Abstract
Workplace temperature screening has become standard practice during the SARS-CoV-2 pandemic. The objective was to determine the consistency of four temperature devices during exposure to simulated and actual environmental conditions reflective of a workplace. An infrared (IR) digital thermometer (accuracy(A)±0.2), IR laser thermometer (A±1), and thermal imaging camera (A±0.3) were used to measure forehead and tympanic (digital only) temperatures. The first experiment was conducted in a controlled simulated environment (−20 to 20 °C) with three participants (32-YOF, 27-YOM, 20-YOF). The second experiment used actual outdoor conditions (−0.48 to 45.6 °C) with two participants (32-YOF, 27-YOM). The tympanic measurement was the least impacted by environmental temperature (mean(±SD)): simulated (36.8(±0.18) °C) and actual (36.9(±0.16) °C). The thermal imaging camera had the lowest RMSE values (0.81–0.97 °C), with outdoor temperatures ranging from 0 to 45 °C. Environmental temperature influenced forehead temperature readings and required a resting period in a thermoneutral environment (5–9 min (−20 to −10 °C) to immediate (15–20 °C)).
Keywords: Screening protocols, Environmental temperature, Infrared camera, Infrared thermometer, Forehead temperature
Abbreviations: CI, confidence interval; ICC, intraclass correlation coefficients; IR, infrared; IRT, infrared thermographs; ITDS, infrared thermal detection systems; M, mean; NCIT, noncontact infrared thermometers; RMSE, root mean square error; SD, standard deviation; SE, standard error
Graphical abstract

1. Introduction
Thermal detection equipment has been in use since the onset of the COVID-19 pandemic to monitor essential workers as primary prevention for limiting the spread of the SARS-CoV-2 virus (Aggarwal et al., 2020; Huang et al., 2020; Ontario-Government, 2020a, 2020b). Similar mass screening entrance protocols were active during previous global outbreaks, including SARS (2003) and the H1N1 pandemic (2009), to detect fevers at international airports and transit terminals (Cho and Yoon, 2014; McBride and Buikstra, 2010; Nishiua and Kamiya, 2011; Perpetuini et al., 2021; Priest et al., 2011). The design of mass screening programs allows for the quick assessment of a large number of people in order to classify them based on the probability that they may present a particular condition of interest (Aragón-Vargas, 2020; Trevethan, 2017); in this context, the condition of interest is a fever, typically defined as a temperature above 37.8–38 °C (Cho and Yoon, 2014; Priest et al., 2011; Zhou et al., 2020).
Workplaces often select infrared (IR) thermal detection systems (ITDS) for mass screening programs to reduce the risk of potential virus transmission and minimize the degree of testing invasiveness (Mercer and Ring, 2009; Nguyen et al., 2010). There are two primary types of IR techniques typically used in this context: noncontact IR thermometers (NCITs) and IR thermographs (IRTs) (Chiappini et al., 2011; Ng and Acharya, 2009; Teran et al., 2012). NCITs and IRTs are passive remote sensing devices that detect mid- and long-wave IR radiation and convert that radiation to temperature without direct contact. In contrast, IRTs detect radiation from the target, typically the human face, and provide a digital image showing temperature distribution (G. Chen et al., 2020; Ng et al., 2004). Both NCITs and IRTs then use point estimation to further convert the measured temperature (i.e. forehead, wrist or tympanic) to a predicted temperature at another body site (i.e. core, oral or rectal) using proprietary algorithms (Aragón-Vargas, 2020).
Humans regulate core temperature within a narrow range between 36.5 and 37.5 °C (Mekjavic et al., 1991), depending on environmental thermal stress and physiological changes (Aragón-Vargas, 2020). This variance in core temperature is also relative to the assessment site; for example, a normal core temperature is considered a rectal temperature of 37.04 °C, the esophageal temperature of 36.8 °C, an axillary temperature of 36.04 °C and tympanic temperature 36.91 °C (Geneva et al., 2019; Oguz et al., 2018). Unfortunately, in mass screening settings, these options are often not available or feasible due to the level of invasiveness. As a result, researchers attempt to determine which factors would allow for the use of IRT and NCITs to estimate the core temperature at more accessible sites (McCarthy and Heusch, 2006).
Based on the influence of the assessment site, the reference value for a fever is, therefore, relative (Rubia-Rubia et al., 2011). For example, a fever would be recognized, using a rectal or tympanic thermometer at 37.8 °C, and using an axillary measure at 37 °C. Numerous studies have aimed to determine comparative values between different assessment sites and NCITs and IRTs (Casa et al., 2007; Ganio et al., 2009; Huggins et al., 2012; Kistemaker et al., 2006; Kocoglu et al., 2002). Unfortunately, these findings are not consistent across devices and often result in underestimating the temperature by 2-4 °C. Therefore, as NCITs and IRTs provide peripheral temperature readings and are merely estimates of actual core temperature (Aragon, 2020), the cut-off values for recognizing a fever need to be considered unique to each device.
Infrared thermography detects IR radiation emitted from the skin surface, and then the temperature distributions are calculated (H.-Y. Chen et al., 2020; Ghassemi et al., 2018). Currently, the only international standards for performance evaluation of IRTs intended for fever screening are IEC 80601-2-59 (2017) and ISO/TR 13154 (2017). This standard is limited for application in workplace entrance screening protocols because it only outlines laboratory characterization test limits for IRTs. For example, the standard requires that the environmental temperature for testing is constant and controlled and that the distance between the device and the subject is unrestricted. Researchers have provided user-friendly summaries of these ISO documents outlining the technical specifications for use (Foster et al., 2021). In Canada, current public health messaging has emphasized the importance of maintaining physical distancing of 2 m (O.Reg.364/20, 2020). As a result, workplace screening programs occur directly at the workplace entrance, with employees lining up outside or staying within their vehicles to ensure distancing is respected. These measurement scenarios create situations where NCIT devices can be misused (Foster et al., 2021).
There is widespread literature simultaneously comparing different IR temperature measurement devices and measurement sites at ambient temperatures (Bijur et al., 2016; Fletcher et al., 2018; Kiekkas et al., 2016; Weng Seng Fong et al., 2020). Environmental conditions, including combinations of air temperature, radiant temperature, humidity, and air velocity, can influence IRT and NCIT devices measurements (Foster et al., 2021; Khaksari et al., 2021). For instance, in a study involving three environmental temperatures (15.5, 21.1 and 26.6 °C) and two humidity conditions (35 and 75%), the variance of each measurement site (rectal, esophageal, tympanic and oral) was found to decrease as the environmental temperature increased (Pascoe and Fisher, 2009). Another study used an IR thermometer to measure tympanic, forehead and wrist temperature with ambient air temperatures ranging from 26 to 37 °C and found no significant effect of air temperature on the body temperature measurement (H.-Y. Chen et al., 2020). For the most part, environmental chamber experiments have focused on heat and increased workloads (through exercise) on the body temperature. In an experiment where participants sat in a cold environment (10 °C, 50% relative humidity) for 20 min, then entered a warm chamber (30 °C, 50% relative humidity) and rested on a chair for 30 min, underestimations of core body temperature occurred for the first 10 min (Kistemaker et al., 2006). To our knowledge, no assessment of the effectiveness of NCITs and IRTs has been reflective of environmental temperatures experienced at Canadian workplaces (Khaksari et al., 2021; Perpetuini et al., 2021). Therefore, the purpose of this study was to establish the consistency of these devices for determining the surface temperature of the skin and ear in both a simulated environmental exposure (controlled temperature and humidity) and an actual environmental exposure (uncontrolled temperature and humidity).
