Abstract
Background
Great saphenous vein (GSV) incompetence, causing varicose veins and venous insufficiency, makes up the majority of lower‐limb superficial venous diseases. Treatment options for GSV incompetence include surgery (also known as high ligation and stripping), laser and radiofrequency ablation, and ultrasound‐guided foam sclerotherapy. Newer treatments include cyanoacrylate glue, mechanochemical ablation, and endovenous steam ablation. These techniques avoid the need for a general anaesthetic, and may result in fewer complications and improved quality of life (QoL). These treatments should be compared to inform decisions on treatment for varicosities in the GSV. This is an update of a Cochrane Review first published in 2011.
Objectives
To assess the effects of endovenous laser ablation (EVLA), radiofrequency ablation (RFA), endovenous steam ablation (EVSA), ultrasound‐guided foam sclerotherapy (UGFS), cyanoacrylate glue, mechanochemical ablation (MOCA) and high ligation and stripping (HL/S) for the treatment of varicosities of the great saphenous vein (GSV).
Search methods
The Cochrane Vascular Information Specialist searched the Cochrane Vascular Specialised Register, CENTRAL, MEDLINE, Embase, CINAHL, and AMED databases, and World Health Organization International Clinical Trials Registry Platform and ClinicalTrials.gov trials registers to 2 November 2020. We undertook reference checking to identify additional studies.
Selection criteria
We included randomised controlled trials (RCTs) treating participants for varicosities of the GSV using EVLA, RFA, EVSA, UGFS, cyanoacrylate glue, MOCA or HL/S. Key outcomes of interest are technical success, recurrence, complications and QoL.
Data collection and analysis
Two review authors independently selected trials, applied Cochrane's risk of bias tool, and extracted data. We calculated odds ratios (ORs) with 95% confidence intervals (CIs) and assessed the certainty of evidence using GRADE.
Main results
We identified 11 new RCTs for this update. Therefore, we included 24 RCTs with 5135 participants. Duration of follow‐up ranged from five weeks to eight years. Five comparisons included single trials. For comparisons with more than one trial, we could only pool data for 'technical success' and 'recurrence' due to heterogeneity in outcome definitions and time points reported. All trials had some risk of bias concerns. Here we report the clinically most relevant comparisons.
EVLA versus RFA
Technical success was comparable up to five years (OR 0.98, 95% CI 0.41 to 2.38; 5 studies, 780 participants; moderate‐certainty evidence); over five years, there was no evidence of a difference (OR 0.85, 95% CI 0.30 to 2.41; 1 study, 291 participants; low‐certainty evidence). One study reported recurrence, showing no clear difference at three years (OR 1.53, 95% CI 0.78 to 2.99; 291 participants; low‐certainty evidence), but a benefit for RFA may be seen at five years (OR 2.77, 95% CI 1.52 to 5.06; 291 participants; low‐certainty evidence).
EVLA versus UGFS
Technical success may be better in EVLA participants up to five years (OR 6.13, 95% CI 0.98 to 38.27; 3 studies, 588 participants; low‐certainty evidence), and over five years (OR 6.47, 95% CI 2.60 to 16.10; 3 studies, 534 participants; low‐certainty evidence). There was no clear difference in recurrence up to three years and at five years (OR 0.68, 95% CI 0.20 to 2.36; 2 studies, 443 participants; and OR 1.08, 95% CI 0.40 to 2.87; 2 studies, 418 participants; very low‐certainty evidence, respectively).
EVLA versus HL/S
Technical success may be better in EVLA participants up to five years (OR 2.31, 95% CI 1.27 to 4.23; 6 studies, 1051 participants; low‐certainty evidence). No clear difference in technical success was seen at five years and beyond (OR 0.93, 95% CI 0.57 to 1.50; 5 studies, 874 participants; low‐certainty evidence). Recurrence was comparable within three years and at 5 years (OR 0.78, 95% CI 0.47 to 1.29; 7 studies, 1459 participants; and OR 1.09, 95% CI 0.68 to 1.76; 7 studies, 1267 participants; moderate‐certainty evidence, respectively).
RFA versus MOCA
There was no clear difference in technical success (OR 1.76, 95% CI 0.06 to 54.15; 3 studies, 435 participants; low‐certainty evidence), or recurrence (OR 1.00, 95% CI 0.21 to 4.81; 3 studies, 389 participants; low‐certainty evidence). Long‐term data are not available.
RFA versus HL/S
No clear difference in technical success was detected up to five years (OR 5.71, 95% CI 0.64 to 50.81; 2 studies, 318 participants; low‐certainty evidence); over five years, there was no evidence of a difference (OR 0.88, 95% CI 0.29 to 2.69; 1 study, 289 participants; low‐certainty evidence). No clear difference in recurrence was detected up to three years (OR 0.93, 95% CI 0.58 to 1.51; 4 studies, 546 participants; moderate‐certainty evidence); but a possible long‐term benefit for RFA was seen (OR 0.41, 95% CI 0.22 to 0.75; 1 study, 289 participants; low‐certainty evidence).
UGFS versus HL/S
Meta‐analysis showed a possible benefit for HL/S compared with UGFS in technical success up to five years (OR 0.32, 95% CI 0.11 to 0.94; 4 studies, 954 participants; low‐certainty evidence), and over five years (OR 0.09, 95% CI 0.03 to 0.30; 3 studies, 525 participants; moderate‐certainty evidence). No clear difference was detected in recurrence up to three years (OR 1.81, 95% CI 0.87 to 3.77; 3 studies, 822 participants; low‐certainty evidence), and after five years (OR 1.24, 95% CI 0.57 to 2.71; 3 studies, 639 participants; low‐certainty evidence).
Complications were generally low for all interventions, but due to different definitions and time points, we were unable to draw conclusions (very‐low certainty evidence). Similarly, most studies evaluated QoL but used different questionnaires at variable time points. Rates of QoL improvement were comparable between interventions at follow‐up (moderate‐certainty evidence).
Authors' conclusions
Our conclusions are limited due to the relatively small number of studies for each comparison and differences in outcome definitions and time points reported. Technical success was comparable between most modalities. EVLA may offer improved technical success compared to UGFS or HL/S. HL/S may have improved technical success compared to UGFS. No evidence of a difference was detected in recurrence, except for a possible long‐term benefit for RFA compared to EVLA or HL/S. Studies which provide more evidence on the breadth of treatments are needed. Future trials should seek to standardise clinical terminology of outcome measures and the time points at which they are measured.
Plain language summary
Which procedures are best for treating varicose veins in the leg?
Key messages
We are uncertain about which treatments are best for varicose veins because we found only a small number of studies that compared the different types of treatment, and because studies differed in how they measured results.
‐ All currently available varicose vein treatments are similar in terms of whether the treatment fully destroys the vein, or stops blood from pooling in the legs, or both (technical success).
‐ We need studies that provide more evidence on all the available treatments.
What are varicose veins?
Varicose veins are bulging, twisty veins close to the skin’s surface that usually occur in the legs. They are caused by chronic venous insufficiency, which is when your veins do not manage to help blood to flow back up to your heart efficiently, and blood pools in your legs. About one‐third of adults are thought to have chronic venous insufficiency. Women are more likely than men to have varicose veins.
Varicose veins can be painful, itchy and unsightly, especially when standing and walking. Occasionally, they may result in skin changes or sores (ulcers) on the leg that take more than two weeks to heal.
How are varicose veins treated?
Varicose veins can be treated using a variety of procedures.
Traditionally, surgery was used to remove the main surface vein (called the ‘great saphenous vein’, which runs from the groin to the ankle) and any connected varicose veins through small openings in the leg. People having this procedure (known as ‘high ligation and stripping’) need to have a general anaesthetic to make them unconscious and stop them from feeling pain or moving while the surgery is done.
More recently, several treatments have emerged where the procedure is done inside the vein (endovenous), using a very fine tube. These treatments involve sealing the main vein in the thigh by deliberately damaging the vein wall. There are two main types of treatment:
‐ heat‐based, where heat energy from lasers, radio waves or steam, is used to damage the vein wall;
‐ chemical‐based, where chemicals (including foam or glue) are used to damage and consequently seal the vein.
These newer treatments are done using a local anaesthetic, meaning you do not feel pain in your legs during the procedure but you remain awake.
What did we want to find out?
We wanted to compare all the currently available treatments for varicose veins to find out which is best in terms of:
‐ short‐ and long‐term technical success (whether the treatment fully destroys the vein, or stops blood from pooling in the legs, or both);
‐ stopping varicose veins from returning (recurrence);
‐ avoiding unwanted effects; and
‐ improving people’s well‐being.
What did we do?
We searched for studies that compared treatments for varicose veins in men and women of any age.
We compared and summarised the results of the studies and rated our confidence in the evidence, based on factors such as study methods and sizes.
What did we find?
We found 24 studies that involved 5135 people with mild to severe varicose veins. The studies followed people for between 5 weeks to 8 years after their treatment. The majority of the people in the studies were women.
The studies took place in private and public clinics and hospitals in 10 different countries: Austria, Denmark, Egypt, Finland, France, Germany, the Netherlands, Turkey, the United Kingdom and the USA.
The studies we found did not investigate all possible treatments for varicose veins, especially newer treatments.
Main results
Technical success
Most treatments are equally likely to fully destroy the vein or prevent blood pooling in the legs, or both. However:
‐ heat‐based endovenous treatment with a laser may be more successful than traditional surgery;
‐ both heat‐based laser treatment and surgery may be more successful than chemical‐based endovenous treatment with a foam chemical.
Recurrence rates
Most treatments were similarly successful at stopping varicose veins from recurring.
Heat‐based radio wave endovenous treatment may be better than both laser endovenous treatment and surgery at preventing varicose veins from recurring in the longer term.
Unwanted effects
Unwanted effects were generally low for all treatments. The studies reported very few serious unwanted effects requiring treatment, both in the short and long term.
Well‐being
People in the studies said they had improved well‐being regardless of the treatment they received.
What are the limitations of the evidence?
Our confidence in the evidence ranges from moderate to very low because of:
‐ concerns over how the studies were carried out (people in most of the studies were aware of which treatment they were getting, as were the researchers assessing treatment data, which could affect the studies’ results);
‐ similar studies did not get the same results; and
‐ only a small number of studies contributed data to each result.
We were not able to reach firm conclusions about which of the treatments compared is best.
How up to date is this evidence?
This Cochrane Review updates our previous review. The evidence is current to November 2020
Summary of findings
Background
Varicose veins of the lower limbs are dilated, tortuous, superficial veins. They can be painful, itchy or unsightly, especially when standing and walking. Occasionally, they may result in skin changes or leg ulcers. Varicose veins have been previously treated with surgery to remove the veins, by stripping them to the level of the knee (high ligation and stripping (HL/S)). Newer, less invasive treatments seal the main leaking vein in the thigh by using heat, chemical irritants (sclerosants) or adhesives (glue). These techniques potentially result in less pain after the procedure, fewer complications, and a quicker return to work and normal activities with improved quality of life. They also avoid the need for a general anaesthetic. The results of these newer treatments need to be compared to high ligation and stripping (HL/S) and to one another.
Description of the condition
The great saphenous vein (GSV) and small saphenous vein (SSV) are the main components of the superficial veins of the leg. The GSV runs from the ankle to the saphenofemoral junction in the groin and is responsible for the majority of varicose veins. The normal venous system relies on a complex mechanism consisting of valves, muscle pumps and pressure changes to overcome the forces of gravity, positional changes and pressure changes within the thorax and abdomen. Disruption of the normal function of the deep or superficial venous system will result in retrograde flow, also known as venous incompetence. Venous incompetence is thought to occur through a number of mechanisms. The ascending valvular incompetence theory describes the failing of valves and the loss of antegrade flow (from the ankle to the heart), of blood from the high‐pressured venous system, venous pooling and resulting venous hypertension (Corcos 1996; Corcos 2000; Trendelenburg 1890). There are other associated mechanisms at play, such as raised ankle venous pressure, inflammation and leakage of blood constituents into the surrounding tissue. These make up the vicious cycle of venous disease as inflammation leads to further venous disruption and failure of the venous mechanisms (Jones 2009; Labropoulos 2005; Pascarella 2005; Takase 2004).
The Clinical, Etiological, Anatomical and Pathophysiological (CEAP) classification for chronic venous disease is used to standardise its reporting. The clinical classes of the CEAP classification are shown in Table 7. The tool is validated in clinical practice and focuses primarily on clinical classification (Carpentier 2003).
1. Clinical, Etiological, Anatomical and Pathophysiological (CEAP) classification.
| C0 | No visible signs of venous disease |
| C1 | Spider veins, telangiectases or reticular veins (diameter < 3 mm) |
| C2 | Varicose veins (with a diameter > 3 mm) |
| C3 | Varicose veins with oedema |
| C4 | Varicose veins with trophic skin lesions secondary to chronic venous insufficiency |
| C4a | Pigmentation, purpura, eczema |
| C4b | Lipodermatosclerosis, atrophie blanche |
| C5 | Healed venous ulcer |
| C6 | Active venous ulcer |
The commonest manifestation of superficial venous incompetence (SVI) is palpable, tortuous, dilated vessels known as varicose veins. Longstanding incompetence, sometimes termed chronic venous insufficiency (CVI), is estimated to affect one third of the adult population (NICE 2013b), 60% to 70% of which is due to saphenofemoral, or GSV, valvular incompetence (Labropoulos 1994). Prevalence of CVI increases with age and risk factors including trauma, history of deep vein thrombosis (DVT), multiple pregnancies, obesity and occupations involving prolonged periods of standing. People may be asymptomatic or complain of mild symptoms such as aching, pain and poor cosmetic appearance. Rabe 2010 reported that in the Bonn Vein Study II 31.8% of people with GSV reflux and C2 disease progressed to more severe disease during 6.6 years of follow up but were not shown to progress to ulcers during the available follow‐up.
Description of the intervention
The traditional treatment of GSV incompetence is by open surgery (Sarin 1992). This involves a small groin incision to perform flush ligation of the saphenofemoral junction (SFJ) and ligation of any tributaries. The GSV is then removed by a process called 'stripping' using a wire or flexible PIN‐stripper. Phlebectomies (small stab incisions) can also then be performed with a vein hook (or through transilluminated powered phlebectomy) to remove any visible or preoperatively‐marked varicosities of the truncal or non‐saphenous veins within the calf or GSV branches within the thigh (Darwood 2008; Nesbitt 2014; Subramonia 2010). The exact role and impact of phlebectomies on the overall outcome for people with venous incompetence are important, but the treatment of choice per se is beyond the scope of this review.
SFJ ligation and stripping (HL/S) is usually performed as a day case procedure, usually under general anaesthesia, within an operating theatre setting. Post‐operative recovery and return to work is usually between two and three weeks; however, in some cases, this may be prolonged up to six weeks (HELP‐1 2011; Subramonia 2010). Overall complication rates following SFJ ligation and stripping are reported as between 17% to 20% (Critchley 1997; HELP‐1 2011). Recognised complications include pain, dysaesthesia, paraesthesia, bruising, haematoma, wound infection, lymphatic leaks, venous thromboembolism (deep vein thrombosis (DVT) and pulmonary embolus (PE)) and damage to major veins, arteries and nerves (CLASS 2014; Critchley 1997; Subramonia 2010). The need for general anaesthesia or spinal anaesthesia also subjects individuals to further risk of complications (i.e. allergic reaction to anaesthetic agents, damage to teeth during intubation, post‐operative nausea and vomiting).
Endovenous treatments
In the past two decades, endovenous procedures for treating SVI have emerged. These procedures rely on a catheter or device inserted into the vein under ultrasound guidance. They are minimally invasive, utilise local anaesthesia and do not require surgical incisions or exposures. These procedures potentially offer more acceptable treatments for GSV varicosities if outcomes are equivalent to or better than conventional surgery. These techniques can be divided into thermal tumescent treatments and non‐thermal non‐tumescent treatments.
Thermal treatments rely on the use of heat energy to damage the vein wall and lead to occlusion and fibrosis. Non‐thermal interventions predominantly rely on the use of a chemical sclerosant or, more recently, a glue that causes inflammatory and chemical damage to the vein wall, which can also be used in combination with mechanical agitation and maceration of the intima.
Thermal tumescent interventions
Endovenous thermal ablation is the use of heat to close the vein. The devices available are endovenous laser ablation (EVLA), radiofrequency ablation (RFA) or steam ablation (EVSA). There are a number of manufacturers, designs and differences within each of these categories. However, for clarity, we have adopted umbrella terminology in this review.
EVLA, RFA and EVSA are performed using tumescent anaesthesia, where local anaesthetic is injected under ultrasound guidance along the length of the vein. The benefit of this approach is four‐fold: (1) analgesia (pain relief): provided during and after the procedure; (2) compression: the perivenous dilute anaesthetic solution compresses the vein wall onto the endovenous catheter due to the increased hydrostatic pressure within the saphenous sheath; (3) hydrodissection: simultaneously, perivenous nervous structures are moved away from heat within the vein by means of hydrodissection, to protect adjacent structures such as nerves; and (4) heat sink: as the fluid is typically cool, it acts as a heat sink, reducing the risk of neurological sequelae and burns (Joh 2014).
Endovenous laser ablation (EVLA) and radiofrequency ablation (RFA) are established interventions with an improved complication profile and reduced recovery time compared to open surgery (CLASS 2014; HELP‐1 2011; LAST 2014; Subramonia 2010). In addition, they do not require general anaesthesia. In 2013, the National Institute for Health and Care Excellence (NICE) recommended the use of endovenous ablation as the first line treatment intervention for duplex ultrasound‐confirmed varicose veins and truncal incompetence (NICE 2013a).
Various types of laser fibres, wavelengths and radial tips are available for EVLA. For the purposes of this clinically‐orientated review, we have grouped these under one category, accepting that there may be nuanced advantages and disadvantages for each laser type. In EVLA, the GSV is cannulated under ultrasound guidance at the most distal point of reflux with an optical laser fibre. This is then advanced to just below the SFJ. The proximity to the junction varies by manufacturer but is typically 2 cm. Tumescent anaesthesia is then infiltrated, surrounding the EVLA catheter under duplex ultrasound (DUS) guidance. Ablation of the vessel occurs as the laser is activated and then slowly withdrawn retrograde (the rate varies depending on manufacturer recommendation). The operator simultaneously compresses the vein, delivering between 60 and 80 J/cm (Darwood 2008). EVLA can be performed using sedation, local or general anaesthesia in addition to tumescence. Complications include phlebitis, pain, bruising, burns and sensory disturbances. Min 2003 showed 93% duplex ultrasound‐proven occlusion at two years following EVLA for GSV varicosities, with all recurrences occurring within the first nine months.
RFA is performed under a similar principle to EVLA; however, luminal occlusion is induced through heat from radiofrequency energy controlled by a thermocouple. As in EVLA, the GSV is cannulated distally and the catheter electrode is positioned just below the SFJ then surrounded with tumescent anaesthesia. The catheter is then withdrawn by segments along the length of the vein whilst under compression. Normal activity following the procedure is encouraged. Complications such as phlebitis, sensory disturbance and burns are uncommon and have reduced since the introduction of tumescence. Arteriovenous fistulation is a recognised but rare complication (< 0.15%) (Rudarakanchana 2012; Weiss 2019).
Endovenous steam ablation (EVSA) works in a similar way to EVLA and RFA, where a catheter is advanced under ultrasound guidance into the target vein. This then allows 'superheated' steam (pressurised) to be pumped into the vein once tumescent has been infiltrated. The result is venous occlusion through thermal damage to the vein wall. Histological examination post intervention shows vein wall fibrosis and inflammation, destruction of endothelium, alterations of elastic and collagen fibres and reduction of the lumen (LAST 2014). Proposed benefits of steam sclerosis include use of lower temperatures (120 oC) compared to EVLA (temperatures of up to 600 oC reported), with fewer thermal injuries and reduced post‐operative pain (LAST 2014). EVSA is reported to not produce potentially harmful exogenous substances and some data on cost‐effectiveness exist (LAST 2014). The catheter in EVSA is also more flexible than those used in RFA and EVLA, which enables access to more tortuous vessels and perforator branches (Van den Bos 2011). Occlusion rates are reported to range from 85 to 100% (Woźniak 2015).
Non‐thermal, non‐tumescent interventions (NTNT)
The initial technique of non‐thermal interventions for GSV incompetence was that of ultrasound‐guided foam sclerotherapy (UGFS). UGFS is the recommended second line technique in the United Kingdom (UK) for the treatment of varicose veins as per NICE guidance (NICE 2013a). Under ultrasound guidance, the vein is cannulated and a foam sclerosant is injected, causing inflammation of the endothelial and subendothelial layers of the wall and hence fibrosis and obliteration of the vein. Various types of foam are available. However, initial success rates have been reported as low and repeated treatments are frequently required (Devereux 2014; Proebstle 2015). The procedure may be associated with poor post‐procedural cosmesis, with skin staining and ‘lumpiness’ reported. There is also a risk of visual disturbances and very low risk of stroke (NICE 2013b). People are also required to wear compression stockings following the procedure. The major advantage of non‐thermal interventions over thermal interventions is that they can be performed in outpatient departments and without any systemic analgesia. In addition, in those with lipodermatosclerosis or ulceration, UGFS can be useful as the infiltration of perivenous tumescence is not required.
More recently, there has been increasing use of other non‐thermal treatments for GSV insufficiency. These also do not require the use of tumescence (which can be painful and itself cause complications). Additionally, they do not subject individuals to the risk of thermal injury and are therefore known as non‐tumescent non‐thermal (NTNT) techniques (Leung 2016; Shepherd 2010).
Mechanochemical ablation (MOCA) is a NTNT technique which obliterates the venous lumen through the use of a rotating catheter tip, causing vasospasm and mechanical damage to endothelial cells. Further chemical injury is induced through the concomitant injection of a liquid sclerosant (Leung 2016; Tang 2017). The procedure only requires local anaesthesia and individuals are encouraged to mobilise immediately following the procedure. MOCA is reported to have lower rates of post‐procedural pain and enhanced recovery times in comparison with other endovenous techniques (Leung 2016). Tang 2017 reported a complication rate of 4.3% (which predominantly consisted of superficial self‐resolving phlebitis), and no major complications were reported. Occlusion rates between 94% to 97% are reported (Tang 2017).
Cyanoacrylate embolisation consists of the injection of cyanoacrylate glue within the vein via a hand‐held delivery gun. Under ultrasound guidance, the incompetent GSV is cannulated distally and a catheter inserted to 5 cm below the SFJ. Cyanoacrylate is then injected with alternating compression and pullback every few minutes for the length of the vein. Cyanoacrylate achieves immediate occlusion by chemically bonding the opposing vein walls together (Morrison 2015). The glue causes fibrotic degradation of the vein via a granulomatous foreign body and inflammatory vein wall reaction (Proebstle 2015). Tumescent anaesthesia is not required and manufacturers state that there no need for people to wear compression stockings post intervention. As the procedure is intraluminal, there is reduced risk of damage to perivenous nervous structures. Side effects predominantly consist of self‐limiting phlebitic reactions and wound infections (Gibson 2017). However, thrombus extension into the deep venous system has been reported with the consequent risk of migration to pulmonary vasculature (Proebstle 2015).
How the intervention might work
All the interventions aim to occlude the incompetent great saphenous vein (GSV). The endovenous interventions outlined above all broadly rely on endoluminal venous damage by means of: thermal energy (EVLA/RFA/EVSA) (Goode 2010; Khilnani 2010; Van den Bos 2011); chemical irritation (UGFS/MOCA) (Mueller 2013; Tessari 2001; Van Eekeren 2014); or adhesion (cyanoacrylate) (Lane 2017).
The outcome is venous endothelial damage which results in venous inflammation and subsequent sclerosis and scarring as the vein heals following the endothelial obliteration. This leads to venous occlusion. All methods described require the application of DUS to enable cannulation of the GSV at the lowest point of reflux, and each method is suitable for the majority of axial venous incompetence.
There has been a large increase in the uptake of these methods and their application in routine practice continues in both the NHS and private sector. The advent of the 2013 NICE guidelines has facilitated a paradigm shift in the management of GSV incompetence (Coughlin 2015; NICE 2013a). Surgery by means of open ligation and stripping is still performed but it is no longer the gold standard intervention. Surgical treatment aims to physically disconnect the GSV from its junction and then remove the length of GSV by stripping. This is an effective treatment but carries a greater morbidity in terms of the need for general anaesthesia, post‐operative complications and a longer recovery.
Why it is important to do this review
This is an update of a Cochrane Review first published in 2011, and previously updated in 2014 (Nesbitt 2011; Nesbitt 2014). Since the previous version of this Cochrane Review was published, new UK NICE guidance (NICE 2013a) and subsequent European guidance on the management of chronic venous incompetence (Wittens 2015) have been published. Furthermore, the development of newer endovenous devices has resulted in a wider range of technologies that can be used to treat this disease. As outlined above, these have varying levels of supporting evidence, and they differ in their underlying application and treatment methods. This has sparked an increase in venous literature comparing existing treatments with newer interventions and reporting on long‐term outcomes. This Cochrane Review considers the full breadth of treatment options for GSV incompetence and compares these options. Therefore, this review has a wider scope compared to previous versions of this review (Nesbitt 2011; Nesbitt 2014). We present the current evidence to provide the venous practitioner and wider healthcare community an up‐to‐date resource to enable accurate, evidence‐based decision‐making that can be tailored to individuals. The review is aimed at highlighting the strengths and weaknesses within the entire field of GSV interventions (open surgery, endovenous thermal and endovenous non‐thermal techniques) in order to answer key questions of day‐to‐day venous practice: which method is currently the most technically effective and which method offers long‐term benefits and lowest recurrence rates.
Objectives
To assess the effects of endovenous laser ablation (EVLA), radiofrequency ablation (RFA), endovenous steam ablation (EVSA), ultrasound‐guided foam sclerotherapy (UGFS), cyanoacrylate glue, mechanochemical ablation (MOCA) and high ligation and stripping (HL/S) for the treatment of varicosities of the great saphenous vein (GSV).
Methods
Criteria for considering studies for this review
Types of studies
We included randomised control trials (RCTs) which compared interventions for treating varicosities of the great saphenous vein (GSV). We excluded studies which:
included participants who underwent a combination of interventions (for instance, endovenous laser ablation (EVLA) or radiofrequency ablation (RFA) with high ligation and stripping (HL/S));
treated all other axes of superficial venous incompetence such as small saphenous vein (SSV), perforating veins or varicosities of tributaries, anterior thigh or accessory GSV veins (AAGSV);
treated telangiectasias or thread veins;
did not provide data (subgroup analysis) for participants who had both GSV and SSV varicosities treated;
included recurrent treatment (i.e. participants underwent previous treatment for GSV varicosities);
included participants who received simultaneous treatment of bilateral GSV insufficiency with different interventions (e.g. one limb treated with EVLA and the other limb with ultrasound‐guided foam sclerotherapy (UGFS);
involved CHIVA and ASVAL, as these are axial‐preserving techniques.
Types of participants
We included men and women of any age, with duplex ultrasound‐proven varicosities of the great saphenous system, who were suitable to undergo any of the treatment interventions. The focus of this review was on the management of C2 to C4 grade varicose veins. People with varicose veins with healed leg ulcer (C5) or active leg ulcer (C6) were excluded from this Cochrane Review. Endovenous thermal ablation for treating venous leg ulcers is evaluated in a separate Cochrane Review (Samuel 2013).
Types of interventions
We included these interventions:
endovenous laser ablation (EVLA);
radiofrequency ablation (RFA);
endovenous steam ablation (EVSA);
ultrasound‐guided foam sclerotherapy (UGFS);
cyanoacrylate glue;
mechanochemical ablation (MOCA);
SFJ ligation and stripping (surgery) (HL/S).
We planned to include these comparisons:
endovenous laser ablation versus radiofrequency ablation;
endovenous laser ablation versus endovenous steam ablation;
endovenous laser ablation versus ultrasound‐guided foam sclerotherapy;
endovenous laser ablation versus cyanoacrylate glue;
endovenous laser ablation versus mechanochemical ablation;
endovenous laser ablation versus SFJ ligation and stripping;
radiofrequency ablation versus endovenous steam ablation;
radiofrequency ablation versus ultrasound‐guided foam sclerotherapy;
radiofrequency ablation versus cyanoacrylate glue;
radiofrequency ablation versus mechanochemical ablation;
radiofrequency ablation versus SFJ ligation and stripping;
endovenous steam ablation versus ultrasound‐guided foam sclerotherapy;
endovenous steam ablation versus cyanoacrylate glue;
endovenous steam ablation versus mechanochemical ablation;
endovenous steam ablation versus SFJ ligation and stripping;
ultrasound‐guided foam sclerotherapy versus cyanoacrylate glue;
ultrasound‐guided foam sclerotherapy versus mechanochemical ablation;
ultrasound‐guided foam sclerotherapy versus SFJ ligation and stripping;
cyanoacrylate glue versus mechanochemical ablation;
cyanoacrylate glue versus SFJ ligation and stripping;
mechanochemical ablation versus SFJ ligation and stripping.
Types of outcome measures
Primary outcomes
Early technical success: defined as complete anatomical obliteration, or absence of reflux, within the GSV at around six weeks, on duplex ultrasound (DUS) (standard criterion of one second of reflux was used)
Long‐term technical success: defined as complete anatomical obliteration, or absence of reflux, within the GSV on DUS at five years or more
Secondary outcomes
Recurrence: clinical definition as reported by the clinician or participant at least one year following intervention. We expanded this outcome to include the term recanalisation. We have outlined the definition where reported by the included studies.
-
Post‐operative complications within three months (early) and beyond three months (late)
Minor complications are defined as those not requiring intervention, such as wound or thigh haematoma, saphenous nerve injury, thermal injury, bruising and phlebitis.
Major complications are defined as those requiring intervention, such as venous thromboembolism (VTE), respiratory distress and wound complications.
Quality of life (QoL): measured by generic QoL scores pre‐ and post‐intervention (e.g. Aberdeen Varicose Vein Symptom Severity score (AVVSS, also referred to as the Aberdeen Varicose Vein Questionnaire, AVVQ), Short Form 36 (SF‐36))
Pain: participant‐reported pain post‐operatively. This could be reported via visual analogue scales or number of analgesic tablets taken.
Venous Clinical Severity Score (VCSS) pre‐ and post‐intervention
Length of procedure
Hospital stay: whether the intervention was performed as a day case procedure or required an inpatient admission
Return to normal activities or work (days)
Search methods for identification of studies
Electronic searches
The Cochrane Vascular Information Specialist conducted systematic searches of the following databases for randomised controlled trials and controlled clinical trials without language, publication year or publication status restrictions.
Cochrane Vascular Specialised Register via the Cochrane Register of Studies (CRS‐Web searched on 2 November 2020).
Cochrane Central Register of Controlled Trials (CENTRAL) Cochrane Register of Studies Online (CRSO 2020, Issue 10).
MEDLINE (Ovid MEDLINE Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE Daily and Ovid MEDLINE) (searched from 1 January 2017 to 2 November 2020).
Embase Ovid (searched from 1 January 2017 to 2 November 2020).
CINAHL Ebsco (searched from 1 January 2017 to 2 November 2020).
AMED Ovid (searched from 1 January 2017 to 2 November 2020).
The Information Specialist modelled search strategies for other databases on the search strategy designed for CENTRAL. Where appropriate, they were combined with adaptations of the highly sensitive search strategy designed by the Cochrane Collaboration for identifying randomised controlled trials and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Chapter 6, Lefebvre 2011). Search strategies for major databases are provided in Appendix 1 and Appendix 2.
The Information Specialist searched these trials registries on 2 November 2020:
World Health Organization International Clinical Trials Registry Platform (who.int/trialsearch);
ClinicalTrials.gov (clinicaltrials.gov).
Searching other resources
We cross‐checked reference lists from relevant RCTs and meta‐analyses to ensure the inclusion of all appropriate studies.
Data collection and analysis
Selection of studies
Two review authors (JW and SN) independently screened the trials identified by the literature search for eligibility. We resolved disagreements by consulting a third review author (GS).
Data extraction and management
Two review authors (JW and SN) independently extracted data. A third review author (CN) then cross‐checked data extraction.
We extracted the following data from the included RCTs.
Methods: aim of study, study design, unit of allocation, start and end date, duration, country, intention‐to‐treat analysis, ethical approval.
Participants: setting, consent, number of participants randomised, number of participants analysed, exclusions post‐randomisation, loss to follow‐up, age (median), sex, co‐morbidities, number of bilateral limbs, inclusion and exclusion criteria.
Interventions: treatment, control, duration, timing, delivery, providers.
Outcomes: primary and secondary outcomes, time points measured and recorded, outcome definition, person measuring, unit of measurement, power.
Other: funding, conflicts of interest.
Assessment of risk of bias in included studies
Two review authors (JW and SN) independently assessed the included studies using Cochrane's risk of bias tool (Higgins 2011). This tool assesses bias in seven different domains (random sequence generation, allocation concealment (selection bias), performance bias, detection bias, attrition bias, reporting bias and other bias), with each domain being assessed as being at high, low or unclear risk of bias, depending on each review author’s judgement. We resolved any disagreements through discussion with a third review author (GS).
Measures of treatment effect
We used odds ratios (OR) with 95% confidence intervals (CI) as the measure of effect for each of the dichotomous outcomes. When data were available, we planned to used mean difference (MD) and standard deviation (SD) to report outcomes with continuous scales of measurement. We also planned to attempt to standardise and combine data where different studies used different scales (i.e. using standardised mean difference (SMD) and SD). We carried out analyses at different time points, as reported by the trials. We based our calculations on an intention‐to‐treat approach.
Unit of analysis issues
We intended to use the participant as the unit of analysis. Where studies used ‘legs or limbs’ as their unit of analysis, we contacted study authors to clarify the number of participants. If we were unable to obtain this information, we used ‘legs/limbs’ as the unit of analysis for technical success, recurrence and VCSS. QoL was reported using a variety of QoL assessment tools.
Dealing with missing data
We contacted study authors to request missing data or answer queries where required.
Assessment of heterogeneity
We noted and explored heterogeneity in the data, using previously identified characteristics of the studies, particularly assessments of risk of bias. The I2 statistic was used to determine heterogeneity. We considered I2 values greater than 50% to indicate the possible presence of heterogeneity, as in the previous version of this review (Nesbitt 2014), and as suggested by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Assessment of reporting biases
We planned to construct funnel plots to evaluate reporting bias, for meta‐analyses including 10 or more studies (Higgins 2011).
Data synthesis
We calculated a summary statistic for each outcome (where there were sufficient data), using Review Manager 5 (Review Manager 2014). We used a fixed‐effect model unless heterogeneity was detected (I2 values greater than 50%), in which case, we planned to use a random‐effects model.
Subgroup analysis and investigation of heterogeneity
We planned to undertake subgroup analyses to examine the stability of the results in relation to a number of factors, including participant type. However, due to the lack of outcome data reported by categories of interest, we did not perform subgroup analysis at this time.
Sensitivity analysis
We planned to exclude from meta‐analysis those studies deemed to have a high risk of bias in four or more bias domains.
Summary of findings and assessment of the certainty of the evidence
We created summary of findings (SOF) tables using the GRADEpro Guideline Development Tool to present the main findings of the review for the time point at which the most relevant data were available from the included studies (Atkins 2004; GRADEpro GDT). The population consisted of people with varicosities of the great saphenous vein (GSV) system. We created one SOF table for comparisons of most clinical relevance and which included data from more than one study. We included in our SOF tables the main outcomes listed under Types of outcome measures that we considered essential for decision‐making; namely, technical success (under and over five years), recurrence (under and over five years), complications, and quality of life. We evaluated the certainty of the evidence using the GRADE approach (Guyatt 2008). We assigned one of four levels of certainty: high, moderate, low or very low, based on overall risk of bias, directness of evidence, inconsistency of results, precision of estimates, and risk of publication bias, as previously described (Higgins 2011).
Results
Description of studies
Results of the search
See Figure 1.
1.
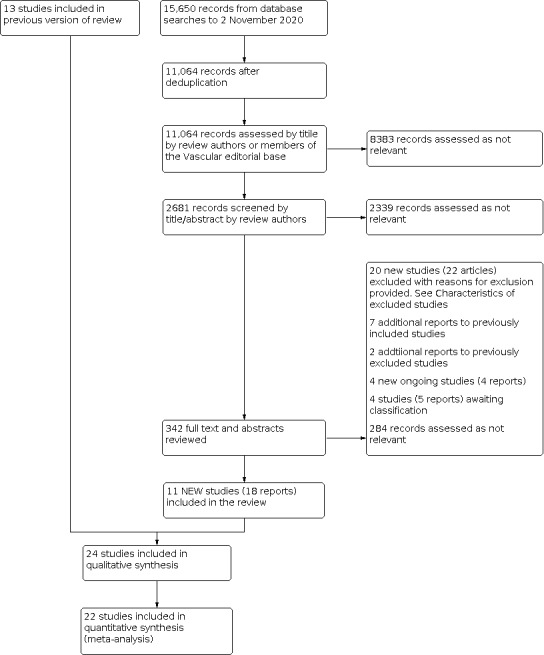
Study flow diagram
Included studies
We included a total of 24 studies in this review. This includes 11 new studies (18 reports) (Calik 2019; Lane 2017; LAST 2014; MARADONA 2019; Morrison 2015; Nordon 2011; Recovery 2009; Shepherd 2010; Syndor 2017; Vähäaho 2019; Vernermo 2016), in addition to the 13 from the previous version of the review (Darwood 2008; EVOLVeS 2003; Flessenkämper 2013; FOAM 2010; Helmy ElKaffas 2011; HELP‐1 2011; Magna 2013; Pronk 2010; Rasmussen 2007; Rasmussen 2011; Rautio 2002; RELACS 2012; Subramonia 2010). We also included additional reports of the long‐term follow‐up (greater than five years) for this update for seven studies (Flessenkämper 2013; FOAM 2010; HELP‐1 2011; Magna 2013; Pronk 2010; Rasmussen 2011; RELACS 2012). See the Characteristics of included studies tables.
All studies were RCTs in single, double and multi‐centre settings. Trials were conducted in a variety of private and public clinics and hospitals in countries including Turkey, Egypt, UK, USA, Finland, Germany, Denmark, Netherlands, Austria and France. The unit of analysis was considered to be the 'participants' in the majority of studies, with six studies reporting 'limbs' or 'legs' as the unit of analysis (Darwood 2008; EVOLVeS 2003; LAST 2014; Magna 2013; Pronk 2010; Rasmussen 2011). Calik 2019 involved a small number of bilaterally treated participants and refers to 'procedures' as their unit of analysis.
The studies included in this review randomised a total of 5135 participants and analysed 4422. Sample sizes in the studies ranged from 33 (Rautio 2002), to 500 participants (Rasmussen 2011); see sample study size in Table 8. In keeping with the epidemiology of venous insufficiency, a female predominance of participants was seen. Participants analysed ranged in age from 18 (Rasmussen 2011), to 86 years old (Syndor 2017). The age and sex of study participants for all trials is given in Table 9.
2. Study sample sizes.
| EVLA versus RFA | ||||
| Study | Participants randomised | Participants analysed | ||
| Overall | Overall | EVLA | RFA | |
| Nordon 2011 | 159 | 157 | 78 | 79 |
| Rasmussen 2011a | 250 292 legs |
213 245 legs |
107 121 legs |
106 124 legs |
| Recovery 2009 | 87 legs | 87 legs | 41 legs | 46 legs |
| Shepherd 2010 | 131 | 115 | 55 | 60 |
| Syndor 2017 | 200 | 153 | 79 | 74 |
| EVLA versus EVSA | ||||
| Study | Participants randomised | Participants analysed | ||
| Overall | Overall | EVLA | EVSA | |
| LAST 2014 | 217 237 legs |
199 legs | 92 legs | 107 legs |
| EVLA versus UGFS | ||||
| Study | Participants randomised | Participants analysed | ||
| Overall | Overall | EVLA | UGFS | |
| Magna 2013a | 160 legs | 155 legs | 78 legs | 77 legs |
| Rasmussen 2011a | 250 289 legs |
214 244 legs |
107 121 legs |
107 123 legs |
| Vernermo 2016a | 159 | 145 | 73 | 72 |
| EVLA versus CA | ||||
| Study | Participants randomised | Participants analysed | ||
| Overall | Overall | EVLA | CA | |
| Calik 2019 | 400 | 355 | 174 | 181 |
| EVLA versus MOCA | ||||
| Study | Participants randomised | Participants analysed | ||
| Overall | Overall | EVLA | MOCA | |
| Vähäaho 2019a | 99 | 88 | 33 | 55 |
| EVLA versus HL/S (surgery) | ||||
| Study | Participants randomised | Participants analysed | ||
| Overall | Overall | EVLA | HL/S (surgery) | |
| Darwood 2008 | 118 136 legs |
95 114 legs |
80 legs | 34 legs |
| Flessenkämper 2013b | 301 | 255 | 127 | 128 |
| HELP‐1 2011 | 280 | 237 | 124 | 113 |
| Magna 2013a | 160 legs | 146 legs | 78 legs | 68 legs |
| Pronk 2010 | 122 130 legs |
130 legs | 62 legs | 68 legs |
| Rasmussen 2007 | 121 137 legs |
88 | 47 | 41 |
| Rasmussen 2011a | 250 287 legs |
204 229 legs |
107 121 legs |
97 108 legs |
| RELACS 2012 | 400 | 316 | 173 | 143 |
| Vernermo 2016a | 152 | 134 | 73 | 61 |
| RFA versus UGFS | ||||
| Study | Participants randomised | Participants analysed | ||
| Overall | Overall | RFA | UGFS | |
| Rasmussen 2011a | 250 292 legs |
213 247 legs |
106 124 legs |
107 123 legs |
| RFA versus CA | ||||
| Study | Participants randomised | Participants analysed | ||
| Overall | Overall | RFA | CA | |
| Morrison 2015 | 222 | 208 | 104 | 104 |
| RFA versus MOCA | ||||
| Study | Participants randomised | Participants analysed | ||
| Overall | Overall | RFA | MOCA | |
| Lane 2017 | 170 | 129 | 60 | 69 |
| MARADONA 2019 | 213 | 200 | 99 | 101 |
| Vähäaho 2019a | 98 | 84 | 29 | 55 |
| RFA versus HL/S (surgery) | ||||
| Study | Participants randomised | Participants analysed | ||
| Overall | Overall | RFA | HL/S (surgery) | |
| EVOLVeS 2003 | 85 86 legs |
80 legs | 44 legs | 36 legs |
| Helmy ElKaffas 2011 | 180 | 162 | 81 | 81 |
| Rasmussen 2011a | 250 290 legs |
203 232 legs |
106 124 legs |
97 108 legs |
| Rautio 2002 | 33 | 28 | 15 | 13 |
| Subramonia 2010 | 93 | 88 | 47 | 41 |
| UGFS versus HL/S (surgery) | ||||
| Study | Participants randomised | Participants analysed | ||
| Overall | Overall | UGFS | HL/S (surgery) | |
| FOAM 2010 | 460 | 390 | 213 | 177 |
| Magna 2013a | 160 legs | 145 legs | 77 legs | 68 legs |
| Rasmussen 2011a | 250 286 legs |
204 231 legs |
107 123 legs |
97 108 legs |
| Vernermo 2016a | 155 | 133 | 72 | 61 |
aStudy includes multiple comparisons of different interventions. bStudy includes third treatment arm not included within this review.
CA: cyanoacrylate glue EVLA: endovenous laser ablation EVSA: endovenous steam ablation HL/S: high ligation and stripping MOCA: mechanochemical ablation RFA: radio frequency ablation UGFS: ultrasound‐guided foam sclerotherapy
3. Age and sex of participants.
| EVLA versus RFA | ||||
| Study | Age (years) | Sex (F:M) | ||
| EVLA | RFA | EVLA | RFA | |
| Nordon 2011 | 46.7 (14.4) mean (SD) |
46.9 (15.1) mean (SD) |
54:26 | 45:34 |
| Rasmussen 2011 | 52 (18 ‐ 74) mean (range) |
51 (23 ‐ 75) mean (range) |
90:35 | 88:37 |
| Recovery 2009 | 51.6 (12.8) mean (SD) |
52.4 (15.3) mean (SD) |
31:10 | 29:17 |
| Shepherd 2010 | 48 (16) mean (SD) |
49 (15) mean (SD) |
42:22 | 47:20 |
| Syndor 2017 | 48.5 (23 ‐ 86) mean (range) |
47 (19 ‐ 86) mean (range) |
77:23 | 80:20 |
| EVLA versus EVSA | ||||
| Study | Age (years) | Sex (F:M) | ||
| EVLA | EVSA | EVLA | EVSA | |
| LAST 2014 | 55 (12) mean (SD) |
56 (13) mean (SD) |
61:45 62:48 (legs) |
73:39 76:41 (legs) |
| EVLA versus UGFS | ||||
| Study | Age (years) | Sex (F:M) | ||
| EVLA | UGFS | EVLA | UGFS | |
| Magna 2013 | 49 (15.03) mean (SD) |
56 (13.30) mean (SD) |
54:24 | 52:25 |
| Rasmussen 2011 | 52 (18 ‐ 74) mean (range) |
51 (18 ‐ 75) mean (range) |
90:35 | 94:30 |
| Vernermo 2016 | 47 (13.4) [20 ‐ 73] mean (SD) [range] |
48.3 (12.7) [20 ‐ 73] mean (SD) [range] |
55:18 | 58:18 |
| EVLA versus CA | ||||
| Study | Age (years) | Sex (F:M) | ||
| EVLA | CA | EVLA | CA | |
| Calik 2019 | 38.4 (11.9) mean (SD) |
38.6 (11.6) mean (SD) |
114:86 | 109:91 |
| EVLA versus MOCA | ||||
| Study | Age (years) | Ses (F:M) | ||
| EVLA | MOCA | EVLA | MOCA | |
| Vähäaho 2019 | 49.5 (11.9) mean (SD) |
50.9 (12.0) mean (SD) |
N/A | N/A |
| EVLA versus HL/S (surgery) | ||||
| Study | Age (years) | Sex (F:M) | ||
| EVLA | HL/S (surgery) | EVLA | HL/S (surgery) | |
| Darwood 2008 | EVLT1: 42 (30.5 ‐ 54.5); EVLT2: 52 (35 ‐ 59); mean (IQR) |
49 (38.5 ‐ 57.5) mean (IQR) |
EVLT1: 22:16 EVLT2: 16:11 |
16:14 |
| Flessenkämper 2013 | 47.4 (12.9) mean (SD) |
47.7 (11.5) mean (SD) |
97:45 | 112:47 |
| HELP‐1 2011 | 49 (14) mean (SD) |
49 (13) mean (SD) |
85:54 | 90:47 |
| Magna 2013 | 49 (15.03) mean (SD) |
52 (15.59) mean (SD) |
54:24 | 46:22 |
| Pronk 2010 | 49 (11.0) mean (SD) |
50 (10.5) mean (SD) |
46:16 | 53:15 |
| Rasmussen 2007 | 53 (26 ‐ 79) mean (range) |
54 (22 ‐ 78) mean (range) |
41:21 | 43:16 |
| Rasmussen 2011 | 52 (18 ‐ 74) mean (SD) |
50 (19 ‐ 72) mean (range) |
90:35 | 95:29 |
| RELACS 2012 | 47.9 (10.9) mean (SD) |
48.0 (10.7) mean (SD) |
113:48 | 124:61 |
| Vernermo 2016 | 47 (13.4) [20 ‐ 73] mean (SD) [range] |
47.3 (11.3) [27 ‐ 75] mean (SD) [range] |
55:18 | 55:10 |
| RFA versus UGFS | ||||
| Study | Age (years) | Sex (F:M) | ||
| RFA | UGFS | RFA | UGFS | |
| Rasmussen 2011 | 51 (23 ‐ 75) mean (range) |
51 (18 ‐ 75) mean (range) |
88:37 | 94:30 |
| RFA versus CA | ||||
| Study | Age (years) | Sex (F:M) | ||
| RFA | CA | RFA | CA | |
| Morrison 2015 | 50.5 (25.6 ‐ 70.1) mean (range) |
49.0 (26.6 ‐ 70.6) mean (range) |
93:21 | 83:25 |
| RFA versus MOCA | ||||
| Study | Age (years) | Sex (F:M) | ||
| RFA | MOCA | RFA | MOCA | |
| Lane 2017 | 58 (median) |
54.5 (median) |
50:33 | 50:37 |
| MARADONA 2019 | 53.4 (22.6 ‐ 77.9) median (range) |
54.9 (16.3 ‐ 18.2) median (range) |
63:43 | 67:40 |
| Vähäaho 2019 | 50.3 (13.9) mean (SD) |
50.9 (12.0) mean (SD) |
N/A | N/A |
| RFA versus HL/S (surgery) | ||||
| Study | Age (years) | Sex (F:M) | ||
| RFA | HL/S (surgery) | RFA | HL/S (surgery) | |
| EVOLVeS 2003 | 49 (4) mean (SD) |
47 (4) mean (SD) |
32:12 | 26:10 |
| Helmy ElKaffas 2011 | 33.1 (2.6) mean (SD) |
34.9 (3.7) mean (SD) |
48:42 | 45:45 |
| Rasmussen 2011 | 51 (23 ‐ 75) mean (range) |
50 (19 ‐ 72) mean (range) |
88:37 | 95:29 |
| Rautio 2002 | 33 (6.7) mean (SD) |
38 (6.8) mean (SD) |
14:1 | 12:1 |
| Subramonia 2010 | 47 (38 ‐ 58) median (IQR) |
45 (37 ‐ 53) median (IQR) |
34:13 | 27:14 |
| UGFS versus HL/S (surgery) | ||||
| Study | Age (years) | Sex (F:M) | ||
| UGFS | HL/S (surgery) | UGFS | HL/S (surgery) | |
| FOAM 2010 | 55.8 (13.4) mean (SD) |
54.6 (13.4) mean (SD) |
175:58 | 162:65 |
| Magna 2013 | 56 (13.30) mean (SD) |
52 (15.59) mean (SD) |
52:25 | 46:22 |
| Rasmussen 2011 | 51 (18 ‐ 75) mean (range) |
50 (19 ‐ 72) mean (range) |
94:30 | 95:29 |
| Vernermo 2016 | 48.3 (12.7) [20 ‐ 73] mean (SD) [range] |
47.3 (11.3) [27 ‐ 75] mean (SD) [range] |
58:18 | 55:10 |
CA: cyanoacrylate glue EVLA: endovenous laser ablation (same as EVLT) EVLT: endovenous laser therapy EVSA: endovenous steam ablation F: female HL/S: high ligation and stripping IQR: interquartile range M: male MOCA: mechanochemical ablation RFA: radio frequency ablation SD: standard deviation UGFS: ultrasound‐guided foam sclerotherapy
Five studies compared endovenous laser ablation (EVLA) to radiofrequency ablation (RFA) (Nordon 2011; Rasmussen 2011; Recovery 2009; Shepherd 2010; Syndor 2017). Only LAST 2014 compared EVLA with endovenous steam ablation (EVSA). Three studies compared EVLA with ultrasound‐guided foam sclerotherapy (UGFS) (Magna 2013; Rasmussen 2011; Vernermo 2016). Calik 2019 was the only study to compare EVLA to cyanoacrylate glue. Only one study compared endovenous laser ablation (EVLA) to mechanochemical ablation (MOCA) (Vähäaho 2019). Nine studies compared EVLA to SFJ ligation and stripping (HL/S; surgery) (Darwood 2008; Flessenkämper 2013; HELP‐1 2011; Magna 2013; Pronk 2010; Rasmussen 2007; Rasmussen 2011; RELACS 2012; Vernermo 2016). The types of laser used in these trials can be found in Table 10. Rasmussen 2011 solely compared RFA with UGFS. Morrison 2015 was the only trial to compare RFA with cyanoacrylate glue. Three studies compared RFA with MOCA (Lane 2017; MARADONA 2019; Vähäaho 2019). Five studies compared RFA with SFJ ligation and stripping (EVOLVeS 2003; Helmy ElKaffas 2011; Rasmussen 2011; Rautio 2002; Subramonia 2010). Ultrasound‐guided foam sclerotherapy was compared with SFJ ligation and stripping in four studies (FOAM 2010; Magna 2013; Rasmussen 2011; Vernermo 2016).
4. Laser technique used.
| Study | Laser | Pulsed/continuous | Energy | Technique |
| Calik 2019 | 1470 nm diode | not stated | 15 W | withdrawn at 2.08 ± 0.6 cm/s |
| Darwood 2008 | 810 nm diode | 1) pulsed | 12 W | 1 s pulses, 1 s intervals |
| 2) continuous | 14 W | withdrawn 2 ‐ 3 mm/s | ||
| Flessenkämper 2013 | 980 nm diode | continuous | 30 W | not indicated |
| HELP‐1 2011 | 810 nm diode | continuous | 14 W | not indicated |
| LAST 2014 | 940 nm diode | continuous | 12 W | not indicated |
| Magna 2013 | 940 nm diode | continuous | not indicated | not indicated |
| Nordon 2011 | 810 nm diode | continuous | 12 W | withdrawn 2 mm/s |
| Pronk 2010 | 980 nm diode | continuous | 12 W | not indicated |
| Rasmussen 2007 | 980 nm diode | pulsed | 12 W | 1.5 s pulses, 1.5 s intervals |
| Rasmussen 2011 | 980 nm diode | 1) pulsed | not indicated | not indicated |
| 2) continuous | ||||
| 1470 nm diode | 1) pulsed | |||
| 2) continuous | ||||
| Recovery 2009 | 980 nm diode | continuous | 12 W | not indicated |
| RELACS 2012 | 810 nm diode | continuous | 20 W | not indicated |
| Shepherd 2010 | 980 nm diode | continuous | 11 W | not indicated |
| Syndor 2017 | 980 nm diode | continuous | 10 W | not indicated |
| Vähäaho 2019 | 1470 nm diode | pulsed | 10 W | 1.5 s impulse |
| Vernermo 2016 | 980 nm diode | pulsed | 12 W | 1.5 s impulse |
| 1470 nm diode | pulsed | 12 W | 1.5 s impulse |
cm: centimetre mm: millimetre nm: nanometre s: seconds W: watts
Four studies compared multiple interventions. Magna 2013 and Vernermo 2016 analysed endovenous laser ablation, ultrasound‐guided foam sclerotherapy and SFJ ligation and stripping against each other. Rasmussen 2011 also analysed these, with the addition of radiofrequency ablation (RFA). Vähäaho 2019 compared EVLA, RFA and MOCA, but it was only powered to compare MOCA against thermal ablation. Hence, we have not included their outcomes for RFA or EVLA within our comparison of these two interventions. Flessenkämper 2013 included a comparison arm which was not included within the scope of this study (EVLA plus high ligation); therefore, we did not include these participants.
We identified no published RCTs which met the inclusion criteria for the following comparisons.
Radiofrequency ablation versus endovenous steam ablation.
Endovenous steam ablation versus ultrasound‐guided foam sclerotherapy.
Endovenous steam ablation versus cyanoacrylate glue.
Endovenous steam ablation versus mechanochemical ablation.
Endovenous steam ablation versus SFJ ligation and stripping.
Ultrasound‐guided foam sclerotherapy versus cyanoacrylate glue.
Ultrasound‐guided foam sclerotherapy versus mechanochemical ablation.
Cyanoacrylate glue versus mechanochemical ablation.
Cyanoacrylate glue versus SFJ ligation and stripping.
Mechanochemical ablation versus SFJ ligation and stripping.
The duration of follow‐up for included trials ranged from five weeks (Subramonia 2010), to eight years (FOAM 2010). The outcome measures for each of the included trials can be found in Table 11.
5. Outcome measures.
| Technique | Study | Outcome measure | ||||||||
|
Technical success |
Complications |
Recurrence/ recanalisation |
Pain |
QoL Score |
VCSS | Duration of procedure | Inpatient/day case | Return to normal activities/work | ||
| EVLA versus RFA | Nordon 2011 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Rasmussen 2011 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Recovery 2009 | ✓ | ✓ | ✓ | ✓ | ||||||
| Shepherd 2010 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Syndor 2017 | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| EVLA versus EVSA | LAST 2014 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| EVLA versus UGFS | Magna 2013 | ✓ | ✓ | ✓ | ✓ | |||||
| Rasmussen 2011 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Vernermo 2016 | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| EVLA versus CA | Calik 2019 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| EVLA versus MOCA | Vähäaho 2019 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
|
EVLA versus HL/S (surgery) |
Darwood 2008 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Flessenkämper 2013 | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| HELP‐1 2011 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Magna 2013 | ✓ | ✓ | ✓ | ✓ | ||||||
| Pronk 2010 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Rasmussen 2007 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Rasmussen 2011 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| RELACS 2012 | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Vernermo 2016 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| RFA versus UGFS | Rasmussen 2011 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| RFA versus CA | Morrison 2015 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| RFA versus MOCA | Lane 2017 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| MARADONA 2019 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Vähäaho 2019 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| RFA versusHL/S(surgery) | EVOLVeS 2003 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Helmy ElKaffas 2011 | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Rasmussen 2011 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Rautio 2002 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Subramonia 2010 | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
|
UGFS versus HL/S (surgery) |
FOAM 2010 | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Magna 2013 | ✓ | ✓ | ✓ | ✓ | ||||||
| Rasmussen 2011 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Vernermo 2016 | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
CA: cyanoacrylate glue EVLA: endovenous laser ablation EVSA: endovenous steam ablation HL/S: high ligation and stripping MOCA: mechanochemical ablation QoL: quality of life RFA: radio frequency ablation UGFS: ultrasound‐guided foam sclerotherapy VCSS: Venous Clinical Severity Score
Excluded studies
We excluded 20 new studies for this update (Basela 2011; Campos 2015; CLASS 2014; De Oliveira 2018; Desai 2009; dos Santos 2020; Eroglu 2018; Honek 2019; Jindal 2018; Karathanos 2019; Kikuchi 2009; Leon 2018; Leung 2019; Mendes 2016; Mozafar 2014; Oster 2018; Ovali 2019; Shadid 2015; Sincos 2018; Tawfik 2020). Due to the wider scope of this update, we included two studies which were previously excluded (Recovery 2009; Shepherd 2010).
The total number of excluded studies is 33 (Basela 2011; Campos 2015; Chant 1972; Christenson 2010; CLASS 2014; Compagna 2010; De Medeiros 2006; De Oliveira 2018; Desai 2009; Disselhoff 2008; dos Santos 2020; Einarsson 1993; Eroglu 2018; Figueiredo 2009; Honek 2019; Jindal 2018; Kalodiki 2012; Karathanos 2019; Kikuchi 2009; Lattimer 2012; Leon 2018; Leung 2019; Lin 2007; Mendes 2016; Mozafar 2014; Oster 2018; Ouvry 2008; Ovali 2019; Shadid 2015; Sincos 2018; Stotter 2005; Tawfik 2020; Wright 2006). See Characteristics of excluded studies table.
A common reason for exclusion was the combination of GSV and small saphenous vein (SSV) participants within the context of a trial. This was the case for CLASS 2014, Eroglu 2018, Figueiredo 2009, Sincos 2018 and Wright 2006. We were unable to obtain GSV data to allow meta‐analysis where applicable. Some studies included techniques not covered within the scope of this review as they are novel or hybrid techniques. These included cryostripping (Disselhoff 2008; Stotter 2005), ligation and axial ablation by foam or EVLA (Compagna 2010; De Medeiros 2006; Kalodiki 2012), RFA plus UGFS (Leon 2018), and ligation of the SFJ only (Mozafar 2014). dos Santos 2020 compared UGFS with UGFS plus tumescence. Honek 2019 compared different types of laser generator in EVLA. Tawfik 2020 performed additional UGFS to EVLA and/or ablated small or accessory veins and/or used foam injections for severely tortuous anterior saphenous vein and superficial varicosities. Lattimer 2012 combined EVLA with phlebectomies versus UGFS. Three studies were excluded as the techniques included liquid sclerotherapy (Chant 1972; Einarsson 1993; Ouvry 2008). Three studies were found not to be randomised controlled trials and therefore were not included (Basela 2011; Ovali 2019; Shadid 2015). Three studies were found to offer simultaneous treatment to both limbs and therefore were excluded (Christenson 2010; Jindal 2018; Mendes 2016). Campos 2015, De Oliveira 2018, and Leung 2019 were excluded due to the inclusion of participants with CEAP C5 or C6 disease, or both. Karathanos 2019 and Oster 2018 included participants with CEAP class C2 to C6. Two studies were conference abstracts only with no data available after contacting authors (Desai 2009; Kikuchi 2009). One study was found to be in a language besides English and despite translation, no meaningful data could be extracted (Lin 2007).
Ongoing studies
We identified four new ongoing studies for this update (Belramman 2018; Cho 2020; NCT04526626; NCT04534244). See Characteristics of ongoing studies.
Studies awaiting classification
We identified four studies from a top‐up search and will incorporate these into the next version of this review (Belramman 2020; Morrison 2020; Rai 2019; Vähäaho 2021). See Characteristics of studies awaiting classification.
Risk of bias in included studies
Risk of bias within each of the included studies is discussed in the Characteristics of included studies section and illustrated by Figure 2 and Figure 3. In summary, there was a significant risk of bias in the majority of included studies that limited our certainty in the evidence. The greatest areas of weakness included the lack of both study personnel and participant blinding that may have introduced observer and performance bias. It is accepted, however, that a number of these interventions differ significantly in the way in which they are performed. It would be impossible to blind a participant to a general anaesthesia open surgical operation compared to a local anaesthesia endovenous procedure. However, some of these difficulties could be mitigated by study personnel blinding.
2.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies
3.

