Abstract
We consider the relationship between stationary distributions for stochastic models of reaction systems and Lyapunov functions for their deterministic counterparts. Specifically, we derive the well known Lyapunov function of reaction network theory as a scaling limit of the non-equilibrium potential of the stationary distribution of stochastically modeled complex balanced systems. We extend this result to general birth-death models and demonstrate via example that similar scaling limits can yield Lyapunov functions even for models that are not complex or detailed balanced, and may even have multiple equilibria.
1. Introduction
Reaction network models are ubiquitous in the study of various types of population dynamics in biology. For example, they are used in modeling subcellular processes in molecular biology [7, 13, 24, 40], signaling systems [42, 43], metabolism [12], as well as the spread of infectious diseases [1] and interactions between species in an ecosystem [35, 41]. Depending upon the relevant scales of the system, either a deterministic or stochastic model of the dynamics is utilized.
This paper studies the connection between deterministic and stochastic models of reaction systems. In particular, for the class of so-called “complex balanced” models, we make a connection between the stationary distribution of the stochastic model and the classical Lyapunov function used in the study of the corresponding deterministic models. Specifically, we show that in the large volume limit of Kurtz [31, 32], the non-equilibrium potential of the stationary distribution of the scaled stochastic model converges to the standard Lyapunov function of deterministic reaction network theory. Further, we extend this result to birth-death processes.
In 1972, Horn and Jackson [28] introduced a Lyapunov function for the study of complex balanced systems, and remarked on a formal similarity to Helmholtz free energy functions. Since then the probabilistic interpretation of this Lyapunov function for complex balanced systems has remained obscure. For detailed balanced systems, which form a subclass of complex balanced systems, a probabilistic interpretation for the Lyapunov function is known — see, for example, the work of Peter Whittle [44, Section 5.8] — though these arguments appear to be little known in the mathematical biology community. The key ingredient that enables us to extend the analysis pertaining to detailed balanced systems to complex balanced systems comes from [4], where Anderson, Craciun, and Kurtz showed that the stationary distribution for the class of complex balanced reaction networks can be represented as a product of Poisson random variables; see equation (1) below.
While there are myriad results pertaining to either stochastic or deterministic models, there are relatively few making a connection between the two. Perhaps the best known such connections come from the seminal work of Thomas Kurtz [31, 32, 33], which details the limiting behavior of classically scaled stochastic models on finite time intervals, and demonstrates the validity of the usual deterministic ODE models on those intervals. There is even less work on the connection between the deterministic and stochastic models on infinite time horizons, that is, on the long term behavior of the different models, though two exceptions stand out. As alluded to above, Anderson, Craciun, and Kurtz showed that a stochastically modeled complex balanced system — for which the deterministically modeled system has complex balanced equilibrium1 c — has a stationary distribution of product form,
| (1) |
where Γ is the state space of the stochastic model and ZΓ > 0 is a normalizing constant [4]. On the other hand, in [5], Anderson, Enciso, and Johnston provided a large class of networks for which the limiting behaviors of the stochastic and deterministic models are fundamentally different, in that the deterministic model has special “absolutely robust” equilibria whereas the stochastic model necessarily undergoes an extinction event.
In the present paper, we return to the context of complex balanced models studied in [4], and show that the usual Lyapunov function of Chemical Reaction Network Theory (CRNT),
| (2) |
can be understood as the limit of the non-equilibrium potential of the distribution (1) in the classical scaling of Kurtz. We extend this result to the class of birth-death models. We then demonstrate through examples that Lyapunov functions for an even wider class of models can be constructed through a similar scaling of stationary distributions. It is not yet clear just how wide the class of models for which this specific scaling limit provides a Lyapunov function is, and we leave this question open. Similar (non-mathematically rigorous) results have been pointed out in the physics literature though the generality of these results remain unclear [39]. See also [25] for recent mathematical work pertaining to the ergodicity of stochastically modeled reaction systems and [37] for earlier related work pertaining to the irreducibility and recurrence properties of stochastic models.
Before proceeding, we provide a key definition.
Definition 1.
Let π be a probability distribution on a countable set Γ such that π(x) > 0 for all x ∈ Γ. The non-equilibrium potential of the distribution π is the function defined by
We close the introduction with an illustrative example.
Example 2. Consider the catalytic activation-inactivation network
| (3) |
where A and B represent the active and inactive forms of a protein, respectively. The usual deterministic mass-action kinetics model for the concentrations (xA, xB) of the species A and B is
where κ1 and κ2 are the corresponding reaction rate constants for the forward and reverse reactions in (3). For a given total concentration , these equations have a unique stable equilibrium
| (4) |
which can be shown to be complex balanced.
