Abstract
Objective
Corticosteroids are a common option used in sepsis treatment. However, the efficacy and potential risk of corticosteroids in septic patients have not been well assessed. This review was performed to assess the efficacy and safety of corticosteroids in patients with sepsis.
Methods
PubMed, Embase, and Cochrane library databases were searched from inception to March 2021. Randomized controlled trials (RCTs) that evaluated the effect of corticosteroids on patients with sepsis were included. The quality of outcomes in the included articles was evaluated using the Grading of Recommendations Assessment, Development, and Evaluation methodology. The data were pooled by using risk ratio (RR) and mean difference (MD). The random-effects model was used to evaluate the pooled MD or RR and 95% confidence intervals (CIs).
Results
Fifty RCTs that included 12,304 patients with sepsis were identified. Corticosteroids were not associated with the mortality in 28-day (RR, 0.94; 95% CI, 0.87–1.02; evidence rank, moderate) and long-term mortality (>60 days) (RR, 0.96; 95% CI, 0.88–1.05) in patients with sepsis (evidence rank, low). However, corticosteroids may exert a significant effect on the mortality in the intensive care unit (ICU) (RR, 0.9; 95% CI, 0.83–0.97), in-hospital (RR, 0.9; 95% CI, 0.82–0.99; evidence rank, moderate) in patients with sepsis or septic shock (evidence rank, low). Furthermore, corticosteroids probably achieved a tiny reduction in the length of hospital stay and ICU. Corticosteroids were associated with a higher risk of hypernatremia and hyperglycemia; furthermore, they appear to have no significant effect on superinfection and gastroduodenal bleeding.
Conclusions
Corticosteroids had no significant effect on the 28-day and long-term mortality; however, they decreased the ICU and hospital mortality. The findings suggest that the clinical corticosteroids may be an effective therapy for patients with sepsis during the short time.
Systematic Review Registration
https://inplasy.com/wp-content/uploads/2021/05/INPLASY-Protocol-1074-4.pdf
Keywords: corticosteroids, sepsis, mortality, meta-analysis, systematic review
Introduction
Sepsis is a life-threatening organ dysfunction, which is caused by a dysregulated host response to infection (1, 2) that culminates in systematic hypoperfusion and considerable organ dysfunction. The main therapies to treat sepsis in the early phase are antibiotic administration and perfusion restoration (3). Early and aggressive treatment is associated with a mortality rate of 30%–50% in critically ill patients admitted to the intensive care unit (ICU) and induces more than 5 million deaths each year across the world (3, 4). Therefore, further investigation for the treatment of sepsis is crucial.
The pathology of sepsis is marked by a dysregulated host response to infection; therefore, immunomodulatory therapies have been used in sepsis treatment that may be effective (5). Doctors have started using corticosteroids as an adjuvant therapy for sepsis since the middle of the twentieth century (3). Corticosteroids were used to treat sepsis, especially the septic shock therapy; numerous randomized clinical trials (RCTs) were performed to evaluate the safety and efficacy of corticosteroids. However, the results of these RCTs varied. Thus, many systematic reviews have been performed to assess the safety and efficacy of corticosteroids in patients with sepsis. However, the results of the most recent reviews remain controversial (6, 7). Subsequently, several studies have further assessed whether the combination of corticosteroids, vitamin C, and thiamine as compared with corticosteroids or placebo improved the survival duration, increased the vasopressor-free time over 7 days, and reduced organ injury (8, 9). These results suggest that the use of corticosteroids in combination with other drugs did not affect the safety and efficacy of corticosteroids in patients with sepsis. Hence, resolution of this controversy regarding the latest reviews that have assessed the efficacy of corticosteroids in patients with sepsis is currently the primary problem in sepsis treatment. Therefore, this systematic review and meta-analysis were performed based on the latest reviews to reintegrate the relevant data to evaluate the effects and safety of corticosteroids in patients with sepsis.
Methods
The protocol of this systematic review and meta-analysis was registered on INPLASY (ID: INPLASY2020110122). The methodology of this study was according to items of the Cochrane Collaboration, and each outcome was assessed using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) guidelines (10).
Study Searches
This meta-analysis was performed based on the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) criteria. Moreover, the PRISMA 2020 checklist is shown in Supplemental Table 1 . PubMed, Embase, and Cochrane library databases were searched for relevant data from inception to March 2021, update to 5 July 2021, to identify RCTs that have evaluated the effect of corticosteroids on patients with sepsis. The MeSH/Emtree and title/abstract keyword combination were used to identify the eligible articles; the keyword search terms used for the English literature included the words corticosteroids and sepsis (detailed search strategy in Supplemental Table 2 ). It is noteworthy that we also conducted a manual search for the references of the relevant articles (study search flowchart in Figure 1 ).
Figure 1.
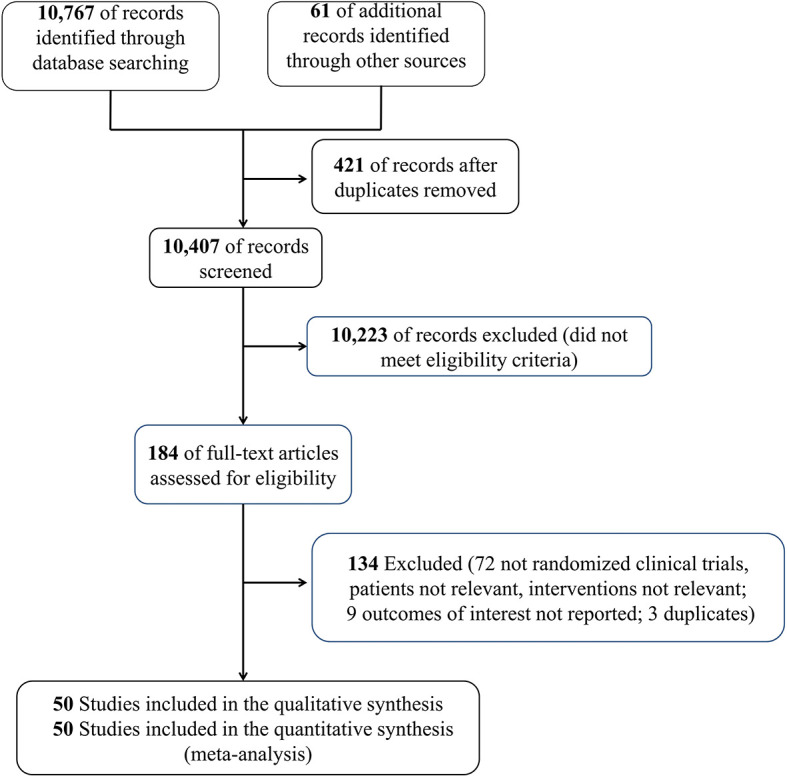
Flowchart of the search strategy in this meta-analysis.
Study Selection
Before the potential articles were searched and screened, the eligibility criteria and exclusion criteria were identified. Articles may be eligible according to the inclusion criteria in this study if they meet all of the following conditions: (1) adult patients diagnosed with sepsis, severe sepsis, or septic shock, as per the inclusion criteria during the study (11–13) [studies reporting adult patients with acute respiratory distress syndrome (ARDS) and sepsis were included]; (2) the study compared the use of corticosteroids (including hydrocortisone, methylprednisolone, betamethasone, fludrocortisone, and dexamethasone) with no use of corticosteroids; (3) the study measured and reported the outcomes in terms of 28-day and long-term mortality (>60 days), ICU mortality, in-hospital mortality, length of stay in hospital and ICU, vasopressor-free days, ventilation-free time, shock reversal at days 7 and 28, time for resolution of shock, Sequential Organ Failure Assessment (SOFA) scores at day 7, hypernatremia, hyperglycemia, superinfection, and gastroduodenal bleeding; (4) the study was an RCT or abstract and was published in English. Furthermore, the study design including case reports, case series, and observational studies or the previous unpublished studies that required the author to be contacted were excluded. All the available articles were searched by two searchers, respectively, and when disagreements occurred during the process, the third investigator should resolve these disagreements. Reviewers performed reviews in pairs to screen all relevant citations and references as per the search strategy, and the screening process included the following two stages: initial evaluation of titles and abstracts and skimming of the full text to identify the eligible studies.
Data Extraction
Researchers conducted data extraction, respectively, and in duplicate based on the eligibility and exclusion criteria. In case of disagreements, the third reviewer resolved the issue. Relevant data, including the study title, first author, study type, study period, the therapy in treatment and control groups, reported outcomes, sepsis definition, and so forth were collected. The data only for the studies that we searched including the previous review (6) were abstracted. The risk of bias for this meta-analysis was assessed by two investigators independently for every abstracted data of each article based on the Cochrane Collaboration (14) and domains including allocation concealment, blinding of participants and staff, blinding of outcome assessors, incomplete outcome data, selective outcome reporting, and other biases. Additionally, the GRADE framework was used to evaluate the overall evidence rank for every outcome (15). The studies with more than six, four, to six and fewer than six items were considered high, fair, and poor quality, respectively. Importantly, the GRADE was used to assess the evidence rank of mortality and adverse events. According to the risk of bias, inconsistency, indirectness, imprecision, and publication bias, the studies were evaluated as low, moderate, or high quality.
Statistical Analyses
Mantel–Haenszel (M-H) or DerSimonian Laird (DL) methods with random-effects meta-analyses were conducted for the eligible RCTs. All the relevant data were assessed using the Review Manager (RevMan), version 5.3 (Cochrane Collaboration), STATA 16.0 (StataCorp, College Station, TX, USA). Risk ratio (RR) and mean difference (MD) were used to present the dichotomous and continuous outcomes, with 95% CI. Moreover, a Funnel plot was used to examine the potential for some small effects if the outcome included more than 10 trials, and the possibility of publication bias was assessed using the Funnel plot and Egger regression test (16). The chi-square test, I 2, and visual inspection of the forest plots were used to evaluate heterogeneity among the eligible studies; when I 2 was >50%, the heterogeneity was considered substantial. In addition, we performed the subgroup analyses based on the following variables: sepsis subtype [sepsis, septic shock, sepsis and ARDS, sepsis and community-acquired pneumonia (CAP), and severe COVID-19], type of corticosteroids (hydrocortisone or hydrocortisone plus fludrocortisone or methylprednisolone or prednisone or betamethasone or dexamethasone), and type of ICU [surgical, medical (internal) or surgical/medical ICU], searching the source of heterogeneity. Additionally, as the unit dose of the corticosteroids varied, relevant included studies about the use of corticosteroids were collected based on catecholamine use for qualitative analysis.
