Abstract
Selective serotonin reuptake inhibitors (SSRIs) have anti‐inflammatory properties that may have clinical utility in treating severe pulmonary manifestations of COVID‐19. SSRIs exert anti‐inflammatory effects at three mechanistic levels: (a) inhibition of proinflammatory transcription factor activity, including NF‐κB and STAT3; (b) downregulation of lung tissue damage and proinflammatory cell recruitment via inhibition of cytokines, including IL‐6, IL‐8, TNF‐α, and IL‐1β; and (c) direct suppression inflammatory cells, including T cells, macrophages, and platelets. These pathways are implicated in the pathogenesis of COVID‐19. In this review, we will compare the pathogenesis of lung inflammation in pulmonary diseases including COVID‐19, ARDS, and chronic obstructive pulmonary disease (COPD), describe the anti‐inflammatory properties of SSRIs, and discuss the applications of SSRIS in treating COVID‐19‐associated inflammatory lung disease.
Keywords: ARDS, COVID‐19, lung inflammation, NF‐κB, selective serotonin reuptake inhibitor
Selective serotonin reuptake inhibitors (SSRIs) have anti‐inflammatory effects on transcription factor activity, cytokine expression profiles, and suppression of innate and adaptive immune cells. These anti‐inflammatory properties may be useful in treating pulmonary inflammation, especially in the case of COVID‐19.
1. INTRODUCTION
The COVID‐19 pandemic has led to press need for treatments and preventative strategies to manage acute and chronic lung disease. Given the rapid spread of COVID‐19, it is expeditious to utilize medications that are already FDA‐approved and that are known to have limited side effects. Selective serotonin reuptake inhibitors (SSRIs) have been explored as anti‐inflammatory agents in the context of autoimmune and inflammatory diseases, and research suggests that SSRIs may inhibit inflammatory pathways implicated in acute and chronic lung disease. In this review, we will explore the utility of SSRIs in treatment and prevention of inflammatory lung disease and discuss the application of these findings to COVID‐19.
2. PATHOGENESIS OF LUNG INFLAMMATION
COVID‐19 is caused by the SARS‐CoV‐2 virus, an enveloped, single‐stranded positive‐sense RNA betacoronavirus.1, 2 Alveolar macrophages detect viral components, leading to a T‐cell‐mediated immune response.1, 2, 3, 4 Cells infected with the virus also stimulate interferon and cytokine release via interferon regulatory factor and transcription factor NF‐κB activation,5 and recruit more immune cells to the site of infection.1 These immune cells propagate release of more proinflammatory molecules, leading to pulmonary and systemic disease.4
COVID‐19 presents a variety of problems to clinicians, including rapid onset of severe disease, various manifestations of pathology,6, 7, 8 and paucity of demonstrably effective treatments.9, 10, 11, 12 SARS‐CoV‐2 infection initially manifests as fever, cough, and fatigue, among other symptoms, which can progress to severe pneumonia and hypoxemic respiratory failure.13 On imaging, patients with COVID‐19 have bilateral ground‐glass opacities7 and upper lobe infiltrates associated with dyspnea and hypoxemia.13, 14 These severe manifestations are mediated by several proinflammatory cytokines, including IL‐6, TNF‐α, IL‐17, GM‐CSF, and G‐CSF.1, 7, 15, 16, 17, 18, 19, 20 Cytokines involved in COVID‐19 pathogenesis are summarized in Table 1.
TABLE 1.
Cytokines in the pathogenesis of lung disease and COVID‐19
| COVID‐19 | ARDS | COPD | Pneumonia | |
|---|---|---|---|---|
| ProInflammatory cytokines | ||||
| IL‐1β | Increased7, 15, 17 | Increased34, 171, 172, 173, 174 | Increased175 | Increased176 |
| IL‐2 | Increased7, 16, severe disease7 | Increased174 Low in serum, high in BAL177 | Increased178 | Increased179, 180 |
| IL‐6 | Increased3,7, 15, 17 | Increased3, 7, 34, 171, 172, 173, 174, 181, 182 | Increased175, 178, 183, 184 Acute COPD exacerbation183 | Increased179, 185, 186, 187 |
| IL‐7 | Increased7, 16, severe disease7 | Increased174 | Increased188 | Protects against bacterial infection189 |
| IL‐8 | Increased7, 15 | Increased34, 171, 172, 173, 174 | Increased175, 178, 184, 190, 191 Acute COPD exacerbation190 | Increased179, 180, 186, 187, 192 |
| IFN‐γ | Increased7, 17 | Increased174, 182 | Increased178 | Increased193, 194 |
| G‐CSF | Increased, severe disease7, 15, 16 | Increased174, 181 | Increased195, 196 | Increased in atypical pneumonia187 |
| GM‐CSF | Increased15 | Increased174, 197 | Increased196 | Increased198, 199 |
| TNF‐α | Increased3, severe disease7, 15 | Increased34, 171, 173, 174 | Increased178, 191, 200 | Increased176, 179 |
| Chemokines | ||||
| IP10 | Increased, severe disease7, 15, 16 | Increased174 | Increased201 | Increased187 |
| MCP1 | Increased, severe disease7, 15, 16 | Increased174 | Increased in emphysema184 | Increased202 |
| MIP1α | Increased, severe disease7, 15, 16 | Increased174, 203 | Increased204 | Increased205 |
| Anti‐inflammatory cytokines | ||||
| IL‐4 | Increased7 | Increased174 | Increased191 | Increased in viral pneumonia206, ventilator‐associated pneumonia207, but not atypical bacterial pneumonia208 |
| IL‐10 | Increased3, 7, severe disease7 | Increased172, 173, 174 | Increased178 | Increased179 |
Cytokine storm in COVID‐19 infection can cause acute respiratory distress syndrome (ARDS), an inflammatory state in which increased vascular permeability leads to pulmonary edema and tissue destruction.21, 22 A major cause of ARDS is sepsis secondary to bacterial pneumonia,23, 24, 25, 26, 27 and influenza A28 and coronaviruses29, 30, 31ARDS is characterized by a primary insult, such as infection or trauma, leading to a secondary insult of inflammation and tissue damage. These insults cause capillary leakage in lung parenchyma which ultimately impairs oxygenation.32, 33 Macrophages, endothelial cells, epithelial cells, and neutrophils release proinflammatory signaling molecules including IL‐6, IL‐8, TNF‐α, and IL‐1β, further increasing vascular permeability.34, 35, 36 More inflammatory cells are recruited, become activated and propagate the inflammatory response.37, 38 Many cells and cytokines involved in the inflammatory response in ARDS pathology are implicated in COVID‐19 infection39 and are summarized in Table 1.
Comorbid pulmonary disease, specifically chronic obstructive pulmonary disease (COPD), is an important risk factor for poor outcomes in COVID‐19.40 Interestingly, patients with COVID‐19 rarely reported comorbid COPD overall, potentially due to underdiagnosis.41 COPD and COVID‐19 cause lung damage through a shared mechanism of increased inflammation, dysregulated immunity, and impaired repair function.42, 43, 44, 45 These effects are summarized in Table 1 and Figure 1.
FIGURE 1.
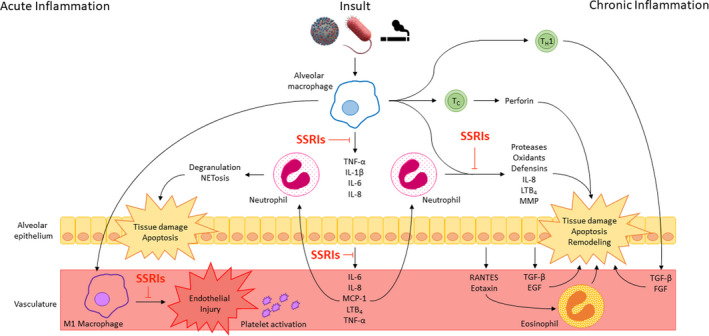
Mechanism of SSRI modulation in pulmonary inflammatory disease. Infectious and inflammatory insults stimulate NF‐κB translocation and cytokine release in alveolar macrophages and epithelial cells. These cytokines recruit neutrophils to the lung, leading to tissue damage and apoptosis. In acute inflammatory disease, M1 macrophages stimulate platelet activation and endothelial injury, and activated platelets recruit neutrophils and promote NET formation, mediating further tissue damage. In chronic disease, T cells lead to direct and indirect tissue damage and promote remodeling associated with decreased pulmonary function. SSRIs reduce pulmonary inflammation in each of these pathways by inhibiting 1 NF‐κB activity, (2) downstream cytokine release, and (3) cellular activity by impairing serotonin reuptake by SERT
3. ANTI‐INFLAMMATORY PROPERTIES OF SSRIS
Selective serotonin reuptake inhibitors (SSRIs) were first used in the 1980s,46 where they found success in treating depression by blocking reuptake and subsequent degradation of serotonin at the synaptic cleft and potentiating serotonin signal transduction at the postsynaptic neuron.47, 48, 49 SSRIs were hailed as a breakthrough medication for depression with fewer side effects than tricyclic antidepressants and without the addictive potential of benzodiazepines.46, 50 More recently, the anti‐inflammatory properties of SSRIs have been explored. SSRIs inhibit inflammation‐induced lung tissue destruction at three mechanistic levels: inhibition of proinflammatory transcription factors,51 reduced production of inflammatory cytokines through canonical serotonergic mechanisms,52 and inhibition of inflammatory cellular responses53, 54 (Figure 1).
