Abstract
Background
A dissection of the aorta is a separation or tear of the intima from the media. This tear allows blood to flow not only through the original aortic flow channel (known as the true lumen), but also through a second channel between the intima and media (known as the false lumen). Aortic dissection is a life‐threatening condition which can be rapidly fatal. There is debate on the optimal surgical approach for aortic arch dissection. People with ascending aortic dissection have poor rates of survival. Currently open surgical repair is regarded as the standard treatment for aortic arch dissection. We intend to review the role of hybrid and open repair in aortic arch dissection.
Objectives
To assess the effectiveness and safety of a hybrid technique of treatment over conventional open repair in the management of aortic arch dissection.
Search methods
The Cochrane Vascular Information Specialist searched the Cochrane Vascular Specialised Register, CENTRAL, MEDLINE, Embase, CINAHL and AMED databases and World Health Organization International Clinical Trials Registry Platform and ClinicalTrials.gov trials registers to 8 February 2021. We also undertook reference checking for additional studies.
Selection criteria
We included randomised controlled trials (RCTs) and clinical controlled trials (CCTs), which compared the effects of hybrid repair techniques versus open surgical repair of aortic arch dissection. Outcomes of interest were dissection‐related mortality and all‐cause mortality, neurological deficit, cardiac injury, respiratory compromise, renal ischaemia, false lumen thrombosis (defined by partial or complete thrombosis) and mesenteric ischaemia.
Data collection and analysis
Two review authors independently screened all records identified by the literature searches to identify those that met our inclusion criteria. We planned to undertake data collection and analysis in accordance with recommendations described in the Cochrane Handbook for Systematic Reviews of Interventions. We planned to assess the certainty of the evidence using GRADE.
Main results
We identified one ongoing study and two unpublished studies that met the inclusion criteria for the review. Due to a lack of study data, we could not compare the outcomes of hybrid repair to conventional open repair for aortic arch dissection.
Authors' conclusions
This review revealed one ongoing RCT and two unpublished RCTs evaluating hybrid versus conventional open repair for aortic arch surgery. Observational data suggest that hybrid repair for aortic arch dissection could potentially be favourable, but conclusions can not be drawn from these studies, which are highly selective, and are based on the clinical status of the patient, the presence of comorbidities and the skills of the operators. However, a conclusion about its definitive benefit over conventional open surgical repair cannot be made from this review without published RCTs or CCTs.
Future RCTs or CCTs need to have adequate sample sizes and follow‐up, and assess clinically‐relevant outcomes, in order to determine the optimal treatment for people with aortic arch dissection. It must be noted that this may not be feasible, due to the reasons mentioned.
Plain language summary
Hybrid versus conventional open repair for aortic arch dissection
Background
A dissection of the aorta is a separation or tear of the intima from the media. This tear allows blood to flow not only through the original aortic flow channel (known as the true lumen), but also through a second channel between the intima and media (known as the false lumen). A dissection can then develop along the artery, secondary to the blood flowing into the space. Aortic dissection is a life‐threatening condition which can rapidly be fatal. Aortic dissection that affects the ascending aorta, aortic arch and the descending aorta is a challenge for physicians. There is debate on the best surgical approach for aortic arch dissection. People with ascending aortic dissection have poor rates of survival. Currently, open surgical repair is regarded as the standard treatment for aortic arch dissection.
Study characteristics and key results
We searched medical databases for clinical trials that compared the use of a hybrid technique versus open surgical technique for people who suffered from arterial dissection of the aortic arch (last search February 2021). We identified one ongoing study and two unpublished studies, which met the inclusion criteria for the review. However, due to a lack of published study data, we could not compare the outcomes of hybrid repair to conventional open repair for aortic arch dissection.
Certainty of the evidence
In the absence of study data for those identified as eligible for inclusion in the review, it was not possible to assess the certainty of the evidence.
Conclusion
There is an absence of data for patients with this type of condition. Reasons may include its acute nature, and the need to intervene quickly in a surgical environment; patients with aortic arch dissection often suffer from many other conditions, which prevent them having particular surgeries; and often centres and surgical expertise may be lacking in this area, leading to a culture of using conventional rather than contemporary methods.
Future studies need to have adequate sample sizes and follow‐up, and assess clinically relevant outcomes, in order to determine the best treatment for people with aortic arch dissection. It must be noted that this may not be feasible due to the reasons mentioned.
Background
See Appendix 1 for Glossary of terms.
Description of the condition
The aorta is the main artery in the body. It originates in the heart and supplies blood to all parts of the body. The aorta consists of three layers: the intima, which is the innermost layer; the media, which is the middle layer; and the adventitia, which is the outermost layer. A dissection of the aorta is a separation or tear of the intima from the media. This tear allows blood to flow not only through the original aortic flow channel (known as the true lumen), but also through a second channel along the medial layers (known as the false lumen) (Tran 2009). A dissection can then propagate along the artery, secondary to the blood flowing into the space. Aortic dissection is a life‐threatening condition which can be rapidly fatal. It occurs more frequently in men, and uncontrolled blood pressure (hypertension) is a leading risk factor (Nienaber 2004). Predominate risk factors include genetic or familial aortopathies and connective tissue disorders such as Loeys‐Dietz syndrome, Marfan syndrome, and Ehlers‐Danlos syndrome (Murphy‐Ryan 2010).
According to the reporting standards for thoracic endovascular aortic repair, the aorta is divided into 12 treatment zones, zone 0 to zone 11. Aortic arch dissection occurs between zone 0 and zone 4 (Fillinger 2010). Zone 0 refers to an area between the aortic sinus and the brachiocephalic artery origin; zone 1 is distal to the brachiocephalic artery but proximal to the left common carotid artery origin; zone 2 is distal to the left common carotid artery but proximal to the subclavian artery; zone 3 is within 2 cm of the left subclavian artery without covering it; and zone 4 refers to an area 2 cm or more distal to the left subclavian artery and ends within the proximal half of the descending thoracic aorta (Fillinger 2010).
There are two classification systems for aortic dissection:
the Stanford classification, which categorises dissection into Type A and Type B (Daily 1970; DeBakey 1966). Type A occurs in the ascending aorta or aortic arch, or both, with possible involvement of the descending aorta. Type B occurs in the descending aorta, beyond the left subclavian artery; and
the DeBakey classification, which categorises dissection into Type I, Type II, and Type III. Type I involves the ascending and descending aorta (Stanford Type A), Type II involves the ascending aorta only (Stanford Type A), and Type III involves the descending aorta only, beginning after the left subclavian artery (Stanford Type B) (Daily 1970; DeBakey 1966).
Aortic dissection is also classified based on the age of the dissection (chronicity), as the mortality rates vary with chronicity (Wong 2008). These classifications are, from the onset of symptoms: less than 24 hours (hyper‐acute); less than 2 weeks (acute); 2 to 6 weeks (sub‐acute); and more than 6 weeks (chronic) (Nienaber 2011). As the dissection progresses in chronicity, the separated arterial layers that divide the true and false lumen (the intraluminal septum) increase in rigidity and reduce in elasticity and mobility, causing the septum to become stiff.
Description of the intervention
Aortic dissection that affects the ascending aorta, aortic arch and the descending aorta is a challenging pathology for physicians. People with this type of aortic disease pose a surgical challenge in this area of continuing development and innovation (Cochennec 2013; Kurimoto 2015; Lu 2013). Treatment of aortic dissection can be by open repair, endovascular repair, or a hybrid repair (Antoniou 2010; Cao 2012; Cochennec 2013; Murphy 2012; O'Callaghan 2014). There is debate on the optimal surgical approach for aortic arch dissection. People with ascending aortic dissection have poor rates of survival. Currently, open surgical repair (OSR) is regarded as the standard treatment for aortic arch dissection (DeBakey 1966; Suzuki 2003).
Open surgical repair
Current treatment for complex aortic arch dissection depends on the distal extent of the dissection, the location of the intimal tear, the diameter of the distal aortic arch and the relative fitness of the patient. The mainstay of treatment for type A dissection is complete resection of the intimal tear and replacement of the ascending aorta with a prosthetic graft. However, if the dissection extends into the aortic arch more extensive resections and aortic graft replacement may be required. A hemi‐arch repair can be undertaken if the dissection does not extend beyond the proximal arch, if the intimal tear is on the inner curve and does not involve the supra‐aortic vessels, if the distal aortic arch is not aneurysmal, or if the patient is unfit for extensive repair (Yang 2019). This involves removal of the ascending portion of the aorta and the proximal aortic arch, and replacement and open proximal and distal anastomosis (connection) with a surgical graft. This is carried out under artificially‐induced circulatory arrest (a method of temporarily stopping the blood flow completely, to create a bloodless field) with varying degrees of hypothermia (cooling of core body temperature), and a selection of cerebral protection techniques, including antegrade or retrograde cerebral perfusion, or deep hypothermia alone. Potential complications of open surgical repair include stroke, cardiac arrhythmia (irregular heartbeat), coagulopathy (failure to clot, or inappropriate clotting of blood), and hypokalaemia (lower than normal level of potassium in the blood) (Groysman 2011). This type of repair is high‐risk and carries a mortality risk of 21.6%, even with the use of circulatory arrest and cerebral perfusion techniques (Patel 2011).
In cases where the intimal dissection cannot be adequately treated by replacement of a hemi‐arch, or where the arch is aneurysmal or there is malperfusion of the supra‐aortic vessels, a more aggressive total arch replacement is warranted (Yang 2019). Surgical grafts with sidearms for the supra‐aortic branches and a perfusion branch reduce the number of surgical anastomoses required. In cases in which there is no evidence of supra‐aortic malperfusion and the supra‐aortic arteries are not dissected, the supra‐aortic vessel can be reimplanted en bloc (island) to the aortic graft, reducing the number of individual anastomoses (Shrestha 2014). Although total arch repair operative time is longer and requires more experience and surgical skill, it is associated with a reduced risk of aortic rupture, a lower stroke rate and a reduction in aortic re‐intervention rates (Smith 2017).
Hybrid repair
Hybrid techniques use a combination of endovascular approaches (intervention through the arteries using wires to carry grafts) and open surgical approaches to treat arch pathologies. These methods are designed to be less invasive than conventional open techniques and may permit more extensive arch repair in those unfit for open total arch repair (Smith 2017). The aorta is treated with a surgical graft in combination with the less invasive approach of endovascular implantation of an aortic stent endograft. Purely endovascular implantation of an endograft in the aorta is made through peripheral arterial access sites such as the femoral arteries, with no invasive surgical intervention. However, techniques for total endovascular repair, although promising, are still in their infancy (Nordon 2012), and reports estimate that in anatomical terms only 30% to 50% of patients with Stanford Type A aortic dissection are suitable for total endovascular repair with current technologies (Moon 2011; Sobocinski 2011).
