Abstract
Chemosensory impairments have been established as a specific indicator of COVID-19. They affect most patients and may persist long past the resolution of respiratory symptoms, representing an unprecedented medical challenge. Since the SARS-CoV-2 pandemic started, we now know much more about smell, taste, and chemesthesis loss associated with COVID-19. However, the temporal dynamics and characteristics of recovery are still unknown. Here, capitalizing on data from the Global Consortium for Chemosensory Research (GCCR) crowdsourced survey, we assessed chemosensory abilities after the resolution of respiratory symptoms in participants diagnosed with COVID-19 during the first wave of the pandemic in Italy. This analysis led to the identification of two patterns of chemosensory recovery, partial and substantial, which were found to be associated with differential age, degrees of chemosensory loss, and regional patterns. Uncovering the self-reported phenomenology of recovery from smell, taste, and chemesthetic disorders is the first, yet essential step, to provide healthcare professionals with the tools to take purposeful and targeted action to address chemosensory disorders and their severe discomfort.
Subject terms: Health care, Quality of life
Introduction
According to the World Health Organization, COVID-19 has been confirmed in more than 113 million cases across 223 countries, leading to more than 4.1 million deaths (https://www.who.int/emergencies/diseases/novel-coronavirus-2019, Last update: July 20, 2021). Recent estimates indicate that up to 98% of individuals diagnosed with COVID-19 developed forms of chemosensory disorders, most prominently smell loss1–8. Data collected before the COVID-19 pandemic showed that up to 49% of the population report an episode of olfactory loss over their lifetime, with 5% of them reporting complete smell loss (anosmia)9–11. Population-based epidemiological studies before COVID-19 provide prevalence estimates of smell loss ranging from 2.7 to 24.5%12–16 and taste disorders ranging from 0.6 to 20%14,16. Moreover, in older adults the prevalence of olfactory impairment increases11.
Reports to date reveal that the COVID-19 pandemic has already significantly increased the prevalence of chemosensory disorders worldwide, especially among younger cohorts1,17, yet the global estimates on chemosensory disorders may be markedly underestimated.
Chemosensory disorders are both early and specific symptoms of COVID-1918–20. Previous studies indicated that the timeframe for a full or partial recovery (in particular of the sense of smell) seems to be highly variable, spanning from 8 days to even 8 weeks8,21–28. For the vast majority of patients (up to 85%), chemosensory issues resolve along with Covid-Like-Illness (CLI) symptoms, in approximately 3 weeks22,27–29. Nevertheless, approximately from 7 to 37% of patients continue to report chemosensory loss as their main neurological sequelae, which persists after the resolution of CLI symptoms8,22. Therefore, if patients affected by COVID-19 are initially very concerned about the development of the infection and the severity of the illness, in a later stage, they develop serious concerns for a prompt resolution of smell and taste loss. Persistent smell and taste loss are unexpected and invisible disorders associated with a significant reduction in a person's quality of life30–32, including increased depressive symptoms30, anxiety33, sexual desire34, nutritional35–38, and safety issues30,39. It is important to note that these side effects are not COVID-specific but characterize patients' experience affected by smell and taste loss because of a variety of etiologies30,39. During the COVID-19 pandemic, smell and taste loss took center stage, exposing the reduced awareness of the national healthcare systems worldwide which were not well prepared to address the needs of patients who suffer from smell loss long-term.
Italy has been the first European country to be massively hit by COVID-1940,41 (http://www.salute.gov.it/imgs/C_17_notizie_4403_0_file.pdf), the Lombardy region was particularly affected, reaching the highest death toll of the first wave (28 K on 25 February, 2021; https://www.statista.com/statistics/1099389/coronavirus-deaths-by-region-in-italy/)42. As a result, throughout the national territory, and in particular in the most affected regions, the need to address COVID-19 long-haulers with chemosensory symptomatology has emerged early and prominently43–46. The Italian National Healthcare system currently lacks capillary specialized assistance for patients with smell and taste loss. Approximately 5500 otolaryngologists operate in the country, of which only a minority is specialized (approximately 5% of them) in taste and smell disorders (interview with Carmelo Zappone, president of Associazione Italiana ORL Libero Professionisti, https://www.aiolp.it/). Taste and smell specialists are mostly located in clinics and centers within hospitals, yet the emergency measures undertaken in response to the COVID-19 pandemic have drastically reduced ENT outpatient activities47,48.
Therefore, to face the exponential increase of patients with taste and smell disorders, a greater number of healthcare professionals, including general practitioners as well as frontline healthcare workers, would need tools (both objective and subjective tests) to recognize and validate the individual chemosensory experience of patients to refer them to specialists. Although psychophysical tests (i.e., Sniffin’Sticks, UPSIT, Taste Strips) are important clinical tools to address chemosensory disorders, here we propose that studying the phenomenology of recovery from chemosensory loss using patients’ self-reports is the first, yet essential step, to shape a purposeful and targeted action to address chemosensory loss and its significant discomfort in patient’s lives. Specifically, we tested our pre-registered hypotheses on the self-reports of the Italian participants collected via the crowdsourced GCCR survey49 detailing the phenomenology of self-reported chemosensory abilities before, during, and after COVID-19 diagnosis. First, we aimed to describe the patterns of recovery of smell, taste, and chemesthetic abilities, individually and in combination, in relation to the timeline of other CLI symptoms. We set out to confirm that the chemosensory recovery would be more advanced the farthest from CLI symptom onset and for limited losses during the disease; we explored the pattern of recovery based on the different profiles of chemosensory loss during the disease. Second, we assessed whether specific demographic information, COVID-19 symptoms and/or prior medical conditions constitute risk factors for lengthy or no recovery from chemosensory loss within 6 months.
