Abstract
Cancer comprises a collection of diseases that occur in almost any tissue and it is characterized by an abnormal and uncontrolled cell growth that results in tumor formation and propagation to other tissues, causing tissue and organ malfunction and death. Despite the undeniable improvement in cancer diagnostics and therapy, there is an urgent need for new therapeutic and preventive strategies with improved efficacy and fewer side effects. In this context, purinergic signaling emerges as an interesting candidate as a cancer biomarker or therapeutic target. There is abundant evidence that tumor cells have significant changes in the expression of purinergic receptors, which comprise the G-protein coupled P2Y and AdoR families of receptors and the ligand-gated ion channel P2X receptors. Tumor cells also exhibit changes in the expression of nucleotidases and other enzymes involved in nucleotide metabolism, and the concentrations of extracellular nucleotides are significantly higher than those observed in normal cells. In this review, we will focus on the potential role of purinergic signaling in the ten most lethal cancers (lung, breast, colorectal, liver, stomach, prostate, cervical, esophagus, pancreas, and ovary), which together are responsible for more than 5 million annual deaths.
Keywords: Purinergic receptors, Ectonucleotidases, ATP, Adenosine, UTP, UDP, Lung cancer, Breast cancer, Gastric cancer, Hepatocellular cancer, Colorectum cancer, Prostate cancer, Cervicouterine cancer, Esophagous cancer, Pancreas cancer, Ovarian cancer
Purines—an overview
Purines are a family of structurally related molecules formed by the fusion of two nitrogen-based heterocyclic rings, one containing six (pyrimidine) and the other five atoms (imidazole). During the Hadean aeon of prebiotic evolution, purines spontaneously appeared as molecules derived from HCN and H2CO under reductive atmospheric conditions, intermittent cycles of freezing temperatures, and alkaline mediums [1]. Delocalized electrons within the p orbitals of the conjugate/aromatic system give purine molecules high molecular stability by resonance equilibrium of tautomeric structures (amino–imino and keto–enol) [2]. These structural properties underpin the plethora of informational, metabolic, signaling, vascular, and hypnogenic roles that purines perform as nucleosides and nucleotides, which are N-glycosides of β-d-ribofuranose and β-d-deoxyribofuranose, respectively [3].
Purines are a set of diverse molecules, with guanine (2-amino-6-oxypurine) and adenine (6-aminopurine) as their main representatives. This review will only cover the physiological and pathological aspects of adenine-related purines, like the nucleoside adenosine (ADO) and the nucleotides AMP, ADP, and ATP (adenosine mono-, di-, and triphosphate, respectively).
Purines in the extracellular milieu
Most cells release purines (primarily ATP) by cytoplasmic leakage after cell injury, or by pannexin-related mechanisms; in some instances, cells also release ADO [4]. Therefore, there is another dynamic environment outside the cell, where the interconversion and proportion of ATP, ADP, AMP, and ADO have the potential to regulate various cellular responses by coordinated signaling mechanisms [5]. ATP, ADO, and sometimes ADP function as ligands of specific membrane receptors (see the corresponding sections). ATP recognizes a family of trimeric receptor channels (P2XRs) (formed by P2X 1-7 subunits) and eight G protein-coupled receptors (P2YRs) (actually had been described the subtypes P2Y 1,2,4,6,11-14), whereas ADO is the ligand of four G protein-coupled receptors (AdoR 1,2A,2B,3) [6]. Hence, purinergic signaling shows the distinctive property that the potential transformation of a primary ligand (ATP) into a secondary ligand (ADO) underlies the sequential and coordinated cellular responses in various physiological and pathological systems. The coincidence of two related ligands in a given cellular system is especially relevant since ATP and ADO signaling often play antagonistic roles (yin/yang effect), as in inflammatory and cancerous processes [7]. A key element allowing the formation of ADO derived from ATP degradation is a family of ecto-enzymes with nucleotidase/phosphatase activities.
Four major families of ectonucleotidases are recognized, namely E-NTPDases (ectonucleoside triphosphate diphosphohydrolases), alkaline phosphatases, NPP-type ectophosphodiesterases (nucleotide pyrophosphatase/phosphodiesterase), and 5′-nucleotidases [8]. Studies have reported that adenosine deaminase (ADA), an enzyme that removes ADO by turning it into inosine, is active in the extracellular space [9].
E-NTPDase is a family of membrane-attached Ca2+/Mg2+ enzymes with catalytic apyrase domains that hydrolyze ATP/ADP to generate AMP. E-NTPDase isoforms show different affinities for ATP and ADP; for example, CD39 (cluster of differentiation 39) forms AMP equally from either ATP or ADP. CD39L1 shows more affinity for ADP, whereas CD39L3 hydrolyzes ATP more than ADP [10].
NPP1 and NPP3 are members of the E-NPP family and can transform ATP into AMP and pyrophosphate [11]. Alkaline phosphatases are a set of glycophosphatidylinositol (GPI)-anchored enzymes that metabolize AMP into ADO and pyrophosphate into inorganic phosphate [12]. CD73 is another ecto-nucleotidase that turns AMP into ADO. It is a GPI-anchored protein formed by two glycoprotein subunits linked by a disulfide bond. CD73 is strongly inhibited by ATP and ADP [13, 14].
Exosomes are extracellular vesicles and an effective intercellular communication system widely expressed in diverse tissues and organs. They play physiological roles, modulating tissue differentiation and repair, pregnancy, and immune surveillance; but exosomes are also recognized as markers of pathological processes such as cancer [15]. In this context, studies have identified CD39 and CD73 in exosomes derived from cancerous tissues [16]. This finding further supports the importance of purines in the physio-pathological regulation of cellular responses at short and long range.
Purinome
The purinome is a dynamic interplay between intra- and extracellular purines (e.g., adenine nucleotides and ADO) and the thousands of proteins that utilize purine cofactors, including enzymes, structural proteins, membrane receptors, and transporters. All of these elements are present at intracellular level with an active traffic with the extracellular environment [17]. The purinome consists of genotypic and phenotypic characteristics that are unique in each cellular system and variable over time. For example, cellular differentiation dictates the expressed purinergic-related genes to accomplish metabolic networks and signaling communication. In this context, the balance between glycolysis and oxidative phosphorylation determines the energetic status of the cell. The set of ATP and ADO membrane receptors commands the purinergic signaling, while the type of ecto-nucleotidases influences the coordination between ATP and ADO-promoted responses. Overall, the cellular phenotype influences the purine function at intracellular (adenylate energy charge) and extracellular milieus (purinergic signaling). The impact of non-transcriptional factors is also determinant in purines proportion and their related functions; for example, the dynamic variation of the ATP/AMP ratio regulates the activity of allosteric enzymes and ion channels. Calcium dynamics modulates mitochondrial membrane potential and ATP synthesis. The transit of exosomes containing ectonucleotidases allows the target tissues to acquire the ability to modulate the proportion of extracellular adenine nucleotides and ADO. On the other hand, it is interesting to remark that, in certain cellular systems, purinergic receptors are segregated in different cellular domains, reinforcing the concept of signalosome [17].
To understand the integrated view of the purinome, it is important to consider that intracellular and extracellular milieus are connected in the transit of purine molecules, mainly by the action of two processes that control the purinergic signaling: (1) Release of ATP and ADO (high proportion of ATP and low proportion of ADO) and uptake of ADO by nucleoside transporters. ATP can reach the extracellular space by exocytotic release, micro-vesicle-mediated release through connexin, pannexin, and ABC transporters and the P2X7 receptor (P2X7R) [18]. (2) Purines return to the interior of the cell as ADO by the transporters of the solute carrier families (SLC28 and SLC29) which are specialized membrane proteins sensitive for inhibition by nitrobenzylthioinosine and dipyridamole [19].
Cancer is a collection of pathologies characterized by loss of genetic and metabolic checkpoints. It has been proposed that a set of hallmarks assist to conceptualize the principal features distinguishing cancerous from non-cancerous cells [20]. The most accepted cancerogenic elements are metabolic rewiring, immune modulation, altered stress response, invasion and metastasis, vascularization, selective growth and proliferative advantage, and an inciting microenvironment. Purines and pyrimidines can influence several hallmarks, acting as signaling molecules or metabolic intermediates. In the present review, we will focus on the role of purinergic signaling in the ten most lethal cancers worldwide according to Globocan (Fig. 1, Tables 1, 2, and 3), indicated by their age-standardized ratio (ASR): lung, breast, colorectal, liver, stomach, prostate, cervical, esophagus, pancreatic, and ovarian cancer.
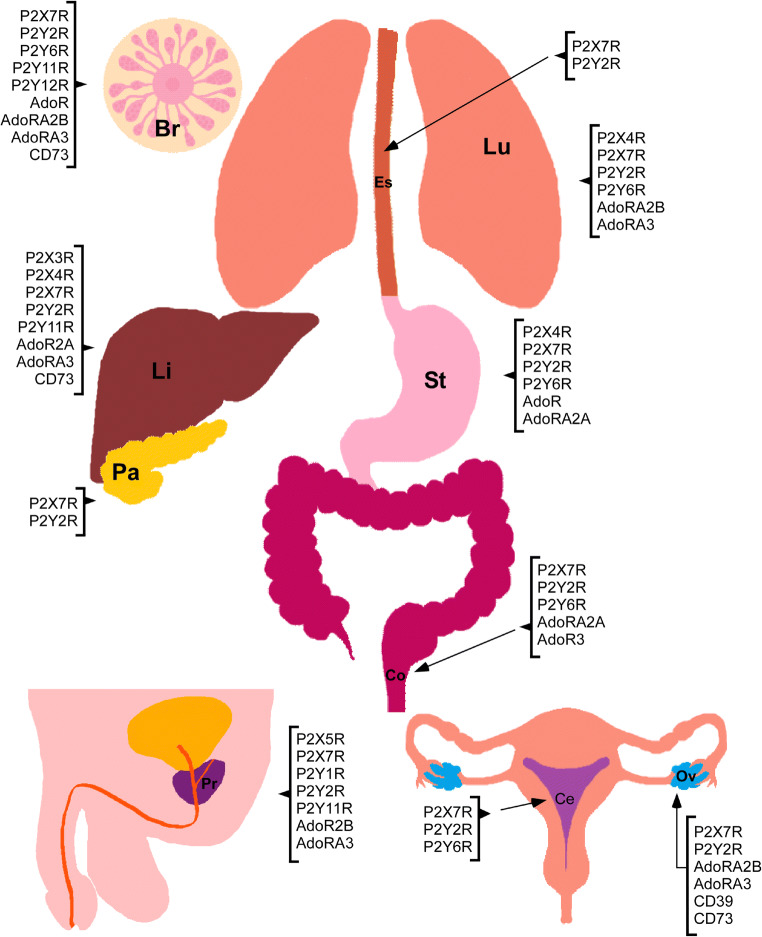
Fig. 1.
Purinergic elements described in the organs suffering the most lethal cancers. In the figure: breast (Br), cervix (Ce), colon (Co), esophagus (Es), lung (Lu), liver (Li), ovary (Ov), pancreas (Pa), prostate (Pr), and stomach (St)
Table 1.
Expression and process regulated by of purinergic elements in lung cancer
| Lung cancer | |||
|---|---|---|---|
| Purinergic element | Effect | Model | References |
| P2YRs | Activation of the Gq/PLC/ Ca2+ pathway | A549 cell line | [21] |
| P2Y2R and P2Y6R | Increase in cell proliferation | A549 cell line | [22] |
| P2X4R | Expression of this receptor, role not elucidated | A549 cell line | [23] |
| P2Y2R | ATP and mucin release after P2Y2R activation | Calu-3 cell line | [24] |
| P2X7R | Increased expression in tumoral cells | A549, PC-9 and H292 cell lines Biopsies from NSCLC patients | [25–27] |
| P2X7R | Decreased expression and higher cell survival by regulation of [Ca2+]i /Bcl-2/Bax ratio | A549 and H23 cell lines | [28, 29] |
| P2X7R | JNK and Rho kinase activation, cell migration | H292 cell line | [26] |
| P2X7R | EMT transition | A549 cell line | [30] |
| P2X7R | Receptor inhibition with A438079, decreased cell migration | HTB183 and HTB177 cell lines | [31] |
| P2X7R | Receptor inhibition with AZ10606120, decreased cell proliferation | A549 cell line | [25] |
| AdoR2B | Angiogenesis promoter, immune response suppressor | Lewis lung carcinoma isograft model | [32] |
| AdoR2B | Regulation of EMT | Human epithelial lung cells | [33] |
| AdoR3 | Decreased cell proliferation apoptosis induction | A549 cell line | [34] |
Table 2.
Expression and process regulated by of purinergic elements in the most lethal cancers from the reproductive system
| Purinergic element | Effect | Model | Reference |
|---|---|---|---|
| Breast carcinoma | |||
| P2X7R | Hypoxia promotes P2X7R over expression and activity of this receptor induces an invasive phenotype. |
MDA-MB-231 MCF-7 |
[35] |
| P2X7R | The receptor is over expressed, its activation increments cell migration and invasion and induces EMT markers. | T47D | [36] |
| P2X7R | Loss of function mutation (E496A) in host, induces fail in chemotherapy with anthracyclins | Tumors from xenotransplanted EL4 lymphoma cells in P2X7−/− mouse | [37] |
| P2X7R and P2Y11R | Reduction of vascular endothelial permeability | Tumor vascular endothelial cells associated to breast cancer (BTEC) | [38] |
| P2Y2R | Favors the formation of the pre-metastatic niche and supports metastasis | MDA-MB-231 cells xenotransplanted in nude mice. | [39] |
| P2Y2R | Modulation of estrogen-induced proliferation and induction of cell migration. | MCF-7 cells | [40, 41] |
| P2Y2R | Induction of cell proliferation, migration, invasion and EMT. | MDA-MB-231 cells | [40–43] |
| P2Y2R | This receptor increments the expression of the NLRC4 inflammasome components as well as IL-1β in response to radiotherapy. | Radiotherapy resistant or normal MDA-MB-231 cells | [44] |
| P2Y6R | The receptor is over expressed and its activity favors metastasis induction |
Tumor biopsies MDA-MB-231 cells xenotransplanted in nude mice. |
[45] |
| P2Y12R | The receptor facilitates the interaction between platelets and breast cancer cells, contributing to metastasis | MCF-7, MDA-MB-468, MDA-MB-231 | [46] |
| AdoR1 | Induction of cell proliferation and inhibition of apoptosis |
MDA-MB-438 MCF-7 |
[47, 48] |
| CD73 | Production of ADO by this enzyme repress maturation and cytotoxic actions of NK cells | 4T1.2 breast cells xenotrasplanted in mice | [49, 50] |
| CD73 | Favors EMT and induce metastasis | MDA-MB-231 and 4T1 | [51] |
| AdoR2B | Stimulation of cell proliferation, migration and metastases | MDA-MB-231 | [52, 53] |
| AdoR2B | Antagonism of this receptor inhibits CSC selection; furthermore, in previously selected CSC antagonists induce cell death. | Selection of CSC by sphere formation from MDA-MB-231 or MCF-7 cells | [54] |
| AdoR2B | Its activity favors the enrichment of cultures with cells expressing CSC markers | Selection of CSC by hypoxia in MCF-7, MDA-MB-231 and SUM-149 lines. | [55] |
| AdoR3 | Receptor over expression in tumor tissue | Carcinoma biopsies | [56] |
| AdoR3 | Induce the reduction of cell proliferation and migration | MCF-7 and MDA-MB-231 | [57, 58] |
| AdoR3 | Promotes bone metastasis | MRMT-1 cells injected in tibia of rats | [59] |
| AdoR3 | Its pharmacological activation inhibits CSC selection to form mammospheres | MCF-7 and MDA-MB-231 | [60] |
| CD73 | Its overexpression leads to immune evasion and is correlated with the induction of other immune check point proteins such as PDL1. | Murine triple negative breast cancer | [61] |
| CD73 | Neutralizing antibodies against this enzyme enhance the effect of chemotherapy and inhibit cell migration, invasion and autophagy. In vivo inhibits lung metastasis and enhances the effect of trastuzumab, a Her2 neutralizing antibody. |
MDA-MB-231 and MDA-MB-468. Her2+ breast cancer |
[62, 63] |
| Prostate | |||
| P2X5R | Its activity inhibits of cell growth and induces apoptosis | PC3 and DU145 cells | [64] |
| P2X7R | It is overexpressed in biopsies of PCa but is not detectable in non-cancerous tissue | Human biopsies | [65] |
| P2X7R | It is highly expressed, its activation induce increment in [Ca2+]i, invasiveness and EMT. Also favors metastases to lymphatic nodes and kidney. | 1E8 (highly metastatic) and 2B4 (non-metastatic) clones derived from PC3 line in vitro or xenotransplanted in nude mice. | [66] |
| P2X7R | A variant lacking “megapore” formation function named nfP2X7R is expressed. Its deletion promotes apoptosis. | PC3, DU145, and LNCaP lines | [67] |
| P2Y11 | Its function inhibits cell proliferation | PC3 and DU145 cells | [68] |
| P2Y1 | Its activation with MRS2365 and new ligands on based on 1-indolinoalkyl 2-phenolic inhibits cell proliferation and induce apoptosis. | PC3 and DU145 cells | [69, 70] |
| P2Y2R | Its activity promotes cell migration and EMT | 1E8 (highly metastatic) and 2B4 (non-metastatic) clones derived from PC3 line, through transactivation of EGFR | [71, 72] |
| AdoR3 | Its activity induce apoptosis | PC3, DU145 and LNCaP cells | [73] |
| AdoR2B | This receptor promotes proliferation and inhibition of apoptosis | PC3 cells | [74] |
| Cervix | |||
| P2X7R | Its function induces apoptosis | CaSki cells | [75] |
| P2X7JR | This variant of P2X7R lacks the second transmembrane, showing a dominant negative effect on channel function, inhibiting apoptosis induction by wt receptor | CaSki cells | [76, 77] |
| P2X7R | It is well expressed in normal tissue but downregulated in cancerous tissue, this downregulation prevents activation of apoptosis | Human biopsies (42 normal and 47 cancerous) | [78] |
| P2Y1R | Promotes proliferation transactivating EGFR through a pathway that involves PKC, Src and cell surface metalloproteases | HeLa cells | [167 |
| P2Y2R | Its activation induced Ca2+ mobilization, c-Fos expression, ERK phosphorylation and cell proliferation. Also regulates Na+/K+-ATPase activity. | HeLa cells | [79–81] |
| P2Y6R | Induce Ca2+ mobilization, activation of conventional and atypical PKC’s and cell proliferation | HeLa cells | [82] |
| Ovarian carcinoma | |||
| P2X7R | Is over expressed in cancerous tissue from different carcinoma types. Its activity supports cell proliferation and viability. |
Human biopsies SKOV-3 and CAOV-3 cells |
[83, 84] |
| P2Y2R | Its activity induces intracellular Ca2+ mobilization and down regulation of cell proliferation | EFO-21, EFO-27, | [85] |
| P2Y2R | Its activity induces intracellular Ca2+ mobilization and favors cell proliferation | IOSE29 (preneoplasic) and IOSE29EC (neoplasic)cells | [86] |
| P2Y2R | Induces increment of cell migration and EMT | SKOV-3 cells | [87] |
| AdoR2B AdoR3 | Its stimulation reduces cell viability and activates apoptosis | CAOV-4 and OVCAR-3 cells | [88, 89] |
| CD73 | Through a meta-analysis it was proposed as a poor prognosis factor |
High degree serous carcinoma (more than 1500 patients) |
[90, 91] |
| CD39 and CD73 | Both contribute to immune evasion of the tumor | Intratumor stromal cells from biopsies. | [92] |
| CD39 and CD73 | Attraction of myeloid cells to be differentiated in TAM. | Skov-3 cells | [93] |
Table 3.
