Abstract
Background
Mind‐body interventions are based on the holistic principle that mind, body and behaviour are all interconnected. Mind‐body interventions incorporate strategies that are thought to improve psychological and physical well‐being, aim to allow patients to take an active role in their treatment, and promote people's ability to cope. Mind‐body interventions are widely used by people with fibromyalgia to help manage their symptoms and improve well‐being. Examples of mind‐body therapies include psychological therapies, biofeedback, mindfulness, movement therapies and relaxation strategies.
Objectives
To review the benefits and harms of mind‐body therapies in comparison to standard care and attention placebo control groups for adults with fibromyalgia, post‐intervention and at three and six month follow‐up.
Search methods
Electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (Ovid), EMBASE (Ovid), PsycINFO (Ovid), AMED (EBSCO) and CINAHL (Ovid) were conducted up to 30 October 2013. Searches of reference lists were conducted and authors in the field were contacted to identify additional relevant articles.
Selection criteria
All relevant randomised controlled trials (RCTs) of mind‐body interventions for adults with fibromyalgia were included.
Data collection and analysis
Two authors independently selected studies, extracted the data and assessed trials for low, unclear or high risk of bias. Any discrepancy was resolved through discussion and consensus. Continuous outcomes were analysed using mean difference (MD) where the same outcome measure and scoring method was used and standardised mean difference (SMD) where different outcome measures were used. For binary data standard estimation of the risk ratio (RR) and its 95% confidence interval (CI) was used.
Main results
Seventy‐four papers describing 61 trials were identified, with 4234 predominantly female participants. The nature of fibromyalgia varied from mild to severe across the study populations. Twenty‐six studies were classified as having a low risk of bias for all domains assessed. The findings of mind‐body therapies compared with usual care were prioritised.
There is low quality evidence that in comparison to usual care controls psychological therapies have favourable effects on physical functioning (SMD ‐0.4, 95% CI ‐0.6 to ‐0.3, ‐7.5% absolute change, 2 point shift on a 0 to 100 scale), pain (SMD ‐0.3, 95% CI ‐0.5 to ‐0.2, ‐3.5% absolute change, 2 point shift on a 0 to 100 scale) and mood (SMD ‐0.5, 95% CI ‐0.6 to ‐0.3, ‐4.8% absolute change, 3 point shift on a 20 to 80 scale). There is very low quality evidence of more withdrawals in the psychological therapy group in comparison to usual care controls (RR 1.38, 95% CI 1.12 to 1.69, 6% absolute risk difference). There is lack of evidence of a difference between the number of adverse events in the psychological therapy and control groups (RR 0.38, 95% CI 0.06 to 2.50, 4% absolute risk difference).
There was very low quality evidence that biofeedback in comparison to usual care controls had an effect on physical functioning (SMD ‐0.1, 95% CI ‐0.4 to 0.3, ‐1.2% absolute change, 1 point shift on a 0 to 100 scale), pain (SMD ‐2.6, 95% CI ‐91.3 to 86.1, ‐2.6% absolute change) and mood (SMD 0.1, 95% CI ‐0.3 to 0.5, 1.9% absolute change, less than 1 point shift on a 0 to 90 scale) post‐intervention. In view of the quality of evidence we cannot be certain that biofeedback has a little or no effect on these outcomes. There was very low quality evidence that biofeedback led to more withdrawals from the study (RR 4.08, 95% CI 1.43 to 11.62, 20% absolute risk difference). No adverse events were reported.
There was no advantage observed for mindfulness in comparison to usual care for physical functioning (SMD ‐0.3, 95% CI ‐0.6 to 0.1, ‐4.8% absolute change, 4 point shift on a scale 0 to 100), pain (SMD ‐0.1, CI ‐0.4 to 0.3, ‐1.3% absolute change, less than 1 point shift on a 0 to 10 scale), mood (SMD ‐0.2, 95% CI ‐0.5 to 0.0, ‐3.7% absolute change, 2 point shift on a 20 to 80 scale) or withdrawals (RR 1.07, 95% CI 0.67 to 1.72, 2% absolute risk difference) between the two groups post‐intervention. However, the quality of the evidence was very low for pain and moderate for mood and number of withdrawals. No studies reported any adverse events.
Very low quality evidence revealed that movement therapies in comparison to usual care controls improved pain (MD ‐2.3, CI ‐4.2 to ‐0.4, ‐23% absolute change) and mood (MD ‐9.8, 95% CI ‐18.5 to ‐1.2, ‐16.4% absolute change) post‐intervention. There was no advantage for physical functioning (SMD ‐0.2, 95% CI ‐0.5 to 0.2, ‐3.4% absolute change, 2 point shift on a 0 to 100 scale), participant withdrawals (RR 1.95, 95% CI 1.13 to 3.38, 11% absolute difference) or adverse events (RR 4.62, 95% CI 0.23 to 93.92, 4% absolute risk difference) between the two groups, however rare adverse events may include worsening of pain.
Low quality evidence revealed that relaxation based therapies in comparison to usual care controls showed an advantage for physical functioning (MD ‐8.3, 95% CI ‐10.1 to ‐6.5, ‐10.4% absolute change) and pain (SMD ‐1.0, 95% CI ‐1.6 to ‐0.5, ‐3.5% absolute change, 2 point shift on a 0 to 78 scale) but not for mood (SMD ‐4.4, CI ‐14.5 to 5.6, ‐7.4% absolute change) post‐intervention. There was no difference between the groups for number of withdrawals (RR 4.40, 95% CI 0.59 to 33.07, 31% absolute risk difference) and no adverse events were reported.
Authors' conclusions
Psychological interventions therapies may be effective in improving physical functioning, pain and low mood for adults with fibromyalgia in comparison to usual care controls but the quality of the evidence is low. Further research on the outcomes of therapies is needed to determine if positive effects identified post‐intervention are sustained. The effectiveness of biofeedback, mindfulness, movement therapies and relaxation based therapies remains unclear as the quality of the evidence was very low or low. The small number of trials and inconsistency in the use of outcome measures across the trials restricted the analysis.
Keywords: Adult; Female; Humans; Male; Attention; Biofeedback, Psychology; Exercise Movement Techniques; Fibromyalgia; Fibromyalgia/therapy; Mind‐Body Therapies; Mind‐Body Therapies/methods; Mindfulness; Pain Management; Pain Management/methods; Psychotherapy; Psychotherapy/methods; Randomized Controlled Trials as Topic; Relaxation Therapy
Plain language summary
Interventions focusing on the link between the mind and body for adults with fibromyalgia
Research question
What are the effects of mind and body therapy for fibromyalgia on pain, physical function, mood and side effects?
What problems does fibromyalgia cause? People with fibromyalgia have chronic, widespread body pain, and often have fatigue (feeling tired), stiffness, depression and problems sleeping.
What are mind‐body interventions?
Mind‐body interventions include treatments such as biofeedback, mindfulness, movement therapies, psychological therapy and relaxation therapies. Biofeedback is when you are connected to electrical sensors that help you receive information about your body to make subtle changes in your body, such as relaxing. Mindfulness means having awareness of thoughts, feelings and bodily sensations. All mind‐body therapies make the link between thoughts, behaviour and feelings to help people to cope with their symptoms.
Study characteristics
We conducted a review of the effect of mind‐body therapies for adults with fibromyalgia. After searching for all relevant studies until October 2013, we found 61 studies including 4234 adults.
‐ Many studies only included female participants, but some males were included in a few studies. ‐ Participants had mild to severe fibromyalgia. ‐ Mind‐body interventions were compared to 'usual care', such as medication use. Secondary analysis also compared findings in comparison to an 'attention control therapy' which involved receiving information for the same amount of time as the mind‐body therapy.
Key results at the end of treatment
‐ Low quality evidence revealed that psychological therapies improved physical functioning, pain, mood and side effects compared to usual care. More people withdrew from the psychological therapy group compared to usual care.
‐ There was little or no difference in physical functioning, pain and mood between people receiving biofeedback and usual care but this may have happened by chance. More people withdrew from the biofeedback than the usual care group. No studies reported any side effects.
‐ There was little or no difference in physical functioning, pain, mood and the number of withdrawals between people receiving mindfulness therapy and usual care. No studies reported any adverse events.
‐ We are uncertain whether movement therapies improve physical functioning, pain, mood, side effects or the number of people who withdrew from the treatment. There were improvements in pain and mood for people receiving movement therapies but the quality of the evidence was very low. More people withdrew and two participants reported experiencing increased pain in the intervention group.
‐ We are uncertain whether relaxation therapies improve physical functioning and pain compared to usual care because the quality of evidence was very low. There was little or no difference in mood and withdrawal from treatment between people receiving relaxation therapies and those receiving usual care. No adverse events were reported.
Best estimates of what happens at the end of treatment in people with fibromyalgia when they use mind‐body therapies
The main findings on the use of psychological therapies are summarised below.
‐ Physical functioning after 1 to 25 weeks (higher scores mean greater limitations)
People who used psychological therapies rated their physical functioning as 2 points lower on a scale of 0 to 100 compared to those who received usual care (7.5% absolute improvement).
‐ Pain after 3 to 14 weeks (higher scores mean worse or more severe pain)
People who used psychological therapies rated their pain as 2 points lower on a scale of 0 to 100 compared to those who received usual care (3.5% absolute improvement).
‐ Mood (higher scores mean worse or more severe pain)
People who used psychological therapies rated their mood as 3 points lower on a scale of 20 to 80 compared to those who received usual care (4.8% absolute improvement).
‐ Withdrawing from the treatment for any reason
A total of 204 out of 1000 people withdrew from psychological therapies compared with 148 out of 1000 from usual care (6% absolute improvement).
‐ Side effects
Nineteen people out of 1000 who received psychological therapies experienced a side effect compared with 51 out of 1000 who had usual care (4% absolute improvement). This may have happened by chance.
We do not have precise information about side effects and complications of mind‐body therapies. Rare adverse events may include worsening of pain.
Summary of findings
Summary of findings for the main comparison. Psychological therapies compared to usual care for fibromyalgia.
| Psychological therapies compared to usual care for fibromyalgia | ||||||
| Patient or population: patients with fibromyalgia Settings: outpatients Intervention: psychological therapies Comparison: usual care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of p (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Usual care | Psychological therapies | |||||
| Functioning as assessed post‐intervention Fibromyalgia Impact Questionnaire. Scale from: 0 to 100 Follow‐up: 1 to 5 weeks | The mean functioning as assessed post‐intervention in the control groups was 6.77 | The mean functioning as assessed post‐intervention in the intervention groups was 0.43 standard deviations lower (0.57 to 0.28 lower) | 733 (10 studies) | ⊕⊕⊝⊝ low1,2 | SMD ‐0.4 (95% CI ‐0.6 to ‐0.3). Absolute change ‐7.5% (95% CI ‐9.9 to ‐4.9), 2 point shift on a scale of 0‐100. Relative improvement ‐10.8% (95% CI ‐5.8 to ‐14.3) NNT 5 (95% CI 4 to 7) |
|
|
Pain as assessed post‐intervention
100 point visual analog scale. Scale from: 0 to 100 Follow‐up: 3 to 14 weeks |
The mean pain as assessed post‐intervention in the control groups was 7.48 | The mean pain as assessed post‐intervention in the intervention groups was 0.33 standard deviations lower (0.52 to 0.15 lower) | 453 (9 studies) | ⊕⊕⊝⊝ low3,4 | SMD ‐0.3 (95% CI ‐0.5 to ‐0.2) Absolute change ‐3.5% (95% CI ‐5.4 to ‐1.6), 2 point shift on a scale of 0‐100 Relative improvement ‐5.3% (95% CI ‐7.0 to ‐8.3) NNT 6 (95% CI 4 to 14) |
|
| Mood as assessed post‐intervention State Trait Anxiety Inventory ‐ State Scale. Scale from: 20 to 80 Follow‐up: 1 to 25 weeks | The mean mood as assessed post‐intervention in the control groups was 7.8 | The mean mood as assessed post‐intervention in the intervention groups was 0.45 standard deviations lower (0.64 to 0.26 lower) | 492 (8 studies) | ⊕⊕⊝⊝ low5,6 | SMD ‐0.5 (95% CI ‐0.6 to ‐0.3). Absolute change ‐4.8 (95% CI ‐6.8 to ‐2.8), 3 point shift on a scale of 20‐80 Relative improvement ‐10.8% (95% CI ‐2.5 to ‐6.3) NNT 5 (95% CI 3 to 8) |
|
| All cause attrition post‐intervention Number of people withdrawing from the study before completing the intervention Follow‐up: 1 to 25 weeks | Study population | RR 1.38 (1.12 to 1.69) | 1687 (22 studies) | ⊕⊝⊝⊝ very low7,8 | Absolute risk difference 6% (95% CI 0.0 to 0.1) Relative per cent change 38% (95% CI 12 to 69) NNTH 18 (95% CI 10 to 55) |
|
| 148 per 1000 | 204 per 1000 (165 to 249) | |||||
| Adverse events post‐intervention Number of people reporting an adverse event before completing the intervention Follow‐up: 4 to 6 weeks | Study population | RR 0.38 (0.06 to 2.5) | 126 (2 studies) | ⊕⊕⊝⊝ low9,10 | Absolute risk difference 4% (95% CI ‐0.1 to 0.0) Relative per cent change 62% (95% CI ‐94 to 150) Not statistically significant |
|
| 51 per 1000 | 19 per 1000 (3 to 127) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded one level due to risk of bias: For some studies allocation concealment was unclear and there was a high risk of selective reporting in one study 2Downgraded one level due to inconsistency: There was diversity in the duration of intervention delivery between studies 3Downgraded one level due to risk of bias: For some studies allocation concealment was unclear and there was a high risk of selective reporting in one study 4Downgraded one level due to inconsistency: There was diversity in the duration of intervention delivery between studies 5Downgraded one level due to risk of bias: For some studies allocation concealment, blinding of participants and selective reporting were unclear 6Downgraded one level due to inconsistency: There was diversity in the duration of intervention delivery between studies 7Downgraded two levels due to risk of bias: Two studies were classified as having a high risk of outcome data and 3 studies were classified as having a high risk of selective reporting bias. Some studies were classified as having an unclear risk of sequence generation, allocation concealment, blinding of outcome assessors and outcome data. 8Downgraded one level due to inconsistency: There was diversity in the duration of intervention delivery between studies 9Downgraded one level due to risk of bias: Some studies were classified as having an unclear risk of sequence generation, allocation concealment and one study was classified as having a high risk of selective reporting 10Downgraded one level due to imprecision: There were less than 200 participants in the analysis
Summary of findings 2. Biofeedback compared to usual care for fibromyalgia.
| Biofeedback compared to usual care for fibromyalgia | ||||||
| Patient or population: patients with fibromyalgia Settings: outpatients Intervention: biofeedback Comparison: usual care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Usual care | Biofeedback | |||||
| Functioning as assessed post‐intervention Fibromyalgia Impact Questionnaire Revised. Scale from: 0 to 100 Follow‐up: 8 to 24 weeks | The mean functioning as assessed post‐intervention in the control groups was 17.16 | The mean functioning as assessed post‐intervention in the intervention groups was 0.06 standard deviations lower (0.44 lower to 0.33 higher) | 106 (2 studies) | ⊕⊝⊝⊝ very low1,2,3 | SMD ‐0.1 (95% CI ‐0.4 to 0.3) Absolute change ‐1.2% (95% CI ‐8.8 to 6.6) Relative improvement 2.2% (95% CI ‐16.3 to12.2) Not statistically significant |
|
| Pain as assessed post‐intervention 100 point visual analog scale. Scale from: 0 to 100 Follow‐up: mean 8 weeks | The mean pain as assessed post‐intervention in the control groups was 1.3 | The mean pain as assessed post‐intervention in the intervention groups was 2.6 lower (91.29 lower to 86.09 higher) | 65 (1 study) | ⊕⊝⊝⊝ very low4,5 | MD ‐2.6 (95% CI ‐91.3 to 86.1) Absolute change ‐2.6% (95% CI ‐91.0 to 86.0) Relative improvement ‐4.0% (95% CI ‐1.0 to1.0) Not statistically significant |
|
| Mood as assessed post‐intervention The Symptom Checklist‐90 Revised. Scale from: 0 to 90 Follow‐up: 8 to 24 weeks | The mean mood as assessed post‐intervention in the control groups was 7.3 | The mean mood as assessed post‐intervention in the intervention groups was 0.13 standard deviations higher (0.26 lower to 0.52 higher) | 104 (2 studies) | ⊕⊝⊝⊝ very low6,7,8 | SMD 0.1 (95% CI ‐0.3 to 0.5) Absolute change 1.9% (95% CI ‐3.7 to 7.4) Relative improvement 3.6% (95% CI ‐7.2 to 14.5) Not statistically significant |
|
| All cause attrition post‐intervention Number of people withdrawing from the study before completing the intervention Follow‐up: 4 to 24 weeks | Study population | RR 4.08 (1.43 to 11.62) | 125 (3 studies) | ⊕⊝⊝⊝ very low9,10,11 | Absolute risk difference 20% (95% CI 0.8 to 0.3) Relative per cent change 308% (95% CI 43 to 1062) NNTH 7 (95% CI 3 to 41) |
|
| 63 per 1000 | 259 per 1000 (91 to 738) | |||||
| Adverse events post‐intervention ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not estimable |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded one level due to risk of bias: Random sequence generation and allocation concealment was unclear for one study 2Downgraded one level due to inconsistency: There was diversity in the duration of intervention delivery 3Downgraded one level due to imprecision: There were less than 200 participants in this analysis 4Downgraded one level due to risk of bias: Random sequence generation and allocation concealment was unclear for one study 5Downgraded one level due to imprecision: There were less than 100 participants in the analysis 6Downgraded one level due to imprecision: Downgraded one level due to risk of bias: Random sequence generation and allocation concealment was unclear for one study 7Downgraded one level due to inconsistency: There was diversity in the duration of intervention delivery 8Downgraded one level due to imprecision: There were less than 200 participants in the analysis 9Downgraded one level due to risk of bias: Random sequence generation and allocation concealment was unclear for one study 10Downgraded one level due to inconsistency: There was diversity in the duration of intervention delivery 11Downgraded one level due to imprecision: There were less than 200 participants in the analysis
Summary of findings 3. Mindfulness compared to usual care for fibromyalgia.
| Mindfulness compared to usual care for fibromyalgia | ||||||
| Patient or population: patients with fibromyalgia Settings: outpatients Intervention: mindfulness Comparison: usual care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Usual care | Mindfulness | |||||
| Functioning as assessed post‐intervention Fibromyalgia Impact Questionnaire. Scale from: 0 to 100 Follow‐up: mean 8 weeks | The mean functioning as assessed post‐intervention in the control groups was 17.22 | The mean functioning as assessed post‐intervention in the intervention groups was 0.26 standard deviations lower (0.6 lower to 0.09 higher) | 128 (2 studies) | ⊕⊕⊝⊝ low1 | SMD ‐0.3 (95% CI ‐0.6 to 0.1) Absolute change ‐4.8% (95% CI ‐11.2 to 1.7%) Relative improvement ‐8.5% (95% CI ‐19.3 to 3.5) Not statistically significant |
|
| Pain as assessed post‐intervention Visual analog scale 0 to 100. Scale from: 0 to 10. Follow‐up: mean 8 weeks | The mean pain as assessed post‐intervention in the control groups was 0.21 | The mean pain as assessed post‐intervention in the intervention groups was 0.09 standard deviations lower (0.44 lower to 0.26 higher) | 128 (2 studies) | ⊕⊕⊝⊝ low2,3 | SMD ‐0.09 (95% CI ‐0.4 to 0.3) Absolute change ‐1.28% (95% CI ‐6.2 to 3.7) Relative improvement ‐2.3% (95% CI ‐11.1 to 6.6) Not statistically significant |
|
| Mood as assessed post‐intervention State Trait Anxiety Inventory State Scale. Scale from: 0 to 60. Follow‐up: mean 8 weeks | The mean mood as assessed post‐intervention in the control groups was 10.28 | The mean mood as assessed post‐intervention in the intervention groups was 0.24 standard deviations lower (0.51 lower to 0.03 higher) | 218 (3 studies) | ⊕⊕⊕⊝ moderate4 | SMD ‐0.24 (95% CI ‐0.5 to 0.0) Absolute change ‐3.7% (95% CI ‐7.9 to 0.5) Relative improvement ‐8.7% (95% CI ‐18.5 to 1.2) Not statistically significant |
|
| All cause attrition post‐intervention Number of people withdrawing from the study before completing the intervention Follow‐up: mean 8 weeks | Study population | RR 1.07 (0.67 to 1.72) | 195 (3 studies) | ⊕⊕⊕⊝ moderate5 | Absolute risk difference 2% (95% CI ‐0.10 to 0.14) Relative per cent change 98% (95% CI ‐90 to ‐86) Not statistically significant |
|
| 223 per 1000 | 239 per 1000 (150 to 384) | |||||
| Adverse events post‐intervention ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded two levels due to imprecision: There were less than 200 participants in the analysis 2Downgraded one level due to risk of bias: One study was classified as having a high risk of blinding of the outcome assessors 3Downgraded one level due to imprecision: There were less than 200 participants in the analysis 4Downgraded one level due to risk of bias: One study was classified as having a high risk of blinding of the outcome assessors 5Downgraded one level due to risk of bias: One study was classified as having a high risk of blinding of the outcome assessors with one study classified as having an unclear risk of sequence generation, allocation concealment and blinding of the outcome assessors
Summary of findings 4. Movement therapies compared to usual care for fibromyalgia.
| Movement therapies compared to usual care for fibromyalgia | ||||||
| Patient or population: patients with fibromyalgia Settings: outpatients Intervention: movement therapies Comparison: usual care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Usual care | Movement therapies | |||||
| Functioning as assessed post‐intervention Fibromyalgia Impact Questionnaire ‐ Revised. Scale from: 0 to 100. Follow‐up: 8 to 14 weeks | The mean functioning as assessed post‐intervention in the control groups was 13.3 | The mean functioning as assessed post‐intervention in the intervention groups was 0.19 standard deviations lower (0.53 lower to 0.15 higher) | 143 (4 studies) | ⊕⊝⊝⊝ very low1,2,3 | SMD ‐0.19 (95% CI ‐0.5 to 0.2). Absolute change ‐3.4% (95% CI ‐9.4 to 2.7) 2 point change on 0 to 100 scale Relative improvement ‐6.8% (95% CI ‐19.1 to 5.5) Not statistically significant |
|
| Pain as assessed post‐intervention 10 point visual analog scale. Scale from: 0 to 10 Follow‐up: mean 8 weeks | The mean pain as assessed post‐intervention in the control groups was ‐0.37 | The mean pain as assessed post‐intervention in the intervention groups was 2.3 lower (4.19 to 0.41 lower) | 28 (1 study) | ⊕⊝⊝⊝ very low4,5 | MD ‐2.3 (95% CI ‐4.2 to ‐0.4) Absolute change ‐23.0% (95% CI ‐42.0 to ‐4.0) Relative improvement ‐3.0% (95% CI ‐6 to ‐0.6) NNT 3 (95% CI 2 to 41) |
|
| Mood as assessed post‐intervention Center for Epidemiologic Studies Depression Scale. Scale from: 0 to 60 Follow‐up: mean 8 weeks | The mean mood as assessed post‐intervention in the control groups was 0.41 | The mean mood as assessed post‐intervention in the intervention groups was 9.84 lower (18.51 to 1.17 lower) | 29 (1 study) | ⊕⊝⊝⊝ very low6,7 | MD ‐9.8 (95% CI ‐18.5 to ‐1.2) Absolute change ‐16.4% (95% CI ‐31.0 to ‐2.0) Relative improvement ‐0.7% (95% CI ‐1.3 to ‐0.1) NNT 3 (95% CI 2 to 34) |
|
| All cause attrition post‐intervention Number of people withdrawing from the study before completing the intervention Follow‐up: 8 to 24 weeks | Study population | RR 1.95 (1.13 to 3.38) | 240 (5 studies) | ⊕⊝⊝⊝ very low8,9 | Absolute risk difference 11% (95% CI 0.0 to 0.2) Relative per cent change 95% (95% CI 13 to 238) NNTH 13 (95% CI 5 to 105) |
|
| 106 per 1000 | 206 per 1000 (119 to 357) | |||||
| Adverse events post‐intervention Number of people reporting an adverse event before completing the intervention Follow‐up: 8 to 24 weeks | Study population | RR 4.62 (0.23 to 93.72) | 98 (1 study) | ⊕⊝⊝⊝ very low11,12,13 | Absolute risk difference 4% (95% CI ‐0.0 to 0.1) Relative per cent change 362% (95% CI ‐77 to 9272) Not statistically significant |
|
| 0 per 1000 | 40 per 100010 (0 to 0) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded one level due to risk of bias: One study was classified as having a high risk of allocation concealment and blinding of outcome assessors 2Downgraded one level due to inconsistency: There was diversity in the duration of intervention delivery 3Downgraded one level due to imprecision: There were less than 200 participants in the analysis 4Downgraded one level due to risk of bias: One study was classified as having a high risk of allocation concealment and blinding of outcome assessors 5Downgraded one level due to imprecision: There were less than 100 participants in the analysis 6Downgraded one level due to risk of bias: One study was classified as having a high risk of allocation concealment and blinding of outcome assessors 7Downgraded one level due to imprecision: There were less than 200 participants in the analysis 8Downgraded one level due to risk of bias: One study was classified as having a high risk of allocation concealment and blinding of outcome assessors and one study had a high risk of selective reporting 9Downgraded one level due to inconsistency: There was diversity in the duration of intervention delivery 10 Absolute effect calculated from risk difference
11Downgraded one level due to risk of bias: One study was classified as having a high risk of selective reporting and unclear sequence generation and allocation concealment 12Downgraded one level due to inconsistency: There was diversity in the duration of intervention delivery 13Downgraded one level due to imprecision: There were less than 200 participants in the analysis
Summary of findings 5. Relaxation compared to usual care for fibromyalgia.
| Relaxation compared to usual care for fibromyalgia | ||||||
| Patient or population: patients with fibromyalgia Settings: outpatients Intervention: relaxation Comparison: usual care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of p (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Usual care | Relaxation | |||||
| Functioning as assessed post‐intervention Fibromyalgia Impact Questionnaire. Scale from: 0 to 80 Follow‐up: 6 to 10 weeks | The mean functioning as assessed post‐intervention in the control groups was 3.16 | The mean functioning as assessed post‐intervention in the intervention groups was 1.63 standard deviations lower (10.14 to 6.53 lower) | 67 (2 studies) | ⊕⊝⊝⊝ very low1,2 | MD ‐8.3 (95% CI ‐10.1 to ‐6.5). Absolute change ‐10.4% (95% CI ‐13.0 to ‐8.0), 5 point shift on 0 to 80 scale Relative improvement ‐20.0% (95% CI ‐0.2 to ‐0.2) NNT 2 (95% CI 1 to 2) |
|
| Pain as assessed post‐intervention Short Form ‐ McGill Pain Questionnaire Total Score. Scale from: 0 to 78 Follow‐up: 6 to 10 weeks | The mean pain as assessed post‐intervention in the control groups was 1.86 | The mean pain as assessed post‐intervention in the intervention groups was 1.02 standard deviations lower (1.55 to 0.5 lower) | 67 (2 studies) | ⊕⊝⊝⊝ very low3,4 | SMD ‐1.0 (95% CI ‐1.6 to ‐0.5). Absolute change ‐3.5% (95% CI ‐5.3 to ‐1.7), 2 point shift on a scale of 0 to 8 Relative improvement ‐9.5% (95% CI ‐14.5 to ‐4.8) NNT 2 (95% CI 1 to 4) |
|
| Mood as assessed post‐intervention Center for Epidemiologic Disease Depression Scale. Scale from: 0 to 60 Follow‐up: mean 6 weeks | The mean mood as assessed post‐intervention in the control groups was ‐1.9 | The mean mood as assessed post‐intervention in the intervention groups was 4.44 lower (14.46 lower to 5.58 higher) | 19 (1 study) | ⊕⊝⊝⊝ very low5,6 | MD ‐4.4 (95% CI ‐14.5 to 5.6) Absolute change ‐7.4% (95% CI ‐24 to 9) Relative improvement ‐27% (95% CI ‐0.9 to ‐0.3) Not statistically significant |
|
| All cause attrition post‐intervention Number of people withdrawing from the study before completing the intervention Follow‐up: mean 6 weeks | Study population | RR 4.4 (0.59 to 33.07) | 21 (1 study) | ⊕⊝⊝⊝ very low7,8 | Absolute risk difference 31% (95% CI ‐0.0 to 0.7) Relative per cent change 340% (95% CI ‐41 to 3207) Not statistically significant |
|
| 91 per 1000 | 400 per 1000 (54 to 1000) | |||||
| Adverse events post‐intervention ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not estimable |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded one level due to risk of bias: One study was classified as having an unclear risk of blinding of outcome assessors 2Downgraded one level due to imprecision: There were less than 100 participants in the analysis 3Downgraded one level due to risk of bias: One study was classified as having an unclear risk of blinding of outcome assessors 4Downgraded one level due to imprecision: There were less than 100 participants in the analysis 5Downgraded one level due to risk of bias: One study was classified as having an unclear risk of blinding of outcome assessors 6Downgraded one level due to imprecision: There were less than 100 participants in the analysis 7Downgraded one level due to risk of bias: One study was classified as having an unclear risk of blinding of outcome assessors 8Downgraded one level due to imprecision: There were less than 100 participants in the analysis
Background
Description of the condition
Fibromyalgia (FM) is a complex, chronic condition, which is characterised by widespread persistent pain, fatigue, cognitive impairment and sleep disturbances that make it difficult for people to engage in everyday activities (Arnold 2011; Bennett 2007; Wolfe 1990). Fibromyalgia has been associated with high individual and societal healthcare costs (Berger 2007; Sicras‐Mainar 2009) with many patients reporting reduced physical functioning and poor quality of life (Burckhardt 1991). The term fibromyalgia (FM) is used in this review in accordance with Cochrane convention.
Estimates suggest that FM affects between 2% to 5% of the general population (Branco 2010; Wolfe 1995). There is a higher prevalence in females (female:male ratio of 9 to 10:1) (MacFarlane 2002; Wolfe 1990; Yunus 2001), with prevalence rising to 8% in women between 55 and 64 years of age (White 1999). Emerging evidence suggests that the condition is linked to dysregulation of the central and sympathetic nervous systems (Mease 2005) that results from neurochemical imbalances leading to both an amplification of pain signals and reduced ability to inhibit the pain response (Ceko 2011; Clauw 2011).
A diagnosis of fibromyalgia is usually based on the exclusion of other potential causes of symptoms and through clinical evaluation. The American College of Rheumatology (ACR) criteria stipulate that pain must be distributed across the four quadrants of the body (that is pain above the waist, below the waist, on the left and right sides of the body) and in the axial skeleton, with tenderness in 11 or more of the 18 specific sites known as tender points during digital palpation (using 4 kg pressure) or dolorimetry (Wolfe 1990). There has been considerable debate regarding the diagnostic accuracy of FM as the ACR criteria have proven problematic, with no objective standardised test. Changes to the criteria have recently been proposed that do not require tender point examination and include a severity rating scale for fibromyalgia symptoms (Wolfe 2011). The revised criteria show potential in refining the diagnostic criteria for FM; however, as the criteria remain preliminary and further evidence of the validity, acceptance reliability and consistent implementation of the new criteria is required, this review classified FM based on the ACR criteria that have been widely implemented since 1990 (Wolfe 2010; Wolfe 2011; Wolfe 2011b).
Description of the intervention
Non‐pharmacological interventions have received increasing attention for helping patients to manage the demands of complex conditions such as FM. Indeed, it has been revealed that people with neurological conditions use complementary therapies more than other therapeutic approaches (Wells 2010). Mind‐body therapies have been defined as focusing on the interactions among the brain, mind, body and behaviour. The aim of mind‐body therapy is to enhance the capacity for self‐knowledge, self‐care and to provide tools that can improve coping, mood and quality of life (NCCAM 2005Appendix 1; Wahbeh 2008). Mind‐body interventions are considered to be a type of approach that falls under the umbrella of complimentary and alternative medicine, which also includes manipulative therapies and herbal products. The National Center for Complementary and Alternative Medicine (NCCAM 2005) describes mind‐body interventions as treatment approaches that are based on the holistic principle that mind, body and behaviour are all interconnected, incorporate strategies that are thought to improve psychological and physical well‐being, and aim to allow patients to take an active role in their treatment and to promote people's ability to cope. Mind and body interventions include a range of treatments (NCCAM 2012). Examples of mind and body therapies include biofeedback (use of technology to give audio or visual feedback on physiological processes such as heart rate to assist people in being able to gain more control over their bodies); mindfulness (a way of looking at the world in a non‐judgemental manner); movement therapies (use of physical movement to stimulate mental clarity, such as yoga, tai chi, qi‐gong); psychological therapies (use of techniques to help people become aware of their own thoughts and behaviours, such as written emotional disclosure and cognitive behaviour therapy); and relaxation strategies (techniques to help calm the mind and relax the body, such as breathing techniques, visual imagery, guided imagery, progressive muscle relaxation).
How the intervention might work
FM is a complex condition and psychological, social and lifestyle factors have all been found to play an important role in the symptom experience (Bergman 2005; Nicassio 2002; Theadom 2008). Interventions that aim to improve well‐being, self esteem, coping ability and reduce stress may therefore improve physical symptoms and quality of life for people with FM. The relevance of mind‐body interventions to FM is also supported by emerging evidence of the interactions between the central nervous, endocrine, immune, and peripheral autonomic nervous systems, suggesting "a mechanism by which mind–body medicine could influence physical health" (Vitetta 2005).
Why it is important to do this review
Symptom‐specific medication has been the primary method of treatment for FM with many patients prescribed tricyclic antidepressants (TCAs), selective serotonin uptake inhibitors (SSRIs), simple analgesics and serotonin norepinephrine reuptake inhibitors (SNRIs), which have demonstrated efficacy for reducing pain (Dworkin 2003; Hauser 2013; Moore 2009). Medications previously used in the treatment of epilepsy such as gabapentin and pregabalin are now more widely used for FM, however many people report side effects and continue to experience symptoms despite using the medication (Moore 2009; Moore 2011). Additionally, a recent review of guidelines on the management of FM (Hauser 2010) highlights the need for a multidimensional approach including a combination of non‐pharmacological and pharmacological therapies.
A review on psychological therapies for the management of chronic pain (excluding headache) in adults revealed that psychological therapies had weak effects in improving pain but that cognitive behaviour therapy and behaviour therapy improved low mood with some evidence of improvements being maintained at six months, in comparison to usual care and attention controls. Whilst this review included participants with FM, the impact of interventions may vary between different pain populations. Previous Cochrane reviews have explored the evidence for the use of exercise and resistance training for FM and found that supervised aerobic exercise and resistance training have beneficial effects on pain and physical functioning (Busch 2007; Busch 2013). A recent review has also found that cognitive behaviour therapy shows a small benefit in comparison to control in reducing pain, negative mood and disability in people with FM (Bernardy 2013).
There is evidence that mind‐body therapies are more effective in comparison to waiting list or placebo control groups on self efficacy and quality of life outcomes for FM (Hadhazy 2000). Since the publication of Hadhazy's review in 2000, a wealth of studies have since been published in this area. The present review aims to provide evidence of the efficacy of mind‐body therapies for adults with FM.
Objectives
To review the benefits and harms of mind‐body therapies in comparison to standard care and attention placebo control groups for adults with fibromyalgia (FM), post‐intervention and at three and six month follow‐up.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) that aimed to explore the benefits or harm for people diagnosed with FM who received a mind‐body intervention in comparison to usual care or a treatment that was not thought to have therapeutic effects but was delivered by an equivalent therapist and for the same amount of time as the mind‐body therapy group (known as an attention control) were included in the review. Case studies, clinical observations and quasi‐randomised controlled trials were excluded from the review in order to minimise bias.
Types of participants
All persons 18 years of age or older with a clinical diagnosis of FM (as defined by the ACR 1990 criteria) (Wolfe 1990). If people with FM were recruited into a trial in addition to participants with other medical conditions, the study was only included if the data for people with FM were available separately.
Types of interventions
Interventions incorporating at least one type of mind‐body therapy were included. Based on the definition of mind‐body interventions proposed by the Centre for Complementary and Alternative Medicine (NCCAM 2005), six criteria were established to determine whether an intervention met the definition of a mind and body intervention for this review.
The criteria specified that the intervention must: 1) be based on the principle that the mind and body are interconnected; 2) aim to increase self knowledge; 3) aim to increase people’s ability to self‐manage their health and consequences of ill‐health; 4) actively engage and involve the participant in the intervention delivery; and 5) provide tools to improve coping and self‐management of the condition. As mind and body interventions are often incorporated with other techniques the sixth criterion, that 6) at least 80% of the total intervention delivery must include components meeting the aforementioned five principles, was added to prevent the findings from trials including only a small mind‐body component influencing the results.
Due to the wide diversity of available mind‐body therapies, interventions were categorised into broad groups to enable comparison.
Psychological therapies (including cognitive behaviour therapy (CBT), psychoanalytic and humanistic approaches).
Biofeedback (providing immediate feedback on bodily functions, such as muscle tension, to raise the patient's awareness and enable the possibility of conscious control of those functions.
Mindfulness meditation therapies (being aware of the present moment in a non‐judgemental and accepting way).
Movement therapies (e.g. yoga, tai chi, qi‐gong).
Relaxation based therapies (e.g. breathing techniques, visual imagery, guided imagery, progressive muscle relaxation).
Interventions delivered in all settings including community, primary care or hospital were included in the review to facilitate the generalisability of the review findings. Exercise based interventions for FM have been subject to their own Cochrane review (Busch 2007) and were therefore not included. Only movement therapies that met the definition of a mind‐body therapy were included in the review. Interventions delivered to a participant manually by a therapist (such as massage, acupuncture, physiotherapy) were not included within the review as participants are not actively engaged in the treatment, a key criterion of mind‐body interventions according to the NCCAM 2005 definition of mind‐body therapy.
Eligible comparative interventions included both usual care, which involved the treatment that people would usually receive (such as medication), or wait‐list conditions or attention control interventions involving participants receiving similar levels of contact with researchers or therapists in a similar format as the experimental intervention (such as sham therapy or peer group support).
Types of outcome measures
Major outcomes
The five major outcomes for this review were:
self‐reported physical functioning (ability to complete everyday tasks e.g. scores on the Fibromyalgia Impact Questionnaire (Bennett 2009));
self‐reported levels of pain (e.g. pain intensity numerical rating scale). A 30% or two point reduction in a 10 point numerical rating scale has been reported to be a relevant clinical outcome in evaluating trials in chronic pain (Farrar 2001);
mood, encompassing both anxiety and depression (e.g. Hospital Anxiety and Depression Scale (Zigmond 1983));
participant withdrawals;
adverse events (e.g. increased pain).
Data on all outcome measures assessed post‐intervention and at three and six month follow‐up were extracted for the review.
Minor outcomes
Minor outcomes were assessed post‐intervention and at three and six month follow‐up. These included:
fatigue (e.g. scores on the Multidimensional Assessment of Fatigue scale (Smets 1995));
sleep (e.g. Pissburgh Sleep Quality Index (Buysse 1989));
self efficacy (perceived ability to manage their overall health e.g. Chronic Pain Self‐Efficacy Scale (Anderson 1995));
tender point score (measured by dolorimetry or digital palpitation);
quality of life (e.g. Short Form Medical Outcome Study (Hays 1993)).
Search methods for identification of studies
Electronic searches
The electronic searches were conducted by the Trial Search Co‐ordinator of the Musculoskeletal Group: Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (2013, Issue 10), MEDLINE (Ovid) (1950 to October 2013), EMBASE (Ovid) (1974 to October 2013), PsycINFO (Ovid) (1806 to October 2013), AMED (EBSCO, Allied and Complementary Medicine) (1985 to October 2013) and CINAHL (Ovid, 1982 to 2008; EBSCO, 2008 to October 2013). The search strategy is shown in Appendix 2.
Searching other resources
The reference lists of relevant articles were searched for additional relevant trials. Authors were also contacted to identify any other unpublished or published studies. The lists of identified articles were then combined and duplicate references were deleted.
Data collection and analysis
Selection of studies
Three review authors (AT, AK, SM) independently assessed all citations and identified abstracts of relevance to the review against the core inclusion criteria using a pre‐designed study selection form. Core criteria included being a RCT, inclusion of participants with FM, diagnosis based on the ACR criteria (Wolfe 1990), included participants aged over 18 years, inclusion of an intervention likely to meet the mind‐body criteria (for example articles on exercise, massage, use of treatment devices or medication or supplements were excluded) and availability of an abstract describing the trial. Full text articles were acquired for any citations meeting the core inclusion criteria for the review or where additional information was required to determine eligibility.
All full text articles were then re‐assessed against the core inclusion criteria and against the additional six inclusion criteria defining what constituted a mind‐body intervention for this review (see criteria described in type of interventions). Reasons for inclusion or exclusion were recorded in an electronic spreadsheet and on the hard copy data extraction form. The results were compared between the review authors and any disagreement resolved through discussion and consensus. Where resolution was not possible through discussion, the full review team was consulted until a consensus decision was reached. Where information was not available in the full article, trial authors were contacted for further details to clarify eligibility for the review.
Data extraction and management
Three review authors (AT, AK, SM) were involved in extracting data from the included trials, with two review authors allocated to each trial to independently extract the data. Any disputes were resolved through discussion. The data were extracted using a hard copy data extraction standard form designed specifically for this review. The data extraction form recorded information on the type of intervention (such as length of programme, therapeutic components and therapist details), setting, study procedures (such as blinding of outcome assessors and treatment allocation), details of participants and outcome measure data. Where the information needed was insufficient or incomplete, multiple attempts were made to contact the trial authors. Data were extracted from graphs if this could be accurately measured with 100% agreement by two independent researchers.The data extracted from the included trials was entered into RevMan 5.
Endpoint versus change data
Continuous data collected from self‐report questionnaires were extracted if the measure explicitly aimed to assess one of the primary or secondary outcomes and was used in its standardised form. Endpoint scores were extracted from the trial articles. Group means were used throughout the analysis (Higgins 2011).
Skewed data
Data collected using questionnaires to measure clinical and psychological outcomes often does not reveal a normal distribution. To avoid the influence of skewed data on the analyses, data were only analysed if: 1) both means and standard deviations could be derived from the data provided in the article or provided by the trial authors; and 2) if the standard deviation was less than half the mean (Altman 1996).
Assessment of risk of bias in included studies
The studies included in the review were assessed for possible risk of bias using the Cochrane Collaboration tool for assessing the risk of bias (Higgins 2011). The methods of each study were assessed independently by two review authors (AT and MC) to ascertain if the procedures applied in the study were adequate. Any disagreement identified between the review authors was resolved through discussion or through the involvement of a third review author.
These components of trials forming the risk of bias assessment included:
1) sequence generation (e.g. was the sequence generation process truly random);
2) concealment of treatment allocation;
3) blinding of the outcome assessor;
4) completeness of outcome data (e.g. participant attrition rates post‐intervention and withdrawal rates between groups);
5) selective reporting bias (e.g. were all pre‐specified outcomes reported).
Other risks of bias such as design‐specific risks were not considered in this review, which only included randomised controlled trials. No studies reported early stopping. For each component the trials were classified as low risk of bias, high risk of bias or unclear (if there was insufficient information provided in the article to make a decision). If information on the procedures used within the trial were unclear, the authors of the article were contacted to yield the necessary information. If the necessary information could not be retrieved, the potential risk was classified as unclear. To assess the direction and magnitude of the risk of bias and the possible impact this may have on the findings, sensitivity analysis was conducted. The ’blinding of participants’ was not applied in this review as it would be extremely difficult to blind people delivering the intervention or participants in accordance with other Cochrane reviews (Bernardy 2013; Williams 2012). As mind‐body interventions require the participant to actively participate in the treatment, it was considered that it was not possible to blind the participant to their treatment allocation. However, it was considered to be both feasible and desirable to randomise participants to their treatment condition, so evidence of randomisation was an important criterion for inclusion in this review. Blinding of the outcome assessors was considered as part of the risk of bias assessment of the included studies.
Measures of treatment effect
For continuous data, the weighted mean difference in endpoint scores between groups (using the same version and scoring method for outcome measure on each of the outcome domains) was calculated with the 95% confidence interval (CI). Standardised mean differences (SMD) were used for continuous outcome data measuring the same outcome variable but using different: 1) scales or subscales; 2) versions published in different languages; or 3) scored using a different approach, due to the likelihood that there would be differences in measurement between the outcome measures (Puhan 2006). For binary data, standard estimation of the risk ratio (RR) and its 95% CI were used. The P < 0.05 significance level and 95% CIs were used as the conventional significance level (Higgins 2011). All outcome data were transformed, if necessary, before analysis to ensure that high scores on each measure reflected poorer health outcomes (by subtracting the mean from the maximum score on the measure). Numbers of withdrawals between the groups post‐intervention and adverse events reported were described in terms of frequencies.
Unit of analysis issues
Cross‐over trials
Cross‐over trials were excluded from this review as there is no evidence to suggest a suitable duration of a washout period following a mind‐body intervention and it is likely that some components (such as increased knowledge) may be sustained or retained over time.
Multiple treatment arms
Where a given trial presented relevant control data for more than one group (for example if a treatment group had both a usual care and an attention placebo comparison group), each set of data were used for the respective separate analyses. If an additional treatment group that met the criteria was presented, this was included separately in the analysis (as long as the control group data were not used more than once in a given comparison).
Dealing with missing data
Missing outcome data not reported
Where possible, trial authors were contacted to request any data of potential relevance to the review that was not presented in the article. For example, requests were made when the trial authors reported that an outcome measurement was conducted at follow‐up but the data were not presented, or if means or standard deviations were not able to be derived from the information provided. For studies where standard deviations were not available for outcome data but CIs were provided, the lower CI was used in addition to the mean to calculate the variance, using the Revman calculator.
Attrition
As high rates of attrition can influence the credibility of outcome data and observation of any treatment effect, any studies with attrition rates higher than 40% (calculated as the number of participants at follow‐up divided by the number of participants randomised x 100) were not included in the analyses but were included in the attrition analyses. This decision was based on evidence that overall completion rates of between 50% to 80% are considered to be acceptable (Altman 2000; Fewtrell 2008). Four studies were found to have high (above 40%) attrition rates (Astin 2003; Brattberg 2008; Edinger 2005; Vlaeyen 1996) and were excluded from the analysis.
Assessment of heterogeneity
Statistical heterogeneity
The statistical heterogeneity of trials was assessed using the I2 statistic, calculated using RevMan 5. A cut‐off point of I2 > 50% and a P value of < 0.10 from the Mantel‐Haenszel Chi2 test were used to determine if statistically significant heterogeneity was found between the trials (Higgins 2011).
Visual inspection of the graphs
All graphs were inspected by the review team to investigate the possibility of heterogeneity. Where differences in the findings were evident, the methodology of the studies included in the analysis were reviewed for potential reasons for heterogenous findings for example clinical heterogeneity or influence of different subtypes of therapy.
Assessment of reporting biases
Funnel plots were not reported due to the low numbers of trials included in the analyses (< 10), which may prevent adequate detection of publication bias (Lau 2006). Approaches to reduce publication bias such as searching for unpublished studies and setting clear inclusion and appraisal criteria were implemented to reduce the impact of possible publication bias on the review findings, however the possibility of publication bias remained. The risk of publication bias was considered in the grading of evidence in the summary of findings tables.
Data synthesis
In the absence of statistical heterogeneity a fixed‐effect model of meta‐analysis was used for combining data. If heterogeneity was found, a sensitivity analysis was completed.
Main comparisons
The main comparisons were conducted at the post‐intervention time point in this review.
Psychological therapies versus usual care.
Psychological therapies versus attention control.
Biofeedback versus usual care.
Biofeedback versus attention control.
Mindfulness meditation therapies versus usual care.
Mindfulness meditation therapies versus attention control.
Movement therapies versus usual care.
Movement therapies versus attention control.
Relaxation based therapies versus usual care.
Relaxation based therapies versus attention control.
It was evident that some interventions applied more than one mind‐body approach within the intervention, so interventions were categorised based on the primary focus or the largest component of the intervention, or both. In one study (Astin 2003) both mindfulness and a movement therapy were applied equally within the intervention and so the data were described but not included in the analyses as the primary focus could not be determined.
Subgroup analysis and investigation of heterogeneity
Subanalyses of longer‐term outcomes of mind‐body interventions including the short‐term (one to three months post‐intervention, where data closest to three months were used) and the medium‐term (three to six months post‐intervention, where data closest to six months were used) were calculated where outcome data were available.
Heterogeniety was investigated if there was observed inconsistency in the findings resulting from the main analyses and subanalyses. If heterogeneity was observed, firstly the accuracy of data entry was checked. Secondly any outliers were specifically investigated to determine if there was a possible explanation for the different findings for example different mode, duration or type of intervention, or risk of bias. Sensitivity analyses were planned to explore the effect of heterogeneity on the findings, where possible.
Sensitivity analysis
A cut‐off point of I2 > 50% and a P value of < 0.10 from the Mantel‐Haenszel Chi2 test were used to determine if statistically significant heterogeneity was found between the trials (Higgins 2011). Sensitivity analyses were completed to explore any potential effect of the intervention content or duration, and inclusion of studies classified as having a high risk of bias.
Grading of evidence and summary of findings tables
The data are presented in the summary of findings tables (Higgins 2011), conducted using GRADEpro software. The primary outcomes of self‐reported functioning and pain were included in the summary of findings tables. Data on adverse events were used only for the groups included in the analysis. Studies were downgraded based on assessments of risk of bias, inconsistency (for example differences in treatment duration), indirectness (for example if no males were included in the analysis to enhance generalisability to the fibromyalgia population), imprecision (studies were downgraded ‐1 if there were < 200 participants in the analysis and ‐2 if < 100 participants in the analysis).
Results
Description of studies
See: 'Characteristics of included studies'; 'Characteristics of excluded studies'; 'Characteristics of studies awaiting classification'.
Results of the search
The search elicited 2083 citations, with 2009 citations excluded as the studies did not meet the inclusion criteria for this review (Figure 1).
1.

