Abstract
Purpose
To evaluate the ability of the new in vivo corneal indentation device (CID) to measure corneal biomechanical properties.
Methods and Results
In total, 186 eyes from 46 healthy subjects, 107 patients with primary open-angle glaucoma, and 33 patients with ocular hypertension were enrolled in a cross-sectional study. Measurements were performed using corneal visualization Scheimpflug technology (Corvis ST) and the CID. The deformation amplitude (DA), inward applanation time, inward applanation velocity (A1V), outward applanation time (A2T), outward applanation velocity (A2V), highest concavity time, DA ratio, max inverse radius (MIR), integrated radius, and stiffness parameter A1 were included as Corvis ST parameters, and stiffness and modulus were included as CID parameters. Associations between the Corvis ST and CID parameters and correlations between central corneal thickness and corneal biomechanical parameters were analyzed. The stiffness was significantly correlated with all the Corvis ST parameters (P < 0.05). The modulus was significantly correlated with the DA, A1V, A2T, A2V, highest concavity time, and MIR (P < 0.05). The DA, inward applanation time, A1V, A2T, A2V, DA ratio, MIR, integrated radius, and stiffness parameter A1 values and both CID-derived values were significantly correlated with central corneal thickness (P < 0.05).
Conclusions
Parameters derived from the CID and Corvis ST demonstrated agreement in the measurement of corneal biomechanical properties. The stiffness and modulus can characterize in vivo corneal biomechanical properties.
Translational Relevance
Agreeing with the Corvis ST regarding the assessment of corneal biomechanical properties, the CID can be a novel clinical tool for biomechanical evaluation of the cornea.
Keywords: corneal biomechanics, glaucoma, ocular hypertension, Corvis ST, corneal indentation device
Introduction
Corneal biomechanical properties describe the inherent characteristics of the cornea. They are based on ocular tissue structure and biologically function as mechanotransducers of stress. Corneal biomechanical properties have gained increasing research attention and have demonstrated differences across various ocular diseases, such as glaucoma, myopia, and ectatic corneal diseases, possibly related to the occurrence and development of the disease.1–4 Additionally, corneal biomechanical measurement promotes the characterization of susceptibility to ectasia in refractive surgery.
Currently, several instruments are available to assess ocular biomechanical properties in vivo. Substantial clinical use has been reported for diseases, including glaucoma and myopia.3,5 An accurate description of in vivo corneal biomechanical properties may help to better understand the underlying mechanism and the genesis and development of ocular diseases. The most frequently used systems to measure corneal biomechanics in vivo include the ocular response analyzer (Reichert Ophthalmic Instruments, Buffalo, NY) and Corvis ST (Oculus, Wetzlar, Germany), which implements corneal visualization Scheimpflug technology.6,7 They are both noncontact, air-jet tonometers based on the theory of dynamic bidirectional applanation that measure corneal deformation. The Corvis ST has two fundamental differences from the ocular response analyzer. First, instead of using the reflection of the infrared beam to monitor the deformation of the cornea, it uses an ultra-high-speed Scheimpflug camera that takes 140 horizontal 8-mm frames over a period of 31 ms. This approach allows a more detailed evaluation of the deformation process. Also, unlike the ocular response analyzer, the Corvis ST yields a fixed maximal peak pressure for the air puff in each examination. However, they do not output traditional parameters directly based on the tissue, such as the Young's modulus, which has been assessed by tensile, inflation and indentation testing in ex vivo studies. Partially in common with other air puff devices, they are easily affected by interfering factors.8–10
Recently, a new device designed and developed to measure corneal stiffness and modulus in vivo was introduced, the corneal indentation device (CID; Patent No. WO2012163080 A1).11,12 The CID implements a noninvasive corneal indentation method to measure the force required to deform the cornea to a certain depth. This device has been validated by comparison with a universal testing machine.11,13 The repeatability and diurnal variation of the novel parameters have been studied in human subjects. The parameters are repeatable and reproducible,12 while stable throughout the day.14 This method can also determine natural intraocular pressure (IOP) by decreasing the confounding effect of corneal properties.15 Although previous small sample clinical studies have shown that the CID parameters are relevant to a few ocular disorders, including myopia,16,17 no study has yet investigated the agreement in the corneal biomechanical properties as measured by the CID and Corvis ST, which is currently the most widely used device for performing these measurements.
This study aimed to elucidate the application of the CID in measuring corneal biomechanical properties in vivo and evaluate the agreement among the parameters derived from the CID and Corvis ST.
