Abstract
Background and Objectives:
Candidiasis and pityriasis versicolor are opportunistic fungal infections that are caused by Candida spp. and Malassezia spp. yeasts. Conventional drugs like azole and amino derivatives are known to treat fungal skin diseases. However, drawbacks like long-term side effects and drug resistance lead to investigate on antifungal properties of phytochemicals as an alternative to available synthetic drugs.
Materials and Methods:
The herbal nano hydrogel was successfully synthesized from Quince Seed extract followed by ultrasonic treatment and it has been formulated using a mixture of essential oils. We evaluated the antifungal in vitro assay for a mixture of essential oils in combination with herbal nano hydrogel against Candida albicans and Malasezia furfur strains by micro dilution method.
Results:
The results indicated that essential oils possess antifungal activity with the MIC value of 12.5 and 6.24 mg/ml against C. albicans and M. furfur, respectively. No fungicidal effect was reported for the herbal hydrogel before nanofabrication while it shown some antifungal activity after ultrasonic treatment for 5 and 10 minutes. As anticipated; the antifungal property of essential oil mixture was appreciably improved when it combined with herbal nano hydrogel where the highest level of inhibition was observed at concentration of 3.125 mg/ml for both strains. The loss in biological activity observed when the ultrasonic treatment on herbal nano hydrogel performed for longer time.
Conclusion:
The proposed plant-based nano formulation shown promising in vitro antifungal activities against C. albicans and M. furfur strains and its antifungal properties were comparable with commercially available agents like clotrimazole. The new formulation expected to be safe with minimum long-term side effects. Further investigations are underway to confirm the safety and the mechanism of the action of this new herbal formulation.
Keywords: Herbal nano hydrogel, Essential oils, Antifungal activity, Candida albicans, Malassezia furfur
INTRODUCTION
Superficial fungal infections are one of the most common types of infections that affect skin, hair and mucous membranes in various forms. Many opportunistic infections like Candidiasis and pityriasis versicolor are caused by Candida and Malassezia yeasts (1).
Malassezia species are considered as medically important yeasts because of their involvement in the etiology of some important skin disorders including pityriasis versicolor, folliculitis, seborrheic dermatitis, dandruff, and psoriasis (2, 3). Candida albicans (C. albicans) is an opportunistic fungus that lives in the gastrointestinal tract, vaginal mucosa, urinary tract, skin and under the nails (4, 5).
Azoles such as clotrimazole and amino compounds like naftinine and terbinafine are promising synthetics drugs to treat skin and oral fungal diseases. However, they have long-term side effects, like burning, stinging, redness, swelling, tenderness, and allergic reaction (6, 8). Moreover, in some cases, resistance of some strains to the available drugs has been reported (9). Therefore, there is still a need to develop more effective alternative medications in clinical practice. Numerous phytochemical studies showed that medicinal plants possess considerable antifungal and antibacterial activities against a wide range of microorganisms. In addition, the essential oils of these plants, as significant alternative treatment, can simultaneously influence various parts of the cell membrane of the bacteria and fungi (10). For example, extracts from Plumbago europaea aerial parts exhibited promising anti-Candida properties and the main components isolated from Nigella Sativa (Black cumin), Citrus × sinensis (Orange) and Cinnamon verum (Cinnamon) including thymoquinone, limonene and cinnamaldehyde respectively, reported to own remarkable antifungal activities (11, 14).
Topical gel formulation provides a suitable delivery system for drugs because they are less greasy and can be easily removed from the skin. Gel formulation moreover, provides better application property and stability in comparison to cream and ointments due to its better penetration (15). Nanotechnology on the other hand, opens a large scope of novel application in the fields of biotechnology and many researchers tried to enhance the efficacy of plant compounds using biocompatible nanomaterials (16).
Nanogels are a class of nanomaterials with crosslinked polymer networks which facilitate hydrophilic or hydrophobic drug delivery in a more sustainable fashion. In addition, with a significant enhancement in cellular penetration of mucosal and transdermal drug delivery systems in one hand and their bioavailability and biocompatibility on the other hand, Nanogels can augment the herbal medicine therapeutic effects. Although the efficiency of various Nanogels in herbal medicine has been extensively studied but to the best of our knowledge, utilizing plant-based mucilage nanoparticles for herbal medicine delivery has not been reported. Mucilage is biocompatible and bio acceptable due to its natural origin and it is not harmful to the skin (17, 18). Owing to wide range of valuable pharmaceuticals, Quince Seed and its extracted mucinous material reported to possess valuable medicinal properties (19, 20).