Hybrid repair involves surgical arch debranching of the supra‐aortic vessels, thereby creating a proximal landing zone of adequate length, followed by endovascular stent graft insertion in the surgically‐constructed landing zone within the aortic arch.
Complete debranching of the aortic arch consists of revascularisation (restoring blood to the vessel) of at least the brachiocephalic artery and the left common carotid artery via a prosthetic bypass from the ascending aorta. After induction of pharmacologic hypotension (inducing a state of low blood pressure to reduce blood loss), the ascending aorta is clamped tangentially and the proximal end of a prosthetic graft sutured in an end‐to‐side anastomosis (Tominaga 2003). The left subclavian artery is revascularised through the sternotomy (division of the chest bone) or through an incision above the clavicle (collar bone). Aortic arch branch vessels can be bypassed with a singular, bifurcated (two branches) or trifurcated (three branches) tube graft.
Alternatively, cervical debranching can be performed through cervicotomies (incision in the neck) and consists of retro‐oesophageal right common carotid‐to‐left common carotid artery bypass using a Dacron graft. Depending on the surgeon's preference, the left subclavian artery can be ligated (tied up) or revascularised via a transposition into the left common carotid artery or a carotid artery bypass (Cochennec 2013).
During hybrid repair the endovascular intervention can be carried out in isolation or concurrently with the surgical intervention. In people with extensive disease of the thoracic arch and descending aorta, a single‐stage approach under circulatory arrest shows promising results (Jakob 2011; Sun 2011).
Hybrid approaches are classified into three types according to the extent of the aortic arch lesion and presence of the proximal and distal landing zones (Moulakakis 2013):
Type I: the debranching procedure consists of brachiocephalic bypass and endovascular repair of the aortic arch. This approach is reserved for people with isolated disease exhibiting an adequate proximal landing zone in the ascending aorta and a distal landing zone in the descending thoracic aorta (Stanford Type A/DeBakey Type II);
Type II: an open ascending aorta reconstruction that creates an appropriate proximal landing zone, supra‐aortic vessel revascularisation, and endoluminal dissection coverage. This approach is designed for people with ascending aortic lesions with a limited extension into the distal arch (Stanford Type A/DeBakey Type I); and
Type III: an elephant trunk procedure with a complete endovascular repair of the thoracoabdominal aorta. This technique is reserved for people with extensive aortic lesions that involve the ascending, transverse arch, and descending thoracic aorta (Stanford Type A/DeBakey Type I). This type III hybrid approach requires total open arch replacement and so falls outside the remit of this review, and is described here for the sake of complete description of the Moulakakis classification.
How the intervention might work
Although to date trial results using hybrid repair techniques for aortic arch dissection are promising, opinion is divided on its efficacy among the wider vascular surgery community (Kurimoto 2015). The aim of both hybrid repair and OSR is to stop further dissection progression in the aortic artery by covering the dissection entry points and also by promoting false lumen thrombosis; OSR is regarded as the standard for aortic arch dissection. Intervention for aortic arch dissection with a hybrid approach could reduce the incidence of highly invasive surgery when compared to OSR, while duration of cardiopulmonary bypass, hypothermic circulatory arrest and antegrade/retrograde cerebral perfusion can be reduced. Cardiopulmonary bypass is a technique that temporarily takes over the function of the heart and lungs during surgery, maintaining the circulation of blood and oxygen in the body. Hypothermic circulatory arrest temporarily suspends blood flow under very cold body temperatures. Antegrade cerebral perfusion involves sewing a small graft to the axillary/brachiocephalic artery or left common carotid artery. The graft is connected to a heart‐lung machine, and allows blood to flow through the brain during complex surgery of the aorta. Retrograde cerebral perfusion requires cannulation of the vena cava with perfusion pressures not exceeding 25 mmHg. Antegrade perfusion permits blood flow through the arterial system, allowing for varying temperature control. Retrograde perfusion permits blood flow through the venous system. The high associated risks using these methods including mortality (death) (6.6% to 9.9%), stroke (2.7% to 6.6%), paraplegia (18%), cardiac arrhythmia (irregular heartbeat), venous congestion and cerebral oedema would therefore be reduced or negated by using hybrid repair (Estrera 2003; Kamiya 2007; Okita 2001).
Why it is important to do this review
To date, no Cochrane Review has assessed the effectiveness of hybrid repair compared to the standard OSR. There is an agreement that intervention is necessary for aortic arch dissection, but complex open aortic arch repair still carries a high degree of health risks and death due to the use of cardiopulmonary bypass, hypothermic circulatory arrest, and antegrade or retrograde cerebral perfusion during the procedure (Lu 2013; Murphy 2012; Rampoldi 2007; Vohra 2012). Deciding if a person will undergo a hybrid versus an open repair depends on their fitness and comorbidity, surgical skill and physician preference, the overall quality of the supra‐aortic vessels (the brachiocephalic artery, the left common carotid artery, and subclavian artery) and the ability to clamp them, and whether cerebral perfusion can be maintained adequately.
We undertook this review as there is a critical need within the cardiovascular community for a synthesis of high‐quality evidence to inform decisions on optimal management of aortic arch dissection. Our systematic review focuses on aortic arch dissection treatments (specifically of Stanford Type A, i.e. DeBakey Type I and Type II) using hybrid and open repair.
Objectives
To assess the effectiveness and safety of a hybrid technique of treatment over conventional open repair in the management of aortic arch dissection.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) and controlled clinical trials (CCTs) assessing the effects of hybrid repair techniques compared to open surgical repair (OSR) of aortic arch dissection.
Types of participants
We include all participants with a diagnosis of aortic arch dissection. This includes classifications of dissection according to Stanford Type A (DeBakey Type I and Type II). Diagnosis was made by relevant diagnostic modalities, i.e. computed tomography (CT) or magnetic resonance imaging (MRI), or both. There was no limitation by participant gender, age, ethnicity, treatment setting (e.g. elective versus emergency repair), or dissection chronicity (acute or chronic). We excluded patients requiring a concomitant aortic valve repair.
Types of interventions
We include the following comparisons:
Type I hybrid repair versus OSR;
Type II hybrid repair versus OSR.
Types of outcome measures
Outcomes were guided and defined by the International Aortic Arch Surgery Study Group (Yan 2014; see also Table 1 for more details).
1. Definition of outcome measures (Yan 2014).
| Types of outcome measures | Defined by | Including |
| Primary outcomes | ||
| Mortality | Dissection related and all causes | (Grade V) All deaths at 30 days and 12 months |
| Neurological deficit | Global events | (Grade I ‐ IV) Postoperative agitation, delirium, obtundation, or myoclonic movements, without localised cerebral neurological signs |
| Focal events | (Grade I ‐ IV) Lateralising sensory or motor deficit or focal seizure activity |
|
| Spinal neurological events | (Grade I ‐ IV) Paraplegia, paraparesis |
|
| Cardiac injury | Myocardial ischaemia | (Grade I ‐ IV) |
| Low cardiac output syndrome | (Grade I ‐ IV) | |
| Arrhythmia | (Grade I ‐ IV) | |
| Pericardial effusion | (Grade I ‐ IV) | |
| Respiratory compromise | Parenchymal complications | (Grade I ‐ IV) Atelectasis, pneumonia, pulmonary oedema, and acute respiratory distress syndrome |
| Pleural complications | (Grade I ‐ IV) Pneumothorax, pleural effusion |
|
| Renal ischaemia | Modified RIFLE classification (Bellomo 2004): Risk (I), Injury (II), Failure (III), Loss/End‐Stage Kidney Dysfunction (IV) |
(Grade I ‐ IV) Serum creatinine increase, glomerular filtration rate (GFR) decrease, anuria, haemodialysis |
| Secondary outcomes | ||
| False lumen thrombosis | Partial or complete thrombosis | ‐ |
| Mesenteric ischaemia | Gut complications | (Grade I ‐ IV) Ileus or gastric paresis, gut ischaemia manifested as metabolic acidosis or increased lactate |
| Grades as defined by Yan 2014: Grade I: any deviation from the normal postoperative course but self‐limiting or requiring simple therapeutic regimens (including antiemetics, antipyretics, analgesics, electrolytes, and physiotherapy); Grade II: complications requiring pharmacological treatment for resolution; Grade III: complications requiring surgical, endoscopic, or radiological intervention but not requiring regional or general anaesthesia or requiring interdisciplinary intervention; Grade IV: complications requiring surgical, endoscopic, or radiological intervention under regional or general anaesthesia, or requiring new intensive care unit (ICU) admission or ongoing ICU management for > 7 days or hospitalisation for > 30 days, or causing secondary organ failure; Grade V: death caused by a complication. | ||
Primary outcomes
Dissection‐related mortality and all‐cause mortality at 30 days and 12 months (Grade V)
Neurological deficit (defined by global, focal and spinal events, Grade I to IV)
Cardiac injury (defined by myocardial ischaemia, low cardiac output syndrome, arrhythmia, pericardial effusion, Grade I to IV)
Respiratory compromise (defined by parenchymal and pleural complications, Grade I to IV)
Renal ischaemia (defined by RIFLE classification Bellomo 2004, Grade I to IV)
Secondary outcomes
False lumen thrombosis (defined by partial or complete thrombosis)
Mesenteric ischaemia (defined by gastrointestinal complications, Grade I to IV)
Search methods for identification of studies
Electronic searches
The Cochrane Vascular Information Specialist conducted systematic searches of the following databases for randomised controlled trials and controlled clinical trials without language, publication year or publication status restrictions.
the Cochrane Vascular Specialised Register via the Cochrane Register of Studies (CRS‐Web searched from inception to 8 February 2021).
the Cochrane Central Register of Controlled Trials (CENTRAL) Cochrane Register of Studies Online (CRSO 2021, Issue 1).
MEDLINE (Ovid MEDLINE® Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE® Daily and Ovid MEDLINE®) (searched to 8 February 2021).
Embase Ovid (searched 8 February 2021).
CINAHL Ebsco (searched to 8 February 2021).
AMED Ovid (searched to 8 February 2021).
The Information Specialist modelled search strategies for other databases on the search strategy designed for CENTRAL. Where appropriate, they were combined with adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Chapter 6, Lefebvre 2011). Search strategies for major databases are provided in Appendix 2.
The Information Specialist also searched the following trials registries on 8 February 2021.
ClinicalTrials.gov (clinicaltrials.gov).
World Health Organization International Clinical Trials Registry Platform (who.int/trialsearch).
For the purpose of this review, we also included studies published as abstracts only, if we could extract sufficient information. In cases where insufficient data were published, we first contacted the trial authors to access the required information. If data remained insufficient after contacting the trial authors, we excluded the study from our review.
Searching other resources
We searched the reference lists of all included studies.
