Abstract
Background:
Despite early attempts to salvage myocardium-at-risk with percutaneous coronary intervention (PCI), changes in myocardial wall stress (MWS) leads to ventricular dilatation and dysfunction after acute ST-elevation myocardial infraction (STEMI). Whether this is transient or leads to long-term adverse outcomes (MACE) is not known. We studied the association between MWS and MACE in patients after a successful PCI for acute STEMI.
Objectives:
To study the MWS in percutaneously revascularized STEMI patients in relation to all-cause mortality and MACE.
Methods:
We prospectively enrolled 142 patients who presented to our tertiary care hospital with acute STEMI requiring emergent PCI. In addition to the standard clinical biomarkers, both end-systolic and end-diastolic MWS was calculated using our recently validated Echocardiographic indices. Patients were then prospectively followed up to an average of 16.5 (± 12.0) months to assess all-cause mortality and MACE.
Results:
During the follow-up period, 9% of the patients died and 17% developed MACE. Patients who died had significantly elevated end-systolic WS compared to those who survived (mean ESWS, 80.01 ± 36.86 vs 59.28 ± 27.68). There was no significant difference in end-diastolic WS, left ventricular systolic function and peak troponin levels among survivors vs. non-survivors. Elevated ESWS (>62.5Kpa) and age remained the significant predictors of mortality on multivariate logistic analysis (OR: 7.75, CI: 1.33-73.86, p= 0.03; OR: 1.16, CI: 1.06-1.31, p=0.002).
Conclusion:
Elevated ESWS measured by Echocardiogram is associated with increased odds of long-term mortality in STEMI patients who have undergone emergent PCI. This finding can help clinicians to risk stratify high-risk patients.
Keywords: Myocardial wall stress, STEMI, Mortality, MACE
Introduction
An acute ST segment elevation myocardial infarction (STEMI) can result in significant immediate and chronic hemodynamic changes of the left ventricle (LV) (1, 2). The ventricular adaptation begins with myocyte necrosis and subsequent increase in the LV pressure and volume overload (3). Development of new metrics to identify post-ischemic changes in patients with high morbidity and mortality risk would help to attenuate the high frequency and acuity of hospitalization and improve quality of life.
Myocardial wall stress is considered as one of the most important factors driving the change in myocardial geometry and function. Increase in LV load triggers a cascade of adaptive mechanisms that lead to progressive eccentric LV dilatation (4, 5). These changes are likely contributed by matrix expansion with change in collagen scaffolding, and thereby further accelerating increase in endo-myocardial wall stress (6). This collagen deposition has been found to occur within hours of myocyte injury eventually leading to infarct expansion, defined by Hutchins and Bulkley as acute dilation and thinning of infarcted area not explained by additional myocardial necrosis which may explain the LV dysfunction (7). Depending on the extent of infarcted non-contractile myocardium, there is proportionate reduction of LV ejection fraction and LV stroke volume (8, 9). To compensate for the reduction in stroke volume, left ventricular adaptation ensues, with progressive increase in LV cavitary dilation and increased wall tension (10–14).
Prior studies have reported changes in the left ventricular wall stress after acute MI (15). In 2018, we published data from 81 STEMI patients, and reported end-diastolic wall stress as a prognostic tool for short-term risk-stratification (1) . Current study expands on our previous research by studying larger patient cohort with a longer duration of follow up. This is important because post-ischemic cardiac adaptation involves chronic myocardial adaptation, and the therapeutic goals may need to be adapted accordingly (1, 16, 17). Therefore, we have aimed to a) evaluate the clinical utility of echocardiography based ventricular wall stress algorithms in long-term risk-stratification of patients with post-ischemic myocardial dysfunction, and b) evaluate the additive utility of echocardiogram-derived ventricular wall stress over other common demographic and clinical parameters such as age and the extent of coronary artery disease.
Methods
Patient Selection
Patients who presented between March 2016 to January 2017 to our tertiary care hospitals (Buffalo General Hospital and Gates Vascular Institute, Buffalo, NY, USA) with an acute STEMI and underwent PCI within 90 minutes from first medical contact were prospectively enrolled. These patients were followed up to January 2019 for clinical outcomes. Patients with documented trauma, acute myocardial infarction, sudden cardiac arrest or cardiac surgery within 3 months prior to presentation and those with active malignancies were excluded from the study. Patients without a pre-discharge echocardiogram or those with limited-to-poor quality echocardiograms were also excluded from the study. The study was approved by the Institutional review board of The State University of New York at Buffalo, and appropriate informed consent were obtained.
Echocardiographic and Cardiac MRI Measurements
Echocardiographic images were taken by certified sonographers, within the first 72-hours from the time of PCI, prior to the hospital discharge. The LV dimensions and volumetric measurements were done in the standard transthoracic views. The Synapse Cardiovascular (FujiFilm, Valhalla, NY, USA) Software was used to postprocess and interpret the echocardiograms.
Cardiac MRI was acquired within 72 hours after echocardiography acquisition. A GE 1.5-T scanner with technical parameters recommended by the manufacturer was used. Scout images in coronal, sagittal and axial planes; fast spin-echo (FSE) axial slices; short-axis and two, three, and four-chamber steady-state free precession (SSFP) sequences; T2-weighted triple-inversion recovery images; T1-weighted FSE sequence, and delayed enhanced images 7–10 minutes after gadolinium (Omniscan 0.1 mmol/kg) injection were acquired. LV dimensions and measurements were performed by using Segment version 3.2 R8531 (http://segment.heiberg.se), as described previously by our group (18).
Left Ventricular Wall Stress:
Both diastolic and systolic left ventricular wall stress were calculated using volume and pressure-based parameters with a modified LaPlace’s equation adapted by Mirskey et al (19–21). The following equation was used to calculate the left ventricular wall stress.