3.1. Selective serotonin reuptake inhibitors modulation of inflammatory transcription factors
Selective serotonin reuptake inhibitors alter the transcriptional regulation of genes encoding non‐serotonergic neurotransmitter systems and inflammatory factors.51 Serotonin receptor activation decreases activity of signal transducer and activator of transcription 3 (STAT3)55 and NF‐κB,53, 56 leading to reduced downstream expression of proinflammatory markers TNF‐α, IL‐1β, IL‐6, and cyclooxygenase‐2.57, 58, 59 The inhibitory effects of SSRIs on inflammatory transcription factors may have implications for treating inflammation‐mediated damage caused by SARS‐CoV‐2 infection.60, 61, 62 In lung tissue, STAT3 and NF‐κB are implicated in a variety of inflammatory processes including pathogen‐induced acute lung injury, pulmonary inflammation, pulmonary fibrosis, and pulmonary vascular remodeling.63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75 SSRIs may decrease inflammation by suppressing the proinflammatory activities of STAT3, NF‐κB, or both.
3.2. Selective serotonin reuptake inhibitors modulation of inflammatory cytokines
Patients with depression have been found to have increased levels of inflammatory cytokines at baseline,76, 77, 78, 79, 80, 81, 82, 83, 84, 85 and cytokines modulate the hypothalamic‐pituitary‐adrenocortical (HPA) axis leading to increased production of corticotropin releasing hormone and glucocorticoid receptor resistance.83, 86 Loss of negative feedback at glucocorticoid receptors leads to dysregulated proinflammatory response.87, 88 Fluoxetine inhibited HPA axis‐mediated inflammatory edema in a rat model,89 and clinical studies have demonstrated the ability of antidepressants to modulate glucocorticoid receptor function in humans.90
Several specific proinflammatory cytokines are implicated in pathogenesis of depression. Levels of TNF‐α, IL‐6, and IFN‐γ, among others, are significantly higher in patients with depression when compared to non‐depressed controls.84, 91, 92, 93, 94, 95 IL‐1β, TNF‐α, and IFN‐γ reduce serotonin production and increase tryptophan and serotonin uptake in the brain, leading to overall depletion of serotonin96 and depression‐like behavior.97 Conversely, serotonin influences macrophage activity in a dose‐dependent manner, increasing production of IL‐1 at physiologic concentrations of serotonin and inhibiting proinflammatory activity at elevated concentrations.98 Serotonin can, therefore, influence proinflammatory cytokine pathways, and SSRIs have been explored as immune modulators.
Selective serotonin reuptake inhibitors directly inhibit proinflammatory pathways. Administration of SSRIs inhibit TNF‐α production in a mouse model of inflammation99, 100 and impair TNF‐α release from monocytes101 and microglia.102 TNF‐α has neuromodulatory effects on norepinephrine secretion that are reversible with antidepressant administration.103 INF‐α and IL‐2 treatment induced reversible depressive symptoms in patients,86, 104 and administration of serotonin reduced TNF‐α and IL‐6 in human blood.105 SSRIs were able to inhibit LPS‐induced IL‐6 production106 and NLRP3 inflammasome activation and downstream IL‐1β production in macrophages.107 Acute administration of SSRIs leads to release of proinflammatory 5‐HT,108 whereas chronic administration leads to overall depletion of serotonin.109, 110, 111 Effects of SSRIs on proinflammatory cytokines are summarized in Figure 1.
3.3. Selective serotonin reuptake inhibitors modulation of inflammatory cellular responses
Selective serotonin reuptake inhibitors inhibit presynaptic reuptake of serotonin to increase extracellular serotonin concentrations, thereby ameliorating depressive symptoms,112 and they also inhibit serotonin uptake by peripheral cells.110, 113, 114, 115 Ninety five percent of the body's serotonin is produced by enterochromaffin cells in the gut and plays a variety of secretory, sensorimotor, homeostatic, and immunologic roles.116, 117 SSRIs block the serotonin transporter (SERT), expressed on platelets,118 T cells,119 macrophages,115 and other immune cells.117 SSRIs increase extracellular serotonin concentrations to exert indirect (serotonergic) activation of serotonin receptors.120 Platelets take up peripheral serotonin produced in enterochromaffin cells via SERT,121, 122, 123 and intracellular transport of serotonin molecules leads to platelet activation and aggregation.124, 125 Release of platelet‐derived serotonin modulates proinflammatory responses and activation of monocytes and T cells.126 Administration of SSRIs increased SERT expression on T cells in patients with depression, which inhibited T‐cell proliferation and promoted apoptosis.119 SSRIs also directly suppress antigen‐presenting cells.127 SSRIs also exert direct (non‐serotonergic) activation of serotonin receptors through direct binding. Fluoxetine binds with high affinity to 5‐HT2B serotonin receptors to induce antidepressant effects that are abrogated in 5‐HT2B knockouts.128 Fluoxetine transitions macrophages from a proinflammatory M1 phenotype to an anti‐inflammatory M2 phenotype.129
4. SELECTIVE SEROTONIN REUPTAKE INHIBITORS : IMPLICATIONS FOR COVID‐19
Strategies to combat COVID‐19 continue to develop, and treatments currently under investigation include antiviral and antimalarial agents,12, 130, 131 immunosuppressant medications,6, 132 and anti‐IL‐6 modulators.17, 133, 134 Olanzapine, an atypical antipsychotic and potent H1 antagonist, has been proposed as a therapeutic IL‐6 modulator for COVID‐19 infection.135 The inflammatory processes implicated in the pathogenesis of COVID‐19 overlap with mechanisms in acute and chronic lung disease, and SSRIs modulate these pathways at several distinct points. Chronic administration of SSRIs reduce levels of IL‐6 and TNF‐α136 to the degree that decreased IL‐6 can be used as a marker for SSRI efficacy.137 SSRIs, therefore, may have clinical utility in targeting IL‐6 to treat COVID‐19.
Selective serotonin reuptake inhibitors have been studied as modulators in lung disease. Fluoxetine was found to be protective against asthma and depression in a rat model,138 and patients with comorbid asthma and depression had improved asthma outcomes when treated with a SSRI.139 Fluoxetine also protects against chronic methamphetamine‐induced pulmonary inflammation.140 Patients with COPD reported improvements in dyspnea when a SSRI was added,141, 142, 143, 144 and improved walking distances correlated with improvements in depressive symptoms over time.145, 146, 147 SSRIs have therapeutic utility in pulmonary arterial hypertension (PAH), a common sequela of COPD associated with pulmonary vascular remodeling.148 Fluoxetine prevents and reverses PAH in mice149 and inhibits remodeling and inflammation in rat lung tissue.150 SSRI use correlated with 50% reduction in risk of death in patients with PAH,151 and baseline SSRI use was associated with a reduced incidence of PAH and decreased mortality in PAH.152 However, one study found increased morbidity and mortality among elderly adults who were newly started on a SSRI medication.153 A Cochrane review was unable to determine the efficacy and safety of SSRIs in COPD and recommends further study.142
Patient appropriateness for the use of SSRIs is generally very broad, but caution should be used with certain comorbid illnesses and certainly patients should be aware of common side effects and less frequent serious risks. Significantly, patients on SSRIs for treatment of COVID‐19 symptoms would likely require treatment for periods of days to weeks, rather than the treatment of months or years seen for their primary disease indications of depression and anxiety. SSRIS are among the most prescribed medications in the United States and have very benign safety profiles. Most side effects associated with SSRIs (including sexual dysfunction, drowsiness, weight gain, and insomnia) are mild and many resolve within a few weeks of initiating treatment.154 Other documented side effects include malaise and diminished mental energy,155 diarrhea, diaphoresis, syndrome of inappropriate antidiuretic hormone and hyponatremia,156 movement disorders,157, 158 and cardiac QT prolongation159 although these are exceedingly rare. Chronic use of SSRIs has also been linked, but with very low incidence, to interstitial lung disease in elderly patients, especially women.160, 161 SSRIs interact with antiplatelet medications including aspirin and clopidogrel162, 163 and nonsteroidal anti‐inflammatory drugs (NSAIDs) to prolong bleeding times in some patients.164, 165 SSRIs have been linked to increased incidence of gastrointestinal and other bleeding incidents and should not be used in patients with an active life‐threatening bleed.166 Many of the life‐threatening complications of COVID‐19 and ARDS are associated with blood clots and so these patients often receive antiplatelet and anticoagulant medications.167, 168 SSRIS should not be coadministered with linezolid antibiotic treatment or other monoamine oxidase inhibitors due to the high risk for serotonin syndrome and caution should be used when combining SSRIs with other serotonergic medications including meperidine, tricyclic antidepressants, and serotonin‐norepinephrine reuptake inhibitors.169
Recent evidence strongly points to a role for acute brief use of SSRIs in COVID‐19 positive patients to prevent serious complications such as hospitalization, intubation, and death. Ultimately, more clinical studies are needed to understand the potential risks and benefits associated with SSRI use in COVID‐19.