Effects and processes regulated by purinergic elements in the most lethal gastrointestinal malignancies
| Purinergic element | Effect | Model | Reference |
|---|---|---|---|
| Hepatocellular carcinoma | |||
| P2X4R and P2X7R | Both receptors are overexpressed | Biopsies of HCC, adenocarcinoma and ampullary carcinoma | [94] |
| P2X4R | The HCV structural protein E1E2 induce overexpression of this receptor which is up regulated in HCV+ hepatocarcinoma biopsies | Huh-7 cell line and HCV+ hepatocarcinoma biopsies | [95, 96] |
| P2X7R | Incremented expression of this receptor in the peritumoral region after 5 years of resection indicates a poor prognosis | HCC biopsies and no cancerous peritumor regions | [97] |
| P2X7R | Polymorphisms in P2RX7 locus (1513A>C, 946G>A, and 1068G>A) indicates susceptibility to suffer HCC | Chinese population | [98] |
| P2X3R | It is overexpressed in HCC biopsies when compared with healthy tissues; in Huh-7 line, induce proliferation | Biopsies from USA and Korea population | [99] |
| P2Y2R | This receptor is overexpressed in HCC lines; its activation promotes cell proliferation and migration. Hypoxia contributes to its high expression level | Hep-G2 and BEL-7404 | [29, 100] |
| P2Y2R | Tumor growth depend on its expression | Tumors induced by DEN injection | [101] |
| P2Y11 | It is overexpressed in HCC lines and its activity increment cell migration | Huh-7 and HepG2 lines | [102] |
| CD73 | This enzyme is overexpressed in HCC biopsies compared with healthy tissues; supports tumor growth, EMT and metastatic invasion. | Human biopsies | [103, 104] |
| AdoR2A | Its pharmacological antagonism with KW-6002 impairs tumor growth | Xenografted HCC cells in nude mice | [104] |
| AdoR3 | It is expressed in HCC biopsies, its stimulation impairs tumor growth | N1C1 cells | [105] |
| Gastric cancer | |||
| P2Y2R | Increased expression in tumor biopsies, increases cell proliferation | Human biopsies, AGS, MKN-45 and MKN-74 cell lines | [106, 107] |
| P2X4R | Decreases cell proliferation | AGS, MKN-45, and MKN-74 cell lines | [107] |
| P2Y6R | Decreases cell proliferation and tumor growth | SGC-7901 and MKN-45 cells, gastric cancer xenografts | [108] |
| P2X7R | Increases cell proliferation | HGC-27, AGS and SGC-7901 cell lines | [109] |
| P1Rs | ATP-induced inhibition of cell growth, apoptosis induction | GT3-TKB and HGC-27 cell lines | [110, 111] |
| AdoR2A | Induction of EMT, activation of Pi3K-Akt-mTor pathway | MKN-45, MGC803, and AGS cell lines | [112] |
| Esophagus cancer | |||
| P2Y2R | Activation by ATP, UTP, and ATPγS leads to cell cycle arrest, apoptosis and cell proliferation decrease | Human biopsies, and Kyse-140 cell line | [113] |
| P2X7R | High expression and activation via ATP reduces cell proliferation and migration | Kyse-30, Kyse-450, and Kyse-520 cell lines | [114] |
| Colon cancer | |||
| P2Y2R | Activation using ATP or ATPγS decreases cell proliferation and induces apoptosis via intracellular Ca2+ and cAMP signaling pathways crosstalk | HT-29 and Colo 320 DM cell lines | [115, 116] |
| P2Y2R | Activation via autocrine or paracrine ATP release induces apoptosis evasion via Src/p38/COX-2 axis activation | HT-29 cell line | [117] |
| P2Y6R | Overexpression and activation by agonists like MRS2693 induces XIAP levels and promotes tissue dysplasia | HT-29 and HT-29/shP2Y6R cell lines, and P2Y6 −/− mice | [118] |
| P2X7R | Overexpression is associated higher tumor size, malignancy stage, metastatic potential, and patient survival, being all of these effects explained via Akt and NF-κB p53 pathways activation | Colorectal cancer biopsies, and NCM460, HCT116, SW480 and SW620 cell lines | [119, 120] |
| AdoR3 | Cell proliferation and liver metastasis reduction using the synthetic agonists CF101 and IB-MECA, being these effects synergistic with 5-FU due to adenylate cyclase inhibition. | HCT166 cell line and murine xenografts | [121, 122] |
| AdoR3 | Pharmacological agonist IB-MECA induces cell proliferation and inhibits apoptotic response | HT-20 cell line | [122] |
| Pancreatic cancer | |||
| P2Y2R | High protein and mRNA levels, activation using UTP or MRS2768, and inhibition by siRNA can promote cell survival, proliferation and migration via PI3K/Akt | Human biopsies, Panc-1 cell line | [123, 124] |
| P2Y2R | Inhibition using AR-C118925XX can reduce tumor growth and progression | Orthotopic pancreatic cancer xenografts on athymic mice | [125] |
| P2X7R | ATP concentrations up to 100 μM promotes cell migration and invasion via PKC-ERK1/2-JNK pathway induction | AsPC-1, BxPC-3, Capan-1, Panc-1 and MiaPaCa-2 cell lines | [177, 179 |
| P2X7R | ATP concentrations higher than 100 μM promotes cell death | Human biopsies, and AsPC-1, BxPC-3, Capan-1, Panc-1, and MiaPaCa-2 cell lines | [126] |
| P2X7R | Using AZ10606120 decreases cell migration and PDAC/PSC interaction | PancTu-1 cell line and murine PSC co-cultures | [127] |
| P2X7R | Inhibition by AZ10606120 reduces tumor growth | Xenografted NMRI-Foxn1nu mice | [127] |
| P2X7R | Pharmacological inhibition with A438079 and AZ10606120 induces tumor spread, pancreatic cancer progression, and apoptosis avoidance | Xenografted p48Cre/+-LSL-KrasG12D/+ mice | [128] |
Purinergic signaling in cancer
Lung
With an ASR of 18/100,000 inhabitants and a total of 1.79 million deaths in 2020, lung cancer is the leading cause of cancer-induced deaths worldwide (Globocan, https://gco.iarc.fr/). Lung cancer can be divided in two main types: (i) small cell lung cancer (SCLC), that occurs mainly in heavy smokers, and (ii) non-small cell lung cancer (NSCLC), which is more common than SCLC. NSCLC is an umbrella term for several types of lung cancers; for example, squamous cell carcinoma, adenocarcinoma, and large cell carcinoma. Conventional therapy for lung cancer includes surgery, chemotherapy, and radiotherapy, with surgery being the most effective treatment [129]. Novel therapies against lung cancer include monoclonal antibodies, tyrosine kinase inhibitors, and mammalian target of rapamycin (mTOR) inhibitors. However, because of the relevance of lung cancer, it is still desirable to find new signaling pathways that will help to improve the current therapies.
Regarding the role of purinergic signaling in lung cancer (Table 1), initial studies used the A549 cell line and found that these cells expressed functional P2YRs, coupled to Gq, PLC activation, and [Ca2+]i increase [21]. Later, it was shown that A549 cells express functional P2Y2R and P2Y6R, which after specific activation by purinergic agonists mediate an increase in cell proliferation [22]. Moreover, these cells are capable of releasing ATP and UTP in a calcium-dependent mechanism [130]. A549 cells express P2X4R, although the particular role of this receptor in lung cancer has not been established [23]. In other lung cancer-derived cell lines, ATP inhibits cell proliferation; that is the case of H460, a cell line derived from human large-cell lung carcinoma; H441 cells, derived from human papillary lung adenocarcinoma; H520 cells, from human squamous cell lung carcinoma; GLC4 cells, from human SCLC; and MER082 cells, derived from a human mesothelioma [131]. A study isolating Calu-3 cells from the pleural effusion of a patient with a lung adenocarcinoma found that these cells released ATP and mucin in response to P2Y2R activation [24]. In addition, a role has been proposed for adenosine through AdoR2B, expressed in host immune cells, as an angiogenesis promoter and suppressor of immune responses; this has been corroborated in ADORA2B knockout mice, which showed a decrease in tumor growth using a Lewis lung carcinoma isograft model [32]. It has been shown that AdoR2B modulates epithelial-mesenchymal transition (EMT) in human epithelial lung cells by triggering changes in the balance of cAMP/PKA and MAPK/ERK pathways [33]. On the other hand, activation of AdoR3 by 3-Deoxyadenosine inhibited tumor growth using a Lewis lung carcinoma mouse model [132]. Similarly, the AdoR3 agonist thio-2-chloro-N6-(3-iodobenzyl)-5-N-ethylcarboxamidoadenosine (thio-Cl-IB-MECA) inhibited A549 cell proliferation through mechanisms related to cell-cycle arrest and apoptosis induction [34].
Several studies have shown that P2X7R plays a role in lung cancer and can be a potential therapeutic target. The expression of this receptor is increased in lung cancer, and P2X7R has been observed in several lung cancer-derived cell lines including A549, PC-9, and H292, but not in the non-tumoral BEAS-2B cell line, which is derived from bronchial epithelia [25, 26]. Moreover, high P2X7R expression has been found in patients with metastatic NSCLC [27]. However, other studies have reported that P2X7R is almost absent in lung cancer cells, which causes cell survival via the regulation of [Ca2+]i and the Bcl-2/Bax ratio [28]. This apparent contradiction can be explained by the fact that P2X7Rs expressed in the tumor microenvironment, but not in cancer cells, could be acting as promoters of cell migration, invasion, and metastasis [133]. In addition, the activation of mitogen-activating kinases, such as JNK and Rho, is a signal that promotes cell migration and actin remodeling, and this pathway can be triggered by P2X7R activation [134]. In H292 cells, TGF-β1 induces the release of ATP and activation of P2X7R-downstream signaling and cell migration [26]. Extracellular ATP can also promote EMT after P2X7R activation [30]. This transition is related to the upregulation of mesenchymal markers and downregulation of epithelial markers. Because of their possible role as modulators of lung cancer progression, P2X7R antagonists such as BBG, KN62, oATP, A740003, A438079, or AZ10606120 are being proposed as anticancer agents [135, 136]. For example, A438079 reduced cell migration in HTB183 and HTB177 [31], and AZ10606120 diminished lung cancer cell proliferation and migration [25]. Although P2X7R polymorphisms have not been directly related to cancer progression in NSCLC [137, 138], it seems that rs1718125 gene polymorphism is associated with pain after lung cancer surgery [139]. Related to P2X7R splicing variants, Benzaquen et al. reported that in samples from patients affected by lung adenocarcinoma, overexpression of the P2RX7B splice variant is associated with a reduction of immune infiltration of B and T lymphocytes, and an increase in myeloid cells infiltration. These findings portray P2R7XB as a prospective biomarker for lung cancer treatment and diagnostics [140]. Furthermore, recent studies showed that the positive P2X7R modulator HEI3090 in combination with anti-PD1 antibody induces tumor regression and increases tumor immunogenicity on in vitro and in vivo models of NSCLC [141].
Breast
Breast cancer ranks first in incidence and second in mortality worldwide. In 2020, a total of 2,261,419 new cases and a mortality of 13.6/100,000 inhabitants were reported (Globocan, https://gco.iarc.fr/). Breast cancer is a group of malignancies with a complex classification. From the histological perspective, breast carcinomas can be invasive ductal or invasive lobular. Tumors are also classified according to the differentiation grade—a score of histological characteristics such as nuclear pleomorphism and mitotic count [142]. In addition, different molecular markers have been used for breast cancer classification. The two main biomarkers are estrogen receptor (ER) and human epidermal growth factor receptor type 2 (HER2). These and other molecular markers, including progesterone receptors (PR) and the proliferation marker Ki67 (Ki67), have also been extensively used to define specific treatments [143]. Recently, transcriptomic, proteomic, epigenetic, and metabolomic data have been used for personalized diagnoses [142]. Actually, breast cancerous tumors are classified in luminal A (positive to ER or/and PR and negative to HER2 and Ki67), luminal B (positive to ER or/and PR, and positive to Ki67 and negative to HER2), luminal-HER2 (positive to ER or/and PR, and positive to HER2), HER2-enriched (positive to HER2), and basal-like (Cytokeratin 5/6 or EGFR-positive and triple-negative (negative to ER, PR, and HER2)) [144].
One of the most studied purinergic components is P2X7R. In tumor cells, hypoxia induces necrotic cell death and activity of HIF-1α, a key adaptive mechanism. In metastatic triple-negative MDA-MB-231 and estrogen-dependent non-metastatic MCF-7 breast cancer cells, the activation of HIF-1α induces an increment in P2X7R expression. Concomitantly, ATP released by dying cells acts as a damage-associated molecular pattern (DAMP) binding to this receptor, whose activity promotes ERK and AKT phosphorylation resulting in NFkB nuclear translocation. This action positively feedbacks the expression of P2X7R and facilitates a highly invasive phenotype [35]. In agreement, incremented expression of P2X7R was also described in T47D breast cancer cells, and its stimulation increased cell migration, invasion ability, and EMT installation through a pathway that also involves AKT phosphorylation [36].
Successful chemotherapy depends on the competence of the host to maintain an immune anti-tumor response. The first evidence supporting this proposal was developed by studying the high mobility group B1 protein (HMGB1), a DAMP acting through the toll-like receptor 4 (TLR-4) [145]. The role of ATP as a DAMP is also important for chemotherapy-associated response, where a substantial increment in extracellular ATP has been observed as a consequence of treatment-induced cell death. Thus, individuals with breast cancer, carrying a loss-of-function mutation in the P2RX7 locus (Glu496Ala, rs3751143), did not respond to anthracycline chemotherapy [37]. Mechanistically, P2X7R is necessary to activate the Nlrp3 inflammasome in dendritic cells, which induce IL-1β release. IL-1β is a cytokine that primes the production of effector CD8+ T lymphocytes (interferon producers) to support an anti-tumor response [37]. These observations contributed to the deduction of one of the most important action of purinergic system in cancer, the modulation of TME composition. This idea has been reinforced by observation in other systems. Thus, xenotransplantation of B16 melanoma cells expressing P2X7R in a P2RX7-deficient host induced a decrease in the number of CD8+ cells and incremented the number of Treg cells [146], suggesting that P2X7R is essential for anti-tumor immune response. The nature of ATP regulation on immune ambient in TME can be dependent of particular conditions; in acute myeloid leukemia, ATP release induced immunosuppressive effects, increasing Treg and tolerogenic DC [147]; these results suggest a fine and specific regulation of ATP over immune cells in TEM of a particular cancer. The ability of P2X7R as regulator of TME is also supported by the fact that P2X7R activation regulates stabilization of HIF-1α transcription factor and secretion of factors of VEGF, two important messengers regulating growth [148].
On the other hand, in tumor vascular endothelial cells associated with breast cancer tumor (BTEC) but not in normal endothelial cells, ATP (> 20 mM) inhibited cell migration acting through P2X7R and P2Y11R by a signaling pathway that involved adenylyl cyclase 10 (Ca2+-dependent), cAMP synthesis, and Epac activity. Moreover, ATP incremented the attraction of pericytes by BTEC, a response that favored the reduction of endothelial permeability [38].
Regarding P2YRs, it has been described that P2Y2R is involved in the formation of the pre-metastatic niche (PMN), a region in a distant organ that facilitates tumor establishment and progression. PMN is characterized by the crosslinking of extracellular matrix proteins catalyzed by the lysyl oxidase enzyme (LOX) and recruitment of bone marrow-derived cells (BMDC). In vitro approaches with MDA-MB-231 cells showed that in hypoxic conditions, ATP released from dying cells activates P2Y2R, which induces HIF-1α activity and LOX release. Besides, transplantation of MDA-MB-231 cells in immunosuppressed mice induces the emergence of PMN-like regions in the lung, an effect that was suppressed by knocking down P2Y2R in the transplanted cells [39]. Other studies also support a role for P2Y2R in metastasis facilitation. Tumor growth and metastasis of MDA-MB-231 xenotransplanted cells in nude mice to other organs were markedly reduced if P2Y2R expression was inhibited [149].
P2Y2R was investigated as a modulator of estrogen actions in cell proliferation. In estrogen receptor alpha (ER-α) positive MCF-7 cells, P2Y2R stimulation reduced the proliferation rate; interestingly, pharmacological activation of ER-α, but not ER-β, downregulated the expression level of P2Y2R. Since ER-α activity in MCF-7 induced cell proliferation, it was proposed that the mechanism involved in this response is the inhibition of P2Y2R constitutive activity by downregulating its expression [40]. This is an important crosstalk considering that a large group of breast tumors are estrogen dependent. Furthermore, in the MCF-7 cell line, P2Y2R activation with UTP or 2-thio-UTP (both at 10 mM) elicited an increment in the [Ca2+]i, dependence of phospholipase C and incremented cell migration but without proliferation [41].
On the other hand, in the highly metastatic triple-negative MDA-MB-231 cells, P2Y2R activation induced cell proliferation and increased cell migration [40]. The incremented invasion ability of MDA-MD-231 in response to P2Y2R activity, mediated by self-released ATP, correlated with a reduction of the mesenchymal markers Vimentin and N-cadherin and an incremented phosphorylation level of ERK and PKC activity, supporting a relationship between P2Y2R signaling and EMT induction [42]. Also, in MCF-7 and MDA-MB-231-cells, P2Y2R upregulated β-catenin expression, which in turn enhanced its nuclear translocation to induce EMT [43].
The purinergic system exhibits adaptive changes in response to radiotherapy. MDA-MB-231 cells that were resistant to radiotherapy (RR-MDA-MB-231) showed increments in basal and TNF-α-induced ATP release and IL-1β secretion [44]. P2Y2R activation induced an accumulation of the constituents in the NLRC4 inflammasome (NLRC4, ASC, and caspase-1) in both MDA-MB-231 and RR-MDA-MB-231 lines, and increased IL-1β and VEGF-A release [150]. Furthermore, transplantation of RR-MDA-MB-231 cells bearing knocking down of the P2RY2 transcript in immune-deficient mice showed diminished tumor growth and intratumor MMP-9 expression [44].
P2Y6R has been associated with metastasis induction in breast cancer. P2Y6R is overexpressed in tumor biopsies and correlates with a bad prognosis; using pharmacological tools, researchers have demonstrated that P2Y6R controls cell migration and invasiveness and favors metastasis through ERK and NfkB signaling pathways [45].
Platelet activation is regulated by P2Y12R and promotes the metastatic potential of cancer cells. Hence, a role for P2Y12R in metastatic induction is feasible. To analyze this possibility, platelets were treated with the P2Y12R antagonist ticagrelor, which induced a decrease in the interaction between platelets and MCF-7, MDA-MB-468, and MDA-MB-231 breast carcinoma cell lines. In in vivo experiments, ticagrelor incremented the survival of the host by reducing lung metastases [46], indicating that cellular interaction between platelets and tumor cells was necessary for metastatic progression.
Receptors for the ADO nucleoside are also present in breast cancer. Incremented expression of AdoR1 was reported in breast carcinoma cell lines [47, 48]. Depletion of ADORA1 transcript by siRNA and pharmacological blocking with 1,3-dipropyl-8-cyclopentylxanthine (DPCPX) in MDA-MB-438 cells attenuated cell proliferation [47] and induced apoptotic cell death in the MCF-7 line [48]. Furthermore, stimulation of AdoR1 with N6-Cyclopentyladenosine (CPA) in this cell line induced cell proliferation and downregulated the expression of p53 [48].
