ABSTRACT
Intestinal catheters have been used for decades in human nutrition, physiology, pharmacokinetics, and gut microbiome research, facilitating the delivery of compounds directly into the intestinal lumen or the aspiration of intestinal fluids in human subjects. Such research provides insights about (local) dynamic metabolic and other intestinal luminal processes, but working with catheters might pose challenges to biomedical researchers and clinicians. Here, we provide an overview of practical and technical aspects of applying naso- and oro-intestinal catheters for delivery of compounds and sampling luminal fluids from the jejunum, ileum, and colon in vivo. The recent literature was extensively reviewed, and combined with experiences and insights we gained through our own clinical trials. We included 60 studies that involved a total of 720 healthy subjects and 42 patients. Most of the studies investigated multiple intestinal regions (24 studies), followed by studies investigating only the jejunum (21 studies), ileum (13 studies), or colon (2 studies). The ileum and colon used to be relatively inaccessible regions in vivo. Custom-made state-of-the-art catheters are available with numerous options for the design, such as multiple lumina, side holes, and inflatable balloons for catheter progression or isolation of intestinal segments. These allow for multiple controlled sampling and compound delivery options in different intestinal regions. Intestinal catheters were often used for delivery (23 studies), sampling (10 studies), or both (27 studies). Sampling speed decreased with increasing distance from the sampling syringe to the specific intestinal segment (i.e., speed highest in duodenum, lowest in ileum/colon). No serious adverse events were reported in the literature, and a dropout rate of around 10% was found for these types of studies. This review is highly relevant for researchers who are active in various research areas and want to expand their research with the use of intestinal catheters in humans in vivo.
Keywords: intestinal catheter, small intestine, ileum, colon, aspiration, delivery, human, trials
Introduction
Intestinal catheters have been used for decades in physiology, nutrition, microbiology, and pharmacokinetics research. Studies involving catheters have helped to shed light on the functioning of the human gastrointestinal (GI) tract. For example, researchers have learned about digestion and absorption of (macro)nutrients, secretion, and flow rates (1–3), as well as intestinal physiology, including digestion and absorption processes (4–6), luminal and adherent microbiome compositions (7), and metabolite production (8) in vivo. In vitro GI-tract models, animal models, or measurements in fecal samples are not directly representative of intraluminal (patho)physiological processes or bacteria in the human GI tract in vivo (9). These models lack essential aspects of a host interaction, and do not capture the variation in response between human subjects. Intestinal catheters can be used to aspirate intestinal fluids to examine intestinal luminal processes. Intestinal catheters can also be used to deliver compounds inside the intestinal lumen (10, 11). This provides valuable information about dynamic metabolic effects after targeted intestinal delivery of a test compound (12), and allows researchers to study changes in systemic metabolisms relevant to health and disease.
As opposed to feeding tubes used to deliver enteral nutrition inside the stomach or the proximal small intestine of patients, working with intestinal catheters that need to be placed in the more distal small intestine or proximal colon poses more challenges to biomedical researchers and clinicians. In-depth information about working with intestinal catheters, including positioning catheters, aspirating intestinal fluids, and standardizing delivery and sampling, as well as a summary of state-of-the-art intestinal catheter designs, is currently lacking in the literature. The objective of this review is to provide an overview of the practical and technical aspects of applying naso- and oro-intestinal catheters to human subjects for delivery of compounds and sampling luminal fluids from the jejunum, ileum, and colon in vivo. For this review, not only did we examine the available literature from experts in this field, but we also included insights from our own clinical trials with intestinal catheters: thus, this review extends beyond the boundaries of a conventional systematic review. This information will be helpful for researchers in setting up and performing trials.
Search Strategy and Inclusion Criteria
A search strategy was developed for PubMed. Combinations of 3 grouped search terms were used to find papers that described clinical trials using intestinal catheters in human subjects (Supplemental Table 1). Papers written in English and published after 1960 were included. Detailed information about the search strategy can be found in Supplemental Table 1. The search terms were adapted accordingly for a search in the Web of Science. Both searches (PubMed and Web of Science) were conducted in March 2020. This resulted in 6338 papers. After removing 971 duplicates, 5367 papers were screened by our team based on titles and abstracts (Figure 1). The inclusion criteria were: 1) full-text clinical trials with human subjects; 2) original research articles; and 3) the use of an intestinal catheter where the tip of the catheter was placed in the jejunum, ileum, or colon. The exclusion criteria were: 1) studies where the catheter was not placed to answer study questions (observational study); 2) studies with catheters that were inserted via the rectum/anus; 3) studies that used manometry catheters or focused on motility and motor complex functions; 4) studies where catheters were not used to deliver compounds to the intestine or to sample intestinal fluids; 5) studies with gastric or duodenal catheters; and 6) studies that did not conform to the inclusion criteria. After excluding 5060 irrelevant papers, 293 papers remained, which were split into 2 groups: papers published before and after 2000. The list of papers published before the year 2000 is provided in the Supplemental Information. The full texts of the 81 papers published after 2000 were examined for state-of-the-art methodology. Finally, 58 papers were included in this review.
FIGURE 1.
Flowchart of the literature search for articles that used an intestinal catheter where the tip was in the jejunum, ileum, or colon for research purposes in human subjects. Searches were performed up to 16 March 2020.
Insights From Our Own Selected Clinical Trials
We also included in-depth information from several of our own (un)published clinical trials. We included (unpublished) insights from one of our studies that applied catheters for simultaneous aspiration of duodenal, jejunal, and ileal content [the so called “CRIB study'', registered at clinicaltrials.gov as NCT02018900 (13)]. Insights obtained during our FiberKinetics study were also described, where a catheter was used for delivery and aspiration in the distal ileum [registered at clinicaltrials.gov as NCT04013607; unpublished study]. Also included were observations from the ileal brake study (14), where nutrients were introduced via the ileum, and an iron oxidation study (15) in which a 40-cm segment of the proximal small intestine was perfused and fluid samples were collected. All studies were approved by an Institutional Review Board (METC Wageningen University or Maastricht University, the Netherlands). All subjects gave written informed consent.
The Use of Intestinal Catheters in Research
General overview of the included studies
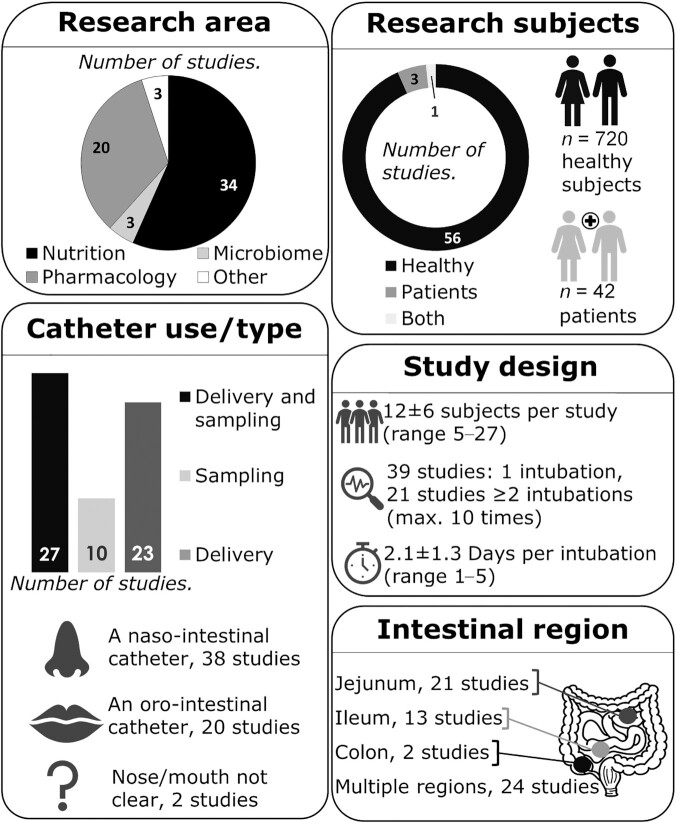
We included 58 studies, as well as 2 unpublished studies from our research groups reviewing methods for the use of intestinal catheters (summarized in Supplemental Table 2). Most studies focused on nutrition and (intestinal) physiology research (34 studies), followed by pharmacological research (20 studies), microbiome research (3 studies), and another category (3 studies; Figure 2). Most of these studies investigated multiple intestinal regions (24 studies), followed by studies investigating only the jejunum (21 studies), ileum (13 studies), or colon (2 studies). There were 56 studies including healthy subjects, 1 study including both healthy subjects and patients, and 3 studies including patients. Studies involved a total of 720 healthy subjects and 42 patients [with type 2 diabetes (16), slow transit constipation (17), ulcerative colitis (18), or cystinosis (19)]. The mean number of subjects per trial was 12 ± 6 (range, 5–27 subjects). In most studies, the subjects were intubated once (39 studies) and in the other 21 studies, subjects were intubated ≥2 times [maximum 10 times (20)] in a cross-over fashion with a wash-out period in between. In general, catheters placed in the distal regions of the GI tract (ileum, colon) required a longer intubation period. The mean number of days per intubation was 2.1 ± 1.3 (range, 1–5 days).
FIGURE 2.
An overview of the general characteristics of the 60 studies included that used an oro- or naso-intestinal catheter placed in the jejunum, ileum, or colon in human subjects.
Recruitment and inclusion
It is important to note that in more invasive studies, recruitment and inclusion are generally more challenging compared with noninvasive studies. This should be considered when setting the study inclusion and exclusion criteria, although it is of paramount importance to include sample sizes that have enough statistical power. For the CRIB study, 49 people responded to our recruitment efforts over a period of 6 months (13). For the FiberKinetics study, 20 people replied within 1 month after the recruitment started (21).
Considerations in the catheter design
A summary of state-of-the-art intestinal catheter designs in the included studies is provided in Supplemental Table 2.
Catheter length and outer diameter
The total catheter length differed depending on the targeted intestinal region. Jejunal catheters had a mean length of 286.3 ± 120.4 cm (range, 150–500 cm), ileal catheters had a mean length of 307.4 ± 34.6 cm (range, 270–400 cm), and colonic catheters had a mean length of 466.7 ± 23.6 cm (range, 450–500 cm). To keep track of the length of the catheter inserted into the GI tract, it is useful to have centimeter markings along the full length of the tube (Figure 3A, B, and D). The intestinal catheters placed via the nose had a mean outer diameter of 2.9 ± 1.2 mm (range, 0.6–4.2 mm), whereas the intestinal catheters placed via the mouth had a bigger outer diameter of 4.7 ± 0.9 mm (range, 2.5–6.3 mm). Generally, catheters with a bigger outer diameter are easier to place, owing to their increased stiffness, and intestinal delivery and/or sampling becomes easier. The diameter of the aspiration channel should be large enough to sample intestinal content at the site of interest. The sample homogeneity and viscosity differ between sampling locations (e.g., small intestine compared with proximal colon) and depending on the condition of the study participant (fasted or after consumption of liquid or solid foods) (22). The proximal colon contains more thick, less homogeneous material, so sampling from this region can be improved by using a catheter with a larger diameter. We provide more information about sampling volumes/rates from various sampling sites using catheters with different diameters below in the section titled “Sampling of intestinal content.” However, a bigger outer diameter might be less comfortable for participants. In the only study reporting on outer diameters in relation to tolerability, nasally placed tubes with outer diameters of both 2.1 and 3.8 mm did not result in increased postprandial supine gastroesophageal reflux in 8 healthy subjects (23). In our experience, intestinal catheters with an outer diameter of maximally 3.5 mm and made from soft materials, such as silicone, were generally well tolerated by subjects (10, 13, 14, 24), whereas oral intubations were less well tolerated and were not used for prolonged measurements. Toleration is a combination of the burden caused by tube insertion and the burden and duration of the transfer of the tube to the target location. The latter is often less comfortable for tubes inserted via the mouth as compared to the nose. A trade-off between practical considerations and participant (dis)comfort must be made when designing and using intestinal catheters.
FIGURE 3.
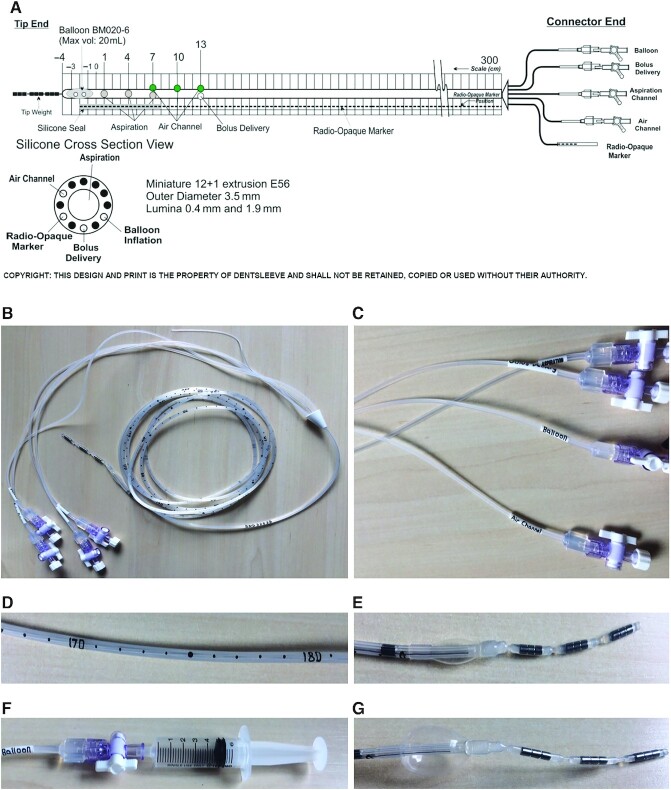
An example of a naso-ileal catheter design that can be used for intestinal aspiration and delivery in human subjects. (A) The schematic design includes the cross-sectional view of the different lumina (reproduced with permission from Mui Scientific, Ontario, Canada). Photos show the (B) naso-intestinal catheter with 300-cm tubing, excluding the connector end; (C) multiple lumina, closed with a stopcock and lid; (D) the centimeter indications on the tubing; (E) the deflated balloon and the 3 tip weights; (F) the inflation of the balloon via the balloon channel using a syringe; and (G) the balloon that is inflated with 5 mL of air (maximum capacity 20 mL).
