Abstract
Background
Pulmonary rehabilitation benefits patients with chronic obstructive pulmonary disease (COPD), but gains are not maintained over time. Maintenance pulmonary rehabilitation has been defined as ongoing supervised exercise at a lower frequency than the initial pulmonary rehabilitation programme. It is not yet known whether a maintenance programme can preserve the benefits of pulmonary rehabilitation over time. Studies of maintenance programmes following pulmonary rehabilitation are heterogeneous, especially regarding supervision frequency. Furthermore, new maintenance models (remote and home‐based) are emerging.
Objectives
To determine whether supervised pulmonary rehabilitation maintenance programmes improve health‐related quality of life (HRQoL), exercise performance, and health care utilisation in COPD patients compared with usual care. Secondly, to examine in subgroup analyses the impact of supervision frequency and model (remote or in‐person) during the supervised maintenance programme.
Search methods
We searched the Cochrane Airways Trials Register, CENTRAL, MEDLINE, Embase, PEDro, and two additional trial registries platforms up to 31 March 2020, without restriction by language or type of publication. We screened the reference lists of all primary studies for additional references. We also hand‐searched conference abstracts and grey literature through the Cochrane Airways Trials Register and CENTRAL.
Selection criteria
We included only randomised trials comparing pulmonary rehabilitation maintenance for COPD with attention control or usual care. The primary outcomes were HRQoL, exercise capacity and hospitalisation; the secondary outcomes were exacerbation rate, mortality, direct costs of care, and adverse events.
Data collection and analysis
Two review authors independently screened titles and abstracts, extracted data, and assessed the risk of bias. Results data that were similar enough to be pooled were meta‐analysed using a random‐effects model, and those that could not be pooled were reported in narrative form. Subgroup analyses were undertaken for frequency of supervision (programmes offered monthly or less frequently, versus more frequently) and those using remote supervision (e.g. telerehabilitation versus face‐to‐face supervision). We used the GRADE approach to assess the certainty of evidence.
Main results
We included 21 studies (39 reports) with 1799 COPD patients. Participants ranged in age from 52 years to 88 years. Disease severity ranged from 24% to 88% of the predicted forced expiratory volume in one second. Programme duration ranged from four weeks to 36 months. In‐person supervision was provided in 12 studies, and remote supervision was provided in six studies (telephone or web platform). Four studies provided a combination of in‐person and remote supervision. Most studies had a high risk of performance bias due to lack of blinding of participants, and high risk of detection, attrition, and reporting bias.
Low‐ to moderate‐certainty evidence showed that supervised maintenance programmes may improve health‐related quality of life at six to 12 months following pulmonary rehabilitation compared to usual care (Chronic Respiratory Questionnaire total score mean difference (MD) 0.54 points, 95% confidence interval (CI) 0.04 to 1.03, 258 participants, four studies), with a mean difference that exceeded the minimal important difference of 0.5 points for this outcome. It is possible that supervised maintenance could improve six‐minute walk distance, but this is uncertain (MD 26 metres (m), 95% CI ‐1.04 to 52.84, 639 participants, 10 studies). There was little to no difference between the maintenance programme and the usual care group in exacerbations or all‐cause hospitalizations, or the chance of death (odds ratio (OR) for mortality 0.73, 95% CI 0.36 to 1.51, 755 participants, six studies). Insufficient data were available to understand the impact of the frequency of supervision, or of remote versus in‐person supervision. No adverse events were reported.
Authors' conclusions
This review suggests that supervised maintenance programmes for COPD patients after pulmonary rehabilitation are not associated with increased adverse events, may improve health‐related quality of life, and could possibly improve exercise capacity at six to 12 months. Effects on exacerbations, hospitalisation and mortality are similar to those of usual care. However, the strength of evidence was limited because most included studies had a high risk of bias and small sample size. The optimal supervision frequency and models for supervised maintenance programmes are still unclear.
Plain language summary
Is supervised maintenance after pulmonary rehabilitation effective compared to usual treatment in people with chronic obstructive pulmonary disease?
Background
Pulmonary rehabilitation is helpful for people with chronic obstructive pulmonary disease (COPD). Pulmonary rehabilitation can give significant improvements in symptoms, quality of life, exercise tolerance, and reduces hospitalizations. This review considers whether maintenance rehabilitation programmes are helpful. Maintenance rehabilitation is supervised exercise training that is offered after a completed pulmonary rehabilitation programme. Typical maintenance rehabilitation programmes are supervised
Search date
The evidence is current to 31 March 2020.
Study characteristics
This review included 21 studies involving 1799 people with COPD. Maintenance rehabilitation programmes were supervised at monthly or shorter intervals, but two studies had longer intervals between sessions. All the programmes were supervised, but some were supervised over the telephone, some were face‐to‐face, and some were a mixture of both. All maintenance programmes in this review gave people supervised exercise training. Some of the programmes also included education sessions. Most programmes lasted between six and 12 months.
Key results
At six to 12 months follow‐up, compared with usual care, supervised maintenance programmes did appear to improve quality of life and physical function by a small amount. The studies did not show reduced hospitalizations, exacerbations, or mortality. There were no adverse events related to maintenance programmes, suggesting that maintenance programmes are safe.
Certainty of the evidence
Many studies showed a high risk of bias because participants knew whether they were in the intervention group or the control group. Some outcomes were based on a small number of studies, and patients, resulting in inconsistent and imprecise results. This means that these results may not apply to all supervised maintenance programme models of delivery.
Summary of findings
Summary of findings 1. Supervised maintenance programme following pulmonary rehabilitation compared to usual care for chronic obstructive pulmonary disease.
| Supervised maintenance programme following pulmonary rehabilitation compared to usual care for chronic obstructive pulmonary disease | ||||||
| Patient or population: chronic obstructive pulmonary disease Setting: home or centre‐based Intervention: supervised maintenance programme following pulmonary rehabilitation Comparison: usual care | ||||||
|
Outcomes Follow‐up from 6 to 12 months. |
Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with usual care | Risk with supervised maintenance programme following pulmonary rehabilitation | |||||
| HRQoL: CRQ total score |
The mean HRQoL CRQ total score ranged from 4 to 4.35 points | MD 0.54 points higher (0.04 higher to 1.03 higher) | ‐ | 258 (4 RCTs) | ⊕⊕⊝⊝ LOW 1 | MID: increase of 0.5 points (Wijkstra 1994) Scale from: 20 to 140 Higher score = better |
| Exercise capacity: 6MWD | The mean exercise capacity: 6MWD ranged from 323 to 453.7 metres | MD 25.90 metres higher (1.04 lower to 52.84 higher) | ‐ | 639 (10 RCTs) | ⊕⊕⊝⊝ LOW 2 | MID: for 6MWD for COPD is 30 metres (Holland 2014) Higher distance = better exercise capacity |
| Hospital admission (all‐cause): number of people admitted to hospital |
56 per 1,000 | 40 per 1000 (8 lower to 180 higher) | OR 0.71 (0.14 to 3.69) | 142 (2 RCTs) | ⊕⊕⊝⊝ LOW 3 | Lower number = better |
| Hospital admission (all‐cause): mean number of hospital admissions per person | The mean varied from 0.3 to 0.6 | The mean varied from 0.2 to 0.9 | ‐ | 332 (3 RCTs) | The data from the three studies have not been pooled or assessed for certainty and have been presented as a range of means in both treatment groups | |
| Exacerbation: number of people experiencing one or more exacerbation | 336 per 1,000 | 270 per 1000 (116 lower to 505 higher) | OR 0.73 (0.26 to 2.02) | 281 (3 RCTs) | ⊕⊕⊝⊝ LOW 3 | Lower number = better |
| Exacerbations: mean number of exacerbations per person | The mean varied from 0.6 to 3.1 |
The mean varied from 0.6 to 3.6 | ‐ | 342 (3 RCTs) | The data from the three studies have not been pooled or assessed for certainty and have been presented as a range of means in both treatment groups | |
| Mortality | 52 per 1,000 | 14 fewer people (19 lower to 76 higher) | OR 0.73 (0.36 to 1.51) | 755 6 studies |
⊕⊕⊕⊝ MODERATE4 |
|
| Adverse events | Information regarding AEs was available descriptively in six studies, none of which reported AEs during the follow‐up period. Two studies reported no AEs during the intervention period, which was less than six months. One study reported no AEs during follow‐up periods of 24 and 36 months. | ‐ | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). 6MWD: 6‐minute walk distance; AEs: adverse events; CI: Confidence interval; CRQ: Chronic Respiratory Questionnaire Health‐related quality of life; HRQoL: health‐related quality of life; MID: minimal important difference; MD: mean difference; OR: Odds ratio; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded by two points for inconsistency due to a high degree of heterogeneity, and high risk of bias for performance and attrition bias
2Downgraded by two points for inconsistency due to high degree of heterogeneity and high risk of bias for performance and attrition bias
3Downgraded by two points for imprecision and high risk of bias for performance and attrition bias.
4Downgraded by one point for high risk of bias for performance and attrition bias.
Background
Description of the condition
Chronic obstructive pulmonary disease (COPD) is currently the third leading cause of death across the world (Lozano 2013), and the fifth cause of death in countries with a high sociodemographic index (GBD 2018). Although COPD is under‐reported, there are an estimated 328 million people in the world living with this condition (Eisner 2011). Moreover, the prevalence of COPD is projected to increase over the decades, due to exposure to tobacco, air pollution and the ageing of the world's population. There is considerable personal, social and economic burden associated with COPD (GOLD 2019). The US Centers for Disease Control and Prevention report that people with COPD have an almost five‐fold increase in incapacity to work, a four‐fold increase in activity limitation due to health problems, and a greater than three‐fold increase in difficulty with daily physical activity, including walking or climbing stairs (Wheaton 2015).
People with COPD experience dyspnoea (breathlessness) on exertion, which generally increases as the disease progresses. The 'dyspnoea spiral' model suggests that, to avoid dyspnoea, people with COPD adopt a sedentary lifestyle, which leads to a reduction in the aerobic capacity of the peripheral muscles (Polkey 2006). This reduces functional exercise capacity, making it more difficult to undertake regular daily activities such as walking up hills, carrying heavy loads or getting dressed. In addition to sedentarism, there is evidence that the etiology of exercise limitation in people with COPD is multifactorial, involving factors such as deconditioning, hypoxia or systemic hypercapnia (or both), nutritional depletion, chronic or repetitive corticosteroid use, age, hormonal dysfunction and systemic inflammation (Maltais 2014).
Description of the intervention
Pulmonary rehabilitation is defined as a "comprehensive intervention based on a thorough patient assessment followed by the patient‐tailored therapies that include, but are not limited to, exercise training, education, and behavior change, designed to improve the physical and psychological condition of people with chronic respiratory disease and to promote the long‐term adherence to health‐enhancing behaviors" (Spruit 2013). It is recommended that people with COPD undertake pulmonary rehabilitation in order to improve peripheral muscle function, optimise self‐management, reduce symptoms, improve exercise capacity, enhance quality of life and reduce hospitalisation (Spruit 2013).
Typically, pulmonary rehabilitation is a short‐term intervention, with the American Thoracic Society/European Respiratory Society (ATS/ERS) statement suggesting a duration of at least eight weeks (Spruit 2013), although longer programmes have been reported (Wijkstra 1994; Guell 2000). The components of pulmonary rehabilitation include aerobic physical training as well as strength training for upper limbs and lower limbs at least twice a week, in addition to health education. Aerobic training involves up to 30 minutes of walking or cycle ergometer, or both, based on intensity prescribed by assessing functional exercise capacity. Strength training is prescribed with an intensity of 60% to 70% of the maximum load determined by one repetition maximum test (Spruit 2013). There is strong evidence supporting the benefits of pulmonary rehabilitation for people with COPD (McCarthy 2015). However, exercise capacity and health‐related quality of life diminish in the 12 months following programme completion (McCarthy 2015). As a result, there is growing interest in the role of maintenance programmes.
Maintenance pulmonary rehabilitation has been defined as ongoing supervised exercise at a lower frequency than the initial pulmonary rehabilitation programme (Alison 2017). A number of different maintenance models have been conducted for people with COPD, ranging from once weekly supervision to monthly or less frequent supervision (Alison 2017). In addition to physical training, some programmes also include continued self‐management education, strategies to improve adherence to ongoing exercise and the reduction of barriers to long‐term exercise training (Spencer 2019). The optimal frequency of contact between health professionals and patients during maintenance pulmonary rehabilitation has not been established. Remotely supervised maintenance strategies have also been tested, including the use of diaries, telephone calls or text messages, and pedometers to encourage participants to maintain the frequency and intensity of the exercise prescription (Nguyen 2009; Vasilopoulou 2017). However, the effectiveness of these monitoring and motivation strategies for maintenance benefits is still unclear. It is not known whether in‐person supervision is essential to successful maintenance pulmonary rehabilitation programmes, or whether remote strategies can also be effective (e.g. telerehabilitation, where supervision is provided via videoconferencing or via the telephone). The role of technology to support long‐term maintenance of gains following pulmonary rehabilitation has not been defined (Holland 2017). The costs of delivering maintenance programmes are likely to vary across different models, but these costs have not been well documented and the impact of maintenance programmes on other healthcare costs (e.g. hospitalisation, primary care visits) is not totally known. One previous systematic review and meta‐analysis showed that supervised maintenance exercise was effective in reducing the rate of respiratory cause hospital admissions (Jenkins 2018). However, other outcomes such as direct care costs during the follow‐up period, adverse events, exercise capacity and quality of life were not reported. An additional systematic review reported that supervised exercise programmes after pulmonary rehabilitation were more effective than usual care in maintaining exercise capacity in the medium term; however, the small number of studies precluded conclusions about longer‐term outcomes (Beauchamp 2013). Additional studies have since been published, providing the opportunity to better understand the effects of maintenance rehabilitation across a broader range of outcomes.
How the intervention might work
The aim of a maintenance pulmonary rehabilitation programme is to maintain physical capacity, quality of life and avoid hospital admissions in the long term (Guell 2017). It is generally assumed that 'graduates' of pulmonary rehabilitation can exercise and manage their health with a degree of independence, such that less‐frequent supervision is sufficient. It is likely that the efficacy of maintenance pulmonary rehabilitation programmes depends on the ability to support long‐term adherence to exercise training and health‐enhancing behaviours, such that physical fitness can be maintained and effective self‐management employed. However, the essential components of maintenance pulmonary rehabilitation, and their mode of action, have not been established.
Why it is important to do this review
The benefits of pulmonary rehabilitation for people with COPD are clinically important, but research suggests that they do not last (Beauchamp 2013). It is important to identify effective, accessible methods that sustain the benefits of pulmonary rehabilitation over time using a cost‐effective method (Nici 2019). Effective strategies that could maintain the benefits of pulmonary rehabilitation would be valuable for patients, their communities and the health system. However, there is currently no consensus on what should happen after pulmonary rehabilitation is finished. There are a growing number of randomised controlled trials (RCTs) investigating maintenance pulmonary rehabilitation but their heterogeneity (particularly with regard to the degree of supervision provided) and variable quality makes it difficult to apply their findings. New models of maintenance are also emerging (e.g. telerehabilitation and home‐based programmes), but their effects are not clear. Pulmonary rehabilitation guidelines for Australia and New Zealand concluded that programmes offered monthly or less were ineffective in maintaining the gains post pulmonary rehabilitation, but there was insufficient evidence to draw conclusions about maintenance programmes that were offered more frequently (Alison 2017). There is an urgent need to clarify the effects of maintenance programmes following pulmonary rehabilitation, including the most effective supervision strategy.
Objectives
To determine whether supervised pulmonary rehabilitation maintenance programmes improve health‐related quality of life (HRQoL), exercise performance, and health care utilisation in COPD patients compared with usual care. Secondly, we examined the impact of supervision frequency and model (remote or in‐person) during the supervised maintenance programme in subgroup analyses.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs. Cluster‐randomised trials were also eligible for inclusion, but no trials of this design were identified. We included studies reported in full text. To avoid publication bias, we also included those published as an abstract only, as well as unpublished data, and attempted to contact study authors to request more information, if necessary. We did not include crossover trials, due to the potential for carry over effects in behavioural interventions such as maintenance programmes.
Types of participants
We included adults (aged 18 years and older) with a diagnosis of COPD (e.g. according to Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria, or the study author’s definition), who had undertaken a pulmonary rehabilitation programme.
We included studies that incorporated a mix of chronic diseases only where outcomes for participants with COPD were reported separately.
We excluded participants who had a primary diagnosis of another chronic pulmonary disease such as asthma, bronchiectasis, cystic fibrosis, and interstitial lung disease.
Types of interventions
We included studies that compared maintenance pulmonary rehabilitation to attention control or usual care.
Maintenance pulmonary rehabilitation was defined as supervised exercise training at a lower frequency than the initial pulmonary rehabilitation program, with or without other components such as education and self‐management training (Alison 2017). Supervision could be provided in‐person or remotely. Remote supervision could be provided by using, for instance, technology such as telephone or Internet. Maintenance programmes of any duration were included.
Attention control was defined as an additional contact with the patient, whereby the patient receives some aspect of attention, time or expectation (e.g. a telephone call), but without supervised training.
Usual care was the group in which participants received only standard treatment for COPD. Usual care did not involve a supervised exercise training programme.
Types of outcome measures
We have analyzed the following outcomes in the review, but we did not use them as a basis for including or excluding studies.
Primary outcomes
HRQoL, measured via disease‐specific questionnaires (e.g. St George's Respiratory Questionnaire, Chronic Respiratory Disease Questionnaire) or generic health questionnaires (i.e. 36‐item Short Form, Euro‐QoL)
Exercise capacity measured by field exercise tests (e.g. 6‐minute walk test, shuttle walk tests) or laboratory exercise tests (e.g. cardiopulmonary exercise test)
All‐cause hospitalisation, as defined by trialists. We extracted the number of participants who required hospital admission, or the hospitalisation rate, or both.
Secondary outcomes
Exacerbation rate, as defined by trialists, we extracted the number of participants experiencing one or more exacerbation, or the exacerbation rate, or both. We reported severe exacerbations (requiring hospitalisation or emergency room admission) separately to moderate exacerbations (requiring initiation of oral antibiotics or corticosteroids, or both, but without hospital/emergency room admission) (GOLD 2019).
Mortality (all‐cause), measured as the incidence or rate of death, assessed at the longest time available
Direct costs of care during the follow‐up period, including intervention costs as defined by trialists
Adverse events (all causes), as defined by the study authors (i.e. the number of participants with adverse events)
Because maintenance programmes are long‐term interventions, all outcomes were assessed at:
six to 12 months following completion of pulmonary rehabilitation;
greater than 12 months following completion of pulmonary rehabilitation.
Search methods for identification of studies
We searched the following databases to 31 March 2020.
Electronic searches
We identified studies from searches of the following databases and trial registries:
Cochrane Airways Trials Register (Cochrane Airways 2019), via the Cochrane Register of Studies, all years to 31 March 2020;
Cochrane Central Register of Controlled Trials (CENTRAL), via the Cochrane Register of Studies, all years to 31 March 2020;
MEDLINE (Ovid SP) ALL 1946 to 31 March 2020;
Embase (Ovid SP) 1974 to 31 March 2020;
PEDro (Physiotherapy Evidence Database) all years to 31 March 2020;
US National Institutes of Health Ongoing Trials Register, ClinicalTrials.gov (www.clinicaltrials.gov); all years to 31 March 2020;
World Health Organisation International Clinical Trials Registry Platform (apps.who.int/trialsearch) (searched via CENTRAL); all years to 31 March 2020.
The search strategy was developed in MEDLINE and adapted for use in the other databases. All the search strategies are listed in Appendix 1. The Cochrane Airways Information Specialist developed the search strategy in collaboration with the protocol authors, and conducted the searches.
We searched all databases and trials registries from their inception, and there were no restriction on language or type of publication. We identified hand searched conference abstracts and grey literature through the Cochrane Airways Trials Register and the CENTRAL database.
Searching other resources
We checked the reference lists of all primary studies for additional references.
On 7 May 2021, we searched PubMed for errata or retractions from included studies published in full text.
Data collection and analysis
We followed the methods recommended by the Cochrane Handook for Systematic Reviews of Interventions (Higgins 2011).
Selection of studies
We used Cochrane’s Screen4Me workflow to help assess the search results. Screen4Me comprises three components: known assessments – a service that matches records in the search results to records that have already been screened in Cochrane Crowd and been labeled as an RCT or as Not an RCT; the RCT classifier – a machine learning model that distinguishes RCTs from non‐RCTs (Marshall 2018). Two review authors (CM, SJ) independently screened titles and abstracts of the search results using Covidence software (Covidence), and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. We retrieved the full‐text study reports of all potentially eligible studies and two review authors (CM, SJ) independently screened them for inclusion, recording the reasons for exclusion of ineligible studies. Any disagreements were resolved by consensus or the determination of a third review author (AEH). We identified and excluded duplicates and collated multiple reports of the same study so that each study, rather than each report, is the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and 'Characteristics of excluded studies' table (Moher 2009).
Data extraction and management
We used a standardized data collection form for study characteristics and outcome data, which was piloted on at least one study in the review. Two review authors (CM, SJ) independently extracted the following study characteristics from included studies.
Methods: study design, duration of the intervention, length of follow‐up, study location, study setting, withdrawals, date of study.
Participant characteristics: number, mean age, age range, gender, diagnosis, severity of condition, diagnostic criteria, number of comorbidities, baseline lung function, smoking history, inclusion criteria, exclusion criteria.
Interventions: intervention, comparison, concomitant medications.
Outcomes: primary and secondary outcomes specified and collected (at baseline and at time of intervention completion) and follow‐up measures at six to 12 months or greater than 12 months.
Notes: funding for studies and notable conflicts of interest of trial authors.
We noted in the 'Characteristics of included studies' table if outcome data were not reported in a usable way. We resolved disagreements by consensus or by involving a third person/review author (AEH). One review author (CM) transferred data into Review Manager 5 (Review Manager 2014). We double‐checked that data were entered correctly by comparing the data presented in the systematic review with the study reports. A second review author (SDC) spot‐checked study characteristics entered into Review Manager 5 for accuracy against the study report.
Assessment of risk of bias in included studies
Two review authors (CM, SDC) assessed risk of bias independently for each study included using the criteria outlined in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements by discussion or by involving another review author (AEH). We assessed the risk of bias using the Cochrane 'Risk of bias' tool according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias.
We judged each potential source of bias as high, low or unclear and provided a quote from the study report together with a justification for our judgment in the 'Risk of bias' table.
We summarised the 'Risk of bias' judgments across different studies for each of the domains listed and summarised results in the 'Risk of bias' table.
We have considered blinding separately for different key outcomes where necessary (e.g. for unblinded outcome assessment, risk of bias for adverse events may be very different than for a patient‐reported outcome). Due to the nature of the intervention, it was not possible to blind participants or personnel to the intervention. We took this into account in the risk of bias and GRADE assessment and considered the potential impact of lack of blinding on a case‐by‐case basis (e.g. subjective outcomes are likely to be more at risk than objective outcomes). Where information on risk of bias related to unpublished data or correspondence with a trialist, we noted this in the 'Risk of bias' table. When considering treatment effects, we considered the risk of bias for the studies that contributed to that outcome.
Assessment of bias in conducting the systematic review
We conducted the review according to the protocol and noted any deviations from it in the 'Differences between protocol and review' section of the systematic review.
Measures of treatment effect
We analyzed data for each outcome, irrespective of reported participant dropout (intention‐to‐treat analysis; ITT). We analyzed dichotomous data (hospitalisation, exacerbation, mortality, adverse events) as odds ratios (OR) and continuous data as the mean difference (MD) with 95% confidence intervals (CI). We analyzed hospitalisation rates and exacerbation rates using risk ratio (incidence). We reported direct costs as costs in each group, or the difference between groups, as reported by the study authors. If data from rating scales were combined in a meta‐analysis, we ensured they were entered with a consistent direction of effect (e.g. lower scores always indicate improvement).
We undertook meta‐analyses only where this was meaningful; that is, if the treatments, participants and the underlying clinical question were similar enough for pooling to make sense.
If data reported in trials were skewed and reported in a way that did not allow for meta‐analysis (e.g. as medians and interquartile ranges for each group), we described the results narratively, rather than excluding the data completely.
Where multiple trial arms were reported in a single study, we included only the relevant arms in analyses. We have listed the other arms in the 'Characteristics of included studies' table. If two comparisons (i.e. maintenance approach one versus usual care and maintenance approach two versus usual care) were combined in the same meta‐analysis, we either combined the active arms or halved the control group to avoid double‐counting.
If adjusted analyses were available (e.g. analysis of variance (ANOVA) or analysis of covariance (ANCOVA)), we used these preferentially in our meta‐analyses. If both change from baseline and endpoint scores were available for continuous data, we used change from baseline unless there was a low correlation between measurements in individuals. If a study reported outcomes at multiple time points, we used the data closest to the primary time point of interest (12 months).
We used ITT or 'full analysis set' analyses where they were reported (i.e. those where data have been imputed for participants who were randomly assigned but did not complete the study) instead of completer or per‐protocol analyses.
Unit of analysis issues
Where studies randomly allocated individual participants to a maintenance intervention or control or sham, we considered the participant as the unit of analysis. We had intended to only meta‐analyse data from cluster‐RCTs if the available data had been adjusted (or could be adjusted), to account for the clustering, but no cluster RCTs were identified. We excluded crossover trials in this review due to the potential carry‐over effects of behavioural interventions.
Dealing with missing data
We contacted investigators or study sponsors to verify key study characteristics and obtain missing numerical outcome data where possible (e.g. when a study was identified as an abstract only). Where this was not possible, and the missing data were thought to introduce serious bias, we took this into consideration in the GRADE rating for affected outcomes.
Assessment of heterogeneity
We used the I2 statistic to measure heterogeneity among the studies in each analysis according to the guidance in the Cochrane Handbook (Higgins 2011). If we identified substantial heterogeneity (I2 of 40% or higher), we reported it and explored the possible causes by prespecified subgroup analysis.
Assessment of reporting biases
If we had been able to pool more than 10 studies, we would have created and examined a funnel plot to explore possible small‐study and publication biases, however an insufficient number of studies were identified.
Data synthesis
We used a random‐effects model, with the assumption that the included studies may have had heterogeneous, but related, intervention effect estimates (due to the clinical nature of the intervention). We performed a sensitivity analysis using a fixed‐effect model to determine whether the result was robust.
Subgroup analysis and investigation of heterogeneity
We compared the effectiveness of the interventions between:
maintenance programmes offered monthly or less frequently, compared to those offered more frequently;
maintenance programmes using remote supervision (e.g. telerehabilitation) versus face‐to‐face supervision.
We used the following outcomes in subgroup analyses:
health‐related quality of life;
exercise capacity;
hospitalizations.
We used the formal test for subgroup interactions in Review Manager 5 (Review Manager 2014).
Sensitivity analysis
We examined the effects of methodological quality on the pooled estimate by removing studies that were at high or unclear risk of bias for the domains of blinding and incomplete outcome data. We compared the results from a fixed‐effect model, using the random‐effects model.
Summary of findings and assessment of the certainty of the evidence
We created a 'Summary of findings' table using the following outcomes.
Health‐related quality of life (generic or disease‐specific).
Exercise capacity: maximal or submaximal, measured directly or by a standardized field test.
All‐cause hospitalisation measured as the incidence or rate of hospitalisation, defined according to study authors.
Mortality.
Adverse events.
We presented effect size with 95% CIs for each outcome as well as absolute effects (generated by GRADEpro GDT software). We used the five GRADE considerations (risk of bias, consistency of effect, imprecision, indirectness and publication bias) to assess the overall certainty of a body of evidence (low, moderate or high certainty) as it related to the studies that contributed data for the prespecified outcomes. We used the methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook (Higgins 2011; Schünemann 2017), using GRADEpro software (GRADEpro GDT). We justified all decisions to downgrade the quality of studies using footnotes and we made comments to aid the reader's understanding of the review where necessary.
Results
Description of studies
All studies are detailed in the following sections: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; and Characteristics of ongoing studies.
Results of the search
The searches were conducted on 31 March 2020. The electronic database searches identified 5,087 records, from which we removed 2,312 duplicate records. From the remaining 2,775 references, 567 were excluded by the Screen4Me assessment, and we removed 51 additional duplicates and 2,086 irrelevant records. This resulted in 71 records (articles, conference abstracts, and a doctoral thesis), the full texts of which we assessed for eligibility. After this full‐text review, we excluded a further 31 records and one additional duplicate because they did not meet our inclusion criteria. Finally, 21 studies (39 references considering multiple records) were kept after careful inspection (Figure 1).
1.
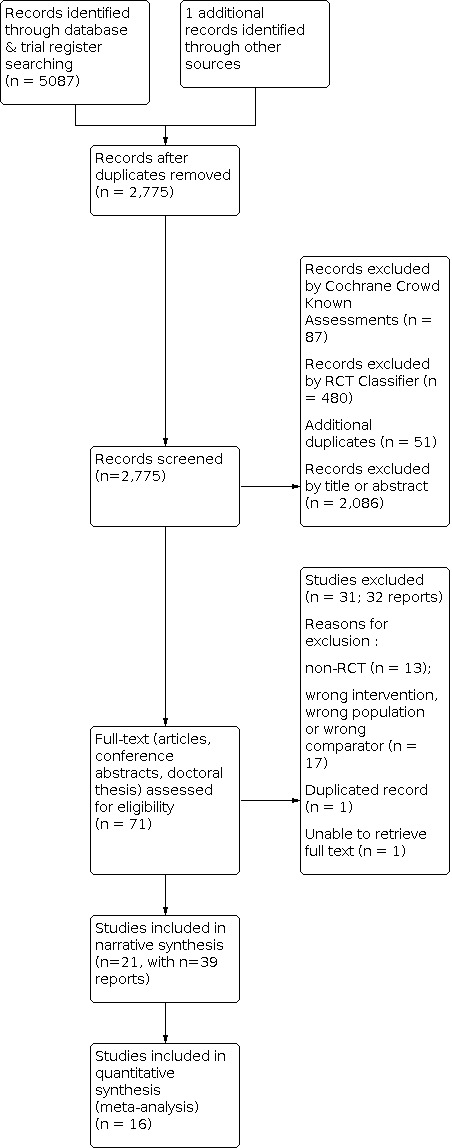
Study flow diagram.
Included studies
Design
This review included 21 randomised controlled trials, four of which were reported only in abstract form (Johnson 2001; Abbink 2004; Hill 2004; Arbane 2010) and one as a thesis (Murphy 2005).
Participants
The 21 studies included involved 1799 participants with COPD, with sample sizes ranging from 20 to 172. All studies included clinically stable participants (Brooks 2002; Johnson 2001; Ries 2003; Abbink 2004; Hill 2004; Murphy 2005; Steele 2008; Du Moulin 2009; Arbane 2010; Ringbaek 2010; Spencer 2010; Linneberg 2012; Roman 2013; Wong 2014; Burns 2016; Guell 2017; Bernocchi 2018; Li 2018; Wootton 2018; Galdiz 2020; Jiménez‐Reguera 2020)
COPD was diagnosed on the basis of spirometry and medical history in 19 studies (Brooks 2002; Johnson 2001; Ries 2003; Abbink 2004; Murphy 2005; Steele 2008; Du Moulin 2009; Ringbaek 2010; Spencer 2010; Linneberg 2012; Roman 2013; Wong 2014; Burns 2016; Guell 2017; Bernocchi 2018; Li 2018; Wootton 2018; Galdiz 2020; Jiménez‐Reguera 2020), and two studies did not report diagnostic criteria (Hill 2004; Arbane 2010).
The age range of participants was 52 to 88 years in 19 studies (Brooks 2002; Johnson 2001; Ries 2003; Abbink 2004; Murphy 2005; Steele 2008; Du Moulin 2009; Ringbaek 2010; Spencer 2010; Linneberg 2012; Roman 2013; Wong 2014; Burns 2016; Guell 2017; Bernocchi 2018; Li 2018; Wootton 2018; Galdiz 2020; Jiménez‐Reguera 2020); two studies did not report age values (Hill 2004; Arbane 2010).
Disease severity was reported in 19 studies according to spirometry, with forced expiratory volume in one second (FEV1) ranging from 23.8% to 88.1% of predicted values (Brooks 2002; Johnson 2001; Ries 2003; Abbink 2004; Murphy 2005; Steele 2008; Du Moulin 2009; Ringbaek 2010; Spencer 2010; Linneberg 2012; Roman 2013; Wong 2014; Burns 2016; Guell 2017; Bernocchi 2018; Li 2018; Wootton 2018; Galdiz 2020; Jiménez‐Reguera 2020). Two studies did not report severity (Hill 2004; Arbane 2010).
Most of the studies (Brooks 2002; Ries 2003; Murphy 2005; Steele 2008; Du Moulin 2009; Ringbaek 2010; Spencer 2010; Linneberg 2012; Roman 2013; Wong 2014; Burns 2016; Guell 2017; Bernocchi 2018; Li 2018; Wootton 2018; Galdiz 2020; Jiménez‐Reguera 2020) reported that included patients were clinically stable for at least four weeks prior to attending an initial pulmonary rehabilitation programme.
Intervention
Nineteen studies delivered maintenance pulmonary rehabilitation at a higher frequency (supervision more often than once a month) (Brooks 2002; Johnson 2001; Ries 2003; Abbink 2004; Hill 2004; Murphy 2005; Steele 2008; Du Moulin 2009; Arbane 2010; Ringbaek 2010; Spencer 2010; Roman 2013; Wong 2014; Guell 2017; Bernocchi 2018; Li 2018; Wootton 2018; Galdiz 2020; Jiménez‐Reguera 2020) while two studies delivered a less frequent intervention (monthly or less frequently) (Linneberg 2012; Burns 2016).
Supervision was provided in‐person in 11 studies (Johnson 2001; Abbink 2004; Hill 2004; Murphy 2005; Arbane 2010; Ringbaek 2010; Spencer 2010; Linneberg 2012; Roman 2013; Burns 2016; Guell 2017), either by home‐visit or by attending training sessions in the rehabilitation centre. Remote supervision was provided by telephone call or web‐based platform in six studies (Du Moulin 2009; Wong 2014; Bernocchi 2018; Wootton 2018; Galdiz 2020; Jiménez‐Reguera 2020). Four studies used a combination of in‐person and remote supervision (Brooks 2002; Ries 2003; Steele 2008; Li 2018).
In 19 studies, the control group that received usual care also received advice to maintain physical activity at discharge after completion of the pulmonary rehabilitation program, however, they were not supervised (Brooks 2002; Ries 2003; Hill 2004; Murphy 2005; Steele 2008; Du Moulin 2009; Arbane 2010; Ringbaek 2010; Spencer 2010; Linneberg 2012; Roman 2013; Wong 2014; Burns 2016; Guell 2017; Bernocchi 2018; Li 2018; Wootton 2018; Galdiz 2020; Jiménez‐Reguera 2020).
Four studies (abstracts) did not report details for the control intervention (Johnson 2001; Abbink 2004; Hill 2004; Arbane 2010).
The duration of the maintenance programme ranged from 4 weeks to 156 weeks (36 months). Seven studies (Johnson 2001; Hill 2004; Murphy 2005; Steele 2008; Arbane 2010; Linneberg 2012; Bernocchi 2018) only reported outcomes at less than six months of the maintenance program, which did not meet our pre‐specified criteria for the time point of measurement. These outcomes were not meta‐analysed but were reported narratively.
Table 2 presents a summary of the description of the intervention and frequency of supervision of the included studies.
1. Intervention characteristics, control, and supervision frequency of the included studies.
| Study | Number of participants | Intervention | Supervision frequency | Control | Country |
| Abbink 2004 | 51 | Not defined | Weekly visit | Usual care | Netherlands |
| Arbane 2010 | 31 | Not defined |
Weekly visit | Usual care | United Kingdom |
| Bernocchi 2018 | 112 | Aerobic exercise and resistance exercise training plus educational intervention | Weekly phone contacts | Usual care | Italy |
| Brooks 2002 | 85 | Aerobic exercise, upper extremity exercise and breathing exercise | Fortnightly phone contacts and weekly visit | Usual care | Canada |
| Burns 2016 | 148 | Aerobic exercise including walking, cycling, standing from sitting, arm exercises using dumbbells and step‐ups. | One visit session every 3 months (3, 6 and 9 months) | Usual care | United Kingdom |
| Du Moulin 2009 | 20 | Aerobic exercise based on the walk a distance equivalent to 125% of their last 6MWT 3 times a day with each training walk not exceeding 15 min. Alternatively, these 3 training walks could be combined to one training walk a day |
Phone contacts every 4 weeks | Usual care | Germany |
|
Galdiz 2020 |
94 | Exercise training using weight lifting and leg cycle ergometry |
Weekly contact by telerehabilitation via Web‐based platform |
Usual care | Spain |
|
Guell 2017 |
138 | Chest physiotherapy, arm training with weight‐lifting and leg training with cycle ergometer |
Weekly contact with either a phone call or a visit | Usual care | Spain |
| Hill 2004 | 61 | Lower limb endurance, upper limb exercises using free weights, and warm‐up and cool‐down exercises once a week at community plus home exercise programme. |
Weekly visit | Usual care | Australia |
| Jiménez‐Reguera 2020 | 44 | Aerobic and breathing exercise daily using a pulmonary care web‐based application. | Weekly remote contact | Usual care | Spain |
| Johnson 2001 | 30 | Not defined | Weekly visit | Usual care | United Kingdom |
| Li 2018 | 82 | Aerobic (mini‐ergometer, pedometer‐based walking), callisthenic and muscle reinforcement exercises |
Step 1: fortnightly visit for 2 months; Step 2: visit every 4 weeks and phone contacts weekly for the next 4 months; Step 3: Phone contacts weekly for the next 6 months. | Usual care | China |
| Linneberg 2012 | 118 | Aerobic exercise based on the same speed of the endurance shuttle walk test and cycle training, and four exercises of strengthening legs, arms, abdominal and thoracic muscles. | Six visits at week 9,11,13,28,26 and 52. | Usual care | Denmark |
| Murphy 2005 | 56 | Aerobic exercise (walking on the treadmill, stationary cycling, stair climbing) and weight training (free weights) |
Weekly visit | Usual care | Australia |
| Ries 2003 | 172 | Aerobic exercise of walking at an appropriate pace and upper limb lifting training | Weekly phone contacts and monthly visit | Usual care | USA |
| Ringbaek 2010 | 96 | Aerobic exercise of walking at a level equal to 85% of a predicted VO2 peak as calculated from the Incremental Shuttle Walk Test, cycling, and educational session. | Weekly visit during the first 6 months, and fortnightly visit during the next 6 months | Denmark | |
| Roman 2013 | 48 | Respiratory physiotherapy, isolated conditioning of peripheral skeletal muscle groups, and educational session |
Weekly visit | Usual care | Spain |
| Spencer 2010 | 59 | Aerobic exercise: on the day per week at gymnasium: 20 min walking (track or treadmill), 20 min cycling, 10 min arm cycling, and upper and lower limb strength training exercises using weight equipment and free weights. At home exercise consisted of 30 min of walking plus 30 min of upper and lower limb strengthening exercises using free weights and body weight. | Weekly visit | Usual care | Australia |
| Steele 2008 | 111 | Aerobic exercise based on walking and monitoring devices | Weekly phone contacts and visit over 3 months | Usual care | USA |
| Wong 2014 | 79 | Flexibility, cardiovascular endurance, resistance training, and breathing exercises. | 8 visit sessions over 6 months | Usual care | Canada |
| Wootton 2018 | 95 | Aerobic exercise based on a walking programme with progressive goal setting. | Phone contacts fortnightly for the first 3 months and then once a month until 14 months | Usual care | Australia |
6MWT: six‐minute walk test; USA: United States of America.
Excluded studies
From the 71 full‐text papers reviewed, we excluded 31 studies (n = 32 reports). We excluded studies that had the wrong study design (n = 13), wrong population (n = 1), or wrong comparator (n = 3). In 13 studies, the intervention did not meet inclusion criteria. We were unable to retrieve the full text of one study. Full reasons for exclusion are provided in the Characteristics of excluded studies table. A further two studies are ongoing (Ongoing studies) and five are awaiting classification (Studies awaiting classification). These will be assessed for eligibility in further updates of this review.
Risk of bias in included studies
Details on our judgements on the potential risks of bias are summarised in Figure 2, with full details in the Characteristics of included studies table.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
For random sequence generation, ten studies had a low risk of bias (Brooks 2002; Ries 2003; Spencer 2010; Roman 2013; Burns 2016; Guell 2017; Bernocchi 2018; Wootton 2018; Galdiz 2020; Jiménez‐Reguera 2020). Insufficient information was available to provide a decision for 11 studies (Johnson 2001; Abbink 2004; Hill 2004; Murphy 2005; Steele 2008; Du Moulin 2009; Arbane 2010; Ringbaek 2010; Linneberg 2012; Wong 2014; Li 2018). For allocation concealment, six studies reported the use of either sealed, opaque envelopes or a method of concealment was used for allocation (Ries 2003; Murphy 2005; Spencer 2010; Bernocchi 2018; Galdiz 2020; Jiménez‐Reguera 2020); 15 studies did not provide sufficient information to inform a decision (Johnson 2001; Brooks 2002; Abbink 2004; Hill 2004; Steele 2008; Du Moulin 2009; Arbane 2010; Ringbaek 2010; Linneberg 2012; Roman 2013; Wong 2014; Burns 2016; Guell 2017; Li 2018; Wootton 2018).
Blinding
Five studies did not provide sufficient information regarding the blinding of participants and personnel delivering the interventions (Johnson 2001;Brooks 2002; Abbink 2004; Hill 2004; Li 2018). Given the nature of the intervention (exercise training), it is unlikely that participants or personnel could be blinded to the intervention. A high risk of performance bias was rated in 16 studies (Ries 2003; Murphy 2005; Steele 2008; Du Moulin 2009; Arbane 2010; Ringbaek 2010; Spencer 2010; Linneberg 2012; Roman 2013; Wong 2014; Burns 2016; Guell 2017; Bernocchi 2018; Wootton 2018; Galdiz 2020; Jiménez‐Reguera 2020).
Seven trials were judged as having a low risk of detection bias because the outcome assessors were blinded (Ries 2003; Du Moulin 2009; Roman 2013; Burns 2016; Guell 2017; Wootton 2018; Jiménez‐Reguera 2020). Ten trials were judged as having an unclear risk of detection bias because there was no clear mention of independent assessors (Johnson 2001; Brooks 2002; Abbink 2004; Hill 2004; Steele 2008; Arbane 2010; Ringbaek 2010; Wong 2014; Li 2018; Galdiz 2020). The remaining four trials were judged as having a high risk of detection bias due to non‐blinding of outcome assessors (Murphy 2005; Spencer 2010; Linneberg 2012; Bernocchi 2018). The overall low risk of detection bias accounted for more than one third of trials, the unclear risk was almost half of trials, and the high risk was less than one quarter of studies.
Incomplete outcome data
Ten studies reported no dropouts, or very few dropouts, and were rated at low risk of attrition bias (Ries 2003; Murphy 2005; Ringbaek 2010; Linneberg 2012; Wong 2014; Burns 2016; Bernocchi 2018; Wootton 2018; Galdiz 2020; Jiménez‐Reguera 2020), and three abstracts (Johnson 2001; Hill 2004; Arbane 2010) were rated at unclear risk. Nine studies had a high risk of bias, with attrition imbalanced between the groups in five studies (Steele 2008; Du Moulin 2009; Spencer 2010; Guell 2017; Li 2018), and in the other three studies the loss to follow‐up was high but with near‐equal percentages in both groups (Brooks 2002; Abbink 2004; Roman 2013).
Selective reporting
Five studies (Spencer 2010; Roman 2013; Burns 2016; Bernocchi 2018; Wootton 2018) had registered and published a trial protocol and the outcomes were reported as planned. Twelve studies (Brooks 2002; Ries 2003; Abbink 2004; Hill 2004; Murphy 2005; Steele 2008; Du Moulin 2009; Arbane 2010; Ringbaek 2010; Linneberg 2012; Wong 2014; Li 2018) were at unclear risk of reporting bias. Four studies (Johnson 2001; Guell 2017; Galdiz 2020; Jiménez‐Reguera 2020) were rated at high risk, mostly because there were discrepancies between trial registration and publications, e.g. outcomes had not been reported as planned.
Other potential sources of bias
We assessed two RCTs to be of low risk with respect to other sources of bias (Ries 2003; Wootton 2018). Three studies were determined as high risk with respect to other sources of bias (Brooks 2002; Bernocchi 2018; Jiménez‐Reguera 2020). One study had retrospective registration, declared conflicts of interest where two authors were financed by the company that developed the intellectual property of the application used in the study, and participants in the control group did not have access to smart devices for an appropriate sham (Jiménez‐Reguera 2020). In one study, participants had a combined diagnosis of COPD and chronic heart failure (Bernocchi 2018). In one study, there was a baseline difference in exercise capacity between the intervention group and the control group (Brooks 2002). The remaining studies were determined to have an unclear risk of other sources of bias associated with retrospective registration, exclusion criteria which meant that patients may not have been representative of the population, variations in components of the intervention or control conditions between study sites, or conflicts of interest noted for the authors.
Effects of interventions
See: Table 1
Supervised maintenance programmes compared to usual care
The certainty of the evidence for this comparison can be found in the Table 1.
Primary outcomes
Health‐related quality of life
Health‐related quality of life was assessed using the St George's Respiratory Questionnaire (SGRQ) in nine studies (Brooks 2002; Murphy 2005; Arbane 2010; Ringbaek 2010; Spencer 2010; Linneberg 2012; Wong 2014; Wootton 2018; Jiménez‐Reguera 2020). The pooled analysis of studies (Brooks 2002; Spencer 2010; Wong 2014; Wootton 2018; Jiménez‐Reguera 2020) using the total SGRQ score showed there was probably little to no difference between maintenance and usual care groups at six to 12 months, with an MD of ‐1.57 points (95% CI ‐4.93 to 1.78; 276 participants, 5 studies; Analysis 1.1; Figure 3). There was low‐certainty evidence and no heterogeneity across the studies (I2 = 0%). The other two studies (Ringbaek 2010; Linneberg 2012) did not provide means and dispersion measures for each group but reported no difference between the maintenance and usual care groups for SGRQ. Two studies (Murphy 2005; Arbane 2010) evaluated SGRQ at less than six months; however, no differences were reported between the maintenance and usual care groups for this quality of life measure.
1.1. Analysis.