ADO is most known in cancer for suppressing the anti-tumor immune response [151, 152]. This inhibition has been attributed to ADO action over its specific membrane receptors in immune cells and is highly dependent of ectonucleotidase CD73 activity that converts ATP into ADO. The administration of an AdoR2A antagonist, in mice bearing tumors induced by xenotransplant of the highly metastatic 4T1.2 breast cancer cell line, which show a constitutive expression of CD73, reduced the number of lung metastases. To explain this observation, it was proposed that AdoR2A in NK cells represses their maturation and cytotoxic function [49]. In agreement, it was demonstrated that in mouse tumors expressing high levels of CD73, the use of an AdoR2A antagonist in combination with neutralizing antibodies against the immune checkpoint protein PD-1 improved the metastatic burden and survival of the mice. This study also detected NK cell dependency in the metastatic process [50].
AdoR2B was identified in MDA-MB-231 but not MCF-7 cell lines. Interestingly, it was described that the receptor was coupled to the Gs and Gq proteins, and consequently, at both synthesis of cAMP and phosphoinositides-Ca2+ pathways [153]. In addition, AdoR2B activation in MDA-MB-231 cells induced cell proliferation and migration by a cAMP-dependent pathway [52]. Controversially, it was shown that in MDA-MB-231, AdoR2B stimulation induced a reduction of ERK phosphorylation by both cAMP and phosphoinositides-Ca2+ pathways that converge in MAPK phosphatase-1 (MKP-1) activation [154].
In mice transplanted with 4T1 cells, a line from the mouse mammary gland, intratumor injection of antagonists for ADO receptors delayed the tumor growth and reduced lung metastasis by 85%; these effects were not present in ADORA2B−/− mice. It was determined that AdoR2B blocking induced dendritic cell activation and CXCR3 anti-tumor responses [155]. Also, it was proposed that the transcription factor Fos-related antigen-1 (Fra-1) is a master regulator of metastasis, with AdoR2B being one of its main targets. Fra-1 and AdoR2B constitute a pathway that promotes mesenchymal phenotype and metastatic induction of breast cancer cells [53].
Expression of AdoR2B has been detected in cancer stem cells (CSCs) derived from carcinoma cell lines MCF-7 and MDA.MB-231. To enrich the cell lines with CSCs, a sphere formation protocol was used. The addition of the AdoR2B agonist BAY606583 to the culture medium inhibited sphere formation, that is to say the selection of CSCs from the parental cell line [54]. Moreover, the addition of BAY606583 to isolated CSC induced cell cycle arrest and apoptotic cell death by downregulating ERK phosphorylation [54]. On the other hand, Lan et al. [55] found in MCF-7 and SUM149 cells that hypoxia upregulated ADORA2B expression and induced CSC enrichment (ALDH+); pharmacological blocking or knockdown of the ADORA2B transcript with shRNAs in MDA-MB-213 cells abolished this effect. Furthermore, AdoR2B activation with ADO in MCF-7, MDA-MB-231, and SUM149 increased the number of cells expressing CSC markers by a pathway involving protein kinase C-δ that phosphorylates Stat3 to promote a positive regulation of NANOG and IL-6, two mediators of the CSC phenotype. Lan and colleagues concluded that AdoR2B was required for hypoxia-induced CSC enrichment [55].
Regarding AdoR3, it was demonstrated that human tumor biopsies express higher levels of the ADORA3 transcript than adjacent non-cancerous tissue or normal tissue; in addition, metastatic nodes showed the highest ADORA3 transcript levels. This was also observed for AdoR3 protein expression. Breast tumors showed incremented expression of this ADO receptor in transformed zones in comparison with non-cancerous adjacent tissue [56]. AdoR3 has also been detected in MCF-7 and MDA-MB-231 cells, in which the selective agonist N6-(3-iodobenzyl)-N-methyl-5′-carbamoyladenosine (IB-MECA) (1–10 mM) induced a reduction in cell proliferation [57]. Furthermore, in MDA-MB-231, AdoR3 activation with ADO and the selective agonist IB-MECA reduced the cellular migration [58]. Since bone is one of the most invaded organs by breast-derived secondary tumors, the potential role of AdoR3 in bone-residing metastatic breast tumors was evaluated. MRMT-1 cells (from rat mammary gland) expressing AdoR3 were injected into the tibia of rats, and tumor development and bone damage were evaluated. Intra-peritoneal administration of Cl-IB-MECA reduced the tumor growth and attenuated bone destruction in a dose-dependent way and, importantly, without white blood cell reduction—a serious side effect of chemotherapy that was significant in cisplatin-treated control mice [59]. These results suggested that AdoR3 could be a target against breast-derived bone secondary tumor development. Activation of AdoR3 with CI-IB-MECA also inhibited the formation of carcinoma-derived mammospheres—cellular bodies enriched in CSCs from MCF-7 and MDA-MB-231 cells—indicating that AdoR3 prevented the CSC selective process. Moreover, in previously selected CSCs, AdoR3 stimulation induced cell cycle arrest and apoptotic cell death [60].
Important actions of extracellular ATP in the tumor microenvironment (TME) depend on its transformation into ADO, being CD73 the main enzyme catalyzing the transformation of AMP into ADO. This protein has received growing attention in recent oncologic research for the relation between CD73 and anti-tumor response suppression [151, 152]. Importantly, it was observed in murine triple-negative breast cancer that chemotherapy with carboplatin, doxorubicin, gemcitabine, and paclitaxel promoted the expression of the immune checkpoints CD47, CD73, and programmed death ligand (PDL1), whose increment favored the tumor immune evasion; these effects were dependent on HIF-1α activity [61]. Similar anti-tumor response mechanisms have been described for immune cells; thus, tumor-infiltrating NK cells upregulated CD73 expression, and this rise correlated with an incremented expression of other immune checkpoints such as programmed death (PD-1) and PDL1 [61].
Furthermore, in other cancer models, the importance of P2X7R in the regulation of TME has been reinforced; thus, in P2RX7 null mice bearing tumors induced by the transplantation of melanoma cell lines, the expression level of CD39 and CD73 ectonucleotidases increased, inducing a low anti-tumor response [146]. CD39 is also a metastasis regulator; its inhibition with a neutralizing antibody reduces the metastasis of induced tumors of different origins in mice. The mechanism of this effect involves the participation of myeloid and NK cells and requires the activity of P2X7R and NALP3-inflammasone downstream of CD39 inhibition [156, 157]. Taken together, the evidence supports a role for extracellular ATP in TME modeling.
In addition, neutralizer antibodies against CD73 have been used as a therapeutic tool. Thus, the anti-CD73 monoclonal antibody (clone TY/23) was utilized to affect the activity of PD-1 and cytotoxic T lymphocyte antigen-4 (CTLA-4); anti-CD73 enhanced the therapeutic activity of anti-PD-1 and anti-CTLA-4 monoclonal antibodies against the established metastasis of 4T1.2 cells originally inoculated in a mammary fat pad of BALB/c mice [158]. In another study, the anti-CD73 monoclonal antibody (3F7) enhanced the cytotoxic effect of doxorubicin and inhibited the migration, invasion, and autophagy of MDA-MB-231 and MDA-MB-468 triple-negative breast cancer cells; besides, 3F7 administered in vivo inhibited lung metastasis in xenotransplanted 4T1 breast cells of BALB/c mice [62]. Interestingly, patients with HER2+ breast cancer and low expression of CD73 showed the better distant relapse-free survival in response to a HER3/ErbB2 neutralizing antibody (trastuzumab) than those expressing high levels of CD73. This effect was not observed with other immune checkpoints such as the PDL-1/PD-1 system. In agreement, CD73 neutralized by antibodies potentiated the trastuzumab effect in grafted tumors modeling HER2+ breast cancer [63].
Evidence demonstrates that CD73 expression regulates breast cancer cells in the TME and other sites. It was observed that 4T1 cells that blocked CD73 expression inhibited cell proliferation, arrested the cell cycle, and induced downregulation of molecules related to angiogenesis as VEGF-A, VEGF-R2, and TGF-β. Accordingly, nanoparticles loaded with cd73 siRNA in mice bearing tumors induced by 4T1 cell xenotransplantation elicited tumor regression and downregulation of pro-angiogenic factors [159]. Moreover, in triple-negative MDA-MB-231 or 4T1 breast cancer lines, pharmacological inhibition, or genetic deletion of CD73, decreased cell viability and migration in normoxia, and suppressed hypoxia-dependent increment of cell viability and phenotypic changes related with facilitation of cell migration. Furthermore, organoids derived from 4T1 cells, where CD73 expression was suppressed, shown less invasive and metastatic ability. These changes were related with EMT regulation [51]
Breast biopsies revealed an incremented expression of CD73 that correlated with enhanced tumor malignity. In vitro evidence indicated that CD73 supported tumor growth through the AKT/GSK3β/β-catenin/Cyclin-D pathway [160]. To understand the clinical relevance of CD73 expression, researchers analyzed the expression level of this ectoenzyme in 122 samples of triple-negative breast cancer of a phase III clinical trial, and the data were correlated with patient survival after 120 months: high expression of CD73 was associated with reduced disease-free survival and poor overall survival [161]. Recently, the possibility of targeting purinergic signaling components in breast cancer was reviewed [162], showing interesting possibilities, as the inhibition of ADO formation with blockers of the ectonucleotidase pathway or pharmacologic or genetic inhibition of AdoR function. Table 2 shows a summary of purinergic elements characterized in breast carcinoma.
Colorectum
With 1,931,590 cases and 935,173 deaths in 2020, colorectal cancer is considered a major health concern worldwide (Globocan, https://gco.iarc.fr/). It has been established as the third leading cause of cancer-related deaths, with an ASR of 9.0/100,000 inhabitants. Colonoscopy is the most accurate method for early diagnosis. Therefore, novel biomarkers that could help to provide a more efficient diagnosis and/or treatment are highly desired [163]. In this regard, the potential role of purinergic signaling in colorectal cancer has been described (Table 3), although further research is required [164].
Purinergic receptors, especially P2YRs, are widely expressed in the gastrointestinal tract where they exert various functions including neurotransmission, gland secretion, contraction, and smooth muscle relaxation [165]. Colorectal cancer response to nucleotides has been characterized by several works. P2Y2R is one of the first receptors described for this malignancy. Studies have identified its overexpression in human cell lines and primary cultures, and described antiproliferative and pro-apoptotic effects on HT-29 and Colo320 DM cells stimulated with ATP and ATPγS via a crosstalk between intracellular Ca2+ and cAMP signaling pathways. These effects are synergic with 5-FU [115, 116]. Conversely, later research by Limami et al. [117] has shown that P2Y2R induces apoptosis resistance in HT-29 cells exposed to ursolic acid (UA), a pentacyclic triterpenoid molecule with anti-tumoral effects. According to these authors, UA treatment induced an increase of intracellular ATP that activated P2Y2R para- or autocrinally, triggering apoptosis evasion via the Src/p38/COX-2 axis [117].
Other P2 receptors described in colorectal cancer are P2Y6R and P2X7R. An antiapoptotic effect has been shown for P2Y6R in HT-29 cells, shP2RY6 cells, and P2RY6−/− mice. P2Y6R activation via MRS2693 induced X-linked inhibitor of apoptosis protein (XIAP) levels, promoting colorectal tissue dysplasia and disrupting the antiproliferative response of traditional chemotherapeutic agents like 5-FU, which suggests that P2Y6R may function as a chemoresistance factor in colorectal cancer [118].
P2X7R has been linked to its value as a clinical prognosis biomarker. Reports show that P2X7R expression in human colorectal cancer cell lines and tumor biopsies is upregulated and associated with tumor size, metastatic potential, a high level of carcinoembryonic antigen, tumor malignancy stage, postoperative survival, and overall survival. These can be potentially explained by the activation of Akt and NF-κB p53 pathways [119, 120]. Recent studies on in vitro models of colorectal cancer support this idea, and also associate P2X7R activation with epithelial-mesenchymal transition (EMT) induction, finding that ATP and BzATP promoted cell proliferation, increased the expression of fibronectin, Snail and Vimentin, and reduced the expression of the epithelial adhesion factor E-cadherin, being these effects of P2X7R mediated by the PI3K/Akt/GSK-3β axis, as could be evidenced by the use of P2X7R antagonists (A438079 and AZD9056), P2X7R siRNA, and the PI3K/Akt inhibitor LY294002, which reduced cell proliferation and conserved the epithelial phenotype of SW620 and HCT116 colorectal cancer cell lines [166].
Concerning adenosine receptors, the role of AdoR3 in the HCT-166 human colon carcinoma cell line and murine xenografts has been described as antiproliferative in primary cancer cells. AdoR3 also reduces liver metastasis after treatment with CF101, a synthetic AdoR3 agonist, at nanomolar concentration range (10–1000 nM). Additionally, experiments on mice demonstrated a synergistic effect between CF101 and 5-FU, improving the cancer response to 5-FU and reducing its typical myelotoxicity [121]. Further investigation on this subject showed that synergy with 5-FU and antiproliferative effects of another AdoR3 agonist, IB-MECA, on HCT-166 cells were due to apoptosis induction at 1 μM. These effects were related to adenylate cyclase inhibition and an intracellular AMPc decrease, and were reversed by AdoR2A activation. The latter promoted cell survival in this cell model. Despite the roles described for HCT-166 cells, the authors also found that IB-MECA induced cell proliferation and inhibited apoptosis in HT-29, another human colon carcinoma cell line, highlighting the significance of genetic profiling to describe the response of different types of cancer to AdoR3 synthetic agonists [122].
Hepatocellular carcinoma
The liver is responsible for a broad group of metabolic processes including fatty acid, purine and triacylglycerol synthesis, glycogen metabolism, gluconeogenesis, and urea cycle. Simultaneously, the liver detoxifies xenobiotics and performs exocrine functions. This organ is exposed to many metabolic and environmental toxics and infectious agents that could eventually promote chronic hepatic disease (CHD), which initiates as fibrosis and can progress into hepatocellular carcinoma (HCC) [167]. HCC represents 80–90% of hepatic cancer patients. Moreover, it ranks seventh in terms of incidence and fourth in mortality worldwide, with an ASR of 8.7/100,000 inhabitants. In 2020, HCC caused 830,1801 deaths (Globocan, https://gco.iarc.fr/).
Early studies in H-35, an HCC cell line, showed that ATP induced the formation of inositol triphosphate (IP3) and intracellular Ca2+ release in a concentration-dependent manner [168]. These observations, made before purinergic receptors were cloned, indicated the presence of ATP-sensitive membrane receptors in transformed liver cells.
P2X4R and P2X7R expression was demonstrated in rat hepatocytes. Interestingly, P2X4R was enriched in the canalicular domain. Pharmacological approaches by patch clamp and Ca2+ imaging have evidenced their functionality [169]. The expression levels of P2X4R and P2X7R were analyzed in a comparative study that included HCC, adenocarcinoma, and ampullary carcinoma human biopsies. Both subunits were overexpressed in the three types of cancers if compared with healthy liver samples [94]. However, their pathophysiological implications were not investigated.
In the human HCC Huh-7 line, one study described the presence of P2X3R, P2X4R, P2X5R, and P2X6R transcripts; interestingly, the exogenous expression of hepatitis C virus (HCV) structural proteins E1 and E2 in these cells promoted an increment in the expression of P2X4 subunit (6.2-fold), suggesting that P2X4R can exert the changes induced by HCV infection [95]. Moreover, in HCC biopsies from a Pakistani population, P2X4R expression was upregulated in tumors infected by HCV [96]. Thus, there is a relationship between the incremented expression of P2X4R and HCV-related HCC; although its meaning remains elusive, it must represent a diagnostic or therapeutic potential to be investigated.
The prognostic value of P2X7R expression level over patient surveillance was investigated in HCC biopsies after surgical rejection (2 groups of 273 patients from Shanghai, China). Paired samples from tumor and peri-tumor areas were evaluated for P2X7R expression and correlated with surveillance data after 5 years. The incremented expression in the peri-tumor region correlated with an unfavorable prognostic of global surveillance [97]. Furthermore, P2RX7R polymorphisms were related to cancer in some organs [135]. A study on the effect of P2RX7R gene polymorphisms on susceptibility to HCC in a Chinese Han population (with nearly 600 individuals) found a correlation between three polymorphisms of this gene (1513A>C, 946G>A, and 1068G>A) and an increased susceptibility to suffer HCC [98]. These data unveil the potential of the P2RX7R locus for HCC diagnosis.
Likewise, purinergic receptor expression was analyzed in two independent cohorts of HCC (42 patients from the Texas Medical Center and 188 from Korean institutions). The expression levels of P2XRs and P2YRs were compared between tumor biopsies against non-cancerous regions of the same liver or against samples of normal livers. Interestingly, 60–70% of tumor biopsies (vs. normal zones or samples from non-cancer livers) showed elevated expression of P2X3R, which correlated with poor recurrence-free survival. Moreover, it was demonstrated that P2X3R is a positive regulator of cell proliferation in the Huh-7 cell line [99].
Regarding P2YRs, a significant increment of P2Y2R expression was observed in the HCC-cultured cells and the Hep-G2 and BEL-7404 lines in comparison with primary cultured hepatocytes or the normal hepatocyte line LO2. In HCC-derived cells, P2Y2R stimulation elicited an increment in the intracellular concentration of Ca2+ coupled to the activation of store-operated calcium signaling (SOCs) and increased cellular proliferation and migration [100]. Furthermore, it was described that hypoxia, a common cellular condition in solid tumors, induced overexpression of P2Y2R through the activity of HIF-1α; this suggested that the increment in P2Y2R expression is a mechanism through which cancerous cells face low O2 conditions [29]. In addition, the induction of hepatic tumors by diethylnitrosamine (DEN) injection was notably reduced in the P2RY2−/− mice in comparison with wild-type individuals. This decreased tumorigenic response was related to low proliferation rates in the liver of P2RY2−/− mice after DEN injection. Ablation of P2Y2R also declined the DNA damage levels—a characteristic of DEN toxicity [101]. These observations suggested that P2Y2R regulates the mechanism mediating hepatocellular damage by carcinogenic toxics.
The actions of extracellular ATP in HCC are also mediated by P2Y11R. It has been demonstrated that the expression of this receptor is incremented in HCC biopsies and HCC lines Huh-7 and HepG2 in comparison to normal tissues. P2Y11R activity elicited an intracellular Ca2+ increment and induced cellular migration, indicating that this receptor is another mediator of purinergic signaling in the cancerous context [102].
An enzyme that has been studied in the HCC context is CD73. IHCC biopsies showed high levels of this ectonucleotidase in comparison to normal peritumoral tissues [103, 104]. Accordingly, time of recurrence and overall surveillance after surgical rejection of the tumor were significantly reduced in patients with high expression of CD73 [104]. Furthermore, HCC xenotransplant growth was repressed in CD73−/− mice (Shali et al. 2019) by the CD73 inhibitor adenosine 5′-(α,β-methylene)diphosphate (APCP) and the AdoR2A antagonist KW-6002 in nude mice [104]. In HCC, CD73 supports cancer progression, EMT induction, and metastatic invasion [103, 104]. AdoR2A is associated with ADO action in HCC, but AdoR3 has also been reported in HCC human biopsies. The stimulation of N1C1 cells with CF102, an agonist of this ADO receptor, decreased cell proliferation and favored apoptosis through crosstalk with NF-kB and WNT pathways [105]. A summary of purinergic effects on liver carcinoma is shown in Table 3.
Stomach
Gastric or stomach cancer is a globally relevant disease [170] that caused more than 750,000 deaths in 2020. It is the fifth most lethal type of cancer, with an ASR of 7.7/100,000 inhabitants (Globocan, https://gco.iarc.fr/). This disease is generally asymptomatic and diagnosed late, resulting in metastasis that can progress to advanced and terminal stages [171]. Five-year survival rates are 50% and 95% in patients in advanced and early stages, respectively [172]. Risk factors include infection by Helicobacter pylori, age, high-salt diet, and high consumption of processed food. Gastric cancer is histologically diagnosed after endoscopic biopsy and staged using diverse techniques, such as computed tomography, endoscopic ultrasound, Positron emission tomography, and laparoscopy [173].