Methodological quality summary: review authors' judgements about methodological quality for each domain for each included study
Allocation
Nine studies were at unclear risk of bias as it was unclear whether their methods were truly random or they gave insufficient descriptions of generation methods used (EVOLVeS 2003; Helmy ElKaffas 2011; HELP‐1 2011; Rasmussen 2011; Recovery 2009; RELACS 2012; Subramonia 2010; Syndor 2017; Vernermo 2016). The remaining 15 studies thoroughly reported their random sequence methods so were at low risk (Calik 2019; Darwood 2008; Flessenkämper 2013; FOAM 2010; Lane 2017; LAST 2014; Magna 2013; MARADONA 2019; Morrison 2015; Nordon 2011; Pronk 2010; Rasmussen 2007; Rautio 2002; Shepherd 2010; Vähäaho 2019).
Risk of bias due to allocation concealment was deemed to be high within three studies, as methods of concealment were not described (Helmy ElKaffas 2011; MARADONA 2019; RELACS 2012). The single‐blinded Recovery 2009 study was deemed to be at unclear risk, as they only stated that they did not discuss the allocated treatment with the participant. Shepherd 2010 was also deemed to be at unclear risk of allocation bias as they only stated they used Internet randomisation. The other 19 studies were judged to be at low risk of allocation concealment bias as methods of allocation concealment were adequately described (Calik 2019; Darwood 2008; EVOLVeS 2003; Flessenkämper 2013; FOAM 2010; HELP‐1 2011; Lane 2017; LAST 2014; Magna 2013; Morrison 2015; Nordon 2011; Pronk 2010; Rasmussen 2007; Rasmussen 2011; Rautio 2002; Subramonia 2010; Syndor 2017; Vähäaho 2019; Vernermo 2016).
Blinding
Syndor 2017 was the only double‐blinded RCT amongst the included studies and was therefore deemed to be at low risk of performance and detection bias. The Recovery 2009 and Shepherd 2010 studies were single‐blinded trials (participants were blinded but the assessors were not blinded), thus conferring a low risk of bias in performance bias and high risk for detection. In Nordon 2011, the participants were blinded (low risk of performance bias) but assessor was blinded until the three‐month follow‐up scan, so this was judged to be at unclear risk of detection bias. Lane 2017 and MARADONA 2019 were deemed at high risk of performance bias as participants were not blinded but deemed at low risk of detection bias because of blinded duplex ultrasound scanning. The remaining 18 studies were all deemed to have a high risk of performance and detection bias, as none of the participants or assessors were blinded (Calik 2019; Darwood 2008; EVOLVeS 2003; Flessenkämper 2013; FOAM 2010; Helmy ElKaffas 2011; HELP‐1 2011; LAST 2014; Magna 2013; Morrison 2015; Pronk 2010; Rasmussen 2007; Rasmussen 2011; Rautio 2002; RELACS 2012; Subramonia 2010; Vähäaho 2019; Vernermo 2016). It is appreciated that some interventions compared do not lend themselves to participant blinding.
Incomplete outcome data
Four studies were determined to be at high risk of attrition bias (Calik 2019; EVOLVeS 2003; Pronk 2010; Recovery 2009). Calik 2019 did not always state the number of participants analysed for outcomes at follow‐up intervals, and they did not provide a cohort diagram. EVOLVeS 2003 provided details on all missing data. However, we noted an imbalance in the study treatment groups. There were also discrepancies between missing outcomes and explanations for these in the two‐year follow‐up paper. Pronk 2010 stated that two participants were lost at six weeks' follow‐up, but gave no explanations. There was also an unexplained discrepancy between study groups and participant follow‐up at one year. Recovery 2009 did not discuss their dropouts and the number of participants analysed for outcomes at follow‐up was not given. Six studies were deemed to be at unclear risk of attrition bias as dropouts were reported but no explanation given (Flessenkämper 2013; LAST 2014; MARADONA 2019; Nordon 2011; Syndor 2017; Vähäaho 2019). The remaining 14 studies were deemed to be at low risk of attrition bias (Darwood 2008; FOAM 2010; Helmy ElKaffas 2011; HELP‐1 2011; Lane 2017; Magna 2013; Morrison 2015; Rasmussen 2007; Rasmussen 2011; Rautio 2002; RELACS 2012; Shepherd 2010; Subramonia 2010; Vernermo 2016).
Selective reporting
The majority of studies had low risk of reporting bias as all predefined outcomes were reported. Magna 2013 did not report on several complications outlined in their methods, whilst Morrison 2015 did not report on analgesia use as planned, so we judged these studies to be at unclear risk of reporting bias. Calik 2019 did not explicitly state the outcome measures they intended to report.
Other potential sources of bias
The majority of studies (as shown in Table 12) used concomitant phlebectomies in their treatment groups, often at the discretion of the treating practitioner. This potentially introduces bias into outcomes such as life measures, pain and return to work. Some studies, including Calik 2019, Darwood 2008, LAST 2014 and Recovery 2009, tried to mitigate this potential source of bias by offering phlebectomies several weeks or months after the initial index procedure.
6. Additional phlebectomies.
| EVLA versus RFA | ||
| Study | Additional phlebectomies | |
| EVLA | RFA | |
| Nordon 2011 | yes | yes |
| Rasmussen 2011 | yes | yes |
| Recovery 2009 | after 30 days | after 30 days |
| Shepherd 2010 | yes | yes |
| Syndor 2017a | yes | yes |
| EVLA versus EVSA | ||
| Study | Additional phlebectomies | |
| EVLA | EVSA | |
| LAST 2014 | after 3 months | after 3 months |
| EVLA versus UGFS | ||
| Study | Additional phlebectomies | |
| EVLA | UGFS | |
| Magna 2013 | yes | yes |
| Rasmussen 2011 | yes | yes |
| Vernermo 2016 | yes | no |
| EVLA versus CA | ||
| Study | Additional phlebectomies | |
| EVLA | CA | |
| Calik 2019 | after 3 months | after 3 months |
| EVLA versus MOCA | ||
| Study | Additional phlebectomies | |
| EVLA | MOCA | |
| Vähäaho 2019 | yes | yes |
| EVLA versus HL/S (surgery) | ||
| Study | Additional phlebectomies | |
| EVLA | HL/S (surgery) | |
| Darwood 2008 | yes ‐ at 6 weeks | yes |
| Flessenkämper 2013 | yes | yes |
| HELP‐1 2011 | yes | yes |
| Magna 2013 | yes | yes |
| Pronk 2010 | yes | yes |
| Rasmussen 2007 | yes | yes |
| Rasmussen 2011 | yes | yes |
| RELACS 2012 | yes | yes |
| Vernermo 2016 | yes | yes |
| RFA versus UGFS | ||
| Study | Additional phlebectomies | |
| RFA | UGFS | |
| Rasmussen 2011 | yes | yes |
| RFA versus CA | ||
| Study | Additional phlebectomies | |
| RFA | CA | |
| Morrison 2015 | no | no |
| RFA versus MOCA | ||
| Study | Additional phlebectomies | |
| RFA | MOCA | |
| Lane 2017 | yes | yes |
| MARADONA 2019 | no | yes |
| Vähäaho 2019 | yes | yes |
| RFA versus HL/S (surgery) | ||
| Study | Additional phlebectomies | |
| RFA | HL/S (surgery) | |
| EVOLVeS 2003 | yes | yes |
| Helmy ElKaffas 2011 | yes | yes |
| Rasmussen 2011 | yes | yes |
| Rautio 2002 | yes | yes |
| Subramonia 2010 | yes | yes |
| UGFS versus HL/S (surgery) | ||
| Study | Additional phlebectomies | |
| UGFS | HL/S (surgery | |
| FOAM 2010 | yes | yes |
| Magna 2013 | yes | yes |
| Rasmussen 2011 | yes | yes |
| Vernermo 2016 | no | yes |
aParticipants were offered ambulatory phlebectomy or UGFS.
CA: cyanoacrylate glue EVLA: endovenous laser ablation EVSA: endovenous steam ablation HL/S: high ligation and stripping RFA: radio frequency ablation MOCA: mechanochemical ablation UGFS: ultrasound‐guided foam sclerotherapy
Only Rautio 2002 and Lane 2017 were found to be at low risk of other potential sources of bias. Calik 2019 was found to be at high risk of bias. The remaining 21 trials had potential sources of bias which were deemed to be of unclear risk.
In the Calik 2019 study, bilateral limbs were evaluated. The study authors made no attempt to account for the impact this may have had on outcomes such as pain and return to work. It was not explicitly stated whether each limb received the same treatment. Although the study population was 400 participants, study authors had performed no power analysis. Also, Calik 2019 did not specify definitions for occlusion, partial and total recanalisation and used the Wong‐Baker FACES pain scale, which is a paediatric pain assessment scale.
Darwood 2008 were unable to meet their necessary sample size. Therefore, the study authors declared that their sample size was insufficient to permit statistical testing for equivalence. The study also included participants who underwent bilateral treatment: these were allocated the same treatment on both limbs; however, they were not stratified within the results. Participants who underwent SFJ ligation and stripping also underwent concomitant phlebectomies. Those who were allocated to EVLA could request injection sclerotherapy for residual varicosities at six weeks. There was no stratification for these participants, and this could potentially add a risk of bias to participant satisfaction and QoL scores. We also noted that one participant randomised to SFJ ligation and stripping underwent EVLA, and was followed up in the EVLA cohort, showing no analysis with intention‐to‐treat.
The EVOLVeS 2003 study received financial support from VNUS Medical Technologies (manufacturers of RFA catheters). The trial centres were also proctored by the company, introducing a potential source of bias. The trial also included one participant who underwent treatment of both limbs. The participant was only randomised once and each limb was treated as a separate episode after a period of three months.
Flessenkämper 2013 calculated that 469 participants were required in the trial, but only 449 were randomised, meaning the study is potentially underpowered. A further source of bias is the admission of a number of participants undergoing concomitant phlebectomies within their respective treatments arms. This procedure could impact upon pain scores, QoL and return to work.
Mini‐phlebectomies were also performed at the operating surgeon's discretion in the FOAM 2010 study in both the SFJ ligation and stripping and UGFS arms. Although the numbers of such participants were given, this procedure could alter the pain and other outcomes.
In Helmy ElKaffas 2011, it was unclear whether participants undergoing bilateral treatment were included or excluded. Concomitant phlebectomies were performed in both the RFA and SFJ ligation and stripping groups. Although the numbers of such procedures were given for both groups, there was no analysis of the impact that this could have had on outcomes such as complications, length of procedure and hospital stay, so this omission introduces a potential source of bias. In addition, some participants required UGFS for persistent varicosities following RFA. However, the timeframe for the additional procedure was not discussed and the only subanalysis of this group was a financial one.
As with other studies, the concomitant use of phlebectomies within HELP‐1 2011 introduced a potential source of bias. The study was also possibly underpowered: a power calculation described a need for 120 participants in each group, but only 113 were available for follow‐up in the surgery group.
LAST 2014 was also underpowered: power calculations required a total of 116 participants per study group, but there were only 92 and 107 participants in the EVLA and EVSA arms, respectively, due to dropouts. In addition, the protocol for the amount of energy required for EVSA was changed during the trial. In LAST 2014 the legs of participants with bilateral GSV incompetence were included separately, provided that there was at least 3 months between the two treatments.
The Magna 2013 trial also included simultaneously treated bilateral limbs. The study authors did not indicate how they analysed the impact of this on quality of life and other measures, conferring a potential risk of bias. The methods stated the intention of performing additional phlebectomies at the time of the initial procedure, but in several cases, the procedure was undertaken at three months. There was no subanalysis for this group of participants. The trial was also possibly underpowered: their power calculation stated that 240 participants would be required, but only 223 were analysed.
Trialists stopped enrolling participants in the MARADONA 2019 study earlier than planned. This was because reimbursement of MOCA treatment was suspended for treatments for class CEAP C3 disease and lower. The study was therefore only able to recruit 46% of the calculated required number of participants and was significantly underpowered for the anatomic success outcome measure. The trial also consequently included a higher proportion of participants with more severe chronic venous insufficiency compared to other such trials.
Additional sources of potential bias within the Morrison 2015 trial included the fact that the authors of the study were paid consultants of Sapheon, a company which manufactures cyanoacrylate glue. However, independent evaluation of ultrasound images was undertaken. The study stated that there were 31 missing or uninterruptible ultrasound scan (USS) reports. Attempts to account for this were made by the study authors by analysing the outcomes via various models for inputting missing data.
In order to blind their participants, Nordon 2011 performed RFA and EVLA under general anaesthesia, whilst all other studies performed these interventions under spinal or conscious sedation. The use of general anaesthesia could have an impact on pain scores, duration of hospital stay, QoL scores and expose participants to risk of anaesthetic‐related complications avoided when the procedure is performed under block or local techniques. The use of general anaesthetic in these procedures is not standard practice, and thus potentially confers a risk of bias.
Pronk 2010 performed both EVLA and SFJ ligation and stripping under tumescent anaesthesia. Other studies evaluating SFJ ligation and stripping have not uniformly used this anaesthetic modality; therefore, it may confer an advantage in the Pronk 2010 trial and impact upon outcomes such as participant‐reported post‐operative pain, QoL, hospital stay and return to normal activities. The study was potentially underpowered: power calculations described a need for 120 participants in each treatment arm, yet only 113 participants were available for follow‐up in the surgery group. The study is unclear about participants who underwent simultaneous bilateral intervention. The study authors claimed participants were only randomised to an intervention once, but the number randomised is reported as legs (130) and not participants (n = 122).
The inclusion of participants with simultaneous bilateral varicosities, with no subsequent stratification within the results, introduces a possible further source of bias in Rasmussen 2007. However, all participants with bilateral disease received the same intervention.
Contrary to the inclusion criteria of this review, Rasmussen 2011 also included a small number of participants who had had previous SFJ ligation on the basis that they had recanalised their GSV and had a patent, refluxing SFJ and GSV. There was no stratification of these participants within the study's results or amongst the treatment arms, conferring a potential source of bias. The technique for EVLA was not uniform within Rasmussen 2011, with different methods, energies and diodes used amongst the trial centres. The trialists also analysed their results by limbs not participants.
We judged the Recovery 2009 study to have an unclear risk of further bias as it was sponsored by VNUS Medical Technologies, who manufacture radiofrequency ablation catheters.
In the RELACS 2012 study, there was no clear consensus on the number of additional phlebectomies, thereby impacting upon the outcomes of pain, QoL and return to normal activities. After three months, those with apparent residual varices and perforators could be treated with additional phlebectomies or sclerotherapy. This trial was also possibly underpowered: a total of 180 participants per treatment group was calculated, but after dropouts and losses to follow‐up, the EVLA group had 173 and SFJ ligation and stripping group had 143.
Shepherd 2010 allowed for additional phlebectomies at the time of the procedure, as well as treatment for SSV and anterior thigh vein incompetence. The study authors stated that pain analysis was subsequently adjusted to make allowances for this. The study included participants undergoing concurrent treatment of bilateral disease. The most symptomatic limb (participant‐reported) was randomised and both limbs received the same intervention. However, this approach impacts on pain and return to normal activities, suggesting a possible risk of bias.
The Subramonia 2010 trial included five participants with recurrent varicose veins, but there was no stratification of these individuals in the results. This could introduce potential bias into results such as pain, return to normal activities and QoL. The trial also Included 12 participants with bilateral varicose veins (randomised on one occasion to the same treatment, with a minimum of six weeks between treatment of the limbs, thus treating each limb as a separate case).
In Syndor 2017, it was noted that there was a vast range in the time frames at which participants were being followed up. For instance, the initial follow‐up review ranged from one to 29 days, and participants who were followed up at one year were being included within the analysis of outcome measures at the six‐week review. Therefore, potentially, participants who could have had complications at six weeks were being missed as they were only seen at one year, by which point, the complication may have resolved ‐ this introduces a risk of bias. Participants underwent concomitant phlebectomies and UGFS, an approach not undertaken in other studies, thereby impacting on the risk of bias for outcomes such as pain, QoL and return to normal activities. No power calculations were performed.
Vähäaho 2019 did not manage to recruit the calculated required sample size (132 instead of 160 participants). Concomitant phlebectomies were also performed, which could impact upon pain and complications such as saphenous nerve injury.
The authors of Vernermo 2016 state that, "Owing to the operating surgeon's preference, five patients originally randomised to EVLA were treated with surgery but, because the analysis was made according to intention to treat, these patients were analysed in EVLA group". There was no further clarification why the surgeon preferred to undertake surgery in these individuals, and no subanalysis. The EVLA diode was also changed from a 980‐nm diode to a 1470‐nm diode during the course of the trial. In comparison to other trials, the sclerosant used in the UGFS arm was more concentrated (air to sclerosant ratio 2:1).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6
Summary of findings 1. Endovenous laser ablation (EVLA) compared to radiofrequency ablation (RFA) for great saphenous vein (GSV) incompetence.
| EVLA compared to RFA for GSV incompetence | ||||||
| Patient or population: people with GSV incompetence Setting: hospital Intervention: EVLA Comparison: RFA | ||||||
| Outcomes | Anticipated absolute effects * (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with RFA | Risk with EVLA | |||||
|
Technical success (< 5 years) |
Study population | OR 0.98 (0.41 to 2.38) | 780 (5 studies) | ⊕⊕⊕⊝ moderatea | ||
| 975 per 1000 | 974 per 1000 (940 to 989) | |||||
|
Technical success (> 5 years) |
Study population | OR 0.85 (0.30 to 2.41) | 291 (1 study) | ⊕⊕⊝⊝ lowb | ||
| 952 per 1000 | 944 per 1000 (857 to 980) | |||||
|
Recurrence (< 5 years) |
Study population | OR 1.53 (0.78 to 2.99) | 291 (1 study) | ⊕⊕⊝⊝ lowb | ||
| 116 per 1000 | 167 per 1000 (93 to 281) | |||||
|
Long‐term recurrence (> 5 years) |
Study population | OR 2.77 (1.52 to 5.06) | 291 (1 study) | ⊕⊕⊝⊝ lowb | ||
| 129 per 1000 | 291 per 1000 (184 to 429) | |||||
|
Complications (up to 8 years) |
See comment | ⊕⊝⊝⊝ very lowc | Analysis was prevented as studies reported minor and major complications using different definitions and at varying time points. Results of individual studies were inconsistent with each other so we are not able to draw any conclusions. | |||
|
QoL (up to 8 years) |
See comment | ⊕⊕⊝⊝ moderatea | The majority of studies for this comparison showed no difference in QoL scores between the two variables. Nordon 2011 showed no difference in improvement using AVVQ and EQ‐5D at three months. There was no difference in AVVQ or SF‐12 (in either the physical or mental component SF‐12) at 6 months in Shepherd 2010. Rasmussen 2011 found no difference in SF‐36 at 1 month or AVVQ at 3 years. Recovery 2009 reported improved global QoL scores in RFA at 7 and 14 days post‐operation but comparable by 1 month. Syndor 2017 did not measure QoL. | |||
| * The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; EVLA: endovenous laser ablation; GSV: great saphenous vein; OR: odds ratio; QoL: quality of life; RFA: radiofrequency ablation | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aWe downgraded by one level due to risk of bias concerns. bWe downgraded by two levels due to risk of bias concerns and possible imprecision. cWe downgraded by three levels due to risk of bias concerns, inconsistency, imprecision and possible publication bias.
Summary of findings 2. Endovenous laser ablation (EVLA) compared to ultrasound‐guided foam sclerotherapy (UGFS) for great saphenous vein (GSV) incompetence.
| EVLA compared to UGFS for GSV incompetence | ||||||
| Patient or population: people with GSV incompetence Setting: hospital Intervention: EVLA Comparison: UGFS | ||||||
| Outcomes | Anticipated absolute effects * (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with UGFS | Risk with EVLA | |||||
|
Technical success (< 5 years) |
Study population | OR 6.13 (0.98 to 38.27) | 588 (3 studies) |
⊕⊕⊝⊝ lowa | ||
| 802 per 1000 | 961 per 1000 (799 to 994) | |||||
|
Technical success (> 5 years) |
Study population | OR 6.47 (2.60 to 16.10) | 534 (3 studies) | ⊕⊕⊝⊝ lowa | ||
| 626 per 1000 | 915 per 1000 (813 to 964) | |||||
|
Recurrence (< 5 years) |
Study population | OR 0.68 (0.20 to 2.36) | 443 (2 studies) | ⊕⊝⊝⊝ very lowb | ||
| 186 per 1000 | 134 per 1000 (44 to 350) | |||||
|
Long‐term recurrence (> 5 years) |
Study population | OR 1.08 (0.40 to 2.87) | 418 (2 studies) | ⊕⊝⊝⊝ verylowb | ||
| 232 per 1000 | 246 per 1000 | |||||
|
Complications (up to 8 years) |
See comment | ⊕⊝⊝⊝ verylowc | All three studies reported on this outcome but using different definitions and at varying time points. Rasmussen 2011 reported more phlebitis and hyperpigmentation rates amongst the UGFS group. In Vernermo 2016, skin pigmentation was more common in the UGFS group but haematomas were seen more often after EVLA compared to UGFS at 1 month. Magna 2013 reported two cases of hyperpigmentation in EVLA participants compared to one case in UGFS at 3 months. |
|||
|
QoL (up to 8 years) |
See comment | ⊕⊕⊕⊝ moderated |
Magna 2013 reported no significant differences between EVLA and UGFS at 3 months and 1 year in CIVIQ2 and EQ‐5D scores. In Rasmussen 2011, UGFS was deemed to be better for bodily pain and physical functioning in the SF‐36 score initially. AVVSS showed no difference between comparisons at 1 month. Vernermo 2016 found no significant difference in median AVVSS between the treatment groups at 1 year |
|||
| * The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; EVLA: endovenous laser ablation; GSV: great saphenous vein; OR: odds ratio; QoL: quality of life; UGFS: ultrasound‐guided foam sclerotherapy | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aWe downgraded by two levels due to risk of bias concerns and inconsistency. bWe downgraded by three levels due to risk of bias concerns, inconsistency and imprecision. cWe downgraded by three levels due to risk of bias concerns, inconsistency, imprecision and possible publication bias. dWe downgraded by one level due to risk of bias concerns.
Summary of findings 3. Endovenous laser ablation (EVLA) compared to SFJ ligation and stripping (HL/S) for great saphenous vein (GSV) incompetence.
| EVLA compared to HL/S for GSV incompetence | ||||||
| Patient or population: people with GSV incompetence Setting: hospital Intervention: EVLA Comparison: HL/S (surgery) | ||||||
| Outcomes | Anticipated absolute effects * (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with HL/S (surgery) | Risk with EVLA | |||||
|
Technical success (< 5 years) |
Study population | OR 2.31 (1.27 to 4.23) | 1051 (6 studies) | ⊕⊕⊝⊝ lowa | ||
| 933 per 1000 | 970 per 1000 (947 to 983) | |||||
|
Technical success (> 5 years) |
Study population | OR 0.93 (0.57 to 1.50) | 874 (5 studies) | ⊕⊕⊝⊝ lowa | ||
| 917 per 1000 | 911 per 1000 (863 to 943) | |||||
|
Recurrence (< 5 years) |
Study population | OR 0.78 (0.47 to 1.29) | 1459 (7 studies) | ⊕⊕⊕⊝ moderateb |
||
| 179 per 1000 | 146 per 1000 (93 to 220) | |||||
|
Long‐term recurrence (> 5 years) |
Study population | OR 1.09 (0.68 to 1.76) | 1267 (7 studies) | ⊕⊕⊕⊝ moderateb |
||
| 328 per 1000 | 347 per 1000 (249 to 462) | |||||
|
Complications (up to 8 years) |
See comment | ‐ | ⊕⊝⊝⊝ very lowc | Analysis was prevented as studies reported minor and major complications using different definitions and at varying time points. Slightly higher rates of early haematomas and wound problems were possibly seen with HL/S (surgery); and EVLA may be associated with slightly more phlebitis. | ||
|
QoL (up to 8 years) |
See comment | ‐ | ⊕⊕⊕⊝ moderateb |
Rates of improvement in QoL were comparable between both treatment groups in all studies. | ||
| * The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; EVLA: endovenous laser ablation; GSV: great saphenous vein; HL/S; SFJ ligation and stripping; OR: odds ratio; QoL: quality of life; UGFS: ultrasound‐guided foam sclerotherapy | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aWe downgraded by two levels due to risk of bias concerns and imprecision. bWe downgraded by one level due to risk of bias concerns. cWe downgraded by three levels due to risk of bias concerns, inconsistency, imprecision and possible publication bias.
Summary of findings 4. Radiofrequency ablation (RFA) compared to mechanochemical ablation (MOCA) for great saphenous vein incompetence.
| RFA compared to MOCA for GSV incompetence | ||||||
| Patient or population: people with GSV incompetence Setting: hospital Intervention: RFA Comparison: MOCA | ||||||
| Outcomes | Anticipated absolute effects * (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with MOCA | Risk with RFA | |||||
|
Technical success (< 5 years) |
Study population | OR 1.76 (0.06 to 54.15) | 435 (3 studies) | ⊕⊕⊝⊝ lowa | ||
| 983 per 1000 | 990 per 1000 (776 to 1000) | |||||
|
Technical success (> 5 years) |
See comment | ‐ | ‐ | ‐ | Data for this time point are not yet available. | |
|
Recurrence (< 5 years) |
Study population | OR 1.00 (0.21 to 4.81) | 389 (3 studies) | ⊕⊕⊝⊝ lowa | ||
| 117 per 1000 | 117 per 1000 (27 to 390) | |||||
|
Long‐term recurrence (≥ 5 years) |
See comment | ‐ | ‐ | ‐ | Data for this time point are not yet available. | |
|
Complications (up to 1 year) |
See comment | ‐ | ‐ | ⊕⊝⊝⊝ very lowb | Analysis was prevented as studies reported minor and major complications using different definitions and at varying time points, but rates were similar between treatment groups. | |
|
QoL (AVVQ, EQ‐5D) (up to 1 year) |
See comment | ‐ | ‐ | ⊕⊕⊕⊝ moderatec |
No differences detected between groups at any time point during the studies. | |
| * The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; GSV; great saphenous vein; MOCA: mechanochemical ablation; OR: odds ratio; QoL: quality of life; RFA: radiofrequency ablation | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aWe downgraded by two levels due to risk of bias concerns and inconsistency. bWe downgraded by three levels due to risk of bias concerns, inconsistency and possible publication bias. cWe downgraded by one level due to risk of bias concerns.
Summary of findings 5. Radiofrequency ablation (RFA) compared to SFJ ligation and stripping (HL/S) for great saphenous vein (GSV) incompetence.
| RFA compared to HL/S for GSV incompetence | ||||||
| Patient or population: people with GSV incompetence Setting: hospital Intervention: RFA Comparison: HL/S (surgery) | ||||||
| Outcomes | Anticipated absolute effects * (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with HL/S (surgery) | Risk with RFA | |||||
|
Technical success (< 5 years) |
Study population | OR 5.71 (0.64 to 50.81) | 318 (2 studies) | ⊕⊕⊝⊝ lowa |
||
| 974 per 1000 | 995 per 1000 (960 to 999) | |||||
|
Technical success (> 5 years) |
Study population | OR 0.88 (0.29 to 2.69) | 289 (1 study) | ⊕⊕⊝⊝ lowb |
||
| 958 per 1000 | 952 per 1000 (868 to 984) | |||||
|
Recurrence (< 5 years) |
Study population | OR 0.93 (0.58 to 1.51) | 546 (4 studies) | ⊕⊕⊕⊝ moderatec |
||
| 147 per 1000 | 138 per 1000 (91 to 206) | |||||
|
Long‐term recurrence (> 5 years) |
Study population | OR 0.41 (0.22 to 0.75) | 289 (1 study) | ⊕⊕⊝⊝ lowb |
||
| 268 per 1000 | 130 per 1000 (74 to 215) | |||||
|
Complications (up to 8 years) |
See comment | ⊕⊝⊝⊝ very lowd | Analysis was prevented as studies reported minor and major complications using different definitions and at varying time points. Overall the number of complications was low, but surgery may be associated with slightly higher rates of wound problems, haematomas and saphenous nerve injuries and more phlebitis was seen after RFA. | |||
|
QoL (up to 8 years) |
See comment | ⊕⊕⊕⊝ moderatec |
None of the studies detected a difference between treatment arms by four months. | |||
| * The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; GSV; great saphenous vein; HL/S; SFJ ligation and stripping; OR: odds ratio; QoL: quality of life; RFA: radiofrequency ablation | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aWe downgraded by two levels due to risk of bias concerns and inconsistency. bWe downgraded by two levels due to risk of bias concerns and imprecision. cWe downgraded by one level due to risk of bias concerns. dWe downgraded by three levels due to risk of bias concerns, inconsistency, imprecision and possible publication bias.
Summary of findings 6. Ultrasound‐guided foam sclerotherapy (UGFS) compared to SFJ ligation and stripping (HL/S) for great saphenous vein (GSV) incompetence.
| UGFS compared to HL/S for GSV incompetence | ||||||
| Patient or population: people with GSV incompetence Setting: hospital Intervention: UGFS Comparison: HL/S (surgery) | ||||||
| Outcomes | Anticipated absolute effects * (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with HL/S (surgery) | Risk with UGFS | |||||
|
Technical success (< 5 years) |
Study population | OR 0.32 (0.11 to 0.94) | 954 (4 studies) | ⊕⊕⊝⊝ lowa | ||
| 888 per 1000 | 718 per 1000 (467 to 882) | |||||
|
Technical success (> 5 years) |
Study population | OR 0.09 (0.03 to 0.30) | 525 (3 studies) | ⊕⊕⊕⊝ moderateb |
||
| 929 per 1000 | 542 per 1000 (283 to 798) | |||||
|
Recurrence (< 5 years) |
Study population | OR 1.81 (0.87 to 3.77) | 822 (3 studies) | ⊕⊕⊝⊝ lowc | ||
| 168 per 1000 | 267 per 1000 (149 to 431) | |||||
|
Long‐term recurrence (≥ 5 years) |
Study population | OR 1.24 (0.57 to 2.71) | 639 (3 studies) | ⊕⊕⊝⊝ lowc | ||
| 380 per 1000 | 432 per 1000 (259 to 624) | |||||
|
Complications (up to 8 years) |
See comment | 639 (3 studies) |
⊕⊝⊝⊝ very lowd | Analysis was prevented as studies reported minor and major complications using different definitions and at varying time points. | ||
|
QoL (up to 8 years) |
See comment | 930 (4 studies) |
⊕⊕⊕⊝ moderateb |
None of the five included studies showed evidence of a difference in QoL scores between the two treatment groups. | ||
| * The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; GSV; great saphenous vein; HL/S: SFJ ligation and stripping; OR: odds ratio; QoL: quality of life; UGFS: ultrasound‐guided foam sclerotherapy | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aWe downgraded by two levels due to risk of bias concerns and inconsistency. bWe downgraded by one level due to risk of bias concerns. cWe downgraded by two levels due to risk of bias concerns and inconsistency. dWe downgraded by three levels due to risk of bias concerns, inconsistency and possible publication bias.
Study authors reported on outcomes using different definitions and at different time points, which impacted our ability to carry out analyses. We provide a brief description of how studies reported on outcomes below. We then present the results by comparison to allow consistent reporting between analyses and the summary of findings tables.
Technical success
We defined technical success as complete anatomical obliteration or absence of reflux within the GSV at six weeks on duplex ultrasound (DUS; standard criterion of 1 second (s) of reflux on DUS). This was evaluated in 18 studies at different time points (Calik 2019; Darwood 2008; FOAM 2010; HELP‐1 2011; Lane 2017; LAST 2014; Magna 2013; MARADONA 2019; Morrison 2015; Nordon 2011; Rasmussen 2007; Rasmussen 2011; Rautio 2002; Recovery 2009; Shepherd 2010; Syndor 2017; Vähäaho 2019; Vernermo 2016). Three trials reported technical success at four weeks (Calik 2019; Recovery 2009; Morrison 2015). As Calik 2019 and Morrison 2015 were the sole studies for their comparison, their data were included. Vähäaho 2019 reported on technical success at 30 days. The results of these studies are shown in Table 13. Rasmussen 2011 reported on technical failure which they defined as "an open refluxing segment of 10cm or more at follow up". We were therefore able to extrapolate their technical success rate from the figures they presented. The primary outcome in Flessenkämper 2013 was inguinal reflux, which they defined as 'any reflux from the SFJ into the GSV lasting > 0.5 seconds'. Due to the manner in which they reported these data, we were unable to extrapolate this and include it within our technical success analysis. We defined long‐term technical success as complete anatomical obliteration, or absence of reflux, within the GSV on DUS at five years or greater. Five trials reported on this (HELP‐1 2011; Magna 2013; Rasmussen 2007; Rasmussen 2011; Vernermo 2016).
7. Technical success.
| EVLA versus RFA | ||
| Study ‐ time point | Technical success (%) | |
| EVLA | RFA | |
| Nordon 2011 3 months | 65/68 (96) | 68/70 (97) |
|
Rasmussen 2011 1 month 5 yr |
143/144 (99) 136/144 (94) |
148/148 (100) 140/147 (95) |
| Recovery 2009 1 month | 41/41 (100) | 46/46 (100) |
| Shepherd 2010 6 months | 50/54 (93) | 50/56 (89) |
| Syndor 2017 6 months | 77/79 (97) | 72/74 (97) |
| EVLA versus EVSA | ||
| Study ‐ time point | Technical success (%) | |
| EVLA | EVSA | |
| LAST 2014 1 yr | 88/92 (96) | all 93/107 (87) higha 68/74 (92) |
| EVLA versus UGFS | ||
| Study ‐ time point | Technical success (%) | |
| EVLA | UGFS | |
|
Magna 2013 1 yr 5 yr |
69/78 (88) 49/63 (77) |
56/77 (73) 15/67 (23) |
|
Rasmussen 2011 1 month 5 yr |
143/144 (99) 136/144 (94) |
142/144 (99) 124/144 (86) |
|
Vernermo 2016 1 yr 5 yr |
71/73 (93) 51/57 (89) |
37/72 (51) 30/59 (51) |
| EVLA versus CA | ||
| Study ‐ time point | Technical success (%) | |
| EVLA | CA | |
| Calik 2019 1 yr | 203/204 (99) | 208/208 (100) |
| EVLA versus MOCA | ||
| Study ‐ time point | Technical success (%) | |
| EVLA | MOCA | |
| Vähäaho 2019 30 days | 33/33 (100) | 55/55 (100) |
| EVLA versus HL/S (surgery) | ||
| Study ‐ time point | Technical success (%) | |
| EVLA | HL/S (surgery) | |
| Darwood 2008b 3 months | EVLT1. 41/42 (97) EVLT2. 26/29 (89) |
28/32 (87) |
| HELP‐1 2011 1 yr | 136/137 (99) 100/108 (92) |
122/137 (89) 94/110 (85) |
|
Magna 2013 1 yr 5 yr |
69/78 (88) 49/63 (78) |
60/68 (88) 53/63 (85) |
|
Rasmussen 2007 1 month 5 yr |
66/69 (96) 66/69 (96) |
66/68 (97) 66/68 (97) |
|
Rasmussen 2011 1 month 5 yr |
143/144 (99) 136/144 (94) |
139/142 (98) 136/142 (96) |
|
Vernermo 2016 1 yr 5 yr |
71/73 (93) 51/57 (89) |
59/61 (97) 48/50 (96) |
| RFA versus UGFS | ||
| Study ‐ time point | Technical success (%) | |
| RFA | UGFS | |
|
Rasmussen 2011 1 month 5 yr |
148/148 (100) 140/147 (95) |
142/144 (99) 124/144 (86) |
| RFA versus CA | ||
| Study ‐ time point | Technical success (%) | |
| RFA | CA | |
| Morrison 2015 1 month | 95/110 (85) | 115/115 (100) |
| RFA versus MOCA | ||
| Study ‐ time point | Technical success (%) | |
| RFA | MOCA | |
| Lane 2017 6 months | 67/68 (98) | 77/77 (100) |
| MARADONA 2019 30 days | 103/103 (100) | 99/103 (96) |
| Vähäaho 2019 30 days | 29/29 (100) | 55/55 (100) |
| RFA versus HL/S (surgery) | ||
| Study ‐ time point | Technical success (%) | |
| RFA | HL/S (surgery) | |
|
Rasmussen 2011 1 months 5 yr |
148/148 (100) 140/147 (95) |
139/142 (98) 136/142 (96) |
| Rautio 2002 mean 50 days | 15/15 (100) | 12/13 (92) |
| UGFS versus HL/S (surgery) | ||
| Study ‐ time point | Technical success (%) | |
| UGFS | HL/S (surgery) | |
| FOAM 2010 2 yr | 139/213 (65) | 140/177 (79) |
|
Magna 2013 1 yr 5 yr |
56/77 (73) 15/67 (22) |
60/68 (88) 53/63 (84) |
|
Rasmussen 2011 1 month 5 yr |
142/144 (99) 124/144 (86) |
139/142 (98) 136/142 (96) |
|
Vernermo 2016 1 yr 5 yr |
37/72 (51) 30/59 (51) |
59/61 (97) 48/50 (96) |
aHigh dose of steam bReported as limbs and not participants
CA: cyanoacrylate glue EVLA: endovenous laser ablation (same as EVLT) EVLT: endovenous laser therapy EVSA: endovenous steam ablation HL/S: high ligation and stripping MOCA: mechanochemical ablation RFA: radiofrequency ablation UGFS: ultrasound‐guided foam sclerotherapy yr: year(s)
Recurrence
We used the clinical definition reported by the clinician or the participant themselves. Definitions of recurrence used by the individual studies varied and are provided in the Characteristics of included studies tables. Fifteen studies reported recurrence (Calik 2019; EVOLVeS 2003; Flessenkämper 2013; FOAM 2010; Helmy ElKaffas 2011; HELP‐1 2011; Lane 2017; MARADONA 2019; Magna 2013; Pronk 2010; Rasmussen 2007; Rasmussen 2011; Rautio 2002; RELACS 2012; Vähäaho 2019). See Table 14 for recurrence data.
8. Recurrence.
| EVLA versus RFA | ||
| Study ‐ final time point | Recurrence noted at final time point (%) < 5 yr | |
| EVLA | RFA | |
| Nordon 2011 | No results | |
| Rasmussen 2011 3 yr | 24/144 (17) | 17/147 (12) |
| Recovery 2009 | No results | |
| Shepherd 2010 | No results | |
| Syndor 2017 | Mention 'recurrent symptoms' but do not specify what this entails | |
| EVLA versus EVSA | ||
| Study ‐ final time point | Recurrence noted at final time point (%) < 5 yr | |
| EVLA | EVSA | |
| LAST 2014 | No results | |
| EVLA versus UGFS | ||
| Study ‐ final time point | Recurrence noted at final time point (%) < 5 yrs | |
| EVLA | UGFS | |
| Magna 2013 1 yr | 9/78 (12) | 21/77 (27) |
| Rasmussen 2011 3 yr | 24/144 (17) | 20/144 (14) |
| Vernermo 2016 | No results | |
| EVLA versus CA | ||
| Study ‐ final time point | Recurrence noted at final time point (%) < 5 yrs | |
| EVLA | CA | |
| Calik 2019 1 yr | 5/204 (2) | 2/208 (1) |
| EVLA versus MOCA | ||
| Study ‐ final time point | Recurrence noted at final time point (%) < 5 yrs | |
| EVLA | MOCA | |
| Vähäaho 2019 1 yr | 0/33 (0) | 10/55 (18) |
| EVLA versus HL/S (surgery) | ||
| Study ‐ final time point | Recurrence noted at final time point (%) < 5 yrs | |
| EVLA | HL/S (surgery) | |
| Darwood 2008a 1 yr | No results | |
| Flessenkämper 2013 2 yr | 20/112 (17.8) | 11/94(11.7) |
| HELP‐1 2011 1 yr | 5/124 (4) | 23/113 (20) |
| Magna 2013 1 yr | 9/78 (12) | 6/68 (9) |
| Pronk 2010 1 yr | 5/62 (8) | 5/68 (7) |
| Rasmussen 2007 2 yr | 18/69 (26) | 25/68 (37) |
| Rasmussen 2011a 3 yr | 24/144 (17) | 22/143 (15) |
| RELACS 2012 2 yr | 28/173 (16) | 33/143 (23) |
| Vernermo 2016 | no results | |
| RFA versus UGFS | ||
| Study ‐ final time point | Recurrence noted at final time point (%) < 5 yrs | |
| RFA | UGFS | |
| Rasmussen 2011 | 17/147 (12) | 20/144 (14) |
| RFA versus CA | ||
| Study ‐ final time point | Recurrence noted at final time point (%) < 5 yrs | |
| RFA | CA | |
| Morrison 2015 | no results | |
| RFA versus MOCA | ||
| Study ‐ final time point | Recurrence noted at final time point (%) < 5 yrs | |
| RFA | MOCA | |
| Lane 2017 | 4/68 (6) | 3/77 (4) |
| MARADONA 2019 2 yr | 21/76 (28) | 12/81 (15) |
| Vähäaho 2019 1 yr | 0/32 (0) | 10/55 (18) |
| RFA versus HL/S (surgery) | ||
| Study ‐ final time point | Recurrence noted at final time point (%) < 5 yrs | |
| RFA | HL/S (surgery) | |
| EVOLVeS 2003 2 yr | 5/36 (14) | 6/29 (21) |
| Helmy ElKaffas 2011 2 yr | 12/81 (15) | 9/81 (11) |
| Rasmussen 2011a 3 yr | 17/148 (11) | 22/143 (15) |
| Rautio 2002 2 yr | 5/15 (33) | 2/13 (15) |
| Subramonia 2010 | not reported | |
| UGFS versus HL/S (surgery) | ||
| Study ‐ final time point | Recurrence noted at final time point (%) < 5 yrs | |
| UGFS | HL/S (surgery) | |
| FOAM 2010 2 yr | 75/213 (35) | 37/177 (21) |
| Magna 2013 1 yr | 21/77 (27) | 6/68 (9) |
| Rasmussen 2011a 3 yr | 20/144 (14) | 22/143 (15) |
| Vernermo 2016 | not reported | |
aReported as limbs and not participants
CA: cyanoacrylate glue EVLA: endovenous laser ablation EVSA: endovenous steam ablation HL/S: high ligation and stripping MOCA: mechanochemical ablation RFA: radio frequency ablation UGFS: ultrasound‐guided foam sclerotherapy yr: year(s)
Five year and longer‐term follow‐up data of Flessenkämper 2013, FOAM 2010, HELP‐1 2011, Magna 2013, Pronk 2010, Rasmussen 2007, Rasmussen 2011 and RELACS 2012 were also available (see Table 15).
9. Five‐year recurrence.
| EVLA versus RFA | ||
| Study ‐ final time point | Recurrence noted at final time point (%) | |
| EVLA | RFA | |
| Rasmussen 2011a | 42/144 (29) | 19/147 (13) |
| EVLA versus EVSA ‐ no data | ||
| EVLA versus UGFS | ||
| Study ‐ final time point | Recurrence noted at final time point (%) | |
| EVLA | UGFS | |
| Magna 2013 | 14/63 (22) | 21/67 (31) |
| Rasmussen 2011a | 42/144 (29) | 28/144(19) |
| EVLA versus CA ‐ no data | ||
| EVLA versus MOCA ‐ no data | ||
| EVLA versus HL/S (surgery) | ||
| Study ‐ final time point | Recurrence noted at final time point (%) | |
| EVLA | HL/S (surgery) | |
| Flessenkämper 2013 | 11/45 (24) | 14/53 (26) |
| HELP‐1 2011 | 29/108 (27) | 47/110 (43) |
| Magna 2013 | 14/63 (22) | 8/63 (13) |
| Pronk 2010 | 19/61 (31) | 4/60 (7) |
| Rasmussen 2007 | 25/69 (36) | 24/68 (35) |
| Rasmussen 2011a | 42/144 (29) | 38/142 (27) |
| RELACS 2012 | 69/152 (45) | 70/129 (54) |
| RFA versus UGFS | ||
| Study ‐ final time point | Recurrence noted at final time point (%) | |
| RFA | UGFS | |
| Rasmussen 2011a | 19/147 (13) | 28/144 (19) |
| RFA versus CA ‐ no data | ||
| RFA versus MOCA ‐ no data | ||
| RFA versus HL/S (surgery) | ||
| Study ‐ final time point | Recurrence noted at final time point (%) | |
| RFA | HL/S (surgery) | |
| Rasmussen 2011a | 19/147 (13) | 38/142 (27) |
| UGFS versus HL/S (surgery) | ||
| Study ‐ final time point | Recurrence noted at final time point (%) | |
| UGFS | HL/S (surgery) | |
| FOAM 2010 8 yr | 86/120 (72) | 71/103 (69) |
| Magna 2013 | 21/67 (31) | 8/63 (13) |
| Rasmussen 2011a | 28/144 (19) | 38/142 (27) |
aReported as limbs and not participants
CA: cyanoacrylate glue EVLA: endovenous laser ablation EVSA: endovenous steam ablation HL/S: high ligation and stripping MOCA: mechanochemical ablation RFA: radiofrequency ablation UGFS: ultrasound‐guided foam sclerotherapy
Rates of recurrence were not reported for the comparisons EVLA versus EVSA and RFA versus cyanoacrylate glue.
Post‐operative complications
All 24 included studies reported rates of post‐operative complications. Unfortunately, we could not perform meta‐analysis due to the considerable array of different terms used within the studies to report adverse events (for instance, 'paraesthesia', 'numbness', 'regional neurological sensory deficit' and 'saphenous nerve injury' were reported separately amongst trials). There was a lack of uniformity in the time points at which these events were measured. Therefore, we divided post‐operative adverse events into minor (i.e. not requiring intervention) and major (i.e. requiring intervention) within the first three months (early) and beyond three months (late) for this review. Within minor complications, we collated rates of haematoma, saphenous nerve injury, thermal injury or inflammation, wound problems (groin/stab), bruising and pigmentation and phlebitis from the included studies. Major complications included wound problems and 'other', further described in the footnotes to the tables. Complication rates are shown in Table 16 (early ≤ 3 months) and Table 17 (late > 3 months). Where complications were recorded at multiple time points during and after the first three months (e.g. in EVOLVeS 2003; Nesbitt 2014), we documented the highest rate of said event.
10. Post‐operative complications within three months.
| Early post‐operative complications (within three months) | ||||||||||||||||
| EVLA versus RFA | ||||||||||||||||
| Study | Minor (not requiring intervention) (%) | Major (requiring intervention) (%) | ||||||||||||||
| Adverse event | Haematoma (wound or thigh) | Saphenous nerve injury | Thermal injury/ inflammation | Wound problems (groin/stab) | Bruising and pigmentation | Phlebitis | Wound problems | Other | ||||||||
| Technique | EVLA | RFA | EVLA | RFA | EVLA | RFA | EVLA | RFA | EVLA | RFA | EVLA | RFA | EVLA | RFA | EVLA | RFA |
| Nordon 2011 | 2/78 (2.6) |
1/77 (1.3) |
2/78 (2.6) |
1/76 (1.3) |
0/78 (0) |
0/76 (0) |
2/78 (2.6) |
1/76 (1.3) |
0/78 (0) |
0/76 (0) |
||||||
| Rasmussen 2011a | 1/125 (0.8) |
0/121 (0) |
3/125 (2.4) |
6/121 (5) |
3/125 (2.4) |
8/121 (6.6 |
4/125 (3.2) |
12/121 (9.9) |
0/125 (0) |
1/121 (0.8) |
0/125 (0) |
0/121 (0) |
||||
| Recovery 2009 | 2/41 (4.9) |
1/46 (2.2) |
0/41 (0) |
1/46 (2.2) |
6/41 (14.6) |
0/46 (0) |
1/41 (2.2) |
0/46 (0) |
||||||||
| Shepherd 2010 | 2/64 (3) |
0/67 (0) |
5/64 (8) |
8/67 (12) |
1/64 (2) |
2/67 (3) |
2/64 (3) |
6/67 (9) |
5/64 (7) |
5/67 (7) |
2/64 (3) |
4/67 (6) |
0/64 (0) |
1/67 (1) PE |
||
| Syndor 2017 | 9/96 (9.4) |
13/97 (13.7) |
0/96 (0) |
0/97 (0) |
3/96 (3.1) |
3/97 (3.1) |
1/96 (1) |
1/97 (1.0) |
3/96 (3.1) |
2/97 (2.1) |
0/96 (0) |
0/97 (0) |
||||
| EVLA versus EVSA | ||||||||||||||||
| Study | Minor (not requiring intervention) (%) | Major (requiring intervention) (%) | ||||||||||||||
| Adverse event | Haematoma (wound or thigh) | Saphenous nerve injury | Thermal injury/ inflammation | Wound problems (groin/stab) | Bruising and pigmentation | Phlebitis | Wound problems | Other | ||||||||
| Technique | EVLA | EVSA | EVLA | EVSA | EVLA | EVSA | EVLA | EVSA | EVLA | EVSA | EVLA | EVSA | EVLA | EVSA | EVLA | EVSA |
| LAST 2014 | 0/109 (0) |
1/117 (0.9) |
0/109 (0) |
0/117 (0) |
0/109 (0) |
0/117 (0) |
10/109 (9.2) |
10/117 (8.5) |
0/109 (0) |
0/117 (0) |
1/109 (0.9) DVT |
0/117 (0) |
||||
| EVLA versus UGFS | ||||||||||||||||
| Study | Minor (not requiring intervention) (%) | Major (requiring intervention) (%) | ||||||||||||||
| Adverse event | Haematoma (wound or thigh) | Saphenous nerve injury | Thermal injury/ inflammation | Wound problems (groin/stab) | Bruising and pigmentation | Phlebitis | Wound problems | Other | ||||||||
| Technique | EVLA | UGFS | EVLA | UGFS | EVLA | UGFS | EVLA | UGFS | EVLA | UGFS | EVLA | UGFS | EVLA | UGFS | EVLA | UGFS |
| Magna 2013 | 2/78 (2.6) |
1/77 (1.3) |
2/78 (2.6) |
1/77 (1.3) |
0/78 (0) |
0/77 (0) |
0/78 (0) |
0/77 (0) |
||||||||
| Rasmussen 2011a | 1/125 (0.8) |
1/124 (0.8) |
3/125 (2.4) |
2/124 (1.6) |
3/125 (2.4) |
8/124 (6.5) |
4/125 (3.2) |
17/124 (13.7) |
0/125 (0) |
4/124 (3.2) |
0/125 (0) |
1/124 (0.8) |
||||
| Vernermo 2016 | 31/73 (42) |
14/72 (20) |
1/73 (1) |
2/72 (2) |
3/73 (5) |
50/72 (67) |
3/73 (4) |
0/72 (0) |
||||||||
| EVLA versus CA | ||||||||||||||||
| Study | Minor (not requiring intervention) (%) | Major (requiring intervention) (%) | ||||||||||||||
| Adverse event | Haematoma (wound or thigh) | Saphenous nerve injury | Thermal injury/ inflammation | Wound problems (groin/stab) | Bruising and pigmentation | Phlebitis | Wound problems | Other | ||||||||
| Technique | EVLA | CA | EVLA | CA | EVLA | CA | EVLA | CA | EVLA | CA | EVLA | CA | EVLA | CA | EVLA | CA |
| Calik 2019b | 28/200 (11) |
6/200 (3) |
63/200 (31) |
31/200 (15.5) |
14/200 (7) |
7/200 (3.5) |
2/200 (1) |
|||||||||
| EVLA versus MOCA | ||||||||||||||||
| Study | Minor (not requiring intervention) (%) | Major (requiring intervention) (%) | ||||||||||||||
| Adverse event | Haematoma (wound or thigh) | Saphenous nerve injury | Thermal injury/ inflammation | Wound problems (groin/stab) | Bruising and pigmentation | Phlebitis | Wound problems | Other | ||||||||
| Technique | EVLA | MOCA | EVLA | MOCA | EVLA | MOCA | EVLA | MOCA | EVLA | MOCA | EVLA | MOCA | EVLA | MOCA | EVLA | MOCA |
| Vähäaho 2019 | 4/34 (12) |
0/65 (0) |
0/34 (0) |
1/65 (1.5) |
||||||||||||
| EVLA versus HL/S (surgery) | ||||||||||||||||
| Study | Minor (not requiring intervention) (%) | Major (requiring intervention) (%) | ||||||||||||||
| Adverse event | Haematoma (wound or thigh) | Saphenous nerve injury | Thermal injury/ inflammation | Wound problems (groin/stab) | Bruising and pigmentation | Phlebitis | Wound problems | Other | ||||||||
| Technique | EVLA | HL/S | EVLA | HL/S | EVLA | HL/S | EVLA | HL/S | EVLA | HL/S | EVLA | HL/S | EVLA | HL/S | EVLA | HL/S |
| Darwood 2008a | 0/80 (0) |
0/32 (0) |
1/80 (1) |
4/32 (13) |
1/80 (1) |
2/32 (6) |
9/80 (11) |
0/32 (0) |
0/80 (0) |
2/32 (6) |
0/80 (0) |
1/32c (3) |
||||
| Flessenkämper 2013 | 24/142 (17) |
23/159 (15) |
68/142 (48) |
108/159 (68) |
1/142 (0.7) |
1/159 (0.6) |
||||||||||
| HELP‐1 2011 | 1/137 (0.7) |
11/133 (8.3) |
4/137 (2.9) |
13/133 (9.8) |
5/137 (3.6) |
3/133 (2.2) |
4/137 (2.9) |
6/133 (4.5) |
2/137 (1.5) |
8/133 (6) |
||||||
| Magna 2013 | 2/78 (2.6) |
4/68 (5.9) |
2/78 (2.6) |
0/68 (0) |
0/78 (0) |
3/68 (4.4) |
0/78 (0) |
0/68 (0) |
||||||||
| Pronk 2010 | 2/62 (3) |
1/68 (1) |
||||||||||||||
| Rasmussen 2007a | 3/69d (5) |
5/68 (8) |
1/69 (2) |
1/68 (5.9) |
0/69 (0) |
0/68 (0) |
0/69 (0) |
1/68 (2) |
7/69 (11) |
15/68 (25) |
2/69 (3) |
2/68 (3) |
0/69 (0) |
1/68e (2) |
0/69 (0) |
0/68 (0) |
| Rasmussen 2011a | 1/125 (0.8) |
1/119 (0.8) |
3/125 (2.4) |
5/119 (4.2) |
3/125 (2.4) |
6/119 (5) |
4/125 (3.2) |
5/119 (4.2) |
0/125 (0) |
0/119 (0.8) |
0/125 (0) |
1/119 (0.8) |
||||
| RELACS 2012 | 169/185 (91) |
145/161 (90) |
20/185 (10.8) |
4/161 (2.5) |
1/185 (0.5) |
0/161 (0) |
3/185 (1.6) |
1/161 (0.6) |
||||||||
| Vernermo 2016 | 31/73 (42) |
40/65 (62) |
1/73 (1) |
2/65 (3) |
3/73 (4) |
3/65 (4) |
3/73 (4) |
3/65 (4) |
||||||||
| RFA versus UGFS | ||||||||||||||||
| Study | Minor (not requiring intervention) (%) | Major (requiring intervention) (%) | ||||||||||||||
| Adverse event | Haematoma (wound or thigh) | Saphenous nerve injury | Thermal injury/ inflammation | Wound problems (groin/stab) | Bruising and pigmentation | Phlebitis | Wound problems | Other | ||||||||
| Technique | RFA | UGFS | RFA | UGFS | RFA | UGFS | RFA | UGFS | RFA | UGFS | RFA | UGFS | RFA | UGFS | RFA | UGFS |
| Rasmussen 2011a | 0/121 (0) |
1/124 (0.8) |
6/121 (0.8) |
2/124 (1.6) |
8/121 (6.6) |
8/124 (6.5) |
12/121 (9.9) |
17/124 (13.7) |
1/121 (0.8) |
4/124 (3.2) |
0/121 (0) |
1/124 (0.8) |
||||
| RFA versus CA | ||||||||||||||||
| Study | Minor (not requiring intervention) (%) | Major (requiring intervention) (%) | ||||||||||||||
| Adverse event | Haematoma (wound or thigh) | Saphenous nerve injury | Thermal injury/ inflammation | Wound problems (groin/stab) | Bruising and pigmentation | Phlebitis | Wound problems | Other | ||||||||
| Technique | RFA | CA | RFA | CA | RFA | CA | RFA | CA | RFA | CA | RFA | CA | RFA | CA | RFA | CA |
| Morrison 2015 | 3/114 (3) |
3/108 (3) |
1/114 (1) |
0/108 (0) |
16/114 (14) |
22/108 (20) |
1/114 (1) |
1/114 (1) |
0/114 (0) |
0/108 (0) |
||||||
| RFA versus MOCA | ||||||||||||||||
| Study | Minor (not requiring intervention) (%) | Major (requiring intervention) (%) | ||||||||||||||
| Adverse event | Haematoma (wound or thigh) | Saphenous nerve injury | Thermal injury/ inflammation | Wound problems (groin/stab) | Bruising and pigmentation | Phlebitis | Wound problems | Other | ||||||||
| Technique | RFA | MOCA | RFA | MOCA | RFA | MOCA | RFA | MOCA | RFA | MOCA | RFA | MOCA | RFA | MOCA | RFA | MOCA |
| Lane 2017 | ||||||||||||||||
| MARADONA 2019 | 15/104 (14) |
14/105 (13) |
3/104 (3) |
1/105 (1) |
0/104 (0) |
0/105 (0) |
0/105 (0) |
1/104 (1) |
2/104 (2) |
7/105 (7) |
8/104 (8) |
12/105 (11) |
3/104 (3) |
|||
| Vähäaho 2019 | 1/65 (1.5) |
0/65 (0) |
||||||||||||||
| RFA versus HL/S (surgery) | ||||||||||||||||
| Study | Minor (not requiring intervention) (%) | Major (requiring intervention) (%) | ||||||||||||||
| Adverse event | Haematoma (wound or thigh) | Saphenous nerve injury | Thermal injury/ inflammation | Wound problems (groin/stab) | Bruising and pigmentation | Phlebitis | Wound problems | Other | ||||||||
| Technique | RFA | HL/S | RFA | HL/S | RFA | HL/S | RFA | HL/S | RFA | HL/S | RFA | HL/S | RFA | HL/S | RFA | HL/S |
| EVOLVeS 2003 | 6/44 (14) |
18/36 (50) |
10/44 (23) |
5/36 (4.2) |
14/44 (32) |
23/36 (64) |
0/44 (0) |
2/36 (6)f |
0/44 (0) |
0/36 (0) |
||||||
| Helmy ElKaffas 2011 | 1/90 (1.1) |
30/90 (33.3) |
9/90 (10) |
3/90 (3.3) |
0/90 (0) |
0/90 (0) |
6/90 (6.6) |
0/90 (0) |
0/90 (0) |
3/90 (3.3) |
0/90 (0) |
1/90 (1.1) |
||||
| Rasmussen 2011a | 0/121 (0) |
1/119 (0.8) |
6/121 (4.9) |
5/119 (4.2) |
8/121 (6.6) |
6/119 (5) |
12/121 (9.9) |
5/119 (4.2) |
1/121 (0.8) |
1/119 (0.8) |
0/121 (0) |
1/119 (0.8) |
||||
| Rautio 2002 | 1/15 (7) |
4/13 (31) |
2/15 (13) |
3/13 (23) |
1/15 (7) |
0/13 (0) |
0/15 (0) |
0/13 (0) |
0/15 (0) |
0/13 (0) |
3/15 (20) |
0/13 (0) |
0/15 (0) |
0/13 (0) |
0/15 (0) |
0/13 (0) |
| Subramonia 2010 | 0/47 (0) |
0/47 (0) |
9/47 (19) |
20/41 (49) |
0/47 (0) |
0/41 (0) |
0/47 (0) |
7/41 (17) |
5/47 (11) |
0/41 (0) |
0/47 (0) |
0/41 (0) |
0/47 (0) |
0/41 (0) |
0/47 (0) |
0/41 (0) |
| UGFS versus HL/S (surgery) | ||||||||||||||||
| Study | Minor (not requiring intervention) (%) | Major (requiring intervention) (%) | ||||||||||||||
| Adverse event | Haematoma (wound or thigh) | Saphenous nerve injury | Thermal injury/ inflammation | Wound problems (groin/stab) | Bruising and pigmentation | Phlebitis | Wound problems | Other | ||||||||
| Technique | UGFS | HL/S | UGFS | HL/S | UGFS | HL/S | UGFS | HL/S | UGFS | HL/S | UGFS | HL/S | UGFS | HL/S | UGFS | HL/S |
| FOAM 2010 | 0/217 (0) |
3/176 (1.7) |
0/217 (0) |
6/176 (3.4) |
17/217 (7.8) |
0/176 (0) |
0/217 (0) |
4/176 (2.3) |
0/176 (0) |
0/217 (0.9) |
||||||
| Magna 2013 | 1/77 (1.3) |
4/68 (5.9) |
1/77 (1.3) |
0/68 (0) |
0/77 (0) |
3/68 (4.4) |
0/77 (0) |
0/68 (0) |
||||||||
| Rasmussen 2011a | 1/124 (0.8) |
1/119 (0.8) |
2/124 (1.6) |
5/119 (4.2) |
8/124 (6.5) |
6/119 (5) |
17/124 (14) |
5/119 (4.2) |
4/124 (3.2) |
1/119 (0.8) |
1/124 (0.8) |
1/119 (0.8) |
||||
| Vernermo 2016 | 14/72 (20) |
40/65 (62) |
2/72 (2) |
2/65 (3) |
5/72 (7) |
3/65 (4) |
0/72 (0) |
3/65 (4) |
||||||||
aResults only available per limb, not per participant bTwo participants developed DVTs. cPost‐operative acute respiratory distress syndrome (requiring seven days intensive therapy unit (ITU) support) following aspiration post‐operatively dIn one participant, the saphenous thrombus extended into the femoral vein; it resolved without intervention. eGroin infection requiring antibiotics fIncludes one participant who required debridement and intravenous antibiotics for a 'thigh and calf infection'
CA: cyanoacrylate glue DVT: deep vein thrombosis EVLA: endovenous laser ablation EVSA: endovenous steam ablation HL/S: high ligation and stripping MOCA: mechanochemical ablation PE: pulmonary embolism RFA: radio frequency ablation UGFS: ultrasound‐guided foam sclerotherapy
11. Post‐operative complications after three months.
| Late post‐operative complications (after three months) | ||||||||||||||||
| EVLA versus RFA | ||||||||||||||||
| Study | Minor (not requiring intervention) (%) | Major (requiring intervention) (%) | ||||||||||||||
| Adverse event | Haematoma (wound or thigh) | Saphenous nerve injury | Thermal injury/ inflammation | Wound problems (groin/stab) | Bruising and pigmentation | Phlebitis | Wound problems | Other | ||||||||
| Technique | EVLA | RFA | EVLA | RFA | EVLA | RFA | EVLA | RFA | EVLA | RFA | EVLA | RFA | EVLA | RFA | EVLA | RFA |
| Nordon 2011 | ||||||||||||||||
| Rasmussen 2011a | ||||||||||||||||
| Recovery 2009 | ||||||||||||||||
| Shepherd 2010 | ||||||||||||||||
| Syndor 2017 | 8/79 (10.3) |
6/74 (8.33) |
0/79 (0) |
0/74 (0) |
10/79 (12.66) |
6/74 (8.11) |
0/79 (0) |
2/74 (2.7) |
0/79 (0) |
0/74 (0) |
0/79 (0) |
0/74 (0) |
||||
| EVLA versus EVSA | ||||||||||||||||
| Study | Minor (not requiring intervention) (%) | Major (requiring intervention) (%) | ||||||||||||||
| Adverse event | Haematoma (wound or thigh) | Saphenous nerve injury | Thermal injury/ inflammation | Wound problems (groin/stab) | Bruising and pigmentation | Phlebitis | Wound problems | Other | ||||||||
| Technique | EVLA | EVSA | EVLA | EVSA | EVLA | EVSA | EVLA | EVSA | EVLA | EVSA | EVLA | EVSA | EVLA | EVSA | EVLA | EVSA |
| LAST 2014 | 0/98 (0) |
2/107 (1.9) |
0/98 (0) |
3/107 (2.8) |
||||||||||||
| EVLA versus UGFS | ||||||||||||||||
| Study | Minor (not requiring intervention) (%) | Major (requiring intervention) (%) | ||||||||||||||
| Adverse event | Haematoma (wound or thigh) | Saphenous nerve injury | Thermal injury/ inflammation | Wound problems (groin/stab) | Bruising and pigmentation | Phlebitis | Wound problems | Other | ||||||||
| Technique | EVLA | UGFS | EVLA | UGFS | EVLA | UGFS | EVLA | UGFS | EVLA | UGFS | EVLA | UGFS | EVLA | UGFS | EVLA | UGFS |
| Magna 2013 | 0/78 (0) |
1/77 (1.3) |
1/78 (1.3) |
1/77 (1.3) |
||||||||||||
| Rasmussen 2011a | ||||||||||||||||
| Vernermo 2016 | ||||||||||||||||
| EVLA versus CA | ||||||||||||||||
| Study | Minor (not requiring intervention) (%) | Major (requiring intervention) (%) | ||||||||||||||
| Adverse event | Haematoma (wound or thigh) | Saphenous nerve injury | Thermal injury/ inflammation | Wound problems (groin/stab) | Bruising and pigmentation | Phlebitis | Wound problems | Other | ||||||||
| Technique | EVLA | CA | EVLA | CA | EVLA | CA | EVLA | CA | EVLA | CA | EVLA | CA | EVLA | CA | EVLA | CA |
| Calik 2019 | 13/200 (7) |
2/200 (1.1) |
3/200 (1.6) |
1/200 (0.5) |
0/200 (0) |
0/200 (0) |
2/200 (1.1) |
0/200 (0) |
||||||||
| EVLA versus MOCA | ||||||||||||||||
| Study | Minor (not requiring intervention) (%) | Major (requiring intervention) (%) | ||||||||||||||
| Adverse event | Haematoma (wound or thigh) | Saphenous nerve injury | Thermal injury/ inflammation | Wound problems (groin/stab) | Bruising and pigmentation | Phlebitis | Wound problems | Other | ||||||||
| Technique | EVLA | MOCA | EVLA | MOCA | EVLA | MOCA | EVLA | MOCA | EVLA | MOCA | EVLA | MOCA | EVLA | MOCA | EVLA | MOCA |
| Vähäaho 2019 | 3/33 (11) |
0/55 (0) |
3/33 (11) |
6/55 (11) |
||||||||||||
| EVLA versus HL/S (surgery) | ||||||||||||||||
| Study | Minor (not requiring intervention) (%) | Major (requiring intervention) (%) | ||||||||||||||
| Adverse event | Haematoma (wound or thigh) | Saphenous nerve injury | Thermal injury/ inflammation | Wound problems (groin/stab) | Bruising and pigmentation | Phlebitis | Wound problems | Other | ||||||||
| Technique | EVLA | HL/S | EVLA | HL/S | EVLA | HL/S | EVLA | HL/S | EVLA | HL/S | EVLA | HL/S | EVLA | HL/S | EVLA | HL/S |
| Darwood 2008a | 0/80 (0) |
0/34 (0) |
0/80 (0) |
1/34 (3) |
0/80 (0) |
0/34 (0) |
0/80 (0) |
0/34 (0) |
0/80 (0) |
0/34 (0) |
0/80 (0) |
0/34 (0) |
0/80 (0) |
0/34 (0) |
0/80 (0) |
0/34 (0) |
| Flessenkämper 2013 | 23/127 (18) |
5/128 (4) |
12/127 (9.4) |
14/128 (11) |
||||||||||||
| HELP‐1 2011 | ||||||||||||||||
| Magna 2013 | 0/78 (0) |
1/68 (1.5) |
1/78 (1.3) |
0/68 (0) |
||||||||||||
| Pronk 2010 | ||||||||||||||||
| Rasmussen 2007a | 0/96 (0) |
0/68 (0) |
0/96 (0) |
1/68 (2) |
0/96 (0) |
0/68 (0) |
0/96 (0) |
0/68 (0) |
0/96 (0) |
0/68 (0) |
0/96 (0) |
0/68 (0) |
0/96 (0) |
0/68 (0) |
0/96 (0) |
0/68 (0) |
| Rasmussen 2011a | ||||||||||||||||
| RELACS 2012 | ||||||||||||||||
| Vernermo 2016 | ||||||||||||||||
| RFA versus UGFS | ||||||||||||||||
| Study | Minor (requiring intervention) (%) | Major (requiring intervention) (%) | ||||||||||||||
| Adverse event | Haematoma (wound or thigh) | Saphenous nerve injury | Thermal injury/ inflammation | Wound problems (groin/stab) | Bruising and pigmentation | Phlebitis | Wound problems | Other | ||||||||
| Technique | RFA | UGFS | RFA | UGFS | RFA | UGFS | RFA | UGFS | RFA | UGFS | RFA | UGFS | RFA | UGFS | RFA | UGFS |
| Rasmussen 2011a | ||||||||||||||||
| RFA versus CA | ||||||||||||||||
| Study | Minor (requiring intervention) (%) | Major (requiring intervention) (%) | ||||||||||||||
| Adverse event | Haematoma (wound or thigh) | Saphenous nerve injury | Thermal injury/ inflammation | Wound problems (groin/stab) | Bruising and pigmentation | Phlebitis | Wound problems | Other | ||||||||
| Technique | RFA | CA | RFA | CA | RFA | CA | RFA | CA | RFA | CA | RFA | CA | RFA | CA | RFA | CA |
| Morrison 2015 | 0/84 (0) |
1/86 (1.2) |
||||||||||||||
| RFA versus MOCA | ||||||||||||||||
| Study | Minor (not requiring intervention) (%) | Major (requiring intervention) (%) | ||||||||||||||
| Adverse event | Haematoma (wound or thigh) | Saphenous nerve injury | Thermal injury/ inflammation | Wound problems (groin/stab) | Bruising and pigmentation | Phlebitis | Wound problems | Other | ||||||||
| Technique | RFA | MOCA | RFA | MOCA | RFA | MOCA | RFA | MOCA | RFA | MOCA | RFA | MOCA | RFA | MOCA | RFA | MOCA |
| Lane 2017 | ||||||||||||||||
| MARADONA 2019 | ||||||||||||||||
| Vähäaho 2019 | 2/29 (6.9) |
0/55 (0) |
4/29 (13.8) |
|||||||||||||
| RFA versus HL/S (surgery) | ||||||||||||||||
| Study | Minor (not requiring intervention) (%) | Major (requiring intervention) (%) | ||||||||||||||
| Adverse event | Haematoma (wound or thigh) | Saphenous nerve injury | Thermal injury/ inflammation | Wound problems (groin/stab) | Bruising and pigmentation | Phlebitis | Wound problems | Other | ||||||||
| Technique | RFA | HL/S | RFA | HL/S | RFA | HL/S | RFA | HL/S | RFA | HL/S | RFA | HL/S | RFA | HL/S | RFA | HL/S |
| EVOLVeS 2003 | 0/43 (0) |
3/34 (9) |
0/43 (0) |
0/34 (0) |
0/43 (0) |
0/34 (0) |
0/43 (0) |
0/34 (0) |
0/43 (0) |
1/34 (3) |
0/43 (0) |
2/34 (6) |
0/43 (0) |
0/34 (0) |
0/43 (0) |
0/34 (0) |
| Helmy ElKaffas 2011 | ||||||||||||||||
| Rasmussen 2011a | ||||||||||||||||
| Rautio 2002 | 0/15 (0) |
0/13 (0) |
1/15 (0) |
5/13 (38) |
0/15 (0) |
0/13 (0) |
0/15 (0) |
0/13 (0) |
0/15 (0) |
0/13 (0) |
0/15 (0) |
0/13 (0) |
0/15 (0) |
0/13 (0) |
0/15 (0) |
0/13 (0) |
| Subramonia 2010 | ||||||||||||||||
| UGFS versus HL/S (surgery) | ||||||||||||||||
| Study | Minor (not requiring intervention) (%) | Major (requiring intervention) (%) | ||||||||||||||
| Adverse event | Haematoma (wound or thigh) | Saphenous nerve injury | Thermal injury/ inflammation | Wound problems (groin/stab) | Bruising and pigmentation | Phlebitis | Wound problems | Other | ||||||||
| Technique | UGFS | HL/S | UGFS | HL/S | UGFS | HL/S | UGFS | HL/S | UGFS | HL/S | UGFS | HL/S | UGFS | HL/S | UGFS | HL/S |
| FOAM 2010 | 12/213 (5.6) |
2/177 (1.1) |
||||||||||||||
| Magna 2013 | 1/77 (1.3) |
1/68 (1.5) |
||||||||||||||
| Rasmussen 2011a | ||||||||||||||||
| Vernermo 2016 | ||||||||||||||||
aResults only available per limb, not per participant
CA: cyanoacrylate glue EVLA: endovenous laser ablation EVSA: endovenous steam ablation HL/S: high ligation and stripping MOCA: mechanochemical ablation RFA: radio frequency ablation UGFS: ultrasound‐guided foam sclerotherapy
Quality of life (QoL)
Twenty‐two studies reported on QoL (Calik 2019; Darwood 2008; EVOLVeS 2003; Flessenkämper 2013; FOAM 2010; HELP‐1 2011; Lane 2017; LAST 2014; Magna 2013; MARADONA 2019; Morrison 2015; Nordon 2011; Pronk 2010; Rasmussen 2007; Rasmussen 2011; Rautio 2002; Recovery 2009; RELACS 2012; Shepherd 2010; Subramonia 2010; Vähäaho 2019; Vernermo 2016). Meta‐analysis was not possible due to different questionnaires being used at different time points. See Table 18.
12. Quality of life scores.
| Technique | Study | Quality of life score | |||||||
| V‐Q/SymQ | AVVSS | CIVIQ2 | SF‐12 | SF‐36 | RAND‐36 | EQ‐5D | SF‐6D | ||
| EVLA versus RFA | Nordon 2011 | ✓ | |||||||
| Rasmussen 2011 | ✓ | ✓ | |||||||
| Recovery 2009 | ✓ | ||||||||
| Shepherd 2010 | ✓ | ||||||||
| EVLA versus EVSA | LAST 2014 | ✓ | |||||||
| EVLA versus UGFS | Magna 2013 | ✓ | ✓ | ||||||
| Rasmussen 2011 | ✓ | ✓ | |||||||
| Vernermo 2016 | ✓ | ||||||||
| EVLA versus CA | Calik 2019 | ✓ | |||||||
| EVLA versus MOCA | Vähäaho 2019 | ✓ | |||||||
|
EVLA versus HL/S (surgery) |
Darwood 2008 | ✓ | |||||||
| Flessenkämper 2013 | ✓ | ||||||||
| HELP‐1 2011 | ✓ | ✓ | ✓ | ✓ | |||||
| Magna 2013 | ✓ | ✓ | |||||||
| Pronk 2010 | ✓ | ||||||||
| Rasmussen 2007 | ✓ | ✓ | |||||||
| Rasmussen 2011 | ✓ | ✓ | |||||||
| RELACS 2012 | ✓ | ||||||||
| Vernermo 2016 | ✓ | ||||||||
| RFA versus UGFS | Rasmussen 2011 | ✓ | ✓ | ||||||
| RFA versus CA | Morrison 2015 | ✓ | ✓ | ||||||
| RFA versus MOCA | Lane 2017 | ✓ | ✓ | ||||||
| MARADONA 2019 | ✓ | ✓ | |||||||
| Vähäaho 2019 | ✓ | ||||||||
| RFA versus HL/S (surgery) | EVOLVeS 2003 | ✓ | |||||||
| Rasmussen 2011 | ✓ | ✓ | |||||||
| Rautio 2002 | ✓ | ||||||||
| Subramonia 2010 | ✓ | ✓ | |||||||
| RFA versus UGFS | Rasmussen 2011 | ✓ | ✓ | ||||||
|
UGFS versus HL/S (surgery) |
FOAM 2010 | ✓ | |||||||
| Magna 2013 | ✓ | ✓ | |||||||
| Rasmussen 2011 | ✓ | ✓ | |||||||
| Vernermo 2016 | ✓ | ||||||||
AVVSS: Aberdeen Varicose Vein Symptom Severity Score CA: cyanoacrylate glue CIVIQ2: Chronic Venous Insufficiency Quality of Life Questionnaire EQ‐5D: EuroQol‐5D EVLA: endovenous laser ablation EVSA: endovenous steam ablation HL/S: high ligation and stripping MOCA: mechanochemical ablation RAND‐36: Short term RAND‐36 (validated for Finland) RFA: radiofrequency ablation SF‐12: Medical Outcomes Study Short Form 12 SF‐36: Medical Outcomes Study Short Form 36 SF‐6D: variation of the Medical Outcomes Study Short Form 36 UGFS: ultrasound‐guided foam sclerotherapy V‐Q/SymQ: VEINES‐QoL/Sym questionnaire
Venous Clinical Severity Score (VCSS)
Thirteen studies reported on VCSS (Calik 2019; EVOLVeS 2003; FOAM 2010; Lane 2017; LAST 2014; MARADONA 2019; Morrison 2015; Rasmussen 2007; Rasmussen 2011; Rautio 2002; Recovery 2009; Shepherd 2010; Syndor 2017). However, meaningful meta‐analysis was prevented for each comparison by the limited studies available and by the different time points measured. Some studies presented the mean baseline and final score without calculating the mean difference. Other studies gave only the change in scores pre‐ and post‐intervention. We have collated and presented the results of the included studies in Table 19.
13. Change in Venous Clinical Severity Score (VCSS).
| EVLA versus RFA | ||||||
| Study ‐ final time point | Initial VCSS | Final VCSS | Change in VCSS | |||
| EVLA | RFA | EVLA | RFA | EVLA | RFA | |
| Recovery 2009 mean (SD) 1 month | 4.9 (2.8) | 4.7 (3.1) | 3.2 (1.8) | 2.7 (2.2) | ||
| Rasmussen 2011 mean (SD) 3 yr | 2.68 (2.25) | 2.95 (2.06) | 0.34 (1.3) | 0.44 (1.82) | 3.3 | 3.7 |
| Shepherd 2010 mean (SD) 6 months | 4.7 (2.1) | 5.1 (2.1) | 1.4 (1.8) | 1.4 (1.7) | ||
| Syndor 2017 median (range) 6 months | 5 (2 ‐ 26) | 5 (1 ‐ 20) | 1 (0 ‐ 18) | 1 (0 ‐ 6) | ||
| EVLA versus EVSA | ||||||
| Study ‐ final time point | Initial VCSS | Final VCSS | Change in VCSS | |||
| EVLA | EVSA | EVLA | EVSA | EVLA | EVSA | |
|
LAST 2014 change after 12 weeks (95% CI) |
not given | not given | not given | not given | ‐2.5 (‐2.1 to ‐2.93) |
all ‐2.9 (‐2.4 to ‐3.5) hIgha ‐2.69 (‐2.34 to ‐3.04) |
| EVLA versus UGFS | ||||||
| Study ‐ final time point | Initial VCSS | Final VCSS | Change in VCSS | |||
| EVLA | UGFS | EVLA | UGFS | EVLA | UGFS | |
| Rasmussen 2011 mean (SD) 3 yr | 2.68 (2.25) | 2.66 (1.45) | 0.34 (1.3) | 0.15 (0.4) | ||
| EVLA versus CA | ||||||
| Study ‐ final time point | Initial VCSS | Final VCSS | Change in VCSS | |||
| EVLA | CA | EVLA | CA | EVLA | CA | |
| Calik 2019 mean (SD) 1 yr | 5.8 (1.9) | 5.7 (1.9) | 1.3 (0.9) | 1.3 (0.9) | ||
| EVLA versus MOCA ‐ no data | ||||||
| EVLA versus HL/S (surgery) | ||||||
| Study ‐ final time point | Initial VCSS | Final VCSS | Change in VCSS | |||
| EVLA |
HL/S (surgery) |
EVLA |
HL/S (surgery) |
EVLA |
HL/S (surgery) |
|
| Rasmussen 2007 mean (SD) 5 yr | 2.8 (1.7) | 2.4 (1.4) | 0.4 (0.9) | 2.4 (1.4) | ||
| Rasmussen 2011 mean (SD) 3 yr | 2.68 (2.25) | 2.75 (1.62) | 0.34 (1.3) | 0.3 (0.5) | ||
| RFA versus UGFS | ||||||
| Study ‐ final time point | Initial VCSS | Final VCSS | Change in VCSS | |||
| RFA | UGFS | RFA | UGFS | RFA | UGFS | |
| Rasmussen 2011 mean (SD) 3 yr | 2.95 (2.06) | 2.66 (1.45) | 0.44 (1.82) | 0.15 (0.4) | ||
| RFA versus CA | ||||||
| Study ‐ final time point | Initial VCSS | Final VCSS | Change in VCSS | |||
| RFA | CA | RFA | CA | RFA | CA | |
| Morrison 2015 mean (SD) 3 months | 5.6 (2.6) | 5.5 (2.6) | 2.0 (2.0) | 1.9 (1.6) | ||
| RFA versus MOCA | ||||||
| Study ‐ final time point | Initial VCSS | Final VCSS | Change in VCSS | |||
| RFA | MOCA | RFA | MOCA | RFA | MOCA | |
| Lane 2017 median (range) 6 months | 5 | 6 | 2 (1 ‐ 5) | 2 (1 ‐ 4) | ||
| MARADONA 2019 median (IQR) 2 yr | individually reported |
individually reported |
individually reported |
individually reported |
4 (3 ‐ 5) | 3 (2 ‐ 5) |
| RFA versus HL/S (surgery) | ||||||
| Study ‐ final time point | Initial VCSS | Final VCSS | Change in VCSS | |||
| RFA | HL/S (surgery) | RFA | HL/S (surgery) | RFA | HL/S (surgery) | |
| EVOLVeS 2003 mean (SD) 2 yr | 4.8 (0.34) | 4.39 (0.38) | unable to read from graph | |||
| Rasmussen 2011 mean (SD) 3 yr | 2.95 (2.06) | 2.75 (1.62) | 0.44 (1.82) | 0.3 (0.5) | ||
|
Rautio 2002 median (range) 3 yr change ‐ mean (SD) |
4 (4 ‐ 6) | 5 (4 ‐ 9) | ‐ 4.3 (2.3) | ‐4 (‐1.2) | ||
| UGFS versus HL/S (surgery) | ||||||
| Study ‐ final time point | Initial VCSS | Final VCSS | Change in VCSS | |||
| UGFS | HL/S (surgery) | UGFS | HL/S (surgery) | UGFS | HL/S (surgery) | |
| FOAM 2010 mean (SD) 2 yr | 3.2 (1.9) | 3.5 (2.2) | 1.7 (1.2) | 1.9 (1.4) | ‐1.49 | ‐1.75 |
| Rasmussen 2011 mean (SD) 3 yr | 2.66 (1.45) | 2.75 (1.62) | 0.15 (0.4) | 0.3 (0.5) | ||
aHigh dose of steam
CA: cyanoacrylate glue CI: confidence interval EVLA: endovenous laser ablation EVSA: endovenous steam ablation HL/S: high ligation and stripping IQR: interquartile range MOCA: mechanochemical ablation RFA: radiofrequency ablation SD: standard deviation UGFS: ultrasound‐guided foam sclerotherapy yr: year(s)
Length of procedure
Eleven studies reported on length of procedure (Calik 2019; EVOLVeS 2003; Helmy ElKaffas 2011; HELP‐1 2011; MARADONA 2019; Morrison 2015; Nordon 2011; Rasmussen 2011; Rautio 2002; Subramonia 2010; Syndor 2017). The results of the length of procedure for the reporting trials are shown in Table 20. What defines the start and end points of a 'procedure' is ambiguous, which is reflected in the various ways the included trials have reported length of procedure. Syndor 2017 presents the median ablation and procedure time with the range; Rasmussen 2011 reports on the mean 'surgeon's time' and range; Subramonia 2010 on theatre and procedure times with the median values and the interquartile range; whilst Rautio 2002 gives the mean operating time, room time and recovery time with standard deviation (SD).
14. Length of procedure or operative time.
| EVLA versus RFA | ||
| Study | Time (min) | |
| EVLA | RFA | |
| Nordon 2011 median (range) | 30 (10 ‐ 60) | 30 (15 ‐ 60) |
| Rasmussen 2011 mean (range)a | 26 (12 ‐ 80) | 27 (12 ‐ 80) |
| Syndor 2017 median (range) | total procedure 23.5 (8 ‐ 95) total ablation time 5 (1 ‐ 18) |
total procedure 21 (6 ‐ 64) total ablation time 4 (1 ‐ 14) |
| EVLA versus EVSA ‐ no data | ||
| EVLA versus UGFS | ||
| Study | Time (min) | |
| EVLA | UGFS | |
| Rasmussen 2011 mean (range)a | 26 (12 ‐ 80) | 19 (5 ‐ 145) |
| EVLA versus CA | ||
| Study | Time (min) | |
| EVLA | CA | |
| Calik 2019 mean (SD) | 31.7 (8.8) | 13 (3.4) |
| EVLA versus MOCA ‐ no data | ||
| EVLA versus HL/S (surgery) | ||
| Study | Time (min) | |
| EVLA | HL/S (surgery) | |
| HELP‐1 2011 mean (SD) | 61 (14) | 67 (16) |
| Rasmussen 2011 mean (range)a | 26 (12 ‐ 80) | 32 (15 ‐ 80) |
| Vernermo 2016 mean (SD) [range] | 83 (17) [50 ‐ 139] | 95 (19) [62 ‐ 155] |
| RFA versus UGFS | ||
| Study | Time (min) | |
| RFA | UGFS | |
| Rasmussen 2011 mean (range)a | 27 (12 ‐ 80) | 19 (5 ‐ 145) |
| RFA versus CA | ||
| Study | Time (min) | |
| RFA | CA | |
| Morrison 2015 mean (range) | 19 (5 ‐ 46) | 24 (11 ‐ 40) |
| RFA versus MOCA | ||
| Study | Time (min) procedural time | |
| RFA | MOCA | |
| MARADONA 2019 mean (IQR) | 13 (4 ‐ 85) | 12 (5 ‐ 45) |
| RFA versus HL/S (surgery) | ||
| Study | Time (min) | |
| RFA | HL/S (surgery) | |
| EVOLVeS 2003 mean (SD) | 74 (10) | 89 (12) |
| Helmy ElKaffas 2011 mean (SD) | 40 (12) | 45 (13) |
| Rasmussen 2011 mean (range)a | 27 (12 ‐ 80) | 32 (15 ‐ 80) |
| Rautio 2002 mean (SD) | Operating time: 75 (16.6) Operating room time: 115 (18.3) Recovery room time: 227 (57.6) |
Operating time: 57 (11) Operating room time: 99 (12.9) Recovery room time: 198 (40.7) |
| Subramonia 2010 median (IQR) | Theatre time: 82 (73 ‐ 91) Procedure time: 76 (67 ‐ 84) |
Theatre time: 55 (48 ‐ 63) Procedure time: 48 (39 ‐ 54) |
| UGFS versus HL/S (surgery) | ||
| Study | Time (min) | |
| UGFS | HL/S (surgery) | |
| Rasmussen 2011 mean (range)a | 19 (5 ‐ 145) | 32 (15 ‐ 80) |
aSurgeon's time
CA: cyanoacrylate glue EVLA: endovenous laser ablation EVSA: endovenous steam ablation HL/S: high ligation and stripping IQR: interquartile range min: minutes MOCA: mechanochemical ablation RFA: radiofrequency ablation SD: standard deviation UGFS: ultrasound‐guided foam sclerotherapy
Duration of hospital stay
The majority of the included studies reported that procedures were performed in day case surgical units or outpatient settings. Ten studies explicitly stated whether all participants were discharged home the same day or whether some required inpatient admission post‐intervention (Darwood 2008; EVOLVeS 2003; Flessenkämper 2013; FOAM 2010; Helmy ElKaffas 2011; HELP‐1 2011; Morrison 2015; Pronk 2010; Rasmussen 2007; Shepherd 2010). See Table 21 for details. For the most part, these procedures were performed as day cases.
15. Duration of hospital stay.
| EVLA versus RFA | ||
| Study | Length of hospital stay % day case | |
| EVLA | RFA | |
| Shepherd 2010 | 98a | 95.5b |
| EVLA versus EVSA ‐ no data | ||
| EVLA versus UGFS ‐ no data | ||
| EVLA versus CA ‐ no data | ||
| EVLA versus MOCA ‐ no data | ||
| EVLA versus HL/S (surgery) | ||
| Study | Length of hospital stay % day case | |
| EVLA | HL/S (surgery) | |
| Darwood 2008 | 100 | 100 |
| Flessenkämper 2013 | ~100 | ~100 |
| HELP‐1 2011 | 100 | 78.8 |
| Pronk 2010 | 100 | 100 |
| Rasmussen 2007 | 100 | 100 |
| RFA versus UGFS ‐ no data | ||
| RFA versus CA | ||
| Study | Length of hospital stay % day case | |
| RFA | CA | |
| Morrison 2015 | 100 | 100 |
| RFA versus MOCA ‐ no data | ||
| RFA versus HL/S (surgery) | ||
| Study | Length of hospital stay % day case | |
| RFA | HL/S (surgery) | |
| EVOLVeS 2003 | 95c | 86d |
|
Helmy ElKaffas 2011 hours in hospital mean (SD) [range] |
14 (SD 3.6) [12 to 18] | 30 (SD 11.5) [18 to 48] |
| Rautio 2002 | 93.3 | 92.3 |
| UGFS versus HL/S (surgery) | ||
| Study | Length of hospital stay % day case | |
| UGFS | HL/S (surgery) | |
| FOAM 2010 | not indicated | 100 |
aOne participant required overnight admission due to post‐operative nausea. bOne participant required overnight admission for pain requiring opioids, one for nausea and one for hypotension secondary to general anaesthesia. cTwo participants kept overnight dFive participants kept overnight
CA: cyanoacrylate glue EVLA: endovenous laser ablation EVSA: endovenous steam ablation HL/S: high ligation and stripping MOCA: mechanochemical ablation RFA: radiofrequency ablation UGFS: ultrasound‐guided foam sclerotherapy
Return to normal activities (days)
Table 22 illustrates the time taken by participants to return to work or normal activities following the intervention within their respective trials. Studies have presented this outcome as either parametric or non‐parametric data (mean, median, range, interquartile range (IQR)), or in the case of Shepherd 2010, the percentage of participants to return to work within a certain time frame. We were not able to perform meta‐analysis.
16. Time to return to work and normal activities.
| EVLA versus RFA | ||||
| Study | Time to return to work (days) | Time to return normal activities (days) | ||
| EVLA | RFA | EVLA | RFA | |
| Nordon 2011 median (range) | 7 (1 ‐ 60)a | 9 (0 ‐ 28) | ||
| Rasmussen 2011 median (range) | 3.6 (0 ‐ 46) | 2.9 (0 ‐ 14) | 3.6 (0 ‐ 46) | 1 (0 ‐ 30) |
| Shepherd 2010 | n returned to work at 3 days 14 (41%) 7 days 27 (71%) |
n returned to work at 3 days 15 (37%) 7 days 29 (71%) |
n returned to normal at 3 days 25 (50%) 7 days 37 (74%) |
n returned to normal at 3 days 37 (60%) 7 days 48 (77%) |
| EVLA versus EVSA | ||||
| Study | Time to return to work (days) | Time to return normal activities (days) | ||
| EVLA | EVSA | EVLA | EVSA | |
| LAST 2014 mean (95% CI) | 4.2 (3.4 ‐ 5) | all 1.6 (1 ‐ 2.1) highb 1.6 (0.9 ‐ 2.3) |
||
| EVLA versus UGFS | ||||
| Study | Time to return to work (days) | Time to return normal activities (days) | ||
| EVLA | UGFS | EVLA | UGFS | |
| Rasmussen 2011 median (range) | 3.6 (0 ‐ 46) | 2.9 (0 ‐ 33) | 2 (0 ‐ 25) | 1 (0 ‐ 30) |
| Vernermo 2016 mean (SD) [range] | 8 (5) [0 ‐ 29] | 1 (3) [0 ‐ 21] | ||
| EVLA versus CA | ||||
| Study | Time to return to work (days) | Time to return normal activities (days) | ||
| EVLA | CA | EVLA | CA | |
| Calik 2019 mean (SD) | 2.9 (1.8) | 1.5 (0.6) | ||
| EVLA versus MOCA | ||||
| Study | Time to return to work (days) | Time to return normal activities (days) | ||
| EVLA | MOCA | EVLA | MOCA | |
| Vähäaho 2019c mean | actual 5.3 perceived 8.6 |
actual 4.3 perceived 7.8 |
||
| EVLA versus HL/S (surgery) | ||||
| Study | Time to return to work (days) | Time to return normal activities (days) | ||
| EVLA | HL/S (surgery) | EVLA | HL/S (surgery) | |
| Darwood 2008d median (IQR) | EVLT1: 4 (2.5 ‐ 7) EVLT2: 4 (1 ‐ 12) |
17 (7.25 ‐ 33.25) | EVLT1: 2 (0 ‐ 7) EVLT2: 2 (0 ‐ 7) |
7 (2 ‐ 26) |
| HELP‐1 2011 median (range) | 4 (2 ‐ 14) | 14 (13 ‐ 28) | 3 (1 ‐ 10) | 14 (7 ‐ 25) |
| Pronk 2010 mean (SD) | 4.38 (5.43) | 4.15 (3.72) | 3.16 (4.34) | 3.20 (4.01) |
| Rasmussen 2007 mean (SD) | 7 (6) | 7.6 (4.9) | 6.9 (7) | 7.7 (6.1) |
| Rasmussen 2011 median (range) | 3.6 (0 ‐ 46) | 4.3 (0 ‐ 42) | 2 (0 ‐ 25) | 4 (0 ‐ 30) |
| RELACS 2012 mean | 10.4 | 11.8 | 4.8 | 4 |
| Vernermo 2016 mean (SD) [range] | 8 (5) [0 ‐ 29] | 12 (6) [0 ‐ 33] | ||
| RFA versus UGFS | ||||
| Study | Time to return to work (days) | Time to return normal activities (days) | ||
| RFA | UGFS | RFA | UGFS | |
| Rasmussen 2011 median (range) | 2.9 (0 ‐ 14) | 2.9 (0 ‐ 33) | 1 (0 ‐ 30) | 1 (0 ‐ 30) |
| RFA versus CA ‐ no data | ||||
| RFA versus MOCA | ||||
| Study | Time to return to work (days) | Time to return normal activities | ||
| RFA | MOCA | RFA | MOCA | |
| Lane 2017 median (IQR) | 2 (2 ‐ 7) | 3 (1 ‐ 7) | 2 (1 ‐ 7) | 2 (1 ‐ 4) |
| MARADONA 2019 mean (range) | 2.98 (0 ‐ 15) | 2.28 (0 ‐13) | 1.43 (0 ‐ 6) | 1 (0 ‐ 6) |
| Vähäaho 2019c mean | actual 4.7 perceived 6.4 |
actual 4.3 perceived 7.8 |
||
| RFA versus HL/S (surgery) | ||||
| Study | Time to return to work (days) | Time to return normal activities | ||
| RFA | HL/S (surgery) | RFA | HL/S (surgery) | |
| EVOLVeS 2003e mean | 4.7 | 12.4 | 1.15 | 3.89 |
| Helmy ElKaffas 2011 mean (SD) | 3 (3) | 7 (2.6) | ||
| Rasmussen 2011 median (range) | 2.9 (0 ‐ 14) | 4.3 (0 ‐ 42) | 1 (0 ‐ 30) | 4 (0 ‐ 30) |
| Rautio 2002c mean (SD) | actual: 6.5 (3.3) perceived: 6.1 (4.4) |
actual: 15.6 (6) perceived: 19.2 (10) |
no data | no data |
| Subramonia 2010 median (IQR) | 10 (4 ‐ 13) | 18.5 (11 ‐ 28) | 3 (0 ‐ 7) | 12.5 (4 ‐ 21) |
| UGFS versus HL/S (surgery) | ||||
| Study | Time to return to work (days) | Time to return normal activities | ||
| UGFS | HL/S (surgery) | UGFS | HL/S (surgery) | |
| Rasmussen 2011 median (range) | 2.9 (0 ‐ 33) | 4.3 (0 ‐ 42) | 1 (0 ‐ 30) | 4 (0 ‐ 30) |
| Vernermo 2016 mean (SD) [range] | 1 (3) [0 ‐ 21] | 12 (6) [0 ‐ 33] | ||
aThree outliers at 42, 60, 60 days bHigh dose of steam cSick leave days taken and participant's own perception of required sick leave dPresented both laser techniques separately eAdjusted according to the number of phlebectomies performed, and the type of anaesthetic used
CA: cyanoacrylate glue CI: confidence interval EVLA: endovenous laser ablation (same as EVLT) EVLT: endovenous laser therapy EVSA: endovenous steam ablation HL/S: high ligation and stripping IQR: interquartile range MOCA: mechanochemical ablation RFA: radiofrequency ablation SD: standard deviation UGFS: ultrasound‐guided foam sclerotherapy
Endovenous laser ablation (EVLA) versus radiofrequency ablation (RFA)
See Table 1.
Five studies compared EVLA to RFA (Nordon 2011; Rasmussen 2011; Recovery 2009; Shepherd 2010; Syndor 2017).
Technical success
These studies reported technical success at one month, three months, six weeks and six months, respectively. Pooling the data from these studies showed little or no differences to success rates within five years (OR 0.98, 95% CI 0.41 to 2.38; I 2 = 0%; 5 studies, 780 participants; moderate‐certainty evidence; Analysis 1.1).
1.1. Analysis.