P = Pressure during Diastole or Systole; Vlum= Volume of LV Lumen during Diastole or Systole; Vmyo= Volume of Myocardium.
Determination of end-systolic and end-diastolic pressures:
The systolic blood pressure measured at the time of echocardiogram was used to calculate the systolic wall stress. For diastolic wall stress, pulmonary capillary wedge pressure (PCWP), a surrogate of end-diastolic pressure was calculated using the Nagueh’s formula utilizing the E/e’ based echocardiographic parameters as described previously (22).
E = Early mitral inflow velocity; E’ = tissue doppler of the mitral annulus.
Quantification of LV volume:
Left ventricular luminal volume was measured using the Simpson’s Biplane Method, also recommended by American Society of Echocardiography (ASE) (23). Myocardial Volume was calculated using the LV mass and specific gravity of the myocardium which was previously reported to be 1.05g/cc.
Quantification of LV mass:
Left ventricular mass was calculated using the following formula as recommended by the ASE(23):
LVEDD = LV end diastolic diameter in cm; IVSd = interventricular septal thickness at the end of diastole; PWd = Posterior wall diameter at the end of diastole.
Follow-up and clinical outcomes:
The primary outcomes for this study was all-cause mortality. The secondary outcomes included MACE (defined as cardiovascular mortality, stroke and recurrent MI/revascularization). The baseline clinical history and laboratory data were obtained by electronic medical records review. The all-cause mortality and MACE outcomes were obtained either by telephone interview of the patient or family members, and electronic medical records review when applicable.
Statistical Analysis
Results are presented as mean ± SD for continuous variables and as percentage (%) for categorical variables. A Chi-square (χ2) test or Fisher exact test and Mann-Whitney U or student t-tests were used to compare categorical and continuous variables, respectively as appropriate. Logistic regression analysis was performed to establish the predictors of all-cause mortality. Variables that were significant in univariate analysis were then used in multivariate logistic regression analysis. The odds ratio (OR) and 95% confidence interval (CI) were calculated for each independent variable.
To assess the ability of the significant variables to predict all-cause mortality, a receiver operating characteristic (ROC) curve was generated. The area under the ROC curve (AUC) along with the corresponding 95% confidence interval (CI) is reported. For those markers with a significant AUC, optimal cut-points for predicting event were identified. For significant predictors obtained from ROC curve, cumulative survival curves were constructed for time to event using the Kaplan-Meier methods and tested for significance using log-rank statistics. Agreement between the quantitative measurements of ESWS calculated by using Echocardiography and cardiac MRI were presented with Bland-Altman plot and correlation analysis. Statistical significance was set at P < 0.05. The statistical analysis was performed using JMP pro, version 14 (SAS Inc).
Results
The initial validation of echocardiography-based myocardial wall stress calculation was performed in 4 subjects who had also undergone cardiac MRI within 72 hours. The graphic representation of the comparative analytical approaches of ESWS calculation using echocardiography vs. cardiac MRI are shown in figure 1 (A and B). Echocardiography and MRI-based MWS were in agreement with each other (Echocardiography, 14.8±1.9 kPa; MRI, 14.7±2.1 kPa).
Figure 1: Analytical approaches for the comparison of end-systolic wall stress calculated by echocardiogram vs. cardiac MRI.
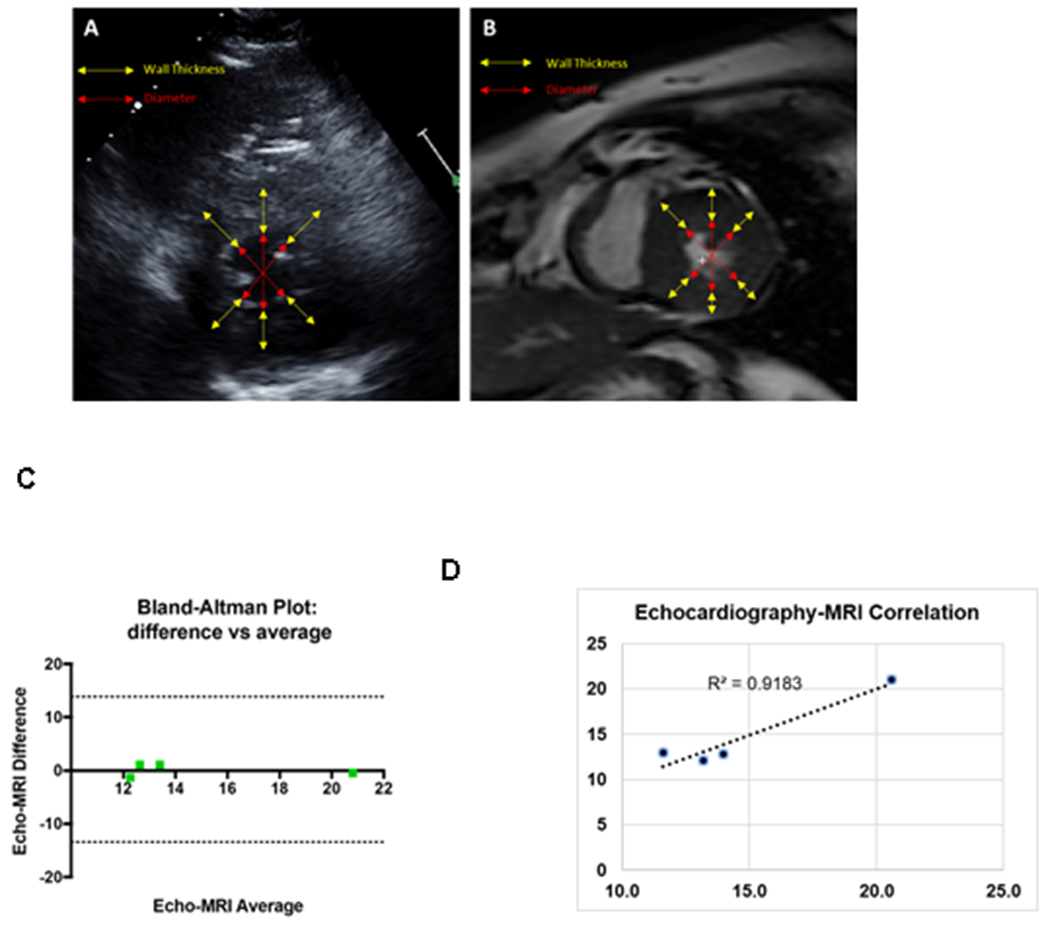
A. Representative echocardiography picture of a patient. B. Representative cardiac MRI picture of the same patient. MRI and echocardiographic acquisitions were obtained within the same day (3-hour apart). C. Dot plots showing the correlation between the ESWS calculated by echocardiography vs. cardiac MRI. D. Bland-Altman plot comparing the differences between the echocardiography vs. cardiac MRI-derived ESWS plotted against the averages of the two techniques.