Methods
Subjects
Forty-six healthy subjects, 107 patients with primary open-angle glaucoma (POAG) and 33 patients with ocular hypertension (OHT) were enrolled in this cross-sectional study from September 2018 to December 2019 at Zhongshan Ophthalmic Center, Sun Yat-Sen University in Guangzhou, China. The Medical Ethics Committee of Zhongshan Ophthalmic Center, Sun Yat-Sen University approved the study. The participants provided written informed consent, and the study followed the tenets of the Declaration of Helsinki.
POAG and OHT were diagnosed under the European Glaucoma Society classification guidelines. POAG was defined by the presence of an open angle on gonioscopy, glaucomatous abnormalities of the optic nerve head, and glaucomatous visual field defects. OHT was defined as an open anterior chamber angle, a normal optic disc, and a visual field with an untreated IOP of greater than 21 mm Hg as measured by a Goldmann applanation tonometer (GAT). Healthy controls had healthy discs, an untreated GAT IOP of less than 21 mm Hg and no ocular pathologies except for clinically insignificant senile cataract and myopia. Normative eyes comprised eyes screened for glaucoma, eyes of the hospital staff, and the patients’ relatives.
Exclusion criteria included any history of ocular trauma, corneal diseases, corneal scarification, uveitis or retina disease, any history of corneal laser treatment or ocular or intraocular surgery, any rigid lens wear, any topical cortisone use, pregnancy, and an inability to cooperate with the ocular examinations.
Ophthalmologic Examinations
All patients underwent a complete ophthalmic examination, including corneal biomechanical measurements using the Corvis ST and CID. The patients were examined by anterior segment optical coherence tomography first. The air-puff, indentation, and use of topical anesthetic in the following measurements would cause interference between each other and lead to systematic bias with a fix measuring order. To avoid this event, we conducted the GAT, Corvis ST, and CID measurements randomly with an interval of 15 minutes. Additionally, patients with glaucoma and patients with OHT received a complete glaucoma workup.
GAT (D-KAT; Keeler Ltd, Windsor, UK) IOP measurements were performed by two trained doctors (YX and YW) using a topical anesthetic (ALCAINE 0.5% eye drops; Alcon Laboratories Inc., Fort Worth, TX). Two GAT IOP readings were taken, and the mean result was analyzed.
Central corneal thickness (CCT) was measured by swept-source anterior segment optical coherence tomography using two-dimensional anterior segment scans with a CASIA SS-1000 (Tomey Corp., Aichi, Japan), which was found to have a good agreement with ultrasound pachymetry.18 Automatic measurements were obtained for each scan while the subject focused on a central target inside the instrument.
Corvis ST Tonometer Measurements
Corvis ST (software version 1.6r2015; Oculus, Wetzlar, Germany) measurements were performed three times for each eye. The patients were allowed to rest for at least 1 minute after each test. All the Corvis ST measurements were captured by automatic release on alignment with the corneal apex and had sufficient reliability according to the “OK” quality index displayed on the Corvis ST monitor. The deformation amplitude (DA), inward applanation time (A1T), inward applanation velocity (A1V), outward applanation time (A2T), outward applanation velocity (A2V), highest concavity time (HCT), DA ratio, max inverse radius (MIR), integrated radius (IR), and stiffness parameter A1 (SPA1) were included as the Corvis ST parameters in the study.
CID Measurements
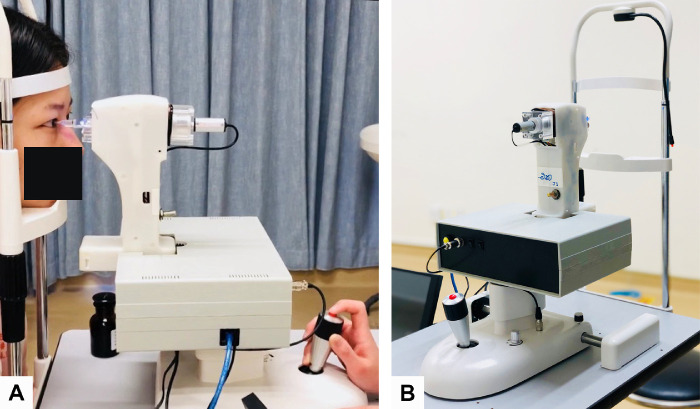
The current prototype of CID contains a main unit, a 2-mm flat-faced indentation probe, a camera, a reset button, a switch button, and a slit lamp as the base device (Fig. 1). The probe was disinfected with povidone iodine and rinsed with 0.9% normal saline. The eyes were anesthetized using ALCAINE 0.5% eye drops before the test. The patient was comfortably positioned with proper placement of the chin and forehead. A frontal view camera was mounted for positioning the indenter at the central cornea. A routine measurement would take approximately 2.7 seconds for indentation. As described in detail previously,11,14,16 the preload was stabilized when the indenter contacted the central cornea (as confirmed by an audible sound). Next, the indenter moved forwards at 12 mm/s to indent the cornea by 1 mm (Fig. 2). Finally, corneal stiffness could be read from the screen. Antibiotic eyedrops (Neomycin; Zhongshan Ophthalmic Center, Sun Yat-Sen University, Guangzhou, China) were used after contact measurement. Three measurements were taken for each eye, and the mean result was analyzed.
