Abstract
Background
Thoracic aortic arch aneurysms (TAAs) can be a life‐threatening condition due to the potential risk of rupture. Treatment is recommended when the risk of rupture is greater than the risk of surgical complications. Depending on the cause, size and growth rate of the TAA, treatment may vary from close observation to emergency surgery. Aneurysms of the thoracic aorta can be managed by a number of surgical techniques. Open surgical repair (OSR) of aneurysms involves either partial or total replacement of the aorta, which is dependent on the extent of the diseased segment of the aorta. During OSR, the aneurysm is replaced with a synthetic graft. Hybrid repair (HR) involves a combination of open surgery with endovascular aortic stent graft placement. Hybrid repair requires varying degrees of invasiveness, depending on the number of supra‐aortic branches that require debranching. The hybrid technique that combines supra‐aortic vascular debranching with stent grafting of the aortic arch has been introduced as a therapeutic alternative. However, the short‐ and long‐term outcomes of HR remain unclear, due to technical difficulties and complications as a result of the angulation of the aortic arch as well as handling of the arch during surgery.
Objectives
To assess the effectiveness and safety of HR versus conventional OSR for the treatment of TAAs.
Search methods
The Cochrane Vascular Information Specialist searched the Cochrane Vascular Specialised Register, CENTRAL, MEDLINE, Embase, CINAHL and AMED databases and World Health Organization International Clinical Trials Registry Platform and ClinicalTrials.gov trials registers to 22 March 2021. We also searched references of relevant articles retrieved from the electronic search for additional citations.
Selection criteria
We considered for inclusion in the review all published and unpublished randomised controlled trials (RCTs) and controlled clinical trials (CCTs) comparing HR to OSR for TAAs.
Data collection and analysis
Two review authors independently screened all titles and abstracts obtained from the literature search to identify those that met the inclusion criteria. We retrieved the full text of studies deemed as potentially relevant by at least one review author. The same review authors screened the full‐text articles independently for inclusion or exclusion.
Main results
No RCTs or CCTs met the inclusion criteria for this review.
Authors' conclusions
Due to the lack of RCTs or CCTs, we were unable to determine the safety and effectiveness of HR compared to OSR in people with TAAs, and we are unable to provide high‐certainty evidence on the optimal surgical intervention for this cohort of patients. High‐quality RCTs or CCTs are necessary, addressing the objective of this review.
Plain language summary
Hybrid versus conventional open surgical repair for thoracic aortic arch aneurysms
Background
An aortic aneurysm is an abnormal bulge or swelling that occurs in the wall of the aorta. An aneurysm can be classified by its shape, location (sometimes reported as zone) and size. A thoracic aortic arch aneurysm (TAA) is a swelling in the upper portion of the thoracic aorta. The estimated annual incidence (number of new cases in a population over a particular period of time) of TAAs is between 5.6 and 10.4 cases per 100,000 patient‐years. TAAs affect people mostly in the sixth and seventh decade of life and affect men and women equally. Management of TAAs differs by the extent and location of the aneurysm along the aortic arch and the patient’s medical history. TAAs can be treated with either open surgical repair (a surgical procedure requiring partial or total replacement of the diseased aortic arch) or hybrid repair (a surgical procedure which is a less invasive form of open surgical repair). So far, there is no consensus on which type of operation offers the best clinical outcomes in people with TAAs.
This review aimed to assess the safety and effectiveness of hybrid repair compared to open surgical repair in treating TAAs.
Study characteristics and key results
We searched the literature for randomised controlled trials (RCTs) and controlled clinical trials (CCTs) to evaluate the effectiveness and safety of HR compared to OSR for treating TAAs. Our search, current up to March 2021, did not identify any trials that met our inclusion criteria. High‐quality trials are needed to help inform healthcare professionals, policy makers, and patients about the best possible treatment option for people with TAAs.
Certainty of the evidence
We found no RCTs and CCTs that addressed the review objective.
Conclusion
High‐quality RCT and CCTs are required to assess the safety and effectiveness of HR compared to OSR effectively.
Background
See Appendix 1 for Glossary of terms
Description of the condition
The aorta is the largest artery in the body, delivering oxygenated blood to all organs through the entire body (Bhimji 2012). The wall of the aorta is made up of three layers, the intima, the media and the adventitia. The intima is the innermost layer. This layer has a smooth surface for blood to flow past. The media is the middle layer made up of muscle and elastic fibres. This layer allows the aorta to expand and tighten. The adventitia is the outermost layer and provides additional support and shape to the aorta.
The aorta is divided into five sections depending on their location: the ascending aorta, aortic arch, descending thoracic aorta, thoracoabdominal aorta and abdominal aorta. The ascending aorta rises from the heart. The aortic arch begins close to the brachiocephalic artery (BA) and ends at the T4 vertebra. Three major vessels, BA, left common carotid artery (LCCA) and left subclavian artery (LSA) arise from the aortic arch (Imran 2020). These three vessels supply blood to the neck, arms and head. The descending aorta begins at the level of the fourth thoracic vertebra as a continuation of the aortic arch and ends at the diaphragm. The abdominal aorta begins at the diaphragm and then divides into the left and right common iliac arteries.
Based on guidelines published by the Society of Vascular Surgery (SVS) for endovascular repair of the aorta, the aorta is divided into 12 zones, from 0 to 11 (Czerny 2019; Fillinger 2010). This zone classification is also used to provide further information on the exact location of an aneurysm along the aorta.
The zones of the aorta are categorised as follows:
Zone 0 involves the proximal ascending aorta to the BA origin; Zone 1 is distal to the BA but proximal to the LCCA; Zone 2 is distal to the LCCA but proximal to the subclavian artery; Zone 3 is distal to the LSA artery and travels 2 cm beyond the subclavian artery; Zone 4 proximal extent of the endograft is 2 cm distal to the LSA and ends within the proximal half of the descending thoracic aorta (T6 approximates the midpoint of the descending thoracic aorta); Zone 5 starts in the distal half of the descending thoracic aorta but ends proximal to the celiac artery; Zone 6 involves the celiac origin to the top of the superior mesenteric artery (SMA); Zone 7 covers the SMA; Zone 8 covers at least one of the renal arteries; Zone 9 covers below the renal arteries to the bifurcation of the common iliac arteries; Zone 10 comprises the common iliac arteries; and Zone 11 covers the external iliac arteries.
Thoracic aortic arch aneurysms (TAAs) are a dilatation or widening of the thoracic aortic arch, reaching at least 1.5 times the normal diameter (Czerny 2019; Elefteriades 2010). Thoracic aortic aneurysms can involve one or more sections of the thoracic aorta that include the aortic root (60% of thoracic aortic cases), ascending aorta (60% of cases), aortic arch (10% of cases) and a portion of the descending aorta (40% of cases) (Clare 2016; Czerny 2019; Elefteriades 2002; Ince 2007; Isselbacher 2005; Olsson 2006). The Ishimaru classification further categorises the aneurysm location based on zones (Mitchell 2002). The thoracic aorta is divided into five zones, each zone corresponding to the site of the aneurysm. Zone 0 involves the proximal ascending aorta to the brachiocephalic artery. Zone 1 comprises the aortic arch between the brachiocephalic and left common carotid artery. Zone 2 involves the aortic arch between the left common carotid artery and the left subclavian artery. Zone 3 involves the proximal descending thoracic aorta distal to the left subclavian artery, and Zone 4 involves the mid‐descending thoracic aorta (Mitchell 2002; Moulakakis 2013). This zone classification can be used to provide further information on the exact location of an aneurysm along the aorta.
The natural history of TAA is a slow expansion with an exponential increase in the risk of rupture at larger diameters. The expansion rates for TAAs are generally less than those of abdominal aortic aneurysms (AAAs) (Masuda 1992; Oladokun 2016). The rate of expansion depends on the location of the aneurysm, its aetiology and diameter. TAAs are associated with high rates of morbidity and mortality. Most TAAs are degenerative in nature, resulting from alterations in the vascular wall, which lead to loss of structural integrity and wall strength (Gleason 2005; Pannu 2005; Wheeler 2014). The underlying causes of degenerative TAAs are not fully elucidated but are associated with underlying conditions such as bicuspid aortic valve disease, hypertension, and presence of aneurysms located in other sections of the aorta. TAAs may also develop following an aortic dissection, from trauma or from a genetic predisposition that is either familial or related to an inherited connective tissue disorder (Gleason 2005; Pannu 2005; Wheeler 2014). Connective tissue disorders associated with TAA include Marfan syndrome, Ehlers Danlos syndrome and Loeys Dietz syndrome (Clare 2016). The outcome after surgical repair differs for each form of TAA, depending on the specific anatomical location, patient comorbidity and the integrity of the connective tissue of the aortic wall.
Description of the intervention
Current surgical techniques in use for the repair of TAAs are OSR and HR (Clouse 1998; Czerny 2019; Hiraoka 2015; Hiratzka 2010; Moulakakis 2013; Ouzounian 2013; Patel 2016). Total endovascular repair (TEVAR) is another surgical technique for treating TAA, but this technique is a relatively new intervention for the aortic arch. TEVAR can be undertaken, even if TAAs involve aortic arch branch vessels, by using specialised endovascular fenestration or branching covered stents, or both. This requires meticulous operative planning and expert operator skill. These types of repair are therefore limited to centres that perform a reasonable number of these procedures (Haulon 2013; Preventza 2014).
OSR of aortic arch aneurysms is a complex surgical procedure requiring partial or total replacement of the diseased aortic arch. Patients offered open surgery are highly selected based on their comorbidity status. The use of cardiopulmonary bypass, hypothermic circulatory arrest and cerebral protection, have resulted in improvement in neurological events such as stroke, but mortality remains high (Bachet 2018; Estrera 2008; Hiraoka 2015).
The hybrid approach involves a debranching procedure, which is a less invasive form of OSR combined with TEVAR, and is suitable for complex cases. This approach has been reported to improve outcomes for high‐risk patients who are unsuitable for OSR, but postoperative complications (particularly stroke) are still a major concern (Vallabhajosyula 2013). Hybrid procedures can be performed as a single‐stage procedure or can be performed as a two‐stage procedure where the open component is performed first and then the endovascular component at a later point.
All surgical interventions of the aorta arch require cerebral protection. Cardiopulmonary bypass is necessary for surgical repair of the aneurysm involving the thoracic aortic arch. Cardiopulmonary bypass provides extracorporeal oxygenation of the patients’ blood, allowing for isolation of the diseased aortic segment while still providing perfusion to the rest of the body. In addition to circulatory arrest, the body is put under moderate hypothermic conditions (a drop in body temperature) in order to reduce metabolic rate. While hypothermia is an important factor for cerebral protection during circulatory arrest, it is not sufficient of itself to provide adequate cerebral protection. There are three methods for cerebral protection during hypothermic circulatory arrest. These three methods include further cooling by deep hypothermic circulatory arrest (DHCA), retrograde cerebral perfusion (RCP) and antegrade cerebral perfusion (ACP). The choice of cerebral protection is institution‐driven and also determined based on surgeons' expertise and preference.
Deep hypothermic circulatory arrest (DHCA) is used for the replacement of the aortic arch during OSR to help re‐anastomosis of the supra aortic vessels. DHCA involves cooling the body slowly over 30 to 35 minutes to a temperature of 20 °C for hemi‐arch replacement or 18 °C for total arch replacement, so blood flow can be stopped temporarily, reducing oxygen requirement and metabolic demands of the brain and vital organs (Conolly 2010; Damberg 2017). This allows surgery on the aorta to be safely performed while preventing organ ischaemia during circulatory arrest (Conolly 2010; Damberg 2017; Ziganshin 2014). DHCA can only be used for short periods of circulatory arrest. Once surgery is complete, patients are gradually rewarmed before the cardiopulmonary bypass is stopped.
Antegrade cerebral perfusion (ACP) and retrograde cerebral perfusion (RCP) are two methods used to ensure cerebral protection. RCP uses cold or moderately‐cold oxygenated blood (15 – 24 °C) administered into the superior vena cava during the circulatory arrest period (Pacini 2007; Safi 2011). Since its introduction in the 1980s, antegrade cerebral perfusion (ACP) is typically used for perfusion during OSR (Mills 1980; Ouzounian 2013; Patel 2016). ACP is typically performed through a cannulation of the brachiocephalic and left common carotid arteries. ACP permits longer periods of circulatory arrest then with just DHCA alone. The lower temperature (12 °C to 16 °C) used in ACP reduces the incidence of blood clots seen with RCP (Ouzounian 2013; Patel 2016). The choice of ACP or RCP is dependent on surgeon expertise and preference as well as institution availability, with acceptable results for both options (Di Bartolomeo 2017; Estrera 2008; Imran 2020; Ouzounian 2013, Shrestha 2017; Stein 2010)
Open surgical repair
Open surgical repair (OSR) involves reconstruction of the aortic arch with a synthetic surgical graft and multiple anastomoses (connections between adjacent blood vessels) (Czerny 2019). Techniques of OSR vary depending on the extent of the thoracic aortic pathology.
In people with a proximal arch aneurysm, a hemi‐arch replacement is performed. The ascending aorta is replaced with a synthetic graft and the arch vessels are left intact.
In people presenting with extensive thoracic aneurysms, a total arch replacement is performed. Total arch replacement is the gold standard for aortic arch repair (Imran 2020; Wallen 2018). This involves removal of the brachiocephalic, left common carotid and left subclavian arteries from the aortic arch (Ouzounian 2013; Patel 2016). The three branching arch vessels are either attached to the synthetic island graft using a patch from the aorta containing the origins of the three vessels, or reimplanted individually using a synthetic graft containing three to four branches. The proximal and distal ends of the graft are attached to normal segments of the ascending and descending aorta (Hiratzka 2010; Ouzounian 2013; Patel 2016).
In cases where the aneurysm extends beyond zone 4, and further staged surgery is expected, a technique called elephant trunk can be used, whereby surplus intravascular graft length can be used to facilitate subsequent operations on the downstream aorta (Heinemann 1995).
Hybrid repair
HR was introduced to simplify and reduce the invasiveness of OSR. Hybrid aortic arch repair involves debranching of the main three vessels (brachiocephalic, left common carotid and left subclavian arteries) using synthetic bypass grafting (Antoniou 2010). This is followed by placing an endovascular graft traversing the aortic arch and landing distally in the descending aorta. This approach is associated with lower mortality and morbidity rates in comparison to OSR, but endoleaks (persistent blood flow outside the lumen of the stent graft within the aneurysm sac, resulting from inadequate sealing between the endograft and the wall of the aorta, fabric defects or retrograde flow from patent aortic side branches) and graft migration remain its main drawbacks (Czerny 2013; Fillinger 2010; Metzger 2014). HR has proven beneficial in cases with extensive disease that also affects the distal aorta (Cao 2012; Clough 2013; Jakob 2012; Jakob 2017). The diseased section of the descending aorta is repaired using an endovascular stent graft (Harris 2013). The decision to perform debranching is multifactorial, depending on the anatomy, patient fitness and comorbid status, as well as surgical expertise and hospital facilities. Hybrid approaches are classified into three types according to the extent of aortic lesion and the presence of the proximal and distal landing zone (Moulakakis 2013) (see Figure 1).
1.

(A) The aortic arch divided into four landing zones for the proximal end of the endograft. (B) Type I: debranching using brachiocephalic bypass grafting and endovascular repair of the aortic arch. This approach is reserved for cases with isolated aortic arch aneurysm that have adequate proximal landing zone 0 in the ascending aorta and distal landing zone in the descending thoracic aorta. (C) Type II: involves an open repair of the ascending aorta and revascularisation of the three branching vessels to create a proximal landing zone for an endovascular graft, which is then deployed to exclude the aneurysm. (D) Type III: consists of an elephant trunk procedure with surgical reconstruction of the aortic arch and revascularisation of the branching vessels of the aortic arch. The surgical graft used to repair the aortic arch is extended in to the descending aorta, where is functions as a landing zone for an endovascular stent graft. This procedure is reserved for patients with extensive aortic lesions involving the ascending aorta, transverse arch and the descending thoracic aorta. "Copyright © [2017] [Oxford University Press on behalf of the European Association for Cardio‐Thoracic Surgery]: reproduced with permission. All rights reserved."
