Abstract
Background
Children and adolescents diagnosed with cancer are at high risk of experiencing severe side effects from cancer treatment, many of which are amenable to physical therapy. These side effects can negatively impact a child's quality of life and ability to participate in daily activities (e.g. play and attendance at school). Researchers have evaluated physical therapy interventions in children with cancer and childhood cancer survivors. However, factors such as small sample sizes, varying intervention protocols and differences in cancer types among trials make it difficult to draw conclusions about efficacy.
Objectives
The primary aim of this review was to evaluate the efficacy of physical therapy interventions ‐ with a specific focus on symptom relief and compensation of therapy‐related side effects ‐ on the quality of life of children and adolescents diagnosed with cancer. Participants must be between the ages of 0 and 19 years at the time of the physical therapy intervention study. The intervention may occur prior to, during or following cancer treatment. The intervention must be compared to a control group of children receiving standard care, no physical therapy intervention or a comparison intervention. We have excluded general physical exercise studies where the primary aim was to improve physical fitness through aerobic, anaerobic, resistance exercise or combined physical exercise training regimens (i.e. combined aerobic and resistance exercise regimens). We have also intended to record the occurrence of any adverse effects resulting from physical therapy interventions.
The secondary aims were to evaluate the efficacy of physical therapy on impairments of pain, peripheral neuropathy, balance, gait, functional abilities and mobility, motor function and performance, range of motion, strength and fatigue.
Search methods
We searched CENTRAL, MEDLINE, Embase, CINAHL, PEDro, ongoing trial registries, conference proceedings and the reference lists of relevant studies and reviews in March 2020. We also contacted oncology rehabilitation researchers working in paediatrics in March 2020 to identify additional studies.
Selection criteria
The review included randomised controlled trials (RCTs), cross‐over trials, and controlled clinical trials (CCTs) that compared the effects of physical therapy interventions to a control group, and involved children and adolescents diagnosed with cancer between the ages of 0 and 19 years at the time of the intervention. We excluded studies examining general physical exercise interventions where the primary aim was to improve physical fitness through aerobic exercise, resistance exercise or combined physical exercise training regimens (i.e. combined aerobic and resistance exercise regimens).
Data collection and analysis
We used standard methodological procedures expected by Cochrane.
Main results
We found no RCTs, cross‐over trials or CCTs comparing the effects of physical therapy interventions with a focus on symptom relief and compensation of therapy‐related side effects for children and adolescents between the ages of 0 and 19 years.
Authors' conclusions
Results demonstrate that the evidence to date is inadequate to inform clinical practice. Recommendations for future research include the need for large‐scale, high‐quality designs that examine: (1) paediatric populations with same cancer types; (2) similar intervention protocols; (3) long‐term outcomes; (4) physical therapy interventions (e.g. electrophysical modalities and sensory interventions); and (5) outcomes commonly impaired in children with cancer (e.g. peripheral neuropathy and gait deficits).
Plain language summary
What are the effects of physical therapy interventions before, during, and after childhood cancer treatment?
Review question
We reviewed the evidence on the effects of physical therapy interventions, other than general physical exercise interventions, on the quality of life of children and adolescents diagnosed with cancer compared to a control group of children receiving standard care, no physical therapy intervention or a comparison intervention. We also reviewed the occurrence of any harms (adverse effects) resulting from the physical therapy interventions. For the purpose of this review, physical therapy interventions of interest had to have a focus on symptom relief or address therapy‐related side effects (symptoms and impairments). We excluded studies examining general physical exercise interventions where the primary aim was to improve physical fitness through aerobic exercise, resistance exercise or combined physical exercise training regimens (i.e. combined aerobic and resistance exercise regimens).
Background
Children and adolescents with cancer often have side effects from cancer and its treatments. These side effects can negatively impact a child's quality of life and ability to participate in daily activities such as play. Researchers have carried out studies that examine physical therapy interventions in children with cancer. However, the benefits of physical therapy are unclear.
Study characteristics
The evidence is current to March 2020. We did not identify any eligible studies.
Key results
We did not identify any studies that examined on the effects of physical therapy interventions on the quality of life of children and adolescents diagnosed with cancer, compared to a control group of children receiving standard care, no physical therapy intervention or a comparison intervention. Thus, no conclusions can be made. Our results show that further research is needed examining the effects of physical therapy interventions in children and adolescents with cancer.
Summary of findings
Summary of findings 1. Physical Therapy compared to standard care, no physical therapy intervention or a comparison intervention in children and adolescents before, during and following cancer treatment.
| Physical therapy compared to usual care in children and adolescents before, during and following cancer treatment | |
| Patient or population: children and adolescents before, during and following cancer treatment Setting: any Intervention: physical therapy Comparison: standard care, no physical therapy intervention or a comparison intervention. | |
| Outcomes | Comments |
| Quality of Life | No studies included |
| Fatigue | No studies included |
| Pain | No studies included |
| Balance | No studies included |
| Range of motion | No studies included |
| Strength | No studies included |
| Adverse events | No studies included |
Background
Description of the condition
It is estimated that, globally, around 300,000 children and adolescents between the ages of 0 and 19 years will be diagnosed with cancer each year (Kids Cancer Care 2020; WHO 2020). Over 175,000 (70%) will be children under the age of 15 years (Ward 2014). Progress in cancer treatments has resulted in improved survival rates for children and adolescents with cancer, now approaching or exceeding 80% for five‐year post‐diagnosis survival (Gatta 2014; Noone 2018). Thus, there is an increased awareness of the need for survivorship care plans, including medical follow‐up and surveillance for long‐term effects of cancer treatment (Buckner 2014; CCS 2020; Robison 2009).
Two‐thirds of children who have been diagnosed with cancer will also develop at least one chronic or long‐term side effect after the cancer treatment (CCS 2020; Skinner 2012). Long‐term and late effects are expected health complications resulting from the cancer or cancer therapy (chemotherapy, radiation therapy, surgery and stem cell transplant), that never resolve or emerge months or years following treatment completion, and impact overall health and quality of life (Green 2012). These effects include impairments such as pain, fatigue, weakness, peripheral neuropathy, limitations in range of motion, and deficits in balance and gait (Baggott 2009; Beulertz 2016b; Gilchrist 2016; Gilchrist 2018; Hartman 2008; Ness 2013; Robison 2009; Skinner 2012; Van Cleve 2012). All of these may negatively affect a child’s overall function, quality of life and ability to participate in age‐appropriate activities including play (Moody 2006; Pruitt 2009).
Cancer treatments can negatively impact the major body systems including musculoskeletal, cardiorespiratory, and neurological systems (Pruitt 2009). The risk of long‐term side effects are dependent on the tumour type and tumour‐related factors (e.g. location within the body and extent of the cancer); the type of cancer treatment administered (e.g. type of surgery, chemotherapy type and dosage, radiation therapy type, location and dosage); as well as patient‐related factors (e.g. the child’s gender, age, overall health pre‐cancer diagnosis and developmental stage at time of diagnosis) (Pruitt 2009; NCI 2020). The focus of this review was on the outcomes associated with physical interventions for musculoskeletal and neurological effects of cancer and cancer treatment.
Musculoskeletal System
Specific long‐term effects from cancer treatment on the musculoskeletal system include effects on muscle and soft tissues (myopathies including proximal muscle weakness, soft tissue contracture and radiation fibrosis syndrome), as well as effects on bone resulting in scoliosis or kyphosis, limb length discrepancies and osteoporosis (NCI 2020; Pruitt 2009). The impact of surgery such as amputation and limb‐salvage intervention may result in chronic pain, gait and balance dysfunction, and impact overall activity (Krivitzky 2015; Pruitt 2009). Effects involving the musculoskeletal system are more likely to occur in cancers such as acute lymphoblastic leukaemia, osteosarcoma, and brain and spinal cord tumours; and in those children who have undergone a stem cell transplant (NCI 2020; Pruitt 2009).
Neurological System
Specific long‐term effects involving the neurological system include motor and sensory deficits (loss of fine motor skills, impairments in coordination and balance, movement disorders and peripheral nerve damage in the hands and feet) (Krivitzky 2015; NCI 2020; Pruitt 2009). Chronic peripheral neuropathy, a condition that may result from the use of neurotoxic agents such as vincristine and cisplatin, is a common long‐term effect experienced by both childhood and especially adult survivors (Kandula 2016; Pruitt 2009; Wickham 2007).
Description of the intervention
Focused physical therapy interventions may help children with the late and long‐term physical effects resulting from cancer treatments, particularly prolonged cancer treatments (Stubblefield 2013). Physical therapists aim to restore and optimise function, mobility and quality of life of individuals of all ages (Punzalan 2009). In oncology rehabilitation, physical therapists work with individuals to manage musculoskeletal and neuromuscular impairments (Punzalan 2009). Rehabilitation for cancer patients include treatments to address acute, late and long‐term effects, as well as those associated with palliative care (Punzalan 2009), with the ultimate aim of improving quality of life.
Physical therapists perform assessments to determine physical function, joint mobility, muscle strength and flexibility. Findings of the assessment are used to inform an appropriate, tailored intervention for the child (Punzalan 2009). Physical therapy for children with cancer aims to regain function through interventions to reduce pain in soft tissues (muscles, tendons and ligaments), increase muscle strength, improve soft tissue and joint flexibility, range of motion and functional mobility. Treatment can be delivered before (prehabilitation), during and after cancer treatment completion (rehabilitation) (Krivitzky 2015). Prehabilitation services include interventions that are administered between the time of diagnosis and cancer treatment initiation. Prehabilitation interventions may be prescribed to enhance a child’s physical functioning and general health status to enable improved tolerance to cancer treatments, overall outcomes and recovery from the prospective cancer treatment. Rehabilitation services delivered during or following cancer treatment are defined as services to decrease adverse effects of treatment and to enhance recovery of functional abilities. Importantly, focused and timely physical therapy intervention may help to prevent the development of late effects and attenuate the severity of long‐term effects (Krivitzky 2015).
The interventions considered in this review included physical therapy techniques such as manual therapy, therapeutic range of motion and strengthening exercises for a joint or muscle region, balance retraining, gait re‐education and electrophysical modalities provided with the aim of addressing impairments related to cancer treatment to ultimately improve quality of life. For this review, physical therapy could have been delivered as prehabilitation or rehabilitation interventions. The children and adolescents participating in the included studies needed to have been between 0 and 19 years old at the time of receipt of the physical therapy intervention in the studies.
Why it is important to do this review
To date, the majority of research trials in cancer rehabilitation have involved adult cancer survivors. Researchers have reported positive results from physical therapy interventions, primarily in the area of breast cancer (Cho 2016; De Groef 2015; McNeely 2010; Nilsen 2015; Pergolotti 2015).
