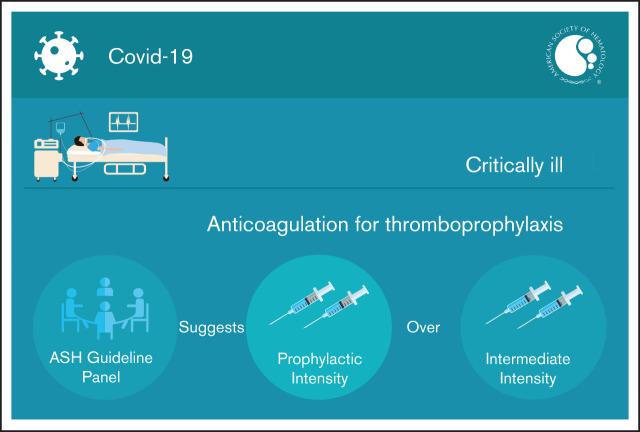
Visual Abstract
Abstract
Background: COVID-19–related critical illness is associated with an increased risk of venous thromboembolism (VTE).
Objective: These evidence-based guidelines of the American Society of Hematology (ASH) are intended to support patients, clinicians, and other health care professionals in making decisions about the use of anticoagulation for thromboprophylaxis in patients with COVID-19–related critical illness who do not have confirmed or suspected VTE.
Methods: ASH formed a multidisciplinary guideline panel that included 3 patient representatives and applied strategies to minimize potential bias from conflicts of interest. The McMaster University Grading of Recommendations Assessment, Development and Evaluation (GRADE) Centre supported the guideline development process by performing systematic evidence reviews (up to 5 March 2021). The panel prioritized clinical questions and outcomes according to their importance for clinicians and patients. The panel used the GRADE approach to assess evidence and make recommendations, which were subject to public comment. This is an update on guidelines published in February 2021.
Results: The panel agreed on 1 additional recommendation. The panel issued a conditional recommendation in favor of prophylactic-intensity over intermediate-intensity anticoagulation in patients with COVID-19–related critical illness who do not have confirmed or suspected VTE.
Conclusions: This recommendation was based on low certainty in the evidence, which underscores the need for additional high-quality, randomized, controlled trials comparing different intensities of anticoagulation in critically ill patients. Other key research priorities include better evidence regarding predictors of thrombosis and bleeding risk in critically ill patients with COVID-19 and the impact of nonanticoagulant therapies (eg, antiviral agents, corticosteroids) on thrombotic risk.
Summary of recommendations
Recommendation 1a
The American Society of Hematology (ASH) guideline panel suggests using prophylactic-intensity over intermediate-intensity anticoagulation in patients with COVID-19–related critical illness who do not have suspected or confirmed venous thromboembolism (VTE) (conditional recommendation based on low certainty in the evidence about effects ⨁⨁◯◯).
Remarks:
The ASH guideline panel plans to continue to update this recommendation when the full results of other trials become available. Clinicians should weigh the benefits and harms based on the most up-to-date evidence in caring for their patients.
A now-expired recommendation published on 27 October 2020 compared therapeutic-intensity or intermediate-intensity with prophylactic-intensity anticoagulation in patients with COVID-19–related critical illness. With the emergence of new evidence, this recommendation has now been split into 2 recommendations: a recommendation comparing intermediate-intensity with prophylactic-intensity anticoagulation (Recommendation 1a) and a separate recommendation comparing therapeutic-intensity with prophylactic-intensity anticoagulation (Recommendation 1b), whereby the latter remains unchanged for now, but, as with other recommendations in this guideline, is subject to review and revision as new evidence becomes available that meets prespecified criteria for updating.
Patients with COVID-19–related critical illness are defined as those suffering from an immediately life-threatening condition who would typically be admitted to an intensive care unit (ICU). Examples include patients requiring hemodynamic support, ventilatory support, and renal replacement therapy.
An individualized assessment of the patient’s risk of thrombosis and bleeding is important when deciding on anticoagulation intensity. Risk assessment models to estimate thrombotic and bleeding risk in hospitalized patients are available, but they have not been prospectively validated in patients with COVID-19.
At present, there is no direct high-certainty evidence comparing different types of anticoagulants. The selection of a specific agent (eg, low molecular weight heparin [LMWH], unfractionated heparin [UFH]) may be based on availability, resources required, familiarity, and the aim of minimizing the use of personal protective equipment or exposure of staff to COVID-19–infected patients as well as patient-specific factors (eg, renal function, history of heparin-induced thrombocytopenia, concerns about gastrointestinal tract absorption).
This recommendation does not apply to patients who require anticoagulation to prevent thrombosis of extracorporeal circuits such as those on extracorporeal membrane oxygenation or continuous renal replacement therapy.
Background
There is a high incidence of thrombotic complications in critically ill patients with COVID-19.1 VTE is the most common thrombotic complication and has been reported in up to 23% of critically ill patients with COVID-19.2 Consequently, there has been intense clinical and research interest in establishing whether intensified thromboprophylaxis regimens are needed in this population.3 However, critically ill patients may be at increased risk for bleeding complications, which may also occur in patients with COVID-19–related critical illness.4-8 The optimal strategy for thromboprophylaxis that balances these thrombosis and bleeding risks remains uncertain.
