Abstract
Background: Ambient temperature is predicted to rise in Saudi Arabia, and how this will impact the health of its population has not been investigated. Saudi Arabia is one of the top ten countries with the highest prevalence of diabetes. The current study investigates the correlation between ambient temperature and HbA1c levels in a group of Saudis in Riyadh. Methods: Age, gender, and HbA1c data for six years were obtained from patients’ records. The maximum daily temperature of Riyadh city for the same period was obtained. Results: A total of 168,614 patient records were obtained. There was a statistically significant positive correlation between ambient temperature and HbA1c levels, where for each 1°C increase in average weekly temperature HbA1c increased by 0.007%. Patients were at higher risk of having HbA1c ≥ 7% in high and moderate temperature than in low temperature (P < 0.001, odds ratio (OR): 1.134, and P < 0.001, odds ratio (OR): 1.034; respectively). The mean of HbA1c in females (7.27±1.96) was significantly lower than in males (7.40±1.86), and the probability of males having HbA1c ≥ 7% was about 17.4% higher than females. However, the HbA1c levels in females were significantly more affected by rising temperature compared to males (B = 0.003, P = 0.008). Conclusion: Overall, rise in ambient temperature is associated with worsening HbA1c, which could be harmful to the health of Saudis suffering from diabetes. Possible reasons for the increase in HbA1c could include reduced physical activity, reduced sunlight exposure, and dehydration during hot weather. More research on the relationship between climate change and public health in Saudi Arabia is needed.
Keywords: Diabetes, HbA1c, temperature, weather
Introduction
According to the International Diabetes Federation (IDF), the prevalence of diabetes mellitus (DM) in adult Saudis in 2017 was 18.6%, and is estimated to reach 23.8% by 2045 [1]. There is a linkage between genetic and risk factors such as obesity, physical inactivity, and unhealthy diet, and the development of type 2 diabetes mellitus (T2DM) [2]. There is a worldwide concern that the global increase in temperature may adversely affect health [3,4]. In Saudi Arabia, there was an increase in the rate of maximum temperature of 0.8°C per decade over 1979 to 2009 [5], and this is predicted to increase by 1.2°C-1.6°C during 2025-2044 [6]. The impacts of climate change on the health of Saudis have not been thoroughly investigated, and there are no studies that relate ambient temperature with health of diabetics in this population. There are studies in the literature on other populations that have shown the relationship between ambient temperature and the health of diabetics [7-13]. The prevalence of T2DM was found to be positively associated with increase in the ambient temperature in a study conducted across the mainland USA [14].
Glycated haemoglobin (HbA1c) is a primary marker for the diagnosis of diabetes; it mirrors the average of plasma glucose over the previous 8-12 weeks [15]. It has been reported that plasma glucose levels reflect 50% of the HbA1c levels during the past 30 days [16]. Indeed, for each 1% decrease in HbA1c level, myocardial infarction declines by 14%, microvascular complications reduce by 37%, and diabetes-related deaths decrease by 21% [17]. Different factors have been reported to modulate HbA1c levels, such as weather (seasons) [7-13], and social events and holidays [18-20].
The main aim of this study was to investigate the impacts of ambient temperature on HbA1c levels in a Saudi population from Riyadh city. This was carried out by correlating temperature with HbA1c levels over six years.
Methodology
Study setting and design
This was a retrospective study conducted at the Security Forces Hospital in Riyadh, Saudi Arabia. Data of all patients who had their HbA1c analysed between the period from 15 November 2012 to 09 September 2018 were retrieved from the patients’ medical records. These data covered six Hijri years. Hijri year consists of 12 lunar months in a year (354 or 355 days). The collected data contained information regarding gender, age, nationality, HbA1c results, and the result date. The data were grouped in Hijri months, and then each month was grouped to four weeks. Only data from adult (≥ 18 years) Saudis were included in the analysis. HbA1c results are expressed in percentage (%) and were measured using a high-performance liquid chromatography instrument Tosoh G8 (G8 HPLC Analyzer, Tosoh Bioscience Inc., CA, USA). Additionally, the maximum daily temperature in degrees Celsius (°C) for Riyadh city was obtained from the General Authority of Meteorology and Environmental Protection (GAMEP), Saudi Arabia, for the study period. The obtained ambient temperature was grouped in Hijri months and each month was grouped to four weeks. From this, the average weekly maximum temperature (AWMT) was calculated.
Data analysis
Data were analysed using SPSS software version 25 (IBM Corporation, Armonk, NY, USA). Descriptive statistics analysis was reported for the study outcomes where mean ± standard deviation (SD) was used for continuous variables, while counts and percentages were used for the categorical variables. HbA1c data were non-normally distributed, and therefore were transformed using a two-step transformation approach suggested by Templeton to assume adequate normality [21]. The transformed HbA1c variable was used in all analysis. The average weekly maximum temperature was categorized into three equal cut-off points: high (> 35.8°C), moderate (26.1°C-35.8°C), and low (< 26.1°C). Welch’s ANOVA with post hoc analysis using Games-Howell tests were performed to determine if there were differences in the mean of HbA1c values between months and different temperature categories. Unpaired t-tests were used to assess the differences between groups and subgroups. Multiple linear regression model was carried out to determine the association between HbA1c as dependent variable, and the AWMT, age, gender, and interaction terms as independent variables. Interaction between age and AWMT (age*AWMT), and gender and AWMT (gender*AWMT) were added to the previous linear regression model to evaluate how age and gender moderate the AWMT as a predictor of HbA1c level. Prior to this model, mean age, mean gender, and mean AWMT were centred to decrease the possibility of multicollinearity between interaction terms included in this model. These centred means were used in the multiple linear regression model. Subsequently, a logistic regression model was used to find the odds ratio (OR) and 95% confidence intervals (95% CIs) for the chance to have HbA1c ≥ 7% (poor glycaemic control). In this model, HbA1c ≥ 7% was set as the dependent variable, and AWMT (categorical variable), age, and gender as independent variables. Chi-square test was used to assess the significant variances between groups and subgroups of the categorical variables. Correlations between continuous variables were assessed by using Pearson Correlation. P < 0.05 (two-tailed) was considered significant for all tests.