Type I involves debranching using brachiocephalic bypass grafting and endovascular repair of the aortic arch. This approach is reserved for cases with isolated aortic arch aneurysm that have adequate proximal landing zone in the ascending aorta and distal landing zone in the descending thoracic aorta (see Figure 1 B).
Type II involves an open reconstruction of the ascending aorta and revascularisation of the three branching vessels to create a proximal landing zone for an endovascular graft, which is then deployed to exclude the aneurysm (see Figure 1 C).
Type III consists of an elephant trunk procedure with surgical reconstruction of the aortic arch and revascularisation of the branching vessels of the aortic arch. The surgical graft used to repair the aortic arch is extended in to the descending aorta, where it functions as a landing zone for an endovascular stent graft. This procedure is reserved for patients with extensive aortic lesions involving the ascending aorta, transverse arch and the descending thoracic aorta (Moulakakis 2013) (see Figure 1 D).
How the intervention might work
Despite improved standards of perioperative care, operative techniques and use of additional protective measures, OSR and HR are associated with considerable morbidity and mortality rates (Chakos 2018; Hiraoka 2015; Moulakakis 2013; Miao 2017; Preventza 2015). Although OSR is regarded as standard therapy for TAAs, the associated morbidity and mortality is significant, with reported rates ranging between 3.7% and 14% for mortality and 4% to 10% for neurological events (Bachet 2018; Chakos 2018; Hanif 2018; Hori 2017; Khullar 2017; Stone 2006; Tanaka 2014). Intervention for TAAs using a hybrid approach reduces the invasiveness of surgery and can remove the need for cardiopulmonary bypass and antegrade cerebral perfusion. The availability of off‐the‐shelf stent grafts that can be easily delivered and deployed in complex aortic arch anatomies has encouraged more surgeons to treat complicated cases using the hybrid approach.
Why it is important to do this review
It is estimated that 3% to 4% of patients over the age of 65 years are affected by TAAs (Elefteriades 2010; Saliba 2015). It is anticipated that the incidence and prevalence of TAAs will accelerate with an increasingly ageing population (Elefteriades 2010). In the event of rupture, sudden death is almost certain; and although the risk of mortality is reduced with repair of the aneurysm, the risk of perioperative morbidity and mortality is still considerable. Management of people with TAAs represents a continuing formidable challenge and is an area of ongoing research and development (Wong 2011). The introduction of HR, a single stage or two‐stage surgical procedure consisting of open debranching of the supra‐aortic arch vessels followed by endovascular repair of the distal aorta (zone 4), has shown potential to reduce rates of mortality and morbidity (Benedetto 2013; Chakos 2018; Miao 2017; Preventza 2015; Tokuda 2016). Although there have been major advances in the quality of treatment of TAAs, to date, there is currently no clinical consensus on which operative approach offers the best clinical outcomes in people with TAAs. We undertook this review in order to guide surgeons on the optimal surgical intervention for TAAs.
Objectives
This review aimed to assess the effectiveness and safety of HR versus conventional OSR for the treatment of TAAs.
Methods
Criteria for considering studies for this review
Types of studies
We considered randomised controlled trials (RCTs) and clinical controlled trials (CCTs) comparing HR to OSR for TAAs for inclusion in the review.
Types of participants
All participants with TAAs in zones 0 to 4, diagnosed using conventional methods such as computed tomography (CT) or magnetic resonance imaging (MRI), or both, were included in the review. We applied no limitation by participants' gender, age, ethnicity or treatment setting (e.g. elective versus emergency). We considered all morphologies (e.g. fusiform and saccular) for inclusion. Based on the American College of Cardiology and American Heart Association (ACC/AHA) 2010 guidelines and the 2014 European Society of Cardiology (ESC) guidelines for the management of TAAs, TAAs larger than 5.5 cm in diameter or with an annual growth rate of more than 0.5 cm requiring surgical intervention by HR or OSR were considered for inclusion (Erbel 2014; Hiratzka 2010). We excluded people with aortic aneurysms that required concomitant aortic valve replacement, TAAs secondary to aortic dissection, traumatic TAAs or infective TAAs. We also excluded cases treated with purely or total endovascular technique.
Types of interventions
We planned to include the following comparisons:
Type I hybrid technique versus OSR;
Type II hybrid technique versus OSR; and
Type III hybrid technique versus OSR.
Types of outcome measures
The primary and secondary outcomes were guided by the International Aortic Arch Surgery Study Group (IAASSG) (Yan 2014).
Primary outcomes
Aneurysm‐related mortality at 30 days
Aneurysm‐related mortality at 12 months
Neurological deficit (stroke or paraplegia)
Cardiovascular event (myocardial ischaemia, heart failure, low cardiac output syndrome, arrhythmia, pericardial effusion)
Respiratory compromise (parenchymal and pleural complications)
Secondary outcomes
Graft patency
Reintervention rate (defined as secondary intervention after the primary hybrid or OSR repair)
Search methods for identification of studies
Electronic searches
The Cochrane Vascular Information Specialist conducted systematic searches of the following databases for randomised controlled trials and controlled clinical trials without language, publication year or publication status restrictions.
The Cochrane Vascular Specialised Register via the Cochrane Register of Studies (CRS‐Web searched from inception to 22 March 2021).
The Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies Online (CRSO 2021, Issue 2).
MEDLINE (Ovid MEDLINE® Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE® Daily and Ovid MEDLINE® 1946 to present) (searched 22 March 2021).
Embase Ovid (searched 22 March 2021).
CINAHL Ebsco (searched 22 March 2021).
AMED Ovid (searched 22 March 2021).
The Information Specialist modelled search strategies for other databases based on the search strategy designed for CENTRAL. Where appropriate, they were combined with adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Chapter 6; Lefebvre 2011). Search strategies for major databases are provided in Appendix 2.
The information Specialist also searched the following trials registries on 22 March 2021.
The World Health Organization International Clinical Trials Registry Platform (who.int/trialsearch).
ClinicalTrials.gov (clinicaltrials.gov).
Searching other resources
We searched references of relevant articles retrieved from the electronic search for additional citations.
Data collection and analysis
Selection of studies
Two review authors (AE and EPK) independently screened and assessed all titles and abstracts identified from the literature search. We retrieved the full text of studies identified as potentially relevant by at least one review author. The same review authors independently screened the full‐text articles for inclusion or exclusion. We resolved any disagreements by discussion or, when necessary, we consulted a third review author (NH). All studies excluded at the full‐text stage are listed as excluded studies, with reasons for their exclusion presented in the Characteristics of excluded studies table. The screening and selection processes are presented using the adapted PRISMA flowchart (Liberati 2009).
Data extraction and management
Had we included studies, we planned for two review authors (AE and EPK) to independently extract data from eligible studies using an adapted data extraction form provided by Cochrane Vascular. Any disagreements would have been resolved by discussion or if necessary, we would have consulted a third review author (NH). We had planned for one review author (AE) to enter extracted data into Review Manager 5 (Review Manager 2020), and we planned for a second review author (NH), to check for accuracy and consistency against the data extraction sheets.
Assessment of risk of bias in included studies
Had we included studies, we planned that two review authors (AE and EPK) would assess each study independently for risks of bias according to the following criteria, as recommended by the Cochrane Handbook (Higgins 2011):
Random sequence generation (selection bias);
Allocation concealment (selection bias);
Blinding of participants and personnel (performance bias);
Blinding of outcome assessment (detection bias);
Incomplete outcome data (attrition bias);
Selective reporting (reporting bias); and
other potential sources of bias.
We planned to judge all included studies as having low, high or unclear risk of bias, based on these criteria. We planned to resolve disagreements by discussion or if necessary by consulting with a third review author (NH).
Measures of treatment effect
Dichotomous data
Had we included studies, we planned to express the results for dichotomous outcome measures using a risk ratio (RR) and its associated 95% confidence interval (CI) to reflect uncertainty of the point estimate of effects.
Continuous data
For continuous outcome measures, we had planned to calculate the mean difference and standard deviation with its corresponding 95% CI. We had planned to use the standardised mean difference (SMD) with its 95% CI to combine outcomes from trials that measure the same outcome using different scales (Higgins 2011).
Time‐to‐event data
In relation to survival analysis, we planned to report time‐to‐event data and the intervention effect expressed as a hazard ratio and its associated 95% CI. Methods used to analyse time‐to‐event data would have been guided by those described by Parmar 1998 and Tierney 2007.
Unit of analysis issues
Had we included studies, the unit of analysis would have been each individual participant.
Dealing with missing data
For studies with missing data, we contacted the corresponding study authors to try to obtain additional information. We planned to record missing and unclear data for each included study. We also aimed to perform all analyses using an intention‐to‐treat approach, that is, we had planned to analyse all participants and their outcomes within the groups to which they were allocated, regardless of whether they received the intervention or not.
Assessment of heterogeneity
Had we included studies, we planned to assess the degree of heterogeneity by visual inspection of forest plots and by examining the Chi2 test for heterogeneity. We planned to assess heterogeneity of the overall results for the main outcomes by use of Chi2, I2 and Tau2 statistics, according to the Cochrane Handbook (Higgins 2011).
We planned to regard statistical heterogeneity as substantial if an I2 was greater than 50% and either the T2 was greater than zero, or there was a low P value (less than 0.10) in the Chi2 test for heterogeneity.
Assessment of reporting biases
If 10 or more studies were included in the review, we had planned to investigate publication bias using funnel plots, as recommended by the Cochrane Handbook (Higgins 2011).
Data synthesis
Had we included studies, we planned to record data and carry out statistical analysis using Review Manager 5 (Review Manager 2020), using a fixed‐effect meta‐analysis for synthesising data where it was reasonable to assume that trials were estimating the same underlying treatment effect. If clinical heterogeneity was sufficient to expect that the underlying treatment effects differed between trials, or if we detected substantial statistical heterogeneity, we had planned to use a random‐effects meta‐analysis to produce an overall summary where the average treatment effect was clinically meaningful. If we detected substantial clinical, methodological or statistical heterogeneity across the included trials, we had planned not to report pooled results from the meta‐analysis but instead to use a narrative approach to data synthesis.
Subgroup analysis and investigation of heterogeneity
We had planned subgroup analyses limited to primary outcomes. Our planned subgroup analyses included:
Classification based on proximal treatment zone and the extent of the disease (number of zones into which the aneurysm extends);
Connective tissue disease versus degenerative disease;
Previous aortic valve repair;
Elective versus emergency;
Gender (men versus women); and
Length of stay in intensive care unit (HR versus OSR).
Sensitivity analysis
For the purpose of this review, and had we included studies, we had planned to classify trials judged as ‘low risk of bias’ for sequence generation and allocation concealment as high‐quality trials. We had planned to repeat the analyses to include only high‐quality trials. We had also planned to repeat the analyses including only RCTs.
Summary of findings and assessment of the certainty of the evidence
Had we included studies, we planned to prepare 'Summary of findings' tables to present the findings from this review using GRADEproGDT (GRADEpro GDT). We had also planned to create one table for each comparison (Type I hybrid technique versus OSR; Type II hybrid technique versus OSR; Type III hybrid technique versus OSR). We planned to include all seven outcomes as detailed in ‘Types of outcome measures’. We would have graded the certainty of the evidence for each outcome using criteria devised by GRADE (GRADE Working Group 2004). We had planned to assess the certainty of the evidence as high, moderate, low or very low, based on risk of bias, inconsistency, indirectness, imprecision and publication bias (Atkins 2004; Guyatt 2008; Higgins 2011; Schünemann 2010).
We include a draft version of the 'Summary of findings' table in this review (see Table 1).
1. What is the comparative effectiveness and safety of hybrid repair versus open surgical repair of thoracic aortic arch aneurysms?
| Hybrid repair versus conventional open repair for thoracic aortic arch aneurysms | ||||||
|
Patient or population: patients with a diagnosis of thoracic aortic arch aneurysms Settings: hospital, elective and emergency Intervention: hybrid repair Comparison: conventional open surgical repair | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with conventional open surgical repair | Risk with hybrid repair | |||||
|
Aneurysm related mortality 30 days Follow‐up: median N |
Study population |
HR N (N to N) |
N (N) | |||
|
N per 1000 (N to N) |
N per 1000 (N to N) |
|||||
|
Aneurysm related mortality 12 months Follow‐up: median N |
Study population |
HR N (N to N) |
N (N) | |||
|
N per 1000 (N to N) |
N per 1000 (N to N) |
|||||
|
Neurological deficita Follow‐up: median N |
Study population |
RR N (N to N) |
N (N to N) |
|||
| N per 1000 |
N per 1000 (N to N) |
|||||
|
Cardiovascular eventb Follow‐up: median N |
Study population |
RR N (N to N) |
N (N to N) |
|||
|
N per 1000 (N to N) |
N per 1000 (N to N) |
|||||
|
Respiratory compromise Follow‐up: median N |
Study population |
RR N (N to N) |
N (N to N) |
|||
|
N per 1000 (N to N) |
N per 1000 (N to N) |
|||||
|
Graft patency Follow‐up: median N |
Study population |
RR N (N to N) |
N (N to N) |
|||
|
N per 1000 (N to N) |
N per 1000 (N to N) |
|||||
|
Reintervention Follow‐up: median N |
Study population |
RR N (N to N) |
N (N to N) |
|||
|
N per 1000 (N to N) |
N per 1000 (N to N) |
|||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HR: hazard ratio; N: number; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aA neurological deficit event includes stroke or paraplegia. bA cardiovascular event includes myocardial ischaemia or heart failure, or low cardiac output syndrome, or arrhythmia, or pericardial effusion.
Results
Description of studies
Results of the search
The database searches resulted in 1959 reports of trials, of which 322 were duplicates. There were no RCTs or CCTs relevant to this review (see Figure 2).
2.
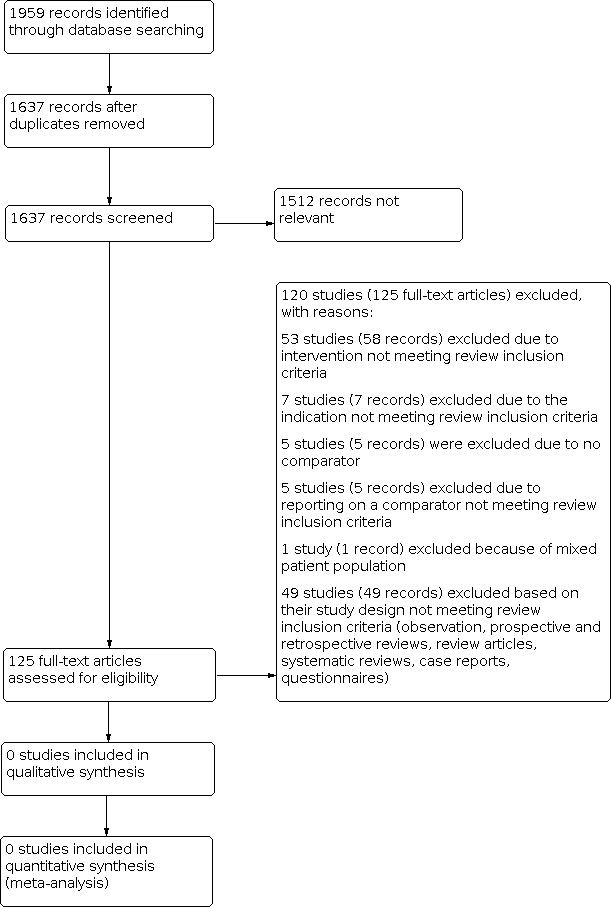
Study flow diagram.