5. CONCLUSION
Selective serotonin reuptake inhibitors modulate inflammatory pathways that are shared in acute and chronic lung inflammation. SSRIs have therapeutic utility in pulmonary arterial hypertension (PAH), a common sequela of COPD associated with pulmonary vascular remodeling.148 Fluoxetine prevents and reverses PAH in mice149 and inhibits remodeling and inflammation in rat lung tissue.150 SSRI use correlated with 50% reduction in risk of death in patients with PAH,151 and baseline SSRI use was associated with a reduced incidence of PAH and decreased mortality in PAH.152 However, one study found increased morbidity and mortality among elderly adults who were newly started on an SSRI medication.153 A Cochrane review was unable to determine the efficacy and safety of SSRIs in COPD and recommends further study.142
Clinicians must consider potential detrimental effects of medications. Most side effects associated with SSRIs (including sexual dysfunction, drowsiness, weight gain, and insomnia) are mild and resolve within a few weeks of initiating treatment.154 Other documented side effects include malaise and diminished mental energy,155 diarrhea, diaphoresis, syndrome of inappropriate antidiuretic hormone and hyponatremia,156 movement disorders,157, 158 and cardiac QT prolongation159 although these are exceedingly rare. SSRIs have also been linked to interstitial lung disease in elderly patients, especially women.160, 161 SSRIs interact with antiplatelet medications including aspirin and clopidogrel162, 163 and nonsteroidal anti‐inflammatory drugs (NSAIDs)164, 165 and can cause serotonin syndrome when combined with other serotonergic medications including tricyclic antidepressants, monoamine oxidase inhibitors, and serotonin‐norepinephrine reuptake inhibitors.169 These are important considerations for patients presenting with COVID‐19, especially as antiplatelet and anticoagulant medications are sometimes used in treatment of ARDS.167, 168 Notably, however, high‐quality randomized trials demonstrate SSRIs are not associated with increased bleeding events.170 Ultimately, clinical studies are needed to understand the potential risks and benefits associated with SSRI use in COVID‐19.
CONFLICT OF INTERESTS
The authors have no funding or disclosures for this study. The authors have no competing financial interested in relation to this work.
AUTHOR CONTRIBUTIONS
Claire Kyung Sun Meikle wrote and edited the manuscript. Justin Fortune Creeden wrote and edited the manuscript. Cheryl McCullumsmith wrote and edited the manuscript. Randall G. Worth edited the manuscript.
Meikle CKS, Creeden JF, McCullumsmith C, Worth RG. SSRIs: Applications in inflammatory lung disease and implications for COVID‐19. Neuropsychopharmacol Rep. 2021;41:325–335. 10.1002/npr2.12194
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
REFERENCES
- 1.Li G, Fan Y, Lai Y, Han T, Li Z, Zhou P, et al. Coronavirus infections and immune responses. J Med Virol. 2020;92:424–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ye Q, Wang B, Mao J. The pathogenesis and treatment of the `Cytokine Storm' in COVID‐19. J Infect. 2020;80:607–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diao B, Wang C, Tan Y, Chen X, Liu Y, Ning L, et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID‐19). Front Immunol. 2020;11:827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarzi‐Puttini P, Giorgi V, Sirotti S, Marotto D, Ardizzone S, Rizzardini G, et al. COVID‐19, cytokines and immunosuppression: what can we learn from severe acute respiratory syndrome? Clin Exp Rheumatol. 2020;38:337–42. [PubMed] [Google Scholar]
- 5.Bohrer H, Qiu F, Zimmermann T, Zhang Y, Jllmer T, Männel D, et al. Role of NFkappaB in the mortality of sepsis. J Clin Investig. 1997;100:972–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li R, Tian J, Yang F, Lv L, Yu J, Sun G, et al. Clinical characteristics of 225 patients with COVID‐19 in a tertiary Hospital near Wuhan. China. J Clin Virol. 2020;127:104363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet. 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang JJ, Dong X, Cao Y‐Y, Yuan Y‐D, Yang Y‐Bin, Yan Y‐Q, et al. Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Allergy. 2020;75:1730–41. [DOI] [PubMed] [Google Scholar]
- 9.Rabby MII. Current drugs with potential for treatment of COVID‐19: A literature review. J Pharm Pharm Sci. 2020;23:58–64. [DOI] [PubMed] [Google Scholar]
- 10.Agrawal S, Goel AD, Gupta N. Emerging prophylaxis strategies against COVID‐19. Monaldi Arch Chest Dis. 2020;90:169–172. [DOI] [PubMed] [Google Scholar]
- 11.Ahn DG, , Shin H‐J, Kim M‐H, Lee S, Kim H‐S, Myoung J, et al. Current status of epidemiology, diagnosis, therapeutics, and vaccines for novel coronavirus disease 2019 (COVID‐19). J Microbiol Biotechnol. 2020;30:313–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cunningham AC, Goh HP, Koh D. Treatment of COVID‐19: old tricks for new challenges. Crit Care. 2020;24:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID‐19) outbreak. J Autoimmun. 2020;109:102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phan LT, Nguyen TV, Luong QC, Nguyen TV, Nguyen HT, Le HQ, et al. Importation and human‐to‐human transmission of a novel coronavirus in Vietnam. N Engl J Med. 2020;382:872–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schett G, Sticherling M, Neurath MF. COVID‐19: risk for cytokine targeting in chronic inflammatory diseases? Nat Rev Immunol. 2020;20:271–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henderson LA, Canna SW, Schulert GS, Volpi S, Lee PY, Kernan KF, et al. On the alert for cytokine storm: immunopathology in COVID‐19. Arthritis Rheumatol. 2020;72:1059–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henry BM, de Oliveira MHS, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID‐19): a meta‐analysis. Clin Chem Lab Med. 2020;58:1021–8. [DOI] [PubMed] [Google Scholar]
- 19.Lagunas‐Rangel FA, Chavez‐Valencia V. High IL‐6/IFN‐gamma ratio could be associated with severe disease in COVID‐19 patients. J Med Virol. 2020;92:1789–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wan S, Yi Q, Fan S, Lv J, Zhang X, Guo L, et al. Relationships among lymphocyte subsets, cytokines, and the pulmonary inflammation index in coronavirus (COVID‐19) infected patients. Br J Haematol. 2020;189:428–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bauer TT, Ewig S, Rodloff AC, Muller EE. Acute respiratory distress syndrome and pneumonia: a comprehensive review of clinical data. Clin Infect Dis. 2006;43:748–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huppert LA, Matthay MA, Ware LB. Pathogenesis of acute respiratory distress Syndrome. Semin Respir Crit Care Med. 2019;40:31–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Estenssoro E, Dubin A, Laffaire E, Canales H, Sáenz G, Moseinco M, et al. Incidence, clinical course, and outcome in 217 patients with acute respiratory distress syndrome. Crit Care Med. 2002;30:2450–6. [DOI] [PubMed] [Google Scholar]
- 24.Valta P, Uusaro A, Nunes S, Ruokonen E, Takala J. Acute respiratory distress syndrome: frequency, clinical course, and costs of care. Crit Care Med. 1999;27:2367–74. [DOI] [PubMed] [Google Scholar]
- 25.Luhr OR, Antonsen K, Karlsson M, Aardal S, Thorsteinsson A, Frostell C, et al. Incidence and mortality after acute respiratory failure and acute respiratory distress syndrome in Sweden, Denmark, and Iceland. The ARF Study Group. Am J Respir Crit Care Med. 1999;159:1849–61. [DOI] [PubMed] [Google Scholar]
- 26.Esteban A, Anzueto A, Alía I, Gordo F, Apezteguía C, Pálizas F, et al. How is mechanical ventilation employed in the intensive care unit? An international utilization review. Am J Respir Crit Care Med. 2000;161:1450–8. [DOI] [PubMed] [Google Scholar]
- 27.Roupie E, Lepage E, Wysocki M, Fagon J‐Y, Chastre J, Dreyfuss D, et al. Prevalence, etiologies and outcome of the acute respiratory distress syndrome among hypoxemic ventilated patients. SRLF Collaborative Group on Mechanical Ventilation. Societe de Reanimation de Langue Francaise. Intensive Care Med. 1999;25:920–9. [DOI] [PubMed] [Google Scholar]
