Abstract
The association of the cystic fibrosis transmembrane conductance regulator (CFTR) and epithelial sodium channel (ENaC) in the pathophysiology of cystic fibrosis (CF) is controversial. Previously, we demonstrated a close physical association between wild-type (WT) CFTR and WT ENaC. We have also shown that the F508del CFTR fails to associate with ENaC unless the mutant protein is rescued pharmacologically or by low temperature. In this study, we present the evidence for a direct physical association between WT CFTR and ENaC subunits carrying Liddle’s syndrome mutations. We show that all three ENaC subunits bearing Liddle’s syndrome mutations (both point mutations and the complete truncation of the carboxy terminus), could be coimmunoprecipitated with WT CFTR. The biochemical studies were complemented by fluorescence lifetime imaging microscopy (FLIM), a distance-dependent approach that monitors protein-protein interactions between fluorescently labeled molecules. Our measurements revealed significantly increased fluorescence resonance energy transfer between CFTR and all tested ENaC combinations as compared with controls (ECFP and EYFP cotransfected cells). Our findings are consistent with the notion that CFTR and ENaC are within reach of each other even in the setting of Liddle’s syndrome mutations, suggestive of a direct intermolecular interaction between these two proteins.
Keywords: CFTR, cystic fibrosis, ENaC, FLIM, Liddle’s syndrome
INTRODUCTION
Cystic fibrosis (CF) is an inherited monogenic disease resulting from loss-of-function mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) protein (1–3), a product of the CFTR gene. Mutations of this protein compromise ion transport in multiple epithelia, including those of the sweat glands, airways, gastrointestinal, and reproductive systems (4). With advances in modulators (correctors and potentiators), enzyme replacement, and nutritional support, persistent lung disease is now the major factor in CF mortality (5). Interestingly, it has been shown that not only the loss-of-function of CFTR protein (6) but also gain-of-function of a second protein epithelial sodium channel (ENaC) contributes to CF airway disease (7, 8). ENaC is a member of the degenerin/ENaC superfamily of ion channels, found in a variety of epithelial and nonepithelial tissues, and plays a critical role in water and sodium homeostasis (9, 10). In addition to CF (8), the improper functioning of ENaC has been linked to pseudohypoaldosteronism type I (11–13), pulmonary edema (14), and Liddle’s syndrome (15). The gain-of-function of ENaC, which leads to the Na+ hyperabsorption, is believed to result from inability of mutated CFTR to regulate ENaC (8); this view, however, is controversial and still debated (16–21). Recent studies also identified association between ENaC encoding genes with CF-related phenotypes (22, 23).
Clinically, patients with CF display a wide spectrum of phenotypic presentation, complications, and survival. However, allelic variations in CFTR account only partially for the phenotypic variability in the disease (24–27). In addition, the correlation between CFTR genotype and lung function appears to be minimal (28–30). These findings suggested that other factors, in addition to mutations in CFTR, might contribute to the progression of CF lung disease (29, 30), and have led to the concept that modifier genes (31, 32) may affect genotype-phenotype correlations and disease progression in CF (32). There is a possibility that ENaC encoded genes might serve as one of such modifier genes (33, 34). There is a well-described reciprocal interaction between CFTR and ENaC (8, 35–45). This was further supported by the observation that overexpression of βENaC in mice resulted in the animals developing lung disease with features of CF, whereas knockout of CFTR alone did not (46). Notably, overexpression of αENaC and/or γENaC alone or in combination did not result in sodium hyperabsorption, and overexpression of βENaC in combination with other subunits correlated with more severe lung phenotype (47). Interestingly, the generation of the congenic mouse (the double mutant lacking CFTR and overexpressing βENaC) produced more severe lung pathology than either defect alone (48). Moreover, both gain-of-function (46, 49, 50) and loss-of-function mutations (51, 52) in ENaC subunits may contribute to CF pathophysiology, with some ENaC mutations thought to play a role in patients developing CF-like pulmonary symptoms in the absence of any mutation in CFTR (49, 51–54).
Gain-of-function mutations in ENaC subunits have been described in patients suffering from an autosomal-dominant form (Liddle’s syndrome) of hypertension (15, 55–57). Liddle’s syndrome is characterized by early onset severe hypertension, metabolic alkalosis, and renal potassium wasting; despite these clear signs of hyperaldosteronism, the levels of plasma renin and aldosterone are very low (58). These gain-of-function mutations interfere with ubiquitination and retrieval of ENaC (59–61) from the apical surface of the renal principal cell, leading to retention of channels in the plasma membrane, which, in turn, leads to unrestrained sodium reabsorption and salt-sensitive hypertension (62–64). Interestingly, patients with Liddle’s syndrome (65) or mice carrying the Liddle’s mutation (66) do not suffer from lung disease (67). On the other hand, mice overexpressing βENaC in the airways develop a distinct CF-like lung disease (46). It has been proposed that the presence of CFTR may protect patients of Liddle’s syndrome (67) from CF-like lung disease as a result of downregulation of ENaC activity by wild-type (WT) CFTR (36, 38, 43). Although previous studies have demonstrated that CFTR restricts sodium transport via ENaC (35–40, 43, 68), and that failure of mutated CFTR to inhibit ENaC leads to sodium hyperabsorption (36, 69), this view is still somewhat controversial (17, 21).
We have previously demonstrated the close association between WT CFTR and ENaC (35, 37, 68); a deletion of phenylalanine at position of 508 (F508del-CFTR) disrupted this link unless F508del-CFTR was partially corrected by low temperature or chemical correctors (44). This study examines the converse scenario, examining the role of disease-causing mutations in ENaC on its close association with WT CFTR to help understand the pathophysiology of CF lung disease. We have combined both an independent biophysical approach, fluorescence lifetime imaging measurements (FLIM), and surface coimmunoprecipitation (IP) to determine if there is a physical basis for the interaction between WT CFTR and ENaC subunits carrying Liddle’s syndrome mutations. Our FLIM results showed a significant increase in fluorescence resonance energy transfer (FRET) efficiencies between WT CFTR and mutated ENaC subunits, as compared with control. Furthermore, WT CFTR coimmunoprecipitated all three ENaC subunits carrying Liddle’s syndrome mutations [both the point mutation (PM) and the complete truncation of C-terminus]. In addition, the presence of these mutations enhanced the surface coimmunoprecipitation signal between ENaC and WT CFTR. Our results suggest that CFTR can associate with ENaC even in the setting of Liddle’s syndrome mutations.
EXPERIMENTAL PROCEDURES
Cell Culture
The human embryonic kidney 293T (HEK293T) cells (kind gift of Drs. K. Kirk and W. Wang, University of Alabama at Birmingham) were maintained (35) at 37°C with 5% CO2, 95% air atmosphere in Dulbecco’s modified Eagle’s medium (Invitrogen) supplemented with 10% fetal bovine serum (HyClone, Logan, UT), penicillin (100 IU/mL, Invitrogen), and streptomycin (100 μg/mL, Invitrogen).
Transient Transfection
A day before transfection, cells were trypsinized (Mediatech, Herndon, VA) and seeded onto glass bottom dishes (In Vitro Scientific, Sunnyvale, CA) coated with poly-l-lysine (Sigma, St. Louis, MO). The transient transfections were carried out at room temperature. Lipofectamine 2000 (Invitrogen) was used for transient transfections according to the manufacturer’s protocol as described previously (35, 44), using a 0.2 μg/1 μL—DNA/Lipofectamine 2000 ratio in 200 μL Opti-MEM I (Invitrogen). The diluted DNA constructs and Lipofectamine 2000 reagent were combined together following a 5-min incubation. The transfection solution was added to the cells in Opti-MEM I (without fetal bovine serum and antibiotics). Cells were incubated at 37°C in a 5% CO2-95% air incubator; after 5 h the transfection solution was changed to the regular growth medium without antibiotics but including 10% FBS and incubated for 48 h. An identical protocol was used to transfect the cells for coimmunoprecipitation experiments, using a DNA/Lipofectamine 2000 ratio of 1 μg/3 μL.
Generation of Fluorophore-Tagged CFTR and ENaC cDNAs
Generation of fluorophore tagged WT CFTR and WT hENaC cDNAs was described previously (35, 70). The CFTR construct N-terminally tagged with the enhanced green fluorescent protein (EGFP-CFTR) was a kind gift of Dr. B. Stanton, Dartmouth Medical School (71). To generate the CFTR construct tagged at its N-terminus with the enhanced cyan fluorescent protein (ECFP-CFTR), the EGFP was exchanged with ECFP or enhanced yellow fluorescent protein (EYFP) following Nhe I/Xho I (New England Biolabs, Ipswich, MA or Promega, Madison, WI) digestion and ligation (35). The human chloride channel 1 with the ECFP fused to the N-terminus [(ECFP-ClC-1, (72)] was a kind gift of Dr. Christoph Fahlke (Institut für Neurophysiologie Medizinische Hochschule Hannover, Hannover, Germany). The Quickchange II XL kit (Agilent Technologies, La Jolla, CA) was utilized to generate constructs of ENaC subunits carrying Liddle’s syndrome mutations and truncations. To truncate the C-terminal end of each ENaC subunit tagged with EYFP at N-teminus (70), a stop codon was introduced right after the second transmembrane domain of each subunit: EYFP-αhENaC R586X, EYFP-βhENaC A557X, EYFP-γhENaC A562X. The point mutations were introduced into C-termini of each ENaC subunits tagged with EYFP (35, 70) at the C-terminus: αhENaC-EYFP Y644A, βhENaC-EYFP Y620A, γhENaC-EYFP Y627A. Each ENaC construct was subjected to PCR with sense and antisense primers with the necessary base changes to result in the desired amino acid mutations. The PCR product was digested overnight at 37°C with DpnI to remove nonmutated DNA, and it was transformed into XL-10 Gold Escherichia coli following the manufacturer’s instructions. Transformed E. coli were plated on Luria Bertani (LB) plates with 50 μg/mL kanamycin and grown for 16 h at 37°C. DNA was isolated from 5 mL LB + kanamycin cultures of each colony using a miniprep kit (Qiagen, Valencia, CA), and the presence of the mutations was confirmed by sequencing (Heflin Genetics Center, UAB). Colonies were then grown into 250 mL LB + kanamycin cultures, and DNA was isolated with a maxiprep kit (Qiagen). The presence of the correct mutation was confirmed by sequencing again after this step.
ENaC Antibodies
Rabbit polyclonal antibodies (Abs) were generated (35) against synthetic peptides in collaboration with Drs. Mark Knepper and Patricia A. Gonzales (NIH, Bethesda, MD).
Western Blot Analysis of CFTR and ENaC
HEK293T cells were transfected with 2 μg ECFP-CFTR construct. Forty-eight hours post-transfection, cells were lysed with RadioImmuno Precipitation Assay buffer (Pierce, Rockford, IL) complemented with Complete protease inhibitor cocktail (Roche Biochemical, Mannheim, Germany) at 4°C (73, 74). After centrifugation (15,800 g for 10 min at 4°C) nonsoluble material was discarded. Total cell lysate (100 μg) was subjected to 6% SDS-PAGE (Invitrogen), followed by transfer to polyvinylidene difluoride (PVDF) membrane (Bio-Rad, Hercules, CA) that was probed with 1:10,000 diluted polyclonal Ab against the second nucleotide binding domain of CFTR (UAB Cystic Fibrosis Research Center). Detection was accomplished using a secondary anti-rabbit antibody conjugated to horseradish peroxidase (HRP) (Dako, Denmark) and chemiluminescence. Western blot analysis of tagged ENaC constructs was as described previously (35).