Included studies
No RCTs or CCTs were eligible for inclusion (see Figure 2).
Excluded studies
We assessed 120 studies (125 records) by full‐text eligibility, and excluded them with reasons (see Figure 2). See Characteristics of excluded studies for the full list of excluded studies and reasons for exclusion.
Fifty‐three studies (58 records) studies were excluded due to intervention not meeting review inclusion criterion (see Figure 2), (Acher 1993; Bockler 2016; ChiCTR‐IPR‐15006372; Fairman 2008; Fernandez 2016; Huang 2011; JPRN‐UMIN000005430; JPRN‐UMIN000007213; JPRN‐UMIN000024202; Karashima 2016; Kaushik 2017; Matsumura 2014; NCT00110201; NCT00409344; NCT00508118; NCT00583817; NCT00590759; NCT00691756; NCT00757003; NCT01033214; NCT01181947; NCT01524211; NCT01756911; NCT01772537; NCT01839695; NCT01920594; NCT02010892; NCT02089607; NCT02253082; NCT02291718; NCT02294435; NCT02323581; NCT02365454; NCT02365467; NCT02471781; NCT02554032; NCT02625324; NCT02652949; NCT02678728; NCT02777528; NCT02777593; NCT02818972; NCT03024554; NCT03111459; NCT03207568; NCT03208504; NCT03214601; Novikova 2011; Patel 2016; Rahe‐Meyer 2013; Spear 2016; Svensson 2001; Svensson 2015).
Forty‐nine studies (49 records) were excluded based on their study design not meeting review inclusion criterion after inspection of full text (observation, prospective and retrospective reviews, review articles, systematic reviews, case reports, questionnaires, drug trials) (see Figure 2). Ten of these studies were excluded because they were systematic reviews (Abraha 2016; Alsawas 2017; Andrasi 2010; Bakoyiannis 2010; Elhelali 2016; Hanif 2018; Li 2018; Miao 2017; Pinto 2017; NCT02644681). We excluded six observational studies (NCT02735720; NCT01480206; NCT03093857; NCT00739557; NCT02111668; Schanzer 2017). We excluded seven review articles (Hongku 2016; Khullar 2017; Radak 2017; Rousseau 2014; Saeyeldin 2017; Safi 1996; Treasure 2016). We excluded one questionnaire (NCT03414866) and one case report (Robinson 2016). We excluded twenty‐three retrospective reviews (Benrashid 2016; Canaud 2017; Carrel 2005; Clare 2016; Coselli 2017; Di Bartolomeo 2017; Hirano 2020; Hirnle 2016; Hori 2017; Kawatou 2017; Liang 2017; Marrocco‐Trischitta 2017; Mougin 2017; Narita 2016; Okita 1996; Patterson 2016; Preventza 2017; Ramanan 2016; Sailer 2016; Settepani 2016; Shrestha 2017; Tokuda 2016; Yoshitake 2017) and one non‐randomised single arm trial (NCT01107366).
Seven studies (7 records) were excluded due to not meeting review inclusion criterion, which included evaluating the abdominal aortic aneurysms and mycotic aneurysms (Fernandes 2017; Karner 1988; NCT02101463; NCT03075748; Piazza 2016; Rodrigues‐Pinto 2016; Schroeder 2009). Five studies (5 records) were excluded due to no comparator (Moldovan 2017; NCT00413231; NCT00488696; NCT00597870; NCT04747626) and five studies (5 records) were excluded due to reporting on a comparator not meeting review inclusion criterion (ACTRN12620000123943; ChiCTR1800018803; Farber 2017; NCT01889498; Safi 1994). We excluded one study (1 record) due to mixed population (data included both aneurysm and dissections) (Tsukui 2002). We contacted the study authors on three different occasions to obtain additional information but we received no response (Tsukui 2002).
Risk of bias in included studies
It was not possible to assess the risks of bias due to the absence of eligible studies for inclusion.
Effects of interventions
Due to the lack of published and unpublished studies eligible for inclusion, it was not possible to examine the effectiveness and safety of HR versus OSR for TAAs.
Discussion
Summary of main results
Aneurysms of the aortic arch pose a formidable challenge. OSR patients are highly selected, based on patient comorbid status and surgeon’s preference and expertise. In spite of advancements in OSR, complications remain high with mortality rates ranging from 3.7% to 14% and neurological events as high as 10% (Estrera 2008; Hiraoka 2015; Harrington 2004; Tanaka 2014). With continuous improvement of technology, HR has evolved as a treatment of TAAs. Improved outcomes in high‐risk populations treated with HR have been reported, but postoperative complications are noteworthy (Benrashid 2016; Chakos 2018; Mestres 2013; Murphy 2009; Preventza 2014; Slisatkorn 2014; Vallabhajosyula 2013). However, current clinical information is based on prospective studies, case series and observational studies (Benrashid 2016; Chakos 2018; Estrera 2008; Hiraoka 2015; Harrington 2004; Mestres 2013; Murphy 2009; Preventza 2014; Slisatkorn 2014; Tanaka 2014; Vallabhajosyula 2013).
We found no published or unpublished RCTs or CCTs addressing the objective of this review. We cannot draw any conclusions about the safety and effectiveness of HR compared with OSR in people with TAAs.
Overall completeness and applicability of evidence
We found no published or unpublished RCTs or CCTs addressing the objective of this review. Studies reporting on outcomes of HR and OSR for treating TAAs are small observational studies or single‐armed prospective studies (Benrashid 2016; Kawatou 2017; Khullar 2017; Murphy 2009; Narita 2016; Preventza 2014; Yoshitake 2017). Randomised trials or controlled clinical trials or both are needed to effectively compare hybrid techniques and conventional OSR for treating TAAs. There are various challenges that make it difficult to conduct RCTs for surgical interventions and thus prevent surgeons from undertaking such trials. Some aspects of surgery present special difficulties for randomised trials, such as standardising surgical intervention, varying surgeon expertise and variability in patient anatomies. Standardising any surgical technique can be very difficult, especially in multicentre studies (Perry 2014); surgeons possess varying surgical skills with different training levels, resulting in inconsistent outcomes for a surgical technique among surgeons (Demange 2011). Additionally, the large variability in patients with arch aneurysms make standardising surgical techniques difficult; which is another significant limitation to performing surgical RCTs. Taking account of these constraints and challenges, it is possible to perform surgical RCTs (Perry 2014). Surgeon expertise can be accounted for in the study design by randomising surgeons of comparable expertise or providing information on how the existence of a learning curve is addressed during the trial design (Demange 2011). Additionally, documenting in detail each step of the surgical intervention during the trial design may help to reduce heterogeneous delivery of interventions in surgical RCTs. Whenever possible, RCTs should be conducted to provide the highest level of evidence, while acknowledging the challenges that need to be overcome in their design.
Quality of the evidence
It was not possible to assess the methodological quality or the certainty of the evidence in the absence of studies eligible for inclusion.
Potential biases in the review process
We found no studies relevant for inclusion in this review. By establishing inclusion and exclusion criteria from the outset and performing an extensive literature search, we reduced the risk of bias.
Agreements and disagreements with other studies or reviews
The use of HR is increasing. Current available evidence suggests that an increase in use seems to be pointing towards improved or equal clinical outcomes to OSR (Benedetto 2013; Chakos 2018; Hori 2017; Miao 2017; Murphy 2009; Preventza 2014; Preventza 2015; Tokuda 2016; Vallabhajosyula 2013). Improved outcomes in high‐risk populations treated with hybrid aortic repair have been reported, but global neurological events are still noteworthy, particularly stroke rate ranging from 4% to 16% (Bachet 2018; Chakos 2018; Hori 2017; Liakopoulos 2020; Mestres 2013; Preventza 2014; Tokuda 2016; Vallabhajosyula 2013). A meta‐analysis of seven retrospective cohort studies evaluating hybrid arch repair versus open surgical repair of aortic arch aneurysm (Miao 2017) reported no significant difference between HR and OSR in relation to neurological complications, late mortality and renal failure. Yet Miao 2017 reported that HR required high rates of reintervention in comparison to OSR. Likewise, in a retrospective review, Tokuda 2016 compared the outcomes of HR to OSR, and found no significant difference between HR and OSR, and advised to use HR only for high‐risk cases. A meta‐analysis comparing hybrid repair to OSR reported lower risk of spinal cord injury in the OSR than the HR group (Chakos 2018). Chakos 2018 recommends that more data are required in order to effectively compare long‐term survival data between the two procedures. Furthermore, in a meta‐analysis looking at four observational studies comparing OSR with HR in a total of 378 participants, HR did not improve surgical deaths (OR 0.67, P = 0.92) (Benedetto 2013). Benedetto 2013 also reported no significant increase in permanent neurologic deficit or late mortality with HR compared to OSR.
Despite the advent of newer technologies such as HR over OSR, there is still no clear consensus for the most appropriate intervention in the aortic arch. There are potential benefits and harms to the use of each, and the optimum may depend on the comorbid status of the patient, the nature of the pathology and the experience of the operator and centre. In the absence of RCTs and CCTs, it is not possible to draw any conclusions to support one treatment over the other.
Authors' conclusions
Implications for practice.
No randomised controlled trials or controlled clinical trials were available to inform decisions on the benefits and harms of HR compared to OSR for treating TAAs. Clinicians and patients should continue to take decisions on the optimal mode based on patient co‐morbid status and on the aneurysms size, expansion rate and location.
Implications for research.
There is a need for high‐quality randomised trials evaluating the effectiveness of hybrid repair versus open surgical repair. Such trials will need to consider the identification, assessment and control of factors that may affect findings, such as surgeon expertise, outcome measures, treatment setting (elective versus emergency) and hospital facilities (Demange 2011).
History
Protocol first published: Issue 1, 2018
Notes
Parts of the Methods section of this review are based on a standard template established by the Cochrane Vascular Group and based on a template developed by our group.
Acknowledgements
We would like to acknowledge Cochrane Vascular for the guidance and support they provided during the preparation of this review. We would like to thank the Health Research Board (HRB) Ireland for funding the completion of this review and for their support. We would also like to thank the National University of Ireland, Galway for their support.
The review authors, and the Cochrane Vascular editorial base, wish to thank the following peer reviewers for their comments: Thomas Martens, MD, Department of Cardiac Surgery, University Hospital Ghent, Belgium; Mr RTA Chalmers, Director of Scottish National Service for the treatment of thoraco‐abdominal aortic aneurysms, Royal Infirmary of Edinburgh, UK
Appendices
Appendix 1. Glossary of terms
A
Antegrade cerebral perfusion (ACP) is a cardiopulmonary bypass technique that uses cannulation procedures to supply blood to only the brain during aortic arch surgical repair.
Aortic arch debranching involves rerouting (debranching) of the aortic arch vessels from the aortic arch using a bypass graft and then an endograft stent is placed to treat the aortic aneurysm. This procedure does not require cardiopulmonary bypass.
C
Cardiopulmonary bypass often referred to as the heart‐lung machine, is a technique that temporally takes over the function of the heart and lungs during aortic arch surgical repair. It maintains blood flow circulation and oxygen content within the body.
Comorbidity is defined as a medical condition that co‐occurs with another medical condition.
E
Endovascular repair is a less invasive technique to open surgical repair and hybrid repair. It involves a small incision in the groin. The catheter is used to guide and deliver the stent graft into the aortic arch aneurysm. The device is deployed in to the aorta to seal the aortic aneurysm from the blood flow.
H
Hemi‐arch replacement involves repair or replacement of the proximal arch beyond the level of the brachiocephalic artery although it does not involve the arch vessels.
Hypothermic circulatory arrest temporarily stops blood flow under extremely cold body temperature to safely allow repair of the aorta for up to 40 minutes.
R
Retrograde cerebral perfusion (RCP) is a neuroprotective technique carried out through the superior vena cava cannula. It decreases the risk of brain injury by maintaining blood flow to the brain, providing back washing of toxic metabolites and possible blood clots and or air bubbles and reduces blood cell microaggregation.
T
Thoracic Aortic Arch Aneurysm (TAA) is a swelling located in the upper portion of the aorta.
References used for glossary:
Ergin 1994; Fraser 2008; Griepp 2013; Hongku 2016; Poon 2016.