Comparison 1: Supervised maintenance programme vs usual care, Outcome 1: QoL SGRQ total at time point six to 12 months following completion of pulmonary rehabilitation
3.

Forest plot of comparison: 1 Supervised maintenance programme vs usual care; outcome: 1.6 QoL SGRQ total at time point six to 12 months following completion of pulmonary rehabilitation (points).
For SGRQ domain scores at six to 12 months (Spencer 2010; Wong 2014; Wootton 2018; Jiménez‐Reguera 2020) showed there was probably little to no difference between maintenance and usual care groups for Symptoms with MD of ‐0.29 points (95% CI ‐6.32 to 5.73; 234 participants, 4 studies; Analysis 1.2); Activity with MD of ‐2.57 points (95% CI ‐8.05 to 2.90; 234 participants, 4 studies; Analysis 1.3); and Impact with MD of ‐2.08 points (95% CI ‐7.39 to 3.23; 234 participants, 4 studies; Analysis 1.4). The mean change did not reach the minimal important difference of ‐4 points (Jones 2005). All domains showed low‐certainty evidence, and there was no significant heterogeneity across studies (I2 across 0% and 25%).
1.2. Analysis.

Comparison 1: Supervised maintenance programme vs usual care, Outcome 2: QoL SGRQ Symptoms at time point six to 12 months following completion of pulmonary rehabilitation
1.3. Analysis.

Comparison 1: Supervised maintenance programme vs usual care, Outcome 3: QoL SGRQ Activity at time point six to 12 months following completion of pulmonary rehabilitation
1.4. Analysis.

Comparison 1: Supervised maintenance programme vs usual care, Outcome 4: QoL SGRQ Impact at time point six to 12 months following completion of pulmonary rehabilitation
The Chronic Respiratory Questionnaire (CRQ) total score was used at six to twelve months (Ries 2003; Abbink 2004; Du Moulin 2009; Wootton 2018) with the mean difference (MD) between supervised maintenance and usual care of 0.54 points (95% CI 0.04 to 1.03, 258 participants, 4 studies, Analysis 1.5; Figure 4) with low‐certainty evidence. This mean difference reaches the minimum important difference for total CRQ of 0.5 points (Regueiro 2013). There was marked heterogeneity across the studies (I2= 69%). One study (Burns 2016) presented the CRQ total score combined with quality‐adjusted life‐years (CRQ/QALYs), making it impossible to compare with other studies.
1.5. Analysis.

Comparison 1: Supervised maintenance programme vs usual care, Outcome 5: QoL CRQ total score at time point six to 12 months following completion of pulmonary rehabilitation
4.