Partial or substantial chemosensory recovery from COVID-19
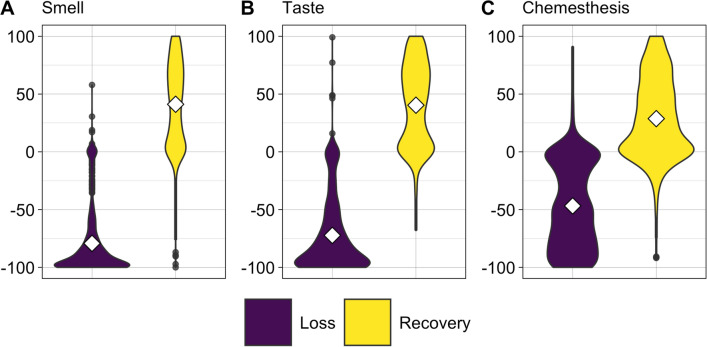
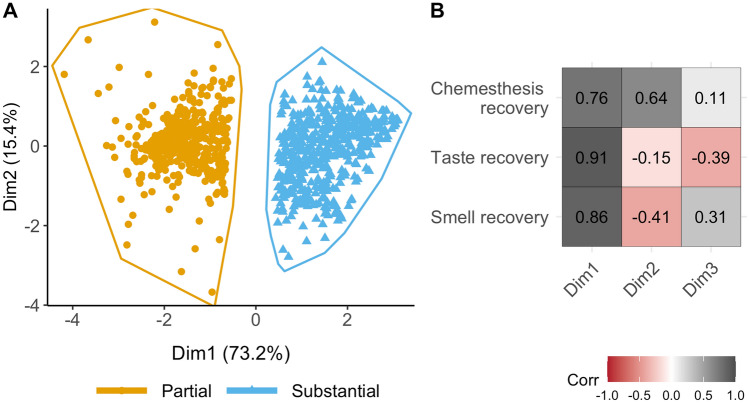
Data from a final sample of the 974 Italian residents who participated in the GCCR online survey between 10th of April 2020 and 17th of October 2020 and who reported partial or full recovery from CLI was used to determine profiles of chemosensory recovery patterns. With the goal of limiting the number of questions that a healthcare professional should ask to determine the state of chemosensory recovery, we focused on rating scales, which proved to be the most accessible way to identify chemosensory loss in individuals positive for COVID-1918. We, therefore, selected the ratings on 0–100 scales given to smell (i.e., the ability to perceive the smell of flowers, soap, or garbage but not the flavor of food in the mouth), taste (i.e., the ability to perceive sweetness, sourness, saltiness, bitterness in the mouth), and chemesthesis abilities (i.e., the ability to perceive the spiciness of chili peppers, the cooling of menthol and the carbonation in soda) after the disease minus their ratings during the disease. Ratings significantly differed among before, during, and after the disease for smell [F(2, 2919) = 2451, p < 0.001], taste [F(2, 2919) = 1989, p < 0.001], and chemesthesis [F(2, 2919) = 793.5, p < 0.001]. Indeed, participants reported to have significantly lost their sense of smell (mean = 11.90, SD = 23.47, Fig. 1A), taste (mean = 20.39, SD = 28.07, Fig. 1B) and chemesthesis (mean = 40.81, SD = 32.97, Fig. 1C) during COVID-19 as compared to before COVID-19 started [smell: mean = 91.14, SD = 16.82, p < 0.001; taste: mean = 92.74, SD = 13.71, p < 0.001; chemesthesis: mean = 87.63, SD = 17.30, p < 0.001]. After the resolution of CLI symptoms, on average smell (mean = 53.05, SD = 32.22, p < 0.001, Fig. 1A); taste (mean = 60.75, SD = 30.89, p < 0.001, Fig. 1B), and chemesthesis abilities (mean = 69.52, SD = 25.80, p < 0.001, Fig. 1C) improved. However, such post-CLI improvement is not homogenous. An exploratory cluster analysis (k-means, bootstrapped stability = 0.98) revealed two chemosensory recovery groups: partial (N = 471, 48.36% of the sample; centroids: smell = 13.4, taste = 10.07, chemesthesis = 7.55) and substantial (N = 503, 51.64% of the sample; centroids: smell = 67.2, taste = 68.1, chemesthesis = 48.5; Fig. 2A, B). The three chemosensory modalities contributed equally to Dimension 1 that explained 73.2% of the variance while chemesthesis recovery was the major contributor to Dimension 2 (Fig. 2B).
Figure 1.
Loss (during—before ratings; violet) and recovery (after—during ratings; yellow) of smell (A), taste (B), and chemesthesis (C). Violin plots represent the smoothed distribution of data; white diamonds indicate the mean; whiskers indicate maximum and minimum values.
Figure 2.
(A) Clusters of participants on chemosensory recovery identified by k-means clustering. The scatterplot shows each participant's loading on Dimension 1 (Dim1) and Dimension 2 (Dim2) of the Principal Component Analysis. Partial = smell, taste, and chemesthesis partial recovery; Substantial = smell, taste, and chemesthesis substantial recovery. (B) Correlations between the three principal components with respect to recovery in smell, taste, and chemesthesis. Gray color indicates a positive correlation, whereas shades of red indicate negative correlations. Darker shades indicate stronger correlations.
Among other characteristics (Table 1), participants who only partially recovered their chemosensory abilities at the time of survey completion were older (mean = 43.16, SD = 11.74) and reported to have contracted the disease earlier (mean = 43.15 days, SD = 23.87) than those who substantially recovered [age: mean = 39.63, SD = 10.75, t(972) = 4.89, p < 0.001; days from COVID-19 symptom onset: mean = 40.17, SD = 15.41, t(972) = 2.33, p = 0.02].
Table 1.
Characteristics of the total sample and the clusters based on chemosensory recovery.
| Variable | Full sample (N = 974) | Partial chemosensory recovery (N = 471) Mean (SD) or N |
Substantial chemosensory recovery (N = 503) Mean (SD) or N |
Statistic |
|---|---|---|---|---|
| Smell recovery | 41.14 (35.69) | 13.37 (24.97) | 67.16 (21.97) | t = − 35.59, p < 0.0001 |
| Taste recovery | 40.35 (35.58) | 10.74 (21.58) | 68.07 (20.62) | t = − 42.32, p < 0.0001 |
| Chemesthesis recovery | 28.71 (34.87) | 7.55 (24.08) | 48.52 (31.63) | t = − 22.83, p < 0.0001 |
| Region of residency (Lombardy) | 653 (67%) | 292 (61.9%) | 361 (71.8%) | χ2 = 2.02, p = 0.15 |
| Gender (female) | 675 (69.3%) | 329 (48.7%) | 346 (51.2%) | χ2 = 0.0002, p = 0.98 |
| Age | 41.33 (11.37), range = 19–78 | 43.16 (11.74), range = 19–75 | 39.63 (10.75), range = 19–78 | t = 4.88, p < 0.0001 |
| Onset of symptoms (days) | 41.61(20), range = 3–177 | 43.15 (23.87), range = 3–177 | 40.17 (15.41), range = 7–152 | t = 2.30, p = 0.02 |
| COVID-19 diagnosis | 591 self-diagnosed (60.6%), 196 lab tested (20.1%), 187 clinical assessment (19.2%) | 279 self-diagnosed (59.2%), 107 lab tested (22.7%), 85 clinical assessment (18.02%) | 312 self-diagnosed (62.02%), 89 lab tested (17.7%), 102 clinical assessment (20.3%) | χ2 = 0.81, p = 0.66 |
| Smokers (yes) | 427 (43.8%) | 194 (41.18%) | 233 (46.3%) | χ2 = 0.35, p = 0.55 |
| Prior medical conditions (% based on presence of at least one prior medical condition) | 311 (31.9%) | 155 (32.9%) | 156 (31.01%) | χ2 = 0.02, p = 0.89 |
Significant differences between the two recovery groups are marked in bold.
Paths from chemosensory loss to recovery
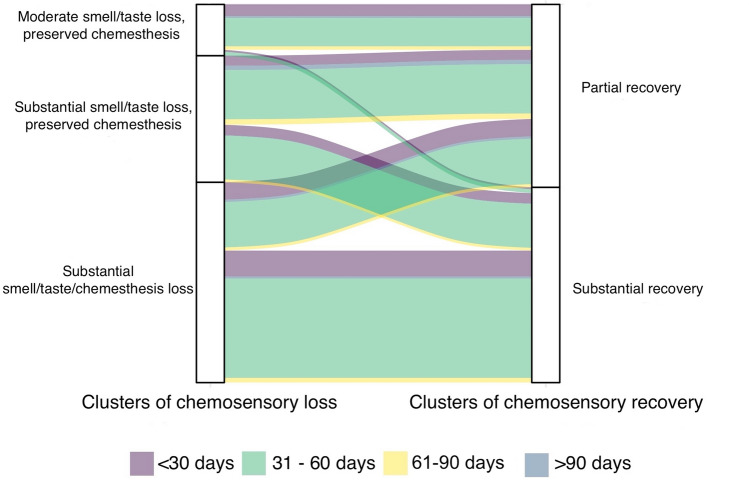
To understand whether partial or substantial recovery from chemosensory loss is dependent on the specific chemosensory loss experienced during COVID-19, we investigated the relationships between clusters of chemosensory loss and recovery (Fig. 3; see also Table 1s in the supplemental material for frequencies of single categories). The best clustering profile for chemosensory loss in this dataset resulted to be 3 (bootstrapped stability = 0.93): Cluster 1) moderate smell/taste loss and preserved chemesthesis (N = 132; centroids: smell = − 20.21, taste = − 19.80, chemesthesis = − 10.71) ; Cluster 2) substantial smell, taste, and chemesthesis loss (N = 516; centroids: smell = − 89.4, taste = − 90.16, chemesthesis = -76.61); Cluster 3) substantial smell and taste loss, but preserved chemesthesis (N = 326; centroids: smell = − 87.03, taste = − 65.41, chemesthesis = − 14.27).