Appendix 2. Database searches
| Source | Search strategy | Hits retrieved |
| Vascular Register | #1 ThAARepair AND INREGISTER #2 thoracic aortic aneurysm AND INREGISTER #3 #1 OR #2 |
13.2.18 ‐ 22 11.2.19 ‐ 1 18.2.20 ‐ 4 22.3.21 ‐ 2 |
| CENTRAL | #1 MESH DESCRIPTOR Aortic Aneurysm 105 #2 MESH DESCRIPTOR Aortic Aneurysm, Thoracic EXPLODE ALL TREES 59 #3 MESH DESCRIPTOR Aorta, Thoracic EXPLODE ALL TREES WITH QUALIFIERS SU 42 #4 (aortic arch):TI,AB,KY 229 #5 TAA:TI,AB,KY 172 #6 #1 OR #2 OR #3 OR #4 OR #5 565 #7 hybrid:TI,AB,KY 1839 #8 debranch*:TI,AB,KY 4 #9 supraaortic:TI,AB,KY 10 #10 rerouting:TI,AB,KY 7 #11 MESH DESCRIPTOR Endovascular Procedures EXPLODE ALL TREES 7282 #12 MESH DESCRIPTOR Stents EXPLODE ALL TREES 3650 #13 MESH DESCRIPTOR Blood Vessel Prosthesis EXPLODE ALL TREES 429 #14 MESH DESCRIPTOR Blood Vessel Prosthesis Implantation EXPLODE ALL TREES 431 #15 endovasc*:TI,AB,KY 2174 #16 endostent*:TI,AB,KY 1 #17 endoluminal:TI,AB,KY 151 #18 endoprosthe*:TI,AB,KY 281 #19 (graft or endograft*):TI,AB,KY 16587 #20 percutaneous*:TI,AB,KY 12621 #21 stent*:TI,AB,KY 9578 #22 TEVAR:TI,AB,KY 43 #23 branched:TI,AB,KY 802 #24 fenestrated:TI,AB,KY 58 #25 (elephant trunk):TI,AB,KY 5 #26 (landing zone):TI,AB,KY 18 #27 #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 40044 #28 #6 AND #27 111 |
13.2.18 ‐ 111 11.2.19 ‐ 29 18.2.20 ‐ 75 22.3.21 ‐ 28 |
| Clinicaltrials.gov | thoracic aortic aneurysm | 13.2.18 ‐ 99 11.2.19 ‐ 15 18.2.20 ‐ 18 22.3.21 ‐ 24 |
| ICTRP Search Portal | thoracic aortic aneurysm AND stent OR stents OR TEVAR OR graft OR grafts OR Blood Vessel Prosthesis |
13.2.18 ‐ 130 11.2.19 ‐ 11 18.2.20 ‐ 3 22.3.21 ‐ 1 |
| MEDLINE | 1 Aortic Aneurysm/ 20264 2 exp Aortic Aneurysm, Thoracic/ 10598 3 Aorta, Thoracic/su [Surgery] 8001 4 aortic arch.ti,ab. 14628 5 TAA.ti,ab. 4513 6 or/1‐5 49561 7 hybrid.ti,ab. 130652 8 debranch*.ti,ab. 1387 9 supraaortic.ti,ab. 372 10 rerouting.ti,ab. 827 11 exp Endovascular Procedures/ 104033 12 exp STENTS/ 67082 13 exp Blood Vessel Prosthesis/ 27086 14 exp Blood Vessel Prosthesis Implantation/ 20234 15 endovasc*.ti,ab. 39997 16 endostent*.ti,ab. 33 17 endoluminal.ti,ab. 3991 18 endoprosthe*.ti,ab. 6517 19 (graft or endograft*).ti,ab. 197594 20 percutaneous*.ti,ab. 125821 21 stent*.ti,ab. 85999 22 TEVAR.ti,ab. 1179 23 branched.ti,ab. 30902 24 fenestrated.ti,ab. 3403 25 elephant trunk.ti,ab. 726 26 landing zone.ti,ab. 572 27 or/7‐26 630362 28 6 and 27 15697 29 randomized controlled trial.pt. 453387 30 controlled clinical trial.pt. 92150 31 randomized.ab. 402555 32 placebo.ab. 186351 33 drug therapy.fs. 1991172 34 randomly.ab. 284804 35 trial.ab. 417887 36 groups.ab. 1762137 37 or/29‐36 4136794 38 exp animals/ not humans.sh. 4424200 39 37 not 38 3573765 40 28 and 39 1327 41 (2018* or 2017*).ed. 1018622 42 40 and 41 131 |
13.2.18 ‐ 131 11.2.19 – 92 18.2.20 ‐ 145 22.3.21 ‐ 202 |
| EMBASE | 1 Aortic Aneurysm/ 981 2 exp Aortic Aneurysm, Thoracic/ 5994 3 Aorta, Thoracic/su [Surgery] 783 4 aortic arch.ti,ab. 14475 5 TAA.ti,ab. 5091 6 or/1‐5 25382 7 hybrid.ti,ab. 108960 8 debranch*.ti,ab. 1421 9 supraaortic.ti,ab. 433 10 rerouting.ti,ab. 853 11 exp Endovascular Procedures/ 29107 12 exp STENTS/ 142449 13 exp Blood Vessel Prosthesis/ 6107 14 exp Blood Vessel Prosthesis Implantation/ 78407 15 endovasc*.ti,ab. 56572 16 endostent*.ti,ab. 46 17 endoluminal.ti,ab. 4970 18 endoprosthe*.ti,ab. 4774 19 (graft or endograft*).ti,ab. 219768 20 percutaneous*.ti,ab. 153902 21 stent*.ti,ab. 133734 22 TEVAR.ti,ab. 1839 23 branched.ti,ab. 26170 24 fenestrated.ti,ab. 3187 25 elephant trunk.ti,ab. 817 26 landing zone.ti,ab. 943 27 or/7‐26 694931 28 randomized controlled trial/ 438541 29 controlled clinical trial/ 408648 30 random$.ti,ab. 1127633 31 randomization/ 68530 32 intermethod comparison/ 219639 33 placebo.ti,ab. 215167 34 (compare or compared or comparison).ti. 325074 35 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 1555721 36 (open adj label).ti,ab. 60358 37 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 153580 38 double blind procedure/ 119348 39 parallel group$1.ti,ab. 18874 40 (crossover or cross over).ti,ab. 69979 41 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 240522 42 (assigned or allocated).ti,ab. 281106 43 (controlled adj7 (study or design or trial)).ti,ab. 252378 44 (volunteer or volunteers).ti,ab. 167706 45 trial.ti. 206079 46 or/28‐45 3359024 47 6 and 27 and 46 804 48 (2018* or 2017*).dc. 1995415 49 47 and 48 137 |
13.2.18 ‐ 137 11.2.19 ‐ 177 18.2.20 ‐ 231 22.3.21 ‐ 221 |
| CINAHL | S35 S26 AND S33 AND S34 13 S34 EM 2017 OR EM 2018 258,997 S33 S27 OR S28 OR S29 OR S30 OR S31 OR S32 971,700 S32 TX randomly 42,638 S31 TX "treatment as usual" 741 S30 TX "double‐blind*" 770,445 S29 TX "single‐blind*" 8,798 S28 TX trial 242,668 S27 MH "Clinical Trials" 92,660 S26 S6 AND S25 992 S25 S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 51,817 S24 TX landing zone 70 S23 TX elephant trunk 54 S22 TX fenestrated 174 S21 TX branched 540 S20 TX TEVAR 113 S19 TX stent* 13,030 S18 TX percutaneous* 19,300 S17 TX (graft or endograft*) 17,854 S16 TX endoprosthe* 410 S15 TX endoluminal 323 S14 TX endostent* 2 S13 TX endovasc* 4,629 S12 (MH "Blood Vessel Prosthesis") 1,005 S11 (MH "Stents+") 9,881 S10 TX rerouting 42 S9 TX supraaortic 5 S8 TX debranch* 28 S7 TX hybrid 3,623 S6 (S1 OR S2 OR S3 OR S4 OR S5) 3,426 S5 TX TAA 234 S4 TX aortic arch 838 S3 (MH "Aorta, Thoracic") 1,241 S2 (MH "Aortic Aneurysm, Thoracic") 780 S1 (MH "Aortic Aneurysm" 1,002 |
13.2.18 ‐ 13 11.2.19 ‐ 19 18.2.20 ‐ 22 22.3.21 ‐ 53 |
| AMED | 1 exp Aortic aneurysm/ 2 aortic arch.ti,ab. 3 TAA.ti,ab. 4 hybrid.ti,ab. 5 debranch*.ti,ab. 6 supraaortic.ti,ab. 7 rerouting.ti,ab. 8 exp Stents/ 9 endovasc*.ti,ab. 10 endostent*.ti,ab. 11 endoluminal.ti,ab. 12 endoprosthe*.ti,ab. 13 (graft or endograft*).ti,ab. 14 percutaneous*.ti,ab. 15 stent*.ti,ab. 16 TEVAR.ti,ab. 17 branched.ti,ab. 18 fenestrated.ti,ab. 19 elephant trunk.ti,ab. 20 landing zone.ti,ab. 21 or/1‐3 22 or/4‐20 23 21 and 22 24 exp CLINICAL TRIALS/ 25 RANDOM ALLOCATION/ 26 DOUBLE BLIND METHOD/ 27 Clinical trial.pt. 28 (clinic* adj trial*).tw. 29 ((singl* or doubl* or trebl* or tripl*) adj (blind* or mask*)).tw. 30 PLACEBOS/ 31 placebo*.tw. 32 random*.tw. 33 PROSPECTIVE STUDIES/ 34 or/24‐33 35 23 and 34 |
13.2.18 ‐ 3 11.2.19 ‐ 0 18.2.20 ‐ 0 22.3.21 ‐ 0 |
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abraha 2016 | Study design does not meet review inclusion criteria ‐ systematic review |
| Acher 1993 | Study design does not meet review inclusion criteria ‐ this is a retrospective review of cases. The intervention, comparator and outcomes do not match the inclusion criteria. The study does not look at surgical intervention, rather it compares only cerebral spinal fluid drainage and naloxone in the reduction of paraplegia during aneurysm repair |
| ACTRN12620000123943 | Excluded due to reporting on a comparator not meeting review inclusion criteria ‐ comparison of two frozen elephant trunk prostheses in the treatment of thoracic aortic disease |
| Alsawas 2017 | Study design does not meet review inclusion criteria ‐ this is a systematic review |
| Andrasi 2010 | Study design does not meet review inclusion criteria ‐ this is a systematic review |
| Bakoyiannis 2010 | Study design does not meet review inclusion criteria ‐ this is a systematic review |
| Benrashid 2016 | Study design does not meet review inclusion criteria ‐ retrospective study |
| Bockler 2016 | Study design does not meet review inclusion criteria ‐ this study was excluded as it only examines TEVAR and does not compare to any other intervention type |
| Canaud 2017 | Study design does not meet review inclusion criteria ‐ this study is a retrospective review which examines hybrid repair and revascularisation of the LSA |
| Carrel 2005 | Study design does not meet review inclusion criteria ‐ this is a retrospective review and was excluded based on its study design as it was not a CCT or RCT |
| ChiCTR1800018803 | Excluded due to reporting on a comparator not meeting review inclusion criteria ‐ same intervention in both arms, total arch replacement and stented elephant trunk implantation |
| ChiCTR‐IPR‐15006372 | Different Intervention does not meet review inclusion criteria ‐ the study examined the effect of dexmedtomidine on early stage of renal function in the chest or abdominal aortic aneurysm (aortic dissection) patients undergoing endovascular repair operation |
| Clare 2016 | Study design does not meet review inclusion criteria ‐ this is a retrospective review and was excluded based on its study design as it was not a CCT or RCT |
| Coselli 2017 | Study design does not meet review inclusion criteria ‐ retrospective review |
| Di Bartolomeo 2017 | Study design does not meet review inclusion criteria ‐ retrospective review |
| Elhelali 2016 | Study design does not meet review inclusion criteria ‐ systematic review |
| Fairman 2008 | Different intervention does not meet review inclusion criteria‐ this study was excluded as it compared TEVAR repair to retrospective OSR data |
| Farber 2017 | Did not meet inclusion for review comparator ‐ this study was excluded as it compared TEVAR repair to prospective and retrospective OSR data |
| Fernandes 2017 | Did not meet inclusion for review indication ‐ this study examined the circuit used for hybrid repair. In addition, it did not compare HR results with OSR |
| Fernandez 2016 | Intervention does not meet review inclusion criteria ‐ this prospective study was excluded as it only used thoracic endovascular repair |
| Hanif 2018 | Study design does not meet review inclusion criteria ‐ systematic review |
| Hirano 2020 | Study design does not meet review inclusion criteria ‐ retrospective review |
| Hirnle 2016 | Study design does not meet review inclusion criteria ‐ retrospective review |
| Hongku 2016 | Study design does not meet review inclusion criteria ‐ review article |
| Hori 2017 | Study design does not meet review inclusion criteria ‐ retrospective review |
| Huang 2011 | Intervention does not meet review inclusion criteria ‐ this study examined TEVAR for aortic repair. It examined the impact of stent graft positioning between rapid artificial cardiac pacing induced hypotension and sodium nitroprusside induced hypotension during thoracic endovascular aortic repair |
| JPRN‐UMIN000005430 | Intervention does not meet review inclusion criteria ‐ this review only reported on TEVAR repair |
| JPRN‐UMIN000007213 | Intervention does not meet review inclusion criteria ‐ compared TEVAR devices only |
| JPRN‐UMIN000024202 | Intervention does not meet review inclusion criteria ‐ this review reported on TEVAR repair and the effects of tranexamic acid suppression fibrinolysis for endoleak before endovascular repair for aortic aneurysm |
| Karashima 2016 | Intervention compared TEVAR to total arch repair ‐ does not meet review inclusion criteria |
| Karner 1988 | Indication does not meet review inclusion criteria ‐ reported on abdominal aortic aneurysms |
| Kaushik 2017 | Intervention does not meet review inclusion criteria ‐ this review reported on TEVAR repair |
| Kawatou 2017 | Study design does not meet review inclusion criteria ‐ retrospective review |
| Khullar 2017 | Study design does not meet review inclusion criteria ‐ retrospective review |
| Li 2018 | Study design does not meet review inclusion criteria ‐ systematic review |
| Liang 2017 | Study design does not meet review inclusion criteria ‐ retrospective review |
| Marrocco‐Trischitta 2017 | Study design does not meet review inclusion criteria ‐ retrospective review |
| Matsumura 2014 | Intervention does not meet review inclusion criteria ‐ compared TEVAR to OSR |
| Miao 2017 | Study design does not meet review inclusion criteria ‐ systematic review and meta‐analysis |
| Moldovan 2017 | No comparator does not meet review inclusion criteria ‐ examines hybrid repair in aortic aneurysms but with no comparator |
| Mougin 2017 | Study design does not meet review inclusion criteria ‐ retrospective review |
| Narita 2016 | Study design does not meet review inclusion criteria ‐ retrospective review |
| NCT00110201 | Intervention does not meet review inclusion criteria ‐ this study was excluded as it looked at the effects of Nesiritide a drug on thoracic aortic repair |
| NCT00409344 | Study design does not meet review inclusion criteria ‐ drug trial ‐ compared dexmedetomidine to normal saline during thoracic aneurysm repair |
| NCT00413231 | No comparator, does not meet review inclusion criteria ‐ this study does not compare TEVAR to either HR or OSR |
| NCT00488696 | No comparator, does not meet review inclusion criteria ‐ this study does not compare TEVAR to either HR or OSR |
| NCT00508118 | Intervention does not meet review inclusion criteria ‐ this study was excluded as it compared two drugs during aortic surgery. The study was conducted to determine whether an infusion of nicardipine is able to reduce the time taken to achieve electrocerebral silence during cardiopulmonary bypass for aortic surgery |
| NCT00583817 | Intervention does not meet review inclusion criteria ‐ this study does not compare TEVAR to either HR or OSR |
| NCT00590759 | Intervention does not meet review inclusion criteria ‐ this study only examined the effects of TEVAR in aortic repair |
| NCT00597870 | No comparator, does not meet review inclusion criteria ‐ this study was excluded as it only examines TEVAR devices |
| NCT00691756 | Study intervention, does not meet review inclusion criteria ‐ this study does not look at surgical outcomes but compares effectiveness of two forms of renal artery perfusion for the prevention of postoperative renal dysfunction |
| NCT00739557 | Study design does not meet review inclusion criteria ‐ observational study |
| NCT00757003 | Intervention does not meet review inclusion criteria ‐ this study does not compare TEVAR to either HR or OSR |
| NCT01033214 | Intervention does not meet review inclusion criteria ‐ this study was excluded as it was a single arm study looking at endovascular repair |
| NCT01107366 | Study design does not meet review inclusion criteria – this non‐RCT study examined a new HR device; they do not compare it to OSR. This study was withdrawn (the study never started since the Medical Ethics Committee did not approve it) |
| NCT01181947 | Intervention does not meet review inclusion criteria ‐ this study does not compare TEVAR to either HR or OSR |
| NCT01480206 | Study design does not meet review inclusion criteria ‐ this is an observational study looking at 3D overlay for stent placement in HR |
| NCT01524211 | Intervention does not meet review inclusion criteria ‐ this review reported on TEVAR repair |
| NCT01756911 | Different intervention and patient population does not meet review inclusion criteria ‐ this study was excluded as it only examines endovascular repair of thoracoabdominal aortic aneurysms and does not compare this surgical technique with other surgical interventions |
| NCT01772537 | Intervention and study population does not meet review inclusion criteria. This review examined the effects of anaesthesia (propofol vs isoflurane) in TEVAR repair in thoracic aneurysm, cardiopulmonary bypass, thoracoabdominal repair and abdominal aortic aneurysm repair) |
| NCT01839695 | Intervention does not meet review inclusion criteria ‐ this study does not compare TEVAR to either HR or OSR |
| NCT01889498 | Comparator did not meet study inclusion criteria ‐ looking at drug to reduce paralysis during aneurysm repair. The authors do not classify the type of aneurysm being treated and what intervention is being used |
| NCT01920594 | Intervention does not meet review inclusion criteria ‐ this study was excluded as it compared GSK1278863 to a placebo during thoracic aortic aneurysm repair. This study did not assess the effects of HR vs OSR in thoracic aortic aneurysm repair |
| NCT02010892 | Intervention does not meet review inclusion criteria ‐ this prospective cohort study was excluded as it compared TEVAR to OSR |
| NCT02089607 | Intervention does not meet review inclusion criteria ‐ this study was excluded as it compared TEVAR devices only |
