Abstract
Hearing loss is one of the most common symptoms of neurofibromatosis type 2 (NF2) caused by vestibular schwannomas (VSs). Fibrosis in the VS tumor microenvironment (TME) is associated with hearing loss in patients with NF2. We hypothesized that reducing the fibrosis using losartan, an FDA-approved antihypertensive drug that blocks fibrotic and inflammatory signaling, could improve hearing. Using NF2 mouse models, we found that losartan treatment normalized the TME by (i) reducing neuroinflammatory IL-6/STAT3 signaling and preventing hearing loss, (ii) normalizing tumor vasculature and alleviating neuro-edema, and (iii) increasing oxygen delivery and enhancing efficacy of radiation therapy. In preparation to translate these exciting findings into the clinic, we used patient samples and data and demonstrated that IL-6/STAT3 signaling inversely associated with hearing function, that elevated production of tumor-derived IL-6 was associated with reduced viability of cochlear sensory cells and neurons in ex vivo organotypic cochlear cultures, and that patients receiving angiotensin receptor blockers have no progression in VS-induced hearing loss compared with patients on other or no antihypertensives based on a retrospective analysis of patients with VS and hypertension. Our study provides the rationale and critical data for a prospective clinical trial of losartan in patients with VS.
INTRODUCTION
Neurofibromatosis type 2 (NF2) is a dominantly inherited neoplasia syndrome resulting from germline mutation of the NF2 tumor suppressor gene (1). The hallmark of NF2 is bilateral vestibular schwannomas (VSs), which progressively enlarge leading to sensorineural hearing loss (SNHL) that translates to social impairment and clinical depression (2). VSs can cause brainstem compression resulting in severe morbidity and mortality (3). Standard treatments for growing VSs include surgery and radiation therapy (RT); however, both carry the risk of nerve damage that can result in deafness, facial palsy, facial numbness, stroke, and even death (4–6). No drug is currently U.S. Food and Drug Administration (FDA)–approved to treat VS or the associated hearing loss. Treatment with bevacizumab, a humanized monoclonal antibody that specifically recognizes vascular endothelial growth factor A (VEGF-A), has been shown to improve hearing in some patients (7). However, not all patients with NF2 with hearing loss respond to bevacizumab monotherapy, and even in those who respond, the effect was not durable. Furthermore, bevacizumab may induce serious adverse effects, such as hypertension and proteinuria (8), rendering some patients unable to tolerate long-term bevacizumab treatment. The development of more effective therapies with enhanced efficacy on hearing preservation and minimal toxicity is urgently needed for VS.
The greatest barrier to managing NF2-related auditory impairment is our incomplete understanding of how schwannomas cause hearing loss. Mechanisms that lead to hearing loss are multifactorial, including tumor-mediated mechanical compression of the cochlear nerve, ischemia to the hearing apparatus due to impaired cochlear blood flow, intratumoral bleeding, tumor-secreted ototoxic molecules and extracellular vesicles, intrinsic differences in genetic landscape of tumors associated with good versus poor hearing, and inflammation and NLRP3 inflammasome activation within the tumor microenvironment (TME) (9). A previous study of 274 patients with VS showed that hemorrhage-related tumor fibrosis correlates with hearing loss (10). Fibrosis is due to the excessive deposition of extracellular matrix (ECM) components, such as collagen and hyaluronan (HA), and is also a well-known sequela of RT-induced tissue damage (11). In highly desmoplastic malignant cancers, tumor-associated fibrosis contributes to high solid stress—a physical force exerted by cancer cells, stromal cells, and the dense ECM (12–16). Because tumor blood vessels are structurally abnormal, they collapse under this high pressure, resulting in reduced blood flow that impairs drug and oxygen delivery and fuels a hypoxic TME (12, 14, 17). Hypoxic cells are more aggressive and more resistant to RT and chemotherapeutics, which require oxygen to be effective (18, 19). The role of the TME in nonmalignant tumor progression and response to therapy, such as NF2 VS, is less well studied. Whether reducing baseline and RT-induced fibrosis can prevent tumor-induced hearing loss is not known.
In this study, we used losartan—a widely prescribed, FDA-approved antihypertensive drug that blocks fibrogenic and inflammatory angiotensin signaling—to reprogram the VS TME. We showed that losartan prevented tumor-induced hearing loss in a mouse model of VS by (i) decreasing inflammatory [interleukin-6 (IL-6)/signal transducer and activator of transcription 3 (STAT3) and Toll-like receptor 4 (TLR4)] signaling in macrophages and (ii) reducing nerve edema via normalizing the tumor vasculature. By analyzing 74 VS samples from patients with different hearing ability, we found that IL-6/STAT3 signaling inversely associated with hearing function and viability of cochlear sensorineural cells. Furthermore, our retrospective analysis revealed that patients with VS who have hypertension and are treated with angiotensin receptor blockers (ARBs) have no progression in VS-induced hearing loss compared with patients on other or no antihypertensives. To improve NF2 therapy, we show that by normalizing the tumor vasculature and alleviating tumor hypoxia, adding losartan to RT enhanced the treatment outcome and reduced the dose of RT needed to control tumor growth, in both syngeneic VS models and in a patient-derived VS xenograft model. Our study provides rationale and critical data for the clinical evaluation of the effect of losartan on hearing function and combining losartan with RT in patients with VS.
RESULTS
Losartan treatment reduces matrix content, improves vessel perfusion, and decreases hypoxia in schwannoma models
First, in archived human VS samples, we confirmed that matrix molecules, collagen I and HA, are abundantly expressed in schwannomas. Collagen I is the most abundant collagen in tumor ECM, and HA is a glycoprotein; both molecules play an important structural role in the tumor-associated ECM (20). We also confirmed that (i) angiotensinogen (AGT), a precursor molecule for angiotensin II (AngII), and (ii) AngII receptor 1 (AT1), the target of losartan, are expressed in patient VS tissues (Fig. 1A) as well as in Nf2−/− and SC4 mouse schwannoma cell lines (fig. S1A).
Fig. 1. Losartan treatment reduces matrix content, improves vessel perfusion, and decreases hypoxia in schwannoma models.
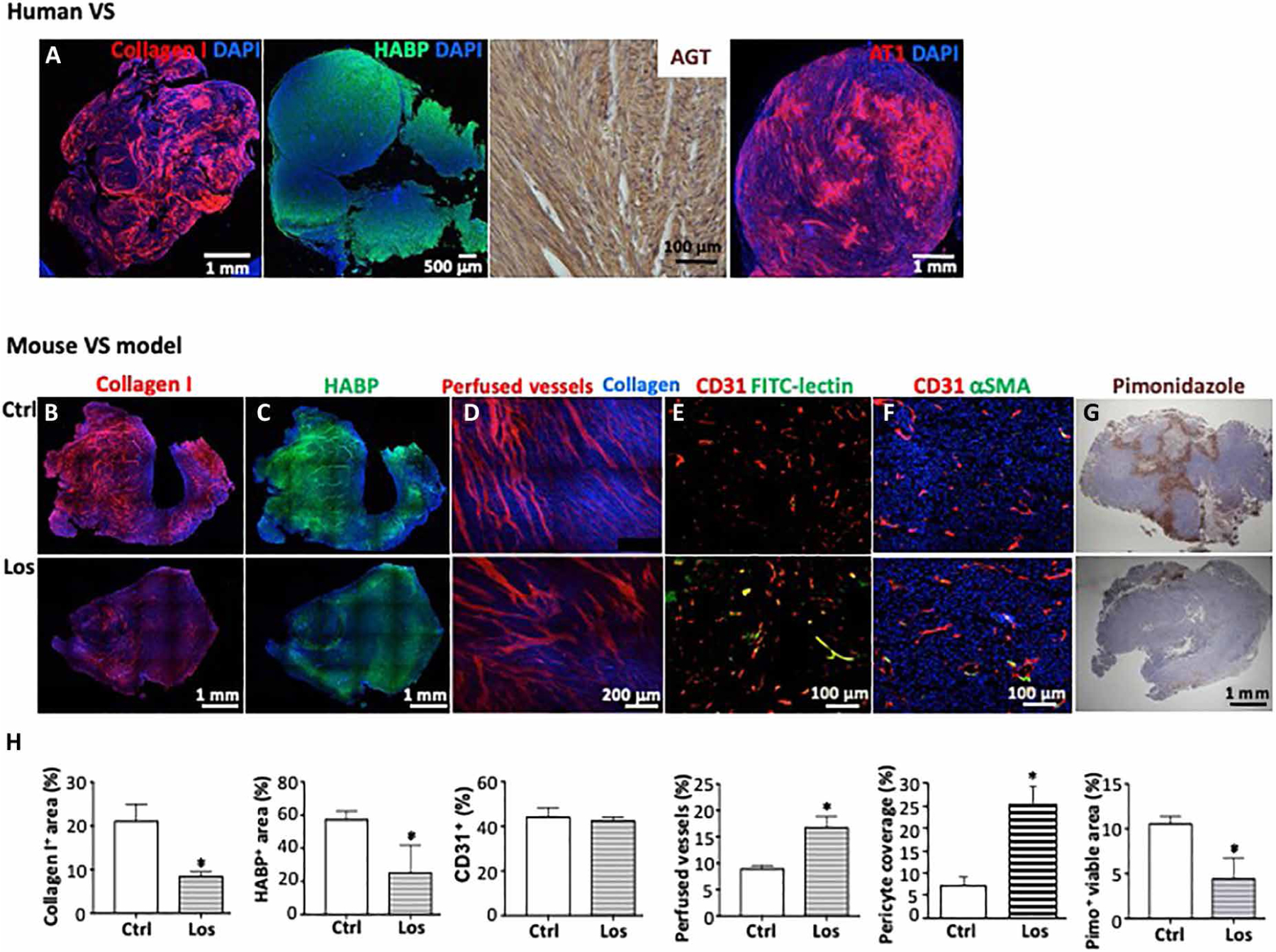
(A) Representative images of histological staining for collagen I, hyaluronic acid binding protein (HABP), AGT, and AT1 receptor in archived human VS tissue. N = 23. For patient demographics, please see table S4. Mice bearing Nf2−/− tumors in the CPA model were treated with saline (control, Ctrl) or losartan (Los, 40 mg/kg per day). Representative staining images of (B) collagen I (red), (C) HABP (green), and DAPI (4′,6-diamidino-2-phenylindole; blue). (D) Simultaneous visualization of collagen fibers (blue) using second harmonic generation (SHG) and blood vessel perfusion (marked with rhodamine dextran perfusion tracer, red) using Doppler optical coherence tomography (OCT). (E) Representative immunofluorescence (IF) staining images of perfused vessels (FITC-lectin+, green and yellow) and total blood vessels (CD31+, red). (F) Representative IF staining images of pericytes (αSMA+, green) covering blood vessels (CD31+, red) and DAPI (blue). (G) Representative images of immunohistochemistry stained hypoxic tissue (pimonidazole+, brown). (H) Quantification of immunostained images using ImageJ software. Control, n = 4 mice; losartan, n = 5 mice; 20 random areas were imaged and quantified. Data are presented as means ± SD. *P < 0.05.
In the cerebellopontine angle (CPA) model of NF2 that faithfully reproduces tumor-induced hearing loss (21), losartan treatment reduced the amount of collagen I and HA (Fig. 1, B, C, and H). To characterize the effects of losartan on schwannoma tumor vasculature, we simultaneously visualized blood vessel perfusion [using Doppler optical coherence tomography (OCT)] and collagen fibers [using second harmonic generation (SHG)]. We found that control tumors exhibited high densities of collagen and small-diameter, compressed blood vessels; losartan treatment reduced fibrillar collagen density and enlarged blood vessel diameter (Fig. 1D), resulting in increased perfusion measured by histological analysis (Fig. 1, E and H). Losartan treatment did not change Vegf RNA expression (fig. S1B) and tumor microvessel density but increased pericyte coverage on blood vessels (Fig. 1, F and H). Because of this vessel structural and perfusion normalization, the fraction of the hypoxic area decreased (Fig. 1, G and H). These studies suggest that losartan normalizes the ECM and vasculature in the NF2 TME.
