Abstract
A family history of alcoholism (FH) increases risk for alcohol use disorder (AUD), yet many at-risk individuals never develop alcohol use problems. FH is associated with intermediate levels of risk phenotypes, whereas distinct, compensatory brain changes likely promote resilience. Although several cognitive, behavioral, and personality factors have been associated with AUD, the relative contributions of these processes and their neural underpinnings to risk or resilience processes remains less clear. We examined whole-brain resting-state functional connectivity (FC) and behavioral metrics from 841 young adults from the Human Connectome Project, including healthy controls, individuals with AUD, and their unaffected siblings. First, we identified functional connections in which unaffected siblings were intermediate between controls and AUD, indicating AUD risk, and those in which siblings diverged, indicating resilience. Canonical correlations relating brain risk and resilience FC to behavioral patterns revealed AUD risk and resilience phenotypes. Risk phenotypes primarily implicated frontal-parietal networks corresponding with executive function, impulsivity, externalizing behaviors, and social-emotional intelligence. Conversely, resilience-related phenotypes were underpinned by networks of medial prefrontal, striatal, temporal, brainstem and cerebellar connectivity, which associated with high trait attention and low antisocial behavior. Additionally, we calculated “polyphenotypic” risk and resilience scores, to investigate how the relative load of risk and resilience phenotypes influenced the probability of an AUD diagnosis. Polyphenotypic scores predicted AUD in a dose-dependent manner. Moreover, resilience phenotypes interacted with risk phenotypes, reducing their effects. The hypothesis-generating results revealed interpretable AUD-related phenotypes and offer brain-informed targets for developing more effective interventions
Keywords: Alcohol use disorder, Family history, Risk, Resilience, Intermediate phenotypes, Human connectome project
1. Introduction
In the US, 9% of young adults aged 18–25 met criteria for alcohol use disorder (AUD) in 2019 (SAMHSA, 2019). Approximately half of the risk for AUD is heritable (Agrawal and Lynskey, 2008, Kendler et al., 2012a), and a family history of alcoholism (FH) substantially increases an individual’s risk for AUD (Anda et al., 2002, Kendler et al., 2012b). AUD risk is driven by the combination and interaction of multiple genetic and environmental risk and protective factors, which vary across affected individuals (Ducci and Goldman, 2008), producing multiple pathways to AUD (Sher et al., 2005).
Due to the polygenic nature and heterogeneous expression of AUD, studies of intermediate phenotypes have been instrumental to the study of AUD risk (Ducci and Goldman, 2008). Here, we use the term intermediate phenotype to refer to markers of risk for AUD that exist somewhere between genetic/environmental factors and the disorder itself, including personality traits and behavioral measures, as well as their neural substrates. By definition, intermediate phenotypes related to AUD risk are not only present in individuals with AUD, but also, to a lesser extent, in their unaffected family members. Behavioral and personality phenotypes linked to AUD include sensation-seeking (Lange et al., 2010), antisocial personality (Oreland et al., 2018), delay discounting (Mitchell, 2011), impulsivity (Dick et al., 2010), sweet liking (Lange et al., 2010), and subjective response to alcohol (Morean and Corbin, 2010). Brain functional connectivity studies have also identified brain alterations in FH (Cservenka et al., 2015, Cservenka et al., 2014a, Cservenka et al., 2014b, Herting et al., 2011, Martz et al., 2019, Spadoni et al., 2013a, Spadoni et al., 2013b, Vaidya et al., 2019, Vaidya et al., 2019b), including alterations in functional connections within and between particular neural systems (Cservenka et al., 2015). For example, FH-positive adolescents demonstrate diminished integration within and reduced segregation between reward and cognitive control regions (Cservenka et al., 2014a), as well as reduced FC integration of motor regions (Vaidya et al., 2019). FH is also associated with reduced cerebellar connectivity with both amygdala and frontal cortex (Cservenka et al., 2014b, Herting et al., 2011), as well as altered frontal-parietal FC (Spadoni et al., 2013a, Me et al., 2019). However, few putative FC-based intermediate phenotypes for AUD are currently supported by consistent evidence of intermediate levels in FH relative to AUD and healthy comparison subjects. Thus, the examination of a large sample including FH, AUD, and healthy controls has the potential to establish the neurobehavioral basis for heritable risk for AUD.