We now turn to a stochastic model for the network depicted in (3), that tracks the molecular counts for species A and B. Letting V be a scaling parameter, which can be thought of as Avogadro’s number multiplied by volume, see section 2.3.1, the standard stochastic mass-action kinetics model can be described in several different ways. For example, the Kolmogorov forward equations governing the probability distribution of the process are
| (5) |
where are the molecular counts of A and B, respectively, and pμ(xA, xB, t) denotes the probability that the system is in state (xA, xB) at time t given an initial distribution of μ. Note that there is one such differential equation for each state, (xA, xB), in the state space. In the biological context the forward equation is typically referred to as the chemical master equation.
Assume that the initial distribution for the stochastic model has support on the set , where M > 0 is fixed and V is selected so that VM is an integer. Hence, the total number of molecules is taken to scale in V. The stationary distribution can then be found by setting the left hand side of the forward equation (5) to zero and solving the resulting system of equations (one equation for each (xA, xB) ∈ ΓV). Finding such a solution is typically a challenging, or even impossible task. However, results in [4] imply that for this particular system the stationary distribution is (almost) a binomial distribution and is of the form (1),
| (6) |
where ZV is the normalizing constant
The distribution is not binomial since the state (xA, xB) = (0, VM) cannot be realized in the system.
In order to make a connection between the stochastic and deterministic models, we convert the stochastic model to concentrations by dividing by V. That is, for we let . Letting denote the stationary distribution of the scaled process, we find that
where . We now consider the non-equilibrium potential of scaled by V
Stirling’s formula says that
| (7) |
Assuming that , and after some calculations, equation (7) yields
Recalling that , we may rewrite in the following useful way
Remarkably, this is exactly the function we would obtain if we were to write the standard Lyapunov function of CRNT, given in (2), for this model. □
The first goal of this paper is to show that the equality between the scaling limit calculated for the stochastic model above, and the Lyapunov function for the corresponding deterministic model is not an accident, but in fact holds for all complex balanced systems. We will also demonstrate that the correspondence holds for a wider class of models.
The remainder of this article is organized as follows. In Section 2, we briefly review some relevant terminology and results. In Section 3, we derive the general Lyapunov function of Chemical Reaction Network Theory for complex balanced systems as a scaling limit of the non-equilibrium potential of the corresponding scaled stochastic model. In Section 4, we discuss other, non-complex balanced, models for which the same scaling limit gives a Lyapunov function for the deterministic model. In particular, we characterize this function when the corresponding stochastic system is equivalent to a stochastic birth-death process.
2. Reaction systems and previous results
2.1. Reaction networks
We consider a system consisting of d species, {S1, …, Sd}, undergoing transitions due to a finite number, m, of reactions. For the kth reaction, we denote by νk, the vectors representing the number of molecules of each species consumed and created in one instance of the reaction, respectively. For example, for the reaction S1 +S2 → S3, we have νk = (1, 1, 0)T and , if there are d = 3 species in the system. Each νk and is termed a complex of the system. The reaction is denoted by , where νk is termed the source complex and is the product complex. A complex may appear as both a source complex and a product complex in the system.
Definition 3.
Let , , and denote the sets of species, complexes, and reactions, respectively. The triple is a reaction network.
Definition 4.
The linear subspace is called the stoichiometric subspace of the network. For we say is a stoichiometric compatibility class, is a non-negative stoichiometric compatibility class, and is a positive stoichiometric compatibility class.
2.2. Dynamical system models
2.2.1. Stochastic models
The most common stochastic model for a reaction network treats the system as a continuous time Markov chain whose state X is a vector giving the number of molecules of each species present with each reaction modeled as a possible transition for the chain. The model for the kth reaction is determined by the source and product complexes of the reaction, and a function λk of the state that gives the transition intensity, or rate, at which the reaction occurs. In the biological and chemical literature, transition intensities are referred to as propensities.
Specifically, if the kth reaction occurs at time t the state is updated by addition of the reaction vector and
The most common choice for intensity functions is to assume the system satisfies the stochastic version of mass-action kinetics, which states that the rate functions take the form
| (8) |
for some constant κk > 0, termed the rate constant, and where νk = (νk1, …, νkd)T. Under the assumption of mass-action kinetics and a non-negative initial condition, it follows that the dynamics of the system is confined to a particular non-negative stoichiometric compatibility class given by the initial value X(0), namely .
The number of times that the kth reaction occurs by time t can be represented by the counting process
where the {Yk, k ∈ {1, …, m}} are independent unit-rate Poisson processes (see [6, 7, 34], or [15, Chapter 6]]). The state of the system then satisfies the equation , or
| (9) |
where the sum is over the reaction channels. Kolmogorov’s forward equation for this model is
| (10) |
where Pμ(x, t) represents the probability that given an initial distribution of μ and λk(x−ζk) = 0 if . So long as the process is non-explosive, the two representations for the processes, the stochastic equation (9) and the Markov process with forward equation (10), are equivalent [6, 15].
