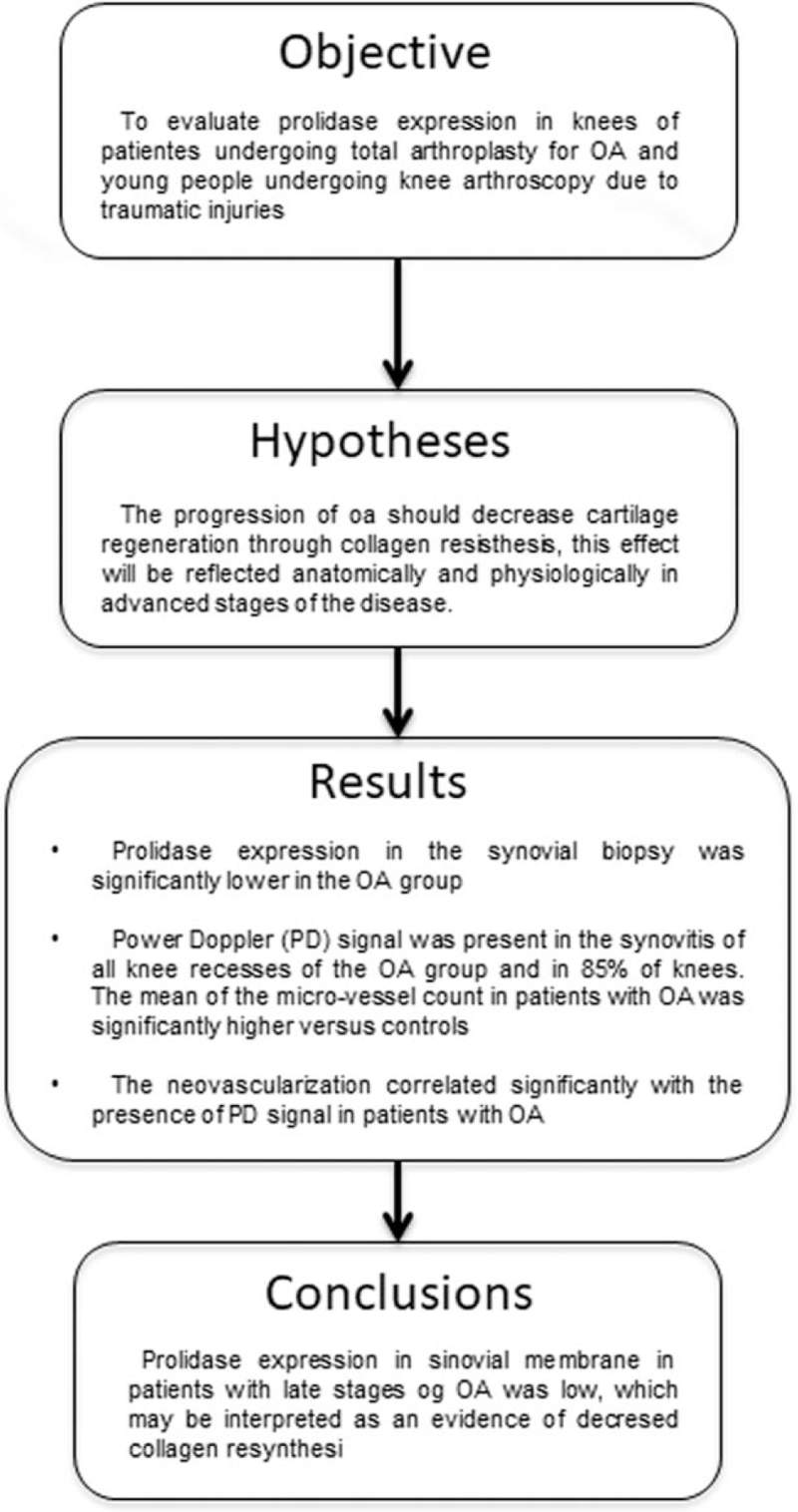
Abstract
Prolidase enzyme activity is important for collagen resynthesis. In late stages of osteoarthritis (OA) its activity is decreased.
To evaluate prolidase expression in knees of patients undergoing total arthroplasty for OA, and compare with young people undergoing knee arthroscopy due to traumatic injuries.
In this cross-sectional study we included 20 patients with OA grade IV who underwent total knee arthroplasty and 20 controls of young patients who underwent arthroscopy for another reason besides OA. All participants were evaluated by knee ultrasound before the procedure. During the procedure, synovial tissue biopsies were taken and analyzed by immunofluorescence to search inflammation. Measures of central tendency, dispersion measures and position measures were used for the case of quantitative variables. Student t test or Mann–Whitney U test, and the logistic regression of Cox, was used.