Figure 1.
Images of a CID and measurement. (A) CID measurement after disinfection, reset procedures. (B) CID comprises a 2-mm circular flat surface indenter, a main unit, and a computer. Prototype from Hong Kong University of Science and Technology (Patent No. WO2012163080 A1).
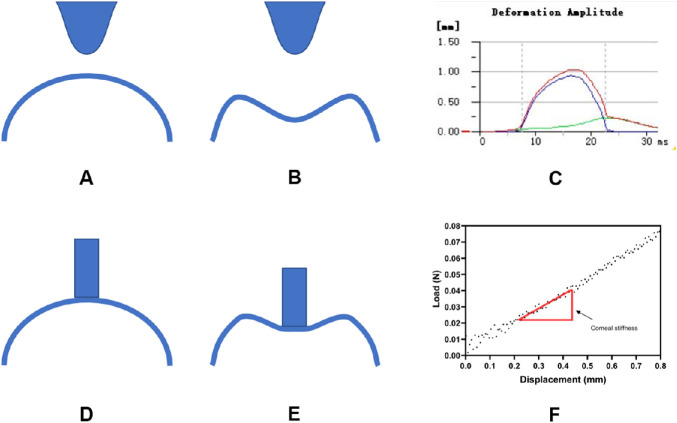
Figure 2.
Measurement difference between Corvis ST and CID. (A) Initial state of Corvis ST measurement. (B) Corvis ST deforms cornea by air-puff. (C) Corneal deformation result by Corvis ST. (D) Initial state of CID measurement with indenter fully contact cornea. (E) CID's indenter moves 1 mm inward. (F) A real force–displacement curve from corneal indentation.)
A previous study demonstrated the good repeatability of the measurements from the CID in normal human subjects.12 The parameters demonstrated good intrasession and intersession repeatability at the same time after one week. In our study, all CID measurements were obtained by a trained doctor (YX), and the intraoperator repeatability was evaluated before the study. Details of the test–retest reliability evaluation of the CID are provided in the Supplementary Material (Fig. S1).
The CID was designed to indent the cornea using a flat punch indenter. The loads and displacements were recorded during corneal indentation. The force–displacement relationship was analyzed after each acquisition. Corneal stiffness was defined as the rate of change of force under a corneal displacement between 0.3 and 0.6 mm, where a high linearity was achieved in the full contact region. The corneal tangent modulus referred to an instantaneous slope at a specific load on a stress-strain curve. It was calculated using a series of mathematical equations that modeled corneal deformation with several metrics, including the CCT, mean corneal radius, and corneal stiffness.14
Statistical Analysis
A sample size of 164 was required to achieve 90% power assuming a Pearson's correlation of 0.25 or higher between two measures for corneal biomechanical parameters using a two-sided test at a significance level of 0.05. The sample size was calculated using PASS 16.0 (NCSS, LLC, Kaysville, UT).
The normality of the data was assessed by specific tests (histogram, Shapiro-Wilk test, and Q–Q plot). The correlation coefficients of corneal biomechanical parameters between both eyes were calculated. Spearman's correlation analysis was performed among the Corvis ST parameters (DA, A1T, A1V, A2T, A2V, HCT, DA ratio, MIR, IR, and SPA1), CID parameters (stiffness and modulus), and CCT. For all tests, the level of significance was set at a P value of less than 0.05. Statistical analysis and randomization were performed using SPSS 21.0 software (IBM SPSS, Armonk, NY). Graphical representation was performed using GraphPad Prism 5.01 (GraphPad Software Inc., La Jolla, CA).
Results
Patient Clinical Characteristics
One hundred eighty-six individuals (81 females and 105 males) aged 38 ± 15 years (range, 18–79 years) had undergone the different assessments. All the correlation coefficients of corneal biomechanical parameters between both eyes were 0.81 to 0.98. One eye was randomly selected from each subject.
One hundred eighty-six eyes were eligible, with 46 eyes (25%) classified as healthy, 107 eyes (57%) classified as having POAG, and 33 eyes (18%) classified as having OHT. The POAG and OHT groups involved patients under treatment. The mean CCT was 538.7 ± 33.2 µm, and the mean IOP was 16.0 ± 4.1 mm Hg (Table 1).