Forest plot of comparison: 1 Supervised maintenance programme vs usual care; outcome: 1.1 QoL CRQ total score at time point six to 12 months following completion of pulmonary rehabilitation (points).
CRQ domain scores at six to 12 months (Brooks 2002; Du Moulin 2009; Wootton 2018; Galdiz 2020) showed little or no difference between maintenance and usual care groups for the domain of Dyspnoea, with MD 0.28 points (95% CI ‐0.51 to 1.06, 210 participants, 4 studies, Analysis 1.6) with substantial heterogeneity across the studies (I2 = 83%). There was a difference favouring maintenance compared to the usual care group for Fatigue with MD 0.38 points (95% CI 0.04 to 0.73; 210 participants, 4 studies; Analysis 1.7) with minimal heterogeneity across the studies (I2 = 4%); for Emotion the MD was 0.57 points (95% CI 0.18 to 0.96; 210 participants, 4 studies; Analysis 1.8) with moderate heterogeneity across the studies (I2 = 39%). For Mastery, the MD was 0.54 points (95% CI 0.05 to 1.04; 210 participants, 4 studies; Analysis 1.9) with moderate heterogeneity across the studies (I2 = 58%). The mean change in the Emotion and Mastery domain reached the minimal important difference of 0.5 points (Regueiro 2013). The analysis of domain scores showed very low‐certainty evidence. Three other studies (Roman 2013; Burns 2016; Guell 2017) did not report data from CRQ domains as measures of central tendency and dispersion at the time of interest but reported finding no mean difference between maintenance and usual care groups for all CRQ domains.
1.6. Analysis.

Comparison 1: Supervised maintenance programme vs usual care, Outcome 6: QoL CRQ Dyspnea time point six to 12 months following completion of pulmonary rehabilitation
1.7. Analysis.

Comparison 1: Supervised maintenance programme vs usual care, Outcome 7: QoL CRQ Fatigue at time point six to 12 months following completion of pulmonary rehabilitation
1.8. Analysis.

Comparison 1: Supervised maintenance programme vs usual care, Outcome 8: QoL CRQ Emotion at time point six to 12 months following completion of pulmonary rehabilitation
1.9. Analysis.

Comparison 1: Supervised maintenance programme vs usual care, Outcome 9: QoL CRQ Mastery at time point six to 12 months following completion of pulmonary rehabilitation
Two studies (Johnson 2001; Hill 2004) evaluated the CRQ domain at a time point of less than six months. Hill (2004) observed improvement in the fatigue domain, and Johnson (2001) observed improvement in the dyspnoea domain between the maintenance and usual care group.
One study (Guell 2017) involving 82 participants developed a long‐term maintenance programme with assessment at 24 and 36 months using CRQ domains. This study did not provide dispersion measures but reported no difference between maintenance and usual care groups at 24 months for all domains with low‐certainty evidence (Dyspnoea MD = ‐0.1, P = 0.61; Fatigue MD = ‐0.1 points, P = 0.38; Emotion MD = ‐0.3, P = 0.73; and Mastery MD = ‐0.6 points, P = 0.10). There were similar findings at 36 months (Dyspnoea MD = 0.1 points, P = 0.28; Fatigue MD = ‐0.3 points, P = 0.19; Emotion MD = ‐0.5 points, P = 0.27; and Mastery MD = ‐0.2 points, P = 0.89; low‐certainty evidence).
For generic HRQoL using the Short‐Form 36 (SF‐36) (Ries 2003; Galdiz 2020) at six to 12 months showed there was probably little to no difference between maintenance and usual care for both the Physical domain with MD of ‐0.72 points (95% CI ‐3.86 to 2.43; 219 participants, 2 studies; Analysis 1.10) and the Mental domain with MD of ‐0.42 points (95% CI ‐3.56 to 2.72; 219 participants, 2 studies; Analysis 1.11). For both domains, there was low‐certainty evidence and no heterogeneity was observed across studies (I2 = 0). One study (Guell 2017) involving 103 participants did not provide dispersion measures for the SF‐36 but reported no mean difference between the maintenance and usual care groups for the Physical (MD = 0 points, P = 0.61) and Mental (MD = ‐1 point, P = 0.31) domains. No studies using the SF‐36 presented data for domains other than Physical and Mental.
1.10. Analysis.

Comparison 1: Supervised maintenance programme vs usual care, Outcome 10: QoL SF36 Physical at time point six to 12 months following completion of pulmonary rehabilitation
1.11. Analysis.

Comparison 1: Supervised maintenance programme vs usual care, Outcome 11: QoL SF36 Mental at time point six to 12 months following completion of pulmonary rehabilitation
One study (Guell 2017) showed little to no difference between the maintenance and usual care groups for the Physical and Mental domains using the SF‐36 after 12 months. Guell 2017 involved 82 participants and did not provide dispersion measures but reported no difference between the Physical (MD ‐2 points, P = 0.78) and Mental (MD = ‐3 points, P = 0.20) domains.
Only one study (Steele 2008) conducted the intervention duration of less than six months reported improvement in the SF‐36 physical domain between the maintenance and usual care groups.
Studies reported data using EuroQoL at six to 12 months (Burns 2016; Jiménez‐Reguera 2020), showing little to no difference between the maintenance and usual care groups with MD = 0.00 point (95% CI ‐0.08 to 0.08; 150 participants, 2 studies; Analysis 1.12), with moderate‐certainty evidence. The mean change did not reach the minimal important difference of 0.07 points (Walters 2005). No heterogeneity was observed across these studies (I2 = 0).
1.12. Analysis.

Comparison 1: Supervised maintenance programme vs usual care, Outcome 12: QoL EuroQoL‐5D at time point six to 12 months following completion of pulmonary rehabilitation
Studies (Li 2018; Jiménez‐Reguera 2020) using COPD assessment test (CAT) at six to 12 months found a difference favouring maintenance compared to the usual care group. The MD was ‐5.88 points (95% CI ‐8.39 to ‐3.37; 147 participants, 2 studies; Analysis 1.13) and with low‐certainty evidence, which exceeded the minimal important difference (MID) of ‐3.8 points, reported for COPD (Smid 2017), with no significant heterogeneity across studies (I2 = 20%). One study (Bernocchi 2018) evaluated CAT at less than six months and reported improvement in favour of maintenance compared to the usual care group.
1.13. Analysis.

Comparison 1: Supervised maintenance programme vs usual care, Outcome 13: QoL CAT at time point six to 12 months following completion of pulmonary rehabilitation
Exercise capacity
Exercise capacity was measured using a six‐minute walk test (6MWT) in 15 studies (Brooks 2002; Ries 2003; Abbink 2004; Hill 2004; Steele 2008; Du Moulin 2009; Spencer 2010; Roman 2013; Wong 2014; Guell 2017; Bernocchi 2018; Li 2018; Wootton 2018; Galdiz 2020; Jiménez‐Reguera 2020), while the incremental shuttle walk test was used in five studies (Johnson 2001; Murphy 2005; Arbane 2010; Spencer 2010; Wootton 2018), the endurance shuttle walk test in four studies (Ringbaek 2010; Spencer 2010; Linneberg 2012; Wootton 2018), and cardiopulmonary exercise tests in two studies (Ries 2003; Abbink 2004;).
A pooled analysis of studies (Brooks 2002; Ries 2003; Abbink 2004; Du Moulin 2009; Spencer 2010; Wong 2014; Li 2018; Wootton 2018; Galdiz 2020; Jiménez‐Reguera 2020) using the 6MWT at six to 12 months showed an effect that may have favoured maintenance over usual care, with an MD of 25.90 metres (m) (95% CI ‐1.04 to 52.84; 639 participants, 10 studies, Analysis 1.14; Figure 5), low‐certainty evidence, with marked heterogeneity across studies (I2= 67%). The mean change did not reach the minimal important difference of 30 metres (Holland 2014).
1.14. Analysis.

Comparison 1: Supervised maintenance programme vs usual care, Outcome 14: 6MWD at time point six to 12 months following completion of pulmonary rehabilitation
5.

Forest plot of comparison: 1 Supervised Maintenance Program vs Usual Care, outcome: 1.14 6MWD at time point six to 12 months following completion of pulmonary rehabilitation [meters].
One study (Roman 2013) did not provide means or dispersion measures for each group and reported no difference between the maintenance and usual care groups for 6MWD, which was ‐0.5 m (95% CI ‐43.02 to 41.9 m; 48 participants), with low‐certainty evidence. Three studies (Hill 2004; Steele 2008; Bernocchi 2018) evaluated exercise capacity using the 6MWT at less than six months. Studies by Bernocchi 2018, Hill 2004, and Steele 2008 reported improvement in six‐minute walk distance (6MWD) in favour of the maintenance group rather than the usual care group.
One study (Guell 2017) used the 6MWT at 24 and 36 months and showed a difference favouring maintenance over usual care (MD = ‐34 m, P = 0.04 and MD = ‐29 m, P = 0.001, respectively), with low‐certainty evidence. Measures of dispersion were not reported.
Studies (Spencer 2010; Wootton 2018) using the incremental shuttle walk test (ISWT) at six to 12 months showed little to no difference between maintenance and usual care groups with MD = 4.15 m (95% CI ‐44.93 to 53.23; 111 participants, 2 studies; Analysis 1.15 ), with low‐certainty evidence and no heterogeneity (I2 = 0). The mean change did not reach the minimal important difference of 47.5 metres (Singh 2014).
1.15. Analysis.

Comparison 1: Supervised maintenance programme vs usual care, Outcome 15: ISWT at time point six to 12 months following completion of pulmonary rehabilitation
The endurance shuttle walk test (ESWT) was assessed (Ringbaek 2010; Spencer 2010; Burns 2016; Linneberg 2012; Wootton 2018) at six to 12 months. There was probably little to no difference between the maintenance and usual care groups with MD = 26.87 seconds (95% CI ‐60.58 to 114.33; 369 participants, 5 studies; Analysis 1.16), with low‐certainty evidence and no significant heterogeneity (I2 = 0%). The mean change did not reach the minimal important difference of 65 seconds (Singh 2014).
1.16. Analysis.

Comparison 1: Supervised maintenance programme vs usual care, Outcome 16: ESWT at time point six to 12 months following completion of pulmonary rehabilitation
Only one study (Abbink 2004) reported the maximum workload in cardiopulmonary exercise testing on a cycle ergometer at 12 months, and there was little to no difference between the maintenance programme and usual care group with MD = 0 m (95% CI ‐14.82 to 14.82; 31 participants), with very low‐certainty evidence.
One study (Ries 2003) reported peak oxygen uptake (VO2 peak) at 12 months and showed little to no difference between maintenance and usual care groups with MD = 0.03 L/min (95% CI ‐0.12 to 0.18; 138 participants).
All‐cause hospitalisation
Hospitalisation (all‐cause) was reported in seven studies (Ries 2003; Murphy 2005; Spencer 2010; Roman 2013; Burns 2016; Bernocchi 2018; Galdiz 2020).
Studies (Roman 2013; Galdiz 2020) presented the data as an odds ratio, with little to no difference between maintenance and usual care groups at six to 12 months with OR 0.71 (95% CI 0.14 to 3.69; 142 participants, 2 studies; Analysis 1.17) with low‐certainty evidence, and no important heterogeneity (I2 = 15%). For three studies (Ries 2003; Spencer 2010; Burns 2016) involving 332 participants, mean difference between maintenance and usual care in all‐cause hospitalisation ranged from ‐0.20 to 0.30 at six to 12 months (Analysis 1.18).
1.17. Analysis.

Comparison 1: Supervised maintenance programme vs usual care, Outcome 17: Hospital admission (all‐causes) odds time point at six to 12 months following completion of pulmonary rehabilitation
1.18. Analysis.

Comparison 1: Supervised maintenance programme vs usual care, Outcome 18: Hospital admission (all‐causes) rate at time point six to 12 months following completion of pulmonary rehabilitation
In another two studies (Murphy 2005; Bernocchi 2018), the duration of the intervention was less than six months. Bernocchi 2018 presented a Kaplan–Meier survival analysis of time‐to‐event (hospitalisation for all causes) that shows the difference between groups, in favour of the maintenance group rather than the usual care group. Murphy 2005 reported no difference in the rate of hospital admission between the maintenance group and usual care group.
No reports regarding hospital admission were made after 12 months.
Secondary outcomes
Exacerbations
Studies assessed exacerbation at six to 12 months (Ries 2003; Spencer 2010; Roman 2013; Burns 2016; Li 2018; Galdiz 2020). Some studies presented the data as an odds ratio (OR) (Roman 2013; Li 2018; Galdiz 2020) and showed little to no difference between maintenance and usual care groups with OR 0.73 (95% CI 0.26 to 2.02; 280 participants, 3 studies; Analysis 1.19) with low‐certainty evidence and substantial heterogeneity across the studies (I2 = 66%). Others studies presented exacerbations as continuous data (Ries 2003; Spencer 2010; Burns 2016) with little or no difference between maintenance and usual care groups. The mean difference ranged from ‐0.01 to 0.90 across three studies, 342 participants (Analysis 1.20)..
1.19. Analysis.

Comparison 1: Supervised maintenance programme vs usual care, Outcome 19: Exacerbation odds at time point six to 12 months following completion of pulmonary rehabilitation
1.20. Analysis.

Comparison 1: Supervised maintenance programme vs usual care, Outcome 20: Exacerbation rate at time point six to 12 months following completion of pulmonary rehabilitation
Mortality
Studies (Ries 2003; Spencer 2010; Burns 2016; Guell 2017; Li 2018; Wootton 2018) have contributed data on mortality showing little to no difference in the chance of death between the maintenance programme and the usual care group with OR 0.73 (95% CI 0.36 to 1.51; 755 participants, 6 studies; Analysis 1.21; Figure 6), with moderate‐certainty evidence. The results indicated no heterogeneity across studies (I2 = 0). One study (Ringbaek 2010) involving 93 participants reported no mortality in either the maintenance or usual care groups.
1.21. Analysis.

Comparison 1: Supervised maintenance programme vs usual care, Outcome 21: Mortality time point six to 12 months following completion of pulmonary rehabilitation
6.

Forest plot of comparison: 1 Supervised maintenance programme vs usual care; outcome: 1.19 Mortality time point six to12 months following completion of pulmonary rehabilitation.
Only one study (Guell 2017) reported mortality at 24 and 36 months, showing for 24 months an OR of 1.03 (95% CI 0.20 to 5.29; 82 participants) and for 36 months an OR of 0.53 (95% CI 0.17‐1.69; 65 participants), with low‐certainty evidence for both.
Direct costs
Direct costs of care during the follow‐up period were reported in two studies (Steele 2008; Burns 2016).
Steele 2008 reported that the duration of intervention was less than six months (three months), and the total mean cost of intervention for each subject in the adherence intervention group was USD 134.36; the range of costs was between USD 76 and USD 221, including phone calls and travel expenses. The usual care group did not include any planned contact with pulmonary rehabilitation participants after the programme so there was no related cost.
Another study (Burns 2016) reported data on the direct costs of care during the 12 months of the maintenance programme. The cost of intervention for the maintenance group was GBP 43, with no cost for the usual care group.
No reports of direct costs of care were made after 12 months.
Adverse events (all causes)
Information regarding adverse events at six to 12 months was available descriptively in six studies (Brooks 2002; Spencer 2010; Burns 2016; Guell 2017; Wootton 2018; Galdiz 2020), none of which reported adverse events during the follow‐up period. Two studies (Johnson 2001; Bernocchi 2018) reported no adverse events during the intervention period, which was less than six months.
Only one study (Guell 2017) reported no adverse effects during follow‐up periods of 24 and 36 months.
Subgroup analyses
The subgroup analyses were performed with primary outcomes and at the six to 12‐month time point, as few studies have shown outcomes beyond 12 months.
Supervised maintenance programmes offered monthly or less frequently compared to those offered more frequently
Two studies (Linneberg 2012; Burns 2016) offered supervision monthly or less frequently and 14 studies (Brooks 2002; Ries 2003; Abbink 2004; Steele 2008; Du Moulin 2009; Arbane 2010; Ringbaek 2010; Spencer 2010; Roman 2013; Wong 2014; Guell 2017; Li 2018; Wootton 2018; Galdiz 2020) offered supervision more frequently than monthly.
Health‐related quality of life
All studies that used the CRQ, SGRQ, SF‐36, CAT to measure health‐related quality of life offered supervised maintenance with a frequency greater than one month, not allowing comparisons with less frequent supervision study, except for the EuroQoL.
Quality of life measured using the EuroQoL (Analysis 2.1) showed little or no differences between the subgroups, with a mean difference between groups for the more frequent supervision subgroup of 0.0 units (95% CI ‐0.13 to 0.13; 36 participants,1 study; Jiménez‐Reguera 2020) and the less frequent supervision subgroup of 0.00 unit (95% CI ‐0.11 to 0.11; 114 participants, 1 study; Burns 2016); test for subgroup differences: P = 1.00 and no heterogeneity across the studies (I2 = 0%).
2.1. Analysis.

Comparison 2: Effectiveness of the maintenance programme offered monthly or less frequently compared to those offered more frequently, Outcome 1: QoL EuroQoL
Exercise capacity
All studies that assessed exercise capacity through the 6MWT, ISWT and cardiopulmonary exercise test offered supervised maintenance with a frequency greater than one month. However, these tests were not assessed when supervision was less frequently than one month, which did not allow comparisons between these subgroups. The comparison between subgroups of higher or lower frequency of supervision for the exercise capacity outcome was possible only with the ESWT.
Exercise capacity for the ESWT (Analysis 2.2) showed a mean difference between groups for more frequent supervision of 26.72 seconds (95% CI ‐110.85 to 164.29; 190 participants, 3 studies; Ringbaek 2010; Spencer 2010; Wootton 2018), and less frequent supervision of ‐5.12 seconds (95% CI ‐168.53 to 158.29; 179 participants, 2 studies; Linneberg 2012; Burns 2016); test for subgroup differences: P = 0.77 and no heterogeneity across the studies (I2 =0%).
2.2. Analysis.

Comparison 2: Effectiveness of the maintenance programme offered monthly or less frequently compared to those offered more frequently, Outcome 2: Exercise capacity: ESWT
All‐cause hospitalisation
The analysis for hospitalisation as odds ratio was not possible for this subgroup because both studies (Roman 2013; Galdiz 2020) offered supervised maintenance with a frequency greater than one month.
The rate of hospital admission (Analysis 2.3) showed no difference in risk rate for all causes of hospitalisation between the subgroups that offered the maintenance programme with supervision more frequently (MD ranged from ‐0.20 to 0.30; 184 participants; 2 studies, Ries 2003; Spencer 2010) and less frequent supervision (MD ‐ 0.10, 160 participants, 1 study, Burns 2016); test for subgroup differences: P= 0.78 and no heterogeneity across the studies (I2 = 0%).
2.3. Analysis.

Comparison 2: Effectiveness of the maintenance programme offered monthly or less frequently compared to those offered more frequently, Outcome 3: Hospital admission rate (all causes) for subgroups only
Supervised maintenance programmes using in‐person supervision versus remote supervision
Eleven studies provided in‐person supervision (Johnson 2001; Abbink 2004; Hill 2004; Murphy 2005; Arbane 2010; Ringbaek 2010; Spencer 2010; Linneberg 2012; Roman 2013; Burns 2016; Guell 2017) and six studies provided remote supervision (Du Moulin 2009; Wong 2014; Bernocchi 2018; Wootton 2018; Galdiz 2020; Jiménez‐Reguera 2020). Studies that used a combination of in‐person and remote supervised maintenance (Brooks 2002; Ries 2003; Steele 2008; Li 2018) were not included in this subgroup analysis.
Health‐related quality of life
The analysis of quality of life using the total SGRQ score (Analysis 3.1) showed the mean difference between groups for in‐person supervision subgroup was 1.00 units (95% CI ‐8.37 to 10.37; 48 participants, 1 study; Spencer 2010), and remote supervision was ‐2.36 units (95% CI ‐7.93 to 3.21, 186 participants, 2 studies; Wong 2014; Wootton 2018; Jiménez‐Reguera 2020); test for subgroup differences P = 0.55, and no heterogeneity across the studies (I2 = 0%). For the SGRQ Symptoms domain (Analysis 3.2), the mean difference between groups for in‐person supervision showed an MD of 3.00 (95% CI ‐10.03 to 16.03, 48 participants, 1 study; Spencer 2010), and remote supervision showed an MD of ‐1.19 (95% CI ‐7.98 to 5.61; 186 participants, 3 studies; Wong 2014; Wootton 2018; Jiménez‐Reguera 2020); test for subgroup differences P = 0.58, and no heterogeneity across the studies (I2 = 0%). For the Activity domain (Analysis 3.3), the in‐person supervision showed an MD of 1.00 (95% CI ‐11.25 to 13.25, 48 participants, 1 study; Spencer 2010), and remote supervision showed an MD of ‐3.46 (95% CI ‐9.58 to 2.65; 186 participants, 3 studies; Wong 2014; Wootton 2018; Jiménez‐Reguera 2020); test for subgroup differences P = 0.52, and no heterogeneity across the studies I = 0%. For the Impact domain (Analysis 3.4), the in‐person supervision showed an MD of 0.00 (95% CI ‐9.44 to 9.44; 48 participants, 1 study; Spencer 2010), and remote supervision showed an MD of ‐2.68 (95% CI ‐9.94 to 4.59, 186 participants, 3 studies; Wong 2014; Wootton 2018; Jiménez‐Reguera 2020); test for subgroup differences P = 0.66, and no heterogeneity across the studies (I2 = 0%).
3.1. Analysis.

Comparison 3: Effectiveness of the maintenance programmes using in‐person supervision versus remote supervision, Outcome 1: QoL SGRQ total score
3.2. Analysis.

Comparison 3: Effectiveness of the maintenance programmes using in‐person supervision versus remote supervision, Outcome 2: QoL SGRQ Symptoms
3.3. Analysis.

Comparison 3: Effectiveness of the maintenance programmes using in‐person supervision versus remote supervision, Outcome 3: QoL SGRQ Activity
3.4. Analysis.

Comparison 3: Effectiveness of the maintenance programmes using in‐person supervision versus remote supervision, Outcome 4: QoL SGRQ Impact
Analysis of the quality of life using the total CRQ score (Analysis 3.5) showed a mean difference between groups for in‐person supervision subgroup of 0.90 units (95% CI ‐0.00 to 1.80; 31 participants, 1 study; Abbink 2004) and remote supervision subgroup of 0.69 units (95% CI ‐0.09 to 1.48, 89 participants, 2 studies; Du Moulin 2009; Wootton 2018); test for subgroup differences P = 0.73, and no heterogeneity across the studies (I2 = 0%).
3.5. Analysis.

Comparison 3: Effectiveness of the maintenance programmes using in‐person supervision versus remote supervision, Outcome 5: QoL CRQ total score
For the quality of life using EuroQoL (Analysis 3.6), there was an MD in the in‐person supervision subgroup of 0.00 units (95% CI ‐0.11 to 0.11; 114 participants, 1 study; Burns 2016), and the remote supervision subgroup of 0.00 units (95% CI ‐0.13 to 0.13, 36 participants, 1 study; Jiménez‐Reguera 2020); test for subgroup differences P = 1.00, and no heterogeneity between the studies (I2 = 0%).
3.6. Analysis.

Comparison 3: Effectiveness of the maintenance programmes using in‐person supervision versus remote supervision, Outcome 6: QoL EuroQoL
Exercise capacity
For exercise capacity using the 6MWT (Analysis 3.7), an MD in the in‐person supervision subgroup of 22.39 m was observed (95% CI ‐51.75 to 96.53, 79 participants, 2 studies; Abbink 2004; Spencer 2010), and the remote supervision subgroup of 27.07 m (95% CI ‐10.99 to 65.14, 270 participants, 5 studies; Du Moulin 2009; Wong 2014; Wootton 2018; Galdiz 2020; Jiménez‐Reguera 2020); test for subgroup differences P = 0.91, and low heterogeneity across the studies (I2 = 27.4%).
3.7. Analysis.

Comparison 3: Effectiveness of the maintenance programmes using in‐person supervision versus remote supervision, Outcome 7: Exercise Capacity: 6MWT
For exercise capacity using the ISWT (Analysis 3.8), a MD in the in‐person supervision subgroup of ‐6.00 m was observed (95% CI ‐75.94 to 63.94, 48 participants, 1 study; Spencer 2010), and the remote supervision subgroup of 14.00 m (95% CI ‐54.88 to 82.88, 63 participants, 1 study; Wootton 2018); test for subgroup differences P = 0.69, and no heterogeneity across the studies (I2 = 0%).
3.8. Analysis.

Comparison 3: Effectiveness of the maintenance programmes using in‐person supervision versus remote supervision, Outcome 8: Exercise Capacity: ISWT
The analysis of exercise capacity for the ESWT (Analysis 3.9) showed an MD in the in‐person supervision subgroup of 9.24 seconds (95% CI ‐89.68 to 108.16, 305 participants, 4 studies; Ringbaek 2010; Spencer 2010; Linneberg 2012; Burns 2016), and the remote supervision subgroup of 90.00 seconds (95% CI ‐97.16 to 277.16, 64 participants, 1 study; Wootton 2018); test for subgroup differences P = 0.45, and no heterogeneity across the studies (I2 = 0%).
3.9. Analysis.

