Abstract
Fetal growth restriction (FGR) is a complication of pregnancy that reduces birth weight, markedly increases infant mortality and morbidity and is associated with later-life cardiometabolic disease. No specific treatment is available for FGR. Placentas of human FGR infants have low abundance of sodium-coupled neutral amino acid transporter 2 (Slc38a2/SNAT2), which supplies the fetus with amino acids required for growth. We determined the mechanistic role of placental Slc38a2/SNAT2 deficiency in the development of restricted fetal growth, hypothesizing that placenta-specific Slc38a2 knockdown causes FGR in mice. Using lentiviral transduction of blastocysts with a small hairpin RNA (shRNA), we achieved 59% knockdown of placental Slc38a2, without altering fetal Slc38a2 expression. Placenta-specific Slc38a2 knockdown reduced near-term fetal and placental weight, fetal viability, trophoblast plasma membrane (TPM) SNAT2 protein abundance, and both absolute and weight-specific placental uptake of the amino acid transport System A tracer, 14C-methylaminoisobutyric acid (MeAIB). We also measured human placental SLC38A2 gene expression in a well-defined term clinical cohort and found that SLC38A2 expression was decreased in late-onset, but not early-onset FGR, compared with appropriate for gestational age (AGA) control placentas. The results demonstrate that low placental Slc38a2/SNAT2 causes FGR and could be a target for clinical therapies for late-onset FGR.
Keywords: amino acid transport system A, fetal growth restriction, lentivirus, maternal-fetal exchange, MeAIB, syncytiotrophoblast
Introduction
Fetal growth restriction (FGR) is a pregnancy complication characterized by abnormal placental function and low birth weight [1]. FGR severely compromises neonatal survival [2] and infant lifelong health [3,4]. There is currently no cure and the underlying mechanisms remain poorly understood. FGR is associated with reduced placental amino acid transport capacity and reduced umbilical concentrations of amino acids [5–8], which provide substrate for fetal tissue accretion [9–11] and stimulate the secretion of growth hormones in utero [12,13]. In particular, System A amino acid transport capacity is low in the maternal-facing syncytiotrophoblast microvillous plasma membrane of human FGR placentas [14–16], suggesting that impaired placental System A transport contributes to the deficit in fetal nutrient supply.
System A transporters mediate sodium-dependent, active accumulation of small neutral amino acids from the maternal blood into the syncytiotrophoblast epithelium (reviewed in [17]). Active transport of amino acids into the placenta is critical for their transepithelial transfer to the fetus, where amino acid concentrations are higher than in the mother [6,7,18]. Amino acids accumulated by System A also drive the placental uptake of large, branched-chain amino acids, including essential amino acids like leucine, via microvillous membrane System L exchangers [19–21]. System A activity is attributed to three sodium-coupled neutral amino acid transporter (SNAT) protein isoforms: SNAT1, SNAT2 and SNAT4, which have all been localized to the human placental microvillous membrane [22–25]. Human FGR has been linked to reduced placental SNAT2 abundance [22,26].
Experimental manipulations that specifically reduce plasma membrane SNAT2 abundance in primary human trophoblast cells inhibit System A activity in vitro [27], and impaired placental System A activity temporally precedes FGR in undernourished rats and baboons [28–30], suggesting that SNAT2 deficiency mechanistically contributes to the development of FGR. In pregnant rodents, systemic pharmacological inhibition of System A activity, using a non-metabolizable, competitive synthetic substrate, reduces fetal weight [31]. Moreover, in mouse embryos, placental and fetal size are both diminished by global deletion of either Slc38a1, Slc38a2 or Slc38a4, which encode SNAT1, SNAT2 and SNAT4, respectively [32]. Global deletion of Slc38a2 also reduces perinatal survival in mouse pups [33]. Other genetic, environmental and hormonal manipulations that reduce intrauterine growth in mice frequently tend to decrease placental SNAT2 abundance and System A activity, measured by unidirectional maternal–fetal clearance of the tracer methylaminoisobutyric acid (MeAIB) [34–38]. However, to date, there has been no demonstration of a cause-and-effect relationship between low placental SNAT2 abundance and reduced fetal growth in vivo.
FGR is also strongly associated with lower fetal insulin, glucose-stimulated insulin secretion (GSIS), islet size and β-cell number [39]. Decreased amino acid transfer from the placenta to the fetus is concomitant with defective fetal pancreatic islet structure and function in FGR sheep fetuses while chronic fetal amino acid infusion restores fetal GSIS, pancreatic vascularity, islet size and β-cell mass [40,41]. However, there is no definitive in vivo genetic evidence of placental amino acid transporter involvement in regulating fetal islet function.
Clinically, FGR is increasingly recognized as either early- or late-onset disease. By definition, early- and late-onset FGR are diagnosed before and after 32 weeks of gestation, respectively, and have distinct phenotypes [42–44]. Early-onset FGR is strongly associated with pre-eclampsia, maternal vascular malperfusion of the placenta, abnormal umbilical artery Doppler measurements, fetal hypoxia, preterm delivery and fetal demise [44–46]. Contrastingly, late-onset FGR is less commonly associated with pre-eclampsia, vascular abnormalities or fetal mortality but affects a larger proportion of pregnancies, is a common cause of stillbirth and carries a similar burden of poor long-term neurodevelopmental and cardiometabolic outcomes in the neonate [44]. The respective contribution of placental amino acid transport to early- and late-onset FGR has not been investigated, and the etiology of late-onset FGR is particularly unclear.
We hypothesized that placenta-specific Slc38a2 knockdown causes FGR and impairs fetal islet GSIS in mice. We used a lentiviral vector to deliver a small hairpin (sh) RNA (shRNA) gene silencing construct to the blastocyst trophectoderm in early gestation [47–51] and subsequently determined placental and fetal weight, in vivo placental amino acid transport capacity and placental amino acid transporter abundance near term. We also measured GSIS in isolated fetal islets from fetuses with placenta-specific Slc38a2 knockdown. Finally, we quantified human placental SLC38A2 gene expression in placentas from pregnancies with early- or late-onset FGR, compared with appropriate for gestational age (AGA) control placentas.
Materials and methods
Lentiviral vectors
The shRNA gene silencing plasmid, targeting the mouse Slc38a2 mRNA (Slc38a2KD), was based upon the pLKO.1 vector [52] and obtained from a commercial vendor (VectorBuilder Inc., Chicago, IL, U.S.A.). Targeting sequence (TCACGGTCCCAGTAGTTATTT), hairpin loop (CTCGAG) and guide sequence (AAATAACTACTGGGACCGTGA) were inserted in series into the plasmid backbone, downstream of the human U6 small nuclear 1 promoter. The plasmid backbone also contained an enhanced green fluorescent protein (EGFP) open reading frame expressed from the human phosphoglycerate kinase 1 promoter and an ampicillin resistance gene. A control plasmid (SCR), with scrambled targeting sequence (CCTAAGGTTAAGTCGCCCTCG), was constructed similarly. Escherichia coli glycerol stocks of both plasmids were amplified in overnight cultures of LB broth with 100 μg.ml−1 ampicillin, then DNA was isolated using a commercially available kit (Plasmid Maxi kit, Qiagen). Targeting and control plasmids were then packaged into vesicular stomatitis virus G pseudotyped lentiviral particles by co-transfecting 293FT cells with the isolated DNA (440 ng.ml−1, with Qiagen Polyfect 1% v/v in culture medium), together with packaging (330 ng.ml−1 pCMV delta 8.9) and envelope plasmids (140 ng.ml−1 pCMV-VSV-G [53]), obtained from the Functional Genomics Shared Resource at the University of Colorado Cancer Centre. Human 293FT (female embryonic kidney epithelial) cells were purchased from Thermo Fisher Scientific and cultured at 37°C in complete medium (Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum, 0.1 mM non-essential amino acids, 6 mM glutamine, 1 mM sodium pyruvate and 1% pen–strep). Lentiviral particles were isolated from the filtered culture medium 72 h after transfection by ultracentrifugation over 20% sucrose (22000 rpm, 2 h) then resuspended in PBS. The functional titer of each batch of lentivirus, in transforming units per ml, was quantified by transducing 293FT cells with serial dilutions of the viral suspension then determining the percentage of EGFP fluorescent cells using flow cytometry.
Lentiviral transduction of mouse blastocysts and surgical embryo transfer
All animal procedures were carried out at the University of Colorado with approval from the Institutional Animal Care and Use Committee (Protocol #344). B6D2F1 mice were purchased from Charles River Laboratories (Wilmington, MA, U.S.A.) and CD-1 mice were purchased from Jackson Laboratories (Bar Harbor, ME, U.S.A.). All animals were maintained under standard 14-h:10-h light:dark conditions with ad libitum access to food and water. Female B6D2F1 mice (n=140), aged <4 weeks, were injected with pregnant mare serum gonadotrophin (5 I.U., i.p., Prospec, East Brunswick, NJ, U.S.A.) and human chorionic gonadotrophin (5 I.U., i.p., Sigma–Aldrich, St Louis, MO, U.S.A.) to induce superovulation, then mated overnight with stud B6D2F1 males. Successfully mated females were identified by the presence of a copulatory plug the following morning, which was designated embryonic day (E) 0.5 (term ≈ E19.5). On E3.5, pregnant females were killed by CO2 asphyxiation and cervical dislocation, their uteri excised and each horn flushed with 5 ml of pre-warmed M2 medium (M7167, Sigma–Aldrich, St. Louis, MO, U.S.A.). Flushed blastocysts were denuded of the zona pellucida by serially incubating in three drops of acidic Tyrodes solution (∼10 s each), then washed and incubated in embryo culture medium (EmbryoMax Advanced KSOM Embryo medium, MR-101-D, Millipore). The trophectoderm was then transduced with either Slc38a2KD or SCR by incubating batches of five to ten blastocysts with either 5 × 105 or 5 × 106 transforming units of lentivirus in a total volume of 20 µl, for 4 h. Blastocysts were subsequently washed in 12 drops of embryo culture medium and surgically transferred to pseudopregnant female CD-1 recipients. All in vitro manipulations were performed at 37°C and under low-light conditions.
Female CD-1 embryo recipient mice (n=74) were mated overnight with vasectomized B6D2F1 males, to induce pseudopregnancy, and underwent surgical embryo transfer 2.5 days post-copulation. Recipients were given pre-operative analgesia (meloxicam, 1 mg/kg, i.p.) then anesthetized with isofluorane (2%, inhaled). With the animal in sternal recumbency and under aseptic conditions, a 0.5-cm incision was made in the skin of the flank overlying the right ovary, 1 cm caudal to the rib cage. Ovary, oviduct and distal uterine horn were exteriorized through the body wall and a 26-ga needle used to puncture the uterus below the oviductal junction. Lentivirus-transduced blastocysts [7–12] were then gently inserted into the uterine horn in a minimal volume of culture medium. The uterus and oviducts were then returned to the body cavity, the body wall closed with a single absorbable suture (5.0 vicryl) and the skin closed with a 9-mm wound clip. The procedure was then repeated for the left ovary, with Slc38a2KD and SCR transduced blastocysts transferred to contralateral horns of the same uterus and randomized to left and right in each recipient. Post-operatively, recipient females were recovered from anesthesia on a heated mat and housed in pairs.