Impairment‐based cancer rehabilitation for children and adolescents with cancer is a growing area of research and clinical practice. Researchers have evaluated physical therapy interventions in children with cancer and childhood cancer survivors. However, factors such as small sample sizes, varying intervention protocols and differences in cancer types among trials make it difficult to draw conclusions about efficacy.
A recent Cochrane Review examined the effects of general exercise training interventions for children and adolescents with cancer (Braam 2016). The review included six studies involving 171 participants, all of whom were being treated for acute lymphoblastic leukaemia. Preliminary findings support benefit from general physical exercise training for body composition, flexibility and cardiorespiratory fitness. To date, however, no systematic reviews have been performed examining the benefits of physical therapy interventions for specific impairments related to cancer treatment. Thus, the main distinctions between this review and that of Braam 2016 were: (1) the type of intervention (physical therapy versus general physical exercise); and (2) the focus of the intervention (amelioration of specific iatrogenic impairments versus physical fitness).
Objectives
Primary objective
To evaluate the efficacy of physical therapy interventions ‐ with a specific focus on symptom relief and compensation of therapy‐related side effects ‐ on the quality of life of children and adolescents who have been diagnosed with cancer. Participants must be between the ages of 0 and 19 years at the time of the physical therapy intervention study. The intervention may occur prior to, during or following cancer treatment. The intervention must be compared to a control group of children receiving standard care, no physical therapy intervention or a comparison intervention. We have excluded general physical exercise studies where the primary aim was to improve physical fitness through aerobic, anaerobic, resistance exercise or combined physical exercise training regimens (i.e. combined aerobic and resistance exercise regimens). We have also intended to record the occurrence of any adverse effects resulting from physical therapy interventions.
Secondary objectives
To evaluate the efficacy of physical therapy interventions on pain, peripheral neuropathy, balance, gait, functional abilities and mobility, motor function and performance, range of motion, strength and fatigue.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs), cross‐over trials (when data were available prior to the cross‐over), and controlled clinical trials (CCTs) examining the effects of physical therapy interventions for children and adolescents between the ages of 0 and 19 years.
Types of participants
Children and adolescents aged 0 to 19 years at the time of the physical therapy intervention. All childhood cancer types were eligible for inclusion in the review. We considered studies that also included adults (20 years old or more) with cancer only if the results of the subgroup of children with cancer (0 to 19 years of age) were available or reported separately.
Types of interventions
We included studies comparing physical therapy interventions (such as manual therapy techniques, therapeutic range of motion and strengthening for a specific joint or impaired body region, balance and gait retraining), and electrophysical modalities to address a specific symptom (e.g. pain), impairment (e.g. gait dysfunction) or body region (e.g. shoulder). For the purpose of this review, physical therapy interventions of interest had to have a focus on symptom relief and compensation of therapy‐related side effects. The intervention may have been delivered before (prehabilitation), during or following cancer treatment. The intervention had to be compared to a control group receiving standard care, no intervention or a comparison intervention (assuming the effect of the physical therapy intervention can be isolated).
The physical therapy intervention had to be delivered or supervised by a physical therapist or healthcare professional (e.g. nurse or occupational therapist). The programme could have been offered as an individualised treatment or a group intervention, and performed in any setting or location (e.g. hospital, outpatient hospital or physical therapy clinic, home, or elsewhere). The duration of the physical therapy intervention period had to be at least four weeks. The time spent per physical therapy session had to be reported or sufficiently described, and delivery of the intervention had to last at least 15 minutes (Hanson 2015; Ospina 2019).
Exclusion criteria
Studies where the primary focus of the intervention was aerobic capacity or general physical fitness alone.
Studies where the intervention comprised a general exercise or physical activity prescription, described in terms of frequency, intensity, type and time.
Types of outcome measures
Primary and secondary outcomes listed below were not used as criteria for including studies, but rather as a list of outcomes of interest within the included studies.
Primary outcomes
The primary outcomes of this review were quality of life and adverse events.
Quality of life: measured by scales such as the Pediatric Quality of Life Inventory (PedsQL), Pediatric Quality of Life (PedsQL Core), Child Health Questionnaire (CHQ), and DISABKIDS or other validated questionnaires.
Adverse events related to the physical therapy intervention such as falls, fractures, soft tissue injuries, or any worsening of impairments (e.g. pain) requiring withdrawal from the study.
Secondary outcomes
Secondary outcomes of the review are as follows.
Pain measured by Visual Analog Scale (VAS), or other valid instruments.
Peripheral neuropathy measured by a validated scale such as the Pediatric Modified Total Peripheral Neuropathy Score (ped‐mTNS), Total Neuropathy Score‐Pediatric Vincristine (TNS‐PV), or Total Neuropathy Score (TNS).
Balance assessed by a validated scale such as the Bruininks Osteretsky Test of Motor Proficiency (BOTMP) Balance Subtest, Bruininks Osteretsky Test of Motor Proficiency Second Edition (BOT‐2) Balance Subtest, Pediatric Berg Balance Scale (BBS), Movement Assessment Battery for Children (Movement ABC‐2), the Flamingo Balance Test or equivalent.
Motor development and performance measured by a validated scale such as Bruininks Osteretsky Test of Motor Proficiency (BOTMP), Bruininks Osteretsky Test of Motor Proficiency Second Edition (BOT‐2), Alberta Infant Motor Scale (AIMS), Movement Assessment Battery for Children (Movement ABC‐2), Peabody Developmental Motor Scales (PDMS‐2), Miller Function and Participation Scales (MFUN‐PS), Gross Motor Function Measure (GMFM), or another valid instrument.
Functional abilities and mobility assessed by a validated scale such as the Functional Mobility Assessment (FMA) tool, Timed Up and Down Stairs (TUDS), Timed Up and Go (TUG), Functional Independence Measure for Children (WeeFIM), Pediatric Evaluation of Disability Inventory (PEDI), Vineland Adaptive Behaviour Scale, or another valid instrument.
Gait assessed descriptively or by use of a computerised or electronic gait analysis.
Fatigue assessed by a validated scale such as the PedsQL Multidimensional Fatigue Scale, Childhood Cancer Fatigue Scale (CCFS), or the Fatigue Scale for a child (FS‐C), the same scale for adolescents (FS‐A), and for parents (FS‐P), or equivalent valid instrument.
Range of motion measured by goniometry, or another valid instrument.
Strength assessed with a hand‐held dynamometer, use of a Biodex/Cybex, spring scale, lateral step‐up test, sit‐to‐stand test, up‐and‐down stairs test, minimum chair height test, incremental shuttle walking test, or another valid instrument.
Search methods for identification of studies
In 2018, Cochrane Childhood Cancer ran the searches in CENTRAL, MEDLINE and Embase. In 2020, the review authors ran all searches (CENTRAL, MEDLINE, Embase, CINAHL, PEDro, ongoing trial registries, conference proceedings, reference lists of relevant articles, and personal communication with researchers). No language restrictions were applied.
Electronic searches
We searched the following electronic databases: the Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library — we used the latest issue; MEDLINE Ovid (from 1946 to 15 March 2020); Embase Ovid (from 1947 to 15 March 2020); CINAHL/EBSCO (1937 to 15 March 2020); and Physiotherapy Evidence Database PEDro (from 1929 to 15 March 2020) (www.pedro.org.au). We modified electronic searches from the recommended Cochrane Childhood Cancer methods used in reviews (Kremer 2016).
The search strategies for the different electronic databases (using a combination of controlled vocabulary and text words) are shown in the appendices (Appendix 1; Appendix 2; Appendix 3; Appendix 4; Appendix 5).
Searching other resources
Bibliographic searching
We searched for trials not registered in CENTRAL, MEDLINE, Embase, CINAHL and PEDro, either published or unpublished, by searching the reference lists of relevant articles and review articles. We searched the five latest editions (2015‐2019) of conference proceedings of the International Society for Paediatric Oncology (SIOP), the American Society of Clinical Oncology (ASCO), the American Society of Pediatric Hematology/Oncology (ASPHO), and the Multinational Association for Supportive Care in Cancer (MASCC). We scanned the ISRCTN Registry (www.isrctn.com), the National Institutes of Health (NIH) Register (www.clinicaltrials.gov), and the World Health Organization portal (http://apps.who.int./trialsearch) for ongoing trials on 14 March 2020.
The search strategies for other resources are shown in the appendices (Appendix 6; Appendix 7).
Personal communication
We contacted oncology rehabilitation researchers working in paediatrics to verify details of any outstanding clinical trials and any relevant unpublished data or study information.
Data collection and analysis
Selection of studies
Two authors independently undertook identification of studies meeting the inclusion criteria. In most cases, we resolved discrepancies between authors by consensus. The authors resolved disagreements through discussion, or if necessary, by involving a third review author to reach consensus. We obtained any study seemingly meeting the inclusion criteria on the grounds of the title, abstract, or both, in full for closer inspection. We clearly stated details of reasons for exclusion of any study considered for review. We would have noted duplicate publications of the same study, and the study would have been counted only once.
We provided a flow diagram for the selection of studies in our review (Figure 1).
1.
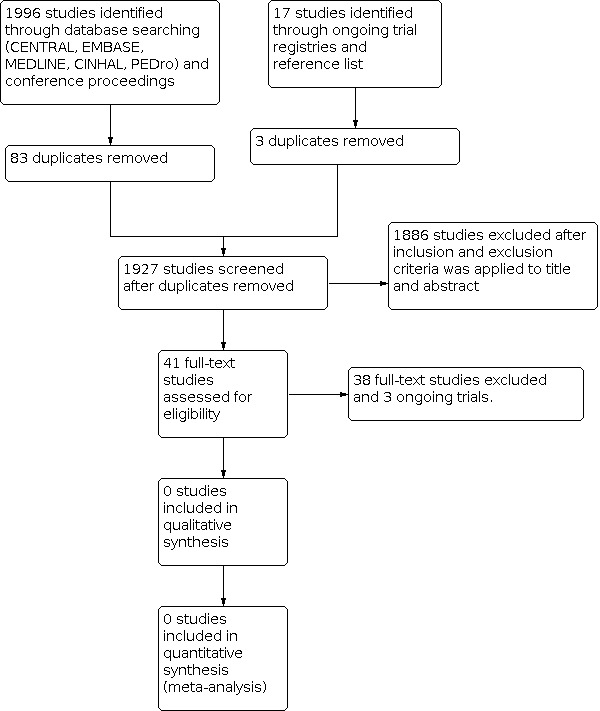
Study flow diagram
Data extraction and management
Two review authors would have extracted the characteristics for each included trial using a data extraction form. If disagreements had arisen and were not resolved by consensus, a third review author would have resolved the discrepancies. In case of missing data or relevant information, we would have contacted study authors.
Should we have had more than one publication for a study, we would have used the primary publication and referenced the other publication, if used, for supplementary information.
Items that we would have included on the data extraction form are reported in the protocol of this review (Ospina 2018).