| NCT02101463 | Indication does not meet review inclusion criteria ‐ abdominal aortic aneurysm were treated using a fenestrated branched stent‐grafts |
| NCT02111668 | Study design does not meet review inclusion criteria ‐ observational study |
| NCT02253082 | Study design does not meet review inclusion criteria ‐ drug trial. Furthermore it reports on the wrong patient population ‐ looking at dissection not aneurysms |
| NCT02291718 | Intervention does not meet review inclusion criteria ‐ excluded as it compared contrast agents and imaging techniques and not HR to OSR |
| NCT02294435 | Intervention does not meet review inclusion criteria ‐ this study only examined the effects of TEVAR in aortic repair |
| NCT02323581 | Intervention does not meet review inclusion criteria ‐ this study only examined the effects of TEVAR in aortic aneurysms |
| NCT02365454 | Intervention does not meet review inclusion criteria ‐ this study only examined the effects of TEVAR in aortic aneurysms |
| NCT02365467 | Intervention does not meet review inclusion criteria ‐ this study only examined the effects of TEVAR in aortic aneurysms |
| NCT02471781 | intervention does not meet review inclusion criteria ‐ this study only examined the effects of TEVAR in aortic aneurysms |
| NCT02554032 | Intervention does not meet review inclusion criteria ‐ this study examines cannulation during cerebral protection and does not report on surgical interventions |
| NCT02625324 | Intervention does not meet review inclusion criteria ‐ this study only examined the effects of TEVAR in aortic aneurysms |
| NCT02644681 | Study design does not meet review inclusion criteria ‐ meta‐analysis |
| NCT02652949 | Intervention does not meet review inclusion criteria ‐ this study only examined the effects of TEVAR in aortic aneurysms |
| NCT02678728 | Intervention does not meet review inclusion criteria ‐ this study was excluded as it compared dexmedetomidine to normal saline during thoracic aortic repair |
| NCT02735720 | Study design does not meet review inclusion criteria ‐ observational study |
| NCT02777528 | Intervention does not meet review inclusion criteria does not meet review inclusion criteria ‐ this study only examined the effects of TEVAR in aortic repair |
| NCT02777593 | Intervention does not meet review inclusion criteria ‐ this study only examined the effects of TEVAR in aortic repair |
| NCT02818972 | Intervention does not meet review inclusion criteria ‐ this study was excluded as it only examines TEVAR and does not compare to any other intervention type |
| NCT03024554 | Intervention does not meet review inclusion criteria ‐ this study was excluded as it only examines TEVAR repair and does not compare to any other intervention type |
| NCT03075748 | Indication does not meet review inclusion criteria ‐ wrong section of the aorta is being treated and is not looking at zones 0 to 4 |
| NCT03093857 | Study design does not meet review inclusion criteria ‐ observational study of diagnostic tools |
| NCT03111459 | Intervention does not meet review inclusion criteria ‐ this study only examined the effects of TEVAR in aortic repair |
| NCT03207568 | Intervention does not meet review inclusion criteria ‐ this study only examined the effects of TEVAR in aortic aneurysms |
| NCT03208504 | Intervention does not meet review inclusion criteria ‐ this study was excluded as it only looked at TEVAR for aneurysm repair |
| NCT03214601 | Intervention does not meet review inclusion criteria ‐ this study was excluded as it only examines TEVAR and does not compare to any other intervention type |
| NCT03414866 | Study design does not meet review inclusion criteria ‐ questionnaire |
| NCT04747626 | Excluded due to no comparator |
| Novikova 2011 | Intervention does not meet review inclusion criteria ‐ this study only reports on gut protection. In addition, wrong outcomes were being assessed |
| Okita 1996 | Study design does not meet review inclusion criteria ‐ retrospective review. No data available from review author |
| Patel 2016 | Intervention does not meet review inclusion criteria ‐ this study was excluded as it compared TEVAR devices only |
| Patterson 2016 | Study design does not meet review inclusion criteria ‐ retrospective review of prospectively maintained database |
| Piazza 2016 | Indication does not meet review inclusion criteria ‐ this study looked at the effect of endovascular repair in infra‐renal AAAs |
| Pinto 2017 | Study design does not meet review inclusion criteria ‐ systematic review |
| Preventza 2017 | Study design does not meet review inclusion criteria ‐ retrospective review |
| Radak 2017 | Study design does not meet review inclusion criteria ‐ review article |
| Rahe‐Meyer 2013 | Study design does not meet review inclusion criteria ‐ drug trial and wrong study outcomes being assessed |
| Ramanan 2016 | Study design does not meet review inclusion criteria ‐ this study was excluded as it is a retrospective review and looked at TEVAR devices only |
| Robinson 2016 | Study design does not meet review inclusion criteria ‐ case report |
| Rodrigues‐Pinto 2016 | Indication does not meet review inclusion criteria ‐ reports on mycotic aneurysms which are excluded from this review |
| Rousseau 2014 | Study design does not meet review inclusion criteria ‐ review paper |
| Saeyeldin 2017 | Study design does not meet review inclusion criteria ‐ review paper |
| Safi 1994 | Different comparator, does not meet review inclusion criteria ‐ retrospective review looking at OSR in thoracoabdominal aneurysms |
| Safi 1996 | Study design does not meet review inclusion criteria ‐ review paper |
| Sailer 2016 | Study design does not meet review inclusion criteria ‐ retrospective review |
| Schanzer 2017 | Study design does not meet review inclusion criteria ‐ this observational cohort study only examined the effects of TEVAR in aortic repair in abdominal and thoracoabdominal aneurysms |
| Schroeder 2009 | Indication does not meet review inclusion criteria ‐ this study examined stent grafts in AAAs |
| Settepani 2016 | Study design does not meet review inclusion criteria ‐ retrospective review |
| Shrestha 2017 | Study design does not meet review inclusion criteria ‐ retrospective review |
| Spear 2016 | Intervention does not meet review inclusion criteria ‐ this study only examined the effects of TEVAR in aortic repair |
| Svensson 2001 | Intervention does not meet review inclusion criteria ‐ the main focus of this study is brain protection and methods used and they do not report on the surgical interventions |
| Svensson 2015 | Intervention does not meet review inclusion criteria ‐ this randomised trial examined brain protection during total aortic arch replacement. The authors do not report on the surgical interventions |
| Tokuda 2016 | Study design does not meet review inclusion criteria ‐ retrospective review |
| Treasure 2016 | Study design does not meet review inclusion criteria ‐ commentary review paper |
| Tsukui 2002 | Mixed patient population ‐ combined patients with thoracic aneurysms and dissections. Authors were contacted to obtain additional information and we received no response |
| Yoshitake 2017 | Study design does not meet review inclusion criteria ‐ retrospective review |
AAA: abdominal aortic aneurysm CCT: controlled clinical trial HR: hybrid repair LSA: left subclavian artery OSR: open surgical repair RCT: randomised controlled trial TEVAR: thoracic endovascular aortic repair
Differences between protocol and review
We updated the subgroups: associated aortic valve repair was changed to previous aortic valve repair and subgroup for intensive care unit stay was changed to ICU length of stay (HR versus OSR), as all cases whether they are treated with HR or OSR, will require ICU stay postoperatively.
Contributions of authors
AE: designing and drafting protocol and full review, acquiring trial reports, trial selection, review drafting and future review updates. NH: designing and drafting protocol and full review, acquiring trial reports, study selection, review drafting and future review updates. DD: designing and drafting protocol and full review, review methodology, review drafting and future review updates. SS: designing and drafting protocol and full review, review drafting and future review updates. EPK: Acquiring trial reports, study selection, review drafting and future review updates. LM: designing and drafting protocol and full review, data interpretation, review drafting and future review updates. DV: designing and drafting protocol and full review, data interpretation, review drafting and future review updates. FJ: designing and drafting protocol and full review, review drafting and future review updates.
Sources of support
Internal sources
-
HRB Cochrane Fellowship Scheme, Ireland
The Cochrane Fellowships aims to build capacity on the island of Ireland in conducting systematic reviews for inclusion in the Cochrane Library. The scheme is funded by the Health Research Board Ireland
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK
The Cochrane Vascular Group editorial base is supported by the Chief Scientist Office.
Declarations of interest
AE: Has received funding from Health Research Board (Ireland) under the HRB Cochrane Ireland Fellowship Scheme to undertake a Cochrane Systematic Review (Grant number CTF‐2016‐1863). NH: Has received payment for consultation on Regulatory Documents (Versono Ltd and Integer) and for medical device design at Boston Scientific (Enterprise Ireland Bioinnovate Fellow). Her institution has received payment for provision of training on endovascular aortic repair from Gore Medical. She is investigator in the INSIGHT Post Market Surveillance Trial of the Incraft AAA device (Cordis/Cardinal Health). Her Institution has received payment for an Aortic Fellowship grant (Jotec/Cryolife), and Research fellowship grants (Gore Medical and Medtronic). She declares no competing interests, relationships, conditions or circumstances, which will conflict with this review. DD: None known. SS: Has received payment for training physicians on endovascular aortic repair from Gore Medical and is the Principal Investigator in the INSIGHT post Market Surveillance Trial of the Incraft abdominal aortic endograft (Cordis/Cardinal health). He has declared he has no conflict of interest, which will affect this review. EPK: None known. LM: None known. DV: None known. FJ: Institution received funding from the Health Research Board (Ireland) for a Cochrane Training Fellowship to enable me to undertake a Cochrane Systematic Review over 24 months. This training grant provides me with funding to attend Cochrane Training Programmes/conferences over the two year period of my fellowship.
New
References
References to studies excluded from this review
Abraha 2016 {published data only}
- Abraha I, Romagnoli C, Montedori A, Cirocchi R. Thoracic stent graft versus surgery for thoracic aneurysm. Cochrane Database of Systematic Reviews 2016, Issue 6. Art. No: CD006796. [DOI: 10.1002/14651858.CD006796.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Acher 1993 {published and unpublished data}
- Acher CW, Wynn MM, Hoch JR, Popic PM, Turnipseed WD. Combined use of spinal fluid drainage and naloxone reduces the risk of neurologic deficit in thoracoabdominal aneurysm repair. Journal of Vascular Surgery 1993;17(6):1125. [DOI] [PubMed] [Google Scholar]
ACTRN12620000123943 {published and unpublished data}
- ACTRN12620000123943. What frozen elephant trunk prosthesis is safe and effective in the treatment thoracic aortic disease. anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12620000123943 (first received 28 November 2019).
Alsawas 2017 {published and unpublished data}
- Alsawas M, Zaiem F, Larrea-Mantilla L, Almasri J, Erwin PJ, Upchurch GR, et al. Effectiveness of surgical interventions for thoracic aortic aneurysms: a systematic review and meta-analysis. Journal of Vascular Surgery 2017;66(4):1258-68.e8. [DOI] [PubMed] [Google Scholar]
Andrasi 2010 {published and unpublished data}
- Andrasi TB, Grossmann M, Zenker D, Danner BC, Schondube FA. Supra-aortic interventions for endovascular exclusion of the entire aortic arch. Journal of Vascular Surgery 2010;66(1):281-97. [DOI] [PubMed] [Google Scholar]
Bakoyiannis 2010 {published and unpublished data}
- Bakoyiannis CN, Economopoulos KP, Georgopoulos S, Klonaris C, Shialarou M, Kafeza M, et al. Fenestrated and branched endografts for the treatment of thoracoabdominal aortic aneurysms: a systematic review. Journal of Endovascular Therapy 2010;17(2):201-9. [DOI] [PubMed] [Google Scholar]
Benrashid 2016 {published and unpublished data}
- Benrashid E, Wang H, Andersen ND, Keenan JE, McCann RL, Hughes GC. Complementary roles of open and hybrid approaches to thoracoabdominal aortic aneurysm repair. Journal of Vascular Surgery 2016;64(5):1228-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bockler 2016 {published and unpublished data}
- Bockler D, Brunkwall J, Taylor PR, Mangialardi N, Husing J, Larzon T, et al. Thoracic endovascular aortic repair of aortic arch pathologies with the conformable thoracic aortic graft: early and 2 year results from a European multicentre registry. European Journal of Vascular and Endovascular Surgery 2016;51(6):791-800. [DOI] [PubMed] [Google Scholar]
Canaud 2017 {published data only}
- Canaud L, Ziza, V, Ozdemir BA, Berthet JP, Marty-Ane CH, Alric P. Outcomes of left subclavian artery transposition for hybrid aortic arch debranching. Annals of Vascular Surgery 2017;40:94-7. [DOI] [PubMed] [Google Scholar]
Carrel 2005 {published data only}
- Carrel TP, Do DD, Triller J, Schmidli J. A less invasive approach to completely repair the aortic arch. Annals of Thoracic Surgery 2005;80(4):1475-8. [DOI] [PubMed] [Google Scholar]
ChiCTR1800018803 {published and unpublished data}
- ChiCTR1800018803. Treatment of complex aortic arch pathologies with a new surgical strategy. chictr.org.cn/showproj.aspx?proj=31803 (first received 10 October 2018).
ChiCTR‐IPR‐15006372 {unpublished data only}
- ChiCTR-IPR-15006372. Renal function after endovascular repair operation in aortic aneurysm (aortic dissection) patients using dexmedetomidine. apps.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR-IPR-15006372 (first received 4 May 2015).
Clare 2016 {published data only}
- Clare R, Jorgensen J, Brar SS. Open versus endovascular or hybrid thoracic aortic aneurysm repair. Current Atherosclerosis Reports 2016;18(10):60. [DOI] [PubMed] [Google Scholar]
Coselli 2017 {published data only}
- Coselli JS, Amarasekara HS, Green SY, Price MD, Preventza O, De la Cruz KI, et al. Open repair of thoracoabdominal aortic aneurysm in patients 50 years old and younger. Annals of Thoracic Surgery 2017;103(6):1849-57. [DOI] [PubMed] [Google Scholar]
Di Bartolomeo 2017 {published data only}
- Di Bartolomeo R, Berretta P, Pantaleo A, Murana G, Cefarelli M, Alfonsi J, et al. Long-term outcomes of open arch repair after a prior aortic operation: our experience in 154 patients. Annals of Thoracic Surgery 2017;103(5):1406-12. [DOI] [PubMed] [Google Scholar]
Elhelali 2016 {published data only}
- Elhelali A, Hynes N, Kavanagh EP, Jordan F, Sultan S. Open surgical repair versus hybrid and endovascular repair for aortic arch pathologies: systematic review. Vascular 2016;185(24):S88. [Google Scholar]
Fairman 2008 {published data only}
- Fairman RM, Criado F, Farber M, Kwolek C, Mehta M, White R, et al. Pivotal results of the Medtronic vascular talent thoracic stent graft system: the VALOR trial. Journal of Vascular Surgery 2008;48(3):546-54. [DOI] [PubMed] [Google Scholar]
- Matsumoto AH, Angle JF, Secic M, Carlson GA, Fisher L, Fairman RM. Secondary procedures following thoracic aortic stent grafting in the first 3 years of the VALOR test and VALOR II trials. Journal of Vascular and Interventional Radiology 2014;25(5):685-92. [DOI] [PubMed] [Google Scholar]
- NCT00604799. VALOR: The talent thoracic stent graft system clinical study. clinicaltrials.gov/show/NCT00604799 (first received 30 January 2008).