Results
Characteristics of Eligible Studies
We initially identified 10,828 records, and 10,407 citations remained after the duplicate trials were removed; 184 RCTs were eligible after preliminary screening by title and abstract. Finally, 50 RCTs (17–66) that included 12,304 patients with sepsis were included in this meta-analysis ( Figure 1 ). The characteristics of the included RCTs are listed in Table 1 . Twenty-five RCTs (18, 20–23, 25–29, 34, 35, 37, 39, 40, 43, 45, 51, 54, 56–60, 64) on 8,400 patients with septic shock, 8 RCTs (36, 38, 40, 42, 47, 52, 65, 66) on 936 patients with sepsis, 4 RCTs (17, 44, 46, 62) on 390 patients with sepsis and ARDS, 8 RCTs (24, 30, 33, 48, 50, 53, 55, 63) on 1,699 patients with sepsis and community-acquired pneumonia, and 4 RCTs (19, 31, 32, 61) on 748 patients with severe COVID-19 were included. Additionally, 27 RCTs (19, 23, 25, 27, 28, 30–32, 34, 35, 37–41, 43, 45, 47, 49–53, 56, 58, 59, 64) (6,981 patients) of which were treated with hydrocortisone, 4 RCTs (18, 20–22) (2,082 patients) with hydrocortisone plus fludrocortisone, 10 RCTs (17, 26, 32, 33, 36, 44, 46, 54, 57, 63) (1,245 patients) with methylprednisolone, 4 RCTs (33, 55, 65, 66) (364 patients) with prednisolone, 3 RCTs (29, 48, 61) (408 patients) with dexamethasone, and only 1 RCT (42) with betamethasone (85 patients). Furthermore, 6 RCTs (39, 51, 60, 62, 65, 66) recruited eligible patients into the medical (internal) ICU and 3 studies (23, 29, 54) into the surgical ICU, and the remaining 40 studies were reported in the medical/surgical ICU or ICU. Moreover, 24 studies (17, 20–23, 25, 27–29, 31, 34, 35, 39, 41, 43, 45, 47, 48, 51, 56–58, 60, 61) showed corticosteroids use based on catecholamine in patients with septic shock. Specifically, 16 RCTs (20–23, 31, 34, 35, 39, 41, 43, 45, 56, 58, 60, 61) reported corticosteroids dose to be not more than 200 mg/day or 50 mg every 6 h; 8 RCTs (17, 25, 27–29, 47, 48, 51) showed a dose more than 200 mg/day (most of which were 200–300 mg/day). In addition, 21 studies (26, 27, 32, 33, 35–37, 39, 42, 44, 45, 47, 48, 51, 54, 55, 57, 62, 63, 65, 66) showed the time of corticosteroids administration in patients with sepsis, 16 RCTs (26, 27, 32, 33, 35–37, 39, 42, 44, 45, 51, 54, 55, 57, 65) of which reported the time of corticosteroids administration within 2 h for prognosis or randomization or as soon as possible and 5 RCTs (47, 48, 62, 63, 66) at 12 h or more after admission.
Table 1.
Characteristics of the included studies in the in adult patients with sepsis.
| Study | Study Type | S/M Center | Study Period | Total Patients/Patients in CS No. | Mean Age, Years | Female/Male of Patient No. | Type of Patient Population | Sepsis or Septic Shock Definition | The time of CS Administration | Experimental Intervention | Reported Outcomes | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annane et al. (21) | RCT | M | NA | 1241/614 | CS: 66 PC: 66 | 415/826 | Septic shock | Sepsis-3 | NA | 50 mg/6 h hydrocortisone intravenously + fludrocortisone 50 µg for 7 days | 28, 90, and 180 days, ICU and hospital discharge, etc. | |||
| Venkatesh et al. (64) | RCT | M | 03/2013 −04/2017 |
3,658/1,832 | CS: 62.3 PC: 62.7 | 1,399/2,259 | Septic shock | Sepsis-3 | NA | 200 mg/day hydrocortisone intravenous infusion for 7 days | 90- and 28-day mortality; ICU/hospital stay time, etc. | |||
| Annane et al. (22) | RCT | M | 10/1995 −02/1999 |
300/151 | CS: 62 PC: 60 |
200/100 | Septic shock | Sepsis-2 | NA | 50 mg/6 h hydrocortisone bolus and 50 μg fludrocortisone orally/24 h for 7 days |
28-day mortality | |||
| Lv et al. (45) | RCT | S | 09/2015–09/2016 | 118/58 | CS: 68.8 PC: 64.8 |
70/68 | Septic shock | NA | With vasoactive drugs initiating | 200 mg/day hydrocortisone for 6 days | 28-day mortality; reversal of shock; hospital mortality; ICU/hospital stay |
|||
| Klastersky et al. (42) | RCT | S | NA | 85/46 | NA | 47/38 | Severe sepsis | NA | With antibacterial agents | Betamethasone 0.5 mg/kg every 12 h for 3 days | 30-day mortality | |||
| Bone et al. (26) | RCT | M | 11/1982–12/1985 | 382/191 | CS:53.0 PC: 53.6 |
147/235 | Septic shock | NA | 2 h from entry | Methylprednisolone bolus (30 mg/kg) repeated every 6 h for 24 h |
Shock incidence; shock reversal; overall mortality; 14-day mortality |
|||
| Schumer et al. (54) | RCT | S | 1967–1975 | 172/86 | 50 | 5/167 | Septic shock | NA | At the time of diagnosis | Methylprednisolone (30 mg/kg) dose was repeated once in both groups after 4 h | Mortality; shock associated mortality; organ injury associated mortality |
|||
| Sprung et al. (57) | RCT | M | 08/1979–02/1982 | 59/43 | CS: 58 PC: 55 |
13/46 | Septic shock | NA | After the onset of shock | Methylprednisolone (30 mg/kg); |
Shock reversal; hospital mortality; blood cultures; adverse events |
|||
| Yildiz et al. (65) | RCT | S | 05/1997–04/1999 | 40/20 | CS: 57.8 PC: 56.5 |
16/24 | Sepsis | Sepsis-1 | Within 2 h after randomization | Prednisolone intravenous blouses 2 times/day at 6:00 a.m. (5 mg) and at 6:00 p.m. (2.5 mg) for 10 days | 28-day mortality; sepsis-related organ dysfunction | |||
| Vasscsg et al. (36) | RCT | M | 10/1983–04/1986 | 223/112 | CS: 60.9 PC: 60.6 |
NA | Sepsis | NA | Within 2 h of diagnosis | Methylprednisolone bolus (30 mg/kg) repeated every 6 h for 24 h | 14-day mortality; adverse occurrences |
|||
| Luce et al. (44) | RCT | S | 09/1983–08/1986 | 75/38 | NA | NA | Sepsis and ARDS | NA | After patients inclusion | Methylprednisolone (30 mg/kg) every 6 h, 4 times | ARDS incidence; all-cause mortality; adverse events |
|||
| Bollaert et al. (25) | RCT | M | NA | 41/22 | CS: 66 PC: 56 |
14/27 | Septic shock | Sepsis-1 | NA | Hydrocortisone bolus (100 mg) every 8 h for 5 days, then tapered over 6 days | 7 days reversal of shock; 28 days reversal of shock; 28-day mortality; adverse events |
|||
| Tilouch et al. (60) | RCT | S | 04/2013–06/2016 | 70/33 | CS (continuous infusion): 69 CS (bolus): 70 |
43/27 | Septic shock | NA | NA | Hydrocortisone 200 mg/days by continuous infusion for 7 days; Hydrocortisone 50 mg intravenously every 6 h for 7 days | Shock reversal at day 7; 28-day mortality; vasopressor-free days; ICU and hospital length of stay; occurrence of superinfection |
|||
| Huang et al. (38) | RCT | S | 12/2010–12/2012 | 60/20 | CS: 53.9 PC: 55.7 |
25/35 | Sepsis | Sepsis-2 | NA | Hydrocortisone (300 mg) daily as a continuous infusion for 7 days | 28-day mortality; 3 days shock reversal |
|||
| Briegel et al. (27) | RCT | S | NA | 40/20 | CS: 47 PC: 51 |
19/21 | Septic shock | Sepsis-1 | Within 30 min | Hydrocortisone bolus (100 mg), followed by a continuous infusion of 0.18 mg/kg per hour until shock reversal, then tapered off | Shock reversal; hemodynamic variables; MOSD |
|||
| Chawla et al. (28) | RCT | S | NA | 44/23 | NA | NA | Septic shock | NA | NA | Hydrocortisone (100 mg)/8 h for 3 days | Shock reversal | |||
| Loisa et al. (43) | RCT | S | 07/2005–04/2006 | 48/NA | NA | NA | Septic shock | Sepsis-2 | NA | Hydrocortisone intravenous bolus of 50 mg/6 h for 5 days | Reversal of shock | |||
| Confalonieri et al. (30) | RCT | M | 07/2000–03/2003 | 46/23 | CS: 60.4 PC: 66.6 |
14/32 | Sepsis and CAP | NA | NA | Hydrocortisone bolus (200 mg), followed by a continuous infusion of 10 mg/h for 7 days, then tapered off over 4 days |
MODS score by Study Day 8 and development of delayed septic shock; duration of mechanical ventilation; length of ICU/RIU hospital stay; survival to hospital discharge and to 60 days |
|||
| Sprung et al. (56) | RCT | M | 03/2002–11/2005 | 499/251 | CS: 63 PC: 63 |
167/332 | Septic shock | Sepsis-1 | NA | Hydrocortisone (50 mg)/6 h for 5 days, then 50 mg/12 h for 3 days, then 50 mg/day for 3 days | 28-day mortality | |||
| Keh et al. (40) | RCT | S | 03/1997–09/2000 | 40/20 | 52 | 26/14 | Septic shock | Sepsis-1 | NA | Hydrocortisone bolus (100 mg) followed by a continuous infusion of 10 mg/h for 3 days | Plasma cortisol | |||
| Arabi et al. (23) | RCT | S | 04/2004–10/2007 | 75/39 | CS: 44 PC: 44 |
33/42 | Cirrhosis and septic shock | Sepsis-2 | NA | Hydrocortisone bolus (50 mg)/6 h until shock resolution | 28-day mortality;shock reversal; ventilation-free days; length of stay in ICU; length of stay in hospital | |||
| Fernandez-Serrano et al. (33) | RCT | S | NA | 56/28 | CS: 61 PC: 66 |
NA | Severe CAP | NA | 30 min before starting the antibiotic treatment | Methylprednisolone as an intravenous bolus of 200 mg administered 30 min followed by 29 mg/6 h for 3 days, then 20 mg/12 h for 3 days, and finally 20 mg/days for another 3 days | Need for mechanical ventilation; time to resolution of morbidity score ICU length of stay; hospital length of stay | |||
| Rinaldi et al. (52) | RCT | S | NA | 40/20 | CS: 68 PC: 66 |
NA | Severe sepsis | Sepsis-1 | NA | Hydrocortisone (300 mg/day) continuous infusion for 6 days | Mortality; SOFA score |
|||
| Cicarelli et al. (29) | RCT | S | 11/2004–12/2005 | 29/14 | CS: 69 PC: 61 |