Study flow diagram.
Included studies
There were 61 distinct trials identified from 74 publications, each of which met the inclusion criteria for the review (see 'Characteristics of included studies' table). Studies were conducted across 13 countries including; USA (22 studies), Spain (11 studies), Sweden (8 studies), Germany (4 studies), Canada (3 studies), Netherlands (3 studies), Norway (3 studies), Turkey (2 studies), Brazil (1 study), France (1 study), Italy (1 study), India (1 study), UK (1 study).
Interventions
The types of mind‐body interventions encompassed by the identified articles included in this review were classified into different mind‐body therapy categories.
There were five biofeedback studies (Babu 2007; Bakker 1995; Baumuller 2009; Kayiran 2010; Van Santen 2002).
There were three mindfulness studies (Parra‐Delgado 2013; Schmidt 2011; Sephton 2007).
There were 11 movement therapy interventions in total including three tai chi studies (Wang 2010; Calandre 2009;Jones 2012), three yoga studies (Carson 2010; Carson 2012; Holmer 2004), three qi‐gong studies (Liu 2012; Lynch 2012; Mannerkorpi 2004), one study on dance therapy (Bojner‐Horwitz 2003) and one study on pilates (Altan 2009).
The majority of studies (N = 35) were classified as involving a psychological therapy, which included two emotional freedom interventions (Brattberg 2008; Connais 2009), one study using the Resserguier approach (Maddali‐Bongh 2010), one study using the written emotional disclosure paradigm (Gillis 2006), one study using Accceptance Commitment Therapy (Wicksell 2013) and one study using psychotherapy (Scheidt 2013). There were 17 studies based on the cognitive behaviour therapy approach (Alda 2011; Ang 2010; Ang 2013; Castel 2009; Castel 2012; Edinger 2005; Falcao 2008; Garcia 2006; Hamnes 2012; Jensen 2012; Langford 2009; Lera 2009; Martinez‐Valero 2008; Miro 2011; Thieme 2006; Vlaeyen 1996; Williams 2002; Woolfolk 2012) and 11 studies based on psychoeducation (Burckhardt 1994; de Souza 2008; Fontaine 2010; Hammond 2006; Hsu 2010; Luciano 2011; Oliver 2001; Soares 2002; Stuifbergen 2010; Wigers 1996; Williams 2010).
Three studies looked at relaxation using the guided imagery approach (Fors 2000; Menzies 2006; Riedel 2012).
Four studies included interventions that were not able to be classified into the pre‐determined categories but were deemed to meet the inclusion criteria for a mind‐body intervention including music therapy (Oneva‐Zafra 2010), hypnosis (Picard 2013) and multi‐component interventions (Astin 2003; Castel 2009).
The overall length of treatment ranged between 1 day to 25 weeks. The average treatment duration was 17 hours. Mind‐body interventions were implemented in a range of settings, with over half of the studies (34 studies, 55.7%) conducted in a healthcare setting such as in a hospital or primary care clinic. Thirteen studies (21.3%) were conducted in a community setting such as in the person's home, with 7 studies (11.5%) conducted in a university or academic research centre. For 7 studies the type of setting where the intervention was delivered was not clear.
Most interventions (44.3%) were facilitated by a healthcare professional and 27.9% by a trained specialist in the particular therapy. Just over half (54.1%) of the studies included in this review involved only female participants. The mode of delivery of the intervention varied between trials with 54.1% of interventions delivered within a group based format, 37.7% delivered on an individual basis, and 6.6% using both a group and individual format for different elements of the intervention. For 1.6% of the interventions the mode of administration was unable to be clearly determined from the intervention description.
One study reported findings on multiple treatment arms (Thieme 2006) which included both a cognitive behaviour therapy intervention group and an operant behavioural therapy experimental group. Given there were no other studies in the review which also looked at the effectiveness of operant behavioural therapy and because both experimental groups would be in the same analysis (psychological therapies), and only one control group was available, only the cognitive behavioural group and control group (attention placebo) were included in the analyses.
Excluded studies
There were two studies that met the inclusion criteria of the review but that were still ongoing at the time of data extraction. These have be specified in the list of ongoing studies and should be included in future updates of this review (Garcia‐Campayo 2009; Miles 2010). Articles that met the inclusion criteria of the review but were excluded based on the six mind‐body intervention criteria for inclusion are outlined in the table 'Characteristics of excluded studies', with reasons for exclusion described.
Risk of bias in included studies
Allocation
All trials included in this review were described as randomised controlled trials or it was stated that a random component of participant allocation to the treatment group had been implemented. As shown in Figure 2, one study utilising a randomisation approach was classified as having a high risk of bias in accordance with the recommendations by Higgins 2011. As shown in Figure 3, a number of studies (13.0%) were classified as having an unclear risk of selection bias as insufficient details of the randomisation procedure were provided. Two studies (Connais 2009; Holmer 2004) were classified as having a high risk for allocation concealment as participants were alternately allocated to treatment groups and the researchers may have been able to foresee treatment allocation.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Blinding
Due to the nature of delivering mind and body interventions, where it would be clear to participants which group they were in, it was not expected that the included studies would be able to be double blinded (blinding of participants). With regard to blinding of outcome assessors most studies were rated as having a low risk of bias. Six trials (Calandre 2009; Fontaine 2010; Holmer 2004; Lera 2009; Parra‐Delgado 2013; Picard 2013) were classified as having a high risk of detection bias as the outcome assessors were not blind to treatment allocation.
Incomplete outcome data
Additional data or clarification of study procedures were obtained from the authors of 36 studies included in this review (Altan 2009; Astin 2003; Babu 2007; Bakker 1995; Baumuller 2009; Brattberg 2008; Burckhardt 1994; Calandre 2009; Carson 2010; Carson 2012; Castel 2007; Castel 2009; Connais 2009; de Souza 2008; Falcao 2008; Fontaine 2010; Gillis 2006; Holmer 2004; Hsu 2010; Kayiran 2010; Lera 2009; Lynch 2012; Maddali‐Bongh 2010; Mannerkorpi 2004; Martinez‐Valero 2008; Menzies 2006; Miro 2011; Oliver 2001; Oneva‐Zafra 2010; Parra‐Delgado 2013; Scheidt 2013; Soares 2002; Stuifbergen 2010; Wang 2010; Wigers 1996; Williams 2010).
Most included studies (N = 48, 78.7%) were rated as having a low risk of attrition bias. Six studies (Bakker 1995; Burckhardt 1994; Connais 2009; Garcia 2006; Lynch 2012; Williams 2002) were rated as having a high risk of attrition bias since we were unable to extract the means and standard deviations from the information provided, precluding inclusion in the meta‐analysis. Three studies (Astin 2003; Edinger 2005; Vlaeyen 1996) were classified as having a high risk of attrition bias as they reported attrition rates over 40%. One study (Brattberg 2008) was classified as being at high risk of bias as a large number of participants (40%) did not undertake or complete the intervention sessions but completed the outcome assessments. Details of reasons for attrition were often not provided.
Selective reporting
Forty‐seven (77.0%) studies were classified as having a low risk of reporting bias as data on the outcome measures of relevance to the review were provided. Seven studies (Bojner‐Horwitz 2003; Jones 2012; Liu 2012; Luciano 2011; Soares 2002; Williams 2002; Woolfolk 2012) were classified as having a high risk of reporting bias as data were not reported on specified outcome measures. It was not always clear from the reports whether measures were planned on being used as outcome measures or that their purpose was solely to provide baseline information or to act as covariates in the analysis.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
The primary outcome assessment time point was post‐intervention (up to one month following intervention delivery). This would provide the greatest opportunity to determine if any treatment effect was evident as any effects were most likely to be at their strongest immediately following a mind‐body intervention. Outcomes were also assessed in the short term (within one to three months post‐intervention) and medium term (greater than three to six months post‐intervention). If an outcome assessment was made at three months this was classified as a short‐term outcome.
Comparison 1. Psychological therapies versus usual care
There were 18 studies with data available for this comparison. Data were unable to be extracted from eight trials (Burckhardt 1994; Connais 2009; Edinger 2005; Garcia 2006; Martinez‐Valero 2008; Vlaeyen 1996; Williams 2002; Woolfolk 2012) exploring psychological therapies in comparison to usual care or were unable to be incorporated due to very high attrition rates (> 40%). Two studies (Falcao 2008; Soares 2002) revealed standard deviations that were more than half the mean on a specific outcome measure, indicating that the mean was unlikely to accurately reflect the centre‐point of the distribution for that variable (Altman 1996). As skewed data is less likely to be problematic if the data set is large, the sample sizes of these two studies were considered. As both studies had sample sizes of less than 100 participants it was decided to exclude the data from the analyses for variables where the standard deviation was more than half the mean. The data from a trial were included in the analyses for variables where this was not the case.
Major outcomes
1.1 Self‐reported physical functioning
Ten trials explored psychological therapies in comparison to usual care on physical functioning outcomes (Alda 2011; Castel 2009; Castel 2012; Falcao 2008; Hamnes 2012; Luciano 2011; Maddali‐Bongh 2010; Scheidt 2013; Soares 2002; Wicksell 2013). There was an advantage for psychological therapies observed post‐intervention (N = 733, SMD ‐0.43, 95% CI ‐0.57 to ‐0.28, Analysis 1.1), at 3 month follow‐up (N = 148, SMD ‐0.54, 95% CI ‐0.87 to ‐0.21, Analysis 1.2) and at 6 month follow‐up (N = 112, MD ‐3.66, 95% CI ‐7.29 to ‐0.03, Analysis 1.3).
1.1. Analysis.

Comparison 1 Psychological therapies versus usual care, Outcome 1 Functioning as assessed post‐intervention.
1.2. Analysis.

Comparison 1 Psychological therapies versus usual care, Outcome 2 Functioning as assessed at 3 month follow‐up.
1.3. Analysis.

Comparison 1 Psychological therapies versus usual care, Outcome 3 Functioning as assessed at 6 month follow‐up.
1.2 Self‐reported pain
Data from nine trials (Alda 2011; Castel 2009; Castel 2012; de Souza 2008; Hsu 2010; Jensen 2012; Maddali‐Bongh 2010; Soares 2002; Wigers 1996) revealed a difference between groups receiving psychological therapy and usual care that favoured psychological therapy post‐intervention (N = 453, SMD ‐0.33, 95% CI ‐0.52 to ‐0.15, Analysis 1.4). The advantage for psychological therapies over usual care was not observed at 3 month follow‐up (Falcao 2008; Castel 2012) (N = 115, MD ‐0.85, 95% CI ‐1.76 to ‐0.06, Analysis 1.5) but was observed at 6 months (N = 371, SMD ‐0.51, 95% CI ‐0.72 to ‐0.30, Analysis 1.6).
1.4. Analysis.

Comparison 1 Psychological therapies versus usual care, Outcome 4 Pain as assessed post‐intervention.
1.5. Analysis.
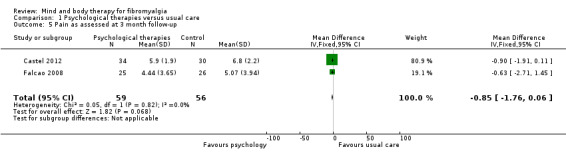
Comparison 1 Psychological therapies versus usual care, Outcome 5 Pain as assessed at 3 month follow‐up.
1.6. Analysis.
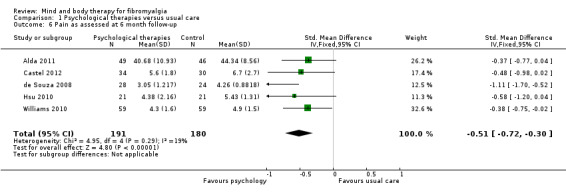
Comparison 1 Psychological therapies versus usual care, Outcome 6 Pain as assessed at 6 month follow‐up.
1.3 Mood
There was an advantage for psychological therapies in comparison to usual care post‐intervention, evident in eight trials (Alda 2011; Castel 2012; Falcao 2008; Hamnes 2012; Jensen 2012; Scheidt 2013; Wicksell 2013; Wigers 1996) (N = 492, SMD ‐0.45, 95% CI ‐0.64 to ‐0.26, Analysis 1.7). There was high heterogeneity between studies; removing the study by Castel 2012, which had a longer intervention delivery, reduced the I2 value to 7%. The advantage of psychological therapies post‐intervention remained (SMD ‐0.29, 95% CI ‐0.48 to ‐0.10). The advantage of psychological therapies was still evident at 3 months (N = 182, SMD ‐1.15, 95% CI ‐1.50 to ‐0.80). There was high heterogeneity observed. Removing the study by Castel 2012 reduced the I2 value to 0%. The advantage for psychology over usual care remained. At 6 months there was no advantage of psychological therapies over usual care (N = 213, SMD ‐0.17, 95% CI ‐0.44 to 0.10, Analysis 1.9).
1.7. Analysis.

Comparison 1 Psychological therapies versus usual care, Outcome 7 Mood as assessed post‐intervention.
1.9. Analysis.

Comparison 1 Psychological therapies versus usual care, Outcome 9 Mood as assessed at 6 month follow‐up.
1.4 Participant withdrawals
The RR of withdrawing from the study was statistically higher in the psychological therapy group in comparison to the control group (RR 1.38, 95% CI 1.12 to 1.69, Analysis 1.10).
1.10. Analysis.

Comparison 1 Psychological therapies versus usual care, Outcome 10 All cause attrition post‐intervention.
1.5 Adverse events
There was no difference between the number of adverse events in the psychological therapy and control groups (RR 0.38, 95% CI 0.06 to 2.50, Analysis 1.11). Only one study reported one person experiencing a worsening of symptoms in the psychological therapy group but it was not clear if this was directly related to the intervention or not (Vlaeyen 1996).
1.11. Analysis.

Comparison 1 Psychological therapies versus usual care, Outcome 11 Adverse events post‐intervention.
Minor outcomes
1.6 Fatigue
Only two studies presented data on fatigue following intervention delivery (Hsu 2010; Williams 2010). There was no advantage for psychological therapies in comparison to usual care at post‐intervention (N = 82, SMD ‐0.09, 95% CI ‐0.53 to 0.34, Analysis 1.12) nor at 6 month follow‐up (N = 160, SMD ‐0.07, 95% CI ‐0.38 to 0.24, Analysis 1.13). No follow‐up data were available for the 3 month follow‐up time point. Moderate heterogeneity was observed in the findings post‐intervention; neither study included in the analysis had a high risk of bias and the heterogeneity may have been reflective of the different psychological interventions included in the analysis, with one trial implementing a self‐awareness intervention and the other a stress management intervention (Analysis 2.3).
1.12. Analysis.

Comparison 1 Psychological therapies versus usual care, Outcome 12 Fatigue as assessed post‐intervention.
1.13. Analysis.

Comparison 1 Psychological therapies versus usual care, Outcome 13 Fatigue as assessed at 6 months post‐intervention.
2.3. Analysis.
Comparison 2 Psychological therapies versus usual care sensitivity analyses, Outcome 3 Fatigue as assessed post‐intervention.
1.7 Sleep
Data on sleep outcomes were presented by five trials (Castel 2012; Hsu 2010; Maddali‐Bongh 2010; Soares 2002; Wigers 1996). There was an advantage observed for psychological therapies in comparison to usual care for sleep post‐intervention (N = 222, SMD ‐0.52, 95% CI ‐0.80 to ‐0.25, Analysis 1.19). High heterogeneity was observed. Removing the study by Castel 2012, which was delivered over a much longer duration than the other trials, reduced the heterogeneity however the advantage of psychological therapies over usual care was no longer observed (N = 158, SMD ‐0.18, 95% CI ‐0.50 to 0.13). At 3 month follow‐up, one study revealed an advantage for psychological therapy over usual care (N = 64, MD ‐11.30, 95% CI ‐15.44 to ‐7.16). At 6 month follow‐up three studies provided data for analysis. No advantage for psychological therapies was observed (N = 224 , SMD ‐0.15, 95% CI ‐0.42 to 0.12, Analysis 1.21). High heterogeneity was observed within the data. Removing the study with a longer intervention duration (Castel 2012) reduced the heterogeneity, however there remained no advantage of psychological therapies over usual care (N = 160, SMD = 0.22, 95% CI ‐0.09 to 0.53, Analysis 2.4).
1.19. Analysis.

Comparison 1 Psychological therapies versus usual care, Outcome 19 Sleep as assessed post‐intervention.
1.21. Analysis.

Comparison 1 Psychological therapies versus usual care, Outcome 21 Sleep as assessed at 6 month follow‐up.
2.4. Analysis.

Comparison 2 Psychological therapies versus usual care sensitivity analyses, Outcome 4 Sleep as assessed post‐intervention.
1.8 Self efficacy
There were four trials that assessed self efficacy as an outcome (Brattberg 2008; Hamnes 2012; Soares 2002; Wicksell 2013). No advantage was found for psychological therapy in comparison to usual care post‐intervention (N = 255, SMD ‐0.25, 95% CI ‐0.50 to ‐0.00, Analysis 1.14). One study (Wicksell 2013) conducted a 3 month follow‐up and found that no difference between groups was observed (N = 23, MD ‐15.10, 95% CI ‐44.95 to 14.75).
1.14. Analysis.
Comparison 1 Psychological therapies versus usual care, Outcome 14 Self‐efficacy as assessed post‐intervention.
1.9 Tender points
No data were able to be extracted from trials assessing tender point count post‐intervention or at 3 month follow‐up. One trial (Hsu 2010) presented data at 6 month follow‐up. There was no advantage for psychological therapies over usual care at 6 months (N = 42, MD 0.38, 95% CI ‐0.12 to 0.88, Analysis 1.15).
1.15. Analysis.

Comparison 1 Psychological therapies versus usual care, Outcome 15 Tender point count as assessed at 6 month follow‐up.
1.10 Quality of life
Six trials presented data on quality of life post‐intervention (Brattberg 2008; Falcao 2008; Hsu 2010; Maddali‐Bongh 2010; Scheidt 2013; Wicksell 2013). There was no difference between groups on quality of life post‐intervention (N = 276, SMD ‐0.19, 95% CI ‐0.44 to 0.06, Analysis 1.16). Moderate heterogeneity was observed. Removing the study by Scheidt 2013 (that had a longer intervention delivery period) reduced the I2 value to 28% and an advantage of psychological therapies was observed (Analysis 1.18). At 3 months only one study provided data and the advantage for psychological therapies remained (N = 33, MD ‐15.16 95% CI ‐21.90 to ‐8.30). At 6 months the advantage for psychological therapies was no longer evident (N = 42, MD ‐2.50, 95% CI ‐7.95 to 2.95).
1.16. Analysis.

Comparison 1 Psychological therapies versus usual care, Outcome 16 Quality of life as assessed post‐intervention.
1.18. Analysis.

Comparison 1 Psychological therapies versus usual care, Outcome 18 Quality of life as assessed at 6 month follow‐up.
Comparison 2. Psychological therapies versus attention control
There were seven studies with data available for this comparison (Fontaine 2010; Gillis 2006; Langford 2009; Lera 2009; Miro 2011; Stuifbergen 2010; Thieme 2006).
Major outcomes
2.1 Self‐reported physical functioning
Seven studies reported data on functioning as an outcome (Fontaine 2010; Gillis 2006; Langford 2009; Lera 2009; Miro 2011; Stuifbergen 2010; Thieme 2006). There was no advantage of psychological therapy in comparison to an attention control post‐intervention (N = 561, SMD ‐0.10, 95% CI ‐0.27 to 0.07, Analysis 3.1) or in the short term (3 months) (N = 447, SMD 0.02, 95% CI ‐0.17 to 0.20, Analysis 3.2) or medium term (6 month follow‐up) (N = 326, SMD 0.00, 95% CI ‐0.22 to 0.23, Analysis 3.3). Moderate heterogeneity was observed within the findings for functioning post‐intervention; removing the study at high risk of bias for blinding of outcome assessors reduced the heterogeneity (Analysis 4.1). High heterogeneity was also observed at 6 month follow‐up, and a review of the forest plot indicated that the findings by Thieme 2006 were outliers. This may reflect that risk of bias was categorised as unclear as there was insufficient information in the article to determine level of risk, which may be reflective of trial quality. Removing the findings by Thieme 2006 reduced the heterogeneity; there remained no difference between groups at 6 month follow‐up (Analysis 4.2).
3.1. Analysis.

Comparison 3 Psychological therapies versus attention control, Outcome 1 Functioning as assessed post‐intervention.
3.2. Analysis.

Comparison 3 Psychological therapies versus attention control, Outcome 2 Functioning as assessed at 3 month follow‐up.
3.3. Analysis.

Comparison 3 Psychological therapies versus attention control, Outcome 3 Functioning as assessed at 6 month follow‐up.
4.1. Analysis.

Comparison 4 Psychological therapies versus attention control sensitivity analyses, Outcome 1 Functioning as assessed post‐intervention.
4.2. Analysis.
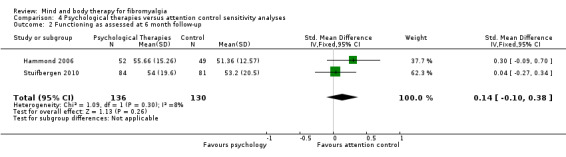
Comparison 4 Psychological therapies versus attention control sensitivity analyses, Outcome 2 Functioning as assessed at 6 month follow‐up.
2.2 Self‐reported pain
An advantage was found when psychological therapy was compared to an attention control post‐intervention (N = 324, SMD ‐0.28, 95% CI ‐0.51 to ‐0.06, Analysis 3.4), however this advantage was not sustained in the short term (N = 115, SMD 0.13, 95% CI ‐0.24 to 0.50, Analysis 3.5), or medium term (N = 60, MD ‐0.34, 95% CI ‐0.89 to 0.21, Analysis 3.6). Moderate heterogeneity was observed within the data for pain outcomes post‐intervention. On review of the type of interventions incorporated within the analysis it became apparent that three trials implemented CBT and one trial implemented written emotional disclosure as an intervention. Removing the written emotional disclosure intervention (Gillis 2006) from the analysis reduced the heterogeneity. An advantage for psychological therapy remained consistent following the sensitivity analysis (Analysis 4.3).
3.4. Analysis.

Comparison 3 Psychological therapies versus attention control, Outcome 4 Pain as assessed post‐intervention.
3.5. Analysis.

Comparison 3 Psychological therapies versus attention control, Outcome 5 Pain as assessed at 3 month follow‐up.
3.6. Analysis.

Comparison 3 Psychological therapies versus attention control, Outcome 6 Pain as assessed at 6 month follow‐up.
4.3. Analysis.
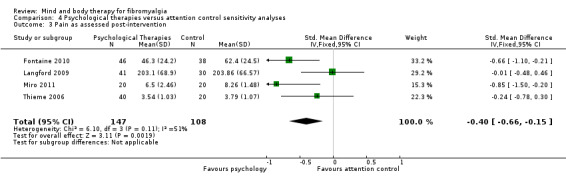
Comparison 4 Psychological therapies versus attention control sensitivity analyses, Outcome 3 Pain as assessed post‐intervention.
2.3 Mood
No advantage was observed for psychological therapy post‐intervention (N = 330, SMD ‐0.12, 95% CI ‐0.33 to 0.10, Analysis 3.7) and at 3 month follow‐up (N = 115, SMD 0.24, 95% CI ‐0.13 to 0.61, Analysis 3.8). No follow‐up data were available at 6 month follow‐up to explore the medium‐term outcomes.
3.7. Analysis.

Comparison 3 Psychological therapies versus attention control, Outcome 7 Mood as assessed post‐intervention.
3.8. Analysis.
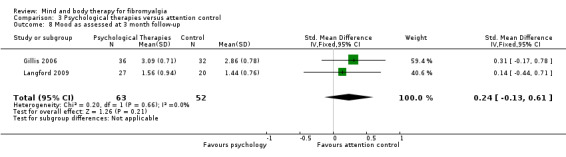
Comparison 3 Psychological therapies versus attention control, Outcome 8 Mood as assessed at 3 month follow‐up.
2.4 Participant withdrawals
The RR of withdrawing from the study for any reason was statistically higher in the control group than the psychological therapies group (RR 0.68, 95% CI 0.54 to 0.87, Analysis 3.9).
3.9. Analysis.

