ABSTRACT
Background
Diet, in particular the Mediterranean diet, has been associated with better cognitive function and less cognitive decline in older populations.
Objectives
To quantify associations of a healthy diet, defined by adherence to either the Mediterranean diet, the WHO guidelines, or Dutch Health Council dietary guidelines, with cognitive function and cognitive decline from middle age into old age.
Methods
From the Doetinchem Cohort Study, a large population-based longitudinal study, 3644 participants (51% females) aged 45–75 y at baseline, were included. Global cognitive function, memory, processing speed, and cognitive flexibility were assessed at 5-y time intervals up to 20-y follow-up. Adherence to the Mediterranean diet was measured with the modified Mediterranean Diet Score (mMDS), adherence to the WHO dietary guidelines with the Healthy Diet Indicator (HDI), and adherence to the Dutch Health Council dietary guidelines 2015 with the modified Dutch Healthy Diet 2015 index (mDHD15-index). The scores on the dietary indices were classified in tertiles (low, medium, high adherence). Linear mixed models were used to model level and change in cognitive function by adherence to healthy diets.
Results
The highest tertiles of the mMDS, HDI, and mDHD15-index were associated with better cognitive function compared with the lowest tertiles (P values <0.01), for instance at age 65 y equal to being 2 y cognitively younger in global cognition. In addition, compared with the lowest tertiles, the highest tertiles of the mMDS, HDI, and mDHD15-index were statistically significantly associated with 6–7% slower global cognitive decline from age 55 to 75 y, but also slower decline in processing speed (for mMDS: 10%; 95% CI: 2, 18%; for mDHD15: 12%; 95% CI: 6, 21%) and cognitive flexibility (for mDHD15: 10%; 95% CI: 4, 18%).
Conclusions
Healthier dietary habits, determined by higher adherence to dietary guidelines, are associated with better cognitive function and slower cognitive decline with aging from middle age onwards.
Keywords: dietary patterns, dietary guidelines, prevention, cognitive decline, dementia
Introduction
In 2018, ∼50 million people were suffering from dementia and it is expected that this number will triple by 2050 (1, 2). Dementia puts a high burden on the individual, their caregivers, and society as a whole (3). Because there is no curative treatment available yet, preventive interventions are needed at an early stage in life to slow down cognitive decline and therefore lower the risk of dementia in the future older population (4). Nutrition is a promising lifestyle factor influencing cognitive decline and the development of dementia (4–9). A number of studies have evaluated effects of single nutrients, such as vitamin antioxidants or omega-3 fatty acids, on cognitive function and cognitive decline (10–12). The human diet consists of many different foods, with macronutrients, micronutrients, and nonnutritive components, and these elements in the diet can interact in their association with health and disease. Therefore, the focus has shifted toward the effect of dietary patterns on cognitive function (13, 14).
Most studies have investigated the Mediterranean diet, characterized by a limited consumption of animal-derived and processed foods and a high consumption of plant foods and olive oil, a moderate-to-high consumption of fish, and moderate consumption of alcohol (15). These studies showed that high adherence to the Mediterranean diet is associated with better cognitive function, slower cognitive decline, and lower risk of dementia in older populations (13, 16–20). However, these associations were not always confirmed in randomized controlled trials, probably due to too short follow-up time (21). Associations between the Mediterranean diet and cognitive decline in middle-aged populations have not yet been studied. Middle-aged persons are in general still on a healthy level of cognitive function and thus are an appropriate target group for the prevention of cognitive decline. In addition, only a few studies have looked at habitual adherence to the Mediterranean diet in relation to (changes in) cognitive function over a long time period (22, 23).
Because many countries have issued guidelines for a healthy diet, it is also important to know to what extent adherence to these guidelines benefits cognitive function. In addition to the Mediterranean diet, we studied 2 dietary guidelines: the worldwide WHO guidelines (24) and the Dutch Health Council dietary guidelines 2015 (25). The WHO guidelines do not primarily focus on reducing the risk of cognitive decline and dementia (24). Two cross-sectional studies have shown higher adherence to the WHO guidelines to be associated with lower odds of cognitive impairment in older people (26, 27), whereas 1 longitudinal study did not find any relation (28). The purpose of the Dutch dietary guidelines is to prevent chronic diseases, including dementia and cognitive decline (25), but adherence to these guidelines has not been studied in relation to cognitive function before.
The aim of the present study was to quantify associations between adherence to the Mediterranean diet, the WHO guidelines, and the Dutch dietary guidelines and cognitive function and cognitive decline.
Methods
Study population
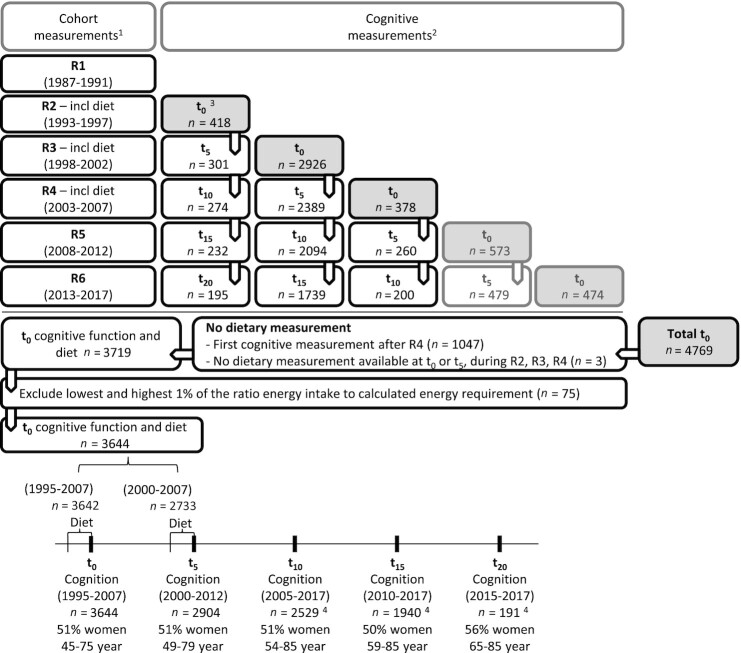
Data were used from the Doetinchem Cohort Study, an ongoing prospective study in which participants are re-examined every 5 y (29, 30). This study has been approved according to the guidelines of the Helsinki Declaration by the external Medical Ethics Committee of the Netherlands Organization for Applied Scientific Research (first 4 rounds) and the Medical Ethics Committee of the University of Utrecht (later rounds). All participants gave written informed consent. A general population sample of 7769 males and females aged 20 to 59 y was examined in 1987–91 (first round) in Doetinchem, a town in the eastern part of The Netherlands and re-examined in second (1993–1997), third (1998–2002), fourth (2003–2007), fifth (2008–2012), and sixth rounds (2013–2017). Three dietary assessments took place (second, third, and fourth rounds). Cognitive testing was introduced halfway through the second round in participants aged ≥45 y. From 1995 onwards, 4769 participants aged ≥45 y took part in cognitive testing for the first time. In the present study, participants’ first cognitive measurement was considered as baseline measurement (t0, 1995–2017). We included participants who had at least a baseline cognitive assessment, and ≥1 dietary measurement at baseline (t0) and/or at 5-y follow-up (t5) (n = 3719, 51% females) (Figure 1).
FIGURE 1.
Overview of the cognitive measurements in participants aged 45+ y in the Doetinchem Cohort Study. 1Cohort measurement rounds are denoted as R1, R2, R3, R4, R5, and R6. 2Cognitive measurement rounds are denoted as t0, t5, t10, t15, and t20, where t0 refers to the first cognitive measurement and therefore baseline measurement in the present study. 3Cognitive testing started as a pilot halfway through R2, from 1995. 4Participants who had their t10, t15, or t20 cognitive measurement between 2013 and 2017 could not have another follow-up measurement yet.