Comparison 1: Endovenous laser ablation versus radiofrequency ablation, Outcome 1: Technical success < 5 years
Only Rasmussen 2011 provided data for five years or beyond, and no evidence of a difference in success rates was seen (OR 0.85, 95% CI 0.30 to 2.41; I 2 = 0%; 1 study, 291 participants; low‐certainty evidence; Analysis 1.2).
1.2. Analysis.

Comparison 1: Endovenous laser ablation versus radiofrequency ablation, Outcome 2: Long‐term technical success > 5 years
We downgraded the certainty of the evidence from high to moderate due to risk of bias concerns.
Recurrence
Only Rasmussen 2011 reported on recurrence in the comparison EVLA versus RFA. At three years, there was no clear difference in recurrence between the groups (OR 1.53; 95% CI 0.78 to 2.99; 291 participants; low‐certainty evidence; Analysis 1.3).
1.3. Analysis.

Comparison 1: Endovenous laser ablation versus radiofrequency ablation, Outcome 3: Recurrence
Rasmussen 2011 also reported five‐year recurrence rates, which favoured RFA (OR 2.77; 95% CI 1.52 to 5.06; 291 participants; low‐certainty evidence; Analysis 1.4). We downgraded the certainty of the evidence from high to low due to risk of bias concerns and possible imprecision as a result of wide CIs.
1.4. Analysis.

Comparison 1: Endovenous laser ablation versus radiofrequency ablation, Outcome 4: Long‐term recurrence > 5 years
Post‐operative complications
We were not able to undertake meta‐analysis for post‐operative complications due to different definitions and time points used. Nordon 2011 evaluated complications at one week and reported a 2.6% rate of skin burns, a 1.3% rate of paraesthesia and a 2.6% rate of thrombophlebitis with EVLA, compared to rates of 1.3%, 2.6% and 1.3%, respectively, with RFA. Rasmussen 2011 reported phlebitis in 12 of their RFA participants compared to four in EVLA participants at one month. There were six cases of paraesthesia and eight cases of hyperpigmentation, with three of each seen with EVLA. Recovery 2009 reported complications at 48 hours, one week, two weeks and one month: 22% of EVLA participants had a complication compared with 4% of RFA participants at one of these time points. Six participants (14.6%) had phlebitis with EVLA compared with zero with RFA. Two participants (4.9%) reported paraesthesia following EVLA (2.2% with RFA), and there was one case of deep vein thrombosis (DVT) following EVLA. In Shepherd 2010, there was a higher rate of complications within the RFA group. Eight participants (12%) developed paraesthesia after RFA compared to five (8%) in EVLA; six participants (9%) had skin staining after RFA compared to two (3%) in EVLA; and one participant developed pulmonary embolism (PE) two weeks after RFA. Syndor 2017 showed comparable rates of phlebitis in EVLA and RFA (1.04% and 1.03%) and hyperpigmentation (3.16% and 3.13%). More paraesthesia was seen following RFA (13.68%) compared to EVLA (9.38%). Results of individual studies were inconsistent with each other, so we are not able to draw any conclusions (very low‐certainty evidence). See Table 16 and Table 17.
Quality of life (QoL)
Due to the variety of different QoL questionnaires used and scores recorded at different time points amongst the included trials, we decided it was inappropriate to combine these for meta‐analysis (see Table 18). The majority of studies for this comparison showed no clear difference in QoL scores between the two treatments compared. Nordon 2011 found no difference in improvement in QoL using the Aberdeen Varicose Vein Questionnaire (AVVQ) and EuroQol‐5D (EQ‐5D) at three months between EVLA and RFA. The mean (SD) AVVQ reduction in the EVLA group was 5.9 (6.1) and 6.2 (5.9) in the RFA group (P = 0.12). The mean improvement in EQ‐5D was 0.22 (0.3) in EVLA and 0.16 (0.3) with RFA (P = 0.66). Shepherd 2010 showed comparable improvements in QoL between treatment groups at six months: mean (SD) AVVQ improved in EVLA from 18.9 (9.8) to 10.9 (8.7) at six months; and from 20.6 (9.4) to 10.2 (9.4) in RFA. The mean (SD) SF‐12 physical component score (PCS) improved from 48.1 (10.1) in EVLA to 51.4 (9.6) and from 48.9 (9.5) to 51.7 (9.3) in RFA at six months. Rasmussen 2011 found that the Aberdeen Varicose Vein Symptom Severity Score (AVVSS) improved in all groups from three days onwards (P < 0.001) with no difference between the groups at any time point. Mean (SD) AVVSS at baseline was 17.94 (9) in EVLA and 18.74 in RFA, improving to 4.61 (5.8) and 4.43 (6.58), respectively, at three years. Rasmussen 2011 reported no difference in Medical Outcomes Study Short Form 36 (SF‐36) at one month. Recovery 2009 reported changes in mean (SD) global QoL scores were better in RFA at 7 and 14 days post operation; (RFA 27.7 (11.5) and 23 (6.1) compared to EVLA 33.7 (13.7) and 29.5 (8.5), respectively). By one month, they were comparable (RFA 22.7 (5) versus EVLA 22.2 (3.3)). Syndor 2017 did not evaluate QoL measures in their study. We assessed the certainty of the evidence for this outcome as moderate, downgrading for concerns regarding risk of bias.
Pain
All studies reported reduced pain in the RFA groups compared to EVLA. Nordon 2011 showed RFA participants took less analgesia during the week after the procedure (median 0 mg ibuprofen; range 0 to 600 mg, compared to median of 200 mg; range 0 to 1050 mg in EVLA group). Median post‐procedural pain scores were higher in EVLA than in RFA: reporting at day one (28 versus 9.5 (P = 0.001)); day three (23.5 versus 6 (P = 0.001)); and day seven (13.5 versus 0 (P = 0.001)), respectively. Recovery 2009 reported significantly lower mean pain levels (SD) on visual analogue system (VAS) at 48 hours in participants who had RFA (0.7 (0.9) versus 1.9 (1.6); P < 0.001); one week (0.2 (0.6) versus 1.8 (1.8) P < 0.001), and two weeks (0.1 (0.4) versus 1.2 (1.7) P < 0.001). In Rasmussen 2011, mean pain scores on VAS at 10 days in EVLA and RFA were 2.58 (2.4) and 1.21 (1.72), respectively. Shepherd 2010 reported that participants who had RFA reported less pain over the first 10 days with mean (SD) VAS score of 22 (19.8) compared to 34.3 (21.1) in EVLA. Also, participants who underwent RFA took fewer analgesic tablets with a mean (SD) consumption of 8.8 (9.5) tablets over three days compared to 14.2 (10.7) in the EVLA group. In Syndor 2017, the median post‐procedure pain score (on a scale of one to ten) was five in the EVLA group compared to two in RFA on initial evaluation (median day of evaluation was five in EVLA (range 1 to 29 days) and six in RFA (range 1 to 9 days).
Venous Clinical Severity Score (VCSS)
Four trials reported on change in VCSS, showing comparable rates between groups at final follow‐up (Rasmussen 2011; Recovery 2009; Shepherd 2010; Syndor 2017). Rasmussen 2011 reported that the VCSS improved significantly in all groups (P < 0.001) with no difference between groups at any evaluated time point through three years. Mean (SD) VCSS at baseline was 2.68 (2.25) in EVLA and 2.95 (2.06) in RFA; at three years, this was 0.34 (1.3) and 0.44 (1.82), respectively. Recovery 2009 reported no difference between treatment groups at baseline. In the RFA group, mean VCSS scores were reduced compared with EVLA at 48 hours (4.7 versus 5.3, P < 0.001), one week (4.2 versus 5.9, P < 0.001); and two weeks (4 vs 5.3; P = 0.0035); there was no difference by one month (2.7 versus 3.2; P = 0.28). In Shepherd 2010, VCSS was comparable between the two groups at six months, with mean improvement of 3.3 in EVLA (initial 4.7 and 1.4 at six months) and 3.7 in RFA (initial 5.1 and 1.4 at six months). Syndor 2017 found participants in both groups demonstrated a reduction in VCSS at six months from baseline. Median (range) VCSS improved from 5 (2 to 26) at baseline to 1 (0 to 18) at six months in EVLA and from 5 (1 to 20) to 1 (0 to 6) with RFA. See Table 19.
Length of procedure
The duration of the procedure was similar between treatment groups. However, the reporting trials used different time points, metrics and terminology, thus impeding analysis. Nordon 2011 reported the median procedural time (range) was 30 minutes (10 to 60 minutes) with EVLA and 30 minutes (15 to 60 minutes) with RFA. In Rasmussen 2011, mean (range) surgeon's time was 26 minutes (12 to 80 minutes) for EVLA and 27 minutes (12 to 80 minutes) with RFA. In Syndor 2017, median (range) total procedure time was 23.5 minutes (8 to 95 minutes) with EVLA and 21 minutes (6 to 64 minutes) with RFA. See Table 20.
Duration of hospital stay
Shepherd 2010 explicitly stated all procedures were day case procedures. Despite their intention to perform all procedures as day case procedures, four participants (3.1%) required overnight admission: three participants in the RFA groups for nausea, hypotension secondary to general anaesthesia or pain requiring opoid analgesia, and one participant in the EVLA group for post‐operative nausea. See Table 21.
Return to normal activities
Three trials reported on return to work and normal activities (Nordon 2011; Rasmussen 2011; Shepherd 2010). Results were comparable but studies evaluated this outcome by different means. Nordon 2011 reported median (range) return to work was seven days (1 to 60 days) after EVLA compared with nine days after RFA (1 to 28 days). In Rasmussen 2011, median (range) return to normal activities was 2 days (0 to 25 days) and to work was 3.6 days (0 to 46 days) compared with 1 day (0 to 30 days) and 2.9 days (0 to 14 days) with RFA. Shepherd 2010 reported 74% of participants had returned to normal activities and 71% had returned to work at seven days following EVLA. This was comparable with RFA, with 77% of participants at normal levels of activity and 71% back at work by seven days. See Table 22.
Endovenous laser ablation (EVLA) versus endovenous steam ablation (EVSA)
Only LAST 2014 compared EVLA with EVSA.
Technical success
In LAST 2014, no clear difference in success was seen between the groups (OR 1.94, 95% CI 0.53 to 7.15; 1 study, 166 participants; Analysis 2.1). There were no reports of data for five years or beyond.
2.1. Analysis.

Comparison 2: Endovenous laser ablation versus endovenous steam ablation, Outcome 1: Technical success < 5 years
Recurrence
LAST 2014 did not report this outcome.
Post‐operative complications
Complication profiles were similar between the two groups. Participants had similar rates of thrombophlebitis following treatment (10 participants in each group at two weeks) and one participant in the EVLA group developed a DVT. Two participants within the EVSA group had nerve injury reported at two weeks. See Table 16 and Table 17.
Quality of life
LAST 2014 reported that the EQ‐5D and EQ visual analogue scale scores were comparable for EVSA and EVLA at 12 weeks.
Pain
LAST 2014 reported that the EVSA group had less post‐procedural pain (mean VAS score in EVLA of 5.6 and 2.6 in EVSA; P < 0.001); and a shorter duration of analgesic use (mean 0.9 days compared with 3.3 days in EVLA; P < 0.001).
Venous Clinical Severity Score (VCSS)
Changes in VCSS between baseline and 12 weeks were similar between the two treatment arms: ‐2.69 (95% CI ‐2.34 to ‐3.04) in ESVS and ‐2.51 (95% CI ‐2.10 to ‐2.93) in the EVLA group. See Table 19.
Length of procedure
LAST 2014 did not report this outcome.
Duration of hospital stay
LAST 2014 did not report this outcome.
Return to normal activities
Convalescence was measured as the number of days lost from work or normal activities. Participants undergoing EVSA had a mean return to normal activity of 1.6 days (95% CI 1 to 2.1), compared to 4.2 days (95% CI 3.4 to 5) with EVLA. See Table 22.
Endovenous laser ablation (EVLA) versus ultrasound‐guided foam sclerotherapy (UGFS)
See Table 2.
Three studies compared EVLA with UGFS (Magna 2013; Rasmussen 2011; Vernermo 2016).
Technical success
Three studies evaluated EVLA compared to UGFS for technical success up to five years (Magna 2013; Rasmussen 2011; Vernermo 2016). Two of these also reported data for greater than five‐year follow‐up (Magna 2013; Vernermo 2016). Meta‐analysis showed technical success may be improved in those undergoing EVLA up to five years (OR 6.13, 95% CI 0.98 to 38.27; 3 studies, 588 participants; low‐certainty evidence; Analysis 3.1); and over five years follow‐up (OR 6.47, 95% CI 2.60 to 16.10; 3 studies, 534 participants; low‐certainty evidence; Analysis 3.2) noting the wide CIs. Heterogeneity was detected at up to and over five years so a random‐effects method was used (I2 = 78% and I2 = 68%, respectively). We downgraded the certainty of the evidence from high to low due to risk of bias concerns and inconsistency.
3.1. Analysis.

Comparison 3: Endovenous laser ablation versus ultrasound‐guided foam sclerotherapy, Outcome 1: Technical success < 5 years
3.2. Analysis.

Comparison 3: Endovenous laser ablation versus ultrasound‐guided foam sclerotherapy, Outcome 2: Technical success > 5 years
Recurrence
Two studies compared recurrence in EVLA and UGFS at one and three years, respectively (Magna 2013; Rasmussen 2011), and showed no clear difference between the groups (OR 0.68, 95% CI 0.20 to 2.36; 2 studies, 443 participants; very low‐certainty evidence; Analysis 3.3). Five‐year recurrence rates were also available for both studies and again no clear differences were seen (OR 1.08, 95% CI 0.40 to 2.87; 2 studies, 418 participants; very low‐certainty evidence; Analysis 3.4). Heterogeneity was detected so a random‐effects model was used (I2 = 82% and 76%, respectively). We downgraded the certainty of the evidence due to risk of bias concerns, inconsistency and imprecision.
3.3. Analysis.

Comparison 3: Endovenous laser ablation versus ultrasound‐guided foam sclerotherapy, Outcome 3: Recurrence
3.4. Analysis.

Comparison 3: Endovenous laser ablation versus ultrasound‐guided foam sclerotherapy, Outcome 4: Long‐term recurrence > 5 years
Post‐operative complications
All three studies reported on post‐operative complications. However, meta‐analysis was impeded by the different definitions of complications used amongst trials and the varying time points at which complications were assessed. At one month, Rasmussen 2011 reported an iliac vein thrombosis with subsequent pulmonary embolism in one participant who had undergone UGFS one week prior. Phlebitis rates were higher amongst the UGFS group and were seen in 17 participants compared to 4 in the EVLA group. UGFS also had higher rates of hyperpigmentation at one month with eight cases compared to three within the EVLA arm. In Vernermo 2016, skin pigmentation was common in the UGFS arm at one month ‐ seen in 67% of participants compared to 4% in the EVLA group. Vernermo 2016 found haematomas in 42% of participants undergoing EVLA compared to 20% of UGFS participants at one month. Magna 2013 reported two cases of hyperpigmentation in EVLA participants compared to one case in UGFS at three months. We downgraded to very‐low certainty evidence due to risk of bias concerns, inconsistency, imprecision and possible publication bias. See Table 16 and Table 17.
Quality of life
All three studies reported on this outcome but evaluated QoL using different questionnaires at different time points. Magna 2013 reported no significant differences between EVLA and UGFS at three months and one year in Chronic Venous Insufficiency Quality of Life Questionnaire (CIVIQ2) and EQ‐5D scores. While in Rasmussen 2011, UGFS was deemed to be better with regard to bodily pain and physical functioning in the SF‐36 score initially, but showed no difference between comparisons at one month. Vernermo 2016 found no significant difference in median AVVSS between the treatment groups at one year. We assessed the overall certainty of evidence for QoL as moderate, downgrading by one step due to risk of bias concerns.
Pain
Two studies evaluated pain scores, with both reporting lower post‐procedural pain with UGFS compared to EVLA treatment, but we were not able to undertake meta‐analysis as data were not reported for both studies (Rasmussen 2011; Vernermo 2016). Vernermo 2016 reported pain after treatment was significantly reduced (lower VAS score) both at the time of discharge, and one week following UGFS treatment compared with EVLA. In Rasmussen 2011, less pain was reported during the first ten days after UGFS treatment (mean (SD) VAS score was 1.6 (2.04) in the UGFS group and 2.58 (2.4) in the EVLA group).
Venous Clinical Severity Score (VCSS)
VCSS was only analysed by Rasmussen 2011, who found that VCSS improved in all groups from baseline, with no difference between treatment arms at any evaluated time point. Initial mean (SD) VCSS improved from 2.68 (2.25) to 0.34 (1.3) in EVLA compared to 2.66 (1.45) to 0.15 (0.4) in UGFS. See Table 19.
Length of procedure
Rasmussen 2011 was the only study which evaluated length of procedure as surgeon's time. Mean surgeon's time was 26 minutes in the EVLA group (range 12 to 80 minutes) and 19 minutes in the UGFS group (range 5 to 145 minutes).
Duration of hospital stay
No studies reported on duration of hospital stay.
Return to normal activities
Vernermo 2016 reported the mean duration of sick leave, and this was eight days in the EVLA group (range 0 to 29 days) and one day in the UGFS (range 0 to 21 days).
Endovenous laser ablation (EVLA) versus cyanoacrylate glue
Calik 2019 was the sole trial to evaluate this comparison. We assessed it as having a high risk of bias in five bias categories, but we included it as it was the sole RCT found for this comparison.
Technical success
Calik 2019 evaluated technical success at 1‐, 3‐, 6‐ and 12‐month follow‐up. As the one‐month data is the closest to our definition of technical success (complete anatomical obliteration, or absence of reflux, within the GSV around six weeks on DUS) we have used this time point in our analysis. Occlusion rates showed no evidence of a difference between the treatment groups (OR 0.33, 95% CI 0.01 to 8.03; 1 study, 412 participants; Analysis 4.1). At 12 months, there was no clear difference in recurrence between groups (OR 2.59, 95% CI 0.50 to 13.49; 1 study, 412 participants). These participants had no clinically significant symptoms. There were no long‐term data available.
4.1. Analysis.