Figure 3.
Alluvial plot showing the pattern of chemosensory loss and recovery clusters in relation to days between the date of onset and completion of the questionnaire. On the left 3 clusters of chemosensory loss while on the right 2 clusters of chemosensory recovery. The flows between the blocks depict the path from loss to recovery. The different colors of flows represent the different time intervals (in days) between the date of onset and completion of the questionnaire. The width of the flow represents the fraction of participants experiencing the recovery either partial or substantial.
The majority of individuals with moderate smell/taste loss and preserved chemesthesis (χ2 (2) = 26.92, p < 0.001; post-hoc p < 0.001) reported a partial recovery (88.6%, N = 117), and only the 11.4% (N = 15) reported substantial recovery. On the contrary, most of the individuals with substantial loss of smell, taste, and chemesthesis showed the highest rate of recovery (65.9%, N = 340; partial recovery: 34.1%, N = 176; post-hoc p < 0.001). Among the individuals who reported substantial smell and taste loss, but preserved chemesthesis there was no significant difference in the reported recovery (substantial recovery: 45.4%, N = 148; partial recovery: 54.6%, N = 178; post-hoc p = 0.38; see Fig. 3). Noteworthy, the clusters “moderate smell/taste loss and preserved chemesthesis” and “substantial smell and taste loss, but preserved chemesthesis” together account for the 62% of the partial recovery cluster, while only for the 32% of the substantial one (χ2 (2) = 46, p < 0.001).
Most participants (71.45%, N = 696) reported the onset of the symptoms within a 31–60 days time frame before the completion of the questionnaire. There was no significant difference in the distribution of the chemosensory recovery groups or the chemosensory loss groups on the different time frames of the onset of symptoms (recovery groups: χ2 (3) = 3.35, p = 0.34; loss groups: χ2 (6) = 4.87, p = 0.56; see Fig. 3).
Association of chemosensory recovery with demographic and clinical predictors
To account for individual differences in baseline chemosensory abilities, and in the use of rating scales, we use as indicators of the status of the chemosensory functions, the “recovery” as the difference between ratings of patients’ chemosensory abilities after- and during- the respiratory illness (Table 2; see the “Method” section).
Table 2.
Summary of the linear regressions on smell, taste, and chemesthesis recovery.
| F value | Pr( >|t|) | |
|---|---|---|
| Smell recovery | ||
| (Intercept) | 2.308 | < 0.001 |
| Region | 8.155 | 0.004 |
| Type of diagnosis | 2.056 | 0.128 |
| Number of symptoms | 4.274 | 0.039 |
| Time from onset | 0.159 | 0.689 |
| Smoking | 2.104 | 0.147 |
| Age | 10.505 | 0.001 |
| Region: time from onset | 5.647 | 0.017 |
| Type of diagnosis: number of symptoms | 2.242 | 0.106 |
| Residual standard error: 32.53 on 962 degrees of freedom | ||
| Multiple R-squared: 0.1787, adjusted R-squared: 0.1693 | ||
| F(11, 962) = 19.03, p-value: < 0.001 | ||
| Taste recovery | ||
| (Intercept) | 17.29 | < 0.001 |
| Region | 20.562 | < 0.001 |
| Number of symptoms | 3.539 | 0.060 |
| Time from onset | 13.95 | < 0.001 |
| Smoking | 5.514 | 0.019 |
| Before rating | 10.607 | < 0.001 |
| Age | 24.247 | < 0.001 |
| Region: time from onset | 11.783 | < 0.001 |
| Residual standard error: 34.47 on 966 degrees of freedom | ||
| Multiple R-squared: 0.06, adjusted R-squared: 0.06 | ||
| F(7, 966) = 10.11, p-value: < 0.001 | ||
| Chemesthesis recovery | ||
| (Intercept) | 1.490 | 0.222 |
| Region | 3.885 | 0.050 |
| Type of diagnosis | 1.790 | 0.167 |
| Number of symptoms | 0.007 | 0.93 |
| Time from onset | 7.72 | 0.005 |
| Smoking | 4.82 | 0.028 |
| Before rating | 25.095 | < 0.001 |
| Age | 4.921 | 0.026 |
| Type of diagnosis: number of symptoms | 2.334 | 0.097 |
| Residual standard error: 34.07 on 963 degrees of freedom | ||
| Multiple R-squared: 0.055, adjusted R-squared: 0.045 | ||
| F(10, 963) = 5.61, p-value: < 0.001 | ||
Significant differences are marked in bold.
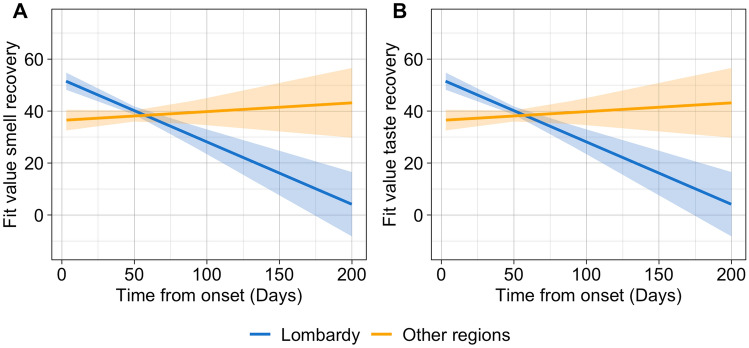
The model on smell recovery (Table 2) showed a significant main effect of regions (Lombardy, Other Regions), indicating that participants living in Lombardy reported higher levels of smell recovery (mean = 42.90, sd = 35.90) compared to participants living in other regions (mean = 37.58, sd = 35.04); a significant main effect of age, with younger participants reporting higher smell recovery; a significant main effect of the number of symptoms (as the total sum of the reported symptoms experienced with the respiratory illness), indicating higher smell recovery when a higher number of symptoms are reported; and a significant main effect of time from onset (number of days from the reported date of the symptoms onset of respiratory illness, and the date of survey completion) indicating lower smell recovery when the time from the onset of the disease is longer (see Fig. 1s of the Supplemental material for the visualization of main effects). Lastly, there was a significant interaction between regions (Lombardy, Other Regions) and time from onset (Fig. 4A). Post-hoc tests showed that in participants residing in Lombardy, when the time from the onset of the disease is longer it is associated to lower smell recovery [t(1) = − 3.10, p < 0.001]; such effect is not present in participants from other Italian regions [t(1) = 0.40, p = 0.69].