In vivo transplacental amino acid clearance and tissue collection
Fetal and placental phenotype were assessed on E17.5 (n=16) or E18.5 (n=58). System A and System L-mediated placental amino acid transport capacity were measured in a subset of E18.5 pregnant recipient females (n=16), by measuring maternal–placental and maternal–fetal clearance of non-metabolizable, radiolabeled 14C-methylaminoisobutryic acid (14C-MeAIB) and 3H-leucine, respectively. Dams were anesthetized using ketamine (60 mg/kg) and xylazine (6 mg/kg, both i.p.) and placed on a heated mat. The lateral tail vein was cannulated and flushed with heparinized saline using a 28-ga needle attached to a 0.5-ml insulin syringe, via polyethylene (PE20) tubing (0.38 mm I.D., ∼12 cm length, B.D. Intramedic 427405, Becton Dickinson, NJ, U.S.A.). A combined bolus of 14C-MeAIB (50 µCi/kg, specific activity 58.7 mCi/mmol) and 3H-leucine (250 µCi/kg, specific activity 60000 mCi/mmol) was delivered to the tail vein, with flushing. At exactly 1.5, 3 or 4.5 min later, the dam was killed with sodium pentobarbital (390 mg/ml, 100 µl, i.v.) and a cardiac blood sample was rapidly collected into a heparinized syringe. The blood was then centrifuged (12000 rpm, 4 min, 4°C) and the supernatant plasma separated and frozen. The uterus was exposed via laparotomy and the number of viable fetuses and non-viable resorptions counted in each horn. Fetuses and placentas were dissected from the uterus and membranes, blotted briefly and weighed. Individual fetuses and placentas were then solubilized in Biosol (1 ml for placenta, 3 ml for fetus, National Diagnostics) at 55°C, overnight. Aliquots of maternal plasma and fetal digestate were subsequently cleared with hydrogen peroxide and their radioactivity determined by liquid scintillation counting. Initial experiments determined that the ratio of fetal to maternal plasma radioactivity per unit volume increased linearly with respect to time until at least 3 min after injection of both tracers, indicating unidirectional maternal–fetal flux in this time period (Supplementary Figure S5). Therefore, a clearance curve of maternal plasma radioactivity per ml versus time from tracer injection was constructed from these experiments and used to calculate net maternal–placental and maternal–fetal clearance of 14C-MeAIB and 3H-leucine for all pregnant dams killed at 1.5 or 3 min after tracer injection, as described [54]. The remaining recipient dams that did not undergo tracer measurements (n=58) were killed by CO2 asphyxiation and cervical dislocation and fetuses and placentas counted, weighed and snap-frozen in liquid N2. In a subset of litters (n=6), fetal liver, heart, brain and skeletal muscle were dissected and pooled for SCR and Slc38a2KD fetuses, within each litter. In a different subset of litters (n=3), placentas were dissected into their constituent labyrinthine and junctional zones and frozen for gene expression analysis. In all cases, embryo implantation rate was determined as the sum of viable and resorbed conceptuses divided by number of embryos transferred, while viability rate was determined as the number of viable conceptuses divided by number of embryos transferred.
Gene expression analysis
RNA was extracted from frozen placentas and fetal tissues and reverse transcribed using commercially available kits (RNeasy Plus Mini kit, Qiagen and High-Capacity cDNA RT kit, Invitrogen). The expression of Slc38a1, Slc38a2 and Slc38a4 was determined by SYBR Green qRT-PCR using the relative standard curve method, relative to RNA28S. Primer sequences are given in Supplementary Table S4.
Trophoblast plasma membrane isolation
For analyses of transporter and signaling protein abundance, pools of SCR and Slc38a2KD placentas were created from multiple litters. Pools contained between 3 and 11 individual placentas and were matched such that each Slc38a2KD pool (n=10) comprised placentas from the same litters as the corresponding SCR pool (n=10). Pooled frozen tissue was homogenized in buffer D (250 mM sucrose, 10 mM Hepes-Tris and 1 mM EDTA (pH 7.4)) with protease and phosphatase inhibitors. Trophoblast plasma membrane (TPM) vesicles were isolated from the homogenate using differential ultracentrifugation and Mg2+ precipitation, as previously described [55]. Briefly, homogenates were serially centrifuged at 10000×g (10 min, 4°C) and 125000 rpm (30 min, 4°C) to remove tissue debris and nuclei. The resultant pellet was resuspended and the TPM precipitated by addition of MgCl2 (12 mM) with stirring, on ice. Precipitated TPM was then isolated by further ultracentrifugation (33000 rpm, 30 min, 4°C), resuspended and vesiculated using a Dounce homogenizer. Homogenate and TPM protein content were determined by bicinchoninic acid assay and the enrichment of the preparation determined by the ratio of alkaline phosphatase activity, per unit protein in the TPM compared with crude homogenate. Average TPM enrichment ratios were similar in SCR (8.9 ± 1.2) and Slc38a2KD (7.7 ± 0.8) pools (P=0.275).
Fetal pancreatic islet isolation and measurement of glucose‐stimulated insulin secretion
Fetal islet function was measured in a subset of litters at E18.5 (n=7). Fetuses were dissected, glucose concentrations in trunk blood measured using a hand-held glucometer and fetal pancreata dissected and pooled for SCR and Slc38a2KD conceptuses within each litter. Pancreata were digested at 37°C for 10–15 min with Hanks’ balanced salt solution (Life Technologies) containing 2.5% bovine serum albumin (BSA) (wt/vol; Sigma), 0.35 g/l NaHCO3 (Sigma) and 2 mg/ml Collagenase P (Roche). Digested tissue was then washed in the cold, supplemented Hanks’ balanced salt solution without collagenase. Islets were isolated by histopaque gradient centrifugation and washed. Isolated islets were snap-frozen in liquid N2 or cultured (50/sample) in RPMI medium for 2 h, preconditioned in Krebs–Ringer bicarbonate buffer for 90 min and then incubated for 60 min with either 2.8 or 10.0 mM glucose in the same buffer. Subsequently, the supernatant was collected and stored at −80°C for later analysis of insulin content by ELISA (Alpco, NH, U.S.A.).
Immunoblotting
SNAT2 abundance in TPM (n=9 paired SCR and Slc38a2KD pools) and Pdx1 (pancreatic and duodenal homobox 1) abundance in fetal islet lysates (n=6 paired SCR and Slc38a2KD pools) were determined by Western blot. Equal amounts of protein from each sample pool were resolved on a polyacrylamide gel, under reducing, denaturing conditions, then transferred to polyvinylidene fluoride membrane, overnight. Membranes were probed with rabbit polyclonal antibodies raised against SNAT2 [56] or a commercially available monoclonal Pdx1 antibody (#5679, Cell Signaling Technology) and visualized using an HRP-conjugated secondary antibody and enhanced chemiluminescence reaction, in an automated gel imaging system. Band intensities were quantified by densitometry and normalized for total protein load, determined by amido black staining. Amino acid response and mechanistic target of rapamycin (mTOR) signaling pathway activity in crude placental homogenates was also determined by Western blot for total and phosphorylated forms of eIF2α (Ser51), S6 (Ser235/236), 4EBP1 (Thr37/46) and Akt (Ser473).
Analysis of human placental SLC38A2 expression in FGR and AGA pregnancies
Placentas were collected from pregnant women who were prospectively recruited at University College London Hospital, London, U.K. All women had their Estimated Date of Delivery (EDD) calculated from their last menstrual period (LMP) confirmed by ultrasound in the first trimester. Women with pregnancies affected by early-onset FGR were defined by ultrasound-assessed estimated fetal weight (EFW) < 600 g and <3rd centile, between 20 and 26 + 6 weeks of gestation [46,57]. Women with pregnancies affected by late-onset FGR were defined as having a normal sized fetus at their mid-gestation anomaly scan (EFW ≥ 10th centile for gestation) but had an EFW < 10th centile diagnosed after 32 weeks, and delivered at term. Women with early FGR delivered either preterm (<37 weeks of gestation) or at term (≥37 weeks of gestation). Women with AGA fetuses (10th–95th centile) delivered at term and were recruited as part of a case–control study [58–60]. Exclusion criteria included multiple pregnancy, maternal age < 18 years, fetal structural or chromosomal abnormalities, premature preterm rupture of membranes or maternal bacterial or viral infection.
At delivery of liveborn infants, placental villous tissue was sampled from two points midway between the umbilical cord insertion and margin, dissected free of decidua and chorionic plate and snap-frozen in liquid nitrogen, or placed in RNAlater. RNA was subsequently extracted from tissue stored at −80°C, using a commercially available kit (RNeasy Fibrous Tissue Mini Kit, Qiagen). Following reverse transcription, placental SLC38A2 expression was quantified using Taqman RT-qPCR, normalized to the geometric mean of 18S (ribosomal 18S RNA), GAPDH (glyceraldehyde-3-phosphate dehydrogenase), YWHAZ (tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein ζ) and B2M (β-2-microglobulin).
Statistics
Results from experimental analyses in mice are mean ± SEM unless indicated otherwise. Categorical data on implantation and viability rate were analyzed by Fisher’s exact test. Discontinuous data on litter size were analyzed by non-parametric Mann–Whitney test. Gene expression, morphometric and placental transport data were determined for every viable conceptus within each litter, then a litter mean value for SCR and Slc38a2KD conceptuses was calculated and used for statistical analysis. SCR and Slc38a2KD litter means were compared by two-tailed Student’s t test, paired within each litter. The linear relationship of individual fetal weights with placental Slc38a2 expression or amino acid transport was determined by Pearson’s product–moment correlation. Transporter and signaling protein abundances determined for matched pools of SCR and Slc38a2KD placentas were also compared by paired Student’s t test.
Results from human subjects are median (interquartile range) for continuous data, or number (% of group total) for categorical data. Analyses were based on comparison of subjects within term-delivered AGA, late- and early-onset FGR, and preterm-delivered early-onset FGR study groups. For categorical data, proportions of each group total were compared across study groups by chi-squared test. For continuous data, normality of residuals was assessed by Shapiro–Wilk test then intergroup comparisons were made by one-way ANOVA with Sidak’s multiple comparisons post-hoc or by Kruskal–Wallis test with Dunn’s multiple comparisons post-hoc, as appropriate. Post-hoc comparisons were conducted in a planned manner, between term AGA, late- and early-onset FGR subjects, then separately between term and preterm early-onset FGR subjects. Correlation and regression analyses were performed for subjects delivering at term only. Interdependence of continuous variables was assessed by Pearson’s correlation. The effect of FGR status on placental SLC38A2 expression, adjusted for potential confounding continuous and categorical variables, was determined using main effects multiple linear regression, with SLC38A2 as the dependent variable. In all cases, significance was at P<0.05. Statistical details of experiments can be found in figure and table legends.