Data collection and analysis
Selection of studies
Two review authors (EPK and AE) independently assessed the titles and abstracts of each identified study. Both review authors (EPK and AE) assessed the full texts of all studies categorised as included or unclear at title/abstract screening. If the review authors disagreed on the inclusion or exclusion of a study, we discussed the reasons. If there was no agreement between the two review authors, then we discussed with a third review author (NH). We recorded reasons for exclusions in the Characteristics of excluded studies table. We describe the selection process in an adapted PRISMA flow chart (Liberati 2009).
Data extraction and management
We obtained full‐text reports of the studies selected, and two review authors (EPK and AE) independently extracted data using an adapted data extraction form provided by Cochrane Vascular. If there was disagreement between the two review authors, we resolved issues by discussion with a third review author (NH). For studies with duplicate or multiple publications (or both), we collated all available data, and presented them as one study data set.
We aimed to describe the studies according to the following:
trial design;
diagnosis of aortic arch dissection;
demographic characteristics of participants;
type of intervention (hybrid and open repair); and
frequency of primary and secondary outcomes.
Assessment of risk of bias in included studies
Two review authors (EPK and AE) planned to independently assess the potential risks of bias in all included RCTs and CCTs using the Cochrane risk of bias tool (Higgins 2011). We planned to judge each domain as low risk, high risk, or unclear risk of bias and provide a statement to support each judgement. If there was disagreement between the two review authors, we planned to resolve these by consensus, and when necessary, by discussion with a third review author (NH).
We assessed the risk of bias in the following domains:
selection bias (random sequence generation and allocation concealment);
performance bias (blinding of participants and personnel);
detection bias (blinding of outcome assessors);
attrition bias (incomplete outcome data);
reporting bias (selective outcome reporting); and
other sources of bias.
Measures of treatment effect
Dichotomous data
We planned to express the results for dichotomous outcomes as risk ratios (RRs) with 95% confidence intervals (CIs), to reflect uncertainty of the point estimate of effects.
Continuous data
We planned to express the results for continuous scales of measurement as mean differences (MDs), standard deviations (SDs) and associated 95% CIs. Where there was a difference in scales for the same outcome, we used the standardised mean difference (SMD) with a 95% CI to combine the outcomes.
Time‐to‐event data
We aimed to use survival analysis to present time‐to‐event data expressed as hazard ratios (HRs) with 95% CIs. Methods used to analyse time‐to‐event outcomes were guided by those described by Parmar 1998 and Tierney 2007, and as detailed in Chapter 7, section 7.7.6 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Unit of analysis issues
We considered the unit of analysis within each trial to be each participant.
Dealing with missing data
In studies that had incomplete data, we contacted the study authors to seek additional data. For all outcomes, we aimed to carry out analyses as far as possible on an intention‐to‐treat basis (i.e. based on the initial treatment assignment and not on the treatment eventually received).
Assessment of heterogeneity
We planned to evaluate clinical heterogeneity based on participant data, the intervention and outcomes of each study. Our aim was to assess the degree of heterogeneity by visual inspection of forest plots and by examining the Chi2 test for heterogeneity. We planned to use the I2 statistic, Tau2 statistic and Chi2 test to determine statistical heterogeneity among studies, according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We would have rated statistical heterogeneity as substantial if an I2 were greater than 50% and either the Tau2 was greater than zero, or there was a low P value (less than 0.10) in the Chi2 test for heterogeneity. If we had identified substantial heterogeneity, we planned to explore possible reasons using subgroup analyses.
We did not undertake an assessment of heterogeneity, since we identified one ongoing study and two unpublished studies only, so results could not be pooled.
Assessment of reporting biases
We planned to address publication bias and other reporting biases (such as multiple publication bias) using funnel plots, in line with Cochrane Vascular guidelines, if there were 10 or more included studies (Higgins 2011).
This review has no included studies, so we did not investigate publication bias or other reporting bias using funnel plots.
Data synthesis
We planned to enter the collected data into Review Manager 5 software (Review Manager 2020). We planned to use a fixed‐effect meta‐analysis for synthesising data where it is reasonable to assume that trials are estimating the same underlying treatment effect. If clinical heterogeneity was sufficient to expect that the underlying treatment effects differ between trials, or if we detected substantial statistical heterogeneity, we planned to use a random‐effects meta‐analysis to produce an overall summary where the average treatment effect is clinically meaningful. If we identified clinical, methodological or statistical heterogeneity across included trials sufficient to cause concerns about the appropriateness of pooling results, we did not report pooled results from the meta‐analysis but instead planned to use a narrative approach to data synthesis. We planned to create a forest plot for each treatment effect, in accord with Cochrane Vascular guidelines (Higgins 2011).
We did not conduct a data synthesis in the form of a meta‐analysis or a narrative approach, since we did not identify published studies that met the inclusion criteria.
Subgroup analysis and investigation of heterogeneity
If we had found considerable heterogeneity within the included studies, we planned to carry out subgroup analyses to investigate possible reasons for this heterogeneity. We also planned to perform the following subgroup analyses, which were guided by DISSECT, a mnemonic‐based approach to the categorisation of aortic dissection (Dake 2013).
Duration of disease (i.e. acute dissection (less than 14 days) versus chronic dissection (14 days or more))
Intimal tear location (i.e. ascending aorta versus aortic arch)
Segmental extent of the disease (i.e. DeBakey Type I versus DeBakey Type II)
Size of the dissected aorta (i.e. maximum diameter less than 5.5 cm versus 5.5 cm or more (Pape 2007))
Presence or absence of complication
Thrombosis of aortic false lumen
Presence or absence of connective tissue disorder
Gender (Nienaber 2004)
Age (i.e. less than 70 years versus 70 years or older (Trimarchi 2010))
We did not undertake subgroup analysis or investigate heterogeneity because we did not conduct a data synthesis.
Sensitivity analysis
We aimed to perform sensitivity analyses on the following:
High‐quality trials, defined as studies with a low risk of bias for sequence generation and allocation concealment; and
RCTs compared with CCTs.
However, we did not perform a sensitivity analysis because we did not conduct a meta‐analysis of data.
Summary of findings and assessment of the certainty of the evidence
We planned to prepare a summary of findings table according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We intended to use GRADE profiler software to create the tables (GRADEproGDT 2015). For each comparator, we planned to include all primary and secondary outcomes as described in the Types of outcome measures section. Using the GRADE approach, we planned to assess the certainty of the body of evidence for the primary and secondary outcomes prespecified in our Cochrane protocol (Kavanagh 2018), as high, moderate, low or very low, based on the criteria of risk of bias, inconsistency, indirectness, imprecision and publication bias (GRADE Working Group 2004; Guyatt 2008a; Guyatt 2008b; Schünemann 2006). A draft version of the summary of findings table is included in this review (see Table 2).
2. Draft Summary of Findings table.
| Summary of findings for the main comparison: Hybrid repair versus conventional open repair for aortic arch dissection | ||||||
|
Patient or population: patients with a diagnosis of aortic arch dissection Settings: hospital Intervention: hybrid repair Comparison: open repair | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with open repair | Risk with hybrid repair | |||||
| Mortality, Follow‐up: median N (months) |
Study population |
HR N (N to N) |
N (N) | ⊕⊝⊝⊝
very low ⊕⊕⊝⊝ low ⊕⊕⊕⊝ moderate ⊕⊕⊕⊕ high |
||
| N per 1000 | N per 1000 (N to N) | |||||
| Neurological deficit, Follow‐up: median N (months) |
Study population |
RR N (N to N) |
N (N) | ⊕⊝⊝⊝
very low ⊕⊕⊝⊝ low ⊕⊕⊕⊝ moderate ⊕⊕⊕⊕ high |
||
| N per 1000 | N per 1000 (N to N) | |||||
| Cardiac injury, Follow‐up: median N (months) |
Study population |
RR N (N to N) |
N (N) | ⊕⊝⊝⊝
very low ⊕⊕⊝⊝ low ⊕⊕⊕⊝ moderate ⊕⊕⊕⊕ high |
||
| N per 1000 | N per 1000 (N to N) | |||||
| Respiratory compromise, Follow‐up: median N (months) |
Study population |
RR N (N to N) |
N (N) | ⊕⊝⊝⊝
very low ⊕⊕⊝⊝ low ⊕⊕⊕⊝ moderate ⊕⊕⊕⊕ high |
||
| N per 1000 | N per 1000 (N to N) | |||||
| Renal ischaemia, Follow‐up: median N (months) |
Study population |
RR N (N to N) |
N (N) | ⊕⊝⊝⊝
very low ⊕⊕⊝⊝ low ⊕⊕⊕⊝ moderate ⊕⊕⊕⊕ high |
||
| N per 1000 | N per 1000 (N to N) | |||||
| False lumen thrombosis, Follow‐up: median N (months) |
Study population |
RR N (N to N) |
N (N) | ⊕⊝⊝⊝
very low ⊕⊕⊝⊝ low ⊕⊕⊕⊝ moderate ⊕⊕⊕⊕ high |
||
| N per 1000 | N per 1000 (N to N) | |||||
| Mesenteric ischaemia, Follow‐up: median N (months) |
Study population |
RR N (N to N) |
N (N) | ⊕⊝⊝⊝
very low ⊕⊕⊝⊝ low ⊕⊕⊕⊝ moderate ⊕⊕⊕⊕ high |
||
| N per 1000 | N per 1000 (N to N) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; N: number; HR: hazard ratio; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
Results
Description of studies
See Figure 1.
1.
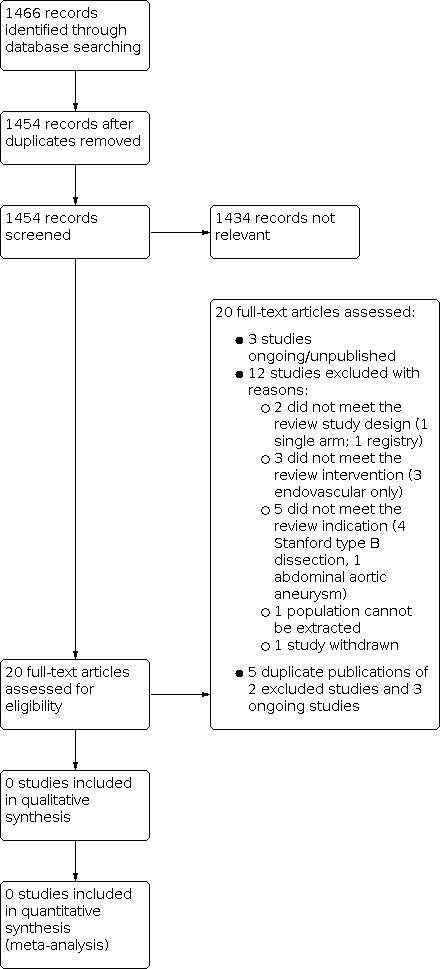
Study flow diagram.