Figure 4.
Representation of significant interaction effects of the regression models. Fitted lines of the time from onset and region interaction effects on (A) smell recovery and (B) taste recovery.
The model on taste recovery (Table 2) showed a significant main effect of regions (Lombardy, Other Regions), indicating that participants living in Lombardy reported higher levels of smell recovery (mean = 43.02, sd = 34.97) compared to participants living in other regions (mean = 34.91, sd = 36.24); ; a significant main effect of time from onset indicating lower taste recovery when the time from the onset of the disease is longer; a main effect of age, indicating that older participants reported less taste recovery and a significant main effect of before rating, consistent with higher recovery when participants reported higher taste perception before the disease. Effect of Smoking (yes, no) was significant as well, suggesting that smokers reported higher taste recovery (mean = 43.04, sd = 34.98) compared to non-smokers (mean = 38.24, sd = 35.93; Fig. 2s in the Supplemental material for visualization of main effects). Moreover, a significant interaction between regions and time from onset (Fig. 4B) was observed. Post-hoc analyses showed that a longer time from onset of respiratory symptoms was associated with lower taste recovery only in participants from the Lombardy region [t(1) = − 3.74, p < 0.001]; such effect is not present in participants from other Italian regions [t(1) = 1.25, p = 0.21].
Results on chemesthesis recovery (Table 2) indicate a significant main effect of age, with older participants reporting a smaller index of recovery than younger participants; a significant main effect of before rating, with higher recovery in cases it was reported better chemesthesis perception before the disease; a significant main effect of time from onset (number of days from the reported date of the symptoms onset of respiratory illness, and the date of survey completion) indicating lower chemesthesis recovery when the time from the onset of the disease is longer; and a significant main effect of smoking, with smokers reporting higher level of chemesthesis recovery (Fig. 3s in the Supplemental material for visualization of main effects).
Discussion
Since the beginning of the COVID-19 pandemic, an increased number of patients with taste and smell loss has been reported, and increased evidence emerged in the literature reporting chemosensory deficits as a salient feature of the disease1. The present study aimed to characterize on a larger scale the persistence and recovery process of chemosensory deficits associated with SARS-CoV-2 infection, attempting to delineate the expectations of recovery for the patients as well as predict/identify groups of patients in need of additional post-viral care.
Current standardized evaluations of smell and taste for clinical purposes, although best practices, require lengthy and maskless, thus unsafe, testing sessions50,51. Additionally, they are not commonly known among first-line healthcare professionals. Therefore, in order to recognize early in the disease and characterize over time these extremely common long-term consequences of COVID-19, it is of paramount importance to add to the first patients’ assessment a set of well-framed informed questions on smell and taste loss and recovery. A direct comparison of the objective and subjective chemosensory assessment showed that subjective methods (self-reports) might underestimate chemosensory loss in COVID-19 patients1, nevertheless, self-reports can provide a first-aid tool to estimate chemosensory loss among the population. The identification and diagnosis of chemosensory impairments are the first, yet important, step to make patients fully aware of the problem and its less obvious consequences, but also to design strategies to improve their quality of life39.
A first indication emerging from our analysis is that asking the patient to rate their smell, taste, and ability to perceive chemical irritation (chemesthesis) on a 0–100 visual analog scale (VAS) before, during, and after the resolution of the respiratory symptoms is a first and important step to understand the recovery. Importantly, chemesthesis, primarily mediated by the trigeminal nerves, is not only responsible for the detection of chemical irritants but it is also involved in inflammatory responses. Most recent reports suggest that this inflammatory response is a possible contributor to the exacerbation of the tissue damage induced by viral SARS-CoV-2 infection52,53. In patients experiencing chemesthesis loss, inflammatory responses might be reduced or impaired, decreasing the probability of further damage to the olfactory epithelium. Interestingly, 62.2% of the subjects experiencing partial chemosensory recovery showed no chemesthesis loss (37.8% moderate smell/taste loss, and 24.8% substantial smell/taste loss), supporting the hypothesis of a contribution of the inflammatory response to long term chemosensory loss. It emerges that the evaluation of chemesthesis function, which has been mostly neglected outside of the GCCR efforts18,49, might provide healthcare professionals not only with an indication of the outcome of the chemosensory recovery process, but also inform the design of better strategies for early treatment of post-viral symptoms.
As observed previously21,24–26,28,54,55, a demographic factor that should be considered is age. We performed a first exploratory cluster analysis (Fig. 2A) that suggested two different patterns of chemosensory recovery: one is characterized by moderate smell, taste, and chemesthesis recovery; and a second one by a substantial smell, taste, and chemesthesis recovery. These two clusters significantly differ for age of the subjects, with the first group on average older than the second. Our analysis then confirms the role of age in the recovery from the chemosensory deficits, showing that younger participants are associated with a better chemosensory recovery prognosis than older ones for all three chemosensory modalities. Although age-related differences in chemosensory abilities are well known, in the case of COVID-19 this relationship is less clear and still controversial. Results from several studies24,28,55 did not find any age-related difference, while Moein et al.8, with an analytic approach similar to ours, found that older age had a negative impact on smell recovery, which is in agreement with our results on this dataset.
Another interesting aspect of our analysis is that smokers report greater recovery rates for taste and chemesthesis than non-smokers. This observation is controversial56,57, as recent data suggest, smokers risk a more severe course of the disease56,58,59. However, we cannot exclude that this effect could be the result of the temporary abstinence from smoking during the disease or the overall limited severity of the disease of participants responding to a survey online. It has been reported that the effects of smoking on chemosensory function are short-term60. While being a smoker could thus be a confounding question to ask, its statistical link with taste and chemesthesis recovery could improve the prognosis.
Finally, our analysis of demographic and clinical predictors for recovery of each sensory modality reveals that being resident in Lombardy was predictive of greater smell recovery. Indeed, Lombardy was the epicenter of the first wave of the COVID-19 pandemic in Italy, with an overall earlier date of onset and the registering of the highest number of cases (and survey participants) in comparison to the rest of Italy. Differences that emerged between Lombardy and other regions could be due to differences in the regional management of the pandemic, but also to the delayed spreading of the disease in the other regions which registered a relatively low number and later onset cases in comparison to Lombardy in the time frame we analyzed49,58. Alternatively, the differences in chemosensory loss among Lombardy and the other regions could be due to the diffusion of the SARS-CoV-2 D614G haplotype, a variant that by March 1st was predominant in Lombardy and may be responsible for the higher rate of chemosensory impairments compared to other regions61,62.
In Lombardy, the time of onset of the disease is predictive of a worst prognosis of chemosensory recovery, confirming the presence of a group of patients whose recovery from any of the symptoms does not occur within 4–6 weeks from their onsets63. This result could be related to the high incidence that Lombardy experienced during the first wave42,64, however, this group deserves further investigation, given that the sequela from non-COVID-19 post-viral chemosensory loss can last on average 1 year65. While regional differences that emerged from our analysis could not be used as a first-aid tool to understand the recovery directly by local healthcare professionals, they could help in understanding the epidemiological scenario of the pandemic.
Strengths and limitations
To account for individual differences in baseline chemosensory abilities, and in the use of rating scales, we suggest using, as indicators of the status of the chemosensory functions, the "recovery" as the difference between ratings of patients' chemosensory abilities after- and during- the respiratory illness and the "loss'' as the difference between ratings of their chemosensory abilities during- and before-the respiratory illness. One caveat could be that of the recovery index suffering from ceiling/floor effect whereby smaller loss might have smaller index of recovery. This problem could be solved by comparing the index with a single reference (i.e., comparing to the rating before the disease).
