health workers (CHWs) who deliver services to people who injected drugs (PWID) in Vietnam INTERVENTION (commune health workers, n = 150) (PWID, n = 461) Name: Communication Training of Community Health Workers on Service Delivery to People Who Inject Drugs Delivered by: LHWs Title/name of PW and number: local health educators ‐ 6 to 8 1. Selection: recruited by investigators 2. Educational background: some medical training 3. Training (contents, duration, by whom): trained by investigators for 1 week 4. Supervision: supervisor attended each session, filled out an evaluation checklist, and provided feedback Intervention details: conducted by local health educators; aimed at commune health workers 1. Duration/frequency: commune health workers received 3 weekly 90‐minute sessions and 2 monthly booster sessions. PWID received 3 one‐hour sessions. Total duration of intervention was 12 months 2. Content of intervention (by types of health workers and per patients/carers): sessions aimed at commune health workers covered roles and responsibilities of commune health workers in HIV and drug control, challenges of working with PWID and possible solutions, stages of behavioural change and client‐centred goal‐setting, and motivational communication tools and communication skills through group discussions, games, role‐play, homework, and practice. Commune health workers delivered individual sessions to PWID using the tools and skills they had learnt. Content of these sessions focused on improving PWID physical and mental health, engaging PWD in harm reduction and HIV services, and enhancing family and social support in positive behavioural change CONTROL: enhanced usual care (commune health workers, n = 150) (PWID, n = 439) Commune health workers in control group received 1 group lecture by local health officials on topics related to drug use CO‐INTERVENTIONS: nil |
|
| Outcomes |
Patients 1. Drug avoidance self‐efficacy 2. Current heroin use (positive urine morphine test) Carers 1. Provider‐client interaction 2. Negative attitude towards drug users Process/health workers Nil Economic outcomes Nil (asterisk for study's primary outcomes; star: outcomes that we have not reported in this review) Time points post end of intervention: 2 months, 5 months, 8 months, 11 months (not clear in the paper how long the intervention took. Assessments were done 3, 6, 9, and 12 months after baseline) |
|
| Notes |
Source of funding: National Institutes of Health Notes on validation of instruments (screening and outcomes): provider‐client interaction (12‐item) instrument was developed and tested in a previous study. Cronbach’s alpha value was provided. Cronbach’s alpha value was also provided for negative attitude towards drug users scale (7‐item) and for drug avoidance self‐efficacy scale; the latter has been validated by other trial authors Additional information: trial protocol is available; personal communication with trial author; declarations of interest ‐ none Handling the data: nil Prospective trial registration number: NCT0213092.1 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: method of random sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: method of allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not performed; lack of blinding of participants may have influenced outcomes, as outcomes were self‐reported |
| Blinding of outcome assessment (detection bias) all outcomes | High risk | Blinding was not performed in this study. 3 out of 4 outcome measures ‐ provider‐client interaction, negative attitude towards PWIDs, and drug avoidance self‐efficacy ‐ were all self‐reported. The only objective outcome measure was urine tests for morphine, which showed no significant differences between control and intervention groups |
| Baseline outcome measurements similar | Low risk | For both community health workers and people who injected drugs, baseline outcome measures were similar in intervention and control groups |
| Baseline characteristics similar? | Low risk | Among community health workers, intervention and control groups were similar in all baseline characteristics except for time at commune health centre (≤ 5 years, 5 to 10 years, 10 to 20 years, > 20 years). However, all CHWs had a similar mean duration at the commune health centre. Among people who inject drugs, intervention and control group subjects were similar in all baseline characteristics except for marital status |
| Incomplete outcome data (attrition bias) Efficacy data | Low risk | Dropout rate was low (among CHWs, none; among PWIDs, 35/611 = 5.7% in intervention and 4.2% in control) and was within the attrition rate expected by trial authors. Therefore subjects who had dropped out are unlikely to have affected the outcome of the study even if they had not dropped out |
| Incomplete outcome data (attrition bias) Safety data (e.g. adverse events) | Unclear risk | More deaths, incarcerations, and hospitalisations occurred in the intervention group than in the control group. Causes of these adverse events were not explained |
| Protection against contamination | Low risk | Judgement comment: cluster‐randomised study, with physical distance between intervention and control groups |
| Selective reporting (reporting bias) | Unclear risk | All outcomes planned in methods section were reported in results section. Unable to locate clinical trial outline |
| Other bias | Low risk | No other sources of bias were found |
Ling 1999.
| Study characteristics | ||
| Methods |
Study design: prospective RCT Duration of study: 1 year. Recruitment at the start of 1994. Intervention lasting 6 months. Last assessment done 1 year after baseline |
|
| Participants |
Country: China Income classification: low income between 1994 and 1996 and 1998. Lower‐middle income in 1997 and 1999. Likely low income during the study period Geographical scope: urban Healthcare setting: home Mental health condition: schizophrenia Population 1. Age: none specified (mean age 42+ years old) 2. Gender: both 3. Socioeconomic background: not specified 4. Inclusion criteria a. Fulfilling Chinese classification of mental disorders (CCMD)‐2 and ICD‐10 criteria for schizophrenia b. Living in the community c. In remission d. No or reduced symptoms e. BPRS ≤ 30 f. Social function normal or mildly reduced g. Generally in good condition h. Carer is first‐ or second‐degree relative who lives with patient 5. Exclusion criteria a. Severe organic brain disease or physical disease b. Drug addiction c. Alcoholism |
|
| Interventions |
Stated purpose: to determine the effectiveness of family intervention for schizophrenic individuals in remission in the community INTERVENTION (n = 60) Name: family intervention Delivered by: PHP Title/name of PW and number: no details available; likely to be a professional, as in China no LHWs in primary care 1. Selection: not specified 2. Educational background: not specified 3. Training: 2 months followed by 1 week every 6 months, by psychiatrists 4. Supervision: not specified Intervention details: family intervention 1. Duration/frequency: 6 months, consisting of group family education with weekly lectures for 5 weeks, intensive intervention for 10 weeks consisting of 1‐ to 2‐weekly home visits, maintenance intervention with 4‐weekly home visits for 10 weeks, and 2 multi‐family sessions during the treatment course 2. Content: based on principles of “Crucial family mediating mechanism” and cognitive‐behavioural therapy, group education covered normal and diseased mental state, symptoms and causes of schizophrenia, pharmacotherapy (antipsychotics), psychotherapy, medication adverse effects and management, prevention of relapse, reproductive and marriage issues, active survival and recovery, intensive intervention focused on communication, recognition of medication adverse effects, symptoms and management of relapse, problem‐solving skills, and maintenance phase focusing on addressing queries and easing the subject out of the intervention. Multi‐family session attendees (subjects and family carers) discussed their successes, experiences, and problems, and built a supportive network amongst themselves CONTROL (n = 60) Usual care in community (presumed as psychiatric follow‐up) CO‐INTERVENTIONS: participants in both groups in principle maintained their original psychiatric medication |
|
| Outcomes |
Patients 1. BPRS: Total, Depression‐Anxiety, Anergia, Thought disorder, Activity, Hostility‐Suspicion 2. PSE: Total, SDSS; Total, SCL‐90; Total, Disease Status (improvement, stable, worsened) 3. Relapse in past 6 months 4. Occupation function 5. Initiative at work 6. Attitude towards medication# 7. Medication adherence# 8. Medication refusal/refusal to report# Carers Nil Process/health workers 1. Attendance by patients and family members# 2. Assessment of patients’ and carers’ understanding# Economic outcomes Nil (asterisk for study's primary outcomes; star: outcomes that we have not reported in this review) Time points: 0 and 6 months post intervention |
|
| Notes | Article in Chinese Source of funding: not specified Notes on validation of instruments (screening and outcomes): BPRS, PSE, and SDSS validated Additional information: declarations of interest ‐ none Handling the data: nil Prospective trial registration number: nil |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: 120 patients who met inclusion/exclusion criteria and agreed to study were randomised, but randomisation method was not described |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: unit of allocation was patient at start of the study, but method of allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Patients were not blinded; unclear how this may have influenced outcomes |
| Blinding of outcome assessment (detection bias) all outcomes | High risk | There is no specification whether assessors were blinded or not |
| Baseline outcome measurements similar | Low risk | Total BPRS score, BPRS: Depression‐anxiety score, BPRS: Apathy score, BPRS: Thought disorder score, BPRS: Activation score, BPRS: Hostility score, total PSE score, total SDSS score, total SCL‐90 score similar in the 2 groups |
| Baseline characteristics similar? | Low risk | In the 2 groups, all baseline characteristics ‐ age, age at onset of disease, duration of disease, gender, marital status, educational level, occupation ‐ were similar |
| Incomplete outcome data (attrition bias) Efficacy data | Low risk | No attrition |
| Incomplete outcome data (attrition bias) Safety data (e.g. adverse events) | Low risk | No attrition |
| Protection against contamination | Low risk | Intervention comprised group education and home visits. Contamination between groups was unlikely |
| Selective reporting (reporting bias) | Unclear risk | No clinical trial protocol is available. All outcomes planned in methods section were reported in results section |
| Other bias | Low risk | No other sources of bias have been found |
Lund 2020.
| Study characteristics | ||
| Methods |
Study design: RCT Duration of study: 1 year 9 months (recruited at first antenatal visit, last assessment at 12 months postpartum). Recruitment October 2013 to October 2014. Last follow‐up May 2016 |
|
| Participants |
Country: South Africa Income classification: upper‐middle income between 2013 and 2016 Geographical scope: peri‐urban settlement of Khayelitsha in Cape Town, South Africa ‐ an area marked by high HIV prevalence, high levels of poverty, and unemployment Healthcare setting: PC facility: antenatal clinics Mental health condition: perinatal depression Population (mention whether patient, carer, or dyad) 1. Age: 18+ years 2. Gender: female 3. Socioeconomic background: relatively equal numbers in all categories of economic status. Majority had studied from grade 0 to 11 (> 56%). Over 44% were employed 4. Inclusion criteria (including threshold cutoff score of measurement tool) a. Women recruited during their first antenatal visit b. Eligible if 18 years of age or older c. Spoke isiXhosa d. Resident in Khayelitsha e. No more than 28 weeks' pregnant f. Did not require urgent medical or psychiatric attention g. Able to give informed consent h. EDPS score ≥ 13 5. Exclusion criteria a. Not pregnant b. Not isiXhosa‐speaking c. Women with diagnosis of schizophrenia or bipolar disorder |
|
| Interventions |
Stated purpose: to determine the effectiveness of a task‐sharing psychological treatment for perinatal depression using non‐specialist community health workers (CHWs) in South Africa. Secondary objectives were to assess predictors of response to, and cost‐effectiveness of, task‐shared psychological treatment INTERVENTION (n = 148) Name: structured 6‐session psychological treatment Delivered by: LHW Title/name of PW and number: CHWs ‐ 6 1. Selection: recruited from local non‐governmental organisation (NGO) and worked full‐time on the study 2. Educational background: not specified 3. Training (contents, duration, by whom): CHWs received 5 days of training by a clinical social worker in basic counselling and delivery of the intervention 4. Supervision: CHWs received weekly group‐based supervision from the clinical social worker. A fidelity checklist was developed by the trial team and included 10 items, divided into 3 sections: (i) introduction to each session, (ii) exploration of the topic of each session, and (iii) ending. Each item on the checklist was scored by a 3‐tiered scoring system: “not done” = 0, “needs improvement” = 1, “well done” = 2 Incentives/remuneration: not specified Intervention details (according to PHWs/CWs and whether aimed at carers and/or patients) 1. Duration/frequency: intended duration of sessions was between 45 and 60 minutes 2. Content of intervention (by types of health workers and per patients/carers): sessions included psychoeducation, problem‐solving, behavioural activation, healthy thinking, relaxation training, and birth preparation. Content of the counselling manual included specific idioms of distress that had been identified in the formative research (mentioned above). At each session, participants' health and suicidal risk were assessed with the use of a checklist. Sessions were provided in addition to routine antenatal health care provided by the clinic CONTROL (n = 187) Participants allocated to control arm received enhanced usual care (EUC), which involved monthly phone calls for 3 months, in addition to routine antenatal health care provided by the clinic. Phone calls followed a set protocol with use of a checklist, which included items such as participant health, major life changes, mental health support received, and experience of depressive symptoms or suicidal ideation. Two CHWs recruited from another NGO were trained to conduct phone calls but were not trained in any counselling techniques used in the intervention arm CO‐INTERVENTIONS: none |
|
| Outcomes |
Patients 1. HDRS 2. EDPS 3. WHODAS 2.0 4. AUDIT 5. MINI‐Diagnosis 6. MINI‐Suicide Risk 7. Functional assessment instrument 8. MSPSS, response* 9. Recovery 10. Adverse events Carers None Process/health workers Number of sessions attended Economic outcomes 1. Service utilisation patterns 2. Unit costs 3. Health system costs 4. Patient costs 5. Total costs 6. Incremental cost‐effectiveness ratios (Supplementary Tables 6, 7, and 8) (asterisk for study's primary outcomes; star: outcomes that we have not reported in this review) Time points post intervention: baseline, post intervention (3 months post baseline) |
|
| Notes |
Source of funding: National Institute of Mental Health of the National Institutes of Health Notes on validation of instruments (screening and outcomes): validated instruments. Additional information: declarations of interest ‐ study authors declared no conflicts of interest Handling the data: as per footnotes in data and analysis Prospective trial registration number: Clinical Trials (ClinicalTrials.gov): NCT01977326, registered on 24/10/2013; Pan African Clinical Trials Registry (www.pactr.org): PACTR201403000676264, registered on 11/10/2013 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: protocol: a random number list will be generated by the data management system with individual numbers automatically allocated to each participant Individual RCT: randomisation was conducted with a computer‐generated random number sequence stratified by clinic of recruitment in blocks of 60 (30 control, 30 intervention) |
| Allocation concealment (selection bias) | Low risk | Quote: "ally allocated to each participant; "investigators and staff do not have access to this list or allocation system"; "after allocation by the system" Quote: "after allocation by the system, a text message will be sent to one of the six intervention arm CHW counsellors (for those women allocated to the intervention) using a round robin approach for the six CHW counsellors, or to the two control CHWs conducting the phone calls for the control (enhanced usual care) arm. When the fieldworker completes the baseline assessment, she will inform the participant that they will either receive an appointment to meet the CHW for an initial counselling session at the clinic, or that they will receive a phone call from a CHW to check on their progress. Further descriptions of the intervention and enhanced usual care conditions are provided below" Judgement comment: blinding of researchers and fieldworkers |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Individual RCT: fieldwork supervisor, counselling trainer/supervisor, and CHWs were the only team members who were unblinded to arm allocation. Investigators were blinded to allocation arm until completion of final follow‐up assessments, finalisation of data analysis plan, and lockdown of data assessed blindly |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | Individual RCT: fieldwork supervisor, counselling trainer/supervisor, and CHWs were the only team members who were unblinded to arm allocation. Investigators were blinded to allocation arm until completion of final follow‐up assessments, finalisation of data analysis plan, and lockdown of data assessed blindly |
| Baseline outcome measurements similar | Low risk | Individual RCT: baseline outcome measurements were similar ‐ Table 1 |
| Baseline characteristics similar? | Low risk | Individual RCT: no differences were noted between the 2 arms in baseline demographic, socioeconomic, or clinical measures at either recruitment site; baseline characteristics were similar (table 1, Lund 2020) |
| Incomplete outcome data (attrition bias) Efficacy data | Low risk | "After randomisation, six participants (1.4%) were excluded from the study as they were found to not fit some inclusion criteria (not pregnant and not isiXhosa‐speaking). The allocated intervention and assessments were discontinued for a further 35 participants (8.2%) across both arms, due to miscarriage or baby death. The analysis was conducted among the remaining 384 participants recruited, referred to as the “modified intention‐to‐treat” population (200 and 184 participants in the control and intervention arms, respectively). These participants did not differ from those excluded from the analysis, besides reporting lower baseline levels of functioning (Supplementary Table 2)" Some attrition/loss to follow‐up: fairly low |
| Incomplete outcome data (attrition bias) Safety data (e.g. adverse events) | Low risk | Adverse event data were reported |
| Protection against contamination | Low risk | Quote: "CHWs involved in the study will be trained in the importance of adhering to the study protocol and random checks will be undertaken by the project manager within the experimental and control arms to minimize contamination" Judgement comment: minimal risk of contamination |
| Selective reporting (reporting bias) | Low risk | Outcomes mentioned in the protocol were reported |
| Other bias | Low risk | No additional risks of bias |
Malakouti 2015.
| Study characteristics | ||
| Methods |
Study design: RCT, single‐blind (outcome assessors were blinded to participant randomisation) Duration of study: 12 months. Participants were recruited between December 2007 and March 2008 and were followed up until 12 months post baseline |
|
| Participants |
Country: Iran Income classification: lower‐middle income between 2007 and 2008; upper‐middle income in 2009 Geographical scope: urban Healthcare setting: participants’ homes Mental health condition: schizophrenia or bipolar disorder Population (patient, carer) 1. Age: 18 and older 2. Gender: both 3. Socioeconomic background: more than 50% of participants were unemployed 4. Inclusion criteria Patients (1) Diagnosis of bipolar or schizophrenia spectrum disorder (2) Hospitalised at least twice in the past 2 years (3) Poor compliance 5. Exclusion criteria Patients (1) Acute phase of illness (2) Mental retardation (3) Organic brain problem (4) Addiction to psychoactive substances (5) Receiving the same services contemporarily from other sources |
|
| Interventions |
Stated purpose: to compare the effectiveness and cost‐effectiveness of home visit clinical case management services provided by trained nurses and general practitioners (GPs) compared to usual treatment INTERVENTION 1 (n = 46) Name: home visit by GP Delivered by: PHP Title/name of PW and number: general practitioners (doctors) ‐ 3 1. Selection: after training, written and oral evaluation was performed, and 3 out of 5 trainees were selected 2. Educational background: medical degree 3. Training: trained in multiple courses for 24 hours a. Manual provided b. Trainer not specified 4. Supervision: every 2 weeks for first 3 months, then once every month, by main investigator (psychiatrist) Intervention details: case management services and GP care 1. Duration/frequency: monthly home visits, 45 minutes per session, for 1 year 2. Content of intervention (by types of health workers and per patients/carers): check for symptoms and drug side effects, educate patients and family members, inject depot antipsychotic if prescribed, serve as point of contact for patients or family in case of emergency, inform psychiatrist or study supervisors when necessary or during emergency. Psychotherapeutic (new or existing) medicine could be prescribed INTERVENTION 2 (n = 52) Name: home visit by nurse Delivered by: nurse Title/name of PHW/CW and number: nurses ‐ 3 1. Selection: after training, written and oral evaluation was performed and 3 out of 6 trainees were selected 2. Educational background: Bachelor’s degree 3. Training: trained for 33 hours; trainer not specified 4. Supervision: every 2 weeks for first 3 months, then once every month, by main investigator (psychiatrist) Intervention details: case management 1. Duration/frequency: monthly home visits, 45 minutes per session, for 1 year 2. Content of intervention (by types of health workers and per patients/carers): check for symptoms and drug side effects, educate patients and family members, inject depot antipsychotic if prescribed, serve as point of contact for patients or family in case of emergency, inform psychiatrist or study supervisors when necessary or during emergency CONTROL (n = 54) Referred to outpatient clinic; during exacerbations, referred to psychiatric hospitals or wards for admission CO‐INTERVENTIONS: nil |
|
| Outcomes |
Patients 1. Young Mania Rating Scale 2. PANSS 3. Kohlman Evaluation of Living Skills (KELZ) 4. QALY (calculated from SF‐36) 5. Hospital admission during past 12 months 6. Client questionnaire satisfaction# Carers 1. GHQ‐28 2. Caregiver burden 3. Knowledge questionnaire# Process/health workers Nil Economic outcomes 1. Cost of home visit 2. Total costs of study participants 3. Cost‐effectiveness ratio 4. ICER (Table 4 and 7th page of article, first 2 paragraphs) 5. Mean difference in utility score# 6. Incremental mean cost# 7. Incremental effectiveness# (asterisk for study's primary outcomes; star: outcomes that we have not reported in this review) Time point post intervention: 0 months |
|
| Notes |
Source of funding: Shahid Beheshti University, Research of University of Welfare and Rehabilitation Sciences Notes on validation of instruments: validated Additional information: declarations of interest ‐ study authors declared no conflicts of interest Handling the data: nil Prospective trial registration number: IRCT138807251959N3 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "who attended selective hospitals according to the study criteria and were randomly allocated into three groups by block randomization method and using random..." |
| Allocation concealment (selection bias) | Low risk | Quote: "the study instruments were completed by trained psychiatric residents or clinical psychologists with master’s degree who were blind to the randomization of the participants before and after the intervention" Judgement comment: randomisation was performed at the start of the study and was concealed from outcome assessors |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding due to nature of the intervention; unlikely to have influenced outcomes |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | Study instruments were completed by trained psychiatric residents or clinical psychologists with master’s degree who were blind to randomisation of participants before and after the intervention |
| Baseline outcome measurements similar | Low risk | Baseline outcome measures were similar in all 3 groups |
| Baseline characteristics similar? | Low risk | The 3 groups were similar in all demographic features (Tables 1 and 2), but the educational level of participants in GP and nurse groups was higher than that of participants in the control group |
| Incomplete outcome data (attrition bias) Efficacy data | High risk | 20/66 = 30.3% in the GP group were lost to follow‐up; this may have impacted the results |
| Incomplete outcome data (attrition bias) Safety data (e.g. adverse events) | High risk | Significant rate of attrition in the GP group may have influenced hospital admission data |
| Protection against contamination | Low risk | Judgement comment: intervention was delivered at home. Control group received usual care; therefore unlikely to have communicated with nurses or GPs |
| Selective reporting (reporting bias) | Low risk | All outcomes described in trial registry (IRCT138807251959N3) are reported in the paper |
| Other bias | Low risk | No other risks of bias present |
Marais 2011.
| Study characteristics | ||
| Methods |
Study design: cluster‐RCT; allocation by clinic; no blinding of patients, interventionists, or assessors; 4 clinics in each arm (8 total) Duration of study: March 2007 to February 2008 |
|
| Participants |
Country: South Africa Income classification: upper‐middle income Geographical scope: rural Healthcare setting: 8 state health clinics Mental health condition: alcohol use disorder Population (mention whether patient, carer, or dyad) 1. Age: 15 and older 2. Gender: women 3. Socioeconomic background: majority were poor seasonal employees 4. Inclusion criteria (including threshold cutoff score of measurement tool) a. Adult pregnant women attending clinic for antenatal services 5. Exclusion criteria: none |
|
| Interventions |
Stated purpose: to determine the effects of a series of BIs on alcohol drinking behaviour of pregnant women in a high‐risk rural district in the Western Cape Province of South Africa INTERVENTION (n = 97) Name: brief intervention Delivered by: CP Title/name of PW and number: trained fieldworkers ‐ 2 1. Selection: previously trained in brief intervention 2. Educational background: 1 a social worker, 1 a social scientist; both are enrolled in MPhil in social science research 3. Training (contents, duration, by whom): not specified 4. Supervision: none Intervention details 1. Duration/frequency: 4 sessions: first lasting an hour and performed at baseline, second and third sessions lasting 20 minutes and performed at 41 and 47 days, and fourth and last sessions on 58th day, lasting an unspecified duration of time 2. Content of intervention: first session – questionnaire, AUDIT (Alcohol Use Disorders Identification Test), explaining the meaning of AUDIT results, BI with setting drinking goals, and making notes in a take‐home alcohol booklet. Second and third sessions ‐ BIs consisted of feedback on drinking behaviour, negotiations, goal‐setting, and reinforcement, followed by a questionnaire. Final session ‐ BI and feedback on drinking behaviour, questionnaire, AUDIT CONTROL (n = 82) Initial interview: questionnaire, AUDIT, take‐home alcohol booklet; second interview: AUDIT, questionnaire on changes in drinking behaviour CO‐INTERVENTIONS: usual antenatal care |
|
| Outcomes |
Patients Post‐intervention AUDIT scores (overall*, no drinking group, unconfirmed drinking group, and confirmed drinking group) Carers None Process/health workers Nnone Economic outcomes None (asterisk for study's primary outcomes; star: outcomes that we have not reported in this review) Time point post intervention: 0 days |
|
| Notes |
Source of funding: Western Cape Department of Social Development Notes on validation of instruments (screening and outcomes): validated Additional information (e.g. provided by authors, existence of a published study protocol): personal communication regarding background of fieldworkers. Declaration of interests ‐ none Handling the data: as per footnotes in data and analysis Prospective trial registration number: none Study also included in prevention review, as unsure about the population (roughly half the intervention group has mental distress or a mental disorder at baseline; thus the intervention may be a treatment, whereas for the other half, it could be a prevention strategy) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomisation of clinics was done by a statistician as independent researcher in the study" Judgement comment: 8 clinics in the area studied (Western Cape Province, South Africa) were randomised by a statistician independent from the rest of the study team |
| Allocation concealment (selection bias) | Low risk | Quote: "four clinics were randomised to each arm of the intervention" Judgement comment: allocation of clinics to intervention or control was performed at the beginning of the study by an independent statistician |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding was not performed |
| Blinding of outcome assessment (detection bias) all outcomes | High risk | Blinding was not performed |
| Baseline outcome measurements similar | Low risk | Page 469: "the baseline AUDIT score was not considered different for the analysed and total group (7.3 vs 6.9, Table 1)" |
| Baseline characteristics similar? | Unclear risk | Groups differed in proportion close to anybody with an alcohol problem, language group Afrikaans, coloured, employed, heard about FAS, and in relationship/not living with partner |
| Incomplete outcome data (attrition bias) Efficacy data | Low risk | Follow‐up rate for intervention group 99%. Follow‐up rate for control group 85% |
| Protection against contamination | Low risk | Judgement comment: randomisation by clinics |
| Selective reporting (reporting bias) | Unclear risk | No available protocol for comparison of intended and reported outcomes |
| Other bias | Low risk | No other risk of bias identified |
Matsuzaka 2017.
| Study characteristics | ||
| Methods |
Study design: RCT Duration of study: 1 May 2013 to 30 April 2015 |
|
| Participants |
Country: Brazil Income classification: upper‐middle income Geographical scope: RCT was conducted in the FHS Unidade Básica de Saúde Iaçapé, Sapopemba, District of São Paulo – Brazi Healthcare setting: PC facility (free standing) Mental health condition: depression Population: Brazilian primary care patients in Sao Paolo with major depressive disorder (MDD) diagnosed on MINI 1. Age: ≥ 18 years 2. Gender: both 3. Socioeconomic background: mean household monthly income (US$): mean (SD) 687.74 (603.79) 4. Inclusion criteria a. Aged 18 or older b. Positive screening for probable depressive disorder using the Zung Scale administered by community health workers, with scale score confirmed by research psychologists c. Diagnosis by a research psychologist of current MDD or dysthymia using the Mini‐International Neuropsychiatric Interview (MINI), a structured clinical diagnostic instrument based on the Diagnostic and Statistical Manual of Mental Disorders Fourth Edition 5. Exclusion criteria a. Ongoing treatment with antidepressants or psychotherapy b. Suicide risk evaluated by the MINI c. Current/previous episodes of mania, hypomania d. Current/previous psychotic symptoms e. Alcohol or psychoactive substance use disorder according to the MINI |
|
| Interventions |
Stated purpose: to evaluate the effectiveness of task shifting interpersonal counselling for depression INTERVENTION (n = 39) Name: interpersonal counselling (IPC) Delivered by: LHW Title/name of PW and number: community health workers ‐ *20 ‐ *20 were selected out of 42 who were trained 1. Selection: employed at the Health Unit bit in the region where the intervention was being delivered 2. Educational background: not mentioned 3. Training: 3‐day training to the 42 community health workers employed at the Health Unit, divided into 3 groups. Two of the study authors (CTM and RFB, interpersonal therapists) facilitated training using the Revised IPC Manual. Training included research ethics and confidentiality, depression education with interactive activities, and role‐playing of IPC techniques. Although all 42 community health workers had participated in the training, 20 were selected, according to motivation and empathy skills observed by facilitators 4. Supervision: supervised through the trial by the same trainers in 2 different groups at a 2‐hour‐long twice‐a‐month supervision meeting. Supervisors were also available by telephone, mobile messages, or email 5. Incentives/remuneration: these selected community health workers received monetary compensation for each session completed Intervention details 1. Duration/frequency: IPC comprised a 1‐hour session per week, with 3 to 4 sessions in total. Sessions were provided at the clinic or in household visits based on the individual’s preference 2. Content of intervention: IPC seeks to address patients’ current psychological and social problems within 4 interpersonal problem areas: prolonged grief, interpersonal disputes, role transitions, and interpersonal deficits CONTROL: enhanced treatment as usual (E‐TAU) (n = 40) Individuals randomised to E‐TAU were provided case management by off‐site research psychologists funded by the study who were not trained in IPC. Research psychologists reported cases to FHS and facilitated referrals to specialised mental healthcare centres within the public system, where IPC is not provided, to receive pharmacological or psychological treatment. The assigned research psychologist made 2 to 3 phone calls to the patient to check on referral status and to ensure follow‐up. We considered E‐TAU as received when a patient followed the task to complete the referral, even if there was a wait‐list for treatment CO‐INTERVENTIONS: nil |
|
| Outcomes |
Patients Screening for inclusion 1. Zung self‐rating depression scale. 2. Mini‐International Neuropsychiatric Interview (MINI) Outcome measures 1. Health Questionnaire 9‐item screen (PHQ‐9) 2. Hamilton Depression Rating Scale (HDRS‐17) 3. Self‐Reporting Questionnaire (SRQ‐20) 4. Clinical Global Impression instrument (CGI) 5. Full or partial remission of depressive disorder (full remission = HDRS‐17 score ≤ 8, partial remission = HDRS‐17 score ≤ 12) Carers Nil Process/health workers Nil Economic outcomes Nil Time points: baseline, 2 months after intervention |
|
| Notes |
Source of funding: Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, São Paulo Research Foundation); contract grant number: 2012/17485–4 (to Dr. Mello). CTM had scholarship funded by Conselho Nacional de Desenvolvimento Cientifico e Tecnologico (CNPq) and Instituto Lemann. EVH had scholarship funded by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Instituto Lemann. Salary support was provided for ANP and ACS by the US National Institute of Mental Health (K01 MH104514; T32‐MH19139) Notes on validation of instruments (screening and outcomes): validated Additional information: declarations of interest ‐ study authors declared no competing interests Handling the data: as per footnotes in data and analysis Prospective trial registration number: trial was registered at Brazilian Clinical Trials, number RBR‐5qhmb5 (http://www.ensaiosclinicos.gov.br/rg/RBR‐5qhmb5/). Registration date: 10 December 2014 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization (allocation ratio 1:1) was stratified by gender, age (17–34 vs. ≥35), and depression severity (Zung score 45–59 vs. ≥60). A statistician not involved in the recruitment process carried out the randomization using a computer algorithm based on Aitchison’s compositional distance" Judgement comment: random allocation using a computer programme |
| Allocation concealment (selection bias) | Unclear risk | Quote: "(Zung score 45–59 vs. ≥60)"; "a statistician not involved in the recruitment process carried out the randomization using a computer algorithm based on Aitchison’s compositional distance. Only the research assistant knew the group allocation"; "evaluators at follow‐up were..." Judgement comment: only the research assistant knew the group allocation. Evaluators at follow‐up were blinded to which intervention was received |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not blinded; however unlikely to have influenced outcomes |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | Blinding of "evaluators" who assessed outcomes at follow‐up |
| Baseline outcome measurements similar | Low risk | Several measures; most no difference between groups, except for higher percentage of MDD in IPC group. Unlikely to bias estimates |
| Baseline characteristics similar? | Low risk | Similar baseline characteristics (Table 1) |
| Incomplete outcome data (attrition bias) Efficacy data | Low risk | Higher percentage of people in E‐TAU group did not complete treatment, but few were lost to follow‐up and data were analysed using ITT approach, so low risk of bias. Post IPC follow‐up 90% (n = 39); post E‐TAU follow‐up 92% (n = 40) |
| Protection against contamination | Low risk | Judgement comment: IPC by community health workers; TAU by research psychologists |
| Selective reporting (reporting bias) | Unclear risk | Contacted study author: study authors retrospectively registered the wider study of which this trial was part, but not the trial itself. So there is no trial registration by which to check outcomes. No evidence of selective outcome reporting was found in the paper |
| Other bias | Low risk | No other risks of bias were identified |
Meffert 2014.
| Study characteristics | ||
| Methods |
Study design: pilot randomised controlled trial Duration of study: May 2008 through September 2008 |
|
| Participants |
Country: Egypt Income classification: lower‐middle income Geographical scope: urban Healthcare setting: community group ‐ Ma'an Organization office (Ma’an Organization was founded and is run by Sudanese. Its aim is to raise the health, social, and legal awareness of Sudanese refugees in Cairo through programmes that address youth, adolescents, men, and women) Mental health condition: PTSD Population 1. Age: > 18 years 2. Gender: both, but 81% women 3. Socioeconomic background: Sudanese refugees 4. Inclusion criteria a. Sudanese refugees living in Cairo b. HTQ (first 16 items = PTSD) score ≥ 2.3 5. Exclusion criteria a. Alcohol or drug dependence |
|
| Interventions |
Stated purpose: to evaluate the effectiveness of IPT twice a week for 3 weeks for Sudanese refugees living in Cairo vs a wait‐list control group using non‐specialists INTERVENTION (n = 11) Name: interpersonal therapy Delivered by: LHW Title/name of PW and number: community therapists ‐ 5 1. Selection: Sudanese community member, over the age of 18 years, fluency in oral and written English and Sudanese Arabic, and previous work with refugee populations. The first study author’s impressions of candidate therapists’ emotional intelligence, interpersonal skills, and interest in learning and applying psychotherapy for traumatized refugees were considered in the therapist selection process 2. Educational background: fluency in oral and written English and in Sudanese Arabic 3. Training (contents, duration, by whom): training by Ma'an Organisation (expertise in community training) and PIs 4. Supervision: formal group supervision of IPT cases occurred twice per week by first study author. Informal supervision occurred nearly daily through interactions related to screening of participants and administration of measures. Therapists were encouraged to contact the first study author by telephone with any questions or concerns between supervision times Intervention details (according to PHW/CWs and whether aimed at carers and/or patients) 1. Duration/frequency: 6 IPT sessions occurred twice per week. Intervention was 3 weeks long 2. Content of intervention: IPT foci were limited to interpersonal disputes, role transitions, or grief. The first 2 sessions were devoted to obtaining the “interpersonal inventory” ‐ an important starting point of IPT. Middle sessions worked through the identified focus (dispute, role transition, or grief) in the manner specified by IPT. Final sessions focused on emotional and interpersonal accomplishments during IPT treatment, as well as goals for the future CONTROL: usual care (n = 8) Individuals assigned to wait‐list condition were offered IPT treatment at the conclusion of therapy in the intervention group CO‐INTERVENTIONS: nil |
|
| Outcomes |
Patients 1. Harvard Trauma Questionnaire* 2. Beck's Depression Inventory – II* 3. STAXI (State‐Trait Anger Expression Inventory)° 4. Conflict Tactics Scale (CTS)° Carers None Process/health workers None Economic outcomes (and where these can be found, e.g. ref or table number) None (asterisk for study's primary outcomes; star: outcomes that we have not reported in this review) Time points post intervention: 0 (baseline), post intervention (3 weeks after baseline) |
|
| Notes |
Source of funding: University of California, San Francisco Academic Senate Research Grant Notes on validation of instruments (screening and outcomes): validated outcomes Additional information: declaration of interests ‐ study authors declared no conflicts of interest Handling the data: as per footnotes in data and analysis Prospective trial registration number: not specified |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "participants were randomly assigned IPT or the waitlist. Pure randomization was used with a random allocation sequence" Quote: "participants were randomly assigned to IPT or waitlist control groups using a computer‐generated random allocation sequence" Judgement comment: participants were randomly assigned using a computer programme |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: unclear whether unit of allocation was concealed from researchers |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "The administrators of the measurement were the future (or former) therapists of the participants. Therapists were not blind to group status. Intervention provider conducted assessments and therefore was not blind to allocation" |
| Blinding of outcome assessment (detection bias) all outcomes | High risk | Intervention provider conducted assessments and therefore was not blind to allocation |
| Baseline outcome measurements similar | Low risk | Participant outcomes were measured prior to the intervention; no important differences were present across study groups |
| Baseline characteristics similar? | Low risk | Similar baseline characteristics were reported |
| Incomplete outcome data (attrition bias) Efficacy data | Low risk | "13 IPT: 1 dropped out, 1 lost to follow‐up; 9 wait list control: 1 dropped out". Proportion of missing data was similar in intervention and control groups; therefore low risk of bias |
| Incomplete outcome data (attrition bias) Safety data (e.g. adverse events) | Low risk | "There were no adverse events" No missing data due to adverse events; therefore low risk |
| Protection against contamination | Unclear risk | Judgement comment: control group received intervention from therapists after intervention group received intervention. Unclear whether there was a prior level of communication between control and therapists |
| Selective reporting (reporting bias) | Unclear risk | All relevant outcomes in the methods section are reported in the results section. No published trial protocol is available |
| Other bias | Low risk | No evidence of other biases |
Mertens 2014.
| Study characteristics | ||
| Methods |
Study design: single‐blind parallel‐group randomised controlled clinical trial (not cluster) (outcome assessors were blinded) Duration of study: recruitment March 2008 to November 2008. Followed up to 3 months post baseline |
|
| Participants |
Country: South Africa Income classification: upper‐middle income Geographical scope (including if rural or urban): urban Healthcare setting: large public sector primary healthcare clinic Mental health condition: alcohol use disorder and/or substance abuse Population 1. Age: young adults 18 to 24 2. Gender: both 3. Socioeconomic background: high poverty and unemployment area. Almost three‐fourths of participants were unemployed. 16% had no access to piped water, 6% had no electricity in their homes, and 20% lived in shacks or traditional dwellings 4. Inclusion criteria (including threshold cutoff score of measurement tool) a. Patients who visited the clinic who screened positive for problematic alcohol use or substance abuse using a screening question for each 5. Exclusion criteria a. Too ill to participate b. Did not have a phone |
|
| Interventions |
Stated purpose: to determine whether brief motivational intervention by nurse practitioners was effective in improving alcohol and drug use outcomes INTERVENTION (n = 190) Name: brief motivational intervention Delivered by: PHP Title/name of PW and number: primary care nurse practitioners ‐ 3 1. Selection: not specified 2. Educational background: not specified 3. Training (contents, duration, by whom): 3‐day training in brief motivational intervention by experienced practitioner and trainer 4. Supervision: weekly for first 6 weeks, monthly thereafter by trainer Intervention details (according to PHWs/CWs and whether aimed at carers and/or patients) 1. Duration/frequency: 10‐minute (on average) single session, after which a referral resource list was given 2. Content of intervention (by types of health workers and per patients/carers): nurse practitioner‐delivered brief motivational intervention and resource list CONTROL: primary care and resource list only (n = 173) CO‐INTERVENTIONS: none |
|
| Outcomes |
Patients 1. Percent decrease in Total ASSIST score* 2. Total ASSIST score 3. Alcohol ASSIST score 4. Per cent decrease in Alcohol ASSIST score 5. Prevalence of at‐risk Alcohol ASSIST score 6. Odds of at‐risk alcohol use 7. Prevalence of heavy drinking 8. Odds of heavy drinking 9. Per cent decrease in Alcohol ASSIST score in men and women 10. Cannabis ASSIST score 11. Methamphetamine ASSIST score 12. Prevalence of at‐risk cannabis use 13. Prevalence of at‐risk methamphetamine use 14. Prevalence of at‐risk sedative use 15. Prevalence of at‐risk methaqualone use 16. Odds of at‐risk cannabis use 17. Odds of at‐risk methamphetamine use Carers None Process/health workers None Economic outcomes (and where these can be found, e.g. ref or table number) None (asterisk for study's primary outcomes; star: outcomes that we have not reported in this review) Time point post intervention: 3 months |
|
| Notes |
Source of funding: National Institute on Drug Abuse Notes on validation of instruments (screening and outcomes): ASSIST developed and validated in primary care clinics including in South Africa Additional information: declaration of interests ‐ no conflicts declared Handling the data (e.g. imputed values, other calculations we have made): nil Prospective trial registration number: nil |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: sealed envelopes were used, but how random sequence was generated is not described |
| Allocation concealment (selection bias) | Low risk | Quote: "information and follow‐up interview date"; "the study recruited 403 patients and implemented the following protocol: patients were interviewed by a research assistant about their demographics, alcohol and drug use and problems, and readiness to change alcohol and drug use (see Measures below). Following the interview, research assistants opened a sealed envelope which contained the randomization result for the patient assigning them to either nurse practitioner‐delivered Brief Motivational Intervention plus a brief intervention for young adult" Judgement comment: sealed envelopes were used and were opened following the screening interview |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not blinded; unclear how this may have influenced outcomes |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | This was a single‐blind, parallel‐group RCT. Research interviewers conducting FU were blinded to randomisation status. Assessors were blinded to patient allocation |
| Baseline outcome measurements similar | Low risk | Table 1 ‐ no significant differences for prevalence of at‐risk use, baseline ASSIST scores, or SOCRATES raw score |
| Baseline characteristics similar? | Low risk | Baseline characteristics were similar in both groups (age, gender, race/ethnicity, education, employment, marital status, socioeconomic status, religious activities, children, language). Only religion was significantly different between the 2 groups |
| Incomplete outcome data (attrition bias) Efficacy data | Unclear risk | 16 patients from the intervention group (16/206, 7%) and 24 patients from the control group (24/197, 12%) were not contactable for follow‐up assessment and were excluded from analysis. This may have had an impact on the results, had they not dropped out of the study |
| Protection against contamination | Unclear risk | Quote: "setting is a large public‐sector primary health care clinic" Judgement comment: setting in 1 clinic, but unlikely control group will have received a BI session |
| Selective reporting (reporting bias) | Unclear risk | No clinical trial protocol was posted. In the methods section, ASSIST was stated as the primary outcome measure. Results of ASSIST tests were reported. SOCRATES score was assessed at baseline but not at follow‐up |
| Other bias | Unclear risk | Initial assessment was long, and we have no minimal assessment group to examine effects of assessment reactivity. This can result in decreases in reported substance use and problems in both intervention and control groups |
Milani 2015.
| Study characteristics | ||
| Methods |
Study design: RCT Duration of study: 6 weeks. Recruitment April 2012 to September 2012. Followed up until 6 weeks after baseline |
|
| Participants |
Country: Iran Income classification: upper‐middle income Geographical scope: urban Healthcare setting: delivered at home using telephone Mental health condition: perinatal disorders Population 1. Age: unknown 2. Gender: female 3. Socioeconomic background: more than 86% were housewives 4. Inclusion criteria (including threshold cutoff score of measurement tool) a. Term pregnancy b. Live birth c. Depression score (Edinburgh Postnatal Depression Scale (EPDS score)) > 10 to < 14 5. Exclusion criteria a. History of mental disorder b. Episodes of mental disorders during pregnancy or in the last 12 months that necessitated use of medicines c. Non‐viable foetus d. History of PPD e. Current use of prescribed psychiatric drugs f. EPDS score ≥ 14 |
|
| Interventions |
Stated purpose: to study the effects of health volunteers' (women who interacted with families in primary health care (PHC) and acted as bridges between health centres and the community in Iran) telephone‐based support on postpartum depression INTERVENTION: psychoeducation (n = 22) Name: psychoeducation Delivered by: LHW Title/name of PW and number: health volunteers 1. Selection: women 2. Educational background: grassroots community members with high potential for communication 3. Training (contents, duration, by whom): trained at a workshop to enable them to communicate effectively and accurately with mothers and to manage their problems. Volunteers managed the problem according to their training; if needed, assistance was given by a principal researcher 4. Supervision: principal researcher gave assistance if needed 5. Incentives/ remuneration: no information provided Intervention details (according to PHW/CWs and whether aimed at carers and/or patients) 1. Duration/frequency: each trained volunteer called 3 to 4 mothers at intervals of 2 to 3 times per week until 6 weeks after childbirth 2. Content of intervention (by types of health workers and per patients/carers): during each phone call, after a greeting, they asked the mother about her health status, newborn’s condition, complaints, and the mother’s relationship with her newborn or husband, and whether there was any problem CONTROL: usual care (n = 24) CO‐INTERVENTIONS: routine postpartum care |
|
| Outcomes |
Patients: Edinburgh Postnatal Depression Scale (EPDS) Carers: None Process/health workers: None Economic outcomes: None Time points post‐intervention: baseline, 1 month |
|
| Notes |
Source of funding: none Notes on validation of instruments (screening and outcomes): all instruments were validated Additional information: declarations of interest ‐ study authors declared no conflicts of interest Handling the data: as per footnotes in data and analysis Prospective trial registration number: Iranian Registry of Clinical Trials (IRCT) registration ID: IRCT201202159027N1 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "these cases were recruited and randomly assigned into the intervention and control groups" Judgement comment: not specified what method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not specified how allocation concealment is maintained |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Non‐blinding of participants was unlikely to affect outcomes |
| Blinding of outcome assessment (detection bias) all outcomes | High risk | Staff doing the intervention also performed outcomes assessment |
| Baseline outcome measurements similar | Low risk | Appropriate analysis was performed, and no important differences were present across study groups (Table 3) |
| Baseline characteristics similar? | Low risk | All baseline characteristics were similar (see P values) |
| Incomplete outcome data (attrition bias) Efficacy data | Unclear risk | 5/27 (i.e. 18%) and 3/27 (11%) dropout rates in I and C arms, respectively. As small numbers then, this dropout may affect results, so marked as unclear |
| Protection against contamination | Low risk | Judgement comment: individual allocation |
| Selective reporting (reporting bias) | Unclear risk | No access to protocol, so unclear |
| Other bias | Low risk | No other sources of bias were found |
Momeni 2016.
| Study characteristics | ||
| Methods |
Study design: RCT Duration of study: 2014 |
|
| Participants |
Country: Iran Income classification: upper‐middle income Geographical scope: urban, District 3 of the city of Kermanshah Healthcare setting: school Mental health condition: child mental disorders Population: hyperactive‐aggressive primary school students (60 eligible, 24 sampled and randomised) 1. Age: primary school; aged 8 to12 years 2. Gender: male 3. Socioeconomic background: low‐income areas 4. Inclusion criteria a. ADHD based on Connors Teacher Rate Scale b. Diagnosis by a psychiatrist c. Parental consent d. Participation in classes (it is implied that these are school classes, but not explicitly mentioned) for at least 4 months e. Hyperactivity/aggression based on Overt and Relational Aggression Questionnaire of School Children 5. Exclusion criteria a. Concurrent intervention |
|
| Interventions |
Stated purpose: to examine the effectiveness of group drawing on aggression reduction of boy students with attention deficit/hyperactivity disorder (ADHD) INTERVENTION (n = 12) Name: group drawing Delivered by: CP Title/name PW and number: teachers 1. Selection: not mentioned 2. Educational background: not mentioned 3. Training: no mention of training for the intervention. Teachers were trained on how to properly fill in questionnaires 4. Supervision: not mentioned 5. Incentives/remuneration: not mentioned Intervention details 1. Duration/frequency: nine 45‐ to 60‐minute sessions held twice a week 2. Content of intervention: intervention was based on Martin's protocol (2009), where in the first session, 1 student held a torch, another made a shadow, and a third drew the shadow; in the second session, students lay down and took turns tracing each other's outlines; in the third session, students drew a selected topic; in the fourth, student pairs drew each other's faces; in the fifth, co‐operative face drawing as a group; in the sixth, 1 student posed as a model for the rest of the group to draw; in the seventh, students each decorated a square of fabric (these were later joined into a quilt); in the eighth, each student drew on 1 side of a box something related to the person whom the box belongs to; in the ninth, all artwork was displayed CONTROL: usual care (n = 12) CO‐INTERVENTIONS: nil |
|
| Outcomes |
Patients 1. Connors Questionnaire for Teachers (Connors, 1997) 2. Relational and Overt Aggression Questionnaire for primary school children Carers Nil Process/health workers Nil Economic outcomes Nil Time points: baseline, post intervention (time not specified) |
|
| Notes |
Source of funding: not mentioned. Notes on validation of instruments (screening and outcomes): validated Additional information: declarations of interest ‐ none Handling the data: as per footnotes in data and analysis Prospective trial registration number: none recorded |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: 5 schools were selected from Zone 3 of the city using multi‐step sampling. The Connors Teacher Rate Scale was used to identify students who were eligible for inclusion. 60 were identified; 24 were selected and randomly put into the control or the intervention group; no indication of randomisation process |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Teachers are measuring the impact with tools, as well as delivering the intervention; no blinding |
| Blinding of outcome assessment (detection bias) all outcomes | High risk | Teachers are measuring the impact with tools, as well as delivering the intervention; no blinding |
| Baseline outcome measurements similar | Low risk | Outcomes were measured prior to the intervention in both control and intervention groups (mean and SD, Table 2). Baseline outcome measurements are similar |
| Baseline characteristics similar? | Unclear risk | No data are provided on baseline characteristics of participants in control and intervention groups |
| Incomplete outcome data (attrition bias) Efficacy data | Unclear risk | No mention is made of dropouts or incomplete follow‐up. 100% follow‐up is not explicitly mentioned in the text |
| Protection against contamination | Unclear risk | Judgement comment: allocation is not based by institution, but by intervention is to individuals. There is unlikely contamination, as the intervention itself is drawing. Randomisation appears to have been of patients, not of professionals |
| Selective reporting (reporting bias) | Unclear risk | No protocol is available, so unable to judge this |
| Other bias | Unclear risk | There is little information on which to assess risk of bias, but as numbers are low, this could affect the reliability of outcomes (only 32 people randomised to the study) |
Murphy 2020.
| Study characteristics | ||
| Methods |
Study design: modified stepped‐wedge cluster‐randomised controlled trial. Unit of allocation: commune; 16 in each arm (32 in total) Duration of study: data collection took place between July 2016 and November 2017 |
|
| Participants |
Country: Vietnam Income classification: lower‐middle income Geographical scope: community and primary care settings at 32 communes in 8 provinces in Vietnam Healthcare setting: intervention delivered in participants' homes Mental health condition: depression Population: Vietnamese primary care patients with depression (SRQ > 7) recruited from primary care and from the community 1. Age: adults aged ≥ 18 years 2. Gender: both 3. Socioeconomic background: middle and lower socioeconomic strata 4. Inclusion criteria a. Score > 7 on SRQ‐20, indicating depression b. Consent to participate and complete all measures 5. Exclusion criteria a. Cognitive disturbance b. Psychotic symptoms |
|
| Interventions |
Stated purpose: to test the effectiveness of a supported self‐management (SSM) intervention to reduce symptoms of depression among adults compared with enhanced treatment as usual in community‐based and primary care settings in Vietnam INTERVENTION (n = 190) Name: supported self‐management intervention Delivered by: LHW Title/name of PW and number: social collaborators ‐ lay social workers based in the community to support families and provide services 1. Selection: social collaborators may be recruited to their role due to existing community involvement and leadership (e.g. as village care workers, Red Cross volunteers, Women’s Union staff) 2. Educational background: varied 3. Training: social collaborators received 3 days of training by the study team, a psychiatrist from the provincial psychiatric hospital, and district‐level representatives from both health and social services sectors. Training components included screening for depression using the SRQ‐20 and in delivery of the coaching intervention 4. Supervision: during the 2‐month course of the intervention, each social collaborator received 2 visits from a provincial‐level social worker during the coaching session with patients to provide supervision and support and to assess fidelity 5. Incentives/remuneration: social collaborators do not receive a monthly salary but may be provided a stipend for specific tasks, including screening for depression and delivering SSM Intervention details 1. Duration/frequency: 2 months 2. Content of intervention: supported self‐management intervention consists of bibliotherapy (Antidepressant Skills Workshop) that is provided to a patient by a health or social worker. Patients are supported over the course of 2 months in the use of skills found in the workbook. Skills in the workbook are based on the principles of cognitive‐behavioural therapy. As part of the SSM intervention, social collaborators provided one‐on‐one coaching on use of the ASW at the homes of participants over the course of 2 months. Six to ten social collaborators per commune delivered the intervention, with numbers varying by commune size. Coaching sessions took place every 2 weeks, during which the social collaborator consulted with the patient on progress, reviewed concepts in the ASW, and helped to create a plan for the subsequent 2 week period CONTROL (n = 185) Treatment as usual (TAU), which consists of a leaflet with information about depression and regular care as provided by primary care centres. Control arm will receive the intervention after the intervention arm has completed the intervention period |
|
| Outcomes |
Patients Primary outcome measures 1. Self‐Reporting Questionnaire 20 (SRQ‐20) continuous 2. SRQ‐20>7 Secondary outcome measures World Health Organization Disability Assessment Scale (WHODAS 2.0) Carers None Process/health workers None Economic outcomes None Time points: baseline, 2 months |
|
| Notes |
Source of funding: Grand Challenges Canada (Grant no. 0762‐05) with matched funds provided by MOLISA Notes on validation of instruments (screening and outcomes): all validated Additional information: declarations of interest ‐ "NVH is a representative of MOLISA, which provided matched funding for the study. He did not influence the study design, data collection or interpretation of results. All other authors have no conflict to declare" Handling the data: as per footnotes in data and analysis Prospective trial registration number: NCT03001063 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation sequence will be developed and controlled by an individual (CHG at Simon Fraser University) not otherwise involved in the study to ensure fidelity |
| Allocation concealment (selection bias) | Low risk | Judgement comment: randomisation is done in permuted blocks to conceal allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study was cluster‐randomised, but non‐blinding of participants was unlikely to affect outcomes |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | Health workers do baseline assessment, but follow‐up is done by outcome assessors |
| Baseline outcome measurements similar | Low risk | Similar baseline outcome measures (table 4, Murphy 2020) |
| Baseline characteristics similar? | Low risk | Baseline characteristics were well balanced in the 2 randomised groups (Table 4). Chi² tests for categorical variables and 2‐sample t‐tests were used to compare the distributions of baseline variables between the 2 randomisation groups; no group difference was found to be statistically significant at the 0.05 level for any baseline variables listed in Table 4 |
| Incomplete outcome data (attrition bias) Efficacy data | Low risk | 10% loss to follow‐up in intervention group and 28% in control group. mainly due to people not wanting to participate. Not sure about their characteristics or whether likely to affect results. Fairly low dropout rate, so overall unlikely |
| Incomplete outcome data (attrition bias) Safety data (e.g. adverse events) | Low risk | Committee met 3 times during the trial and identified no concerns regarding safety or adverse events. No adverse events were reported |
| Protection against contamination | Low risk | Judgement comment: "as randomization occurred at the commune level, participants in the immediate intervention and control (delayed intervention) groups accessed services in different locations, minimizing the risk of contamination" (Murphy 2020) Low risk of contamination |
| Selective reporting (reporting bias) | Low risk | Same outcomes in protocol and in trial results |
| Other bias | Low risk | No other risk of bias |
Murray 2015.
| Study characteristics | ||
| Methods |
Study design: RCT; unit of allocation: individual Duration of study: August 2012 to December 2013 |
|
| Participants |
Country: Zambia Income classification: lower‐middle income Geographical scope: urban; Lusaka, Zambia Healthcare setting: the 5 study sites included a home‐based care programme, a programme for street children, a government health clinic, a public school, and a school or residential programme Mental health condition: PTSD Population (mention whether patient, carer, or dyad) 1. Age: 5 to 18 years 2. Gender: both male and female 3. Socioeconomic background: not specified 4. Inclusion criteria (including threshold cutoff score of measurement tool) a. Age of 5 through 18 years b. Living within a site catchment area c. History of at least 1 traumatic event d. Significant trauma‐related symptoms (mean item score ≥ 1.0 on the locally validated UCLA Posttraumatic Stress Disorder Reaction Index (PTSD‐RI)) 5. Exclusion criteria a. Child or legal caregiver not mentally competent to give consent or currently receiving psychiatric treatment |
|
| Interventions |
Stated purpose: to evaluate the effectiveness of lay counsellor–provided trauma‐focused cognitive‐behavioural therapy (TF‐CBT) to address trauma and stress‐related symptoms among OVC in Lusaka, Zambia INTERVENTION (n = 131) Name: TF‐CBT Delivered by: LHW Title/name of PW and number: adult counsellors ‐ 20 1. Selection: not specified 2. Educational background: at least a high school education and basic communication and social skills 3. Training (contents, duration, by whom): via the apprenticeship model: a 10‐day on‐site training of counsellors and supervisors, followed by weekly meetings of local supervisors with groups of counsellors and weekly supervisor consultation with TF‐CBT experts 4. Supervision: counsellors documented how they provided each component according to specific steps detailed in the manual. Supervisors elicited (from counsellors) and recorded session details (techniques used and homework assigned) during weekly supervision meetings. If a component (e.g. relaxation) or a component step (e.g. assigning homework) was missed, supervisor requested completion in the next session. Local supervisor and counsellor discussed and/or role‐played the component and planning for the next session. A TF‐CBT expert (L.K.M., S.S., or S.D.) recorded detailed notes from supervisors’ weekly verbal reports, checking that all TF‐CBT components were provided with proper technique or, if not, asked for those components to be provided again Intervention details: trauma‐focused CBT aimed at patients 1. Duration/frequency: TF‐CBT is typically conducted in weekly 60‐ to 90‐ minute sessions with the child and caregiver (if available) and involves provision of 9 components. 2. Content of intervention: components: psychoeducation, programme information (duration, content, expectations), normalisation of symptoms and problems, parenting skills, praise, rewards, one‐on‐one time. Relaxation: strategies to reduce physiological tension and stress. Affective modulation: identifying feelings, linking them to situations, rating intensity of emotions. Cognitive coping: distinguishing and connecting thoughts, feelings, and behaviours, evaluating and restructuring thoughts to be more accurate and/or helpful. Trauma narrative (imaginal gradual exposure): facing feared and/or avoided traumatic memories through writing or drawing, identifying related thoughts and feelings, restructuring unhelpful thoughts from the trauma narrative. In vivo exposure: facing innocuous triggers or reminders in the client’s environment. Conjoint session: sharing the trauma narrative and restructured thoughts between child and supportive caregiver (if available). Enhancing safety skills: developing a safety plan linked to the trauma experience and possible future challenges CONTROL: enhanced usual care (n = 126) Barefeet (community outreach) psychosocial counselling: peer education, prevention of HIV and AIDS. City of Hope (community school or residence): education and assistance (school fees), nutrition, room and board for adolescent girls. Kaunda Square Ministry of Health Clinic: primary healthcare services. St Paul’s School Education: psychosocial and guidance counselling, Ngombe home‐based care, medical support and referrals, support groups, community outreach CO‐INTERVENTIONS: none |
|
| Outcomes |
Patients 1. Post‐traumatic stress disorder reaction index* (PTSD‐RI) 2. Functional Impairment* Carers Caretakers of child participants were asked to complete the Child Behavior Checklist Process/health workers None Economic outcomes None (asterisk for study's primary outcomes; star: outcomes that we have not reported in this review) Time points post intervention: baseline, within 1 month (intervention), 4 months (control) |
|
| Notes |
Source of funding: grant GHS‐A‐00‐09‐00004 Mod 6 from US Agency for International Development Displaced Children's and Orphans Fund Notes on validation of instruments (screening and outcomes): all validated and translated into 3 Zambian languages Additional information: study protocol in supplementary materials; declarations of interest ‐ none Handling the data (e.g. imputed values, other calculations we have made): none Prospective trial registration number: NCT01624298 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Completion of the trial. Randomisation was conducted immediately following completion of baseline assessment. Study assessor opened a sealed envelope that was attached to the consent form only after completion of assessment |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: this was a single‐blind study. At post assessment, assessors were masked; however, participants were aware of their own study status and could have divulged this or been biased based on expectations (see Murray 2015) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not possible to blind participants and personnel to the intervention; unlikely to affect outcomes |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | Post assessments were completed within 1 month after TF‐CBT completion (for TAU participants, approximately 4 months after baseline). To ensure masked assessment, assessors from a site other than where the participant received services completed the post assessment |
| Baseline outcome measurements similar | Low risk | The only variable that appeared different between groups was length of time between baseline and post assessments, which was longer on average for the TF‐CBT group (150.83 days) compared with the TAU group (116.03 days). Otherwise, baseline characteristics were similar between groups (Table 1). Mean baseline trauma symptom scores (1.88 in TF‐CBT group and 1.75 in TAU group) and functional impairment scores (0.85 in TF‐CBT group and 0.79 in TAU group) were comparable between groups (From Murray 2015) |
| Baseline characteristics similar? | Low risk | The only variable that appeared different between groups was length of time between baseline and post assessments, which was longer on average for the TF‐CBT group (150.83 days) compared with the TAU group (116.03 days). Otherwise, baseline characteristics were similar between groups (Table 1). Mean baseline trauma symptom scores (1.88 in TF‐CBT group and 1.75 in TAU group) and functional impairment scores (0.85 in TF‐CBT group and 0.79 in TAU group) were comparable between groups. Mean number of trauma events reported was 5.07; Figure 2 summarises the types. See Table 1 and Figure 2 in Murray 2015 |
| Incomplete outcome data (attrition bias) Efficacy data | Unclear risk | Proportion of missing data was similar in intervention and control groups (25 vs 22 lost to follow‐up or refused post assessment). However, see Figure 1 in Murray 2015 ‐ 30 (TF‐CBT) and 20 (usual care) participants lost to follow‐up |
| Protection against contamination | Unclear risk | Judgement comment: individual randomisation |
| Selective reporting (reporting bias) | Low risk | (NvG added on 6/5/20) No reporting of ASSIST scores or HIV risk scores; explanation satisfactory; inconsistent responses and small numbers, so no additional analysis performed |
| Other bias | Low risk | No other sources of bias were found |
Nadkarni 2017.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Duration of study: participants were recruited between October 2013 and July 2015, and data were locked in September 2016 |
|
| Participants |
Country: India Income classification: lower‐middle income Geographical scope: urban Healthcare setting: 10 primary health centres (PHCs) Mental health condition: alcohol use disorder Population 1. Age: 18 to 65 years 2. Gender: male 3. Socioeconomic background: about 70% worked as unskilled manual labour 4. Inclusion criteria a. Male primary care attendees found to have harmful drinking upon screening with AUDIT (12 to 19) 5. Exclusion criteria a. Needed emergency medical treatment or inpatient admission b. Unable to communicate clearly c. Intoxicated at time of screening |
|
| Interventions |
Stated purpose: to assess the effectiveness and cost‐effectiveness of CAP treatment when used in primary care INTERVENTION (n = 188) Name: counselling for alcohol problems (CAP) Delivered by: LHW Title/name of PW and number: lay counsellors ‐ 11 1. Selection: no professional training in mental health, fluent in vernacular languages used in study settings. Applicants first needed to first pass an interview involving role‐plays, then had to undergo a 2‐week classroom training and pass the competency assessment, then had to complete a 6‐month internship followed by a multiple choice examination and a skills test involving role‐plays with standardised vignettes and quality ratings of actual CAP sessions delivered 2. Educational background: at least secondary school education 3. Training (contents, duration, by whom): intensive 2‐week classroom training and 6‐month internship with supervision of cases by experts 4. Supervision: weekly peer‐led supervision and twice‐monthly individual supervision by peers and experts Intervention details 1. Duration/frequency: 30 to 45 minutes per session, maximum 4 sessions, weekly to fortnightly 2. Content of intervention: manualised psychological treatment involving detailed assessment and personalised feedback, cognitive and behavioural skills and techniques, and learning to manage potential or actual relapses using these skills CONTROL (n = 189) Usual care with PHC physician and provision of screening results and WHO Mental Health Gap Action Programme guidelines for harmful drinking, including when and where to refer patients for specialist care to PHC physician CO‐INTERVENTIONS: none |
|
| Outcomes |
Patients 1. Remission (AUDIT score < 8)* 2. Abstinent in the past 14 days 3. Ethanol consumption 4. SIP score 5. WHODASII score 6. Days unable to work 7. Number of attempted suicides 8. Perpetration of IPV amongst married participants 9. Timeline follow‐back generated 10. % of days abstinent and % of days of heavy drinking 11. Deaths 12. Unplanned hospital admission 13. PHQ‐9 14. Suicidal behaviour (suicidal thoughts (PHQ item) + suicidal attempts in past 3 months) Carers Nil Process/health workers None Economic outcomes 1. Health system costs (doctor consultations, admissions, laboratory tests, medicines, total public health care costs, CAP treatment) 2. Time costs to service users and families 3. Productivity losses 4. Total costs from health system perspective 5. Total costs from societal perspective 6. QALYs gained (Tables 3 and 4) (asterisk for study's primary outcomes; star: outcomes that we have not reported in this review) Time points post intervention: 1 to 2 months, 9 to 10 months |
|
| Notes |
Source of funding: Wellcome Trust Notes on validation of instruments (screening and outcomes): validated Additional information: study protocol ‐ Systematic development and pilot randomised evaluation of counselling for alcohol problems, a lay counsellor‐delivered psychological treatment for harmful drinking in primary care in India: the PREMIUM study. Nadkarni A, Velleman R, Dabholkar H, Shinde S, Bhat B, et al. Alcoholism, clinical and experimental research. 2015;39(3):522‐31; 12 month outcomes reported in Sustained effectiveness and cost‐effectiveness of Counselling for Alcohol Problems, a brief psychological treatment for harmful drinking in men, delivered by lay counsellors in primary care: 12‐month follow‐up of a randomised controlled trial. Nadkarni A, Weiss HA, Weobong B, McDaid D, Singla DR, et al. PLoS Medicine/Public Library of Science 2017;14(9):e1002386. Declarations of interest ‐ DMD has received honoraria for lectures not related to this work from Otsuka Pharmaceuticals, Janssen‐Cilag, and H Lundbeck. CGF holds a Principal Research Fellowship from the Wellcome Trust Handling the data (e.g. imputed values/other calculations we have made); nil Prospective trial registration number: ISRCTN registry ISRCTN76465238 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A randomisation list in randomly sized blocks (four to six), stratified by PHC, was generated by a statistician independent of the trial" |
| Allocation concealment (selection bias) | Low risk | "The randomisation code was concealed and allocated by trained health assistants based at the primary health centres at the individual level after completion of the baseline assessments using sequential numbered opaque sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Physicians providing enhanced usual care (EUC) were masked to allocation status" Interventionists were not blinded to allocation; this is unlikely to have influenced results |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | Outcomes were assessed blindly by independent assessors |
| Baseline outcome measurements similar | Low risk | Baseline AUDIT scores were similar in both groups |
| Baseline characteristics similar? | Low risk | Baseline age, marital status, occupation, education, and expectation of usefulness of counselling were similar in both groups |
| Incomplete outcome data (attrition bias) Efficacy data | Unclear risk | 10.8% of participants initially randomised were lost to follow‐up, within the expected 15% attrition rate |
| Protection against contamination | Unclear risk | Participants in both groups lived in the same area and may have shared information |
| Selective reporting (reporting bias) | Unclear risk | PHQ‐9 and change in marital status were planned as secondary outcomes in published protocol but were not reported in this paper (generic protocol for a number of studies?) |
| Other bias | Low risk | "SIP mean score was changed to a secondary outcome to reduce multiplicity of the primary outcomes. Two additional secondary outcomes that were not prespecified (percentage of days abstinent and percentage of days of heavy drinking generated from the Timeline Followback) were also added to bring the trial in line with recommendations of the National Institute on Alcohol Abuse and Alcoholism" These measures were unlikely to have introduced bias to the study |
Nadkarni 2019.
| Study characteristics | ||
| Methods |
Study design: RCT; single‐blind (outcome assessors), parallel‐arm Duration of study: October 2013 to August 2016 |
|
| Participants |
Country: India Income classification: lower‐middle income Geographical scope: urban Healthcare setting: 10 primary health centres Mental health condition: alcohol dependence Population 1. Age: 18 to 65 years 2. Gender: male 3. Socioeconomic background: more than 80% of participants were employed 4. Inclusion criteria (including threshold cutoff score of measurement tool) a. Attendees at primary health centres b. 18‐ to 65‐year‐old males c. Residing in the PHC catchment area d. Intending to reside at the same address for at least 12 months e. Able to communicate clearly f. Able to comprehend one of the programme’s 4 languages g. Scoring ≥ 20 on the AUDIT 5. Exclusion criteria a. Presenting with an emergency medical condition |
|
| Interventions |
Stated purpose: to examine the feasibility of identifying and recruiting men with probable AD in primary care and of delivering a brief treatment for AD by lay counsellors in primary care, as well as acceptability and safety of treatment and preliminary cost‐effectiveness of treatment INTERVENTION (n = 59) Name: Counselling for Alcohol Problems (CAP) Delivered by: LHW Title/name of PW and number: lay counsellors ‐ 11 1. Selection: no professional training in mental health, fluent in vernacular languages used in study settings. Applicants first needed to pass an interview involving role‐plays, then had to undergo a 2‐week classroom training and pass the competency assessment, then had to complete a 6‐month internship followed by a multiple choice examination and a skills test involving role‐plays with standardised vignettes and quality ratings of actual CAP sessions delivered 2. Educational background: completed at least high school 3. Training (contents, duration, by whom): intensive 2‐week classroom training and 6‐month internship with supervision of cases by experts, by 5 local specialists previously trained by international experts and who continued to receive once‐monthly supervision via Skype. Manuals available online 4. Supervision: weekly peer‐led supervision and twice‐monthly individual supervision by peers and experts Intervention details: lay counsellors delivered CAP 1. Duration/frequency: maximum of four 30‐ to 45‐minute sessions at weekly to fortnightly intervals 2. Content of intervention (by types of health workers and per patients/carers): manualised psychological treatment involving detailed assessment, personalised feedback, helping patients to develop cognitive and behavioural skills and techniques, and learning how to manage potential or actual relapses using the skills acquired CONTROL: enhanced usual care (n = 62) Comprised consultation with physician, providing screening results, and referral to a de‐addiction centre (options included outpatient and inpatient settings, public hospitals, and private rehabilitation centres) CO‐INTERVENTIONS: patients in intervention group also had enhanced usual care: consultation with physician, providing screening results, and referral to a local de‐addiction centre |
|
| Outcomes |
Patients 1. Remission defined as AUDIT score < 8* 2. Mean daily ethanol consumption (g) in past 14 days* 3. Percentage of days abstinent (PDA) 4. Percentage of days of heavy drinking (PDHD) 5. Recovery defined as AUDIT < 8 at both 3 and 12 months 6. PHQ‐9 7. Short inventory of problems 8. WHO‐DAS score 9. Total days unable to work 10. Perpetration of intimate partner violence 11. Unplanned hospitalisation in past 12 months 12. Death due to any cause in past 12 months 13. Suicidal behaviour 14. Uptake of detoxification services# Carers Nil Process/health workers 1. Acceptability and feasibility indicators – number of sessions# 2. Duration of sessions# 3. Homework completion# 4. Significant other involvement# 5. Planned discharge# 6. Referrals# Economic outcomes 1. Doctor consultations 2. Detoxification service costs 3. Admissions 4. Laboratory tests 5. Medicines 6. Total health service utilisation costs 7. CAP treatment 8. Time costs to service users and families 9. Productivity losses 10. Total costs from health system perspective 11. Total costs from societal perspective (Table 6) (asterisk for study's primary outcomes; star: outcomes that we have not reported in this review) Time points post intervention: 1 to 2 months, 10 to 11 months |
|
| Notes |
Source of funding: Wellcome Trust Senior Research Fellowship grant Notes on validation of instruments (screening and outcomes): validated Additional information (e.g. provided by authors, existence of a published study protocol): selection and training of lay counsellors described in Nadkarni A; Weobong B; Weiss HA; McCambridge J; Bhat B, et al. Counselling for Alcohol Problems (CAP), a lay counsellor‐delivered brief psychological treatment for harmful drinking in men, in primary care in India: a randomised controlled trial. Lancet 2017;389(10065):186‐95. Declarations of interest ‐ none Handling the data (e.g. imputed values, other calculations we have made): nil Prospective trial registration number: ISRCTN76465238 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation list in randomly sized blocks (2 to 4), stratified by PHC, was generated by a statistician independent of the trial |
| Allocation concealment (selection bias) | Low risk | Randomisation code was concealed and consenting participants were randomised at the individual level by trained health assistants based at primary health centres in a 1:1 allocation scheme to either of 1 intervention arms (enhanced usual care (EUC) or EUC plus CAP) after completion of baseline assessments, using sequentially numbered opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Physicians providing EUC were masked to allocation status. Participants and interventionists in experimental arm were not blinded, but this is unlikely to have influenced outcomes |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | Assessors who performed outcome assessments were blinded to participants' allocation status. All study authors, apart from the data manager (B.B.), were masked |
| Baseline outcome measurements similar | Low risk | See Table 1 |
| Baseline characteristics similar? | Low risk | Baseline characteristics were similar and are reported in Table 1 |
| Incomplete outcome data (attrition bias) Efficacy data | Low risk | Attrition rates are similar between groups. A total 112 participants at 12‐month follow‐up: 58 (11 missing) in the intervention group vs 54 (14 missing) in the control group |
| Incomplete outcome data (attrition bias) Safety data (e.g. adverse events) | Low risk | Attrition is similar between treatment and control groups |
| Protection against contamination | Low risk | Reasonable protection against contamination |
| Selective reporting (reporting bias) | Low risk | All primary outcomes planned in published clinical trial protocol were reported. All planned secondary outcomes were reported, except change in marital status and in employment status (non‐clinical outcomes) |
| Other bias | Low risk | No other sources of bias were found |
Neuner 2008.
| Study characteristics | ||
| Methods |
Study design: randomised (although partly random and partly alternate sequence), parallel‐group assessor‐blinded 3‐armed controlled clinical trial Duration of study: 2003 to 2004 |
|
| Participants |
Country: Uganda Income classification: low Geographical scope: Nakivale refugee settlements in Uganda for Somali and Rwandan refugees; semi‐rural (2 refugee camps close to base hospital) Healthcare setting: home‐based care Mental health condition: PTSD Population: patients 1. Age: > 18 years; mean age 34 to 36 years (SD 12 to 14 years) in the 3 groups 2. Gender: both 3. Socioeconomic background: refugees from Somalia and Rwanda 4. Inclusion criteria a. Fulfilling DSM‐IV criteria for PTSD (assessed using the PDS) b. Consent to participate 5. Exclusion criteria a. Drug abuse b. Obvious mental retardation c. Psychosis |
|
| Interventions |
Stated purpose: to evaluate whether trained counsellors from the local afflicted population can effectively deliver a manual‐based approach to counselling victims of civil war trauma, and to compare structured manual‐based approach vs a more flexible approach or no specific intervention INTERVENTION 1 Name: narrative exposure therapy (NET) ‐ 111 people Delivered by: LHW (residents of refugee camps trained in counselling for the study) Title/name of PW and number: counsellors (9 in total; Somali and Rwandan refugees; 5 women, 4 men; mean age 27 years) 1. Selection: literacy in English and in mother tongue; ability to empathise with clients; strong motivation 2. Educational background: secondary school (7); primary school (1); university (1) 3. Training: 6 weeks of general counselling skills; NET and TC given by 5 post‐doc and doctoral university personnel from Germany and Uganda; used the NET manual and case discussions. 5 trainees had PTSD (3 lifetime, 2 current) and were given individual NET by trainees 4. Supervision: weekly case and personal supervision by trainers; treatment adherence monitored by case discussions during supervision, direct observation of treatment sessions, and review of patient testimonies and treatment protocols 5. Incentives/remuneration: not stated in this report Intervention details 1. Duration/frequency: 6 sessions (2 per week for 3 weeks); 1 to 2 hours' duration 2. Content of intervention: manualised, structured reconstruction of chronology of biography incorporating traumatic events into a coherent narrative; emphasis on reliving and describing emotional, physiological, cognitive, and behavioural reactions to traumatic events; habituation of reactions. Final narrative report (psychoeducation about PTSD in initial sessions; written rationale about relationship between PTSD and multiple past trauma; written chronological autobiography of traumatic experiences given to participant) INTERVENTION 2 Name: trauma counselling (TC) ‐ 111 people Delivered by: lay PHW (LHW) ‐ residents of refugee camps trained in counselling for the study; same as those who gave NET Title/name of PHW/CW and number: counsellors (9 in total; Somali and Rwandan refugees; 5 women, 4 men; mean age 27 years) 1. Selection: as above 2. Educational background: as above 3. Training: flexible, less directive approach than NET; developed through discussions with trainees by experienced senior counsellors from Uganda; training sessions focused on psychological and social needs, conflicts, and current life problems of clients; related current problems to past traumatic experiences; counsellors also trained in non‐directive active listening; problem‐solving; exploring coping skills and grief interventions 4. Supervision: weekly supervision assisted by experienced senior Ugandan counsellor 5. Incentives/remuneration: not stated Intervention details 1. Duration/frequency: 6 sessions (2 per week for 3 weeks) of 1 to 2 hours' duration 2. Content of intervention: not manualised but used a flexible approach focusing on current psychological and social needs of clients; NET considered a part of this approach but not mandatory. Psychoeducation about PTSD in initial sessions; written rationale about relationship between PTSD and multiple past trauma developed; final report in mother tongue of participant included current and past problems discussed with the counsellor and possible solutions and coping strategies CONTROL: monitoring group (no treatment) who were told they would be eligible for NET or TC if they proved effective ‐ 55 people CO‐INTERVENTIONS: not stated |
|
| Outcomes |
Patients 1. PDS (Foa 2005; contains 17 items of DSM‐IV for PTSD; translated and linguistically adapted; standard methods to translate and back‐translate from Afsomali and Kinyaruwanda ‐ methods published separately; used to make DSM‐IV diagnoses of PTSD at baseline, 3 months, and 6 months by 12 trained research assistants blind to allocation 2. Expert evaluation: using PTSD section of Composite International Diagnostic Interview (WHO 1997), by PhD level psychologists or graduate students (number not stated) at 9 months; blind to allocation 3. Physical health checklist: sum of scores of symptoms of common illnesses over last 4 weeks (not validated) Carers Not applicable Process/health workers Not reported Economic outcomes (and where these can be found, e.g. ref or table number) Not reported Time points: baseline for all; 3 and 6 months for intervention groups; 3 and 6 months for monitoring group |
|
| Notes |
Source of funding: German funding agencies (DFG; BMZ) Notes on validation of instruments (screening and outcomes): psychological outcomes validated; physical symptoms checklist not validated Additional information (e.g. provided by authors, existence of a published study protocol): translation of instruments published. Declaration of interest ‐ none Handling the data: as per footnotes in data and analysis Prospective trial registration number: not prospectively registered |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote from report: "the list of participants was ordered randomly; the first 4 were consecutively assigned to NET (narrative exposure therapy), TC (trauma counselling). NET, TC and the fifth was assigned to the MG (monitoring) group.This procedure was repeated until all 277 participants were assigned" Comment: alternate assignment; prone to prediction of next allocation and to high risk of bias; baseline imbalances in nationalities due to lack of stratification |
| Allocation concealment (selection bias) | High risk | Comment: allocation not concealed; participants approached at home and allocated to treatments after randomisation; baseline imbalances in prognostic variables evident |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: open‐label trial; group supervision of cases also precludes effective blinding; counsellors used both interventions; risk of contamination present, as well as of differential interventions |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | Comment: outcome assessors were blind to allocation |
| Baseline outcome measurements similar | Unclear risk | Comment: mean (SD) for PTS diagnostic scale in NET and TC groups were similar at baseline (25.9 (13.2) and 26.7 (12.5), respectively); however, it was lower in the control group (21.3 (10.3)). Unclear if this is a significant difference |
| Baseline characteristics similar? | High risk | Comment: baseline differences in proportions of Somali and Rwandan refugees in intervention groups, with highest % of Rwandan nationals in monitoring group (79%), and lowest in NET group (32%) (P < 0.01). Somali participants had more trauma than Rwandan participants; analyses in report were adjusted for this difference, but this is unlikely to have eliminated risk of bias |
| Incomplete outcome data (attrition bias) Efficacy data | High risk | Comment: dropouts > 65% in all groups; significantly high differential dropout rates |
| Protection against contamination | High risk | Comment: contamination likely, as same therapists used NET and TC; NET was a manualised treatment and TC is expected to incorporate NET; it is also possible that participants discussed treatments among themselves in the refugee camps, further contaminating the fidelity of the interventions |
| Selective reporting (reporting bias) | Unclear risk | Comment: trial not prospectively registered; protocol not available; yet we could detect no evidence of selective reporting |
| Other bias | Low risk | Comment: none detected |
Niemi 2016.