Comparison 3: Effectiveness of the maintenance programmes using in‐person supervision versus remote supervision, Outcome 9: Exercise Capacity: ESWT
All‐cause hospitalisation
The analysis for hospitalisation as the rate was not possible for this subgroup because both studies (Burns 2016; Spencer 2010) offered supervised maintenance in person.
The analysis of hospital admission for studies that presented dichotomous data (Analysis 3.10) showed an OR in the in‐person supervision subgroup of 0.44 (95% CI 0.09 to 2.12; 48 participants, 1 study; Roman 2013), and the remote supervision subgroup of 3.20 (95% CI 0.13 to 80.52; 94 participants, 1 study; Galdiz 2020); test for subgroup differences P = 0.28, and no important heterogeneity across the studies (I2 = 14.3%).
3.10. Analysis.

Comparison 3: Effectiveness of the maintenance programmes using in‐person supervision versus remote supervision, Outcome 10: Hospital admission odds (all‐causes) for subgroup only
Sensitivity analysis
For the sensitivity analysis, studies that did not specify blinding of the outcome measures or had incomplete outcome data (tendency to attrition) were excluded. As a result, only four studies (Ries 2003; Burns 2016; Wootton 2018; Jiménez‐Reguera 2020) could be considered for the comparison between supervised maintenance programmes and usual care regarding the primary outcomes. The sensitivity analysis can be found in the additional Table 3.
2. Sensitivity analysis ‐ any risk of bias vs. low risk of bias.
| Outcome | Effect estimate from analysis with all studies | Effect estimate from sensitivity analysis with studies removed |
| CRQ Total (HRQoL) | MD 0.54, 95% CI 0.04 to 1.03 (N = 258; S = 4) |
MD 0.16, 95% CI ‐0.13 to 0.46 (N = 211; S = 2) |
| CRQ Dyspnoea (HRQoL) | MD 0.28, 95% CI‐0.52 to 1.08 (N = 210; S = 4) |
MD ‐0.20, 95% CI ‐0.80 to 0.40 (N = 73; S = 1) |
| CRQ Fatigue (HRQoL) | MD 0.40, 95% CI 0.06 to 0.74 (N = 210; S = 4) |
MD 0.25, 95% CI ‐0.46 to 0.96 (N = 73; S = 1) |
| CRQ Emotion (HRQoL) | MD 0.60, 95% CI 0.22 to 0.98 (N = 210; S = 4) |
MD 0.29, 95% CI ‐0.31 to 0.89 (N = 73; S = 1) |
| CRQ Mastery (HRQoL) | MD 0.57, 95% CI 0.08 to 1.05 (N = 210; S = 4) |
MD 0.25, 95% CI ‐0.33 to 0.83 (N = 73; S = 1) |
| SGRQ Total (HRQoL) | MD ‐1.55, 95% CI ‐4.91 to 1.81 (N = 276; S = 5) |
MD ‐5.06, 95% CI ‐11.55 to 1.44 (N = 107; S = 2) |
| SGRQ Symptoms (HRQoL) | MD ‐0.29, 95% CI ‐6.32 to 5.73 (N = 234; S = 4) |
MD ‐1.73, 95% CI ‐10.55 to 7.09 (N = 107; S = 2) |
| SGRQ Activity (HRQoL) | MD ‐2.57, 95% CI ‐8.04 to 2.90 (N = 234; S = 4) |
MD ‐5.22, 95% CI ‐12.90 to 2.45 (N = 107; S = 2) |
| SGRQ Impact (HRQoL) | MD ‐2.15, 95% CI ‐6.70 to 2.40 (N = 234; S = 4) |
MD ‐6.20, 95% CI ‐12.93 to 0.53 (N = 107; S = 2) |
| SF‐36 Physical (HRQoL) | MD ‐0.72, 95% CI ‐3.86 to 2.43 (N = 219; S = 2) |
MD ‐0.30, 95% CI ‐3.60 to 3.00 (N = 138; S = 1) |
| SF‐36 Mental (HRQoL) | MD ‐0.42, 95% CI ‐3.56 to 2.72 (N = 219; S = 2) |
MD ‐0.70, 95% CI ‐4.04 to 2.64 (N = 138; S = 1) |
| CAT (HRQoL) | MD‐6.08, 95% CI ‐8.07 to‐4.09 (N = 147; S = 2) |
MD ‐3.60, 95% CI ‐8.37 to 1.17 (N = 36; S = 1) |
| EuroQoL‐5D (HRQoL) | MD 0.01, 95% CI ‐0.07 to 0.09 (N = 150; S = 2) |
MD 0.01, 95% CI ‐0.07 to 0.09 (N = 150; S = 2) |
| 6MWD (Exercise capacity) | MD 25.91, 95% CI ‐1.03 to 52.85 (N = 639; S = 10) |
MD 18.73, 95% CI ‐10.73 to 48.20 (N = 236; S = 3) |
| ISWT (Exercise capacity) | MD 4.15, 95% CI ‐44.93 to 53.23 (N = 111; S = 2) |
MD 14.00, 95% CI ‐54.88 to 82.88 (N = 63; S = 1) |
| ESWT (Exercise capacity) | MD 26.90, 95% CI ‐60.56 to 114.36 (N = 369; S = 5) |
MD 37.71, 95% CI ‐91.61 to 167.03 (N = 147; S = 2) |
| Hospital admission (subgroup) | RR ranged from ‐0.20 to 0.30 (N = 332; S = 3) |
RR ranged from ‐0.06 to 0.30 (N = 284; S = 2) |
| Hospital admission (subgroup) | OR 0.71, 95% CI 0.14 to 3.69 (N = 142; S = 2) |
‐ |
6MWD: six‐minute walk distance; CAT: COPD assessment test; CRQ: chronic respiratory questionnaire; ESWT: endurance shuttle walk test; HRQoL: health‐related quality of life; ISWT: incremental shuttle walk test; MD: mean difference; N: number of participants; OR: odds ratio; RR: risk ratio; S: number of studies; SF‐36: Short Form 36; SGRQ: St George's Respiratory Questionnaire.
The sensitivity analysis, including only those studies with assessor blinding and more complete outcome data, showed a smaller magnitude of treatment effect. In the sensitivity analysis, there was little or no difference between the maintenance and usual care groups for the CRQ total, CRQ domain scores for Fatigue, Emotion, and Mastery and also for CAT, which lost their effect. The other outcomes did not change the pattern of findings, or insufficient data were available to perform a sensitivity analysis to examine the effects of the supervised maintenance programme compared to usual care.
Discussion
This review has synthesised the available evidence for supervised maintenance programmes compared to usual care following pulmonary rehabilitation for chronic obstructive pulmonary disease. Supervised maintenance programmes presented a wide range of frequency and forms of supervision offers (in‐person or remote) that have been used so far.
Summary of main results
Low‐certainty evidence suggests that compared to usual care, supervised maintenance programmes may improve the primary outcome of health‐related quality of life at six to 12 months after completion of pulmonary rehabilitation; but this finding was very uncertain. The CRQ Total score, Emotion and Mastery domains and CAT showed clinically important effects on health‐related quality of life favouring supervised maintenance at six to 12 months (Smid 2017); however, results were not consistent across other health‐related quality of life measures, including the SGRQ. The certainty of the evidence for quality of life was downgraded because of high risk of bias and imprecision.
Low‐certainty evidence suggests that effects on exercise capacity measured by 6MWD may have favoured supervised maintenance pulmonary rehabilitation over usual care at six to 12 months following completion of pulmonary rehabilitation, but it is not possible to be sure. The certainty of the evidence for the 6MWD was downgraded because of the high risk of bias and inconsistency.
There are only two studies that have looked at health‐related quality of life and exercise capacity at a time point greater than 12 months following completed pulmonary rehabilitation; when comparing the supervised maintenance programme and usual care, there were no differences in health‐related quality of life and exercise capacity with very low‐ to low‐certainty evidence.
Low‐certainty evidence showed that compared to usual care, supervised maintenance programmes do not reduce the risk and the rate of hospitalisation at six to 12 months after completion of pulmonary rehabilitation. The certainty of the hospitalisation evidence was downgraded because of the high risk of bias in blinding and attrition.
Low‐certainty evidence suggests that compared to usual care, supervised maintenance programmes probably do not reduce the odds of exacerbation at six to 12 months after completion of pulmonary rehabilitation. The certainty of the exacerbation evidence was downgraded because of the high risk of bias in blinding and attrition. Moderate‐certainty evidence suggests that compared to usual care, supervised maintenance programmes probably do not reduce exacerbation risk at six to 12 months after completion of pulmonary rehabilitation. The certainty of the exacerbation evidence was downgraded because of the high risk of bias in blinding.
Moderate‐certainty evidence suggests that compared with usual care, supervised maintenance programmes do not reduce the chance of death within six to 12 months after completion of pulmonary rehabilitation. The certainty of the mortality evidence was downgraded because of the high risk of attrition bias.
Comparison of direct costs of care during the follow‐up period was only observed in two studies (Steele 2008; Burns 2016), which resulted in increased costs for the maintenance group and no cost for the usual care group.
Information regarding adverse events was available only descriptively in six studies (Brooks 2002; Spencer 2010; Burns 2016; Guell 2017; Wootton 2018; Galdiz 2020), none of which reported any adverse events during the follow‐up period. Consistent methods for reporting serious or rare adverse events in the context of pulmonary rehabilitation are needed. Currently, no standardized assessment is used in clinical trials to detect adverse events during pulmonary rehabilitation in people with COPD. In general, details of adverse events were poorly reported.
Overall completeness and applicability of evidence
Study design
All studies involved participants with stable COPD who had completed an outpatient pulmonary rehabilitation programme. The average age of the participants was 67.3 years, with a variation in severity by FEV1 of 23.8% to 88.1% of the predicted value. Therefore, the population was representative of the general population of people with COPD who are usually referred for pulmonary rehabilitation.
Outcomes
Of the 21 included studies, only 16 offered maintenance pulmonary rehabilitation programmes with a duration of at least six months. These studies were considered for the meta‐analysis comparisons because we considered maintenance as a long‐term intervention. There is evidence that the effect of pulmonary rehabilitation on exercise capacity decays to levels comparable to pre‐rehabilitation within six months of programme completion (Troosters 2000).
The primary outcomes studied were HRQoL, exercise capacity, and hospitalisation, which are of the greatest interest or concern for people with COPD, practitioners, and researchers. The secondary outcomes were exacerbation, mortality, costs of care, and adverse events, which are also important outcomes of concern for people involved in providing rehabilitation, as well as funding organisations and policymakers.
Intervention
All participants in the intervention group underwent a supervised maintenance programme at the centre, home‐based, or in the community, but with considerable variation in the frequency of supervision, from weekly until every three months. All control groups received the usual care standards. Most trials reported supervision performed in‐person, and fewer trials were offered remotely. Usually, maintenance programmes are provided by practitioners at a lower frequency than the initial pulmonary rehabilitation programme. The Australian and New Zealand Pulmonary Rehabilitation Guidelines made a weak recommendation that supervised maintenance programmes provided monthly or less frequently are insufficient to maintain clinical benefits compared with standard care (Alison 2017). In our systematic review, remotely supervised maintenance showed no difference in outcomes in comparison to in‐person supervision, but the number of studies was very small. Both frequency and forms (in‐person or remote) of providing supervised maintenance programmes require more research to determine an effective strategy.
Overall completeness
In this review, the analyses were based on the available data. The rate of attrition was high in many studies, due to the fact that maintenance pulmonary rehabilitation is a long‐term intervention with less frequent supervision than the initial pulmonary rehabilitation program, In addition, some studies did not contribute data to meta‐analyses, but were considered in non‐statistical syntheses of findings.
Quality of the evidence
Most of the evidence was of low certainty, which was primarily due to statistical imprecision, high risk of performance bias, failure to use intention‐to‐treat analyses (attrition bias), and inconsistency (heterogeneity). As the nature of the maintenance programme intervention includes physical training, it is expected that blinding of participants and personnel would be limited. To assess whether study quality affected the conclusions of the review, we conducted sensitivity analyses based on primary outcomes (HRQoL, exercise capacity, and hospitalisation). However, a small number of studies were available for each outcome in the sensitivity analysis, with the majority of the outcomes encompassing one or two studies, and a maximum of four studies. Overall, although all included studies were RCTs, the evidence base for supervised maintenance of pulmonary rehabilitation remains insufficient.
Statistical heterogeneity was present in many analyses. Several factors could have contributed to the heterogeneity of evidence in this review, including variations in the components of the intervention or control conditions among studies, such as patient education and self‐care and respiratory muscle training. Other factors included the severity of COPD, and the duration or effects of the initial pulmonary rehabilitation programme before the maintenance programme.
Potential biases in the review process
The review was based on a published protocol (Malaguti 2020) and deviations are reported in the Differences between protocol and review. There are some potential sources of bias in this review. We found four studies available only in abstract form (Johnson 2001; Abbink 2004; Hill 2004; Arbane 2010). These publications provided limited data for our review. We made extensive efforts to contact all authors to collect additional data of available reports, but only one author (Hill 2004) was able to provide additional information.
Given that the maintenance programme is a long‐term intervention, a high dropout rate was observed in almost half of the studies, and thus the incomplete outcome data may have influenced results. As physical training is an essential physical intervention in the maintenance program, it is understandable that blinding of the participants is difficult in these studies. Many studies have not blinded assessors to the outcomes. Furthermore, in many domains, the risk of bias is unclear. Therefore, all trials included in this meta‐analysis were classified as having a medium or high risk of bias. As a result, the quality of the general evidence from the present review was low.
Agreements and disagreements with other studies or reviews
The results of this review for supervised maintenance programmes compared to usual care shows some consistency and inconsistency with other previous systematic reviews.
Beauchamp 2013 included only six studies (619 participants) to investigate the effectiveness of supervised maintenance programmes for exercise capacity and quality of life. Our updated review included 22 studies, from which 16 contributed to meta‐analyses. Beauchamp 2013 specified medium (six months) and long‐term (12 months) intervention time points, while time points in our study were from six to 12 months and more than 12 months. The present review, which includes a meta‐analysis of 10 studies (639 participants), suggests that compared to usual care, a supervised maintenance intervention after pulmonary rehabilitation may improve exercise capacity at six to 12 months. This finding is consistent with Beauchamp 2013, who reported results obtained in five studies that acquired data in six months (433 participants), showing that supervised maintenance programmes improved exercise capacity at this time point; however, this effect was not observed after 12 months of the supervised maintenance programme. On the other hand, our review showed a clinically significant effect of the supervised maintenance programme on the overall quality of life and also for the domains of Emotion and Mastery, which is different from results obtained by Beauchamp 2013. In their review, quality of life did not improve as much at six months as for 12 months of the supervised maintenance programme. This difference can be attributed to the fact that the latter grouped different quality of life instruments in the analysis and presented the data in terms of the standardized mean difference (SMD). In our review, however, we analyzed the results of quality of life from different instruments separately and presented it as the mean difference, allowing us to interpret the clinical significance of findings using the MID.
Jenkins 2018 focused on health care utilisation and included six relevant trials in the meta‐analysis. However, the authors also did not consider data from time points used in our review (i.e. six to 12 months). Our review included five trials (474 participants) for hospital admission for all causes, with moderate‐certainty evidence for no observed effect on this outcome, whereas Jenkins 2018 found from three trials (195 participants) a reduction in the rate of hospital admissions for respiratory causes. Our findings on mortality rates are consistent with those shown by Jenkins 2018. We did not observe a reduction in the mortality rate, using data from six trials (755 participants), similar to the findings in Jenkins 2018 with two trials (207 participants).
Soysa 2012 investigated the effectiveness of supervised maintenance programmes on exercise capacity and quality of life. Eight published studies were included. However, the studies presented different methodological quality. Two studies (Wijkstra 1994; Berry 2003) delivered supervision for both intervention and control groups after the initial pulmonary rehabilitation program; therefore, we did not include these in our review. Based on five studies (495 participants), Soysa 2012 observed that supervised maintenance programmes increased exercise capacity using the 6MWD compared to the usual care. However, this effect did not reach the minimum clinically important difference of 30 metres (Holland 2014). In agreement, our review also showed a potential effect in the 6MWD and also in the quality of life between supervised maintenance programmes and usual care at six to 12 months time points.
A recent systematic review by Imamura 2020 investigated the effectiveness of low‐frequency maintenance programmes on exercise capacity and quality of life in COPD patients at 12 months after completion of pulmonary rehabilitation, and performed a meta‐analysis with six studies and a small number of participants. However, the authors included only published studies (publication bias) and did not consider mandatory pulmonary rehabilitation before the supervised maintenance programme (Engström 1999; Bestall 2003). In our review, all participants had performed pulmonary rehabilitation before the supervised maintenance programme or usual care. Consistent with our findings, Imamura 2020 also showed that the supervised maintenance programme improved the distance in the 6MWT; however, the clinically important minimum difference of 30 metres was not reached (Holland 2014). Furthermore, similar to our findings, there was no effect of maintenance programmes on the total score of the SGRQ and its domains, although we have observed differences in the analysis of other quality of life instruments.
Authors' conclusions
Implications for practice.
Evidence from our systematic review suggests that maintenance programmes that include physical exercise (aerobic or resistance training or both) may improve health‐related quality of life (HRQoL) at six to 12 months of people with chronic obstructive pulmonary disease (COPD) following pulmonary rehabilitation. A benefit for exercise capacity at six to 12 months may also occur, with low certainty of evidence. However, our findings suggest (with low to moderate certainty of evidence) that maintenance programmes confer no additional benefits for reducing exacerbations and hospitalisations, over and above that provided by pulmonary rehabilitation. Our review did not suggest mortality benefits from participating in maintenance pulmonary rehabilitation; however, longer‐term, observational evidence would be required to be confident in this finding.
Limited data from this review indicate that maintenance programmes are inexpensive to deliver and not associated with increased adverse events in people with COPD. For some people with COPD, it might be reasonable to refer to maintenance for its effect on HRQoL and exercise capacity, but it is likely that some patients may experience no noticeable benefits. It is uncertain whether there would be benefits to the health system, such as reduced costs related to hospitalisation and also the commissioning of maintenance programmes after pulmonary rehabilitation.
Implications for research.
To be confident regarding the benefits of maintenance programmes after pulmonary rehabilitation, and to identify the optimal format of such programmes, future studies are required. Studies with larger samples are needed to improve our certainty regarding the size of observed effects. Given the long term nature of maintenance interventions, studies must be appropriately powered to allow for potential attrition, while appropriate methods must be used to account for dropouts in the analysis, especially to assess outcomes such as hospitalisation and mortality events. Future trials should also guarantee blinding of assessors. It is worth noting that many studies presented inadequate reporting or insufficient information about their methodology, which limited the certainty of the findings of this review. We recommend that future clinical trials follow the Consolidated Standards of Reporting Trials (CONSORT) checklist to improve research methodology in this field (Schulz 2010). Use of consistent outcome measures, particularly for commonly used outcomes such as health‐related quality of life, would facilitate data synthesis in future systematic reviews. The optimum maintenance pulmonary rehabilitation programme has not been established and requires further investigation. The optimal supervision frequency and programme duration are not yet known and should be investigated in future trials. Future trials should also seek to identify whether responses to supervised maintenance vary according to COPD severity, or other patient characteristics. The impact of interventions delivered alongside exercise training, such as self‐efficacy interventions, should be examined. The impact of remote supervision approaches, such as telerehabilitation, should be investigated. Cost‐effectiveness analysis will be critical to future trials of supervised maintenance interventions. It may also be useful to examine alternative approaches to maintenance programmes, including repeating pulmonary rehabilitation at regular intervals (e.g. 12‐monthly), or ‘top‐up’ programmes, delivered at an intensity similar to the initial pulmonary rehabilitation programme.
What's new
| Date | Event | Description |
|---|---|---|
| 12 April 2022 | Amended | Fixed the graph labels on some forest plots. |
History
Protocol first published: Issue 4, 2020 Review first published: Issue 8, 2021
Acknowledgements
The Background and Methods sections of this review are based on a standard template used by Cochrane Airways.
The authors and Airways Editorial Team are grateful to the following peer reviewers for their time and comments: Arwel W Jones (Australia), Kate Jolly (UK) and Bernard McCarthy (Ireland).
This project was funded by the National Institute for Health Research Systematic Reviews Programme (project number 16/114/21). This project was also supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Airways Group. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Health Research Systematic Reviews Programme, NIHR, National Health Service or the Department of Health and Social Care.
Appendices
Appendix 1. Database search strategies
| Database/search platform/date of last search | Search strategy | Results |
| Airways Register (via Cochrane Register of Studies) Date of most recent search: 31 March 2020 | 1 MESH DESCRIPTOR Lung Diseases, Obstructive AND INREGISTER 2 MESH DESCRIPTOR Pulmonary Disease, Chronic Obstructive EXPLODE ALL AND INREGISTER 3 emphysema AND INREGISTER 4 chronic* NEAR2 bronchiti* AND INREGISTER 5 (obstruct* NEAR3 (pulmonary or lung* or airway* or airflow* or bronch$ or respirat*)) AND INREGISTER 6 (COPD or AECOPD or AECB):ti,ab AND INREGISTER 7 #1 OR #2 OR #3 OR #4 OR #5 OR #6 8 MESH DESCRIPTOR Physical Therapy Modalities AND INREGISTER 9 MESH DESCRIPTOR Physical Fitness EXPLODE ALL AND INREGISTER 10 MESH DESCRIPTOR Physical Endurance EXPLODE ALL AND INREGISTER 11 MESH DESCRIPTOR Exercise Therapy EXPLODE ALL AND INREGISTER 12 MESH DESCRIPTOR Physical Exertion AND INREGISTER 13 MESH DESCRIPTOR Exercise Test EXPLODE ALL AND INREGISTER 14 MESH DESCRIPTOR Exercise EXPLODE ALL AND INREGISTER 15 ((pulmonary or respiratory) NEAR3 rehabilitation*):ti,ab AND INREGISTER 16 exercis*:ti,ab AND INREGISTER 17 (physical* NEAR3 (activit* or train* or fitness* or therap*)):ti,ab AND INREGISTER 18 interval train*:ti,ab AND INREGISTER 19 #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 20 (maintain* or maintenance):ti,ab AND INREGISTER 21 MESH DESCRIPTOR Follow‐Up Studies AND INREGISTER 22 follow up*:ti,ab AND INREGISTER 23 support*:ti,ab AND INREGISTER 24 remind*:ti,ab AND INREGISTER 25 repeat*:ti,ab AND INREGISTER 26 refresh*:ti,ab AND INREGISTER 27 #26 OR #25 OR #24 OR #23 OR #22 OR #21 OR #20 28 #6 AND #19 AND #27 | March 2020 = 788 |
| CENTRAL (via Cochrane Register of Studies) Date of most recent search: 31 March 2020 | 1 MESH DESCRIPTOR Lung Diseases, Obstructive AND CENTRAL:TARGET 2 MESH DESCRIPTOR Pulmonary Disease, Chronic Obstructive EXPLODE ALL AND CENTRAL:TARGET 3 emphysema AND CENTRAL:TARGET 4 chronic* NEAR2 bronchiti* AND CENTRAL:TARGET 5 (obstruct* NEAR3 (pulmonary or lung* or airway* or airflow* or bronch$ or respirat*)) AND CENTRAL:TARGET 6 (COPD or AECOPD or AECB):ti,ab AND CENTRAL:TARGET 7 #1 OR #2 OR #3 OR #4 OR #5 OR #6 AND CENTRAL:TARGET 8 MESH DESCRIPTOR Physical Therapy Modalities AND CENTRAL:TARGET 9 MESH DESCRIPTOR Physical Fitness EXPLODE ALL AND CENTRAL:TARGET 10 MESH DESCRIPTOR Physical Endurance EXPLODE ALL AND CENTRAL:TARGET 11 MESH DESCRIPTOR Exercise Therapy EXPLODE ALL AND CENTRAL:TARGET 12 MESH DESCRIPTOR Physical Exertion AND CENTRAL:TARGET 13 MESH DESCRIPTOR Exercise Test EXPLODE ALL AND CENTRAL:TARGET 14 MESH DESCRIPTOR Exercise EXPLODE ALL AND CENTRAL:TARGET 15 ((pulmonary or respiratory) NEAR3 rehabilitation*):ti,ab AND CENTRAL:TARGET 16 exercis*:ti,ab AND CENTRAL:TARGET 17 (physical* NEAR3 (activit* or train* or fitness* or therap*)):ti,ab AND CENTRAL:TARGET 18 interval train*:ti,ab AND CENTRAL:TARGET 19 #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 AND CENTRAL:TARGET 20 (maintain* or maintenance):ti,ab AND CENTRAL:TARGET 21 MESH DESCRIPTOR Follow‐Up Studies AND CENTRAL:TARGET 22 follow up*:ti,ab AND CENTRAL:TARGET 23 support*:ti,ab AND CENTRAL:TARGET 24 remind*:ti,ab AND CENTRAL:TARGET 25 repeat*:ti,ab AND CENTRAL:TARGET 26 refresh*:ti,ab AND CENTRAL:TARGET 27 #26 OR #25 OR #24 OR #23 OR #22 OR #21 OR #20 AND CENTRAL:TARGET 28 #6 AND #19 AND #27 AND CENTRAL:TARGET | March 2020 = 1220 |