Prolidase expression in the synovial biopsy was significantly lower in the OA group than in the controls (0.017 ± 0.009 vs 0.062 ± 0.094, P < .05). Power Doppler (PD) signal was present in the synovitis of all knee recesses of the OA group in grayscale and in 17 (85%) of knees. The mean of the micro-vessel count in patients with OA was significantly higher vs controls (11 + 5.3 vs 4 + 2.1, P = .001). The neovascularization correlated significantly with the presence of PD signal in patients with OA (1.16, 95% CI, 1.02–1.34, P = .02).
The prolidase expression in the synovial membrane evaluated by immunofluorescence, in patients with late stages of knee OA, is low, which may be interpreted as an evidence of decreased collagen resynthesis.
Keywords: inmunofluorescence, knee osteoarthritis, oxidative stress, prolidase
1. Introduction
Osteoarthritis (OA) is the most common joint disease in the world, affecting up to 10% of men and 13% of women.[1,2] The knee is the main affected joint.[3,4] Its etiology is multifactorial. One possible cause of OA is oxidative stress due to levels of pro-inflammatory mediators, such as reactive oxygen species (ROS), that are elevated in this condition.[5] It is known that ROS stimulate pro-inflammatory cytokines such as interleukin-1 and tumor necrosis factor, which play an important role in cartilage matrix degradation.[1] Additionally, ROS reduce cartilage repair capacity and induce cell death in the extracellular matrix.[6] The oxidative stress has been related to a decreased collagen metabolism, which has a role in etiopathogenesis and/or in the progression of OA.[7]
Collagen is the most abundant structural macromolecule of the extracellular matrix of articular cartilage and makes up approximately 60% of the dry weight of cartilage. Collagen type II is the most abundant since it represents 90% of the collagen in the extracellular matrix of cartilage and forms fibrils and fibers interlaced with aggregates of proteoglycans.[8] For collagen biosynthesis, prolidase enzyme activity is needed for breakdown.[9,10] Prolidase is a cytosolic exopeptidase. Its main function is to participate in the last step of collagen degradation.[11] This enzyme has an important role in the recycling of proline from imidodipeptides derived from degradation products of collagen and other proline-containing proteins for collagen re-synthesis.[12] If prolidase enzyme activity is reduced, it would affect the recycling of proline, which is necessary for collagen biosynthesis.
Significant decrease in serum prolidase activity in patients with OA has been described, which may be interpreted as evidence of decreased collagen re-synthesis.[4] However, it is not known whether or not prolidase expression is decreased at the joint level in patients with OA. Thus, the aim of this study was to evaluate prolidase expression in knee joints from patients undergoing total knee arthroplasty for OA and to contrast it with healthy individuals undergoing knee arthroscopy due to sport-related injuries.
2. Patients and methods
A cross-sectional prospective study was carried out. Study population were patients from the traumatology clinic of the National Institute of Rehabilitation “Luis Guillermo Ibarra Ibarra,” with knee OA (KOA) diagnosis according to the American College of Rheumatology criteria.[12] The severity of KOA was classified according to the Kellgren and Lawrence radiographic scoring system.[13] All patients underwent total knee arthroplasty with synovectomy. The control group consisted of healthy subjects, who underwent elective knee arthroscopy due to sport-related injuries (non-systemic causes), such as meniscal pathology, rupture of the cruciate ligaments, patellar syndrome and extraction of joint loose bodies.
2.1. Ethical approval
All procedures performed in the study involving human participants were in accordance with the ethical standards of the institutional committee and with the 1964 Helsinki declaration and its later amendments. The study was approved by the Ethics and Research Committee of the National Institute of Rehabilitation “Luis Guillermo Ibarra Ibarra” (Ref. number 42/13).
2.2. Informed consent
Informed consent was obtained from all the individual participants included in the study. All participants were formally informed about the synovial tissue sample study and they gave their written consent to participate.
2.3. Patients
Patients with an established diagnosis of KOA and grade 4 Kellgren–Lawrence severity scale[14,15] who were hospitalized 24 hours prior to knee replacement surgery. None of them received non-steroidal anti-inflammatory drugs during the week previous to the surgical procedure, to avoid modifying the inflammatory process of OA. Patients received paracetamol in the case of joint pain.
2.4. Ultrasonography
Knee ultrasonography (US) examination was performed in all patients and controls 24 hours before surgical procedure, by an ultrasonographer with 10 years of experience blinded to clinical characteristics, with a Siemens Acuson Antares device (IM-USX-USDX-02), equipped with a linear array transducer of 13 to 5 MHz. All images were saved and then interpreted by 2 ultrasonographers.