Dietary assessment and adherence scores
Up to and including 2007, participants completed validated semiquantitative FFQs on their average consumption over the previous year (31, 32). The FFQ assessed the habitual frequency and amount of consumption of 178 food items. Total energy intake and the consumption of food groups were computed, as described previously (31). In order to exclude extreme under- or overreporting, participants with the lowest and highest 1% of the ratio energy intake to calculated energy requirement (33) (n = 75) were excluded. As a result, 3644 participants were included in the analyses (Figure 1). Average consumption over the 2 dietary measurements at t0 and t5 was calculated as a measure of long-term dietary habits and to reduce random measurement error (34, 35). If only 1 dietary measurement was available at the first (t0) or second (t5) cognitive measurement (n = 913), that dietary measurement was used. Three dietary indices were computed: the modified Mediterranean Diet Score (mMDS), the Healthy Diet Indicator (HDI), and the modified Dutch Healthy Diet 2015 index (mDHD15-index). These scores were categorized in tertiles, in order to have distinct high and low groups to compare, with the lowest tertile as reference.
Modified Mediterranean Diet Score
The mMDS, as proposed by Trichopoulou et al. (36), was used to measure adherence to the Mediterranean diet. The following 9 food groups were included: vegetables, legumes and nuts, fruits, grains, fish and seafood, meat and meat products including poultry, dairy, alcohol, and the ratio of unsaturated to saturated fatty acids. According to the mMDS, for alcohol consumption, a score of 1 was assigned for males and females with low to moderate alcohol consumption, that is, 10–50 and 5–25 g/d, respectively, otherwise a score of 0 was assigned. For the other food groups, a score of 1 was assigned if the consumption was above the sex-specific population median, and a score of 0 if it was below the sex-specific median, except for meat and dairy, for which the scoring was just the other way around (Supplemental Table 1). This resulted in an overall mMDS score ranging from 0 to 9.
Healthy Diet Indicator
The HDI score is based on WHO dietary recommendations for preventing chronic diseases and consists of 6 nutrients and 1 food group: intake of SFAs, PUFAs, cholesterol, protein, dietary fiber, free sugars, and the consumption of fruits and vegetables (24). For each component, participants were assigned a score of 1 when their intake was within the recommended range; otherwise a score of 0 was assigned (Supplemental Table 1). These scores were summed into a final HDI score ranging from 0 to 7, with a higher score representing a higher adherence to the WHO recommendations.
Modified Dutch Healthy Diet 2015 index
Based on the latest Dutch Health Council dietary guidelines (25) the Dutch Healthy Diet 15-index (37) consists of 15 components: vegetables, fruits, wholegrain products, legumes, nuts, dairy products, fish, tea, replacing fats by oils, replacing unfiltered coffee by filtered coffee, red meat, processed meat, sweetened beverages and fruit juices, alcohol, and sodium (Supplemental Table 1). For each component, a score of 10 is given for consumption within the recommended range. For consumption outside the recommended range, the score linearly decreases to 0 points. This results in an overall DHD15-index ranging from 0 to 150 (37). Because our FFQ did not distinguish between filtered and unfiltered coffee, and did not assess added sodium intake, coffee and salt were excluded from the index. Therefore, our modified DHD15-index ranged from 0 (no adherence) to 130 (maximal adherence).
Cognitive assessment
Our primary outcome variable was cognitive function, repeatedly measured over time. The following specific cognitive domains were measured: global cognitive function, memory function, processing speed, and cognitive flexibility. For measuring these domains, 4 subtests were used: the 15-Word Verbal Learning Test (38), the Stroop Color Word Test (39), the Verbal Fluency Test (animal naming) (40), and the Letter Digit Substitution Test (41) (Supplemental Table 2). To be able to combine the scores on the different tests, all test scores were standardized based on the mean and SD at baseline. A previous study describes the subtests and formulae for calculating the score on each of the cognitive domains (42) (see also Supplemental Table 2). All cognitive domain scores were computed over standardized test scores, based on clustering used in former studies (43–45).
Other measures
Information on sociodemographic characteristics, lifestyle factors, medical history of chronic diseases, and medication use was obtained from self-administered standardized questionnaires at each measurement. Educational level was classified into 5 categories: primary school, lower vocational, intermediate secondary, intermediate vocational/higher secondary, and higher vocational/university. Marital status was dichotomized into married (including living together) compared with not married.
Smoking status was dichotomized into current smoker compared with nonsmoker (including former smoker and smoking <1 cigarette per month). Physical activity was measured by an extensive validated questionnaire on (the duration of) physical activities at work and during leisure time. Information obtained from these questions was classified according to the Cambridge Physical Activity Index into the following 4 categories: inactive, moderately inactive, moderately active, and active (46).
Symptoms of depression were assessed using the Dutch version (47) of the Medical Outcomes Study 36-item short form Health Survey (SF-36) (48). The categories “Mental health” (feelings of depression) and “Vitality” (feelings of liveliness) indicate depressive symptoms. Scores in both categories range from 0 to 100, with higher scores representing better (mental) health. Height, weight, blood pressure, and waist circumference were measured during a physical examination at the research center. BMI was computed by dividing body weight by height squared (kg/m2), waist circumference was measured to the nearest centimeter, and both were used as a continuous measure. Hypertension (yes/no) was included in the data analyses and defined as a systolic pressure ≥140 mmHg and/or diastolic pressure ≥90 mmHg, and/or use of blood pressure–lowering medication. Nonfasting blood samples were obtained to determine serum total cholesterol, HDL cholesterol, blood glucose concentration, and C-reactive protein [CRP; available up to and including 2012 (49)] (29). The total cholesterol to HDL cholesterol ratio was calculated. These variables were used as continuous measures. A past cerebrovascular accident (CVA) was self-reported. Diabetes was self-reported (validated for most participants) or considered present when random blood glucose concentration was >11 mmol/L, and both CVA and diabetes were used as dichotomous measures (yes/no). DNA was extracted from buffy coat samples and used for determining apoE4 genotype (presence of 0, 1, or 2 ε4 alleles), because carrying the ε4 allele is associated with increased risk of Alzheimer disease (50).
Statistical methods
Baseline characteristics are presented as means (SD) for normally distributed variables, and medians (IQR) for nonnormally distributed variables. Percentages are used to present categorical variables. Baseline characteristics were compared between tertiles of the dietary indices (mMDS, HDI, and mDHD15-index) using the χ2 test for categorical variables, 1-factor ANOVA for normally distributed continuous variables, and the Kruskal–Wallis test for nonnormally distributed variables. To see to what extent these indices measure the same healthy diet, the correlation between the mMDS, the HDI, and the mDHD15-index was investigated using a Pearson correlation test.
The z-scores on all cognitive domains were analyzed as continuous outcome measures in all analyses. To adjust for learning effects, cognitive domain scores were adjusted for the number of cognitive measurements. Based on repeated cognitive measurements, linear mixed model analysis with a random intercept and a random slope (to account for the variability in cognitive function between individuals) was used to investigate the association of adherence to dietary guidelines with level of cognitive function. Mixed model analysis can deal with missing data; all available data are included in analyses. To compare change in cognitive function with aging (in this article also referred to as cognitive decline) between tertiles of the dietary indices, an interaction term of calendar age and tertiles of the dietary indices was added to the models. The interaction term was considered statistically significant at P values <0.05, and refers to a significant association between the dietary indices and cognitive decline.
Four models were used, with model I adjusted for age at cognitive measurement (linear as well as quadratic), sex, educational level, and apoE4 genotype, model II with additional adjustment for lifestyle factors (physical activity, smoking), energy intake, marital status, and symptoms of depression, and an explanatory model III, with additional adjustment for cardiovascular risk factors (cholesterol ratio, diabetes, hypertension, waist circumference) and CVA (model IIIa), or CRP as a chronic inflammation marker (model IIIb). In model IIIb, plasma CRP concentrations >10 mg/L were excluded, because these values can indicate an acute-phase response to infection (51). Because plasma CRP was not analyzed in the last measurement round, the last observation was carried forward to be able to analyze the complete follow-up period. Repeated measurements of all covariates were included in the analyses as time-dependent variables. Sex, level of education, dietary intake (average of t0 and t5), and apoE4 genotype were included as time-independent variables. Age was also included as a quadratic term, because cognitive function was better described as a quadratic function of ageing. Effect modification by sex, educational level, and apoE4 genotype for the associations between the highest and lowest tertiles of the dietary indices and global cognitive function was tested. Regarding effect modification, a P value <0.05 for the interaction term was considered significant. For model I, 10,719–10,823 observations from 3504–3518 participants could be included, due to missing values on apoE4 (n = 120), level of education (n = 2), and/or cognitive function (number of participants and observations depending on domain; see also Supplemental Table 3). For model II, 10,601–10,702 observations from 3496–3510 participants could be included; for model IIIa 10,443–10,540 observations from 3483–3497 participants, and for model IIIb 10,056–10,152 observations from 3416–3430 participants.