| MEDLINE (Ovid) ALL Date of most recent search: 31 March 2020 | 1 Lung Diseases, Obstructive/ 2 exp Pulmonary Disease, Chronic Obstructive/ 3 emphysema$.tw. 4 (chronic$ adj3 bronchiti$).tw. 5 (obstruct$ adj3 (pulmonary or lung$ or airway$ or airflow$ or bronch$ or respirat$)).tw. 6 (COPD or AECOPD or AECB).tw. 7 or/1‐6 8 Physical Therapy Modalities/ 9 exp Physical Fitness/ 10 exp Physical endurance/ 11 exp Exercise Therapy/ 12 Physical Exertion/ 13 exp Exercise Test/ 14 exp Exercise/ 15 ((pulmonary or respiratory) adj3 rehabilitation$).ti,ab. 16 exercis$.ti,ab. 17 (physical$ adj3 (activit$ or train$ or fitness$ or therap$)).ti,ab. 18 interval train$.ti,ab. 19 or/8‐18 20 7 and 19 21 (maintain$ or maintenance).tw. 22 Follow‐Up Studies/ 23 follow up$.tw. 24 support$.tw. 25 remind$.tw. 26 repeat$.tw. 27 refresh$.tw. 28 or/21‐27 29 20 and 28 30 (controlled clinical trial or randomized controlled trial).pt. 31 (randomized or randomised).ab,ti. 32 placebo.ab,ti. 33 randomly.ab,ti. 34 trial.ab,ti. 35 groups.ab,ti. 36 or/30‐35 37 Animals/ 38 Humans/ 39 37 not (37 and 38) 40 36 not 39 41 29 and 40 | March 2020 = 1214 |
| Embase (Ovid) Date of most recent search: 31 March 2020 | 1 Chronic Obstructive Lung Disease/ 2 Obstructive Airway Disease/ 3 Chronic Bronchitis/ 4 emphysema$.ti,ab. 5 chronic bronchitis.ti,ab. 6 (chronic adj2 obstruct$ adj2 (pulmonary or lung$ or airway$ or airflow$ or bronch$ or respirat$)).ti,ab. 7 (COPD or AECOPD or AECB).ti,ab. 8 or/1‐7 9 exp physiotherapy/ 10 fitness/ 11 endurance/ 12 exp kinesiotherapy/ 13 exp exercise/ 14 exp exercise test/ 15 ((pulmonary or respiratory) adj3 rehabilitation$).ti,ab. 16 exercis$.ti,ab. 17 (physical$ adj3 (activit$ or train$ or fitness$ or therap$)).ti,ab. 18 interval train$.ti,ab. 19 or/9‐18 20 (maintain$ or maintenance).ti,ab. 21 follow up/ 22 follow up$.ti,ab. 23 support$.ti,ab. 24 remind$.ti,ab. 25 repeat$.ti,ab. 26 refresh$.ti,ab. 27 or/20‐26 28 Randomized Controlled Trial/ 29 randomization/ 30 controlled clinical trial/ 31 Double Blind Procedure/ 32 Single Blind Procedure/ 33 Crossover Procedure/ 34 (clinica$ adj3 trial$).tw. 35 ((singl$ or doubl$ or trebl$ or tripl$) adj3 (mask$ or blind$ or method$)).tw. 36 exp Placebo/ 37 placebo$.ti,ab. 38 random$.ti,ab. 39 ((control$ or prospectiv$) adj3 (trial$ or method$ or stud$)).tw. 40 (crossover$ or cross‐over$).ti,ab. 41 or/28‐40 42 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 43 human/ or normal human/ or human cell/ 44 42 and 43 45 42 not 44 46 41 not 45 47 8 and 19 and 27 and 46 | March 2020 = 1821 |
|
PEDro Date of most recent search: 31 March 2020 |
Title & abstract: maintenance pulmonary rehabilitation Method: clinical trial |
March 2020 = 22 |
| ClinicalTrials.gov Date of most recent search: 31 March 2020 | Study type: Interventional
Condition: COPD Intervention: maintenance pulmonary rehabilitation |
March 2020 = 13 |
Data and analyses
Comparison 1. Supervised maintenance programme vs usual care.
Comparison 2. Effectiveness of the maintenance programme offered monthly or less frequently compared to those offered more frequently.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 QoL EuroQoL | 2 | 150 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.08, 0.08] |
| 2.1.1 Maintenance program offered more frequently | 1 | 36 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.13, 0.13] |
| 2.1.2 Maintenance program offered less frequently | 1 | 114 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.11, 0.11] |
| 2.2 Exercise capacity: ESWT | 5 | 369 | Mean Difference (IV, Random, 95% CI) | 26.87 [‐60.58, 114.33] |
| 2.2.1 Maintenance program offered more frequently | 3 | 190 | Mean Difference (IV, Random, 95% CI) | 26.72 [‐110.85, 164.29] |
| 2.2.2 Maintenance program offered less frequently | 2 | 179 | Mean Difference (IV, Random, 95% CI) | ‐5.12 [‐168.53, 158.29] |
| 2.3 Hospital admission rate (all causes) for subgroups only | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.3.1 Maintenance program offered more frequently | 2 | 184 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.49, 0.43] |
| 2.3.2 Maintenance program offered less frequently | 1 | 148 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.30, 0.10] |
Comparison 3. Effectiveness of the maintenance programmes using in‐person supervision versus remote supervision.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 QoL SGRQ total score | 4 | 234 | Mean Difference (IV, Random, 95% CI) | ‐1.63 [‐6.10, 2.84] |
| 3.1.1 Maintenance programmes using in‐person supervision | 1 | 48 | Mean Difference (IV, Random, 95% CI) | 1.00 [‐8.37, 10.37] |
| 3.1.2 Maintenance programmes using remote supervision | 3 | 186 | Mean Difference (IV, Random, 95% CI) | ‐2.36 [‐7.93, 3.21] |
| 3.2 QoL SGRQ Symptoms | 4 | 234 | Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐6.32, 5.73] |
| 3.2.1 Maintenance programs using in‐person supervision | 1 | 48 | Mean Difference (IV, Random, 95% CI) | 3.00 [‐10.03, 16.03] |
| 3.2.2 Maintenance programmes using remote supervision | 3 | 186 | Mean Difference (IV, Random, 95% CI) | ‐1.19 [‐7.98, 5.61] |
| 3.3 QoL SGRQ Activity | 4 | 234 | Mean Difference (IV, Random, 95% CI) | ‐2.57 [‐8.05, 2.90] |
| 3.3.1 Maintenance programmes using in‐person supervision | 1 | 48 | Mean Difference (IV, Random, 95% CI) | 1.00 [‐11.25, 13.25] |
| 3.3.2 Maintenance programmes using remote supervision | 3 | 186 | Mean Difference (IV, Random, 95% CI) | ‐3.46 [‐9.58, 2.65] |
| 3.4 QoL SGRQ Impact | 4 | 234 | Mean Difference (IV, Random, 95% CI) | ‐2.08 [‐7.39, 3.23] |
| 3.4.1 Maintenance programmes using in‐person supervision | 1 | 48 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐9.44, 9.44] |
| 3.4.2 Maintenance programmes using remote supervision | 3 | 186 | Mean Difference (IV, Random, 95% CI) | ‐2.68 [‐9.94, 4.59] |
| 3.5 QoL CRQ total score | 3 | 120 | Mean Difference (IV, Random, 95% CI) | 0.74 [0.19, 1.28] |
| 3.5.1 Maintenance programmes using in‐person supervision | 1 | 31 | Mean Difference (IV, Random, 95% CI) | 0.90 [‐0.00, 1.80] |
| 3.5.2 Maintenance programmes using remote supervision | 2 | 89 | Mean Difference (IV, Random, 95% CI) | 0.69 [‐0.09, 1.48] |
| 3.6 QoL EuroQoL | 2 | 150 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.08, 0.08] |
| 3.6.1 Maintenance programmes using in‐person supervision | 1 | 114 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.11, 0.11] |
| 3.6.2 Maintenance programmes using remote supervision | 1 | 36 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.13, 0.13] |
| 3.7 Exercise Capacity: 6MWT | 7 | 349 | Mean Difference (IV, Random, 95% CI) | 25.38 [‐5.63, 56.38] |
| 3.7.1 Maintenance programmes using in‐person supervision | 2 | 79 | Mean Difference (IV, Random, 95% CI) | 22.39 [‐51.75, 96.53] |
| 3.7.2 Maintenance programmes using remote supervision | 5 | 270 | Mean Difference (IV, Random, 95% CI) | 27.07 [‐10.99, 65.14] |
| 3.8 Exercise Capacity: ISWT | 2 | 111 | Mean Difference (IV, Random, 95% CI) | 4.15 [‐44.93, 53.23] |
| 3.8.1 Maintenance programmes using in‐person supervision | 1 | 48 | Mean Difference (IV, Random, 95% CI) | ‐6.00 [‐75.94, 63.94] |
| 3.8.2 Maintenance programmes using remote supervision | 1 | 63 | Mean Difference (IV, Random, 95% CI) | 14.00 [‐54.88, 82.88] |
| 3.9 Exercise Capacity: ESWT | 5 | 369 | Mean Difference (IV, Random, 95% CI) | 26.87 [‐60.58, 114.33] |
| 3.9.1 Maintenance programmes using in‐person supervision | 4 | 305 | Mean Difference (IV, Random, 95% CI) | 9.24 [‐89.68, 108.16] |
| 3.9.2 Maintenance programmes using remote supervision | 1 | 64 | Mean Difference (IV, Random, 95% CI) | 90.00 [‐97.16, 277.16] |
| 3.10 Hospital admission odds (all‐causes) for subgroup only | 2 | 142 | Odds Ratio (M‐H, Random, 95% CI) | 0.71 [0.14, 3.69] |
| 3.10.1 Maintenance programmes using in‐person supervision | 1 | 48 | Odds Ratio (M‐H, Random, 95% CI) | 0.44 [0.09, 2.12] |
| 3.10.2 Maintenance programmes using remote supervision | 1 | 94 | Odds Ratio (M‐H, Random, 95% CI) | 3.20 [0.13, 80.52] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abbink 2004.
| Study characteristics | ||
| Methods | RCT, parallel group. Setting: Netherlands. Study duration: 12 months | |
| Participants | 51 participants Intervention: n = 26, Control: n = 22 Males: n = 37, Female: n = 14 Age, mean: Intervention: 66, Control: 64 FEV1% predicted, mean and SD: Intervention: 34.2 ± 6.4, Control: 32.9 ± 9.0 Inclusion criteria: COPD patients who participated in outpatient PR of 12 weeks. Exclusion criteria: unclear |
|
| Interventions | Intervention group Components: not reported Supervision: weekly maintenance programme. Control group: not reported |
|
| Outcomes | Exercise capacity: 6MWD, maximal workload on a cycloergometer (watts) and HRQoL: CRQ (global score) | |
| Notes | No study sponsor. It is a conference abstract. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information. Reported as randomised, but no further information “COPD patients were randomized to RC or to MP” |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. Method of concealment not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Percentage attrition was higher for intervention group and control group (around 30%). |
| Selective reporting (reporting bias) | Unclear risk | No paper publication and no information on trial registry. |
| Other bias | Unclear risk | Any self‐reported outcomes are likely to be affected any bias |
Arbane 2010.
| Study characteristics | ||
| Methods | RCT, parallel group. Setting: 2 respiratory departments, UK. Study duration: 4 weeks | |
| Participants | 31 participants Age: not reported Gender: not reported Severity: not reported Inclusion criteria: Participants with stable COPD diagnosis, that had successfully completed PR 6 months Exclusion criteria: not reported |
|
| Interventions | Intervention group: Components: not reported Supervision: short intense bout of physical training provided with weekly supervision of one hour. Control group: usual care |
|
| Outcomes | Exercise capacity: ISWT and HRQoL: SGRQ (global score), LCADL (London Chest Activity Daily Living) | |
| Notes | This study was supported by Wandsworth PCT. There are two conference abstracts. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as randomised, but no further information. "... patients with stable COPD were randomized into 4 weeks of PAT exercise performed once a week for one hour or usual care". |
| Allocation concealment (selection bias) | Unclear risk | No further information. Method of concealment not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No further information, but assumed "It was not possible to blind patients or personnel" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Overall 25% did not complete treatment, but it was not specify from which group was the lost. |
| Selective reporting (reporting bias) | Unclear risk | No paper publication and no information on trial registry. |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists. |
Bernocchi 2018.
| Study characteristics | ||
| Methods | RCT, parallel group; setting: Italy; Study duration: 4 months | |
| Participants | 112 participants Intervention: n = 56, Control: n = 56 Males: n = 92, Female: n = 20 Age, mean and SD: Intervention: 71 ± 9, Control:70 ± 9.5 FEV1% predicted, mean and SD: Intervention: 66.6 ± 18.6, Control: 66.1 ± 16.4. GOLD Intervention: IB: 19 (33.9%); IC: 1 (1.8%); IIB: 20 (35.7%); IIC: 2 (3.6%); IIIC: 2 (3.6%); IIID: 2 (3.6%); IVB: 1 (1.8%); IVD: 9 (16.0%), Control: IB: 15 (26.7%); IC: 0; IIB: 26 (46.4%); IIC: 0; IIIC: 0; IIID2; IVB:1 (1.8%); IVD: 9 (16.1%). Inclusion criteria: participants with COPD (B, C and D GOLD class) had to be documented by a spirometry examination performed within the previous 12 months. Exclusion criteria: if they did not return to home after hospitalisation, they had physical activity limitations due to non‐pulmonary conditions, limited life expectancy ( < 6 months), or severe cognitive impairments (Mini Mental Test Examination < 16). |
|
| Interventions | Intervention group: Components: telerehabilitation home‐based programme with aerobic and strength training Personalised exercise programme for each patient who were provided with mini‐ergometer, pedometer and diary. The number/intensity of training sessions according to patients’ progress were adjusted during 4 months or in the case of problems. The ‘basic level’ of programme consisted of 15–25 min of exercise with mini‐ergometer without load and 30 min of callisthenic exercises, performed three times/week and free walking twice a week. The ‘high level’ consisted of 30–45 min of mini‐ergometer with incremental load (from 0 to 60 W), 30–40 min of muscle reinforcement exercises using 0.5 kg weights and pedometer‐based walking, performed from 3 to 7 days/week Supervision: participants were followed during the Telereab‐HBP, and the PT made a weekly phone call to each patient, verified the training level of physical activity performed and planned the rehabilitation targets for the following week and gave extra reinforcement on the value of lifestyle changes and the importance of exercise. Control group: Usual care and advise about physical activity. |
|
| Outcomes | Exercise capacity: 6MWD, HRQoL: CAT, Symptoms MRC dyspnoea, physical activity profile (PASE); impairment/disability (Barthel), hospitalisation and mortality. | |
| Notes | "This study was supported by Ministero della Salute Italian Ministry of Health (http://www.ccm‐network.it/
home.html; CCM2011; project no. 14, Modelli innovativi di gestione integrata telegestita ospedale‐territorio del malato cronico a fenotipo complesso: studio di implementazione, validazione e impatto)." This trial is registered at NCT02269618. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation sequence was generated using a computer programme. |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed in sequentially numbered opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Reported that was not possible to blind patients and healthcare personnel to intervention ( study limitation). |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "Due to the nature of the intervention, neither the patients nor the physicians were blinded to patients' group allocation..." (PROTOCOL) "Due to the nature of the trial, it was not possible to blind patients and healthcare personnel to intervention." (PAPER) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only one participant was lost to follow up in the intervention arm. There was no loss to follow up in the control group. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as planned. Protocol was published in trial registry. |
| Other bias | High risk | This study showed bias due to retrospective recording. |
Brooks 2002.
| Study characteristics | ||
| Methods | RCT, parallel group. Setting: Canada. Study duration: 12 months | |
| Participants | 85 participants Intervention: n = 37, Control: n = 48 Males: n = 50, Female: n = 35 Age, mean and SD: Intervention: 68 ± 1.1, Control: 68 ± 1.1 FEV1% predicted, mean and SD: Intervention: 32 ± 1.9, Control: 32 ± 1.6; FEV1/FVC, mean and SD: Intervention: 38.1 ± 1.7; Control: 35.6 ± 1.4. Inclusion criteria: Severe stable COPD (FEV1 < 40% predicted, FEV1/FVC < 0.70); completion of inpatient or outpatient rehabilitation; nonsmoker for a minimum of 6 months; aged 49–85 yrs. Exclusion criteria: coexisting conditions that might limit exercise tolerance or cognitive functioning; noncompliance with respiratory rehabilitation; mechanical ventilatory support for any part of the day; inability to communicate in English; living too far away to participate. |
|
| Interventions | Intervention group:
Components: exercise and education.
Supervision: monthly 2‐hour group sessions in‐person, led by a physical therapist, and between visits (2 weeks after the group session), participants received a phone call from a different physical therapist who asked standardized questions regarding adherence to their programme and discussed any of their concerns. The first hour was spent discussing any concerns they had regarding their home maintenance programme and in the second hour participants performed components of their home programme (choice of the participant) under the therapists supervision. During visits to the centre and during telephone conversations, participants were encouraged to continue their exercises as well as their general health habits. Control group: visited the physiotherapist every 3 months for a year. They received usual care and counselling on physical activity without supervision. |
|
| Outcomes | Exercise capacity: 6MWD and HRQoL: CRQ (global score), SF‐36 (global score) and SGRQ (global score). | |
| Notes | This study was supported in part by the West Park Foundation, the Physiotherapy Foundation of Canada and the Ontario Respiratory. Only CRQ are reported for HRQoL, the SF‐36 and SGRQ are presented only in graphics. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised to control or study group using a random number table. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. Method of concealment not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Percentage attrition was 51% for intervention group and 52% in control group. Both groups lost to follow up were more than 20%. |
| Selective reporting (reporting bias) | Unclear risk | No paper publication and no information on trial registry. |
| Other bias | High risk | There are baseline difference in exercise capacity outcome between intervention group and control group |
Burns 2016.
| Study characteristics | ||
| Methods | RCT, parallel group. Setting: UK. Study duration: 12 months | |
| Participants | 148 participants Intervention: n = 73, Control: n = 75 Males: n = 91, Female: n = 57 Age, mean and SD: Intervention: 67.3 ± 15.1, Control: 69.3 ± 8.9 FEV1% predicted: All: 41 ± 16. Inclusion criteria: aged over 35 years, had a physician labelled diagnosis of COPD, emphysema or chronic bronchitis with a > 20 pack‐year smoking history and a FEV1 < 80%. Participants had to have completed at least 60% of the sessions in the initial PR programme. Exclusion criteria: if the participant had a respiratory infection within 4 weeks of randomization or other co‐morbidities considered severe enough to affect the study outcome, serious pulmonary disease other than COPD, or history of myocardial infarction within 6 months of baseline. |
|
| Interventions | Intervention group: Components: The exercise training comprised supervised strength and endurance training including walking, cycling, standing from sitting, arm exercises using dumbbells and step‐ups. The training was individually tailored to the participant's abilities and participant received a written report on their progress with positive re‐enforcement. Supervision: one hour of education and one hour of structure exercise in addition to stand care conducted every 3 months at 3, 6 and 9 months. Participants received a reminder letter 2 weeks before the session and a reminder phone call 1 week before the session. Control group: usual care and the standard advice to undertake strength and endurance exercises at home with no supervision. |
|
| Outcomes | HRQoL: Exercise capacity: ESWT, HRQoL: CRQ (global score and domains), EQ5D, hospitalisation, exacerbation rate, mortality and direct costs. | |
| Notes | This paper presents independent research funded by the National Institute for Health Research (NIHR) under its Research for Patient Benefit (RfPB) Programme (Grant Reference Number PB‐PG‐0408‐16225). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. This trial was registered as NCT00925171. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised using a computer generated sequence. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. Method of concealment not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study was reported as single blind. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Analysis was performed blind to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of the 148 patients randomised, 86 % provided responses and recorded resource use at the point of randomization and at 12 months. The number of patients are the same pre and post for control and intervention group. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as planned. |
| Other bias | Unclear risk | Different number of patients for the outcomes ESWT, BMI, Body fat, HADS, EQ‐5D, Activity in relation to age, and CRQ domains. |
Du Moulin 2009.
| Study characteristics | ||
| Methods | RCT, parallel group. Setting: Germany. Study duration: 6 months | |
| Participants | 20 participants Intervention: n = 10, Control: n = 10 Males: n = 14, Female: n = 6 Age, mean (95% CI): Intervention: 67 (63 ‐ 72), Control: 72 (69 ‐ 77) FEV1% predicted, mean (95% CI): Intervention: 58.6 (53.8 ‐ 63.5), Control: 62.5 (57.7 ‐ 67.3) Inclusion criteria: Participants diagnosed with moderate COPD and that completed a comprehensive, multidisciplinary, 3‐week outpatient pulmonary rehabilitation programme. Only participants not planning to attend some other form of structured maintenance programme were included. Exclusion criteria: Participants who were not capable of carrying out the exercise training because of cardiac, pulmonary or orthopedic problems. |
|
| Interventions | Intervention group: Components: This group received an individualized training plan, based on their last 6MWT. Participants were instructed to quickly walk a distance equivalent to 125% of their last 6MWT three times a day with each training walk not exceeding 15 min. Alternatively, these three training walks could be combined to one training walk a day. The training was carried out independently in a home‐based setting. Participants were given a pedometer so that the training could be better incorporated into daily activities. Patients kept a training diary. The diary was returned by post and was renewed every 4 weeks. Supervision: Participants were contacted by telephone every 4 weeks. Control group: Usual care. No instructions were given regarding physical activities during the period following rehabilitation. |
|
| Outcomes | Exercise capacity: 6MWD and HRQoL: CRQ domains | |
| Notes | No study sponsor. Trial is registered at the Hamburg Chamber of Physicians (No. 2617) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as randomised, but no further information. |
| Allocation concealment (selection bias) | Unclear risk | For randomization purposes, were prepared 20 envelopes, 10 with a plus, indicating intervention group, and 10 with a minus, indicating control group. Patients drew these envelopes themselves. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This trial only observer was blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Observer was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Percentage attrition was high in both groups: intervention group: 50% and control group 60%. |
| Selective reporting (reporting bias) | Unclear risk | Could not find in trial registry, and could not find a protocol so unclear if outcomes were reported as planned. |
| Other bias | Unclear risk | Any self‐reported outcomes are likely to be affected any bias |
Galdiz 2020.
| Study characteristics | ||
| Methods | RCT, parallel group. Setting: Spain. Study duration: 12 months | |
| Participants | 94 participants Intervention: n = 46, Control: n = 48 Males: n = 63, Female: n = 31 Age, mean and SD: Intervention: 62.3 ± 8.2, Control: 63 ± 6.6 FEV1% predicted, mean and SD: Intervention: 42.8 ± 14.6, Control: 45.8 ± 17.2 Inclusion criteria: Diagnosis of COPD, according to the GOLD guidelines with spirometric grade II ‐ IV severity; age between 18 and 75 years; non‐smoker and ex‐smoker or smoker with an intention to quit; BODE index value between 3 ‐ 7, and no acute exacerbation event during the last 4 weeks prior to enrolment. Exclusion criteria: COPD participants with a bronchodilator response (FEV1 increase > 15% of the baseline value after 200 μg of inhaled bronchodilator), a clinical diagnosis of respiratory disease other than COPD, a history of severe coronary artery disease, orthopaedic diseases seriously limiting mobility, life expectancy of less than 2 years, or inability to co‐operate. |
|
| Interventions | Intervention group: Components: The home‐based telerehabilitation programme included the following components a) individualized action plan; b) access to the call centre (enabling them to speak to a system technician if any technical problem occurred with the data management platform); and c) arm and leg exercises. They were provided with a telerehabilitation kit (mobile phone, pulse oximeter, dumbbells, and exercise bicycle). Supervision: The home exercise was periodically monitored by the physiotherapist, using the web‐based platform, to provide feedback to patients regarding activity levels and exercise training load. The exercises were adjusted according to compliance and the participant’s reported symptoms and recorded vital sign parameters. Control group: Usual care and counselling to keep active and exercise regularly. No supervision of their level of activity was organized. |
|
| Outcomes | Exercise capacity: 6MWD; HRQoL: CRQ (domains) and SF‐36 (domains), BODE and exacerbation rate (hospitalisation). | |
| Notes | No study sponsor. This trial is registered at ClinicalTrials.gov Identifier: NCT03247933 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was implemented through the generation of a computer‐based random allocation sequence. Allocation was stratified by centre and based on randomly permuted blocks of variable size with a 1:1 allocation ratio. (Supplement). |
| Allocation concealment (selection bias) | Low risk | Clinical epidemiology staff kept the centralised allocation lists concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This trial was open‐label. Not possible to blind participants to intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across groups, similar reasons for missing data. |
| Selective reporting (reporting bias) | High risk | Outcomes in paper are slightly different from Clinical trial registration. Paper do not reported Compliance and Adherence data, and other outcome measure as the cost effectiveness. |