In the stomach, P2YRs can regulate gastric acid secretion, smooth muscle contraction/relaxation, and neurotransmission mediated by extrinsic and intrinsic neural systems. Although the specific P2YRs involved in these functions have not been completely identified, it has been shown that UTP and UDP, agonists of P2Y2R, P2Y4R, and P2Y6R, can induce contraction of gastric smooth muscle [174]. In addition, adenosine, through the activation of P1 receptors, regulates gastric acid secretion [164]. ATP also induces contraction of gastric smooth muscle in guinea pigs by activating P2Y receptors and increasing intracellular calcium [175]. Although different purinergic receptor subtypes are expressed in cancer cells and primary cancer tissues of the digestive system (Table 3), there is a lack of information about purinergic signaling in stomach/gastric cancer.
The first studies in this field have reported that adenosine and ATP could inhibit cell growth and induce apoptosis in gastric cancer-derived cell lines GT3-TKB and HGC-27, acting mainly through adenosine P1 receptors [110, 111]. In addition, adenosine promotes cell invasion, metastasis, and the expression of genes related to cell stemness and EMT, probably due to the activation and upregulation of the adenosine receptor AdoR2A, which is associated with poor prognosis and survival in gastric cancer, and the subsequent PI3K-Akt-mTOR signaling pathway enhancement [112].
Regarding P2 receptors, it has been shown by cDNA microarray analysis and RT-PCR that higher levels of P2RY2 transcripts are present in fresh gastric cancer biopsies compared to adjacent healthy tissue samples [106]. Our group studied the role of purinergic signaling in gastric cancer pathophysiology, finding that whereas the activation of P2Y2R promotes cell proliferation, P2X4R activation decreases cell proliferation. Changes in the expression patterns of these receptors, as observed in gastric cancer-derived cell lines and primary cultures, can direct ATP and nucleotide signaling from antiproliferative effects in healthy tissues to proliferative effects in cancer, and therefore constitute a potential target for new gastric cancer therapies [107]. According to these results, high expression levels of P2Y2R are found in most of the studied gastric cancer-derived cell lines, making this receptor a candidate for further investigation.
Another study has shown that the activation of the UDP-preferring P2Y6R decreases gastric cancer proliferation and tumor growth, an opposite effect to those observed in colorectal and pancreatic cancer. This P2Y6R-dependent mechanism involves store-operated calcium entry (SOCE) and calcium-dependent inhibition of the β-catenin pathway [108], which is different from the canonical SOCE/calcium-induced apoptosis that has been observed in other types of cancer. Concordantly, the expression of this receptor is downregulated in gastric cancer-derived cells and gastric tumors [108].
A recent study has shown an increase of P2X7R expression in gastric cancer-derived tissue, as compared to normal gastric tissue [109]. Moreover, Kaplan-Meier analysis showed that this increase was associated with lower survival of gastric cancer patients. It was suggested that these effects of P2X7R in gastric cancer were related to the activation of ERK1/2 and Akt pathways and the increase of the EMT markers N-cadherin, vimentin, and snail [109].
Prostate
Prostate cancer (PCa) is ranked second in incidence, with 30.7/100,000 cases, and sixth in mortality worldwide, with 7.7/100,000 deaths (Globocan, https://gco.iarc.fr/). PCa includes epithelial, mesenchymal, neuroendocrine, hematolymphoid, and miscellaneous tumors, but the epithelial type is the most abundant; specifically, acinar adenocarcinoma [176]. The search for molecular markers is in progress, and purinergic elements represent good candidates (Table 2).
In 1992, Fang and collaborators were among the first researchers to study hormone refractory prostate cancer cells (HRPCs) before P2 receptors were cloned [177]. They observed that ATP, in a range of 100 nM to 100 mM, elicited transient increments in [Ca2+]i. The nucleotide also incremented inositol phosphate turnover, suggesting that the effects were mediated by Gαq proteins. Moreover, ATP also induced strong inhibition of cell proliferation (greater than 90%) [177], opening the possibility for the purinergic system to be considered as a therapeutic target for PCa.
From these early observations, purinergic signaling has been thoroughly described in diverse PCa-derived cells. Thus, P2XRs expression has been investigated in HRPCs including PC3 and DU145. PC3 cells express P2X3R, P2X4R, P2X5R, and P2X7R, whereas DU145 cells express P2X4R and P2X5R [68]. Incubation of PC3 or DU145 cells with ATP (100 mM) inhibited cell growth and induced apoptotic cell death in a way that is sensitive to the antagonists suramin and PPADS [178, 179]. Interestingly, ATP did not inhibit cell growth effectively in PNT2, a prostatic non-cancerous cell line [178]. Cell growth inhibition was not induced by UTP or ADO, but BzATP mimicked the effect in both cell lines, suggesting the participation of P2XRs; pharmacological characterization suggested the specific participation of P2X5R [64].
P2X7R expression was investigated in human normal or carcinoma prostate biopsies [65]. This receptor was absent in normal prostate epithelium but was consistently detected in the 116 carcinoma samples. Besides, high P2X7R expression was detected in 1E8 (highly metastatic) and 2B4 (non-metastatic) prostate cancer cell lines (clones derived from the PC3 line), while the non-malignant prostate epithelial cell line BPH1 showed extremely low levels of this receptor [66]. Stimulation of P2X7R with ATP (1 mM) or BzATP (100 mM) in both 1E8 or 2B4 cells induced an increment in [Ca2+]i and promoted in vitro invasiveness; concomitantly, an increment in the expression of EMT induction-related genes (Snail, IL-8, and MMP-3) and a reduction of the epithelial markers (E-Cadherin and Claudin-1) were detected. Furthermore, the degree of tumor invasion in the kidney or lymph nodes by xenografted prostate carcinoma cells in nude mice was attenuated when P2X7R was silenced in transplanted cells [66].
A study using PC3, DU145, and LNCaP cells, which express a P2X7R variant named not-pore function P2X7R (nfP2X7R), which is a loss of function variant of the channel, described that knocking down this receptor induces apoptotic cell death, suggesting that nfP2X7R is essential for the survival of these prostatic cancer cells [67]. Regarding P2YRs, PC3 cells express P2Y1R, P2Y2R, P2Y4R, P2Y6R, whereas DU145 cells express P2Y11R [64, 178, 179]. As mentioned, ATP inhibited the growth of both cell lines. Pharmacological data also suggest that P2Y11R participates in this effect [68]. Androgen-sensitive LNCaP cells also express P2Y2R, P2Y6R, and P2Y11R [64].
Stimulation with MRS2365, a selective agonist of P2Y1R, induced an increment in the [Ca2+]i, inhibited cell proliferation, and triggered apoptotic cell death in PC3 cells. These responses were blocked by the corresponding selective antagonist MRS2500 or by knocking down P2Y1R [69]. In addition, PC3 and Du-145 PCa incubation with new agonists based on the 1-indolinoalkyl 2-phenolic compound that binds P2Y1R promoted caspase 3/7 induction and the ROS signaling pathway [70]. All together, these observations suggest that P2Y1R ligands are potential therapeutic targets.
On the other hand, in the PC3 cell subclones, 1E8 and 2B4, it was described that P2YRs stimulation with ATP induced cell invasiveness through a pathway involving ERK1/2 and p38 kinases; this action was blocked by suramin [180]. In these cell lines, in contrast with P2Y1R actions, it was shown that P2Y2R activation induced an increment in cell migration and invasion by inducing EMT [71]. This effect was accomplished through a pathway that involved transactivation of epidermal growth factor receptor (EGFR) and the downstream actions of ERK1/2 which induced overexpression of IL-18 [72].
The effects mediated by ADO have also been investigated in DU-145, PC3, and LNCaP-FGC10 cells. These cell lines express transcripts corresponding to the 4 subtypes of AdoR (ADORA1, ADORA2A, ADORA2B, and ADORA3). Stimulation with ADO for 48 h reduced cell proliferation in a concentration-dependent manner by arresting the cell cycle in the G0/G1 phase and induced apoptotic cell death; the latter was inhibited by preincubation with the AdoR3 antagonist MRS1220 [73]. Studies comparing receptor abundance in DU-145 and PC3 lines determined that the most enriched was AdoR2B [74]. Functional experiments in PC3 cells revealed that stimulation with NECA or BAY606583 induced an increment in cAMP. NECA also diminished apoptosis induced by TNF-α and cycloheximide and promoted cell proliferation; all these effects were blocked by the antagonist PSB603 [74].
One of the mechanisms elicited by ADO receptors to induce apoptosis in PCa cells, but not normal prostate cells, is the increased generation of ROS concomitant with the decreased expression of the antioxidant peptide glutathione. Thus, in androgen-independent and -sensitive cells (PC3 and LNCaP), the stimulation with 2-chloroadenosine, a non-selective agonist of AR, induced the reduction of viability and apoptotic cell death modifying ROS and glutathione levels. In primary prostate cells, 2-chloroadenosine did not show any effect [181].
On the other hand, in PC3 cells, low μM concentrations of ADO reduced cell migration and invasiveness by a receptor-independent action. The inhibitory mechanism involved ADO uptake and derivative metabolites, its intracellular conversion into ADP and ATP, and regulation of intracellular pathways (e.g., phospho-AMPK1α activation) [182].
Interestingly, it was observed that nucleotide hydrolysis incremented in the serum of PCa subjects compared with normal subjects. AMP hydrolysis was sensible at the CD73 inhibitor adenosine 5′-(α,β-methylene)diphosphate (APCP) and the alkaline phosphatase inhibitor levamisole. Besides, it was noted that patients who received radiotherapy displayed higher AMP hydrolysis, whereas patients at late clinical stages presented low ATP hydrolysis in comparison with patients at early clinical stages [183]. These observations reveal that ADO is systemically elevated in PCa; however, its implications should be further explored.
Cervix
Cervicouterine cancer is the seventh most lethal type of cancer in the world. It has an ASR of 7.3/100,000 and caused 341,831 deaths in 2020 (Globocan, https://gco.iarc.fr/). Cervical carcinogenesis is related to specific types of papillomavirus (HPV) infection of cervical epithelium cells [184]; however, multiple factors including purines can contribute to the establishment of a tumor microenvironment that favors cancer development.
Initial approaches exploring the effects of purinergic agonists on the cervical epithelium were performed in normal human ectocervical epithelial cells (hECE). Placing hECE in culture promoted spontaneous apoptotic cell death, which was blocked by the ectonucleotidase apyrase, indicating that purinergic signaling was involved. Researchers using pharmacological approaches determined that spontaneous apoptotic hECE induction was mediated by a pathway initiated by P2X7R, which induced the activation of the mitochondrial-dependent apoptotic machinery by incrementing [Ca2+]i. It was further determined that this pathway is active in the metastatic carcinoma cervix line CaSki, and that both hECE and CaSki lines release ATP to the extracellular media [75]. Interestingly, a truncated variant of P2X7R in CaSki cells was isolated. This isoform, known as P2X7-J, lacks the second transmembrane domain and carboxy terminus, displays diminished ligand binding, and is unable to form pores and induce apoptosis [76, 77]. However, P2X7-J reaches the plasmatic membrane and interacts with wild-type P2X7R, possibly forming heteromers and modulating P2X7R function. Notably, P2X7-J levels are similar in normal and cancer epithelial cervix cells, but P2X7R displays a higher expression in normal tissues than in cancerous ones, suggesting that in cancer cells P2X7-J negatively modulates the function of P2X7R by dampening its apoptotic function in cervix carcinoma cells [76, 77].
A study by Li et al. [78] analyzed the expression of P2X7R in human cervix biopsies (42 normal and 47 cancerous samples). This receptor was expressed in epithelial cells of normal endometrial, endocervical, and ectocervical tissue, but in cancer samples (endocervical adenocarcinoma or cervical squamous cell carcinoma) P2X7R was not detected. In addition, the relative abundance of P2X7R, protein and transcript, showed a good correlation to distinguish healthy tissue (high expression) from cancer tissue (null expression) [78]; thus, it was suggested that the absence of P2X7R can be a biomarker of cervix carcinoma.
Regarding to P2YRs, first it was shown that HeLa cells respond to extracellular nucleotides (ATP, UTP, ADP, and UDP) with intracellular Ca2+ oscillations; pharmacological approaches indicated that these responses were elicited by P2YR. By RT-PCR, it was demonstrated the expression of P2Y2R, P2Y4R, and P2Y6R, physiological experiments suggested a role for P2Y4R and P2Y6R in cell proliferation induction [79]. Other studies have shown that P2Y2R stimulation induces c-Fos and Erk1/2 activity and through this pathway, it promotes proliferation of HeLa cells [80]; moreover, P2Y2R and P2Y6R activation in this cell line inhibits the activity of Na+/K+-ATPase [81]. Activation of P2Y6R expressed in HeLa cells with UDP also promotes intracellular Ca2+ mobilization, and activation of conventional and atypical PKCs to induce cell proliferation [82]. P2Y6R activation with, thymidine 5′-Omonophosphorothioate (TMPS), a partial agonist, also reproduce this effect [185]. P2Y1R has also been described in HeLa cells; its ligation transactivates epidermal growth factor receptor (EGFR) to promote cell proliferation throughout a pathway that involves PKC, Src, and cell surface metalloproteases. Moreover, incubation of HeLa with apyrase or with a P2Y1R antagonist reduced basal proliferation [186].
On the other hand, it was proposed that the cytotoxic effect of extracellular ATP on the cervix carcinoma cell lines (SiHa, HeLa, and C33A) required the conversion of ATP into ADO, and that the accumulation of the nucleoside promoted the cellular response [187]. Interestingly, SiHa, HeLa, and C33A lines express ectonucleotidases and display low nucleotide hydrolysis activity, which is consistent with a reduced expression of E-NTPDase. Also, the NTPDases 5 and 6, ecto-5-nucleotidase/CD73, and NPP1, 2, and 3 were detected, indicating that these cells can transform ATP into ADO [188]. These groups of enzymes are relevant because their activity impacts tumor microenvironment composition and modulates purinergic signaling. In HeLa and SiHa cells, treatment with ADO inhibited migration and invasion and favored the epithelial phenotype inducing an increment in E-cadherin expression and low of N-cadherin and fibronectin. Moreover, it was showed that Ado induced apoptotic cell death of this cell lines, by a mitochondria-dependent pathway [189].
It has been proposed that in the stroma of the solid tumors, around 0.01% of cells are tumor mesenchymal stem cells (tMSC), which are multipotent cells with high differentiation capacities that regulate tumor cell proliferation, EMT induction, as well as angiogenesis and metastasis [190]. Cervical cancer-derived tMSC (cctMSC) express higher levels of CD39 and CD73 ectonucleotidases if compared with MSC from healthy tissue. The expression of these enzymes promotes the synthesis of high levels of ADO that suppress the function of cytotoxic T cells, thus inhibiting the anti-tumor immune response [191]. A recently published review also looked at the current understanding of purinergic signaling and cervical cancer [192]. A summary of purinergic elements characterized in the cervix is shown in Table 2.
Esophagus
Esophageal cancer is the eight most lethal cancer worldwide, with an ASR of 5.6/100,000 inhabitants and 544,076 deaths in 2020 (Globocan, https://gco.iarc.fr/). Incidence rates vary in different geographic locations. In some regions, higher rates of esophageal cancer may be attributed to tobacco and alcohol use or nutritional habits and obesity.
As in pancreatic cancer, research about purinergic signaling in esophageal malignancies has centered on the role of P2Y2R and P2X7 receptors (Table 3). According to the little information available, P2Y2R activation via ATP, UTP, and ATPγS can lead to cell cycle arrest, apoptosis induction, and reduced cell proliferation of human esophageal cancer primary cultures and Kyse-140, a human squamous esophageal cancer cell line [113]. On the contrary, P2X7R activation by ATP leads to a decrease in cell number, proliferation, and migration in Kyse-30, Kyse-450, and Kyse-520 cells, which have different P2XRs and P2YRs expression patterns [114], highlighting the need for further investigation about the functions of P2X7R and P2Y2R in each environment in order to be used for esophageal cancer therapies.
Pancreas
Pancreatic cancer has an ASR of 4.5/100,000 inhabitants and caused 466,003 deaths in 2020 (Globocan, https://gco.iarc.fr/). Its estimated 5-year survival rate is below 5% in developed and underdeveloped economies. Furthermore, its response to traditional chemotherapy is mostly inefficient, and diagnostic tools fail to detect this disease in the early stages. Thus, pancreatic cancer research has focused on describing novel signaling pathways involved that could be used as novel therapeutic or diagnostic agents.
Early reports of purinergic signaling in pancreatic cancer have shown that tissue samples from patients undergoing partial pancreaticoduodenectomy had higher protein and mRNA levels of P2X7R and P2Y2R in comparison to their healthy counterparts [123]. Recent investigations have elucidated the potential role of P2X7R and P2Y2R in the onset, survival, migration, and invasion of pancreatic cancer (Table 3).
Authors have reported that P2X7R participates in different cell processes in pancreatic cancer. Many reports have centered on human pancreatic ductal adenocarcinoma (PDAC), which is one of the deadliest forms of pancreatic cancer, describing that P2X7R promotes cell migration and invasion, and contributes to cell proliferation/cell death equilibrium in PDAC in vitro models like AsPC-1, BxPC-3, Capan-1, Panc-1, and MiaPaCa-2 cells [193]. Reports using pancreatic stellate cells (PSC) showed a two-sided effect of P2X7R, promoting cell proliferation using ATP concentrations up to 100 μM, but inducing cell death at higher concentrations. This effect on PSC is interesting, because these cells are an essential component to sustain PDAC and its chemoresistance [126]. The effects of P2X7R activation via ATP on the increase of cell proliferation were explained in MiaPaCa-2 cells by PKC-ERK1/2-JNK pathway induction [194], supporting the idea of using P2X7R as a target for novel therapeutics. To further explore this goal, NMRI-Foxn1nu mice were transplanted with cells from the PancTu-1 human PDAC cell line, and used to test the in vivo effects of AZ10606120, a P2X7R allosteric inhibitor. These orthotopic xenografts formed poorly differentiated tumors with high expression levels of P2X7R and EGFR, and AZ10606120 reduced tumor growth but did not have significant effects suppressing cell invasion and metastasis development [127]. When these authors evaluated AZ10606120 effects in vitro using co-cultures of PancTu-1 cells with murine PSC, they found a decrease on cell migration and a reduction on PDAC/PSC interaction, an essential feature in pancreatic cancer, where P2X7R hyperstimulation can lead to an abnormal secretion of extracellular matrix proteins (e.g., collagen and laminin) by PSC, which contributes to an increase of PDAC hardness, a factor that reduce the efficacy of chemotherapeutic agents [127]. Dissimilar results on in vivo models were observed by Mohammed et al., who found that two P2X7R antagonists (A438079 and AZ10606120) induced cancer spread, suppressed apoptotic mediators, and elicited pancreatic cancer progression in orally treated p48Cre/+-LSL-KrasG12D/+ mice [128]. These reports clarify the need for further investigation about the role of P2X7R in pancreatic cancer. A proper understanding of the signaling pathways involved could lead to the development of successful strategies for treatment or therapeutic approaches that could consider P2X7R inhibitors more as coadjuvant drugs than as first-line standalone agents.
Regarding P2Y2R, some reports show that it stimulates Panc-1 cell proliferation after treatment with UTP or MRS2768. This effect is dependent on the PI3K/Akt pathway and reversed using anti-P2Y2R siRNA [124]. These data were supported by works on human PDAC samples that found P2Y2R hyperexpression. Stimulation of P2Y2R promoted cell proliferation and triggered PI3K/AKT–mTOR crosstalk with PDGFR via Yes1. Furthermore, P2Y2R inhibition by AR-C118925XX reduced tumor growth and progression on a subcutaneous and orthotopic xenograft model in athymic male nu/nu mice. These in vivo effects were synergized using an association of AR-C118925XX and gemcitabine, resulting in better prognosis for the evaluated mice [125].