Losartan treatment prevents hearing loss in the CPA schwannoma model
Using our NF2 CPA model, we evaluated the effect of losartan on hearing by measuring distortion product otoacoustic emissions (DPOAEs), which reflect outer hair cell (OHC) function, and auditory brainstem evoked response (ABR), which represents the summed activity of the auditory nerve and central auditory nuclei. In mice bearing similarly sized tumors (Fig. 2A), we found that losartan treatment lowered both DPOAE and ABR thresholds to the normal values of non–tumor-bearing mice (Fig. 2B), indicating that losartan treatment prevented tumor-induced hearing loss.
Fig. 2. Losartan treatment prevents hearing loss in the CPA schwannoma model and reduces neuronal edema and inflammatory response.
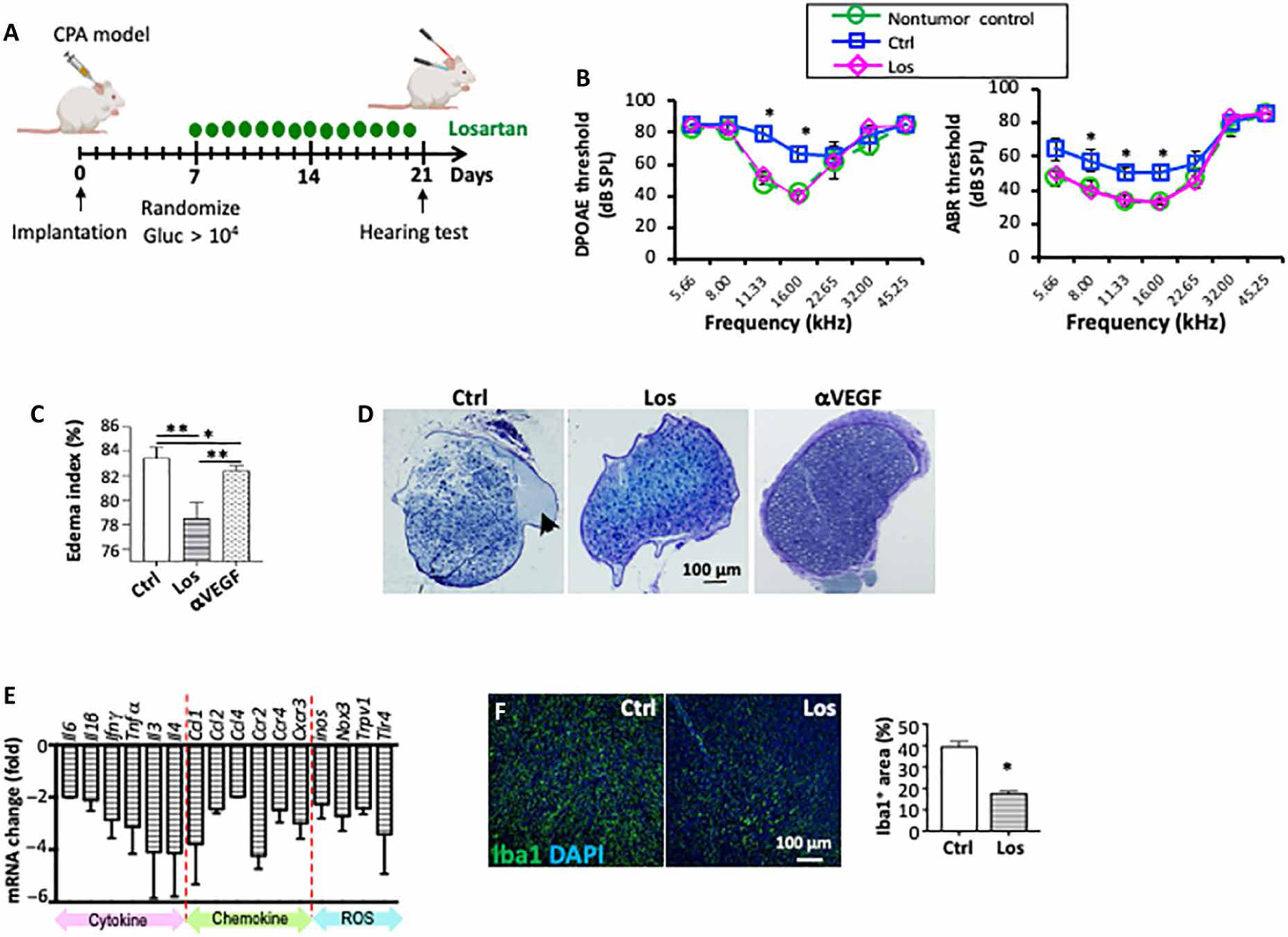
(A) Schematics of treatment and hearing test. Groups of mice bearing Nf2−/− tumor in the CPA model were randomized by blood Gluc measurement to be treated with saline or losartan. Hearing function test was carried out in mice bearing similarly sized tumors [blood Gluc concentration: Ctrl, 8.5 × 105 ± 1.4 × 105 relative light units (RLU); Los, 7.65 × 105 ± 3.9 × 105 RLU]. (B) DPOAE thresholds and ABR thresholds as a function of frequency in non–tumor-bearing mice (n = 5) and in Nf2−/− tumor–bearing mice ipsilateral to the tumor. Tumor-bearing mice were treated with saline (Ctrl, n = 10) or losartan (Los, n = 7). *P < 0.01. (C) Quantification of tumor tissue edema as measured using wet/dry weight method. Anti-VEGF treatment with B20 (5 mg/kg, i.p., once a week) was included as a positive control. Data are presented as means ± SD. *P < 0.01 and **P < 0.005. (D) Tibial nerves from mice bearing Nf2−/− tumors in the sciatic nerve were collected when tumors reached 1 cm in diameter. Representative images of toluidine blue staining of the tibial nerve, arrow points to edema and focal fluid collection in the perineuronal region. (E) After losartan treatment, changes in the mRNA level of inflammatory cytokines, chemokines, and receptors, as well as ROS signaling–related molecules, were assessed by real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR) analysis. Data are plotted relative to saline-treated controls. For primer sequences, please see table S7. (F) Representative IF staining images and quantification of tumor-infiltrating macrophage (Iba1+, green) in control (n = 10) and losartan-treated tumors (n = 10) (DAPI, blue). Data are presented as means ± SD. *P < 0.05.
Losartan treatment reduces neuronal edema
Previously, we showed that normalization of the tumor vasculature by anti-VEGF treatment improves neurological function via reducing nerve edema in NF2 models (19). Therefore, we assessed the effect of losartan treatment on brain and peripheral nerve edema. From mice bearing Nf2−/− tumors in the CPA, we collected the entire tumor-bearing brain and found that losartan treatment reduced brain edema compared to control and anti-VEGF treatment (Fig. 2C). From mice bearing Nf2−/− tumors in the sciatic nerve, we collected the tibial nerve, which is distal to the tumor implantation site. The tibial nerve in the control group presented with substantial edema, which was evident as a large amount of focal fluid in perineuronal areas. Losartan treatment decreased perineuronal edema (Fig. 2D). These data suggest that losartan may improve hearing function by reducing edema, thereby relieving compression on the eighth cranial nerve.
Losartan treatment reduces VS inflammatory response
Inflammation is an important mediator of noise- and tumor-induced hearing loss (22–24). In Nf2−/− tumors, we observed that losartan treatment reduced the expression of a panel of inflammatory molecules that play a role in hearing loss (22, 25–30), including the following: (i) secreted cytokines that regulate inflammation, such as IL-6, IL-1β, interferon-γ (IFN-γ), tumor necrosis factor–α (TNF-α), IL-3, and IL-4; (ii) chemokines and their receptors that recruit inflammatory cells, such as CCL1, CCL2, CCL4, CCR2, CCR4, and CXCR3; and (iii) reactive oxygen species (ROS) signaling–related molecules that are central factors causing hearing loss and radiation-induced fibrosis (31–34), such as inducible nitric oxide synthase, NADPH oxidase 3, transient receptor potential cation channel subfamily V member 1, and TLR4 (Fig. 2E).
CCL1, CCL2, and CCL4 are key chemokines that regulate migration and infiltration of macrophages (35). As a result of losartan-reduced Ccl-1, Ccl-2, and Ccl-4 expression, we observed a decreased number of infiltrating tumor-associated macrophages (TAMs) in losartan-treated Nf2−/− tumors (Fig. 2F). Considering the broad role of TAMs in matrix production (35), tumor progression, and possibly in orchestrating the secretion of ototoxic VS-secreted factors (27, 36), we next focused on characterizing the effects of losartan on TAMs.
Losartan treatment reduces IL-6/STAT3 and TLR4 signaling in TAMs
Using bulk tumor RNA, we did not observe changes in macrophage polarization markers (fig. S2). Therefore, we next sorted TAMs from control or losartan-treated Nf2−/− tumors and performed RNA sequencing (RNA-seq)–based transcriptional profiling. We defined a core losartan response gene signature in TAMs composed of 125 differentially expressed genes (DEGs); losartan treatment down-regulated 55 genes and up-regulated 70 genes [false discovery rate (FDR)–adjusted P < 0.05 and log2 fold change (Log2FC) > 2; table S1]. Overview CluGo pie chart showed the highest enrichment for myeloid dendritic cell chemotaxis, ECM and adhesion, and angiogenesis (Fig. 3A). Functional analysis revealed inhibition of multiple drivers of tumor growth and hearing loss in losartan-treated TAMs, including inflammation, fibrosis, angiogenesis, signal transduction, and hearing function–related pathways (Fig. 3B). Gene set enrichment analysis (GSEA) confirmed that down-regulated genes were enriched in the fibrogenic SMAD2 and SMAD3 signaling, neuroinflammation, IL-6/Janus kinase (JAK)/STAT signaling, and ROS synthesis pathways (Fig. 3C).
Fig. 3. Losartan treatment reduces IL-6/STAT3 and TLR4 signaling in TAMs.