Despite a small sample size, the correlation analysis showed a strong correlation between the echocardiography-based vs. cardiac MRI-derived wall stress parameters (R2=0.92) (Figure 1 C). Bland–Altman plots showed no systematic outliers or random fluctuations around mean, suggesting an agreement among the two different measurements techniques (Figure 1 D)
For our prospective follow-up study, a total of 142 patients who presented with an acute STEMI and underwent PCI within the 90 minutes of first medical contact were enrolled in this study. Out of 142 patients, 26 were excluded from the study as they did not meet the criteria for enrollment and/or inability to calculate myocardial wall stress due to poor echocardiographic images. The remaining 116 patients were followed up for an average of 16.7 (± 12.0) months to assess for all-cause mortality and MACE as outlined in figure 2. Upon completion of the follow up, 9% had died and 17% had developed MACE. Among the patients who developed MACE, the frequency of CV death, stroke and recurrent MI/revascularization were at 1.8%, 2.7% and 12.5%, respectively.
Figure 2: Overview of the research protocol.
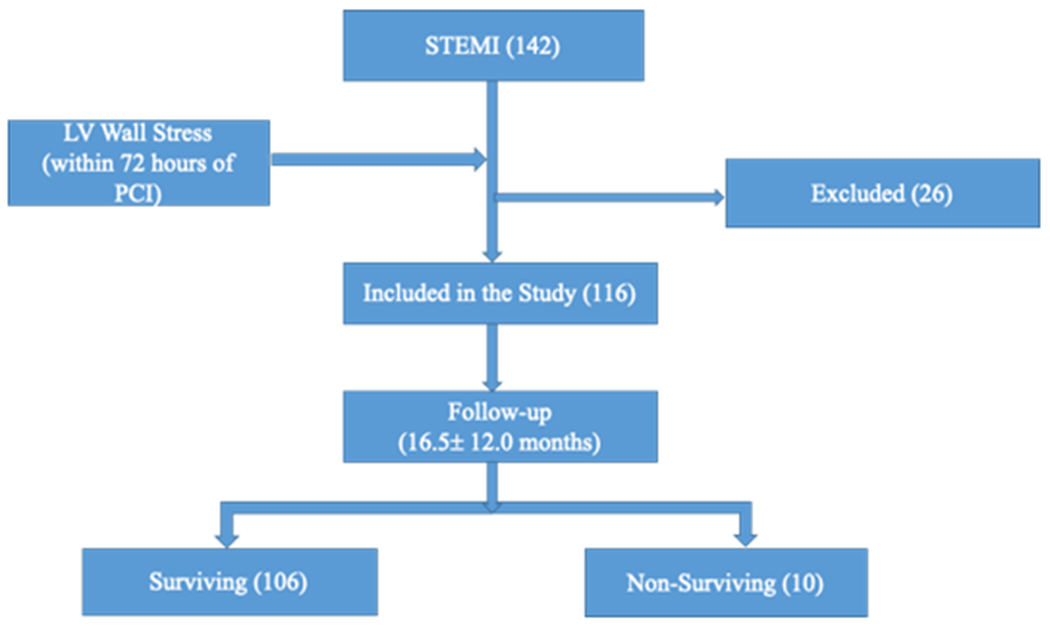
STEMI, ST elevation myocardial infraction
Table 1 outlines the baseline clinical characteristics of all the patients and comparisons among the surviving and non-surviving groups. Overall, non-survivors were older than survivors (non-surviving: 77.30±9.21; survivors: 60.03±12.87; P<0.0001). However, there were no statistically significant differences among other baseline characteristics including sex, ethnicity and existing medical comorbidities. The ESWS was found to be significantly higher in the non-surviving group compared to the surviving group (non-survivor: 80.00±36.86 vs survivor: 59.28±27.68; P = 0.03). There were no differences in peak troponin levels, LV ejection fraction (LVEF) and EDWS measured after myocardial infraction, when compared between the survivors and non-survivors.