Assessment of risk of bias in included studies
If studies had met our eligibility criteria, two review authors would have independently assessed risk of bias in the RCTs, cross‐over trials and CCTs, rating each risk of bias item as 'low', 'unclear' or 'high' risk of bias. We would have used the 'risk of bias' criteria as described in the module of Cochrane Childhood Cancer (Kremer 2016), and based on recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The two review authors would have resolved any disagreements through discussion, or if necessary, by involving a third review author to reach consensus. Further details can be found in the protocol of this review (Ospina 2018).
Measures of treatment effect
If studies had met our eligibility criteria, we would have analyzed continuous data (QoL, adverse events, fatigue, pain, gait, peripheral neuropathy, balance, range of motion, strength) as mean differences either weighted or standardised using a random‐effects model. We would use difference in means (MD) for continuous variables when data are provided using the same units, measurement methods or outcome measure. We would use the standardised mean difference (SMD) for continuous variables when trials report results using different measurement units, measurement methods or outcome measures. We would analyze dichotomous outcomes (e.g. adverse event rates, outcomes reported as dichotomous variables) using risk ratio (RR). All results would be presented with corresponding 95% confidence intervals (CIs).
Unit of analysis issues
If studies had met our eligibility criteria, the only unit of analysis issue we anticipated is with cross‐over designs, in which we would use only first‐cycle data (prior to cross‐over).
Dealing with missing data
If studies had met our eligibility criteria, when information relevant to study selection, data extraction or assessment of risk of bias was missing, we would attempt to contact the authors to obtain the missing data. When applicable, we would extract data by allocated intervention, irrespective of compliance with the allocated intervention, to allow an intention‐to‐treat analysis. We would state if this is not possible and will perform an 'as treated' analysis.
Assessment of heterogeneity
If studies had met our eligibility criteria, we would assess heterogeneity by visual inspection of the forest plots and by the use of the statistical test for heterogeneity I² statistic (Higgins 2011). I² values ranging from 0% (homogeneity) to 100% (heterogeneity) will be calculated to quantify variability in study effect. An I² value greater than 50% would be considered the cutpoint for significant heterogeneity (Higgins 2011). Where possible, subgroup analyses would be performed to explore and explain heterogeneity among studies.
Assessment of reporting biases
In addition to the evaluation of reporting bias as described in the Assessment of risk of bias in included studiessection, we planned to assess reporting bias by constructing a funnel plot if there are a sufficient number of included studies (at least 10 studies included in a meta‐analysis), otherwise the power of the tests is too low to distinguish chance from real asymmetry (Higgins 2011).
To minimise the effect of publication bias, we searched the grey literature and contacted authors of trials.
Data synthesis
We did not identify any eligible studies. As a result, data analyses could not be performed. If studies had met our eligibility criteria, we would enter data of the included studies into Review Manager 5 software (Review Manager 2014) and undertake analyses according to the guidelines of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We would pool the results of studies, (1) if there are at least three studies with the same outcome measure (or measurement) for the given primary or secondary outcome; and (2) if appropriate, after consideration of heterogeneity between the trials. We will pool outcomes when sufficient data are available in the papers or from the trialists' data sets using the random‐effects model. We will describe outcomes that we cannot pool in narrative form in the Results section. We would create a 'Summary of findings' table, including post‐intervention results as well as short‐term follow‐up results (3 to 6 months after the intervention completion) and long‐term follow‐up results (1 year or more after the intervention completion).
In a multi‐arm study we would include the intervention groups as separate comparisons if each arm meets the criteria for inclusion, and would split the 'shared' control/comparison group for analyses. We would note all the intervention groups in the table of 'Characteristics of the included studies'. However, we would only describe and analyse the intervention groups relevant to the review (Higgins 2011).
Subgroup analysis and investigation of heterogeneity
If studies had met our eligibility criteria, we would have analyzed, a priori subgroup analyses would include examining the pooled effect estimate by the type of physical therapy intervention, the timing of the intervention (i.e. prior to, during, or following cancer treatment), and cancer type.
Where possible, we would perform subgroup analyses to assess if the observed effect of an intervention is consistent across participants based on subgroups of (1) age of the participant (continuous co‐variate) and (2) the location of the physical therapy intervention (inpatient hospital, outpatient clinic or home), and (3) by study design (RCT, CCT, cross‐over).
Sensitivity analysis
If studies had met our eligibility criteria, for any outcomes for which pooling was possible we would perform sensitivity analyses for 'Risk of bias' criteria separately. We would exclude studies with a high risk of bias and unclear risk of bias in the sensitivity analyses, and compare the results of studies with a low risk of bias with the results of all available studies. Sensitivity analyses would only be done when there remain at least two studies with a low risk of bias in the analyses
Summary of findings and assessment of the certainty of the evidence
For each comparison we prepared a 'Summary of findings' table using the GRADE profiler software (Guyatt 2008), in which we presented the following outcomes: QoL, fatigue, pain, balance, range of motion, strength, adverse events.
We did not identify any eligible studies. As a result, GRADE assessments could not be performed. If studies had met our eligibility criteria, For each outcome two review authors would independently assess the quality of the evidence by using the five GRADE considerations, i.e. study limitations, inconsistency, indirectness, imprecision and publication bias, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Results
Description of studies
Results of the search
We conducted searches of CENTRAL, MEDLINE, Embase, CINAHL, PEDro, ongoing trial registries, conference proceedings (SIOP, ASCO, ASPHO, and MASCC), and reference lists of relevant studies and reviews. We also contacted oncology rehabilitation researchers working in paediatrics for additional studies. These searches resulted in the retrieval of 2013 references (Figure 1).
After removal of duplicates, the search yielded 1927 potentially eligible articles (Figure 1). After initial screening of titles and abstracts, we excluded 1886 references as they did not meet eligibility criteria. We obtained, read and assessed for eligibility the full‐text versions of 41 studies. Thirty‐eight of these studies did not meet eligibility criteria (refer to Characteristics of excluded studies), and we excluded three studies as they were protocols or reported preliminary results of ongoing trials (refer to Characteristics of ongoing studies). No studies were eligible for the review (refer to Characteristics of included studies).
Included studies
We identified no trials that matched our inclusion cirteria.
Excluded studies
Reasons for exclusions can be found in Characteristics of excluded studies.
Risk of bias in included studies
We found no RCTs, cross‐over trials or CCTs comparing the effects of physical therapy interventions, with a focus on symptom relief and compensation of therapy‐related side effects, for children and adolescents between the ages of 0 and 19 years, and risk of bias assessment is thus not applicable.
Effects of interventions
See: Table 1
We found no RCTs, cross‐over trials or CCTs comparing the effects of physical therapy interventions for children and adolescents between the ages of 0 and 19 years, and thus the effects of physical therapy interventions on the quality of life of children and adolescents diagnosed with cancer remain unclear.
Discussion
Summary of main results
A growing number of studies have investigated the effects of physical therapy in adults with cancer (Cho 2016; Cormie 2017; De Groef 2015; McNeely 2010; Nilsen 2015; Pergolotti 2015). Conversely, literature examining the effects of physical therapy in childhood cancer is just beginning to emerge. Unfortunately, the vast majority of the research examining the effects of physical therapy in childhood cancer comprises lower‐quality study designs (e.g. single‐subject designs, case‐control studies) not eligible for this review (Ospina 2019). No eligible studies could be included.
Although no studies met our eligibility criteria, it is promising that further research in paediatric oncology physical therapy is underway. Our review identified three ongoing clinical trials that might be eligible for this review upon completion (Tanner 2016; Tanriverdi 2017; Xipell 2019).
Overall completeness and applicability of evidence
As demonstrated in this review, there is a paucity of high‐quality research evidence from studies examining physical therapy interventions with a focus on symptom relief and compensation of therapy‐related side effects in children and adolescents with cancer. Of note, chemotherapy‐induced peripheral neuropathy (CIPN) is one of the most common side effects in children undergoing cancer treatment, causing debilitating consequences such as balance issues, gait impairments, muscle weakness and sensory impairments. Despite the lack of research examining the effects of physical therapy for commonly occurring side effects in childhood cancer, it is promising that clinical trials are currently in progress (Weiner 2019; Wickham 2007).
Quality of the evidence
We identified no eligible studies for inclusion.
Potential biases in the review process
We developed comprehensive search strategies to cover the main databases relevant to our research question, including: CENTRAL, MEDLINE, Embase, CINAHL and PEDro. It is possible that we missed studies. However, our search results retrieved repeats of many of the same studies across databases, suggesting that a comprehensive search was performed. Also, we searched reference lists and conference proceedings to minimise the risk of missing relevant literature. Finally, we excluded studies from this review ‐ even though many incorporated a physical therapy intervention ‐ due to an overlap with the review of Braam 2016.
Agreements and disagreements with other studies or reviews
To our knowledge, this is the first systematic review specifically focused on physical therapy interventions, other than general physical exercise interventions, in children and adolescents before, during and following treatment for cancer. Other reviews have been conducted exploring general exercise interventions and non‐pharmacological interventions in childhood cancer survivors. Braam 2016 published an updated version of their previous systematic review (Braam 2013), examining the effects of physical exercise in childhood cancer. Braam 2016 included six studies (n = 171), of which five were RCTs and one was a controlled clinical trial. None of the studies included in their review ‐ De Macedo 2010, Hartman 2009, Marchese 2004, Moyer‐Mileur 2009, Tanir 2013, and Yeh 2011 ‐ met the eligibility criteria of this review either because (1) physical activity was the primary interest of these studies, or (2) the effect of the physical therapy intervention could not be isolated from the physical activity or exercise intervention. Braam 2016 concluded that overall findings of the benefits of physical exercise interventions were not convincing possibly due to the small number of participants and lack of high quality evidence, but it can also be that this type of intervention is not as effective as in adult cancer patients. However, Braam and colleagues did identify positive intervention effects for body composition, flexibility, cardiorespiratory fitness, muscle strength and health‐related quality of life (cancer‐related items). They also reported that the quality of the evidence was low, suggesting that further research is needed using larger‐scale studies.
Wacker 2017 conducted a systematic review on nonsurgical, nonpharmacologic, rehabilitation interventions on physical impairments and functional mobility limitations for children and adolescents undergoing treatment for non‐central nervous system cancers. They included a total of 22 studies in their review. No restrictions on study design were applied; thus, uncontrolled studies and case‐control studies were included. Their findings comprised a general overview of interventions in the field, but did not consider quality and risk of bias. The authors identified that the majority of the studies were focused on increasing physical activity, with few studies evaluating physiotherapeutic interventions for other outcomes.