| Study characteristics | ||
| Methods |
Study design: cluster‐RCT superiority trial. Unit of allocation: commune; 11 in intervention group, 10 in control group Duration of study: July 2013 to January 2014 |
|
| Participants |
Country: Vietnam Income classification: lower‐middle income Geographical scope: Ha Nam Province, Vietnam; rural Healthcare setting: PC facility Mental health condition: depression Population: patients who sought care at Binh Luc District Hospital in the Ha Nam Province, Binh Luc District, Vietnam 1. Age: ≥ 18 years 2. Gender: both 3. Socioeconomic background: not mentioned 4. Inclusion criteria a. All patients aged ≥ 18 years who sought care at the district hospital – with somatic or psychological complaints – were screened for depression b. Those with moderate depressive symptoms were eligible for the intervention (PHQ‐9 score 10 to 19) 5. Exclusion criteria a. Psychotic or infective symptoms b. Impaired consciousness c. Emergency cases |
|
| Interventions |
Stated purpose: to evaluate the effectiveness of a collaborative stepped‐care community‐based intervention for depression management, including psychoeducation and yoga, which was successful in reducing the level and severity of depression INTERVENTION (n = 28) Name: psychoeducation and yoga Delivered by: PHP Title/name of PW and number: community nurses and primary care physicians 1. Selection: nurses at community health stations and in the district 2. Educational background: not mentioned 3. Training: 4 training courses were held for health staff at the intervention district. The first course consisted of an introduction on depression as an illness and the intervention model. This included the screening instrument PHQ‐9, correct use of the questionnaire, treatment methods, assessment and management of suicidal risk, and monitoring and supervision of patients with depression. The second course was carried out only for nurses and focused on the principles of psychoeducation and counselling and on developing communication skills. Nurses, doctors, and assistant doctors participated in the third course. They were trained in psychoeducation and counselling and in the 8‐week yoga course. Yoga practices consisted of slow movements and breathing exercises, and providers were trained for 3 full days in teaching the content of all 8 sessions in succession. Postures and exercises were taught for each session, with illustrations and written instructions provided as a complement. The last course was provided for doctors and assistant doctors and focused on antidepressant medication and other pharmacological treatments used for depression in primary health care 4. Supervision: nurses at community health stations and at the district hospital and general doctors at the district health centre were in charge of supervising intervention implementation on a daily basis 5. Incentives/remuneration: not mentioned Intervention details 1. Duration/frequency: 1 session/week for 8 weeks 2. Content of intervention: psychoeducation counselling manuals developed for the MANAS intervention were used to train local healthcare staff in Vietnam. The MANAS programme is manualised and has previously been implemented and assessed in Goa, India, where it was found to be successful when provided by non‐specialists. In addition, nurses and physicians at the primary healthcare level were trained to provide yoga training. The yoga course lasted 8 weeks, with 1 session each week. The form of yoga that was taught in the intervention is used in psychiatric hospitals in Vietnam and includes some components derived from ‘Qigong’, which is a Vietnamese mode of awareness‐building movement practice. All patients in the intervention group received an 8‐week course of psychoeducation in a group setting from a trained nurse using the MANAS manual and an 8‐week yoga course in a group setting. In case a patient did not respond to the intervention, he or she was to be referred to specialist services for pharmacological treatment CONTROL (n = 17) Usual/standard primary care (no information provided for what standard primary care consisted of) CO‐INTERVENTIONS: nil |
|
| Outcomes |
Patients PHQ‐9 Carers Nil Process/health workers Nil Economic outcomes Nil Time points: baseline, 8 weeks post intervention |
|
| Notes |
Source of funding: Swedish International Development Cooperation Agency Notes on validation of instruments (screening and outcomes): all validated Additional information: declaration of interests ‐ none Handling the data: as per footnotes in data and analysis Prospective trial registration number: nil |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "healthcare authorities supporting the project; within the catchment area of the district hospital, 11 communes were randomly selected as the intervention and 10 were randomly selected as the control. The selection was made in accordance with a random number sequence, from a list of the 21 communes in the district. Intervention psychoeducation counselling manuals developed" Judgement comment: Yen ‐ LOW: 21 communes within catchment area of the district hospital were randomised to intervention (10) and control (11) groups by a random number sequence Ujala ‐ UNCLEAR: It is not fully clear how the random sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment Yen ‐ UNCLEAR: randomisation was performed at the start of the study, but there is no indication of how allocation was concealed Ujala ‐ LOW: allocation was by healthcare centre; however It is not clear who performed the randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | This was a cluster‐randomised trial, but non‐blinding of participants was unlikely to affect outcomes |
| Blinding of outcome assessment (detection bias) all outcomes | High risk | Yen ‐ HIGH: blinding was not utilised through this study Ujala ‐ UNCLEAR: knowledge of allocated interventions could not be assessed blindly, as intervention allocation was by healthcare centres. However, it is unclear whether healthcare staff conducting screening were also the ones to deliver the intervention and conduct post assessments |
| Baseline outcome measurements similar | Unclear risk | Yen ‐ LOW: intervention and control groups had similar median PHQ‐9 scores at baseline (Table I) Ujala ‐ UNCLEAR: unequal numbers of patients in intervention and control groups; therefore baseline outcome measurements are not similar |
| Baseline characteristics similar? | Unclear risk | Yen ‐ LOW: intervention and control groups were similar in age and gender (Table I) Ujala ‐ UNCLEAR: unequal numbers of depressed patients in control and intervention groups |
| Incomplete outcome data (attrition bias) Efficacy data | Unclear risk | Yen ‐ HIGH: attrition rate was 6/34 = 17.7% in intervention group and 5/28 = 22.8% in control group. This may have an impact on results had the patients who dropped out stayed on in the study. This shows high risk of bias, as the dropout was 11/56 Ujala ‐ LOW: missing data were adjusted for during analyses. Joint decision: unclear as high risk was adjusted for in the analyses |
| Protection against contamination | Low risk | Judgement comment: cluster‐randomised trial |
| Selective reporting (reporting bias) | Unclear risk | PHQ‐9 and determination of presence/absence of depression using the PHQ‐9 score was the only outcome measure planned in methods section and reported; could not find trial registry online, therefore unclear whether outcomes described in trial registry are reported in the paper |
| Other bias | Low risk | No other biases were present |
Noknoy 2010.
| Study characteristics | ||
| Methods |
Study design: RCT Duration of study: 2003 to 2004 |
|
| Participants |
Country: Thailand Income classification: lower‐middle income Geographical scope: rural Healthcare setting: PCUs ‐ 7 in northeast Thailand and 1 in central Thailand Mental health condition: hazardous drinking Population: hazardous drinkers 1. Age: 18 to 65 years 2. Gender: both, but majority (91%) male 3. Socioeconomic background: predominantly primary and secondary education; married 4. Inclusion criteria a. 18 to 65 years old b. AUDIT score ≥ 8 5. Exclusion criteria a. Alcohol‐dependent patients (DSM‐IV criteria) b. History of liver disease c. History of or regular early morning drinking d. Recent extremely high consumption (> 120 g for men, > 80 g for women) e. Neurological and psychiatric disorders f. Pregnant women g. Outside age range |
|
| Interventions |
Stated purpose: to determine the effectiveness of Motivational Enhancement Therapy (MET) for hazardous drinkers in PCU settings INTERVENTION (n = 55) Name: MET Delivered by: PHP Title/name of PW and number: nurses ‐ 8 1. Selection: nurses from each of the selected PCUs (only 1 nurse per PCU) 2. Educational background: nursing degree 3. Training (contents, duration, by whom): 6 hours' training by a psychiatrist, consisting of understanding the standard drink measurement, the stage of change, and MET 4. Supervision: not specifically planned but nurses could contact main study author (GP working in PC) by telephone for any difficulties or clarifications 5. Incentives/remuneration: none Intervention details 1. Duration/frequency: 3 scheduled sessions: on day 1, at 2 weeks, and at 6 weeks after baseline evaluation. Each session comprised ∼15 minutes of counselling 2. Content of intervention: evaluation of patient's ability to change drinking habits according to the stage of change. For patients in the pre‐contemplation stage, the main technique was feedback, using reflection and questioning skills to elicit self‐motivational statements. If change was contemplated, the study nurse would work with the patient's ambivalence using a pros and cons technique. At the same time, an empathic counselling style and encouragement of the patient's self‐efficacy were used to support change in drinking behaviour. Subsequently, each participant's readiness to change drinking behaviour was assessed if, in the determination stage, options on how to reduce drinking behaviour were provided CONTROL (n = 53) Patients without MET intervention, who were told that the trial focused on health behaviours, which included questions on smoking, exercise, eating behaviour, weight, and alcohol use (to minimise intervention effects on health behaviour) CO‐INTERVENTIONS: none |
|
| Outcomes |
Patients 1. AUDIT tool (for screening) 2. Outcome measures: health survey questionnaire that included amount of alcohol consumption in the previous week*, measured in 4 ways (mean drinking/per drinking day/previous week, hazardous drinking/drinking day/previous week, mean drinking/per week, hazardous drinking/per week), and number of episodes of binge drinking in 7 days 3. Frequency of accidents and traffic accidents 4. Frequency of being drunk in the last month 5. GGT: blood test for evaluation of current drinking severity§ 6. Honesty/accuracy of patient information assessed through collateral informant interviews§ Carers None Process/health workers None Economic outcomes None (*: primary outcomes of the study; §: outcomes that we have not reported in this review) Time points: baseline, 6 weeks (post intervention), 3 months, 6 months |
|
| Notes |
Source of funding: Thai Health Promotion Foundation Notes on validation of instruments: AUDIT is validated, but not the health survey Additional information: provided by study authors for characteristics of NSHWs and intervention. Declarations of interest ‐ none Handling the data: as per footnotes in data and analysis Prospective trial registration number: no protocol |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the unit of randomisation was the individual patient. Randomization of subjects to the intervention and control groups was carried out from the Coordinating Centre in Phramong‐Kutklao Hospital in Bangkok using a standard randomisation table. Each PCU had both control and intervention groups. In order to keep both groups of similar size, random allocation was done in blocks. On average, the trial was to have 6–8 participants in each study condition in each PCU" |
| Allocation concealment (selection bias) | Low risk | Quote: "randomization codes were distributed to each PCU in sealed envelopes. Eligible study participants were enrolled by health personnel when subjects" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "in order to minimize the intervention effect of the research procedures, the subjects randomised into the control condition were told that the trial focused on health behaviours, which included questions on smoking, exercise, eating behaviour, weight and alcohol use. The study interviewers at follow‐up visits were not aware of the assignment allocation of the study participants" |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | Quote: "the study interviewers at follow‐up visits were not aware of the assignment allocation of the study participants" |
| Baseline outcome measurements similar | Low risk | Comment: all similar |
| Baseline characteristics similar? | Low risk | Comment: all similar |
| Incomplete outcome data (attrition bias) Efficacy data | High risk | Comment: there is a < 20% dropout rate in intervention between baseline and last follow‐up; however, dropout rate is > 20% in the control group. May affect outcomes. No mention about analysis of control dropouts; therefore classified as high risk |
| Protection against contamination | Low risk | Quote: "in order to minimize the intervention effect of the research procedures, the subjects randomised into the control condition were told that the trial focused on health behaviours, which included questions on smoking, exercise, eating behaviour, weight and alcohol use" Comment: also unlikely contamination between groups, as dispersed communities (clinics) are not aware of what the intervention was regarding alcohol necessarily |
| Selective reporting (reporting bias) | Unclear risk | Comment: all outcomes in methods section were reported; no published clinical trial protocol is available |
| Other bias | Unclear risk | Comment: trial showed an increase in GGT at 6 months in both groups, possibly because baseline data were collected just after 'Kao Pansaa' ‐ a 3‐month Buddhist retreat, where it is customary for people to avoid wrongdoing; measures include reducing drinking |
O'Callaghan 2013.
| Study characteristics | ||
| Methods |
Study design: single‐centre equal‐randomisation single‐blind parallel‐group RCT; unit of allocation: individual Duration of study: data collection May 2011 to October 2011 |
|
| Participants |
Country: Democratic Republic of Congo Income classification: low income Geographical scope: urban; intervention took place in Beni, a small town in North Kivu, with an estimated population of 100,000 Healthcare setting: schools Mental health condition: PTSD Population 1. Age: 12 to 17 years 2. Gender: female 3. Socioeconomic background: all were rescued from brothels by NGO 4. Inclusion criteria (including threshold cutoff score of measurement tool) a. War‐affected girls aged 12 to 17 years who had witnessed or had personal experience of rape or sexual abuse with post‐traumatic stress, depression, anxiety and conduct problems, and prosocial behaviour (screened with Traumatic Life Events Scale) 5. Exclusion criteria a. Intellectual disabilities b. Psychosis c. Severe emotional and behavioural problems that prevented group participation |
|
| Interventions |
Stated purpose: to assess the efficacy of TF‐CBT in reducing PTS, depression, and anxiety and conduct problems and in increasing prosocial behaviour in a group of war‐affected, sexually exploited girls INTERVENTION (n = 24) Name: trauma‐focused CBT Delivered by: CP Title/name of PW and number: social workers ‐ number not specified 1. Selection: female 2. Educational background: not specified 3. Training (contents, duration, by whom): social workers (female facilitators); facilitators received the manualised intervention in French to study before each session and to raise any questions or suggest any cultural adaptations required before delivering the session. Daily pre‐intervention and post‐intervention meetings took place with facilitators and lead study authors (who had previous experience delivering CBT interventions with young people in Northern Ireland) to ensure that module content was understood, to discuss cultural adaptations, and to address logistical problems (e.g. time management); trained by lead researcher 4. Supervision: lead researcher, who speaks Swahili, monitored each session to ensure treatment integrity and to check that examples, activities, and teaching points discussed at the pre‐intervention meeting were addressed Intervention details: group‐based, culturally modified, TF‐CBT intervention Duration/frequency: 15 sessions that ran for 2 hours per day, 3 days per week, for 5 weeks Content of intervention (by types of health workers and per patients/carers): intervention group received a 15‐session, manualised, culturally modified, trauma‐focused cognitive‐behavioural therapy intervention. It included the following modules: introduction (ground rules, psychoeducation on rape and trauma, and a safe place); stress management (controlled breathing, progressive muscle relaxation, and thought stopping); feelings (affect, expression, and modulation); cognitive coping (cognitive triangle, relationship between thoughts, feelings, and behaviours); trauma narratives; identifying and changing inaccurate or unhelpful cognitions. All modules were delivered in a group setting, with the exception of module 5, for which 3 individual sessions were provided. There was one intervention group with sessions that ran for 2 hours per day, 3 days per week, for 5 weeks, in a hall in the local secondary school. Three caregiver sessions took place for parents/guardians of girls in the intervention group to explain the intervention, talk about the impact of trauma, sensitise parents about children’s rights, and discuss what caregivers can do to foster healthy relationships at home CONTROL: wait‐list control group (n = 28) CO‐INTERVENTIONS: 3 caregiver sessions took place for parents/guardians of girls in the intervention group to explain the intervention, talk about the impact of trauma, sensitise parents about children’s rights, and discuss what caregivers can do to foster healthy relationships at home |
|
| Outcomes |
Patients 1. UCLA‐PTSD RI: PTS* 2. AYPA subscale: depression/anxiety* 3. AYPA subscale: conduct problem Carers None Process/health workers None Economic outcomes (and where these can be found, e.g. ref or table number) None (asterisk for study's primary outcomes; star: outcomes that we have not reported in this review) Time points: baseline, 7 weeks, 3 months |
|
| Notes |
Source of funding: World Vision and CERAO Notes on validation of instruments (screening and outcomes): all validated and translated Additional information: trial protocol is available on request from the lead study author. Declarations of interest ‐ study authors declared no conflicts of interest Handling the data: as per footnotes in data and analysis Prospective trial registration number: NCT01483261 Study also included in prevention review, as unsure about the population (roughly half the intervention group has mental distress or a mental disorder at baseline; thus the intervention may be a treatment, whereas for the other half, it could be a prevention strategy) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "participants were randomly assigned to either the intervention or control group using a computer‐generated random sequence of numbers" |
| Allocation concealment (selection bias) | Low risk | Quote: "selection bias was reduced by ensuring that treatment allocation was concealed from those responsible for participant enrollment and by ensuring that the person responsible for assigned participants had met none of the participants before the group allocation" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not possible to blind participants and personnel to the intervention; unlikely to affect outcomes |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | "The outcome assessors were blinded to the intervention allocation" Knowledge of allocated interventions was adequately prevented during the study. This involved withholding the randomisation sequence from the interviewers, having no overlap between interviewers and intervention facilitators, and ensuring that no interviewers attended or participated in any of the intervention sessions. At the post‐intervention interviews, assessors were told not to ask which group the girls they were interviewing had been in |
| Baseline outcome measurements similar | Low risk | "No statistically significant baseline differences were found between the intervention and control groups on any of the four variables" All outcomes were measured at baseline and were reported in the table |
| Baseline characteristics similar? | Low risk | Baseline characteristics are presented in table 1; no differences were observed |
| Incomplete outcome data (attrition bias) Efficacy data | Low risk | Proportions of missing data were similar in intervention and control groups |
| Protection against contamination | Unclear risk | Judgement comment: wait‐list control received intervention afterwards; however it is not clear whether there was communication between intervention participants and control participants |
| Selective reporting (reporting bias) | Low risk | All outcomes described in trial registry NCT01483261 are reported |
| Other bias | Low risk | No other risks of bias were present |
O'Callaghan 2014.
| Study characteristics | ||
| Methods |
Study design: pilot RCT; unit of allocation: individual Duration of study: March 2012 to July 2012 |
|
| Participants |
Country: Democratic Republic of Congo Income classification: low income Geographical scope: rural. Li‐May and Kiliwa, 2 small villages in Dungu Territory, in Haut Uele Province, with an estimated combined population of fewer than 1000 inhabitants Healthcare setting: lay facilitators working in humanitarian NGO delivered intervention in the Church Mental health condition: PTSD Population: war‐affected youth (no inclusion criteria for mental health problems but at very high risk with high scores at baseline; included in both treatment and prevention reviews) 1. Age: 7 to 18 years 2. Gender: both male and female 3. Socioeconomic background: not specified 4. Inclusion criteria (including threshold cutoff score of measurement tool) a. War‐affected children and caregivers: "As such, it was not designed as a mental health intervention to treat specific psychiatric conditions and so no symptom cut‐off points were used for eligibility. Instead inclusion criteria were broad: children ages 7–18 and their caregivers living in a war‐affected community facing current risks of attack/abduction by armed groups. These criteria was chosen on the basis that all youth in the two villages faced a credible and current risk of abduction and/or attack and so could benefit from a programme that aimed to reduce psychological and behavioural problems and improve interpersonal skills through improving communication and developing conflict resolution strategies and building life skills" 5. Exclusion criteria a. None |
|
| Interventions |
Stated purpose: to develop and evaluate a community participative psychosocial intervention involving life skills and relaxation training and mobile cinema screenings with a war‐affected population under threat INTERVENTION (n = 79) Name: family‐focused psychosocial intervention Delivered by: LHW Title/name of PW and number: 3 male and 3 female; local lay facilitators 1. Selection: living in Dungu and working for SAIPED, a Dungu‐based humanitarian NGO 2. Educational background: not specified 3. Training: facilitators were given a copy of the manualised intervention in French and met for 3 hours with the lead researcher the day before delivering each module 4. Supervision: facilitators met for 3 hours with the lead researcher the day before delivering each module to review the previous module taught and prepare for the subsequent module; a translator was hired, so the lead researcher could monitor the teaching components of the intervention, provide on‐site clinical supervision during sessions, and ensure that each section in each module of the manual was covered in the intervention Intervention details: psychosocial intervention involving life skills and relaxation training and Mobile Cinema screenings 1. Duration/frequency: thrice‐weekly, 2‐hour, group‐based, 8‐session 2. Content of intervention: manualised psychosocial intervention where each participant was invited to choose 1 caregiver to attend the entire intervention. Intervention manual was based on 3 components: chuo cha maisha (youth life skill leadership programme), Mobile Cinema clips, and relaxation technique script used in trauma‐focused CBT. The Chuo Cha Maisha programme comprised the majority of the intervention manual and was selected to boost prosocial behaviour and reduce conduct problems through its modules on effective communication and conflict resolution strategies. The 8 sessions covered (1) psychoeducation (e.g. normalising traumatic stress responses, explaining the role of the family and the community in ameliorating these responses, introducing the concept of stigma), (2) relaxation techniques (progressive muscle relaxation, deep breathing, and mental imagery), (3) brainstorming major problems in families (e.g. drug‐taking, neglect of children, poor school attendance) and how they could be resolved (better parental supervision, parents ensuring their children go to school instead of to the local brewery to buy beer for them), (4) improving interpersonal communication in families (how important it is for parents to listen to their children, how to listen effectively using smiling, nodding, appropriate questions, etc.), (5) major problems in the community (e.g. war, hunger, lack of education) and how they could be resolved (e.g. civil leaders to seek a political solution to the LRA problem, increased cultivation of fields near the village, opening a secondary school in the village), (6) conflict resolution within the community (conflict resolution styles, e.g. authoritarian, permissive, denial of problem, authoritative), (7a) effective parenting (what are the strengths, weaknesses, threats, and opportunities facing parents in the community, what positive qualities did the parents of the 2 children in the video clip have, and what could be learned from them) or (7b) youth contribution to the community (youth leadership styles, implementing a youth community service project), and (8) summary and recap of sessions and a graduation celebration. The intervention used participant modelling of relaxation techniques, small group discussion, drama, whole‐group brainstorming, and 4 Mobile Cinema assessments. During the intervention, participants were split into 4 groups based on gender (male/female) and age (18 and over/under 18) to discuss topics together and to feed their answers back to the whole group during the module. Children chose representatives from among their groups to feed back their views to community members attending the intervention CONTROL (n = 80) Wait‐list control group who received the intervention later (different villages) CO‐INTERVENTIONS: none |
|
| Outcomes |
Patients 1. CRIES‐8* 2. AYPA conduct* 3. AYPA depression/anxiety* 4. AYPA prosocial* 5. AYPA conduct rated Carers None Process/health workers None Economic outcomes (and where these can be found, e.g. ref or table number) None Time points: baseline, 4 weeks, 3 months |
|
| Notes |
Source of funding: a donor who wishes to remain anonymous and who financed the costs of the intervention through the NGO, Discover the Journey Notes on validation of instruments (screening and outcomes): all instruments validated and translated Additional information (e.g. provided by authors, existence of a published study protocol): trial protocol available on request. Declarations of interest ‐ none Handling the data (e.g. imputed values, other calculations we have made): comparisons were conducted using an analysis of covariance Prospective trial registration number: NCT01542398 Study also included in prevention review, as unsure about the population (roughly half the intervention group has mental distress or a mental disorder at baseline; thus the intervention may be a treatment, whereas for the other half, it could be a prevention strategy). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "pair was randomly assigned to either the treatment or control group using a computer generated random sequence (www.random.org)" Judgement comment: random sequence was generated using computer software |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was ensured by ensuring person responsible for allocation did not meet participants |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not possible to blind participants and personnel to the intervention; unlikely to affect outcomes |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | Data were collected by the same (blinded) outcome assessors at pre‐intervention (March 2012), 4 weeks later at post intervention (April 2012), and at a 3‐month follow‐up (July 2012) Data were collected by assessors who were blind to intervention allocation |
| Baseline outcome measurements similar | Low risk | "No important differences found between groups on the outcome measures at pre‐intervention" |
| Baseline characteristics similar? | Unclear risk | Characteristics are mentioned in text, but no data are presented |
| Incomplete outcome data (attrition bias) Efficacy data | Low risk | Incomplete data were adjusted for in analysis |
| Protection against contamination | Low risk | Control group was a wait‐list control; therefore it is unlikely that there was contamination before the intervention was received |
| Selective reporting (reporting bias) | Low risk | All outcomes described in trial registry (NCT01542398) were reported |
| Other bias | Low risk | No other risk of bias was present |
O'Callaghan 2015.
| Study characteristics | ||
| Methods |
Study design: RCT; single‐blinded parallel. Unit of allocation: individual Duration of study: October 2011 to April 2012 |
|
| Participants |
Country: Democratic Republic of Congo Income classification: low income Geographical scope: village of Mwenga, with approximately 10,000 inhabitants, located in the mineral‐rich region of South Kivu, in the Democratic Republic of the Congo (DRC). It is about 120 km by road from the provincial city of Bukavu Healthcare setting: schools Mental health condition: PTSD Population: war‐affected minors. Mental distress is not an inclusion criterion however due to very high mean scores in baseline characteristics included in treatment review (as well as prevention review) 1. Age: 14 to 17 years 2. Gender: both male and female 3. Socioeconomic background: not specified 4. Inclusion criteria (including threshold cutoff score of measurement tool) a. Aged over 7 b. Prior exposure to traumatic war‐related violence c. Ability to attend a 9‐session intervention. 5. Exclusion criteria: nil |
|
| Interventions |
Stated purpose: to compare an evidence‐based, trauma‐focused intervention ‐ Trauma‐Focused Cognitive‐Behavioural Therapy (TF‐CBT) ‐ with an under‐researched, yet widely used, psychosocial intervention ‐ Child Friendly Spaces (CFS) INTERVENTION 1: TF‐CBT (n = 26) Name: TF‐CBT Delivered by: CP Title/name of PW and number: facilitators ‐ number not specified 1. Selection: not specified 2. Educational background: teacher who had delivered 3 TF‐CBT interventions with similar groups of youth in the DRC 3. Training (contents, duration, by whom): both TF‐CBT and CFS facilitators received a minimum of 6 training sessions on how to deliver their particular interventions 4. Supervision: all members of both teams had received prior ‘in‐the‐field’ supervision while delivering their specific interventions Intervention details: TF‐CBT is a component‐based intervention that combines cognitive therapy aimed at changing the way a person thinks and behavioural therapy, which aims to change the way a person acts. It helps an individual come to terms with trauma through exposure to memories of the event 1. Duration/frequency: each intervention ran for 9 sessions (3 sessions per week), and each session was approximately 1.5 hours 2. Content of intervention: this intervention contained 8 modules: (1) introductions; ice breakers; ground rules; psychoeducation on trauma, normalising stress reactions, intrusive memories, and establishing a safe place; (2) imagery; auditory and olfactory techniques to change pictures, sounds, or smells of a traumatic event in the mind; dual‐attention tasks; (3) controlled breathing, progressive muscle relaxation, positive self‐talk, and sleep hygiene; (4) identifying, rating, and productively expressing feelings; (5) cognitive triangle; identifying and re‐framing unhelpful or inaccurate thoughts; (6) graded exposure, using taught techniques during an imagery exposure task, good and bad avoidance; (7) trauma processing via artwork and individual sharing of narratives with a facilitator; and (8) challenging unhelpful and inaccurate cognitions via role‐play; exploring responsibility and advice given to other youth in overcoming traumatic events. All sessions began with culturally familiar games and songs, and after each session, homework was set to practise the concepts learned that day INTERVENTION 2: CFS (n = 24) Title/name of PHW/CW and number: facilitators ‐ numbers not given 1. Selection: not specified 2. Educational background: trained animators 3. Training (contents, duration, by whom): both TF‐CBT and CFS facilitators received a minimum of 6 training sessions on how to deliver their particular interventions 4. Supervision: all members of both teams had received prior ‘in‐the‐field’ supervision while delivering their specific interventions Intervention details: CFS is a psychosocial intervention that improves resilience and well‐being of youth through community‐based, structured activities held in a safe, child‐friendly environment. Unlike TFCBT, CFS does not focus on processing past traumas or re‐framing inaccurate or unhelpful cognitions, but uses creative, expressive, and discursive activities to learn about common dangers young people face and how to avoid them 1. Duration/frequency: each intervention ran for 9 sessions (3 sessions per week); each session was approximately 1.5 hours 2. Content of intervention: this 8‐module intervention explored the following: (1) child protection, i.e. identifying specific risks in the village and how to avoid them such as collecting firewood in groups, not accepting gifts or money from older men, etc.; (2) sexually transmitted diseases, including HIV/AIDS; how they affect people and how to avoid contracting them; (3) child rights under international and Congolese law, with particular focus on child labour and risks of working in nearby mining zones; (4) the Tree of Life, where participants draw a diagram of their own personal skills and resources (leaves) and people in their lives who can help them (trunk and branches) or have helped them (roots) achieve their goals; (5) the Journey of Life, which is a pictorial representation of challenges youth face in life (e.g. drug‐taking, sexually transmitted diseases, lack of school fees, unemployment) and how they can be overcome; (6) to (8) preparing and acting out a play on how to protect yourself as a young person from drug‐taking, violence, and sexual abuse CONTROL: no care; wait‐list group (n = 22) CO‐INTERVENTIONS: caregiver sessions: two 90‐minute sessions took place for the caregivers of both TF‐CBT and CFS intervention groups. These sessions briefly explained the 2 interventions being run, the psychological impact of war and violence on young people, how child rights can be better protected and respected, and how parents can improve communication and interaction with their children at home. Sessions were delivered by a panel and included TF‐CBT facilitators, CFS animators, the lead researcher, social workers, and religious and civil representatives |
|
| Outcomes |
Patients 1. UCLA PTSD‐RI* 2. African Youth Psychosocial Assessment Instrument (AYPA)* ‐ depression, conduct problems, prosocial behaviour, adverse life events Carers None Process/health workers None Economic outcomes (and where these can be found, e.g. ref or table number) None (asterisk for study's primary outcomes; star: outcomes that we have not reported in this review) Time points: baseline, 3 weeks, 6 months |
|
| Notes |
Source of funding: jointly funded by the Doctorate in Educational, Child and Adolescent Psychology at Queen’s University and the Transcultural Psychosocial Organisation (TPO), which receive financial support from UNICEF, DRCongo Notes on validation of instruments (screening and outcomes): AYPA is the only African‐developed and validated instrument of psychosocial functioning. Congolese Swahili version of the PTSD‐RI was used. Due to concerns with cross‐cultural applicability of a PTSD diagnosis in a non‐western population, this measure was used to record post‐traumatic stress symptoms but not to diagnose PTSD Additional information Previous and similar studies by authors 1. O'Callaghan P, McMullen J, Shannon C, Rafferty H. Comparing a trauma focused and non trauma focused intervention with war affected Congolese youth: a preliminary randomised trial. Intervention 2015;13(1):28‐44. https://doi.org/10.1097/WTF.0000000000000054 2. O’Callaghan P, McMullen J, Shannon C, Rafferty H, Black A. A single‐site, parallel design, randomized controlled trial with sexually exploited, war‐affected Congolese girls. Journal of the American Academy of Child and Adolescent Psychiatry 2013;52(4):359‐369 Declarations of interest ‐ none Handling the data: nil Prospective trial registration number: NCT01509872 Study also included in prevention review, as unsure about the population (roughly half the intervention group has mental distress or a mental disorder at baseline; thus the intervention may be a treatment, whereas for the other half, it could be a prevention strategy) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The lead author randomised eligible participants on their posttraumatic stress (PTS) score to either the TF‐CBT group or the CFS group using a computer generated random sequence supplied by one of the research team of site (CS)" Randomisation generated through computer programme; therefore low risk |
| Allocation concealment (selection bias) | Low risk | Group allocation was concealed from those responsible for participant enrolment (RECOPE) by ensuring that the person responsible for assigning participants met none of them prior to group allocation" Allocation was concealed from those enrolling participants; therefore low risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | This was a single‐centre, equal‐randomisation, single‐blind (outcome assessors), parallel‐group intervention. Not possible to blind participants and personnel to the intervention. Unlikely to affect outcomes |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | "The interviewers (outcome assessors) were blinded to the intervention allocation. This involved withholding the randomisation sequence from the interviewers, having no overlap between interviewers and intervention facilitators, and by ensuring no interviewers attended nor participated in any of the intervention sessions" Knowledge of allocation to interventions was prevented during the study; therefore low risk |
| Baseline outcome measurements similar | Low risk | Participant outcomes were measured prior to the intervention; no important differences were present |
| Baseline characteristics similar? | Low risk | "Randomisation resulted in no significant difference in age, number of traumatic events nor any pre‐intervention symptom scores" Baseline characteristics of participants presented in table. No significant differences between characteristics; therefore low risk |
| Incomplete outcome data (attrition bias) Efficacy data | Low risk | Missing outcome measures were unlikely to bias the results. Low attrition rates ‐ unlikely to bias results "All participants interviewed at the start of the study were included in the outcome analysis i.e. post intervention and follow‐up analysis was by intention‐to‐treat, using a last observation carried forward‐procedure. This means that if a participant was unavailable for the post intervention follow‐up, then their pre‐test score was used for the purposes of data analysis" |
| Incomplete outcome data (attrition bias) Safety data (e.g. adverse events) | Low risk | Low attrition rates ‐ similar for intervention and control groups; therefore unlikely to bias results |
| Protection against contamination | Unclear risk | Control was wait‐list; therefore unlikely to have contact with facilitators. It is unknown whether there was contact between facilitators of both intervention groups. This is not fully clear |
| Selective reporting (reporting bias) | Low risk | All measures described in trial registry (NCT01509872) have been reported |
| Other bias | Low risk | No other risk of bias was present |
Oladeji 2015.
| Study characteristics | ||
| Methods |
Study design: cluster‐RCT. Unit of allocation: primary healthcare centres; 3 in each arm, 6 in total Duration of study: estimated start date 01/11/2013; end date 31/10/2016 (from Trial registry) |
|
| Participants |
Country: Nigeria Income classification: lower‐middle income Geographical scope: Oyo State, one of the 6 states in the Southwest geopolitical zone of Nigeria, with a population of about 4.5 million Healthcare setting: study was carried out at 6 primary healthcare centres (PHCCs); randomly selected from 2 local government areas (LGAs) ‐ 1 rural, the other urban, in Oyo State. In Oyo State, primary care service is delivered mainly by non‐physician primary care providers consisting of nurses, community health officers, and community health extension workers. Each of these categories of providers has a minimum of 3 years of post‐secondary education and is certified by the respective board. Supervision for all clinics in each LGA is provided by 1 general practitioner employed by the government and designated as the primary healthcare co‐ordinator for the local government Mental health condition: depression Population 1. Age: ≥ 18 years 2. Gender: both 3. Socioeconomic background: mean of 8.2 years of education 4. Inclusion criteria a. Adults, aged 18 years or over b. Score ≥ 11 on the Patient Health Questionnaire (PHQ‐9) c. Confirmed 5th edition of Diagnostic and Statistical Manual of Mental Disorders (DSM‐V) diagnosis of depression using the Composite International Diagnostic Interview (CIDI) d. Provided informed consent 5. Exclusion criteria a. Immediate need for medical attention b. Pregnant c. Actively suicidal d. Presence of bipolar or psychotic disorder or severe substance dependence e. Unlikely to be in the neighbourhood in the following 12 months |
|
| Interventions |
Stated purpose: to explore the feasibility, acceptability, and potential effectiveness of a multi‐component stepped‐care intervention package in primary care INTERVENTION (n = 165) Name: Stepped Care Package for Depression Delivered by: PHP and LHW Title/name of PW and number: primary care health workers (PCHWs) ‐ 18 (6 nurses, 3 community health officers, 9 community health extension workers) 1. Selection: selected by primary healthcare co‐ordinators at each of the selected local government areas (doctors) and by matrons in charge of each of the selected clinics 2. Educational background: nurses, community health officers, and community health extension workers with minimum of 3 years of post‐secondary education and certified by their respective boards 3. Training: initial 3‐day training, focused on identification and treatment of depression. Training on how to manage depression using psychoeducation, activity scheduling, and problem‐solving treatment. Workers had a further 3‐day top‐up training about a month into the study to reinforce the treatment modalities they had been trained in, and to identify any difficulties they had in administering treatments. Training consisted of didactic lectures enhanced with clinical demonstrations and role‐playing exercises. Training was provided by local psychiatrists 4. Supervision: consultation with supervising doctors via mobile phone. The doctor similarly had access to the psychiatrist for consultation for difficult cases. Ongoing support and supervision for the PHCW delivering interventions were provided by the team. A member of the team scheduled visits to sit in with providers to observe some treatment sessions, to listen to recordings of other sessions, and to provide feedback to individual PHCWs 5. Incentives/remuneration: not mentioned Intervention details 1. Duration/frequency: initial 8 weekly sessions, then further 8 weekly or fortnightly sessions, depending on response to treatment 2. Content of intervention: psychoeducation, activity scheduling, problem‐solving treatment, antidepressant medication (amitriptyline). STEPPED care approach depending on response (assessed with PHQ‐9 < 5, then 4 fortnightly follow‐on sessions from initial 8). If > 5 suicidal thoughts: CMHW consults doctor and decides whether or not to initiate medication and add more therapy (details: diagnosis of depression is explained to the patient in simple language using local expressions while avoiding labelling of ‘mental illness/disorder’. Patients are helped to understand that symptoms being experienced are not the result of laziness or supernatural forces, but an ailment that is common and amenable to treatment. Patients are encouraged to ask questions and to express feelings. Patients are encouraged to carry out more activities that are important or pleasurable to them. They used a local adaptation of Problem‐Solving Treatment for Primary Care (PST‐PC), a 7‐step commonsense talk therapy that aims to help patients solve troublesome problems that contribute to causing or prolonging the depressive episode. It includes identification and exploration of the problems currently faced by the patient and aiding the patient to develop and implement practical solutions. PHCWs are allowed to prescribe antidepressant medication under the supervision of a primary care physician. In the first step of the intervention package, all patients with a PHQ‐9 score between 8 and 14 receive 8 weekly sessions of individual talking therapy delivered by the PHCW. For participants whose PHQ‐9 score is 15 or higher at the outset, or who express suicidal ideation, the PHCW consults with the doctor by phone immediately. The doctor decides whether to see and review the patient or gives instruction on the prescription of amitriptyline to the patient. Participants who are prescribed antidepressant medication nevertheless also receive weekly sessions of talking therapy, in addition to the antidepressant medication. Following completion of 8 weeks of treatment, the PHCW administers the PHQ‐9 to assess level of improvement and decides on interventions for the second step. Participants who improve, as indicated by a PHQ‐9 score ≤ 5 or less than half of baseline score, receive 4 fortnightly top‐up talking therapy sessions over an additional 8 weeks. Those who do not improve are reviewed by the doctor and are considered for medication, if none has been prescribed in the earlier step, or medication is reviewed, if already on antidepressants. Such participants are also offered weekly talking therapy sessions for 8 weeks CONTROL (n = 69) PHCWs from control clinics received 2 days of training on identification and standard treatment of depression. They were provided with manuals detailing diagnosis of and treatment for depression. However, the intervention was selected at the discretion of the healthcare provider CO‐INTERVENTIONS: nil |
|
| Outcomes |
Patients 1. Patient’s Health Questionnaire (PHQ‐9) 2. WHO quality of Life instrument (WHOQOL‐Bref) 3. WHO disability assessment schedule (WHODAS). Carers Nil Process/health workers For the intervention arm 1. Proportion of patients who received at least the first psychoeducation session 2. Proportion of patients who received at least 2 sessions of talking therapy 3. Proportion of patients who completed at least 6 sessions of talking therapy 4. Proportion of patients who received antidepressants 5. Proportion of patients receiving antidepressants who completed at least 3 months of treatment 6. Proportion of patients for whom telephone contact to doctor was made 7. Proportion of patients referred to the doctor 8. Proportion of patients referred to psychiatrist Economic outcomes Nil (pilot study only) Economic outcomes Reported as part of full trial in Gureje 2019 Time points: baseline, *3 and 6 months (from intervention) *As per methods section, but only 6‐month results reported in the paper |
|
| Notes |
Source of funding: funding for the pilot of Stepped Care Intervention for Depression in Primary Care in Nigeria (STEPCARE) was provided by the Wellcome Trust Notes on validation of instruments (screening and outcomes): validated Additional information: trial protocol; declaration of interests ‐ study authors declared no competing interests Handling the data: as per footnotes in data and analysis Prospective trial registration number: ISRCTN46754188 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A listing of all eligible PHCCs was obtained (eligible clinics were those with a full complement of primary care workers and providing a broad range of clinical service). From this listing, 3 clinics were randomly allocated to the intervention arm and 3 to the control arm. Allocations were done by an independent statistician using a table of random numbers |
| Allocation concealment (selection bias) | Low risk | Allocation was by institution at the start of the study |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not possible to blind participants and personnel to the intervention; unlikely to affect outcomes |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | Patients and interventionists were not blinded, but assessors were blinded |
| Baseline outcome measurements similar | Low risk | Similar PHQ‐9 scores in both groups at baseline |
| Baseline characteristics similar? | Low risk | Groups were similar in age, years of education, and gender (Table 2) |
| Incomplete outcome data (attrition bias) Efficacy data | Unclear risk | Weng ‐ LOW: Figure 1 Yen ‐ HIGH: greater proportion of dropouts (16.9%) in intervention than in control (7.2%). Dropouts differed from those who did not drop out in demographics |
| Incomplete outcome data (attrition bias) Safety data (e.g. adverse events) | Unclear risk | 16.9% of participants in the intervention group dropped out; 7.2% of participants in the control group dropped out. There were 7 deaths in the intervention group and 1 in the control group. No further details are given |
| Protection against contamination | Low risk | Cluster‐randomised trial |
| Selective reporting (reporting bias) | Low risk | From the clinical trial protocol: recovery from depression will be assessed at 12 months. Changes in cost‐effectiveness, disability, and quality of life will also be measured. In this report, PHQ‐9 (depression), WHO‐QOL (QOL), and WHO‐DAS (disability) were presented. Cost‐effectiveness data were not presented (not a clinical outcome) |
| Other bias | Low risk | No other sources of bias were found |
Papas 2011.
| Study characteristics | ||
| Methods |
Study design: randomised gender‐stratified parallel‐group open‐label controlled clinical trial Duration of study: February to December 2009 |
|
| Participants |
Country: Kenya Income classification: low income Geographical scope: urban; HIV clinic affiliated with Moi Teaching and Referral Hospital, Eldoret, Kenya Healthcare setting: outpatient clinic Mental health condition: hazardous use of alcohol or binge‐drinking Population: patients 1. Age: ≥ 18 years 2. Gender: both 3. Socioeconomic background: not specified 4. Inclusion criteria a. ≥ 18 years b. Enrolment as an AMPATH HIV outpatient attending the Eldoret Clinic affiliated with Moi Teaching and Referral Hospital c. Hazardous or binge drinking criteria (score ≥ 3 on AUDIT‐C, or more than 6 drinks per occasion at least monthly) d. Any alcohol use in the past 30 days e. Antiretroviral eligible or antiretroviral initiated in the past 12 months f. Spoken knowledge of Kiswahili g. Living within 1 hour's travelling distance from the clinic h. No plans to move farther away during the study period i. Available during weekly group time 5. Exclusion criteria a. Active psychosis or suicidal b. Attendance in the past year at an existing AMPATH alcohol peer support group c. Participation in the study’s group CBT pre‐pilot development |
|
| Interventions |
Stated purpose: to use CBT due to empirical evidence of success in reducing risky behaviours in African HIV‐infected people and its structured format, which makes it feasible to train paraprofessionals INTERVENTION Name: CBT; 42 people Delivered by: LHW Title/name of PW and number: CBT counsellors ‐ 2 (1 male, 1 female) 1. Selection: knowledge of English and Kswahili; essays and role‐plays to assess empathy, emotional perceptiveness; good communication skills and analytical abilities; meeting certification criteria for CBT training (adherence and competence) 2. Educational background: high school 3. Training (contents, duration, by whom): trained by study personnel; 175 hours of training; classes, role‐plays, videotaped feedback with medical students as simulated patients; assessment of adherence and competency using the YACS 4. Supervision: 300 hours of supervision prior to trial; during trial, all CBT group sessions were videotaped and monitored weekly by 1 experienced CBT supervisor. Supervision was conducted via telephone during latter stages of trial. 50% of sessions with men and women, respectively (18 sessions), were selected randomly, translated into English, with random back‐translation verification, and were rated by 2 highly experienced YACS raters from the Yale Psychotherapy Development Center 5. Incentives/remuneration: not stated Intervention details 1. Duration/frequency: 6 weekly, gender‐stratified 90‐minute group CBT sessions; 7 participants per group; delivered by same‐sex CBT counsellor 2. Content of intervention: manual‐based CBT. Abstinence from alcohol was set as a goal and a quit date was decided during the second session; behavioural analysis; risky behaviours; alcohol refusal skills reinforced CONTROL: routine medical care provided by the clinic (33 people) CO‐INTERVENTIONS: not reported |
|
| Outcomes |
Patients 1. Percentage of drinking days* 2. Mean drinks per drinking days 3. Abstinence at longest follow‐up § 4. Adherence to CBT sessions § Carers Not applicable Process/health workers Adherence and competence to CBT Economic outcomes Not reported (*: primary outcomes of the study; §: outcomes that we have not reported in this review) Time points: 30 days, 60 days, 90 days post treatment |
|
| Notes |
Source of funding: National Institute on Alcohol Abuse and Alcoholism‐funded grant (R21AA016884), USAID‐AMPATH Partnership from the United States Agency for International Development (President’s Emergency Plan for AIDS Relief and P50DA09241) Notes on validation of instruments (screening and outcomes): validated outcome tools Additional information: declaration of interests ‐ none Handling the data: as per footnotes in data and analysis Prospective trial registration number: clinicaltrials.gov identifier: NCT00792519 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a stratified simple randomization procedure was used to form gender‐stratified cohorts. Within gender‐based cohorts, participants were assigned randomly until a minimum was achieved of seven CBT and five usual care participants, thereby creating some waiting time. A group of seven was required for CBT to enhance participation, while fewer were required for the individual usual care condition to minimize waiting time before treatment initiation" |
| Allocation concealment (selection bias) | Low risk | Quote: "each participant was randomized after she or he drew from a jar a paper with the name of the condition. The papers were prepared by study administrators to conceal the name of the condition during the drawing, which was supervised by staff" Comment: allocation was possibly concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: not blinded, but this was not possible; no likely effect on outcomes |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | Quote: "non‐blinded research assistants both recruited and interviewed participants; none delivered study interventions" Comment: unlikely that they did not deliver the intervention. Three alcohol saliva tests came back positive during treatment phase. This showed concordance with patient's self‐reported or scored outcomes |
| Baseline outcome measurements similar | Low risk | Comment: All similar |
| Baseline characteristics similar? | Low risk | Comment: All similar |
| Incomplete outcome data (attrition bias) Efficacy data | Low risk | Comment: 36/42 completed intervention; 32/33 stayed in control (completers), i.e. less than 20% dropout rate |
| Protection against contamination | Low risk | Comment: RCT occurring in just 1 clinic. Lessons from CBT therapy could therefore have been shared within the population between controls and those in CBT intervention. However a large number of people were enrolled at the clinic, suggesting its geographical remit is very wide |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes mentioned in methods section were reported. Trial was prospectively registered and primary outcomes were identical: quantity and frequency of alcohol use |
| Other bias | Low risk | Comment: none detected |
Papas 2020.
| Study characteristics | ||
| Methods |
Study design: RCT Duration of study: July 2012 to August 2016 |
|
| Participants |
Country: Kenya Income classification: low income from 2012 to 2013; lower‐middle income from 2014 to 2016 Geographical scope: urban Healthcare setting: a large HIV outpatient clinic affiliated with the Academic Model Providing Access to Healthcare (AMPATH) collaboration Mental health condition: alcohol use disorder Population 1. Age: 18 and older 2. Gender: both 3. Socioeconomic background: median annual income USD $240 4. Inclusion criteria (including threshold cutoff score of measurement tool) a. Age 18 and older b. Enrolment as an outpatient attending any of 4 AMPATH HIV clinics c. Score ≥ 3 on the Alcohol Use Disorders Identification Test ‐ Consumption (AUDIT‐C) or binge‐drinking (≥ 6 drinks per occasion at least monthly) d. Alcohol use in the past 30 days e. Verbal working knowledge of Kiswahili f. Living within 1 hour's travel distance from the Eldoret Clinic affiliated with MTRH, where the study was conducted g. Available during weekly group time 5. Exclusion criteria a. Participation in CBT pilot study b. Cohabitation or regular contact with a current study participant c. Impaired physical mobility d. Active psychosis or active suicidality |
|
| Interventions |
Stated purpose: to evaluate a culturally adapted group cognitive‐behavioural therapy (CBT) intervention delivered by paraprofessionals to reduce alcohol use among HIV‐infected outpatients in Eldoret, Kenya INTERVENTION (n = 312) Name: cognitive‐behavioural therapy Delivered by: LHW (paraprofessional counsellors) Title/name of PW and number: paraprofessional counsellors ‐ 8 1. Selection: first screened with essays and role‐play; after classroom training, assessed through role‐play with simulated patients; if satisfactory (frequency rating of 4 on at least 50% of YACS items, with a skill rating of 4 on these items; all on a 7‐point scale), subsequently assessed through 6 videotaped sessions with pilot groups. Certification to participate in the randomised trial required minimum delivery of 1 group in English and 1 group in Kiswahili 2. Educational background: 2‐year post high school counselling diploma 3. Training (contents, duration, by whom): classroom work, role‐play, videotaped feedback, ethics and basic health education training 4. Supervision: every session was videotaped and reviewed by a US clinician and a Kenyan counselling manager (trained during pilot study) during the first half of the study, and by the Kenyan manager alone for the remainder of the study Intervention details: by paraprofessional counsellors 1. Duration/frequency: 6 weekly 90‐minute closed gender‐separated group sessions led by a same‐sex counsellor and delivered in Kiswahili 2. Content of intervention (by types of health workers and per patients/carers): structured, manualised protocol with abstinence as the goal, including HIV/alcohol education; discussing reasons to drink and to quit; preparing to quit; setting a quit day; coping with triggers, urges, and high‐risk situations; problem‐solving; alcohol refusal skills; long‐term planning; practice exercises CONTROL: time‐ and attention‐controlled Healthy Lifestyles education intervention (n = 302) 6 weekly 90‐minute gender‐separated closed group sessions conducted in Kiswahili and led by 1 same‐sex counsellor to teach healthy lifestyle behaviour, including HIV‐alcohol education CO‐INTERVENTIONS: nil |
|
| Outcomes |
Patients 1. Timeline follow‐back derived percent drinking days* 2. Drinks per drinking day (14 g ethanol per drink)* 3. Saliva tests for alcohol 4. Adherence to antiretrovirals# 5. HIV viral load# 6. Tobacco use# 7. Marijuana use# 8. Khat use# 9. Risky sex# 10. Medication for withdrawal symptoms# Carers Nil Process/health workers 1. Adherence and competence 2. Working Alliance Inventory short form (participant self‐report of working alliance in counselling relationship)# 3. Homework adherence# Economic outcomes (Galárraga et al. Task‐shifting alcohol interventions for HIV+ persons in Kenya: a cost‐benefit analysis. BMC Health Services Research 2017;17:239) 1. Anticipated supervisor costs 2. Trainer costs 3. Trainee costs 4. Training materials costs 5. Conference centre costs 6. Furniture costs 7. Equipment costs (Table 2, training costs) 8. Detailed scaled‐up costs per site (Table 3) (asterisk for study's primary outcomes; star: outcomes that we have not reported in this review) Time points post intervention: 1 month, 9 months |
|
| Notes |
Source of funding: US National Institute on Alcohol Abuse and Alcoholism (NIAAA), United States Agency for International Development Notes on validation of instruments (screening and outcomes): validated Additional information: declaration of interests ‐ study authors reported no conflicts of interest Training Papas RK, Sidle JE, Martino S, Baliddawa JB, Songole R, Omolo OE, Gakinya BN, Mwaniki MM, Adina JO, Nafula T, Owino-Ong'or WD, Bryant KJ, Carroll KM, Goulet JL, Justice AC, Maisto SA. Systematic cultural adaptation of cognitive‐behavioural therapy to reduce alcohol use among HIV‐infected outpatients in western Kenya. AIDS Behav. 2010;14(3):669‐78. doi: 10.1007/s10461‐009‐9647‐6 Anticipated cost‐benefit analysis Galárraga et al. Task‐shifting alcohol interventions for HIV+ persons in Kenya: a cost‐benefit analysis. BMC Health Services Research 2017;17:239 Handling the data: nil Prospective trial registration number: NCT01503255 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: a stratified block randomisation procedure with random block sizes of 2 and 4 was generated by an analyst to balance gender and antiretroviral (ARV) use (yes/no) across the 2 intervention conditions. Equal allocation was intended between conditions. Within gender‐ and ARV‐based cohorts, participants were randomly assigned to a condition until a target recruitment of 7 individuals per group was achieved. Attendance in groups generally consisted of 5 to 7 individuals. Assigned condition was not revealed to participants until they arrived for first intervention sessions. Gender stratification was conducted to avoid reinforcing the secondary status of women in Kenya. ARV stratification was conducted to balance any potential behavioural or medical factors associated with greater severity of HIV/AIDS |
| Allocation concealment (selection bias) | Low risk | Judgement comment: individual assignment based on block was printed in sealed envelopes, which were handed to the RA by the study co‐ordinator after each enrolment. Assigned condition was not revealed to participants until they arrived for first intervention sessions |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not possible to blind participants and personnel to the intervention; unlikely to affect outcomes |
| Blinding of outcome assessment (detection bias) all outcomes | High risk | interviewers who did assessments were not blinded to participants' assignment but were not involved in providing the intervention |
| Baseline outcome measurements similar | Low risk | Similar DDD and PDD values at baseline in both groups |
| Baseline characteristics similar? | Low risk | Control participants had a greater proportion with CIWA‐Ar greater than 10 (10.6% vs 6.4%). Groups were well matched in other baseline characteristics |
| Incomplete outcome data (attrition bias) Efficacy data | Low risk | Study authors anticipated 15% attrition and calculated sample size accordingly (minimum 336 participants). In the intervention group, 312 were randomised and 269 were present at the last follow‐up assessment (86%). In the control group, 302 were randomised and 251 were present at the last follow‐up assessment (83%). Thus the attrition rate was well within the anticipated range. Sensitivity analysis was done and was presented to account for participants who had dropped out, assuming that they had reverted to baseline drinking levels |
| Incomplete outcome data (attrition bias) Safety data (e.g. adverse events) | Low risk | A total of 2 deaths in the intervention group and 6 deaths in the control group were reported, but all were deemed unrelated |
| Protection against contamination | Unclear risk | Judgement comment: as this is an individually randomised clinical trial, contamination was possible, although study authors tried to minimise this by excluding partners or close contacts of those who were already recruited |
| Selective reporting (reporting bias) | Low risk | Timeline follow‐back alcohol use (percent drinking days) was the only outcome planned in the clinical trial protocol. In the report, both PDD and DDD were reported, but the same technique was used to collect both |
| Other bias | Low risk | No other sources of bias were found |
Patel 2010.
| Study characteristics | ||
| Methods |
Study design: RCT (cluster trial ‐ unit allocation ‐ health facility (PHC or GP), 12 clusters in each arm, 24 in total; analysis ‐ individual) Duration of study: April 2007 to September 2009 |
|
| Participants |
Country: India Income classification: lower‐middle income Geographical scope: urban and rural Healthcare setting: PC facilities, i.e. all facilities with space and privacy for LHCs; regular outpatient clinics not involved in preliminary phases of the project. There were government PHC facilities and private GP settings Mental health condition: common mental disorders Population: patients 1. Age: > 17 2. Gender: both 3. Socioeconomic background: predominantly female; married and one‐third widower; nearly half with < 1 year of education or illiterate 4. Inclusion criteria a. Adults > 17 years of age b. Speaking Konkani, Marathi, Hindi, English c. Not needing medical attention d. Did not have difficulty with hearing, speaking, cognition e. Not already screened in previous weeks f. Not receiving intervention g. Screened positive for common mental disorders with GHQ‐12 with previously validated cutoff > 5) h. Expected to be resident of Goa for subsequent 12 months 5. Exclusion criteria a. Cognitive or sensory impairment that made participation in evaluation difficult b. Not speaking Konkani, Marathi, Hindi, English |
|
| Interventions |
Stated purpose: to test the effectiveness of an intervention led by LHCs in PC settings to improve outcomes of people with these disorders INTERVENTION (n = 1160) Name: collaborative stepped‐care intervention ‐ phase 1 (12 government PHCs) and phase 2 (12 private GP facilities) Delivered by: LHW and PHP Title/name of PW and number: LHCs (lay health counsellors); GP and PHC physicians 1. Selection: LHC: a woman fluent in local languages, with excellent communication skills, and available for consultations on a regular basis in the clinics; GP/PHC physician: those located at selected facilities 2. Educational background: LHC: graduates ‐ locally recruited, graduate non‐medical workers; GP/PHC physician: registered medical GP as per a priori eligibility criteria 3. Training: LHC: training component included how to deliver various treatments, including counselling skills, psychoeducation, yoga, and IPT. Training was based on a draft manual developed for the intervention. Duration: 2 months' training. Trained by research team. GP/PHC physician: half day of training and given a manual 4. Supervision: LHCs and GPs/PHC physicians: clinical specialist (psychiatrist) visited about once a month and was available for consultation on the telephone to discuss cases 5. Incentives/remuneration: not mentioned Intervention details 1. Duration/frequency: both phases carried out consecutively between April 2007 and September 2009 2. Content of intervention: LHCs provided psychoeducation: psychoeducation taught patients strategies to alleviate symptoms, such as breathing exercises for anxiety symptoms and scheduling activities for symptoms of depression. Encouraging adherence to treatments for these disorders and providing information about social and welfare organisations when needed were other key components of psychoeducation. Individual (not group) IPT was also provided by the LHC as the psychological treatment of choice. Focus on interpersonal problems such as grief, disputes, and role transitions. Minimum of 6 sessions, with an optimum of 8 and a maximum of 12 sessions, was offered to each eligible participant. Interpersonal psychotherapy was reserved for patients who had moderate or severe common mental disorders, and was offered as an alternative to, or in addition to, antidepressant drugs for those who did not respond to antidepressant treatment. Physician/GP roles: prescribe antidepressants according to a protocol for moderate to severe depression (private GPs could prescribe their drug of choice, PHC doctors had to use fluoxetine 20 to 40 mg/d). Other key roles of physicians were to encourage patients to meet the LHC, to avoid use of unnecessary drugs, and to provide usual care for any co‐existing physical health problems. Referral: Referral to the clinical specialist was reserved for patients who were assessed as having high suicide risk at any stage, were unresponsive to earlier treatments, posed diagnostic dilemmas, had substantial comorbidity with alcohol dependence, or had other associated substantial medical problems, or for whom the PC physician requested a consultation CONTROL (n = 1269) Enhanced usual care: physicians and patients in usual care practices received screening results and were given the treatment manual prepared for PC physicians. Physicians were allowed to start treatments of their choice CO‐INTERVENTIONS: none |
|
| Outcomes |
Patients 1. Screening: GHQ‐12 2. Primary outcome: CIS‐R*: generates 2 outputs ‐ an ICD‐10 diagnosis derived from a computer algorithm and a total score indicating overall severity of symptoms Carers None Process/health workers None Economic outcomes None (*: primary outcomes of the study) Time points: baseline; follow‐up at 6 months, 12 months |
|
| Notes |
Source of funding: MANAS project was funded by a Wellcome Trust fellowship in clinical sciences Notes on validation of instruments (screening and outcomes): validated GHQ in Goa setting but not specified for the CIS‐R Additional information (e.g. provided by authors, existence of a published study protocol): yes; declaration of interests ‐ all study authors’ expenses related to this trial were paid for by the Wellcome Trust grant through partner institutions. Study authors declared no other conflicts of interest Handling the data: as per footnotes in data and analysis Prospective trial registration number: NCT00446407 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote 1: "facilities were stratified into three strata: urban with a visiting psychiatrist (VP), rural with a VP, rural without a VP. Two intervention and two control PHCs were selected at random from each stratum, using on‐line software by the MANAS trial statistician (HW). A given seed number was used to enable the randomisation procedure to be reproduced. This guards against mis‐allocation or changes in allocation at a later stage" Quote 2: "for phase 1, 17 facilities in Goa met these inclusion criteria, of which 12 were randomly selected for inclusion in the trial. PHC facilities were first stratified by the presence or absence of a visiting psychiatrist and then randomised within four strata defined by size" Quote 3: "12 of the 22 eligible GP facilities were randomly selected for phase 2 of the trial. The 12 GP facilities were randomised within two strata defined by size. For both phases, facilities were randomly allocated within each stratum to either the intervention or control arm using a 1:1 allocation ratio using a computer‐generated randomisation sequence" |
| Allocation concealment (selection bias) | Low risk | Quote 1: "randomly allocating unique patient IDs [identification number] so that there is no association between the ID number and the facility identity" Quote 2: "assessing the efficacy of blinding (through asking assessors to guess which arm the participant is allocated to) at the end of the trial" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: this was a cluster‐randomised trial, but non‐blinding of participants was unlikely to affect outcomes |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | Quote 1: "health assistant completes baseline CIS‐R schedule" Quote 2: "masking of the research assessor maximised by; undertaking assessment at home; randomly allocating clinic identification numbers to patients so that there was no association between their number and identity of the facility; outcome assessment by an independent association and undertaking primary outcome assessment before all assessment" Quote 3: "emphasizing to assessors that all patients are receiving an intervention (not specifying whether this is enhanced care or Collaborative Stepped Care) and that there is genuine equipoise about which is better. but also: health assistant completes baseline CIS‐R schedule" |
| Baseline outcome measurements similar | Low risk | Quote 1: "we recorded little intra‐cluster correlation (0.03), and the coefficient of variation (k) for prevalence of these disorders at baseline in all patients who screened positive was 0.08" Quote 2: "although participants in the enhanced usual care group were more likely to have depression, the proportion of patients with these disorders according to ICD‐10 and mean CIS‐R scores were similar" |
| Baseline characteristics similar? | Low risk | Quote 1: "characteristics of patients differed by clinic type" Quote 2: "distribution of these disorders between groups was similar; although participants in the enhanced usual care group were more likely to have depression, the proportion of patients with these disorders according to ICD‐10 and mean CISR scores were similar" Comment: baseline characteristics were dissimilar but were adjusted for in the analysis |
| Incomplete outcome data (attrition bias) Efficacy data | Unclear risk | Quote: "1160 participants (85%) in the collaborative stepped‐care group and 1269 (88%) in the control group completed the 6‐month outcome assessment" Comment: low risk at 6 months, but high risk at 12 months: significant difference in attrition between collaborative care and control groups (81% vs 77%; P = 0.01), which may not be clinically significant; nevertheless no reasons stated for this variation in dropout |
| Incomplete outcome data (attrition bias) Safety data (e.g. adverse events) | Low risk | Quote 1: "no stopping rules are proposed because serious adverse events are not expected in the trial since none of the treatments being offered are experimental or associated with serious outcomes" Quote 2: "there were seven serious adverse events (three deaths and four suicide attempts) in the collaborative stepped‐ care group and 12 in the enhanced usual care group (six deaths and six suicide attempts). None of the deaths were from suicide" |
| Protection against contamination | Low risk | Quote: "we do not anticipate a significant risk of contamination, i.e. patients moving from an Enhanced usual care control facility to an intervention facility, due to the geographical spread of facilities, and because no publicity will be produced regarding the availability of the intervention in other facilities" |
| Selective reporting (reporting bias) | Low risk | Comment: clinical trial protocol also stated disability outcomes, which were not reported in this paper. However, disability scores were used to generate QALY results (to calculate ICERs), which were reported in a separate paper (Buttorf 2012) that focused on economic outcomes |
| Other bias | Low risk | Comment: no other bias detected |
Patel 2017.
| Study characteristics | ||
| Methods |
Study design: RCT Duration of study: 28 October 2013 and 30 August 2016 |
|
| Participants |
Country: India Income classification: lower‐middle income Geographical scope: Goa, India Healthcare setting: PC facility Mental health condition: depression Population 1. Age: 18 to 65 years 2. Gender: both 3. Socioeconomic background: lower SEC 4. Inclusion criteria a. Above the age of 18 but below the age of 65 b. Reside within the geographic area selected for the PHC c. Plan to stay at the same address for at least 12 months d. Able to speak one of the following languages: Konkani/Hindi/Marathi/English e. Must not have been screened in the previous 3 months f. PHQ‐9 score > 14 5. Exclusion criteria a. Pregnant women b. Patients with drinking problems c. Patients who need urgent medical attention (defined as needing emergency treatment and/or in‐patient admission) d. Patients unable to communicate clearly (e.g. due to speech or hearing disability) e. In receipt of PREMIUM counselling treatment f. Patient lives together in the same household with previously recruited patient or is in regular contact with previously recruited patient |
|
| Interventions |
Stated purpose: to assess the effectiveness and cost‐effectiveness of a brief psychological treatment (Healthy Activity Program (HAP)) for delivery by lay counsellors to patients with moderately severe to severe depression in primary healthcare settings INTERVENTION (n = 245) Name: Healthy Activity Program (HAP) Delivered by: LHW Title/name of PW and number: lay counsellors ‐ 11 1. Selection: trained and met competency standards 2. Educational background: not mentioned 3. Training: an international expert in behavioural activation (SD) trained and provided ongoing supervision for 5 local specialists, who in turn provided onsite training and supervision for lay counsellors. Training of lay counsellors involved a 3‐week participatory workshop covering both HAP and CAP treatments, followed by an internship phase of 6 months, in which trainee counsellors delivered treatment to eligible patients in primary healthcare clinics, combined with peer‐led group supervision as trainees gained experience in delivery of treatment. 11 counsellors who met competency standards as assessed by standardised role‐plays and therapy quality measures participated in the trial 4. Supervision: weekly peer‐led supervision in groups of 4 to 6 that involved rating of a randomly selected 10% of recorded sessions on the HAP Therapy Quality Scale (TQS)‐26 and individual supervision twice monthly 5. Incentives/remuneration: not mentioned. Intervention details 1. Duration/frequency: 6 to 8 individual 30– to 40‐minute sessions, with initial sessions at weekly intervals 2. Content of intervention: EUC plus HAP. HAP is a manualised psychological treatment based on behavioural activation that includes the following strategies: psychoeducation, behavioural assessment, activity monitoring, activity structuring and scheduling, activation of social networks, and problem‐solving. Additional strategies used in response to specific needs consisted of behavioural strategies to improve interpersonal communication skills and decrease rumination, advice regarding sleep problems and tobacco cessation, and relaxation training CONTROL: enhanced usual care (n = 248) EUC comprised routine consultation with the PHC physician, enhanced by providing PHQ‐9 screening results to both the PHC physician and the patient, and providing copies of a contextualised version of the WHO Mental Health Gap Action Programme (mhGAP) guidelines to the PHC physician, which included information on when and where to refer for psychiatric care. EUC was available to all trial participants CO‐INTERVENTIONS: nil |
|
| Outcomes |
Patients Primary outcomes 1. Depression symptom severity on Beck Depression Inventory version II 2. Remission from depression (PHQ‐9 score < 10) at 3 months in the intention‐to‐treat population Secondary outcomes 1. Disability 2. Days unable to work 3. Behavioural activation 4. Suicidal thoughts or attempts 5. Intimate partner violence 6. Resource use and costs of illness Adverse events 1. Deaths 2. Suicide attempts 3. Unplanned admissions to hospital from any cause Carers Nil Process/health workers Nil Economic outcomes Cost per QALY gained (from health system and societal perspectives) Time points: baseline, 3 months post intervention |
|
| Notes |
Source of funding: Wellcome Trust Senior Research Fellowship Notes on validation of instruments (screening and outcomes): all validated Additional information: Patel V, Weobong B, Nadkarni A, et al. The effectiveness and cost effectiveness of lay counsellor‐delivered psychological treatments for harmful and dependent drinking and moderate to severe depression in primary care in India: PREMIUM study protocol for randomized controlled trials. Trials 2014;15:101. Declarations of interest ‐ DM received honoraria for lectures not related to this work from Otsuka Pharmaceuticals, Janssen‐Cilag, and H Lundbeck. CGF holds a Principal Research Fellowship from the Wellcome Trust. All other study authors declare no competing interests Handling the data: as per footnotes in data and analysis Prospective trial registration number: International Society for the Registration of Clinical Trials (ISRCTN95149997) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "an independent statistician generated a randomisation list in randomly sized blocks (block size four to six [two to four for men because we anticipated relatively fewer men on the basis of the epidemiology of the prevalence of depression and did not want imbalance between groups]), stratified by PHC and sex. Assignments..." Judgement comment: random sequence generation done |
| Allocation concealment (selection bias) | Low risk | Judgement comment: cluster‐RCT but appropriate block randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Cluster allocation but different PHCs, so low risk of contamination? Protocol: quality indicators will be assessed through ratings of 10% of audio‐recording transcripts of all sessions by independent experts blind to outcome data using respective PT quality assessment scales. Participants will be purposively recruited after completion of 12‐month outcome assessments by independent statistician (to maintain blinding) to ensure balance of arms, recovery status, and PHCs. Baseline assessments will be carried out by health assistants in the PHC before randomisation |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | Outcomes were assessed blindly: "the three‐ and 12‐month outcome assessments will be carried out by an independent team of field workers who have no contact with the PHCs and who will be entirely community based (that is, assessments will be done at home, to minimize the risk of unmasking)" |
| Baseline outcome measurements similar | Low risk | Table 1: baseline outcome data similar in terms of PHQ‐9 score |
| Baseline characteristics similar? | Low risk | Baseline characteristics similar |
| Incomplete outcome data (attrition bias) Efficacy data | Low risk | All outcome data from protocol reported |
| Incomplete outcome data (attrition bias) Safety data (e.g. adverse events) | Low risk | All adverse events reported (suicide attempts and relapse and suicidal behaviour) |
| Protection against contamination | Low risk | Contamination unlikely between PHC groups as patients attending practice usually will be from the locality |
| Selective reporting (reporting bias) | Low risk | No evidence of selective outcome reporting. All outcomes planned in published trial protocol were reported |
| Other bias | Unclear risk | Some scores reported at 12 months are not in original trial published; however they were in the 'analysis plan' (e.g. PHQ‐9 score at 12 months (mean); relapse scores, recovery scores). This has little impact potentially on outcomes (other than PHQ‐9 is not validated as a good score in India (Patel 2008) |
Peltzer 2013.
| Study characteristics | ||
| Methods |
Study design: cluster‐RCT, allocation by primary care clinic, 20 clinics in each arm (40 in total), single blind (outcome assessors) Duration of study: participants were recruited between April and October 2011 and were followed up for 6 months |
|
| Participants |
Country: South Africa Income classification: upper‐middle Geographical scope: urban and rural Healthcare setting: public primary healthcare facilities Mental health condition: alcohol use disorder Population (mention whether patient, carer, or dyad) 1. Age: ≥ 18 2. Gender: both 3. Socioeconomic background: majority of participants scored medium on the poverty index 4. Inclusion criteria (including threshold cutoff score of measurement tool) a. TB patients (new and re‐treatment) b. AUDIT ≥ 8 (men)/≥ 7 (women) 5. Exclusion criteria: none specified |
|
| Interventions |
Stated purpose: to assess the effectiveness of the Screening and Brief Interventions (SBI) among tuberculosis patients found to be misusing alcohol INTERVENTION (n = 584) Name: brief intervention Delivered by: LHW and PHP (lay HIV counsellors with the assistance of trained nurses) Title/name of PW and number: lay HIV counsellors (number not specified) and up to 4 nurses per study clinic 1. Selection: lay HIV counsellors from study clinics who spoke the predominant languages, namely, English, Afrikaans, i‐Zulu, i‐Xhosa, and Tswana, in the respective areas. Nurses from study clinics 2. Educational background: not specified 3. Training: 3 days for counsellors and 2 days for nurses. Training comprised 4 elements: orientation to relevant practice, standardised PowerPoint presentation, tape‐recorded simulated consultations with trained actors, ongoing supervision. Counsellors were assessed for adherence to treatment protocol and were supervised and trained until a required standard of practice had been reached 4. Supervision: bi‐weekly, by project trainers Intervention details: HIV lay counsellors delivered the intervention, and trained nurses assisted when necessary 1. Duration/frequency: two 15‐ to 20‐minute sessions on Day 1 and within 1 month later 2. Content of intervention: goals of brief intervention were (1) to identify alcohol‐related problems mentioned in the interview, (2) to emphasise sensible drinking limits and make sure that patients realise they are in the risk drinking category, (3) to provide feedback on the relationship between alcohol and TB treatment, (4) to work through the first 3 sections of the problem‐solving manual while mentioning the value of reviewing other sections, (5) to describe drinking diary cards, and (6) to identify a helper. The intervention was guided by the Information‐Motivation‐Behaviour Skills model CONTROL: received a health education leaflet on responsible drinking (n = 269) CO‐INTERVENTIONS: TB treatment |
|
| Outcomes |
Patients 1. AUDIT score* 2. Percentage of participants in high risk or dependent category 3. Percentage of participants in high risk category 4. Percentage of participants in dependent category 5. Percentage of participants engaging in heavy episodic drinking 6. TB treatment outcome# 7. Daily or almost daily tobacco use# 8. TB treatment cure or completion# 9. Death Carers None Process/health workers None Economic outcomes None Time points post intervention: 2 months, 5 months |
|
| Notes |
Source of funding: Department of Health of South Africa Notes on validation of instruments (screening and outcomes): validated Additional information: declaration of interests ‐ study authors declared no competing interests Handling the data (e.g. imputed values/other calculations we have made): nil Prospective trial registration number: PACTR201105000297151 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomisation was conducted using a secure remote randomization service" Judgement comment: although a randomisation service carried out randomisation, it is unclear how this randomisation was conducted (i.e. computer software) |
| Allocation concealment (selection bias) | Low risk | Quote: "randomisation was stratified by clinic type (clinic and community health centre) and TB case load. The standby and reallocated clinics were subsequently randomly allocated in a similar..." Judgement comment: unit of allocation is clearly described. Randomisation was conducted by a secure randomisation service; therefore concealment was ensured |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and interventionists were not blind to their allocation status, but this is unlikely to have influenced outcomes |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | "to protect against information biases in the reporting of alcohol use and TB treatment adherence behaviour, the data collection team who assessed the outcomes were blinded to the clinic’s status as intervention or control group" Outcome assessors were blinded to allocation of intervention |
| Baseline outcome measurements similar | Low risk | Statistical analysis was intention‐to‐treat. All outcomes were measured and presented before and after intervention. Differences were adjusted for during analysis |
| Baseline characteristics similar? | Low risk | Baseline characteristics are reported in Table 1; differences were accounted for during analyses |
| Incomplete outcome data (attrition bias) Efficacy data | Low risk | Although there was a difference in dropout, this was accounted for and was analysed during analyses of results |
| Incomplete outcome data (attrition bias) Safety data (e.g. adverse events) | Low risk | Missing data were adjusted for during analyses |
| Protection against contamination | Low risk | Judgement comment: allocation was by clinic; therefore it is unlikely that there was contamination between groups |
| Selective reporting (reporting bias) | Low risk | All outcomes described in trial registry ‐ PACTR201105000297151 ‐ are reported |
| Other bias | Unclear risk | McCambridge and Kypri reviewed that simply answering questions on drinking in brief intervention trials appears to alter subsequent self‐reported behaviour. This potentially generates bias by exposing non‐intervention control groups to an integral component of the intervention |
Peltzer 2019.