Table 1.
Ocular Information of Healthy Eyes and Eyes With OHT or POAG
| Group | ||||
|---|---|---|---|---|
| Overall | Healthy | OHT | POAG | |
| No. of eyes | 186 | 46 | 33 | 107 |
| CCT (µm) | ||||
| Mean ± SD (range) | 538.7 ± 33.2 (463–627) | 536.0 ± 37.9 (463–607) | 547.0 ± 30.1 (487–615) | 537.3 ± 31.8 (472–627) |
| IOP (mm Hg) | ||||
| Mean ± SD (Range) | 16.0 ± 4.1 (8.3–35.5) | 13.8 ± 3.1 (8.3–20.1) | 19.2 ± 3.4 (11.0–30.0) | 15.8 ± 4.1 (9.0–35.5) |
SD, Standard deviation.
Corneal Biomechanical Characteristics Measured by the Corvis ST and CID
The mean ± standard deviation of DA was 1.09 ± 0.11 mm in the healthy eyes, 1.02 ± 0.14 mm in the POAG eyes, and 0.94 ± 0.09 mm in the OHT eyes. The stiffness was 0.076 ± 0.013 N/mm in the healthy eyes, 0.080 ± 0.018 N/mm in the POAG eyes, and 0.087 ± 0.016 N/mm in the OHT eyes. The modulus of the healthy eyes was 0.614 ± 0.138 MPa, that of the POAG eyes was 0.641 ± 0.148 MPa, and that of the OHT eyes was 0.671 ± 0.154 MPa. The other Corvis ST and CID parameters of the healthy eyes, eyes with OHT and eyes with POAG are summarized in Table 2.
Table 2.
Corneal Biomechanical Parameters Derived From CID and Corvis ST in Healthy Eyes and Eyes With OHT or POAG
| Group | ||||
|---|---|---|---|---|
| Overall | Healthy | OHT | POAG | |
| Corvis ST parameters | ||||
| DA (mm) | 1.02 ± 0.13 | 1.09 ± 0.11 | 0.94 ± 0.09 | 1.02 ± 0.14 |
| A1T (ms) | 7.74 ± 0.51 | 7.51 ± 0.38 | 8.06 ± 0.41 | 7.74 ± 0.53 |
| A1V (m/s) | 0.14 ± 0.02 | 0.15 ± 0.02 | 0.12 ± 0.02 | 0.14 ± 0.02 |
| A2T (ms) | 21.99 ± 0.60 | 22.35 ± 0.41 | 21.66 ± 0.40 | 21.93 ± 0.64 |
| A2V (m/s) | −0.241 ± 0.043 | −0.262 ± 0.033 | −0.214 ± 0.038 | −0.241 ± 0.045 |
| HCT (ms) | 17.32 ± 0.38 | 17.40 ± 0.32 | 17.28 ± 0.39 | 17.29 ± 0.39 |
| MIR (mm−1) | 0.170 ± 0.017 | 0.170 ± 0.017 | 0.163 ± 0.014 | 0.172 ± 0.018 |
| DA Ratio | 4.26 ± 0.46 | 4.40 ± 0.52 | 3.96 ± 0.32 | 4.29 ± 0.43 |
| IR (mm−1) | 7.80 ± 1.11 | 8.20 ± 1.10 | 7.03 ± 0.84 | 7.87 ± 1.08 |
| SPA1 | 113.8 ± 19.2 | 104.9 ± 19.5 | 126.7 ± 18.4 | 113.6 ± 17.2 |
| CID parameters | ||||
| Stiffness (N/mm) | 0.080 ± 0.017 | 0.076 ± 0.013 | 0.087 ± 0.016 | 0.080 ± 0.018 |
| Modulus (MPa) | 0.640 ± 0.147 | 0.614 ± 0.138 | 0.671 ± 0.154 | 0.641 ± 0.148 |
Values are mean ± standard deviation.