2. Methodology
These experiments were performed in accordance with the research ethics board from Laurentian University (REB#: 6020885). All university-specific safety protocols and guidelines for Covid-19 exposure were followed, including adherence to physical distancing and mask guidelines.
2.1. Temperature detection equipment
Indirect (non-contact) forehead surface temperature was measured using a seven-inch forehead temperature detection facial recognition terminal (SEK-SVBFF07, Provix Inc., New Lowell, Ontario, Canada) with a body temperature measurement range of 34-42 °C (Table 1 ) was used with the participant's toes at a distance of 0.5 m from the terminal. The thermal imaging camera sensor (MLX90640ESF-BAx-000-TU, Melexis, Ypres, Belgium) was a factory calibrated 32 × 24 pixels thermal IR array with a 4-lead TO39 package and digital interface. To calculate the forehead temperature, an ambient sensor is integrated to measure the ambient temperature of the chip and a separate supply sensor uses the IR pixel array to calculate the forehead temperature. The device was left in its default mode, where the measurement frame rate was 2 Hz, with an emissivity coefficient of 1, and the chess pattern of pixel analysis was used. Once the measurement is completed with the IR pixel array algorithm, the forehead temperature displays on the screen and an automated voice announces the temperature.
Table 1.
Summary of the temperature measurements range, accuracy, and resolution for the equipment used throughout these studies.
| Equipment | Measurement Range (oC) | Accuracy (oC) | Resolution (oC) |
|---|---|---|---|
| IR Tympanic and Forehead Digital Contact Probe | 32.00 to 42.89 | ±0.2 | 0.1 |
| iButton® | −40 to 85 | ±0.5 | 0.5 |
| IR Laser Thermometer | −30 to 650 | ±1 | 0.1 |
| Thermal Imaging Camera | 34 to 42 | ±0.3 | 0.1 K RMS @1 Hz refresh ratea |
Indicates the resolution for the thermal imaging camera has been provided in noise equivalent temperature difference (NETD).
An IR laser thermometer (FLIR TG54, FLIR Systems Inc., Portland, USA) with a 24:1 (inch) distance to spot ratio was used at distances of 0.61, 1.22, and 1.83 m, as instructed in the user manual (Table 1). The right tympanic membrane temperature was measured using a combined IR tympanic and forehead digital contact probe (Wellworks, Model #10092) as it has previously demonstrated good agreement with rectal thermometry for measurement of core body temperature (Dzarr et al., 2009) and is considered less invasive (Yeoh et al., 2017). Direct (contact) forehead surface temperature was measured using the IR tympanic and forehead digital contact probe without the tympanic probe attachment and an iButton® (Embedded Data Systems, DS1923-F5#-Hygrochron) directly secured on the forehead with medical tape (Hasselberg et al., 2013).
The IR laser thermometer (FLIR TG54) was calibrated on July 21, 2020 (Cert.: C344614-00-01). The FLIR TG54 was calibrated at an ISO 17025 laboratory using blackbodies techniques from ITM instruments Inc. It was calibrated using a tolerance of ± 1 °C or 1%, whichever is greater (Table 1). Four different black bodies were used (50, 100, 200, 400 °C), and the device passed with a reading of 50.7, 100.6, 200.4 and 399.8 °C for each corresponding blackbody. All other equipment has standard factory calibrations with the previously mentioned temperature measurement ranges, accuracy and resolutions.
2.2. Environmental chamber
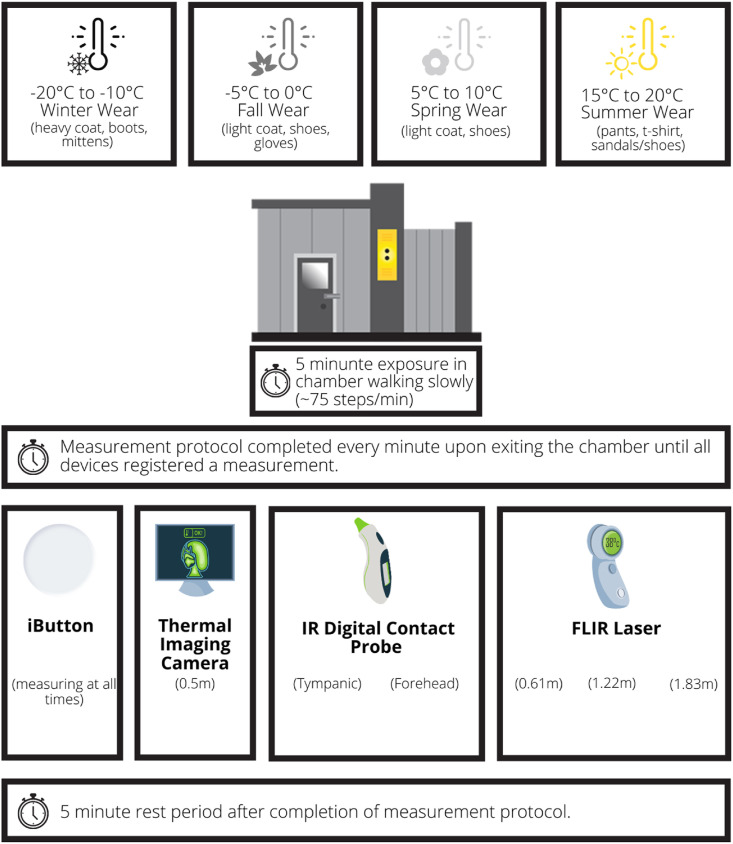
A walk-in environmental chamber (Labworks International Inc.) was used to replicate the desired combination of temperature and humidity for the controlled environment experiment (Fig. 1 ). The chamber can operate from −20 to 40 °C, with humidity variable up to 100% (temperature dependent).
Fig. 1.
Walk-in environmental chamber (Labworks Internationals Inc.) used to create simulated environmental conditions by controlling temperature and humidity.
2.3. Simulated environmental exposure protocol
In this experiment, three participants (32-YOF, 27-YOM, 20-YOF) were exposed to temperature changes at 5 °C increments from −20 to 20 °C (humidity range: 27–52%) for a total of nine exposures. Participants wore weather-appropriate clothing for each exposure, except a hat to avoid confounding factors during the forehead surface measurement. While inside the environmental chamber, participants were advised to walk at the same pace used when they walk into work (~75 steps per minute) to simulate the brief commute between car and workplace entrance and ensure thermal comfort. An iButton® was placed at the measurement location outside the environmental chamber, and a second iButton® was fastened directly on the participant's forehead using medical tape for the duration of the experiment. Once each temperature range was achieved, the participant would leave the chamber and temperature measurements were taken with the forehead temperature detection facial recognition terminal (Fig. 2 ), the IR tympanic and digital forehead thermometer, and the IR laser thermometer. The tympanic and forehead surface measurements were taken immediately after the terminal measurement, and then the IR laser thermometer was used at distances of 0.61 m, 1.22 m, and 1.83 m (Fig. 3 ) as instructed by the manufacturer. The protocol at each temperature range was considered complete when all devices were able to detect a skin temperature measurement (Fig. 4 ). After the exposure and measurement protocol was completed for each temperature, participants took a 5-min break.