WT-CFTR and ENaC Coimmunoprecipitation Experiments
The standard coimmunoprecipitation protocol was described in great detail previously (35, 44, 74).
Surface Coimmunoprecipitation
The surface biotinylation and coimmunoprecipitation was combined as previously described (44, 75, 76) to detect the surface expression changes following the C-terminal truncation/mutations in ENaC subunits. Briefly, the HEK293T cells were cotransfected with WT-CFTR and all three ENaC subunits, under conditions where one of the three subunits in αβγ-ENaC was tagged with EYFP; α-EYFP-βγ, αβ-EYFP-γ, and αβγ-EYFP. The cells were used 48–72 h post-transfection. Cells were washed three times with ice-cold PBS containing (in mM) 1 MgCl2 and 0.1 CaCl2 and incubated with sulfosuccinimidyl-2-(biotinamido)-ethyl-1,3′-dithiopropionate (Pierce; 1.5 mg/mL in ice cold PBS) for 30 min at 4°C. Then, the cells were washed twice with freshly prepared quenching solution (50 mM glycine in PBS) and lysed by radioimmunoprecipitation assay buffer (150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, and 50 mM Tris; pH 7.4) with protease inhibitor cocktail tablets. The lysed cells were homogenized by passing several times through a 22-g needle and centrifuged at 13,200 rpm for 30 min at 4°C. The bicinchoninic acid (BCA) protein assay was used to determine the supernatant protein concentration. CFTR was immunoprecipitated by monoclonal anti-mouse CFTR antibody clone 24-1 (R&D Systems, Minneapolis, MN, 4 µg to 2,000 µg of protein cell lysate) with an overnight incubation at 4°C, using protein G beads (100 µL, Pierce, washed three times with lysis buffer) and the samples were incubated overnight at 4°C. The samples were centrifuged at 5,000 rpm for 5 min, and the beads were collected and washed three times with lysis buffer. The supernatant was discarded, and the bead-IP-CFTR interactions were disrupted by incubating with 150 µL of 1% SDS for 2 h at 37°C. Following the centrifugation at 5,000 rpm for 5 min, the supernatant was mixed with 450 µL of lysis buffer. The streptavidin beads (100 µL; Pierce, washed three times with lysis buffer) were added to the samples. After an overnight incubation at 4°C, the streptavidin beads were collected as described, and the supernatant was discarded. Then, the samples were heated for 6 min at 95°C in 1X Laemmli sample buffer (25% glycerol, 2% SDS, 0.01% bromophenol blue, 10% β-mercaptoethanol, 62.5 mM Tris HCl, pH 6.8) and subjected to SDS-PAGE over 10% separating gels. After the transfer, the samples were blocked with 5% bovine serum albumin (BSA) in Tris-buffered saline [100 mM Tris (pH 7.5), 150 mM NaCl], with Tween-20 (0.1%: Bio-Rad; TBS-T) at room temperature for 1 h. The samples were probed overnight at 4°C with mouse anti-GFP monoclonal antibody (Abgent, San Diego, CA) at 1:2,000 in 5% BSA in TBS-T.
FLIM Imaging
FLIM imaging was performed as previously described (44). Briefly, HEK293T cells were seeded on a glass bottom dishes (In Vitro Scientific, Sunnyvale, CA) and transfected with appropriate constructs as described. FLIM measurements in a time domain mode were carried out using a Zeiss 710 confocal system (Carl Zeiss MicroImaging, Thornwood, New York) outfitted with Becker & Hickl GmbH (Berlin, Germany) FLIM system. A 405-nm picosecond pulsed diode laser integrated into the Zeiss confocal system with 50 MHz repetition rate and a pulse width of around 70 ps was used as an excitation source. The apparent mean lifetime of the donor was calculated from a double exponential fit of the fluorescence decay curves with the SPC Image software (Becker & Hickl). FRET efficiency (E) was calculated using SPC Image software: E = 1 − (TFRET/TECFP-CFTR). The Student’s two-tailed test was used to calculate the difference between groups; each experimental set was compared with negative control, and the significance was set at P < 0.05.
RESULTS
ENaC Subunits with Liddle’s Mutations Interact with CFTR by Immunoprecipitation
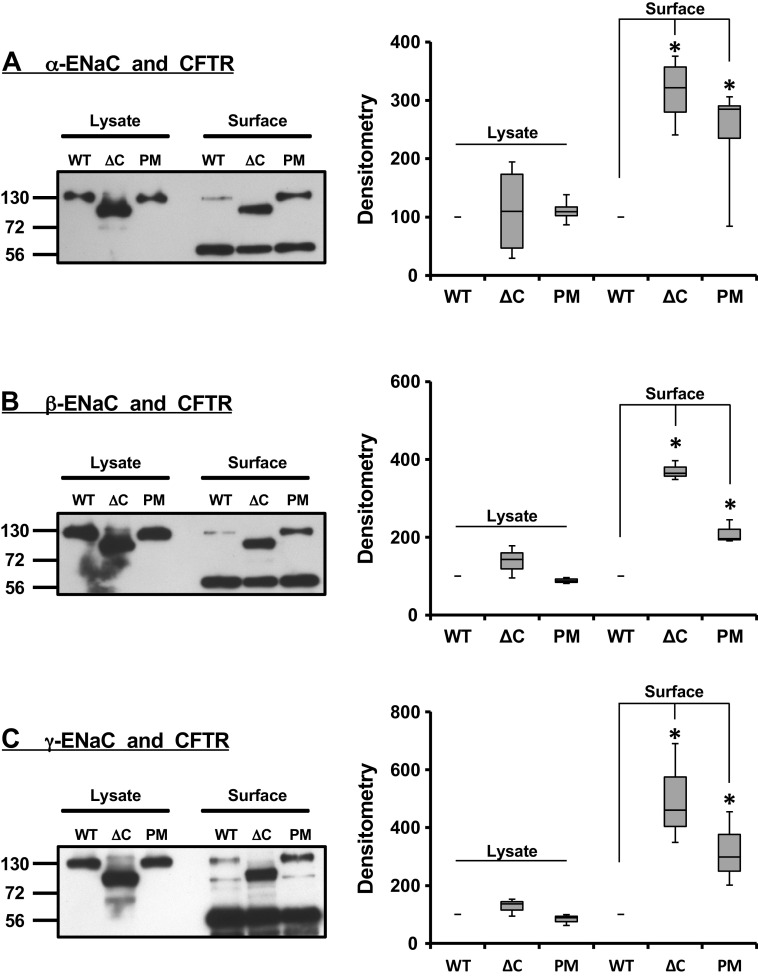
Previous studies demonstrated that the fusion of a fluorescent tag to the WT or mutated CFTR and ENaC subunits did not affect protein expression (35, 44, 71), trafficking, or channel function (35, 70, 71, 77, 78). When WT-CFTR and mutated α-, β-, and γ-ENaC were coexpressed, each ENaC subunit coimmunoprecipitated with CFTR (Fig. 1, left side of each blot). These findings are consistent with CFTR and mutated ENaC subunits being in close proximity. As expected, the C-terminally truncated subunits had a lower molecular mass (Fig. 1, left side of each blot, the lanes denoted as ΔC) as compared with the WT (lanes labeled WT), and the point mutation (lanes labeled PM). Liddle’s syndrome mutations are known to lead to an increase in the surface expression of ENaC subunits. Therefore, we tested the hypothesis that these mutations also can lead to enhanced coimmunoprecipitation signal between CFTR and ENaC subunits. Figure 1 summarizes the outcome of these experiments. As expected, both the point mutation (PM) and the complete truncation of the C-terminus (ΔC) enhanced the coimmunoprecipitation signal between CFTR and ENaC as compared with the WT ENaC subunits (Fig. 1, right side of each blot). This increase in coimmunoprecipitation signal was statistically significant compared with WT (Fig. 1, corresponding densitometry analyses). Taken together, our results indicate that CFTR interacts with individual ENaC subunits carrying Liddle’s syndrome mutations, and that an increase in the amount of surface ENaC significantly enhances the total amount of ENaC that can be immunoprecipitated by CFTR. These results suggest that CFTR and ENaC interact at the plasma membrane.
Figure 1.
Surface coimmunoprecipitation of mutated epithelial sodium channel (ENaC) subunits with cystic fibrosis transmembrane conductance regulator (CFTR). Surface biotinylation was combined with coimmunoprecipitation to monitor changes in surface expression of CFTR and α-ENaC (A), CFTR and β-ENaC (B), and CFTR and γ-ENaC (C). The cells were cotransfected with CFTR and all three ENaC subunits: in each case one of three subunits in the αβγ-ENaC complex was tagged with enhanced yellow fluorescent protein (EYFP). In all blots and graphs, the wild-type construct of ENaC subunit with EYFP at C-terminus is denoted as WT, the subunit truncated at C-terminus and N-terminally tagged with EYFP designated as ΔC, and the subunit with single-point mutation at C-terminus and C-terminally tagged with EYFP indicated as PM. Both the C-terminal truncation and single-point mutation at C-terminus of each individual ENaC subunit enhanced the coimmunoprecipitation signal and was statistically significant (*the corresponding densitometry bar graphs. Statistical significance was calculated using the Student’s t test). The changes in densitometry values following the C-terminal truncation and single point mutation were normalized to that of WT (100%). The bands at 56 kDa in each panel indicate the high-molecular weight immunoglobulin band. These experiments were repeated at least five times with similar results.
FRET Measurements by FLIM Microscopy
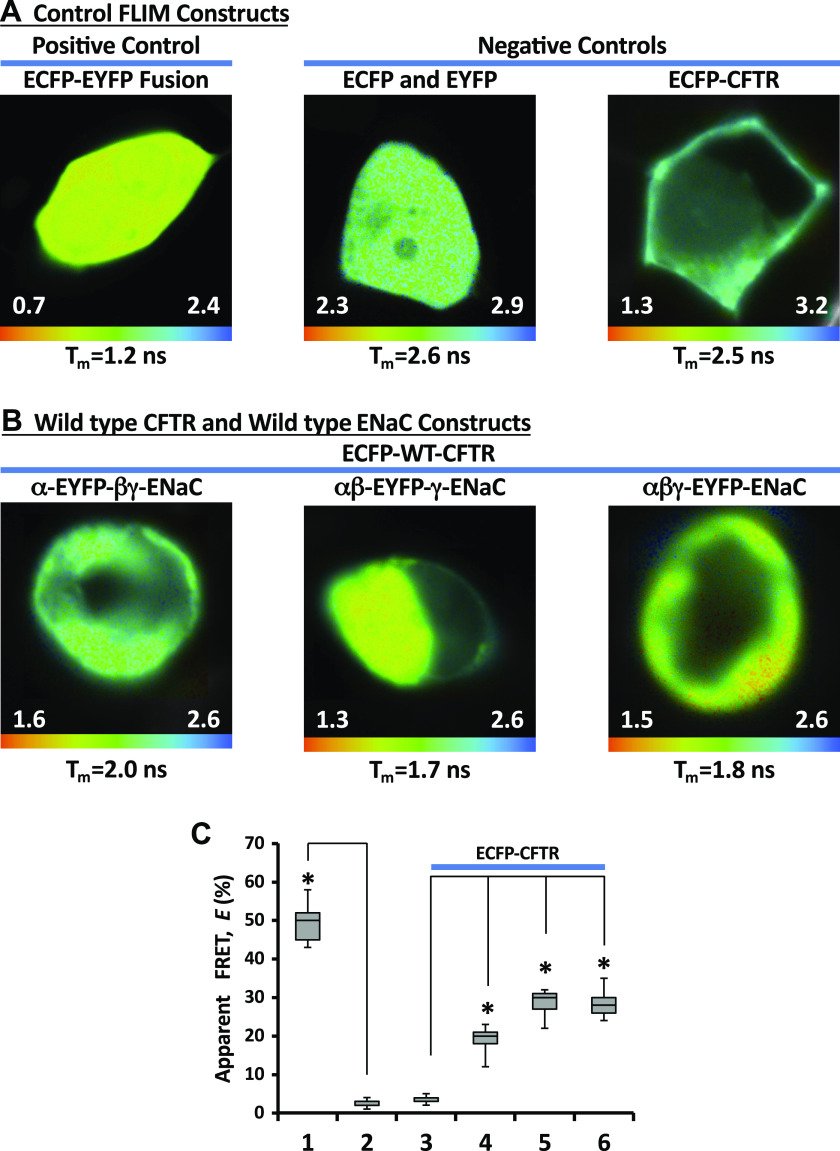
Next, we assessed the proximity of WT-CFTR and ENaC subunits carrying Liddle’s syndrome mutations using FLIM imaging. FLIM measures the average time the fluorophore spends in an excited state; a decrease in a donor fluorophore lifetime in the presence of an acceptor fluorophore indicates that they are less than 10 nm apart (79–81). An apparent FRET efficiency is calculated from the ratio of the donor lifetimes in the presence and in the absence of the acceptor. To obtain control FLIM measurements, the cells were transfected with our negative control constructs, (unlinked ECFP and EYFP) or ECFP tagged to CFTR. As shown in Fig. 2A (right), these negative controls exhibited an average donor lifetime (τECFP) of ∼2.5 ns as reported previously (84). On the other hand, ECFP directly fused with EYFP via a 7 amino acid linker (ECFP-EYFP), which served as a positive control, had an average donor lifetime of ∼1.2 ns (Fig. 2A, left). This decrease in fluorescence lifetime reflects an energy transfer from ECFP to EYFP, with an average efficiency (E) of around ∼55% (Fig. 2C).