| Study characteristics | ||
| Methods |
Study design: 2‐arm cluster‐randomised controlled feasibility trial; unit of allocation was community health centre, 6 clusters in each arm (12 in total) Duration of study: recruited between 10 April 2014 and 30 January 2017; follow‐up until 12 months' postpartum |
|
| Participants |
Country: South Africa Income classification: upper‐middle income from 2014 to 2018 Geographical scope: Mpumalanga Province of South Africa (rural) Healthcare setting: 12 community health clinics Mental health condition: perinatal mental disorders Population: HIV‐infected pregnant women with male partners 1. Age: ≥ 18 years 2. Gender: female 3. Socioeconomic background: 36.4% with income < USD21/month 4. Inclusion criteria a. HIV‐seropositive pregnant women with partners b. 8 to 24 weeks pregnant (typical time of entry into antenatal clinic care) c. Age > 18 d. In phase 2 (couples’ phase), both women and their partners will be enrolled (1) For the purposes of this study, primary male partners are defined as (1) husband, (2) current baby's father, or (3) current sexual partner 5. Exclusion criteria a. Persons actively psychotic (auditory or visual hallucination) or intoxicated (e.g. under the influence of alcohol or illegal drugs) (1) Not eligible and referred for treatment (2) Following resolution of symptoms, these persons will be eligible for the study N.B.: Any person presenting for sessions actively psychotic or intoxicated will be referred for treatment and will not be eligible to participate in sessions until symptoms are resolved due to reduced likelihood of benefit from sessions |
|
| Interventions |
Stated purposes: to test the effectiveness of a behavioural intervention for increasing Prevention of Mother‐to‐Child Transmission of HIV (PMTCT) protocol uptake among South African HIV‐positive pregnant women; to determine whether participation of male partners will have additional positive impact on PMTCT uptake INTERVENTION (phase 1; n = 198) (phase 2, female; n = 243) (phase 2, male; n = 125) Name: "Protect Your Family" intervention Delivered by: LHW Title/name of PW and number: lay health care workers (n not mentioned) 1. Selection: not specified 2. Educational background: not mentioned 3. Training: study staff at CHC sites underwent formal training on the study protocol, informed consent, protection of human subjects, recruitment, assessment and use of ACASI technology, with in‐depth review of the meaning of each item in assessment instruments presented by ACASI, presented by University of Miami (UM) and HSRC investigators. Experimental condition staff attended a 5‐day training course that included an intensive review of the "Protect Your Family" intervention manual, the PMTCT protocol, and use of cognitive‐behavioural (CB) intervention strategies in the intervention, as well as how to manage sensitive issues (such as serostatus disclosure, IPV, gender dynamics, sexual risk reduction, and safer conception practices). Following training, all staff received additional supervision at CHC sites on the study protocol for data collection. In addition, experimental condition staff will receive guided training and practice on the intervention under supervision of the intervention co‐ordinator, who will act as leader and then co‐leader of the intervention at each experimental CHC site for the first 2 cohorts. Thus, each experimental clinic staff person is currently conducting 2 sequences of group sessions and individual counselling sessions under supervision of the HSRC co‐ordinator 4. Supervision: experimental condition staff will receive additional guided training and practice on the intervention under supervision of the intervention co‐ordinator, who will act as leader and then co‐leader of the intervention at each experimental CHC site for the first 2 cohorts. Thus, each experimental clinic staff person is currently conducting 2 sequences of group sessions and individual counselling sessions under supervision of the HSRC co‐ordinator 5. Incentives/remuneration: not mentioned Intervention details 1. Duration/frequency: 2 (phase 1 and 2, females only) to 4 (phase 2, males only) group sessions during the antenatal period, followed by 3 individual (phase 1)/couple (phase 2) sessions at 32 weeks' pregnancy and at 6 weeks' and 3 months' postpartum 2. Content of intervention: "Protect Your Family" intervention is a manual, closed, structured behavioural risk reduction programme targeting prevention of vertical transmission; the importance of adherence to PMTCT and medication use; HIV testing of family members and prevention of transmission of HIV; stigma; sero status disclosure; partner communication; IPV; safe infant feeding; safer conception; family planning; and dual‐method sexual barrier use CONTROL: enhanced usual care (EUC) (phase 1; n = 226) (phase 2, females; n = 290) (phase 2, males; n = 237) Control condition staff received an identical 1‐day training session on use of ACASI technology and 4‐hour orientation to the protocol to enable them to conduct time‐equivalent group sessions comprising childhood disease prevention and adult health hazard videotapes (e.g. measles, diarrhoea management, immunisations) Control condition participants receive PMTCT standard of care plus a time‐equivalent, group‐administered video presentation on health promotion and disease prevention (e.g. measles, diarrhoeal management, dysentery and dehydration, immunisations and vaccinations) in 3 group sessions, followed by 1 individual and 2 couple or individual women’s sessions on disease prevention and health promotion CO‐INTERVENTIONS: nil |
|
| Outcomes |
Patients Edinburgh Postnatal Depression Scale (EPDS)‐10 Carers Nil Process/health workers Nil Economic outcomes Nil Time points: baseline, 3 months and 9 months post intervention (i.e. 6 months and 12 months postpartum) |
|
| Notes |
Source of funding: National Institute of Child Health and Human Development, R01HD078187, US National Institutes of Health, with support from the Miami CFAR, NIH grant numberP30 AI073961 Notes on validation of instruments (screening and outcomes): locally validated Additional information: declaration of interests ‐ study authors reported no conflicts of interest Study also included in prevention review, as unsure about the population (roughly half the intervention group has mental distress or a mental disorder at baseline; thus the intervention may be a treatment, whereas for the other half, it could be a prevention strategy) Handling the data: as per footnotes in data and analysis Prospective trial registration number: NCT02085356 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "twelve CHCs were matched in a 1:1 ratio according to patient census and HIV rates and randomized to intervention or control condition after stratification by case load in the upper 50th percentile of MTCT rates at study onset (> 13%) (more details in Jones et al. (2014))" Judgement comment: from protocol: "the twelve CHCs were matched in a 1:1 ratio according to patient census and average ANC volume, and one clinic in each pair was randomly assigned to the experimental or control condition using a computer program written by the data manager. The matched clinics were then assigned to the opposite condition. The randomization process was carried out by four people. The first conducted the computer‐generated randomization assignments stratified by clinic size (selected a seed for the random number generator, ran the program, and completed the table of condition assignments)."adequate random sequence generation" |
| Allocation concealment (selection bias) | Low risk | Judgement comment: allocation was done by institution. Cluster‐RCT |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | From Jones 2014 protocol paper: "only the Human Sciences Research Council (HSRC) study staff activating and overseeing the sites were aware of site assignment. All assessments will be conducted using an audio computer‐assisted self‐interview (ACASI) program. As such, participants enter their data themselves and are blind to their assignment. Following randomization, clinic sites were activated individually, and clinic staff are blinded to the condition. Training for clinic study staff was conducted by condition, and clinic study staff conducting the study are also blind to clinic randomization status. Finally, data analysis to evaluate study outcomes will be blinded to the clinic’s status as an intervention or control intervention arm" |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | All assessments will be conducted using an audio computer‐assisted self‐interview (ACASI) programme. As such, participants enter their data themselves and are blind to their assignment |
| Baseline outcome measurements similar | Low risk | Baseline measurements of outcomes were similar; no statistical differences |
| Baseline characteristics similar? | High risk | There was a significant difference in the poverty line, wealth (greater percentage in the experimental group were poor (44.5%; 312 rand or more) vs control (55.5%)). SImilarly increased alcohol use experimental 58.8% vs control 41.2%. Also in experimental group: poorer adherence to HIV treatment, more stigma, and more physical and psychological intimate partner violence. This may affect results; It seems that baseline scores in experimental group were higher than in control group, but this may not be significant |
| Incomplete outcome data (attrition bias) Efficacy data | High risk | Fewer men were initially recorded at baseline antenatal assessment after women were randomised (intervention 222 vs control 319). Also there is larger dropout of men at group sessions (intervention 117/222 vs control 234/319). The fact that fewer men and smaller proportion of men are included in the intervention group (and we know this group is poorer) will possible bias results |
| Incomplete outcome data (attrition bias) Safety data (e.g. adverse events) | Low risk | Maternal deaths reported and similar in both arms; not discussed whether or not investigators thought this was linked to intervention |
| Protection against contamination | Low risk | Judgement comment: CRCT; there has been no unit of analysis error |
| Selective reporting (reporting bias) | High risk | In the protocol, postpartum depression outcomes are listed as co‐variates; primary outcomes are infant HIV status In the final Peltzer paper (2019), it is suggested that postpartum depression was the main focus; not able to find infant HIV status/transmission in this paper |
| Other bias | Low risk | No other risks of bias identified |
Pengpid 2013.
| Study characteristics | ||
| Methods |
Study design: RCT; single blind (outcome assessors) Duration of study: August 2011 to November 2012 |
|
| Participants |
Country: South Africa Income classification: upper‐middle income Geographical scope: urban Healthcare setting: university Mental health condition: alcohol use disorder Population (mention whether patient, carer, or dyad) 1. Age: ≥ 18 2. Gender: both 3. Socioeconomic background: 43% to 45.3% wealthy/quite well‐off categories 4. Inclusion criteria (including threshold cutoff score of measurement tool) a. University students age ≥ 18 who visited public recruitment centres and scored ≥ 8 on AUDIT 5. Exclusion criteria a. Pregnant b. Under treatment for alcohol use disorder c. Scored < 8 on AUDIT |
|
| Interventions |
Stated purpose: to determine the efficacy of a brief alcohol intervention to reduce alcohol use by problem drinkers among university students INTERVENTION (n = 79) Name: brief intervention Delivered by: PHP Title/name of PW and number: assistant nurse counsellors ‐ number not specified 1. Selection: not specified 2. Educational background: not specified 3. Training (contents, duration, by whom): 5‐day workshop involving role‐playing and general skills training 4. Supervision: bi‐weekly, by project manager Intervention details: issuing a health education leaflet, simple advice, and brief counselling 1. Duration/frequency: single 20‐minute session 2. Content of intervention (by types of health workers and per patients/carers): Information‐Motivation‐Behavioural Skills (IMB) model was used to guide the alcohol reduction intervention. Major steps were (1) to identify any alcohol‐related problems, (2) to introduce the sensible drinking leaflet, emphasise the idea of sensible limits, and make sure that patients realise they are in the medium‐risk drinking category, (3) to work through the first 3 sections of the problem‐solving manual while mentioning the value of reviewing the other sections, (4) to describe drinking diary cards, and (5) to identify a helper CONTROL (n = 68) Feedback on initial alcohol screening. Health leaflet on responsible drinking CO‐INTERVENTIONS: nil |
|
| Outcomes |
Patients 1. AUDIT score 2. Percentage of participants in high‐risk drinking group 3. Percentage of participants with alcohol dependence 4. Heavy episodic drinking score 5. Cannabis use in the last month 6. Self‐rated health 7. PTSD score 8. CES‐D: Depression, Drinking norms score# 9. Attitude towards risky alcohol use behaviour score# 10. Tobacco use#. Carers Nil Process/health workers Nil Economic outcomes Nil (asterisk for study's primary outcomes; star: outcomes that we have not reported in this review) Time points post intervention: 6 months, 12 months |
|
| Notes |
Source of funding: Directorate General for Development Cooperation (DGDC) through the Flemish Interuniversity Council (VLIR‐UOS) Notes on validation of instruments (screening and outcomes): validated Additional information: declarations of interest ‐ study authors declare no conflicts of interest Handling the data (e.g. imputed values/other calculations we have made): calculate percentage of participants who became AUDIT < 8 at follow‐up (recovery) Prospective trial registration number: nil |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Students were randomised using sequentially numbered opaque sealed envelopes prepared according to a computer‐generated (prepared using Stata version 10) randomisation allocation sequence" |
| Allocation concealment (selection bias) | Low risk | Students were randomised using sequentially numbered opaque sealed envelopes prepared according to a computer‐generated (prepared using Stata version 10) randomisation allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | RA nurses and students were not blind to the intervention. Not possible to blind participants and personnel to the intervention. Unlikely to affect outcomes |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | Data collection team who assessed outcomes were blind to client status as intervention arm |
| Baseline outcome measurements similar | Unclear risk | Despite randomisation, there was evidence of inequality between control and intervention groups with regard to severity of alcohol use |
| Baseline characteristics similar? | Low risk | "The study groups were equivalent on all characteristics apart from AUDIT levels of alcohol use" |
| Incomplete outcome data (attrition bias) Efficacy data | Low risk | Similar proportions of missing data |
| Protection against contamination | Unclear risk | Judgement comment: allocated by student; high risk of contamination |
| Selective reporting (reporting bias) | Unclear risk | No published clinical trial protocol available. All outcomes mentioned in methods section reported |
| Other bias | Unclear risk | All measures were self‐reported |
Petersen 2014.
| Study characteristics | ||
| Methods |
Study design: cluster‐RCT Duration of study: intervention was conducted in a 6‐month period between 2012 and 2013 |
|
| Participants |
Country: South Africa Income classification: upper‐middle income between 2012 and 2014 Geographical scope: study was conducted at a public clinic in the KwaZuluNatal Province in a periurban area outside of Durban in the eThekwini District, situated on the eastern seaboard of South Africa Healthcare setting: PC facility Mental health condition: depression Population 1. Age: ≥ 18 years 2. Gender: both 3. Socioeconomic background: 70% to 80% not working 4. Inclusion criteria a. Participants attending the dedicated ART (HIV anti‐retroviral treatment) clinic for treatment b. 18 years age or older c. Not requiring urgent medical attention d. No difficulty with hearing, speaking, or cognition that would make interviewing difficult e. Screened ≥ 8 on the SRQ‐20 f. Diagnosis of major depressive disorder (MDD) g. Structured clinical interview 5. Exclusion criteria: not mentioned |
|
| Interventions |
Stated purpose: to conduct a pilot randomised controlled trial (RCT) to evaluate potential effectiveness of an adapted group‐based HIV counsellor‐delivered intervention for treating depression in people living with HIV/AIDS INTERVENTION (n = 17) Name: group‐based counselling adapted from a local group‐based interpersonal therapy (IPT) intervention Delivered by: LHW Title/name of PW and number: lay HIV counsellors ‐ 6 1. Selection: lay HIV counsellors, historically funded by US President's Emergency Plan for AIDS Relief (PEPFAR) to provide health counselling and testing (HCT); based in most primary healthcare (PHC) clinics in South Africa 2. Educational background: not mentioned 3. Training: training was conducted by a clinical psychologist and a clinical psychology trainees. It took place over 4 days. The first 2 days involved training in micro‐counselling skills as well as in different ways of helping, viz psychoeducation, problem management, health thinking, and getting active. The second 2 days involved training in group‐based sessions that drew on the techniques learned during the first 2 days 4. Supervision: adopting the apprenticeship model, which has been shown to be the most appropriate training model within a task‐shifting approach in LMIC (Murray et al, 2011), lay HIV counsellors were supported through via weekly supervision sessions with clinical psychology trainees for the first 2 months, then on a monthly basis. Exposure to the intervention was measured through an attendance register 5. Incentives/remuneration: not mentioned Intervention details 1. Duration/frequency: weekly for 8 weeks (8 sessions) 2. Content of intervention: each session comprised a number of steps starting with introducing a common trigger or exacerbating factor using a vignette. The second step involved asking participants who identify with the story to share their problem. The third step drew on problem management to address the triggers of depression and cognitive‐behavioural techniques for exacerbating factors, promoting healthy thinking in the case of negative intrusive thoughts, and managing behavioural activation for social isolation. The fourth step involved getting participants to identify problems that they were going to work on in the next week CONTROL: treatment as usual (n = 17) Non‐treatment group received normal standard of care, which included counselling services provided by HIV counsellors CO‐INTERVENTIONS: nil |
|
| Outcomes |
Patients 1. PHQ‐9 2. HSCL‐25 3. MSPSS Carers Nil Process/health workers 1. Qualitative process evaluation, e.g. to explore poor uptake, dropout, and loss to follow‐up rates Economic outcomes Nil Time points: baseline, 3 months *post baseline (*1 month post intervention, as the intervention was 8 weeks long) |
|
| Notes |
Source of funding: Health Economics and HIV/AIDS Research Division (HEARD) at University of KwaZulu‐Natal, South Africa Notes on validation of instruments (screening and outcomes): all validated, with resulting item reductions: PHQ‐9 item 8 removed, HSCL‐25 items 13,15, 23, 24 removed, and MSPSS items 8, 12 removed, but not sure how this impacted scoring and cutoffs Additional information: declarations of interest ‐ study authors declared no conflicts of interest Handling the data: as per footnotes in data and analysis Prospective trial registration number: none stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Following recruitment of the final sample, participants were allocated to an intervention and control arm using computer generated random allocation by the third author, who had no knowledge of the participant scores" |
| Allocation concealment (selection bias) | Low risk | Study author who performed random sequence generation did not have knowledge of participants' baseline scores |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not possible to blind participants and personnel to the intervention; unlikely to affect outcomes |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | "Follow up outcome evaluation...was administered by 3 independent fieldworkers who were not informed whether the participants were fewer in the intervention or control arms" |
| Baseline outcome measurements similar | Low risk | Independent sample t‐tests showed no significant differences at baseline between intervention and control group mean scores on PHQ‐9, HSCL‐25, and MSPSS measures |
| Baseline characteristics similar? | Low risk | Chi‐square (χ2 ) analysis did not reveal any significant differences in demographic characteristics between intervention and control arms |
| Incomplete outcome data (attrition bias) Efficacy data | High risk | Around half of initial participants were lost to follow‐up |
| Protection against contamination | Unclear risk | Study takes place in 1 clinic |
| Selective reporting (reporting bias) | Unclear risk | No published clinical trial protocol available; outcomes declared at beginning of the study are reported at the end |
| Other bias | Unclear risk | This is a pilot study; the sample size is small |
Pradeep 2014.
| Study characteristics | ||
| Methods |
Study design: cluster‐RCT. Unit of allocation: village (3 in each arm, 6 total) Duration of study: August 2006 to September 2009 |
|
| Participants |
Country: India Income classification: low income in 2006; lower‐middle income between 2007 and 2009 Geographical scope: 6 villages from rural Bangalore Healthcare setting: PC facility Mental health condition: depression Population 1. Age: 18 to 65 years 2. Gender: female 3. Socioeconomic background: lower SEC (> 90% unemployed; around 22% low SES) 4. Inclusion criteria a. Age ≥ 18 years 2. GHQ ≥ 5 3. MINI plus diagnosis of major depression 4. Treatment‐naïve and did not receive any treatment in the previous 6 months 5. Exclusion criteria: not mentioned |
|
| Interventions |
Stated purposes: to evaluate whether enhanced care resulted in greater numbers of treatment‐naïve women with depression living in the community seeking help from the primary care centre, and to examine whether adherence to antidepressant medication would be better in women receiving enhanced care compared to those given treatment as usual. Secondary objective included whether there was a change in the severity of depression and in quality of life (QOL) before and after treatment intervention INTERVENTION (n = 138) Name: enhanced care Delivered by: LHW and PHP Title/name of PW and number: community health worker (CHW) and primary care physician 1. Selection: CHWs were all women from the local community, had studied up to 10th standard, and had previous experience of working in community mental health programmes 2. Primary care physicians practising at primary care centres 3. Educational background: CHWs: studied up to 10th standard (age 15 to 16 years) 4. Training: PHC physician and CHW trained; details not specified 5. Supervision: not mentioned 6. Incentives/remuneration: not mentioned Intervention details 1. Duration/frequency: monthly for 16 weeks 2. Content of intervention: (1) MONTHLY meetings at PHC with primary care physician for antidepressant treatment initiation (amitriptyline or fluoxetine) and follow‐up. (2) CHWs visited patients immediately following first medical consultation, educated patients and family members about depression and its treatment. They also emphasised taking antidepressant medication and continuing the treatment regimen. This was followed by another visit in the subsequent week to enquire about any possible side effects of medication and clarification of any doubts concerning medical treatment of depression. This pattern of visits was maintained after every monthly consultation with the physician in the TI group. In addition, CHWs visited patients who discontinued medication and/or those who did not visit the PHC for an initial consultation and encouraged them to resume treatment in the intervention group CONTROL: treatment as usual (n = 122) In the TAU group, patients diagnosed with depression were encouraged to seek help from the physician at PHC with no additional input from the CHW CO‐INTERVENTIONS: nil |
|
| Outcomes |
Patients 1. Changes in severity of depression on Hamilton Depression Rating Scale (HDRS‐17) 2. Changes in quality of life on WHO‐QOL (Brev) Scale. Carers Nil Process/health workers 1. Number of women who sought and completed treatment 2. Number of clinic visits 3. Duration of treatment with antidepressant Economic outcomes Nil Time points: baseline, 6 months post intervention |
|
| Notes |
Source of funding: Anuradha Foundation, Los Altos, CA, USA Notes on validation of instruments (screening and outcomes): all validated Additional information: declarations of interest ‐ none Handling the data: as per footnotes in data and analysis Prospective trial registration number: none stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "the six villages covered by the PHC were randomized into two groups of three villages each namely ‘Treatment as usual (TAU)’ and ‘Treatment intervention (TI)’ groups. Cluster randomized analysis was used. Village was taken as the unit of randomization and the analysis was done at the participant level" Judgement comment: no information on random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: no information. Contacted study author. Reply 03/03: "the research assistant who performed the various assessments was blind to the allocation of participants to the intervention groups. In addition, the participants were reviewed by a trained physician at the PHC and [were] blind to the treatment randomization" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not possible to blind participants and personnel to the intervention; unlikely to affect outcomes |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | Research assistant blind |
| Baseline outcome measurements similar | High risk | Higher percentage of recurrent depressive disorders in intervention arm. Higher risk of suicide in control arm. Much higher percentage of lifetime panic disorder in control arm |
| Baseline characteristics similar? | Unclear risk | There are differences in employment levels although low (3.6% in intervention group vs 9% in control group). There seem to have been no dropouts? So unlikely to affect results |
| Incomplete outcome data (attrition bias) Efficacy data | High risk | No dropouts reported in PRISMA diagram, but in text it appears majority of women did not complete treatment; very high number of dropouts |
| Protection against contamination | Low risk | Judgement comment: cluster‐randomisation; CHW only in some villages. Unlikely contamination by other villages, unless a woman from another village may have been opportunistically present during CHW visit in the intervention village to an included participant |
| Selective reporting (reporting bias) | Unclear risk | No protocol available, but measures reported in methods are reported in results. Contacted study authors: no online trial registration |
| Other bias | Low risk | None found |
Rahman 2008.
| Study characteristics | ||
| Methods |
Study design: cluster‐RCT single‐blind study with 2 parallel groups (unit of allocation ‐ union council clusters, 20 clusters per arm, 40 in total; unit of analysis ‐ individual) Duration of study: enrolments between April 2005 and March 2006 |
|
| Participants |
Country: Pakistan Income classification: low‐income country Geographical scope: rural area of Pakistan where there was subsistence farming Healthcare setting: home Mental health condition: antenatal depression in third trimester Population 1. Age: 16 to 45 years 2. Gender: female 3. Socioeconomic background: 68% of cases and controls were poor; nearly 40% relying on well without pump; 55% relying on the field for toilets and "subsistence farming, supplemented by one or more of the men serving in the armed forces or working as government employees, or as semi‐skilled or unskilled labourers in the cities". "Male and female literacy rates are 79.6% and 48.6%, respectively". "Infant mortality rates are 84 per 1000 live births" 4. Inclusion criteria a. Women in the 40 Union Councils who were aged 16 to 45 years, married, and in the third trimester of pregnancy (1) They were enrolled from lists of participants compiled from official registers kept with the Lady Health Workers 5. Exclusion criteria a. Women with a diagnosed serious medical condition requiring inpatient or outpatient treatment b. Pregnancy‐related illness (except for common conditions, such as anaemia) c. Substantial physical or learning disability d. Postpartum or other form of psychosis |
|
| Interventions |
Stated purpose: to develop and deliver a psychological intervention to depressed mothers and their infants through non‐specialist village‐based health workers INTERVENTION (n = 418) Name: thinking healthy programme Delivered by: LHW Title/name of PW and number: lady health workers ‐ 40 1. Selection: existing staff in union councils were trained to deliver the intervention 2. Educational background: completed secondary schools 3. Training: 2‐day workshop and 1‐day refresher 3 months after first training were all given by study team psychiatrist. Here and now problem‐solving CBT was used with a manual that used culturally appropriate illustrations. Included in the training were the 3 steps that helped in avoiding direct confrontation with mothers and managing illiterate mothers 4. Supervision: research team meetings in which "health workers brainstorm for solutions and discuss their successes and failures in a supportive environment" 5. Incentives/remuneration: not mentioned Intervention details 1. Duration/frequency: session every week for 4 weeks in the last month of pregnancy, 3 sessions in the first postnatal month, and nine 1‐monthly sessions thereafter 2. Content of intervention: 3‐step approach: (1) identify unhealthy unhelpful thinking styles and behaviours; (2) replace these with helpful or healthy thinking; (3) provide activities and 'homework' to help mothers practice healthy thinking CONTROL: enhanced usual care (n = 400) "Control clusters received an equal number of visits in exactly the same way as those in the intervention group, but by routinely trained Lady Health Workers (two for each Union Council)" CO‐INTERVENTIONS: none |
|
| Outcomes |
Patients 1. Infant weight and height at 6 and 12 months*§ 2. Maternal depression 3. Exclusive breastfeeding§ 4. Number of diarrhoeal episodes in infants in the 2 weeks before interview§ 5. Records of immunisation§ (with or without up‐to‐date immunisation status) 6. Use of contraception§ 7. Whether both parents set aside time every day to play with their infant§ Carers (mothers) 1. Structured clinical interview for DSM‐IV diagnosis (screening) 2. HDRS (for outcomes) 3. Brief disability questionnaire§ 4. Global assessment of functioning questionnaire 5. Multi‐dimensional scale for perceived social support§ Process/health workers None Economic outcomes None (*: primary outcomes of the study; §: outcomes that we have not reported in this review) Time points post intervention: baseline, 3 months (6 months postnatally), 9 months (12 months postnatally) |
|
| Notes |
Source of funding: this research was funded by a career development fellowship awarded to Atif Rahman by Wellcome Trust, UK Notes on validation of instruments (screening and outcomes): Structured Clinical Interview for DSM‐IV diagnosis and HDRS are internationally validated, but not specified if validated for Pakistani settings. Other mother outcome scales not validated. Child outcome tools validated Additional information: study protocol is not present. Declarations of interest ‐ study authors declared no conflicts of interest Handling the data: as per footnotes in data and analysis Prospective trial registration number: ISRCTN65316374 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "40 Union Councils in the two subdistricts of the study area. These subdistricts were geographically contiguous and ethnically, culturally, and socioeconomically homogeneous. All the units were eligible for randomisation, which was done by an independent trial centre in Islamabad, before recruitment of participants. These administrative units were assigned by random allocation with a table of random numbers by a researcher who was not involved in the study and who was unaware of the identity of the Union Councils. Lady Health Workers from each Union Council were enrolled to participate in the study before randomisation" Comment: adequate |
| Allocation concealment (selection bias) | Low risk | Quote: "the interviewers were unaware of the allocation status of the Union Councils (because they had no contact with the team that did the randomisation), and we took care to ensure they remained so; none of the interviewers resided in the study area, and throughout the duration of the study they had no contact with the Lady Health Workers or any other health personnel in the study area. Mothers were asked not to tell the interviewers anything about their sessions with Lady Health Workers" Comment: adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "mothers in the control clusters received an equal number of visits in exactly the same way as those in the intervention group, but by routinely trained Lady Health Workers (two for each Union Council). These health workers in both groups received monthly supervision, and were monitored by the research team to ensure that they were attending the scheduled visits. In practice, the Lady Health Workers seldom provide such structured and monitored care in the community. The control group thus received what would be regarded as ideal care, which we called enhanced routine care" |
| Blinding of outcome assessment (detection bias) all outcomes | Unclear risk | Subjective outcomes: Quote: "the interviewers were unaware of the allocation status of the Union Councils (because they had no contact with the team that did the randomisation), and we took care to ensure they remained so; none of the interviewers resided in the study area, and throughout the duration of the study they had no contact with the Lady Health Workers or any other health personnel in the study area. Mothers were asked not to tell the interviewers anything about their sessions with Lady Health Workers" Comment: likely low risk, although small risk that mothers may have told aspects of their interactions with LHWs to interviewers Objective outcomes: Quote: "all infant outcomes were assessed by researchers unaware of the psychiatric status of the mother" Comment: > 80% of participants deemed low risk |
| Baseline outcome measurements similar | Low risk | Comment: all similar |
| Baseline characteristics similar? | Low risk | Comment: all similar |
| Incomplete outcome data (attrition bias) Efficacy data | Low risk | Comment: all outcomes stated to be collected are reported |
| Protection against contamination | Low risk | Quote: "normally one Basic Health Unit provides primary health care for one Union Council and all affiliated Lady Health Workers work in villages within that Union Council only. Supervision of health workers takes place in the Union Council. Thus the risk of contamination of the control group with the intervention is negligible" |
| Selective reporting (reporting bias) | Low risk | Comment: no selective reporting |
| Other bias | Low risk | Comment: no other obvious sources of bias |
Rahman 2016.
| Study characteristics | ||
| Methods |
Study design: 2‐arm single‐blind randomised controlled trial Duration of study: 1 November 2014 through 28 January 2016 |
|
| Participants |
Country: Pakistan Income classification: lower‐middle income Geographical scope: conflict affected city of Peshawar Healthcare setting: 3 primary care centres, covering population of 30,000 to 50,000 individuals each, are government facilities staffed by physician, midwife, paramedic staff, and community Mental health condition: prodromal/distress Population: primary care attendees scoring ≥ 2 on GHQ and exhibiting functional impairment 1. Age: 18 to 60 years 2. Gender: both 3. Socioeconomic background: not mentioned 4. Inclusion criteria a. Score ≥ 3 on 12‐item General Health Questionnaire (GHQ‐12) AND b. Score ≥ 17 on WHO Disability Assessment Schedule 2.0 (WHODAS 2.0) 5. Exclusion criteria a. Imminent risk of suicide b. Severe mental disorder (e.g. psychotic disorders, substance dependence) c. Severe cognitive impairment (e.g. severe intellectual disability, dementia) |
|
| Interventions |
Stated purpose: to test the effectiveness of a multi‐component behavioural intervention delivered by lay health workers to adults with psychological distress in primary care settings INTERVENTION (n = 146) Name: problem management plus Delivered by: LHW Title/name of PW and number: lay healthcare workers ‐ 9 1. Selection: not specified 2. Educational background: 12 to 16 years' education 3. Training: 8 days' training, compromising education about common mental disorders, basic counselling skills, delivery of intervention strategies, self‐care provided by local mental health specialists who had received 6 days training from master trainer 4. Supervision: supervised in 2 groups on a weekly basis (2 hours). One in‐country supervisor (N.R.A.) directly observed a randomly selected sample of 10% of health workers’ sessions (n = 80; 10 sessions per health worker) and used a checklist to systematically assess fidelity to the intervention. In‐country supervisors were supervised (1 to 2 hours per month by Skype) by the master trainer, building their skills in the intervention and in training and supervision of others 5. Incentives/remuneration: not mentioned Intervention details 1. Duration/frequency: 5 weekly 90‐minute individual sessions 2. Content of intervention: (a) intervention was transdiagnostic (i.e. targeted symptoms across a range of conditions rather than diagnosis‐specific); (b) session 1 oriented participants to the intervention with motivational interviewing techniques to improve engagement, provided information about common reactions to adversity, and taught participants a basic stress management strategy (slow breathing). This strategy was practised at the conclusion of every session to enhance learning; (c) session 2 addressed a participant‐selected problem using problem‐solving techniques and introduced behavioural activation, during which individuals were encouraged to re‐engage gradually with pleasant and task‐oriented activities to improve mood and functionality; (d) sessions 3 and 4 supported participants’ continued application of problem‐solving, behavioural activation, and stress management and introduced strategies to strengthen social support networks; and (e) in session 5, education about retaining treatment gains was provided and all learned strategies were reviewed CONTROL: enhanced usual care (EUC) (n = 160) Participants randomised to enhanced usual care were seen at least once by their primary care physician, who had received 5‐day training by the Lady Reading Hospital as part of the national community mental health programme. Study participants and their accompanying family members were provided psychoeducation and the opportunity to talk about their health in a supportive environment CO‐INTERVENTIONS: nil |
|
| Outcomes |
Patients 1. HADS 2. PCL‐5 3. WHODAS 2.0 4. PHQ‐9 Carers Nil Process/health workers Nil Economic outcomes Nil Time points: baseline, 6 months and 12 months post intervention |
|
| Notes |
Source of funding: Enhanced Learning and Research for Humanitarian Assistance’s Research for Health in Humanitarian Crises initiative funded by the UK Department for International Development and Wellcome Trust Notes on validation of instruments (screening and outcomes): locally validated Additional information: trial protocol; declaration of interests ‐ study authors reported no conflicts of interest Handling the data: as per footnotes in data and analysis Prospective trial registration number: ACTRN12614001235695 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was performed using computerized software on 1:1 basis" |
| Allocation concealment (selection bias) | Low risk | Randomisation was conducted by an independent research assistant located off‐site |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not possible to blind participants and personnel to the intervention; unlikely to affect outcomes |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | All assessments were performed by trained research assistants blind to allocation status of participants |
| Baseline outcome measurements similar | Low risk | Baseline HADS‐A, HADS‐D, PCL‐5, PSYCHLOPS, and PHQ‐9 scores were similar in both groups. Only baseline WHODAS score was slightly different (difference of ‐1.45; 95% CI ‐2.83 to ‐0.06) between groups |
| Baseline characteristics similar? | Low risk | There were no differences between groups in demographic characteristics, except in education, with more participants in the control group having no education |
| Incomplete outcome data (attrition bias) Efficacy data | Low risk | "We were able to access only about 40% of the sample at 1‐week post‐treatment follow‐up...however, the risk of bias due to this limitation is likely to be small: there were similar rates of attrition across both groups of the trial; there was a high response rate at the subsequent 3‐month follow‐up when the situation was relatively stable; and the use of mixed models in the context of repeated outcome measurement analyses using the random‐effects model adjusts for bias induced by missing values" Similar attrition rates in intervention and control groups |
| Incomplete outcome data (attrition bias) Safety data (e.g. adverse events) | Low risk | No adverse outcomes happened. Missing data were similar in both groups |
| Protection against contamination | Unclear risk | "Possible contamination of the control group with elements of intervention cannot be entirely ruled out as the health workers and participants attended the same health care facilities and may have interacted" |
| Selective reporting (reporting bias) | Low risk | All outcomes described in trial registry have been reported |
| Other bias | Low risk | No other sources of bias were found |
Rahman 2019.
| Study characteristics | ||
| Methods |
Study design: single‐blind, cluster‐RCT. Unit of allocation: lady health worker (LHW) community cluster ‐ 17 in each arm, 34 total Duration of study: January to August 2016 (enrolled between 11 January 2016 and 21 August 2016; 1 week after intervention, follow‐up results; primary outcomes 3 months after intervention) |
|
| Participants |
Country: Pakistan Income classification: lower‐middle income between 2016 and 2017 Geographical scope: 2 rural union councils of Swat, Odigram, and Ghalegay, in community clusters (defined as a neighbourhood of about 150 households covered by an LHW) (estimated population of about 2 million) Healthcare setting: community‐based (delivered through community‐based groups) Mental health condition: adults (women) with anxiety, depression, PTSD Population 1. Age: 18 to 60 years 2. Gender: female 3. Socioeconomic background: more than 85% live in rural areas; 2% no education; 85% housewives 4. Inclusion criteria a. Scored ≥ 3 on General Health Questionnaire‐12 (GHQ‐12) ‐ a screening questionnaire for common mental health disorders ‐ and ≥ 17 on the WHO Disability Assessment Schedule 2·0 (WHODAS) ‐ a questionnaire for functional impairments 5. Exclusion criteria a. Women with severe mental health disorders (e.g. psychotic disorders, substance dependence) b. Severe cognitive impairment (e.g. severe intellectual disability) c. At risk of imminent suicide |
|
| Interventions |
Stated purpose: to establish the effectiveness of WHO's Problem Management Plus group intervention in a conflict‐affected setting INTERVENTION Name: WHO’s Problem Management Plus (PM+) intervention Delivered by: LHW Title/name of PW and number: for treatment arms of trials, PM+ treatments were delivered by 17 lady health workers (LHWs) and 6 facilitators (therapists) 1. Selection: these LHWs were community health workers, each responsible for a community cluster of approximately 1000 people or 150 homes, visiting 5 to 7 homes per day. Facilitators were members of the local community 2. Educational background: facilitators were local graduates with bachelor’s degrees without mental healthcare experience 3. Training: facilitators received 7 days of intervention training by a master trainer (KSD). Intervention training included education on adversity and its effects on mental health, basic helping skills, delivery of intervention strategies, skills in group facilitation, and facilitator self‐care. To assess competency, all facilitators delivered 1 practice group each at an accelerated rate (5 sessions in 2 weeks) with participants living outside the trial area and under intensive supervision (10 hours' supervision over 2 weeks), who were assessed for their competency using a specially developed checklist that evaluated basic counselling skills and their use of intervention strategies through direct observation 4. Supervision: supervision of facilitators was done through 2 hours of weekly group session by experienced Islamabad‐based supervisors via telecommunication software (Skype Technologies, Palo Alto, CA, USA). In turn, supervisors received 1·5 hours' fortnightly supervision via Skype by the master trainer in Sydney, NSW, Australia. Supervision included review of participants’ progress and individual case management, refresher training on strategies, and rehearsal of skills through role‐play 5. Incentives/remuneration: facilitators received a small honorarium of USD100 per month Intervention details 1. Duration/frequency: 5 group sessions per week, with approximately 6 to 8 participants per group, each session lasting for approximately 2 hours (excluding breaks) 2. Content of intervention: first session included psychoeducation, goal‐setting, and brief motivational interviewing. Sessions 1 to 4 introduced strategies for stress management, problem‐solving, behavioural activation, and strengthening of social support. Each strategy was reviewed in every subsequent session, and the final session involved revision of learning, education on preventing relapse, and a closing ceremony. Given that many participants were non‐literate, the intervention included locally relevant pictorial materials and adopted a narrative format. The groups, facilitated by local women, gave participants a safe space to share their feelings and to learn from one another’s experiences, allowing a degree of empowerment and control over their lives as they problem‐solved together CONTROL: EUC for all participants comprised feedback about assessment results, the offer of psychoeducation for themselves and accompanying family members, the opportunity to talk about their health with their LHW (who received a half‐day training programme in psychoeducation and supportive communication), and information about options for seeking care for distress (i.e. through the PHC centre or the tertiary healthcare centre). PHC providers received a half‐day training in detection and management of mental health problems and referral pathways for care CO‐INTERVENTIONS: none |
|
| Outcomes |
Patients 1. Hospital Anxiety and Depression Scale (HADS) 2. 14‐item scale consisting of 2 subscales—HADS‐A (anxiety, 7 items, range 0 to 21) and HADS‐D (depression, 7 items, range 0 to 21) 3. PTSD symptoms recorded using the 20‐item PTSD checklist of the Diagnostic and Statistical Manual of Mental Disorders‐5 (PTSD checklist‐5) 4. WHODAS (WHO Disability Assessment Schedule) 5. Patient Health Questionnaire‐9 (PHQ‐9) Carers None Process/health workers None Economic outcomes Direct cost: cost of acute treatment/care (* study's primary and secondary outcomes) Time points: baseline, 1 week, 3 months |
|
| Notes |
Source of funding: Office of Foreign Disaster Assistance to WHO and a small travel grant from the University of Liverpool Overseas Development Agency Seed Fund Notes on validation of instruments (screening and outcomes): all instruments validated, not mentioned in WHODAS validation Additional information: declaration of interests ‐ study authors declared no competing interests Handling the data: as per footnotes in data and analysis Prospective trial registration number: Australian New Zealand Clinical Trials Registry: 12616000037404 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "permuted‐block randomisation method was used to generate the randomisation code, with a block size of six. Allocation of clusters was done by an independent statistician based at the University of Liverpool, Liverpool, UK, using a computerised randomisation sequence" |
| Allocation concealment (selection bias) | Low risk | Quote: "however, the researchers responsible for identifying participants, obtaining consent, enrolment, and outcome assessments were masked to allocation. All assessors resided outside the study area and had no interaction with the intervention team" Judgement comment: assessors and researchers are masked and are not part of the community |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not possible to blind participants and personnel to the intervention; unlikely to affect outcomes |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | Researchers responsible for identification of participants, receipt of consent, enrolment, and outcome assessments were masked to allocation |
| Baseline outcome measurements similar | Low risk | See Table 2 (baseline outcome measurements are similar) |
| Baseline characteristics similar? | Low risk | Table 1: similar baseline characteristics |
| Incomplete outcome data (attrition bias) Efficacy data | Low risk | Attrition rate (about 5% to 6% in both arms) was lower than what was provided for (20%) in the sample size calculation |
| Incomplete outcome data (attrition bias) Safety data (e.g. adverse events) | Low risk | Safety data collected; no adverse events. Attrition rates do not differ significantly |
| Protection against contamination | Low risk | Judgement comment: LHW community clusters in 2 union councils were block‐randomised; intervention is 1‐to‐1, so contamination unlikely |
| Selective reporting (reporting bias) | Low risk | Same outcomes as in protocol, other than costs, which are not published yet |
| Other bias | Low risk | No comments |
Rojas 2007.
| Study characteristics | ||
| Methods |
Study design: single‐blind parallel RCT Duration of study: June 2004 to 2006 |
|
| Participants |
Country: Chile Income classification: upper‐middle income Geographical scope: urban ‐ deprived urban area of Santiago Healthcare setting: 3 PHC clinics Mental health condition: postnatal depression Population: women 1. Age: mean (SD): 26.7 (SD 6.4) 2. Gender: female 3. Socioeconomic background: low‐income women; majority were housewives 4. Inclusion criteria a. Score ≥ 10 on EPDS at 2‐week intervals b. Age ≥ 18 years c. Women with children younger than 1 year of age d. Meeting DSM‐IV criteria for major depression 5. Exclusion criteria a. Women who received treatment for depression during current postnatal period if they were pregnant b. Psychotic symptoms c. Serious suicide risks d. History of mania e. Alcohol or drug abuse |
|
| Interventions |
Stated purpose: to compare the effectiveness of a multi‐component intervention with usual care to treat postnatal depression in low‐income mothers in primary care clinics in Santiago, Chile (protocol mentioned they would look at infant outcomes; no infant outcomes are mentioned in the protocol, nor have they been reported in the results paper) INTERVENTION (n = 114) Name: multi‐component intervention Delivered by: PHP Title/name of PW and number: physician doctor, group leaders (midwives) and nurse, and designated trained non‐professional person 1. Selection: doctor ‐ from PHC selected; group leaders and non‐professionals ‐ not specified 2. Educational background: doctor ‐ medical degree, group leaders, and non‐professionals ‐ not specified 3. Training: group leaders ‐ 8 hours of training. Non‐professional ‐ not specified 4. Supervision: doctor ‐ 1 hour of supervision every week by research psychiatrist, group leaders ‐ supervision every week by the doctor and by non‐professionals ‐ not specified 5. Incentives/remuneration: not specified Intervention details 1. Duration/frequency: group sessions consisted of 1 session per week for 8 weeks (maximum 20 attendants, with every session lasting 50 minutes). Women received medical appointments at 2 and 4 weeks, and subsequently every month for the first 6 months 2. Content of intervention (by types of health workers and per patients/carers):Doctors: protocol: first choice of drug was fluoxetine (20 to 40 mg/d) but sertraline (50 to 100 mg/d) was also available for those who did not respond to fluoxetine or were breastfeeding. All medication was supplied free for both groups. Group leaders: psychoeducation intervention, which consisted of information about symptoms and treatments, problem‐solving and simple behavioural activation, and cognitive techniques. All topics were presented with examples relevant to the postnatal period. Groups consisted of 1 session per week for 8 weeks (maximum 20 attendants), with every session lasting 50 minutes. Groups followed a structured format, with every session covering something different but with plenty of time for sharing experiences. There was poor attendance of these psychoeducational sessions: "mean number of multicomponent intervention group sessions attended was 2·7 of eight (SD 3·1), and attendance was not associated with the EPDS score"; "women taking medication attended slightly more sessions". Group leaders delivered sessions but had no further contact with patients. Doctor was ultimately responsible for the group. Non‐professionals: designated trained, non‐professional persons monitored attendance at consultations and group sessions and provided support and advice about antidepressant use following a structured format. If any problems were detected, patients were advised to see their doctors, and some assistance was provided to obtain medical appointments sooner if deemed essential CONTROL: usual care (n = 116) "Usual care included all services normally available in the clinics, including antidepressant drugs, brief psychotherapeutic interventions, medical consultations, or external referral for specialty treatment. Although all these options are potentially part of usual care, in reality medication and consultation remain the main treatment methods; psychotherapy and specialty referrals are rarely offered. Doctors in the usual care group were informed of the baseline assessment but no further information was provided" CO‐INTERVENTIONS: none |
|
| Outcomes |
Patients 1. EPDS* ‐ used twice for screening (2 weeks apart) 2. MINI (used to establish inclusion and exclusion criteria in people screened twice) 3. SF‐36 ‐ secondary outcome (has 4 dimensions ‐ mental health, social functioning, emotional role, and vitality) Carers NA Process/health workers No process Economic outcomes None (*: primary outcomes of the study) Time points: baseline, 3 months, 6 months |
|
| Notes |
Source of funding: Fondo Nacional de Desarrollo Científico y Tecnológico, Chile Notes on validation of instruments (screening and outcomes): all validated, but not specified if EPDS and MINI were translated/validated in that setting Additional information (e.g. provided by authors, existence of a published study protocol): declarations of interest ‐ study authors declared no conflicts of interest Handling the data: as per footnotes in data and analysis Prospective trial registration number: NCT00518830 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from the paper: "the clinics participating in the trial were chosen for practical reasons rather than randomly selected, which could affect the generalisability of our findings" Comment: (1) But these are deemed fairly representative of PHCs in deprived urban areas in Santiago; (2) the numbers were computer‐generated random numbers; therefore, low risk of bias |
| Allocation concealment (selection bias) | Low risk | Quote: "allocations were kept in numbered sealed envelopes in every clinic, opened by a person" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: neither control nor intervention groups were blinded to the intervention. The usual care group could receive medical consultation, and so differential interventions were unlikely |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | Quote: "staff recruiting patients were neither involved in nor aware of the procedure used to generate allocations" |
| Baseline outcome measurements similar | Low risk | Comment: yes, baseline outcome measurement similar |
| Baseline characteristics similar? | Low risk | Comment: yes, all were similar |
| Incomplete outcome data (attrition bias) Efficacy data | Low risk | Comment: 90% of women randomly assigned and completed their 6‐month assessment in both groups, but not adjusted for in analysis |
| Protection against contamination | Low risk | Quote: "since women in both groups attended the same centres, some degree of contamination could possibly have occurred, but we tried to reduce this possibility by allocating patients in each group to different doctors. Our previous experience with clinical trials in these settings shows that after a few weeks, the pressure of work is so intense that participating clinicians only remember things when constantly reminded. If there were contamination it would have been more likely in early stages of the study, which is when we found the largest differences, than in late stages" Comment: although there is no wait‐list control, study authors have tried to minimise contamination by allocating patients to different doctors; unlikely to be that much contamination |
| Selective reporting (reporting bias) | Low risk | Comment: trial was prospectively registered; all pre‐stated outcomes are reported |
| Other bias | Low risk | Comment: no other bias |
Shaw 2018.
| Study characteristics | ||
| Methods |
Study design: RCT; non‐blinded Duration of study: enrolled during 3 months of 2016 ‐ from randomisation to final assessment: 7 months |
|
| Participants |
Country: Malaysia Income classification: upper‐middle income country Geographical scope: urban (Kuala Lumpur) Healthcare setting: community organisation group/clinic; health care programme based at the Malaysian Social Research Institute (during open clinic days) Mental health condition: post‐traumatic stress and common mental disorders Population 1. Age: ≥ 18 years 2. Gender: female 3. Socioeconomic background: female refugees from Afghanistan living in Kuala Lumpur; no specific info. 50% experienced food insecurity, 20% experienced homelessness 4. Inclusion criteria (including threshold cutoff score of measurement tool) a. Refugee Health Screening score ≥ 12 on items 1 to 14 (1) Age ≥ 18 years (2) Female (3) Residence in Malaysia (4) Refugee or asylum‐seeker (5) Dari speaking (6) Symptomatic for emotional distress (Refugee Health Screening score ≥ 12 on items 1 to 14) or other mental health symptoms 5. Exclusion criteria: not mentioned |
|
| Interventions |
Stated purpose: to examine the effectiveness of an 8‐week culturally adapted CBT group among women from Afghanistan living in Kuala Lumpur, Malaysia INTERVENTION (n = 20) Name: somatic‐focused cognitive‐behavioural therapy Delivered by: LHW Title/name of PW and number: Afghan therapist ‐ 1 1. Selection: not specified 2. Educational background: completed secondary education but did not have training in mental health service provision 3. Training (contents, duration, by whom): intensive training on facilitation techniques, group content, and research procedures 4. Supervision: intensive training and supervision by the PI (mental health professional) and the co‐PI in Malaysia (medical doctor) 5. Incentives/remuneration: not described Intervention details 1. Duration/frequency: 8 weekly sessions 2. Content of intervention: emotional regulation, stretching and relaxation, breathing, mindfulness, visualisation, and managing anger and worry. Education about trauma‐related disorders and emotions. Cognitive restructuring, interoceptive exposure, emotion regulation, decreasing negative affect, and increasing psychological flexibility. Intervention group delivered alongside PI. First group was implemented jointly with trained and licensed mental health professional (PI), where the facilitator primarily interpreted. Second group was implemented jointly, where the facilitator primarily conducted the group and the PI was there to help and support. Third and fourth groups were conducted by the facilitator alone. A non‐randomised group received the intervention at the same time as patients in the wait‐list control group (not included in this review) CONTROL (n = 9) Wait‐list control. Afghan therapist delivered intervention after comparison assessment was completed CO‐INTERVENTIONS: nil |
|
| Outcomes |
Patients 1. Refugee Health Screener‐15 (RHS‐15)* 2. Hopkins Symptom Checklist‐25* 3. Harvard Trauma Questionnaire* 4. Emotional/informational support subscale of the MOS Social Support Survey° Carers None Process/health workers None Economic outcomes (and where these can be found, e.g. ref or table number) None (asterisk for study's primary outcomes; star/circle: outcomes that we have not reported in this review) Time points post intervention G1 (intervention group): baseline, 0 months (immediately post intervention), 3 months G2 (control group): baseline, 0 months (immediately post G1 intervention), 2 months (immediately post G2 intervention), 5 months (3 months post G2 intervention) |
|
| Notes |
Source of funding: Centre of Excellence for Research in AIDS, University of Malaya Research Grant, Carefugees, Malaysia, and Brigham Young University, USA Notes on validation of instruments (screening and outcomes): validated instruments in Farsi (which is almost identical to Dari in written form) Additional information: no protocol; information on training/supervision received from study authors. Declarations of interest ‐ none Handling the data (e.g. imputed values/other calculations we have made): as per footnotes in data and analysis Prospective trial registration number: not specified |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "random assignment was done through online software" |
| Allocation concealment (selection bias) | Unclear risk | Participants were individually randomised to intervention or control group via computer programme. However, a third group who had expressed interest were assigned to a separate group; therefore not concealed. Furthermore, there was lack of research assistant blinding |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not possible to blind participants and personnel to the intervention; unlikely to affect outcomes |
| Blinding of outcome assessment (detection bias) all outcomes | High risk | Research assistant was not blind to group assignment. Possible bias due to researcher not blinded to allocation |
| Baseline outcome measurements similar | Low risk | Participant outcomes were measured prior to the intervention; no important differences were present across study groups ''There were no significant differences in emotional distress, anxiety, and social support by participant group. However participants in G3 (non‐randomized group) had higher rates of depression and PTSD symptoms when compared with G1 and G2" |
| Baseline characteristics similar? | Low risk | Baseline characteristics of intervention and control groups are reported and similar |
| Incomplete outcome data (attrition bias) Efficacy data | Low risk | Missing data were few, with no missing data in any group at baseline measurement; no missing data in wait‐list control group for all measurements, and no more than 3 participants missing in treatment groups at post‐treatment and 3‐month follow‐up measurements. Therefore, for linear growth analyses ‐ in which the “known class” option in Mplus was utilised ‐ missing data were handled using Missing Data Theory |
| Protection against contamination | Unclear risk | Quote: "G1 comprised 2 separate groups, which were facilitated jointly by Stacey A. Shaw and the Afghan therapist, whereas the Afghan therapist alone conducted G2 and G3" Judgement comment: although the Aghan therapist delivered intervention to control and non‐randomised groups after delivery of intervention to intervention group, it is unclear whether there was communication prior to intervention delivery |
| Selective reporting (reporting bias) | Unclear risk | No published clinical trial protocol available; all relevant outcomes in the methods section reported in results section |
| Other bias | Low risk | Group 3 was non‐randomised; should not be included in analysis. Other than this, no risks of bias were found |
Shen 2016.
| Study characteristics | ||
| Methods |
Study design: RCT Duration of study: participants recruited between August 2011 and August 2012 and followed up for 1 year |
|
| Participants |
Country: China Income classification: upper‐middle income between 2011 and 2013 Geographical scope: urban Healthcare setting: clubhouse set up for day rehabilitation Mental health condition: schizophrenia Population 1. Age: mean age about 45 ± 8 years 2. Gender: both 3. Socioeconomic background: not specified 4. Inclusion criteria a. Diagnosis of schizophrenia according to Chinese Classification of Mental Disorders diagnostic criteria b. Newly diagnosed and in recovery phase 5. Exclusion criteria a. Other psychiatric or serious physical disease b. Patients who withdrew during the study (claimed to have randomised 111 and analysed 111, but this statement suggests patients who withdrew were excluded from analysis) |
|
| Interventions |
Stated purpose: to investigate effects of a clubhouse day rehabilitation model on clinical status, psychological functioning, and social functioning of patients with newly diagnosed schizophrenia in the first recovery phase INTERVENTION (n = 81) Name: clubhouse model day rehabilitation Delivered by: LHW Title/name of PW and number: clubhouse employees ‐ number not specified 1. Selection: not specified 2. Educational background: not specified 3. Training (contents, duration, by whom): not specified 4. Supervision: not specified Intervention details 1. Duration/frequency: 1 year, 5 days a week for 8 hours 2. Content of intervention: participants became voluntary lifelong club members at no charge and worked and managed the clubhouse with equal status to employees. Jobs such as meal preparation, cleaning, newsletter, news‐stand and reception, cashier, storage, inventory, etc; encouraging socialising according to social rules and volunteering in libraries and old folks’ homes, etc; weekly cultural/entertainment activities such as picnics, visiting scenic spots, visiting the zoo or botanical gardens, etc; and music/dance/chess/card game clubs and classes based on participants’ interests CONTROL: routine community intervention (n = 30) Psychiatrists and associated nurses gave monthly 2‐hour treatments, including seminars and medication education, encouraging outdoor and social/family activities and education on recognising emotional distress and relapse and bringing in for treatment when needed CO‐INTERVENTIONS: both groups were given usual maintenance medication. Intervention group also received routine community intervention |
|
| Outcomes |
Patients 1. BPRS 2. SDSS 3. SDS 4. SAS Carers Nil Process/health workers Nil Economic outcomes Nil (asterisk for study's primary outcomes; star: outcomes that we have not reported in this review) Time points post intervention: 0 months |
|
| Notes | Article in Chinese Source of funding: Shenzhen City Science and Technology Project Notes on validation of instruments (screening and outcomes): validated Additional information: declaration of interests ‐ none Handling the data (e.g. imputed values/other calculations we have made): nil Prospective trial registration number: nil |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: using a random number table |
| Allocation concealment (selection bias) | Low risk | Judgement comment: assigned at the start of the study |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No mention of masking or blinding |
| Blinding of outcome assessment (detection bias) all outcomes | High risk | No mention of masking or blinding |
| Baseline outcome measurements similar | Low risk | Baseline BPRS, SDSS, SDS, SAS scores are similar in both groups |
| Baseline characteristics similar? | Unclear risk | Table of characteristics includes only age and gender |
| Incomplete outcome data (attrition bias) Efficacy data | Unclear risk | Patients who did not continue with intervention/control were excluded from the study |
| Protection against contamination | Low risk | Judgement comment: participants in intervention and control groups were treated in different settings |
| Selective reporting (reporting bias) | Unclear risk | No published clinical trial protocol available; all outcomes planned in methods section reported in results section |
| Other bias | Low risk | No comments |
Sherman 2009.
| Study characteristics | ||
| Methods |
Study design: RCT Duration of study: 12‐month trial between April 2005 and June 2006 |
|
| Participants |
Country: Thailand Income classification: lower‐middle income country Geographical scope: urban, Chiang Mai, Northern Thailand Healthcare setting: in the community, done in 'unmarked building', which was a drug treatment centre Mental health condition: methamphetamine use Population: adults; initial index participants recruited, who then also brought in 'network' participants 1. Age: 18 to 25 years old (median 19 years, interquartile range 18 to 20)) 2. Gender: both male and female (75% male) 3. Socioeconomic background: about one‐third worked, one‐third were students, one‐third were unemployed; primarily Buddhist (97.1%) and ethnically Thai (99.2%). A majority (63.8%) reported living with their parents. Participants' education level was low, with only 39% reporting they were currently in school and a median of 9 (interquartile range 9 to 11) years of schooling 4. Inclusion criteria a. Index participants (1) Between the ages of 18 and 25 years at screening (2) Used methamphetamine at least 3 times (3) Had sex at least 3 times in the past 3 months (4) Able to enrol at least 1 of their sex or drug network members in the study within 45 days of screening b. Network participants (1) Between the ages of 18 and 25 years at screening (2) Had used methamphetamine at least 3 times or had sex with the index participant at least 3 times in the last 3 months 5. Exclusion criteria a. Refused to have blood drawn or to provide urine b. Were enrolled in another prevention study c. Refused to provide locator information |
|
| Interventions |
Stated purpose: to compare the efficacy of a peer educator, network‐oriented intervention ("peer education" condition) with a best practice standard life skills curriculum ("life skills" condition) for methamphetamine use, sexual risks, and incident STIs INTERVENTION (n = 442) Name: peer education condition Delivered by: LHW Title/name of PW and number: peer educators ‐ 6 1. Selection: 2 facilitators with 1 backup (totalling 6 facilitators), who were in their early 20s and had been a part of the ethnography team in the study's first phase 2. Educational background: not specified 3. Training: facilitators were trained by the study's first and third authors in an intensive 1‐week‐long training session. Curriculum was implemented using a manual. Copies of manuals for peer education and life skills conditions are available in Thai and in English from the study authors 4. Supervision: not specified 5. Incentives/remuneration: not specified Intervention details 1. Duration/frequency: seven 2‐hour sessions for each group undertaken by facilitators over 1 month with twice‐weekly sessions. Participants in the peer education condition also attended 2 booster sessions that occurred 3 months and 6 months after study entry 2. Content of intervention: "peer education condition was based on theory, informed by an extensive 18‐month formative research phase, and built upon our previous intervention experience in Thailand and USA". "The peer education condition aimed to teach participants to think critically about and reduce their methamphetamine use and sexual risk behaviours. Participants were taught communication skills that they practised in role‐plays during the sessions and used to convey methamphetamine and risk reduction messages to specific social network members that were identified through a social network inventory administered at baseline. The first session aimed to build group cohesion and identity, through having the group establish its own 'group rules' to follow during the ensuing sessions. During this session, participants delineated how methamphetamine affected themselves, their social network, and their family. The second session focused on social influences in initiating methamphetamine use and taught participants a set of communication tools that were reinforced and used throughout the subsequent sessions in designated role plays and videos. The third and fourth sessions focused on sexual risk reduction, sexually transmitted infections (STIs), and communication skills in sexual situations. The fifth session focused on stigma and examined methamphetamine's effects on participants' families and the broader community. Because of the intervention's focus on creating a positive and constructive role for participants, the sixth session was dedicated to participants being involved in a community service project, which was chosen by each group. These projects lasted two to four hours and included painting or cleaning temples, garbage clean‐up in villages, renovating a village playground, and weeding a community garden. During the seventh and final session, participants reviewed the content from the previous sessions and graduated from the project. Sessions were comprised of interactive teaching modules, instructive games, and problem‐solving activities. Sessions ended with assigning peer education homework in which participants would discuss a specific issue with specific peers (MA‐using and/or sexual partners), which was reviewed at the beginning of the next session" CONTROL (n = 440) Best practice intervention: a life skills building approach based on a skills building approach. "It was largely derived from cognitive‐behavioural psychology, which is widely used with youth in drug treatment and juvenile justice settings in Thailand. Juvenile justice staff were consulted throughout the development of the life skills condition. The sessions focused on the causes and consequences of methamphetamine use at the individual level, with specific attention to stress in the role of drug use. 1 session focused on STIs and sex risk behaviours. The sessions placed no emphasis on communicating the session content to social network members. The first session focused on examining the role of methamphetamine in participants' lives. The second session reviewed problem‐solving tools and friendships. The third session focused on the physiological effects of methamphetamine use. The fourth session addressed STIs and safer sex practices. The fifth session considered stress and coping. The sixth session focused on managing emotions and self‐worth. The last session reviewed the intervention and participants graduated" CO‐INTERVENTIONS: prevention of STIs as part of what peer educator sessions comprised |
|
| Outcomes |
Patients 1. 2 behavioural outcomes and 1 biological outcome a. Methamphetamine use during the 3 months before the interview* b. Use of condom for either vaginal or anal sex*§ c. Presence of a laboratory‐confirmed STI*§ 2. In German (2012), the main outcome is depression scores (using CES‐D scale) Carers None Process/health workers Not mentioned Economic outcomes Not mentioned (*: primary outcomes of the study; §: outcomes that we have not reported in this review) Time points: baseline, 3 months, 6 months, 9 months, 12 months |
|
| Notes |
Source of funding: National Institutes of Health (1 R01 DA14702) Notes on validation of instruments (screening and outcomes): no screening instruments were used. "Methods to enhance the reliability of self‐reported behaviours included (1) using unique study ID's to maintain confidentiality during data collection; and (2) using a brief recall period (3 months and 30 days). In addition, STI testing at the 12‐month visit provided a biological outcome measure". CES‐D validated in Thai setting (Trangkasombat and Nukhew 1998) with a cutoff score of 22 (range 0 to 60) Additional information: none; declarations of interest ‐ none Handling the data: as per footnotes in data and analysis Prospective trial registration number: registration not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "nonrandom sampling recruitment methods"; "some risk of sampling bias due to recruitment time periods and locations" Quote: "randomization of index members occurred at the end of the baseline visit. Indexes were randomised to either the peer education or the life skills condition within 45 days of their baseline visit. Randomization occurred in blocks (cohorts) once a minimum of 16 and a maximum of 24 index participants had been enrolled, and randomisation sequences for each cohort were generated by a computer program. Scheduling for the first session occurred within two weeks of randomisation. In total, 21 cohorts were randomised over a period of 15 months. As this was a peer network intervention and we were interested in examining the effects of index participants on their network members' risk behaviours, network members were not randomised to attend the peer education or life skills sessions. Their involvement was limited to the baseline and four follow‐up visit assessments" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "randomisation of index members occurred at the end of the baseline visit. Indexes were randomised to either the peer education or the life skills condition within 45 days of their baseline visit. Randomisation occurred in blocks (cohorts) once a minimum of 16 and a maximum of 24 index participants had been enrolled, and randomisation sequences for each cohort were generated by a computer programme" Comment: allocation of randomisation in blocks. Not mentioned if this was done with sealed envelopes, etc., among those excluded (as found in CONSORT diagram, 6 were randomised but attended the wrong arm of the trial) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: participants and personnel not blinded to intervention; this is unlikely to affect outcomes |
| Blinding of outcome assessment (detection bias) all outcomes | High risk | Quote: "behavioral data were collected through self‐report and it is possible that social desirability influenced participants' responses, particularly in light of the recent 'war on drugs'" Quote 2: "interviewers were blind to the participant's group allocation" Comment: behavioural data self‐reporting is likely to bias outcome assessment |
| Baseline outcome measurements similar | High risk | Quote: "there were few significant differences in demographic or reported drug use patterns between participants randomised to the peer education compared to the life skills condition. A significantly higher percentage of participants in the peer education condition compared to those in the life skills condition reported drinking problems (77% vs 71%, P < 0.05), condom use at last vaginal sex act (38% vs 31%, P < 0.05), and "always" using condoms in the past 30 days (22% vs 16%, P < 0.05)" |
| Baseline characteristics similar? | High risk | Quote: "there were few significant differences in demographic or reported drug use patterns between participants randomised to the peer education compared to the life skills condition. A significantly higher percentage of participants in the peer education condition compared to those in the life skills condition reported drinking problems (77% vs 71%, P < 0.05)" Comment: sociodemographic details similar, but differences in drinking problems |
| Incomplete outcome data (attrition bias) Efficacy data | Low risk | Quote: "at each of the four follow‐up visits, follow‐up was greater or equal to 90% (range: 89% – 95%) for index participants and 86% (range: 85% – 91%) for network participants in both arms. Among index and network members in both arms, there was at least an 89% retention rate at the 12‐month follow‐up" Comment: mean 10% dropout (11% in intervention group, 9% in control group) reported, but reasons not specified; however, low dropout rate, so unlikely to affect outcomes |
| Protection against contamination | High risk | Quote: "there is the possibility that tight social networks were randomised to both control and intervention arms, leading to a high degree of contamination that resulted in a bias towards the null" Quote 2: "it is highly probable that contamination occurred between the two study arms. Based on our observation at the study house, many participants enrolled in the study with or were referred to the study by their friends who could have been randomized to different study arms" |
| Selective reporting (reporting bias) | Unclear risk | Comment: all stated outcomes reported; no protocol to check if pre‐specified outcomes are reported |
| Other bias | High risk | Quote: "session attendance and follow‐up rates were consistently high in both arms, indicating a high level of interest; 'perhaps the comparison arm was too similar to the intervention with its parallel, albeit not as intense'; 'current study occurred in the wake of the Thai government's 'war on drugs', ... which resulted in the arrest and forced treatment of thousands of drug users, as well as the extrajudicial killings of over 2500 people. In this context, it was difficult not to provide a comparison condition that was meaningful to the study participants and that provided them with important risk reduction information delivered in a humane and respectful manner" Comment: intervention and comparisons were too similar |
Sikander 2019.
| Study characteristics | ||
| Methods |
Study design: single‐blind, cluster‐randomised controlled trial; unit of allocation: village cluster Duration of study: screening between 15 October 2014 and 25 February 2016. Last assessment at 6 months after childbirth; total duration for each participant is up to 9 months |
|
| Participants |
Country: Pakistan Income classification: lower‐middle income between 2014 and 2016 Geographical scope: Kallar Syedan, a rural subdistrict of Rawalpindi, Pakistan Healthcare setting: individual THPP sessions were delivered by Razakaars at participants’ homes, and group sessions were delivered at the LHW’s health house or at a nearby place that was convenient for participants Mental health condition: perinatal MD Population 1. Age: 18+ years 2. Gender: women 3. Socioeconomic background: over 93% did not work; over 52% had no formal education 4. Inclusion criteria a. Women aged 18 years or older b. In third trimester of pregnancy c. Registered with local LHWs d. Intended to stay in the study area for at least 1 year e. Had scored ≥ 10 on Patient Health Questionnaire‐9 (PHQ‐9). 5. Exclusion criteria a. Did not speak Urdu, Punjabi, or Potohari b. Needed immediate medical or psychiatric inpatient care |
|
| Interventions |
Stated purpose: to evaluate effectiveness and cost‐effectiveness of the adapted Thinking Healthy Programme peer‐delivered (THPP) compared with enhanced usual care (EUC) in Pakistan INTERVENTION (n = 227) Name: the Thinking Healthy Programme (peer‐delivered) Delivered by: LHW Title/name of PW and number: Razakaars, volunteer peers (lay women from the community) ‐ 66 1. Selection: local volunteers; married women, around ages 30 to 35 years, with good communication skills. Possible Razakaars were identified by LHWs and by community elders 2. Educational background: not specified, but stated similar educational background to participants 3. Training (contents, duration, by whom): peers received brief classroom training and field training, and were able to deliver the intervention to satisfactory fidelity. A high proportion of women (78%) completed ≥ 10 sessions of the possible 14 sessions, indicating the acceptability of peers in delivering these sessions 4. Supervision: brief classroom training of peers was supplemented with regular group training and field supervision by local THPP trainers, who were not mental health specialists; these THPP trainers were supervised by a specialist therapist, generating a cascade model of training and supervision 5. Incentives/remuneration: peers received no financial remuneration for this work Intervention details 1. Duration/frequency: THPP consisted of 10 individual and 4 group sessions, each of which lasted 30 to 45 minutes, from third trimester of pregnancy to 6 months after childbirth 2. Content of intervention (by types of health workers and per patients/carers): THPP sessions were front‐loaded (i.e. greater frequency of sessions, intensity, and content were delivered during pregnancy until the third month after childbirth); 10 of the 14 sessions were delivered during pregnancy and in the first 3 months after childbirth. This approach was taken to ensure an early reduction in maternal depressive symptoms during this crucial phase of infant care CONTROL: enhanced usual care (n = 226) Standard care from lady health workers (LHWs), which did not include treatment of perinatal depression. In addition, participants and LHWs who had registered them were informed of their screening results, and all doctors and midwives at primary healthcare centres were given the adapted mental health Gap Action Programme treatment guidelines for perinatal depression, which included information on how to refer patients with severe depression and those at high risk of suicide to specialist mental healthcare facilities; participants were provided with an information sheet that included details on where to seek appropriate health care during pregnancy and beyond CO‐INTERVENTIONS: enhanced usual care was given to participants from both groups |
|
| Outcomes |
Patients 1. Symptom severity (PHQ‐9) at 6 months postpartum* 2. Remission (PHQ‐9 < 5) at 6 months postpartum* 3. PHQ‐9 at 3 months# 4. Remission at 3 months# 5. Recovery (PHQ‐9 < 5 at 3 and 6 months) 6. WHODAS 7. Number of days unable to work 8. Perceived social support# 9. Exclusive breastfeeding# 10. Infant weight‐for‐age and height‐for‐age scores# 11. Serious adverse events (death of participant due to any cause, death of the child, suicide attempts, hospital admissions, experiences of physical violence, infant abuse or neglect, social stigmatisation regarding the study, violence towards others) Carers None Process/health workers Proportion of women attending at least 10 of 14 sessions Economic outcomes 1. Average duration of health service use 2. Average time to access services 3. Any health service use, unit costs, health system costs, productivity costs, societal costs (asterisk for study's primary outcomes; star: outcomes that we have not reported in this review) Time points post intervention: baseline, during intervention (3 months' postpartum), end of intervention (6 months' postpartum) |
|
| Notes |
Source of funding: National Institute of Mental Health (USA) Notes on validation of instruments (screening and outcomes): all instruments were validated Additional information: declarations of interest ‐ study authors declared no competing interests Handling the data: as per footnotes in data and analysis Prospective trial registration number: trial was registered with ClinicalTrials.gov (NCT02111915) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: randomisation was conducted using a 'computerised randomisation sequence' |
| Allocation concealment (selection bias) | Low risk | "Randomisation list for village clusters, which was stratified by 11 union councils (the smallest administrative unit of the subdistrict), was prepared by an independent statistician (HAW, who had no subsequent involvement in the trial) by use of a computerised randomisation sequence"; "all members of the Trial Steering Committee, except for the data manager (AZ), remained masked to allocation status until the data were unmasked after interpretation of the results" Assignment to groups was concealed and conducted by an independent researcher |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Single‐blind, cluster‐randomised controlled trial. Participants and personnel not blinded to intervention; this is unlikely to affect outcomes |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | "Outcome assessors were masked to treatment allocation of participants during baseline and follow‐up assessments, had no interaction with the intervention team, and resided outside of the study area" Outcomes were assessed blindly by assessors, and precautions were taken to ensure masking |
| Baseline outcome measurements similar | Low risk | "There was no evidence of a difference in baseline characteristics in women for whom we had 6‐month outcome data and those for whom we did not, except for a slightly longer time between screening and childbirth for those from whom we were missing primary outcome data (which was therefore adjusted for in outcome analyses), and there was no difference in the baseline characteristics of those who had a study visit during the protocol‐defined window compared with those who did not" Baseline outcome measurements were similar between groups, and any differences were adjusted for in analyses |
| Baseline characteristics similar? | Low risk | "There were no major imbalances between the groups, except a slightly higher proportion of women with duration of depression of more than 12 weeks in the intervention group versus the control group (which was therefore adjusted for in outcome analyses)" Any differences in baseline characteristics were adjusted for in analysis |
| Incomplete outcome data (attrition bias) Efficacy data | Low risk | Missing outcome data were adjusted for during analysis "Sensitivity analyses for primary outcomes included random‐effects models to account for missing outcome data with multiple imputation (which assumed data were missing at random) and alternative models for PHQ‐9 score" |
| Incomplete outcome data (attrition bias) Safety data (e.g. adverse events) | Low risk | "Overall, 43 (15%) women in the intervention group and 47 (16%) women in the control group had at least one serious adverse event, which were evenly distributed between the groups (P = 0·72; appendix). The most common serious adverse events were death of the child, hospital admissions (mainly of the child), and experience of physical violence; however, there was no evidence of any difference between the groups" Adverse events were similar between groups; there was no difference |
| Protection against contamination | Low risk | "To minimise contamination, we used eligible village clusters that were geographically separate" Precautions were taken to prevent contamination "We assumed a more conservative effect size to allow for the possibility of contamination between groups" However, this was also adjusted for during analyses |
| Selective reporting (reporting bias) | Low risk | Outcomes described in trial registry: remission (i.e. recovery from depression) measured with Patient Health Questionnaire‐9 (PHQ‐9). Remission (i.e. recovery from depression) measured with Patient Health Questionnaire‐9 (PHQ‐9). Maternal disability measured with World Health Organization Disability Assessment Schedule (WHO‐DAS). Maternal support measured with Multidimensional Scale of Perceived Social Support (MSPSS). Breastfeeding rates, infant height, all outcomes described in trial registry were reported |
| Other bias | Low risk | No other evidence of bias |
Sorsdahl 2015.
| Study characteristics | ||
| Methods |
Study design: RCT Duration of study: March 2012 to March 2013 |
|
| Participants |
Country: South Africa Income classification: upper‐middle income Geographical scope: urban Healthcare setting: PC facilities in hospital (3 emergency departments in Cape Town) and at community health centres Mental health condition: substance abuse including alcohol Population 1. Age: 18 to 75 years 2. Gender: both 3. Socioeconomic background: 55.6% were unemployed 4. Inclusion criteria (including threshold cutoff score of measurement tool) a. ≥ 18 years of age b. Moderate to high risk for substance use problems, as measured by the Alcohol, Smoking and Substance Involvement Screening Test (> 10 for alcohol, > 3 for drugs) 5. Exclusion criteria a. Severely altered mental status b. Physically incapable of participating due to severe illness c. Without any detailed locator information |
|
| Interventions |
Stated purpose: to determine if a blended motivational interviewing (MI) – problem‐solving therapy (PST) intervention, a brief MI intervention, or simple alcohol screening and psychoeducation would lead to reductions in alcohol consumption and improve quality of life INTERVENTION 1 (n = 70) Name: motivational interviewing intervention Delivered by: LHWs (peer counsellors) Title/name of PW and number: peer counsellors ‐ 5 1. Selection: originated from communities served by selected emergency services 2. Educational background: bachelors‐level education or equivalent experience 3. Training (contents, duration, by whom): 18 hours of training with proficiency testing in MI by an MI‐certified trainer, and 3 half‐day booster training sessions; 12 hours of training with proficiency testing in PST; training in substance use and associated risks; ASSIST scoring; research procedures 4. Supervision: bi‐weekly Intervention details: motivational interviewing 1. Duration/frequency: one 20‐minute session 2. Content of intervention: ASSIST‐linked brief intervention INTERVENTION 2 (n = 46) Name: blended motivational interviewing – problem‐solving therapy intervention Delivered by: community workers, peer counsellors Title/name of PHW/CW and number: peer counsellors ‐ 5 1. Selection: originated from communities served by selected emergency services 2. Educational background: bachelors‐level education or equivalent experience 3. Training (contents, duration, by whom): 18 hours of training with proficiency testing in MI by an MI‐certified trainer, and 3 half‐day booster training sessions; 12 hours of training with proficiency testing in PST; training in substance use and associated risks; ASSIST scoring; research procedures 4. Supervision: bi‐weekly Intervention details: blended motivational interviewing and problem‐solving therapy 1. Duration/frequency: one 20‐minute session followed by 4 weekly 45‐ to 60‐minute sessions 2. Content of intervention: first session ASSIST‐linked brief intervention; subsequent 4 sessions focused on identifying problems in participants’ lives and teaching participants a structured problem‐solving approach to address them CONTROL: enhanced care (n = 66) Participants were provided with a brochure giving information on effects of substance use CO‐INTERVENTIONS: nil |
|
| Outcomes |
Patients 1. CES‐D score 2. ASSIST substance use* 3. Healthcare visits 4. Injuries 5. Verbal arguments 6. Physical fights 7. Police interactions Carers Nil Process/health workers Nil Economic outcomes 1. Cost of screening 2. Intervention cost 3. Transportation 4. intervention cost incremental effectiveness ratio (Dwommoh 2018, Tables 1, 4, 5) (asterisk for study's primary outcomes; star: outcomes that we have not reported in this review) Time point: 3 months post intervention |
|
| Notes |
Source of funding: Western Cape Department of Health Notes on validation of instruments (screening and outcomes): validated Additional information: Dwommoh R, Sorsdahl K, Myers B, Asante KP, Naledi T, Stein DJ, Cleary S. Brief interventions to address substance use among patients presenting to emergency departments in resource poor settings: a cost‐effectiveness analysis. Cost Effectiveness and Resource Allocation 2018;6:24 Declarations of interest ‐ study authors declare no competing interests Handling the data: nil Prospective trial registration number: Pan‐African Clinical Trial Registry (PACTR201308000591418) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "treatment allocation was by numbered sealed, opaque envelopes, which were generated by random number tables" |
| Allocation concealment (selection bias) | Low risk | Quote: "treatment allocation was by numbered sealed, opaque envelopes, which were generated by random number tables by a research worker not involved in the delivery of the intervention..." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and personnel not blinded to intervention; this is unlikely to affect outcomes |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | Peer counsellors who delivered interventions did not conduct the follow‐up assessment, and interviewers were blinded to treatment allocation. No knowledge of allocated interventions when follow‐up assessments were conducted; therefore, low risk |
| Baseline outcome measurements similar | Low risk | "Outcome comparisons between intervention arms were addressed by Helmert contrast under an ANCOVA model" Low risk of bias; differences in outcome measures were addressed during analyses |
| Baseline characteristics similar? | Low risk | Baseline characteristics of the study and of the control group are reported and similar |
| Incomplete outcome data (attrition bias) Efficacy data | Low risk | "We replaced missing data using multiple imputation by chained equations, as described by Royston [31] in SPSS. We incorporated baseline ASSIST and CES‐D scores, age, gender, and race in the imputation model to estimate the missing data in the primary and secondary outcomes. Data were imputed 10 times and on each imputed data file, the analyses were performed. The ten sets of outcomes were then pooled to get a single set of results using multiple imputation inference [32]" |
| Protection against contamination | Unclear risk | Individually allocated |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes in the published clinical trial protocol are reported in the results section |
| Other bias | Low risk | No other risk of bias was found |
Tan 2005.
| Study characteristics | ||
| Methods |
Study design: RCT Duration of study: May 2003 to June 2004 |
|
| Participants |
Country: China Income classification: lower‐middle income Geographical scope: rural Healthcare setting: community health service centre and participants’ homes Mental health condition: schizophrenia Population 1. Age: 24 to 63 years 2. Gender: both 3. Socioeconomic background: not specified 4. Inclusion criteria a. Patients who met criteria for schizophrenia who received care in a community area in Enshi City between 2003 and 2005 and between 2004 and 2006 5. Exclusion criteria a. Severe debility b. Comorbid physical illness |
|
| Interventions |
Stated purpose: to study the impact of community management and economic factors on efficacy of treatment for patients with schizophrenia and the burden on their families INTERVENTION (n = 80) Name: community observational group Delivered by: PHP (community doctor) Title/name of PW and number: community doctors ‐ number not specified 1. Selection: from community health service centre 2. Educational background: medical degree 3. Training (contents, duration, by whom): not specified 4. Supervision: not specified Intervention details: collaborative involving mental health professionals (psychiatrists and therapists), community doctor, and caregiver 1. Duration/frequency: 12 months/monthly follow‐up 2. Content of intervention (by types of health workers and per patients/carers): community doctors ensured that participants’ rehabilitation plans were followed and solved routine problems as they arose. Mental health professionals developed care plan including pharmacological treatment using convenient and effective therapies such as depot or oral antipsychotics, with medication dosages equivalent to chlorpromazine at 238 mg ± 9.67mg/d, gave monthly educational seminars, and individualised family therapy; they were on hand for guidance and questions CONTROL (n = 80) Participants went to hospital for treatment when their disease became active, where they received medications (dosages equivalent to chlorpromazine 242 mg ± 10.53 mg/d) CO‐INTERVENTIONS: both groups received similar types and dosages of medications |
|
| Outcomes |
Patients 1. BPRS 2. SDSS 3. Medication compliance (#) 4. Relapse Carers Nil Process/health workers Nil Economic outcomes 1. Healthcare costs of prevention and treatment of schizophrenia 2. Economic losses caused by missed work (patients and family members reported separately) due to health (Table 2) (asterisk for study's primary outcomes; star: outcomes that we have not reported in this review) Time point: 0 months |
|
| Notes | Article in Chinese Source of funding: National Science Program of Hebei Department of Education Notes on validation of instruments (screening and outcomes): validated Additional information: declarations of interest ‐ none Handling the data (e.g. imputed values/other calculations we have made): nil Prospective trial registration number: nil |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No mention of blinding in the paper |
| Blinding of outcome assessment (detection bias) all outcomes | High risk | No mention of blinding in the paper |
| Baseline outcome measurements similar | Low risk | 2 groups had similar baseline BPRS scores, SDSS scores, and medication compliance rates |
| Baseline characteristics similar? | Low risk | 2 groups were similar in gender distribution, age, and duration of disease |
| Incomplete outcome data (attrition bias) Efficacy data | Low risk | No attrition |
| Incomplete outcome data (attrition bias) Safety data (e.g. adverse events) | Low risk | No attrition |
| Protection against contamination | Low risk | No risk of contamination between groups (different setting) |
| Selective reporting (reporting bias) | Unclear risk | No clinical trial protocol available; all outcomes described in methods section reported in results section |
| Other bias | Low risk | No comments |
Tol 2008.