Herein, a novel herbal nano formulation has been evaluated for its antifungal activities against Malassezia furfur and C. albicans species. For this purpose, the Quince Seeds mucilage hydrogel extracted and it has been fabricated to nano hydrogel. The resulted herbal nano hydrogel (HNHG) has been formulated by adding black cumin seed, Orange and Cinnamon essential oils. This new herbal formulation exhibited appreciable antifungal properties and can be used as a reference for the next stage in drug design.
MATERIALS AND METHODS
Microbial strains and culture media.
C. albicans (ATCC 10231) and M. furfur (ATCC 14521) used to test the antifungal properties of the hydrogels were obtained from the Iranian Research Organization for Science and Technology. Both strains were sub-cultured on Sabouraud Dextrose Agar (SDA) (Merck, Germany) and grown at 37°C for 24 h. The antifungal experiments were carried out using RPMI and Potato Dextrose Agar (PDA) culture media.
Preparation of quince seeds mucilage hydrogel.
The quince seeds were purchased from the local herbal medicine market of Shahroud, Iran. They were washed and disinfected using ultraviolet germicidal irradiation (Honeywell RUVLAMP1, USA). The seeds were dried in the oven at 50°C for 8 hours prior to grinding and filtering through sieve (1.18 mm). The crushed seeds (10 g) were mixed with distilled water (200 mL) and warmed in a water bath at 80°C for 40 minutes to form mucilage hydrogel (5% w/v). The hydrogel was precipitated from 1:1 v/v ratio of ethanol: water solution and collected (21). Unused hydrogel was stored at 4°C.
Essential oil extraction.
The essential oils from the seeds of Nigella sativa, the peels of Cinammon verum and Citrus sinensis were extracted using a Clevenger apparatus, in accordance to the method of Karakaya et al. (23). Briefly, 50 g dried powder of each plant materials was placed in a 500 ml Clevenger apparatus containing 300 ml of distilled water and brought to the boiling point. The extraction process was completed in 2.5 hours. Equal volume mixture of each essential oil was used for antifungal experiments.
Loading of essential oils into hydrogel.
The quince seeds mucilage hydrogel (2.5% w/v), with 2.5% (v/v) essential oil was vortexed for 30s prior to ultrasonic treatment (22). Ultrasonic treatment of the hydrogel mixture was performed at different time points (5, 10 and 15 minutes) at 19 MHz using an ultrasonic probing machine (model UP400A, Iran). In order to obtain the optimized conditions for the desired nanostructure, the treated samples were analyzed using scanning electron microscopic technique (SEM). The Fourier-transform infrared spectroscopy (FTIR) analysis was also used to ensure that the polymeric structure of the mucilage hydrogel has not been decomposed after nanofabrication.
Preparation of stock solutions for antifungal assay.
To evaluate the antifungal properties of essential oils and mucilage hydrogel, stock solutions were prepared by dissolving each sample in minimum amount of DMSO according to the standard procedure (24).
As summarized in Table 1, clotrimazole, essential oils mixture, hydrogel, and hydrogel - essential oils combinations were labelled as Standard, A, B and BA respectively. In addition, the ultrasonic wave treatment time was stated in the bracket for each treated sample.
Table 1.
Stock solutions for antifungal assay
| Entry | Stocks | Components | Concentration |
|---|---|---|---|
| 1 | Standard | Clotrimazole | 50 mg/ml |
| 2 | A | Essential oils | 5% v/v |
| 3 | B | Untreated hydrogel | 5% w/v |
| 4 | B (5) | Hydrogel (5 minutes ultrasonic treatment) | 5% w/v |
| 5 | B (10) | Hydrogel (10 minutes ultrasonic treatment) | 5% w/v |
| 6 | B (15) | Hydrogel (15 minutes ultrasonic treatment) | 5% w/v |
| 7 | BA | Hydrogel–essential oils (Untreated) | 2.5% w/v |
| +2.5% v/v | |||
| 8 | BA (5) | Hydrogel–essential oils (5 minutes ultrasonic treatment) | 2.5% w/v |
| +2.5% v/v | |||
| 9 | BA (10) | Hydrogel–essential oils (10 minutes ultrasonic treatment) | 2.5% w/v |
| +2.5% v/v | |||
| 10 | BA (15) | Hydrogel–essential oils (15 minutes ultrasonic treatment) | 2.5% w/v |
| +2.5% v/v |
Antifungal assay: Minimal inhibitory concentration (MIC).