| Other bias | Unclear risk | Any self‐reported outcomes are likely to be affected any bias. |
Guell 2017.
| Study characteristics | ||
| Methods | RCT, parallel group. Setting: Spain. Study duration: 36 months | |
| Participants | 138 participants Intervention: n = 68, Control: n = 50 Age, mean and SD: Intervention: 64 ± 9, Control: 64 ± 8 FEV1% predicted, mean and SD: Intervention: 36 ± 10, Control: 37 ± 11. Inclusion criteria: Diagnosis of COPD, according to the GOLD guidelines with spirometric grade II ‐ IV severity, between 18 and 75 years old ; ex‐smokers or with intention to quit; BODE index value between 3‐10, and no acute exacerbation during the last four weeks prior to enrolment. Exclusion criteria: COPD participants showing a bronchodilator response (FEV1 increment > 15% of the baseline value after 200 μg of inhaled bronchodilator), a clinical diagnosis of respiratory disease other than COPD, severe coronary artery disease, orthopedic diseases seriously limiting mobility, life expectancy lower than 2 years, or inability to co‐operate. |
|
| Interventions | Intervention group: Components: exercise (weight and cycle ergometre). This group was asked to continue at home with a programme similar to that conducted at the hospital. Supervision: the physiotherapist called the IG participants every 15 days, and participants attended the hospital for a training session, every 15 days alternatively (the participants had weekly contact with either a phone call or a hospital visit). On the alternate week, the participant attended the hospital for a supervised training session. The participant never increased the load of any of the exercises at home without supervision by the physiotherapist. The cycle ergometres were provided by the team. Control group: usual care and counselling on physical activity without supervision. |
|
| Outcomes | Exercise capacity: 6MWD, HRQoL: SF‐36 (domains) CRQ (domains), BODE and mortality. | |
| Notes | No study sponsor. Trial is registered at NCT 01090999 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using a computer programme by an independent statistician. The process was stratified in a 1:1 allocation with permuted blocks of varying size. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. Method of concealment not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Reported as open‐label on trials registry, and it is not possible to blind participants to intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blind to allocation group at baseline and follow up time points |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Higher attrition rate in the control group (30%) compared to the intervention group (24%). |
| Selective reporting (reporting bias) | High risk | Outcomes in the manuscript are different from those described in the Clinical trial registration. Clinical trial: dyspnoea (CRQ), 6MWT, BODE index, economical: direct costs (program), indirect costs (exacerbations/admissions), comparison of GR1 and GR2 costs. For all, time frame 1.5 yrs. Paper: Time frame: 12 mo, 24 mo, and 36 mo. Missing outcomes: economical: direct costs (program), indirect costs (exacerbations/admissions), comparison of GR1 and GR2 costs. New outcomes: SF36f, SF36m, CRQ (fatigue, emotional factor, mastery). One of the reasons to consider high risk is "One or more reported primary outcomes were not pre‐specified". |
| Other bias | Unclear risk | Any self‐reported outcomes are likely to be affected any bias. |
Hill 2004.
| Study characteristics | ||
| Methods | RCT, parallel group. Setting: Australia. Study duration: 3 months | |
| Participants | 61 participants Intervention: n = 31, Control: n = 30 Age: not reported FEV1: not reported Inclusion criteria: COPD participants who participated in 6‐week outpatient PR Exclusion criteria: not reported |
|
| Interventions | Intervention group: Components: exercise (aerobic and strength training). Exercise consisted of 30 minutes of lower limb endurance training (walking on a 20‐metre track, steps and pedals), upper limb exercises using free weights, and warm up and cool down exercises. Supervision: Participants in the maintenance group attended a weekly one‐hour exercise session at the community centre for twelve weeks. The session was supervised by a physiotherapist from the community health service and was based on the exercise training the subjects had been doing at the outpatient PR programme. Participants were encouraged to continue a home exercise program, maintain a home exercise diary and were invited to attend the monthly support group meetings. Control group: Usual care: They received ongoing medical care from their primary care provider. They were encouraged to continue a home exercise programme and maintain a home exercise diary. Participants were invited to attend the monthly “Huff and Puff” support group meetings held at the community centre. |
|
| Outcomes | Exercise capacity: 6MWD and HRQoL: CRQ (global score) | |
| Notes | No study sponsor. It is a conference abstract. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as randomised, but no further information. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. Method of concealment not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information. |
| Other bias | Unclear risk | There may be a risk of bias, but there is either: Insufficient information to assess whether an important risk of bias exists. |
Jiménez‐Reguera 2020.
| Study characteristics | ||
| Methods | RCT, parallel group. setting: Spain. Study duration: 12 months | |
| Participants | 44 participants Intervention: n = 20, Control: n = 24 Male: n = 21, Female: n = 23 Age, mean and SD: Intervention: 68.1 ± 6.6, Control: 68.1 ± 7 FEV1% predicted, mean and SD: Intervention: 45.0 ± 15.3, Control: 43.1 ± 13.6 Inclusion criteria: Participants with COPD, age between 55 and 85 years old, with degree of severity II, III or IV of the GOLD scale, in a stable clinical situation (no exacerbations in the last 6 weeks). Exclusion criteria: Participants with unstable cardiovascular disease, muscular, osteoarticular, auditory, visual or central or peripheral nervous system impairment, which prevented the performance of the rehabilitation programme or evaluation tests and cognitive impairment that made it difficult to understand the education programme and to manage the HappyAir™ system. |
|
| Interventions | Intervention group: telerehabilitation program Components: exercise Participants were advised to perform physical activity and breathing exercises daily, followed an integrated care plan using a mobile device with the pulmonary care web‐based application (HappyAir™ App) and were instructed in its use. The HappyAir™ App daily reminded daily to use the application, indicating that they record medication intake, daily exercise time (min), level of tiredness after the exercises (good, little tired, very tired, or exhausted) and daily mood (happy, little sad, sad or very sad). Supervision: the therapeutic educators had access to the platform to supervise the evolution of this group during the follow‐up period, collect data, see the results of clinical evaluations, record weekly and monthly goals and to contact the physician responsible for the participants (in case they detected warning signs of possible exacerbations or relapses). The Happyair™ application was a means of participant‐educator interaction. Pulmonologist and physiotherapist of the hospital had access, by login, to the data collection platform to enter the clinical data, record the results of the evaluations, see the evolution of participants. Control group: they received usual care and counselling to perform physical activity and breathing exercises daily, without receiving integrated supervision or using the HappyAir™ App. |
|
| Outcomes | Exercise capacity: 6MWD, HRQoL: CAT, SGRQ (domains and global score), EuroQoL‐5D. | |
| Notes | This study was supported by the Board of Lovexair Foundation (HappyAir™) Intellectual Property of the Lovexair Foundation). Trial is registered at NCT04479930. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An online programme was used to generate the randomization sequence. |
| Allocation concealment (selection bias) | Low risk | "Sequentially numbered and coded to ensure the confidentiality of participants and to ensure masking of professionals who performed the rehab protocol" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patients could not be blinded for group. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessment of outcome measures of both groups was carried out by a blinded assessor. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Percentage attrition was lower for both groups (intervention = 15% and control = 20%) |
| Selective reporting (reporting bias) | High risk | Clinical trial registration seems to have been submitted after the end of the study.
NCT 04479930. First posted: 21 July 2020. Last update posted: 21 July 2020
Actual study start date: Dec 2015. Study completion date: May 2017. Some outcomes are different between paper and protocol. |
| Other bias | High risk | It was identified late registration, declared conflicts of interest which two authors are financed by the company that developed the intellectual property of the application used in the study, and with participants in the control group did not have access to smart devices for an appropriate sham. |
Johnson 2001.
| Study characteristics | ||
| Methods | RCT, parallel group. Setting: UK. Study duration: 2 months | |
| Participants | 30 participants Male: n = 20, Female: n = 10 Age, mean and SD: All: 65 (range: 52 ‐ 77) FEV1% predicted, mean and SD: All: 32 ± 13.6 Inclusion criteria: Participant with severe COPD after participation in a hospital based PR programme Exclusion criteria: not reported |
|
| Interventions | Intervention group: Maintenance of PR at a local leisure centre. Supervision: by a fitness instructor, 5 ‐ 8 sessions during 8 weeks of maintenance programme. Control group: home exercise counselling. Supervision: not reported |
|
| Outcomes | Exercise capacity: ISWT and HRQoL: CRQ (domains) | |
| Notes | This study was supported by British Lung Foundation/Trevor Clay Memorial Grant. It is a conference abstract. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but no further information. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. Method of concealment not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear how many participants in each arm at baseline, unclear how many completed treatment. |
| Selective reporting (reporting bias) | High risk | Number of participants in each group not reported at baseline or endpoint. Duration of post‐PR intervention/control not reported. Could not find a publication or protocol on trial registry. |
| Other bias | Unclear risk | Any self‐reported outcomes are likely to be affected any bias. |
Li 2018.
| Study characteristics | ||
| Methods | RCT, parallel group. Setting: China. Study duration: 12 months | |
| Participants | 151 participants Intervention: n = 82, Control: n = 69 Male: n = 126, Female: n = 25 Age, mean and SD: Intervention: 65.1 ± 8.7, Control: 66 ± 9.3 FEV1% predicted, mean and SD: Intervention: 48.7 ± 11.7, Control: 49.2 ± 11.0 Inclusion criteria: Diagnosis of COPD according to GOLD guideline, clinically stable in the first 4 weeks, able to complete the PR programme and questionnaire survey successfully and independently, willing to participate in the PR and maintenance program, and willing to sign informed consent. Exclusion criteria: Participants having asthma, obstructive sleep apnea syndrome, or underdiagnosis of cancer; diagnosed with Alzheimer’s disease or depression and anxious disorder; unavailable for exercise even during the stable stage; having severe dysfunction of the heart, liver, or kidney; and suffering emotional trauma in the previous 6 months such as relative death and divorce. The exit criteria included exacerbation during PR and maintenance, frequent accident events during PR and maintenance, and death. |
|
| Interventions | Intervention group: Components: exercise and education home‐based Supervision: Step 1: Home‐visit once every 2 weeks to conduct rehabilitation exercise strategy and provide health education for 2 months. Step 2: Home‐visit every 4 weeks and phone contact once a week for 4 months. Step 3: Phone contact once a week for 6 months. Phone contact once a week for 6 months. Control group: usual care. |
|
| Outcomes | Exercise capacity: 6MWD, HRQoL: CAT, mortality and exacerbation rate. | |
| Notes | Grants from the National Natural Science Foundation of China (No. 81270144, 81570084, 30800507), and the Science and Technology Plan Project of Tianjin (13ZCZDSY02000). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as randomised, but no further information. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. Method of concealment not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Percentage attrition was 17% for intervention group and 37% in control group. There were more participants lost to follow up in the control group compared to the intervention group |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registry information found, unclear if outcomes were reported as planned. |
| Other bias | Unclear risk | Any self‐reported outcomes are likely to be affected any bias. |
Linneberg 2012.
| Study characteristics | ||
| Methods | RCT, parallel group. Setting: Denmark. Study duration: 12 months | |
| Participants | 118 participants Intervention: n = 59, Control: n = 59 Male: n = 45, Female: n = 73 Age, years (%) Intervention: 60 (11.9%), 60 – 69 (30.5%), 70 – 79 (40.7%) and > 80 (16.9%); Control: < 60 (10.2%), 60 – 69 (27.1%), 70 – 79 (54.2%) > 80 (8.5%) FEV1% predicted, mean: Intervention: 42.5, Control: 41.9 Inclusion criteria: Participants should be able to walk, no significant cognitive impairment, no symptomatic ischaemic heart disease, should be able to understand Danish. No exacerbation of COPD within 4 weeks prior to start of rehabilitation. Exclusion criteria: Participants in need of supplementary oxygen. |
|
| Interventions | Intervention group: Components: aerobic and strength training These supplemental exercise sessions were similar to the ones that were part of the initial 7‐week rehabilitation programme. The participants were encouraged to train and walk every day at home and the walking time was recorded in a diary. The sessions started with a warm‐up exercise followed by individually planned walking and cycle training. In addition, the participants were taught about four exercise activities aimed to strengthen legs, arms, abdominal and thoracic muscles. The participants were encouraged to train and walk every day at home and the walking time was recorded in a diary. Supervision: Manteinance group received six supervised exercise sessions at week 9, 11, 13, 18, 26 and 52. These supplemental exercise sessions were similar to the ones that were part of the initial 7‐week rehabilitation programme. The intervention group was asked to complete a diary with home‐based training time, and this was checked by the study nurses. Control group: Usual care without receive any additional exercise sessions, were not further encouraged to do home‐based exercise and not asked to complete a diary. |
|
| Outcomes | Exercise capacity: ESWT and HRQoL: SGRQ (global score) | |
| Notes | No study sponsor. This trial is registered at ClinicalTrials.gov (Protocol ID: KA20060179) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were randomised (in blocks of 20) for the intervention group or the control group. No further information. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. Method of concealment not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar attrition in both groups (17% in the intervention arm and 15% in the control arm). |
| Selective reporting (reporting bias) | Unclear risk | There is a register of CT. Reported the primary outcomes, all outcomes reported as planned except for hospital data not reported. |
| Other bias | Unclear risk | Any self‐reported outcomes are likely to be affected any bias. |
Murphy 2005.
| Study characteristics | ||
| Methods | RCT, parallel group. Setting: Australia. Study duration: 4 months | |
| Participants | 56 participants Intervention: n = 28, Control: n = 28 Male: n = 27, Female: n = 29 Age, mean and SD: Intervention: 67 ± 11, Control: 69 ± 10 FEV1% predicted, mean and SD: All: 40 ± 15 Inclusion criteria: PR programme participants who completed their week 7 assessment. Criteria for enrolled at PR were: undertake (or have had) a Respiratory Function Test (RFT) within the 12 months prior to trial entry to confirm the diagnosis of COPD; intact cognitive function (to enable self completion of questionnaires); reside or work in the catchment area of Hospital A; to enable a sample set of people with a similar sociodemographic profile; had experienced a moderate level of self reported dyspnoea as described by the MRC Dyspnoea scale; clinically stable and/or discharged from hospital for a minimum of four weeks before trial entry, (to ensure that participants were sufficiently well to engage in the intervention); literate in English (in order to be able to participate in the programme and/or enable completion of self administered questionnaires at assessment time). Exclusion criteria: Participants who completed a pulmonary rehabilitation programme in the previous six months, (to ensure the absence of any potential ‘carry over’ effect from earlier interventions). Any medical condition that could place a patient at greater risk during the assessment procedure or gymnasium programme. Medical conditions considered an absolute contraindication to exercise include: malignancy, aortic stenosis, known aneurysm or acute infections. Other conditions reported as relative contraindications to exercise testing include electrolyte abnormalities, end stage renal failure, unstable angina and, poorly controlled hypertension or, metabolic conditions. |
|
| Interventions | Intervention group: Components: endurance and weight training (stretching, treadmill, stationary cycling, stair climbing) according to prescription. Followed the same format as the six week PR programme intervention with the frequency, intensity and duration adjusted to the patient’s capacity and progress. The maintenance was structured weekly pulmonary rehabilitation class for 19 weeks. Control group: usual care. |
|
| Outcomes | Exercise capacity: ISWT, HRQoL: SGRQ (global score), Symptoms: MRC, hospitalisation, adverse event. | |
| Notes | This study was supported by HARP Funding. This study is registered at ACTRN12606000082505 There are two reports: one conference abstracts and one Thesys. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were randomised. No further information. |
| Allocation concealment (selection bias) | Low risk | Allocation of the randomization sequence was placed in opaque envelopes. Participants and nurses were blinded. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open label study. "Due to study constraints, the candidate facilitated the assessments and interventions" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only one participant was lost to follow up in the intervention arm. There was no loss to follow up in the control group. |
| Selective reporting (reporting bias) | Unclear risk | Data from a doctoral thesis. There is not a paper published. Protocol was published in trial registry. This study doesn't reported all outcomes for maintenance phase compared to usual care. |
| Other bias | Unclear risk | Any self‐reported outcomes are likely to be affected any bias. |
Ries 2003.
| Study characteristics | ||
| Methods | RCT, parallel group. Setting: USA. Study duration: 12 months | |
| Participants | 172 participants Intervention: n = 87, Control: n = 85 Male: n = 89, Female: n = 75 Age, mean and SD: All: 67.1 ± 8.2 FEV1% predicted, mean and SD: All: 45 Inclusion criteria: Participants with lung diseases who completed an 8‐week, 12‐session comprehensive pulmonary rehabilitation programme. Selection criteria for the pulmonary rehabilitation programme included: clinical diagnosis of chronic lung disease (obstructive) confirmed by history, physical examination, pulmonary function tests, and chest roentgenograms; chronic symptoms and perceived disability from disease; stable on an acceptable medical regimen under the care of a primary care physician; no other significant medical or psychiatric conditions that would interfere with full participation in the program; and commitment to abstain from smoking. Exclusion criteria: not reported. |
|
| Interventions | Intervention group: Components: walking and arm strength training Based on the results of initial exercise testing and used methods easily adapted to the home setting. Supervised walking exercise was begun on a treadmill and subsequently translated into an appropriate walking pace for home training. The training programme emphasized increasing endurance up to 20 to 30 minutes of continuous walking with increase in level as tolerated by symptom‐limits. In addition, participants performed an upper extremity training programme of arm lifts developed for pulmonary patients. Diaries were reviewed regularly during scheduled programme sessions. During supervised rehabilitation sessions, participants were also able to build upper extremity strength and endurance through the use of isokinetic arm cycle ergometers. Supervision: weekly telephone calls and monthly supervised reinforcement sessions. Control group: A written daily home programme was provided for each participant and sent to their physician detailing individual recommendations for exercise training and other treatments developed within the rehabilitation programme. Supervision: subjects were invited to regular monthly alumni group meetings. |
|
| Outcomes | Exercise capacity: VO2 max with an incremental symptom‐limited treadmill test and 6MWD, HRQoL: SF‐36 (domains), CRQ (global score), Quality of well‐being scale‐QWB, hospitalisation and mortality. | |
| Notes | Supported by National Institutes of Health grant R01 HD/HL 30912. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using a random number generator. |
| Allocation concealment (selection bias) | Low risk | Allocation was achieved using the Moses‐Oakford assignment algorithm 1:1, sealed in sequentially numbered identical opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Reported that "assessments were performed without identification". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar rates of attrition in each treatment arm. |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registry information found, unclear if outcomes were reported as planned. |
| Other bias | Low risk | No other sources of bias were identified |
Ringbaek 2010.
| Study characteristics | ||
| Methods | RCT, parallel group. Setting: Denmark. Study duration: 12 months | |
| Participants | 96 participants Intervention: n = 55, Control: n = 41 Male: n = 33, Female: n = 63 Age, mean and SD: Intervention: 66.7 ± 10.6, Control: 69.2 ± 8.5 FEV1% predicted, mean and SD: Intervention: 35.6 ± 14.0, Control: 36.2 ± 16.0. Inclusion criteria: Participants with stable chronic obstructive pulmonary disease (COPD, FEV1 of less than 80% and FEV1/FVC of less than 70%); motivation for pulmonary rehabilitation; and completion of a 7‐week rehabilitation programme. Exclusion criteria: If they presented significant musculoskeletal, cardiac, or cognitive problems. |
|
| Interventions | Intervention group: Components: aerobic training Initial programme consisted of supervised walking and cycling twice a week for 7 weeks combined with unsupervised daily training at home patients was instructed to continue the training at home with periodic supervision. Supervision: weekly supervised training sessions during the first 6 months and every second week during the next 6 months and finally no supervised training for the last 6 months. Control group: Usual care. They were advised to keep unsupervised daily training at home. |
|
| Outcomes | Exercise capacity: ESWT; HRQoL: SGRQ (global score). | |
| Notes | No study sponsor. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as randomised, but no further information. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. Method of concealment not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants to intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across groups, similar reasons for missing data. |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registry information found, unclear if outcomes were reported as planned. |
| Other bias | Unclear risk | Any self‐reported outcomes are likely to be affected any bias. |
Roman 2013.
| Study characteristics | ||
| Methods | RCT, parallel group. Setting: Spain. Study duration: 12 months | |
| Participants | 48 participants Intervention: n = 32, Control: n = 33 Male: n = 39, Female: n = 9 Age, mean (95% CI): Intervention: 64.9 (62.1 ‐ 67.7), Control: 64.1 (59.9 ‐ 68.2) FEV1% predicted, mean (95% CI): Intervention: 60.9 (56.3 ‐ 65.5); Control: 59.9 (54.9 ‐ 64.8). Inclusion criteria: if they were 35 to 74 years old, had moderate COPD according to the GOLD criteria and had post‐bronchodilator results on most recent spirometry of FEV1/FVC < 0.7, FEV1 values between 50% and 80%, and were either smokers or non‐smokers. Exclusion criteria: if they presented with any musculoskeletal conditions that prevented exercising and walking test assessments, or terminal illness or other severe disease at the time of enrolment. |
|
| Interventions | Intervention group: Components: respiratory physiotherapy and peripheral muscle training Respiratory physiotherapy: included a series of exercises, lasting a total of 15 minutes and including self‐conscious breathing control, diaphragmatic breathing control, and exercises for the chest wall and abdominal muscle walls. Low intensity peripheral muscle training included abdominal and upper and lower limb exercises, shoulder and full arm circling, weight‐lifting and other exercises. Each exercise was repeated 8– 10 times over 45 minutes. Supervision: weekly‐session maintenance programme until the end of the programme at 12 months. Control group: Usual care. |
|
| Outcomes | Exercise capacity: 6MWD, HRQoL: CRQ (domains), Symptoms MRC (dyspnoea), hospitalisation, exacerbation rate and mortality. | |