To visualize the differences between the tertiles of the dietary indices, including the age effect on cognitive function, results of the linear mixed model analyses were plotted as a function of calendar age, based on model II. The z-score of the lowest tertile was set at zero at the age of 45 y. If diet is associated not only with level of cognitive function, but also with change in cognitive function, and cognitive function is not a linear function of calendar age, it depends on the calendar age how much difference is observed between the extreme tertiles and to how many cognitive years this corresponds. To facilitate interpretation of the z-scores and to quantify the associations, the difference in cognitive age between the lowest and highest adherence tertiles was expressed as the difference in calendar years at which the cognitive function in the highest tertile equals the cognitive function in the lowest tertile at age 65 y. So, if the cognitive function in the lowest tertile at age 65 y equals the cognitive function in the highest tertile at age 68 y, the difference in cognitive age is 3 y. The difference in cognitive decline was expressed as the percentage difference in decline from age 55 to 75 y of participants in the highest compared with the lowest tertile.
To evaluate possible information bias and reverse causation, sensitivity analyses were performed by excluding participants who had a score in the lowest 2.5% on global cognitive function at baseline (n = 90). Persons with worse cognitive function might not be able to recall their habitual diet over the last year. In a second sensitivity analysis, participants with only a baseline cognitive measurement (n = 607) were excluded, because these participants do not contribute to change in cognitive function with aging. In addition, decline in cognitive function is likely to occur after individuals have suffered a CVA (52). For this reason, a third sensitivity analysis was performed excluding participants who had suffered a CVA (n = 177). All analyses were performed using SAS 9.4 (SAS Institute, Inc.).
Results
The number of included respondents with cognitive measurements at each timepoint was 3644 at baseline (t0, 1995–2007), 2904 at 5-y follow-up (t5, 2000–2012), 2529 at 10-y follow-up (t10, 2005–2017), 1940 at 15-y follow-up (t15, 2010–2017), and 191 participants at 20-y follow-up (t20, 2015–2017). For 607 participants, only a baseline measurement was available, 433 participants had 5-y follow-up data, 656 had 10-y follow-up data, 1757 participants had 15-y follow-up data, and 191 participants had 20-y follow-up data. Hence, median follow-up was 15 y (range 0–20 y). Average cognitive z-scores at baseline were (per definition) 0.00 and declined over follow-up for all domains (see also Supplemental Table 3 for number of valid cognitive data and average cognitive scores per measurement round).
Participants were on average 56 ±7 y old at the first cognitive measurement, half of the participants were female (51%), and one-third (34%) had not achieved an educational level beyond lower vocational education. About three-quarters (73%) of the population was physically active, and 24% were current smokers.
The mMDS score was categorized in tertiles as low adherence (mMDS 0–3), medium adherence (mMDS 4–5), and high adherence (mMDS 6–9). Scores in the tertiles for HDI were 0–2 (low), 3 (medium), and 4–6 (high adherence), and for the mDHD15-index 22–62 (low), 62–73 (medium), and 73–110 (high adherence). In general, participants in the highest adherence tertiles of the dietary indices were more often highly educated and physically active, were less often smokers, and had a lower BMI. Baseline characteristics according to tertiles of the mMDS are presented in Table 1. Baseline characteristics according to tertiles of HDI and mDHD15-index are presented in Supplemental Tables 4 and 5.
TABLE 1.
Baseline characteristics by tertiles of the modified Mediterranean Diet Score1
| Low | Middle | High | |
|---|---|---|---|
| (0–3) | (4–5) | (6–9) | |
| n = 1064 | n = 1582 | n = 998 | |
| Age, y | 56.4 ± 7.3 | 55.5 ± 7.1 | 55.5 ± 7.1 |
| Women, % | 54 | 50 | 48 |
| Educational level (% low)2 | 41 | 35 | 23 |
| Married, % | 83 | 83 | 84 |
| BMI, kg/m2 | 26.7 ± 4.1 | 26.5 ± 3.7 | 26.2 ± 3.8 |
| Current smoking, % | 30 | 22 | 20 |
| Physically active,3 % | 67 | 73 | 79 |
| Cardiovascular risk factors | |||
| Systolic blood pressure, mmHg | 133 ± 19 | 131 ± 18 | 131 ± 18 |
| Hypertension, % | 41 | 37 | 38 |
| HDL cholesterol, mmol/L | 1.3 ± 0.4 | 1.4 ± 0.4 | 1.4 ± 0.4 |
| Diabetes, % | 3.4 | 2.8 | 2.3 |
| CVA, % | 1.9 | 1.7 | 1.6 |
| Mental health4 | 80 (68–88) | 80 (68–88) | 80 (72–88) |
| Vitality4 | 70 (55–80) | 70 (55–80) | 70 (60–80) |
| apoE4 carriers, % | 27 | 29 | 29 |
| Components of the mMDS, g/d | |||
| Vegetables | 98 ± 34 | 114 ± 41 | 133 ± 41 |
| Fruits | 119 (67–184) | 166 (97–245) | 235 (156–293) |
| Legumes and nuts | 17 (11–25) | 23 (14–33) | 29 (20–40) |
| Grains | 173 ± 59 | 201 ± 70 | 223 ± 75 |
| Fish and seafood | 8 (3–12) | 12 (6–17) | 17 (13–23) |
| Meat and meat products | 117 ± 45 | 109 ± 49 | 98 ± 51 |
| Dairy | 446 ± 234 | 385 ± 211 | 344 ± 203 |
| UFA:SFA ratio5 | 1.3 ± 0.2 | 1.5 ± 0.2 | 1.6 ± 0.2 |
| Alcohol consumption, g/d | 4 (1–15) | 9 (2–20) | 13 (6–23) |
| HDI (0–7) | 2.1 ± 0.9 | 2.5 ± 1.0 | 3.0 ± 1.1 |
| mDHD15-index (0–130) | 61 ± 12 | 67 ± 12 | 75 ± 12 |
| Total energy intake, MJ/d | 8.4 ± 1.9 | 8.7 ± 2.1 | 9.1 ± 2.2 |
| Cognitive function (z-scores) | |||
| Global cognitive function | −0.14 ± 1.02 | −0.00 ± 0.98 | 0.16 ± 0.99 |
| Memory function | −0.11 ± 1.00 | 0.01 ± 0.98 | 0.11 ± 1.01 |
| Cognitive processing speed | −0.14 ± 1.06 | 0.01 ± 0.98 | 0.13 ± 0.95 |
| Cognitive flexibility | −0.11 ± 1.04 | −0.01 ± 1.00 | 0.14 ± 0.94 |
| VLT—delayed recall (no words) | 7.6 ± 2.9 | 7.9 ± 2.8 | 8.1 ± 2.9 |
| Stroop ink color, s | 46.3 ± 16.7 | 44.8 ± 15.7 | 42.7 ± 13.0 |
| LDST (no correct) | 31.7 ± 7.6 | 32.7 ± 7.1 | 33.5 ± 6.9 |
| Fluency (no animals) | 23.4 ± 6.0 | 23.9 ± 6.0 | 24.8 ± 6.0 |
Values are mean ± SD, median (IQR), or percentage. CVA, cerebrovascular accident; HDI, Healthy Diet Indicator; LDST, letter digit substitution test; mDHD15-index, modified Dutch Healthy Diet 2015 index; mMDS, modified Mediterranean Diet Score; UFA, unsaturated fatty acid; VLT, verbal learning test.