Fig. 2.
Example of participants using the forehead temperature detection facial recognition terminal (SEK-SVBFF07, Provix Inc., New Lowell, Ontario, Canada).
Fig. 3.
Temperature measurement distances from facial recognition terminal (0 m), to the location for forehead temperature detection (green line at 0.5 m), and to the three locations used for the IR laser forehead measurements (red lines at 0.61 m, 1.22 m and 1.83 m). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 4.
Schematic of the simulated environmental exposure protocol using a walk-in environmental chamber (Labworks Internationals Inc.).
2.4. Actual environmental exposure protocol
Two participants (32-YOF, 27-YOM), took temperature measurements outdoors in an unshaded and shaded (outdoor pop-up tent) location every half an hour from 6:30 am to 7:30 pm The same measurement protocol (Fig. 3) was used, except for the IR forehead measurements distance being set at 0.61 m. An additional iButton® was placed outside in the measurement area to capture the environmental temperature and humidity.
2.5. Statistical analysis
Descriptive statistics, including: mean, standard deviation (SD), standard error (SE), and the lower and upper bounds of the 95% confidence interval (CI) for mean, were assessed for each temperature measurement device, in both the simulated and actual environmental experiments, using SPSS (Version 27, IBM). Intraclass correlation coefficients (ICCs) were calculated individually for each participant at each environmental chamber exposure temperature using a two-way random-effects model for absolute agreement (Dzarr et al., 2009). The ICCs indicate a level of agreement on temperatures measured using the FLIR laser temperature monitor at different distances (0.61, 1.22, 1.83 m from the skin surface) (Koo and Li, 2016; Landers, 2015) for the simulated environmental chamber experiment. Interpretation of ICCs has been identified as values between 0.5 and 0.75 signify moderate reliability, between 0.75 and 0.9 signify good reliability, and values greater than 0.9 signify excellent reliability (Koo and Li, 2016).
Regression analysis was conducted on the actual environmental exposure data to determine whether the outdoor environmental temperature influenced the skin surface temperature measurement. Root mean square error (RMSE), calculated as the square root of the average of squared errors, was used to quantify the difference between the forehead surface skin temperature measurements and the tympanic temperature (control). Lower RMSE is considered better and was previously found to be 0.439 °C for a similar thermal unit (Lin et al., 2019). The overall RMSE for all data points (N = 177) was calculated and RMSE for groupings of data over specified temperature ranges (0–5, 5–10, 10-15 °C, …). Homogenous subset testing on the difference between means and 95% CI was conducted to determine which equipment performed differently and determine whether any equipment could be grouped for similar performance.
3. Results
3.1. Simulated environmental exposure
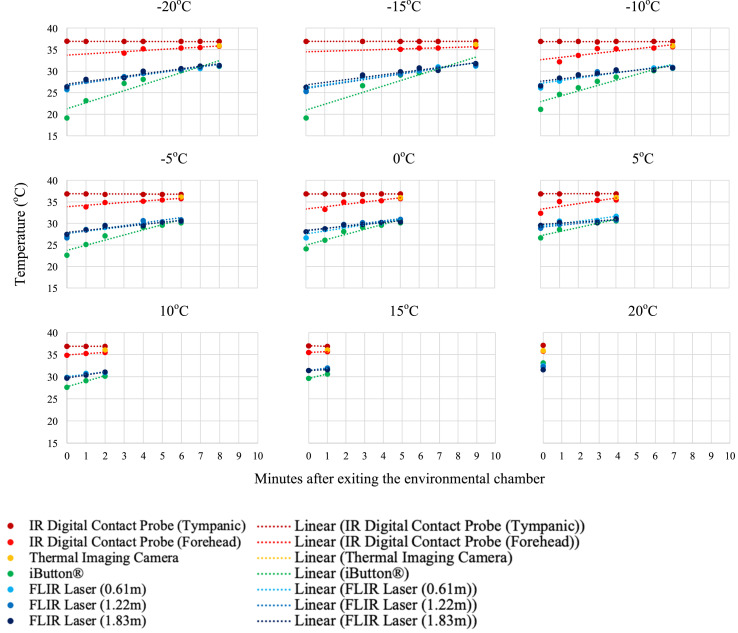
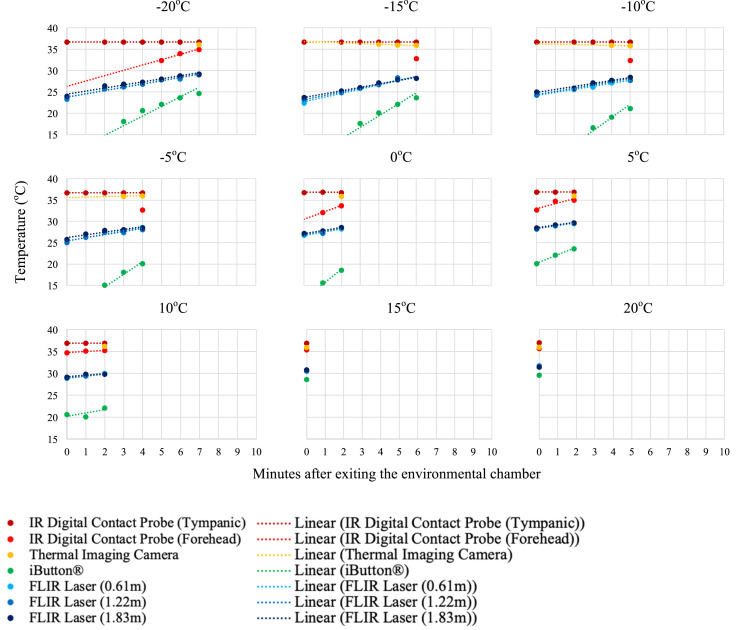
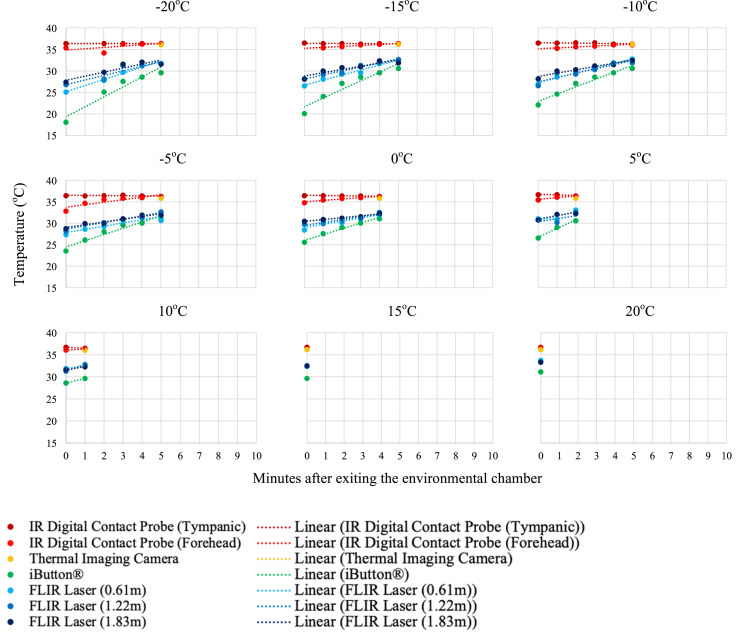
Individual temperature results of the three participants are presented in Fig. 5a, Fig. 5b, Fig. 5c a–c . As anticipated, after exiting the environmental chamber and the participant resting in the ambient temperature (21 °C) room, the skin surface temperature measurements increased.