Catheter material
Intestinal catheters are often made from polyurethane, silicone, or polyvinyl chloride. Polyurethane and silicone tubes, also called fine-bore tubes, are softer and more flexible than polyvinyl chloride tubes and, therefore, they are more comfortable for the subject and easier to place along the curves of the small intestine. On the other hand, a higher flexibility increases the risk of curling and coiling inside the GI tract upon introducing the catheter, mainly in the stomach, which hampers positioning of the catheter postpylorus. In the included studies, the materials used were silicone (10 studies), silicone rubber (12 studies), polyvinyl chloride (14 studies), polyethylene (3 studies), or not mentioned (21 studies). The use of stiffener or guidewire (see the section below titled “The use of a guidewire, stiffener, and/or endoscope”) results in a more rigid catheter, which can especially be of added value to silicone catheters due to their high flexibility. Fine-bore tubes can better withstand GI circumstances (25) such as gastric acid, bile acid, and other GI secretions. Thus, reusable fine-bore tubes (silicon or polyurethane) will likely last longer than polyvinyl chloride tubes.
Multi-lumen catheters with side holes
In some studies, several different catheters were synchronously introduced, with each separate catheter tip located in another intestinal region [(26–29), published prior to 2007]. Introducing several catheters at once increases the burden during positioning and the subsequent experiment because the outer diameter of the combined catheters is substantially larger than that of only a single catheter. In 1 study, the same tube was used for collection of intestinal content from various intestinal regions (30), which may have led to contamination of luminal contents from 1 intestinal region to the other. Nowadays, these issues are resolved using multi-lumen catheters (in 41 studies, ranging from 2 to 23 lumina per catheter). Multi-lumen means that multiple so-called “lumen” or “channels” with various dimensions are combined in 1 tube construction (Figure 3A, cross-section view). The numerous lumina are labeled according to their proposed function and separately protrude at the connector end (Figure 3B and C). The different lumina within 1 catheter can serve different purposes, such as simultaneous sampling/delivery, sometimes from various intestinal regions (e.g., the duodenum and ileum), or inflating or deflating balloons. The diameter of a specific lumen can be adapted according to the use (aspiration compared with delivery) or intestinal region (proximal compared with distal). Often, 1 lumen is used for balloon inflation/deflation, as further explained below, and another lumen is used to transport radio-opaque material. A lumen for air inflation may be included in the design to prevent the catheter from adhering to the intestinal wall due to creation of a local vacuum during fluid sampling, which may cause intestinal sampling issues. When lumina are not in use, they can be closed at the connector end with a stopcock and/or lid (Figure 3C). Side holes can be positioned in 1 or more lumina to enable targeted exposure of specific intestinal segments. In Figure 3A, 3 side holes in the catheter aspiration channel used to obtain aspirates of a ∼7-cm intestinal segment can be seen. Multiple side holes in 1 lumen reduce the risk of obtaining no sample due to a potential blockage of 1 side hole or the catheter sticking to the intestinal wall.
The use of inflatable balloons and weighted tips
Most of the catheters were equipped with an inflatable balloon (35 studies) or bag (3 studies). These inflatable accessories or a capsule [30 mm × 10 mm (31–33)] attached to the distal tip are often used to enhance catheter progression, making it move distally with peristaltic contractions. The bags and balloons can be inflated via a specific inflation channel (Figure 3E–G) with water, saline, or air. These balloons have a maximum inflation capacity [5 mL (19), 10 mL (13, 14, 16, 34–36), or 20 mL (21)]. Since water or saline is heavier than the same volume of air, this might beneficially impact progression of the catheter when the person is positioned in such a way that gravity can exert its normal downward pulling action. However, whether the balloon or bag can be filled with liquid depends on the material and on cleaning/sterilization protocols of reusable catheters. For example, our silicone catheters and the encased balloon had to be completely free of water before sterilization; therefore, the balloon was filled with air. In 1 study, a rubber balloon was first filled with 1.5 mL of mercury and, after reaching the duodenum, the mercury was replaced by 15 mL of air to facilitate further progression into the small intestine (15). Other studies used a rubber bag with 30 g of mercury (37), an inflatable mercury bag (38), a lead weight (30), or a finger cot with 2 mL of mercury (39) at the tip. Although using mercury has the advantage of increasing the weight of the balloon and therefore speeding up progression, it is a highly toxic compound that is dangerous if it leaks from the balloon. Nowadays, to avoid the use of heavy metals, (tungsten) weights/pellets are often encased at the distal tip to promote movement from the stomach to the duodenum.
Catheter design for perfusion experiments
Another reason that inflatable balloons were incorporated into catheter design was to isolate a closed segment of the intestine or to isolate proximal and distal segments from each other (40–43). Such catheters can be used for intestinal perfusion experiments (infusion and sampling within 1 test segment) (44, 45). Perfusion experiments using occluding balloons allow researchers to investigate the net absorption/transport or secretion of water or solutes in a segment of the intestine, for instance. The occluding balloons were placed at a variable distance apart from each other (1, 46, 47) to create independent segments of 10 cm (48) or 20 cm (49–52). Balloons were inflated with a relatively high quantity of air [∼30–45 mL (43, 51, 52) to maintain a constant pressure of ∼25 mm Hg] as compared to balloons used for progression. Air was added until pressure sensations (i.e., without inducing discomfort) were experienced by the subjects (43), to achieve total occlusion of the intestinal segment and to prevent progression of the catheter by peristalsis. To achieve this inflation, balloons were bigger: namely, 5–10 cm long (42, 43, 49–52). After inflation, pressure was continuously monitored to ensure sustained inflation (43). Nonabsorbable markers, such as phenol red (phenolsulfonphthalein), can be used to check for leakages from the intestinal segment. The occluding balloon proximal to the study segment prevents endogenous secretions, with an unknown quantity of salts and water, from contaminating the test segment (44). Semi-open perfusion systems/segments can also be used by inflating the distal balloon with 26–30 mL of air (48, 53, 54). Another option is to use an open perfusion system without balloons, with an infusion port located a few centimeters proximally from the sampling port(s), to determine absorption or secretion between the infusion site and the aspiration site(s). This method aims to not to influence the flow rate at the sampling point, but to calculate the real liquid flow rate using a marker. Nonabsorbable markers, such as polyethylene glycol (PEG), have to be added to infusions in known concentrations to correct for the dilution.
Placement, progression, and removal of the catheter
Catheter insertion
Intestinal catheters were positioned via the nose in 38 studies or via the mouth in 20 studies (Figure 2). In 2 studies, placement was not mentioned. Endoscopic insertion via the nose was tolerated significantly better in comparison to conventional oral gastroscopy in 181 consecutive outpatients (55). Before placement, the tube is lubricated with a medical-grade lubrication gel, sometimes also containing a local anesthetic, to reduce friction. Moreover, local anesthesia (if preferred by the subject) can be applied to the nasal mucosa [e.g., lidocaine 10% spray/Xylocain (10)] or the upper throat/pharynx [e.g., using lidocaine spray (51, 53, 54)] to reduce potential pain and discomfort during insertion. Previously, topical pharyngeal anesthesia given before endoscopy was effective in reducing discomfort in 201 patients undergoing the procedure for the first time (56). In 1 study, a topical anesthetic (1 mL 4% lidocaine) was administered to prevent a gag reflex (20). Moreover, in a randomized controlled trial in 100 patients, infusion of 100 mL of sodium chloride (NaCl) 0.9% with 10 mg metoclopramide 15 minutes before the procedure significantly reduced overall discomfort, nausea, and vomiting (57). However, the use of medication other than lubrication gel before the start of a study should be thoroughly thought out, because it could potentially affect the study outcomes. The potential impact of medication on the outcomes is higher when experimental tests take place shortly after the intubation procedure. After lubrication and local anesthesia, the tube was inserted via the nose or oral cavity and pushed through the esophagus to the stomach. During this procedure, the subject was asked to drink sips of water, to ensure closure of the esophagus by the epiglottis.
Transpyloric migration
Different strategies for transpyloric migration were described in the studies. One strategy was to inflate the balloon inside the stomach [with ∼3 mL saline (58) or 1.5 mL mercury (15)], facilitating progression of the catheter tip towards the pylorus by peristalsis. To stimulate peristalsis, participants were offered snacks (58) or were placed in a supine position on their right side with their upper body lifted 45° and their feet raised (15). By positioning the subject on the right side, the pyloric sphincter was in the lowest part of the stomach. Gravity forced the mercury balloon to migrate towards the pyloric sphincter, facilitating catheter progression into the small intestine. In 2 other studies, the subjects were also intubated while in the supine position (16, 59). In several studies, the tube was allowed to pass into the duodenum without inflating the balloon inside the stomach (31–35, 42, 43, 60, 61), and 3 of these studies used a tube with a capsule attached at the tip to stimulate progression (31–33). In 30 healthy subjects, 87% of the inflated ballooned tubes passed the pyloric sphincter with the aid of normal peristaltic movements after 6 hours, compared to 67% of the noninflated ballooned tubes (62). Thus, balloon inflation in the stomach improves spontaneous transpyloric migration. Theoretically, the inter-digestive migrating motor complex, responsible for letting particles larger than a few millimeters pass the pylorus, only occurs in the fasting state (63), which could imply that postpyloric placement after overnight fasting is good timing. In practice, however, simultaneous feeding could stimulate transpyloric migration of the catheter. An intravenous dose of erythromycin, a prokinetic, significantly increased successful postpyloric tube placement in randomized controlled trials (64). Another strategy was to guide manual catheter progression from the stomach into the proximal small intestine under (freeze-frame) intermittent fluoroscopic control (1, 10, 13, 14, 20, 21, 24, 26–29, 59) or static X-rays (65).
The use of a guidewire, stiffener, and/or endoscope
Stiffeners allow for more control while manually introducing a catheter into the GI tract, facilitating progression from the stomach to the duodenum/jejunum. In 4 studies, guidewires (66) [coated with polytetrafluoroethylene (47) or Teflon (48, 53)] were used to facilitate passage into the small intestine, sometimes monitored by fluoroscopy. Guidewires were mostly used to insert shorter tubes (i.e., duodenum and jejunum tubes) 150–200 cm long. In 1 study, 1 channel within a silicone catheter was filled with a guidewire stiffener to facilitate passage (58). Wilms et al. (13) used an assembly stiffener (0.3 mm, in the center lumen) within a silicone catheter, which resulted in a catheter with increased rigidity that was still flexible enough to pass easily along the curves of the small intestine. In 1 study, a guidewire was first inserted 60 cm distally from the pylorus with the aid of an endoscope. The endoscope was then retracted, leaving the guidewire in place. Upon removal of the endoscope, the guidewire could be retracted back into the stomach (67). After retracting the endoscope, the correct positioning of the guidewire needs to be confirmed using radiography. Only in 1 other study was the use of an endoscope mentioned (17). The catheter was subsequently introduced over this guidewire into the proximal small intestine, all under fluoroscopic guidance (68). After the catheter was correctly positioned, the guidewire was removed. An advantage of this procedure was the rapid placement in the proximal small intestine, with a median time of 18 minutes (range 12–45 minutes) in 22 patients (69), due to easy detection of the pylorus.
Other ways to monitor gastric and postpyloric placement
Smithard et al. (70) described the use of electromagnetic access systems for tube placement. The average time of postpyloric placement using these devices was 16 minutes, whereas blind placement took 42 minutes on average (70). A pH measurement of the aspirate could be used to determine the position of the tube, since the pH level in the stomach is 3 to 4. To move the catheter from the stomach into the duodenum, 6 studies used the antral and duodenal transmucosal potential difference gradient (TMPD) for continuous monitoring of the catheter position (antral TMPD < −20 mV; duodenal TMPD > −15 mV; difference >15 mV). To establish this gradient, isotonic sodium chloride was perfused via the catheter infusion channels at the gastroduodenal junction (16, 49–52, 60). The disadvantage of this method is the fact that manometry equipment is required for monitoring to ensure correct placement (60).