Although it has been shown that subjective ratings are a good proxy for the understanding of chemosensory loss during the COVID-19 pandemic43,49 (and summarized by66), in relation to complete and sudden smell loss, these measurements are known to have limitations since they might suffer from under- and over-reporting biases8,26,67–69 and possible arbitrary scale usage. Participants who experienced a more severe chemosensory loss might tend to overestimate their recovery67.
Nevertheless, the results of our study are comparable to those obtained with objective testing methods, which also observed a similar dependency between loss and recovery, strengthening the evidence that a greater olfactory improvement post-infection is more likely in patients experiencing sudden anosmia or ageusia during the viral infection than in those experiencing hyposmia and hypogeusia23,25.
Despite the afore-mentioned limitations18,49, the analysis of self-reports of patients’ chemosensory abilities is to date the most effective strategy to target the largest number of patients that could not be otherwise reached due to the safety policies implemented during the COVID-19 pandemic, as well as the lack of widespread routine chemosensory testing and the lack of healthcare providers able to rigorously (and reliably) perform them70.
Conclusions
With the SARS-CoV-2 pandemic, the number of patients affected by chemosensory loss substantially increased. Our work provides indications on the recovery process on which we shaped a scientific-based approach for healthcare professionals to characterize the clinical picture of patients reporting chemosensory loss due to COVID-19 infection. We further provide indexes such as loss and recovery that would be extremely useful for single ENT doctors to have a starting point for further diagnosis and prognosis. Three different profiles of chemosensory loss were identified: substantial loss of all the three chemosensory modalities, substantial loss of only smell and taste, and moderate loss of only smell and taste. Clinicians must take into account demographic factors that influence chemosensory recovery, among them the age as we showed that older adults had a longer recovery period. Uncovering the self-reported phenomenology of recovery from smell, taste, and chemesthetic disorders is the first, yet essential step, to provide healthcare professionals with the tools to take purposeful and targeted action to address chemosensory disorders and its severe discomfort.
Method
The GCCR online survey
The data utilized in this study is part of the GCCR survey49, which was developed as a global, crowdsourced online study, and deployed in 35 languages. The survey aimed to measure self-reported smell, taste, and chemesthesis function, and nasal blockage, amongst other variables, in participants with recent (within the past 2 weeks) or current respiratory illness, including COVID-19. Participants were asked to rate their ability to smell, taste, and perceive cooling, tingling, and burning sensations (chemesthesis) before, during, and, in case of recovery, after their respiratory illness, using 100-point visual analog scales (VAS). The online survey was approved as an exempt study by the Institutional Review Board (IRB) of The Pennsylvania State University (STUDY00014904) in accordance with the revised Declaration of Helsinki. Informed consent was obtained from all participants.
Participants
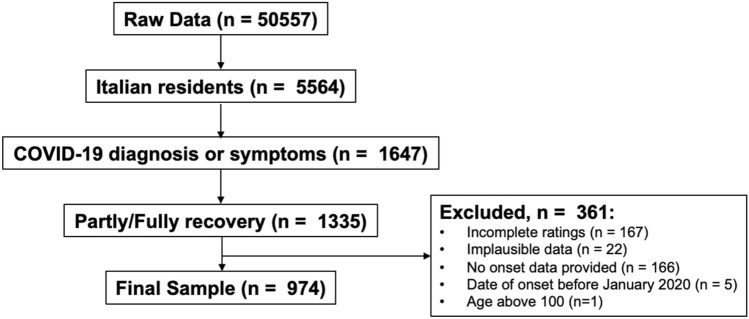
The entry criterion for participation in the GCCR survey was a recent or current respiratory illness (symptoms present in the past 2 weeks). Accordingly, only participants who answered "Yes" to Question 6, "Within the past 2 weeks, have you been diagnosed with or suspect that you have a respiratory illness?" were allowed to complete the survey (see Appendix 1 of Parma et al.49 for all survey questions). In the present study, were included only participants who reported to be resident in Italy (n = 5564) and a COVID-19 diagnosis or symptoms [Question 8 "Have you been diagnosed with COVID-19?", answers "No-I was not diagnosed, but I have symptoms" (self-diagnosed group), "Yes-diagnosed based on symptoms only" (Clinical assessment group), "Yes-diagnosed with viral swab", "Yes-diagnosed with another lab test", (Lab tested group)] (n = 1647). In order to investigate chemosensation after the recovery from COVID-19, we included only participants who answered “Yes—partly” or “Yes—fully” to Question 28 “Have you recovered from your recent respiratory Illness or diagnosis? (For example, you no longer have a cough, fever, or shortness of breath.)” (n = 1335). Other exclusion criteria were: incomplete ratings (n = 167), no date of onset of respiratory illness symptoms provided (n = 166; Question 7: “What date did you first notice symptoms of your recent respiratory illness?”), inconsistent responses in questions on smell changes (n = 22; specifically, selecting changes in smell in Question 10 “Have you had any of the following symptoms with your recent respiratory illness or diagnosis?”, reporting a difference in Question 13 “Rate your ability to smell before your recent respiratory illness or diagnosis” and/or select at least one answer from Question 15 “Have you experienced any of the following changes in smell with your recent respiratory illness diagnosis?”), age above 100 (n = 1), reported date of onset of respiratory symptoms after the date of participation or before January 2020 (n = 5). The final sample included 974 participants (see Fig. 5).
Figure 5.
Flow diagram presenting the selection of the observations included in the present study.
Indices
To standardize statistical analyses, some measures were combined into indices. We defined the time from onset as the number of days from the reported date of symptoms onset of respiratory illness and the date of survey completion. We defined the number of symptoms as the total sum of the reported symptoms experienced with the respiratory illness (“Have you had any of the following symptoms with your recent respiratory illness or diagnosis?” Question 10) and the prior conditions as the total sum of the reported medical conditions experienced in the 6 months prior to the onset of the respiratory illness (“Did you have any of the following in the 6 months prior to your recent respiratory illness or diagnosis?” Question 38). Moreover, indices of loss for smell, taste, and chemesthesis was computed by subtracting ratings “before illness” (Question 14 “Rate your ability to smell BEFORE your recent respiratory illness or diagnosis”) from ratings “during illness” (Question 13 “Rate your ability to smell DURING your recent respiratory illness or diagnosis”). Finally, indices of recovery of each sense (Smell, taste, and chemesthesis) were computed by subtracting ratings “during illness” from ratings “after illness” (Question 29 “Rate your ability to smell AFTER your recovery”).