It is of interest to characterize the long-term behavior of the process. Let be a closed component of the state space; that is, Γ is closed under the transitions of the Markov chain. A probability distribution π(x), x ∈ Γ, is a stationary distribution for the chain on Γ if
| (11) |
for all x ∈ Γ. (If x − ζk ∉ Γ then π(x − ζk) is put to zero.) If in addition Γ is irreducible, that is, any state in Γ can be reached from any other state in Γ (for example, ΓV in Example 2 is an irreducible component) and π exists, then π is unique [30].
Solving equation (11) is in general a difficult task, even when we assume each λk is determined by mass-action kinetics. However, if in addition there exists a complex balanced equilibrium for the associated deterministic model, then equation (11) can be solved explicitly, see Theorem 6 below.
2.2.2. Deterministic models and complex balanced equilibria
For two vectors we define and adopt the convention that 00 = 1.
Under an appropriate scaling limit (see Section 2.3.1) the continuous time Markov chain model described in the previous section becomes
| (12) |
where
| (13) |
and κk > 0 is a constant. We say that the deterministic system (12) has deterministic mass-action kinetics if the rate functions fk have the form (13). The system (12) is equivalent to the system of ordinary differential equations (ODEs) with a given initial condition x0 = x(0),
| (14) |
The trajectory with initial condition x0 is confined to the non-negative stoichiometric compatibility class .
Some mass-action systems have complex balanced equilibria [27, 28],2 which have been shown to play an important role in many biological mechanisms [9, 20, 29, 42]. An equilibrium point is said to be complex balanced if and only if for each complex we have
| (15) |
where the sum on the left is over reactions for which z is the product complex and the sum on the right is over reactions for which z is the source complex. For such an equilibrium the total inflows and the total outflows balance out at each complex [16, 23].
In [28] it is shown that if there exists a complex balanced equilibrium for a given model then
There is one, and only one, positive equilibrium point in each positive stoichiometric compatibility class.
Each such equilibrium point is complex balanced.
Each such complex balanced equilibrium point is locally asymptotically stable relative to its stoichiometric compatibility class.
Whether or not each complex balanced equilibrium is globally asymptotically stable relative to its positive stoichiometric compatibility class is the content of the Global Attractor Conjecture, which has received considerable attention [2, 3, 8, 11, 21, 36]. The local asymptotic stability is concluded by an application of the Lyapunov function (2).
2.2.3. Lyapunov functions
Definition 5.
Let be an open subset of and let . A function is called a (strict) Lyapunov function for the system at x0 ∈ E if x0 is an equilibrium point for f, that is, f(x0) = 0, and
for all x ≠ x0, x ∈ E and V (x0) = 0
, for all x ∈ E, with equality if and only if x = x0, where denotes the gradient of .
If these two conditions are fulfilled then the equilibrium point x0 is asymptotically stable [38]. If the inequality in (2) is not strict for x0 ≠ x then x0 is stable and not necessarily asymptotically stable. If the inequality is reversed, , then the equilibrium point is unstable [38].
We will see that in many cases the large volume limit of the non-equilibrium potential of a stochastically modeled system is a Lyapunov function defined on the interior of the nonnegative stoichiometric subspace.
2.3. Product form stationary distributions
The following result from [4], utilized in (6), provides a characterization of the stationary distributions of complex balanced systems. See also [18, 26] for related work.
Theorem 6.
Let be a reaction network and let {κk} be a choice of rate constants. Suppose that, modeled deterministically, the system is complex balanced with a complex balanced equilibrium . Then the stochastically modeled system with intensities (8) has a stationary distribution on consisting of the product of Poisson distributions,
| (16) |
If is irreducible, then (16) is the unique stationary distribution. If is not irreducible, then the stationary distribution, πΓ, of an irreducible component of the state space is
and πΓ(x) = 0 otherwise, where ZΓ is a positive normalizing constant.
Each irreducible component of the state space is necessarily contained in a single non-negative stoichiometric compatibility class (Definition 4). The choice of the complex balanced equilibrium point c in the theorem is independent of Γ and the particular stoichiometric compatibility class containing it [4]. Since , it follows that
| (17) |
2.3.1. The classical scaling
We may convert from molecular counts to concentrations by scaling the counts by V, where V is the volume of the system times Avogadro’s number. Following [4], define . Let {κk} be a set of rate constants and define the scaled rate constants, , for the stochastic model in the following way,
| (18) |
(see [45, Chapter 6]). Let be an arbitrary state of the system and denote the intensity function for the stochastic model by
Note that gives the concentrations in moles per unit volume and that if (that is, if x = Θ(V)), then by standard arguments
where the final equality determines λk, and justifies the definition of deterministic mass-action kinetics in (13).