Study approval
Human subjects were recruited with written informed consent and ethical approval from U.K. National Health Service Research Ethics Committees (London: Hampstead Research Ethics Committee, REC reference 15/LO/1488 and Stanmore Research Ethics Committee, REC reference 13/LO/1254).
Results
Placenta-specific lentiviral transduction of mouse embryos
B6D2F1 mouse blastocysts were transduced with a lentiviral shRNA vector targeting Slc38a2 (Slc38a2KD), on day (E) 3.5 post-conception, to silence the Slc38a2 mRNA specifically in the trophectoderm cells, which form the definitive placenta [61] (see ‘Materials and methods’ section). Control blastocysts were transduced with a scrambled, non-targeting vector (SCR). Both lentiviral vectors expressed a green fluorescent protein (GFP) reporter driven by the human phosphoglycerate kinase 1 promoter. Irrespective of shRNA construct, blastocysts transduced with either 5 × 105 or 5 × 106 transforming units of lentivirus, but not with lower viral titers, expressed GFP immediately after the 4-h transduction period and 24 h later (Supplementary Figure S1A). When blastocysts were surgically transferred to pseudopregnant CD-1 recipient female mice, those embryos transduced with 5 × 106 transforming units produced fewer viable fetuses near term (1%, 2 fetuses/159 embryos transferred, 10 recipients) and fewer total implantations (8%, 12 implantation sites/159 embryos transferred) than embryos transduced with 5 × 105 transforming units (27% viability rate, 336 fetuses/1255 embryos and 33% implantation rate, 410 implantation sites/1255 embryos; 64 recipients P<0.001 Fisher’s exact test, E17.5 and E18.5 combined). Therefore, subsequent analyses of intrauterine growth used blastocysts incubated with 5 × 105 transforming units of lentivirus. SCR and Slc38a2KD transduced blastocysts were transferred to contralateral horns of each recipient uterus. Ninety-five percent of viable conceptuses (329/336) exhibited placenta-specific GFP reporter expression at term (Supplememtary Figure S1B) and GFP negative conceptuses (5%, 17/336) were excluded from morphometric analyses.
Placenta-specific Slc38a2 knockdown reduces fetal and placental weight
On E17.5, implantation and viability rate were similar in SCR and Slc83a2KD conceptuses (Supplementary Table S1). Slc38a2 expression was 26% lower in Slc38a2KD than SCR placentas (Supplementary Figure S2A). Placenta weight (−15%), but not fetal weight, was lower in Slc38a2KD conceptuses than SCR conceptuses (Supplementary Figure S2B,C). However, there was a trend for fetal weight to be lower by 7% (P=0.059). We therefore sought to determine the effect of placental Slc38a2 knockdown closer to term, at E18.5, because the mouse fetus grows rapidly over this period, gaining weight by two- to three-fold between E16.5 and E18.5 [62].
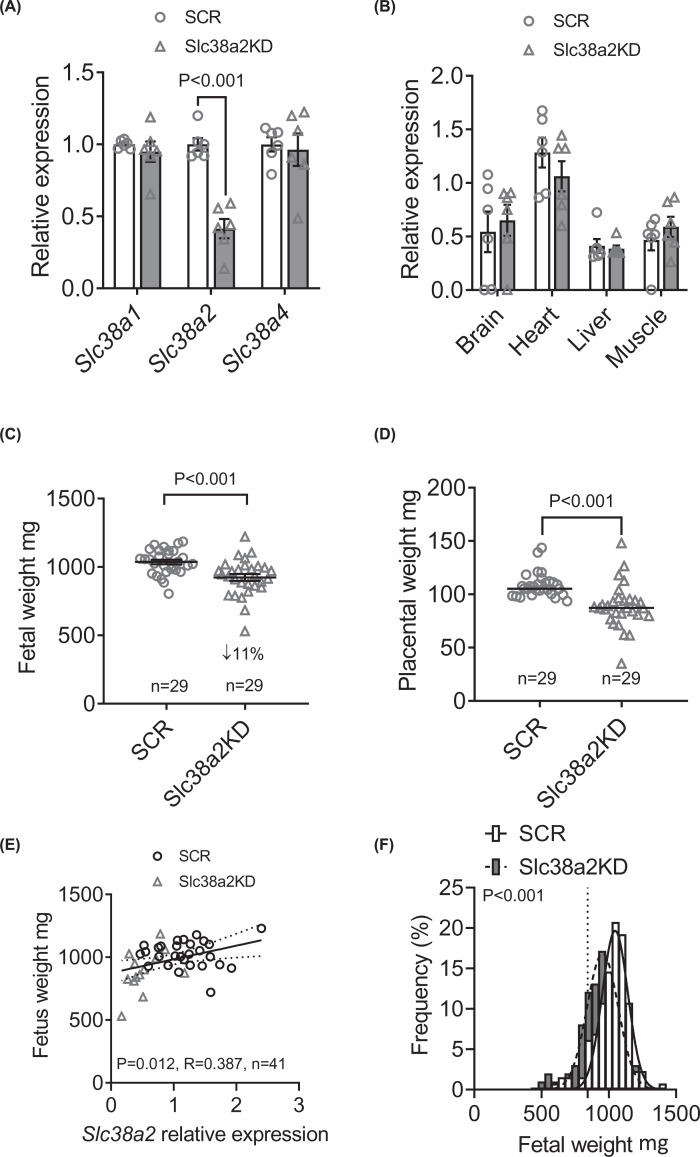
On E18.5, implantation rate was similar in SCR and Slc38a2KD embryos (Table 1). However, a smaller proportion of the Slc38a2KD embryos gave rise to viable fetuses, resulting in fewer viable Slc38a2KD conceptuses than SCR conceptuses per uterine horn (Table 1). Only litters of at least four viable fetuses in total, and at least one viable fetus transduced with each virus, were used for subsequent analyses (median number of fetuses per horn in this subset: SCR, 4; Slc38a2KD, 3, P=0.009, n=29 litters). Placental Slc38a2 mRNA expression was 59% lower in Slc38a2KD conceptuses than SCR conceptuses, whereas Slc38a1 and Slc38a2 expression were similar in the two groups, as expected (Figure 1A). Similarly, when placentas were dissected to separate the nutrient-transporting labyrinthine zone from the hormone-producing junctional zone, Slc38a2KD reduced Slc38a2 mRNA expression in both zones, without altering Slc38a1 or Slc38a2 expression, even though basal expression of all three transporters was higher in the labyrinth than in the junctional zone (Supplementary Figure S3). Slc38a2 mRNA expression in non-placental tissues did not differ between SCR and Slc38a2KD conceptuses, in either the fetal liver, heart, brain or skeletal muscle (Figure 1B), consistent with trophoblast-specific knockdown of the target gene.
Table 1. Implantation and viability rates for SCR and Slc38a2KD transduced embryos at E18.5.
| SCR | Scl38a2KD | P-value | |
|---|---|---|---|
| Total embryos transferred (n) | 550 | 538 | |
| Implanted (n) | 195 | 165 | |
| % | 35% | 31% | 0.0941 |
| Viable fetus at E18.5 | 178 | 113 | |
| % | 32% | 21% | <0.0011 |
| Number of fetuses per uterine horn (median, min-max) | 4 (0–9) | 2 (0–12) | <0.0012 |
Embryos were transferred to 58 recipient dams, of which 47 were pregnant, with any number of implantations, and 11 were non-pregnant at E18.5. SCR and Slc38a2 were compared by 1Fisher’s exact test or 2Mann–Whitney U-test.
Figure 1. Placenta-specific Slc38a2 knockdown reduces placental and fetal weights at E18.5.
(A) Gene expression of Slc38a1, Slc38a2 and Slc38a4 in placentas of SCR and Slc38a2KD conceptuses, n=6 litters (28 SCR placentas, 13 Slc38a2KD placentas). (B) Slc38a2 expression in selected fetal tissues from SCR and Slc38a2 conceptuses, pooled from n=6 litters (27 SCR fetuses, 15 Slc38a2KD fetuses). (C) Fetal weight and (D) placental weight in SCR and Slc38a2KD conceptuses from n=29 litters (130 SCR conceptuses and 99 Slc38a2KD conceptuses). Points represent litter means. (A–D) Litter mean values for SCR and Slc38a2 compared by paired Student’s t test. P-values for statistically significant differences (P<0.05) given in figure. Mean ± SEM. (E) Correlation of fetal weight with placental Slc38a2 expression for individual SCR (n=28) and Slc38a2KD (n=13) conceptuses. Points represent individual conceptuses. Pearson correlation coefficient (R) and P-value given in figure. Linear regression line with 95% confidence intervals shown. (F) Frequency distribution of individual fetal weights in SCR (n=130) and Slc38a2KD conceptuses (n=99). Gaussian curves fitted by least-squares non-linear regression and compared by extra sum-of-squares F-test (P-value given in figure). Best-fit values for curves: SCR amplitude 18.7 ± 1.2%, mean 1034 ± 7 mg, SD 101 ± 7 mg; Slc38a2KD amplitude 17.2 ± 1.3%, mean 952 ± 10 mg, SD 113 ± 10 mg. Dotted vertical line indicates 10th percentile of SCR fetal weights.
Placenta-specific Slc38a2 knockdown reduced fetal weight by 11% compared with SCR controls (Figure 1C), consistent with our original hypothesis, and despite fewer Slc38a2KD fetuses per litter. Slc38a2KD also reduced placental weight by 18% (Figure 1D). When SCR and Slc38a2KD conceptuses were combined, fetal weight correlated with placental Slc38a2 expression (Figure 1E), whereas placental weight did not (R = 0.305, P=0.052, n=41). The frequency distribution of fetal weights in Slc38a2KD conceptuses was left-shifted, compared with SCR conceptuses (Figure 1F) such that a greater proportion of Slc38a2KD fetuses (22%) were below the 10th centile of control fetal weight (854 g, P=0.015, Fisher’s exact test), consistent with the FGR clinical phenotype.
To investigate the mechanism linking placenta-specific Slc38a2 silencing with reduced placental weight, we also determined the activity of the nutrient-sensing amino acid response and mTOR signaling pathways in SCR and Slc38a2KD placentas, using Western blotting. Slc38a2KD affected neither abundance nor phosphorylation of eIF2α (Ser51), which is responsive to amino acid deprivation, or the read-outs of mTOR activity, S6 ribosomal protein (Ser235/236), eukaryotic translation initiation factor 4E-binding protein (4EBP1, Thr37/46) and protein kinase B (Akt, Ser473) (Supplemenatary Figure S4).