Table 1:
Baseline clinical characteristics among survivals and non-survivals
| Parameters | All Patients (n=116) | Surviving (N=106) | Non-surviving (N=10) | P value |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age, years | 61.3 ± 13.5 | 60.0 ± 12.9 | 77.3 ± 9.2 | <0.0001 |
| Female, % | 32 | 29.5 | 60 | 0.07 |
| Whites, % | 92 | 93.8 | 80 | 0.12 |
| HTN, % | 52 | 49.1 | 80 | 0.09 |
| DM, % | 24 | 22.3 | 40. | 0.24 |
| Smoking, % | 62 | 62.2 | 60 | 1.00 |
| CHF, % | 5.1 | 5.4 | 0 | 1.00 |
| CAD, % | 18 | 16.1 | 40 | 0.08 |
| Meds | ||||
| ASA, % | 28 | 25.9 | 50 | 0.13 |
| Statin, % | 34 | 33 | 60 | 0.16 |
| BB, % | 24 | 21.4 | 40 | 0.23 |
| ACEi/ARB, % | 28 | 25.9 | 50 | 0.13 |
| Diuretics, % | 9 | 8.9 | 10 | 1.00 |
| Labs | ||||
| Peak Troponin, ng/ml | 104.9 ± 127.0 | 106.1 ± 131.6 | 105.5 ± 82.2 | 0.47 |
| Anterior STEMI on ECG, % | 38 | 40.5 | 20 | 0.31 |
| Echocardiogram | ||||
| LVEF, % | 50.0 ± 12.0 | 50.4±11.9 | 45.6 ± 12.4 | 0.24 |
| LVEF < 35%, % | 14.6 | 20.0 | 14.15 | 0.63 |
| EDWS, Kpa | 12.5 ± 6.5 | 12.3 ± 6.3 | 14.6 ± 9.1 | 0.34 |
| ESWS, Kpa | 60.9 ± 28.9 | 59.3 ± 27.7 | 80.0 ± 36.9 | 0.03 |
| LV EDV, ml | 92.9 ± 41.3 | 92.8 ± 40.7 | 93.2 ± 48.9 | 0.97 |
| LV ESV, ml | 47.9 ± 34.7 | 47.4 ± 35.16 | 54.2 ±30.7 | 0.55 |
| LV SV, ml | 44.9 ± 17.9 | 45.5 ± 17.2 | 39.0 ± 24.5 | 0.27 |
| Coronary Angiogram | ||||
| PCI of: | ||||
| LAD, % | 43 | 44.4 | 30 | 0.81 |
| LCx, % | 9 | 8.3 | 10 | |
| RCA, % | 44 | 42.6 | 60 | |
| 3 Vessel dis, % | 24 | 23.4 | 30 | 0.70 |
| >50% LM stenosis, % | 5.1 | 2.8 | 30 | 0.007 |
| LV EDP, mmHg | 21.9 ± 8.0 | 22.2 ± 7.9 | 19.5 ± 8.4 | 0.34 |
| LV SP, mmHg | 119.8 ± 17.5 | 118.9 ± 16.5 | 129.8 ± 25.5 | 0.06 |
| Outcomes | ||||
| Hospital LOS, hrs | 96.8 ± 137.2 | 96.4 ± 142 | 101.7 ± 46.7 | 0.15 |
| MACE, % | 17 | 16.5 | 30 | 0.37 |
HTN, hypertension; DM, diabetes mellitus; CHF, congestive heart failure; CKD, chronic kidney disease; STEMI, ST-elevation myocardial infarction; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin-receptor blocker; EDWS, end-diastolic wall stress; ESWS, end-systolic wall stress; LVEF, left ventricular ejection fraction; PCI, percutaneous intervention; LOS, length of stay; MACE, major adverse cardiac events. LV SV, LV stroke volume; LV EDV, LV end-diastolic volume; LVEDP, LV end-diastolic pressure; LVSP, LV systolic pressure.
The incidence of significant left main disease (defined as > 50% stenosis) was higher in the non-survivors compared to survivors (non-survivors: 30% vs survivors: 2.70%; P = 0.007). There were no differences in the types of coronary intervention, compared between the surviving and non-surviving groups.
Univariate analysis to establish the possible predictors for all-cause mortality identified age (OR: 1.14, 95% CI: 1.07-1.23, P= 0.0001), ESWS (OR 1.02, 95% CI: 1.00-1.04, P=0.01) and significant left main disease (OR: 14.12, 95% CI: 3.50-56.80, P=0.0002) as the independent predictors of mortality (table 2). Receiver operating characteristics (ROC) of the variables significant on univariate analysis (age, elevated ESWS and >50% left main (LM) disease) are shown figure 3. On multivariate nominal logistic regression including the predictors identified on univariate analysis, identified age (OR: 1.16, 95% CI: 1.06-1.31, P=0.0002) and ESWS over 62.5 kpa (OR 7.75, 95% CI:1.33-73.86, P=0.008) as the only independent predictors for all-cause mortality as shows in Table 3.
Table 2:
Univariate logistic regression analysis for all-cause mortality
| OR | 95 % CI | P value | |
|---|---|---|---|
| Clinical parameters | |||
| Age, per unit change | 1.2 | 1.1 - 1.2 | <0.0001 |
| Female | 2.9 | 0.8 - 10.4 | 0.09 |
| AA vs Whites | 3.3 | 0.7 - 15.5 | 0.13 |
| HTN | 3.6 | 0.8 - 16.9 | 0.10 |
| DM | 2.0 | 0.6 - 7.2 | 0.27 |
| CAD | 3.2 | 0.9 - 11.3 | 0.07 |
| Smoking | 0.7 | 0.2 - 2.6 | 0.61 |
| CHF | 1.6 | 0.7 - 3.6 | 0.30 |
| Medications | |||
| ASA | 2.4 | 0.7 - 8.5 | 0.15 |
| Statin | 2.9 | 0.8 - 10.1 | 0.10 |
| BB | 1.1 | 0.5 - 6.3 | 0.80 |
| ACEi/ARB | 2.2 | 0.6 - 7.6 | 0.21 |
| Diuretics | 1.2 | 0.1 - 9.3 | 0.87 |
| Echocardiogram | |||
| LVEF, per unit change | 0.9 | 0.9 - 1.0 | 0.15 |
| LVEF <35% | 1.5 | 0.3 - 7.8 | 0.61 |
| ESWS, per unit change | 1.0 | 1.0 - 1.0 | 0.01 |
| EDWS, per unit change | 1.0 | 0.9 - 1.1 | 0.38 |
| LV SV, per unit change | 0.9 | 0.9 – 1.0 | 0.27 |
| LV EDV, per unit change | 1.0 | 0.9 – 1.0 | 0.97 |
| Coronary Angiogram | |||
| 3 Vessel dis, % | 1.6 | 0.4 - 6.3 | 0.48 |
| >50% LM stenosis | 14.1 | 3.5 - 56.8 | 0.02 |
| Proximal LAD stenosis | 0.5 | 0.1 - 2.6 | 0.43 |
| LVEDP, per unit change | 0.9 | 0.8 - 1.0 | 0.34 |
| LVSP, per unit change | 1.0 | 0.9 - 1.1 | 0.06 |
AA, African American; HTN, hypertension; DM, diabetes mellitus; CHF, congestive heart failure; CKD, chronic kidney disease; STEMI, ST-elevation myocardial infarction; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin-receptor blocker; EDWS, end-diastolic wall stress; ESWS, end-systolic wall stress; LVEF, left ventricular ejection fraction; LM, left main; LAD, left anterior coronary artery; LV SV, LV stroke volume; LV EDV, LV end-diastolic volume; LVEDP, LV end-diastolic pressure; LVSP, LV systolic pressure.