Results
A total of 168,614 patient records were eligible and included for the analysis in this study. Table 1 describes the demographics of the study population. In term of gender, 56.44% (n = 95162) belonged to females and 43.56% (n = 73452) were males. Patients’ age ranged from 18 to 119 years old, and the mean age ± SD was 54.21±15.60 years. The overall mean HbA1c (%) ± SD in all patients was 7.32±1.92 (male = 7.40±1.86; female = 7.27±1.96). The mean HbA1c in females was significantly lower than in males (P < 0.001). The distribution of HbA1c categories significantly differed between genders (P < 0.001).
Table 1.
Baseline characteristics of patient records (n = 168,614)
| Total (n=168,614) | Gender (Mean ± SD or n (%)) | P Value | ||
|---|---|---|---|---|
|
| ||||
| Female (n = 95,162) | Male (n = 73,452) | |||
| Age (years) | 54.21±15.60 | 53.94±15.61 | 54.56±15.58 | < 0.001a |
| 18-24 | 9,419 (5.59) | 5,457 (3.24) | 3,962 (2.35) | < 0.001b |
| 25-39 | 19,283 (11.44) | 10,834 (6.43) | 8,449 (5.01) | |
| 40-49 | 29,261 (17.35) | 16,897 (10.02) | 12,364 (7.33) | |
| 50-59 | 48,005 (28.47) | 27,695 (16.43) | 20,310 (12.05) | |
| 60-69 | 36,220 (21.48) | 19,806 (11.75) | 16,414 (9.73) | |
| 70-79 | 18,760 (11.13) | 10,272 (6.09) | 8,488 (5.03) | |
| ≥ 80 | 7,666 (4.55) | 4,201 (2.49) | 3,465 (2.05) | |
| HbA1c % | 7.32±1.92 | 7.27±1.96 | 7.40±1.86 | < 0.001a |
| < 5.7 | 31649 (18.77) | 19368 (11.49) | 12281 (7.28) | < 0.001b |
| 5.7-6.49 | 26566 (15.76) | 15255 (9.05) | 11311 (6.71) | |
| 6.5-8.99 | 78575 (46.60) | 42558 (25.24) | 36017 (21.36) | |
| 9-11.99 | 30360 (18.17) | 17313 (10.27) | 13317 (7.90) | |
| ≥ 12 | 1194 (0.71) | 668 (0.40) | 526 (0.31) | |
P values based on unpaired t-test.
Chi-square test was used.
SD, standard deviation.
The AWMT ranged from 16.26°C to 45.59°C during the study period, with an overall mean ± SD of 32.68±8.64°C. Figure 1 shows a significant positive correlation between the mean of HbA1c and the mean of AWMT over consecutive six years in Riyadh city (r = 0.031, P < 0.001). The mean of HbA1c gradually increased from low-temperature months to high-temperature months. However, this relationship was disrupted by the month of Ramadan even though this month coincided with a high-temperature period. The mean of HbA1c declined during the month of Ramadan reaching the lowest level compared to other months of the Hijri year.
Figure 1.
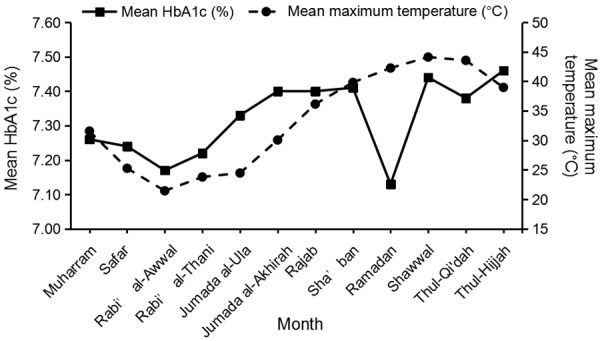
Correlation between the mean of monthly HbA1c levels and the mean of monthly maximum temperature.
Table 2 presents the means of HbA1c and temperature in each month. Comparisons of HbA1c levels between different temperature categories were conducted using Welch’s ANOVA test (assuming heterogeneous variances which assessed by Levine’s test [P < 0.05]). Welch’s ANOVA test revealed that there were significant differences in HbA1c levels between different temperature levels (F(2, 102919.88) = 99.13, P < 0.001). Post hoc analysis using Games-Howell tests indicated that the lowest mean HbA1c was observed during the low-temperature period. The mean of HbA1c in low temperatures was significantly lower than during high temperatures and non-significantly lower than in moderate temperatures, (P < 0.001 and P = 0.143; respectively). Mean of HbA1c in moderate temperatures was significantly lower than in the high-temperature category (P < 0.001). Figure 2 shows the relationship between the mean of HbA1c and different levels of temperature. The mean difference in HbA1c between high temperature and low temperature was 0.14%, between moderate temperature and low temperature was 0.02%, and between high temperature and moderate temperature was 0.12%.
Table 2.