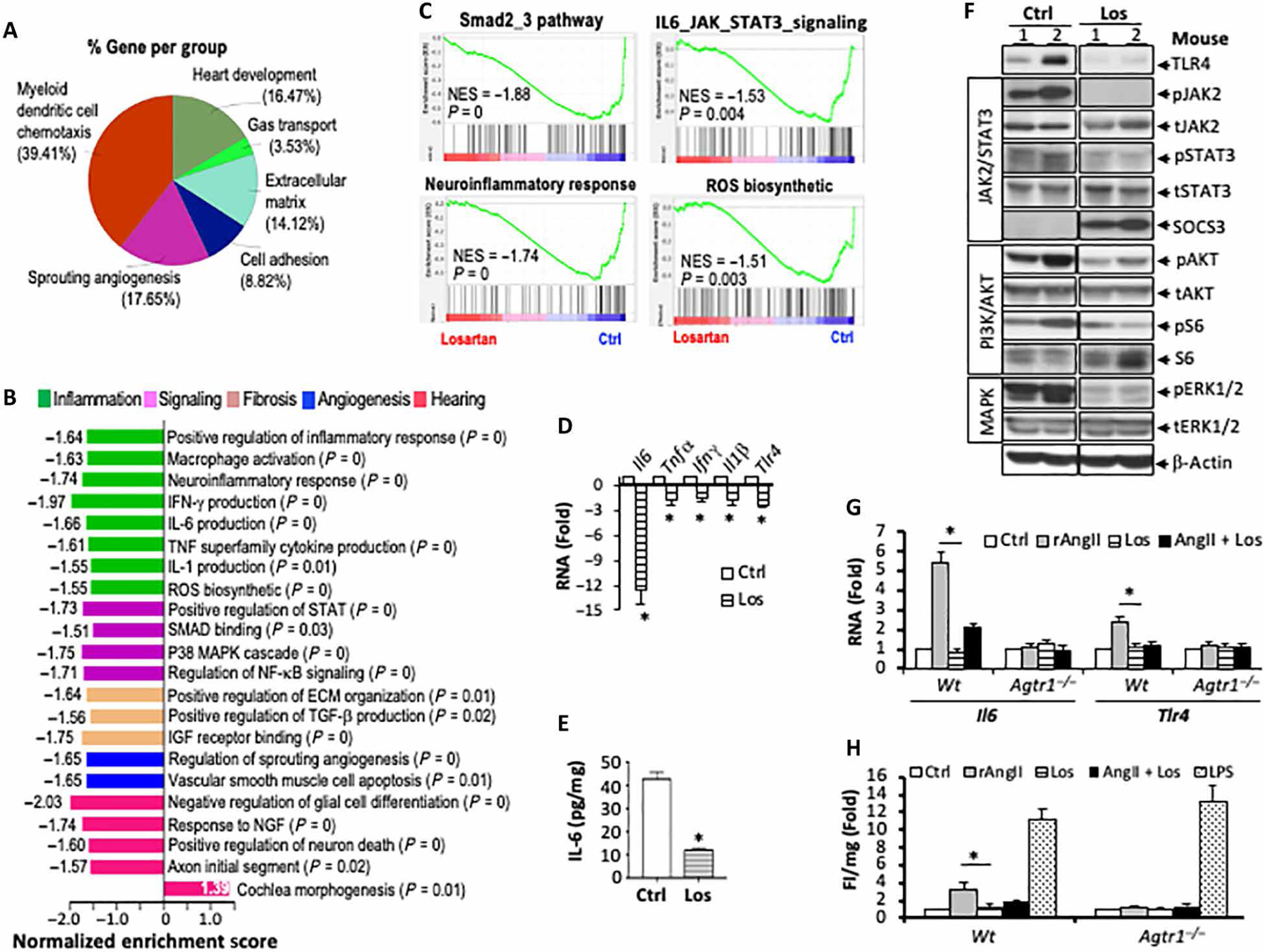
RNA-seq analysis was performed to compare the transcriptional profile between TAMs isolated from control and losartan-treated tumors. (A) Functional annotation enrichment analysis of the DEGs using CluGo charts. (B) Normalized enrichment scores (NES) indicate the distribution of Gene Ontology categories. (C) GSEA enrichment plots (80), showing gene sets negatively enriched in TAMs from losartan-treated tumors. N = 3 per each arm. (D) qRT-PCR analysis of inflammatory cytokine mRNA in TAMs isolated from control or losartan-treated tumors. Data are presented as means ± SD. *P < 0.01. In control and losartan-treated Nf2−/− tumors, (E) IL-6 protein level was measured by ELISA, N = 3 per each arm, *P < 0.01, and (F) TLR4 protein and JAK2/STAT3, PI3K/Akt, and ERK1/2 MAPK phosphorylation were evaluated by Western blot. Peritoneal macrophages isolated from wild-type or Agtr1−/− mice were treated with recombinant AngII (0.1 μM), losartan (1 μM), or AngII + Los for 6 hours. (G) Changes in Il-6 and Tlr4 RNA were evaluated by qRT-PCR. *P < 0.01. For primer sequences, please see table S7. (H) ROS production was measured by dihydroethidium (DHE) fluorescent intensity using a plate reader. *P < 0.01. Triplicate in each treatment group.
On the basis of the results of the unbiased transcriptional profiling, we confirmed that Il6, Tnfα, Ifnγ, Ilβ, and Tlr4 mRNA were reduced in TAMs isolated from losartan-treated tumors compared to those from the control group (Fig. 3D). IL-6 mediates inner ear damage in noise- and chemotherapy-induced hearing loss (25, 26), and TLR4 is known to activate IL-6 transcription (37, 38). Therefore, we further demonstrated that, in tumor tissue, losartan treatment (i) reduced IL-6 and TLR4 protein quantity; (ii) reduced IL-6 downstream JAK2 and STAT3 phosphorylation while increasing SOCS3, a negative regulator of JAK/STAT pathway; and (iii) reduced phosphoinositide 3-kinase (PI3K)/Akt and extracellular signal–regulated kinase 1/2 (ERK1/2) mitogen-activated protein kinase (MAPK) pathways (Fig. 3, E and F).
To examine whether the angiotensin pathway directly regulates macrophage IL-6 and TLR4 signaling, we isolated peritoneal macrophages from wild-type (Wt) or AngII receptor 1 knockout mice (Agtr1−/−). We treated these macrophages with recombinant AngII, losartan, or AngII + losartan. In macrophages from Wt mice, AngII induced both IL-6 and TLR4 mRNA expressions, and the induction was abrogated by losartan treatment. In macrophages from Agtr1−/− mice, treatment with AngII failed to induce either IL-6 or TLR4 mRNA (Fig. 3G).
In cardiovascular disease models, IL-6 and TLR4 activation in response to AngII was reported to cause oxidative stress characterized by overproduction of ROS (39–41). Oxidative stress is considered a key cause of hearing loss (31–33) and radiation-induced pulmonary fibrosis (34). In macrophages from Wt mice, treatment with recombinant AngII induced macrophage ROS production, and losartan treatment abrogated this induction. In macrophages from Agtr1−/− mice, treatment with AngII failed to induce ROS production (Fig. 3H).
Elevated IL-6/STAT3 signaling inversely correlates with hearing function in NF2 patients with VS
Next, we validate our preclinical findings in archived human VS tissue samples. To eliminate the differences in hearing loss that may stem from differences in tumor size, we analyzed size-matched tumors from VS patients with good or poor hearing function (tables S3 and S4). There were no differences in the expression of collagen I, HA, AT1 (fig. S3), and CCL2 (Fig. 4A) between poor and good hearing groups, but the expression of these proteins was higher in VSs compared to normal nerves. In patients with poor hearing compared to those with good hearing or normal nerves, we found (i) a trend of higher number of infiltrating TAMs (Fig. 4B), (ii) more IL-6 protein and STAT3 phosphorylation (Fig. 4, C and D), and (iii) higher TLR4 expression (Fig. 4E).
Fig. 4. Elevated IL-6/STAT3 signaling inversely correlates with hearing function in patients with NF2 VS.
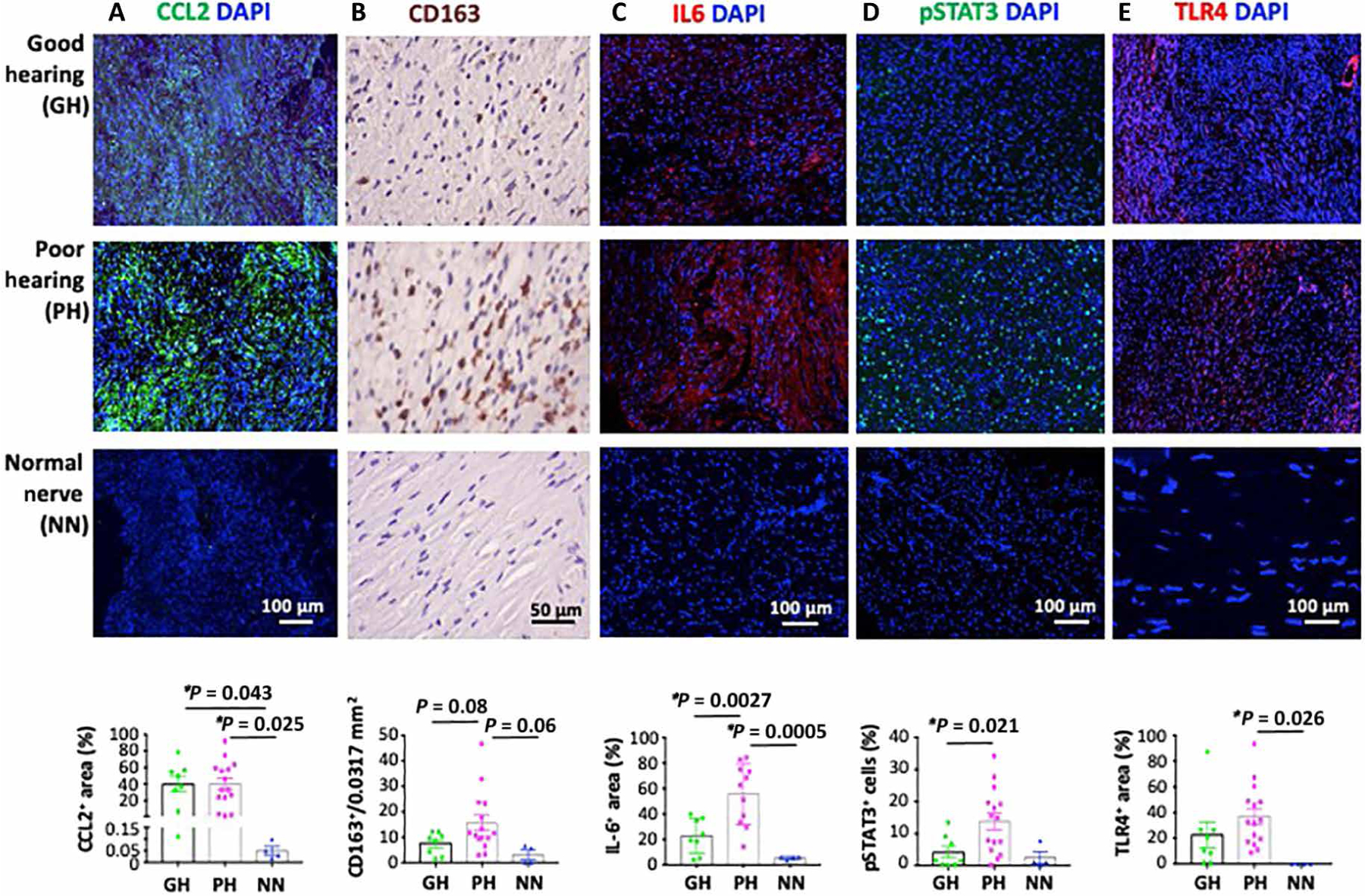
Archived paraffin-embedded tumors from patients with VS who had good hearing [pure-tone average (PTA) ≤ 30 dB HL and word recognition score (WRS) ≥ 70%, n = 8], poor hearing (n = 15), and normal nerve (n = 4) were immunostained for (A) CCL2 (green), (B) TAMs (CD163+, brown), (C) IL-6 (red), (D) phosphorylated STAT3 (green), and (E) TLR4 (red) (DAPI, blue). The number of TAMs per 0.0317-mm2 field was counted manually. Fluorescent images were analyzed using ImageJ software.
Elevated tumor-derived IL-6 is associated with reduced viability of cochlear sensory cells and neurons
We collected 57 fresh tumor pieces from indicated surgeries of VS patients with good (n = 10) or poor (n = 47) hearing function (tables S3 and S5). There was no difference (P = 0.61) in released IL-6 from tumor samples isolated from patients with poor hearing (10.05 ± 1.31 ng/ml; n = 47) compared to those from patients with good hearing (6.59 ± 0.93 ng/ml; n = 10) (Fig. 5A). Although this trend did not meet our criterion for statistical significance using the Mann-Whitney test, there was a statistically significant negative correlation between IL-6 quantity in VS-conditioned medium (VS-CM) and viability of hair cells (P = 0.01) and neurites (P = 0.03) when VS-CM was applied to neonatal murine cochlear explants (Fig. 5, B to D). We previously established an organotypic neonatal murine cochlear explant culture model for the assessment of ototoxic and neurotoxic factors using patient VS-CM (27). Here, we found that culture of cochlear explants in patient VS-CM with higher concentration of IL-6 was associated with reduced viability of OHCs and neurites in the basal turn (Fig. 5, B and C). However, no effect of losartan on AngII-induced IL-6 production was observed in human primary VS cells (fig. S4). The enhanced susceptibility of the cochlear base, which encodes for high frequencies, to ototoxic and neurotoxic effects in VS-CM is consistent with the clinical observation that patients with VS typically present with high-frequency SNHL. Together, these data suggest that tumor-derived IL-6 may be involved in cochlear damage and associated hearing loss.