| Study characteristics | ||
| Methods |
Study design: cluster RCT; schools as unit of allocation, 7 in each arm, 14 total; individuals as unit of analysis Duration of study: March to December 2006 |
|
| Participants |
Country: Indonesia Income classification: lower‐middle Geographical scope: rural, in Poso District of Central Sulawesi Healthcare setting: school Mental health condition: PTSD symptoms Population: patients 1. Age: 8 to 13 years (80% between 9 and 11 years) 2. Gender: both (50/50 boys and girls in sample) 3. Socioeconomic background: 25% of population in province below poverty line and living off agriculture, 20% intervention group and 30% control group displaced, most houses had 4.5 household members, most suffered about 4 violent event types on average. 31% Muslim; 47% Protestant 4. Inclusion criteria a. Children screened for exposure to traumatic events, PTSD symptoms, or depressive anxiety symptoms, with the use of symptom checklists 5. Exclusion criteria a. Serious psychopathology and psychiatric disorders (mutism, retardation, psychotic symptoms) b. Incapability to function in a group (conduct disorders, harming others), as judged by local psychosocial counsellors |
|
| Interventions |
Stated purpose: to assess the efficacy of a school‐based intervention designed for conflict‐exposed children, implemented in a low‐income setting INTERVENTION (n = 182) Name: CBI Delivered by: LHW Title/name of PW and number: paraprofessional interventionists ‐ number not specified 1. Selection: selected from local target communities, based on selection procedure assessing social skills through role‐plays 2. Educational background: at least a high school education, without former mental health background but with some experience as volunteers in humanitarian programmes 3. Training: 2‐week training programme; trained by national staff working for partnering humanitarian organisation Church World Services, based on a manual developed by the Centre for Trauma Psychology in Boston, which conforms to current expert‐based consensus and similar school‐based interventions 4. Supervision: unspecified 5. Incentives/remuneration: unspecified Intervention details 1. Duration/frequency: 15 sessions with groups of 15 children over 5 weeks; manualised CBI 2. Content of intervention: manualised CBI, CBT, and creative‐expressive techniques in a structured format: week 1: psychoeducation; week 2: stabilisation awareness self‐esteem; weeks 3 and 4: trauma narrative; week 5: reconnecting child and group to social context/ resiliency, etc., and sharing trauma stories CONTROL: usual care (wait‐list control) (n = 221) CO‐INTERVENTIONS: none |
|
| Outcomes |
Patients Primary outcomes 1. PTSD (CPSS) 2. Depressive symptoms (DSRS)* Secondary outcomes 1. Anxiety (SCARED) 2. Aggression (Children's Aggression Scale for Parents)§ 3. Daily functioning (Children's Function Impairment) 4. Social support (Social Support Inventory Scheme; SSIS)§ 5. Coping (Kidcope)§ 6. Functioning (impairment in functioning) 7. Hope (Children's Hope Scale)§ Carers NA Process/health workers None reported Economic outcomes 1. Treatment outcome 2. Treatment satisfaction 3. Therapist burden 4. Level of selection to care 5. Care package cost (see Jordans 2011) (*: primary outcomes of the study; §: outcomes that we have not reported in this review) Time points: baseline, 1 week, 6 months |
|
| Notes |
Source of funding: PLAN Netherlands Notes on validation of instruments: validated in local context: "to measure internal reliability, we used a Cronbach Alpha and for 2‐week test‐re‐test reliability, the Spearman‐Brown coefficient"; screening measure was a self‐developed symptom checklist, which was not validated against clinical interview Additional information:www.controlled-trials.com/ISRCTN25172408; declarations of interest ‐ none reported Handling the data: as per footnotes in data and analysis Prospective trial registration number: ISRCTN25172408 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: randomisation using government‐provided list of schools; excluded single religious and private schools; random selection using SPSS function |
| Allocation concealment (selection bias) | Low risk | Comment: SPSS allocation function Quote: "select exact amount of cases randomisation" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: not possible to blind participants or personnel, but unlikely to affect outcomes |
| Blinding of outcome assessment (detection bias) all outcomes | High risk | Quote: "assessors were not blinded to treatment status, and this could have biased results" Comment: child self ratings with help of assessors who were not blinded to treatment condition |
| Baseline outcome measurements similar | Low risk | Comment: no differences, except parent‐rated aggression was higher in wait‐list control group (P = 0.03) |
| Baseline characteristics similar? | Low risk | Comment: differences in gender, age, and % displaced controlled for in analyses |
| Incomplete outcome data (attrition bias) Efficacy data | Low risk | Comment: good follow‐up data (> 90%) for 1 week and 6 months for both intervention and control |
| Protection against contamination | Low risk | Comment: randomisation done by school; in addition, there is a wait‐list control, so unlikely for groups to share information |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes reported in methods and in online trial protocol are reported in results |
| Other bias | Low risk | Comment: ICC done and adjustment for clustering; intervention fidelity assessed (89.76% adherence) |
Tol 2012.
| Study characteristics | ||
| Methods |
Study design: cluster‐RCT; randomised by district divisions, 1 division per arm, 2 in total; 10 schools in 1 arm and 9 schools in the other arm (19 total); unit of analysis: individuals Intracluster correlation calculation was based on each school as a cluster Duration of study: September 2007 to March 2008 |
|
| Participants |
Country: Sri Lanka Income classification: lower‐middle Geographical scope: urban and rural, Tellippallai and Uduvil Divisions of the Jaffna District of northern Sri Lanka Healthcare setting: school‐based group intervention Mental health condition: PTSD Population: children/adolescents 1. Age: 9 to 12 years 2. Gender: both 3. Socioeconomic background: war‐traumatised area with rationed food and other essential supplies, curfews, road blocks, disappearances, extra judicial killings; "in August 2006, a peace agreement that had been observed since 2002 was abandoned, followed by closure of the only land road into the Jaffna peninsula. The subsequent period was characterized by rationed food and other essential supplies, curfews, road blocks, disappearances, extra judicial killings, and skirmishes between the army and Liberation Tigers" 4. Inclusion criteria a. Those who scored positive using the Child Psychosocial Distress Screener (CPDS) b. Age 9 to 12 years c. Children reporting severe mental problems (1) they were provided individual supportive counselling in addition to being enrolled in the study (19 children; 4.8%) 5. Exclusion criteria: not specified |
|
| Interventions |
Stated purpose: to examine outcomes, moderators, and mediators of a preventive school‐based mental health intervention implemented by paraprofessionals in a war‐affected setting in northern Sri Lanka INTERVENTION (n = 199) Name: school‐based group intervention Delivered by: LHW Title/name of PW and number: non‐specialised personnel ‐ number not specified 1. Selection: locally identified 2. Educational background: at least a high school diploma and were selected for their affinity and capacity to work with children as demonstrated in role‐plays and interview 3. Training (contents, duration, by whom): trained 1 year before intervention, manualised intervention; not specified by whom 4. Supervision: some supervision but no details mentioned 5. Incentives/remuneration: not specified Intervention details 1. Duration/frequency: 5 weeks, 15 sessions (about 60‐minute sessions). Intervention followed a specific structure within and between sessions, with the following foci: information, safety, and control in week 1 (sessions 1 to 3); stabilisation, awareness, and self‐esteem in week 2 (sessions 4 to 6); trauma narrative in week 3 (sessions 7 to 9); resource identification and coping skills in week 4 (sessions 10 to 12); and reconnection with social context and future planning in week 5 (sessions 13 to 15). Each session is divided into 4 parts, starting and ending with structured movement, songs, and dance with the use of a 'parachute' (i.e. a large circular coloured fabric). The second part is based on a 'central activity' focused on the main theme of that week (e.g. a drama exercise to identify social supports in the environment, drawing of traumatic events), and the third part is a co‐operative game (i.e. a game in which all children had to participate to promote group cohesion 2. Content of intervention: manualised intervention consisted of cognitive‐behavioural techniques (psychoeducation, strengthening coping, and guided exposure to past traumatic events through drawing) and creative expressive elements (co‐operative games, structured movement, music, drama, and dance) with groups of around 15 children; aimed at decreasing symptoms of common mental disorders and strengthening protective factors CONTROL: wait‐list control (n = 200) CO‐INTERVENTIONS: intervention was part of a larger public mental health programme for children affected by war, including primary and tertiary prevention approaches |
|
| Outcomes |
Patients 1. CPSS* 2. DSRS (depression scale)* 3. SCARED‐5 (anxiety)* 4. SDQ§ 5. Psychological complaints 6. Functional impairment scale 7. Exposure to violence and daily stressors local scale§ 8. KIDCOPE (daily stressors)§ Carers NA Process/health workers None Economic outcomes 1. Treatment outcome 2. Treatment satisfaction 3. Therapist burden 4. Level of selection to care 5. Care package cost (see Jordans 2011) (*: primary outcomes of the study; §: outcomes that we have not reported in this review) Time points: baseline, 1 week, 3 months |
|
| Notes |
Source of funding: PLAN Netherlands Notes on validation of instruments (screening and outcomes) Primary outcome measures: primary outcome measures for PTSD, depression, and anxiety have unknown local criterion validity Secondary outcome measures 1. SDQ: validation in tamil (Lukumar 2008) 2. Psychological complaints: not validated 3. Functional impairment scale: validated in Tol 2011a 4. Exposure to violence and daily stressors local scale: not mentioned if validated 5. KIDCOPE (daily stressors): validated in Spirito 1988 Additional information: declarations of interest ‐ none Handling the data: as per footnotes in data and analysis Prospective trial registration number: none given |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "we used a two‐step randomisation procedure. First, within district divisions, we randomly allocated each division to either the intervention or waitlist control condition (see Figure 1). Second, we randomly selected schools for inclusion in the study. All schools on the government‐provided list were eligible" Comment: random sequence generation not specified |
| Allocation concealment (selection bias) | Unclear risk | Comment: not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: not blinded but unlikely to affect outcomes |
| Blinding of outcome assessment (detection bias) all outcomes | Unclear risk | Quote 1: "group of assessors not involved in service delivery" Quote 2: "assessors were not informed about which schools received intervention" Quote 3: "although we did not disclose study condition to assessors and we selected research assessors external to intervention activities, we were not able to control possible disclosure of study condition by children participating in the study" Comment: may have impacted outcome assessment |
| Baseline outcome measurements similar | Low risk | Comment: similar |
| Baseline characteristics similar? | Low risk | Quote: "we compared demographic characteristics (gender, religion, type of house, occupation caregiver, household size), exposure to violence, ongoing war‐related stressors, and scores on outcome measures, and found no statistically significant differences between study conditions. The sample consisted of more boys (61.4%) than girls, was dominantly of Hindu religion (81.0%), and children were between 9 and 12 years old (mean 11.03 ± 1.05)" Comment: similar baseline characteristics from what text says (although sociodemographics not present in a table) |
| Incomplete outcome data (attrition bias) Efficacy data | Low risk | Comment: very small dropout rate (only 1/200 in each of control and intervention groups) |
| Incomplete outcome data (attrition bias) Safety data (e.g. adverse events) | Low risk | Quote: "for girls, we found an unintended harmful effect, such that girls in the waitlist condition showed larger improvements in PTSD symptoms than girls in the intervention condition" Comment: adverse effects looked for via the intervention |
| Protection against contamination | Low risk | Comment: cluster trial, so low risk of contamination |
| Selective reporting (reporting bias) | Unclear risk | Comment: do not have protocol to check against prespecified outcomes; however, outcomes similar to other studies by the same authors |
| Other bias | Low risk | Comment: none detected |
Tol 2020.
| Study characteristics | ||
| Methods |
Study design: cluster‐RCT; unit of allocation: village in refugee settlement, 7 in each arm, 14 total; single‐blind, parallel group Duration of study: May 2016 to January 2018 |
|
| Participants |
Country: Uganda Income classification: low income Geographical scope: rural; Rhino Camp settlement in northwestern Uganda, which hosts mainly South Sudanese refugees Healthcare setting: tent structures erected specifically for this programme Mental health condition: psychological distress Population 1. Age: any age (mean 31 ± 10 years) 2. Gender: female 3. Socioeconomic background: 46% homemakers, 35% unemployed, 7% farmers, 6% self‐employed 4. Inclusion criteria a. Juba‐Arabic speaking b. Female c. Resided in household approached randomly by investigators d. Kessler 6 (K6) score ≥ 5 or more; if there were multiple eligible women in a household, 1 was selected randomly by drawing slips (1) Screening continued until 2 groups of 20 to 30 participants had been formed in each village, or after every household in the village had been approached in smaller villages 5. Exclusion criteria a. Imminent risk of suicide b. Observable signs of severe mental disorder (e.g. psychosis) c. Not able to understand basic instructions |
|
| Interventions |
Stated purpose: to assess the effectiveness of a facilitator‐guided, group‐based, self‐help intervention (Self‐Help Plus) to reduce psychological distress in female refugees INTERVENTION (n = 283) Name: Self‐Help Plus Delivered by: LHW (facilitator) Title/name of PW and number: facilitators ‐ 8 1. Selection: female; 7 were Ugandans residing in the area, 1 was a South Sudanese refugee; all finished secondary education; all had experience working in the settlement; all were proficient in Juba‐Arabic and English 2. Educational background: none had formal mental health training nor work experience; all had finished secondary education 3. Training (contents, duration, by whom): 4 facilitators were trained by master trainers, who were 2 of the study authors, for 5 days before the pilot trial and 4 days before the feasibility trial. 4 new facilitators were trained by listening to audio recordings, taking part in practice sessions led by the intervention team leader, who was one of the experienced facilitators, for 4 days and training in Self‐Help Plus facilitation skills for 4 days 5. Supervision: by Ugandan social worker, who was available for questions, and by the intervention team leader, who held the debriefs and provided supervision every 2 weeks. Additional supervision was requested from the master trainer if necessary (< 2 hours every month) Intervention details: Self‐Help Plus workshops 1. Duration/frequency: 5 weekly 2‐hour sessions 2. Content of intervention: participants attended workshop sessions in groups of 20 to 25 people, comprising presentation of pre‐recorded audio presentations, experiential exercises, and small group discussions. They were given a locally adapted illustrated self‐help book. Workshops were delivered by pairs of facilitators, whose role was limited to playing the audio recording, responding to questions and disruptions, and facilitating highly scripted individual exercises and small group discussions. Based on acceptance and commitment therapy, sessions were centred around the themes of grounding (present‐centred awareness), unhooking (from difficult thoughts and feelings), values (personal values and engaging in value‐guided actions), and being kind (to others and self) and making room (accepting difficult thoughts and feelings) CONTROL: enhanced usual care (n = 330) Participants had a 30‐minute meeting with trained community health worker, who provided psychoeducation using a structured script, and were provided information on where to access mental health services including weekly multi‐disciplinary mental health services at primary health care centres and a network of trained refugee community health workers CO‐INTERVENTIONS: both study groups received the control intervention |
|
| Outcomes |
Patients 1. K6 score* 2. PSYCHLOPS score 3. PCL‐6 score 4. PHQ‐9 score 5. Explosive anger 6. WHO‐DAS 7. WHO‐5# 8. AAQ‐II 9. Adverse events 10. Inter‐ethnic relationship score# Carers Nil Process/health workers Nil Economic outcomes Nil (asterisk for study's primary outcomes; star: outcomes that we have not reported in this review) Time points post end of intervention: 1 week, 3 months |
|
| Notes |
Source of funding: Research for Health in Humanitarian Crises (R2HC) Programme Notes on validation of instruments (screening and outcomes): validated Additional information: Brown FL, Carswell K, Augustinavicius J, Adaku A, Leku MR, White RG, Ventevogel P, Kogan CS, Garcia‐Moreno C, Bryant RA, Musci RJ, van Ommeren M, Tol WA. Self Help Plus: study protocol for a cluster‐randomised controlled trial of guided self‐help with South Sudanese refugee women in Uganda. Global Mental Health 2018;5:e27 Declarations of interest ‐ study authors declared no competing interests Handling the data: nil Prospective trial registration number: ISRCTN50148022 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generation done by an independent epidemiologist at Johns Hopkins University using Stat 14 |
| Allocation concealment (selection bias) | Low risk | Judgement comment: both reviewers rated "low" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Single‐blind cluster‐RCT ‐ lack of blinding unlikely to affect analysis |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | Allocation sequence was hidden from assessors |
| Baseline outcome measurements similar | Low risk | No comments |
| Baseline characteristics similar? | Low risk | Yen ‐ study conditions were largely similar with regards to sociodemographics, except ethnicity and length of time in the refugee settlement. These were included as covariates in effectiveness analyses Ujala ‐ baseline characteristics are reported in table 1. Study conditions were largely similar with regard to sociodemographics and baseline scores on outcomes, with the exception of ethnicity and length of time in refugee settlement |
| Incomplete outcome data (attrition bias) Efficacy data | Low risk | Yen ‐ attrition rate was 15% in the intervention group and 10% in the control group. If they had stayed on in the study, they could have influenced the magnitude of the results. However, the actual attrition rate was lower than that allowed for by the researchers in their sample size calculation Ujala ‐ we followed an intention‐to‐treat approach; we analysed all participants randomly assigned to either study group, regardless of level of intervention participation. For participants lost at follow‐up, we used list‐wise deletion (or complete case analysis), an acceptable approach when the level of missing data is minimal Missing data were adjusted for during analyses |
| Incomplete outcome data (attrition bias) Safety data (e.g. adverse events) | Low risk | Yen ‐ participants who dropped out of the study may have influenced the safety analysis if they had stayed on in the study Ujala ‐ with regard to safety considerations, the independent data safety management board responded to 6 adverse events, and none were evaluated to be concerns in response to the intervention |
| Protection against contamination | Low risk | Judgement comment: both reviewers rated "low" |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in trial registry ISRCTN, number 50148022, are reported in the paper |
| Other bias | Low risk | No comments |
Weiss 2015.
| Study characteristics | ||
| Methods |
Study design: RCT (parallel 2‐site 2‐arm (1:1 allocation) single‐blinded wait‐list randomised controlled trial) Duration of study: March 2011 to July 2012 |
|
| Participants |
Country: Iraq Income classification: lower‐middle income in 2011; upper‐middle income in 2012 Geographical scope: areas surrounding the cities of Karbala, Najaf, and Hilla (CETA), and around Basra/Nassariyah (CPT), in Southern Iraq Healthcare setting: treatment was provided at Ministry of Health primary health care centres unless there was insufficient privacy; if the client found it difficult to travel. In these situations, another mutually convenient and private place was chosen (e.g. client’s home) Mental health condition: PTSD, depression, anxiety Population 1. Age: ≥ 18 years 2. Gender: both 3. Socioeconomic background: 30% to 40% not working; 15% to 25% irregular or daily working; 30% to 40% regular stable employment 4. Inclusion criteria a. Exposure to torture (1) Referred to CMHWs by physicians at the health centre where they worked, from local prisoners’ associations, and through self‐referral after learning of services through public service announcements or by word of mouth. Survivors were defined as persons who had experienced or witnessed physical torture or militant attacks b. PTSD (1) Based on item analysis, trauma symptoms on the locally validated HTQ, along with several additional local trauma symptoms, were used to create a trauma scale. This screening instrument was used by the CMHW both to determine a client’s eligibility for the trial and, if recruited, as their baseline assessment. The instrument had a section on dysfunction, a section on depression and anxiety symptoms, a section on trauma symptoms, a section on problems of torture survivors, and a section with demographic questions. A score of 36 or higher on the 29‐question trauma section was the cutoff used for study eligibility. An earlier validity study found that this cutoff was optimal for discriminating those individuals diagnosed with PTSD from those without PTSD c. ≥ 18 years old 5. Exclusion criteria a. Children b. Mentally incompetent to understand therapy c. Currently psychotic d. Those who were a danger to themselves or to others |
|
| Interventions |
Stated purpose: to test the effectiveness of 1 counselling therapies in Southern Iraq in addressing multiple mental health problems among survivors of systematic violence: (1) a transdiagnostic intervention (Common Elements Treatment Approach, or CETA); and (2) cognitive processing therapy (CPT) INTERVENTION 1 (n = 99) Name: Common Elements Treatment Approach (CETA) Delivered by: PHP Title/name of PW and number: CMHWs ‐ community mental health workers ‐ 12 (8 males, 4 females) 1. Selection: CMHW working in and around the cities of Karbala, Najaf, and Hilla, south of Baghdad. CMHWs are non‐mental health professionals (medics or nurses who worked in rural Ministry of Health) who are trained to provide mental health services locally (i.e. the mental health equivalent of community health workers, or CHWs); must have some background/experience in counselling or mental health 2. Educational background: from Protocol: CMHWs had received training in non‐specific counselling methods some years before from our partner international non‐governmental organisation (Heartland Alliance International) and continued to provide these services part‐time 3. Training: Heartland Alliance (HA) has trained these CHMWs in non‐specific counselling interventions and currently provides ongoing supervision. Training provided by US‐based clinical psychologists 4. Supervision: weekly 1‐hour group supervision meetings 5. Incentives/remuneration: not mentioned Intervention details 1. Duration/frequency: CETA was designed to include approximately 8 to 12 weekly individual sessions 50 to 60 minutes in length. Expected time between sessions is 1 week. Total time per client is expected to last about 4 to 5 months to complete the 12 therapy sessions 2. Content of intervention: transdiagnostic treatment approach for delivery by lay counsellors in low‐resource settings with few mental health professionals; designed to treat symptoms of common mental health disorders INTERVENTION 2 (n = 129) Name: Cognitive Processing Therapy (CPT) Delivered by: PHW Title/name of PW and number: community mental health workers (CMHWs) ‐ 17 1. Selection: community mental health workers (medics or nurses who worked in rural Ministry of Health) working in and around the cities of Basra and Nassariyah in the far south of Iraq, trained to provide mental health services locally, with some background/experience in counselling or mental health 2. Educational background: non‐mental health professionals, trained to provide mental health services locally; some background/experience in counselling or mental health 3. Training: trained by US‐based clinical psychologists in Heartland Alliance (HA) in non‐specific counselling interventions 4. Supervision: weekly 1‐hour group supervision meetings by Heartland Alliance (HA) supervisors 5. Incentives/remuneration: not mentioned Intervention details 1. Duration/frequency: therapy was 12 sessions, usually 1 week apart 2. Content of intervention: cognitive‐behavioural psychotherapy originally developed for treatment of PTS or PTS with comorbid depression CONTROL: wait‐list enhanced care (CETA trial control, n = 50) (CPT trial control, n = 64) Participants received monthly telephone calls from the CMHWs who enrolled them into the study to assess their safety and to find out whether they needed referral to psychiatric care (i.e. were a danger to self or others or presented with psychosis) CO‐INTERVENTIONS: nil |
|
| Outcomes |
Patients Primary outcome 1. Trauma symptoms, assessed by a trauma scale score representing the mean of scores given to responses on the locally validated HTQ (Harvard Trauma Score) Secondary outcomes 1. Dysfunction, assessed by mean item scores for gender‐specific items on the locally developed dysfunction scale (function scale) 2. Anxiety and depression, assessed using mean item score on the locally validated HSCL‐25 (HSCL anxiety score HSCL depression score) Carers Nil Process/health workers Nil Economic outcomes Nil Time points: baseline, 3.5 to 4 months post interventions |
|
| Notes |
Source of funding: USAID Victims of Torture fund (VOT) Notes on validation of instruments (screening and outcomes): validated Additional information: declarations of interest ‐ study authors declare no conflicts of interest Handling the data: as per footnotes in data and analysis Prospective trial registration number: NCT01177072 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: "the assignment was generated using a random number generator in Excel, with a 2 to 1 probability of assignment to the intervention vs. the waitlist" |
| Allocation concealment (selection bias) | Low risk | "A piece of paper indicating the treatment assignment (intervention or waitlist) was stapled directly to the back of the study consent forms that were pre‐numbered with the participant identification number. This paper could only be read if removed from the consent form" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding of participants or personnel, but unlikely to affect outcomes |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | Quote: "interviewers at baseline and follow‐up did not know to which study arm the interviewees belonged Quote: "baseline assessments were conducted by CMHWs as part of the recruitment process prior to randomization and who were therefore blind to the assignment of study participants to intervention or WLC. These CMHWs treated those persons they had recruited who were randomly assigned to treatment. Therefore, to maintain blinding, follow‐up interviews were done by a different CMHW than the one who recruited the participant so they were unaware of the participant’s assignment. The supervisors and the study participants were not blind to the treatment condition" |
| Baseline outcome measurements similar | Low risk | No important differences were present across study groups |
| Baseline characteristics similar? | Low risk | "There were no apparent differences between intervention and control clients in demographic characteristics (Table 2)" |
| Incomplete outcome data (attrition bias) Efficacy data | Low risk | 107/129 completed intervention in CPT arm vs 97/99 in CETA arm, but unlikely to affect results |
| Incomplete outcome data (attrition bias) Safety data (e.g. adverse events) | Unclear risk | Adverse events reported, but not as outcomes; not reported per intervention |
| Protection against contamination | High risk | Judgement comment: allocated by patients |
| Selective reporting (reporting bias) | Low risk | All outcomes planned in clinical trial registry record were reported |
| Other bias | Low risk | No other risk of bias found. Adverse events were reported but not as outcomes "The research, and the services program provided by Heartland Alliance, was solely funded by the USAID Victims of Torture fund (VOT) Award DFD‐A‐00‐08‐00308‐00. This was the sole source of funding for each author and for manuscript preparation. USAID/VOT was not involved in the research or program design or implementation, or in the management or analysis of the data" |
Wu 2016.
| Study characteristics | ||
| Methods |
Study design: RCT Duration of study: June 2011 to June 2015 |
|
| Participants |
Country: China Income classification: upper‐middle income Geographical scope: urban Healthcare setting: participants’ homes Mental health condition: schizophrenia Population 1. Age: 18 to 59 years 2. Gender: both 3. Socioeconomic background: not specified 4. Inclusion criteria (including threshold cutoff score of measurement tool) a. Stable chronic schizophrenia patients with disease > 12 months with positive and negative symptoms at time of diagnosis b. Living with family carer c. Receiving continuing care by community health centre 5. Exclusion criteria a. Presence of serious physical comorbidity |
|
| Interventions |
Stated purpose: to determine the influence of Orem self‐care theory combined with collaborative care intervention on community‐based rehabilitation of patients with stable mental illness who have returned to their families INTERVENTION (n = 68) Name: Orem self‐care theory model combined with collaborative care intervention Delivered by: multi‐disciplinary team of PHPs Title/name of PW and number: doctors, nurses, and therapists ‐ number not specified 1. Selection: not specified 2. Educational background: not specified 3. Training: all trained; no details given 4. Supervision: by manager Intervention details: therapist performed counselling and corrected behaviour of both carers and patients. Nurse performed self‐care assessment and phone interviews of both carers and patients. Doctors issued medication to patients. Doctor and nurse with/without therapist performed home visits 1. Duration/frequency: phone interviews twice a month, 15‐ to 30‐minute home visits once a month 2. Content of intervention: comprised medication, counselling, evaluation of self‐care, home visits. Self‐care model involved needs evaluation, functional evaluation, psychological evaluation, beliefs and capability to handle stress, and was based on individual needs, teaching and demonstration of self‐care techniques. Home visits involved practice and immediate feedback. Participants were guided to participate in social activities or to work if capable, and their progress monitored. Participants were encouraged to express themselves, and their families were encouraged to treat them respectfully and to refrain from expressing annoyance and resentment. Participants were encouraged whenever they made incremental progress in their work ability CONTROL (n = 68) Nurse‐led usual community psychiatric care, comprising at least 3 monthly phone interview and outpatient visits in which a 17‐item “Serious mental disorder patient record” is filled, with referral to a doctor when necessary CO‐INTERVENTIONS: nil |
|
| Outcomes |
Patients 1. Self‐Care ADL 2. Instrumental ADL 3. WHOQOL‐BREF Physical health domain, Psychological domain, Social Relationships domains, and Environment domain 4. Relapse 5. Re‐admission 6. Compliance with daily life activities# 7. Medicine adherence# Carers Nil Process/health workers Nil Economic outcomes Nil (asterisk for study's primary outcomes; star: outcomes that we have not reported in this review) Time point post intervention: 0 months |
|
| Notes | Article in Chinese Source of funding: Guangzhou City Yuexiu District Notes on validation of instruments (screening and outcomes): validated Additional information: declaration of interest ‐ none Handling the data: as per footnotes in data and analysis Prospective trial registration number: nil |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation based on patients' case numbers. it is not clear whether this was a random sequence or sequential |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: allocation performed by study team at the start of the study, but concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Yen ‐ unclear: blinding was not stated explicitly in the paper Nadja ‐ unclear: no information |
| Blinding of outcome assessment (detection bias) all outcomes | High risk | No form of blinding was described in this article |
| Baseline outcome measurements similar | Low risk | Baseline outcomes are similar in all tables |
| Baseline characteristics similar? | Unclear risk | Groups were comparable in terms of age and gender; no other characteristics were compared |
| Incomplete outcome data (attrition bias) Efficacy data | Low risk | No attrition according to the paper |
| Incomplete outcome data (attrition bias) Safety data (e.g. adverse events) | Low risk | Adverse events are reported |
| Protection against contamination | Low risk | Intervention performed by multi‐disciplinary team not involved in treatment of control group |
| Selective reporting (reporting bias) | Unclear risk | Study protocol is not available |
| Other bias | Low risk | No comments |
Xie 2019.
| Study characteristics | ||
| Methods |
Study design: RCT Duration of study: March 2014 to March 2015 |
|
| Participants |
Country: China Income classification: upper‐middle income Geographical scope: Yankou Town is a representative town of Lengshuijiang City of Hunan Province, where many young couples have moved to the cities to work; their parents are typically left behind without care Healthcare setting: local community health centre Mental health condition: depression Population: ‘left behind’ elders with GDS score 11 to 25 in Hunan Province 1. Age: ≥ 65 years 2. Gender: both 3. Socioeconomic background: economic support < 500 RMB/month 3. Inclusion criteria a. GDS score 11 to 25 AND b. Over 65 years of age c. Only 1 participant from each family d. Left behind for longer than 6 months 5. Exclusion criteria a. Psychiatric and medical comorbidities that are potentially life threatening or expected to severely limit client participation or adherence b. Currently seeing a cognitive‐behavioural therapist, psychotherapist, or counsellor c. People unable to understand and fill out the questionnaires |
|
| Interventions |
Stated purpose: to evaluate the effectiveness of a modified behavioural activation treatment (MBAT) intervention on reducing depressive symptoms in rural left‐behind elderly INTERVENTION (n = 37) Name: Modified Behavioral Activation Treatment (MBAT) and regular care Delivered by: LHW and PHP Title/name of PW and number: facilitators ‐ 4, volunteers ‐ 10 1. Selection: 4 postgraduate nursing students served as facilitators. In addition, 10 local nurses familiar with the dialect were recruited as volunteers and were trained over a period of 2 days on test procedures and questionnaire items. These 10 volunteers were primarily responsible for the survey and for regional dialect co‐ordination 2. Educational background: postgraduate nursing students, local nurses 3. Training: no details of training provided. Facilitators were trained by the investigator to administer treatments. Volunteers were trained over a period of 2 days on test procedures and questionnaire items 4. Supervision: each facilitator worked with 1 of the 4 groups of participants in the intervention period in close collaboration with the investigator. To ensure competent provision of intervention, all facilitators met for weekly individual supervision sessions with the investigator 5. Incentives/remuneration: not mentioned Intervention details 1. Duration/frequency: 8 weekly sessions, each lasting 2 hours 2. Content of intervention: before the first session, participants in the intervention group were divided into 4 groups. Then, a treatment rationale and outline for BA for depression were provided to participants by the Co‐PI. The daily monitoring form (a blank calendar of the hours of each day of a week) was typically assigned as homework but was started in session to ensure that participants understood the concepts and expectations. Strategies such as enlisting a supportive family member to help with written assignments were encouraged. After submitting the assignment, local volunteers translated the log and confirmed it with participants CONTROL: usual care ("regular care") (n = 36) Included regular physical examinations, such as checking temperature, blood pressure, heart, and breathing rate, and reviewing current health symptoms depending on health needs, and receiving education (knowledge on prevention of and treatments for chronic diseases, infectious diseases, etc.), delivered by village doctors weekly during the 8‐week intervention period CO‐INTERVENTIONS: both groups received regular care |
|
| Outcomes | (Participants were given 30 to 45 minutes to finish the questionnaires; reading assistance was provided when necessary by nurse volunteers) Patients 1. Beck Anxiety Inventory (BAI) (score ≥ 45 was defined as positive for anxiety) 2. Oxford Happiness Questionnaire (OHQ) 3. Geriatric depression scale (long form) (remission of depression symptoms was defined as score < 11 on the GDS) Carers Nil Process/health workers Nil Economic outcomes Nil Time points: baseline, 3 months post‐intervention |
|
| Notes |
Source of funding: China Family Foundation Health Fellowship Program of Yale‐China Association and the National Natural Science Foundation of China (NO.81502701) Notes on validation of instruments (screening and outcomes): all locally validated Additional information: declarations of interest ‐ none Handling the data: as per footnotes in data and analysis Prospective trial registration numbers: ChiCTR‐IOR‐17011289 and NCT02785211 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random number table was used to allocate the 80 participants randomly to 2 groups of 40 each |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: both reviewers rated unclear; no mention of any effort made to conceal allocation of participants. Participants were allocated to their groups at the start of the study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Outcomes were self‐assessed. Facilitators read out questions to participants while participants filled out their responses. Participants are aware of their treatment assignment and may choose to answer favourably if they are in the intervention group because of pressure from attending the intervention or from facilitators who are translating and reading out questions on the questionnaires |
| Blinding of outcome assessment (detection bias) all outcomes | High risk | Outcomes were self‐assessed. Facilitators read out questions to participants while participants filled out their responses. Participants are aware of their treatment assignment and may choose to answer favourably if they are in the intervention group because of pressure from attending the intervention or from facilitators who are translating and reading out questions on the questionnaires |
| Baseline outcome measurements similar | Low risk | Baseline GDS, BAI, and OHQ score were similar in both groups |
| Baseline characteristics similar? | Low risk | Partipants in the 2 groups were similar in age, gender, education, economic means, visit frequency by children, previous exposure to psychoeducation or counselling, and need for psychoeducation or counselling |
| Incomplete outcome data (attrition bias) Efficacy data | Low risk | Yen ‐ unclear: dropout numbers were small (3/80 immediately post intervention and 7/80 3 months post intervention), but because effect sizes are small, it is possible for participants who had dropped out to have an influence on study results if they had stayed on Rakesh ‐ low |
| Protection against contamination | Unclear risk | Participants in both groups lived in the same area and may have shared information with each other |
| Selective reporting (reporting bias) | Unclear risk | No clinical trial registry data available. Outcomes planned in methods section were reported in results section |
| Other bias | Low risk | No comment |
Yao 2014.
| Study characteristics | ||
| Methods |
Study design: RCT; “double‐blind”; outcome assessors were blinded to patients’ allocation Duration of study: recruitment was conducted in June 2012, and participants were followed up for 12 months |
|
| Participants |
Country: China Income classification: upper‐middle income Geographical scope: urban Healthcare setting: community day rehabilitation centre Mental health condition: schizophrenia Population 1. Age: 23 to 55 2. Gender: both 3. Socioeconomic background: not specified 4. Inclusion criteria a. Fulfilled Chinese Classification of Mental Disorders (CCMD) 3 diagnostic criteria for schizophrenia b. Clinically stable c. Age ≥ 15 d. Lives with family 5. Exclusion criteria a. Dementia b. Deafness c. Blindness d. Other physical handicaps e. Severe physical illness f. Delirium caused by organic brain disorder or organ dysfunction g. Mood disorder or neurosis |
|
| Interventions |
Stated purpose: to determine the efficacy of day nursing care in community on improving the social function of patients with chronic schizophrenia INTERVENTION (n = 40) Name: day nursing care in community Delivered by: PHP and psychiatrists (collaborative care) Title/name of PW and number: general practice physicians, 3 community nurses, and preventive health workers 1. Selection: nurses: nursing supervisor grade 2. Educational background: nurse in charge attained National Counselling Grade 2 certificate 3. Training (contents, duration, by whom): not specified 4. Supervision: by nurse in charge Intervention details: participants attended a community day rehabilitation centre free of charge 1. Duration/frequency: day rehabilitation for 12 months. Health education sessions every 1 to 2 months 2. Content of intervention: nurse with counselling certificate delivered supportive psychotherapy, psychiatrist delivered health education, nurses demonstrated content and assisted in role‐play, local disability service workers facilitated group training activities. Doctors (general practice physicians) responded to carers’ (accompanying family or disability service worker) concerns about early signs of deterioration in participants, performing a preventive role. Psychiatrists delivered health education and medical care to the intervention group CONTROL (n = 40) Routine care of mentally ill patients in the community comprising medication and (not specified psychiatric or community) nursing follow‐up, with monthly home visits, 3‐monthly risk assessments, health education, and advice on medication. Participants who developed irregular symptoms were promptly referred CO‐INTERVENTIONS: participants in both groups received psychiatric medication (antipsychotics), with modifications to their original regimen as needed |
|
| Outcomes |
Patients 1. BPRS: total, NORS: total, SDSS: total, Occupational role, Social withdrawal, Social activities, Participation in household activities, Family functioning, Self‐care, Interest in outside world, Responsibility or planning, Marital role, and Parental role Carers Nil Process/health workers Nil Economic outcomes Nil (asterisk for study's primary outcomes; star: outcomes that we have not reported in this review) Time point post intervention: 0 months |
|
| Notes | Article in Chinese Source of funding: Pudong Health Bureau of Shanghai Notes on validation of instruments (screening and outcomes): validated Additional information: declarations of interest ‐ none Handling the data: nil Prospective trial registration number: nil |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: using a random number table, participants were randomly selected and grouped into intervention and control groups |
| Allocation concealment (selection bias) | Low risk | Allocation by study team at start of study |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel were not blinded, but this may not have influenced outcomes |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | Primary outcome variables were assessed by 2 psychiatrists blinded to participants' allocation groups |
| Baseline outcome measurements similar | Low risk | Baseline NORS, BPRS, and SDSS scores ‐ no significant differences between groups |
| Baseline characteristics similar? | Low risk | Age, education, disease course, marital status, medication type and dosage compared between the 2 groups ‐ no significant differences |
| Incomplete outcome data (attrition bias) Efficacy data | Low risk | No dropouts |
| Protection against contamination | Low risk | Judgement comment: intervention carried out at community day rehab centre. Control participants did not attend this day rehab centre |
| Selective reporting (reporting bias) | Unclear risk | No clinical trial registry data available. All outcomes planned in methods section were reported in results section |
| Other bias | Low risk | No comments |
Yeomans 2010.
| Study characteristics | ||
| Methods |
Study design: RCT (3‐arm trial) Duration of study: spring 2007 ‐ ? |
|
| Participants |
Country: Burundi Income classification: low income Geographical scope: 2 rural communities in north‐central Burundi, country that suffered a civil war in which over 300,000 people were killed Healthcare setting: community groups Mental health condition: PTSD Population: patients 1. Age: mean age 38.6 years (SD 12.8) 2. Gender: both; 44.4% female 3. Socioeconomic background: 48.3% lived in camps; only 5% of sample completed > 6 years of education; ethnic composition was 52% Hutu and 47.6% Tutsi; almost all were directly victimised by violence during or since conflict onset in 1993; most were not fully literate 4. Inclusion criteria a. Among future participants of 2 trauma workshops offered by internally displaced people camps 5. Exclusion criteria: not specified |
|
| Interventions |
Stated purpose: to evaluate the effects of PTSD psychoeducation within a larger trauma healing and reconciliation intervention in a rural region of Burundi INTERVENTION 1 (n = 38) Name: workshop with psychoeducation Delivered by: LHW Title/name of PW: Burundian facilitators ‐ number not specified 1. Selection: "chosen by the nonprofit organisation for their extensive experience with trauma workshop facilitation and for having demographics comparable to participants" 2. Educational background: "rural, poor, many without substantial formal education, and balanced in gender and ethnicity" 3. Training: "all facilitators had a full day of training dedicated to the modification of the standard workshop to accommodate planned differences in condition"; not specified by whom 4. Supervision: not specified 5. Incentives/remuneration: not specified Intervention details 1. Duration/frequency: standard intervention included 2 phases. 6 groups of approximately 20 participants gathered for 3 days, and 1 month later, each workshop group reconvened for a full‐day follow‐up session, during which major workshop components were reinforced 2. Content of intervention: 3‐day workshop used discussion, experiential exercises aimed at fostering interpersonal exchange, and games to explore themes of trauma, loss, anger, trust, and the roots of violence; the Healing and Reconciling Our Communities workshop manual (African Great Lakes Initiative of the Friends Peace Teams, 2006) emphasised that recovery from trauma lies in the restoration of relations between community members, and in understanding how trauma can affect these relationships and individuals. The Healing and Reconciling Our Communities programme integrates theoretical frames as described by Herman 1997 and Staub 2005. Each of Herman's 3 stages of recovery from trauma was incorporated within the Healing and Reconciling Our Communities workshop design. There was emphasis on the need for personal recovery and interpersonal reconciliation by means of "a neighbour‐to‐neighbour healing process, which must include cognitive and affective engagement with experience in the context of interpersonal support". Psychoeducational content on the first day of the workshop included a 90‐minute presentation and discussion of the 17 specific symptoms of PTSD. Orientation to and solicitation of potential Criterion A (according to the DSM) events were also included. These ideas were reviewed again in the afternoon, and participants shared how they had been affected by the traumatic events they had experienced (1 hour additional). Coping with trauma was addressed in terms of teaching relaxation skills with substantial emphasis on repairing relationships with community members INTERVENTION 2 (n = 37) Name: workshop without psychoeducation Delivered by: LHWs Title/name of PWs: Burundian facilitators ‐ number not specified 1. Selection: "chosen by the nonprofit organisation for their extensive experience with trauma workshop facilitation and for having demographics comparable to participants" 2. Educational background: "rural, poor, many without substantial formal education, and balanced in gender and ethnicity" 3. Training (contents, duration, by whom): "all facilitators had a full day of training dedicated to the modification of the standard workshop to accommodate planned differences in condition"; not specified by whom 4. Supervision: not specified 5. Incentives/remuneration: not specified Intervention details 1. Duration/frequency: standard intervention included 2 phases. 6 groups of approximately 20 participants gathered for 3 days, and 1 month later, each workshop group reconvened for a full‐day follow‐up session, during which major workshop components were reinforced 2. Content of intervention: active workshop condition with no psychoeducation was identical to that described in intervention 1, with 2 exceptions. First, this condition did not include the introduction of PTSD psychoeducational content. Second, to ensure that both workshop conditions were of equal length, additional time was devoted to an exercise in which participants formed pairs and answered questions provided to them. Assigned topics facilitated communication around perspectives on trust, safety, sense of security, and interethnic relations in the community (e.g. "someone I trust and why", "a time I overcame fear"). It is important to note that participants were encouraged to discuss how they have been affected by events during the war, but unlike in the workshop with the psychoeducation condition, facilitators did not augment this discussion with any PTSD psychoeducational content CONTROL: wait‐list control (n = 38) Received workshops after second assessment period CO‐INTERVENTIONS: none |
|
| Outcomes |
Patients 1. HSCL‐25 plus 10 somatic symptoms from HSCL‐58 comprised a hybrid HSCL instrument (anxiety and depression and global measure of emotional distress) 2. HTQ Part IV (Trauma) 3. HTQ‐b (guilt, loneliness, shame, betrayal, and rumination)§ Carers NA Process/health workers Facilitators completed a report after each workshop in reference to the integrity of the condition. Reports indicated that workshop components were consistent as planned and true to treatment condition. Facilitators did report 3 instances (in the course of over 2500 participant‐hours) in which a participant proposed the concept of 'trauma' during a brainstorm about the consequences of the war. As previously instructed, facilitators acknowledged the statement, but did not foster discussion on it§ Economic outcomes None (*: primary outcomes of the study; §: outcomes that we have not reported in this review) Time points: 6 weeks before intervention, 2 weeks after intervention |
|
| Notes |
Source of funding: not specified Notes on validation of instruments (screening and outcomes):HSCL : for depression scale, sensitivity of 0.88 and specificity of 0.73; proven to be culturally sensitive with samples around the world and demonstrated sufficient validity and reliability (Fox 2002); HTQ Part IV and HTQ‐b : not validated in local setting; Trauma Discourse exposure interview : not validated in local setting Additional information: declarations of interest ‐ none Handling the data: as per footnotes in data and analysis Prospective trial registration number: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "participants were blocked according to ethnicity and gender and randomly assigned to condition" |
| Allocation concealment (selection bias) | Low risk | Quote: "in each community, using a computerized random‐number generator, participants were assigned to condition according to stratified randomisation (by gender and ethnicity) to either workshop with psychoeducation, workshop without psychoeducation, or waitlist control" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "participants and interviewers (at pre‐ and post‐test) were blind to condition assignment" |
| Blinding of outcome assessment (detection bias) all outcomes | Low risk | Quote: "participants and interviewers (at pre‐ and post‐test) were blind to condition assignment" Comment: adequate assessor blinding |
| Baseline outcome measurements similar | Low risk | Quote: "there were no significant baseline differences between the three treatment groups across age, gender, ethnicity, symptoms, education level, traumatic events experienced, or on prior exposure to trauma discourse" |
| Baseline characteristics similar? | Low risk | Quote: "there were no significant baseline differences between the three treatment groups across age, gender, ethnicity, symptoms, education level, traumatic events experienced, or on prior exposure to trauma discourse" |
| Incomplete outcome data (attrition bias) Efficacy data | Low risk | Quote: "participants received a small reimbursement for transportation expense only" Comment: only a few participants not available at follow‐up points |
| Protection against contamination | Low risk | Comment: study done before workshops were delivered later in communities; assessed for prior discourse on trauma |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol |
| Other bias | Low risk | Quote: "participants received a small reimbursement for transportation expenses only" Comment: unlikely to affect outcomes |
Zhong 2015.
| Study characteristics | ||
| Methods |
Study design: RCT Duration of study: participants were recruited between December 2008 and February 2009, and were followed up for 12 months |
|
| Participants |
Country: China Income classification: lower‐middle income in 2008 to 2009; upper‐middle income in 2010 Geographical scope: urban Healthcare setting: community centre Mental health condition: substance abuse (heroin) Population 1. Age: 18 to 60 years 2. Gender: both 3. Socioeconomic background: about one‐third (31.1%) had been employed in the past 1 year 4. Inclusion criteria a. Met Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition criteria for heroin dependence b. Had just been released from mandatory 2‐year rehabilitation at a treatment centre c. Between 18 and 60 years of age d. At least 9 years of education e. Permanent Shanghai residents 5. Exclusion criteria a. Involved in another treatment programme, such as taking methadone or buprenorphine b. Significant withdrawal symptoms c. Presence of schizophrenia, bipolar disorder, and other severe psychological or physical disorders |
|
| Interventions |
Stated purpose: to investigate whether comprehensive psychosocial rehabilitation would be effective in improving participants' mental health and quality of life, reducing risk of relapse, and reducing drug use. INTERVENTION (n = 90) Name: Comprehensive Psychosocial Intervention (CPI) Delivered by: CP Title/name of PW and number: social workers ‐ number not specified 1. Selection: experienced social workers 2. Educational background: not specified 3. Training: trained between January 2009 and February 2009 (2 months) on the principles of comprehensive psychosocial intervention, assessment measurements, and skills of dealing with persons with opioid use disorders, by substance abuse research team from Shanghai Mental Health Centre 4. Supervision: on‐site supervision by supervisor with level II psychological counselling certification; group sessions supervised by a psychologist. Intervention details: directed at participants. Encompassed cognitive‐behaviour therapy theory, motivational interview techniques, case management, and urine testing, using both individual therapy and group therapy settings 1. Duration/frequency: weekly 60‐minute individual sessions, 2‐monthly 100‐minute group activities for 8 to 10 participants each time, for 1 year 2. Content of intervention: engaged participants' intrinsic motivations; psychoeducation; identification of risk factors and triggers for relapse; techniques to prevent relapse; HIV, HCV, and high‐risk sexual behaviour education; identifying and coping with negative emotions; interpersonal skills training; time management; developing healthy alternative habits; using community resources to help participants CONTROL: usual community care (n = 90) Participants received monthly visit by social worker, urine tests, and simple advice regarding life events CO‐INTERVENTIONS: on relapse, participants in intervention group were referred to medical institutions for treatment with methadone, buprenorphine, or extended‐release naltrexone |
|
| Outcomes |
Patients 1. SCL‐90 ‐ Somatisation, Obsessive‐Compulsive, Interpersonal, Depression, Anxiety, Hostility, Phobic Anxiety, Paranoid, Psychoticism, Total 2. SF36 ‐ Physical functioning, Physical role limitation, Social functioning, Emotional role functioning, Mental Health (Quality of Life Related With Health), Pain, (Quality of Life Related With Health), General Health Perceptions 3. Self‐reported heroin use in last 12 months and past 30 days 4. Urine drug screen (morphine) 5. Urine drug screen (methamphetamine) 6.Relapse 7. Self‐reported alcohol use in last 12 months and past 30 days 8. Self‐reported amphetamine use in past 12 months and past 30 days Carers Nil Process/health workers Nil Economic outcomes Nil (asterisk for study's primary outcomes; star: outcomes that we have not reported in this review) Time point post intervention: 0 months |
|
| Notes |
Sources of funding: Ministry of Science and Technology Project, Chinese National Basic Science Foundation, Shanghai One Hundred Talent Project Notes on validation of instruments (screening and outcomes): validated Additional information: declarations of interest ‐ study authors declared no conflicts of interest Handling the data (e.g. imputed values/other calculations we have made): possible error in reporting urine morphine screen and relapse, as text and tables did not match. Entered data from text only Prospective trial registration number: nil |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not described |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: participants were randomised at the start of the study, but it is not specified where randomisation was performed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding was not described in the paper |
| Blinding of outcome assessment (detection bias) all outcomes | High risk | Social workers who may provide the intervention performed outcomes assessment |
| Baseline outcome measurements similar | Low risk | No comments |
| Baseline characteristics similar? | Low risk | No comments |
| Incomplete outcome data (attrition bias) Efficacy data | Low risk | No comments |
| Protection against contamination | Unclear risk | Judgement comment: intervention and control group participants lived in the same communities and could have shared information and skills learnt in their respective groups |
| Selective reporting (reporting bias) | High risk | Addiction severity index was described in methods section, but no outcomes were reported in results section |
| Other bias | Low risk | No comments |
AIDS: acquired immunodeficiency syndrome; AMPATH: Academic Model for Providing Access to Healthcare; ART: antiretroviral therapy; AUDIT: Alcohol Use Disorders Identification Test; BDI: Beck Depression Inventory; CBA: controlled before‐and‐after; CBI: classroom‐based intervention; CBCL: Child Behavior Checklist; CBT: cognitive‐behavioural therapy; CDI: Children's Depression Inventory; CES‐D: Center for Epidemiological Studies Depression Scale; CHA: community health aide; CHW: community health worker; CIS‐R: revised Clinical Interview Schedule; CP: community professional (teacher, non‐health professional, social worker, etc.); CPSS: Child Post‐traumatic Stress Scale; CPTSD‐RI: Child Post‐traumatic Stress Reaction Index; CTS2S: Revised Conflict Tactics Scales, short form; DEMQOL: Dementia Quality of Life; DIS: Diagnostic Interview Schedule; DSM:Diagnostic and Statistical Manual of Mental Disorders; DSRS: Depression Self‐Rating Scale; EPDS: Edinburgh Postnatal Depression Scale; ESB: economic skill‐building; FBIS: Family Burden Interview Schedule; GGT: gamma‐glutamyl transferase; GHQ‐12: General Health Questionnaire; GP: general practitioner; GSE: general self‐efficacy; HCA: home care advisor; HDRS: Hamilton Depression Rating Scale; HIV: human immunodeficiency virus; HSCL: Hopkins Symptom Checklist; HTQ: Harvard Trauma Questionnaire; ICC: intracluster correlation; ICD: International Classification of Diseases; ICDP: International Child Development Programme; ID: intellectual disability; IES: Impact of Events Scale; IPT: interpersonal therapy; IPT‐G: interpersonal therapy group; IPV: intimate partner violence; ITT: intention‐to‐treat; LHC: lay health counsellor; LHW: lay health worker; MDD: major depressive disorder; MET: motivational enhancement therapy; MINI: Mini International Neuropsychiatry Interview; MOS: Medical Outcomes Study; NET: narrative exposure therapy; NGO: non‐government organisation; NPI‐S: Neuropsychiatric Inventory ‐ Severity; PC: primary care; PCU: primary care unit; PW: primary‐level worker; PDS: Post‐traumatic stress; PHC: primary health care; PHP: primary health professional; PhD: doctor of philosophy; PSS: Parental Support Scale; PTSD: post‐traumatic stress disorder; QLDS: Quality of Life in Depression Scale; QoL: quality of life; RCT: randomised controlled trial; SA‐SCAT: Social Capital Assessment Tool, short adapted version; SCARED: Screen for Child Anxiety Related Disorders; SCL: Symptom Checklist; SD: standard deviation; SDQ: Strengths and Difficulties Questionnaire; SEI: Self‐Esteem Inventory; SES: socioeconomic status; SF: Short Form; SRQ: Self‐Reporting Questionnaire; STI: sexually transmitted infection; TC: trauma counselling; UCLA: University of California, Los Angeles; UNICEF: United Nations Children's Fund; UNRWA: United Nations Relief and Works Agency; USAID: United States Agency for International Development; VABS: Vineland Adaptive Behavior Scales; WHO: World Health Organization; WHODAS: World Health Organization Disability Assessment Scale; WHOQOL‐BREF: World Health Organization Quality of Life‐BREF; YACS: Yale Adherence and Competence Scale; ZBI: Zarit Burden Interview; ZBS: Zarit Burden score.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adina 2017 | Wrong intervention |
| Agberotimi 2017 | Wrong intervention |
| Akol 2018 | Wrong outcomes (no mental health outcomes) |
| Alexander 2013 | Wrong intervention |
| Assanangkornchai 2012 | Wrong intervention |
| Avci 2016 | Wrong intervention |
| Bagaiolo 2017 | Wrong outcomes |
| Baker 2005a | Moved to prevention review |
| Bass 2012 | Wrong study design |
| Botha 2018 | Wrong intervention |
| Brown 2009 | Wrong study design |
| Cai 2015 | Wrong intervention |
| Chaowanee 2018 | Wrong intervention |
| Chawarski 2008 | Wrong intervention |
| Chen 2000 | Wrong setting |
| Chien 2017 | Wrong intervention |
| Conde 2018 | Wrong intervention |
| Damra‐Jalal 2014 | Wrong intervention |
| Danaee‐Far 2016 | Wrong outcomes |
| Dawson 2018 | Wrong comparator |
| Firn 2019 | Wrong intervention |
| Fleischmann 2008 | Wrong intervention. A mixed group of specialists (psychologists) and non‐specialists (general doctors, nurses) were delivering care at the primary level in this trial (relative weights of roles of specialists vs non‐specialists were not described |
| Green 2019 | Wrong intervention |
| Gu 2013 | Wrong intervention |
| Guerra 2011 | Specialist‐delivered intervention (junior psychologist and social workers) |
| Guo 2019a | Article was retracted |
| Hamadani 2006 | Prevention study |
| Hamdani 2017a | Wrong intervention |
| Han 2013 | Wrong intervention |
| Hassanzadeh 2010 | Wrong intervention |
| Hegde 2012 | Wrong intervention |
| Huang 2017 | Wrong intervention |
| Huang 2018 | Wrong intervention |
| IRCT2016042527584N1 2016 | Wrong intervention |
| ISRCTN72875710 2014 | Wrong intervention |
| Jacob 2014 | Wrong intervention |
| Jacobs 2016 | Wrong intervention |
| Kauye 2014 | Wrong intervention |
| Knettel 2019 | Wrong intervention |
| Lara 2010 | Wrong intervention |
| Layne 2008 | Wrong intervention |
| Li 1989 | Study was included in the last review, but as we have now excluded epilepsy and other neurological disorders, it has been removed |
| Li 2017 | Wrong intervention |
| Li 2017a | Wrong intervention |
| Li 2018a | Wrong intervention |
| Li 2018b | Wrong intervention |
| Liao 2018 | Wrong intervention |
| Liu 2005 | Wrong intervention |
| Liu 2018 | Wrong intervention |
| Long 2015 | Wrong intervention |
| Loughry 2006 | Wrong study design |
| Manwong 2018 | Wrong intervention |
| Marchira 2018 | Wrong intervention |
| Marchira 2019 | Wrong intervention |
| McMullen 2013 | Wrong intervention |
| Mehrabi 2011 | Wrong intervention |
| Miranda‐Castillo 2013 | Wrong intervention |
| Molazem 2014 | Wrong intervention |
| Moshki 2014 | Wrong intervention |
| Mousavi 2019 | Wrong intervention |
| Mushtaq 2017 | Wrong intervention |
| Mutiso 2018 | Wrong intervention |
| Naeem 2011 | Wrong intervention |
| Nagarajaiahnull 2012 | Wrong intervention |
| Naidoo 2014 | Wrong intervention |
| Nakimuli‐Mpungu 2014 | Wrong intervention |
| NCT00539123 2007 | Wrong intervention |
| NCT01523444 2016 | Study was withdrawn |
| NCT02598024 2015 | Study was withdrawn |
| NCT03754829 2018 | Wrong intervention |
| NCT03884933 2020 | Wrong intervention |
| NCT03912753 2019 | Wrong intervention |
| NCT03966885 2020 | Wrong intervention |
| Nwobi 2018 | Wrong intervention |
| Orang 2018 | Wrong intervention |
| Orawan 2018 | Wrong intervention |
| Oveisi 2010 | Wrong outcomes |
| PACTR201310000589295 2013 | Wrong intervention |
| Paddick 2017 | Wrong intervention |
| Pahlavanzadeh 2010 | Wrong intervention |
| Palavras 2015 | Wrong intervention |
| Pang 2002 | Wrong intervention |
| Prince 2007 | Wrong intervention |
| Qouta 2018 | Wrong intervention |
| Rahman 2019a | Wrong intervention |
| Ran 2003 | Specialist‐led intervention (mainly conducted by trained psychiatrists) |
| Razali 2000 | Wrong intervention |
| Reddy 2019 | Wrong intervention |
| Ross 2013 | Wrong intervention |
| Rossouw 2018 | Wrong intervention (non‐specialists delivered intervention in both arms) |
| Samaraweera 2007 | Wrong intervention |
| Schade 2011 | Wrong intervention |
| Schapira 2010 | Wrong intervention |
| Senanarong 2004 | Wrong intervention |
| Sharifi 2012 | Wrong intervention |
| Sheikh 2017 | Wrong patient population |
| Shen 2018 | Not a task‐shifting intervention nor an MH intervention (expressive writing vs controlled writing) |
| Shin 2009 | Included in 2013 review but now moved to prevention study due to participant inclusion criteria; does not include child with mental disorder (prevention of mental ill health for disabled children) |
| Shin 2013 | Wrong population (hospitalised in TB ward). Included in ongoing studies from last review (Greenfield 2010) |
| Shin 2017 | Wrong outcomes |
| Soares 2018 | Wrong intervention |
| Steinert 2017 | Wrong intervention |
| Sumathipala 2008 | Wrong intervention |
| Tao 2015 | Wrong patient population |
| Thabet 2005 | Wrong study design |
| Thomas 2017 | Wrong intervention |
| Tian 2005 | Wrong intervention |
| Tian 2006 | Wrong intervention |
| Tiwari 2005 | Specialist‐led intervention (midwife with a master's degree in counselling) |
| Tiwari 2010 | Wrong setting |
| Tomlinson 2015 | Wrong outcomes |
| Tripathy 2010 | No mental health intervention. Confirmed with study author (VP) that mental health outcomes were included after the trial had started, as an add‐on |
| Ugwoke 2018 | Wrong intervention |
| Unterhitzenberger 2014 | Wrong intervention |
| Visagie 2015 | Wrong intervention |
| Vitriol 2010 | Wrong intervention |
| Vreeman 2018 | Wrong intervention |
| Wandera 2017 | Wrong patient population |
| Wang 2008 | Wrong intervention |
| Wang 2013 | Wrong intervention |
| Wang 2017 | Wrong intervention |
| Wei 2013 | Wrong intervention |
| Wolmer 2005 | Wrong study design |
| Xiang 2006 | Wrong intervention |
| Xu 2019 | Wrong intervention |
| Yildirim 2013 | Wrong intervention |
| Yildiz 2004 | Wrong patient population |
| Zambori 2002 | This study was in the last review, but as we have now excluded epilepsy and other neurological disorders, it has been removed |
| Zhang 2015 | Wrong intervention |
| Zhao 1999 | Wrong intervention |
| Zhao 2015 | Wrong intervention |
| Zhou 2014 | Wrong intervention |
CBA: controlled before‐and‐after; EPOC: Effective Practice and Organisation of Care; HIV: human immunodeficiency virus; IPT: interpersonal therapy; IQ: intelligence quotient; ITS: interrupted time series; LMIC: low‐ and middle‐income country; MNS: mental, neurological, and substance abuse; NSHW: non‐specialist health worker; PHC: primary health care; RCT: randomised controlled trial.
Characteristics of studies awaiting classification [ordered by study ID]
Ali 2020.
| Methods | Randomised controlled clinical trial |
| Participants | Patients with depression and diabetes in India Inclusion criteria 1. Age ≥ 35 years 2. Confirmed diagnosis of diabetes (documented glucose tolerance test or 2 venous glucose levels) 3. PHQ‐9 score ≥ 10 4. ≥ 1 poorly controlled CVD risk factor (HbA1c ≥ 8.0% or SBP ≥ 140 mmHg or LDL ≥ 130 mg/dL), irrespective of medications used 5. Willingness to consent to randomisation Exclusion criteria 1. Patient reports a "3" on the PHQ‐9 questionnaire suicide item (Item No: 9) or the patient's PHQ‐9 score is above 23, indicating severe depression requiring immediate referral 2. Any participant reporting a "2" on the PHQ‐9 suicide item (Item No: 9) will be reviewed carefully and, if considered too high risk, the participant will be excluded from enrolment in the trial and referred for more intensive psychiatric care 3. Already in psychiatrist's care or using antipsychotic or mood stabiliser medication or with diagnosed dementia or bipolar disorder or schizophrenia (based on bipolar and schizophrenia modules of the MINI) 4. Diabetes secondary to uncommon causes (e.g. chronic pancreatitis) 5. Pregnancy or breast‐feeding 6. Documented CVD event (MI, stroke) in past 12 months 7. End‐stage renal disease awaiting transplant 8. Malignancy or life‐threatening disease with death probable in 3 years 9. Alcohol or drug abuse 10. No fixed address or contact details |
| Interventions |
Intervention Comprises: 1. Patient education and behavioural activation Delivered by non‐specialist providers 2. Supporting self‐care Care coordinators will (a) meet with intervention arm patients and collaboratively set treatment goals; (b) provide verbal education regarding diabetes and depression self‐care; (c) use motivational interviewing and self‐efficacy enhancement strategies to promote monitoring of depressive symptoms, glucose, BP; (d) proactively follow up to externally monitor depression symptoms and CVD indicators; (e) enter updated patient indicators into decision support electronic health record and utilise software outputs to prioritise patients for review; (g) convene case review meetings with supervising physicians; and (h) communicate physician‐recommended treatment changes to patients and their routine providers 3. Psychiatrist and diabetologist reviews Senior psychiatrist and endocrinologist/diabetologist will be involved in weekly off‐line case review meetings with care co‐ordinators. Physicians will recommend treatment changes (initiation, increase, or simplification of medication regimens), which will be communicated by care co‐ordinators to patients and their usual care providers 4. Decision support electronic health record system The decision support electronic health record will store patient indicators entered by Nurse Case Managers (NCMs) and will provide diabetes and depression care prompts based on an evidence‐based treatment algorithm developed from recommended guidelines for control of diabetes and depression, Indian formularies, and TeamCare investigators. The decision support electronic health record (DS‐EHR) will prioritise patients (new; poorly controlled; or well controlled but not reviewed ≥ 3 months) for case review meetings and will promote accountability (physicians must justify rejecting electronic care prompts) Control Participants will receive existing standard care and treatment for diabetes provided routinely at each clinic site, and their care provider will be notified regarding their depressive symptoms. Physicians treating the control arm will also be provided with training regarding identification and care for people with depression. Control participants will have no contact with care co‐ordinators and will be contacted only at 6‐monthly intervals for assessment by the blinded outcomes assessor |
| Outcomes |
Primary outcome measures 1. Percentage of Combined Improvement of Depressive Symptoms and CVD Risk Factors [Time Frame: 24 months post intervention] 2. Sustained (24‐month) percentage (%) of participants achieving the outcome in each arm for combined depression and CVD risk factor improvements (≥ 50% reduction in SCL‐20 score AND ≥ 1 of ≥ 0.5% reduction in HbA1c, ≥ 5 mmHg reduction in systolic blood pressure (SBP), or ≥ 10 mg/dL reduction in LDL‐C) Secondary outcome measures 1. Measures of "Common Effect" [Time Frame: 12 months post intervention, 24 months post intervention, 36 months post intervention (post hoc follow‐up)] a. Measure of whether intervention had a similar beneficial effect on 4 targets: SCL‐20 score, HbA1c, SBP, and LDL 2. Proportion of Participants Achieving All 3 CVD Risk Factor Targets in the Two Groups [Time Frame: 12 months post intervention, 24 months post intervention, 36 months post intervention (post hoc follow‐up)] a. HbA1c ≤ 7.0% and SBP ≤ 130 mmHg and LDL ≤ 100 mg/dL 3. Mean Changes in Each of the Four Main Targets (SCL‐20 Score, HbA1c, SBP, LDL‐C) [Time Frame: 12 months post intervention, 24 months post intervention, 36 months post intervention (post hoc follow‐up)] 4. Proportion of Participants Achieving Treatment Targets or Significant Reductions in Individual Risk Factors [Time Frame: 12 months post intervention, 24 months post intervention, 36 months post intervention (post hoc follow‐up)] a. HbA1c ≤ 7.0% or ≥ 0.5% reduction [SBP ≤ 130 mmHg or ≥5 mmHg reduction] [LDL ≤ 100 mg/dL or ≥ 10 mg/dL reduction] 5. Mean Treatment Satisfaction Scores [Time Frame: 12 months post intervention, 24 months post intervention, 36 months post intervention (post hoc follow‐up)] a. Diabetes Treatment Satisfaction Questionnaire (DTSQ) 6. Mean Health Expenditures (Direct Medical Costs) [Time Frame: 12 months post intervention, 24 months post intervention, 36 months post intervention (post hoc follow‐up)] a. Include direct medical costs for consultations, diagnostic tests, medications, hospital admissions, and/or surgeries or procedures 7. Cost Utility in the Treatment Arm and in Usual Care Arms [Time Frame: 12 months post intervention, 24 months post intervention, 36 months post intervention (post hoc follow‐up)] a. Ratio of total costs, which include health expenditures by participants plus clinic or study costs to deliver the intervention and relative gain or loss in health utilities (measured by health utilities index) |
| Notes |
Anjara 2019.
| Methods | Cluster‐randomised trial |
| Participants | Clusters were community health centres (Puskesmas) in Yogyakarta, Indonesia. The study was deemed to be partially randomised, as centres in the control arm came from 2 out of the 5 possible districts Inclusion criteria 1. Adult primary care patients attending Puskesmas 2. GHQ‐12 ≥ 2 Exclusion criteria Ongoing treatment for mental health issues |
| Interventions |
Intervention 1. mhGAP by GPs a. GPs in intervention arms underwent training in mhGAP modules. Participants’ screening results were given to GPs, who planned medications and/or psychosocial therapy; further therapy sessions were given as needed Control 1. Care by specialists co‐located in primary care 2. Participants were referred by treating GPs to a psychologist. Clinical psychologists then treated participants in a separate consultation room in the Pukesmas. Further therapy sessions were based on participants’ needs |
| Outcomes |
Primary outcomes at 6 months Health of the Nation Outcome Scale Secondary at 6 months 1. WHO Disability Assessment Schedule 2.0 2. 3‐Level European Quality of Life Scale (EQ‐5D‐3L) 3. Client service receipt inventory 4. Total CIS‐R score 5. Resource use and costs |
| Notes |
Asadzadeh 2020.
| Methods | Randomised controlled trial |
| Participants | Pregnant women attending 3 government antenatal clinics in Zanjan City, Iran, were screened Inclusion criteria 1. 18 to 35 years old 2. Speak and read Persian 3. Last trimester of pregnancy 4. Single embryo Exclusion criteria 1. Score ≥ 10 on Edinburgh Postnatal Depression Scale 2. History of abortion and infertility 3. Mental or physical chronic disease 4. Taking medicine that causes symptoms of depression 5. History of postpartum depression in first‐degree relatives 6. Major stressful event during the past year Eligible women were followed up until childbirth, after which those who met DSM‐5A criteria for diagnosis of traumatic childbirth were randomised into intervention and control groups |
| Interventions |
Intervention Participants received a face‐to‐face counselling session by a midwife (first study author), who had been trained by a psychologist, within 72 hours after childbirth and a telephone counselling session 4 to 6 weeks after childbirth. Each session lasted 40 to 60 minutes. Participants could contact their midwife between sessions by telephone. Sessions emphasised the therapeutic relationship between midwife and participant, accepting and working with perceptions, reviewing labour management, increasing social support, reinforcing positive coping approaches, and exploring solutions Control Routine postpartum care |
| Outcomes | 1. Edinburgh Postnatal Depression Scale 2. Hamilton’s Anxiety Rating Scale 3. PCL‐5 |
| Notes |
Dorsey 2020.
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria 1. Age 7 to 13 years 2. Death of 1 or both parents 3. Residing in a family home 4. Child and guardian willing to participate 5. Ability to speak Kisawahili 6. Score ≥ 18 on child PTSD Symptom Scale and/or score ≥ 35 on 28‐item Inventory of Complicated Grief Exclusion criteria 1. Parent(s) died when child was 3 years old or before 2. Parent(s) died within previous 6 months 3. Substantial cognitive impairment |
| Interventions |
Intervention Trauma‐focused cognitive‐behavioural therapy (TF‐CBT): 12 group sessions and 3 to 4 individual sessions for 12 consecutive weeks for children and guardians, involving psychoeducation, coping strategies, behaviour management skills, imaginal exposure (“trauma narrative”), cognitive processing, emotional support, planning for trauma reminders, sharing, and grief‐specific elements. Sessions were delivered by counsellors who received 2 weeks of in‐person training using the apprenticeship model and booster training sessions, and who were supervised weekly. Supervision and training were conducted by Tanzanian lay counsellors from a previous study. These in turn were supervised by study authors Control Usual care: participants accessed usual services such as education and mental health care |
| Outcomes |
Primary outcome measures 1. Post‐traumatic Stress Syndrome (PTSS) [Time Frame: 15 months] a. Measured using the Child PTSD Symptoms Scale (CPSS). Caregiver and Child reported separately 2. Childhood Traumatic Grief [Time Frame: 15 months] a. Measured using the Inventory of Complicated Grief (ICG). Child report only Secondary outcome measures 1. Behavioural Difficulties [Time Frame: 15 months] a. Measured using the Child Behavior Checklist (CBCL), Youth Self‐Report (YSR), and items developed locally that are culturally specific Other outcome measures 1. Depression [Time Frame: 15 months] 2. Child Functioning [Time Frame: 15 months] 3. Locally developed tool used to measure functional impairment and improvement over time a. Child‐Guardian Relationship [Time Frame: 15 months] b. Measured using the Child‐Parent Relationship Scale (CPRS) |
| Notes | Registered at ClinicalTrials.gov as NCT01822366 Funded by National Institute of Mental Health |
Gureje 2020a.
| Methods | Cluster‐RCT |
| Participants | Each cluster consists of a primary care clinic and its neighbouring complementary alternative health provider (CAP) facilities Participants were patients with psychosis in Nigeria Inclusion criteria 1. Aged 18 years or over and speaking the study language of Yoruba (Nigeria) or Twi (Ghana) 2. An inpatient at participating CAP facilities in selected clusters with a confirmed diagnosis of non‐organic psychosis as assessed by research interviewers using the Structured Clinical Interview for Diagnostic and Statistical Manual version IV (SCID) 3. Symptomatic at the time of recruitment as indicated by a minimum score of 60 on the total Positive and Negative Symptoms Scale (PANSS) Exclusion criteria 1. Serious physical illness and in need of urgent medical attention as determined by research assistants (RAs), a PHCP, or the independent social worker during the process of assessing for capacity to consent 2. Serious cognitive impairment that may interfere with research assessments, such as a prior history of intellectual or learning disability as assessed clinically by an independent social worker 3. Persons who would not be in the study area for at least 6 months following recruitment 4. Women who are pregnant or attempting to become pregnant during the study period. Pregnancy testing will be conducted on all eligible female potential trial patients by appropriately trained research staff 5. Eligible female participants who decline to have pregnancy test will be excluded |
| Interventions |
Collaborative shared care Conventional primary healthcare providers (PHCPs) are trained to collaborate with and support traditional and faith healers (complementary alternative providers ‐ CAPs) in the care of patients with psychosis. PHCPs are purposively trained to deliver evidence‐based treatment for psychosis and to conduct scheduled and on‐request visits to facilities of the CAPs to collaborate in the treatment of patients with psychosis through joint decision‐making and clinical management. Overall care of patients remains the responsibility of the healers. The role of the PHCP is to support healers in delivering safe and acceptable care to patients, including promotion of and respect for human rights and avoidance of harmful practices in the care of patients Control Complementary alternative providers deliver intervention for patients with psychosis without active or formal collaboration with conventional primary healthcare providers. Primary healthcare providers in this arm nevertheless receive training on evidence‐based treatment of psychosis |
| Outcomes |
Primary 1. Symptom severity on Positive and Negative Symptoms Scale (PANSS) Secondary 1. Disability (WHODAS) 2. Experience of harmful treatment practices and human rights abuse (e.g. beating, shackling, scarification, harm from herbs, sexual abuse) 3. Experience of victimisation by caregivers 4. Experience of stigma (Internalised stigma of mental illness, ISMI, scale) 5. Family Burden interview Schedule (FBIS) 6. Service utilisation questionnaire |
| Notes |
Hartmann 2020.
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria (couples)
1. Female partner age 18 to 40 years 2. Married 3. Both partners speak Kannada 4. Female partner has ever experienced psychological, physical, or sexual violence perpetrated by her male partner 5. Consent from both partners Exclusion criteria 1. Male partner severely alcohol dependent or at risk of severe withdrawal symptoms 2. Female partner reporting ≥ 6 occurrences of severe physical or sexual violence in past 6 months 3. One or both members fearing the intervention may increase violence |
| Interventions |
Intervention arm 1 Incentives and behavioural couples therapy (BCT): same as incentives arm, with addition of 4 weekly 1‐hour BCT sessions on topics such as alcohol use and communication. Visual materials and assignments given. Sessions delivered by lay counsellors who had prior social work experience and had been trained on BCT facilitation and were supervised by a senior clinical psychologist Intervention arm 2 1. Incentives only 2. Every other day breathalyzer test for 4 weeks 3. Monetary incentive for each negative BrAC score as well as participation fee 4. Financial goal‐setting exercise Control 1. Every other day breathalyzer test for 4 weeks 2. Participation fee given |
| Outcomes |
Primary outcome Proportion of negative BrAC (alcohol use assessed by breathalyzer) tests (<0.01 g/dL) Secondary outcomes 1. Proportion of participants sober per day 2. Longest duration of abstinence 3. Women’s report of partner’s alcohol use 4. Indian Family Violence and Control Scale (IFVCS), with sexual violence domain omitted |
| Notes |
Jordans 2020.
| Methods | Randomised controlled trial |
| Participants | Unit of randomisation: 24 health facilities in Chitwan, Nepal All health facilities participating in the Program for Improving Mental Health Care (PRIME) scale‐up phase were eligible, excluding health facilities in the same village and urban centres with overlapping catchment areas |
| Interventions |
Intervention Use of Community Informant Detection Tool (CIDT): community health volunteers used the CIDT, a proactive case‐finding tool to promote mental health help‐seeking; to identify people with potential depression, psychosis, alcohol use disorder, and epilepsy; and when such a problem was identified, to encourage the person to seek help at the health facility Control Standard practices: female community health volunteers in both arms were trained in awareness‐raising activities over 2 days, and those in the CIDT arm received additional training on making proactive community referrals and on potential risks of this approach. Bi‐monthly supervision was provided to all community health volunteers |
| Outcomes | 1. Number of patients with depression, psychosis, alcohol use disorder, and epilepsy recorded at each health facility 2. Number of patients for whom treatment was recorded at each health facility relative to the facility’s population catchment 3. Number of patients for whom treatment was recorded at each health facility relative to number of trained community health volunteers per facility |
| Notes |
Kamal 2020.