Two pathogenic fungi namely, C. albicans (ATCC 10231) and M. furfur (ATCC 14521) were used in this study. They were maintained and cultured according to the CLSI recommended methods. The micro dilution method was used to determine the MIC of tested samples (24). A stock solution of 1.0 g/ml for each tested sample was prepared in dimethyl sulfoxide. These stock solutions were two fold serially diluted with the RPMI 1640 medium (Life Technologies, Gibco®) into five different concentrations (50, 25, 12.5, 6.45, 3.125, 1.562 mg/ml). Subsequently, these tested samples in different concentrations were evaluated in 96-well plate for antifungal activity. Briefly, 40 μl of the tested samples were placed in each well and 10 μl of fungal suspension was added to each well. The positive control comprised of 40 μL of RPMI medium and 10 μL of fungal suspension, whereas the negative control contained 50 μL of RPMI medium. The micro plates were incubated at 37°C for 24 h (24). After that, 10 μL p-iodonitrotetrazolium violet (Sigma Aldrich, USA) (2 mg/mL, in water) was added to each well and the micro plates were further incubated at 37°C for 48 h. MIC was defined as the lowest concentration that inhibited the colour change of p-iodonitrotetrazolium violet (25).
Minimal fungicidal concentration (MFC).
From each tube, 20 μL of culture was inoculated onto Potato Dextrose Agar (PDA) plate and incubated at 37°C for 48 h. The plates were observed and the MFC was determined as the lowest concentration of essential oil completely inhibiting the growth of C. alibans and M. furfur (26).
RESULTS
Characterization of HNHG.
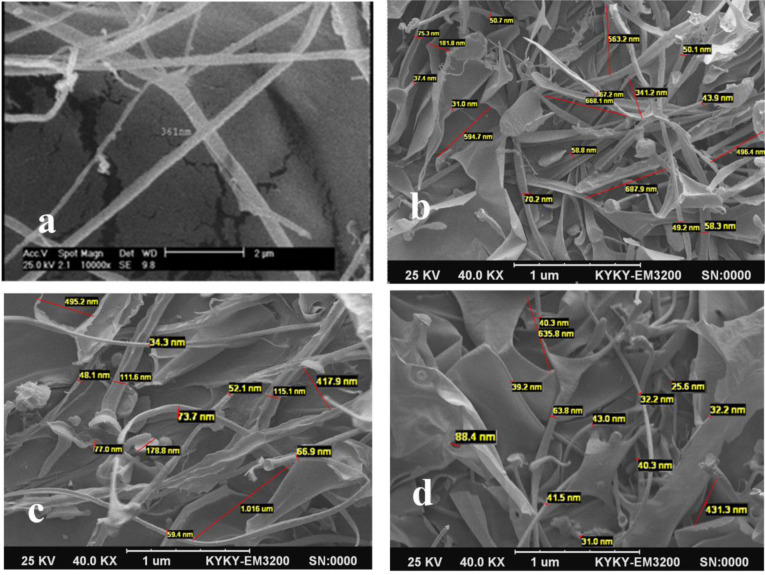
Morphological characterization of HNHG has been performed using SEM analysis. Fig. 1 demonstrated the SEM images of Quince Seeds mucilage extract before and after ultrasonic treatment. Existence of cellulose microfibrils in Quince Seeds mucilage extract has been documented in the literature (27). As expected, in all studied samples, structure transformation observed after ultrasonic treatments (22). SEM images confirmed the presence of nanofibers in samples B, B (5) and B (10). The size of nanofibers however, reduced from 361 nm to 31 nm after 5 minutes ultrasonic treatment. While the co-occurrence of nanofibers and nanoparticles observed after 10 minutes treatment, the nanofibers converted to nanoparticles eventually after 15 min ultrasonic treatment.
Fig. 1.
SEM image of mucilage before ultrasonic treatment is a, for the mucilage after five minutes ultrasonic treatment B (5) is b, for the mucilage after ten minutes ultrasonic treatment B (10) is c and for the mucilage after fifteen minutes ultrasonic treatment B (15) is d
FT-IR analysis.