Results of the search
We identified three studies that met the inclusion criteria. One study is ongoing (ChiCTR‐IPR‐16009372), while the remaining two studies are unpublished, with study data not available (ChiCTR‐TRC‐11001828; ChiCTR‐TRC‐13003857). The study investigators have been contacted, but with no response. These studies will be assessed further during subsequent review updates. See Characteristics of ongoing studies.
Ongoing studies
The study ChiCTR‐IPR‐16009372 is a randomised parallel controlled trial, which compared and evaluated different therapeutic procedures of the aortic arch for acute Stanford type A aortic dissection, with the aim of elucidating the optimal therapeutic strategy for the aortic arch. The target sample size was 280 participants, over four groups:
Total arch replacement (TAR group);
Arch reserved procedure (ARP group);
Hybrid procedure (Hybrid group); and
Triple‐branched stent graft (TBSG group).
The populations included participants diagnosed with Stanford type A aortic dissection by computed tomography angiography (CTA) imaging, with an onset time of less than two weeks. The primary source of funding was Renji Hospital, School of Medicine, Shanghai Jiao Tong University (China). The study describes its recruitment status as pending/not yet recruiting. This study is a registered clinical trial but no trial results are available. The study investigators have been contacted for clarification, and possible results, but we have received no response.
ChiCTR‐TRC‐11001828 is a randomised parallel controlled trial comparing the outcome of the two different operational methods to treat the aortic dissection: replacing ascending aorta plus reconstructing aortic arch with triple‐branched stent graft, and replacing ascending aorta plus replacing half aortic arch to treat the aortic dissection. The target sample size was 100 participants. The population included aortic dissection: type A with an onset time of less than two weeks. Primary outcomes were operation time, length of stay and aortic angiography computed tomography (CT). There were no secondary outcomes. The primary source of funding was the Scientific Research Department of The Affiliated Union Hospital, Fujian Medical University (China). This study is a registered clinical trial but no trial results are available. The study investigators have been contacted for clarification, and possible results, but we have received no response.
ChiCTR‐TRC‐13003857 is a prospective, multicentre, randomised parallel controlled trial, which evaluated the safety and efficacy of Xuper Open Surgery Stent Graft System (Lifetech Scientific, Shenzhen, China) for the surgical treatment of Stanford type A aortic dissection. The intervention was the Xuper Open Surgery Stent Graft System, while the comparator was open surgical repair using the Intergard graft (Getinge AB, Stockholm, Sweden). The population included those diagnosed with Stanford type A aortic dissection. The target sample sizes were 60 and 30, respectively. Primary outcomes included the duration of circulatory arrest. Secondary outcomes included: the incidence of major adverse events (death, paraplegia, brain complications); stent implantation successful (stent in place and successfully released); operation time; cardiopulmonary bypass time; arterial anastomosis time; aortic occlusion time; intraoperative blood loss and blood transfusion volume; and treatment success (12 months after operation). The primary source of funding was Lifetech Scientific (Shenzhen) Co. Ltd. This study is a registered clinical trial but no trial results are available. The study investigators have been contacted for clarification, and possible results, but we have received no response.
We will assess these studies further during subsequent review updates. See Characteristics of ongoing studies.
Included studies
There are no studies with sufficient available information to include
Excluded studies
We excluded 12 studies, upon inspection of the full text, for the following reasons.
-
2 studies were excluded because they did not meet the review study design
1 single arm (NCT02724072)
1 registry (NCT01500395)
-
3 studies were excluded because they did not meet the review intervention
3 endovascular (NCT00583817; NCT02201589; NCT03322033)
-
5 were excluded because they did not meet the review indication
4 Stanford type B dissection (NCT00526487; NCT01568320; NCT02094300; NCT02464943)
1 abdominal aortic aneurysm (NCT01704391)
1 study was excluded because the specific population could not be extracted, due to the type and extent of the lesion (Tsukui 2002)
1 study was excluded because it was withdrawn (NCT01107366)
We identified duplicate publications for two excluded studies (NCT02201589; NCT03322033) and three ongoing studies (ChiCTR‐IPR‐16009372; ChiCTR‐TRC‐11001828; ChiCTR‐TRC‐13003857).
A list of excluded studies and the reasons for exclusion are detailed in the Characteristics of excluded studies table.
Risk of bias in included studies
It was not possible to assess the risks of bias due to lack of included studies.
Effects of interventions
It was not possible to study the effect of hybrid repair versus conventional open repair for aortic arch dissection due to lack of included studies.
Discussion
Summary of main results
Patients with acute type A aortic dissection who are left untreated have a mortality rate of approximately 1% per hour, which increases dramatically to 90% within 30 days (Hagan 2000). Surgery of the ascending aorta and aortic arch is particularly complex in nature, owing to the need for patients to undergo either cardiopulmonary bypass, hypothermic circulatory arrest or selective perfusion in order to maintain blood flow to the brain and body during the procedure. These patients may also have significant progression of their disease into the thoracic aorta, meaning a return for secondary operation. The advent of stented grafts to replace conventional fabric grafts has allowed hybrid procedures to now be performed in one single operation, which treats not only the ascending/aortic arch dissection, but also treats further extensive thoracic dissection or potential for further dissection in the form of re‐entry tears. Hybrid surgery also gives an option for patients who were previously deemed unfit to undergo conventional open surgery.
This review demonstrates that while we found three RCTs meeting the inclusion criteria, there was a lack of study data as one RCT is ongoing and two RCTs are unpublished. We therefore could not assess the comparison between hybrid and conventional open repair for aortic arch dissection, for dissection‐related and all‐cause mortality, or for adverse complications. A review of the literature revealed a general lack of RCTs and CCTs, often in favour of prospective single‐arm or observational studies. Aortic arch dissection is relatively new in nature. This further reduces the ability of investigators to perform RCTs and CCTs without compromising overall treatment of the patient. Patients can be randomised to a specific treatment arm that may be otherwise clinically unsuitable. However, although RCTs will be challenging in this area, they are not unrealistic and there is precedent for RCTs in the setting of aortic rupture, such as the UK Improve trial (IMPROVE 2015). They will need to be confined to high‐volume centres that have both open and endovascular expertise to hand. There are well‐established aortic teams at many centres of excellence around the world, and in Europe these teams are encouraged to work together on aortic arch disease as specified in the consensus statement jointly published by the European Association for Cardio‐Thoracic Surgery (EACTS) and the European Society for Vascular Surgery (ESVS) (Czerny 2019).
Overall completeness and applicability of evidence
Overall completeness and applicability of the evidence comparing hybrid and conventional open repair for aortic arch dissection, with relation to dissection‐related and all‐cause mortality, and adverse complications could not be assessed, as we identified no studies for inclusion.
Quality of the evidence
It was not possible to review methodological quality or the certainty of the evidence in the absence of study data for those eligible for inclusion in the review.
Potential biases in the review process
The Cochrane Vascular Information Specialised performed a comprehensive search of the literature and we selected studies according to the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2011). Two review authors (EPK and AE) independently assessed studies for potential inclusion (Figure 1). We resolved any disagreements by discussion, and included the third review author (NH) where necessary.
Agreements and disagreements with other studies or reviews
Data could not be gathered from the three studies identified according to the inclusion criteria (ChiCTR‐TRC‐11001828; ChiCTR‐TRC‐13003857; ChiCTR‐IPR‐16009372). One of these studies, ChiCTR‐TRC‐13003857, describes a Xuper Open Surgery Stent Graft System (Lifetech Scientific, Shenzhen, China), which is a hybrid graft consisting of three parts: a proximal polyester surgical graft, a primary stent graft with two super branches set 3 mm apart, and a delivery system. The Xuper Open Surgery Stent Graft system is used for hybrid arch repair, whereby it is anastomosed to an ascending aortic graft. The side branches from the stent graft are deployed in the left carotid and left subclavian arteries, and the innominate artery is transected and anastomosed to the ascending aortic graft. Although the results of the trial were not available, we did identify a case series of 21 patients in whom the device was used (Yu 2019). Yu 2019 reported that the system was found to be safe and effective for total arch repair in acute aortic dissection with an in‐hospital mortality rate of 4.8% and no need for a re‐intervention at an average follow‐up time of 35.2 ± 2.1 months (range 15 to 42 months).
We found two systematic reviews, which illustrate the current trends for treatment of aortic arch dissection (Mussa 2016; Smith 2017). These are not pertinent to our conclusions, but provide additional information to the reader.
A systematic review which included 82 studies (2 RCTs and 80 observational) (Mussa 2016), reviewed the current evidence relating to diagnosis and treatment of acute aortic syndromes. It should be noted that the two RCTs included in this systematic review evaluated acute and chronic uncomplicated descending aortic dissection. Available data suggested that open surgical repair is optimal for treating Stanford type A (ascending aorta). Thirty‐day mortality for people treated with open surgery was 13% to 17%. The authors also concluded that there is a significant lack of RCTs relating to acute aortic syndromes. While evidence shows there have not been any RCTs or CCTs carried out that compare hybrid repair to conventional open surgical repair for aortic arch dissection, the overall clinical status of the patient, as well as the presence of preoperative complications, often dictates the decision to intervene surgically or to use medical management.
Similarly, a systematic review and meta‐analysis of 'extended' or hybrid arch repair for acute Stanford type A dissection carried out on literature between 1946 and August 2015 found no RCTs have been conducted on this topic (Smith 2017). The study compiled demographics and outcomes on a number of hybrid surgeries, including:
total arch replacement ± standard elephant trunk without descending thoracic aortic stent grafting;
total arch replacement and descending thoracic aortic stent grafting with frozen stent graft placed under circulatory arrest;
hemi‐arch replacement and descending thoracic aortic stent grafting with the stent graft placed under circulatory arrest; and
total arch replacement with stent graft placed after coming off cardiopulmonary bypass and with the use of fluoroscopy to identify landing zones.
This review of the literature by Smith and colleagues revealed 38 studies, which included 2140 participants who had an extended/hybrid arch repair as defined above. Overall hospital mortality was 8.6% (95% CI 7.2 to 10.0), stroke rate was 5.7% (95% CI 3.6 to 8.2) and spinal cord ischaemia rate was 2.0% (95% CI 1.2 to 3.0) (Smith 2017). Although the purpose of the review by Smith 2017 was not to compare the hybrid surgical techniques, it is still interesting to note the breakdown between groups. Hospital mortality was 11.9 % (95% CI 7.0 to 17.8), 8.6% (95% CI 7.0 to 10.2), 6.3% (95% CI 4.5 to 8.3 and 5.5% (95% CI 3.3 to 8.3), respectively. Stroke rate was 7.7% (95% CI 4.9 to 10.8), 3.7% (95% CI 2.1 to 5.7), 3.0% (95% CI 1.5 to 5.0) and 1% (95% CI 0.0 to 0.1), respectively, and spinal cord ischaemia rate was 1.6% (95% CI 0.1 to 4.3), 1.95% (95% CI 1.04 to 3.12), 2.9% (95% CI 0.8 to 6.4) and 1% (95% CI 0.0 to 0.1), respectively. It is interesting to consider that the highest death and stroke rates occurred with conventional repair, and that using stent grafts in the descending thoracic aorta reduced these complications without an increase in spinal cord ischaemia rates. Of note, the procedure which theoretically should reduce spinal cord ischaemia by reducing manipulation and coverage of the subclavian arteries, was actually associated with a higher spinal cord ischaemia rate. There are of course many confounding factors, such as stent graft length or patient haemodynamic stability which are not taken into account.