- 28.de Roux A, Marcos MA, Garcia E, Mensa J, Ewig S, Lode H, et al. Viral community‐acquired pneumonia in nonimmunocompromised adults. Chest. 2004;125:1343–51. [DOI] [PubMed] [Google Scholar]
- 29.Chen CY, Lee C‐H, Liu C‐Y, Wang J‐H, Wang L‐M, Perng R‐P. Clinical features and outcomes of severe acute respiratory syndrome and predictive factors for acute respiratory distress syndrome. J Chin Med Assoc. 2005;68:4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peiris JS, Chu CM, Cheng VCC, Chan KS, Hung IFN, Poon LLM, et al. Clinical progression and viral load in a community outbreak of coronavirus‐associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sung JJ, Wu A, Joynt GM, Yuen KY, Lee N, Chan PK, et al. Severe acute respiratory syndrome: report of treatment and outcome after a major outbreak. Thorax. 2004;59:414–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yadav H, Kor DJ. Platelets in the pathogenesis of acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol. 2015;309:L915–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–49. [DOI] [PubMed] [Google Scholar]
- 34.Matthay MA, Zemans RL, Zimmerman GA, Arabi YM, Beitler JR, Mercat A, et al. Acute respiratory distress syndrome. Nat Rev Dis Primers. 2019;5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oehadian A, Koide N, Mu MM, Hassan F, Islam S, Yoshida T, et al. Interferon (IFN)‐beta induces apoptotic cell death in DHL‐4 diffuse large B cell lymphoma cells through tumor necrosis factor‐related apoptosis‐inducing ligand (TRAIL). Cancer Lett. 2005;225:85–92. [DOI] [PubMed] [Google Scholar]
- 36.Yamagishi S, Ohnishi M, Pawankar R. IL‐1 and TNF‐alpha‐mediated regulation of IL‐6, IL‐8, and GM‐CSF release from cultured nasal epithelial cells. Nihon Jibiinkoka Gakkai Kaiho. 2000;103:829–35. [DOI] [PubMed] [Google Scholar]
- 37.Song C, Li H, Li Y, Dai M, Zhang L, Liu S, et al. NETs promote ALI/ARDS inflammation by regulating alveolar macrophage polarization. Exp Cell Res. 2019;382:111486. [DOI] [PubMed] [Google Scholar]
- 38.Greco E, Lupia E, Bosco O, Vizio B, Montrucchio G. Platelets and multi‐organ failure in sepsis. Int J Mol Sci. 2017;18:E2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang X, Xiu H, Zhang S, Zhang G. The role of macrophages in the pathogenesis of ALI/ARDS. Mediators Inflamm. 2018;2018:1264913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS, Li YM, et al. Comorbidity and its impact on 1590 patients with COVID‐19 in China: a nationwide analysis. Eur Respir J. 2020;55:2000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fang L, Gao P, Bao H, Tang X, Wang B, Feng Y, et al. Chronic obstructive pulmonary disease in China: a nationwide prevalence study. Lancet Respir Med. 2018;6:421–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boyd AR, Orihuela CJ. Dysregulated inflammation as a risk factor for pneumonia in the elderly. Aging Dis. 2011;2:487–500. [PMC free article] [PubMed] [Google Scholar]
- 43.Morris A, George MP, Crothers K, Huang L, Lucht L, Kessinger C, et al. HIV and chronic obstructive pulmonary disease: is it worse and why? Proc Am Thorac Soc. 2011;8:320–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Edmond K, Scott S, Korczak V, Ward C, Sanderson C, Theodoratou E, et al. Long term sequelae from childhood pneumonia; systematic review and meta‐analysis. PLoS One. 2012;7:e31239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beers MF, Morrisey EE. The three R's of lung health and disease: repair, remodeling, and regeneration. J Clin Investig. 2011;121:2065–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hillhouse TM, Porter JH. A brief history of the development of antidepressant drugs: from monoamines to glutamate. Exp Clin Psychopharmacol. 2015;23:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wong DT, Perry KW, Bymaster FP. Case history: the discovery of fluoxetine hydrochloride (Prozac). Nat Rev Drug Discov. 2005;4:764–74. [DOI] [PubMed] [Google Scholar]
- 48.Stahl SM. Mechanism of action of serotonin selective reuptake inhibitors. Serotonin receptors and pathways mediate therapeutic effects and side effects. J Affect Disord. 1998;51:215–35. [DOI] [PubMed] [Google Scholar]
- 49.Walker FR. A critical review of the mechanism of action for the selective serotonin reuptake inhibitors: do these drugs possess anti‐inflammatory properties and how relevant is this in the treatment of depression? Neuropharmacology. 2013;67:304–17. [DOI] [PubMed] [Google Scholar]
- 50.Wong DT, Bymaster FP, Engleman EA. Prozac (fluoxetine, lilly 110140), the first selective serotonin uptake inhibitor and an antidepressant drug: Twenty years since its first publication. Life Sci. 1995;57:411–41. [DOI] [PubMed] [Google Scholar]
- 51.Kroeze Y, Zhou H, Homberg JR. The genetics of selective serotonin reuptake inhibitors. Pharmacol Ther. 2012;136:375–400. [DOI] [PubMed] [Google Scholar]
- 52.Galecki P, Mossakowska‐Wojcik J, Talarowska M. The anti‐inflammatory mechanism of antidepressants ‐ SSRIs. SNRIs. Prog Neuropsychopharmacol Biol Psychiatry. 2018;80:291–4. [DOI] [PubMed] [Google Scholar]
- 53.Talmon M, Rossi S, Pastore A, Cattaneo CI, Brunelleschi S, Fresu LG. Vortioxetine exerts anti‐inflammatory and immunomodulatory effects on human monocytes/macrophages. Br J Pharmacol. 2018;175:113–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Abajo FJ. Effects of selective serotonin reuptake inhibitors on platelet function: mechanisms, clinical outcomes and implications for use in elderly patients. Drugs Aging. 2011;28:345–67. [DOI] [PubMed] [Google Scholar]
- 55.Taler M, Gil‐Ad I, Lomnitski L, Korov I, Baharav E, Bar M, et al. Immunomodulatory effect of selective serotonin reuptake inhibitors (SSRIs) on human T lymphocyte function and gene expression. Eur Neuropsychopharmacol. 2007;17:774–80. [DOI] [PubMed] [Google Scholar]
- 56.Jin Y, Lim C‐M, Kim S‐W, Park J‐Y, Seo J‐S, Han P‐L, et al. Fluoxetine attenuates kainic acid‐induced neuronal cell death in the mouse hippocampus. Brain Res. 2009;1281:108–16. [DOI] [PubMed] [Google Scholar]
- 57.Lim CM, Kim S‐W, Park J‐Y, Kim C, Yoon SH, Lee J‐K. Fluoxetine affords robust neuroprotection in the postischemic brain via its anti‐inflammatory effect. J Neurosci Res. 2009;87:1037–45. [DOI] [PubMed] [Google Scholar]
- 58.Liu T, Zhang L, Joo D, Sun SC. NF‐kappaB signaling in inflammation. Signal Transduct Target Ther. 2017;2:17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu D, Wang Z, Liu S, Wang F, Zhao S, Hao A. Anti‐inflammatory effects of fluoxetine in lipopolysaccharide(LPS)‐stimulated microglial cells. Neuropharmacology. 2011;61:592–9. [DOI] [PubMed] [Google Scholar]
- 60.Tay MZ, Poh CM, Renia L, MacAry PA, Ng LFP. The trinity of COVID‐19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wong CK, Lam CWK, Wu AKL, Ip WK, Lee NLS, Chan IHS, et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol. 2004;136:95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou Y, Hou Y, Shen J, Huang Y, Martin W, Cheng F. Network‐based drug repurposing for novel coronavirus 2019‐nCoV/SARS‐CoV‐2. Cell Discovery. 2020;6:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao J, Yu H, Liu Y, Gibson SA, Yan Z, Xu X, et al. Protective effect of suppressing STAT3 activity in LPS‐induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2016;311:L868–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang H, Sha J, Feng X, Hu X, Chen Y, Li B, et al. Dexmedetomidine ameliorates LPS induced acute lung injury via GSK‐3beta/STAT3‐NF‐kappaB signaling pathway in rats. Int Immunopharmacol. 2019;74:105717. [DOI] [PubMed] [Google Scholar]
- 65.Lin MH, Chen MC, Chen TH, Chang HY, Chou TC. Magnolol ameliorates lipopolysaccharide‐induced acute lung injury in rats through PPAR‐gamma‐dependent inhibition of NF‐kB activation. Int Immunopharmacol. 2015;28:270–8. [DOI] [PubMed] [Google Scholar]