Figure 2.
Fluorescence resonance energy transfer (FRET) measurement by fluorescence lifetime imaging microscopy. Enhanced cyan fluorescent protein (ECFP)-enhanced yellow fluorescent protein (EYFP) fusion protein separated by a 7 amino acid linker served as a positive control (A, left) while separately coexpressed ECFP and EYFP, or ECFP-CFTR expressed alone served as a negative control (A, right). The images in B are shown for the cells transfected with wild type (WT)-ECFP-cystic fibrosis transmembrane conductance regulator (CFTR) and WT αβγ-epithelial sodium channel (ENaC) (in αβγ-ENaC one of the three subunits were tagged at the C-terminus with EYFP). The images were taken with Becker and Hickl FLIM system attached to Zeiss confocal microscope. The lifetime images are shown in pseudocolors (nanoseconds). The apparent mean lifetime for each image is shown at the bottom of each image. Note: each image has separate lifetime range (ns). C: apparent FRET efficiencies, E, obtained with fluorescence lifetime imaging microscopy (FLIM) in the cells cotransfected with vectors encoding ECFP-CFTR and αβγ-ENaC. One subunit in ENaC complex was tagged with EYFP: α-EYFP (4), β-EYFP (5), and γ-EYFP (6). ECFP-EYFP fusion construct (1) served as a positive control while separately coexpressed ECFP and EYFP (2), and ECFP-CFTR expressed alone served as a negative controls. *Statistically significant changes compared with negative controls (2 or 3) and was calculated using the Student’s t test. These experiments were repeated at least five times with similar results.
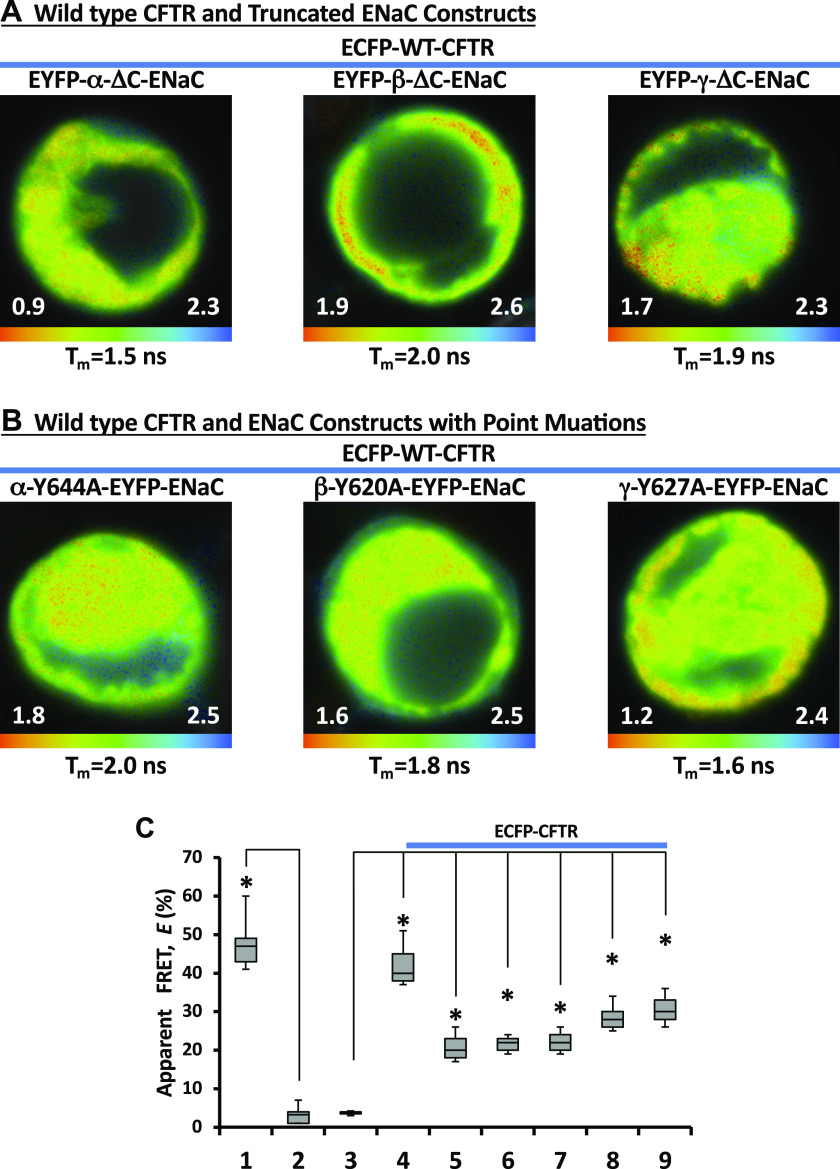
We initially performed FLIM measurements between WT CFTR and WT ENaC subunits. The cells were cotransfected with CFTR N-terminally tagged with ECFP and αβγ-ENaC; one subunit in the αβγ-ENaC complex carried EYFP as a tag at its C-terminus. Figure 2B demonstrates a set of the lifetime images acquired from these cells. We found an appreciable decrease in the mean ECFP lifetime with all combinations of ENaC and CFTR (Fig. 2B), which indicates an energy transfer from ECFP to EYFP. The E averaged ∼20% (E of the negative controls was <5%, Fig. 2C). These data are consistent with energy transfer between WT CFTR and WT ENaC subunits and puts the N-terminus of CFTR close to the C-termini of the ENaC subunits. Next, we carried out the same set of the experiments with C-terminally truncated ENaC subunits, with one subunit in each ENaC complex being tagged with EYFP (EYFP-α-ΔC-βγ-ENaC, EYFP-β-ΔC-αγ-ENaC, or EYFP-γ-ΔC-αβ-ENaC, respectively). As can be seen from Fig. 3A, we observed a decrease in the mean ECFP lifetime with all combinations of ENaC and CFTR. The calculated E value for all combinations (Fig. 3C) was comparable with those of WT ENaCs and CFTR (Fig. 2C). It has been reported that Liddle’s syndrome genotypes include not only truncation but also point mutations in ENaC subunits (15, 55, 85). Therefore, we performed FLIM between ECFP-CFTR and ENaC subunits carrying the point mutations mimicking the Liddle’s syndrome genotype. The cells were cotransfected with ECFP-CFTR and αβγ-ENaCs as before; one subunit in αβγ-ENaC was mutated at the C-terminus and C-terminally tagged with EYFP (α-Y644A-EYFP-βγ-ENaC, β-Y620A-EYFP-αγ-ENaC, or γ-Y627A-EYFP-αβ-ENaC, respectively). Again, we observed a decrease in ECFP lifetime with all combinations (Fig. 3B). These FLIM measurements are consistent with interaction between CFTR and the mutated ENaC proteins.
Figure 3.
Fluorescence lifetime imaging microscopy (FLIM) images of the cells cotransfected with enhanced cyan fluorescent protein (ECFP)-cystic fibrosis transmembrane conductance regulator (CFTR) and epithelial sodium channel (ENaC) subunits with C-terminal mutations. The cells were cotransfected with ECFP-CFTR and αβγ-ENaC. One subunit in αβγ-ENaC complex was truncated at the C-terminus and N-terminally tagged with enhanced yellow fluorescent protein (EYFP) (A) or mutated at the C-terminus and C-terminally tagged with EYFP (B). The lifetime images are shown in pseudocolors (nanoseconds). The apparent mean lifetime for each image is shown at the bottom of each image. Note: each image has separate lifetime range (ns). C: apparent fluorescence resonance energy transfer (FRET) efficiencies, E, in the cells cotransfected with ECFP-CFTR and αβγ-ENaC. One subunit in αβγ-ENaC complex was truncated at the C-terminus and N-terminally tagged with EYFP: EYFP-α-ΔC (4), EYFP-β-ΔC (5), or EYFP-γ-ΔC (6). The ENaC subunit with a point at the C-terminus was C-terminally tagged with EYFP: α-Y644A-EYFP (7), β-Y620A-EYFP (8), or γ-Y627A-EYFP (9). ECFP-EYFP fusion (1) served as a positive control while separately coexpressed ECFP and EYFP (2), and ECFP-CFTR expressed alone served as a negative controls. *Statistically significant changes compared with negative controls (2 or 3) and was calculated using the Student’s t test. These experiments were repeated at least five times with similar results.