The monthly mean of HbA1c, and maximum temperature for six years in a Saudi population from Riyadh city (n = 168,614)
| Month | Mean HbA1c (%) ± SD | Temperature (°C) (mean ± SD) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Total | Female | Male | Year one | Year two | Year three | Year four | Year five | Year six | ||
| Muharram | 7.26±2.01 | 7.18±2.06 | 7.36±1.94 | 7.59±1.79 | 7.56±1.77 | 7.46±2.13 | 7.02±1.86 | 6.84±2.14 | 7.71±1.78 | 31.57±4.70 |
| Safar | 7.24±1.92 | 7.18±1.95 | 7.33±1.86 | 7.69±1.76 | 7.52±1.77 | 7.26±1.84 | 7.07±1.89 | 6.98±2.07 | 7.36±1.86 | 25.27±4.90 |
| Rabi’ al-Awwal | 7.17±2.02 | 7.08±±2.07 | 7.28±1.95 | 7.59±1.85 | 7.75±1.74 | 6.71±2.16 | 6.86±2.08 | 7.02±2.07 | 7.42±1.81 | 21.45±2.51 |
| Rabi’ al-Thani | 7.22±1.99 | 7.17±2.03 | 7.28±1.94 | 7.95±1.75 | 7.63±1.90 | 6.76±2.04 | 6.97±2.01 | 7.11±2.06 | 7.39±1.79 | 23.80±3.16 |
| Jumada al-Ula | 7.33±1.96 | 7.28±2.00 | 7.40±1.91 | 7.83±2.05 | 7.85±1.67 | 6.97±1.95 | 7.18±1.90 | 7.13±2.10 | 7.39±1.85 | 24.47±4.84 |
| Jumada al-Akhirah | 7.40±1.92 | 7.32±1.96 | 7.50±1.87 | 8.00±1.97 | 7.65±1.72 | 7.13±1.98 | 7.24±1.96 | 7.37±1.93 | 7.32±1.91 | 30.07±4.08 |
| Rajab | 7.40±1.85 | 7.36±1.90 | 7.46±1.79 | 7.73±1.97 | 7.53±1.72 | 7.06±1.90 | 7.39±1.97 | 7.48±1.80 | 7.36±1.81 | 36.15±3.25 |
| Sha’ban | 7.41±1.81 | 7.36±1.84 | 7.49±1.77 | 7.67±1.80 | 7.47±1.75 | 7.35±1.83 | 7.29±1.93 | 7.46±1.76 | 7.36±1.81 | 39.83±2.86 |
| Ramadan | 7.13±1.98 | 7.06±2.03 | 7.21±1.92 | 7.52±1.78 | 7.41±1.92 | 7.29±1.98 | 6.70±2.25 | 7.21±1.90 | 6.85±1.89 | 42.26±1.95 |
| Shawwal | 7.44±1.80 | 7.39±1.86 | 7.49±1.72 | 7.80±1.70 | 7.45±1.71 | 7.45±1.78 | 7.43±1.89 | 7.50±1.83 | 7.12±1.81 | 44.12±0.92 |
| Thul-Qi’dah | 7.38±1.80 | 7.35±1.85 | 7.42±1.74 | 7.66±1.73 | 7.46±1.74 | 7.15±1.84 | 7.43±1.87 | 7.64±1.72 | 7.02±1.84 | 43.56±2.08 |
| Thul-Hijjah | 7.46±1.89 | 7.39±1.93 | 7.54±1.83 | 7.61±1.77 | 7.84±1.75 | 7.26±1.89 | 7.16±2.06 | 7.73±1.80 | 6.97±1.92 | 38.98±3.35 |
| Total | 7.32±1.92 | 7.27±1.96 | 7.40±1.86 | 7.74±1.84 | 7.60±1.76 | 7.14±1.97 | 7.16±1.97 | 7.26±1.97 | 7.30±1.85 | 32.68±8.64 |
SD, standard deviation.
Figure 2.
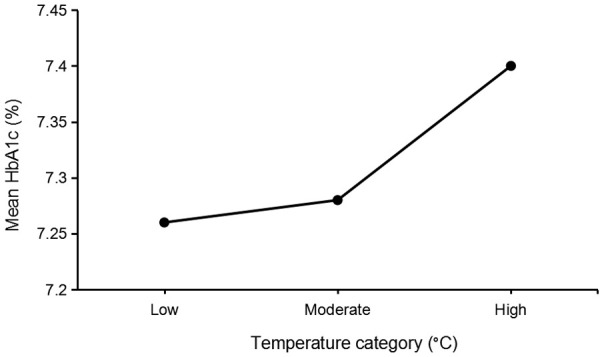
Relationship between the mean of HbA1c and different temperature levels [high (> 35.8°C), moderate (26.1°C-35.8°C), and low (< 26.1°C)].
Multiple linear regression model was carried out to determine the association between HbA1c levels and the average weekly maximum temperature. This revealed that 8.2% (R2 = 0.082) of the variance in HbA1c was explained by the regression model. The AWMT significantly contributed to the model (B = 0.007, P < 0.001). This indicates that for each 1°C increase in the AWMT, the HbA1c increases by 0.007%. For further statistical analysis, HbA1c values were divided into two groups (HbA1c < 7% and HbA1c ≥ 7%, good glycaemic control and poor glycaemic control; respectively) [22]. Subsequently, binary logistic regression models were conducted to determine the odds ratio (OR) for the patients at risk of having an HbA1c ≥ 7 with the different levels of AWMT as a categorical variable. The binary logistic regression analysis showed that the high-temperature category and the moderate temperature category were significant predictors for HbA1c variability of having an HbA1c ≥ 7% [Chi-Square= 13651.31, df = 4 and P < 0.001]. All predictors explained 10.4% (Nagelkerke R2) of the variability of HbA1 for HbA1c ≥ 7%. High temperature significantly contributed to the model (P < 0.001), and the odds ratio (OR) was 1.134 (95% CI 1.107-1.162) to have HbA1c ≥ 7% compared to low temperature. Similarly, moderate temperature significantly contributed to the model (P = 0.012), and the odds ratio (OR) was 1.034 (95% CI 1.007-1.062).