Figure 3: Receiver operating characteristics of the variables significant on univariate analysis.
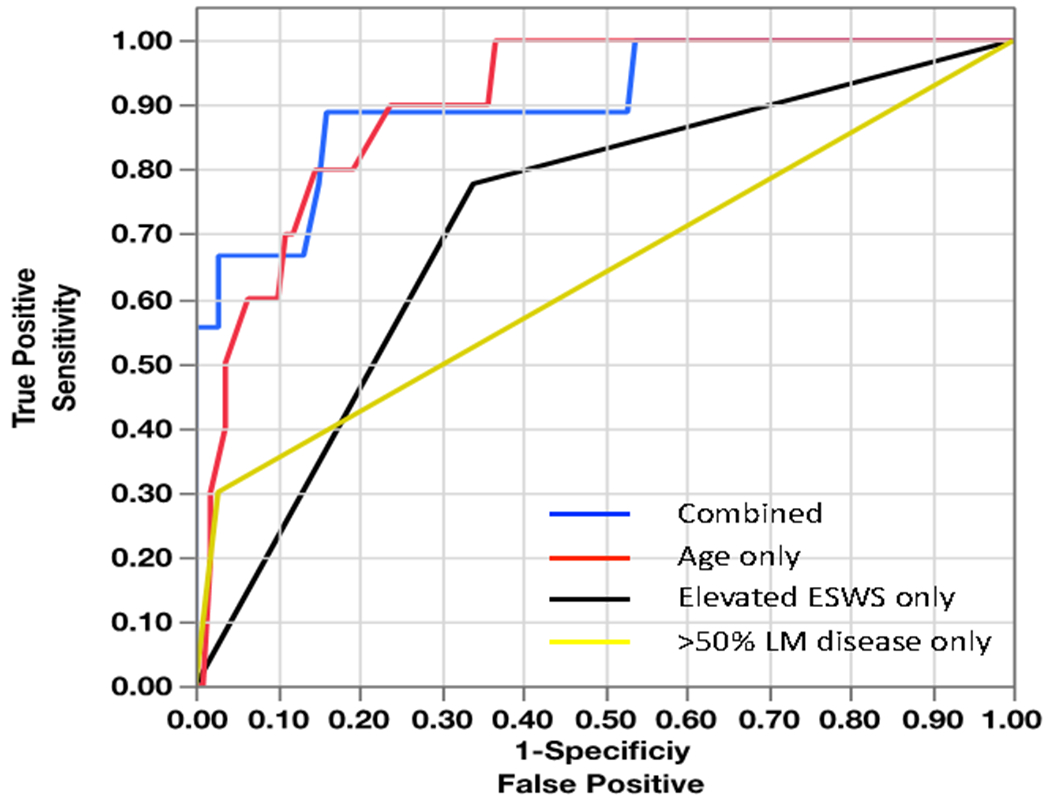
Combined AUC: 0.89; Age only: 0.86; Elevated ESWS>62.5 only: 0.73, LM disease only: 0.58; ESWS-End systolic wall stress, LM-left main.
Table 3:
Multivariate logistic regression analysis for all-cause mortality
| Parameters | OR | 95 % CI | P value |
|---|---|---|---|
| Age, per unit change | 1.2 | 1.1 - 1.3 | 0.0002* |
| ESWS (>62.5 Kpa) | 7.8 | 1.3 - 73.9 | 0.008* |
| >50% LM disease | 5.7 | 0.3 - 108.4 | 0.22 |
ESWS, end-systolic wall stress; LM, left main.
statistically significant
Figure 4 shows the Kaplan Meir survival plot showing elevated ESWS greater than 62.5 kpa to be associated with increased mortality long-term follow-up (log rank: 5.73, p=0.01). Figure 5 shows the distribution of ESWS at various ranges of LVEF. In particular, the non-survivors with only mildly reduced LVEF had very high LV ESWS (> 62.5 Kpa). Univariate analysis for MACE outcome did not identify independent predictors of long-term outcomes as shown in supplemental table 1.
Figure 4: Kaplan Meier Survival plot of patients with and without elevated ESWS (>62.5 Kpa).