Correlations Between the Corvis ST and CID Parameters
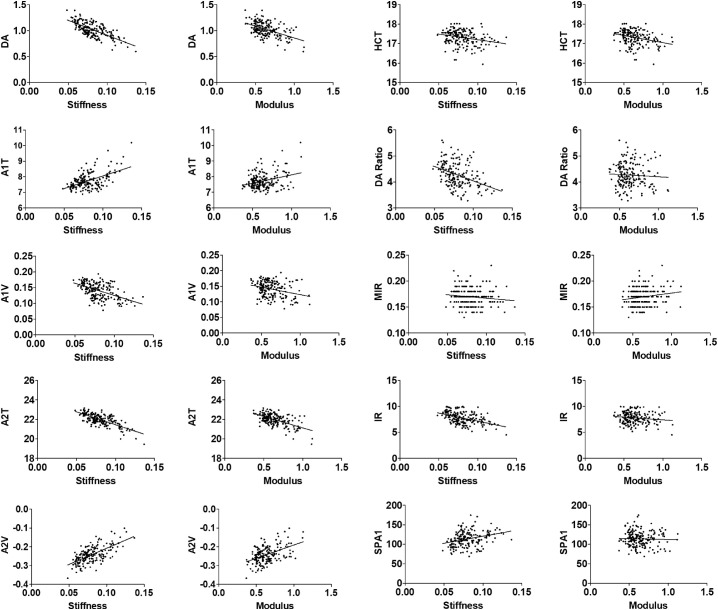
The correlation between the CID parameters (stiffness and modulus) and Corvis ST corneal response parameters was analyzed (Table 3). Across all 186 eyes, stiffness was negatively correlated with the Corvis ST variables DA, A1V, A2T, HCT, DA ratio, MIR, and IR, and positively associated with A1T, A2V, and SPA1. Additionally, the modulus was inversely related to the Corvis ST variables DA, A1V, A2T, and HCT, and correlated positively with A2V and MIR. Figure 3 shows scatterplots between the CID parameters (stiffness and modulus) and Corvis ST parameters (DA, A1T, A1V, A2T, A2V, HCT, DA ratio, MIR, IR, and SPA1). The Corvis ST-derived DA was moderately related to the CID-derived stiffness (γ = −0.659, P < 0.001) and modulus (γ = −0.422, P < 0.001). The DA ratio and SPA1 were associated with stiffness (γ = −0.407, P < 0.001; γ = 0.341, P < 0.001, respectively) but not significantly related to the modulus (P > 0.05).
Table 3.
Correlation Between Corvis ST Parameters and CID Parameters of Overall Subjects
| Stiffness | Modulus | |||
|---|---|---|---|---|
| γ | P Value | γ | P Value | |
| Direct indicators | ||||
| DA | −0.659 | <.001 | −0.422 | <.001 |
| A1T | 0.409 | <.001 | 0.143 | .052 |
| A1V | −0.492 | <.001 | −0.238 | .001 |
| A2T | −0.634 | <.001 | −0.467 | <.001 |
| A2V | 0.601 | <.001 | 0.397 | <.001 |
| HCT | −0.271 | <.001 | −0.304 | <.001 |
| Aggregative indicators | ||||
| DA ratio | −0.407 | <.001 | −0.030 | .689 |
| MIR | −0.174 | .018 | 0.151 | .039 |
| IR | −0.457 | <.001 | −0.100 | .175 |
| SPA1 | 0.341 | <.001 | −0.037 | .614 |
Figure 3.
Correlation between CID parameters (Stiffness, Modulus) and Corvis ST parameters (DA, A1T, A1V, A2T, A2V, HCT, DA Ratio, MIR, IR, SPA1).
Correlations Between Corneal Biomechanical Parameters and CCT
The correlations between Corvis ST- and CID-measured corneal biomechanical parameters and CCT were analyzed (Table 4). Across all 186 eyes, the Corvis ST variables DA, A1V, A2T, DA ratio, MIR, and IR were inversely related to the CCT, whereas the A1T, A2V, and SPA1 were positively associated with the CCT. The stiffness correlated positively with the CCT, whereas the modulus was negatively related to the CCT.
Table 4.
Correlation Between Corneal Biomechanical Parameters and CCT of Overall Subjects
| CCT | ||
|---|---|---|
| γ | P Value | |
| Corvis ST parameters | ||
| DA | −0.334 | <.001 |
| A1T | 0.392 | <.001 |
| A1V | −0.398 | <.001 |
| A2T | −0.218 | .003 |
| A2V | 0.281 | <.001 |
| HCT | 0.079 | .287 |
| DA ratio | −0.657 | <.001 |
| MIR | −0.609 | <.001 |
| IR | −0.590 | <.001 |
| SPA1 | 0.654 | <.001 |
| CID parameters | ||
| Stiffness | 0.171 | .019 |
| Modulus | −0.385 | <.001 |
Discussion
Because corneal biomechanics have a potential impact on many aspects of ophthalmic practice and research, we evaluated whether the CID, a novel indentation instrument, can describe the in vivo biomechanical properties of the cornea. In the current study, we obtained measurements using the Corvis ST and CID from 186 eyes of 46 healthy participants, 107 patients with POAG, and 33 patients with OHT. The findings revealed good agreement between the CID and Corvis ST in assessing corneal biomechanical properties, indicating that the stiffness and modulus from the CID can characterize in vivo corneal biomechanical properties.