16/13 | Septic shock | NA | NA | Dexamethasone (0.2 mg/kg) given 3 times at 36 h | SOFA score 7-day mortality; 28-day mortality lactate evolution |
|||
| Aboab et al. (18) | RCT | S | NA | 23/10 | CS: 55 PC: 56 |
9/14 | Septic shock | Sepsis-1 | NA | Hydrocortisone bolus (50 mg) 6 h and fludrocortisone (50 μg) for 7 days | Blood pressure, etc. | |||
| Annane et al. (20) | RCT | M | 01/2006–01/2009 | 518/264 | CS: 63.9 PC: 64.3 |
195/323 | Septic shock | NA | NA | Hydrocortisone 50 mg/6 h; fludrocortisone orally in 50 µg tablets/day, each for 7 days | In-hospital mortality mechanical ventilation-free within 28 days; length of stay in ICU |
|||
| Yildiz et al. (66) | RCT | S | 04/2005–05/2008 | 55/27 | CS: 75 PC: 64 |
36/19 | Sepsis | Sepsis-1 | Within 24 h after admission | Prednisolone intravenous boluses 3 times daily at 6 a.m. (10 mg), 2 p.m. (5 mg), and 10 p.m. (5 mg) for 10 days | 28-day mortality; hospital stay |
|||
| Meduri et al. (46) | RCT | M | 04/1997–04/2002 | 91/63 | CS: 59.1 PC: 54.5 |
44/47 | ARDS and sepsis | NA | NA | Methylprednisolone loading dose of 1 mg/kg, followed by continuous infusion of 1 mg/kg per day then 0.5 mg/kg per day from 15–21 days | Length of ICU stay; hospital stay; ICU mortality; hospital mortality |
|||
| Snijders et al. (55) | RCT | S | 08/2005–07/2008 | 213/104 | CS: 63.0 PC: 64.0 |
89/124 | Sepsis and CAP | NA | After randomization | Prednisolone (40 mg) intravenous once daily for 7 days | Treatment failure at 7 and 30 days; treatment cure at 7 and 30 days | |||
| Meijvis et al. (48) | RCT | M | 11/2007–09/2010 | 304/151 | CS: 64.5 PC: 62.5 |
133/171 | Sepsis and CAP | NA | Within a maximum of 12 h from admission | Dexamethasone (5 mg) intravenously for 4 days | Length of hospital stay; hospital mortality; adverse events |
|||
| Mirea et al. (49) | RCT | S | NA | 112/54 | CS (200): 64.3 CS(300): 65.1 |
NA | Septic shock | NA | NA | Hydrocortisone 50 mg intravenous bolus per 6 h or 200 mg per day as a continuous infusion for a maximum of 7 days | Mean serum sodium values over 7 days; short-term mortality, etc. |
|||
| Rezk et al. (17) | RCT | S | 10/2011–10/2012 | 27/18 | NA | 4/23 | Sepsis and ARDS | NA | NA | 1 mg/kg methylprednisolone, followed by continuous infusion of 1 mg/kg per day from day 1 to 14, 0.5 mg/kg per day from day 15 to 21, 0.25 mg/kg per day from day 22–25, and 0.125 mg/kg per day from day 26 to 28 | Mortality; extubation from mechanical ventilation |
|||
| Gordon et al. (34) | RCT | M | 10/2010–03/2012 | 61/31 | CS: 61 PC: 60 |
25/36 | Septic shock | Sepsis-1 | NA | Hydrocortisone (50 mg) every 6 h for the first 5 days, 50 mg every 12 h for the next 3 days | Mortality; organ failure-free days |
|||
| Tongyoo et al. (62) | RCT | S | 12/2010–12/2014 | 197/98 | CS: 64.5 PC: 64.3 |
96/101 | Sepsis and ARDS | Sepsis-1 | Within 12 h | Hydrocortisone (50 mg) every 6 h for 7 days | 28-day mortality; mechanical ventilation-free days; 60-day mortality; adverse events |
|||
| Blum et al. (24) | RCT | M | 12/2009–05/2014 | 800/400 | CS: 74 PC: 73 | NA | CAP | NA | NA | Prednisone 50 mg per day for 7 days | All-cause mortality 30 and 180 days | |||
| Oppert et al. (51) | RCT | S | NA | 41/18 | CS: 59 PC: 47 |
9/32 | Septic shock | Sepsis-1 | After inclusion | Hydrocortisone bolus (50 mg), followed by continuous infusion of 0.18 mg/kg per hour up to cessation of vasopressor for ≥1 h | Vasopressor-free time; 28 days survival; SOFA score; adrenal reserve |
|||
| Angus et al. (19) | RCT | M | 03/2020–06/2020 | 379/278 | CS: 60.4 PC: 65.9 |
111/273 | Severe COVID-19 | NA | NA | Intravenous hydrocortisone (50 mg or 100 mg every 6 h) for 7 days | Organ support-free and mortality within 21 days | |||
| Torres et al. (63) | RCT | M | 06/2004–02/2012 | 120/61 | CS: 64.5 PC: 66.1 |
46/74 | CAP and sepsis | NA | Within 36 h of hospital admission | Methylprednisolone intravenous bolus of 0.5 mg/kg/12 h for 5 days started within 36 h of hospital admission |
Length of ICU and hospital; in-hospital mortality |
|||
| Gordon et al. (35) | RCT | M | 02/2013–05/2015 | 409/202 | 66 | 171/238 | Septic shock | Sepsis-1 | After inclusion | Hydrocortisone (50 mg) every 6 h for the first 5 days | Mortality; serious adverse events |
|||
| Edalatifard et al. (32) | RCT | S | 04/2020–06/2020 | 62/34 | CS: 55.8 PC: 61.7 |
23/39 | Severe COVID-19 | NA | After inclusion | Methylprednisolone intravenous injection, 250 mg/day for 3 days | Time of clinical improvement or death | |||
| Keh et al. (41) | RCT | M | 01/2009–08/2013 | 353/177 | CS: 65.5 PC: 64.6 |
124/229 | Severe sepsis | Sepsi-2 | NA | Hydrocortisone bolus (50 mg) followed by a continuous infusion of 200 mg daily for 3 days | Mortality in ICU or hospital; adverse events |
|||
| Doluee et al. (58) | RCT | S | 08/2014–04/2015 | 160/NA | NA | NA | Septic shock | NA | NA | Hydrocortisone (50 mg intravenous bolus every 6 h for 7 days) | 28-day mortality shock termination |
|||
| Nafae et al. (50) | RCT | S | NA | 80/NA | NA | NA | Severe CAP | NA | NA | Hydrocortisone 200 mg as intravenous bolus followed by infusion at 10 mg/h for 7 days | In-hospital mortality; serious adverse events | |||
| Dequin et al. (31) | RCT | M | 03/2020–06/2020 | 149/76 | CS: 63.1 PC: 66.3 |
45/104 | Severe COVID-19 | NA | NA | Hydrocortisone at aninitial dose of 200 mg/day continued at 200 mg/day until day 7 | Duration of mechanical ventilation; hospital length of stay | |||
| Meduri et al. (47) | RCT | S | NA | 80/NA | NA | NA | Sepsis | NA | Severe sepsis <48 h ICU entry | Hydrocortisone as a bolus of 300 mg | Mortality at 7 days and at 28 days, etc. | |||
| Tandan et al. (59) | RCT | S | NA | 51 | 51 | NA | Septic shock and adrenal insufficiency | NA | NA | Hydrocortisone (stated low dose but actual dose and duration NR) | 28-day mortality; the survival of hospital discharge | |||
| Hyvernat et al. (39) | RCT | M | 11/2008–07/2010 | 122/63 | CS(200): 64.3 CS(300): 65.1 |
80/42 | Septic shock | Sepsis-2 | When patients presenting septic shock | Hydrocortisone 50 mg/6 h for 5 days |
28 days all-cause mortality; free of vasopressor; free of mechanical ventilation | |||
| Tomazini et al. (61) | RCT | M | 04/2020–07/2020 | 299/151 | CS: 60.1 PC: 62.7 |
112/187 | COVID-19-associated ARDS | NA | NA | 20 mg dexamethasone for 5 days, 10 mg dexamethasone for 5 days or until ICU discharge | Ventilator-free and 28 days all-cause mortality; ICU-free days | |||
| Hu et al. (37) | RCT | S | 02/2007–01/2009 | 77/34 | CS: 56 PC: 54 |
48/29 | Septic shock | Sepsis-2 | After randomization | Hydrocortisone 50 mg/6 h for the first 7 days, 50 mg every 8 h for the next 3 days | Mortality; length of ICU stay |
|||
| Sabry et al. (53) | RCT | M | 07/2010–01/2011 | 80/40 | 63 | 22/58 | Sepsis and CAP | NA | NA | Hydrocortisone bolus (200 mg) followed by intravenous dose of 300 mg daily for 7 days | Duration of the mechanical ventilation | |||
RCT, randomized controlled trial; M, multicenter; S, single-center; ARDS, acute respiratory distress syndrome; CS, corticosteroids; PC, placebo or control; ICU, intensive care unit; NA, not acquired; MODS, multiple organ dysfunction syndrome; SOFA, sepsis-related organ failure assessment; IL, interleukin; COVID-19, coronavirus disease 2019; CAP, community-acquired pneumonia.
Primary Outcome
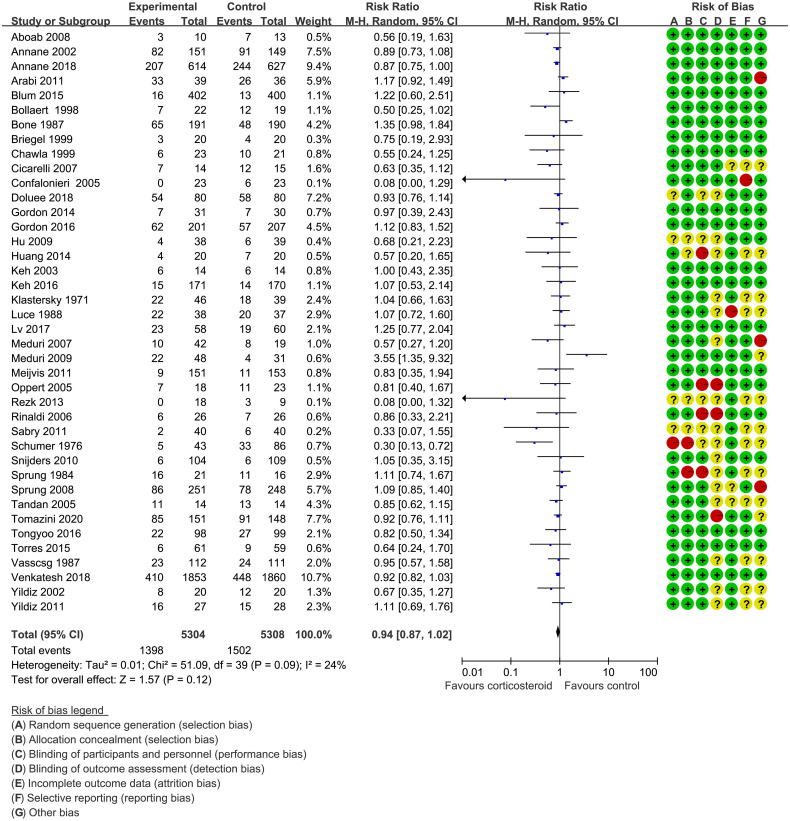
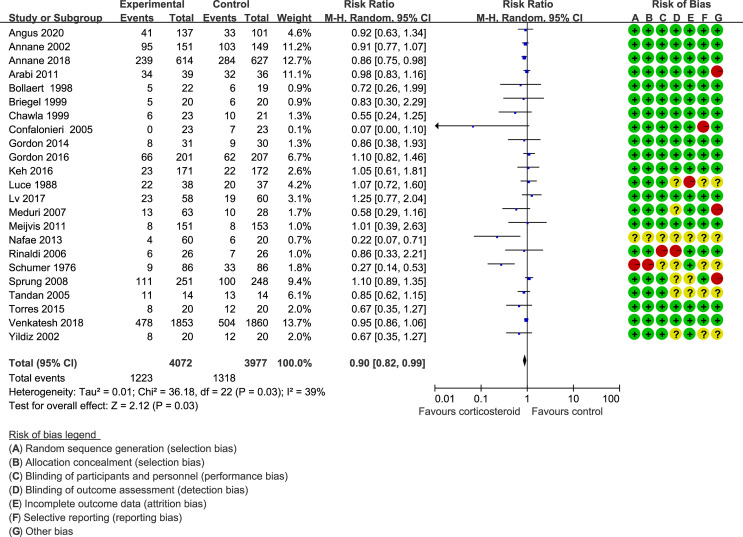
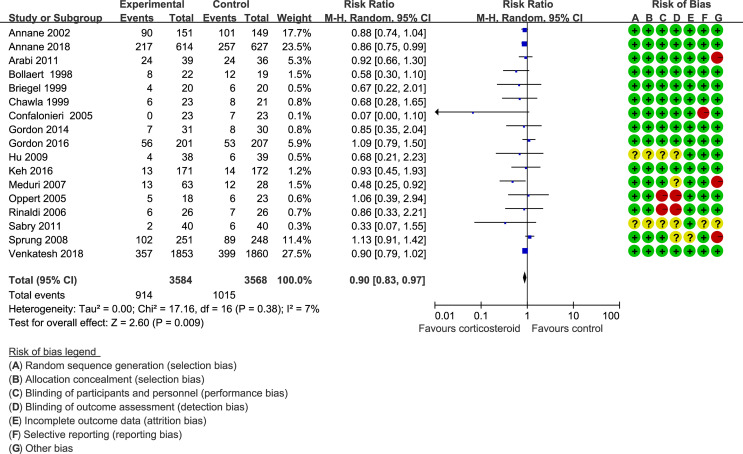
Forty trials (17, 18, 21–30, 34–38, 40–42, 44–48, 51–59, 61–66) (10,612 patients), 23 trials (21–23, 25, 27, 28, 30, 33–35, 41, 44–48, 50, 52, 56, 59, 63–65) (11,579 patients), and 17 trials (21–23, 25, 27, 28, 30, 34, 35, 37, 41, 46, 52, 53, 56, 63, 64) (7,175 patients) were included in this meta-analysis for assessing the 28-day mortality, in-hospital mortality, and ICU mortality, respectively. We used the random-effects model with RRs to assess the pooled results. Corticosteroids therapy showed no difference in the 28-day mortality (RR, 0.94; 95% CI, 0.87–1.02; evidence rank, moderate; Figure 2 ), with low heterogeneity among the trials (I 2 = 24%). However, corticosteroids treatment resulted in a significant decrease in the in-hospital mortality (RR, 0.90; 95% CI, 0.82–0.99; evidence rank, moderate; Figure 3 ) and ICU mortality (RR, 0.90; 95% CI, 0.83–0.97; evidence rank, high; Figure 4 ) with low heterogeneity (I 2 = 39% and I 2 = 7%, respectively). The Funnel plot and Egger test showed no publication bias in the 28-day mortality (p = 0.11), but in-hospital mortality (p = 0.028) and ICU mortality (p = 0.054) showed potential publication bias ( Supplementary Figures 1 – 3 ). The results of sensitivity analysis showed that the models of the 28-day mortality, in-hospital mortality, and ICU mortality were credible ( Supplementary Figures 4 – 6 ). Furthermore, L’Abbé plot reported that the mortality in the placebo group increased significantly than the corticosteroids group, suggesting the potential effects of corticosteroids in patients with sepsis ( Supplementary Figures 7 – 9 ).