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria 1. Students 18 to 22 years 2. AUDIT score 8 to 19 Exclusion criteria 1. Intoxicated 2. Severe withdrawal symptoms from alcohol/drugs 3. AUDIT score < 8 or ≥ 20 |
| Interventions |
Intervention Brief intervention: feedback on AUDIT score and single 15‐ to 20‐minute brief intervention session using motivational interviewing techniques including goal‐setting, given by nurse who had completed online training on Alcohol Brief Interventions from NHS, UK, and had undergone induction and experiential training by a psychiatrist. Education material was given. Psychiatrist provided supervision monitoring and feedback throughout study Control 1. General advice 2. Feedback on AUDIT score given 3. Uniform general advice given |
| Outcomes |
Primary outcome 1. Reduction in mean AUDIT score after 90 days Secondary outcome 1. Change in frequency of binge‐drinking after 90 days |
| Notes |
Markkula 2019.
| Methods | Randomised controlled trial |
| Participants | Adults visiting Sisaniya and Gadawa health posts were invited to participate Inclusion criteria 1. Age 16 years or older 2. Scoring ≥ 6 on the General Health Questionnaire (GHQ‐12) 3. Fluent in Nepali 4. Residence in Dang for the next 10 months Exclusion criteria 1. Severe illness or condition requiring urgent attention 2. Psychotic symptoms 3. Suicidality |
| Interventions |
Intervention Psychosocial counselling Participants received five 45‐minute sessions over 4 weeks, which focused on problem‐solving, emotional support, and coping strategies and skills. Sessions were delivered by lay workers who had minimum 12 years of education and minimum 3 years of experience in counselling, who were trained for 6 months in psychosocial counselling. Training programme acknowledged and encouraged traditional practices and local explanatory models and idioms of distress. Supervision was provided in‐person and by telephone 1 to 3 times a week as needed and every 2 months. Telephone consultation with senior counsellors, project coordinator, a psychologist, or a medical doctor was available as needed Control Enhanced usual care Primary health workers who had received a 5‐day training and a 3‐day refresher training gave usual care to participants attending both health posts. Psychotropic medication was supposedly available according to government guidelines, although in practice the supply was inconsistent |
| Outcomes |
Primary outcome 1. Response to treatment: reduction of minimum 50% in Beck Depression Inventory 21‐item version (BDI) score from T0 (beginning of intervention) to T2 (after 6 months) Secondary outcomes 1. Mean reduction in BDI 2. Mean reduction in Beck Anxiety Inventory (BAI) 3. Mean reduction in WHO Disability Assessment Schedule (WHODAS) |
| Notes | Registered in clinicaltrials.gov as NCT03544450 |
Maselko 2020.
| Methods | Cluster‐randomised controlled trial |
| Participants | Conducted in rural Pakistan in the rural sub‐district of Kallar Syedan, Rawalpindi, Pakistan THPP+ is conducted in the same 40 village clusters as the THPP trial. The same study population of perinatally depressed women is invited to consent to participate in THPP+. An equal number of perinatally non‐depressed women were also recruited from each village cluster Inclusion criteria 1. Female 2. 18 years and older 3. Currently enrolled in THPP trial 4. Married 5. Residing in study area for the long term Exclusion criteria 1. Requiring immediate medical attention 2. Development of psychotic or manic episode 3. Broken mother‐child dyad |
| Interventions |
Intervention THPP+ As part of THPP+, the intervention will continue from 6 months postnatal through 36 months postnatal and so will consist of an additional 30 months of lower‐intensity services that are unique to THPP+. THPP+ will include additional group sessions to be held every other month for a total of 18 over the intervention duration. Content will be a continuation of previous THPP sessions with continuing emphasis on self‐care as well as the baby's health and development. The perinatally non‐depressed women in the intervention arm do not receive the THPP+ intervention Control Enhanced usual care Women in control clusters who were depressed prenatally have been receiving enhanced usual care (EUC). At the time of screening, women, their Lady Health Workers, and their local primary health care facility were informed of the diagnosis, and women were given an information sheet about depression and how to access care. No new EUC protocols were put in place postpartum as part of the THPP+ |
| Outcomes |
Primary outcome measures 1. Depression (PHQ‐9 instrument) [Time Frame: 36 months postpartum] 2. Infant Socioemotional Development (Strengths and Difficulties Questionnaire) [Time Frame: 36 months] Secondary outcome measures 1. Disability (WHO Disability Assessment Schedule) [Time Frame: 36 months postpartum] 2. Infant Cognitive Development (Bayley Scales of Infant Development) [Time Frame: 36 months] 3. Depression (SCID Major Depressive Episode) [Time Frame: 36 months postpartum] 4. Structured Clinical Interview for DSM‐IV Diagnosis/Major Depressive Episode Other outcome measures 1. Infant Physical Development [Z‐Scores (based on WHO criteria)] [Time Frame: 36 months] 2. Weight‐for‐age and height‐for‐age Z‐scores (based on WHO criteria) |
| Notes |
Michelson 2020.
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria 1. Student age 12 to 20 years (grades 9 through 12) 2. SDQ Total Difficulties Scale ≥19 for boys and ≥ 20 for girls 3. SDQ Impact Scale ≥ 2 4. Written assent (age < 18 years) 5. Written consent (age ≥ 18; parent or guardian) Exclusion criteria 1. Needing urgent medical attention 2. Receiving another mental health intervention 3. Previously taken part in PRemIum for aDolEscents research programme (PRIDE) feasibility studies 4. Receptive or expressive difficulties |
| Interventions |
Intervention Brief counsellor‐led problem‐solving intervention with supporting printed materials: participants received 4 to 5 face‐to‐face 30‐minute problem‐solving sessions over 2 to 3 weeks on school premises in private rooms or behind screens in relatively quiet areas. Principles of therapy included problem identification (“P”), option generation (“O”), and implementation (“Do it”). Accompanying booklets and assignments were given. Sessions were delivered by lay counsellors ‐ Hindi‐speaking college graduates with no previous formal mental health training who had gone through a 5‐day training session and a 6‐week period of supervised practice, and 3 additional monthly training sessions during the trial. They were supervised weekly by a counsellor, a psychologist, and a supervisor Control Problem‐solving via printed booklets alone: participants received problem‐solving booklets and were encouraged to read through and complete the assignments. No additional counsellor input was given |
| Outcomes |
Primary outcomes (adolescent‐reported) 1. Severity of mental health symptoms: SDQ total difficulties score at 6 weeks 2. Severity of self‐defined psychosocial problems: Youth Top Problems score at 6 weeks Secondary outcomes (adolescent‐reported) 1. SDQ total difficulties score at 12 weeks 2. YTP score at 12 weeks 3. Distress and functional impairment (SDQ Impact score) at 12 weeks 4. Peer relationship problems and emotional symptoms (SDQ Internalising symptoms subscale score) at 12 weeks 5. Conduct problems and hyperactivity and inattention (SDQ Externalising symptoms subscale score) at 12 weeks 6. Perceived stress (Perceived Stress Scale ‐ 4‐item version) 7. Well‐being (Short Warwick‐Edinburgh Mental Well‐being Scale) at 12 weeks 8. Remission (defined as becoming SDQ < 19 for boys and < 20 for girls and becoming SDQ Impact score < 2) at 12 weeks Exploratory outcomes 1. Caregiver‐reported SDQ scores (total difficulties, impact, internalising sub‐scale, externalising sub‐scale) 2. Adolescent‐reported SDQ prosocial sub‐scale score |
| Notes | Registered under ClinicalTrials.gov as NCT03630471 Funding by Wellcome Trust |
Nakimuli‐Mpungu 2020.
| Methods | Cluster‐randomised controlled trial |
| Participants | Clusters were 30 primary care health centres (PHCs) situated in 3 districts in northern Uganda (Gulu, Kitgum, and Pader) Inclusion criteria 1. HIV positive 2. Aged 19 to 80 years 3. Met Mini International Neuropsychiatric Interview (MINI) criteria for major depression 4. Live or work in villages served by participating lay health worker 5. Antidepressant naive Exclusion criteria 1. High suicide risk 2. Severe medical disorder such as pneumonia or active tuberculosis 3. Psychotic symptoms 4. Hearing or visual impairment |
| Interventions |
Intervention Group support psychotherapy. 8 sessions conducted weekly, each lasting 2 to 3 hours. A culturally sensitive intervention that aims to treat depression by enhancing social support and teaching coping skills and income‐generating skills. Group sessions were led by lay health workers who had undergone a 5‐day training course Control Group HIV education. 8 sessions conducted weekly, each lasting 2 hours. Consists of health education talks on pertinent HIV topics. Group sessions were led by lay health workers who had undergone a 2‐day training course |
| Outcomes |
Primary outcomes 1. Difference in follow‐up proportions of participants with Mini International Neuropsychiatric Interview criteria for major depression 2. Difference in follow‐up function scores of participants in intervention and control arms 6 months after end of treatment Secondary outcomes 1. Emotional outcomes: depression; PTSD symptoms; self‐esteem 2. Social outcomes: alcohol use and perceived social support; coping skills; HIV‐related stigma; enacted stigma (discrimination) 3. HIV treatment outcomes: adherence to antiretroviral therapy (ART); viral load 4. Economic outcomes: poverty index scores; disability days 5. Cost‐effectiveness |
| Notes |
Pan 2019.
| Methods | Randomised controlled trial |
| Participants | Family caregivers of patients with dementia (PWD) recruited from community health centres and an 880‐bed tertiary hospital (discharge lists of neurological units) in a city in southeastern China Inclusion criteria 1. 20 years of age or older 2. Serving as primary caregiver for a family member diagnosed with Alzheimer's disease or vascular dementia or with a Mini Mental Status Examination (MMSE) score < 17 3. Living in the target city or its metropolitan area 4. Depressive symptom score ≥ 10 but < 20 5. Able to understand and speak Mandarin Exclusion criteria Individuals who showed signs of suicidal tendencies, history or evidence of a psychotic disorder, or intellectual deficits, or were being treated at a mental health clinic |
| Interventions |
Intervention Nurses were trained in the intervention protocol and in relevant psychosocial skills and were instructed to follow the protocol. A Wechat group (Tencent, Shenzhen, China) allowed nurse interventionists to discuss procedural issues to maintain intervention consistency. The cognitive‐behavioural intervention consisted of 5 monthly 60‐minute, face‐to‐face, individual sessions with a 20‐ to 30‐minute telephone consultation after each session. Face‐to‐face sessions were conducted by nurse interventionists early in the month. Details were flexible and were tailored to the culture of family caregivers of PWD. Telephone consultations were conducted to obtain participant feedback, to reinforce strategies, and to answer questions Control Participants in the control group received 5 monthly, short, general conversations from nurses early in the month at participants' homes, in the hospital units during medical visits, or via telephone contact. The conversation was a 5‐ to 10‐minute casual chat about daily life and health. Nurses responded naturally by showing concern but with no in‐depth instructions. Participants did not receive any additional interventions, with the possible exception of regularly scheduled medical visits |
| Outcomes | 1. Caregiver depression: CES‐D 10 2. Caregiver coping (20‐item simplified coping scale) 3. PWD’s Activities of Daily Living Scale (14‐item) 4. PWD’s Cognitive Function Scale (MMSE) |
| Notes |
Rodriguez 2019.
| Methods | Randomised controlled trial |
| Participants | 54 students from 36 universities across China reporting at least mild stress, anxiety, and/or depression |
| Interventions |
Intervention Brief (4‐week) online mindfulness intervention (MIND) plus non‐specialist provider (NSP; e.g. nurses, clergy, community members) support (MIND+) NSPs received 1 day of training, were instructed to provide 6 brief (15‐ to 20‐minute) weekly meetings, with the intention of supporting and encouraging participants in their completion of the online intervention, and received weekly online group supervision Control Brief (4‐week) online mindfulness intervention (MIND) |
| Outcomes | 1. Daily monitoring of mindfulness practice and mood 2. Baseline and post‐treatment self‐report packet assessing depression, anxiety, and stress symptoms and trait mindfulness |
| Notes |
Soares 2020.
| Methods | Randomised controlled trial |
| Participants | Setting: basic health unit (BHU) in Sao Paolo, Brazil Inclusion criteria 1. Over 18 years old 2. Able to attend intervention at scheduled times 3. Scored in Zone II (8 to 15 points) or III (16 to 19 points) on AUDIT 4. Could read and write Exclusion criteria 1. Intoxicated 2. Visible behavioural changes 3. Scored in Zone I (0 to 7 points) or IV (≥ 20 points) on AUDIT 4. Not available for follow‐up |
| Interventions |
Intervention Research team: 4 nurses from the Center of Studies and Researchers in Nursing in Addictions – alcohol and other drugs ‐ who were trained in screening and group brief intervention Intervention: Group Brief Intervention (GBI) Participants received feedback on their AUDIT score, an educational leaflet on problems related to alcohol use, and an invitation to participate in 4 weekly GBI sessions. GBIs, based on the combination of brief individual intervention and guided self‐help technique and aimed at behavioural change and reduction of alcohol use, were conducted in a room provided by the BHU and lasted 60 to 120 minutes per session. On the fourth session, participants were invited to attend the follow‐up evaluation Control Feedback and educational leaflet only. Participants received feedback on their AUDIT score and an educational leaflet on problems related to alcohol use and an invitation for 2 phone evaluations |
| Outcomes | 1. Alcohol Use Disorders Identification Test (AUDIT) 2. Ruler for Readiness for Change |
| Notes | Funding by CAPES – Coordenação de Aperfeicoamento de Pessoal de Nível Superior (Coordination for the Improvement of Higher Education Personnel) |
Ward 2020.
| Methods | Randomised controlled trial |
| Participants | Study was performed in Cape Town, South Africa Child‐caregiver dyads; screened through door‐to‐door visits or by referral Adults’ inclusion criteria 1. Older than age 18 2. Primary caregiver of child age 2 to 9 years 3. Resides with child > 4 nights a week 4. Reports > 15 problem behaviours on the ECBI problem scale |
| Interventions |
Intervention Parenting programme. Facilitators ‐ paraprofessional community members with high school level education trained for this study visited each family to explore goals, focused on positive relationship‐building, taught limit‐setting and non‐violent discipline strategies, and practised with role‐play, feedback, and problem‐solving. A total of 12 three‐hour sessions were held Control Services as usual |
| Outcomes |
Primary outcomes 1. Positive parenting: setting limits and supporting positive behaviour sub‐scales of the Parenting Young Children Scale 2. Non‐violent psychological and physical punishment: ICAST‐Parent Report 3. Child behaviour: ECBI intensity and problem scales 4. Dyadic parent‐child interaction of video‐recording of structured observational task Secondary outcomes 1. Parenting stress: Parenting Stress Index 2. Depression: Beck Depression Inventory 3. Exposure to IPV: Revised Conflict Tactics Scale 4. Monitoring and supervision: Alabama Parenting Scale 5. Social support: Medical Outcomes Study Social Support Survey 6. Substance misuse |
| Notes |
GP: general practitioner; ITS: interrupted time series; NSHW: non‐specialist health worker; RCT: randomised controlled trial.
Characteristics of ongoing studies [ordered by study ID]
Akhtar 2020.
| Study name | Group problem management plus (gPM+) in the treatment of common mental disorders in Syrian refugees in a Jordanian camp: study protocol for a randomized controlled trial |
| Methods | Randomised controlled trial |
| Participants | This study is to be carried out in Azraq Refugee Camp in Jordan Inclusion criteria 1. Syrian refugees 2. Psychological distress (K10 scores > 15) 3. Impaired functioning (WHODAS > 16) 4. Has a child age 10 to 16 years 5. Age 18 to 50 years Exclusion criteria 1. At risk of self‐harm or harming others 2. Plan to return to Syria in the next 6 months |
| Interventions |
Intervention Group Problem Management Plus Group‐administered once‐weekly 90‐minute sessions over 5 weeks. Include psychoeducation, problem‐solving, arousal management, behavioural activation, and instruction in social support. Therapy is provided by local health workers Control Enhanced treatment as usual Referral to local psychosocial services; basic education about common psychological problems |
| Outcomes |
Primary outcomes 1.Anxiety (Hopkins Symptom Checklist) 2. Depression (Hopkins Symptom Checklist) Secondary outcomes 1. Post‐traumatic stress disorder (Post‐traumatic Stress Checklist) 2. Prolonged grief (Prolonged Grief‐13, adapted) 3. Functional impairment (WHODAS) 4. Personally identified problems (PSYCHLOPS) 5. Prodromal psychotic symptoms (Brief Prodromal Questionnaire) 6. Children’s mental health (Pediatric Symptom Checklist) 7. Parenting skills (Alabama Parenting Questionnaire) |
| Starting date | 14 October 2019 |
| Contact information | r.bryant@unsw.edu.au |
| Notes | Anticipated last data collection: 15 April 2021 |
Asher 2016.
| Study name | RISE (Rehabilitation Intervention for People With Schizophrenia in Ethiopia) |
| Methods | Cluster‐randomised controlled trial |
| Participants | People with schizophrenia receiving treatment in primary care Inclusion criteria 1. Age ≥ 18 years 2. Diagnosis of schizophrenia spectrum disorder (schizophrenia, schizoaffective disorder, or schizophreniform disorder) using DSM‐IV criteria 3. Evidence of severe, enduring, or disabling illness 5. Resident in kebele for > 6 months and no immediate plans to leave the kebele 6. Has a primary caregiver who is willing to participate in the study Exclusion criteria 1. No specific criteria |
| Interventions | Facility‐based care plus community rehabilitation vs facility care alone |
| Outcomes |
Primary outcome Disability as measured by the WHODAS 2.0 score Secondary outcomes 1. Change in symptom severity 2. Economic activity 3. Physical restraint 4. Discrimination 5. Caregiver burden |
| Starting date | September 2015 |
| Contact information |
Principal Investigator Mary De Silva, PhD, MSc London School of Hygiene and Tropical Medicine Principal Investigator Abebaw Fekadu Addis Ababa University, Department of Psychiatry |
| Notes | ClinicalTrials.gov Identifier: NCT02160249 |
Brown 2019.
| Study name | STRENGTHS‐consortium. Early Adolescent Skills for Emotions (EASE) intervention for the treatment of psychological distress in adolescents: study protocol for randomised controlled trials in Lebanon and Jordan |
| Methods | 2 separate randomised controlled trials: 1 in Jordan, 1 in Lebanon |
| Participants | Participants will be identified through door‐to‐door screening Inclusion criteria 1. Age between 10 and 14 years 2. Reside with caregiver who is able to provide consent 3. Able and willing to attend weekly EASE sessions 4. Screens positive for psychological distress during screening: a. Jordan: score > 15 on the Pediatric Symptom Checklist‐17 (PSC‐17) b. Lebanon: score > 12 on the PSC‐17 (1) Syrian refugee (Jordan only) Exclusion criteria 1. Unaccompanied minor 2. Caregiver not a family member 3. Significant cognitive or neurological impairment or developmental difficulties 4. Imminent risk of suicide 5. Married 6. Child protection concerns 7.Sibling in study (Jordan only) |
| Interventions |
Intervention EASE A group psychological intervention consisting of seven 90‐minute sessions for adolescents and three 90‐minute sessions for their caregivers. Adolescent sessions involve psychoeducation, problem‐solving, stress management, behavioural activation, and relapse prevention. Caregiver sessions involve psychoeducation, active listening, quality time, praise, self‐care, and relapse prevention. Interventions are conducted by trained facilitators who have undergone 8 days of training and will receive regular supervision Control Enhanced treatment as usual Provision of a single‐session psychoeducation home visit of approximately 30 minutes in which feedback on youth had indicated psychological distress; advice on self‐care strategies and seeking services from local mental health and psychological support services is given. Control facilitators would have undergone 3 days of training and will also be supervised |
| Outcomes |
Primary outcome 1. Youth‐reported psychological distress (PSC‐35) Secondary outcomes 1. Youth‐reported depression symptoms (PHQ‐9) 2. Youth‐reported traumatic stress symptoms (CRIES‐13) 3. Youth‐reported functional impairment (custom Impairment of Daily Functioning Questionnaire) 4. Youth‐reported well‐being (Warwick Edinburgh Mental Well‐being Scale) 5. (Jordan only) Youth‐reported Psychological Sense of School Membership 6. (Lebanon only) Youth‐reported child Strategy Use Questionnaire (SUQ) 7. Caregiver‐reported PSC‐35 in children 8. Caregiver psychological distress (Kessler Psychological Distress Scale) (K6) 9. Caregiver‐reported Alabama Parenting Questionnaire (APQ‐42) 10. (Lebanon only) Caregiver‐reported SUQ Other outcomes Traumatic event checklist |
| Starting date | 1 March 2019 |
| Contact information | mark.jordans@warchild.nl |
| Notes | Registered as ISRCTN75375136 (Lebanon) and ACTRN12619000341123 (Jordan) |
Chen 2018.
| Study name | Protocol of an ongoing randomized controlled trial of care management for comorbid depression and hypertension: the Chinese Older Adult Collaborations in Health (COACH) study |
| Methods | Cluster‐RCT |
| Participants | Older adult Chinese primary care patients with depression and hypertension in Zhejiang Province |
| Interventions |
Intervention COACH intervention for 12 months The COACH intervention applies chronic disease management principles to treatment of both depression and HTN by a team consisting of the village primary care provider (PCP), an Aging Worker (AW), and a Psychiatrist. PCPs in village clinics have on average 3 years of medical education after completing high school. They receive systematic training in the diagnosis and treatment of common chronic medical illnesses; however, they receive little training in the diagnosis or treatment of mental disorders. An AW typically has a middle‐school education, knows the residents well, and is well connected with the village leadership. Although the AWs have no special training in social work, they receive in‐service training from the Bureau of Civil Affairs and have experience in addressing the villagers’ social needs. Their responsibilities for the COACH intervention include baseline and ongoing assessment of social stressors and supports, fostering social connectedness, behavioural activation, adherence support, facilitating communications with the PCP, and education of the patient, family, and community about depression and HTN and their management. Psychiatrists have on average 5 years of medical training after high school followed by 2 years of specialty training. Their scope of practice includes psychiatric diagnosis and management of patients of all ages with mental illness from across the county. Their role in COACH is to provide baseline diagnosis and initial prescription of depression treatment, and to provide ongoing consultation to the team in its algorithm‐driven management of the patient’s illness Control Enhanced care as usual for 12 months PCPs are told when study participants screen positive for depression and are provided with copies of depression practice guidelines developed for the study |
| Outcomes |
Primary outcomes 1. Depression symptom severity measured by the Hamilton Depression Rating Scale 2. Hypertension control 3. Adherence to antidepressant and antihypertensive medication recommendations Secondary outcomes 1. QOL measured by WHOQOL‐BREF 2. Costs |
| Starting date | January 2014 |
| Contact information |
Principal Investigator Yeates Conwell, MD University of Rochester Medical Center University of Rochester Rochester, New York, United States 14642 Principal Investigator Shulin Chen, MD, PhD Department of Psychology Zhejiang University Hangzhou, Zhejiang, China 310058 |
| Notes | Completed 2 January 2019 |
Chinoda 2020.
| Study name | Effectiveness of a peer‐led adolescent mental health intervention on HIV virological suppression and mental health in Zimbabwe: protocol of a cluster‐randomised trial |
| Methods | Cluster‐randomised clinical trial |
| Participants | Health centres (“clinics”) are randomised into intervention and control arms Inclusion criteria 1. Adolescents living with HIV age 10 to 19 eligible for ART 2. SSQ‐14 score ≥ 7 3. Able to provide consent (ages 18 and 19) 4. Ablel to provide assent (ages 10 to 17) and caregiver provides consent Exclusion criteria 1. Unable to comprehend study in English, Shona, or Ndebele 2. Currently in psychiatric care 3. End‐stage AIDS 4. Current psychosis, intoxication, and/or dementia |
| Interventions |
Intervention CATS‐PST Participants receive 4 to 6 PST sessions conducted by Community Adolescent Treatment Supporters (CATS) ‐ trained, mentored peer supporters ages 18 to 24 ‐ where CATS help participants understand and share their experiences and feelings, work with participants on 1 problem of their choice and develop an action plan, and invite participants to join a 6‐week‐long support group programme. Supervision is provided weekly by nurses and monthly by a mental health specialist Control Zvandiri standard care Participants receive ART, adherence support and counselling from clinic‐based nurses and primary counsellors, counselling and home‐based support from CATS, monthly support groups, weekly text messages, and weekly home visits |
| Outcomes |
Primary outcome 1. Proportion of adolescents with viral failure (viral load > 100 copies/mL) or death at 48 weeks Secondary outcomes 1. Proportion of adolescents with CMD (SSQ‐14 ≥ 8) 2. Mean SSQ‐14 scores 3. Mean PHQ‐9 scores 4. Proportion of adolescents with depressive symptoms (PHQ‐9 ≥ 11) 5. Mean total score for health‐related quality of life (EQ‐5D) |
| Starting date | January 2018 |
| Contact information | silindi.chinoda@friendshipbench.io |
| Notes |
Clair 2016.
| Study name | The Computer‐based Drug and Alcohol Training Assessment in Kenya (eDATA K) |
| Methods | Randomised controlled trial |
| Participants | Adults with alcohol use disorder Inclusion criteria 1. 18 years of age or older 2. ASSIST alcohol score of 11 to 26 Exclusion criteria 1. Being pregnant 2. Reaching a score ≥ 27 in ≥ 1 substance (other than tobacco or cannabis) 3. Attended an alcohol treatment programme in the last year 4. Reporting symptoms of suicide 5. Severe neurological or psychiatric impairment (such as overt psychosis) |
| Interventions | 1. Brief intervention vs screening only in both private and public clinics 2. Brief intervention is administered by clinicians trained on the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST)‐linked brief intervention (BI) through the NextGenU.org model of training 3. Investigators hypothesise that the NextGenU.org model of online training with mentor and peer activities is an effective way to train clinicians to deliver the ASSIST‐linked brief intervention |
| Outcomes |
Primary outcome measure 1. Mean difference in alcohol consumption a. Difference in alcohol consumption between intervention and control (average grams/week in previous 7 and 14 days' use collected through a timeline follow‐back assessment) Secondary outcome measures 1. Change from baseline in frequency of drinking ‐ mean change in number of days per week of drinking between various time frames, in control and intervention participant groups 2. Change from baseline in alcohol consumption ‐ mean change in grams of alcohol consumed per week between various time frames, in control and intervention participant groups 3. Change from baseline in frequency of binge‐drinking ‐ mean change in number of days of drinking more than 6 drinks for men, and more than 4 drinks for women, between various time frames, in control and intervention participant groups 4. Change from baseline in stigma related to alcohol use ‐ change over time in control and intervention groups in stigma score 5. Difference between groups in stigma related to alcohol use ‐ difference at each time point in control and intervention groups in stigma score 6. Depression and suicidality ‐ change (over time) and difference (between groups at any given time) in Beck Depression score and in Beck Suicidality score 7. Perceived Quality of Life WHO Quality of Life‐BREF (WHOQOL‐BREF) ‐ change over time (inside group) and differences over time (between groups) in the WHOQOL‐BREF. The WHOQOL‐BREF is a shorter version of the original instrument that is convenient for use in large research studies or clinical trials Other outcome measures Treatment outcomes ‐ to assess changes over time in 1 group and between groups at various time points of other potential treatment effects such as health services utilisation; housing; involvement in criminal activities; aggressive behaviours (perpetration or victim of); employment; other substance use (amount per day average in last 4 weeks); self‐reported health status (using analogue scale) for psychological health, physical health, and overall quality of life; satisfaction with life; self‐reported general health status; self‐reported general mental health; self‐reported daily activity stress; sense of belonging to community; number of sexual partners (last month and lifetime); sexually transmitted disease dx in the past |
| Starting date | September 2014 |
| Contact information |
Principal Investigator David M Ndetei, PhD Africa Mental Health Foundation Principal Investigator Erica Frank, MD, MPH University of British Columbia, NextGenU.org Principal Investigator Victoria N Mutiso, PhD Africa Mental Health Foundation Principal Investigator Veronic Clair, MD University of British Columbia |
| Notes | ClinicalTrials.gov Identifier: NCT02388243 Recruitment completed December 2015 |
Fairall 2018.
| Study name | Collaborative care for the detection and management of depression among adults receiving antiretroviral therapy in South Africa |
| Methods | Cluster‐randomised controlled trial |
| Participants | Clusters: 40 nurse‐led primary care clinics providing ART in the Dr Kenneth Kaunda and Bojonala districts of the North West Province, South Africa Inclusion criteria 1. Age ≥ 18 years 2. Receiving ART at time of enrolment 3. Depressive symptoms, as indicated by total score ≥ 9 or more on the PHQ‐9 4. Planning to reside in the area for the next year 5. Capable of actively engaging in an interviewer‐administered questionnaire at the time of recruitment and 3, 6, and 12 months later 6. Written consent to participate in the study Exclusion criteria 1. Clinics a. Clinics that participate in formative research and piloting of the intervention 2. Patients a. Inability to meet the above inclusion criteria |
| Interventions |
Intervention Primary Care 101 Plus Supplementary training in PC101+ focusing on (1) increasing nurse capacity to detect and manage non‐communicable diseases, specifically, depression, cardiovascular disease, diabetes, and alcohol misuse among patients receiving lifelong ART; and (2) providing a group counselling intervention for depression for these participants Control Primary Care 101 Provision of the standard version of the PC101 guideline, excluding mental health and standard training |
| Outcomes |
Primary outcome 1. PHQ‐9 response defined as ≥ 50% improvement in baseline score Secondary outcomes 1. PHQ‐9 including response at 12 months 2. PHQ‐9 mean scores at 6 and 12 months 3. Remission, defined as PHQ‐9 score ≤ 5 at 12 months 4. Category of depression severity based on PHQ‐9 (mild, moderate, severe) at 6 and 12 months 5. Treatment for depression, including initiation or intensification of antidepressant medication, referral to a counsellor for depression counselling, number of contacts with a counsellor, and referral to a mental health specialist (clinical psychologist, psychiatrist, or secondary care services) 6. Perceived Stress Scale 7.HIV viral suppression at 12 months defined as viral load < 400 copies/mL, virologic failure defined as 2 consecutive viral load values > 1000 copies/mL, change in viral load values over time 8. Antiretroviral therapy programme retention 9. Appropriate maintenance on enrolment ART regimen 10. ART regimen switched to second line 11. Internalised AIDS Related Stigma Scale 12. Adherence to ART medication (30‐day VAS self‐reported measure) 13. Risk factors for cardiovascular diseases (blood pressure, weight, smoking status) 14. Detection and treatment of other chronic disease 15. Mortality (mortality reported at loss to follow‐up or through the South African Population register) 16. Hospital admissions (number and duration of overnight hospital stays) 17. Care utilisation and resource use 18. Provision of integrated care from patient perspective will be assessed using the Patient Assessment of Care for Chronic Conditions (PACIC) |
| Starting date | 16/4/2015 |
| Contact information | lara.fairall@uct.ac.za |
| Notes | Also registered as NCT02407691 at ClinicalTrials.gov |
Fawzi 2020.
| Study name | Healthy Options: study protocol and baseline characteristics for a cluster‐randomised controlled trial of group psychotherapy for perinatal women living with HIV and depression in Tanzania |
| Methods | Cluster‐randomised controlled trial |
| Participants | Clusters were primary healthcare facilities with a reproductive and child health (RCH) clinic that provided PMTCT services and satellite facilities Inclusion criteria 1. Women age 18 years and older 2. Up to 30 weeks' gestation 3. HIV‐positive receiving ART 4. Symptoms consistent with major depressive disorder on the PHQ‐9, scoring ≥ 9 5. Planning to continue postpartum care at study facility Exclusion criteria 1. Plan to harm self, including suicide |
| Interventions |
Intervention Healthy Options intervention Includes components from problem‐solving therapy and cognitive‐behavioural therapy approaches. Six psychosocial group therapy sessions and 1 orientation session prior to delivery provided problem‐solving therapy. Following delivery, women who scored ≥ 9 on the PHQ‐9 are invited to participate in 8 cognitive‐behavioural group sessions. Psychosocial support group sessions are facilitated by lay community‐based healthcare workers experienced in providing HIV‐related peer counselling and support, who have been trained for 2 weeks. Group sessions are 2 to 3 hours long and are held in health facilities, schools, or religious sites Control Improved standard of care A 1‐day training session offered to clinical staff of the RCH clinics based on WHO mhGAP guidelines. Patients cared for in control group facilities are given access to counselling and antidepressant treatment when necessary, including immediate care if presenting with acute suicidal ideation |
| Outcomes |
Primary outcomes 1. PHQ‐9 score Secondary outcomes 1. Intimate partner violence ‐ DHS 2. Social support – Duke University‐UNC Functional Social Support Questionnaire 3. Self‐efficacy – General Self‐Efficacy Scale 4. Hope – local scale 5. HIV‐related stigma – HIV Stigma Scale 6. ART adherence 7. Food insecurity – custom composite measure |
| Starting date | May 2015 |
| Contact information | mksfawzi@msn.com |
| Notes | Registered under clinicaltrials.gov as NCT02039973 |
Gureje 2020b.
| Study name | Responding to the challenge of Adolescent Perinatal Depression (RAPiD): protocol for a cluster randomized hybrid trial of psychosocial intervention in primary maternal care |
| Methods | Cluster‐randomised trial |
| Participants | Unit of randomisation: primary maternal care clinics in Oyo State, Nigeria Inclusion criteria 1. Adolescents age < 20 years 2. Score ≥ 12 on the Edinburgh Postnatal Depression Scale 3. Fetal gestational age < 36 weeks 4. Consent (with parent/guardian consent if < 16 years old) Exclusion criteria 1. Immediate need for medical attention 2. Actively suicidal 3. Unlikely to be available for follow‐up |
| Interventions |
Intervention Psychosocial intervention Providers in the clinic are trained at a 3‐day workshop focused on the delivery of behaviour activation, problem‐solving treatment, and parenting skills training ‐ how to engage with and enlist the involvement of the “neighbourhood mother” by psychiatrists. A 1‐day refresher training was provided 3 months into the trial. Participants receive a manualised package of care that consists of behavioural activation and problem‐solving treatment delivered in 6 sessions by clinic providers, first weekly, then fortnightly, for those with EPDS score 12 to 17, or weekly for those with EPDS score > 17, with supplemental sessions if needed, parental skills training during problem‐solving sessions and through phone calls and text messages during the postnatal period, and social and parenting skills support provided by a “neighbourhood mother” identified by the participant Control Usual care Includes psychoeducation, reactivation of social network, and addressing current psychosocial stressors In both arms, providers in the clinics have been trained to use the mhGAP‐IG |
| Outcomes |
Primary outcomes at 6 months postnatal 1. Difference in EPDS score between groups 2. Level of parenting skills (HOME‐IT total and sub‐scale scores) Secondary outcomes (3 or 6 months) 1. Rate of remission of depression (EPDS < 6) 2. WHODAS score 3. Maternal Adjustment and Maternal Attitude Scale (MAMAS) 4. WHOQoL‐BREF score 5. Postnatal Bonding Questionnaire score 6. Perinatal Infant Care Social Support Scale 7. Infant weight, height, head circumference, nutrition, vaccinations, social, cognitive and physical developmental milestones (birth, 3 and 6 months of age) 8. Process evaluation |
| Starting date | 15 May 2018 |
| Contact information | Correspondence: ogureje@com.ui.edu.ng Trial registration: ISRCTN16775958. Registered on 30 April 2019 |
| Notes | Funded by the International Development Research Centre |
Hanlon 2016.
| Study name | Task sharing for the care of severe mental disorders in a low‐income country (TaSCS): study protocol for a randomised, controlled, non‐inferiority trial |
| Methods | Randomised controlled trial |
| Participants | Criteria Inclusion criteria for phase 1 1. 25 years of age and older 2. Participant in the ongoing Butajira, Ethiopia, SMD cohort study (at baseline (between 1998 and 2001), cohort participants were aged between 15 and 49 years, resident in the area for at least 6 months and with a DSM‐IV (SCAN) diagnosis of schizophrenia or schizoaffective disorder, bipolar disorder, or major depressive disorder 3. Ongoing need for continuing mental health care due to a. Being on psychotropic medication at assessment or b. Not on medication but symptomatic at the time of assessment or c. Have experienced partial or full relapse within the 2 years preceding the assessment 4. Stable clinical condition: either in remission from SMD or with residual symptoms that have been stable over the preceding 3 months 5. Planning to stay resident in the area for 18 months 6. Able to communicate in Amharic, the official language of Ethiopia 7. Willing to be randomised to either of the service models as described in the protocol 8. Has capacity to consent to participation or permission given by guardian and not refusing to participate 9. Resident in catchment area of TaSCS health centres (excluding Butajira health centre) Exclusion criteria for phase 1 1. Suicide attempt within preceding 3 months 2. Current active suicide intent 3. Prescribed thioridazine, valproate, lithium, or second‐generation antipsychotic medications (risperidone and olanzapine), as these medications are not available in psychiatric nurse‐led units or PHC settings in Ethiopia. Within the Butajira SMD cohort, only people who have received care from psychiatrist‐led units in the capital city, Addis Ababa, might be receiving these medications. At present, fewer than 10 patients are known to be taking one of these medications 4. Prescribed depot medication 5. Complex or unstable medical condition interfering with management of psychiatric disorder or requiring ongoing medical treatment from Butajira Hospital 6. Alcohol or khat dependence or abuse within the last 12 months 7. Pregnant or breast‐feeding 8. Restrained at home 9. Refusing to participate in the study Inclusion criteria for phase 2 As for phase 1, but if we are unable to recruit enough participants from the existing Butajira SMD cohort, then we will expand recruitment to people with SMD attending the psychiatric outpatient clinic at Butajira Hospital. A semi‐structured diagnostic interview will be carried out to determine diagnostic eligibility (DSM‐IV diagnosis of schizophrenia or schizoaffective disorder, bipolar disorder, or major depressive disorder). For participants recruited from Butajira Hospital psychiatric outpatient clinic, the minimum age will be 25 years and participants should have had their first contact with specialist mental health services at least 2 years prior to recruitment into the trial to ensure comparability with the Butajira SMD cohort sample Exclusion criteria for phase 2 1. Current active suicide intent 2. Prescribed thioridazine, valproate, lithium or second‐generation antipsychotic medications (risperidone and olanzapine) 3. Pregnant or breast‐feeding and prescribed depot 4. Refusing to participate in the study 5. Medical condition requiring ongoing medical treatment from Butajira Hospital |
| Interventions |
Intervention Integrated mental health in primary care A task‐shared model of collaborative mental health care integrated into the primary care setting. Participants in the intervention arm will receive a task‐sharing model of locally delivered mental health care integrated into primary health care. General health workers (health officers, nurses, and community‐based health extension workers) will be given brief training using the WHO mental health Gap Action Programme and ongoing supervision to deliver mental health care to people with severe mental disorders Control Psychiatric nurse‐led specialist care A centralised, psychiatric nurse‐led, hospital outpatient service with outreach from project outreach workers. Participants in the active control arm will receive an established model of centralised, specialist mental health care delivered by psychiatric nurses at an outpatient clinic within Butajira General Hospital and supported by outreach from project workers |
| Outcomes |
Primary outcome
Brief Psychiatric Rating Scale Secondary outcomes 1. WHODAS 2. Local functioning scale 3. Relapse of mental disorder (Life Chart Schedule) 4. Patient service satisfaction 5. Body mass index 6. Service utilisation (Client Service Receipt Inventory) 7. Medication side effects 8. Medication adherence 9. Stigma (Family Interview Schedule) 10. Restraint (proportion chained, restrained, or confined in the past month) 11. Adverse events 12. Quality of clinical care 13. Acceptance and feasibility |
| Starting date | March 2015 |
| Contact information | Charlotte Hanlon, BM BS, PhD, Addis Ababa University and King's College London |
| Notes | Completed 16 November 2017, but not published yet ClinicalTrials.gov Identifier: NCT02308956 |
Hoffmann 2016.
| Study name | Child psychopathology after treatment of maternal depression in primary care in Brazil |
| Methods | Randomised controlled trial |
| Participants | Mothers with children age 6 to 14 with depression (based on Hamilton Rating Scale for Depression ‐ HRSD) in a low‐income primary care setting |
| Interventions |
Intervention Interpersonal therapy counselling for 3 weeks Control Enhanced treatment as usual |
| Outcomes | 1. Psychiatric symptoms in children of depressed mothers (based on Child Behavior Checklist) 2. Change in maternal depressive symptoms (HRSD score) 3. Remission of maternal depression (HRSD ≤ 7) 4. Response of maternal depression (decrease ≥ 50% of baseline HRSD score) |
| Starting date | NA |
| Contact information | Elis Viviane Hoffmann, Department of Psychiatry, Federal University of Sao Paulo, Sao Paulo, Brazil |
| Notes | Completed, abstract published. |
ISRCTN16463016 2017.
| Study name | 'PROCUIDA‐Dementia' a feasibility Mexican study investigating an optimised person‐centred intervention to reduce antipsychotic medication and improve the quality of life of older people living in care homes. |
| Methods | Cluster‐randomised controlled trial |
| Participants | Clusters are care homes providing residential care to persons with dementia, their residents, staff, and residents’ relatives Care Home inclusion criteria 1. 20% of residents suspected with a diagnosis of neurodegenerative disease (DSM‐V criteria) of mild stages (Clinical Dementia Rating/CDR) 2. Care Homes that are not taking similar training on psychosocial interventions, or who are currently reviewing antipsychotic medication 3. Care Homes with ≥ 1 spacious and ventilated room designated for PROCUIDA‐Dementia Study activities 4. Care Homes with manager and proprietor willing to be involved in the programme and allow staff to take part in the training 5. Psychologist and GP to support residents in case of crisis 6. Health and safety protocols in place Residents inclusion criteria 1. Residents living permanently in the care home 2. Dementia diagnosis 3. SPPB screening measure, cutoff point 10 to prevent risk of falls 4. Informed consent to take part in the study 5. Participants must speak and understand Spanish; alternative English consent forms will be given if the participant is not Spanish‐native speaker 6. Preferably on current antipsychotic medication prescription Staff inclusion criteria 1. Consent to participate in the PROCUIDA‐Dementia study 2. Available for the 3‐day staff training delivered by PIs to ensure that interventions are being implemented in the care homes 3. Commitment to support the study whether or not allocated to intervention or control group for 24 weeks. Staff willing to facilitate musical and dancing sessions do not require professional dance/musical experience, but a rhythmic response to music is required Relatives inclusion criteria 1. Consent to participate in the PROCUIDA‐Dementia activities organised by the care home 2. Consent to participate in the Focus Groups targeting family members at the end of the study 3. Visit at least once a month, to witness the staff‐resident intervention activities 4. Read/Speak Spanish |
| Interventions |
Intervention 12‐Week staff training programme involving reviewing antipsychotic medication and enhancing its prescription to treat stress and distress in dementia, person‐centred care therapy, and psychosocial interventions for people with dementia Control Wait‐list control |
| Outcomes |
Primary outcome 1. Quality of life is measured by applying the Quality of Life‐Alzheimer’s Disease (QoL‐AD) questionnaire at baseline, 12 and 24 weeks 2. Cognition is measured by using Addenbrooke’s Cognitive Examination Revised (ACE‐R) at baseline, 12 and 24 weeks 3. Behaviour is measured by using the Neuropsychiatry Inventory‐Nursing Home (NPI‐NH) at baseline, 12 and 24 weeks 4. Burnout is measured using Maslach Burnout Inventory (MBI) at baseline, 12 and 24 weeks 5. Staff attitudes towards people with dementia are measured by applying the Approaches to Dementia Questionnaire (ADQ) at baseline, 12 and 24 weeks 6. Care staff’s sense of competence is measured by using the Sense of Competence in Dementia Care (SCIDS) questionnaire at baseline, 12 and 24 weeks Secondary outcome None |
| Starting date | 1 June 2016 |
| Contact information | saratorrescastro@gmail.com azucena.guzman@ed.ac.uk |
| Notes | Trial registered as ISRCTN16463016 |
ISRCTN57805470 2019.
| Study name | A collaborative care psychosocial intervention to improve late life depression in socioeconomically deprived areas of Guarulhos, Brazil: the PROACTIVE study |
| Methods | Cluster‐randomised trial |
| Participants | Clusters are 80 Family Health Teams (FHTs) across 20 Family Health Units (FHUs). An additional 16 FHTs over 4 FHUs will be recruited as reserve Inclusion criteria 1. 60 years or older 2. Registered with selected Family Health Teams (FHTs) 3. Score ≥ 10 on the PHQ‐9 Exclusion criteria 1. Partner or another person in the same household included in study 2. Acute suicidal risk 3. Completely deaf 4. Unable to communicate 5. Unable to engage in trial for the period of 12 months |
| Interventions |
Intervention Psychosocial intervention The FHTs' community health workers (CHWs) visit homes regularly and work collaboratively with other members from their FHT. Usual care is given. For the psychosocial intervention, the intensity of care management is tailored according to the needs of participants (Stepped Care). The intervention is based on measurement‐based (with the PHQ‐9) care, psychoeducation, behavioural activation, relapse prevention, and use of a tablet application. Intervention lasts for 17 weeks with an option to extend to 22 weeks. The initial 3 weeks include 3 weekly meetings. In the next phase, participants can have access to either low‐ or high‐intensity regimens, based on their PHQ‐9 score. CHWs receive training prior to delivering the intervention, in 3 group sessions of 6 hours each, and are supervised by a psychologist, first weekly, then fortnightly or less frequently, depending on need during the intervention Control Enhanced usual care Research group will send to the FHUs information about participants’ level of depression based on their PHQ‐9 scores. The research team will not interfere with usual care such as prescription of medications, CHW monthly home visits, or access to family doctors or specialists |
| Outcomes |
Primary outcome 1. Proportion of depression recovery (PHQ‐9 < 10) at 8 months' follow‐up Secondary outcomes 1. Proportion of depression recovery (PHQ‐9 < 10) at 12 months 2. European Quality of Life 5‐dimensional questionnaire (EQ‐5D‐5L) 3. ICEpop CAPability measure for Older people (ICECAP‐O) 4. Behavioural Activation for Depression Scale (BADS) 5. Stressful life events (SLEs) 6. General Anxiety Disorder‐7 (GAD‐7) 7. Lubben Social Network Scale‐6 (LSNS‐6) 8. Alcohol Use Disorders Identification Test‐Consumption (AUDIT‐C) 9. Loneliness Scale (3‐item UCLA) 10. Cost‐effectiveness of intervention at 12 months |
| Starting date | 6 May 2019 |
| Contact information | cpqfmusp@usp.br |
| Notes | Recruitment ended on 30 September 2020 Registered on the ISRCTN registry as ISRCTN57805470 |
ISRCTN62728852 2019.
| Study name | ImPROving TB outcomes by modifying LIFE‐style behaviours through a brief motivational intervention followed by short text messages |
| Methods | Prospective multi‐centre 2‐arm randomised controlled trial |
| Participants | Adult participants (aged ≥ 18 years) with drug‐sensitive pulmonary TB who are current smokers and/or report harmful or hazardous alcohol use, enrolled at 27 government TB clinics in 3 different health districts in South Africa Inclusion criteria 1. adult patients (aged ≥ 18 years) with drug‐sensitive (bacteriologically or clinically confirmed) PTB 2. Initiating TB treatment or on TB treatment for < 1 month (these include both ‘new’ and ‘retreatment’ patients) 3. Current smokers and/or hazardous/harmful drinkers who are not alcohol dependent (Alcohol Use Disorders Identification Test (AUDIT) score ≥ 8 for men or ≥ 7 for women but < 20) 4. Access to a functional mobile phone 5. Understands 1 of the 4 languages used for the trial (Sesotho, Setswana, Isizulu, or English) Exclusion criteria 1. Alcohol‐dependent participants (AUDIT score ≥ 20) 2. Extrapulmonary TB without PTB 3. Resistance to ≥ 1 TB drugs at baseline |
| Interventions |
Intervention Motivational interviewing counselling strategy delivered by lay health workers augmented with subsequent text messages (ProLife programme): participants will receive 3 MI counselling sessions 1 month apart. Each MI session will be followed by twice‐weekly SMS messages targeting treatment adherence, alcohol use, and tobacco smoking Control Usual TB treatment and support offered to TB patients |
| Outcomes |
Primary outcome TB treatment success Secondary outcomes 1. Sputum conversion 2. Smoking cessation (self‐report, exhaled CO) 3. Reduction in alcohol use (AUDIT) 4. TB medication and antiretroviral treatment adherence (assessed using the ACTG) 5. Proportion of HIV patients on ART 6. CES‐D 7. Economic evaluation |
| Starting date | 13 November 2018 |
| Contact information | Correspondence: andrewmoriarty1@doctors.org.uk, Andrew Stephen Moriarty, Department of Health Sciences and the Hull York Medical School, University of York, York, UK |
| Notes |
ISRCTN86760765 2017.
| Study name | Integrating depression management in HIV care in Uganda |
| Methods | Cluster‐randomised trial |
| Participants | Clusters are public healthcare facilities that provide HIV care in the 3 trial districts of Wakiso, Masaka, and Kalungu Inclusion criteria 1. Adult patients living with HIV attending the study clinics 2. Aged >18 years 3. An established resident of the study area 4. Medically stable (not too ill to require emergency admission) 5. Conversant in Luganda (the predominant language spoken in the study area) 6. ‘Screen positive’ for a depressive disorder (DD) (score ≥ 10 on the PHQ‐9) Exclusion criteria 1. Adults who screen below the DD threshold score on the PHQ‐9 2. Those who have severe cognitive or sensory impairments that hinder engagement with research procedures |
| Interventions |
Intervention HIV+ D intervention, which will consist of the following 4 steps: Step 1 (initiation of treatment): patients screened to have ‘significant’ depressive disorder (DD) scores (score ≥ 10 on the depression screening instrument, Patient Health Questionnaire ‐ PHQ‐9) will be advised about results from the screening questionnaire and offered psychoeducation Step 2 (management of moderate to severe cases): patients who have moderate to severe DD (PHQ‐9 score 15 to 19) at first consultation or those who remain symptomatic at follow‐up (PHQ‐9 score ≥ 5) despite Step 1. These will be given the choice of either Healthy Activity Program (HAP, a brief psychological treatment for depression that is based on behavioural activation, will consist of 6 sessions over a 12‐week period) administered by a lay health worker or antidepressant medication (fluoxetine 20 mg/d for 6 months) administered by attending clinician Step 3 (monitoring outcomes): patients who remain symptomatic at follow‐up despite Step 2. These will be given both HAP and antidepressant medication concurrently Step 4 (referral to specialist mental health worker): patients who remain symptomatic at follow‐up despite taking Step 3 or with severe DD (PHQ‐9 score ≥ 20) at treatment initiation or at high risk for suicide at any time (including treatment initiation). Continue all existing treatments and referred to mental health specialist Control The control arm of the trial will receive enhanced usual care (EUC), which will consist of usual care plus the provision of screening results and mhGAP treatment guidelines to attending clinicians with the option of referral to specialist mental health services at the regional hospital |
| Outcomes |
Primary outcomes 1. Mean depressive disorder (DD) symptom severity scores, assessed using the PHQ‐9 at 6 months 2. Proportion of participants who fail to achieve remission from DD; proportion with PHQ‐9 scores ≥ 5 at 6 months Secondary outcomes 1. Primary outcome measures at 12 months 2. Proportion of participants who are on ART at baseline and who have virological failure (defined as a viral load ≥ 1000 copies/mL) at 6 and 12 months 3. Proportion of participants who report having missed ≥ 1 dose of cotrimoxazole prophylaxis in the past 3 days, assessed at 6 and 12 months 4. Mean CD4 count at 6 and 12 months 5. Proportion of participants who experience a new WHO stage 3 or 4 event or death over the 12 months 6. Quality‐adjusted life‐years (QALYs), measured using the SF‐6D (the Short‐Form Six‐Dimension) at quarterly intervals for 12 months 7. Days out of work and functional impairment, measured using the WHO‐DAS29 at 6 and 12 months 8. Failure to recover from DD; proportion whose PHQ‐9 scores are ≥ 5 on 2 consecutive occasions 3 months apart, and who relapse after recovery |
| Starting date | 02/02/2017 |
| Contact information | Mr Richard Mpango 51‐59 Nakiwogo Street Entebbe PO Box 49 Entebbe Uganda |
| Notes | Expected to end 01/02/2022 Funded by Wellcome Trust (UK) |
Joag 2020.
| Study name | Atmiyata, a community‐led intervention to address common mental disorders: study protocol for a stepped wedge cluster randomized controlled trial in rural Gujarat, India |
| Methods | Stepped Wedge Cluster Randomized Controlled Trial |
| Participants | Adults with CMDs residing in rural villages in the Mehsana district Inclusion criteria 1. Age ≥ 18 and < 65 2. GHQ ≥ 3 Exclusion criteria 1. Terminal medical condition 2. Suicidal ideation or plans for suicide at the baseline interview 3. Cannot provide consent 4. Decline participation |
| Interventions | Atmiyata intervention: a complex psychosocial intervention involving 2 tiers of community volunteers ‐ Mitras from different caste and religion‐based sections of the village ‐ who are trained to identify persons in mental distress; Champions, who are well‐known and approachable community members of the village with leadership and communication skills who are trained to identify and provide structured counselling to persons with significant mental distress. Champions are trained by community facilitators who typically have a masters’ degree in social work or related fields and are locally based and aware of community dynamics. Mitras are trained by Champions. Champions deliver 4 to 6 counselling sessions to patients, which are based on active listening, activity scheduling, and problem‐solving techniques. Each session is to last 20 to 40 minutes, and sessions are to be held over 6 to 12 weeks and held at the participant’s home or at a community location preferred by the participant Control group: received enhanced usual care, where participants are given information on the impact of distress on physical and mental health, available public mental healthcare services, and active support if identified to be in crisis. Due to the stepped wedge design, all participants will have received the intervention by the end of the trial |
| Outcomes |
Primary outcome 1. Improvement in CMD using the GHQ‐12 score (Gujarati version), both continuous and categorical (≥ 3 defined as a case, < 3 defined as a non‐case) Secondary outcomes 1. QOL (EQ‐5D, Gujarati version) 2. SRQ, Gujarati version 3. WHODAS‐12, Gujarati version 4. PHQ‐9, Gujarati version 5. Client Satisfaction Questionnaire (intervention group only) 6. Social Participation Scale 7. Economic questionnaire 8. Accuracy of Champions identifying persons with CMD using the GHQ‐12 |
| Starting date | April 2017 |
| Contact information | kaustubh@cmhlp.org |
| Notes | Recruitment completed August 2019 |
Joska 2020.
| Study name | Nurse‐delivered cognitive‐behavioural therapy for adherence and depression among people living with HIV (the Ziphamandla study): protocol for a randomized controlled trial |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria 1. HIV seropositive, enrolled at primary HIV care clinics in periurban areas of Cape Town 2. Not attained viral suppression (viral load > 400 copies/mL) 3. Diagnosis of major depression disorder (MINI) Exclusion criteria 1. Unable or unwilling to consent 2. Active untreated major mental illness (psychosis, mania, high suicide risk) 3. Received cognitive‐behavioural therapy for depression 4. Age < 18 years |
| Interventions |
Intervention Cognitive‐behavioural therapy for adherence and depression (CBT‐AD) Nurse interventionists who have been trained for 1 week and have undergone further training as required as well as yearly booster sessions will deliver 8 sessions of CBT and optional additional sessions that incorporate motivational interviewing, psychoeducation, adherence counselling, adherence problem‐solving, plan for coping, systematic programme pleasure‐ and mastery‐based activities in their lives, relaxation training, and relapse prevention Control Enhanced treatment as usual The clinic nurse provides feedback to the participant and the clinic doctor about the major depressive disorder diagnosis. All participants undergo standard adherence counselling in the clinic |
| Outcomes |
Primary outcomes 1. Depression symptoms: HAM‐D 2. Antiretroviral therapy adherence: Wisepill electronic adherence monitoring system Secondary outcomes 1. HIV viral load 2. CD4 count 3. Psychosocial self‐report assessment battery 4. Medications |
| Starting date | August 2015 |
| Contact information | |
| Notes | Registered at ClinicalTrials.gov as NCT02696824 Enrollment completed in June 2019 |
Kohrt 2018.
| Study name | Reducing stigma among healthcare providers to improve mental health services (RESHAPE): protocol for a pilot cluster randomized controlled trial of a stigma reduction intervention for training primary healthcare workers in Nepal |
| Methods | Pilot cluster‐randomised controlled trial |
| Participants | Clusters are primary healthcare facilities. The direct beneficiaries are the primary healthcare workers at these facilities. The indirect beneficiaries are their patients Inclusion criteria Primary care workers 1. Participating in either prescriber or non‐prescriber PRIME trainings 2. 21 to 65 years of age 3. Recruitment will attempt to balance gender distribution in recruitment health clusters 4. Nepali language competency, actively engaged in care provision in the health cluster, with a valid certificate of practice from the Ministry of Health 5. Permission from the health supervisor to attend the entire duration of training Patients 1. Receiving PRIME services including persons with diagnosis of depression, psychosis, harmful drinking, or epilepsy. Providers make the diagnosis based on mhGAP criteria 2. 21 to 65 years of age 3. Fluency in Nepali Exclusion criteria 1. Primary care trainees will be excluded if they have any prior citations on their clinical practice licensure 2. Patients who cannot provide consent |
| Interventions |
Intervention PRIME/mhGAP + RESHAPE = Mental health services users and primary care workers who have previously completed training are trained using PhotoVoice and other techniques to participate as co‐facilitators. They participate in introductions to the intervention, myth busting, recovery stories, psychosocial communication role‐plays, and collaborative activities addressing challenges and barriers to task‐sharing/task‐shifting mental health services in primary care Other name: social contact anti‐stigma behavioural intervention Control Treatment as usual (PRIME/mhGAP). Primary care workers are trained using the mental health Global Action Programme (mhGAP) to identify and treat mental disorders in primary care. Primary care "prescribers" (those who can administer psychotropic medication) are trained to treat disorders including depression, alcohol use disorder, psychosis/schizophrenia, and epilepsy. "Non‐prescribers" (primary care workers not authorised to dispense medications) are trained to deliver psychosocial and psychological interventions |
| Outcomes |
Primary outcomes (health provider) Change in stigmatising attitudes, as measured by the Social Distance questionnaire Secondary outcomes (health provider) 1. Change in clinical knowledge, as measured by the mhGAP knowledge assessment 2. Change in implicit attitudes, as measured by the Implicit Association Test (IAT) [Time Frame: baseline, +4 months, + 16 months] 3. Change in stigmatising attitudes, as measured by the mhGAP Attitudes Questionnaire 4. Change in clinical competence, as measured by Enhancing Assessment of Common Therapeutic Factors Secondary outcomes (patient) 1. Change in patient functioning, as measured by the World Health Organization Disability Assessment Scale (WHODAS) [Time Frame: baseline, 6 months] 2. Change in patient‐perceived stigma as a barrier to accessing care, as measured by the Barriers to Access to Care Evaluation (BACE) [Time Frame: baseline, 6 months] 3. Change in patient depression, as measured by the Patient Health Questionnaire (PHQ‐9) [Time Frame: baseline, 6 months] |
| Starting date | 8 June 2016 |
| Contact information | bkohrt@gwu.edu |
| Notes | Recruitment completed |
Madhombiro 2017.
| Study name | A cluster randomised controlled trial protocol of an adapted intervention for alcohol use disorders in people living with HIV and AIDS: impact on alcohol use, general functional ability, quality of life and adherence to HAART |
| Methods | Cluster‐randomised controlled trial |
| Participants | Clusters are HIV care clinics in Zimbabwe Patients with HIV on HAART and with alcohol use disorder Inclusion criteria 1. Age 18 to 75 2. Clients must be on antiretroviral treatment for ≥ 3 months; male clients must score ≥ 8 on the WHO Alcohol Use Disorders Identification Test, and females must score ≥ 7 on the same screener Exclusion criteria 1. Score on AUDIT > 21 2. On another alcohol treatment programme 3. Abusing other drugs 3. Having a psychotic disorder 4. Unable to follow and participate in therapy |
| Interventions |
Intervention An adapted motivational interviewing cognitive‐behavioural therapy intervention. 4 sessions per month at baseline, 3, 6, and 12 months; 45 minutes every session. Using principle of motivational interviewing, the client is moved into readiness to change in use of alcohol. When ready to change, the client is taken through process that will sustain the change. This involves building coping skills and assertiveness Control WHO mental health GAP. 4 sessions per month at baseline, 3, 6, and 12 months; 45 minutes every session. Clients will be assessed as to their alcohol use; if they are dependent on alcohol, they will be referred for specialist care. If they are at risk ‐ hazardous or harmful users of alcohol ‐ then they go for the brief intervention |
| Outcomes |
Primary outcome AUDIT score Secondary outcomes 1. Viral load 2. CD4 3. WHODAS2 score |
| Starting date | 1/7/2016 |
| Contact information | Munyaradzi Madhombiro, mmadhombiro@gmail.com, 263773064519, 36 East Road, Avondale, Harare, HG 841, Zimbabwe |
| Notes |
Myers 2018.
| Study name | Comparing dedicated and designated models of integrating mental health into chronic disease care |
| Methods | Cluster‐randomised trial with 3 arms |
| Participants | Clusters are primary healthcare facilities in Western Cape Province of South Africa. Participants are patients with HIV or diabetes attending these clinics Inclusion criteria 1. ≥ 18 years of age From HIV clinics: 1. ≥ 18 years old 2. Taking ART for their HIV disease 3. Screen at risk for depression, with CES‐D score ≥ 16 and/or screen at risk for hazardous or harmful alcohol use (with AUDIT score ≥ 8) 4. Providing written informed consent to participate in the study 5. Agree to comply with all study requirements From diabetes clinics: 1. ≥ 18 years old 2. Taking medication for diabetes disease (type 1 or type 2) 3. Screening at risk for depression, with CES‐D score ≥ 16 and/or screening at risk for hazardous or harmful alcohol use (with AUDIT score ≥ 8) 4. Providing written informed consent to participate in the study 5. Agreeing to comply with all study requirements Exclusion criteria 1. Currently receiving treatment (counselling or medication) for a mental disorder 2. Participating in another research study |
| Interventions |
Intervention 1 Dedicated arm ‐ a 3‐session blended motivational interviewing‐problem‐solving therapy (MI‐PST) intervention delivered by community health workers who will work across both HIV and diabetes clinics. They will be dedicated staff who will have no other chronic disease duties and who will be responsible only for delivering this mental health intervention package.1 hour per session Intervention 2 Designated arm ‐ in clinics allocated to this arm, participants will receive a 3‐session blended MI‐PST intervention delivered by community health workers who will work across both HIV and diabetes services. They will be designated to provide this mental health care package and referrals to additional specialised services (if indicated) in addition to their other chronic disease‐related duties. 1 hour per session Control Screening and referral ‐ in CHCs assigned to the TAU arm, participants will receive the standard package of care when a health provider suspects a patient may have a common mental disorder. This includes screening for a common mental disorder and referring patients for further assessment and treatment to onsite or offsite mental health nurses or social workers for further assessment and treatment. One 1‐hour session |
| Outcomes |
Primary outcomes
1. Depression score (CES‐D) 2. Hazardous alcohol use (AUDIT) Secondary outcomes 1. HBA1C 2. HIV RNA viral load |
| Starting date | 26/7/2017 |
| Contact information | Bronwyn Myers, bmyers@mrc.ac.za |
| Notes |
NCT02185482 2014.
| Study name | Physician‐led counselling in management of depression in type 2 diabetes mellitus |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria 1. Eligible patients are ambulatory 2. Adequate hearing to complete a telephone interview 3. Diabetic patients screened for depression 4. Age 35 to 60 years Exclusion criteria 1. Currently in care with a psychiatrist 2. Diagnosis of bipolar disorder or schizophrenia 3. Use of antipsychotic or mood stabiliser medication 4. Mental confusion on interview, suggesting significant dementia |
| Interventions |
Intervention Physician‐supported care An individualised, stepped care depression treatment provided by a depression clinical specialist primary care physician Control Usual care Primary care physicians prescribe antidepressant medication and refer patients to Mental Health Services |
| Outcomes |
Primary outcome (baseline to 6 months) 1. Change in depression using SCL‐90 depression scale Secondary outcomes (baseline to 6 months) 1. Change in glycaemic control (glycosylated haemoglobin levels) 2. Global assessment of improvement (Patient Global Impression) |
| Starting date | August 2014 |
| Contact information |
Principal Investigator Naresh C Kumar, MD Kaithal Civil Hospital |
| Notes | Completed November 2015 |
NCT03378544 2019.
| Study name | Engaging traditional birth attendants to reduce maternal depression in rural Kenya |
| Methods | Cluster‐randomised controlled trial |
| Participants | Clusters are 2 sub‐counties in Makueni County (rural Kenya). Participants are pregnant mothers age 14 to 50 years seeking the services of traditional birth attendants (TBAs) Inclusion criteria 1. Gestational age between 16 and 26 weeks 2. Positive score on EPDS 3. Confirmed diagnosis of depression using the Mini International Psychiatric Interview 4. Provision of informed consent Exclusion criteria 1. Actively suicidal 2. Severe mental disorder and/or medical condition requiring immediate medical attention 3. Negative score on EPDS 4. Pregnancy‐related complications 5. Decline to provide informed consent |
| Interventions |
Intervention Patients with suicidal ideation and depression will receive psychosocial interventions adapted from the WHO mental health Global Action Programme Intervention Guide (mhGAP‐IG). The intervention will involve psychoeducation for patients on the importance of maintaining interest in activities that they used to do, regular sleep cycles, physical activity, and social activity Control Patients with suicidal ideation and depression will be referred to the nearest health centre for further treatment |
| Outcomes |
Primary outcome measure 1. Reduction in depressive symptoms [Time Frame: 0, 3, 6, and 9 months] using the Edinburgh Postnatal Depression Scale Secondary outcome measures 1. Quality of life of mothers with depression [Time Frame: 0, 3, 6, and 9 months] using the World Health Organization Quality of Life questionnaire 2. Satisfaction level [Time Frame: 0, 3, 6, and 9 months] a. Change in satisfaction levels among patients seeking TBA services 3. Suicidality [Time Frame: 0, 3, 6, and 9 months]. The proportion of patients with reduced suicidal behaviours such as ideations or attempts as measured on Beck's Suicidality Scale 4. Disability [Time Frame: 0, 6, and 9 months] using the World Health Organization Disability Assessment Schedule |
| Starting date | 9 April 2018 |
| Contact information |
Principal Investigator Christine W Musyimi, Africa Mental Health Foundation |
| Notes |
NCT03430622 2019.
| Study name | Effectiveness of a telephone‐based learning through play (LTP) plus interpersonal psychotherapy (IPT) for depressed mothers (Telemothercare) |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria 1. 18‐ to 44‐year‐old mothers with children between 0 and 30 months of age 2. Diagnosis of major depressive disorder using the Structured Clinical Interview for DSM‐V 3. Ability to complete a baseline assessment 4. Participating mother with telephone/mobile phone access for at least 1 hour per week Exclusion criteria 1. Any medical illness that will prevent participation in the clinical trial 2. Current or past diagnosis of bipolar depression 3. Currently using antidepressants or receiving any kind of psychotherapy 4. Active suicidal ideation 5. Any other severe physical or mental disorder 6. No access to telephone/mobile |
| Interventions |
Intervention LTP Plus (Psychosocial intervention) LTP Plus group participants will receive intervention over the telephone for 3 months 1 session per week for 2 months and the rest of the sessions fortnightly by trained graduates, expert in delivering LTP plus intervention. LTP Plus comprises 2 components: learning through play (LTP) and interpersonal psychotherapy (IPT). LTP Plus is a low‐literacy, sustainable programme intended to provide parents with information on the healthy growth and development of their young children. LTP research‐based activities enhance children's development while simultaneously promoting attachment security by building parents' ability to read and being sensitive to their children's cues and through active involvement in their children's development. The interpersonal psychotherapy (IPT) intervention comprises a supportive element, an educational element, a parenting element, and an interpersonal relationship element. Intervention goals include helping mothers feel supported, empowered, and confident about their parenting abilities, which would directly influence reduction in depressive symptoms as well as resolution of interpersonal conflicts Control Treatment as usual (TAU) TAU group will receive routine care and follow‐up will be done after completion of the intervention, then at 6 months post randomisation |
| Outcomes |
Primary outcome measures Patient Health Questionnaire (PHQ‐9) to check severity of depression Secondary outcome measures 1. Generalised anxiety disorder (GAD) 7 to screen for and measure severity of generalised anxiety disorder 2. Postpartum Bonding Questionnaire (PBQ) to detect disturbance in the mother‐child relationship 3. Experiences in Close Relationships‐Revised (ECR‐R). The ECR‐R measures individuals on 2 sub‐scales of attachment: Avoidance and Anxiety. In general, avoidant individuals find discomfort with intimacy and seek independence, whereas anxious individuals tend to fear rejection and abandonment 4. Dyadic Adjustment Scale (DAS) to assess how each partner perceives his/her relationship 5. Client Service Receipt Inventory. We will collect information about the use of other health services (including the informal sector, faith healers/Imams) using CSRI based on our previous work in Pakistan 6. Infant development: Ages and Stages Questionnaire will be used to measure child development. Parents will report on their child's communication, gross and fine motor skills, problem‐solving, and personal‐social development at different time points 7. Ages and Stages Social‐Emotional Questionnaire to obtain maternal report on the child's social and emotional development. The questionnaire is already translated in Urdu and will be used in the proposed trial 8. World Health Organization Quality of Life (WHO 2004). A self‐report scale comprising 26 items that measures physical health, psychological health, social relationships, and environment Learning Through Play (LTP) KAP Questionnaire to measure change in Knowledge, Attitude and Practices (KAP) at different time points |
| Starting date | 20 February 2018 |
| Contact information |
Principal Investigator Nasim Chaudhry Pakistan Institute of Living & Learning |
| Notes |
NCT03587896 2018.
| Study name | Implementation of Self Help Plus in adult Syrian refugees in Turkey (RE‐DEFINE) |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria 1. Age 18 or above 2. Able to speak and understand 1 Arabic 3. Asylum seeker or refugee or person under temporary protection 4. Presence of psychological distress, as shown by a score ≥ 3 on the 12‐item GHQ‐12 5. Both oral and written informed consent to enter the study Exclusion criteria 1. Presence of any mental disorders according to DSM‐5 and ICD‐10, as shown by a positive MINI 2. Acute medical condition contraindicating study participation 3. Clinical evidence of imminent suicide risk or suicide risk scored as ‘moderate or high’ (or a positive suicidality behaviour disorder) by the MINI (section Suicidality) 4. Clinical evidence that decision‐making capacity is impaired |
| Interventions |
Intervention SH+ programme A pre‐recorded audio course delivered by briefly trained, non‐specialist facilitators with a refugee or migrant background in a group setting of up to 30 people across five 2‐hour sessions complemented by an illustrated self‐help book. Based on acceptance and commitment therapy, SH+ includes techniques that aim to help people cope with stress, respond compassionately to themselves and others, and live in accordance with their values Control Enhanced treatment as usual Social support and/or care following local regulations; baseline and follow‐up assessments; information about freely available health and social services, and community networks that provide support to refugees and asylum seekers |
| Outcomes | 1. Psychological distress (GHQ‐12) 2. Psychiatric diagnosis (MINI) 3. Symptoms of PTSD (PCL‐5) 4. Depressive symptoms (PHQ‐9) 5. Subjective well‐being (WHO‐5) 6. Health‐related quality of life (EQ‐5D‐3L) 7. Self‐defined psychosocial goals (PSYCHLOPS) 8. Post‐migration stress difficulties (post‐migration living difficulties ‐ PMLDs) 9. Psychological functioning (WHODAS 2.0) 10. Cost‐effectiveness (CSSRI‐EU) |
| Starting date | 1 September 2018 |
| Contact information | marianna.purgato@univr.it |
| Notes | Terminated due to logistical reasons |
NCT03887312 2019.
| Study name | Phone‐delivered psychological intervention (t‐CETA) for mental health problems in 8‐ to 17‐year‐old Syrian refugee children (t‐CETA) |
| Methods | Randomised controlled trial |
| Participants | This study is conducted in Lebanon Inclusion criteria 1. Ages 8 to 17 2. Lives with parent or legal guardian 3. Child and/or parent identifies that child has mental health difficulties and requests services 4. At high risk of having a mental disorder by falling in the top 40% of the distribution in any one of the following child‐report questionnaires: (1) Screen for Child Anxiety Related Emotional Disorders (SCARED), (2) Center for Epidemiological Studies Depression Scale for Children (CES‐DC), (3) Child PTSD Symptom Scale (CPSS); AND falling in the top 40% of the distribution in the following parent report questionnaire: Strengths and Difficulties Questionnaire (SDQ) total difficulties 5. Confirmation of significant level of symptoms and functional impairment on clinical interview (MINI KID) as indicated by (1) meeting full or probable diagnostic criteria for ANY of the following: any category of mood disorder, any category of anxiety disorder, PTSD, conduct disorder, or oppositional defiant disorder; AND (2) Clinical Global Impression severity (CGI‐S) score > 3 (Criterion 5 takes precedence over Criterion 4 when both are available) 6. Consent Exclusion criteria 1. Problem for which t‐CETA would not be appropriate, including psychiatric disorders for which CETA treatment is not recommended (e.g. bipolar disorder, psychosis), severe distress (e.g. acute suicidal ideation), or problems that would preclude delivery over the telephone (e.g. selective mutism) 2. Parent or guardian is not able to provide consent 3. Child protection issues |
| Interventions |
Intervention t‐CETA Telephone‐delivered common elements treatment approach (t‐CETA) sessions of up to 30 minutes are delivered 1 to 2 times per week for 8 to 12 weeks. CETA is a cognitive‐behavioural therapy‐based approach with components for anxiety, depression, PTSD, conduct problems, substance abuse, and safety issues. A tailored treatment package is produced for each child, and there are components for both child and caregiver Control Medecins du Monde treatment as usual Case manager‐led care with referral to a psychotherapist or psychiatrist as necessary. Based on a collaboration between mental health‐trained case managers and psychotherapists |
| Outcomes |
Primary outcomes 1. Emotional and behavioural problem composite score 2. WHO Disability Assessment Schedule for Children (WHODAS‐Child, adapted) Secondary outcomes 1. Child PTSD Symptom Scale (CPSS) 2. Center of Epidemiological Studies Depression Scale for Children (CES‐DC) 3. Screen for Child Anxiety Related Emotional Disorders (SCARED) 4. Externalising behaviour problems score 5. WHO‐5 Well‐Being Index (WHO‐5) 6. Youth Life Orientation Test (YLOT) 7. PSYCHLOPS 8. Client Monitoring Form (CMF, custom) |
| Starting date | 1 May 2019 |
| Contact information | Michael Pluess, PhD Queen Mary University of London |
| Notes | Completed 31 January 2020 |
NCT03912077 2019.
| Study name | Implementing psychosocial interventions to Syrian refugee women who are exposed to psychological trauma |
| Methods | Randomised controlled trial |
| Participants | Setting: Turkey Inclusion criteria 1. 18 years old and older 2. Syrian woman under temporary protection who resides in Istanbul 3. Able to speak and understand Arabic 4. Psychological distress symptoms, as shown by a score ≥ 1.75 on the Hopkins Symptoms Checklist‐25 (HSCL‐25 ≥ 1.75) Exclusion criteria 1. Imminent risk of suicide 2. Severe mental disorder (psychotic disorders, substance dependence) 3. Severe cognitive impairment (severe intellectual disability or dementia) |
| Interventions |
Intervention Culturally adapted cognitive‐behavioural therapy Seven group therapy sessions that have been adapted to Syrian culture and the needs of Syrian refugee women Control Treatment as usual Routine social support and/or care |
| Outcomes |
Primary outcomes 1. Psychological distress symptoms (Hopkins Symptoms Checklist – 25) Secondary outcomes 1. Psychological trauma symptoms (Harvard Trauma Questionnaire) 2. Depression symptoms (Beck Depression Inventory II) 3. Distress symptoms related to post‐migration events (Post‐Migration Living Difficulties) |
| Starting date | 9 March 2019 |
| Contact information |
Principal Investigator Halime Sevde Eskici Istanbul Sehir University |
| Notes | Completed 20 June 2019 |
NCT03919695 2019.
| Study name | Development of an intervention to reduce heavy drinking and improve HIV care engagement among fisherfolk in Uganda |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria 1. Occupation of working in the fishing industry or industry supporting the fishing industry 2. HIV+ 3. On ART for at least 1 month 4. Missed ≥ 1 dose of ART in the prior 2 weeks 5. Consume ≥ 5 drinks per occasion ≥ 2 times in the prior month or AUDIT‐C score ≥ 4 6. Not planning to move from the area within the next 6 weeks 7. Have their own mobile phone and can be reached via phone Exclusion criteria 1. Currently receiving a majority of income for work via mobile money 2. Does not speak Luganda or English 3. Unable to read basic Luganda or English 4. Occupation of boat or engine owner |
| Interventions |
Intervention Behavioural and structural intervention (KISOBOKA) Alcohol screening, financial literacy training, counselling, goal‐setting, alcohol use, HIV care engagement. The mode of work payment is changed from cash to mobile money Control Screening and referral Brief feedback on AUDIT‐C score, referral for alcohol counselling, and brief discussion of the importance of HIV care engagement and adherence |
| Outcomes |
Primary outcomes 1. Change from baseline in frequency of heavy/binge drinking 2. HIV care engagement 3. ART adherence Secondary outcomes 1. Phosphatidylethanol level 2. Change in HIV viral load from baseline |
| Starting date | July 2020 |
| Contact information |
Principal Investigator Susan M Kiene San Diego State University |
| Notes | Estimated completion date July 2021 |
NCT03922425 2019.
| Study name | Large‐scale implementation of community‐based mental health care for people with severe and enduring mental ill health in Europe |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria 1. Age 18 to 65 years 2. Current service users, with severe and enduring mental disorder 3. Severe mental illness ‐ bipolar disorder, severe depression, or schizophrenia Exclusion criteria 1. Patients who do not consent to their data being collected 2. Patients with acute somatic disorders 3. Patients who are under the age of 18 at the start of the study |
| Interventions |
Intervention Community Mental Health Team (CMHT) Multi‐disciplinary CMHTs (comprising nurses, social workers, psychiatrists, psychologists, and a “peer expert”) will provide home‐based treatment including crisis resolution, early recognition of subclinical psychosis and bipolar disorder, intensive case management, and care integrating health and social services. Family‐based interventions, motivational interviewing, cognitive‐behavioural techniques, medication management, employment identification and support, recovery groups, and housing opportunities are given Control No intervention beyond standard care |
| Outcomes |
Primary outcome Change in daily functioning (WHODAS‐2) Secondary outcome Change in health‐related quality of life (EuroQoL 5D‐3L) |
| Starting date | 1 January 2018 |
| Contact information | Nikola Shipkovenky, Mental Health Center, Sofia, Bulgaria |
| Notes | Estimated completion date: 1 June 2022 Sponsors and collaborators National Center for Public Health and Analyzes, Bulgaria Stichting Trimbos Institut, Netherlands |
NCT03960892 2019.