Fig. 5a.
Temperature measurements and linear trend lines for participant 1 (32 YOF) after environmental exposure to −20 to 20 °C at 5 °C increments. Measurements were taken every minute after exiting the environmental chamber until a measurement could be registered by the device (i.e. lower temperatures needed longer for skin surface measurements to register).
Fig. 5b.
Temperature measurements and linear trend lines for participant 2 (27 YOM) after environmental exposure to −20 to 20 °C at 5 °C increments. Measurements were taken every minute after exiting the environmental chamber until a measurement could be registered by the device (i.e. lower temperatures needed longer for skin surface measurements to register).
Fig. 5c.
Temperature measurements and linear trend lines for participant 3 (20 YOF) after environmental exposure to −20 to 20 °C at 5 °C increments. Measurements were taken every minute after exiting the environmental chamber until a measurement could be registered by the device (i.e. lower temperatures needed longer for skin surface measurements to register).
The descriptive statistics for the first measurement upon exiting the environmental chamber are documented for each device (Table 2 ). The IR digital thermometer tympanic measurement was the most consistent (mean(±SD) = 36.8 (±0.18)°C), regardless of the participant exposure temperature. The same instrument measuring the forehead demonstrated changeability with the environmental exposure temperature (35.0 (±1.31)°C). The iButton® registered the lowest forehead skin surface temperatures with the most variability (21.9 (±7.66)°C), as it was affixed to the participant's forehead for the duration of the testing protocol.
Table 2.
Descriptive statistics for the first measurement immediately after exiting the environmental chamber.
|
Equipment |
N | Mean(oC) | SD(oC) | SE(oC) | 95% CI for Mean(oC) |
|
|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||
| IR Digital Contact Probe (Tympanic) | 27 | 36.78 | 0.1847 | 0.03554 | 36.70 | 36.85 |
| IR Digital Contact Probe (Forehead) | 15 | 34.98 | 1.3094 | 0.3381 | 34.25 | 35.76 |
| Thermal Imaging Camera | 5 | 36.04 | 0.1516 | 0.06782 | 35.85 | 36.23 |
| iButton® | 27 | 21.87 | 7.661 | 1.4743 | 18.84 | 24.90 |
| FLIR Laser (0.61 m) | 27 | 28.09 | 3.040 | 0.5851 | 26.88 | 26.88 |
| FLIR Laser (1.22 m) | 27 | 28.33 | 2.831 | 0.5447 | 27.21 | 27.21 |
| FLIR Laser (1.83 m) | 27 | 28.66 | 2.572 | 0.4951 | 27.65 | 27.65 |
| Total | 155 | 29.58 | 6.213 | 0.4951 | 28.60 | 28.60 |
The ICC tests between the FLIR laser thermometer used at distances of 0.61, 1.22, 1.83 m from the first participant, showed ICC values ranging from excellent reliability ICC(2, 1) = 0.98 at −20 °C to good reliability ICC(2, 1) = 0.79 at 15 °C (Table 3 ). Measurement results for participant 2 showed a similar trend: ICC(2, 1) = 0.97 at −20 °C and ICC(2, 1) = 0.88 at 10 °C. Participant 3 had ICC results ranging from an excellent at −10 °C (ICC(2, 1) = 0.91) to moderate at 5 °C (ICC(2, 1) = 0.65).
Table 3.
Intraclass correlation (ICC) results for FLIR laser thermometer at measurement distances of 0.61, 1.22, 1.83 m from the participant.
| Environmental Chamber Temperature (oC) | Intraclass Correlation (ICC) |
||
|---|---|---|---|
| Participant 1 | Participant 2 | Participant 3 | |
| −20 | 0.984a | 0.972a | 0.871b |
| −15 | 0.968a | 0.972a | 0.84b |
| −10 | 0.972a | 0.924a | 0.909a |
| −5 | 0.939a | 0.917a | 0.821b |
| 0 | 0.893b | 0.906a | 0.736 |
| 5 | 0.8228b | 0.962a | 0.648 |
| 10 | 0.948a | 0.878b | 0.837b |
| 15 | 0.786b | N/A | N/A |
| 20 | N/A | N/A | N/A |
Indicates excellent reliability (ICC>0.9).
Indicates good reliability (ICC = 0.75–0.9).
3.2. Actual environmental exposure
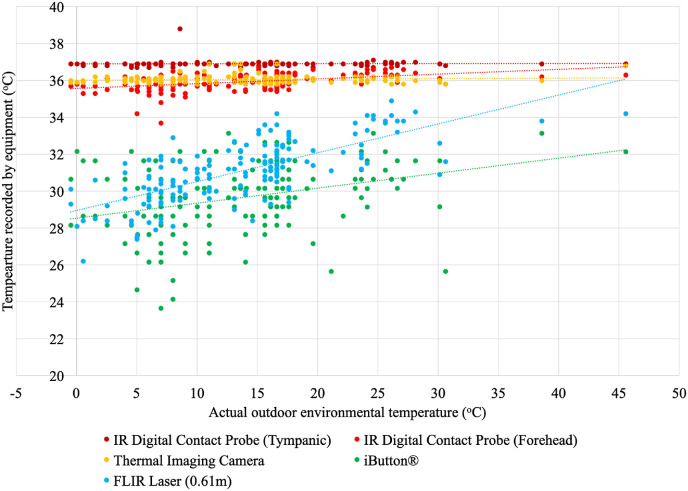
Two participants recorded temperature measurements every 30 min from 6:30 a.m. to 7:30 p.m., over four days in August and September. A total of 177 sets of measurements were recorded using each device. The outdoor temperature ranged from −0.48 to 45.6 °C, and the participants remained in a room temperature environment (~21 °C) between the outdoor temperature measurements. The iButton® registered a minimum forehead surface temperature (23.7 °C) and a maximum temperature was recorded with the IR digital thermometer in the tympanic location (38.8 °C) (Fig. 6 ). Generally, the IR digital thermometer and thermal imaging camera were unaffected by the outdoor temperature. However, the iButton® and IR laser thermometer had positive regressions, where the lower the outdoor temperature, the lower the skin surface temperature measurement, and the higher the outdoor temperature, the higher the surface temperature measurement (Fig. 6).