Statistical analyses
Data was pre-processed and analyzed using the software R71. Statistical analyses were pre-registered at the Open Science Framework (OSF, https://osf.io/vun72/) before the data became available. First, repeated-measures ANOVA tests (aov function from the R default stats package) were performed on smell, taste, and chemesthesis rating with time of rating (before, during, and after the disease) as an independent variable. Post-hoc comparisons were performed with Tukey's test (TukeyHSD function from the R default stats package). Then, to investigate whether chemosensory profiles of recovery exist and if they followed the profiles found for chemosensory loss49, we extended the cluster analysis of Parma et al.49 on the Italian dataset, that only partially overlapped with the data previously analyzed (594 Italian residents49). Cluster analyses were performed based on the similarities and differences in indexes of smell, taste, and chemesthesis loss, and recovery using the k-means function from the R default stats package. The optimal number of clusters was determined with NbCluster72, which tests 30 methods that vary the combinations of cluster numbers and distance measures for the k-means clustering. Cluster stability was estimated through a bootstrapping approach (100 iterations) with the bootcluster package73. Descriptive analyses on the resulting clusters were run using t-tests (stats package74) and chi-square tests (chisq.test function of the stats package74). Pairwise comparisons after chi-square tests were performed using the chisq.multcomp function of the RVAideMemoire package. Next, smell, taste, and chemesthesis recovery were investigated through three separate multiple linear regression models (lm function of stats package) with the same predictors. Predictors included continuous and categorical variables. The former included: age, number of symptoms, time from onset, prior conditions, and the rating of the dependent variable (e.g., smell for smell recovery) related to before the disease (“Before rating”); the latter included: region of residence (Lombardy, Other regions), type of diagnosis (Self-diagnosed, Clinical assessment, or lab tested), smoking (yes, no; also including e-cigarette). In order to explore the recovery profile and region specificity, in the models, we included interaction between these variables: region of residence, type of diagnosis, number of symptoms, and time from onset. To avoid overly complicated and uninterpretable models, only second-level interactions were included. To ensure that each predictor improved the models’ fit, the function step (stats package) was used to perform automatic backward elimination, which relies on the AIC criterion75. Factors that did not significantly improve the models’ fit were removed. AIC values of the initial and final models were calculated using the ANOVA function (stats package74). Collinearity was calculated with the Variance Inflation Factors (VIF) using the vif function of the car package76. Interactions including continuous factors were analyzed according to Aiken & West’s method77. RStudio software and package ggplot78 were used to build all the graphs in the manuscript and all the scripts along with information on the computational environment and dependencies will be found, upon acceptance of the manuscript, at OSF (https://osf.io/vun72/).
In the light of recent studies from the GCCR dataset18,49,79, additions to the pre-registered linear models were necessary: (1) smell, taste and chemesthesis ratings were not analyzed as repeated measures (before, during, after) but rather index of recovery was computed and used as dependent variable and the before rating was included in the model as independent variable to better characterize the degree of changes; (2) since in cluster analyses age was significantly different between the two clusters, it was included as fixed and not anymore as a random factor; (3) gender and type of recovery were removed because they did not improve the models’ fit. Due to the particular spread of the pandemic in Italy, the region of residence was also included as a predictor.
Supplementary Information
Author contributions
Conceived, designed the study, and wrote the paper: C.C., A.D.P., F.G., V.P., M.D. Data acquisition and curation: C.C., A.D.P., F.G., O.C., A.M., K.O., S.S., M.C.F., P.V.J., A.M., E.C., C.D., M.P.C., A.D.E., C.M., V.P., M.D. Performed analysis: C.C., A.D., M.D. Edited and approved the final manuscript: C.C., A.D.P., F.G., O.C., A.M., K.O., A.D., S.S., M.C.F., P.V.J., A.M., E.C., C.D., M.P.C., A.D.E., C.M., V.P., MD.
Funding
CC is supported by a grant from MIUR (Dipartimenti di Eccellenza DM 11/05/2017 n. 262) to the Department of General Psychology. FG is supported by NIH/NIDCD grant R21DC018358. PVJ is supported by the National Institute of Alcohol Abuse and Alcoholism and the National Institute of Nursing Research and by the Office of Workforce Diversity, National Institutes of Health, and the Rockefeller University Heilbrunn Nurse Scholar Award. MD is supported by Rita Levi Montalcini Award from Italian Ministry of Education, University, and Research (DM100915_685).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-96987-0.
References
- 1.Hannum ME, et al. Objective sensory testing methods reveal a higher prevalence of olfactory loss in COVID-19–positive patients compared to subjective methods: A systematic review and meta-analysis. Chem. Senses. 2020;45:865–874. doi: 10.1093/chemse/bjaa064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hornuss D, Lange B, Schröter N, Rieg S, Kern WV, Wagner D. Anosmia in COVID-19 patients. Clin. Microbiol. Infect. 2020;26(10):1426–1427. doi: 10.1016/j.cmi.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lechien JR, et al. Objective olfactory evaluation of self-reported loss of smell in a case series of 86 COVID-19 patients. Head Neck. 2020;42:1583–1590. doi: 10.1002/hed.26279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lechien JR, et al. Psychophysical olfactory tests and detection of COVID-19 in patients with sudden onset olfactory dysfunction: A prospective study. Ear Nose Throat J. 2020;99:579–583. doi: 10.1177/0145561320929169. [DOI] [PubMed] [Google Scholar]
- 5.Moein ST, et al. Smell dysfunction: A biomarker for COVID-19. Int. Forum Allergy Rhinol. 2020;10:944–950. doi: 10.1002/alr.22587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vaira LA, et al. Objective evaluation of anosmia and ageusia in COVID-19 patients: Single-center experience on 72 cases. Head Neck. 2020;42:1252–1258. doi: 10.1002/hed.26204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaira LA, et al. Validation of a self-administered olfactory and gustatory test for the remotely evaluation of COVID-19 patients in home quarantine. Head Neck. 2020;42:1570–1576. doi: 10.1002/hed.26228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moein ST, Hashemian SM, Tabarsi P, Doty RL. Prevalence and reversibility of smell dysfunction measured psychophysically in a cohort of COVID-19 patients. Int. Forum Allergy Rhinol. 2004;10(10):1127–1135. doi: 10.1002/alr.22680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Landis BN, Konnerth CG, Hummel T. A study on the frequency of olfactory dysfunction. Laryngoscope. 2004;114:1764–1769. doi: 10.1097/00005537-200410000-00017. [DOI] [PubMed] [Google Scholar]