Fig. 5. Elevated expression of tumor-derived IL-6 is associated with reduced viability of cochlear sensory cells and neurons.
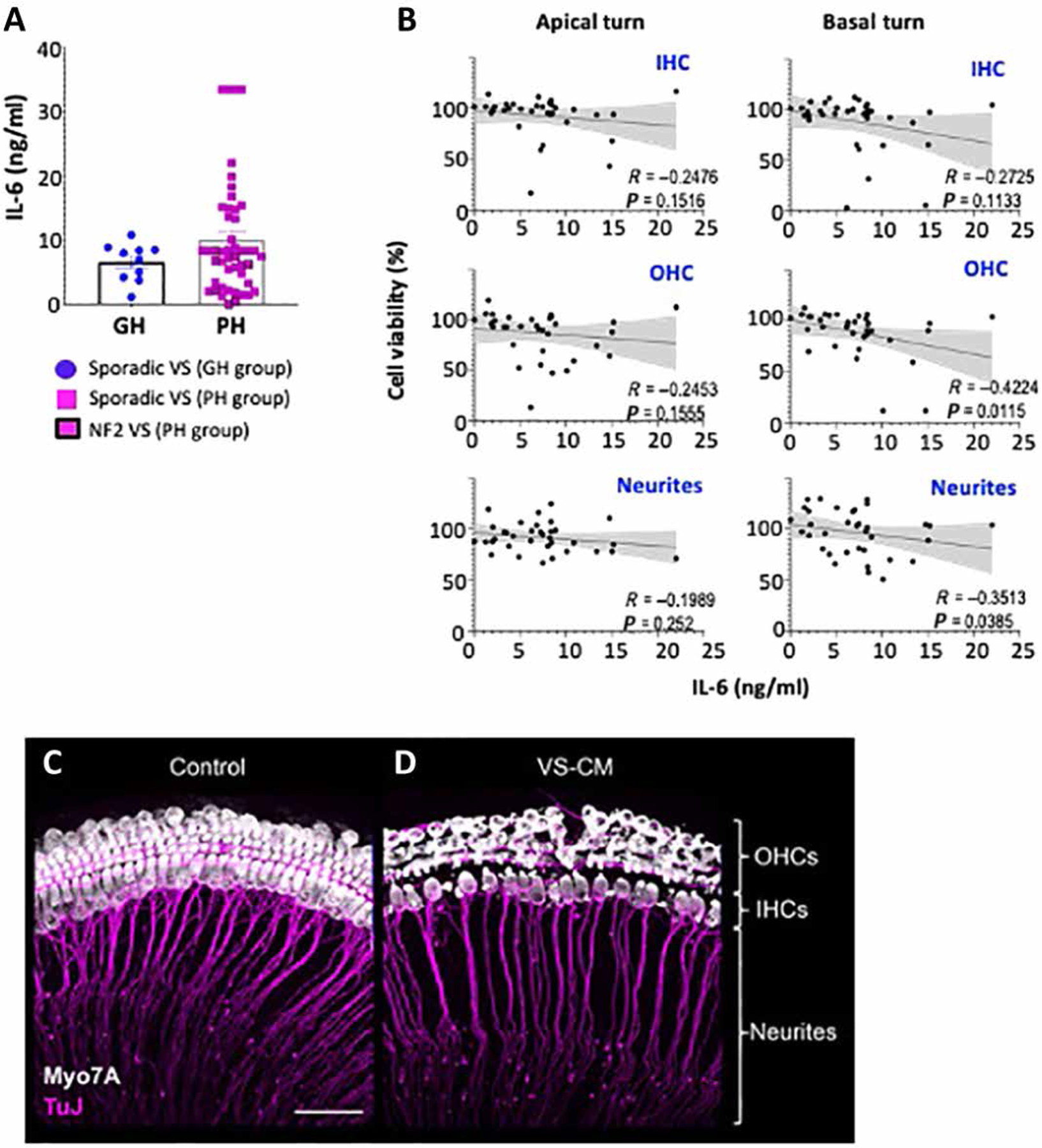
(A) Measurement of IL-6 quantity in fresh VS tumor pieces surgically removed from patients with good (n = 10) or poor (n = 47) hearing. Tumor pieces were cultured for 72 hours in serum-free media to generate VS-conditioned media (VS-CM). Values shown are means ± SEM. P = 0.61. (B) Quantification of inner hair cell (IHC), outer hair cell (OHC), and neurite viability as a function of IL-6 concentration in VS-CM. Spearman nonparametric correlation coefficient and P value are shown. Representative confocal images of cochlear explants treated with (C) control media or (D) VS-CM from a patient with poor hearing for 48 hours and then immunostained for Myo7A to reveal hair cells (white) and β-tubulin to reveal auditory nerve fibers (pink). Cellular debris (white dots) closely resembled apoptotic bodies of OHCs. Scale bar, 50 μm.
Combining losartan with RT more effectively inhibits growth of Nf2−/− schwannomas than RT alone
Because losartan treatment increases oxygenation and oxygen is a potent radiosensitizer, we next tested whether combined losartan treatment enhances RT efficacy (Fig. 6A). In the Nf2−/− sciatic nerve and CPA models, combined losartan and RT inhibited tumor growth and extended mouse survival to a greater extent as compared with RT monotherapy, with mice living ~50% longer (Fig. 6, B and C). The same effect of losartan-enhanced RT efficacy was also observed in SC4 schwannoma model (fig. S5A).
Fig. 6. Combining losartan with RT more effectively inhibits tumor growth in syngeneic schwannoma models.
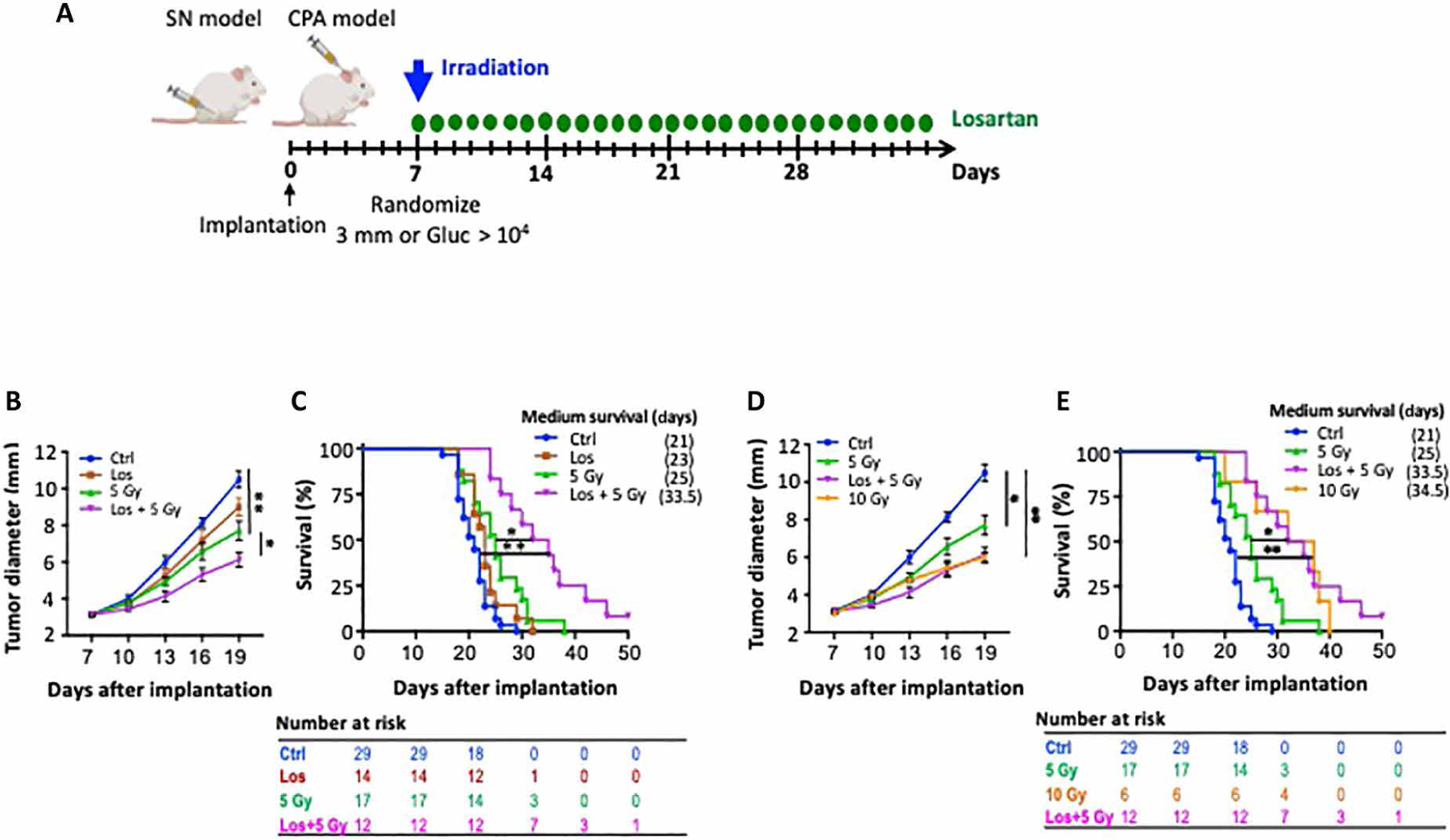
(A) Schematic of experimental design. SN, sciatic nerve; CPA, cerebellopontine angle. (B) Growth curve of Nf2−/− tumor in the sciatic nerve model. Tumor-bearing mice were treated with control, radiation (5 Gy), losartan (40 mg/kg), or combined RT + losartan treatment. (C) Kaplan-Meier survival curve of mice implanted with Nf2−/− tumor cells in the CPA model. Tumor-bearing mice were treated with the same protocol as in (B). (D) Growth curve of Nf2−/− tumor in the sciatic nerve model. Tumor-bearing mice were treated with control, 5 Gy, 10 Gy, or losartan combined with 5 Gy. (E) Kaplan-Meier survival curve of Nf2−/− tumor implanted in the CPA model. Tumor-bearing mice were treated with the same protocol as in (B). All animal studies presented are representative of at least three independent experiments, n = 8 per group in each experiment. All data presented are means ± SEM. *P < 0.01 and **P < 0.05.
Fibrosis is known to be a long-term adverse effect of RT (11, 42), and TAMs are key players in matrix production (43). In in vitro experiments using a macrophage cell line, RT (i) increased the expression of collagen I and αSma as well as fibrogenic Ctgf, Pdgfβ, and Tgfβ mRNA (fig. S5B); (ii) activated fibrogenic transforming growth factor–β (TGF-β)/Smad2 phosphorylation (fig. S5C); and (iii) increased ROS production (fig. S5D). In in vivo Nf2−/− schwannoma model, RT increased ECM content in tumors (fig. S5E). However, at 3 weeks after RT, we did not observe changes in ROS production or hearing function (fig. S5, F and G).
Losartan treatment combined with low-dose radiation is as effective as high-dose radiation monotherapy
We next tested whether losartan treatment could reduce the required dose and thus adverse effects of RT. In both sciatic nerve and CPA models, we found high-dose RT [10 grays (Gy)] to be more effective than the low-dose 5 Gy. However, when combined with losartan treatment, 5-Gy RT was as effective as 10-Gy RT alone (Fig. 6, D and E).
Losartan normalizes the TME
Immunohistochemistry staining confirmed that combined treatment decreased the number of proliferating (Ki67+) tumor cells and increased the number of apoptotic (TUNEL+) tumor cells compared to RT alone (fig. S6, A and B). In in vitro studies, losartan did not directly affect tumor cell viability as measured by MTT assay (fig. S6C) and DNA damage repair after RT as evaluated by γH2AX staining (fig. S6, D and E).