- 66.Liang Y, Yang N, Pan G, Jin B, Wang S, Ji W. Elevated IL‐33 promotes expression of MMP2 and MMP9 via activating STAT3 in alveolar macrophages during LPS‐induced acute lung injury. Cell Mol Biol Lett. 2018;23:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tang X, Sun L, Wang G, Chen B, Luo F. RUNX1: a regulator of NF‐Kb signaling in pulmonary diseases. Curr Protein Pept Sci. 2018;19:172–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sun SC. The non‐canonical NF‐kappaB pathway in immunity and inflammation. Nat Rev Immunol. 2017;17:545–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Patel H, Zaghloul N, Lin K, Liu SF, Miller EJ, Ahmed M. Hypoxia‐induced activation of specific members of the NF‐kB family and its relevance to pulmonary vascular remodeling. Int J Biochem Cell Biol. 2017;92:141–7. [DOI] [PubMed] [Google Scholar]
- 70.Mitchell S, Vargas J, Hoffmann A. Signaling via the NFkappaB system. Wiley Interdiscip Rev Syst Biol Med. 2016;8:227–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu B, Rong Y, Sun D, Li W, Chen H, Cao B, et al. Costunolide inhibits pulmonary fibrosis via regulating NF‐kB and TGF‐beta1/Smad2/Nrf2‐NOX4 signaling pathways. Biochem Biophys Res Comm. 2019;510:329–33. [DOI] [PubMed] [Google Scholar]
- 72.Lee H, Herrmann A, Deng JH, Kujawski M, Niu G, Li Z, et al. Persistently activated Stat3 maintains constitutive NF‐kappaB activity in tumors. Cancer Cell. 2009;15:283–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hayden MS, West AP, Ghosh S. NF‐kappaB and the immune response. Oncogene. 2006;25:6758–80. [DOI] [PubMed] [Google Scholar]
- 74.Hayden MS, Ghosh S. NF‐kappaB in immunobiology. Cell Res. 2011;21:223–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cui Y, Jiang L, Ruitao Y, Shao Y, Mei L, Tao Y. beta‐carboline alkaloids attenuate bleomycin induced pulmonary fibrosis in mice through inhibiting NF‐kb/p65 phosphorylation and epithelial‐mesenchymal transition. J Ethnopharmacol. 2019;243:112096. [DOI] [PubMed] [Google Scholar]
- 76.Nishuty NL, Khandoker MMH, Karmoker JR, Ferdous S, Shahriar M, Qusar MMAS, et al. Evaluation of serum interleukin‐6 and C‐reactive protein levels in drug‐naive major depressive disorder patients. Cureus. 2019;11:e3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Maes M, Bosmans E, Meltzer HY, Scharpe S, Suy E. Interleukin‐1 beta: a putative mediator of HPA axis hyperactivity in major depression? Am J Psychiatry. 1993;150:1189–93. [DOI] [PubMed] [Google Scholar]
- 78.Maes M, Scharpé S, Meltzer HY, Bosmans E, Suy E, Calabrese J, et al. Relationships between Interleukin‐6 Activity, Acute‐Phase Proteins, and Function of the Hypothalamic‐Pituitary‐Adrenal Axis in Severe Depression. Psychiat Res. 1993;49:11–27. [DOI] [PubMed] [Google Scholar]
- 79.Maes M, Scharpé S, Meltzer HY, Okayli G, Bosmans E, D'Hondt P, et al. Increased neopterin and interferon‐gamma secretion and lower availability of L‐tryptophan in major depression ‐ further evidence for an immune‐response. Psychiat Res. 1994;54:143–60. [DOI] [PubMed] [Google Scholar]
- 80.Griffiths J, Ravindran AV, Merali Z, Anisman H. Neuroendocrine measures and lymphocyte subsets in depressive illness: influence of a clinical interview concerning life experiences. Psychoneuroendocrinology. 1997;22:225–36. [DOI] [PubMed] [Google Scholar]
- 81.Owen BM, Eccleston D, Ferrier IN, Young AH. Raised levels of plasma interleukin‐1beta in major and postviral depression. Acta Psychiatr Scand. 2001;103:226–8. [DOI] [PubMed] [Google Scholar]
- 82.Musselman DL, Miller AH, Porter MR, Manatunga A, Gao F, Penna S, et al. Higher than normal plasma interleukin‐6 concentrations in cancer patients with depression: preliminary findings. Am J Psychiatry. 2001;158:1252–7. [DOI] [PubMed] [Google Scholar]
- 83.Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee CH, Giuliani F. The role of inflammation in depression and fatigue. Front Immunol. 2019;10:1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Maes M. A review on the acute phase response in major depression. Rev Neurosci. 1993;4:407–16. [DOI] [PubMed] [Google Scholar]
- 86.Dunn AJ, Swiergiel AH, de Beaurepaire R. Cytokines as mediators of depression: what can we learn from animal studies? Neurosci Biobehav Rev. 2005;29:891–909. [DOI] [PubMed] [Google Scholar]
- 87.Raison CL, Miller AH. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress‐related disorders. Am J Psychiatry. 2003;160:1554–65. [DOI] [PubMed] [Google Scholar]
- 88.Carvalho LA, Pariante CM. In vitro modulation of the glucocorticoid receptor by antidepressants. Stress. 2008;11:411–24. [DOI] [PubMed] [Google Scholar]
- 89.Bianchi M, Sacerdote P, Panerai AE. Fluoxetine reduces inflammatory edema in the rat: involvement of the pituitary‐adrenal axis. Eur J Pharmacol. 1994;263:81–4. [DOI] [PubMed] [Google Scholar]
- 90.Carvalho LA, Garner BA, Dew T, Fazakerley H, Pariante CM. Antidepressants, but not antipsychotics, modulate GR function in human whole blood: an insight into molecular mechanisms. Eur Neuropsychopharmacol. 2010;20:379–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Goldsmith DR, Rapaport MH, Miller BJ. A meta‐analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry. 2016;21:1696–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. A meta‐analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–57. [DOI] [PubMed] [Google Scholar]
- 93.Maes M, Leonard B, Fernandez A, Kubera M, Nowak G, Veerhuis R, et al. (Neuro)inflammation and neuroprogression as new pathways and drug targets in depression: from antioxidants to kinase inhibitors. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:659–63. [DOI] [PubMed] [Google Scholar]
- 94.Maes M. Evidence for an immune response in major depression: a review and hypothesis. Prog Neuropsychopharmacol Biol Psychiatry. 1995;19:11–38. [DOI] [PubMed] [Google Scholar]
- 95.Köhler CA, Freitas TH, Stubbs B, Maes M, Solmi M, VeroneseN, et al. Peripheral alterations in cytokine and chemokine levels after antidepressant drug treatment for major depressive disorder: systematic review and meta‐analysis. Mol Neurobiol. 2018;55:4195–206. [DOI] [PubMed] [Google Scholar]
- 96.Zhu CB, Blakely RD, Hewlett WA. The proinflammatory cytokines interleukin‐1beta and tumor necrosis factor‐alpha activate serotonin transporters. Neuropsychopharmacology. 2006;31:2121–31. [DOI] [PubMed] [Google Scholar]
- 97.Moreau M, André C, O’Connor JC, Dumich SA, Woods JA, Kelley KW, et al. Inoculation of Bacillus Calmette‐Guerin to mice induces an acute episode of sickness behavior followed by chronic depressive‐like behavior. Brain Behav Immun. 2008;22:1087–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sternberg EM, Trial J, Parker CW. Effect of serotonin on murine macrophages: suppression of Ia expression by serotonin and its reversal by 5‐HT2 serotonergic receptor antagonists. J Immunol. 1986;137:276–82. [PubMed] [Google Scholar]
- 99.Tynan RJ, Weidenhofer J, Hinwood M, Cairns MJ, Day TA, Walker FR. A comparative examination of the anti‐inflammatory effects of SSRI and SNRI antidepressants on LPS stimulated microglia. Brain Behav Immun. 2012;26:469–79. [DOI] [PubMed] [Google Scholar]
- 100.Ohgi Y, Futamura T, Kikuchi T, Hashimoto K. Effects of antidepressants on alternations in serum cytokines and depressive‐like behavior in mice after lipopolysaccharide administration. Pharmacol Biochem Behav. 2013;103:853–9. [DOI] [PubMed] [Google Scholar]
- 101.Roumestan C, Michel A, Bichon F, Portet K, Detoc M, Henriquet C, et al. Anti‐inflammatory properties of desipramine and fluoxetine. Respir Res. 2007;8:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lu Y, Xu X, Jiang T, Jin L, Zhao XD, Cheng JH, et al. Sertraline ameliorates inflammation in CUMS mice and inhibits TNF‐alpha‐induced inflammation in microglia cells. Int Immunopharmacol. 2019;67:119–28. [DOI] [PubMed] [Google Scholar]
- 103.Ignatowski TA, Noble BK, Wright JR, Gorfien JL, Heffner RR, Spengler RN. Neuronal‐associated tumor necrosis factor (TNF alpha): its role in noradrenergic functioning and modification of its expression following antidepressant drug administration. J Neuroimmunol. 1997;79:84–90. [DOI] [PubMed] [Google Scholar]