Based on corneal deformation, the CID and Corvis ST estimate corneal biomechanical properties via different technologies. The Corvis ST produces an air pulse at a fixed pressure and measures corneal displacement by recording the shape of the corneal deformation with a high-speed camera19,20; corneas with different biomechanical properties have different degrees of deformation under the same air pressure. The CID uses a load cell to measure the exact force exerted when the indenter fully contacts the central cornea and moves a certain distance forward. This force can be described in Newtons for a fixed displacement, and the stress–strain relationship can then be plotted and analyzed afterward.13,15 From the perspective of design principles, the CID's stress–strain relationship is theoretically more accurate than the Corvis ST to characterize corneal biomechanical properties.
Although they both characterize corneal biomechanical properties, the parameters obtained using the Corvis ST and CID are derived according to different theories. The Corvis ST describes corneal biomechanical properties using the DA, A1T, A1V, A2T, A2V, DA ratio, IR, and SPA1, among others. These parameters are obtained by analyzing video frames and detailing the shape of corneal deformation, making the Corvis ST an acceptable device for indirectly measuring corneal biomechanical properties. Although these amplitude, time, and velocity parameters are related to biomechanical tissue features, little evidence suggests that they can be used as standard parameters of tissue elasticity or viscosity.21 Furthermore, these metrics rely on images and are thus highly dependent on the camera shooting quality and are easily influenced by confounding factors, including corneal irregularities, fixation, blinking, the size of the eyelids, or eye movement.22 CID parameters are directly measured from the cornea or derived from mechanical algorithms. Stiffness indicates the force–displacement association of the corneal tissue during indentation and represents the rigidity of the cornea. The modulus is calculated as the rate of change at a specific pressure of the nonlinear stress–strain curve and represents the elastic property of the tissue.11,23,24 These parameters are widely used in physics-based biomechanical studies. For example, the ex vivo corneal biomechanical properties in human cadaver tissue have been described by the modulus of elasticity in studies both on inflation testing25 and atomic force microscopy.26,27 With the CID, this metric is available for further in vivo studies.
As a novel device for assessing indentation to measure corneal biomechanical properties, the CID must be evaluated for measurement credibility. Currently, the most widely used in vivo corneal biomechanics assessment device is the Corvis ST. By assessing the degree of agreement in the corneal biomechanical properties measured between the two devices, we can evaluate whether the CID-derived parameters—stiffness and modulus—can be used to characterize in vivo corneal biomechanical properties. In the present study, the CID metrics (stiffness and modulus) were closely related to the Corvis ST parameters. A stiff cornea presented with a low DA and DA ratio and a high SPA1 from the Corvis ST and a high stiffness from the CID. This finding agrees with previous studies showing that a higher DA ratio and lower SPA1 suggest a softer cornea, including those with keratoconus.6,28 From this perspective, the stiffness value agrees with the DA, DA ratio, and SPA1 when describing corneal biomechanical properties. Meanwhile, the modulus was correlated with the DA. To some extent, agreement exists between the parameters derived from the CID and Corvis ST in the measurement of corneal biomechanical properties. Therefore, the stiffness and modulus can describe in vivo corneal biomechanical properties.
In previous ex vivo studies, thicker corneas tended to be stiffer, with more collagen fibers and greater ground substance, resulting in smaller deformations under the same amount of pressure.29 In previous Corvis ST studies, parameters such as the A1T, A1V, A2V, DA, and SPA1 were correlated significantly with the CCT, indicating that thicker corneas required a greater force to indent.30,31 Thus, in our current study, the DA and DA ratios decreased with increasing CCT, whereas the SPA1 was positively correlated with the CCT, demonstrating that thick corneas are less deformable. According to the results of the CID, stiffness increases with increasing CCT. The correlation coefficient was low, but the correlation was statistically significant, indicating that a thick cornea requires more force to produce unit deformation. The modulus was inversely correlated with CCT, suggesting that thick corneas are less elastic. In this regard, the CID-derived stiffness and Corvis ST metrics described elsewhere in this article were in accordance, whereas the modulus showed the opposite relationship. Unlike stiffness, which involves measuring the required force for deformation and is structure dependent, the modulus is an intensive property of the material. This result follows the law of Laplace32 in that the tangential tensile stress is higher with a lower CCT under the same range of IOPs, thus increasing the modulus. In ex vivo studies, the corneal modulus was increased after laser ablation surgeries33,34 with a decrease in CCT, in agreement with our study results. Because the Corvis ST lacks parameters that reflect the elastic properties of tissue, the CID is more likely a more comprehensive device than the Corvis ST in assessing biomechanical properties.