Ovarian cancer
The ovary is the organ responsible of folliculogenesis and the secretion of steroid hormones [195]. In each follicular cycle culminating in ovulation, the ovary undergoes changes in architecture, which involves many control elements. This constant tissue remodeling makes the ovary susceptible to carcinogenesis. In 2020, ovarian cancer (OCa) was the eighth most frequent and tenth most lethal cancer in the world (Globocan, https://gco.iarc.fr/). OCa is a group of diseases with heterogeneous origin and characteristics: epithelial ovarian cancer (EOC) represents more than 90% of tumors in the ovary; they are classified as serous, mucinous, endometrioid, and clear cell [196]. It has been proposed that EOC is originated by (a) a malignant transformation in ovarian surface epithelium (OSE) and (b) tumor cells originated from precursor lesions in the endometrium and uterine horn which eventually colonize the ovary [197]. Considering its phenotypic diversity, ovarian cancer has multiple origins.
Although the physiological role of purines is not completely understood in the ovary, it is clear that this organ expresses functional purinergic receptors in the main cellular types of the follicle, theca, and granulosa cells, as well as in the OSE [198]. Early studies about purinergic actions in ovarian cancer cells were based in carcinoma-derived cell lines (CdCL) obtained from ascitic bodies, which are cellular bodies detached from the primary tumor that are disseminated to the abdominal cavity [199]. To date, several purinergic elements have been identified in ovarian carcinoma (Table 2).
In initial studies centered on the ability of purines to induce intracellular Ca2+ mobilization, the stimulation with ATP (mM range) of SKOV-3 and OVCAR-3 lines promoted a biphasic increment in [Ca2+]i. This response showed two features: (1) a fast and transient increment and (2) a slow and sustained phase. The last phase was abrogated by intracellular Ca2+ chelation, indicating the role played by intracellular reservoirs, while the fast transient was originated by Ca2+ influx. These observations revealed the presence of both P2YRs and P2XRs. Moreover, ATP stimulation of SKOV-3 and OVCAR-3 induced cell proliferation [200, 201].
UTP-sensitive P2Y2Rs were identified in the EFO21 and EFO27 CdCL, where their stimulation elicited an increment in [Ca2+]i [85] as well as in the OSE derived lines: IOSE29 (preneoplastic) and IOSE29EC (neoplastic) [86]. However, the effects on cell proliferation were antagonistic, since ATP downregulated basal and fetal serum-induced cell proliferation in EFO21 and EFO27 [85], whereas in IOSE29 and IOSE29EC, ATP favored cell proliferation by a pathway that involved MAPK/ERK1/2 activity [86].
The mechanisms supporting the cellular invasiveness ability are important in cancer biology. In OCa, groups of cells within the tumor undergo EMT, acquiring migratory capability; these cells can form cell bodies that separate from the primary tumor and are scattered toward the peritoneal cavity where they become part of the ascitic fluid and can invade other organs [199]. The activity of P2Y2R in the SKOV-3 cell line enhanced its migration capability, while P2Y2R knockdown abolished cell migration. Cell migration induced by the P2Y2R-selective agonist UTP was accompanied by enhanced expression of Snail and Twist transcripts and an accumulation of the intermediate filament vimentin, evidencing EMT induction. Also, it was shown that these effects depended on EGFR transactivation [87]. In contrast, ADO accumulation in the extracellular space favored an epithelial phenotype in SKOV-3 cells [202].
Regarding P2XRs, it was demonstrated that P2X7R exhibits an incremented and scattered expression in cancerous ovaries [83, 84]. A detailed analysis showed that P2X7R was strongly expressed in transformed zones from mucinous, borderline, and endometrial tumors. In SKOV-3 and CAOV-3 cell lines, the blocking of P2X7R with AZ10606120 and oxATP reduced cell viability; furthermore, stimulation of P2X7R elicited a concentration-dependent phosphorylation of ERK and AKT proteins. Altogether, these data suggested a tumor growth supporting role for this receptor in OCa [203].
Expression of AdoR1, AdoR2A, and AdoR2B, but not AdoR3, was described in A2780 and HEY lines. It was also reported that ADO showed moderate cytotoxicity over these CdCL, inducing low viability and apoptotic cell death at high concentrations (hundreds of mM levels). ADO showed the ability to increase the sensitivity to cisplatin in both CdCL. Surprisingly, the effects were not sensible to the AdoR1 or AdoR2B antagonist but to dipyridamole. These actions are receptor-independent [204]. In addition, the expression of all four AdoR was reported in CAOV-4, SKOV-3, and OVCAR-3 lines, being AdoR2B and AdoR3 the most abundant [88]. In CAOV-4 and OVCAR-3, NECA, a non-selective AdoR agonist, reduced cell viability and induced apoptotic cell death [89].
Besides, it is well known that the main role of ADO in cancer is the induction of immune evasion, mainly by acting through receptors expressed in specialized cells [152]. In a recent meta-analysis encompassing 13 independent data sets including more than 1500 patients, Stagg’s group concluded that high expression of CD73 ectonucleotidase is a poor prognosis factor for patients with C1/mesenchymal high-grade serous carcinoma when it is expressed in cancerous cells and stromal regions, mainly in tumor-associated fibroblast [90, 91].
Evidence generated in biopsies and SKOV-3 and OaW42 CdCL indicates that CD39 and CD73 expressed in OCa and tumor stroma cells contribute to the anti-tumor immune response by favoring T regulatory (Treg) accumulation and, consequently, inhibiting CD4+ T cells and natural killer (NK) cell activity [92]. From these observations, it was proposed that antibodies against CD39 and CD73 could be used as immunotherapy strategies in ovarian cancer [205]. Moreover, carcinoma cells expressing CD39 and CD73 ectonucleotidases showed the ability to attract myeloid cells (blood monocytes), which depended on the activity of these ecto-enzymes. In contact with CdCL, myeloid cells differentiate into M2 tumor-associated macrophages (TAMs), which also express CD39 and CD73. TAMs that can reduce CD4+ T cell proliferation in the tumor microenvironment contribute to tumor immunosuppression [93].
Concluding remarks
Cancer is a complex disease that affects thousands of people around the world. Intense efforts have been made to understand the defining characteristics of cancer cells and the process that enables their implantation, growth, and spread in the host organism. It has been defined that outstanding characteristics of cancer cells, such as proliferation rate, phenotypic plasticity, and the ability to modify the environment, are highly regulated by autocrine-paracrine mechanisms. As shown in this review of the ten most lethal cancers, purines have emerged as important intercellular messengers that mediate autocrine-paracrine signaling, with a ubiquitous presence in cancer, regardless of the affected tissue and that contribute with cancer progression.
From the analysis of purinergic signaling effects on all cancer types throughout this review, we highlighted the regulatory effects of the purinergic system on the composition of TME. Importantly, purinergic signaling mediates the social behavior of tumor cells and regulates the interaction with the host, modulating immune signals; this can be translated into whether the host will develop an anti-tumor response. In this context, four purinome elements attract our attention: (1) ectonucleotidase CD73, (2) ectonucleotidase CD39, (3) AdoR2A, and (4) P2X7R, as shown in the text, the coordinated actions of this molecules state the nature of the microenvironment with decisive consequences for cancer progression. Future research in diverse types of cancers will confirm whether it is a general mechanism to generate favorable conditions for tumor growth and spread. In addition to these four proteins, there are other interesting candidates as potential purinergic targets in cancer therapies, one of them is the P2Y2R, that is overexpressed and contributes to tumor-cell proliferation in several types of cancer including the ten most lethal that we have reviewed in the present work.
On the other hand, an important cell population in tumor are CSCs; these cells are related to carcinogenesis, resistance to chemotherapy and radiotherapy, and tumor recurrence; as shown in this review, purinergic signaling in breast cancer CSCs [54] exerts regulatory actions that can be exploited for new therapies; however, there is a lack of information in other types of cancer that must be cover in the next years. This field of study in expansion is promissory for the emergence of new general concepts in cancer understanding and for developing new therapies.
Therefore, the study of the processes regulated by purinergic system in cancer models represents opportunities to understand the mechanisms underlying the disease progression and also to find new pharmacological targets to improve the existing anti-cancer therapies or introduce novel treatments.
Acknowledgements
This work was funded by grants from Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica-UNAM (PAPIIT-UNAM) #IN202620 to F.G.V.-C., #IN202121 to M.D.-M.; Consejo Nacional de Ciencia y Tercnología (CONACYT-México) #284-557 to M.D.M.; Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT-Chile) Regular Grant # 1161490, FONDEQUIP-Chile EQM140100; and Millennium Nucleus for the Study of Pain (MiNuSPain) to C.C. MiNuSPain is a Millennium Nucleus supported by the Millennium Science Initiative of the Ministry of Science, Technology, Knowledge and Innovation (Chile). We are grateful to Jessica González Norris for proofreading and to Biol. Jazmín Vázquez-Méndez for the art in Fig. 1.
Code availability
Not applicable.
Mauricio Reyna-Jeldes
is a Pharmaceutical Chemist graduated from the Universidad de Valparaíso (2012) and a Master in Bioactivity of Natural and Synthetic Products from the same University (2016). He started working on regenerative medicine, describing the involvement of TGF-β3 on wound healing, and developing biocompatible scaffolds to be used with autologous stem cells for treating skin injuries on human patients. His main focus is on describing the molecular mechanisms behind the antiproliferative effects of natural extracts, like rapeseed neutral pectins, and natural and hemisynthetic molecules, like DHA and chalcone derivates, on several types of cancer cell lines. Since 2019, he has been working as Lab Manager on the Purinergic Signalling Lab from the Universidad Católica del Norte, describing the role of receptors P2X2, P2X4 and P2Y2 on the proliferation, invasiveness and dedifferentiation of gastric cancer.

Author’s contribution
C.C. and F.G. V. C, conceived the paper. All the authors wrote and reviewed the paper.
Funding
PAPIIT-UNAM #IN202620 and #IN202121; CONACYT#284-557; FONDECYT# 1161490, FONDEQUIP EQM140100 and MiNuSPain, MiNuSPain is a Millennium Nucleus supported by the Millennium Science Initiative of the Ministry of Science, Technology, Knowledge and Innovation (Chile).
Data availability
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All the authors are in agreement with publish this paper.
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
C. Coddou, Email: ccoddou@ucn.cl
F. G. Vázquez-Cuevas, Email: fvazquez@comunidad.unam.mx
References
- 1.Kitadai NMS. Origins of building blocks of life: a review. Geosci Front. 2018;9:1117–1153. doi: 10.1016/j.gsf.2017.07.007. [DOI] [Google Scholar]
- 2.Raczynska EDKB. Prototopy and π-electron delocalization for purines and its radical ions—DFT studies. J Phys Org Chem. 2010;23:828–835. doi: 10.1002/poc.1668. [DOI] [Google Scholar]
- 3.Henderson JFPA (1973) Chapter 2—configuration and conformation of nucleosides and nucleotides. Nucleotide metabolism—an introduction Academic Press, New York:22–27
- 4.Nguyen MD, Lee ST, Ross AE, Ryals M, Choudhry VI, Venton BJ. Characterization of spontaneous, transient adenosine release in the caudate-putamen and prefrontal cortex. PLoS One. 2014;9(1):e87165. doi: 10.1371/journal.pone.0087165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burnstock G. Purine and purinergic receptors. Brain Neurosci Adv. 2018;2:2398212818817494. doi: 10.1177/2398212818817494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacobson KA, Muller CE. Medicinal chemistry of adenosine, P2Y and P2X receptors. Neuropharmacology. 2016;104:31–49. doi: 10.1016/j.neuropharm.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng LL, Cai YQ, Zhu MC, Xing LJ, Wang X. The yin and yang functions of extracellular ATP and adenosine in tumor immunity. Cancer Cell Int. 2020;20:110. doi: 10.1186/s12935-020-01195-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stefan C, Jansen S, Bollen M. Modulation of purinergic signaling by NPP-type ectophosphodiesterases. Purinergic Signal. 2006;2(2):361–370. doi: 10.1007/s11302-005-5303-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zimmermann FF, Altenhofen S, Kist LW, Leite CE, Bogo MR, Cognato GP, Bonan CD. Unpredictable chronic stress alters adenosine metabolism in zebrafish brain. Mol Neurobiol. 2016;53(4):2518–2528. doi: 10.1007/s12035-015-9270-7. [DOI] [PubMed] [Google Scholar]
- 10.Novitskaya T, Chepurko E, Covarrubias R, Novitskiy S, Ryzhov SV, Feoktistov I, Gumina RJ. Extracellular nucleotide regulation and signaling in cardiac fibrosis. J Mol Cell Cardiol. 2016;93:47–56. doi: 10.1016/j.yjmcc.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 11.Stefan C, Jansen S, Bollen M. NPP-type ectophosphodiesterases: unity in diversity. Trends Biochem Sci. 2005;30(10):542–550. doi: 10.1016/j.tibs.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Zimmermann H, Zebisch M, Strater N. Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal. 2012;8(3):437–502. doi: 10.1007/s11302-012-9309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colgan SP, Eltzschig HK, Eckle T, Thompson LF. Physiological roles for ecto-5'-nucleotidase (CD73) Purinergic Signal. 2006;2(2):351–360. doi: 10.1007/s11302-005-5302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strater N. Ecto-5'-nucleotidase: Structure function relationships. Purinergic Signal. 2006;2(2):343–350. doi: 10.1007/s11302-006-9000-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colombo M, Moita C, van Niel G, Kowal J, Vigneron J, Benaroch P, Manel N, Moita LF, Thery C, Raposo G. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci. 2013;126(Pt 24):5553–5565. doi: 10.1242/jcs.128868. [DOI] [PubMed] [Google Scholar]
- 16.Whiteside TL. The potential of tumor-derived exosomes for noninvasive cancer monitoring. Expert Rev Mol Diagn. 2015;15(10):1293–1310. doi: 10.1586/14737159.2015.1071666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Volonte C, D'Ambrosi N. Membrane compartments and purinergic signalling: the purinome, a complex interplay among ligands, degrading enzymes, receptors and transporters. FEBS J. 2009;276(2):318–329. doi: 10.1111/j.1742-4658.2008.06793.x. [DOI] [PubMed] [Google Scholar]
- 18.Di Virgilio F, Adinolfi E. Extracellular purines, purinergic receptors and tumor growth. Oncogene. 2017;36(3):293–303. doi: 10.1038/onc.2016.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dos Santos-Rodrigues A, Grane-Boladeras N, Bicket A, Coe IR. Nucleoside transporters in the purinome. Neurochem Int. 2014;73:229–237. doi: 10.1016/j.neuint.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 20.Fouad YA, Aanei C. Revisiting the hallmarks of cancer. Am J Cancer Res. 2017;7(5):1016–1036. [PMC free article] [PubMed] [Google Scholar]
- 21.Clunes MT, Kemp PJ. P2u purinoceptor modulation of intracellular Ca2+ in a human lung adenocarcinoma cell line: down-regulation of Ca2+ influx by protein kinase C. Cell Calcium. 1996;20(4):339–346. doi: 10.1016/s0143-4160(96)90039-1. [DOI] [PubMed] [Google Scholar]
- 22.Schafer R, Sedehizade F, Welte T, Reiser G. ATP- and UTP-activated P2Y receptors differently regulate proliferation of human lung epithelial tumor cells. Am J Phys Lung Cell Mol Phys. 2003;285(2):L376–L385. doi: 10.1152/ajplung.00447.2002. [DOI] [PubMed] [Google Scholar]
- 23.Zhao DM, Xue HH, Chida K, Suda T, Oki Y, Kanai M, Uchida C, Ichiyama A, Nakamura H. Effect of erythromycin on ATP-induced intracellular calcium response in A549 cells. Am J Phys Lung Cell Mol Phys. 2000;278(4):L726–L736. doi: 10.1152/ajplung.2000.278.4.L726. [DOI] [PubMed] [Google Scholar]
- 24.Kreda SM, Okada SF, van Heusden CA, O'Neal W, Gabriel S, Abdullah L, Davis CW, Boucher RC, Lazarowski ER. Coordinated release of nucleotides and mucin from human airway epithelial Calu-3 cells. J Physiol. 2007;584(Pt 1):245–259. doi: 10.1113/jphysiol.2007.139840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takai E, Tsukimoto M, Harada H, Sawada K, Moriyama Y, Kojima S. Autocrine regulation of TGF-beta1-induced cell migration by exocytosis of ATP and activation of P2 receptors in human lung cancer cells. J Cell Sci. 2012;125(Pt 21):5051–5060. doi: 10.1242/jcs.104976. [DOI] [PubMed] [Google Scholar]