Low educated was defined as primary school or lower vocational education as highest attained level.
Active was defined as the 2 highest of 4 categories of the Cambridge physical activity index (46).
A score ranging from 0 to 100, with a higher score indicating better (mental) health.
Ratio total UFAs/SFAs.
The 3 dietary indices were moderately correlated: the mMDS and the HDI (r = 0.37, P < 0.01), the HDI and the mDHD15-index (r = 0.31, P < 0.01), and the mMDS and the mDHD15-index (r = 0.44, P < 0.01).
Level of cognitive function
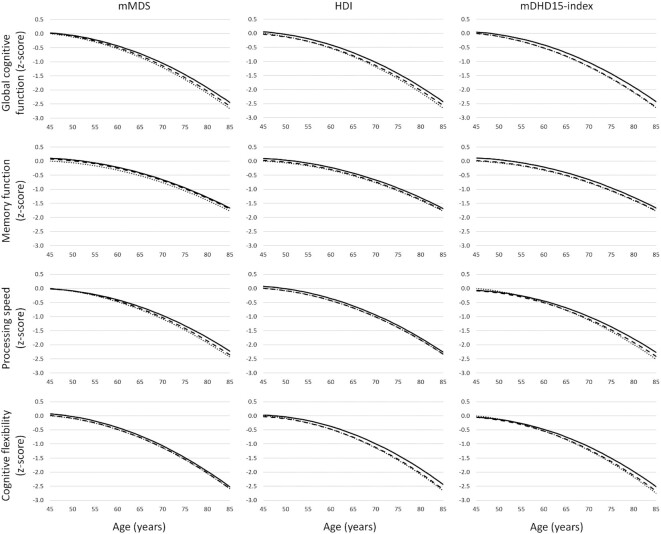
For all 3 dietary indices, the average level of cognitive functioning was higher in the highest adherence tertile compared with the lowest tertile (Table 2, Supplemental Tables 6–8), when not taking differences in cognitive decline into account (models without interaction term of tertiles × age). Based on the models including cognitive decline (models including interaction term of tertiles × age), global cognitive function at age 65 y in the lowest adherence tertiles was equal to the cognitive function of 67-y-olds in the highest adherence tertiles (Table 3). Differences at age 65 were largest for memory function, equal to a difference of 2.4 y (95% CI: 0.9, 4.0 y) in favor of the highest compared with the lowest mDHD15-index tertile (Table 3). The difference at age 65 y was used as an example. The differences between the tertiles, expressed in calendar age, depend not only on the relation between dietary indices and cognitive function, but also on that between age and cognitive function. Therefore, although the absolute difference between the tertiles increases with aging, the difference expressed in calendar age might not increase. Differences between the tertiles over the studied age range are visualized in Figure 2.
TABLE 2.
Level and change in global cognitive function by adherence to healthy diets1
| Model I | Model II | Model IIIa | Model IIIb | |||
|---|---|---|---|---|---|---|
| Dietary score | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | ||
| mMDS | Level2 | Medium | 0.05 (−0.02, 0.11) | 0.04 (−0.02, 0.10) | 0.03 (−0.03, 0.09) | 0.04 (−0.03, 0.10) |
| High | 0.11 (0.04, 0.18) | 0.10 (0.03, 0.17) | 0.09 (0.02, 0.16) | 0.08 (0.02, 0.15) | ||
| Change3 | Medium | 0.01 (−0.07, 0.09) | 0.02 (−0.07, 0.10) | 0.02 (−0.06, 0.10) | 0.00 (−0.08, 0.08) | |
| Medium × age | 0.00 (−0.00, 0.01) | 0.00 (−0.00, 0.01) | 0.00 (−0.00, 0.00) | 0.00 (−0.00, 0.01) | ||
| High | 0.03 (−0.06, 0.12) | 0.03 (−0.06, 0.12) | 0.03 (−0.06, 0.12) | 0.02 (−0.08, 0.11) | ||
| High × age | 0.01 (0.00, 0.01) | 0.00 (0.00, 0.01) | 0.00 (−0.00, 0.01) | 0.00 (0.00, 0.01) | ||
| HDI | Level2 | Medium | 0.01 (−0.05, 0.06) | 0.02 (−0.04, 0.07) | −0.00 (−0.06, 0.06) | 0.01 (−0.05, 0.07) |
| High | 0.10 (0.03, 0.17) | 0.12 (0.05, 0.19) | 0.09 (0.02, 0.16) | 0.11 (0.04, 0.18) | ||
| Change3 | Medium | −0.04 (−0.12, 0.04) | −0.03 (−0.11, 0.05) | −0.04 (−0.12, 0.04) | −0.03 (−0.11, 0.05) | |
| Medium × age | 0.00 (−0.00, 0.01) | 0.00 (−0.00, 0.01) | 0.00 (−0.00, 0.01) | 0.00 (−0.00, 0.01) | ||
| High | 0.04 (−0.05, 0.13) | 0.05 (−0.04, 0.15) | 0.04 (−0.06, 0.13) | 0.05 (−0.04, 0.14) | ||
| High × age | 0.00 (0.00, 0.01) | 0.00 (0.00, 0.01) | 0.00 (−0.00, 0.01) | 0.00 (−0.00, 0.01) | ||
| mDHD15-index | Level2 | Medium | 0.02 (−0.05, 0.08) | 0.01 (−0.06, 0.07) | −0.01 (−0.07, 0.05) | −0.01 (−0.07, 0.06) |
| High | 0.12 (0.06, 0.19) | 0.11 (0.04, 0.17) | 0.08 (0.01, 0.14) | 0.10 (0.04, 0.17) | ||
| Change3 | Medium | 0.01 (−0.07, 0.09) | −0.00 (−0.09, 0.08) | −0.02 (−0.10, 0.06) | −0.02 (−0.10, 0.07) | |
| Medium × age | 0.00 (−0.00, 0.00) | 0.00 (−0.00, 0.00) | 0.00 (−0.00, 0.00) | 0.00 (−0.00, 0.00) | ||
| High | 0.06 (−0.03, 0.14) | 0.04 (−0.04, 0.13) | 0.03 (−0.06, 0.12) | 0.05 (−0.04, 0.14) | ||
| High × age | 0.00 (0.00, 0.01) | 0.00 (0.00, 0.01) | 0.00 (−0.00, 0.01) | 0.00 (−0.00, 0.01) |
1Estimates are calculated in contrast to cognitive function in the lowest tertile, using linear mixed model analyses with random intercept and random slope, including the following covariates: model I: age (centered at 45), age2 (centered at 45), sex, level of education, and apoE4 gene variant; model II: model I + physical activity, smoking, marital status, energy intake, vitality, and mental health; model IIIa: model II + cholesterol ratio, diabetes, hypertension, waist circumference, and CVA; model IIIb: model II + CRP. Number of subjects (observations) in the models were as follows: I, 3504 (10,719); II, 3496 (10,601); IIIa, 3483 (10,443); and IIIb, 3416 (10,056). Negative estimates denote worse cognitive function or faster cognitive decline compared with the cognitive function or cognitive decline in the lowest tertile. CRP, C-reactive protein; CVA, cerebrovascular accident; HDI, Healthy Diet Indicator; mDHD15-index, modified Dutch Healthy Diet 2015 index; mMDS, modified Mediterranean Diet Score.
2Estimates in the “level model” for differences in level of cognitive function between tertiles are from models without interaction terms of tertiles × age.
Estimates in the “change model” for differences in cognitive function and cognitive decline between tertiles are from models with interaction terms of tertiles × age.
TABLE 3.