Fig. 6.
Linear regression of the actual outdoor environmental temperature (°C) with the temperature recorded by each device (°C) every 30 min from 6:30am to 7:30pm (EST) (latitude 46.5oN).
The IR digital thermometer tympanic measurements were the most consistent with a mean(±SD) of 36.9(±0.15)°C (Table 4 ). On average, the iButton® forehead surface measurements were the lowest with the most variability (29.6(±1.88)°C), and the forehead temperature detection facial recognition terminal measurements were the highest with the least variability (36.1(±0.20)°C), after the tympanic measurements (Table 4).
Table 4.
Descriptive statistics of 177 temperature measurements (°C) taken in outdoor temperatures ranging from −0.48 to 45.6 °C.
|
Equipment |
N | Mean(oC) | SD(oC) | SE(oC) | 95% CI for Mean(oC) |
|
|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||
| IR Digital Contact Probe (Tympanic) | 177 | 36.90 | 0.1561 | 0.01173 | 36.88 | 36.92 |
| IR Digital Contact Probe (Forehead) | 177 | 35.92 | 0.4183 | 0.03144 | 35.85 | 35.98 |
| Thermal Imaging Camera | 177 | 36.05 | 0.2037 | 0.01531 | 36.02 | 36.08 |
| iButton® | 177 | 29.64 | 1.878 | 0.1412 | 29.37 | 29.92 |
| FLIR Laser (0.61 m) | 177 | 31.10 | 1.630 | 0.1225 | 30.86 | 31.34 |
| Total | 885 | 33.92 | 3.166 | 0.1064 | 33.71 | 34.13 |
The literature regarding the average adult temperatures suggests the tympanic measurement should be 37.0 °C for non-febrile patients, and the forehead skin surface temperature typically measures approximately a degree lower (~36 °C) (Aragón-Vargas, 2020; Geneva et al., 2019). The RMSE of the forehead temperature measurement equipment (IR digital contact probe, thermal imaging camera, iButton®, and FLIR laser (0.61 m)) was calculated from the measured tympanic temperature (Table 5 ). The lowest RMSE values were calculated for the thermal imaging camera (RMSE = 0.81–0.97 °C), and the iButton® had the highest RMSE ranging from 6.36 to 8.59 °C.
Table 5.
Root mean square error (RMSE) values at different temperature ranges, calculated as the difference between tympanic temperature and forehead surface skin measurements (N = 177) using four pieces of equipment.
| Root mean square error (RMSE) |
||||
|---|---|---|---|---|
| Temperature Range (oC) | IR Digital Contact Probe (Forehead) | Thermal Imaging Camera | iButton® | FLIR Laser (0.61 m) |
| 0-5 | 1.31 | 0.84 | 7.77 | 7.99 |
| 5.1–10 | 1.30 | 0.97 | 8.59 | 6.89 |
| 10.1–15 | 1.08 | 0.81 | 7.32 | 6.19 |
| 15.1–20 | 0.94 | 0.87 | 6.91 | 5.27 |
| 20.1–25 | 0.66 | 0.85 | 6.86 | 4.37 |
| 25.1–45 | 0.67 | 0.90 | 6.36 | 3.63 |
| Overall | 1.04 | 0.86 | 7.31 | 5.72 |
On average, the iButton® −7.26 (95% CI [-6.93, −7.59]) and FLIR laser thermometer −5.80 (95% CI [-5.47, −6.13]) recorded temperatures well below the IR digital thermometer tympanic measurement. While the IR digital thermometer used on the forehead −0.98 (95% CI [-0.65, −1.31]) and thermal imaging camera −0.84 (95% CI [-0.52, −1.18]) recorded temperatures within a degree of the IR digital tympanic thermometer measurement, on average. Homogenous subset testing (alpha = .05) revealed the only two pieces of equipment to perform similarly were the IR digital thermometer on the forehead (M = 35.92 °C) and thermal imaging camera (M = 36.05 °C). The IR digital thermometer used on the tympanic membrane (M = 36.90 °C), FLIR laser thermometer (M = 31.10 °C), and iButton® (M = 29.64 °C) were all in their own subset.
4. Discussion
The implementation of temperature measurements to detect febrile individuals before entering a public space or private workplace has only been researched in a limited variety of workplaces in the field (Aggarwal et al., 2020; Aragón-Vargas, 2020; Khaksari et al., 2021; Perpetuini et al., 2021). This research used two experiments to test four temperature measurement devices, where participants were exposed to both a simulated environmental exposure (temperature range: 20 to 20 °C) and actual outdoor environmental exposure (temperature range: 0.48 to 45.6 °C). Normal axillary temperatures measured within a room temperature environment (~21 °C) are typically 35.97 °C; on average approximately a degree lower than rectal (37.04 °C) and tympanic (36.64 °C) temperatures (Geneva et al., 2019), with rectal temperature being considered the more accurate measurement of core body temperature (Casa et al., 2007; Parsons, 2002; Yeoh et al., 2017).
At outdoor temperatures of −15 to −20 °C it was found that it can take approximately 5–9 min before the skin surface can register a temperature measurement on all devices. At these temperatures, the forehead temperature detection facial recognition terminal and the IR digital forehead thermometer took the longest to register a measurement (i.e. these devices did not work right away). When the exposure temperature was 15 °C and 20 °C, all devices registered a forehead surface temperature measurement immediately after the participant exited the environmental chamber. When using controlled environmental temperatures, the devices registered mean forehead temperatures from 28.09 °C (IR laser thermometer) to 36.04 °C (thermal imaging camera) on the first measurement when the participants exited the chamber (Table 2). When the participants came from an indoor environment (~21 °C) to be screened in an outdoor environment (−0.48 to 45.6 °C), the temperature detection equipment performed similarly, with mean forehead temperatures from 29.64 °C (iButton®) to 36.05 °C (thermal imaging camera). This finding should be immediate cause for concern as the outdoor temperatures tested are typical in Northern countries like Canada and demonstrate an approximate 9 °C underestimation of the forehead skin temperature, with a commonly used screening mechanism (IR laser thermometer).
Generally, RMSE was lowest for all equipment in the 20.1–25 °C range, which can be attributed to skin surface temperature measurements remaining the same as the change from room temperature (~21 °C) to this outdoor temperature range is small. The IR digital thermometer tympanic measurement was the most consistent and had the lowest RMSE regardless of the environmental temperature, as this site is the least sensitive to ambient disturbances (Rubia-Rubia et al., 2011; Yeoh et al., 2017). The IR tympanic membrane had a mean(±SD) measurement of 36.78 (±0.19)°C in the simulated environment experiment and 36.9 (±0.16)°C in the actual environment experiment. These results are comparable to tympanic membrane measurements after a brief 5 min period of exercise 37.0 °C (Yeoh et al., 2017), a convenience sample of 2006 hospital patients 36.4 (±0.6)°C (Sund Levander and Grodzinsky, 2017), and 659 randomly selected citizens after sitting in a measurement room for 5 min 36.91 (±0.26)°C (H.-Y. Chen et al., 2020). Although the tympanic measurement was the most consistent in the context of workplace screening protocols during a pandemic, its practicality is limited by the need for equipment sanitization in between every use and the requirement for close contact.