- 26.Takai E, Tsukimoto M, Harada H, Kojima S. Autocrine signaling via release of ATP and activation of P2X7 receptor influences motile activity of human lung cancer cells. Purinergic Signal. 2014;10(3):487–497. doi: 10.1007/s11302-014-9411-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmid S, Kubler M, Korcan Ayata C, Lazar Z, Haager B, Hossfeld M, Meyer A, Cicko S, Elze M, Wiesemann S, Zissel G, Passlick B, Idzko M. Altered purinergic signaling in the tumor associated immunologic microenvironment in metastasized non-small-cell lung cancer. Lung Cancer. 2015;90(3):516–521. doi: 10.1016/j.lungcan.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Song S, Jacobson KN, McDermott KM, Reddy SP, Cress AE, Tang H, Dudek SM, Black SM, Garcia JG, Makino A, Yuan JX (2016) ATP promotes cell survival via regulation of cytosolic [Ca2+] and Bcl-2/Bax ratio in lung cancer cells. Am J Phys Cell Phys 310(2):C99–C114. 10.1152/ajpcell.00092.2015 [DOI] [PMC free article] [PubMed]
- 29.Tak E, Jun DY, Kim SH, Park GC, Lee J, Hwang S, Song GW, Lee SG. Upregulation of P2Y2 nucleotide receptor in human hepatocellular carcinoma cells. J Int Med Res. 2016;44(6):1234–1247. doi: 10.1177/0300060516662135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cao Y, Wang X, Li Y, Evers M, Zhang H, Chen X. Extracellular and macropinocytosis internalized ATP work together to induce epithelial-mesenchymal transition and other early metastatic activities in lung cancer. Cancer Cell Int. 2019;19:254. doi: 10.1186/s12935-019-0973-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schneider G, Glaser T, Lameu C, Abdelbaset-Ismail A, Sellers ZP, Moniuszko M, Ulrich H, Ratajczak MZ. Extracellular nucleotides as novel, underappreciated pro-metastatic factors that stimulate purinergic signaling in human lung cancer cells. Mol Cancer. 2015;14:201. doi: 10.1186/s12943-015-0469-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ryzhov S, Zaynagetdinov R, Goldstein AE, Novitskiy SV, Blackburn MR, Biaggioni I, Feoktistov I. Effect of A2B adenosine receptor gene ablation on adenosine-dependent regulation of proinflammatory cytokines. J Pharmacol Exp Ther. 2008;324(2):694–700. doi: 10.1124/jpet.107.131540. [DOI] [PubMed] [Google Scholar]
- 33.Giacomelli C, Daniele S, Romei C, Tavanti L, Neri T, Piano I, Celi A, Martini C, Trincavelli ML. The A2B Adenosine Receptor Modulates the Epithelial- Mesenchymal Transition through the Balance of cAMP/PKA and MAPK/ERK Pathway Activation in Human Epithelial Lung Cells. Front Pharmacol. 2018;9:54. doi: 10.3389/fphar.2018.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim SJ, Min HY, Chung HJ, Park EJ, Hong JY, Kang YJ, Shin DH, Jeong LS, Lee SK. Inhibition of cell proliferation through cell cycle arrest and apoptosis by thio-Cl-IB-MECA, a novel A3 adenosine receptor agonist, in human lung cancer cells. Cancer Lett. 2008;264(2):309–315. doi: 10.1016/j.canlet.2008.01.037. [DOI] [PubMed] [Google Scholar]
- 35.Tafani M, Schito L, Pellegrini L, Villanova L, Marfe G, Anwar T, Rosa R, Indelicato M, Fini M, Pucci B, Russo MA. Hypoxia-increased RAGE and P2X7R expression regulates tumor cell invasion through phosphorylation of Erk1/2 and Akt and nuclear translocation of NF-{kappa}B. Carcinogenesis. 2011;32(8):1167–1175. doi: 10.1093/carcin/bgr101. [DOI] [PubMed] [Google Scholar]
- 36.Xia J, Yu X, Tang L, Li G, He T. P2X7 receptor stimulates breast cancer cell invasion and migration via the AKT pathway. Oncol Rep. 2015;34(1):103–110. doi: 10.3892/or.2015.3979. [DOI] [PubMed] [Google Scholar]
- 37.Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, Vermaelen K, Panaretakis T, Mignot G, Ullrich E, Perfettini JL, Schlemmer F, Tasdemir E, Uhl M, Genin P, Civas A, Ryffel B, Kanellopoulos J, Tschopp J, Andre F, Lidereau R, McLaughlin NM, Haynes NM, Smyth MJ, Kroemer G, Zitvogel L. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15(10):1170–1178. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- 38.Avanzato D, Genova T, Fiorio Pla A, Bernardini M, Bianco S, Bussolati B, Mancardi D, Giraudo E, Maione F, Cassoni P, Castellano I, Munaron L. Activation of P2X7 and P2Y11 purinergic receptors inhibits migration and normalizes tumor-derived endothelial cells via cAMP signaling. Sci Rep. 2016;6:32602. doi: 10.1038/srep32602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joo YN, Jin H, Eun SY, Park SW, Chang KC, Kim HJ. P2Y2R activation by nucleotides released from the highly metastatic breast cancer cell MDA-MB-231 contributes to pre-metastatic niche formation by mediating lysyl oxidase secretion, collagen crosslinking, and monocyte recruitment. Oncotarget. 2014;5(19):9322–9334. doi: 10.18632/oncotarget.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li HJ, Wang LY, Qu HN, Yu LH, Burnstock G, Ni X, Xu M, Ma B. P2Y2 receptor-mediated modulation of estrogen-induced proliferation of breast cancer cells. Mol Cell Endocrinol. 2011;338(1-2):28–37. doi: 10.1016/j.mce.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 41.Chadet S, Jelassi B, Wannous R, Angoulvant D, Chevalier S, Besson P, Roger S. The activation of P2Y2 receptors increases MCF-7 breast cancer cells migration through the MEK-ERK1/2 signalling pathway. Carcinogenesis. 2014;35(6):1238–1247. doi: 10.1093/carcin/bgt493. [DOI] [PubMed] [Google Scholar]
- 42.Eun SY, Ko YS, Park SW, Chang KC, Kim HJ. P2Y2 nucleotide receptor-mediated extracellular signal-regulated kinases and protein kinase C activation induces the invasion of highly metastatic breast cancer cells. Oncol Rep. 2015;34(1):195–202. doi: 10.3892/or.2015.3972. [DOI] [PubMed] [Google Scholar]
- 43.Zhang JL, Liu Y, Yang H, Zhang HQ, Tian XX, Fang WG. ATP-P2Y2-beta-catenin axis promotes cell invasion in breast cancer cells. Cancer Sci. 2017;108(7):1318–1327. doi: 10.1111/cas.13273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jin H, Ko YS, Kim HJ. P2Y2R-mediated inflammasome activation is involved in tumor progression in breast cancer cells and in radiotherapy-resistant breast cancer. Int J Oncol. 2018;53(5):1953–1966. doi: 10.3892/ijo.2018.4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ma X, Pan X, Wei Y, Tan B, Yang L, Ren H, Qian M, Du B. Chemotherapy-induced uridine diphosphate release promotes breast cancer metastasis through P2Y6 activation. Oncotarget. 2016;7(20):29036–29050. doi: 10.18632/oncotarget.8664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gareau AJ, Brien C, Gebremeskel S, Liwski RS, Johnston B, Bezuhly M. Ticagrelor inhibits platelet-tumor cell interactions and metastasis in human and murine breast cancer. Clin Exp Metastasis. 2018;35(1-2):25–35. doi: 10.1007/s10585-018-9874-1. [DOI] [PubMed] [Google Scholar]
- 47.Mirza A, Basso A, Black S, Malkowski M, Kwee L, Pachter JA, Lachowicz JE, Wang Y, Liu S. RNA interference targeting of A1 receptor-overexpressing breast carcinoma cells leads to diminished rates of cell proliferation and induction of apoptosis. Cancer Biol Ther. 2005;4(12):1355–1360. doi: 10.4161/cbt.4.12.2196. [DOI] [PubMed] [Google Scholar]
- 48.Dastjerdi MN, Valiani A, Mardani M, Ra MZ. Adenosine A1 receptor modifies P53 expression and apoptosis in breast cancer cell line Mcf-7. Bratisl Lek Listy. 2016;117(4):242–246. doi: 10.4149/bll_2016_046. [DOI] [PubMed] [Google Scholar]
- 49.Beavis PA, Milenkovski N, Stagg J, Smyth MJ, Darcy PK. A2A blockade enhances anti-metastatic immune responses. Oncoimmunology. 2013;2(12):e26705. doi: 10.4161/onci.26705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mittal D, Young A, Stannard K, Yong M, Teng MW, Allard B, Stagg J, Smyth MJ. Antimetastatic effects of blocking PD-1 and the adenosine A2A receptor. Cancer Res. 2014;74(14):3652–3658. doi: 10.1158/0008-5472.CAN-14-0957. [DOI] [PubMed] [Google Scholar]
- 51.Petruk N, Tuominen S, Akerfelt M, Mattsson J, Sandholm J, Nees M, Yegutkin GG, Jukkola A, Tuomela J, Selander KS. CD73 facilitates EMT progression and promotes lung metastases in triple-negative breast cancer. Sci Rep. 2021;11(1):6035. doi: 10.1038/s41598-021-85379-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fernandez-Gallardo M, Gonzalez-Ramirez R, Sandoval A, Felix R, Monjaraz E. Adenosine stimulate proliferation and migration in triple negative breast cancer cells. PLoS One. 2016;11(12):e0167445. doi: 10.1371/journal.pone.0167445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Desmet CJ, Gallenne T, Prieur A, Reyal F, Visser NL, Wittner BS, Smit MA, Geiger TR, Laoukili J, Iskit S, Rodenko B, Zwart W, Evers B, Horlings H, Ajouaou A, Zevenhoven J, van Vliet M, Ramaswamy S, Wessels LF, Peeper DS. Identification of a pharmacologically tractable Fra-1/ADORA2B axis promoting breast cancer metastasis. Proc Natl Acad Sci U S A. 2013;110(13):5139–5144. doi: 10.1073/pnas.1222085110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jafari SM, Joshaghani HR, Panjehpour M, Aghaei M. A2B adenosine receptor agonist induces cell cycle arrest and apoptosis in breast cancer stem cells via ERK1/2 phosphorylation. Cell Oncol (Dordr) 2018;41(1):61–72. doi: 10.1007/s13402-017-0359-z. [DOI] [PubMed] [Google Scholar]
- 55.Lan J, Lu H, Samanta D, Salman S, Lu Y, Semenza GL. Hypoxia-inducible factor 1-dependent expression of adenosine receptor 2B promotes breast cancer stem cell enrichment. Proc Natl Acad Sci U S A. 2018;115(41):E9640–E9648. doi: 10.1073/pnas.1809695115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Madi L, Ochaion A, Rath-Wolfson L, Bar-Yehuda S, Erlanger A, Ohana G, Harish A, Merimski O, Barer F, Fishman P. The A3 adenosine receptor is highly expressed in tumor versus normal cells: potential target for tumor growth inhibition. Clin Cancer Res. 2004;10(13):4472–4479. doi: 10.1158/1078-0432.CCR-03-0651. [DOI] [PubMed] [Google Scholar]
- 57.Panjehpour M, Karami-Tehrani F. An adenosine analog (IB-MECA) inhibits anchorage-dependent cell growth of various human breast cancer cell lines. Int J Biochem Cell Biol. 2004;36(8):1502–1509. doi: 10.1016/j.biocel.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 58.Ledderose C, Hefti MM, Chen Y, Bao Y, Seier T, Li L, Woehrle T, Zhang J, Junger WG. Adenosine arrests breast cancer cell motility by A3 receptor stimulation. Purinergic Signal. 2016;12(4):673–685. doi: 10.1007/s11302-016-9531-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Varani K, Vincenzi F, Targa M, Paradiso B, Parrilli A, Fini M, Lanza G, Borea PA. The stimulation of A(3) adenosine receptors reduces bone-residing breast cancer in a rat preclinical model. Eur J Cancer. 2013;49(2):482–491. doi: 10.1016/j.ejca.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 60.Jafari SM, Panjehpour M, Aghaei M, Joshaghani HR, Enderami SE. A3 adenosine receptor agonist inhibited survival of breast cancer stem cells via GLI-1 and ERK1/2 pathway. J Cell Biochem. 2017;118(9):2909–2920. doi: 10.1002/jcb.25945. [DOI] [PubMed] [Google Scholar]
- 61.Samanta D, Park Y, Ni X, Li H, Zahnow CA, Gabrielson E, Pan F, Semenza GL. Chemotherapy induces enrichment of CD47(+)/CD73(+)/PDL1(+) immune evasive triple-negative breast cancer cells. Proc Natl Acad Sci U S A. 2018;115(6):E1239–E1248. doi: 10.1073/pnas.1718197115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qiao Z, Li X, Kang N, Yang Y, Chen C, Wu T, Zhao M, Liu Y, Ji X (2019) A novel specific anti-CD73 antibody inhibits triple-negative breast cancer cell motility by regulating autophagy. Int J Mol Sci 20(5). 10.3390/ijms20051057 [DOI] [PMC free article] [PubMed]
- 63.Turcotte M, Allard D, Mittal D, Bareche Y, Buisseret L, Jose V, Pommey S, Delisle V, Loi S, Joensuu H, Kellokumpu-Lehtinen PL, Sotiriou C, Smyth MJ, Stagg J. CD73 Promotes Resistance to HER2/ErbB2 Antibody Therapy. Cancer Res. 2017;77(20):5652–5663. doi: 10.1158/0008-5472.CAN-17-0707. [DOI] [PubMed] [Google Scholar]
- 64.Janssens R, Boeynaems JM. Effects of extracellular nucleotides and nucleosides on prostate carcinoma cells. Br J Pharmacol. 2001;132(2):536–546. doi: 10.1038/sj.bjp.0703833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Slater M, Danieletto S, Pooley M, Cheng Teh L, Gidley-Baird A, Barden JA. Differentiation between cancerous and normal hyperplastic lobules in breast lesions. Breast Cancer Res Treat. 2004;83(1):1–10. doi: 10.1023/B:BREA.0000010670.85915.0f. [DOI] [PubMed] [Google Scholar]
- 66.Qiu Y, Li WH, Zhang HQ, Liu Y, Tian XX, Fang WG. P2X7 mediates ATP-driven invasiveness in prostate cancer cells. PLoS One. 2014;9(12):e114371. doi: 10.1371/journal.pone.0114371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gilbert SM, Oliphant CJ, Hassan S, Peille AL, Bronsert P, Falzoni S, Di Virgilio F, McNulty S, Lara R. ATP in the tumour microenvironment drives expression of nfP2X7, a key mediator of cancer cell survival. Oncogene. 2019;38(2):194–208. doi: 10.1038/s41388-018-0426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shabbir M, Ryten M, Thompson C, Mikhailidis D, Burnstock G. Characterization of calcium-independent purinergic receptor-mediated apoptosis in hormone-refractory prostate cancer. BJU Int. 2008;101(3):352–359. doi: 10.1111/j.1464-410X.2007.07293.x. [DOI] [PubMed] [Google Scholar]
- 69.Wei Q, Costanzi S, Liu QZ, Gao ZG, Jacobson KA. Activation of the P2Y1 receptor induces apoptosis and inhibits proliferation of prostate cancer cells. Biochem Pharmacol. 2011;82(4):418–425. doi: 10.1016/j.bcp.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Le HTT, Rimpilainen T, Konda Mani S, Murugesan A, Yli-Harja O, Candeias NR, Kandhavelu M. Synthesis and preclinical validation of novel P2Y1 receptor ligands as a potent anti-prostate cancer agent. Sci Rep. 2019;9(1):18938. doi: 10.1038/s41598-019-55194-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li WH, Qiu Y, Zhang HQ, Liu Y, You JF, Tian XX, Fang WG. P2Y2 receptor promotes cell invasion and metastasis in prostate cancer cells. Br J Cancer. 2013;109(6):1666–1675. doi: 10.1038/bjc.2013.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li WH, Qiu Y, Zhang HQ, Tian XX, Fang WG. P2Y2 Receptor and EGFR cooperate to promote prostate cancer cell invasion via ERK1/2 pathway. PLoS One. 2015;10(7):e0133165. doi: 10.1371/journal.pone.0133165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aghaei M, Karami-Tehrani F, Panjehpour M, Salami S, Fallahian F. Adenosine induces cell-cycle arrest and apoptosis in androgen-dependent and -independent prostate cancer cell lines, LNcap-FGC-10, DU-145, and PC3. Prostate. 2012;72(4):361–375. doi: 10.1002/pros.21438. [DOI] [PubMed] [Google Scholar]
- 74.Wei Q, Costanzi S, Balasubramanian R, Gao ZG, Jacobson KA. A2B adenosine receptor blockade inhibits growth of prostate cancer cells. Purinergic Signal. 2013;9(2):271–280. doi: 10.1007/s11302-012-9350-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang Q, Wang L, Feng YH, Li X, Zeng R, Gorodeski GI. P2X7 receptor-mediated apoptosis of human cervical epithelial cells. Am J Phys Cell Phys. 2004;287(5):C1349–C1358. doi: 10.1152/ajpcell.00256.2004. [DOI] [PubMed] [Google Scholar]
- 76.Feng YH, Li X, Zeng R, Gorodeski GI. Endogenously expressed truncated P2X7 receptor lacking the C-terminus is preferentially upregulated in epithelial cancer cells and fails to mediate ligand-induced pore formation and apoptosis. Nucleosides Nucleotides Nucleic Acids. 2006;25(9-11):1271–1276. doi: 10.1080/15257770600890921. [DOI] [PubMed] [Google Scholar]
- 77.Feng YH, Li X, Wang L, Zhou L, Gorodeski GI. A truncated P2X7 receptor variant (P2X7-j) endogenously expressed in cervical cancer cells antagonizes the full-length P2X7 receptor through hetero-oligomerization. J Biol Chem. 2006;281(25):17228–17237. doi: 10.1074/jbc.M602999200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li X, Zhou L, Feng YH, Abdul-Karim FW, Gorodeski GI. The P2X7 receptor: a novel biomarker of uterine epithelial cancers. Cancer Epidemiol Biomark Prev. 2006;15(10):1906–1913. doi: 10.1158/1055-9965.EPI-06-0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Okuda A, Furuya K, Kiyohara T. ATP-induced calcium oscillations and change of P2Y subtypes with culture conditions in HeLa cells. Cell Biochem Funct. 2003;21(1):61–68. doi: 10.1002/cbf.992. [DOI] [PubMed] [Google Scholar]
- 80.Muscella A, Elia MG, Greco S, Storelli C, Marsigliante S. Activation of P2Y2 receptor induces c-FOS protein through a pathway involving mitogen-activated protein kinases and phosphoinositide 3-kinases in HeLa cells. J Cell Physiol. 2003;195(2):234–240. doi: 10.1002/jcp.10242. [DOI] [PubMed] [Google Scholar]
- 81.Muscella A, Elia MG, Greco S, Storelli C, Marsigliante S. Activation of P2Y2 purinoceptor inhibits the activity of the Na+/K+-ATPase in HeLa cells. Cell Signal. 2003;15(1):115–121. doi: 10.1016/s0898-6568(02)00062-1. [DOI] [PubMed] [Google Scholar]
- 82.Muscella A, Greco S, Elia MG, Storelli C, Marsigliante S. Differential signalling of purinoceptors in HeLa cells through the extracellular signal-regulated kinase and protein kinase C pathways. J Cell Physiol. 2004;200(3):428–439. doi: 10.1002/jcp.20033. [DOI] [PubMed] [Google Scholar]
- 83.Adinolfi E, Raffaghello L, Giuliani AL, Cavazzini L, Capece M, Chiozzi P, Bianchi G, Kroemer G, Pistoia V, Di Virgilio F. Expression of P2X7 receptor increases in vivo tumor growth. Cancer Res. 2012;72(12):2957–2969. doi: 10.1158/0008-5472.CAN-11-1947. [DOI] [PubMed] [Google Scholar]
- 84.Vazquez-Cuevas FG, Cruz-Rico A, Garay E, Garcia-Carranca A, Perez-Montiel D, Juarez B, Arellano RO. Differential expression of the P2X7 receptor in ovarian surface epithelium during the oestrous cycle in the mouse. Reprod Fertil Dev. 2013;25(7):971–984. doi: 10.1071/RD12196. [DOI] [PubMed] [Google Scholar]
- 85.Schultze-Mosgau A, Katzur AC, Arora KK, Stojilkovic SS, Diedrich K, Ortmann O. Characterization of calcium-mobilizing, purinergic P2Y(2) receptors in human ovarian cancer cells. Mol Hum Reprod. 2000;6(5):435–442. doi: 10.1093/molehr/6.5.435. [DOI] [PubMed] [Google Scholar]