Figure 2.
The 28-day mortality of patients with sepsis based on the corticosteroids treatment. The pooled effects in the forest plot were calculated by the M-H method with the random-effects model.
Figure 3.
In-hospital mortality of patients with sepsis based on the corticosteroids treatment. The pooled effects in the forest plot were calculated by the M-H method with the random-effects model.
Figure 4.
ICU mortality of patients with sepsis based on the corticosteroids treatment. The pooled effects in the forest plot were calculated by the M-H method with the random-effects model.
Secondary Outcomes
Supplementary Figures 10 – 22 present the assessment of the secondary outcomes. Corticosteroids achieved a small reduction in length of stay in hospital (MD, −1.38; 95% CI, −2.28 to −0.49; I 2 = 5%; evidence rank, high), SOFA scores at day 7 (MD, −0.90; 95% CI, −1.72 to −0.09; I 2 = 93%; evidence rank, low), and time to resolution of shock (MD, −1.35; 95% CI, −1.79 to −0.92; I 2 = 68%; evidence rank, low) for patients with sepsis. Conversely, corticosteroids resulted in higher risk of hypernatremia (RR, 1.51; 95% CI, 1.10–2.07; I 2 = 0%; evidence rank, moderate) and hyperglycemia (RR, 1.19; 95% CI, 1.10–1.29; I 2 = 49%; evidence rank, high). Furthermore, corticosteroids increased the vasopressor-free days (MD, 1.93; 95% CI, 0.76–3.09; I 2 = 0%; evidence rank, moderate), ventilation-free time (MD, 1.46; 95% CI, 0.27–2.65; I 2 = 21%; evidence rank, moderate), and shock reversal at day 7 (RR, 1.16; 95% CI, 1.06–1.27; I 2 = 72%; evidence rank, moderate) and day 28 (RR, 1.07; 95% CI, 1.01–1.13; I 2 = 12%; evidence rank, moderate). Additionally, corticosteroids achieve no reduction in the long-term mortality (>60 days) (RR, 0.96; 95% CI, 0.88–1.05; I 2 = 54%; evidence rank, low), length of stay in ICU (MD, −0.89; 95% CI, −1.80–0.03; I 2 = 47%; evidence rank, moderate), superinfection (RR, 1.06; 95% CI, 0.92–1.22; I 2 = 13%; evidence rank, moderate), and gastroduodenal bleeding (RR, 1.07; 95% CI, 0.85–1.36; I 2 = 0%; evidence rank, high).
The Funnel plot and Egger test showed no publication bias in the length of stay in hospital (p = 0.99), SOFA scores at day 7 (p = 0.86), hyperglycemia (p = 0.98), the shock reversal at day 7 (p = 0.285), length of stay in ICU (p = 0.334), superinfection (p = 0.231), gastroduodenal bleeding (p = 0.867), and shock reversal at day 28 (p = 0.414) ( Supplementary Figures 23 – 30 ). The results of the sensitivity analysis showed that the models of the abovementioned outcomes, including length of stay in hospital, SOFA scores at day 7, hyperglycemia, shock reversal at day 7, length of stay in ICU, superinfection, gastroduodenal bleeding, and shock reversal at day 28 were credible ( Supplementary Figures 31 – 38 ).
Importantly, the risk of bias was reported in the first plot of each outcome, and the evidence rank is shown in Table 2 .
Table 2.
The findings and evidence rank of the included studies in patients with sepsis.
| Pooled results | No. of Patients (No. of Studies) | Relative Effect, RR, or MD (95% CI) | Heterogeneity I2,% | Absolute effect (95%CI) | Evidence rank |
|---|---|---|---|---|---|
| Primary outcomes | |||||
| 28 d mortality | 10,612 (40) | 0.94 (0.87, 1.02) | 24 | 17 fewer per 1000 (from 37 fewer to 6 more) | Moderate1 |
| In-hospital mortality | 8049 (23) | 0.90 (0.82, 0.99) | 39 | 33 fewer per 1000 (from 3 fewer to 60 fewer) | Moderate1 |
| ICU mortality | 7,152 (17) | 0.90 (0.83,0.97) | 7 | 28 fewer per 1000 (from 9 fewer to 48 fewer) | High |
| Secondary outcomes | |||||
| Long-term mortality | 6,254 (9) | 0.96 (0.88, 1.05) | 54 | 24 fewer per 1000 (from 48 fewer to 20 more) | Low2,3 |
| Shock reversal at 7 d | 6,738 (16) | 1.16 (1.06,1.27) | 72 | 105 more per 1000 (from 39 more to 178 more) | Moderate2 |
| Shock reversal at 28 d | 2,526 (12) | 1.07 (1.01,1.13) | 12 | 48 more per 1000 (from 7 fewer to 89 more) | Moderate2 |
| Gastroduodenal bleeding | 5,128 (24) | 1.07 (0.85,1.36) | 0 | 3 more per 1000 (from 7 fewer to 17 more) | High |
| Superinfection | 5,375 (24) | 1.06 (0.92, 1.22) | 13% | 10 more per 1000 (from 13 fewer to 36 more) | Moderate2 |
| Hypernatremia | 4,569 (3) | 1.51 (1.10,2.07) | 0 | 12 more per 1000 (from 2 more to 24 more) | Moderate2 |
| Hyperglycemia | 8,787 (20) | 1.19 (1.10,1.29) | 49% | 49 more per 1000 (from 24 more to 76 more) | High |
| Vasopressor-free days | 1,316 (2) | 1.93 (0.76, 3.09) | 0 | 1.93 more per 1000 (from 0.76 more to 3.09 more) | Moderate2 |
| Ventilation-free days | 1,812 (4) | 1.46 (0.27, 2.65) | 21 | 1.46 more per 1000 (from 0.27 more to 2.65 more) | Moderate2 |
| Length of stay in hospital | 8,383 (19) | -1.38(-2.28, -0.49) | 5 | 1.38 fewer per 1000 (from 2.28fewer to 0.49 fewer) | High |
| Length of stay in ICU | 8,166 (22) | -0.89 (-1.80, 0.03) | 47 | 0.89 fewer per 1000 (from 1.8 fewer to 0.03 more) | High |
| Time to resolution of shock | 4,091 (5) | -1.35(-1.79, -0.92) | 68 | 1.35 fewer per 1000 (from 1.79 fewer to 0.92 fewer) | Low2,3 |
| SOFA score at day 7 | 3,076 (13) | -0.90 (-1.72, -0.09) | 93 | 0.9 fewer per 1000 (from 1.72 fewer to 0.08 fewer) | Low2,3 |
RR, risk ratio; MD, mean difference; ICU, intensive care unit.
1Inconsistencies. 2Imprecisions. 3Risk of bias.
Subgroup Analysis
We performed subgroup analysis based on the sepsis subtype or type of corticosteroids used for the primary outcomes or I 2 >75% in the secondary outcomes with more than 10 trials for each outcome. The results of the subgroup analysis showed no effect on the 28-day mortality; however, the in-hospital and ICU mortality were significantly improved in the hydrocortisone plus fludrocortisone treatment and in the patients with septic shock, sepsis, and community-acquired pneumonia ( Supplementary Figures 39 – 44 ). Moreover, the result of the subgroup in SOFA scores at day 7 represented that the main original heterogeneity may be from the trials with smaller samples who were given hydrocortisone treatment or trials on patients with sepsis shock ( Supplementary Figures 45 and 46 ). Additionally, the subgroup based on the patients that were recruited into the surgical, medical, or surgical/medical ICU showed that corticosteroids were not associated with a 28-day mortality, SOFA scores at day 7, and in-hospital mortality but were related to lower ICU mortality in surgical/medical ICU patients ( Supplementary Table 3 ). Importantly, the subgroup in the corticosteroids based on catecholamine use for qualitative analysis showed that 19 RCTs (21–23, 25, 28, 29, 34, 35, 39–41, 43, 45, 51, 56–58, 60, 61) reported 28-day mortality, but it was not associated with the reduced 28-day mortality, no matter what the corticosteroids dose. Moreover, 11 RCTs (21–23, 25, 27, 28, 34, 35, 41, 45, 56) showed in-hospital mortality, 8 RCTs (21–23, 34, 35, 41, 45, 56) of which reported that the dose of corticosteroids was 200 mg/day or 50 mg every 6 h; only 1 (21) showed that corticosteroids may be associated with lower in-hospital mortality. Eleven RCTs (21–23, 25, 27, 28, 34, 35, 41, 51, 56) reported ICU mortality, seven studies (21–23, 34, 35, 41, 56) of which reported the corticosteroids dose was 200 mg/day or 50 mg every 6 h; only one (21) study showed that corticosteroids may be associated with the lower ICU mortality. Furthermore, three RCTs (25, 27, 28) showed that the corticosteroids dose was more than 200 mg/day; corticosteroids was not associated with the ICU mortality. However, two RCTs (21, 23) showed that corticosteroids dose of 200 mg/day or 50 mg every 6 h may reduce the time of vasopressors use.
Discussion
This meta-analysis included 50 RCTs (12,304 patients) and demonstrated that corticosteroids failed to improve the 28-day and long-term mortality; however, there was a small reduction in the in-hospital mortality and ICU mortality. To our knowledge, this systematic review and meta-analysis is the most comprehensive review of many new RCTs; the precision of the pooled effect estimates how sepsis could be increased substantially.
We found that the corticosteroids therapy for sepsis increased the incidence of the vasopressor-free days, ventilation-free time, shock reversal at days 7 and 28, and adverse events, such as hyperglycemia and hypernatremia. Corticosteroids were associated with a decreased risk of the time for shock resolution and length of stay in the hospital. However, our study failed to report a decreased risk of corticosteroids on the length of ICU stay and adverse events, such as superinfection and gastroduodenal bleeding. Ascertainment of the adverse events in the eligible trials was also vulnerable, which may induce the evidence rank to be low. Moreover, a quantitative analysis for the effect of the time of corticosteroids administration on septic patients was made. The effect of the different time of corticosteroid administration on septic patients cannot be compared because the time of corticosteroid administration was indistinct in the included studies. Therefore, further clinical studies should explore the time of corticosteroid administration for septic patients and ensure whether it is the same as antibiotics, which is the earlier the better.
Subgroup analyses in this review showed that the results did not identify any credible effect of modification in sepsis subtype and type of corticosteroids used. Much evidence comes from the trials with hydrocortisone or methylprednisolone treatment. Our subgroup analysis results showed that the efficacy of corticosteroids on in-hospital, ICU, and short-time mortality was mainly due to the hydrocortisone plus fludrocortisone.