Comparison 4: Endovenous laser ablation versus cyanoacrylate glue, Outcome 1: Technical success < 5 years
Recurrence
There were two recanalisations in the cyanoacrylate glue group and five within the EVLA group, and results showed no evidence of a difference in recanalisation rates at one year (OR 2.59, 95% CI 0.50 to 13.49; 1 study, 412 participants; Analysis 4.2). There were no long‐term data available.
4.2. Analysis.

Comparison 4: Endovenous laser ablation versus cyanoacrylate glue, Outcome 2: Recurrence
Post‐operative complications
Higher rates of post‐procedural induration, bruising and paraesthesia were reported following EVLA at one week, but there was no difference by the three‐month time point except for paraesthesia, which was reported in 13 EVLA participants and 2 cyanoacrylate glue participants (P < 0.001). Two DVTs were found within the EVLA group. See Table 16 and Table 17.
Quality of life
Quality of life was evaluated via the CIVIQ2 score. The mean CIVIQ2 scores demonstrated meaningful improvement in all groups at follow‐up (P < 0.001) with no clear difference between cyanoacrylate glue and EVLA groups reported. The mean pre‐procedural score was 41.4 in the EVLA group, improving to 12.8 at one year. In the cyanoacrylate glue group, the mean pre‐procedural score was 40.6 and 12.3 at one year.
Pain
Calik 2019 evaluated participant‐reported pain using the Wong‐Baker FACES pain rating scale. At one week, participants who had undergone EVLA had a higher mean pain score (5.4 (SD 3.7)) than participants who underwent cyanoacrylate glue (2.8 (SD 3.1); P < 0.001). However, at three months, there was no evidence of a difference between the mean pain scores 0.7 (SD 0.5) and 0.6 (SD 0.4), respectively (P < 0.46).
Venous Clinical Severity Score (VCSS)
At one year, VCSS (SD) had declined from 5.8 (1.9) to 1.3 (0.9) (P < 0.001) for the EVLA group, and from 5.7 (1.9) to 1.3 (0.9) (P < 0.001) for the cyanoacrylate glue group, with no evidence of difference between groups. See Table 19.
Length of procedure
The mean operative time (SD) was longer for the EVLA group (31.7 (8.8) minutes) than for cyanoacrylate glue group (13 (3.4) minutes) (P < 0.001). See Table 20.
Duration of hospital stay
Calik 2019 did not evaluate this outcome.
Return to normal activities
Amongst the cyanoacrylate glue group, there was a faster return to daily activities (1.5 days) compared to participants who underwent EVLA (2.9 days; P < 0.001). Results are summarised below under 'Narrative summaries' and detailed within Table 22.
Endovenous laser ablation (EVLA) versus mechanochemical ablation (MOCA)
One study compared EVLA with MOCA (Vähäaho 2019).
Technical success
At one month, all treated great saphenous veins were occluded, regardless of treatment modality (Analysis 5.1). There were no long‐term data available.
5.1. Analysis.

Comparison 5: Endovenous laser ablation versus mechanochemical ablation, Outcome 1: Technical success < 5 years
Recurrence
Ten participants within the MOCA treatment group had ultrasound‐proven recanalisation at one year compared to none in the EVLA group (OR 0.06, 95% CI 0.00 to 1.14; 1 study, 88 participants; Analysis 5.2). There were no long‐term data available.
5.2. Analysis.

Comparison 5: Endovenous laser ablation versus mechanochemical ablation, Outcome 2: Recurrence
Post‐operative complications
Three participants in the EVLA group reported sensory disturbance at one year; no nerve injuries were seen in the MOCA group. There was one superficial infection seen in the MOCA treatment group. See Table 16 and Table 17.
Quality of life
Mean AVVQ at baseline was 16.1 in EVLA group and 15.8 in the MOCA group. By year one, all had improved and there was no evidence of a difference between the treatment groups reported by the study authors (mean AVVQ in EVLA was 5.3, and in MOCA 6.2 (P = 0.9).
Pain
Vähäaho 2019 evaluated pain using the visual analogue system (VAS) and recorded scores as zero to ten. During the procedure, the mean VAS pain score was 3.9 for EVLA and 4.6 for MOCA (P = 0.12). The study authors reported that use of extra periprocedural sedative (propofol) was less in participants undergoing MOCA than in participants undergoing thermal ablation (P < 0.001). The use of fentanyl and diazepam periprocedurally did not differ between treatment groups (P = 0.12 and P = 0.41, respectively). Prior to discharge, pain scores were found to be similar between interventions (P = 0.18), as well as at one week (P = 0.92). The amount of post‐operative analgesia consumed by participants did not differ (P = 0.12).
Venous Clinical Severity Score (VCSS)
Vähäaho 2019 did not report on this outcome.
Length of procedure
Vähäaho 2019 did not report on this outcome.
Duration of hospital stay
Vähäaho 2019 did not explicitly mention whether all procedures were performed as day case surgery.
Return to normal activities
Participants undergoing EVLA took a mean of 5.3 days sick leave compared to 4.3 days in those undergoing MOCA. See Table 22.
Endovenous laser ablation (EVLA) versus SFJ ligation and stripping (HL/S, surgery)
See Table 3.
Nine studies compared EVLA with SFJ ligation and stripping (Darwood 2008; Flessenkämper 2013; HELP‐1 2011; Magna 2013; Pronk 2010; Rasmussen 2007; Rasmussen 2011; RELACS 2012; Vernermo 2016).
Technical success
A total of six studies compared technical success in EVLA and SFJ ligation and stripping (Darwood 2008; HELP‐1 2011; Magna 2013; Rasmussen 2007: Rasmussen 2011; Vernermo 2016); with five studies also reporting five‐year data (HELP‐1 2011; Magna 2013; Rasmussen 2007: Rasmussen 2011; Vernermo 2016).
There was a possible benefit in technical success at less than five years in the EVLA group (OR 2.31, 95% CI 1.27 to 4.23; 6 studies, 1051 participants; low‐certainty evidence; Analysis 6.1). There was no clear difference seen at five years and beyond (OR 0.93, 95% CI 0.57 to 1.50; 5 studies, 874 participants; low‐certainty evidence; Analysis 6.2). We downgraded the certainty of the evidence from high to low due to risk of bias concerns and imprecision. See Table 3.
6.1. Analysis.

Comparison 6: Endovenous laser ablation versus SFJ ligation and stripping (HL/S, surgery), Outcome 1: Technical success < 5 years
6.2. Analysis.

Comparison 6: Endovenous laser ablation versus SFJ ligation and stripping (HL/S, surgery), Outcome 2: Technical success > 5 years
Recurrence
Seven studies reported on recurrence (one to three years) between EVLA and SFJ ligation and stripping (Flessenkämper 2013; HELP‐1 2011; Magna 2013; Pronk 2010; Rasmussen 2007; Rasmussen 2011; RELACS 2012). We were able to pool these data. Meta‐analysis showed no clear difference in recurrence rate between the EVLA or surgery group up to 5 years (OR 0.78, 95% CI 0.47 to 1.29; 7 studies, 1459 participants; moderate‐certainty evidence; Analysis 6.3).
6.3. Analysis.

Comparison 6: Endovenous laser ablation versus SFJ ligation and stripping (HL/S, surgery), Outcome 3: Recurrence
Five‐year data was available also from seven studies (Flessenkämper 2013; HELP‐1 2011; Magna 2013; Pronk 2010; Rasmussen 2007; Rasmussen 2011; RELACS 2012). Pooling showed no clear difference in recurrence rates (OR 1.09, 95% CI 0.68 to 1.76; 7 studies, 1267 participants; moderate‐certainty evidence; Analysis 6.4). Heterogeneity was detected so a random‐effects model was used (I2 = 62% and I2 = 70%, respectively). We downgraded from high to moderate certainty due to the lack of blinding inherent within these studies.
6.4. Analysis.

Comparison 6: Endovenous laser ablation versus SFJ ligation and stripping (HL/S, surgery), Outcome 4: Long‐term recurrence > 5 years
Post‐operative complications
We were not able to undertake meta‐analysis for post‐operative complications because the included trials used different definitions and time points. Darwood 2008 reported higher rates of phlebitis amongst EVLA participants (11%) compared to SFJ ligation and stripping (0%). Neurosensory loss was reported in 13% of SFJ ligation and stripping participants compared to 1% in EVLA. One participant undergoing SFJ ligation and stripping developed acute respiratory distress syndrome after aspirating on extubation and required intensive care unit (ICU) care for seven days. Flessenkämper 2013 reported similar rates of saphenous nerve injury between groups at two months (15% and 17%, respectively); early bruising and pigmentation was higher in the SFJ ligation and stripping group (68%) compared to the EVLA group (47.9%). HELP‐1 2011 reported higher rates of sensory disturbance (9.8%), haematoma (8.3%) and infection (8%) following SFJ ligation and stripping compared to EVLA (4%, 1% and 1.5%, respectively). Magna 2013 reported low rates of complications at three months' follow‐up. Paraesthesia was reported in 5.9% of SFJ ligation and stripping participants compared to 2.6% with EVLA; 2.6% of EVLA participants had hyperpigmentation, none was seen with SFJ ligation and stripping. Pronk 2010 reported low levels of complications in their trial: paraesthesia was seen in 3% of their EVLA participants and 1% of SFJ ligation and stripping participants. Rasmussen 2007 reported higher rates of bruising at 12 days following SFJ ligation and stripping (25%) compared to EVLA (11%) (P > 0.05). Paraesthesia was slightly higher following SFJ ligation and stripping (4.2%) than EVLA (2.4%) as was hyperpigmentation (5% compared to 2.45) in Rasmussen 2011. High levels of bruising were reported with each treatment group (90.1% in both) in RELACS 2012. Phlebitis was more pronounced in the EVLA group (10.8% versus 2.5%) as was pigmentation (32% versus 12%). At one month, higher rates of haematoma were seen with SFJ ligation and stripping (62%) compared with EVLA (42%). We downgraded to very low‐certainty evidence due to risk of bias concerns, inconsistency, imprecision and possible publication bias. See Table 16 and Table 17.
Quality of life
Darwood 2008 reported that the AVVSS improved at three months and was similar between groups (P = 0.694). At baseline, AVVSS (SD) was 11.76 (9.81 ‐ 19.44), improving to 5.6 (1.45 ‐ 8.2) at three months in the EVLA group, while in the SFJ ligation and stripping group, baseline AVVSS was 14.02 (9.49 ‐ 19.16), improving to 5.32 (1.03 ‐ 7.66) at three months. HELP‐1 2011 found that AVVSS, EQ‐5D and several domains of the SF‐36 showed deterioration within the first post‐operative week for both treatment groups (P < 0.001). However, these scores improved for the rest of the duration of the follow‐up period (P < 0.001), with no statistical difference seen between either groups at any time point for AVVSS and EQ‐5D, and none after four weeks in the SF‐36. For the SF‐6D (a variation of SF‐36), the EVLA group was seen to have significantly better scores than the surgical group (P = 0.003). Magna 2013 showed improvement in both CIVIQ2 and EQ‐5D scores at three months but no significant difference in score was seen between either groups. Within the EVLA group in Pronk 2010, the EQ‐5D scores for daily activity were better than for those in the surgery group on day one (P = 0.01). However, the EVLA group had lower mobility scores on days seven and ten (P < 0.01, P = 0.01, respectively) than the surgery group. At six months and five years, there was no change in EQ‐5D in either EVLA or surgery groups. Rasmussen 2007 showed significant improvements at five years in the AVVSS and SF‐36 scores with no significant differences in outcomes between the groups. Rasmussen 2011 showed the AVVSS improved from baseline from day three onwards (P > 0.001) with no difference at any evaluated time point. SF‐36 scores showed improvement in all domains at some time point with no difference between groups. The RELACS 2012 study demonstrated that CIVIQ scores remained stable up to five years after treatment, without significant differences between the two groups. Vernermo 2016 reported that the AVVSS was improved from baseline with no difference between EVLA and SFJ ligation and stripping. We downgraded the certainty of the evidence from high to moderate due to risk of bias concerns.
Pain
We were not able to undertake meta‐analysis for pain because the included trials used different definitions, methods of measuring the outcome and evaluation times. Darwood 2008 evaluated daily pain scores through use of an ungraded visual analogue pain score over the first week, and found no difference between interventions at any time point. Median (IQR) duration of analgesic use was six days (3 to 7) with EVLA, and four days (1 to 7) with SFJ ligation and stripping. Flessenkämper 2013 reported no difference in pain during the first five days following intervention (P = 0.12). The HELP‐1 2011 study reported that the EVLA group reported less pain from day one compared with the SFJ ligation and stripping group (P = 0.004 to P < 0.001), with a resultant increase in the latter group's analgesic consumption over the same period (P = 0.012 to P = 0.001). Pronk 2010 demonstrated higher mean pain scores (SD) following EVLA compared with SFJ ligation and stripping at day 7 (3.74 (2.72) versus 1.78 (1.94), P < 0.01), day 10 (2.65 (2.21) versus 1.18 (1.49), P < 0.01), and day 14 (1.66 (2.04) versus 0.77 (1.46), P = 0.01). However, periprocedural pain scores were higher with SFJ ligation and stripping, with a mean (SD) periprocedural pain score of 3.39 (2.57) versus EVLA pain score of 2.21 (2.4); P = 0.02. The higher pain in EVLA could possibly be attributed to the use of tumescent analgesia with SFJ ligation and stripping. Within the Rasmussen 2007 trial, VAS pain scores were not significantly statistically different between groups (P < 0.01). No difference in the mean use of analgesia was found, with 12 tablets consumed in the EVLA group and 12.9 in the SFJ ligation and stripping group. Rasmussen 2011 reported no difference in mean pain score (SD) within ten days, with a score of 2.58 (2.41) in EVLA and 2.25 (2.23) with SFJ ligation and stripping. RELACS 2012 reported similar mean (SD) VAS pain scores during the first post‐operative week between EVLA (1.6 (0.8)) and SFJ ligation and stripping (1.3 (0.6)) (P = 0.005). Duration of pain (SD) was 8 (6) days in EVLA and 17 (20) days in SFJ ligation and stripping.
Venous Clinical Severity Score (VCSS)
Four studies reported on VCSS with comparable improvements in scores between interventions. Darwood 2008 reported that, following treatment, VCSS improved from a median (IQR) of 4 (1 ‐ 3) to 0 (0 ‐ 1) (P < 0.001). HELP‐1 2011 reported that both groups showed a similar improvement in VCSS from a median of 4 (3 ‐ 5) to 1 (0 ‐ 3) by three months (P < 0.001). This was maintained up to a year with no difference between interventions at any evaluation point. Rasmussen 2007 found mean VCSS (SD) improved from baseline from 2.8 (1.7) to 0.4 (0.9) at five years in EVLA and from 2.4 (1.4) to 2.4 (1.4) with SFJ ligation and stripping. Scores were not seen to differ between interventions at any time point. Rasmussen 2011 reported improvement in both groups (P > 0.001), with no difference at any time point over three years. The mean (SD) VCSS at baseline was 2.68 (2.25) for EVLA and 2.75 (1.62) for SFJ ligation and stripping. This had improved to 0.34 (1.3) and 0.3 (0.5) by three years. See Table 19.
Length of procedure
Three studies reported on length of procedure. HELP‐1 2011 reported that EVLA took longer, with a mean time (SD) of 67 minutes (16) compared to 61 minutes (14) with SFJ ligation and stripping. Rasmussen 2011 reported a mean surgeon's time (range) of 26 minutes (12 to 80) with EVLA and 32 minutes (15 to 80) with SFJ ligation and stripping. Vernermo 2016 reported a mean (SD) duration of treatment of 83 (17) minutes (range 50 to 139 minutes) in EVLA compared to 95 (19) minutes (range 62 to 155 minutes) with SFJ ligation and stripping. See Table 20.
Duration of hospital stay
Darwood 2008, Flessenkämper 2013, Pronk 2010 and Rasmussen 2007 stated that all their procedures were undertaken in an outpatient setting. HELP‐1 2011 reported that 21.2% of their participants undergoing SFJ ligation and stripping required inpatient admission due to their unsuitability for day case general anaesthesia. See Table 21.
Return to normal activities
Seven studies evaluated return to normal activities and work. The majority of studies demonstrated that participants undergoing EVLA returned to work faster. Darwood 2008 found that participants undergoing EVLA returned to work faster than with SFJ ligation and stripping, with a median time to return to work (IQR) of four days (2.5 to 7) in the EVLA group compared to 17 days (7.25 to 33.25) with SFJ ligation and stripping. Median (IQR) return to normal activities was two days (0 to 7) and seven days (2 to 26), respectively. HELP‐1 2011 reported a median (range) return to work of four days (2 to 14 days) and a median (range) return to normal activities of three days (1 to 10 days) with EVLA, compared to 14 days (13 to 28) and 14 days (7 to 25), respectively with SFJ ligation and stripping. Mean return to work was comparable between interventions in Pronk 2010, with a mean return (SD) of 4.38 (5.43) in EVLA and 4.15 (3.72) SFJ ligation and stripping. Mean (SD) return to normal activities was 3.16 days (4.34) in EVLA and 3.20 days (4.01) with SFJ ligation and stripping. In Rasmussen 2007, mean (SD) return to normal activities (6.9 days (7) versus 7.7 days (6.1)), and mean (SD) time to resume work (7 days (6) versus 7.6 days (4.9)) was comparable between EVLA and SFJ ligation and stripping. Rasmussen 2011 reported no difference between EVLA and SFJ ligation and stripping concerning return to normal activities and work (P = 0.18 and P = 0.26, respectively). The median time to return to work (range) was 3.6 days (0 to 46 days) in EVLA and 4.3 days (0 to 42 days) with SFJ ligation and stripping. Median time to return to normal activities was 2 days (0 to 25 days) and 4 days (0 to 30 days). RELACS 2012 reported a mean return to basic activity of 4 days with EVLA and 4.8 days with SFJ ligation and stripping; the ability to work or perform comparable tasks was achieved after 10.4 days and 11.8 days, respectively, for the two groups. Vernermo 2016 reported a mean (range) length of sick leave of 8 days (0 to 29 days) after EVLA and 12 days (0 to 33 days) following SFJ ligation and stripping. See Table 22.
Radiofrequency ablation (RFA) versus ultrasound‐guided foam sclerotherapy (UGFS)
Only Rasmussen 2011 compared RFA with UGFS.
Technical success
There was no clear benefit to either treatment in technical success up to 5 years (OR 5.21, 95% CI 0.25 to 109.48; 1 study, 292 participants; Analysis 7.1) with a notably wide CI.
7.1. Analysis.

Comparison 7: Radiofrequency ablation versus ultrasound‐guided foam sclerotherapy, Outcome 1: Technical success < 5 years
Rasmussen 2011 also reported on long‐term technical success with a possible benefit to RFA treatment detected (OR 3.23, 95% CI 1.32 to 7.89; 1 study, 291 participants; Analysis 7.2).
7.2. Analysis.

Comparison 7: Radiofrequency ablation versus ultrasound‐guided foam sclerotherapy, Outcome 2: Long‐term technical success > 5
Recurrence
Rasmussen 2011 evaluated recurrence in RFA against UGFS at three years, and results show no clear difference (OR 0.81, 95% CI 0.41 to 1.62; 1 study, 291 participants; Analysis 7.3).
7.3. Analysis.

Comparison 7: Radiofrequency ablation versus ultrasound‐guided foam sclerotherapy, Outcome 3: Recurrence
Five‐year comparison data was also available and again showed no clear difference (OR 0.61, 95% CI 0.33 to 1.16; 1 study, 291 participants; Analysis 7.4).
7.4. Analysis.

Comparison 7: Radiofrequency ablation versus ultrasound‐guided foam sclerotherapy, Outcome 4: Long‐term recurrence > 5 years
Post‐operative complications
One participant developed an iliac vein thrombosis and subsequent pulmonary embolus one week post‐UGFS. Equal levels of hyperpigmentation were seen between groups. More episodes of phlebitis were recorded in the UGFS group than in RFA (12 versus 17). See Table 16 and Table 17.
Quality of life
Rasmussen 2011 did not present data but reported that "for all groups in all domains there was statistically significant improvement in most scores from pre‐treatment to one year. At three days participants treated with UGFS and RFA had significantly better scores for bodily pain, physical functioning and role‐physical, this difference went by one month".
Pain
In Rasmussen 2011, the mean (SD) pain score (VAS) for the first 10 days post‐procedure was 1.21 (1.72) and 1.6 (2.04) in RFA and UGFS, respectively.
Venous Clinical Severity Score (VCSS)
Mean (SD) VCSS at baseline was 2.95 (2.06) in RFA and 2.06 (1.45) in UGFS, reducing to 0.44 (1.82) and 0.15 (0.4), respectively, at three years. See Table 19.
Length of procedure
The length of procedure was recorded as 'surgeon's time' within the trial. The median surgeon's time for RFA was 27 minutes (range of 12 to 80 minutes) compared to 19 minutes (range of 5 to 145 minutes) with UGFS. See Table 20.
Duration of hospital stay
Rasmussen 2011 did not report upon duration of hospital stay.
Return to normal activities
The median time to return to normal activities was one day in both groups, with a range of 0 to 30 days. The median time to return to work was 2.9 days in both groups, with a range of 0 to 14 days in the RFA group and 0 to 33 days in the UGFS group. See Table 22.
Radiofrequency ablation (RFA) versus cyanoacrylate glue
Morrison 2015 was the only trial to compare RFA with cyanoacrylate glue.
Technical success
Morrison 2015 reported technical success at one and three months. We report the one‐month results in this review as these are closest to the primary outcome of six‐week technical success. There were increased occlusions in the cyanoacrylate glue group compared to RFA (OR 0.03, 95% CI 0.00 to 0.54; 1 study, 215 participants; Analysis 8.1). The two‐year follow‐up results (n = 171) found there to be equivalent technical success for cyanoacrylate glue: 82/86 (95.3%) and RFA: (94.0% (79/84). Follow‐up data were also available for 36 months, and the study authors reported that at this time point, occlusion was comparable between cyanoacrylate glue (94.4%, 68/72) and RFA (91.9%, 68/74) (P = 0.75).
8.1. Analysis.

Comparison 8: Radiofrequency ablation versus cyanoacrylate glue, Outcome 1: Technical success < 5 years
Recurrence
The two‐year follow‐up identified 12/86 recanalisations in the cyanoacrylate glue group and only 1/84 in the RFA group. This was non‐inferior.
Post‐operative complications
Within the first three months, three participants in each treatment group were reported to have paraesthesia. There were 16/84 episodes of phlebitis with RFA and 22/86 with cyanoacrylate glue. Between three and twelve months, there was one DVT within the RFA arm, one case of endovenous heat‐induced thrombosis with RFA, and one participant with chronic phlebitis who had undergone cyanoacrylate glue. See Table 16 and Table 17.
Quality of life
Morrison 2015 demonstrated that at one year, QoL, as measured by the EQ‐5D, increased by small and similar amounts in both RFA and cyanoacrylate glue groups (P = 0.12). At 36 months, there was no statistical difference between cyanoacrylate glue and RFA in both AVVQ (P = 0.45) and EQ‐5D (P = 0.4). See Table 18.
Pain
Morrison 2015 found there was no difference in the pain experienced between the two treatment arms during the 24 hours before the day three visit (P = 0.36).
Venous Clinical Severity Score (VCSS)
VCSS was evaluated at baseline and was to found to have improved by approximately 3.5 points at three months (P > 0.01). Initial VCSS was 5.6 in RFA and 5.5 in cyanoacrylate glue, improving to 2 and 1.9, respectively. There was no difference between treatment groups. See Table 19.
Length of procedure
Mean procedural time was five minutes longer for cyanoacrylate glue (24 minutes) than RFA (19 minutes) (P < 0.01). See Table 20.
Duration of hospital stay
All interventions were undertaken as day case procedures.
Return to normal activities
Morrison 2015 did not evaluate post‐operative return to activity.
Radiofrequency ablation (RFA) versus mechanochemical ablation (MOCA)
See Table 4.
Three studies compared RFA with MOCA (Lane 2017; MARADONA 2019; Vähäaho 2019).
Technical success
All three studies compared technical success rates in RFA and MOCA (Lane 2017; MARADONA 2019; Vähäaho 2019). Both Vähäaho 2019 and MARADONA 2019 reported on technical success at 30 days, while Lane 2017 reported technical success rates at 6 months. Following discussion between all review authors it was felt inclusion in meta‐analysis was warranted. Meta‐analysis showed no clear evidence of a benefit for RFA over MOCA (OR 1.76, 95% CI 0.06 to 54.15; 3 studies, 435 participants; low‐certainty evidence; Analysis 9.1), noting the wide CI. We downgraded by two levels due to risk of bias concerns and inconsistency. A random‐effects model was used as heterogeneity was detected (I2 = 60%).
9.1. Analysis.

Comparison 9: Radiofrequency ablation versus mechanochemical ablation, Outcome 1: Technical success < 5 years
No long‐term data were available.
Recurrence
All three studies compared recurrence rates for RFA versus MOCA (Lane 2017; MARADONA 2019; Vähäaho 2019). Meta‐analysis did not show a clear benefit for one intervention over the other (OR 1.00, 95% CI 0.21 to 4.81; 3 studies, 389 participants; low‐certainty evidence; Analysis 9.2). We downgraded by two levels due to risk of bias concerns and inconsistency. A random‐effects model was used as heterogeneity was detected (I2 = 67%).
9.2. Analysis.

Comparison 9: Radiofrequency ablation versus mechanochemical ablation, Outcome 2: Recurrence
No long‐term data were available.
Post‐operative complications
All three studies reported on complication rates, which were similar between treatment arms. In the MARADONA 2019 trial, there was one DVT at one year in the RFA group. Lane 2017 showed equal rates of DVTs between groups. In Vähäaho 2019, two participants who had undergone RFA were found to have sensory disturbance; none was seen in the MOCA group. We were unable to perform meta‐analysis because the trials used different definitions and evaluated complications at different time points. We downgraded to very‐low certainty evidence due to risk of bias concerns, inconsistency and possible publication bias. See Table 16 and Table 17.
Quality of life
All studies evaluated quality of life scores. For disease‐specific quality of life (AVVQ), the Lane 2017 study authors report that there was no difference at any time point during the study. At one month, mean AVVQ was 12.1 (7.3 to 21.2) for MOCA versus 12.9 (6.6 to 20.4) for RFA (P = 0.80); and 11.8 (7.2 to 20.5) for MOCA versus 9.4 (3.6 to 21.4) for RFA at six months (P = 0.51). Between groups, there was no significant difference in EQ‐5D QoL at one month (MOCA 0.76 (0.659 to 1.00) versus RFA –0.76 (0.69 to 1) (P = 0.94)); or at six months (MOCA 0.76 (0.69 to 1.00) versus RFA 0.76 (0.49 to 1.00) (P = 0.13)).
The MARADONA 2019 trial reported "no difference were observed between groups in drawn blocks and total AVVQ scores at 1‐ and 2‐year follow‐up". AVVQ improvement at one year was 90% in MOCA and 78% RFA (P = 0.19). At two years, this was 88% and 89%, respectively (P = 0.90). Participants who underwent RFA demonstrated an improvement in physical functioning at one year on the SF‐36, whilst in MOCA, there were significant improvements in physical and social functioning, both physical and emotional role functioning, mental health and pain. In Vähäaho 2019, the mean AVVQ at baseline was 16.1 in EVLA participants and 15.8 in MOCA. By year one, all had improved and there was no statistically difference (mean AVVQ 5.3 in EVLA and 6.2 in MOCA; P = 0.90).
We downgraded the certainty of evidence by one level due to risk of bias concerns.
Pain
This was the primary outcome for Lane 2017. The study authors reported that the maximum periprocedural pain score, measured on a visual analogue scale, was significantly lower following MOCA (median 15 mm (IQR 7 mm to 36 mm)) compared with the score following RFA (34 mm (IQR 16 mm to 34 mm)) (P = 0.003). In the MARADONA 2019 trial, lower pain scores were seen in the first two weeks after MOCA. Median pain score in this group was 0.2 with a range of 0 to 0.8; and in participants undergoing RFA, the median pain score was 0.5 with a range of 0.2 to 1.3 (P = 0.01). However, the analgesic requirement was similar. Pain was evaluated using a visual analogue system (VAS) in Vähäaho 2019 and ranked from 0 to 10. During the procedure, the mean VAS pain score was 3.5 for RFA and 4.6 for MOCA (P = 0.12). The use of extra periprocedural sedative (propofol) was found to be significantly less in participants undergoing MOCA (P < 0.001) than in participants undergoing RFA. The use of fentanyl and diazepam periprocedurally did not differ between treatment groups (P = 0.11 and P = 0.41, respectively). Prior to discharge, pain scores were found to be similar between interventions (P = 0.18) as well as at one week (P = 0.92). The amount of post‐operative analgesia consumed by participants did not differ (P = 0.12).
Venous Clinical Severity Score (VCSS)
Both Lane 2017 and MARADONA 2019 reported on VCSS. The Lane 2017 study authors reported that, between groups, there was no significant difference for VCSS at either one month (MOCA 2 (1 to 4) versus RFA 3 (1 to 5), P = 0.1); or six months (MOCA 2 (1 to 4) versus RFA 2 (1 to 5), P = 0.54)). MARADONA 2019 reported on the components of VCSS individually, precluding meta‐analysis. They found no difference in VCSS between groups at baseline. Absolute VCSSs were similar in both arms at one and two years with a comparable improvement compared to baseline (P = 0.05). See Table 19.
Length of procedure
Only the MARADONA 2019 trial reported on this outcome, and showed that times were similar, with RFA taking an average of 13 minutes (range 4 to 85 minutes) and MOCA taking 12 minutes (range 5 to 45 minutes). See Table 20.
Duration of hospital stay
No study explicitly stated their rates of day case or inpatient procedures. See Table 21.
Return to normal activities
All studies reported on return to daily activities or work, with no difference found between participants within the RFA or MOCA arms. See Table 22.
Radiofrequency ablation (RFA) versus SFJ ligation and stripping (HL/S, surgery)
See Table 5.
Five studies compared RFA with SFJ ligation and stripping (EVOLVeS 2003; Helmy ElKaffas 2011; Rasmussen 2011; Rautio 2002; Subramonia 2010).
Technical success
Three studies comparing RFA with SFJ ligation and stripping reported on this outcome (EVOLVeS 2003; Rasmussen 2011; Rautio 2002). The EVOLVeS 2003 trial reported that in "many cases the GSV was completely obliterated by the intervention"; however, authors did not give actual figures to allow inclusion into the meta‐analysis. Combining the under five year data from Rasmussen 2011 and Rautio 2002 showed no clear difference in the technical success of the two procedures (OR 5.71, 95% CI 0.64 to 50.81; 2 studies, 318 participants; low‐certainty evidence; Analysis 10.1). We downgraded the certainty of the evidence from high to low due to risk of bias concerns and inconsistency, reflected in the wide CI.
10.1. Analysis.

Comparison 10: Radiofrequency ablation versus SFJ ligation and stripping (HL/S, surgery), Outcome 1: Technical success < 5 years
Rasmussen 2011 reported data for over five years and no evidence of a difference was demonstrated (OR 0.88, 95% CI 0.29 to 2.69; 1 study, 289 participants; low‐certainty evidence; Analysis 10.2).
10.2. Analysis.

Comparison 10: Radiofrequency ablation versus SFJ ligation and stripping (HL/S, surgery), Outcome 2: Technical success > 5 years
Recurrence
Four studies assessed recurrence at two and three years for RFA versus SFJ ligation and stripping (EVOLVeS 2003; Helmy ElKaffas 2011; Rasmussen 2011; Rautio 2002). No clear difference was detected between the groups (OR 0.93, 95% CI 0.58 to 1.51; 4 studies, 546 participants; moderate‐certainty evidence; Analysis 10.3). We downgraded the certainty of the evidence from high to moderate due to risk of bias concerns.
10.3. Analysis.

Comparison 10: Radiofrequency ablation versus SFJ ligation and stripping (HL/S, surgery), Outcome 3: Recurrence
Rasmussen 2011 also reported long‐term data, and a possible benefit to RFA treatment was seen (OR 0.41, 95% CI 0.22 to 0.75; 1 study, 289 participants; low‐certainty evidence; Analysis 10.4).
10.4. Analysis.

Comparison 10: Radiofrequency ablation versus SFJ ligation and stripping (HL/S, surgery), Outcome 4: Long‐term recurrence > 5 years
Post‐operative complications
All five studies reported complications. We were not able to undertake meta‐analysis for complications because the trials used different definitions and time points. While the number of complications was low in the studies, surgery was associated with higher rates of wound problems, haematomas and saphenous nerve injuries within both the early and late comparisons. More phlebitis was seen with RFA. EVOLVeS 2003 reported more paraesthesia in participants undergoing RFA at one week (23.3%) compared to SFJ ligation and stripping (13.9%). In the SFJ ligation and stripping group, two participants developed wound infections; one settled with antibiotics while the other required surgical debridement and admission for intravenous antibiotic therapy. Helmy ElKaffas 2011 found more cases of paraesthesia with RFA (10%) compared with SFJ ligation and stripping (3%), and more episodes of thrombophlebitis (six cases compared to none with SFJ ligation and stripping). There was one iliofemoral DVT with SFJ ligation and stripping and higher rates of haematoma (seen in 30 participants compared to one with RFA). Three participants developed groin infections requiring parenteral antibiotics. Rasmussen 2011 reported one case of popliteal vein thrombosis with SFJ ligation and stripping at one month. There were more cases of phlebitis following RFA (12) compared to SFJ ligation and stripping (five). Rautio 2002 reported more saphenous nerve injuries (23%) with SFJ ligation and stripping than RFA (13%), higher rates of haematomas were also seen (31% compared to 7% with RFA). Among the RFA group, 20% developed clinical thrombophlebitis and 7% had thermal skin injuries; no cases of either these complications were seen with SFJ ligation and stripping. Subramonia 2010 reported numbness in 49% of participants undergoing SFJ ligation and stripping at one week compared to 19% of those undergoing RFA. Groin wound problems were present in 17% of SFJ ligation and stripping participants while 11% of RFA participants had hyperpigmentation at initial follow‐up. We downgraded the certainty of the evidence to very low due to risk of bias concerns, inconsistency, imprecision and possible publication bias. See Table 16 and Table 17.
Quality of life
Four studies evaluated QoL scores (EVOLVeS Study; Rasmussen 2011; Rautio 2002; Subramonia 2010). Rautio 2002 demonstrated improved QoL scores within all subgroups of RAND‐36 (a validation version of the SF‐36 for Finland), and reported that physical functioning was restored faster in the RFA group. Median difference from baseline for physical functioning/role functioning was 0 in RFA and five with SFJ ligation and stripping at four weeks. Subramonia 2010 showed significant improvement in AVVSS QoL scores following treatment, with no difference between the groups (mean improvement in QoL score was ‐9.12 in RFA compared to ‐8.24 with SFJ ligation and stripping). Using the Venous Insufficiency Epidemiological and Economics Study (VEINES)‐QoL/Sym questionnaire (V‐Q/SymQ) at five weeks, improvement was reported with RFA compared with SFJ ligation and stripping (mean improvement 12.62 versus 9.94; 95% CI ‐1.65 to 7.01; P = 0.22). The EVOLVeS Study reported significant improvement via the CIVIQ2 QoL tool (global score and bodily pain) in participants undergoing RFA at 72 hours and one week, with the mean difference in global score ‐3 and ‐9.2 in RFA compared with 13.3 and 3.7 with SFJ ligation and stripping. However, the magnitude of the difference was negligible by four months. The EVOLVeS Study adjusted their figures for the number of adjunctive procedures undertaken. Rasmussen 2011 found improved AVVQ from day three onwards, with no difference between groups at any time point (mean (SD) AVVSS at baseline was 18.74 (8.63) for RFA and 19.3 (8.46) for SFJ ligation and stripping, reduced to 4.43 (6.58) and 4.0 (4.87), respectively, at three years). Their SF‐36 results demonstrated comparable short‐ and medium‐term benefits overall. However, participants who underwent SFJ ligation and stripping had poorer bodily pain and physical function domains compared to participants in the RFA group in the three‐day follow‐up. This difference was not seen at one month. We downgraded the certainty of the evidence from high to moderate due to risk of bias concerns.
Pain
Four studies comparing RFA with SFJ ligation and stripping reported less post‐operative pain and analgesic consumption within the RFA arm (EVOLVeS 2003; Rasmussen 2011; Rautio 2002; Subramonia 2010). The EVOLVeS 2003 study reported statistically significant differences in the pain scores recorded at 72 hours and one week post‐intervention (P < 0.001, for both time points). Rautio 2002 found less ibuprofen consumption in RFA participants compared to surgical participants (average daily number of 600 mg ibuprofen tablets (SD) 0.4 (0.49) versus 1.3 (1.09); P = 0.004). Mean pain scores at rest, standing and walking in RFA participants were reported as lower than surgical participants. This was especially so between the fifth to fourteenth post‐operative day. The average VAS (SD) score at rest was 0.7 (0.5) for RFA and 1.7 (1.3) for SFJ ligation and stripping; on standing, 1.3 (0.7) versus 2.6 (1.9), respectively; and on walking, 1.8 (0.8) versus 3 (1.8), respectively (Rautio 2002). In Rasmussen 2011, the mean pain (SD) score for the first ten days was 1.21 (1.72) in RFA and 2.25 (2.23) in surgery. The number of phlebectomies did not affect pain scores. In Subramonia 2010, the median pain score during the first week post‐intervention was higher in surgical participants (P = 0.001), whilst the duration of analgesic consumption was lower for RFA participants (P = 0.001).
Venous Clinical Severity Score (VCSS)
Three studies reported on change in VCSS and demonstrated comparable rates of improvement between RFA and surgery (EVOLVeS 2003; Rasmussen 2011; Rautio 2002). We were not able to undertake meta‐analysis for VCSS because the trials used different time points. EVOLVeS 2003 found improved changes in VCSS for RFA over SFJ ligation and stripping at 72 hours (P > 0.05) and one week (P > 0.5). This difference disappeared at subsequent follow‐ups. In Rautio 2002, the average decrease (SD) in VCSS at three years was 4.3 (2.3) in RFA and 4 (1.2) after surgery (P = 0.7). Rasmussen 2011 reported that VCSS improved in all groups with no difference between groups at any time point. See Table 19.
Length of procedure
Five studies reported on the length of procedure but we were not able to undertake meta‐analysis for length of procedure because the studies defined the procedure differently (EVOLVeS 2003; Helmy ElKaffas 2011; Rasmussen 2011; Rautio 2002; Subramonia 2010). EVOLVeS 2003 reported mean treatment time (SD) as 74 minutes (10) and 89 minutes (12) for RFA and SFJ ligation and stripping, respectively. In Helmy ElKaffas 2011, the mean (SD) procedure time was 40 (10) minutes for RFA and 45 (13) minutes for SFJ ligation and stripping. Rasmussen 2011 recorded the mean surgeon's time (range) as 27 (12 to 80) minutes for RFA and 32 (15 to 80) minutes for SFJ ligation and stripping. In Subramonia 2010, median theatre time (IQR) was 82 (73 to 91) minutes for RFA and 55 (48 to 63) minutes for SFJ ligation and stripping; procedural time was 76 (67 to 84) minutes for RFA and 48 (39 to 54) minutes for SFJ ligation and stripping. Mean operating time (SD) in Rautio 2002 was 75 (16.6) minutes in RFA and 57 (11) minutes for SFJ ligation and stripping. See Table 20.
Duration of hospital stay
Three studies reported on duration of hospital stay (EVOLVeS 2003; Helmy ElKaffas 2011; Rautio 2002). EVOLVeS 2003 reported that 95% of their RFA procedures were day case compared to 86% with SFJ ligation and stripping. In Rautio 2002, one participant in each treatment group stayed overnight for social reasons; 93.3% of RFA procedures were undertaken as day case and 92.3% with SFJ ligation and stripping. Helmy ElKaffas 2011 reported that RFA participants stayed in hospital for 14 hours (SD 3.6 hours (range 12 to 18 hours)), as compared to 30 hours (SD 11.5 hours (range 18 to 48 hours)) with SFJ ligation and stripping. See Table 21.
Return to normal activities
Five studies reported on return to normal activities, but we were not able to undertake meta‐analysis due to differing measurements used (EVOLVeS 2003; Helmy ElKaffas 2011; Rasmussen 2011; Rautio 2002; Subramonia 2010). In EVOLVeS 2003, mean return to normal activities was adjusted for the type of anaesthetic and number of adjunctive procedures. The study reported that participants who were given general anaesthesia took longer to return to work. Mean return to normal activities was 1.15 days with RFA and 3.89 days with SFJ ligation and stripping; return to work was 4.74 and 12.4 days, respectively. In Helmy ElKaffas 2011, time to return to normal physical activity was three (SD 3) days for RFA and seven (SD 2.6) days for SFJ ligation and stripping. Median time to resume work in Rasmussen 2011 was 2.9 days (range 0 to 14 days) for RFA compared to 4.3 days (range 0 to 42 days) in SFJ ligation and stripping; return to normal activities was one day (range 0 to 30 days) in RFA and four days (0 to 30 days) with SFJ ligation and stripping. Rautio 2002 found mean sick leave was shorter with RFA, with a mean of 6.5 (SD 3.3) days taken compared with 15.6 (SD 6) days with SFJ ligation and stripping. In Subramonia 2010, mean return to work and normal activities was 10 days (IQR 4 to 13 days) and three days (IQR 0 to 7) with RFA compared to 18.5 days (IQR 11 to 28) and 12.5 days with SFJ ligation and stripping of GSV respectively. See Table 22.
Ultrasound‐guided foam sclerotherapy (UGFS) versus SFJ ligation and stripping (HL/S, surgery)
See Table 6.
Four studies compared ultrasound‐guided foam sclerotherapy to SFJ ligation and stripping (FOAM 2010; Magna 2013; Rasmussen 2011; Vernermo 2016).
Technical success
All four studies assessed technical success between UGFS and SFJ ligation and stripping (FOAM 2010 (two years); Magna 2013 (one year and five years); Rasmussen 2011 (one month and five years); and Vernermo 2016 (one year and five years). Pooling the early data shows a possible benefit for SFJ ligation and stripping compared to UGFS (OR 0.32, 95% CI 0.11 to 0.94; 4 studies, 954 participants; low‐certainty evidence; Analysis 11.1). This indicates UGFS may be inferior to surgery. Heterogeneity was detected so a random‐effects model was used (I2 = 78%). We downgraded the certainty of the evidence from high to low due to risk of bias concerns and inconsistency.
11.1. Analysis.

Comparison 11: Ultrasound‐guided foam sclerotherapy versus SFJ ligation and stripping (HL/S, surgery), Outcome 1: Technical success < 5 years
Three studies reported data for over five years (Magna 2013; Rasmussen 2011; Vernermo 2016). In the more than five‐year follow‐up, the probability of technical success was lower in the UGFS than the SFJ ligation and stripping group (OR 0.09, 95% CI 0.03 to 0.30; 3 studies, 525 participants; moderate‐certainty evidence; Analysis 11.2). Heterogeneity was detected so a random‐effects model was used (I2 = 73%). We downgraded the certainty of the evidence due to risk of bias concerns.
11.2. Analysis.

Comparison 11: Ultrasound‐guided foam sclerotherapy versus SFJ ligation and stripping (HL/S, surgery), Outcome 2: Technical success > 5 years
Recurrence
Three trials compared recurrence in UGFS and SFJ ligation and stripping between one and three years (FOAM 2010; Magna 2013; Rasmussen 2011). Pooling the data did not show a clear difference (OR 1.81, 95% CI 0.87 to 3.77; 3 studies, 822 participants; low‐certainty evidence; Analysis 11.3).
11.3. Analysis.

Comparison 11: Ultrasound‐guided foam sclerotherapy versus SFJ ligation and stripping (HL/S, surgery), Outcome 3: Recurrence
Five‐year data were also available from these studies and, again, no clear difference was detected (OR 1.24, 95% CI 0.57 to 2.71; 3 studies, 639 participants; low‐certainty evidence; Analysis 11.4).
11.4. Analysis.