Given the lack of RCTs or CCTs comparing open to hybrid repair for aortic arch dissection, the International Registry of Acute Aortic Dissections (IRAD) was established in 1996 to assess the aetiological factors, modes of presentation, clinical features, treatment, and hospital outcomes of people with acute aortic dissection from around the world. IRAD is a consortium of research centres and currently has 30 large referral centres in 11 countries participating in the registry. Information such as dates and times of symptom onset, presentation, diagnosis, haemodynamic signs of aortic dissection, initial and chronic medical therapy, diagnostic imaging chosen, and surgical and medical management is being studied. IRAD investigators echo other studies and conclude that extensive versus conservative (i.e. open versus hybrid) surgical management of aortic arch dissection should be determined case‐by‐case, and on the basis of the clinical status of the patient, their specific aortic anatomy, i.e. anatomy of great vessels and location of entry and re‐entry tears, and the specific experience of the operator (Di Eusanio 2014). A review of the IRAD data demonstrated a mortality of 25.1%, with a higher mortality in those classified as unstable (Trimarchi 2005). The authors concluded that risk factors associated with Stanford type A aortic dissection will considerably alter the treatment outcome, rather than the choice of treatment alone.
Authors' conclusions
Implications for practice.
This review revealed one ongoing RCT and two unpublished RCTs evaluating hybrid versus conventional open repair or aortic arch surgery. Observational data suggest that hybrid repair for aortic arch dissection could potentially be favourable, but the studies are highly selective, and decisions on patient care are based on the clinical status of the patient, the presence of comorbidities and the skills of the operators. However, a conclusion on its definitive benefit over conventional open surgical repair cannot be made from this review without published RCTs or CCTs. Until high‐certainty evidence becomes available for people with aortic arch dissection, clinicians should continue to assign treatment on a strict case‐by‐case basis. Results from the currently ongoing study (ChiCTR‐IPR‐16009372) will inform practice in the future.
Implications for research.
This review revealed one ongoing RCT (ChiCTR‐IPR‐16009372) that evaluates hybrid versus conventional open aortic arch surgery. This RCT assesses outcomes such as 30‐day mortality, major adverse cardio‐cerebral events, renal complications, paraplegia, ICU/hospital length of stay, and hospitalisation costs. Future RCTs/CCTs need to have adequate sample sizes and follow‐up, and to assess clinically relevant outcomes, in order to determine the optimal treatment for people with aortic arch dissection.
History
Protocol first published: Issue 1, 2018
Notes
Parts of the Methods section of this review are based on a standard template established by Cochrane Vascular.
Acknowledgements
We are very grateful to Cochrane Vascular for their support and guidance in the preparation of this review.
The review authors and the Cochrane Vascular editorial base are grateful to the following peer reviewers for their time and comments: Piergiorgio Cao FRCS, Senior Professor of Vascular Surgery, University of Perugia; Consultant Vascular Surgeon, Ospedale Bel Colle Viterbo, Clinica Mater Dei Roma, Rome, Italy; Mr Stephen Badger, Belfast Health & Social Care Trust, Belfast, UK.
Appendices
Appendix 1. Glossary of terms
A
Anastomosis is a connection made surgically between adjacent blood vessels.
Antegrade cerebral perfusion (ACP) is a method of supplying blood to the brain during surgery, while the function of the heart and lungs is temporarily stopped.
Aortic dissection is a separation or tear of the intima layer from the media layer of the aorta.
B
Bifurcated refers to a division in an object in to two objects, e.g. one part into two parts.
C
Cardiacarrhythmia is an irregular heart beat.
Cardiopulmonary bypass is a technique that temporarily takes over the function of the heart and lungs during surgery, maintaining the circulation of blood and oxygen in the body.
Cervical is an anatomical term used for a section of the spine in the neck (cervical spine).
Circulatory arrest is an artificially induced method of slowing the blood flow around the body during surgical interventions.
Clavicle is an anatomical term for the collar bone.
Coagulopathy is a failure in the blood to clot, leading to excessive bleeding.
D
Distal refers to a point that is farthest away from the centre of the body.
E
Elephant trunk is a vascular technique used to repair patients with extensive disease in their aorta. It consists of two stages, 1) open surgery to replace a portion of the ascending aorta, while leaving a section of graft hanging within the descending aorta. 2) This graft section can then be used to place an endovascular stent (known as stented elephant trunk technique). This technique can also be carried out as a single‐stage (known as frozen elephant trunk technique).
Endovascular repair involves intervention through the arteries using wires to carry grafts to the area of interest to be repaired.
H
Hypokalaemia is related to the status of potasium in the blood, specifically when the level is lower than normal.
Hypothermia refers to cooling of core body temperature.
Hypothermic circulatory arrest temporarily suspends blood flow under very cold body temperatures.
L
Landing zone refers to the zone of landing for a graft in the aorta.
Lesion is a region in an organ or tissue which has suffered damage through injury or disease, for example a wound, ulcer, abscess, or tumour.
M
Mortality is also known as death.
O
Open surgical repair (OSR) involves surgical intervention through a large incision made through the skin, revealing the inner organs to be repaired. It also involves induced circulatory arrest or hypothermia, and methods of brain protection.
P
Peripheral arterial access is the point of access to the blood in an artery, specifically in the limbs of the body, e.g. the arms or the legs.
Pharmacologic hypotension refers to a method of inducing a state of low blood pressure using a drug(s) during surgery, in order to reduce the amount of blood lost.
Proximal refers to a point that is closest to the centre of the body.
R
Retrograde Cerebral Perfusion (RCP) is a method of supplying blood to the brain during surgery, while the function of the heart and lungs is temporarily stopped. The blood receives oxygen outside the body, and is washed of toxins, and blood clots, and is cannulated back into the body through a vein.
Revascularisation is a process of restoring blood to a vessel or organ following a state of deprivation.
S
Stroke occurs when the blood flow to the brain is obstructed, resulting in cellular death.
T
Transposition is a term used when a vessel is transferred onto another vessel.
Trifurcated refers to division in an object in to three objects, e.g. one part into three parts.
Appendix 2. Database searches
| Source | Search strategy | Hits retrieved |
| VASCULAR REGISTER IN CRSW | AORTDISSECT | 19.2.18 ‐ 22 11.2.19 ‐ 24 19.2.20 ‐ 5 08.2.21 ‐ 26 |
| CENTRAL via CRSO | #1 MESH DESCRIPTOR Aneurysm, Dissecting 70 #2 MESH DESCRIPTOR Aorta WITH QUALIFIERS SU 105 #3 (aortic arch):TI,AB,KY 229 #4 (aort* near4 dissect*):TI,AB,KY 241 #5 (aort* near4 tear*):TI,AB,KY 4 #6 (aort* near4 trauma*):TI,AB,KY 20 #7 deBakey:TI,AB,KY 10 #8 (de Bakey):TI,AB,KY 2 #9 Stanford:TI,AB,KY 603 #10 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 1194 #11 hybrid:TI,AB,KY 1839 #12 debranch*:TI,AB,KY 4 #13 supraaortic:TI,AB,KY 10 #14 rerouting:TI,AB,KY 7 #15 MESH DESCRIPTOR Endovascular Procedures EXPLODE ALL TREES 7282 #16 MESH DESCRIPTOR Stents EXPLODE ALL TREES 3650 #17 MESH DESCRIPTOR Blood Vessel Prosthesis EXPLODE ALL TREES 429 #18 MESH DESCRIPTOR Blood Vessel Prosthesis Implantation EXPLODE ALL TREES 431 #19 endovasc*:TI,AB,KY 2174 #20 endostent*:TI,AB,KY 1 #21 endoluminal:TI,AB,KY 151 #22 endoprosthe*:TI,AB,KY 281 #23 (graft or endograft*):TI,AB,KY 16587 #24 percutaneous*:TI,AB,KY 12621 #25 stent*:TI,AB,KY 9578 #26 TEVAR:TI,AB,KY 43 #27 branched:TI,AB,KY 802 #28 fenestrated:TI,AB,KY 58 #29 (elephant trunk):TI,AB,KY 5 #30 (landing zone):TI,AB,KY 18 #31 #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 40044 #32 #10 AND #31 172 |
19.2.18 ‐ 172 11.2.19 ‐ 16 19.2.20 ‐ 75 08.2.21 ‐ 94 |
| Clinicaltrials.gov | dissecting Aneurysm OR Aorta | Stents OR Blood Vessel Prosthesis OR TEVAR | 19.2.18 ‐ 199 11.2.19 ‐ 18 19.2.20 ‐ 29 08.2.21 ‐ 50 |
| ICTRP Search Portal | Aneurysm OR Aorta AND Stents OR Blood Vessel Prosthesis OR TEVAR | 19.2.18 ‐ 169 11.2.19 ‐ 13 19.2.20 ‐ 17 08.2.21 ‐ N/A |
| Ovid MEDLINE(R) Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) 1946 to Present | 1 Aneurysm, Dissecting/ 15515 2 AORTA/su [Surgery] 7260 3 aortic arch.ti,ab. 14642 4 deBakey.ti,ab. 902 5 de Bakey.ti,ab. 81 6 Stanford.ti,ab. 6136 7 (aort* adj4 dissect*).ti,ab. 15282 8 (aort* adj4 tear*).ti,ab. 555 9 (aort* adj4 trauma*).ti,ab. 2201 10 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 47567 11 hybrid.ti,ab. 130820 12 debranch*.ti,ab. 1388 13 supraaortic.ti,ab. 372 14 rerouting.ti,ab. 827 15 exp Endovascular Procedures/ 104049 16 exp STENTS/ 67095 17 exp Blood Vessel Prosthesis/ 27088 18 exp Blood Vessel Prosthesis Implantation/ 20235 19 endovasc*.ti,ab. 40036 20 endostent*.ti,ab. 33 21 endoluminal.ti,ab. 3992 22 endoprosthe*.ti,ab. 6521 23 (graft or endograft*).ti,ab. 197756 24 percutaneous*.ti,ab. 125928 25 stent*.ti,ab. 86095 26 TEVAR.ti,ab. 1179 27 branched.ti,ab. 30930 28 fenestrated.ti,ab. 3406 29 elephant trunk.ti,ab. 726 30 landing zone.ti,ab. 574 31 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 630918 32 10 and 31 13958 33 randomized controlled trial.pt. 453520 34 controlled clinical trial.pt. 92155 35 randomized.ab. 402996 36 placebo.ab. 186469 37 drug therapy.fs. 1991613 38 randomly.ab. 285102 39 trial.ab. 418372 40 groups.ab. 1763857 41 or/33‐40 4139506 42 exp animals/ not humans.sh. 4424690 43 41 not 42 3576406 44 (2017* or 2018*).ed. 1023755 45 32 and 43 and 44 101 |