AngII acts via two receptors, AT1 and AT2, and they exert counteracting effects on cellular growth, vascular tone, and inflammation (44). By competing for binding to AT1 receptor, losartan can make more AngII available to bind to AT2 receptor (45), and AT2 receptor activation attenuates the growth of several tumors (46). To determine whether this is a potential mechanism of action for losartan, we treated mice with losartan and the AT2 receptor blocker, PD123319. We observed that both losartan and PD123319 had minimal effects on tumor growth, and combined PD123319 treatment did not change the losartan effects (fig. S7A).
To determine the contribution of macrophage AT1 signaling to tumor progression and response to treatment, we generated bone marrow chimeric mice that lack AT1 receptor expression on hematopoietic cells (fig. S7B). After successful generation of bone marrow chimera (fig. S7, C and D), Nf2−/− tumors were implanted in Wt mice and in bone marrow–transplanted mice, and groups of mice were treated with losartan, RT, or losartan + RT. We found that compared to Wt mice, replacing hematopoietic cells from Agtr1−/− donors did not change tumor growth nor did it affect the losartan and radiation treatment response (fig. S7E). In TAMs sorted from these tumors, we observed that AT1 knockout did not change IL-6 RNA expression; however, losartan treatment reduced Il6 to a lesser extent compared to those in tumor grown in Wt mice (fig. S7F). This finding is consistent with our data showing that losartan reduces TAM IL-6 expression via AT1 signaling in vivo. In summary, these data suggest that losartan does not exert direct antitumor effects to enhance the efficacy of RT in vivo but functions via normalizing the TME.
Establishment of patient-derived VS model to study the effects of losartan
Last, to provide a more representative delineation of the therapeutic response in patients, we established six patient-derived primary VS cells from patients with NF2 (table S6). We first examined their transcriptional profiles and compared them to those of normal Schwann cells (NCs) using RNA-seq analysis. We found elevated expression of 2382 genes and reduced expression of 941 genes in VSs compared to NCs (FDR-adjusted P < 0.001 and Log2FC > 2; Fig. 7A and table S2). Gene classification analysis was performed using DAVID (david.ncifcrf.gov), and the DEGs were assigned to the cell cycle group. Functional analysis of the DEGs indicated that genes in response to auditory stimulus, Schwann and glial cell differentiation, negative regulation of inflammation, and IL-6 production were down-regulated in VSs, and genes in the signaling pathways for fibrosis and tumor growth were enriched in VSs (Fig. 7, B and C).
Fig. 7. Characterize losartan effects in VS patient samples and data.
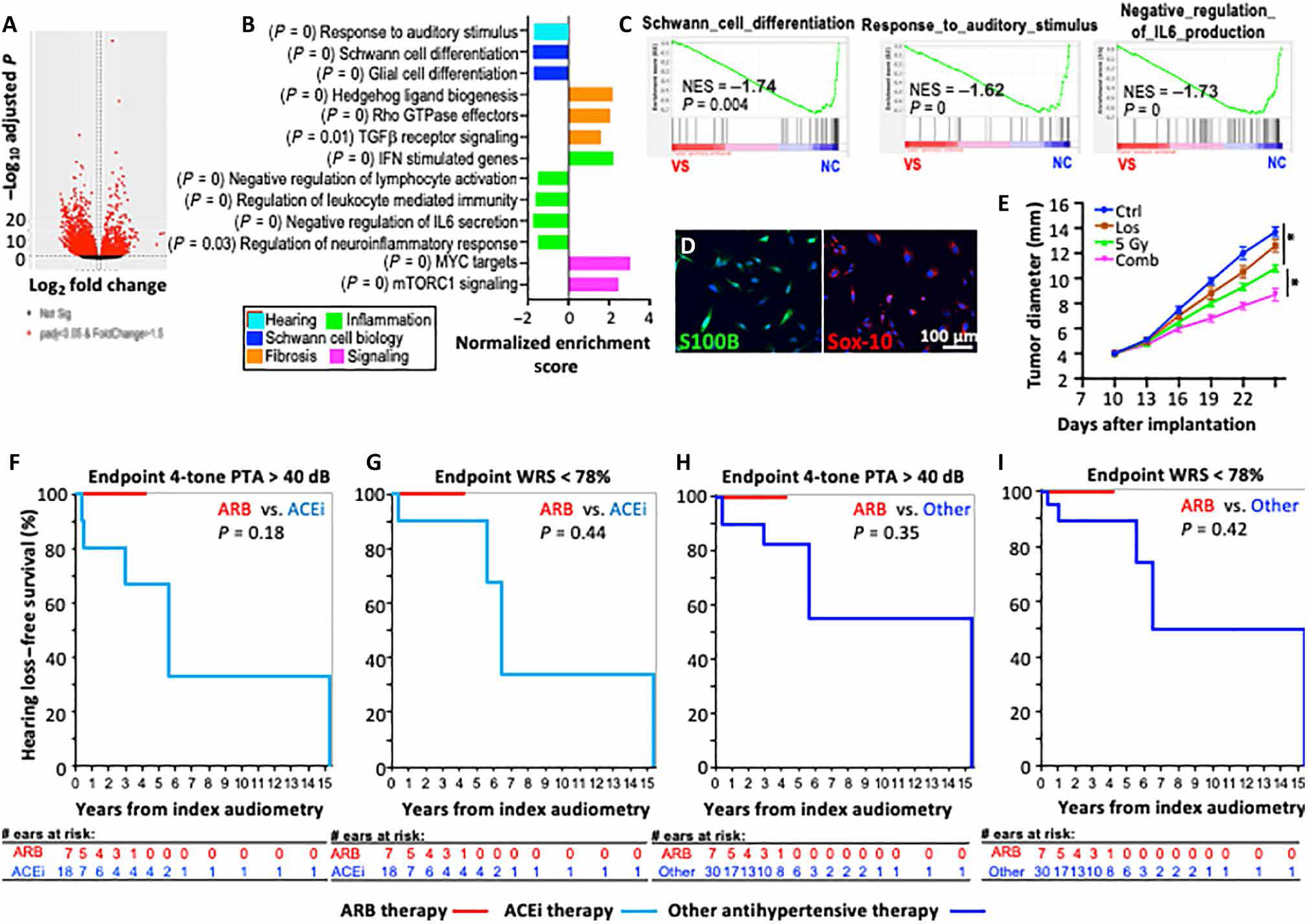
Transcriptome comparison between human NF2 VSs with NCs by RNA-seq analysis. (A) Volcano plot depicts DEGs. (B) Normalized enrichment scores (NES) indicate the distribution of Gene Ontology categories in patient NF2 VSs versus NCs. (C) GSEA analysis showing pathway enrichment in patients with NF2 VSs as compared to normal Schwann cell. NF2 VS, n = 6 tumors; normal Schwann cell (NC), n = 3. (D) Representative images of IF staining for Schwann cell markers, S100B (green) and Sox-10 (red), in patient-derived NF2 VS4 cell line. (E) Tumor diameter as a function of time after losartan, RT, and combined treatment in mice implanted with patient-derived VS4 cells into the sciatic nerve. Representative of three independent experiments, n = 8 per group in each experiment. All data are presented as means ± SEM. *P < 0.01. Retrospective analysis of hearing preservation in hypertensive VS patients with normal baseline hearing in VS-ipsilateral ear treated with ARB therapy or other antihypertensive medications. (F) Endpoint by threshold criteria with four-tone PTA > 40 dB. (G) Endpoint by word understanding with WRS < 78%. Hearing loss progression in patients with diagnosis of both VS and hypertension based on ARB versus any other antihypertensive therapy. (H) Endpoint by threshold criteria with four-tone PTA > 40 dB. (I) Endpoint by word understanding with WRS < 78%.
Then, we implanted the patient-derived VS cells in the murine sciatic nerve model. Among the six patient-derived VS primary cells, only one cell line formed tumors in immunodeficient NOD scid gamma mice. Expression of S100B and Sox-10 confirmed its Schwann cell origin (Fig. 7D). Using this patient-derived xenograft model, we confirmed our finding that losartan combined with RT inhibits tumor growth to a greater extent as compared to RT alone (Fig. 7E).
Hypertensive patients with VS on ARB therapy tend to have more preserved hearing than patients taking other antihypertensive medications
Hypertension is a leading comorbidity in VS surgical cases (35% comorbidity cases) (47). Considering the protective role of losartan in our NF2 mouse model, we retrospectively analyzed a cohort of VS patients with hypertension treated at the Massachusetts Eye and Ear from January 1994 to October 2018. A total of 45 VS patients with hypertension met the requirement of normal baseline hearing with four-tone pure-tone average (PTA) ≤ 25 dB and word recognition score (WRS) ≥ 92% in the VS-ipsilateral ear and had sequential audiometry available to track long-term hearing outcomes. Of these patients, 7 were either already taking an ARB medication at the time of initial VS diagnosis or started on ARBs within 1 year of diagnosis; 30 were either already taking another antihypertensive medication or started on such a medication within 1 year of VS diagnosis, and 8 were not on any medical therapy for hypertension. Hearing loss progression was evaluated by Kaplan-Meier analysis, with the endpoint defined as a substantial change in hearing from baseline, either by thresholds (PTA > 40 dB) or word understanding (WRS < 78%). Median follow-up time for patients taking an ARB medication was 2.7 years, with maximum follow-up of 4.2 years (tables S9 to S11). Zero patients taking ARB therapy showed any change in hearing over the course of follow-up, whether by thresholds or word understanding. Other patient groups with both VS and hypertension, regardless of whether on antihypertensive medical therapy or not, invariably showed greater hearing loss progression over time (Fig. 7, F to I, and fig. S8).
DISCUSSION
Developing effective therapeutics to preserve hearing function in patients with NF2 VS is an urgent unmet medical need. On the basis of a previous report that tumor fibrosis correlates with hearing loss in patients with NF2 (10), we set out to investigate whether anti-fibrotic treatment using losartan can improve hearing in NF2 models. In malignant cancers, it is well documented that the abnormal desmoplastic TME fuels disease progression and treatment resistance (15, 48). In nonmalignant NF2 VS, the role of TME in disease progression has not been fully characterized. Schwannomas are composed predominantly of neoplastic Schwann cell, which is a major producer of myelin sheath and ECM that support neuronal survival and axonal growth (49). We found that ECM components are elevated in the schwannomas. To reduce the schwannoma ECM, we used losartan, a widely prescribed antihypertensive ARB. The renin-angiotensin system (RAS), initially found for its pivotal role in maintaining cardiovascular homeostasis as well as fluid and electrolyte balance, is now known to stimulate the accumulation of ECM components (50). Two major RAS blockers commonly used in the clinic are angiotensin-converting enzyme inhibitors (ACEi) and ARBs (50). Blocking AngII signaling at the AT1 receptor with losartan can be more effective compared to blocking AngII production by ACEi, because (i) enzymes other than ACE can generate AngII (51), (ii) AngII can be generated independently of AngI and ACE but directly from Ang-(1–12), a fragment of AGT, and (iii) AT1 can be activated independently of AngII binding (52). Moreover, a previous study showed that losartan is more effective than ACEi (lisinopril) in reducing “solid stress” in tumors (12). Therefore, we chose to block AT1 receptor using losartan in our study. The advantage of ARB over ACEi was supported by our retrospective study that showed ARBs to be more beneficial in preventing hearing loss than ACEi. In the NF2 mouse models, we showed that losartan effectively normalized the TME: (i) reduced schwannoma ECM, (ii) normalized tumor vasculature, and (iii) increased oxygen delivery (fig. S9). As a result of the normalized TME, losartan prevented hearing loss and enhanced RT efficacy.
We found that one of the direct consequences of matrix and vessel normalization is that losartan reduced perineuronal edema. Previously, in NF2 mouse models, we showed that anti-VEGF treatment improved neurological function by normalizing the tumor vasculature and reducing nerve edema (19). In the current study, losartan treatment did not change VEGF expression; instead, it normalized the vasculature by (i) reducing the ECM content and can thus relieve the compressive pressure on the vessels to improve perfusion and (ii) normalizing the vessel structure by increasing pericyte coverage of the endothelial cells. In patients with NF2, hearing improvement after bevacizumab is not durable. Furthermore, anti-VEGF treatment can cause substantial fibrosis, which, in the long-term, may further damage hearing function (53–55). It remains to be determined whether losartan is more powerful in preserving hearing than bevacizumab and whether combined losartan and anti-VEGF is a better strategy to preserve hearing function.