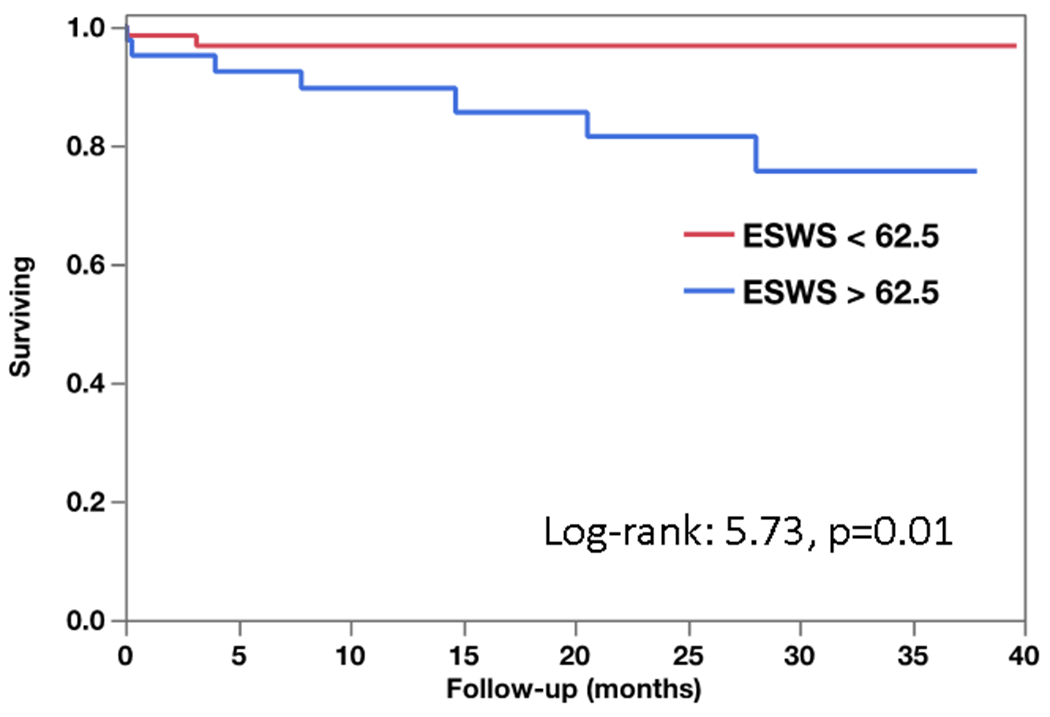
ESWS-End systolic wall stress.
Figure 5: Distribution of ESWS based on the LVEF among Survivors and Non-Survivors.
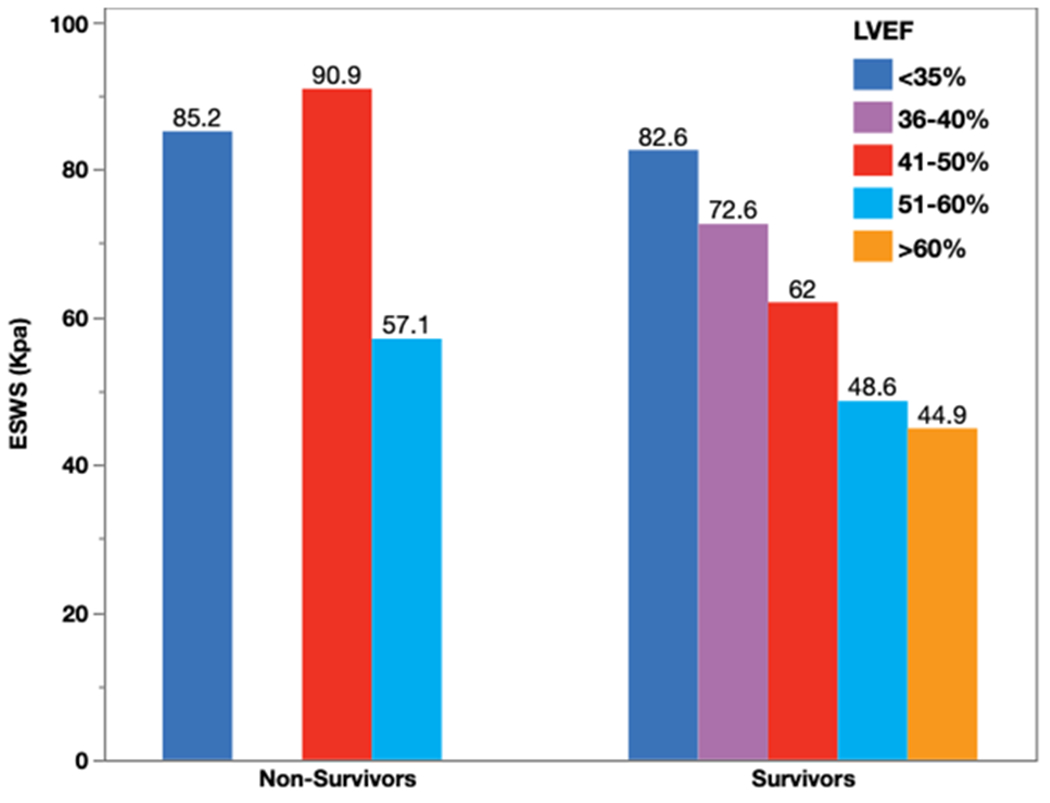
ESWS-End systolic wall stress, LVEF- Left ventricle ejection fraction.
Discussion
This goal of this study was study was to validate new echocardiogram derived wall stress parameters for the long-term risk-prediction of cardiac deaths in patients with ischemic cardiomyopathy. Our study shows elevated ESWS to be associated with increased mortality in patients who underwent primary PCI after STEMI. We found that an elevated ESWS of greater than 62.5 kpa is associated with eight-fold higher odds of mortality compared to those with ESWS of less than 62.5 kpa.
Acute ischemia results into hemodynamic alteration and progressive changes of myocardial geometry and function. In pre-clinical models, an acute ischemic insult results in substantial increase in the myocardial systolic wall stress at the infarcted region (24). This increase in systolic wall stress was also found to be associated with increased expression of matrix metalloproteinase-9, higher macrophage density and increased collagen content (24). The increase in systolic wall stress was sustained and progressive for up to three weeks following the infarction. Earlier studies have also found strong association between left ventricular wall stress and subsequent development of heart failure (16, 17). However, change in wall stress in relation to the prognostication and risk stratification after acute MI was not well studied. We have found no other studies that have comprehensively examined myocardial wall stress in relation to increased mortality in patients with had undergone PCI after acute MI.