Progression towards the distal intestine
To assist with tube passage through the GI tract, inflatable accessories such as balloons or bags at the catheter tip can be inflated with water [7 mL (20)], saline [∼3 mL (58)], or air [5 mL (10, 13), ∼6 mL (71), 8 mL (21, 66), 10 mL (60, 72), or 15 mL (15)]. In many studies, the balloon was inflated once it passed the pyloric valve (15, 21, 49–52, 60, 73–76) or after passing the ligament of Treitz (19, 34–36, 66, 77). In 5 studies, the participants were instructed to inflate and deflate the balloon every other hour and inflate the balloon upon waking up to advance further passage of the tube (10, 13, 14, 24, 77), to ensure the inflated balloon did not block the passage of food or GI excretions. In some studies, the catheter progressed with gravity/peristalsis and no balloon inflation was mentioned (30–32, 59, 78). Keeping the balloon continuously inflated directly after reaching the duodenum until it reaches the region of interest will result in optimal progression, but the risk of full intestinal occlusion for a longer period should be avoided. Occlusion may result in symptoms such as abdominal pain, nausea, and vomiting (79). A 2.1-cm balloon inflated with 5 mL of air is equivalent to a spherical volume of 4.8 mL (equation: V = (4/3)πr3). The balloon is also soft and malleable, so it is unlikely that (full) occlusion will occur when it is kept inflated. Moreover, the small intestine is known to dilate (80) after administration of a (food) bolus (81) and even allows passage of solid objects. In our studies, the balloon gradually deflated spontaneously over time (after >30 minutes) ex vivo. In the FiberKinetics study, progression was not successful in 2 subjects, as the balloon was not continuously inflated during manual insertion (10 cm/hour) to promote further progression, which resulted in a coiled tube inside the stomach (21). On the subsequent test days, the subjects had to empty and refill the balloon every hour to ensure sustained inflation, since apparently this was crucial for successful catheter positioning in our study. When applying reusable catheters, the balloon should be checked for leakages ex vivo before each intubation.
Along with inflating and deflating the balloon, the subjects needed to further insert the catheter into the nose or mouth to advance the tube. In our studies, the tube was manually inserted either at a rate of 10 cm/hour starting after the tube passed the ligament of Treitz (21) or at a rate of 5–10 cm/hour starting ∼2 hour after placement (13). In the study of Zarate et al. (66), the tube was inserted at a rate of ∼10 cm/30 min after the tip passed the ligament of Treitz. Pulling at the nares might be caused by coiling of the tube within the stomach/small intestine (58); however, pulling at the nares is usually a sign of spontaneous catheter progression inside the intestine. When an excessive length of the tube is quickly inserted into the stomach, there is a risk of tube knot formation (82). Therefore, very fast insertion of catheters should be prevented by adhering to a maximum rate per hour, such as 10 cm/hour.
Practical procedures to stimulate catheter progression
To stimulate progression, participants can be offered drinks (e.g., tea/coffee) or food (13, 34, 36) and be encouraged to walk around/move periodically (13, 20) during the day of intubation to stimulate peristalsis. Due to the upright posture, progression will also benefit due to gravity (13, 20). In 1 study, when the tip did not reach the desired location within the scheduled time, an intravenous injection of metoclopramide (Primperan; 10 mg in 2 mL) was given to increase GI motility (33). Other examples of (intravenous) drugs to stimulate GI motility are domperidone (83, 84) and erythromycin (85). Erythromycin is a motilin agonist and initiates the interdigestive migrating contractions (86), the effects were previously mostly seen on gastric contractions (87, 88). The use of pro-motility drugs may influence the test procedures.
Hospitalization or home-based stay?
Some study participants were hospitalized during the full intubation period when progression took more than 1 day (53, 71, 75, 89). Subjects could also be instructed to assist the passage of the catheter at home (36, 90), which was the case in our own studies. With the catheter inserted, participants could perform daily activities such as eating, drinking, showering, moving, and sleeping. We provide information about various aspects to consider when deciding whether to hospitalize participants or not in the Supplemental Information.
Progression time
Placement of the tube into the stomach takes between 10 and 15 minutes (21, 58). Transpyloric migration of the tip can take ∼10 minutes (66), although in our experience manual transpyloric tube placement using fluoroscopy took between 10 and 45 minutes (21). Automatic postpyloric migration with a 1.5-mL mercury-inflated balloon took 1–2 hours after catheter ingestion (15). Moreover, jejunal catheters were positioned mostly on the same day as the experimental tests took place (1, 33, 52, 53, 61), and positioning the tube in the (proximal) jejunum took ∼1 hour (1, 53), 2.2 ± 0.2 hours (52), or 1–3 hours (61).
Ileum and colon intubations, however, require more time due to a longer progression period. In (terminal) ileum intubation studies, the subjects always visited the research facilities the day prior to the experimental tests for catheter insertion, allowing the catheter to progress over time (a full 24-hour period). The next day, the subjects returned between 07:30 and 10:00 (10, 13, 14, 21, 39, 58, 73, 75, 76) to check the position of the tip and, when appropriately placed, start the experiments. Depending on the insertion procedures, within 24 hours the catheter tip could be located ∼100–120 cm distal to the ligament of Treitz (CRIB study) or 240–250 cm from the nose (13, 21). In some other studies, an intubation 120 cm distal from Treitz (34) or ≥175 cm from the nose (35, 36) took less than 24 hours (34–36, 66). In the study of Borg et al. (16), the terminal ileum was intubated within 5 hours. To reach the terminal ileum, a progression period of 20 hours (including overnight) might be too short in some subjects. Colonic intubations may take slightly more time compared to ileum intubations, but in most studies, the subjects were also intubated in ∼24 hours (33, 78, 90). In 1 study, the catheter progressed from the jejunum to the proximal colon within 18 hours, or from the mouth to cecum within 20–48 hours (72). In the study of Dohil et al. (19), the ballooned tube entered the proximal small intestine after 3 days and the cecum after 5 or 7 days in all subjects, whereas in another study, the tube reached the cecum after only 6 to 10 hours [balloon filled with 30 g mercury (37)].
After reaching the correct intestinal segment
After reaching the correct GI segment, the tube can be held in place by fixing it to the face and deflating the balloon (37, 60, 72). In 1 study, a residual volume of 3 mL was kept inside the balloon to avoid retraction (66). Marteau et al. (37) flushed the sampling lumen with nitrogen in the ileum and colon, likely to ensure no residual oxygen present in the sampling lumen disturbed the anaerobic environment in the colon. Moreover, after correct positioning the catheter, subjects remained in a semi-recumbent position (37, 38, 73–75) or a semi-reclining position (72) to avoid further progression of the tube. Since the tube can still progress further (47) over time, especially in studies that take a number of days, the position of the tube should be verified before important measurements are carried out.
Removal and cleaning (of reusable catheters)
Before removal of the catheter, the inflatable bag or balloon must be completely deflated. After deflation, the catheter can be removed by pulling it out gently, either by the subjects (15) or by medical staff. In 2 studies, the tube was cut off near the nose and allowed to leave the body naturally in the feces (31, 32). When colonic catheters are removed via the nose or mouth, the colonic bacteria could potentially contaminate the small intestine (91). Moreover, spasming of the ileo-cecal valve caused by retracting the colonic catheter may cause severe discomfort or pain. In this case, the use of spasmolytic intravenous glucagon or hyoscine butylbromide (buscopan) is recommended. We used custom-made, multi-use catheters that could be sterilized up to 50 times (13, 21). For cleaning, the tube can be flushed with alkaline enzymatic detergent used for cleaning medical devices (MediClean Forte, Dr. Weigert, Hamburg, Germany) (21) or with enzymatic presoak detergents and disinfectants (20). The tube is then manually flushed with water using a syringe and completely dried before sterilization. It is advised to check with the manufacturer regarding the compatibility between cleaning reagents and the material, and with the central sterilization department in the hospital regarding standard cleaning procedures.
Determination of the catheter location
Radiography
There are multiple methods to determine the catheter location during placement, progression, and final positioning (during test days). Most studies made use of imaging techniques using radiation (46 out of 60 studies), such as plain abdominal X-ray static pictures or fluoroscopy. Freeze-frame fluoroscopy—intermittent periods of fluoroscopy instead of continuous real-time monitoring—is often applied to minimize radiation exposure during insertion of the catheter and when verifying the location (66). Radio-opaque markers integrated into the catheter design enable visualization by fluoroscopy or static X-rays. Radio-opaque material can be added close to infusion ports or to (all) side holes (10) to select the appropriate infusion channel from multi-lumen catheters for an intestinal segment (24). Radio-opaque material can also be added to the tip of the tube (19, 92), as a capsule at the distal end (31), or every 10 cm to measure the length in cm distal to Treitz. Tungsten weights at the tip can also be visualized with radiation. Another approach is to fill 1 lumen inside the catheter with radio-opaque material to visualize the complete tube length (21, 58, 65, 73, 76). The marker along the catheter can assist with tube placement using fluoroscopy, since visualizing the marker distribution throughout the intestine shows whether the catheter tip passes the pyloric sphincter and the ligament of Treitz (Figure 4A) or whether the catheter is coiled or looped in the stomach or small intestine (58, 77).
FIGURE 4.
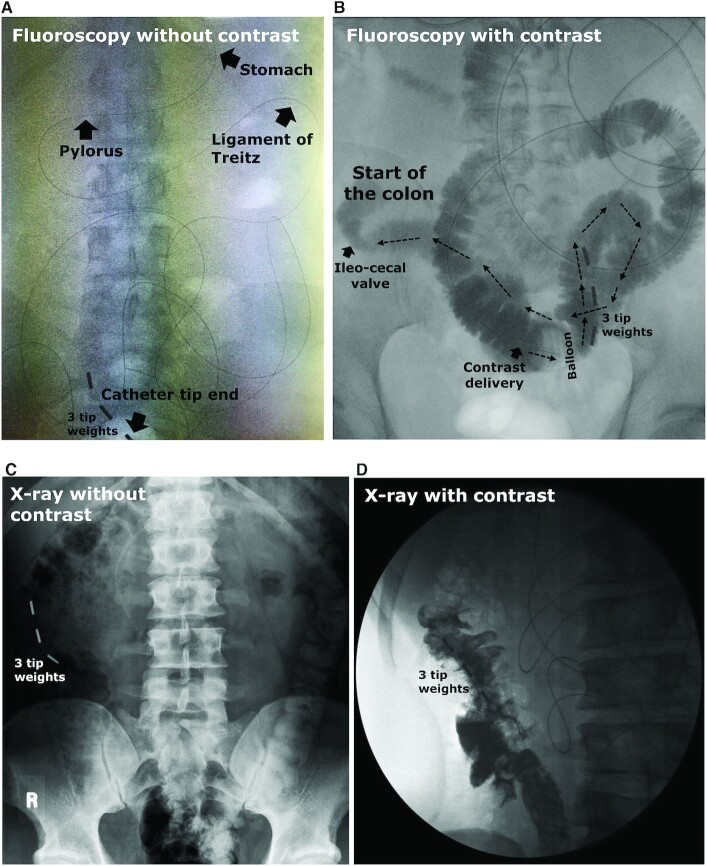
Examples of visualization of the catheter tip location using fluoroscopy or X-rays with/without the delivery of contrast liquid in human subjects. Fluoroscopy pictures are (A) without or (B) in combination with the delivery of a contrast liquid, where the contrast liquid appears in black and will follow the direction of the arrows towards the colon. Abdominal X-ray pictures are (C) without or (D) in combination with the delivery of a contrast liquid. Panels C and D were reproduced with permission from Dohil et al. (19). The black line in the pictures is the radio-opaque marker, and 3 small metal (or radio-opaque) weights/markers are located at the tip of the catheter.
Radiography in combination with contrast liquid
Only after infusion of contrast fluid via the most distal catheter lumen can X-ray and fluoroscopy examination determine the catheter position more precisely with respect to intestinal anatomical structures such as the ileocecal valve (19, 36, 71, 77). Studies used diluted barium sulphate (71), meglumine-ioxitalamate [Telebrix GASTRO, Guerbet, France; 50 mL in total, diluted 1:2 with water (21)], or Gastrografin [Bayer, Berkshire, UK; 20 mL (66)] as contrast fluid. Contrast can be delivered directly via a catheter lumen into the intestine, facilitating the visualization of a small segment of the intestine within a few minutes [30–60 cm (77)]. Contrast liquid allows researchers to discriminate between the small and large intestine (Figure 4A and C compared with Figure 4B and D) and determine the catheter tip position (in centimeter distance) in relation to the ileocecal valve (19, 21). If the side holes allow, contrast can be delivered distally from the inflated balloon to prevent backflow into the more proximal intestine. When contrast can only be delivered via side holes located proximal to the balloon, it is best to deflate the balloon for optimal visualization of the more distal intestine. Knowing the exact position of the catheter can be important for delivery or aspiration at the right location, as well as standardization and interpretation of the research outcomes. Gastrografin and Telebrix GASTRO are both water-soluble, hyperosmolar contrast media, and therefore draw water into the lumen, which may impact study outcomes such as the microbiome composition. Thus, delivering contrast media after completing experimental tests may be better for accurate test results. Gastrografin might cause diarrhea (93), whereas barium sulfate is not hyperosmolar and is almost insoluble in water. Overall, radiography allows accurate verification of the catheter location but exposes participants to radiation. We describe the radiation effective doses measured during our studies, as well as other aspects related to radiation exposure and safety, in the Supplemental Information. The total radiation exposure for fluoroscopy can be considered low (<0.1 mSv). A conventional abdominal static X-ray will result in a higher radiation exposure as compared to fluoroscopy, but results in better-quality images (Figure 4A and B compared with Figure 4C and D).
Other methods to monitor catheter location
Another method to estimate the location of the catheter tip is the use of centimeter marks or color marks (32, 33) along the tube, indicating the distance from nose to catheter tip (the total tube length). This, often in combination with fluoroscopy or X-ray, gives an indication of the tube location (30, 34, 92). A summary of distances (cm) for the different assumed intestinal regions used by previous studies is provided in Table 1. The tip near the pylorus was estimated at a ∼70-cm distance from the nose (58). However, distances can vary between individuals. Body height is not significantly correlated to small intestine length, so the tube distance cannot be predicted based on height (94, 95). The length of the small intestine is negatively correlated with age, and was found to be longer in males as compared to females (94).
TABLE 1.