These guidelines are based on updated and original systematic reviews of evidence conducted under the direction of the McMaster University Grading of Recommendations Assessment, Development and Evaluation (GRADE) Centre with international collaborators. This is an update of the previous ASH guideline published in February 2021,9 and it focuses on intermediate-intensity vs prophylactic-intensity anticoagulation. The panel followed best practices for guideline development recommended by the Institute of Medicine and the Guidelines International Network.10-12 The panel used the GRADE approach13-19 to assess the certainty of the evidence and formulate recommendations. The recommendation is listed in Table 1.
Table 1.
Recommendations
| Recommendation | Remarks |
|---|---|
| Recommendation 1a. The ASH guideline panel suggests using prophylactic-intensity over intermediate-intensity anticoagulation in patients with COVID-19–related critical illness who do not have suspected or confirmed VTE (conditional recommendation based on low certainty in the evidence about effects ⨁⨁◯◯). | • The ASH guideline panel plans to continue to update this recommendation when the full results of other trials become available. Clinicians should weigh the potential benefits and harms based on the most up-to-date evidence in caring for their patients. • A now-expired recommendation published on 27 October 2020 compared therapeutic-intensity or intermediate-intensity with prophylactic-intensity anticoagulation in patients with COVID-19–related critical illness. With the emergence of new evidence, this recommendation has now been split into 2 recommendations: a recommendation comparing intermediate-intensity vs prophylactic-intensity anticoagulation (Recommendation 1a) and a separate recommendation comparing therapeutic-intensity vs prophylactic-intensity anticoagulation (Recommendation 1b), whereby the latter remains unchanged for now, but, as with other recommendations in this guideline, is subject to review and revision as new evidence becomes available that meets prespecified criteria for updating. • Patients with COVID-19–related critical illness are defined as those suffering from an immediately life-threatening condition who would typically be admitted to an ICU. Examples include patients requiring hemodynamic support, ventilator support, and renal replacement therapy. • An individualized assessment of the patient’s risk of thrombosis and bleeding is important when deciding on anticoagulation intensity. Risk assessment models to estimate thrombotic and bleeding risk in hospitalized patients are available, but they have not been prospectively validated in patients with COVID-19. • At present, there is no direct high-certainty evidence comparing different types of anticoagulants. The selection of a specific agent (eg, LMWH, UFH) may be based on availability, resources required, familiarity, and the aim of minimizing the use of personal protective equipment or staff exposure to COVID-19–infected patients as well as patient-specific factors (eg, renal function, history of heparin-induced thrombocytopenia, concerns about gastrointestinal tract absorption). • This recommendation does not apply to patients who require anticoagulation to prevent thrombosis of extracorporeal circuits such as those on extracorporeal membrane oxygenation or continuous renal replacement therapy. |
Values and preferences
The guideline panel identified all-cause mortality, pulmonary embolism (PE), deep vein thrombosis (DVT), and major bleeding as critical outcomes and placed a high value on avoiding these outcomes with the interventions assessed.
Panel members noted that there was possible uncertainty and variability in the relative value that patients place on avoiding major bleeding events compared with reducing thrombotic events.
Explanations and other considerations
Please refer to the original ASH guideline on thromboprophylaxis in patients with COVID-19.9
Interpretation of strong and conditional recommendations
Please refer to the original ASH guideline on thromboprophylaxis in patients with COVID-19.9
Introduction
Aims of these guidelines and specific objectives
Please refer to the original ASH guideline on thromboprophylaxis in patients with COVID-19.9
Description of the health problem
The COVID-19 pandemic has had a significant public health impact. As of 2 May 2021, more than 152 million cases and 3.2 million deaths had been attributed to COVID-19–related illness globally.20 It is estimated that 5% to 20% of infected patients require hospital admission, of whom 5% to 15% may develop critical illness requiring intensive care support.21-23
Patients with critical illness resulting from COVID-19 may develop a severe inflammatory response and endothelial dysfunction, which may result in platelet activation, activation of the coagulation cascade, and a hypercoagulable state.24 Several laboratory predictors of thrombosis in hospitalized patients with COVID-19 have been reported, including elevated D-dimer, C-reactive protein, and erythrocyte sedimentation rate.5,25 VTE has emerged as an important complication in critically ill patients with COVID-19, occurring in up to 23% of such patients.2 This was observed in the very early days of the pandemic, but it remains an important issue, even with the introduction of improved treatments in subsequent waves of the pandemic.26,27 In addition, arterial thrombotic complications including stroke have been noted.28,29 Microvascular thrombosis, which may involve the pulmonary vasculature and other organs, has been reported in autopsy studies, although its impact on the development of respiratory and multiorgan failure remains unclear.30,31 Patients who are critically ill may also be at increased bleeding risk, which may be a result of platelet dysfunction, thrombocytopenia, organ dysfunction, or consumptive coagulopathy.4,5
The optimal thromboprophylaxis strategy that balances thrombotic and bleeding risk in critically ill patients with COVID-19 remains uncertain.32-35 This uncertainty has led to variability in clinical practice regarding empiric thromboprophylaxis regimens, and several randomized trials are in progress.36,37 Although COVID-19–associated coagulopathy seems to be marked primarily by thrombotic complications, patients may develop major bleeding complications when receiving anticoagulation therapy, which can have an impact on the safety of intensified thromboprophylaxis regimens.5,8,38 In this living guideline update, the role of intermediate- vs prophylactic-intensity anticoagulation in critically ill patients with COVID-19 is addressed.