Other work has begun to identifiy brain and behavioral markers of protection from AUD, including FC changes (Martz et al., 2019) and cognitive factors (Kim-Spoon et al., 2016, Wills et al., 2008). However, recent work in the neuroscience of resilience suggests resilience is not simply reduced, intermediate levels of risk, but involves separate brain connections offering protection against coexisting vulnerability-related alterations (Garavan and Albaugh, 2019, Ohashi et al., 2019). Support for resilience relies on evidence of interactions between risk factors and outcomes (Roosa, 2000), suggesting particular brain functional connections differentiate resilient individuals not only from their affected siblings (Doucet et al., 2017), but also from healthy controls (Luthar et al., 2006). Studies of reilience in AUD populations have primarily focused on the buffering effects of cognitive abilities on the risk for AUD associated with heightened reward sensitivity (Kim-Spoon et al., 2016) and environmental factors (Wills et al., 2008). One recent study identified enhanced FC between a dorsolateral prefrontal seed and the posterior cingulate cortex among FH adolescents with limited prior substance use, versus those self-reporting substance use, indicating resilience (ME et al., 2019). However, brain-based phenotypes of resilience to AUD in the context of familial risk remain scarce. Recent findings of brain functional connectivity markers of resilience in other psychiatric disorders (Doucet et al., 2017, Ersche et al., 2020) suggest the potential for functional connectivity to similarly reveal the neural basis of resilience in AUD.
Given the polygenic and heterogeneous nature of AUD, we hypothesized that 1) patterns of brain FC would associate with multiple, distinct neurobehavioral phenotypes of vulnerability or resilience to AUD and 2) the number of risk and/or resilience phenotypes an individual expressed would influence their likelihood of having AUD. We leveraged 841 resting-state fMRI data sets from the Human Connectome Project (HCP) young adult study (Van Essen et al., 2013) to examine brain differences between healthy controls, individuals with AUD, and their unaffected siblings. First, a data-driven canonical correlation analysis (CCA) related brain FC differences between groups to patterns of behaviors in those same individuals. Confirming our first hypothesis, CCA identified multiple risk and resilience phenotypes represented in human brain function that associated with interpretable behavioral profiles. Secondly, we tested the utility of “polyphenotypic” risk and resilience scores, predicting that risk and resilience phenotypes would combine to increase or decrease AUD risk. Indeed, the number of risk phenotypes expressed predicted AUD in a dose-dependent manner, whereas the number of resilience phenotypes expressed reduced the probability of AUD but also moderated effects of risk phenotypes, supporting interactive models of resilience (Brook et al., 1990, Garmezy et al., 1984, Rutter, 1985). The results provide brain-informed targets for AUD interventions and suggest how interventions targeting either risk or resilience phenotypes could impact AUD.
2. Methods
Data were downloaded from the Human Connectome Project Young Adult 1200 dataset repository (https://www.humanconnectome.org/study/hcp-young-adult, 2017), which includes 1206 highly-characterized adults, aged 22–35, recruited to participate in fMRI scanning sessions and behavioral assessments. The demographic and substance use characteristics of each group are summarized in Table 1. Group differences were examined for significance with one-way analyses of variance (ANOVAs) for age, handedness, and alcoholic drinks in the past 7 days. Group differences in the distribution of sexes and nicotine dependence symptoms were assessed with chi-squared tests. Data collection and analysis was carried out in accordance with the ethical standards of the Declaration of Helsinki.
Table 1.