In two temperature screening experiments involving 782 participants sitting at room temperature for 5 min before the screening, it was found that the difference between the tympanic temperature and the forehead temperature ranges from 2.0 to 2.2 °C (H.-Y. Chen et al., 2020). Therefore it has been suggested that a forehead temperature greater than 36.0 °C should be used as an indication of possible fever. It is important to note that these authors did not consider the temperature from which their participants had entered the screening protocol. As our experiments involved environmental temperature manipulation and not strictly ambient room temperature, the difference between the tympanic temperature and forehead temperatures can be greater than 2 °C, depending on the device. The mean differences between the tympanic and forehead temperatures in the simulated environment experiment at 20 °C were: IR digital contact probe (forehead) (0.9 °C), thermal imaging camera (0.9 °C), iButton® (5.6 °C), FLIR laser (0.61 m) (4.4 °C), FLIR laser (1.22 m) (4.5 °C), and FLIR laser (1.83 m) (4.8 °C). Additionally, the actual environment experiment (at 19-23 °C) found mean differences between the tympanic temperature and IR digital contact probe (forehead) (0.35 °C), thermal imaging camera (0.55 °C), FLIR laser (0.61 m) (4.6 °C) and iButton® (7.55 °C). Therefore, if using an IR laser thermometer for forehead skin surface temperature, it should be tested against a device with a known degree of accuracy, such as a tympanic thermometer, to determine the threshold value for fever indication. The iButton® that was used in this study has previously been determined to accurately measure body temperature in laboratory and clinical settings (Hasselberg et al., 2013). However, our results determined the iButton® experienced the greatest variation in forehead temperature measurement (mean (±SD)) with simulated environmental exposures (21.9 (±7.6)°C) and actual environmental exposures (29.6 (±1.9) °C). The iButton® is very easy to use in a laboratory setting but is not suggested for use in workplace temperature screening with multiple participants. Its purpose in this study was to remain on the participant and measure forehead temperature throughout the experiments.
Traditionally, IR forehead surface probes have been used and experimentally tested in hospitals or clinical settings. A systematic review advised health care professionals to approach the use of IR forehead thermometry in adults with caution as the accuracy and precision of the readings can exceed recommended levels by 0.3 °C (Kiekkas et al., 2016). In a study designed to specifically test an IR forehead surface probe (Sensortouch thermometer), forehead surface temperatures were found to be 36.3 °C (at 10 °C) and increased to 36.9 °C after 5 min in the chamber (at 30 °C) (Kistemaker et al., 2006). Both of our experiments varied the environmental temperature to a greater degree, and the IR forehead surface probe had mean (±SD) temperatures of 34.98 (±1.31)°C and 35.92 (±0.42)°C, suggesting increased environmental temperature variation can cause more significant variation of the IR forehead surface probe. In an outdoor exercise in the heat experiment, forehead temperature has previously been found to depend on the setting in which it was measured (Casa et al., 2007). Previous protocols for IR forehead surface probe suggest incorporating a 10 min waiting period to allow for acclimatization of the body to thermal equilibrium (Kistemaker et al., 2006). Our results would also suggest using an acclimatization period for the skin, depending on the environmental exposure the worker is coming from before using devices for forehead surface measurement.
Infrared laser forehead temperature measurements are very appealing for workplace screening protocols as they are non-invasive for the workers (Foster et al., 2021) and do not require direct contact with the worker, eliminating the need for individual sanitization equipment. This study found that with this particular IR laser (FLIR TG54), there were no differences between measurements taken from different distances (0.61, 1.22, 1.83 m) as the ICCs indicated excellent-to good reliability between measures. This insinuates that the IR laser can be used at a distance of 2 m from a worker to maintain physical distancing requirements. However, in this study, the IR laser underestimated the forehead skin surface temperature in the simulated (28.09 (±3.04)°C) and actual (31.10 (±1.63)°C) environmental experiments. To address the temperature underestimation of IR laser thermometers on forehead skin measurements, evidence suggests that an indoor acclimatization period is still required when exposed to ambient temperatures to improve the accuracy of the measurement (H.-Y. Chen et al., 2020; Dell'Isola et al., 2021). Knowing that the IR laser thermometers also underestimate skin temperatures when exposed to varying outdoor conditions (Fig. 6), it can be hypothesized that in order to provide a more reliable estimation of the temperature and increase effectiveness in fighting the COVID-19 pandemic spread, a correction factor or uncertainty budget (Dell'Isola et al., 2021) needs to be applied to the registered temperature. Additionally, these findings support consideration for adding a secondary screening protocol, whereby if a high temperature is detected on an IR laser, a follow-up measure should be taken on a tympanic thermometer (Dell'Isola et al., 2021).
The lowest RMSE between the tympanic and forehead skin temperature values were calculated for the thermal imaging camera (RMSE = 0.81–0.97 °C), and a similar thermal detection unit to the thermal imaging camera was found to have an RMSE of 0.439 °C (Lin et al., 2019). These findings suggest that IR thermal imaging cameras are the most reliable of these devices for fever screening (Howell et al., 2020). The increased reliability of the thermal imaging cameras is due to the fact that they measure the entire face and are not limited to single spot emissive irregularities from temperature nonuniformity on the face (Dell'Isola et al., 2021). However, IR thermal imaging cameras are commonly misused (Mercer and Ring, 2009). For instance, the International Standards Organization (IEC-80601-2-59, 2017) has specifically outlined that these devices should simultaneously conduct single measurements and multiple measurements. Much like any other form of medical thermography (i.e. used for breast cancer detection, diagnosis of diabetic neuropathy and vascular disorders, and dental and dermatologic applications (Lahiri et al., 2012), strict protocols are critical to reliability when using IR thermography for temperature screening purposes (Mercer and Ring, 2009). The thermal imaging camera proved to be an effective means of taking reliable forehead temperature measurements but requires participant acclimatization to the environment and consideration for the environmental temperature to which the device itself is exposed. The thermal imaging camera does not record a forehead surface temperature when the exposure temperature is 10 °C or lower.
This study had limited participants due to the Covid-19 pandemic and health and safety protocols deployed by the Canadian Government and Universities to prevent the spread of Covid-19. The Canadian workforce is diverse in age, natural pigmentation of the skin due to genetics and exposure to the sun, and as such, the sample is not representative. Nevertheless, the results of this study using only three participants are in line with previous experiments conducted with between 600 and 1500 participants (G. Chen et al., 2020; Weng Seng Fong et al., 2020). It is also important to acknowledge that the data collection was conducted in August and September, but outdoor environmental temperatures well below zero are typical in Canada in the winter months, as are wind chill and air velocity, which were not collected in this study. Future studies should also address the effects of implementing an acclimatization period before using any of these devices to determine the period length based on environmental exposure to increase the accuracy of the temperature measurements. Further, the difference between skin surface temperature measurements and core body temperatures must be rigorously studied to provide an appropriate temperature threshold.