Comparison 3 Psychological therapies versus attention control, Outcome 9 All cause attrition post‐intervention.
2.5 Adverse events
No studies reported data on any adverse events observed.
Minor outcomes
2.6 Fatigue
Only two studies reported data on fatigue (Fontaine 2010; Gillis 2006). No advantage was observed for psychological therapy post‐intervention (N = 153, SMD ‐0.12, 95% CI ‐0.44 to 0.20, Analysis 3.10) or at 3 month follow‐up (N = 69, MD ‐0.18, CI ‐0.73 to 0.37, Analysis 3.11). No studies reported data at 6 month follow‐up.
3.10. Analysis.

Comparison 3 Psychological therapies versus attention control, Outcome 10 Fatigue as assessed post‐intervention.
3.11. Analysis.
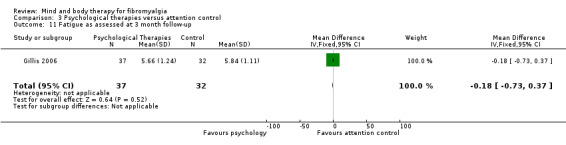
Comparison 3 Psychological therapies versus attention control, Outcome 11 Fatigue as assessed at 3 month follow‐up.
2.7 Sleep
No differences were observed in group outcomes for sleep when assessed post‐intervention (N = 109, SMD ‐0.12, 95% CI ‐0.50 to 0.25, Analysis 3.19) and at 3 month follow‐up (N = 69, MD 0.01, 95% CI ‐0.45 to 0.47, Analysis 3.20). No data were available for analysis at 6 month follow‐up. Moderate heterogeneity was observed in the findings for sleep outcomes. The two trials utilised different interventions, which may explain the heterogeneity, with one trial implementing CBT and one trial implementing a written emotional disclosure intervention (Analysis 4.4).
3.19. Analysis.

Comparison 3 Psychological therapies versus attention control, Outcome 19 Sleep as assessed post‐intervention.
3.20. Analysis.

Comparison 3 Psychological therapies versus attention control, Outcome 20 Sleep as assessed at 3 month follow‐up.
4.4. Analysis.

Comparison 4 Psychological therapies versus attention control sensitivity analyses, Outcome 4 Sleep as assessed post‐intervention.
2.8 Self efficacy
Only Langford 2009 reported outcomes with regard to self efficacy post‐intervention. No advantage was observed for psychological therapy in comparison to attention control (N = 105, MD 0.48, 95% CI ‐0.27 to 1.23, Analysis 3.12). Further data were available at the 3 month follow‐up time point (Hammond 2006; Langford 2009), however no differences in group outcomes were observed (N = 151, SMD ‐0.27, 95% CI ‐0.59 to 0.05, Analysis 3.13). One trial reported outcomes at 6 month follow‐up with no differences between the groups observed (N = 36, MD 0.01, 95% CI ‐1.31 to 1.33, Analysis 3.14).
3.12. Analysis.

Comparison 3 Psychological therapies versus attention control, Outcome 12 Self‐efficacy as assessed post‐intervention.
3.13. Analysis.

Comparison 3 Psychological therapies versus attention control, Outcome 13 Self efficacy as assessed at 3 month follow‐up.
3.14. Analysis.

Comparison 3 Psychological therapies versus attention control, Outcome 14 Self‐efficacy as assessed at 6 month follow‐up.
2.9 Tender points
There was no advantage observed for psychological therapies in comparison to attention control with regards to the tender point count post‐intervention (N = 150, MD ‐0.80, 95% CI ‐1.62 to 0.02, Analysis 3.15). No short or medium‐term follow‐up data were available.
3.15. Analysis.

Comparison 3 Psychological therapies versus attention control, Outcome 15 Tender point score as assessed post‐intervention.
2.10 Quality of life
Three trials reported data on quality of life (Langford 2009; Lera 2009; Stuifbergen 2010). No advantage was observed for psychological therapies at any endpoint: post‐intervention (N = 308, SMD ‐0.13, 95% CI ‐0.35 to ‐0.10, Analysis 3.16), 3 month follow‐up (N = 218, SMD ‐0.05, 95% CI ‐0.31 to 0.22, Analysis 3.17), 6 month follow‐up (N = 171, SMD ‐0.04, 95% CI ‐0.34 to 0.26, Analysis 3.18).
3.16. Analysis.

Comparison 3 Psychological therapies versus attention control, Outcome 16 Quality of life as assessed post‐intervention.
3.17. Analysis.

Comparison 3 Psychological therapies versus attention control, Outcome 17 Quality of life as assessed at 3 month follow‐up.
3.18. Analysis.

Comparison 3 Psychological therapies versus attention control, Outcome 18 Quality of life as assessed at 6 month follow‐up.
Comparison 3. Biofeedback versus usual care
There were two studies with data available for this comparison (Baumuller 2009; Van Santen 2002).
Major outcomes
3.1 Self‐reported physical functioning
Two studies provided data post‐intervention. No advantage was observed when biofeedback was compared to usual care post‐intervention (N = 106, SMD ‐0.06, 95% CI ‐0.44 to 0.33, Analysis 5.1). Only one study provided data at 3 month follow‐up (Baumuller 2009) revealing no advantage for biofeedback in the short to medium term (N = 36, MD ‐0.41, 95% CI ‐8.88 to 8.06, Analysis 5.2).
5.1. Analysis.

Comparison 5 Biofeedback versus usual care, Outcome 1 Functioning as assessed post‐intervention.
5.2. Analysis.
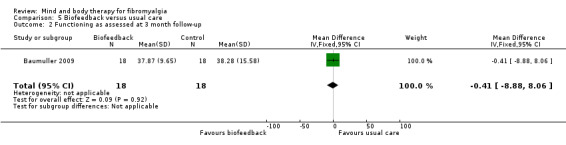
Comparison 5 Biofeedback versus usual care, Outcome 2 Functioning as assessed at 3 month follow‐up.
3.2 Self‐reported pain
Only one study provided data on pain post‐intervention (Van Santen 2002). It was revealed that there was no effect of biofeedback on pain in comparison to usual care (N = 65, MD ‐2.60, 95% CI ‐91.29 to 86.09, Analysis 5.3).
5.3. Analysis.

Comparison 5 Biofeedback versus usual care, Outcome 3 Pain as assessed post‐intervention.
3.3 Mood
There was no overall effect favouring biofeedback when compared to usual care post‐intervention (N = 104, SMD 0.13, 95% CI ‐0.26 to 0.52, Analysis 5.4) and at 3 month follow‐up (N = 36, MD 4.61, 95% CI ‐0.16 to 9.38, Analysis 5.5).
5.4. Analysis.
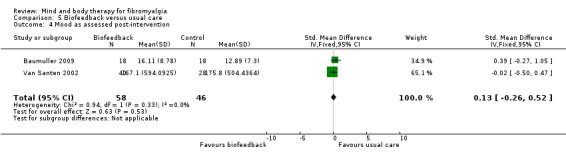
Comparison 5 Biofeedback versus usual care, Outcome 4 Mood as assessed post‐intervention.
5.5. Analysis.
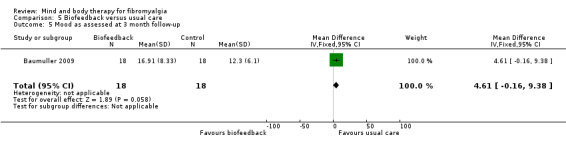
Comparison 5 Biofeedback versus usual care, Outcome 5 Mood as assessed at 3 month follow‐up.
3.4 Participant withdrawals
The RR of withdrawing from the study for any reason was statistically lower in the control group than the intervention group (RR 4.08, 95% CI 1.43 to 11.62, Analysis 5.6).
5.6. Analysis.

Comparison 5 Biofeedback versus usual care, Outcome 6 All cause attrition post‐intervention.
3.5 Adverse events
No studies reported data on any adverse events observed.
Minor outcomes
3.6 Fatigue
No data were available for analysis
3.7 Sleep
No data were available for analysis.
3.8 Self efficacy
No data were available for analysis.
3.9 Tender points
No effect in favour of biofeedback was observed post‐intervention (N = 101, MD ‐0.92, 95% CI ‐2.29 to 0.45, Analysis 5.7) or at 3 month follow‐up (N = 36, MD ‐0.09, 95% CI 0.09 to 0.62, Analysis 5.8).
5.7. Analysis.
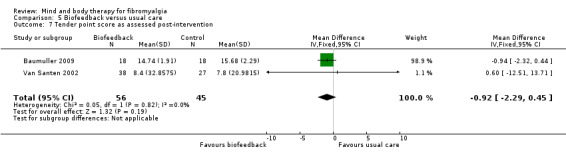
Comparison 5 Biofeedback versus usual care, Outcome 7 Tender point score as assessed post‐intervention.
5.8. Analysis.

Comparison 5 Biofeedback versus usual care, Outcome 8 Tender point score as assessed at 3 month follow‐up.
3.10 Quality of life
Only one study (Baumuller 2009) presented data on quality of life as an outcome, as assessed by the German version of the Short Form 36 (SF36). There was an overall effect for the vitality domain post‐intervention (N = 36, MD ‐13.43, 95% CI ‐24.06 to ‐2.80, Analysis 5.13) but this was not sustained at 3 month follow‐up (Analysis 5.22).
5.13. Analysis.
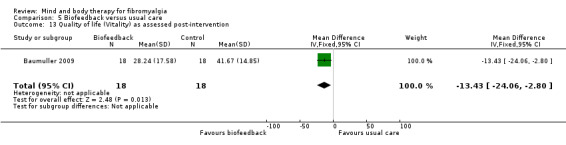
Comparison 5 Biofeedback versus usual care, Outcome 13 Quality of life (Vitality) as assessed post‐intervention.
5.22. Analysis.

Comparison 5 Biofeedback versus usual care, Outcome 22 Quality of life (Vitality) as assessed at 3 month follow‐up.
There was no overall effect for biofeedback over usual care post‐intervention on seven out of the eight outcome domains (Analysis 5.9; Analysis 5.10; Analysis 5.11; Analysis 5.12; Analysis 5.14; Analysis 5.15; Analysis 5.16), nor at 3 month follow‐up (Analysis 5.17; Analysis 5.18; Analysis 5.19; Analysis 5.21Analysis 5.20; Analysis 5.23; Analysis 5.24). There was an overall effect for the vitality domain post‐intervention (N = 36, MD ‐13.43, 95% CI ‐24.06 to ‐2.80, Analysis 5.13) but this was not sustained at 3 month follow‐up (Analysis 5.22).
5.9. Analysis.

Comparison 5 Biofeedback versus usual care, Outcome 9 Quality of life (Physical functioning) as assessed post‐intervention.
5.10. Analysis.
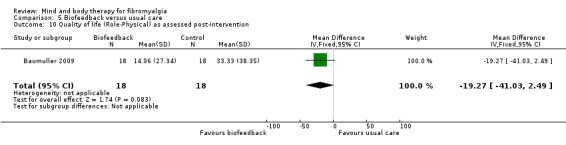
Comparison 5 Biofeedback versus usual care, Outcome 10 Quality of life (Role‐Physical) as assessed post‐intervention.
5.11. Analysis.

Comparison 5 Biofeedback versus usual care, Outcome 11 Quality of life (Bodily Pain) as assessed post‐intervention.
5.12. Analysis.

Comparison 5 Biofeedback versus usual care, Outcome 12 Quality of life (General Health) as assessed post‐intervention.
5.14. Analysis.
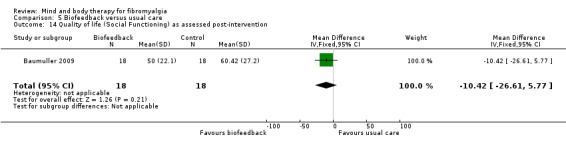
Comparison 5 Biofeedback versus usual care, Outcome 14 Quality of life (Social Functioning) as assessed post‐intervention.
5.15. Analysis.

Comparison 5 Biofeedback versus usual care, Outcome 15 Quality of life (Role‐Emotional) as assessed post‐intervention.
5.16. Analysis.

Comparison 5 Biofeedback versus usual care, Outcome 16 Quality of life (Mental Health) as assessed post‐intervention.
5.17. Analysis.

Comparison 5 Biofeedback versus usual care, Outcome 17 Quality of life (Physical functioning) as assessed at 3 month follow‐up.
5.18. Analysis.

Comparison 5 Biofeedback versus usual care, Outcome 18 Quality of life (Role‐Physical) as assessed at 3 month follow‐up.
5.19. Analysis.

Comparison 5 Biofeedback versus usual care, Outcome 19 Quality of life (Bodily Pain) as assessed at 3 month follow‐up.
5.21. Analysis.

Comparison 5 Biofeedback versus usual care, Outcome 21 Quality of life (General Health) as assessed at 3 month follow‐up.
5.20. Analysis.
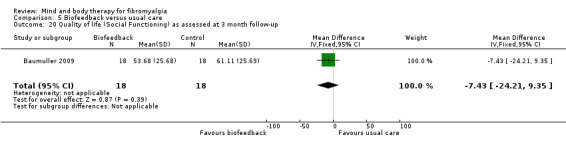
Comparison 5 Biofeedback versus usual care, Outcome 20 Quality of life (Social Functioning) as assessed at 3 month follow‐up.
5.23. Analysis.

Comparison 5 Biofeedback versus usual care, Outcome 23 Quality of life (Role‐Emotional) as assessed at 3 month follow‐up.
5.24. Analysis.

Comparison 5 Biofeedback versus usual care, Outcome 24 Quality of life (Mental Health) as assessed at 3 month follow‐up.
Comparison 4. Biofeedback versus attention control
Only one study presented data using an attention placebo control group (Babu 2007). Outcome assessments were only completed post‐intervention for this study therefore three and six month data were not available.
Major outcomes
4.1 Self‐reported physical functioning
There was a difference in Fibromyalgia Impact Questionnaire scores between biofeedback and sham attention control post‐intervention favouring biofeedback (N = 30, MD 13.60, 95% CI 1.05 to 26.13, Analysis 6.1).
6.1. Analysis.

Comparison 6 Biofeedback versus attention control, Outcome 1 Functioning as assessed post‐intervention.
4.2 Self‐reported pain
An advantage was observed for biofeedback in comparison to sham attention control post‐intervention on a 100 point visual analog scale for pain post‐intervention (N = 30, MD 2.66, 95% CI 1.21 to 5.71, Analysis 6.2).
6.2. Analysis.

Comparison 6 Biofeedback versus attention control, Outcome 2 Pain as assessed post‐intervention.
4.3 Mood
No data were available for analysis.
4.4 Participant withdrawals
The RR of withdrawing from the study for any reason did not differ between the biofeedback group and control group (RR 3.46, 95% CI 0.44 to 27.19, Analysis 6.3).
6.3. Analysis.

Comparison 6 Biofeedback versus attention control, Outcome 3 All cause attrition post‐intervention.
4.5 Adverse events
No studies reported data on any adverse events observed.
Minor outcomes
4.6 Fatigue
No data were available for analysis.
4.7 Sleep
No data were available for analysis.
4.8 Self efficacy
No data were available for analysis.
4.9 Tender points
Data available for the tender point count post‐intervention revealed an advantage for biofeedback over a sham attention control group (N = 30, MD 2.93, 95% CI 0.15 to 5.71, Analysis 6.4).
6.4. Analysis.
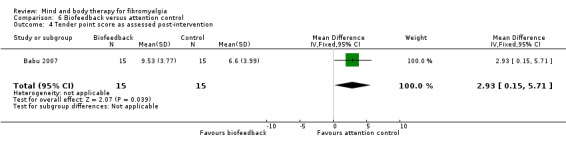
Comparison 6 Biofeedback versus attention control, Outcome 4 Tender point score as assessed post‐intervention.
4.10 Quality of life
No data were available for analysis.
Comparison 5. Mindfulness meditation therapies versus usual care
Only three studies reported data that could be extracted for this analysis (Parra‐Delgado 2013; Schmidt 2011; Sephton 2007).
Major outcomes
5.1 Self‐reported physical functioning
Two studies reported data relating to self‐reported physical functioning following a mindfulness intervention in comparison to a usual care control group (Parra‐Delgado 2013; Schmidt 2011). There were no differences between the mindfulness and the wait‐list control groups post‐intervention (N = 128, SMD ‐0.26, 95% CI ‐0.60 to 0.09, Analysis 7.1) or at short‐term follow‐up (N = 103, MD ‐0.06, 95% CI ‐1.78 to 0.66, Analysis 7.2). No statistical or clinical heterogeneity was observed in this comparison.
7.1. Analysis.
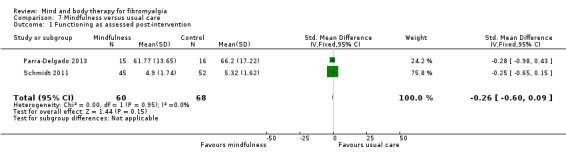
Comparison 7 Mindfulness versus usual care, Outcome 1 Functioning as assessed post‐intervention.
7.2. Analysis.
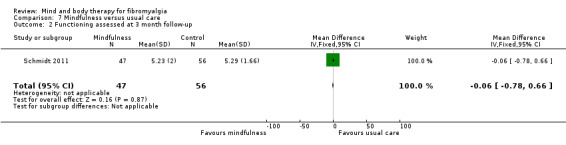
Comparison 7 Mindfulness versus usual care, Outcome 2 Functioning assessed at 3 month follow‐up.
5.2 Self‐reported pain
Two studies reported data on pain as an outcome measure (Parra‐Delgado 2013; Schmidt 2011). There was no advantage of mindfulness in comparison to a wait‐list control group post‐intervention (N = 128, SMD ‐0.09, 95% CI ‐0.44 to 0.26, Analysis 7.3) and at short‐term follow‐up (N = 103, MD ‐0.28, 95% CI ‐2.37 to 1.81, Analysis 7.4). No statistical or clinical heterogeneity was observed in this comparison.
7.3. Analysis.
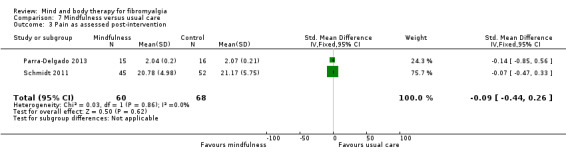
Comparison 7 Mindfulness versus usual care, Outcome 3 Pain as assessed post‐intervention.
7.4. Analysis.

Comparison 7 Mindfulness versus usual care, Outcome 4 Pain as assessed at 3 month follow‐up.
5.3 Mood
Three trials reported data relating to mood as an outcome measure (Parra‐Delgado 2013; Schmidt 2011; Sephton 2007). There was no difference between the mindfulness and wait‐list control groups post‐intervention (N = 218, SMD ‐0.24, 95% CI ‐0.51 to 0.03, Analysis 7.5) and at short‐term follow‐up (N = 193, SMD ‐0.21, 95% CI ‐0.50 to 0.07, Analysis 7.6). There was a moderate level of heterogeneity observed at the 3 month follow‐up for mood. On review of the included studies it became evident that one study (Parra‐Delgado 2013) was classified as having a high risk of bias for outcome assessment. Following removal of this study there remained no difference between participants receiving the mindfulness intervention and controls (Analysis 4.3).
7.5. Analysis.
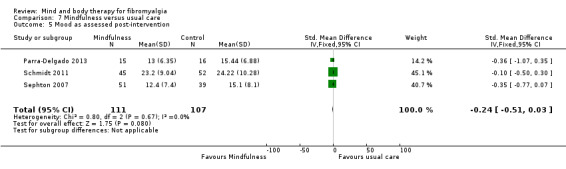
Comparison 7 Mindfulness versus usual care, Outcome 5 Mood as assessed post‐intervention.
7.6. Analysis.

Comparison 7 Mindfulness versus usual care, Outcome 6 Mood as assessed at 3 month follow‐up.
5.4 Participant withdrawals
There was no difference in participant withdrawals between the intervention and control groups (RR 1.07, CI 0.67 to 1.72, Analysis 7.7), however as the CI included one there was uncertainty in the estimate.
7.7. Analysis.
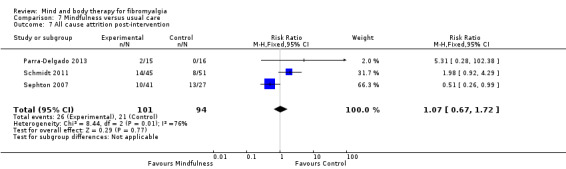
Comparison 7 Mindfulness versus usual care, Outcome 7 All cause attrition post‐intervention.
5.5 Adverse events
No studies reported data on any adverse events observed.
Minor outcomes
5.6 Fatigue
No data were available for this analysis.
5.7 Sleep
Only one study (Schmidt 2011) reported on sleep as an outcome (assessed using the Pittsburgh Sleep Quality Index). There was no advantage of mindfulness in comparison to usual care post‐intervention (N = 97, MD ‐0.64, 95% CI ‐2.27 to 0.99, Analysis 7.8) or at short‐term follow‐up (N = 103, MD ‐0.36, 95% CI ‐1.91 to 1.19, Analysis 7.9).
7.8. Analysis.

Comparison 7 Mindfulness versus usual care, Outcome 8 Sleep as assessed post‐intervention.
7.9. Analysis.

Comparison 7 Mindfulness versus usual care, Outcome 9 Sleep as assessed at 3 month follow‐up.
5.8 Self efficacy
No data were available for this analysis.
5.9 Tender points
No data were available for this analysis.
5.10 Quality of life
No data were available for this analysis.
Comparison 6. Mindfulness meditation therapies versus attention control
There was no data available for this comparison.
Comparison 7. Movement therapies versus usual care
Data were available for four studies exploring a movement therapy in comparison to a usual care control group (Carson 2010; Carson 2012; Holmer 2004; Mannerkorpi 2004).
Major outcomes
7.1 Self‐reported physical functioning
Four studies providing data on functioning post‐intervention (Carson 2010; Carson 2012; Holmer 2004; Mannerkorpi 2004). There was an advantage for movement therapies over usual care (N = 124, SMD ‐0.19, 95% CI ‐0.5 to ‐0.2, Analysis 9.1). However, high statistical heterogeneity was observed. To explore reasons for this heterogeneity two sensitivity analyses were completed, one to explore the effect of removing one trial classified as having a high risk of bias (Holmer 2004) and the other to explore potential differences between movement therapy types by removing the two studies looking at qi‐gong interventions (as the other three studies looked at the effect of yoga). Despite completing these two sensitivity analyses high heterogeneity remained and no other reasons for the heterogeneity were observed. In the short and medium‐term follow‐ups, there remained an advantage for movement therapies over usual care (N = 143, MD ‐0.65, 95% CI ‐1.08 to ‐0.22, P < 0.01 at 3 months; MD ‐11.21, 95% CI ‐19.13 to ‐3.29, P < 0.01 at 6 month follow‐up).
9.1. Analysis.

Comparison 9 Movement therapies versus usual care, Outcome 1 Functioning as assessed post‐intervention.
7.2 Self‐reported pain
One study (Holmer 2004) reported data on pain post‐intervention. There was an advantage for movement therapies in comparison to usual care (N = 28, MD ‐2.30, 95% CI ‐4.19 to ‐0.41, Analysis 9.2).
9.2. Analysis.

Comparison 9 Movement therapies versus usual care, Outcome 2 Pain as assessed post‐intervention.
7.3 Mood
Data were available for one study that assessed mood post‐intervention (Holmer 2004). An overall effect was observed for movement therapy over usual care (N = 29, MD ‐9.84, 95% CI ‐18.51 to ‐1.17, P = 0.03, Analysis 9.3).
9.3. Analysis.
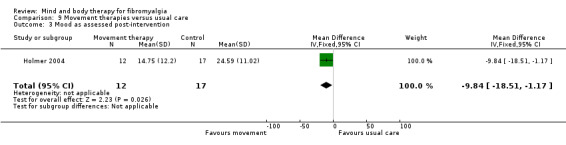
Comparison 9 Movement therapies versus usual care, Outcome 3 Mood as assessed post‐intervention.
7.4 Participant withdrawals
The RR of withdrawing from the study for any reason was statistically lower in the control group than the movement therapy group (RR 1.95, 95% CI 1.13 to 3.38, Analysis 9.4).
9.4. Analysis.

Comparison 9 Movement therapies versus usual care, Outcome 4 All cause attrition post‐intervention.
7.5 Adverse events
There was no difference between adverse events in the movement therapy and control group (RR 4.62, 95% CI 0.23 to 93.92, Analysis 9.5). The CIs were large, which may reflect that only one study (Lynch 2012) reported on adverse events that occurred. In this study two people reported experiencing increased pain in the intervention group.
9.5. Analysis.

Comparison 9 Movement therapies versus usual care, Outcome 5 Adverse events post‐intervention.
Minor outcomes
7.6 Fatigue
The data presented on fatigue (Holmer 2004), as assessed using the Multidimensional Assessment of Fatigue scale, revealed that there was an advantage of movement therapy in comparison to usual care (N = 29, MD ‐10.80, 95% CI ‐18.57 to ‐1.17, Analysis 9.6).
9.6. Analysis.
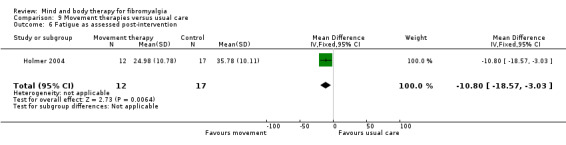
Comparison 9 Movement therapies versus usual care, Outcome 6 Fatigue as assessed post‐intervention.
7.7 Sleep
One study reported data assessing sleep post‐intervention (Holmer 2004). Sleep was assessed using the Pittsburgh Sleep Quality Index. There was a difference observed in sleep quality post‐intervention, with those receiving a movement therapy intervention revealing improved outcomes (N = 29, MD ‐4.68, 95% CI ‐8.14 to ‐1.22, Analysis 9.8).
9.8. Analysis.
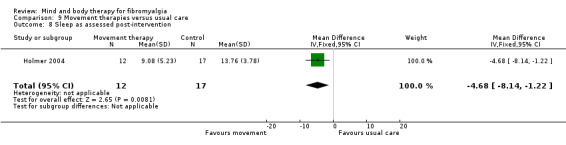
Comparison 9 Movement therapies versus usual care, Outcome 8 Sleep as assessed post‐intervention.
7.8 Self efficacy
No data were available.
7.9 Tender points
Data were only available for two studies which incorporated a tender point count as an outcome measure (Carson 2010; Carson 2012). There was no advantage observed for movement therapy over usual care (N = 93, SMD 0.18, 95% CI ‐0.25 to 0.60, Analysis 9.7). High heterogeneity was observed (91%) but no reasons for the heterogeneity were apparent in terms of intervention delivery or study quality.
9.7. Analysis.

Comparison 9 Movement therapies versus usual care, Outcome 7 Tender point count as assessed post‐intervention.
7.10 Quality of life
No data were available.
Comparison 8. Movement therapies versus attention control
There were three studies with data available for this comparison. One study (Wang 2010) revealed standard deviations that were more than half the mean on a specific outcome measure, indicating that the mean was unlikely to accurately reflect the centre‐point of the distribution for that variable (Altman 1996). Due to the low number of studies that could be analysed for each outcome domain, it was not possible to complete a sensitivity analyses with and without these data, as planned. As skewed data were less likely to be problematic if the data set was large, and the study had a sample size of less than 100 participants, it was decided to exclude the data from the analyses for variables where the standard deviation was more than half the mean (but the data from the trial were included in the analyses for variables where this was not the case).
Major outcomes
8.1 Self‐reported physical functioning
There was an advantage observed from three studies (Altan 2009; Calandre 2009; Wang 2010) for movement therapy over an attention control group on functioning post‐intervention (N = 191, SMD ‐0.65, 95% CI ‐0.94 to ‐0.35, Analysis 12.1) and this was sustained at 3 month follow‐up (N = 189, SMD ‐0.53, 95% CI ‐0.82 to ‐0.23, Analysis 12.2). Removing the data from one trial with inadequate blinding of outcome assessors (Calandre 2009) reduced the heterogeneity and the advantage for movement therapy remained (Analysis 13.1; Analysis 13.2).
12.1. Analysis.

Comparison 12 Movement therapies versus attention control, Outcome 1 Functioning as assessed post‐intervention.
12.2. Analysis.
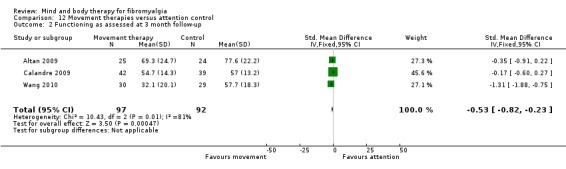
Comparison 12 Movement therapies versus attention control, Outcome 2 Functioning as assessed at 3 month follow‐up.
13.1. Analysis.

Comparison 13 Movement therapies versus attention control ‐ sensitivity analyses, Outcome 1 Functioning as assessed post‐intervention.
13.2. Analysis.
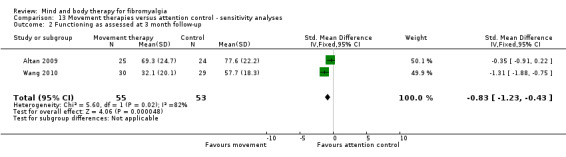
Comparison 13 Movement therapies versus attention control ‐ sensitivity analyses, Outcome 2 Functioning as assessed at 3 month follow‐up.
8.2 Self‐reported pain
Three studies assessed pain as an outcome using a 10 point visual analog scale (Altan 2009; Calandre 2009; Wang 2010). An advantage was revealed for movement therapy over attention control post‐intervention (N = 172, MD ‐1.45, 95% CI ‐2.08 to ‐0.81, Analysis 12.3) that was sustained at 3 month follow‐up (N = 165, MD ‐1.19, 95% CI ‐1.87 to ‐0.52, Analysis 12.4). When one trial (Calandre 2009) showing inadequate blinding of outcome assessors was removed from the analysis statistical heterogeneity was reduced and an advantage for movement therapies remained (Analysis 13.3; Analysis 13.4).
12.3. Analysis.

Comparison 12 Movement therapies versus attention control, Outcome 3 Pain as assessed by a 10‐point VAS scale post‐intervention.
12.4. Analysis.

Comparison 12 Movement therapies versus attention control, Outcome 4 Pain as assessed by a 10‐point VAS scale at 3 month follow‐up.
13.3. Analysis.
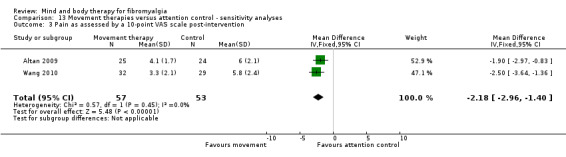
Comparison 13 Movement therapies versus attention control ‐ sensitivity analyses, Outcome 3 Pain as assessed by a 10‐point VAS scale post‐intervention.
13.4. Analysis.

Comparison 13 Movement therapies versus attention control ‐ sensitivity analyses, Outcome 4 Pain as assessed by a 10‐point VAS scale at 3 month follow‐up.
8.3 Mood
Two studies presented data on mood as an outcome (Calandre 2009; Wang 2010). There was a difference in mood scores between movement therapy and the attention control groups favouring movement therapy post‐intervention (N = 141, SMD ‐0.49, 95% CI ‐0.83 to ‐0.15, Analysis 12.5). The group difference remained evident at 3 months (N = 140, SMD ‐0.35, 95% CI ‐0.69 to ‐0.01, Analysis 12.6). After removing one trial from the analysis due to the high risk of bias identified (due to inadequate blinding of outcome assessors) differences in group outcomes remained, favouring movement therapy (Analysis 13.5; Analysis 13.6).
12.5. Analysis.

Comparison 12 Movement therapies versus attention control, Outcome 5 Mood as assessed post‐intervention.
12.6. Analysis.

Comparison 12 Movement therapies versus attention control, Outcome 6 Mood as assessed at 3 month follow‐up.
13.5. Analysis.

Comparison 13 Movement therapies versus attention control ‐ sensitivity analyses, Outcome 5 Mood as assessed post‐intervention.
13.6. Analysis.
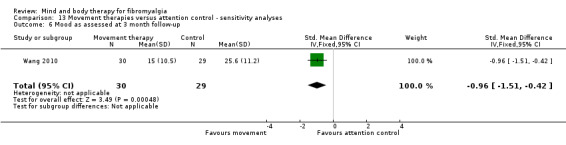
Comparison 13 Movement therapies versus attention control ‐ sensitivity analyses, Outcome 6 Mood as assessed at 3 month follow‐up.
8.4 Participant withdrawals
There was no difference between the rates of participant withdrawals for the movement therapy and control groups (RR 1.16, 95% CI 0.65 to 2.09, Analysis 12.7).
12.7. Analysis.

Comparison 12 Movement therapies versus attention control, Outcome 7 All cause attrition post‐intervention.
8.5 Adverse events
There was no difference between the number of adverse events in the movement therapy and control groups (RR 7.00, 95% CI 0.37 to 131.17, Analysis 12.8). The CIs were large, which may reflect that only one study (Calandre 2009) reported on adverse events. In this study three people experienced adverse events in the intervention group including one person who reported being hypersensitive to chlorine (as the intervention was conducted in a pool) and two participants who reported increased pain.
12.8. Analysis.

Comparison 12 Movement therapies versus attention control, Outcome 8 Adverse events post‐intervention.
Minor outcomes
8.5 Fatigue
No studies reported data that could be used in this analysis. Calandre 2009 reported data on the fatigue questions of the Fibromyalgia Impact Questionnaire, but as these data was included in the total score for self‐reported physical functioning variable the data were not presented here.
8.7 Sleep
The Pittsburgh Sleep Quality Index was used to assess sleep quality in two trials (Calandre 2009; Wang 2010). There was an advantage for movement therapy in comparison to attention control for sleep post‐intervention (N = 141, MD ‐1.88, 95% CI ‐3.27 to ‐0.48, Analysis 12.15), but this was not evident at 3 month follow‐up (N = 140, MD ‐1.35, 95% CI ‐2.77 to 0.07, Analysis 12.16).
12.15. Analysis.

Comparison 12 Movement therapies versus attention control, Outcome 15 Sleep quality as assessed by the Pittsburgh Sleep Quality Index post‐intervention.
12.16. Analysis.

Comparison 12 Movement therapies versus attention control, Outcome 16 Sleep quality as assessed by the Pittsburgh Sleep Quality Index at 3 month follow‐up.
8.8 Self efficacy
One trial presented data on self efficacy for movement therapy versus an attention control group (Wang 2010). There was an advantage observed for movement therapies post‐intervention (N = 60, MD ‐45.20, 95% CI ‐46.14 to ‐44.22, Analysis 12.9) and this was sustained at 3 month follow‐up (N = 59, MD 1.20, 95% CI 0.15 to 2.25, Analysis 12.10).
12.9. Analysis.

Comparison 12 Movement therapies versus attention control, Outcome 9 Self‐efficacy as assessed post‐intervention.
12.10. Analysis.

Comparison 12 Movement therapies versus attention control, Outcome 10 Self‐efficacy as assessed at 3 month follow‐up.
8.9 Tender points
There was no advantage revealed for movement therapies in the short (N =130, MD 0.09, 95% CI ‐1.16 to 1.33, Analysis 12.11) or medium term (N = 130, MD ‐0.39, 95% CI ‐1.63 to 0.85, Analysis 12.12) across two trials (Altan 2009; Calandre 2009).
12.11. Analysis.

Comparison 12 Movement therapies versus attention control, Outcome 11 Tender points as assessed post‐intervention.
12.12. Analysis.

Comparison 12 Movement therapies versus attention control, Outcome 12 Tender points as assessed at 3 month follow‐up.
8.10 Quality of life
Two studies presented data from quality of life assessments (Altan 2009; Wang 2010). An advantage was observed for movement therapies in comparison to attention control post‐intervention (N = 109, SMD ‐0.70, 95% CI ‐1.09 to ‐0.31, Analysis 12.13) and at 3 month follow‐up (N = 108, SMD ‐0.52, 95% CI ‐0.91 to ‐0.14, Analysis 12.14).
12.13. Analysis.
Comparison 12 Movement therapies versus attention control, Outcome 13 Quality of life as assessed post‐intervention.
12.14. Analysis.
Comparison 12 Movement therapies versus attention control, Outcome 14 Quality of life as assessed at 3 month follow‐up.
Comparison 9. Relaxation based therapies versus usual care
There were two studies with data available for this comparison (Menzies 2006; Riedel 2012).
Major outcomes
9.1 Self‐reported physical functioning
Two trials (Menzies 2006; Riedel 2012) presented data relating to functioning post‐intervention, which was assessed using the Fibromyalgia Impact Questionnaire. There was an advantage for relaxation in comparison to usual care (N = 67, MD ‐8.34, 95% CI ‐10.14 to ‐6.53, Analysis 14.1). No follow‐up data were available to determine short and medium‐term effectiveness and no statistical heterogeneity was observed.
14.1. Analysis.