Mechanistically, corticosteroids could inhibit the nuclear factor kappa B (NF-κB) activation and the extensive inflammatory factors release, finally improving the inflammatory response of sepsis or pneumonia. Our previous studies reported that corticosteroids were associated with a decreased risk of ARDS and length of the disease in patients with CAP (67). Previous reviews have assessed the efficacy and safety of corticosteroids in patients with sepsis. Unfortunately, the conclusions were contradictory owing to the small number of trials included. One meta-analysis included 20 RCTs and showed no reduction in the 28-day mortality, hospital mortality, and ICU mortality in patients with severe sepsis and sepsis shock on corticosteroids treatment (68). Subsequently, a Cochrane systematic review further conducted to search the effect of corticosteroids on mortality of patients with sepsis, including a total of 33 RCTs, found a small reduction in 28-day mortality on the corticosteroids treatment (69). Simultaneously, another study included 35 RCTs and showed a converse result that corticosteroids failed to decrease the mortality (70). In 2018, Rochwerg et al. (7) examined 42 RCTs including 10,194 patients, wherein corticosteroids achieved no reduction in the short-term (28–31 days) mortality and may have a little effect on the long-term mortality. In 2019, Fang et al. (71) included 37 RCTs; this trial suggested that corticosteroids use was associated with a decrease in the 28-day mortality, ICU mortality, and in-hospital mortality. In parallel, Annane et al. (6) published a Cochrane systematic review on 40 RCTs and achieved a reduction in the 28-day mortality in patients with sepsis on the corticosteroids therapy.
The results of this meta-analysis showed that corticosteroids treatment failed to improve the 28-day mortality, in contrast with results from the previous meta-analysis. The difference in part may be due to the result reported by Annane et al. (6), in which corticosteroids therapy showed an increased risk of 28-day mortality, while the CI contained the null effect line, suggesting that corticosteroids had no effect on sepsis based on the statistics. More importantly, we included four RCTs about severe COVID-19 and showed that there was no significant difference in 28-day mortality with corticosteroids use. The data were extracted from the latest RCTs and may have helped in reinforcing the conclusions, decreasing the heterogeneity among the studies, and improving the precision with more comprehensive assessment for the therapeutic effects of corticosteroids treatment.
In this meta-analysis, the result of the qualitative analysis showed that 200 mg/day or less may have a clinical benefit, such as increasing the vasopressor-free time, improving tissue oxygen supply, and restoring circulatory homeostasis in catecholamine-dependent septic shock (21, 23, 37). More importantly, the earlier study (25) reported that supraphysiological doses of hydrocortisone could improve hemodynamics and enhance the vascular sensitivity to catecholamines, thereby reducing the dose of catecholamine (dopamine >10 µg/kg/min) in the patients with septic shock. The subsequent studies (40) also showed that under the dose of dopamine ≥6 µg/kg/min, low-dose hydrocortisone could be a better maintenance of hemodynamics by increasing vascular sensitivity to catecholamines. Furthermore, Ibarra-Estrada et al. (72) suggested that compared with bolus infusion of hydrocortisone, continuous infusion may restore the vascular sensitivity to catecholamines better. Similarly, an experimental study (73) found that fludrocortisone combined with hydrocortisone therapy dose dependently increased phenylephrine with cumulative increasing concentrations, which caused concentration-dependent contraction of isolated mesenteric arteries from septic rats. Contrarily, a prospective cohort study (74) showed that after hydrocortisone therapy, there was a significant reduction in norepinephrine in survivors, whereas higher catecholamine dosages were required for the non-survivors. However, a latest retrospective cohort study (75) showed that higher norepinephrine (24.6 mcg/min) in early hydrocortisone therapy could improve reduction in ICU mortality compared with the late hydrocortisone therapy with norepinephrine (21.3 mcg/min) in patients with sepsis shock. Based on the abovementioned results, the potential mechanisms of corticosteroids restoring the vascular sensitivity to catecholamines have been reported as follows: (1) in the septic shock, as the excess production of nitric oxide causes host catecholamine resistance (76, 77), corticosteroids could inhibit inducible NOS formation and production restoring the vascular sensitivity to catecholamines; (2) desensitization and/or downregulation of β-adrenergic receptors (78) and possibly α-adrenergic receptors (79) maybe due to the downregulation by endogenous catecholamine production in septic shock, whereas the corticosteroids may reverse receptor desensitization (80) and further allow reduced catecholamine dosage (25). Given that the evidence of the relationship between catecholamines and corticosteroids is currently inconsistent, future clinical studies should be conducted to further research the dependence of catecholamine administration on the effect of cortisone administration.
Additionally, to explore which septic patients were more responsive to the corticosteroids therapy, the ICU subgroup analysis after the type of disease and corticosteroids subgroup was conducted. The results showed that with corticosteroids use, there was no difference in the 28-day mortality, in-hospital mortality, and SOFA scores at day 7 among the surgical ICU, medical ICU, and surgical/medical ICU, but there was lower ICU mortality in patients with sepsis from surgical/medical ICU. The results of the subgroup analysis may not provide useful information mainly because ICU description is too vague in the included studies. Thus, details cannot be determined. Therefore, future clinical research should distinguish patients based on ICU type (e.g., surgical or medical ICU) to explore which sepsis primary cause is the corticosteroids therapy effective.
Corticosteroids have already been used for adjuvant therapy in sepsis for more than half a century. However, credible evidence is still lacking to guide the choice of patients, the time of corticosteroids administration, or the dose of corticosteroids for catecholamine-dependent patients. With the definition of sepsis that varies from sepsis-1.0 to sepsis-3.0, the accuracy of sepsis diagnosis has significantly improved. However, corticosteroids use also varied from sepsis-1.0 to sepsis-3.0. Specifically, only patients with septic shock used corticosteroids and suggested the use of flumetasone (50 µg/day) in sepsis-1.0 (81). The use of hydrocortisone was suggested only in children with suspected or confirmed absolute adrenal insufficiency, which was a more stringent use of corticosteroids compared with sepsis-1, in sepsis-2.0 (82), and the use of hydrocortisone (200 mg/day) was suggested only in patients with refractory septic shock wherein appropriate fluid resuscitation and vasopressor therapy cannot restore hemodynamic stability in sepsis-3.0 (3), The proposals from sepsis-1.0, sepsis-2.0, and sepsis-3.0 lack credible evidence to support the clinical use of corticosteroids. Analysis of all relevant data from available RCTs showed that the effect of corticosteroids therapy for septic patients was not consistent. However, the latest studies showed that corticosteroids may not reduce mortality in septic patients compared with the control group. Importantly, this study suggests that corticosteroids administration may not reduce the 28-day mortality, long-term mortality, and length of ICU stay but may be associated with ICU mortality, in-hospital mortality, length of hospital stay, SOFA scores at day 7, and time to shock resolution, and increased shock reversal at days 7 and 28 and vasopressor- and ventilation-free days. Furthermore, this study suggested that corticosteroids may be an effective therapy with a low dose and long-term course. However, future studies need to appropriately study the time of corticosteroids administration, the primary infection source, and dose of corticosteroids use for septic shock patients who are dependent on catecholamine in the treatment of sepsis.
This meta-analysis has several strengths. First, this study is the most comprehensive trial to assess the efficacy of corticosteroids treatment on patients with sepsis to date. Second, we performed a thorough literature search including unpublished sources, using the GRADE methodology, to evaluate the evidence rank in overall RR, a predefined illustration of potential effect variables including direction of effect and subsequent subgroup analysis to search the effect variables, and illustration including the relative and absolute effects. Third, the primary outcomes showed low or no heterogeneity among the studies, suggesting that the results were not variable. Furthermore, the heterogeneity of SOFA scores on day 7 was high, and the subgroup analysis showed that the source of heterogeneity may be the inclusion of trials with small size on patients who were given hydrocortisone treatment. Finally, the results of the sensitivity analysis for this study suggested that these conclusions were robust and reliable.
However, this meta-analysis also has certain limitations, including the significant methodological or clinical heterogeneity among the included studies, especially with respect to the SOFA score on day 7. All the included RCTs enrolled patients with sepsis as per the previous sepsis definition criteria; however, we do not know whether the efficacy and safety of corticosteroids would change using the Sepsis-3 definition criteria. Hence, the defined mortality may be essential, but the certainty is limited due to the imprecision of the included studies.
Conclusions
This is the most comprehensive systematic review and meta-analysis to describe the efficacy and safety of corticosteroids in patients with sepsis. The findings demonstrate that corticosteroids failed to reduce the 28-day, 90-day, and long-term mortalities; however, they could reduce the in-hospital and ICU mortalities. Importantly, our subgroup analyses results indicated that this efficacy of corticosteroids in patients with sepsis may be associated with the hydrocortisone plus fludrocortisone treatment. Therefore, the results suggest that corticosteroids could not improve the 28-day mortality in adult patients with sepsis.
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding authors.
Author Contributions
All the authors contributed equally to the work presented in this article. TS, HuL conceived the idea of this study. XD, HoL, and GS contributed to the data extraction. SH, RZ, and XD computed and evaluated the pooled outcomes. HuL and SH contributed to the study protocol and wrote the article. QK and TS revised the article. QK and TS had full access to all of the data, and the final responsibility for the decision to submit this article for publication. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the United Fund of National Natural Science Foundation of China (Grant No. U2004110) and Leading Talents Fund in Science and Technology Innovation in Henan Province (Grant No. 194200510017).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.709155/full#supplementary-material
The Funnel plot assessed the potential publication bias of pooled effect in 28-day mortality for corticosteroids vs. placebo treatment in patients with sepsis.
The Funnel plot assessed the potential publication bias of pooled effect in in-hospital mortality for corticosteroids vs. placebo treatment in patients with sepsis.
The Funnel plot assessed the potential publication bias of pooled effect in ICU mortality for corticosteroids vs. placebo treatment in patients with sepsis.
The sensitivity analysis evaluated the robustness of the pooled effect model in 28-day mortality for this meta-analysis.
The sensitivity analysis evaluated the robustness of the pooled effect model in in-hospital mortality for this meta-analysis.
The sensitivity analysis evaluated the robustness of the pooled effect model in ICU mortality for this meta-analysis.
L’Abbe plot according to the corticosteroids therapy. 40 RCTs of corticosteroids and 28-day mortality in patients with sepsis were presented in a L’Abbe plot.
L’Abbe plot according to the corticosteroids therapy. 23 RCTs of corticosteroids and in-hospital mortality in patients with sepsis were presented in a L’Abbe plot.
L’Abbe plot according to the corticosteroid therapy. 17 RCTs of corticosteroids and ICU mortality in patients with sepsis were presented in a L’Abbe plot.
The Forest plot showed the pooled effect of length of stay in hospital in patients with sepsis based on the corticosteroids treatment for this meta-analysis.
The Forest plot showed the pooled effect of SOFA scores at day 7 in patients with sepsis based on the corticosteroids treatment for this meta-analysis.
The Forest plot showed the pooled effect of time to resolution of shock in patients with sepsis based on the corticosteroids treatment for this meta-analysis.
The Forest plot showed the pooled effect of hypernatremia in patients with sepsis based on the corticosteroids treatment for this meta-analysis.
The Forest plot showed the pooled effect of hyperglycemia in patients with sepsis based on the corticosteroids treatment for this meta-analysis.
The Forest plot showed the pooled effect of vasopressor-free days in patients with sepsis based on the corticosteroids treatment for this meta-analysis.
The Forest plot showed the pooled effect of ventilation-free time in patients with sepsis based on the corticosteroids treatment for this meta-analysis.
The Forest plot showed the pooled effect of shock reversal at day 7 in patients with sepsis based on the corticosteroids treatment for this meta-analysis.
The Forest plot showed the pooled effect of shock reversal at day 28 in patients with sepsis based on the corticosteroids treatment for this meta-analysis.