Farber 2017 {published data only}
- Farber MA, Lee WA, Szeto WY, Panneton JM, Kwolek CJ. Initial and midterm results of the Bolton Relay Thoracic Aortic Endovascular Pivotal Trial. Journal of Vascular Surgery 2017;65(6):1556-66. [DOI] [PubMed] [Google Scholar]
Fernandes 2017 {published data only}
- Fernandes P, Walsh G, Walsh S, O'Neil M, Gelinas J, Chu MW. Whole body perfusion for hybrid aortic arch repair: evolution of selective regional perfusion with a modified extracorporeal circuit. Perfusion 2017;32(3):230-7. [DOI] [PubMed] [Google Scholar]
Fernandez 2016 {published data only}
- Fernandez CC, Sobel JD, Gasper WJ, Vartanian SM, Reilly LM, Chuter TA, et al. Standard off-the-shelf versus custom-made multibranched thoracoabdominal aortic stent grafts. Journal of Vascular Surgery 2016;63(5):1208-15. [DOI] [PubMed] [Google Scholar]
Hanif 2018 {published data only}
- Hanif H, Dubois L, Ouzounian M, Peterson MD, El-Hamamsy I, Dagenais F. Aortic arch reconstructive surgery with conventional techniques vs frozen elephant trunk: a systematic review and meta-analysis. Canadian Journal of Cardiology 2018;34(3):262-73. [DOI] [PubMed] [Google Scholar]
Hirano 2020 {published data only}
- Hirano K, Tokui T, Nakamura B, Inoue R, Inagaki M, Hirano R, et al. Impact of the frozen elephant trunk technique on total aortic arch replacement. Annals of Vascular Surgery 2020;65:206-21. [DOI] [PubMed] [Google Scholar]
Hirnle 2016 {published data only}
- Hirnle T, Stankiewicz A, Matlak K, Frank M, Trzcinski R, Lejko A, et al. Single-centre experience in surgery of acute aortic type A dissection and true aortic arch aneurysm. Kardiologia Polska 2016;74(9):994-1001. [DOI] [PubMed] [Google Scholar]
Hongku 2016 {published data only}
- Hongku K, Dias NV, Sonesson B, Resch TA. Total aortic endovascular repair. Journal of Cardiovascular Surgery 2016;57(6):784-805. [PubMed] [Google Scholar]
Hori 2017 {published data only}
- Hori D, Okamura H, Yamamoto T, Nishi S, Yuri K, Kimura N, et al. Early and mid-term outcomes of endovascular and open surgical repair of non-dissected aortic arch aneurysm+. Interactive Cardiovascular and Thoracic Surgery 2017;24(6):944-50. [DOI] [PubMed] [Google Scholar]
Huang 2011 {published data only}
- Huang WH, He PC, Luo JF, Liu Y, Chen JY, Tan N, et al. A randomized controlled trial of rapid artificial cardiac pacing in thoracic endovascular aortic repair. Zhonghua Yi Xue Za Zhi 2011;91(24):1668-72. [PubMed] [Google Scholar]
JPRN‐UMIN000005430 {published data only}
- JPRN-UMIN000005430. Inoue stentraft implantation for aortic aneurysm and dissection. upload.umin.ac.jp/cgi-open-bin/ctr/ctr.cgi?function=brows&action=brows&recptno=R000006443&type=summary&language=E (first received 13 April 2011).
JPRN‐UMIN000007213 {published data only}
- JPRN-UMIN000007213. Next-gen fenestrated endgraft to seek indication expansion for arch aneurysm treatment. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000008490 (first received 2 February 2012).
JPRN‐UMIN000024202 {published data only}
- JPRN-UMIN000024202. The study of tranexamic acid suppression fibrinolysis for endoleak before endovascular repair for aortic aneurysm. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000027828 (first received 30 September 2016).
Karashima 2016 {published data only}
- Karashima C, Kagiyama N, Ohara M, Hayashida A, Hirohata A, Banba K, et al. O2-3 - The change of cardiovascular function after Total Arch Replacement (TAR) or Thoracic Endovascular Aortic Repair (TEVAR) for thoracic aortic aneurysm. Journal of Cardiac Failure 2016;22:S168. [Google Scholar]
Karner 1988 {published data only}
- Karner J, Schemper M, Teleky B, Kretschmer G, Piza F, Polterauer P. Aorto-Y-bifurcation graft: Dacron versus PTFE. Preliminary results of a randomized prospective study. International Surgery 1988;73(4):218-20. [PubMed] [Google Scholar]
Kaushik 2017 {published data only}
- Kaushik S, Ramanan B, Gasper W, Vartanian S, Reilly L, Chuter T, et al. The impact of prior aortic surgery on outcomes after multibranched endovascular repair of thoracoabdominal and pararenal aortic aneurysms. Journal of Vascular Surgery 2017;65(6 Supplement 1):29S-30S. [DOI] [PubMed] [Google Scholar]
Kawatou 2017 {published data only}
- Kawatou M, Minakata K, Sakamoto K, Nakatsu T, Tazaki J, Higami H, et al. Comparison of endovascular repair with branched stent graft and open repair for aortic arch aneurysm. Interactive Cardiovascular and Thoracic Surgery 2017;25(2):246-53. [DOI] [PubMed] [Google Scholar]
Khullar 2017 {published data only}
- Khullar V, Schaff HV, Dearani JA, Daly RC, Greason KL, Joyce LD, et al. Open surgical repair remains the gold standard for treating aortic arch pathology. Annals of Thoracic Surgery 2017;103(5):1413-20. [DOI] [PubMed] [Google Scholar]
Li 2018 {published data only}
- Li Y, Hu Z, Wang J, Zhang Y, Chen Z, Zhang H. Endovascular chimney technique for aortic arch pathologies treatment: a systematic review and meta-analysis. Annals of Vascular Surgery 2018;47:305-15. [DOI] [PubMed] [Google Scholar]
Liang 2017 {published data only}
- Liang NL, Yuo TH, Al-Khoury GE, Hager ES, Makaroun MS, Singh MJ. High mortality rates after both open surgical and endovascular thoracic aortic interventions in patients with end-stage renal disease. Journal of Vascular Surgery 2017;66(4):991-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marrocco‐Trischitta 2017 {published data only}
- Marrocco-Trischitta MM, De Beaufort HW, Secchi F, Van Bakel TM, Ranucci M, Van Herwaarden JA, et al. A geometric reappraisal of proximal landing zones for thoracic endovascular aortic repair according to aortic arch types. Journal of Vascular Surgery 2017;65(6):1584-90. [DOI] [PubMed] [Google Scholar]
Matsumura 2014 {published data only}
- Matsumura JS, Cambria RP, Dake MD, Greenberg R, Moore RD, Svensson LG. Early results of an international controlled trial of TEVAR. Vascular Annual Meeting 2007.
- Matsumura JS, Cambria RP, Dake MD, Moore RD, Svensson LG, Snyder S, et al. International controlled clinical trial of thoracic endovascular aneurysm repair with the Zenith TX2 endovascular graft: 1-year results. Journal of Vascular Surgery 2008;47(2):247-57. [DOI] [PubMed] [Google Scholar]
- Matsumura JS, Melissano G, Cambria RP, Dake MD, Mehta S, Svensson LG, et al. Five-year results of thoracic endovascular aortic repair with the Zenith TX2. Journal of Vascular Surgery 2014;60(1):1-10. [DOI] [PubMed] [Google Scholar]
- NCT00111176. STARZ-TX2 clinical study: study of thoracic aortic aneurysm repair with the Zenith TX2 endovascular graft (STARZ-TX2). clinicaltrials.gov/show/NCT00111176 (first received 18 May 2005).
Miao 2017 {published data only}
- Miao L, Song L, Sun SK, Wang ZG. Meta-analysis of open surgical repair versus hybrid arch repair for aortic arch aneurysm. Interactive Cardiovascular and Thoracic Surgery 2017;24(1):34-40. [DOI] [PubMed] [Google Scholar]
Moldovan 2017 {published data only}
- Moldovan H, Niculescu R, Balanescu S, Spanu C, Popescu D, Craciun M, et al. Current approaches for complex aortic arch diseases-TEVAR. MFM and hybrid procedures. Vascular 2017;25(1 Supplement 1):11-2. [Google Scholar]
Mougin 2017 {published data only}
- Mougin J, Sobocinski J, Hertault A, Azzaoui R, Delloye M, Haulon S. Applicability of a model of standardized branched aortic stentgraft for the management of the distal lesions of the aortic arch requiring the coverage of the left artery subclavian artery. Annals of Vascular Surgery 2017;44:23-4. [Google Scholar]
Narita 2016 {published data only}
- Narita H, Komori K, Usui A, Yamamoto K, Banno H, Kodama A, et al. Postoperative outcomes of hybrid repair in the treatment of aortic arch aneurysms. Annals of Vascular Surgery 2016;34:55-61. [DOI] [PubMed] [Google Scholar]
NCT00110201 {published data only}
- NCT00110201. The use of Nesiritide in thoracic aneurysm repair to prevent acute renal failure. clinicaltrials.gov/show/NCT00110201 (first received 5 May 2005).
NCT00409344 {published data only}
- NCT00409344. Dexmedetomidine for postoperative sedation in patients undergoing repair of thoracoabdominal aortic aneurysms. clinicaltrials.gov/show/NCT00409344 (first received 8 December 2006).
NCT00413231 {published data only}
- NCT00413231. Valor II: the Valiant thoracic stent graft system clinical study. clinicaltrials.gov/show/NCT00413231 (first received 19 December 2006).
NCT00488696 {published data only}
- NCT00488696. Branched aortic arch study. clinicaltrials.gov/show/NCT00488696 (first received 20 June 2007).
NCT00508118 {published data only}
- NCT00508118. NICardipine Neuroprotection in AortiC Surgery (NICNACS). clinicaltrials.gov/show/NCT00508118 (first received 27 July 2007).
NCT00583817 {published data only}
- NCT00583817. Endovascular treatment of thoracic aortic disease. clinicaltrials.gov/show/NCT00583817 (first received 31 December 2007).
NCT00590759 {published data only}
- NCT00590759. Evaluation of the GORE TAG thoracic endoprosthesis in the treatment of aneurysms. clinicaltrials.gov/show/NCT00590759 (first received 11 January 2008).
NCT00597870 {published data only}
- NCT00597870. Physician-sponsored IDE for the talent endoluminal stent graft system for the treatment of thoracic lesions. clinicaltrials.gov/show/NCT00597870 (first received 18 January 2008).
NCT00691756 {published and unpublished data}
- NCT00691756. A comparison of renal perfusion in thoracoabdominal aortic aneurysm (TAAA) repair. clinicaltrials.gov/show/NCT00691756 (first received 5 June 2008).
NCT00739557 {published data only}
- NCT00739557. A pilot study to evaluate the systemic inflammatory response of thoracoabdominal aortic aneurysm repair. clinicaltrials.gov/show/NCT00739557 (first received 21 August 2008).
NCT00757003 {published data only}
- NCT00757003. To evaluate the safety and efficacy for GORE TAG thoracic endoprosthesis in the treatment of thoracic aortic disease. clinicaltrials.gov/show/NCT00757003 (first received 22 September 2008).
NCT01033214 {published data only}
- NCT01033214. ENTRUST - TAArget® thoracic stent graft clinical trial. clinicaltrials.gov/show/NCT01033214 (first received 16 December 2009).
NCT01107366 {unpublished data only}
- NCT01107366. ATLANTIS: extensive type A dissections and thoracic/thoraco-abdominal aneurysms repair with LupiAe Hybrid TechNique. clinicaltrials.gov/show/NCT01107366 (first received 21 April 2010).
NCT01181947 {published data only}
- NCT01181947. VALIANT CAPTIVIA Post-market Registry (VCOUS). clinicaltrials.gov/show/NCT01181947 (first received 13 August 2010).
NCT01480206 {published data only}
- NCT01480206. Overlay of 3D scans on live fluoroscopy for endovascular procedures in the hybrid OR. clinicaltrials.gov/show/NCT01480206 (first received 28 November 2011).
NCT01524211 {published data only}
- NCT01524211. Evaluation of branch endografts in the treatment of aortic aneurysms. clinicaltrials.gov/show/NCT01524211 (first received 1 February 2012).
NCT01756911 {unpublished data only}
- NCT01756911. Evaluation of the safety and efficacy of the multilayer stent (STRATO). clinicaltrials.gov/show/NCT01756911 (first received 28 December 2012).
NCT01772537 {published data only}
- NCT01772537. The effects of anesthesia on patients undergoing surgery for repair of a thoracoabdominal aneurysm. clinicaltrials.gov/show/NCT01772537 (first received 24 January 2013).
NCT01839695 {published data only}
- NCT01839695. Safety and efficacy of Valiant Mona LSA stent graft system. clinicaltrials.gov/show/NCT01839695 (first received 25 April 2013).
NCT01889498 {published data only}
- NCT01889498. Effectiveness of acetazolamide in reducing paralysis of the leg in patients undergoing aortic aneurysm surgery surgery (AZATAAR). clinicaltrials.gov/show/NCT01889498 (first received 28 June 2013).
NCT01920594 {published data only}
- NCT01920594. Study of GSK1278863 to reduce ischemic events in patients undergoing thoracic aortic aneurysm repair. clinicaltrials.gov/show/NCT01920594 (first received 12 August 2013).
NCT02010892 {published data only}
- NCT02010892. Effective treatments for thoracic aortic aneurysms (ETTAA Study): a prospective cohort study. clinicaltrials.gov/show/NCT02010892 (first received 13 December 2013).
NCT02089607 {published data only}
- NCT02089607. Thoracoabdominal aortic aneurysms with fenestrated and branched stent grafts. clinicaltrials.gov/show/NCT02089607 (first received 18 March 2014).
NCT02101463 {published data only}
- NCT02101463. MOSTEGRA TRIAL:MO-(Dified) STE-(nt) GRA(-ft): surgeon-modified fenestrated-branched stent-grafts. clinicaltrials.gov/show/NCT02101463 (first received 2 April 2014).
NCT02111668 {published data only}
- NCT02111668. Thoracic aortic dilatation syndromes. clinicaltrials.gov/show/NCT02111668 (first received 11 April 2014).
NCT02253082 {published data only}
- NCT02253082. Vasculopathic injury and plasma as endothelial rescue - OCTAplas Trial (EudraCT no. 2014-000452-28) (VIPER-OCTA). clinicaltrials.gov/show/NCT02253082 (first received 1 October 2014). [EudraCT no. 2014-000452-28]
NCT02291718 {published data only}
- NCT02291718. Thoracoabdominal arortic CTA study. clinicaltrials.gov/show/NCT02291718 (first received 14 November 2014).
NCT02294435 {published data only}
- NCT02294435. Visceral manifold and unitary device study for the repair of thoracoabdominal aortic aneurysms. clinicaltrials.gov/show/NCT02294435 (first received 19 November 2014).
NCT02323581 {published data only}
- NCT02323581. Feasibility and safety of endovascular thoracoabdominal aortic aneurysm repair using a standard-configuration stent graft with branches and fenestrations in patients at high risk for open surgical repair (TAAA IDE). clinicaltrials.gov/show/NCT02323581 (first received 23 December 2014).
NCT02365454 {published data only}
- NCT02365454. NEXUS™ aortic arch stent graft system first in man study. clinicaltrials.gov/show/NCT02365454 (first received 19 February 2015).
NCT02365467 {published data only}
- NCT02365467. Evaluation of Valiant Mona LSA. clinicaltrials.gov/show/NCT02365467 (first received 19 February 2015).
NCT02471781 {published data only}
- NCT02471781. Zenith® TX2® low profile endovascular graft. clinicaltrials.gov/show/NCT02471781 (first received 15 June 2015).
NCT02554032 {published data only}
- NCT02554032. The Aortic surgery Cerebral protection Evaluation (ACE) randomized CardioLink-3 trial. clinicaltrials.gov/show/NCT02554032 (first received 18 September 2015).
NCT02625324 {published data only}
- NCT02625324. Valiant Evo international clinical trial. clinicaltrials.gov/show/NCT02625324 (first received 9 December 2015).
NCT02644681 {published data only}
- NCT02644681. Neurophysiological Intraoperative Monitoring during Aortic Surgery (NIMAS). clinicaltrials.gov/show/NCT02644681 (first received 1 January 2016).
NCT02652949 {published data only}
- NCT02652949. Valiant Evo US clinical trial (VEVO). clinicaltrials.gov/show/NCT02652949 (first received 12 January 2016).