Comparison 11: Ultrasound‐guided foam sclerotherapy versus SFJ ligation and stripping (HL/S, surgery), Outcome 4: Long‐term recurrence (≥ 5 years)
Heterogeneity was detected so a random‐effects model was used (I2 = 72% and I2 = 76%, respectively). We downgraded the certainty of the evidence from high to low due to risk of bias concerns and inconsistency.
Post‐operative complications
All four studies reported on complication rates (FOAM 2010; Magna 2013; Rasmussen 2011; Vernermo 2016). FOAM 2010 reported a higher rate of phlebitis with UGFS (17 participants out of 230) compared to none with SFJ ligation and stripping. Six of 200 participants who underwent SFJ ligation and stripping developed paraesthesia compared to none with UGFS. There was one DVT and one PE in the UGFS group one week post‐procedure. At two years, hyperpigmentation was seen in 12 of the 213 UGFS participants and in two of 200 in the SFJ ligation and stripping group. In Magna 2013, the frequency of reported complications was low, with one reported case of paraesthesia following UGFS and four with SFJ ligation and stripping at three months; and one reported case in each group at one year. Magna 2013 reported three cases of wound infection in the SFJ ligation and stripping arm and none in the UGFS arm. Rasmussen 2011 reported one DVT in each group at one month. Rates of phlebitis were higher with UGFS (17 cases compared to 12 in SFJ ligation and stripping), whilst more participants who underwent SFJ ligation and stripping had paraesthesia at one month (six participants versus two with UGFS). Vernermo 2016 reported at one month that skin pigmentation was more common after UGFS (67%) compared to SFJ ligation and stripping (5%); rates of paraesthesia were comparable (2% vs 3%); and 91% of participants who underwent UGFS had palpable lumps compared to 54% with SFJ ligation and stripping. We downgraded the certainty of the evidence from high to very low due to risk of bias concerns, inconsistency and possible publication bias. See Table 16 and Table 17.
Quality of life
None of the four included studies showed any difference in QoL scores between the two treatment groups. The FOAM 2010 study found no difference in improvement between EQ‐5D scores at two years. The change from baseline to two years was 0.064 and 0.061 in UGFS and SFJ ligation and stripping, respectively (P = 0.89). Magna 2013 excluded participants who had undergone bilateral interventions from their analysis, and reported that CIVIQ and EQ‐5D improved in all groups with no difference seen at two years. Rasmussen 2011 reported no significant difference between groups in the improvement of the SF‐36 score at one month. Vernermo 2016 reported no difference between treatment groups in AVVSS at one year, and similarly at five years, the mean AVVSS was 11.2 (95% CI 8.5 to 14) in the UGFS group and 8.7 (95% CI 6.7 to 10.7) in the SFJ ligation and stripping (P = 0.64). We downgraded the certainty of the evidence from high to moderate due to risk of bias concerns.
Pain
Three studies evaluated pain between UGFS and SFJ ligation and stripping treatment groups (FOAM 2010; Rasmussen 2011; Vernermo 2016). Rasmussen 2011 reported that participants who underwent UGFS had less post‐operative pain than those who had surgery: mean (SD) score during the first ten days was 1.6 (2.04) in UGFS, and 2.25 (2.23) for surgery (P < 0.001). The number of phlebectomies was not found to alter pain scores. Vernermo 2016 also showed participants had a lower VAS pain score after UGFS, both at discharge and one week post‐procedure. FOAM 2010 found that the intervention did not greatly influence pain, with similar scores for 'more', 'stable' or 'less' pain at 3, 12 and 24 months, for both surgery and UGFS.
Venous Clinical Severity Score (VCSS)
Two studies reported on change in VCSS (FOAM 2010; Rasmussen 2011). In FOAM 2010, no difference was detected at different time points. At baseline, the mean (SD) VCSS was 3.2 (1.9) in UGFS and 3.5 (2.2) in SFJ ligation and stripping. This score had improved in both groups to 1.7 (1.2) and 1.9 (1.4), respectively, at two years. By eight years, VCSS had deteriorated to 5.4 (3.3) and 4.6 (2.9) in each group, showing regression to worse scores when compared to baseline. Rasmussen 2011 reported that the VCSS score improved in both groups, with no difference between groups at any time point over three years. See Table 19.
Length of procedure
Rasmussen 2011 was the sole study to evaluate length of procedure as surgeon's time. Mean surgeon's time (range) in the UGFS group was 19 (5 to 145) minutes compared to 32 (15 to 80) minutes in SFJ ligation and stripping. See Table 20.
Duration of hospital stay
Only FOAM 2010 reported duration of hospital stay, with 100% of cases undertaken as day cases. See Table 21.
Return to normal activities
Two trials reported on return to normal activities, with participants undergoing UGFS possibly returning to normal activities faster. Rasmussen 2011 reported a median (range) return to work of one (0 to 21) day with UGFS, and 12 (0 to 33) days with SFJ ligation and stripping. Vernermo 2016 reported median (range) sick leave of 2.9 (0 to 33) days with UGFS and 4.3 (0 to 42) days with SFJ ligation and stripping. See Table 22.
Reporting bias and subgroup analysis
As none of the analyses included more than the ten studies required to create meaningful funnel plots, we could not evaluate reporting bias. None of the studies presented outcome data by the predefined variables of interest, so we did not perform subgroup analysis.
Sensitivity analysis
We planned to carry out sensitivity analyses by excluding studies that had a high risk of bias in four or more bias domains. Only one study, Calik 2019, had four or more bias domains at high risk. As this study was the only study in the comparison 'EVLA versus cyanoacrylate glue', we were unable to carry out this analysis.
Discussion
Summary of main results
This Cochrane Review included 24 studies with a total of 5135 randomised participants. Some studies involved multiple comparisons of interventions (Magna 2013; Rasmussen 2011; Vähäaho 2019; Vernermo 2016), or a comparison group not included in our analysis (Flessenkämper 2013). The duration of follow‐up ranged from five weeks (Subramonia 2010), to eight years (FOAM 2010). We did not find studies to provide results for all possible comparisons, especially newer treatments (see Types of interventions). Single studies provided evidence for five comparisons. When more than one study reported on a particular comparison, we were only able to pool the outcomes of technical success and recurrence due to heterogeneity in how the studies defined outcomes and reported time points. All studies had some risk of bias concerns. This has limited our ability to draw firm conclusions. Below, we report on the clinically most relevant comparisons. Details for all comparison and outcomes can be found in the Effects of interventions section.
EVLA versus RFA
See Table 1.
Five studies reported on technical success (Nordon 2011; Rasmussen 2011; Recovery 2009; Shepherd 2010; Syndor 2017). Their data demonstrated that the rate of technical success was comparable between RFA and EVLA to five years (OR 0.98, 95% CI 0.41 to 2.38; 5 studies, 780 participants; moderate‐certainty evidence; Analysis 1.1).
Only Rasmussen 2011 provided data for long‐term technical success, and no evidence of a difference in success rates was seen (OR 0.85, 95% CI 0.30 to 2.41; 291 participants; low‐certainty evidence; Analysis 1.2).
Only Rasmussen 2011 reported on recurrence and there was no clear difference between the groups at three years (OR 1.53, 95% CI 0.78 to 2.99; 291 participants; low‐certainty evidence; Analysis 1.3). Five‐year recurrence rates were also reported and favoured RFA (OR 2.77, 95% CI 1.52 to 5.06; 291 participants; low‐certainty evidence; Analysis 1.4).
Complication rates were recorded by all five studies using different definitions and time points, which prevented meta‐analysis. Results of individual studies were inconsistent with each other, so we are not able to draw any conclusions (very low‐certainty evidence).
The included trials used different QoL questionnaires at different time points, so we decided it was inappropriate to combine these for meta‐analysis. Improvement in QoL scores over follow‐up were similar between the two procedures in Nordon 2011, Rasmussen 2007 and Shepherd 2010. Recovery 2009 reported improved global QoL scores in the RFA group at 7 and 14 days post‐operation compared to EVLA, but by one month they were comparable. Syndor 2017 did not evaluate QoL measures in their study. We assessed the certainty of the evidence for this outcome as low.
All studies reported reduced pain in the RFA groups compared to EVLA. Nordon 2011 showed RFA participants took less analgesia during the week post‐procedure and post‐procedural pain scores were less following RFA at days one, three and seven. In Rasmussen 2011, mean pain scores on VAS at ten days were less in the RFA group compared to EVLA. Recovery 2009 reported significantly lower pain levels on VAS in participants who had RFA, at 48 hours, one week and two weeks. Shepherd 2010 reported lower mean (SD) VAS in RFA over the first ten days compared to EVLA, and a lower consumption of analgesic tablets over three days. In Syndor 2017, the median post‐procedural pain scores on a scale of one to ten were worse in the EVLA group compared to RFA, on initial evaluation.
Four trials reported on change in VCSS, showing comparable rates between both groups at final follow‐up (Rasmussen 2011; Recovery 2009; Shepherd 2010; Syndor 2017). Rasmussen 2011 reported that the VCSS improved significantly in all groups (P < 0.001), with no difference between groups at any evaluated time point through three years. Recovery 2009 reported no difference between treatment groups at baseline, and reduced VCSS scores in the RFA group compared with EVLA at 48 hours, one week and two weeks, but no difference was detected by one month. In Shepherd 2010, VCSS was comparable between the two groups at six months. Syndor 2017 found participants in both groups demonstrated a reduction in VCSS at six months from baseline. See Table 19 .
The duration of the procedure was similar in the three reporting studies. However, the reporting trials used different time points, metrics and terminology, impeding analysis (Nordon 2011; Rasmussen 2011; Syndor 2017). See Table 20 .
Shepherd 2010 was the sole trial to explicitly state that all procedures were intended to be day cases. However, 3.1% of participants required inpatient admission. See Table 21.
Three trials reported on return to work and normal activities (Nordon 2011; Rasmussen 2011; Shepherd 2010). Results were comparable between treatment groups but studies evaluated this outcome by different means, making it difficult to draw conclusions. See Table 22.
It is worth noting that we compared studies on a statistical front only. There are a number of radiofrequency devices historically available, and the same is true for laser devices. We did not sub‐define these modalities.
EVLA versus EVSA
Only one study compared EVLA and EVSA (LAST 2014 ). At one year, rates of technical success were comparable between high dose EVSA and EVLA (OR 1.94, 95% CI 0.53 to 7.15; 166 participants; Analysis 2.1). No long‐term data were available.
Complication profiles were similar between both treatment groups, as were reports of QoL. For QoL, LAST 2014 evaluated AVVQ, EQ‐5D and EQ VAS at baseline and after 12 weeks; improvement in scores were found to be comparable between EVLA and EVSA groups.
Participants who underwent EVSA reported less post‐procedural pain and had a shorter duration of analgesic consumption than participants who had EVLA. Convalescence was measured as the number of days lost from work or normal activities, with participants in the EVSA group returning to normal activity faster than those in the EVLA group. Rates of recurrence, length of procedure and duration of hospital stay were not reported.
EVLA versus UGFS
See Table 2.
Three studies compared EVLA with UGFS (Magna 2013; Rasmussen 2011; Vernermo 2016). Technical success may be improved in participants undergoing EVLA, both up to five years (OR 6.13, 95% CI 0.98 to 38.27; 3 studies, 588 participants; low‐certainty evidence; Analysis 3.1), and over five years' follow‐up (OR 6.47, 95% CI 2.60 to 16.10; 3 studies, 534 participants; low‐certainty evidence; Analysis 3.2).
Two studies evaluated recurrence (Magna 2013; Rasmussen 2011), and showed no clear difference between the groups (OR 0.68, 95% CI 0.20 to 2.36; 2 studies, 443 participants; very low‐certainty evidence; Analysis 3.3). Five‐year recurrence rates were also available for both studies, and again no clear differences were seen (OR 1.08, 95% CI 0.40 to 2.87; 2 studies, 418 participants; very low‐certainty evidence).
All three studies reported on post‐operative complications. However, meta‐analysis was impeded because the studies used different definitions of complications and assessed complications at varying time points. Rasmussen 2011 reported more phlebitis and hyperpigmentation rates amongst the UGFS group compared to the EVLA group. In Vernermo 2016, skin pigmentation was more common in the UGFS arm compared to EVLA, but haematomas were seen more often after EVLA compared to UGFS at one month. Magna 2013 reported two cases of hyperpigmentation in EVLA participants compared to one case in UGFS at three months (very low‐certainty evidence).
Each of the three studies evaluated QoL using different questionnaires at different time frames. No differences were detected beyond one month by any measurement (Magna 2013; Rasmussen 2011; Vernermo 2016). We assessed the certainty of the evidence for this outcome as moderate.
Two studies evaluated pain scores, with both reporting lower post‐procedural pain with UGFS compared to EVLA treatment (Rasmussen 2011; Vernermo 2016).
Only Rasmussen 2011 analysed VCSS, finding no difference between treatment arms at any evaluated time point.
Again, Rasmussen 2011 was the sole study which evaluated length of procedure as surgeon's time. Mean surgeon's time was 26 minutes in EVLA (range 12 to 80 minutes) and 19 minutes in UGFS (range 5 to 145 minutes).
Participants undergoing UGFS returned to work faster in the two studies which reported this outcome. Rasmussen 2011 reported the median time to return to work (range) as 3.6 days (0 to 46 days) in the EVLA group and 2.9 days (0 to 42 days) in the UGFS group. The mean duration of sick leave in Vernermo 2016 was eight days in EVLA (range 0 to 29 days) and one day in UGFS (range 0 to 21 days). No studies reported on duration of hospital stay.
EVLA versus cyanoacrylate glue
Calik 2019 was the sole trial to evaluate EVLA against cyanoacrylate glue. The trial analysed occlusion rates at one, three, six and twelve months. There was no evidence of a difference in occlusion rates at one month (OR 0.33, 95% CI 0.01 to 8.03; 412 participants; Analysis 4.1). Similarly, results showed no evidence of difference in recanalisation rates at one year (OR 2.59, 95% CI 0.50 to 13.49; 412 participants; Analysis 4.2).
Higher rates of post‐procedural induration, bruising and paraesthesia were seen following EVLA at one week compared to cyanoacrylate glue, but there was no difference by the three‐month time point, except for paraesthesia which was more common after EVLA. Two DVTs were found within the EVLA group. Both groups demonstrated improved QoL at follow‐up, but there was no clear difference between the groups.
Calik 2019 evaluated periprocedural pain levels using the Wong‐Baker FACES pain score. Pain scores were lower in the cyanoacrylate glue group at one week, but by three months they were comparable. There were improvements in VCSS in both groups post‐operatively, although there was no evidence of a difference between groups.
The operative time was longer for EVLA than for cyanoacrylate glue, and there was a faster return to daily activities in the cyanoacrylate glue group. Calik 2019 did not evaluate duration of hospital stay.
EVLA versus MOCA
Vähäaho 2019 was the only trial which compared EVLA to MOCA. At one month, they found 100% occlusion rates of the GSV via DUS amongst both treatment groups. There were no long‐term data available.
At one year, 100% of the participants who underwent EVLA treatment still had GSV occlusion, while ten participants in the MOCA treatment group showed recanalisation of the GSV (OR 0.06, 95% CI 0.00 to 1.14; 88 participants; Analysis 5.2).
Three participants in the EVLA group reported sensory disturbance at one year; no nerve injuries were seen in the MOCA group. There was one superficial infection seen in the MOCA treatment group. There was no evidence of a difference between the treatment groups in QoL at one year. The VAS pain score prior to discharge and at one week post‐procedure was similar between treatment modalities, and there was no difference between the amount of painkillers required. Participants undergoing EVLA took a mean of 5.3 days sick leave compared to 4.3 days in those undergoing MOCA. Vähäaho 2019 did not report change in VCSS, duration of procedure and duration of hospital stay.
EVLA versus SFJ ligation and stripping (HL/S, surgery)
See Table 3.
Nine trials compared EVLA with SFJ ligation and stripping. There was a possible benefit to technical success at less than five years in the EVLA group (OR 2.31, 95% CI 1.27 to 4.23; 6 studies, 1051 participants; low‐certainty evidence; Analysis 6.1). No clear difference in results were seen at five years and beyond (OR 0.93, 95% CI 0.57 to 1.50; 5 studies, 874 participants; low‐certainty evidence; Analysis 6.2). We downgraded the certainty of the evidence from high to low due to risk of bias concerns and imprecision. See Table 3.
Seven studies analysed recurrence, showing it to be comparable between groups within three years post‐intervention (OR 0.78, 95% CI 0.47 to 1.29; 7 studies, 1459 participants; moderate‐certainty evidence; Analysis 6.3). Similar results were seen with five year data (OR 1.09, 95% CI 0.68 to 1.76; 7 studies, 1267 participants; moderate‐certainty evidence; Analysis 6.4).
All studies reported on complications. However, the reporting studies used different definitions and evaluation time points, impeding accurate comparison of post‐operative complications. Slightly higher rates of early haematomas and wound problems may be seen with SFJ ligation and stripping. EVLA may be associated with slightly higher rates of phlebitis. We assessed the certainty of the evidence for this outcome as very low.
All studies evaluated QoL scores using a variety of different questionnaires at variable time points, impeding accurate comparison. Rates of improvement were comparable between interventions in all studies (moderate‐certainty evidence).
The studies analysed pain in a wide variety of ways, precluding accurate meta‐analysis. The majority of studies reported comparable post‐operative pain scores between interventions (Darwood 2008; Flessenkämper 2013; Rasmussen 2007; Rasmussen 2011; RELACS 2012 ). HELP‐1 2011 reported higher pain scores and analgesic consumption with SFJ ligation and stripping. Pronk 2010 reported higher mean post‐operative pain scores with EVLA.
Four trials reported change in VCSS, with comparable improvements in scores between interventions (Darwood 2008; HELP‐1 2011; Rasmussen 2007; Rasmussen 2011).
Three studies measured length of procedure (non‐comparably), using various different definitions and metrics, with no clear difference seen in the times taken (HELP‐1 2011; Rasmussen 2011; Vernermo 2016).
Four trials conducted all their procedures as day case (Darwood 2008; Flessenkämper 2013; Pronk 2010; Rasmussen 2007). One study reported that 21% of participants required admission following SFJ ligation and stripping (HELP‐1 2011).
Seven studies reported on time to return to work or normal activity. EVLA was associated with a quicker return to work and normal activity in three of the trials (Darwood 2008; HELP‐1 2011; Vernermo 2016). Four studies reported comparable rates of return to work and normal activity (Pronk 2010; Rasmussen 2007; Rasmussen 2011; RELACS 2012). The disparity in methodology, definitions and metrics within the studies should be borne in mind before drawing conclusions.
RFA versus UGFS
Rasmussen 2011 was the sole study comparing these interventions. Technical success rates did not clearly favour one treatment compared to another, at up to 5 years (OR 5.21, 95% CI 0.25 to 109.48; 1 study, 292 participants; Analysis 7.1). There may be a benefit for RFA at five years (OR 3.23, 95% CI 1.32 to 7.89; 1 study, 291 participants; Analysis 7.2). Three‐ and five‐year recurrence rates showed no clear difference between the treatment groups (OR 0.81, 95% CI 0.41 to 1.62; 1 study, 291 participants; Analysis 7.3; and OR 0.61, 95% CI 0.33 to 1.16; 1 study, 291 participants; Analysis 7.4, respectively).
One participant developed an iliac vein thrombosis and subsequent pulmonary embolus one week post‐UGFS. Equal levels of hyperpigmentation were seen between groups. More episodes of phlebitis were recorded in the UGFS group than in RFA (12 versus 17). Rasmussen 2011 evaluated quality of life using the SF‐36. There was no evidence of a difference in the mean (SD) pain score (VAS) between the RFA and UGFS groups during the first ten days post‐procedure. It was noted that the number of concomitant phlebectomies did not alter the pain scores. The VCSS score improved in both groups, with no difference between groups at any time point over three years. No clear differences were detected in pain between groups. The VCSS reduced from baseline in both groups, with no clear difference between groups by three years. Rasmussen 2011 recorded the length of procedure as 'surgeon's time' within the trial, and there was no clear difference in procedure time between treatment groups. The return to normal activities and return to work time was comparable between groups. Rasmussen 2011 did not report upon duration of hospital stay.
RFA versus cyanoacrylate glue
Morrison 2015 was the only included trial comparing these interventions. There were increased occlusions in the cyanoacrylate glue group compared to the RFA group after one month (OR 0.03, 95% CI 0.00 to 0.54; 1 study, 215 participants; Analysis 8.1). The study reported a final time point of 36 months, showing comparable occlusions in cyanoacrylate glue (94.4%, 68/72) compared to RFA (91.9%, 68/74) (P = 0.75).
The two‐year follow‐up identified 12 recanalisations in the cyanoacrylate glue group and only one in the RFA group. Within the first three months, there were similar reports of paraesthesia and phlebitis between RFA and cyanoacrylate glue groups. QoL scores were seen to improve throughout the trial duration, and by three years, there was no clear difference between RFA and cyanoacrylate glue in both AVVQ (P = 0.45) and EQ‐5D (P = 0.4). There was no difference in the pain experienced between the two treatment arms, or in VCSS between treatment groups. Mean procedural time was five minutes longer for cyanoacrylate glue (24 minutes) than for RFA (19 minutes) (P < 0.01). All interventions were undertaken as day case procedures. Morrison 2015 did not evaluate post‐operative return to activity.
RFA versus MOCA
See Table 4.
Three trials compared RFA to MOCA (Lane 2017; MARADONA 2019; Vähäaho 2019). All three trials reported on technical success. Pooling the data showed no clear evidence of a benefit for RFA over MOCA (OR 1.76, 95% CI 0.06 to 54.15; 3 studies, 435 participants; low‐certainty evidence; Analysis 9.1). No long‐term data were available.
The evaluation of recurrence rates amongst the trials did not show a clear benefit for one intervention over the other (OR 1.00, 95% CI 0.21 to 4.81; 3 studies, 389 participants; low‐certainty evidence; Analysis 9.2). No long‐term data were available.
All three studies reported on complication rates, which were similar between treatment arms (very low‐certainty evidence). All three studies reported on QoL and found no significant difference between treatment arms (moderate‐certainty evidence).
All three studies evaluated rates of post‐procedural pain, but the differing time points and assessment modalities prevented formal meta‐analysis. Lane 2017 reported on maximum pain experienced (measured by VAS) and reported that it was significantly less in the MOCA group. MARADONA 2019 and Vähäaho 2019 showed similar rates of analgesic consumption post‐operatively. The MARADONA 2019 study demonstrated lower median pain scores for MOCA during the first two post‐operative weeks, while Vähäaho 2019 reported similar scores between the groups, using VAS, in the first post‐operative week. Lane 2017 evaluated VCSS at one and six months, and MARADONA 2019 at one and two years. Both trials showed comparable improvement in VCSS between modalities. Only MARADONA 2019 reported on the duration of procedures, which showed they were similar. No study explicitly stated their rates of day case or inpatient procedures. All studies reported on return to daily activities or work, with no difference found between participants within the RFA or MOCA arms.
RFA versus SFJ ligation and stripping (HL/S, surgery)
See Table 5.
Five studies compared RFA with surgery (EVOLVeS 2003; Helmy ElKaffas 2011; Rasmussen 2011; Rautio 2002; Subramonia 2010).
Two studies reported data for technical success up to five years (Rasmussen 2011; Rautio 2002). No clear difference in technical success was detected between groups (OR 5.71, 95% CI 0.64 to 50.81; 2 studies, 318 participants; low‐certainty evidence; Analysis 10.1). Rasmussen 2011 reported data for over five years, and no evidence of a difference was demonstrated (OR 0.88, 95% CI 0.29 to 2.69; 1 study, 289 participants; low‐certainty evidence; Analysis 10.2).
Four studies compared recurrence rates between RFA and surgery (EVOLVeS 2003; Helmy ElKaffas 2011; Rasmussen 2011; Rautio 2002). No clear difference was detected between the groups at two and three years (OR 0.93, 95% CI 0.58 to 1.51; 4 studies, 546 participants; moderate‐certainty evidence; Analysis 10.3). Long‐term data were also reported by Rasmussen 2011, and a possible benefit to RFA treatment was seen (OR 0.41, 95% CI 0.22 to 0.75; 1 study, 289 participants; low‐certainty evidence; Analysis 10.4).
All five studies reported complications, but meta‐analysis was impeded because the studies used different definitions and evaluated complications at different time points. While the number of complications was low for all studies, surgery may be associated with slightly higher rates of wound problems, haematomas and saphenous nerve injuries within both the early and late comparisons. More phlebitis was seen after RFA (very low‐certainty evidence). See Table 16 and Table 17.
Four studies evaluated QoL scores. The EVOLVeS Study reported improvement via the CIVIQ2 QoL tool (global score and bodily pain) in RFA over SFJ ligation and stripping at 72 hours and one week, but this difference was negligible by four months. Rautio 2002 demonstrated improved QoL scores within all subgroups of RAND‐36 (a validation version of the SF‐36 for Finland), and reported that physical functioning was restored faster in the RFA group. Subramonia 2010 did not demonstrate a clear difference in groups using V‐Q/SymQ or AVVSS. Rasmussen 2011 found no difference in improvement between groups using the AVVQ and SF‐36 by one month, but reported poorer bodily pain and physical function domains with SFJ ligation and stripping initially. Overall, we assessed QoL evidence as moderate‐certainty.
Four studies reported on post‐operative pain, with higher pain scores and analgesic consumption with SFJ ligation and stripping compared to RFA (EVOLVeS 2003; Rasmussen 2011; Rautio 2002; Subramonia 2010).
Three studies reported on change in VCSS, and demonstrated comparable rates of improvement between RFA and surgery (EVOLVeS 2003; Rasmussen 2011; Rautio 2002).
Three studies indicated that RFA may be faster to perform, while two studies found surgery was faster. The discrepancy between the five trials reporting length of procedure may be due to the discrepancy in the definitions used (EVOLVeS 2003; Helmy ElKaffas 2011; Rasmussen 2011; Rautio 2002; Subramonia 2010). We cannot draw any conclusions.
Three studies reported on duration of hospital stay, and all five reported more hospital admissions for SFJ ligation and stripping (EVOLVeS 2003; Helmy ElKaffas 2011; Rautio 2002).
All five studies indicated that time to return to work and normal activities was shorter amongst participants who underwent RFA (EVOLVeS 2003; Helmy ElKaffas 2011; Rasmussen 2011; Rautio 2002; Subramonia 2010). However, this conclusion is tentative due to the lack of standardisation in the measurement of this outcome by reporting studies.
UGFS versus SFJ ligation and stripping (HL/S, surgery)
See Table 6.
Four studies compared ultrasound‐guided foam sclerotherapy to SFJ ligation and stripping (FOAM 2010; Magna 2013; Rasmussen 2011; Vernermo 2016).
Pooling the early data shows a possible benefit for SFJ ligation and stripping compared to UGFS in technical success (OR 0.32, 95% CI 0.11 to 0.94; 4 studies, 954 participants; low‐certainty evidence; Analysis 11.1). Similarly, in the more than five years follow‐up, the probability of technical success is lower in the UGFS than the surgery group (OR 0.09, 95% CI 0.03 to 0.30; 3 studies, 525 participants; moderate‐certainty evidence; Analysis 11.2).
Three trials compared recurrence in UGFS and SFJ ligation and stripping (FOAM 2010; Magna 2013; Rasmussen 2011). No clear difference was detected between the procedures at one and three years (OR 1.81, 95% CI 0.87 to 3.77; 3 studies, 822 participants; low‐certainty evidence; Analysis 11.3), or after five years (OR 1.24, 95% CI 0.57 to 2.71; 3 studies, 639 participants; low‐certainty evidence; Analysis 11.4).
All four studies reported on complication rates. We were not able to pool the data and cannot draw any conclusions because the trials reported different complications and time points (very low‐certainty evidence).
The studies used a variety of QoL scores and time points to evaluate the interventions, preventing meta‐analysis. No difference in QoL was detected between treatment groups in any of the studies during follow‐up (moderate‐certainty evidence).
Of the three studies evaluating pain between UGFS and SFJ ligation and stripping, two studies reported lower post‐operative pain after UGFS (Rasmussen 2011; Vernermo 2016), and the other found pain was comparable between UGFS and SFJ ligation and stripping groups (FOAM 2010).
Two studies reported on change in VCSS, with no differences detected between groups at any time points in either study (FOAM 2010; Rasmussen 2011).
Rasmussen 2011 was the sole study which evaluated length of procedure as 'surgeon's time', with no clear difference detected between groups.
FOAM 2010 was the only study to report duration of hospital stay, with 100% of cases undertaken as day case.
Two trials reported on return to normal activities, with participants undergoing UGFS possibly returning to normal activities sooner (Rasmussen 2011; Vernermo 2016).
Overall completeness and applicability of evidence
We identified no RCTs for ten of the comparisons we hoped to review. We found only one RCT for the following comparisons: EVLA versus EVSA, EVLA versus cyanoacrylate glue, EVLA versus MOCA, RFA versus UGFS and RFA versus cyanoacrylate glue. The trial for EVSA did not report on recurrence rates. We identified no new trials for the comparison of RFA and SFJ ligation and stripping of GSV. This update included long‐term follow‐up data (greater than five years) on recurrence and technical success, which was not available in earlier versions of this Cochrane Review. The validity of this review has been hampered by lack of standardisation in the reporting of outcomes methods with regard to follow‐up time points, metrics and terminology used by the included trials. This has significantly impeded our ability to perform accurate meta‐analysis for the majority of outcomes, echoing the sentiments of the previous version of the review in 2014 (Nesbitt 2014). This review focused on the management of C2 to C4 grade varicose veins. We excluded varicose veins with healed ulcers (C5) or active ulcers (C6) from this Cochrane Review.
A number of studies included interventions for bilateral GSV incompetence, and this fact also hampered meta‐analysis (Calik 2019; Darwood 2008; EVOLVeS 2003; LAST 2014; Magna 2013; Pronk 2010; Rasmussen 2007; Rasmussen 2011; Recovery 2009; Shepherd 2010; Subramonia 2010). In Darwood 2008, Pronk 2010, Rasmussen 2007, Rasmussen 2011 and Shepherd 2010, participants were randomised and received the same treatment on the same day, but trialists made no separate stratification of bilateral and unilateral participants. EVOLVeS 2003, LAST 2014 and Subramonia 2010 waited over six weeks (three months in LAST 2014) from the initial procedure to randomise the other limb. This reality brings into question the reliability of the results for these participants, as ongoing disease in the second untreated limb may have impacted on the QoL outcomes, and results cannot accurately represent the outcome of the intervention.
Studies reported different complications, used different definitions to describe complications (such as symptomatic DVT) and measured complications at different time points. In addition, the complications reported can vary by the extent of venous treatment or stripping. This Cochrane Review did not assess this variation, but it should be noted as an impact on the strength of the complication results.
Quality of life and patient‐reported outcome measures are valuable metrics for assessing interventional success. Unfortunately, the studies included in this review employed a variety of quality of life tools, and reported them in different ways, meaning we could not pool the results. This represents a significant limitation to the patient‐level power of this review. Technical outcomes can be useful in guiding practitioners and patients alike, but more consistent and rigorous quality of life assessment would be of value in future venous literature.
Three studies allowed the inclusion of participants who had residual ultrasound‐proven SFJ reflux despite previous surgery (Rasmussen 2007; Rasmussen 2011; Subramonia 2010). In Rasmussen 2011 and Rasmussen 2007, 5.6% and 16% of randomised limbs had recurrence, respectively. Subramonia 2010 gave no breakdown of participants. None of the studies provided stratification of these participants.
Some varicosities are not amenable to endovenous treatments (i.e. they are too tortuous or are greater than 1.2 cm in diameter with extensive superficial varicosities). These can only be treated with open surgical methods. Conversely, not all participants are able to undergo general anaesthesia and open surgery. Tumescent and non‐tumescent techniques are now a feasible option in the treatment of venous insufficiency within this participant group.
Quality of the evidence
See Table 1; Table 2; Table 3; Table 4; Table 5; Table 6.
Overall, the lack of standardisation amongst trials for reporting their findings led to a lack of comparable data. This prevented meta‐analysis for many outcomes. We downgraded the certainty of the evidence for all outcomes as a result of concerns about detection and performance bias arising from a lack of blinding in the majority of the included studies. Other risk of bias concerns arose from attrition bias (missing data not explained by the study authors) or other potential risk of bias concerns (reasons included evaluation of bilateral limbs, underpowered studies, participants also underwent phlebectomies, etc.; see Risk of bias in included studies). We downgraded some outcomes further for imprecision as they involved limited numbers of participants from a small number of studies. Where studies reported conflicting or heterogenous results for an outcome, we downgraded for inconsistency. For the outcome of complications, studies reported different complications, used different definitions to describe complications (such as symptomatic DVT) and measured these at different time points. It was not unusual for different studies to have effects in opposite directions for the same complication, or have wide confidence intervals. Therefore, for each comparison, we downgraded the certainty of the evidence for outcome complications by three levels (risk of bias concerns, inconsistency, imprecision and possible publication bias).
Potential biases in the review process
We excluded several trials as they treated both GSV and SSV but provided no subgroup analysis (See Excluded studies for further details).
Within this review, we used the number of participants analysed for meta‐analysis as opposed to the number of participants randomised (as in the intention‐to‐treat method). This was due to discrepancies between the two numbers. Trials often noted that participants would drop out following randomisation as they were unhappy with the treatment arm to which they had been allocated (predominantly surgery).
As none of the studies which included bilateral treatment of varicose veins provided any stratification, we were unable to exclude them from this review. This has introduced a potential bias as simultaneous bilateral treatment of varicose veins impacts on outcome measures, such as procedural time, quality of life scores, pain and duration of hospital stay.
Agreements and disagreements with other studies or reviews
National Institute for Health and Care Excellence (NICE) guidelines recommend an hierarchical approach, with endothermal ablation preferred. According to the guidelines, if endothermal ablation is unsuitable, “offer ultrasound‐guided foam sclerotherapy", and if "ultrasound‐guided foam sclerotherapy is unsuitable, offer surgery” (NICE 2013a). These recommendations are based on cost‐effectiveness analysis. The Gloviczki 2012 review of guidelines, recommended by the Society for Vascular Surgery (SVS) and the American Venous Forum (AVF) Venous Guideline Committee, reported that endovenous thermal ablation (EVLA or RFA) is preferential to SFJ ligation and stripping for the treatment of GSV incompetence (recommendation: GRADE 1 (strong), level of evidence: B (medium quality)). They did not support the use of one endothermal technique over another. UGFS was also suggested as an option to treat the incompetent saphenous vein; however, the recommendation for this was weak and based on low‐ to very low‐quality evidence.
A meta‐analysis by Kheirelseid 2018 compared long‐term recurrence rates after conventional surgery versus endovenous treatments. This analysis included nine RCTs, including three trials rejected for this Cochrane Review because their comparisons did not meet the inclusion criteria of this Cochrane review (Disselhoff 2008; Disselhoff 2011; Kalteis 2015). In keeping with this Cochrane Review, Kheirelseid 2018 found no statistical difference between EVLA and surgery for recurrence (36.6% versus 33.3%, respectively; pooled RR 1.35, 95% CI 0.76 to 2.37; P = 0.3). UGFS had a higher recurrence rate than EVLA (68.6% versus 24.4%; RR 6.08, 95% CI 1.62 to 22.82; P = 0.007). Recurrence was lower in surgery participants compared to UGFS (68.6% versus 18.1%; RR 8.88, 95% CI 1.67 to 47.14; P < 0.01). UGFS was also found to be inferior to RFA. They, too, were unable to comment on QoL measures due to the heterogeneity of how this outcome was reported.
A meta‐analysis by Hamann 2017 compared the five‐year efficacy of surgery, endovenous laser therapy (EVLT, equivalent to EVLA) and UGFS. Their primary outcome was anatomical success, and secondary outcomes were recurrent reflux rate and changes in disease‐specific QoL (AVVQ, CIVIQ). They included three RCTs and ten follow‐ups of RCTs. Of these, seven are included within this review. In an attempt to overcome the wide variation of definitions of anatomical success and recurrent reflux, Hamann 2017 standardised the definitions to be able to pool the data together, which could significantly impact on the results. As reported in this review, UGFS was found to be inferior at five years compared to EVLA and HL/S with regard to anatomical success. Hamann 2017 demonstrated high rates of recurrent reflux. VCSS scores were comparable between EVLA and SFJ ligation and stripping.
Authors' conclusions
Implications for practice.
Our conclusions are limited due to the small numbers of studies available for each comparison, especially newer treatments, and by differences in definitions used and time points reported. Technical success was broadly comparable between most modalities. EVLA may offer improved technical success compared to UGFS (low‐certainty), or surgery (low‐certainty); and surgery may have improved success compared to UGFS both under (low‐certainty) and over five years (moderate‐certainty evidence). Similarly, no evidence of a difference in recurrence rates was detected, except for a possible long‐term benefit for RFA compared to EVLA (low‐certainty), or compared to surgery (low‐certainty evidence).
In the absence of better evidence, it is not currently possible to reach firm conclusions as to which of the methods reviewed are to be preferred in treating GSV varicosities. As well as variation in individual venous anatomy and vein size, there will also be significant variation in individual and surgeon preferences as to which procedure is preferred. More evidence is required before treatment modality recommendations for individuals with GSV varicosities can be made.
Implications for research.
We identified no RCTs that met the inclusion criteria for 10 of our comparisons. This was particularly so for the newer therapies, with only one RCT for EVSA (which lacked data on recurrence rates) and a paucity of long‐term data for MOCA and cyanoacrylate glue. Further research comparing these novel treatments with more conventional tumescent techniques, with longer‐term follow‐up and the inclusion of recurrence rates, is required.
The high recurrence rate after foam sclerotherapy seems to have been confirmed. Further research should be conducted on the requirement for re‐treatment after foam sclerotherapy within the follow‐up periods, and the subsequent cost implications of this, as this recurrence rate may reflect initial under‐treatment. Some trials have reported on re‐intervention rates for their comparisons, which could be included within subsequent updates of this review.
The vast majority of studies either performed phlebectomies or foam sclerotherapy to visible varicosities in addition to the intervention, either concomitantly or at a later date. This obviously impacted on the outcomes from the included trials. Further research is required on the optimum time to perform these procedures, and which groups need phlebectomies, if not all do.
Future trials should seek to standardise the clinical terminology of their outcome measures and the time points at which they are measured. Although we included 24 trials in this review, the ability to perform accurate meta‐analysis of the majority of outcome measure (namely, complications, pain, VCSS, quality of life scores, return to normal function, duration of procedure and inpatient stay) was impeded due to lack of consistency in how they were reported. Only one trial was double‐blinded. To improve the quality of the outcome measures, future trials should seek to blind the post‐operative assessors to which intervention the participant has undergone, and to include the participant and not 'legs' with varicose veins, for clarity.
What's new
| Date | Event | Description |
|---|---|---|
| 2 November 2020 | New search has been performed | New search run. Eleven new studies included and 20 new studies excluded. Four new ongoing studies identified. Four studies awaiting classification. |
| 2 November 2020 | New citation required and conclusions have changed | New search run. Eleven new studies included and 20 new studies excluded. Four new ongoing studies identified. Four studies awaiting classification. New authors joined review team. Scope amended to reflect the range of interventions currently available. Text amended to reflect current Cochrane standards, 'Summary of findings' tables added. Conclusions changed. |
History
Protocol first published: Issue 1, 2006 Review first published: Issue 10, 2011
| Date | Event | Description |
|---|---|---|
| 3 June 2014 | New citation required but conclusions have not changed | Searches re‐run. Eight additional included studies and 12 additional excluded studies identified. Review text updated accordingly. New author joined review team. |
| 3 June 2014 | New search has been performed | Searches re‐run. Eight additional included studies and 12 additional excluded studies identified. |
Acknowledgements
We thank the Cochrane Vascular editorial base and editors for their input.
The review authors, and the Cochrane Vascular editorial base, are grateful to the following peer reviewers for their time and comments.
Sophie Blaise MD PhD, Department of Vascular Medicine, Grenoble ‐ Alpes University Hospital, France;
Mr Harvey Chant FRCS MD Royal Cornwall Hospital Trust, UK;
Dr Elrasheid Kheirelseid, Beaumont Hospital and the Royal College of Surgeons in Ireland, Dublin, Ireland;
Neil M Khilnani MD, Division of Interventional Radiology, New York Presbyterian Hospital ‐ Weill Cornell Medicine, Weill Cornell Vein Center, USA;
Eyelin Ahmadi, Iran;
Mbaka Fon Nji, Cameroon.
Appendices
Appendix 1. Database searches Nov 2017 and Jan 2018
Search 1: EVLA and foam sclerotherapy vs open surgery
| Source | Search strategy | Hits retrieved |
| VASCULAR REGISTER IN CRS WEB | great saphenous vein AND VVeins* | 14 Nov 2017: 136 |
| CENTRAL via CRSO | #1 MESH DESCRIPTOR Sclerotherapy EXPLODE ALL TREES 447 #2 MESH DESCRIPTOR Sclerosing Solutions EXPLODE ALL TREES 385 #3 sclero*:TI,AB,KY 10085 #4 (tetradecyl adj2 (sulfate or sulphate)):TI,AB,KY 62 #5 MESH DESCRIPTOR Sodium Tetradecyl Sulfate EXPLODE ALL TREES 37 #6 MESH DESCRIPTOR Saline Solution, Hypertonic EXPLODE ALL TREES 442 #7 MESH DESCRIPTOR Ethanolamines 1499 #8 (polydocanol or polidocanol):TI,AB,KY 216 #9 saline:TI,AB,KY 20656 #10 (ethanolamine adj2 oleate):TI,AB,KY 65 #11 (sodium adj2 morrhuate):TI,AB,KY 19 #12 sotradecol:TI,AB,KY 6 #13 (aetoxisclerol or aethoxysclerol):TI,AB,KY 15 #14 Turbofoam:TI,AB,KY 2 #15 (foam* or microfoam*):TI,AB,KY 1440 #16 varisolve:TI,AB,KY 2 #17 MESH DESCRIPTOR Laser Therapy EXPLODE ALL TREES 3452 #18 (endovenous or EVLA or EVLT or radiofrequency or laser* or ablation* or obliteration* or RFA):TI,AB,KY 16990 #19 MESH DESCRIPTOR Catheter Ablation EXPLODE ALL TREES 1211 #20 aetoxiskerol or aethoxyskerol 1 #21 21 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 49890 #22 MESH DESCRIPTOR Vascular Surgical Procedures EXPLODE ALL TREES 13303 #23 MESH DESCRIPTOR Ligation EXPLODE ALL TREES 559 #24 (surg* or ligat* or strip* or phlebectomy):TI,AB,KY 149800 #25 #22 or #23 or #24 155224 #26 #21 and #25 15863 #27 MESH DESCRIPTOR Varicose Veins EXPLODE ALL TREES 803 #28 MESH DESCRIPTOR Saphenous Vein EXPLODE ALL TREES WITH QUALIFIERS SU 207 #29 (varic* or incomp* or insuffici* or tortuous or sapheno* or GSV or CVI):TI,AB,KY 23616 #30 MESH DESCRIPTOR Venous Insufficiency EXPLODE ALL TREES 405 #31 #27 or #28 or #29 or #30 23650 #32 #26 and #31 1011 #33 01/01/2014 TO 13/11/2017:CD 370986 #34 #32 AND #33 402 |
13 Nov 2017: 402 |
| Clinicaltrials.gov | Varicose Veins OR VARICES | ablation OR foam | First posted from 01/01/2014 to 11/14/2017 | 14 Nov 2017: 35 |
| ICTRP Search Portal | Varicose Veins OR VARICES | ablation OR foam | 01/01/2014 to 11/14/2017 | 14 Nov 2017: 17 |
| MEDLINE VIA OVID | 1 exp Sclerotherapy/ 5411 2 exp Sclerosing Solutions/ 10832 3 sclero*.ti,ab. 180324 4 (tetradecyl adj2 (sulfate or sulphate)).ti,ab. 455 5 exp Sodium Tetradecyl Sulfate/ 460 6 exp Saline Solution, Hypertonic/ 5645 7 Ethanolamines/ 11813 8 (polydocanol or polidocanol).ti,ab. 752 9 saline.ti,ab. 170166 10 (ethanolamine adj2 oleate).ti,ab. 332 11 (sodium adj2 morrhuate).ti,ab. 179 12 sotradecol.ti,ab. 55 13 (aetoxisclerol or aethoxysclerol).ti,ab. 50 14 (aetoxiskerol or aethoxyskerol).ti,ab. 1 15 Turbofoam.ti,ab. 2 16 (foam* or microfoam*).ti,ab. 23577 17 varisolve.ti,ab. 2 18 exp Laser Therapy/ 60498 19 (endovenous or EVLA or EVLT or radiofrequency or laser* or ablation* or obliteration* or RFA).ti,ab. 341596 20 exp Catheter Ablation/ 31195 21 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 743792 22 exp Vascular Surgical Procedures/ 242140 23 exp Ligation/ 23767 24 (surg* or ligat* or strip* or phlebectomy).ti,ab. 1911449 25 22 or 23 or 24 2073066 26 21 and 25 97099 27 exp Varicose Veins/ 17830 28 exp Venous Insufficiency/ 7384 29 exp Saphenous Vein/su [Surgery] 3241 30 (varic* or incomp* or insuffici* or tortuous or sapheno* or GSV or CVI).ti,ab. 445791 31 27 or 28 or 29 or 30 455898 32 26 and 31 6917 33 randomized controlled trial.pt. 505234 34 controlled clinical trial.pt. 100418 35 randomized.ab. 441461 36 placebo.ab. 205236 37 drug therapy.fs. 2146561 38 randomly.ab. 304739 39 trial.ab. 464951 40 groups.ab. 1882251 41 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 4444149 42 32 and 41 1865 43 2017*.ed. 946724 44 42 and 43 76 45 from 44 keep 1‐76 76 |
14 Nov 2017: 76 |
| EMBASE | 1 exp Sclerotherapy/ 8991 2 exp Sclerosing Solutions/ 7438 3 sclero*.ti,ab. 184740 4 (tetradecyl adj2 (sulfate or sulphate)).ti,ab. 412 5 exp Sodium Tetradecyl Sulfate/ 1098 6 exp Saline Solution, Hypertonic/ 138036 7 Ethanolamines/ 1673 8 (polydocanol or polidocanol).ti,ab. 755 9 saline.ti,ab. 161243 10 (ethanolamine adj2 oleate).ti,ab. 297 11 (sodium adj2 morrhuate).ti,ab. 96 12 sotradecol.ti,ab. 57 13 (aetoxisclerol or aethoxysclerol).ti,ab. 41 14 (aetoxiskerol or aethoxyskerol).ti,ab. 1 15 Turbofoam.ti,ab. 3 16 (foam* or microfoam*).ti,ab. 23376 17 varisolve.ti,ab. 5 18 exp Laser Therapy/ 18342 19 (endovenous or EVLA or EVLT or radiofrequency or laser* or ablation* or obliteration* or RFA).ti,ab. 307984 20 exp Catheter Ablation/ 27168 21 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 741625 22 exp Vascular Surgical Procedures/ 332738 23 exp Ligation/ 43062 24 (surg* or ligat* or strip* or phlebectomy).ti,ab. 1839209 25 22 or 23 or 24 2038357 26 21 and 25 112846 27 exp Varicose Veins/ 31021 28 exp Venous Insufficiency/ 6660 29 exp Saphenous Vein/su [Surgery] 261 30 (varic* or incomp* or insuffici* or tortuous or sapheno* or GSV or CVI).ti,ab. 407337 31 27 or 28 or 29 or 30 421472 32 26 and 31 9311 33 randomized controlled trial/ 434019 34 controlled clinical trial/ 407541 35 random$.ti,ab. 1124569 36 randomization/ 67995 37 intermethod comparison/ 222704 38 placebo.ti,ab. 213793 39 (compare or compared or comparison).ti. 324804 40 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 1550509 41 (open adj label).ti,ab. 59603 42 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 152470 43 double blind procedure/ 118472 44 parallel group$1.ti,ab. 18844 45 (crossover or cross over).ti,ab. 69732 46 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 239509 47 (assigned or allocated).ti,ab. 280620 48 (controlled adj7 (study or design or trial)).ti,ab. 251185 49 (volunteer or volunteers).ti,ab. 167273 50 trial.ti. 204542 51 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 3351318 52 32 and 51 2503 53 2017*.dc. 1594452 54 52 and 53 176 |
13 Nov 2017: 179 |
| CINAHL | S39 (EM 2017) AND (S37 AND S38) (15) S38 EM 2017 (170,959) S37 S29 AND S36 (235) S36 S30 OR S31 OR S32 OR S33 OR S34 OR S35 (950,269) S35 TX randomly (41,411) S34 TX "treatment as usual" (707) S33 TX "double‐blind*" (754,745) S32 TX "single‐blind*" (8,635) S31 TX trial (235,674) S30 MH "Clinical Trials" (90,720) S29 S19 AND S23 AND S28 (538) S28 S24 OR S25 OR S26 OR S27 (42,333) S27 varic* or incomp* or insuffici* or tortuous or sapheno* or GSV or CVI (40,779) S26 (MH "Saphenous Vein/SU") (120) S25 (MH "Venous Insufficiency+") (678) S24 (MH "Varicose Veins+") (2,342) S23 S20 OR S21 OR S22 (284,406) S22 surg* or ligat* or strip* or phlebectomy (284,406) S21 (MH "Ligation") (629) S20 vascular surgical procedures (42) S19 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 (54,423) S18 (MH "Catheter Ablation") (6,617) S17 (endovenous or EVLA or EVLT or radiofrequency or laser* or ablation* or obliteration* or RFA) (22,989) S16 (MH "Laser Therapy+") (5,466) S15 varisolve (0) S14 foam* or microfoam* (2,350) S13 Turbofoam (0) S12 aetoxisclerol or aethoxysclerol (0) S11 sotradecol (2) S10 sodium n2 morrhuate (11) S9 ethanolamine n2 oleate (13) S8 saline (8,211) S7 polydocanol or polidocanol (33) S6 (MH "Ethanolamines") (123) S5 (MH "Saline Solution, Hypertonic") (527) S4 TX tetradecyl N2 (sulfate or sulphate) (10) S3 TX sclero* (21,277) S2 (MH "Sclerosing Solutions") (181) S1 (MH "Sclerotherapy") (365) |
14 Nov 2017: 15 |
| AMED | 1 sclero*.ti,ab. 2212 2 (tetradecyl adj2 (sulfate or sulphate)).ti,ab. 0 3 (polydocanol or polidocanol).ti,ab. 3 4 saline.ti,ab. 528 5 (ethanolamine adj2 oleate).ti,ab. 0 6 (sodium adj2 morrhuate).ti,ab. 1 7 sotradecol.ti,ab. 0 8 (aetoxisclerol or aethoxysclerol).ti,ab. 0 9 (aetoxiskerol or aethoxyskerol).ti,ab. 0 10 Turbofoam.ti,ab. 0 11 (foam* or microfoam*).ti,ab. 269 12 varisolve.ti,ab. 0 13 exp Laser Therapy/ 168 14 (endovenous or EVLA or EVLT or radiofrequency or laser* or ablation* or obliteration* or RFA).ti,ab. 1116 15 (surg* or ligat* or strip* or phlebectomy).ti,ab. 11043 16 exp Varicose Veins/ 63 17 exp Venous Insufficiency/ 49 18 (varic* or incomp* or insuffici* or tortuous or sapheno* or GSV or CVI).ti,ab. 2784 19 or/1‐14 4120 20 or/16‐18 2845 21 15 and 19 and 20 7 22 "2017".yr. 47 23 21 and 22 0 |
13 Nov 2017: 0 |
Search 2: Cyanoacrylate Glue, OR Mechanochemical endovenous Ablation (MOCA) OR Steam treatment versus open surgery for great saphenous vein varices
| Source | Search strategy | Hits retrieved |
| VASCULAR REGISTER IN CRS WEB | #1 STEAM AND INREGISTER #2 Cyanoacrylate AND INREGISTER #3 Mechanochemical AND INREGISTER #4 #1 OR #2 OR #3 |
20 Nov 2017: 13 |
| CENTRAL via CRSO Issue 10, 2017 |
#1 MESH DESCRIPTOR Vascular Surgical Procedures EXPLODE TREES 1 13304 #2 MESH DESCRIPTOR Ligation EXPLODE ALL TREES 559 #3 (surg* or ligat* or strip* or phlebectomy):TI,AB,KY 151275 #4 #1 OR #2 OR #3 156699 #5 MESH DESCRIPTOR Varicose Veins EXPLODE ALL TREES 803 #6 MESH DESCRIPTOR Venous Insufficiency EXPLODE ALL TREES 405 #7 MESH DESCRIPTOR Saphenous Vein EXPLODE ALL TREES WITH QUALIFIERS SU 207 #8 (varic* or incomp* or insuffici* or tortuous or sapheno* or GSV or CVI):TI,AB,KY 23850 #9 #5 OR #6 OR #7 OR #8 23884 #10 MESH DESCRIPTOR Cyanoacrylates EXPLODE ALL TREES 178 #11 ("tissue adhesive*"):TI,AB,KY 722 #12 Cyanoacrylate*:TI,AB,KY 314 #13 Enbucrilate:TI,AB,KY 83 #14 VenaSeal:TI,AB,KY 3 #15 VariClose:TI,AB,KY 0 #16 VeClose:TI,AB,KY 5 #17 Histoacryl:TI,AB,KY 39 #18 ("Mechanochemical endovenous Ablation"):TI,AB,KY 6 #19 ("Mechanochemical Ablation"):TI,AB,KY 8 #20 MOCA:TI,AB,KY 265 #21 ClariVein:TI,AB,KY 9 #22 steam:TI,AB,KY 184 #23 #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 1349 #24 #4 AND #9 AND #23 70 |
20 Nov 2017: 70 |
| Clinicaltrials.gov | varicose OR "Venous Insufficiency" | "tissue adhesives" OR Cyanoacrylates OR Enbucrilate OR Histoacryl OR MOCA OR steam OR Mechanochemical | 20 Nov 2017: 19 |
| ICTRP Search Portal | varicose OR "Venous Insufficiency" | "tissue adhesives" OR Cyanoacrylates OR Enbucrilate OR Histoacryl OR MOCA OR steam OR Mechanochemical | 20 Nov 2017: 5 |
| MEDLINE VIA OVID | 1 exp Vascular Surgical Procedures/ 242206 2 exp Ligation/ 23775 3 (surg* or ligat* or strip* or phlebectomy).ti,ab. 1912818 4 1 or 2 or 3 2074481 5 exp Varicose Veins/ 17833 6 exp Venous Insufficiency/ 7385 7 exp Saphenous Vein/su [Surgery] 3242 8 (varic* or incomp* or insuffici* or tortuous or sapheno* or GSV or CVI).ti,ab. 445981 9 5 or 6 or 7 or 8 456089 10 randomized controlled trial.pt. 505454 11 controlled clinical trial.pt. 100423 12 randomized.ab. 441797 13 placebo.ab. 205350 14 drug therapy.fs. 2147141 15 randomly.ab. 304986 16 trial.ab. 465386 17 groups.ab. 1883715 18 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 4446580 19 exp Cyanoacrylates/ 4846 20 "tissue adhesive*".ti,ab. 1862 21 Cyanoacrylate*.ti,ab. 4342 22 Enbucrilate.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 1682 23 VenaSeal.ti,ab. 8 24 Enbucrilate.ti,ab. 34 25 VariClose.ti,ab. 6 26 VeClose.ti,ab. 3 27 Histoacryl.ti,ab. 508 28 NBCA.ti,ab. 457 29 "Mechanochemical endovenous Ablation".ti,ab. 20 30 "Mechanochemical Ablation".ti,ab. 25 31 MOCA.ti,ab. 1410 32 ClariVein.ti,ab. 31 33 "Endovenous steam".ti,ab. 9 34 steam.ti,ab. 7050 35 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 16144 36 4 and 9 and 18 and 35 106 |
20 Nov 2017: 106 |
| EMBASE | 1 exp Vascular Surgical Procedures/ 410518 2 exp Ligation/ 53587 3 (surg* or ligat* or strip* or phlebectomy).ti,ab. 2359292 4 1 or 2 or 3 2609911 5 exp Varicose Veins/ 49426 6 exp Venous Insufficiency/ 9594 7 exp Saphenous Vein/su [Surgery] 1007 8 (varic* or incomp* or insuffici* or tortuous or sapheno* or GSV or CVI).ti,ab. 541810 9 exp Cyanoacrylates/ 1798 10 "tissue adhesive*".ti,ab. 2040 11 Cyanoacrylate*.ti,ab. 5292 12 Enbucrilate.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word] 3938 13 VenaSeal.ti,ab. 24 14 Enbucrilate.ti,ab. 38 15 VariClose.ti,ab. 11 16 VeClose.ti,ab. 5 17 Histoacryl.ti,ab. 712 18 NBCA.ti,ab. 793 19 "Mechanochemical endovenous Ablation".ti,ab. 23 20 "Mechanochemical Ablation".ti,ab. 41 21 MOCA.ti,ab. 3509 22 ClariVein.ti,ab. 36 23 "Endovenous steam".ti,ab. 12 24 STEAM.ti,ab. 9350 25 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 22590 26 randomized controlled trial/ 483088 27 controlled clinical trial/ 453499 28 random$.ti,ab. 1264147 29 randomization/ 76355 30 intermethod comparison/ 232411 31 placebo.ti,ab. 264252 32 (compare or compared or comparison).ti. 459073 33 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 1680000 34 (open adj label).ti,ab. 61263 35 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 203375 36 double blind procedure/ 145498 37 parallel group$1.ti,ab. 21082 38 (crossover or cross over).ti,ab. 90507 39 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 272867 40 (assigned or allocated).ti,ab. 321579 41 (controlled adj7 (study or design or trial)).ti,ab. 283958 42 (volunteer or volunteers).ti,ab. 219487 43 trial.ti. 240867 44 or/26‐43 3905395 45 5 or 6 or 7 or 8 565240 46 4 and 25 and 44 and 45 210 |
20 Nov 2017: 210 |
| CINAHL | S31 S23 AND S30 41 S30 S24 OR S25 OR S26 OR S27 OR S28 OR S29 951,453 S29 TX randomly 41,603 S28 TX "treatment as usual" 707 S27 TX "double‐blind*" 755,453) S26 TX "single‐blind*" 8,658 S25 TX trial 236,190 S24 MH "Clinical Trials" 90,793 S23 S4 AND S8 AND S22 72 S22 S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 2,012 S21 TX steam 736 S20 TX ClariVein 2 S19 TX MOCA 470 S18 TX "Mechanochemical Ablation" 3 S17 TX "Mechanochemical endovenous Ablation" 1 S16 TX NBCA 22 S15 TX Histoacryl 25 S14 TX VeClose 0 S13 TX VariClose 0) S12 TX VenaSeal 0 S11 TX Enbucrilate 2 S10 "tissue adhesive*" 593 S9 TX Cyanoacrylates 263 S8 S5 OR S6 OR S7 46,250 S7 TX (varic* or incomp* or insuffici* or tortuous or sapheno* or GSV or CVI 44,693 S6 (MH "Venous Insufficiency+") 678 S5 (MH "Varicose Veins+") 2,349 S4 S1 OR S2 OR S3 392,631 S3 TX surg* or ligat* or strip* or phlebectomy 386,026 S2 (MH "Ligation") 630 S1 (MH "Vascular Surgery+") 13,726 |
20 Nov 2017: 41 |
| AMED | 1 (surg* or ligat* or strip* or phlebectomy).ti,ab. 11043 2 exp Varicose Veins/ 63 3 exp Venous Insufficiency/ 49 4 (varic* or incomp* or insuffici* or tortuous or sapheno* or GSV or CVI).ti,ab. 2784 5 "tissue adhesive*".ti,ab. 2 6 Cyanoacrylate*.ti,ab. 3 7 Enbucrilate.mp. [mp=abstract, heading words, title] 1 8 Enbucrilate.ti,ab. 1 9 Histoacryl.ti,ab. 2 10 MOCA.ti,ab. 6 11 steam.ti,ab. 72 12 2 or 3 or 4 2845 13 5 or 6 or 7 or 8 or 9 or 10 or 11 85 14 1 and 12 and 13 0 |
20 Nov 2017: 0 |
Search 3: Endovenous ablation, Foam Sclerotherapy, Glue, MOCA, Steam
| Source | Search strategy | Hits retrieved |
| VASCULAR REGISTER IN CRSW | #1 Varicose Veins AND INREGISTER #2 foam AND INREGISTER #3 mechanochemical endovenous ablation AND INREGISTER #4 MOCA AND INREGISTER #5 sclerotherapy AND INREGISTER #6 sclerosing solutions AND INREGISTER #7 Laser AND INREGISTER #8 Ablation AND INREGISTER #9 Mechanochemical Ablation AND INREGISTER #10 Cyanoacrylates AND INREGISTER #11 steam AND INREGISTER #12 #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 #13 #1 AND #12 |
9 Jan 2018: 209 |
| CENTRAL Issue 12, 2017 |