The anatomical areas scanned were knee recesses (suprapatellar, medial parapatellar and lateral). With the patient in supine position, the knee flexed at 30°, placing the probe longitudinally with its distal edge on the patella, in the insertion of the quadriceps tendon of the patella, to explore suprapatellar recess. The medial and lateral parapatellar recesses were evaluated with the knee in neutral position. The vertical edge along the medial and lateral margins of the knee cap (contracted femoral biceps) was identified by scanning.
Power Doppler (PD) settings were as follows: frequency 8.9 MHz, PRF 610 Hz and low wall filter. Gains were adjusted according to the scanned region to avoid signals below the bony cortex.
OMERACT definitions were used for synovitis (hypertrophy and synovial effusion).[16] Synovitis was recorded in a dichotomous manner, and PD signal was scored in a semi-quantitative way: grade 1, less than 3 signs or positive dots; grade 2, more than 3 dots in less than 50% of the area examined; and grade 3, PD signal more than 50% of the area of interest evaluated.[17]
2.5. Histological analysis
The synovial tissue samples obtained during the joint surgical procedure were fixed in buffered formalin 10%. After fixative procedure, the sample was removed and dehydrated with gradual series of aqueous solutions, from lower to higher dehydrating agent, starting with 50% alcohol and progressing until 100% was reached, in order to eliminate water. Once the tissue was dehydrated, it was transferred to a xylose solution so that fine cuts could be obtained to be evaluated under a microscope; then, the tissues were included and wrapped in molten paraffin at 60° C, placing the sample in a stove for 30 minutes at 6 hours maintaining the temperature at 60° C. This piece, and a little-molten paraffin, were placed in a mould of rectangular shape and it was allowed to solidify at room temperature, forming a solid block of paraffin with the piece of tissue included. The cuts were placed on slides to which a small amount of albumin was added, which acts as an adhesive. The paraffin was removed in an organic solvent, again they were included in xylol (Sigma-Aldrich, USA), and the sample was rehydrated by passing it through a series of decreasing graduations of ethyl alcohol (Sigma-Aldrich, USA) until it reached a 100% water solution. Once rehydrated, the tissue was stained with hematoxylin and eosin (Sigma-Aldrich, USA), and then dehydrated again, so that it could be fixed permanently with the cover for assembly. One experienced anatomo-pathologist, blinded to the clinical data of both groups, performed the tissue analysis.
2.6. Immunohistochemical analysis
The immuno-detection of blood vessels was performed by immunohistochemistry (IHC) of endothelial cells with anti-factor VIII monoclonal antibody (Dakopoliclonales, Dako, Santa Barbara, CA), using as standard an immunoperoxidase technique. Once stained, a selection of areas with greater neovascularization was made. Later, they were examined by light microscopy and classified subjectively on a scale of 1 to 4+ for individual microvessels, in fields of 100x and 200x.
2.7. Expression of prolidase by immunofluorescence
Immunohistochemistry stains were made on blank lamellae obtained from the histopathological study with polyclonal primary antibodies for the enzyme prolidase and prolylhydroxylase from Abcam (ab111851). The lamellae were fixed with absolute methanol at 4 ° C for 7 minutes, then washed with a phosphate buffer saline phosphate-buffered saline (Gibco, USA) at pH 7.2. Samples were permeabilized with a 0.2% Triton X-100 solution for 20 minutes. Synovial membrane staining was performed with a primary anti-mouse antibody prolidase (1: 100) or anti-prolylhydroxylase (1:100) Abcam (ab108980) to characterize the synovial membrane for 1 hour. Subsequently, a secondary anti-mouse antibody Alexa-488 (1: 400) Abcam (ab150113) was added for 45 minutes. The samples were then washed with Bovine serum albumin solution and mounted with 4′,6-diamidino-2-phenylindole °(Thermofisher, USA) or propidium iodide (Sigma-Aldrich, USA), to stain in red the nuclei. The microscopic observation of the morphology and the positive staining of prolyl-hydroxylase in the samples helped us to discard sections of tissue that were not easily identifiable as a synovial membrane for the analysis of prolidase. Images were captured to 200x zoom in a cell Floid epifluorescence system (Thermofisher, USA) and analyzed with the imageJ software (http://imagej.nih.gov/ij/, 1997–2014), and the data was expressed as unit arbitrary fluorescence (UAF). The average fluorescence of 5 fields observed at 200x of patients and controls was analyzed.
2.8. Sample's size calculation
The G∗ Power 3.1.9.6 software was used for calculating the sample size in order to find the difference between groups by comparing 2 means with effect size d = 0.99, an α value of 0.05 and β: 80%, the number to recruit was 18 subjects per group. We included 20 per group (Power = 86.22%).