Group Characteristics.
| CON | FH | AUD | p-value | |
|---|---|---|---|---|
| n | 433 | 208 | 200 | – |
| Age | 28.7 | 28.6 | 28.8 | p = 0.88 |
| Sex (females, %) | 253 (58%) | 123 (59%) | 74 (37%) | p < 0.001 |
| Handedness (left = -100, right = 100) | 67.1 | 71.8 | 66.2 | p = 0.31 |
| Drinks past 7 days | 2.8 | 4.7 | 9.2 | p < 0.001 |
| Cannabis abuse/dependence | – | – | 57 (29%) | – |
| Nicotine tolerance, withdrawal, or difficulty quitting | 47 (11%) | 27 (13%) | 72 (36%) | p < 0.001 |
Demographic and substance use measures are presented for controls (CON), individuals with a family history of alcohol use disorder (FH), and their siblings with an alcohol use disorder (AUD). P-values indicate the significance of group differences according to one-way analyses of variance or chi-squared tests.
2.1. Experimental design and statistical analysis
Three groups of subjects were defined, including a healthy control group (CON), individuals with AUD, and their unaffected siblings (FH), as described in detail in the Inclusion/Exclusion section. CCA were conducted in SAS 9.4 using the Cancorr procedure and are described in the Canonical Correlation Analysis (CCA) section. To test the associations of risk and resilience phenotypes, including polyphenotypic scores, and the probability of AUD, we employed logistic regression analyses (SAS PROC LOGISTIC) with AUD as a binary outcome variable; these analyses are described in the Associations of Risk and Resilience Phenotypes with AUD section.
2.2. Inclusion/exclusion
Inclusion criteria for the AUD group was meeting DSM-IV criteria for either alcohol abuse or dependence based on Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA) interview. FH group inclusion required a sibling in the dataset meeting criteria for the AUD group but no personal AUD or other substance use disorder. CON group exclusion criteria were personal, sibling, or parental alcohol or substance use disorder, based on SSAGA interview. Exclusion criteria for all groups included positive breathalyzer test, positive urine drug screen on the day of the scan, or incomplete resting-state data. This selection criteria produced 841 subjects, including 433 CON (253 females), 208 FH (123 females), and 200 AUD (74 females).
2.3. Functional connectivity analyses
To examine resting-state FC, we downloaded the “netmats” partial correlation matrices for each of the available independent component parcellations (i.e. 15, 25, 50, 100, 200, and 300). Preliminary tests examined the similarities between CCA results across parcellations. When comparing the first canonical brain variate from each parcellation, the combination of functional connections from all parcellations tended to be more strongly correlated with each parcellation CCA result than any independent parcellation (Supplementary Table 1). Therefore, the analysis considered all six parcellations, totaling 71,330 connections. Effects of several nuisance variables were removed based on estimates from linear regressions that simultaneously controlled for group: age, sex, handedness, years of education, nicotine dependence, and cannabis dependence. The resulting residual FC values were used in subsequent analyses.
Next, we identified functional connections consistent with risk or resilience. Functional connections representing risk were identified as those for which the group mean FC values followed the pattern CON < FH < AUD or CON > FH > AUD, consistent with an intermediate phenotype. Similarly, resilience was defined as connections in which siblings diverged (i.e., FH < CON < AUD or FH > CON > AUD), indicating protection from AUD. To ensure reliable estimates, the probability that a connection followed patterns of either risk or resilience was calculated with 10,000 bootstrap iterations, based on resampling observations within each group with replacement. We also calculated the probability that a connection would fit the pattern of risk or resilience by chance using random permutation of group labels. Using this method, all 71,330 connections were designated as possible risk connections, possible resilience connections, or neither, depending on whichever gave the greatest probability above chance. We did not apply statistical thresholds at this step, but rather performed an additional data-reduction step (i.e., principal component analysis) to isolate risk and resilience signals from this large set of connections prior to entering FC data into the CCA (see below). 25,354 functional connections were designated as possible risk connections and 23,687 functional connections were designated as possible resilience connections.
2.4. Canonical correlation analysis (CCA)
CCA is a multivariate correlation analysis that identifies linear combinations of two sets of variables, known as canonical variates, that maximize their correlation while remaining orthogonal to other variate pairs. In this analysis, we correlated brain FC variables with behavioral variables. We conducted two separate analyses: one for risk brain FC and one for resilience brain FC. The goal of this analysis was to identify brain FC-derived neurobehavioral phenotypes of risk or resilience for AUD. We predicted that CCA would identify multiple significant brain-behavior correlations.