#1 MESH DESCRIPTOR Varicose Veins EXPLODE ALL TREES 809 #2 MESH DESCRIPTOR Venous Insufficiency EXPLODE ALL TREES 406 #3 (varic* or incomp* or insuffici* or tortuous or sapheno* or GSV or CVI):TI,AB,KY 24143 #4 #1 OR #2 OR #3 24177 #5 MESH DESCRIPTOR Laser Therapy EXPLODE ALL TREES 3510 #6 MESH DESCRIPTOR Catheter Ablation EXPLODE ALL TREES 1237 #7 (endovenous or EVLA or EVLT or radiofrequency or laser* or ablation* or obliteration* or RFA):TI,AB,KY 17365 #8 NBCA:TI,AB,KY 13 #9 (Mechanochemical endovenous Ablation):TI,AB,KY 6 #10 (Mechanochemical Ablation):TI,AB,KY 8 #11 #5 OR #6 OR #7 OR #8 OR #9 OR #10 17417 #12 MESH DESCRIPTOR Sclerotherapy EXPLODE ALL TREES 448 #13 MESH DESCRIPTOR Sclerosing Solutions EXPLODE ALL TREES 385 #14 sclero*:TI,AB,KY 10391 #15 (tetradecyl near2 (sulfate or sulphate)):TI,AB,KY 63 #16 MESH DESCRIPTOR Sodium Tetradecyl Sulfate EXPLODE ALL TREES 37 #17 MESH DESCRIPTOR Saline Solution, Hypertonic EXPLODE ALL TREES 446 #18 MESH DESCRIPTOR Ethanolamines 1503 #19 (polydocanol or polidocanol):TI,AB,KY 217 #20 saline:TI,AB,KY 20996 #21 (ethanolamine near2 oleate):TI,AB,KY 66 #22 (sodium near2 morrhuate):TI,AB,KY 19 #23 sotradecol:TI,AB,KY 6 #24 (aetoxisclerol or aethoxysclerol):TI,AB,KY 15 #25 (aetoxiskerol or aethoxyskerol):TI,AB,KY 1 #26 Turbofoam:TI,AB,KY 2 #27 (foam* or microfoam*):TI,AB,KY 1464 #28 varisolve:TI,AB,KY 2 #29 #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 34198 #30 MESH DESCRIPTOR Cyanoacrylates EXPLODE ALL TREES 181 #31 ("tissue adhesive*"):TI,AB,KY 730 #32 Cyanoacrylate*:TI,AB,KY 317 #33 Enbucrilate:TI,AB,KY 83 #34 VenaSeal:TI,AB,KY 3 #35 VariClose:TI,AB,KY 0 #36 VeClose:TI,AB,KY 5 #37 Histoacryl:TI,AB,KY 39 #38 #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 905 #39 MOCA:TI,AB,KY 278 #40 ClariVein:TI,AB,KY 9 #41 #39 OR #40 284 #42 (Endovenous steam):TI,AB,KY 1 #43 steam:TI,AB,KY 186 #44 #42 OR #43 186 #45 #11 AND #19 34 #46 #11 AND #38 50 #47 #11 AND #41 16 #48 #11 AND #44 16 #49 #29 AND #38 70 #50 #29 AND #41 17 #51 #29 AND #44 11 #52 #38 AND #41 1 #53 #38 AND #44 0 #54 #41 AND #44 1 #55 #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52 OR #53 OR #54 184 #56 #4 AND #55 95 |
9 Jan 2018: 95 |
| Clinicaltrials.gov | Varicose Veins | steam OR ablation OR sclerotherapy OR Cyanoacrylates OR GLUE OR foam | 9 Jan 2018: 99 |
| ICTRP Search Portal | Varicose Veins | steam OR ablation OR sclerotherapy OR Cyanoacrylates OR GLUE OR foam | 9 Jan 2018: 68 |
| MEDLINE 2017 and 2018 only |
1 exp Varicose Veins/ 17950 2 exp Venous Insufficiency/ 7467 3 (varic* or incomp* or insuffici* or tortuous or sapheno* or GSV or CVI).ti,ab. 454946 4 or/1‐3 464353 5 exp Laser Therapy/ 61085 6 exp Catheter Ablation/ 31949 7 (endovenous or EVLA or EVLT or radiofrequency or laser* or ablation* or obliteration* or RFA).ti,ab. 347334 8 NBCA.ti,ab. 475 9 "Mechanochemical endovenous Ablation".ti,ab. 21 10 "Mechanochemical Ablation".ti,ab. 25 11 or/5‐10 364051 12 exp Sclerotherapy/ 5483 13 exp Sclerosing Solutions/ 10961 14 sclero*.ti,ab. 185374 15 (tetradecyl adj2 (sulfate or sulphate)).ti,ab. 461 16 exp Sodium Tetradecyl Sulfate/ 461 17 exp Saline Solution, Hypertonic/ 5748 18 Ethanolamines/ 11982 19 (polydocanol or polidocanol).ti,ab. 770 20 saline.ti,ab. 173305 21 (ethanolamine adj2 oleate).ti,ab. 337 22 (sodium adj2 morrhuate).ti,ab. 179 23 sotradecol.ti,ab. 54 24 (aetoxisclerol or aethoxysclerol).ti,ab. 50 25 (aetoxiskerol or aethoxyskerol).ti,ab. 1 26 Turbofoam.ti,ab. 2 27 (foam* or microfoam*).ti,ab. 24095 28 varisolve.ti,ab. 2 29 or/12‐28 403167 30 exp Cyanoacrylates/ 4934 31 "tissue adhesive*".ti,ab. 1886 32 Cyanoacrylate*.ti,ab. 4419 33 Enbucrilate.ti,ab. 35 34 VenaSeal.ti,ab. 9 35 VariClose.ti,ab. 6 36 VeClose.ti,ab. 3 37 Histoacryl.ti,ab. 513 38 or/30‐37 7716 39 MOCA.ti,ab. 1488 40 ClariVein.ti,ab. 32 41 or/39‐40 1510 42 "Endovenous steam".ti,ab. 9 43 steam.ti,ab. 7174 44 or/42‐43 7174 45 11 and 29 8205 46 11 and 38 850 47 11 and 41 42 48 11 and 44 235 49 29 and 38 540 50 29 and 41 58 51 29 and 44 153 52 38 and 41 2 53 38 and 44 1 54 41 and 44 6 55 or/45‐54 9670 56 4 and 55 1693 57 randomized controlled trial.pt. 516039 58 controlled clinical trial.pt. 101743 59 randomized.ab. 453171 60 placebo.ab. 210619 61 drug therapy.fs. 2199170 62 randomly.ab. 312199 63 trial.ab. 477783 64 groups.ab. 1925728 65 or/57‐64 4548008 66 56 and 65 427 |
9 Jan 2018: 25 |
| EMBASE 2017 and 2018 only |
1 exp Varicose Veins/ 30954 2 exp Venous Insufficiency/ 6648 3 (varic* or incomp* or insuffici* or tortuous or sapheno* or GSV or CVI).ti,ab. 404745 4 or/1‐3 418741 5 exp Laser Therapy/ 18166 6 exp Catheter Ablation/ 27123 7 (endovenous or EVLA or EVLT or radiofrequency or laser* or ablation* or obliteration* or RFA).ti,ab. 305281 8 NBCA.ti,ab. 777 9 "Mechanochemical endovenous Ablation".ti,ab. 24 10 "Mechanochemical Ablation".ti,ab. 40 11 or/5‐10 315178 12 exp Sclerotherapy/ 8990 13 exp Sclerosing Solutions/ 7375 14 sclero*.ti,ab. 185006 15 (tetradecyl adj2 (sulfate or sulphate)).ti,ab. 410 16 exp Sodium Tetradecyl Sulfate/ 1078 17 exp Saline Solution, Hypertonic/ 137265 18 Ethanolamines/ 1670 19 (polydocanol or polidocanol).ti,ab. 760 20 saline.ti,ab. 160073 21 (ethanolamine adj2 oleate).ti,ab. 299 22 (sodium adj2 morrhuate).ti,ab. 97 23 sotradecol.ti,ab. 57 24 (aetoxisclerol or aethoxysclerol).ti,ab. 42 25 (aetoxiskerol or aethoxyskerol).ti,ab. 1 26 Turbofoam.ti,ab. 3 27 (foam* or microfoam*).ti,ab. 22781 28 varisolve.ti,ab. 5 29 or/12‐28 432105 30 exp Cyanoacrylates/ 1015 31 "tissue adhesive*".ti,ab. 1263 32 Cyanoacrylate*.ti,ab. 4139 33 Enbucrilate.ti,ab. 27 34 VenaSeal.ti,ab. 26 35 VariClose.ti,ab. 11 36 VeClose.ti,ab. 5 37 Histoacryl.ti,ab. 519 38 or/30‐37 5714 39 MOCA.ti,ab. 3454 40 ClariVein.ti,ab. 40 41 or/39‐40 3483 42 "Endovenous steam".ti,ab. 12 43 steam.ti,ab. 7068 44 or/42‐43 7068 45 11 and 29 10224 46 11 and 38 1029 47 11 and 41 70 48 11 and 44 367 49 29 and 38 666 50 29 and 41 126 51 29 and 44 224 52 38 and 41 10 53 38 and 44 6 54 41 and 44 10 55 or/45‐54 12009 56 4 and 55 2299 57 randomized controlled trial/ 434283 58 controlled clinical trial/ 407173 59 random$.ti,ab. 1117832 60 randomization/ 68256 61 intermethod comparison/ 218276 62 placebo.ti,ab. 213565 63 (compare or compared or comparison).ti. 322736 64 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 1540369 65 (open adj label).ti,ab. 59678 66 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 152435 67 double blind procedure/ 118292 68 parallel group$1.ti,ab. 18718 69 (crossover or cross over).ti,ab. 69418 70 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 238152 71 (controlled adj7 (study or design or trial)).ti,ab. 249992 72 (volunteer or volunteers).ti,ab. 166514 73 trial.ti. 203926 74 or/57‐73 3255961 75 56 and 74 561 |
9 Jan 2018: 65 |
| AMED 2017 and 2018 only |
1 exp Varicose Veins/ 64 2 exp Venous Insufficiency/ 50 3 (varic* or incomp* or insuffici* or tortuous or sapheno* or GSV or CVI).ti,ab. 2794 4 or/1‐3 2855 5 exp Laser Therapy/ 169 6 exp Catheter Ablation/ 0 7 (endovenous or EVLA or EVLT or radiofrequency or laser* or ablation* or obliteration* or RFA).ti,ab. 1122 8 NBCA.ti,ab. 0 9 "Mechanochemical endovenous Ablation".ti,ab. 0 10 "Mechanochemical Ablation".ti,ab. 0 11 or/5‐10 1133 12 exp Sclerotherapy/ 0 13 exp Sclerosing Solutions/ 0 14 sclero*.ti,ab. 2225 15 (tetradecyl adj2 (sulfate or sulphate)).ti,ab. 0 16 exp Sodium Tetradecyl Sulfate/ 0 17 exp Saline Solution, Hypertonic/ 0 18 Ethanolamines/ 0 19 (polydocanol or polidocanol).ti,ab. 3 20 saline.ti,ab. 532 21 (ethanolamine adj2 oleate).ti,ab. 0 22 (sodium adj2 morrhuate).ti,ab. 1 23 sotradecol.ti,ab. 0 24 (aetoxisclerol or aethoxysclerol).ti,ab. 0 25 (aetoxiskerol or aethoxyskerol).ti,ab. 0 26 Turbofoam.ti,ab. 0 27 (foam* or microfoam*).ti,ab. 270 28 varisolve.ti,ab. 0 29 or/12‐28 3024 30 exp Cyanoacrylates/ 0 31 "tissue adhesive*".ti,ab. 2 32 Cyanoacrylate*.ti,ab. 3 33 Enbucrilate.ti,ab. 1 34 VenaSeal.ti,ab. 0 35 VariClose.ti,ab. 0 36 VeClose.ti,ab. 0 37 Histoacryl.ti,ab. 2 38 or/30‐37 7 39 MOCA.ti,ab. 6 40 ClariVein.ti,ab. 0 41 or/39‐40 6 42 "Endovenous steam".ti,ab. 0 43 steam.ti,ab. 73 44 or/42‐43 73 45 11 and 29 14 46 11 and 38 0 47 11 and 41 0 48 11 and 44 1 49 29 and 38 0 50 29 and 41 0 51 29 and 44 0 52 38 and 41 0 53 38 and 44 0 54 41 and 44 0 55 or/45‐54 15 56 4 and 55 2 |
9 Jan 2018: 0 |
| CINAHL 2017 and 2018 only |
S62 S4 AND S54 AND S61 78 S61 S55 OR S56 OR S57 OR S58 OR S59 OR S60 958,825 S60 TX randomly 42,126 S59 TX "treatment as usual" 724 S58 TX "double‐blind*" 760,703 S57 TX "single‐blind*" 8,730 S56 TX trial 238,589 S55 MH "Clinical Trials" 91,184 S54 S45 OR S46 OR S47 OR S48 OR S49 OR S50 OR S51 OR S52 OR S53 840 S53 S38 AND S44 0 S52 S38 AND S41 0 S51 S29 AND S44 22 S50 S29 AND S41 9 S49 S29 AND S38 45 S48 S11 AND S44 40 S47 S11 AND S41 5 S46 S11 AND S38 51 S45 S11 AND S29 696 S44 S42 OR S43 749 S43 TX steam 749 S42 TX "Endovenous steam" 0 S41 S39 OR S40 482 S40 TX ClariVein 2 S39 TX MOCA 482 S38 S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 822 S37 TX Histoacryl 25 S36 TX VeClose 0 S35 TX VariClose 0 S34 TX VenaSeal 0 S33 TX Enbucrilate 2 S32 TX Cyanoacrylate* 270 S31 TX "tissue adhesive*" 610 S30 TX Cyanoacrylates 270 S29 S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 38,921 S28 TX varisolve 0 S27 TX foam* or microfoam* 2,605 S26 TX Turbofoam 0 S25 TX aetoxiskerol or aethoxyskerol 0 S24 TX aetoxisclerol or aethoxysclerol 0 S23 TX sotradecol 2 S22 TX sodium N2 morrhuate 12 S21 TX ethanolamine N2 oleate 13 S20 TX saline 8,727 S19 TX polydocanol or polidocanol 33 S18 (MH "Ethanolamines+") 6,503 S17 (MH "Saline Solution, Hypertonic") 533 S16 TX Sodium Tetradecyl Sulfate 6 S15 TX (tetradecyl N2 (sulfate or sulphate)) 10 S14 TX sclero* 21,475 S13 (MH "Sclerosing Solutions") 182 S12 (MH "Sclerotherapy") 365 S11 S5 OR S6 OR S7 OR S8 OR S9 OR S10 24,433 S10 TX "Mechanochemical Ablation" 3 S9 TX "Mechanochemical endovenous Ablation" 1 S8 TX NBCA 22 S7 TX endovenous or EVLA or EVLT or radiofrequency or laser* or ablation* or obliteration* or RFA 24,345 S6 (MH "Catheter Ablation") 6,674 S5 (MH "Laser Therapy+") 5,572 S4 (S1 OR S2 OR S3) 46,802 S3 TX varic* or incomp* or insuffici* or tortuous or sapheno* or GSV or CVI 45,221 S2 (MH "Venous Insufficiency+") 686 S1 (MH "Varicose Veins+") 2,378 |
9 Jan 2018: 1 |
Appendix 2. Database searches Jan 2019 and Nov 2020
| Source | Search strategy | Hits retrieved |
| VASCULAR REGISTER IN CRSW | #1 Venous Insufficiency AND #2 Varicose Veins AND #3 Saphenous Vein AND #4 varic* or incomp* or insuffici* or tortuous or sapheno* or GSV or CVI #5 #1 OR #2 OR #3 OR #4 #6 Catheter Ablation AND #7 CYANOACRYLATES AND #8 ETHANOLAMINES AND #9 Laser Therapy AND #10 LIGATION AND #11 Solution, Hypertonic #12 Sclerosing Solutions AND #13 SCLEROTHERAPY AND #14 Sodium Tetradecyl Sulfate AND #15 Vascular Surgical Procedures AND #16 Mechanochemical Ablation AND #17 endovenous or EVLA or EVLT or radiofrequency or laser* or ablation* or obliteration* or RFA AND #18 foam* or microfoam* AND #19 steam #20 #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 #21 #20 AND #5 |
25 Jan 2019: 27 2 Nov 2020: 93 |
| CENTRAL | #1 MESH DESCRIPTOR Varicose Veins EXPLODE ALL TREES 1025 #2 MESH DESCRIPTOR Venous Insufficiency EXPLODE ALL TREES 514 #3 MESH DESCRIPTOR Saphenous Vein EXPLODE ALL TREES 651 #4 ((varic* or incomp* or insuffici* or tortuous or sapheno*) and (vein* or venous)).TI,AB,KY 26520 #5 #1 OR #2 OR #3 OR #4 26570 #6 MESH DESCRIPTOR Catheter Ablation EXPLODE ALL TREES 1325 #7 MESH DESCRIPTOR CYANOACRYLATES EXPLODE ALL TREES 194 #8 MESH DESCRIPTOR ETHANOLAMINES EXPLODE ALL TREES 12584 #9 MESH DESCRIPTOR Laser Therapy EXPLODE ALL TREES 3698 #10 MESH DESCRIPTOR LIGATION EXPLODE ALL TREES 581 #11 MESH DESCRIPTOR Saline Solution, Hypertonic EXPLODE ALL TREES 471 #12 MESH DESCRIPTOR Sclerosing Solutions EXPLODE ALL TREES 409 #13 MESH DESCRIPTOR SCLEROTHERAPY EXPLODE ALL TREES 460 #14 MESH DESCRIPTOR Sodium Tetradecyl Sulfate EXPLODE ALL TREES 41 #15 MESH DESCRIPTOR Vascular Surgical Procedures EXPLODE ALL TREES 14037 #16 (Mechanochemical Ablation):TI,AB,KY 11 #17 (Mechanochemical endovenous Ablation):TI,AB,KY 5 #18 (tissue adhesive*):TI,AB,KY 824 #19 (aetoxisclerol or aethoxysclerol):TI,AB,KY 15 #20 (aetoxiskerol or aethoxyskerol):TI,AB,KY 1 #21 (endovenous or EVLA or EVLT or radiofrequency or laser* or ablation* or obliteration* or RFA):TI,AB,KY 20245 #22 (ethanolamine adj2 oleate):TI,AB,KY 68 #23 (foam* or microfoam*):TI,AB,KY 1724 #24 (polydocanol or polidocanol):TI,AB,KY 254 #25 (sodium adj2 morrhuate):TI,AB,KY 21 #26 (surg* or ligat* or strip* or phlebectomy):TI,AB,KY 174252 #27 (tetradecyl adj2 (sulfate or sulphate)):TI,AB,KY 65 #28 ClariVein:TI,AB,KY 14 #29 Cyanoacrylate*:TI,AB,KY 369 #30 Enbucrilate:TI,AB,KY 74 #31 Histoacryl:TI,AB,KY 42 #32 MOCA:TI,AB,KY 308 #33 NBCA:TI,AB,KY 10 #34 saline:TI,AB,KY 21059 #35 sclero*:TI,AB,KY 12043 #36 sotradecol:TI,AB,KY 7 #37 steam:TI,AB,KY 184 #38 Turbofoam:TI,AB,KY 2 #39 VariClose:TI,AB,KY 0 #40 varisolve:TI,AB,KY 2 #41 VeClose:TI,AB,KY 6 #42 VenaSeal:TI,AB,KY 6 #43 #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 228556 #44 #5 AND #43 8028 #45 VenaSeal:TI,AB,KY AND 01/01/2018 TO 25/01/2019:CD 5 |
25 Jan 2019: 5 Nov 2020: 751 |
| Clinicaltrials.gov | Varicose Veins OR Venous Insufficiency OR Saphenous Vein OR varic* OR incomp* OR tortuous OR sapheno* | surg* OR ligat* OR strip* OR foam OR ablation* OR endovenous OR glue OR SCLERO* | 25 Jan 2019: 12 2 Nov 2020: 25 |
| ICTRP Search Portal | Varicose Veins OR Venous Insufficiency OR Saphenous Vein OR varic* OR incomp* OR tortuous OR sapheno*AND surg* OR ligat* OR strip* OR foam OR ablation* OR endovenous OR glue OR SCLERO* | 25 Jan 2019: 5 2 Nov 2020: N/A |
| MEDLINE | 1 exp Varicose Veins/ 2 exp Venous Insufficiency/ 3 exp Saphenous Vein/su 4 ((varic* or incomp* or insuffici* or tortuous or sapheno*) and (vein* or venous)).ti,ab. 5 or/1‐4 6 exp Catheter Ablation/ 7 exp CYANOACRYLATES/ 8 exp ETHANOLAMINES/ 9 exp Laser Therapy/ 10 exp LIGATION/ 11 Saline Solution, Hypertonic/ 12 exp Sclerosing Solutions/ 13 exp SCLEROTHERAPY/ 14 exp Sodium Tetradecyl Sulfate/ 15 exp Vascular Surgical Procedures/ 16 Mechanochemical Ablation.ti,ab. 17 Mechanochemical endovenous Ablation.ti,ab. 18 tissue adhesive*.ti,ab. 19 (aetoxisclerol or aethoxysclerol).ti,ab. 20 (aetoxiskerol or aethoxyskerol).ti,ab. 21 (endovenous or EVLA or EVLT or radiofrequency or laser* or ablation* or obliteration* or RFA).ti,ab. 22 (ethanolamine adj2 oleate).ti,ab. 23 (foam* or microfoam*).ti,ab. 24 (polydocanol or polidocanol).ti,ab. 25 (sodium adj2 morrhuate).ti,ab. 26 (surg* or ligat* or strip* or phlebectomy).ti,ab. 27 (tetradecyl adj2 (sulfate or sulphate)).ti,ab. 28 ClariVein.ti,ab. 29 Cyanoacrylate*.ti,ab. 30 Enbucrilate.ti,ab. 31 Histoacryl.ti,ab. 32 MOCA.ti,ab. 33 NBCA.ti,ab. 34 saline.ti,ab. 35 sclero*.ti,ab. 36 sotradecol.ti,ab. 37 steam.ti,ab. 38 Turbofoam.ti,ab. 39 VariClose.ti,ab. 40 varisolve.ti,ab. 41 VeClose.ti,ab. 42 VenaSeal.ti,ab. 43 or/6‐42 44 5 and 43 45 randomized controlled trial.pt. 46 controlled clinical trial.pt. 47 randomized.ab. 48 placebo.ab. 49 drug therapy.fs. 50 randomly.ab. 51 trial.ab. 52 groups.ab. 53 or/45‐52 54 exp animals/ not humans.sh. 55 53 not 54 56 44 and 55 57 (2018* or 2019*).ed. 58 56 and 57 |
25 Jan 2019: 1054 2 Nov 2020: 1890 |
| EMBASE | 1 exp varicosis/ 2 exp vein insufficiency/ 3 exp saphenous vein/ 4 ((varic* or incomp* or insuffici* or tortuous or sapheno*) and (vein* or venous)).ti,ab. 5 or/1‐4 6 exp catheter ablation/ 7 exp cyanoacrylate derivative/ 8 exp ethanolamine derivative/ 9 exp low level laser therapy/ 10 exp ligation/ 11 exp sclerosing agent/ 12 exp sclerotherapy/ 13 exp tetradecyl sulfate sodium/ 14 exp vascular surgery/ 15 exp ablation therapy/ 16 tissue adhesive*.ti,ab. 17 (aetoxisclerol or aethoxysclerol).ti,ab. 18 (aetoxiskerol or aethoxyskerol).ti,ab. 19 (endovenous or EVLA or EVLT or radiofrequency or laser* or ablation* or obliteration* or RFA).ti,ab. 20 (ethanolamine adj2 oleate).ti,ab. 21 (foam* or microfoam*).ti,ab. 22 (polydocanol or polidocanol).ti,ab. 23 (sodium adj2 morrhuate).ti,ab. 24 (surg* or ligat* or strip* or phlebectomy).ti,ab. 25 (tetradecyl adj2 (sulfate or sulphate)).ti,ab. 26 ClariVein.ti,ab. 27 Cyanoacrylate*.ti,ab. 28 Enbucrilate.ti,ab. 29 Histoacryl.ti,ab. 30 MOCA.ti,ab. 31 NBCA.ti,ab. 32 saline.ti,ab. 33 sotradecol.ti,ab. 34 steam.ti,ab. 35 Turbofoam.ti,ab. 36 VariClose.ti,ab. 37 varisolve.ti,ab. 38 VeClose.ti,ab. 39 VenaSeal.ti,ab. 40 or/6‐39 41 5 and 40 42 randomized controlled trial/ 43 controlled clinical trial/ 44 random$.ti,ab. 45 randomization/ 46 intermethod comparison/ 47 placebo.ti,ab. 48 (compare or compared or comparison).ti. 49 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 50 (open adj label).ti,ab. 51 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 52 double blind procedure/ 53 parallel group$1.ti,ab. 54 (crossover or cross over).ti,ab. 55 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 56 (assigned or allocated).ti,ab. 57 (controlled adj7 (study or design or trial)).ti,ab. 58 (volunteer or volunteers).ti,ab. 59 trial.ti. 60 or/42‐59 61 41 and 60 62 (2018* or 2019*).em. 63 61 and 62 64 from 63 keep 3001‐3437 |
25 Jan 2019: 3437 2 Nov 2020: 4403 |
| AMED | 1 exp Varicose veins/ 2 exp Venous insufficiency/ 3 (varic* or incomp* or insuffici* or tortuous or sapheno* or GSV or CVI).ti,ab. 4 or/1‐3 5 tissue adhesive*.ti,ab. 6 (aetoxisclerol or aethoxysclerol).ti,ab. 7 (aetoxiskerol or aethoxyskerol).ti,ab. 8 (endovenous or EVLA or EVLT or radiofrequency or laser* or ablation* or obliteration* or RFA).ti,ab. 9 (ethanolamine adj2 oleate).ti,ab. 10 (foam* or microfoam*).ti,ab. 11 (polydocanol or polidocanol).ti,ab. 12 (sodium adj2 morrhuate).ti,ab. 13 (surg* or ligat* or strip* or phlebectomy).ti,ab. 14 (tetradecyl adj2 (sulfate or sulphate)).ti,ab. 15 ClariVein.ti,ab. 16 Cyanoacrylate*.ti,ab. 17 Enbucrilate.ti,ab. 18 Histoacryl.ti,ab. 19 MOCA.ti,ab. 20 NBCA.ti,ab. 21 saline.ti,ab. 22 sotradecol.ti,ab. 23 steam.ti,ab. 24 Turbofoam.ti,ab. 25 VariClose.ti,ab. 26 varisolve.ti,ab. 27 VeClose.ti,ab. 28 VenaSeal.ti,ab. 29 or/5‐28 30 4 and 29 31 exp CLINICAL TRIALS/ 32 RANDOM ALLOCATION/ 33 DOUBLE BLIND METHOD/ 34 Clinical trial.pt. 35 (clinic* adj trial*).tw. 36 ((singl* or doubl* or trebl* or tripl*) adj (blind* or mask*)).tw. 37 PLACEBOS/ 38 placebo*.tw. 39 random*.tw. 40 PROSPECTIVE STUDIES/ 41 or/31‐40 42 30 and 41 43 ("2018" or "2019").yr. 44 42 and 43 |
25 Jan 2019: 1 2 Nov 2020: 1 |
| CINAHL | S56 S53 AND S54 S55 S53 AND S54 S54 EM 2018 OR EM 2019 S53 S39 AND S52 S52 S40 OR S41 OR S42 OR S43 OR S44 OR S45 OR S46 OR S47 OR S48 OR S49 OR S50 OR S51 S51 MH "Random Assignment" S50 MH "Single‐Blind Studies" or MH "Double‐Blind Studies" or MH "Triple‐Blind Studies" S49 MH "Crossover Design" S48 MH "Factorial Design" S47 MH "Placebos" S46 MH "Clinical Trials" S45 TX "multi‐centre study" OR "multi‐center study" OR "multicentre study" OR "multicenter study" OR "multi‐site study" S44 TX crossover OR "cross‐over" S43 AB placebo* S42 TX random* S41 TX trial* S40 TX "latin square" S39 S5 AND S38 S38 S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 S37 TX VenaSeal S36 TX VeClose S35 TX varisolve S34 TX VariClose S33 TX Turbofoam S32 TX steam S31 TX sotradecol S30 TX sclero* S29 TX saline S28 TX NBCA S27 TX MOCA S26 TX Histoacryl S25 TX Enbucrilate S24 TX Cyanoacrylate* S23 TX ClariVein S22 TX tetradecyl N2 (sulfate or sulphate) S21 TX surg* or ligat* or strip* or phlebectomy S20 TX sodium N2 morrhuate S19 TX polydocanol or polidocanol S18 TX foam* or microfoam* S17 TX ethanolamine N2 oleate S16 TX endovenous or EVLA or EVLT or radiofrequency or laser* or ablation* or obliteration* or RFA S15 TX aetoxisclerol or aethoxysclerol S14 TX aetoxiskerol or aethoxyskerol S13 TX aetoxisclerol or aethoxysclerol S12 TX tissue adhesive* S11 TX Mechanochemical endovenous Ablation S10 TX Mechanochemical Ablation S9 (MH "Sclerotherapy") S8 (MH "Sclerosing Solutions") S7 (MH "Saline Solution, Hypertonic") S6 (MH "Catheter Ablation") S5 S1 OR S2 OR S3 OR S4 S4 TX varic* or incomp* or insuffici* or tortuous or sapheno* or GSV or CVI S3 (MH "Saphenous Vein/SU") S2 (MH "Venous Insufficiency") S1 (MH "Varicose Veins+") |
25 Jan 2019: 493 2 Nov 2020: 699 |
Data and analyses
Comparison 1. Endovenous laser ablation versus radiofrequency ablation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Technical success < 5 years | 5 | 780 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.41, 2.38] |
| 1.2 Long‐term technical success > 5 years | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.3 Recurrence | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.4 Long‐term recurrence > 5 years | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 2. Endovenous laser ablation versus endovenous steam ablation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Technical success < 5 years | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 3. Endovenous laser ablation versus ultrasound‐guided foam sclerotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Technical success < 5 years | 3 | 588 | Odds Ratio (M‐H, Random, 95% CI) | 6.13 [0.98, 38.27] |
| 3.2 Technical success > 5 years | 3 | 534 | Odds Ratio (M‐H, Random, 95% CI) | 6.47 [2.60, 16.10] |
| 3.3 Recurrence | 2 | 443 | Odds Ratio (M‐H, Random, 95% CI) | 0.68 [0.20, 2.36] |
| 3.4 Long‐term recurrence > 5 years | 2 | 418 | Odds Ratio (M‐H, Random, 95% CI) | 1.08 [0.40, 2.87] |
Comparison 4. Endovenous laser ablation versus cyanoacrylate glue.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Technical success < 5 years | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.2 Recurrence | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 5. Endovenous laser ablation versus mechanochemical ablation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Technical success < 5 years | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.2 Recurrence | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 6. Endovenous laser ablation versus SFJ ligation and stripping (HL/S, surgery).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Technical success < 5 years | 6 | 1051 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.31 [1.27, 4.23] |
| 6.2 Technical success > 5 years | 5 | 874 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.57, 1.50] |
| 6.3 Recurrence | 7 | 1459 | Odds Ratio (M‐H, Random, 95% CI) | 0.78 [0.47, 1.29] |
| 6.4 Long‐term recurrence > 5 years | 7 | 1267 | Odds Ratio (M‐H, Random, 95% CI) | 1.09 [0.68, 1.76] |
Comparison 7. Radiofrequency ablation versus ultrasound‐guided foam sclerotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Technical success < 5 years | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.2 Long‐term technical success > 5 | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.3 Recurrence | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.4 Long‐term recurrence > 5 years | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 8. Radiofrequency ablation versus cyanoacrylate glue.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Technical success < 5 years | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 9. Radiofrequency ablation versus mechanochemical ablation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Technical success < 5 years | 3 | 435 | Odds Ratio (M‐H, Random, 95% CI) | 1.76 [0.06, 54.15] |
| 9.2 Recurrence | 3 | 389 | Odds Ratio (M‐H, Random, 95% CI) | 1.00 [0.21, 4.81] |
Comparison 10. Radiofrequency ablation versus SFJ ligation and stripping (HL/S, surgery).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 Technical success < 5 years | 2 | 318 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.71 [0.64, 50.81] |
| 10.2 Technical success > 5 years | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.3 Recurrence | 4 | 546 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.58, 1.51] |
| 10.4 Long‐term recurrence > 5 years | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 11. Ultrasound‐guided foam sclerotherapy versus SFJ ligation and stripping (HL/S, surgery).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 11.1 Technical success < 5 years | 4 | 954 | Odds Ratio (M‐H, Random, 95% CI) | 0.32 [0.11, 0.94] |
| 11.2 Technical success > 5 years | 3 | 525 | Odds Ratio (M‐H, Random, 95% CI) | 0.09 [0.03, 0.30] |
| 11.3 Recurrence | 3 | 822 | Odds Ratio (M‐H, Random, 95% CI) | 1.81 [0.87, 3.77] |
| 11.4 Long‐term recurrence (≥ 5 years) | 3 | 639 | Odds Ratio (M‐H, Random, 95% CI) | 1.24 [0.57, 2.71] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Calik 2019.
| Study characteristics | ||
| Methods |
Study design: single centre, prospective comparative (but used randomisation) Country: Turkey Setting/Location: Erzurum Regional Training and Research Hospital Source of funding: not stated Intention‐to‐treat analysis: not stated |
|
| Participants |
No of participants randomised: n = 400 participants, 412 procedures (EVLA = 200 (204 procedures) CA = 200 (208 procedures)) No of participants analysed: no cohort diagram EVLA = 200 (204 procedures) CA = 200 (208 procedures) Exclusions post‐randomisation: not stated Losses to follow‐up: "The 1, 3, 6 and 12 month follow‐up visits and CDUS examinations were done in 181 (90.5%) patients in the CAA group and 174 (87%) in the EVLA group" Age ‐ mean years (SD): EVLA 38.4 (11.9) CA 38.6 (11.6) Sex ‐ F/M: EVLA 114/86 CA 109/91 No. bilateral limbs randomised: EVLA 4 CA 8 Inclusion criteria: aged 18‐75 years with symptomatic varicose veins; CEAP C2‐C5; GSV insufficiency 0.5 sec determined by CDUS; could come to follow‐up examinations and mentally healthy to approve Exclusion criteria: saphenous vein duplication or accessory saphenous vein with venous insufficiency; advanced tortuous GSV; saphenous vein under 3 mm and over 15 mm diameter, history of DVT; active thrombophlebitis in deep or superficial veins; arterial insufficiency history or ABPI < 0.9; significant femoral or popliteal vein insufficiency; history of saphenous vein intervention (surgical, thermal or chemical ablation) |
|
| Interventions |
Treatment(s): EVLA ‐ performed under mild sedation. 1470 nm radial tip laser inserted 2 cm below SFJ. TA administrated. EVLA catheter withdrawn at 2.08 cm ± 0.6 cm/sec, 15 W power applied with external pressure from the ultrasound probe. Elastic bandage applied for 1 to 4 days. Then compression stockings (20 ‐ 30 mmHg) for one month. Control: CA ‐ performed under mild sedation. GSV punctured, 0.035' guidewire placed, delivery catheter (CA delivery system (CADS)) inserted and placed 3 cm distal to SFJ, CA injected, delivery catheter pulled back at 2 cm/sec whilst compression with ultrasound probe applied. Injection/retraction process repeated until whole segment sealed. Elastic bandage (20‐30 mmHg) applied for one day following. Duration: follow‐up was at 1 day, week 1 and at 1, 3, 6, and 12 months |
|
| Outcomes |
Primary outcomes: it is not clear from the paper what the specific primary or secondary outcomes were. Calik states the aims of the study were to assess the safety and efficacy of the CA in GSV in comparison to EVLA, and to present both anatomic and clinical results of 12 months follow‐up. They have reported on occlusion rates, recanalisation rates, post‐procedural complications, pain scores procedural time, VCSS, quality of life measures via the CIVIQ and time to return to daily activity. Recurrence definition: reported on recanalisation but definition not given |
|
| Notes | Additional phlebectomies and treatment with UGFS allowed after 3 months Use of bilateral procedures which could impact upon outcome measures such as pain, quality of life and return to work |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Consecutive treatment methods were blindly assigned by using block randomisation. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of assessors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No consort diagram, losses to follow‐up not discussed, difficult to decipher how many participants were analysed at each of the time frames. |
| Selective reporting (reporting bias) | High risk | The specific outcomes of the study were not explicitly stated. |
| Other bias | High risk | No power calculation performed Use of bilateral procedures which could impact upon outcome measures such as pain, quality of life and return to work. No subanalysis of these participants performed. Definitions for occlusion, partial and total recanalisation never specified Use of the Wong‐Baker FACES pain scale, which is a paediatric pain assessment scale. |
Darwood 2008.
| Study characteristics | ||
| Methods |
Study design: prospective, RCT Country: UK Setting/Location: hospital Source of funding: Promed (Bluntisham, UK) ‐ sponsor had no input in study design, data collection, data analysis/interpretation or preparation of the manuscript Intention‐to‐treat analysis: no (one surgery participant had EVLT1 and was followed up in the laser cohort) |
|
| Participants |
No of participants randomised: total n = 118 participants (136 legs) (EVLT1 49 legs; EVLT2 42 legs; HL/S 45 legs) No of participants analysed: total n = 95 participants (114 legs) (EVLT1 42 legs; EVLT2 29 legs; HL/S 32 legs) Exclusions post‐randomisation: seven participants (11 legs) withdrew from the study as not happy with their treatment allocation. Six participants were treated outside the study interval and were also excluded. Losses to follow‐up: total n = 11 participants (EVLT1 5 legs; EVLT2 4 legs; HL/S 2 legs) Age ‐ median years (IQR): EVLT1: 42 (30.5 ‐ 54.5); EVLT2: 52 (35 ‐ 59); HL/S: 49 (38.5 ‐ 57.5) Sex ‐ F/M : EVLT1: 22/16; EVLT2: 16/11; HL/S: 16/14 No bilateral limbs randomised: EVLT1 9, EVLT2 6, HL/S 4 Inclusion criteria: > 18 years of age; symptomatic varicose veins and primary SFJ incompetence (confirmed on DUS) Exclusion criteria: on warfarin; unsuitable for EVLT (tortuous GSV, large incompetent anterior accessory saphenous vein) |
|
| Interventions |
Treatment(s): 2 EVLT techniques: EVLT1: 12 W power with 1s laser pulses and 1s intervals between pulses; laser fibre withdrawn 2 ‐ 3 mm during intervals EVLT2: 14 W continuous power and continuous laser withdrawal Both procedures performed with EVLT; Diomed, Andover, Massachusetts, USA Control: HL/S ‐ open surgery; SFJ ligation, GSV stripping to knee level and multiple phlebectomies of varicosities Duration: follow‐up at 1, 6, 12 weeks and 1 yr after treatment |
|
| Outcomes |
Primary outcomes: abolition of reflux in the treated segment of GSV and improvement in disease‐specific QoL 3 months after treatment Secondary outcomes: post‐procedure pain, time to return to normal activity and work, cosmesis, overall satisfaction at 3 months Recurrence definition: study authors state "This short‐term study was not designed to assess recurrence rates" |
|
| Notes | Participants with bilateral veins were randomised once and received the same treatment simultaneously on each leg. Study authors reported difficulty recruiting participants to the study. They did not meet the sample sizes for their study groups to make their desired power calculations. Statistical tests for equivalence were therefore not performed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation using sealed envelopes. Randomisation was stratified by consultant 'to allow for any minor variations in technique'. No clear details on how this stratification was achieved |
| Allocation concealment (selection bias) | Low risk | Used sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind investigators or participants. No blinding of participants |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data are balanced across the groups, with similar reasons given for the missing data |
| Selective reporting (reporting bias) | Low risk | The pre‐specified outcomes in the study protocol were reported in the pre‐specified way |
| Other bias | Unclear risk | Study authors reported difficulty recruiting participants to the study. They declared that their sample sizes were insufficient to permit statistical testing for equivalence One participant randomised to surgery underwent laser, and was followed up in the laser cohort showing no analysis of intention to treat Some participants received bilateral treatment. Study authors stated these participant would receive the same treatment on both limbs. These participants who received bilateral treatment were not stratified in the results Some outcome measures can be affected by bilateral treatment e.g. QoL, pain, time to return to work etc. therefore introducing a bias Participants who underwent laser did not have concomitant mini‐phlebectomies. This adds a potential confounding bias when analysing results of post‐operative pain, time to return to work etc. |
EVOLVeS 2003.
| Study characteristics | ||
| Methods |
Study design: multicentre, prospective, RCT Country: France, Austria and USA Setting/Location: hospitals Source of funding: VNUS medical technologies provided financial support for data collection, clinical monitors and disposable catheters (RFA) free of charge Intention‐to‐treat analysis: no |
|
| Participants |
No of participants randomised: total n = 85 participants (86 limbs); RFA n = 45 (46 limbs); HL/S n = 40 (40 limbs) No of participants analysed: at 72 hs, total 80 legs (RFA 44 legs; HL/S 36 legs) at 4 months, total n = 79 (77 legs) (RFA 43 legs; HL/S 34 legs) at 2 ys, total n = 65 (65 legs) (RFA 36 legs; HL/S 29 legs) Exclusions post‐randomisation: 3 participants refused surgery, 1 participant repeatedly DNA, 2 participants excluded from RFA due to inclusion criteria violation Losses to follow‐up: yes: (a) clinical examination: 2 surgery and 1 RFA no follow‐up at 4 months; (b) QoL questionnaires: surgery: 1 at 72 hrs, 4 at 4 months not completed. RFA: 1 at 72 hrs, 1 week, 3 weeks and 4 months not completed; at 1 yr 19 limbs in RFA and 16 limbs in HL/S were lost but at 2 yrs it improved with only 8 RFA and 7 HL/S losses Age ‐ mean years (SD): RFA 49 (4); HL/S 47 (4) Sex ‐ F/M: RFA 32/12; HL/S 26/10 No bilateral limbs randomised: RFA 1, HL/S 0 Inclusion criteria: reverse flow in GSV lasting > 0.5 s in standing position; age 21 ‐ 80; CEAP classification C2, C3, C4; ambulatory status; segmental deep reflux allowable; saphenous vein diameter ≤ 1.2 cm in supine position; availability for follow‐up visits (72 hrs, 1 week, 3 weeks, 4 months) Exclusion criteria: saphenous vein diameter > 1.2 cm or < 0.2 cm; duplication of saphenous trunk or incompetent accessory branch; SSV reflux; varices of the thigh; previous DVT; ABI < 0.9; axial deep venous reflux from groin through popliteal vein; tortuosity of GSV segment to be treated on basis of appearance and USS as unsuitable for catheterisation |
|
| Interventions |
Treatment(s): GSV obliteration with RFA without high ligation of SFJ: used the Closure catheter and system (VNUS Medical Technologies) Control: HL/S: vein stripping (from knee or upper calf to the SFJ) with high ligation of SFJ Duration: follow‐up was at 72 hrs, 1 week, 3 weeks, 4 months, 1 yr and 2 yrs |
|
| Outcomes |
Primary outcomes: it is not clear from the paper what the specific primary or secondary outcomes were. EVOLVeS was designed to compare procedure‐related complications, participant recuperation and QoL outcomes Secondary outcomes: although it was not initially declared, the EVOLVeS trials later presented rates of neovascularisation in the groin and recurrence at 2 yrs Recurrence definition: new varicose veins below the knee |
|
| Notes | Two investigators audited the study's raw data handling and storage methods, data processing accuracy, and presentation of specific results. They reported all was in order and that the raw data reflected the results accurately. This was done at 4 months and 2 yrs post‐data collection | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Randomisation was allocated via Internet' ‐ no further details were given |
| Allocation concealment (selection bias) | Low risk | Allocation performed via the Internet |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants or operators. No blinding of participants |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of assessors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Details were provided on all missing outcome data; however, it led to an imbalance in the study treatment group There is also discrepancy with the missing outcome data and explanations of these missing data compared to the published two‐year follow‐up |
| Selective reporting (reporting bias) | Low risk | The pre‐specified outcomes in the study protocol were reported in the pre‐specified way |
| Other bias | Unclear risk | The RFA treatment cohort included one participant who underwent treatment of both limbs with a 3‐month gap between treatments. The participant was only randomised once and each limb treated as a separate episode. All centres were established centres in the use of RFA and the company funded the research. No subjective data were reported. However, as in all of these studies, surgical technique and ultrasonographic results are operator‐dependent. |
Flessenkämper 2013.
| Study characteristics | ||
| Methods |
Study design: multicentre, prospective, RCT Country: Germany Setting/Location: 1) Centre for Vascular Medicine, Helios Klinikum Emil von Behring, Berlin 2) Centre for Venous Diseases, Frieburg 3) Centre for Venous Diseases, Saarlois Source of funding: sponsored by Deutschen Gessellschaft für Phlebologie (DGP) Intention‐to‐treat analysis: not indicated |
|
| Participants |
No of participants randomised: total n = 449 (EVLT n = 142; EVLT+HL n = 148; HL/S n = 159). Details of the EVLT+HL group are reported here but were not used in this review. No of participants analysed: 100% at 2 months; 86% at 6 months; total n = 385 (EVLT n = 127; EVLT+HL n = 133; HL/S n = 128) Exclusions post‐randomisation: not indicated Losses to follow‐up: at 6 months EVLT n = 15; EVLT+HL n = 15; HL/S n = 39 Age ‐ mean years (SD): EVLT 47.4 (12.9); EVLT+HL 48.7 (12.0); HL/S 47.7 (11.5) Sex ‐ M/F: EVLT 45/97; EVLT+HL 37/111; HL/S 47/112 Inclusion criteria: people between 18 and 72 years old with clinical signs or symptoms of superficial venous insufficiency with proven reflux into GSV, with a life expectancy of more than 5 years; all people suitable for open and endoluminal therapy with diameter of GSV not exceeding 16 mm at a point 5 cm distal to the SFJ Exclusion criteria: previous surgery of the GSV was the only reported exclusion criteria |
|
| Interventions |
Treatment(s): EVLT: laser therapy with a 980 nm diode laser, used local tumescent and general anaesthesia EVLT with high ligation (EVLT+HL): EVLT performed combined with HL, under general anaesthesia. Both EVLT procedures performed with instruments from Biolitec Jena, Germany (30 W) Control: HL/S: resection of all branches down to the dorsal level of the femoral vein; under general anaesthesia Duration: follow‐up at 2, 6, 12 and 24 months for re‐examination, then followed participants as long as possible |
|
| Outcomes |
Primary outcomes: inguinal venous reflux after 2 yrs Secondary outcomes: peri‐operative technical success rate, rate of hyperpigmentation and matting, neurological compilations, duration of compression therapy and lymphoedema, complications, post‐operative ecchymosis, pain (visual analogue scale 1 ‐10) or discomfort, duration of disability, participant satisfaction, clinical severity (CEAP, VCSS, Hach classification, VDS) Recurrence definition: any reflux more than 0.5 s from the SFJ into the GSV, which was assessed by physicians by DUS ultrasound at 2‐yr follow‐up |
|
| Notes | May 2005 to July 2009 Reflux was defined as retrograde flow of > 0.5 s duration after Valsalva manoeuvre or manual compression and decompression of distal vein Nearly all participants were treated as inpatients All 3 groups had simultaneous mini‐phlebectomies, as required |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used lottery ticket box at central office and telephone randomisation |
| Allocation concealment (selection bias) | Low risk | Used central office and telephone randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of assessor. "Because of the scars, blinding for the follow‐up was not possible" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts reported but no reasons given |
| Selective reporting (reporting bias) | Low risk | All outcomes reported on |
| Other bias | Unclear risk | Possibly underpowered; power calculation described a need for 469 participants, but only 449 were randomised Number of participants needing additional phlebectomies was not recorded, which could affect post‐operative pain, QoL, etc. |
FOAM 2010.
| Study characteristics | ||
| Methods |
Study design: multicentre, prospective RCT Country: the Netherlands Setting/Location: hospital outpatient dermatology and surgery departments (n = 3) Source of funding: the Netherlands Organization for Health Research and Development (ZonMw); sponsor had no input in study design, data collection, data analysis/interpretation or preparation of the manuscript Intention‐to‐treat analysis: no: "Only patients who underwent the allocated intervention were included in the analysis" |
|
| Participants |
No of participants randomised: total n = 460 (UGFS n = 233; HL/S n = 227) No of participants analysed: total n = 390 (UGFS n = 213; HL/S n = 177) Exclusions post‐randomisation: UGFS n = 3; HL/S n = 27 Losses to follow‐up: UGFS n = 17; HL/S n = 23 Age ‐ mean years (SD): UGFS 55.8 (13.4); HL/S 54.6 (13.4) Sex (F/M): UGFS 175/58; HL/S 162/65 Inclusion criteria: people with primary GSV incompetence, presence of one or more venous symptoms in combination with incompetence of the SFJ and GSV; reflux time of more than 0.5 s; normal deep venous system on DUS imaging Exclusion criteria: people with an incompetent deep venous system; sign of a previous DVT on DUS imaging; an active ulcer; contraindication to the use of polidocanol |
|
| Interventions |
Treatment(s): UGFS: sclerosing foam was prepared with the double‐syringe technique, applying a 1:4 ratio of sclerosant:air; the treatment was considered successful when the proximal GSV was completely filled with foam and maximal venospasm was achieved Control: HL/S: performed as day‐case procedure under general or spinal anaesthesia; SFJ was ligated and the GSV divided and stripped to just below the knee Duration: follow‐up at 3 months, 1 and 2 yrs. An eight year follow‐up was performed but this was not in the original study protocol |
|
| Outcomes |
Primary outcomes: recurrence Secondary outcomes: recurrent reflux (irrespective of symptoms), reduction of symptoms, QoL (EQ‐5D), adverse events, direct hospital costs, participant satisfaction Recurrence definition: defined as reflux longer than 0.5 s by DUS, combined with the presence of one or more venous symptoms |
|
| Notes | October 2005 to December 2007 Phlebectomies: UGFS ‐ as needed; HL/S ‐ at discretion of the surgeon (UGFS n = 26; HL/S n = 87) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "… assigned randomly to UGFS or surgery using a computer‐generated randomisation scheme with random permuted blocks of eight" |
| Allocation concealment (selection bias) | Low risk | Used computer‐generated randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | In initial study (two‐year follow‐up), there was no blinding of the outcome assessors. At the subsequent eight‐year follow‐up, the vascular technicians performing the DUS examinations were blinded to previous treatments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts and reasons were thoroughly reported |
| Selective reporting (reporting bias) | Low risk | All outcomes reported on |
| Other bias | Unclear risk | Mini‐phlebectomies were performed at the operating surgeon's discretion and 26 people in the UGFS group received phlebectomies compared to 7 in the surgery treatment group; this could alter the pain and other outcomes. As seen in the commentary letter from MJ Gough at the end of the British Journal of Surgery publication (Gough 2012), there are concerns with the high incidence of recurrence in the surgery treatment group and the definition of recurrence solely as reflux; caution should be taken when interpreting data from this study. |
Helmy ElKaffas 2011.
| Study characteristics | ||
| Methods |
Study design: prospective, RCT Country: Egypt Setting/Location: not indicated Source of funding: not indicated Intention‐to‐treat analysis: not indicated |
|
| Participants |
No of participants randomised: total n = 180 (RFA n = 90; HL/S n = 90) No of participants analysed: at 24 months: total n = 162 (RFA n = 81; HL/S n = 81) Exclusions post‐randomisation: it appears 2 were excluded from the RFA group, but no explanation; none excluded from HL/S Losses to follow‐up: RFA n = 7; HL/S n = 9 Age ‐ mean years (SD): RFA 33.1 (2.6); HL/S 34.9 (3.7) Sex ‐ M/F: RFA 42/48; HL/S 45/45 Inclusion criteria: people with SFJ and great saphenous reflux on DUS, either in response to Valsalva manoeuvre or with standing manual compression and release Exclusion criteria: people with DVT or superficial venous thrombosis; people on anticoagulants; those with concomitant PAD, pacemakers or serious systemic disease; pregnant women; people with GSV lumen more than 18 mm in the thigh or extremely tortuous veins |
|
| Interventions |
Treatment(s): UGFS: RFA Closure system, using local (tumescent) anaesthesia, managed as day patients; ClosureSystem VNUS Medical Technologies Inc Control: standard surgical treatment (HL/S): saphenofemoral high ligation and great saphenous stripping at ankle (in 40 participants) and at knee level (in 50 participants), using general anaesthesia, managed as inpatients Duration: followed up after 1 week, 1 month, 6‐month intervals for 24 months |
|
| Outcomes |
Primary outcomes: operative time, hospital stay, costs, short‐term and mid‐term complications, recurrence Recurrence definition: not provided |
|
| Notes | Conducted between May 2006 and January 2009 No information was given on the inclusion or exclusion of participants with bilateral treatment; study authors have been contacted, but no response received Adjuvant stab phlebectomies were performed in n = 15 participants in RFA and n = 39 in the surgical group; all phlebectomies took place at the primary intervention. In addition, n = 24 participants required foam sclerotherapy for persistent veins following RFA; n = 0 required foam following HL/S. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of sequence generation methods |
| Allocation concealment (selection bias) | High risk | Allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding or participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts were reported and similar between groups, although reasons were not given |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported on |
| Other bias | Unclear risk | Two operators performed RFA and just one performed HL/S; this could have led to enhanced outcomes; no indication was given about whether bilaterally treated participants were included or excluded, and how many between groups (study authors were contacted). |
HELP‐1 2011.
| Study characteristics | ||
| Methods |
Study design: single centre, prospective, RCT Country: UK Setting/Location: tertiary referral vascular surgical department Source of funding: internal university funding; Diomed/Angiodynamics provided 50% of a research nurse's salary over 12 months; sponsor had no input in study design, data collection, data analysis/interpretation or preparation of the manuscript Intention‐to‐treat analysis: yes |
|
| Participants |
No of participants randomised: total n = 280 (EVLT n = 140; HL/S n = 140) No of participants analysed: total n = 237 (EVLT n = 124; HL/S n = 113) Exclusions post‐randomisation: EVLT n = 1; HL/S n = 3 Losses to follow‐up: EVLT n = 15; HL/S n = 24 Age ‐ mean years (SD): EVLT 49 (14); HL/S 49 (13) Sex ‐ F/M: EVLT 85/54; HL/S 90/47 Inclusion criteria: primary, symptomatic unilateral varicose veins with isolated SFJ incompetence, leading to reflux into the GSV; incompetence was defined as reflux of at least 1 s on spectral Doppler analysis; both surgeon and participant had to occupy position of equipoise for either procedure Exclusion criteria: previous treatment for ipsilateral varicose veins; deep venous incompetence or obstruction; age less than 18 years; pregnancy; impalpable foot pulses; inability to give informed consent |
|
| Interventions |
Treatment(s): EVLT (810 nm, bare‐tipped): performed under local tumescent anaesthesia within an outpatient department; GSV was cannulated percutaneously; cannulation was performed laterally at the lowest point of demonstrable reflux; catheter positioned at the SFJ, aiming for a flush occlusion; bar‐tipped 600 nm laser fibre was introduced and delivered energy using an 810 nm diode laser generator set to 14 W; Diomed/Angiodynamics, Cambridge UK Control: HL/S: all participants received general anaesthesia; flush SFJ ligation followed by ligation of all tributaries to second branch; inversion stripping of the GSV to the knee Duration: assessed at 1 week, 6 weeks, 3 months, 1 yr and 5 yrs. Have ethical approval up to 10‐year time point |
|
| Outcomes |
Primary outcomes: QoL (UK SF‐36 V1); recurrence Secondary outcomes: QoL (EQ‐5D), AVVQ, severity of venous disease by CEAP and VCSS, post‐operative pain scores (0 ‐ 10 VAS scale), time to return to normal activity and work, participant satisfaction (0 ‐ 10 scale) Recurrence definition: clinically evident varicose veins at least 3 mm in diameter and not present at 1 week or 6 weeks |
|
| Notes | September 2004 to March 2009 Concomitant phlebectomies were performed via stab incisions (EVLT n = 7; HL/S n = 10) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomised equally into two groups by means of sealed opaque envelopes, receiving either surgery or EVLA. Patients selected their own envelope in the clinic under the supervision of a research nurse". Does not adequately describe sequence generation |
| Allocation concealment (selection bias) | Low risk | Used sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts and reasons were thoroughly reported |
| Selective reporting (reporting bias) | Low risk | All outcomes reported on |
| Other bias | Unclear risk | Possibly underpowered; power calculation described a need for 120 participants in each group, but only 113 were available for follow‐up in the surgery group |
Lane 2017.
| Study characteristics | ||
| Methods |
Study design: multicentre, randomised control trial Country: UK Setting/Location: Charing Cross Hospital (Imperial College Healthcare NHS Trust) and Northwick Park Hospital (London North West Healthcare NHS Trust) in London, UK Source of funding: the study author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: "This study was supported by a research grant from the Clarivein device manufacturer, Vascular Insights and an educational research grant from the Graham‐Dixon Charitable Trust. Vascular Insights provided funding for Clarivein devices, patient follow‐up and DUS. Case funding was not used in this study. All trial particulars (design, data collection, analysis, discussion and data access) were performed independently of the funding bodies and the trial’s research sponsor was Imperial College London." Intention‐to‐treat analysis: yes |
|
| Participants |
No of participants randomised: 170 No of participants analysed (n, %): 1 month follow‐up 69 MOCA and 60 RFA (129, 79%), 6 months 121, 71% Exclusions post‐randomisation: 0 Losses to follow‐up (n, %): 1 month follow‐up 69 MOCA and 60 RFA (129, 79%), 6 months 62 MOCA and 59 RFA (121, 71%) Age ‐ median: 50 overall; MOCA 54.5, RFA 58 Sex ‐ percent female: MOCA 57.5%, RFA 60.2% Inclusion criteria: people with symptomatic primary varicose veins with either great saphenous vein (GSV) incompetence (> 0.5 s reflux on colour DUS) Exclusion criteria: people with recurrent varicose veins, current deep vein thrombosis, arterial disease (ankle brachial pressure index < 0.8), veins < 3 mm in diameter or hypercoagulability were excluded from participation. Additionally, people unable or unwilling to complete questionnaires or to participate were also excluded. |
|
| Interventions |
Treatment(s): MOCA (Clarivein, Vascular Insights, USA), DUS guided cannulation. MOCA chemical‐ablative catheter. Control: RFA Closure system, using local (tumescent) anaesthesia, managed as day patients; ClosureSystem VNUS Medical Technologies Inc Duration: assessed at 1 month and 6 months. |
|
| Outcomes |
Primary outcomes: degree of pain experience during endovenous ablation using a validated patient‐reported VAS and 0–10 number scale, prior to completion of any phlebectomies Secondary outcomes: improvement in patient‐reported quality of life, both disease specific (Aberdeen Varicose Vein Questionnaire – AVVQ) and generic (EuroQol 5 Domain 3 Level – EQ‐5D‐3L and EuroQol VAS), clinical scores (Venous Clinical Severity Score – VCSS, Venous Disability Score – VDS and Clinical Etiology Anatomy Pathology score) and time taken to return to normal activities and work Recurrence definition: clinically‐evident varicose veins at least 3 mm in diameter and not present at 1 month. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Consenting participants were then randomised on the day of treatment to either MOCA (group one) or RFA (group two), using an online computerised randomisation software (SealedEnvelope, London, UK)" |
| Allocation concealment (selection bias) | Low risk | Methods of allocation concealment adequately described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Given the nature of the interventions, blinding of the participant to the intervention allocated would be impossible. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "blinded venous duplex ultrasound scanning" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts were reported and similar between groups, although reasons were not given |
| Selective reporting (reporting bias) | Low risk | All outcomes reported on |
| Other bias | Low risk | No other potential risk detected |
LAST 2014.
| Study characteristics | ||
| Methods |
Study design: prospective, multicentre, RCT Country: the Netherlands Setting/Location: Department of Dermatology, Erasmus MC University Medical Centre Source of funding: Erasmus Medical Centre Intention‐to‐treat analysis: no, per‐protocol analysis |
|
| Participants |
No of participants randomised: 237 legs (in 217 participants); EVLA n = 119/EVSA n = 118 No of participants analysed: EVLA = 110/EVSA = 117 Exclusions post‐randomisation: 5 technical failures: 4 EVLA 1 EVSA, 5 EVLA withdrew after allocation Losses to follow‐up: EVLA: 2 weeks = 1 (n = 109); 12 weeks = 6 (n = 104); 1 year = 18 (n = 92) EVSA: 2 weeks = 0 (n = 117); 12 weeks = 3 (n = 114); 1 year = 10 (n = 107) Age ‐ mean years (SD): EVLA 55 (12); EVSA 56 (13) Sex (legs) ‐ F/M: EVLA 62/48; EVSA 76/41 Inclusion criteria: > 18 years, informed consent and symptomatic primary incompetence of the GSV with reflux time exceeding 0.5 s and diameter 5 mm or more (at mid‐thigh level) according to DUS examination Exclusion criteria: acute DVT or superficial vein thrombosis, agenesis of the deep venous system, vascular malformation or syndrome, PTS of the obstruction type, pregnancy, immobility, allergy to lidocaine and arterial insufficiency (ABI < 0.9) |
|
| Interventions |
Treatment(s): EVLA: tumescent anaesthesia, 940 nm diode laser using a bare fibre, a power setting of 12 W, delivering approximately 60 J/cm; medical elastic compression stockings for 1 week and to mobilise immediately Control: EVSA: tumescent anaesthesia, Steam Vein Sclerosis (SVS) system (cermaVEIN, Archamps, France). "For the first 36 procedures the treatment protocol was to apply 1 pulse/cm in veins smaller than 7 mm, 2 pulses/cm in veins of 7–10 mm, and 3 pulses/cm in veins larger than 10 mm. With insight and after temperature experiments, this was increased to 2, 3 and 4 pulses/cm respectively during the study." Duration: 2, 12 and 52 weeks post‐intervention |
|
| Outcomes |
Primary outcomes: treatment success "Obliteration of the GSV and/or absence of reflux (more than 0.5 s of retrograde flow) along the treated segment of the GSV, according to DUS", VCSS Secondary outcomes: pain (VAS and 0 ‐ 10 duration of painkiller use (ds)), satisfaction, convalescence, complications, changes in Health‐related QoL (AVVQ), EQ‐5D |
|
| Notes | November 2009 to 2011 Limbs not participants. "The legs of patients with bilateral GSV incompetence were included separately, provided that there was at least 3 months between the two treatments" "When needed, tributaries were treated with phlebectomies at least 3 months after EVLA or EVSA" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Consenting patients were randomised to either EVLA or EVSA, using a computerized randomisation list" |
| Allocation concealment (selection bias) | Low risk | Used a computerised randomisation list |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts reported, no reasons given |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Unclear risk | Possibly underpowered; needs 116 per study group – with dropouts had 92 and 107 in EVLA and EVSA, respectively Changed protocol for EVSA during study – increased amount of energy |
Magna 2013.
| Study characteristics | ||
| Methods |
Study design: sIngle‐centre*, prospective, RCT (* second centre was added in May 2009 due to slow inclusion rate) Country: the Netherlands Setting/Location: Departments of Dermatology and Vascular Surgery in two hospitals Source of funding: Erasmus Medical Centre listed under Sponsors and Collaborators Intention‐to‐treat analysis: yes |
|
| Participants |
No of participants randomised: total n = 240 legs (EVLT n = 80 legs; UGFS n = 80 legs; HL/S n = 80 legs) No of participants analysed: total n = 223 legs (EVLT n = 78 legs; UGFS n = 77 legs; HL/S n = 68 legs) Exclusions post‐randomisation: not indicated Losses to follow‐up: total n = 1 (EVLT n = 0; UGFS n = 1; HL/S n = 0) Age ‐ mean years (SD): EVLT 49 (15.03); UGFS 56 (13.30); HL/S 52 (15.59) Sex ‐ M/F: EVLT 24/54; UGFS 25/52; HL/S 22/46 No bilateral limbs randomised: EVLT 16, UGFS 19, HL/S 17 Inclusion criteria: adults with symptomatic primary incompetent GSV at least above the knee with a diameter ≥ 0.5 cm; with an incompetent SFJ (incompetence defined as reflux ≥ 0.5 s at colour DUS Exclusion criteria: previous treatment of the ipsilateral GSV; deep venous incompetence or obstruction; agenesis of the deep system; vascular malformations; use of anticoagulant; pregnancy; heart failure; contraindication for one of the treatments; immobility; arterial insufficiency; age under 18 yrs; inability to provide written informed consent |
|
| Interventions |
Treatment(s): EVLT (940 nm diode laser): performed under UG tumescent anaesthetic UGFS: prepared foam made with 1 cc aethoxysclerol 3%, 3 cc air; if considered necessary procedure could be repeated after 3 months; no manufacturer information given Control: HL/S: high ligation with short (above knee) stripping; performed under spinal or general anaesthesia Duration: evaluated at 3 months, 12 months and 5 yrs |
|
| Outcomes |
Primary outcomes: anatomic success according to DUS, neovascularisation Secondary outcomes: C of the CEAP classification; type and frequency of complications; QoL (CIVIQ and EuroQol‐5D) Recurrence definition: for the UGFS and EVLT groups ‐ flow or reflux of the GSV at midthigh; for surgery ‐ presence of the GSV in the saphenous compartment at thigh level (both groups evaluated by clinical examination and DUS) |
|
| Notes | January 2007 to May 2010 Intention for additional phlebectomies was to perform during initial treatment, but in several cases were performed after 3 months (during initial treatment: EVLT n = 15; UGFS n = 0; HL/S n = 18; after 3 months: EVLT n = 12; UGFS n = 15; HL/S n = 11) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...randomised using a computerized list by an independent research nurse." |
| Allocation concealment (selection bias) | Low risk | "...randomised using a computerized list by an independent research nurse." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No indication of blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No indication of blinding of assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts and reasons were thoroughly reported |
| Selective reporting (reporting bias) | Unclear risk | For complications, study authors stated they would report on migraine, skin burns, skin necrosis, and anaphylactic shock. No data were presented for these outcomes. |
| Other bias | Unclear risk | Possibly underpowered; power calculation described a need for 240 participants, but only 223 were analysed. Also unclear how QoL was evaluated for bilaterally treated participants ‐ study authors do not clarify |
MARADONA 2019.
| Study characteristics | ||
| Methods |
Study design: multicentre randomised control trial (single‐blinded) Country: the Netherlands Setting/Location: Department of surgery, Rijinstate Arnhem; Department of surgery, OLVG; Department of surgery, BovenIJ Hospital Amsterdam; Department of vascular surgery, St Antonius Hospital Nieuwegein; and the division of vascular surgery, UMCG, Groningen Source of funding: ‘investigator‐initiated study supported by Vascular Insights Ltd’ Intention‐to‐treat analysis: yes |
|
| Participants |
No of participants randomised: n = 213 (using an online randomisation module with block randomisation per site); MOCA n = 107; RFA n =106 No of participants analysed: MOCA 1 yr analysed ITT n = 101 PP n = 99, 2 yr ITT n = 95, PP n = 93 RFA = 1 yr analysed ITT n = 99, PP n = 99, 2 yr ITT n = 97, PP n = 97 Exclusions post‐randomisation: MOCA n = 5, RFA n = 3 Losses to follow‐up: MOCA 1 yr n = 20, 2 yr n = 19; RFA 1 yr n = 32. 2 yr n = 16 Age ‐ median years (IQR): MOCA 54.9 (16.3 ‐ 81.2); RFA 53.4 (22.6 – 77.9) Sex percent female: MOCA 62.4; RFA 59.3 Inclusion criteria: GSV incompetence (> 3 mm and < 12 mm) with CEAP C2 to C5 Exclusion criteria: active ulcer, previous surgery or treatment of ipsilateral GSV, use of oral anticoagulants, pregnancy or lactation, previous DVT, immobilisation, contraindication or known allergy to sclerosant, coagulation disorders or increased risk of thromboembolism, severe renal or liver insufficiency and severe peripheral artery disease. |