The observed decline in HbA1c during the month of Ramadan was investigated by conducting the Welch’s ANOVA test between months, which indicated that there were significant differences in the mean of HbA1c between months, Welch’s F (11, 168602) = 40.19, P < 0.001. Then, post hoc comparisons, using the Games-Howell post hoc measure, shown that the mean of HbA1c during the month of Ramadan was significantly lower than all other months except with the months of Rabi’ al-Awwal and Rabi’ al-Thani where the reductions were non-significant (P = 0.997 and P = 0.188; respectively).
There was a significant positive correlation between the mean HbA1c and age (r = 0.282, N = 168,614, P < 0.001). Age contributed significantly to the multiple linear regression model (B = 0.035, P < 0.001), and for each extra year in age, HbA1c increases by 0.035%. The interaction between age and AWMT did not contribute significantly to multiple linear regression (P = 0.252). This reveals that age did not moderate the association between AWMT and HbA1c level. For each additional year in age, the odds of having HbA1c ≥ 7% are higher by an odds ratio (OR) of 1.039 (95% CI 1.038-1.040). Interestingly, the mean of HbA1c in males (M = 7.40, SD = 1.86) was significantly higher than in females (M = 7.27, SD = 1.96), P < 0.001, with mean difference 0.13%. Gender contributed significantly to the multiple linear regression model (B = -0.112, P < 0.001). This indicates that if the gender was female, the HbA1c decreased by 0.112%. The interaction between gender and AWMT significantly contributed to the multiple linear regression model (B = 0.003, P = 0.008). This shows that if the gender was female, HbA1c increased by 0.003% more than in males for each 1°C increase in AWMT. Additionally, the binary logistic regression model indicated that gender was a significant predictor for having an HbA1c ≥ 7% (P < 0.001). The odds ratio (OR) for males to have HbA1c ≥ 7% was 1.174 (95% CI 1.151-1.198) more than females.
Discussion
Recently, researchers have shown an increased interest in assessing the impact of climate change on health [3,23,24], including diabetes [8,25-27]. It has been reported that Saudi Arabia is predicted to see an increase in temperature, but as yet the impact of this on the health of its population has not been investigated. To the best of our knowledge, this is the first study to assess the impact of ambient temperature on HbA1c levels for a population in Saudi Arabia.
The findings of the current study suggest that ambient temperature has a significant impact on HbA1c levels in the study population with worsening of HbA1c levels at higher temperature. The findings of this study are consistent with previous studies. For example, Blauw et al. [25] reported that the global prevalence of glucose intolerance and age-adjusted diabetes incidence in the USA increased with increase in outdoor temperature. A recent study demonstrated that fasting plasma glucose, two hours of plasma glucose, and insulin resistance were positively correlated with ambient temperature [26]. However, some studies have reported different patterns of the seasonal variations in HbA1c. For example, low HbA1c levels or fasting plasma glucose were observed in high temperatures [7,9-12,27,28]. This apparent contradiction with our study could be explained by the fact Riyadh has a much hotter climate compared to the regions investigated in these previous studies. There are two types of studies reported in the literature regarding temperature-induced effects on HbA1c levels in humans and these are discussed below.
One type of study investigated HbA1c levels in different climatic zones for different populations. For example, Blauw et al. [25] analysed diabetes incidence and the prevalence of glucose intolerance in different temperature zones represented by different countries as well as different states of the USA. They found that diabetes incidence and the prevalence of glucose intolerance was higher in locations with a higher ambient temperature. Additionally, a positive correlation between ambient temperature and the prevalence of deglycation and insulin resistance was reported in different parts of Spain [26].
The other type of studies monitored the HbA1c levels for the same population residing in the same region, but the HbA1c levels were determined in different seasons [7,9-12,27,28]. These latter studies are similar to ours and yet show contradictory results. This could be explained by the fact that our study location has a much higher ambient temperature than the regions investigated in the latter studies. For example, the average hot temperature in Riyadh for our study was 41.4°C while in some settings of these contrary studies it was 26°C [12] in China, 28.4°C in Taiwan [9], 22.3°C in Australia (Wollongong city) [10] and 25.7°C in Portugal [28]. The common reason provided in most of these contrary studies, for temperature-induced changes in HbA1c levels, was variations in physical activity. Physical activity increases the usage of glucose by body cells and muscles to produce energy that can result in a decrease in blood glucose which reflects HbA1c levels. The finding of our study suggests that as the temperature gets extremely hot, people are less physically active in Riyadh, and this contributes toward raised HbA1c levels. Therefore, the impact of hot weather on health is modulated by the extremes of temperature. In a city such as Riyadh, where the temperature in the summer can reach as high as 46°C, outdoor activities are much lower in hot weather compared to the cooler periods [29]. Indeed, this explanation is consistent with previous studies that found the weather is one of the obstacles to physical activity. Amin et al. [29] found, in a study conducted on Saudis, that the weather was a barrier for performing physical activity in 65.9% of the participants. The weather, especially extremely hot weather, has been reported as one of the most common barriers to physical activity in Arabia adults [30].
In extreme hot ambient temperatures, as in this study where maximum weather temperature in summer can reach 46°C, people may avoid exposure to sunlight. In a recent cohort study, exposure to bright sunlight has been found to be negatively correlated with insulin resistance, where insulin sensitivity increased with increased time of exposure to bright sunlight and beta-cell function was improved [31]. Therefore, the extremely hot temperatures might prevent exposure to bright sunlight, which may lead to elevated HbA1c levels, and this could be a second reason for the observed high HbA1c levels in hot temperatures. Dehydration is common in hot weather, which could be another factor for the elevated HbA1c levels that were observed in hot temperatures. The reason for the elevated HbA1c levels in hot temperatures is that the dehydration might be due to increase in blood glucose that results from glycogenolysis in the liver induced by the vasopressin hormone [32], which can be induced by dehydration [33,34]. A further possible reason is the thermogenic brown adipose tissue (BAT) which utilises glucose for energy production, and improved insulin sensitivity and glucose homeostasis [35,36]. The BAT was found to be affected by ambient temperature changes and becomes more active in a cold environment [37-41], which can support our findings that low HbA1c levels were observed in the low-temperature period.