Comparison 14 Relaxation versus usual care, Outcome 1 Functioning as assessed post‐intervention.
9.2 Self‐reported pain
Menzies 2006 and Riedel 2012 reported on pain as an outcome following a relaxation intervention in comparison to usual care. There was an advantage observed for relaxation post‐intervention (N = 67, SMD 1.02, 95% CI ‐1.55 to 0.50, Analysis 14.2). No follow‐up data were available. Statistical heterogeneity was observed between the two studies but no major methodological reasons were identified.
14.2. Analysis.

Comparison 14 Relaxation versus usual care, Outcome 2 Pain as assessed post‐intervention.
9.3 Mood
Riedel 2012 presented data on depression following intervention delivery as assessed by the Center for Epidemiological Studies Depression Scale. There were no differences observed between the experimental and control groups post‐intervention (N = 19, MD ‐4.44, 95% CI ‐14.46 to 5.58).
9.4 Participant withdrawals
There was no difference between participant withdrawal rates for the relaxation and control groups (RR 4.40, 95% CI 0.59 to 33.07, Analysis 14.4).
14.4. Analysis.

Comparison 14 Relaxation versus usual care, Outcome 4 All cause attrition post‐intervention.
9.5 Adverse events
No studies reported data on any adverse events observed.
Minor outcomes
9.6 Fatigue
Only one study (Riedel 2012) presented data on fatigue post‐intervention. There were no differences observed between relaxation and control participants (N = 19, MD ‐0.82, 95% CI ‐2.91 to 1.27).
9.7 Sleep
The study by Riedel 2012 presented information on sleep quality as assessed by the Pittsburgh Sleep Quality Index. There were no differences observed between the experimental and control groups post‐intervention (N = 19, MD 1.03, 95% CI ‐2.23 to 4.29).
9.8 Self efficacy
There was an advantage observed for relaxation over usual care on self efficacy as assessed by two studies post‐intervention (Menzies 2006; Riedel 2012) (N = 67, SMD ‐1.54, 95% CI ‐2.13 to ‐0.95, Analysis 14.5). No follow‐up data were available.
14.5. Analysis.

Comparison 14 Relaxation versus usual care, Outcome 5 Self‐efficacy as assessed post‐intervention.
9.9 Tender points
No data were available for this analysis.
9.10 Quality of life
No data were available for this analysis.
Comparison 10. Relaxation based therapies versus attention control
There was only one study with data available for this comparison (Fors 2000).
Major outcomes
10.1 Self‐reported physical functioning
No data were available for this analysis.
10.2 Self‐reported pain
One trial presented data on pain, which was assessed by a 100 point visual analog scale (Fors 2000). There was an advantage identified for the relaxation group in comparison to an education control group (N = 39, MD ‐23.17, 95% CI ‐36.73 to ‐9.61, Analysis 15.1). No follow‐up assessment data were available.
15.1. Analysis.

Comparison 15 Relaxation versus attention control, Outcome 1 Pain as assessed post‐intervention.
10.3 Mood
The data presented by Fors 2000, which assessed mood using a 100 point visual analog scale for anxiety, found an improvement with the use of relaxation based therapies in comparison to an education control (N = 39, MD ‐32.10, 95% CI ‐46.35 to ‐17.85, Analysis 15.2). No follow‐up data were available.
15.2. Analysis.

Comparison 15 Relaxation versus attention control, Outcome 2 Mood as assessed post‐intervention.
10.4 Participant withdrawals
As no participants were reported to have withdrawn from the Fors 2000 study estimates could not be derived for this outcome.
10.5 Adverse events
No studies reported data on any adverse events observed.
Minor outcomes
10.6 Fatigue
No data were available for this analysis.
10.7 Sleep
No data were available for this analysis.
10.8 Self efficacy
No data were available for this analysis.
10.9 Tender points
No data were available for this analysis.
10.10 Quality of life
No data were available for this analysis.
Discussion
Summary of main results
Moderate, low or very low quality of evidence from 61 trials (including a total of 4234 participants) was analysed. Mind‐body interventions were analysed based on the type of intervention including biofeedback, movement therapies, psychological therapies, relaxation based therapies and mindfulness.
There was no advantage observed for biofeedback in comparison to usual care controls and no studies reported any adverse events, however the quality of the evidence was very low so we cannot be certain if there is any effect or not. There was also no advantage observed for mindfulness in comparison with usual care, There was no difference in withdrawals between groups. Adverse events were not reported.
There was no advantage observed for mindfulness in comparison to usual care for physical functioning, pain or mood post‐intervention. However the quality of the evidence was very low. There was uncertainty as to whether there were statistical differences in withdrawals between the two groups. No studies reported any adverse events.
There were improved outcomes for movement therapies over usual care and attention controls for physical functioning, pain and mood post‐intervention. However the risk of increased pain reported by one trial suggests caution is needed in interpreting the results and we cannot be certain of any effect due to the very low quality of evidence.
Results for the main analyses on the use of psychological therapies in comparison to usual care controls revealed low quality evidence from 10 trials (733 participants) suggesting that psychological therapies provide a small improvement in physical functioning, pain and mood at the end of treatment. Low quality evidence revealed that improvements in physical functioning and mood were sustained at three month follow‐up and at six month follow up for physical functioning. There was very low quality evidence for the secondary outcomes resulting from psychological therapies.
Relaxation based therapies showed an advantage over usual care for physical functioning and pain outcomes post‐intervention; for pain however the quality of the evidence was very low. No differences in withdrawals or adverse events were reported for relaxation based therapies.
The small number of studies that provided short to medium‐term (three to six month follow‐up) data in this review is a concern and limited evidence was available to determine the short to medium‐term impact of mind‐body interventions for adults with FM.
Overall completeness and applicability of evidence
Overall completeness
In the search strategy we included efforts to identify and include unpublished data to reduce the possible impact of publication bias. Whilst some unpublished data have been included in the review, we cannot rule out the possibility that negative study results may not have been published or identified for inclusion by this review.
The applicability of the evidence included in this review is considered to be strong for a number of reasons. Firstly, the review includes a number of mind‐body interventions delivered across a range of contexts including hospital settings, primary care centres and in the community. Secondly, many samples included both male as well as female adults with FM, which is important as although FM predominantly affects women it can affect men; however, due to the low numbers no study performed a subgroup analysis for male participants. Thirdly, many trials included participants with additional co‐morbidities (not serious or life‐threatening), such as depression, which commonly occur in adults with FM.
Due to the diversity of symptoms experienced by people with FM, this review analysed the evidence on a wide range of outcomes including the major outcomes of self‐reported physical functioning, pain, withdrawals and adverse events, and minor outcomes such as fatigue and self efficacy. Outcomes such as walk time, self confidence, use of medication and healthcare visits may be important but we were not able to incorporate them within the scope of this review as there is a limit to the number of outcomes that can reliably be studied within the context of a Cochrane review.
This review aimed to quantitatively summarise the effects of mind‐body interventions for FM. Whilst this review was targeted at one specific population (adults with FM), the findings may have relevance to other populations where complex symptomology presents. Within the context of current practice, many chronic pain programmes already implement components of mind‐body therapies, such as the use of guided imagery. Mind‐body interventions that require specialist expertise to deliver, such as tai chi or cognitive behaviour therapy (CBT), may be more challenging to incorporate into practice without additional resources.
Quality of the evidence
The evidence presented in this review was extracted from trials published in academic journals and was requested from the trial authors. The overall quality of the evidence was moderate, low or very low (see summary of findings tables). Trial quality was reduced by unclear details or high risk of allocation concealment, non‐blinding of outcome assessors or risk of bias from selective reporting; however, sensitivity analyses revealed that the findings were not influenced by the removal of studies with a high risk of bias from the analyses. The sample size of the included trials was often small and even in the meta‐analyses participant numbers were as low as N = 19, increasing to N = 733. Few studies reported information on any adverse events arising from the interventions. It is important to record any instances where there is a decrease in health or well‐being, such as increased levels of pain or fatigue, that may have been exacerbated by the intervention.
Potential biases in the review process
Despite efforts to reduce the impact of publication bias in the review, the possibility remains that some studies (with positive or negative findings) may not have been identified by the search. Where further clarification of study methodology could not be obtained from the authors, there is the possibility that the risk of bias for the studies may have been overestimated. Whilst contacting authors for additional information assisted in the accuracy of the information reported in most cases, this may have introduced a 'response bias' into the risk of bias assessment. Some values needed to be imputed for missing data (such as variability estimates when the lower confidence interval was used) using the Revman calculator. Data were unable to be extracted accurately for several trials that presented their findings graphically, thus limiting the generalisability of the findings. The small number of trials included in some analyses further reduces the robustness of these findings.
In many cases determining the assessment time point was difficult, as it was not always clear if the timeframe was post‐randomisation or intervention delivery and the window within which assessments were completed was rarely documented.
Agreements and disagreements with other studies or reviews
One previous systematic review of mind‐body therapies for adults with FM was found (Hadhazy 2000). The review included 13 trials of 802 participants and searched the literature until 1999. The findings of the review support the findings of this current review suggesting that there is some limited evidence of the effectiveness of mind‐body therapies in comparison to placebo or attention control for self‐reported pain and physical functioning.
Reviews examining different types of mind‐body interventions include a Cochrane review of cognitive behaviour therapy (CBT) for adults and children with FM, conducted until August 2013 (Bernardy 2013). The results from this current review were more negative than for the review conducted by Bernardy 2013, which revealed small effects for pain, mood and disability that were sustained at follow‐up. By including CBTs in comparisons of treatment effect with other psychological therapies may have obscured unique effects of different types of interventions. The inclusion of children in the other review may also have increased the observed treatment effect.
Another systematic review explored the effectiveness of qi‐gong interventions for adults and children with FM (Chan 2012). The review completed a search of studies until February 2011 and revealed that it was too early to draw conclusions as to the efficacy of qi‐gong and that further robust RCTs were warranted. The current review supports these findings that there is currently insufficient evidence on movement therapies to draw any firm conclusions.
The authors of a Cochrane review on psychological therapies for chronic pain (excluding headache) searched the literature until September 2011 (Williams 2012). This review revealed that people receiving psychological therapies experienced small improvements in functioning, pain and mood when compared to usual care but not when compared to attention control participants. These findings are comparable to the findings in the current review.
Other systematic reviews in this field have been published outside of The Cochrane Library. Findings revealed some inconsistencies which may be due to differences in the inclusion criteria set and outcomes domains explored. In a review of mindfulness based relaxation studies for FM (Lauche 2013) the authors revealed that mindfulness group participants showed reductions in pain and improved quality of life post‐intervention in comparison to controls. In contrast, this review found no difference between the experimental and control groups on any of the major outcomes including pain and quality of life. This disparity in the findings may reflect the inclusion of non‐randomised trials in the review by Lauche 2013. A review of guided imagery for FM that was conducted by Bernardy 2011 revealed similar findings to the relaxation analysis conducted as part of this Cochrane review, where both studies included in the relaxation analysis used a guided imagery intervention. Both reviews revealed that participants receiving guided imagery showed reductions in pain but not quality of life post‐intervention. This Cochrane review also identified that participants receiving biofeedback demonstrated improved physical functioning post‐intervention compared to controls. In a review of biofeedback for people with FM conducted by Glombiewski 2013, it was revealed that participants receiving biofeedback reported reductions in pain post‐intervention in comparison to controls. This Cochrane review was unable to detect a difference between participants receiving biofeedback and controls. This may be due to the inclusion of trials using additional interventions such as cognitive strategies or exercise. In contrast, for inclusion in this review biofeedback needed to be the primary focus of the intervention (constituting at least 80% of the intervention) to ensure any effects detected were due to the biofeedback rather than inclusion of other treatments. A consistent finding between these three reviews published outside of Cochrane and the current review is that the quality of the available evidence in this area is poor.
Authors' conclusions
Implications for practice.
Mind‐body interventions are becoming increasingly incorporated into treatment programmes for fibromyalgia (FM). The findings of this review indicate that psychological therapies may improve physical functioning, pain and mood following treatment but highlight that the quality of evidence is low. The observed effects were not sustained at six months follow‐up. There was wide variation in intervention mode of delivery and there were an insufficient number of trials to enable calculation of the effect of different modes of delivery on outcomes. Psychological therapies were delivered over a period of between one and 25 weeks (mean 11 weeks), with greater effects observed with longer duration. There was low reporting on the presence or absence of adverse events, however equivalent rates of dropout between the treatment and control groups indicate that the risks to people receiving psychological interventions are low.
There is insufficient evidence to determine the use of biofeedback, mindfulness, movement therapies or relaxation based therapies for adults with FM.
Implications for research.
Evidence
The evidence for outcomes in this review was limited by the low or very low quality of the trials identified. To enable recommendations on the use of mind‐body therapies for adults with fibromyalgia to be determined, robust randomised controlled trials are needed. Following the findings of this review, trials should take into account the need to accurately report on randomisation procedures and allocation concealment processes and to clearly report data including measures of variance for all outcomes at all time points assessed. There is no need for further low quality trials (trials with high risk of bias) in this area.
Population
As fibromyalgia predominantly affects females, many studies only included female participants in order to reduce heterogeneity, however this limits the applicability of the findings for males with fibromyalgia. Subgroup analyses could help explore the impact of gender on treatment effect and future studies should consider inclusion of male and female participants. This study only presents data for adults over 18 years and future reviews are needed to review the evidence of mind‐body therapies for children.
Intervention
There was wide heterogeneity in the interventions delivered within the groups specified, this was particularly evident within the sensitivity analyses of the data. For example, psychological therapies encompassed written therapies, educational based approaches as well as specific therapeutic techniques such as the Resseguier approach. There was also variability in the mode of intervention delivery with therapies being self‐administered or delivered by a therapists on an individual basis or within a group. Future reviews would benefit from having a narrower focus to ensure that the effective elements of the specific components of mind‐body therapies can be identified.
Comparison
Trials used a combination of usual care and attention control groups for comparison. Greater differences between groups were observed when the intervention was compared to usual care, suggesting that therapeutic attention or a placebo effect may have been observed.
Outcomes
It was often difficult to determine why participants withdrew from the included trials and at which time point. Higher numbers of withdrawals from the intervention group can indicate difficulties with the feasibility of the intervention or mode of delivery and should be reported; although this was not found to be the case for studies in our analyses where this information could be determined. CONSORT diagrams outlining reasons for withdrawal between groups over time are a useful way of presenting this information for future studies.
Few studies described any adverse events experienced by participants and this information is critical for ensuring the safety and feasibility of interventions in clinical practice, and should be reported. Declarations that no adverse events were experienced, if this was the case, would also facilitate interpretation of the results.
A wide range of outcomes and outcome measures were reported between trials. As recommended by Choy 2009 and Bernardy 2013, a core set of outcome measures that should be assessed and reported across clinical trials needs to be established by consensus to facilitate pooling of trial data and the comparison of study findings. As recommended by Choy 2009, the Fibromyalgia Impact Questionnaire should be considered to be the primary outcome measures for all fibromyalgia randomised controlled trials, with secondary outcomes of self‐reported pain, fatigue and sleep.
Time
This review presents data identified up to October 2013, and further updates will be required as new evidence emerges. More trials with follow‐up at three and six months are needed to determine if the effects of mind‐body interventions are sustained.
Feedback
Comment, 29 June 2015
Summary
Why would you discriminate between standard care and attention control as comparators, ie separate out sham therapy since this halves the number of eligible studies, to just a few in many cases? Why is Toussaint awaiting assessment ‐ it appears it should be ruled out due to dropouts ? I'd also expect Ide & Tanaka et al to appear as a movement therapy (Effect of aquatic respiratory exercise‐based program in patients with fibromyalgia, APLAR '08)
Reply
Many thanks for your contribution. External feedback helps to improve review, and we appreciate your continuous attention to our reviews. “Why would you discriminate between standard care and attention control as comparators, ie separate out sham therapy since this halves the number of eligible studies, to just a few in many cases?” We usually separate the two, as one is a no treatment control group (usual care), and attention control is more akin to a sham‐intervention, thus the comparators are heterogeneous. Attention control is considered more rigorous than no treatment, and at least allows the possibility of blinding the participant, and thus allows for a lower risk of both performance bias and detection bias for self‐reported subjective outcomes. “Why is Toussaint awaiting assessment ‐ it appears it should be ruled out due to dropouts?” Awaiting assessment is used when the author team have not yet been able to determine eligibility for the review. It is a useful interim service for the reader because it indicates that there are possibly studies that might influence the results of the review. In this case, Toussaint 2012 (as well as Thorsell 2011 and Wang 2012) are awaiting assessment as it was not possible to ascertain if the diagnosis of fibromyalgia was done according to the ACR criteria (one of the inclusion criteria). This information is available on the table of studies awaiting assessment. We do not exclude studies on the basis of numbers of dropouts. “I'd also expect Ide & Tanaka et al to appear as a movement therapy (Effect of aquatic respiratory exercise‐based program in patients with fibromyalgia, APLAR '08)” We assume that you refer to IDE, M. R., LAURINDO, I. M. M., RODRIGUES‐JÚNIOR, A. L. and TANAKA, C. (2008), Effect of aquatic respiratory exercise‐based program in patients with fibromyalgia. International Journal of Rheumatic Diseases, 11: 131–140. doi: 10.1111/j.1756‐185X.2008.00348.x The inclusion criteria of the reviews specifies that the intervention needs to follow the following principles: 1) be based on the principle that the mind and body are interconnected; 2) aim to increase self knowledge; 3) aim to increase people’s ability to self‐manage their health and consequences of ill‐health; 4) actively engage and involve the participant in the intervention delivery; and 5) provide tools to improve coping and self‐management of the condition, 6) at least 80% of the total intervention delivery must include components meeting the aforementioned five principles. This study did not seem to fit with any of the pre‐specified inclusion criteria.Although the study mentions yoga as the rationale for the inclusion of the breathing techniques, it does not reflect on the principle that mind and body are interconnected.
Contributors
Geoff Kirwood Researcher, FnMyalgia.com
What's new
| Date | Event | Description |
|---|---|---|
| 3 August 2015 | Feedback has been incorporated | Clarification on some aspects of the review requested, as well as on the potential inclusion of a new study. |
History
Protocol first published: Issue 1, 2000 Review first published: Issue 4, 2015
| Date | Event | Description |
|---|---|---|
| 10 April 2015 | Amended | Typo amended |
| 25 April 2008 | Amended | Converted to new review format CMSG ID: C138‐P |
| 24 January 2008 | New search has been performed | Change in authorship |
Acknowledgements
The review authors would like to thank the Cochrane Musculoskeletal editorial team for their comments and helpful editorial suggestions.
Amy King (AK) and Sue Mahon (SM) for their assistance in identifying relevant trials for the review.
We would like to acknowledge funding to support the completion of this review from Auckland University of Technology, Faculty of Health and Environmental Sciences.
Appendices
Appendix 1. National Center for Complementatry and Alternative Medicine Overview Statement
The National Center for Complementary and Alternative Medicine Overview Statement on mind‐body medicine that "mind‐body medicine focuses on interactions among the brain, mind, body and behavior, and the powerful ways in which emotional, mental, social and spiritual and behavioral factors can directly affect health. It regards as fundamental an approach that respects and enhances each person's capacity for self‐knowledge and self‐care, and it emphasizes techniques that are grounded in this approach. Mind‐body medicine typically focuses on intervention strategies that are thought to promote health, such as relaxation, yoga, biofeedback, tai chi, qi gong and cognitive behavioral therapies. NCCAM 2005 page 1.
Appendix 2. Full search strategy
Search Strategies: Cochrane Library Issue 10, 2013
#1 (fibromyalgia):ti,ab,kw or (fibromyalg*):ti,ab,kw or (muscular rheumatism):ti,ab,kw or (fibrositi*):ti,ab,kw
#2 (mind near body):ti,ab,kw or (hypnosis):ti,ab,kw or (meditat*):ti,ab,kw or (relax*):ti,ab,kw or (mindful*):ti,ab,kw
#3 (yoga):ti,ab,kw or (tai chi):ti,ab,kw or (breath* near exercise*):ti,ab,kw or (massage*):ti,ab,kw or (imagery):ti,ab,kw
#4 (biofeedback):ti,ab,kw or (hypno*):ti,ab,kw or (suggest*):ti,ab,kw or (autosuggest*):ti,ab,kw or (aromatherapy):ti,ab,kw
#5 MeSH descriptor Mind‐Body Therapies explode all trees
#6 MeSH descriptor Yoga explode all trees
#7 MeSH descriptor Biofeedback, Psychology explode all trees
#8 MeSH descriptor Counseling explode all trees
#9 MeSH descriptor Aromatherapy explode all trees
#10 MeSH descriptor Tai Ji explode all trees
#11 MeSH descriptor Cognitive Therapy explode all trees
#12 MeSH descriptor Psychotherapy explode all trees
#13 MeSH descriptor Relaxation explode all trees
#14 MeSH descriptor Relaxation Therapy explode all trees
#15 (#2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14)
#16 (#1 AND #15)
(OVID) AMED (Allied and Complementary Medicine) <1985 to 31 October 2013>
1 exp Fibromyalgia/ (1437)
2 fibrositi$.tw. (21)
3 fibromyalgia.tw. (1604)
4 (chronic adj2 pain).tw. (2457)
5 or/1‐4 (3805)
6 Psychosomatic therapies/ (1468)
7 (mind adj2 body).tw. (767)
8 Yoga/ (335)
9 exp Tai chi/ (202)
10 Exercise therapy/ (4804)
11 Tai ji.tw. (6)
12 autosuggestion.tw. (6)
13 exp Suggestion/ (129)
14 exp Hypnosis/ (3515)
15 hypnoti$.tw. (1398)
16 exp Imagery/ (162)
17 exp Visualization/ (171)
18 exp Mental healing/ (420)
19 guided imagery.tw. (99)
20 exp Relaxation/ (936)
21 relax$.tw. (2677)
22 ((relax$ or relaxation) and (training or therapy or technique$)).ti,ab. (875)
23 exp Mind body relations/ (277)
24 exp Counseling/ (1589)
25 counsel$.tw. (2681)
26 exp Biofeedback/ (1005)
27 biofeedback.tw. (1194)
28 exp Aroma therapy/ (531)
29 aromatherap$.tw. (390)
30 or/6‐29 (17221)
31 5 and 30 (448)
(OVID) Embase Classic+Embase <1947 to 31 October 2013>
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 exp fibromyalgia/ (10187)
2 fibromyalg$.tw. (7904)
3 exp fibromatosis/ (3124)
4 fibrositi$.tw. (733)
5 muscular rheumatism.tw. (82)
6 or/1‐5 (14475)
7 exp autogenic training/ (1411)
8 guided imagery/ (407)
9 relaxation training/ (7541)
10 imagery/ (4040)
11 exp suggestion/ (2495)
12 exp hypnosis/ (14958)
13 hypnoti$.tw. (16211)
14 exp meditation/ (2747)
15 meditat$.tw. (3100)
16 (auto adj2 suggestion).tw. (25)
17 (mind adj2 body).tw. (2861)
18 exp Tai Chi/ (876)
19 tai ji.tw. (12)
20 ((relax or relaxation) and (therapy or training or technique$)).tw. (16976)
21 directive counseling/ or patient counseling/ or counseling/ (59696)
22 biofeedback.tw. (6026)
23 awareness/ (25450)
24 bodywork/ (48)
25 exp massage/ (10249)
26 mindful$.tw. (2274)
27 or/7‐26 (157692)
28 random$.tw. (693382)
29 factorial$.tw. (18501)
30 crossover$.tw. (42003)
31 cross over.tw. (19245)
32 cross‐over.tw. (19245)
33 placebo$.tw. (172180)
34 (doubl$ adj blind$).tw. (129772)
35 (singl$ adj blind$).tw. (11667)
36 assign$.tw. (195232)
37 allocat$.tw. (65592)
38 volunteer$.tw. (158289)
39 crossover procedure/ (31641)
40 double blind procedure/ (106689)
41 randomized controlled trial/ (296329)
42 single blind procedure/ (14559)
43 or/28‐42 (1170580)
44 6 and 27 and 43 (170)
(OVID) MEDLINE(R) In‐Process & Other Non‐Indexed Citations and Ovid MEDLINE(R) <1948 to 31 October 2013>
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 Fibromyalgia/ (5292)
2 fibromyalg$.tw. (5521)
3 muscular rheumatism.tw. (38)
4 fibrositi$.tw. (484)
5 exp Mind‐Body Therapies/ (37799)
6 (mind adj2 body).tw. (2139)
7 exp Psychophysiology/ (573224)
8 Relaxation/ (1670)
9 Relaxation Therapy/ (5474)
10 relax$.tw. (110666)
11 exp Meditation/ (1112)
12 meditat$.tw. (2330)
13 mindful.tw. (855)
14 exp Breathing Exercises/ (2457)
15 (breathing adj3 exercises).tw. (456)
16 respiratory muscle training.tw. (170)
17 (progressive adj muscle).tw. (1127)
18 exp Massage/ (4261)
19 massag$.tw. (6607)
20 exp "Imagery (Psychotherapy)"/ (951)
21 imagery.tw. (7207)
22 exp Biofeedback, Psychology/ (6311)
23 biofeedback.tw. (4492)
24 aromatherap$.tw. (488)
25 exp Aromatherapy/ (455)
26 essential oils.tw. (2920)
27 exp Hypnosis/ (10256)
28 hypnoti$.tw. (10876)
29 hypnosis.tw. (5787)
30 hypnotherap$.tw. (876)
31 exp suggestion/ (2986)
32 autosuggest$.tw. (33)
33 ((mind or mental) adj heal$).tw. (63093)
34 exp Yoga/ (1173)
35 yoga.tw. (1287)
36 exp Tai Ji/ (458)
37 (tai ji or tai chi).tw. (615)
38 (qigong or (qi adj gong) or (gi adj gong)).tw. (343)
39 (chi adj kung).tw. (6)
40 exp Psychotherapy/ (137463)
41 psychotherap$.tw. (28690)
42 exp Cognitive Therapy/ (12610)
43 cbt.tw. (3704)
44 (behav$ adj2 therap$).tw. (11218)
45 (cognitive adj2 therap$).tw. (8028)
46 exp Counseling/ (29617)
47 counsel$.tw. (58972)
48 exp Counseling/ (29617)
49 Directive Counseling/ (827)
50 (supportive adj2 (therap* or psychotherap*)).tw. (3914)
51 humanistic.tw. (1715)
52 randomized controlled trial.pt. (323095)
53 controlled clinical trial.pt. (84091)
54 randomized.ab. (239369)
55 placebo.ab. (135087)
56 clinical trials as topic.sh. (159645)
57 randomly.ab. (175198)
58 trial.ti. (102635)
59 or/52‐58 (776419)
60 exp animals/ not humans.sh. (3722514)
61 59 not 60 (718473)
62 or/1‐4 (6807)
63 or/5‐51 (954295)
64 61 and 62 and 63 (439)
(OVID) PsycINFO <1806 to 31 October 2013>
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 exp Fibromyalgia/ (902)
2 fibrositi$.tw. (44)
3 fibromyalg$.tw. (1813)
4 muscular rheumatism.tw. (3)
5 (chronic adj2 pain).ti,ab. (9336)
6 or/1‐5 (10671)
7 exp Autogenic Training/ (591)
8 exp RELAXATION THERAPY/ (3274)
9 exp Guided Imagery/ (551)
10 exp HYPNOTHERAPY/ (4264)
11 exp MEDITATION/ (2405)
12 exp YOGA/ (777)
13 autosuggestion.tw. (185)
14 exp HYPNOSIS/ (6685)
15 exp Meditation/ (2405)
16 meditat$.tw. (4718)
17 auto suggestion.tw. (95)
18 Posthypnotic Suggestions/ (241)
19 exp Biofeedback Training/ or exp Biofeedback/ (4577)
20 exp Counseling/ (60325)
21 ((relax$ or relaxation) and (training or therapy or technique$)).ti,ab. (7026)
22 Dualism/ (2474)
23 (mind adj2 body).tw. (5676)
24 or/7‐23 (92232)
25 6 and 24 (719)
EbscoHost CINAHL 1981 to 31 October 2013
S16 S3 and S15
S15 S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14
S14 (MM "Autogenic Training (Iowa NIC)")
S13 (MM "Counseling")
S12 (MM "Massage")
S11 (MM "Tai Chi")
S10 (MM "Yoga")
S9 (MM "Meditation")
S8 (MM "Relaxation")
S7 (MM "Biofeedback")
S6 (MM "Hypnosis")
S5 (MM "Guided Imagery") OR (MM "Simple Guided Imagery (Iowa NIC)") S4 (MM "Mind Body Techniques")
S3 S1 or S2
S2 (MM "Chronic Pain/DH/NU/PR/PC/PF/RT/RH/TH")
S1 (MH "Fibromyalgia") OR "fibromyalgia"
Data and analyses
Comparison 1. Psychological therapies versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Functioning as assessed post‐intervention | 10 | 733 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.43 [‐0.57, ‐0.28] |
| 2 Functioning as assessed at 3 month follow‐up | 3 | 148 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.54 [‐0.87, ‐0.21] |
| 3 Functioning as assessed at 6 month follow‐up | 1 | 112 | Mean Difference (IV, Fixed, 95% CI) | ‐3.66 [‐7.29, ‐0.03] |
| 4 Pain as assessed post‐intervention | 9 | 453 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.33 [‐0.52, ‐0.15] |
| 5 Pain as assessed at 3 month follow‐up | 2 | 115 | Mean Difference (IV, Fixed, 95% CI) | ‐0.85 [‐1.76, 0.06] |
| 6 Pain as assessed at 6 month follow‐up | 5 | 371 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.51 [‐0.72, ‐0.30] |
| 7 Mood as assessed post‐intervention | 8 | 492 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.45 [‐0.64, ‐0.26] |
| 8 Mood as assessed at 3 month follow‐up | 4 | 182 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐1.15 [‐1.50, ‐0.80] |
| 9 Mood as assessed at 6 month follow‐up | 2 | 213 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐0.44, 0.10] |
| 10 All cause attrition post‐intervention | 22 | 1687 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.12, 1.69] |
| 11 Adverse events post‐intervention | 2 | 126 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.06, 2.50] |
| 12 Fatigue as assessed post‐intervention | 2 | 82 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.53, 0.34] |
| 13 Fatigue as assessed at 6 months post‐intervention | 2 | 160 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.38, 0.24] |
| 14 Self‐efficacy as assessed post‐intervention | 4 | 255 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐0.50, ‐0.00] |
| 15 Tender point count as assessed at 6 month follow‐up | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐0.38 [‐0.88, 0.12] |
| 16 Quality of life as assessed post‐intervention | 6 | 276 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐0.44, 0.06] |
| 17 Quality of life as assessed at 3 month follow‐up | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | ‐15.10 [‐21.90, ‐8.30] |
| 18 Quality of life as assessed at 6 month follow‐up | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐2.50 [‐7.95, 2.95] |
| 19 Sleep as assessed post‐intervention | 5 | 222 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.52 [‐0.80, ‐0.25] |
| 20 Sleep as assessed at 3 month follow‐up | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | ‐11.30 [‐15.44, ‐7.16] |
| 21 Sleep as assessed at 6 month follow‐up | 3 | 224 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐0.42, 0.12] |
| 22 Self‐efficacy as assessed at 3 month follow‐up | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | ‐15.10 [‐44.95, 14.75] |
1.8. Analysis.
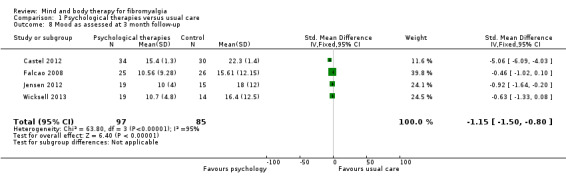
Comparison 1 Psychological therapies versus usual care, Outcome 8 Mood as assessed at 3 month follow‐up.
1.17. Analysis.

Comparison 1 Psychological therapies versus usual care, Outcome 17 Quality of life as assessed at 3 month follow‐up.
1.20. Analysis.

Comparison 1 Psychological therapies versus usual care, Outcome 20 Sleep as assessed at 3 month follow‐up.
1.22. Analysis.

Comparison 1 Psychological therapies versus usual care, Outcome 22 Self‐efficacy as assessed at 3 month follow‐up.
Comparison 2. Psychological therapies versus usual care sensitivity analyses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mood as assessed post‐intervention | 7 | 428 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.29 [‐0.48, ‐0.10] |
| 2 Mood as assessed at 3 month follow‐up | 3 | 118 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.63 [‐1.00, ‐0.26] |
| 3 Fatigue as assessed post‐intervention | 1 | 42 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.42 [‐1.04, 0.19] |
| 4 Sleep as assessed post‐intervention | 4 | 158 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.18 [‐0.50, 0.13] |
| 5 Sleep as assessed at 6 month follow‐up | 2 | 160 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.22 [‐0.09, 0.53] |
2.1. Analysis.

Comparison 2 Psychological therapies versus usual care sensitivity analyses, Outcome 1 Mood as assessed post‐intervention.
2.2. Analysis.
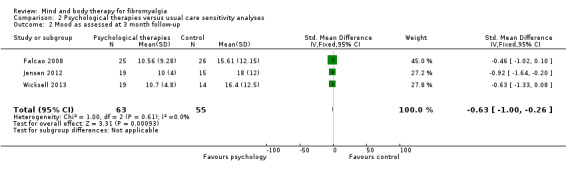
Comparison 2 Psychological therapies versus usual care sensitivity analyses, Outcome 2 Mood as assessed at 3 month follow‐up.
2.5. Analysis.