- 10.Mullol J, et al. Furthering the understanding of olfaction, prevalence of loss of smell and risk factors: A population-based survey (OLFACAT study) BMJ Open. 2012;2:e001256. doi: 10.1136/bmjopen-2012-001256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murphy C, et al. Prevalence of olfactory impairment in older adults. JAMA. 2002;288:2307–2312. doi: 10.1001/jama.288.18.2307. [DOI] [PubMed] [Google Scholar]
- 12.Desiato VM, et al. The prevalence of olfactory dysfunction in the general population: A systematic review and meta-analysis. Am. J. Rhinol. Allergy. 2021;35(2):195–205. doi: 10.1177/1945892420946254. [DOI] [PubMed] [Google Scholar]
- 13.Hoffman HJ, Cruickshanks KJ, Davis B. Perspectives on population-based epidemiological studies of olfactory and taste impairment. Ann. N. Y. Acad. Sci. 2009;1170:514. doi: 10.1111/j.1749-6632.2009.04597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu G, Zong G, Doty RL, Sun Q. Prevalence and risk factors of taste and smell impairment in a nationwide representative sample of the US population: A cross-sectional study. BMJ Open. 2008;6(11):e013246. doi: 10.1136/bmjopen-2016-013246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nordin S, Brämerson A, Brömerson A. Complaints of olfactory disorders: Epidemiology, assessment and clinical implications. Curr. Opin. Allergy Clin. Immunol. 2008;8:10–15. doi: 10.1097/ACI.0b013e3282f3f473. [DOI] [PubMed] [Google Scholar]
- 16.Rawal S, Hoffman HJ, Bainbridge KE, Huedo-medina TB, Duffy VB. Prevalence and risk factors of self-reported smell and taste alterations: Results from the 2011–2012 US National Health and Nutrition Examination Survey (NHANES) Chem Senses. 2016;41:69–76. doi: 10.1093/chemse/bjv057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saniasiaya J, Islam MA, Abdullah B. Prevalence of olfactory dysfunction in coronavirus disease 2019 (COVID-19): A meta-analysis of 27,492 patients. The Laryngoscope. 2021;131(4):865–878. doi: 10.1002/lary.29286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerkin, R. C. et al. GCCR Group Author. Recent smell loss is the best predictor of COVID-19 among individuals with recent respiratory symptoms. Chem. Senses46, bjaa081. 10.1093/chemse/bjaa081 (2021). [DOI] [PMC free article] [PubMed]
- 19.Menni C, et al. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat. Med. 2020;26:1037–1040. doi: 10.1038/s41591-020-0916-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mizrahi B, et al. Longitudinal symptom dynamics of {COVID}-19 infection. Nat. Commun. 2020;11:1–10. doi: 10.1038/s41467-020-20053-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lechien JR, et al. Prevalence and 6-month recovery of olfactory dysfunction: A multicentre study of 1363 COVID-19 patients. J. Intern. Med. 2021;290(2):451–461. doi: 10.1111/joim.13209. [DOI] [PubMed] [Google Scholar]
- 22.Chiesa-Estomba CM, et al. Patterns of smell recovery in 751 patients affected by the COVID-19 outbreak. Eur. J. Neurol. 2020;27:2318–2321. doi: 10.1111/ene.14440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iannuzzi L, et al. Gaining back what is lost: Recovering the sense of smell in mild to moderate patients after COVID-19. Chem. Senses. 2020;45:875–881. doi: 10.1093/chemse/bjaa066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Bon SD, et al. Psychophysical evaluation of chemosensory functions 5 weeks after olfactory loss due to COVID-19: A prospective cohort study on 72 patients. Eur. Arch. Otorhinolaryngol. 2021;278(1):101–108. doi: 10.1007/s00405-020-06267-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niklassen AS, et al. COVID-19: Recovery from chemosensory dysfunction. A multicentre study on smell and taste. Laryngoscope. 2021 doi: 10.1002/lary.29383. [DOI] [PubMed] [Google Scholar]
- 26.Otte MS, Eckel HNC, Poluschkin L, Klussmann JP, Luers JC. Olfactory dysfunction in patients after recovering from COVID-19. Acta Otolaryngol. 2020;140:1032–1035. doi: 10.1080/00016489.2020.1811999. [DOI] [PubMed] [Google Scholar]
- 27.Reiter ER, Coelho DH, Kons ZA, Costanzo RM. Subjective smell and taste changes during the COVID-19 pandemic: Short term recovery. Am. J. Otolaryngol. 2020;41:102639. doi: 10.1016/j.amjoto.2020.102639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vaira LA, et al. Smell and taste recovery in coronavirus disease 2019 patients: A 60-day objective and prospective study. J. Laryngol. Otol. 2020;134:703–709. doi: 10.1017/S0022215120001826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakalli E, et al. Ear nose throat-related symptoms with a focus on loss of smell and/or taste in COVID-19 patients. Am. J. Otolaryngol. 2020;41:102622. doi: 10.1016/j.amjoto.2020.102622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Croy I, Nordin S, Hummel T. Olfactory disorders and quality of life—An updated review. Chem. Senses. 2014;39:185–194. doi: 10.1093/chemse/bjt072. [DOI] [PubMed] [Google Scholar]
- 31.Erskine SE, Philpott CM. An unmet need: Patients with smell and taste disorders. Clin. Otolaryngol. 2020;45:197–203. doi: 10.1111/coa.13484. [DOI] [PubMed] [Google Scholar]
- 32.Neuland C, Bitter T, Marschner H, Gudziol H, Guntinas-Lichius O. Health-related and specific olfaction-related quality of life in patients with chronic functional anosmia or severe hyposmia. Laryngoscope. 2011;121:867–872. doi: 10.1002/lary.21387. [DOI] [PubMed] [Google Scholar]
- 33.Nordin S, et al. Effects of smell loss on daily life and adopted coping strategies in patients with nasal polyposis with asthma. Acta Otolaryngol. 2011;131:826–832. doi: 10.3109/00016489.2010.539625. [DOI] [PubMed] [Google Scholar]
- 34.Schäfer L, et al. Sexual desire after olfactory loss: Quantitative and qualitative reports of patients with smell disorders. Physiol. Behav. 2019;201:64–69. doi: 10.1016/j.physbeh.2018.12.020. [DOI] [PubMed] [Google Scholar]
- 35.de Jong N, Mulder I, de Graaf C, van Staveren WA. Impaired sensory functioning in elders: The relation with its potential determinants and nutritional intake. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 1999;54:B324–B331. doi: 10.1093/gerona/54.8.B324. [DOI] [PubMed] [Google Scholar]
- 36.Duffy VB, Backstrand JR, Ferris AM. Olfactory dysfunction and related nutritional risk in free-living, elderly women. J. Am. Diet. Assoc. 1995;95:876–879. doi: 10.1016/S0002-8223(95)00244-8. [DOI] [PubMed] [Google Scholar]
- 37.Henkin RI. Effects of smell loss (hyposmia) on salt usage. Nutrition. 2014;30:690–695. doi: 10.1016/j.nut.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 38.Toussaint N, de Roon M, van Campen JPCM, Kremer S, Boesveldt S. Loss of olfactory function and nutritional status in vital older adults and geriatric patients. Chem. Senses. 2015;40:197–203. doi: 10.1093/chemse/bju113. [DOI] [PubMed] [Google Scholar]
- 39.Boesveldt S, et al. Anosmia—A clinical review. Chem. Senses. 2017;42:513–523. doi: 10.1093/chemse/bjx025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nadeau SA, Vaughan TG, Scire J, Huisman JS, Stadler T. The origin and early spread of SARS-CoV-2 in Europe. Proc. Natl. Acad. Sci. USA. 2021;118(9):e2012008118. doi: 10.1073/pnas.2012008118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Remuzzi A, Remuzzi G. COVID-19 and Italy: What next? Lancet. 2020;395(10231):1225–1228. doi: 10.1016/S0140-6736(20)30627-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Odone A, Delmonte D, Scognamiglio T, Signorelli C. COVID-19 deaths in Lombardy, Italy: Data in context. Lancet Public Health. 2020;5:e310. doi: 10.1016/S2468-2667(20)30099-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giacomelli A, et al. Self-reported olfactory and taste disorders in SARS-CoV-2 patients: A cross-sectional study. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spinato G, et al. Alterations in smell or taste in mildly symptomatic outpatients with SARS-CoV-2 infection. JAMA. 2020;323:2089–2090. doi: 10.1001/jama.2020.6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walker A, Hopkins C, Surda P. Use of Google Trends to investigate loss-of-smell-related searches during the COVID-19 outbreak. Int. Forum Allergy Rhinol. 2020;10(7):839–847. doi: 10.1002/alr.22580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Capelli M, Gatti P. Anosmia and COVID-19 in south Lombardy: Description of the first cases series in Europe. B-ENT. 2020;16:86–90. doi: 10.5152/B-ENT.2020.20129. [DOI] [Google Scholar]