A recent systematic review by Morales 2018 investigated RCTs that examined the effects of exercise training on physical capacity‐related endpoints, survival, disease relapse and adverse effects in children after cancer diagnosis. They included a total of eight studies (n = 283) in their review. Results demonstrated that exercise training during childhood cancer treatment significantly improved Timed Up and Down Stairs (TUDS) scores (SMD ‐0.73, P < 0.001). These findings, however, were based on pooling of data that also included exercise‐based studies not eligible for inclusion in our review.
Authors' conclusions
Implications for practice.
Results obtained in this review demonstrate that research evidence is lacking to inform clinical practice. Since research data are lacking, physical therapists should consider other approaches to exchange expertise, such as workshops, meetings, online platforms, interviews and Delphi techniques.
Implications for research.
To date, there are no data to support the safety or efficacy of physical therapy interventions for children with cancer. Many studies have focused on general exercise interventions to improve physical fitness. Recommendations for future research include the need for large‐scale, multi‐centre, high‐quality research designs (e.g. RCTs and CCTs) with adequate power to analyse results. These studies should: (1) include paediatric populations comprising the same cancer type; (2) adopt similar intervention protocols; (3) deliver physical therapy interventions including, but not limited to, gait retraining, use of electrophysical modalities, manual therapy and sensory interventions; (4) assess outcomes such as chemotherapy‐induced peripheral neuropathy (CIPN), cancer‐related fatigue, balance and gait, which are commonly impaired in children with cancer; and (5) include long‐term follow‐up assessments of physical therapy interventions.
History
Protocol first published: Issue 1, 2018
Acknowledgements
We thank FS van Etten‐Jamaludin (clinical librarian at the Medical Library of the Academic Medical Center, Amsterdam, the Netherlands) for running the searches in CENTRAL, MEDLINE and Embase on 28 February 2019. We would like to thank the Editorial Base of Cochrane Childhood Cancer for their advice and support. The Editorial Base of Cochrane Childhood Cancer has been funded by Stichting Kinderen Kankervrij (KiKa) and is located in the Princess Máxima Center for Pediatric Oncology, Utrecht, the Netherlands. We would like to acknowledge the assistance of the University of Alberta Health Sciences Librarian Liz Dennett for her assistance in developing the search strategy. We thank the peer reviewers Simon Turner, Monash University School of Public Health and Preventive Medicine, Melbourne, Australia; Miriam Götte, University Hospital Essen, Pediatric III, Essen, Germany; and P. van der Torre, Princess Máxima Center for Pediatric Oncology, Utrecht, the Netherlands. We also thank the paediatric oncology researchers who provided information on their studies by means of personal communication.
Appendices
Appendix 1. Search strategy for Central Register of Controlled Trials (CENTRAL)
For Children the following thesaurus terms and text words were used:
#1.[mh ^adolescent] or [mh child] or [mh infant]
#2.(infan* or neonat* or newborn or baby or babies or child* or schoolchild* or kid or kids or toddler* or adoles* or teen* or boy* or girl* or minor* or underag* or (under NEXT ag*) or juvenil* or youth* or kindergar* or puber* or pubescen* or prepubescen* or prepuberty* or pediatric* or paediatric* or peadiatric* or school* or preschool* or highschool* or "high school"):ti,ab,kw
#3.(pediatric* or paediatric* or child* or adolesc*):so
#4.#1 or #2 or #3
For Cancer the following thesaurus terms and text words were used:
#5.([mh Neoplasms] or (oncolog* or neoplas* or carcinom* or tumor* or tumour* or cancer* or malignan* or "hemato‐oncologic*" or hematolo* or "haemato‐oncologic*" or haematolo or bone marrow transplant* or leukemi* or leukaemi* or AML or lymphom* or hodgkin* or "T‐cell" or "B‐cell" or "non‐hodgkin" or sarcom* or Ewing* or osteosarcom* orwilms* or nephroblastom* or neuroblastom* or rhabdomyosarcom* or teratom* or hepatom* or hepatoblastom* or medulloblastom* or PNET* or retinoblastom* or meningiom* or gliom*):ti,ab,kw) not (breast*:ti or [mh "breast neoplasms"])
For Physical therapy the following thesaurus terms and text words were used:
#6.[mh "Physical Therapy Modalities"] or [mh ^"physical therapy specialty"] or [mh ^"physical and rehabilitation medicine"] or [mh "range of motion, articular"] or [mh ^gait] or [mh ^proprioception]
#7.[mh ^"postural balance"] or [mh ^"muscle stretching exercises"]
#8.[mh "ultrasonic therapy"] or [mh ^Cryotherapy]
#9.[mh ^"short‐wave therapy"]
#10.#6 or #7 or #8 or #9
#11.((exercis* NEAR/4 (therap* or strength or balance or gait or stretch*)) or (manual NEXT therap*) or (physical NEXT therap*) or physiotherap* or "stability training" or "muscle training" or "strength training" or "resistance training" or locomotion* or (functional NEXT therap*) or "weight lifting" or kinesiotherap* or manipulation* or "short‐wave‐therap*" or cryotherap* or electrotherap* or "ultraso* therap*" or (rehab* NEAR/6 physical)):ti,ab,kw
#12.#4 and #5 and #10 and #11
^: denotes a non‐exploded subject heading
mh: subject heading
[so]: Word in journal title
[tw]: text word (title and abstract)
near/6: up to 6 words can intervene between the word(s) to the left of the operator and the word(s) to the right.
*=zero or more characters
Appendix 2. Search strategy for MEDLINE Ovid
1 For Children the following MeSH headings and text words were used:
1. adolescent/ or exp child/ or exp infant/
2. (infan* or neonat* or newborn or baby or babies or child* or schoolchild* or kid or kids or toddler* or adoles* or teen* or boy* or girl* or minor* or underag* or under ag* or juvenil* or youth* or kindergar* or puber* or pubescen* or prepubescen* or prepuberty* or pediatrics or pediatric* or paediatric* or peadiatric* or school* or preschool* or highschool*).mp.
3. (pediatric* or paediatric* or child* or adolesc*).jw.
4. or/1‐3
2 For Cancer the following MeSH headings and text words were used:
1. exp Neoplasms/
2. (oncolog* or neoplas* or carcinom* or tumor* or tumour* or cancer* or malignan* or hemato‐oncological or hematolo* or bone marrow transplant* or leukemi* or leukaemi* or AML or lymphom* or hodgkin* or T‐cell or B‐cell or non‐hodgkin or sarcom* or Ewing* or osteosarcom* or wilms* or nephroblastom* or neuroblastom* or rhabdomyosarcom* or teratom* or hepatom* or hepatoblastom* or medulloblastom* or PNET* or retinoblastom* or meningiom* or gliom*).mp.
3. 1 or 2
4. breast*.ti. or exp breast neoplasms/
5. 3 not 4
3 For Physical therapy the following MeSH headings and text words were used:
1. exp Physical Therapy Modalities/
2. physical therapy specialty/
3. "physical and rehabilitation medicine"/
4. "range of motion, articular"/
5. gait/
6. proprioception/ or postural balance/
7. muscle stretching exercises/
8. short‐wave therapy/ or exp ultrasonic therapy/
9. Cryotherapy/
10. ((exercis* adj4 (therapeutic or strength or balance or gait or stretch*)) or manual therap* or physical therap* or physiotherap* or stability training or muscle training or strength training or locomotion* or functional therap* or weight lifting or kinesiotherap* or manipulation* or short‐wave‐therap* or cryotherap* or electrotherap* or ultraso* therap* or (rehab* adj6 physical)).tw,kf.
11. or/1‐10
4 For RCTs and CCTs the following MeSH headings and text words were used:
1. exp clinical trial/
2. control groups/
3. double‐blind method/
4. random allocation/
5. cross‐over studies/
6. (random* or quasi‐random* or quasi‐experiment* or cross‐over or placebo or trial or groups or double blind).mp.
7. randomized controlled trial/
8. or/1‐7
Final search: 1 and 2 and 3 and 4
/=MeSH term, *=zero or more characters
adj4, adj6, adj#= up to # words can intervene between the word(s) to the left of the operator and the word(s) to the right
[mp]= multiple places (title, abstract, subject headings, author keywords)
[tw]= text word (title and abstract)
[kf]= author keyword
[jw]= Word in journal title
The child search was based on the CCG standard search strategy for children (Leclercq 2013).
Appendix 3. Search strategy for Embase Ovid
1 For Children the following Emtree terms and text words were used:
1. adolescent/ or exp child/ or exp infant/
2. (infan* or neonat* or newborn or baby or babies or child* or schoolchild* or kid or kids or toddler* or adoles* or teen* or boy* or girl* or underag* or under ag* or juvenil* or youth* or kindergar* or puber* or pubescen* or prepubescen* or prepuberty* or pediatrics or pediatric* or paediatric* or peadiatric* or school* or preschool* or highschool*).mp.
3. (pediatric* or paediatric* or child* or adolesc*).jx.
4. or/1‐13
2 For Cancer the following Emtree terms and text words were used:
1. exp neoplasm/
2. (oncolog* or neoplas* or carcinom* or tumor* or tumour* or cancer* or malignan* or hemato‐oncological or hematolo* or bone marrow transplant* or leukemi* or leukaemi* or AML or lymphom* or hodgkin* or T‐cell or B‐cell or non‐hodgkin or sarcom* or Ewing* or osteosarcom* or wilms* or nephroblastom* or neuroblastom* or rhabdomyosarcom* or teratom* or hepatom* or hepatoblastom* or medulloblastom* or PNET* or retinoblastom* or meningiom* or gliom*).mp.
3. 1 or 2
4. breast*.ti. or exp breast tumor/
5. 3 not 4
3 For Physical therapy the following Emtree terms and text words were used:
1. exp physiotherapy/
2. physical medicine/ or electrostimulation therapy/ or exp kinesiotherapy/ or exp manipulative medicine/ or exp ultrasound therapy/
3. "range of motion"/
4. gait/
5. proprioception/
6. exp body equilibrium/
7. stretching exercise/
8. exp diathermy/
9. cryotherapy/
10. ((exercis* adj4 (therapeutic or strength or balance or gait or stretch*)) or manual therap* or physical therap* or physiotherap* or stability training or muscle training or strength training or locomotion* or functional therap* or weight lifting or kinesiotherap* or manipulation* or short‐wave‐therap* or cryotherap* or electrotherap* or ultraso* therap* or (rehab* adj6 physical)).tw,kw.
11. or/6‐15
12. adolescent/ or exp child/ or exp infant/
13. (infan* or neonat* or newborn or baby or babies or child* or schoolchild* or kid or kids or toddler* or adoles* or teen* or boy* or girl* or underag* or under ag* or juvenil* or youth* or kindergar* or puber* or pubescen* or prepubescen* or prepuberty* or pediatrics or pediatric* or paediatric* or peadiatric* or school* or preschool* or highschool*).mp.
14. (pediatric* or paediatric* or child* or adolesc*).jx.