The observed sharp drop in the mean of HbA1c during the month of Ramadan could be due to fasting during Ramadan, which is strikingly consistent with numerous studies that have shown that Ramadan fasting gives a definite reduction in HbA1c values of diabetic patients [42-44]. However, Ramadan can be associated with some alterations in the lifestyle such as dietary intake, sleeping and physical activity. Previously, non-significant differences have been found in physical activity or food consumption between Ramadan and non-Ramadan periods in a study conducted on healthy men in Riyadh [45], and in a study conducted on individuals with T2DM in Riyadh [46]. Furthermore, a significant decline in HbA1c was reported in non-insulin-dependent diabetic Jordanians who did not change their dietary intake [47]. Therefore, the decline in HbA1c during Ramadan might be due to the positive impact of fasting on health and particularly HbA1c.
Intermittent fasting and low calorie diet have been found to be effective in the management of type 2 diabetes mellitus in both human and animals [48-50]. In a recent study, alternate day fasting in healthy middle-aged people was found to be safe and beneficial in improving cardiovascular markers, reducing fat mass, and improving other markers of general health [51]. The month of Ramadan involves a type of intermittent fasting when adult Muslims fast (abstaining from eating foods, drinking, smoking etc.) from dawn till dusk for 29-30 days. Therefore, the marked reduction in Hba1c levels during the month of Ramadan is interesting and suggests that fasting and other activities performed during this month may offer health benefits that need further investigation in the future.
The current study demonstrated that the mean of HbA1c positively correlated with age, and that the mean HbA1c in males was significantly higher than in females. These findings agree with previous studies [52,53]. The former finding is expected because age is one of the risk factors for T2DM [54], while the gender variation in HbA1c could be as previously discussed in our previous study due to the menstrual cycle in women [55]. Although the mean of HbA1c in females was significantly lower than males, the negative impact of ambient temperature on HbA1c in females was significantly higher than in males. This impact because the sweating rate is lower in females than in men [56,57] making females more susceptible to the effects of heat [58,59]. This agrees with a recent study by Achebak et al. [60] who reported that the effect of warm temperatures on cardiovascular deaths was higher for women compared to men in all age groups. Further possible reasons could be due to: females being generally less active than males [61], especially in hot temperature; and avoidance of sun exposure for cosmetic purposes. Avoidance of exposure to sunlight for beauty purposes was considered a likely cause of the higher prevalence of vitamin D deficiency that was found among females than males in Riyadh [62]. Therefore, females are likely to have lower exposure to the sun than males in Riyadh, that becomes worse with increasing temperature. This could increase the negative impact of hot ambient temperature on HbA1c in females compared to males.
Strengths and limitations
To the best of our knowledge, this study is the first to investigate the impact of climate on health outcomes for a Saudi Arabia. The study is limited by the fact the data used are heterogeneous, containing HbA1c results of both diabetics and non-diabetics. If more clinical information such as BMI, diabetes duration, and treatment regimens were available and included in this study, the impact of other confounding factors could be investigated.
Conclusion
This study shows that a rise in ambient temperature correlates with a worsening of HbA1c levels. This is a cause for concern since Saudi Arabia already has one of the highest prevalences of diabetes, and temperature in the region is predicted to rise 1.6°C by 2044. Therefore, the rising temperature could further increase the prevalence of this disease and adversely affect those who already have the disease. Possible causes for the elevated HbA1c levels, observed in the hot weather, include reduced physical activity, reduced sunlight exposure, and dehydration. There were positive effects of Ramadan fasting on HbA1c levels even though Ramadan occurred during the hot weather period. The marked reduction in Hba1c levels during the month of Ramadan is interesting and suggests that fasting and other activities performed during this month may offer health benefits.
Acknowledgements
The authors thank the General Directorate of Medical Services of the Interior Ministry in Saudi Arabia for their financial support. We acknowledge the information technology team in the security forces hospital program in Riyadh for their assistance in extracting data from patient medical records. The authors would like to thank the Health and Life science Faculty, De Montfort University, and the security forces hospital program in Riyadh for their support and ethical approvals. We also thank the General Authority of Meteorology and Environmental Protection (GAMEP) in Saudi Arabia for providing the weather data.
Disclosure of conflict of interest
None.