Differences in level of cognitive function between the highest and lowest tertile of adherence to healthy diets at age 65 y1
| Global cognitive function | Memory function | Information processing speed | Cognitive flexibility | |
|---|---|---|---|---|
| Dietary indices | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) |
| mMDS | 2.0 (0.8, 3.4) | 2.3 (0.7, 3.9) | 1.7 (0.3, 3.4) | 1.1 (0.0, 2.2) |
| HDI | 2.2 (1.0, 3.6) | 2.1 (0.5, 3.7) | 1.2 (−0.008, 2.5) | 1.6 (0.5, 2.7) |
| mDHD15-index | 2.0 (0.9, 3.4) | 2.4 (0.9, 4.0) | 1.5 (0.2, 3.1) | 1.5 (0.3, 2.9) |
Differences were calculated as the difference in calendar age [mean (95% CI)] between the highest and lowest adherence tertiles where cognitive function was at the level of 65-y-olds in the lowest tertile. Calculations are based on associations between adherence to the dietary indices and cognitive function in linear mixed model analyses, adjusted for age, age2, sex, level of education, apoE4 gene variant, physical activity, smoking, marital status, energy intake, vitality, and mental health, and, only if significant, the interaction term between adherence tertiles and calendar age. Positive differences denote a higher level of cognitive function in the highest adherence tertile compared with the lowest adherence tertile at age 65 y. HDI, Healthy Diet Indicator; mDHD15-index, modified Dutch Healthy Diet 2015 index; mMDS, modified Mediterranean Diet Score.
FIGURE 2.
Cognitive domains (z-scores) as a function of calendar age by tertiles of modified Mediterranean Diet Score (mMDS), Healthy Diet Index (HDI), and modified Dutch Heathy Diet 15 index (mDHD15-index). Values were adjusted for sex, level of education, apoE4 genotype, marital status, physical activity, smoking, total energy intake, mental health, and vitality (model II).
Significant interaction by apoE4 status was observed for the association between the highest and lowest mDHD15-index tertiles and global cognitive function: differences in cognitive function between the highest and lowest tertile were larger for participants with 2 ε4 alleles compared with participants without (P = 0.01) or with only 1 ε4 allele (P = 0.03). For participants with 0 or 1 ε4 allele, global cognitive function at age 65 y in the lowest adherence tertile was equal to that of 66–67-y-olds in the highest adherence group (difference no ε4 allele: 2.1; 95% CI: 0.7, 3.9; 1 ε4 allele: 1.2; 95% CI: −0.5, 3.6), for participants with 2 ε4 alleles it was equal to that of 70-y-olds in the highest adherence group (difference 5.6; 95% CI: 1.1, 17.1).
Additional adjustment for cardiovascular risk factors and CVA (model IIIa) or CRP (model IIIb) did not essentially change the associations between the dietary indices and cognitive function (Table 2, Supplemental Tables 6–8).
Change in cognitive function
High scores on all 3 dietary indices were associated with slower decline in global cognitive function (Table 2, models including interaction term of tertiles × age). Difference in cognitive decline between the highest and lowest tertiles was ∼7% between ages 55 and 75 y (Table 4; for mMDS = 7.4%; 95% CI: 1.0, 14.9%; for HDI = 6.5%; 95% CI: 0.3, 13.7%; and for DHD15-index = 6.5%; 95% CI: 0.6, 13.6%). High scores on the mMDS were also associated with slower decline in processing speed (Supplemental Table 7), equal to a 9.5% (95% CI: 2.1, 18.4%) difference (Table 4). High scores on the mDHD15-index were associated with slower decline in both processing speed (12.4% difference; 95% CI: 5.5, 20.8%) and cognitive flexibility (10.3% difference; 95% CI: 3.7, 18.3%), compared with the lowest tertile (Table 4). None of the dietary indices was associated with change in memory function (Supplemental Table 6).
TABLE 4.
Differences in cognitive decline from age 55 to 75 y between the highest and lowest tertile of adherence to healthy diets1
| Global cognitive function | Memory function | Information processing speed | Cognitive flexibility | |
|---|---|---|---|---|
| Dietary indices | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) |
| mMDS | 7.4% (1.0, 14.9) | — | 9.5% (2.1, 18.4) | — |
| HDI | 6.5% (0.3, 13.7) | — | — | — |
| mDHD15-index | 6.5% (0.6, 13.6) | — | 12.4% (5.5, 20.8) | 10.3% (3.7, 18.3) |
Differences expressed in percentages [mean (95% CI)]. Calculations are based on associations between adherence to the dietary indices and cognitive function, adjusted for age, age2, tertiles × age, sex, level of education, apoE4 genotype, physical activity, smoking, marital status, energy intake, vitality, and mental health. Figures are only shown if differences in cognitive decline between highest and lowest tertiles were statistically significant (P < 0.05). Positive differences denote less cognitive decline in the highest tertile compared with the lowest tertile. HDI, Healthy Diet Indicator; mDHD15-index, modified Dutch Healthy Diet 2015 index; mMDS, modified Mediterranean Diet Score.
Additional adjustment for cardiovascular risk factors and CVA (model IIIa) or CRP (model IIIb) did not essentially change the associations between the dietary indices and cognitive decline (Table 2, Supplemental Tables 6–8).
Sensitivity analysis
Sensitivity analyses, excluding participants who had a score in the lowest 2.5% on global cognitive function at baseline, participants with only a baseline cognitive measurement, or participants who had suffered a CVA, showed associations of similar strength as in our primary results (Supplemental Table 9).
Discussion
We found that higher adherence to a healthy diet, independent of its operationalization, was associated with better cognitive function and less severe global cognitive decline.
Our findings are in agreement with several longitudinal, cross-sectional, and experimental studies showing that a healthy dietary pattern is associated with better cognitive function (16–20, 26, 27, 53). A recent review concluded that adherence to the Mediterranean diet was more consistently associated with cognitive decline in the Mediterranean region than in non-Mediterranean regions (17). Our results contribute to the evidence that in a non-Mediterranean region also, better adherence is associated with both better cognitive function (54) and slower global cognitive decline. These results were not limited to adherence to the Mediterranean diet, but were also observed for higher adherence to the WHO dietary guidelines and the Dutch guidelines. Although the 3 dietary indices were only moderately correlated, the magnitudes of the associations between the indices and (change in) cognitive function were similar.
The observation that dietary indices were associated with level of memory function, but not with change in memory function might indicate that a healthy diet plays a role in the development of memory function (cognitive reserve), but less in the maintenance of memory function with aging.
Possible explanations of findings
Possible mechanisms underlying the associations between adherence to healthy diets and (change in) cognitive function include effects on inflammation levels and oxidative stress in the brain (55, 56) and effects on cardiovascular risk factors (57). The healthy diets are characterized by higher consumption of vegetables and fruits and a higher unsaturated to saturated fatty acid ratio and are therefore high in antioxidants, such as vitamin C and E, β-carotene, and polyphenols, and anti-inflammatory nutrients, such as n–3 PUFAs (58). These nutrients decrease inflammation and oxidative stress in the brain (59), processes that affect neuronal pathways and disturb physiological mediators involved in cognitive processes (60). Midlife cardiovascular risk factors are associated with accelerated cognitive decline at midlife (61). However, adjusting for CRP or cardiovascular risk factors did not essentially alter the results, suggesting that these factors are not the main mechanisms in the associations, or that the dietary indices are not strongly enough associated with these factors. In participants carrying 2 apoE4 alleles, being at higher risk of developing Alzheimer's disease, we observed larger cognitive benefits of adherence to the Dutch Healthy Diet 2015 Index. So, for genetically predisposed persons, consuming a healthy diet might be one way to partly counteract one's higher risk of accelerated cognitive decline.
Scores on dietary indices were based not only on different guidelines, but also on different scoring methods: for the mMDS, cutoff values are based on population-based sex-specific medians; for the HDI and mDHD15-index, predefined cutoff values are used based on absolute intake values. For the mMDS and HDI, only values 0 and 1 are given for (non) adherence on each component; for the mDHD15-index, a continuous scale (0–10) was used to indicate whether or not participants complied with components of the guideline. This makes mMDS and HDI less sensitive scores than the mDHD15-index. However, the FFQ used is valid to rank persons based on their dietary intake, and not to assess their absolute dietary intake (31). Therefore, scores based on absolute intake (HDI and mDHD15-index) will introduce more noise in classification than scores based on ranking (mMDS). Based on the 3 scores, we explored relative adherence to dietary guidelines by comparing the highest and the lowest adherence tertiles. Despite misclassification, for all 3 dietary indices significant differences in both cognitive function as well as cognitive decline were observed between the highest and lowest adherence tertiles, even in our population where general adherence to guidelines was relative low. Less misclassification and wider ranges of dietary intake in the population probably will yield even larger cognitive differences between high- and low-adherence groups.