- 104.Niiranen A, Laaksonen R, livanainen M, Mattson K, Färkkilä M, Cantell K. Behavioral assessment of patients treated with alpha‐interferon. Acta Psychiatr Scand. 1988;78:622–6. [DOI] [PubMed] [Google Scholar]
- 105.Kubera M, Maes M, Kenis G, Kim YK, Lason W. Effects of serotonin and serotonergic agonists and antagonists on the production of tumor necrosis factor alpha and interleukin‐6. Psychiatry Res. 2005;134:251–8. [DOI] [PubMed] [Google Scholar]
- 106.Durairaj H, Steury MD, Parameswaran N. Paroxetine differentially modulates LPS‐induced TNFalpha and IL‐6 production in mouse macrophages. Int Immunopharmacol. 2015;25:485–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Du RH, Tan J, Sun X‐Y, Lu M, Ding J‐H, Hu G. Fluoxetine inhibits NLRP3 inflammasome activation: implication in depression. Int J Neuropsychopharmacol. 2016;19:pii: pyw037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sadoughi A, Roberts KE, Preston IR, Lai GP, McCollister DH, Farber HW, et al. Use of selective serotonin reuptake inhibitors and outcomes in pulmonary arterial hypertension. Chest. 2013;144:531–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Siesser WB, Sachs BD, Ramsey AJ, Sotnikova TD, Beaulieu JM, Zhang X, et al. Chronic SSRI treatment exacerbates serotonin deficiency in humanized Tph2 mutant mice. ACS Chem Neurosci. 2013;4:84–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schlienger RG, Meier CR. Effect of selective serotonin reuptake inhibitors on platelet activation: can they prevent acute myocardial infarction? Am J Cardiovasc Drugs. 2003;3:149–62. [DOI] [PubMed] [Google Scholar]
- 111.Celada P, Dolera M, Alvarez E, Artigas F. Effects of acute and chronic treatment with fluvoxamine on extracellular and platelet serotonin in the blood of major depressive patients. Relationship to clinical improvement. J Affect Disord. 1992;25:243–9. [DOI] [PubMed] [Google Scholar]
- 112.Cowen PJ, Browning M. What has serotonin to do with depression? World Psychiatry. 2015;14:158–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hergovich N, Aigner M, Eichler HG, Entlicher J, Drucker C, Jilma B. Paroxetine decreases platelet serotonin storage and platelet function in human beings. Clin Pharmacol Ther. 2000;68:435–42. [DOI] [PubMed] [Google Scholar]
- 114.Alderman CP, Moritz CK, Ben‐Tovim DI. Abnormal platelet aggregation associated with fluoxetine therapy. Ann Pharmacother. 1992;26:1517–9. [DOI] [PubMed] [Google Scholar]
- 115.Rudd ML, Nicolas AN, Brown BL, Fischer‐Stenger K, Stewart JK. Peritoneal macrophages express the serotonin transporter. J Neuroimmunol. 2005;159:113–8. [DOI] [PubMed] [Google Scholar]
- 116.Banskota S, Ghia JE, Khan WI. Serotonin in the gut: Blessing or a curse. Biochimie. 2019;161:56–64. [DOI] [PubMed] [Google Scholar]
- 117.Wu H, Denna TH, Storkersen JN, Gerriets VA. Beyond a neurotransmitter: The role of serotonin in inflammation and immunity. Pharmacol Res. 2019;140:100–14. [DOI] [PubMed] [Google Scholar]
- 118.Mercado CP, Kilic F. Molecular mechanisms of SERT in platelets: regulation of plasma serotonin levels. Mol Interv. 2010;10:231–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fazzino F, Urbina M, Cedeno N, Lima L. Fluoxetine treatment to rats modifies serotonin transporter and cAMP in lymphocytes, CD4+ and CD8+ subpopulations and interleukins 2 and 4. Int Immunopharmacol. 2009;9:463–7. [DOI] [PubMed] [Google Scholar]
- 120.Tavoulari S, Forrest LR, Rudnick G. Fluoxetine (Prozac) binding to serotonin transporter is modulated by chloride and conformational changes. J Neurosci. 2009;29:9635–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Holinstat M. Normal platelet function. Cancer Metastasis Rev. 2017;36:195–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mustard JF, Packham MA. Factors influencing platelet function: adhesion, release, and aggregation. Pharmacol Rev. 1970;22:97–187. [PubMed] [Google Scholar]
- 123.Brenner B, Harney JT, Ahmed BA, Jeffus BC, Unal R, Mehta JL, et al. Plasma serotonin levels and the platelet serotonin transporter. J Neurochem. 2007;102:206–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cerrito F, Lazzaro MP, Gaudio E, Arminio P, Aloisi G. 5ht2‐Receptors and serotonin release ‐ their role in human platelet‐aggregation. Life Sci. 1993;53:209–15. [DOI] [PubMed] [Google Scholar]
- 125.Brunk I, Blex C, Rachakonda S, Höltje M, Winter S, Pahner I, et al. The first luminal domain of vesicular monoamine transporters mediates G‐protein‐dependent regul;ation of transmitter uptake. J Biol Chem. 2006;281:33373–85. [DOI] [PubMed] [Google Scholar]
- 126.Ali RA, Wuescher LM, Worth RG. Platelets: essential components of the immune system. Curr Trends Immunol. 2015;16:65–78. [PMC free article] [PubMed] [Google Scholar]
- 127.Bhat R, Mahapatra S, Axtell RC, Steinman L. Amelioration of ongoing experimental autoimmune encephalomyelitis with fluoxetine. J Neuroimmunol. 2017;313:77–81. [DOI] [PubMed] [Google Scholar]
- 128.Peng L, Gu L, Li B, Hertz L. Fluoxetine and all other SSRIs are 5‐HT2B agonists ‐ importance for their therapeutic effects. Curr Neuropharmacol. 2014;12:365–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Roman A, Kusmierczyk J, Klimek E, Rogoz Z, Nalepa I. Effects of co‐administration of fluoxetine and risperidone on properties of peritoneal and pleural macrophages in rats subjected to the forced swimming test. Pharmacol Rep. 2012;64:1368–80. [DOI] [PubMed] [Google Scholar]
- 130.Simsek Yavuz S, Unal S. Antiviral treatment of COVID‐19. Turk J Med Sci. 2020;50:611–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) and coronavirus disease‐2019 (COVID‐19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55:105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhai P, Ding Y, Wu X, Long J, Zhong Y, Li Y. The epidemiology, diagnosis and treatment of COVID‐19. Int J Antimicrob Agents. 2020;55:105955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Buonaguro FM, Puzanov I, Ascierto PA. Anti‐IL6R role in treatment of COVID‐19‐related ARDS. J Transl Med. 2020;18:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Liu B, Li M, Zhou Z, Guan X, Xiang Y. Can we use interleukin‐6 (IL‐6) blockade for coronavirus disease 2019 (COVID‐19)‐induced cytokine release syndrome (CRS)? J Autoimmun. 2020;111:102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Altschuler EL, Kast RE. Dapsone, colchicine and olanzapine as treatment adjuncts to prevent COVID‐19 associated adult respiratory distress syndrome (ARDS). Med Hypotheses. 2020;141:109774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lu Y, Ho CS, Liu X, Chua AN, Wang W, McIntyre RS, et al. Chronic administration of fluoxetine and pro‐inflammatory cytokine change in a rat model of depression. PLoS One. 2017;12:e0186700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yasui T, Yamada M, Uemura H, Ueno S‐I, Numata S, Ohmori T, et al. Changes in circulating cytokine levels in midlife women with psychological symptoms with selective serotonin reuptake inhibitor and Japanese traditional medicine. Maturitas. 2009;62:146–52. [DOI] [PubMed] [Google Scholar]
- 138.Sherkawy MM, Abo‐Youssef AM, Salama AAA, Ismaiel IE. Fluoxetine protects against OVA induced bronchial asthma and depression in rats. Eur J Pharmacol. 2018;837:25–32. [DOI] [PubMed] [Google Scholar]
- 139.Brown ES, Sayed N, Van Enkevort E, Kulikova A, Nakamura A, Khan DA, et al. A randomized, double‐blind, placebo‐controlled trial of escitalopram in patients with asthma and major depressive disorder. J Allergy Clin Immunol Pract. 2018;6:1604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang Y, Gu YH, Liu M, Bai Y, Wang HL. Fluoxetine protects against methamphetamineinduced lung inflammation by suppressing oxidative stress through the SERT/p38 MAPK/Nrf2 pathway in rats. Mol Med Rep. 2017;15:673–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Smoller JW, Pollack MH, Systrom D, Kradin RL. Sertraline effects on dyspnea in patients with obstructive airways disease. Psychosomatics. 1998;39:24–9. [DOI] [PubMed] [Google Scholar]