Description of the target populations
The target population, patients with COVID-19–related critical illness, is described in Table 2.
Table 2.
Definition of target population
| Target population | Definition |
|---|---|
| Critically ill | Patients with COVID-19 who develop respiratory or cardiovascular failure normally requiring advanced clinical support in the ICU or critical care unit (CCU) but could include admission to another department if the ICU/CCU was over capacity. ICU/CCU capacity and admission criteria could vary according to the specific setting. |
Methods
This updated guideline recommendation on the use of intermediate-intensity anticoagulation in critically ill patients was developed in the living phase of the ASH 2021 living guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID-19. The ASH guideline panel generated Recommendation 1a on 30 March 2021 before asking for public comments.
We followed the same methods as published in the initial guideline,9 with the following important updates and differences for the recommendation reported here:
Organization, panel composition, planning, and coordination: With one exception, we retained the same panel members because no conflicts of interest emerged that would require exclusion of panel members.
Guideline funding and management of conflicts of interest: Supplement 4 provides updated “Participant Information Forms” for all panel members, detailing financial and nonfinancial interests, as well as the ASH conflict-of-interest policies agreed to by each individual. Supplement 5 provides the updated complete Participant Information Forms for researchers on the Systematic Review Team who contributed to these guidelines.
Formulating specific clinical questions and determining outcomes of interest: This updated manuscript focuses on 1 question: In patients with COVID-19–related critical illness who do not have confirmed or suspected VTE, should we use direct oral anticoagulants, LMWH, UFH, fondaparinux, argatroban, or bivalirudin at intermediate intensity or prophylactic intensity? There were no changes in the definitions for population (Table 2), anticoagulation intensity, or outcomes.9
Evidence review and development of recommendations: A new evidence-to-decision (EtD) framework was created for Recommendation 1a (see Recommendations) that uses any applicable evidence and information from the EtD framework for the initial Recommendation 1, and it will be updated with new evidence and considerations specifically for Recommendation 1a. The systematic review to identify comparative antithrombotic studies for the entire guideline was updated until 5 March 2021, the literature search strategy (Supplement 6) was modified only to add search terms for antiplatelet agents for another guideline question, and the protocol (Supplement 9) was modified to focus on inclusion of only randomized controlled trials for the guideline after the initial phase. Baseline risk estimates for outcomes in patients with COVID-19–related critical illness were not updated. The decision to create this updated guideline recommendation was based on publication of the INSPIRATION trial,39 which was not yet included in the systematic literature searches but was identified by expert panel members, critically assessed by the evidence synthesis team, and determined to increase the certainty of the evidence for several critical outcomes.
Document review: The draft recommendation was reviewed by all members of the panel, revised, and then made available online from 21 to 28 April 2021 for external review by stakeholders, including allied organizations, other medical professionals, patients, and the public. Two individuals or organizations submitted responses that did not require changes to the document. On 1 June 2021, the ASH Guideline Oversight Subcommittee and the ASH Committee on Quality verified that the defined guideline development process was followed, and on 3 June 2021, the officers of the ASH Executive Committee approved submission of the updated guideline manuscript for publication under the imprimatur of ASH. The updated guideline manuscript was then subjected to peer review by Blood Advances.
How to use these guidelines: We refer readers to the description in the initial guideline publication from February 2021,9 as well as the user guide for the ASH clinical practice guidelines.40
Recommendations
Recommendation 1a
Should direct oral anticoagulants, LMWH, UFH, fondaparinux, argatroban, or bivalirudin at intermediate-intensity vs prophylactic-intensity be used for patients with COVID-19–related critical illness who do not have suspected or confirmed VTE?
Recommendation 1a
The ASH guideline panel suggests using prophylactic-intensity over intermediate-intensity anticoagulation in patients with COVID-19–related critical illness who do not have suspected or confirmed VTE (conditional recommendation based on low certainty in the evidence about effects ⨁⨁◯◯).
Remarks:
The ASH guideline panel plans to continue to update this recommendation when the full results of other trials become available. Clinicians should weigh the benefits and harms based on the most up-to-date evidence in caring for their patients.
A now-expired recommendation published on 27 October 2020 compared therapeutic-intensity or intermediate-intensity with prophylactic-intensity anticoagulation in patients with COVID-19–related critical illness. With the emergence of new evidence, this recommendation has now been split into 2 recommendations: a recommendation comparing intermediate-intensity with prophylactic-intensity anticoagulation (Recommendation 1a) and a separate recommendation comparing therapeutic-intensity with prophylactic-intensity anticoagulation (Recommendation 1b), whereby the latter remains unchanged for now but, as with other recommendations in this guideline, is subject to review and revision as new evidence becomes available that meets prespecified criteria for updating.
Patients with COVID-19–related critical illness are defined as those suffering from an immediately life-threatening condition who would typically be admitted to an intensive care unit. Examples include patients requiring hemodynamic support, ventilatory support, and renal replacement therapy.
An individualized assessment of the patient’s risk of thrombosis and bleeding is important when deciding on anticoagulation intensity. Risk assessment models to estimate thrombotic and bleeding risk in hospitalized patients are available, but they have not been prospectively validated in patients with COVID-19.