- 47.Elli F, et al. Changes in the use of Otorhinolaryngology Emergency Department during the COVID-19 pandemic: Report from Lombardy, Italy. Eur. Arch. Oto-Rhino-Laryngol. 2020;277:3525–3528. doi: 10.1007/s00405-020-06119-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ralli M, et al. Impact of COVID-19 on otolaryngology in Italy: A commentary from the COVID-19 task force of the Young Otolaryngologists of the Italian Society of Otolaryngology. Eur. Rev. Med. Pharmacol. Sci. 2020;24:7516–7518. doi: 10.26355/eurrev_202007_21925. [DOI] [PubMed] [Google Scholar]
- 49.Parma V, et al. More than smell-COVID-19 is associated with severe impairment of smell, taste, and chemesthesis. Chem. senses. 2020;45(7):609–622. doi: 10.1093/chemse/bjaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen R, et al. The spatial and cell-type distribution of SARS-CoV-2 receptor ACE2 in human and mouse brain. Front. Neurol. 2021;11:1860. doi: 10.3389/fneur.2020.573095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Whitcroft KL, Hummel T. Olfactory dysfunction in COVID-19: Diagnosis and management. JAMA. 2020;323:2512–2514. doi: 10.1001/jama.2020.8391. [DOI] [PubMed] [Google Scholar]
- 52.Cazzolla AP, et al. Taste and smell disorders in COVID-19 patients: Role of Interleukin-6. ACS Chem. Neurosci. 2020;11:2774–2781. doi: 10.1021/acschemneuro.0c00447. [DOI] [PubMed] [Google Scholar]
- 53.Wang H, Zhou M, Brand J, Huang L. Inflammation and taste disorders: Mechanisms in taste buds. Ann. N. Y. Acad. Sci. 2020;1170:596–603. doi: 10.1111/j.1749-6632.2009.04480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Konstantinidis I, et al. Short-term follow-up of self-isolated COVID-19 patients with smell and taste dysfunction in Greece: Two phenotypes of recovery. ORL. 2020;82:295–303. doi: 10.1159/000511436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nguyen, N. N., Hoang, V. T., Lagier, J. C., Raoult, D., & Gautret, P. Long-term persistence of olfactory and gustatory disorders in COVID-19 patients. Clin. Microbiol. Infect.27(6), 931–932. 10.1016/j.cmi.2020.12.021 (2021). [DOI] [PMC free article] [PubMed]
- 56.Leung, J. M., & Sin, D. D. Smoking, ACE-2 and COVID-19: Ongoing controversies. Eur. Respir. J.56(1), 2001759. 10.1183/13993003.01759-2020 (2020). [DOI] [PMC free article] [PubMed]
- 57.Vardavas, C. I., & Nikitara, K. COVID-19 and smoking: A systematic review of the evidence. Tob. Induc. Dis.18, 20. 10.18332/tid/119324 (2020). [DOI] [PMC free article] [PubMed]
- 58.Alqahtani JS, et al. Prevalence, severity and mortality associated with COPD and smoking in patients with COVID-19: A rapid systematic review and meta-analysis. PLoS ONE. 2020;15:e0233147. doi: 10.1371/journal.pone.0233147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Russo, P. et al. COVID-19 and smoking: Is nicotine the hidden link? Eur. Respir. J.55(6), 2001116. 10.1183/13993003.01116-2020 (2020). [DOI] [PMC free article] [PubMed]
- 60.Vennemann MM, Hummel T, Berger K. The association between smoking and smell and taste impairment in the general population. J. Neurol. 2008;255:1121–1126. doi: 10.1007/s00415-008-0807-9. [DOI] [PubMed] [Google Scholar]
- 61.Korber B, et al. Tracking changes in SARS-CoV-2 spike: Evidence that D614G increases infectivity of the COVID-19 virus. Cell. 2020;182:812–827. doi: 10.1016/j.cell.2020.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.von Bartheld, C. S., Mathew, D., & Butowt, R. New study on prevalence of anosmia in COVID-19 implicates the D614G virus mutation as a major contributing factor to chemosensory dysfunction. Eur. Arch. Otorhinolaryngol.278(9), 3593–3594. 10.1007/s00405-021-06759-9 (2021). [DOI] [PMC free article] [PubMed]
- 63.Klein H, et al. Onset, duration and unresolved symptoms, including smell and taste changes, in mild COVID-19 infection: A cohort study in Israeli patients. Clin. Microbiol. Infect. 2021;27:769–774. doi: 10.1016/j.cmi.2021.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Usuelli M. The Lombardy region of Italy launches the first investigative COVID-19 commission. Lancet. 2020;396:e86–e87. doi: 10.1016/S0140-6736(20)32154-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Seiden AM. Postviral olfactory loss. Otolaryngol. Clin. N. Am. 2004;37:1159–1166. doi: 10.1016/j.otc.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 66.Pellegrino R, et al. Coronaviruses and the chemical senses: Past, present, and future. Chem. Senses. 2020;45:415–422. doi: 10.1093/chemse/bjaa031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boscolo-Rizzo, P. et al. Six-month psychophysical evaluation of olfactory dysfunction in patients with COVID-19. Chem. Senses46, bjab006. 10.1093/chemse/bjab006 (2021). [DOI] [PMC free article] [PubMed]
- 68.Dalton P, Hummel T. Chemosensory function and response in idiopathic environmental intolerance. Occup. Med. 2000;15:539–556. [PubMed] [Google Scholar]
- 69.Oleszkiewicz A, Kunkel F, Larsson M, Hummel T. Consequences of undetected olfactory loss for human chemosensory communication and well-being. Philos. Trans. R. Soc. B. 2020;375:20190265. doi: 10.1098/rstb.2019.0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lötsch J, Hummel T. Clinical usefulness of self-rated olfactory performance—A data science-based assessment of 6000 patients. Chem. Senses. 2019;44:357–364. doi: 10.1093/chemse/bjz029. [DOI] [PubMed] [Google Scholar]
- 71.RStudio Team. RStudio: Integrated Development Environment for R (RStudio, PBC, Boston, MA, 2020). http://www.rstudio.com/.
- 72.Charrad M, Ghazzali N, Boiteau V, Niknafs A. NbClust: An RPackage for determining the relevant number of clusters in a data set. J. Stat. Softw. 2014;61:1–36. doi: 10.18637/jss.v061.i06. [DOI] [Google Scholar]
- 73.Yu, H. Bootcluster: Bootstrapping Estimates of Clustering Stability. https://cran.r-project.org/package=bootcluster (2017).
- 74.R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna, Austria, 2017). https://www.R-project.org.
- 75.Bolker BM, et al. Generalized linear mixed models: A practical guide for ecology and evolution. Trends Ecol. Evol. 2009;24:127–135. doi: 10.1016/j.tree.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 76.Fox, J., Weisberg, S., & Price, B. carData: Companion to Applied Regression Data Sets. R package version 3.0-4. https://CRAN.R-project.org/package=carData (2020).
- 77.Aiken LS, West SG, Reno RR. Multiple Regression: Testing and Interpreting Interactions. Sage; 1991. [Google Scholar]
- 78.Wickham H. ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag New York; 2016. [Google Scholar]
- 79.Pierron D, et al. Smell and taste changes are early indicators of the {COVID}-19 pandemic and political decision effectiveness. Nat. Commun. 2020;11:1–8. doi: 10.1038/s41467-020-18963-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.