NCT02678728 {published data only}
- NCT02678728. Effect of intraoperative dexmedetomidine on lung protection following thoracic aorta surgery with hypothermic circulatory arrest: a randomized clinical trial. clinicaltrials.gov/show/NCT02678728 (first received 10 February 2016).
NCT02735720 {published data only}
- NCT02735720. The CardiOvascular Remodeling following Endovascular aortic repair (CORE) Study. clinicaltrials.gov/show/NCT02735720 (first received 13 April 2016).
NCT02777528 {published data only}
- NCT02777528. Evaluation of the GORE® TAG® Thoracic Branch Endoprosthesis (TBE Device) in the treatment of lesions of the aortic arch and descending thoracic aorta (Zone 0/1). clinicaltrials.gov/show/NCT02777528 (first received 19 May 2016).
NCT02777593 {published data only}
- NCT02777593. Evaluation of the GORE® TAG® Thoracic Branch Endoprosthesis (TBE Device) in the treatment of lesions of the aortic arch and descending thoracic aorta (Zone 2). clinicaltrials.gov/show/NCT02777593 (first received 19 May 2016).
NCT02818972 {unpublished data only}
- NCT02818972. RelayPro thoracic stent-graft in subjects with thoracic aortic aneurysms and penetrating atherosclerotic ulcers (RelayPro-A). clinicaltrials.gov/show/NCT02818972 (first received 30 June 2016).
NCT03024554 {unpublished data only}
- NCT03024554. Evaluation of safety and efficacy of the Bifurcated Multilayer Flow Modulator (BMFM®). clinicaltrials.gov/show/NCT03024554 (first received 19 January 2017).
NCT03075748 {published data only}
- NCT03075748. Visceral manifold study for the repair of TAAA. clinicaltrials.gov/show/NCT03075748 (first received 9 March 2017).
NCT03093857 {published data only}
- NCT03093857. Investigation of the cerebrospinal fluid and further tissue samples for biomarker indicating spinal ischemia and organ failure in patients with thoracoabdominal aortic aneurysm (TAA). clinicaltrials.gov/show/NCT03093857 (first received 28 March 2017).
NCT03111459 {published data only}
- NCT03111459. Repair of thoracoabdominal aortic aneurysms. clinicaltrials.gov/show/NCT03111459 (first received 12 April 2017).
NCT03207568 {published data only}
- NCT03207568. RE-GENERATION: the safety and performance of the Relay Pro and Relay NBS Pro stent-graft devices in the European Union (EU). clinicaltrials.gov/show/NCT03207568 (first received 5 July 2017).
NCT03208504 {published data only}
- NCT03208504. The Single Branch NEXUS™ clinical study. clinicaltrials.gov/show/NCT03208504 (first received 5 July 2017).
NCT03214601 {unpublished data only}
- NCT03214601. Early feasibility study of the RelayBranch thoracic stent-graft system (RelayBranch). clinicaltrials.gov/show/NCT03214601 (first received 11 July 2017).
NCT03414866 {published data only}
- NCT03414866. Thoraflex hybrid post-market study. clinicaltrials.gov/show/NCT03414866 (first received 30 January 2018).
NCT04747626 {published and unpublished data}
- NCT04747626. B-SAFER: Branched stented anastomosis frozen elephant trunk repair. clinicaltrials.gov/ct2/show/NCT04747626 (first received 10 February 2021).
Novikova 2011 {published data only}
- Novikova O, Yavorovsky A, Guleshov V, Morozov Y, Trekova NX. Methods of gut protection during thoracoabdominal aortic aneurysm repair. Journal of Cardiothoracic and Vascular Anesthesia 2011;25(3 Supplement 1):S11-2. [Google Scholar]
Okita 1996 {published data only}
- Okita Y, Takamoto S, Ando M, Morota T, Yamaki F, Kawashima Y. Is use of aprotinin safe with deep hypothermic circulatory arrest in aortic surgery? Investigations on blood coagulation. Circulation 1996;94(Supplement 9):II177-81. [PubMed] [Google Scholar]
Patel 2016 {published data only}
- Patel HJ, Dake MD, Bavaria JE, Singh MJ, Filinger M, Fischbein MP, et al. Branched endovascular therapy of the distal aortic arch: preliminary results of the feasibility multicenter trial of the Gore thoracic branch endoprosthesis. Annals of Thoracic Surgery 2016;102(4):1190-8. [DOI] [PubMed] [Google Scholar]
Patterson 2016 {published data only}
- Patterson BO, Vidal-Diez A, Holt PJ, Scali ST, Beck AW, Thompson MM. Predicting mid-term all-cause mortality in patients undergoing elective endovascular repair of a descending thoracic aortic aneurysm. Annals of Surgery 2016;264(6):1162-7. [DOI] [PubMed] [Google Scholar]
Piazza 2016 {published data only}
- Piazza M, Squizzato F, Zavatta M, Menegolo M, Ricotta JJ, Lepidi S, et al. Outcomes of endovascular aneurysm repair with contemporary volume-dependent sac embolization in patients at risk for type II endoleak. Journal of Vascular Surgery 2016;63(1):32-8. [DOI] [PubMed] [Google Scholar]
Pinto 2017 {published data only}
- Pinto C, Garas G, Harling L, Darzi A, Casula R, Athanasiou T. Is endovascular treatment with multilayer flow modulator stent insertion a safe alternative to open surgery for high-risk patients with thoracoabdominal aortic aneurysm? Annals of Medicine and Surgery 2017;15:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Preventza 2017 {published data only}
- Preventza O, Coselli JS, Akvan S, Kashyap SA, Garcia A, Simpson KH, et al. The impact of temperature in aortic arch surgery patients receiving antegrade cerebral perfusion for >30 minutes: How relevant is it really? Journal of Thoracic and Cardiovascular Surgery 2017;153(4):767-76. [DOI] [PubMed] [Google Scholar]
Radak 2017 {published data only}
- Radak D, Djukic N, Tanaskovic S, Obradovic M, Cenic-Milosevic D, Isenovic ER. Should we be concerned about the inflammatory response to endovascular procedures? Current Vascular Pharmacology 2017;15(3):230-7. [DOI] [PubMed] [Google Scholar]
Rahe‐Meyer 2013 {published data only}
- Rahe-Meyer N, Hanke A, Schmidt DS, Hagl C, Pichlmaier M. Fibrinogen concentrate reduces intraoperative bleeding when used as first-line hemostatic therapy during major aortic replacement surgery: results from a randomized, placebo-controlled trial. Journal of Thoracic and Cardiovascular Surgery 2013;145(3 Supplement):S178-85. [DOI] [PubMed] [Google Scholar]
Ramanan 2016 {published data only}
- Ramanan B, Fernandez CC, Sobel JD, Gasper WJ, Vartanian SM, Reilly LM, et al. Low-profile versus standard-profile multibranched thoracoabdominal aortic stent grafts. Journal of Vascular Surgery 2016;64(1):39-45. [DOI] [PubMed] [Google Scholar]
Robinson 2016 {published data only}
- Robinson WP, Schuksz M. Surgical and antimicrobial management of a thoracic aortic aneurysm due to Q fever: a case report and brief review. Vascular and Endovascular Surgery 2016;50(4):290-4. [DOI] [PubMed] [Google Scholar]
Rodrigues‐Pinto 2016 {published data only}
- Rodrigues-Pinto E, Baron TH. 1047 Endoscopic ultrasound-guided transesophageal drainage of a mycotic aneurysm after aortic stent graft placement. Gastrointestinal Endoscopy 2016;83(5):AB196-7. [DOI] [PubMed] [Google Scholar]
Rousseau 2014 {published data only}
- Rousseau H, Mokrane T, Lions C, Saint Lebes B. Thoracic aortic aneurysm. Cardiovascular and Interventional Radiology 2014;37(2 Supplement 1):S140-1. [Google Scholar]
Saeyeldin 2017 {published data only}
- Saeyeldin AA, Velasquez CA, Mahmood SU, Brownstein AJ, Zafar MA, Ziganshin BA, et al. Thoracic aortic aneurysm: unlocking the "silent killer" secrets. General Thoracic and Cardiovascular Surgery 2017;1(1):1-11. [DOI: 10.1007/s11748-017-0874-x] [DOI] [PubMed] [Google Scholar]
Safi 1994 {published data only}
- Safi HJ, Bartoli S, Asimacopoulos PJ, Hess KR. Spinal cord protection during thoracoabdominal aortic aneurysm repair: preliminary results in 144 patients. Proceedings of the European Society for Vascular Surgery 8th Annual Meeting 1994;21(6):970-5. [Google Scholar]
Safi 1996 {published data only}
- Safi HJ. Role of the BioMedicus pump and distal aortic perfusion in thoracoabdominal aortic aneurysm repair. Artificial Organs 1996;20(6):694-9. [PubMed] [Google Scholar]
Sailer 2016 {published data only}
- Sailer A, Nelemans P, Berlo C, Yazar O, Haan M, Fleischmann D, et al. Endovascular treatment of complex aortic aneurysms: prevalence of acute kidney injury and effect on long-term renal function. European Radiology 2016;26(6):1613-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schanzer 2017 {published data only}
- Schanzer A, Simons JP, Flahive J, Durgin J, Aiello FA, Doucet D, et al. Outcomes of fenestrated and branched endovascular repair of complex abdominal and thoracoabdominal aortic aneurysms. Journal of Vascular Surgery 2017;66(3):687-94. [DOI] [PubMed] [Google Scholar]
Schroeder 2009 {published data only}
- Schroeder TV, Eldrup N, Just S, Hansen M, Nyhuus B, Sillesen H. Dilatation of aortic grafts over time: what to expect and when to be concerned. Seminars in Vascular Surgery 2009;22(2):119-24. [DOI] [PubMed] [Google Scholar]
Settepani 2016 {published data only}
- Settepani F, Cappai A, Basciu A, Barbone A, Tarelli G. Outcome of open total arch replacement in the modern era. Journal of Vascular Surgery 2016;63(2 Suppl):537-45. [DOI] [PubMed] [Google Scholar]
Shrestha 2017 {published data only}
- Shrestha M, Martens A, Kaufeld T, Beckmann E, Bertee S, Krueger H, et al. Single-centre experience with the frozen elephant trunk technique in 251 patients over 15 years. European Journal of Cardiothoracic Surgery 2017;52(5):858-66. [DOI] [PubMed] [Google Scholar]
Spear 2016 {published data only}
- Spear R, Haulon S, Ohki T, Tsilimparis N, Kanaoka Y, Milne CP, et al. Editor's choice - Subsequent results for arch aneurysm repair with inner branched endografts. European Journal of Vascular and Endovascular Surgery 2016;51(3):380-5. [DOI] [PubMed] [Google Scholar]
Svensson 2001 {published data only}
- Svensson LG, Nadolny EM, Penney DL, Jacobson J, Kimmel WA, Entrup MH, et al. Prospective randomized neurocognitive and S-100 study of hypothermic circulatory arrest, retrograde brain perfusion, and antegrade brain perfusion for aortic arch operations. Annals of Thoracic Surgery 2001;71(6):1905-12. [DOI] [PubMed] [Google Scholar]
Svensson 2015 {published data only}
- Svensson LG, Blackstone EH, Apperson-Hansen C, Ruggieri PM, Ainkaran P, Naugle RI, et al. Implications from neurologic assessment of brain protection for total arch replacement from a randomized trial. Journal of Thoracic and Cardiovascular Surgery 2015;150(5):1140-7. [DOI] [PubMed] [Google Scholar]
Tokuda 2016 {published data only}
- Tokuda Y, Oshima H, Narita Y, Abe T, Mutsuga M, Fujimoto K, et al. Extended total arch replacement via the L-incision approach: single-stage repair for extensive aneurysms of the aortic arch. Interactive Cardiovascular and Thoracic Surgery 2016;22(6):750-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Treasure 2016 {published data only}
- Treasure T. A 'compare and contrast' exercise: wrapping versus personalised external aortic root support (PEARS). Journal of Cardiothoracic Surgery 2016;11(1):104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tsukui 2002 {published data only}
- Tsukui H, Aomi S, Tomioka H, Nonoyama M, Koyanagi H, Nagasawa C, et al. Arch-first technique for aortic arch operation using branched graft. Asian Cardiovascular & Thoracic Annals 2002;10(4):318-21. [DOI] [PubMed] [Google Scholar]
Yoshitake 2017 {published data only}
- Yoshitake A, Okamoto K, Yamazaki M, Kimura N, Hirano A, Iida Y, et al. Comparison of aortic arch repair using the endovascular technique, total arch replacement and staged surgery. European Journal of Cardiothoracic Surgery 2017;51(6):1142-8. [DOI] [PubMed] [Google Scholar]
Additional references
Antoniou 2010
- Antoniou GA, El Sakka K, Hamady M, Wolfe JH. Hybrid treatment of complex aortic arch disease with supra-aortic debranching and endovascular stent graft repair. European Journal of Vascular and Endovascular Surgery 2010;39(6):683-90. [DOI] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bachet 2018
- Bachet J. Open repair techniques in the aortic arch are still superior. Annals of Cardiothoracic Surgery 2018;7(3):328. [DOI] [PMC free article] [PubMed] [Google Scholar]
Benedetto 2013
- Benedetto U, Melina G, Angeloni E, Codispoti M, Sinatra R. Current results of open total arch replacement versus hybrid thoracic endovascular aortic repair for aortic arch aneurysm: a meta-analysis of comparative studies. Journal of Thoracic and Cardiovascular Surgery 2013;145(1):305-6. [DOI] [PubMed] [Google Scholar]
Bhimji 2012
- Bhimji S, Hoynak B, Hale KL, Forker AD, Talavera F, Anker A. Aortic aneurysm. WebMD 2012.