- 86.Choi KC, Tai CJ, Tzeng CR, Auersperg N, Leung PC. Adenosine triphosphate activates mitogen-activated protein kinase in pre-neoplastic and neoplastic ovarian surface epithelial cells. Biol Reprod. 2003;68(1):309–315. doi: 10.1095/biolreprod.102.006551. [DOI] [PubMed] [Google Scholar]
- 87.Martinez-Ramirez AS, Garay E, Garcia-Carranca A, Vazquez-Cuevas FG. The P2RY2 receptor induces carcinoma cell migration and EMT through cross-talk with epidermal growth factor receptor. J Cell Biochem. 2016;117(4):1016–1026. doi: 10.1002/jcb.25390. [DOI] [PubMed] [Google Scholar]
- 88.Hajiahmadi S, Panjehpour M, Aghaei M, Mousavi S. Molecular expression of adenosine receptors in OVCAR-3, Caov-4 and SKOV-3 human ovarian cancer cell lines. Res Pharm Sci. 2015;10(1):43–51. [PMC free article] [PubMed] [Google Scholar]
- 89.Hajiahmadi S, Panjehpour M, Aghaei M, Shabani M. Activation of A2b adenosine receptor regulates ovarian cancer cell growth: involvement of Bax/Bcl-2 and caspase-3. Biochem Cell Biol. 2015;93(4):321–329. doi: 10.1139/bcb-2014-0117. [DOI] [PubMed] [Google Scholar]
- 90.Turcotte M, Spring K, Pommey S, Chouinard G, Cousineau I, George J, Chen GM, Gendoo DM, Haibe-Kains B, Karn T, Rahimi K, Le Page C, Provencher D, Mes-Masson AM, Stagg J. CD73 is associated with poor prognosis in high-grade serous ovarian cancer. Cancer Res. 2015;75(21):4494–4503. doi: 10.1158/0008-5472.CAN-14-3569. [DOI] [PubMed] [Google Scholar]
- 91.Gaudreau PO, Allard B, Turcotte M, Stagg J. CD73-adenosine reduces immune responses and survival in ovarian cancer patients. Oncoimmunology. 2016;5(5):e1127496. doi: 10.1080/2162402X.2015.1127496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hausler SF, Montalban del Barrio I, Strohschein J, Chandran PA, Engel JB, Honig A, Ossadnik M, Horn E, Fischer B, Krockenberger M, Heuer S, Seida AA, Junker M, Kneitz H, Kloor D, Klotz KN, Dietl J, Wischhusen J. Ectonucleotidases CD39 and CD73 on OvCA cells are potent adenosine-generating enzymes responsible for adenosine receptor 2A-dependent suppression of T cell function and NK cell cytotoxicity. Cancer Immunol Immunother. 2011;60(10):1405–1418. doi: 10.1007/s00262-011-1040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Montalban Del Barrio I, Penski C, Schlahsa L, Stein RG, Diessner J, Wockel A, Dietl J, Lutz MB, Mittelbronn M, Wischhusen J, Hausler SFM. Adenosine-generating ovarian cancer cells attract myeloid cells which differentiate into adenosine-generating tumor associated macrophages - a self-amplifying, CD39- and CD73-dependent mechanism for tumor immune escape. J Immunother Cancer. 2016;4:49. doi: 10.1186/s40425-016-0154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Asif A, Khalid M, Manzoor S, Ahmad H, Rehman AU. Role of purinergic receptors in hepatobiliary carcinoma in Pakistani population: an approach towards proinflammatory role of P2X4 and P2X7 receptors. Purinergic Signal. 2019;15(3):367–374. doi: 10.1007/s11302-019-09675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Manzoor S, Idrees M, Ashraf J, Mehmood A, Butt S, Fatima K, Akbar H, Rehaman IU, Qadri I. Identification of ionotrophic purinergic receptors in Huh-7 cells and their response towards structural proteins of HCV genotype 3a. Virol J. 2011;8:431. doi: 10.1186/1743-422X-8-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Khalid M, Manzoor S, Ahmad H, Asif A, Bangash TA, Latif A, Jaleel S. Purinoceptor expression in hepatocellular virus (HCV)-induced and non-HCV hepatocellular carcinoma: an insight into the proviral role of the P2X4 receptor. Mol Biol Rep. 2018;45(6):2625–2630. doi: 10.1007/s11033-018-4432-0. [DOI] [PubMed] [Google Scholar]
- 97.Liu H, Liu W, Liu Z, Liu Y, Zhang W, Xu L, Xu J. Prognostic value of purinergic P2X7 receptor expression in patients with hepatocellular carcinoma after curative resection. Tumour Biol. 2015;36(7):5039–5049. doi: 10.1007/s13277-015-3155-2. [DOI] [PubMed] [Google Scholar]
- 98.Duan S, Yu J, Han Z, Cheng Z, Liang P. Association between P2RX7 gene and hepatocellular carcinoma susceptibility: a case-control study in a Chinese Han population. Med Sci Monit. 2016;22:1916–1923. doi: 10.12659/msm.895763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Maynard JP, Lee JS, Sohn BH, Yu X, Lopez-Terrada D, Finegold MJ, Goss JA, Thevananther S. P2X3 purinergic receptor overexpression is associated with poor recurrence-free survival in hepatocellular carcinoma patients. Oncotarget. 2015;6(38):41162–41179. doi: 10.18632/oncotarget.6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xie R, Xu J, Wen G, Jin H, Liu X, Yang Y, Ji B, Jiang Y, Song P, Dong H, Tuo B. The P2Y2 nucleotide receptor mediates the proliferation and migration of human hepatocellular carcinoma cells induced by ATP. J Biol Chem. 2014;289(27):19137–19149. doi: 10.1074/jbc.M113.540047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schulien I, Hockenjos B, van Marck V, Ayata CK, Follo M, Thimme R, Hasselblatt P. Extracellular ATP and purinergic P2Y2 receptor signaling promote liver tumorigenesis in mice by exacerbating DNA damage. Cancer Res. 2020;80(4):699–708. doi: 10.1158/0008-5472.CAN-19-1909. [DOI] [PubMed] [Google Scholar]
- 102.Khalid M, Brisson L, Tariq M, Hao Y, Guibon R, Fromont G, Mortadza SAS, Mousawi F, Manzoor S, Roger S, Jiang LH. Carcinoma-specific expression of P2Y11 receptor and its contribution in ATP-induced purinergic signalling and cell migration in human hepatocellular carcinoma cells. Oncotarget. 2017;8(23):37278–37290. doi: 10.18632/oncotarget.16191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shali S, Yu J, Zhang X, Wang X, Jin Y, Su M, Liao X, Yu J, Zhi X, Zhou P. Ecto-5'-nucleotidase (CD73) is a potential target of hepatocellular carcinoma. J Cell Physiol. 2019;234(7):10248–10259. doi: 10.1002/jcp.27694. [DOI] [PubMed] [Google Scholar]
- 104.Ma XL, Shen MN, Hu B, Wang BL, Yang WJ, Lv LH, Wang H, Zhou Y, Jin AL, Sun YF, Zhang CY, Qiu SJ, Pan BS, Zhou J, Fan J, Yang XR, Guo W. CD73 promotes hepatocellular carcinoma progression and metastasis via activating PI3K/AKT signaling by inducing Rap1-mediated membrane localization of P110beta and predicts poor prognosis. J Hematol Oncol. 2019;12(1):37. doi: 10.1186/s13045-019-0724-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bar-Yehuda S, Stemmer SM, Madi L, Castel D, Ochaion A, Cohen S, Barer F, Zabutti A, Perez-Liz G, Del Valle L, Fishman P. The A3 adenosine receptor agonist CF102 induces apoptosis of hepatocellular carcinoma via de-regulation of the Wnt and NF-kappaB signal transduction pathways. Int J Oncol. 2008;33(2):287–295. [PubMed] [Google Scholar]
- 106.Aquea G, Bresky G, Lancellotti D, Madariaga JA, Zaffiri V, Urzua U, Haberle S, Bernal G. Increased expression of P2RY2, CD248 and EphB1 in gastric cancers from Chilean patients. Asian Pac J Cancer Prev. 2014;15(5):1931–1936. doi: 10.7314/APJCP.2014.15.5.1931. [DOI] [PubMed] [Google Scholar]
- 107.Hevia MJ, Castro P, Pinto-Irish K, Reyna-Jeldes M, Rodríguez-Tirado F, Robles-Planells C, Ramírez-Rivera S, Madariaga JA, Gutiérrez F, López J, Barra M, De La Fuente-Ortega E, Bernal G, Coddou C (2019) Differential effects of purinergic signaling in gastric cancer derived cells through P2Y and P2X receptors. Frontiers in Pharmacology in press. 10.3389/fphar.2019.00612 [DOI] [PMC free article] [PubMed]
- 108.Wan H, Xie R, Xu J, He J, Tang B, Liu Q, Wang S, Guo Y, Yang X, Dong TX, Carethers JM, Yang S, Dong H. Anti-proliferative Effects of Nucleotides on Gastric Cancer via a Novel P2Y6/SOCE/Ca(2+)/beta-catenin Pathway. Sci Rep. 2017;7(1):2459. doi: 10.1038/s41598-017-02562-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lili W, Yun L, Tingran W, Xia W, Yanlei S. P2RX7 functions as a putative biomarker of gastric cancer and contributes to worse prognosis. Exp Biol Med (Maywood) 2019;244(9):734–742. doi: 10.1177/1535370219846492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Saitoh M, Nagai K, Nakagawa K, Yamamura T, Yamamoto S, Nishizaki T. Adenosine induces apoptosis in the human gastric cancer cells via an intrinsic pathway relevant to activation of AMP-activated protein kinase. Biochem Pharmacol. 2004;67(10):2005–2011. doi: 10.1016/j.bcp.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 111.Wang MX, Ren LM. Growth inhibitory effect and apoptosis induced by extracellular ATP and adenosine on human gastric carcinoma cells: involvement of intracellular uptake of adenosine. Acta Pharmacol Sin. 2006;27(8):1085–1092. doi: 10.1111/j.1745-7254.2006.00342.x. [DOI] [PubMed] [Google Scholar]
- 112.Shi L, Wu Z, Miao J, Du S, Ai S, Xu E, Feng M, Song J, Guan W. Adenosine interaction with adenosine receptor A2a promotes gastric cancer metastasis by enhancing PI3K-AKT-mTOR signaling. Mol Biol Cell. 2019;30(19):2527–2534. doi: 10.1091/mbc.E19-03-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Maaser K, Hopfner M, Kap H, Sutter AP, Barthel B, von Lampe B, Zeitz M, Scherubl H. Extracellular nucleotides inhibit growth of human oesophageal cancer cells via P2Y(2)-receptors. Br J Cancer. 2002;86(4):636–644. doi: 10.1038/sj.bjc.6600100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Santos AA, Jr, Cappellari AR, de Marchi FO, Gehring MP, Zaparte A, Brandao CA, Lopes TG, da Silva VD, Pinto LFR, Savio LEB, Moreira-Souza ACA, Coutinho-Silva R, Paccez JD, Zerbini LF, Morrone FB. Potential role of P2X7R in esophageal squamous cell carcinoma proliferation. Purinergic Signal. 2017;13(3):279–292. doi: 10.1007/s11302-017-9559-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hopfner M, Maaser K, Barthel B, von Lampe B, Hanski C, Riecken EO, Zeitz M, Scherubl H. Growth inhibition and apoptosis induced by P2Y2 receptors in human colorectal carcinoma cells: involvement of intracellular calcium and cyclic adenosine monophosphate. Int J Color Dis. 2001;16(3):154–166. doi: 10.1007/s003840100302. [DOI] [PubMed] [Google Scholar]
- 116.Nylund G, Hultman L, Nordgren S, Delbro DS. P2Y2- and P2Y4 purinergic receptors are over-expressed in human colon cancer. Auton Autacoid Pharmacol. 2007;27(2):79–84. doi: 10.1111/j.1474-8673.2007.00389.x. [DOI] [PubMed] [Google Scholar]
- 117.Limami Y, Pinon A, Leger DY, Pinault E, Delage C, Beneytout JL, Simon A, Liagre B. The P2Y2/Src/p38/COX-2 pathway is involved in the resistance to ursolic acid-induced apoptosis in colorectal and prostate cancer cells. Biochimie. 2012;94(8):1754–1763. doi: 10.1016/j.biochi.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 118.Placet M, Arguin G, Molle CM, Babeu JP, Jones C, Carrier JC, Robaye B, Geha S, Boudreau F, Gendron FP. The G protein-coupled P2Y(6) receptor promotes colorectal cancer tumorigenesis by inhibiting apoptosis. Biochim Biophys Acta Mol basis Dis. 2018;1864(5 Pt A):1539–1551. doi: 10.1016/j.bbadis.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 119.Qian F, Xiao J, Hu B, Sun N, Yin W, Zhu J. High expression of P2X7R is an independent postoperative indicator of poor prognosis in colorectal cancer. Hum Pathol. 2017;64:61–68. doi: 10.1016/j.humpath.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 120.Zhang Y, Ding J, Wang L. The role of P2X7 receptor in prognosis and metastasis of colorectal cancer. Adv Med Sci. 2019;64(2):388–394. doi: 10.1016/j.advms.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 121.Ohana G, Bar-Yehuda S, Arich A, Madi L, Dreznick Z, Rath-Wolfson L, Silberman D, Slosman G, Fishman P. Inhibition of primary colon carcinoma growth and liver metastasis by the A3 adenosine receptor agonist CF101. Br J Cancer. 2003;89(8):1552–1558. doi: 10.1038/sj.bjc.6601315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sakowicz-Burkiewicz M, Kitowska A, Grden M, Maciejewska I, Szutowicz A, Pawelczyk T. Differential effect of adenosine receptors on growth of human colon cancer HCT 116 and HT-29 cell lines. Arch Biochem Biophys. 2013;533(1-2):47–54. doi: 10.1016/j.abb.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 123.Kunzli BM, Berberat PO, Giese T, Csizmadia E, Kaczmarek E, Baker C, Halaceli I, Buchler MW, Friess H, Robson SC. Upregulation of CD39/NTPDases and P2 receptors in human pancreatic disease. Am J Physiol Gastrointest Liver Physiol. 2007;292(1):G223–G230. doi: 10.1152/ajpgi.00259.2006. [DOI] [PubMed] [Google Scholar]
- 124.Choi JH, Ji YG, Lee DH. Uridine triphosphate increases proliferation of human cancerous pancreatic duct epithelial cells by activating P2Y2 receptor. Pancreas. 2013;42(4):680–686. doi: 10.1097/MPA.0b013e318271bb4b. [DOI] [PubMed] [Google Scholar]
- 125.Hu LP, Zhang XX, Jiang SH, Tao LY, Li Q, Zhu LL, Yang MW, Huo YM, Jiang YS, Tian GA, Cao XY, Zhang YL, Yang Q, Yang XM, Wang YH, Li J, Xiao GG, Sun YW, Zhang ZG. Targeting purinergic receptor P2Y2 prevents the growth of pancreatic ductal adenocarcinoma by inhibiting cancer cell glycolysis. Clin Cancer Res. 2019;25(4):1318–1330. doi: 10.1158/1078-0432.CCR-18-2297. [DOI] [PubMed] [Google Scholar]
- 126.Haanes KA, Schwab A, Novak I. The P2X7 receptor supports both life and death in fibrogenic pancreatic stellate cells. PLoS One. 2012;7(12):e51164. doi: 10.1371/journal.pone.0051164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Giannuzzo A, Saccomano M, Napp J, Ellegaard M, Alves F, Novak I. Targeting of the P2X7 receptor in pancreatic cancer and stellate cells. Int J Cancer. 2016;139(11):2540–2552. doi: 10.1002/ijc.30380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mohammed A, Janakiram NB, Madka V, Pathuri G, Li Q, Ritchie R, Biddick L, Kutche H, Zhang Y, Singh A, Gali H, Lightfoot S, Steele VE, Suen CS, Rao CV. Lack of chemopreventive effects of P2X7R inhibitors against pancreatic cancer. Oncotarget. 2017;8(58):97822–97834. doi: 10.18632/oncotarget.22085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hirsch FR, Scagliotti GV, Mulshine JL, Kwon R, Curran WJ, Jr, Wu YL, Paz-Ares L. Lung cancer: current therapies and new targeted treatments. Lancet. 2017;389(10066):299–311. doi: 10.1016/S0140-6736(16)30958-8. [DOI] [PubMed] [Google Scholar]
- 130.Tatur S, Kreda S, Lazarowski E, Grygorczyk R. Calcium-dependent release of adenosine and uridine nucleotides from A549 cells. Purinergic Signal. 2008;4(2):139–146. doi: 10.1007/s11302-007-9059-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Agteresch HJ, Burgers SA, van der Gaast A, Wilson JH, Dagnelie PC. Randomized clinical trial of adenosine 5'-triphosphate on tumor growth and survival in advanced lung cancer patients. Anti-Cancer Drugs. 2003;14(8):639–644. doi: 10.1097/00001813-200309000-00009. [DOI] [PubMed] [Google Scholar]
- 132.Nakamura K, Yoshikawa N, Yamaguchi Y, Kagota S, Shinozuka K, Kunitomo M. Antitumor effect of cordycepin (3'-deoxyadenosine) on mouse melanoma and lung carcinoma cells involves adenosine A3 receptor stimulation. Anticancer Res. 2006;26(1A):43–47. [PubMed] [Google Scholar]
- 133.Fang WG, Tian XX. Identification of a new pro-invasion factor in tumor microenvironment: progress in function and mechanism of extracellular ATP. Beijing Da Xue Xue Bao. 2017;49(2):188–195. [PubMed] [Google Scholar]
- 134.Humphreys BD, Rice J, Kertesy SB, Dubyak GR. Stress-activated protein kinase/JNK activation and apoptotic induction by the macrophage P2X7 nucleotide receptor. J Biol Chem. 2000;275(35):26792–26798. doi: 10.1074/jbc.M002770200. [DOI] [PubMed] [Google Scholar]
- 135.De Marchi E, Orioli E, Dal Ben D, Adinolfi E. P2X7 receptor as a therapeutic target. Adv Protein Chem Struct Biol. 2016;104:39–79. doi: 10.1016/bs.apcsb.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 136.Park JH, Williams DR, Lee JH, Lee SD, Lee JH, Ko H, Lee GE, Kim S, Lee JM, Abdelrahman A, Muller CE, Jung DW, Kim YC. Potent suppressive effects of 1-piperidinylimidazole based novel P2X7 receptor antagonists on cancer cell migration and invasion. J Med Chem. 2016;59(16):7410–7430. doi: 10.1021/acs.jmedchem.5b01690. [DOI] [PubMed] [Google Scholar]
- 137.Boldrini L, Giordano M, Ali G, Melfi F, Romano G, Lucchi M, Fontanini G. P2X7 mRNA expression in non-small cell lung cancer: MicroRNA regulation and prognostic value. Oncol Lett. 2015;9(1):449–453. doi: 10.3892/ol.2014.2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Boldrini L, Giordano M, Ali G, Servadio A, Pelliccioni S, Niccoli C, Mussi A, Fontanini G. P2X7 protein expression and polymorphism in non-small cell lung cancer (NSCLC) J Negat Results Biomed. 2014;13:16. doi: 10.1186/1477-5751-13-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ma J, Li W, Chai Q, Tan X, Zhang K. Correlation of P2RX7 gene rs1718125 polymorphism with postoperative fentanyl analgesia in patients with lung cancer. Medicine (Baltimore) 2019;98(7):e14445. doi: 10.1097/MD.0000000000014445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Benzaquen J, Dit Hreich SJ, Heeke S, Juhel T, Lalvee S, Bauwens S, Saccani S, Lenormand P, Hofman V, Butori M, Leroy S, Berthet JP, Marquette CH, Hofman P, Vouret-Craviari V. P2RX7B is a new theranostic marker for lung adenocarcinoma patients. Theranostics. 2020;10(24):10849–10860. doi: 10.7150/thno.48229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Douguet L, Janho Dit Hreich S, Benzaquen J, Seguin L, Juhel T, Dezitter X, Duranton C, Ryffel B, Kanellopoulos J, Delarasse C, Renault N, Furman C, Homerin G, Feral C, Cherfils-Vicini J, Millet R, Adriouch S, Ghinet A, Hofman P, Vouret-Craviari V. A small-molecule P2RX7 activator promotes anti-tumor immune responses and sensitizes lung tumor to immunotherapy. Nat Commun. 2021;12(1):653. doi: 10.1038/s41467-021-20912-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Heng YJ, Lester SC, Tse GM, Factor RE, Allison KH, Collins LC, Chen YY, Jensen KC, Johnson NB, Jeong JC, Punjabi R, Shin SJ, Singh K, Krings G, Eberhard DA, Tan PH, Korski K, Waldman FM, Gutman DA, Sanders M, Reis-Filho JS, Flanagan SR, Gendoo DM, Chen GM, Haibe-Kains B, Ciriello G, Hoadley KA, Perou CM, Beck AH. The molecular basis of breast cancer pathological phenotypes. J Pathol. 2017;241(3):375–391. doi: 10.1002/path.4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rakha EA, Green AR. Molecular classification of breast cancer: what the pathologist needs to know. Pathology. 2017;49(2):111–119. doi: 10.1016/j.pathol.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 144.Voduc KD, Cheang MC, Tyldesley S, Gelmon K, Nielsen TO. Kennecke H. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol. 2010;28(10):1684–1691. doi: 10.1200/JCO.2009.24.9284. [DOI] [PubMed] [Google Scholar]