| Study name | Implementation of group problem management Plus (PM+) in adult Syrian refugees in Turkey: RCT (STRENGTHS) |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria 1. Adults ≥ 18 years of age 2. Syrian under temporary protection status 3. Arabic‐speaking 4. Elevated levels of psychological distress (K10 > 15) and reduced psychosocial functioning (WHODAS 2.0 > 16) Exclusion criteria 1. Acute medical condition 2. Imminent suicide risk or expressed acute needs/protection risks 3. Severe mental disorder (psychotic disorder, substance dependence) 4. Severe cognitive impairment (e.g. severe intellectual disability, dementia) |
| Interventions |
Intervention Enhanced care as usual (E‐CAU) with group problem management Plus (PM+) 190 participants will be randomly assigned to E‐CAU with Group PM+. The PM+ is developed by the World Health Organization (WHO) especially for communities exposed to adversity. PM+ (Dawson et al, 2015) belongs to a set of programmes that are low‐intensity, shorter, less expensive and transdiagnostic (i.e. not condition‐specific, but targeted at a broader set of symptoms of common mental disorders) programmes to reduce common mental health symptoms (including depression, anxiety, and stress symptoms) and improve psychosocial functioning. This intervention is based on WHO treatment guidelines for conditions related to stress (WHO, 2013). It is a 5‐session intervention that is delivered by trained non‐specialists and is available in individual and group delivery formats for both children and adults. PM+ includes evidence‐based techniques such as problem‐solving, stress management, behavioural activation, and access to social support. It was proved effective by 2 randomised controlled trials (RCTs) in Kenya and Pakistan (Bryant, Dawson, Schafer, Sijbrandij, & van Ommeren, 2016; Rahman, Hamdani, Awan, Bryant, Dawson, Khan, Mukhtar‐ul‐Haq Azeemi, et al, 2016). Participants in the experimental arm will receive Group PM+ by trained, non‐specialist peer refugees in addition to E‐CAU Control Enhanced care as usual (E‐CAU) only 190 participants will be randomly assigned to E‐CAU group. CAU ranges from the free health services government provides to Refugee and Asylum Seekers Assistance and Solidarity Association's (RASASA) mental health services, which are provided by the Psychological Support Unit (MHPSS Support Unit), which includes counselling as well. The enhanced care arm (CAU, with the addition of a leaflet that will include information on the services they can get from RASASA and other public services) is to be used as a benchmark for measuring the effectiveness of STRENGTHS's intervention, which is Problem Management Plus (PM+) |
| Outcomes |
Primary outcome measures 1. Hopkins Symptom Checklist‐25 (HSCL‐25) [Time Frame: Change from baseline assessment, at 1 week post‐intervention assessment (6 weeks after baseline), change from post‐assessment at 3 months post‐intervention assessment (4 to 4.5 months after baseline), and change from 12 months post intervention assessment] The aim is to measure the change in psychological distress. Sub‐scales can be calculated for depression (13 items) and anxiety symptoms (10 items). Two items are related to somatic symptoms. The items are rated on a 1 to 4‐point Likert scale, with a well‐validated cutoff score of 1.75. The current study will primarily look at the total score at which lower values represent a better outcome. Furthermore, aim is to examine changes in case‐ness in depression; a cutoff score for the depression subscale of 2.1 will be used Secondary outcome measures 1. PTSD Checklist for DSM‐5 (PCL‐5) [Time Frame: Change from baseline assessment, at 1 week post‐intervention assessment (6 weeks after baseline), change from post‐assessment at 3 months post‐intervention assessment (4 to 4.5 months after baseline), and change at 12 months post intervention assessment] 2. Psychological Outcome Measures (PSYCHLOPS) [Time Frame: Change from baseline assessment, at 1 week post‐intervention assessment (6 weeks after baseline), change from post‐assessment at 3 months post‐intervention assessment (4 to 4.5 months after baseline), and change at 12 months post intervention assessment] a. The aim is to assess the change in self‐identified problems. PSYCHLOPS consists of 4 questions. It contains 3 domains: 2 questions on problems, 1 question on function, and 1 question on well‐being. Participants are asked to give free text responses to the problem and function domains. Responses are scored on a 0 to 5 scale, producing a maximum score of 20 (6 points per domain). The current study will primarily look at the total score at which lower values represent a better outcome (1) Client Service Receipt Inventory (CSRI) [Time Frame: Change from baseline assessment, at 1 week post‐intervention assessment (6 weeks after baseline), change from post‐assessment at 3 months post‐intervention assessment (4 to 4.5 months after baseline), and change at 12 months post intervention assessment] b. The aim is to measure the change in the cost of care. CSRI was developed for the collection of data on service utilisation and related characteristics of people with mental disorders, as the basis for calculating the costs of care for mental health cost‐effectiveness research. The current study will assess the health service utilisation and productivity impact (1) Access to health care: own questionnaire [Time Frame: Change from baseline assessment, at 1 week post‐intervention assessment (6 weeks after baseline), change from post‐assessment at 3 months post‐intervention assessment (4 to 4.5 months after baseline), and change at 12 months post intervention assessment] c. The aim is to assess the change in access to health care. The questionnaire will include 70 questions on perceived access to health care. 57 of those questions will be 2‐way closed‐ended questions (yes or no) with the addition of the items "don't know" and "refused to answer". 13 of these questions are asking the type of help received (counselling/psychotherapy, psychological support, and medicines) with the addition of the items "don't know" and "refused to answer" (1) Sociodemographic information and disability: WHODAS [Time Frame: Change from baseline assessment, at 1 week post‐intervention assessment (6 weeks after baseline), change from post‐assessment at 3 months post‐intervention assessment (4 to 4.5 months after baseline), and change at 12 months post intervention assessment] This questionnaire includes 12 questions scored on a 5‐point Likert scale ranging from 0 (none) to 4 (extreme), before summation (range 0 to 48). Higher scores indicate worse functional impairment Other outcome measures 1. Traumatic experiences questionnaire: own questionnaire [Time Frame: Change from baseline assessment, at 1 week post‐intervention assessment (6 weeks after baseline), and change at 12 months post intervention assessment] a. The aim of the questionnaire is to assess trauma exposure. The questionnaire has 28 items from the Harvard Trauma Questionnaire (HTQ) and the Post‐traumatic Diagnostic Scale (PDS), and specific traumatic experiences of Syrian refugees. The items are scored as 1 (yes) or 0 (no), ranging from 0 to 28 |
| Starting date | 29 December 2018 |
| Contact information |
Principal Investigator Zeynep Ceren Acartürk Assoc. Prof., Koç University |
| Notes |
NCT03966833 2019.
| Study name | Using group interpersonal psychotherapy to improve the well‐being of adolescent girls |
| Methods | Randomised controlled trial |
| Participants | This study is carried out in Kampala, Uganda Inclusion criteria 1. 13 to 19 years old 2. Female 3. Score ≥ 10 on the Primary Health Questionnaire (PHQ‐8) Exclusion criteria 1. Male 2. Score < 10 on the PHQ‐8 |
| Interventions |
Intervention arm 1 Group‐based interpersonal therapy (IPT‐G) 14 weeks of group interpersonal therapy delivered by trained community members as mentors at Empowerment and Livelihood for Adolescents (ELA) clubs Intervention arm 2 IPT‐G with unconditional cash transfer IPT‐G and a 1‐time lump sum of 200,000 UGX (equivalent to USD 54) near or at the conclusion of the 14‐week therapy Control No intervention ELA clubs function as normal |
| Outcomes |
Primary outcomes (6, 12, 24 months) 1. PHQ‐8 ≤ 10 2. General Health Questionnaire (GHQ)‐12 ≥ 3 3. Rosenberg self‐esteem score 4. Child and Youth Resilience Measure‐Revised Secondary outcomes 1. Self‐reported school enrolment 2. Pregnancy 3. Child marriage 4. Math skills 5. Self‐reported condom use |
| Starting date | 26 May 2019 |
| Contact information | munshi.sulaiman@brac.net |
| Notes | Estimated completion date: 30 September 2021 |
NCT04018391 2019.
| Study name | The TENDAI study: task shifting to treat depression and HIV medication non‐adherence in low resource settings |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria 1. Initiated on ART for ≥ 6 months 2. Clinically significant depression symptoms score ≥ 10 on the Patient Health Questionnaire‐9 3. Viral non‐suppression in past 2 months per local clinic standard (VL > 1000 copies/mL) 4. Able to provide informed consent 5. If prescribed antidepressants, on stable regimen for at least 2 months Exclusion criteria 1. Unable to provide informed consent 2. Active major mental illness (e.g. untreated psychosis or mania, actively suicidal), major untreated or undertreated mental illness, or advanced physical disease or severe cognitive impairment assessed using the psychosis module of the MINI, the PHQ‐9, and the International HIV Dementia Scale, which would interfere with engagement in PST‐AD 3. Has ever received PST or CBT for depression 4. Younger than 18 years of age |
| Interventions |
Intervention Stepped Care 6 sessions of problem‐solving therapy and adherence intervention sessions based on a culturally adapted LifeSteps and Problem Solving Therapy for Depression. If a participant’s depression score remains above threshold in Session 6, participant is referred to a research mental health nurse for a psychopharmacological assessment to prescribe an antidepressant Control Enhanced usual care Participants undergo clinic‐provided adherence counselling and access to providers trained in mhGAP, and receives a letter to medical provider detailing the depression diagnosis |
| Outcomes |
Primary outcome 1. Viral suppression [Time Frame: 12‐month post randomization study visit] (proportion of participants who achieve viral suppression (< 1000 copies/mL)) Secondary outcomes 1. Depression severity measured as total score on the Patient Health Questionnaire (PHQ‐9) [Time Frame: 12‐months post randomisation study visit] 2. Adherence to ART medication [Time Frame: 4 months post‐randomisation study visit] ‐ assessed as proportion of the sample achieving ≥ 90% adherence in the past month via pharmacy refill data 3. Adherence to ART medication [Time Frame: 12 months post randomisation study visit] 4. Adherence to ART medication [Time Frame: 8 months post randomisation study visit] 5. Self‐reported adherence to ART medication [Time Frame: 4 months post randomisation study visit] 6. Self‐reported adherence to ART medication [Time Frame: 12 months post randomisation study visit] 7. Self‐reported adherence to ART medication [Time Frame: 8 months post randomisation study visit] 8. Viral load copies/mL [Time Frame: 12 months post randomisation study visit] Other outcome measures 1. Cost‐effectiveness of TENDAI intervention [Time Frame: 12 months] |
| Starting date | 12 July 2019 |
| Contact information | Dr Melanie Abas, Associate Professor in Global Mental Health, King's College London |
| Notes | Estimated study completion date: 31 March 2023 |
NCT04110405 2019.
| Study name | Scaling‐up Stepped Care for women's mental health in primary care in an LMIC |
| Methods | Randomised controlled clinical trial |
| Participants |
Inclusion criteria 1. Female Tajik citizen between 18 and 45 years old 2. Score >16 on HAM‐D 3. No current or past substance use 4. Willing to participate in intervention and research procedures 5. Able to give written informed consent Exclusion criteria 1. Women who are older or younger than 18 to 45 years 2. Women who do not score > 16 on HAM‐D |
| Interventions |
Intervention Stepped Care Step 1: peer and nurse co‐led 8‐session group therapy Step 2: peer‐ or nurse‐led individual therapy sessions based on interpersonal therapy Step 3: primary care physician‐led medication treatment (amitriptyline) Control Standard of care plus Healthy lifestyle Standard outpatient care supplemented with literature on healthy lifestyles |
| Outcomes |
Primary outcomes 1. Change in Hamilton Depression Rating Scale 2. Change in Texas Christian University Organizational Readiness for Change Secondary outcomes 1. Change in Hamilton Anxiety Rating Scale 2. Change in PTSD Checklist for DSM‐5 (PCL‐5) 3. Change in Evidence‐based Practice Attitude Scale 4. Number of Stepped Care interventions initiated and completed |
| Starting date | 14 August 2020 |
| Contact information | Stevan Weine, Professor of Psychiatry, University of Illinois at Chicago |
| Notes | Estimated completion date: 1 October 2024 |
NCT04143009 2019.
| Study name | Adaptation of the friendship bench intervention for HIV‐infected perinatal women in Lilongwe (Periscope) |
| Methods | Randomised controlled clinical trial |
| Participants |
Inclusion criteria 1. Female 2. ≥ 18 years of age 3. Pregnant ≤ 30 weeks 4. HIV‐infected 5. Initiating or re‐initiating ART treatment 6. Screened positive for depression (score > 8 on SRQ‐20) Exclusion criterion 1. Women successfully established on ART |
| Interventions |
Intervention 1 Adapted Friendship Bench (AFB). 4 individual prenatal counselling sessions and 2 group postnatal counselling sessions. Problem‐solving therapy‐based ART adherence support is integrated into counselling sessions. Participants are given opportunities to address HIV‐specific concerns Intervention 2 Enhanced Friendship Bench. All elements of AFB with the addition of retention strategies to support engagement. 1 support session at which participants are invited to bring a person of their choice to support them in managing HIV and/or depression. A trained psychosocial counsellor conducts up to 6 home visits, to deliver medications and conduct a counselling session Control Enhanced Standard Care. Standard mental health care in public facilities (primary nurse, clinic psychiatric nurse, mental health clinic, psychiatric units of tertiary care hospitals) and mental health evaluation, brief supportive counselling, information, education, support and facilitation of referral to the clinic’s psychiatric nurse or mental health clinic, by trained study research assistant |
| Outcomes |
Primary outcomes 1. Feasibility 2. Acceptability 3. Fidelity Secondary outcomes 1. Composite outcome – proportion of women retained in HIV care (HIV visit in last 30 days), virally suppressed (HIV RNA < 1000 copies), with improved depression (≥ 50% improvement in SRQ‐20 from baseline) 6 months postpartum 2. Proportion of women reporting SRQ‐20 ≥ 8 six months postpartum 3. Proportion of women retained in HIV care 4. Proportion of kept visits through 6 months postpartum 5. Proportion of women virally suppressed 6. Proportion of women whose infants received an HIV viral load test |
| Starting date | 10 December 2019 |
| Contact information | smphonda@unclilongwe.org |
| Notes | Estimated completion date: June 2021 |
NCT04254393 2020.
| Study name | Early Adolescent Skills for Emotions (EASE) ‐ pilot cluster randomized controlled trial (cRCT) in public schools of rural Pakistan |
| Methods | Cluster‐randomised controlled trial |
| Participants | Clusters are schools Inclusion criteria 1. Adolescents ages 13 to 15 2. Self‐reported Pediatric Symptoms Checklist score ≥ 28 Exclusion criteria 1. High risk of abuse or harm to self or others 2. Ongoing medical or psychiatric care |
| Interventions |
Intervention Early Adolescent Skills for Emotion (EASE) Programme Group psychological intervention programme based on cognitive‐behavioural therapy techniques to manage symptoms of depression, anxiety, and distress in adolescents, to be delivered by non‐specialists in low resource settings. Includes 7 group adolescent sessions, each lasting 90 minutes, involving psychoeducation, problem‐solving, stress management, behavioural activation, and relapse prevention, and 3 caregiver group sessions, each lasting 120 minutes, involving psychoeducation, active listening, quality time, praise, caregiver self‐care, and relapse prevention Control Treatment as usual Participants access routine services available in school settings |
| Outcomes |
Primary outcome measure Pediatric Symptoms Checklist (PSC) [Time Frame: At 3 months post‐intervention] Secondary outcome measures 1. Somatic‐symptom questionnaire [Time Frame: At baseline, immediate and 3 months post‐intervention] 2. Social Problem‐Solving Inventory ‐ Revised Short Form [Time Frame: At baseline, immediate and 3 months post‐intervention] 3. Perceived Emotional/Personal Support Scale [Time Frame: At baseline, immediate and 3 months post‐intervention] 4. Patient Health Questionnaire (PHQ‐9) ‐ adapted for adolescents [Time Frame: At baseline, immediate and 3 months post‐intervention] 5. Pediatric Quality of Life (PedsQL) [Time Frame: At baseline, immediate and 3 months post‐intervention] 6. Pediatric Quality of Life (Peds‐QL)‐Family impact module [Time Frame: At baseline, immediate and 3 months post‐intervention] 7. Alabama Parenting Scale [Time Frame: At baseline, immediate and 3 months post‐intervention] 8. Health services utilisation [Time Frame: At baseline and 3 months post‐intervention] 9. Short Warwick Edinburgh Well‐being Scale (SWEWS) [Time Frame: At baseline, immediate and 3 months post‐intervention] |
| Starting date | 7 February 2020 |
| Contact information |
Principal Investigator Atif Rahman, PhD University of Liverpool |
| Notes | Sponsored by Human Development Research Foundation, Pakistan |
NCT04385498 2020.
| Study name | Primary care intervention for PTSD in Ethiopia |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria 1. For all participants: 18 years of age or older 2. For all participants: able and willing to provide informed consent to participate in the study 3. For all participants: able to complete procedures in Amharic or English 4. For patients: treatment at a Sodo district primary care clinic for severe mental illness (SMI) 5. For patients: identified as having: a. Experienced a traumatic event, b. Associated PTSD symptoms defined as scores on the PTSD Checklist DSM‐5 (PCL‐5), AND c. Any associated functional impairment on the WHODAS‐2 6. For healthcare providers: providers working at target health centres who administer, provide care for, or supervise the care of patients with mental health concerns 7. For caregivers: identified by the patient as a close family member or friend whom the patient gives permission to be involved in the study Exclusion criteria 1. Current high risk of suicide as measured by the Composite International Diagnostic Interview (CIDI) Suicide module 2. Inability to participate in treatment, as determined by the psychiatric nurse |
| Interventions |
Intervention BREATHE Intervention Five 20 to 30‐minute sessions focusing on breathing re‐training, psychoeducation, and positive coping, with additional sessions as needed Control Treatment as usual Typical primary care treatment including medication management and follow‐up |
| Outcomes |
Primary outcomes 1. Change in PTSD Knowledge Test score [Time Frame: Day 0 baseline, 3 months] 2. Change in PTSD Related Stigma measured by the Internalized Stigma of Mental Illness Scale [Time Frame: Day 0 baseline, 3 months] 3. Change in trauma‐related cognitions assessed by the Post‐Traumatic Cognitions Inventory [Time Frame: Day 0 baseline, 3 months] 4. Change in self‐reported arousal measured with Self‐Assessment Manikin [Time Frame: Day 0 baseline, 3 months] 5. Change in stress management strategy use as measured by self‐ and caregiver reports [Time Frame: Day 0 baseline, 3 months] 6. Change in physiological arousal measured by heart rate variability (HRV) [Time Frame: Day 0 baseline, 3 months] Secondary outcomes 1. Change in PTSD symptoms measured by the PTSD Checklist for Diagnostic Statistical Manual‐5 [Time Frame: Day 0 baseline, 3 months] 2. Change in depression symptoms measured by the Patient Health Questionnaire [Time Frame: Day 0 baseline, 3 months] 3. Change in anxiety symptoms measured by the Generalized Anxiety Disorder‐7 [Time Frame: Day 0 baseline, 3 months] 4. Change in functional impairment assessed by the WHO Disability Assessment Schedule II [Time Frame: Day 0 baseline, 3 months] |
| Starting date | Estimated start date: December 2020 |
| Contact information |
laurenng@ucla.edu abebaw.fekadu@aau.edu.et |
| Notes |
Nusrat 2016.
| Study name | Change your life with seven sheets of paper: a pilot randomised controlled trial for postnatal depression (CREATOR) |
| Methods | Randomised controlled trial |
| Participants | Participants with postnatal depression in Pakistan |
| Interventions |
Intervention Cognitive‐behavioural therapy delivered by trained traditional birth attendants through 8 group sessions Control Not stated in abstract |
| Outcomes | 1. Edinburgh Postnatal Depression Scale 2. Patient Health Questionnaire‐9 3. World Health Organization Quality of Life |
| Starting date | |
| Contact information | |
| Notes |
Onu 2016.
| Study name | Interpersonal psychotherapy for depression and post‐traumatic stress disorder among HIV‐positive women in Kisumu, Kenya: study protocol for a randomised controlled trial |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria 1. HIV+ women enrolled at Family AIDS Care Education and Services, Kisumu, Nyanza, Kenya (FACES‐Kisumu) 2. Diagnosis with depression (MDD, dysthymia, minor depression) and PTSD secondary to gender‐based violence (GBV) on the CIDI 3. Ability to attend weekly therapy sessions and repeated measures 4. Age ≥ 18 years 5. Ability to give informed consent. Exclusion criteria 1. Cognitive dysfunction requiring a higher level of care or compromising ability to participate in interpersonal therapy (IPT) 2. Severe thought or mood disorder symptoms requiring a higher level of care or interfering with ability to participate in IPT 3. Current drug and alcohol dependence requiring substance use treatment |
| Interventions |
Intervention Interpersonal psychotherapy Interpersonal psychotherapy is an evidence‐based structured, brief psychotherapy that focuses on improving relationships to improve mood and reduce anxiety Control Treatment as usual Usual clinic psychosocial treatment |
| Outcomes | Primary outcome measures 1. Depression, PTSD [Time Frame: Change from 0 to 12 weeks, 12 to 24 weeks, and 24 to 36 weeks] 2. Structured clinical interview: Composite International Diagnostic Interview (CIDI) Secondary outcome measure 1. ARV adherence [Time Frame: Change from 0 to 12 weeks, 12 to 24 weeks, and 24 to 36 weeks] Visual analogue scale self‐report 1. HIV viral load [Time Frame: 12 weeks, 24 weeks, 36 weeks] Other outcome measures 1. Cost analyses [Time Frame: Cost: 12 weeks; benefits (DALYS and productivity): Change from 0 to 12 weeks, 12 to 24 weeks, and 24 to 36 weeks] 2. Neurocognitive outcomes [Time Frame: Change from 0 to 12 weeks, 12 to 24 weeks, and 24 to 36 weeks] |
| Starting date | September 2015 |
| Contact information | Susan M Meffert, MD, MPH University of California, San Francisco |
| Notes | Completed 31 January 2019 |
PACTR201812802696820.
| Study name | Piloting WHO's parent skills training in a low‐income setting: a case study in Ethiopia |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria 1. Long‐term caring responsibility for a child aged 2 to 9 for whom a developmental disorder or delay (DD) has been identified 2. Living within easy travelling distance of training site 3. Able to attend all sessions 4. Sufficient contact time during the week with child with DD 5. Able to speak Amhari 6. Willing to give feedback to programme 7. ≥ 18 years of age 8. Consent Exclusion criteria 1. Child with DD is acutely disturbed and in need of specialist medical attention 2. Child with DD has severe visual or hearing impairment |
| Interventions |
Intervention Caregiver skills training Participants receive 9 weekly group sessions and 3 home visits. Programme lasts a total of 12 weeks. In each 3‐hour group session, caregivers are trained on strategies to engage their child in communication and play and promote adaptive behaviours and learning. Home visits are used for goal‐setting, evaluating progress, trouble‐shooting, and identifying possible support needs Control Wait‐list Treatment as usual |
| Outcomes |
Primary outcome 1. Caregiver child interaction Secondary outcomes 1. Caregiver knowledge and skills 2. Adapted Family Interview Schedule 3. Autism Treatment and Evaluation Checklist 4. Child Behaviour Checklist 5. Communication Profile‐Adapted 6. Patient Health Questionnaire‐9 (PHQ‐9) 7. PedsQL parent‐reported Family Impact module |
| Starting date | 9 December 2018 |
| Contact information | Rosa.Hoekstra@kcl.ac.uk |
| Notes | Recruiting |
Petersen 2018.
| Study name | Collaborative care for the detection and management of depression among adults with hypertension in South Africa: study protocol for the PRIME‐SA randomised controlled trial |
| Methods | Cluster‐randomised controlled trial |
| Participants |
Inclusion criteria 1. Twenty (20) largest clinics providing chronic care to patients 2. Receiving hypertensive treatment at time of enrolment 3. Depressive symptoms as indicated by total score ≥ 9 on PHQ‐9 4. Planning to reside in area for the next year 5. Capable of actively engaging in interviewer‐administered questionnaire at time of recruitment and 6 months and 12 months later 6. Written consent to participate in the study 7. Participants 18 to 80 years of age 8. Receiving treatment for hypertension 9. Screening positive for depression with total score ≥ 9 on the PHQ‐9 Exclusion criteria 1. Clinics that do not provide integrated chronic disease management 2. Small (< 10,000 attendances/year) 3. Mobile or satellite 4. Participated in piloting of intervention and data collection 5. Inability to meet the above inclusion criteria |
| Interventions |
Intervention PC101 + mental health facility‐based stepped care intervention combining stress and depression case detection and management by non‐physician clinicians and referral pathways for antidepressant medication and/or group/individual counselling delivered by lay health workers for patients with depression Control PC101 enhanced usual primary health care where non‐physician clinicians have been equipped with the basic skills to identify stress and depression/anxiety but with limited access to doctors authorised to prescribe antidepressant medication, and with no specific psychosocial interventions |
| Outcomes |
Primary outcome Reduction in PHQ‐9 score ≥ 50% compared with baseline evaluated at 6 months Secondary outcomes 1. Response at 12 months defined as 50% reduction in score on the PHQ‐9 2. Remission at 12 months defined as score < 5 on the PHQ‐9 3. Mean PHQ‐9 scores at 6 and 12 months 4. Antidepressant treatment, including initiation or intensification of antidepressant medication 5. Referral to a counsellor for depression counselling 6. Referral to a mental health specialist (clinical psychologist, psychiatrist, or secondary care services) 7. Blood pressure at 6 and 12 months 8. Disability measured at 12 months using the WHO Disability Assessment Schedule version 2.0 (WHODAS 2) (12‐item interviewer‐administered version) 9. Stress symptoms measured at 12 months using the Perceived Stress Scale (PSS) 10. Patient assessment of quality of chronic illness care received measured at 12 months using the Patient Assessment of Care for Chronic Conditions (PACIC) Scale (locally adapted 10‐item scale) 11. Health care utilisation including clinic visits and hospital admissions 12. Resource use and economic outcomes measured using a Service Use Questionnaire and 13. All‐cause mortality measured through follow‐up at the clinic and linkage to the South African population register |
| Starting date | 22 April 2015 |
| Contact information | peterseni@ukzn.ac.za |
| Notes | Completed 31 July 2017 |
Priebe 2019.
| Study name | A research study in Bosnia and Herzegovina to test an intervention called Volunteer Support, designed to improve care for people living in the community with severe mental illness |
| Methods | Randomised controlled trial |
| Participants | ISRCTN51290984 Inclusion criteria 1. Primary diagnosis of severe mental illness (ICD‐10 F2) 2. Aged ≥ 18 years 3. Capacity to provide informed consent 4. Score ≤ 5 on the MANSA Scale 5. Illness longer than 6 months Exclusion criteria 1. Does not meet inclusion criteria 2. Primary diagnosis of substance‐use disorder, learning disability, dementia, organic psychosis 3. Diagnosis of bipolar disorder 4. Inpatient at the time of recruitment 5. Participating in another study conducted by this or another research group |
| Interventions | Intervention group participants have 6 or 7 1‐to‐1 meetings doing joint social activities with an unpaid volunteer over 6 months and treatment as usual Control group participants receive treatment as usual over only 6 months |
| Outcomes |
Primary outcome measure Quality of life, measured using the Manchester Short Assessment of Quality of Life (MANSA) at baseline, 6 months (post intervention), and 12 months Secondary outcome measures 1. Symptoms, measured using the Brief Psychiatric Rating Scale (BPRS) at baseline, 6 and 12 months 2. Quality of life, measured using the Manchester Short Assessment of Quality of Life (MANSA) at baseline, 6 and 12 months 3. Objective social situation, measured using the Objective Social Outcomes Index (SIX) at baseline, 6 and 12 months 4. Service use, measured using adapted Client Service Receipt Inventory (CSRI) at baseline, 6 and 12 months 5. Self‐esteem, measured using the Self‐Esteem Rating Scale at baseline, 6 and 12 months 6. Stigma, measured using the Internalized Stigma of Mental Illness Inventory (ISMI) at baseline, 6 and 12 months |
| Starting date | 1/8/2017 |
| Contact information | f.vanloggerenberg@qmul.ac.uk |
| Notes | Planned to end 31/3/2021 |
Sangraula 2018.
| Study name | Protocol for a feasibility study of group‐based focused psychosocial support to improve the psychosocial well‐being and functioning of adults affected by humanitarian crises in Nepal: group Problem Management Plus (PM+) |
| Methods | Cluster‐RCT |
| Participants | Clusters were Village Development Committees in Sindhuli district, Nepal, which was impacted by 2 earthquakes in 2015 Inclusion criteria 1. ≥ 18 years of age 2. Score > 2 on General Health Questionnaire (dichotomous item scoring method) 3. Score > 16 on World Health Organization Disability Assessment Scale Exclusion criteria 1. Presence of a severe mental disorder (e.g. psychosis) 2. Alcohol use disorder (score > 16 on Alcohol Use Disorders Identification Test (AUDIT)) |
| Interventions |
Intervention Group problem management plus Five sessions of group low‐intensity psychological intervention including stress management, behavioural activation, problem‐solving, and strengthening social support Delivered by community‐based psychosocial workers (CPSWs) Control Enhanced treatment as usual Referral to primary healthcare workers trained in mental health Gap Action Programme |
| Outcomes |
Primary outcomes 1. Depression ‐ Patient Health Questionnaire [Time Frame: 1 week post‐intervention] 2. 9‐Item measure of depression symptoms, culturally and clinically validated in Nepal Secondary outcomes 1. Daily functioning ‐ World Health Organization Disability Assessment Scale [Time Frame: 1 week post‐intervention] a. 12‐Item assessment ability to engage in daily activities, previously used in numerous studies in Nepal (1) General psychological distress ‐ General Health Questionnaire [Time Frame: 1 week post‐intervention] b. 12‐Item measure of general psychological distress, previously validated for use in Nepal (1) Post‐traumatic stress disorder ‐ Post‐traumatic Stress Disorder Checklist [Time Frame: 1 week post‐intervention] c. 8‐Item measure of post‐traumatic stress symptoms validated for use in Nepal (1) Personalized measure of distress ‐ Psychological Outcome Profiles [Time Frame: 1 week post‐intervention] d. 3‐Item measure of personalised distress and problems, 4‐items post‐treatment (1) Culture‐specific general psychological distress ‐ Nepali Psychosocial and Mental Health Problems [Time Frame: 1 week post‐intervention] e. 5‐Item measure of somatic symptoms of psychosocial and mental health problems validated in Nepal (1) Reducing Tension Checklist for Problem Management Plus Skills [Time Frame: 1 week post‐intervention] f. 12‐Item measure of behavioural and psychosocial skills related to coping mechanisms |
| Starting date | 17 December 2017 |
| Contact information | sangraulamanaswi@gmail.com |
| Notes | Full trial is now ongoing |
Schulte 2020.
| Study name | Feasibility of alcohol screening and brief intervention in primary health care in Kazakhstan: study protocol of a pilot cluster randomised trial |
| Methods | Pilot cluster‐RCT |
| Participants | Individuals attending primary care clinics. Age between 18 and 69 years |
| Interventions |
Intervention Performed by primary care physicians trained in screening procedures and in motivational interviewing for 3 hours with a 1‐hour booster session by local research staff, which includes role‐play, activities, and a post‐training questionnaire. Participating individuals are screened with the AUDIT‐C, and a full AUDIT is done for comparison. Those identified with hazardous or harmful alcohol use identified on the AUDIT‐C (≥ 4 for women, ≥ 5 for men) receive face‐to‐face brief behavioural intervention to prevent the development of alcohol‐related problems including dependent drinking for 5 minutes. Alcohol‐dependent individuals (AUDIT ≥ 20) will be excluded and referred to a specialist. The remaining participants receive short verbal feedback based on their alcohol consumption and a leaflet reinforcing the benefits of low‐risk alcohol use Control Primary care physicians are trained in screening procedures but not in motivational interviewing. All patients receive simple feedback about their alcohol consumption and a leaflet |
| Outcomes | Feasibility and acceptance (among physicians) |
| Starting date | August 2018 |
| Contact information | u.verthein.at.uke.de |
| Notes | This study was expected to end in November 2019 |
Sharifi 2019.
| Study name | Collaborative care for child and youth mental health problems in a middle‐income country: study protocol for a randomised controlled trial training general practitioners |
| Methods | Cluster‐randomised controlled clinical trial |
| Participants | Clusters are general practitioners supported by community mental health centres in a collaborative care network, Tehran, Iran Participants are children and youths visiting general practitioners for non‐emergent medical, emotional, or behavioural concerns inclusion criteria 1. Age 5 to 15 years 2. Screening positive on Strengths and Difficulties Questionnaire (SDQ, score ≥ 17) Exclusion criteria 1. In pain 2. Acutely medically ill 3. Actively treated at community mental health centre 4. Do not speak Farsi |
| Interventions |
Intervention GPs are trained over 2.5 days to identify child and youth emotional and behavioural problems, engage families in care, provide first‐line interventions including psychoeducation, and refer to community mental health centre if needed or desired. A training manual is used. Role‐plays with standardised patients and case discussions are used during training Control GPs receive a 1‐day child/youth mental health refresher course that focuses on identification of child and youth emotional and behavioural problems and discussion with families about treatment options at community mental health centres. Training includes role‐plays with standardised patients Both arms GPs receive compensation for training and completing study instruments and incentive payments for successfully managed cases (monthly visits for the first 3 months, and follow‐up visits 3‐monthly for patients who are stable). Mental health centre staff provide feedback and telephone consultations. Control GPs are advised to refer for assessment and care, and intervention GPs are given advice on whether patients can be treated in primary care and on the recommended treatment |
| Outcomes |
Primary outcome 1. Total problems score on the SDQ Secondary outcomes 1. Functional status (functional assessment score on SDQ) 2. Parental mental health (General Health Questionnaire (GHQ)) 3. Parental functional status (EuroQol EQ‐5D‐5L) 4. Parent satisfaction with care 5. Use of treatment from GP and community mental health centre 6. Use of mental health or counselling services outside the network Other outcomes 1. Fidelity 2. Changes in GPs’ confidence in managing child/youth mental health problems 3. Changes in attitude (burden sub‐scale of Physicians’ Belief Scale) 4. Quality of care by GP |
| Starting date | 1 August 2017 |
| Contact information | lwissow@uw.edu |
| Notes | Trial registered as NCT03144739 Completed 30 September 2019 |
Shepherd 2020.
| Study name | Supervised treatment in outpatients for schizophrenia plus (STOPS+): protocol for a cluster‐randomised trial of a community‐based intervention to improve treatment adherence and reduce the treatment gap for schizophrenia in Pakistan |
| Methods | Cluster‐RCT |
| Participants | Clusters are primary healthcare centres Inclusion criteria 1. Diagnosis of schizophrenia or schizoaffective disorder 2. Age between 17 and 65 years 3. Not in remission 4. Capacity for and able to give informed consent 5. Family member willing to participate in trial and supervise treatment after training Exclusion criteria 1. Serious or unstable medical illness 2. Learning disability 3. Severe drug dependence requiring treatment or detoxification 4. Pregnant or breast‐feeding |
| Interventions |
Intervention STOPS+ ETAU Plus 1. Supervision by trained family member for dispensing and administering medication. Family member is trained by a multi‐purpose technician from each primary healthcare (PHC) centre 2. Treatment for schizophrenia at PHC by PHC physician under supervision of treating psychiatrist with the help of mobile smartphone technology 3. Monitoring the availability of essential psychotropic medication and side effects 4. Assessment of barriers and facilitators of intervention 5. Community engagement Control Enhanced treatment as usual (ETAU) Treatment received by patients’ in routine healthcare setting (e.g. hospital outpatient psychiatric clinic); brief counselling about treatment and outcome of disorder; PHC physicians trained in mhGAP; regular and reliable provision of psychotropic drugs at the PHC, which patients can access for medication if needed; PHC physician may liaise with treating physician |
| Outcomes |
Primary outcomes 1. Level of functioning (Global Assessment of Functioning Scale) 2. Adherence to treatment regimen Secondary outcomes 1. Mental state and psychiatric symptoms (Brief Psychiatric Rating Scale) 2. Caregivers’ burden (Family Burden Scale) 3. Perceived stigma (Internalised Stigma of Mental Illness) 4. Side effects of psychotropic medication (Glasgow Antipsychotic Side‐effects Scale) 5. Cost of care (Client Service Receipt Inventory) 6. Quality of life and cost‐effectiveness (EuroQol EQ‐5D) 7. Illness severity (Clinical Global Impression Scale) 8. Drug use (DAST Drug Screening Tool) 9. Depression (Patient Health Questionnaire) 10. Suicide Ideation (Suicide Behaviours Questionnaire Revised) |
| Starting date | 1 September 2019 |
| Contact information | s.farooq@keele.ac.uk |
| Notes | Recruitment ended 31 August 2020 |
Sijbrandij 2016.
| Study name | Problem management plus (PM+) in the treatment of common mental disorders in women affected by gender‐based violence and urban adversity in Kenya |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria 1. Adult ≥ 18 years or older 2. Female 3. Score > 2 on a screening questionnaire for psychological distress (General Health Questionnaire‐12; GHQ‐12) and 4. Score > 16 on a screening questionnaire for functional impairments (WHO Disability Assessment Schedule 2.0; WHODAS) Exclusion criteria 1. Male gender 2. Acute medical condition 3. Risk of suicide 4. Severe mental disorder or cognitive impairment (e.g. severe intellectual disability, dementia, psychosis) |
| Interventions |
Intervention Problem Management Plus Therapy is administered once weekly over 5 weeks on an individual 60‐minute basis. Problem Management Plus includes skills in identifying emotional and practical problems that can be managed and strategies to reduce and cope with these problems. Attendance at sessions will be monitored via session attendance records. Adherence to strategies will be monitored by checklists of strategies employed. The study for any participant will conclude after immediate post‐trial follow‐up assessment, resulting in participation duration of 1.5 months. Therapy is provided by local health workers Control Enhanced treatment as usual comprises normal treatment provided by local primary care providers (usually nurses) who have received training in supportive counselling and psychological first aid. Treatment as usual involves non‐directive counselling about daily problems reported by participants, as well as provision of basic education about common psychological problems. Attendance at sessions will be monitored via session attendance records. Adherence to strategies will be monitored by checklists of strategies employed. The study for any participant will conclude after immediate post‐trial follow‐up assessment, resulting in participation duration of 1.5 months |
| Outcomes |
Primary outcome Level of psychological distress as measured with the GHQ‐12 at 3‐month follow‐up (i.e. 3 months after the fifth PM+ session) Secondary outcomes 1. General functioning as measured with the WHODAS 2. Post‐traumatic stress disorder (PTSD) symptoms will be measured using the PCL‐5, which is a 20‐item checklist corresponding with the 20 DSM‐5 PTSD symptoms. Items are rated on a 0 to 4 scale and add up to a total severity score of 80. The PCL‐5 will be adapted to ask for symptoms in the last week (rather than month) to enhance sensitivity to change. PSYCHLOPS will be administered at all assessments and at the beginning of each PM+ session to assess progress on problems for which the person seeks help. It consists of 4 questions that encompass 3 domains: problems (2 questions), functioning (1 question), and well‐being (1 question). Participants are asked to give free text responses to the problem and function domains. Responses are scored on an ordinal 6‐point scale, producing a maximum score of 20 (5 points per question). The PSYCHLOPs version administered at post‐treatment and at follow‐up also includes an overall valuation question (determining self‐rated outcome ranging from “much better” to “much worse”). PSYCHLOPS has been validated in primary care populations across several countries Other measures 1. Previous stressor exposure will be assessed using the LEC. This is a widely used list of 17 experienced or witnessed events, such as rape, serious injury, combat exposure, or the sudden death of a loved one. A Kiswahili version of this list is available. At post‐test, question phrasing will be adapted to capture life events that have occurred since the trial commenced Five key questions of the Swahili version of the WHOVAW as developed for use in the WHO Multi‐Country Study on Women’s Health and Domestic Violence are administered. Indicators of economic impact will be assessed using the WHODAS question on days out of role and by selected SRI questions on healthcare service use Process evaluation 1. Feasibility and difficulties and successes in carrying out research and intervention activities will be explored through semi‐structured interviews with 20 key informants, including 5 CHWs 2. The burden of completing assessments and PM+ on the time and effort of participants, satisfaction with the intervention, and barriers and facilitators to adherence will be explored through semi‐structured interviews with a sample of 5 participants (including participants that have dropped out) 3. In addition, 5 decision‐makers with responsibilities for developing or implementing health policy, including heads of relevant clinics, and 5 PHC staff (clinical officers/nurses) will be interviewed to obtain their perceptions of the benefits and challenges of integrating PM+ into the CHW routine services provision |
| Starting date | 15/4/2015 |
| Contact information | r.bryant@unsw.edu.au |
| Notes | Trial registration: ACTRN12616000032459 |
SLCTR/2018/008.
| Study name | Integrating mental health into primary care for post‐conflict populations in Northern Sri Lanka |
| Methods | Cluster‐randomised trial Multi‐centre stepped wedge cluster‐randomised trial |
| Participants |
Inclusion criteria Primary care practitioners (PCPs) 1. Full registration with the Sri Lankan Medical Council 2. ≥ 6 months until the next transfer rotation (this applies only for PCPs working in government facilities) or 6 months to retirement Public health personnel (PHP) 1. Nurse, attendant, or health service assistant within each facility 2. ≥ 6 months left on transfer rotation (this applies only for PHPs working in government facilities) or 6 months to retirement Community representatives (CRs) 1. Located within the catchment area of each selected facility Patients 1. ≥ 18 years of age or older 2. Attending selected facilities 3. Belonging to internally displaced persons (IDPs) or post‐conflict populations Exclusion criteria 1. Primary care practitioners 2. Public health professionals and community representatives if they have secondary mental health training 3. Patients if they are younger than 18 years of age or diagnosed with mental disorders other than depression and/or anxiety |
| Interventions |
Intervention 1. Primary care practitioners: primary care practitioners (PCPs) at randomly selected facilities will be monitored for 1 month prior to commencement of training. They will receive 3 days of training, 6 hours a day, 1‐on‐1 training using locally adapted WHO mhGAP intervention guide. Once training is completed, PCPs will use the mhGAP guide to diagnose, treat, and refer primary care patients with possible mental health problems 2. Public health professionals (PHPs): 2 days of training in groups of 3 or 6 hours a day using locally adapted educational material on mental health awareness and stigma reduction. Once training is completed, PHPs will engage in specific activities outlined during their training, with the patient population attending their respective facilities 3. Community representatives (CRs): 1 day of training in groups of 2 or more, 6 hours total, using locally adapted educational material on mental health awareness and stigma reduction. Community representatives (CR) will not be randomised. CRs within the catchment area of each randomly selected primary care facility will be identified and approached for inclusion. Once training is completed, CRs will engage in specific activities outlined in their training within their respective communities, such as creating awareness, finding local resources, and organising mental health promotional activities 4. Patients attending primary care facilities who are identified by mhGAP‐trained primary care practitioners will be asked to answer a brief sociodemographic questionnaire at point of recruitment along with the Hopkins Symptoms Checklist (for depression and anxiety) at point of recruitment and at 3 and 6 months of follow‐up. They will also be re‐examined by a psychiatrist to confirm the primary care practitioner diagnosis 5. All training interventions will be based on the World Health Organization mhGAP 2.0 training module. Key modules informed by previous work on depression, stress‐related disorders, medically unexplained symptoms, alcohol/drug use disorder, and others will be used. All materials have been locally translated and adapted 6. After training is completed, PCPs, PHPs, and CRs will be monitored for a period of 1 month Pre‐ and post‐knowledge tests and refresher courses will be carried out at 3 and 6 months after initial training Control PCPs, PHPs, and CRs who have not yet received training, and their patients, will act as controls and will continue treatment as usual |
| Outcomes |
Primary outcome Successful uptake of mhGAP 2.0 training measured using minimum 40% concurrence increase of primary care practitioners who correctly diagnose patients with common mental disorders as verified by specialist psychiatrists Secondary outcome Successful integration of mental health services into primary care measured by reaching a 40% rate of mhGAP 2.0 trained primary care practitioners in the Northern Province at 1 year |
| Starting date | 14/3/2018 |
| Contact information | shannon.doherty@anglia.ac.uk |
| Notes |
Trial registration SLCTR/2018/008 ISRCTN62598070 |
Srinivasan 2018.
| Study name | Improving mental health through integration with primary care in rural Karnataka: study protocol of a cluster‐randomised control trial |
| Methods | Multi‐level cluster‐randomised controlled trial |
| Participants | Patients with depression or anxiety and comorbid diabetes or cardiovascular disease |
| Interventions |
Intervention Collaborative care. PHC staff are trained in mental health diagnosis, treatment, and the collaborative care model. Involves community‐based “Healthy Living groups” that use cognitive‐behavioural strategies to promote healthy behaviour Control Enhanced standard care. Includes referrals for mental health needs |
| Outcomes |
Primary outcomes 1. Severity of common mental disorder – GAD7 (anxiety) 2. PHQ‐9 (depression) Secondary outcomes 1. Diabetes 2. Cardiovascular risk 3. Staff knowledge 4. Patient perception 5. Kessler‐10 for screening and MINI to confirm diagnosis (for recruitment) Not stated primary or secondary 1. Fagerstrom Test for Nicotine Dependence (FTND) and FTND‐Smokeless Tobacco 2. Alcohol Use Disorders Identification Test (AUDIT) 3. Food Frequency 4. International Physical Activity (IPAQ) 5. Medication adherence (VAS) 6. Diabetes self‐management questionnaire 7. World Health Organization (WHO) Disability Assessment Schedule 2.0 8. Social support 9. WHO Quality of Life (WHOQOL‐BREF) 10. Integrated Stigma for Mental Illness Scale (ISMI) |
| Starting date | 8 December 2014 |
| Contact information | maria.ekstrand@ucsf.edu |
| Notes | Completed 9 June 2020 Trial registry information: CTRI/2018/04/013001; NCT02310932 |
Surkan 2020.
| Study name | Happy Mother‐Healthy Baby: an anxiety‐focused early prenatal intervention for the prevention of common mental disorders in Pakistan |
| Methods | Randomised controlled trial |
| Participants | Setting: Holy Family Hospital, Pakistan Inclusion criteria 1. Ability to understand spoken Urdu 2. Pregnant, ≤ 22 weeks' gestation 3. Age ≥ 18 years 4. Residence ≤ 20 km of Holy Family Hospital 5. intent to reside in study areas until completion of the study 6. Score ≥ 8 for anxiety on the Hospital Anxiety and Depression Scale (HADS) Exclusion criteria 1. Current major depressive episode (MDE on SCID) or life‐threatening health conditions including e.g. active severe depression or suicidal ideation 2. Self‐report of past or current significant learning disability 3. Self‐report of past or current psychiatric disorder (e.g. bipolar disorder, schizophrenia) or psychiatric care (e.g. current use of anxiolytic drug and/or other psychotropic drug) 4. Medical disorder or severe maternal morbidity requiring inpatient management that would preclude participation 5. ICU admission indicated by diagnosis (not only for assessment) |
| Interventions |
Intervention Happy Mother‐Healthy Baby (HMHB) HMHB is a cognitive‐behavioural therapy‐based facility‐based psychosocial intervention with 6 core and 6 booster sessions delivered by non‐specialist providers, aimed at raising psychosocial awareness; facilitating positive change, interpersonal well‐being, social support, and bonding with baby during pregnancy; employing empathetic listening, behaviour activation, problem management, and family involvement; and addressing relapse prevention, planning for baby, and managing emotional challenges in the postnatal period. Family member(s) are invited to attend 3 core sessions Control Enhanced usual care 8 antenatal visits depending on gestational week; hospital staff trained in mental health treatment and counselling; transportation to appointments; medically indicated ultrasound |
| Outcomes |
Primary outcome Combined Common Mental Disorder (through Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders) Secondary outcomes 1. Preterm birth (< 37 weeks' gestation) 2. Small‐for‐gestational‐age (10th percentile for gestational age) 3. Low birth weight (< 2500 g) |
| Starting date | 16 April 2019 |
| Contact information | psurkan@jhu.edu |
| Notes | Estimated completion date: 30 June 2022 |
Tol 2017.
| Study name | Nguvu: a randomised controlled trial of an integrated intervention to reduce intimate partner violence and psychological distress in adult, female Congolese refugees in Tanzania |
| Methods | Cluster‐randomised pilot trial |
| Participants | Clusters are local women’s groups in Nyarugusu, a refugee camp in northwest Tanzania in the Kasulu district, Kigoma Province, hosting over 60,000 Congolese refugees Inclusion criteria 1. ≥ 18 years of age 2. Female 3. Refugee from the Democratic Republic of the Congo (DRC) currently residing in Nyarugusu Refugee Camp, Tanzania 4. Participating in local women’s groups 5. Past‐year's history of intimate partner violence: endorsing any past‐year intimate partner violence on a modified version of the Abuse Assessment Screen (AAS) 6. Experiencing moderate to severe psychological distress: average score moderate to severe (≥ 1.75 out of 4) on items assessing depressive or anxiety symptoms; or ≥ 1.0 out of 3 on items assessing post‐traumatic stress symptoms. Depressive and anxiety symptoms were measured using the 25‐item Hopkins Symptom Checklist (HSCL). Post‐traumatic stress symptoms were measured using the 16‐item Harvard Trauma Questionnaire (HTQ) Exclusion criteria 1. Serious mental illness 2. Substance use disorder 3. Imminent risk of suicide |
| Interventions |
Intervention (Nguvu) 8‐week programme that integrates advocacy and empowerment counselling with cognitive processing therapy. Weekly sessions (1 individual session followed by 7 group sessions). Sessions 1 and 8 are advocacy sessions. Sessions 2 through 7 are cognitive processing therapy sessions. Intervention will be delivered by trained lay facilitators ‐ a single facilitator delivers individual sessions, and a pair of facilitators delivers group sessions. Facilitators will be supported by a psychologist onsite and will be supervised by telephone or in‐person weekly Comparison Participants will receive standard mental health and protection services during the trial period provided by the International Rescue Committee in Nyarugusu camp and the Tanzania Red Cross |
| Outcomes | Participants in both groups are followed up after 9 and 20 weeks Primary outcome measures 1. Relevance of the intervention and research protocols 1.1. Prevalence of psychological distress as a priority 1.2. Validity and reliability of mental health, violence, and functioning outcome measures 2. Acceptability of the intervention and research protocols 2.1. Participant retention in intervention sessions and research interviews 2.2. Serious adverse events related to study participation 2.3. Safety and ethical concerns 3. Feasibility of the intervention and research protocols 3.1. Fidelity of intervention implementation 3.2. Recruitment 3.3. Balanced randomisation 3.4. Implementation challenges 3.5.Research protocol deviations Secondary outcome measures 1. Recurrence of intimate partner violence as measured using the Domestic Violence Module of the Demographic and Health Survey at baseline and post‐treatment assessments 2. Psychological distress symptoms as measured using the 25‐item Hopkins Symptom Checklist (HSCL‐25) and PTSD symptom items in the Harvard Trauma Questionnaire at baseline and post‐treatment assessments 3. Functional impairment as measured using 12 items developed from qualitative data at baseline and post‐treatment assessments |
| Starting date | 1/01/2014 |
| Contact information | wtol@jhu.edu |
| Notes |
Wagner 2019.
| Study name | Maternal depression treatment in HIV (M‐DEPTH): study protocol for a cluster‐randomised controlled trial |
| Methods | Cluster‐randomised controlled trial |
| Participants | 8 antenatal clinics in Uganda will be randomised Inclusion criteria 1. Pregnant up to 24 weeks' gestation, attending antenatal clinic 2. HIV positive, on ART for ≥ 4 weeks 3. Age ≥ 15 years 4. Patient Health Questionnaire (PHQ‐9) > 4 Exclusion criteria 1. Unstable health (about to start ART or on ART < 4 weeks; active, untreated opportunistic infection) |
| Interventions |
Intervention Maternal depression treatment in HIV (M‐DEPTH) Stepped care approach. Usual care, problem‐solving therapy through manualised individual counselling sessions by trained peer mothers and antidepressant treatment (fluoxetine, imipramine, or amitriptyline) for severe or refractory depression after the first trimester prescribed by midwife nurses and supervised by the doctor‐in‐charge. Participants with clinical depression (defined as PHQ‐9 > 9) will be offered problem‐solving therapy (PST) or antidepressant therapy (ADT), but those with moderate to moderately severe depression will be recommended PST, and those with severe depression will be recommended ADT. Participants with sub‐threshold depressive symptoms (PHQ‐9 5 to 9) will receive depression psychoeducation and continued depressive monitoring. Treatment is discontinued if symptoms are in remission (PHQ‐0 < 5) for 6 months and patient is at least 6 months past completion of pregnancy. Supervision of peer mothers and midwife nurses will be conducted 1‐on‐1 and in groups, and will be weekly for the first 2 months, bi‐weekly for the next 4 months, and monthly thereafter. Supervisors are available 24/7 for suicide and emergency consultations Control Usual care Referral to mental health specialist at district or regional hospital for care of depression. Access to the Family Support Group programme for care of HIV in pregnancy, including psychosocial support, instruction and education to support pregnancy, and postpartum care including PMTCT adherence through 24 two‐hour monthly group sessions. Pill counts and adherence counselling as needed |
| Outcomes |
Primary outcomes 1. Rate of maternal HIV viral suppression 1 month post pregnancy 2. Mean maternal antiretroviral adherence from study enrolment to 1 month post pregnancy 3. Rate of prevention of mother‐to‐child transmission care retention through study completion 4. Rate of delivery in health facility 1 month post pregnancy 5. Rate of infant use of ART in first 6 weeks of life 6. Rate of universal infant feeding in first 6 months of life 7. Rate of complete infant HIV testing 18 months after birth Secondary outcomes 1. Pregnancy outcome 2. Inventory of functional status after childbirth self‐report 3. New baby sub‐scale of the Postpartum Adjustment Questionnaire 4. Child’s weight, height 5. Ages and Stages Questionnaire 6. Child HIV status at month 18 7. PHQ‐9 8. Rate of depression alleviation or response to depression treatment (PHQ‐9 < 5) 9. Depression treatment uptake and attendance 10. Other mental health services received Others 1. Process evaluation 2. Provider experience 3. Patient experience |
| Starting date | 8 July 2019 |
| Contact information | gwagner@rand.org |
| Notes | Estimated completion date: December 2022 Registered at clinicaltrials.gov as NCT03892915 |
CBA: controlled before‐and‐after; HIV: human immunodeficiency virus; HSCL: Hopkins Symptom Checklist; MINI: Mini International Neuropsychiatry Interview; NSHW: non‐specialist health worker; RCT: randomised controlled trial; STI: sexually transmitted infection.
Differences between protocol and review
Differences between the 2013 review, the 2019 protocol to this review, and this final update
Three review authors (Nadja van Ginneken, Simon Lewin, and Paul Garner) were keen to ensure that this review update should be as relevant and valuable for implementers and policy makers as possible.
We therefore performed the following.
A group face‐to‐face consultation with seven LMIC clinicians who are mature students/masters students or PhD students at the Liverpool School of Tropical Medicine (December 2018).
An online consultation with seven implementers, academics, and policy makers from LMICs, and four written answers from further stakeholders by email (Feb to April 2019).
An updated literature review of mental health terminology and description.
The overall message that emanated from this was to broaden the review to include preventative strategies, in line with the Lancet Commission Mental Illness re‐framing document (Patel 2018). This provides an explanation that mental ill health is on a continuum from at‐risk, to distress, to sub‐syndromal symptoms, to actual classifiable disorders.
However it is clear from the literature that interventions still fall broadly into two categories: those focused on prevention and mental health promotions, and those aiming to address various forms of psychological distress and diagnostic (and transdiagnostic) categories.
This Cochrane Review Update on treatment interventions for mental disorders is therefore being re‐framed to include the spectrum of mental ill health as broader than just diagnostic categories (this was already partly included in the previous version of the review but was not made explicit). The prevention/promotion review features in a separate protocol (Purgato 2021). The comparison of review aims and outcomes can be found in Figure 2.
Other amendments
Authors
We have changed many authors compared to the previous review ‐ van Ginneken 2013 ‐ due to previous author unavailability and due to the large scope of this review and the need for involvement of more people. Review authors were chosen from those with expertise in different areas of mental health care research in LMICs (and for some, from and living in LMICs) and according to their interest in mental health. We also worked with authors who will be taking over leadership of the parallel prevention review (Purgato 2021).
Terminology of health workers
We received feedback in our consultations that the terms non‐specialist health workers (NSHWs) and other professionals with health roles (OPHRs), used in the previous version of this review to describe the types of included mental health care providers (van Ginneken 2013), are difficult to understand. We therefore used the following terms in this update, also taking into account that it was easier to have an overarching term for both clinical and non‐clinical workers: primary‐level workers (PWs). We subdivided these into lay health workers (LHWs), community professionals (CPs), and primary health professionals (PHPs). See the main text, Figure 1, and Table 16 for definitions.
Methods
Population ‐ mental disorders and distress
As mentioned above, the mental disorders and distress included in this review will therefore include any mental illness symptoms and expressions of distress, not just mental disorders, in line with the transdiagnostic approach.
Due to now having a parallel review on mental prevention and promotion of mental health (Purgato 2021), we have moved some of the previously included studies to the prevention review (Shin 2011 and Baker Henningham 2015 ‐ in excluded studies).
Also we have removed ‘neurological disorders’ from this review (thus Li 2009 epilepsy was excluded) but we have kept substance abuse, as treatment needs for neurological disorders are likely to be different, with more pharmacological treatments. For this update, we focus on the spectrum of mental disorders and distress seen frequently in primary care. We have updated the ICD‐10 codes to the ICD‐11 codes in the table of definitions of mental disorders (Table 16).
Setting ‐ definition of LMICs
The definition of LMICs was refined to include the World Bank historical criteria, to be more consistent about what was and was not included as an LMIC. This meant that some interventions were re‐classified and excluded (e.g. those from HongKong, Taiwan (Chen 2000 and Tiwari 2010), were previously included as they were considered to be in China, but they were stand‐alone economies in the World Bank classification and are considered HICs).
Interventions
We make clear that the intervention does not include trials that only compare training competencies or methods, which do not have any aim of assessing patient outcomes.
We wish to specify that we have included only studies that have one of our primary outcomes and not only those with our secondary outcomes.
Outcomes
Outcomes have been adjusted to first make health service utilisation a primary outcome (due to relevance for policy and practice, as recommended by the stakeholders we consulted). We had originally in the 2019 protocol to this review update ‐ van Ginneken 2019 ‐ changed the prevalence outcome to a recovery outcome. However during analysis, we realised a lot of the older papers had prevalence data that could not be transformed to recovery data, principally due to sub‐syndromal symptoms. In the 2013 review, we had also transformed some continuous outcome data into prevalence data (e.g. Rojas 2007), but on looking through studies this time, we found this was very subjective and thus we could not identify the cutoff for having depression. Although it is possible to convert continuous to dichotomous data, because we used these continuous data in symptom severity outcomes, we decided not to transform continuous data to dichotomous data for this review.
In addition, compared to the 2013 review (van Ginneken 2013), we have simplified secondary outcomes to include direct and indirect costs to patients and health services. We have elaborated on what is included within these costs by defining opportunity costs better as also including effects on patient employment, income, retention, etc. (see below). Compared to the 2019 protocol to this review (van Ginneken 2019), we have further refined how we present data for these by specifying which costs and cost‐effectiveness data are presented in these reviews, along with which are resource use and societal costs (see amended section under secondary outcomes in methods).
Comparison groups
We amended the 2019 protocol ‐ van Ginneken 2019 ‐ to try to divide the various comparison groups due to the heterogeneity of availability of ‘usual care’ (i.e. into trained versus untrained PWs, no care, usual care, enhanced care). However when it came to sub‐grouping studies, we realised that these comparison groups were not always so obvious to tease out. We therefore combined them with no care, broadened the definition, and simplified the comparison categories to usual care (with combination with no care and enhanced care) (see definitions and methods above). Although we intended to subgroup the different comparison groups, we found too few studies to make these subgroups meaningful.
Study design
We decided based on the 2013 review findings that the studies that were not RCTs did not contribute any additional information to the review. Therefore we decided to exclude CBAs, NRCTs, and ITSs (i.e. the following studies were excluded for being CBAs: Bass 2012, Brown 2009, Loughry 2006 CBA, Lyketsos 1999, Paranthanam 2010, Scholte 2011, Thabet 2005, Wolmer 2005, and Zambori 2002, in van Ginneken 2013).
Foreign language papers
We have been more specific about our management of double data extraction with foreign language papers, as we have included several foreign language papers in this review (van Ginneken 2013 included only one). Foreign language reviewers extracted and translated RoB comments/justifications and sent them to a second reviewer in English (withholding their own assessment of risk) so that the second reviewer could then make his or her own judgement. The foreign language reviewer also sent a translated legend of anything in the outcomes tables that was not clear without translation. Occasionally, some papers necessitated more extensive translation (e.g. if the full paper was around costs) so the health economists could extract the data. The foreign language reviewer then reviewed each paper verbally over the phone with the second reviewer to ensure concordance and to check the accuracy of extraction.
Time points
We grouped outcomes into three sets of time points to indicate the stability of remission.
Post intervention (0 to 1 month after intervention) (to detect illness remission = immediate remission/immediate symptom reduction of the intervention).
1 month to 6 months post intervention (to detect sustained remission/sustained symptom reduction).
7 to 24 months post intervention (which indicates medium‐ to long‐term remission, I.e. avoidance of recurrence and chronicity or long‐term symptom reduction) (with subgroup for the 1‐ to 2‐year ones if needed).
This differs from the previous review ‐ van Ginneken 2013 ‐ 0 to 2 months, 4 to 6 months, and 8 to 12 months ‐ as we wanted to capture better the difference between post intervention and remission and to include a measure of long‐term outcomes too.
A recent literature review summarises that the duration criteria for declaring remission and recovery seem unnecessary. Depressive remission can be defined as the asymptomatic state after a depressive episode, without any duration criterion applied. Stability of remission is then relatively low on the first day but increases gradually with duration. The term 'recovery' is then used as a concept that would consist of more than absence of symptoms and would also include better social functioning or subjective well‐being, possibly including the absence of significant treatment, as this would better fit the concept of recovery from a patient’s perspective (de Zwart 2019).
This review does not attempt to present illness recovery outcomes as one outcome, although individual studies will include some of the information pertaining to illness recovery (such as social functioning).
Search methods
Electronic databases searched were identical to those searched for the 2013 review, although EPOC search strategies now are not run through Science Citation Index and Social Sciences Citation Index, ISI Web of Knowledge, as they pick up very little if any new information. LILACS was not searched separately, as it is now included in the WHO Global Health Library search.
Trial registries searched this time were the Clinical Trials Register (clinicaltrials.gov) instead of the metaRegister of Controlled Trials, as this latter register has now been pooled with the former.
Subgroup analyses
In the 2013 review and in the 2019 protocol, we suggested that we would do subgroup analyses (1) by category of health worker (health professionals (PHPs): e.g. doctors, nurses; community non‐health professionals (CPs): e.g. social workers, teachers; and non‐professionals (LHWs); (2) by type of community intervention (e.g. collaborative versus psychological interventions); and (3) by setting (e.g. government versus non‐government). However health worker categories and community interventions became planned as the main analyses of our comparisons due to a sufficient number of studies for these groups, and because we believed this would be most relevant to policy makers. We dropped analyses by setting, as it was difficult to disentangle this information within most of the studies.
Assessment of risk of bias
We simplified the detection bias to consist of just one category, whereas in the 2013 review (van Ginneken 2013), we had added two extra categories for risk of bias assessment (i.e. detection bias that had been divided into assessing subjective and objective outcomes was assessed blindly). In the 2019 protocol (van Ginneken 2019), we had removed blinding of outcome assessment, but at analysis stage, we re‐integrated this. We have retained the split of attrition bias that we used in the last review and have kept it divided into how incomplete or not two types of outcomes are: efficacy outcomes and safety outcomes (e.g. adverse events).
Consensus on Health Economic Criteria (CHEC) list criteria: this was adapted in the last review and was retained, with more questions: (1) Was there a comparison between two more groups receiving different interventions? (2) Is the perspective/viewpoint** of the analysis explicitly stated? If yes, give details. (3) Are costs measured? If yes, give details of costs measured. (4). Were outcomes measured? If yes, give details of outcomes measured. and (5) Were sensitivity analyses undertaken? If yes, give details on the forms of sensitivity analysis. We included a total of 24 questions (Appendix 2).
GRADE
Since the 2013 review, Cochrane has requested greater rigour in assessing the certainty of evidence. Therefore our GRADE assessments are stricter in this review (most results were low as opposed to moderate in the 2013 review) and apply the more stringent criteria for assessment (Guyatt 2011). For example, this has affected how we judged the dementia outcomes, even though no additional evidence has contributed to this since the last review.
Contributions of authors
NvG, WYC, SL, and PG contributed to development of the protocol. All review authors (except SL) extracted data and assessed risk of bias. NvG, WYC, and YCL checked these and resolved conflicts, also with SL when needed. NvG, WYC, and YCL performed GRADE assessments, checked by NH, LYC, and SL. NH and LYC performed the statistical analysis with help from UMA and RS for statistical queries and re‐extraction/checking. NvG, WYC, and YCL co‐wrote the review. UMA performed extraction, analysis, and writing of all economic components, assisted by NvG and SL. HSF helped with aspects of economic risk of bias assessment, checking, and referencing. All review authors commented on and approved the final version.
Sources of support
Internal sources
No sources of support provided
External sources
-
Norwegian Agency for Development Cooperation (Norad), Norway
Funding through the Norwegian EPOC satellite for support from Cochrane Response for meta‐analysis and other analysis/grading
-
Indian Council of Medical Research, India
Funding for the Prof. BV Moses & ICMR Centre for Advanced Research and Training in Evidence‐Informed Healthcare, which hosts the South Asian Cochrane Network & Centre (previous version of review)
-
Wellcome Trust, UK
Clinical PhD Fellowship awarded to NvG (previous version of review)
-
Wellcome Trust, UK
Senior Research Fellowship awarded to VP
-
UKaid (Department of International Development), UK
Funding for the South Asian Cochrane Centre via a grant‐in‐aid of developing countries through the Effective Health Care Research Consortium led by the Liverpool School of Tropical Medicine (Paul Garner). PT is a programme partner of this Consortium (previous version of review)
-
Foreign, Commonwealth and Development Office, UK
Project number 300342‐104
Declarations of interest
YCL, AURS, US, MP, AR, EU, SM, HSF, ATPLR, AB, LYC: none known
NvG: honorarium for undertaking the review (WHO). I work as a general practitioner
WYC: Sub‐contracted to contribute to the review (The University of Liverpool)
NH: Since 2016 I have been employed by Cochrane Response, an evidence consultancy initiative from Cochrane. Cochrane Response was commissioned by the Norwegian EPOC satellite and the Cochrane CET to perform tasks on this review.
SL: Joint Coordinating Editor for the Cochrane EPOC Group; was not involved in the editorial process for this review. My time is partly funded by the Norwegian Agency for Development Cooperation (NORAD)
These authors contributed equally to this work
These authors contributed equally to this work
These authors contributed equally to this work
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Abas 2018 {published data only}
- Abas M, Nyamayaro P, Bere T, Saruchera E, Mothobi N, Simms V, et al. Feasibility and acceptability of a task-shifted intervention to enhance adherence to HIV medication and Improve depression in people living with HIV in Zimbabwe, a low income country in sub-Saharan Africa. AIDS and Behavior 2018;22(1):86-101. [DOI: 10.1007/s10461-016-1659-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bere T, Nyamayaro P, Magidson JF, Chibanda D, Chingono A, Munjoma R, et al. Cultural adaptation of a cognitive-behavioural intervention to improve adherence to antiretroviral therapy among people living with HIV/AIDS in Zimbabwe: Nzira Itsva. Journal of Health Psychology 2017;22(10):1265-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chibanda D, Verhey R, Gibson LJ, Munetsi E, Machando D, Rusakaniko S, et al. Validation of screening tools for depression and anxiety disorders in a primary care population with high HIV prevalence in Zimbabwe. Journal of Affective Disorders 2016;198:50-5. [DOI] [PubMed] [Google Scholar]
- PACTR201511001150307. TENDAI STUDY: treatment to reduce depression and improve adherence to anti-retroviral therapy in Harare, Zimbabwe. http://www.who.int/trialsearch/Trial2.aspx?TrialID=PACTR201511001150307; 2015.
Adewuya 2019 {published data only}
- Adewuya AO, Ola BA, Coker O, Atilola O, Fasawe A, Ajomale T. A stepped care intervention for non-specialist health workers' management of depression in the Mental Health in Primary Care (MeHPriC) project, Lagos, Nigeria: a cluster randomised controlled trial. General Hospital Psychiatry 2019;60:76-82. [DOI: 10.1016/j.genhosppsych.2019.07.012] [DOI] [PubMed] [Google Scholar]
- ISRCTN66243738. Management of depression by primary care health workers in Lagos, Nigeria. http://www.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN66243738; 2017.
Ali 2003 {published data only}
- Ali BS, Rahbar MH, Naeem S. The effectiveness of counselling on anxiety and depression by minimally trained counsellors: a randomised controlled trial. American Journal of Psychotherapy 2003;57(3):324-36. [DOI] [PubMed] [Google Scholar]
- Gul A, Ali BS. The onset and duration of benefit from counselling by minimally trained counsellors on anxiety and depression in women. Journal of the Pakistan Medical Association 2004;54(11):549-52. [PubMed] [Google Scholar]
Araya 2003 {published data only}
- Araya R, Flynn T, Rojas G, Fritsch R, Simon G. Cost-effectiveness of a primary care treatment program for depression in low-income women in Santiago, Chile. American Journal of Psychiatry 2006;163(8):1379-87. [DOI] [PubMed] [Google Scholar]
- Araya R, Rojas G, Fritsch R, Gaete J, Rojas M, Simon G, et al. Treating depression in primary care in low-income women in Santiago, Chile: a randomised controlled trial. Lancet 2003;361(9362):995-1000. [DOI] [PubMed] [Google Scholar]
Arjadi 2018 {published data only}
- Arjadi R, Nauta MH, Scholte WF, Hollon SD, Chowdhary N, Suryani AO, et al. Internet-based behavioural activation with lay counsellor support versus online minimal psychoeducation without support for treatment of depression: a randomised controlled trial in Indonesia. Lancet Psychiatry 2018;5(9):707-16. [DOI: 10.1016/S2215-0366(18)30223-2] [DOI] [PubMed] [Google Scholar]
- Arjadi R, Nauta MH, Scholte WF, Hollon SD, Chowdhary N, Suryani AO, et al. Guided Act and Feel Indonesia (GAF-ID) - Internet-based behavioral activation intervention for depression in Indonesia: study protocol for a randomized controlled trial. Trials [electronic resource] 2016;17(1):455. [DOI: 10.1186/s13063-016-1577-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ayoughi 2012 {published data only}
- Ayoughi S, Missmahl I, Weierstall R, Elbert T. Provision of mental health services in resource-poor settings: a randomised trial comparing counselling with routine medical treatment in North Afghanistan (Mazar-e-Sharif). BMC Psychiatry 2012;12:14. [DOI: 10.1186/1471-244x-12-14] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barfar 2017 {published data only}
- Barfar E, Sharifi V, Amini H, Mottaghipour Y, Yunesian M, Tehranidoost M, et al. Cost-effectiveness analysis of an aftercare service vs treatment-as-usual for patients with severe mental disorders. Journal of Mental Health Policy and Economics 2017;20(3):101-10. [PubMed] [Google Scholar]
- Hajebi A, Sharifi V, Ghadiri VM, Moradi-Lakeh M, Tehranidoost M, Yunesian M, et al. A multicenter randomized controlled trial of aftercare services for severe mental illness: study protocol. BMC Psychiatry 2013;13:178. [DOI: 10.1186/1471-244X-13-178] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barron 2013 {published data only}
- Barron IG, Abdallah G, Smith P. Randomized control trial of a CBT trauma recovery program in Palestinian schools. Journal of Loss & Trauma 2013;18(4):306-21. [DOI: 10.1080/15325024.2012.688712] [DOI] [Google Scholar]
Barron 2016 {published data only}
- Barron I, Abdallah G, Heltne U. Randomized control trial of teaching recovery techniques in rural occupied Palestine: effect on adolescent dissociation. Journal of Aggression, Maltreatment & Trauma 2016;25(9):955-73. [DOI: 10.1080/10926771.2016.1231149] [DOI] [Google Scholar]
Bass 2013 {published data only}
- Bass JK, Annan J, Murray SM, Kaysen D, Griffiths S, Cetinoglu T, et al. "Controlled trial of psychotherapy for Congolese survivors of sexual violence": erratum. New England Journal of Medicine/Boston Medical & Surgical Journal 2014;370(26):2547. [DOI: ] [DOI] [PubMed] [Google Scholar]
- Bass JK, Annan J, Murray SM, Kaysen D, Griffiths S, Cetinoglu T, et al. Controlled trial of psychotherapy for Congolese survivors of sexual violence. New England Journal of Medicine 2013;368(23):2182-91. [DOI: 10.1056/NEJMoa1211853] [DOI] [PubMed] [Google Scholar]
- Hall BJ, Bolton PA, Annan J, Kaysen D, Robinette K, Cetinoglu T, et al. The effect of cognitive therapy on structural social capital: results from a randomized controlled trial among sexual violence survivors in the Democratic Republic of the Congo. American Journal of Public Health 2014;104(9):1680-6. [DOI: 10.2105/ajph.2014.301981] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray SM, Augustinavicius J, Kaysen D, Rao D, Murray LK, Wachter K, et al. The impact of cognitive processing therapy on stigma among survivors of sexual violence in eastern Democratic Republic of Congo: results from a cluster randomized controlled trial. Conflict & Health [electronic resource] 2018;12:1. [DOI: 10.1186/s13031-018-0142-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bass 2016 {published data only}
- Bass J, Murray SM, Mohammed TA, Bunn M, Gorman W, Ahmed AM, et al. A randomized controlled trial of a trauma-informed support, skills, and psychoeducation intervention for survivors of torture and related trauma in Kurdistan, Northern Iraq. Global Health, Science and Practice 2016;4(3):452-66. [DOI: 10.9745/GHSP-D-16-00017] [DOI] [PMC free article] [PubMed] [Google Scholar]
Berger 2009 {published data only}
- Berger R, Gelkopf M. School-based intervention for the treatment of tsunami-related distress in children: a quasi-randomized controlled trial. Psychotherapy and Psychosomatics 2009;78:364-71. [DOI] [PubMed] [Google Scholar]
Betancourt 2014 {published data only}
- Betancourt TS, McBain R, Newnham EA, Akinsulure-Smith AM, Brennan RT, Weisz JR, et al. A behavioral intervention for war-affected youth in Sierra Leone: a randomized controlled trial. Journal of the American Academy of Child and Adolescent Psychiatry 2014;53(12):1288-97. [DOI: 10.1016/j.jaac.2014.09.011] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bolton 2003 {published data only}
- Bass J, Neugebauer R. Group interpersonal psychotherapy for depression in rural Uganda: 6-month outcomes randomised controlled trial. British Journal of Psychiatry 2006;188:567-73. [DOI] [PubMed] [Google Scholar]
- Bolton P, Neugebauer R, Ndogoni L. Prevalence of depression in rural Rwanda based on symptom and functional criteria. Journal of Nervous and Mental Disease 2002;190(9):631-7. [DOI] [PubMed] [Google Scholar]
- Bolton P. Cross-cultural validity and reliability testing of a standard psychiatric assessment instrument without a gold standard. Journal of Nervous and Mental Disease 2001;189(4):238-42. [DOI] [PubMed] [Google Scholar]
- Bolton P. Group interpersonal psychotherapy for depression in rural Uganda: a randomized controlled trial. JAMA 2003;289(23):3117-24. [DOI] [PubMed] [Google Scholar]
- Clougherty KF, Verdeli H, Weissman MM. Interpersonal psychotherapy for group in Uganda (IPTG-U). Unpublished Manual 2002.
- Verdeli H, Clougherty K, Bolton P, Speelman L, Lincoln N, Bass J, et al. Adapting group interpersonal psychotherapy for a developing country: experience in rural Uganda. World Psychiatry 2003;2(2):114-20. [PMC free article] [PubMed] [Google Scholar]
Bolton 2007 {published data only}
- Betancourt TS, Newnham EA, Brennan RT, Verdeli H, Borisova I, Neugebauer R, et al. Moderators of treatment effectiveness for war-affected youth with depression in Northern Uganda. Journal of Adolescent Health 2012;51(6):544-50. [DOI: 10.1016/j.jadohealth.2012.02.010] [DOI] [PubMed] [Google Scholar]
- Bolton P, Bass J. Interventions for depression symptoms among adolescent survivors of war and displacement in northern Uganda. JAMA 2007;298(5):519-27. [DOI] [PubMed] [Google Scholar]
- Bolton P, Betancourt T, Bass J, Speelman S, Onyango G. A randomized controlled trial of group interpersonal psychotherapy and creative play for psychosocial problems in war-affected Acholi adolescents. Final Programme Report 2006.
- Bolton P, Wilk CM, Ndogoni L. Assessment of depression prevalence in rural Uganda using symptom and function criteria. Social Psychiatry and Psychiatric Epidemiology 2004;38(6):442-7. [DOI] [PubMed] [Google Scholar]
Bolton 2014 (Iraq) {published data only}
- Bolton P, Bass JK, Zangana GA, Kamal T, Murray SM, Kaysen D, et al. A randomized controlled trial of mental health interventions for survivors of systematic violence in Kurdistan, Northern Iraq. BMC Psychiatry 2014;14:360. [DOI: 10.1186/s12888-014-0360-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magidson JF, Lejuez CW, Kamal T, Blevins EJ, Murray LK, Bass JK, et al. Adaptation of community health worker-delivered behavioral activation for torture survivors in Kurdistan, Iraq. Global Mental Health 2015;2:e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00925262. A controlled trial of mental health interventions for common mental health problems experienced by torture survivors living in Kurdistan, Iraq. https://clinicaltrials.gov/ct2/show/NCT00925262 2009.
Bolton 2014 (Thailand) {published data only}
- Bolton P, Lee C, Haroz EE, Murray L, Dorsey S, Robinson C, et al. A transdiagnostic community-based mental health treatment for comorbid disorders: development and outcomes of a randomized controlled trial among Burmese refugees in Thailand. PLOS Medicine/Public Library of Science 2014;11(11):e1001757. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bonilla‐Escobar 2018 {published data only}
- Bonilla-Escobar FJ, Fandino-Losada A, Martinez-Buitrago DM, Santaella-Tenorio J, Tobon-Garci D, Munoz-Morales EJ, et al. Randomized controlled trial of a transdiagnostic cognitive-behavioral intervention for Afro-descendants' survivors of systemic violence in Colombia. PLOS ONE 2018;13(12):e0208483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01856673. Evaluation of two community-based mental health interventions for violence-displaced Afro-descendants in Colombia. https://clinicaltrials.gov/show/NCT01856673; 2013.