Cao 2012
- Cao P, De Rango P, Czerny M, Evangelista A, Fattori R, Nienaber C, et al. Systematic review of clinical outcomes in hybrid procedures for aortic arch dissections and other arch diseases. Journal of Thoracic and Cardiovascular Surgery 2012;144(6):1286-300. [DOI] [PubMed] [Google Scholar]
Chakos 2018
- Chakos A, Jbara D, Yan TD, Tian DH. Long-term survival and related outcomes for hybrid versus traditional arch repair—a meta-analysis. Annals of Cardiothoracic Surgery 2018;7(3):319. [DOI] [PMC free article] [PubMed] [Google Scholar]
Clough 2013
- Clough R, Lotfi S, Powell J, Lee A, Taylor P. Hybrid aortic arch repair. Annals of Cardiothoracic Surgery 2013;2(3):300-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Clouse 1998
- Clouse WD, Hallett JW Jr, Schaff HV, Gayari MM, Ilstrup DM, Melton LJ 3rd. Improved prognosis of thoracic aortic aneurysms: a population-based study. JAMA 1998;280(22):1926-9. [DOI] [PubMed] [Google Scholar]
Conolly 2010
- Conolly S, Arrowsmith JE, Klein AA. Deep hypothermic circulatory arrest. Continuing Education in Anaesthesia Critical Care & Pain 2010;10(5):138–42. [DOI: 10.1093/bjaceaccp/mkq024] [DOI] [Google Scholar]
Czerny 2013
- Czerny M, Schmidli J, Carrel T, Grimm M. Hybrid aortic arch repair. Annals of Cardiothoracic Surgery 2013;2(3):372-7. [DOI: 10.3978/2013] [DOI] [PMC free article] [PubMed] [Google Scholar]
Czerny 2019
- Czerny M, Schmidli J, Adler S, Van den Berg JC, Bertoglio L, Carrel T, et al. Editor's Choice–Current options and recommendations for the treatment of thoracic aortic pathologies involving the aortic arch: an expert consensus document of the European Association for Cardio-Thoracic Surgery (EACTS) & the European Society for Vascular Surgery (ESVS). European Journal of Vascular and Endovascular Surgery 2019;57(2):165-98. [DOI] [PubMed] [Google Scholar]
Damberg 2017
- Damberg A, Carino D, Charilaou P, Peterss S, Tranquilli M, Ziganshin BA, et al. Favorable late survival after aortic surgery under straight deep hypothermic circulatory arrest. Journal of Thoracic and Cardiovascular Surgery 2017;154(6):1831-9. [DOI] [PubMed] [Google Scholar]
Demange 2011
- Demange MK, FregniII F. Limits to clinical trials in surgical areas. Clinics 2011;66(1):159-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elefteriades 2002
- Elefteriades JA. Natural history of thoracic aortic aneurysms: indications for surgery, and surgical versus nonsurgical risks. Annals of Thoracic Surgery 2002;74(5):S1877-80. [DOI] [PubMed] [Google Scholar]
Elefteriades 2010
- Elefteriades JA, Farkas EA. Thoracic aortic aneurysm: clinically pertinent controversies and uncertainties. Journal of American College of Cardiology 2010;55(9):841-57. [DOI: 10.1016/j.jacc.2009.08.084] [DOI] [PubMed] [Google Scholar]
Erbel 2014
- Erbel R, Aboyans V, Boileau C, Bossone E, Bartolomeo RD, Eggebrecht H, et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). European Heart Journal 2014;35(41):2873-926. [DOI] [PubMed] [Google Scholar]
Ergin 1994
- Ergin MA, Galla JD, Lansman SL, Quintana C, Bodian C, Griepp RB. Hypothermic circulatory arrest in operations on the thoracic aorta. Journal of Thoracic and Cardiovascular Surgery 1994;107(3):788-99. [PubMed] [Google Scholar]
Estrera 2008
- Estrera AL, Miller CC 3rd, Lee TY, Shah P, Safi HJ. Ascending and transverse aortic arch repair. Circulation 2008;118(Suppl 1):S160-6. [DOI: 10.1161/CIRCULATIONAHA.107.757419] [DOI] [PubMed] [Google Scholar]
Fillinger 2010
- Fillinger MF, Greenberg RK, McKinsey JF, Chaikof E, Society for Vascular Surgery Ad Hoc Committee on TEVAR Reporting Standards. Reporting standards for thoracic endovascular aortic repair (TEVAR). Journal of Vascular Surgery 2010;52(2):1022-33. [DOI] [PubMed] [Google Scholar]
Fraser 2008
- Fraser CD Jr, Andropoulos DB. Principles of antegrade cerebral perfusion during arch reconstruction in newborns/infants. Seminars in Thoracic and Cardiovascular Surgery. Pediatric Cardiac Surgery Annual 2008;11(1):61-8. [DOI: 10.1053/j.pcsu.2007.12.005] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gleason 2005
- Gleason TG. Heritable disorders predisposing to aortic dissection. Seminars in Thoracic and Cardiovascular Surgery 2005;17(3):274-81. [DOI: 10.1161/CIRCULATIONAHA.105.537340] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. GRADEpro GDT, Version accessed 4 January 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2018. Available at gradepro.org.
GRADE Working Group 2004
- GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Griepp 2013
- Griepp RB, Griepp EA. Perfusion and cannulation strategies for neurological protection in aortic arch surgery. Annals of Cardiothoracic Surgery 2013;2(2):159-62. [DOI: 10.3978/j.issn.2225-319X.2013.03.12] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924-6. [DOI: 10.1136/bmj.39489.470347.AD] [DOI] [PMC free article] [PubMed] [Google Scholar]
Harrington 2004
- Harrington DK, Lilley JP, Rooney SJ, Bonser RS. Nonneurologic morbidity and profound hypothermia in aortic surgery. Annals of Thoracic Surgery 2004;78(2):596-601. [DOI] [PubMed] [Google Scholar]
Harris 2013
- Harris R, Croce B, Tian DH. Hybrid aortic arch surgery. Annals of Cardiothoracic Surgery 2013;2(5):680. [PMC free article] [PubMed] [Google Scholar]
Haulon 2013
- Haulon S, Barillà D, Tyrrell M, Tsilimparis N, Ricotta II JJ. Debate: whether fenestrated endografts should be limited to a small number of specialized centers. Journal of Vascular Surgery 2013;57(3):875-82. [DOI] [PubMed] [Google Scholar]
Heinemann 1995
- Heinemann MK, Buehner B, Jurmann MJ, Borst HG. Use of the "Elephant Trunk Technique" in Aortic Surgery. Annals of Thoracic Surgery 1995;60(1):2-7. [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Hiraoka 2015
- Hiraoka A, Chikazawa G, Tamura K, Totsugawa T, Sakaguchi T, Yoshitaka H. Clinical outcomes for different approaches to aortic arch disease. Journal of Vascular Surgery 2015;61(1):88-95. [DOI: 10.1016/j.jvs.2014.06.121] [DOI] [PubMed] [Google Scholar]
Hiratzka 2010
- Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey Jr DE, et al. ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation 2010;121(13):e266-e369. [DOI] [PubMed] [Google Scholar]
Imran 2020
- Imran M, Zafar MA, Chkhikvadze T, Ziganshin BA, Elefteriades JA. Aortic arch aneurysms. In: Cardiac Surgery. Springer, Cham, 2020:529-44. [DOI: 10.1007/978-3-030-24174-2_57] [DOI] [Google Scholar]
Ince 2007
- Ince H, Nienaber CA. Etiology, pathogenesis and management of thoracic aortic aneurysm. Nature Reviews Cardiology 2007;4(8):418. [DOI] [PubMed] [Google Scholar]
Isselbacher 2005
- Isselbacher EM. Thoracic and abdominal aortic aneurysms. Circulation 2005;111(6):816-28. [DOI] [PubMed] [Google Scholar]
Jakob 2012
- Jakob H, Dohle DS, Piotrowski J, Benedik J, Thielmann M, Marggraf G, et al. Six-year experience with a hybrid stent graft prosthesis for extensive thoracic aortic disease: an interim balance. European Journal of Cardiothroacic Surgery 2012;42:1018-25. [DOI] [PubMed] [Google Scholar]
Jakob 2017
- Jakob H, Dohle D, Benedik J, Jánosi RA, Schlosser T, Wendt D, et al. Long-term experience with the E-vita Open hybrid graft in complex thoracic aortic disease. European Journal of Cardiothroacic Surgery 2017;51(2):329-38. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Liakopoulos 2020
- Liakopoulos OJ, Merkle J, Wahlers TC. Hybrid aortic arch repair. In: Cardiac Surgery. Springer, Cham, 2020:545-51. [DOI: 10.1007/978-3-030-24174-2_58] [DOI] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Götzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLOS Medicine 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Masuda 1992
- Masuda Y, Takanashi K, Takasu J, Morooka N, Inagaki Y. Expansion rate of thoracic aortic aneurysms and influencing factors. Chest 1992;102(2):461-6. [DOI] [PubMed] [Google Scholar]
Mestres 2013
- Mestres CA, Tsagakis KB, Pacini D, Di Bartolomeo R, Grabenwöger M, Borger M, et al, the IEOR Registry Group. One-stage repair in complex multisegmental thoracic aneurysmal disease: results of a multicentre study. European Journal of Cardio-Thoracic Surgery 2013;44:e325-e331. [DOI] [PubMed] [Google Scholar]
Metzger 2014
- Metzger PB, Rossi FH, Moreira SM, Issa M, Izukawa NM, Dinkhuysen JJ, et al. Hybrid treatment of aortic arch disease [Tratamento híbrido das doenças do arco aórtico]. Brazilian Journal of Cardiovascular Surgery 2014;29(4):527-36. [DOI: 10.5935/1678-9741.20140056] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mills 1980
- Mills NL, Ochsner JL. Massive air embolism during cardiopulmonary bypass. Causes, prevention, and management. Journal of Thoracic and Cardiovascular Surgery 1980;80(5):708-17. [PubMed] [Google Scholar]
Mitchell 2002
- Mitchell RS, Ishimaru S, Ehrlich MP. First International Summit on Thoracic Aortic Endografting: round table on thoracic aortic dissection as an indication for endografting. Journal of Endovascular Therapy 2002;9(Suppl 2):98-105. [PubMed] [Google Scholar]
Moulakakis 2013
- Moulakakis KG, Mylonas SN, Markatis F, Kotsis T, Kakisis J, Liapis CD. A systematic review and meta-analysis of hybrid aortic arch replacement. Annals of Cardiothoracic Surgery 2013;2(3):247-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Murphy 2009
- Murphy EH, Beck AW, Clagett GP, DiMaio JM, Jessen ME, Arko FR. Combined aortic debranching and thoracic endovascular aneurysm repair (TEVAR) effective but at a cost. Archives of Surgery 2009;3(144):222-7. [DOI: 10.1001/archsurg.2009.3] [DOI] [PubMed] [Google Scholar]
Oladokun 2016
- Oladokun D, Patterson BO, Sobocinski J, Karthikesalingam A, Loftus I, Thompson MM, et al. Systematic review of the growth rates and influencing factors in thoracic aortic aneurysms. European Journal of Vascular and Endovascular Surgery 2016;51(5):674-81. [DOI] [PubMed] [Google Scholar]
Olsson 2006
- Olsson C, Thelin S, Ståhle E, Ekbom A, Granath F. Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14 000 cases from 1987 to 2002. Circulation 2006;114(24):2611-8. [DOI] [PubMed] [Google Scholar]
Ouzounian 2013
- Ouzounian M, LeMaire SA, Coselli JS. Open aortic arch repair: state-of-the-art and future perspectives. Seminars in Thoracic and Cardiovascular Surgery 2013;2(3):247-60. [DOI] [PubMed] [Google Scholar]
Pacini 2007
- Pacini D, Leone A, Di Marco L, Marsilli D, Sobaih F, Turci S, et al. Antegrade selective cerebral perfusion in thoracic aorta surgery: safety of moderate hypothermia. European Journal of Cardio-thoracic Surgery 2007;31(4):618-22. [DOI] [PubMed] [Google Scholar]
Pannu 2005
- Pannu H, Fadulu VT, Chang J, Lafont A, Hasham SN, Sparks E, et al. Mutations in transforming growth factor-beta receptor type II cause familial thoracic aortic aneurysms and dissections. Circulation 2005;112(4):513-20. [DOI: 10.1161/CIRCULATIONAHA.105.537340] [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815-34. [DOI] [PubMed] [Google Scholar]
Perry 2014
- Perry DC, Griffin XL, Parsons N, Costa ML. Designing clinical trials in trauma surgery: overcoming research barriers. Bone & Joint Research 2014;3(4):123-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Poon 2016
- Poon SS, Theologou T, Harrington D, Kuduvalli M, Oo A, Field M. Hemiarch versus total aortic arch replacement in acute type A dissection: a systematic review and meta-analysis. Annals of Cardiothoracic Surgery 2016;5(3):156-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Preventza 2014
- Preventza O, Mohammed S, Cheong BY, Gonzalez L, Ouzounian M, Livesay JJ, et al. Endovascular therapy in patients with genetically triggered thoracic aortic disease: applications and short- and mid-term outcomes. European Journal of Cardio-Thoracic Surgery 2014;46(2):248-53. [DOI] [PubMed] [Google Scholar]
Preventza 2015
- Preventza O, Garcia A, Cooley DA, Haywood-Watson RJ, Simpson K, Bakaeen FG, et al. Total aortic arch replacement: a comparative study of zone 0 hybrid arch exclusion versus traditional open repair. Journal of Thoracic and Cardiovascular Surgery 2015;150(6):1591-600. [DOI] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- The Cochrane Collaboration Review Manager (RevMan 5). Version Version 5.4.1. The Cochrane Collaboration, 2020.
Safi 2011
- Safi HJ, Miller CC 3rd, Lee TY, Estrera A. Repair of ascending and transverse aortic arch. Journal of Thoracic and Cardiovascular Surgery 2011;142(3):630-3. [DOI] [PubMed] [Google Scholar]
Saliba 2015
- Saliba E, Sia Y. The ascending aortic aneurysm: when to intervene? International Journal of Cardiology. Heart & Vasculature 2015;6:91-100. [DOI: 10.1016/j.ijcha.2015.01.009] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2010
- Schünemann H, Brozek J, Guyatt G, Oxman A, editors. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendation. Version 3.2 (updated March 2009) edition. The GRADE Working Group, 2010. [Google Scholar]
Slisatkorn 2014
- Slisatkorn W. Comparison outcomes of hybrid and open surgery in aortic arch aneurysm. In: Journal of Thoracic and Cardiovascular Surgery. New York: American Association for Thoracic Surgery, 2014.
Stein 2010
- Stein LH, Elefteriades JA. Protecting the brain during aortic surgery: an enduring debate with unanswered questions. Journal of Cardiothoracic and Vascular Anesthesia 2010;24(2):316-21. [DOI] [PubMed] [Google Scholar]
Stone 2006
- Stone DH, Brewster DC, Kwolek CJ, LaMuraglia GM, Conrad MF, Chung TK, et al. Stent-graft versus open-surgical repair of the thoracic aorta: mid-term results. Journal of Vascular Surgery 2006;44(6):1188-97. [DOI] [PubMed] [Google Scholar]
Tanaka 2014
- Tanaka Y, Mikamo A, Suzuki R, Kurazumi H, Kudo T, Takahashi M, et al. Mortality and morbidity after total aortic arch replacement. Annals of Thoracic Surgery 2014;97(5):1569-75. [DOI] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8(1):1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vallabhajosyula 2013
- Vallabhajosyula P, Szeto W, Desai N, Bavaria J. Type I and Type II hybrid aortic arch replacement: postoperative and mid-term outcome analysis. Annals of Cardiothoracic Surgery 2013;2(3):280-7. [DOI: 10.3978/1998] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wallen 2018
- Wallen TJ, Bavaria JE, Vallabhajosyula P. Hybrid arch surgery challenges other forms of arch treatment. Journal of Cardiovascular Surgery 2018;59(4):554-8. [DOI] [PubMed] [Google Scholar]
Wheeler 2014
- Wheeler JB, Ikonomidis JS, Jones JA. Advances in Experimental Medicine and Biology. Vol. 802. Springer, 2014. [DOI: 10.1007/978-94-007-7893-1_8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wong 2011
- Wong DR, Parenti JL, Green SY, Chowdhary V, Liao JM, Zarda S, et al. Open repair of thoracoabdominal aortic aneurysm in the modern surgical era: contemporary outcomes in 509 patients. Journal of American College of Surgery 2011;212(4):579-81. [DOI: 10.1016/j.jamcollsurg.2010.12.041] [DOI] [PubMed] [Google Scholar]
Yan 2014
- Yan TD, Tian DH, LeMaire SA, Hughes GC, Chen EP, Misfeld M, et al. Standardizing clinical end points in aortic arch surgery: a consensus statement from the International Aortic Arch Surgery Study Group. Circulation 2014;129(15):1610-6. [DOI: 10.1161/CIRCULATIONAHA.113.006421] [DOI] [PubMed] [Google Scholar]
Ziganshin 2014
- Ziganshin BA, Rajbanshi BG, Tranquilli M, Fang H, Rizzo JA, Elefteriades JA. Straight deep hypothermic circulatory arrest for cerebral protection during aortic arch surgery: safe and effective. Journal of Thoracic and Cardiovascular Surgery 2014;148(3):888-98. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Elhelali 2018
- Elhelali A, Hynes N, Devane D, Sultan S, Kavanagh EP, Morris L, et al. Hybrid repair versus conventional open repair for thoracic aortic arch aneurysms. Cochrane Database of Systematic Reviews 2018, Issue 1. Art. No: CD012923. [DOI: 10.1002/14651858.CD012923] [DOI] [PMC free article] [PubMed] [Google Scholar]