The mMDS has some limitations regarding external validity, because it is based on a relative ranking within the study population, and therefore the absolute cutoff values can be quite different between populations. It can be argued that the Mediterranean dietary pattern defined in our study is not truly Mediterranean (62). When the mMDS was calculated in elderly persons in several European countries, the average score in The Netherlands was 2.92, in contrast to an average score of 6.25 in Greece (36). In addition, the average intake of vegetables, fruits, and fish in our cohort was lower, and the consumption of dairy products somewhat higher than the average intake in the European elderly (36). More recent scores as measures for adherence to the Mediterranean diet, such as Pyramid (63) and the Mediterranean Diet Adherence Score (MEDAS) (64), are based on predefined cutoff values for dietary intake. Therefore the use of these scores yields better external validity. However, because these scores are highly correlated to the mMDS as we used, conclusions would not change.
Diet is culturally determined and age-specific. However, the HDI and DHD15 are based on absolute intakes and our analyses have been adjusted for many potential confounders. In terms of foods and nutrients, dietary guidelines in Europe and the United States are comparable. Moreover, comparable results have been found for MDS in other cohorts/populations. We believe that associations we observed can be generalized to other populations with comparable intake ranges. Therefore, conclusions will also hold for, at least, other populations with predominantly Western dietary habits.
Strengths and limitations
Major strengths of this study are its repeated measures of cognitive function, diet and covariates, large sample size, relatively young population, 3 dietary indices based on an extensive, validated FFQ, a prospective design with a long follow-up period of ≤20 y, and a low loss to follow-up rate (<20% per 5 y). Another strength of our study is the use of a battery of sensitive cognitive tests. Previous studies on the association between adherence to dietary patterns and cognition mainly used a more crude and less sensitive measure of cognitive function based on the Mini-Mental State Examination (65–67).
In addition, we were able to adjust for a wide variety of potential confounders. Higher scores on our dietary indices could simply be markers for an overall healthy lifestyle, education, and wealth, each of which is associated with beneficial effects on health. In our analyses, we adjusted for several lifestyle factors and level of education, but the associations between dietary indices and cognition remained. Especially for level of cognition premorbid intelligence is a strong confounder. However, in our study and others (68) level of education (as a proxy for premorbid intelligence) or Intelligence Quotient less affected change in cognitive function. We realize that level of education is only a proxy measure for premorbid intelligence, and residual confounding can be present. On the other hand, most important determinants of cognitive function are age, sex, and level of education. These variables are objectively measured, leaving less room for residual confounding.
A limitation is that completing an FFQ strongly depends on memory and therefore is vulnerable to recall bias, which might be stronger for participants who already have symptoms of cognitive impairment or dementia. However, sensitivity analyses on a sample excluding participants with the lowest 2.5% score on global cognitive function at baseline yielded comparable results.
Conclusion
In this prospective cohort study we observed that, compared with lowest adherence, highest adherence to the dietary guidelines was associated with being 2 y cognitively younger at age 65 y, and 7% less global cognitive decline between the ages of 55 and 75 y. Adherence to a healthy diet could help to maintain a healthy cognitive function with aging.
Supplementary Material
Acknowledgments
We thank the respondents and the epidemiologists and fieldworkers of the Municipal Health Service in Doetinchem for their contribution to the data collection for this study. The principal investigator is WMMV, and project leader is HSJP. Logistic management was provided by J Steenbrink and P Vissink, and administrative support by EP van der Wolf. Data management was provided by A Blokstra, AWD van Kessel, and PE Steinberger.
The authors’ responsibilities were as follows—ACJN and JMAB: designed research; WMMV and HSJP: had overall oversight of Doetinchem Cohort Study; ACJN, BY, and LGH: performed statistical analysis; BY, LGH, ACJN, and SB: wrote the paper; ACJN: had primary responsibility for final content; and all authors: critically reviewed the manuscript, and read and approved the final manuscript. The authors report no conflicts of interest.
Notes
The Doetinchem Cohort Study is financially supported by the Ministry of Health, Welfare and Sport of The Netherlands and the National Institute for Public Health and the Environment. The data up to and including 1997, including the dietary assessment method, were additionally financially supported by the Europe against Cancer programme of the European Commission (DG SANCO). ACJN and WMMV received funding in the context of Deltaplan Dementie from ZonMW Memorabel (Netherlands Consortium of Dementia Cohorts project 73305095005) and Alzheimer Nederland.
Supplemental Tables 1–9 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: CRP, C-reactive protein; CVA, cerebrovascular accident (stroke); HDI, Healthy Diet Indicator; mDHD15-index, modified Dutch Healthy Diet 2015 index; mMDS, modified Mediterranean Diet Score.
Contributor Information
Astrid C J Nooyens, Centre for Nutrition, Prevention, and Health Services, National Institute for Public Health and the Environment (RIVM), Bilthoven, The Netherlands.
Berivan Yildiz, Centre for Nutrition, Prevention, and Health Services, National Institute for Public Health and the Environment (RIVM), Bilthoven, The Netherlands; Department of Public Health, Erasmus University Medical Center, Rotterdam, The Netherlands.
Lisa G Hendriks, Centre for Nutrition, Prevention, and Health Services, National Institute for Public Health and the Environment (RIVM), Bilthoven, The Netherlands.
Sharell Bas, Centre for Nutrition, Prevention, and Health Services, National Institute for Public Health and the Environment (RIVM), Bilthoven, The Netherlands.
Martin P J van Boxtel, Department of Psychiatry and Neuropsychology, Maastricht University, Maastricht, The Netherlands.
H Susan J Picavet, Centre for Nutrition, Prevention, and Health Services, National Institute for Public Health and the Environment (RIVM), Bilthoven, The Netherlands.
Jolanda M A Boer, Centre for Nutrition, Prevention, and Health Services, National Institute for Public Health and the Environment (RIVM), Bilthoven, The Netherlands.
W M Monique Verschuren, Centre for Nutrition, Prevention, and Health Services, National Institute for Public Health and the Environment (RIVM), Bilthoven, The Netherlands; Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht University, Utrecht, The Netherlands.
Data Availability
Data described in the manuscript, code book, and analytic code are available upon request pending application and approval.