| Notes | This study was supported by This study was supported by a grant from the Marató de TV3 (nº 04–2610), by the University Institute of Health Sciences (IUNICS). In addition, the study has received support from the Heath Promotion and Preventive Activities Network RD06/0018/0036 accredited by the Instituto Carlos III of the Ministry of Health. This trial was registered at ISRCTN94514482. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Subjects were randomly assigned to one of the three groups using a centrally administered, computer‐generated block randomisation scheme using blocks of 6 with EPIDAT, stratified according to participating site. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. Method of concealment not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "The case manager subsequently informed patients of their group allocation at the end of the baseline visit". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Health staff members involved in follow‐up were blinded to patient assignment". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Percentage attrition was 27% for intervention group and 31% control group. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as planned, trial was registered. |
| Other bias | Unclear risk | Any self‐reported outcomes are likely to be affected any bias |
Spencer 2010.
| Study characteristics | ||
| Methods | RCT, parallel group. Setting: Australia. Study duration:12 months | |
| Participants | 59 participants Intervention: n = 31, Control: n = 28 Male: n = 27, Female: n = 32, Age, mean and SD: Intervention: 65 ± 8, Control: 67 ± 7 FEV1% predicted, mean and SD: Intervention: 57 ± 21, Control: 60 ± 16. Inclusion criteria: COPD participants with a FEV1/FVC < 70% and FEV1 < 80% predicted following the successful completion of an 8‐week pulmonary rehabilitation programme. Exclusion criteria: if they had experienced an exacerbation of COPD in the previous month, required supplemental oxygen or had comorbidities, such as severe cardiovascular, neurological or musculoskeletal conditions, that would prevent them performing functional exercise tests. |
|
| Interventions | Intervention group: Components: aerobic and strength training. This included 20 min walking (track or treadmill), 20 min cycling, 10 min arm cycling, and upper and lower limb strength training exercises using weight equipment and free weights. Unsupervised home exercise consisted of 30 min of walking plus 30 min of upper and lower limb strengthening exercises using free weights and body weight. The home exercises were practiced during the pulmonary rehabilitation programme and all participants had an illustrated home exercise booklet to guide them, plus a diary for recording sessions completed. Supervision: one day per week in the Pulmonary Rehabilitation Physiotherapy Gymnasium, where they had completed their initial programme, plus unsupervised home exercise on four other days. Control group: They received the home exercise booklet and diary with advice to perform unsupervised home exercise 5 days per week. |
|
| Outcomes | Exercise capacity: 6MWT, ISWT, ESWT and HRQoL: SGRQ (domains), hospitalisation, exacerbations rate and mortality. | |
| Notes | No study sponsor. This trial was registered at ACTRN012605000678695. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Reported that "randomization (performed using a computerised number generation)". |
| Allocation concealment (selection bias) | Low risk | The allocation was concealed in opaque envelopes prepared by an investigator not directly involved in the study. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded to allocation group. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessor was not blind to allocation. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Percentage attrition was 22% for intervention group and 14% control group. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as planned, trial was registered. |
| Other bias | Unclear risk | Any self‐reported outcomes are likely to be affected any bias. |
Steele 2008.
| Study characteristics | ||
| Methods | RCT, parallel group. Setting: USA. Study duration: 12 months | |
| Participants | 111 participants Intervention: n = 54, Control: n = 57 Gender: not reported Age, mean: All: 67 FEV1% predicted, mean and SD: Intervention: 45 ± 17 Control: 41 ± 17. Inclusion criteria: participants with chronic lung disease and shortness of breath with self‐reported diminished physical functioning related to a pulmonary problem, pulmonary function impairment, ambulatory, able to read and speak English, and at least 45 years old. Exclusion criteria: inpatient admission within the past 2 months, asthma with episodic abnormality in pulmonary function, pulmonary exacerbation within the past 2 weeks, unstable cardiopulmonary or sensorimotor problems, daily use of a motorized cart, already exercising at least 30 minutes 3 or more days a week, impairment of cognition or communication, active malignancy or rapidly declining clinical course with expected survival of less than 1 year, suboptimal medical management, and/or history of drug or alcohol treatment within the past 6 months. |
|
| Interventions | Intervention group: Components: aerobic and strength training The exercise intervention was divided into 2 phases: (1) weeks 1 through 4: establishing a home‐ and community‐based exercise programme with emphasis on walking, including skills development in exercise equipment and monitoring devices and (2) weeks 5 through 12: implementing a regular programme of exercise, at least 20 minutes, 4 days a week. In addition, the experimental group received a digital pedometer for self‐monitoring and an exercise handbook that described aspects of the programme in more detail. Supervision: weekly phone calls and 1 home visit over 3 months, including counselling on establishing, monitoring, and problem‐solving in maintaining a home exercise programme. Control group: usual care and counselling on physical activity without supervision. |
|
| Outcomes | Exercise capacity: 6MWD, HRQoL: SF‐36v (domains), Symptom: SOLDQ (dyspnoea), and walking self‐efficacy. | |
| Notes | This study was supported by the Department of Veterans Affairs, Veterans Health Administration, Health Services Research and Development Service (grant no. NRI 98‐194) and the University of Washington School of Nursing (research and intramural funding grant). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised using a block design of 10 subjects per block. Unlcear how the randomization sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. Method of concealment not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Participants were not blinded to their group randomisations and received considerable contact from study staff". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Slightly higher attrition in the intervention arm (22%) compared with 17.5% in the control arm. |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registry information found, unclear if outcomes were reported as planned. |
| Other bias | Unclear risk | Any self‐reported outcomes are likely to be affected any bias. |
Wong 2014.
| Study characteristics | ||
| Methods | RCT, parallel group. Setting: Canada. Study duration: 6 months | |
| Participants | 79 participants Intervention: n = 41, Control: n = 38 Male: n = 40, Female: n = 39 Age, mean and SD: Intervention: 70.2 ± 8.6, Control: 65.7 ± 10.5 FEV1% predicted, mean and SD: Intervention: 47.3 ± 23.5, Control: 48.7 ± 21. Inclusion criteria: Participants with COPD confirmed by lung function testing and patient history and that attended sessions twice per week for eight weeks, or three times per week for six weeks throughout the PR programme. Exclusion criteria: if they were deemed to have unstable cardiovascular disease and/or dementia. |
|
| Interventions | Intervention group: Components: endurance, strength and breathing training encouragement All participants were given an Exercises Action Plan prior to completion of RP. This exercise action plan encouraged exercise three to five days per week, including four components: flexibility, cardiovascular endurance, resistance training, and breathing exercises. The type, intensity, and duration of exercise included in the action plan addressed individual abilities, limitations, and needs, including the use of aides such as walkers and supplemental oxygen. Peers were encouraged to counsel participants toward overcoming barriers to exercise based on their own personal experiences; however, if other health concerns were raised, peers instructed the participant to speak to a healthcare provider or offer to have a respiratory therapist contact the patient. Supervision: participants received 8 sessions of Peer Educators support at regular intervals via telephone during 6 months period. Control group: Usual care and counselling to follow their exercise action plan. Supervision: they did not receive any phone calls in the six month period following PR |
|
| Outcomes | Exercise capacity: 6MWD and HRQoL: SGRQ (domains) | |
| Notes | This study was supported by Alberta Health Services (Capital Health) and Department of Medicine, University of Alberta, and the Social Sciences and Humanities Research Council of Canada. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised but no further information. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. Method of concealment not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar attrition in both groups (14% vs 18%). |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registry information found, unclear if outcomes were reported as planned. |
| Other bias | Unclear risk | Any self‐reported outcomes are likely to be affected any bias. |
Wootton 2018.
| Study characteristics | ||
| Methods | RCT, parallel group. Setting: Australia. Study duration: 12 months | |
| Participants | 95 participants Intervention: n = 49, Control: n = 46 Male: n = 55, Female: n = 40 Age, mean and SD: Intervention: 70 ± 7, Control: 69 ± 9 FEV1% predicted, mean and SD: Intervention: 43 ± 15, Control: 43 ± 15. Inclusion criteria: if they had a medical diagnosis of moderate, severe or very severe COPD according to the GOLD spirometric classification FEV1/ FVC < 0.7 and FEV1 < 80% predicted, were in a stable clinical state and had a smoking history of > 10 pack‐years. Exclusion criteria: prescription of long‐term oxygen therapy, morbid obesity (body mass index.35 kg/m2), use of a walking aid, comorbidities likely to adversely affect exercise performance, or participation in supervised exercise training in the past 12 months. |
|
| Interventions | Intervention group: Components: walking training programme. Supervision: Telephone contact was made every two weeks for the first three months and then once a month until 14 months. Biofeedback provided via a pedometer and progressive goal‐setting (based on pedometer data) Control group: They received no feedback. Diaries were provided for participants in both groups to record details of completed walking sessions. |
|
| Outcomes | Exercise capacity: 6MWD, ISWT, ESWT and HRQoL: SGRQ (domains) and mortality. | |
| Notes | This study was supported by an Australian National Health and Medical Research Council project grant: 570814. This study was registered at ACTRN 12609000472279. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "independent telephone randomization using computerised random number generator sequence". |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. Method of concealment not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessor was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Percentage attrition was 7.9% for control group and 9.1% intervention group. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as planned, trial was registered. |
| Other bias | Low risk | No other sources of bias were identified. |
6MWD: six‐minute walk distance; BODE: body mass index airflow obstruction dyspnoea and exercise capacity index score; CAT: COPD assessment test; COPD: chronic obstructive pulmonary disease; CRQ: chronic respiratory questionnaire; EQ5D: EuroQol 5 Dimension; ESWT: endurance shuttle walk test; FEV1: forced expiratory volume in one second; FVC: forced vital capacity; HBP: home‐based telerehabilitation; HRQoL: health‐related quality of life; ISWT: incremental shuttle walk test; LCADL: London Chest Activity Daily Living; MRC: Medical Research Council; PASE: physical activity profile; PR: pulmonary rehabilitation; PT: physiotherapist tutor; RCT: randomised controlled trial; SD: standard deviation; SF‐36: Short Form 36; SGRQ: St George's Respiratory Questionnaire; SOLDQ: Seattle Obstructive Lung Disease Questionnaire; VO2 max: maximum oxygen uptake.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Andrews 2017 | The frequency of supervision was the same as in the initial rehabilitation program, different from what we defined about maintenance of rehabilitation being offered at a lower frequency of supervision. |
| Atabaki 2015 | Retrospective study |
| Barberan‐Garcia 2014 | Not an RCT. |
| Bauldoff 2003 | Wrong intervention. |
| Beauchamp 2013 | Review paper. |
| Bentley 2020 | Wrong intervention. |
| Carr 2009 | Wrong intervention. |
| Cockcroft 1981 | Non‐supervised and wrong population (mix population). |
| Cruz 2016 | Wrong intervention. |
| Desveaux 2016 | Protocol study. |
| Elliott 2004 | Wrong intervention. |
| Foglio 2001 | Wrong intervention. |
| Gomes 2006 | Protocol study. |
| Grosbois 1999 | Not an RCT. |
| Kaltsakas 2015 | Not an RCT. |
| Kotrach 2016 | Wrong intervention. |
| Loeckx 2018 | Wrong intervention (not supervised). |
| Lopes 2020 | Protocol study. |
| Lorenzo 2018 | Observational study. |
| Moullec 2010 | Not an RCT. |
| Moy 2015 | Protocol study. |
| Ng 2011 | Wrong intervention. |
| Nguyen 2009 | Wrong intervention. |
| Peters 1999 | Wrong comparator. |
| Pleguezuelos 2013 | Wrong intervention. |
| Puente‐Maestu 2003 | Wrong comparator. |
| Saini 2017 | Wrong intervention. |
| Spencer 2013 | Survey study. |
| Swerts 1990 | Unable to retrieve full text. |
| Vasilopoulou2017 | Wrong comparator. |
| Wadell 2005 | Not an RCT. |
Characteristics of studies awaiting classification [ordered by study ID]
NCT00419289.
| Methods | RCT |
| Participants | 120 COPD patients |
| Interventions | rehabilitation follow‐up and control follow‐up |
| Outcomes | HRQoL, work capacity and number of days admitted to hospital |
| Notes | Status: unknown |
NCT01235481.
| Methods | RCT |
| Participants | 90 COPD patients |
| Interventions | Device: Exercise DVD for maintenance vs usual care |
| Outcomes | Exercise capacity: 6MWT, QoL: CRQ, exercise compliance, self‐efficacy and satisfaction |
| Notes | Status: completed |
NCT01942499.
| Methods | RCT |
| Participants | 98 COPD patients |
| Interventions | Community‐based rehabilitation vs usual care |
| Outcomes | Exercise capacity: 6MWT, Sit to stand test, QoL:CRQ, Duke activities status, Health utility index and self‐efficacy |
| Notes | Status: completed |
NCT04284865.
| Methods | interventional |
| Participants | 30 COPD patients |
| Interventions | Web platform for 12 months combined with a monthly phone follow‐up. |
| Outcomes | Adherence, exercise capacity:6MWT, symptoms: CAT, MMRC, hospitalisation and exacerbation rate. |
| Notes | Status: not yet recruiting |
NL3827 (NTR4009).
| Methods | RCT |
| Participants | 100 COPD patients |
| Interventions | Self‐management support via internet using the Patient Coach‐platform. |
| Outcomes | HRQoL: CRQ, exercise capacity, actual activity, lung function, self‐management skills and health education impact, illness perceptions, patient utilities, exacerbations, costs. |
| Notes | Status: Stop date 2015‐02‐01 |
Characteristics of ongoing studies [ordered by study ID]
NCT03443817.
| Study name | Feasibility and effect of a follow‐up tele‐rehabilitation program for chronic obstructive lung disease vs. standard follow‐up (2‐TELEKOL) |
| Methods | RCT |
| Participants | 54 COPD patients |
| Interventions | Tele‐rehabilitation programme vs usual care |
| Outcomes | Exercise capacity: 6MWT, QoL: SGRQ, Generalised Anxiety Disorder Assessment and costs of the telerehabilitation |
| Starting date | March 1, 2018 |
| Contact information | joscer@rm.dk |
| Notes | Status: recruiting |
NCT04299165.
| Study name | Smartphone‐app as maintenance program in COPD (AMOPUR) |
| Methods | RCT |
| Participants | 104 COPD patients |
| Interventions | Smartphone Application (KAIA COPD‐App) in combination with activity monitoring vs usual care |
| Outcomes | Steps per day, dyspnoea:CAT, QoL:SAS‐CRQ, Exercise capacity: sit to stand test, sleep efficacy, motivation, usability, rate, number and severity of adverse events, Anxiety and Depression: HADS |
| Starting date | March 6, 2020 |
| Contact information | pawan.kaurbollinger@kaiahealth.com |
| Notes | Status: recruiting |
Differences between protocol and review
For the subgroup analysis of the maintenance programmes' effectiveness using face‐to‐face supervision versus remote supervision, we also considered, the maintenance programmes that provided a combination of face‐to‐face and remote supervision.
Contributions of authors
CM: drafting of background and methods of protocol, sifting, data extraction, risk of bias assessment and write‐up of full review.
SDC: drafting of background and methods of protocol, sifting and write‐up of full review.
SJ: critical review of protocol, sifting, data extraction, risk of bias assessment, critical review of final draft of full review.
AH: conceptual and clinical advice, drafting of background and methods of protocol, arbitrating conflicts, analysis and interpretation, approval of final draft of full review.
Contributions of editorial team
Rebecca Fortescue (Co‐ordinating Editor): checked the data, edited the review, signed off the review for publication.
Alexander Mathioudakis (Contact Editor): edited the review; advised on content.
Emma Dennett (Managing Editor): co‐ordinated the editorial process; advised on content; edited the review.
Emma Jackson (Assistant Managing Editor): conducted peer review; edited the references and other sections of the review.
Elizabeth Stovold (Information Specialist): designed the search strategy.
Sources of support
Internal sources
-
All authors, Other
The authors declare that no such funding was received for this systematic review
External sources
-
National Institute for Health Research, UK
Cochrane Programme Grant 16/114/21: NHS priorities in the management of chronic respiratory disease
-
SDC, Brazil
is supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (Process 306531/2018‐6) and Sao Paulo Research Foundation (SPRINT 17/50273‐4)
-
CM, Brazil
is partially supported researcher by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (process number: 200042/2019‐0), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superi – Brazil (CAPES) – Finance Code 001.
Declarations of interest
CM: none.
SDC: none.
SJ: is a systematic reviewer at Cochrane Airways and is funded by a National Institute for Health Research (NIHR) Programme Grant to complete work on this review.
AH: none.
Edited (no change to conclusions)
References
References to studies included in this review
Abbink 2004 {published data only}
- Abbink JJ, Bruinings AL, Braak I, Willems LK.Maintenance after pulmonary rehabilitation in COPD a randomised trial preliminary results. European Respiratory Journal 2004;24((Suppl48)):245s. [Google Scholar]
Arbane 2010 {published data only}
- Arbane G, Douiri A, Enright L, Haggis L, Poulter T, Garrod R.Effects of physical activity top up 'pat on the back' programme on exercise capacity and healthcare utilisation for people with chronic obstructive pulmonary disease (COPD). Thorax 2010;65(Suppl 4):A96. [DOI: 10.1136/thx.2010.150961.46] [DOI] [Google Scholar]
- Arbane G, Douiri A, Enright L, Haggis L, Poulter T, Garrod R.Physical activity top up—does it affect exercise tolerance for people with chronic obstructive pulmonary disease (COPD)? WPT Research Report Abstracts 2011;97:eS395. [DOI: 10.1016/j.physio.2011.04.002] [DOI] [Google Scholar]
Bernocchi 2018 {published data only}
- Bernocchi P, Vitacca M, La Rovere MT, Volterrani M, Galli T, Baratti D, et al.Home-based telerehabilitation in older patients with chronic obstructive pulmonary disease and heart failure: a randomised controlled trial. Age and Ageing 2018;47(1):82-8. [DOI: 10.1093/ageing/afx146] [DOI] [PubMed] [Google Scholar]
- Paneroni M, Scalvini S, Bernocchi P, Galli T, Baratti D, La Rovere MT, et al.Home telerehabilitation maintenance program for patients affected by COPD and CHF. European Respiratory Journal 2016;48((Suppl 60)):OA268. [DOI: 10.1183/13993003.congress-2016.OA268] [DOI] [Google Scholar]
Brooks 2002 {published data only}
- Brooks D, Krip B, Mangovski-Alzamora S, Goldstein RS.The effect of post rehabilitation programmes among individuals with chronic obstructive pulmonary disease. European Respiratory Journal 2002;20(1):20-9. [DOI: 10.1183/09031936.02.01852001] [DOI] [PubMed] [Google Scholar]
Burns 2016 {published data only}
- Browne P, Olive S, Staunton L, Clark A, Wilson E, Galey P, et al.The effects of maintenance schedules following pulmonary rehabilitation in patients with chronic obstructive pulmonary disease. Thorax 2013 2013;A16:S25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns DK, Wilson ECF, Browne P, Olive S, Clark A, Galey P, et al.The cost-effectiveness of maintenance schedules following pulmonary rehabilitation in patients with chronic obstructive pulmonary disease: an economic evaluation alongside a randomised controlled trial. Applied Health Economics and Health Policy 2016;14(1):105-15. [DOI: 10.1007/s40258-015-0199-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson AM, Browne P, Olive S, Clark A, Galey P, Dix E, et al.The effects of maintenance schedules following pulmonary rehabilitation inpatients with chronic obstructive pulmonary disease: a randomised controlled trial. BMJ Open 2015;5(3):e005921. [DOI: 10.1136/bmjopen-2014-005921] [DOI] [PMC free article] [PubMed] [Google Scholar]
Du Moulin 2009 {published data only}
- du Moulin M, Taube K, Wegscheider K, Behnke M, den Bussche H.Home-based exercise training as maintenance after outpatient pulmonary rehabilitation. Respiration 2009;77(2):139-45. [DOI: 10.1159/000150315] [DOI] [PubMed] [Google Scholar]
Galdiz 2020 {published data only}
- Galdiz JB, Gómez A, Rodriguez D, Guell R, Cebollero P, Hueto J, et al.Telerehabilitation programme as a maintenance strategy for COPD patients: a 12-month randomized clinical trial. Archivos de Bronconeumología 2020;57(3):195-204. [DOI: ] [DOI] [PubMed] [Google Scholar]
- Iturri JBG, Gomez A, Guell R, Cebollero P, Rodriguez D, Cejudo P, et al.A respiratory telerehabilitation program as maintenance in patients with chronic obstructive pulmonary disease. European Respiratory Journal 2018;52(Suppl 62):PA2049. [DOI: 10.1183/13993003.congress-2018.PA2049] [DOI] [Google Scholar]
Guell 2017 {published data only}
- Cejudo P, Galdiz B, Puy C, Martinez Indart L, Ortega F, Vazquez-Sanchez R, et al.Outcomes evaluation of a maintenance 3-year follow-up pulmonary rehabilitation program. European Respiratory Journal 2014;44(58):1708. [Google Scholar]
- Cejudo P, Galdiz B, Puy C, Martinez Indart L, Ortega F, Vazquez-Sanchez R, et al.Outcomes evaluation of a maintenance 3-year follow-up pulmonary rehabilitation program (Abstract). American Journal of Respiratory and Critical Care Medicine 2014;189:A3645. [Google Scholar]
- Cejudo P, Trigo GR, Puy C, Indart LM, Galdiz JB, Bdeir K, et al.Maintenance programme after COPD pulmonary rehabilitation (PR): differences in long-term BODE index between responders and non-responders. European Respiratory Journal 2011;38:p3642. [Google Scholar]
- Guell MR, Cejudo P, Ortega F, Puy MC, Rodriguez-Trigo G, Pijoan JI, et al.Benefits of long-term pulmonary rehabilitation maintenance program in severe COPD patients: 3 year follow-up. American Journal of Respiratory and Critical Care Medicine 2017;195(5):622-9. [DOI: 10.1164/rccm.201603-0602OC] [DOI] [PubMed] [Google Scholar]
- Rodriguez-Trigo G, Cejudo P, Puy C, Galdiz JB, Bdeir K, Gorostiza A, et al.Long term pulmonary rehabilitation programs for chronic obstructive pulmonary disease (COPD). Two years follow-up. European Respiratory Journal 2011;38(55):655s. [DOI] [PubMed] [Google Scholar]
Hill 2004 {published data only}
- Hill C, McDonal C.A maintenance program post pulmonary rehabilitation improved exercise tolerance in patients with COPD. Respirology 2004;9(2):A8. [Google Scholar]
Jiménez‐Reguera 2020 {published data only}
- Jiménez-Reguera B, Maroto López E, Fitch S, Juarros-Monteagudo L, Sánchez-Cortés M, Rodríguez-Hermosa JL, et al.Development and preliminary evaluation of the effects of an mHealth web-based platform (HappyAir™) on adherence to a maintenance program after pulmonary rehabilitation in COPD patients: randomized controlled trial. JMIR Mhealth Uhealth 2020;8:7. [DOI: 10.2196/18465] [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnson 2001 {published data only}
- Johnson LC, Backley J, Gray B, Moxham J.A controlled pilot study of a community based maintenance class for patients with severe COPD following pulmonary rehabilitation (PR). Thorax 2003;Suppl 3:iii37. [Google Scholar]
Li 2018 {published data only}
- Li Y, Feng J, Jia W, Qian H.A new pulmonary rehabilitation maintenance strategy through home-visiting and phone contact in COPD. Patient Preference and Adherence 2018;12:97-104. [DOI: 10.2147/PPA.S150679] [DOI] [PMC free article] [PubMed] [Google Scholar]
Linneberg 2012 {published data only}
- Linneberg A, Rasmussen M, Buch TF, Wester A, Malm L, Fannikke G, et al.A randomised study of the effects of supplemental exercise sessions after a 7-week chronic obstructive pulmonary disease rehabilitation program. Clinical Respiratory Journal 2012;6(2):112-9. [DOI: 10.1111/j.1752-699X.2011.00256.x] [DOI] [PubMed] [Google Scholar]
Murphy 2005 {published data only}
- Murphy MC, Berlowitz DJ, Saunders JE, Campbell M, Jackson B.A randomised trial to compare pulmonary rehabilitation (PRP) & the Stanford model chronic disease self management program (CDSMP) in COPD with 12 month follow-up. Respirology 2005;10(Suppl):A61. [Google Scholar]
- Murphy MC, Campbell M, Saunders JE, Jackson B.A randomized trial to compare the outcomes of a pulmonary rehabilitation program(PRP) weekly maintenance and the Stanford Model Chronic Disease SelfManagement Program (CDSMP) in COPD. Annals of the American Thoracic Society 2005;2:A26. [Google Scholar]