One of our most important findings is that losartan treatment prevented hearing loss by reducing neuroinflammation. Neuroinflammation plays a key role in SNHL (26, 27, 56–59), and treatment with ARBs decreases brain inflammation and is neuroprotective in rodent models of central nervous system inflammatory diseases (60–66); however, whether ARBs have hearing benefit is not known. Here, we found that losartan treatment reduced the chemotaxis of macrophages. Cochlear macrophages are responsible for the inflammatory response that leads to damaged and dying cochlear hair cells (26, 67, 68). Combining RNA-seq analysis of TAMs and the use of macrophages from Agtr1−/− mice, we demonstrated that macrophage AT1 signaling is critical for the production of ototoxic IL-6, TLR4, and ROS in response to AngII. Further mechanistic studies into whether TAMs and other immune cells migrate into the inner ear and what inflammatory cytokines are produced by these immune cells will help better define the anti-inflammatory strategies to control hearing loss.
In patients with VS, hearing loss occurs in about half of the patients after photon radiotherapy, a percentage that continues to increase with longer duration of follow-up (69–73). A recent clinical study (NCT01199978) that aimed to determine the long-term effects of fractionated proton radiotherapy on hearing function in patients with sporadic VS showed that although fractionated radiation has 100% tumor control, hearing function deteriorated at 6 months after treatment (74). These data suggest that hearing loss is not a result of tumor growth but of RT-associated ototoxicity. Fibrosis is a late complication of RT but a substantial contributor to patient morbidity (75). Radiation activates highly fibrogenic TGF-β signaling (11, 76) and increases TLR4 expression leading to increased ROS production (77), which could directly damage the hearing function. In our model, RT increased fibrogenic signaling leading to increased ECM quantity. However, we did not see any effect from RT on hearing function in the mouse model, likely because (i) we used a relatively low radiation dose of 5 Gy, which was sufficient in controlling tumor growth in the mouse model, and (ii) the longest period of time between RT and hearing assessment was only 3 weeks because the tumor-bearing mice became moribund. Because RT-associated ototoxicity is a long-term toxic effect, the Nf2 knockout mouse model would be well suited to study the long-term RT effect on hearing.
In preparation to translate our findings into the clinic, we performed the following studies: (i) We confirmed that IL-6/STAT and TLR4 expression in archived human VS samples negatively associated with hearing function; (ii) we showed that elevated production of human VS–derived IL-6 was associated with reduced viability of cochlear sensory cells and neurons in a cochlear explant culture model; (iii) we found that patients on ARB therapy do not show progression in hearing loss compared to patients who were treated with other or no antihypertensive medications based on retrospective analysis; (iv) we confirmed that losartan enhanced RT efficacy in two mouse NF2 models and a patient-derived VS model. Because most VSs have a deletion on chromosome 22 similar to what is seen in the genomes of patients with NF2, results from nonsyndromic patients and their samples are likely generalizable to patients with NF2. Together, our findings provide the rationale and critical data for the potential clinical translation of combining losartan with RT in patients with VSs.
There are several limitations in the current work. First, it remains to be determined whether losartan affects tinnitus in our NF2 mouse model. Besides hearing loss, tinnitus is common among patients with VS, affecting 65 to 75% of people with unilateral VS (78). Losartan has been suggested as a possible therapy for tinnitus because losartan prevented maladaptive axonal sprouting in the cochlear nucleus after cochlear ablation in rats (79). Whether losartan can alleviate tinnitus and other neurological symptoms in patients with VS needs to be investigated in the future. Second, it remains to be addressed whether losartan can benefit patients with preexisting hearing loss. Currently, we are technically limited by our animal model to investigate this question because in the CPA hearing mouse model, after tumor implantation, there is only a 2- to 3-week treatment window that allows losartan to work. After 3 weeks, mice start to develop neurological deficits (such as ataxia) and need to be sacrificed. When considering patient outcomes, a larger retrospective study population with hypertension and VS-induced hearing loss and treated with losartan could reasonably be expected to demonstrate effects within a relatively short time. Third, a quantitative evaluation of the IL-6/Stat3 signaling in human VS tissue would complement our measurements of IL-6 in VS-CM. Addressing this issue will require future collection of additional size-matched VS tumor samples.
In summary, our study demonstrates that losartan prevents hearing loss and enhances radiation efficacy in rodents. As one of the most commonly prescribed drugs for hypertension, the safety and low cost of losartan warrant rapid translation of our research to patients with VS-induced SNHL.
MATERIALS AND METHODS
Study design
The overall objective of the study was to test the hypothesis that reducing the fibrosis using losartan, an FDA-approved antihypertensive drug that blocks fibrotic and inflammatory signaling, prevents hearing loss. All experiments and animal protocols were approved by the Massachusetts General Hospital and Massachusetts Eye and Ear in accordance with the guidelines of Public Health Service Policy on Humane Care of Laboratory Animals. Using NF2 mouse models, we first examined the losartan effects on reducing ECM, normalizing tumor vasculature, and changing hearing function. Then, we characterized the mechanisms of action by transcriptomic profiling using single-cell RNA-seq and in vitro molecular studies. Last, we confirmed the preclinical findings in archived patient samples by histological analysis; in fresh, surgically obtained VS tumor samples by RNA-seq, enzyme-linked immunosorbent assay (ELISA), and organotypic cochlear cultures; and by retrospective analysis of hearing outcomes in patients with VS and hypertension. Animals were randomly assigned to experimental groups such that the mean tumor burden was similar in each group to avoid biased results. For the hearing test, the experimenters were blinded; for the efficacy, molecular mechanism, and clinical patient sample studies, the experimenters were not blinded. Sample sizes were not predetermined with a power calculation, more than eight animals per group were typically used for treatment efficacy studies, and the experiments were independently repeated three times. Figure legends contain sample sizes and replicate information. Primary data are reported in data file S1.
Statistical analyses
Spearman’s rho correlation coefficient was calculated. We determined whether growth curves significantly differed from each other by log-transforming the data, fitting a linear regression to each growth curve, and comparing the slopes of the regression lines [using an equivalent of analysis of variance (ANOVA)]. Significant differences between two groups were analyzed using Student’s t test (two-tailed) or Mann-Whitney U test (two-tailed). All calculations were done using the GraphPad Prism software 6.0 and Microsoft Excel software 2010. Differences in hearing response between multiple groups were evaluated with ANOVAs. Welch’s t test was used for comparison between two groups. Benjamini-Hochberg correction for multiple comparisons was applied. Histological analysis of gene expression in patient schwannoma tissues was statistically analyzed using statistics software (Statistical Package for the Social Sciences Statistics, version 20.0; IBM, Armonk, NY) with a priori significant P value of <0.05. Correlation of IL-6 quantity and viability of sensorineural cells in mouse cochlear explants treated with VS-CM was analyzed using Spearman nonparametric correlation test (GraphPad Prism software 6.0).
Supplementary Material
Acknowledgments:
We thank M. Duquette, A. Khachatryan, and C. Smith for technical support; S. Roberge, M. K. Selig, and P. Huang for assisting in animal studies, tumor implantation, and cranial window generation; I. L.G.D. Santos for helpful discussion; and J. L. W. Da for editorial help with the manuscript.
Funding:
This study was supported by the Department of Defense New Investigator award (W81XWH-16-1-0219, to L.X.) and Investigator Initiated Research award (W81XWH-20-1-0222, to L.X.); Children’s Tumor Foundation Drug Discovery Initiative (to L.X.); P01-CA080124, P50-CA165962, R01-CA129371, R01-CA208205, and U01-CA 224348 (to R.K.J.); NCI Outstanding Investigator award (R35-CA197743, to R.K.J.); Advanced Medical Research Foundation (to R.K.J.); Jane’s Trust Foundation (to R.K.J.); the Lustgarten Foundation, the Ludwig Center at Harvard, the National Foundation for Cancer Research, and the Gates Foundation (to R.K.J.); Cancer Research Institute award (to J.R.); American Association of Cancer Research Fellowship (19-40-50-DATT, to M.D.); NIDCD grant R01DC015824 (to K.M.S.); Nancy Sayles Day Foundation (to K.M.S.); Lauer Tinnitus Research Center (to K.M.S.); the Barnes Foundation (to K.M.S.); the Zwanziger Foundation (to K.M.S.); and Sheldon and Dorothea Buckler (to K.M.S.).
Competing interests:
R.K.J. received honorarium from Amgen; consultant fees from Chugai, Elpis, Merck, Ophthotech, Pfizer, SPARC, SynDevRx, and XTuit; owns equity in Accurius, Enlight, Ophthotech, and SynDevRx; and serves on the Boards of Trustees of Tekla Healthcare Investors, Tekla Life Sciences Investors, Tekla Healthcare Opportunities Fund, and Tekla World Healthcare Fund. Neither any reagent nor any funding from these organizations was used in this study.
Footnotes
SUPPLEMENTARY MATERIALS
stm.sciencemag.org/cgi/content/full/13/602/eabd4816/DC1
Materials and Methods
Figs. S1 to S9
Tables S1 to S11
Data file S1
Data and materials availability:
All data associated with this study are present in the paper or the Supplementary Materials.