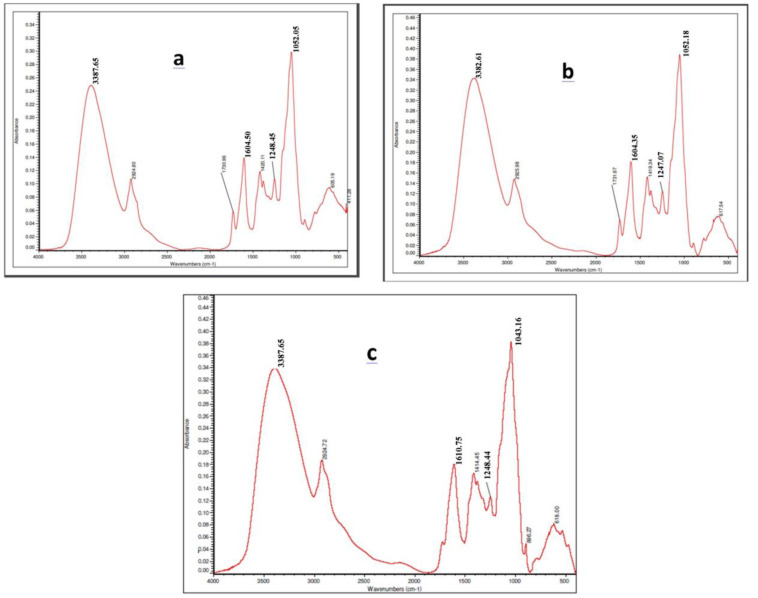
FT-IR spectroscopy technique utilized to investigate on generic chemical composition and ensure that the protein structure of mucilage hydrogel has not been disturbed during the nanofabrication process. As reported by Ritzoulis and coworkers, Quince Seeds hydrogel extraction showed characteristic peaks for its functional groups (20). Presenting sharp picks at 3387.65, 1604.50, 1248.45 and 1052.05 cm−1 correspond to amine, carbonyl and alcoholic functional groups respectively. Likewise, in case of Fig. 2, graphs a, b and c, similar frequencies were observed which correspond to the same functional groups presented in B (5), B (10) and B (15). As observed, the FTIR data for HNHG samples are in agreement with the mucilage hydrogel. Hence, the protein structure of mucilage hydrogel has not been disturbed during nanofabrication.
Fig. 2.
FT-IR spectra for samples B (5) is a; B (10) is b and B (15) is c
In vitro antifungal activity.
The result of microdilution tests demonstrated in Table 2. The essential oils possessed antifungal activity against C. albicans and M. furfur strains. MIC value for essential oil combination (A) was at 12.5, 6.24 mg/ml against C. albicans and M. furfur, respectively.
Table 2.
Antifungal activity of essential oil against C. albicans and M. furfur
| Entry | Stocks | C. albicans | M. Furfur | ||
|---|---|---|---|---|---|
|
MIC
(mg/ml) |
MFC
(mg/ml) |
MIC
(mg/ml) |
MFC
(mg/ml) |
||
| 1 | Standard | 25 | 50 | 12.5 | 25 |
| 2 | A | 12.5 | 25 | 6.25 | 12.5 |
| 3 | B | - | - | - | - |
| 4 | B (5) | 25 | 50 | 25 | 50 |
| 5 | B (10) | 25 | 50 | 25 | 50 |
| 6 | B (15) | - | - | - | - |
| 7 | BA | 12.5 | 25 | 12.5 | 25 |
| 8 | BA (5) | 6.25 | 12.5 | 12.5 | 25 |
| 9 | BA (10) | 3.125 | 3.125 | 3.125 | 6.25 |
| 10 | BA (15) | 25 | 50 | 25 | 50 |
Minimum inhibitory concentration, MFC: Minimal fungicidal concentration, Standard: Clotrimazole
No fungicides were reported for samples B and B (15) stocks. While for B (5) and B (10), the same antifungal activity has been observed on both strains.
The antifungal activity of essential oil mixture observed to be enhanced compare to the standard clotrimazole. No sign of activity reported for herbal hydrogel before ultrasonic treatment. To our surprise, the MIC and MFC values for M. furfur strain diminished from 6.25 to 12.5 and 12.5 to 25 mg/ml respectively when the untreated hydrogel was used in combination with essential oils. However, the antifungal property of essential oil mixture was appreciably improved when it combined with HNHG, where the highest level of inhibition was observed for BA (10); at concentration of 3.125 mg/ml for both strains. This dramatic improvement in antifungal activity can prove the effectiveness of our formulated potential herbal medicine. Nevertheless, the inhibitory activity obtained from stock BA (15), where the nanofibers transformed to nanoparticles, diminished to 25 mg/ml for both strains.