References
- 1.International Diabetes Federation. IDF diabetes atlas. Brussels, Belgium: International Diabetes Federation; 2017. [Google Scholar]
- 2.Cowie CC, Harris MI, Silverman RE, Johnson EW, Rust KF. Effect of multiple risk factors on differences between blacks and whites in the prevalence of non-insulin-dependent diabetes mellitus in the United States. Am J Epidemiol. 1993;137:719–732. doi: 10.1093/oxfordjournals.aje.a116732. [DOI] [PubMed] [Google Scholar]
- 3.Guo Y, Gasparrini A, Armstrong BG, Tawatsupa B, Tobias A, Lavigne E, Coelho M, Pan X, Kim H, Hashizume M, Honda Y, Guo YL, Wu CF, Zanobetti A, Schwartz JD, Bell ML, Scortichini M, Michelozzi P, Punnasiri K, Li S, Tian L, Garcia SDO, Seposo X, Overcenco A, Zeka A, Goodman P, Dang TN, Dung DV, Mayvaneh F, Saldiva PHN, Williams G, Tong S. Heat wave and mortality: a multicountry, multicommunity study. Environ Health Perspect. 2017;125:087006. doi: 10.1289/EHP1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gasparrini A, Guo Y, Hashizume M, Lavigne E, Zanobetti A, Schwartz J, Tobias A, Tong S, Rocklov J, Forsberg B, Leone M, De Sario M, Bell ML, Guo YL, Wu CF, Kan H, Yi SM, de Sousa Zanotti Stagliorio Coelho M, Saldiva PH, Honda Y, Kim H, Armstrong B. Mortality risk attributable to high and low ambient temperature: a multicountry observational study. Lancet. 2015;386:369–375. doi: 10.1016/S0140-6736(14)62114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Almazroui M, Islam MN, Jones PD, Athar H, Rahman MA. Recent climate change in the Arabian Peninsula: seasonal rainfall and temperature climatology of Saudi Arabia for 1979-2009. Atmospheric Research. 2012;111:29–45. [Google Scholar]
- 6.Tarawneh Q, Chowdhury S. Trends of climate change in Saudi Arabia: implications on water resources. Climate. 2018:6. [Google Scholar]
- 7.Hou L, Li M, Huang X, Wang L, Sun P, Shi R, Ding L, Pang S. Seasonal variation of hemoglobin A1c levels in patients with type 2 diabetes. Int J Diabetes Dev Ctries. 2016;37:432–436. [Google Scholar]
- 8.Sakura H, Tanaka Y, Iwamoto Y. Seasonal fluctuations of glycated hemoglobin levels in Japanese diabetic patients. Diabetes Res Clin Pract. 2010;88:65–70. doi: 10.1016/j.diabres.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 9.Tien KJ, Yang CY, Weng SF, Liu SY, Hsieh MC, Chou CW. The impact of ambient temperature on HbA1c in Taiwanese type 2 diabetic patients: the most vulnerable subgroup. J Formos Med Assoc. 2016;115:343–349. doi: 10.1016/j.jfma.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 10.Moses RG, Wong VC, Lambert K, Morris GJ, San Gil F. Seasonal changes in the prevalence of gestational diabetes mellitus. Diabetes Care. 2016;39:1218–1221. doi: 10.2337/dc16-0451. [DOI] [PubMed] [Google Scholar]
- 11.Higgins T, Saw S, Sikaris K, Wiley CL, Cembrowski GC, Lyon AW, Khajuria A, Tran D. Seasonal variation in hemoglobin A1c: is it the same in both hemispheres? J Diabetes Sci Technol. 2009;3:668–671. doi: 10.1177/193229680900300408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang L, Li W, Xian T, Pan Q, Li M, Guo L. Seasonal variations of hemoglobin A1c in residents of Beijing, China. Int J Clin Exp Pathol. 2016;9:9429–9435. [Google Scholar]
- 13.Tseng CL, Brimacombe M, Xie M, Rajan M, Wang H, Kolassa J, Crystal S, Chen TC, Pogach L, Safford M. Seasonal patterns in monthly hemoglobin A1c values. Am J Epidemiol. 2005;161:565–574. doi: 10.1093/aje/kwi071. [DOI] [PubMed] [Google Scholar]
- 14.Speakman JR, Heidari-Bakavoli S. Type 2 diabetes, but not obesity, prevalence is positively associated with ambient temperature. Sci Rep. 2016;6:30409. doi: 10.1038/srep30409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nathan DM, Turgeon H, Regan S. Relationship between glycated haemoglobin levels and mean glucose levels over time. Diabetologia. 2007;50:2239–2244. doi: 10.1007/s00125-007-0803-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tahara Y, Shima K. Kinetics of HbA, c, glycated albumin, and fructosamine and analysis of their weight functions against preceding plasma glucose level. Diabetes Care. 1995;18:440–7. doi: 10.2337/diacare.18.4.440. [DOI] [PubMed] [Google Scholar]
- 17.Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hawkins RC. Circannual variation in glycohemoglobin in Singapore. Clin Chim Acta. 2010;411:18–21. doi: 10.1016/j.cca.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 19.Holstein A, Wohland T, Patzer OM, Trachte F, Kovacs P, Holstein JD. Accumulation of severe hypoglycemia at weekends and in warm seasons in patients with type 1 diabetes but not with type 2 diabetes. J Diabetes Complications. 2016;30:1308–1314. doi: 10.1016/j.jdiacomp.2016.05.026. [DOI] [PubMed] [Google Scholar]
- 20.Chen HS, Wu TE, Jap TS, Chen RL, Lin HD. Effects of health education on glycemic control during holiday time in patients with type 2 diabetes mellitus. Am J Manag Care. 2008;14:45–51. [PubMed] [Google Scholar]
- 21.Templeton GF. A two-step approach for transforming continuous variables to normal: implications and recommendations for IS research. Communications of The Association for Information Systems. 2011;28:41–58. [Google Scholar]
- 22.American Diabetes Association. 6. Glycemic targets: standards of medical care in diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S61–S70. doi: 10.2337/dc19-S006. [DOI] [PubMed] [Google Scholar]