2.9. Statistical analysis
Measures of central tendency, dispersion measures and position measures were used in the case of quantitative variables, as well as the frequencies observed in the qualitative variables. In the inferential phase, the comparisons were made at 95% reliability, using the Student t test, or its non-parametric alternative, Mann–Whitney U test. To estimate the synovial tissue neovascularization with objectivity and transform it into a continuous quantitative variable, the logistic regression of Cox was used. Inter-reading reliability was calculated with Cohen's kappa for cualitative variables, according to Landis and Koch.[18] Statistical analyses were performed using SPSS 22 (SPSS INC, CHIGAGO IL).
3. Results
The aim of this study was to evaluate prolidase expression in knee joints from patients undergoing total knee arthroplasty for OA (see flow diagram in Fig. 1). A total of 20 patients and 20 controls were included. The demographic, anthropometric characteristics and comorbidities of participants are presented in Table 1.
Figure 1.
Study flow diagram.
Table 1.
Demographic characteristics of patients with KOA and controls.
| Patients | Healthy | |
| Age years M + SD | 70 ± 7.7 | 36 ± 9.8 |
| Gender male/female | 4/16 | 20/0 |
| Duration disease years M + SD | 11 ± 4.3 | NA |
| Weight kg M + SD | 77.5 ± 8.3 | 71 ± 7.6 |
| Height Kg M + SD | 1.6 ± 0.1 | 1.7 ± 0.7 |
| BMI M + SD | 31.6 ± 3.2 | 27 ± 2.6 |
| Comorbidities | NA | |
| High blood pressure n (%) | 8 (40) | |
| Diabetes mellitus n (%) | 3 (15) |
BMI = body mass index, M = media, NA = no applicable, SD = standard deviation.
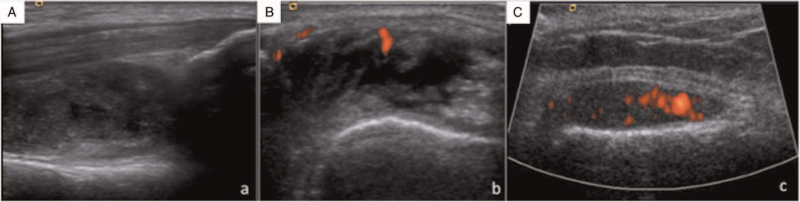
Related to US findings, we found synovitis in all knee recesses from the OA group in grayscale and in 17 (85%) knees PD signal was present (Fig. 2), while it was absent in controls (P = .05).
Figure 2.
Presence of synovitis in (A) suprapatellar recess, (B) medial parapatellar recess and (C) lateral parapatellar recess in grayscale and PD.
Table 2 shows the elementary lesions identified in the ultrasonic study, highlighting the changes of synovial hyperplasia and the synovial state documented in 100% of the cases in the 3 knee joints studied. On the other hand, the frequency of the Doppler signal in intermediate and lateral media stands out, while in the previous 1, the suprapatellar mean is exceeded with statistical significance (P < .05).
Table 2.
Frequecy of sinovial hyperplasy, and sinovial spill according to articular recess evaluated by ultrasound in patients with KOA (N = 20).
| Variable | SPR | MPR | LPR | Value of P∗ |
| Synovial Hypertrophy | 20 (100%) | 20 (100%) | 20 (100%) | N/A |
| Synovial Effusion | 20 (100%) | 20 (100%) | 20 (100%) | N/A |
| Present (1 a 3) | 1 (5%) | 9 (45%) | 7 (35%) | <.05 |
| Absent (0) | 19 (95%) | 11 (55%) | 65%) |
MR = medial parapatelar recess, PLR = lateral parapatellar recess, SPR = suprapatellar recess.
Calculated with Chi Squared test.