The Forest plot showed the pooled effect of long-term mortality in patients with sepsis based on the corticosteroids treatment for this meta-analysis.
The Forest plot showed the pooled effect of length of stay in ICU in patients with sepsis based on the corticosteroids treatment for this meta-analysis.
The Forest plot showed the pooled effect of superinfection in patients with sepsis based on the corticosteroids treatment for this meta-analysis.
The Forest plot showed the pooled effect of gastroduodenal bleeding in patients with sepsis based on the corticosteroids treatment for this meta-analysis.
The Funnel plot assessed the potential publication bias of pooled effect in length of stay in hospital in patients with sepsis for this meta-analysis.
The Funnel plot assessed the potential publication bias of pooled effect in SOFA scores at day 7 in patients with sepsis for this meta-analysis.
The Funnel plot assessed the potential publication bias of pooled effect in hyperglycemia in-hospital in patients with sepsis for this meta-analysis.
The Funnel plot assessed the potential publication bias of pooled effect in the shock reversal at day 7 in patients with sepsis for this meta-analysis.
The Funnel plot assessed the potential publication bias of pooled effect in length of stay in ICU in patients with sepsis for this meta-analysis.
The Funnel plot assessed the potential publication bias of pooled effect in superinfection in patients with sepsis for this meta-analysis.
The Funnel plot assessed the potential publication bias of pooled effect in gastroduodenal bleeding in patients with sepsis for this meta-analysis.
The Funnel plot assessed the potential publication bias of pooled effect in shock reversal at day 28 in patients with sepsis for this meta-analysis.
The sensitivity analysis evaluated the robustness of pooled effect model in length of stay in hospital for this meta-analysis.
The sensitivity analysis evaluated the robustness of pooled effect model in SOFA scores at day 7 for this meta-analysis.
The sensitivity analysis evaluated the robustness of pooled effect model in hyperglycemia for this meta-analysis.
The sensitivity analysis evaluated the robustness of pooled effect model in shock reversal at day 7 for this meta-analysis.
The sensitivity analysis evaluated the robustness of pooled effect model in length of stay in ICU for this meta-analysis.
The sensitivity analysis evaluated the robustness of pooled effect model in superinfection for this meta-analysis.
The sensitivity analysis evaluated the robustness of pooled effect model in gastroduodenal bleeding for this meta-analysis.
The sensitivity analysis evaluated the robustness of pooled effect model in shock reversal at day 28 for this meta-analysis.
Subgroup analysis of 28-day mortality of patients with sepsis based on the corticosteroids type.
Subgroup analysis of in-hospital mortality of patients with sepsis based on the corticosteroids type.
Subgroup analysis of ICU mortality of patients with sepsis based on the corticosteroids type.
Subgroup analysis of 28-day mortality of patients with sepsis based on the sepsis subtype.
Subgroup analysis of in-hospital mortality of patients with sepsis based on the sepsis subtype.
Subgroup analysis of ICU mortality of patients with sepsis based on the sepsis subtype.
Subgroup analysis of SOFA scores at day 7 of patients with sepsis based on the corticosteroids type.
Subgroup analysis of SOFA scores at day 7 of patients with sepsis based on the sepsis subtype.
Abbreviations
RR, risk ratio; CI, confidence interval; RCTs, randomized controlled trials; MD, mean difference; CAP, community-acquired pneumonia; ICU, intensive care unit; ARDS, acute respiratory distress syndrome; GRADE, Grading of Recommendations Assessment, Development and Evaluation; SOFA, Sequential Organ Failure Assessment.
References
- 1. Seymour CW, Liu VX, Iwashyna TJ, Brunkhorst FM, Rea TD, Scherag A, et al. Assessment of Clinical Criteria for Sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA (2016) 315(8):762–74. doi: 10.1001/jama.2016.0288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. DeMerle KM, Angus DC, Baillie JK, Brant E, Calfee CS, Carcillo J, et al. Sepsis Subclasses: A Framework for Development and Interpretation. Crit Care Med (2021) 49(5):748–59. doi: 10.1097/CCM.0000000000004842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med (2017) 43(3):304–77. doi: 10.1007/s00134-017-4683-6 [DOI] [PubMed] [Google Scholar]
- 4. Rhee C, Dantes R, Epstein L, Murphy DJ, Seymour CW, Iwashyna TJ, et al. Incidence and Trends of Sepsis in US Hospitals Using Clinical vs Claims Data, 2009-2014. JAMA (2017) 318(13):1241–9. doi: 10.1001/jama.2017.13836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Annane D, Pastores SM, Arlt W, Balk RA, Beishuizen A, Briegel J, et al. Critical Illness-Related Corticosteroid Insufficiency (CIRCI): A Narrative Review From a Multispecialty Task Force of the Society of Critical Care Medicine (SCCM) and the European Society of Intensive Care Medicine (ESICM). Intensive Care Med (2017) 43(12):1781–92. doi: 10.1007/s00134-017-4914-x [DOI] [PubMed] [Google Scholar]
- 6. Annane D, Bellissant E, Bollaert PE, Briegel J, Keh D, Kupfer Y, et al. Corticosteroids for Treating Sepsis in Children and Adults. Cochrane Database Systematic Rev (2019) 12(12):Cd002243. doi: 10.1002/14651858.CD002243.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rochwerg B, Oczkowski SJ, Siemieniuk RAC, Agoritsas T, Belley-Cote E, D’Aragon F, et al. Corticosteroids in Sepsis: An Updated Systematic Review and Meta-Analysis. Crit Care Med (2018) 46(9):1411–20. doi: 10.1097/CCM.0000000000003262 [DOI] [PubMed] [Google Scholar]
- 8. Fujii T, Luethi N, Young PJ, Frei DR, Eastwood GM, French CJ, et al. Effect of Vitamin C, Hydrocortisone, and Thiamine vs Hydrocortisone Alone on Time Alive and Free of Vasopressor Support Among Patients With Septic Shock: The VITAMINS Randomized Clinical Trial. JAMA (2020) 323(5):423–31. doi: 10.1001/jama.2019.22176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sevransky JE, Rothman RE, Hager DN, Bernard GR, Brown SM, Buchman TG, et al. Effect of Vitamin C, Thiamine, and Hydrocortisone on Ventilator- and Vasopressor-Free Days in Patients With Sepsis: The VICTAS Randomized Clinical Trial. JAMA (2021) 325(8):742–50. doi: 10.1001/jama.2020.24505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schünemann HJ. What is “Quality of Evidence” and Why is it Important to Clinicians? BMJ (Clinical Res ed) (2008) 336(7651):995–8. doi: 10.1136/bmj.39490.551019.BE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for Sepsis and Organ Failure and Guidelines for the Use of Innovative Therapies in Sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest (1992) 101(6):1644–55. doi: 10.1378/chest.101.6.1644 [DOI] [PubMed] [Google Scholar]
- 12. Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med (2003) 31(4):1250–6. doi: 10.1097/01.CCM.0000050454.01978.3B [DOI] [PubMed] [Google Scholar]
- 13. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA (2016) 315(8):801–10. doi: 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Higgins JPT GS. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 [Updated March 2011]. Cochrane Collaboration (2011). [Google Scholar]
- 15. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: An Emerging Consensus on Rating Quality of Evidence and Strength of Recommendations. BMJ (Clinical Res ed) (2008) 336(7650):924–6. doi: 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ (Clinical Res ed) (2011) 343:d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Abdelsalam Rezk N, Mohamed Ibrahim A. Effects of Methyl Prednisolone in Early ARDS. Egyptian J Chest Dis Tuberculosis (2013) 62(1):167–72. doi: 10.1016/j.ejcdt.2013.02.013 [DOI] [Google Scholar]
- 18. Aboab J, Polito A, Orlikowski D, Sharshar T, Castel M, Annane D. Hydrocortisone Effects on Cardiovascular Variability in Septic Shock: A Spectral Analysis Approach. Crit Care Med (2008) 36(5):1481–6. doi: 10.1097/CCM.0b013e31816f48f2 [DOI] [PubMed] [Google Scholar]
- 19. Angus DC, Derde L, Al-Beidh F, Annane D, Arabi Y, Beane A, et al. Effect of Hydrocortisone on Mortality and Organ Support in Patients With Severe COVID-19: The REMAP-CAP COVID-19 Corticosteroid Domain Randomized Clinical Trial. JAMA (2020) 324(13):1317–29. doi: 10.1001/jama.2020.17022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Annane D, Cariou A, Maxime V, Azoulay E, D’Honneur G, Timsit JF, et al. Corticosteroid Treatment and Intensive Insulin Therapy for Septic Shock in Adults: A Randomized Controlled Trial. JAMA (2010) 303(4):341–8. doi: 10.1001/jama.2010.2 [DOI] [PubMed] [Google Scholar]
- 21. Annane D, Renault A, Brun-Buisson C, Megarbane B, Quenot JP, Siami S, et al. Hydrocortisone Plus Fludrocortisone for Adults With Septic Shock. N Engl J Med (2018) 378(9):809–18. doi: 10.1056/NEJMoa1705716 [DOI] [PubMed] [Google Scholar]
- 22. Annane D, Sébille V, Charpentier C, Bollaert PE, François B, Korach JM, et al. Effect of Treatment With Low Doses of Hydrocortisone and Fludrocortisone on Mortality in Patients With Septic Shock. JAMA (2002) 288(7):862–71. doi: 10.1001/jama.288.7.862 [DOI] [PubMed] [Google Scholar]
- 23. Arabi YM, Aljumah A, Dabbagh O, Tamim HM, Rishu AH, Al-Abdulkareem A, et al. Low-Dose Hydrocortisone in Patients With Cirrhosis and Septic Shock: A Randomized Controlled Trial. CMAJ: Can Med Assoc J = J l’Association medicale Can (2011) 182(18):1971–7. doi: 10.1503/cmaj.090707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Blum CA, Nigro N, Briel M, Schuetz P, Ullmer E, Suter-Widmer I, et al. Adjunct Prednisone Therapy for Patients With Community-Acquired Pneumonia: A Multicentre, Double-Blind, Randomised, Placebo-Controlled Trial. Lancet (Lond Engl) (2015) 385(9977):1511–8. doi: 10.1016/S0140-6736(14)62447-8 [DOI] [PubMed] [Google Scholar]
- 25. Bollaert PE, Charpentier C, Levy B, Debouverie M, Audibert G, Larcan A. Reversal of Late Septic Shock With Supraphysiologic Doses of Hydrocortisone. Crit Care Med (1998) 26(4):645–50. doi: 10.1097/00003246-199804000-00010 [DOI] [PubMed] [Google Scholar]
- 26. Bone RC, Fisher CJ, Jr, Clemmer TP, Slotman GJ, Metz CA, Balk RA. A Controlled Clinical Trial of High-Dose Methylprednisolone in the Treatment of Severe Sepsis and Septic Shock. N Engl J Med (1987) 317(11):653–8. doi: 10.1056/NEJM198709103171101 [DOI] [PubMed] [Google Scholar]
- 27. Briegel J, Forst H, Haller M, Schelling G, Kilger E, Kuprat G, et al. Stress Doses of Hydrocortisone Reverse Hyperdynamic Septic Shock: A Prospective, Randomized, Double-Blind, Single-Center Study. Crit Care Med (1999) 27(4):723–32. doi: 10.1097/00003246-199904000-00025 [DOI] [PubMed] [Google Scholar]