First, we reduced the number of brain FC variables for this analysis, performing parallel analyses for risk and resilience. We started with all possible risk (or resilience) connections that were identified by bootstrapping (see Section 2.3) and then isolated sources of risk- (or resilience-) related signals from the noise. To do this, we converted FC values to z-scores and then performed a principal component analysis on the set of risk (or resilience) functional connections. This analysis identified patterns of brain FC that accounted for the greatest variance among the included connections. We discarded any components that did not exhibit patterns consistent with risk (or resilience) as irrelevant to the analysis. Although a previous CCA using this dataset retained the first 100 FC components for analysis (Smith et al., 2015), we heeded concerns of overfitting with higher ratios of variables to observations and retained the first 50 components for CCA. Because the principal component analysis was performed on functional connections identified from all six parcellations, and thus identified potentially shared, as well as unique, sources of variance across parcellations, this strategy enabled us to utilize signals relating to between-network and within-network connectivity simultaneously.
We also limited the number of behavioral variables entered in the CCA, following a similar process as previously published (Smith et al., 2015). We excluded variables for which more than 5% of values were missing, as well as discrete variables in which more than 90% of values were identical. Other variables not clearly related to behavioral and personality factors of AUD were not evaluated, such as sleep quality, motor skills, and sensory abilities. Notably, we also excluded substance use measures so that results would be driven by intermediate measures of risk or resilience to AUD rather than by alcohol use itself. The final selection of 76 variables are provided in Supplementary Table 2. CCA cannot handle missing data; therefore, missing values were imputed with the group-specific mean value. Behavioral values underwent a rank-based inverse normal transformation to account for departures from normality. Prior to entering values into the CCA, effects of age, sex, handedness, years of education, nicotine dependence, and cannabis dependence were removed from behavioral data following identical nuisance regression procedures as described for the brain FC data.
To correct for the identification of multiple canonical correlations, we calculated the distribution of the maximum correlation across 1000 CCAs using random permutations of the data. A comparison of each canonical correlation calculated with the original data to this distribution provided family-wise error-rate corrected p-values.
We next tested the validity of the identified risk and resilience canonical variates. To ensure the identified canonical correlations represented risk or resilience phenotypes based on evidence for group differences, post-hoc analyses examined group effects on behavioral variates in one-way analyses of variance (ANOVA) in SAS 9.4 using PROC ANOVA. Because reversing the signs of the canonical coefficients/scores does not alter the interpretation of the correlation, we reversed signs as necessary based on group effects to be consistent with interpretations of greater vulnerability (i.e., CON < FH < ALC) or greater resilience (i.e., FH > CON > ALC).
Risk and resilient brain variates were mapped onto a brain anatomical image for visualization purposes. We first conducted ridge regressions (i.e., linear regression with L2 regularization to limit overfitting) using the ridge function in MATLAB to test associations between the set of risk and resilient connections and the corresponding canonical variable scores for the brain risk or resilience brain variates, using a ridge parameter value of 0.01. The purpose of mapping regularized regression coefficients, rather than simple correlations, was to highlight brain regions most directly related with the phenotypes, rather than those regions that were indirectly correlated. Then, the coefficients were mapped onto the brain for each of the risk and resilience canonical variable scores, separately for each component parcellation (i.e., 15, 25, 50, 100, 200, 300). Specifically, for each component, we calculated a measure of strength of that component’s association with a given brain variate by averaging the absolute coefficient values for all functional connections of that component. The strength measure was then multiplied by the corresponding component spatial map, which we had normalized to values of 0–1 after discarding negative values. These strength-weighted component maps were then summed across all components from that parcellation and then normalized by the sum of unweighted component maps. Finally, the normalized strength maps were averaged across the six parcellations and then converted to z-scores.