At present, there is no direct high-certainty evidence comparing different types of anticoagulants. The selection of a specific agent (eg, LMWH or UFH) may be based on availability, resources required, familiarity, and the aim of minimizing the use of personal protective equipment or staff exposure to COVID-19–infected patients as well as patient-specific factors (eg, renal function, history of heparin-induced thrombocytopenia, concerns about gastrointestinal tract absorption).
This recommendation does not apply to patients who require anticoagulation to prevent thrombosis of extracorporeal circuits such as those on extracorporeal membrane oxygenation or continuous renal replacement therapy.
Summary of the evidence.
We rated the certainty in the evidence as moderate for the outcomes of ventilator-free days and length of ICU stay owing to serious imprecision, and as low for all other outcomes owing to very serious imprecision (see evidence profile and EtD online at https://guidelines.ash.gradepro.org/profile/iqf3QTwLZt0). We found no systematic reviews that addressed this question. There was 1 randomized controlled trial that provided evidence related to this question.39 Supplement 10 presents the characteristics of the included study.
One randomized controlled trial reported the effect of intermediate-intensity anticoagulation on all-cause mortality, PE, DVT, VTE, major bleeding, renal replacement therapy, ischemic stroke, intracranial hemorrhage, ventilator-free days, length of ICU stay, and myocardial infarction.39 No studies reported the effect of intermediate-intensity anticoagulation on multiorgan failure or limb amputation.
Benefits.
Intermediate-intensity anticoagulation may reduce the risk of PE but the evidence is uncertain (odds ratio [OR], 0.41; 95% confidence interval [CI], 0.08-2.13); this corresponds to 55 fewer (from 89 fewer to 90 more) PEs per 1000 patients; low certainty.39 Taking PE and DVT together, intermediate-intensity anticoagulation may reduce the risk of VTE, but the evidence is uncertain (OR, 0.93; 95% CI, 0.37-2.32); this corresponds to 8 fewer (from 78 fewer to 127 more) VTE events per 1000 patients; low certainty. Intermediate-intensity anticoagulation may reduce the length of ICU stay, but the evidence is uncertain (mean difference, 1 day fewer; 95% CI, 4 days fewer to 3 days more); moderate certainty. Intermediate-intensity anticoagulation had no effect on ventilator-free days, but the evidence is uncertain (mean difference, 0 days; 95% CI, 0-0 days); moderate certainty.
Harms and burden.
Intermediate-intensity anticoagulation may increase the risk of all-cause mortality, but the evidence is uncertain (OR, 1.09; 95% CI, 0.78-1.53); this corresponds to 16 more (from 42 fewer to 85 more) deaths per 1000 patients; low certainty.39 Intermediate-intensity anticoagulation may increase the risk of DVT, but the evidence is uncertain (OR, 1.46; 95% CI, 0.46-4.66); this corresponds to 42 more (from 54 fewer to 250 more) DVTs per 1000 patients; low certainty. Intermediate-intensity anticoagulation may increase the risk of major bleeding, but the evidence is uncertain (OR, 1.83; 95% CI, 0.53-5.93); this corresponds to 60 more (from 38 fewer to 268 more) major bleeding events per 1000 patients; low certainty. Intermediate-intensity anticoagulation may increase the use of renal replacement therapy, but the evidence is uncertain (OR, 1.49; 95% CI, 0.58-3.86); this corresponds to 125 more (from 48 fewer to 230 more) uses of renal replacement therapy per 1000 patients; low certainty. The effect of intermediate-intensity anticoagulation on the outcomes of ischemic stroke, intracranial hemorrhage, and myocardial infarction was very uncertain because only 1 ischemic stroke, 1 intracranial hemorrhage, and no myocardial infarctions occurred in the trial.
Other EtD criteria and considerations.
The guideline panel noted that there was possible uncertainty and variability in the relative value patients place on reducing thrombotic events compared with avoiding major bleeding events. The panel agreed that the use of intermediate-intensity anticoagulation would be acceptable to patients and health care providers. However, given the low certainty in the evidence, there may be regional variation in the acceptability of intermediate-intensity anticoagulation, particularly in regions where baseline VTE risk may be lower (eg, Asian populations).41,42
The panel recognized that COVID-19 disproportionately affects certain racial and ethnic groups, including Black and Hispanic individuals. However, the use of intermediate-intensity anticoagulation was judged to probably not have a differential impact on health equity relative to the use of prophylactic-intensity anticoagulation. Although intermediate-intensity anticoagulation would result in a higher cost for drugs, the panel judged this difference to be negligible relative to the total costs of providing critical care.
Conclusions.
The panel judged that there was low certainty evidence in the desirable and undesirable effects of intermediate-intensity anticoagulation in patients with COVID-19–related critical illness. There was a suggestion of a reduction in PE with intermediate-intensity anticoagulation, but the opposite was observed for DVT, and the evidence for both outcomes was of low certainty.