Placenta-specific Slc38a2 knockdown reduces placental System A amino acid transport
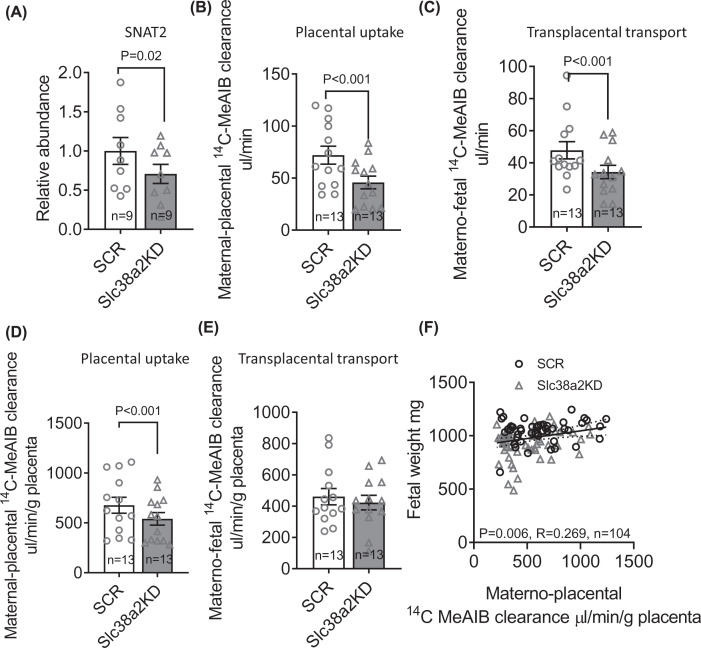
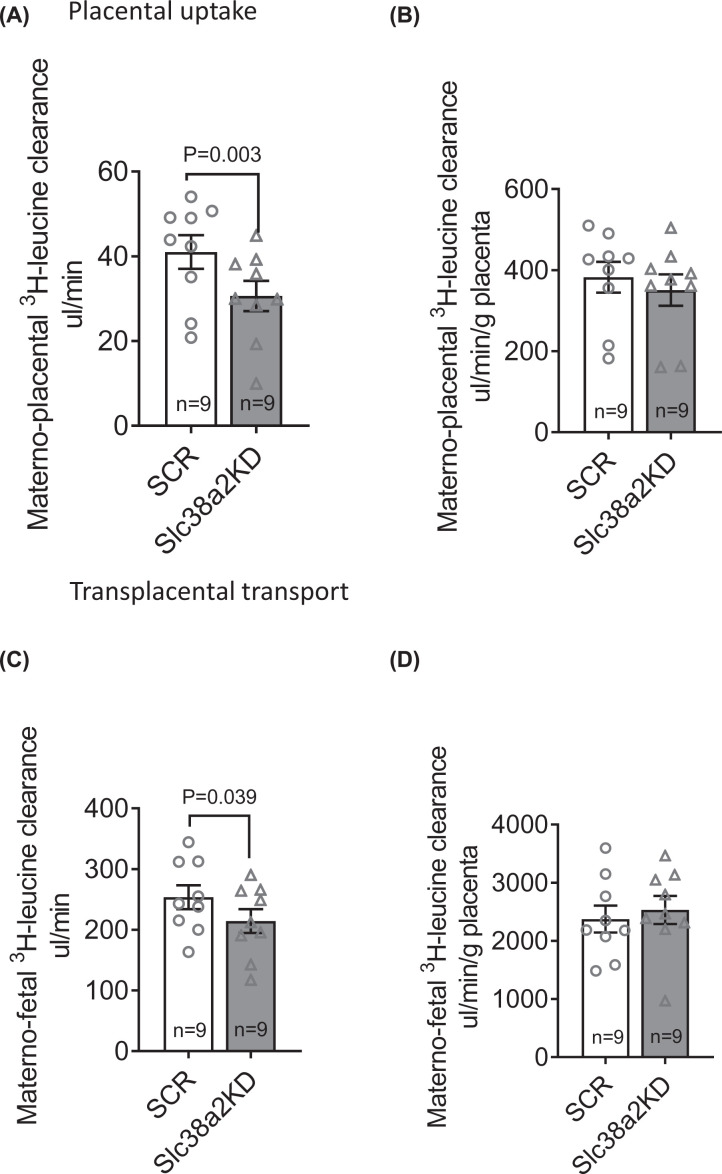
Next, we used immunoblotting to determine SNAT2 protein abundance in the isolated TPM of E18.5 SCR and Slc38a2KD placentas, because membrane translocation of the transporter is required for cellular amino acid accumulation and known to be post-translationally regulated in trophoblasts [26]. TPM SNAT2 was 30% lower in Slc38a2KD than in SCR placentas, in line with Slc38a2 mRNA expression (Figure 2A). Placental System A amino acid transport capacity was then determined by the in vivo unidirectional maternal–placental clearance (placental uptake) and maternal–fetal clearance (transplacental transport) of non-metabolizable 14C-MeAIB (see ‘Materials and methods’ section). Slc38a2KD reduced both placental uptake and transplacental transport of 14C-MeAIB, consistent with an overall reduction in placental System A transport capacity (Figure 2B,C). Placental uptake of 14C-MeAIB was also lower in Slc38a2KD than SCR conceptuses when expressed per gram of placental weight (Figure 2D). However, there was no significant difference between SCR and Slc38a2KD conceptuses when transplacental 14C-MeAIB transport was expressed per gram of placenta (Figure 2E), indicating that the mass-specific System A transport activity of the placenta was maintained, despite its smaller size. Fetal weight was positively correlated with placental uptake of 14C-MeAIB, when each conceptus within each litter was considered as an individual (Figure 2F). We similarly determined maternal–placental and maternal–fetal clearance of 3H-leucine, as read-outs of placental System L exchanger activity. Both placental uptake and transplacental clearance of 3H-leucine were lower in Slc38a2KD than SCR conceptuses as absolute values, but not when expressed per gram of placenta (Figure 3).
Figure 2. Placenta-specific Slc38a2 knockdown reduces TPM SNAT2 abundance and placental System A amino acid transport.
(A) SNAT2 protein abundance in isolated TPMs from pooled SCR and Slc38a2KD placentas, determined by Western blot. Representative blot includes human placental microvillous membrane (MVM) sample, as positive control. (B–E) Placental uptake and transplacental transport of 14C-MeAIB in SCR (n=13 litters, representing 46 conceptuses) and Slc38a2KD conceptuses (n=13 litters, representing 58 conceptuses), expressed as absolute values (B,C) and per gram of placenta (D,E). Litter mean values for SCR and Slc38a2KD conceptuses compared by paired t test. P-values for significant differences (P<0.05) given in figure. Mean + SEM. (F) Correlation of fetal weight with placental 14C-MeAIB uptake for SCR (n=46) and Slc38a2KD (n=58) conceptuses. Points represent individual conceptuses. Pearson correlation coefficient (R) and P-value given in figure. Linear regression line with 95% confidence intervals shown.
Figure 3. Placental System L amino acid transport in SCR and Slc38a2KD conceptuses.
(A,B) Placental uptake and (C,D) transplacental transport of 3H-leucine, expressed as absolute values (A,C) or per gram placenta (B,D). n=9 litters (33 SCR conceptuses, 29 Slc38a2KD conceptuses). Litter mean values for SCR and Slc38a2KD conceptuses compared by paired Student’s t test. P-values for significant differences (P<0.05) given in figure. Mean + SEM
Placenta-specific Slc38a2 knockdown decreases fetal islet GSIS
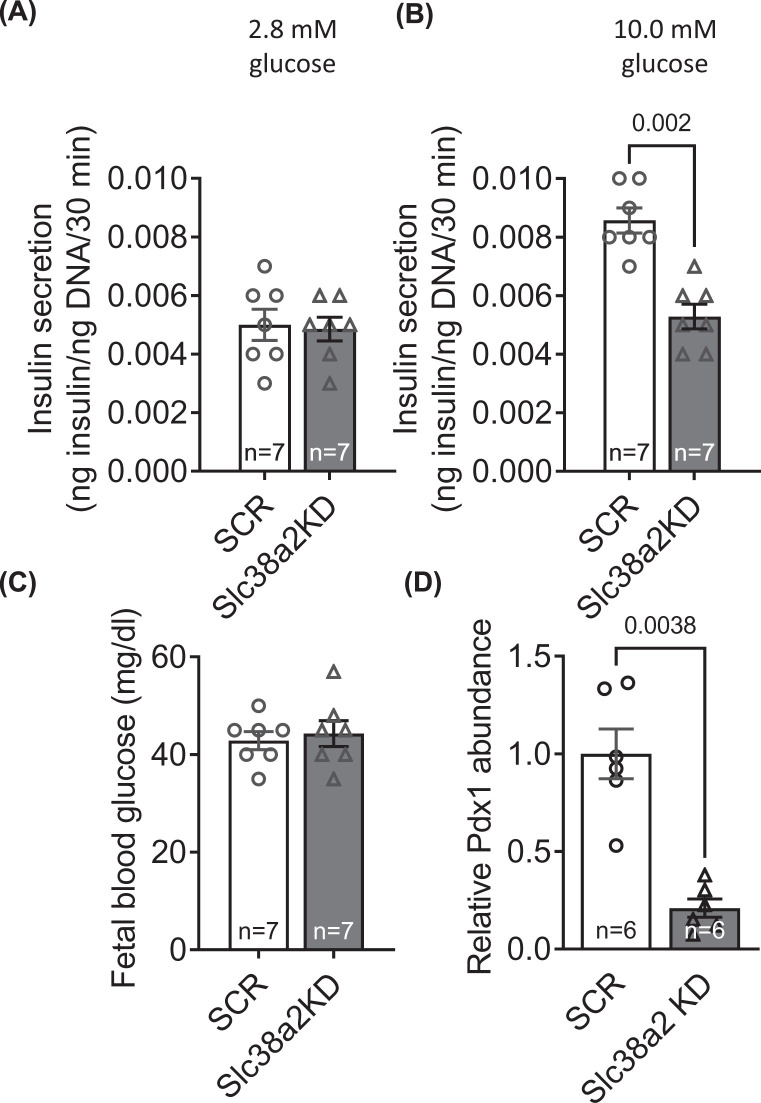
GSIS was measured ex vivo in pancreatic islets isolated from a subset of Slc38a2KD and SCR fetuses at E18.5. Islet GSIS did not differ between Slc38a2KD and SCR fetuses in the presence of 2.8 mM glucose but was significantly lower in Slc38a2KD fetuses than SCR fetuses at 10.0 mM glucose (Figure 4A,B). Fetal glucose concentrations were similar in the two groups (Figure 4C). Placenta-specific Slc38a2 knockdown reduced fetal islet abundance of the transcription factor Pdx1, a master regulator of β cell differentiation (Figure 4D).
Figure 4. Placenta-specific Slc38a2 knockdown decreases fetal islet GSIS.
GSIS from mouse fetal islet cells. Pancreatic fetal islets were isolated, and GSIS determined in response to stimulation with (A) 2.8 mM and (B) 10.0 mM glucose for 60. (C) Fetal blood glucose was measured in trunk blood. n=7 litters/group. GSIS and blood glucose for SCR and Slc38a2KD conceptuses compared by paired Student’s t test. P-values for significant differences (P<0.05) given in the figure. Mean + SEM.