Comparison 2 Psychological therapies versus usual care sensitivity analyses, Outcome 5 Sleep as assessed at 6 month follow‐up.
Comparison 3. Psychological therapies versus attention control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Functioning as assessed post‐intervention | 7 | 561 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.27, 0.07] |
| 2 Functioning as assessed at 3 month follow‐up | 4 | 447 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.17, 0.20] |
| 3 Functioning as assessed at 6 month follow‐up | 3 | 326 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.22, 0.23] |
| 4 Pain as assessed post‐intervention | 5 | 324 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.28 [‐0.51, ‐0.06] |
| 5 Pain as assessed at 3 month follow‐up | 2 | 115 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.24, 0.50] |
| 6 Pain as assessed at 6 month follow‐up | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.34 [‐0.89, 0.21] |
| 7 Mood as assessed post‐intervention | 5 | 330 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.33, 0.10] |
| 8 Mood as assessed at 3 month follow‐up | 2 | 115 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.24 [‐0.13, 0.61] |
| 9 All cause attrition post‐intervention | 8 | 669 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.54, 0.87] |
| 10 Fatigue as assessed post‐intervention | 2 | 153 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.44, 0.20] |
| 11 Fatigue as assessed at 3 month follow‐up | 1 | 69 | Mean Difference (IV, Fixed, 95% CI) | ‐0.18 [‐0.73, 0.37] |
| 12 Self‐efficacy as assessed post‐intervention | 1 | 105 | Mean Difference (IV, Fixed, 95% CI) | 0.48 [‐0.27, 1.23] |
| 13 Self efficacy as assessed at 3 month follow‐up | 2 | 151 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.27 [‐0.59, 0.05] |
| 14 Self‐efficacy as assessed at 6 month follow‐up | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐1.31, 1.33] |
| 15 Tender point score as assessed post‐intervention | 2 | 150 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐1.62, 0.02] |
| 16 Quality of life as assessed post‐intervention | 3 | 308 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.35, 0.10] |
| 17 Quality of life as assessed at 3 month follow‐up | 2 | 218 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.31, 0.22] |
| 18 Quality of life as assessed at 6 month follow‐up | 1 | 171 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.34, 0.26] |
| 19 Sleep as assessed post‐intervention | 2 | 109 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.50, 0.25] |
| 20 Sleep as assessed at 3 month follow‐up | 1 | 69 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.45, 0.47] |
Comparison 4. Psychological therapies versus attention control sensitivity analyses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Functioning as assessed post‐intervention | 6 | 390 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐0.45, ‐0.05] |
| 2 Functioning as assessed at 6 month follow‐up | 2 | 266 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.14 [‐0.10, 0.38] |
| 3 Pain as assessed post‐intervention | 4 | 255 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.66, ‐0.15] |
| 4 Sleep as assessed post‐intervention | 1 | 40 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.51 [‐1.14, 0.12] |
Comparison 5. Biofeedback versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Functioning as assessed post‐intervention | 2 | 106 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.44, 0.33] |
| 2 Functioning as assessed at 3 month follow‐up | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐0.41 [‐8.88, 8.06] |
| 3 Pain as assessed post‐intervention | 1 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐2.60 [‐91.29, 86.09] |
| 4 Mood as assessed post‐intervention | 2 | 104 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.26, 0.52] |
| 5 Mood as assessed at 3 month follow‐up | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | 4.61 [‐0.16, 9.38] |
| 6 All cause attrition post‐intervention | 3 | 125 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.08 [1.43, 11.62] |
| 7 Tender point score as assessed post‐intervention | 2 | 101 | Mean Difference (IV, Fixed, 95% CI) | ‐0.92 [‐2.29, 0.45] |
| 8 Tender point score as assessed at 3 month follow‐up | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.85, 0.67] |
| 9 Quality of life (Physical functioning) as assessed post‐intervention | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐4.92 [‐19.30, 9.46] |
| 10 Quality of life (Role‐Physical) as assessed post‐intervention | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐19.27 [‐41.03, 2.49] |
| 11 Quality of life (Bodily Pain) as assessed post‐intervention | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | 6.27 [‐4.49, 17.03] |
| 12 Quality of life (General Health) as assessed post‐intervention | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐8.14 [‐20.47, 4.19] |
| 13 Quality of life (Vitality) as assessed post‐intervention | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐13.43 [‐24.06, ‐2.80] |
| 14 Quality of life (Social Functioning) as assessed post‐intervention | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐10.42 [‐26.61, 5.77] |
| 15 Quality of life (Role‐Emotional) as assessed post‐intervention | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐9.49 [‐39.26, 20.28] |
| 16 Quality of life (Mental Health) as assessed post‐intervention | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐9.32 [‐22.93, 4.29] |
| 17 Quality of life (Physical functioning) as assessed at 3 month follow‐up | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐13.73, 13.73] |
| 18 Quality of life (Role‐Physical) as assessed at 3 month follow‐up | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐5.21 [‐24.28, 13.86] |
| 19 Quality of life (Bodily Pain) as assessed at 3 month follow‐up | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | 0.71 [‐8.15, 9.57] |
| 20 Quality of life (Social Functioning) as assessed at 3 month follow‐up | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐7.43 [‐24.21, 9.35] |
| 21 Quality of life (General Health) as assessed at 3 month follow‐up | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐0.94 [‐12.33, 10.45] |
| 22 Quality of life (Vitality) as assessed at 3 month follow‐up | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐10.17 [‐20.57, 0.23] |
| 23 Quality of life (Role‐Emotional) as assessed at 3 month follow‐up | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐23.84 [‐53.57, 5.89] |
| 24 Quality of life (Mental Health) as assessed at 3 month follow‐up | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐6.44 [‐18.27, 5.39] |
Comparison 6. Biofeedback versus attention control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Functioning as assessed post‐intervention | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 13.60 [1.05, 26.15] |
| 2 Pain as assessed post‐intervention | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 2.66 [1.21, 4.11] |
| 3 All cause attrition post‐intervention | 2 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.46 [0.44, 27.19] |
| 4 Tender point score as assessed post‐intervention | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 2.93 [0.15, 5.71] |
Comparison 7. Mindfulness versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Functioning as assessed post‐intervention | 2 | 128 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.26 [‐0.60, 0.09] |
| 2 Functioning assessed at 3 month follow‐up | 1 | 103 | Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.78, 0.66] |
| 3 Pain as assessed post‐intervention | 2 | 128 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.44, 0.26] |
| 4 Pain as assessed at 3 month follow‐up | 1 | 103 | Mean Difference (IV, Fixed, 95% CI) | ‐0.28 [‐2.37, 1.81] |
| 5 Mood as assessed post‐intervention | 3 | 218 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.24 [‐0.51, 0.03] |
| 6 Mood as assessed at 3 month follow‐up | 2 | 193 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐0.50, 0.07] |
| 7 All cause attrition post‐intervention | 3 | 195 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.67, 1.72] |
| 8 Sleep as assessed post‐intervention | 1 | 97 | Mean Difference (IV, Fixed, 95% CI) | ‐0.64 [‐2.27, 0.99] |
| 9 Sleep as assessed at 3 month follow‐up | 2 | 134 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.24 [‐0.59, 0.10] |
Comparison 8. Mindfulness versus usual care ‐ sensitivity analyses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mood as assessed at 3 month follow‐up | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐1.5 [‐4.77, 1.77] |
8.1. Analysis.

Comparison 8 Mindfulness versus usual care ‐ sensitivity analyses, Outcome 1 Mood as assessed at 3 month follow‐up.
Comparison 9. Movement therapies versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Functioning as assessed post‐intervention | 4 | 143 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐0.53, 0.15] |
| 2 Pain as assessed post‐intervention | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐2.3 [‐4.19, ‐0.41] |
| 3 Mood as assessed post‐intervention | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | ‐9.84 [‐18.51, ‐1.17] |
| 4 All cause attrition post‐intervention | 5 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.95 [1.13, 3.38] |
| 5 Adverse events post‐intervention | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.62 [0.23, 93.72] |
| 6 Fatigue as assessed post‐intervention | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | ‐10.8 [‐18.57, ‐3.03] |
| 7 Tender point count as assessed post‐intervention | 2 | 93 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.18 [‐0.25, 0.60] |
| 8 Sleep as assessed post‐intervention | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | ‐4.68 [‐8.14, ‐1.22] |
Comparison 10. Movement therapies versus usual care ‐ sensitivity analyses intervention type.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Functioning as assessed post‐intervention | 3 | 121 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.26 [‐0.63, 0.11] |
10.1. Analysis.

Comparison 10 Movement therapies versus usual care ‐ sensitivity analyses intervention type, Outcome 1 Functioning as assessed post‐intervention.
Comparison 11. Movement therapies versus usual care ‐ sensitivity analyses quality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Functioning as assessed post‐intervention | 3 | 115 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.44, 0.31] |
11.1. Analysis.
Comparison 11 Movement therapies versus usual care ‐ sensitivity analyses quality, Outcome 1 Functioning as assessed post‐intervention.
Comparison 12. Movement therapies versus attention control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Functioning as assessed post‐intervention | 3 | 191 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.65 [‐0.94, ‐0.35] |
| 2 Functioning as assessed at 3 month follow‐up | 3 | 189 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.53 [‐0.82, ‐0.23] |
| 3 Pain as assessed by a 10‐point VAS scale post‐intervention | 3 | 172 | Mean Difference (IV, Fixed, 95% CI) | ‐1.45 [‐2.08, ‐0.81] |
| 4 Pain as assessed by a 10‐point VAS scale at 3 month follow‐up | 3 | 165 | Mean Difference (IV, Fixed, 95% CI) | ‐1.19 [‐1.87, ‐0.52] |
| 5 Mood as assessed post‐intervention | 2 | 141 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.49 [‐0.83, ‐0.15] |
| 6 Mood as assessed at 3 month follow‐up | 2 | 140 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.35 [‐0.69, ‐0.01] |
| 7 All cause attrition post‐intervention | 5 | 279 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.65, 2.09] |
| 8 Adverse events post‐intervention | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.0 [0.37, 131.17] |
| 9 Self‐efficacy as assessed post‐intervention | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐1.60 [‐2.54, ‐0.66] |
| 10 Self‐efficacy as assessed at 3 month follow‐up | 1 | 59 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐2.25, ‐0.15] |
| 11 Tender points as assessed post‐intervention | 2 | 130 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐1.16, 1.33] |
| 12 Tender points as assessed at 3 month follow‐up | 2 | 130 | Mean Difference (IV, Fixed, 95% CI) | ‐0.39 [‐1.63, 0.85] |
| 13 Quality of life as assessed post‐intervention | 2 | 109 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐1.09, ‐0.31] |
| 14 Quality of life as assessed at 3 month follow‐up | 2 | 108 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.52 [‐0.91, ‐0.14] |
| 15 Sleep quality as assessed by the Pittsburgh Sleep Quality Index post‐intervention | 2 | 141 | Mean Difference (IV, Fixed, 95% CI) | ‐1.88 [‐3.27, ‐0.48] |
| 16 Sleep quality as assessed by the Pittsburgh Sleep Quality Index at 3 month follow‐up | 2 | 140 | Mean Difference (IV, Fixed, 95% CI) | ‐1.35 [‐2.77, 0.07] |
Comparison 13. Movement therapies versus attention control ‐ sensitivity analyses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Functioning as assessed post‐intervention | 2 | 110 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.99 [‐1.39, ‐0.59] |
| 2 Functioning as assessed at 3 month follow‐up | 2 | 108 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.83 [‐1.23, ‐0.43] |
| 3 Pain as assessed by a 10‐point VAS scale post‐intervention | 2 | 110 | Mean Difference (IV, Fixed, 95% CI) | ‐2.18 [‐2.96, ‐1.40] |
| 4 Pain as assessed by a 10‐point VAS scale at 3 month follow‐up | 2 | 108 | Mean Difference (IV, Fixed, 95% CI) | ‐1.94 [‐2.77, ‐1.10] |
| 5 Mood as assessed post‐intervention | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐1.28 [‐1.84, ‐0.72] |
| 6 Mood as assessed at 3 month follow‐up | 1 | 59 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.96 [‐1.51, ‐0.42] |
Comparison 14. Relaxation versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Functioning as assessed post‐intervention | 2 | 67 | Mean Difference (IV, Fixed, 95% CI) | ‐8.33 [‐10.14, ‐6.53] |
| 2 Pain as assessed post‐intervention | 2 | 67 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐1.02 [‐1.55, ‐0.50] |
| 3 Mood as assessed post‐intervention | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | ‐4.44 [‐14.46, 5.58] |
| 4 All cause attrition post‐intervention | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.4 [0.59, 33.07] |
| 5 Self‐efficacy as assessed post‐intervention | 2 | 67 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐1.54 [‐2.13, ‐0.95] |
| 6 Fatigue as assessed post‐intervention | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | ‐0.82 [‐2.91, 1.27] |
| 7 Sleep as assessed post‐intervention | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | 1.03 [‐2.23, 4.29] |
14.3. Analysis.
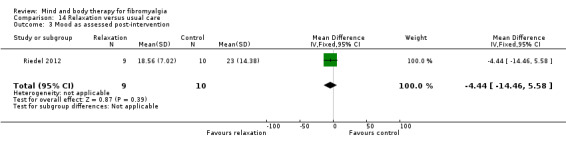
Comparison 14 Relaxation versus usual care, Outcome 3 Mood as assessed post‐intervention.
14.6. Analysis.

Comparison 14 Relaxation versus usual care, Outcome 6 Fatigue as assessed post‐intervention.
14.7. Analysis.