19.2.18 ‐ 101 11.2.19 ‐ 87 19.2.20 ‐ 127 08.2.21 ‐ 260 |
| EMBASE | 1 dissecting aneurysm/ 5385 2 aorta/su [Surgery] 1839 3 aortic arch.ti,ab. 18998 4 (aort* adj4 dissect*).ti,ab. 19789 5 (aort* adj4 tear*).ti,ab. 710 6 (aort* adj4 trauma*).ti,ab. 2613 7 deBakey.ti,ab. 1146 8 de Bakey.ti,ab. 117 9 Stanford.ti,ab. 8465 10 or/1‐9 50787 11 hybrid.ti,ab. 131196 12 debranch*.ti,ab. 1656 13 supraaortic.ti,ab. 584 14 rerouting.ti,ab. 1014 15 exp endovascular surgery/ 29196 16 exp stent/ 146772 17 exp blood vessel prosthesis/ 13097 18 exp blood vessel transplantation/ 97311 19 endovasc*.ti,ab. 58168 20 endostent*.ti,ab. 47 21 endoluminal.ti,ab. 5492 22 endoprosthe*.ti,ab. 7671 23 (graft or endograft*).ti,ab. 271072 24 percutaneous*.ti,ab. 184355 25 stent*.ti,ab. 139867 26 TEVAR.ti,ab. 1849 27 branched.ti,ab. 34323 28 fenestrated.ti,ab. 4313 29 elephant trunk.ti,ab. 851 30 landing zone.ti,ab. 949 31 or/11‐30 833027 32 10 and 31 13503 33 randomized controlled trial/ 487494 34 controlled clinical trial/ 454734 35 random$.ti,ab. 1269278 36 randomization/ 77055 37 intermethod comparison/ 229341 38 placebo.ti,ab. 266628 39 (compare or compared or comparison).ti. 459288 40 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 1686482 41 (open adj label).ti,ab. 61981 42 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 204557 43 double blind procedure/ 146460 44 parallel group$1.ti,ab. 21189 45 (crossover or cross over).ti,ab. 90873 46 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 273848 47 (assigned or allocated).ti,ab. 322159 48 (controlled adj7 (study or design or trial)).ti,ab. 285465 49 (volunteer or volunteers).ti,ab. 219940 50 trial.ti. 242339 51 or/33‐50 3914529 52 32 and 51 1302 53 (2017* or 2018*).dc. 2043204 54 52 and 53 169 55 from 54 keep 1‐169 169 |
19.2.18 ‐ 169 11.2.19 ‐ 139 19.2.20 ‐ 210 08.2.21 ‐ 357 |
| CINAHL | S44 S30 AND S43 S43 S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 S42 (MH "Random Assignment") S41 (MH "Single‐Blind Studies") or (MH "Double‐Blind Studies") or (MH "Triple‐Blind Studies") S40 (MH "Crossover Design") S39 (MH "Factorial Design") S38 (MH "Placebos") S37 (MH "Clinical Trials") S36 TX "multi‐centre study" OR "multi‐center study" OR "multicentre study" OR "multicenter study" OR "multi‐site study" S35 TX crossover OR "cross‐over" S34 AB placebo* S33 TX random* S32 TX trial* S31 TX "latin square" S30 S10 AND S29 S29 (S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28) S28 TX landing zone S27 TX elephant trunk S26 TX fenestrated S25 TX branched S24 TX TEVAR S23 TX stent* S22 TX percutaneous* S21 TX (graft or endograft*) S20 TX endoprosthe* S19 TX endoluminal S18 TX endostent* S17 TX endovasc* S16 (MH "Blood Vessel Prosthesis") S15 (MH "Stents+") S14 TX rerouting S13 TX supraaortic S12 TX debranch* S11 TX hybrid S10 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 S9 TX Stanford S8 TX de Bakey S7 TX deBakey S6 TX aort* n4 trauma* S5 TX aort* n4 tear* S4 TX aort* n4 dissect* S3 TX aortic arch S2 (MH "Aorta/SU") S1 (MH "Aneurysm, Dissecting") |
19.2.18 ‐ 27 11.2.19 ‐ 50 19.2.20 ‐ 46 08.2.21 ‐ 99 |
| AMED | 1 exp Aneurysm/ 2 exp Aorta/ 3 aortic arch.ti,ab. 4 de Bakey.ti,ab. 5 deBakey.ti,ab. 6 Stanford.ti,ab. 7 (aort* adj4 dissect*).ti,ab. 8 (aort* adj4 tear*).ti,ab. 9 (aort* adj4 trauma*).ti,ab. 10 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 11 hybrid.ti,ab. 12 debranch*.ti,ab. 13 supraaortic.ti,ab. 14 rerouting.ti,ab. 15 exp Stents/ 16 Blood Vessel Prosthesis.ti,ab. 17 endovasc*.ti,ab. 18 endostent*.ti,ab. 19 endoluminal.ti,ab. 20 endoprosthe*.ti,ab. 21 (graft or endograft*).ti,ab. 22 percutaneous*.ti,ab. 23 stent*.ti,ab. 24 TEVAR.ti,ab. 25 branched.ti,ab. 26 fenestrated.ti,ab. 27 elephant trunk.ti,ab. 28 landing zone.ti,ab. 29 or/11‐28 30 10 and 29 |
19.2.18 ‐ 0 11.2.19 ‐ 0 19.2.20 ‐ 0 08.2.21 ‐ 0 |
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| NCT00526487 | Did not meet review indication (descending thoracic dissection) |
| NCT00583817 | Did not meet review intervention (endovascular) |
| NCT01107366 | Study withdrawn. The study was never started as was unable to obtain ethical approval. |
| NCT01500395 | Did not meet review study design (registry) |
| NCT01568320 | Did not meet review indication (patients with acute complicated type B aortic dissections) |
| NCT01704391 | Did not meet review indication (abdominal aortic aneurysm) |
| NCT02094300 | Did not meet review indication (Stanford type B, deBakey type III) |
| NCT02201589 | Did not meet review intervention (endovascular) |
| NCT02464943 | Did not meet review indication (patients with acute, complicated type B aortic dissection) |
| NCT02724072 | Did not meet review study design (single arm study) |
| NCT03322033 | Did not meet review intervention (TEVAR) |
| Tsukui 2002 | Specific population cannot be extracted (mixture of lesion type and extent) |
TEVAR: thoracic endovascular aortic repair
Characteristics of ongoing studies [ordered by study ID]
ChiCTR‐IPR‐16009372.
| Study name | Therapeutic strategy of aortic arch for acute type A aortic dissection |
| Methods | Randomised parallel controlled |
| Participants | Participants diagnosed with type A aortic dissection by CTA imaging, onset time less than 2 weeks Age minimum: 18; age maximum: 70 Gender: both |
| Interventions | TAR group: total arch replacement ARP group: arch reserved procedure Hybrid group: hybrid procedure TBSG group: triple‐branched stent graft |
| Outcomes | Primary outcomes: postoperative 30‐day mortality; postoperative 30‐day major adverse cardiocerebral events (MACCE); postoperative 1‐year mortality; postoperative 1‐year major adverse cardiocerebral events (MACCE) Secondary outcomes: acute kidney injury; renal replacement therapy; hypoxaemia; liver failure; paraplegia; mediastinal infection; reopening for bleeding; ICU length of stay; postoperative hospital length of stay; hospitalisation expense; follow‐up CTA imaging |
| Starting date | 11 January 2017 |
| Contact information | Name: Song Xue Address: 160 Pujian Road, Shanghai 200127 Telephone: +86 13501754558 Email: xuesong64@163.com Affiliation: Renji Hospital, School of Medicine, Shanghai Jiao Tong University, CHINA |
| Notes | The study investigators have been contacted for clarification, and possible results. No response has been received |
ChiCTR‐TRC‐11001828.
| Study name | The contrast of the outcome between replacing ascending aorta + reconstructing aortic arch with triple‐branched stent graft and replacing ascending aorta + replacing half aortic arch to treat the aortic dissection (the contrast of the outcome of the two different operational methods to treat the aortic dissection) |
| Methods | Randomised parallel controlled trial |
| Participants | Participants diagnosed with aortic dissection; type A; onset time < 2 weeks |
| Interventions | Test group: place triple‐branched stent graft into aortic arch to reconstruct |
| Outcomes | Primary outcomes: operation time; length of stay; aortic angiography CT |
| Starting date | 01 February 2009 |
| Contact information | Name: Chen Liang‐Wan Address: 29 Xinquan Road, Fuzhou, Fujian 350001 Telephone: +86 13358255333 Email: chenliangwan@tom.com Affiliation: The Affiliated Union Hospital, Fujian Medical University, CHINA |
| Notes | The study investigators have been contacted for clarification, and possible results. No response has been received |
ChiCTR‐TRC‐13003857.
| Study name | Evaluate the safety and efficacy of Xuper open surgery stent graft system for the surgical of type A aortic dissection: a prospective, multi‐center clinical trial |
| Methods | Randomised parallel controlled trial |
| Participants | Inclusion criteria
Exclusion criteria:
Age minimum: 18 Age maximum: 65 Gender: Both |
| Interventions | Test group: Xuper Open Surgery Stent Graft System (Lifetech Scientific, Shenzhen, China) Control group: open surgical repair using the Intergard artificial graft (Getinge AB, Stockholm, Sweden) |
| Outcomes | Primary outcomes: duration of circulatory arrest Secondary outcomes: incidence of major adverse events (death, paraplegia, brain complications); stent implantation successful (stent in place and successfully released); operation time; cardiopulmonary bypass time; arterial anastomosis time; aortic occlusion time; intraoperative blood loss and blood transfusion volume; treatment success (12 months after operation) |
| Starting date | 31 May 2013 |
| Contact information | Name: Zhiyun Xu
Address: 168 Changhai Road, Yangpu District, Shanghai 200433
Telephone: +21 81871114
Email: zhiyunx@hotmail.com
Affiliation: Changhai Hospital of Second Military Medical University Name: Xiangman Zhang Address: Cybio Electronic Building, Langshan 2nd Street, North Area of High‐tech Park, Nanshan District, Shenzhen, Guangdong, CHINA 518057 Telephone: +86 13817024547 Email: zhangxiangman@lifetechmed.com Affiliation: Lifetech Scientific (Shenzhen) Co.Ltd |
| Notes | The study investigators have been contacted for clarification, and possible results. No response has been received |
ARP: arch reserved procedure; CT: Computed tomography; CTA: Computed tomography angiography; MACCE: major adverse cardiocerebral events; TAR: total arch replacement; TBSG: triple‐branched stent graft
Differences between protocol and review
There are no differences between the protocol and review.