A limitation of the present study is the narrow study sample. We only included patients with glaucoma, which is a common disease relevant to corneal biomechanics. However, whether consistent results can be obtained with other diseases (eg, high myopia or keratoconus) is unknown, and further studies are needed. Additionally, among the glaucomatous samples, the corneal biomechanical properties for different types and stages of glaucoma were not considered, particularly for POAG and normal tension glaucoma. The relationship between IOP and corneal biomechanical properties was not measured, and future studies should also address these limitations.
In conclusion, the parameters derived from the CID and Corvis ST were in agreement regarding the measurement of corneal biomechanical properties. The stiffness and modulus from the CID can characterize in vivo corneal biomechanical properties.
Supplementary Material
Acknowledgments
Supported by the Hong Kong Innovation and Technology Fund (Grant No. MRP/039/18X).
Disclosure: Y. Xu, None; Y. Ye, None; I.T. Chong, None; Z. Chen, None; J. Xu, None; Y. Yang, None; K. Yu, None; D.C.C. Lam, None; M. Yu, None
References
- 1.Susanna CN, Diniz-Filho A, Daga FB, et al.. A prospective longitudinal study to investigate corneal hysteresis as a risk factor for predicting development of glaucoma. Am J Ophthalmol. 2018; 187: 148–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Susanna BN, Ogata NG, Jammal AA, Susanna CN, Berchuck SI, Medeiros FA.. Corneal biomechanics and visual field progression in eyes with seemingly well-controlled intraocular pressure. Ophthalmology. 2019; 126: 1640–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chansangpetch S, Panpruk R, Manassakorn A, et al.. Impact of myopia on corneal biomechanics in glaucoma and nonglaucoma patients. Invest Ophthalmol Vis Sci. 2017; 58: 4990–4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ambrosio R Jr., Lopes BT, Faria-Correia F, et al.. Integration of Scheimpflug-based corneal tomography and biomechanical assessments for enhancing ectasia detection. J Refract Surg. 2017; 33: 434–443. [DOI] [PubMed] [Google Scholar]
- 5.Salvetat ML, Zeppieri M, Tosoni C, Felletti M, Grasso L, Brusini P.. Corneal deformation parameters provided by the Corvis-ST pachy-tonometer in healthy subjects and glaucoma patients. J Glaucoma. 2015; 24: 568–574. [DOI] [PubMed] [Google Scholar]
- 6.Roberts CJ, Mahmoud AM, Bons JP, et al.. Introduction of two novel stiffness parameters and interpretation of air puff-induced biomechanical deformation parameters with a dynamic Scheimpflug analyzer. J Refract Surg. 2017; 33: 266–273. [DOI] [PubMed] [Google Scholar]
- 7.Luce DA.Determining in vivo biomechanical properties of the cornea with an ocular response analyzer. J Cataract Refract Surg. 2005; 31: 156–162. [DOI] [PubMed] [Google Scholar]
- 8.Roberts CJ.Concepts and misconceptions in corneal biomechanics. J Cataract Refract Surg. 2014; 40: 862–869. [DOI] [PubMed] [Google Scholar]
- 9.Kling S, Hafezi F.. Corneal biomechanics - a review. Ophthalmic Physiol Opt. 2017; 37: 240–252. [DOI] [PubMed] [Google Scholar]
- 10.Sit AJ, Lin SC, Kazemi A, McLaren JW, Pruet CM, Zhang X.. In vivo noninvasive measurement of young's modulus of elasticity in human eyes: a feasibility study. J Glaucoma. 2017; 26: 967–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ko MW, Leung LK, Lam DC, Leung CK.. Characterization of corneal tangent modulus in vivo. Acta Ophthalmol. 2013; 91: e263–269. [DOI] [PubMed] [Google Scholar]
- 12.Lam AK, Hon Y, Leung LK, Lam DC.. Repeatability of a novel corneal indentation device for corneal biomechanical measurement. Ophthalmic Physiol Opt. 2015; 35: 455–461. [DOI] [PubMed] [Google Scholar]
- 13.Ko MW, Leung LK, Lam DC.. Comparative study of corneal tangent elastic modulus measurement using corneal indentation device. Med Eng Phys. 2014; 36: 1115–1121. [DOI] [PubMed] [Google Scholar]
- 14.Hon Y, Wan K, Chen GZ, Lu SH, Lam DC, Lam AK.. Diurnal variation of corneal tangent modulus in normal Chinese. Cornea. 2016; 35: 1600–1604. [DOI] [PubMed] [Google Scholar]