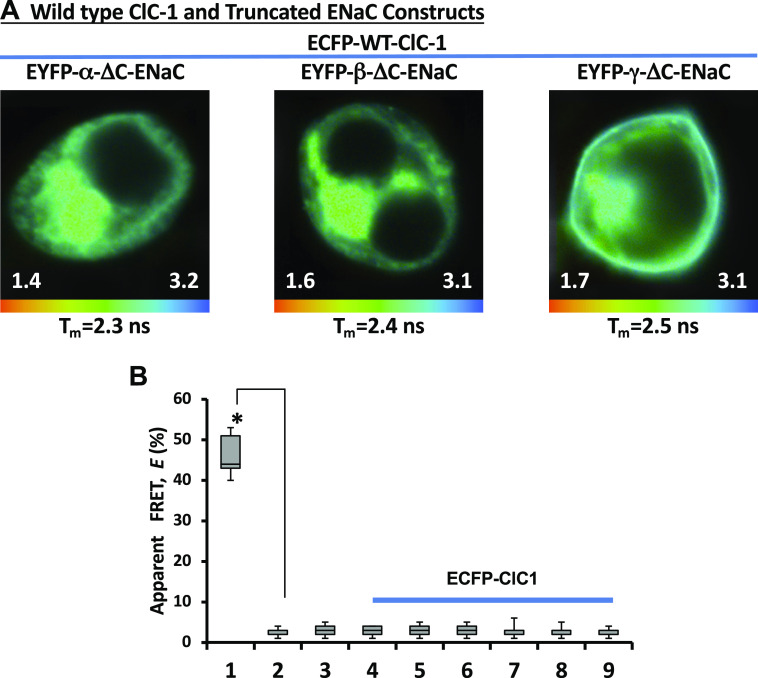
In our previous studies (35, 44), we have shown that the FRET between CFTR and ENaC subunits was specific, as we were not able to observe FRET when CFTR was replaced with another chloride channel, ClC-1. We repeated our FLIM measurements between ENaC subunits with Liddle’s syndrome mutations and ClC-1 tagged with ECFP. ECFP tagged to the ClC-1 (Fig. 4A, top) had an average donor lifetime (τECFP) of ∼2.5 ns which is not different from the lifetimes of our negative control constructs (Fig. 4B). The same was true with FLIM measurements between ClC-1 and ENaC subunits with point mutations; an average lifetime of ECFP was comparable with those of our negative control measurements (Fig. 4B). The absence of FRET between ClC-1 and ENaC constructs strengthen the specificity of energy transfer between CFTR and ENaC subunits with Liddle’s syndrome mutations.
Figure 4.
Fluorescence lifetime imaging microscopy (FLIM) images of the cells cotransfected with enhanced cyan fluorescent protein (ECFP)-chloride channel 1 (ClC-1) and epithelial sodium channel (ENaC) subunits with C-terminal mutations. The cells were cotransfected with ECFP-ClC-1 and αβγ-ENaC. One subunit in αβγ-ENaC complex was truncated at the C-terminus and N-terminally tagged with enhanced yellow fluorescent protein (EYFP) (A). The lifetime images are shown in pseudocolors (nanoseconds). The apparent mean lifetime for each image is shown at the bottom of each image. Note: each image has separate lifetime range (ns). B: apparent FRET efficiencies, E, in the cells cotransfected with ECFP-ClC-1 and αβγ-ENaC. One subunit in αβγ-ENaC complex was truncated at the C-terminus and N-terminally tagged with EYFP; EYFP-α-ΔC (4), EYFP-β-ΔC (5), or EYFP-γ-ΔC (6). The ENaC subunit with a point at the C-terminus was C-terminally tagged with EYFP; α-Y644A-EYFP (7), β-Y620A-EYFP (8), or γ-Y627A-EYFP (9). ECFP-EYFP fusion (1) served as a positive control while separately coexpressed ECFP and EYFP (2), and ECFP-ClC-1 expressed alone served as a negative controls. *Statistically significant changes (Student’s t test) compared with negative controls (2 or 3). These experiments were repeated at least five times with similar results.
DISCUSSION
In the present study, we have revisited the issue of direct interaction between CFTR and ENaC using both biochemical and microscopy-based approaches in mammalian cells. We have previously used coimmunoprecipitation to demonstrate a physical interaction between ENaC and WT CFTR and importantly, a lack of interaction between ENaC and another chloride channel, ClC1, suggesting that there is specificity to the interaction (35). The current results reconfirm our earlier data and show that CFTR can coimmunoprecipitate with all ENaC subunits carrying Liddle’s mutations (Fig. 1). However, coimmunoprecipitation experiments, especially when conducted in overexpression systems, should not be considered in isolation. Thus, we have also used FLIM microscopy to examine potential interactions. FLIM has an advantage over more conventional FRET in that FLIM is insensitive to the concentration of the fluorophore or the intensity of excitation (86, 87); hence potential artifacts due to overexpression and close-packing of the molecules are significantly reduced. Furthermore, FLIM provides greatly increased temporal and spatial resolution. FRET occurring between two fluorophores significantly decreases the FLIM signal and suggests that the two fluorophores are in close (<10 nm) proximity (79, 88). Taken together, the findings in this study further support the theory of direct interaction and regulation of ENaC by CFTR (36).
The regulation of ENaC by CFTR has been controversial since the initial observations of increased open probability of amiloride-sensitive sodium channels in human nasal epithelial cells derived from a patient with CF were made more than twenty-five years ago (8, 89). Although hyperactivity of sodium channels in CF epithelia has been widely described since the initial observations, the exact mechanism underlying this finding has been vigorously debated and has been attributed to a direct effect of CFTR to downregulate ENaC activity (35, 37, 40, 68, 90), CFTR-mediated protection of ENaC from proteolytic cleavage (91), an effect of intracellular chloride to downregulate the channel (92, 93), changes in electrical driving force, and artifacts due to inefficient voltage clamping (94). We have previously reported that CFTR can downregulate ENaC activity both in a cell-free lipid bilayer system and under conditions of heterologous expression (35, 37, 40, 41, 68). However, one outstanding question has remained; if one role of CFTR is to curb excessive sodium absorption from airway surface fluid, then why do patients with Liddle’s syndrome not have the dehydrated airway symptoms associated with CF? Although feedback inhibition of ENaC by intracellular sodium could account for this observation (95–97), this does not seem to occur in channels containing the mutations associated with Liddle’s syndrome (98). One potential explanation is that in humans sufficient CFTR is expressed in the airways to downregulate even a hyperactive Liddle’s ENaC, whereas in the kidney, where CFTR expression seems to be greatest in the proximal tubule (99), lower CFTR expression in renal principal cells could lead to less restriction of ENaC activity and result in the characteristic symptoms of hypertension and end-stage renal disease. However, in the murine lung, even very low levels of CFTR have been reported to effectively regulate ENaC activity (100). An alternative explanation is that CFTR does not regulate ENaC at all, as suggested by Welsh and coworkers (19) who showed that transepithelial sodium conductance and 22[Na] absorption were not increased in CF as compared with normal human airway epithelia. Furthermore, studies in Xenopus oocytes coexpressing both CFTR and ENaC have produced conflicting results, showing both that Liddle’s mutations prevent functional and physical association of the two channels, and that interaction between these two membrane proteins is not hindered by the presence of these mutations (41, 101).
Our current FLIM-FRET data show that ENaC subunits bearing Liddle’s mutations are capable of interacting with WT CFTR and support the concept of direct physical contact between the two channels. Thus, CFTR could effectively downregulate mutated ENaCs, restricting sodium absorption and preventing airway dehydration. These data are consistent with the findings of Mall et al. (67), who showed that CFTR could limit the increased sodium absorption in the airways of Liddle’s syndrome mice. Lack of regulation of a Liddle’s ENaC channel by CFTR as was observed in earlier studies in Xenopus oocytes (41), may reflect poor surface expression of CFTR, as has been postulated to account for the lack of electrophysiological evidence for a Liddle’s phenotype in nasal but not tracheal epithelia in mice (67).
An additional possibility is that the interaction identified in the current study is between CFTR and the quiescent uncleaved population of sodium channels. Previous studies have shown that the Liddle’s syndrome mutations are associated not only with an increase in the surface expression of ENaC, but also with an increase in the fraction of total cleaved ENaC within the cell (102). Furthermore, it has been postulated that the interaction of WT CFTR with ENaC prevents the cleavage of the α and γENaC subunits, thus limiting the fraction of active sodium channel on the cell surface (91). The coimmunoprecipitation data shown in Fig. 1, while consistent with increased surface trafficking of the Liddle’s subunits, do not exhibit evidence of cleavage in the αENaC subunit, and show only evidence of a weak cleavage in γENaC. This could reflect both potential interference of the large YFP tag with the cleavage mechanism and/or interaction of CFTR with the uncleaved channel population. However, an N-terminal GFP-tagged αENaC has been previously associated with increased amiloride-sensitive short-circuit currents under conditions where the smaller tagged cleavage fragment was not detected (103), suggesting that the tag does not interfere with cleavage.
As mentioned earlier, Liddle’s syndrome mutations target PY motifs of ENaC subunits (15, 55, 56, 104, 105) that increase channel surface expression/channel open probability (106–109) by interfering with its association with Nedd4 (82, 102, 108, 110, 111). Furthermore, Liddle’s syndrome mutations specifically increase the fractions of proteolytically cleaved ENaC subunits (102). It is clear so far from the discussion of our results that increase in surface expression, decrease in internalization, or perhaps a combination of both as a result of ENaC subunits mutations contribute to the complex pathophysiology of Liddle’s syndrome (59). At this point it is not quite clear why some patients and mice carrying Liddle’s syndrome mutation do not develop an overt lung disease phenotype, whereas mice with constitutive or conditional deletion of Nedd4, which leads to gain of function of ENaC in the lung via a mechanism similar to the Liddle’s mutation, causes severe lung disease in mice (112–114). A number of possible mechanisms might account for these observations. A simple and tempting explanation would be that the disruption of Nedd4 signaling pathway (113, 114) has a much more profound effect than the Liddle’s syndrome mutations that contained within ENaC subunits structure. Nedd4 has been implicated in the regulation of many receptors, transporters, and the components of different signaling pathways (115). It is a possibility that the disrupted regulation of other proteins in addition to ENaC as a result of disruption in Nedd4 pathway contributes to the inflammatory lung phenotype. Furthermore, the disruption of Nedd4 signaling might unleash a proinflammatory/profibrotic transforming growth factor (TGF)-β signaling (116) that contributes to the lung disease phenotype (113) whereas patients and mice carrying Liddle’s syndrome mutations is shielded from a consequence of hyperactive TGF-β signaling due to intact Nedd4 pathway. It is interesting to note that Duerr et al. (113) observed increased ENaC-mediated sodium reabsorption as a result of conditional deletion of Nedd4 several months before elevated levels of TGF-β. This speaks to the possibility that sodium hyperabsorption might play an important role in triggering the pathophysiological cascade that is further sustained by TGF-β signaling. The picture is further complicated by the fact that the disruption of Nedd4 signaling pathway interferes with sodium self-inhibition of ENaC (98, 117) and the findings that some of the biophysical characteristics of ion channels are not invariant but depend upon the biochemical state of the channel and/or its associated proteins (118, 119). On the other hand, ENaC subunits carrying Liddle’s syndrome mutations did retain some inhibitory influence of CFTR (37, 101) which perhaps may explain, at least in part, an unobservable lung phenotype in some patients suffering from Liddle’s disease. Furthermore, there is a reported difference between a distal airway expression of CFTR in mice and humans. Again, compared with humans, the expression of CFTR in mice is much lower (67) and may not be sufficient to suppress the increased levels of ENaC following overexpression of its β-subunit (46, 47) or the disruption of Nedd4 (112–114). Recently, an interesting case of a family with Liddle’s syndrome phenotype was reported (85). The siblings carried a gain-of-function mutation that affected the second cysteine reach domain of an extracellular loop of α-ENaC that mainly increased open probability of the channel but not its surface expression (85). The clinical picture of the siblings was consistent with Liddle’s syndrome: resistant hypertension, hypokalemia, metabolic alkalosis, and decrease in the levels of plasma renin and aldosterone; no lung phenotype was reported. This work again supports the likelihood that the CFTR regulatory influence was sufficient, at least in part, to restrain the sodium reabsorption through ENaC. The aforemention discussion points to the complex nature of the Liddle’s syndrome mutations on ENaC channels and its regulatory network (59). We speculate that our data are consistent with the notion that the presence of WT-CFTR might be sufficient enough to exert a negative inhibitory influence, which in turn might prevent the sodium hyperabsorption via ENaC subunits bearing Liddle’s syndrome mutations.