Comparison 14 Relaxation versus usual care, Outcome 7 Sleep as assessed post‐intervention.
Comparison 15. Relaxation versus attention control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain as assessed post‐intervention | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | ‐23.17 [‐36.73, ‐9.61] |
| 2 Mood as assessed post‐intervention | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | ‐32.1 [‐46.35, ‐17.85] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alda 2011.
| Methods | Randomised controlled multi‐centre trial | |
| Participants | Participants who fulfil (ACR) criteria for primary fibromyalgia were recruited by doctors working in primary care centres Aged 18 to 65 years Total participants N = 169 randomised (141 completed) N = 159 female, N = 10 male Exclusions: unable to understand or read Spainish; undergone psychological treatment in previous two years, receiving pharmacological treatment or unwilling to discontinue treatment two weeks before start of study; no informed consent provided; patients with severe axis I or axis II disorders and other medical disorders that from the clinician's point of view prevented the patient from following the treatment protocol; women who were pregnant or nursing and those declining to participate |
|
| Interventions | 1) Cognitive behavioural therapy consisting of two components; cognitive restructuring and coping 90 minute sessions, held weekly for 10 weeks, delivered by trained therapists 2) Treatment as usual 3) Pharmacological treatment (data not included in this review) |
|
| Outcomes | Measures relevant to this review: Fibromylagia impact questionnaire, pain visual analog scale, Hamilton rating scale for depression and EuroQol 5D Assessment time points: baseline, post‐intervention and 6 month follow‐up |
|
| Notes | The study was funded by a grant from the Carlos III Health Institute of the Spainish Ministry of Health and Consumption (ETES P107/90959). The authors declared no competing interests | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component is included in the sequence generation process used |
| Allocation concealment (selection bias) | Low risk | "Each patient was assigned to one of the three groups by a computer‐generated random number sequence." "The allocation sequence was generated by a member of the research group who was not involved in the study." |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | Low risk | "Study personnel who conducted psychological assessments (RM and YLdH) were blinded to participants' treatment conditions" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A 16% total attrition rate was reported. No indication of imbalance evident between groups at follow‐up |
| Selective reporting (reporting bias) | Low risk | All of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
Altan 2009.
| Methods | Randomised prospective controlled trial | |
| Participants | Women who had a diagnosis of fibromyalgia according to the ACR criteria and who had admitted to rheumatology clinic were invited to participate in the study Aged 24 to 63 years Total participants N = 50 randomised (49 completed) N = 50 female Exclusions: rheumatoid disease; unstable hypertension; severe cardiopulmonary problems; any other psychiatric disorder that could affect patient compliance. All participants were instructed to discontinue nonsteroidal anti‐inflammatory drugs during the study period. Patients were able to continue taking antidepressant or sedative drugs |
|
| Interventions | 1) Pilates exercise programme consisting of postural education, antalgic and stretching exercises, and breathing education 1 hour sessions, delivered 3 x per week for 12 weeks, by a certified trainer 2) Relaxation and stretching home exercise programme consisting of active and passive stretching Conducted for 1 hour 3 x per week for 12 weeks |
|
| Outcomes | Measures relevant to this review: pain visual analog scale, Fibromyalgia impact Questionnaire, tender point count, Nottingham health profile Assessment time points: baseline, post‐intervention and 3 month follow‐up |
|
| Notes | The authors report no financial interest in the results of the research | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component is included in the sequence generation process used |
| Allocation concealment (selection bias) | Low risk | A random number table was used |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | Low risk | Assessors were blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant withdrew due to medical reasons (2% total attrition rate) |
| Selective reporting (reporting bias) | Low risk | All of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
Ang 2010.
| Methods | Randomised controlled trial | |
| Participants | Female participants who met the ACR classification criteria for fibromyalgia confirmed by a rheumatologist. To be eligible participants needed to be experiencing moderate symptoms (FIQ pain score > 3 and FIQ physical impairment score of ≥ 2) Total participants = 32 randomised (29 completed) Exclusions: non‐stable doses of pain‐related medication; peripheral neuropathy, diabetes mellitus, demyelinating disorders and inflammatory rheumatic diseases |
|
| Interventions | 1) Cognitive behaviour therapy delivered by telephone by a trained therapist (psychology graduate student) with an accompanying workbook Components included: time‐contingent activity, pacing, activity scheduling, relaxation, automatic thoughts and cognitive restructuring and stress management 30 to 40 minute sessions, delivered weekly, for 6 weeks 2) Usual care as provided by treating physicians |
|
| Outcomes | Measures relevant to this review: Fibromyalgia Impact Questionnaire, Patient Health Questionnaire Assessment time points: baseline, post‐intervention and 3 month follow‐up |
|
| Notes | Dr Ang reports to have received consulting fees (less than ($100,000) from Eli Lilly | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated that participants were randomised, but no details of the random component provided |
| Allocation concealment (selection bias) | Unclear risk | No details of the randomisation procedure provided |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | Low risk | Outcome assessors were blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A 13% total attrition rate with reasons for attrition provided. No clear imbalance between groups |
| Selective reporting (reporting bias) | Low risk | All of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
Ang 2013.
| Methods | Randomised controlled trial | |
| Participants | Participants who met the ACR classification criteria for fibromyalgia were referred into the study by a rheumatology clinic. To be eligible participants needed to have a weekly average pain intensity score more than or equal to 4, to be on a stable medication regime for over one month and be between 18 and 65 years old Total participants = 58 randomised (49 completed) Exclusions: current use of selective serotonin reuptake inhibitor, milnacipran or tricyclic antidepressant, uncontrolled hypertension, suicidal ideation, planned elective surgery, inflammatory rheumatic condition, active psychosis, pregnancy and previous cognitive behavioural therapy |
|
| Interventions | 1) Cognitive behavioural therapy delivered by telephone by psychology graduate students including education, progressive muscle relaxation, cognitive restructuring, pacing and anger management 8 x 35 minute sessions 2) Usual care (treatment with milnacipran) |
|
| Outcomes | Measures relevant to this review: Fibromyalgia impact questionnaire, 10 point visual analog scale for pain, Patient Health Questionnaire Depression Scale Assessment time‐points: Baseline 3 months and 5 month follow‐up |
|
| Notes | The study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (Grant number: 1R21AR056046‐01A2). The Forest Research Institute provided the active drug and placebo. The authors report no conflict of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified in the manuscript and no other details able to be obtained |
| Allocation concealment (selection bias) | Unclear risk | Not specified in the manuscript and no other details able to be obtained |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | Unclear risk | Not specified in the manuscript and no other details able to be obtained |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A 15.5% attrition rate with reasons for withdrawal provided |
| Selective reporting (reporting bias) | Unclear risk | All of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
Astin 2003.
| Methods | Randomised controlled trial | |
| Participants | Individuals with a diagnosis of fibromyalgia were recruited by newspaper and radio advertising and local physicians Aged 18 to 70 years Total participants N =128 randomised (65 completed) N = 63 female, N = 2 male Exclusions: unable to read and speak English fluently, unable to attend group sessions;unable to give informed consent; pregnancy, substance abuse; major psychiatric disorder, involvement in impending litigation; uncontrolled hypertension; diabetes, congestive heart failure; or other severe medical condition judged by the clinician to place the patient at risk of possible severe consequences of his or her disease |
|
| Interventions | 1) Mindfulness based stress reduction and qi‐gong. Mindfulness involved learning two meditation practices (a body scan and meditation) and application of mindfulness in context of chronic pain. Qi‐gong consisted of physical postures, breathing techniques and focused intention taught by a qi‐gong master 2.5 hour sessions, delivered weekly for 8 weeks, in a group based format 2) Education control 2.5 hour sessions, delivered weekly, for 8 weeks in a group based format Short lectures of stress, exercise, pain, emotions, sleep, work intimacy and review of current research in addition to group discussion |
|
| Outcomes | Measures relevant to this review; Fibromyalgia Impact questionnaire, SF36 pain subscale, Beck depression inventory, tender point count Assessment time points: baseline, post‐intervention and 4 month follow‐up |
|
| Notes | The study was funded by a grant from the National Center for Complementary and Alternative Medicine, National Institutes for Health (5 P50AT00084‐03) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component is included in the sequence generation process used |
| Allocation concealment (selection bias) | Low risk | Blocks of 2, 4 or 6 groups randomly assigned by computer |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | Low risk | Outcome assessors were blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High attrition rate (49% total attrition rate) |
| Selective reporting (reporting bias) | Low risk | All of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
Babu 2007.
| Methods | Randomised controlled trial | |
| Participants | Participants attending the outpatient department who fulfilled the ACR criteria were recruited Mean age = 39 years Total participants N = 30 randomised (30 completed) N = 21 female, N= 9 male Exclusions: major psychiatric disorders, malignancies, osteomalacia, recent stroke or myocardial infarction, renal failure or neuropathic pain |
|
| Interventions | 1) Biofeedback 45 minute sessions, delivered daily, for 6 consecutive days 2) Sham control. Participants were provided with constant visual feedback irrespective of muscle activity At the end of both group interventions participants received a home programme of gentle stretching and aerobic training |
|
| Outcomes | Measures relevant to this review; Fibromyalgia Impact Questionnaire, pain visual analog scale, number of tender points Assessment time points: baseline and post‐intervention |
|
| Notes | The study was funded by a Fluid Research Grant | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component is included in the sequence generation process used |
| Allocation concealment (selection bias) | Low risk | Participants were randomly allocated to groups through concealed envelopes prepared using block randomisation |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | Low risk | Study was described as being a double‐blinded placebo controlled trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data was reported in figure 1 (0% attrition rate) |
| Selective reporting (reporting bias) | Low risk | All of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review were reported in the pre‐specified way |
Bakker 1995.
| Methods | Randomised controlled trial | |
| Participants | Female participants meeting the ACR criteria Total participants = 85 randomised (74 completed) Aged 18 to 60 years |
|
| Interventions | 1) Biofeedback 20 minutes sessions, held twice weekly, for 8 weeks 2) Attention control included personal communications with participants 3) Low impact fitness training including aerobic and stretching exercises (data not included in review) |
|
| Outcomes | Measures of relevant to this review; Sickness Impact profile, Dutch AIMS, Health Assessment Questionnaire, 100 mm VAS pain scale Assessment time points: baseline and post‐intervention |
|
| Notes | The study was supported in part by 'Het Nationaal Reumafonds' of the Netherlands and the Health Insurance Executive Board (Investigations in Medicine, Grant number 0G90‐018) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component is included in the sequence generation process used |
| Allocation concealment (selection bias) | Low risk | Random numbers generated by computer confirmed in an e‐mail |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | Low risk | Asessors were blind to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | A 13% total attrition rate. Unable to extract means and standard deviations from data reported |
| Selective reporting (reporting bias) | Unclear risk | Findings on all outcomes reported but unable to extract means and standard deviations from data reported |
Baumuller 2009.
| Methods | Randomised controlled trial | |
| Participants | Female participants diagnosed with fibromyalgia according to the ACR criteria were recruited from a list of patients referred to a hospital rehabilitation programme Age range = 18 to 65 years Total participants = 40 randomised (36 completed) Exclusions: unable to read or understand German, to provide informed consent or have major comorbid medical disorders including cancer, chronic heart failure, psychosis of major affective disorders, substance abuse, co‐medication with opiates or benzodiazepines, shift‐work or trans‐meridian flight in last weeks |
|
| Interventions | 1) Biofeedback 15 minute sessions, three sessions per week for the first 3 weeks followed by one sessions per week for the following five weeks 2) Usual care |
|
| Outcomes | Measures relevant to this review; SF36 health related quality of life, Beck Depression Inventory, SCL‐90, tender point score, Fibromyalgia Impact Questionnaire Assessment time points: baseline, post‐intervention and 3 month follow‐up |
|
| Notes | There was no reference to sources of funding or conflicts of interest declared in the article | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component is included in the sequence generation process used |
| Allocation concealment (selection bias) | Low risk | Randomised by a block randomisation of two or four to treatment or control group using computer and placed into sealed envelopes |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | Low risk | Assessor blinded controlled trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A 10% total attrition rate and no clear imbalance evident between groups |
| Selective reporting (reporting bias) | Low risk | All of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
Bojner‐Horwitz 2003.
| Methods | Randomised controlled trial | |
| Participants | Female participants met the ACR criteria for fibromyalgia Total participants = 36 randomised (number withdrawn not stated) Mean age 57 years (SD 7.2 years) |
|
| Interventions | 1) Dance and movement therapy consisted of four main themes including; awareness of the body; movement expressions; movement, feeling, image; and differentiation of feelings and integration 1 hour session, held weekly for 6 months 2) Control group participants received the intervention on completion of the study |
|
| Outcomes | Measures relevant to this review; VAS 0 to 100 pain scale, Montgomery Asberg Depression Rating Scale Follow‐up time points: baseline and month 14 (not able to be included in the review) |
|
| Notes | The study was funded by the Order of Carpenters in Sweden | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated that patients were randomly allocated but details not provided |
| Allocation concealment (selection bias) | Unclear risk | Details of randomisation procedure not provided |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | Unclear risk | Details not provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Details not provided |
| Selective reporting (reporting bias) | High risk | Outcome data not reported for pain VAS and the Montgomery Asberg Depression Rating Scale |
Brattberg 2008.
| Methods | Randomised controlled trial | |
| Participants | Women with a diagnosis of fibromyalgia for less than 5 years and who had been on sick leave for at least 3 months for the condition were included in the study. Other requirements were access to the Internet and willingness to train in and perform emotional freedom techniques Aged 20 to 65 years Total participants = 86 randomised (66 completed) Exclusions: individuals undergoing or having received rehabilitation in the previous 6 months |
|
| Interventions | 1) Emotional freedom involved holding a disturbing memory, emotion or sensation in focus and simultaneously using the fingers to tap on a series of 13 specific points on the body that correspond to meridians used in Chinese medicine The intervention comprised of a set up phase to build acceptance and affirmation, a tapping phase and the ganut procedure which involves performing brain stimulating actions (such as moving the eyes) Emotional freedom was practiced daily by participants for 8 weeks 2) Waiting list control |
|
| Outcomes | Measures relevant to the review: SF36 health related quality of life, Hospital Anxiety and Depression Scale, General Self‐efficacy scale Assessment time points: baseline and post‐intervention |
|
| Notes | There was no reference to sources of funding or conflicts of interest declared in the article | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component is included in the sequence generation process used |
| Allocation concealment (selection bias) | Unclear risk | A lottery drawing by study leader who was blindfolded but exact details of procedure unclear |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | Low risk | Self administered outcome assessments so non‐blinding of assessors not deemed to bias outcome |
| Incomplete outcome data (attrition bias) All outcomes | High risk | A 23% total attrition rate. A high number (40%) of participants did not complete the intervention |
| Selective reporting (reporting bias) | Low risk | All of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
Burckhardt 1994.
| Methods | Radomised controlled clinical trial | |
| Participants | Women who had been diagnosed with fibromyalgia by physicians using the ACR criteria through occupational health and primary health clinics Total participants = 99 randomised (86 completed) Age range = 24 to 69 years Exclusions: unable to understand the Swedish language or have a severe medical condition (e.g. severe osteoarthiritis) |
|
| Interventions | 1) Multi‐component psychological intervention focusing on self‐management; 1.5 hour sessions were held weekly for 6 weeks in a group based format 2) Waiting list control 3) Education and physical therapy (data not included in this review) |
|
| Outcomes | Measures relevant to this review: Fibromyalgia Impact Questionnaire, quality of life scale, self efficacy scale, tender point count, Beck Depression Inventory Assessment time points: baseline and 3 months |
|
| Notes | Funding was provided by Riksforbundet mot Reumatism and the Ragnar och Lisa Stenberg's fund | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component is included in the sequence generation process used |
| Allocation concealment (selection bias) | Low risk | Confirmed in e‐mail that an independent person randomly assigned participants after pre‐testing |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | Low risk | E‐mail confirmation received that outcome assessors were blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | A 13% total attrition rate. No measure of variance for outcome measures provided |
| Selective reporting (reporting bias) | Unclear risk | Means for all outcome measures were reported but no standard deviations |
Calandre 2009.
| Methods | Prospective randomised controlled trial | |
| Participants | Patients who had a diagnosis of fibromyalgia according to the ACR criteria were recruited through a University Hospital Pain Unit Total participants = 81 randomised (57 completed) N = 73 female, N = 8 male Age range 32 to 69 years Exclusions: patients who had never attended a swimming pool as well as those suffering any co‐concomitant disease susceptible to worsen with warm water exercise were excluded |
|
| Interventions | 1) Tai chi was performed in a pool with water heated at 36 ° and was preceded by a shower with warm water to condition patients' bodies. A trained physiotherapist adjusted the movement intensity to meet individual needs and participants were taught the 16 movements which constitute tai chi therapy 2) Stretching was facilitated using supportive aids such as long wooden sticks, flexible strings and tubes to stretch muscles in the cervical, upper and lower extremities and trunk Both groups received 18 sessions of 60 minutes, delivered 3 times per week for 6 weeks |
|
| Outcomes | Measures relevant to this review: Fibromyalgia Impact Questionnaire, Pittsburgh Sleep Quality Index, Beck Depression Inventory, State and Trait Anxiety Inventory, SF12 Health Survey, tender point count Assessment time points: baseline, post‐intervention, one and three month follow‐up |
|
| Notes | There was no reference to sources of funding or conflicts of interest declared in the article | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component is included in the sequence generation process used |
| Allocation concealment (selection bias) | Low risk | Computer generated table of random numbers |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | High risk | Assessors were not blind to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | A 29% total attrition rate; 3 adverse events were reported in the intervention group participants but not for controls, unclear if pain exacerbations directly related to intervention |
| Selective reporting (reporting bias) | Low risk | All of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
Carson 2010.
| Methods | Pilot randomised controlled trial | |
| Participants | Women who had been diagnosed with fibromyalgia according to the ACR criteria for at least one year and were on a stable regimen of treatment Total participants = 53 randomised (48 completed) Mean age = 53.7 (SD 11.5) years Exclusions: residing > 70 miles from the research site, unavailable to attend the intervention at one of the schedule times, currently engaged in yoga practice, actively contemplating suicide, currently undergoing disability application, or litigation, schedule for elective surgery during the study period, physically disabled in a manner that precluded meaningful participation in the intervention, unwilling to forgo changing any voluntary treatments for the length of this study and those unable to speak English |
|
| Interventions | 1) Yoga consisted of 2 hour sessions, held weekly for 8 weeks in a group based format led by a certified, experienced yoga teacher. The intervention included meditation, breathing exercises, study of the application of yoga principles to optimal coping and gentle stretching poses and group discussions 2) Usual care, wait list |
|
| Outcomes | Measures relevant to this review: Fibromyalgia Impact Questionnaire, tender point score Assessment time points: baseline and post‐intervention |
|
| Notes | The study was supported by a grant from the Oregan Health and Science University Medical Research Foundation and resources supplied by the Fibromyalgia Information Foundation. The authors report no conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component is included in the sequence generation process used |
| Allocation concealment (selection bias) | Low risk | Randomised assignments were generated by an individual not involved in the study using a random numbers table |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | Low risk | The outcome assessors were blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A 9% total attrition rate. There was no imbalance evident between groups |
| Selective reporting (reporting bias) | Low risk | All of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
Carson 2012.
| Methods | Randomised controlled trial | |
| Participants | Female participants who had been diagnosed according to the ACR criteria for fibromyalgia syndrome for at least one year. To be eligible participants needed to be on a stable regimen of pharmacological or non‐pharmacological treatment for more than or equal to 3 months before study enrolment Total participants = 53 randomised (39 completed) Exclusions: residing > 70 miles from research site or unable to attend the intervention, engaged in intensive yoga practice, actively contemplating suicide, Undergoing disability assessment, or litigation, scheduled for elective surgery, physically disabled as to preclude meaningful participation in the intervention, unwilling to change treatment for duration of the study and non‐English speaking |
|
| Interventions | 1) Yoga delivered within group sessions by a certified yoga instructor 120 minute sessions, delivered weekly over 8 weeks 2) Wait‐list control group |
|
| Outcomes | Measures relevant to this review: Fibromyalgia Impact Questionnaire Revised, tender point score Assessment time points: baseline and post‐intervention |
|
| Notes | The study was supported by a grant from the Oregan Health and Science University Medical Research Foundation and resources supplied by Fibromyalgia Information Foundation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component is included in the sequence generation process used |
| Allocation concealment (selection bias) | Low risk | "Randomisation assignments were generated by an individual not involved in the study using a random number table. Assignments were concealed in envelopes until completion of the baseline assessment" |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | Low risk | "Research Assistants who collected assessment data were kept blind with regard to condition" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A 24% total attrition rate, no imbalance evident between groups post‐intervention |
| Selective reporting (reporting bias) | Low risk | All of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
Castel 2007.
| Methods | Randomised controlled trial | |
| Participants | Patients attending the University Hospital diagnosed with fibromyalgia by a rheumatologist following the ACR criteria were recruited if they had experienced pain for at least 6 months Total participants = 45 randomised (45 completed) Age range = 25 to 68 years N = 39 female, N = 6 male Exclusions: non‐specified |
|
| Interventions | 1) Hypnosis with relaxation. Participants were invited to lie down on a comfortable, reclining chair with arm rests and engaged in hypnosis and relaxation strategies were facilitated by a trained therapist. The hypnosis session lasted for 20 minutes and was a single session. Participants were asked to stare at an external stimulus and at a particular moment to close their eyes. A chain of suggestions were made using palpebral catalepsy, catalepsy of the vocal chords and the raising of an arm 2) Relaxation comprised of participants being shown how to relax various parts of the body beginning with the feet and finishing with the head followed by diaphragmatic breathing. The session lasted for 20 minutes and was a single session 3) Hypnosis with analgesia (data not included in this review) |
|
| Outcomes | Measures relevant to this review: McGill Pain Questionnaire, pain 10 point visual analog scale Assessment time points: baseline and post‐intervention |
|
| Notes | There was no reference to sources of funding or conflicts of interest declared in the article | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component is included in the sequence generation process used |
| Allocation concealment (selection bias) | Low risk | Participants were randomised using a 1:1:1 ratio based on a random numbers tables generated by computer |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | Low risk | Confirmation that outcome assessors were blind to treatment allocation received by e‐mail |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data were reported at follow‐up (0% total attrition rate) |
| Selective reporting (reporting bias) | Low risk | All of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
Castel 2009.
| Methods | Randomised pilot controlled trial | |
| Participants | Participants who had been experiencing pain for at least 6 months and had a diagnosis of fibromyalgia based on the ACR criteria were recruited Total participants = 47 randomised (39 completed) Age range = 18 to 60 years N = 37 female, N = 2 male Exclusions: 6 years education, severe chronic medical pain conditions, significant suicidal ideation, severe psychopathology, moderate to severe cognitive impairment and presence of pending litigation |
|
| Interventions | 1) Cognitive behaviour therapy (CBT) sessions were delivered in groups and included didactic presentations on fibromyalgia and the theory of pain perceptions, cognitive restructuring, assertiveness training, behavioural goal setting, problem solving and maintaining gains. This was followed by 20 minutes of relaxation training beginning with the feet and ending with the head by means of sensation awareness and diaphragmatic breathing. Sessions were supported by an audio relaxation exercise for practice at home 90 minutes sessions, held weekly, for 12 weeks 2) CBT = hypnosis (data not included in review) 3) Usual care |
|
| Outcomes | Measures relevant to review: Fibromyalgia Impact Questionnaire, pain 10 point visual analog scale, McGill Pain Questionnaire | |
| Notes | There was no reference to sources of funding or conflicts of interest declared in the article | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component is included in the sequence generation process used |
| Allocation concealment (selection bias) | Unclear risk | Participants were assigned a number and randomised using a 1:3 ratio by exact randomisation procedure unclear |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | Low risk | Confirmed in an e‐mail that the outcome assessor was blind to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A 17% total attrition rate. There was an indication of higher withdrawal in the control group with less participants reported as attending the second session. This may possibly be due to lack of efficacy of the control intervention |
| Selective reporting (reporting bias) | Low risk | All of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
Castel 2012.
| Methods | Randomised controlled trial | |
| Participants | Participants aged between 18 and 65 years old with a diagnosis of FMS according to the ACR criteria Total participants = 93 randomised (71 completed) Exclusions: 1 or more additional severe chronic medical pain conditions or moderate to severe cognitive impairment |
|
| Interventions | 1) Cognitive behaviour therapy delivered using group and individual sessions 120 minute sessions, delivered weekly for 14 weeks 2) Usual care |
|
| Outcomes | Measures relevant to the review: Fibromyalgia Impact Questionnaire, 10 point Numerical Pain Rating Scale, Hospital Anxiety and Depression Scale, Medical Outcomes Study Sleep Scale Assessment time points: baseline, post‐intervention and 3 month follow‐up |
|
| Notes | The authors report no conflicts of interest with this study. No sources of funding were declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component is included in the sequence generation process used |
| Allocation concealment (selection bias) | Low risk | It was confirmed in an e‐mail from the author that "patients were randomly assigned in a 1:1:1 ratio in blocks of 18 according to a computer‐generated random number table" |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | Low risk | "All outcome measures were administered by a Psychologist who was blinded to the participant's group assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A 19% attrition rate with equal balance between groups |
| Selective reporting (reporting bias) | Low risk | All of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
Connais 2009.
| Methods | Randomised controlled trial | |
| Participants | Female participants were recruited via flyers and word of mouth and referrals from treating practitioners Total participants = 6 randomised (6 completed) Age range = 48 to 60 years Exclusions; non‐specified |
|
| Interventions | 1) Emotional freedom was delivered by research team members trained in delivering the intervention. The concept of energy in the body and how pain be a manifestation of the body's energy being out of alignment and included a tapping sequence of a series of 13 specific points on the body whilst focusing on a disturbing thought or traumatic memory and included the four steps of emotional freedom including the setup, the sequence, the gamut procedure (tapping og the meridian points) followed by the sequence again. The practice took approximately 5 minutes 2) Wait‐list control |
|
| Outcomes | Measures relevant to the review: Fibromyalgia Impact Questionnaire, tender point scores Assessment time points: baseline and post‐intervention |
|
| Notes | There was no reference to sources of funding or conflicts of interest declared in the article | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Sequence generated by rule based on attendance |
| Allocation concealment (selection bias) | High risk | Alternate nature of allocation to treatment groups could lead to investigators being able to foresee treatment allocation |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | Low risk | Confirmation that outcome assessors were blind to treatment allocation received by e‐mail |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No missing data were reported at follow‐up (0% total attrition rate). Means and standard deviations were unable to be extracted accurately from the graphs provided and no measure of variance provided |
| Selective reporting (reporting bias) | Unclear risk | Data were reported on all outcome measures relevant to review but unable to be extracted from the graphs provided |
de Souza 2008.
| Methods | Randomised controlled trial | |
| Participants | Women were recruited who had been diagnosed with fibromyalgia for > 6 months and who were had been on a stable treatment regime for > 3 months Total participants = 60 randomised (59 completed) Age range = 20 to 65 years Exclusions: pregnancy or breast feeding, cancer, serious depression associated with suicidal thoughts, rheumatic arthritis and uncontrolled cardiopatia |
|
| Interventions | 1) The Interdisciplinary group intervention was delivered in a group based format by allied health professionals. The sessions included information provision on symptoms and the cycle of pain, exercise, relaxation techniques, pacing, nutrition, negative thoughts, and maintenance 9 x 2 hour sessions were delivered over 11 weeks 2) Usual care |
|
| Outcomes | Measures relevant to this review: Multidimensional Pain Inventory, pain visual analog scales on intensity, affective and interference | |
| Notes | Financial agencies included the Co‐ordination of Improvement of People of (Castrate) and Canadian Institutes of Health Research (CIHR) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component is included in the sequence generation process used |
| Allocation concealment (selection bias) | Low risk | Concealed allocation by block randomisation stratified by FIQ score |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | Low risk | Confirmation that outcome assessors were blind to treatment allocation received by e‐mail |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low total attrition (8.3%) rate unlikely to influence outcome data |
| Selective reporting (reporting bias) | Low risk | All of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
Edinger 2005.
| Methods | Randomised parallel group clinical trial | |
| Participants | Participants diagnosed with fibromyalgia according to the ACR criteria with sleep disturbance as assessed by a structured interview criteria for insomnia were recruited through newspaper advertisements Total participants = 47 randomised (20 completed) Age range = 21 to 65 years N = 41 female, N = 6 male Exclusions: pregnancy or breastfeeding, co‐morbid sleep disruptive medical condition, Axis I depressive, anxious or substance abuse disorder, severe hypnotic dependence, diagnosed sleep disorder as assessed by polysomnography |
|
| Interventions | 1) Cognitive behaviour therapy was delivered by two experienced male clinical psychologists. Individual sessions included cognitive restructuring, stimulus control, sleep restriction and sleep education 60 minute sessions, were delivered weekly for 6 weeks 2) Wait‐list control. Participants met weekly with a study co‐ordinator to provide sleep logs and actigraphy data |
|
| Outcomes | Measures relevant to review: McGill Pain Questionnaire, Profile of Mood States, SF36 health related quality of life, Insomia Symptoms Questionnaire, Brief Pain inventory Assessment time points: baseline, post‐intervention and 6 month follow‐up |
|
| Notes | The study was supported by a grant from the National Institute of Arthritis and Musuculoskeletal and Skin Diseases. Dr Edinger reports to have received honoraria from Fisson Communications, Sepracor and Axis Healthcare. Dr Rice reports having provided expert testimony and medical record review as a defence expert in FM for several attorneys | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study claimed to be a randomised clinical trial but no details of sequence generation process provided |
| Allocation concealment (selection bias) | Unclear risk | No details of the randomisation procedure provided |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | Unclear risk | Study presented as a single blind study and unclear if single blind referred to participants or assessors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | A 57% total attrition rate at 6 months |
| Selective reporting (reporting bias) | Low risk | All of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
Falcao 2008.
| Methods | Randomised controlled trial | |
| Participants | Female patients diagnosed with fibromyalgia based on the ACR criteria were consecutively recruited from university outpatient clinics Total participants = 60 randomised (51 completed) Age range = 18 to 65 years Exclusions: < 4 years of elementary school, receiving no other treatment, rheumatic disease, hypersensitivity to amitriptyline, cyclobenzaprine or paracetamol, use of psychotropic medication or psychiatric disease |
|
| Interventions | 1) Cognitive behavioural therapy was delivered in a group based format combining progressive muscle relaxation, diaphragmatic breathing, cognitive restructuring and stress management, facilitated by keeping of a diary 3 hour sessions were held weekly for 10 weeks 2) Usual care Both groups were prescribed amitriptyline 12.5 mg per day increasing to 25 mg the following week and were seen by a medical practitioner weekly for 10 weeks |
|
| Outcomes | Measures relevant to this review: Fibromyalgia Impact Questionnaire, 10 point visual analog pain scale, Medical Outcomes Survey, SF36, State and Trait Anxiety Inventory, Beck Depression Inventory Assessment time points: baseline, post‐intervention and 3 month follow‐up |
|
| Notes | There was no reference to sources of funding or conflicts of interest declared in the article | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component is included in the sequence generation process used |
| Allocation concealment (selection bias) | Low risk | Randomized by drawing lots with concealed allocation |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | Low risk | Confirmation that outcome assessors blind to treatment allocation received by e‐mail |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A 15% total attrition rate |
| Selective reporting (reporting bias) | Low risk | All of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
Fontaine 2010.
| Methods | Randomised controlled trial | |
| Participants | Minimally active people diagnosed with fibromyalgia according to the ACR criteria were recruited through advertisements in newsletters, newspapers and via clinical trial recruitment websites Total participants = 84 randomised (73 completed) N = 88 female, N = 8 male Mean age = 47.7 years (SD 10.7) Exclusions: cancer, coronary heart disease, those where there was intended change of medication planned that might affect mood and those unwilling to make the required time commitment |
|
| Interventions | 1) Cogniitve behaviour therapy based Lifestyle Physical Activity encompassed dealing with pain and fatigue, fear of physical activity, self monitoring, goal setting problem solving and identifying ways of increasing short bouts of physical activity throughout the day. Six, 60 minute group based sessions were delivered over 12 weeks 2) Education control consisted of monthly group meetings of 90 to 120 minutes over 3 months. Sessions included presentations on fibromyalgia, facilitating time to discussion and the opportunity for question and answer |
|
| Outcomes | Measures relevant to this review: 100 visual analog pain scale, Fatigue Severity Scale, Center for Epidemiologic Studies Depression Scale, tender point count Assessment time points: baseline and post‐intervention |
|
| Notes | The study was funded by a NIH/NIAMS grant number AR053168. Dr Clauw reports acting as a consultant for Pfizer, Lilly, Forest Laboratories, Cyprus Biosciences, Pierre Fabre, UCB and Wyeth and has received grant support from Pfizer, Cypress Bioscience and Forest. The other authors report no conflict of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component is included in the sequence generation process used |
| Allocation concealment (selection bias) | Low risk | Randomised via a coin toss at 1:1 allocation ratio |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | High risk | Assesors not blind to treatment allocation (confirmed by e‐mail) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A 13% total attrition rate |
| Selective reporting (reporting bias) | Low risk | All of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
Fors 2000.
| Methods | Randomised clinical trial | |
| Participants | Female participants were recruited through the fibromyalgia association and had been diagnosed with fibromyalgia by medical specialists Total participants = 58 randomised (58 completed) Age range = 21 to 68 years Exclusions: male gender |
|
| Interventions | 1) Guided imagery was delivered via an audio recording that guided participants through a visual imagery exercise that encouraged visualisation of the natural environment with a focus on relaxation without the presence of a researcher 2) Education group (data excluded from this review) listened to an audio recording guiding them to visualise functioning pain killing systems in the body 3) Pain related talk group attended one session talking about their fibromyalgia with a therapist |
|
| Outcomes | Measures relevant to this review: visual analog scale 0 to 100 for pain and anxiety Assessment time points: baseline and post‐intervention |
|
| Notes | There was no reference to sources of funding or conflicts of interest declared in the article | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component is included in the sequence generation process used |
| Allocation concealment (selection bias) | Low risk | Participants were randomised after a lottery with 3 possibilities |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | Low risk | Confirmation that outcome assessors were blinded to treatment outcome received by e‐mail |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A 0% total attrition rate reported |
| Selective reporting (reporting bias) | Low risk | All of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
Garcia 2006.
| Methods | Randomised controlled clinical trial | |
| Participants | Participants were identified through searching case records of people from a pain treatment unit. All patients had their diagnosis of fibromyalgia verified by a rheumatologist before commencing the study Total participants = 28 randomised (28 completed) N = 27 female, N = 1 male Age range = 30 to 76 years Exclusions: being treated by medication, in process of lawsuit for disability, unable to participate in the sessions due to psychological or physical impairments |
|
| Interventions | 1) Multi‐component treatment programme based on cognitive behaviour therapy which included education about stress, developing coping skills and cognitive techniques and relaxation training 2) Usual care |
|
| Outcomes | Measures relevant to this review: Fibromyalgia Impact Questionnaire, tender point count Assessment time points: baseline, post‐intervention and 3 month follow‐up |
|
| Notes | There was no reference to sources of funding or conflicts of interest declared in the article | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component is included in the sequence generation process used |
| Allocation concealment (selection bias) | Unclear risk | Homogenous blocks method used to randomise participants but exact details of procedure unclear |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | Low risk | Physician blinded to treatment allocation for assessment of tender points. Other outcomes self administered and therefore non‐blinding not considered to bias results |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data were unable to be accurately extracted from the graphs provided and no measure of variance was provided. No missing data or attrition reported (0% total attrition rate) |
| Selective reporting (reporting bias) | Unclear risk | All of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way (although no measure of variance provided) |
Gillis 2006.
| Methods | Randomised controlled trial | |
| Participants | Participants diagnosed with fibromyalgia were recruited via flyers in rheumatology clinics, patient support organisations and newsletters Total participants = 83 randomised (72 completed) N = 70 female, N = 2 male Age range = 23 to 72 years Exclusions: autoimmune rheumatic disease, unable to read or write in English |
|
| Interventions | 1) Written emotional disclosure. Participants were sent a writing pack including instructions to identify a stressful experience that continues to bother them and to write about that situation and their deepest feelings about the experience 2) The control writing condition also received a writing pack which included instructions to write about different time periods of the day including what they did and their planned actions |
|
| Outcomes | Measures relevant to this review: Fibromyalgia Impact Questionnaire, Arthritis Impact Measurement Scale‐II Fatigue severity scale, 10 point sleep visual analog scale, Positive and Negative Affect Scale Assessment time points: baseline, post‐intervention and 3 month follow‐up |
|
| Notes | The study was funded by a dissertation grant from the Blue Cross Blue Shield of Michigan Foundation and in part by a Postdoctoral Fellowship Award and Cinical Science Award from the Arthritis Foundation and by a National Institute of Health grant number R01 AR049059 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component is included in the sequence generation process used |
| Allocation concealment (selection bias) | Low risk | Pre‐prepared packs numbered with a unique identifier and randomised via a random numbers table |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | Low risk | Outcome measures were self‐administered and therefore non‐blinding unlikely to bias results |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A 13% total attrition rate |
| Selective reporting (reporting bias) | Low risk | All of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
Hammond 2006.
| Methods | Randomised controlled trial | |
| Participants | People diagnosed with fibromyalgia according to the ACR critiera were recruited through a hospital based rheumatology clinic Total participants = 183 randomised (133 completed) N = 120 female, N = 13 male Mean age = 48.53 (10.89) years Exclusions: alternative medical diagnosis could explain symptoms, undergoing current medical investigation, ongoing psychological problems requiring the care of a mental health practitioner or severe medical conditions affecting the person's ability to participate in exercise safely |
|
| Interventions | 1) Cognitive behavioural group based exercise and education intervention. 2 hour sessions, were held weekly for 10 weeks and included information on the physiological basis of symptoms, activity pacing, sleep hygiene, relaxation, problem solving, pain and stress management, cognitive restructuring, postural training, stretching exercises and tai chi. A tai chi DVD was provided to facilitate practice at home 2) Relaxation group based programme (which acted as an attention control), 1 hour sessions were held weekly for 10 weeks. Information on fibromyalgia and relaxation techniques were outlined with time allocated for group discussion |
|
| Outcomes | Measures relevant to this review: Fibromyalgia Impact Questionnaire, Arthritis Self‐efficacy Scale Assessment time points: baseline, 3 and 6 months |
|
| Notes | The study was funded by the Derbyshire Royal Infirmary Rheumatology Charitable Trust Fund (Derby Hospitals NHS Foundation Trust). The authors declare no conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component is included in the sequence generation process used |
| Allocation concealment (selection bias) | Low risk | Computer generated random numbers in pre‐prepared sealed numbered envelopes |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | Low risk | Self administered outcome measures used, assessor not believed to be able to bias findings |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A 27% total attrition rate |
| Selective reporting (reporting bias) | Low risk | All of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
Hamnes 2012.
| Methods | Randomised controlled trial | |
| Participants | Participants aged between 20 and 70 years, diagnosed with FMS according to the ACR criteria were recruited through the Lillehammer Hospital for Rheumatic Diseases. To be eligible participants needed to be able to speak Norweigen and be willing to give informed consent Total participants = 147 randomised (118 completed) Exclusions: previous participation in a self‐management programme, cognitive impairment or hearing problems or serious mental health disorders |
|
| Interventions | 1) Self‐management programme based on cognitive behaviour therapy run by a multidisciplinary team using group based sessions in hospital 2) Wait‐list control |
|
| Outcomes | Measures relevant to this review: Fibromyalgia Impact Questionnaire, General Health Questionnaire, Arthritis Self‐Efficacy Scale Assessment time points: baseline and post‐intervention |
|
| Notes | The study was funded by the Hospital for Rheumatic Diseases, Lillehammer and was also supported by the Norwegian Rheumatism Association, The Norwegian Nurses Organisation and Per Ryghs Legacy, University of Oslo. The authors declare no conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component is included in the sequence generation process used |
| Allocation concealment (selection bias) | Unclear risk | No details specified |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | Low risk | Questionnaires were self administered |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A 20% attrition rate with equal balance of withdrawals between groups |
| Selective reporting (reporting bias) | Unclear risk | All of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
Holmer 2004.
| Methods | Randomised controlled trial | |
| Participants | Participants had been diagnosed with fibromyalgia based on the ACR criteria Total participants = 28 randomised (22 completed) Age range 18 to 65 years N = 26 female, N = 3 male Exclusions: none specified |
|
| Interventions | 1) Yoga delivered by a certified yoga instructor 2) Waiting list control |
|
| Outcomes | Measures relevant to this review: Multidimensional Assessent of Fatigue Scale, Fibromyalgia Impact Assessment ‐ pain scale, Arthritis Impact Measuresment Scale ‐ II, anxiety subscale, Center for Epidemiology Scale ‐ Depression, Pittsburgh Sleep Quality Index, visual analog scale for pain Assessment time points: baseline and post‐intervention |
|
| Notes | There was no reference to sources of funding or conflicts of interest declared in the article | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component is included in the sequence generation process used |
| Allocation concealment (selection bias) | High risk | Alternate group assignment method was employed (informed by e‐mail) |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | High risk | Outcome assessors were not blind to treatment allocation (confirmed by e‐mail) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A 21% total attrition rate |
| Selective reporting (reporting bias) | Low risk | All of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
Hsu 2010.
| Methods | Randomised controlled trial | |
| Participants | Female participants were recruited through advertisements distributed in the local area and to physicians and through presentations to patient support groups. Diagnosis of fibromyalgia based on the ACR criteria was confirmed by an assessment by a rheumatologist Total participants = 45 randomised (42 completed) Age range = 25 to 66 years Exclusions: serious co‐morbid medical conditions that could confound results in the next 6 months e.g. cancer, heart disease, current serious psychiatric disorder, recent suicide risk or substance abuse and changes in pain medication within 1 month |