|
| Interventions |
Treatment(s): MOCA USS guidance, Clarivein tip placed 5 mm below orifice of superficial epigastric vein or 2 cm below the SFJ. Wire activated for 10 sec, device withdrawn at speed of 7 s/cm while liquid sclerosant continuously injected using 2 mL of 3% polidocanol for first 10 cm ‐ 15 cm and 1.5% for remainder. Control: RFA, Closure fast device positioned as above, TA (500 mL of NaCl including 20 mL of 8.4% sodium bicarbonate and 50 mL of lidocaine 1% with epinephrine 1:200,000 injected along entire segment) every 20 sec 7 cm segment of GSV treated after pullback. Most proximal segment treated twice. Both groups had compression stockings continuously for first 24 hr then daily for first 2 weeks Duration: 30 days (± 7 days), 1 yr (± 1 month), 2 yrs (± 2 months) |
|
| Outcomes |
Primary outcomes: post‐procedural pain evaluated using 100‐point visual analogue scale two weeks post procedure. Anatomic success at one year. Secondary outcomes: anatomic success, clinical success using VCSS, 30‐day morbidity, disease‐specific quality of life (AVVQ), general health‐related (HR) QoL (SF‐36), time to return to daily activities/work, re‐intervention rate and additional varicose vein treatment during 2‐year follow‐up. Recurrence definition: recanalisation (failure of treatment) which could be complete or partial (> 10 cm) Success definitions: initial success of the procedure (i.e. catheter placed at defined location and GSV treated without technical problems). Anatomic success was occlusion of the treated GSV segment, objective by DUS. Failure of treatment is recanalisation which could be complete or partial (> 10 cm). Clinical success was defined as improvement in the VCSS of > 1 point. Duration: two years |
|
| Notes |
Phlebectomies: ‘No concomitant phlebectomy or sclerotherapy was scheduled to be performed unless indicated by the treating physician’. In the MOCA group. 1 participant had phlebectomies, none did in the RFA arm Notes: Only managed to randomise 46.3% of intended. Reimbursement of MOCA was suspended and enrolment was stopped at the end of 2014; this was not reinstated for over a year. Trial was therefore advised by the ethics committee to terminate the study. In both groups, 6 participants had adjunctive therapies; however, these were reported and sub‐analysis done (median pain score similar). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation performed using online randomisation module with block randomisation per site |
| Allocation concealment (selection bias) | High risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | DUS was performed by vascular technicians who were blinded for treatment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts recorded, no reasons provided; reported as ‘unknown why’ |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Unclear risk | Sample size calculation to assess anatomical and clinical success rate at one year showed the need for 230 participants in each arm (accounting for a 10% dropout rate). Study only managed to recruit 46% of this number. |
Morrison 2015.
| Study characteristics | ||
| Methods |
Study design: prospective, multicentre RCT for incompetent GSV (Veclose)
Country: USA
Setting/Location: 10 vein, dermatology and vascular clinics
Source of funding: sponsored by Sapheon. Multiple authors work as consultants for Sapheon Intention‐to‐treat analysis: yes |
|
| Participants |
No of participants randomised total: n = 122; CA = 108; RFA = 114
No of participants analysed: CA = 108; RFA = 114
Exclusions post‐randomisation: CA: 2 withdrawn; RFA: 4 withdrawn
Losses to follow‐up: CA = 2; RFA = 6
Intention‐to‐treat analysis: yes
Age years (range): CA 49.0 (26.6 ‐ 70.6); RFA 50.5 (25.6 ‐ 70.1)
Sex F/M: CA 83/25; RFA 93/21 No bilateral limbs randomised: 0 Inclusion criteria: adults 21 ‐ 70, symptomatic moderate to severe varicosities (CEAP C2 to C4B), incompetent GSV (reflux at least 0.5 s), ability to walk unassisted, able to attend follow‐up, provide informed consent Exclusion criteria: haemodynamically significant reflux of SSV or anterior accessory vein, prior treatment of GSV, prior treatment of target GSV, symptomatic PAD (ABI < 0.89), history of DVT, PE, aneurysm of target GSV > 12 mm, life expectancy 1 yr, malignant disease, anticoagulation, known hypercoagulable states, pregnancy, peoples who require bilateral treatment within 3 months, people who require further ipsilateral treatment within 3 months |
|
| Interventions | Treatment description: "Catheter inserted under high resolution US guidance to 5 cm below SFJ. Proximal GSV ultrasound compression applied. 2 injections of 0.10 mL CA given 1 cm apart, with 3 min compression. This is repeated along length of the vein with 30 s of compression in between. Patients wore compression stockings for 3 ds following. Performed in an outpatient clinic". Control description: "Closure fast system used. Perivenous tumescent anaesthesia used double cycles of RF. Performed in outpatient clinics and compression stockings worn for 3 days following." Duration: day 3, 1 month, 3 months, 12 months, 24 months and 36 months | |
| Outcomes | Primary outcomes: complete closure at target GSV on DUS at 3 months (closure of entire length with no discrete segments of patency > 5 cm) (day 3 and 1 month also performed) Secondary outcomes: pain during procedure rated 0 ‐ 10 on numerical scale, number of analgesia taken in the 24 hrs prior to day 3 review, investigator‐rated ecchymosis at day 3, changes in VCSS, AVVQ, EQ‐5D ‐ baseline, day 3, 1 month and 3 months | |
| Notes | No additional phlebectomies | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised 1:1 to CA or RFA. Stratified by study site. Random block 4 or 6. Assignments were given by an interactive voice response system linked to web bound database |
| Allocation concealment (selection bias) | Low risk | Randomised 1:1 to CA or RFA. Stratified by study site. Random block 4 or 6. Assignments were given by an interactive voice response system linked to web bound database |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of assessors: "could not blind assessors due to characteristic appearances of CA, to reduce bias both groups wore stockings" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts reported |
| Selective reporting (reporting bias) | Unclear risk | Study authors indicated they would report on analgesia use for pain; this was not reported in the results |
| Other bias | Unclear risk | "...there were 31 missing or uninterruptible USS reports at 3/12 (14%)" Primary end point analysed under various models for imputing missing data were performed Study authors work for Sapheon |
Nordon 2011.
| Study characteristics | ||
| Methods |
Study design: prospective, double‐blind RCT
Country: UK Setting/Location: a National Health Service vascular unit Source of funding: St George's, University of London Charitable Trust (UK) Intention‐to‐treat analysis: yes |
|
| Participants | No of participants randomised: 159 randomised No of participants analysed: EVLA = 78; RFA = 79 Exclusions post‐randomisation: 2 EVLA failed cannulation Losses to follow‐up: EVLA = 10; RFA = 9 Age years mean (SD): EVLA 46.7 (± 14.4); RFA 46.9 (± 15.1) Sex ‐ F/M: EVLA 54/26; RFA 45/34 Inclusion criteria: 18 – 80 yrs, primary varicose veins, GSV territory, symptomatic varicose veins and able to attend follow‐up Exclusion criteria: unable to provide consent, pregnancy, age < 18 or > 80 yrs, tortuosity of GSV not amenable to endovenous treatment, recurrent varicose veins, recent DVT/PE, on anticoagulants, intolerance of nonsteroidal anti‐inflammatory drugs, SSV reflux, deep venous reflux, people with bilateral symptomatic varicose veins and people not fit for day case were excluded | |
| Interventions |
Treatment description: EVLA : performed under general anaesthesia. Vari‐Lase Bright tip laser fibre (810 nm diode laser, EVLT, Pyramed, Vascular Solutions). The fibre was withdrawn continuously at 2 mm/s (12 W power) with a target 80 J/cm energy. Standard tumescent anaesthesia used. Full‐length compression wool (Formflex, Natural, Lantor, UK) and crepe bandage (Multi‐crepe, Frontier Multigate, UK) applied post‐operatively then exchanged for thigh‐length compression hosiery after 24 hrs, worn for a minimum of 2 weeks The median energy delivery in the EVLT group was 77.1 J/cm Phlebectomy hooks were used for simultaneous avulsion of infragenicular varicosities that had been marked before surgery Control description: RFA: performed under general and tumescent anaesthesia VNUSClosureFAST (Covidien, USA) segmental RFA. Double treatment of the most proximal segment was performed. External compression of the treated segment was applied with target power less than 20 W at 120 ◦C. Bandaging and compression stocking applied as above. Duration: 1 week and 3 months |
|
| Outcomes | Primary outcomes: GSV occlusion at 7 days and GSV occlusion at 3 months, DUS was performed to confirm GSV occlusion. Disease recurrence/treatment failure was defined as a 5 cm segment of GSV with reflux, identified on DUS. Performed by blinded physician. Secondary outcomes: post‐operative pain – pain diary visual analogue pain chart (0 – 100) daily (validated) and the number of analgesics taken at 1 week. Percentage area of bruising based on 2 views. Complications at 1 week and QoL score pre‐operatively (AVVQ and EQ‐ 5D) and 3 months. Return to work assessed at 3 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was performed on the day of surgery. Patients were randomised to receive either laser therapy or RFA" Envelopes were ordered using binary random number tables. |
| Allocation concealment (selection bias) | Low risk | By sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were blinded to the endovenous treatment received |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Physician performing post‐procedural DUS blinded until after scan at 3 months |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts recorded, no reasons provided |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Unclear risk | At 80% power (α‐error 5%, β‐error 20%), 138 participants were required. Assuming a 10% dropout rate, a minimum of 152 participants were to be recruited In contrast to other studies, Nordon 2011 performed all interventions under general anaesthesia in addition to tumescent anaesthesia |
Pronk 2010.
| Study characteristics | ||
| Methods |
Study design: single‐centre, prospective, non‐blinded, RCT Country: the Netherlands Setting/Location: outpatient clinic specialising in venous disease Source of funding: in article as 'None' Intention‐to‐treat analysis: unclear ‐ but most likely as analyses 68 in the HL/S group, although two were lost to follow‐up |
|
| Participants |
No of participants randomised: total n = 122; legs = 130 (EVLT legs = 62; HL/S legs = 68) No of participants analysed: total n = 122; legs = 130 (EVLT legs = 62; HL/S legs = 68) Exclusions post‐randomisation: not indicated Losses to follow‐up: total = 9 (EVLT n = 1; HL/S n = 8) Age ‐ mean years (SD): EVLT 49 (11.0); HL/S 50 (10.5) Sex ‐ M/F: EVLT 16/46; HL/S 15/53 No bilateral limbs randomised: EVLT 8, HL/S 0 Inclusion criteria: age > 18 years at randomisation,CEAP classification C2, GSV and SFJ incompetence defined as reflux > 0.5 s seen on DUS imaging with an intrafascial length of at least 15 cm measured from the SFJ downwards, and GSV diameter between 0.3 and 1.5 cm Exclusion criteria: previous surgical treatment of the GSV; intrafascial GSV reflux length ≤ 15 cm measure from SFJ downwards; GSV diameter ≤ 0.3 or ≥ 1.5 cm; pregnancy; immobility; intolerance of lidocaine; active superficial phlebitis; previous or active DVT; deep venous insufficiency |
|
| Interventions |
Treatment(s): EVLT (980 nm diode laser; Biolitec): DUS‐guided; perivenous tumescent anaesthesia under ultrasonographic guidance Control: HL/S of the GSV; perivenous tumescent anaesthesia; ligation of GSV followed by ligation of all tributaries then stripping by PIN‐stripper through small incision just below or above the knee Duration: followed up at 1 week and 6 weeks, 6 months, 1 yr, 3 yrs and 5 yrs |
|
| Outcomes |
Primary outcomes: recurrent varicose veins in follow‐up of 10 yrs (current publication only focuses on 1 yr results) Secondary outcomes: QoL (EQ‐5D), post‐operative pain (visual analogue scale from 0 ‐ 10) and complications Recurrence definition: visible, palpable varicosities in the area of the treated GSV, classified as CEAP greater than or equal to C2; after surgery a new refluxing vein less than 3 mm and clinically visible was also considered recurrent; after EVLT a recurrent varicose vein on DUS was defined as the ability to compress the GSV, or as reflux > 0.5 s in a vein originating in the groin and connected with the femoral vein |
|
| Notes | June 2007 to December 2008 "Patients with bilateral GSV incompetence were randomised only once" "Directly after SFL/S and EVLA treatment, sclerotherapy (Aethoxysclerol 0.5 ‐ 3.0%, Kreussler) of residual superficial varicose veins was performed by a phlebologist" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used computer randomisation, per participant |
| Allocation concealment (selection bias) | Low risk | Used computer randomisation (1:1) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of assessors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Although it was stated that two participants were lost to follow‐up at six weeks, there is no explanation of the numbers used to analyse the one‐year outcomes or the participant satisfaction outcome |
| Selective reporting (reporting bias) | Low risk | All outcomes reported on |
| Other bias | Unclear risk | Possibly underpowered; power calculation described a need for 120 participants in each group, but only 113 were available for follow‐up in the surgery group. Also, study authors say participants with dual incompetencies were only randomised once, but the number randomised (130) is legs, and not participants, which is n = 122. This is confusing and possibly misleading. All procedures performed under local tumescent anaesthesia. |
Rasmussen 2007.
| Study characteristics | ||
| Methods |
Study design: RCT Country: Denmark Setting/Location: office‐based setting, private clinic Source of funding: grant from the Public Health Insurance Research Foundation of Denmark; EVLT catheters provided, in part, by Biolitec AG (Bonn, Germany) and Micronmed (Kristianstad, Sweden) Intention‐to‐treat analysis: yes |
|
| Participants |
No of participants randomised: total n = 121 participants (137 legs) (EVLT n = 62 (69 legs); HL/S n = 59 (68 legs) No of participants analysed: at 6 months: total n = 88 (EVLT n = 47; HL/S n = 41) (all meta‐analyses performed on an ITT basis by all legs randomised) Exclusions post‐randomisation: none Losses to follow‐up: 12 days ‐ EVLT 2, HL/S 0; 1 month ‐ EVLT 4, HL/S 2; 3 months ‐ EVLT 6, HL/S 5; 6 months ‐ EVLT 15, HL/S 18 Age ‐ mean years (range): EVLT 53 (26 ‐ 79); HL/S 54 (22 ‐ 78) Sex ‐ (M/F): EVLT 21/41; HL/S 16/43 No bilateral limbs randomised: EVLT 7; HL/S 9 Inclusion criteria: CEAP class C2 to C4 (Ep, As, Pr); informed consent; age 18 ‐ 80; GSV incompetence confirmed by > 0.5 s reflux on DUS Exclusion criteria: duplication of GSV or incompetent anterior accessory GSV; SSV reflux (or < 3 months since surgery for SSV incompetence); previous DVT; ABI < 0.9 or Hx arterial disease; femoral or popliteal insufficiency; tortuous GSV |
|
| Interventions |
Treatment(s): EVLT (DUS guided) 980 nm diode laser, 1.5 s pulses, 1.5 s pause, 12 W energy; EVLT Ceralas D 980 Biolitec, Bonn, Germany Control: high tie strip and multiple stab avulsion (HL/S) Duration: follow‐up 12 ds, 1, 3, and 6 months, 2 and 5 yrs post‐procedure |
|
| Outcomes |
Primary outcomes: it is not clear what their specific primary or secondary outcomes were. Rasmussen 2007 set out to assess safety, efficacy, post‐operative morbidity, sick leave, QoL and costs. They reported results on: absence from work and normal activity; AVVSS; SF‐36 score; VVSS; pain VAS; complications (minor e.g. required no treatment versus major e.g. required treatment, hospitalisation, permanent sequelae or death); cost (procedure and days off sick from work) Recurrence definition: veins which had not been observed before or not previously marked by the participant on the AVVSS form |
|
| Notes | 8 participants in each group had previous high ligation i.e. were recurrent. They were permitted as they had a patent refluxing SFJ and GSV. Study author contacted and further information on randomisation process given: "A block of 10 envelopes would ensure that a sufficient number of each treatments were available, i.e. 5 of each all the time. This is like tossing a coin but easier to document. The envelopes were kept in a basket, but the basket was filled by a research nurse when the patients were not present. All envelopes were alike. There was no chance of bias." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | After contacting the study author, further details on the random sequence generation were confirmed: 'Blocks of 10 envelopes kept in a basket, the basket was filled by a research nurse when the patients were not present. All envelopes were alike.' |
| Allocation concealment (selection bias) | Low risk | After contacting the study author, further details on allocation concealment were confirmed: 'The envelopes were kept in a basket, but the basket was filled by a research nurse when the patient were not present. All envelopes were alike.' |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind operator or participants to treatment. No blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No mention that outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up did not have an impact on the outcome measures; the two treatment groups remained similar in numbers despite losses |
| Selective reporting (reporting bias) | Low risk | The pre‐specified outcomes in the study protocol were reported in the pre‐specified way Additional outcome measures were reported in a subsequent publication (2‐year results) reporting recurrence rates, which were not a pre‐specified outcome measure. However, this does not introduce any bias or inaccuracy into the trial. |
| Other bias | Unclear risk | 121 participants (137 limbs): included 16 participants with bilateral varicose veins; no stratification of these participants in the results; all bilaterally treated participants received the same treatment on both legs. |
Rasmussen 2011.
| Study characteristics | ||
| Methods |
Study design: two‐centre, prospective, RCT Country: Denmark Setting/Location: two private surgical centres under contract to the national healthcare system in Denmark: Danish Vein Centre, Naestved, Surgical Centre Roskilde, Denmark Source of funding: financed by a grant from the Public Health Insurance Research Foundation of Denmark. Radiofrequency equipment was provided by VNUS Medical Technologies Intention‐to‐treat analysis: not indicated |
|
| Participants |
No of participants randomised: total n = 500 (580 legs); (EVLT n = 125 (144 legs); RFA n = 125 (148 legs); UGFS n = 125 (145 legs); HL/S n = 125 (143 legs)) No of participants analysed: at 3 days: total n = 494 (573 legs); (EVLT n = 124 (143 legs); RFA n = 124 (146 legs); UGFS n = 123 (143 legs); HL/S n = 123 (141 legs) at 1 month: total n = 489 (564 legs); (EVLT n = 125 (144 legs); RFA n = 121 (141 legs); UGFS n = 124 (144 legs); HL/S n = 119 (135 legs) At 1 yr: total n = 417 (476 legs); (EVLT n = 107 (121 legs); RFA n = 106 (124 legs); UGFS n = 107 (123 legs); HL/S n = 97 (108 legs) Exclusions post‐randomisation: total n = 2 (EVLT n = 0; RFA n = 0; UGFS n = 1; HL/S n = 1) Losses to follow‐up: at 3 days 4 losses; (1 in EVLT, 1 in RFA (2 legs), 1 in UGFS, 1 in HLS group), at 3 months 9 losses; (4 (7 legs) from RFA, 5 (7 legs) from HLS). At 1 yr 81 losses; (18 (23 legs) from EVLT, 19 (24 legs) RFA, 17 (21 legs) UGFS, 27 (34 legs) HLS groups) Age ‐ mean years (range): EVLT 52 (18 ‐ 74); RFA 51 (23 ‐ 75); UGFS 51 (18 ‐ 75); HL/S 50 (19 ‐ 72) Sex ‐ percent women: EVLT 72%; RFA 70%; UGFS 76%; HL/S 77% No bilateral limbs randomised: EVLT 19, RFA 23, UGFS 20, HL/S 18 Inclusion criteria: age 18 ‐ 75; symptomatic varicose veins; CEAP class C2 to C4 (Ep As Pr); GSV incompetence defined by reflux time of more than 0.5 s on DUS; informed consent provided Exclusion criteria: duplication of the saphenous trunk or an incompetent anterior accessory saphenous vein; SSV reflux (until 3 months after removal of such a vein); previous DVT; history of arterial insufficiency or ABPI < 0.9, or both; axial deep venous insufficiency (femoral, popliteal or both); tortuous GSV rendering the vein unsuitable for endovenous treatment |
|
| Interventions |
Treatment(s): all performed under tumescent anaesthesia, 'most' with a light sedative EVLT: duplex guidance ‐ 980 nm diode for the first 17 participants, 1470 nm diode for the rest, in one centre (Roskilde) pulse mode was used and continuous mode was used in the other centre; Ceralas D 980 Biolitec, Jeno, Germany and 1470 Ceralas D RFA: performed according to the manufacturer's recommendations; VNUS Medical Technologies Inc USGF: participant in reverse Trendelenburg position, GSV cannulated (5‐Fr) just above the knee, foam was 3% polidocanol (2 mL and 8 mL air mix), before injection the table was put into the Trendelenburg position, foam was injected under USS guidance; re‐treatment was permitted within 1 month Control: HLS: under tumescent anaesthesia and 'most with sedation'. Standard groin incision, flush ligation of GSV, division of all tributaries, GSV stripped with a PIN‐stripper to below the knee Duration: follow‐up at 3 days, 1 month, 1, 3, 4 and 5 yrs after treatment |
|
| Outcomes |
Primary outcomes: GSV closure (closed or absent GSV with lack of flow) Secondary outcomes: pain, absence from work and normal activity, QoL (SF‐36, AVVSS) and VCSS and recurrence rates, costs Recurrence definition: not provided |
|
| Notes | Randomisation took place between February 2007 to July 2009 Bilateral treatment permitted, but both legs received same treatment Mini‐phlebectomies performed in all treatment groups to remove varicose veins (mean (range)): EVLT 14 (1 ‐ 43); RFA 16 (10 ‐ 80); UGFS 15 (1 ‐ 43); HL/S 15 (1 ‐ 48)) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Consecutive patients referred for varicose vein treatment by the family physician were randomised in the two sites in blocks of 12 sealed envelopes to one of the four treatments". Insufficient description of random sequence generation |
| Allocation concealment (selection bias) | Low risk | Used sealed envelopes, although do not specify whether these were opaque |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts and reasons were thoroughly reported |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported on |
| Other bias | Unclear risk | Different methods, energies and diodes used for EVLT in the two centres, the procedure technique was not uniform; used limbs and not participants in analysis; participants with ‘recurrent varicose veins’ were also included if the GSV was preserved to the groin on DUS: no report on number of recurrent varicose veins in each group; all procedures performed under local tumescent anaesthesia |
Rautio 2002.
| Study characteristics | ||
| Methods |
Study design: RCT Country: Finland Setting/Location: University of Oulu Source of funding: Grant from University of Oulu, Finland Intention‐to‐treat analysis: one participant was excluded after randomisation but not withdrawn from the study, indicating some intention‐to‐treat process, but which group this participant retired from is not made clear |
|
| Participants |
No of participants randomised: total n = 33 (RFA n = 16; HL/S n = 17) No of participants analysed: total n = 28 (RFA n = 15; HL/S n = 13) Exclusions post‐randomisation: 3 participants left as found schedule unsuitable, further 4 refused chosen treatment and 1 excluded due to pregnancy Losses to follow‐up: no Age ‐ mean years (SD): RFA 33 (6.7); HL/S 38 (6.8) Sex ‐ M/F: RFA 1/14; HL/S 1/12 Inclusion criteria: confirmed reflux (USS > 2 s GSV reflux); person suitable for day case; symptomatic previously untreated uncomplicated GSV tributary varicosities and isolated unilateral SFJ incompetence Exclusion criteria: coagulopathies; pregnancy; multiple, tortuous (> 90 degree bend) large‐diameter GSV trunks; bilateral varicose veins; concomitant SSV varicosities |
|
| Interventions |
Treatment(s): RFA: VNUS Closure system, inserted into GSV at ankle level; no ligation of SFJ, EVLA, UGFS Control: HL/S:open surgery; SFJ ligation of all tributaries and stripping of GSV to just below knee Duration: follow‐up for 3 yrs |
|
| Outcomes |
Primary outcomes: it is not clear what their specific primary or secondary outcomes were, aimed to evaluate outcome in terms of pain, sick leave, health‐related QoL and cost Secondary outcomes: assessed further outcomes at 3 yrs including recurrence, satisfaction, VCSS, VSDS and the VDS, patency of GSV and presence of neovascularisation Recurrence definition: not provided |
|
| Notes | Study author contacted 2 February 2010 Replied 8 February 2010: "The 36 patients had their preoperative diagnostic done in an earlier trial (Accuracy of HHD in planning the operating for primary varicose veins. Eur J Vasc Endovasc Surg 2002). After examining these patients and ensuring their suitability to the study they were included. The patients were given the study information and after getting informed consent from all of them, I put 36 named tags to identical envelopes, which were sealed. After shuffling the envelopes I numbered them randomly. List of numbers for randomisation was done earlier according to instructions of the biostatistician of our department. I opened the envelopes in numerical order. Randomisation was done this way, because our strict schedule. Resource allocations (operating theatres, angiography suites etc.) forced us to perform the operations and procedures during a period of two weeks. We also thought, that it was better to inform the result of randomisation to patients in good time beforehand. Four patients withdrew because of the disappointment of having been assigned to the stripping group. Three patients discontinued the study because of an unsuitable schedule. One patient was excluded because of pregnancy. As a result we missed three patients from the RFA group and five patients from the stripping group. I do not see any chance of bias because of selection process itself. Withdrawal of eight patients might have had some influence to the results." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | After contacting the study author, the sequence generation details were clarified: "....36 named tags to identical envelopes, which were sealed. After shuffling the envelopes they were numbered randomly. List of numbers for randomisation was done earlier according to instructions of the biostatistician of our department. The envelopes were opened in numerical order" |
| Allocation concealment (selection bias) | Low risk | As above |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind operator or participants to treatment. No blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No mention that post‐operative assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In a later publication (three‐year outcome measures), the study authors claim 'all patients also underwent 3 year follow up'; They report no long‐term losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | The pre‐specified outcomes in the study protocol were reported in the pre‐specified way. Additional outcome measures were reported in a subsequent publication (3yr results): recurrence rates, an additional outcome which was not a pre‐specified outcome measure. However, this does not introduce any bias or inaccuracy into the trial. |
| Other bias | Low risk | States "competition of interest: nil" Small study groups |
Recovery 2009.
| Study characteristics | ||
| Methods |
Study design: prospective, single‐blinded, RCT comparing Laser (EVL) and RFA
Country: USA (5 centres) Germany (1 centre)
Setting/Location: Miami Vein Center; Dotter Interventional Institute, Oregon; Venenzentrum Elsterpark Germany; Midwest Institute for Minimally Invasive Therapies, Illinois; Vein Solutions, Virginia; Community Surgical Associates, Montana
Source of funding: VNUS Medical Technologies provided financial support for data collection and clinical monitoring and participated in the protocol design Intention‐to‐treat analysis: not reported |
|
| Participants | No of participants randomised: 87 veins in 69 participants No of participants analysed: 46 limbs were randomised to undergo RFA treatment and 41 to undergo EVLA Exclusions post‐randomisation : not reported Losses to follow‐up: none reported Intention‐to‐treat analysis: not reported Age: mean years (SD): EVLA: 51.6 (12.8); RFA: 52.4 (15.3) Sex F/M: EVLA 31:10 ; RFA 29:17 Inclusion criteria: age 18 ‐ 80 yrs with incompetent GSV documented on DUS (US; B‐mode and colour Doppler imaging) NB: reflux = significant if reversal of flow > 0.5 s after distal compression in the standing position Exclusion criteria: thrombus in the vein of interest, previous GSV treatment, pregnancy, known malignancy, and use of anticoagulant medication except low dose aspirin | |
| Interventions |
Treatment description: EVLA US‐guided percutaneous access followed by perivenous tumescent anaesthesia (0.1% lidocaine with epinephrine): EVLA with 980 nm wavelength in continuous mode at 12 W of power with a linear endovenous energy density of 80 J/cm. After treatment: limbs wrapped in compression bandages and class II compression stockings for 24 – 72 hrs then compression stockings for 2 weeks
Control description: RFA US‐guided percutaneous access followed by perivenous tumescent anaesthesia (0.1% lidocaine with epinephrine): RFA was performed with an intraluminally placed Closure‐FAST 7‐cm tip device placed 2 cm from SFJ, segmental energy delivery at 120 °C in 20 s cycles. 2 cycles to proximal GSV, then 1 cycle to the remaining GSV. After treatment: limbs wrapped in compression bandages and class II compression stockings for 24 – 72 hrs then compression stockings for 2 weeks Duration: 48 hrs, 1 week, 2 weeks, and 1 month after treatment |
|
| Outcomes |
Primary outcomes: post‐operative pain (measured by the subject on a validated VAS (0 (no pain) to 10 (most severe pain)); ecchymosis measured by clinic staff (0 (no ecchymosis) to 5 (ecchymosis over the entire segment and extension above or below the treatment segment)); incidence of adverse procedural sequelae (e.g. DVT, paraesthesia, phlebitis, hyperpigmentation, and infection) Secondary outcomes: technical success: DUS assessment of which veins were closed within 3 cm of the SFJ at 48 hs and 1 month; reflux = present if reversal of flow > 0.5 s after distal compression in the standing position; VCSS was recorded during each follow‐up visit; limb tenderness (scale: 0 (no tenderness) to 10 (acutely severe tenderness)) the use of periprocedural analgesic agents (limited to ibuprofen with a maximum dose of 800 mg twice daily) QoL using CIVIQ |
|
| Notes | March to December 2007 Phlebectomy was not permitted until at least 30 ds had elapsed after the procedure Results reported as number of limbs NOT number of participants which is confusing Phlebitis was defined as induration and erythema along the course of the target vein Small study sample size |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomization was performed within 24 hours before the procedure and was accomplished by the investigators accessing a Web site and downloading the procedure to be performed." |
| Allocation concealment (selection bias) | Unclear risk | Study authors mention the actual "treatment procedure was not discussed with the participants." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were blinded. ".....the actual treatment procedure was not discussed with the participants", thus maintaining the "single‐blind nature of the study" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of assessors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts were not discussed |
| Selective reporting (reporting bias) | Low risk | All pre‐defined outcomes were reported |
| Other bias | Unclear risk | Use of private centres and sponsorship by VNUS Technologies (RFA manufacturers). Limbs rather than participants reported |
RELACS 2012.
| Study characteristics | ||
| Methods |
Study design: two‐centre, prospective, RCT Country: Germany Setting/Location: university dermatology department (EVLT‐treated group) and a specialised vein clinic (HL/S‐treated group); Homburg and Bad Bertrich vascular centres Source of funding: not indicated Intention‐to‐treat analysis: not indicated |
|
| Participants |
No of participants randomised: total n = 400 (EVLT n = 200; HL/S n = 200) No of participants analysed: total n = 316 (EVLT n = 173; HL/S n = 143) Exclusions post‐randomisation: total n = 54 (EVLT n = 15; HL/S n = 39). All declined to participate. Losses to follow‐up: total n = 30 (EVLT n = 12; HL/S n = 18); at 5 yrs n = 65 (EVLA n = 33; HL/S n = 32) Age ‐ mean years (SD): EVLT 47.9 (10.9); HL/S 48.0 (10.7) Sex ‐ percent female: EVLT 67%; HL/S 70% Inclusion criteria: GSV insufficiency with saphenofemoral incompetence and reflux at least down to the knee level; CVI and/or symptoms caused by GSV incompetence and or severe clinical finding at risk of varicose vein bleeding, thrombophlebitis or DVT; age 18 to 65 yrs; performance status (according to criteria of the American Society of Anesthesiologists, of class I ‐ II) Exclusion criteria: previous surgical interventions in the groin area with the exception of inguinal herniotomy; anterior or posterior accessory saphenous vein incompetence; small saphenous vein insufficient requiring treatment at the same limb; acute DVT or PTS; known thrombophilia associated with a high risk of thromboembolism; arterial occlusive disease classified as at least Fontaine stage IIA, and/or ABI below 0.8; active malignant disease (diagnosed during the past 5 yrs); poor compliance or inability to understand the study‐related procedures; women who are pregnant or nursing |
|
| Interventions |
Treatment(s): EVLT (810 nm bare fibres): laser power delivered in a continuous pull‐back fashion, performed with tumescent local anaesthetic and sedation at surgeon's discretion; model 435 MedArt A/S Hvidovre, Denmark Control: HL/S: transection of all tributaries, flush ligation of SFJ with non‐absorbable Ethibond 0‐0 suture and neoreflux protection with an invaginating continuous Prolene 4‐0 stump suture followed by invagination of GSV to just below the knee. Performed under tumescent local anaesthetic and sedation at surgeon's discretion Duration: follow‐up at 1 week (days 1 to 7), 3 months, 1 yr, 2 yrs and 5 yrs |
|
| Outcomes |
Primary outcomes: 5‐year clinical recurrence‐free rate according to the classification of REVAS Secondary outcomes: 5‐year DUS recurrence‐free rate at the SFJ, treatment‐related adverse effects, HVVSS, QoL (CIVIQ‐2), participant satisfaction, cosmetic outcome and recovery using questionnaires and VAS (range 1 ‐ 5) Recurrence definition: REVAS criteria, which defined recurrence as the presence of any new visible or palpable varicosity on the study leg noticed by the examining clinician, originating form the operated site linked to a saphenofemoral recurrence, to an incompetent GSV or perforator at medial thigh level with medical indication for re‐operation |
|
| Notes | Randomisation took place between September 2004 to March 2007 One limb per participant was randomised (for participants with both limbs being eligible, the one more affected by CVI was chosen for study participation) Incompetent perforators were ligated and peripheral side branches were removed with multiple stab avulsions. After 3 months, those with apparent residual varices and perforators could be treated with additional phlebectomies or sclerotherapy (exclusively at this time point). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient description of random sequence generation ‐ only described as "blocks of 10" but: "Independent randomisation was conducted via fax from a remote site" |
| Allocation concealment (selection bias) | High risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and personnel reported |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of assessors reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts and reasons were thoroughly reported |
| Selective reporting (reporting bias) | Low risk | All outcomes reported on |
| Other bias | Unclear risk | EVLT and HL/S performed at 2 separate clinics; possibly underpowered: needed 180 participants per group but after dropouts and losses to follow‐up, the EVLT group had only 173 and HL/S had 143; no agreed protocol on number of additional phlebectomies ‐ this could affect pain, cosmesis, QoL etc; all procedures performed under local tumescent anaesthesia |
Shepherd 2010.
| Study characteristics | ||
| Methods | Study design: single centre, single‐blinded, RCT Country: UK Setting/Location: NHS hospital London Source of funding: "funded by the Mason Medical Research Foundation (registered charity), the Royal Society of Medicine Venous Forum and Imperial College London; these bodies had no input into the study design, data collection, data analysis, manuscript preparation or publication decisions. The authors declare no conflict of interest" Intention‐to‐treat analysis: yes | |
| Participants | No of participants randomised: n = 131; RFA = 67; EVLA = 64 No of participants analysed: RFA 3 day analysis n = 66; 10 day analysis n = 59; 6 week analysis n = 60; EVLA 3 day analysis n = 61; 10 day analysis n = 51; 6 week analysis n = 55 Exclusions post‐randomisation: RFA 3 day excluded n = 1; 10 day analysis excluded n = 8; 6 week analysis excluded n = 7 EVLA 3 day analysis excluded n = 3; 10 day analysis excluded n = 13; 6 week analysis excluded n = 9 Losses to follow‐up: RFA 3 day analysis lost to follow‐up n = 1; 10 day analysis lost to follow‐up n = 1; 6 week analysis lost to follow‐up n = 7 EVLA 3 day analysis lost to follow‐up n = 2; 10 day analysis lost to follow‐up n = 0; 6 week analysis lost to follow‐up n = 8 Age years (SD): RFA 49 (15); EVLA 48 (16) Sex M/F: RFA 47:20 EVLA 42:22 Inclusion criteria: people over 18 yrs of age with primary GSV incompetence were invited to participate Exclusion criteria: people with current DVT, significant arterial disease (ABI below 0.8) or who were unsuitable for general anaesthesia were excluded | |
| Interventions | Treatment: VNUS ClosureFAST (RFA), in an operating theatre under general anaesthesia, performed by 1 of 3 vascular surgeons. Standard tumescent local anaesthesia (50 mL 1% lidocaine with 1:200,000 adrenaline (epinephrine) in 1000 mL normal saline). In participants treated with segmental RFA, the first segment was treated with two RFA cycles, and the remainder of the vein was treated with one RFA cycle per 7‐cm segment. Extrinsic pressure was applied over the vein during treatment cycles. TED stocking continuously for 1 week Control: 980‐nm laser (EVLA) using a bare fibre, in an operating theatre under general anaesthesia, performed by 1 of 3 vascular surgeons. Standard tumescent local anaesthesia (50 mL 1% lidocaine with 1:200,000 adrenaline (epinephrine) in 1000 mL normal saline). In patients who had EVLA, the laser was continually withdrawn with the aim to deliver energy greater than 60 J/cm; power setting 11 W. TED stocking continuously for 1 week Duration: 3 and 10 days, 6 weeks, 6 months | |
| Outcomes | Primary outcomes: mean post‐procedural pain over the first 3 days (number of tablets taken, ratings on 100 mm visual analogue scale) first 3 days Secondary outcomes: AVVQ and SF‐12 at 6 weeks (compare with baseline). The VCSS at week 6, complications at 1 week and 6 weeks. Assessment of vein occlusion rates 6 months after the intervention | |
| Notes | 12 months from July 2008 to July 2009 Participants with additional small saphenous or anterior thigh vein incompetence were treated with the allocated treatment modality at the same sitting. This will impact on QoL scores and return to work. "Patients with varicosities were treated with concomitant phlebectomies using a standard technique with an Oesch hook and all phlebectomy sites were sutured with 6/0 polypropylene." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used an Internet randomisation service |
| Allocation concealment (selection bias) | Unclear risk | Used an Internet randomisation service |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts and reasons were thoroughly reported |
| Selective reporting (reporting bias) | Low risk | All outcomes reported on |
| Other bias | Unclear risk | Phlebectomies also performed as was SSV and anterior thigh vein incompetence as required. Report pain analysis was adjusted to make allowances for this. RFA 36 bilateral limbs EVLA 30 performed concurrently ‐ subsequently impacts on pain and return to work. In participants with bilateral disease, the leg that was most symptomatic according to the participant was randomised, and both legs received the same treatment. |
Subramonia 2010.
| Study characteristics | ||
| Methods |
Study design: RCT Country: UK Setting/Location: hospital Source of funding: VNUS Medical Technologies funded some of the Closure PLUS radiofrequency ablation catheters used in the trial. They were not involved in the running of the trial, data collection, interpretation or analyses. Intention‐to‐treat analysis: "There was no crossover of patients between the treatment arms after randomisation and before treatment." However, authors do not explicitly state if there was going to be ITT analysis. |
|
| Participants |
No of participants randomised: total n = 93 (RFA n = 48; HL/S n = 45) No of participants analysed: total n = 88 (RFA n = 47; HL/S n = 41) Exclusions post‐randomisation: 2 RFA participants (1 taken off waiting list, 1 did not receive any treatment); 4 surgery (1 taken off waiting list, 1 developed atrial fibrillation, 1 developed hypertension, 1 operated on a non‐trial list) Losses to follow‐up: none at 6 weeks; 53 participants (61 limbs) available at 20 months Age ‐ median years (IQR): RFA 47 (38 ‐ 58); HL/S 45 (37 ‐ 53) Sex ‐ M/F: RFA 13/34; HL/S 14/27 No bilateral limbs randomised: no bilateral limbs were included Inclusion criteria: age 18 ‐ 70 yrs; primary or recurrent GSV reflux on DUS; DUS confirmed suitable for RFA; fit for GA; physical condition allowing ambulation after surgery; can give informed consent; individual and surgeon agree intervention is required; availability for follow‐up Exclusion criteria: varicose veins without GSV incompetence on DUS; associated small saphenous or deep venous incompetence; tortuous GSV unsuitable for RFA; GSV diameter < 3 mm and > 12 mm in supine position; GSV thrombus; people with permanent pacemaker or internal defibrillator; concomitant PVD (ABI < 0.9); pregnancy; unable to complete QoL questionnaire due to poor English language skills |
|
| Interventions |
Treatment(s): RFA; the VNUS Closure PLUS intravascular catheter with bipolar electrodes Control: HL/S ‐ open surgery Duration: 1 week and 5 weeks follow‐up |
|
| Outcomes |
Primary outcomes: time taken to return to normal household activities Secondary outcomes: intraoperative complications; duration of the procedure; post‐operative morbidity (pain, analgesic requirements, sensory abnormalities, wound problems, phlebitis, skin burns, pigmentation); time to return to driving; participant satisfaction and QoL Recurrence definition: not evaluated |
|
| Notes | Article was written and designed by two vascular surgeons who perform both procedures regularly and both authors declare no personal conflict of interests in either treatment. The study authors standardised their anaesthetic and inter‐operator variability thus reducing bias. Age and sex variables were controlled in the randomisation process, thus reducing potential confounding. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Age and sex were 'judged most likely to influence outcome in the two groups'. Study author contacted for further details: "A web‐based randomisation method was used (with assistance from the Institute of Health and Society, Newcastle University, UK) with stratification to ensure appropriate balance between the arms with respect to variables that might influence outcome in the two groups and to minimise the risk of confounding. The method used two stratification variables, age and sex, that were judged most likely to influence the outcome in the two groups. Two levels of each stratification variable were employed: Age: ≤ 50 years and > 50 years Sex: male or female Simple randomisation without stratification does not guarantee equivalence between the two groups and several levels of stratification can make the randomisation system more complicated and also result in some small strata. The same procedure was allocated to those with bilateral varicose veins both of which were suitable for the trial with a minimum period of 3 months between the procedures. Access to the web site was protected by password and the file server maintained by the University of Newcastle had high security protocols. The researcher alone had knowledge of the password to access the web site. No problems were encountered either in accessing the web site or in randomising patients during the trial." |
| Allocation concealment (selection bias) | Low risk | Used web‐based randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unable to blind surgeon or participant to treatment. No blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No mention that assessors post‐operatively were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data fully reported and balanced in numbers across intervention groups; all participants were followed up at 5 weeks. |
| Selective reporting (reporting bias) | Low risk | The pre‐specified outcomes in the study protocol were reported in the pre‐specified way |
| Other bias | Unclear risk | Included five participants with recurrent varicose veins. No stratification of these participants in the results. This could introduce a potential bias into results such as pain, time to return to normal activities, QoL, etc. Included 12 participants with bilateral varicose veins (randomised on one occasion to the same treatment, but had their limbs treated with a minimum of 6 weeks in between treatments, thus treating each limb as a separate case). |
Syndor 2017.
| Study characteristics | ||
| Methods | Study design: prospective, single‐blinded, RCT Country: USA Setting/Location: clinic Source of funding: not stated, study authors received no financial support Intention‐to‐treat analysis: not stated | |
| Participants | No of participants randomised: 200 participants; RFA = 100; EVLA = 100 No of participants analysed: RFA = 100, EVLA = 100 Exclusions post‐randomisation: nil Losses to follow‐up: 6 weeks RFA = 97, EVLA = 96; 6 months EVLA = 79, RFA = 74 Age mean (range): RFA = 47 (19 ‐ 86); EVLA = 48.5 (23 ‐ 86) Sex ‐ F/M: EVLA 77/23; RFA 80/20 Inclusion criteria: CVI symptoms caused by GSV reflux (reversed flow in GSV > 0.5 s after calf compression in a standing position); CEAP > 2; prior attempt of at least 6 weeks of compression stockings for CVI Exclusion criteria: previous vein surgery/EVTA/phlebectomy in target extremity excluding sclerosant injection for spider veins; active or prior DVT; active or prior hypercoagulability; people who are breastfeeding; people who are non‐ambulatory; age < 18 yrs; prisoners | |
| Interventions |
Treatment description: RFA: office‐based majority without conscious sedation, tumescent anaesthesia. Heat (120 oC) segmentally 20 s cycles spaced 6.5 cm apart via VNUS ClosureFAST technology (2 consecutive cycles delivered 1 ‐ 2 cm distal to the SFJ and all other segments were treated with 1 cycle. Stockings continuously 24 hrs then during day for 14 days
Control description: EVLA: office‐based majority without conscious sedation, tumescent anaesthesia. 980 nm diode laser system (AngioDynamics, Qeensbury NY) at a fluence range of 50 ‐ 80 J and power back power of 10 W with a constant continuous pullback. Stockings continuously 24 hrs then during day for 14 days. Duration: post‐procedure review within 7 days, 6 weeks, 6 months |
|
| Outcomes | Primary outcomes: technical success (closure of GSV with no new reflux, neovascularity or other refluxing truncal veins) Secondary outcomes: pain during procedure (1 ‐ 10), haematoma, paraesthesia, thermal injury, overall satisfaction, satisfaction within 7 days, 6 weeks and 6 months | |
| Notes | Participants were offered ambulatory phlebectomies or UGFS Conscious sedation commonly administered when adjunctive ambulatory phlebectomy performed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was performed in blocks of two, four or six participants |
| Allocation concealment (selection bias) | Low risk | "Objective data recorded by nurse practitioner blinded with regards to which EVTA procedure the patient had undergone. All patient charts with photographs were kept in a locked office and the patient database was kept in a secure password protected format. Patients were blinded" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and personnel blinded. "Objective data recorded by nurse practitioner blinded with regards to which EVTA procedure the patient had undergone. All patient charts with photographs were kept in a locked office and the patient database was kept in a secure password protected format. Patients were blinded" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Objective data recorded by nurse practitioner blinded with regards to which EVTA procedure the patient had undergone. All patient charts with photographs were kept in a locked office and the patient database was kept in a secure password protected format." Participants were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts reported, reasons not given |
| Selective reporting (reporting bias) | Low risk | All predefined outcomes reported |
| Other bias | Unclear risk | Phlebectomies and UGFS performed concomitantly Conscious sedation used Wide range in follow‐up dates (i.e. some 6‐week reviews were done at 1 yr; initial follow‐up ranged from 1 to 29 days) No power analysis |
Vähäaho 2019.
| Study characteristics | ||
| Methods |
Study design: prospective single centre RCT Country: Finland Setting/Location: Helsinki University Hospital Source of funding: none stated Intention‐to‐treat analysis: yes |
|
| Participants |
No of participants randomised: total n = 132; MOCA n = 65; EVLA n = 34; RFA = 33 No of participants analysed: total n = 124; MOCA n = 55; EVLA n = 33; RFA = 29 Exclusions post‐randomisation: n = 7; MOCA n = 6; EVLA n = 0; RFA n = 1 Losses to follow‐up: total n = 8; MOCA n = 4; EVLA n = 1; RFA = 3 Age ‐ mean years (SD): MOCA 50.9 (12); EVLA 49.5 (11.9); RFA 50.3 (13.9) Sex ‐ M/F: not stated No bilateral limbs randomised: N/A Inclusion criteria: C2 to C4, US verified reflux in the GSV, mean GSV diameter in thigh 5 mm to 12 mm, age 20 to 75 years, informed consent provided Exclusion criteria: BMI > 40 kg/m2, PAD, lymphoedema, pregnancy, allergy to either sclerosant or lidocaine, severe general illness, malignancy, previous DVT, previous varicose vein intervention in the same leg, coagulation disorder |
|
| Interventions |
Treatment(s): MOCA Clarivein inserted to ‐2 cm below SFJ, rotation for 2‐3 secs at highest setting. Whilst wire rotating, simultaneous injection of sclerosant (Sotradecol 1.5%) inserted. First 10 cm rechecked with ultrasound and further second treatment given if necessary. Additional phlebectomies under tumescent anaesthesia performed if required Control: EVLA: performed under local TA using 0.1% lidocaine in ringer’s acetate (150 mL to 500 mL used) and light sedative (pre‐operative diazepam and if required propofol +/‐ fentanyl). Ultrasound guidance, 1470 nm diode laser comprising 1.5 sec impulse and 10 W energy with a protocol to apply 70 J/cm. Additional phlebectomies under TA. Class 2 compression stockings for 48 h then daily for 2 weeks RFA: performed under local TA using 0.1% lidocaine in ringer’s acetate (150 mL to 500 mL used) and light sedative (pre‐operative diazepam and if required propofol ± fentanyl). Ultrasound guidance VNUS closure FAST catheter. 120 oC for 20 s per segment. The first segment is ablated twice. Additional phlebectomies under TA. Class 2 compression stockings for 48 h then daily for 2 weeks Duration: one year |
|
| Outcomes |
Primary outcomes: occlusion rate of the GSV at 1 yr Secondary outcomes: disease‐specific QoL, perceived pain during and after treatment, duration of sick leave, amount of pain medication consumed during and after treatment, 30‐day occlusion rate and complications. Recurrence definition: partial recanalisation defined as the presence of at least 5 cm of compressible patent GSV |
|
| Notes | Additional phlebectomies – yes in both EVLA, RFA and MOCA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation done by study nurse after appointment and sealed envelopes into EVLA, RFA and MOCA 1:1:2 |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts reported, no reasons given |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Unclear risk | Sample size calculations indicated 160 participants would be required. Due to slow recruitment was terminated at 132. |
Vernermo 2016.
| Study characteristics | ||
| Methods |
Study design: prospective, multicentre RCT
Country: Finland Setting/Location: 2 Finnish hospitals Source of funding: not stated, study authors declare no conflict of interest Intention‐to‐treat analysis: yes |
|
| Participants | No of participants randomised: 233 participants (233 legs) No of participants analysed: 214 participants: HL/S = 65; EVLA = 73; UGFS =76 Exclusions post‐randomisation: 19 randomised participants were excluded from the study before treatment Losses to follow‐up: 1 yr: HL/S = 4, EVLA = 0, UGFS = 4 Age mean (SD, range): HL/S 47.3 (11.3, 27 ‐ 75); EVLA 47 (13.4, 20 ‐ 73); UGFS 48.3 (12.7, 20 ‐ 73) Sex ‐ F/M: HL/S 55/10; EVLA 55/18; UGFS 58/18 Inclusion criteria: unilateral symptomatic, uncomplicated varicose veins (CEAP clinical classification C2 to C4), DUS‐verified reflux in the GSV, mean diameter of the GSV in the thigh 5 – 10 mm, and age 20 – 70 yrs Exclusion criteria: PAD, lymphoedema, BMI exceeding 40 kg/m2, pregnancy, allergy to the sclerosant or lidocaine, severe general illness, malignancy, previous DVT and coagulation disorder | |
| Interventions |
Treatment description: HL/S ‐ "SFJ was exposed in the groin and side branches were ligated back to the femoral vein. Retrograde invagination stripping of the GSV was done, usually down to below the knee. Tumescent solution was injected into the tunnel of the stripped. Hook phlebectomies performed. Most patients had general anaesthesia". Compression stockings Treatment description: EVLA ‐ tumescent local anaesthesia under UG. A light sedative was administered before (diazepam) and during the procedure. 980 nm diode laser (Ceralas D 980; Biolitec, Bonn, Germany) was used initially, but during the study replaced with a 1470 nm radial laser (ELVes; Biolitec). Pulsed mode, with a 1.5 s impulse and 12 W of energy, with the aim of applying 70 J/cm to the GSV. The EVLA catheter tip was positioned 1.5 – 2 cm below the SFJ using UG. Hook phlebectomies performed. Same compression bandage protocol used Control description: UGFS ‐ "The GSV was cannulated under ultrasound guidance, usually at proximal thigh level and immediately below the knee. The sclerosant foam was prepared with a double‐syringe technique with a sclerosant to air ratio of 1:2. The sclerosants used were polidocanol 1% (Aetoxysclerol®; Kreussler, Wiesbaden, Germany) and sodium tetradecyl sulphate (STS) 1% and 3% (Fibrovein™; STD Pharmaceutical Products, Hereford, UK). A compression stocking was applied with the instruction to wear it continuously for 3 ds, followed by day time use for 11 ds. At 1 mt follow‐up, a duplex ultrasound examination was done and, if any reflux was observed, a second treatment with foam was carried out. These patients were seen again 4 weeks after the second treatment, and the need for a possible third treatment was checked by duplex imaging". Duration: follow‐up at 1 week, 1 month, 1 year, and 5 years after treatment |
|
| Outcomes | Primary outcomes: "1 year occlusion (or absence) rate of GSV on routine duplex imaging, changes in disease‐specific quality of life according to the Aberdeen Varicose Vein Severity Score (AVVSS). The diameter of the GSV 20 cm below the groin was also measured and compared with preoperative values." Secondary outcomes: perioperative pain measured using a visual analogue scale (VAS) at the time of discharge and at 1 week after the procedure; duration of sick leave; and rate of complications such as haematoma, pigmentation, thrombophlebitis and paraesthesia | |
| Notes | November 2007 to May 2010 "Owing to the operating surgeon's preference, five patients originally randomised to EVLA were treated with surgery but, because the analysis was made according to intention to treat, these patients were analysed in EVLA group" Changed from 980 nm diode to a 1470 nm diode Foam used was more concentrated (air to sclerosant ratio 2:1) than in other studies Phlebectomies performed in HL/S and EVLA arms. Some 33% also had foam injected into varicose tributaries during UGFS |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Block randomisation. No further information |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts rates and reasons given and not analysed |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Unclear risk | "Owing to the operating surgeon's preference, five patients originally randomised to EVLA were treated with surgery but, because the analysis was made according to intention to treat, these patients were analysed in EVLA group". The EVLA diode was also changed from a 980 nm diode to a 1470 nm diode during the course of the trial. |
ABI: ankle brachial index ABPI: ankle‐brachial pressure index AVVQ: Aberdeen Varicose Vein Questionnaire (also known as AVVSS) AVVSS: Aberdeen Varicose Vein Symptom Severity Score BMI: body mass index CA: cyanoacrylate glue CAA: cyanoacrylate ablation cc: cubic centimetre CDUS: colour duplex ultrasound CEAP: Clinical, Etiological, Anatomical, Pathological classification score CIVIQ: Chronic Venous Insufficiency Quality of Life Questionnaire cm: centimetre CVI: chronic venous insufficiency ds: days DNA: did not attend DUS: duplex ultrasound DVT: deep vein thrombosis EQ‐5D: EuroQol 5D EVLA: endovenous laser ablation (same as EVLT) EVLT: endovenous laser therapy GA: general anaesthetic GSV: great saphenous vein HHD: hand‐held Doppler HL: high ligation HL/S: high ligation and stripping hrs: hours HVVSS: Homburg Varicose Vein Severity Score Hx: history IQR: interquartile range ITT: intention‐to‐treat mins: minutes mL: millilitre mm: millimetre MOCA: mechanochemical endovenous ablation MRFA: monopolar radiofrequency ablation N/A: not applicable no: number nm: nanomole PAD: peripheral artery disease PE: pulmonary embolism PP: per‐protocol PTS: post‐thrombotic syndrome PVD: peripheral vascular disease QoL: quality of life RCT: randomised controlled trial REVAS: Recurrent Varices After Surgery RFA: radiofrequency ablation s: second SD: standard deviation SF‐36: Medical Outcomes Short Form‐36 SFJ: saphenofemoral junction SFL/S: saphenofemoral ligation and stripping (equivalent to HL/S) SSV: small saphenous vein TA: tumescent anaesthesia TED: thrombo‐embolic‐deterrent TCSS: Total Clinical Severity Score UG: ultrasound guidance UGFS: ultrasound‐guided foam sclerotherapy USS: ultrasound scan VAS: visual analogue scale VCSS: Venous Clinical Severity Score VDS: Venous Disability Score W: watts yr(s): year(s)
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Basela 2011 | Claim randomisation yet a retrospective study |
| Campos 2015 | Inclusion of participants with CEAP C5 and C6 disease |
| Chant 1972 | Used liquid sclerotherapy ‐ not foam sclerotherapy |
| Christenson 2010 |
Christenson 2010 included the treatment of 200 limbs, randomised to receive SFJ ligation and stripping or EVLA. After contacting the study author, it was confirmed that 40 participants underwent bilateral varicose vein treatment. It was also confirmed that participants' 'limbs' were randomised, not participants. In fact, eight participants underwent SFJ ligation and stripping on one limb and EVLA on the other. All participants with bilateral varicose veins were treated on the same day. The high proportion of bilaterally treated participants affects pain scores and post‐operative QoL scores. Time to return to work is also published, but limbs cannot return to work independently of one another and, consequently, these results are not suitable for this Cochrane Review. |
| CLASS 2014 | Combined GSV and SSV; data not stratified by GSV despite contacting study authors |
| Compagna 2010 | Comparison of UGFS and SFJ ligation against HL/S and SFJ ligation; the former is not standard practice |