Human placental SLC38A2 gene expression is decreased in late- but not early-onset FGR
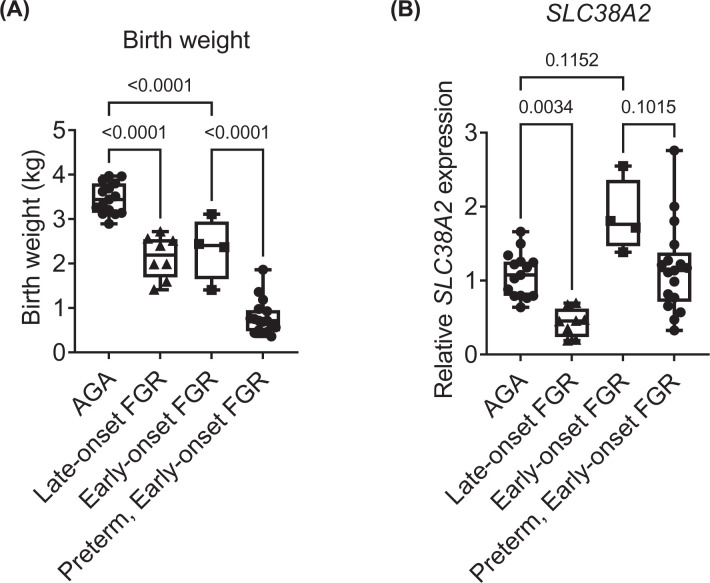
Finally, we quantified SLC38A2 expression in human placentas collected at term from AGA, early- and late-onset FGR neonates, with informed consent in a U.K. hospital setting. Because early-onset FGR is frequently associated with preterm delivery, we separately compared SLC38A2 expression in early-onset FGR placentas delivered at term with those delivered preterm (<37 weeks of gestation). Maternal age and pre-pregnancy body mass index (BMI) and the proportional distributions of fetal sex and delivery mode were similar in AGA and FGR groups, irrespective of gestational age at FGR onset and at delivery (Table 2). Maternal ethnicity differed between study groups, with the late-onset FGR group containing the largest proportion of Asian, Black or Mixed ethnicity subjects and the AGA group containing the smallest proportion of subjects of these ethnicities. Women with FGR fetuses were more likely than women with AGA fetuses to be diagnosed with a hypertensive disorder of pregnancy, defined as pregnancy-induced hypertension, pre-eclampsia or HELLP syndrome. Hypertensive disorder of pregnancy was highest in the preterm, early-onset FGR group. As expected, women in the preterm, early-onset FGR group delivered earlier than the other three groups, by ∼10 weeks on average. Placental weight differed significantly between AGA and FGR groups but was not recorded for all subjects (Table 2). Both birth weight and placental SLC38A2 expression were lower in late-onset FGR pregnancies than AGA pregnancies, by 38 and 60%, respectively (Figure 5). Contrastingly, early-onset FGR pregnancies delivered at term had birth 1weights that were 33% lighter than AGA control birthweights, but their placental SLC38A2 expression levels did not differ significantly, and tended to be higher than control values (Figure 5B). Placental SLC38A2 expression also tended to be higher in term-delivered early-onset FGR pregnancies compared with their preterm-delivered counterparts, which had even lower birth weights (Figure 5A,B).
Table 2. Demographic characteristics of human AGA and FGR study participants.
| Term delivery | Preterm delivery | P-value | |||
|---|---|---|---|---|---|
| AGA | Late-onset FGR | Early-onset FGR | Early-onset FGR | ||
| n=15 | n=8 | n=4 | n=17 | ||
| Maternal age (years) | 38.0 (31.0–39.0) | 32.5 (31.0–34.8) | 32.0 (25.3–39.5) | 35.0 (31.0–39.5) | 0.4591 |
| Maternal BMI (kg/m2) | 23.0 (22.0–28.0) | 22.0 (22.0–31.0) | 23.0 (21.5–32.0) | 24.0 (23.0–28.0) | 0.4452 |
| Ethnicity (number Asian, Black or Mixed) | 2 (13%) | 6 (75%) | 1 (25%) | 5 (29%) | 0.0253 |
| Hypertensive disorder of pregnancy (number with any diagnosis) | 0 (0%) | 1 (13%) | 1 (25%) | 7 (41%) | 0.0343 |
| Fetal sex (number male) | 10 (67%) | 4 (50%) | 1 (25%) | 6 (35%) | 0.2563 |
| Mode of delivery (number vaginal) | 4 (27%) | 1 (13%) | 2 (50%) | 1 (6%) | 0.1483 |
| Gestational age (weeks) | 39.1 (39.0–39.6) | 37.5 (36.9–38.5 | 38.4 (37.6–40.5) | 28.05 (27.1–31.0) | <0.0012 |
| Placental weight (g)6 | 491 (428–521) | 3514 (240–365) | 584 | 1425 (135–185) | <0.0011 |
Continuous variables are given as median (interquartile range) and compared by one-way ANOVA1 with Sidak’s multiple comparison post-hoc or by Kruskal–Wallis test2 with Dunn’s multiple comparison post-hoc. 4Significantly different from AGA, 5significantly different from early-onset FGR delivered at term. Categorical variables are given as number (% of n) and compared by chi-squared test3.
6Placental weights were collected for a subset of study participants: AGA n=9, late-onset FGR n=6, term early-onset FGR n=1, preterm early-onset FGR n=5.
Figure 5. Human placental SLC38A2 expression is decreased in late- but not early-onset FGR.
(A) Birth weights and (B) placental relative SLC38A2 expression levels in AGA and FGR neonates, delivered at term (≥37 weeks gestation) or preterm (<37 weeks gestation, early-onset FGR only). Birth weights were statistically compared between groups by one-way ANOVA (P<0.001) with planned post-hoc comparisons using Sidak’s multiple comparisons test. SLC38A2 expression was statistically compared between groups using Kruskal–Wallis test (P<0.001) with planned post-hoc comparisons using Dunn’s multiple comparisons test. P-values for post-hoc tests given in figure. AGA n=15, late-onset FGR n=8, term early-onset FGR n=4, preterm early-onset FGR n=17. Boxes are median with IQR, whiskers are range.
Within subjects delivering at term, placental SLC38A2 expression correlated with placental weight (P=0.005, R = 0.664, n=16) and gestational age at delivery (P=0.008, R = 0.501, n=27) but did not correlate with any of the other continuous independent variables (Supplementary Table S2). When FGR status and potential confounding variables (gestational age, maternal ethnicity and hypertensive disorder) were entered into a multiple linear regression model to predict placental SLC38A2 expression at term, the overall effect of FGR status remained significant (main effect: P<0.001; regression model: P<0.001, R2 = 0.769, degrees of freedom = 21). Regression coefficients for the adjusted effect of each type of FGR, compared with AGA, further indicated decreased SLC38A2 expression in late-onset FGR (P=0.058, β= −0.389) and increased placental SLC38A2 expression in early-onset FGR (P<0.001, β = 0.869) (Supplementary Table S3).
Discussion
The present study shows, for the first time, that placenta-specific knockdown of the System A amino acid transporter Slc38a2/SNAT2 causes FGR in mice, consistent with our original hypothesis. Lower fetal weight in Slc38a2KD transduced conceptuses was accompanied by diminished fetal viability, lower placental weight, lower maternal–fetal MeAIB clearance and lower fetal islet GSIS near term, compared with control transduced conceptuses. The study also provides the first demonstration that human placental SLC38A2 expression is reduced at term in FGR pregnancies compared with AGA pregnancies, specifically when FGR is late in onset, occurring after 32 weeks of gestation, but not when it is diagnosed before. Our study therefore proves that normal trophoblast Slc38a2/SNAT2 expression and function are necessary for normal fetal growth and supports the concept that impaired placental System A amino acid transport mechanistically contributes to human late-onset FGR.
The 11% reduction in fetal weight with placenta-specific Slc38a2 knockdown was similar in magnitude to that observed in mouse embryos with global Slc38a2 deletion [32] and in rat embryos following inhibition of System A amino acid transport using unlabeled MeAIB infusion [31]. These findings are all consistent with the physiological role of SNAT2 as an active transporter of amino acids across the rodent placenta, where it is abundantly expressed in the labyrinthine TPM and correlates with fetal size [28,63,64]. FGR in our study was attributable to a deficiency of trophoblast membrane SNAT2 and overall functional reduction in System A-mediated amino acid delivery to the umbilical circulation, because transplacental MeAIB clearance was 23% lower in Slc38a2KD than SCR conceptuses. However, Slc38a2 silencing also caused placental growth restriction, which preceded FGR, alongside diminished placental System A amino acid uptake per unit mass of tissue. The fact that transplacental MeAIB clearance per gram of placenta was not changed therefore suggests that the fetal effect of Slc38a2 knockdown was partly mediated by smaller placental size, and consequently reduced exchange surface area, as well as lower SNAT2 transporter density in the TPM. Lower placental weight in Slc38a2KD conceptuses was not related to inhibition of the nutrient-sensing amino acid response and mTOR signaling pathways, which regulate trophoblast growth in vitro [65,66]. Instead, SNAT2 deficiency in the TPM probably directly reduced intracellular amino acid availability for cellular accretion or proliferation. Indeed, mouse placentas genetically lacking Slc38a4/SNAT4 exhibit mid-gestation hypoplasia, with reduced expression of cell cycle-related genes and lower numbers of proliferating trophoblasts in the chorionic plate and labyrinthine zone [32]. In contrast with our findings, global genetic deletion of Slc38a2 does not alter placental weight at term [32]. This may suggest that compensatory processes maintain placental size during differentiation of the trophectoderm when Slc38a2 is deleted by zygotic CRISPR-Cas9 injection, albeit the number of Slc38a2-deficient placentas analysed in the previous study was very small.
Near-term fetal viability rate was reduced by 33% in Slc38a2KD embryos, whereas implantation rates were similar to controls, in line with the effect of global Slc38a2 deletion [32,33]. Therefore, a greater proportion of Slc38a2KD embryos died after implantation but before term. This finding reflects the increased incidence of stillbirth and perinatal death seen in FGR human fetuses [67–69] and supports the importance of placental SNAT2 for normal fetal development. It indicates that SNAT2 may be involved in post-implantation development, earlier in gestation. Certainly, SNAT2 is abundantly expressed in the peri-implantation mouse embryo and plays a role in embryonic stem cell differentiation, although its role in extraembryonic cells in early gestation is not well established [70,71].