- Murphy MC.A comparison of the Stanford model chronic disease self management program with pulmonary rehabilitation on health outcomes for people with chronic obstructive pulmonary disease in the northern and western suburbs of Melbourne [PhD thesis]. ACU Research Bank, 2007. [DOI: 10.4226/66/5a95dbdfc67d8] [DOI] [Google Scholar]
Ries 2003 {published data only}
- Ries AL, Kaplan RM, Myers R, Prewitt LM.Maintenance after pulmonary rehabilitation in chronic lung disease: a randomized trial. American Journal of Respiratory and Critical Care Medicine 2003;167(6):880-8. [DOI: 10.1164/rccm.200204-318OC] [DOI] [PubMed] [Google Scholar]
- Santiago PB.Adherence to exercise following pulmonary rehabilitation of chronic obstructive pulmonary disease [PhD thesis]. University of California and San Diego State University, 2004. [Google Scholar]
Ringbaek 2010 {published data only}
- Ringbaek T, Brondum E, Martinez G, Thogersen J, Lange P.Long-term effects of 1-year maintenance training on physical functioning and health status inpatients with COPD: a randomized controlled study. Journal of Cardiopulmonary Rehabilitation and Prevention 2010;30(1):47-52. [DOI: 10.1097/HCR.0b013e3181c9c985] [DOI] [PubMed] [Google Scholar]
Roman 2013 {published data only}
- Roman M, Larraz C, Gomez A, Ripoll J, Mir I, Miranda EZ, et al.Efficacy of pulmonary rehabilitation in patients with moderate chronic obstructive pulmonary disease: a randomized controlled trial. BMC Family Practice 2013;14(1):21. [DOI: 10.1186/1471-2296-14-21] [DOI] [PMC free article] [PubMed] [Google Scholar]
Spencer 2010 {published data only}
- Spencer L, Alison J, McKeough Z.Maintenance of exercise capacity following pulmonary rehabilitation in COPD: randomised controlled trial. In: European Respiratory Society 19th Annual Congress; 2009 Sep 12-15; Vienna. 2009.
- Spencer L, McKeough Z, Alison J.Where are they now? Four years after the completion of a maintenance exercise programme in people with COPD. Respirology 2012;17(1):15. [Google Scholar]
- Spencer LM, Alison JA, McKeough ZJ.Do supervised weekly exercise programs maintain functional exercise capacity and quality of life, twelve months after pulmonary rehabilitation in COPD? BMC Pulmonary Medicine 2007;7:1-7. [DOI: 10.1186/1471-2466-7-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer LM, Alison JA, McKeough ZJ.Maintaining benefits following pulmonary rehabilitation: a randomised controlled trial. European Respiratory Journal 2010;35(3):571-7. [DOI: 10.1183/09031936.00073609] [DOI] [PubMed] [Google Scholar]
- Spencer LM, Alison JA, McKeough ZJ.Maintaining benefits following pulmonary rehabilitation: a randomised controlled trial. Journal of Cardiopulmonary Rehabilitation and Prevention 2009;29(6):411. [DOI] [PubMed] [Google Scholar]
Steele 2008 {published data only}
- Steele BG, Belza B, Cain KC, Coppersmith J, Lakshminarayan S, Howard JE, et al.A randomized clinical trial of an activity and exercise adherence intervention in chronic pulmonary disease. Archives of Physical Medicine and Rehabilitation 2008;89(3):404-12. [DOI: 10.1016/j.apmr.2007.11.003] [DOI] [PubMed] [Google Scholar]
Wong 2014 {published data only}
- Hamir R, Simmonds LG, Pratley M, Stickland MK, Rodgers W, Wong EYL.A novel patient support system to further improve health-related quality of life through self management after pulmonary rehabilitation. American Journal of Respiratory and Critical Care Medicine 2010;181:A1215. [Google Scholar]
- Hamir R, Simmonds LG, Yee B, Pratley M, Todosijczuk I, Rodgers WM, et al.Patient evaluation of a peer educator Vs. Respiratory therapist support program aimed at maintaining physical activity following pulmonary rehabilitation. American Journal of Respiratory and Critical Care Medicine 2012;185:A4883. [Google Scholar]
- Wong EY, Jennings CA, Rodgers WM, Selzler AM, Simmonds LG, Hamir R, et al.Peer educator vs. respiratory therapist support: which form of support better maintains health and functional outcomes following pulmonary rehabilitation? Patient Education and Counseling 2014;95(1):118-25. [DOI: 10.1016/j.pec.2013.12.008] [DOI] [PubMed] [Google Scholar]
Wootton 2018 {published data only}
- Wootton SL, McKeough Z, Ng CLW, Jenkins S, Hill K, Eastwood PR, et al.Effect on health-related quality of life of ongoing feedback during a 12-month maintenance walking programme in patients with COPD: a randomized controlled trial. Respirology 2018;23(1):60-7. [DOI: 10.1111/resp.13128] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Andrews 2017 {published data only}
- Andrews SM, Deoghare HV, Mills PK, Van Gundy K, Vempilly JJ, Jain VV.Pulmonary rehabilitation maintenance program may prevent accelerated FEV 1 decline in patients with COPD.. Clinical Pulmonary Medicine 2017;24(4):143-8. 2017;24(4):143-8.. [Google Scholar]
Atabaki 2015 {published data only}
- Atabaki A, Fine J, Haggerty M, Marolda C, Wakefield D, Yu A, et al.Effectiveness of repeated courses of pulmonary rehabilitation on functional exercise capacity in patients with COPD. Journal of Cardiopulmonary Rehabilitation and Prevention 2015;35(4):272-7. [DOI: 10.1097/HCR.0000000000000115] [DOI] [PubMed] [Google Scholar]
- Atabaki A, Haggerty M, Marolda C, Fine J, ZuWallack R, et al.Effectiveness of repeated courses of pulmonary rehabilitation on functional exercise capacity in patients with COPD. Chest 2012;142(4 Suppl 1):788A. [DOI] [PubMed] [Google Scholar]
Barberan‐Garcia 2014 {published data only}
- Barberan-Garcia A, Vogiatzis I, Solberg HS, Vilaro J, Rodriguez DA, Garasen HM, et al.Effects and barriers to deployment of telehealth wellness programs for chronic patients across 3 European countries. Respiratory Medicine 2014;108(4):628-37. [DOI: ] [DOI] [PubMed] [Google Scholar]
Bauldoff 2003 {published data only}
- Bauldoff GS, Hoffman LA, Diaz PT.Distractive auditory stimuli effect on home-based maintenance exercise in persons with COPD. In: National COPD Conference; 2003 Nov 14-15; Arlington. 2003:1072.
Beauchamp 2013 {published data only}
- Beauchamp MK, Rachael E, Janaudis-Ferreira T, Goldstein RS, Brooks D.Systematic review of supervised exercise programs after pulmonary rehabilitation in individuals with COPD. Chest 2013;144:1124-33. [10.1378/chest.12-2421] [DOI] [PubMed] [Google Scholar]
Bentley 2020 {published data only}
- Bentley CL, Powell L, Potter S, Parker J, Mountain GA, Bartlett YK, et al.The use of a smartphone app and an activity tracker to promote physical activity in the management of chronic obstructive pulmonary disease: randomized controlled feasibility study. JMIR Mhealth Uhealth 2020;8(6):e16203. [DOI: 10.2196/16203.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Carr 2009 {published data only}
- Carr SJ, Hill K, Brooks D, Goldstein RS.Pulmonary rehabilitation after acute exacerbation of chronic obstructive pulmonary disease in patients who previously completed a pulmonary rehabilitation program. Journal of Cardiopulmonary Rehabilitation and Prevention 2009;29(5):318-24. [DOI: 10.1097/HCR.0b013e3181ac7bb8] [DOI] [PubMed] [Google Scholar]
Cockcroft 1981 {published data only}
- Cockcroft AE, Saunders MJ, Berry G.Randomised controlled trial of rehabilitation in chronic respiratory disability. Thorax 1981;3:200-3. [DOI: 10.1136/thx.36.3.200] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cruz 2016 {published data only}
- Cruz J, Brooks D, Marques A.Walk2Bactive: a randomized controlled trial of a physical activity-focused behavioural intervention beyond pulmonary rehabilitation in chronic obstructive pulmonary disease. Chronic Respiratory Disease 2016;13(1):57-66. [DOI: 10.1177/1479972315619574] [DOI] [PMC free article] [PubMed] [Google Scholar]
Desveaux 2016 {published data only}
- Desveaux L, Beauchamp MK, Lee A, Ivers N, Goldstein R, Brooks D.Effects of a community-based, post-rehabilitation exercise program in COPD: protocol for a randomized controlled trial with embedded process evaluation. JMIR Research Protocols 2016;5(2):e63. [DOI: 10.2196/resprot.5435] [DOI] [PMC free article] [PubMed] [Google Scholar]
Elliott 2004 {published data only}
- Elliott M, Watson C, Wilkinson E, Musk AW, Lake FR, Musk A W, et al.Short- and long-term hospital and community exercise programmes for patients with chronic obstructive pulmonary disease. Respirology 2004;9(3):345-51. [DOI: 10.1111/j.1440-1843.2004.00595.x] [DOI] [PubMed] [Google Scholar]
Foglio 2001 {published data only}
- Foglio K, Bianchi L, Ambrosino N.Is it really useful to repeat outpatient pulmonary rehabilitation programs in patients with chronic airway obstruction? A 2-year controlled study. Chest 2001;119(6):1696-1704. [DOI: 10.1378/chest.119.6.1696] [DOI] [PubMed] [Google Scholar]
Gomes 2006 {published data only}
- Gómez A, Román M, Concepción L, Esteva M, Mir I, Vicenç T, et al.Efficacy of respiratory rehabilitation on patients with moderate COPD in primary care and maintenance of benefits at 2 years. Aten Primaria 2006;38(4):230-3. [DOI: 10.1157/13092346] [DOI] [PMC free article] [PubMed] [Google Scholar]
Grosbois 1999 {published data only}
- Grosbois JM, Lamblin C, Lemaire B, Chekroud H, Dernis JM, Douay B, et al.Long-term benefits of exercise maintenance after outpatient rehabilitation program in patients with chronic obstructive pulmonary disease. Journal of Cardiopulmonary Rehabilitation 1999;19(4):216-25. [DOI: 10.1097/00008483-199907000-00002] [DOI] [PubMed] [Google Scholar]
Kaltsakas 2015 {published data only}
- Kaltsakas G, Papaioannou AI, Vasilopoulou M, Spetsioti S, Gennimata SA, Palamidas AF, et al.Effectiveness of home maintenance telerehabilitation on COPD exacerbations. Thorax 2015;70((Suppl 3)):A56. [DOI: ] [Google Scholar]
Kotrach 2016 {published data only}
- Kotrach K, Dajczman E, Baltzan MA, Wardini R, Levitz S, Rotaple M, et al.A randomized controlled pilot study using a virtual game system (VGS) as a home-based exercise modality following pulmonary rehabilitation (PR) in patients with COPD. American Journal of Respiratory and Critical Care Medicine 2016;193:A4530. [Google Scholar]
Loeckx 2018 {published data only}
- Loeckx M, Rodrigues F, Demeyer H, Janssens W, Troosters T.Improving physical activity to obtain sustainable benefits in extra-pulmonary consequences ofCOPD after pulmonary rehabilitation: a randomized controlled trial. American Journal of Respiratory and Critical Care Medicine 2018;197:A2452. [Google Scholar]
Lopes 2020 {published data only}
- Lopez D, Cecins N, Cockram J, Collins A, Landers H, Sanfilippo F, et al.Maintaining quality of life in patients with chronic obstructive pulmonary disease (COPD) by extending the maintenance phase of community-based pulmonary rehabilitation: protocol for a randomised controlled trial (ComEx3 Study). BMJ Open Respiratory Research 2020;7(1):e000548. [DOI: 10.1136/bmjresp-2019-000548] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lorenzo 2018 {published data only}
- Lorenzo S, Rai S, Joao S, Stone IS, Buxton M.Effects of supervised maintenance exercise following pulmonary rehabilitation on exercise capacity and symptoms in COPD: a one year observational prospective study. Thorax 2018;73(S4):A158. [Google Scholar]
Moullec 2010 {published data only}
- Moullec G, Ninot G.An integrated programme after pulmonary rehabilitation in patients with chronic obstructive pulmonary disease: effect on emotional and functional dimensions of quality of life. Clinical Rehabilitation 2010;24(2):122-36. [DOI: 10.1177/0269215509346088] [DOI] [PubMed] [Google Scholar]
Moy 2015 {published data only}
- Moy ML, Wayne PM, Litrownik D, Beach D, Klings ES, Davis RB, et al.Long-term exercise after pulmonary rehabilitation (LEAP): design and rationale of a randomized controlled trial of Tai Chi. Contemporary Clinical Trials 2015;45:458-67. [DOI: 10.1016/j.cct.2015.09.004] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ng 2011 {published data only}
- Ng BH, Tsang HW, Jones AY, So CT, Mok TY.Functional and psychosocial effects of health qigong in patients with COPD: a randomized controlled trial. Journal of Alternative Complementary Medicine 2011;17(3):243-51. [DOI: 10.1089/acm.2010.0215] [DOI] [PubMed] [Google Scholar]
Nguyen 2009 {published data only}
- Nguyen HQ, Gill DP, Wolpin S, Steele BG, Benditt JO.Pilot study of a cell phone-based exercise persistence intervention post-rehabilitation for COPD. International Journal of Chronic Obstructive Pulmonary Disease 2009;4:301-13. [DOI: 10.2147/copd.s6643] [DOI] [PMC free article] [PubMed] [Google Scholar]
Peters 1999 {published data only}
- Peters ML.The effects of follow-up treatment following a multi-disciplinary pulmonary rehabilitation program on dyspnea, functional status, psychological symptoms and adherence to discharge recommendations for patients with COPD [dissertation]. Bell & Howell Information and Learning, 1999. [Google Scholar]
Pleguezuelos 2013 {published data only}
- Pleguezuelos E, Perez ME, Guirao L, Samitier B, Costea M, Ortega P, et al.Improving physical activity in patients with severe COPD with urban walking circuits. A randomised trial. European Respiratory Journal 2013;42(Suppl 57):302s. [DOI] [PubMed] [Google Scholar]
Puente‐Maestu 2003 {published data only}
- Puente-Maestu L, Luisa Sanz M, Sanz P, Ona RJ, Arnedillo A, Casaburi R.Long-term effects of a maintenance program after supervised or self-monitored training programs in patients with COPD. Lung 2003;181(2):67-78. [10.1007/s00408-003-1007-0] [DOI] [PubMed] [Google Scholar]
Saini 2017 {published data only}
- Saini PK, Priori R, Barretto C, Delbressine J, Van Genugten L, Dekker M, et al.Activity maintenance after pulmonary rehabilitation-first results of an online coaching program. American Journal of Respiratory and Critical Care Medicine 2017;195:A4942. [Google Scholar]
Spencer 2013 {published data only}
- Spencer LM, Alison JA, McKeough ZJ.A survey of opinions and attitudes toward exercise following a 12-month maintenance exercise program for people with COPD. Cardiopulmonary Physical Therapy Journal 2103;24(3):30-5. [PMC free article] [PubMed] [Google Scholar]
Swerts 1990 {published data only}
- Swerts PM, Kretzers LM, Terpstra-Lindeman E, Verstappen FT, Wouters EF.Exercise reconditioning in the rehabilitation of patients with chronic obstructive pulmonary disease: a short- and long-term analysis.. Arch Phys Med Rehabil 1990;71(8):580-3. [PubMed] [Google Scholar]
Vasilopoulou2017 {published data only}
- Vasilopoulou M, Papaioannou AI, Kaltsakas G, Louvaris Z, Chynkiamis N, Spetsioti S, et al.Home-based maintenance tele-rehabilitation reduces the risk for acute exacerbations of COPD, hospitalisations and emergency department visits. European Respiratory Journal 2017;25(49):1602129. [DOI: 10.1183/13993003.02129-2016] [DOI] [PubMed] [Google Scholar]
Wadell 2005 {published data only}
- Wadell K, Henriksson-Larsén K, Lundgren R, Sundelin G.Group training in patients with COPD - long-term effects after decreased training frequency. Disability Rehabilitation 2005;17(10):571-81. [DOI: 10.1080/09638280400018627] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
NCT00419289 {unpublished data only}
- Vest 2007.Rehabilitation of COPD patients: can the effect be prolonged by follow-up and continued rehabilitation [Vest S]. NCT00419289 2007.
NCT01235481 {unpublished data only}
- Hernandez P.Validation of an exercise DVD for maintenance after 12-week outpatient pulmonary rehabilitation program: a randomized clinical trial. NCT01235481 2015.
NCT01942499 {unpublished data only}
- Goldstein R.A randomized controlled trial of a post-rehabilitation community-based exercise program for individuals with COPD. NCT01942499;2018.
NCT04284865 {unpublished data only}
- Marquis N.Optimizing maintenance for patients with chronic obstructive pulmonary disease following pulmonary rehabilitation via a web platform. NCT04284865 2020.
NL3827 (NTR4009) {unpublished data only}
- Sont JK.Pulmonary rehabilitation of COPD: a trial of sustained internet based self-management support. Trial NL3827 (NTR4009) 2013.
References to ongoing studies
NCT03443817 {unpublished data only}
- Cerdan J.Feasibility and effect of a follow-up tele-rehabilitation program for chronic obstructive lung disease vs. standard follow-up. NCT03443817 2018.
NCT04299165 {unpublished data only}
- Spielmanns M.Impact of a smartphone application (KAIA COPD-App) in combination with activity monitoring as maintenance program following pulmonary rehabilitation in COPD: an international multi-centered randomized controlled trial. NCT04299165 2020. [DOI] [PMC free article] [PubMed]
Additional references
Alison 2017
- Alison JA, McKeough ZJ, Johnston K, McNamara RJ, Spencer LM, Jenkins SC, et al.Lung Foundation Australia and the Thoracic Society of Australia and New Zealand. Australian and New Zealand Pulmonary Rehabilitation Guidelines. Respirology 2017;22:800-19. [DOI: 10.1111/resp.13025] [DOI] [PubMed] [Google Scholar]
Berry 2003
- Berry MJ, Rejeski WJ, Adair NE, Ettinger WH Jr, Zaccaro DJ, Sevick MA.A randomized, controlled trial comparing long-term and short-term exercise in patients with chronic obstructive pulmonary disease. Journal of Cardiopulmonary Rehabilitation 2003;23(1):60-8. [DOI: doi: 10.1097/00008483-200301000-00011.] [DOI] [PubMed] [Google Scholar]
Bestall 2003
- Bestall JC, Paul EA, Garrod R, Garnham R, Jones RW, Wedzicha AJ.Longitudinal trends in exercise capacity and health status after pulmonary rehabilitation in patients with COPD. Respiratory Medicine 2003;97(2):173-80. [PMID: doi: 10.1053/rmed.2003.1397] [DOI] [PubMed] [Google Scholar]
Cochrane Airways 2019
- Cochrane Airways Trials Register. Available from airways.cochrane.org/trials-register (accessed 7 May 2019).
Covidence [Computer program]
- Covidence.Version (accessed prior to 21 August 2020). Melbourne, Australia: Veritas Health Innovation, 10 January 2017. Available at covidence.org.
Eisner 2011
- Eisner MD, Anthonisen N, Coultas D, Kuenzli N, Perez-Padilla R, Postma D, et al.An official American Thoracic Society public policy statement: novel risk factors and the global burden of chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2011;182(5):693-718. [DOI: 10.1164/rccm.200811-1757ST] [DOI] [PubMed] [Google Scholar]
Engström 1999
- Engström CP, Persson LO, Larsson S, Sullivan M.Long-term effects of a pulmonary rehabilitation programme in outpatients with chronic obstructive pulmonary disease: a randomized controlled study. Scandinavian Journal of Rehabilitation Medicine 1999;31(4):207-13. [PMID: doi: 10.1080/003655099444371] [DOI] [PubMed] [Google Scholar]
GBD 2018
- Global Burden of Disease 2017 Causes of Death Collaborators.Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1736-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
GOLD 2019
- Singh D, Augusti A, Anzueto A, Barnes PJ, Bourbeau J, Celli BR, et al.Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD Science Committee report 2019. European Respiratory Journal 2019;53(5):1900164. [DOI: 10.1183/13993003.00164-2019] [DOI] [PubMed]
GRADEpro GDT [Computer program]
- GRADEpro GDT.Version accessed 16 September 2019. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Guell 2000
- Guell R, Casan P, Belda J, Sangenis M, Morante F, Guyatt GH, et al.Long-term effects of outpatient rehabilitation of COPD: a randomized trial. Chest 2000;117:976-83. [DOI: 10.1378/chest.117.4.976] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA.Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Holland 2014
- Holland AE, Spruit MA, Troosters T, Puhan MA, Pepin V, Saey D, et al.An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. European Respiratory Journal 2014;44(6):1428-46. [DOI: doi: 10.1183/09031936.00150314] [DOI] [PubMed] [Google Scholar]
Holland 2017
- Holland AE, Cox NS.Telerehabilitation for COPD: could pulmonary rehabilitation deliver on its promise? Respirology 2017;22(4):626-7. [DOI: 10.1111/resp.13028] [DOI] [PubMed] [Google Scholar]
Imamura 2020
- Imamura S, Inagaki T, Terada J, Nagashima K, Katsura H, Tatsumi K.Long-term efficacy of pulmonary rehabilitation with home-based or low frequent maintenance programs in patients with chronic obstructive pulmonary disease: a meta-analysis. Annals of Palliative Medicine 2020;9(5):2606-15. [DOI: ] [DOI] [PubMed] [Google Scholar]
Jenkins 2018
- Jenkins AR, Gowler H, Curtis F, Holden NS, Bridle C, Jones AW.Efficacy of supervised maintenance exercise following pulmonary rehabilitation on health care use: a systematic review and meta-analysis. International Journal of Chronic Obstructive Pulmonary Disease 2018;13:257-73. [DOI: 10.2147/COPD.S150650] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 2005
- Jones PW.St. George's Respiratory Questionnaire: MCID. COPD 2005;2(1):75-9. [DOI: 10.1081/copd-200050513] [DOI] [PubMed] [Google Scholar]
Lozano 2013
- Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al.Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2013;380(9859):2095-128. [DOI: 10.1016/S0140-6736(12)61728-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Maltais 2014
- Maltais F, Decramer M, Casaburi R, Barreiro E, Burelle Y, Debigaré R, et al.An official American Thoracic Society/European Respiratory Society statement: update on limb muscle dysfunction in chronic obstructive pulmonary disease. American Journal of Respiratory Critical Care Medicine 2014;189(9):e15-62. [DOI: 10.1164/rccm.201402-0373ST] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marshall 2018
- Marshall IJ, Noel-Storr A, Kuiper J, Thomas J, Wallace BC.Machine learning for identifying randomised controlled trials: an evaluation and practitioner's guide. Res Synth Methods 2018;9(4):602-14. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
McCarthy 2015
- McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y.Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2015, Issue 2. Art. No: CD003793. [DOI: 10.1002/14651858.CD003793.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman D.Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nici 2019
- Nici L, Singh SJ, Holland AE, ZuWallack RL.Opportunities and challenges to expanding pulmonary rehabilitation into the home and community. American Journal of Respiratory Critical Care Medicine 2019;200(7):822-7. [DOI: 10.1164/rccm.201903-0548PP] [DOI] [PubMed] [Google Scholar]
Polkey 2006
- Polkey MI, Moxham J.Attacking the disease spiral in chronic obstructive pulmonary disease. Clinical Medicine 2006;6(2):90-6. [DOI: 10.7861/clinmedicine.11-5-461] [DOI] [PMC free article] [PubMed] [Google Scholar]
Regueiro 2013
- Regueiro EM, Burtin C, Baten P, Langer D, Van Remoortel H, Di Lorenzo VA, et al.The minimal important difference of the pulmonary functional status and dyspnea questionnaire in patients with severe chronic obstructive pulmonary disease. Respiratory Research 2013;14(1):58. [DOI: 10.1186/1465-9921-14-58] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Review Manager (RevMan).Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schulz 2010
- Schulz KF, Altman DG, Moher D.CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. British Medical Journal 2010;23(349):c332. [DOI: 10.1136/bmj.c332] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2017
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al.Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). Cochrane, 2017. Available from training.cochrane.org/handbook.
Singh 2014
- Singh SJ, Puhan MA, Andrianopoulos V, Hernandes NA, Mitchell KE, Hill CJ, et al.An official systematic review of the European Respiratory Society/American Thoracic Society: measurement properties of field walking tests in chronic respiratory disease. European Respiratory Journal 2014;44(6):1447-78. [DOI: 10.1183/09031936.00150414] [DOI] [PubMed] [Google Scholar]
Smid 2017
- Smid DE, Franssen FM, Houben-Wilke S, Vanfleteren LE, Janssen DJ, Wouters EF, et al.Responsiveness and MCID estimates for CAT, CCQ, and HADS in patients with COPD undergoing pulmonary rehabilitation: a prospective analysis. Journal of the American Medical Directors Association 2017;18(1):53-8. [DOI] [PubMed] [Google Scholar]
Soysa 2012
- Soysa S, McKeough Z, Spencer L, Alison J.Effects of maintenance programs on exercise capacity and quality of life in chronic obstructive pulmonary disease. Physical Therapy Reviews 2012;17(5):335-45. [DOI: 10.1179/1743288X12Y.0000000033] [DOI] [Google Scholar]
Spencer 2019
- Spencer LM, McKeough ZJ.Maintaining the benefits following pulmonary rehabilitation: achievable or not? Respirology 2019;24:909-15. [DOI: 10.1111/resp.13518] [DOI] [PubMed] [Google Scholar]
Spruit 2013
- Spruit MA, Singh SJ, Garvey C, ZuWallack R, Nici L, Rochester C, et al.An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. American Journal of Respiratory Critical Care Medicine 2013;188(8):e13-64. [DOI: 10.1164/rccm.201309-1634ST] [DOI] [PubMed] [Google Scholar]
Troosters 2000
- Troosters T, Gosselink R, Decramer M.Short- and long-term effects of outpatient rehabilitation in patients with chronic obstructive pulmonary disease: a randomized trial.. American Journal of Medicine 2000;109(3):207-12. [DOI: 10.1016/s0002-9343(00)00472-1] [DOI] [PubMed] [Google Scholar]
Vasilopoulou 2017
- Vasilopoulou M, Papaioannou AI, Kaltsakas G, Louvaris Z, Chynkiamis N, Spetsioti S, et al.Home-based maintenance telerehabilitation reduces the risk for acute exacerbations of COPD, hospitalisations and emergency department visits. European Respiratory Journal 2017;49(5):1602129. [DOI: ] [DOI] [PubMed] [Google Scholar]
Walters 2005
- Walters SJ, Brazier JE.Comparison of the minimally importantdifference for two health state utility measures: EQ-5D and SF-6D. Quality of Life Research 2005;14:1523e32. [DOI: 10.1007/s11136-004-7713-0] [DOI] [PubMed] [Google Scholar]
Wheaton 2015
- Wheaton AG, Cunningham TJ, Ford ES, Croft JB.Employment and activity limitations among adults with chronic obstructive pulmonary disease–United States, 2013.. Morbidity and Mortality Weekly Report. 2015;64((11)):289–295. [PMC free article] [PubMed] [Google Scholar]
Wijkstra 1994
- Wijkstra PJ, TenVergert EM, Van Altena R, Otten V, Postma DS, Kraan J, et al.Reliability and validity of the chronic respiratory questionnaire (CRQ). Thorax 1994;49(5):465-7. [DOI: 10.1136/thx.49.5.465.] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Malaguti 2020
- Malaguti C, Dal Corso S, Janjua S, Holland AE.Supervised maintenance programs following pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2020, Issue 4. Art. No: CD013569. [DOI: 10.1002/14651858.CD013569] [DOI] [PMC free article] [PubMed] [Google Scholar]