Denote the stochastic process determining the abundances by XV(t) (see (9)). Then, normalizing the original process XV by V and defining yields
Since the law of large numbers for the Poisson process implies V−1Y (V u) ≈ u, we may conclude that a good approximation to the process is the function x = x(t) defined as the solution to the ODE
which is (14). For a precise formulation of the above scaling argument, termed the classical scaling, see [7].
The following is an immediate corollary to Theorem 6, and can also be found in [4]. The result rests upon the fact that if c is a complex balanced equilibrium for a given reaction network with rates {κk}, then V c is a complex balanced equilibrium for the reaction network endowed with rates of (18).
Theorem 7.
Let be a reaction network and let {κk} be a choice of rate constants. Suppose that, modeled deterministically, the system is complex balanced with a complex balanced equilibrium . For some V > 0, let be related to {κk} via (18). Then the stochastically modeled system with intensities (8) and rate constants has a stationary distribution on consisting of the product of Poisson distributions,
| (19) |
If is irreducible, then (19) is the unique stationary distribution. If is not irreducible, then the stationary distribution, , of an irreducible component of the state space is
| (20) |
and otherwise, where is a positive normalizing constant.
Note that Theorem 7 implies that a stationary distribution for the scaled model is
| (21) |
3. Complex balanced systems
We are ready to state and prove our first result. For an increasing series of volumes Vi, i = 1, 2, …, we consider converging sequences of points in . To ease the notation we omit the index i and write, for example, instead of .
Theorem 8.
Let be a reaction network and let be a choice of rate constants. Suppose that, modeled deterministically, the system is complex balanced. For V > 0, let be related to {κk} via (18). Fix a sequence of points for which . Further let c be the unique complex balanced equilibrium within the positive stoichiometric compatibility class of .
Let π V be given by (19) and let be as in (21) , then
where satisfies (2) . In particular, is a Lyapunov function (Definition 5).
Further, suppose is an irreducible component of the state space for the Markov model with rate constantssuch that: Let be given by(20). For , define , then
| (22) |
and
| (23) |
where satisfies(2). In particular, is a Lyapunov function (Definition 5).
Proof. We prove the second statement. The proof of the first is the same with the exception that .
We first consider the limit (22). Begin by supposing that there is a sequence for which . In this case,
where the first inequality follows from (17) and the third from an application of Stirling’s formula (C is a constant). Taking the logarithm and dividing by V, it follows that . Thus, the limit (22) will be shown so long as we can prove the existence of the sequence converging to the complex balanced equilibrium c.
For M > 0, define the set . for each i ∈ {1, …, d}}. From the remark below Lemma 4.1 in [37], there is an M0 > 0 so that for all V large enough
| (24) |
Thus, for V large enough, ΓV has constant positive density on its stoichiometric compatibility class. Let be the unique complex balanced equilibrium in the positive stoichiometric compatibility class of . It follows that is the unique complex balanced equilibrium in the positive stoichiometric compatibility class of , from which we may conclude that [10]. Finally, define via the relation
where is a minimizer of over the set . Note that . From (24), we see that , which, when combined with , gives the desired result.
We now turn to (23). We have
Applying Stirling’s formula (7) to the final term and performing some algebra yields
The sum is the usual Lyapunov function , and the result is shown after letting V → ∞, utilizing (22), and recalling that . □
The theorem above can be applied to Example 2. The unique equilibrium point given in (4) is easily seen to fulfil the complex balanced condition in (15).
4. Non-complex balanced systems
4.1. Birth-death processes and reaction networks
In this section we will study reaction networks that also are birth-death processes. Many results are known for birth-death processes. In particular, a characterization of the stationary distribution can be accomplished [30].
Let be a reaction network with one species only, , and assume all reaction vectors are either ζk = (−1) or ζk = (1). This implies that the number of molecules of S goes up or down by one each time a reaction occurs. For convenience, we re-index the reactions and the reaction rates in the following way. By assumption, a reaction of the form nS → n′S will either have n′ = n + 1 or n′ = n − 1. In the former case we index the reaction by n and denote the rate constant by κn and in the latter case by −n and κ−n, respectively. Note that this stochastically modeled reaction network can be considered as a birth-death process with birth and death rates
| (25) |
for i ≥ 0, respectively.
If the stochastically modeled system has absorbing states (i.e. states for which pi = qi = 0) we make the following modification to the intensity functions of the system. Let be the smallest value such that (i) all birth rates of i0 are non-zero, that is, λn(i0) > 0 for n ≥ 0, and (ii) all death rates of i0 + 1 are non-zero, that is, λn(i0 + 1) > 0 for n < 0. We modify the system by letting λn(i0) = 0 for n < 0. Note that the modified system has a lowest state i0, which is not absorbing.