| De Medeiros 2006 | Comparison of EVLA and SFJ ligation against HL/S and SFJ ligation; the former is not standard practice |
| De Oliveira 2018 | Wrong participant population; evaluated people with CEAP C6 |
| Desai 2009 | Conference presentation, not enough information to extrapolate data. Study author previously contacted by Cochrane Vascular but has not provided data |
| Disselhoff 2008 | Used cryostripping |
| dos Santos 2020 | Wrong intervention: evaluated UGFS with tumescence against UGFS |
| Einarsson 1993 | Used liquid sclerotherapy ‐ not foam ‐ in their comparison to surgery |
| Eroglu 2018 | Combined GSV and SSV; data not stratified by GSV despite contacting study authors |
| Figueiredo 2009 | Combined GSV and SSV; data not stratified by GSV |
| Honek 2019 | Comparing EVLA with different types of laser generator (1060 nm Nd‐Yag crystal compared to 1470 nm diode laser generator) |
| Jindal 2018 | All participants had bilateral varicose veins and underwent both MOCA and RFA |
| Kalodiki 2012 | This paper compared foam sclerotherapy plus ligation to open surgery. Foam plus ligation does not represent 'standard' foam sclerotherapy |
| Karathanos 2019 | Study used inclusion criteria CEAP C2 and above; included C5‐C6 participants; data not stratified |
| Kikuchi 2009 | Conference abstract only, not enough information provided to determine inclusion |
| Lattimer 2012 | Comparisons included the use of combined interventions (EVLA with phlebectomies vs UGFS) |
| Leon 2018 | Wrong participant population; compared radiofrequency venous ablation and sclerotherapy with polidocanol foam 3% versus only radiofrequency venous ablation in saphenous veins of 1.5 cm of diameter or more |
| Leung 2019 | Inclusion of participants with CEAP C5 and C6 disease and participants with SSV |
| Lin 2007 | This paper was written in Chinese. Unable to extract any meaningful data despite complete translation |
| Mendes 2016 | Simultaneously treated bilateral legs. Each participant was treated with RFA on one leg and SFJ ligation and stripping on the contralateral limb |
| Mozafar 2014 | Surgical arm comprised of SFJ ligation only which is not standard practice |
| Oster 2018 | Included participants with C2 to C6 disease; data not stratified |
| Ouvry 2008 | Compares two different types of foam, no comparison to different treatment techniques |
| Ovali 2019 | Not an RCT |
| Shadid 2015 | Not an RCT |
| Sincos 2018 | Combined GSV and SSV; data not stratified by GSV despite contacting study authors |
| Stotter 2005 | Surgical arm involved cryostripping |
| Tawfik 2020 | Used combined techniques: EVLA with complementary UGFS to treat incompetent perforating veins and superficial varicosities, and MOCA. In all participants, study authors ablated small saphenous veins and straight accessory saphenous veins or used foam injections for severely tortuous anterior saphenous vein, superficial varicosities, and below knee segments |
| Wright 2006 | Participants randomised by medical staff based on severity of symptoms. Study also combined GSV and SSV, data not stratified by GSV |
EVLA: endovenous laser ablation (equivalent to EVLT) EVLT: endovenous laser therapy GSV: great saphenous vein MOCA: mechanochemical ablation RCT: randomised controlled trial RFA: radiofrequency ablation SFJ: saphenofemoral junction SPJ: saphenopopliteal junction SSV: small saphenous vein UGFS: ultrasound‐guided foam sclerotherapy vs: versus
Characteristics of studies awaiting classification [ordered by study ID]
Belramman 2020.
| Methods | Randomised controlled trial to assess pain resulting from MOCA compared with cyanoacrylate adhesive |
| Participants | People who have primary GSV or SSV vein reflux > 0.5 s on DUS scanning and who are aged over 18 years will be included. Exclusion criteria are: current deep vein thrombosis; recurrent varicose veins; arterial disease (ABPI < 0.8); venous diameter < 3 mm; people who are unwilling to participate; inability or unwillingness to complete questionnaires; adverse reaction to sclerosant or cyanoacrylate or involvement in another venous trial in the past 6 months. |
| Interventions | Participants are randomised to undergo either MOCA or cyanoacrylate adhesive truncal ablation, followed by treatment of any varicosities. Participants are required to wear compression stockings for 4 days post intervention. |
| Outcomes | "The primary end point is pain score immediately following completion of truncal ablation, measured by a 100mm visual analogue scale (VAS). The secondary end points include entire treatment pain scores, clinical scores and quality of life scores. Additional assessments also include ecchymosis scores, occlusion rates, time to return to usual activities/work at two weeks. Patients are reviewed at 2 weeks, 3 months, 6 months and 12 months" |
| Notes | Two references, additional references to ongoing study Belramman 2018, were identified from a top‐up search and will be incorporated in the next update |
Morrison 2020.
| Methods | 60‐month extension study of the randomised VeClose study |
| Participants | Participants with symptomatic moderate to severe varicosities (CEAP class C2 ‐ C4b) and symptomatic GSV incompetence |
| Interventions | Randomly assigned (1:1) to either CAC or RFA |
| Outcomes | The primary outcome measure of this 60‐month extension study was complete closure of the target vein, with planned exploratory analysis of noninferiority Secondary outcomes included CEAP class; completion of the VCSS, EuroQol‐Five Dimension survey, and Aberdeen Varicose Vein Questionnaire; participant satisfaction with treatment; AEs related to target GSV; and details of adjunctive procedures |
| Notes | This reference, an additional publication presenting follow‐up data of included study Morrison 2015, was identified from a top‐up search and will be incorporated in the next update |
Rai 2019.
| Methods | Parallel single‐blinded randomised clinical trial |
| Participants | 60 adults with primary varicose veins due to incompetent GSV (CEAP classes C2 to C4 (Ep As Pr)) |
| Interventions | RFA or foam sclerotherapy |
| Outcomes | HRQoL was assessed by the Short Form 36, and the AVVQ was applied to assess the impact of varicose veins on quality of life of the participants. In addition, pain severity after the procedures was investigated by a VAS (range, 0 to 10). The participants were followed at 1 week, 1 month, 3 months, and 6 months post‐operation. GSV reflux and recurrence was assessed by colour DUS examination after 6 months |
| Notes | This reference was identified from a top‐up search and will be incorporated in the next update |
Vähäaho 2021.
| Methods | Randomised, three‐arm clinical study |
| Participants | Venous outpatient clinic patients with varicose veins (CEAP class C2 ‐ C4) caused by GSV insufficiency (132 participants) |
| Interventions | 2:1:1 for MOCA, EVLA, and RFA, respectively |
| Outcomes | "The state of the GSV with duplex Doppler ultrasound examination and the disease‐specific quality of life were assessed at 1 month, 1 year, and 3 years after the treatment" |
| Notes | This reference, an additional publication presenting follow‐up data of included study Vähäaho 2019, was identified from a top‐up search and will be incorporated in the next update |
AVVQ: Aberdeen Varicose Vein Questionnaire ABPI: ankle‐brachial pressure index AE: adverse events CAC: cyanoacrylate closure CEAP: Clinical, Etiological, Anatomical and Pathophysiological DUS: duplex ultrasound EVLA: endovenous laser ablation GSV: great saphenous vein HRQoL: health‐related quality of life MOCA: mechanochemical ablation RFA: radiofrequency ablation SSV: short saphenous vein VAS: visual analogue scale VCSS: Venous Clinical Severity Score
Characteristics of ongoing studies [ordered by study ID]
Belramman 2018.
| Study name | Randomised controlled trial of mechanochemical ablation versus cyanoacrylate adhesive for the treatment of varicose veins |
| Methods | Prospective, multicentre, randomised, double‐blind, parallel assignment trial |
| Participants | 180 participants |
| Interventions | Procedure 1: Mechanochemical ablation (MOCA). MOCA using the ClariVein mechanochemical ablation (MOCA) device (Vascular Insights, Madison, CT, USA). Procedure 2: Cyanoacrylate adhesive. Cyanoacrylate using the VenaSealTM Closure System (Medtronic, Minneapolis, Minnesota, USA). |
| Outcomes | Primary outcome
Secondary outcomes
|
| Starting date | 6 November 2017 |
| Contact information | Amjad Belramman: mailto:a.belramman17%40imperial.ac.uk?subject=NCT03392753, 17/LO/1457, Mechanochemical Ablation Compared to Cyanoacrylate Adhesive Roshan Bootun: mailto:r.bootun%40imperial.ac.uk?subject=NCT03392753, 17/LO/1457, Mechanochemical Ablation Compared to Cyanoacrylate Adhesive |
| Notes |
Cho 2020.
| Study name | CASS (CyanoAcrylate closure versus Surgical Stripping for incompetent saphenous veins) study: a randomized controlled trial comparing clinical outcomes after cyanoacrylate closure and surgical stripping for the treatment of incompetent saphenous veins |
| Methods | Open‐label, multicenter, prospective, randomised controlled trial evaluating the non‐inferior clinical outcomes of cyanoacrylate closure compared to surgical stripping for the treatment of incompetent saphenous veins |
| Participants | Participants must have identifiable reflux in the GSV for greater than 0.5 s after distal compression and release or Valsalva’s maneuver in the standing or reverse Trendelenburg position. Participants must also have a CEAP classification score of C2 through C5. |
| Interventions | CAC closure or surgical stripping and followed up for a total of 24 months after treatment |
| Outcomes | Primary outcome: complete closure of the target vein (defined as vein closure along the entire treated vein segment with no discrete segments of patency exceeding 5 cm after cyanoacrylate closure, and the absence of venous reflux or residual venous tissue after surgical stripping) Secondaryoutcomes: perioperative pain, post‐operative ecchymosis, VCSS score, AVVQ, and EQ‐5D at each scheduled follow‐up visit; all adverse events during the 24‐month follow‐up period; and the complete closure rate and absence of venous reflux or residual venous tissue at the 12‐ and 24‐month follow‐ups |
| Starting date | 2 April 2018 |
| Contact information | In Mok Jung. Department of Surgery, Seoul Metropolitan Government‐Seoul National University Boramae Medical Center, Seoul National University College of Medicine, Seoul, South Korea |
| Notes | Esimated completion of recruitment 29 February 2020 |
NCT04526626.
| Study name | Endovenous radiofrequency ablation versus high ligation and stripping for treatment of varicose veins: a prospective controlled trial |
| Methods | Single group assignment. No details on allocation. This study was to investigate the outcomes of RFA and stripping for varicose veins |
| Participants | 300 participants with varicose veins |
| Interventions | High ligation and stripping or RFA for treatment of lower limb varicose veins (ClosureFast, Medtronic) |
| Outcomes | Technical success, complications, recurrence |
| Starting date | 1 February 2020 |
| Contact information | Principal Investigator: Hailei Li, MD, PhD; University of Hong Kong Shenzhen Hospital |
| Notes | Estimated completion 30 June 2022 |
NCT04534244.
| Study name | Management of tributary veins in superficial venous insufficiency of the lower limbs: impact of endovenous steam treatment versus phlebectomy on quality of life (INVOLVE) |
| Methods | Randomised, parallel, open label. This study aims to compare two surgical techniques for the treatment of superficial chronic venous insufficiency of the lower limbs: phlebectomy, the gold‐standard technique, and endovenous steam treatment |
| Participants | 134 participants with venous insufficiency of the leg |
| Interventions | Experimental group: endovenous steam treatment of the tributary veins (VBox Hybrid) Control group: treatment of the tributary veins by phlebectomy |
| Outcomes | QoL, occlusion, return to activity |
| Starting date | October 2020 |
| Contact information | No contact details provided. Centre Hospitalier Universitaire de Besancon |
| Notes | Estimated completion February 2023 |
AVVQ: Aberdeen Varicose Vein Questionnaire CAC: cyanoacrylate closure CEAP: Clinical, Etiological, Anatomical and Pathophysiological classification score CIVIQ‐14: Chronic Venous Insufficiency Questionnaire CVI: chronic venous insufficiency EQ‐5D: EuroQol 5‐domain Utility Index GSV: great saphenous vein MOCA: mechanochemical ablation QoL: quality of life VAS: visual analogue scale VCSS: Venous Clinical Severity Score
Differences between protocol and review
The title of this review was changed from 'Endovenous ablation (radiofrequency and laser) and foam sclerotherapy versus open surgery for great saphenous vein varices' to 'Interventions for great saphenous vein incompetence'. This was to reflect a widening of scope to include the range of interventions currently available for GSV incompetence. Additional comparisons are listed in Types of interventions. To reflect current clinical relevance, we rearranged outcomes and clarified inclusion and exclusion criteria. Due to new Cochrane Vascular guidelines, we did not perform cost analysis of the interventions within this review, and we included summary of findings tables to present the certainty of the evidence.
Contributions of authors
JW: conducted the review, analysed studies for inclusion, selected trials for inclusion, assessed methodological quality of trials and extracted data, entered the data, developed the analysis plan for the update and drafted the review update, wrote the manuscript and contributed significantly to the overall process (joint first author) SN: conducted the review of included studies, arbitrated on the selection of trials, assisted with data extraction, assessed methodological quality and assisted in drafting the final review, reviewed and wrote the manuscript, contributed significantly to the overall process (joint first author) CN: conducted the previous review, contributed to the included studies' analysis, dealt with discrepancies and reviewed the manuscript GS: conceived the original idea for this review, supervised the review, developed the protocol, helped with analysis, wrote and proofread this current review
Sources of support
Internal sources
No sources of support provided
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK
The Cochrane Vascular editorial base is supported by the Chief Scientist Office.
Declarations of interest
JW: none known SN: none known CN: none known GS: none known
These authors contributed equally to this work
These authors contributed equally to this work
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Calik 2019 {published data only}
- Calik ES, Arslan U, Erkut B. Ablation therapy with cyanoacrylate glue and laser for refluxing great saphenous veins - a prospective randomised study. VASA. Zeitschrift fur Gefasskrankheiten. Journal for Vascular Diseases 2019;48(5):405-12. [DOI] [PubMed] [Google Scholar]
Darwood 2008 {published data only}
- Beale R, Theivacumar N, Mavor AID, Gough MJ. Endovenous Laser Treatment (EVLT) or surgery of varicose veins? A randomised controlled trial in patients with saphenofemoral and long saphenous incompetence. The Vascular Society of Great Britain and Ireland Yearbook 2005:77.
- Darwood RJ, Theivacumar N, Dellagrammaticas D, Mavor AI, Gough MJ. Randomized clinical trial comparing endovenous laser ablation with surgery for the treatment of primary great saphenous varicose veins. British Journal of Surgery 2008;95(3):294-301. [DOI] [PubMed] [Google Scholar]
EVOLVeS 2003 {published data only}
- Lurie F, Creton D, Eklof B, et al. Prospective randomised study of endovenous radiofrequency obliteration (closure) versus ligation and vein stripping (EVOLVeS): 2-year follow-up. Journal of Vascular Surgery 2005;42(1):178. [DOI] [PubMed] [Google Scholar]
- Lurie F, Creton D, Eklof B, Kabnick L, Kistner R, Pichot O, et al. Prospective randomised study of endovenous radiofrequency obliteration (closure) versus ligation and stripping (evolves study): early results and one year follow-up. In: European Society for Vascular Surgery, Programme and Abstract Book, XVII Annual Meeting and Course on Vascular Surgical Techniques. Dublin, Ireland, 2003:74-5.
- Lurie F, Creton D, Eklof B, Kabnick LS, Kistner RL, Pichot O, et al. Prospective randomised study of endovenous radiofrequency obliteration (closure) versus ligation and vein stripping (EVOLVeS): two-year follow-up. European Journal of Vascular and Endovascular Surgery 2005;29(1):67-73. [DOI] [PubMed] [Google Scholar]
- Lurie F, Creton D, Eklof B, Kabnick LS, Kistner RL, Pichot O, et al. Prospective randomized study of endovenous radiofrequency obliteration (closure procedure) versus ligation and stripping in a selected patient population (EVOLVeS Study). Journal of Vascular Surgery 2003;38(2):207-14. [DOI] [PubMed] [Google Scholar]
Flessenkämper 2013 {published data only}
- Flessenkämper I, Hartmann M, Hartmann K, Stenge D, Roll S. Endovenous laser ablation with and without high ligation compared to high ligation and stripping for treatment of great saphenous varicose veins: results of a multicentre randomised controlled trial with up to 6 years follow-up. Phlebology 2016;31(1):23-33. [DOI: 10.1177/0268355514555547] [DOI] [PubMed] [Google Scholar]
- Flessenkämper I, Hartmann M, Stenger D, Roll S. Endovenous laser ablation with and without high ligation compared with high ligation and stripping in the treatment of great saphenous varicose veins: initial results of a multicentre randomized controlled trial. Phlebology 2013;28(1):16-23. [DOI] [PubMed] [Google Scholar]
- Flessenkaemper I, Hartmann M, Stenger D. RCT for differentiated open and endoluminal therapies for degenerative disease of the great saphenous vein. Union Internationale de Phlebologie World Congress 2009.
- Flessenkamper I, Stenger D, Hartmann M, Roll S. Endovenous laser therapy vs. high ligation/stripping for varicosity of the great saphenous vein: clinical and sonographic findings. Phlebologie 2013;42(1):7-11. [Google Scholar]
FOAM 2010 {published data only}
- Lam L, Lawson JA, Toonder IM, Shadid NH, Sommer A, Veenstra M, et al. Eight-year follow-up of a randomized clinical trial comparing ultrasound-guided foam sclerotherapy with surgical stripping of the great saphenous vein. British Journal of Surgery 2018;105:692-98. [DOI] [PubMed] [Google Scholar]
- NCT01103258. Cost minimization study comparing surgery versus duplex guided foam sclerotherapy of varicose veins (FOAM Study). clinicaltrials.gov/ct2/show/NCT01103258 (first received 14 April 2010).
- Shadid N, Ceulen R, Nelemans P, Dirksen C, Veraart J, Schurink GW, et al. Randomized clinical trial of ultrasound-guided foam sclerotherapy versus surgery for the incompetent great saphenous vein. British Journal of Surgery 2012;99(8):1062-70. [DOI] [PubMed] [Google Scholar]
- Shadid N, Nelemans P, Sommer A. Duplex guided foam sclerotherapy vs surgery for the incompetent great saphenous vein: a randomised controlled trial. Phlebology 2010;25:306-7. [Google Scholar]
Helmy ElKaffas 2011 {published data only}
- Helmy ElKaffas K, ElKashef O, ElBaz W. Great saphenous vein radiofrequency ablation versus standard stripping in the management of primary varicose veins - a randomized clinical trial. Angiology 2011;62(1):49-54. [DOI] [PubMed] [Google Scholar]
HELP‐1 2011 {published data only}
- Carradice D, Mekako A, Hatfield J, Chetter I. Recurrent varicose veins are more common following surgery than EVLT - results of a randomised controlled trial. The Vascular Society of Great Britain and Ireland Yearbook 2009:86.
- Carradice D, Mekako AI, Hatfield J, Chetter IC. A randomised trial of EVLT vs surgery for varicose veins. The Vascular Society of Great Britain and Ireland Yearbook 2008:91.
- Carradice D, Mekako AI, Mazari FA, Samuel N, Hatfield J, Chetter IC. Clinical and technical outcomes from a randomized clinical trial of endovenous laser ablation compared with conventional surgery for great saphenous varicose veins. British Journal of Surgery 2011;98(8):1117-23. [DOI] [PubMed] [Google Scholar]
- Carradice D, Mekako AI, Mazari FA, Samuel N, Hatfield J, Chetter IC. Randomized clinical trial of endovenous laser ablation compared with conventional surgery for great saphenous varicose veins. British Journal of Surgery 2011;98(4):501-10. [DOI] [PubMed] [Google Scholar]
- Carradice D, Wallace T, Samuel N, Gohil R, Chetter I. A comparison of the effectiveness of treating those with and without the complications of superficial venous insufficiency. Vascular Society of Great Britain and Ireland Yearbook 2012:38. [DOI] [PubMed]
- Wallace T, El-Sheikha J, Nandhra S, Leung C, Mohamed A, Harwood A, et al. Long-term outcomes of endovenous laser ablation and conventional surgery for great saphenous varicose veins. British Journal of surgery 2018;105:1759-67. [DOI] [PubMed] [Google Scholar]
Lane 2017 {published data only}
- Bootun R, Lane TR, Dharmarajah B, Lim CS, Najem M, Renton S, et al. Intra-procedural pain score in a randomised controlled trial comparing mechanochemical ablation to radiofrequency ablation: the multicentre Venefit TM versus ClariVein® for varicose veins trial. Phlebology 2016;31(1):61-5. [DOI] [PubMed] [Google Scholar]
- Lane T, Bootun R, Dharmarajah B, Lim CS, Najem M, Renton S, et al. A multi-centre randomised controlled trial comparing radiofrequency and mechanical occlusion chemically assisted ablation of varicose veins - final results of the Venefit versus Clarivein for varicose veins trial. Phlebology 2017;32(2):89-98. [DOI] [PubMed] [Google Scholar]
LAST 2014 {published data only}
- NCT02046967. Steam ablation versus endovenous laser ablation for the treatment of great saphenous veins (LAST). clinicaltrials.gov/show/NCT02046967 (first received 28 January 2014).
- Van den Bos RR, Malskat WS, De Maeseneer MG, Roos KP, Groeneweg DA, Kockaert MA, et al. Randomized clinical trial of endovenous laser ablation versus steam ablation (LAST trial) for great saphenous varicose veins. British Journal of Surgery 2014;101(9):1077-83. [DOI] [PubMed] [Google Scholar]
Magna 2013 {published data only}
- Biemans AA, Kockaert M, Akkersdijk GP, Van den Bos RR, Maeseneer MG, Cuypers P, et al. Comparing endovenous laser ablation, foam sclerotherapy, and conventional surgery for great saphenous varicose veins. Journal of Vascular Surgery 2013;58(3):727-34. [DOI] [PubMed] [Google Scholar]
- Biemans AA, Kockaert M, Van den Bos RR, Cuypers P, Neumann HA, Nijsten TE. A randomized comparative study of the three most commonly performed treatments for varicose veins, results after one year. Nederlands Tijdschrift voor Dermatologie en Venereologie 2012;22(2):78-84. [Google Scholar]
- NCT00529672. Surgery or non invasive therapy for varicose veins (Magna). clinicaltrials.gov/ct2/show/NCT00529672 (first received 14 September 2014).
- Van der Velden SK, Biemans AA, De Maeseneer MG, Kockaert MA, Cuypers PW, Hollestein LM, et al. Five-year results of a randomized clinical trial of conventional surgery, endovenous laser ablation and ultrasound-guided foam sclerotherapy in patients with great saphenous varicose veins. British Journal of Surgery 2015;102(10):1184-94. [DOI] [PubMed] [Google Scholar]
MARADONA 2019 {published data only}
- Holewijn S, Van Eekeren RJ, Vahl A, De Vries JP, Reijnen MM. Two year results of a multicentre randomised control trial comparing mechanochemical endovenous ablation to radiofrequency ablation in the treatment of primary great saphenous vein incompetence (MARADONA trial). Journal of Vascular Surgery 2019;7(3):364-74. [DOI] [PubMed] [Google Scholar]
- NCT01936168. MOCA versus RFA in the treatment of primary great saphenous varicose veins (MARADONA). clinicaltrials.gov/show/NCT01936168 (first received 5 September 2013).
Morrison 2015 {published data only}
- Morrison N, Gibson K, McEnroe S, Goldman M, King T, Weiss R, et al. Randomized trial comparing cyanoacrylate embolization and radiofrequency ablation for incompetent great saphenous veins (VeClose). Journal of Vascular Surgery 2015;61(4):985-94. [DOI] [PubMed] [Google Scholar]
- Morrison N, Kolluri R, Vasquez M, Madsen M, Jones A, Gibson K. Comparison of cyanoacrylate closure and radiofrequency ablation for the treatment of incompetent great saphenous veins: 36-month outcomes of the VeClose randomized controlled trial. Phlebology 2019;34(6):380-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nordon 2011 {published data only}
- Nordon IM, Hinchliffe RJ, Brar R, Moxey P, Black S, Thompson M, et al. A prospective double-blind randomised controlled trial of radiofrequency versus laser treatment of great saphenous varicose veins. Cardiovascular and Interventional Radiology 2011;34:503-4. [DOI] [PubMed] [Google Scholar]
- Nordon IM. A prospective double-blind randomized controlled trial of radiofrequency versus laser treatment of the great saphenous vein in patients with varicose veins. Annals of Surgery 2011;254:876-81. [DOI] [PubMed] [Google Scholar]
Pronk 2010 {published data only}
- Gauw SA, Lawson JA, Van Vlijmen-Van Keulen CJ, Pronk P, Gaastra MT, Mooij MC. Five-year follow-up of a randomized, controlled trial comparing saphenofemoral ligation and stripping of the great saphenous vein with endovenous laser ablation (980 nm) using local tumescent anesthesia. Journal of Vascular Surgery 2016;63(2 Suppl):420-8. [DOI] [PubMed] [Google Scholar]
- Pronk P, Gauw SA, Mooij MC, Gaastra MT, Lawson JA, Van Goethem AR, et al. Randomised controlled trial comparing sapheno-femoral ligation and stripping of the great saphenous vein with endovenous laser ablation (980 nm) using local tumescent anaesthesia: one year results. European Journal of Vascular and Endovascular Surgery 2010;40(5):649-656 e61. [DOI] [PubMed] [Google Scholar]
Rasmussen 2007 {published data only}
- ISRCTN16747172. Randomized controlled study comparing endovenous laser ablation with stripping, in patients with varicose veins due to greater saphenous vein insufficiency. isrctn.com/ISRCTN16747172 (first received 15 November 2005).
- Lawaetz M, Bjoern L, Rasmussen L. Randomised clinical trial comparing endovenous laser ablation with stripping of the great saphenous vein. Clinical outcome and recurrence after 5 years. Scientific Programme and Book of Abstracts of the 14th Annual Meeting of the European Venous Forum 2013:41. [DOI] [PubMed]
- Rasmussen L, Lawaetz M, Bjoern L, Blemings A, Eklof B. Randomized clinical trial comparing endovenous laser ablation and stripping of the great saphenous vein with clinical and duplex outcome after 5 years. Journal of Vascular Surgery 2013;58(2):421-6. [DOI] [PubMed] [Google Scholar]
- Rasmussen LH, Bjoern L, Lawaetz B, Blemings A, Eklof B. Randomised clinical trial comparing endovenous laser ablation with stripping of the great saphenous vein: clinical outcome and recurrence after 2 years. European Journal of Vascular and Endovascular Surgery 2010;39(5):630-5. [DOI] [PubMed] [Google Scholar]
- Rasmussen LH, Bjoern L, Lawaetz M, Blemings A, Lawaetz B, Eklof B. Randomized trial comparing endovenous laser ablation of the great saphenous vein with high ligation and stripping in patients with varicose veins: short-term results. Journal of Vascular Surgery 2007;46(2):308-15. [DOI] [PubMed] [Google Scholar]
- Rasmussen LH, Lawaetz M, Bjoern L, Lawaetz B, Blemings A, Eklof B. Medium-term follow-up of a randomised trial comparing laser ablation with stripping of the great saphenous vein. Recurrence rate and pattern after two years. Phlebology 2009;24:231.
Rasmussen 2011 {published data only}
- ISRCTN08060326. Endovenous laser ablation (EVLA), radio frequency (RF), foam sclerotherapy and stripping for treatment of varicose veins. isrctn.com/ISRCTN08060326 (first received 31 July 2009).
- Lawaetz M, Rasmussen LH, Bjoern L, Blemings A, Eklof B. Randomized trial comparing RF, laser, foam sclerotherapy and stripping in varicose veins. Phlebology 2010;25:307. [DOI] [PubMed] [Google Scholar]
- Lawaetz M, Serup J, Lawaetz B, Bjoern L, Blemings A, Eklof B, et al. Comparison of endovenous ablation techniques, foam sclerotherapy and surgical stripping for great saphenous varicose veins. Extended 5-year follow-up of a RCT. International Angiology 2017;36(3):281-8. [DOI] [PubMed] [Google Scholar]
- Rasmussen L. Randomised trial shows higher technical failure rate with foam. Vascular News 2010;May:33. [Google Scholar]
- Rasmussen LH, Lawaetz M, Bjoern L, Vennits B, Blemings A, Eklof B. Randomized clinical trial comparing endovenous laser ablation, radiofrequency ablation, foam sclerotherapy and surgical stripping for great saphenous varicose veins. British Journal of Surgery 2011;98(8):1079-87. [DOI] [PubMed] [Google Scholar]
Rautio 2002 {published data only}
- Perala J, Rautio T, Biancari F, Ohtonen P, Wiik H, Heikkinen T, et al. Radiofrequency endovenous obliteration versus stripping of the long saphenous vein in the management of primary varicose veins: 3-year outcome of a randomized study. Annals of Vascular Surgery 2005;19(5):669-72. [DOI] [PubMed] [Google Scholar]
- Rautio T, Ohinmaa A, Perala J, Ohtonen P, Heikkinen T, Wiik H, et al. Endovenous obliteration versus conventional stripping operation in the treatment of primary varicose veins: a randomized controlled trial with comparison of the costs. Journal of Vascular Surgery 2002;35(5):958-65. [DOI] [PubMed] [Google Scholar]
Recovery 2009 {published data only}
- Almeida JI, Kaufman J, Gockeritz O, Chopra P, Evans MT, Hoheim DF, et al. Radiofrequency endovenous ClosureFAST versus laser ablation for the treatment of great saphenous reflux: a multicenter, single-blinded, randomized study (RECOVERY study). Journal of Vascular and Interventional Radiology 2009;20(6):752-9. [DOI] [PubMed] [Google Scholar]
RELACS 2012 {published data only}
- Rass K, Frings N, Glowacki P, Graber S, Tilgen W, Vogt T. Same site recurrence is more frequent after endovenous laser ablation compared with high ligation and stripping of the great saphenous vein: 5 year results of a randomized clinical trial (RELACS Study). European Journal of Vascular and Endovascular Surgery 2015;50(5):648-56. [DOI] [PubMed] [Google Scholar]
- Rass K, Frings N, Glowacki P, Hamsch C, Graber S, Vogt T, et al. Comparable effectiveness of endovenous laser ablation and high ligation with stripping of the great saphenous vein: two-year results of a randomized clinical trial (RELACS study). Archives of Dermatology 2012;148(1):49-58. [DOI] [PubMed] [Google Scholar]
Shepherd 2010 {published data only}
- Shepherd AC, Gohel MS, Brown LC, Metcalfe MJ, Hamish M, Davies AH. Randomized clinical trial of VNUS ClosureFAST radiofrequency ablation versus laser for varicose veins. British Journal of Surgery 2010;97(6):810-8. [DOI] [PubMed] [Google Scholar]
- Shepherd AC, Ortega-Ortega M, Gohel MS, Epstein D, Brown LC, Davies AH. Cost-effectiveness of radiofrequency ablation versus laser for varicose veins. International Journal of Technology Assessment in Health Care 2015;31(5):289-96. [DOI] [PubMed] [Google Scholar]
Subramonia 2010 {published data only}
- Balakrishnan A, Mylankal K, Nalachandran S, Subramonia S, Lees T. A randomized controlled trial of radiofrequency ablation and conventional surgery for primary long saphenous varicose veins. Phlebology 2008;23(4):198. [Google Scholar]
- Subramonia S, Lees T. Radiofrequency ablation vs conventional surgery for varicose veins - a comparison of treatment costs in a randomised trial. European Journal of Vascular and Endovascular Surgery 2010;39:104-11. [DOI] [PubMed] [Google Scholar]
- Subramonia S, Lees T. Randomized clinical trial of radiofrequency ablation or conventional high ligation and stripping for great saphenous varicose veins. British Journal of Surgery 2010;97(3):328-36. [DOI] [PubMed] [Google Scholar]
Syndor 2017 {published data only}
- Sydnor M, Mavropoulos J, Slobodnik N, Wolfe L, Strife B, Komorowski D. A randomized prospective long-term (>1 year) clinical trial comparing the efficacy and safety of radiofrequency ablation to 980 nm laser ablation of the great saphenous vein. Phlebology 2017;32(6):415-24. [DOI] [PubMed] [Google Scholar]
Vähäaho 2019 {published data only}
- Vähäaho S, Mahmoud O, Halmesmäki K, Albäck A, Noronen K, Vikatmaa P, et al. Randomized clinical trial of mechanochemical and endovenous thermal ablation of great saphenous varicose veins. British Journal of Surgery 2019;106(5):548-54. [DOI] [PubMed] [Google Scholar]
Vernermo 2016 {published data only}
- Vähäaho S, Halmesmäki K, Albäck A, Saarinen E, Venermo M. Five-year follow-up of a randomized clinical trial comparing open surgery, foam sclerotherapy and endovenous laser ablation for great saphenous varicose veins. British Journal of Surgery 2018;105:686-91. [DOI] [PubMed] [Google Scholar]
- Venermo M, Saarinen J, Eskelinen E, Vähäaho S, Saarinen E, Railo M, et al. Randomized clinical trial comparing surgery, endovenous laser ablation and ultrasound-guided foam sclerotherapy for the treatment of great saphenous varicose veins. British Journal of Surgery 2016;103(11):1438-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Basela 2011 {published data only}
- Basela H, Aydina C, Aya Y, Inana B, Ekimb H, Goyac C, et al. Endovenous laser ablation (EVLA) versus high ligation and striping (HL/S): two-years follow up. Eastern Journal of Medicine 2012;17(2):83-7. [Google Scholar]
Campos 2015 {published data only}
- Campos W, Torres IO, da Silva ES, Casella IB, Puech-Leão P. A prospective randomised study comparing polidocanol foam sclerotherapy with surgical treatment of patients with primary chronic venous insufficiency and ulcer. Annals of Vascular Surgery 2015;29(6):1128-35. [DOI] [PubMed] [Google Scholar]
Chant 1972 {published data only}
- Beresford SA, Chant AD, Jones HO, Piachaud D, Weddell JM. Varicose veins: a comparison of surgery and infection/compression sclerotherapy. Five-year follow-up. Lancet 1978;1(8070):921-4. [DOI] [PubMed] [Google Scholar]
- Chant AD, Jones HO, Weddell JM. Varicose veins: a comparison of surgery and injection/compression sclerotherapy. Lancet 1972;2(7788):1188-91. [DOI] [PubMed] [Google Scholar]
Christenson 2010 {published data only}
- Christensen JT, Gueddi S, Gemayel G, Bounameaux H. Prospective randomized trial comparing endovenous laser ablation and surgery for treatment of primary great saphenous varicose veins with a 2-year follow-up. Journal of Vascular Surgery 2010;52(5):1234-41. [DOI] [PubMed] [Google Scholar]
CLASS 2014 {published data only}
- Brittenden J, Cotton SC, Elders A, Ramsay CR, Norrie J, Burr J, et al. A randomized trial comparing treatments for varicose veins. New England Journal of Medicine 2014;371(13):1218-27. [DOI] [PubMed] [Google Scholar]
Compagna 2010 {published data only}
- Compagna R, De Vito D, Rossi R, Fappiano F, De Magistris L, Sodano L, et al. A prospective randomised controlled trial comparing foam sclerotherapy combined with sapheno-femoral ligation to surgical treatment of varicose veins. European Surgical Research 2010;45(3-4):267. [Google Scholar]
De Medeiros 2006 {published data only}
- De Medeiros CA, Luccas GC. Comparison of endovenous treatment with an 810 nm laser versus conventional stripping of the great saphenous vein in patients with primary varicose veins. Dermatologic Surgery 2005;31(12):1685-94. [DOI] [PubMed] [Google Scholar]
- De Medeiros CAF. Comparison of endovenous laser therapy vs conventional stripping of the great saphenous vein: midterm results. Jornal Vascular Brasileiro 2006;5(4):277-87. [Google Scholar]
De Oliveira 2018 {published data only}
- De Oliveira AC, Sobreira ML, Bertanha M, Jaldin RG, Rosa FD, Angeleli P, et al. Comparison of the efficacy and safety of endovenous radiofrequency ablation, endovenous laser ablation, foam sclerotherapy, and elastic compression for the treatment of severe chronic venous insufficiency (CEAP 6). Vascular and Endovascular Surgery 2018;52(8 Suppl 1):S44-45. [DOI: 10.1177/1538574418797220] [DOI] [Google Scholar]
Desai 2009 {published data only}
- Desai DJ, Pedgoankar P, Sekhar R. Endovascular venous laser vs oesch pin stripper in management of primary varicosities of the great saphenous vein: a randomized control trial. ANZ Journal of Surgery 2009;79(S1):A88-91. [Google Scholar]
Disselhoff 2008 {published data only}
- Disselhoff BC, Buskens E, Kelder JC, Kinderen DJ, Moll FL. Randomised comparison of costs and cost-effectiveness of cryostripping and endovenous laser ablation for varicose veins: 2-year results. European Journal of Vascular and Endovascular Surgery 2009;37(3):357-63. [DOI] [PubMed] [Google Scholar]
- Disselhoff BC, Kinderen DJ, Kelder JC, Moll FL. Five-year results of a randomized clinical trial comparing endovenous laser ablation with cryostripping for great saphenous varicose veins. British Journal of Surgery 2011;98(8):1107-11. [DOI] [PubMed] [Google Scholar]
- Disselhoff BC, Kinderen DJ, Kelder JC, Moll FL. Randomized clinical trial comparing endovenous laser ablation of the great saphenous vein with and without ligation of the sapheno-femoral junction: 2-year results. European Journal of Vascular and Endovascular Surgery 2008;36(6):713-8. [DOI] [PubMed] [Google Scholar]
- Disselhoff BC, der-Kinderen DJ, Kelder JC, Moll FL. Randomized clinical trial comparing endovenous laser with cryostripping for great saphenous varicose veins. British Journal of Surgery 2008;95(10):1232-8. [DOI] [PubMed] [Google Scholar]
dos Santos 2020 {published data only}
- dos Santos JB, Júnior WC, Porta RM, Puggina J, da Silva DF, Puech-Leao P, et al. Catheter-directed foam sclerotherapy with tumescence of the great saphenous vein versus ultrasound-guided foam sclerotherapy: a randomized controlled trial. Phlebology 2020;35(2):84-91. [DOI] [PubMed] [Google Scholar]
Einarsson 1993 {published data only}
- Einarsson E, Eklof B, Neglen P. Sclerotherapy or surgery as treatment for varicose veins: a prospective randomized study. Phlebology 1993;8:22-6. [Google Scholar]
Eroglu 2018 {published data only}
- Eroglu E, Yasim A. A randomised clinical trial comparing N-butyl cyanoacrylate, radiofrequency ablation and endovenous laser ablation for the treatment of superficial venous incompetence: two year follow up results. European Journal of Vascular and Endovascular Surgery 2018;56(4):553-60. [DOI] [PubMed] [Google Scholar]
Figueiredo 2009 {published data only}
- Figueiredo M, Araujo S, Barros N Jr, Miranda F Jr. Results of surgical treatment compared with ultrasound-guided foam sclerotherapy in patients with varicose veins: a prospective randomised study. European Journal of Vascular and Endovascular Surgery 2009;38(6):758-63. [DOI] [PubMed] [Google Scholar]
Honek 2019 {published data only}
- Honek T, Horvath M, Horvath V, Slais M, Kneifl T, Honek J, et al. Catheter laser ablation of superficial veins of the lower extremities in the symptomatic treatment of venous reflux comparison of the immediate results of two types of laser [Katetrizacni laserova ablace povrchovych zil dolnich koncetin v lecbe symptomatickeho zilniho refluxu s varixy - porovnani bezprostredni ucinnosti dvou typu laserovych]. Rozhledy v chirurgii: mesicnik Ceskoslovenske chirurgicke spolecnosti 2019;98(6):248-51. [PubMed] [Google Scholar]
Jindal 2018 {published data only}
- Jindal R. A single center randomized controlled trial comparing radiofrequency and mechanical occlusion chemically assisted ablation of varicose veins in patients with bilateral involvement: Initial experience. International Angiology 2018;37(1 Suppl 1):20. [Google Scholar]
Kalodiki 2012 {published data only}
- Kalodiki E, Azzam M, Schnatterbeck P, Geroulakos G, Lattimer CR. The Discord Outcome Analysis (DOA) as a reporting standard at three months and five years in randomised varicose vein treatment trials. European Journal of Vascular and Endovascular Surgery 2019;57(2):267-74. [DOI] [PubMed] [Google Scholar]
- Kalodiki E, Lattimer CR, Azzam M, Shawish E, Bountouroglou D, Geroulakos G. Long-term results of a randomized controlled trial on ultrasound-guided foam sclerotherapy combined with saphenofemoral ligation vs standard surgery for varicose veins. Journal of Vascular Surgery 2012;55(2):451-7. [DOI] [PubMed] [Google Scholar]
Karathanos 2019 {published data only}
- Karathanos C, Spanos K, Nana P, Batzalexis K, Kouvelos G, Giannoukas A. A randomized clinical study of radiofrequency ablation versus 1470nm laser for great saphenous vein reflux. European Journal of Vascular and Endovascular Surgery 2019;58(6 (Suppl 3)):e705-6. [Google Scholar]
Kikuchi 2009 {published data only}
- Kikuchi R, Neto EA, Carnevale F. Clinical comparison of radiofrequency ablation (RFA) versus endovenous laser ablation (EVLA) in great saphenous vein insufficiency treatment. In: XVI World Congress of the Union Internationale de Phlebologie Abstract Book. Vol. 14. 2009.
Lattimer 2012 {published data only}
- Lattimer CR, Azzam M, Geroulakos G, Kalodiki E. The anterior accessory saphenous vein in the treatment of a great saphenous vein reflux with laser versus foam: five-year results of a randomized controlled trial. Vasomed 2017;29(5):222-3. [Google Scholar]
- Lattimer CR, Azzam M, Kalodiki E, Shawish E, Trueman P, Geroulakos G. Cost and effectiveness of laser with phlebectomies compared with foam sclerotherapy in superficial venous insufficiency. Early results of a randomised controlled trial. European Journal of Vascular and Endovascular Surgery 2012;43(5):594-600. [DOI] [PubMed] [Google Scholar]
- Lattimer CR, Kalodiki E, Azzam M, Makris GC, Somiayajulu S, Geroulakos G. Interim results on abolishing reflux alongside a randomized clinical trial on laser ablation with phlebectomies versus foam sclerotherapy. International Angiology 2013;32(4):394-403. [PubMed] [Google Scholar]
Leon 2018 {published data only}
- Leon I, Blando JS, Alarcon H. Randomized, comparative and prospective study between radiofrequency venous ablation and sclerotherapy with polidocanol foam 3% versus only radiofrequency venous ablation in saphenous veins of 1.5 cm. of diameter or more. Phlebology 2018;33(10):NP3-NP4. [DOI: 10.1177/0268355518804484] [DOI] [Google Scholar]
Leung 2019 {published data only}
- Leung C, Carradice D, Mohamed A, Wallace T, Chetter I. The Lama Trial - a randomized controlled trial comparing endovenous laser ablation versus mechanochemical ablation in the treatment of superficial venous incompetence. European Journal of Vascular and Endovascular Surgery 2019;58(6 Suppl 1):e118-9. [Google Scholar]
- Leung CC, Carradice D, Wallace T, Chetter IC. Endovenous laser ablation versus mechanochemical ablation with ClariVein® in the management of superficial venous insufficiency (LAMA trial): study protocol for a randomised controlled trial. Trials 2016;17(1):421. [DOI: 10.1186/s13063-016-1548-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lin 2007 {published data only}
- Lin Y, Ye CS, Huang XL, Ye JL, Yin HH, Wang SM. A random, comparative study on endovenous laser therapy and saphenous veins stripping for the treatment of great saphenous vein incompetence. Chung-Hua i Hsueh Tsa Chih [Chinese Medical Journal] 2007;87(43):3043-6. [PubMed] [Google Scholar]
Mendes 2016 {published data only}
- Mendes CA, Martins AA, Fukuda JM, Parente JB, Munia MA, Fioranelli A, et al. Randomized trial of radiofrequency ablation versus conventional surgery for superficial venous insufficiency: if you don't tell, they won't know. Clinics (Sao Paulo, Brazil) 2016;71(11):650-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mozafar 2014 {published data only}
- Mozafar M, Atqiaee K, Haghighatkhah H, Taheri MS, Tabatabaey A, Lotfollahzadeh S. Endovenous laser ablation of the great saphenous vein versus high ligation: long-term results. Lasers in Medical Science 2014;29(2):765-71. [DOI] [PubMed] [Google Scholar]
Oster 2018 {published data only}
- Öster M, Nelzén O. Venous insufficiency treatment and the effect on quality of life. Phlebology 2018;33(10):NP14-5. [Google Scholar]
Ouvry 2008 {published data only}
- Ouvry P, Allaert FA, Desnos P, Hamel-Desnos C. Efficacy of polidocanol foam versus liquid in sclerotherapy of the great saphenous vein: a multicentre randomised controlled trial with a 2-year follow-up. European Journal of Vascular and Endovascular Surgery 2008;36(3):366-70. [DOI] [PubMed] [Google Scholar]
Ovali 2019 {published data only}
- Ovali C, Sevin MB. Twelve-month efficacy and complications of cyanoacrylate embolization compared with radiofrequency ablation for incompetent great saphenous veins. Journal of Vascular Surgery: Venous and Lymphatic Disorders 2019;7(2):210-216. [DOI: 10.1016/j.jvsv.2018.10.019] [DOI] [PubMed] [Google Scholar]
Shadid 2015 {published data only}
- NCT02304146. Long-term ultrasound guided foam sclerotherapy versus classical surgical stripping study. clinicaltrials.gov/show/NCT02304146 2014 (first received 1 December 2014).
- Shadid N, Nelemans P, Lawson J, Sommer A. Predictors of recurrence of great saphenous vein reflux following treatment with ultrasound-guided foam sclerotherapy. Phlebology 2015;30(3):194-9. [DOI] [PubMed] [Google Scholar]
Sincos 2018 {published data only}
- Sincos IR, Puggina J, Campos W, Baptista-Sincos AP, Porta RM, De Luccia N, et al. Effects of saphenous and perforating vein radiofrequency ablation in ulcer healing: midterm results of a randomized controlled trial (VUERT). Journal of Vascular Surgery 2018;67(6):e81. [Google Scholar]
Stotter 2005 {published data only}
- Stotter L, Schaaf I, Bockelbrink A, Baurecht HJ. Radiofrequency obliteration, invagination or cryostripping: which is the best tolerated treatment by the patients? Phlébologie 2005;34(1):19-24. [Google Scholar]
Tawfik 2020 {published data only}
- Tawfik AM, Sorour WA, El-Laboudy ME. Laser ablation versus mechanochemical ablation in the treatment of primary varicose veins: a randomized clinical trial. Journal of Vascular Surgery 2020;8(2):211-5. [DOI] [PubMed] [Google Scholar]
Wright 2006 {published data only}
- Wright D, Gobin JP, Bradbury AW, Coleridge-Smith P, Spoelstra H, Berridge D, et al. Varisolve polidocanol microfoam compared with surgery or sclerotherapy in the management of varicose veins in the presence of trunk vein incompetence: European randomized controlled trial. Phlebology 2006;21(4):180-90. [Google Scholar]
- Wright D. European randomized controlled trial of Varisolve® PD microfoam compared with alternative therapy in management of moderate-to-severe varicose veins: preliminary results. In: Abstracts from the UIP World Congress Chapter Meeting, 2003 Aug 27-31; San Diego, California. 2003.
References to studies awaiting assessment
Belramman 2020 {published data only}
- Belramman A, Bootun R, Tang TY, Lane TR, Davies AH. Early results of a randomised clinical trial of mechanochemical ablation versus cyanoacrylate adhesive for the treatment of varicose veins (MOCCA). European Journal of Vascular and Endovascular Surgery 2019;58(6 Suppl 3):E703. [Google Scholar]
- Belramman A, Bootun R, Tang TY, Lane TR, Davies AH. Randomized clinical trial of mechanochemical ablation versus cyanoacrylate adhesive for the treatment of varicose veins. Journal of Vascular Surgery: Venous and Lymphatic Disorders 2020;8(2):315-6. [Google Scholar]
Morrison 2020 {published data only}
- Morrison N, Gibson K, Vasquez M, Weiss R, Jones A. Five-year extension study of patients from a randomized clinical trial (VeClose) comparing cyanoacrylate closure versus radiofrequency ablation for the treatment of incompetent great saphenous veins. Journal of Vascular Surgery: Venous and Lymphatic Disorders 2020;8(6):978-89. [DOI] [PubMed] [Google Scholar]
Rai 2019 {published data only}
- Rai A, Porsalman M, Khatony A, Sobhiyeh M. Comparison of foam sclerotherapy versus radiofrequency ablation in the treatment of primary varicose veins due to incompetent great saphenous vein: randomized clinical trial. Journal of Vascular Nursing 2019;37(4):226-31. [DOI] [PubMed] [Google Scholar]
Vähäaho 2021 {published data only}
- Vähäaho S, Halmesmäki K, Mahmoud O, Albäck A, Noronen K, Venermo M. Three-year results of a randomized controlled trial comparing mechanochemical and thermal ablation in the treatment of insufficient great saphenous veins. Journal of Vascular Surgery: Venous and Lymphatic Disorders 2021;9(3):652-9. [DOI: 10.1016/j.jvsv.2020.08.007] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Belramman 2018 {published data only}
- Belramman A, Bootun R, Tang TY, Lane TR, Davies AH. Mechanochemical ablation versus cyanoacrylate adhesive for the treatment of varicose veins: study protocol for a randomised controlled trial. Trials 2018;19:428. [DOI: 10.1186/s13063-018-2807-0] [DOI] [PMC free article] [PubMed]
Cho 2020 {published data only}
- Cho S, Park HS, Lee T, Byun SJ, Yun WS, Yang SS, et al. CASS (CyanoAcrylate closure versus Surgical Stripping for incompetent saphenous veins) study: a randomized controlled trial comparing clinical outcomes after cyanoacrylate closure and surgical stripping for the treatment of incompetent saphenous veins. Trials 2020;21:460. [DOI: 10.1186/s13063-020-04393-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT04526626 {published data only}
- NCT04526626. Endovenous radiofrequency ablation versus high ligation and stripping for treatment of varicose veins: a prospective controlled trial. ClinicalTrials.gov/show/NCT04526626 (first received 26 August 2020).
NCT04534244 {published data only}
- NCT04534244. Management of tributary veins in superficial venous insufficiency of the lower limbs: impact of endovenous steam treatment versus phlebectomy on quality of life (INVOLVE). ClinicalTrials.gov/show/NCT04534244 (first received 1 September 2020).
Additional references
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carpentier 2003
- Carpentier PH, Cornu-Thénard A, Uhl JF, Partsch H, Antignani PL. Appraisal of the information content of the C classes of CEAP clinical classification of chronic venous disorders; a multi centre evaluation. Journal of Vascular Surgery 2003;37(4):827–33. [DOI] [PubMed] [Google Scholar]
Corcos 1996
- Corcos L, Procacci T, Peruzzi G, Dini M, De Anna D. Sapheno-femoral valves. Histopathological observations and diagnostic approach before surgery. Dermatologic Surgery 1996;22(10):873-80. [PubMed] [Google Scholar]
Corcos 2000
- Corcos L, De Anna D, Dini M, Macchi C, Ferrari PA, Dini S. Proximal long saphenous vein valves in primary venous insufficiency. Journal des Maladies Vasculaires 2000;25(1):27-36. [PubMed] [Google Scholar]
Coughlin 2015
- Coughlin PA, Berridge DC. Is there a continuing role for traditional surgery? Phlebology 2015;30(2 Suppl):29-35. [DOI: 10.1177/0268355515589248] [DOI] [PubMed] [Google Scholar]
Critchley 1997
- Critchley G, Handa A, Maw A, Harvey A, Harvey MR, Corbett CR. Complications of varicose vein surgery. Annals of the Royal College of Surgeons of England 1997;79(2):105-10. [PMC free article] [PubMed] [Google Scholar]
Devereux 2014
- Devereux N, Recke AL, Westermann L, Recke A, Kahle B. Catheter-directed foam sclerotherapy of great saphenous veins in combination with pre-treatment reduction of the diameter employing the principals of perivenous tumescent local anesthesia. European Journal of Vascular and Endovascular Surgery 2014;47:187-195. [DOI] [PubMed] [Google Scholar]
Disselhoff 2011
- Disselhoff BC, Kinderen DJ, Kelder JC, Moll FL. Five-year results of a randomised clinical trial of endovenous laser ablation of the great saphenous vein with and without ligation of the saphenofemoral junction. European Journal of Vascular and Endovascular Surgery 2011;41(5):685-90. [DOI] [PubMed] [Google Scholar]
Gibson 2017
- Gibson K, Ferris B. Cyanoacrylate closure of incompetent great, small and accessory saphenous veins without the use of post-procedure compression: initial outcomes of a post-market evaluation of the VenaSeal System (the WAVES Study). Vascular 2017;25(2):149-56. [DOI] [PubMed] [Google Scholar]
Gloviczki 2012
- Gloviczki P, Gloviczki ML. Guidelines for the management of varicose veins. Phlebology 2012;27 (Suppl 1):2-9. [DOI: 10.1258/phleb.2012.012s28] [DOI] [PubMed] [Google Scholar]
Goode 2010
- Goode SD, Chowdhury A, Crockett M, Beech A, Simpson R, Richards T, et al. Laser and radiofrequency ablation study (LARA study): a randomised study comparing radiofrequency ablation and endovenous laser ablation (810 nm). European Journal of Vascular and Endovascular Surgery 2010;40(2):246-53. [DOI] [PubMed] [Google Scholar]
Gough 2012
- Gough MJ. Comment on randomized clinical trial of ultrasound-guided foam sclerotherapy versus surgery for the incompetent great saphenous vein. British Journal of Surgery 2012;99(8):1071. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 27 October 2020. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al, GRADE working group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924-926. [DOI: 10.1136/bmj.39489.470347.AD] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hamann 2017
- Hamann AS, Giang J, De Maeseneer M, Nijsten T, Van den Bos R. Editor's choice – five year results of great saphenous vein treatment: a meta-analysis. European Journal of Vascular and Endovascular Surgery 2017;54(4):760-770. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Joh 2014
- Joh JH, Kim WS, Jung IM, Park KH, Lee T, Kang JM, et al. Consensus for the treatment of varicose vein with radiofrequency ablation. Vascular Specialist International 2014;30(4):105-12. [DOI: 10.5758/vsi.2014.30.4.105] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 2009
- Jones GT, Grant MW, Thomson IA, Hill BG, Van Rij AM. Characterization of a porcine model of chronic superficial varicose veins. Journal of Vascular Surgery 2009;49(6):1554-61. [DOI] [PubMed] [Google Scholar]
Kalteis 2015
- Kalteis M, Adelsgruber P, Messie-Werndl S, Gangl O, Berger I. Five-year results of a randomized controlled trial comparing high ligation combined with endovenous laser ablation and stripping of the great saphenous vein. Dermatologic Surgery 2015;41(5):579-86. [DOI] [PubMed] [Google Scholar]
Kheirelseid 2018
- Kheirelseid EA, Crowe G, Sehgal R, Liakopoulos D, Bela H, Mulkern E, et al. Systematic review and meta-analysis of randomized controlled trials evaluating long-term outcomes of endovenous management of lower extremity varicose veins. Journal of Vascular Surgery: Venous Lymphatic Disorders 2018;6(2):256–70. [DOI] [PubMed] [Google Scholar]
Khilnani 2010
- Khilnani NM, Grassi CJ, Kundu S, D'Agostino HR, Khan AA, McGraw JK, et al. Multi-society consensus quality improvement guidelines for the treatment of lower-extremity superficial venous insufficiency with endovenous thermal ablation from the Society of Interventional Radiology, Cardiovascular Interventional Radiological Society of Europe, American College of Phlebology and Canadian Interventional Radiology Association. Journal of Vascular and Interventional Radiology 2010;21(1):14-31. [DOI] [PubMed] [Google Scholar]
Labropoulos 1994
- Labropoulos N, Leon M, Nicolaides AN, Giannoukas AD, Volteas N, Chan P. Superficial venous insufficiency: correlation of anatomic extent of reflux with clinical symptoms and signs. Journal of Vascular Surgery 1994;20(6):953-8. [DOI] [PubMed] [Google Scholar]
Labropoulos 2005
- Labropoulos N, Leon L, Kwon S, Tassiopoulos A, Gonzalez-Fajardo JA, Kang SS, et al. Study of the venous reflux progression. Journal of Vascular Surgery 2005;41(2):291-5. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration. Available from handbook.cochrane.org 2011.
Leung 2016
- Leung C, Carradice D, Wallace T, Chetter IC. Endovenous laser ablation versus mechanochemical ablation with ClariVein® in the management of superficial venous insufficiency (LAMA trial): study protocol for a randomised controlled trial. Trials 2016;17(1):421. [DOI] [PMC free article] [PubMed] [Google Scholar]
Min 2003
- Min RJ, Khilnani N, Zimmet SE. Endovenous laser treatment of saphenous vein reflux: long-term results. Journal of Vascular and Interventional Radiology 2003;14(8):991-6. [DOI] [PubMed] [Google Scholar]
Mueller 2013
- Mueller RL, Raines JK. ClariVein mechanochemical ablation: background and procedural details. Vascular and Endovascular Surgery 2013;47(3):195-206. [DOI] [PubMed] [Google Scholar]
NICE 2013a
- National Institute for Health and Care Excellence (NICE). Varicose veins in the legs: the diagnosis and management of varicose veins. Clinical guideline CG168; July 2013. nice.org.uk/guidance/CG168 (accessed 14 July 2014). [PubMed]
NICE 2013b
- National Institute for Health and Care Excellence (NICE). Ultrasound-guided foam sclerotherapy for varicose veins. Interventional procedures guidance, IPG440; February 2013. nice.org.uk/guidance/ipg440 (accessed 18 January 2019).
Pascarella 2005
- Pascarella L, Schmid-Schonbein GW, Bergan J. An animal model of venous hypertension: the role of inflammation in venous valve failure. Journal of Vascular Surgery 2005;41(2):303-11. [DOI] [PubMed] [Google Scholar]
Proebstle 2015
- Proebstle TM, Alm J, Dimitri S, Rasmussen L, Whiteley M, Lawson J, et al. The European multicenter cohort study on cyanoacrylate embolization of refluxing great saphenous veins. Journal of Vascular Surgery 2015;3(1):2-7. [DOI] [PubMed] [Google Scholar]
Rabe 2010
- Rabe E, Pannier F, Ko A, Berboth G, Hoffmann B, Hertel S. Incidence of varicose veins, chronic venous insufficiency, and progression of the disease in the Bonn Vein Study II. Journal of Vascular Surgery 2010;51(3):P791. [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rudarakanchana 2012
- Rudarakanchana N, Berland TL, Chasin C, Sadek M, Kabnick LS. Arteriovenous fistula after endovenous ablation for varicose veins. Journal of Vascular Surgery 2012;55(5):1492-4. [DOI] [PubMed] [Google Scholar]
Samuel 2013
- Samuel N, Carradice D, Wallace T, Smith GE, Chetter IC. Endovenous thermal ablation for healing venous ulcers and preventing recurrence. Cochrane Database of Systematic Reviews 2013, Issue 10. Art. No: CD009494. [DOI: 10.1002/14651858.CD009494.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sarin 1992
- Sarin S, Scurr JH, Coleridge Smith PD. Assessment of stripping the long saphenous vein in treatment of primary varicose veins. British Journal of Surgery 1992;79(9):889-93. [DOI] [PubMed] [Google Scholar]
Takase 2004
- Takase S, Pascarella L, Bergan JJ, Schmid-Schonbein GW. Hypertension-induced venous valve remodeling. Journal of Vascular Surgery 2004;39(6):1329-34. [DOI] [PubMed] [Google Scholar]
Tang 2017
- Tang TY, Kam JW, Gaunt ME. ClariVein® – early results from a large single-centre series of mechanochemical endovenous ablation for varicose veins. Phlebology 2017;32(1):6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tessari 2001
- Tessari L, Cavezzi A, Frullini A. Preliminary experience with a new sclerosing foam in the treatment of varicose veins. Dermatologic Surgery 2001;27(1):58-60. [PubMed] [Google Scholar]
Trendelenburg 1890
- Trendelenburg F. Via the connection of the great saphenous vein to the lower leg varices [Uber die tunderbindung der vena saphena magna bie unterschenkel varicen]. Beitrage zur Klinischen Chirurgie 1890;7:195. [Google Scholar]
Van den Bos 2011
- Van den Bos RR, Milleret R, Neumann M, Nijsten T. Proof-of-principle study of steam ablation as novel thermal therapy for saphenous varicose veins. Journal of Vascular Surgery 2011;53(1):181-6. [DOI] [PubMed] [Google Scholar]
Van Eekeren 2014
- Van Eekeren RR, Hillebrands JL, Van der Sloot K, Vries JP, Zeebregts CJ, Reijnen MM. Histological observations one year after mechanochemical endovenous ablation of the great saphenous vein. Journal of Endovascular Therapy 2014;21(3):429-33. [DOI] [PubMed] [Google Scholar]
Weiss 2019
- Weiss M, Maitland Noell CM, Weiss R. Radiofrequency ablation for varicose veins; October 2019. emedicine.medscape.com/article/1085800-overview#a1 (accessed 18 January 2019).
Wittens 2015
- Wittens C, Davies AH, Bækgaard N, Broholm R, Cavezzi A, Chastanet S, et al. Editor’s Choice - Management of chronic venous disease: clinical practice guidelines of the European Society for Vascular Surgery (ESVS). Europpean Journal of Vascular and Endovascular Surgery 2015;49:678-737. [DOI: 10.1016/j.ejvs.2015.02.007] [DOI] [PubMed] [Google Scholar]
Woźniak 2015
- Woźniak W, Mlosek RK, Ciostek P. Assessment of the efficacy and safety of steam vein sclerosis as compared to classic surgery in lower extremity varicose vein management. Videosurgery and Other Mini-invasive Techniques 2015;10(1):15-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Eifell 2006
- Eifell RK, Bhattacharya V, Stansby GP. Endovenous ablation (radiofrequency and laser) and foam sclerotherapy versus conventional surgery for long saphenous vein varices. Cochrane Database of Systematic Reviews 2006, Issue 1. Art. No: CD005624. [DOI: 10.1002/14651858.CD005624] [DOI] [PubMed] [Google Scholar]
Nesbitt 2011
- Nesbitt C, Eifell RK, Coyne P, Badri H, Bhattacharya V, Stansby G. Endovenous ablation (radiofrequency and laser) and foam sclerotherapy versus conventional surgery for great saphenous vein varices. Cochrane Database of Systematic Reviews 2011, Issue 10. Art. No: CD005624. [DOI: 10.1002/14651858.CD005624.pub2] [DOI] [PubMed] [Google Scholar]
Nesbitt 2014
- Nesbitt C, Bedenis R, Bhattacharya C, Stansby G. Endovenous ablation (radiofrequency and laser) and foam sclerotherapy versus open surgery for great saphenous vein varices. Cochrane Database of Systematic Reviews 2014, Issue 7. Art. No: CD005624. [DOI: 10.1002/14651858.CD005624.pub3] [DOI] [PubMed] [Google Scholar]