Bryant 2017 {published data only}
- Bryant RA, Schafer A, Dawson KS, Anjuri D, Mulili C, Ndogoni L, et al. Effectiveness of a brief behavioural intervention on psychological distress among women with a history of gender-based violence in urban Kenya: a randomised clinical trial. PLOS Medicine/Public Library of Science 2017;14(8):e1002371. [DOI: 10.1371/journal.pmed.1002371] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson KS, Schafer A, Anjuri D, Ndogoni L, Musyoki C, Sijbrandij M, et al. Feasibility trial of a scalable psychological intervention for women affected by urban adversity and gender-based violence in Nairobi. BMC Psychiatry 2016;16(1):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chatterjee 2014 {published data only}
- Balaji M, Chatterjee S, Koschorke M, Rangaswamy T, Chavan A, Dabholkar H, et al. The development of a lay health worker delivered collaborative community based intervention for people with schizophrenia in India. BMC Health Service Research 2012;12:42. [DOI: 10.1186/1472-6963-12-42] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S, Naik S, John S, Dabholkar H, Balaji M, Koschorke M, et al. Effectiveness of a community-based intervention for people with schizophrenia and their caregivers in India (COPSI): a randomised controlled trial. Lancet (London, England) 2014;383(9926):1385-94. [DOI: 10.1016/S0140-6736(13)62629-X] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S, Leese M, Koschorke M, McCrone P, Naik S, John S, et al. Collaborative community based care for people and their families living with schizophrenia in India: protocol for a randomised controlled trial. Trials 2011;12(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koschorke M, Padmavati R, Kumar S, Cohen A, Weiss HA, Chatterjee S, et al. Experiences of stigma and discrimination of people with schizophrenia in India. Social Science and Medicine 2014;123:149-59. [DOI] [PMC free article] [PubMed]
Chen 2015 {published data only}
- Chen S, Conwell Y, He J, Lu N, Wu J. "Depression care management for adults older than 60 years in primary care clinics in urban China: a cluster-randomised trial". Erratum. Lancet Psychiatry 2015;2(5):378. [DOI: 10.1016/S2215-0366%2815%2900162-5] [DOI] [PubMed] [Google Scholar]
- Chen S, Conwell Y, Xu B, Chiu H, Tu X, Ma Y. Depression care management for late-life depression in China primary care: protocol for a randomized controlled trial. Trials [electronic resource] 2011;12 CNO-00792546:121. [DOI: 10.1186/1745-6215-12-121] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Conwell Y, He J, Lu N, Wu J. Depression care management for adults older than 60 years in primary care clinics in urban China: a cluster-randomised trial. Lancet Psychiatry 2015;2(4):332-9. [DOI: 10.1016/S2215-0366(15)00002-4] [DOI] [PubMed] [Google Scholar]
- Chen S, Fang Y, Li L, Fan H. Collaborative late-life depression care management in urban China primary care. Asia-Pacific Psychiatry 2012;4:97. [DOI: 10.1111/appy.12004] [DOI] [Google Scholar]
Chibanda 2014 {published data only}
- Chibanda D, Shetty AK, Tshimanga M, Woelk G, Stranix-Chibanda L, Rusakaniko S. Group problem-solving therapy for postnatal depression among HIV-positive and HIV-negative mothers in Zimbabwe. Journal of the International Association of Providers of AIDS Care 2014;13(4):335-41. [DOI: 10.1177/2325957413495564] [DOI] [PubMed] [Google Scholar]
Chibanda 2016 {published data only}
- Chibanda D, Bowers T, Verhey R, Rusakaniko S, Abas M, Weiss HA, et al. The Friendship Bench programme: a cluster randomised controlled trial of a brief psychological intervention for common mental disorders delivered by lay health workers in Zimbabwe. International Journal of Mental Health Systems 2015;9(1):21. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chibanda D, Weiss HA, Verhey R, Simms V, Munjoma R, Rusakaniko S, et al. Effect of a primary care-based psychological intervention on symptoms of common mental disorders in Zimbabwe: a randomized clinical trial. JAMA 2016;316(24):2618-26. [DOI: 10.1001/jama.2016.19102] [DOI] [PubMed] [Google Scholar]
Christoff 2015 {published data only}
- Christoff AO, Boerngen-Lacerda R. Reducing substance involvement in college students: a three-arm parallel-group randomized controlled trial of a computer-based intervention. Addictive Behaviors 2015;45:164-71. [DOI: 10.1016/j.addbeh.2015.01.019] [DOI] [PubMed] [Google Scholar]
Connolly 2011 {published data only}
- Connolly S, Sakai C. Brief trauma intervention with Rwandan genocide-survivors using thought field therapy. International Journal of Emergency Mental Health 2011;13(3):161-72. [PubMed] [Google Scholar]
Dawson 2016 {published data only}
- Dawson KS, Schafer A, Anjuri D, Ndogoni L, Musyoki C, Sijbrandij M, et al. Feasibility trial of a scalable psychological intervention for women affected by urban adversity and gender-based violence in Nairobi. BMC Psychiatry 2016;16(1):410. [DOI: 10.1186/s12888-016-1117-x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dias 2008 {published data only}
- Dias A, Dewey ME, D'Souza J, Dhume R, Motghare DD, Shaji KS, et al. The effectiveness of a home care program for supporting caregivers of persons with dementia in developing countries: a randomised controlled trial from Goa, India. PLOS One 2008;3(6):e2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
Divan 2019 {published data only}
- Divan G, Vajaratkar V, Cardozo P, Huzurbazar S, Verma M, Howarth E, et al. The feasibility and effectiveness of PASS Plus, a lay health worker delivered comprehensive intervention for Autism Spectrum Disorders: pilot RCT in a rural low and middle income country setting. Autism Research 2019;12(2):328-39. [DOI: 10.1002/aur.1978] [DOI] [PubMed] [Google Scholar]
- ISRCTN10260663. Autism identification and moving towards treatments (Swamagnata Aakalan and Upacharachi Disha [SAAD]). http://www.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN10260663; 2016.
Dybdahl 2001 {published data only}
- Dybdahl R. Child Development and Impact of Stress on Young Children. An Intervention Programme for Mothers. Prepared for the UNICEF/Centre for Crisis Psychology Psychosocial Project 1999.
- Dybdahl R. Children and mothers in war. Child Development 2001;72(4):1214-30. [DOI] [PubMed] [Google Scholar]
Ertl 2011 {published data only}
- Ertl V, Pfeiffer A, Schauer A, Elbert T, Neuner F. Community-implemented trauma therapy for former child soldiers in Northern Uganda: a randomised controlled trial. JAMA 2011;306(5):503-12. [DOI] [PubMed] [Google Scholar]
Fritsch 2007 {published data only}
- Fritsch R, Araya R, Solis J, Montt E, Pilowsky D, Rojas G. A randomised trial of pharmacotherapy with telephone monitoring to improve treatment of depression in primary care in Santiago, Chile. Revista Medica de Chile 2007;135(5):587-95. [PubMed] [Google Scholar]
Fuhr 2019 {published data only}
- Fuhr DC, Weobong B, Lazarus A, Vanobberghen F, Weiss HA, Singla DR, et al. Delivering the Thinking Healthy Programme for perinatal depression through peers: an individually randomised controlled trial in India. Lancet Psychiatry 2019;6(2):115-27. [DOI: 10.1016/S2215-0366(18)30466-8] [DOI] [PubMed] [Google Scholar]
Gavrilova 2009 {published data only}
- Gavrilova SI, Ferri CP, Mikhaylova N, Sokolova O, Banerjee S, Prince M. Helping carers to care - the 10/66 dementia research group's randomised control trial of a caregiver intervention in Russia. International Journal of Geriatric Psychiatry 2009;24(4):347-54. [DOI] [PubMed] [Google Scholar]
Gordon 2008 {published data only}
- Gordon JS, Staples JK, Blyta A, Bytyqi M, Wilson AT. Treatment of posttraumatic stress disorder in postwar Kosovar adolescents using mind-body skills groups: a randomised controlled trial. Journal of Clinical Psychiatry 2008;69(9):1469-76. [DOI] [PubMed] [Google Scholar]
- Gordon JS, Staples JK, Blyta A, Bytyqi M. Treatment of posttraumatic stress disorder in postwar Kosovo high school students using mind-body skills groups: a pilot study. Journal of Traumatic Stress 2004;17(2):143-7. [DOI] [PubMed] [Google Scholar]
Gureje 2019 (EXPONATE) {published data only}
- Gureje O, Oladeji BD, Araya R, Montgomery AA, Kola L, Kirmayer L, et al. Expanding care for perinatal women with depression (EXPONATE): study protocol for a randomized controlled trial of an intervention package for perinatal depression in primary care. BMC Psychiatry 2015;15:136. [DOI: 10.1186/s12888-015-0537-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gureje O, Oladeji BD, Montgomery AA, Araya R, Bello T, Chisholm D, et al. High- versus low-intensity interventions for perinatal depression delivered by non-specialist primary maternal care providers in Nigeria: cluster randomised controlled trial (the EXPONATE trial). British Journal of Psychiatry 2019;215(3):528-35. [DOI: 10.1192/bjp.2019.4] [DOI] [PubMed] [Google Scholar]
Gureje 2019 (STEPCARE) {published data only}
- Gureje O, Oladeji BD, Araya R, Montgomery AA. A cluster randomized clinical trial of a stepped care intervention for depression in primary care (STEPCARE) - study protocol. BMC Psychiatry 2015;15:148. [DOI: 10.1186/s12888-015-0542-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gureje O, Oladeji BD, Montgomery AA, Bello T, Kola L, Ojagbemi A, et al. Effect of a stepped-care intervention delivered by lay health workers on major depressive disorder among primary care patients in Nigeria (STEPCARE): a cluster-randomised controlled trial. Lancet Global Health 2019;7(7):e951-60. [DOI: 10.1016/S2214-109X(19)30148-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
HuisIntVeld 2019 {published data only}
- Huis In 't Veld D, Skaal L, Peltzer K, Colebunders R, Ndimande JV, Pengpid S. The efficacy of a brief intervention to reduce alcohol misuse in patients with HIV in South Africa: study protocol for a randomized controlled trial. Trials [electronic resource] 2012;13(1):190. [DOI: 10.1186/1745-6215-13-190] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huis in ‘t Veld D, Ensoy-Musoro C, Pengpid S, Peltzer K, Colebunders R. The efficacy of a brief intervention to reduce alcohol use in persons with HIV in South Africa: a randomized clinical trial. PLOS ONE 2019;14(8):e0220799. [DOI] [PMC free article] [PubMed] [Google Scholar]
Humeniuk 2012 {published data only}
- Humeniuk R, Ali R, Babor T, Souza-Formigoni ML, Lacerda RB, Ling W, et al. A randomized controlled trial of a brief intervention for illicit drugs linked to the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) in clients recruited from primary health-care settings in four countries. Addiction (Abingdon, England) 2012;107(5):957-66. [DOI: 10.1111/j.1360-0443.2011.03740.x] [DOI] [PubMed] [Google Scholar]
- Humeniuk R, Dennington V, Ali R, on behalf of the WHO ASSIST Phase III Study Group. The Effectiveness of a Brief Intervention for Illicit Drugs Linked to the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) in Primary Health Care Settings: A Technical Report of Phase III Findings of the WHO ASSIST Randomized Controlled Trial. Geneva, Switzerland: WHO Press, World Health Organization, 2008. [Google Scholar]
Indu 2018 {published data only}
- Indu PS, Anilkumar TV, Vijayakumar K, Kumar KA, Sarma PS, Remadevi S, et al. Effectiveness of community-based depression intervention programme (ComDIP) to manage women with depression in primary care - randomised control trial. Asian Journal of Psychiatry 2018;34:87-92. [DOI: 10.1016/j.ajp.2018.04.022] [DOI] [PubMed] [Google Scholar]
Jenkins 2013 {published data only}
- Jenkins R, Othieno C, Okeyo S, Kaseje D, Aruwa J, Oyugi H, et al. Short structured general mental health in service training programme in Kenya improves patient health and social outcomes but not detection of mental health problems - a pragmatic cluster randomised controlled trial. International Journal of Mental Health Systems 2013;7(1):25. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins R, Kiima D, Njenga F, Okonji M, Kingora J, Kathuku D, et al. Integration of mental health into primary care in Kenya. World Psychiatry 2010;9(2):118-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins R, Kiima D, Okonji M, Njenga F, Kingora J, Lock S. Integration of mental health into primary care and community health working in Kenya: context, rationale, coverage and sustainability. Mental Health in Family Medicine 2010;7(1):37-47. [PMC free article] [PubMed] [Google Scholar]
- Jenkins R. Assessment of the impact of a Kenya Medical Training College delivered structured five day training programme on mental health core concepts, skills and competencies on mental health for primary care staff in Kenya. ISRCTN53515024; 2012.
Jiang 2017 {published data only}
- Jiang N, Hu H, Chen Y. Effect of spiritual support model based on cloud platform on mild to moderate depression among community empty nests. Hu Li Yan Jiu [Chinese Nursing Research] 2017;31:2325-8. [DOI: 10.3969/j.issn.1009-6493.2017.19.008] [DOI] [Google Scholar]
Jordans 2010 {published data only}
- Jordans MJ, Komproe IH, Tol WA, Kohrt BA, Luitel NP, Macy RD, et al. Evaluation of a classroom-based psychosocial intervention in conflict-affected Nepal: a cluster randomised controlled trial. Journal of Child Psychology and Psychiatry, and Allied Disciplines 2010;51(7):818-26. [DOI] [PubMed] [Google Scholar]
Jordans 2019 {published data only}
- ISRCTN72875710. An evaluation of the effectiveness of a psychological treatment for moderate to severe depression and harmful or dependent drinking in rural communities in Nepal. https://www.isrctn.com/ISRCTN72875710 2014. [DOI: 10.1186/ISRCTN72875710] [DOI]
- Jordans MJ, Luitel NP, Garman E, Kohrt BA, Rathod SD, Shrestha P, et al. Effectiveness of psychological treatments for depression and alcohol use disorder delivered by community-based counsellors: two pragmatic randomised controlled trials within primary healthcare in Nepal. British Journal of Psychiatry 2019;215(2):485-93. [DOI: 10.1192/bjp.2018.300] [DOI] [PMC free article] [PubMed] [Google Scholar]
Khan 2017 {published data only}
- Khan MN, Dherani M, Chiumento A, Atif N, Bristow K, Sikander S, et al. Evaluating feasibility and acceptability of a local psycho-educational intervention for pregnant women with common mental problems affected by armed conflict in Swat, Pakistan: a parallel randomized controlled feasibility trial. International Journal of Social Psychiatry 2017;63(8):724-35. [DOI: 10.1177/0020764017734001] [DOI] [PubMed] [Google Scholar]
Khan 2019 {published data only}
- Khan MN, Hamdani SU, Chiumento A, Dawson K, Bryant RA, Sijbrandij M, et al. Evaluating feasibility and acceptability of a group WHO trans-diagnostic intervention for women with common mental disorders in rural Pakistan: a cluster randomised controlled feasibility trial. Epidemiology and Psychiatric Sciences 2019;28(1):77-87. [DOI: 10.1017/S2045796017000336] [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2002 {published data only}
- Li X, Li X, Ma X, Ni Y. Effects of social rehabilitation of late-onset schizophrenia. Zhongguo Xin Li Wei Sheng Za Zhi [Chinese Mental Health Journal] 2002;16(10):711-3. [Google Scholar]
Li 2018 {published data only}
- Li L, Hien NT, Liang LJ, Lin C, Lan CW, Lee SJ, et al. Efficacy of communication training of community health workers on service delivery to people who inject drugs in Vietnam: a clustered randomized trial. American Journal of Public Health 2018;108:791-8. [DOI: 10.2105/AJPH.2018.304350] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ling 1999 {published data only}
- Ling S, Zhao C, Yang W, Wang R, Jin Z, Ma T, et al. Efficacy of family intervention on schizophrenics in remission in community: result of one year follow-up study. Zhongguo Xin Li Wei Sheng Za Zhi [Chinese Mental Health Journal] 1999;13(06):325-7. [Google Scholar]
Lund 2020 {published data only}
- Davies T, Schneider M, Nyatsanza M, Lund C. “The sun has set even though it is morning”: experiences and explanations of perinatal depression in an urban township, Cape Town. Transcult Psychiatry 2016;53(3):286-312. [DOI: 10.1177/1363461516632389] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund C, Alem A, Schneider M, Hanlon C, Ahrens J, Bandawe C, et al. Generating evidence to narrow the treatment gap for mental disorders in sub-Saharan Africa: rationale, overview and methods of AFFIRM. Epidemiology and Psychiatric Sciences 2015;24(3):233-40. [DOI: 10.1017/S2045796015000281] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund C, Schneider M, Davies T, Nyatsanza M, Honikman S, Bhana A, et al. Task sharing of a psychological intervention for maternal depression in Khayelitsha, South Africa: study protocol for a randomized controlled trial. Trials [electronic resource] 2014;15(1):457. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund C, Schneider M, Garman EC, Davies T, Munodawafa M, Honikman S, et al. Task-sharing of psychological treatment for antenatal depression in Khayelitsha, South Africa: effects on antenatal and postnatal outcomes in an individual randomised controlled trial. Behaviour Research and Therapy 2020;130:103466. [DOI: 10.1016/j.brat.2019.103466] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munodawafa M, Lund C, Schneider M. A process evaluation exploring the lay counsellor experience of delivering a task shared psycho-social intervention for perinatal depression in Khayelitsha, South Africa. BMC Psychiatry 2017;17(1):236. [DOI: 10.1186/s12888-017-1397-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
- PACTR201403000676264. AFFIRM-SA trial. http://www.who.int/trialsearch/Trial2.aspx?TrialID=PACTR201403000676264; 2013.
Malakouti 2015 {published data only}
- Malakouti SK, Mirabzadeh A, Nojomi M, Ahmadi TA, Nadarkhani F, Mirzaie M, et al. Clinical outcomes and cost effectiveness of two aftercare models provided by general physicians and nurses to patients with severe mental illness. Medical Journal of the Islamic Republic of Iran 2015;29:196. [PMC free article] [PubMed] [Google Scholar]
Marais 2011 {published data only}
- Marais S, Jordaan E, Viljoen D, Olivier L, De Waal J, Poole C. The effect of brief interventions on the drinking behaviour of pregnant women in a high-risk rural South African community: a cluster randomised trial. Early Child Development and Care 2011;181:463-74. [DOI: 10.1080/03004430903450392] [DOI] [Google Scholar]
Matsuzaka 2017 {published data only}
- Matsuzaka CT, Wainberg M, Norcini PA, Hoffmann EV, Coimbra BM, Braga RF, et al. Task shifting interpersonal counseling for depression: a pragmatic randomized controlled trial in primary care. BMC Psychiatry 2017;17(1):225. [DOI: 10.1186/s12888-017-1379-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
Meffert 2014 {published data only}
- Meffert SM, Abdo AO, Alla OA, Elmakki YO, Omer AA, Yousif S, et al. A pilot randomized controlled trial of interpersonal psychotherapy for Sudanese refugees in Cairo, Egypt. Psychological Trauma: Theory, Research, Practice, and Policy 2014;6(3):240-9. [DOI: 10.1037/a0023540] [DOI] [Google Scholar]
Mertens 2014 {published data only}
- Mertens JR, Ward CL, Bresick GF, Broder T, Weisner CM. Effectiveness of nurse-practitioner-delivered brief motivational intervention for young adult alcohol and drug use in primary care in South Africa: a randomized clinical trial. Alcohol and Alcoholism (Oxford, Oxfordshire) 2014;49(4):430-8. [DOI: 10.1093/alcalc/agu030] [DOI] [PMC free article] [PubMed] [Google Scholar]
Milani 2015 {published data only}
- IRCT201202159027N1. The effect of volunteer’s telephone-based support on the effect of telephone-based support on postnatal depression. https://en.irct.ir/trial/9651 2012.
- Milani HS, Azargash E, Beyraghi N, Defaie S, Asbaghi T. Effect of telephone-based support on postpartum depression: a randomized controlled trial. International Journal of Fertility & Sterility 2015;9:247-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Momeni 2016 {published data only}
- Momeni K, Shalani B. The effectiveness of group drawing on aggression reduction in hyperactive-aggressive students. Developmental Psychology: Journal of Iranian Psychologists 2016;13(49):71-7. [Google Scholar]
Murphy 2020 {published data only}
- Murphy J, Goldsmith CH, Jones W, Oanh PT, Nguyen VC. The effectiveness of a supported self-management task-shifting intervention for adult depression in Vietnam communities: study protocol for a randomized controlled trial. Trials [electronic resource] 2017;18(1):209. [DOI: 10.1186/s13063-017-1924-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J, Oanh PT, Goldsmith CH, Jones W, Nguyen VC. Introducing supported self-management for depression to primary care in Vietnam: a feasibility study in preparation for a randomized controlled trial. Families Systems & Health 2018;36(2):210. [DOI] [PubMed] [Google Scholar]
- Murphy JK, Xie H, Nguyen VC, Chau LW, Oanh PT, Nhu TK, et al. Is supported self-management for depression effective for adults in community-based settings in Vietnam? A modified stepped-wedge cluster randomized controlled trial. International Journal of Mental Health Systems 2020;14:8. [DOI: 10.1186/s13033-020-00342-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Murray 2015 {published data only}
- Kane JC, Murray LK, Cohen J, Dorsey S, Skavenski van Wyk S, Galloway Henderson J, et al. Moderators of treatment response to trauma-focused cognitive behavioral therapy among youth in Zambia. Journal of Child Psychology and Psychiatry and Allied Disciplines 2016;57(10):1194-202. [DOI: 10.1111/jcpp.12623] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray L, Kane J, Bolton P, Imasiku M. A randomized effectiveness trial of trauma-focused cognitive-behavioral therapy for trauma affected children in Lusaka, Zambia. In: Journal of the American Academy of Child and Adolescent Psychiatry. Vol. 55. 2016:S279. [DOI: 10.1016/j.jaac.2016.07.201] [DOI]
- Murray LK, Skavenski S, Kane JC, Mayeya J, Dorsey S, Cohen JA, et al. Effectiveness of trauma-focused cognitive behavioral therapy among trauma-affected children in Lusaka, Zambia: a randomized clinical trial. JAMA Pediatrics 2015;169(8):761-9. [DOI: 10.1001/jamapediatrics.2015.0580] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nadkarni 2017 {published data only}
- Nadkarni A, Velleman R, Dabholkar H, Shinde S, Bhat B, McCambridge J, et al. The systematic development and pilot randomized evaluation of counselling for alcohol problems, a lay counselor-delivered psychological treatment for harmful drinking in primary care in India: the PREMIUM study. Alcoholism Clinical and Experimental Research 2015;39(3):522-31. [DOI: 10.1111/acer.12653] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadkarni A, Weobong B, Weiss HA, McCambridge J, Bhat B, Katti B, et al. Counselling for Alcohol Problems (CAP), a lay counsellor-delivered brief psychological treatment for harmful drinking in men, in primary care in India: a randomised controlled trial. Lancet (London, England) 2017;389(10065):186-95. [DOI: 10.1016/S0140-6736(16)31590-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadkarni A, Weiss HA, Weobong B, McDaid D, Singla DR, Park AL, et al. Sustained effectiveness and cost-effectiveness of Counselling for Alcohol Problems, a brief psychological treatment for harmful drinking in men, delivered by lay counsellors in primary care: 12-month follow-up of a randomised controlled trial. PLOS Medicine/Public Library of Science 2017;14(9):e1002386. [DOI: 10.1371/journal.pmed.1002386] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V, Weobong B, Nadkarni A, Weiss HA, Anand A, Naik S, et al. The effectiveness and cost-effectiveness of lay counsellor-delivered psychological treatments for harmful and dependent drinking and moderate to severe depression in primary care in India: PREMIUM study protocol for randomized controlled trials. Trials 2014;15(1):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nadkarni 2019 {published data only}
- Nadkarni A, Weiss HA, Velleman R, McCambridge J, McDaid D, Park AL, et al. Feasibility, acceptability and cost-effectiveness of a brief, lay counsellor-delivered psychological treatment for men with alcohol dependence in primary care: an exploratory randomized controlled trial. Addiction (Abingdon, England) 2019;114:1192-203. [DOI: 10.1111/add.14630] [DOI] [PMC free article] [PubMed] [Google Scholar]
Neuner 2008 {published data only}
- Neuner F, Onyut PL, Ertl V, Odenwald M, Schauer E, Elbert T. Treatment of posttraumatic stress disorder by trained lay counsellors in an African refugee settlement: a randomised controlled trial. Journal of Consulting and Clinical Psychology 2008;76(4):686-94. [DOI: 10.1037/0022-006X.76.4.686] [DOI] [PubMed] [Google Scholar]
- Neuner F, Onyut PL, Ertl V, Odenwald M, Schauer E, Elbert T. Treatment of posttraumatic stress disorder by trained lay counsellors in an African refugee settlement. https://clinicaltrials.gov/show/NCT00550056; 2007. [DOI] [PubMed]
Niemi 2016 {published data only}
- Niemi M, Kiel S, Allebeck P, Hoan le T. Community-based intervention for depression management at the primary care level in Ha Nam province, Vietnam: a cluster-randomised controlled trial. Tropical Medicine & International Health 2016;21(5):654-61. [DOI: 10.1111/tmi.12674] [DOI] [PubMed] [Google Scholar]
Noknoy 2010 {published data only}
- Noknoy S, Rangsin R, Saengcharnchai P, Tantibhaedhyangkul U, McCambridge J. RCT of effectiveness of motivational enhancement therapy delivered by nurses for hazardous drinkers in primary care units in Thailand. Alcohol and Alcoholism (Oxford, Oxfordshire) 2010;45(3):263-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Callaghan 2013 {published data only}
- O'Callaghan P, McMullen J, Shannon C, Rafferty H, Black A. A randomized controlled trial of trauma-focused cognitive behavioral therapy for sexually exploited, war-affected Congolese girls. Journal of the American Academy of Child and Adolescent Psychiatry 2013;52(4):359-69. [DOI: 10.1016/j.jaac.2013.01.013] [DOI] [PubMed] [Google Scholar]
- O'Callaghan P, Shannon C. An RCT of trauma focused-cognitive behavioral therapy with sexually exploited, war-affected girls in the DRC. https://clinicaltrials.gov/ct2/show/NCT01483261; 2011. [DOI] [PubMed]
O'Callaghan 2014 {published data only}
- O'Callaghan P, Branham L, Shannon C, Betancourt TS, Dempster M, McMullen J. A pilot study of a family focused, psychosocial intervention with war-exposed youth at risk of attack and abduction in north-eastern Democratic Republic of Congo. Child Abuse & Neglect 2014;38(7):1197-207. [DOI: 10.1016/j.chiabu.2014.02.004] [DOI] [PubMed] [Google Scholar]
- Shannon C. Is a family-based, life skills focused intervention effective in reducing psychological distress and stigma and improving inter-personal relations and functioning among former LRA abductees and other war-affected children in their community in Dungu, the Democratic Republic of Congo? http://clinicaltrials.gov/show/nct01542398; 2012.
O'Callaghan 2015 {published data only}
- NCT01509872. Psychological and psychosocial intervention with war-affected children. https://clinicaltrials.gov/ct2/show/NCT01509872; 2011.
- O'Callaghan P, McMullen J, Shannon C, Rafferty H. Comparing a trauma focused and non trauma focused intervention with war affected Congolese youth: a preliminary randomised trial. Intervention 2015;13(1):28-44. [DOI: ] [Google Scholar]
Oladeji 2015 {published data only}
- Oladeji BD, Kola L, Abiona T, Montgomery AA, Araya R, Gureje O. A pilot randomized controlled trial of a stepped care intervention package for depression in primary care in Nigeria. BMC Psychiatry 2015;15(1):96. [DOI: 10.1186/s12888-015-0483-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Papas 2011 {published data only}
- NCT00792519. Cognitive behavioral treatment to reduce alcohol use among HIV-infected Kenyans (KHBS). https://clinicaltrials.gov/ct2/show/NCT00792519 2008.
- Papas RK, Sidle JE, Gakinya BN, Baliddawa JB, Martino S, Mwaniki MM, et al. Treatment outcomes of a stage 1 cognitive-behavioral trial to reduce alcohol use among human immunodeficiency virus-infected out-patients in Western Kenya. Addiction 2011;106(12):2156-66. [DOI] [PMC free article] [PubMed]
- Papas RK, Sidle JE, Martino S, Baliddawa JB, Songole R, Omolo OE, et al. Systematic cultural adaptation of cognitive-behavioral therapy to reduce alcohol use among HIV-infected outpatients in western Kenya. AIDS and Behavior 2010;14(3):669-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Papas 2020 {published data only}
- Galárraga O, Gao B, Gakinya BN, Klein DA, Wamai RG, Sidle JE, et al. Task-shifting alcohol interventions for HIV+ persons in Kenya: a cost-benefit analysis. BMC Health Services Research 2017;17(1):239. [DOI: 10.1186/s12913-017-2169-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01503255. A stage 2 cognitive-behavioral trial: Reduce Alcohol First in Kenya intervention. https://clinicaltrials.gov/ct2/show/NCT01503255; 2016.
- Papas RK, Gakinya BN, Mwaniki MM, Lee H, Keter AK, Klein DA, et al. Successful treatment outcomes from a stage 2 randomized clinical trial of CBT to reduce alcohol use among HIV-infected outpatients in Western Kenya. In: Alcoholism: Clinical and Experimental Research. Vol. Conference: 40th Annual Scientific Meeting of the Research Society on Alcoholism. United States. 41. 2017:253A. [DOI: 10.1111/acer.13391] [DOI]
- Papas RK, Sidle JE, Martino S, Baliddawa JB, Songole R, Omolo OE, et al. Systematic cultural adaptation of cognitive-behavioral therapy to reduce alcohol use among HIV-infected outpatients in western Kenya. AIDS Behavior 2010;14(3):669-78. [DOI: 10.1007/s10461-009-9647-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papas RK, Gakinya BN, Mwaniki MM, Lee H, Keter AK, Martino S, et al. A randomized clinical trial of a group cognitive-behavioral therapy to reduce alcohol use among human immunodeficiency virus-infected out-patients in western Kenya [Epub ahead of print]. Addiction 2020;10.1111/add.15112:no pagination. [DOI: 10.1111/add.15112] [DOI] [PMC free article] [PubMed] [Google Scholar]
Patel 2010 {published data only}
- Buttorff C, Hock RS, Weiss HA, Naik S, Araya R, Kirkwood BR, et al. Economic evaluation of a task-shifting intervention for common mental disorders in India. Bulletin of the World Health Organization 2012;90:813-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V, Weiss HA, Chowdhary N, Naik S, Pednekar S, Chatterjee S, et al. Effectiveness of an intervention led by lay health counsellors for depressive and anxiety disorders in primary care in Goa, India (MANAS): a cluster randomised controlled trial. Lancet 2010;376(9758):2086-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel VH, Kirkwood BR, Pednekar S, Araya R, King M, Chisholm D, et al. Improving the outcomes of primary care attenders with common mental disorders in developing countries: a cluster randomised controlled trial of a collaborative stepped care intervention in Goa, India. Trials 2008;9:4. [DOI: 10.1186/1745-6215-9-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinde S, Andrew G, Bangash O, Cohen A, Kirkwood B, Patel V. The impact of a lay counselor led collaborative care intervention for common mental disorders in public and private primary care: a qualitative evaluation nested in the MANAS trial in Goa, India. Social Science & Medicine 2013 Jul 1;88:48-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Patel 2017 {published data only}
- Chowdhary N, Anand A, Dimidjian S, Shinde S, Weobong B, Balaji M, et al. The Healthy Activity Program lay counsellor delivered treatment for severe depression in India: systematic development and randomised evaluation. British Journal of Psychiatry 2016;208(4):381-8. [DOI: 10.1192/bjp.bp.114.161075] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V, Weobong B, Weiss HA, Anand A, Bhat B, Katti B, et al. The Healthy Activity Program (HAP), a lay counsellor-delivered brief psychological treatment for severe depression, in primary care in India: a randomised controlled trial. Lancet (London, England) 2017;389(10065):176-85. [DOI: 10.1016/S0140-6736(16)31589-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V, Araya R, Chowdhary N, King M, Kirkwood B, Nayak S, et al. Detecting common mental disorders in primary care in India: a comparison of five screening questionnaires. Psychological Medicine 2008;38(2):221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weobong B, Weiss HA, McDaid D, Singla DR, Hollon SD, Nadkarni A, et al. Sustained effectiveness and cost-effectiveness of the Healthy Activity Programme, a brief psychological treatment for depression delivered by lay counsellors in primary care: 12-month follow-up of a randomised controlled trial. PLOS Medicine/Public Library of Science 2017;14(9):e1002385. [DOI: 10.1371/journal.pmed.1002385] [DOI] [PMC free article] [PubMed] [Google Scholar]
Peltzer 2013 {published data only}
- Peltzer K, Naidoo P, Louw J, Matseke G, Zuma K, McHunu G, et al. Screening and brief interventions for hazardous and harmful alcohol use among patients with active tuberculosis attending primary public care clinics in South Africa: results from a cluster randomized controlled trial. BMC Public Health 2013;13:699. [DOI: 10.1186/1471-2458-13-699] [DOI] [PMC free article] [PubMed] [Google Scholar]
Peltzer 2019 {published data only}
- Jones D, Peltzer K, Weiss SM, Sifunda S, Dwane N, Ramlagan S, et al. Implementing comprehensive prevention of mother-to-child transmission and HIV prevention for South African couples: study protocol for a randomized controlled trial. Trials 2014;15(1):417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltzer K, Abbamonte JM, Mandell LN, Rodriguez VJ, Lee TK, Weiss SM, et al. The effect of male involvement and a prevention of mother-to-child transmission (PMTCT) intervention on depressive symptoms in perinatal HIV-infected rural South African women. Archives of Women's Mental Health 2020;12(1):101-11. [DOI: 10.1007/s00737-019-00955-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pengpid 2013 {published data only}
- Pengpid S, Peltzer K, Heever H, Skaal L. Screening and brief interventions for hazardous and harmful alcohol use among university students in South Africa: results from a randomized controlled trial. International Journal of Environmental Research and Public Health 2013;10(5):2043-57. [DOI: 10.3390/ijerph10052043] [DOI] [PMC free article] [PubMed] [Google Scholar]
Petersen 2014 {published data only}
- Petersen I, Hanass Hancock J, Bhana A, Govender K. A group-based counselling intervention for depression comorbid with HIV/AIDS using a task shifting approach in South Africa: a randomized controlled pilot study. Journal of Affective Disorders 2014;158:78-84. [DOI: 10.1016/j.jad.2014.02.013] [DOI] [PubMed] [Google Scholar]
Pradeep 2014 {published data only}
- Pradeep J, Isaacs A, Shanbag D, Selvan S, Srinivasan K. Enhanced care by community health workers in improving treatment adherence to antidepressant medication in rural women with major depression. Indian Journal of Medical Research 2014;139(2):236-45. [PMC free article] [PubMed] [Google Scholar]
Rahman 2008 {published data only}
- Rahman A, Malik A, Sikander S, Roberts C, Creed F. Cognitive behaviour therapy-based intervention by community health workers for mothers with depression and their infants in rural Pakistan: a cluster-randomised controlled trial. Lancet 2008;372(9642):902-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A. Challenges and opportunities in developing a psychological intervention for perinatal depression in rural Pakistan - a multi-method study. Archives of Women's Mental Health 2007;10(5):211-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rahman 2016 {published data only}
- Rahman A, Hamdani SU, Awan NR, Bryant RA, Dawson KS, Khan MF, et al. Effect of a multicomponent behavioral intervention in adults impaired by psychological distress in a conflict-affected area of Pakistan: a randomized clinical trial. JAMA 2016;316(24):2609-17. [DOI: 10.1001/jama.2016.17165] [DOI] [PubMed] [Google Scholar]
- Sijbrandij M, Farooq S, Bryant RA, Dawson K, Hamdani SU, Chiumento A, et al. Problem management plus (PM+) for common mental disorders in a humanitarian setting in Pakistan; study protocol for a randomised controlled trial (RCT). BMC Psychiatry 2015;15:232. [DOI: 10.1186/s12888-015-0602-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijbrandij M, Farooq S, Bryant RA, Dawson K, Hamdani SU, Chiumento A, et al. Correction to: Problem management plus (PM+) for common mental disorders in a humanitarian setting in Pakistan; study protocol for a randomised controlled trial (RCT). BMC Psychiatry. 2018;18(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rahman 2019 {published data only}
- Chiumento A, Hamdani SU, Khan MN, Dawson K, Bryant RA, Sijbrandij M, et al. Evaluating effectiveness and cost-effectiveness of a group psychological intervention using cognitive behavioural strategies for women with common mental disorders in conflict-affected rural Pakistan: study protocol for a randomised controlled trial. Trials [electronic resource] 2017;18:190. [DOI: 10.1186/s13063-017-1905-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Hamdani SU, Awan NR, Bryant RA, Dawson KS, Khan MF, et al. Effect of a multicomponent behavioral intervention in adults impaired by psychological distress in a conflict-affected area of Pakistan: a randomized clinical trial. JAMA 2016;316(24):2609-17. [DOI] [PubMed] [Google Scholar]
- Rahman A, Khan MN, Hamdani SU, Chiumento A, Akhtar P, Nazir H, et al. Effectiveness of a brief group psychological intervention for women in a post-conflict setting in Pakistan: a single-blind, cluster, randomised controlled trial. Lancet (London, England) 2019;393(10182):1733-44. [DOI: 10.1016/S0140-6736(18)32343-2] [DOI] [PubMed] [Google Scholar]
- Sijbrandij M, Farooq S, Bryant RA, Dawson K, Hamdani SU, Chiumento A, et al. Correction to: Problem management plus (PM+) for common mental disorders in a humanitarian setting in Pakistan; study protocol for a randomised controlled trial (RCT). BMC Psychiatry 2018;18:331. [DOI: 10.1186/s12888-018-1922-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rojas 2007 {published data only}
- Rojas G, Fritsch R, Solis J, Jadresic E, Castillo C, Gonzalez M, et al. Treatment of postnatal depression in low-income mothers in primary-care clinics in Santiago, Chile: a randomised controlled trial. Lancet 2007;370(9599):1629-37. [DOI] [PubMed] [Google Scholar]
Shaw 2018 {published data only}
- Shaw SA, Ward KP, Pillai V, Hinton DE. A group mental health randomized controlled trial for female refugees in Malaysia. American Journal of Orthopsychiatry 2019;89(6):665-674. [DOI: 10.1037/ort0000346] [DOI] [PubMed] [Google Scholar]
Shen 2016 {published data only}
- Shen Y, Xiong Y, Li G. Application of club day rehabilitation model in patients with episode schizophrenia during recovery period. Hu Li Yan Jiu [Chinese Nursing Research] 2016;30:1453-5. [DOI: 10.3969/j.issn.1009-6493.2016.12.014] [DOI] [Google Scholar]
Sherman 2009 {published data only}
- German D, Sutcliffe CG, Sirirojn B, Sherman SG, Latkin CA, Aramrattana A, et al. Unanticipated effect of a randomised peer network intervention on depressive symptoms among young methamphetamine users in Thailand. Journal of Community Psychology 2012;40(7):799-813. [Google Scholar]
- Sutcliffe C, Srirojn B, Latkin CA, Aramratanna A, Celentano DD, Sherman SG. Evaluation of a peer network intervention trial among young methamphetamine users in Chiang Mai, Thailand. Social Science & Medicine 2009;68(1):69-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sikander 2019 {published data only}
- Atif N, Krishna RN, Sikander S, Lazarus A, Nisar A, Ahmad I, et al. Mother-to-mother therapy in India and Pakistan: adaptation and feasibility evaluation of the peer-delivered Thinking Healthy Programme. BMC Psychiatry 2017;17(1):79. [DOI: 10.1186/s12888-017-1244-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atif N, Nisar A, Bibi A, Khan S, Zulfiqar S, Ahmad I, et al. Scaling-up psychological interventions in resource-poor settings: training and supervising peer volunteers to deliver the 'Thinking Healthy Programme' for perinatal depression in rural Pakistan. Global Mental Health 2019;6:e4. [DOI: 10.1017/gmh.2019.4] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikander S, Ahmad I, Atif N, Zaidi A, Vanobberghen F, Weiss HA, et al. Delivering the Thinking Healthy Programme for perinatal depression through volunteer peers: a cluster randomised controlled trial in Pakistan. Lancet Psychiatry 2019;6(2):128-39. [DOI: 10.1016/S2215-0366(18)30467-X] [DOI] [PubMed] [Google Scholar]
- Sikander S, Lazarus A, Bangash O, Fuhr DC, Weobong B, Krishna RN, et al. The effectiveness and cost-effectiveness of the peer-delivered Thinking Healthy Programme for perinatal depression in Pakistan and India: the SHARE study protocol for randomised controlled trials. Trials 2015;16:534. [DOI: 10.1186/s13063-015-1063-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sorsdahl 2015 {published data only}
- Dwommoh R, Sorsdahl K, Myers B, Asante KP, Naledi T, Stein DJ, et al. Brief interventions to address substance use among patients presenting to emergency departments in resource poor settings: a cost-effectiveness analysis. Cost Effectiveness and Resource Allocation 2018;16:24. [DOI: 10.1186/s12962-018-0109-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers B, Westhuizen C, Naledi T, Stein DJ, Sorsdahl K. Readiness to change is a predictor of reduced substance use involvement: findings from a randomized controlled trial of patients attending South African emergency departments. BMC Psychiatry 2016;16:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorsdahl K, Stein D, Corrigall J, Cuijpers P, Smits N, Naledi T, et al. The efficacy of a blended motivational interviewing and problem solving therapy intervention to reduce substance use among patients presenting for emergency services in South Africa: a randomized controlled trial. Substance Abuse Treatment, Prevention, and Policy 2015;10:46. [DOI: 10.1186/s13011-015-0042-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tan 2005 {published data only}
- Tan B, Xiang EP, He YF. Effect of community prevention and treatment in patients with schizophrenia and their family economic burden [Chinese]. Zhongguo Lin Chuang Kang Fu [Chinese Journal of Clinical Rehabilitation] 2005;9(28):24-6. [Google Scholar]
Tol 2008 {published data only}
- Jordans MJ, Komproe IH, Tol WA, De Jong JT. Screening for psychosocial distress amongst war‐affected children: cross‐cultural construct validity of the CPDS. Journal of Child Psychology and Psychiatry 2009;50(4):514-23. [DOI] [PubMed] [Google Scholar]
- Jordans MJ, Komproe IH, Tol WA, Susanty D, Vallipuram A, Ntamatumba P, et al. Practice-driven evaluation of a multi-layered psychosocial care package for children in areas of armed conflict. Community Mental Health Journal 2011;47(3):267-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordans MJ, Ventevogel P, Komproe IH, Tol WA, Jong JT. Development and validation of the child psychosocial distress screener in Burundi. American Journal of Orthopsychiatry 2008;78(3):290-9. [DOI] [PubMed] [Google Scholar]
- Tol WA, Komproe IH, Jordans MJ, Gross AL, Susanty D, Macy RD, et al. Mediators and moderators of a psychosocial intervention for children affected by political violence. Journal of Consulting and Clinical Psychology 2010;78(6):818-28. [DOI] [PubMed] [Google Scholar]
- Tol WA, Komproe IH, Jordans MJ, Susanty D, De Jong JT. Developing a function impairment measure for children affected by political violence: a mixed methods approach in Indonesia. International Journal for Quality in Health Care 2011;23(4):375-83. [DOI] [PubMed] [Google Scholar]
- Tol WA, Komproe IH, Susanty D, Jordans MJ, Macy RD, De Jong Joop TV. School-based mental health intervention for children affected by political violence in Indonesia: a cluster randomised trial. JAMA 2008;300(6):655-62. [DOI] [PubMed] [Google Scholar]
Tol 2012 {published data only}
- Jordans MJ, Komproe IH, Tol WA, Susanty D, Vallipuram A, Ntamatumba P, et al. Practice-driven evaluation of a multi-layered psychosocial care package for children in areas of armed conflict. Community Mental Health Journal 2011;47(3):267-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tol WA, Komproe IH, Jordans MJ, Vallipuram A, Sipsma H, Sivayokan S, et al. Outcomes and moderators of a preventive school-based mental health intervention for children affected by war in Sri Lanka: a cluster randomised trial. World Psychiatry: Official Journal of the World Psychiatric Association (WPA) 2012;11(2):114-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tol 2020 {published data only}
- Brown FL, Carswell K, Augustinavicius J, Adaku A, Leku MR, White RG, et al. Self Help Plus: study protocol for a cluster-randomised controlled trial of guided self-help with South Sudanese refugee women in Uganda. Global Mental Health 2018;5:e27. [DOI: 10.1017/gmh.2018.17] [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRCTN50148022. Self-help plus (SH+) for South Sudanese refugees in Uganda. https://www.isrctn.com/ISRCTN50148022 2017. [DOI: 10.1186/ISRCTN50148022] [DOI]
- Tol WA, Leku MR, Lakin DP, Carswell K, Augustinavicius J, Adaku A, et al. Guided self-help to reduce psychological distress in South Sudanese female refugees in Uganda: a cluster randomised trial. Lancet Global Health 2020;8(2):e254-63. [DOI: 10.1016/S2214-109X(19)30504-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weiss 2015 {published data only}
- Mahmooth Z, Weiss WM, Zangana GA, Bolton P. Study participant reported outcomes of mental health interventions: results from a randomized controlled trial among survivors of systematic violence in southern Iraq. Global Mental Health 2018;5:e19. [DOI: 10.1017/gmh.2018.11] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss WM, Murray LK, Zangana GA, Mahmooth Z, Kaysen D, Dorsey S, et al. Community-based mental health treatments for survivors of torture and militant attacks in southern Iraq: a randomized control trial. BMC Psychiatry 2015;15:249. [DOI: 10.1186/s12888-015-0622-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 2016 {published data only}
- Wu F, Shi X, Mo Z. Self-care theory with collaborative care model applied in community-based rehabilitation of patients with mental illness. Hu Li Yan Jiu [Chinese Nursing Research] 2016;30:3495-8. [DOI: 10.3969/j.issn.1009-6493.2015.28.012] [DOI] [Google Scholar]
Xie 2019 {published data only}
- NCT02785211. A modified behavioral activation treatment for geriatric depressive symptoms in left-behind elderly in rural China. https://clinicaltrials.gov/show/NCT02785211; 2016.
- Xie J, He G, Ding S, Pan C, Zhang X, Zhou J, et al. A randomized study on the effect of modified behavioral activation treatment for depressive symptoms in rural left-behind elderly. Psychotherapy Research 2019;29(3):372-82. [DOI: 10.1080/10503307.2017.1364444] [DOI] [PubMed] [Google Scholar]
Yao 2014 {published data only}
- Yao ZZ, Xu Q, Wu LF, Fu WZ, Lu Y, Zhao AP. Effects of day nursing care in community on social function of patients with chronic schizophrenia. [Chinese]. Journal of Shanghai Jiaotong University (Medical Science) 2014;34(6):830-5. [DOI: ] [Google Scholar]
Yeomans 2010 {published data only}
- Yeomans PD, Forman EM, Herbert JD, Yuen E. A randomized trial of a reconciliation workshop with and without PTSD psychoeducation in Burundian sample. Journal of Traumatic Stress 2010;23(2):305-12. [DOI] [PubMed] [Google Scholar]
Zhong 2015 {published data only}
- Zhong N, Yuan Y, Chen H, Jiang H, Du J, Sun H, et al. Effects of a randomized comprehensive psychosocial intervention based on cognitive behavioral therapy theory and motivational interviewing techniques for community rehabilitation of patients with opioid use disorders in Shanghai, China. Journal of Addiction Medicine 2015;9(4):322-30. [DOI: 10.1097/ADM.0000000000000139] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adina 2017 {published data only}
- Adina JO, Maritim EK, Sindabi AM, Disiye MA. Effect of cognitive behaviour therapy on depressive symptoms among HIV-infected outpatients in Kenya. International Journal of Psychology & Psychological Therapy 2017;17(2):161-73. [Google Scholar]
Agberotimi 2017 {published data only}
- Agberotimi SF, Osinowo HO, Asagba RB. Efficacy of compassion-focused therapy in a sample of youth with substance use disorder in Ogbomoso, Nigeria. African Journal of Drug and Alcohol Studies 2017;16:107-16. [Google Scholar]
Akol 2018 {published data only}
- Akol A, Babirye JN, Makumbi F, Engebretsen IMS. Effect of primary health care worker training on identification of child-and adolescent mental health conditions: a randomised controlled trial in Uganda. Tropical Medicine & International Health 2017;22:283. [DOI: 10.1111/(ISSN)1365-3156] [DOI] [Google Scholar]
- Akol A, Makumbi F, Babirye JN, Nalugya JS, Nshemereirwe S, Engebretsen IMS. Does mhGAP training of primary health care providers improve the identification of child- and adolescent mental, neurological or substance use disorders? Results from a randomized controlled trial in Uganda. Global Mental Health 2018;5:e29. [DOI: 10.1017/gmh.2018.18] [DOI] [PMC free article] [PubMed] [Google Scholar]
Alexander 2013 {published data only}
- Alexander CL, Arnkoff D, Glass CR, Kaburu AW. Detecting depression in rural primary care clinics in central Kenya: impact of a brief training intervention. International Perspectives in Psychology: Research, Practice, Consultation 2013;2:14-28. [DOI: 10.1037/a0031360] [DOI] [Google Scholar]
Assanangkornchai 2012 {published data only}
- Assanangkornchai S, Nima P. Screening and brief intervention for harmful alcohol and drug use in primary care and community settings in Thailand. Alcoholism: Clinical and Experimental Research 2012;36:24A. [DOI: ] [Google Scholar]
Avci 2016 {published data only}
- Avci D, Kelleci M. Effects of the Anger Coping Programme based on cognitive behavioural techniques on adolescents' anger, aggression and psychological symptoms. International Journal of Nursing Practice 2016;22(2):189-96. [DOI: 10.1111/ijn.12410] [DOI] [PubMed] [Google Scholar]
Bagaiolo 2017 {published data only}
- Bagaiolo LF, Mari JJ, Bordini D, Ribeiro TC, Martone MCC, Caetano SC, et al. Procedures and compliance of a video modeling applied behavior analysis intervention for Brazilian parents of children with autism spectrum disorders. Autism 2017;21(5):603-10. [DOI: 10.1177/1362361316677718] [DOI] [PubMed] [Google Scholar]
Baker 2005a {published data only}
- Baker-Henningham H, Powell C. The effect of early stimulation on maternal depression: a cluster randomised controlled trial. Archives of Disease in Childhood 2005;80:1230-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bass 2012 {published data only}
- Bass J, Poudyal B, Tol W, Murray L, Nadison M, Bolton P. A controlled trial of problem-solving counselling for war-affected adults in Aceh, Indonesia. Social Psychiatry and Psychiatric Epidemiology 2012;47(2):279-91. [DOI] [PubMed] [Google Scholar]
Botha 2018 {published data only}
- Botha UA, Koen L, Mazinu M, Jordaan E, Niehaus DJ. Brief report: a randomized control trial assessing the influence of a telephone-based intervention on readmissions for patients with severe mental illness in a developing country. Community Mental Health Journal 2018;54(2):197-203. [DOI: 10.1007/s10597-016-0069-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2009 {published data only}
- Brown L, Thurman TR. Impact of a mentoring program on psychosocial wellbeing of youth in Rwanda: results of a quasi-experimental study. Vulnerable Children and Youth Studies 2009;4(4):288-99. [Google Scholar]
Cai 2015 {published data only}
- Cai J, Zhu Y, Zhang W, Wang Y, Zhang C. Comprehensive family therapy: an effective approach for cognitive rehabilitation in schizophrenia. Neuropsychiatric Disease and Treatment 2015;11:1247-53. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chaowanee 2018 {published data only}
- Chaowanee L, Darawan T, Ratchneewan R, Wanchai L. The Thai group cognitive behavior therapy intervention program for depressive symptoms among older women: a randomized controlled trial. Pacific Rim International Journal of Nursing Research 2018;22:74-85. [Google Scholar]
Chawarski 2008 {published data only}
- Chawarski MC, Mazlan M, Schottenfeld RS. Behavioral drug and HIV risk reduction counseling (BDRC) with abstinence-contingent take-home buprenorphine: a pilot randomized clinical trial. Drug and Alcohol Dependence 2008;94(1-3):281-4. [DOI: 10.1016/j.drugalcdep.2007.11.008] [DOI] [PubMed] [Google Scholar]
Chen 2000 {published data only}
- Chen CH, Tseng YF, Chou F-H, Wang S-Y. Effects of support group intervention in postnatally distressed women: a controlled study in Taiwan. Journal of Psychosomatic Research 2000;49:395-9. [DOI] [PubMed] [Google Scholar]
Chien 2017 {published data only}
- Chien WT, Bressington D, Yip A, Karatzias T. An international multi-site, randomized controlled trial of a mindfulness-based psychoeducation group programme for people with schizophrenia. Psychological Medicine 2017;47(12):2081-96. [DOI: 10.1017/S0033291717000526] [DOI] [PMC free article] [PubMed] [Google Scholar]
Conde 2018 {published data only}
- Conde K, Brandariz RA, Lichtenberger A, Cremonte M. The effectiveness of a brief intervention for reducing adolescent alcohol consumption. Revista Ciencias de la Salud 2018;16:393-407. [DOI: 10.12804/revistas.urosario.edu.co/revsalud/a.7261] [DOI] [Google Scholar]
Damra‐Jalal 2014 {published data only}
- Damra Jalal KM, Nassar YH, Ghabri Thaer MF. Trauma-focused cognitive behavioral therapy: cultural adaptations for application in Jordanian culture. Counselling Psychology Quarterly 2014;27(3):308-23. [DOI: ] [Google Scholar]
Danaee‐Far 2016 {published data only}
- Danaee-Far M, Maarefvand M, Rafiey H. Effectiveness of a brief home-based social work motivational intervention for male methamphetamine users in Tehran: a randomized clinical trial. Substance Use & Misuse 2016;51(14):1863-9. [DOI: 10.1080/10826084.2016.1200620] [DOI] [PubMed] [Google Scholar]
Dawson 2018 {published data only}
- Dawson K, Joscelyne A, Meijer C, Steel Z, Silove D, Bryant RA. A controlled trial of trauma-focused therapy versus problem-solving in Islamic children affected by civil conflict and disaster in Aceh, Indonesia. Australian and New Zealand Journal of Psychiatry 2018;52(3):253-61. [DOI: 10.1177/0004867417714333] [DOI] [PubMed] [Google Scholar]
Firn 2019 {published data only}
- Firn M, Alonso-Vicente M, Hubbeling D. The Ferrari v the bicycle. Luo X, Law SF, Wang X, et al. Effectiveness of an assertive community treatment program for people with severe schizophrenia in mainland China – a 12-month randomized controlled trial. Psychological Medicine 2018. Cambridge University Press 2019;49:1. [DOI: 10.1017/s0033291718002179] [DOI] [PubMed] [Google Scholar]
Fleischmann 2008 {published data only}
- Fleischmann A, Bertolote JM, Wasserman D, De Leo D, Bolhari J, Botega NJ, et al. Effectiveness of brief intervention and contact for suicide attempters: a randomized controlled trial in five countries. Bulletin of the World Health Organization 2008;86(9):703-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Green 2019 {published data only}
- Green EP, Cho H, Gallis J, Puffer ES. The impact of school support on depression among adolescent orphans: a cluster-randomized trial in Kenya. Journal of Child Psychology and Psychiatry and Allied Disciplines 2019;60(1):54-62. [DOI: 10.1111/jcpp.12955] [DOI] [PubMed] [Google Scholar]
Gu 2013 {published data only}
- Gu J, Lau JT, Xu H, Zhong Y, Hao Y, Zhao Y, et al. A randomized controlled trial to evaluate the relative efficacy of the addition of a psycho-social intervention to standard-of-care services in reducing attrition and improving attendance among first-time users of methadone maintenance treatment in China. AIDS and Behavior 2013;17(6):2002-10. [DOI: 10.1007/s10461-012-0393-9] [DOI] [PubMed] [Google Scholar]
Guerra 2011 {published data only}
- Guerra M, Ferri CP, Fonseca M, Banerjee S, Prince M. Helping carers to care: the 10/66 dementia research group's randomized control trial of a caregiver intervention in Peru. Revista Brasileira de Psiquiatria 2011;33(1):47. [DOI] [PubMed] [Google Scholar]
Guo 2019a {published data only}
- Guo ZH, Li ZJ, Ma Y, Sun J, Guo JH, Li WX, et al. Erratum: brief cognitive-behavioural therapy for patients in the community with schizophrenia: randomised controlled trial in Beijing, China - expression of concern (British Journal of Psychiatry 2017;210:3(223-9). DOI: 10.1192/bjp.bp.116.183285). British Journal of Psychiatry 2019;214:119. [DOI: 10.1192/bjp.2018.198] [DOI] [PubMed] [Google Scholar]
Hamadani 2006 {published data only}
- Hamadani JD, Huda SN, Khatun F, Grantham-McGregor SM. Psychosocial stimulation improves the development of undernourished children in rural Bangladesh. Journal of Nutrition 2006;136(10):2645-52. [DOI] [PubMed] [Google Scholar]
Hamdani 2017a {published data only}
- Hamdani SU, Ahmed Z, Sijbrandij M, Nazir H, Masood A, Akhtar P, et al. Problem Management Plus (PM+) in the management of common mental disorders in a specialized mental healthcare facility in Pakistan; study protocol for a randomized controlled trial. International Journal of Mental Health Systems 2017;11:40. [DOI: 10.1186/s13033-017-0147-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Han 2013 {published data only}
- Han CK, Ssewamala FM, Wang JS. Family economic empowerment and mental health among AIDS-affected children living in AIDS-impacted communities: evidence from a randomised evaluation in southwestern Uganda. Journal of Epidemiology and Community Health 2013;67(3):225-30. [DOI: 10.1136/jech-2012-201601] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hassanzadeh 2010 {published data only}
- Hassanzadeh M, Khajeddin N, Nojomi M, Fleischmann A, Eshrati T. Brief intervention and contact after deliberate self-harm: an Iranian randomized controlled trial. Iranian Journal of Psychiatry and Behavioral Sciences 2010;4(2):5-12. [Google Scholar]
Hegde 2012 {published data only}
- Hegde S, Rao SL, Raguram A, Gangadhar BN. Addition of home-based cognitive retraining to treatment as usual in first episode schizophrenia patients: a randomized controlled study. Indian Journal of Psychiatry 2012;54(1):15-22. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Huang 2017 {published data only}
- Huang KY, Nakigudde J, Rhule D, Gumikiriza-Onoria JL, Abura G, Kolawole B, et al. Transportability of an evidence-based early childhood intervention in a low-income African country: results of a cluster randomized controlled study. Prevention Science 2017;18(8):964-75. [DOI: 10.1007/s11121-017-0822-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Huang 2018 {published data only}
- Huang H-C, Liu S-I, Hwang L-C, Sun F-J, Tjung J-J, Huang C-R, et al. The effectiveness of culturally sensitive collaborative treatment of depressed Chinese in family medicine clinics: a randomized controlled trial. General Hospital Psychiatry 2018;50:96-103. [DOI: 10.1016/j.genhosppsych.2017.10.002] [DOI] [PubMed] [Google Scholar]
IRCT2016042527584N1 2016 {published data only}
- IRCT2016042527584N1. Effect of mindfulness based on acceptance and commitment on mental health of the elderly. http://www.who.int/trialsearch/trial2.aspx? Trialid=irct2016042527584n1; 2016.
ISRCTN72875710 2014 {published data only}
- ISRCTN72875710. An evaluation of the effectiveness of a psychological treatment for moderate to severe depression and harmful or dependent drinking in rural communities in Nepal. http://www.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN72875710; 2014.
Jacob 2014 {published data only}
- Jacob N, Neuner F, Maedl A, Schaal S, Elbert T. Dissemination of psychotherapy for trauma spectrum disorders in postconflict settings: a randomized controlled trial in Rwanda. Psychotherapy and Psychosomatics 2014;83(6):354-63. [DOI: 10.1159/000365114] [DOI] [PubMed] [Google Scholar]
Jacobs 2016 {published data only}
- Jacobs P, Estrada YA, Tapia MI, Quevedo Teran AM, Condo Tamayo C, Alban Garcia M, et al. Familias Unidas for high risk adolescents: study design of a cultural adaptation and randomized controlled trial of a U.S. drug and sexual risk behavior intervention in Ecuador. Contemporary Clinical Trials 2016;47:244-53. [DOI: 10.1016/j.cct.2016.01.014] [DOI] [PubMed] [Google Scholar]
Kauye 2014 {published data only}
- Kauye F, Jenkins R, Rahman A. Training primary health care workers in mental health and its impact on diagnoses of common mental disorders in primary care of a developing country, Malawi: a cluster-randomized controlled trial. Psychological Medicine 2014;44(3):657-66. [DOI: 10.1017/S0033291713001141] [DOI] [PubMed] [Google Scholar]
Knettel 2019 {published data only}
- Knettel BA, Mulawa MI, Knippler ET, Ciya N, Robertson C, Joska JA, et al. Women’s perspectives on ImpACT: a coping intervention to address sexual trauma and improve HIV care engagement in Cape Town, South Africa. AIDS Care - Psychological and Socio-medical Aspects of AIDS/HIV 2019;31(11):1389-96. [DOI: 10.1080/09540121.2019.1587368] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lara 2010 {published data only}
- Lara MA, Navarro C, Navarrete L. Outcome results of a psycho-educational intervention in pregnancy to prevent PPD: a randomized control trial. Journal of Affective Disorders 2010;122(1-2):109-17. [DOI: 10.1016/j.jad.2009.06.024] [DOI] [PubMed] [Google Scholar]
Layne 2008 {published data only}
- Layne CM, Saltzman WR, Poppleton L, Burlingame GM, Pasalic A, Durakovic E, et al. Effectiveness of a school-based group psychotherapy program for war-exposed adolescents: a randomized controlled trial. Journal of the American Academy of Child and Adolescent Psychiatry 2008;47(9):1048-62. [DOI: 10.1097/CHI.0b013e31817eecae] [DOI] [PubMed] [Google Scholar]
Li 1989 {published data only}
- Li SC. A report on a feasibility test of "community control of epilepsy" proposed by WHO. Chinese Journal of Neurology and Psychiatry 1989;22(3):144-7. [PubMed] [Google Scholar]
Li 2017 {published data only}
- Li J. A randomized controlled study to evaluate the efficacy of a positive psychology and social networking intervention in reducing depressive symptoms among HIV-infected men who have sex with men in China. Dissertation Abstracts International: Section B: The Sciences and Engineering 2017;77:No Pagination Specified. [Google Scholar]
Li 2017a {published data only}
- Li R, Shi G, Ji J, Wang H, Wang W, Wang M, et al. A 2-year longitudinal psychological intervention study on the prevention of Internet addiction in junior high school students of Jinan City. Biomedical Research (India) 2017;28(22):10033-8. [Google Scholar]
Li 2018a {published data only}
- Li J, Huang YG, Ran MS, Fan Y, Chen W, Evans-Lacko S, et al. Community-based comprehensive intervention for people with schizophrenia in Guangzhou, China: effects on clinical symptoms, social functioning, internalized stigma and discrimination. Asian Journal of Psychiatry 2018;34:21-30. [DOI: 10.1016/j.ajp.2018.04.017] [DOI] [PubMed] [Google Scholar]
Li 2018b {published data only}
- Li HH, Li CL, Gao D, Pan XY, Du L, Jia FY. Preliminary application of Early Start Denver Model in children with autism spectrum disorder. Zhongguo Dangdai Erke Zazhi 2018;20:793-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liao 2018 {published data only}
- Liao SJ, Chong MC, Tan MP, Chua YP. Tai Chi with music improves quality of life among community-dwelling older persons with mild to moderate depressive symptoms: a cluster randomized controlled trial. Geriatric Nursing (New York, N.Y.) 2019;40(2):154-9. [DOI: 10.1016/j.gerinurse.2018.08.001] [DOI] [PubMed] [Google Scholar]
- Liao SJ, Tan MP, Chong MC, Chua YP. The impact of combined music and tai chi on depressive symptoms among community-dwelling older persons: a cluster randomized controlled trial. Issues in Mental Health Nursing 2018;39(5):398-402. [DOI: 10.1080/01612840.2017.1417519] [DOI] [PubMed] [Google Scholar]
Liu 2005 {published data only}
- Liu H-Z, Zheng Y, Chen X-S, Jia J-P, Ye Y. Effects of early psychosocial intervention on behavior problems of the only child [Chinese]. Chinese Journal of Clinical Rehabilitation 2005;9(32):98-100. [Google Scholar]
Liu 2018 {published data only}
- Liu P, Song R, Zhang Y, Liu C, Cai B, Liu X, et al. Educational and behavioral counseling in a methadone maintenance treatment program in China: a randomized controlled trial. Frontiers in Psychiatry 2018;9(no pagination):113. [DOI: 10.3389/fpsyt.2018.00113] [DOI] [PMC free article] [PubMed] [Google Scholar]
Long 2015 {published data only}
- Long F, Yan J, Hu P, Xia M, Liu H, Gu C. Effect of group counseling on depression, compliance and blood sugar level in diabetic patients. Zhong Nan da Xue Xue Bao. Yi Xue Ban [Journal of Central South University. Medical Sciences] 2015;40(8):879-85. [DOI: 10.11817/j.issn.1672-7347.2015.08.009] [DOI] [PubMed] [Google Scholar]
Loughry 2006 {published data only}
- Loughry M, Ager A, Flouri E, Khamis V, Afana AH, Qouta S. The impact of structured activities among Palestinian children in a time of conflict. Journal of Child Psychology and Psychiatry and Allied Disciplines 2006;47(12):1211-8. [DOI] [PubMed] [Google Scholar]
Manwong 2018 {published data only}
- Manwong M, Lohsoonthorn V, Booranasuksakul T, Chaikoolvatana A. Effects of a group activity-based motivational enhancement therapy program on social media addictive behaviors among junior high school students in Thailand: a cluster randomized trial. Psychology Research & Behavior Management 2018;11:329-39. [DOI: 10.2147/PRBM.S168869] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marchira 2018 {published data only}
- Marchira CR, Suprianto I, Subandi MA, Del Vecchio Good M-J, Good BJ. The effectiveness of a brief interactive psychoeducational intervention for persons living with first episode psychosis and family caregivers in Yogyakarta, Indonesia. Early Intervention in Psychiatry 2018;12:28. [DOI: 10.1111/eip.12722] [DOI] [Google Scholar]
Marchira 2019 {published data only}
- Marchira CR, Supriyanto I, Subandi S, Good MJD, Good BJ. Brief interactive psychoeducation for caregivers of patients with early phase psychosis in Yogyakarta, Indonesia. Early Intervention in Psychiatry 2019;13:469-76. [DOI: 10.1111/eip.12506] [DOI] [PubMed] [Google Scholar]
McMullen 2013 {published data only}
- McMullen J, O'Callaghan P, Shannon C, Black A, Eakin J. Group trauma-focused cognitive-behavioural therapy with former child soldiers and other war-affected boys in the DR Congo: a randomised controlled trial. Journal of Child Psychology and Psychiatry and Allied Disciplines 2013;54(11):1231-41. [DOI: 10.1111/jcpp.12094] [DOI] [PubMed] [Google Scholar]
Mehrabi 2011 {published data only}
- Mehrabi T, Musavi T, Ghazavi Z, Zandieh Z, Zamani A. The impact of group therapy training on social communications of Afghan immigrants. Iranian Journal of Nursing and Midwifery Research 2011;16(2):148-52. [PMC free article] [PubMed] [Google Scholar]
Miranda‐Castillo 2013 {published data only}
- Miranda-Castillo C, Tapia FM, Herrera AR, Ghigliotto FM, Guerra LS. Implementation of a cognitive stimulation program for people with Alzheimer disease: a pilot study in a Chilean elderly sample. Universitas Psychologica 2013;12(2):445-55. [Google Scholar]
Molazem 2014 {published data only}
- Molazem Z, Falahati T, Jahanbin I, Jafari P, Ghadakpour S. The effect of psycho-educational interventions on the quality of life of the family caregivers of the patients with spinal cord injury: a randomized controlled trial. International Journal of Community Based Nursing & Midwifery 2014;2:31-9. [PMC free article] [PubMed] [Google Scholar]
Moshki 2014 {published data only}
- Moshki M, Hassanzade T, Taymoori P. Effect of life skills training on drug abuse preventive behaviors among university students. International Journal of Preventive Medicine 2014;5(5):577-83. [PMC free article] [PubMed] [Google Scholar]
Mousavi 2019 {published data only}
- Mousavi S, Pahlavanzadeh S, Maghsoudi J. Evaluating the effect of a need-based program for caregivers on the stress, anxiety, depression, and the burden of care in families of children with attention deficit-hyperactive disorder. Iranian Journal of Nursing and Midwifery Research 2019;24:96-101. [DOI: 10.4103/ijnmr.IJNMR_11_17] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mushtaq 2017 {published data only}
- Mushtaq A, Lochman JE, Tariq PN, Sabih F. Preliminary effectiveness study of coping power program for aggressive children in Pakistan. Prevention Science 2017;18(7):762-71. [DOI: 10.1007/s11121-016-0721-9] [DOI] [PubMed] [Google Scholar]
Mutiso 2018 {published data only}
- Mutiso V, Tele A, Gitonga I, Musau A, Musyimi C, Ndetei D. Effectiveness of life skills education and psychoeducation on emotional and behavioral problems among adolescents in institutional care in Kenya: a longitudinal study. Child and Adolescent Mental Health 2018;23(4):351-8. [DOI: 10.1111/camh.12232] [DOI] [PubMed] [Google Scholar]
Naeem 2011 {published data only}
- Naeem F, Waheed W, Gobbi M, Ayub M, Kingdon D. Preliminary evaluation of culturally sensitive CBT for depression in Pakistan: findings from developing culturally-sensitive CBT project (DCCP). Behavioural and Cognitive Psychotherapy 2011;39(2):165-73. [DOI: 10.1017/s1352465810000822] [DOI] [PubMed] [Google Scholar]
Nagarajaiahnull 2012 {published data only}
- Nagarajaiah N, Jothimani G, Parthasarathi R, Reddemma K, Giri AT. Efficacy of nursing interventions in reducing social and occupational disabilities among patients with neurosis. Nursing Journal of India 2012;103(6):256-60. [PubMed] [Google Scholar]
Naidoo 2014 {published data only}
- Naidoo SS, Gathiram P, Schlebusch L. Effectiveness of a buddy intervention support programme for suicidal behaviour in a primary care setting. South African Family Practice 2014;56(5):263-70. [DOI: 10.1080/20786190.2014.980159] [DOI] [Google Scholar]
Nakimuli‐Mpungu 2014 {published data only}
- Nakimuli-Mpungu E, Wamala K, Okello J, Alderman S, Odokonyero R, Musisi S, et al. Outcomes, feasibility and acceptability of a group support psychotherapeutic intervention for depressed HIV-affected Ugandan adults: a pilot study. Journal of Affective Disorders 2014;166:144-50. [DOI: ] [DOI] [PubMed] [Google Scholar]
NCT00539123 2007 {published data only}
- NCT00539123. Drug counseling and abstinent-contingent take-home buprenorphine in Malaysia. https://clinicaltrials.gov/show/NCT00539123; 2007.
NCT01523444 2016 {published data only}
- NCT01523444. Advancing adolescent screening and brief intervention protocols in primary care settings. https://clinicaltrials.gov/ct2/show/NCT01523444; 2016.
NCT02598024 2015 {published data only}
- NCT02598024. Treating earthquake in Nepal trauma (TENT) trial 2016. https://clinicaltrials.gov/show/NCT02598024; 2015.
NCT03754829 2018 {published data only}
- NCT03754829. Effectiveness of a web and mobile guided psychological intervention for depressive symptoms in Turkey. https://clinicaltrials.gov/show/nct03754829; 2018. [DOI] [PMC free article] [PubMed]
NCT03884933 2020 {published data only}
- NCT03884933. Large scale implementation of community based mental health care for people with severe and enduring mental ill health in Europe. https://clinicaltrials.gov/ct2/show/NCT03884933; 2019.
NCT03912753 2019 {published data only}
- NCT03912753. Building mobile HIV prevention and mental health support in low-resource settings. https://clinicaltrials.gov/show/nct03912753; 2019.
NCT03966885 2020 {published data only}
- NCT03966885. Zambia common elements treatment approach pilot study. https://clinicaltrials.gov/ct2/show/NCT03966885; 2020.
Nwobi 2018 {published data only}
- Nwobi UA, Eseadi C, Emeka O, Ekwealor N, Ogbonnaya KA, Oboegbulem AI, et al. A stress management intervention for adults living with HIV in Nigerian community settings: an effects study. Medicine 2018;97(44):e12801. [DOI: 10.1097/MD.0000000000012801] [DOI] [PMC free article] [PubMed] [Google Scholar]
Orang 2018 {published data only}
- Orang T, Ayoughi S, Moran JK, Ghaffari H, Mostafavi S, Rasoulian M, et al. The efficacy of narrative exposure therapy in a sample of Iranian women exposed to ongoing intimate partner violence - a randomized controlled trial. Clinical Psychology & Psychotherapy 2018;25(6):827-41. [DOI: 10.1002/cpp.2318] [DOI] [PubMed] [Google Scholar]
Orawan 2018 {published data only}
- Orawan P, Linchong P, Khanokporn S, Totsaporn K. A randomized controlled trial of enhancing positive aspects of caregiving in Thai dementia caregivers for dementia. Pacific Rim International Journal of Nursing Research 2018;22(2):131-43. [Google Scholar]
Oveisi 2010 {published data only}
- Oveisi S, Ardabili HE, Dadds MR, Majdzadeh R, Mohammadkhani P, Rad JA, et al. Primary prevention of parent-child conflict and abuse in Iranian mothers: a randomized-controlled trial. Child Abuse & Neglect 2010;34(3):206-13. [DOI: 10.1016/j.chiabu.2009.05.008] [DOI] [PubMed] [Google Scholar]
PACTR201310000589295 2013 {published data only}
- PACTR201310000589295. Psychosocial treatment for methamphetamine use disorders in South Africa: a pilot study of a cognitive behavioural therapy program. http://www.who.int/trialsearch/Trial2.aspx?TrialID=PACTR201310000589295; 2013.
Paddick 2017 {published data only}
- Paddick S-M, Mkenda S, Mbowe G, Kisoli A, Gray WK, Dotchin CL, et al. Cognitive stimulation therapy as a sustainable intervention for dementia in sub-Saharan Africa: feasibility and clinical efficacy using a stepped-wedge design - ERRATUM. International Psychogeriatrics 2017;29(6):979-89. [DOI: 10.1017/S1041610217000163] [DOI] [PubMed] [Google Scholar]
Pahlavanzadeh 2010 {published data only}
- Pahlavanzadeh S, Heidari FG, Maghsudi J, Ghazavi Z, Samandari S. The effects of family education program on the caregiver burden of families of elderly with dementia disorders. Iranian Journal of Nursing and Midwifery Research 2010;15(3):102. [PMC free article] [PubMed] [Google Scholar]
Palavras 2015 {published data only}
- Palavras MA, Hay P, Touyz S, Sainsbury A, da Luz F, Swinbourne J, et al. Comparing cognitive behavioural therapy for eating disorders integrated with behavioural weight loss therapy to cognitive behavioural therapy-enhanced alone in overweight or obese people with bulimia nervosa or binge eating disorder: study protocol for a randomised controlled trial. Trials [electronic resource] 2015;16(no pagination):578. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pang 2002 {published data only}
- Pang Y, Lin C, Wang S, 庞月岱, 王淑君, 李秀华, et al. The effect of health education on compliance of patients with paranoid schizophrenia in their follow up therapy. Chinese Mental Health Journal 2002;16(5):348-50. [Google Scholar]
Prince 2007 {published data only}
- Prince M. 10/66 Dementia Research Group randomised controlled trial: helping carers to care - Peru. http://www.who.int/trialsearch/trial2.aspx? Trialid=isrctn66355402; 2007.