In this study, we evaluated the potential direct association of CFTR and ENaC subunits carrying Liddle’s syndrome mutations. We exploited the FLIM, a distance-dependent imaging approach, to probe the proximity of CFTR and ENaC to each other. FLIM was complemented by surface biotinylation and coimmunoprecipitation. Our results suggest that mutation in the C-terminal of ENaC does not prevent interaction of the channel with CFTR in a heterologous expression system with known limitations. Whether or not this interaction reflects a functional relationship in terms of a direct regulation of conductance or an interaction required for appropriate biological processing of the channel remains to be determined, and the functional significance of these findings will benefit from studies carried in polarized epithelia.
Limitations of the Study
One of the major limitations of our study is the use of an overexpression system. Unfortunately, this is inevitable when FLIM/FRET-based approach using fluorescently tagged proteins is utilized to evaluate the association between proteins. The high concentration of CFTR and ENaC, as a result of the high levels of expression in a heterologous expression system like ours, can lead to a close packing of fluorescently tagged proteins. This can lead to a nonspecific association between proteins as a result of the mass balance effect. To minimize the possibility that the random association between CFTR and ENaC were responsible for their association, we replaced CFTR with another chloride channel, CLCN1. CLCN1 is not known to associate with ENaC and we did not observe FRET signal between these two proteins. The absence of FRET between CLCN1 and ENaC and the presence of FRET between CFTR and ENaC points to the possibility of direct and specific association between CFTR and ENaC.
Regrettably, an overexpression because of the use of a heterologous expression system can also be an issue in coimmunoprecipitation experiments. Again, the nonspecific associations can occur because of high concentrations of two proteins. We and others have shown convincingly that CFTR and ENaC association is not due to overexpression or random associations between these two proteins (35, 44, 91, 120).
Polarization is a very important step in biogenesis of CFTR and critical in its functioning as a Cl− channel (121, 122). As a complex transmembrane protein, CFTR is bound to spend some time in different compartments of the protein secretory pathway (121) as it travels toward its final destination. In polarized epithelial cells (airway epithelia, Caco-2, HT29, T84), endogenous CFTR predominantly localizes to apical membrane (123, 124). Overexpressed in nonpolarized cells (3T3 cells, HeLa), CFTR is mostly found in intracellular compartments with a limited level of expression on the cell surface (123). On the other hand, wild-type ENaC subunits are poorly trafficked (77, 125) while Liddle’s syndrome mutations increase ENaC surface expression/open probability (37, 101, 109, 110). Furthermore, CFTR has an ability to regulate ENaC surface expression (126). Therefore, it is quite possible that CFTR and ENaC can associate intracellularly and then traffic together to the cell surface membrane. Alternatively, they can travel separately to the cell surface and then associate with each other there; combinations of these scenarios are also a possibility.
At the functional level, the well-described and yet somewhat controversial CFTR and ENaC relationship seems to be intact even in the setting of Liddle’s syndrome mutations; open probability of C-terminally truncated ENaCs were lower in the presence of CFTR (37, 101). We are not sure about the ENaC-mediated increase in CFTR Cl− conductance which is ENaC subunit specific and dependent on the amount of ENaC cRNA injected in oocytes (42). Since the Liddle’s syndrome mutations tend to increase the amount of ENaC protein at the surface of the membrane, it is reasonable to predict that in this case the CFTR will be more active in the presence ENaC subunit(s) harboring the Liddle’s syndrome mutation(s).
GRANTS
This work was supported, in whole or in part, by National Institutes of Health (NIH) Grant R21HL085112 (to B. K. Berdiev), GMS Grant R01GM123971 (to V. Parpura), NIH Grant DK37206 (to C. M. Fuller), NIH Grants P30DK072482 and R474-CR11 [to the University of Alabama at Birmingham (UAB) Cystic Fibrosis (CF) Center; Director Dr. Eric J. Sorscher]. R. Boddu was supported in part by an interdisciplinary training in kidney-related research grant awarded from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (T32 DK007545). A. Agarwal was supported in part by the UAB-UCSD O’Brien Center for Acute Kidney Injury Research funded by the NIDDK (P30 DK079337) and NIH R01 DK059600. This work was also supported by the UAB Health Services Foundation General Endowment Fund 2004622 (to B. K. Berdiev), MBRU-COM Internal Grant Awards MBRU-CM-RG2018-04 and MBRU-CM-RG2018-05 (to M. Uddin and B. K. Berdiev), Sandooq Al Watan Research & Development Grant SWARD-F2018-002 (M. Uddin and B. K. Berdiev), AlMahmeed Collaborative Research Award 2018 (to M. Uddin and B. K. Berdiev), and Al Jalila Foundation Grant AJF201763 (to M. Uddin). Dr. R. Tambi was supported by MBRU Post-Doctoral Fellow Award (MBRU-PD-2020-04).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
V.P., E.J.S., C.M.F., and B.K.B. conceived and designed research; A.K.R., E.B.C., W.L., R.B., and B.K.B. performed experiments; A.K.R., E.C-B., E.B.C., Y.J.Q., W.L., R.B., R.T., M.U., V.P., E.J.S., C.M.F., and B.K.B. analyzed data; A.K.R., E.C-B., E.B.C., Y.J.Q., W.L., R.B., A.A., R.T., M.U., V.P., E.J.S., C.M.F., and B.K.B. interpreted results of experiments; A.K.R., R.T., and B.K.B. prepared figures; Y.J.Q., C.M.F., and B.K.B. drafted manuscript; A.K.R., E.C-B., E.B.C., Y.J.Q., W.L., R.B., A.A., R.T., M.U., V.P., E.J.S., C.M.F., and B.K.B. edited and revised manuscript; A.K.R., E.C-B., E.B.C., Y.J.Q., W.L., R.B., A.A., R.T., M.U., V.P., E.J.S., C.M.F., and B.K.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Melissa McCarthy and Marina Mazur for excellent technical assistance. We also thank Shawn Williams and Edward Phillips (UAB Imaging Facility) for imaging assistance and Firuz B. Berdiev for careful reading of the manuscript. HEK293T cells were a kind gift of Drs. K. L. Kirk and W. Wang (UAB). Wild-type αβγ human ENaC cDNAs were a kind gift of Dr. M. J. Welsh (University of Iowa). EGFP-CFTR construct was a kind gift of Dr. B. Stanton (Dartmouth Medical School). ECFP-ClC-1 construct was a kind gift of Dr. Christoph Fahlke (Medizinische Hochschule Hannover, Hannover, Germany).
Present address of A. K. Rooj: Brigham and Women’s Hospital, Harvard Medical School, Boston MA 02115.
REFERENCES
- 1.Kerem B, Rommens JM, Buchanan JA, Markiewicz D, Cox TK, Chakravarti A, Buchwald M, Tsui LC. Identification of the cystic fibrosis gene: genetic analysis. Science 245: 1073–1080, 1989. doi: 10.1126/science.2570460. [DOI] [PubMed] [Google Scholar]
- 2.Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 245: 1066–1073, 1989[Erratum inScience245: 1437, 1989]. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 3.Rommens JM, Iannuzzi MC, Kerem B, Drumm ML, Melmer G, Dean M, Rozmahel R, Cole JL, Kennedy D, Hidaka N. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science 245: 1059–1065, 1989. doi: 10.1126/science.2772657. [DOI] [PubMed] [Google Scholar]
- 4.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med 352: 1992–2001, 2005. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- 5.Davis PB. Cystic fibrosis since 1938. Am J Respir Crit Care Med 173: 475–482, 2006. doi: 10.1164/rccm.200505-840OE. [DOI] [PubMed] [Google Scholar]
- 6.Quinton PM. Chloride impermeability in cystic fibrosis. Nature 301: 421–422, 1983. doi: 10.1038/301421a0. [DOI] [PubMed] [Google Scholar]
- 7.Knowles MR, Stutts MJ, Spock A, Fischer N, Gatzy JT, Boucher RC. Abnormal ion permeation through cystic fibrosis respiratory epithelium. Science 221: 1067–1070, 1983. doi: 10.1126/science.6308769. [DOI] [PubMed] [Google Scholar]
- 8.Stutts MJ, Canessa CM, Olsen JC, Hamrick M, Cohn JA, Rossier BC, Boucher RC. CFTR as a cAMP-dependent regulator of sodium channels. Science 269: 847–850, 1995. doi: 10.1126/science.7543698. [DOI] [PubMed] [Google Scholar]
- 9.Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev 82: 735–767, 2002. doi: 10.1152/physrev.00007.2002. [DOI] [PubMed] [Google Scholar]
- 10.Kleyman TR, Eaton DC. Regulating ENaC’s gate. Am J Physiol Cell Physiol 318: C150–C162, 2020. doi: 10.1152/ajpcell.00418.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang SS, Grunder S, Hanukoglu A, Rosler A, Mathew PM, Hanukoglu I, Schild L, Lu Y, Shimkets RA, Nelson-Williams C, Rossier BC, Lifton RP. Mutations in subunits of the epithelial sodium channel cause salt wasting with hyperkalaemic acidosis, pseudohypoaldosteronism type 1. Nat Genet 12: 248–253, 1996. doi: 10.1038/ng0396-248. [DOI] [PubMed] [Google Scholar]
- 12.Grunder S, Firsov D, Chang SS, Jaeger NF, Gautschi I, Schild L, Lifton RP, Rossier BC. A mutation causing pseudohypoaldosteronism type 1 identifies a conserved glycine that is involved in the gating of the epithelial sodium channel. EMBO J 16: 899–907, 1997. doi: 10.1093/emboj/16.5.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strautnieks SS, Thompson RJ, Gardiner RM, Chung E. A novel splice-site mutation in the gamma subunit of the epithelial sodium channel gene in three pseudohypoaldosteronism type 1 families. Nat Genet 13: 248–250, 1996. doi: 10.1038/ng0696-248. [DOI] [PubMed] [Google Scholar]
- 14.Hummler E, Barker P, Gatzy J, Beermann F, Verdumo C, Schmidt A, Boucher R, Rossier BC. Early death due to defective neonatal lung liquid clearance in alpha-ENaC-deficient mice. Nat Genet 12: 325–328, 1996. doi: 10.1038/ng0396-325. [DOI] [PubMed] [Google Scholar]
- 15.Shimkets RA, Warnock DG, Bositis CM, Nelson-Williams C, Hansson JH, Schambelan M, Gill JR Jr, Ulick S, Milora RV, Findling JW. Liddle’s syndrome: heritable human hypertension caused by mutations in the beta subunit of the epithelial sodium channel. Cell 79: 407–414, 1994. doi: 10.1016/0092-8674(94)90250-x. [DOI] [PubMed] [Google Scholar]