To test the stability and generalizability of the CCA results, we repeated the analysis on 100 randomly-split samples (Table 2). We randomly divided each of the three groups in half 100 times in order to determine the reliability of results across two independent samples. In this procedure, first each group was divided in half at random. Next, the full set of brain functional connections related to either risk or resilience (as previously determined with the computationally-intensive bootstrapping of the full sample) was entered into a principal component analysis for each of the two groups separately. Then the first 50 components that followed patterns of risk (i.e., CON < FH < ALC or CON > FH > ALC) or resilience (i.e., FH < CON < ALC or FH > CON > ALC) were entered into CCA for each of the two groups independently. We tested the stability of the CCA results between group one and group two. For canonical correlations #1-#5 (for the risk analysis) or #2-#6 (for the resilience analysis), we identified the canonical variate of behavior for the second group (considering the first ten variates, since variate order may differ between samples) that was most similar to that of the first group based on the Pearson correlation. This process was repeated for both risk and resilience variates. We continued this process 100 times, saving correlation values of the corresponding canonical variates. The 95% confidence intervals were calculated across the 100 bootstrapped samples.
Table 2.
Canonical correlations and the stability of their estimates.
| Risk | r | FWE-corrected p | Mean r | 95% CI | |
|---|---|---|---|---|---|
| # 1 | 0.63 | <0.001 | 0.50 | 0.34 | 0.69 |
| # 2 | 0.59 | <0.001 | 0.49 | 0.31 | 0.66 |
| # 3 | 0.57 | <0.001 | 0.49 | 0.32 | 0.69 |
| # 4 | 0.55 | 0.002 | 0.44 | 0.24 | 0.63 |
| Resilience | r | Mean r | 95% CI | ||
| # 2 | 0.57 | <0.001 | 0.52 | 0.32 | 0.67 |
| # 3 | 0.55 | 0.004 | 0.44 | 0.21 | 0.61 |
Canonical correlation values and corresponding p-values for each risk and resilience phenotype are provided. The right column displays the mean and 95% confidence intervals (CI) of correlations between the behavior canonical variates produced on random splits of the sample, repeated 100 times, demonstrating the stability of the results across subsamples.
2.5. Associations of risk and resilience phenotypes with AUD
We next determined how risk and resilience phenotypes predicted AUD diagnosis. First, we set the upper quartile of each brain and behavior variate score as a cut-off to create a binary variable indicating whether a subject expressed that particular phenotype. We then summed the number of phenotypes expressed, separately for brain and behavior, and separately for risk and resilience, creating four scores. To examine relationships between these polyphenotypic scores and the probability of AUD, we employed four separate logistic regression analyses with AUD as the outcome variable and the polyphenotypic scores as ordinal predictors. Due to low frequencies of 4 total risk phenotypes, we truncated risk scores to include categories: 0, 1, 2, and 3+.
We next tested whether resilience phenotypes interacted with risk phenotypes to moderate their influence on AUD risk. Risk scores, resilience scores, and their interaction were entered into a logistic regression as ordinal variables with AUD as a binary outcome variable. Due to even lower cell frequencies when calculating interactions, scores were truncated to 0, 1, or 2+ risk phenotypes.
Finally, we explored whether specific resilience phenotypes moderated effects of specific risk phenotypes. In logistic regression analyses, we estimated effects of a single risk and single resilience phenotype and their interaction on AUD as a binary outcome variable in a pairwise manner. We tested a contrast of expressing (1) versus not expressing (0) each resilience phenotype in the presence of each risk phenotype (1). These exploratory analyses did not correct for multiple comparisons.
3. Results
3.1. Canonical correlation analysis
There were four significant canonical correlations between risk functional connections and behavioral measures after multiple comparison correction (Wilks’ λ(df = 3800/30546): 0.00135, p < 0.001). There were three significant canonical correlations between protective functional connections and behavioral measures (Wilks’ λ(df = 3800/30546): 0.00191, p < 0.001) after multiple comparison correction. Post-hoc analyses using one-way ANOVAs confirmed the presence of significant group effects on behavioral variate scores (all p’s < 0.003), demonstrating the validity of these canonical correlations in representing risk. Similar one-way ANOVAs on behavior variate scores for resilience indicated significant group effects consistent with resilience for the second (p < 0.001) and third (p < 0.001) behavioral variates; there were no significant group differences in the first behavioral variate (p = 0.29). Thus, only canonical variate pairs #2 and #3 were interpreted as resilience phenotypes. Canonical correlations for these variate pairs are in Table 2. The canonical variates produced in half of the sample generally replicated in the other half of the sample with moderate-to-strong correlations (Table 2), demonstrating stability of the results.