The effects of placenta-specific Slc38a2 knockdown on fetal growth were generally milder than those of glucocorticoid excess, hypoxia, protein restriction or nitric oxide deficiency in pregnant rodents, which are similarly associated with reduced System A amino acid transport [28,34–36,38]. This is probably explained by the tendency for these perturbations to reduce fetal delivery of other nutrients and oxygen by altering uteroplacental blood flow. Additionally, some of these insults are associated with placental down-regulation of the other System A amino acid transporters, Slc38a1/SNAT1 or Slc38a4/SNAT4 [29,34,38], which may have additive effects on net transplacental amino acid transport. Placental Slc38a1 and Slc38a4 expression was maintained at normal levels in our study, by design. The relative importance of Slc38a2/SNAT2 compared with the other two placental SNAT transporter isoforms is still being established and appears to vary with species. Global deletion of either Slc38a1, Slc38a2 or Slc38a4 reduces fetal growth in mice [32], while studies in isolated microvillous membrane indicate that SNAT1 and SNAT2 mainly mediate neutral amino acid uptake in rat syncytiotrophoblast [72]. In humans, in vitro experiments demonstrate that SNAT1, SNAT2 and SNAT4 all contribute to trophoblast System A-mediated amino acid uptake, depending on gestational age [23,24,72,73], but placental SNAT2 abundance is most closely related to term fetal growth [22,26]. The present study shows that exclusively reducing placental Slc38a2 expression is sufficient to impair maternal–fetal System A amino acid transport and fetal growth. Notably, FGR occurred even though placental Slc38a2 silencing did not affect net placental uptake or transplacental transport of the essential amino acid, leucine. This finding was unexpected, given that leucine transport is mediated by System L transporters [19], the activity of which is believed to depend on the intracellular concentration of non-essential amino acids accumulated by System A [20]. This observation may be partly explained by computational modeling data suggesting that placental L-type amino acid transporters are not obligate exchangers but can also act as facilitated transporters [74].
Although reduced fetal weight in our study is partly explained by low amino acid availability for tissue accretion, it may also be indirectly attributed to fetal hyperinsulinaemia, due to impaired pancreatic islet function. Our finding of reduced GSIS in Slc38a2KD fetal pancreatic islets reflects that in growth restricted human and sheep fetuses with reduced umbilical amino acid concentration, β-cell mass and pancreatic expression of the transcription factor Pdx1 [75–79]. Pdx1 is a key differentiation initiator in pancreatic progenitor cells and activator of β-cell specific genes, and is regulated by amino acids, including the System A substrate, glutamine [80]. Therefore, at a cellular and molecular level, reduced GSIS in pancreatic islets from Slc38a2KD fetuses is most likely due to deficient β-cell development caused by amino acid deprivation and Pdx1 down-regulation.
The physiological effects of placental Slc38a2 silencing were not in proportion to the degree of knockdown of mRNA expression, which was decreased by more than 50%. This reflects the limitation of using RNAi methodology to study placental membrane-bound transporter function, which is also governed by the rates of translation and, particularly, trafficking of the transporter proteins to the plasma membrane [27]. Furthermore, lower viability in Slc38a2KD fetuses may have contributed to an underestimation of the effect on nutrient transport and fetal growth, because fetal weight in rodents is inversely related to the number of fetuses in each horn [81]. Although the experimental design controlled for effects of overall litter size, by transferring control and Slc38a32KD embryos to contralateral horns of each recipient uterus, the relative difference in fetal weight and amino acid transport would most likely have been larger if we controlled the number of viable fetuses per horn. The full magnitude of the effect of placenta-specific Slc38a2 silencing may also have been masked if those embryos with greatest functional knockdown of SNAT2 were resorbed prior to the E18.5 analyses. Nevertheless, our study demonstrates that lentiviral shRNA-mediated gene silencing is effective as a tool to study placental nutrient transporter function in mice.
This is the first study to determine human placental SLC38A2 expression in well-defined early- and late-onset FGR subjects delivering at term. The reduction in SLC38A2 gene expression in placentas with late-onset FGR was consistent with, but greater in magnitude than, that reported in another cohort, which had a mean gestational age of ∼33 weeks and most likely represented a mixed population of early- and late-onset FGR pregnancies [26]. The data therefore strongly support a mechanistic role for placental SLC38A2 deficiency in late-onset FGR. By contrast, the lack of reduction in SLC38A2 expression in early-onset FGR placentas was unexpected. It may suggest that amino acid transport is causatively less important than factors such as impaired spiral artery remodeling and uteroplacental blood flow in early-onset FGR [44]. Alternatively, early-onset FGR may be underpinned by reduced post-translational trafficking of SNAT2 to the TPM, due to suppressed mTOR signaling [22], rather than decreased SLC38A2 transcription per se. We were unable to measure membrane localized transporter abundance or System A activity using the small amounts of human placental material collected in this study. The analyses of human placentas were also limited by the small number of patients with early onset FGR that reached term for delivery; the lack of preterm AGA control placentas; and our inability to control for possible effects of maternal ethnicity or hypertensive disorders, which differed between AGA and FGR subjects. Nevertheless, the data provide compelling evidence that early- and late-onset FGR are underpinned by different mechanisms, with reduced placental amino acid transport critical in the latter.
Overall, the present study is the first demonstration of a cause-and-effect relationship between placental Slc38a2/SNAT2 expression and fetal growth. Prior to the present study, there was no definitive evidence that SNAT2 deficiency mechanistically contributes to FGR. Given that System A amino acid transporter activity is compromised more severely in human FGR placentas (>70% [22]) than in this study (23%), the results strongly indicate that diminished fetal amino acid supply is an underlying cause. Currently, there is no specific treatment of FGR in the clinic. Our findings suggest that interventions that augment placental System A amino acid transport capacity may improve fetal growth and outcomes in severe FGR. These interventions may be most effective in women diagnosed with late-onset FGR, which constitutes the overwhelming majority of FGR cases.
Clinical perspectives
FGR is an obstetric disease that severely compromises perinatal survival and long-term health of the infant. The biological mechanisms underlying FGR are unknown, preventing us from developing treatments.
We show that experimental knockdown of Slc38a2 in the mouse placenta reduces fetal weight at the end of gestation, demonstrating a cause-and-effect relationship between low placental Slc38a2/SNAT2 expression and FGR in vivo. We also show that human placental Slc38a2 expression is specifically reduced in late-onset FGR.
Our findings support the concept that placental SNAT2 deficiency underpins human FGR and suggest that interventions that augment placental amino acid transport could improve fetal growth and perinatal outcomes in severe FGR.
Supplementary Material
Abbreviations
- AGA
appropriate for gestational age
- EFW
estimated fetal weight
- EGFP
enhanced green fluorescent protein
- eIF2
eukaryotic initiation factor 2
- FGR
fetal growth restriction
- GFP
green fluorescent protein
- GSIS
glucose-stimulated insulin secretion
- HELLP
haemolysis, elevated liver enzymes and low platelet count
- MeAIB
methylaminoisobutyric acid
- mTOR
mechanistic target of rapamycin
- Pdx1
pancreatic and duodenal homobox 1
- SCR
scrambled
- shRNA
small hairpin RNA
- SNAT
sodium-coupled neutral amino acid transporter
- TPM
trophoblast plasma membrane
- 4EBP1
eukaryotic translation initiation factor 4E-binding protein
Data Availability
The authors confirm that the data supporting the findings of the present study are available within the article, its supplementary materials and from the corresponding author [Owen R. Vaughan] upon reasonable request.
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was supported by the National Institute of Health [grant numbers R01HD065007, R01HD068370]; the European Union Seventh Framework Programme (FP7/2007–2013) [grant number 305823]; and U.K. National Institute for Health Research University College London Hospitals Biomedical Research Centre
Open Access
Open access for this article was enabled by the participation of University College London in an all-inclusive Read & Publish pilot with Portland Press and the Biochemical Society under a transformative agreement with JISC.
CRediT Author Contribution
Owen R. Vaughan: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing—original draft, Writing—review and editing. Katarzyna Maksym: Investigation. Elena Silva: Investigation, Methodology. Kenneth Barentsen: Investigation, Methodology. Russel V. Anthony: Methodology. Thomas L. Brown: Methodology. Sara L. Hillman: Investigation. Rebecca Spencer: Investigation. Anna L. David: Funding acquisition, Investigation, Writing—review and editing. Fredrick J. Rosario: Investigation, Writing—original draft. Theresa L. Powell: Supervision, Funding acquisition, Writing—review and editing. Thomas Jansson: Conceptualization, Supervision, Funding acquisition, Writing—review and editing.
References
- 1.McCowan L.M., Figueras F. and Anderson N.H. (2018) Evidence-based national guidelines for the management of suspected fetal growth restriction: comparison, consensus, and controversy. Am. J. Obstet. Gynecol. 218, S855–S868 10.1016/j.ajog.2017.12.004 [DOI] [PubMed] [Google Scholar]
- 2.Ray J.G., Park A.L. and Fell D.B. (2017) Mortality in infants affected by preterm birth and severe small-for-gestational age birth weight. Pediatrics 140, e20171881 10.1542/peds.2017-1881 [DOI] [PubMed] [Google Scholar]
- 3.de Jong F., Monuteaux M.C., van Elburg R.M., Gillman M.W. and Belfort M.B. (2012) Systematic review and meta-analysis of preterm birth and later systolic blood pressure. Hypertension 59, 226–234 10.1161/HYPERTENSIONAHA.111.181784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaillard R., Steegers E.A., Tiemeier H., Hofman A. and Jaddoe V.W. (2013) Placental vascular dysfunction, fetal and childhood growth, and cardiovascular development: the generation R study. Circulation 128, 2202–2210 10.1161/CIRCULATIONAHA.113.003881 [DOI] [PubMed] [Google Scholar]