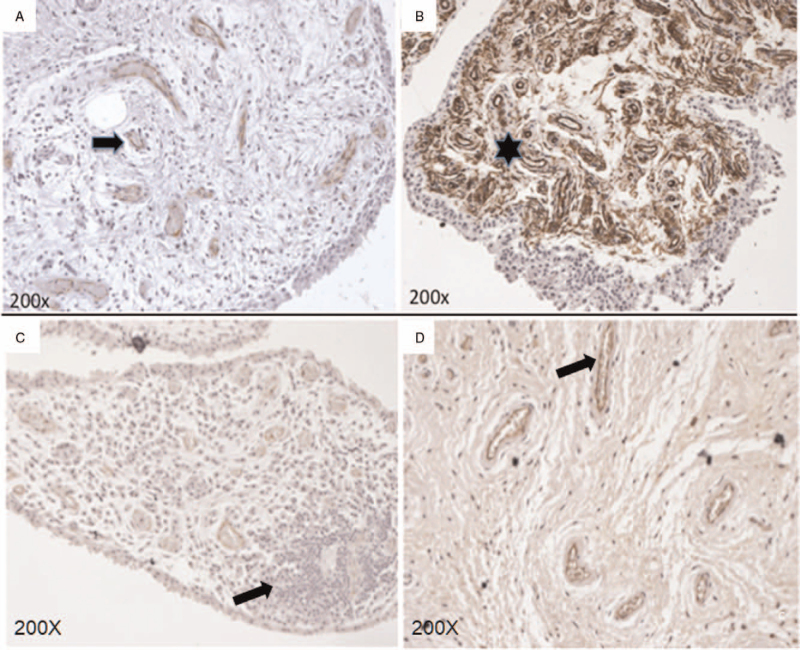
To corroborate the presence of neovascularization and structural alterations in the synovial membrane tissue, hyperplasia was analyzed by IHC (Fig. 3). It showed an increase of vascular pattern that was statistically significant predominantly in OA patients. The mean of the micro-vessel count in patients with OA was significantly higher versus controls (11 + 5.3 vs 4 + 2.1, P = .001). The neovascularization (showed in Fig. 3) correlated significantly with the presence of PD signal in patients with OA (1.16, 95% CI, 1.02–1.34, P = .02).
Figure 3.
Immunohistochemistry synovial membrane vascularization assessment (stained with factor VIII): (A) healthy control (B) OA patient; the arrow shows the blood vessel, and the asterisk the neovascularization zone. The synovial membrane tissue was stained with hematoxylin and eosin. The arrow shows focal infiltration of lymphocytes and monocytes is observed in (C). The arrow shows cells stained with factor VIII (dark color) indicate neovascularization, (D).
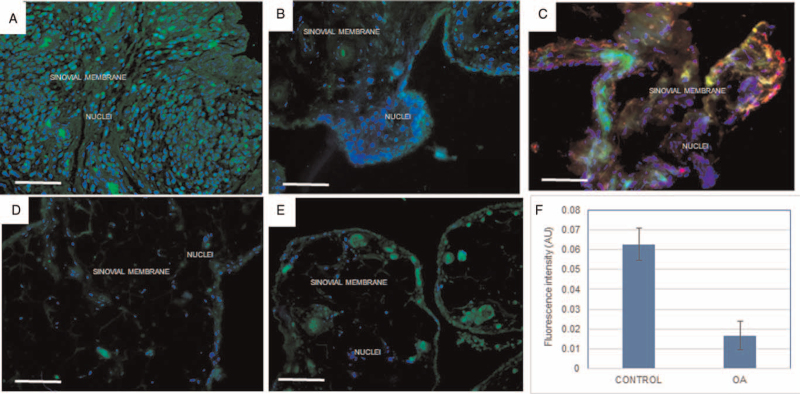
The prolidase enzyme was identified by immunofluorescence in frozen biopsies sections of the synovial membrane of patients with OA and controls (see methods). The analysis of representative synovial membrane images showed a lower presence of prolidase in patients when compared with the control group (0.017 ± 0.009 UAF vs 0.062 ± 0.04 UAF respectively P < .05) (Fig. 4).
Figure 4.
Identification and analysis of prolidase expression. Images (A) and (B) show the presence of prolidase in green and in the membrane of controls, (D) and (E) in patients with OA. (C) shows prolyl hydroxylase stained in the synovial membrane network of controls; nuclei is in blue, stained with DAPI. The analysis expression of prolidase according to fluorescence intensity (AU) is shown in (F). The asterisk indicates statistically significant differences (P < .05) (bar = 100 μm).
4. Discussion
In this study we first reported the analysis of prolidase expression specifically in the area of the synovial membrane by immunofluorescence, these same areas were used for their quantitative analysis and for their comparison with controls. This is important because it allows us to know if it is possible to specifically detect the protein in the synovial membrane and not in the areas of the tissue outside the membrane, due to the variations in the size and integrity of the biopsy. The presence of the enzyme in the membrane with synovitis is important for cellular metabolism and indicates the capacity of tissue response to activate the pathway of collagen recycling and tissue regeneration. Its decrease therefore indicates the gradual loss of that capacity. Our findings indicated lower expression of the protein in patients with respect to controls, when observing the individual data of expression of prolidase of patients and controls, it was evident that no patient with KOA expressed more prolidase than the person with the lowest expression of the controls. This may suggest a difference in the expression of prolidase more due to the advancement of KOA than due to other types of damage to the joint. These results are similar to various reports on the decrease of prolidase activity in serum of patients with OA, and specifically with KOA.[4]
The integrity of the articular cartilage is maintained under moderate load conditions in normal activities, however obesity is a factor that increases the load on the joint with the possibility of causing structural damage, synovitis and alterations of the collagen network.[19–21] Most of our patients had synovitis which was related to the severe degree of KOA, additionally 25% of them were overweight and 75% of them were obese (see supplemental Figure S1). Despite not having measured any biomechanical factor in them, we cannot rule out their participation in the loss of prolidase, it would be important to evaluate the changes in the expression of the enzyme as a possible chronic cellular response to KOA over time.