Meanwhile, there was less uncertainty in the potential undesirable effects of intermediate-intensity anticoagulation with respect to increased risk of major bleeding complications. The panel considered that there was higher-quality indirect evidence from critically ill patients who did not have COVID-19 for a dose-dependent increase in the risk of major bleeding with anticoagulation, although the magnitude of this effect was of low certainty in the population who did have COVID-19.8,43-46 Given that there was low certainty for potential benefit to offset the moderate risk of major bleeding complications, the usual practice of prophylactic-intensity anticoagulation, as used in critically ill patients who did not have COVID-19, was suggested.47
However, the panel noted that an individualized decision is important for each patient based on an assessment of thrombosis and bleeding risk. Dose adjustment of prophylactic-intensity anticoagulation for extremes of body weight or renal impairment may also be considered.48-52
This recommendation does not apply to thrombotic complications related to extracorporeal circuits. Although high rates of circuit-related thrombosis during extracorporeal membrane oxygenation and continuous renal replacement therapy have been reported in patients with COVID-19, this outcome was not prioritized by the guideline panel as part of its systematic review of the evidence.53
What are others saying and what is new in these guidelines?
There are multiple other guidance documents on the use of anticoagulation in patients with COVID-19. These include the 2020 CHEST COVID-19 Guidelines, the Anticoagulation (AC) Forum interim clinical guidance, the International Society on Thrombosis and Haemostasis (ISTH) Scientific and Standardization Committee (SSC) COVID-19 clinical guidance, the National Institutes of Health (NIH) COVID-19 treatment guidelines, and the American College of Cardiology (ACC) clinical guidance.54-58
Major differences between the current ASH guidelines and these other documents include use of high-quality systematic reviews and EtD frameworks, which increase transparency, along with use of marker states to estimate the relative importance to patients as key outcomes of treatment. This ASH guideline is also unique in its “living” format, which enables the inclusion of the recently published INSPIRATION clinical trial39 to inform the current recommendation (most other guidance documents were published before this trial’s results were made available; they may be revised as new evidence becomes available).
Four of the 5 other guidance documents (NIH, ISTH, ACC, and CHEST) suggest that prophylactic-dose heparin (either UFH or LMWH) be used for thromboprophylaxis in critically ill patients with COVID-19 over higher-intensity anticoagulation. They acknowledge that although there is an increased risk of VTE in hospitalized patients with COVID-19, there is insufficient randomized data at this time to recommend increased-intensity anticoagulation. The ISTH guidance document suggests that intermediate-dose LMWH could be considered in high-risk patients, and that patients with obesity could be considered for a 50% empiric dose increase for thromboprophylaxis. The ISTH document also suggests that multimodal prophylaxis with mechanical methods should be considered. This is notable because current guidelines in critically ill patients who do not have COVID-19 suggest using pharmacologic prophylaxis alone over combined pharmacologic and mechanical prophylaxis.47,59
Meanwhile, the guidance document from the AC Forum suggests that in critically ill patients with COVID-19, intermediate or increased intensities of thromboprophylaxis could be used.55 The authors acknowledge that this was based on expert opinion, and this was published before the results of the INSPIRATION randomized trial were made available.39
At the time of this writing, there has been only 1 small published randomized trial in critically ill patients with COVID-19 that compares therapeutic-dose and prophylactic-dose anticoagulation.60 Although this trial demonstrated improvements in gas exchange (PaO2:FiO2 ratio) with therapeutic anticoagulation, definitive conclusions cannot be drawn because only 20 patients were enrolled. A second randomized trial, the ACTION trial, compared therapeutic-intensity vs prophylactic-intensity anticoagulation in hospitalized patients with COVID-19 and elevated D-dimer. Most of the patients did not have critical illness. Therapeutic-intensity anticoagulation did not improve clinical outcomes and was associated with increased bleeding.61 A multiplatform study (ACTIV-4/REMAP-CAP/ATTACC) in critically ill patients with COVID-19 comparing therapeutic- with prophylactic-intensity anticoagulation has also recently been conducted. This study was stopped early because therapeutic anticoagulation met the predefined criteria for futility in the primary outcome of organ support–free days.62 The preprint article for this study suggested that therapeutic anticoagulation did not improve hospital survival or days free of organ support, and a potential for harm could not be excluded. When the results of this study are published, the ASH guidelines will be updated accordingly.
Limitations of this guideline
The limitations of these guidelines are inherent in the low certainty of the evidence we identified for the research questions. There were 2 outcomes that were identified as critical for decision making by the guideline panel for which no direct evidence was available (multiple organ failure and limb amputation).
In addition, the use of treatments other than anticoagulants for management of COVID-19–related critical illness (eg, corticosteroids, anticytokine therapies, ventilatory support) as well as the emergence of different viral variants has changed over the course of the pandemic. These changes may impact the baseline risk of VTE. Evidence collected earlier in the pandemic and included in our systematic reviews may not fully reflect the baseline risk of VTE or the effect of different intensities of anticoagulation in the current state of the pandemic.