- 28. Chawla K, Kupfer Y, Goldman I, Tessler S. Hydrocortisone Reverses Refractory Septic Shock. Crit Care Med (1999) 27(1):33A. doi: 10.1097/00003246-199901001-00022 [DOI] [Google Scholar]
- 29. Cicarelli DD, Benseñor FE, Vieira JE. Effects of Single Dose of Dexamethasone on Patients With Systemic Inflammatory Response. Sao Paulo Med J = Rev paulista medicina (2007) 124(2):90–5. doi: 10.1590/S1516-31802006000200008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Confalonieri M, Urbino R, Potena A, Piattella M, Parigi P, Puccio G, et al. Hydrocortisone Infusion for Severe Community-Acquired Pneumonia: A Preliminary Randomized Study. Am J Respir Crit Care Med (2005) 171(3):242–8. doi: 10.1164/rccm.200406-808OC [DOI] [PubMed] [Google Scholar]
- 31. Dequin PF, Heming N, Meziani F, Plantefève G, Voiriot G, Badié J, et al. Effect of Hydrocortisone on 21-Day Mortality or Respiratory Support Among Critically Ill Patients With COVID-19: A Randomized Clinical Trial. JAMA (2020) 324(13):1298–306. doi: 10.1001/jama.2020.16761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Edalatifard M, Akhtari M. Intravenous Methylprednisolone Pulse as a Treatment for Hospitalised Severe COVID-19 Patients: Results From a Randomised Controlled Clinical Trial. Eur Respir J (2020) 56(6):2002808. doi: 10.1183/13993003.02808-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fernández-Serrano S, Dorca J, Garcia-Vidal C, Fernández-Sabé N, Carratalà J, Fernández-Agüera A, et al. Effect of Corticosteroids on the Clinical Course of Community-Acquired Pneumonia: A Randomized Controlled Trial. Crit Care (Lond Engl) (2011) 15(2):R96. doi: 10.1186/cc10103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gordon AC, Mason AJ, Perkins GD, Stotz M, Terblanche M, Ashby D, et al. The Interaction of Vasopressin and Corticosteroids in Septic Shock: A Pilot Randomized Controlled Trial. Crit Care Med (2014) 42(6):1325–33. doi: 10.1097/CCM.0000000000000212 [DOI] [PubMed] [Google Scholar]
- 35. Gordon AC, Mason AJ, Thirunavukkarasu N, Perkins GD, Cecconi M, Cepkova M, et al. Effect of Early Vasopressin vs Norepinephrine on Kidney Failure in Patients With Septic Shock: The VANISH Randomized Clinical Trial. JAMA (2016) 316(5):509–18. doi: 10.1001/jama.2016.10485 [DOI] [PubMed] [Google Scholar]
- 36. VASSCS G . Effect of High-Dose Glucocorticoid Therapy on Mortality in Patients With Clinical Signs of Systemic Sepsis. N Engl J Med (1987) 317(11):659–65. doi: 10.1056/NEJM198709103171102 [DOI] [PubMed] [Google Scholar]
- 37. Hu B, Li JG, Liang H, Zhou Q, Yu Z, Li L, et al. [The Effect of Low-Dose Hydrocortisone on Requirement of Norepinephrine and Lactate Clearance in Patients With Refractory Septic Shock]. Zhongguo wei zhong bing ji jiu yi xue = Chin Crit Care Med = Zhongguo weizhongbing jijiuyixue (2009) 21(9):529–31. doi: 10.3760/cma.j.issn.1003-0603.2009.09.006 [DOI] [PubMed] [Google Scholar]
- 38. Huang R, Zhang Z, Xu M, Chang X, Qiao Q, Wang L, et al. [Effect of Sini Decoction on Function of Hypothalamic-Pituitary-Adrenal Axis in Patients With Sepsis]. Zhonghua wei zhong bing ji jiu yi xue (2014) 26(3):184–7. doi: 10.3760/cma.j.issn.2095-4352.2014.03.012 [DOI] [PubMed] [Google Scholar]
- 39. Hyvernat H, Barel R, Gentilhomme A, Césari-Giordani JF, Freche A, Kaidomar M, et al. Effects of Increasing Hydrocortisone to 300 Mg Per Day in the Treatment of Septic Shock: A Pilot Study. Shock (Augusta Ga) (2016) 46(5):498–505. doi: 10.1097/SHK.0000000000000665 [DOI] [PubMed] [Google Scholar]
- 40. Keh D, Boehnke T, Weber-Cartens S, Schulz C, Ahlers O, Bercker S, et al. Immunologic and Hemodynamic Effects of “Low-Dose” Hydrocortisone in Septic Shock: A Double-Blind, Randomized, Placebo-Controlled, Crossover Study. Am J Respir Crit Care Med (2003) 167(4):512–20. doi: 10.1164/rccm.200205-446OC [DOI] [PubMed] [Google Scholar]
- 41. Keh D, Trips E, Marx G, Wirtz SP, Abduljawwad E, Bercker S, et al. Effect of Hydrocortisone on Development of Shock Among Patients With Severe Sepsis: The HYPRESS Randomized Clinical Trial. JAMA (2016) 316(17):1775–85. doi: 10.1001/jama.2016.14799 [DOI] [PubMed] [Google Scholar]
- 42. Klastersky J, Cappel R, Debusscher L. Effectiveness of Betamethasone in Management of Severe Infections. A Double-Blind Study. N Engl J Med (1971) 284(22):1248–50. doi: 10.1056/NEJM197106032842206 [DOI] [PubMed] [Google Scholar]
- 43. Loisa P, Parviainen I, Tenhunen J, Hovilehto S, Ruokonen E. Effect of Mode of Hydrocortisone Administration on Glycemic Control in Patients With Septic Shock: A Prospective Randomized Trial. Crit Care (Lond Engl) (2007) 11(1):R21. doi: 10.1186/cc5696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Luce JM, Montgomery AB, Marks JD, Turner J, Metz CA, Murray JF. Ineffectiveness of High-Dose Methylprednisolone in Preventing Parenchymal Lung Injury and Improving Mortality in Patients With Septic Shock. Am Rev Respir Dis (1988) 138(1):62–8. doi: 10.1164/ajrccm/138.1.62 [DOI] [PubMed] [Google Scholar]
- 45. Lv QQ, Gu XH, Chen QH, Yu JQ, Zheng RQ. Early Initiation of Low-Dose Hydrocortisone Treatment for Septic Shock in Adults: A Randomized Clinical Trial. Am J Emergency Med (2017) 35(12):1810–4. doi: 10.1016/j.ajem.2017.06.004 [DOI] [PubMed] [Google Scholar]
- 46. Meduri GU, Golden E, Freire AX, Taylor E, Zaman M, Carson SJ, et al. Methylprednisolone Infusion in Early Severe ARDS: Results of a Randomized Controlled Trial. Chest (2007) 131(4):954–63. doi: 10.1378/chest.06-2100 [DOI] [PubMed] [Google Scholar]
- 47. Meduri GU, Golden E, Umberger R. Prospective Double-Blind Randomized Clinical Trial on the Effects of Low-Dose Hydrocortisone Infusion in Patients With Severe Sepsis. Chest (2009) 136(4):45S. doi: 10.1016/S0012-3692(16)48001-3 [DOI] [Google Scholar]
- 48. Meijvis SC, Hardeman H, Remmelts HH, Heijligenberg R, Rijkers GT, van Velzen-Blad H, et al. Dexamethasone and Length of Hospital Stay in Patients With Community-Acquired Pneumonia: A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet (Lond Engl) (2011) 377(9782):2023–30. doi: 10.1016/S0140-6736(11)60607-7 [DOI] [PubMed] [Google Scholar]
- 49. Mirea L, Ungureanu R, Pavelescu D, Grintescu IC, Dumitrache C, Grintescu I, et al. Continuous Administration of Corticosteroids in Septic Shock can Reduce Risk of Hypernatremia. Crit Care (2014) 18(1):P239. doi: 10.1186/cc13429 [DOI] [Google Scholar]
- 50. Nafae RM, Ragab MI, Amany FM, Rashed SB. Adjuvant Role of Corticosteroids in the Treatment of Community-Acquired Pneumonia. Egyptian J Chest Dis Tuberculosis (2013) 62(3):439–45. doi: 10.1016/j.ejcdt.2013.03.009 [DOI] [Google Scholar]
- 51. Oppert M, Schindler R, Husung C, Offermann K, Gräf KJ, Boenisch O, et al. Low-Dose Hydrocortisone Improves Shock Reversal and Reduces Cytokine Levels in Early Hyperdynamic Septic Shock. Crit Care Med (2005) 33(11):2457–64. doi: 10.1097/01.CCM.0000186370.78639.23 [DOI] [PubMed] [Google Scholar]
- 52. Rinaldi S, Adembri C, Grechi S, De Gaudio AR. Low-Dose Hydrocortisone During Severe Sepsis: Effects on Microalbuminuria. Crit Care Med (2006) 34(9):2334–9. doi: 10.1097/01.CCM.0000233872.04706.BB [DOI] [PubMed] [Google Scholar]
- 53. Sabry NA, Omar EE-D. Corticosteroids and ICU Course of Community Acquired Pneumonia in Egyptian Settings. Pharmacol Pharm (2011) 02(02):73–81. doi: 10.4236/pp.2011.22009 [DOI] [Google Scholar]
- 54. Schumer W. Steroids in the Treatment of Clinical Septic Shock. Ann Surg (1976) 184(3):333–41. doi: 10.1097/00000658-197609000-00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Snijders D, Daniels JM, de Graaff CS, van der Werf TS, Boersma WG. Efficacy of Corticosteroids in Community-Acquired Pneumonia: A Randomized Double-Blinded Clinical Trial. Am J Respir Crit Care Med (2010) 181(9):975–82. doi: 10.1164/rccm.200905-0808OC [DOI] [PubMed] [Google Scholar]
- 56. Sprung CL, Annane D, Keh D, Moreno R, Singer M, Freivogel K, et al. Hydrocortisone Therapy for Patients With Septic Shock. N Engl J Med (2008) 358(2):111–24. doi: 10.1056/NEJMoa071366 [DOI] [PubMed] [Google Scholar]
- 57. Sprung CL, Caralis PV, Marcial EH, Pierce M, Gelbard MA, Long WM, et al. The Effects of High-Dose Corticosteroids in Patients With Septic Shock. A Prospective, Controlled Study. N Engl J Med (1984) 311(18):1137–43. doi: 10.1056/NEJM198411013111801 [DOI] [PubMed] [Google Scholar]
- 58. Talebi Doluee M, Salehi M, Mahmoudi Gharaee A, Jalalyazdi M, Reihani H. The Effect of Physiologic Dose of Intravenous Hydrocortisone in Patients With Refractory Septic Shock: A Randomized Control Trial. J Emergency Pract Trauma (2018) 4(1):29–33. doi: 10.15171/jept.2017.25 [DOI] [Google Scholar]
- 59. Tandan SM GR GN. Corticosteroids for Treating Sepsis in Children and Adultslow Dose Steroids and Adrenocortical Insufficiency in Septic Shock: A Double-Blind Randomised Controlled Trial From India. Proc Am Thoracic Soc Meeting (2005) A24. [Google Scholar]
- 60. Tilouche N, Jaoued O, Ali HBS, Gharbi R, Fekih Hassen M, Elatrous S. Comparison Between Continuous and Intermittent Administration of Hydrocortisone During Septic Shock: A Randomized Controlled Clinical Trial. Shock (Augusta Ga) (2019) 52(5):481–6. doi: 10.1097/SHK.0000000000001316 [DOI] [PubMed] [Google Scholar]
- 61. Tomazini BM, Maia IS, Cavalcanti AB, Berwanger O, Rosa RG, Veiga VC, et al. Effect of Dexamethasone on Days Alive and Ventilator-Free in Patients With Moderate or Severe Acute Respiratory Distress Syndrome and COVID-19: The CoDEX Randomized Clinical Trial. JAMA (2020) 324(13):1307–16. doi: 10.1001/jama.2020.17021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tongyoo S, Permpikul C, Mongkolpun W, Vattanavanit V, Udompanturak S, Kocak M, et al. Hydrocortisone Treatment in Early Sepsis-Associated Acute Respiratory Distress Syndrome: Results of a Randomized Controlled Trial. Crit Care (Lond Engl) (2016) 20(1):329. doi: 10.1186/s13054-016-1511-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Torres A, Sibila O, Ferrer M, Polverino E, Menendez R, Mensa J, et al. Effect of Corticosteroids on Treatment Failure Among Hospitalized Patients With Severe Community-Acquired Pneumonia and High Inflammatory Response: A Randomized Clinical Trial. JAMA (2015) 313(7):677–86. doi: 10.1001/jama.2015.88 [DOI] [PubMed] [Google Scholar]
- 64. Venkatesh B, Finfer S, Cohen J, Rajbhandari D, Arabi Y, Bellomo R, et al. Adjunctive Glucocorticoid Therapy in Patients With Septic Shock. N Engl J Med (2018) 378(9):797–808. doi: 10.1056/NEJMoa1705835 [DOI] [PubMed] [Google Scholar]
- 65. Yildiz O, Doganay M, Aygen B, Güven M, Keleştimur F, Tutuû A. Physiological-Dose Steroid Therapy in Sepsis [ISRCTN36253388]. Crit Care (Lond Engl) (2002) 6(3):251–9. doi: 10.1186/cc1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yildiz O, Tanriverdi F, Simsek S, Aygen B, Kelestimur F. The Effects of Moderate-Dose Steroid Therapy in Sepsis: A Placebo-Controlled, Randomized Study. J Res Med Sciences: Off J Isfahan Univ Med Sci (2011) 16(11):1410–21. [PMC free article] [PubMed] [Google Scholar]