Indications of centimeter insertion of the catheter for the assumed intestinal regions, as found in research papers that described clinical trials applying intestinal catheters in human subjects1
| Intestinal region | Distance from the nose or mouth | Distance from the pylorus | Distance from the ligament of Treitz |
|---|---|---|---|
| Duodenum | 86 ± 5 cm (78) | 5 cm (24) | — |
| — | 5–10 cm (26) | — | |
| — | 12 cm (42) | — | |
| — | 13 cm (16) | — | |
| — | ∼15 cm (58, 60) | — | |
| Jejunum | 100 cm: proximal jejunum (33) | 20 cm (50) | ∼30 cm (2) |
| 167 cm (89) | 40–50 cm (24) | — | |
| — | 50 cm: first segment (96) | — | |
| — | 50 cm: proximal jejunum (43) | — | |
| — | 70 cm (42) | — | |
| — | ∼100 cm (26) | — | |
| — | 100 cm: “mid-jejunum” (60) | — | |
| Ileum | 160–180 cm (13) | ≥120 cm (14, 24, 77), | ≥120 cm (34) |
| ≥175–195 cm (35, 36) | ≥170 cm (58) | — | |
| 180 cm (66) | 190 cm (16) | — | |
| 186 ± 21 cm: terminal ileum (78) | — | — | |
| 214 cm: terminal ileum (89) | — | — | |
| 240–250 cm: terminal ileum (21) | — | — | |
| 260 ± 20 cm: terminal ileum (33) | — | — | |
| 300 cm (59) | — | — | |
| Colon | Ascending colon: 330 cm (33) | — | — |
The centimeter distances were often used in combination with fluoroscopy/X-ray to confirm the location.
The antral and duodenal transmucosal potential difference gradient can be used for continuous monitoring on the catheter position (16, 49–52, 60). Moreover, measurement of pH is also often used as an indirect measure to check tube positioning throughout the experiments. The pH level can be easily determined in aspirates from various locations using pH strips (10). The intraluminal pH level was also measured continuously via pH electrodes that were fitted in the catheter close to the injection port (68), at the tube tip (15), or through 2 pH probes near the tip and 1 probe 35-cm proximal to the tip (72). Another option is real-time confirmation of transpyloric migration using electromagnetic guidance (70), which eliminates or reduces the need for radiography, and thus exposure to radiation and dependence on the hospital radiology department. One example is the Cortrak feeding tub (Viasys Healthcare) for which placement and real-time location information is provided via a Cortrak Enteral Access System. This tube has been used in research to sample inside the stomach and duodenum (97). The maximum length of the Cortrak tube is 140 cm (outer diameter maximum 4 mm) with an electromagnetic transmitting tip; therefore, it cannot be used in studies targeting the ileum or colon. If needed, radio-opaque markers can be integrated into the tube design for precise visualization with contrast liquid and fluoroscopy or X-ray.
Adverse events and drop-outs
There are several tube-related adverse events (AEs) that can occur during the phase of tube placement or maintenance. In the studies with healthy individuals, the residence time of the catheter in situ was a maximum of 5 days (Supplemental Table 2). Related AEs included nasopharyngeal discomfort, such as a sore throat, thirst, dysphagia (25), rhinorrhea (98), nasal bleeding, nausea, or throwing up (98). Sinusitis and laryngitis were also tube-related complications (99) but normally occurred during long-term maintenance in patients (>2 weeks), and therefore are not expected to be relevant in short-term trials. Nasopharyngeal discomfort could be partly prevented by using smaller-diameter and/or softer tubes (25). There is a risk of tube misplacement and dislocation (e.g., endobronchial placement) which is often caused by the lack of a gag, swallowing, and cough reflex or by altered consciousness in patients because they cannot indicate what they feel (25). The lack of these reflexes is generally not a problem in healthy subjects. A proper swallowing reflex contributed significantly to the overall tolerance of catheter placement, as shown in patients who had undergone gastroscopy (100). In general, the presence of an intestinal tube in situ can slow down swallowing in healthy subjects (101). Gastrointestinal perforation during forceful tube insertion or the reinsertion of the guidewire with the tube in situ has been described (25), but in the included articles (n = 762 subjects in total) no cases of perforation were mentioned, likely because the catheter or guidewire tips were soft and rounded. Gastro-esophageal reflux with aspiration is a potential AE that can occur during catheter placement or after insertion, because the tube slightly relaxes the lower esophageal sphincter. The risk during placement can be minimized if participants have fasted (e.g., minimum of 6 hours with no solid foods and minimum of 2 hours with no liquid foods before catheter placement). Overall, in the studies, which included a total of 762 subjects, nothing was mentioned about serious AEs, such as reflux with aspiration or perforation. Some studies specifically mentioned that the study procedures were (well) tolerated without AEs (16, 19, 43, 60, 92). Five other studies described AEs which were (possibly) related to the procedure, including dizziness (n = 1) (31), nausea/vomiting (n = 3) (21, 31), nasal irritation (n = 2) (21, 52), distension discomfort from a balloon inflated with 45 ± 9 mL of air (n = 2) (52), and local throat irritation (n = 14) (72).
A summary of the dropouts in the included studies is shown in Table 2. In total, 52 subjects dropped out due to the use of the intestinal catheter, especially due to discomfort induced by the catheter (24 subjects), problems with catheter positioning (18 subjects), or sampling difficulties (6 subjects). Moreover, 11 subjects dropped out because of other reasons not related to the catheter or had no reason mentioned. Overall, a dropout rate of around ∼10% is expected for these types of studies.
TABLE 2.
A summary of the number of dropouts and reasons for dropping out, as reported in the 60 research papers that described clinical trials applying intestinal catheters in human subjects1
| Number of dropouts | Reason for dropping out | Reference |
|---|---|---|
| Discomfort, n = 24 | ||
| 10 | Due to discomfort induced by the catheter/inability to tolerate the catheter, reasons not mentioned. | (10, 13, 14, 24, 72) |
| 2 | Due to nausea and vomiting. | (21, 42) |
| 9 | Due to various difficulties with the tolerance of the tube (failure of the tube to fit through the nose, pain, vomiting, nonmigration through the pylorus, excessive pulling of the tube at the nares). | (39, 58) |
| 3 | Due to discomfort during catheter positioning (e.g., nausea, vomiting). | (34–36) |
| Catheter positioning, n = 18 | ||
| 5 | Due to difficulties when positioning the catheter, or incorrect positioning of the catheter. | (10, 46, 73) |
| 4 | Due to failure to position the tip of the catheter beyond the ligament of Treitz. | (34–36) |
| 7 | The tube did not progress below the upper small intestine. | (19, 21, 74) |
| 2 | The tube did not reach below the mid-ileum. | (19) |
| Sampling difficulties, n = 6 | ||
| 1 | The aspirated sample volume (jejunum) was not sufficient to allow proper evaluation. | (46) |
| 5 | Experiments did not last for the full period or intestinal samples were not obtained, reasons not mentioned. | (47) |
| Other, n = 11 | ||
| 9 | Other reasons or reasons not mentioned (e.g., not properly following the instructions, vasovagal reaction on blood withdrawal). | (2, 10, 20, 31, 46, 92, 96) |
| 2 | Due to the discomfort of the study procedure, reasons not mentioned. | (42) |
From the 60 studies, involving 720 healthy subjects and 42 patients, 56 studies included healthy subjects, 1 study included both healthy subjects and patients, and 3 studies included patients.
Intestinal catheters as tool for intestinal delivery and sampling
Intestinal catheters are often used for delivery (23 studies), sampling (10 studies), or both delivery and sampling (27 studies).
Delivery of compounds inside the intestine
In order to study local dynamic metabolic changes, as well as absorption and digestion processes, a variety of tools are available for delivery: namely, calibrated volumetric pumps (1), infusion pumps (32, 33, 58, 65), peristaltic pumps (68), calibrated (syringe) pumps (48, 61), and motor-driven syringes (49, 50, 52) for delivery at a constant rate or “normal” syringes (31) for 1 bolus delivery. Some of these devices were equipped with a luer-lock fit between the catheter and the syringe (32, 33) or a device that ensures a leak-free connection and should be considered when designing a catheter (luer-lock at the connector end). Before delivery, compounds were dissolved in water (19) or saline solution (0.9% NaCl) (72, 90). Infusion is often performed at a constant rate, expressed in mL/min, kcal/min, or kJ/min when delivering nutrients (Table 3). In the included studies, solutions were delivered within a set period of time [15 min (52), 60 min (24, 36, 61), 90 min (14, 41, 54), or 195 min (15)] or for the full duration of the experimental tests (73). Constant infusion rates can be used to reach steady-state conditions (15). Moreover, compounds were also administered as a single-dose bolus injection that was completed within 5 minutes, regardless of the bolus viscosity and the length and diameter of the tube (Table 3). If preferred, the delivery time can be reduced by concentrating the compounds as much as possible. However, it must be noted that hypertonic solutions can influence peristalsis and can increase intestinal secretions (102), which in turn dilute the infused compound. Increasing the temperature of solutions to 37°C before infusion (61) could make participants more comfortable than using cold infusions, and warm infusions are less likely to cause GI disturbances (103).
TABLE 3.
An overview of the constant rates and single bolus injections that were used for delivery of compounds in the intestine, and the sample volumes that could be obtained from the duodenum and jejunum, as described in 60 clinical trials applying intestinal catheters in human subjects1
| Intestinal delivery | Intestinal sampling | ||||
|---|---|---|---|---|---|
| Constant rates | Single bolus injection | Sample volume, mL | |||
| mL/min | mL/hour | kcal/min or kJ/min | Volume (mL) and timing (minutes) | Duodenum | Jejunum |
| 0.67 mL/min (35) | 50 mL/h (65) | 0.6 kcal/min (35, 77) | 2 mL (32, 33) in 2–3 min | ∼1 mL (20) | ∼1 mL (20) |
| 1 mL/min (34, 36, 39, 73, 75–78, 89, 104) | 220 mL/h (2) | 0.9 kcal/min (34, 36) | 10 mL (19) in 1–2 min | 1 mL (mucus) (30) | 1 mL (mucus) (30) |
| 2 mL/min (10, 14, 41, 48, 54) | — | 1 kcal/min (60) | 10 mL (72, 92) | 0–10 mL (27) | 4 mL per time point (96) |
| 2.5 mL/min (41, 51) | — | 1.35 kcal/min (77) | 10 mL (21) in 5 min | 5–10 mL (65) | >4 mL pooled sample over 30 min (75) |
| 3 mL/min (24, 31) | — | 2 kcal/min (40) | 15 mL (59) | — | 5 mL aliquots (1) |
| 3.15 mL/min (42) | — | 2.5 kJ/min (35) | 40 mL (16, 90) | — | 0–10 mL (27) |
| 5 mL/min (1) | — | — | — | — | 5–10 mL: 60 cm distal to duodenum (65) |
| 10 mL/min (15, 61, 68) | — | — | — | — | >10 mL (26) |
From the 60 studies included; 50 studies used an intestinal catheter for the delivery of compounds (sometimes in combination with sampling).
After delivery, the tube was flushed with water [10–50 mL (46, 59, 92)], saline [range 1–50 mL (17, 18, 32, 33, 72, 90)], or another dissolvent [3 mL (31)] to ensure that all compounds were delivered inside the intestine. The catheter should be flushed with at least the volume of water or saline to rinse the dead space of the tube (calculated according to the equation of cylindrical volume V = πr2h, where r = radius and h = height). The dead space can be minimized by reducing the tube length and lumen diameter. Another important point is the use of a control infusion in randomized controlled trials. In 2 studies, multiple intestinal regions were infused in a randomized, cross-over fashion. At the time of treatment, the solution was delivered to 1 intestinal site while saline (34, 60) or water (24) was infused in the other site(s), and vice versa. This was done to ensure the blinding of the subjects with regards to the timing and the nature of the infusion.
Sampling of intestinal content
Additionally, intestinal catheters can be applied to aspirate intestinal fluids. Adhesion of analytes of interest to the material could be checked for in ex vivo studies. Syringes were often used as a sampling tool by connecting a syringe to the proximal end of the sampling lumen in the intestinal catheter (13, 20, 21, 27, 30, 38) via manual suction (38, 74). To ensure efficient aspiration, in 2 studies, the catheter drainage channels were connected to a vacuum pump (46, 53), which facilitated sampling from the duodenum and jejunum. In other studies, aspirates were obtained using the catheter lever properties [40-cm segment of the proximal small intestine (68)], gravity [jejunum (1)], or simply by syphoning/slight aspiration through the opening of the tube [ileum (39)]. Zilberstein et al. (30) and Wilms et al. (13) used a 20-mL syringe to take samples from the duodenum, jejunum, and ileum, whereas in 1 of our other studies, gentle aspiration using 5-mL syringes was more efficient for aspiration from the ileum (21). In our experience, applying gentle and regular manual suction (37, 61) worked most optimally, although continuous suction (28, 38) was also applied. Stopcocks attached to the syringe and the aspiration channel can be used for opening and closing before and after sampling to prevent air (i.e., oxygen) from going into the anaerobic environment of the cecum (37). Importantly, catheter patency inside the GI tract (i.e., not being vacuumed against the intestinal wall) should be ensured (27) to avoid damage to the intestinal epithelium. Perfusates can be checked for signs of damage, such as blood contamination (52). Overall, sampling rates are dependent on the diameter and total length of the aspiration channel, the tube stiffness, the pressure applied, and the intestinal region sampled (i.e., viscosity of intestinal fluid).