- 23.D’Ippoliti D, Michelozzi P, Marino C, de’Donato F, Menne B, Katsouyanni K, Kirchmayer U, Analitis A, Medina-Ramon M, Paldy A, Atkinson R, Kovats S, Bisanti L, Schneider A, Lefranc A, Iniguez C, Perucci CA. The impact of heat waves on mortality in 9 European cities: results from the EuroHEAT project. Environ Health. 2010;9:37. doi: 10.1186/1476-069X-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi L, Kloog I, Zanobetti A, Liu P, Schwartz JD. Impacts of temperature and its variability on mortality in New England. Nat Clim Chang. 2015;5:988–991. doi: 10.1038/nclimate2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blauw LL, Aziz NA, Tannemaat MR, Blauw CA, de Craen AJ, Pijl H, Rensen PC. Diabetes incidence and glucose intolerance prevalence increase with higher outdoor temperature. BMJ Open Diabetes Res Care. 2017;5:e000317. doi: 10.1136/bmjdrc-2016-000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valdes S, Doulatram-Gamgaram V, Lago A, Garcia Torres F, Badia-Guillen R, Olveira G, Goday A, Calle-Pascual A, Castano L, Castell C, Delgado E, Menendez E, Franch-Nadal J, Gaztambide S, Girbes J, Gomis R, Ortega E, Galan-Garcia JL, Aguilera-Venegas G, Soriguer F, Rojo-Martinez G. Ambient temperature and prevalence of diabetes and insulin resistance in the Spanish population: Di@bet. es study. Eur J Endocrinol. 2019;180:273–280. doi: 10.1530/EJE-18-0818. [DOI] [PubMed] [Google Scholar]
- 27.Li S, Zhou Y, Williams G, Jaakkola JJ, Ou C, Chen S, Yao T, Qin T, Wu S, Guo Y. Seasonality and temperature effects on fasting plasma glucose: a population-based longitudinal study in China. Diabetes Metab. 2016;42:267–275. doi: 10.1016/j.diabet.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Pereira MT, Lira D, Bacelar C, Oliveira JC, de Carvalho AC. Seasonal variation of haemoglobin A1c in a Portuguese adult population. Arch Endocrinol Metab. 2015;59:231–235. doi: 10.1590/2359-3997000000043. [DOI] [PubMed] [Google Scholar]
- 29.Amin TT, Suleman W, Ali A, Gamal A, Al Wehedy A. Pattern, prevalence, and perceived personal barriers toward physical activity among adult Saudis in Al-Hassa, KSA. J Phys Act Health. 2011;8:775–784. doi: 10.1123/jpah.8.6.775. [DOI] [PubMed] [Google Scholar]
- 30.Benjamin K, Donnelly T. Barriers and facilitators influencing the physical activity of Arabic adults: a literature review. Avicenna. 2013;1:8. [Google Scholar]
- 31.Noordam R, Ramkisoensing A, Loh NY, Neville MJ, Rosendaal FR, Willems van Dijk K, van Heemst D, Karpe F, Christodoulides C, Kooijman S. Associations of outdoor temperature, bright sunlight, and cardiometabolic traits in two European population-based cohorts. J Clin Endocrinol Metab. 2019;104:2903–2910. doi: 10.1210/jc.2018-02532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma GY, Hems DA. Inhibition of fatty acid synthesis and stimulation of glycogen breakdown by vasopressin in the perfused mouse liver. Biochem J. 1975;152:389–392. doi: 10.1042/bj1520389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robertson GL, Shelton RL, Athar S. The osmoregulation of vasopressin. Kidney Int. 1976;10:25–37. doi: 10.1038/ki.1976.76. [DOI] [PubMed] [Google Scholar]
- 34.Moses A, Miller M. Osmotic threshold for vasopressin release as determined by saline infusion and by dehydration. Neuroendocrinology. 1971;7:219–226. doi: 10.1159/000121970. [DOI] [PubMed] [Google Scholar]
- 35.Chondronikola M, Volpi E, Borsheim E, Porter C, Annamalai P, Enerback S, Lidell ME, Saraf MK, Labbe SM, Hurren NM, Yfanti C, Chao T, Andersen CR, Cesani F, Hawkins H, Sidossis LS. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes. 2014;63:4089–4099. doi: 10.2337/db14-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carpentier AC, Blondin DP, Virtanen KA, Richard D, Haman F, Turcotte EE. Brown adipose tissue energy metabolism in humans. Front Endocrinol (Lausanne) 2018;9:447. doi: 10.3389/fendo.2018.00447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 38.Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, Iwanaga T, Miyagawa M, Kameya T, Nakada K, Kawai Y, Tsujisaki M. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58:1526–1531. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen KY, Brychta RJ, Linderman JD, Smith S, Courville A, Dieckmann W, Herscovitch P, Millo CM, Remaley A, Lee P, Celi FS. Brown fat activation mediates cold-induced thermogenesis in adult humans in response to a mild decrease in ambient temperature. J Clin Endocrinol Metab. 2013;98:E1218–1223. doi: 10.1210/jc.2012-4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Au-Yong IT, Thorn N, Ganatra R, Perkins AC, Symonds ME. Brown adipose tissue and seasonal variation in humans. Diabetes. 2009;58:2583–2587. doi: 10.2337/db09-0833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saada AD, Attou SG, Belkacemi L, Chabane AO, Italhi M, Bekada AMA, Kati D, Saada DA, Attou GS, Belkacemi L, Chabane OA, Italhi M, Bekada AMA, Kati D. Effect of Ramadan fasting on glucose, glycosylated haemoglobin, insulin, lipids and proteinous concentrations in women with non-insulin dependent diabetes mellitus. Afr J Biotechnol. 2010;9:87–94. [Google Scholar]