Revision or adaptation of the guideline
Plans for updating these guidelines
The reported recommendation is the first living update of recommendations from the initial guideline publication that will be maintained by ASH through surveillance for new evidence, ongoing review by experts, and regular revisions. See the initial guideline publication for methods of living systematic reviews and recommendations, including considerations for deciding when to reassess and update recommendations.9
Updating or adapting recommendations locally
Adaptation of these guidelines will be necessary in many circumstances. These adaptations should be based on the associated EtD frameworks.17
Priorities for research
On the basis of gaps in evidence identified during the guideline development process, the panel identified the following urgent research priorities in this patient population:
Studies assessing baseline VTE risk in critically ill patients receiving prophylactic-intensity anticoagulation therapy
Randomized controlled trials comparing anticoagulation at differing intensities (prophylactic vs intermediate vs therapeutic)
Studies examining the impact of nonanticoagulant interventions (eg, anticomplement therapy, corticosteroids, antiviral therapies, anticytokine therapies, antiplatelet therapies, monoclonal antibody therapy, convalescent plasma) on thrombotic risk
Development or validation of risk assessment models for thrombosis and bleeding in patients with COVID-19–related critical illness
Studies examining the impact of anticoagulant therapy on thrombosis and bleeding outcomes in critically ill patients of differing race and ethnicity
Studies comparing mortality, thrombosis, bleeding, and functional outcomes with different available anticoagulant agents in critically ill patients
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors acknowledge Rob Kunkle, Eddrika Russell, and Kendall Alexander for their overall coordination of the guideline panel and thank the following members of the knowledge synthesis team for their contributions to this work: Reyad Al Jabiri, Yazan Al Jabiri, Samer G. Karam, and Emma Cain.
D.R.T. was supported by a career development award from the National Institutes of Health, National Heart, Lung, and Blood Institute (1K01HL135466).
Authorship
Contribution: E.K.T., R.N., A.C., R.A.M., and H.J.S. wrote the manuscript; all other authors contributed to critical revisions of the manuscript; and all authors approved of the content. Members of the knowledge synthesis team—R.N., I.B.A., A.B., M.B., R.B.-P., R.C., M.C., K.D., A.J.D., P.K., L.E.C.-L., R.M., G.M.-S., G.P.M., R.Z.M., A.N., B.A.P., T.P., Y.Q., Y.R., F.S., A.S., K.S., and W.W.—searched the literature, extracted data from eligible studies, analyzed the data, and prepared evidence summaries and evidence to decision tables; panel members A.C., E.K.T., P.A., C.B., K.D., J.D., M.T.D., D.D., D.O.G., S.R.K., F.A.K., A.I.L., I.N., A.P., M.R., K.M.S., D.S., M.S., D.R.T., K.T., R.A.M., and H.J.S. assessed the evidence, voted and made judgments within the EtD framework, and discussed and issued the recommendations; the methods leadership team (R.N., R.B.-P., K.D., A.S., K.S., A.C., E.A.A., W.W., R.A.M., and H.J.S.) developed the methods and provided guidance to the knowledge synthesis team and guideline panel; and A.C., R.A.M., and H.J.S. were the co-chairs of the panel and led panel meetings.
Conflict-of-interest disclosure: All authors were members of the guideline panel or members of the systematic review team or both. They completed a disclosure of interest form, which was reviewed by ASH and is available as Supplements 4 and 5.
Correspondence: Adam Cuker, Hospital of the University of Pennsylvania, 3400 Spruce St, Philadelphia, PA 19104; e-mail: adam.cuker@pennmedicine.upenn.edu.
References
- 1.Connors JM, Levy JH.. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nopp S, Moik F, Jilma B, Pabinger I, Ay C.. Risk of venous thromboembolism in patients with COVID-19: A systematic review and meta-analysis. Res Pract Thromb Haemost. 2020;4(7):1178-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flumignan RL, Tinôco JDS, Pascoal PI, et al. Prophylactic anticoagulants for people hospitalised with COVID-19. Cochrane Database Syst Rev. 2020;10:CD013739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iba T, Levy JH, Connors JM, Warkentin TE, Thachil J, Levi M.. The unique characteristics of COVID-19 coagulopathy. Crit Care. 2020;24(1):360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Samkari H, Karp Leaf RS, Dzik WH, et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136(4):489-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Godier A, Clausse D, Meslin S, et al. Major bleeding complications in critically ill patients with COVID-19 pneumonia. J Thromb Thrombolysis. 2021;52(1):18-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mai V, Mainbourg S, Tan BK, Lega J-C, Provencher S.. Significant major bleeding in hospitalized patients with COVID-19 receiving thromboprophylaxis [published online ahead of print on 8 April 2021]. Thromb Haemost. [DOI] [PubMed] [Google Scholar]
- 8.Halaby R, Cuker A, Yui J, et al. Bleeding risk by intensity of anticoagulation in critically ill patients with COVID-19: A retrospective cohort study. J Thromb Haemost. 2021;19(6):1533-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cuker A, Tseng EK, Nieuwlaat R, et al. American Society of Hematology 2021 guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID-19. Blood Adv. 2021;5(3):872-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Institute of Medicine. Clinical Practice Guidelines We Can Trust. Washington, DC: The National Academic Press; 2011. [Google Scholar]