15. or/1‐14
4 For RCTs and CCTs the following Emtree terms and text words were used:
1. exp clinical trial/
2. control groups/
3. double blind procedure/
4. randomization/
5. crossover procedure/
6. (random* or quasi‐random* or quasi‐experiment* or cross‐over or placebo or trial or groups or double blind).mp.
7. randomized controlled trial/
8. or/1‐7
Final search 1 and 2 and 3 and 4
/=Emtree term; *=zero or more characters
adj4, adj6, adj#= up to # words can intervene between the word(s) to the left of the operator and the word(s) to the right
[mp]= multiple places (title, abstract, subject headings, author keywords)
[tw]= text word (title and abstract)
[kw]= author keyword
[jx]= Word in journal title
Appendix 4. Search strategy for CINAHL/EBSCO
1 For Children the following Subject headings and text words were used:
S1. ( (MH "Child+") OR (MH "Adolescence+") ) OR ( infan* or neonat* or newborn or baby or babies or child* or schoolchild* or kid or kids or toddler* or adoles* or teen* or boy* or girl* or minor* or underag* or under ag* or juvenil* or youth* or kindergar* or puber* or pubescen* or prepubescen* or prepuberty* or pediatrics or pediatric* or paediatric* or peadiatric* or school* or preschool* or highschool* ) OR SO ( pediatric* or paediatric* or child* or adolesc* )
2 For Cancer the following Subject headings and text words will be used:
S1. (MH "Neoplasms+") OR ( oncolog* or neoplas* or carcinom* or tumor* or tumour* or cancer* or malignan* or hemato‐oncological or hematolo* or bone marrow transplant* or leukemi* or leukaemi* or AML or lymphom* or hodgkin* or T‐cell or B‐cell or non‐hodgkin or sarcom* or Ewing* or osteosarcom* or wilms* or nephroblastom* or neuroblastom* or rhabdomyosarcom* or teratom* or hepatom* or hepatoblastom* or medulloblastom* or PNET* or retinoblastom* or meningiom* or gliom* )
S2. (MH "Breast Neoplasms+") OR TI breast*
S3. S1 NOT S2
3 For Physical therapy the following Subject headings and text words were used:
S1. (MH "Physical Therapy+")
S2. (MH "Research, Physical Therapy")
S3. (MH "Proprioception+")
S4. (MH "Balance, Postural")
S5. (MH "Range of Motion")
S6. (exercis* n4 (therapeutic or strength or balance or gait or stretch*)) or manual therap* or physical therap* or physiotherap* or stability training or muscle training or strength training or locomotion* or functional therap* or weight lifting or kinesiotherap* or manipulation* or short‐wave‐therap* or cryotherap* or electrotherap* or ultraso* therap* or (rehab* n6 physical)
S7. S1 OR S2 OR S3 OR S4 OR S5 OR S6
4 For RCTs and CCTs the following MeSH headings and text words were used:
S1. ( (MH "Random Sample+") OR (MH "Control Group") OR (MH "Clinical Trials+") OR (MH "Randomized Controlled Trials") OR (MH "Crossover Design") OR (MH "Quasi‐Experimental Studies+") ) OR ( random* or quasi‐random* or quasi‐experiment* or cross‐over or placebo or trial or groups or double blind )
Final search: 1 and 2 and 3 and 4
MH= subject heading TI= title SO= journal title/source
+= denotes that the subject heading was exploded
*= zero or more characters
n4, n6 (n#) = adjacency operator where # is the maximum number of words that two terms can be separated by
Appendix 5. Search strategy for PEDro
Three different search strategies, combining the results with OR were used to search this database:
Search A
1. paediatric* <Abstract & Title> field
2. oncology <Subdiscipline> field
3. stretching, mobilisation, manipulation, massage <Therapy> field.
4. clinical trial <Method> field
Search B
1. child* <Abstract & Title> field
2. oncology <Subdiscipline> field
3. stretching, mobilisation, manipulation, massage <Therapy> field.
4. clinical trial <Method> field
Search C
1. adolescent* <Abstract & Title> field
2. oncology <Subdiscipline> field
3. stretching, mobilisation, manipulation, massage <Therapy> field.
4. clinical trial <Method> field
Appendix 6. Key terms for ongoing trials registries
1. www.isctrn.org (ISCTRN register) We browsed by "Cancer" studies, limited by "Child", and scanned results with the search terms: "physical therapy"; physiotherapy; rehabilitation.
2. www.clinicaltrials.gov(National Institutes of Health (NIH) Register for ongoing trials) We searched at the Advanced search page: Study type: Interventional studies Group: Child (birth‐17yrs) will be checked Condition: cancer OR neoplasm OR oncology Interventions: "physical therapy" OR physiotherapy OR rehab*
3. http://apps.who.int./trialsearch(WHO portal) We will search at the Advanced search page: (cancer OR oncology OR neoplasm) AND (physical therapy OR physiotherapy OR rehabilitation) Check box for clinical trials in Children
Appendix 7. Key terms for conference proceedings
ASCO (American Society of Clinical Oncology) website
We searched the meeting library (meetinglibrary.asco.org) with different search strategies combining key words.
Searches separated by semicolons:
"physical therapy" child; "physical therapy" children; "physical therapy" childhood; "physiotherapy" child; "physiotherapy" children; "physiotherapy" childhood; "rehabilitation" child; "rehabilitation" children; "rehabilitation" childhood.
MASCC (Multinational Association for Supportive Care in Cancer) website
We searched the meeting abstracts (http://www.mascc.org/past‐annual‐meetings) with different key words:
"physical therapy"; physiotherapy; rehabilitation; child; infant
The SIOP (International Society for Paediatric Oncology) and ASPHO (American Society of Pediatric Hematology/Oncology) abstracts were searched by using the "Find text" in pdf documents with the keywords: physical therapy; physiotherapy; rehabilitation
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Beulertz 2016a | Exploratory prospective study that included a general exercise intervention conducted by a sports therapist. |
| Braam 2010 | Physical fitness is the primary objective of the study. |
| Braam 2018 | A combined intervention where the effect of the physical therapy intervention could not be isolated from the physical activity‐based intervention. |
| Casanova‐Garcia 2015 | This is a research proposal and preliminary data were presented as case series. |
| Corr 2017 | A combined intervention where the effect of the physical therapy intervention could not be isolated from the physical activity‐based intervention. |
| Cox 2018 | The effects of the nursing motivation‐based intervention are not isolated from the physical therapy intervention. |
| Cunningham 1986 | Sample combined adults and children. |
| Däggelmann 2017 | Comparison group included healthy controls. |
| De Macedo 2010 | Respiratory rehabilitation intervention, did not meet criteria of our physical therapy interventions of interest. |
| Fiuza‐Luces 2017 | Physical activity‐based intervention. Both groups received physical therapy only as needed. |
| Gohar 2011 | Uncontrolled study. |
| Hartman 2009 | A combined intervention where the effect of the physical therapy intervention could not be isolated from the physical activity‐based intervention. |
| Kim 2019 | Uncontrolled study. Physical activity‐based intervention. |
| Lam 2018 | Behaviour change intervention. |
| Manchola‐Gonzalez 2019 | Physical fitness is the focus of the study. A combined intervention where the effect of the physical therapy intervention could not be isolated from the physical activity‐based intervention. |
| Marchese 2004 | A combined intervention where the effect of the physical therapy intervention could not be isolated from the physical activity‐based intervention. |
| Morales 2019 | Physical exercise intervention. |
| Moyer‐Mileur 2009 | Physical fitness is the primary objective of the study. |
| Müller 2014 | Study outcomes were primarily bone mass and physical activity. |
| Müller 2016 | Uncontrolled study. |
| Müller 2017 | Uncontrolled study. |
| Ouyang 2019 | Physical activity‐based intervention. |
| Rosenhagen 2011 | Case‐control study. Evaluates the integration of sports activity. |
| Sabel 2016 | Physical activity‐based intervention. |
| San Juan 2008 | Physical exercise intervention. Used healthy control group. |
| Speyer 2010 | Physical activity‐based intervention. |
| Tanir 2013 | Physical exercise intervention. |
| Tanner 2015 | Uncontrolled study. |
| Tanner 2017 | Uncontrolled study. |
| Tanner 2018 | A combined intervention where the effect of the physical therapy intervention could not be isolated from the physical activity‐based intervention. |
| Tomasello 2018 | Uncontrolled study. |
| Van Dijk‐Lokkart 2016 | Physical exercise intervention. |
| Wallek 2018 | Primarily physical exercise intervention. Also, it seems that this paper presents secondary analysis of a trial that may already be published or is in process. |
| Winter 2013 | Prospective, cohort study focused on physical activity. |
| Wurz 2019 | Physical activity‐based intervention. |
| Yeh 2011 | Aerobic exercise intervention. |
Characteristics of ongoing studies [ordered by study ID]
Tanner 2016.
| Study name | Impact of an Orthotic Intervention in Children with Peripheral Neuropathy (IOPN) |
| Methods | Type of study: interventional Setting: Children’s Hospitals and Clinics of Minnesota Department: Developmental and Rehabilitation Services Randomisation: randomisation through parallel assignment with each group receiving a different type of orthotic Stratification: not mentioned Timing: children undergoing treatment Study duration: 8 weeks End point measurements: measured at baseline and after study completion (8 weeks) Trial register: NCT03655587 |
| Participants | N = 50 (estimated) Diagnosis: children with a cancer diagnosis Inclusion criteria: children aged 5 to 14 years old demonstrating neuropathy (PedmTNS score > 4), with a normalised step length more than 1 standard deviation below the mean for their age and ankle dorsiflexion passive range < 10 degrees Age at start of study: 5 to 14 years old Exclusion criteria: children with lower extremity sarcomas, children with central nervous system tumours, children with neurological, developmental or genetic disorders, children with osteonecrosis, children with less than antigravity dorsiflexion strength and children with neuroblastomas |
| Interventions | The subjects were randomised between an 8‐week ankle foot orthosis (AFO) or ankle resting night spring (RNS) intervention. The orthotic was fitted with a small temperature sensor that collected data for wearing time of the brace. The solid foot ankle orthotic is made to fit the contour of the patient's foot, ankle and lower leg. The two pull solid ankle AFO is fabricated from a rigid polypropylene outer boot and a more flexible silicone inner boot. It is commonly used in rehabilitation to improve gait in paediatric and adult populations. A certified orthotist fabricated the device. The resting night splint group received an off‐the‐shelf device that provides static sagittal plane dorsiflexion. The RNS was worn nocturnally to provide maximal stretch/length to the gastrocnemius and soleus to maintain or increase dorsiflexion range of motion (ROM) and/or to prevent further regressions in ankle range. |
| Outcomes | Primary outcomes: Step length: 'GAITRite' analysis system Secondary outcomes: Ankle range of motion: passive and active ankle dorsiflexion measured by goniometry Ankle strength: ankle dorsiflexion and plantar flexion strength measured by dynamometer Gait capacity: Six‐minute walk test (6MWT) distance Foot posture: foot posture index |
| Starting date | 20 September 2016 |
| Contact information | Lynn Tanner, telephone: 612‐813‐6274 |
| Notes | Estimated study completion 30 June 2021. On 23 June 2020, no full‐text publication was available. |
Tanriverdi 2017.