- 43.Aziz KM. Effect of fasting ramadan in diabetes control status - application of extensive diabetes education, serum creatinine with HbA1c statistical ANOVA and regression models to prevent hypoglycemia. Recent Pat Endocr Metab Immune Drug Discov. 2013;7:233–251. doi: 10.2174/18715303113139990010. [DOI] [PubMed] [Google Scholar]
- 44.Bener A, A Al-Hamaq AOA, Ozturk M, Catan F, Haris PI, Rajput KU, Omer A. Effect of ramadan fasting on glycemic control and other essential variables in diabetic patients. Ann Afr Med. 2018;17:196–202. doi: 10.4103/aam.aam_63_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Al-Barha NS, Aljaloud KS. The effect of ramadan fasting on body composition and metabolic syndrome in apparently healthy men. Am J Mens Health. 2019;13:1557988318816925. doi: 10.1177/1557988318816925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alghamdi AS, Alghamdi KA, Jenkins RO, Alghamdi MN, Haris PI. Impact of Ramadan on physical activity and sleeping patterns in individuals with type 2 diabetes: the first study using fitbit device. Diabetes Therapy. 2020;11:1331–1346. doi: 10.1007/s13300-020-00825-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khatib FA, Shafagoj YA. Metabolic alterations as a result of Ramadan fasting in non-insulin-dependent diabetes mellitus patients in relation to food intake. Saudi Med J. 2004;25:1858–1863. [PubMed] [Google Scholar]
- 48.Furmli S, Elmasry R, Ramos M, Fung J. Therapeutic use of intermittent fasting for people with type 2 diabetes as an alternative to insulin. BMJ Case Rep. 2018;2018:bcr2017221854. doi: 10.1136/bcr-2017-221854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lean ME, Leslie WS, Barnes AC, Brosnahan N, Thom G, McCombie L, Peters C, Zhyzhneuskaya S, Al-Mrabeh A, Hollingsworth KG, Rodrigues AM, Rehackova L, Adamson AJ, Sniehotta FF, Mathers JC, Ross HM, McIlvenna Y, Stefanetti R, Trenell M, Welsh P, Kean S, Ford I, McConnachie A, Sattar N, Taylor R. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018;391:541–551. doi: 10.1016/S0140-6736(17)33102-1. [DOI] [PubMed] [Google Scholar]
- 50.Cheng CW, Villani V, Buono R, Wei M, Kumar S, Yilmaz OH, Cohen P, Sneddon JB, Perin L, Longo VD. Fasting-mimicking diet promotes Ngn3-driven beta-Cell regeneration to reverse diabetes. Cell. 2017;168:775–788. e712. doi: 10.1016/j.cell.2017.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stekovic S, Hofer SJ, Tripolt N, Aon MA, Royer P, Pein L, Stadler JT, Pendl T, Prietl B, Url J, Schroeder S, Tadic J, Eisenberg T, Magnes C, Stumpe M, Zuegner E, Bordag N, Riedl R, Schmidt A, Kolesnik E, Verheyen N, Springer A, Madl T, Sinner F, de Cabo R, Kroemer G, Obermayer-Pietsch B, Dengjel J, Sourij H, Pieber TR, Madeo F. Alternate day fasting improves physiological and molecular markers of aging in healthy, non-obese humans. Cell Metab. 2019;30:462–476. e466. doi: 10.1016/j.cmet.2019.07.016. [DOI] [PubMed] [Google Scholar]
- 52.Shearer DM, Thomson WM, Broadbent JM, McLean R, Poulton R, Mann J. High-risk glycated hemoglobin trajectories established by mid-20s: findings from a birth cohort study. BMJ Open Diabetes Res Care. 2016;4:e000243. doi: 10.1136/bmjdrc-2016-000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang YC, Lu FH, Wu JS, Chang CJ. Age and sex effects on HbA1c. A study in a healthy Chinese population. Diabetes Care. 1997;20:988–991. doi: 10.2337/diacare.20.6.988. [DOI] [PubMed] [Google Scholar]
- 54.American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2019. Diabetes Care. 2019;42:S13–S28. doi: 10.2337/dc19-S002. [DOI] [PubMed] [Google Scholar]
- 55.Alghamdi AS, Alqadi A, Jenkins RO, Haris PI. The influence of gender and menopausal status on Hba1c variation in a big data study of a Saudi population. Curr Diabetes Rev. 2021;17:365–372. doi: 10.2174/1573399816999200729143238. [DOI] [PubMed] [Google Scholar]
- 56.Bar-Or O, Magnusson LI, Buskirk ER. Distribution of heat-activated sweat glands in obese and lean men and women. Hum Biol. 1968;40:235–248. [PubMed] [Google Scholar]
- 57.Wyndham CH, Morrison JF, Williams CG. Heat reactions of male and female Caucasians. J Appl Physiol. 1965;20:357–364. doi: 10.1152/jappl.1965.20.3.357. [DOI] [PubMed] [Google Scholar]
- 58.Druyan A, Makranz C, Moran D, Yanovich R, Epstein Y, Heled Y. Heat tolerance in women--reconsidering the criteria. Aviat Space Environ Med. 2012;83:58–60. doi: 10.3357/asem.3130.2012. [DOI] [PubMed] [Google Scholar]
- 59.Kenney WL. A review of comparative responses of men and women to heat stress. Environ Res. 1985;37:1–11. doi: 10.1016/0013-9351(85)90044-1. [DOI] [PubMed] [Google Scholar]
- 60.Achebak H, Devolder D, Ballester J. Trends in temperature-related age-specific and sex-specific mortality from cardiovascular diseases in Spain: a national time-series analysis. Lancet Planet Health. 2019;3:e297–e306. doi: 10.1016/S2542-5196(19)30090-7. [DOI] [PubMed] [Google Scholar]
- 61.Al-Nozha MM, Al-Hazzaa HM, Arafah MR, Al-Khadra A, Al-Mazrou YY, Al-Maatouq MA, Khan NB, Al-Marzouki K, Al-Harthi SS, Abdullah M. Prevalence of physical activity and inactivity among Saudis aged 30-70 years. Saudi Med J. 2007;28:559–568. [PubMed] [Google Scholar]
- 62.Al-Mogbel ES. Vitamin D status among adult Saudi females visiting primary health care clinics. Int J Health Sci (Qassim) 2012;6:116. doi: 10.12816/0005987. [DOI] [PMC free article] [PubMed] [Google Scholar]