The low expression does not seem to be related to age, as reported in a previous study where it was shown that, regardless of age (younger vs older than 60 years), the activity of the enzyme was lower than in controls.[4,22] In our patients, although there was a tendency to a lower expression of prolidase in patients older than 70 years, however, it did not reach statistical significance. The severity of the KOA may be associated to low prolidase expression rather than age. Akif Altay M, et al[4] found significant decreases in the levels of prolidase activity in the late stages of KOA, like our patients who had advanced KOA (Kellgren–Lawrence grade 4). The cause of this decreased expression with advancing OA stage is unclear. Nevertheless, in grades 3 and 4 of OA, most of the cartilage has already degenerated.[1] Another possible explanation for decreased prolidase activity in KOA patients has been related to the low physical activity levels of the individuals imposed by the structural damage and impaired joint function, because collagen turnover seems to be positively correlated with the degree of exercise.[23,24] The findings found in our patients with synovitis in late stages of OA only suggest that there could be differences in the presence of prolidase in early stages of KOA, but the presence of synovitis in them should be measured and analyzed.
The importance of the low prolidase expression in synovial membrane is that it could be a good evidence of the decreased collagen resynthesis. Some studies have reported that ROS-generating systems degrade collagen and prevent formation of fibrils by this collagen, suggesting a significant relationship between prolidase metabolism and oxidative stress in the ethiopathogenesis or in the OA progression.[4,7,25] In this study, significant differences were found in the presence of the enzyme, in spite of the difficulty in obtaining frozen sections of the synovium due to its composition and fragility, which decreased significantly in the patients with OA. We do not know if this finding could be due to a decrease in the gene expression or to tissue deterioration in the damaged area by the OA stage of the patients. The observation of the expression of prolidase in the human synovial membrane by immunofluorescence has not been reported.[7] Additionally, it was able to be correlated with the damage or loss of the synovial membrane during the progression of OA. The analysis of prolidase by techniques such as western blot or histochemistry and immunohistochemistry can be used to know the amount of total protein in the tissue of the damaged area and its possible variations. The measurement of enzymatic activity in serum will allow us to know if the decrease in the expression of the protein in the tissue in an early event is a consequence of the inflammatory alteration or by the damage of the synovial membrane.[25,26,27] This information would serve to assess the damage caused by the progression of KOA.
4.1. Synovitis
US constitutes an attractive alternative for visualizing synovial tissue inflammation.[28,29] Like our results, others have shown that synovial inflammation was more prevalent and severe in end-stage knee OA.[30] Synovial angiogenesis in OA is a key component of chronic inflammation.[31] Several factors such as vascular endothelial growth factor and its inducer hypoxia-inducible factor-1a may contribute to angiogenesis in the inflamed synovium.[31,32] In this study, we observed that the presence of a PD signal is significantly correlated with synovial angiogenesis, as observed by Walsh DA and col. who described that angiogenesis indices increased with increasing histological synovitis.[33,34] These findings highlight the importance of ultrasound for the detection of active synovitis, which is correlated with low levels of prolidase.
4.2. Limitations
A limitation of our study is that the expression of prolidase in synovial membrane was not evaluated through genomic tests, which would have allowed the demonstration that the absence or not of the enzyme gene in our patients with advanced KOA is a pathogenic factor. Another limitation is the lack of tissue of another control group from patients with non-advanced degree of OA, in order to demonstrate that low levels of prolidase are related to severity rather than to the age of the patients. The difference of ages between groups represent a bias because the controls have greater expression of the enzyme. It would be ideal to compare to a control group of similar age, however, we know that it is difficult to find controls of this age without changes of OA.
4.3. Strengths of the study
It is the first study that analyzes the expression of prolidase in the synovial membrane at specific sites and this expression is associated with the presence of synovitis, (detected by 2 different techniques: US and IHC).
5. Conclusions
The prolidase expression in synovial membrane evaluated by immunofluorescence in patients with later stages of KOA is low, which may be interpreted as an evidence of decreased collagen resynthesis during joint inflammation. US and IHC are 2 different techniques to evaluate the presence of sinovitis in patients with later stages of KOA, allowing it to be differentiated from people with non-systemic knee injuries without KOA
Author contributions
Conceptualization: Karina Silva Luna.
Data curation: Karina Silva Luna, Ambar López Macay.
Formal analysis: Lucio Ventura Ríos.