5. Conclusion
For people infected with the SARS-CoV-2 virus, up to 98% of hospitalized (i.e. severely symptomatic) patients suffered from a fever at some point in the early stages of the infection (Huang et al., 2020). The present study demonstrates the reliability and consistency of four commonly used workplace temperature screening devices in simulated and actual environmental conditions. The tympanic temperature was the most consistent regardless of the environmental temperature (Weng Seng Fong et al., 2020). The thermal imaging camera provided reliable forehead temperature measurements. It was also found that despite good consistency, some devices provide underestimated temperature values. As expected, the findings also suggest that workplace temperature screening should not be conducted outdoors at temperatures below 20 °C (Foster et al., 2021). When outdoor temperatures are below 20 °C, workplace temperature screening protocols must allow for an indoor acclimatization period: ranging from 2 to 9 min depending on the outdoor temperature. This research is an essential step towards broadening the research into the practical application of infrared screening devices in varying environmental contexts.
Funding
This work was supported by the Centre for Research in Occupational Safety and Health [grant number SG 2020S, 2020].
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We want to express our gratitude to Sara N. Gauthier for her assistance with data collection and the development of knowledge transfer materials through her internship with the Centre for Research in Occupational Safety and Health.
References
- Aggarwal N., Gargantuas M., Dwarakanathan V., Gautam N., Kumar S.S., Jadon R.S., Ray A. Diagnostic accuracy of non-contact infrared thermometers and thermal scanners: a systematic review and meta-analysis. J. Trav. Med. 2020;27(8):1–17. doi: 10.1093/jtm/taaa193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragón-Vargas L.F. Limitations of temporal (forehead) temperature readings as a screening method for covid-19. Pensar en Movimiento: Revista de Ciencias del Ejercicio y la Salud. 2020;18(1):1–10. doi: 10.15517/pensarmov.v18i1.42241. [DOI] [Google Scholar]
- Bijur P.E., Shah P.D., Esses D. Temperature measurement in the adult emergency department: oral, tympanic membrane and temporal artery temperatures versus rectal temperature. Emerg. Med. J. 2016;33:843–847. doi: 10.1136/emermed-2015-205122. [DOI] [PubMed] [Google Scholar]
- Casa D.J., Becker S.M., Ganio M.S., Brown C.M., Yeargin S.W., Roti M.W., Maresh C.M. Validity of devices that assess body temperature during outdoor exercise in the heat. J. Athl. Train. 2007;42(3):333–342. [PMC free article] [PubMed] [Google Scholar]
- Chen G., Xie J., Dai G., Zheng P., Hu X., Lu H., Chen X. Validity of the use of wrist and forehead temperature in screening the general population for COVID-19: a prospective real-world study. Iran. J. Public Health. 2020;49(1):57–66. doi: 10.18502/ijph.v49iS1.3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H.-Y., Chen A., Chen C. Investigation of the impact of infrared sensors on core body temperature monitoring by comparing measurement sites. Sensors. 2020;20(2885):1–17. doi: 10.3390/s20102885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiappini E., Sollai S., Longhi R., Morandini L., Laghi A., Osio C.E., de Martino M. Performance of non-contact infrared thermometer for detecting febrile children in hospital and ambulatory settings. J. Clin. Nurs. 2011;20(9–10):1311–1318. doi: 10.1111/j.1365-2702.2010.03565.x. [DOI] [PubMed] [Google Scholar]
- Cho K.S., Yoon J. Fever screening and detection of febrile arrivals at an international airport in Korea: association among self-reported fever, infrared thermal camera scanning, and tympanic temperature. Epidemiology and Health. 2014;36:1–6. doi: 10.4178/epih/e2014004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'Isola G.B., Cosentini E., Canale L., Ficco G., Dell'Isola M. Noncontact body temperature measurement: uncertainty evaluation and screening decision rule to prevent the spread of COVID-19. Sensors. 2021;21(346):1–20. doi: 10.3390/s21020346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzarr A.A., Kamal M., Baba A.A. A comparison between infrared tympanic thermometry, oral and axilla with rectal thermometry in neutropenic adults. Eur. J. Oncol. Nurs. 2009;13:250–254. doi: 10.1016/j.ejon.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Fletcher T., Whittam A., Simpson R., Machin G. Comparison of non-contact infrared skin thermometers. J. Med. Eng. Technol. 2018;42(2) doi: 10.1080/03091902.2017.1409818. [DOI] [PubMed] [Google Scholar]
- Foster J., Lloyd A.B., Havenith G. Non-contact infrared assessment of human body temperature: the journal Temperature toolbox. Temperature. 2021:1–14. doi: 10.1080/23328940.2021.1899546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganio M.S., Brown C.M., Casa D.J., Becker S.M., Yeargin S.W., McDermott B.P., Maresh C.M. Validity and reliability of devices that assess body temperature during indoor exercise in the heat. J. Athl. Train. 2009;44(2):124–135. doi: 10.4085/1062-6050-44.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geneva I.I., Cuzzo B., Fazili T., Javaid W. Normal body temperature: a systematic review. Open Forum Infectious Diseases. 2019;6(4):1–7. doi: 10.1093/ofid/ofz032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghassemi P., Prefer T.J., Casamento J.P., Simpson R., Wang Q. Best practices for standardized performance testing of infrared thermographs intended for fever screening. PloS One. 2018;13(9):1–24. doi: 10.1371/journal.pone.0203302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselberg M.J., McMahon J., Parker K. The validity, reliability, and utility of the iButton for measurement of body temperature circadian rhythms in sleep/wake research. Sleep Med. 2013;14:5–11. doi: 10.1016/j.sleep.2010.12.011. [DOI] [PubMed] [Google Scholar]
- Howell K.J., Mercer J.B., Smith R.E. Infrared thermography for mass fever screening: repeating the mistakes of the past? Thermology International. 2020;30(1):5–6. [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huggins R., Glaviano N., Negishi N., Casa D.J., Hertel J. Comparison of rectal and aural core body temperature thermometry in hyperthermic exercising individuals: a meta-analysis. J. Athl. Train. 2012;47(3):329–338. doi: 10.4085/1062-6050-47.3.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IEC-80601-2-59 . 2017. Medical Electrical Equipment - Part 2-59: Particular Requirement for the Basic Safety and Essential Performance of Screening Thermographs for Human Febrile Temperature Screening. [Google Scholar]