- 67. Wan YD, Sun TW, Liu ZQ, Zhang SG, Wang LX, Kan QC. Efficacy and Safety of Corticosteroids for Community-Acquired Pneumonia: A Systematic Review and Meta-Analysis. Chest (2016) 149(1):209–19. doi: 10.1378/chest.15-1733 [DOI] [PubMed] [Google Scholar]
- 68. Annane D, Bellissant E, Bollaert PE, Briegel J, Confalonieri M, De Gaudio R, et al. Corticosteroids in the Treatment of Severe Sepsis and Septic Shock in Adults: A Systematic Review. JAMA (2009) 301(22):2362–75. doi: 10.1001/jama.2009.815 [DOI] [PubMed] [Google Scholar]
- 69. Annane D, Bellissant E, Bollaert PE, Briegel J, Keh D, Kupfer Y. Corticosteroids for Treating Sepsis. Cochrane Database Systematic Rev (2015) 2015(12):Cd002243. doi: 10.1002/14651858.CD002243.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Volbeda M, Wetterslev J, Gluud C, Zijlstra JG, van der Horst IC, Keus F. Glucocorticosteroids for Sepsis: Systematic Review With Meta-Analysis and Trial Sequential Analysis. Intensive Care Med (2015) 41(7):1220–34. doi: 10.1007/s00134-015-3899-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Fang F, Zhang Y, Tang J, Lunsford LD, Li T, Tang R, et al. Association of Corticosteroid Treatment With Outcomes in Adult Patients With Sepsis: A Systematic Review and Meta-Analysis. JAMA Internal Med (2019) 179(2):213–23. doi: 10.1001/jamainternmed.2018.5849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ibarra-Estrada MA, Chávez-Peña Q, Reynoso-Estrella CI, Rios-Zermeño J, Aguilera-González PE, García-Soto MA, et al. Timing, Method and Discontinuation of Hydrocortisone Administration for Septic Shock Patients. World J Crit Care Med (2017) 6(1):65–73. doi: 10.5492/wjccm.v6.i1.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Laviolle B, Nesseler N, Massart C, Bellissant E. Fludrocortisone and Hydrocortisone, Alone or in Combination, on In Vivo Hemodynamics and In Vitro Vascular Reactivity in Normal and Endotoxemic Rats: A Randomized Factorial Design Study. J Cardiovasc Pharmacol (2014) 63(6):488–96. doi: 10.1097/FJC.0000000000000072 [DOI] [PubMed] [Google Scholar]
- 74. Winter W, Kamolz L, Donner A, Hoerauf K, Blaicher A, Andel H. Hydrocortisone Improved Haemodynamics and Fluid Requirement in Surviving But Not Non-Surviving of Severely Burned Patients. Burns: J Int Soc Burn Injuries (2003) 29(7):717–20. doi: 10.1016/S0305-4179(03)00148-7 [DOI] [PubMed] [Google Scholar]
- 75. Sacha GL, Chen AY, Palm NM, Duggal A. Evaluation of the Initiation Timing of Hydrocortisone in Adult Patients With Septic Shock. Shock (Augusta Ga) (2021) 55(4):488–94. doi: 10.1097/SHK.0000000000001651 [DOI] [PubMed] [Google Scholar]
- 76. Vincent JL, Zhang H, Szabo C, Preiser JC. Effects of Nitric Oxide in Septic Shock. Am J Respir Crit Care Med (2000) 161(6):1781–5. doi: 10.1164/ajrccm.161.6.9812004 [DOI] [PubMed] [Google Scholar]
- 77. Parker MM. Pathophysiology of Cardiovascular Dysfunction in Septic Shock. New Horizons (Baltimore Md) (1998) 6(2):130–8. [PubMed] [Google Scholar]
- 78. Silverman HJ, Penaranda R, Orens JB, Lee NH. Impaired Beta-Adrenergic Receptor Stimulation of Cyclic Adenosine Monophosphate in Human Septic Shock: Association With Myocardial Hyporesponsiveness to Catecholamines. Crit Care Med (1993) 21(1):31–9. doi: 10.1097/00003246-199301000-00010 [DOI] [PubMed] [Google Scholar]
- 79. McMillan M, Chernow B, Roth BL. Hepatic Alpha 1-Adrenergic Receptor Alteration in a Rat Model of Chronic Sepsis. Circulatory Shock (1986) 19(2):185–93. [PubMed] [Google Scholar]
- 80. Walker BR, Williams BC. Corticosteroids and Vascular Tone: Mapping the Messenger Maze. Clin Sci (London England: 1979) (1992) 82(6):597–605. doi: 10.1042/cs0820597 [DOI] [PubMed] [Google Scholar]
- 81. American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference Definitions for Sepsis and Organ Failure and Guidelines for the Use of Innovative Therapies in Sepsis. Crit Care Med (1992) 20(6):864–74. doi: 10.1097/00003246-199206000-00025 [DOI] [PubMed] [Google Scholar]
- 82. Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving Sepsis Campaign: International Guidelines for Management of Severe Sepsis and Septic Shock, 2012. Intensive Care Med (2013) 39(2):165–228. doi: 10.1007/s00134-012-2769-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The Funnel plot assessed the potential publication bias of pooled effect in 28-day mortality for corticosteroids vs. placebo treatment in patients with sepsis.
The Funnel plot assessed the potential publication bias of pooled effect in in-hospital mortality for corticosteroids vs. placebo treatment in patients with sepsis.
The Funnel plot assessed the potential publication bias of pooled effect in ICU mortality for corticosteroids vs. placebo treatment in patients with sepsis.
The sensitivity analysis evaluated the robustness of the pooled effect model in 28-day mortality for this meta-analysis.
The sensitivity analysis evaluated the robustness of the pooled effect model in in-hospital mortality for this meta-analysis.
The sensitivity analysis evaluated the robustness of the pooled effect model in ICU mortality for this meta-analysis.
L’Abbe plot according to the corticosteroids therapy. 40 RCTs of corticosteroids and 28-day mortality in patients with sepsis were presented in a L’Abbe plot.
L’Abbe plot according to the corticosteroids therapy. 23 RCTs of corticosteroids and in-hospital mortality in patients with sepsis were presented in a L’Abbe plot.
L’Abbe plot according to the corticosteroid therapy. 17 RCTs of corticosteroids and ICU mortality in patients with sepsis were presented in a L’Abbe plot.
The Forest plot showed the pooled effect of length of stay in hospital in patients with sepsis based on the corticosteroids treatment for this meta-analysis.
The Forest plot showed the pooled effect of SOFA scores at day 7 in patients with sepsis based on the corticosteroids treatment for this meta-analysis.
The Forest plot showed the pooled effect of time to resolution of shock in patients with sepsis based on the corticosteroids treatment for this meta-analysis.
The Forest plot showed the pooled effect of hypernatremia in patients with sepsis based on the corticosteroids treatment for this meta-analysis.
The Forest plot showed the pooled effect of hyperglycemia in patients with sepsis based on the corticosteroids treatment for this meta-analysis.
The Forest plot showed the pooled effect of vasopressor-free days in patients with sepsis based on the corticosteroids treatment for this meta-analysis.
The Forest plot showed the pooled effect of ventilation-free time in patients with sepsis based on the corticosteroids treatment for this meta-analysis.
The Forest plot showed the pooled effect of shock reversal at day 7 in patients with sepsis based on the corticosteroids treatment for this meta-analysis.
The Forest plot showed the pooled effect of shock reversal at day 28 in patients with sepsis based on the corticosteroids treatment for this meta-analysis.
The Forest plot showed the pooled effect of long-term mortality in patients with sepsis based on the corticosteroids treatment for this meta-analysis.
The Forest plot showed the pooled effect of length of stay in ICU in patients with sepsis based on the corticosteroids treatment for this meta-analysis.
The Forest plot showed the pooled effect of superinfection in patients with sepsis based on the corticosteroids treatment for this meta-analysis.
The Forest plot showed the pooled effect of gastroduodenal bleeding in patients with sepsis based on the corticosteroids treatment for this meta-analysis.
The Funnel plot assessed the potential publication bias of pooled effect in length of stay in hospital in patients with sepsis for this meta-analysis.
The Funnel plot assessed the potential publication bias of pooled effect in SOFA scores at day 7 in patients with sepsis for this meta-analysis.
The Funnel plot assessed the potential publication bias of pooled effect in hyperglycemia in-hospital in patients with sepsis for this meta-analysis.
The Funnel plot assessed the potential publication bias of pooled effect in the shock reversal at day 7 in patients with sepsis for this meta-analysis.
The Funnel plot assessed the potential publication bias of pooled effect in length of stay in ICU in patients with sepsis for this meta-analysis.
The Funnel plot assessed the potential publication bias of pooled effect in superinfection in patients with sepsis for this meta-analysis.
The Funnel plot assessed the potential publication bias of pooled effect in gastroduodenal bleeding in patients with sepsis for this meta-analysis.
The Funnel plot assessed the potential publication bias of pooled effect in shock reversal at day 28 in patients with sepsis for this meta-analysis.
The sensitivity analysis evaluated the robustness of pooled effect model in length of stay in hospital for this meta-analysis.
The sensitivity analysis evaluated the robustness of pooled effect model in SOFA scores at day 7 for this meta-analysis.
The sensitivity analysis evaluated the robustness of pooled effect model in hyperglycemia for this meta-analysis.
The sensitivity analysis evaluated the robustness of pooled effect model in shock reversal at day 7 for this meta-analysis.
The sensitivity analysis evaluated the robustness of pooled effect model in length of stay in ICU for this meta-analysis.
The sensitivity analysis evaluated the robustness of pooled effect model in superinfection for this meta-analysis.
The sensitivity analysis evaluated the robustness of pooled effect model in gastroduodenal bleeding for this meta-analysis.
The sensitivity analysis evaluated the robustness of pooled effect model in shock reversal at day 28 for this meta-analysis.
Subgroup analysis of 28-day mortality of patients with sepsis based on the corticosteroids type.
Subgroup analysis of in-hospital mortality of patients with sepsis based on the corticosteroids type.
Subgroup analysis of ICU mortality of patients with sepsis based on the corticosteroids type.
Subgroup analysis of 28-day mortality of patients with sepsis based on the sepsis subtype.
Subgroup analysis of in-hospital mortality of patients with sepsis based on the sepsis subtype.
Subgroup analysis of ICU mortality of patients with sepsis based on the sepsis subtype.
Subgroup analysis of SOFA scores at day 7 of patients with sepsis based on the corticosteroids type.
Subgroup analysis of SOFA scores at day 7 of patients with sepsis based on the sepsis subtype.
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding authors.