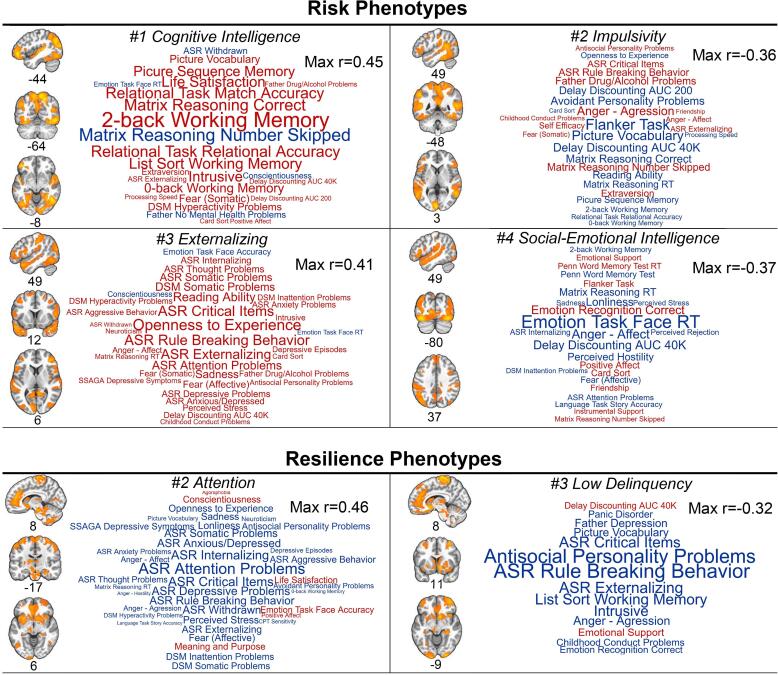
Fig. 1 presents brain maps of the regions involved in the functional connections with the strongest direct influences on each brain canonical variate based on ridge regression analyses. Additionally, word clouds depict behavioral variables that correlated with each behavioral variate with a strength of at least |r| > 0.15 (Fig. 1). We also separately visualized all brain functional connections correlating with each of the brain canonical variates with a strength of at least |r| > 0.2 based on simple Pearson correlations (Supplementary Figure 1).
Fig. 1.
Summary of brain-behavior phenotypes of risk and resilience for alcohol use disorder based on canonical correlation analyses. Functional connectivity data for risk phenotypes (left) and resilience phenotypes (right) were mapped onto brain anatomical images to depict regions for which their functional connections were most strongly associated (averaging across all of the node’s connections) with the brain canonical variate. For these images, we used a regularized linear regression, covarying for all other functional connections, to emphasize regions with direct contributions to the brain variate. Only voxels exceeding a z-score threshold of 1.0 are displayed. Coordinates are in Montreal Neurological Institute (MNI) space. Word clouds were created to display the behavioral variables that correlated with the corresponding behavioral variate. Behavioral variables with a correlation strength of at least |r| > 0.15 are shown, where font size is linearly related to correlation strength and the maximum |r| is indicated. Red indicates the behavior positively correlated with the behavioral variate whereas blue color indicates negative correlations. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2. Relationship between risk or resilience phenotypes and AUD
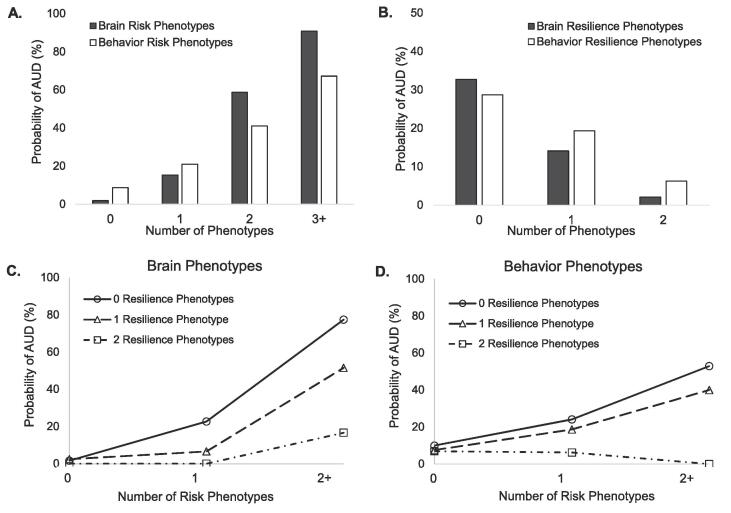
Compared with individuals with no risk phenotypes, individuals with one or more risk phenotypes exhibited an increased probability of meeting AUD criteria in a graded positive relationship (Fig. 2A, Supplementary Table 3), which was observed for both brain and behavior risk scores, although brain relationships were stronger. The number of resilience phenotypes was associated with a graded decrease in probability of AUD. Again, these relationships were observed for both brain and behavior resilience scores (Fig. 2B, Supplementary Table 3), with stronger statistical relationships for brain scores. Independent effects of individual phenotypes are also provided (Supplementary Table 4).