- Khaksari K., Nguyen T., Hill B., Quang T., Perreault J., Gorti V., Gandkbakhche A.H. Review of the efficacy of infrared thermography for screening infectious diseases with application to COVID-19. J. Med. Imag. 2021;8:1–15. doi: 10.1117/1.JMI.8.S1.010901. 010901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiekkas P., Stefanopoulos N., Bakalis N., Kefaliakos A., Karanikolas M. Agreement of infrared temporal artery thermometry with other thermometry methods in adults: systematic review. J. Clin. Nurs. 2016;25:894–905. doi: 10.1111/jocn.13117. [DOI] [PubMed] [Google Scholar]
- Kistemaker J.A., Den Hartog E.A., Daanen H.A.M. Reliability of an infrared forehead skin thermometer for core temperature measurements. J. Med. Eng. Technol. 2006;30(4):252–261. doi: 10.1080/03091900600711381. [DOI] [PubMed] [Google Scholar]
- Kocoglu H., Goksu S., Isik M., Akturk Z., Bayazit Y.A. Infrared tympanic thermometer can accurately measure the body temperature in children in an emergency room setting. Int. J. Pediatr. Otorhinolaryngol. 2002;65(1):39–43. doi: 10.1016/s0165-5876(02)00129-5. [DOI] [PubMed] [Google Scholar]
- Koo T., Li M. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. Journal of Chiropractic Medicine. 2016;15:155–163. doi: 10.1016/j.jcm.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahiri B.B., Bagavathiappan S., Jayakumar T., Philip J. Medical applications of infrared thermography: a review. Infrared Phys. Technol. 2012;55:221–235. doi: 10.1016/j.infrared.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landers R. Computing intraclass correlations (ICC) as estimates of interrupter reliability in SPSS. The Winnower. 2015 doi: 10.15200/winn.143518.81744. [DOI] [Google Scholar]
- Lin J.-W., Lu M.-H., Lin Y.-H. IEEE/CVF International Conference on Computer Vision Workshop (ICCVW) 2019. A thermal camera based continuous body temperature measurement system. Seoul, Korea (South), Korea (South) [Google Scholar]
- McBride W.J.H., Buikstra E. Investigation of febrile passengers detected by infrared thermal scanning at an international airport. Aust. N. Z. J. Publ. Health. 2010;34(1) doi: 10.1111/j.1753-6405.2010.00466x. [DOI] [PubMed] [Google Scholar]
- McCarthy P.W., Heusch A.I. The vagaries of ear temperature assessment. J. Med. Eng. Technol. 2006;30(4):242–251. doi: 10.1080/03091900600711415. [DOI] [PubMed] [Google Scholar]
- Mekjavic I.B., Sundberg C.J., Linnarsson D. Core temperature "null zone". J. Appl. Physiol. 1991;71(4):1289–1295. doi: 10.1152/jappl.1991.71.4.1289. [DOI] [PubMed] [Google Scholar]
- Mercer J.B., Ring E.F.J. Fever screening and infrared thermal imaging: concerns and guidelines. Thermology International. 2009;19(3):67–69. [Google Scholar]
- Ng E.Y.K., Acharya U.R. Remote-sensing infrared thermography. IEEE Eng. Med. Biol. Mag. 2009;28(1):76–83. doi: 10.1109/MEMB/2008.931018. [DOI] [PubMed] [Google Scholar]
- Ng E.Y.K., Kaw G.J.L., Chang W.M. Analysis of IR thermal imager for mass blind fever screening. Microvasc. Res. 2004;68(2):104–109. doi: 10.1016/j.mvr.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Nguyen A.V., Cohen N.J., Lipman H., Brown C.M., Molinari N.-A., Jackson W.L., Fishbein D.B. Comparison of 3 infrared thermal detection systems and self-report for mass fever screening. Emerg. Infect. Dis. 2010;16(11):1710–1717. doi: 10.3201/eid1611.100703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiua H., Kamiya K. Fever screening during the influenza (H1N1-2009) pandemic at narita international airport, Japan. Infectious Diseases. 2011;11(111):1–11. doi: 10.1186/1471-2334-11-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O.Reg.364/20 . 2020. Rules for Areas in Stage 3 (Reopening Ontario (A Flexible Response to COVID-19) Act. Issue. [Google Scholar]
- Oguz F., Yildiz I., Varkal M.A., Hizli Z., Toprak S., Kaymakci K., Unuvar E. Axillary and tympanic temperature measurement in children and normal values for ages. Pediatr. Emerg. Care. 2018;34(3):169–173. doi: 10.1097/PEC.0000000000000693. [DOI] [PubMed] [Google Scholar]
- Ontario-Government . Government of Ontario; Ontario: 2020. COVID-19 Reference Document for Symptoms. [Google Scholar]
- Ontario-Government . Government of Ontario; Ontario: 2020. COVID-19 Screening Tool for Workplaces (Businesses and Organizations) [Google Scholar]
- Parsons K.C. third ed. Taylor & Francis Group; 2002. Human Thermal Environments: the Effects of Hot, Moderate, and Cold Environments on Human Health, Comfort and Performance. [Google Scholar]
- Pascoe D.D., Fisher G. Comparison of measuring sites for the assessment of body temperature. Thermology international. 2009;19(1):35–42. [Google Scholar]
- Perpetuini D., Filippini C., Cardone D., Merla A. An overview of thermal infrared imaging-based screenings during pandemic emergencies. Int. J. Environ. Res. Publ. Health. 2021;18(3286):1–12. doi: 10.3390/ijerph18063286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priest P.C., Duncan A.R., Jennings L.C., Baker M.G. Thermal image scanning for influenza border screening: results of an airport screening study. PloS One. 2011;6(1):1–7. doi: 10.1371/journal.pone.0014490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia-Rubia J., Arias A., Sierra A., Aguirre-Jaime A. Measurement of body temperature in adult patients: comparative study of accuracy, reliability and validity of different devices. Int. J. Nurs. Stud. 2011;48:872–880. doi: 10.1016/j.ijnurstu.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Sund Levander M., Grodzinsky E. Variation in normal ear temperature. Am. J. Med. Sci. 2017;354(4):370–378. doi: 10.1016/j.amjms.2017.05.013. [DOI] [PubMed] [Google Scholar]
- Teran C.G., Torrez-Llanos J., Teran-Miranda T.E., Balderrama C., Shah N.S., Villarroel P. Clinical accuracy of a non-contact infrared skin thermometer in a paediatric practice. Child Care Health Dev. 2012;38(4):471–476. doi: 10.1111/j.1365-2214.2011.01264.x. [DOI] [PubMed] [Google Scholar]
- Trevethan R. Sensitivity, specificity, and predictive values: foundations, pliabilities, and pitfalls in research and practice. Frontiers in Public Health. 2017;5(307):1–7. doi: 10.3389/fpubh.2017.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng Seng Fong W., Khiag Yeo S., Man Chung Fook-Chong S., Kie Phang J., Sim E. Comparison of temperature readings using infrared thermometers at three different sites tympanic, forehead and temporal. Proceedings of Singapore Healthcare. 2020:1–3. doi: 10.1177/2010105820935932. [DOI] [Google Scholar]
- Yeoh W.K., Lee J.K.W., Lim H.Y., Gan C.W., Liang W., Tan K.K. Re-visiting the tympanic membrane vicinity as core body temperature measurement site. PloS One. 2017;12(4):1–21. doi: 10.1371/journal.pone.0174120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Ghassemi P., Chen M., McBride D., Casamento J.P., Pfefer T.J., Wang Q. Clinical evaluation of fever-screening thermography: impact of consensus guidelines and facial measurement location. J. Biomed. Opt. 2020;25(9):1–21. doi: 10.1117/1.JBO.25.9.097002. [DOI] [PMC free article] [PubMed] [Google Scholar]