- 142.Pollok J, van Agteren JE, Carson‐Chahhoud KV. Pharmacological interventions for the treatment of depression in chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2018;12:CD012346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yohannes AM, Connolly MJ. Do antidepressants work in patients with chronic obstructive pulmonary disease with comorbid depression? Expert Rev Respir Med. 2011;5:727–9. [DOI] [PubMed] [Google Scholar]
- 144.Perna G, Cogo R, Bellodi L. Selective serotonin re‐uptake inhibitors beyond psychiatry: are they useful in the treatment of severe, chronic, obstructive pulmonary disease? Depress Anxiety. 2004;20:203–4. [DOI] [PubMed] [Google Scholar]
- 145.Eiser N, Harte R, Spiros K, Phillips C, Isaac MT. Effect of treating depression on quality‐of‐life and exercise tolerance in severe COPD. COPD. 2005;2:233–41. [PubMed] [Google Scholar]
- 146.He Y, Zheng Y, Xu C, Yang H, Wang Z, Zhou L, et al. Sertraline hydrochloride treatment for patients with stable chronic obstructive pulmonary disease complicated with depression: a randomized controlled trial. Clin Respir J. 2016;10:318–25. [DOI] [PubMed] [Google Scholar]
- 147.Lacasse Y, Beaudoin L, Rousseau L, Maltais F. Randomized trial of paroxetine in end‐stage COPD. Monaldi Arch Chest Dis. 2004;61:140–7. [DOI] [PubMed] [Google Scholar]
- 148.Chaouat A, Naeije R, Weitzenblum E. Pulmonary hypertension in COPD. Eur Respir J. 2008;32:1371–85. [DOI] [PubMed] [Google Scholar]
- 149.Guignabert C, Raffestin B, Benferhat R, Raoul W, Zadigue P, Rideau D, et al. Serotonin transporter inhibition prevents and reverses monocrotaline‐induced pulmonary hypertension in rats. Circulation. 2005;111:2812–9. [DOI] [PubMed] [Google Scholar]
- 150.Li XQ, Wang H‐M, Yang C‐G, Zhang X‐H, Han D‐D, Wang H‐L. Fluoxetine inhibited extracellular matrix of pulmonary artery and inflammation of lungs in monocrotaline‐treated rats. Acta Pharmacol Sin. 2011;32:217–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kawut SM, Horn EM, Berekashvili KK, Lederer DJ, Widlitz AC, Rosenzweig EB, et al. Selective serotonin reuptake inhibitor use and outcomes in pulmonary arterial hypertension. Pulm Pharmacol Ther. 2006;19:370–4. [DOI] [PubMed] [Google Scholar]
- 152.Shah SJ, Gomberg‐Maitland M, Thenappan T, Rich S. Selective serotonin reuptake inhibitors and the incidence and outcome of pulmonary hypertension. Chest. 2009;136:694–700. [DOI] [PubMed] [Google Scholar]
- 153.Vozoris NT, Wang X, Austin PC, Stephenson AL, O'Donnell DE, Gershon AS, et al. Serotonergic antidepressant use and morbidity and mortality among older adults with COPD. Eur Respir J. 2018;52. [DOI] [PubMed] [Google Scholar]
- 154.Hu XH, Bull SA, Hunkeler EM, Ming E, Lee JY, Fireman B, et al. Incidence and duration of side effects and those rated as bothersome with selective serotonin reuptake inhibitor treatment for depression: patient report versus physician estimate. J Clin Psychiatry. 2004;65:959–65. [DOI] [PubMed] [Google Scholar]
- 155.Nierenberg AA, Ostacher MJ, Huffman JC, Ametrano RM, Fava M, Perlis RH, et al. A brief review of antidepressant efficacy, effectiveness, indications, and usage for major depressive disorder. J Occup Environ Med. 2008;50:428–36. [DOI] [PubMed] [Google Scholar]
- 156.Labbate LA, Fava M, Rosenbaum JF, Arana GW. in Handbook of Psychiatric Drug Therapy. Philadelphia, PA: Lippincott Williams & Wilkins, 2010; p. 54. [Google Scholar]
- 157.Gerber PE, Lynd LD. Selective serotonin‐reuptake inhibitor‐induced movement disorders. Ann Pharmacother. 1998;32:692–8. [DOI] [PubMed] [Google Scholar]
- 158.Jimenez‐Jimenez FJ, Molina JA. Extrapyramidal symptoms associated with selective serotonin reuptake inhibitors ‐ Epidemiology, mechanisms and management. CNS Drugs. 2000;14:367–79. [Google Scholar]
- 159.Beach SR, Kostis WJ, Celano CM, Januzzi JL, Ruskin JN, Noseworthy PA, et al. Meta‐analysis of selective serotonin reuptake inhibitor‐associated QTc prolongation. J Clin Psychiatry. 2014;75:e441–9. [DOI] [PubMed] [Google Scholar]
- 160.Deidda A, Pisanu C, Micheletto L, Bocchetta A, Del Zompo M, Stochino ME. Interstitial lung disease induced by fluoxetine: systematic review of literature and analysis of Vigiaccess, Eudravigilance and a national pharmacovigilance database. Pharmacol Res. 2017;120:294–301. [DOI] [PubMed] [Google Scholar]
- 161.Rosenberg T, Lattimer R, Montgomery P, Wiens C, Levy L. The relationship of SSRI and SNRI usage with interstitial lung disease and bronchiectasis in an elderly population: a case‐control study. Clin Interv Aging. 2017;12:1977–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Schalekamp T, Klungel OH, Souverein PC, de Boer A. Increased bleeding risk with concurrent use of selective serotonin reuptake inhibitors and coumarins. Arch Intern Med. 2008;168:180–5. [DOI] [PubMed] [Google Scholar]
- 163.Labos C, Dasgupta K, Nedjar H, Turecki G, Rahme E. Risk of bleeding associated with combined use of selective serotonin reuptake inhibitors and antiplatelet therapy following acute myocardial infarction. Can Med Assoc J. 2011;183:1835–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Oka Y, Okamoto K, Kawashita N, Shirakuni Y, Takagi T. Meta‐analysis of the risk of upper gastrointestinal hemorrhage with combination therapy of selective serotonin reuptake inhibitors and non‐steroidal anti‐inflammatory drugs. Biol Pharm Bull. 2014;37:947–53. [DOI] [PubMed] [Google Scholar]
- 165.Shin JY, Park M‐J, Lee SH, Choi S‐H, Kim M‐H, Choi N‐K, et al. Risk of intracranial haemorrhage in antidepressant users with concurrent use of non‐steroidal anti‐inflammatory drugs: nationwide propensity score matched study. BMJ. 2015;351:h3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Laporte S, Chapelle C, Caillet P, Beyens M‐N, Bellet F, Delavenne X, et al. Bleeding risk under selective serotonin reuptake inhibitor (SSRI) antidepressants: A meta‐analysis of observational studies. Pharmacol Res. 2017;118:19–32. [DOI] [PubMed] [Google Scholar]
- 167.Mohananey D, Sethi J, Villablanca PA, Ali MS, Kumar R, Baruah A, et al. Effect of antiplatelet therapy on mortality and acute lung injury in critically ill patients: a systematic review and meta‐analysis. Ann Card Anaesth. 2016;19:626–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wang Y, Zhong M, Wang Z, Song J, Wu W, Zhu D, et al. The preventive effect of antiplatelet therapy in acute respiratory distress syndrome: a meta‐analysis. Crit Care. 2018;22:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352:1112–20. [DOI] [PubMed] [Google Scholar]
- 170.Mead GE, Hsieh CF, Lee R, Kutlubaev MA, Claxton A, Hankey GJ, et al. Selective serotonin reuptake inhibitors (SSRIs) for stroke recovery. Cochrane Database Syst Rev. 2012;11:CD009286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Meduri GU, Kohler G, Headley S, Tolley E, Stentz F, Postlethwaite A. Inflammatory cytokines in the BAL of patients with ARDS. Persistent elevation over time predicts poor outcome. Chest. 1995;108:1303–14. [DOI] [PubMed] [Google Scholar]
- 172.Lee YL, Chen W, Chen LY, Chen CH, Lin YC, Liang SJ, et al. Systemic and bronchoalveolar cytokines as predictors of in‐hospital mortality in severe community‐acquired pneumonia. J Crit Care. 2010;25(176):e177–113. [DOI] [PubMed] [Google Scholar]
- 173.Spadaro S, Park M, Turrini C, Tunstall T, Thwaites R, Mauri T, et al. Biomarkers for Acute Respiratory Distress syndrome and prospects for personalised medicine. J Inflamm (Lond). 2019;16:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Guo J, Huang F, Liu J, Chen Y, Wang W, Cao B, et al. The serum profile of hypercytokinemia factors identified in H7N9‐infected patients can predict fatal outcomes. Sci Rep. 2015;5:10942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Keatings VM, Collins PD, Scott DM, Barnes PJ. Differences in interleukin‐8 and tumor necrosis factor‐alpha in induced sputum from patients with chronic obstructive pulmonary disease or asthma. Am J Respir Crit Care Med. 1996;153:530–4. [DOI] [PubMed] [Google Scholar]