REFERENCES AND NOTES
- 1.Evans DG, Neurofibromatosis type 2 (NF2): A clinical and molecular review. Orphanet J. Rare Dis 4, 16 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evans DG, Moran A, King A, Saeed S, Gurusinghe N, Ramsden R, Incidence of vestibular schwannoma and neurofibromatosis 2 in the North West of England over a 10-year period: Higher incidence than previously thought. Otol. Neurotol 26, 93–97 (2005). [DOI] [PubMed] [Google Scholar]
- 3.Plotkin SR, Merker VL, Muzikansky A, Barker FG 2nd, Slattery W III, Natural history of vestibular schwannoma growth and hearing decline in newly diagnosed neurofibromatosis type 2 patients. Otol. Neurotol 35, e50–e56 (2014). [DOI] [PubMed] [Google Scholar]
- 4.Masuda A, Fisher LM, Oppenheimer ML, Iqbal Z, Slattery WH; Natural History Consortium, Hearing changes after diagnosis in neurofibromatosis type 2. Otol. Neurotol 25, 150–154 (2004). [DOI] [PubMed] [Google Scholar]
- 5.Ammoun S, Hanemann CO, Emerging therapeutic targets in schwannomas and other merlin-deficient tumors. Nat. Rev. Neurol 7, 392–399 (2011). [DOI] [PubMed] [Google Scholar]
- 6.Plotkin SR, Merker VL, Halpin C, Jennings D, McKenna MJ, Harris GJ, Barker FG II, Bevacizumab for progressive vestibular schwannoma in neurofibromatosis type 2: A retrospective review of 31 patients. Otol. Neurotol 33, 1046–1052 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Plotkin SR, Stemmer-Rachamimov AO, Barker FG II, Halpin C, Padera TP, Tyrrell A, Sorensen AG, Jain RK, di Tomaso E, Hearing improvement after bevacizumab in patients with neurofibromatosis type 2. N. Engl. J. Med 361, 358–367 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slusarz KM, Merker VL, Muzikansky A, Francis SA, Plotkin SR, Long-term toxicity of bevacizumab therapy in neurofibromatosis 2 patients. Cancer Chemother. Pharmacol 73, 1197–1204 (2014). [DOI] [PubMed] [Google Scholar]
- 9.Ren Y, Chari D, Vasilijic S, Welling DB, Stankovic KM, New developments in neurofibromatosis type 2 and vestibular schwannoma. Neuro Oncol. Adv 3, vdaa153 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sughrue ME, Kaur R, Kane AJ, Rutkowski MJ, Yang I, Pitts LH, Tihan T, Parsa AT, Intratumoral hemorrhage and fibrosis in vestibular schwannoma: A possible mechanism for hearing loss. J. Neurosurg 114, 386–393 (2011). [DOI] [PubMed] [Google Scholar]
- 11.Straub JM, New J, Hamilton CD, Lominska C, Shnayder Y, Thomas SM, Radiation-induced fibrosis: Mechanisms and implications for therapy. J. Cancer Res. Clin. Oncol 141, 1985–1994 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chauhan VP, Martin JD, Liu H, Lacorre DA, Jain SR, Kozin SV, Stylianopoulos T, Mousa AS, Han X, Adstamongkonkul P, Popovic Z, Huang P, Bawendi MG, Boucher Y, Jain RK, Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat. Commun 4, 2516 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stylianopoulos T, Martin JD, Chauhan VP, Jain SR, Diop-Frimpong B, Bardeesy N, Smith BL, Ferrone CR, Hornicek FJ, Boucher Y, Munn LL, Jain RK, Causes, consequences, and remedies for growth-induced solid stress in murine and human tumors. Proc. Natl. Acad. Sci. U.S.A 109, 15101–15108 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu J, Liao S, Diop-Frimpong B, Chen W, Goel S, Naxerova K, Ancukiewicz M, Boucher Y, Jain RK, Xu L, TGF-β blockade improves the distribution and efficacy of therapeutics in breast carcinoma by normalizing the tumor stroma. Proc. Natl. Acad. Sci. U.S.A 109, 16618–16623 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jain RK, Normalizing tumor microenvironment to treat cancer: Bench to bedside to biomarkers. J. Clin. Oncol 31, 2205–2218 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao Y, Cao J, Melamed A, Worley M, Gockley A, Jones D, Nia HT, Zhang Y, Stylianopoulos T, Kumar AS, Mpekris F, Datta M, Sun Y, Wu L, Gao X, Yeku O, Del Carmen MG, Spriggs DR, Jain RK, Xu L, Losartan treatment enhances chemotherapy efficacy and reduces ascites in ovarian cancer models by normalizing the tumor stroma. Proc. Natl. Acad. Sci. U.S.A 116, 2210–2219 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jain RK, Martin JD, Stylianopoulos T, The role of mechanical forces in tumor growth and therapy. Annu. Rev. Biomed. Eng 16, 321–346 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jain RK, Antiangiogenesis strategies revisited: From starving tumors to alleviating hypoxia. Cancer Cell 26, 605–622 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao X, Zhao Y, Stemmer-Rachamimov AO, Liu H, Huang P, Chin S, Selig MK, Plotkin SR, Jain RK, Xu L, Anti-VEGF treatment improves neurological function and augments radiation response in NF2 schwannoma model. Proc. Natl. Acad. Sci. U.S.A 112, 14676–14681 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shoulders MD, Raines RT, Collagen structure and stability. Annu. Rev. Biochem 78, 929–958 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao Y, Liu P, Zhang N, Chen J, Landegger LD, Zhao F, Zhang J, Fujita T, Stemmer-Rachamimov AO, Zhang Y, Ferraro G, Liu H, Muzikansky A, Plotkin S, Stankovic KM, Jain RK, Xu L, Targeting the cMET pathway augments radiation response without adverse effect on hearing in NF2 schwannoma models. Proc. Natl. Acad. Sci. U.S.A 115, E2077–E2084 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Landegger LD, Vasilijic S, Fujita T, Soares VY, Seist R, Xu L, Stankovic KM, Cytokine levels in inner ear fluid of young and aged mice as molecular biomarkers of noise-induced hearing loss. Front. Neurol 10, 977 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ren Y, Stankovic KM, The role of tumor necrosis factor alpha (TNFα) in hearing loss and vestibular schwannomas. Curr. Otorhinolaryngol. Rep 6, 15–23 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sagers JE, Sahin MI, Moon I, Ahmed SG, Stemmer-Rachamimov A, Brenner GJ, Stankovic KM, NLRP3 inflammasome activation in human vestibular schwannoma: Implications for tumor-induced hearing loss. Hear. Res 381, 107770 (2019). [DOI] [PubMed] [Google Scholar]
- 25.Fujioka M, Kanzaki S, Okano HJ, Masuda M, Ogawa K, Okano H, Proinflammatory cytokines expression in noise-induced damaged cochlea. J. Neurosci. Res 83, 575–583 (2006). [DOI] [PubMed] [Google Scholar]
- 26.Fujioka M, Okano H, Ogawa K, Inflammatory and immune responses in the cochlea: Potential therapeutic targets for sensorineural hearing loss. Front. Pharmacol 5, 287 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dilwali S, Landegger LD, Soares VY, Deschler DG, Stankovic KM, Secreted factors from human vestibular schwannomas can cause cochlear damage. Sci. Rep 5, 18599 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dhukhwa A, Bhatta P, Sheth S, Korrapati K, Tieu C, Mamillapalli C, Ramkumar V, Mukherjea D, Targeting inflammatory processes mediated by TRPVI and TNF-α for treating noise-induced hearing loss. Front. Cell. Neurosci 13, 444 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang M, Li H, Johnson A, Karasawa T, Zhang Y, Meier WB, Taghizadeh F, Kachelmeier A, Steyger PS, Inflammation up-regulates cochlear expression of TRPV1 to potentiate drug-induced hearing loss. Sci. Adv 5, eaaw1836 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frye MD, Ryan AF, Kurabi A, Inflammation associated with noise-induced hearing loss. J. Acoust. Soc. Am 146, 4020–4032 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clerici WJ, DiMartino DL, Prasad MR, Direct effects of reactive oxygen species on cochlear outer hair cell shape in vitro. Hear. Res 84, 30–40 (1995). [DOI] [PubMed] [Google Scholar]
- 32.Henderson D, Bielefeld EC, Harris KC, Hu BH, The role of oxidative stress in noise-induced hearing loss. Ear Hear. 27, 1–19 (2006). [DOI] [PubMed] [Google Scholar]
- 33.Poirrier AL, Pincemail J, Van Den Ackerveken P, Lefebvre PP, Malgrange B, Oxidative stress in the cochlea: An update. Curr. Med. Chem 17, 3591–3604 (2010). [DOI] [PubMed] [Google Scholar]
- 34.Ding NH, Li JJ, Sun LQ, Molecular mechanisms and treatment of radiation-induced lung fibrosis. Curr. Drug Targets 14, 1347–1356 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deshmane SL, Kremlev S, Amini S, Sawaya BE, Monocyte chemoattractant protein-1 (MCP-1): An overview. J. Interf. Cytokine Res 29, 313–326 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cassetta L, Pollard JW, Targeting macrophages: Therapeutic approaches in cancer. Nat. Rev. Drug Discov 17, 887–904 (2018). [DOI] [PubMed] [Google Scholar]
- 37.Kawai T, Akira S, Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 34, 637–650 (2011). [DOI] [PubMed] [Google Scholar]
- 38.Medzhitov R, Horng T, Transcriptional control of the inflammatory response. Nat. Rev. Immunol 9, 692–703 (2009). [DOI] [PubMed] [Google Scholar]
- 39.De Batista PR, Palacios R, Martin A, Hernanz R, Medici CT, Silva MA, Rossi EM, Aguado A, Vassallo DV, Salaices M, Alonso MJ, Toll-like receptor 4 upregulation by angiotensin II contributes to hypertension and vascular dysfunction through reactive oxygen species production. PLOS ONE 9, e104020 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pushpakumar S, Ren L, Kundu S, Gamon A, Tyagi SC, Sen U, Toll-like receptor 4 deficiency reduces oxidative stress and macrophage mediated inflammation in hypertensive kidney. Sci. Rep 7, 6349 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wassmann S, Stumpf M, Strehlow K, Schmid A, Schieffer B, Bohm M, Nickenig G, Interleukin-6 induces oxidative stress and endothelial dysfunction by overexpression of the angiotensin II type 1 receptor. Circ. Res 94, 534–541 (2004). [DOI] [PubMed] [Google Scholar]
- 42.Bentzen SM, Preventing or reducing late side effects of radiation therapy: Radiobiology meets molecular pathology. Nat. Rev. Cancer 6, 702–713 (2006). [DOI] [PubMed] [Google Scholar]
- 43.Afik R, Zigmond E, Vugman M, Klepfish M, Shimshoni E, Pasmanik-Chor M, Shenoy A, Bassat E, Halpern Z, Geiger T, Sagi I, Varol C, Tumor macrophages are pivotal constructors of tumor collagenous matrix. J. Exp. Med 213, 2315–2331 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patel SN, Fatima N, Ali R, Hussain T, Emerging role of angiotensin AT2 receptor in anti-inflammation: An update. Curr. Pharm. Des 26, 492–500 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu F, Mao C, Liu Y, Wu L, Xu Z, Zhang L, Losartan chemistry and its effects via AT1 mechanisms in the kidney. Curr. Med. Chem 16, 3701–3715 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steckelings UM, Kaschina E, Unger T, The AT2 receptor: A matter of love and hate. Peptides 26, 1401–1409 (2005). [DOI] [PubMed] [Google Scholar]
- 47.Hatch JL, Bauschard MJ, Nguyen SA, Lambert PR, Meyer TA, McRackan TR, National trends in vestibular schwannoma surgery: Influence of patient characteristics on outcomes. Otolaryngol. Head Neck Surg 159, 102–109 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nia HT, Munn LL, Jain RK, Physical traits of cancer. Science 370, eaaz0868 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ndubaku U, de Bellard ME, Glial cells: Old cells with new twists. Acta Histochem. 110, 182–195 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pinter M, Jain RK, Targeting the renin-angiotensin system to improve cancer treatment: Implications for immunotherapy. Sci. Transl. Med 9, eaan5616 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simoes ESAC, Teixeira MM, ACE inhibition, ACE2 and angiotensin-(1–7) axis in kidney and cardiac inflammation and fibrosis. Pharmacol. Res 107, 154–162 (2016). [DOI] [PubMed] [Google Scholar]
- 52.Unal H, Karnik SS, Constitutive activity in the angiotensin II type 1 receptor. Adv. Pharmacol 70, 155–174 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hwang JC, Del Priore LV, Freund KB, Chang S, Iranmanesh R, Development of subretinal fibrosis after anti-VEGF treatment in neovascular age-related macular degeneration. Ophthalmic Surg. Lasers Imaging 42, 6–11 (2011). [DOI] [PubMed] [Google Scholar]