Qouta 2018 {published data only}
- Qouta SR, Palosaari E, Diab M, Punamaki R-L. "Intervention effectiveness among war-affected children: a cluster randomized controlled trial on improving mental health": erratum. Journal of Traumatic Stress 2018;31:163-4. [DOI: 10.1002/jts.22225] [DOI] [PubMed] [Google Scholar]
Rahman 2019a {published data only}
- Rahman A, Akhtar P, Hamdani SU, Atif N, Nazir H, Uddin I, et al. Using technology to scale-up training and supervision of community health workers in the psychosocial management of perinatal depression: a non-inferiority, randomized controlled trial. Global Mental Health 2019;6:e8. [DOI: 10.1017/gmh.2019.7] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafar S, Sikander S, Hamdani SU, Atif N, Akhtar P, Nazir H, et al. The effectiveness of technology-assisted cascade training and supervision of community health workers in delivering the Thinking Healthy Program for perinatal depression in a post-conflict area of Pakistan - study protocol for a randomized controlled trial. Trials [electronic resource] 2016;17:188. [DOI: 10.1186/s13063-016-1308-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ran 2003 {published data only}
- Ran M-S, Xiang M-Z, Chan CL-W, Leff J, Simpson P, Huang M-S, et al. Effectiveness of psychoeducational intervention for rural Chinese families experiencing schizophrenia-a randomised controlled trial. Social Psychiatry and Psychiatric Epidemiology 2003;38(2):69-75. [DOI] [PubMed] [Google Scholar]
Razali 2000 {published data only}
- Razali SM, Hasanah CI, Khan UA, Subramaniam M. Psychosocial interventions for schizophrenia. Journal of Mental Health (Abingdon, England) 2000;9(3):283-9. [Google Scholar]
Reddy 2019 {published data only}
- Reddy NV, Omkarappa DB. Cognitive-behavioral therapy for depression among menopausal woman: a randomized controlled trial. Journal of Family Medicine & Primary Care 2019;8:1002-6. [DOI: 10.4103/jfmpc.jfmpc_396_18] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ross 2013 {published data only}
- Ross R, Sawatphanit W, Suwansujarid T, Stidham AW, Drew BL, Creswell JW. The effect of telephone support on depressive symptoms among HIV-infected pregnant women in Thailand: an embedded mixed methods study. Journal of the Association of Nurses in AIDS Care 2013;24(5):e13-24. [DOI: 10.1016/j.jana.2012.08.005] [DOI] [PubMed] [Google Scholar]
Rossouw 2018 {published data only}
- Rossouw J, Yadin E, Alexander D, Mbanga I, Jacobs T, Seedat S. A pilot and feasibility randomised controlled study of prolonged exposure treatment and supportive counselling for post-traumatic stress disorder in adolescents: a third world, task-shifting, community-based sample. Trials [electronic resource] 2016;17(1):548. [DOI: 10.1186/s13063-016-1677-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossouw J, Yadin E, Alexander D, Seedat S. Prolonged exposure therapy and supportive counselling for post-traumatic stress disorder in adolescents: task-shifting randomised controlled trial. British Journal of Psychiatry 2018;213:587-94. [DOI: 10.1192/bjp.2018.130] [DOI] [PubMed] [Google Scholar]
- Rossouw J, Yadin E, Alexander D, Seedat S. Prolonged exposure therapy and supportive counselling for posttraumatic stress disorder in adolescents in a community-based sample, including experiences of stakeholders: study protocol for a comparative randomized controlled trial using task-shifting. BMC Psychiatry 2018;18:288. [DOI: 10.1186/s12888-018-1873-x] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossouw J, Yadin E, Mbanga I, Jacobs T, Rossouw W, Alexander D, et al. Prolonged exposure treatment for PTSD in a third-world, taskshifting, community-based environment. South African Journal of Psychiatry 2015;21:127. [DOI: 10.7196/SAJP.8782] [DOI] [Google Scholar]
- de Water T, Rossouw J, Yadin E, Seedat S. Impediments and catalysts to task-shifting psychotherapeutic interventions for adolescents with PTSD: perspectives of multi-stakeholders. Child & Adolescent Psychiatry & Mental Health [electronic resource] 2017;11:48. [DOI: 10.1186/s13034-017-0187-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
Samaraweera 2007 {published data only}
- Samaraweera S, Sivayogan S, Sumathipala A, Bhugra D, Siribaddana S. RCT of cognitive behaviour therapy in active suicidal ideation - a feasibility study in Sri Lanka. European Journal of Psychiatry 2007;21(3):175-8. [DOI: 10.4321/S0213-61632007000300001] [DOI] [Google Scholar]
Schade 2011 {published data only}
- Schade N, Torres P, Beyebach M. Cost-efficiency of a brief family intervention for somatoform patients in primary care. Families Systems & Health 2011;29(3):197-205. [DOI: 10.1037/a0024563] [DOI] [PubMed] [Google Scholar]
Schapira 2010 {published data only}
- Schapira M, Camera L, Finkelsztein C, Matusevich D, Smietniansky M, Esteban J, et al. A multidisciplinary program for the treatment and follow up of depression in ambulatory elderly. Vertex: Revista Argentina de Psiquiatria 2010;21(92):284-90. [PubMed] [Google Scholar]
Senanarong 2004 {published data only}
- Senanarong V, Jamjumras P, Harmphadungkit K, Klubwongs M, Udomphanthurak S, Poungvarin N, et al. A counseling intervention for caregivers: effect on neuropsychiatric symptoms. International Journal of Geriatric Psychiatry 2004;19(8):781-8. [DOI] [PubMed] [Google Scholar]
Sharifi 2012 {published data only}
- Sharifi V, Tehranidoost M, Yunesian M, Amini H, Mohammadi M, Roudsari MJ. Effectiveness of a low-intensity home-based aftercare for patients with severe mental disorders: a 12-month randomized controlled study. Community Mental Health Journal 2012;48(6):766-70. [DOI: 10.1007/s10597-012-9516-z] [DOI] [PubMed] [Google Scholar]
Sheikh 2017 {published data only}
- Sheikh WA, Paul R, Banda H, Agath K, Luty J. Impact of brief relapse prevention intervention in patients with alcohol dependence in Zambia. Journal of Substance Use 2017;22(1):113-7. [DOI: 10.3109/14659891.2015.1090494] [DOI] [Google Scholar]
Shen 2018 {published data only}
- Shen L, Yang L, Zhang J, Zhang M. Benefits of expressive writing in reducing test anxiety: a randomized controlled trial in Chinese samples. PLOS ONE [electronic resource] 2018;13(2):e0191779. [DOI: 10.1371/journal.pone.0191779] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shin 2009 {published data only}
- Shin JY, Nhan NV, Lee S-B, Crittenden KS, Flory M, Hong HTD. The effects of a home-based intervention for young children with intellectual disabilities in Vietnam. Journal of Intellectual Disability Research 2009;53(4):339-52. [DOI] [PubMed] [Google Scholar]
Shin 2013 {published data only}
- Shin S, Livchits V, Connery HS, Shields A, Yanov S, Yanova G, et al, Tomsk Tuberculosis Alcohol Working Group. Effectiveness of alcohol treatment interventions integrated into routine tuberculosis care in Tomsk, Russia. Addiction (Abingdon, England) 2013;108(8):1387-96. [DOI: 10.1111/add.12148] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shin 2017 {published data only}
- Shin JY, Nguyen Duc S. The effects of a home-based intervention conducted by college students for young children with developmental delays in Vietnam. International Journal of Developmental Disabilities 2017;63(2):110-23. [DOI: 10.1080/20473869.2016.1144316] [DOI] [PMC free article] [PubMed] [Google Scholar]
Soares 2018 {published data only}
- Soares J, Vargas D. Effectiveness of brief group intervention in the harmful alcohol use in primary health care. Revista de Saude Publica 2018;53:4. [DOI: 10.11606/S1518-8787.2019053000498] [DOI] [PMC free article] [PubMed] [Google Scholar]
Steinert 2017 {published data only}
- Steinert C, Bumke PJ, Hollekamp RL, Larisch A, Leichsenring F, Mattheß H, et al. Resource activation for treating post-traumatic stress disorder, co-morbid symptoms and impaired functioning: a randomized controlled trial in Cambodia. Psychological Medicine 2017;47(3):553-64. [DOI: 10.1017/S0033291716002592] [DOI] [PubMed] [Google Scholar]
Sumathipala 2008 {published data only}
- Sumathipala A, Siribaddana S, Abeysingha MR, De Silva P, Dewey M, Prince M, et al. Cognitive-behavioural therapy v. structured care for medically unexplained symptoms: randomised controlled trial. British Journal of Psychiatry 2008;193(1):51-9. [DOI: 10.1192/bjp.bp.107.043190] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tao 2015 {published data only}
- Tao J, Zeng Q, Liang J, Zhou A, Yin X, Xu A. Effects of cognitive rehabilitation training on schizophrenia: 2 years of follow-up. African Journal of Psychiatry (South Africa) 2015;18(SpeciaI Issue):1-4. [DOI: ] [PMC free article] [PubMed] [Google Scholar]
Thabet 2005 {published data only}
- Thabet AA, Vostanis P, Karim K. Group crisis intervention for children during ongoing war conflict. European Child & Adolescent Psychiatry 2005;14(5):262-9. [DOI] [PubMed] [Google Scholar]
Thomas 2017 {published data only}
- Thomas B, Watson B, Senthil EK, Deepalakshmi A, Balaji G, Chandra S, et al. Alcohol intervention strategy among tuberculosis patients: a pilot study from South India. International Journal of Tuberculosis and Lung Disease 2017;21(8):947-52. [DOI: 10.5588/ijtld.16.0693] [DOI] [PubMed] [Google Scholar]
Tian 2005 {published data only}
- Tian S, Li S, Xie Z. Influence of family nursing intervention on therapeutic effect of childhood patients with schizophrenia of first episode. Chinese Nursing Research 2005;19(8B):1551-2. [Google Scholar]
Tian 2006 {published data only}
- Tian B, Zhao ZH, Li XK. Effect of relax training on different kinds of test anxiety. Chinese Journal of Clinical Rehabilitation 2006;10:27-9. [Google Scholar]
Tiwari 2005 {published data only}
- Tiwari A, Leung WC, Leung TW, Humphreys J, Parker B, Ho PC. A randomised controlled trial of empowerment training for Chinese abused pregnant women in Hong Kong. British Journal of Obstetrics and Gynaecology 2005;112(9):1249-56. [DOI] [PubMed] [Google Scholar]
Tiwari 2010 {published data only}
- Tiwari A, Fong DYT, Yuen KH, Yuk H, Pang P, Humphreys J, et al. Effect of an advocacy intervention on mental health in Chinese women survivors of intimate partner violence: a randomised controlled trial. JAMA 2010;304(5):536-43. [DOI] [PubMed] [Google Scholar]
Tomlinson 2015 {published data only}
- Tomlinson M, Rotheram-Borus MJ, Harwood J, le Roux IM, O'Connor M, Worthman C. Community health workers can improve child growth of antenatally depressed, South African mothers: a cluster randomized controlled trial. BMC Psychiatry 2015;15(1):225. [DOI: 10.1186/s12888-015-0606-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tripathy 2010 {published data only}
- Tripathy P, Nair N, Barnett S, Mahapatra R, Borghi J, Rath S, et al. Effect of a participatory intervention with women's groups on birth outcomes and maternal depression in Jharkhand and Orissa, India: a cluster-randomised controlled trial. Lancet 2010;375(9721):1182-92. [DOI] [PubMed] [Google Scholar]
Ugwoke 2018 {published data only}
- Ugwoke SC, Eseadi C, Onuigbo LN, Aye EN, Akaneme IN, Oboegbulem AI, et al. A rational-emotive stress management intervention for reducing job burnout and dysfunctional distress among special education teachers. Medicine (United States) 2018;97(17):e0475. [DOI: 10.1097/MD.0000000000010475] [DOI] [PMC free article] [PubMed] [Google Scholar]
Unterhitzenberger 2014 {published data only}
- Unterhitzenberger J, Rosner R. Lessons from writing sessions: a school-based randomized trial with adolescent orphans in Rwanda. European Journal of Psychotraumatology 2014;5:24917. [DOI: 10.3402/ejpt.v5.24917] [DOI] [PMC free article] [PubMed] [Google Scholar]
Visagie 2015 {published data only}
- Visagie L, Loxton H, Silverman WK. Research protocol: development, implementation and evaluation of a cognitive behavioural therapy-based intervention programme for the management of anxiety symptoms in South African children with visual impairments. African Journal of Disability 2015;4:160. [DOI: 10.4102/ajod.v4i1.160] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vitriol 2010 {published data only}
- Vitriol VG, Ballesteros ST, Florenzano RU, Weil KP, Benadof DF. Evaluation of an outpatient intervention for women with severe depression and a history of childhood trauma SRCH - Ml-09-2009. Psychiatric Services (Washington, D.C.) 2010;60(7):936-42. [DOI: 10.1176/ps.2009.60.7.936] [DOI] [PubMed] [Google Scholar]
Vreeman 2018 {published data only}
- Vreeman R, Nyandiko W, Marete I, Mwangi A, McAteer C, Keter A, et al. A randomized, controlled trial of a patient-centered disclosure counseling intervention for Kenyan children living with HIV. In: Journal of the International AIDS Society. Vol. 21. 2018. [DOI: 10.1002/jia2.25148] [DOI]
Wandera 2017 {published data only}
- Wandera B, Tumwesigye NM, Nankabirwa JI, Mafigiri DK, Parkes-Ratanshi RM, Kapiga S, et al. Efficacy of a single, brief alcohol reduction intervention among men and women living with HIV/AIDS and using alcohol in Kampala, Uganda: a randomized trial. Journal of the International Association of Providers of AIDS Care 2017;16(3):276-85. [DOI: 10.1177/2325957416649669] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2008 {published data only}
- Wang NS, RongQin LI, Zhan LY, Wang GS, Lin LIU, 王年生, et al. The effect of regular health education on rehabilitation of outpatients with schizophrenia in rural areas. Chinese Mental Health Journal 2008;22(10):709-12. [Google Scholar]
Wang 2013 {published data only}
- Wang L, Zhou J, Yu X, Qiu J, Wang B. Psychosocial rehabilitation training in the treatment of schizophrenia outpatients: a randomized, psychosocial rehabilitation training- and monomedication-controlled study. Pakistan Journal of Medical Sciences 2013;29(2):597-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2017 {published data only}
- Wang S-J, Bytyci A, Izeti S, Kallaba M, Rushiti F, Montgomery E, et al. A novel bio-psycho-social approach for rehabilitation of traumatized victims of torture and war in the post-conflict context: a pilot randomized controlled trial in Kosovo. Conflict & Health [electronic resource] 2017;10:34. [DOI: 10.1186/s13031-016-0100-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wei 2013 {published data only}
- Wei SN, Liu L, Bi B, Li HY, Hou JL, Tan SY, et al. An intervention and follow-up study following a suicide attempt in the emergency departments of four general hospitals in Shenyang, China. Journal of Crisis Intervention and Suicide Prevention 2013;34(2):107-15. [DOI: 10.1027/0227-5910/a000181] [DOI] [PubMed] [Google Scholar]
Wolmer 2005 {published data only}
- Wolmer L, Laor N, Dedeoglu C, Siev J, Yazgan Y. Teacher-mediated intervention after disaster: a controlled three-year follow-up of children's functioning. Journal of Child Psychology, Psychiatry and Allied Disciplines 2005;46(11):1161-8. [DOI] [PubMed] [Google Scholar]
Xiang 2006 {published data only}
- Xiang Y, Weng Y, Li W, Gao L, Chen G, Xie L, et al. Training patients with schizophrenia with the community re-entry module: a controlled study. Social Psychiatry and Psychiatric Epidemiology 2006;41(6):464-9. [DOI: 10.1007/s00127-006-0050-6] [DOI] [PubMed] [Google Scholar]
Xu 2019 {published data only}
- Xu DR, Xiao S, He H, Caine ED, Gloyd S, Simoni J, et al. Lay health supporters aided by mobile text messaging to improve adherence, symptoms, and functioning among people with schizophrenia in a resource-poor community in rural China (LEAN): a randomized controlled trial. PLOS Medicine/Public Library of Science 2019;16(4):e1002785. [DOI: 10.1371/journal.pmed.1002785] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yildirim 2013 {published data only}
- Yildirim A, Hacihasanoglu AR, Karakurt P. Effects of a nursing intervention program on the depression and perception of family functioning of mothers with intellectually disabled children. Journal of Clinical Nursing 2013;22(1-2):251-61. [DOI: 10.1111/j.1365-2702.2012.04280.x] [DOI] [PubMed] [Google Scholar]
Yildiz 2004 {published data only}
- Yildiz M, Veznedaroglu B, Eryavuz A, Kayahan B. Psychosocial skills training on social functioning and quality of life in the treatment of schizophrenia: a controlled study in Turkey. International Journal of Psychiatry in Clinical Practice 2004;8(4):219-25. [DOI: 10.1080/13651500410005595] [DOI] [PubMed] [Google Scholar]
Zambori 2002 {published data only}
- Zambori J, Szadoczky E, Rozsa S, Furedi J. Cost-outcome of anxiety treatment intervention in primary care in Hungary. Journal of Mental Health Policy and Economics 2002;5:115-20. [PubMed] [Google Scholar]
Zhang 2015 {published data only}
- Zhang S, Wang H, Chen C, Zhou J, Wang X. Effcacy of Williams LifeSkills Training in improving psychological health of Chinese male juvenile violent offenders: a randomized controlled study. Neuroscience Bulletin 2015;31(1):53-60. [DOI: 10.1007/s12264-014-1492-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhao 1999 {published data only}
- Zhao B, Shen J, Shi Y. A comparative study on family intervention on schizophrenics in community. Chinese Mental Health Journal 1999;13:323-4. [Google Scholar]
Zhao 2015 {published data only}
- Zhao W, Law S, Luo X, Chow W, Zhang J, Zhu Y, et al. First adaptation of a family-based ACT model in Mainland China: a pilot project. Psychiatric Services (Washington, D.C.) 2015;66(4):438-41. [DOI: 10.1176/appi.ps.201400087] [DOI] [PubMed] [Google Scholar]
Zhou 2014 {published data only}
- Zhou B, Gu Y. Effect of self-management training on adherence to medications among community residents with chronic schizophrenia: a singleblind randomized controlled trial in Shanghai, China. Shanghai Jingshen Yixue 2014;26(6):332-8. [DOI: 10.11919/j.issn.1002-0829.214076] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Ali 2020 {published data only}
- Ali MK, Chwastiak L, Poongothai S, Emmert-Fees KM, Patel SA, Anjana RM, et al. Effect of a collaborative care model on depressive symptoms and glycated hemoglobin, blood pressure, and serum cholesterol among patients with depression and diabetes in India: the INDEPENDENT randomized clinical trial. JAMA 2020;324(7):651-62. [DOI: 10.1001/jama.2020.11747] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski AJ, Poongothai S, Chwastiak L, Hutcheson M, Tandon N, Khadgawat R, et al. The INtegrating DEPrEssioN and Diabetes treatmENT (INDEPENDENT) study: design and methods to address mental healthcare gaps in India. Contemporary Clinical trials 2017;60:113-24. [DOI: 10.1016/j.cct.2017.06.013] [DOI] [PMC free article] [PubMed] [Google Scholar]
Anjara 2019 {published data only}
- Anjara SG, Bonetto C, Ganguli P, Setiyawati D, Mahendradhata Y, Yoga BH, et al. Can general practitioners manage mental disorders in primary care? A partially randomised, pragmatic, cluster trial. PLOS ONE 2019;14(11):e0224724. [DOI: 10.1371/journal.pone.0224724] [DOI] [PMC free article] [PubMed] [Google Scholar]
Asadzadeh 2020 {published data only}
- Asadzadeh L, Jafari E, Kharaghani R, Taremian F. Effectiveness of midwife-led brief counseling intervention on post-traumatic stress disorder, depression, and anxiety symptoms of women experiencing a traumatic childbirth: a randomized controlled trial. BMC Pregnancy Childbirth 2020;20(1):142. [DOI: 10.1186/s12884-020-2826-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dorsey 2020 {published data only}
- Dorsey S, Lucid L, Martin P, King KM, O'Donnell K, Murray LK, et al. Effectiveness of task-shifted trauma-focused cognitive behavioral therapy for children who experienced parental death and posttraumatic stress in Kenya and Tanzania: a randomized clinical trial. JAMA Psychiatry 2020;77(5):464-73. [DOI: 10.1001/jamapsychiatry.2019.4475] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gureje 2020a {published data only}
- Gureje O, Makanjuola V, Kola L, Yusuf B, Price L, Esan O, et al. COllaborative Shared care to IMprove Psychosis Outcome (COSIMPO): study protocol for a randomized controlled trial. Trials [electronic resource] 2017;18(1):462. [DOI: 10.1186/s13063-017-2187-x] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gureje O, Appiah-Poku J, Bello T, Kola L, Araya R, Chisholm D, et al. Effect of collaborative care between traditional and faith healers and primary health-care workers on psychosis outcomes in Nigeria and Ghana (COSIMPO): a cluster randomised controlled trial. Lancet 2020;396(10251):612-22. [DOI: 10.1016/S0140-6736(20)30634-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hartmann 2020 {published data only}
- Hartmann M, Datta S, Browne EN, Appiah P, Banay R, Caetano V, et al. A combined behavioral economics and cognitive behavioral therapy intervention to reduce alcohol use and intimate partner violence among couples in Bengaluru, India: results of a pilot study [Epub ahead of print]. Journal of Interpersonal Violence 2020;20:886260519898431. [DOI: doi: 10.1177/0886260519898431] [DOI] [PubMed] [Google Scholar]
Jordans 2020 {published data only}
- Jordans MJ, Luitel NP, Lund C, Kohrt BA. Evaluation of proactive community case detection to increase help seeking for mental health care: a pragmatic randomized controlled trial. Psychiatric Services 2020;71(8):810-5. [DOI: 10.1176/appi.ps.201900377] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kamal 2020 {published data only}
- Kamal K, Sunita S, Karobi D, Abhishek G. Nurse-delivered screening and brief intervention among college students with hazardous alcohol use: a double-blind randomized clinical trial from India. Alcohol and Alcoholism 2020;55(3):284-90. [DOI: 10.1093/alcalc/agaa014] [DOI] [PubMed] [Google Scholar]
Markkula 2019 {published data only}
- Markkula N, Lehti V, Adhikari P, Peña S, Heliste J, Mikkonen E, et al. Effectiveness of non-medical health worker-led counselling on psychological distress: a randomized controlled trial in rural Nepal. Global Mental Health (Cambridge Core) 2019;6:e15. [DOI: 10.1017/gmh.2019.15] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markkula N, Lehti V, Adhikari P, Peña S, Heliste J, Mikkonen E, et al. Effectiveness of non-medical health worker-led counselling on psychological distress: a randomized controlled trial in rural Nepal - ADDENDUM. Global Mental Health (Cambridge Core) 2019;6:e19. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Maselko 2020 {published data only}
- Maselko J, Sikander S, Turner EL, Bates LM, Ahmad I, Atif N, et al. Effectiveness of a peer-delivered, psychosocial intervention on maternal depression and child development at 3 years postnatal: a cluster randomised trial in Pakistan. Lancet Psychiatry 2020;7(9):775-87. [DOI: 10.1016/S2215-0366(20)30258-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maselko J, Sikander S, Turner EL, Bates LM, Ahmad I, Atif N, et al. Effectiveness of a peer-delivered, psychosocial intervention on maternal depression and child development at 3 years postnatal: a cluster randomised trial in Pakistan - Erratum. Lancet Psychiatry 2020;S2215-0366(20):30522-8. [DOI: 10.1016/S2215-0366(20)30522-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02658994. Thinking Healthy Program Peer Delivered Plus. https://clinicaltrials.gov/ct2/show/NCT02658994; 2019.
- Turner EL, Sikander S, Bangash O, Zaidi A, Bates L, Gallis J, et al. The effectiveness of the peer delivered Thinking Healthy Plus (THPP+) Programme for maternal depression and child socio-emotional development in Pakistan: study protocol for a three-year cluster randomized controlled trial. Trials [electronic resource] 2016;17(1):442. [DOI: 10.1186/s13063-016-1530-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
Michelson 2020 {published data only}
- Michelson D, Malik K, Krishna M, Sharma R, Mathur S, Bhat B, et al. Development of a transdiagnostic, low-intensity, psychological intervention for common adolescent mental health problems in Indian secondary schools. Behavior Research and Therapy 2020;130:103439. [DOI: 10.1016/j.brat.2019.103439] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelson D, Malik K, Parikh R, Weiss HA, Doyle AM, Bhat B, et al. Correction to Lancet Child and Adolescent Health 2020;4:571-82. Lancet Child and Adolescent Health 2020;4(9):e35. [DOI: 10.1016/S2352-4642(20)30232-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelson D, Malik K, Parikh R, Weiss HA, Doyle AM, Bhat B, et al. Effectiveness of a brief lay counsellor-delivered, problem-solving intervention for adolescent mental health problems in urban, low-income schools in India: a randomised controlled trial. Lancet Child and Adolescent Health 2020;4(8):571-82. [DOI: 10.1016/S2352-4642(20)30173-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh R, Michelson D, Malik K, Shinde S, Weiss HA, Hoogendoorn A, et al. The effectiveness of a low-intensity problem-solving intervention for common adolescent mental health problems in New Delhi, India: protocol for a school-based, individually randomized controlled trial with an embedded stepped-wedge, cluster randomized controlled recruitment trial. Trials 2020;20(1):568. [DOI: 10.1186/s13063-019-3573-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nakimuli‐Mpungu 2020 {published data only}
- Nakimuli-Mpungu E, Musisi S, Wamala K, Okello J, Ndyanabangi S, Birungi J, et al. Recruitment and baseline characteristics of participants in the social, emotional, and economic empowerment through knowledge of group support psychotherapy study (SEEK-GSP): cluster randomized controlled trial. JMIR Research Protocols 2019;8:e11560. [DOI: 10.2196/11560] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakimuli-Mpungu E, Musisi S, Wamala K, Okello J, Ndyanabangi S, Mojtabai R, et al. The effect of group support psychotherapy delivered by trained lay health workers for depression treatment among people with HIV in Uganda: protocol of a pragmatic, cluster randomized trial. JMIR Research Protocols 2017;6:e250. [DOI: 10.2196/resprot.8925] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakimuli-Mpungu E, Musisi S, Wamala K, Okello J, Ndyanabangi S, Birungi J, et al. Effectiveness and cost-effectiveness of group support psychotherapy delivered by trained lay health workers for depression treatment among people with HIV in Uganda: a cluster-randomised trial. Lancet Global Health 2020;8(3):e387-98. [DOI: 10.1016/S2214-109X(19)30548-0] [DOI] [PubMed] [Google Scholar]
- Nakimuli-Mpungu E, Musisi S, Wamala K, Okello J, Ndyanabangi S, Birungi J, Nanfuet al. Correction to Lancet Global Health 2020;8:e387-98. Lancet Global Health 2020;8(8):e1002. [DOI: 10.1016/S2214-109X(20)30284-9] [DOI] [PubMed] [Google Scholar]
Pan 2019 {published data only}
- Pan Y, Chen R. The effect of a nurse-led cognitive behavioral protocol on depressive symptoms and coping strategies of dementia caregivers. Journal of Nursing Research 2019;27(6):e55. [DOI: 10.1097/jnr.0000000000000327] [DOI] [PubMed] [Google Scholar]
Rodriguez 2019 {published data only}
- Rodriguez MA, Eisenlohr-Moul TA, Su K, Xiao Z, Rosenthal MZ. Using non-specialist providers to improve treatment engagement in an online mindfulness intervention in China. Behaviour Research and Therapy 2020;130:103644. [DOI: ] [Google Scholar]
- Rodriguez MA. The use of task-sharing to improve treatment engagement in an online mindfulness intervention for stress among Chinese college students. Dissertation Abstracts International: Section B: The Sciences and Engineering 2019;80:No Pagination Specified.
Soares 2020 {published data only}
- Soares J, Vargas D. Group brief intervention: effectiveness in motivation to change alcohol intake [Intervención grupal breve: efectividad de motivación para cambios en el consumo de alcohol]. Revista Brasileira Enfermagem [online] 2020;73(1):e20180138. [DOI: 10.1590/0034-7167-2018-0138] [DOI] [PubMed] [Google Scholar]
Ward 2020 {published data only}
- Ward CL, Wessels IM, Lachman JM, Hutchings J, Cluver LD, Kassanjee R, et al. Parenting for lifelong health for young children: a randomized controlled trial of a parenting program in South Africa to prevent harsh parenting and child conduct problems. Journal of Child Psychology and Psychiatry 2020;61(4):503-12. [DOI: 10.1111/jcpp.13129] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Akhtar 2020 {published data only}
- ACTN. Pilot study of testing group psychological help for adult Syrian refugees in Jordan. http://www.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12619000340134; 2019.
- ACTRN. Testing group psychological help for adult Syrian refugees in Jordan. http://www.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12619001386123; 2019.
- Akhtar A, Giardinelli L, Bawaneh A, Awwad M, Naser H, Whitney C, et al. STRENGTHS Consortium. Group problem management plus (gPM+) in the treatment of common mental disorders in Syrian refugees in a Jordanian camp: study protocol for a randomized controlled trial. BMC Public Health 2020;20(1):390. [DOI: 10.1186/s12889-020-08463-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Asher 2016 {published data only}
- Asher L, Fekadu A, Hanlon C, Mideksa G, Eaton J, Patel V, et al. Development of a community-based rehabilitation intervention for people with schizophrenia in Ethiopia. PLOS ONE 2015;10(11):e0143572. [DOI: 10.1371/journal.pone.0143572] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asher L, De Silva M, Hanlon C, Weiss HA, Birhane R, Ejigu DA, et al. Community-based rehabilitation intervention for people with schizophrenia in Ethiopia (RISE): study protocol for a cluster randomised controlled trial. Trials [electronic resource] 2016;17(1):299. [DOI: 10.1186/s13063-016-1427-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asher L, Hanlon C, Birhane R, Habtamu A, Eaton J, Weiss HA, et al. Community-based rehabilitation intervention for people with schizophrenia in Ethiopia (RISE): a 12 month mixed methods pilot study. BMC Psychiatry 2018;18:250. [DOI: 10.1186/s12888-018-1818-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2019 {published data only}
- ACTRN. Pilot study of testing group psychological help for young adolescent Syrian refugees in Jordan. http://www.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12618001917224 2018; 2018.
- Brown FL, Steen F, Taha K, Aoun M, Bryant RA, Jordans MJD, et al. STRENGTHS-consortium. Early Adolescent Skills for Emotions (EASE) intervention for the treatment of psychological distress in adolescents: study protocol for randomised controlled trials in Lebanon and Jordan. ERRATUM. Trials 2019;20(1):617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown FL, Steen F, Taha K, Aoun M, Bryant RA, Jordans MJD, et al. STRENGTHS-consortium. Early Adolescent Skills for Emotions (EASE) intervention for the treatment of psychological distress in adolescents: study protocol for randomised controlled trials in Lebanon and Jordan. Trials 2019;20(1):545. [DOI: 10.1186/s13063-019-3654-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRCTN. Early Adolescent Skills for Emotions (EASE) for young adolescents in Lebanon. http://www.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN75375136 2019; 2019.
Chen 2018 {published data only}
- Chen S, Conwell Y, Xue J, Li LW, Tang W, Bogner HR, et al. Protocol of an ongoing randomized controlled trial of care management for comorbid depression and hypertension: the Chinese Older Adult Collaborations in Health (COACH) study. BMC Geriatrics 2018;18(1):124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chinoda 2020 {published data only}
- Chinoda S, Mutsinze A, Simms V, Beji-Chauke R, Verhey R, Robinson J, et al. Effectiveness of a peer-led adolescent mental health intervention on HIV virological suppression and mental health in Zimbabwe: protocol of a cluster-randomised trial. Global Mental Health (Cambridge Core) 2020;7:e23. [DOI: 10.1017/gmh.2020.14] [DOI] [PMC free article] [PubMed] [Google Scholar]
- PACTR. Operations research to estimate the effectiveness of a peer-led mental health intervention on virological suppression and mental health among adolescents with HIV in Zimbabwe. http://www.who.int/trialsearch/Trial2.aspx?TrialID=PACTR201810756862405 2018; 2018.
Clair 2016 {published data only}
- Clair V, Mutiso V, Musau A, Frank E, Ndetei D. Online learning improves substance use care in Kenya: randomized control trial results and implications. In: Annals of Global Health. Conference: 7th Annual CUGH Conference: bridging to a sustainable future in global health. United States. Conference start: 20160409. Conference end: 20160411. Vol. 82. 2016:320-1.
Fairall 2018 {published data only}
- Fairall L, Petersen I, Zani B, Folb N, Georgeu-Pepper D, Selohilwe O, et al. Collaborative care for the detection and management of depression among adults receiving antiretroviral therapy in South Africa: study protocol for the CobALT randomised controlled trial. Trials 2018;19(1):193. [DOI: 10.1186/s13063-018-2517-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara F. Collaborative care for the detection and management of depression among adults receiving antiretroviral therapy in South Africa. https://pactr.samrc.ac.za/TrialDisplay.aspx?TrialID=1078; 2015. [DOI] [PMC free article] [PubMed]
Fawzi 2020 {published data only}
- Fawzi MC, Siril H, Larson E, Aloyce Z, Araya R, Kaale A, et al. Healthy Options: study protocol and baseline characteristics for a cluster randomized controlled trial of group psychotherapy for perinatal women living with HIV and depression in Tanzania. BMC Public Health 2020;20(1):80. [DOI: 10.1186/s12889-019-7907-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gureje 2020b {published data only}
- Gureje O, Kola L, Oladeji BD, Abdulmalik J, Ayinde O, Zelkowitz P, et al. Responding to the challenge of Adolescent Perinatal Depression (RAPiD): protocol for a cluster randomized hybrid trial of psychosocial intervention in primary maternal care. Trials 2020;21(1):231. [DOI: 10.1186/s13063-020-4086-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hanlon 2016 {published data only}
- Hanlon C, Alem A, Medhin G, Shibre T, Ejigu DA, Negussie H, et al. Task sharing for the care of severe mental disorders in a low-income country (TaSCS): study protocol for a randomised, controlled, non-inferiority trial. Trials [electronic resource] 2016;17:76. [DOI: 10.1186/s13063-016-1191-x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoffmann 2016 {published data only}
- Hoffmann EV, Duarte CS, Mello AF, Matsuzaka CT, Fossaluza V, De Mello MF. Child psychopathology after treatment of maternal depression in primary care in Brazil RTY. In: Journal of the American Academy of Child and Adolescent Psychiatry. Conference: 63rd Annual Meeting of the American Academy of Child and Adolescent Psychiatry. United States. Conference start: 20161024. Conference end: 20161029. Vol. 55. 2016:S255-6. [DOI: 10.1016/j.jaac.2016.09.476] [DOI]
ISRCTN16463016 2017 {published data only}
- Torres-Castro S, Lopez-Ortega M, Martinez-Ruiz A, Gutierrez-Robledo LM, Guzman A. Pilot study and key feasibility factors of a staff training intervention and reduction of antipsychotic prescription practice in Mexican urban care homes: study protocol. Journal of Mental Health and Aging 2018;2(2):47-55. [Google Scholar]
- Torres-Castro S. 'PROCUIDA-dementia': a feasibility Mexican study investigating an optimised person-centred intervention to reduce antipsychotic medication and improve the quality of life of older people living in care homes. http://isrctn.com/isrctn16463016; 2017. [DOI: ]
ISRCTN57805470 2019 {published data only}
- ISRCTN. A collaborative care psychosocial intervention to improve late life depression in socioeconomically deprived areas of Guarulhos, Brazil: the PROACTIVE study. http://www.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN57805470; 2019. [DOI] [PMC free article] [PubMed]
ISRCTN62728852 2019 {published data only}
- ISRCTN262728852. Improving TB outcomes by modifying life-style behaviours through a behavioural intervention comprising motivational interviewing counselling strategy augmented with subsequent short text messaging. http://www.isrctn.com/ISRCTN62728852; 2018. [DOI: ]
- Moriarty AS, Louwagie GM, Mdege ND, Morojele N, Tumbo J, Omole OB, et al. ImPROving TB outcomes by modifying LIFE-style behaviours through a brief motivational intervention followed by short text messages (ProLife): study protocol for a randomised controlled trial. Trials 2019;20:457. [DOI: 10.1186/s13063-019-3551-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
ISRCTN86760765 2017 {published data only}
- ISRCTN86760765. Integrating depression management in HIV care in Uganda. http://www.isrctn.com/ISRCTN86760765; 2017. [DOI: ]
Joag 2020 {published data only}
- Joag K, Kalha J, Pandit D, Chatterjee S, Krishnamoorthy S, Shields-Zeeman L, et al. Atmiyata, a community-led intervention to address common mental disorders: study protocol for a stepped wedge cluster randomized controlled trial in rural Gujarat, India. Trials 2020;21(1):212. [DOI: 10.1186/s13063-020-4133-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaustubh J. Atmiyata Gujarat: promoting wellbeing, reducing distress and improving access to mental health care through self-help groups and farmers clubs in rural Gujarat: transition to scale project. https://apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2017/03/008139; 2017.
Joska 2020 {published data only}
- Joska JA, Andersen LS, Smith-Alvarez R, Magidson J, Lee JS, O'Cleirigh C, et al. Correction: Nurse-delivered cognitive behavioral therapy for adherence and depression among people living with HIV (the Ziphamandla study): protocol for a randomized controlled trial. JMIR Research Protocols 2020;9(9):e24074. [DOI: 10.2196/24074] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joska JA, Andersen LS, Smith-Alvarez R, Magidson J, Lee JS, O'Cleirigh C, et al. Nurse-delivered cognitive behavioral therapy for adherence and depression among people living with HIV (the Ziphamandla study): protocol for a randomized controlled trial. JMIR Research Protocols 2020;9(2):e14200. [DOI: 10.2196/14200] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kohrt 2018 {published data only}
- Kohrt BA, Jordans MJD, Turner EL, Sikkema KJ, Luitel NP, Rai S, et al. Reducing stigma among healthcare providers to improve mental health services (RESHAPE): protocol for a pilot cluster randomized controlled trial of a stigma reduction intervention for training primary healthcare workers in Nepal. Pilot & Feasibility Studies 2018;4:36. [DOI: 10.1186/s40814-018-0234-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Madhombiro 2017 {published data only}
- Madhombiro M, Dube-Marimbe B, Dube M, Chibanda D, Zunza M, Rusakaniko S, et al. A cluster randomised controlled trial protocol of an adapted intervention for alcohol use disorders in people living with HIV and AIDS: impact on alcohol use, general functional ability, quality of life and adherence to HAART. BMC Psychiatry 2017;17:44. [DOI: 10.1186/s12888-017-1208-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Myers 2018 {published data only}
- Myers B, Lund C, Lombard C, Joska J, Levitt N, Butler C, et al. Comparing dedicated and designated models of integrating mental health into chronic disease care: study protocol for a cluster randomized controlled trial. Trials [electronic resource] 2018;19:185. [DOI: 10.1186/s13063-018-2568-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT02185482 2014 {published data only}
- NCT02185482. Physician led counseling in management of depression in type 2 diabetes mellitus. https://clinicaltrials.gov/ct2/show/NCT02185482.
NCT03378544 2019 {published data only}
- Musyimi CW. Engaging traditional birth attendants to reduce maternal depression in rural Kenya. https://clinicaltrials.gov/ct2/show/NCT03378544; 2019.
NCT03430622 2019 {published data only}
- Nasim C. Effectiveness of a telephone based learning through play (LTP) plus interpersonal psychotherapy (IPT) for depressed mothers (Telemothercare). https://clinicaltrials.gov/ct2/show/NCT03430622; 2019.
NCT03587896 2018 {published data only}
- NCT. Refugee emergency: defining and implementing novel evidence-based psychosocial interventions - the Turkey site. https://clinicaltrials.gov/ct2/show/NCT03587896.
- Purgato M, Carswell K, Acarturk C, Au T, Akbai S, Anttila M, et al. Effectiveness and cost-effectiveness of Self-Help Plus (SH+) for preventing mental disorders in refugees and asylum seekers in Europe and Turkey: study protocols for two randomised controlled trials. BMJ Open 2019;9(5):e030259. [DOI: 10.1136/bmjopen-2019-030259] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT03887312 2019 {published data only}
- LBCTR. Phone-delivered psychological intervention (t-CETA) for mental health problems in 8-17 year-old Syrian refugee children. http://www.who.int/trialsearch/Trial2.aspx?TrialID=LBCTR2019040213 2019; 2019.
NCT03912077 2019 {published data only}
- NCT. Implementing psychosocial interventions to Syrian refugee women who are exposed to psychological trauma. https://clinicaltrials.gov/show/NCT03912077 2019; 2019.
NCT03919695 2019 {published data only}
- NCT03919695. Development of an intervention to reduce heavy drinking and improve HIV care engagement among fisherfolk in Uganda. https://clinicaltrials.gov/show/nct03919695 2019; 2019.
NCT03922425 2019 {published data only}
- NCT. Community-based mental health care for people with severe and enduring mental iII health (RECOVER-E). https://clinicaltrials.gov/show/NCT03922425 2019; 2019.
NCT03960892 2019 {published data only}
- Uygun E, Ilkkursun Z, Sijbrandij M, Aker AT, Bryant R, Cuijpers P, et al. STRENGHTS consortium. Protocol for a randomized controlled trial: peer-to-peer Group Problem Management Plus (PM+) for adult Syrian refugees in Turkey. Trials 2020;21(1):283. [DOI: 10.1186/s13063-020-4166-x.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeynep CA, Zeynep İ. Implementation of group problem management plus (PM+) in adult Syrian refugees in Turkey: RCT (STRENGTHS). https://clinicaltrials.gov/ct2/show/NCT03960892; 2019.
NCT03966833 2019 {published data only}
- NCT03966833. Using group interpersonal psychotherapy to improve the well-being of adolescent girls. https://clinicaltrials.gov/ct2/show/NCT03966833; 2019.
NCT04018391 2019 {published data only}
- NCT. The TENDAI study: task shifting to treat depression and HIV medication nonadherence in low resource settings. https://clinicaltrials.gov/ct2/show/NCT04018391; 2019.
NCT04110405 2019 {published data only}
- NCT04110405. Scaling-up stepped care for women's mental health in primary care in an LMIC. clinicaltrials.gov; 2019.
NCT04143009 2019 {published data only}
- NCT04143009. Adaptation of the friendship bench intervention for HIV-infected perinatal women in Lilongwe (Periscope). https://clinicaltrials.gov/ct2/show/NCT04143009.
NCT04254393 2020 {published data only}
- NCT. Early adolescent skills for emotions (EASE) - pilot cluster randomized controlled trial (cRCT) in public schools of rural Pakistan. https://clinicaltrials.gov/show/NCT04254393; 2020.
NCT04385498 2020 {published data only}
- NCT. Primary care intervention for PTSD in Ethiopia. https://clinicaltrials.gov/show/NCT04385498; 2020.
Nusrat 2016 {published data only}
- Nusrat H, Batool F, Tayyeba K, Farah N, Ann M. Change your life with seven sheets of paper: a pilot randomized controlled trial for postnatal depression (CREATOR). European Psychiatry 2016;33(S1):S414. [DOI: 10.1016/j.eurpsy.2016.01.1496] [DOI] [Google Scholar]
Onu 2016 {published data only}
- Onu C, Ongeri L, Bukusi E, Cohen CR, Neylan TC, Oyaro P, et al. Interpersonal psychotherapy for depression and posttraumatic stress disorder among HIV-positive women in Kisumu, Kenya: study protocol for a randomized controlled trial. Trials [electronic resource] 2016;17(1):64. [DOI: 10.1186/s13063-016-1187-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
PACTR201812802696820 {published data only}
- PACTR. Adapting and piloting WHO's parent skills training in a low income setting: a case study in Ethiopia. http://www.who.int/trialsearch/Trial2.aspx?TrialID=PACTR201812802696820; 2018.
Petersen 2018 {published data only}
- Petersen I, Bhana A, Folb N, Thornicroft G, Zani B, Selohilwe O, et al. Collaborative care for the detection and management of depression among adults with hypertension in South Africa: study protocol for the PRIME-SA randomised controlled trial. Trials [electronic resource] 2018;19(1):192. [DOI: 10.1186/s13063-018-2518-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Priebe 2019 {published data only}
- Priebe S, Fung C, Sajun SZ, Alinaitwe R, Giacco D, Gomez-Restrepo C, et al. Resource-oriented interventions for patients with severe mental illnesses in low- and middle-income countries: trials in Bosnia-Herzegovina, Colombia and Uganda. BMC Psychiatry 2019;19:181. [DOI: 10.1186/s12888-019-2148-x RTY - Journal article] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sangraula 2018 {published data only}
- Sangraula M, Van't Hof E, Luitel NP, Turner EL, Marahatta K, Nakao JH, et al. Protocol for a feasibility study of group-based focused psychosocial support to improve the psychosocial well-being and functioning of adults affected by humanitarian crises in Nepal: group Problem Management Plus (PM+). Pilot & Feasibility Studies 2018;4:126. [DOI: 10.1186/s40814-018-0315-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangraula M, Turner EL, Luitel NP, Van't Hof E, Shrestha P, Ghmire R, et al. Feasibility of Group Problem Management Plus (PM+) to improve mental health and functioning of adults in earthquake-affected communities in Nepal. Epidemiology and Psychiatric Sciences 2020;29:e130. [DOI: 10.1017/S2045796020000414] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van't Hof E, Sangraula M, Luitel NP, Turner EL, Marahatta K, Ommeren M, et al. Effectiveness of Group Problem Management Plus (Group-PM+) for adults affected by humanitarian crises in Nepal: study protocol for a cluster randomized controlled trial. Trials 2020;21(1):343. [DOI: 10.1186/s13063-020-04263-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schulte 2020 {published data only}
- DRKS. Reach and effectiveness of alcohol screening and brief intervention in primary health care in Kazakhstan: a pilot cluster randomized trial. http://www.who.int/trialsearch/Trial2.aspx?TrialID=DRKS00015882; 2018.
- Schulte B, O’Donnell A, Lahusen H, Lindemann C, Prilutskaya M, Yussopov O, et al. Feasibility of alcohol screening and brief intervention in primary health care in Kazakhstan: study protocol of a pilot cluster randomised trial. Pilot & Feasibility Studies 2020;6:3. [DOI: 10.1186/s40814-019-0547-x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sharifi 2019 {published data only}
- Sharifi V, Shahrivar Z, Zarafshan H, Ashkezary SB, Stuart E, Mojtabai R, et al. Collaborative care for child and youth mental health problems in a middle-income country: study protocol for a randomized controlled trial training general practitioners. Trials 2019;20(1):405. [DOI: 10.1186/s13063-019-3467-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shepherd 2020 {published data only}
- ISRCTN. Supervised treatment for outpatients with schizophrenia (STOPS+). http://www.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN93243890;2019.
- Shepherd TA, Ul-Haq Z, Ul-Haq M, Khan MF, Afridi A, Dikomitis L, et al. Supervised treatment in outpatients for schizophrenia plus (STOPS+). BMJ Open 2020;10(6):e034709. [DOI: 10.1136/bmjopen-2019-034709] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sijbrandij 2016 {published data only}
- Sijbrandij M, Bryant RA, Schafer A, Dawson KS, Anjuri D, Ndogoni L, et al. Problem management plus (PM+) in the treatment of common mental disorders in women affected by gender-based violence and urban adversity in Kenya; study protocol for a randomized controlled trial. International Journal of Mental Health Systems 2016;10(1):44. [DOI: 10.1186/s13033-016-0075-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijbrandij M, Bryant RA, Schafer A, Dawson KS, Anjuri D, Ndogoni L, et al. "Problem management plus (PM+) in the treatment of common mental disorders in women affected by gender-based violence and urban adversity in Kenya; study protocol for a randomized controlled trial": correction. International Journal of Mental Health Systems 2018;12:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
SLCTR/2018/008 {published data only}
- Doherty S. Integrating mental health into primary care for post-conflict populations in Northern Sri Lanka (COMGAP-S) [Conducting operational research to identify numbers and rates, determine needs, and integrate services to mitigate morbidity and mortality among internally displaced persons affected by emergencies]. https://slctr.lk/trials/slctr-2018-008; 2018.
Srinivasan 2018 {published data only}
- Krishnamachari S. Improving mental health through integration with primary care in rural Karnataka - HOPE. https://apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2018/04/013001; 2018. [DOI] [PMC free article] [PubMed]
- Srinivasan K, Mazur A, Mony PK, Whooley M, Ekstrand ML. Improving mental health through integration with primary care in rural Karnataka: study protocol of a cluster randomized control trial. BMC Family Practice 2018;19:158. [DOI: 10.1186/s12875-018-0845-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
Surkan 2020 {published data only}
- Atif N, Nazir H, Zafar S, Chaudhri R, Atiq M, Mullany LC, et al. Development of a psychological intervention to address anxiety during pregnancy in a low-income country. Frontiers in Psychiatry 2020;10:927. [DOI: 10.3389/fpsyt.2019.00927] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happy Mother-Healthy Baby: an anxiety-focused early prenatal intervention for the prevention of common mental disorders in Pakistan. https://clinicaltrials.gov/ct2/show/NCT03880032.
- Surkan PJ, Hamdani SU, Huma ZE, Nazir H, Atif N, Rowther AA, et al. Cognitive-behavioral therapy-based intervention to treat symptoms of anxiety in pregnancy in a prenatal clinic using non-specialist providers in Pakistan: design of a randomised trial. BMJ Open 2020;10(4):e037590. [DOI: 10.1136/bmjopen-2020-037590] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tol 2017 {published data only}
- Greene MC, Rees S, Likindikoki S, Bonz AG, Joscelyne A, Kaysen D, et al. Developing an integrated intervention to address intimate partner violence and psychological distress in Congolese refugee women in Tanzania. Conflict and Health 2019;13:38. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tol WA, Greene MC, Likindikoki S, Misinzo L, Ventevogel P, Bonz AG, et al. An integrated intervention to reduce intimate partner violence and psychological distress with refugees in low-resource settings: study protocol for the Nguvu cluster randomized trial. BMC Psychiatry 2017;17(1):186. [DOI: 10.1186/s12888-017-1338-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wagner 2019 {published data only}
- Wagner G. Maternal depression treatment in HIV (M-DEPTH). https://ClinicalTrials.gov/show/NCT03892915.
- Wagner GJ, McBain RK, Akena D, Ngo V, Nakigudde J, Nakku J, et al. Maternal depression treatment in HIV (M-DEPTH): study protocol for a cluster randomized controlled trial. Medicine (Baltimore) 2019;98(27):e16329. [DOI: 10.1097/MD.0000000000016329] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Alonso 2018
- Alonso J, Liu Z, Evans‐Lacko S, Sadikova E, Sampson N, et al. Treatment gap for anxiety disorders is global: results of the World Mental Health Surveys in 21 countries. Depression and Anxiety 2018;35:195-208. [DOI: 10.1002/da.22711] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aluko 2021
- Aluko P, Graybill E, Craig D, Henderson C, Drummond M, Wilson ECF, et al. Chapter 20. Economic evidence. In: Higgins JT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. [Google Scholar]
Archer 2012
- Archer J, Bower P, Gilbody S, Lovell K, Richards D, Gask L, et al. Collaborative care for depression and anxiety problems. Cochrane Database of Systematic Reviews 2012 Oct 17, Issue 10. Art. No: CD006525. [DOI: 10.1002/14651858.CD006525.pub2] [PMID: ] [DOI] [PubMed] [Google Scholar]
Asher 2017
- Asher L, Patel V, De Silva MJ. Community-based psychosocial interventions for people with schizophrenia in low and middle-income countries: systematic review and meta-analysis. BMC Psychiatry 2017;17:355. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ballini 2010
- Ballini L, Bero L, Eccles MP, Grimshaw J, Gruen RL, Lewin S, et al. Cochrane Effective Practice and Organisation of Care Group. About the Cochrane Collaboration (Cochrane Review Groups (CRGs)). 3. Art. No.: EPOC. http://www.mrw.interscience.wiley.com/cochrane/clabout/articles/EPOC/frame.html; 2010.
Barbui 2020
- Barbui C, Purgato M, Abdulmalik J, Acarturk C, Eaton J, Gastaldon C, et al. Efficacy of psychosocial interventions for mental health outcomes in low-income and middle-income countries: an umbrella review. Lancet Psychiatry 2020;7(2):162-72. [DOI: 10.1016/S2215-0366(19)30511-5] [DOI] [PubMed] [Google Scholar]
Barnett 2018
- Barnett ML, Gonzalez A, Miranda J, Chavira DA, Lau AS. Mobilizing community health workers to address mental health disparities for underserved populations: a systematic review. Administration and Policy on Mental Health 2018;45(2):195-211. [DOI: 10.1007/s10488-017-0815-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barry 2013
- Barry MM, Clarke AM, Jenkins R, Patel V. A systematic review of the effectiveness of mental health promotion interventions for young people in low and middle income countries. BMC Public Health 2013;13:835. [DOI: 10.1186/1471-2458-13-835] [DOI] [PMC free article] [PubMed] [Google Scholar]
Beck 1974
- Beck AT, Rial WY, Rickels K. Short form of depression inventory: cross validation. Psychological Reports 1974;34:1184-6. [PubMed] [Google Scholar]
Birbeck 2012
- Birbeck GL, Chomba E, Mbewe E, Atadzhanov M, Haworth A, Kansembe H. The cost of implementing a nationwide program to decrease the epilepsy treatment gap in a high gap country. Neurology International 2012;4(3):e14. [DOI: 10.4081/ni.2012.e14] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boer 2005
- Boer PC, Wiersma D, Russo S, Bosch RJ. Paraprofessionals for anxiety and depressive disorders. Cochrane Database of Systematic Reviews 2005, Issue 2. Art. No: CD004688. [DOI: 10.1002/14651858.CD004688] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bolton 2002
- Bolton P, Tang AM. An alternative approach to cross-cultural function assessment. Social Psychiatry and Psychiatric Epidemiology 2002;37:537-43. [DOI] [PubMed] [Google Scholar]
Bower 2006
- Bower P, Gilbody S, Richards D, Fletcher J, Sutton A. Collaborative care for depression in primary care. Making sense of a complex intervention: systematic review and meta-regression. British Journal of Psychiatry 2006;189:484-93. [DOI] [PubMed] [Google Scholar]
Burkey 2018
- Burkey MD, Hosein M, Morton I, Purgato M, Adi A, Kurzrok M, et al. Psychosocial interventions for disruptive behaviour problems in children in low- and middle-income countries: a systematic review and meta-analysis. Journal of Child Psychology and Psychiatry 2018;59(9):982-93. [DOI: ] [DOI] [PubMed] [Google Scholar]
Cardozo 2000
- Cardozo BL, Vergara A, Agani F, Gotway CA. Mental health, social functioning, and attitudes of Kosovar Albanians following the war in Kosovo. JAMA 2000;284(5):569-77. [DOI] [PubMed] [Google Scholar]
CCEMG 2010
- Campbell and Cochrane Economics Methods Group. Training materials. http://www.c-cemg.org/; 2010.
Chisholm 2019
- Chisholm D, Docrat S, Abdulmalik J, Alem A, Gureje O, Gurung D, et al. Mental health financing challenges, opportunities and strategies in low- and middle-income countries: findings from the Emerald project. BJPsych Open. 2019;5(5):e68. [DOI: 10.1192/bjo.2019.24] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chisolm 2000
- Chisholm D, Sekar K, Kumar KK, Saeed K, James S, Mubbashar MH, et al. Integration of mental health care into primary care. British Journal of Psychiatry 2000;176:581-8. [DOI: 10.1192/bjp.176.6.581] [DOI] [PubMed] [Google Scholar]
Clarke 2013
- Clarke K, King M, Prost A. Psychosocial interventions for perinatal common mental disorders delivered by providers who are not mental health specialists in low- and middle-income countries: a systematic review and meta-analysis. PLOS Medicine 2013;10(10):e1001541. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical Power Analysis in the Behavioral Sciences. 2nd edition. Hillsdale (NJ): Lawrence Erlbaum Associates, Inc., 1988. [Google Scholar]
Cuijpers 2018
- Cuijpers P, Karyotaki E, Reijnders M, Purgato M, Barbui C. Psychotherapies for depression in low- and middle-income countries: a meta-analysis. World Psychiatry 2018;17:90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dalgleish 2020
- Dalgleish T, Black M, Johnston D, Bevan A. Transdiagnostic approaches to mental health problems: current status and future directions. Journal of Consulting and Clinical Psychology 2020;88(3):179-95. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
De Silva 2013
- De Silva MJ, Cooper S, Li HL, Lund C, Patel V. Effect of psychosocial interventions on social functioning in depression and schizophrenia: meta-analysis. British Journal of Psychiatry 2013;202:253–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
De Vet 2008
- De Vet HC, Eisinga A, Riphagen II, Aertgeerts B, Pewsner D. Chapter 7. Searching for studies. In: Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy. Version 0.4 (updated September 2008). The Cochrane Collaboration, 28 September 2008:http://srdta.cochrane.org/sites/srdta.cochrane.org/files/uploads/Chapter07-Searching-%28September-2008%29.pdf. [Google Scholar]
de Zwart 2019
- De Zwart PL, Jeronimus BF, De Jonge P. Empirical evidence for definitions of episode, remission, recovery, relapse and recurrence in depression: a systematic review. Epidemiology and Psychiatric Sciences 2019;28(5):544-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Docrat 2019
- Docrat S, Besada D, Cleary S, Daviaud E, Lund C. Mental health system costs, resources and constraints in South Africa: a national survey. Health Policy Planning 2019;34(9):706-19. [DOI: 10.1093/heapol/czz085] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Drummond 2005
- Drummond MF, Sculpher MJ, Torrance GW, O'Brien BJ, Stoddart GL. Methods for the economic evaluation of health care programmes. In: Methods for the Economic Evaluation of Health Care Programmes. 3rd edition. Oxford (UK): Oxford University Press, 2005:11. [Google Scholar]
Dybdahl 1996
- Dybdahl R. Group Work Manual. Sarajevo, Bosnia Herzegovina: UNICEF, 1996.
Dybdahl 1999
- Dybdahl R. Child development and impact of stress on young children. An intervention programme for mothers. Prepared for the UNICEF/Centre for Crisis Psychology Psychosocial Project, 1999.
EPOC 2017a
- Cochrane Effective Practice and Organisation of Care (EPOC). Suggested risk of bias criteria for EPOC reviews. EPOC Resources for Review Authors. epoc.cochrane.org/resources/epoc-resources-review-authors; 2017.
EPOC 2017b
- Higgins JP, Altman DG (editors). Chapter 8. Assessing risk of bias in included studies. In: Higgins JT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions version 5.0.1. Available from http://training.cochrane.org/handbook. The Cochrane Collaboration, 2008. [Google Scholar]
Foa 2005
- Foa EB, Hembree EA, Cahill SP, Rauch SA, Riggs DS, Feeny NC, et al. Randomised trial of prolonged exposure for post traumatic stress disorder with and without cognitive restructuring: outcome at academic and community clinics. Journal of Consulting and Clinical Psychology 2005;73(5):953-64. [DOI] [PubMed] [Google Scholar]
Fox 2002
- Fox S, Tang S. The Sierra Leonean refugee experience: traumatic events and psychiatric sequelae. Journal of Nervous and Mental Disease 2002;188:490-5. [DOI] [PubMed] [Google Scholar]
Fuhr 2014
- Fuhr DC, Salisbury TT, De Silva M, Atif N, Ginneken N, Rahman A, et al. Effectiveness of peer-delivered interventions for severe mental illness and depression on clinical and psychosocial outcomes: a systematic review and meta-analysis. Social Psychiatry and Psychiatric Epidemiology 2014;49:1691-702. [DOI: 10.1007/s00127-014-0857-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gajaria 2018
- Gajaria A, Ravindran AV. Interventions for perinatal depression in low and middle-income countries: a systematic review. Asian Journal of Psychiatry 2018;37:112-20. [DOI] [PubMed] [Google Scholar]
Galvin 2020
- Galvin G, Byansi W. A systematic review of task shifting for mental health in sub-Saharan Africa. International Journal of Mental Health 2020;49(4):336-60. [DOI: 10.1080/00207411.2020.1798720] [DOI] [Google Scholar]
Gamieldien 2020
- Gamieldien F, Galvaan R, Myers B, Sorsdahl K. Exploration of recovery of people living with severe mental illness (SMI) in low-income and middle-income countries (LMIC): a scoping review protocol. BMJ Open 2020;10(2):e032912. [DOI] [PMC free article] [PubMed] [Google Scholar]
GBD 2017
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;10(392):1789-858. [DOI: 10.1016/S0140-6736(18)32279-7] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Goodrich 2013
- Goodrich DE, Kilbourne AM, Nord KM, Bauer MS. Mental health collaborative care and its role in primary care settings. Current Psychiatry Reports 2013;15(8):383. [DOI: 10.1007/s11920-013-0383-2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime, Inc.) GRADEpro Guideline Development Tool [Software]. McMaster University (developed by Evidence Prime, Inc.), 2020. Available from gradepro.org.
Gureje 2007
- Gureje O, Chisholm D, Kola L, Lasebikan V, Saxena S. Cost-effectiveness of an essential mental health intervention package in Nigeria. World Psychiatry 2007;6(1):42-8. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. Rating quality of evidence and strength of recommendations GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. British Medical Journal 2008;336:924. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction - GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [DOI: 10.1016/j.jclinepi.2010.04.026] [DOI] [PubMed] [Google Scholar]
Guyatt 2013
- Guyatt GH, Thorlund K, Oxman AD, Walter SD, Patrick D, Furukawa TA, et al. GRADE guidelines: 13. Preparing summary of findings tables and evidence profiles for continuous outcomes. Journal of Clinical Epidemiology 2013;66:173e183. [DOI] [PubMed] [Google Scholar]
Herman 1997
- Herman J. Trauma and Recovery. New York: Basic Books, 1997. [Google Scholar]
Higgins 2009
- Higgins JP, Altman DG. Assessing risk of bias in included studies. In: Higgins JT, Green S, editors(s). Cochrane Handbook for Systematic Reviews of Interventions, version 5.0.2 (updated September 2009). The Cochrane Collaboration, 2009. Available from www.cochrane-handbook.org. [Google Scholar]
Higgins 2011
- Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0. The Cochrane Collaboration, March 2011. [Google Scholar]
Huntley 2012
- Huntley AL, Araya R, Salisbury C. Group psychological therapies for depression in the community: systematic review and meta-analysis. British Journal of Psychiatry 2012;200(3):184-90. [DOI] [PubMed] [Google Scholar]
Jan 2018
- Jan S, Laba TL, Essue BM, Gheorghe A, Muhunthan J, Engelgau M, et al. Action to address the household economic burden of non-communicable diseases. Lancet 2018;391(10134):2047-58. [DOI: 10.1016/S0140-6736(18)30323-4] [PMID: ] [DOI] [PubMed] [Google Scholar]
Jordans 2011
- Jordans MJ, Komproe IH, Tol WA, Susanty D, Vallipuram A, Ntamatumba P, et al. Practice-driven evaluation of a multi-layered psychosocial care package for children in areas of armed conflict. Community Mental Health Journal 2011;47(3):267-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kakuma 2011
- Kakuma R, Minas H, Ginneken N, Dal Poz MR, Desiraju K, Morris JE, et al. Human resources for mental health care: current situation and strategies for action. Lancet 2011;378(9803):1654-63. [DOI] [PubMed] [Google Scholar]
Keynejad 2018
- Keynejad RC, Dua T, Barbui C, Thornicroft G. WHO Mental Health Gap Action Programme (mhGAP) intervention guide: a systematic review of evidence from low and middle-income countries. Evidence-Based Mental Health 2018;21(1):30-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Klasen 2013
- Klasen H, Crombag AC. What works where? A systematic review of child and adolescent mental health interventions for low and middle income countries. Social Psychiatry and Psychiatric Epidemiology 2013;48(4):595-611. [DOI] [PubMed] [Google Scholar]
Kohrt 2018
- Kohrt BA, Asher L, Bhardwaj A, Fazel M, Jordans MJ, Mutamba BB, et al. The role of communities in mental health care in low- and middle-income countries: a meta-review of components and competencies. International Journal of Environmental Research and Public Health 2018;15(6):1276. [DOI: 10.3390/ijerph15061279] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lassi 2013
- Lassi ZS, Cometto G, Huicho L, Bhutta ZA. Quality of care provided by mid-level health workers: systematic review and meta-analysis. Bulletin of the World Health Organization 2013;91(11):824-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
La Trobe 2008
- Cochrane Consumers & Communication Review Group. Outcomes of interest to the Cochrane Consumers & Communication Review Group [La Trobe University]. http://www.latrobe.edu.au/chcp/assets/downloads/Outcomes.pdf; 2008.
Lavis 2009
- Lavis JN, Oxman AD, Souza NM, Lewin S, Gruen RL, Fretheim A. SUPPORT Tools for evidence-informed health Policymaking (STP): 9. Assessing the applicability of the findings of a systematic review. Health Research Policy and Systems 2009;7(suppl 1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Levin 2016
- Levin C, Chisholm D. Chapter 12. Cost-effectiveness and affordability of interventions, policies, and platforms for the prevention and treatment of mental, neurological, and substance use disorders. In: Patel V, Chisholm D, Dua T, et al, editors(s). Mental, Neurological, and Substance Use Disorders: Disease Control Priorities. 3rd edition. Vol. 4. Washington DC: The World Bank, 2016. [DOI: 10.1596/978-1-4648-0426-7_ch12] [DOI] [PubMed] [Google Scholar]
Lewin 2010
- Lewin S, Munabi-Babigumira S, Glenton C, Daniels K, Bosch-Capblanch X, Van Wyk BE, et al. Lay health workers in primary and community health care for maternal and child health and the management of infectious diseases. Cochrane Database of Systematic Reviews 2010, Issue 3. Art. No: CD004015. [DOI: 10.1002/14651858.CD004015.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lucas 2001
- Lucas CP, Zhang H, Fisher PW, Shaffer D, Regier DA, Narrow WE, et al. The DISC Predictive Scales (DPS): efficiently screening for diagnosis. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:443-9. [DOI] [PubMed] [Google Scholar]
Luengo‐Fernandez 2011
- Luengo-Fernandez R, Leal J, Gray AM. Cost of dementia in the pre-enlargement countries of the European Union. Journal of Alzheimer's Disease 2011;27(1):187-96. [DOI: 10.3233/JAD-2011-102019] [DOI] [PubMed] [Google Scholar]
Lukumar 2008
- Lukumar P, Wijewardana K, Hermansson J, Lindmark G. Validity and reliability of Tamil version of Strengths and Difficulties Questionnaire self-report. Ceylon Medical Journal 2008;53:48-52. [DOI] [PubMed] [Google Scholar]
Lund 2009
- Lund C, Boyce G, Flisher AJ, Kafaar Z, Dawes A. Scaling up child and adolescent mental health services in South Africa: human resource requirements and costs. Journal of Child Psychology and Psychiatry 2009;50(9):1121-30. [DOI: 10.1111/j.1469-7610.2009.02078.x] [DOI] [PubMed] [Google Scholar]
Lund 2016
- Lund C, Tomlinson M, Patel V. Integration of mental health into primary care in low- and middle-income countries: the PRIME mental healthcare plan. British Journal of Psychiatry 2016;208(suppl 56):s1-3. [DOI: 10.1192/bjp.bp.114.153668] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mendenhall 2014
- Mendenhall E, De Silva MJ, Hanlon C, Petersen I, Shidhaye R, Jordans M, et al. Acceptability and feasibility of using non-specialist health workers to deliver mental healthcare: stakeholder perceptions from the PRIME district sites in Ethiopia, India, Nepal, South Africa, and Uganda. Social Science & Medicine 2014;118:33-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mugashi 2017
- Mugisha J, Abdulmalik J, Hanlon C, Petersen I, Lund C, Upadhaya N et al. Health systems context(s) for integrating mental health into primary health care in six Emerald countries: a situation analysis. International Journal of Mental Health Systems 2017;11:7. [DOI: 10.1186/s13033-016-0114-2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Munodawafa 2018
- Munodawafa M, Mall S, Lund C, Schneider M. Process evaluations of task sharing interventions for perinatal depression in low and middle income countries (LMIC): a systematic review and qualitative meta-synthesis. BMC Health Services Research 2018;18(1):205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Murray 2015a
- Murray CJ, Barber RM, Foreman KJ. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: quantifying the epidemiological transition. Lancet 2015;386(10009):2145-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Murthy 2005
- Murthy RS, Kumar KKV, Chisholm D, Thomas T, Sekar K, Chandrashekari CR. Community outreach for untreated schizophrenia in rural India: a follow-up study of symptoms, disability, family burden and costs. Psychological Medicine 2005;35(3):341-51. [DOI: 10.1017/s0033291704003551] [DOI] [PubMed] [Google Scholar]
Mutamba 2013
- Mutamba BB, Ginneken N, Paintain LS, Wandiembe S, Schellenberg D. Roles and effectiveness of lay community health workers in the prevention of mental, neurological and substance use disorders in low and middle income countries: a systematic review. BMC Health Services Research 2013;13:412. [DOI: 10.1186/1472-6963-13-412] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ola 2019
- Ola BA, Atilola O. Task-shifted interventions for depression delivered by lay primary health-care workers in low-income and middle-income countries. Lancet Global Health 2019;7(7):e829-30. [DOI: 10.1016/S2214-109X(19)30197-4] [PMID: ] [DOI] [PubMed] [Google Scholar]
Padmanathan 2013
- Padmanathan P, De Silva MJ. The acceptability and feasibility of task-sharing for mental healthcare in low and middle income countries: a systematic review. Social Science & Medicine 2013;97:82-6. [DOI: 10.1016/j.socscimed.2013.08.004] [DOI] [PubMed] [Google Scholar]
Papastavrou 2010
- Papastavrou E, Charalambous A, Tsangari H, Karayiannis G. The cost of caring: the relative with schizophrenia. Scandinavian Journal of Caring Science 2010;24(4):817-23. [DOI: 10.1111/j.1471-6712.2010.00782.x] [DOI] [PubMed] [Google Scholar]
Parker 2008
- Parker AG, Hetrick SE, Purcell R, Gillies D. Consultation liaison in primary practice for mental health problems. Cochrane Database of Systematic Reviews 2008, Issue 2. Art. No: CD007193. [DOI: 10.1002/14651858.CD007193] [DOI] [PMC free article] [PubMed] [Google Scholar]
Patel 2007
- Patel V, Flisher AJ, Hetrick S, McGorry P. Mental health of young people: a global public-health challenge. Lancet 2007;369(9569):1302-13. [DOI] [PubMed] [Google Scholar]
Patel 2008a
- Patel V, Flisher AJ, Nikapota A, Malhotra S. Promoting child and adolescent mental health in low and middle income countries. Journal of Child Psychology and Psychiatry and Allied Disciplines 2008;49(3):313-34. [DOI] [PubMed] [Google Scholar]
Patel 2016
- Patel V, Chisholm D, Parikh R, Charlson FJ, Degenhardt L, Dua T, et al. Addressing the burden of mental, neurological, and substance use disorders: key messages from Disease Control Priorities, 3rd edition. Lancet 2016;387(10028):1672-85. [DOI: 10.1016/S0140-6736(15)00390-6] [PMID: ] [DOI] [PubMed] [Google Scholar]
Patel 2018
- Patel V, Saxena S, Lund C, Thornicroft G, Baingana F, Bolton P, et al. The Lancet Commission on global mental health and sustainable development. Lancet 2018;392(10157):1553-98. [DOI: 10.1016/s0140-6736(18)31612-x] [DOI] [PubMed] [Google Scholar]
Petersen 2012
- Petersen I, Lund C, Bhana A, Flisher AJ. A task shifting approach to primary mental health care for adults in South Africa: human resource requirements and costs for rural settings. Health Policy Plan 2012;27(1):42-51. [DOI: 10.1093/heapol/czr012] [DOI] [PubMed] [Google Scholar]
PRESS checklist 2016
- McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS – Peer Review of Electronic Search Strategies: 2015 Guideline Explanation and Elaboration (PRESS E&E). Ottawa: CADTH, 2016. [https://www.cadth.ca/sites/default/files/pdf/CP0015_PRESS_Update_Report_2016.pdf] [DOI] [PubMed]
Prince 2004
- Prince M, Graham N, Brodaty H, Rimmer E, Varghese M, Chiu H, et al. Alzheimer Disease International’s 10/66 Dementia Research Group - one model for action research in developing countries. Internation Journal of Geriatric Psychiatry 2004;19(2):178–81. [DOI] [PubMed] [Google Scholar]
Purgato 2018
- Purgato M, Gross AL, Betancourt T, Bolton P, Bonetto C, Gastaldon C, et al. Focused psychosocial interventions for children in low-resource humanitarian settings: a systematic review and individual participant data meta-analysis. Lancet Global Health 2018;6(4):e390-400. [DOI] [PubMed] [Google Scholar]
Purgato 2021
- Purgato M, Abdulmalik JO, Prina E, Ceccarelli C, Tol WA, Ginneken N, et al. Primary-level and community worker interventions for the prevention of mental disorders and the promotion of well-being in low- and middle-income countries. Cochrane Database of Systematic Reviews 2021, Issue 3. Art. No: CD014722. [DOI: 10.1002/14651858.CD014722] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rahman 2013
- Rahman A, Fisher J, Bower P, Luchters S, Tran T, Yasamy MT, et al. Interventions for common perinatal mental disorders in women in low- and middle-income countries: a systematic review and meta-analysis. Bulletin of the World Health Organization 2013;91:593-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rathod 2017
- Rathod S, Pinninti N, Irfan M, Gorczynski P, Rathod P, Gega L, et al. Mental health service provision in low- and middle-income countries. Health Service Insights 2017;10:1178632917694350. [DOI: 10.1177/1178632917694350] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ratzliff 2016
- Ratzliff A, Cerimele J, Katon W, Unützer J. Working as a team to provide collaborative care. In: Integrated Care: Creating Effective Mental and Primary Health Care Teams. 1st edition. Hoboken, NJ: John Wiley & Sons, 2016:1-24. [ISBN: 9781118900031] [Google Scholar]
Razzouk 2017
- Razzouk D. Burden and indirect costs of mental disorders. In: Mental Health Economics. New York, NY: Springer, 2017. [DOI: 10.1007/978-3-319-55266-8_25] [DOI] [Google Scholar]
Reeves 2009
- Reeves BC, Deeks JJ, Higgins JT, Wells GA. Including non-randomized studies. Cochrane Handbook for Systematic Reviews of Interventions, version 5.0.1 (updated September 2009). Available from www.cochrane-handbook.org, 2009.
Rehm 2019
- Rehm J, Shield KD. Global burden of disease and the impact of mental and addictive disorders. Current Psychiatry Reports 2019;21(2):10. [DOI: 10.1007/s11920-019-0997-0] [PMID: ] [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.2.. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Rocks 2020
- Rocks S, Berntson D, Gil-Salmerón A, Kadu M, Ehrenberg N, Stein V, et al. Cost and effects of integrated care: a systematic literature review and meta-analysis. European Journal of Health Economics 2020;21(8):1211-21. [DOI: 10.1007/s10198-020-01217-5] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2009
- Schünemann HJ, Oxman AD, Vist GE, Higgins JT, Deeks JJ, Glasziou P. Interpreting results and drawing conclusions. In: Higgins JT, Green S, editors(s). Cochrane Handbook for Systematic Reviews of Interventions, version 5.0.1 (updated September 2009). Available from www.cochrane-handbook.org. Chichester, UK: Wiley-Blackwell, 2009. [Google Scholar]
Seidman 2017
- Seidman G, Atun R. Does task shifting yield cost savings and improve efficiency for health systems? A systematic review of evidence from low-income and middle-income countries. Human Resources for Health 2017;15(1):29. [DOI: ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Semrau 2019
- Semrau M, Alem A, Ayuso-Mateos JL, Chisholm D, Gureje O, Hanlon C, et al. Strengthening mental health systems in low- and middle-income countries: recommendations from the Emerald programme. BJPsych Open 2019;5(5):e73. [PMID: 10.1192/bjo.2018.90] [PMID: ] [DOI] [PMC free article] [PubMed]
Shahmalak 2019
- Shahmalak U, Blakemore A, Waheed MW, Waheed W. The experiences of lay health workers trained in task-shifting psychological interventions: a qualitative systematic review. International Journal of Mental Health Systems 2019;13:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Singla 2017
- Singla DR, Kohrt BA, Murray LK, Anand A, Chorpita BF, Patel V. Psychological treatments for the world: lessons from low- and middle-Income countries. Annual Review of Clinical Psychology 2017;13:149-81. [DOI: 10.1146/annurev-clinpsy-032816-045217] [DOI] [PMC free article] [PubMed] [Google Scholar]
Siskind 2010
- Siskind D, Araya R, Kim J. Cost-effectiveness of improved primary care treatment of depression in women in Chile. British Journal of Psychiatry 2010;197(4):291-6. [DOI] [PubMed] [Google Scholar]
Snyder 1997
- Snyder CR, Hoza B, Pelham WE, Rapoff M, Ware L, Danovsky M, et al. The development and validation of the Children's Hope Scale. Journal of Pediatric Psychology 1997;22:399-421. [DOI] [PubMed] [Google Scholar]
Spijker 2002
- Spijker J, Graaf R, Bijl RV, Beekman ATF, Ormel J, Nolen WA. Duration of major depressive episodes in the general population: results from The Netherlands Mental Health Survey and Incidence Study (NEMESIS). British Journal of Psychiatry 2002;181:208-13. [DOI] [PubMed] [Google Scholar]
Spirito 1988
- Spirito A, Stark LJ, Williams C. Development of a brief coping checklist for use with paediatric populations. Journal of Pediatric Psychology 1988;13:555-74. [DOI] [PubMed] [Google Scholar]
Staub 2005
- Staub E, Pearlman LA, Gubin A, Hagengimana A. Healing, reconciliation, forgiving, and the prevention of violence after genocide or mass killing: an intervention and its experimental evaluation in Rwanda. Journal of Social and Clinical Psychology 2005;24:297-334. [Google Scholar]
Stuart‐Parrigon 2014
- Stuart-Parrigon K, Stuart S. Perinatal depression: an update and overview. Current Psychiatry Reports 2014;16:468. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Suh 2006
- Suh GH, Knapp M, Kang CJ. The economic costs of dementia in Korea, 2002. International Journal of Geriatric Psychiatry 2006;21(8):722-8. [DOI: 10.1002/gps.1552] [DOI] [PubMed] [Google Scholar]
Sørensen 2017
- Sørensen CW, Bæk O, Kallestrup P, Carlsson J. Integrating mental health in primary healthcare in low-income countries: changing the future for people with mental disorders. Nordic Journal of Psychiatry 2017;71(2):151-7. [DOI: 10.1080/08039488.2016.1245784] [DOI] [PubMed] [Google Scholar]
Thornicroft 2019
- Thornicroft G, Semrau M. Health system strengthening for mental health in low- and middle-income countries: introduction to the Emerald programme. BJPsych Open 2019;5(5):e66. [DOI: 10.1192/bjo.2019.9] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tol 2011
- Tol WA, Barbui C, Galappatti A, Silove D, Betancourt TS, Souza R, et al. Mental health and psychosocial support in humanitarian settings: linking practice and research. Lancet 2011;378(9802):1581-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Trautmann 2016
- Trautmann S, Rehm J, Wittchen HU. The economic costs of mental disorders. EMBO Reports 2016;17(9):1245-9. [DOI: 10.15252/embr.201642951] [DOI] [PMC free article] [PubMed] [Google Scholar]
Turrini 2019
- Turrini G, Purgato M, Acarturk C, Anttila M, Au T, Ballette F, et al. Efficacy and acceptability of psychosocial interventions in asylum seekers and refugees: systematic review and meta-analysis. Epidemiology and Psychiatric Sciences 2019;28:376–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ukoumunne 1999
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JA, Burney PG. Methods for evaluating area-wide and organisation-based interventions in health and health care: a systematic review. Health Technology Assessment 1999;3(5):iii-92. [PubMed] [Google Scholar]
van Ginneken 2021
- Nadja van Ginneken, Weng Yee Chin, Yen Chian Lim, Amin Ussif, Rakesh Singh, Ujala Shahmalak, Simon Lewin. Primary-level worker interventions for the care of mental disorders and distress in low- and middle-income countries - GRADE evidence profiles [Data set]. Zenodo 2021. Available at zenodo.org/record/5031582. [DOI: 10.5281/zenodo.5031582] [DOI] [PMC free article] [PubMed]
van Steenbergen‐Weijenburg 2010
- Steenbergen-Weijenburg KM, Feltz-Cornelis CM, Horn EK, Marwijk HW, Beekman AT, Rutten FF, et al. Cost-effectiveness of collaborative care for the treatment of major depressive disorder in primary care. A systematic review. BMC Health Services Research 2010;10(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO & WONCA 2008
- WHO, WONCA Working Party on Mental Health 2008. What is primary care mental health? Mental Health and Family Medicine 2008;5:9-13. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
WHO 1997
- World Health Organization. Composite International Diagnostic Interview (CIDI). Geneva, Switzerland: WHO, 1997.
WHO 2003
- World Health Organization. The Mental Health Context. Geneva, Switzerland: WHO, 2003. [Google Scholar]
WHO 2016
- WHO. mhGAP Intervention Guide - Version 2.0. World Health Organisation 2016:173. [ISBN: 978 92 4 154979 0]
WHO 2018
- World Health Organization. Mental Health Atlas 2017. Geneva, Switzerland: WHO, 2018. [ISBN: 978-92-4-151401-9]
WHO 2019
- World Health Organization. International Classification of Diseases, 11th revision (ICD-11). Available at homepage https://icd.who.int.
Wiley‐Exley 2007
- Wiley-Exley E. Evaluations of community mental health care in low- and middle-income countries: a 10-year review of the literature. Social Science and Medicine 2007;64(6):1231-41. [DOI] [PubMed] [Google Scholar]
Williams 2019
- Williams F, Moghaddam N, Ramsden S, De Boos D. Interventions for reducing levels of burden amongst informal carers of persons with dementia in the community. A systematic review and meta-analysis of randomised controlled trials. Aging and Mental Health 2019;23:1629-42. [DOI: ] [DOI] [PubMed] [Google Scholar]
Wimo 2007
- Wimo A, Winblad B, Jönsson L. An estimate of the total worldwide societal costs of dementia in 2005. Alzheimer's Dementia 2007;3(2):81-91. [DOI: 10.1016/j.jalz.2007.02.001] [DOI] [PubMed] [Google Scholar]
Woltmann 2012
- Woltmann E, Gorgan-Kaylor A, Perron B, Georges H, Kilbourne AM, Bauer MS. Effectiveness of collaborative chronic care models for mental health conditions across primary, specialty, and behavioral health care settings: systematic review and meta-analysis. American Journal of Psychiatry 2012;169(8):790-804. [DOI] [PubMed] [Google Scholar]
Zilberberg 2010
- Zilberberg MD, Shorr AF. Understanding cost-effectiveness. Clinical Microbiology and Infection 2010;16:1707–12. [DOI: 10.1111/j.1469-0691.2010.03331] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
van Ginneken 2013
- Ginneken N, Tharyan P, Lewin S, Rao GN, Meera SM, Pian J, et al. Non-specialist health worker interventions for the care of mental, neurological and substance-abuse disorders in low- and middle-income countries. Cochrane Database of Systematic Reviews 2013, Issue 11. Art. No: CD009149. [DOI: 10.1002/14651858.CD009149.pub2] [DOI] [PubMed] [Google Scholar]
van Ginneken 2019
- Ginneken N, Chin WY, Lewin S, Garner P, Tharyan P, Patel V, et al. Primary-level worker interventions for the care of mental disorders and distress in low- and middle-income countries (Cochrane review update protocol), version 1. Zenodo, 28 June 2019. [DOI: 10.5281/zenodo.3972778] [WEBSITE LINK: https://zenodo.org/record/3972778#.XyplsxNLj_Q] [DOI]