DISCUSSION
Extracts and essential oils from some aromatic plants have antibacterial, antifungal, antioxidant and anti-cancer properties and can control the growth of pathogens (28). One of the important characteristics of the essential oils and plant components is hydrophobicity that facilitates the penetration of these materials into bacterial cell membrane lipids, which disrupts their structures and increases their permeability. This causes the ejection and leakage of ions and other cellular contents, resulting in cell death (27).
A research was conducted in 2017 to evaluate the potential antifungal effects of different black cumin seed oils. The results indicated a moderate inhibitory effect with some components of the black cumin seed oils and extracts in vivo and in vitro against some pathogenic yeast like C. albicans, dermatophytes, non-dermatophyte molds and some aflatoxin producing fungi (29). Interestingly, N. sativa seeds exhibited high inhibitory effect against candidiasis in mice (29).
Thymoquin that isolated from Nigella Sativa revealed high antifungal activity against Aspergillus niger, Fusarium solani and Scopnlariopsis brevicaulis; and this bioactivity was comparable to the antifungal drug amphotericin-B (29). The methanol and ethanol extracts of the black cumin seed displayed potent inhibition on Aspergillus flavus, Aspergillus fumigates, Issatchenkia orientalis, Cryptococcus laurentii, Cryptococcus albidus, C. parapsilosis, C. albicans and C. tropicalis, and these extracts were more potent than the standard drug Amphotericin-B (30).
In a study conducted in 2017 by Pootong and coworkers, the antifungal activity of cinnamon on the C. albicans was evaluated. Results showed that cinnamaldehyde possessed antifungal activity against C. albicans with a minimum inhibitory concentration of 125 μg/ml. At sub-inhibitory concentrations, cinnamaldehyde significantly reduced germ tube formation, proteinase and phospholipase activities in a dose dependent manner (p<0.01). It also significantly inhibited the adhesion of C. albicans to buccal epithelial cells (p<0.01) (31).
Investigation on the effect of pure citrus essential oils dilution (lemon and orange) from 0.1 to 6.2 mg/ ml was compared with two common chemical drugs on tinea versicolor. The inhibition zone for pure orange essential oil observed at 20 mm while for commercially available drugs like gentamycin and streptomycin were 5.5 and 17 mm, respectively. MIC for orange essential oil reported at 2.2 mg/mg (32).
In a study conducted by Devkatte et al. 38 herbal essential oils as potential inhibitors of C. albicans growth were investigated. Amongst them, cinnamon oil exhibited the best antifungal activity against 4 candida strains (33).
An experiment on the antifungal properties interactions between conventional antifungal drugs and essential oils (Cinnamomum, Melaleuca, Mentha, Origanum, Syzygium) against isolates of Malassezia pachydermatis were conducted. The results indicated the highest and lowest antifungal effect exhibited by Origanum and cinnamon essential oils respectively (34).
In a trial, different doses of citrus essential oil (C. sinensis) were evaluated in an antimycotic assays and the result compared with a chemical drug. The Inhibitory concentration at 50% of growth (IC50) and the Minimum Inhibitory Concentrations (MIC) for orange essential oil against Candida albicans were observed at 8.41 mm and 1.68mm accordingly (35).
CONCLUSION
In the current study, we evaluated the in vitro assay for a mixture of essential oils in combination with herbal nano hydrogel against Candida albicans and Malasezia furfur strains. Although the efficiency of various Nanogels in herbal medicine has been extensively studied but to the best of our knowledge, utilizing plant-based mucilage nanoparticles for herbal medicine delivery has not been reported. Herein, the herbal nano hydrogel was successfully synthesized from Quince Seed extract and it has been decorated with a mixture of essential oils. Although, prior to nanofabrication process, the herbal hydrogel did not show any antifungal activities but it revealed some antifungal activity after ultrasonic treatment for 5 and 10 minutes. Moreover, the antifungal property of essential oil mixture was appreciably improved when it was combined with herbal nano hydrogel. Further investigations are required to confirm the safety and the mechanism of the action of this herbal formulation.