Contributions of authors
EPK: acquiring trial reports, trial selection, data extraction, data analysis, data interpretation, and review drafting, SS: review drafting FJ: data interpretation AE: acquiring trial reports, trial selection, and data extraction DD: data interpretation DV: review drafting NH: trial selection, data interpretation, review drafting, future review updates, and guarantor of the review
Sources of support
Internal sources
No sources of support provided
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK
The Cochrane Vascular editorial base is supported by the Chief Scientist Office.
Declarations of interest
EPK: none known. SS: SS and his institution have received payment from Gore Medical for training physicians on endovascular aortic repair. SS is the Principal Investigator in the INSIGHT post Market Surveillance trial of the INCRAFT abdominal aortic endograft (Cordis/Cardinal health). He reports he has no conflict of interest which will affect this review. FJ: Institution received funding from the Health Research Board (Ireland) for a Cochrane Training Fellowship to enable me to undertake a Cochrane Systematic Review over 24 months. This training grant provides me with funding to attend Cochrane Training Programmes/conferences over the two year period of my fellowship. AE: has received funding from Health Research Board (Ireland) under the HRB Cochrane Ireland Fellowship Scheme to undertake a Cochrane Systematic Review (Elhelali 2021) (Grant number CTF‐2016‐1863). DD: none known. DV: none known. NH: has received payment for consultation on Regulatory Documents (Versono Ltd and Integer) and for working on medical device design at Boston Scientific (Enterprise Ireland Bioinnovate Fellow). Her institution has received payment for provision of training on endovascular aortic repair from Gore Medical. She is investigator in the INSIGHT Post Market Surveillance Trial of the Incraft AAA device (Cordis/Cardinal Health). Her Institution has received payment for an Aortic Fellowship grant (Jotec/Cryolife), and Research fellowship grants (Gore Medical and Medtronic). She declares no competing interests, relationships, conditions or circumstances, which will conflict with this review.
New
References
References to studies excluded from this review
NCT00526487 {published data only}
- NCT00526487. Clinical study to assess safety and effectiveness of the Zenith® dissection endovascular system in patients with aortic dissection. clinicaltrials.gov/ct2/show/NCT00526487 (first received 10 September 2007).
NCT00583817 {published data only}
- NCT00583817. Endovascular exclusion of ascending and thoracic aortic pathology. clinicaltrials.gov/ct2/show/NCT00583817 (first received 31 December 2007).
NCT01107366 {published data only}
- NCT01107366. ATLANTIS: extensive type A dissections and thoracic/ thoraco-abdominal aneurysms repair with LupiAe Hybrid TechNique (ATLANTIS). clinicaltrials.gov/ct2/show/NCT01107366 (first received 21 April 2010).
NCT01500395 {published data only}
- NCT01500395. Hybrid operation in thoracic aortic dissection. clinicaltrials.gov/ct2/show/NCT01500395 (first received 28 December 2011).
NCT01568320 {published data only}
- NCT01568320. Zenith® dissection clinical trial. clinicaltrials.gov/ct2/show/NCT01568320 (first received 2 April 2012).
NCT01704391 {published data only}
- NCT01704391. Haemodynamic response to aortic surgery. clinicaltrials.gov/ct2/show/NCT01704391 (first received 11 October 2012).
NCT02094300 {published data only}
- NCT02094300. Zenith® dissection endovascular system (STABLE I). clinicaltrials.gov/ct2/show/NCT02094300 (first received 14 September 2015).
NCT02201589 {published data only}
- NCT02201589. Feasibility of endovascular repair of ascending aortic pathologies. clinicaltrials.gov/ct2/show/NCT02201589 (first received 28 July 2014).
NCT02464943 {published data only}
- NCT02464943. Zenith® dissection endovascular system in the treatment of patients with aortic dissections. clinicaltrials.gov/ct2/show/NCT02464943 (first received 8 June 2015).
NCT02724072 {published data only}
- NCT02724072. Thoraflex™ Hybrid IDE Study. clinicaltrials.gov/ct2/show/NCT02724072 (first received 31 March 2016).
NCT03322033 {published data only}
- NCT03322033. Feasibility of endovascular repair of ascending aortic pathologies (PS-IDE). clinicaltrials.gov/ct2/show/NCT03322033 (first received 26 October 2017).
Tsukui 2002 {published data only}
- Tsukui H, Aomi S, Tomioka H, Nonoyama M, Koyanagi H, Nagasawa C, et al. Arch-first technique for aortic arch operation using branched graft. Asian Cardiovascular and Thoracic Annals 2002;10(4):318-21. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ChiCTR‐IPR‐16009372 {published data only (unpublished sought but not used)}
- ChiCTR-IPR-16009372. Therapeutic strategy of aortic arch for acute type A aortic dissection. apps.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR-IPR-16009372 (first received 11 October 2016).
ChiCTR‐TRC‐11001828 {published data only (unpublished sought but not used)}
- ChiCTR-TRC-11001828. The contrast of the outcome between replacing ascending aorta + reconstructing aortic arch with triple-branched stent graft and replacing ascending aorta + replacing half aortic arch to treat the aortic dissection (the contrast of the outcome of the two different operational methods to treat the aortic dissection). apps.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR-TRC-11001828 (first received 27 July 2015).
ChiCTR‐TRC‐13003857 {published data only (unpublished sought but not used)}
- ChiCTR-TRC-13003857. Evaluate the safety and efficacy of Xuper open surgery stent graft system for the surgical of type A aortic dissection: a prospective, multi-center clinical trial. apps.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR-TRC-13003857 (first received 31 May 2016).
Additional references
Antoniou 2010
- Antoniou GA, El Sakka K, Hamady M, Wolfe JH. Hybrid treatment of complex aortic arch disease with supra-aortic debranching and endovascular stent graft repair. European Journal of Vascular and Endovascular Surgery 2010;39(6):683-90. [DOI] [PubMed] [Google Scholar]
Bellomo 2004
- Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P Acute Dialysis Quality Initiative Workgroup. Acute renal failure: definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Critical Care 2004;8(4):R204-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cao 2012
- Cao P, De Rango P, Czerny M, Evangelista A, Fattori R, Nienaber C, et al. Systematic review of clinical outcomes in hybrid procedures for aortic arch dissections and other arch diseases. Journal of Thoracic and Cardiovascular Surgery 2012;144(6):1286-300. [DOI] [PubMed] [Google Scholar]
Cochennec 2013
- Cochennec F, Tresson P, Cross J, Desgranges P, Allaire E, Becquemin JP. Hybrid repair of aortic arch dissections. Journal of Vascular Surgery 2013;57(6):1560-7. [DOI] [PubMed] [Google Scholar]
Czerny 2019
- Czerny M, Schmidli J, Adler S, Van den Berg JC, Bertoglio L, Carrel T, et al. Editor’s choice - Current options and recommendations for the treatment of thoracic aortic pathologies involving the aortic arch: an expert consensus document of the European Association for Cardio-Thoracic Surgery (EACTS) & the European Society for Vascular Surgery (ESVS). European Journal of Vascular and Endovascular Surgery 2019;57(2):165-98. [DOI: 10.1016/j.ejvs.2018.09.016] [PMID: ] [DOI] [PubMed] [Google Scholar]
Daily 1970
- Daily PO, Trueblood HW , Stinson EB, Wuerflein RD, Shumway NE. Management of acute aortic dissections. Annals of Thoracic Surgery 1970;10(3):237–47. [DOI] [PubMed] [Google Scholar]
Dake 2013
- Dake MD, Thompson M, Van Sambeek M, Vermassen F, Morales JP, DEFINE Investigators. DISSECT: a new mnemonic-based approach to the categorization of aortic dissection. European Journal of Vascular and Endovascular Surgery 2013;46(2):175-90. [DOI] [PubMed] [Google Scholar]
DeBakey 1966
- DeBakey ME, Beall AC Jr, Cooley DA, Crawford ES, Morris GC Jr, Garrett HE, et al. Dissecting aneurysms of the aorta. Surgical Clinics of North America 1966;46(4):1045-55. [DOI] [PubMed] [Google Scholar]
Elhelali 2021
- Elhelali A, Hynes N, Devane D, Sultan S, Kavanagh EP, Morris L, et al. Hybrid repair versus conventional open repair for thoracic aortic arch aneurysms. Cochrane Database of Systematic Reviews 2021, Issue 6. Art. No: CD012923. [DOI: 10.1002/14651858.CD012923.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Estrera 2003
- Estrera AL, Garami Z, Miller CC 3rd, Sheinbaum R, Huynh TT, Porat EE, et al. Determination of cerebral blood flow dynamics during retrograde cerebral perfusion using power M-mode transcranial Doppler. Annals of Thoracic Surgery 2003;76(3):704–10. [DOI] [PubMed] [Google Scholar]
Fillinger 2010
- Fillinger MF, Greenberg RK, McKinsey JF, Chaikof EL. Society for Vascular Surgery Ad Hoc Committee on TEVAR Reporting Standards. Reporting standards for thoracic endovascular aortic repair (TEVAR). Journal of Vascular Surgery 2010;52(4):1022-33. [DOI] [PubMed] [Google Scholar]
GRADEproGDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 4 January 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available from gradepro.org.
GRADE Working Group 2004
- GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Groysman 2011
- Groysman LI, Emanuel BA, Kim-Tenser MA, Sung GY, Mack WJ. Therapeutic hypothermia in acute ischemic stroke. Neurosurgical Focus 2011;30(6):E17. [DOI] [PubMed] [Google Scholar]
Guyatt 2008a
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schünemann HJ. What is 'quality of evidence' and why is it important to clinicians? BMJ 2008;336(7651):995-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008b
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hagan 2000
- Hagan PG, Nienaber CA, Isselbacher EM, Bruckman D, Karavite DJ, Russman PL, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA 2000;283(7):897-903. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
IMPROVE 2015
- IMPROVE Trial Investigators. Endovascular strategy or open repair for ruptured abdominal aortic aneurysm: one-year outcomes from the IMPROVE randomized trial. European Heart Journal 2015;36(31):2061-9. [DOI: 10.1093/eurheartj/ehv125.] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
IRAD
- International Registry of Acute Aortic Dissection. www.iradonline.org/.