Intestinal sample volumes
The relatively large sample volumes obtained from the duodenum and jejunum indicate that sample collection from the duodenum and jejunum entailed few challenges (Table 3). Flow rates of intestinal contents into fasted test segments were previously estimated to be around 2.2 mL/min in the jejunum and 0.7 mL/min in the ileum in various studies, and flow rates of intestinal contents after a meal were estimated to be 10 mL/min in the proximal jejunum (45). Matsson et al. (46) mentioned that in 1 subject (from the n = 8 in total), the aspirated sample volume from the duodenum and jejunum was not sufficient (<3 mL after 1 hour of sampling time). Jejunal samples were collected at 10–15-minute intervals (15, 48, 50–52, 54) over periods of 1.5 hours (54) to 6 hours (52, 78), and the volumes of the jejunum aspirates ranged from 0 to >10 mL (Table 3). The short sampling intervals of sample collection indicate rapid sampling. In contrast, samples from the ileum were collected in aliquots at longer intervals of 30 minutes (28, 29, 75), 1 hour (38, 104), 2 hours (74), or until enough sample was obtained (37), over a total maximum period of 8 hours (28, 29, 74, 76, 78, 104). When samples are collected at multiple time points, the remaining intestinal fluid in the aspiration channel after sampling can be reinjected into the intestine to minimize contamination caused by sample remainders present in the dead space volume (27). Gaudichon et al. (104) indicated that sample collection in the fasted state (volume not mentioned) from the ileum took 30 minutes; in another study (28), 1 subject was excluded due to practical problems concerning sampling of ileal content (lumen diameter 1.5 mm). For thesup sampling of colonic content in 1 study, >5 mL (5 to 50 mL) was aspirated [(37) diameter 3.5 mm]. After collection, samples needed to be put on (dry) ice to stop enzyme and/or bacterial activity.
Intestinal sampling rates
In our study [(13) unpublished data] duodenum, jejunum, and ileum samples were aspirated using a multi-lumen ileal catheter at several time points. The diameters of the duodenum and jejunum aspiration channels were 0.65 mm, and the diameter of the ileum aspiration channel was 0.9 mm. In a representative selection of 3 subjects, we found that duodenum samples were aspirated at a rate of 0.45 (0.61 [IQR]) mL/min, jejunum samples at 0.37 (0.36 [IQR]) mL/min, and ileum samples at 0.30 (0.17 [IQR]) mL/min. Another study reported a sampling rate of 0.5 mL/min for the proximal and distal jejunum (61). In conclusion, the sampling speed decreases with an increasing distance from the sampling syringe to the specific intestinal segment (i.e., speed is highest in the duodenum and lowest in the ileum), despite the highest diameter being in the ileum aspiration channel. In the FiberKinetics study (21), 2–3-mL distal ileum samples were aspirated per time point using a naso-ileal catheter (300-cm long, 1.9-mm diameter aspiration channel) over a span of 340 minutes. Due to sampling difficulties, it was not possible to aspirate a sample in the fasted state. The ileum samples were aspirated at a rate of 0.35 (0.05 [IQR]) mL/minafter consumption of a drink with water-soluble dietary fibers. In most studies, comprehensive information regarding sampling volumes, rates, and difficulties were lacking (Supplemental Table 2).
Practical procedures to improve sampling rates
Marteau et al. (37) provided a standard meal to subjects before sampling from the colon, because colonic sampling after an overnight fast was not always possible (105). In 1 of our studies (21), it was also not possible to obtain a sample after an overnight fast, but a sample was collected after consumption of a fiber-rich drink. This suggests that providing subjects with a drink including dissolved macronutrients improves sampling. Feeding increases the flow rates of intestinal contents in the jejunum, ileum, and terminal ileum in human subjects (106). Additionally, in the case of unsuccessful sampling, nitrogen gas (5–10 mL) can be flushed through the lumen to ensure catheter patency, followed by gentle suction until a sample is collected (37). For colonic sampling specifically, insertion of nitrogen gas is preferred over ambient air, to not disturb the anaerobic environment of the colon. Marteau et al. (37) obtained a colonic sample (5–50 mL) 2 hours after a meal in 64% of the experiments, and 2.5–3 hours after a meal in the other 36% (aspiration channel 3.5 mm). Another option to improve sampling speed is the delivery of water or saline, preferably with a dilution marker, via the catheter channel directly in the sampling location. However, this should be considered and carried out carefully because the intestinal fluid, and therefore the analytes of interest, may become too diluted for analyses. Troost et al. (15) measured a dilution of ∼100-fold in the intestinal samples. The dilution factor can be measured with the addition of inert, nonabsorbable recovery markers, such as PEG-4000 or phenol red, and can be corrected if needed. Repeated flushing with 10 mL of physiological salt without the addition of compounds through a port of the naso-ileal catheter shifted the relative microbiota composition (107). Alternating the position of the subjects, such as having them sit, lie, or walk, could also improve sampling rates. One study mentioned that sampling took place while the subjects were in a semi-recumbent position (76).
Discussion
We reviewed practical and technical aspects of using naso- and oro-intestinal catheters in human studies for delivering compounds and sampling luminal fluids from the jejunum, ileum, and colon in vivo. An extensive review of the available literature was provided to include experiences of experts in this field. We also included insights obtained during the execution of our own clinical trials. A limited number of studies used colonic catheters as compared to small intestinal catheters. Unfortunately, the catheter design and any related study procedures were often only briefly described. To aid future researchers, we recommend describing all procedures related to the use of the intestinal catheter in detail. This will facilitate comparisons between clinical trials and improve the reproducibility of results, allowing other researchers to benefit from the information when designing and performing new studies.
Naso- and oro-intestinal catheters
Intestinal catheters have been useful tools in studying in vivo processes of the GI tract, greatly advancing existing knowledge in the fields of physiology, gut microbiota, nutrition, and pharmacology. These in vivo studies are superior to in vitro and ex vivo models of the GI tract due to the presence of all complex and relevant physiological processes. Nowadays, tubes can be custom-made, with multiple options for the catheter design (e.g., the number of lumen), which can be used to sample and deliver to very specific sites in the intestinal region of interest. The more advanced catheters are often reusable, reducing costs when using this medical device to study in vivo processes. As seen during early investigations (108), the possibility of including inflatable accessories at the distal tip of the catheter facilitates (rapid) intubation of the distal small intestine and proximal colon. This allows researchers to study these relatively inaccessible regions in people. Applying naso- and oro-intestinal catheters for the study of distal regions of the GI tract is a time-consuming procedure, which can be considered a disadvantage. The practical tools outlined in this paper can be helpful to improve intestinal sampling from the ileum and colon. Although the use of these tools can cause discomfort in participants, resulting in a potentially more challenging recruitment procedure, we are not aware of serious AEs reported in the literature, and the dropout rate seems to be acceptable (∼10%). Most study participants had no direct benefit from the procedure or the study results. Offering a disproportionate financial reimbursement for participating in the study is ethically doubtful; therefore, reimbursement should mainly compensate for the invested time and body measurements. An overview of the main recommendations related to the catheter design, catheter placement, determination of catheter location, and intestinal delivery or sampling is provided in Table 4.
TABLE 4.
A nonexclusive overview of the main recommendations when working with intestinal catheters
| Phase | Recommendations |
|---|---|
| Catheter design (details in section titled “The use of intestinal catheters in research”) | Best tolerated: outer diameter of ≤3.5 mm, soft material (e.g., silicone), intubation via the nose. |
| Dependent on intestinal region of interest, and aim of use, decide on: 1) Total length of the tube; 2) Number and diameter of lumen (delivery compared with sampling); 3) Number of side holes → multiple side holes reduce the risk of obtaining no sample; 4) For distal jejunum/ileum and colon: include inflatable balloon or bags. |
|
| Include radio-opaque markers for visualization. | |
| Stiffeners reduce the risk of tube coiling. | |
| Catheter placement (details in section titled “Placement, progression, and removal of the catheter”) | Use medical lubrication gel. |
| Pro-motility drugs/other medication can be used but may influence study outcomes. | |
| The use of a guidewire/endoscope can assist placement of shorter tubes (often maximally 150–200 cm). | |
| Specific body position of the participant, such as lying on the right side, can assist correct placement. | |
| Stick to a maximum insertion rate to prevent tube coiling. | |
| Inflate the balloon/bag to stimulate catheter progression. | |
| Check the progression regularly (e.g., via radiography or pH). | |
| Stimulate progression by offering drinks/food and encourage participant to move/walk periodically. | |
| Catheter location determination (details in section titled “Determination of the catheter location”) | Fluoroscopy and X-ray enable visualization of radio-opaque markers → fluoroscopy provides lower radiation dosage. |
| Examination with radiography and contrast liquid enables catheter position assessment with respect to intestinal anatomical structures (e.g., ileocecal valve). | |
| The use of centimeter distance of tube insertion, the pH of aspirate, or an enteral access system are not specific. | |
| Intestinal delivery (details in section titled “Intestinal catheters as tool for intestinal delivery and sampling”) | Use of pumps and motor-driven syringes to deliver at a constant rate. |
| Ensure a leak-free connection by luer-lock equipment. | |
| Avoid delivery of a hypertonic solution. | |
| Infusion of a prewarmed solution (body temperature) is more comfortable for the participant. | |
| Use inert nonabsorbable recovery markers to correct for dilution. | |
| Correct for the dead space volume of the tube. | |
| Intestinal sampling (details in section titled “Intestinal catheters as tool for intestinal delivery and sampling”) | Choose the number of time points and intervals of sample collection wisely. |
| Apply gentle and regular manual suction during sampling. | |
| Ensure catheter patency when there are sampling difficulties → inject ambient air (small intestine) or nitrogen gas (colon). | |
| Correct for the dead volume of the tube → e.g., discard dead space volume. | |
| Providing drinks/food, and alternating positioning of subjects (short walks etc.). |
The recommendations are a combination of expert experiences as obtained from the papers included in this literature review and of insights obtained during the execution of our own clinical trials.
Research gaps
Many papers used only the centimeter indication on the sampling tube to locate the catheter in the ileum for delivery and/or sampling. However, the length of the small intestine/ileum can vary substantially between subjects. This makes it difficult to determine beforehand how far the catheter needs to be inserted to reach the distal ileum or proximal colon in a study subject. Currently, there is no best practice for placing the catheter in exactly the same location in the intestine in all subjects in the same trial, which could be of importance when interpreting the study outcomes. Secondly, having an intestinal tube with a latex balloon in situ increased the gastric emptying time and decreased small bowel residence (109), and a small, inflated balloon influenced motor patterns (110). Therefore, we cannot exclude the possibility that intestinal intubations affect GI-tract functioning and the luminal environment, which can be of potential concern. Having a control group with the tube in situ in the study design is important when testing interventions. More research is needed to determine the time period between intestinal intubation and the return to a normal luminal environment. In microbiome studies, potential contamination from the upper GI-tract regions should be considered when using this tool for sample collection from the ileum or colon, although the transit of bacteria can also be considered a physiological aspect. How important the disruption is and how long the bacteria from nonsampling sites (potentially) remain in the sampling site are currently unknown. As the total bacteria load in the ileum or colon is higher when compared to more proximal GI-tract regions (111), the impact is likely minor.
Alternatives for intestinal delivery and sampling in vivo
Alternatives for delivery and sampling in the terminal ileum and colon are colonoscopies and rectal/anal catheters (intra-colonic tubing) (112, 113). To reach the terminal ileum and proximal colon, laxatives are administered to prepare the bowel for a colonoscopy. During this step, the luminal environment may become disturbed, resulting in changes in the microbiota load, diversity, and composition (114–116). Alternatively, enemas were used to clean the distal colon (30–40 cm), leaving the proximal colonic content undisturbed. Reaching these segments can be challenging for the endoscopist, although catheters are normally positioned within ∼45 minutes. The procedure can be invasive for subjects, especially when sampling or infusing at multiple time points. In this case, rectally placed catheters can be attached to the colonic mucosa to secure the position with an endoclip fixation technique (112). Reaching and studying the distal colon is easier as compared to the more proximal colon, since the length of the endoscope/catheter to be introduced is shorter and no laxatives or sedative agents are needed. Endoscopies can be used to obtain epithelial tissue in different anatomical regions of the GI tract, whereas oro- and naso-intestinal catheters only allow sampling of intestinal lumen content or mucus. Compared to the intestinal lumen content, epithelial tissue provides more information about the host. Nonendoscopic biopsy techniques are available or currently in development, such as (wireless) biopsy capsules (117, 118). Intestinal luminal content and tissue samples can be collected from sudden death victims (119) or during surgery, which is mainly limited to patients (120). These kinds of samples cannot easily be combined with an intervention. Noninvasive in vivo studies have been performed in patients with an ostomy bag attached to the small intestine or colon. This allowed researchers to collect samples from the ostomy bag and test for absorption and digestion during oro-ileal transit (121). It is not clear whether these patients are representative of the healthy population, but microbiota encountered in ileostomy effluent resembled microbiota in the proximal small intestine in healthy subjects (122).
For intestinal delivery, capsules coated with a pH‐dependent film have been used (123, 124). Since the delivery is gradual and depends on local pH levels, it is impossible to guarantee a continuous delivery rate (mL/min) to reach steady-state conditions, and researchers have no control over the complete volume that is delivered. Alternatively, more advanced capsules with in vivo real-time monitoring possibilities can be used for targeted intestinal delivery (125). Nowadays, efforts are being put into the development of novel gastrointestinal sampling capsules (126–128), which are described in the review of Tang et al. (127). For future studies, there are multiple possibilities to consider for intestinal delivery and sampling in vivo, all of them with specific advantages and disadvantages.