Currently, limited imaging-based data are available to risk-stratify post-STEMI patients for long-term clinical outcomes, including the follow-up beyond hospital discharge. Our analyses show that elevated ESWS is associated with poor long-term survival. ESWS enhances the predictive utility when combined with other clinical covariates, including age and the extent of coronary artery stenosis. Our previous clinical study published in 2018 identified elevated EDWS as one of the independent predictors of MACE after acute MI (1). The results from the current study, which has larger sample size and longer follow-up interval, suggest that elevated diastolic wall stress is not able to discriminate the long-term prognosis but may still be relevant at an early stage following STEMI. Taken together, the diastolic wall stress may represent the acute ischemic response, and myocardial stunning that persists for a few weeks after acute MI despite a successful PCI. However, long-term, there are progressive changes in the LV geometry and function, in particular, the intracavitary sub-endocardial wall thinning which leads to elevated ESWS. Our study emphasizes the importance of further studying these unique echocardiography-based metrics in larger longitudinal studies.
There has been substantial improvement in the last decade in acute care of the STEMI with goal to reduce the infract size with early aggressive PCI, introduction of potent antiplatelet therapies and advancement in intervention techniques and technologies(25). However, after successful emergent PCI, risk stratification and prognostication still remain to be a challenging task. LVEF still is the main clinical prognostication tool in the contemporary practice (26, 27). Increased mortality and higher risk of sudden cardiac death are reported in patients with severely reduced LVEF. However, when LVEF is normal or mildly reduced, its discrimination power is poor (28, 29). Besides, the current approaches used in the measurement of the LVEF continues to be subjective, with inter-observer variability as high as 14%. In our study, LVEF was not an independent predictor for mortality. This could be due to relatively preserved LVEF in our patient population likely from timely intervention with relatively small infract zone. Besides, in patients with normal or mildly reduced LVEF, ejection fraction may not be a strong discriminator for mortality. Importantly, we found that patients with mildly reduced LVEF with significantly elevated ESWS had higher mortality. Although the odds of mortality risk could not be initially discriminated by the LVEF alone, use of an additional parameter (ESWS > 62.5 Kpa), clearly discerned the higher odds of mortality during follow-up.
The findings of our study need to be considered in light of some limitations. Our sample size for STEMI population was only modest, whereas the comparative analysis of ESWS using echocardiography vs. cardiac MRI was performed in only 4 patients. The quantification of myocardial wall stress can be affected by accurate measurement of the interventricular septum and posterior wall thickness. Additionally, myocardial wall stress is relatively dynamic variable and values may vary depending on the time and circumstances of measurement. In particular, other determinants of clinical outcomes after STEMI including infarct size, time-to-reperfusion and echocardiography-based global longitudinal strains were not included in the multivariate analysis. Additionally, wall stress was calculated within the first 72 hours post PCI which is within a feasible pre-discharge timeframe from the clinical standpoint.
Conclusion
This study has important clinical implications in patients with ischemic cardiomyopathy. We report that elevated end-systolic wall stress is associated with increased long-term mortality in patients who underwent emergent PCI for STEMI. To date, there are no other reliable echocardiographic variables to risk stratify post-ischemic changes of myocardial wall sress. We believe that left ventricular wall stress will provide a better and reliable risk stratification tool by taking in to account multiple indices of myocardial geometry and function. As mentioned above, left ventricular wall stress can be calculated simply from transthoracic echocardiography, which is a routinely performed study in these patients. Therefore, this study will allow us to risk stratify patients using a simple routine test and tailor treatment plan based on their risk status.
Supplementary Material
Funding Sources:
This research was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health (award number UL1TR001412) to the University at Buffalo. UCS also received support from the NIH/NHLBI K08 HL131987 and R01HL152090).
Footnotes
Disclosures:
All authors disclose no conflict of interest.
Data Availability Statement:
The data that support the findings of this study are available on request from the corresponding author.