As an example of the above modification, consider the system with network
| (26) |
This model has rates λ4(x) = κ4x(x − 1)(x − 2)(x − 3) and λ−3(x) = κ−3x(x − 1)(x − 2). The modified system would simply take λ−3(4) = 0.
Let nu (u for ‘up’) be the largest n for which κn is a non-zero reaction rate and similarly let nd (d for ‘down’) be the largest n for which κ−n is a non-zero rate constant. For the network (26), nu = 4 and nd = 3.
Theorem 9.
Let be a reaction network with one species only. Assume that all reaction vectors are of the form ζn = (−1) or ζn = (1), and assume that there is at least one of each form. Let {κn} be a choice of rate constants and assume, for some V > 0, that is related to {κn} via (18). Then a stationary distribution, πV , for the modified birth-death process with rates (25) and rate constants exists on the irreducible component Γ = {i|i ≥ i0} if and only if either of the following holds,
nd > nu, or
nd = nu and ,
in which case such a πVexists for each choice of V > 0.
If either of conditions (1) or (2) holds, and if , where each , then
| (27) |
where is the stationary distribution for the stochastic model scaled by V > 0 and state space (as in (21)), and is a value of (potentially not unique) that maximizes the integral
Further, the function of (27) fulfills condition (2) in Definition 5; that is, decreases along paths of the deterministically modeled system with rate constants {κn}.
Proof. Since all reactions have ζn = (1) or ζn = (−1) it follows that the system is equivalent to a birth-death process with birth and death rates (25). As in the discussion below (25), let i0 be the smallest value the chain may attain. Potentially after modifying the system as detailed above, we have that pi > 0 for all i ≥ i0 and qi > 0 for all i ≥ i0 + 1. Hence, is irreducible and the stationary distribution, if it exists, is given by (see [30])
where the empty product is taken to be equal to 1, and the partition function ZV satisfies
| (28) |
Let δ = nd − nu. Note that for ϵ > 0 arbitrarily small, there exists an m > 0 such that
| (29) |
for all V > 0. Hence,
which is finite if and only if one of the two conditions (1) and (2) in the theorem is fulfilled, in which case it is finite for all V > 0. If δ = 0, one should choose ϵ such that . Since a stationary distribution exists if and only if ZV is finite (see [30]), this concludes the first part of the theorem.
We assume now that the stationary distribution exists, that is, that one of the two conditions (1) and (2) are fulfilled, and consider the non-equilibrium potential. Letting with x ≥ i0, the scaled non-equilibrium potential takes the form
| (30) |
Using the definitions of pi, qi and , the first term in (30) becomes
Noting that this is a Riemann sum approximation, we have for ,
| (31) |
as V → ∞.
We next turn to the second term of (30). First, we consider the infinite series in equation (28). By (29), for ϵ > 0 small enough there is an m > 0 so that if i > mV, then
| (32) |
Let mV = ⎿mV⏌ + 1. Hence, it follows that the tail of the partition function ZV fulfills
| (33) |
Next we bound ZV above, using (33),
| (34) |
with the convention that the empty sum is zero, and where δa(x) is an indicator function that takes the value 1 if x = a, and is zero otherwise. In the last inequality we have used that −δa(x)ln(1 − β) ≤ −ln(1 − β).
Consider the right side of (34). Let xV be the value of x ≤ mV for which the sum attains it maximum. Hence, we have
| (35) |
The sequence has an accumulation point in [0, m + 1] since the interval is compact. Using (31) and mV = ⎿mV⏌ + 1, we obtain from (35)
| (36) |
Note that is a global maximum of the integral on the entire [0, ∞) (though it might not be unique): according to (32), the terms in the inner sum in (34) are negative for x > xV.
To get a lower bound for ZV, we choose a sequence of points , such that as V → ∞. Then, with ,
and consequently,
| (37) |
by arguing as in (31). Combining (36) and (37) yields the desired result that V−1 ln(ZV) → g0 as V → ∞.
Hence, we may conclude that the non-equilibrium potential converges to the function , as stated in the theorem. To conclude the proof, we only need to confirm that g fulfills condition (2) in Definition 5, which we verify by differentiation,
This is strictly negative unless
in which case we are at an equilibrium. □
For this particular class of systems we have
so that the ratio in equation (27) is simply the ratio of the two terms in the equation above. The local minima and maxima of are therefore the equilibrium points of the deterministically modeled system. Further, by inspection, it can be seen that and as . If none of the extrema of are plateaus, then it follows that asymptotically stable and unstable equilibria must alternate and that the largest equilibrium point is asymptotically stable (Definition 5). Around each of the stable equilibria the function is a Lyapunov function.