- 11.Schünemann HJ, Al-Ansary LA, Forland F, et al. Board of Trustees of the Guidelines International Network . Guidelines International Network: Principles for disclosure of interests and management of conflicts in guidelines. Ann Intern Med. 2015;163(7):548-553. [DOI] [PubMed] [Google Scholar]
- 12.Qaseem A, Forland F, Macbeth F, Ollenschläger G, Phillips S, van der Wees P; Board of Trustees of the Guidelines International Network .Guidelines International Network: toward international standards for clinical practice guidelines. Ann Intern Med. 2012;156(7):525-531. [DOI] [PubMed] [Google Scholar]
- 13.Alonso-Coello P, Schünemann HJ, Moberg J, et al. GRADE Working Group . GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016;353:i2016. [DOI] [PubMed] [Google Scholar]
- 14.Alonso-Coello P, Oxman AD, Moberg J, et al. GRADE Working Group . GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016;353:i2089. [DOI] [PubMed] [Google Scholar]
- 15.Atkins D, Eccles M, Flottorp S, et al. GRADE Working Group . Systems for grading the quality of evidence and the strength of recommendations I: critical appraisal of existing approaches The GRADE Working Group. BMC Health Serv Res. 2004;4(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schünemann HJ, Best D, Vist G, Oxman AD; GRADE Working Group .Letters, numbers, symbols and words: how to communicate grades of evidence and recommendations. CMAJ. 2003;169(7):677-680. [PMC free article] [PubMed] [Google Scholar]
- 17.Schünemann HJ, Wiercioch W, Brozek J, et al. GRADE Evidence to Decision (EtD) frameworks for adoption, adaptation, and de novo development of trustworthy recommendations: GRADE-ADOLOPMENT. J Clin Epidemiol. 2017;81:101-110. [DOI] [PubMed] [Google Scholar]
- 18.Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. J Clin Epidemiol. 2011;64(4):395-400. [DOI] [PubMed] [Google Scholar]
- 19.Guyatt GH, Oxman AD, Vist GE, et al. GRADE Working Group . GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johns Hopkins University and Medicine. Coronavirus Resource Center. https://coronavirus.jhu.edu/map.html. Accessed 2 May 2021.
- 21.Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763-1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Government of Canada. COVID-19 daily epidemiology update. https://health-infobase.canada.ca/covid-19/epidemiological-summary-covid-19-cases.html#a7. Accessed 2 May 2021.
- 23.Grasselli G, Pesenti A, Cecconi M.. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA. 2020;323(16):1545-1546. [DOI] [PubMed] [Google Scholar]
- 24.Goshua G, Pine AB, Meizlish ML, et al. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020;7(8):e575-e582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mouhat B, Besutti M, Bouiller K, et al. Elevated D-dimers and lack of anticoagulation predict PE in severe COVID-19 patients. Eur Respir J. 2020;56(4):2001811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dutch COVID & Thrombosis Coalition, Kaptein FHJ, Stals MAM, et al. Incidence of thrombotic complications and overall survival in hospitalized patients with COVID-19 in the second and first wave. Thromb Res. 2021;199:143-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mumoli N, Conte G, Cei M, et al. In-hospital fatality and venous thromboembolism during the first and second COVID-19 waves at a center opting for standard-dose thromboprophylaxis. Thromb Res. 2021;203:82-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fara MG, Stein LK, Skliut M, Morgello S, Fifi JT, Dhamoon MS.. Macrothrombosis and stroke in patients with mild Covid-19 infection. J Thromb Haemost. 2020;18(8):2031-2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fan S, Xiao M, Han F, et al. Neurological manifestations in critically ill patients with COVID-19: a retrospective study. Front Neurol. 2020;11:806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fox SE, Akmatbekov A, Harbert JL, Li G, Quincy Brown J, Vander Heide RS.. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med. 2020;8(7):681-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wichmann D, Sperhake JP, Lütgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med. 2020;173(4):268-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ren B, Yan F, Deng Z, et al. Extremely high incidence of lower extremity deep venous thrombosis in 48 patients with severe COVID-19 in Wuhan. Circulation. 2020;142(2):181-183. [DOI] [PubMed] [Google Scholar]
- 33.Cui S, Chen S, Li X, Liu S, Wang F.. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18(6):1421-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Middeldorp S, Coppens M, van Haaps TF, et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18(8):1995-2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leentjens J, Van Haaps TF, Wessels PF, Schutgens REG, Middeldorp S.. COVID-19-associated coagulopathy and antithrombotic agents-lessons after 1 year. Lancet Haematol. 2021;8(7):e524-e533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Talasaz AH, Sadeghipour P, Kakavand H, et al. Recent randomized trials of antithrombotic therapy for patients with COVID-19: JACC State-of-the-Art Review. J Am Coll Cardiol. 2021;77(15):1903-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paranjpe I, Fuster V, Lala A, et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. 2020;76(1):122-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.INSPIRATION Investigators, Sadeghipour P, Talasaz AH, et al. Effect of intermediate-dose vs standard-dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with COVID-19 admitted to the intensive care unit: the INSPIRATION randomized clinical trial. JAMA. 2021;325(16):1620-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Izcovich A, Cuker A, Kunkle R, et al. A user guide to the American Society of Hematology clinical practice guidelines. Blood Adv. 2020;4(9): 2095-2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang D, Chan P-H, She H-L, et al. Secular trends and etiologies of venous thromboembolism in Chinese from 2004 to 2016. Thromb Res. 2018;166:80-85. [DOI] [PubMed] [Google Scholar]