| Study name | The Effect of Virtual Reality Exercises on Balance in Children with Brain Tumours |
| Methods | Type of study: interventional Setting: Bezmialem Vakif University Department: Physiotherapy and Rehabilitation Randomisation: randomised through parallel assignment Stratification: not mentioned Timing: after surgical intervention Study duration: 8 weeks End point measurements: at baseline and at 8 weeks (study completion) Trial register: NCT03142087 |
| Participants | N = 30 Diagnosis: brain tumour Inclusion criteria: children aged 6 to 18 years old with the diagnosis of a brain tumour Age at start of study: 6 to 18 years old Exclusion criteria: children with conditions with exercise, children with neurological or musculoskeletal disorders |
| Interventions | The study group included in the Nintendo Wii Fit Plus Balance Game exercise program were under the supervision of a physiotherapist for 2 days per week for 8 weeks. The virtual reality exercises were performed on the Nintendo Wii Fit Plus Game Console. Sessions consisted of warm up exercises for 5 to 10 minutes, followed by games (soccer heading, ski jumping, slalom, etc.) for 30 to 40 minutes, and cool down exercises for 5 minutes. The control group was taken to a physiotherapy/rehabilitation exercise program under the supervision of a physiotherapist for 2 days a week, 1 hour a day. The control group program comprised of warm up exercises for 5 to 10 minutes, followed by balance and weight bearing exercises for 30 to 40 minutes and a cool down of 5 minutes. |
| Outcomes | Physical function: Anthropometric evaluations, muscle strength measurement, pain visual analogue scale, observational walking analysis, balanced paediatric functional range test, Timed Up and Go, one foot standing test, Nintendo Wii Fit Plus Balance Assessment, Functional capacity 2 minutes, fatigue with PedsQL Multidimensional Fatigue Scale, daily life activities with WeeFIM. |
| Starting date | 21 March 2017 |
| Contact information | Müberra Tanrıverdi |
| Notes | Study completed in January 2018. On 23 June 2020, no full‐text publication was available. |
Xipell 2019.
| Study name | Horse Assisted Rehabilitation Postoncologic Treatment in Children and Adolescents: Physical and Psychological Effects |
| Methods | Type of study: interventional Setting: Bezmialem Vakif University Department: Physiotherapy and Rehabilitation Randomisation: Randomised through parallel assignment Stratification: not mentioned Timing: over 6 months after surgical intervention Study duration: 6 months End point measurements: baseline, 6‐week, 12‐week, and 25‐week (study completion) Trial register: NCT04070131 |
| Participants | N = 30 Diagnosis: cancer with affectation of the central nervous system Inclusion criteria: children aged 4 to 18 years old with the diagnosis of a brain tumour; with or without motor, functional and/or cognitive deficits or neurological disorders due to their basic problem or as a consequence of the therapeutic procedures, with any degree of disability; more than 6 months after receiving the discharge of oncology (chemotherapy or radiotherapy). Exclusion criteria: children with Immunodepression, hypotonia with severe pelvic instability that does not allow seating on the horse safely, weight greater than 80 kg, and phobia of horses. |
| Interventions | Control group: standard follow‐up Intervention group: horse‐assisted rehabilitation (1 hr per week) and session of hippotherapy. |
| Outcomes |
Primary outcome measures Self‐reported quality of life changes (Pediatric Quality of Life Inventory ‐ Child Self‐Report (PedsQL‐C)) Secondary outcome measures Parent‐proxy‐reported quality of life changes (Pediatric Quality of Life Inventory ‐ Parent‐Proxy Report (PedsQL‐PC)) General health status changes (Barcelona General Health Status Questionnaire 2000) Anxiety changes (State Trait Anxiety Inventory for children (STAI‐CH)) Depression changes (Childhood Depression Inventory (CDI)) Behavior changes ((Behavior Assessment System for Children ‐ Parent Rating Scale (BASC‐PRS)) Self‐reported physical function changes (PedsQL‐C (subscale Health and Activities)) Parent‐reported physical function changes (PedsQL‐PC (subscale Physical Functioning)) Self‐reported emotional function changes ((PedsQL‐C (subscale Feelings)) Parent‐reported emotional function changes (PedsQL‐PC (subscale Emotional Functioning)) Self‐reported sociability function changes (PedsQL‐C (subscale Get Along with Others)) Parent‐reported sociability function changes (PedsQL‐PC (subscale Emotional Functioning)) Balance changes (Pediatric Balance Scale) Position changes (Sitting Assessment Scale) Proprioception‐coordination changes (Developmental Coordination Disorder Questionnaire 2007 (DCDQ'07)) Autonomous nervous system activation function changes (Heart Rate Variability (HRV)) |
| Starting date | 12 October 2019 |
| Contact information | Teresa Xipell Prunés, teresa.xipell@eug.es |
| Notes | Estimated study completion date: September 2020. On 23 June 2020, no full‐text publication was available. |
Differences between protocol and review
We identified an omission in the list of secondary outcomes in the protocol. The categories 'functional abilities and mobility' and 'motor development and performance' were not listed. However, some of the tests for these were included under the category of 'balance'. We have revised to include the category of 'motor development and performance', and we added the following tests: Bruininks Osteretsky Test of Motor Proficiency (BOTMP), Bruininks Osteretsky Test of Motor Proficiency Second Edition (BOT‐2), Alberta Infant Motor Scale (AIMS), Movement Assessment Battery for Children (Movement ABC‐2), Peabody Developmental Motor Scales (PDMS‐2), Miller Function and Participation Scales (MFUN‐PS), Gross Motor Function Measure (GMFM). In the category of 'functional abilities and mobility', we added the following tests: Functional Mobility Assessment (FMA) tool, Timed Up and Down Stairs (TUDS), Timed Up and Go (TUG), Functional Independence Measure for Children (WeeFIM), Pediatric Evaluation of Disability Inventory (PEDI), and Vineland Adaptive Behaviour Scale.
We updated the CENTRAL search strategy with the assistance of the University of Alberta Health Sciences librarian as we identified some minor notation errors that did not allow us to re‐run the search strategy. No content was modified; only the order of statements and punctuation were corrected.
Contributions of authors
Paula A. Ospina, MScPT: responsible for protocol development, database search, screening of articles for inclusion, data extraction, and writing of the final review paper. Alyssa McComb, MScPT: responsible for protocol development, database search, screening of articles for inclusion, data extraction, and contributing to the writing of the final review. Lesley Wiart, PT, PhD: responsible for protocol development, interpretation of findings, and review preparation and conclusions. David Eisenstat, MD, MA, FRCPC: content expert. Responsible for protocol development, interpretation of findings, review preparation and conclusions. Margaret L. McNeely, PT, PhD: methodological expert. Responsible for protocol development, overseeing the database search, third‐party arbitration to reach consensus in disagreements, screening of articles for inclusion, data extraction, interpretation of findings and review conclusions, and writing of the final review paper.
Sources of support
Internal sources
-
Canadian Child Health Clinician Scientist Training Program (Dr. Wiart), Women and Children's Health Research Institute (Ms. Ospina, Dr. Wiart, Dr. Eisenstat, Dr. McNeely), Stollery Children's Foundation (Dr. Wiart), Alberta Policy Wise for Children and Families (Dr. Wiart), Canada
Research project support for Dr. Wiart's research projects
External sources
No sources of support provided
Declarations of interest
None known.