References
- 1.Alzheimer's Disease International, Patterson C . World Alzheimer report 2018. The state of the art of dementia research: new frontiers. Alzheimer's Disease International; 2018. [Google Scholar]
- 2.World Health Organization . World report on ageing and health. WHO; 015. [Google Scholar]
- 3.Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer's disease. Alzheimers Dement (Amst). 2007;3(3):186–91. [DOI] [PubMed] [Google Scholar]
- 4.Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer's disease prevalence. Lancet Neurol. 2011;10(9):819–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caselli RJ, Dueck AC, Locke DE, Sabbagh MN, Ahern GL, Rapcsak SZ, Baxter LC, Yaari R, Woodruff BK, Hoffman-Snyder Cet al. . Cerebrovascular risk factors and preclinical memory decline in healthy APOE epsilon4 homozygotes. Neurology. 2011;76(12):1078–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gu Y, Scarmeas N. Dietary patterns in Alzheimer's disease and cognitive aging. Curr Alzheimer Res. 2011;8(5):510–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharp ES, Gatz M. Relationship between education and dementia: an updated systematic review. Alzheimer Dis Assoc Disord. 2011;25(4):289–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Den Heuvel C, Thornton E, Vink R. Traumatic brain injury and Alzheimer's disease: a review. Prog Brain Res. 2007;161:303–16. [DOI] [PubMed] [Google Scholar]
- 9.Whalley LJ, Duthie SJ, Collins AR, Starr JM, Deary IJ, Lemmon H, Duthie AC, Murray AD, Staff RT. Homocysteine, antioxidant micronutrients and late onset dementia. Eur J Nutr. 2014;53(1):277–85. [DOI] [PubMed] [Google Scholar]
- 10.Rafnsson SB, Dilis V, Trichopoulou A. Antioxidant nutrients and age-related cognitive decline: a systematic review of population-based cohort studies. Eur J Nutr. 2013;52(6):1553–67. [DOI] [PubMed] [Google Scholar]
- 11.Crichton GE, Bryan J, Murphy KJ. Dietary antioxidants, cognitive function and dementia – a systematic review. Plant Foods Hum Nutr. 2013;68(3):279–92. [DOI] [PubMed] [Google Scholar]
- 12.Sydenham E, Dangour AD, Lim WS. Omega 3 fatty acid for the prevention of cognitive decline and dementia. Cochrane Database Syst Rev. 2012(6):CD005379. [DOI] [PubMed] [Google Scholar]
- 13.Knight A, Bryan J, Murphy K. Is the Mediterranean diet a feasible approach to preserving cognitive function and reducing risk of dementia for older adults in Western countries? New insights and future directions. Ageing Res Rev. 2016;25:85–101. [DOI] [PubMed] [Google Scholar]
- 14.Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13(1):3–9. [DOI] [PubMed] [Google Scholar]
- 15.Willett WC, Sacks F, Trichopoulou A, Drescher G, Ferro-Luzzi A, Helsing E, Trichopoulos D. Mediterranean diet pyramid: a cultural model for healthy eating. Am J Clin Nutr. 1995;61(6):1402S–6S. [DOI] [PubMed] [Google Scholar]
- 16.Gardener H, Caunca MR. Mediterranean diet in preventing neurodegenerative diseases. Curr Nutr Rep. 2018;7(1):10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aridi YS, Walker JL, Wright ORL. The association between the Mediterranean dietary pattern and cognitive health: a systematic review. Nutrients. 2017;9(7):674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klimova B, Valis M. Nutritional interventions as beneficial strategies to delay cognitive decline in healthy older individuals. Nutrients. 2018;10(7):905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu L, Sun D. Adherence to Mediterranean diet and risk of developing cognitive disorders: an updated systematic review and meta-analysis of prospective cohort studies. Sci Rep. 2017;7(1):41317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pistollato F, Iglesias RC, Ruiz R, Aparicio S, Crespo J, Lopez LD, Manna PP, Giampieri F, Battino M. Nutritional patterns associated with the maintenance of neurocognitive functions and the risk of dementia and Alzheimer's disease: a focus on human studies. Pharmacol Res. 2018;131:32–43. [DOI] [PubMed] [Google Scholar]
- 21.Radd-Vagenas S, Duffy SL, Naismith SL, Brew BJ, Flood VM, Fiatarone Singh MA. Effect of the Mediterranean diet on cognition and brain morphology and function: a systematic review of randomized controlled trials. Am J Clin Nutr. 2018;107(3):389–404. [DOI] [PubMed] [Google Scholar]
- 22.Koyama A, Houston DK, Simonsick EM, Lee JS, Ayonayon HN, Shahar DR, Rosano C, Satterfield S, Yaffe K. Association between the Mediterranean diet and cognitive decline in a biracial population. J Gerontol A Biol Sci Med Sci. 2015;70(3):354–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tangney CC, Kwasny MJ, Li H, Wilson RS, Evans DA, Morris MC. Adherence to a Mediterranean-type dietary pattern and cognitive decline in a community population. Am J Clin Nutr. 2011;93(3):601–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amine E, Baba NH, Belhadj M, Deureberg-Yap M, Djazayery A, Forrester T, Yoshiike N. Diet, nutrition and the prevention of chronic diseases: report of a joint WHO/FAO expert consultation. WHO; 2002. [Google Scholar]
- 25.Health Council of the Netherlands . Dutch dietary guidelines 2015. The Hague: Health Council of the Netherlands; 2015. Report publication no. 2015/24E. ISBN 978-94-6281-104-1. [Google Scholar]
- 26.Huijbregts P, Feskens EJM, Räsänen L, Fidanza F, Alberti-Fidanza A, Nissinen A, Giampaoli S, Kromhout D. Dietary patterns and cognitive function in elderly men in Finland, Italy and the Netherlands. Eur J Clin Nutr. 1998;52(11):826–31. [DOI] [PubMed] [Google Scholar]
- 27.Correa Leite ML, Nicolosi A, Cristina S, Hauser WA, Nappi G. Nutrition and cognitive deficit in the elderly: a population study. Eur J Clin Nutr. 2001;55(12):1053–8. [DOI] [PubMed] [Google Scholar]
- 28.Berendsen AA, Kang JH, van de Rest O, Jankovic N, Kampman E, Kiefte-de Jong JC, Franco OH, Ikram MA, Pikhart H, Nilsson LMet al. . Association of adherence to a healthy diet with cognitive decline in European and American older adults: a meta-analysis within the CHANCES consortium. Dement Geriatr Cogn Disord. 2017;43(3–4):215–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verschuren WM, Blokstra A, Picavet HS, Smit HA. Cohort profile: the Doetinchem Cohort Study. Int J Epidemiol. 2008;37(6):1236–41. [DOI] [PubMed] [Google Scholar]
- 30.Picavet HSJ, Blokstra A, Spijkerman AMW, Verschuren WMM. Cohort profile update: the Doetinchem Cohort Study 1987–2017: lifestyle, health and chronic diseases in a life course and ageing perspective. Int J Epidemiol. 2017;46(6):1751–1751g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ocke MC, Bueno-de-Mesquita HB, Goddijn HE, Jansen A, Pols MA, van Staveren WA, Kromhout D. The Dutch EPIC food frequency questionnaire. I. Description of the questionnaire, and relative validity and reproducibility for food groups. Int J Epidemiol. 1997;26(90001):37S–48. [DOI] [PubMed] [Google Scholar]
- 32.Ocke MC, Bueno-de-Mesquita HB, Pols MA, Smit HA, van Staveren WA, Kromhout D. The Dutch EPIC food frequency questionnaire. II. Relative validity and reproducibility for nutrients. Int J Epidemiol. 1997;26(90001):49S–58. [DOI] [PubMed] [Google Scholar]
- 33.Schofield WN. Predicting basal metabolic rate, new standards and review of previous work. Hum Nutr Clin Nutr. 1985;39(Suppl 1):5–41. [PubMed] [Google Scholar]
- 34.Hoevenaar-Blom MP, Spijkerman AM, Boshuizen HC, Boer JM, Kromhout D, Verschuren WM. Effect of using repeated measurements of a Mediterranean style diet on the strength of the association with cardiovascular disease during 12 years: the Doetinchem Cohort Study. Eur J Nutr. 2014;53(5):1209–15. [DOI] [PubMed] [Google Scholar]
- 35.Hu FB, Stampfer MJ, Rimm E, Ascherio A, Rosner BA, Spiegelman D, Willett WC. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol. 1999;149(6):531–40. [DOI] [PubMed] [Google Scholar]