|
| Interventions | 1) Mulit‐component group based intervention following an initial 90 min individual consultation. 2 hour group sessions were held were held weekly for 3 weeks and consisted of education, written emotional disclosure, affective awareness and re‐engagement in previously avoided activities 2) Wait‐list control group |
|
| Outcomes | Measures relevant to this review: Medical Outcome Study SF36, Brief Pain Inventory, tender point count, MOS sleep scale, Multidimensional Fatigue Inventory Assessment time points: baseline, post‐intervention and 6 month follow‐up |
|
| Notes | The study was supported in part by the Scott F Nadler DO, Research Grant (Psychiatric Association of Spine, Sports and Occupational Rehabilitation), Michigan Institute of Clinical and Health Research grant number U020912, NICHD/NIH grant numbers T32‐HD007422, K12HD001097, NIAMS/NIH AR049059 and Department of Defence DAMD 17‐00‐2‐0018. Dr Schubiner reports developing the programme which is used in the Providence Hospital and on the Internet. The other authors report no conflicts of interests | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component is included in the sequence generation process |
| Allocation concealment (selection bias) | Low risk | Random assignment of information sheets in opaque sealed envelopes generated by computer confirmed by e‐mail |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | Low risk | Confirmation received by e‐mail that outcome assessors were blind to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A 6% total attrition rate, the 3 participants who withdrew were all in the intervention group but withdrew due to scheduling difficulties rather than as a direct result of the intervention |
| Selective reporting (reporting bias) | Low risk | All of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
Jensen 2012.
| Methods | Randomised controlled trial | |
| Participants | Participants were referred to the study by their primary care physicians. To be eligible for the study participants needed to be diagnosed with fibromyalgia syndrome (FMS) in accordance with the ACR criteria, have an average weekly pain intensity of at least 40 mm. Total participants = 43 randomised (34 completed) Exclusions: being left‐handed, pregnant or breast feeding, those with metal implants or claustrophobia due to fMRI requirements |
|
| Interventions | 1) Cognitive behaviour therapy and acceptance and commitment therapy. The interventions was delivered in group based sessions by psychologists and clinicians in a pain clinic 90 minute sessions, delivered weekly for 12 weeks 2) Wait‐list control |
|
| Outcomes | Measure relevant to this review: 100 point visual analog scale for pain, Beck Depression Inventory and State‐Trait Anxiety Inventory Assessment time points: baseline, post‐intervention and 3 month follow‐up |
|
| Notes | The study was funded Swedish Council for Working LIfe and Social Research and the Swedish Research Council, grant number K2009‐53x‐21070‐01‐3. The authors report no conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component is included in the sequence generation process used |
| Allocation concealment (selection bias) | Low risk | "Presented with the content of a randomisation envelope" |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | Low risk | "An independent researcher (KJ) with no insight or involvement in the treatment intervention performed all pre and post treatment assessments" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A 21% attrition rate |
| Selective reporting (reporting bias) | Unclear risk | All of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
Jones 2012.
| Methods | Randomised controlled trial | |
| Participants | Participants aged 40 years diagnosed with fibromyalgia syndrome or over were recruited with approval of a healthcare practitioner Total participants = 101 randomised (98 completed) Exclusions: practice of of tai chi within past 6 months, exercised more than 30 minutes three times weekly for past 3 months, unable to ambulate without assistive devices, pain severity or interference scores less than 5, planned elective surgery in study period, actively involved in healthcare litigation, unwilling to keep all treatments stable throughout the study duration |
|
| Interventions | 1) Tai chi delivered in a group based format 90 minute sessions delivered twice weekly for 12 weeks 2) Education sessions delivered in a group based format on fibromyalgia , healthy eating, education based CBT strategies, sleep hygiene and lifestyle management 90 minute sessions delivered twice weekly for 12 weeks |
|
| Outcomes | Measures relevant to this review: Fibromyalgia Impact Questionnaire, Brief Pain Inventory, Numerical Rating Scale for pain, Arthritis Self‐Efficacy Scale, Pittsburgh Sleep Quality Index Assessment time points: baseline and post‐intervention |
|
| Notes | The study was funded by the National Institutes of Health/NIAMS grant number 5R21 AR053506, NIH/NCCAM1K23 AT006392‐01. The authors report no conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component is included in the sequence generation process used |
| Allocation concealment (selection bias) | Low risk | "computer generated table of random numbers with block stratification using age in 5‐year intervals" |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | Unclear risk | No details provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A 3% attrition rate although all withdrawals occurred in the control group |
| Selective reporting (reporting bias) | High risk | Means and standard deviations not reported |
Kayiran 2010.
| Methods | Randomised, controlled, rater blind clinical trial | |
| Participants | Consecutive female patients who met the ACR criteria for fibromyalgia admitted to outpatient clinic were recruited Total participants: 40 randomised (36 completed) Age range = 16 to 49 years Exclusions: not currently receiving medication; major health conditions including stroke, diabetes mellitus, coronary heart disease; alcohol abuse and any abnormality in routine laboratory tests |
|
| Interventions | 1) Neurofeedback was provided using Brain Feedback ‐3 EEG biofeedback software. patients were informed about the system and told to follow the continuous feedback process and try to maximise their scores, 20 sessions of 30 minutes in duration of neuro feedback were received over 4 weeks 2) Escitalopram (10 mg) per day for 8 week duration |
|
| Outcomes | Measures relevant to this review: SF36, 10 point visual analog pain and fatigue scales, Hamilton Depression Scale, Hamilton Anxiety Scale, Beck Anxiety Scale Assessment time points: baseline, post‐intervention, 3 and 6 month follow‐up |
|
| Notes | There was no reference to sources of funding or conflicts of interest declared in the article | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component is included in the sequence generation process used |
| Allocation concealment (selection bias) | Low risk | Participants randomised by a coin toss (confirmed by e‐mail) |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | Low risk | Study described as "rater‐blinded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A 10% total attrition rate, no clear imbalance between groups observed |
| Selective reporting (reporting bias) | Low risk | All of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
Langford 2009.
| Methods | Randomised controlled trial | |
| Participants | Female participants diagnosed with fibromyalgia by a rheumatologist were recruited Total participants = 105 randomised (99 completed) Mean age = 52.44 years (SD 9.39) Exclusions: co‐comitant rheumatic medical conditions or other serious illness or unstable medication regime for < 2 months |
|
| Interventions | 1) Cognitive behavioural and interpersonal therapy was delivered in group sessions. Sessions included a review of the gate‐control theory, sleep difficulties, relaxation strategies, identifying and changing cognitions and problem solving techniques. Sessions lasted for 2 hours and were held weekly for 8 weeks 2) Attention control participants received phone calls from a researcher over the course of the 8 week intervention period |
|
| Outcomes | Measures relevant to this review: Fibromyalgia Impact Questionnaire, 0 to 100 numerical pain rating scale, Health Assessment Questionnaire, Arthritis Self‐Efficacy Scale, Symptom Checklist 90‐R, Quality of Life Scale (QoLS) Assessment time points: baseline, post‐intervention and 3 month follow‐up |
|
| Notes | There was no reference to sources of funding or conflicts of interest declared in the article | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component is included in the sequence generation process used |
| Allocation concealment (selection bias) | Low risk | A coin toss was used to determine which member of the matched pair was assigned to which group |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | Low risk | Self administered outcome measures used, assessor not believed to be able to bias findings |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | An 11% total attrition rate |
| Selective reporting (reporting bias) | Low risk | All of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
Lera 2009.
| Methods | Randomised controlled trial | |
| Participants | Female participants were recruited from a hospital rheumatology unit based on a diagnosis of fibromyalgia according to the ACR criteria Total participants = 83 randomised (56 completed) Mean age = 51.1 years (SD 8.7) Exclusions: male gender, severe depression, psychosis or delusional disorder |
|
| Interventions | 1) The multidisciplinary group received multidisciplinary treatment including an appointments with a rheumatologist for pharmacological management of symptoms, and group sessions including information on fibromyalgia, postural advice, exercise and activity pacing led by a physiotherapist. There were 14 group based sessions held for 1 hour per week over 4 months 2) The second group received the multidisciplinary intervention described above in addition to 15 group based sessions of 90 minutes on cognitive behaviour therapy. The sessions were led by a clinical psychologist which included techniques to reduce physiological arousal, improve sleep, well‐being, self esteem, goal planning and modifying negative thoughts |
|
| Outcomes | Measures relevant to this review: Fibromyalgia Impact Questionnaire, Medical Outcomes Study SF36, Symptom Checklist 90‐Revised, tender point count Assessment time points: baseline, post‐intervention and 6 month follow‐up |
|
| Notes | There was no reference to sources of funding or conflicts of interest declared in the article | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component is included in the sequence generation process used |
| Allocation concealment (selection bias) | Low risk | Random sequence generated by a coin toss (confirmed by e‐mail) |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | High risk | The psychologist completing outcome assessments was not blind to treatment allocation (confirmed by e‐mail) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A 32% total attrition rate |
| Selective reporting (reporting bias) | Low risk | All of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
Liu 2012.
| Methods | Randomised controlled trial | |
| Participants | Participants aged between 18 and 70 years with a diagnosis of FMS according to the ACR criteria were recruited from a neurology clinic and support group Total participants = 14 randomised (12 completed) Exclusions: severe psychiatric illness, significant suicide risk, alcohol abuse, use of benzodiazepines, history of behaviour that would prohibit compliance for the duration of the study, co‐morbid medical conditions, severe sleep apnoea, pregnancy or breastfeeding |
|
| Interventions | 1) Qi‐gong delivered in a group based format with home practice in between sessions 15 to 20 minute sessions, held weekly for 6 weeks 2) Sham qi‐gong delivered in a group based format with no meditation or healing sounds 15 to 20 minute sessions, held weekly for 6 weeks |
|
| Outcomes | Measures relevant to the review: Fibromyalgia Impact Questionnaire, McGill Pain Questionnaire, Mulitidimensional Fatigue Inventory, Pittsburgh Sleep Quality Index Assessment time points: baseline and post‐intervention |
|
| Notes | The authors report no conflicts of interest. No sources of funding were declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component is included in the sequence generation process used |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | Unclear risk | No details provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A 14% attrition, both withdrawals were in the treatment group |
| Selective reporting (reporting bias) | High risk | Means and standard deviations for outcome measures not reported |
Luciano 2011.
| Methods | Randomised controlled trial | |
| Participants | Participants were recruited from a database of patients diagnosed with fibromyalgia confirmed by a rheumatologist Total participants = 216 randomised (185 completed) Age range = 18 to 75 years Exclusions: diagnosis not based on the ACR criteria, cognitive impairment, presence of physical, psychiatric limitations that impeded participation in the study assessments, life expectancy of less than 12 months, absence of schooling |
|
| Interventions | 1) Autogenic training and education comprised of 9 sessions with a clinical psychologist. The link between emotions and bodily reactions was highlighted and use of distraction explained and encouraged in addition to use of relaxation techniques that could be practiced at home 2) Usual care |
|
| Outcomes | Measures relevant to this review: Fibromyalgia Impact Questionnaire, State‐Trait Anxiety Inventory Assessment time points: baseline and post‐intervention |
|
| Notes | The study was funded by a grant from the Agencia d/Avaluacio de Technologia i Recerca Mediques grant number AATRM 0077/25/06. Dr Luciano received a postdoctoral contract from the Instituto de Salud Carlos III RD06/0018/0017 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random component is included in randomisation procedure used |
| Allocation concealment (selection bias) | Low risk | Computer generated randomisation list |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | Low risk | The Research Assistant was bind to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A 14% total attrition rate |
| Selective reporting (reporting bias) | High risk | Two measures taken at baseline only (MCSDS, STAI) |
Lynch 2012.
| Methods | Randomised controlled trial | |
| Participants | Participants were recruited through advertisements in local newspapers. To be eligible participants were required to have a diagnosis of FMS according to the ACR criteria, have had a stable medication regime in the past 2 weeks, have an average weekly pain score more than 4 on an 11 point rating scale Total participants = 100 randomised (89 completed) Exclusions: significant medical disorder |
|
| Interventions | 1) Qi‐gong delivered by a psychologist in a group based format in the community 3.5 day workshops held weekly with additional refresher sessions 2) Wait‐list control |
|
| Outcomes | Measures relevant to the review: Fibromyalgia Impact Questionnaire, 11 point numerical rating scale for pain, SF36 Health Survey, Pittsburgh Sleep Quality Index Assessment time points: baseline, post‐intervention and 6 month follow‐up |
|
| Notes | The study was funded by a Pfizer Neuropathic Pain Research Award. Authors CH and DM provide qi‐gong interventions in the community. The other co‐authors report no conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as a randomised controlled trial but no details of the sequence generation process provided |
| Allocation concealment (selection bias) | Low risk | "participants were assigned using computer generated numbers to an immediate Qigong training group or to a control group. Assignments were sealed in opaque white envelopes" |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | Unclear risk | No details specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | An 11% attrition although more withdrawals occurred in the treatment group in comparison to control |
| Selective reporting (reporting bias) | High risk | Data were presented as change scores and were not able to be included in the analyses |
Maddali‐Bongh 2010.
| Methods | Randomised controlled trial | |
| Participants | Consecutive participants diagnosed with FMS were recruited Total participants = 44 randomised (41 completed) N = 38 females, N = 3 males Mean age 45.5 (SD 11.79) years Exclusions: none specified |
|
| Interventions | 1) The Ressegiuer method. Patients are asked to describe painful areas of the body in terms of weight, consistency and symmetry. The method aims to obtain patient awareness and control of bodily perceptions, thus reaching a modulation of responses to pain. Throughout the intervention sessions, the therapist controlled the patient's attention and perception by verbal and manual contacts. and leads to perform bodily and respiratory active and conscious movements “petite gymnastique” of different areas of the body tailored on the patients needs. Sessions were delivered for 60 minutes, once per week for 8 weeks delivered by a trained physiotherapist 2) Waiting list control group |
|
| Outcomes | Measures relevant to this review: Medical Outcomes Survey SF36, Fibromyalgia Impact Questionaire, numerical pain rating scale. Assessment time points: baseline and post‐intervention (no data available for the control group at 6 month follow‐up) |
|
| Notes | There was no reference to sources of funding or conflicts of interest declared in the article | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component is included in the sequence generation process used |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered sealed envelopes prepared by an independent person. |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | Low risk | All assessment examinations were performed by an operator blinded to group assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A 6% total attrition rate. Three participants withdrew from the control group as they did not accept their group allocation which is unlikely to affect the outcome |
| Selective reporting (reporting bias) | Low risk | All of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
Mannerkorpi 2004.
| Methods | A controlled randomised pilot study | |
| Participants | Women fulfilling the ACR criteria for fibromyalgia were recruited Total participants = 36 randomised (22 completed) Age range = 18 to 65 years Exclusions: unable to speak Swedish |
|
| Interventions | 1) Qi‐gong + relaxation, 14 group sessions of 1.5 hours, were held weekly, delivered by a physiotherapist. The treatment included various breathing, relaxation and concentration techniques conducted in a supine or standing position including qi‐gong movements. The movements were individually modified to match the functional limitations of the patients and there was an opportunity for discussion about the movements with the therapist. Participants were encouraged to practice the movements in between sessions 2) Usual care |
|
| Outcomes | Measures relevant to this review: Fibromyalgia Impact Questionnaire Assessment time points: baseline and post‐intervention |
|
| Notes | The study was supported by grants from the Swedish Rheumatism Association and the Swedish Research Council | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component is included in the sequence generation process used |
| Allocation concealment (selection bias) | Low risk | Independent person allocated patients to groups using sealed envelopes |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | Low risk | Outcome assessor was blinded to patients group membership |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A 39% total attrition rate, fell just below cut‐off of 40% used to indicate high risk of attrition bias |
| Selective reporting (reporting bias) | Low risk | All of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
Martinez‐Valero 2008.
| Methods | Randomised controlled trial | |
| Participants | Female participants who were between one and three years post‐diagnosis of fibromyalgia were recruited by a clinician at the hospital Total participants = 6 randomised (6 completed) Age range = 25 to 60 years Exclusions: receiving psychological treatment, co‐morbid psychiatric or medical pathology, receipt of compensation for fibromyalgia |
|
| Interventions | 1) Cognitive behavioural therapy (CBT) was delivered in 1 hour sessions, held weekly for 10 weeks. Each session consisted of a review of homework from previous session, introduction and practice of strategies and new homework exercises. Information about fibromyalgia, coping skills, cognitive restructuring, pacing and planning activities and training in social skills and problem resolution was included 2) Usual care |
|
| Outcomes | Measures relevant to this review: Fibromyalgia Impact Questionnaire, Plates Coop/Wonca, numerical rating scales for pain, fatigue, sleep Assessment time points: baseline, post‐intervention and 3 month follow‐up |
|
| Notes | There was no reference to sources of funding or conflicts of interest declared in the article | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component is included in the sequence generation process used |
| Allocation concealment (selection bias) | Low risk | Randomised by recruiting doctor using pre‐prepared sealed envelopes |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | Low risk | Post treatment and follow ups were administered by an experienced, masked, independent rater |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition or missing data apparent (0% total attrition rate) |
| Selective reporting (reporting bias) | Low risk | All of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
Menzies 2006.
| Methods | Longitudinal, prospective, two‐group, randomised controlled clinical trial | |
| Participants | Participants were recruited through outpatient clinics Total participants = 48 randomised (48 completed) N = 47 female, N = 1 male Mean age = 49.9 years (SD 12.9) Exclusions: Mini‐Mental Status examination score < 25, Fibromyalgia Impact Score of < 20, presence of other systemic rheumatologic conditions or major communicative disorder |
|
| Interventions | 1) Guided imagery was administered using 3 guided imagery audio tapes ranging in length between 12 and 22 minutes. The audio tapes introduced participants to relaxation and release of tension and encouraged an overall sense of well‐being. Participants were also trained to learn a conditioned response to the signal breath to elicit relaxation and were to use techniques such as progressive muscle relaxation and guided imagery of a pleasant scene. Participants were advised to use the tapes at least daily during the 10 week treatment period 2) Usual care |
|
| Outcomes | Measures relevant to this review: Short form McGill Pain Questionnaire, Fibromyalgia Impact Questionnaire, Arthritis Self‐Efficacy Scale Assessment time points: baseline, post‐intervention and 1 month follow‐up |
|
| Notes | The study was funded by a National Center for Complementary and Alternative Medicine T32‐AT‐00052 and K30‐AT‐00060 and the National Institute of Nursing Research F31‐NR‐007696 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component is included in the sequence generation process used |
| Allocation concealment (selection bias) | Low risk | Random number table was used to generate the order of the group assignments |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | Low risk | E‐mail received from author confirming assessors were blind to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition or missing data apparent (0% total attrition rate) |
| Selective reporting (reporting bias) | Low risk | All of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
Miro 2011.
| Methods | Pilot randomised controlled trial | |
| Participants | Female participants meeting the ACR criteria for fibromyalgia and Ameican Psychiatric Association (2000) criteria for insomnia were recruited through a hospital pain unit Total participants = 44 randomised (31 completed) Age range = 25 to 60 years Exclusions: pregnancy, having a significant history of head injury of neurological disorder, major concomitant medical conditions, major depressive disorder with suicidal ideation or other major Axis I diagnoses, symptoms of sleep disruptive co morbidities with insomnia, apnea, hypopnea index or periodic limb movement arousal index of ≥ 15 per hour of sleep, severe hypnotic dependence, being treated with another psychological or physical therapy during the course of the study |
|
| Interventions | 1) Cognitive behavioural therapy (CBT) was delivered in groups for 90 minutes, once a week for 6 weeks by a CBT trained expert based on a treatment manual. Participants received information on sleep and sleep hygiene education, sleep restriction and stimulus control instructions, relaxation training and cognitive restructuring in addition to planning to prevent relapses 2) The sleep hygiene control group received information on environmental and lifestyle factors related to sleep held in groups for 90 minutes, once a week for 6 weeks |
|
| Outcomes | Measures relevant to this review: McGill Pain Questionnaire, Hospital Anxiety and Depression Scale, Pittsburgh Sleep Quality Index, Fibromyalgia Impact Questionnaire Assessment time points: baseline and post‐intervention |
|
| Notes | The study was funded by the Spainish Ministry of Science and Innovation (SEJ2006‐07513, PSI2008‐03595PSIC and PSI2009‐1365PSIC). The authors report no conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component is included in the sequence generation process used |
| Allocation concealment (selection bias) | Low risk | Simple randomisation (1:1) was implemented by a researcher with no clinical involvement in the trial |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | Low risk | Performed by an examiner (CD) who was blinded to group assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A 29% total attrition rate |
| Selective reporting (reporting bias) | Low risk | All of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
Oliver 2001.
| Methods | Randomised controlled trial | |
| Participants | Participants were recruited through members of a health maintenance organisation, advertisements in newspaper, with flyers advertised in waiting rooms, and e‐mails sent to physicians asking them to refer patients into the study. Diagnosis was confirmed by a trained examiner Total participants = 600 randomised (492 completed) Mean age = 54 (SD 11) years Exclusions: not meeting the ACR criteria for fibromyalgia |
|
| Interventions | 1) Social support and education was delivered for 2 hours, held weekly for 10 weeks, followed by 10 monthly meetings. Sessions included group discussions prompted by assigned tasks aimed at promoting empathy and sharing of coping techniques between participants and education provided by a health educator 2) Usual care control group |
|
| Outcomes | Measures relevant to this review: Fibromyalgia Impact Questionnaire, Arthritis Self‐Efficacy Scale, Center for Epidemiological Studies ‐ Depression Scale and Quality of Wellbeing Scale Assessment time points: baseline (12 month follow‐up not included in this review) |
|
| Notes | The study was funded by a National Institutes of Health Grant AR‐44020 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component is included in the sequence generation process used |
| Allocation concealment (selection bias) | Low risk | E‐mail from author received confirming use of random generated numbers placed in sealed envelopes. Participants were asked to select an envelope from a box |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | Low risk | E‐mail from author confirmed that assessors were blind to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | An 18% attrition rate |
| Selective reporting (reporting bias) | Low risk | All of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
Oneva‐Zafra 2010.
| Methods | Randomised controlled clinical trial | |
| Participants | Participants who had experienced fibromyalgia ≥ 3 years were recruited through three fibromyalgia associations Total participants = 60 randomised (55 completed) Age range = 45 to 65 years N = 106 female, N = 4 male Exclusions: major psychiatric condition, inability to understand or follow instructions, inability to read or write Spanish, and deafness |
|
| Interventions | 1) Music therapy was delivered for 1 hour on a compact disc (CD). Participants were asked to play the CD at home ≥ 4 days in the first week and every day in the second week. A second CD with a different compilation was provided for the following two weeks 2) Usual care |
|
| Outcomes | Measures relevant to this review: visual analog scale 0 to 10 for pain, Beck Depression Inventory, McGill Pain Questionnaire ‐ long form Assessment time points: baseline and post‐intervention |
|
| Notes | There was no reference to sources of funding or conflicts of interest declared in the article | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component is included in the sequence generation process used |
| Allocation concealment (selection bias) | Low risk | Computer generated random numbers table by independent researcher. Sealed envelopes chosen on allocation |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | Unclear risk | Not clear if outcome assessor was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | An 8% total attrition rate |
| Selective reporting (reporting bias) | Low risk | All of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
Parra‐Delgado 2013.
| Methods | Randomised controlled trial | |
| Participants | Women were recruited through the Spanish Fibromyalgia Association. To be eligible people needed to be diagnosed with FMS according to the ACR criteria and willing to commit to daily mindfulness practice Total participants = 33 randomised (31 completed) Exclusions: diagnosis with alcohol or substance abuse problems or receiving psychological therapy |
|
| Interventions | 1) Mindfulness based cognitive therapy delivered by rehabilitation clinicians in a group format 2.5 hour sessions held weekly for 8 weeks 2) Usual care |
|
| Outcomes | Measures relevant to this review: Fibromyalgia Impact Questionnaire, 10 point visual analog scale, Beck Depression Inventory Assessment time points: baseline, post‐intervention and 3 month follow‐up |
|
| Notes | There was no reference to sources of funding or conflicts of interest declared in the article | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component is included in the sequence generation process used |
| Allocation concealment (selection bias) | Low risk | "randomly assigned to the MBCT intervention single group or the treatment as usual group using the random number generator programme" |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | High risk | Outcome assessors were not blind to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A 6% attrition, 2 participants withdrew from the treatment group |
| Selective reporting (reporting bias) | Low risk | All of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
Picard 2013.
| Methods | Randomised controlled trial | |
| Participants | Female participants referred to a pain clinic who had experienced FMS for at least 6 months and who had been diagnosed by a rheumatologist according to the ACR criteria were recruited Total participants = 62 randomised (59 completed) Exclusions: diagnosed with chronic inflammatory arthritis or peripheral or central neuropathic pain, taking opioids, severe psychiatric illness or history of substance abuse |
|
| Interventions | 1) Hypnosis delivered by a clinician within a pain clinic and including home practice in between sessions 60 minute sessions held weekly for 5 weeks 2) Wait‐list control |
|
| Outcomes | Measures relevant to this review: Fibromyalgia Impact Questionnaire, Hospital Anxiety and Depression Scale, Medical Outcomes Study Sleep Scale, Mulitdimensional Fatigue Invenory Assessment time points: baseline, post‐intervention and 3 month follow‐up |
|
| Notes | The study was funded by the Foundation de France UB 032115 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | A random component appears to be included in the sequence generation process but details are not provided |
| Allocation concealment (selection bias) | Unclear risk | "preparing envelopes"; it was unclear from the information provided as to whether the investigators would have been able to foresee group assignment |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | High risk | "Follow up assessments were then performed during a consultation, 3 and 6 months post‐randomisation by the same medical doctor who was not blind to study condition" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A 5% attrition with equal distribution between groups |
| Selective reporting (reporting bias) | Low risk | All of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
Riedel 2012.
| Methods | Randomised controlled trial | |
| Participants | Adult participants (aged over 18 years) diagnosed with FMS were referred by clinicians into the study. To be eligible participants were required to have a diagnosis of FMS based on the ACR criteria, be able to read, write, and understand English and to be available for weekly telephone contact Total participants = 24 randomised (17 completed) Exclusions: current or regular use of guided imagery within the last 6 months, Mini‐Mental State Examination (MMSE) score < 25, unstable or severe psychiatric illness |
|
| Interventions | 1) Guided imagery. Audio recording listened to using an MP3 player at the person's home. Audio recordings were of 20 minutes duration and were listened to daily for 14 days 2) Usual care |
|
| Outcomes | Measures relevant to this review: Fibromyalgia Impact Questionnaire, Short Form McGill Pain Questionnaire, Lees Fatigue Inventory, State‐Trait Anxiety Inventory, Center for Epidemiologic Studies Depression Scale, Arthritis Self‐Efficacy Scale, Pittsburgh Sleep Quality Index Assessment time points: baseline and post‐intervention |
|
| Notes | The study was funded by the National Center for Complimentary and Alternative Medicine, National Institutes for Health grant number 5‐T32‐AT000052 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component is included in the sequence generation process used |
| Allocation concealment (selection bias) | Low risk | "table of random numbers with probabilities in a ratio of 1:1" |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | Unclear risk | Details not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A 27% attrition |
| Selective reporting (reporting bias) | Low risk | All of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
Scheidt 2013.
| Methods | Randomised controlled trial | |
| Participants | Women aged 18 to 70 years diagnosed with FMS according to the ACR criteria were recruited Total participants = 47 randomised (35 completed) Exclusions: severe or life threatening illness, cognitive impairment, suicidal ideation, current psychotherapy or participation in other clinical trials |
|
| Interventions | 1) Psychodynamic psychotherapy delivered by a psychologist on an individual basis within a hospital setting 50 to 60 minute sessions, held weekly for 25 weeks 2) Wait‐list control |
|
| Outcomes | Measures relevant to this review: Fibromyalgia Impact Questionnaire, Hospital Anxiety and Depression Scale, Symptom Checklist‐27 and Medical Outcome Study SF36 Assessment time points: baseline, post‐intervention and 12 month follow‐up |
|
| Notes | The study was funded as part of an Interdisciplinary Research Project by the Freiberg Institute of Advanced Studies | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component is included in the sequence generation process used |
| Allocation concealment (selection bias) | Low risk | "Randomised in blocks of 10 to treatment or control group according to a 1;1 schedule" |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | Low risk | Outcome assessors were blind to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | An 11% attrition, equal distribution of withdrawals between groups |
| Selective reporting (reporting bias) | Low risk | All of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
Schmidt 2011.
| Methods | Randomised controlled trial | |
| Participants | Women diagnosed with fibromyalgia defined by the ACR criteria were eligible for the trial Total participants = 177 randomised (168 completed) Age range = 18 to 70 years Exclusions: unable to speak or understand German, life‐threatening disease, evidence of suppressed immune functioning, participation in other clinical trials |
|
| Interventions | 1) Mindfulness was delivered based on the Mindfulness Based Stress Reduction structured programme by Kabat‐Zinn 1990. Groups of up to 12 patients took part in 2.5 hour sessions, held weekly and an additional all‐day session on a weekend day for 8 weeks. Sessions included mindful awareness of dynamic yoga postures, mindfulness during stressful situations and social situations. Participants were encouraged to practice techniques in‐between the weekly sessions at home 2) Wait‐list control group |
|
| Outcomes | Measures relevant to this review: Center for Epidemiological Studies ‐ Depression Scale, State‐Trait Anxiety Inventory, tender point count, Pittsburgh Sleep Quality Index, Pain Perception Scale, Fibromyalgia Impact Questionnaire, The Quality of Life Profile for the Chronically Ill Assessment time points: baseline and post‐intervention |
|
| Notes | The study was supported by the Amueli Institute and the Manfred Kohnlechner Stiftung, Munich, Germany. The authors report no conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component is included in the sequence generation process used |
| Allocation concealment (selection bias) | Low risk | Patients were randomised in blocks using a computer generated algorithm |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | Low risk | All personnel handling data or interacting with the patients stayed blinded until the final analysis |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A 5% total attrition rate |
| Selective reporting (reporting bias) | Low risk | All of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
Sephton 2007.
| Methods | Randomised clinical trial | |
| Participants | Women diagnosed with fibromyalgia according to the ACR criteria were recruited through media broadcasts and newspaper advertisements Total participants = 91 randomised (68 completed) Mean age = 48.2 (SD 10.6) years Exclusions: no confirmation of diagnosis, declining to participate, unavailability to attend 8 week intervention |
|
| Interventions | 1) Mindfulness was delivered based on the Mindfulness Based Stress Reduction programme. Weekly sessions lasted for 2.5 hours and were facilitated by a licensed clinical psychologist. Participants were encouraged to practice the techniques at home daily for 3 to 45 minutes in‐between sessions. A day long meditation retreat was held in addition to 7 weekly sessions. 2) Wait list |
|
| Outcomes | Measures relevant to this review: Fibromyalgia Impact Questionnaire, Beck Depression Inventory, Stanford Sleep Questionnaire Assessment time‐points: Baseline, post‐intervention and 2 month follow‐up |
|
| Notes | This study was supported by an intramural research grant from the University of Louisville, School of Medicine and Office of the Vice President for Research. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It is stated that participants were randomly assigned but no details of randomisation procedure provided |
| Allocation concealment (selection bias) | Unclear risk | No information on the actual randomisation procedure used provided |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | Unclear risk | Only stated that data entry personnel were blinded, not outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A 25% total attrition rate |
| Selective reporting (reporting bias) | Low risk | Two measures were used to yield covariate variables, All of the study’s pre‐specified outcomes that are of interest in the review have been reported in the pre‐specified way |
Soares 2002.
| Methods | Randomised controlled trial | |
| Participants | Female participants diagnosed with fibromyalgia within the previous 2 years were recruited through general practitioners Total participants = 60 randomised (53 completed) Age range 18 to 64 years Exclusions: serious illness (e.g. other rheumatic disease, ongoing alcohol or drug abuse, receipt of other therapies) |
|
| Interventions | 1) The behavioural intervention consisted of five individual sessions of 1 hour each and 15 group sessions of 2 hours delivered by a licensed psychologist or cognitive behavioural specialist. The sessions included training in relaxation, biofeedback, pain and stress management, cognitive restructuring, problem solving and self‐management 2) Wait‐list control group |
|
| Outcomes | Measures relevant to this review: Fibromyalgia Impact Questionnaire, McGill Pain Questionnaire, Symptom Checklist Revised, Arthritis Self‐Efficacy Scale, Karolinska Sleep Questionnaire Assessment time points: baseline and post‐intervention (no control group data available at 6 months as wait‐list control group used) |
|
| Notes | There was no reference to sources of funding or conflicts of interest declared in the article | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component is included in the sequence generation process used |
| Allocation concealment (selection bias) | Low risk | Computerised random procedure reported to have been used so unlikely investigators would have been able to foresee group assignment but exact details unclear |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | Low risk | Authors confirmed that the outcome assessors were blind to treatment allocation in an e‐mail |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | An 11% total attrition rate evident |
| Selective reporting (reporting bias) | High risk | Data were not reported for social support or the SCL‐90 measures |
Stuifbergen 2010.
| Methods | Randomised controlled trial | |
| Participants | Female participants were recruited through advertisements in local newspapers, websites, physician's offices, community sites and support groups Total participants = 234 randomised (165 completed) Age range = 20 to 75 years Exclusions: diagnosis of fibromyalgia within 6 months, unable to attend 8 week intervention, enrolled in other pain management programmes, pregnant, taking medication for which changes in diet and exercise are contraindicated |
|
| Interventions | 1) Lifestyle intervention. The lifestyle counts intervention was adapted from the Wellness Intervention for Women with Multiple Sclerosis programme, 2 hour group sessions were held weekly for 8 weeks, delivered by a clinical nurse specialist. Sessions included information on health promotion, enhancing self efficacy, stress management, intimacy and personal relationships and engaging participants in individualised goal setting and monitoring 2) The attention control group received received 8 sessions on topics related to disease management including information on medication, secondary outcomes of fibromyalgia, enhancing memory and health insurance |
|
| Outcomes | Measures relevant to this review: Medical Outcomes Study short form health survey (SF36), Self‐rated Abilities for Health Practices scale, Fibromyalgia Impact Questionnaire Assessment time points: baseline, post‐intervention, 3 and 6 month follow‐up |
|
| Notes | This study was supported by the National Institutes of Health, National Institute of Child Health and Human Development, Center for Medical Rehabilitation Research R01HD035047. The authors report no conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component is included in the sequence generation process used |
| Allocation concealment (selection bias) | Low risk | Women within each cohort were randomised using a coin toss witnessed by an office staff member |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | Low risk | The staff member was blinded to the class to which the person was assigned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | A 29% total attrition rate. It was reported that 11 women did not attend the intervention sessions and were excluded from the analysis. Reasons for non‐attendance not presented |
| Selective reporting (reporting bias) | Low risk | All of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
Thieme 2006.
| Methods | Randomised controlled trial | |
| Participants | Consecutive female patients diagnosed with fibromyalgia were recruited from outpatient rheumatology clinics Total participants = 125 randomised (100 completed) Age range = 21 to 67 years Exclusions: diagnosis not in accordance with ACR criteria, pain for < 6 months, not of married status, spouse unwilling or unable to participate, inability to complete the assessment questionnaire and understand the treatment components |
|
| Interventions | 1) Cognitive behaviour therapy was delivered in groups by a psychologist and rheumatologist. 2 hour sessions, held weekly, for 15 weeks. The content of the sessions included a focus on the patient's thinking, problem solving, stress and pain management and relaxation techniques. The sessions were supported by weekly homework tasks 2) The attention placebo group received group discussion sessions that lasted for 2 hours and were held weekly for 15 weeks. Sessions were guided by therapists and discussions focused on medical and psychosocial problems resulting from fibromyalgia |
|
| Outcomes | Measures relevant to this review: Fibromyalgia Impact Questionnaire, West Haven‐Yale Multidimensional Pain Inventory, Pain Related Self Statements Scale Assessment time points: baseline, post‐intervention and 6 month follow up (12 month follow‐up outside timeframe of this review) |
|
| Notes | The study was funded by the Deutsche Forschungsgemeinschaft to KT (Th 899‐1/2 and 899‐2/2 and HF (FL 156/26 Clinical Research Unit), Max‐Planck Award for International Cooperation to HF and the National Institutes of Health/National Institute of Arthritis and Musculo and Skin Diseases to DCT (AR44724 and AR 47298). The authors report no conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It is stated that participants were randomly assigned but no details of random component provided |
| Allocation concealment (selection bias) | Unclear risk | No details of the randomisation procedure used were provided |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | Unclear risk | No details of the blinding of the assessors were provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | A 20% total attrition rate, imbalance between attrition rates between groups for deterioration of symptoms evident in the control group |
| Selective reporting (reporting bias) | Low risk | All of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
Van Santen 2002.
| Methods | Randomised controlled trial | |
| Participants | Female participants were recruited from a registry for rheumatic diseases if they lived within 30 km of either treatment centre. Diagnosis of fibromyalgia was verified according to the ACR criteria before commencement of the study Total participants = 85 randomised (65 completed) Age range = 18 to 60 years Exclusions: male gender, known co‐morbidity and localised myalgia |
|
| Interventions | 1) Multi‐component (relaxation and biofeedback). Biofeedback was delivered in individual, 30 sessions, held twice weekly, for 8 weeks . Participants received feedback on their level of relaxation from electrodes placed on the forehead. All participants received training in progressive muscle relaxation from a psychologist or physiotherapist and were encouraged to practice the technique in between sessions using an audio tape with instructions 2) Usual care 3) The fitness training group data were not included in this review |
|
| Outcomes | Measures relevant to this review: 0 to 100 visual analog scale for pain and fatigue, Arthiritis Impact Measurement Scales, tender points, Symptom Checklist‐90 revised Assessment time‐points: Baseline and post‐intervention |
|
| Notes | The study was funded by the Dutch Arthritis Association | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It is stated "after randomisation" however no details of the random component were provided |
| Allocation concealment (selection bias) | Unclear risk | No details of the randomisation procedure used were provided |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | Low risk | Outcome assessors were blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A 23% total attrition rate |
| Selective reporting (reporting bias) | Low risk | All of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
Vlaeyen 1996.
| Methods | Randomised clinical trial | |
| Participants | Participants meeting the ACR criteria for diagnosis of fibromyalgia were referred from the department of rheumatology at the regional general hospital Total participants N = 131 randomised (67 completed) Age range = 18 to 65 years N = 350 female, N = 50 male Exclusions: illiteracy, pregnancy, substance abuse, involvement in any litigation concerning disability income, medical disorders and diseases making immediate treatment necessary and that may prevent participants performing physical exercise, use of supportive equipment for ambulation and severe psychopathology |
|
| Interventions | 1) Cognitive education consisted of 90 minutes, held twice weekly, over 6 weeks. The treatment aimed at decreasing distorted pain perceptions and increasing self efficacy. Applied relaxation and biofeedback techniques were introduced to participants as part of the skills acquisition phase. Tasks to complete in between sessions were given to participants 2) Educational discussion (attention control). Participants were asked to read parts of a book about pain and then to share the information and their thoughts in group discussion sessions. Participants were also asked to listen to music on audiotapes 3) Wait‐list control |
|
| Outcomes | Measures relevant to this review: McGill Pain Questionnaire, Beck Depression Inventory Assessment time points: baseline, post‐intervention, 6 and 12 month follow‐up |
|
| Notes | The study was funded by the Dutch Prevention Fund, grant number 28‐2055 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It is stated that participants were "randomly assigned" but no details of random component provided |
| Allocation concealment (selection bias) | Unclear risk | No details of the randomisation procedure used were provided |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | Unclear risk | No details of the blinding of the assessors were provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | A 48% total attrition rate at 6 months |
| Selective reporting (reporting bias) | Low risk | All of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
Wang 2010.
| Methods | Randomised clinical trial | |
| Participants | Partiicpants meeting the ACR diagnostic criteria for fibromyalgia were recruited through a tertiary academic hospital Total participants = 66 randomised (59 completed) N = 57 female, N = 9 male Age range = 21 years and over Exclusions: Particpation in tai chi in previous 6 months, serious medical conditions that might preclude participation in the trial, co‐morbid medical conditions known to contribute to fibromyalgia symptoms, pregnancy or plans to become pregnant and cognitive impairment (score ≤ 24) |
|
| Interventions | 1)Tai chi consisted of group sessions delivered by a tai chi master. 60 minute sessions, held twice weekly, for 12 weeks. The theory of tai chi was explained and participants practiced 10 forms from the classic Yang style of tai chi. Each session included a warm up, self massage and breathing techniques. Participants were asked to practice the movements in‐between sessions 2) The education and stretching programme consisted of group sessions. 60 minute sessions, held twice weekly, for 12 weeks. Sessions were delivered by a range of health professionals on topics related to fibromyalgia including coping and problem solving strategies, diet and nutrition, sleep disorders, pain management, exercise and medication. Stretches were also completed within the group sessions and held for 15 to 20 seconds |
|
| Outcomes | Measures relevant to this review: Medical Outcomes Study (SF36), visual analog pain scale 0 to 10, Center for Epidemiologic Studies ‐ Depression scale, Fibromyalgia Impact questionnaire, Pittsburgh Sleep Quality Index. Assessment time points: baseline, post‐intervention and 3 month follow‐up |
|
| Notes | The study was funded by the National Center for Complementary and Alternative Medicine of the National Institutes of Health, the American College of Rheumatology Research and Education Foundation Health Professional Investigator Award and the Boston Claude D. Pepper Older Americans Independence Center Research Career Development Award | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component is included in the sequence generation process used |
| Allocation concealment (selection bias) | Low risk | Three randomisation cycles using computer generated numbers placed in opaque sealed envelopes |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | Low risk | Research staff were unaware of the group assignments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A 10% total attrition rate |
| Selective reporting (reporting bias) | Low risk | All of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
Wicksell 2013.
| Methods | Randomised controlled trial | |
| Participants | Female patients aged between 18 and 55 years old diagnosed with FMS according to the ACR criteria with a weekly average pain score of > 40 (on a scale of 0 to 100) were recruited Total participants = 43 randomised (36 completed) Exclusions: left handed, pregnant and breast feeding participants were excluded |
|
| Interventions | 1) Acceptance and commitment therapy delivered by psychologists and clinicians in a group based format 90 minute sessions, held weekly for 12 weeks 2) Wait‐list control |
|
| Outcomes | Measures relevant to this review: Fibromyalgia Impact Questionnaire, Beck Depression Inventory, State‐trait Anxiety Inventory, Short Form Health Survey Assessment time points: baseline, post‐intervention and 3 months |
|