New
References
References to studies excluded from this review
Beulertz 2016a {published data only}
- Beulertz J, Prokop A, Rustler V, Bloch W, Felsch M, Baumann FT. Effects of a 6-month, group-based, therapeutic exercise program for childhood cancer outpatients on motor performance, level of activity, and quality of life. Pediatric Blood & Cancer 2016;63(1):127-32. [DOI] [PubMed] [Google Scholar]
Braam 2010 {published data only}
- Braam KI, Veening MA, Kaspers GJ, Van Dulmen-den Broeder E, Van Dijk EM, Huisman J. Design of the Quality of Life in Motion (QLIM) study: a randomized controlled trial to evaluate the effectiveness and cost-effectiveness of a combined physical exercise and psychosocial training program to improve physical fitness in children with cancer. BMC Cancer 2010;10:624-32. [DOI: 10.1186/1471-2407-10-624] [DOI] [PMC free article] [PubMed] [Google Scholar]
Braam 2018 {published data only}
- Braam KI, Van Dijk-Lokkart EM, Kaspers GJ, Takken T, Huisman J, Buffart LM, et al. Effects of a combined physical and psychosocial training for children with cancer: a randomized controlled trial. BMC Cancer 2018;18(1):1-12. [DOI: 10.1186/s12885-018-5181-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Casanova‐Garcia 2015 {published data only}
- Casanova-García C, Lerma Lara S, Pérez Ruiz M, Ruano Domínguez D, Satana Sosa E. Non-pharmacological treatment for neuropathic pain in children with cancer. Medical Hypotheses 2015;85(6):791-97. [DOI] [PubMed] [Google Scholar]
Corr 2017 {published data only}
- Corr AM, Wilson T, Ness KK, Liu W, Bishop M, Pappo A, et al. Feasibility and functional outcomes of children and adolescents undergoing preoperative chemotherapy prior to a limb-sparing procedure or amputation. Rehabilitation Oncology 2017;35(1):38-45. [PMC free article] [PubMed] [Google Scholar]
Cox 2018 {published data only}
- Cox CL, Zhu L, Kaste SC, Srivastava K, Barnes L, Nathan PC, et al. Modifying bone mineral density, physical function, and quality of life in children with acute lymphoblastic leukemia. Pediatric Blood & Cancer 2018;65(4). [DOI: 10.1002/pbc.26929] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cunningham 1986 {published data only}
- Cunningham BA, Morris G, Cheney CL, Buergel N, Aker SN, Lenssen P. Effects of resistive exercise on skeletal muscle in marrow transplant recipients receiving total parenteral nutrition. Journal of Parenteral and Enteral Nutritition 1986;10(6):558-63. [DOI] [PubMed] [Google Scholar]
Däggelmann 2017 {published data only}
- Däggelmann J, Krauth KA, Mailand P, Nopper S, Renniger M, Bündgen L, et al. Effects of a four-week rehabilitation program on motor performance, quality of life and fatigue in childhood cancer patients and healthy siblings. Die Rehabilitation 2017;56(2):119-26. [DOI] [PubMed] [Google Scholar]
De Macedo 2010 {published data only}
- De Macedo TM, Oliveira KM, Melo JB, De Madeiros MG, De Medeiros Filho WC, Ferreira GM, et al. Inspiratory muscle training in patients with acute leukemia: preliminary results. Revista Paulista de Pediatria 2010;28(4):352-58. [Google Scholar]
Fiuza‐Luces 2017 {published data only}
- Fiuza-Luces C, Padilla JR, Soares-Miranda L, Santana-Sosa E, Quiroga JV, Santos-Lozano A, et al. Exercise intervention in pediatric patients with solid tumors: the Physical Activity in Pediatric Cancer Trial. Medicine & Science in Sports & Exercise 2017;49(2):223-30. [DOI] [PubMed] [Google Scholar]
Gohar 2011 {published data only}
- Gohar SF, Comito M, Price J, Marchese V. Feasibility and parent satisfaction of a physical therapy intervention program for children with acute lymphoblastic leukemia in the first 6 months of medical treatment. Pediatric Blood & Cancer 2011;56(5):799-804. [DOI] [PubMed] [Google Scholar]
Hartman 2009 {published data only}
- Hartman A, Te Winkel ML, Beek RD, Muinck Keizer-Schrama SM, Kemper HC, Hop WC, et al. A randomized trial investigating an exercise program to prevent reduction of bone mineral density and impairment of motor performance during treatment for childhood acute lymphoblastic leukemia. Pediatric Blood & Cancer 2009;53(1):64-71. [DOI] [PubMed] [Google Scholar]
Kim 2019 {published data only}
- Kim Y, Park S. Feasibility and benefits of a combined programme of exercise and play for paediatric cancer survivors: a pilot study. European Journal of Cancer Care 2019;28(5). [DOI: 10.1111/ecc.13111] [DOI] [PubMed] [Google Scholar]
Lam 2018 {published data only}
- Lam KK, Li WH, Chung OK, Ho KY, Chiu SY, Lam HS, et al. An integrated experiential training programme with coaching to promote physical activity, and reduce fatigue among children with cancer: a randomised controlled trial. Patient Education and Counseling 2018;101:1947-56. [DOI] [PubMed] [Google Scholar]
Manchola‐Gonzalez 2019 {published data only}
- Manchola-Gonzalez JD, Bagur-Calafat C, Girabent-Farres M, Serra-Grima JR, Alvarez R, Garnacho-Castano MV, et al. Effects of a home-exercise programme in childhood survivors of acute lymphoblastic leukaemia on physical fitness and physical functioning: results of a randomised clinical trial. Supportive Care in Cancer 2019;28(7):3171-78. [DOI] [PubMed] [Google Scholar]
Marchese 2004 {published data only}
- Marchese VG, Chiarello LA, Lange BJ. Effects of physical therapy intervention for children with acute lymphoblastic leukemia. Pediatric Blood & Cancer 2004;42(2):127-33. [DOI] [PubMed] [Google Scholar]
Morales 2019 {published data only}
- Morales JS, Santana-Sosa E, Santos-Lozano A, Bano-Rodrigo A, Valenzuela PL, Rincon-Castanedo C, et al. Inhospital exercise benefits in childhood cancer: a prospective cohort study. Scandinavian Journal of Medicine & Science in Sports 2020;30:126-34. [DOI] [PubMed] [Google Scholar]
Moyer‐Mileur 2009 {published data only}
- Moyer-Mileur LJ, Ransdell L, Bruggers CS. Fitness of children with standard-risk acute lymphoblastic leukemia during maintenance therapy: response to a home-based exercise and nutrition program. Journal of Pediatric Hematology/Oncology 2009;31(4):259-66. [DOI] [PubMed] [Google Scholar]
Müller 2014 {published data only}
- Müller C, Winter C, Boos J, Goshenger G, Hardes J, Vieth V, et al. Effects of an exercise intervention on bone mass in pediatric bone tumor patients. International Journal of Sports Medicine 2014;35(8):696-703. [DOI] [PubMed] [Google Scholar]
Müller 2016 {published data only}
- Müller C, Krauth K, Gerß K, Rosenbaum D. Physical activity and health-related quality of life in pediatric cancer patients following a 4-week inpatient rehabilitation program. Supportive Care in Cancer 2016;24(9):3793-802. [DOI] [PubMed] [Google Scholar]
Müller 2017 {published data only}
- Müller C, Rosenbaum D, Krauth KA. Prospective evaluation of postural control and gait in pediatric patients with cancer after a 4-week inpatient rehabilitation program. American Journal of Physical Medicine & Rehabilitation 2017;96(9):646-53. [DOI] [PubMed] [Google Scholar]
Ouyang 2019 {published data only}
- Ouyang N, Cai R, Zhou X, Huang H, Qiu X, Liu K. Effects of a group-based physical activity program for pediatric patients with cancer on physical activity and symptom experience: a quasi-experimental study. Pediatric Blood & Cancer 2019;66(11). [DOI: 10.1002/pbc.27965] [DOI] [PubMed] [Google Scholar]
Rosenhagen 2011 {published data only}
- Rosenhagen A, Bernhörster M, Vogt L, Weiss B, Senn A, Arndt S, et al. Implementation of structured physical activity in the pediatric stem cell transplantation. Klinische Padiatrie 2011;223(3):147-51. [DOI] [PubMed] [Google Scholar]
Sabel 2016 {published data only}
- Sabel M, Sjölund A, Broeren J, Arvidsson D, Saury J, Blomgren K. Active video gaming improves body coordination in survivors of childhood brain tumours. Disability & Rehabilitation 2016;38(21):2073-84. [DOI] [PubMed] [Google Scholar]
San Juan 2008 {published data only}
- San Juan AF, Chamorro-Vina C, Moral S, Fernandez del Valle M, Madero L, Ramirez M, et al. Benefits of intrahospital exercise training after pediatric bone marrow transplantation. International Journal of Sports Medicine 2008;29(5):439-46. [DOI] [PubMed] [Google Scholar]
Speyer 2010 {published data only}
- Speyer E, Herbinet A, Vuillemin A, Briançon S, Chastagner P. Effect of adapted physical activity sessions in the hospital on health-related quality of life for children with cancer: a cross-over randomized trial. Pediatric Blood & Cancer 2010;55(6):1160-66. [DOI] [PubMed] [Google Scholar]
Tanir 2013 {published data only}
- Tanir MK, Kuguoglu S. Impact of exercise on lower activity levels in children with acute lymphoblastic leukemia: a randomized controlled trial from Turkey. Rehabilitation Nursing 2013;38:49-59. [DOI] [PubMed] [Google Scholar]
Tanner 2015 {published data only}
- Tanner LR, Hooke MC, Hinshon S, Hansen CR. Effect of an ankle foot orthosis intervention for children with non-central nervous system cancers: a pilot study. Pediatric Physical Therapy 2015;27(4):425-32. [DOI] [PubMed] [Google Scholar]
Tanner 2017 {published data only}
- Tanner L, Sencer S, Hooke MC. The Stoplight Program: a proactive physical therapy intervention for children with acute lymphoblastic leukemia. Journal of Pediatric Oncology Nursing 2017;34(5):347-57. [DOI] [PubMed] [Google Scholar]
Tanner 2018 {published data only}
- Tanner LR, Hooke MC. Improving body function and minimizing activity limitations in pediatric leukemia survivors: the lasting impact of the Stoplight Program. Pediatric Blood & Cancer 2018;65(5). [DOI: 10.1002/pbc.27596] [DOI] [PubMed] [Google Scholar]
Tomasello 2018 {published data only}
- Tomasello C, Pinto RM, Mennini C, Conicella E, Stoppa F, Raucci U. Scrambler therapy efficacy and safety for neuropathic pain correlated with chemotherapy-induced peripheral neuropathy in adolescents: a preliminary study. Pediatric Blood & Cancer 2018;65(7). [DOI: 10.1002/pbc.27064] [DOI] [PubMed] [Google Scholar]
Van Dijk‐Lokkart 2016 {published data only}
- Van Dijk-Lokkart EM, Braam KI, Van Dulmen-den Broeder E, Kaspers GJ, Takken T, Grootenhuis MA, et al. Effects of a combined physical and psychosocial intervention program for childhood cancer patients on quality of life and psychosocial functioning: results of the QLIM randomized clinical trial. Psycho-oncology 2016;25(7):815-22. [DOI] [PubMed] [Google Scholar]
Wallek 2018 {published data only}
- Wallek S, Senn-Malashonak A, Vogt L, Schmidt K, Bader P, Banzer W. Impact of the initial fitness level on the effects of a structured exercise therapy during pediatric stem cell transplantation. Pediatric Blood & Cancer 2018;65(2). [DOI: 10.1002/pbc.26851] [DOI] [PubMed] [Google Scholar]
Winter 2013 {published data only}
- Winter CC, Müller C, Hardes J, Gosheger G, Georg BJ, Rosenbaum D. The effect of individualized exercise interventions during treatment in pediatric patients with a malignant bone tumor. Supportive Care in Cancer 2013;21(6):1629-36. [DOI] [PubMed] [Google Scholar]
Wurz 2019 {published data only}
- Wurz A, Brunet J. Exploring the feasibility and acceptability of a mixed-methods pilot randomized controlled trial testing a 12-week physical activity intervention with adolescent and young adult cancer survivors. Pilot and Feasibility Studies 2019;5(1):1-14. [DOI: 10.1186/s40814-019-0530-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yeh 2011 {published data only}
- Yeh CH, Man Wai JP, Lin US, Chiang YC. A pilot study to examine the feasibility and effects of a home‐based aerobic program on reducing fatigue in children with acute lymphoblastic leukemia. Cancer Nursing 2011;34:3-12. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Tanner 2016 {published data only}
- Tanner L. Impact of an orthotic intervention on physical function in children with chemotherapy-induced peripheral neuropathy. clinicaltrials.gov/ct2/show/NCT03655587 accessed 23 June 2020.
Tanriverdi 2017 {published data only}
- Tanrıverdi M. The effect of virtual reality exercises on balance in children with brain tumors. clinicaltrials.gov/ct2/show/NCT03142087 accessed 23 June 2020.
Xipell 2019 {published data only}
- Xipell T. Horse assisted rehabilitation postoncologic treatment in children and adolescents: physical and psychological effects. clinicaltrials.gov/ct2/show/NCT04070131 accessed 23 June 2020.