- 15.Lu SH, Chong IT, Leung SYY, Lam DCC.. Characterization of corneal biomechanical properties and determination of natural intraocular pressure using CID-GAT. Transl Vis Sci Technol. 2019; 8: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hon Y, Chen GZ, Lu SH, Lam DC, Lam AK.. High myopes have lower normalised corneal tangent moduli (less 'stiff' corneas) than low myopes. Ophthalmic Physiol Opt. 2017; 37: 42–50. [DOI] [PubMed] [Google Scholar]
- 17.Lam AK, Leung SY, Hon Y, et al.. Influence of short-term orthokeratology to corneal tangent modulus: a randomized study. Current Eye Res. 2018; 43: 474–481. [DOI] [PubMed] [Google Scholar]
- 18.Scotto R, Bagnis A, Papadia M, Cutolo CA, Risso D, Traverso CE.. Comparison of central corneal thickness measurements using ultrasonic pachymetry, anterior segment OCT and noncontact specular microscopy. J Glaucoma. 2017; 26: 860–865. [DOI] [PubMed] [Google Scholar]
- 19.Correia FF, Ramos I, Roberts CJ, Steinmueller A, Krug M, Ambrósio R Jr. Impact of chamber pressure and material properties on the deformation response of corneal models measured by dynamic ultra-high-speed Scheimpflug imaging. Arq Brasil Oftalmol. 2013; 76: 278–281. [DOI] [PubMed] [Google Scholar]
- 20.Koprowski R.Automatic method of analysis and measurement of additional parameters of corneal deformation in the Corvis tonometer. Biomed Eng Online. 2014; 13: 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Francis M, Matalia H, Nuijts R, Haex B, Shetty R, Sinha Roy A. Corneal viscous properties cannot be determined from air-puff applanation. J Refract Surg. 2019; 35: 730–736. [DOI] [PubMed] [Google Scholar]
- 22.Lanza M, Iaccarino S, Bifani M.. In vivo human corneal deformation analysis with a Scheimpflug camera, a critical review. J Biophotonics. 2016; 9: 464–477. [DOI] [PubMed] [Google Scholar]
- 23.Boyce BL, Jones RE, Nguyen TD, Grazier JM.. Stress-controlled viscoelastic tensile response of bovine cornea. J Biomech. 2007; 40: 2367–2376. [DOI] [PubMed] [Google Scholar]
- 24.Buzard KA.Introduction to biomechanics of the cornea. Refract Corneal Surg. 1992; 8: 127–138. [PubMed] [Google Scholar]
- 25.Elsheikh A, Alhasso D, Rama P.. Biomechanical properties of human and porcine corneas. Exp Eye Res. 2008; 86: 783–790. [DOI] [PubMed] [Google Scholar]
- 26.Lombardo M, Lombardo G, Carbone G, De Santo MP, Barberi R, Serrao S.. Biomechanics of the anterior human corneal tissue investigated with atomic force microscopy. Invest Ophthalmol Vis Sci. 2012; 53: 1050–1057. [DOI] [PubMed] [Google Scholar]
- 27.Dias JM, Ziebarth NM.. Anterior and posterior corneal stroma elasticity assessed using nanoindentation. Exp Eye Res. 2013; 115: 41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vinciguerra R, Ambrosio R Jr., Elsheikh A, et al.. Detection of keratoconus with a new biomechanical index. J Refract Surg. 2016; 32: 803–810. [DOI] [PubMed] [Google Scholar]
- 29.Liu J, Roberts CJ.. Influence of corneal biomechanical properties on intraocular pressure measurement: quantitative analysis. J Cataract Refract Surg. 2005; 31: 146–155. [DOI] [PubMed] [Google Scholar]
- 30.Valbon BF, Ambrosio R Jr., Fontes BM, Luz A, Roberts CJ, Alves MR. Ocular biomechanical metrics by CorVis ST in healthy Brazilian patients. J Refract Surg. 2014; 30: 468–473. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Wang Y, Li L, et al.. Corneal stiffness and its relationship with other corneal biomechanical and nonbiomechanical parameters in myopic eyes of Chinese patients. Cornea. 2018; 37: 881–885. [DOI] [PubMed] [Google Scholar]
- 32.Basford JR.The law of Laplace and its relevance to contemporary medicine and rehabilitation. Arch Phys Med Rehabil. 2002; 83: 1165–1170. [DOI] [PubMed] [Google Scholar]
- 33.Dupps WJ Jr., Wilson SE. Biomechanics and wound healing in the cornea. Exp Eye Res. 2006; 83: 709–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang X, Li X, Chen W, He R, Gao Z, Feng P.. Effects of ablation depth and repair time on the corneal elastic modulus after laser in situ keratomileusis. Biomed Eng Online. 2017; 16: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.