Jakob 2011
- Jakob H, Tsagakis K, Pacini D, Di Bartolomeo R, Mestres C, Mohr F. The International E-vita Open Registry: data sets of 274 patients. Journal of Cardiovascular Surgery (Torino) 2011;52(5):717-23. [PubMed] [Google Scholar]
Kamiya 2007
- Kamiya H, Hagl C, Kropivnitskaya I, Böthig D, Kallenbach K, Khaladj N, et al. The safety of moderate hypothermic lower body circulatory arrest with selective cerebral perfusion: a propensity score analysis. Journal of Thoracic and Cardiovascular Surgery 2007;133(2):501-9. [DOI] [PubMed] [Google Scholar]
Kurimoto 2015
- Kurimoto Y, Maruyama R, Ujihira K, Nishioka N, Hasegawa K, Iba Y, et al. Thoracic endovascular aortic repair for challenging aortic arch diseases using fenestrated stent grafts from zone 0. Annals of Thoracic Surgery 2015;100(1):24-33. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLOS Medicine 2009;6(7):e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lu 2013
- Lu Q, Feng J, Zhou J, Zhao Z, Bao J, Feng R, et al. Endovascular repair of ascending aortic dissection: a novel treatment option for patients judged unfit for direct surgical repair. Journal of the American College of Cardiology 2013;61(18):1917-24. [DOI] [PubMed] [Google Scholar]
Moon 2011
- Moon MC, Greenberg RK, Morales JP, Martin Z, Lu Q, Dowdall JF, et al. Computed tomography-based anatomic characterization of proximal aortic dissection with consideration for endovascular candidacy. Journal of Vascular Surgery 2011;53(4):942-9. [DOI] [PubMed] [Google Scholar]
Moulakakis 2013
- Moulakakis KG, Mylonas SN, Markatis F, Kotsis T, Kakisis J, Liapis CD. A systematic review and meta-analysis of hybrid aortic arch replacement. Annals of Cardiothoracic Surgery 2013;2(3):247-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Murphy 2012
- Murphy EH, Stanley GA, Ilves M, Knowles M, Dimaio JM, Jessen ME, et al. Thoracic endovascular repair (TEVAR) in the management of aortic arch pathology. Annals of Vascular Surgery 2012;26(1):55-66. [DOI] [PubMed] [Google Scholar]
Murphy‐Ryan 2010
- Murphy-Ryan M, Psychogios A, Lindor NM. Hereditary disorders of connective tissue: a guide to the emerging differential diagnosis. Genetics in Medicine 2010;12(6):344-54. [DOI] [PubMed] [Google Scholar]
Mussa 2016
- Mussa FF, Horton JD, Moridzadeh R, Nicholson J, Trimarchi S, Eagle KA. Acute aortic dissection and intramural hematoma a systematic review. JAMA 2016;316(7):754-63. [DOI] [PubMed] [Google Scholar]
Nienaber 2004
- Nienaber CA, Fattori R, Mehta RH, Richartz BM, Evangelista A, Petzsch M, et al. Gender-related differences in acute aortic dissection. Circulation 2004;109(24):3014-21. [DOI] [PubMed] [Google Scholar]
Nienaber 2011
- Nienaber CA, Powell JT. Management of acute aortic syndromes. European Heart Journal 2011;33(1):26-35. [DOI] [PubMed] [Google Scholar]
Nordon 2012
- Nordon IM, Hinchliffe RJ, Morgan R, Loftus IM, Jahangiri M, Thompson MM. Progress in endovascular management of type A dissection. European Journal of Vascular and Endovascular Surgery 2012;44(4):406-10. [DOI] [PubMed] [Google Scholar]
O'Callaghan 2014
- O'Callaghan A, Mastracci TM, Greenberg RK, Eagleton MJ, Bena J, Kuramochi Y. Outcomes for supra-aortic branch vessel stenting in the treatment of thoracic aortic disease. Journal of Vascular Surgery 2014;60(4):914-20. [DOI] [PubMed] [Google Scholar]
Okita 2001
- Okita Y, Minatoya K, Tagusari O, Ando M, Nagatsuka K, Kitamura S. Prospective comparative study of brain protection in total aortic arch replacement: deep hypothermic circulatory arrest with retrograde cerebral perfusion or selective antegrade cerebral perfusion. Annals of Thoracic Surgery 2001;72(1):72-9. [DOI] [PubMed] [Google Scholar]
Pape 2007
- Pape LA, Tsai TT, Isselbacher EM, Oh JK, O'Gara PT, Evangelista A, et al. Aortic diameter ≥ 5.5 cm is not a good predictor of Type A aortic dissection observations from the International Registry of Acute Aortic Dissection (IRAD). Circulation 2007;116(10):1120-7. [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815-34. [DOI] [PubMed] [Google Scholar]
Patel 2011
- Patel HJ, Nguyen C, Diener AC, Passow MC, Salata D, Deeb GM. Open arch reconstruction in the endovascular era: analysis of 721 patients over 17 years. Journal of Thoracic and Cardiovascular Surgery 2011;141(6):1417-23. [DOI] [PubMed] [Google Scholar]
Rampoldi 2007
- Rampoldi V, Trimarchi S, Eagle KA, Nienaber CA, Oh JK, Bossone E, et al. Simple risk models to predict surgical mortality in acute type A aortic dissection: the International Registry of Acute Aortic Dissection score. Annals of Thoracic Surgery 2007;83(1):55-61. [DOI] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Schünemann 2006
- Schünemann HJ, Jaeschke R, Cook DJ, Bria WF, El-Solh AA, Ernst A, et al. An official ATS statement: grading the quality of evidence and strength of recommendations in ATS guidelines and recommendations. American Journal of Respiratory and Critical Care Medicine 2006;174(5):605-14. [DOI] [PubMed] [Google Scholar]
Shrestha 2014
- Shrestha M, Martens A, Behrendt S, Maeding I, Koigeldiyev N, Haverich A. Is the branched graft technique better than the en bloc technique for total aortic arch replacement? European Journal of Cardiothoracic Surgery 2014;45(1):181-6. [DOI: 10.1093/ejcts/ezt357] [PMID: ] [DOI] [PubMed] [Google Scholar]
Smith 2017
- Smith HN, Boodhwani M, Ouzounian M, Saczkowski R, Gregory AJ, Herget EJ, et al. Classification and outcomes of extended arch repair for acute Type A aortic dissection: a systematic review and meta-analysis. Interactive Cardiovascular and Thoracic Surgery 2017;24(3):450-9. [DOI] [PubMed] [Google Scholar]
Sobocinski 2011
- Sobocinski J, O'Brien N, Maurel B, Bartoli M, Goueffic Y, Sassard T, et al. Endovascular approaches to acute aortic type A dissection: a CT-based feasibility study. European Journal of Vascular and Endovascular Surgery 2011;42(4):442-7. [DOI] [PubMed] [Google Scholar]
Sun 2011
- Sun L, Qi R, Zhu J, Liu Y, Zheng J. Total arch replacement combined with stented elephant trunk implantation: a new “standard” therapy for type a dissection involving repair of the aortic arch? Circulation 2011;123(9):971-8. [DOI] [PubMed] [Google Scholar]
Suzuki 2003
- Suzuki T, Mehta RH, Ince H, Nagai R, Sakomura Y, Weber F, et al. Clinical profiles and outcomes of acute type B aortic dissection in the current era: lessons from the International Registry of Aortic Dissection (IRAD). Circulation 2003;108(10 Suppl 1):II312-7. [DOI] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8(1):1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tominaga 2003
- Tominaga R, Kurisu K, Ochiai Y, Nakashima A, Masuda M, Morita S. Total aortic arch replacement through the L-incision approach. Annals of Thoracic Surgery 2003;75(1):121-5. [DOI] [PubMed] [Google Scholar]
Tran 2009
- Tran TP, Khoynezhad A. Current management of type B aortic dissection. Vascular Health and Risk Management 2009;5(1):53-63. [PMC free article] [PubMed] [Google Scholar]
Trimarchi 2005
- Trimarchi S, Nienaber CA, Rampoldi V, Myrmel T, Suzuki T, Mehta RH, et al. Contemporary results of surgery in acute type A aortic dissection: the International Registry of Acute Aortic Dissection experience. Journal of Thoracic and Cardiovascular Surgery 2005;129(1):112-22. [DOI] [PubMed] [Google Scholar]
Trimarchi 2010
- Trimarchi S, Eagle KA, Nienaber CA, Rampoldi V, Jonker FH, De Vincentiis C, et al. Role of age in acute type A aortic dissection outcome: report from the International Registry of Acute Aortic Dissection (IRAD). Journal of Thoracic and Cardiovascular Surgery 2010;140(4):784-9. [DOI] [PubMed] [Google Scholar]
Vohra 2012
- Vohra HA, Modi A, Barlow CW, Ohri SK, Livesey SA, Tsang GM. Repair of acute type A aortic dissection: results in 100 patients. Asian Cardiovascular and Thoracic Annals 2012;20(2):160-7. [DOI] [PubMed] [Google Scholar]
Wong 2008
- Wong DR, Lemaire SA, Coselli JS. Managing dissections of the thoracic aorta. American Surgeon 2008;74(5):364-80. [PMC free article] [PubMed] [Google Scholar]
Yan 2014
- Yan TD, Tian DH, LeMaire SA, Hughes GC, Chen EP, Misfeld M, et al. Standardizing clinical end points in aortic arch surgery: a consensus statement from the International Aortic Arch Surgery Study Group. Circulation 2014;129(15):1610-6. [DOI] [PubMed] [Google Scholar]
Yang 2019
- Yang B, Norton EL, Shih T, Farhat L, Wu X, Hornsby WE, et al. Late outcomes of strategic arch resection in acute type A aortic dissection. Journal of Thoracic and Cardiovascular Surgery 2019;157(4):1313-21. [DOI: 10.1016/j.jtcvs.2018.10.139] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yu 2019
- Yu B, Liu Z, Xue C, Liu J, Yang J, Jin Z, et al. Total arch repair with open placement of a novel double-branched stent graft for acute Type A aortic dissection: a single-centre experience with 21 consecutive patients. Interactive Cardiovascular and Thoracic Surgery 2019;28(2):262-9. [DOI: 10.1093/icvts/ivy243] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Kavanagh 2018
- Kavanagh EP, Jordan F, Hynes N, Elhelali A, Devane D, Veerasingam D, et al. Hybrid repair versus conventional open repair for aortic arch dissection. Cochrane Database of Systematic Reviews 2018, Issue 1. Art. No: CD012920. [DOI: 10.1002/14651858.CD012920] [DOI] [PMC free article] [PubMed] [Google Scholar]