- 145.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, Yang H, Amigorena S, Ryffel B, Barrat FJ, Saftig P, Levi F, Lidereau R, Nogues C, Mira JP, Chompret A, Joulin V, Clavel-Chapelon F, Bourhis J, Andre F, Delaloge S, Tursz T, Kroemer G, Zitvogel L. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13(9):1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 146.De Marchi E, Orioli E, Pegoraro A, Sangaletti S, Portararo P, Curti A, Colombo MP, Di Virgilio F, Adinolfi E. The P2X7 receptor modulates immune cells infiltration, ectonucleotidases expression and extracellular ATP levels in the tumor microenvironment. Oncogene. 2019;38(19):3636–3650. doi: 10.1038/s41388-019-0684-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lecciso M, Ocadlikova D, Sangaletti S, Trabanelli S, De Marchi E, Orioli E, Pegoraro A, Portararo P, Jandus C, Bontadini A, Redavid A, Salvestrini V, Romero P, Colombo MP, Di Virgilio F, Cavo M, Adinolfi E, Curti A. ATP release from chemotherapy-treated dying leukemia cells elicits an immune suppressive effect by increasing regulatory T cells and tolerogenic dendritic cells. Front Immunol. 2017;8:1918. doi: 10.3389/fimmu.2017.01918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Amoroso F, Capece M, Rotondo A, Cangelosi D, Ferracin M, Franceschini A, Raffaghello L, Pistoia V, Varesio L, Adinolfi E. The P2X7 receptor is a key modulator of the PI3K/GSK3beta/VEGF signaling network: evidence in experimental neuroblastoma. Oncogene. 2015;34(41):5240–5251. doi: 10.1038/onc.2014.444. [DOI] [PubMed] [Google Scholar]
- 149.Jin H, Eun SY, Lee JS, Park SW, Lee JH, Chang KC, Kim HJ. P2Y2 receptor activation by nucleotides released from highly metastatic breast cancer cells increases tumor growth and invasion via crosstalk with endothelial cells. Breast Cancer Res. 2014;16(5):R77. doi: 10.1186/bcr3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jin H, Kim HJ (2020) NLRC4, ASC and caspase-1 are inflammasome components that are mediated by P2Y2R activation in breast cancer cells. Int J Mol Sci 21(9). 10.3390/ijms21093337 [DOI] [PMC free article] [PubMed]
- 151.Allard B, Beavis PA, Darcy PK, Stagg J. Immunosuppressive activities of adenosine in cancer. Curr Opin Pharmacol. 2016;29:7–16. doi: 10.1016/j.coph.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 152.Campos-Contreras ADR, Diaz-Munoz M, Vazquez-Cuevas FG (2020) Purinergic signaling in the hallmarks of cancer. Cells 9(7). 10.3390/cells9071612 [DOI] [PMC free article] [PubMed]
- 153.Panjehpour M, Castro M, Klotz KN. Human breast cancer cell line MDA-MB-231 expresses endogenous A2B adenosine receptors mediating a Ca2+ signal. Br J Pharmacol. 2005;145(2):211–218. doi: 10.1038/sj.bjp.0706180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Koussemou M, Lorenz K, Klotz KN. The A2B adenosine receptor in MDA-MB-231 breast cancer cells diminishes ERK1/2 phosphorylation by activation of MAPK-phosphatase-1. PLoS One. 2018;13(8):e0202914. doi: 10.1371/journal.pone.0202914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Cekic C, Sag D, Li Y, Theodorescu D, Strieter RM, Linden J. Adenosine A2B receptor blockade slows growth of bladder and breast tumors. J Immunol. 2012;188(1):198–205. doi: 10.4049/jimmunol.1101845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yan J, Li XY, Roman Aguilera A, Xiao C, Jacoberger-Foissac C, Nowlan B, Robson SC, Beers C, Moesta AK, Geetha N, Teng MWL, Smyth MJ. Control of metastases via myeloid CD39 and NK cell effector function. Cancer Immunol Res. 2020;8(3):356–367. doi: 10.1158/2326-6066.CIR-19-0749. [DOI] [PubMed] [Google Scholar]
- 157.Li XY, Moesta AK, Xiao C, Nakamura K, Casey M, Zhang H, Madore J, Lepletier A, Aguilera AR, Sundarrajan A, Jacoberger-Foissac C, Wong C, Dela Cruz T, Welch M, Lerner AG, Spatola BN, Soros VB, Corbin J, Anderson AC, Effern M, Holzel M, Robson SC, Johnston RL, Waddell N, Smith C, Bald T, Geetha N, Beers C, Teng MWL, Smyth MJ. Targeting CD39 in cancer reveals an extracellular ATP- and inflammasome-driven tumor immunity. Cancer Discov. 2019;9(12):1754–1773. doi: 10.1158/2159-8290.CD-19-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Allard B, Pommey S, Smyth MJ, Stagg J. Targeting CD73 enhances the antitumor activity of anti-PD-1 and anti-CTLA-4 mAbs. Clin Cancer Res. 2013;19(20):5626–5635. doi: 10.1158/1078-0432.CCR-13-0545. [DOI] [PubMed] [Google Scholar]
- 159.Ghalamfarsa G, Rastegari A, Atyabi F, Hassannia H, Hojjat-Farsangi M, Ghanbari A, Anvari E, Mohammadi J, Azizi G, Masjedi A, Yousefi M, Yousefi B, Hadjati J, Jadidi-Niaragh F. Anti-angiogenic effects of CD73-specific siRNA-loaded nanoparticles in breast cancer-bearing mice. J Cell Physiol. 2018;233(10):7165–7177. doi: 10.1002/jcp.26743. [DOI] [PubMed] [Google Scholar]
- 160.Yu J, Wang X, Lu Q, Wang J, Li L, Liao X, Zhu W, Lv L, Zhi X, Yu J, Jin Y, Zou Q, Ou Z, Liu X, Zhou P. Extracellular 5'-nucleotidase (CD73) promotes human breast cancer cells growth through AKT/GSK-3beta/beta-catenin/cyclinD1 signaling pathway. Int J Cancer. 2018;142(5):959–967. doi: 10.1002/ijc.31112. [DOI] [PubMed] [Google Scholar]
- 161.Buisseret L, Pommey S, Allard B, Garaud S, Bergeron M, Cousineau I, Ameye L, Bareche Y, Paesmans M, Crown JPA, Di Leo A, Loi S, Piccart-Gebhart M, Willard-Gallo K, Sotiriou C, Stagg J. Clinical significance of CD73 in triple-negative breast cancer: multiplex analysis of a phase III clinical trial. Ann Oncol. 2018;29(4):1056–1062. doi: 10.1093/annonc/mdx730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.de Araujo JB, Kerkhoff VV, de Oliveira Maciel SFV, de Resende ESDT (2021) Targeting the purinergic pathway in breast cancer and its therapeutic applications. Purinergic Signal. 10.1007/s11302-020-09760-9 [DOI] [PMC free article] [PubMed]
- 163.Raut JR, Guan Z, Schrotz-King P, Brenner H. Fecal DNA methylation markers for detecting stages of colorectal cancer and its precursors: a systematic review. Clin Epigenetics. 2020;12(1):122. doi: 10.1186/s13148-020-00904-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Burnstock G. Purinergic signalling in the gastrointestinal tract and related organs in health and disease. Purinergic Signal. 2014;10(1):3–50. doi: 10.1007/s11302-013-9397-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wan HX, Hu JH, Xie R, Yang SM, Dong H. Important roles of P2Y receptors in the inflammation and cancer of digestive system. Oncotarget. 2016;7(19):28736–28747. doi: 10.18632/oncotarget.7518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhang WLC, Huang C, Pu F, Zhu J, Zhu Z (2021) PI3K/Akt/GSK-3β signal pathway is involved in P2X7 receptor-induced proliferation and EMT of colorectal cancer cells. Eur J Pharmacol:174041. 10.1016/j.ejphar.2021.174041 [DOI] [PubMed]
- 167.Velazquez-Miranda E, Diaz-Munoz M, Vazquez-Cuevas FG. Purinergic signaling in hepatic disease. Purinergic Signal. 2019;15(4):477–489. doi: 10.1007/s11302-019-09680-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Horstman DA, Tennes KA, Putney JW., Jr ATP-induced calcium mobilization and inositol 1,4,5-triphosphate formation in H-35 hepatoma cells. FEBS Lett. 1986;204(2):189–192. doi: 10.1016/0014-5793(86)80809-2. [DOI] [PubMed] [Google Scholar]
- 169.Emmett DS, Feranchak A, Kilic G, Puljak L, Miller B, Dolovcak S, McWilliams R, Doctor RB, Fitz JG. Characterization of ionotrophic purinergic receptors in hepatocytes. Hepatology. 2008;47(2):698–705. doi: 10.1002/hep.22035. [DOI] [PubMed] [Google Scholar]
- 170.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 171.Subhash VV, Yeo MS, Tan WL, Yong WP. Strategies and advancements in harnessing the immune system for gastric cancer immunotherapy. J Immunol Res. 2015;2015:308574. doi: 10.1155/2015/308574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ferro A, Peleteiro B, Malvezzi M, Bosetti C, Bertuccio P, Levi F, Negri E, La Vecchia C, Lunet N. Worldwide trends in gastric cancer mortality (1980-2011), with predictions to 2015, and incidence by subtype. Eur J Cancer. 2014;50(7):1330–1344. doi: 10.1016/j.ejca.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 173.Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396(10251):635–648. doi: 10.1016/S0140-6736(20)31288-5. [DOI] [PubMed] [Google Scholar]
- 174.Yuan W, Wang Z, Li J, Li D, Liu D, Bai G, Walsh MP, Gui Y, Zheng XL. Uridine adenosine tetraphosphate induces contraction of circular and longitudinal gastric smooth muscle by distinct signaling pathways. IUBMB Life. 2013;65(7):623–632. doi: 10.1002/iub.1171. [DOI] [PubMed] [Google Scholar]
- 175.Ahn SC, Xu WX, So I, Kim KW, Kang TM. Effects of purinergic agonists on mechanical and electrical activities of gastric smooth muscle of guinea-pig. J Smooth Muscle Res. 1995;31(6):407–410. [PubMed] [Google Scholar]
- 176.Humphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE. The 2016 WHO classification of tumours of the urinary system and male genital organs-part B: prostate and bladder tumours. Eur Urol. 2016;70(1):106–119. doi: 10.1016/j.eururo.2016.02.028. [DOI] [PubMed] [Google Scholar]
- 177.Fang WG, Pirnia F, Bang YJ, Myers CE, Trepel JB. P2-purinergic receptor agonists inhibit the growth of androgen-independent prostate carcinoma cells. J Clin Invest. 1992;89(1):191–196. doi: 10.1172/JCI115562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Calvert RC, Shabbir M, Thompson CS, Mikhailidis DP, Morgan RJ, Burnstock G. Immunocytochemical and pharmacological characterisation of P2-purinoceptor-mediated cell growth and death in PC-3 hormone refractory prostate cancer cells. Anticancer Res. 2004;24(5A):2853–2859. [PubMed] [Google Scholar]
- 179.Shabbir M, Thompson C, Jarmulowiczc M, Mikhailidis D, Burnstock G. Effect of extracellular ATP on the growth of hormone-refractory prostate cancer in vivo. BJU Int. 2008;102(1):108–112. doi: 10.1111/j.1464-410X.2008.07578.x. [DOI] [PubMed] [Google Scholar]
- 180.Chen L, He HY, Li HM, Zheng J, Heng WJ, You JF, Fang WG. ERK1/2 and p38 pathways are required for P2Y receptor-mediated prostate cancer invasion. Cancer Lett. 2004;215(2):239–247. doi: 10.1016/j.canlet.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 181.Minelli A, Bellezza I, Tucci A, Rambotti MG, Conte C, Culig Z. Differential involvement of reactive oxygen species and nucleoside transporters in cytotoxicity induced by two adenosine analogues in human prostate cancer cells. Prostate. 2009;69(5):538–547. doi: 10.1002/pros.20900. [DOI] [PubMed] [Google Scholar]
- 182.Virtanen SS, Kukkonen-Macchi A, Vainio M, Elima K, Harkonen PL, Jalkanen S, Yegutkin GG. Adenosine inhibits tumor cell invasion via receptor-independent mechanisms. Mol Cancer Res. 2014;12(12):1863–1874. doi: 10.1158/1541-7786.MCR-14-0302-T. [DOI] [PubMed] [Google Scholar]
- 183.Gardani CFF, Cappellari AR, de Souza JB, da Silva BT, Engroff P, Moritz CEJ, Scholl JN, Battastini AMO, Figueiro F, Morrone FB. Hydrolysis of ATP, ADP, and AMP is increased in blood plasma of prostate cancer patients. Purinergic Signal. 2019;15(1):95–105. doi: 10.1007/s11302-018-9642-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Schiffman M, Wentzensen N, Wacholder S, Kinney W, Gage JC, Castle PE. Human papillomavirus testing in the prevention of cervical cancer. J Natl Cancer Inst. 2011;103(5):368–383. doi: 10.1093/jnci/djq562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Gendaszewska-Darmach E, Szustak M. Thymidine 5'-O-monophosphorothioate induces HeLa cell migration by activation of the P2Y6 receptor. Purinergic Signal. 2016;12(2):199–209. doi: 10.1007/s11302-015-9492-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Buvinic S, Bravo-Zehnder M, Boyer JL, Huidobro-Toro JP, Gonzalez A. Nucleotide P2Y1 receptor regulates EGF receptor mitogenic signaling and expression in epithelial cells. J Cell Sci. 2007;120(Pt 24):4289–4301. doi: 10.1242/jcs.03490. [DOI] [PubMed] [Google Scholar]
- 187.Mello Pde A, Filippi-Chiela EC, Nascimento J, Beckenkamp A, Santana DB, Kipper F, Casali EA, Nejar Bruno A, Paccez JD, Zerbini LF, Wink MR, Lenz G, Buffon A. Adenosine uptake is the major effector of extracellular ATP toxicity in human cervical cancer cells. Mol Biol Cell. 2014;25(19):2905–2918. doi: 10.1091/mbc.E14-01-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Beckenkamp A, Santana DB, Bruno AN, Calil LN, Casali EA, Paccez JD, Zerbini LF, Lenz G, Wink MR, Buffon A. Ectonucleotidase expression profile and activity in human cervical cancer cell lines. Biochem Cell Biol. 2014;92(2):95–104. doi: 10.1139/bcb-2013-0051. [DOI] [PubMed] [Google Scholar]
- 189.Gao ZW, Wang HP, Dong K, Lin F, Wang X, Zhang HZ. Adenosine inhibits migration, invasion and induces apoptosis of human cervical cancer cells. Neoplasma. 2016;63(2):201–207. doi: 10.4149/204_150723N407. [DOI] [PubMed] [Google Scholar]
- 190.Johann PD, Muller I. Multipotent mesenchymal stromal cells: possible culprits in solid tumors? Stem Cells Int. 2015;2015:914632. doi: 10.1155/2015/914632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.De Lourdes M-GM, Garcia-Rocha R, Morales-Ramirez O, Montesinos JJ, Weiss-Steider B, Hernandez-Montes J, Avila-Ibarra LR, Don-Lopez CA, Velasco-Velazquez MA, Gutierrez-Serrano V, Monroy-Garcia A. Mesenchymal stromal cells derived from cervical cancer produce high amounts of adenosine to suppress cytotoxic T lymphocyte functions. J Transl Med. 2016;14(1):302. doi: 10.1186/s12967-016-1057-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Pfaffenzeller MS, Franciosi MLM, Cardoso AM. Purinergic signaling and tumor microenvironment in cervical Cancer. Purinergic Signal. 2020;16(1):123–135. doi: 10.1007/s11302-020-09693-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Giannuzzo A, Pedersen SF, Novak I. The P2X7 receptor regulates cell survival, migration and invasion of pancreatic ductal adenocarcinoma cells. Mol Cancer. 2015;14:203. doi: 10.1186/s12943-015-0472-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Choi JH, Ji YG, Ko JJ, Cho HJ, Lee DH. Activating P2X7 receptors increases proliferation of human pancreatic cancer cells via ERK1/2 and JNK. Pancreas. 2018;47(5):643–651. doi: 10.1097/MPA.0000000000001055. [DOI] [PubMed] [Google Scholar]
- 195.Freeman M. Neuroendocrine control of the ovarian cycle of the rat. In: Neill J, Knobil, Neill's, editors. Physiology of Reproduction 3rd edAcademic Press. 2006. pp. 2328–2388. [Google Scholar]
- 196.Lheureux S, Gourley C, Vergote I, Oza AM. Epithelial ovarian cancer. Lancet. 2019;393(10177):1240–1253. doi: 10.1016/S0140-6736(18)32552-2. [DOI] [PubMed] [Google Scholar]
- 197.Kuhn E, Kurman RJ, Shih IM. Ovarian cancer is an imported disease: fact or fiction? Curr Obstet Gynecol Rep. 2012;1(1):1–9. doi: 10.1007/s13669-011-0004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Martinez-Ramirez AS, Vazquez-Cuevas FG. Purinergic signaling in the ovary. Mol Reprod Dev. 2015;82(11):839–848. doi: 10.1002/mrd.22537. [DOI] [PubMed] [Google Scholar]
- 199.Yousefi M, Dehghani S, Nosrati R, Ghanei M, Salmaninejad A, Rajaie S, Hasanzadeh M, Pasdar A. Current insights into the metastasis of epithelial ovarian cancer—hopes and hurdles. Cell Oncol (Dordr) 2020;43(4):515–538. doi: 10.1007/s13402-020-00513-9. [DOI] [PubMed] [Google Scholar]
- 200.Batra S, Fadeel I. Release of intracellular calcium and stimulation of cell growth by ATP and histamine in human ovarian cancer cells (SKOV-3) Cancer Lett. 1994;77(1):57–63. doi: 10.1016/0304-3835(94)90348-4. [DOI] [PubMed] [Google Scholar]
- 201.Popper LD, Batra S. Calcium mobilization and cell proliferation activated by extracellular ATP in human ovarian tumour cells. Cell Calcium. 1993;14(3):209–218. doi: 10.1016/0143-4160(93)90068-H. [DOI] [PubMed] [Google Scholar]
- 202.Martinez-Ramirez AS, Diaz-Munoz M, Battastini AM, Campos-Contreras A, Olvera A, Bergamin L, Glaser T, Jacintho Moritz CE, Ulrich H, Vazquez-Cuevas FG. Cellular migration ability is modulated by extracellular purines in ovarian carcinoma SKOV-3 Cells. J Cell Biochem. 2017;118(12):4468–4478. doi: 10.1002/jcb.26104. [DOI] [PubMed] [Google Scholar]
- 203.Vazquez-Cuevas FG, Martinez-Ramirez AS, Robles-Martinez L, Garay E, Garcia-Carranca A, Perez-Montiel D, Castaneda-Garcia C, Arellano RO. Paracrine stimulation of P2X7 receptor by ATP activates a proliferative pathway in ovarian carcinoma cells. J Cell Biochem. 2014;115(11):1955–1966. doi: 10.1002/jcb.24867. [DOI] [PubMed] [Google Scholar]
- 204.Sureechatchaiyan P, Hamacher A, Brockmann N, Stork B, Kassack MU. Adenosine enhances cisplatin sensitivity in human ovarian cancer cells. Purinergic Signal. 2018;14(4):395–408. doi: 10.1007/s11302-018-9622-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Hausler SF, Del Barrio IM, Diessner J, Stein RG, Strohschein J, Honig A, Dietl J, Wischhusen J. Anti-CD39 and anti-CD73 antibodies A1 and 7G2 improve targeted therapy in ovarian cancer by blocking adenosine-dependent immune evasion. Am J Transl Res. 2014;6(2):129–139. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.