- 54.Chu SJ, Zhang ZH, Wang M, Xu HF, Effect of bevacizumab on the expression of fibrosis-related inflammatory mediators in ARPE-19 cells. Int. J. Ophthalmol 10, 366–371 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Daniel E, Pan W, Ying GS, Kim BJ, Grunwald JE, Ferris III FL, Jaffe GJ, Toth CA, Martin DF, Fine SL, Maguire MG; Comparison of Age-related Macular Degeneration Treatments Trials, Development and course of scars in the comparison of age-related macular degeneration treatments trials. Ophthalmology 125, 1037–1046 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Satoh H, Firestein GS, Billings PB, Harris JP, Keithley EM, Proinflammatory cytokine expression in the endolymphatic sac during inner ear inflammation. J. Assoc. Res. Otolaryngol 4, 139–147 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Uchida Y, Sugiura S, Ueda H, Nakashima T, Ando F, Shimokata H, The association between hearing impairment and polymorphisms of genes encoding inflammatory mediators in Japanese aged population. Immun. Ageing 11, 18 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Trune DR, Kempton B, Hausman FA, Larrain BE, MacArthur CJ, Correlative mRNA and protein expression of middle and inner ear inflammatory cytokines during mouse acute otitis media. Hear. Res 326, 49–58 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.MacArthur CJ, Pillers DA, Pang J, Kempton JB, Trune DR, Altered expression of middle and inner ear cytokines in mouse otitis media. Laryngoscope 121, 365–371 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saavedra JM, Sanchez-Lemus E, Benicky J, Blockade of brain angiotensin II AT1 receptors ameliorates stress, anxiety, brain inflammation, and ischemia: Therapeutic implications. Psychoneuroendocrinology 36, 1–18 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ando H, Zhou J, Macova M, Imboden H, Saavedra JM, Angiotensin II AT1 receptor blockade reverses pathological hypertrophy and inflammation in brain microvessels of spontaneously hypertensive rats. Stroke 35, 1726–1731 (2004). [DOI] [PubMed] [Google Scholar]
- 62.Lou M, Blume A, Zhao Y, Gohlke P, Deuschl G, Herdegen T, Culman J, Sustained blockade of brain AT1 receptors before and after focal cerebral ischemia alleviates neurologic deficits and reduces neuronal injury, apoptosis, and inflammatory responses in the rat. J. Cereb. Blood Flow Metab 24, 536–547 (2004). [DOI] [PubMed] [Google Scholar]
- 63.Jung KH, Chu K, Lee ST, Kim SJ, Song EC, Kim EH, Park DK, Sinn DI, Kim JM, Kim M, Roh JK, Blockade of AT1 receptor reduces apoptosis, inflammation, and oxidative stress in normotensive rats with intracerebral hemorrhage. J. Pharmacol. Exp. Ther 322, 1051–1058 (2007). [DOI] [PubMed] [Google Scholar]
- 64.Lanz TV, Ding Z, Ho PP, Luo J, Agrawal AN, Srinagesh H, Axtell R, Zhang H, Platten M, Wyss-Coray T, Steinman L, Angiotensin II sustains brain inflammation in mice via TGF-β. J. Clin. Invest 120, 2782–2794 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mertens B, Vanderheyden P, Michotte Y, Sarre S, The role of the central renin-angiotensin system in Parkinson’s disease. J. Renin-Angiotensin-Aldosterone Syst 11, 49–56 (2010). [DOI] [PubMed] [Google Scholar]
- 66.Tsukuda K, Mogi M, Iwanami J, Min LJ, Sakata A, Jing F, Iwai M, Horiuchi M, Cognitive deficit in amyloid-β–injected mice was improved by pretreatment with a low dose of telmisartan partly because of peroxisome proliferator-activated receptor-γ activation. Hypertension 54, 782–787 (2009). [DOI] [PubMed] [Google Scholar]
- 67.Zhang W, Dai M, Fridberger A, Hassan A, Degagne J, Neng L, Zhang F, He W, Ren T, Trune D, Auer M, Shi X, Perivascular-resident macrophage-like melanocytes in the inner ear are essential for the integrity of the intrastrial fluid-blood barrier. Proc. Natl. Acad. Sci. U.S.A 109, 10388–10393 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu W, Molnar M, Garnham C, Benav H, Rask-Andersen H, Macrophages in the human cochlea: Saviors or predators - a study using super-resolution immunohistochemistry. Front. Immunol 9, 223 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Persson O, Bartek J Jr., Shalom NB, Wangerid T, Jakola AS, Forander P, Stereotactic radiosurgery vs. fractionated radiotherapy for tumor control in vestibular schwannoma patients: A systematic review. Acta Neurochir. 159, 1013–1021 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gan HK, Bernstein LJ, Brown J, Ringash J, Vakilha M, Wang L, Goldstein D, Kim J, Hope A, O’Sullivan B, Waldron J, Abdul Razak AR, Chen EX, Siu LL, Cognitive functioning after radiotherapy or chemoradiotherapy for head-and-neck cancer. Int. J. Radiat. Oncol. Biol. Phys 81, 126–134 (2011). [DOI] [PubMed] [Google Scholar]
- 71.Gondi V, Hermann BP, Mehta MP, Tome WA, Hippocampal dosimetry predicts neurocognitive function impairment after fractionated stereotactic radiotherapy for benign or low-grade adult brain tumors. Int. J. Radiat. Oncol. Biol. Phys 85, 348–354 (2013). [DOI] [PubMed] [Google Scholar]
- 72.Hansen CC, Smith JB, Mohamed ASR, Mulcahy CF, Wefel JS, Hutcheson KA, Chrane K, Phan J, Frank SJ, Garden AS, Smith BD, Eichelberger H, Anderson C, McCoy C, Horiates M, Patrick C, Floris S, French C, Beadle BM, Morrison WH, Su SY, Lewis CM, Kupferman ME, Johnson JM, Skinner HD, Lai SY, Hanna EY, Rosenthal DI, Fuller CD, Gunn GB; MD Anderson Head and Neck Cancer Symptom Working Group, Cognitive function and patient-reported memory problems after radiotherapy for cancers at the skull base: A cross-sectional survivorship study using the Telephone Interview for Cognitive Status and the MD Anderson Symptom Inventory-Head and Neck Module. Head Neck 39, 2048–2056 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Koetsier KS, Hensen EF, Wiggenraad R, Lips IM, van Benthem PPG, van Vulpen M, Shih HA, Clinical outcomes and toxicity of proton radiotherapy for vestibular schwannomas: A systematic review. J. Radiat. Oncol 8, 357–368 (2019). [Google Scholar]
- 74.Pike LRG, Horick NK, Yeap BY, Franck KH, Wang I, Loeffler JS, McKenna MJ, Fullerton BC, Shih HA, Early outcomes of hearing preservation following fractionated proton radiation therapy for vestibular schwannoma, in Proceedings of the 58th Annual Conference of the Particle Therapy Co-Operative Group (2019). [Google Scholar]
- 75.Pinter M, Kwanten WJ, Jain RK, Renin-angiotensin system inhibitors to mitigate cancer treatment-related adverse events. Clin. Cancer Res 24, 3803–3812 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Citrin DE, Prasanna PGS, Walker AJ, Freeman ML, Eke I, Barcellos-Hoff MH, Arankalayil MJ, Cohen EP, Wilkins RC, Ahmed MM, Anscher MS, Movsas B, Buchsbaum JC, Mendonca MS, Wynn TA, Coleman CN, Radiation-induced fibrosis: Mechanisms and opportunities to mitigate. Report of an NCI workshop, September 19, 2016. Radiat. Res 188, 1–20 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yoshino H, Chiba K, Saitoh T, Kashiwakura I, Ionizing radiation affects the expression of Toll-like receptors 2 and 4 in human monocytic cells through c-Jun N-terminal kinase activation. J. Radiat. Res 55, 876–884 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Naros G, Sandritter J, Liebsch M, Ofori A, Rizk AR, Del Moro G, Ebner F, Tatagiba M, Predictors of preoperative tinnitus in unilateral sporadic vestibular schwannoma. Front. Neurol 8, 378 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Han KH, Mun SK, Sohn S, Piao XY, Park I, Chang M, Axonal sprouting in the dorsal cochlear nucleus affects gap-prepulse inhibition following noise exposure. Int. J. Mol. Med 44, 1473–1483 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Reimand J, Isserlin R, Voisin V, Kucera M, Tannus-Lopes C, Rostamianfar A, Wadi L, Meyer M, Wong J, Xu C, Merico D, Bader GD, Pathway enrichment analysis and visualization of omics data using g:Profiler, GSEA, Cytoscape and EnrichmentMap. Nat. Protoc 14, 482–517 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wong HK, Lahdenranta J, Kamoun WS, Chan AW, McClatchey AI, Plotkin SR, Jain RK, di Tomaso E, Anti-vascular endothelial growth factor therapies as a novel therapeutic approach to treating neurofibromatosis-related tumors. Cancer Res. 70, 3483–3493 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen J, Landegger LD, Sun Y, Ren J, Maimon N, Wu L, Ng MR, Chen JW, Zhang N, Zhao Y, Gao X, Fujita T, Roberge S, Huang P, Jain RK, Plotkin SR, Stankovic KM, Xu L, A cerebellopontine angle mouse model for the investigation of tumor biology, hearing, and neurological function in NF2-related vestibular schwannoma. Nat. Protoc 14, 541–555 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Diop-Frimpong B, Chauhan VP, Krane S, Boucher Y, Jain RK, Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proc. Natl. Acad. Sci. U.S.A 108, 2909–2914 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chung E, Yamashita H, Au P, Tannous BA, Fukumura D, Jain RK, Secreted Gaussia luciferase as a biomarker for monitoring tumor progression and treatment response of systemic metastases. PLOS ONE 4, e8316 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tannous BA, Gaussia luciferase reporter assay for monitoring biological processes in culture and in vivo. Nat. Protoc 4, 582–591 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kodack DP, Chung E, Yamashita H, Incio J, Duyverman AM, Song Y, Farrar CT, Huang Y, Ager E, Kamoun W, Goel S, Snuderl M, Lussiez A, Hiddingh L, Mahmood S, Tannous BA, Eichler AF, Fukumura D, Engelman JA, Jain RK, Combined targeting of HER2 and VEGFR2 for effective treatment of HER2-amplified breast cancer brain metastases. Proc. Natl. Acad. Sci. U.S.A 109, E3119–E3127 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brown E, McKee T, diTomaso E, Pluen A, Seed B, Boucher Y, Jain RK, Dynamic imaging of collagen and its modulation in tumors in vivo using second-harmonic generation. Nat. Med 9, 796–800 (2003). [DOI] [PubMed] [Google Scholar]
- 88.Xu L, Xie K, Fidler IJ, Therapy of human ovarian cancer by transfection with the murine interferon-β gene: Role of macrophage-inducible nitric oxide synthase. Hum. Gene Ther 9, 2699–2708 (1998). [DOI] [PubMed] [Google Scholar]
- 89.Liao S, Liu J, Lin P, Shi T, Jain RK, Xu L, TGF-β blockade controls ascites by preventing abnormalization of lymphatic vessels in orthotopic human ovarian carcinoma models. Clin. Cancer Res 17, 1415–1424 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xu L, Tong R, Cochran DM, Jain RK, Blocking platelet-derived growth factor-D/platelet-derived growth factor receptor β signaling inhibits human renal cell carcinoma progression in an orthotopic mouse model. Cancer Res. 65, 5711–5719 (2005). [DOI] [PubMed] [Google Scholar]
- 91.Duran-Struuck R, Dysko RC, Principles of bone marrow transplantation (BMT): Providing optimal veterinary and husbandry care to irradiated mice in BMT studies. J. Am. Assoc. Lab. Anim. Sci 48, 11–22 (2009). [PMC free article] [PubMed] [Google Scholar]
- 92.Landegger LD, Sagers JE, Dilwali S, Fujita T, Sahin MI, Stankovic KM, A unified methodological framework for vestibular schwannoma research. J. Vis. Exp 2017, 55827 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Floyd SR, Pacold ME, Huang Q, Clarke SM, Lam FC, Cannell IG, Bryson BD, Rameseder J, Lee MJ, Blake EJ, Fydrych A, Ho R, Greenberger BA, Chen GC, Maffa A, Del Rosario AM, Root DE, Carpenter AE, Hahn WC, Sabatini DM, Chen CC, White FM, Bradner JE, Yaffe MB, The bromodomain protein Brd4 insulates chromatin from DNA damage signalling. Nature 498, 246–250 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Winkler F, Kozin SV, Tong RT, Chae SS, Booth MF, Garkavtsev I, Xu L, Hicklin DJ, Fukumura D, di Tomaso E, Munn LL, Jain RK, Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: Role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell 6, 553–563 (2004). [DOI] [PubMed] [Google Scholar]
- 95.Zhao H, Joseph J, Fales HM, Sokoloski EA, Levine RL, Vasquez-Vivar J, Kalyanaraman B, Detection and characterization of the product of hydroethidine and intracellular superoxide by HPLC and limitations of fluorescence. Proc. Natl. Acad. Sci. U.S.A 102, 5727–5732 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Early S, Rinnooy Kan CE, Eggink M, Frijns JHM, Stankovic KM, Progression of contralateral hearing loss in patients with sporadic vestibular schwannoma. Front. Neurol 11, 796 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Halpin C, Rauch SD, Using audiometric thresholds and word recognition in a treatment study. Otol. Neurotol 27, 110–116 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data associated with this study are present in the paper or the Supplementary Materials.