- 16.Chen JH, Stoltz DA, Karp PH, Ernst SE, Pezzulo AA, Moninger TO, Rector MV, Reznikov LR, Launspach JL, Chaloner K, Zabner J, Welsh MJ. Loss of anion transport without increased sodium absorption characterizes newborn porcine cystic fibrosis airway epithelia. Cell 143: 911–923, 2010. doi: 10.1016/j.cell.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collawn JF, Lazrak A, Bebok Z, Matalon S. The CFTR and ENaC debate: how important is ENaC in CF lung disease? Am J Physiol Lung Cell Mol Physiol 302: L1141–L1146, 2012. doi: 10.1152/ajplung.00036.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horisberger JD. ENaC-CFTR interactions: the role of electrical coupling of ion fluxes explored in an epithelial cell model. Pflugers Arch 445: 522–528, 2003. doi: 10.1007/s00424-002-0956-0. [DOI] [PubMed] [Google Scholar]
- 19.Itani OA, Chen JH, Karp PH, Ernst S, Keshavjee S, Parekh K, Klesney-Tait J, Zabner J, Welsh MJ. Human cystic fibrosis airway epithelia have reduced Cl− conductance but not increased Na+ conductance. Proc Natl Acad Sci USA 108: 10260–10265, 2011. doi: 10.1073/pnas.1106695108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirk KL. Being positive: revisiting the elevated sodium permeability hypothesis in cystic fibrosis. J Physiol 591: 3675–3676, 2013. doi: 10.1113/jphysiol.2013.260406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stoltz DA, Meyerholz DK, Welsh MJ. Origins of cystic fibrosis lung disease. N Engl J Med 372: 351–362, 2015. doi: 10.1056/NEJMra1300109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baker SE, Wong EC, Wheatley CM, Foxx-Lupo WT, Martinez MG, Morgan MA, Sprissler R, Morgan WJ, Snyder EM. Genetic variation of SCNN1A influences lung diffusing capacity in cystic fibrosis. Med Sci Sports Exerc 44: 2315–2321, 2012. doi: 10.1249/MSS.0b013e318266ebc3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramos MD, Trujillano D, Olivar R, Sotillo F, Ossowski S, Manzanares J, Costa J, Gartner S, Oliva C, Quintana E, Gonzalez MI, Vazquez C, Estivill X, Casals T. Extensive sequence analysis of CFTR, SCNN1A, SCNN1B, SCNN1G and SERPINA1 suggests an oligogenic basis for cystic fibrosis-like phenotypes. Clin Genet 86: 91–95, 2014. doi: 10.1111/cge.12234. [DOI] [PubMed] [Google Scholar]
- 24.Brennan ML, Schrijver I. Cystic fibrosis: a review of associated phenotypes, use of molecular diagnostic approaches, genetic characteristics, progress, and dilemmas. J Mol Diagn 18: 3–14, 2016. doi: 10.1016/j.jmoldx.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 25.Cystic Fibrosis Genotype-Phenotype Consortium. Correlation between genotype and phenotype in patients with cystic fibrosis. N Engl J Med 329: 1308–1313, 1993. doi: 10.1056/NEJM199310283291804. [DOI] [PubMed] [Google Scholar]
- 26.Mickle JE, Cutting GR. Genotype-phenotype relationships in cystic fibrosis. Med Clin North Am 84: 597–607, 2000. doi: 10.1016/s0025-7125(05)70243-1. [DOI] [PubMed] [Google Scholar]
- 27.Zielenski J. Genotype and phenotype in cystic fibrosis. Respiration 67: 117–133, 2000. doi: 10.1159/000029497. [DOI] [PubMed] [Google Scholar]
- 28.Collaco JM, Blackman SM, McGready J, Naughton KM, Cutting GR. Quantification of the relative contribution of environmental and genetic factors to variation in cystic fibrosis lung function. J Pediatr 157: 802–807.e1-3,2010. doi: 10.1016/j.jpeds.2010.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cutting GR. Modifier genes in Mendelian disorders: the example of cystic fibrosis. Ann NY Acad Sci 1214: 57–69, 2010. doi: 10.1111/j.1749-6632.2010.05879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kerem E, Corey M, Kerem BS, Rommens J, Markiewicz D, Levison H, Tsui LC, Durie P. The relation between genotype and phenotype in cystic fibrosis—analysis of the most common mutation (delta F508). N Engl J Med 323: 1517–1522, 1990. doi: 10.1056/NEJM199011293232203. [DOI] [PubMed] [Google Scholar]
- 31.Cutting GR. Modifier genetics: cystic fibrosis. Annu Rev Genomics Hum Genet 6: 237–260, 2005. doi: 10.1146/annurev.genom.6.080604.162254. [DOI] [PubMed] [Google Scholar]
- 32.Davies J, Alton E, Griesenbach U. Cystic fibrosis modifier genes. J R Soc Med 98: 47–54, 2005. [PMC free article] [PubMed] [Google Scholar]
- 33.Agrawal PB, Wang R, Li HL, Schmitz-Abe K, Simone-Roach C, Chen J, Shi J, Louie T, Sheng S, Towne MC, Brainson CF, Matthay MA, Kim CF, Bamshad M, Emond MJ, Gerard NP, Kleyman TR, Gerard C. The epithelial sodium channel is a modifier of the long-term nonprogressive phenotype associated with F508del CFTR mutations. Am J Respir Cell Mol Biol 57: 711–720, 2017. doi: 10.1165/rcmb.2017-0166OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stanke F, Becker T, Cuppens H, Kumar V, Cassiman JJ, Jansen S, Radojkovic D, Siebert B, Yarden J, Ussery DW, Wienker TF, Tummler B. The TNFalpha receptor TNFRSF1A and genes encoding the amiloride-sensitive sodium channel ENaC as modulators in cystic fibrosis. Hum Genet 119: 331–343, 2006. doi: 10.1007/s00439-006-0140-2. [DOI] [PubMed] [Google Scholar]
- 35.Berdiev BK, Cormet-Boyaka E, Tousson A, Qadri YJ, Oosterveld-Hut HM, Hong JS, Gonzales PA, Fuller CM, Sorscher EJ, Lukacs GL, Benos DJ. Molecular proximity of cystic fibrosis transmembrane conductance regulator and epithelial sodium channel assessed by fluorescence resonance energy transfer. J Biol Chem 282: 36481–36488, 2007. doi: 10.1074/jbc.M708089200. [DOI] [PubMed] [Google Scholar]
- 36.Berdiev BK, Qadri YJ, Benos DJ. Assessment of the CFTR and ENaC association. Mol Biosyst 5: 123–127, 2009. doi: 10.1039/b810471a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berdiev BK, Shlyonsky VG, Karlson KH, Stanton BA, Ismailov II. Gating of amiloride-sensitive Na+ channels: subunit-subunit interactions and inhibition by the cystic fibrosis transmembrane conductance regulator. Biophys J 78: 1881–1894, 2000. doi: 10.1016/S0006-3495(00)76737-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Donaldson SH, Boucher RC. Sodium channels and cystic fibrosis. Chest 132: 1631–1636, 2007. doi: 10.1378/chest.07-0288. [DOI] [PubMed] [Google Scholar]
- 39.Hobbs CA, Da Tan C, Tarran R. Does epithelial sodium channel hyperactivity contribute to cystic fibrosis lung disease? J Physiol 591: 4377–4387, 2013. doi: 10.1113/jphysiol.2012.240861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ismailov II, Awayda MS, Jovov B, Berdiev BK, Fuller CM, Dedman JR, Kaetzel M, Benos DJ. Regulation of epithelial sodium channels by the cystic fibrosis transmembrane conductance regulator. J Biol Chem 271: 4725–4732, 1996. doi: 10.1074/jbc.271.9.4725. [DOI] [PubMed] [Google Scholar]
- 41.Ji HL, Chalfant ML, Jovov B, Lockhart JP, Parker SB, Fuller CM, Stanton BA, Benos DJ. The cytosolic termini of the beta- and gamma-ENaC subunits are involved in the functional interactions between cystic fibrosis transmembrane conductance regulator and epithelial sodium channel. J Biol Chem 275: 27947–27956, 2000. doi: 10.1074/jbc.M002848200. [DOI] [PubMed] [Google Scholar]
- 42.Jiang Q, Li J, Dubroff R, Ahn YJ, Foskett JK, Engelhardt J, Kleyman TR. Epithelial sodium channels regulate cystic fibrosis transmembrane conductance regulator chloride channels in Xenopus oocytes. J Biol Chem 275: 13266–13274, 2000. doi: 10.1074/jbc.275.18.13266. [DOI] [PubMed] [Google Scholar]
- 43.Kunzelmann K, Schreiber R. CFTR, a regulator of channels. J Membr Biol 168: 1–8, 1999. doi: 10.1007/s002329900492. [DOI] [PubMed] [Google Scholar]
- 44.Qadri YJ, Cormet-Boyaka E, Rooj AK, Lee W, Parpura V, Fuller CM, Berdiev BK. Low temperature and chemical rescue affect molecular proximity of DeltaF508-cystic fibrosis transmembrane conductance regulator (CFTR) and epithelial sodium channel (ENaC). J Biol Chem 287: 16781–16790, 2012. doi: 10.1074/jbc.M111.332031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suaud L, Li J, Jiang Q, Rubenstein RC, Kleyman TR. Genistein restores functional interactions between Delta F508-CFTR and ENaC in Xenopus oocytes. J Biol Chem 277: 8928–8933, 2002. doi: 10.1074/jbc.M111482200. [DOI] [PubMed] [Google Scholar]
- 46.Mall M, Grubb BR, Harkema JR, O’Neal WK, Boucher RC. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat Med 10: 487–493, 2004. doi: 10.1038/nm1028. [DOI] [PubMed] [Google Scholar]
- 47.Livraghi-Butrico A, Wilkinson KJ, Volmer AS, Gilmore RC, Rogers TD, Caldwell RA, Burns KA, Esther CR Jr, Mall MA, Boucher RC, O’Neal WK, Br G. Lung disease phenotypes caused by overexpression of combinations of α-, β-, and γ-subunits of the epithelial sodium channel in mouse airways. Am J Physiol Lung Cell Mol Physiol 314: L318–L331, 2018. doi: 10.1152/ajplung.00382.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Livraghi-Butrico A, Kelly EJ, Wilkinson KJ, Rogers TD, Gilmore RC, Harkema JR, Randell SH, Boucher RC, O’Neal WK, Grubb BR. Loss of Cftr function exacerbates the phenotype of Na+ hyperabsorption in murine airways. Am J Physiol Lung Cell Mol Physiol 304: L469–L480, 2013. doi: 10.1152/ajplung.00150.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Azad AK, Rauh R, Vermeulen F, Jaspers M, Korbmacher J, Boissier B, Bassinet L, Fichou Y, Des Georges M, Stanke F, De Boeck K, Dupont L, Balascakova M, Hjelte L, Lebecque P, Radojkovic D, Castellani C, Schwartz M, Stuhrmann M, Schwarz M, Skalicka V, de Monestrol I, Girodon E, Ferec C, Claustres M, Tummler B, Cassiman JJ, Korbmacher C, Cuppens H. Mutations in the amiloride-sensitive epithelial sodium channel in patients with cystic fibrosis-like disease. Hum Mutat 30: 1093–1103, 2009. doi: 10.1002/humu.21011. [DOI] [PubMed] [Google Scholar]
- 50.Rauh R, Diakov A, Tzschoppe A, Korbmacher J, Azad AK, Cuppens H, Cassiman JJ, Dotsch J, Sticht H, Korbmacher C. A mutation of the epithelial sodium channel associated with atypical cystic fibrosis increases channel open probability and reduces Na+ self inhibition. J Physiol 588: 1211–1225, 2010. doi: 10.1113/jphysiol.2009.180224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huber R, Krueger B, Diakov A, Korbmacher J, Haerteis S, Einsiedel J, Gmeiner P, Azad AK, Cuppens H, Cassiman JJ, Korbmacher C, Rauh R. Functional characterization of a partial loss-of-function mutation of the epithelial sodium channel (ENaC) associated with atypical cystic fibrosis. Cell Physiol Biochem 25: 145–158, 2010. doi: 10.1159/000272059. [DOI] [PubMed] [Google Scholar]