Example 10. Consider the following network which has three equilibria (for appropriate choice of rate constants), two of which may be stable,
The deterministic model satisfies
We have nu = 2 and nd = 3 such that condition (1) of Theorem 9 is fulfilled. Hence, the non-equilibrium potential converges to the function
| (38) |
The stationary distribution of the stochastically modeled system can be obtained in closed form [19],
where
If P = R, then the distribution is Poisson with parameter B and, in fact, the system is complex balanced. In this case, and the Lyapunov function (38) reduces to
in agreement with Theorem 8.
For a concrete example that is not complex balanced, consider the model with rate constants κ0 = 6, κ−1 = 11, κ2 = 6, κ−3 = 1. In this case
and there are two asymptotically stable equilibria at c = 1, 3 and one unstable at c = 2. Hence, the function is a Lyapunov function locally around , and takes the form
| (39) |
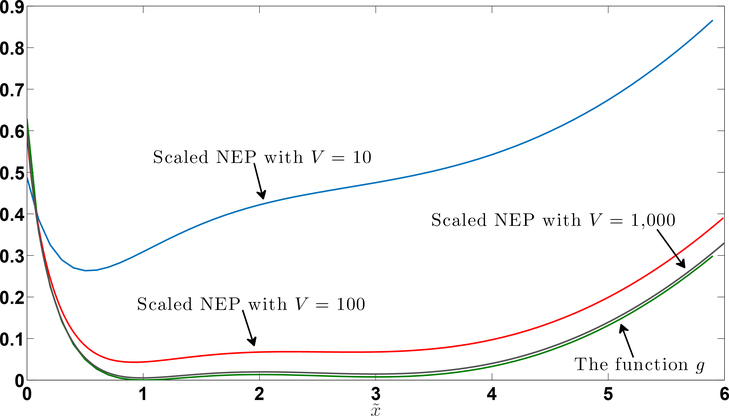
where, for this example, . In Figure 1, we demonstrate the convergence of the scaled non-equilibrium potential, , of the scaled process to of (39).
Figure 1:
Plots of the scaled non-equilibrium potential (NEP), , of the scaled birth-death process of Example 10 are given for V ∈ {10, 102, 103}, as is the function of (39).
Example 11. Consider the reaction network
which is equivalent to a linear birth-death process with absorbing state 0. This model has nu = nu = 1, and so for a stationary distribution to exist the second condition of Theorem 9 must hold. If we put the death rate λ−1(1) to 0 and assume κ−1 > κ1, then condition (2) is fulfilled and
| (40) |
is a Lyapunov function. In fact, the stationary distribution of the modified system is proportional to
which is independent of V. It follows that for ,
in agreement with (40). In this particular case the deterministic system converges to zero – the absorbing state of the stochastic system – though this correspondence will not hold in general for systems with an absorbing state. □
4.2. Other examples
Example 12. Consider the reaction network,
The network is not complex balanced, nor is it a birth-death process, hence the theory developed in the previous sections is not applicable. The stationary distribution with scaled rate constants as in (18) can be given in explicit form [14],
where In(z) is the modified Bessel function of the nth kind. To evaluate the non-equilibrium potential we need two asymptotic results for the modified Bessel functions [22]:
where
and uk(t), k ≥ 1, are functions of t. Note that the sum involving uk(t) decreases proportionally to n−1u1(t) as n gets large (the other terms vanish faster than ).
After some cumbersome calculations using the asymptotic relationships for the modified Bessel function, we obtain that the non-equilibrium potential satisfies
where is defined by
Another straightforward, but likewise cumbersome, calculation, shows that in fact fulfils condition (2) in Definition 5. By differentiation twice with respect to x, we find that , hence is a Lyapunov function. □
Example 13. As a last example consider the reaction network:
It is not weakly reversible, hence not complex balanced for any choice of rate constants. It is not a birth-death process either, as two molecules are created at each “birth” event. It is similar to Example 12, but with the reactions going in the opposite direction.
Let the rate constants {κk} be given and let the scaled rates be given accordingly. The deterministically modeled system takes the form
| (41) |
such that there is a unique equilibrium at . Let so that c = 4a. The stationary distribution exists for all reaction rates and is most easily characterized in the following way (see Supporting Information):
where N1 and N2 are two independent Poisson random variables with intensities 2aV and aV, respectively. Hence, the stationary distribution can be written as
In the Supporting Information it is shown that the limit of the non-equilibrium potential exists as V → ∞ with :
where
(the integral can be solved explicitly, see Supporting Information). The first derivative of g fulfils
and zero if and only if 4a = x. Comparing with (41) yields
and equality only if 4a = x. The second derivative of g is positive for all x. Hence, g(x) is a Lyapunov function.
5. Discussion
We have demonstrated a relationship between the stochastic models for reaction systems and an important Lyapunov function for the corresponding deterministic models. In particular, we showed that this relationship holds for the class of complex balanced systems, which contains the class of detailed balanced systems that have been well studied in both the physics and probability literature [44]. Further, we showed the correspondence holds for a wider class of models including those birth and death systems that can be modeled via reaction systems. It remains open just how wide the class of models satisfying this relationship is.