Conclusions
This extensive review is relevant for researchers active in various research areas during the set-up and execution of experiments using intestinal catheters in human subjects. We provided an overview with technical and practical, expert-based information on the use of intestinal catheters. Catheters are often used for intestinal delivery and fluid sampling to obtain direct data on the human intestinal (patho)physiology. Custom-made state-of-the-art catheters are available with numerous options for the design, and development of new catheters is ongoing. Hence, researchers can control sampling and delivery sites in the intestinal region of interest. The use of intestinal catheters enabled intubations of the distal small intestine and proximal colon, allowing the study of relatively inaccessible regions in humans. Although working with catheters might pose challenges to the researcher, clinician, and study participants, most challenges can be overcome. These challenges do not outweigh the numerous advantages of catheter use. The dropout rate and overall burden to healthy subjects caused by related study procedures seem to be acceptable.
Supplementary Material
Acknowledgments
We thank Tim Klaassen (Division of Gastroenterology and Hepatology, Maastricht University Medical Center, the Netherlands) for information about radiation data measured in his study and for sharing his expertise about naso-ileal catheters, and Bart Vermolen (Medical Physicist, Hospital Gelderse Vallei, the Netherlands) for calculations of the exposure radiation dosage. Furthermore, we thank Maaike Witjes-Kroon (Hospital Gelderse Vallei, the Netherlands) for medical assistance during the FiberKinetics study and for discussions about intestinal catheters. We thank Prof. Dr. Dirk-Jan Reijngoud (University Medical Center Groningen, the Netherlands) for a critical review of the manuscript. We thank members from the Wageningen University Library for their support during the conceptualization of the search terms and search strategy.
The authors’ responsibilities were as follows—MPHvT, EW, FJT, BJMW, and GJEJH: designed the research and search strategy; MPHvT: searched the literature and analyzed the included papers; MPHvT and EW: wrote the original draft; and all authors: wrote, reviewed, and edited the manuscript and read and approved the final manuscript. The authors report no conflicts of interest.
Notes
This work was funded by the public-private partnership “CarboKinetics,” coordinated by the Carbohydrate Competence Center (www.cccresearch.nl). CarboKinetics is financed by participating industrial partners Agrifirm Innovation Center B.V., Nutrition Sciences N.V., Cooperatie Avebe U.A., DSM Food Specialties B.V., VanDrie Holding N.V., and Sensus B.V., and by allowances of The Netherlands Organization for Scientific Research (NWO).
The funders had no role in data collection and analysis, or preparation of the manuscript.
Supplemental Tables 1 and 2 are available from the “Supplemental Information” link in the online posting of the article at https://academic.oup.com/ajcn/.
Abbreviations used: AE, adverse event; GI, gastrointestinal; NaCl, sodium chloride; PEG, polyethylene glycol; TMPD, transmucosal potential difference gradient.
Contributor Information
Mara PH van Trijp, Division of Human Nutrition and Health, Wageningen University, Wageningen, The Netherlands.
Ellen Wilms, Division Gastroenterology-Hepatology, Department of Internal Medicine, NUTRIM School of Nutrition and Translational Research in Metabolism, Maastricht University, Maastricht, The Netherlands.
Melany Ríos-Morales, Laboratory of Pediatrics, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands.
Ad Am Masclee, Division Gastroenterology-Hepatology, Department of Internal Medicine, NUTRIM School of Nutrition and Translational Research in Metabolism, Maastricht University, Maastricht, The Netherlands.
Robert Jan Brummer, Nutrition-Gut-Brain Interactions Research Centre, School of Medical Sciences, Faculty of Medicine and Health, Örebro University, Örebro, Sweden.
Ben Jm Witteman, Division of Human Nutrition and Health, Wageningen University, Wageningen, The Netherlands; Hospital Gelderse Vallei, Department of Gastroenterology and Hepatology, Ede, The Netherlands.
Freddy J Troost, Division Gastroenterology-Hepatology, Department of Internal Medicine, NUTRIM School of Nutrition and Translational Research in Metabolism, Maastricht University, Maastricht, The Netherlands; Food Innovation and Health, Centre for Healthy Eating and Food Innovation, Maastricht University, Maastricht, The Netherlands.
Guido Jej Hooiveld, Division of Human Nutrition and Health, Wageningen University, Wageningen, The Netherlands.
Data Availability
Data described in the manuscript, code book, and analytic code will be made available upon reasonable request
References
- 1.Nagy K, Ramos L, Courtet-Compondu MC, Braga-Lagache S, Redeuil K, Lobo B, Azpiroz F, Malagelada JR, Beaumont M, Moulin Jet al. . Double-balloon jejunal perfusion to compare absorption of vitamin E and vitamin E acetate in healthy volunteers under maldigestion conditions. Eur J Clin Nutr. 2013;67(2):202–6. [DOI] [PubMed] [Google Scholar]
- 2.Luttikhold J, van Norren K, Buijs N, Ankersmit M, Heijboer AC, Gootjes J, Rijna H, van Leeuwen PA, van Loon LJ. Jejunal casein feeding is followed by more rapid protein digestion and amino acid absorption when compared with gastric feeding in healthy young men. J Nutr. 2015;145(9):2033–8. [DOI] [PubMed] [Google Scholar]
- 3.Strocchi A, Levitt MD. Measurement of starch absorption in humans. Can J Physiol Pharmacol. 1991;69(1):108–10. [DOI] [PubMed] [Google Scholar]
- 4.Flourie B, Vidon N, Florent CH, Bernier JJ. Effect of pectin on jejunal glucose absorption and unstirred layer thickness in normal man. Gut. 1984;25(9):936–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hecketsweiler P, Vidon N, Emonts P, Bernier JJ. Absorption of elemental and complex nutritional solutions during a continuous jejunal perfusion in man. Digestion. 1979;19(3):213–7. [DOI] [PubMed] [Google Scholar]
- 6.Silk DB, Perrett D, Webb JP, Clark ML. Absorption of two tripeptides by the human small intestine: A study using a perfusion technique. Clin Sci Mol Med. 1974;46(3):393–402. [DOI] [PubMed] [Google Scholar]
- 7.Singh RK, Chang H-W, Yan D, Lee KM, Ucmak D, Wong K, Abrouk M, Farahnik B, Nakamura M, Zhu THet al. . Influence of diet on the gut microbiome and implications for human health. J Transl Med. 2017;15(1):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chalet C, Rubbens J, Tack J, Duchateau GS, Augustijns P. Intestinal disposition of quercetin and its phase-II metabolites after oral administration in healthy volunteers. J Pharm Pharmacol. 2018;70(8):1002–8. [DOI] [PubMed] [Google Scholar]
- 9.Vasapolli R, Schütte K, Schulz C, Vital M, Schomburg D, Pieper DH, Vilchez-Vargas R, Malfertheiner P. Analysis of transcriptionally active bacteria throughout the gastrointestinal tract of healthy individuals. Gastroenterology. 2019;157(4):1081–1092.e3. [DOI] [PubMed] [Google Scholar]
- 10.Klaassen T, Alleleyn AME, van Avesaat M, Troost FJ, Keszthelyi D, Masclee AAM. Intraintestinal delivery of tastants using a naso-duodenal-ileal catheter does not influence food intake or satiety. Nutrients. 2019;11(2):472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ripken D, van Avesaat M, Troost FJ, Masclee AA, Witkamp RF, Hendriks HF. Intraileal casein infusion increases plasma concentrations of amino acids in humans: a randomized cross over trial. Clin Nutr. 2017;36(1):143–9. [DOI] [PubMed] [Google Scholar]
- 12.Reisz JA, D'Alessandro A. Measurement of metabolic fluxes using stable isotope tracers in whole animals and human patients. Curr Opin Clin Nutr Metab Care. 2017;20(5):366–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilms E, Gerritsen J, Smidt H, Besseling-van der Vaart I, Rijkers GT, Garcia Fuentes AR, Masclee AA, Troost FJ. Effects of supplementation of the synbiotic ecologic(R) 825/FOS P6 on intestinal barrier function in healthy humans: a randomized controlled trial. PLoS One. 2016;11(12):e0167775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Avesaat M, Troost FJ, Ripken D, Hendriks HF, Masclee AA. Ileal brake activation: macronutrient-specific effects on eating behavior?. Int J Obes. 2015;39(2):235–43. [DOI] [PubMed] [Google Scholar]
- 15.Troost FJ, Saris WH, Haenen GR, Bast A, Brummer RJ. New method to study oxidative damage and antioxidants in the human small bowel: effects of iron application. Am J Physiol Gastrointest Liver Physiol. 2003;285(2):G354–9. [DOI] [PubMed] [Google Scholar]
- 16.Borg MJ, Bound M, Grivell J, Sun Z, Jones KL, Horowitz M, Rayner CK, Wu T. Comparative effects of proximal and distal small intestinal administration of metformin on plasma glucose and glucagon-like peptide-1, and gastric emptying after oral glucose, in type 2 diabetes. Diabetes Obes Metab. 2019;21(3):640–7. [DOI] [PubMed] [Google Scholar]
- 17.Ge XL, Tian HL, Ding C, Gu LL, Wei Y, Gong JF, Zhu WM, Li N, Li JS. Fecal microbiota transplantation in combination with soluble dietary fiber for treatment of slow transit constipation: a pilot study. Arch Med Res. 2016;47(3):236–42. [DOI] [PubMed] [Google Scholar]
- 18.Angelberger S, Reinisch W, Makristathis A, Lichtenberger C, Dejaco C, Papay P, Novacek G, Trauner M, Loy A, Erry DB. Temporal bacterial community dynamics vary among ulcerative colitis patients after fecal microbiota transplantation. Am J Gastroenterol. 2013;108(10):1620–30. [DOI] [PubMed] [Google Scholar]
- 19.Dohil R, Fidler M, Barshop BA, Gangoiti J, Deutsch R, Martin M, Schneider JA. Understanding intestinal cysteamine bitartrate absorption. J Pediatr. 2006;148(6):764–9. [DOI] [PubMed] [Google Scholar]
- 20.Seekatz AM, , Schnizlein MK, Koenigsknecht MJ, Baker JR, Hasler WL, Bleske BE, Young VB, Sun D. Spatial and temporal analysis of the stomach and small-intestinal microbiota in fasted healthy humans. mSphere. 2019;4(2):e00126–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. ClinicalTrials.gov [Internet]. National Library of Medicine (US). Identifier NCT04013607, Fiber Fermentation Kinetics Inside the Gut, and Utilization of Bacterial Metabolites; July 10, 2019 [cited 15 March 2020]. Available from: https://www.clinicaltrials.gov/ct2/show/NCT04013607. [Google Scholar]
- 22.Schönfeld J, Evans DF, Wingate DL. Effect of viscous fiber (guar) on postprandial motor activity in human small bowel. Dig Dis Sci. 1997;42(8):1613–7. [DOI] [PubMed] [Google Scholar]
- 23.Kuo B, Castell DO. The effect of nasogastric intubation on gastroesophageal reflux: a comparison of different tube sizes. Am J Gastroenterol. 1995;90(10):1804–7. [PubMed] [Google Scholar]
- 24.van Avesaat M, Ripken D, Hendriks HF, Masclee AA, Troost FJ. Small intestinal protein infusion in humans: evidence for a location-specific gradient in intestinal feedback on food intake and GI peptide release. Int J Obes. 2017;41(2):217–24. [DOI] [PubMed] [Google Scholar]
- 25.Braegger C, Decsi T, Dias JA, Hartman C, Kolacek S, Koletzko B, Koletzko S, Mihatsch W, Moreno L, Puntis Jet al. . Practical approach to paediatric enteral nutrition: a comment by the ESPGHAN Committee on Nutrition. J Pediatr Gastroenterol Nutr. 2010;51(1):110–22. [DOI] [PubMed] [Google Scholar]
- 26.Perez de la Cruz Moreno M, Oth M, Deferme S, Lammert F, Tack J, Dressman J, Augustijns P. Characterization of fasted-state human intestinal fluids collected from duodenum and jejunum. J Pharm Pharmacol. 2010;58(8):1079–89. [DOI] [PubMed] [Google Scholar]
- 27.Brouwers J, Ingels F, Tack J, Augustijns P. Determination of intraluminal theophylline concentrations after oral intake of an immediate- and a slow-release dosage form. J Pharm Pharmacol. 2010;57(8):987–95. [DOI] [PubMed] [Google Scholar]
- 28.Mariotti F, Pueyo ME, Tome D, Berot S, Benamouzig R, Mahe S. The influence of the albumin fraction on the bioavailability and postprandial utilization of pea protein given selectively to humans. J Nutr. 2001;131(6):1706–13. [DOI] [PubMed] [Google Scholar]