References
- 1.Mosleh W, Elango K, Shah T, Chaudhari M, Gandhi S, Kattel S, et al. Elevated end-diastolic wall stress after acute myocardial infarction predicts adverse cardiovascular outcomes and longer hospital length of stay. Echocardiography (Mount Kisco, NY). 2018;35(11):1721–8. [DOI] [PubMed] [Google Scholar]
- 2.Gammill JF, Applegarth JJ, Reed CE, Fernald JD, Antenucci AJ. Hemodynamic changes following acute myocardial infarction using the dye injection method for cardiac output determination. Ann Intern Med 1955;43(1):100–19. [DOI] [PubMed] [Google Scholar]
- 3.Lamas GA, Pfeffer MA. Left ventricular remodeling after acute myocardial infarction: clinical course and beneficial effects of angiotensin-converting enzyme inhibition. Am Heart J 1991;121(4 Pt 1):1194–202. [DOI] [PubMed] [Google Scholar]
- 4.Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101(25):2981–8. [DOI] [PubMed] [Google Scholar]
- 5.Strauer BE. Myocardial oxygen consumption in chronic heart disease: role of wall stress, hypertrophy and coronary reserve. Am J Cardiol 1979;44(4):730–40. [DOI] [PubMed] [Google Scholar]
- 6.Cleutjens JP, Kandala JC, Guarda E, Guntaka RV, Weber KT. Regulation of collagen degradation in the rat myocardium after infarction. J Mol Cell Cardiol 1995;27(6):1281–92. [DOI] [PubMed] [Google Scholar]
- 7.Hutchins GM, Bulkley BH. Infarct expansion versus extension: two different complications of acute myocardial infarction. Am J Cardiol 1978;41(7):1127–32. [DOI] [PubMed] [Google Scholar]
- 8.Feild BJ, Russell RO, Jr., Dowling JT, Rackley CE. Regional left ventricular performance in the year following myocardial infarction. Circulation. 1972;46(4):679–89. [DOI] [PubMed] [Google Scholar]
- 9.Swan HJ, Forrester JS, Diamond G, Chatterjee K, Parmley WW. Hemodynamic spectrum of myocardial infarction and cardiogenic shock. A conceptual model. Circulation. 1972;45(5):1097–110. [DOI] [PubMed] [Google Scholar]
- 10.Pfeffer MA, Pfeffer JM, Fishbein MC, Fletcher PJ, Spadaro J, Kloner RA, et al. Myocardial infarct size and ventricular function in rats. Circ Res 1979;44(4):503–12. [DOI] [PubMed] [Google Scholar]
- 11.Kitamura S, Kay JH, Krohn BG, Magidson O, Dunne EF. Geometric and functional abnormalities of the left ventricle with a chronic localized noncontractile area. Am J Cardiol 1973;31(6):701–7. [DOI] [PubMed] [Google Scholar]
- 12.Lamas GA, Pfeffer MA. Increased left ventricular volume following myocardial infarction in man. Am Heart J 1986;111(1):30–5. [DOI] [PubMed] [Google Scholar]
- 13.Grossman W, Jones D, McLaurin LP. Wall stress and patterns of hypertrophy in the human left ventricle. J Clin Invest 1975;56(1):56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gulch RW, Jacob R. Geometric and muscle physiological determinants of cardiac stroke volume as evaluated on the basis of model calculations. Basic Res Cardiol 1988;83(5):476–85. [DOI] [PubMed] [Google Scholar]
- 15.Prunier F, Brette S, Delepine S, Geslin P, Le Jeune JJ, Furber AP. Three-dimensional MRI assessment of regional wall stress after acute myocardial infarction predicts postdischarge cardiac events. J Magn Reson Imaging 2008;27(3):516–21. [DOI] [PubMed] [Google Scholar]
- 16.Clerfond G, Biere L, Mateus V, Grall S, Willoteaux S, Prunier F, et al. End-systolic wall stress predicts post-discharge heart failure after acute myocardial infarction. Arch Cardiovasc Dis 2015;108(5):310–20. [DOI] [PubMed] [Google Scholar]
- 17.Alter P, Koczulla AR, Nell C, Figiel JH, Vogelmeier CF, Rominger MB. Wall stress determines systolic and diastolic function--Characteristics of heart failure. Int J Cardiol 2016;202:685–93. [DOI] [PubMed] [Google Scholar]
- 18.Karthikeyan B, Sonkawade SD, Pokharel S, Preda M, Schweser F, Zivadinov R, et al. Tagged cine magnetic resonance imaging to quantify regional mechanical changes after acute myocardial infarction. Magn Reson Imaging 2020;66:208–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mirsky I Left ventricular stresses in the intact human heart. Biophys J 1969;9(2):189–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mirsky I, Parmley WW. Assessment of passive elastic stiffness for isolated heart muscle and the intact heart. Circulation research. 1973;33(2):233–43. [DOI] [PubMed] [Google Scholar]
- 21.Alter P, Rupp H, Stoll F, Adams P, Figiel JH, Klose KJ, et al. Increased end diastolic wall stress precedes left ventricular hypertrophy in dilative heart failure--use of the volume-based wall stress index. Int J Cardiol 2012;157(2):233–8. [DOI] [PubMed] [Google Scholar]
- 22.Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Quinones MA. Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. Journal of the American College of Cardiology. 1997;30(6):1527–33. [DOI] [PubMed] [Google Scholar]
- 23.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2015;28(1):1–39.e14. [DOI] [PubMed] [Google Scholar]
- 24.Rohde LE, Aikawa M, Cheng GC, Sukhova G, Solomon SD, Libby P, et al. Echocardiography-derived left ventricular end-systolic regional wall stress and matrix remodeling after experimental myocardial infarction. J Am Coll Cardiol 1999;33(3):835–42. [DOI] [PubMed] [Google Scholar]
- 25.Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, et al. 2015 ACC/AHA/SCAI Focused Update on Primary Percutaneous Coronary Intervention for Patients With ST-Elevation Myocardial Infarction: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention and the 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction. Journal of the American College of Cardiology. 2016;67(10):1235–50. [DOI] [PubMed] [Google Scholar]
- 26.Krumholz HM, Anderson JL, Bachelder BL, Fesmire FM, Fihn SD, Foody JM, et al. ACC/AHA 2008 performance measures for adults with ST-elevation and non-ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures (Writing Committee to develop performance measures for ST-elevation and non-ST-elevation myocardial infarction): developed in collaboration with the American Academy of Family Physicians and the American College of Emergency Physicians: endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation, Society for Cardiovascular Angiography and Interventions, and Society of Hospital Medicine. Circulation. 2008;118(24):2596–648. [DOI] [PubMed] [Google Scholar]
- 27.Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction). Circulation. 2004;110(5):588–636. [DOI] [PubMed] [Google Scholar]
- 28.Hammermeister KE, DeRouen TA, Dodge HT. Variables predictive of survival in patients with coronary disease. Selection by univariate and multivariate analyses from the clinical, electrocardiographic, exercise, arteriographic, and quantitative angiographic evaluations. Circulation. 1979;59(3):421–30. [DOI] [PubMed] [Google Scholar]
- 29.Cleempoel H, Vainsel H, Bernard R, Dramaix M, Lenaers A, Van Kuyk M, et al. Predictors of early death after acute myocardial infarction: two months follow-up. European heart journal. 1986;7(4):305–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.