| Notes | The study was supported by the Swedish Research Council K2009‐53x‐21070‐01‐3, the Stcokholm County Council and the Swedish Rheumatism Association. The authors report no conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component is included in the sequence generation process used |
| Allocation concealment (selection bias) | Low risk | "sealed enveloped with codes for the different study conditions" |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | Unclear risk | No details specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A 10% attrition |
| Selective reporting (reporting bias) | Low risk | All of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
Wigers 1996.
| Methods | Randomised clinical trial | |
| Participants | Participants were recruited through local patient association and a hospital outpatient clinic Total participants = 60 randomised (57 completed) N = 55 female, N = 5 male Age range = 23 to 73 years Exclusions: not meeting ACR criteria for fibromyalgia |
|
| Interventions | 1) Stress management, 90 minute sessions held twice weekly for 6 weeks, followed by once weekly for 8 weeks (30 hours of active treatment) 2) Treatment as usual (aerobic exercise group data not included) |
|
| Outcomes | Measures relevant to this review: 0 to 100 visual analog scales for pain, fatigue, mood and depression Assessment time points: baseline and post‐intervention (4 year follow‐up data not included in this review) |
|
| Notes | The study was supported by the Research Council of Norway 101417/320 and the Norwegian Fibromyalgia Association | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component is included in the sequence generation process used |
| Allocation concealment (selection bias) | Low risk | Randomised by drawing lots |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | Low risk | E‐mail confirmation received from author that outcomes assessors were blind to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A 26% total attrition rate |
| Selective reporting (reporting bias) | Low risk | All of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
Williams 2002.
| Methods | Randomised clinical trial | |
| Participants | Participants who had been in standard medical care for at least 6 months were identified through a patient registry held by a rheumatology clinic specialising in the treatment of fibromyalgia Total participants = 124 randomised (122 completed) N = 130 female and N = 15 men Mean age 47.7 years (SD 11.4) Exclusions: under 18 years of age, severe physical impairment, co‐morbid medical condition causing a worsening in physical functioning, uncontrolled endocrine or allergic disorders, malignancy within 2 years, present psychiatric disorder, current suicide risk or attempt, or substance abuse within 2 years of the study |
|
| Interventions | 1) Cognitive behaviour therapy was delivered in group sessions by a doctoral level clinical psychologist, six one hour sessions, held over 4 weeks. The intervention comprised of information on the gate theory of pain, progressive muscle relaxation, pacing skills, activity scheduling, communication skills and assertiveness training and cognitive restructuring 2) Usual care |
|
| Outcomes | Measures relevant to this review: McGill Pain Questionnaire, Medical Outcomes Study (SF36) Assessment time points: baseline (12 month follow‐up not included in this review) |
|
| Notes | The study was funded by the National Institutes of Health R29MH54877 and DAMD 17‐00‐02‐0018 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detail on randomisation provided |
| Allocation concealment (selection bias) | Unclear risk | No details of the randomisation procedure provided |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | Unclear risk | The questionnaire measures outlined in this study were described as being self assessment tools but it was not clear from manuscript if the questionnaires were indeed self administered by participants or whether they were conducted over the telephone by a researcher where it would be important to know if they were blinded to the treatment allocation of the participant |
| Incomplete outcome data (attrition bias) All outcomes | High risk | A 1% total attrition rate. Means and standard deviations could not be extracted from data provided |
| Selective reporting (reporting bias) | High risk | Findings were provided on both outcome measures (although means and standard deviations could not be extracted from information provided) |
Williams 2010.
| Methods | Randomised clinical trial | |
| Participants | Participants receiving standard care for fibromyalgia were recruited into the study through their treating clinician Total participants = 118 randomised (106 completed) Age range = N = female, N = male Exclusions: no fulfilment of ACR criteria, < 18 years of age, been in receipt of treatment for < 3 months, severe physical impairment, co‐morbid medical condition, psychiatric disorder, receipt of CBT prior to participation in study, pending status with disability compensation or receipt of disability compensation for < 2 years |
|
| Interventions | 1) Cognitive behaviour therapy was delivered using an online resource. The website contained 13 modules encompassing education about fibromyalgia, behavioural and cognitive skills to assist in symptom management. Video lectures, self‐monitoring forms and written summaries were provided 2) Usual care |
|
| Outcomes | Measures relevant to this review: SF36 health survey, Brief Pain Inventory, Multidimensional Fatigue Inventory, Center for Epidemiologic Studies ‐Depression Scale, Medical Outcomes Study Sleep Scale, State‐Trait Anxiety Inventory Assessment time points: baseline and 6 month follow‐up |
|
| Notes | The study was supported by the NIAMS/NIH (R01‐AR050044) and the Department of Defence (DAMD 17‐00‐2‐0018) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component is included in the sequence generation process used |
| Allocation concealment (selection bias) | Low risk | 1:1 ratio. A computerised randomisation program was used |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | Low risk | Outcome assessors were blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A 10% total attrition rate |
| Selective reporting (reporting bias) | Low risk | All of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
Woolfolk 2012.
| Methods | Randomised controlled trial with an additive design | |
| Participants | Participants diagnosed with FMS according to the ACR criteria were referred into the study by their rheumatologist Total participants = 76 randomised (70 completed) Exclusions: pain from traumatic injury, structural or regional disease, rheumatoid arthritis inflammatory arthritis, autoimmune disease, unstable medical or psychiatric illness or active suicidal ideation |
|
| Interventions | 1) Affective cognitive behavioural therapy delivered on an individual basis, 10 sessions were delivered 2) Usual care |
|
| Outcomes | Measures relevant to this review: 10 point visual analog scale, Beck Depression Inventory, Beck Anxiety Inventory, Chronic Pain Efficacy Scale, Medical Outcomes Study SF36 Assessment time points: baseline, post‐intervention and 6 month follow‐up |
|
| Notes | There was no reference to sources of funding or conflicts of interest declared in the article | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random component is included in the sequence generation process used |
| Allocation concealment (selection bias) | Low risk | "Computer generated random number sequence. Neither blocking nor stratification used" |
| Blinding of outcome assessment (detection bias) Questionnaire assessors blind? | Low risk | "Study personnel administering the questionnaires were masked to participant's treatment conditions" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | An 8% attrition |
| Selective reporting (reporting bias) | High risk | Means and standard deviations are not available for the Beck Depression Ivenotry or Beck Anxiety Inventory or for all components of the SF36 |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alvarez‐Nemegyei 2006 | The intervention of providing Ericksonian hypnosis was not deemed to meet the mind and body criteria as participants were not actively engaged in the intervention as the intervention and did not aim to provide a tool for self management |
| Ang 2013b | The exercise component was more substantial than the motivational interviewing component of the intervention and therefore was not deemed to meet the 80% criterion for the intervention to be based on mind‐body principles |
| Arcos‐Carmona 2011 | The relaxation component of the intervention was deemed to be less than the 80% mind‐body focus that was required for inclusion in the review |
| Bieber 2004 | The intervention of providing shared decision making communication programme for clinicians for inclusion as the intervention of providing information was not considered to provide tools or to increase self management directly for patients but as a consultation tool |
| Bieber 2008 | The intervention of providing shared decision making communication programme for clinicians for inclusion as the intervention of providing information was not considered to provide tools or to increase self management directly for patients but as a consultation tool |
| Bosch‐Romero 2002 | The health education sessions were not deemed to meet the 80% criteria for being based on mind and body principles including only two sessions based on mind and body principles |
| Casanueva‐Fernandez 2012 | The intervention was not deemed to meet the 80% criteria for the intervention to be based on mind‐body principles due to the substantial inclusion of massage, thermal therapy and exercise components |
| Castel 2013 | The intervention was based on 50% on cognitive behavioural therapy and 50% on physical therapy and was therefore deemed not to meet the 80% criteria for being based on mind‐body therapy principles |
| Cedraschi 2004 | The intervention programme was not deemed to meet the 80% criteria based on mind‐body principles as 10/22 sessions were based on exercise |
| Gowans 1999 | The intervention of providing exercise and education was not deemed to meet the mind and body criteria as 50% of the intervention was specifically focused on exercise |
| Hochlehnert 2006 | The intervention of providing shared decision making communication programme for clinicians for inclusion as the intervention of providing information was not considered to provide tools or to increase self management directly for patients but as a consultation tool |
| Hunt 2000 | The intervention of providing exercise and education was not deemed to meet the 80% mind and body criteria as there was a substantial focus on exercise |
| King 2002a | The intervention of providing exercise and education was not deemed to meet the 80% mind and body criteria as there was a substantial focus on exercise |
| King 2002b | The intervention was not deemed to meet the 80% mind and body criteria as there was a substantial focus on exercise |
| Kravitz 2006 | The intervention of providing neuro feedback was not deemed to meet the mind and body criteria as participants were not actively engage in the intervention and did not provide a tool for self management |
| Lemstra 2005 | The intervention was not deemed to meet the 80% mind and body criteria as there was a substantial focus on exercise |
| Mannerkorpi 2000 | The intervention was not deemed to meet the 80% mind and body criteria as there was a substantial focus on exercise |
| McBeth 2012 | The intervention was not deemed to meet the 80% mind‐body criteria for intervention content as just under half of the sessions focuses on physical activity |
| McVeigh 2006 | The intervention was not deemed to meet the 80% mind and body criteria as there was a substantial focus on exercise |
| Nelson 2010 | The intervention of low energy neuro feedback was not deemed to meet the mind and body criteria as the participants were not consciously learning to change brain wave activity |
| Van Eijk‐Hustings 2013 | The multidisciplinary programme including psychological, physiological and sociological elements was not deemed to meet the criterion of 80% of the intervention based on mind‐body principles due to a substantial component of physiotherapy |
| van Koulil 2010 | The intervention was not deemed to meet the 80% mind and body therapy criteria as only 50% of the intervention focused on CBT the other 50% focused on exercise |
| Zhang 2009 | The intervention was not deemed to meet the 80% mind and body criteria as there was at lease a 50% focus on massage |
Characteristics of studies awaiting assessment [ordered by study ID]
Thorsell 2011.
| Methods | Randomised controlled trial |
| Participants | Adults participants diagnosed with fibromyalgia (unclear if diagnosis was according to the ACR diagnostic criteria) Total participants N = 115 randomised (55 completed post‐intervention measures) |
| Interventions | 1) Acceptance Commitment Therapy 2 face to face sessions of 90 minutes and 7 30 minutes telephone sessions. Email contact with the therapist was also available 2) Applied relaxation 2 x 90 minutes face to face sessions and 7 weekly sessions of telephone support |
| Outcomes | Measures relevant to this review: Hospital Anxiety and Depression Scale, 10 point Visual Analog Scale Assessment time points: baseline, post‐intervention, 6 and 12 month follow‐up |
| Notes |
Toussaint 2012.
| Methods | Randomised controlled trial |
| Participants | Adults participants diagnosed with fibromyalgia (unclear if diagnosis was according to the ACR diagnostic criteria) Total participants = 57 randomised (21 completed post‐intervention measures) |
| Interventions | 1) Amygdala retraining delivered during a 2.5 hour training programme 2) Usual care including a 1.5 day multi‐disciplinary programme |
| Outcomes | Measures relevant to this review: Multi‐dimensional Fatigue Inventory, Medical Outcomes Study (SF36) Fibromyalgia Impact Questionnaire Assessment time points: baseline and post‐intervention |
| Notes |
Wang 2012.
| Methods | Randomised controlled trial |
| Participants | People diagnosed with fibromyalgia (unclear if diagnosis was according to the ACR diagnostic criteria and the age range of participants was not specified) Total participants (N = 66) |
| Interventions | 1) Yang‐style Tai Chi 2) Wellness education and stretching control |
| Outcomes | Measures relevant to this review: Fibromyalgia Impact Questionnaire, Medical Outcomes Study 36‐item Short Form Health Survey, Visual Analog Scale for pain Assessment time points: baseline to week 24 |
| Notes |
Characteristics of ongoing studies [ordered by study ID]
Garcia‐Campayo 2009.
| Trial name or title | Effectiveness of the psychological and pharmacological treatment of catastrophisation in patients with fibromyalgia |
| Methods | Randomised Controlled Trial |
| Participants | 180 adults (aged 18‐70) diagnosed with fibromyalgia according to the ACR classification criteria for fibromyalgia. To be eligible participants needed to be able to provide informed consent. Total participants = 180 Exclusions: Previous psychological or pharmacological treatment |
| Interventions | 1) Cognitive behaviour therapy delivered in 10 weekly group sessions. 2) Usual care |
| Outcomes | Measures relevant to this review: Hamilton Anxiety Rating Scale, Hamilton Rating Scale for Depression, Fibromyalgia Impact Questionnaire, EuroQol 5D questionnaire. Assessment time‐points: Baseline, post‐intervention, 3 and 6 month follow‐up |
| Starting date | Unknown |
| Contact information | jgarcamp@arrakis.es |
| Notes | The results of this study will be included in the review once completed |
Miles 2010.
| Trial name or title | Effects of gentle yoga versus CBT on fibromyalgia symptoms |
| Methods | Randomised Controlled Trial |
| Participants | Female participants who met the ACR criteria for diagnosis of fibromyalgia |
| Interventions | 1) Gentle yoga 2) Cognitive behaviour therapy |
| Outcomes | Measures relevant to this review: measures not specified but domains include fibromyalgia symptoms, anxiety, depression and self‐efficacy Assessment time‐points: Baseline and post‐intervention |
| Starting date | Unknown |
| Contact information | Not provided |
| Notes | Need to ascertain eligibility of control group. A preliminary report of 10 people has since been published in International Journal of Yoga Therapy 2012. |
Differences between protocol and review
Further refinement of the definition of what constitutes a mind‐body intervention was required to provided clear criteria on what types of interventions would and would not meet the criteria for inclusion in the review.
Outcomes have been presented to facilitate standardisation of outcomes between reviews on fibromyalgia within Cochrane.
Adverse events and withdrawals between groups have been added to the major outcomes to reflect other important potential harmful outcomes of mind‐body interventions.
No studies made reference to early stopping or indicated any variations in intervention delivery, and therefore these other risks of bias were not included in the risk of bias assessment.
Contributions of authors
Alice Theadom has been responsible for co‐ordinating the development of the protocol, assessing eligibility of studies, risk of bias, data extraction and data analysis and writing the draft of the review.
Mark Cropley provided content expertise to the development of the protocol, assessed risk of bias, supported data extraction and contributed to the writing of the review.
Helen Smith contributed her clinical and methodological expertise to the development of the protocol, supported interpretation of the findings and contributed to the writing of the review.
Valery Feigin contributed his clinical and methodological expertise to the interpretation of the analyses and contributed to the writing of the review.
Kathryn McPherson contributed to the interpretation of the analyses and writing of the review.
Sources of support
Internal sources
AUT University, Faculty of Health Student Assistantship Award, New Zealand.
External sources
No sources of support supplied
Declarations of interest
Alice Theadom is a psychologist who specialises in conducting research of psychological interventions in health care.
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
Alda 2011 {published data only}
- Alda M, Luciano JV, Andres E, Serrano‐Blanco A, Rodero B, Lopez del Hoyo Y, et al. Effectiveness of cognitive behaviour therapy for the treatment of catastrophisation in patients with fibromyalgia: a randomised controlled trial. Arthritis Research and Therapy 2011;13(5):R173. [DOI] [PMC free article] [PubMed] [Google Scholar]
Altan 2009 {published and unpublished data}
- Altan L, Korkmaz N, Bingol U, Gunay B. Effect of pilates training on people with fibromyalgia syndrome: a pilot study. Archives of Physical Medicine and Rehabilitation 2009;90:1983‐8. [DOI] [PubMed] [Google Scholar]
Ang 2010 {published and unpublished data}
- Ang DC, Hakr R, Mazzuca S, France CR, Steiner J, Stump T. Cognitive‐behavioral therapy attenuates nociceptive responding in patients with fibromyalgia: a pilot study. Arthritis Care and Research 2010;62(5):618‐23. [DOI] [PubMed] [Google Scholar]
Ang 2013 {published data only}
- And DC, Jensen MP, Steiner JL, Hilligoss JLPN, Gracely RH, Saha C. Combining cognitive‐behavioral therapy and milnacipran for fibromyalgia: a feasibility randomized‐controlled trial. Clinical Journal of Pain 2013;29:747‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Astin 2003 {published and unpublished data}
- Astin JA, Berman BM, Bausell B, Lee WL, Hochberg M, Forys KL. The efficacy of mindfulness meditation plus Gigong movement therapy in the treatment of fibromylagia: a randomized clinical trial. The Journal of Rheumatology 2003;30(10):2257‐62. [PubMed] [Google Scholar]
Babu 2007 {published and unpublished data}
- Babu A, Mathew E, Danda D, Prakash H. Management of patients with fibromyalgia using biofeedback: a randomized control trial. Indian Journal of Medical Sciences 2007;61(8):455‐61. [PubMed] [Google Scholar]
Bakker 1995 {published and unpublished data}
- Bakker C, Linden S, Santen‐Hoeufft M, Bolwijn P, Hidding A. Problem elicitation to assess patient priorities in ankylosing spondylitis and fibromyalgia. The Journal of Rheumatology 1995;22(7):1304‐10. [PubMed] [Google Scholar]
Baumuller 2009 {published data only}
Bojner‐Horwitz 2003 {published data only (unpublished sought but not used)}
- Bojner‐Horwitz E, Theorell T, Anderberg UM. Dance/movement therapy and changes in stress‐related hormones: a study of fibromyalgia patients with video‐interpretation. The Arts in Psychotherapy 2003;30:255‐64. [Google Scholar]
Brattberg 2008 {published and unpublished data}
- Brattberg G. Self‐administered EFT (Emotional Freedom Techniques) in individuals with fibromyalgia: a randomized trial. Integrative Medicine 2008;7(4):30‐5. [Google Scholar]
Burckhardt 1994 {published and unpublished data}
- Burckhardt CS, Mannerkorpi K, Hedenberg L, Bjelle A. A randomized, controlled clinical trial of education and physical training for women with fibromyalgia. The Journal of Rheumatology 1994;21(4):714‐20. [PubMed] [Google Scholar]
Calandre 2009 {published and unpublished data}
- Calandre EP, Rodriquez‐Claro ML, Rico‐Villademoros F, Vilchez JS, Hidalgo J, Delgado‐Rodriquez A. Effects of pool‐based exercise in fibromyalgia symptomatology and sleep quality: a prospective ransomized comparison between stretching and Tai Chi. Clincial and Experimental Rheumatology 2009;27:S21‐8. [PubMed] [Google Scholar]
Carson 2010 {published and unpublished data}
- Carson JW, Carson KM, Jones KD, Bennett RM, Wright CL, Mist SD. A pilot randomized controlled trial of the yoga of awareness program in the management of fibromyalgia. Pain 2010;151:530‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carson 2012 {published and unpublished data}
- Carson JW, Carsib KM, Jones KD, Mist SD, Bennett RM. Follow‐up yoga of awareness for fibromyalgia: Results at 3 months and replication in the wait‐list group. Clinical Journal of Pain 2012;28(9):804‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Castel 2007 {published and unpublished data}
- Castel A, Perez M, Sala J, Padrol A, Rull M. Effect of hypnotic suggestion on fibromyalgic pain: comparison between hypnosis and relaxation. European Journal of Pain 2007;11(4):463‐8. [DOI] [PubMed] [Google Scholar]
Castel 2009 {published and unpublished data}
- Castel A, Salvat M, Sala J, Rull M. Cognitive‐behavioural group treatment with hypnosis: a randomized pilot trial in fibromyalgia. Contemporary Hypnosis 2009;26(1):48‐59. [Google Scholar]
Castel 2012 {published and unpublished data}
- Castel A, Cascon R, Padrol A, Sala J, Rull M. Multicomponent cognitive‐behavioral group therapy with hypnosis for the treatment of fibromyalgia: long‐term outcome. The Journal of Pain 2012;13(3):255‐65. [DOI] [PubMed] [Google Scholar]
Connais 2009 {published and unpublished data}
- Effectiveness of EFT on fibromyalgia. Thesis.
de Souza 2008 {published and unpublished data}
- Souza JB, Bourgault P, Charest J, Marchand S. Interactional school of fibromyalgia: learning to cope with pain ‐ a randomized controlled study. Revista Brasileira de Reumatologia 2008;48(4):218‐25. [Google Scholar]
Edinger 2005 {published data only}
- Edinger JD, Wohlgemuth WK, Krystal AD, Rice JR. Behavioral insomnia therapy for fibromyalgia patients. Archives of Internal Medicine 165;28:2527‐35. [DOI] [PubMed] [Google Scholar]
Falcao 2008 {published and unpublished data}
- Falcao DM, Sales L, Leite JR, Feldman D, Valim V, Natour J. Cognitive behavioral therapy for the treatment of fibromyalgia syndrome: a randomized controlled trial. Journal of Musculoskeletal Pain 2008;16(3):133‐40. [Google Scholar]
Fontaine 2010 {published and unpublished data}
- Fontaine KR, Conn L, Clauw DJ. Effects of lifestyle physical activity on perceived symptoms and physical function in adults with fibromyalgia: results of a randomized trial. Arthritis Research and Therapy 2010;12:R55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fors 2000 {published data only}
- Fors EA, Gotestam KG. Patient education, guided imagery and pain related talk in fibromyalgia coping. European Journal of Psychiatry 2000;14(4):233‐40. [Google Scholar]
Garcia 2006 {published data only}
- Garcia J, Simon MA, Duran M, Canceller J, Aneiros FJ. Differential efficacy of a cognitive‐behavioral intervention versus pharmacological treatment in the management of fibromyalgic syndrome. Psychology, Health and Medicine 2006;11(4):498‐506. [DOI] [PubMed] [Google Scholar]
Gillis 2006 {published and unpublished data}
- Gillis ME, Lumley MA, Mosley‐Williams A, Leisen JCC, Roehrs T. The health effects of at‐home written emotional disclosure in fibromyalgia: a randomized trial. Annals of Behavioral Medicine 2006;32(2):135‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hammond 2006 {published data only}
- Hammond A, Freeman K. Community patient education and exercise for people with fibromyalgia: a parallel group randomized controlled trial. Clinical Rehabilitation 2006;20:835‐46. [DOI] [PubMed] [Google Scholar]
Hamnes 2012 {published data only}
- Hamnes B, Mowinckel P, Kjeken I, Hagen KB. Effects of a one week multidisciplinary inpatient self‐management programme for patients with fibromyalgia: a randomised controlled trial. BMC Musculoskeletal Disorders 2012;13:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
Holmer 2004 {published and unpublished data}
- Holmer ML, Gevirtz R, Spira JL, Greenberg MA. The effects of yoga on symptoms and psychosocial adjustment in fibromyalgia syndrome patients. Conference abstracts from papers presented at the 35th annual meeting. 2004; Vol. 29:302.
Hsu 2010 {published and unpublished data}
- Hsu MC, Schubiner H, Lumley MA, Stracks JS, Clauw DJ, Williams DA. Sustained pain reduction through affective self awareness in fibromyalgia: a randomized controlled trial. Journal of General Internal Medicine 2010;25(10):1064‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jensen 2012 {published data only}
- Jensen KB, Kosek E, Wicksell R, Kemani M, Olsson G, Merle JV, et al. Cognitive behavioral therapy increases pain‐evoked activation of the prefrontal cortex in patients with fibromyalgia. Pain 2012;153:1495‐503. [DOI] [PubMed] [Google Scholar]
Jones 2012 {published and unpublished data}
- Jones KD, Sherman CA, Mist SD, Carson JW, Bennett RM, Li F. A randomized controlled trial of 8‐form tai chi improves symptoms and functional mobility in fibromyalgia patients. Clinical Rheumatology 2012;31:1205‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kayiran 2010 {published and unpublished data}
- Kayiran S, Dursun E, Dursun N, Ermutlu N, Karamursel S. Neurofeedback intervention in fibromyalgia syndrome; a randomized, controlled, rater blind clinical trial. Applied Psychophysiology Biofeedback 2010;35:293‐302. [DOI] [PubMed] [Google Scholar]
Langford 2009 {published and unpublished data}
- Langford MM. The efficacy of a combined cognitive‐behavioural and interpersonal therapy approach to the treatment of fibromyalgia syndrome: a randomized controlled trial. Dissertation.
Lera 2009 {published and unpublished data}
- Lera S, Gelman SM, Lopez M, Abenoza M, Zorrilla JG, Castro‐Fornieles J, et al. Multi‐disciplinary treatment of fibromyalgia: does cognitive behavior therapy increase the response to treatment?. Journal of Psychosomatic Research 2009;67:433‐41. [DOI] [PubMed] [Google Scholar]
Liu 2012 {published and unpublished data}
- Liu W, Zahner L, Cornell M, Le T, Ratner J, Wang Y, et al. Benefit of Qigong exercise in patients with fibromyalgia: a pilot study. International Journal of Neuroscience 2012;122:657‐64. [DOI] [PubMed] [Google Scholar]
Luciano 2011 {published data only}
- Luciano JV, Martinez N, Penarrubia‐Maria MT, Fernandez‐Vergel R, Garcia‐Campayo J, Verduras C, Blanco, et al and the FibroQoL Study Group. Effectiveness of a psychoeducational treatment program implemented in a general practice for fibromyalgia patients. Clinical Journal of Pain 2011;27(5):383‐90. [DOI] [PubMed] [Google Scholar]
Lynch 2012 {published and unpublished data}
- Lynch M, Sawynok J, Hiew C, Marcon D. A randomized controlled trial of qigong for fibromyalgia. Arthritis Research and Therapy 2012;14:R178. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maddali‐Bongh 2010 {published and unpublished data}
- Maddali‐Bongi S, Felice C, Rosso A, Galluccio F, Landi G, Tai G, et al. The efficacy of the Resseguier method in the treatment of fibromyalgia syndrome: a randomised controlled trial. Clinical and Experimental Rheumatology 2010;28:S46‐S50. [PubMed] [Google Scholar]
Mannerkorpi 2004 {published and unpublished data}
- Mannerkorpi K, Arndorw M. Efficacy and feasibility of a combination of body awareness therapy and qigong in patients with fibromyalgia: a pilot study. Journal of Rehabilitation Medicine 2004;36(6):279‐81. [DOI] [PubMed] [Google Scholar]
Martinez‐Valero 2008 {published and unpublished data}
- Martinez‐Valero C, Castel A, Capafons A, Sala J, Espejo B, Cardena E. Hypnotic treatment synergizes the psychological treatement of fibromyalgia: a pilot study. American Journal of Clinical Hypnosis 2008;50(4):311‐21. [DOI] [PubMed] [Google Scholar]
Menzies 2006 {published and unpublished data}
- Menzies V, Taylor AG, Bourguignon C. Effects of guided imagery on outcomes of pain, functional status, and self efficacy in persons diagnosed with fibromyalgia. The Journal of Alternative and Complementary Medicine 2006;12(1):23‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Miro 2011 {published and unpublished data}
- Miro E, Lupianez J, Martinez MP, Snachez AI, Diaz‐Piedra C, Guzman MA, et al. Cognitive‐behavioral therapy for insomnia improves attention function in fibromyalgia syndrome: a pilot randomized controlled trial. Journal of Health Psychology 2011;16(5):770‐82. [DOI] [PubMed] [Google Scholar]
Oliver 2001 {published and unpublished data}
- Oliver K, Cronan TA, Walen HR, Tomita M. Effects of social support and education on health care costs for patients with fibromyalgia. The Journal of Rheumatology 2001;28(12):2711‐9. [PubMed] [Google Scholar]
Oneva‐Zafra 2010 {published and unpublished data}
- Oneva‐Zaffra MD, Castro‐Sanchez AM, Mataran‐Penarrocha GA, Moreno‐Lorenzo C. Effect of music as nursing intervention for people diagnosed with fibromyalgia. Pain Management 2010;14(2):e39‐46. [DOI] [PubMed] [Google Scholar]
Parra‐Delgado 2013 {published and unpublished data}
- Parra‐Delgado M, Latorre‐Postigo JM. Effectiveness of mindfulness‐based cognitive therapy in the treatment of fibromyalgia: a randomised trial. Cognitive Therapy Research 2013;37:1015‐26. [Google Scholar]
Picard 2013 {published data only}
- Picard P, Jusseaume C, Boutet M, Duale C, Mulliez A, Aublet‐Cuvellier B. Hypnosis for management of fibromyalgia. International Journal of Clinical and Experimental Hypnosis 2013;61(1):111‐23. [DOI] [PubMed] [Google Scholar]
Riedel 2012 {published data only}
- Riedel SL, Bluefield WV. Effects of guided imagery in persons with fibromyalgia. Electronic thesis 2012.
Scheidt 2013 {published and unpublished data}
- Scheidt CE, Waller E, Endorf K, Schmidt S, Konig R, Zeeck A, et al. Is brief psychodynamic psychotherapy in primary fibromyalgia syndrome with concurrent depression an effective treatment? A randomized controlled trial. General Hospital Psychiatry 2013;35:160‐7. [DOI] [PubMed] [Google Scholar]
Schmidt 2011 {published data only}
- Schmidt S, Grossman P, Schwarzer B, Jena S, Naumann J, Walach H. Treating fibromyalgia with mindfulness‐based stress reduction: results from a 3‐armed randomized controlled trial. Pain 2010;152(2):361‐9. [DOI] [PubMed] [Google Scholar]
Sephton 2007 {published and unpublished data}
- Sephton SE, Salmon P, Weissbecker I, Ulmer C, Floyd A, Hoover K, et al. Mindfulness meditation alleviates depressive symptoms in women with fibromyalgia: results of a randomized clinical trial. Arthritis Care and Research 2007;15:77‐85. [DOI] [PubMed] [Google Scholar]
- Weissbecker I, Salmon P, Studts JL, Floyd AR, Dedert EA, Sephton SE. Mindfulness‐based stress reduction and sense of coherence among women with fibromyalgia.. Jourrnal of Clinical Psychology in Medical Settings 2002;9(4):297‐305. [Google Scholar]
Soares 2002 {published and unpublished data}
- Soares JJF, Grossi G. A randomized, controlled comparison of educational and behavioural interventions for women with fibromyalgia. Scandinavian Journal of Occupational Therapy 2002;9(1):35‐45. [Google Scholar]
Stuifbergen 2010 {published and unpublished data}
- Stuifbergen AK, Blozis SA, Becker H, Phillips L, Timmerman G, Kullberg V, et al. A randomized controlled trial of a wellness intervention for women with fibromyalgia syndrome. Clinical Rehabilitation 2010;24:305‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thieme 2006 {published data only}
- Thieme K, Flor H, Turk DC. Psychological pain treatment in fibromyalgia syndrome: efficacy of operant behavioural and cognitive behavioural treatments. Arthritis Research and Therapy 2006;8:R121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Santen 2002 {published and unpublished data}
- Santen M, Bolwijn P, Verstappen F, Bakker C, Hidding A, Houben H, et al. A randomized clinical trial comparing fitness and biofeedback training versus basic treatment in patients with fibromyalgia. Journal of Rheumatology 2002;29(3):575‐81. [PubMed] [Google Scholar]
Vlaeyen 1996 {published data only}
- Vlaeyen JWS, Teeken‐Gruben NJG, Goosens MEKB, Rutten van Molken MPMH, Pelt RAGB, Eek H, et al. Cognitive ‐educational treatment of fibromyalgia: a randomized clinical trial. 1. Clinical Effects. The Journal of Rheumatology 1996;23(7):1237‐45. [PubMed] [Google Scholar]
Wang 2010 {published and unpublished data}
- Wang C, Schmid CH, Rones R, Kalish R, Yinh J, Goldenberg DL, et al. A randomized trial of Tai Chi for fibromyalgia. The New England Journal of Medicine 2010;363:743‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wicksell 2013 {published and unpublished data}
- Wicksell RK, Kemani M, Jensen K, Kosek E, Kadetoff D, Sorjonen K, et al. Acceptance and commitment therapy for fibromyalgia: a randomized controlled trial. European Journal of Pain 2012;17:599‐611. [DOI] [PubMed] [Google Scholar]
Wigers 1996 {published and unpublished data}
- Wigers SH, Stiles TC, Vogel PA. Effects of aerobic exercise versus stress management treatment in fibromyalgia. Scandinavian Journal of Rheumatology 1996;25(2):77‐86. [DOI] [PubMed] [Google Scholar]
Williams 2002 {published data only}
- Williams DA, Cary MA, Groner KH, Chaplin W, Glazier LJ, Rodriquez AM, et al. Improving physical functional status in patients with fibromyalgia: a brief cognitive behavioral intervention. The Journal of Rheumatology 2002;29(6):1280‐6. [PubMed] [Google Scholar]
Williams 2010 {published and unpublished data}
- Williams DA, Kuper D, Segar M, Mohan N, Sheth M, Clauw DA. Internet‐enhanced management of fibromyalgia: a randomized controlled trial. Pain 2010;151(3):694‐702. [DOI] [PMC free article] [PubMed] [Google Scholar]
Woolfolk 2012 {published and unpublished data}
- Woolfolk RL, Allen LA, Apter JT. Affective‐cognitive behavioral therapy for fibromyalgia: a randomized controlled trial. Pain Research and Treatment 2012;2012:937873. [DOI: 10.1155/2012/937873] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Alvarez‐Nemegyei 2006 {published data only}
- Alvarez‐Nemegyei J, Negreros‐Castillo A, Nuno‐Gutierrez BL, Alvarez‐Berzunza J, Alcocer‐Martinez LM. Efficacy of Eriksonian hypnosis in women with fibromyalgia syndrome [Eficacia de la hipnosis ericksoniana en el sindrome de fibromialgia en mujeres]. Revista Medica del Instituto Mexicano del Seguro Social 2006;45(4):395‐401. [PubMed] [Google Scholar]
Ang 2013b {published data only}
- Ang DC, Kaleth AS, Bigatti S, Mazzuca SA, Jensen MP, Hilligoss J, et al. Research to encourage exercise for fibromyalgia (REEF): use of motivational interviewing, outcomes from a randomized‐controlled trial. Clinical Journal of Pain 2013;29(4):296‐304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Arcos‐Carmona 2011 {published data only}
- Arcos‐Carmona. Effects of aerobic exercise program and relaxation techniques on anxiety, quality of sleep, depression, and quality of life in patients with fibromyalgia: a randomized controlled trial. Medicina Clinica 2011;137(9):398‐401. [DOI] [PubMed] [Google Scholar]
Bieber 2004 {published data only}
- Bieber C, Müller KG, Blumenstiel K, Schuller‐Roma B, Richter A, Hochlehnert A, et al. Shared decision making (SDM) with chronic pain patients. The patient as a partner in the medical decision making process. Bundesgesundheitsblatt, Gesundheitsforschung, Gesundheitsschutz 2004;47(10):985‐91. [DOI] [PubMed] [Google Scholar]
Bieber 2008 {published data only}
- Bieber C, Müller KG, Blumenstiel K, Hochlehnert A, Wilke S, Hartmann M, et al. A shared decision‐making communication training program for physicians treating fibromyalgia patients: effects of a randomized controlled trial. Journal of Psychosomatic Research 2008;64(1):13‐20. [DOI] [PubMed] [Google Scholar]
Bosch‐Romero 2002 {published data only}
- Bosch Romero PE, Saenz Moya N, Vallis Esteve M, Vinolas Valer S. Study of quality of life of patients with fibromyalgia: impact of a health education programme. Atencion Primaria 2002;30:16‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Casanueva‐Fernandez 2012 {published data only}
- Casanueva‐Fernandez B, Llorca J, Blanch i Rubio J, Rodero‐Fernandez B, Gonzalez‐Gay MA. Efficacy of a multidisciplinary treatment program in patients with severe fibromyalgia. Rheumatology International 2012;32:2497‐502. [DOI] [PubMed] [Google Scholar]
Castel 2013 {published data only}
- Castel A, Fontova R, Montull S, Perinan R, Poveda MJ, Miralles I, et al. Efficacy of a multi‐disciplinary fibromyalgia treatment adapted for women with low educational levels: a randomized controlled trial. Arthritis Care and Research 2013;65(3):421‐31. [DOI] [PubMed] [Google Scholar]
Cedraschi 2004 {published data only}
- Cedraschi C, Desmeules J, Rapiti E, Baumgartner E, Cohen P, Finckh A, et al. Fibromyalgia: a randomised, controlled trial of a treatment programme based on self management. Annals of the Rheumatic Diseases 2004;63(3):290‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gowans 1999 {published data only}
- Gowans SE, deHueck A, Voss S, Richardson M. A randomized, controlled trial of exercise and education for individuals with fibromyalgia. Arthritis Care & Research 1999;12(2):120‐8. [DOI] [PubMed] [Google Scholar]
Hochlehnert 2006 {published data only}
- Hochlehnert A, Richter A, Bludau HB, Bieber C, Blumenstiel K, Mueller K, et al. A computer‐based information‐tool for chronic pain patients. Computerized information to support the process of shared decision‐making. Patient Education and Counseling 2006;61(1):92‐8. [DOI] [PubMed] [Google Scholar]
Hunt 2000 {published data only}
- Hunt J, Bogg J. An evaluation of the impact of a fibromyalgia self‐management programme on patient morbidity and coping. Advances in Physiotherapy 2000;2(4):168‐75. [Google Scholar]
King 2002a {published data only}
- King SJ, Wessel J, Bhambhani Y, Sholter D, Maksymowych W. The effects of exercise and education, individually or combined, in women with fibromyalgia. The Journal of Rheumatology 2002;29(12):2620‐7. [PubMed] [Google Scholar]
King 2002b {published data only}
- King SJ, Wessel J, Bhambhani Y, Sholter D, Maksymowych W. Predictors of success of intervention programs for persons with fibromyalgia. The Journal of Rheumatology 2002;29(5):1034‐40. [PubMed] [Google Scholar]
Kravitz 2006 {published data only}
- Kravitz HM, Esty ML, Katz RS, Fawcett J. Treatment of fibromyalgia syndrome using low‐intensity neurofeedback with the Flexyx Neurotherapy System: a randomized controlled clinical trial. Journal of Neurotherapy 2006;10(2‐3):41‐58. [Google Scholar]
Lemstra 2005 {published data only}
- Lemstra M, Olszynski WP, Lemstra M. The effectiveness of multidisciplinary rehabilitation in the treatment of fibromyalgia: a randomized controlled trial. Clinical Journal of Pain 2005;21(2):166‐74. [DOI] [PubMed] [Google Scholar]
Mannerkorpi 2000 {published data only}
- Mannerkorpi K, Nyberg B, Ahlmén M, Ekdahl C. Pool exercise combined with an education program for patients with fibromyalgia syndrome. A prospective, randomized study. Journal of Rheumatology 2000;27(10):2473‐81. [PubMed] [Google Scholar]
McBeth 2012 {published data only}
- McBeth J, Prescott G, Scotland G, Lovell K, Keeley P, Hannaford P, et al. Cognitive behaviour therapy, exercise or both for treating chronic widespread pain. Archives of Internal Medicine 2012;172(1):48‐57. [DOI] [PubMed] [Google Scholar]
McVeigh 2006 {published data only}
- McVeigh JG, Hurley DA, Basford JR, Sim J, Baxter GD, Finch MB. Effectiveness of a combined pool‐based exercise and education programme compared to usual medical care in fibromyalgia syndrome: a randomised, controlled trial. Physical Therapy Reviews 2006;11(3):217. [Google Scholar]
Nelson 2010 {published data only}
- Nelson DV, Bennett RM, Barkhuizen A, Sexton GJ, Jones KD, Esty ML, et al. Neurotherapy of fibromyalgia?. Pain Medicine 2010;11(6):912‐9. [DOI] [PubMed] [Google Scholar]
Van Eijk‐Hustings 2013 {published data only}
- Eijk‐Hustings Y, Kroese M, Tan F, Boonen A, Bessems‐Beks M, Landewe R. Challenges in demonstrating the effectiveness of multidisciplinary treatment on quality of life, participation and health care utilisation in patients with fibromyalgia: a randomised controlled trial. Clinical Rheumatology 2013;32:199‐209. [DOI] [PubMed] [Google Scholar]
van Koulil 2010 {published data only}
- Koulil S, Lankveld W, Kraaimaat FW, Helmond T, Vedder A, Hoorn H, et al. Tailored cognitive‐behavioral therapy and exercise training for high‐risk patients with fibromyalgia. Arthritis Care & Research 2010;62(10):1377‐85. [DOI] [PubMed] [Google Scholar]
Zhang 2009 {published data only}
- Zhang. Influence of psychological intervention on therapeutic effects of massage therapy for fibromyalgia syndrome. Journal of Guangzhou University of Traditional Chinese Medicine 2009; Vol. 26, issue 1:20‐3.
References to studies awaiting assessment
Thorsell 2011 {published and unpublished data}
- Thorsell J, Finnes A, Dahl JA, Lundgren T, Gybrant M, Gordh T, et al. A comparative study of 2 manual‐based self‐help interventions, acceptance and commitment therapy and applied relaxation for persons with chronic pain. Clinical Journal of Pain 2011;27(8):716‐23. [DOI] [PubMed] [Google Scholar]
Toussaint 2012 {published and unpublished data}
- Toussaint LT, Whipple MO, Abboud LL, Vincent A, Wahner‐Roedler DL. A mind‐body technique for symptoms related to fibromyalgia and chronic fatigue. Explore 2012;8(2):92‐8. [DOI] [PubMed] [Google Scholar]
Wang 2012 {published and unpublished data}
- Wang C. Cost‐effectiveness of tai chi mind‐body exercise for the treatment of fibromyalgia. Explore Meeting. 2013.
References to ongoing studies
Garcia‐Campayo 2009 {published data only}
- Garcia‐Campayo J, Serrano‐Blanco A, Rodero B, Magallon R, Alda M, Andres E, et al. Effectiveness of the psychological and pharmacological treatment of catastrophization in patients with fibromyalgia: a randomized controlled trial. Trials 2009;10(24):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Miles 2010 {published data only}
- Miles. Effects of gentle yoga versus CBT on fibromyalgia symptoms. APA Convention Presentation. 2010:Topic 36.5.
Additional references
Altman 1996
- Altman DG, Bland JM. Detecting skewness from summary information. BMJ 1996;313:1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Altman 2000
- Altman DG. Statistics in medical journals: some recent trends. Statistics in Medicine 2000;19(23):3275‐89. [DOI] [PubMed] [Google Scholar]
Anderson 1995
- Anderson KO, Dowds BN, Pelletz RE, Edwards WT, Peeters‐Asdourian C. Development and initial validation of a scale to measure self‐efficacy beliefs in patients with chronic pain. Pain 1995;63(1):77‐84. [DOI] [PubMed] [Google Scholar]
Arnold 2011
- Arnold LM, Clauw DJ, McCarberg BH. Improving the recognition and diagnosis of fibromyalgia. Mayo Clinic Proceedings 2011;86(5):457‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bennett 2007
- Bennett RM, Jones J, Turk DC, Russell IJ, Matallana L. An Internet survey of 2,596 people with fibromyalgia. BMC Musculoskeletal Disorders. 2007;8(27):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bennett 2009
- Bennett RM, Friend R, Jones KD, Ward R, Han BK, Ross RL. The Revised Fibromyalgia Impact Questionnaire (FIQR): validation and psychometric properties. Arthritis Research and Therapy 2009;11(4):R120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berger 2007
- Berger A, Dukes E, Martin S, Edelsberg J, Oster G. Characteristics and healthcare costs of patients with fibromyalgia syndrome. International Journal of Clinical Practice 2007;61(9):1498‐508. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bergman 2005
- Bergman S. Psychosocial aspects of chronic widespread pain and fibromyalgia. Disability and Rehabilitation 2005;27(12):675‐83. [DOI] [PubMed] [Google Scholar]
Bernardy 2011
- Bernardy K, Füber N, Klose P, Häuser W. Efficacy of hypnosis/guided imagery in fibromyalgia syndrome – a systematic review and meta‐analysis of controlled trials. BMC Musculoskeletal Disorders 2011 Jun 15;12:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bernardy 2013
- Bernardy K, Klose P, Busch AJ, Choy EHS, Hauser W. Cognitive behavioural therapies for fibromyalgia. Cochrane Database of Systematic Reviews 2013, Issue 9. [DOI: 10.1002/14651858.CD009796] [DOI] [PMC free article] [PubMed] [Google Scholar]
Branco 2010
- Branco JC, Bannworth B, Failde I, Abello Carbonell J, Blotman F, Spaeth M, et al. Prevalence of fibromyalgia: a survey in five European countries. Seminars in Arthritis and Rheumatism 2010;39(6):448‐53. [DOI: 10.1016/j.semarthrit.2008.12.003] [DOI] [PubMed] [Google Scholar]
Burckhardt 1991
- Burckhardt CS, Clark SR, Bennett RM. The fibromyalgia impact questionnaire: development and validation. The Journal of Rheumatology 1991;18:728‐33. [PubMed] [Google Scholar]
Busch 2007
- Busch Aj, Barber KA, Overend TJ, Peloso PM, Schacter CL. Exercise for treating fibromyalgia syndrome. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD003786.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Busch 2013
- Busch A, Webber SC, Richards RS, Bidonde J, Schacter CL, Schafer LA, et al. Resistance exercise training for fibromyalgia. Cochrane Database of Systematic Reviews 2013, Issue 12. [DOI: 10.1002/14651858.CD010884] [DOI] [PMC free article] [PubMed] [Google Scholar]
Buysse 1989
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Research 1989;28(2):193‐213. [DOI] [PubMed] [Google Scholar]
Ceko 2011
- Ceko M, Bushnell MC, Gracely RH. Neurobiology underlying fibromyalgia symptoms. Pain Research and Treatment 2011;2012:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chan 2012
- Chan CL, Wang CW, Ho RT, Ng SM, Ziea ET, Wong VT. Qigong exercise for the treatment of fibromyalgia: a systematic review of randomized controlled trials. Journal of Alternative and Complementary Medicine 2012;18(7):641‐6. [DOI] [PubMed] [Google Scholar]
Choy 2009
- Choy EH, Mease PJ. Key symptom domains to be assessed in fibromyalgia (outcome measures in rheumatoid arthritis clinical trials). Rheumatology Diseases Clinics of North America 2009;35(2):329‐37. [DOI] [PubMed] [Google Scholar]
Clauw 2011
- Clauw DJ, Arnold LM, McCarberg BH. The science of fibromyalgia. Mayo Clinic Proceedings 2011;86(9):907‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dworkin 2003
- Dworkin RH, Corbin AE, Young JP, Sharma U, LaMoreaux L, Bockbrader H, et al. Pregabalin for the treatment of postherpetic neuralgia. Neurology 2003;60:1274‐83. [DOI] [PubMed] [Google Scholar]
Farrar 2001
- Farrar JT, Young JP, LaMoreaux L, Werth JL, Poole M. Clinical importance of changes in chronic pain intensity measured on an 11 point numerical pain rating scale. Pain 2001;94:149‐58. [DOI] [PubMed] [Google Scholar]
Fewtrell 2008
- Fewtrell MS, Kennedy K, Singhal A, Martin RM, Ness A, Hadders‐Algra M, et al. How much loss to follow‐up isacceptable in long‐term randomised trials and prospective. Archives of Disease in Childhood 2008;93(6):458‐61. [DOI] [PubMed] [Google Scholar]
Glombiewski 2013
- Glombiewski JA, Bernardy K, Häuser W. Efficacy of EMG‐ and EEG‐Biofeedback in Fibromyalgia Syndrome: A Meta‐Analysis and a Systematic Review of Randomized Controlled Trials. Evidence‐Based Complementary and Alternative Medicine 2013;2013(962741):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hadhazy 2000
- Hadhazy VA, Ezzo J, Creamer P, Berman BM. Mind‐body therapies for the treatment of fibromyalgia. A systematic review. Journal of Rheumatology 2000;27(12):2911‐8. [PubMed] [Google Scholar]
Hauser 2010
- Hauser W, Thieme K, Turk DC. Guidelines on the management of fibromyalgia syndrome ‐ a systematic review. European Journal of Pain 2010;14(1):5‐10. [DOI: 10.1016/j.ejpain.2009.01.006] [DOI] [PubMed] [Google Scholar]
Hauser 2013
- Hauser W, Urrutia, Tort, Uceyler N, Walitt B. Serotonin and noradrenaline reuptake inhibitors (SNRIs) for fibromyalgia syndrome. Cochrane Database of Systematic Reviews 2013, Issue 1. [DOI: 10.1002/14651858.CD010292] [DOI] [PubMed] [Google Scholar]
Hays 1993
- Hays RD, Sherbourne CD. The MOS 36‐item short‐form health survey 1.0. Health Economics 1993;2(3):217‐27. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (Editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration. Available from www.cochrane‐handbook.org 2011.
Lau 2006
- Lau J, Loannidis JPA, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ 2006;16(7568):597–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lauche 2013
- Lauche R, Cramer H, Dobos G, Langhorst J, Schmidt S. A systematic review and meta‐analysis of mindfulness‐based stress reduction for the fibromyalgia syndrome. Journal of Psychosomatic Research 2013;75(6):500‐10. [DOI] [PubMed] [Google Scholar]
MacFarlane 2002
- Macfarlane TV, Blinkhorn A, Worthington HV, Davies RM, Macfarlane GJ. Sex hormonal factors and chronic widespread pain: a population study among women. Rheumatology 2002;41(4):454‐7. [DOI] [PubMed] [Google Scholar]
Mease 2005
- Mease P. Fibromyalgia syndrome: review of clinical presentation, pathogenesis, outcome measures and treatment. The Journal of Rhematology 2005;32(75):6‐21. [PubMed] [Google Scholar]
Moore 2009
- Moore RA, Straube S, Wiffen PJ, Derry S, McQuay HJ. Pregabalin for acute and chronic pain in adults. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD007076.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2011
- Moore, RA. Wiffen PJ. Derry S. McQuay HJ. Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database of Systematic Reviews 2011, Issue 3. [DOI: 10.1002/14651858.CD007938.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCCAM 2005
- National Center for Complementary and Alternative Medicine. Mind‐Body Medicine: an o. National Institutes of Health, US Department of Health and Human Services 2005.
NCCAM 2012
- National Center for Complementary and Alternative Medicine. Mind–body Medicine: an overview. http://nccam.nih.gov/health/backgrounds/mindbody.htm 2012.
Nicassio 2002
- Nicassio PM, Moxham EG, Schuman EC, Gevirtz RN. The contribution of pain, reported sleep quality, and depressive symptoms to fatigue in fibromyalgia. Pain 2002;100:271‐9. [DOI] [PubMed] [Google Scholar]
Puhan 2006
- Puhan MA, Soesilo I, Guyatt GH, Schunemann HJ. Combining scores from different patient reported outcome measures in meta‐analyses: when is it justified?. Health and Quality of Life Outcomes 2006;4:94. [DOI: 10.1186/1477-7525-4-94] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sicras‐Mainar 2009
- Sicras‐Mainar A, Rejas J, Navarro R, Blanca M, Morcillo A, Larios R, et al. Treating patients with fibromyalgia in primary care settings under routine medical practice: a claim database cost and burden of illness study. Arthritis Research and Therapy 2009;11(2):R54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smets 1995
- Smets EM, Garssen B, Bonke B, Haes JC. The multidimensional fatigue inventory (MFI) psychometric qualities of an instrument to assess fatigue. Journal of Psychosomatic Research 1995;39(3):315‐25. [DOI] [PubMed] [Google Scholar]
Theadom 2008
- Theadom A, Cropley M. Dysfunctional beliefs, stress and sleep disturbance in fibromyalgia. Sleep Medicine 2008;9(4):376‐81. [DOI] [PubMed] [Google Scholar]
Vitetta 2005
- Vitetta L, Anton B, Cortizo F, Sali A. Mind–body medicine: stress and its impact on overall health and longevity. Annals of the New York Academy of Sciences 2005;1057:492‐505. [DOI] [PubMed] [Google Scholar]
Wahbeh 2008
- Wahbeh H, Elsas SM, Oken BS. Mind‐body interventions. Neurology 2008;70(24):2321‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wells 2010
- Wells RE, Phillips RS, Schachter SC, McCarthy EP. Complementary and alternative medicine among US adults with common neurological conditions. Journal of Neurology 2010;257(11):1822‐31. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
White 1999
- White KP, Speechley M, Harth M, Ostbye T. The London fibromyalgia epidemiology study: the prevalence of fibromyalgia syndrome in London, Ontario. The Journal of Rheumatology 1999;26(7):1570‐6. [PubMed] [Google Scholar]
Williams 2012
- Williams AC, Eccleston C, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD007407] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wolfe 1990
- Wolfe F, Smythe A, Yunus M. The College of Rheumatology 1990 criteria for the classification of fibromyalgia. Arthritis and Rheumatism 1990;33:160‐72. [DOI] [PubMed] [Google Scholar]
Wolfe 1995
- Wolfe F, Ross K, Anderson J, Russell IJ, Herbert L. The prevalence and characteristics of fibromyalgia in the general population. Arthritis and Rheumatism 1995;38:19‐28. [DOI] [PubMed] [Google Scholar]
Wolfe 2010
- Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease PJ, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care & Research 2010;62(5):600‐10. [DOI] [PubMed] [Google Scholar]
Wolfe 2011
- Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Hauser W, Katz RS, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. The Journal of Rheumatology. 2011;38(6):1113‐22. [DOI] [PubMed] [Google Scholar]
Wolfe 2011b
- Wolfe F. How to use the new American College of Rheumatology fibromyalgia diagnostic criteria. Arthritis Care & Research 2011;63(7):1073‐4. [DOI] [PubMed] [Google Scholar]
Yunus 2001
- Yunus MB. The role of gender in fibromyalgia syndrome. Current Rheumatology Reports 2001;3(2):128‐34. [DOI] [PubMed] [Google Scholar]
Zigmond 1983
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica 1983;67(6):361‐70. [DOI] [PubMed] [Google Scholar]