Investigation: Karina Silva Luna, Lucio Ventura Ríos, Ambar López Macay.
Methodology: Karina Silva Luna, Lucio Ventura Ríos, Ambar López Macay.
Project administration: Karina Silva Luna, Lucio Ventura Ríos.
Resources: Karina Silva Luna.
Software: Ambar López Macay.
Supervision: Lucio Ventura Ríos, Ambar López Macay.
Validation: Karina Silva Luna, Ambar López Macay.
Writing – original draft: Karina Silva Luna, Lucio Ventura Ríos, Ambar López Macay.
Writing – review & editing: Ambar López Macay.
Supplementary Material
Footnotes
Abbreviations: KOA = knee osteoarthritis, OA = osteoarthritis, PD = Power Doppler, ROS = reactive oxygen species, UAF = unit arbitrary fluorescence, US = ultrasonography.
How to cite this article: Silva-Luna K, Ventura-Ríos L, López-Macay A. Prolidase expression in knee osteoarthritis and healthy controls: observational study. Medicine. 2021;100:35(e27059).
This work was supported by CONACYT Clave: Salud 2013-01-202779, Instituto Nacional de Rehabilitación “Luis Guillermo Ibarra Ibarra” (INRLGII).
The authors have no conflicts of interests to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Supplemental digital content is available for this article.
References
- [1].Giwnewer U, Rubin G1, Orbach H, Rozen N. Treatment for osteoarthritis of the knee. Harefuah 2016;155:403–6. [PubMed] [Google Scholar]
- [2].Manlapaz DG, Sole G, Jayakaran P, Chapple CM. Risk factors for falls in adults with knee osteoarthritis: a systematic review. PM R 2019;4: doi: 10.1002/pmrj.12066. [DOI] [PubMed] [Google Scholar]
- [3].Damen J, van Rijn RM, Oei EH, et al. Prevalence and development of hip and knee osteoarthritis according to American College of Rheumatology criteria in the CHECK cohort. Arthritis Res Ther 2019;21:04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Altay MA, Ert ÜrK C, Aksoy N. Evaluation of prolidase activity and oxidative status in patients with knee osteoarthritis: relationships with radiographic severity and clinical parameters. Rheumatol Int 2015;35:1725–31. [DOI] [PubMed] [Google Scholar]
- [5].Franz A, Joseph L, Mayer C, et al. The role of oxidative and nitrosative stress in the pathology of osteoarthritis: novel candidate biomarkers for quantification of degenerative changes in the knee joint. Orthop Rev (Pavia) 2018;10:7460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Brandl A, Hartmann A, Nerlich M, Graf B, Nerlich M, Angele P. Oxidative stress induces senescence in chondrocytes. J Orthop Res 2011;29:1114–20. [DOI] [PubMed] [Google Scholar]
- [7].Iwasa K, Hayashi S, Fujishiro T, et al. PTEN regulates matrix synthesis in adult human chondrocytes under oxidative stress. J Orthop Res 2014;32:231–7. [DOI] [PubMed] [Google Scholar]
- [8].Sophia Fox AJ, Bedi A, Rodeo SA. The basic science of articular cartilage: structure, composition, and function. Sports Health 2009;1:461–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zareba I, Palka J. Prolidase-proline dehydrogenase/proline oxidase-collagen biosynthesis axis as a potential interface of apoptosis/autophagy. Biofactors 2016;42:341–8. [DOI] [PubMed] [Google Scholar]
- [10].Szoka L, Karna E, Morka RP, Palka JA. Enalapril stimulates collagen biosynthesis through prolidase-dependent mechanism in cultured fibroblasts. Naunyn Schmiedebergs Arch Pharmacol 2015;388:677–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Namiduru ES. Prolidase. Bratisl Lek Listy 2016;117:480–5. [DOI] [PubMed] [Google Scholar]
- [12].Karna E, Szoka L, Huynh TY, Palka J. Proline-dependent regulation of collagen metabolism. Cell Mol Life Sci 2020;77:1911–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Skou ST, Koes BW, Grønne DT, Young J, Roos EM. Comparison of three sets of clinical classification criteria for knee osteoarthritis: a cross-sectional study of 13,459 patients treated in primary care. Osteoarthritis Cartilage 2020;28:167–72. [DOI] [PubMed] [Google Scholar]
- [14].Kellgren JH, Lawrence JS. Radiological assessment of osteoarthrosis. Ann Rheum Dis 1957;16:494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Braunschweig R, Spahn G, Regier M, Weber M-A. Osteoarthritis classifications: modification proposal for the criteria of Kellgren and Vallotton Article in German. Radiologe 2019;59:1010–8. [DOI] [PubMed] [Google Scholar]