- 29.Mariotti F, Mahe S, Luengo C, Benamouzig R, Tome D. Postprandial modulation of dietary and whole-body nitrogen utilization by carbohydrates in humans. Am J Clin Nutr. 2000;72(4):954–62. [DOI] [PubMed] [Google Scholar]
- 30.Zilberstein B, Quintanilha AG, Santos MAA, Pajecki D, Moura EG, Alves PRA, Maluf F, de Souza JA, Gama-Rodrigues J. Digestive tract microbiota in healthy volunteers. Clinics. 2007;62(1):47–54. [DOI] [PubMed] [Google Scholar]
- 31.Dahlgren D, Roos C, Lundqvist A, Abrahamsson B, Tannergren C, Hellstrom PM, Sjogren E, Lennernas H. Regional intestinal permeability of three model drugs in human. Mol Pharm. 2016;13(9):3013–21. [DOI] [PubMed] [Google Scholar]
- 32.Seidegard J, Nyberg L, Borga O. Differentiating mucosal and hepatic metabolism of budesonide by local pretreatment with increasing doses of ketoconazole in the proximal jejunum. Eur J Pharm Sci. 2012;46(5):530–6. [DOI] [PubMed] [Google Scholar]
- 33.Seidegard J, Nyberg L, Borga O. Presystemic elimination of budesonide in man when administered locally at different levels in the gut, with and without local inhibition by ketoconazole. Eur J Pharm Sci. 2008;35(4):264–70. [DOI] [PubMed] [Google Scholar]
- 34.Maljaars PW, van der Wal RJ, Wiersma T, Peters HP, Haddeman E, Masclee AA. The effect of lipid droplet size on satiety and peptide secretion is intestinal site-specific. Clin Nutr. 2012;31(4):535–42. [DOI] [PubMed] [Google Scholar]
- 35.Maljaars PW, Peters HP, Kodde A, Geraedts M, Troost FJ, Haddeman E, Masclee AA. Length and site of the small intestine exposed to fat influences hunger and food intake. Br J Nutr. 2011;106(10):1609–15. [DOI] [PubMed] [Google Scholar]
- 36.Maljaars J, Romeyn EA, Haddeman E, Peters HP, Masclee AA. Effect of fat saturation on satiety, hormone release, and food intake. Am J Clin Nutr. 2009;89(4):1019–24. [DOI] [PubMed] [Google Scholar]
- 37.Marteau P, Pochart P, Dore J, Bera-Maillet C, Bernalier A, Corthier G. Comparative study of bacterial groups within the human cecal and fecal microbiota. Appl Environ Microbiol. 2001;67(10):4939–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vesa T, Pochart P, Marteau P. Pharmacokinetics of Lactobacillus plantarum NCIMB 8826, Lactobacillus fermentum KLD, and Lactococcus lactis MG 1363 in the human gastrointestinal tract. Aliment Pharmacol Ther. 2000;14(6):823–8. [DOI] [PubMed] [Google Scholar]
- 39.Oberli M, Marsset-Baglieri A, Airinei G, Sante-Lhoutellier V, Khodorova N, Remond D, Foucault-Simonin A, Piedcoq J, Tome D, Fromentin Get al. . High true ileal digestibility but not postprandial utilization of nitrogen from bovine meat protein in humans is moderately decreased by high-temperature, long-duration cooking. J Nutr. 2015;145(10):2221–8. [DOI] [PubMed] [Google Scholar]
- 40.Wu T, Thazhath SS, Marathe CS, Bound MJ, Jones KL, Horowitz M, Rayner CK. Comparative effect of intraduodenal and intrajejunal glucose infusion on the gut-incretin axis response in healthy males. Nutr Diabetes. 2015;5:e156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takamatsu N, Kim ON, Welage LS, Idkaidek NM, Hayashi Y, Barnett J, Yamamoto R, Lipka E, Lennernas H, Hussain Aet al. . Human jejunal permeability of two polar drugs: cimetidine and ranitidine. Pharm Res. 2001;18(6):742–4. [DOI] [PubMed] [Google Scholar]
- 42.Rigda RS, Trahair LG, Little TJ, Wu T, Standfield S, Feinle-Bisset C, Rayner CK, Horowitz M, Jones KL. Regional specificity of the gut-incretin response to small intestinal glucose infusion in healthy older subjects. Peptides. 2016;86:126–32. [DOI] [PubMed] [Google Scholar]
- 43.Wu TZ, Bound MJ, Standfield SD, Jones KL, Horowitz M, Rayner CK. Effects of taurocholic acid on glycemic, glucagon-like peptide-1, and insulin responses to small intestinal glucose infusion in healthy humans. J Clin Endocrinol Metab. 2013;98(4):E718–E22. [DOI] [PubMed] [Google Scholar]
- 44.Cooper H, Levitan R, Fordtran JS, Ingelfinger FJ. A method for studying absorption of water and solute from the human small intestine. Gastroenterology. 1966;50(1):1–7. [PubMed] [Google Scholar]
- 45.Modigliani R, Rambaud JC, Bernier JJ. The method of intraluminal perfusion of the human small intestine. I. Principle and technique. Digestion. 1973;9(2):176–92. [DOI] [PubMed] [Google Scholar]
- 46.Matsson EM, Eriksson UG, Palm JE, Artursson P, Karlgren M, Lazorova L, Brannstrom M, Ekdahl A, Duner K, Knutson Let al. . Combined in vitro–in vivo approach to assess the hepatobiliary disposition of a novel oral thrombin inhibitor. Mol Pharm. 2013;10(11):4252–62. [DOI] [PubMed] [Google Scholar]
- 47.Knutson L, Koenders DJ, Fridblom H, Viberg A, Sein A, Lennernas H. Gastrointestinal metabolism of a vegetable-oil emulsion in healthy subjects. Am J Clin Nutr. 2010;92(3):515–24. [DOI] [PubMed] [Google Scholar]
- 48.Lennernas H, Knutson L, Knutson T, Hussain A, Lesko L, Salmonson T, Amidon GL. The effect of amiloride on the in vivo effective permeability of amoxicillin in human jejunum: experience from a regional perfusion technique. Eur J Pharm Sci. 2002;15(3):271–7. [DOI] [PubMed] [Google Scholar]
- 49.Glaeser H, Drescher S, Hofmann U, Heinkele G, Somogyi AA, Eichelbaum M, Fromm MF. Impact of concentration and rate of intraluminal drug delivery on absorption and gut wall metabolism of verapamil in humans. Clin Pharmacol Ther. 2004;76(3):230–8. [DOI] [PubMed] [Google Scholar]
- 50.Drescher S, Glaeser H, Murdter T, Hitzl M, Eichelbaum M, Fromm MF. P-glycoprotein-mediated intestinal and biliary digoxin transport in humans. Clin Pharmacol Ther. 2003;73(3):223–31. [DOI] [PubMed] [Google Scholar]
- 51.Glaeser H, Drescher S, van der Kuip H, Behrens C, Geick A, Burk O, Dent J, Somogyi A, Von Richter O, Griese EUet al. . Shed human enterocytes as a tool for the study of expression and function of intestinal drug-metabolizing enzymes and transporters. Clin Pharmacol Ther. 2002;71(3):131–40. [DOI] [PubMed] [Google Scholar]
- 52.von Richter O, Greiner B, Fromm MF, Fraser R, Omari T, Barclay ML, Dent J, Somogyi AA, Eichelbaum M. Determination of in vivo absorption, metabolism, and transport of drugs by the human intestinal wall and liver with a novel perfusion technique. Clin Pharmacol Ther. 2001;70(3):217–27. [DOI] [PubMed] [Google Scholar]
- 53.Bergman E, Forsell P, Persson EM, Knutson L, Dickinson P, Smith R, Swaisland H, Farmer MR, Cantarini MV, Lennernas H. Pharmacokinetics of gefitinib in humans: the influence of gastrointestinal factors. Int J Pharm. 2007;341(1-2):134–42. [DOI] [PubMed] [Google Scholar]
- 54.Persson EM, Nilsson RG, Hansson GI, Lofgren LJ, Liback F, Knutson L, Abrahamsson B, Lennernas H. A clinical single-pass perfusion investigation of the dynamic in vivo secretory response to a dietary meal in human proximal small intestine. Pharm Res. 2006;23(4):742–51. [DOI] [PubMed] [Google Scholar]
- 55.Campo R, Montserrat A, Brullet E. Transnasal gastroscopy compared to conventional gastroscopy: a randomized study of feasibility, safety, and tolerance. Endoscopy. 1998;30(05):448–52. [DOI] [PubMed] [Google Scholar]
- 56.Soma Y, Saito H, Kishibe T, Takahashi T, Tanaka H, Munakata A. Evaluation of topical pharyngeal anesthesia for upper endoscopy including factors associated with patient tolerance. Gastrointest Endosc. 2001;53(1):14–8. [DOI] [PubMed] [Google Scholar]
- 57.Wood C. New strategy to ease the discomfort of insertion of nasogastric tubes. Int J Clin Pract. 2005;59(12):1373–4. [DOI] [PubMed] [Google Scholar]
- 58.Poppitt SD, Shin HS, McGill AT, Budgett SC, Lo K, Pahl M, Duxfield J, Lane M, Ingram JR. Duodenal and ileal glucose infusions differentially alter gastrointestinal peptides, appetite response, and food intake: a tube feeding study. Am J Clin Nutr. 2017;106(3):725–35. [DOI] [PubMed] [Google Scholar]
- 59.Drewe J, Narjes H, Heinzel G, Brickl RS, Rohr A, Beglinger C. Absorption of lefradafiban from different sites of the gastrointestinal tract. Br J Clin Pharmacol. 2000;50(1):69–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chaikomin R, Wu KL, Doran S, Meyer JH, Jones KL, Feinle-Bisset C, Horowitz M, Rayner CK. Effects of mid-jejunal compared to duodenal glucose infusion on peptide hormone release and appetite in healthy men. Regul Pept. 2008;150(1–3):38–42. [DOI] [PubMed] [Google Scholar]
- 61.Coeffier M, Hecketsweiler B, Hecketsweiler P, Dechelotte P. Effect of glutamine on water and sodium absorption in human jejunum at baseline and during PGE1-induced secretion. J Appl Physiol. 2005;98(6):2163–8. [DOI] [PubMed] [Google Scholar]
- 62.Cohen LD, Alexander DJ, Catto J, Mannion R. Spontaneous transpyloric migration of a ballooned nasojejunal tube: a randomized controlled trial. JPEN J Parenter Enteral Nutr. 2000;24(4):240–3. [DOI] [PubMed] [Google Scholar]
- 63.Deloose E, Janssen P, Depoortere I, Tack J. The migrating motor complex: control mechanisms and its role in health and disease. Nat Rev Gastroenterol Hepatol. 2012;9(5):271–85. [DOI] [PubMed] [Google Scholar]
- 64.Jiang Q-J, Jiang C-F, Chen Q-T, Shi J, Shi B. Erythromycin for promoting the postpyloric placement of feeding tubes: a systematic review and meta-analysis. Gastroenterol Res Pract. 2018;2018:1671483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nissinen M, Gylling H, Vuoristo M, Miettinen TA. Micellar distribution of cholesterol and phytosterols after duodenal plant stanol ester infusion. Am J Physiol Gastrointest Liver Physiol. 2002;282(6):G1009–15. [DOI] [PubMed] [Google Scholar]
- 66.Zarate N, Mohammed SD, O'Shaughnessy E, Newell M, Yazaki E, Williams NS, Lunniss PJ, Semler JR, Scott SM. Accurate localization of a fall in pH within the ileocecal region: validation using a dual-scintigraphic technique. Am J Physiol Gastrointest Liver Physiol. 2010;299(6):G1276–G86. [DOI] [PubMed] [Google Scholar]
- 67.Rafferty GP, Tham TC. Endoscopic placement of enteral feeding tubes. World J Gastrointest Endosc. 2010;2(5):155–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Troost FJ, Brummer RJ, Haenen GR, Bast A, van Haaften RI, Evelo CT, Saris WH. Gene expression in human small intestinal mucosa in vivo is mediated by iron-induced oxidative stress. Physiol Genomics. 2006;25(2):242–9. [DOI] [PubMed] [Google Scholar]
- 69.Mahadeva S, Malik A, Hilmi I, Qua C-S, Wong C-H, Goh K-L. Transnasal endoscopic placement of nasoenteric feeding tubes: outcomes and limitations in non–critically ill patients. Nutr Clin Pract. 2008;23(2):176–81. [DOI] [PubMed] [Google Scholar]
- 70.Smithard D, Barrett NA, Hargroves D, Elliot S. Electromagnetic sensor-guided enteral access systems: a literature review. Dysphagia. 2015;30(3):275–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Byrne CS, Blunt D, Burn J, Chambers E, Dagbasi A, Franco Becker G, Gibson G, Mendoza L, Murphy K, Poveda Cet al. . A study protocol for a randomised crossover study evaluating the effect of diets differing in carbohydrate quality on ileal content and appetite regulation in healthy humans. F1000Res. 2019;8:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gervasio JM, Brown RO, Lima J, Tabbaa MG, Abell T, Werkman R, Haberer LJ, Hak LJ. Sequential group trial to determine gastrointestinal site of absorption and systemic exposure of azathioprine. Dig Dis Sci. 2000;45(8):1601–7. [DOI] [PubMed] [Google Scholar]
- 73.Miner-Williams W, Deglaire A, Benamouzig R, Fuller MF, Tome D, Moughan PJ. Endogenous proteins in the ileal digesta of adult humans given casein-, enzyme-hydrolyzed casein- or crystalline amino-acid-based diets in an acute feeding study. Eur J Clin Nutr. 2014;68(3):363–9. [DOI] [PubMed] [Google Scholar]
- 74.Rochet V, Rigottier-Gois L, Levenez F, Cadiou J, Marteau P, Bresson JL, Goupil-Feillerat N, Dore J. Modulation of Lactobacillus casei in ileal and fecal samples from healthy volunteers after consumption of a fermented milk containing Lactobacillus casei DN-114 001Rif. Can J Microbiol. 2008;54(8):660–7. [DOI] [PubMed] [Google Scholar]