- 36.Trichopoulou A, Orfanos P, Norat T, Bueno-de-Mesquita B, Ocke MC, Peeters PH, van der Schouw YT, Boeing H, Hoffmann K, Boffetta Pet al. . Modified Mediterranean diet and survival: ePIC-elderly prospective cohort study. BMJ. 2005;330(7498):991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Looman M, Feskens EJ, de Rijk M, Meijboom S, Biesbroek S, Temme EH, de Vries J, Geelen A. Development and evaluation of the Dutch Healthy Diet index 2015. Public Health Nutr. 2017;20(13):2289–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van der Elst W, van Boxtel MPJ, van Breukelen GJ, Jolles J.. Rey's verbal learning test: normative data for 1855 healthy participants aged 24–81 years and the influence of age, sex, education, and mode of presentation. J Int Neuropsychol Soc. 2005;11(3):290–302. [DOI] [PubMed] [Google Scholar]
- 39.Van der Elst W, van Boxtel MPJ, van Breukelen GJ, Jolles J. The Stroop color-word test: influence of age, sex, and education; and normative data for a large sample across the adult age range. Assessment. 2006;13(1):62–79. [DOI] [PubMed] [Google Scholar]
- 40.Van der Elst W, van Boxtel MPJ, van Breukelen GJ, Jolles J. Normative data for the Animal, Profession and Letter M Naming verbal fluency tests for Dutch speaking participants and the effects of age, education, and sex. J Int Neuropsychol Soc. 2006;12(1):80–9. [DOI] [PubMed] [Google Scholar]
- 41.Van der Elst W, van Boxtel MPJ, van Breukelen GJ, Jolles J. The Letter Digit Substitution Test: normative data for 1,858 healthy participants aged 24–81 from the Maastricht Aging Study (MAAS): influence of age, education, and sex. J Clin Exp Neuropsychol. 2006;28(6):998–1009. [DOI] [PubMed] [Google Scholar]
- 42.Nooyens AC, Bueno-de-Mesquita HB, van Boxtel MP, van Gelder BM, Verhagen H, Verschuren WM. Fruit and vegetable intake and cognitive decline in middle-aged men and women: the Doetinchem Cohort Study. Br J Nutr. 2011;106(5):752–61. [DOI] [PubMed] [Google Scholar]
- 43.van Boxtel MP, Buntinx F, Houx PJ, Metsemakers JF, Knottnerus A, Jolles J. The relation between morbidity and cognitive performance in a normal aging population. J Gerontol A Biol Sci Med Sci. 1998;53A(2):M147–54. [DOI] [PubMed] [Google Scholar]
- 44.Durga J, van Boxtel MP, Schouten EG, Kok FJ, Jolles J, Katan MB, Verhoef P. Effect of 3-year folic acid supplementation on cognitive function in older adults in the FACIT trial: a randomised, double blind, controlled trial. Lancet. 2007;369(9557):208–16. [DOI] [PubMed] [Google Scholar]
- 45.Kalmijn S, van Boxtel MP, Ocke M, Verschuren WM, Kromhout D, Launer LJ. Dietary intake of fatty acids and fish in relation to cognitive performance at middle age. Neurology. 2004;62(2):275–80. [DOI] [PubMed] [Google Scholar]
- 46.Wareham NJ, Jakes RW, Rennie KL, Schuit J, Mitchell J, Hennings S, Day NE. Validity and repeatability of a simple index derived from the short physical activity questionnaire used in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Public Health Nutr. 2003;6(4):407–13. [DOI] [PubMed] [Google Scholar]
- 47.Van der Zee KI, Sanderman R. Het meten van de algemene gezondheidstoestand met de RAND-36: een handleiding [Measuring the general health status with the RAND-36: a manual]. Groningen: Noordelijk Centrum voor Gezondheidsvraagstukken; 1993. [Google Scholar]
- 48.Ware JEJ, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–83. [PubMed] [Google Scholar]
- 49.Hulsegge G, Spijkerman AM, van der Schouw YT, Bakker SJ, Gansevoort RT, Smit HA, Verschuren WM. Trajectories of metabolic risk factors and biochemical markers prior to the onset of cardiovascular disease – the Doetinchem Cohort Study. PLoS One. 2016;11(5):e0155978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jansen WJ, Ossenkoppele R, Knol DL, Tijms BM, Scheltens P, Verhey FRJ, Visser PJ, Amyloid Biomarker Study Group . Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA. 2015;313(19):1924–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yeh E TH, Willerson J T. Coming of age of C-reactive protein. Circulation. 2003;107(3):370–1. [DOI] [PubMed] [Google Scholar]
- 52.Sun JH, Tan L, Yu JT. Post-stroke cognitive impairment: epidemiology, mechanisms and management. Ann Transl Med. 2014;2(8):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Richard EL, Laughlin GA, Kritz-Silverstein D, Reas ET, Barrett-Connor E, McEvoy LK. Dietary patterns and cognitive function among older community-dwelling adults. Nutrients. 2018;10(8):1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shannon OM, Stephan BCM, Granic A, Lentjes M, Hayat S, Mulligan A, Brayne C, Khaw KT, Bundy R, Aldred Set al. . Mediterranean diet adherence and cognitive function in older UK adults: the European Prospective Investigation into Cancer and Nutrition-Norfolk (EPIC-Norfolk) study. Am J Clin Nutr. 2019;110(4):938–48. [DOI] [PubMed] [Google Scholar]
- 55.Dai J, Jones DP, Goldberg J, Ziegler TR, Bostick RM, Wilson PW, Manatunga AK, Shallenberger L, Jones L, Vaccarino V. Association between adherence to the Mediterranean diet and oxidative stress. Am J Clin Nutr. 2008;88(5):1364–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Frisardi V, Panza F, Seripa D, Imbimbo BP, Vendemiale G, Pilotto A, Solfrizzi V. Nutraceutical properties of Mediterranean diet and cognitive decline: possible underlying mechanisms. J Alzheimer Dis. 2010;22(3):715–40. [DOI] [PubMed] [Google Scholar]
- 57.Satija A, Hu FB. Plant-based diets and cardiovascular health. Trends Cardiovasc Med. 2018;28(7):437–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Feart C, Samieri C, Rondeau V, Amieva H, Portet F, Dartigues JF, Scarmeas N, Barberger-Gateau P. Adherence to a Mediterranean diet, cognitive decline, and risk of dementia. JAMA. 2009;302(6):638–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Esfahani A, Wong JM, Truan J, Villa CR, Mirrahimi A, Srichaikul K, Kendall CW. Health effects of mixed fruit and vegetable concentrates: a systematic review of the clinical interventions. J Am Coll Nutr. 2011;30(5):285–94. [DOI] [PubMed] [Google Scholar]
- 60.Scarmeas N, Stern Y, Mayeux R, Luchsinger JA. Mediterranean diet, Alzheimer disease, and vascular mediation. Arch Neurol. 2006;63(12):1709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yaffe K, Bahorik AL, Hoang TD, Forrester S, Jacobs DR Jr, Lewis CE, Lloyd-Jones DM, Sidney S, Reis JP. Cardiovascular risk factors and accelerated cognitive decline in midlife: the CARDIA Study. Neurology. 2020;95(7):e839–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rumawas ME, Dwyer JT, McKeown NM, Meigs JB, Rogers G, Jacques PF. The development of the Mediterranean-style dietary pattern score and its application to the American diet in the Framingham Offspring Cohort. J Nutr. 2009;139(6):1150–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bach-Faig A, Berry EM, Lairon D, Reguant J, Trichopoulou A, Dernini S, Medina FX, Battino M, Belahsen R, Miranda Get al. . Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011;14(12A):2274–84. [DOI] [PubMed] [Google Scholar]
- 64.Estruch R, Ros E, Salas-Salvado J, Covas MI, Corella D, Aros F, Gomez-Gracia E, Ruiz-Gutierrez V, Fiol M, Lapetra Jet al. . Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med. 2018;378(25):e34. [DOI] [PubMed] [Google Scholar]
- 65.Hensel A, Angermeyer MC, Riedel-Heller SG. Measuring cognitive change in older adults. Do reliable change indices of the SIDAM predict dementia?. J Neurol. 2007;254(10):1359–65. [DOI] [PubMed] [Google Scholar]
- 66.Kurlowicz L, Wallace M. The Mini Mental State Examination (MMSE). Director. 1999;7(2):62. [PubMed] [Google Scholar]
- 67.Pendlebury ST, Cuthbertson FC, Welch SJ, Mehta Z, Rothwell PM. Underestimation of cognitive impairment by Mini-Mental State Examination versus the Montreal Cognitive Assessment in patients with transient ischemic attack and stroke: a population-based study. Stroke. 2010;41(6):1290–3. [DOI] [PubMed] [Google Scholar]
- 68.Ritchie SJ, Tucker-Drob EM, Cox SR, Corley J, Dykiert D, Redmond P, Pattie A, Taylor AM, Sibbett R, Starr JMet al. . Predictors of ageing-related decline across multiple cognitive functions. Intelligence. 2016;59:115–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, code book, and analytic code are available upon request pending application and approval.