- 52.Sheridan MB, Fong P, Groman JD, Conrad C, Flume P, Diaz R, Harris C, Knowles M, Cutting GR. Mutations in the beta-subunit of the epithelial Na+ channel in patients with a cystic fibrosis-like syndrome. Hum Mol Genet 14: 3493–3498, 2005. doi: 10.1093/hmg/ddi374. [DOI] [PubMed] [Google Scholar]
- 53.Groman JD, Meyer ME, Wilmott RW, Zeitlin PL, Cutting GR. Variant cystic fibrosis phenotypes in the absence of CFTR mutations. N Engl J Med 347: 401–407, 2002. doi: 10.1056/NEJMoa011899. [DOI] [PubMed] [Google Scholar]
- 54.Mekus F, Ballmann M, Bronsveld I, Dork T, Bijman J, Tummler B, Veeze HJ. Cystic-fibrosis-like disease unrelated to the cystic fibrosis transmembrane conductance regulator. Hum Genet 102: 582–586, 1998. doi: 10.1007/s004390050744. [DOI] [PubMed] [Google Scholar]
- 55.Hansson JH, Nelson-Williams C, Suzuki H, Schild L, Shimkets R, Lu Y, Canessa C, Iwasaki T, Rossier B, Lifton RP. Hypertension caused by a truncated epithelial sodium channel gamma subunit: genetic heterogeneity of Liddle syndrome. Nat Genet 11: 76–82, 1995. doi: 10.1038/ng0995-76. [DOI] [PubMed] [Google Scholar]
- 56.Hansson JH, Schild L, Lu Y, Wilson TA, Gautschi I, Shimkets R, Nelson-Williams C, Rossier BC, Lifton RP. A de novo missense mutation of the beta subunit of the epithelial sodium channel causes hypertension and Liddle syndrome, identifying a proline-rich segment critical for regulation of channel activity. Proc Natl Acad Sci USA 92: 11495–11499, 1995. doi: 10.1073/pnas.92.25.11495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tetti M, Monticone S, Burrello J, Matarazzo P, Veglio F, Pasini B, Jeunemaitre X, Mulatero P. Liddle syndrome: review of the literature and description of a new case. Int J Mol Sci 19: 812, 2018. doi: 10.3390/ijms19030812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scheinman SJ, Guay-Woodford LM, Thakker RV, Warnock DG. Genetic disorders of renal electrolyte transport. N Engl J Med 340: 1177–1187, 1999. doi: 10.1056/NEJM199904153401507. [DOI] [PubMed] [Google Scholar]
- 59.Rotin D, Staub O. Nedd4-2 and the regulation of epithelial sodium transport. Front Physiol 3: 212, 2012. doi: 10.3389/fphys.2012.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Staub O, Abriel H, Plant P, Ishikawa T, Kanelis V, Saleki R, Horisberger JD, Schild L, Rotin D. Regulation of the epithelial Na+ channel by Nedd4 and ubiquitination. Kidney Int 57: 809–815, 2000. doi: 10.1046/j.1523-1755.2000.00919.x. [DOI] [PubMed] [Google Scholar]
- 61.Staub O, Rotin D. Role of ubiquitylation in cellular membrane transport. Physiol Rev 86: 669–707, 2006. doi: 10.1152/physrev.00020.2005. [DOI] [PubMed] [Google Scholar]
- 62.Pavlov TS, Staruschenko A. Involvement of ENaC in the development of salt-sensitive hypertension. Am J Physiol Renal Physiol 313: F135–F140, 2017. doi: 10.1152/ajprenal.00427.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shimkets RA, Lifton RP, Canessa CM. The activity of the epithelial sodium channel is regulated by clathrin-mediated endocytosis. J Biol Chem 272: 25537–25541, 1997. doi: 10.1074/jbc.272.41.25537. [DOI] [PubMed] [Google Scholar]
- 64.Snyder PM. The epithelial Na+ channel: cell surface insertion and retrieval in Na+ homeostasis and hypertension. Endocr Rev 23: 258–275, 2002. doi: 10.1210/edrv.23.2.0458. [DOI] [PubMed] [Google Scholar]
- 65.Baker E, Jeunemaitre X, Portal AJ, Grimbert P, Markandu N, Persu A, Corvol P, MacGregor G. Abnormalities of nasal potential difference measurement in Liddle’s syndrome. J Clin Invest 102: 10–14, 1998. doi: 10.1172/JCI1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pradervand S, Wang Q, Burnier M, Beermann F, Horisberger JD, Hummler E, Rossier BC. A mouse model for Liddle’s syndrome. J Am Soc Nephrol 10: 2527–2533, 1999. doi: 10.1681/ASN.V10122527. [DOI] [PubMed] [Google Scholar]
- 67.Mall MA, Button B, Johannesson B, Zhou Z, Livraghi A, Caldwell RA, Schubert SC, Schultz C, O’Neal WK, Pradervand S, Hummler E, Rossier BC, Grubb BR, Boucher RC. Airway surface liquid volume regulation determines different airway phenotypes in Liddle compared with βENaC-overexpressing mice. J Biol Chem 285: 26945–26955, 2010. doi: 10.1074/jbc.M110.151803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ismailov II, Berdiev BK, Shlyonsky VG, Fuller CM, Prat AG, Jovov B, Cantiello HF, Ausiello DA, Benos DJ. Role of actin in regulation of epithelial sodium channels by CFTR. Am J Physiol Cell Physiol 272: C1077–C1086, 1997. doi: 10.1152/ajpcell.1997.272.4.C1077. [DOI] [PubMed] [Google Scholar]
- 69.Boucher RC. New concepts of the pathogenesis of cystic fibrosis lung disease. Eur Respir J 23: 146–158, 2004. doi: 10.1183/09031936.03.00057003. [DOI] [PubMed] [Google Scholar]
- 70.Meltzer RH, Kapoor N, Qadri YJ, Anderson SJ, Fuller CM, Benos DJ. Heteromeric assembly of acid-sensitive ion channel and epithelial sodium channel subunits. J Biol Chem 282: 25548–25559, 2007. doi: 10.1074/jbc.M703825200. [DOI] [PubMed] [Google Scholar]
- 71.Moyer BD, Loffing J, Schwiebert EM, Loffing-Cueni D, Halpin PA, Karlson KH, Ismailov II, Guggino WB, Langford GM, Stanton BA. Membrane trafficking of the cystic fibrosis gene product, cystic fibrosis transmembrane conductance regulator, tagged with green fluorescent protein in madin-darby canine kidney cells. J Biol Chem 273: 21759–21768, 1998[Erratum inJ Biol Chem273: 26256, 1998]. doi: 10.1074/jbc.273.34.21759. [DOI] [PubMed] [Google Scholar]
- 72.Hebeisen S, Biela A, Giese B, Muller-Newen G, Hidalgo P, Fahlke C. The role of the carboxyl terminus in ClC chloride channel function. J Biol Chem 279: 13140–13147, 2004. doi: 10.1074/jbc.M312649200. [DOI] [PubMed] [Google Scholar]
- 73.Bebok Z, Varga K, Hicks JK, Venglarik CJ, Kovacs T, Chen L, Hardiman KM, Collawn JF, Sorscher EJ, Matalon S. Reactive oxygen nitrogen species decrease cystic fibrosis transmembrane conductance regulator expression and cAMP-mediated Cl− secretion in airway epithelia. J Biol Chem 277: 43041–43049, 2002. doi: 10.1074/jbc.M203154200. [DOI] [PubMed] [Google Scholar]
- 74.Cormet-Boyaka E, Di A, Chang SY, Naren AP, Tousson A, Nelson DJ, Kirk KL. CFTR chloride channels are regulated by a SNAP-23/syntaxin 1A complex. Proc Natl Acad Sci USA 99: 12477–12482, 2002. doi: 10.1073/pnas.192203899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Holman D, Henley JM. A novel method for monitoring the cell surface expression of heteromeric protein complexes in dispersed neurons and acute hippocampal slices. J Neurosci Methods 160: 302–308, 2007. doi: 10.1016/j.jneumeth.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rooj AK, Liu Z, McNicholas CM, Fuller CM. Physical and functional interactions between a glioma cation channel and integrin-beta1 require alpha-actinin. Am J Physiol Cell Physiol 309: C308–C319, 2015. doi: 10.1152/ajpcell.00036.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Helms MN, Liu L, Liang YY, Al-Khalili O, Vandewalle A, Saxena S, Eaton DC, Ma HP. Phosphatidylinositol 3,4,5-trisphosphate mediates aldosterone stimulation of epithelial sodium channel (ENaC) and interacts with γ-ENaC. J Biol Chem 280: 40885–40891, 2005. doi: 10.1074/jbc.M509646200. [DOI] [PubMed] [Google Scholar]
- 78.Staruschenko A, Medina JL, Patel P, Shapiro MS, Booth RE, Stockand JD. Fluorescence resonance energy transfer analysis of subunit stoichiometry of the epithelial Na+ channel. J Biol Chem 279: 27729–27734, 2004. doi: 10.1074/jbc.M404169200. [DOI] [PubMed] [Google Scholar]
- 79.Clegg RM. Fluorescence resonance energy transfer and nucleic acids. Methods Enzymol 211: 353–388, 1992. doi: 10.1016/0076-6879(92)11020-j. [DOI] [PubMed] [Google Scholar]
- 80.Kenworthy AK. Imaging protein-protein interactions using fluorescence resonance energy transfer microscopy. Methods 24: 289–296, 2001. doi: 10.1006/meth.2001.1189. [DOI] [PubMed] [Google Scholar]
- 81.Selvin PR. Fluorescence resonance energy transfer. Methods Enzymol 246: 300–334, 1995. doi: 10.1016/0076-6879(95)46015-2. [DOI] [PubMed] [Google Scholar]
- 82.Abriel H, Loffing J, Rebhun JF, Pratt JH, Schild L, Horisberger JD, Rotin D, Staub O. Defective regulation of the epithelial Na+ channel by Nedd4 in Liddle’s syndrome. J Clin Invest 103: 667–673, 1999. doi: 10.1172/JCI5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim J, Kwon D, Lee J, Pasquier H, Grailhe R. The use of cyan fluorescent protein variants with a distinctive lifetime signature. Mol Biosyst 5: 151–153, 2009. doi: 10.1039/b815445g. [DOI] [PubMed] [Google Scholar]
- 85.Salih M, Gautschi I, van Bemmelen MX, Di Benedetto M, Brooks AS, Lugtenberg D, Schild L, Hoorn EJ. A missense mutation in the extracellular domain of αENaC causes Liddle syndrome. J Am Soc Nephrol 28: 3291–3299, 2017. doi: 10.1681/ASN.2016111163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Becker W. Fluorescence lifetime imaging-techniques and applications. J Microsc 247: 119–136, 2012. doi: 10.1111/j.1365-2818.2012.03618.x. [DOI] [PubMed] [Google Scholar]
- 87.van Munster EB, Gadella TW. Fluorescence lifetime imaging microscopy (FLIM). Adv Biochem Eng Biotechnol 95: 143–175, 2005. doi: 10.1007/b102213. [DOI] [PubMed] [Google Scholar]
- 88.Clegg RM, Holub O, Gohlke C. Fluorescence lifetime-resolved imaging: measuring lifetimes in an image. Methods Enzymol 360: 509–542, 2003. doi: 10.1016/s0076-6879(03)60126-6. [DOI] [PubMed] [Google Scholar]
- 89.Chinet TC, Fullton JM, Yankaskas JR, Boucher RC, Stutts MJ. Sodium-permeable channels in the apical membrane of human nasal epithelial cells. Am J Physiol Cell Physiol 265: C1050–C1060, 1993. doi: 10.1152/ajpcell.1993.265.4.C1050. [DOI] [PubMed] [Google Scholar]
- 90.Kunzelmann K, Kiser GL, Schreiber R, Riordan JR. Inhibition of epithelial Na+ currents by intracellular domains of the cystic fibrosis transmembrane conductance regulator. FEBS Lett 400: 341–344, 1997. doi: 10.1016/s0014-5793(96)01414-7. [DOI] [PubMed] [Google Scholar]