- 75.Deglaire A, Moughan PJ, Airinei G, Benamouzig R, Tome D. Intact and hydrolyzed casein lead to similar ileal endogenous protein and amino acid flows in adult humans. Am J Clin Nutr. 2020;111(1):90–7. [DOI] [PubMed] [Google Scholar]
- 76.Miner-Williams W, Deglaire A, Benamouzig R, Fuller MF, Tome D, Moughan PJ. Endogenous proteins in terminal ileal digesta of adult subjects fed a casein-based diet. Am J Clin Nutr. 2012;96(3):508–15. [DOI] [PubMed] [Google Scholar]
- 77.Maljaars PW, Symersky T, Kee BC, Haddeman E, Peters HP, Masclee AA. Effect of ileal fat perfusion on satiety and hormone release in healthy volunteers. Int J Obes. 2008;32(11):1633–9. [DOI] [PubMed] [Google Scholar]
- 78.Bos C, Juillet B, Fouillet H, Turlan L, Dare S, Luengo C, N'Tounda R, Benamouzig R, Gausseres N, Tome Det al. . Postprandial metabolic utilization of wheat protein in humans. Am J Clin Nutr. 2005;81(1):87–94. [DOI] [PubMed] [Google Scholar]
- 79.Gore RM, Silvers RI, Thakrar KH, Wenzke DR, Mehta UK, Newmark GM, Berlin JW. Bowel obstruction. Radiol Clin North Am. 2015;53(6):1225–40. [DOI] [PubMed] [Google Scholar]
- 80.Jacobs SL, Rozenblit A, Ricci Z, Roberts J, Milikow D, Chernyak V, Wolf E. Small bowel faeces sign in patients without small bowel obstruction. Clin Radiol. 2007;62(4):353–7. [DOI] [PubMed] [Google Scholar]
- 81.Shi H, Liu C, Ding HY, Li CW. Magnesium sulfate as an oral contrast medium in magnetic resonance imaging of the small intestine. Eur J Radiol. 2012;81(3):e370–5. [DOI] [PubMed] [Google Scholar]
- 82.Matsushita M, Mori S, Tahashi Y, Wakamatsu T, Okazaki K. Excessive length of a tube in the stomach: a risk factor for a tangled or knotted tube. Gastrointest Endosc. 2011;74(1):237–8. [DOI] [PubMed] [Google Scholar]
- 83.Barone JA. Domperidone: a peripherally acting dopamine2-receptor antagonist. Ann Pharmacother. 1999;33(4):429–40. [DOI] [PubMed] [Google Scholar]
- 84.Baeyens R, van de Velde E, De Schepper A, Wollaert F, Reyntjens A. Effects of intravenous and oral domperidone on the motor function of the stomach and small intestine. Postgrad Med J. 1979;55(Suppl 1):19–23. [PubMed] [Google Scholar]
- 85.Tack J, Janssens J, Vantrappen G, Peeters T, Annese V, Depoortere I, Muls E, Bouillon R. Effect of erythromycin on gastric motility in controls and in diabetic gastroparesis. Gastroenterology. 1992;103(1):72–9. [DOI] [PubMed] [Google Scholar]
- 86.Kawamura O, Sekiguchi T, Itoh Z, Omura S. Effect of erythromycin derivative EM523L on human interdigestive gastrointestinal tract. Dig Dis Sci. 1993;38(6):1026–31. [DOI] [PubMed] [Google Scholar]
- 87.Lee AL, Kim C-B. The effect of erythromycin on gastrointestinal motility in subtotal gastrectomized patients. J Korean Surg Soc. 2012;82(3):149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Deloose E, Vos R, Corsetti M, Depoortere I, Tack J. Endogenous motilin, but not ghrelin plasma levels fluctuate in accordance with gastric phase III activity of the migrating motor complex in man. Neurogastroenterol Motil. 2015;27(1):63–71. [DOI] [PubMed] [Google Scholar]
- 89.Bos C, Airinei G, Mariotti F, Benamouzig R, Berot S, Evrard J, Fenart E, Tome D, Gaudichon C. The poor digestibility of rapeseed protein is balanced by its very high metabolic utilization in humans. J Nutr. 2007;137(3):594–600. [DOI] [PubMed] [Google Scholar]
- 90.Geboes KP, De Preter V, Luypaerts A, Bammens B, Evenepoel P, Ghoos Y, Rutgeerts P, Verbeke K. Validation of lactose[15N,15N]ureide as a tool to study colonic nitrogen metabolism. Am J Physiol Gastrointest Liver Physiol. 2005;288(5):G994–9. [DOI] [PubMed] [Google Scholar]
- 91.Kastl AJ Jr, Terry NA, Wu GD, Albenberg LG. The structure and function of the human small intestinal microbiota: current understanding and future directions. Cell Mol Gastroenterol Hepatol. 2020;9(1):33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee L, Hossain M, Wang Y, Sedek G. Absorption of rivastigmine from different regions of the gastrointestinal tract in humans. J Clin Pharmacol. 2004;44(6):599–604. [DOI] [PubMed] [Google Scholar]
- 93.Slater A, Betts M, D'Costa H. Laxative-free CT colonography. Br J Radiol. 2012;85(1016):e410–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hounnou G, Destrieux C, Desmé J, Bertrand P, Velut S. Anatomical study of the length of the human intestine. Surg Radiol Anat. 2002;24(5):290–4. [DOI] [PubMed] [Google Scholar]
- 95.Minko E, Pagano A, Caceres N, Adar T, Márquez S. Human intestinal tract length and relationship with body height. FASEB J. 2014;28(S1):916–4. [Google Scholar]
- 96.Hens B, Corsetti M, Brouwers J, Augustijns P. Gastrointestinal and systemic monitoring of posaconazole in humans after fasted and fed state administration of a solid dispersion. J Pharm Sci. 2016;105(9):2904–12. [DOI] [PubMed] [Google Scholar]
- 97.König J, Holster S, Bruins MJ, Brummer RJ. Randomized clinical trial: effective gluten degradation by Aspergillus niger-derived enzyme in a complex meal setting. Sci Rep. 2017;7(1):13100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Long C, Yu Y, Cui B, Jagessar SAR, Zhang J, Ji G, Huang G, Zhang F. A novel quick transendoscopic enteral tubing in mid-gut: technique and training with video. BMC Gastroenterol. 2018;18(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Prabhakaran S, Doraiswamy VA, Nagaraja V, Cipolla J, Ofurum U, Evans DC, Lindsey DE, Seamon MJ, Kavuturu S, Gerlach ATet al. . Nasoenteric tube complications. Scand J Surg. 2012;101(3):147–55. [DOI] [PubMed] [Google Scholar]
- 100.Walmsley RS, Montgomery SM. Factors affecting patient tolerance of upper gastrointestinal endoscopy. J Clin Gastroenterol. 1998;26(4):253–5. [DOI] [PubMed] [Google Scholar]
- 101.Huggins PS, Tuomi SK, Young C. Effects of nasogastric tubes on the young, normal swallowing mechanism. Dysphagia. 1999;14(3):157–61. [DOI] [PubMed] [Google Scholar]
- 102.Abbott WO, Karr WG, Miller TG. Intubation studies of the human small intestine: VII. Factors concerned in absorption of glucose from the jejunum and ileum. Am J Dig Dis. 1937;4(11):742–52. [Google Scholar]
- 103.Kagawa-Busby KS, Heitkemper MM, Hansen BC, Hanson RL, Vanderburg VV. Effects of diet temperature on tolerance of enteral feedings. Nurs Res. 1980;29(5):276–80. [PubMed] [Google Scholar]
- 104.Gaudichon C, Bos C, Morens C, Petzke KJ, Mariotti F, Everwand J, Benamouzig R, Dare S, Tome D, Metges CC. Ileal losses of nitrogen and amino acids in humans and their importance to the assessment of amino acid requirements. Gastroenterology. 2002;123(1):50–9. [DOI] [PubMed] [Google Scholar]
- 105.Marteau P, Flourié B, Cherbut C, Corrèze JL, Pellier P, Seylaz J, Rambaud JC. Digestibility and bulking effect of ispaghula husks in healthy humans. Gut. 1994;35(12):1747–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kerlin P, Zinsmeister A, Phillips S. Relationship of motility to flow of contents in the human small intestine. Gastroenterology. 1982;82(4):701–6. [PubMed] [Google Scholar]
- 107.van den Bogert B. Community and genomic analysis of the human small intestine microbiota. Chapter 7, general discussion. 2013. [Google Scholar]
- 108.Ingelfinger FJ, Abbott WO. Intubation studies of the human small intestine. Am J Dig Dis. 1940;7(11):468–74. [Google Scholar]
- 109.Read NW, Al Janabi MN, Bates TE, Barber DC. Effect of gastrointestinal intubation on the passage of a solid meal through the stomach and small intestine in humans. Gastroenterology. 1983;84(6):1568–72. [PubMed] [Google Scholar]
- 110.Kerlin P, Tucker R, Phillips SF. Rapid intubation of the ileo-colonic region of man. Aust NZ J Med. 1983;13(6):591–3. [DOI] [PubMed] [Google Scholar]
- 111.Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016;14(8):e1002533–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.van der Beek CM, Canfora EE, Lenaerts K, Troost FJ, Damink S, Holst JJ, Masclee AAM, Dejong CHC, Blaak EE. Distal, not proximal, colonic acetate infusions promote fat oxidation and improve metabolic markers in overweight/obese men. Clin Sci. 2016;130(22):2073–82. [DOI] [PubMed] [Google Scholar]
- 113.Wolever TM, Brighenti F, Royall D, Jenkins AL, Jenkins DJ. Effect of rectal infusion of short chain fatty acids in human subjects. Am J Gastroenterol. 1989;84(9):1027–33. [PubMed] [Google Scholar]
- 114.Bucher P, Gervaz P, Egger J-F, Soravia C, Morel P. Morphologic alterations associated with mechanical bowel preparation before elective colorectal surgery: a randomized trial. Dis Colon Rectum. 2006;49(1):109–12. [DOI] [PubMed] [Google Scholar]
- 115.Shobar RM, Velineni S, Keshavarzian A, Swanson G, DeMeo MT, Melson JE, Losurdo J, Engen PA, Sun Y, Koenig L. The effects of bowel preparation on microbiota-related metrics differ in health and in inflammatory bowel disease and for the mucosal and luminal microbiota compartments. Clin Transl Gastroenterol. 2016;7(2):e143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jalanka J, Salonen A, Salojärvi J, Ritari J, Immonen O, Marciani L, Gowland P, Hoad C, Garsed K, Lam C. Effects of bowel cleansing on the intestinal microbiota. Gut. 2015;64(10):1562–8. [DOI] [PubMed] [Google Scholar]
- 117.Crosby WH, Kugler HW. Intraluminal biopsy of the small intestine; The intestinal biopsy capsule. Am J Dig Dis. 1957;2(5):236–41. [DOI] [PubMed] [Google Scholar]
- 118.Otuya DO, Verma Y, Farrokhi H, Higgins L, Rosenberg M, Damman C, Tearney GJ. Non-endoscopic biopsy techniques: a review. Expert Rev Gastroenterol Hepatol. 2018;12(2):109–17. [DOI] [PubMed] [Google Scholar]
- 119.Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28(10):1221–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Vander Noot MR 3rd, Eloubeidi MA, Chen VK, Eltoum I, Jhala D, Jhala N, Syed S, Chhieng DC. Diagnosis of gastrointestinal tract lesions by endoscopic ultrasound-guided fine-needle aspiration biopsy. Cancer. 2004;102(3):157–63. [DOI] [PubMed] [Google Scholar]
- 121.Edwards CH, Grundy MM, Grassby T, Vasilopoulou D, Frost GS, Butterworth PJ, Berry SE, Sanderson J, Ellis PR. Manipulation of starch bioaccessibility in wheat endosperm to regulate starch digestion, postprandial glycemia, insulinemia, and gut hormone responses: a randomized controlled trial in healthy ileostomy participants. Am J Clin Nutr. 2015;102(4):791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zoetendal EG, Raes J, van den Bogert B, Arumugam M, Booijink C, Troost FJ, Bork P, Wels M, de Vos WM, Kleerebezem M. The human small intestinal microbiota is driven by rapid uptake and conversion of simple carbohydrates. ISME J. 2012;6(7):1415–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Boets E, Gomand SV, Deroover L, Preston T, Vermeulen K, De Preter V, Hamer HM, Van den Mooter G, De Vuyst L, Courtin CMet al. . Systemic availability and metabolism of colonic-derived short-chain fatty acids in healthy subjects: A stable isotope study. J Physiol. 2017;595(2):541–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Weerts Z, Keszthelyi D, Vork L, Aendekerk NCP, Frijlink HW, Brouwers J, Neef C, Jonkers D, Masclee AAM. A novel ileocolonic release peppermint oil capsule for treatment of irritable bowel syndrome: a Phase I study in healthy volunteers. Adv Ther. 2018;35(11):1965–78. [DOI] [PubMed] [Google Scholar]
- 125.Munoz F, Alici G, Li W. A review of drug delivery systems for capsule endoscopy. Adv Drug Deliv Rev. 2014;71:77–85. [DOI] [PubMed] [Google Scholar]
- 126.Cui J, Zheng X, Hou W, Zhuang YP, Pi X, Yang J. The study of a remote-controlled gastrointestinal drug delivery and sampling system. Telemed J E Health. 2008;14(7):715–9. [DOI] [PubMed] [Google Scholar]
- 127.Tang Q, Jin G, Wang G, Liu T, Liu X, Wang B, Cao H. Current sampling methods for gut microbiota: a call for more precise devices. Front Cell Infect Microbiol. 2020;10(151). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nejad HR, Oliveira BC, Sadeqi A, Dehkharghani A, Kondova I, Langermans JA, Guasto JS, Tzipori S, Widmer G, Sonkusale SR. Ingestible osmotic pill for in-vivo sampling of gut microbiome. BioRxiv. 2019;1:690982. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon reasonable request