- 42.Cheuk BLY, Cheung GCY, Cheng SWK.. Epidemiology of venous thromboembolism in a Chinese population. Br J Surg. 2004;91(4):424-428. [DOI] [PubMed] [Google Scholar]
- 43.Lauzier F, Arnold DM, Rabbat C, et al. Risk factors and impact of major bleeding in critically ill patients receiving heparin thromboprophylaxis. Intensive Care Med. 2013;39(12):2135-2143. [DOI] [PubMed] [Google Scholar]
- 44.Schulman S, Beyth RJ, Kearon C, Levine MN.. Hemorrhagic complications of anticoagulant and thrombolytic treatment: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest. 2008;133(6 suppl):257S-298S. [DOI] [PubMed] [Google Scholar]
- 45.Anand SS, Yusuf S, Pogue J, Ginsberg JS, Hirsh J; Organization to Assess Strategies for Ischemic Syndromes Investigators .Relationship of activated partial thromboplastin time to coronary events and bleeding in patients with acute coronary syndromes who receive heparin. Circulation. 2003;107(23):2884-2888. [DOI] [PubMed] [Google Scholar]
- 46.Koch A, Bouges S, Ziegler S, Dinkel H, Daures JP, Victor N.. Low molecular weight heparin and unfractionated heparin in thrombosis prophylaxis after major surgical intervention: update of previous meta-analyses. Br J Surg. 1997;84(6):750-759. [PubMed] [Google Scholar]
- 47.Schünemann HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018;2(22):3198-3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miranda S, Le Cam-Duchez V, Benichou J, et al. Adjusted value of thromboprophylaxis in hospitalized obese patients: A comparative study of two regimens of enoxaparin: The ITOHENOX study. Thromb Res. 2017;155:1-5. [DOI] [PubMed] [Google Scholar]
- 49.Rondina MT, Wheeler M, Rodgers GM, Draper L, Pendleton RC.. Weight-based dosing of enoxaparin for VTE prophylaxis in morbidly obese, medically-Ill patients. Thromb Res. 2010;125(3):220-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Castellucci LA, Shaw J, Giulivi A, Edwards C, Carrier M, Patel R.. Determining the safety of enoxaparin prophylaxis in critically ill patients with severe renal insufficiency - The PACER pilot study. Thromb Res. 2016;144:69-71. [DOI] [PubMed] [Google Scholar]
- 51.Douketis J, Cook D, Meade M, et al. Canadian Critical Care Trials Group . Prophylaxis against deep vein thrombosis in critically ill patients with severe renal insufficiency with the low-molecular-weight heparin dalteparin: an assessment of safety and pharmacodynamics: the DIRECT study. Arch Intern Med. 2008;168(16):1805-1812. [DOI] [PubMed] [Google Scholar]
- 52.Mahé I, Aghassarian M, Drouet L, et al. Tinzaparin and enoxaparin given at prophylactic dose for eight days in medical elderly patients with impaired renal function: a comparative pharmacokinetic study. Thromb Haemost. 2007;97(4):581-586. [PubMed] [Google Scholar]
- 53.Helms J, Tacquard C, Severac F, et al. CRICS TRIGGERSEP Group (Clinical Research in Intensive Care and Sepsis Trial Group for Global Evaluation and Research in Sepsis) . High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spyropoulos AC, Levy JH, Ageno W, et al. Subcommittee on Perioperative, Critical Care Thrombosis, Haemostasis of the Scientific, Standardization Committee of the International Society on Thrombosis and Haemostasis . Scientific and Standardization Committee communication: Clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18(8):1859-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barnes GD, Burnett A, Allen A, et al. Thromboembolism and anticoagulant therapy during the COVID-19 pandemic: interim clinical guidance from the anticoagulation forum. J Thromb Thrombolysis. 2020;50(1):72-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moores LK, Tritschler T, Brosnahan S, et al. Prevention, diagnosis, and treatment of VTE in patients with coronavirus disease 2019: CHEST Guideline and Expert Panel Report. Chest. 2020;158(3):1143-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bikdeli B, Madhavan MV, Jimenez D, et al. Global COVID-19 Thrombosis Collaborative Group, Endorsed by the ISTH, NATF, ESVM, and the IUA, Supported by the ESC Working Group on Pulmonary Circulation and Right Ventricular Function . COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;75(23): 2950-2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.National Institutes of Health. Coronavirus Disease 2019 (COVID-19) treatment guidelines. https://www.covid19treatmentguidelines.nih.gov/. Accessed 2 May 2021. [PubMed]
- 59.Arabi YM, Al-Hameed F, Burns KEA, et al. Saudi Critical Care Trials Group . Adjunctive intermittent pneumatic compression for venous thromboprophylaxis. N Engl J Med. 2019;380(14):1305-1315. [DOI] [PubMed] [Google Scholar]
- 60.Lemos ACB, do Espírito Santo DA, Salvetti MC, et al. Therapeutic versus prophylactic anticoagulation for severe COVID-19: A randomized phase II clinical trial (HESACOVID). Thromb Res. 2020;196:359-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lopes RD, de Barros E Silva PGM, Furtado RHM, et al. ACTION Coalition COVID-19 Brazil IV Investigators . Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): an open-label, multicentre, randomised, controlled trial. Lancet. 2021;397(10291):2253-2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.REMAP-CAP, ACTIV-4a, and ATTACC Investigators, Zarychansky R.. Therapeutic anticoagulation in critically ill patients with COVID-19 - preliminary report. medRxiv. Preprint posted online 12 March 2021. https://www.medrxiv.org/content/10.1101/2021.03.10.21252749v1.full.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.