- 5.Cetin I., Corbetta C., Sereni L.P., Marconi A.M., Bozzetti P., Pardi G.et al. (1990) Umbilical amino acid concentrations in normal and growth-retarded fetuses sampled in utero by cordocentesis. Am. J. Obstet. Gynecol. 162, 253–261 10.1016/0002-9378(90)90860-A [DOI] [PubMed] [Google Scholar]
- 6.Cetin I., Ronzoni S., Marconi A.M., Perugino G., Corbetta C., Battaglia F.C.et al. (1996) Maternal concentrations and fetal-maternal concentration differences of plasma amino acids in normal and intrauterine growth-restricted pregnancies. Am. J. Obstet. Gynecol. 174, 1575–1583 10.1016/S0002-9378(96)70609-9 [DOI] [PubMed] [Google Scholar]
- 7.Economides D.L., Nicolaides K.H., Gahl W.A., Bernardini I. and Evans M.I. (1989) Plasma amino acids in appropriate- and small-for-gestational-age fetuses. Am. J. Obstet. Gynecol. 161, 1219–1227 10.1016/0002-9378(89)90670-4 [DOI] [PubMed] [Google Scholar]
- 8.Paolini C.L., Marconi A.M., Ronzoni S., Di Noio M., Fennessey P.V., Pardi G.et al. (2001) Placental transport of leucine, phenylalanine, glycine, and proline in intrauterine growth-restricted pregnancies. J. Clin. Endocrinol. Metab. 86, 5427–5432 10.1210/jcem.86.11.8036 [DOI] [PubMed] [Google Scholar]
- 9.de Boo H.A., van Zijl P.L., Smith D.E., Kulik W., Lafeber H.N. and Harding J.E. (2005) Arginine and mixed amino acids increase protein accretion in the growth-restricted and normal ovine fetus by different mechanisms. Pediatr. Res. 58, 270–277 10.1203/01.PDR.0000169977.48609.55 [DOI] [PubMed] [Google Scholar]
- 10.Liechty E.A., Boyle D.W., Moorehead H., Auble L. and Denne S.C. (1999) Aromatic amino acids are utilized and protein synthesis is stimulated during amino acid infusion in the ovine fetus. J. Nutr. 129, 1161–1166 10.1093/jn/129.6.1161 [DOI] [PubMed] [Google Scholar]
- 11.Meier P.R., Peterson R.G., Bonds D.R., Meschia G. and Battaglia F.C. (1981) Rates of protein synthesis and turnover in fetal life. Am. J. Physiol. 240, E320–E324 10.1152/ajpendo.1981.240.3.E320 [DOI] [PubMed] [Google Scholar]
- 12.Fowden A.L. (1980) Effects of adrenaline and amino acids on the release of insulin in the sheep fetus. J. Endocrinol. 87, 113–121 10.1677/joe.0.0870113 [DOI] [PubMed] [Google Scholar]
- 13.Gadhia M.M., Maliszewski A.M., O'Meara M.C., Thorn S.R., Lavezzi J.R., Limesand S.W.et al. (2013) Increased amino acid supply potentiates glucose-stimulated insulin secretion but does not increase β-cell mass in fetal sheep. Am. J. Physiol. Endocrinol. Metab. 304, E352–E362 10.1152/ajpendo.00377.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glazier J.D., Cetin I., Perugino G., Ronzoni S., Grey A.M., Mahendran D.et al. (1997) Association between the activity of the system A amino acid transporter in the microvillous plasma membrane of the human placenta and severity of fetal compromise in intrauterine growth restriction. Pediatr. Res. 42, 514–519 10.1203/00006450-199710000-00016 [DOI] [PubMed] [Google Scholar]
- 15.Jansson T., Ylvén K., Wennergren M. and Powell T.L. (2002) Glucose transport and system A activity in syncytiotrophoblast microvillous and basal plasma membranes in intrauterine growth restriction. Placenta 23, 392–399 10.1053/plac.2002.0826 [DOI] [PubMed] [Google Scholar]
- 16.Mahendran D., Donnai P., Glazier J.D., D'Souza S.W., Boyd R.D. and Sibley C.P. (1993) Amino acid (system A) transporter activity in microvillous membrane vesicles from the placentas of appropriate and small for gestational age babies. Pediatr. Res. 34, 661–665 10.1203/00006450-199311000-00019 [DOI] [PubMed] [Google Scholar]
- 17.Vaughan O.R., Rosario F.J., Powell T.L. and Jansson T. (2017) Regulation of placental amino acid transport and fetal growth. Prog. Mol. Biol. Transl. Sci. 145, 217–251 10.1016/bs.pmbts.2016.12.008 [DOI] [PubMed] [Google Scholar]
- 18.Holm M.B., Bastani N.E., Holme A.M., Zucknick M., Jansson T., Refsum H.et al. (2017) Uptake and release of amino acids in the fetal-placental unit in human pregnancies. PLoS ONE 12, e0185760 10.1371/journal.pone.0185760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaccioli F., Aye I.L., Roos S., Lager S., Ramirez V.I., Kanai Y.et al. (2015) Expression and functional characterisation of System L amino acid transporters in the human term placenta. Reprod. Biol. Endocrinol. 13, 57 10.1186/s12958-015-0054-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meier C., Ristic Z., Klauser S. and Verrey F. (2002) Activation of system L heterodimeric amino acid exchangers by intracellular substrates. EMBO J. 21, 580–589 10.1093/emboj/21.4.580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prasad P.D., Wang H., Huang W., Kekuda R., Rajan D.P., Leibach F.H.et al. (1999) Human LAT1, a subunit of system L amino acid transporter: molecular cloning and transport function. Biochem. Biophys. Res. Commun. 255, 283–288 10.1006/bbrc.1999.0206 [DOI] [PubMed] [Google Scholar]
- 22.Chen Y.-Y., Rosario Fredrick J., Shehab Majida A., Powell Theresa L., Gupta Madhulika B. and Jansson T. (2015) Increased ubiquitination and reduced plasma membrane trafficking of placental amino acid transporter SNAT-2 in human IUGR. Clin. Sci. 129, 1131–1141 10.1042/CS20150511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Desforges M., Greenwood S.L., Glazier J.D., Westwood M. and Sibley C.P. (2010) The contribution of SNAT1 to system A amino acid transporter activity in human placental trophoblast. Biochem. Biophys. Res. Commun. 398, 130–134 10.1016/j.bbrc.2010.06.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Desforges M., Mynett K.J., Jones R.L., Greenwood S.L., Westwood M., Sibley C.P.et al. (2009) The SNAT4 isoform of the system A amino acid transporter is functional in human placental microvillous plasma membrane. J. Physiol. 587, 61–72 10.1113/jphysiol.2008.161331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.James-Allan L.B., Teal S., Powell T.L. and Jansson T. (2020) Changes in placental nutrient transporter protein expression and activity across gestation in normal and obese women. Reprod. Sci. 27, 1758–1769 10.1007/s43032-020-00173-y [DOI] [PubMed] [Google Scholar]
- 26.Mando C., Tabano S., Pileri P., Colapietro P., Marino M.A., Avagliano L.et al. (2013) SNAT2 expression and regulation in human growth-restricted placentas. Pediatr. Res. 74, 104–110 10.1038/pr.2013.83 [DOI] [PubMed] [Google Scholar]
- 27.Rosario F.J., Kanai Y., Powell T.L. and Jansson T. (2013) Mammalian target of rapamycin signalling modulates amino acid uptake by regulating transporter cell surface abundance in primary human trophoblast cells. J. Physiol. 591, 609–625 10.1113/jphysiol.2012.238014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jansson N., Pettersson J., Haafiz A., Ericsson A., Palmberg I., Tranberg M.et al. (2006) Down-regulation of placental transport of amino acids precedes the development of intrauterine growth restriction in rats fed a low protein diet. J. Physiol. 576, 935–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosario F.J., Jansson N., Kanai Y., Prasad P.D., Powell T.L. and Jansson T. (2011) Maternal protein restriction in the rat inhibits placental insulin, mtor, and stat3 signaling and down-regulates placental amino acid transporters. Endocrinology 152, 1119–1129 10.1210/en.2010-1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pantham P., Rosario F.J., Weintraub S.T., Nathanielsz P., Powell T., Li C.et al. (2016) Down-regulation of placental transport of amino acids precedes the development of intrauterine growth restriction in maternal nutrient restricted baboons. Biol. Reprod. 95, 98 10.1095/biolreprod.116.141085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cramer S., Beveridge M., Kilberg M. and Novak D. (2002) Physiological importance of system A-mediated amino acid transport to rat fetal development. Am. J. Physiol. Cell Physiol. 282, C153–C160 10.1152/ajpcell.2002.282.1.C153 [DOI] [PubMed] [Google Scholar]
- 32.Matoba S., Nakamuta S., Miura K., Hirose M., Shiura H., Kohda T.et al. (2019) Paternal knockout of Slc38a4/SNAT4 causes placental hypoplasia associated with intrauterine growth restriction in mice. Proc. Natl. Acad. Sci. U.S.A. 116, 21047–21053 10.1073/pnas.1907884116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weidenfeld S., Chupin C., Langner D.I., Zetoun T., Rozowsky S. and Kuebler W.M. (2021) Sodium-coupled neutral amino acid transporter SNAT2 counteracts cardiogenic pulmonary edema by driving alveolar fluid clearance. Am. J. Physiol. Lung Cell. Mol. Physiol. 320, L486–L497 10.1152/ajplung.00461.2020 [DOI] [PubMed] [Google Scholar]
- 34.Coan P.M., Vaughan O.R., McCarthy J., Mactier C., Burton G.J., Constancia M.et al. (2011) Dietary composition programmes placental phenotype in mice. J. Physiol. 589, 3659–3670 10.1113/jphysiol.2011.208629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Constancia M., Hemberger M., Hughes J., Dean W., Ferguson-Smith A., Fundele R.et al. (2002) Placental-specific IGF-II is a major modulator of placental and fetal growth. Nature 417, 945–948 10.1038/nature00819 [DOI] [PubMed] [Google Scholar]
- 36.Higgins J.S., Vaughan O.R., Fernandez de Liger E., Fowden A.L. and Sferruzzi-Perri A.N. (2015) Placental phenotype and resource allocation to fetal growth are modified by the timing and degree of hypoxia during mouse pregnancy. J. Physiol. 594, 1341–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kusinski L.C., Stanley J.L., Dilworth M.R., Hirt C.J., Andersson I.J., Renshall L.J.et al. (2012) eNOS knockout mouse as a model of fetal growth restriction with an impaired uterine artery function and placental transport phenotype. Am. J. Physiol. Regul. Integr. Compar. Physiol. 303, R86–R93 10.1152/ajpregu.00600.2011 [DOI] [PubMed] [Google Scholar]
- 38.Vaughan O.R., Sferruzzi-Perri A.N. and Fowden A.L. (2012) Maternal corticosterone regulates nutrient allocation to fetal growth in mice. J. Physiol. 590, 5529–5540 10.1113/jphysiol.2012.239426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Green A.S., Rozance P.J. and Limesand S.W. (2010) Consequences of a compromised intrauterine environment on islet function. J. Endocrinol. 205, 211–224 10.1677/JOE-09-0399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gadhia M.M., Maliszewski A.M., O’Meara M.C., Thorn S.R., Lavezzi J.R., Limesand S.W.et al. (2013) Increased amino acid supply potentiates glucose-stimulated insulin secretion but does not increase beta-cell mass in fetal sheep. Am. J. Physiol. Endocrinol. Metab. 304, E352–E362 10.1152/ajpendo.00377.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brown L.D., Davis M., Wai S., Wesolowski S.R., Hay W.W. Jr, Limesand S.W.et al. (2016) Chronically increased amino acids improve insulin secretion, pancreatic vascularity, and islet size in growth-restricted fetal sheep. Endocrinology 157, 3788–3799 10.1210/en.2016-1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gordijn S.J., Beune I.M., Thilaganathan B., Papageorghiou A., Baschat A.A., Baker P.N.et al. (2016) Consensus definition of fetal growth restriction: a Delphi procedure. Ultrasound Obstet. Gynecol. 48, 333–339 10.1002/uog.15884 [DOI] [PubMed] [Google Scholar]