REFERENCES
- 1.Hay R. Therapy of skin, hair and nail fungal infections. J Fungi (Basel) 2018; 4: 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barac A, Donadu M, Usai D, Tomic Spiric V, Mazzarello V, Zanetti S, et al. Antifungal activity of Myrtus communis against Malassezia sp. isolated from the skin of patients with pityriasis versicolor. Infection 2018; 46:253–257. [DOI] [PubMed] [Google Scholar]
- 3.Gaitanis G, Velegraki A, Mayser P, D Bassukas I. Skin diseases associated with Malassezia yeasts: facts and controversies. Clin Dermatol 2013; 31:455–463. [DOI] [PubMed] [Google Scholar]
- 4.Zida A, Bamba S, Yacouba A, Ouedraogo-Traore R, Guiguemde RT. Anti-Candida albicans natural products, sources of new antifungal drugs: A review. J Mycol Med 2017; 27:1–19. [DOI] [PubMed] [Google Scholar]
- 5.Ludwig A, de Jesus FPK, Dutra V, Candido SL, Alves SH, Santurio JM. Susceptibility profile of Candida rugosa (Diutina rugosa) against antifungals and compounds of essential oils. J Mycol Med 2019; 29:154–157. [DOI] [PubMed] [Google Scholar]
- 6.Mendling W, Atef El Shazly M, Zhang L. Clotrimazole for vulvovaginal Candidosis: more than 45 years of clinical experience. Pharmaceuticals (Basel) 2020; 13: 274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Society of Health-System Pharmacists . “Clotrimazole Monograph for Professionals”. www.drugs.com. Archived from the original on 28 October 2016. Retrieved 28 October 2016.
- 8.Crowley PD, Gallagher HC. Clotrimazole as a pharmaceutical: past, present and future. J Appl Microbiol 2014; 117: 611–617. [DOI] [PubMed] [Google Scholar]
- 9.Schlemmer KB, Jesus FPK, Tondolo JSM, Weiblen C, Azevedo MI, Machado VS, et al. In vitro activity of carvacrol, cinnamaldehyde and thymol combined with antifungals against Malassezia pachydermatis. J Mycol Med 2019; 29: 375–377. [DOI] [PubMed] [Google Scholar]
- 10.Chouhan S. Antimicrobial activity of some essential oils perspectives. Medicines (Basel) 2017; 4: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sobhani M, Abbas-Mohammadi M, Nejad Ebrahimi S, Aliahmadi A. Tracking leading anti-Candida compounds in plant samples; Plumbago europaea. Iran J Microbiol 2018; 10: 187–193. [PMC free article] [PubMed] [Google Scholar]
- 12.Akhtar MS. Antimicrobial activity of essential oils extracted from medicinal plants against the pathogenic microorganisms. A review. Issues Biol Sci Pharm Res 2014; 2: 001–007. [Google Scholar]
- 13.Nazzaro F, Fratianni F, Coppola R, De Feo V. Essential oils and antifungal activity. Pharmaceuticals (Basel) 2017; 10:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bona E, Cantamessa S, Pavan M, Novello G, Massa N, Rocchetti A. Sensitivity of Candida albicans to essential oils: are they an alternative to antifungal agents. J Appl Microbiol 2016; 121: 1530–1545. [DOI] [PubMed] [Google Scholar]
- 15.Aiyalu R, Govindarjan A, Ramasamy A. Formulation and evaluation of topical herbal gel for the treatment of arthritis in animal model. Braz J Pharm Sci 2016; 52: 493–507. [Google Scholar]
- 16.Manzer H, Siddiqui Mohamed H, Al-Whaibi, Firoz Mohammad, Mutahhar AlK. (2015). Role of Nanoparticles in Plants. In: Nanotechnology and Plant Sciences. Springer International Publishing, e book, Library of Congress; Control Number: 2014958574, Switzerland, pp. 19–35. [Google Scholar]
- 17.Sultana F, Manirujjaman, Imran-Ul-Haque M, Arafat M, Sharmin S. An overview of nanogel delivery system. J Appl Pharm Sci 2013; 3 (8 Suppl 1):S95–S105. [Google Scholar]
- 18.Kumar S, Gupta SK. Natural polymers, gums and mucilages as excipients in drug delivery. Polim Med 2012; 42: 191–197. [PubMed] [Google Scholar]