Additional references
Baggott 2009
- Baggott C, Dodd M, Kennedy C, Marina N, Miaskowski C. Multiple symptoms in pediatric oncology patients: a systematic review. Journal of Pediatric Oncology Nursing 2009;26(6):325-39. [DOI] [PubMed] [Google Scholar]
Beulertz 2016b
- Beulertz J, Bloch W, Prokop A, Rustler V, Fitzen C, Herich L, et al. Limitations in ankle dorsiflexion range of motion, gait, and walking efficiency in childhood cancer survivors. Cancer Nursing 2016;39(2):117-24. [DOI] [PubMed] [Google Scholar]
Braam 2013
- Braam KI, Van der Torre P, Takken T, Veening MA, Van Dulmen-den Broeder E, Kaspers GJ. Exercise interventions for children and young adults during and after treatment for childhood cancer. Cochrane Database of Systematic Reviews 2013, Issue 11. Art. No: CD008796. [DOI: 10.1002/14651858.CD008796] [DOI] [PubMed] [Google Scholar]
Braam 2016
- Braam KI, Van der Torre P, Takken T, Veening MA, Van Dulmen-den Broeder E, Kaspers GJ. Physical exercise training interventions for children and young adults during and after treatment for childhood cancer. Cochrane Database of Systematic Reviews 2016, Issue 3. Art. No: CD008796. [DOI: 10.1002/14651858.CD008796.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Buckner 2014
- Buckner TW, Wang J, Dewalt DA, Jacobs S, Reeve BB, Hinds PS. Patterns of symptoms and functional impairments in children with cancer. Pediatric Blood & Cancer 2014;61(7):1282-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
CCS 2020
- Canadian Cancer Society. Childhood Cancer Statistics 2020. www.cancer.ca/en/cancer-information/cancer-101/childhood-cancer-statistics/?region=ab (accessed 08 December 2020).
Cho 2016
- Cho Y, Do J, Jung S, Kwon O, Jeon J, Jeon JY. Effects of a physical therapy program combined with manual lymphatic drainage on shoulder function, quality of life, lymphedema incidence, and pain in breast cancer patients with axillary web syndrome following axillary dissection. Supportive Care In Cancer 2016;24(5):2047-57. [DOI] [PubMed] [Google Scholar]
Cormie 2017
- Cormie P, Zopf EM, Zhang X, Schmitz KH. The impact of exercise on cancer mortality, recurrence, and treatment‐related adverse effects. Epidemiologic Reviews 2017;39(1):71-92. [DOI] [PubMed] [Google Scholar]
De Groef 2015
- De Groef A, Van Kampen M, Dieltjens E, Christiaens M, Neven P, Geraerts I, et al. Effectiveness of postoperative physical therapy for upper-limb impairments after breast cancer treatment: a systematic review. Archives of Physical Medicine and Rehabilitation 2015;96(6):1140-53. [DOI] [PubMed] [Google Scholar]
Gatta 2014
- Gatta G, Botta L, Rossi S, Aareleid T, Bielska-Lasota M, Clavel J, et al. EUROCARE Working Group. Childhood cancer survival in Europe 1999-2007: results of EUROCARE-5--a population-based study. Lancet Oncology 2014;15(1):35-47. [DOI] [PubMed] [Google Scholar]
Gilchrist 2016
- Gilchrist L, Tanner L. Gait patterns in children with cancer and vincristine neuropathy. Pediatric Physical Therapy 2016;28(1):16-22. [DOI] [PubMed] [Google Scholar]
Gilchrist 2018
- Gilchrist LS, Tanner LR. Short-term recovery of balance control: association with chemotherapy-induced peripheral neuropathy in pediatric oncology. Pediatric Physical Therapy 2018;30(2):119-24. [DOI] [PubMed] [Google Scholar]
Green 2012
- Green DM. 11th International Conference on Long-Term Complications of Treatment of Children and Adolescents for Cancer. Forward. Pediatric Blood & Cancer 2012;58(1):111. [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hanson 2015
- Hanson H, Harrington AT, Nixon-Cave K. Implementing treatment frequency and duration guidelines in a hospital-based pediatric outpatient setting: administrative case report. Physical Therapy 2015;95:678-84. [DOI] [PubMed] [Google Scholar]
Hartman 2008
- Hartman A, Van den Bos C, Stijnen V, Pieters R. Decrease in peripheral muscle strength and ankle dorsiflexion as long-term side effects of treatment for childhood cancer. Pediatric Blood & Cancer 2008;50(4):833-7. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook-5-1.cochrane.org.
Kandula 2016
- Kandula T, Park SB, Cohn RJ, Krishnan AV, Farrar MA. Pediatric chemotherapy induced peripheral neuropathy: A systematic review of current knowledge. Cancer Treatment Reviews 2016;50:118-28. [DOI] [PubMed] [Google Scholar]
Kids Cancer Care 2020
- Kids Cancer Care. Stats Childhood Cancer. www.kidscancercare.ab.ca/childhood-cancer/stats (accessed 08 December 2020).
Kremer 2016
- Kremer LC, Leclercq E, Noorman JK, Van Dalen EC. Childhood Cancer Group. About the Cochrane Collaboration (Cochrane Review Groups CRGs) 2016;(12):Art. No.: CHILDCA.
Krivitzky 2015
- Krivitzky LS, Blaufuss MM, Van Den Heuvel S. Rehabilitation consideration in pediatric cancer survivors. In: Mucci GA, Torno LR, editors(s). Handbook of Long Term Care of the Childhood Cancer Survivor. Boston (MA): Springer, 2015:385-95. [Google Scholar]
Leclercq 2013
- Leclercq E, Leeflang MM, Van Dalen EC, Kremer LC. Validation of search filters for identifying pediatric studies. The Journal of Pediatrics 2013;162(3):629-34. [DOI] [PubMed] [Google Scholar]
McNeely 2010
- McNeely ML, Campbell K, Ospina M, Rowe BH, Dabbs K, Klassen TP, et al. Exercise interventions for upper-limb dysfunction due to breast cancer treatment. Cochrane Database of Systematic Reviews 2010, Issue 6. Art. No: CD005211. [DOI: 10.1002/14651858.CD005211.pub2] [DOI] [PubMed] [Google Scholar]
Moody 2006
- Moody K, Meyer M, Mancuso CA, Charlson M, Robbins L. Exploring concerns of children with cancer. Supportive Care in Cancer 2006;14(9):960-66. [DOI] [PubMed] [Google Scholar]
Morales 2018
- Morales JS, Valenzuela PL, Rincón-Castanedo C, Takken T, Fiuza-Luces C, Santos-Lozano A, et al. Exercise training in childhood cancer: a systematic review and meta-analysis of randomized controlled trials. Cancer Treatment Reviews 2018;70:154-67. [DOI] [PubMed] [Google Scholar]
NCI 2020
- National Cancer Institute. Late effects of treatment for childhood Cancer (PDQ®) – patient version. www.cancer.gov/types/childhood-cancers/late-effects-pdq (accessed 08 December 2020). [PubMed]
Ness 2013
- Ness KK, Jones KE, Smith WA, Spunt SL, Wilson CL, Armstrong GT, et al. Chemotherapy-related neuropathic symptoms and functional impairment in adult survivors of extracranial solid tumors of childhood: results from the St. Jude Lifetime Cohort Study. Archives of Physical Medicine and Rehabilitation 2013;94(8):1451-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nilsen 2015
- Nilsen TS, Raastad T, Skovlund E, Courneya KS, Langberg CW, Lilleby W, et al. Effects of strength training on body composition, physical functioning, and quality of life in prostate cancer patients during androgen deprivation therapy. Acta Oncologica 2015;54(10):1805-13. [DOI] [PubMed] [Google Scholar]
Noone 2018
- Noone AM, Howlader N, Krapcho M, Miller D, Brest A, Yu M, et al. SEER Cancer Statistics Review, 1975-2015, National Cancer Institute. https://seer.cancer.gov/csr/1975_2015/ (accessed 08 December 2020) 2018.
Ospina 2019
- Ospina PA, McNeely ML. A scoping review of physical therapy interventions for childhood cancers. Physiotherapy Canada 2019;73(3):287-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pergolotti 2015
- Pergolotti M, Deal AM, Lavery J, Reeve BB, Muss HB. The prevalence of potentially modifiable functional deficits and the subsequent use of occupational and physical therapy by older adults with cancer. Journal of Geriatric Oncology 2015;6(3):194-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pruitt 2009
- Pruitt DW, Nagarajan R. Rehabilitation of the pediatric cancer patient. In: Stubblefield MD, O'Dell MW, editors(s). Cancer Rehabilitation: Principles and Practices. New York (NY): Demos Medical, 2009:855-68. [Google Scholar]
Punzalan 2009
- Punzalan M, Hyden G. The role of physical therapy and occupational therapy in the rehabilitation of pediatric and adolescent patients with osteosarcoma. Cancer Treatment and Research 2009;152:367-84. [DOI] [PubMed] [Google Scholar]
Robison 2009
- Robison LL, Armstrong GT, Boice JD, Chow EJ, Davies S, Donaldson S, et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. Journal of Clinical Oncology 2009;27(14):2308-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Skinner 2012
- Skinner R. Long-term effects of cancer therapy in children - functional effects, late mortality and long-term follow-up. Paediatrics and Child Health 2012;22(6):248-52. [Google Scholar]
Stubblefield 2013
- Stubblefield MD, Schmitz KH, Ness KK. Physical functioning and rehabilitation for the cancer survivors. Seminars in Oncology 2013;12(1):784-95. [DOI] [PubMed] [Google Scholar]
Van Cleve 2012
- Van Cleve L, Munoz CE, Savedra M, Riggs M, Bossert E, Grant M, et al. Symptoms in children with advanced cancer: child and nurse reports. Cancer Nursing 2012;35(2):115-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wacker 2017
- Wacker K, Tanner L, Ovans J, Mason J, Gilchrist L. Improving functional mobility in children and adolescents undergoing treatment for non-central nervous system cancers: a systematic review. Contemporary Issues in Cancer Rehabilitation 2017;9(9S2):S385-97. [DOI] [PubMed] [Google Scholar]
Ward 2014
- Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA: a Cancer Journal for Clinicians 2014;64(2):83-103. [DOI] [PubMed] [Google Scholar]
Weiner 2019
- Weiner L, Brannagan T. Peripheral neuropathy in cancer. In: M Stubblefield, editors(s). Cancer Rehabilitation Principles and Practices. 2nd edition. New York (NY): Springer Publishing Company, 2019:658-76. [Google Scholar]
WHO 2020
- World Health Organization. Improving childhood cancer cure rate. www.who.int/activities/improving-childhood-cancer-cure-rate (accessed 08 December 2020).
Wickham 2007
- Wickham R. Chemotherapy-induced peripheral neuropathy: a review and implications for oncology nursing practice. Clinical Journal of Oncology Nursing 2007;11(3):361-76. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Ospina 2018
- Ospina PA, McComb A, Wiart LE, Eisenstat DD, McNeely ML. Physical therapy interventions, other than general physical exercise interventions, in children and adolescents before, during and following treatment for cancer. Cochrane Database of Systematic Reviews 2018, Issue 1. Art. No: CD012924. [DOI: 10.1002/14651858.CD012924] [DOI] [PMC free article] [PubMed] [Google Scholar]