- [16].Bruyn GA, Iagnocco A, Naredo E, et al. OMERACT definitions for ultrasonographic pathologies and elementary lesions of rheumatic disorders 15 years on. J Rheumatol 2019;46:1388–93. [DOI] [PubMed] [Google Scholar]
- [17].Takase K, Ohno S, Ihata A, et al. Simultaneous evaluation of long-lasting knee synovitis in patients undergoing arthroplasty by power Doppler ultrasonography and contrast-enhanced MRI in comparison with histopathology. Clin Exp Rheumatol 2012;30:85–92. [PubMed] [Google Scholar]
- [18].Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159–74. [PubMed] [Google Scholar]
- [19].Jørgensen AEM, Kjær M, Heinemeier KM. The effect of aging and mechanical loading on the metabolism of articular cartilage. J Rheumatol 2017;44:410–7. [DOI] [PubMed] [Google Scholar]
- [20].Kaplan JT, Neu CP, Drissi H, Emery NC, Pierce DM. Cyclic loading of human articular cartilage: the transition from compaction to fatigue. J Mech Behav Biomed Mater 2017;65:734–42. [DOI] [PubMed] [Google Scholar]
- [21].Schröder A, Nazet U, Muschter D, Grässel S, Proff P, Kirschneck C. Impact of mechanical load on the expression profile of synovial fibroblasts from patients with and without osteoarthritis. Int J Mol Sci 2019;20:585.doi: 10.3390/ijms20030585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hunter DJ, Zhang Y, Niu J, et al. Structural factors associated with malalignment in knee osteoarthritis: the Boston osteoarthritis knee study. J Rheumatol 2005;32:2192–9. [PubMed] [Google Scholar]
- [23].Azukizawa M, Ito H, Hamamoto Y, et al. The effects of well-rounded exercise program on systemic biomarkers related to cartilage metabolism. Cartilage 2019;10:451–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sezen Y, Bas M, Aksoy N, et al. Serum prolidase activity in idiopathic and ischemic cardiomyopathy patients. J Clin Lab Anal 2010;24:213–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Altindag O, Erel O, Aksoy N, Selek S, Celik H, Karaoglanoglu M. Increased oxidative stress and its relation with collagen metabolism in knee osteoarthritis. Rheumatol Int 2007;27:339–44. [DOI] [PubMed] [Google Scholar]
- [26].Griswold AJ, Nuytemans K, Kaplan LD, et al. Transcriptomic analysis of synovial extracellular RNA following knee ttrauma: a pilot study. J Orthop Res 2018;36:1659–65. [DOI] [PubMed] [Google Scholar]
- [27].Altay MA, Ertürk C, Aksoy N, et al. Evaluation of prolidase activity and oxidative status in patients with knee osteoarthritis: relationships with radiographic severity and clinical parameters. Rheumatol Int 2015;35:1725–31. [DOI] [PubMed] [Google Scholar]
- [28].Vreju Fl, Ciurea M, Roşu A, Muşetescu A, Grecu D, Ciurea P. Power Doppler sonography, a non-invasive method of assessment of the synovial inflammation in patients with early rheumatoid arthritis. Rom J Morphol Embryol 2011;52:637–43. [PubMed] [Google Scholar]
- [29].Karim Z, Wakefield RJ, Veale DJ, et al. Validation and reproducibility of ultrasonography in the detection of synovitis in the knee: a comparison with arthroscopy and clinical examination. Arthritis Rheum 2004;50:387–94. [DOI] [PubMed] [Google Scholar]
- [30].de Lange-Brokaar BJE, Ioan-Facsinay A, Andersen SN, et al. Degree of synovitis on MRI by comprehensive whole knee semi-quantitative scoring method correlates with histologic and macroscopic features of synovial tissue inflammation in knee osteoarthritis. Osteoarthritis Cartilage 2014;22:1606–13. [DOI] [PubMed] [Google Scholar]
- [31].Mapp PI, Walsh DA. Mechanisms and targets of angiogenesis and nerve growth in osteoarthritis. Nat Rev Rheumatol 2012;8:390–8. [DOI] [PubMed] [Google Scholar]
- [32].Henrotin Y, Pesesse L, Lambert C. Targeting the synovial angiogenesis as a novel treatment approach to osteoarthritis. Ther Adv Musculoskelet Dis 2014;6:20–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Walsh DA, Bonnet CS, McWilliams DF, et al. Angiogenesis in the synovium and at the osteochondral junction in osteoarthritis. Osteoarthritis Cartilage 2007;15:743–51. [DOI] [PubMed] [Google Scholar]
- [34].Gok M, Erdem H, Gogus F, et al. Relationship of ultrasonographic findings with synovial angiogenesis modulators in different forms of knee arthritides. Rheumatol Int 2013;33:879–85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.