- 19.Hemmati AA, Kalantari H, Jalali A, Rezai S, Haghighi Zadeh H. Healing effect of quince seed mucilage on T-2 toxin-induced dermal toxicity in rabbit. Exp Toxicol Pathol 2012; 64: 181–186. [DOI] [PubMed] [Google Scholar]
- 20.Ritzoulis CH, Marini E, Aslanidou A, Georgiadis N, Karayannakidis P, Koukiotis CH, et al. Hydrocolloids from quince seed: Extraction, characterization, and study of their emulsifying/stabilizing capacity. Food Hydrocoll 2014; 42:1–9. [Google Scholar]
- 21.Vignon MR, Gey C. Isolation, 1H and 13C NMR studies of (4-O-methyl-d-glucurono)-d-xylans from luffa fruit fibers, jute best fibers and mucilage of quince tree seeds. Carbohydr Res 1998; 307: 107–111. [Google Scholar]
- 22.Emi T, Michaud K, Orton E, Santilli G, Linh C, O’Connell M, et al. Ultrasonic generation of pulsatile and sequential therapeutic delivery profiles from calcium-crosslinked zlginate hydrogels. Molecules 2019; 24:1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karakaya S, Nehir El S, Karagozlu N, Sahin S, Sumnu G, Bayramoglu B. Microwave-assisted hydrodistillation of essential oil from rosemary. J Food Sci Technol 2014; 51: 1056–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jersek B, Poklar Ulrih N, Skrt M, Gavaric N, Bozin B, Smole Mozina S. Effects of selected essential oils on the growth and production of ochratoxin A by Penicillium verrucosum. Inhibition of fungal growth by essential oils. Arh Hig Rada Toksikol 2014; 65:199–208. [DOI] [PubMed] [Google Scholar]
- 25.Elof J. Avoiding pitfalls in determining antimicrobial activity of plant extracts and publishing the results. BMC Complement Altern Med 2019; 19:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naeini A, Jalayer Naderi N, Shokri H. Analysis and in vitro anti-Candida antifungal activity of Cuminum cyminum and Salvadora persica herbs extracts against pathogenic Candida strains. J Mycol Med 2014; 24: 13–18. [DOI] [PubMed] [Google Scholar]
- 27.Hakala T, Saikko V, Arola S, Ahlroos T, Helle A, et al. Structural characterization and tribological evaluation of quince seed mucilage. Tribol Int 2014; 77: 24–31. [Google Scholar]
- 28.Al-Mariri A, Safi M. In vitro antibacterial activity of several plant extracts and oils against some Gram-negative bacteria. Iran J Med Sci 2014; 39: 36–43. [PMC free article] [PubMed] [Google Scholar]
- 29.Shokri H. A review on the inhibitory potential of Nigella sativa against pathogenic and toxigenic fungi. Avicenna J Phytomed 2016; 6: 21–33. [PMC free article] [PubMed] [Google Scholar]
- 30.Aljabre SHM, Alakloby OM, Randhawa MA. Dermatological effects of Nigella sativa. J Dermatol Dermatol Surg 2015; 19:92–98. [Google Scholar]
- 31.Pootong A, Norrapong B, Cowawintaweewat S. antifungal activity of cinnamaldehyde against Candida albicans. Southeast Asian J Trop Med Public Health 2017; 48:150–158. [PubMed] [Google Scholar]
- 32.Abd Rashed A, Gunasegavan Rathi DN, Husna Ahmad Nasir NA, Abd Rahman AZ. Antifungal properties of essential oils and their compounds for application in skin fungal infections: conventional and nonconventional approaches. Molecules 2021; 26: 1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Devkatte AN, Zore GB, Karuppayil SM. Potential of plant oils as inhibitors of Candida albicans growth. FEMS Yeast Res 2005; 5: 867–873. [DOI] [PubMed] [Google Scholar]
- 34.Bohmova E, Conkova E, Harcarova M, Sihelska Z. Interactions between Clotrimazole and selected essential oils against Malassezia pachydermatis clinical isolates. Pol J Vet Sci 2019; 22: 173–175. [DOI] [PubMed] [Google Scholar]
- 35.Ruiz-Perez J, Gonzalez-Avila M, Sánchez-Navarrete J, D Toscano-Garibay J, Moreno-Eutimio M, Sandoval-Hernández T, et al. Antimycotic activity and genotoxic evaluation of Citrus sinensis and Citrus latifolia essential oils. Sci Rep 2016; 6: 25371. [DOI] [PMC free article] [PubMed] [Google Scholar]