Supplementary Material
Acknowledgements.
We thank the American Institute of Mathematics for hosting a workshop at which this research was initiated. Anderson was supported by NSF grants DMS-1009275 and DMS-1318832 and Army Research Office grant W911NF-14-1-0401. Craciun was supported by NSF grant DMS1412643 and NIH grant R01GM086881. Wiuf was supported by the Lundbeck Foundation (Denmark), the Carlsberg Foundation (Denmark), Collstrups Fond (Denmark), and the Danish Research Council. Part of this work was carried out while Wiuf visited the Isaac Newton Institute in 2014.
Footnotes
By equilibrium we mean a fixed point of a dynamical system. In particular, what is referred to in the biochemistry literature as a “non-equilibrium steady state” is also included in our use of the term equilibrium.
References
- [1].Allen Linda J.S., An introduction to stochastic processes with applications to biology, Pearson Education; New Jersey, 2003. [Google Scholar]
- [2].Anderson David F., A proof of the Global Attractor Conjecture in the single linkage class case, SIAM J. Appl. Math 71 (2011), no. 4, 1487–1508. [Google Scholar]
- [3].––––, Global asymptotic stability for a class of nonlinear chemical equations, SIAM J. Appl. Math 68 (May 2008), no. 5, 1464–1476. [Google Scholar]
- [4].Anderson David F., Craciun Gheorghe, and Kurtz Thomas G., Product-form stationary distributions for deficiency zero chemical reaction networks, Bull. Math. Biol 72 (2010), no. 8, 1947–1970. [DOI] [PubMed] [Google Scholar]
- [5].Anderson David F., Enciso Germán A., and Johnston Matthew D., Stochastic analysis of biochemical reaction networks with absolute concentration robustness, J. R. Soc. Interface 11 (2014), no. 93, 20130943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Anderson David F. and Kurtz Thomas G., Continuous time Markov chain models for chemical reaction networks, Design and Analysis of Biomolecular Circuits: Engineering Approaches to Systems and Synthetic Biology (Koeppl H et al., ed.), Springer, 2011, pp. 3–42. [Google Scholar]
- [7].––––, Stochastic analysis of biochemical systems, Springer, 2015. [Google Scholar]
- [8].Anderson David F. and Shiu Anne, The dynamics of weakly reversible population processes near facets, SIAM J. Appl. Math. 70 (2010), no. 6, 1840–1858. [Google Scholar]
- [9].Chan Carlo, Liu Xinfeng, Wang Liming, Bardwell Lee, Nie Qing, and Enciso German, Protein scaffolds can enhance the bistability of multisite phosphorylation systems, PLoS computational biology 8 (2012), no. 6, e1002551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Craciun Gheorghe, Dickenstein Alicia, Shiu Anne, and Sturmfels Bernd, Toric dynamical systems, Journal of Symbolic Computation 44 (2009), 1551–1565. [Google Scholar]
- [11].Craciun Gheorghe, Nazarov Fedor, and Pantea Casian, Persistence and permanence of mass-action and power-law dynamical systems, SIAM J. Appl. Math. 73 (2013), no. 1, 305–329. [Google Scholar]
- [12].Duncan Tanya M., Reed Michael C., and Nijhout H. Frederik, A population model of folate-mediated one-carbon metabolism, Nutrients 5 (2013), no. 7, 2457–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Elowitz Michael B., Levin Arnold J., Siggia Eric D., and Swain Peter S., Stochastic gene expression in a single cell, Science 297 (2002), no. 5584, 1183–1186. [DOI] [PubMed] [Google Scholar]
- [14].Engblom Stefan, Spectral approximation of solutions to the chemical master equation, J. Comp. Appl. Math. 229 (2009), 208–221. [Google Scholar]
- [15].Ethier Stewart N. and Kurtz Thomas G., Markov processes: Characterization and convergence, John Wiley & Sons, New York, 1986. [Google Scholar]
- [16].Feinberg Martin, Lectures on chemical reaction networks, Delivered at theMathematics Research Center, Univ. Wisc.-Madison. Available for download at http://crnt.engineering.osu.edu/LecturesOnReactionNetworks, 1979. [Google Scholar]
- [17].––––, Existence and uniqueness of steady states for a class of chemical reaction networks, Arch. Rational Mech. Anal 132 (1995), 311–370. [Google Scholar]
- [18].Gadgil Chetan, Lee Chang Hyeong, and Othmer Hans G., A stochastic analysis of first-order reaction networks, Bull. Math. Bio 67 (2005), 901–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gardiner Crispin W., Handbook of stochastic methods, 2nd edition, Spinger, 1985. [Google Scholar]