- 43.Savchev S., Figueras F., Sanz-Cortes M., Cruz-Lemini M., Triunfo S., Botet F.et al. (2014) Evaluation of an optimal gestational age cut-off for the definition of early- and late-onset fetal growth restriction. Fetal Diagn. Ther. 36, 99–105 10.1159/000355525 [DOI] [PubMed] [Google Scholar]
- 44.Figueras F. and Gratacós E. (2014) Update on the diagnosis and classification of fetal growth restriction and proposal of a stage-based management protocol. Fetal Diagn. Ther. 36, 86–98 10.1159/000357592 [DOI] [PubMed] [Google Scholar]
- 45.Aviram A., Sherman C., Kingdom J., Zaltz A., Barrett J. and Melamed N. (2019) Defining early vs late fetal growth restriction by placental pathology. Acta Obstet. Gynecol. Scand. 98, 365–373 10.1111/aogs.13499 [DOI] [PubMed] [Google Scholar]
- 46.Aughwane R., Mufti N., Flouri D., Maksym K., Spencer R., Sokolska M.et al. (2021) Magnetic resonance imaging measurement of placental perfusion and oxygen saturation in early-onset fetal growth restriction. BJOG 128, 337–345 10.1111/1471-0528.16387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baker C.M., Goetzmann L.N., Cantlon J.D., Jeckel K.M., Winger Q.A. and Anthony R.V. (2016) Development of ovine chorionic somatomammotropin hormone-deficient pregnancies. Am. J. Physiol. Regul. Integr. Comp. Physiol. 310, R837–R846 10.1152/ajpregu.00311.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Georgiades P., Cox B., Gertsenstein M., Chawengsaksophak K. and Rossant J. (2007) Trophoblast-specific gene manipulation using lentivirus-based vectors. BioTechniques 42, 317–8 10.2144/000112341 [DOI] [PubMed] [Google Scholar]
- 49.Kaufman M.R., Albers R.E., Keoni C., Kulkarni-Datar K., Natale D.R. and Brown T.L. (2014) Important aspects of placental-specific gene transfer. Theriogenology 82, 1043–1048 10.1016/j.theriogenology.2014.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee D.S., Rumi M.A., Konno T. and Soares M.J. (2009) In vivo genetic manipulation of the rat trophoblast cell lineage using lentiviral vector delivery. Genesis 47, 433–439 10.1002/dvg.20518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Okada Y., Ueshin Y., Isotani A., Saito-Fujita T., Nakashima H., Kimura K.et al. (2007) Complementation of placental defects and embryonic lethality by trophoblast-specific lentiviral gene transfer. Nat. Biotechnol. 25, 233–237 10.1038/nbt1280 [DOI] [PubMed] [Google Scholar]
- 52.Moffat J., Grueneberg D.A., Yang X., Kim S.Y., Kloepfer A.M., Hinkle G.et al. (2006) A lentiviral RNAi library for human and mouse genes applied to an arrayed viral high-content screen. Cell 124, 1283–1298 10.1016/j.cell.2006.01.040 [DOI] [PubMed] [Google Scholar]
- 53.Stewart S.A., Dykxhoorn D.M., Palliser D., Mizuno H., Yu E.Y., An D.S.et al. (2003) Lentivirus-delivered stable gene silencing by RNAi in primary cells. RNA 9, 493–501 10.1261/rna.2192803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Flexner L.B. and Pohl H.A. (1941) The transfer of radioactive sodium across the placenta of the white rat. J. Cell. Compar. Physiol. 18, 49–59 10.1002/jcp.1030180107 [DOI] [Google Scholar]
- 55.Kusinski L.C., Jones C.J., Baker P.N., Sibley C.P. and Glazier J.D. (2010) Isolation of plasma membrane vesicles from mouse placenta at term and measurement of system A and system beta amino acid transporter activity. Placenta 31, 53–59 10.1016/j.placenta.2009.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ling R., Bridges C.C., Sugawara M., Fujita T., Leibach F.H., Prasad P.D.et al. (2001) Involvement of transporter recruitment as well as gene expression in the substrate-induced adaptive regulation of amino acid transport system A. Biochim. Biophys. Acta 1512, 15–21 10.1016/S0005-2736(01)00310-8 [DOI] [PubMed] [Google Scholar]
- 57.Spencer R., Ambler G., Brodszki J., Diemert A., Figueras F., Gratacós E.et al. (2017) EVERREST prospective study: a 6-year prospective study to define the clinical and biological characteristics of pregnancies affected by severe early onset fetal growth restriction. BMC Pregnancy Childbirth 17, 43 10.1186/s12884-017-1226-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hillman S., Peebles D.M. and Williams D.J. (2013) Paternal metabolic and cardiovascular risk factors for fetal growth restriction: a case-control study. Diabetes Care 36, 1675–1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hillman S.L., Finer S., Smart M.C., Mathews C., Lowe R., Rakyan V.K.et al. (2015) Novel DNA methylation profiles associated with key gene regulation and transcription pathways in blood and placenta of growth-restricted neonates. Epigenetics 10, 50–61 10.4161/15592294.2014.989741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hillman S.L., Kubba T. and Williams D.J. (2017) Delivery of small-for-gestational-age neonate and association with early-onset impaired maternal endothelial function. Ultrasound Obstet. Gynecol. 49, 150–154 10.1002/uog.17342 [DOI] [PubMed] [Google Scholar]
- 61.Hemberger M., Hanna C.W. and Dean W. (2020) Mechanisms of early placental development in mouse and humans. Nat. Rev. Genet. 21, 27–43 10.1038/s41576-019-0169-4 [DOI] [PubMed] [Google Scholar]
- 62.Coan P.M., Ferguson-Smith A.C. and Burton G.J. (2004) Developmental dynamics of the definitive mouse placenta assessed by stereology. Biol. Reprod. 70, 1806–1813 10.1095/biolreprod.103.024166 [DOI] [PubMed] [Google Scholar]
- 63.Novak D., Lehman M., Bernstein H., Beveridge M. and Cramer S. (2006) SNAT expression in rat placenta. Placenta 27, 510–516 10.1016/j.placenta.2005.05.002 [DOI] [PubMed] [Google Scholar]
- 64.Rosario F.J., Kanai Y., Powell T.L. and Jansson T. (2015) Increased placental nutrient transport in a novel mouse model of maternal obesity with fetal overgrowth. Obesity 23, 1663–1670 10.1002/oby.21165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gonzalez I.M., Martin P.M., Burdsal C., Sloan J.L., Mager S., Harris T.et al. (2012) Leucine and arginine regulate trophoblast motility through mTOR-dependent and independent pathways in the preimplantation mouse embryo. Dev. Biol. 361, 286–300 10.1016/j.ydbio.2011.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim J., Song G., Wu G., Gao H., Johnson G.A. and Bazer F.W. (2013) Arginine, leucine, and glutamine stimulate proliferation of porcine trophectoderm cells through the MTOR-RPS6K-RPS6-EIF4EBP1 signal transduction pathway. Biol. Reprod. 88, 113 10.1095/biolreprod.112.105080 [DOI] [PubMed] [Google Scholar]
- 67.Ego A., Monier I., Skaare K. and Zeitlin J. (2020) Antenatal detection of fetal growth restriction and risk of stillbirth: population-based case-control study. Ultrasound Obstet. Gynecol. 55, 613–620 10.1002/uog.20414 [DOI] [PubMed] [Google Scholar]
- 68.Gardosi J., Madurasinghe V., Williams M., Malik A. and Francis A. (2013) Maternal and fetal risk factors for stillbirth: population based study. BMJ 346, f108 10.1136/bmj.f108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Unterscheider J., O’Donoghue K., Daly S., Geary M.P., Kennelly M.M., McAuliffe F.M.et al. (2014) Fetal growth restriction and the risk of perinatal mortality-case studies from the multicentre PORTO study. BMC Pregnancy Childbirth 14, 63 10.1186/1471-2393-14-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tan B.S., Lonic A., Morris M.B., Rathjen P.D. and Rathjen J. (2011) The amino acid transporter SNAT2 mediates L-proline-induced differentiation of ES cells. Am. J. Physiol. Cell Physiol. 300, C1270–C1279 10.1152/ajpcell.00235.2010 [DOI] [PubMed] [Google Scholar]
- 71.Tan B.S.N., Rathjen P.D., Harvey A.J., Gardner D.K. and Rathjen J. (2016) Regulation of amino acid transporters in pluripotent cell populations in the embryo and in culture; novel roles for sodium-coupled neutral amino acid transporters. Mech. Dev. 141, 32–39 10.1016/j.mod.2016.06.003 [DOI] [PubMed] [Google Scholar]
- 72.Takahashi Y., Nishimura T., Maruyama T., Tomi M. and Nakashima E. (2017) Contributions of system A subtypes to α-methylaminoisobutyric acid uptake by placental microvillous membranes of human and rat. Amino Acids 49, 795–803 10.1007/s00726-017-2384-7 [DOI] [PubMed] [Google Scholar]
- 73.Wang H., Huang W., Sugawara M., Devoe L.D., Leibach F.H., Prasad P.D.et al. (2000) Cloning and functional expression of ATA1, a subtype of amino acid transporter A, from human placenta. Biochem. Biophys. Res. Commun. 273, 1175–1179 10.1006/bbrc.2000.3061 [DOI] [PubMed] [Google Scholar]
- 74.Widdows K.L., Panitchob N., Crocker I.P., Please C.P., Hanson M.A., Sibley C.P.et al. (2015) Integration of computational modeling with membrane transport studies reveals new insights into amino acid exchange transport mechanisms. FASEB J. 29, 2583–2594 10.1096/fj.14-267773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Van Assche F.A., Prins F.D., Aerts L. and Verjans M. (1977) The endocrine pancreas in small-for-dates infants. BJOG 84, 751–753 10.1111/j.1471-0528.1977.tb12486.x [DOI] [PubMed] [Google Scholar]
- 76.Nicolini U., Hubinont C., Santolaya J., Fisk N. and Rodeck C. (1990) Effects of fetal intravenous glucose challenge in normal and growth retarded fetuses. Horm. Metab. Res. 22, 426–430 10.1055/s-2007-1004939 [DOI] [PubMed] [Google Scholar]
- 77.Limesand S.W., Rozance P.J., Zerbe G.O., Hutton J.C. and Hay W.W. Jr (2006) Attenuated insulin release and storage in fetal sheep pancreatic islets with intrauterine growth restriction. Endocrinology 147, 1488–1497 10.1210/en.2005-0900 [DOI] [PubMed] [Google Scholar]
- 78.Limesand S.W., Jensen J., Hutton J.C. and Hay W.W. Jr (2005) Diminished β-cell replication contributes to reduced β-cell mass in fetal sheep with intrauterine growth restriction. Am. J. Physiol. Regul. Integr. Compar. Physiol. 288, R1297–R1305 10.1152/ajpregu.00494.2004 [DOI] [PubMed] [Google Scholar]
- 79.Chen X., Rozance P.J., Hay W.W. Jr and Limesand S.W. (2012) Insulin-like growth factor and fibroblast growth factor expression profiles in growth-restricted fetal sheep pancreas. Exp. Biol. Med. (Maywood) 237, 524–529 10.1258/ebm.2012.011375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Corless M., Kiely A., McClenaghan N.H., Flatt P.R. and Newsholme P. (2006) Glutamine regulates expression of key transcription factor, signal transduction, metabolic gene, and protein expression in a clonal pancreatic beta-cell line. J. Endocrinol. 190, 719–727 10.1677/joe.1.06892 [DOI] [PubMed] [Google Scholar]
- 81.Barr M. Jr, Jensh R.P. and Brent R.L. (1970) Prenatal growth in the albino rat: effects of number, intrauterine position and resorptions. Am. J. Anat. 128, 413–428 10.1002/aja.1001280403 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of the present study are available within the article, its supplementary materials and from the corresponding author [Owen R. Vaughan] upon reasonable request.