- 176.Jones MR, Simms BT, Lupa MM, Kogan MS, Mizgerd JP. Lung NF‐kappaB activation and neutrophil recruitment require IL‐1 and TNF receptor signaling during pneumococcal pneumonia. J Immunol. 2005;175:7530–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lesur O, Kokis A, Hermans C, Fülöp T, Bernard A, Lane D. Interleukin‐2 involvement in early acute respiratory distress syndrome: relationship with polymorphonuclear neutrophil apoptosis and patient survival. Crit Care Med. 2000;28:3814–22. [DOI] [PubMed] [Google Scholar]
- 178.Bradford E, Jacobson S, Varasteh J, Comellas AP, Woodruff P, O’Neal W, et al. The value of blood cytokines and chemokines in assessing COPD. Respir Res. 2017;18:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wang H, Zhou Q, Dai W, Feng X, Lu Z, Yang Z, et al. Lung Microbiota and Pulmonary Inflammatory Cytokines Expression Vary in Children With Tracheomalacia and Adenoviral or Mycoplasma pneumoniae Pneumonia. Front Pediatr. 2019;7:265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Reynolds CJ, Quigley K, Cheng X, Suresh A, Tahir S, Ahmed‐JushufF, et al. Lung defense through IL‐8 carries a cost of chronic lung remodeling and impaired function. Am J Respir Cell Mol Biol. 2018;59:557–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wiedermann FJ, Mayr AJ, Hobisch‐Hagen P, Fuchs D, Schobersberger W. Association of endogenous G‐CSF with anti‐inflammatory mediators in patients with acute respiratory distress syndrome. J Interferon Cytokine Res. 2003;23:729–36. [DOI] [PubMed] [Google Scholar]
- 182.Bos LD, Schouten LR, van Vught LA , Wiewel MA, Ong DSY, Cremer O, et al. Identification and validation of distinct biological phenotypes in patients with acute respiratory distress syndrome by cluster analysis. Thorax. 2017;72:876–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bhowmik A, Seemungal TA, Sapsford RJ, Wedzicha JA. Relation of sputum inflammatory markers to symptoms and lung function changes in COPD exacerbations. Thorax. 2000;55:114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Di Stefano A, Coccini T, Roda E, Signorini C, Balbi B, Brunetti G, et al. Blood MCP‐1 levels are increased in chronic obstructive pulmonary disease patients with prevalent emphysema. Int J Chron Obstruct Pulmon Dis. 2018;13:1691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Glynn P, Coakley R, Kilgallen I, Murphy N, O'Neill S. Circulating interleukin 6 and interleukin 10 in community acquired pneumonia. Thorax. 1999;54:51–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Calbo E, Alsina M, Rodriguez‐Carballeira M, Lite J, Garau J. Systemic expression of cytokine production in patients with severe pneumococcal pneumonia: effects of treatment with a beta‐lactam versus a fluoroquinolone. Antimicrob Agents Chemother. 2008;52:2395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Liu M, Li H, Xue CX, Gu L, Qu JX, Yu XM, et al. Differences in inflammatory marker patterns for adult community‐acquired pneumonia patients induced by different pathogens. Clin Respir J. 2018;12:974–85. [DOI] [PubMed] [Google Scholar]
- 188.Caramori G, Adcock IM, Di Stefano A, Chung KF. Cytokine inhibition in the treatment of COPD. Int J Chron Obstruct Pulmon Dis. 2014;9:397–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hassane M, Jouan Y, Creusat F, Soulard D, Boisseau C, Gonzalez L, et al. Interleukin‐7 protects against bacterial respiratory infection by promoting IL‐17A‐producing innate T‐cell response. Mucosal Immunol. 2020;13:128–39. [DOI] [PubMed] [Google Scholar]
- 190.Qiu Y, Zhu J, Bandi V, Atmar RL, Hattotuwa K, Guntupalli KK, et al. Biopsy neutrophilia, neutrophil chemokine and receptor gene expression in severe exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;168:968–75. [DOI] [PubMed] [Google Scholar]
- 191.Vitenberga Z, Pilmane M, Babjoniseva A. An insight into COPD morphopathogenesis: chronic inflammation, remodeling, and antimicrobial defense. Medicina (Kaunas). 2019;55:pii: E496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Tumgor G, Celik U, Alabaz D, Cetiner S, Yaman A, Yildizdas D, et al. Aetiological agents, interleukin‐6, interleukin‐8 and CRP concentrations in children with community‐ and hospital‐acquired pneumonia. Ann Trop Paediatr. 2006;26:285–91. [DOI] [PubMed] [Google Scholar]
- 193.Gomez JC, Yamada M, Martin JR, Dang H, Brickey WJ, Bergmeier W, et al. Mechanisms of interferon‐gamma production by neutrophils and its function during Streptococcus pneumoniae pneumonia. Am J Respir Cell Mol Biol. 2015;52:349–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Bansal S, Yajjalaa VK, Bauer C, Sun K. Influenza‐induced interferon‐γ promotes alveolar macrophage depletion during secondary pneumococcal infection. J Immunol. 2018;200, 1425–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Tsantikos E, Lau M, Castelino CMN, Maxwell MJ, Passey SL, Hansen MJ, et al. Granulocyte‐CSF links destructive inflammation and comorbidities in obstructive lung disease. J Clin Investig. 2018;128:2406–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ponce‐Gallegos MA, Ramirez‐Venegas A, Falfan‐Valencia R. Th17 profile in COPD exacerbations. Int J Chron Obstruct Pulmon Dis. 2017;12:1857–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Goodman ER, Stricker P, Velavicius M, Fonseca R, Kleinstein E, Lavery R, et al. Role of granulocyte‐macrophage colony‐stimulating factor and its receptor in the genesis of acute respiratory distress syndrome through an effect on neutrophil apoptosis. Archiv Surg. 1999;134:1049–1054. [DOI] [PubMed] [Google Scholar]
- 198.Brown RL, Sequeira RP, Clarke TB. The microbiota protects against respiratory infection via GM‐CSF signaling. Nat Commun. 2017;8:1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Liu Y, Zhang X, Wang Y, Zhu C, Fan M, Dou X, et al. The role of granulocyte macrophage colony stimulating factor in hospitalized children with Mycoplasma pneumoniae pneumonia. J Infect Chemother. 2018;24:789–94. [DOI] [PubMed] [Google Scholar]
- 200.Calikoglu M, Şahin G, Unlu A, Ozturk C, Tamer L, Ercan B, et al. Leptin and TNF‐alpha levels in patients with chronic obstructive pulmonary disease and their relationship to nutritional parameters. Respiration. 2004;71:45–50. [DOI] [PubMed] [Google Scholar]
- 201.Norman KC, Freeman CM, Bidthanapally NS, Han MK, Martinez FJ, Curtis JL, et al. Inference of cellular immune environments in sputum and peripheral blood associated with acute exacerbations of COPD. Cell Mol Bioeng. 2019;12:165–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Li YT, Wang YC, Lee HL, Tsao SC, Lu MC, Yang SF. Monocyte chemoattractant protein‐1, a possible biomarker of multiorgan failure and mortality in ventilator‐associated pneumonia. Int J Mol Sci. 2019;20:E2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lin WC, Lin CF, Chen CL, Chen CW, Lin YS. Prediction of outcome in patients with acute respiratory distress syndrome by bronchoalveolar lavage inflammatory mediators. Exp Biol Med (Maywood). 2010;235:57–65. [DOI] [PubMed] [Google Scholar]
- 204.Barczyk A, Pierzchala W, Sozanska E. Levels of CC‐chemokine (MCP‐1 alpha, MIP‐1 beta) in induced sputum of patients with chronic obstructive pulmonary disease and patients with chronic bronchitis. Pneumonol Alergol Pol. 2001;69:40–9. [PubMed] [Google Scholar]
- 205.Driscoll KE. Macrophage inflammatory proteins: biology and role in pulmonary inflammation. Exp Lung Res. 1994;20:473–90. [DOI] [PubMed] [Google Scholar]
- 206.Baurakiades E, Costa VH, Raboni SM, de Almeida VRT, Larsen KSK, Kohler JN, Gozzo PC, et al. The roles of ADAM33, ADAM28, IL‐13 and IL‐4 in the development of lung injuries in children with lethal non‐pandemic acute infectious pneumonia. J Clin Virol. 2014;61:585–9. [DOI] [PubMed] [Google Scholar]
- 207.De Winter FHR, Jongers B, Bielen K, Mancuso D, Timbermont L, Lammens C, et al. Mechanical ventilation impairs IL‐17 cytokine family expression in ventilator‐associated pneumonia. Int J Mol Sci. 2019;20:E5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Medjo B, Atanaskovic‐Markovic M, Nikolic D, Radic S, Lazarevic I, Cirkovic I, et al. Increased Serum Interleukin‐10 but not Interleukin‐4 Level in Children with Mycoplasma pneumoniae Pneumonia. J Trop Pediatr. 2017;63:294–300. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.