- 91.Gentzsch M, Dang H, Dang Y, Garcia-Caballero A, Suchindran H, Boucher RC, Stutts MJ. The cystic fibrosis transmembrane conductance regulator impedes proteolytic stimulation of the epithelial Na+ channel. J Biol Chem 285: 32227–32232, 2010. doi: 10.1074/jbc.M110.155259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kunzelmann K. ENaC is inhibited by an increase in the intracellular Cl− concentration mediated through activation of Cl− channels. Pflugers Arch 445: 504–512, 2003. doi: 10.1007/s00424-002-0958-y. [DOI] [PubMed] [Google Scholar]
- 93.Xie Y, Schafer JA. Inhibition of ENaC by intracellular Cl− in an MDCK clone with high ENaC expression. Am J Physiol Renal Physiol 287: F722–F731, 2004. doi: 10.1152/ajprenal.00135.2004. [DOI] [PubMed] [Google Scholar]
- 94.Nagel G, Barbry P, Chabot H, Brochiero E, Hartung K, Grygorczyk R. CFTR fails to inhibit the epithelial sodium channel ENaC expressed in Xenopus laevis oocytes. J Physiol 564: 671–682, 2005. doi: 10.1113/jphysiol.2004.079046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Anantharam A, Tian Y, Palmer LG. Open probability of the epithelial sodium channel is regulated by intracellular sodium. J Physiol 574: 333–347, 2006. doi: 10.1113/jphysiol.2006.109173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Garty H, Palmer LG. Epithelial sodium channels: function, structure, and regulation. Physiol Rev 77: 359–396, 1997. doi: 10.1152/physrev.1997.77.2.359. [DOI] [PubMed] [Google Scholar]
- 97.Ismailov II, Berdiev BK, Benos DJ. Regulation by Na+ and Ca2+ of renal epithelial Na+ channels reconstituted into planar lipid bilayers. J Gen Physiol 106: 445–466, 1995. doi: 10.1085/jgp.106.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kellenberger S, Gautschi I, Rossier BC, Schild L. Mutations causing Liddle syndrome reduce sodium-dependent downregulation of the epithelial sodium channel in the Xenopus oocyte expression system. J Clin Invest 101: 2741–2750, 1998. doi: 10.1172/JCI2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Crawford I, Maloney PC, Zeitlin PL, Guggino WB, Hyde SC, Turley H, Gatter KC, Harris A, Higgins CF. Immunocytochemical localization of the cystic fibrosis gene product CFTR. Proc Natl Acad Sci USA 88: 9262–9266, 1991. doi: 10.1073/pnas.88.20.9262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lazrak A, Jurkuvenaite A, Chen L, Keeling KM, Collawn JF, Bedwell DM, Matalon S. Enhancement of alveolar epithelial sodium channel activity with decreased cystic fibrosis transmembrane conductance regulator expression in mouse lung. Am J Physiol Lung Cell Mol Physiol 301: L557–L567, 2011. doi: 10.1152/ajplung.00094.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hopf A, Schreiber R, Mall M, Greger R, Kunzelmann K. Cystic fibrosis transmembrane conductance regulator inhibits epithelial Na+ channels carrying Liddle’s syndrome mutations. J Biol Chem 274: 13894–13899, 1999. doi: 10.1074/jbc.274.20.13894. [DOI] [PubMed] [Google Scholar]
- 102.Knight KK, Olson DR, Zhou R, Snyder PM. Liddle’s syndrome mutations increase Na+ transport through dual effects on epithelial Na+ channel surface expression and proteolytic cleavage. Proc Natl Acad Sci USA 103: 2805–2808, 2006. doi: 10.1073/pnas.0511184103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Woollhead AM, Baines DL. Forskolin-induced cell shrinkage and apical translocation of functional enhanced green fluorescent protein-human αENaC in H441 lung epithelial cell monolayers. J Biol Chem 281: 5158–5168, 2006. doi: 10.1074/jbc.M509947200. [DOI] [PubMed] [Google Scholar]
- 104.Inoue J, Iwaoka T, Tokunaga H, Takamune K, Naomi S, Araki M, Takahama K, Yamaguchi K, Tomita K. A family with Liddle’s syndrome caused by a new missense mutation in the β subunit of the epithelial sodium channel. J Clin Endocrinol Metab 83: 2210–2213, 1998. doi: 10.1210/jcem.83.6.5030. [DOI] [PubMed] [Google Scholar]
- 105.Tamura H, Schild L, Enomoto N, Matsui N, Marumo F, Rossier BC. Liddle disease caused by a missense mutation of beta subunit of the epithelial sodium channel gene. J Clin Invest 97: 1780–1784, 1996. doi: 10.1172/JCI118606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Firsov D, Schild L, Gautschi I, Merillat AM, Schneeberger E, Rossier BC. Cell surface expression of the epithelial Na channel and a mutant causing Liddle syndrome: a quantitative approach. Proc Natl Acad Sci USA 93: 15370–15375, 1996. doi: 10.1073/pnas.93.26.15370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schild L, Canessa CM, Shimkets RA, Gautschi I, Lifton RP, Rossier BC. A mutation in the epithelial sodium channel causing Liddle disease increases channel activity in the Xenopus laevis oocyte expression system. Proc Natl Acad Sci USA 92: 5699–5703, 1995. doi: 10.1073/pnas.92.12.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schild L, Lu Y, Gautschi I, Schneeberger E, Lifton RP, Rossier BC. Identification of a PY motif in the epithelial Na channel subunits as a target sequence for mutations causing channel activation found in Liddle syndrome. EMBO J 15: 2381–2387, 1996. [PMC free article] [PubMed] [Google Scholar]
- 109.Snyder PM, Price MP, McDonald FJ, Adams CM, Volk KA, Zeiher BG, Stokes JB, Welsh MJ. Mechanism by which Liddle’s syndrome mutations increase activity of a human epithelial Na+ channel. Cell 83: 969–978, 1995. doi: 10.1016/0092-8674(95)90212-0. [DOI] [PubMed] [Google Scholar]
- 110.Snyder PM. Liddle’s syndrome mutations disrupt cAMP-mediated translocation of the epithelial Na+ channel to the cell surface. J Clin Invest 105: 45–53, 2000. doi: 10.1172/JCI7869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Staub O, Dho S, Henry P, Correa J, Ishikawa T, McGlade J, Rotin D. WW domains of Nedd4 bind to the proline-rich PY motifs in the epithelial Na+ channel deleted in Liddle’s syndrome. EMBO J 15: 2371–2380, 1996. doi: 10.1002/j.1460-2075.1996.tb00593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Boase NA, Rychkov GY, Townley SL, Dinudom A, Candi E, Voss AK, Tsoutsman T, Semsarian C, Melino G, Koentgen F, Cook DI, Kumar S. Respiratory distress and perinatal lethality in Nedd4-2-deficient mice. Nat Commun 2: 287, 2011. doi: 10.1038/ncomms1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Duerr J, Leitz DHW, Szczygiel M, Dvornikov D, Fraumann SG, Kreutz C, Zadora PK, Seyhan Agircan A, Konietzke P, Engelmann TA, Hegermann J, Mulugeta S, Kawabe H, Knudsen L, Ochs M, Rotin D, Muley T, Kreuter M, Herth FJF, Wielputz MO, Beers MF, Klingmuller U, Mall MA. Conditional deletion of Nedd4-2 in lung epithelial cells causes progressive pulmonary fibrosis in adult mice. Nat Commun 11: 2012, 2020. doi: 10.1038/s41467-020-15743-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kimura T, Kawabe H, Jiang C, Zhang W, Xiang YY, Lu C, Salter MW, Brose N, Lu WY, Rotin D. Deletion of the ubiquitin ligase Nedd4L in lung epithelia causes cystic fibrosis-like disease. Proc Natl Acad Sci USA 108: 3216–3221, 2011. doi: 10.1073/pnas.1010334108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Boase NA, Kumar S. NEDD4: The founding member of a family of ubiquitin-protein ligases. Gene 557: 113–122, 2015. doi: 10.1016/j.gene.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gao S, Alarcon C, Sapkota G, Rahman S, Chen PY, Goerner N, Macias MJ, Erdjument-Bromage H, Tempst P, Massague J. Ubiquitin ligase Nedd4L targets activated Smad2/3 to limit TGF-beta signaling. Mol Cell 36: 457–468, 2009. doi: 10.1016/j.molcel.2009.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dinudom A, Harvey KF, Komwatana P, Young JA, Kumar S, Cook DI. Nedd4 mediates control of an epithelial Na+ channel in salivary duct cells by cytosolic Na+. Proc Natl Acad Sci USA 95: 7169–7173, 1998. doi: 10.1073/pnas.95.12.7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ismailov II, Berdiev BK, Benos DJ. Biochemical status of renal epithelial Na+ channels determines apparent channel conductance, ion selectivity, and amiloride sensitivity. Biophys J 69: 1789–1800, 1995. doi: 10.1016/S0006-3495(95)80049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Stutts MJ, Rossier BC, Boucher RC. Cystic fibrosis transmembrane conductance regulator inverts protein kinase A-mediated regulation of epithelial sodium channel single channel kinetics. J Biol Chem 272: 14037–14040, 1997. doi: 10.1074/jbc.272.22.14037. [DOI] [PubMed] [Google Scholar]
- 120.Henry KR, Lee S, Walker D, Zeitlin PL. Direct interactions between ENaC gamma subunit and ClCN2 in cystic fibrosis epithelial cells. Physiol Rep 3: e12264, 2015. doi: 10.14814/phy2.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bertrand CA, Frizzell RA. The role of regulated CFTR trafficking in epithelial secretion. Am J Physiol Cell Physiol 285: C1–C18, 2003. doi: 10.1152/ajpcell.00554.2002. [DOI] [PubMed] [Google Scholar]
- 122.Moyer BD, Denton J, Karlson KH, Reynolds D, Wang S, Mickle JE, Milewski M, Cutting GR, Guggino WB, Li M, Stanton BA. A PDZ-interacting domain in CFTR is an apical membrane polarization signal. J Clin Invest 104: 1353–1361, 1999. doi: 10.1172/JCI7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Denning GM, Ostedgaard LS, Cheng SH, Smith AE, Welsh MJ. Localization of cystic fibrosis transmembrane conductance regulator in chloride secretory epithelia. J Clin Invest 89: 339–349, 1992. doi: 10.1172/JCI115582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Denning GM, Ostedgaard LS, Welsh MJ. Abnormal localization of cystic fibrosis transmembrane conductance regulator in primary cultures of cystic fibrosis airway epithelia. J Cell Biol 118: 551–559, 1992. doi: 10.1083/jcb.118.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Valentijn JA, Fyfe GK, Canessa CM. Biosynthesis and processing of epithelial sodium channels in Xenopus oocytes. J Biol Chem 273: 30344–30351, 1998. doi: 10.1074/jbc.273.46.30344. [DOI] [PubMed] [Google Scholar]
- 126.Rubenstein RC, Lockwood SR, Lide E, Bauer R, Suaud L, Grumbach Y. Regulation of endogenous ENaC functional expression by CFTR and ΔF508-CFTR in airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 300: L88–L101, 2011. doi: 10.1152/ajplung.00142.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]