- [20].Gnacadja Gilles, Univalent positive polynomial maps and the equilibrium state of chemical networks of reversible binding reactions, Advances in Applied Mathematics 43 (2009), no. 4, 394–414. [Google Scholar]
- [21].Gopalkrishnan Manoj, Miller Ezra, and Shiu Anne, A geometric approach to the global attractor conjecture, SIAM J. Appl. Dyn. Syst 13 (2014), no. 2, 758–797. [Google Scholar]
- [22].Gradshteyn IS and Ryzhik IM, Tables of integrals, series, and products, 7nd edition, Academic Press, 2007. [Google Scholar]
- [23].Gunawardena Jeremy, Chemical reaction network theory for in-silico biologists, Notes available for download at http://vcp.med.harvard.edu/papers/crnt.pdf, 2003. [Google Scholar]
- [24].Gunawardena Jeremy, Multisite protein phosphorylation makes a good threshold but can be a poor switch, PNAS 102 (2005), no. 41, 14617–14622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gupta Ankit and Khammash Mustafa, Determining the long-term behavior of cell populations: A new procedure for detecting ergodicity in large stochastic reaction networks, arXiv:1312.2879, 2013. [Google Scholar]
- [26].Heuett William J. and Qian Hong, Grand canonical Markov model: A stochastic theory for open nonequilibrium biochemical networks, J. Chem. Phys. 124 (2006), 044110. [DOI] [PubMed] [Google Scholar]
- [27].Horn Friedrich J. M., Necessary and sufficient conditions for complex balancing in chemical kinetics, Arch. Rat. Mech. Anal 49 (1972), no. 3, 172–186. [Google Scholar]
- [28].Horn Friedrich J. M. and Jackson Roy, General mass action kinetics, Arch. Rat. Mech. Anal. 47 (1972), 81–116. [Google Scholar]
- [29].Kang Hye-Won, Zheng Likun, and Othmer Hans G, A new method for choosing the computational cell in stochastic reaction–diffusion systems, Journal of mathematical biology 65 (2012), no. 6–7, 1017–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Karlin Samuel and Taylor Howard M., A first course in stochastic processes, 2nd edition, Academic Press, 1975. [Google Scholar]
- [31].Kurtz Thomas G., The relationship between stochastic and deterministic models for chemical reactions, J. Chem. Phys 57 (1972), no. 7, 2976–2978. [Google Scholar]
- [32].––––, Strong approximation theorems for density dependent Markov chains, Stoch. Proc. Appl. 6 (1977/78), 223–240. [Google Scholar]
- [33].––––, Representations of markov processes as multiparameter time changes, Ann. Prob 8 (1980), no. 4, 682–715. [Google Scholar]
- [34].––––, Approximation of population processes, CBMS-NSF Reg. Conf. Series in Appl. Math.: 36, SIAM, 1981. [Google Scholar]
- [35].McCredie May Robert, Stability and complexity in model ecosystems, vol. 6, Princeton University Press, 2001. [Google Scholar]
- [36].Pantea Casian, On the persistence and global stability of mass-action systems, SIAM J. Math. Analy 44 (2012), no. 3, 1636–1673. [Google Scholar]
- [37].Paulevé Loïc, Craciun Gheorghe, and Koeppl Heinz, Dynamical properties of discrete reaction networks, Journal of Mathematical Biology 69 (2014), no. 1, 55–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Perko Lawrence, Differential equations and dynamical systems, 3rd edition, Springer, 2000. [Google Scholar]
- [39].Qian Hong, Nonlinear stochastic dynamics of mesoscopic homogeneous biochemical reaction systems—an analytical theory, Nonlinearity 24 (2011), R19–R49. [Google Scholar]
- [40].Shinar Guy and Feinberg Martin, Structural sources of robustness in biochemical reaction networks, Science 327 (2010), no. 5971, 1389–1391. [DOI] [PubMed] [Google Scholar]
- [41].Smith Hal L. and Thieme Horst R., Dynamical systems and population persistence, vol. 118, American Mathematical Soc, 2011. [Google Scholar]
- [42].Sontag Eduardo D., Structure and stability of certain chemical networks and applications to the kinetic proofreading of t-cell receptor signal transduction, IEEE Trans. Auto. Cont 46 (2001), no. 7, 1028–1047. [Google Scholar]
- [43].Tyson John J., Chen Katherine C., and Novak Bela, Sniffers, buzzers, toggles and blinkers: dynamics of regulatory and signaling pathways in the cell, Current opinion in cell biology 15 (2003), no. 2, 221–231. [DOI] [PubMed] [Google Scholar]
- [44].Whittle Peter, Systems in stochastic equilibrium, John Wiley & Sons, Inc., New York, NY, USA, 1986. [Google Scholar]
- [45].Wilkinson Darren J., Stochastic modelling for systems biology, Chapman and Hall/CRC Press, 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.