Fig. 2.
Effect of the number of risk or resilience phenotypes and their interaction on probability of AUD. The prevalence of AUD among subjects having A) 0, 1, 2, or 3 + risk brain or behavior phenotypes and B) 0, 1, or 2 resilience brain or behavior phenotypes is plotted. The prevalence of AUD among subjects having 0, 1, or 2 + risk phenotypes are plotted separately according to the number of resilience phenotypes for C) brain phenotypes and D) behavior phenotypes.
Consistent with an ability of resilience to moderate risk, there was a significant interaction of polyphenotypic risk and resilience scores for both brain (Wald Χ2(5) = 20.4, p = 0.001; Fig. 2C) and behavior (Wald Χ2(5) = 33.4, p < 0.001; Fig. 2D). These patterns were replicated using a median rather than upper-quartile cut-off (Supplementary Figure 2), although absolute AUD probabilities depended on the selected cut-off. The results indicated that subjects expressing more resilience phenotypes exhibited reduced effects of risk phenotypes on probability of AUD.
Finally, logistic regressions indicated specific brain and behavioral resilience phenotypes may be particularly effective at moderating specific risk phenotypes (Table 3). Again, brain phenotypes demonstrated stronger effects than behavior phenotypes.
Table 3.
Results of logistic regression analyses testing interactions between risk and resilience phenotypes on probability of alcohol use disorder.
| Brain Phenotypes | #2 Attention | #3 Low Delinquency |
|---|---|---|
| #1 Cognitive Intelligence | CON = −1.28 CI:(−1.99, −0.57) p < 0.001 | CON = −1.04 CI:(−1.81, −0.27) p = 0.008 |
| #2 Impulsivity | CON = −1.26 CI:(−1.95, −0.57) p < 0.001 | CON = −0.67 CI:(−1.41, 0.06) p = 0.071 |
| #3 Externalizing | CON = −0.52 CI:(−1.50, 0.57) p = 0.30 | CON = −1.29 CI:(−1.96, −0.62) p < 0.001 |
| #4 Social−Emotional Intelligence | CON = −1.19 CI:(−1.86, −0.51) p < 0.001 | CON = −0.99 CI:(−2.01, 0.03) p = 0.056 |
| Behavior Phenotypes | #2 Attention | #3 Low Delinquency |
| #1 Cognitive Intelligence | CON = −1.24 CI:(−2.00, −0.48) p = 0.001 | CON = −0.59 CI:(−1.33, 0.14) p = 0.11 |
| #2 Impulsivity | CON = −0.73 CI:(−1.46, −0.01) p = 0.048 | CON = −0.13 CI:(−0.94, 0.67) p = 0.74 |
| #3 Externalizing | CON = −0.63 CI:(−2.22, 0.97) p = 0.44 | CON = −0.82 CI:(−1.47, −0.18) p = 0.012 |
| #4 Social-Emotional Intelligence | CON = −0.38 CI:(−1.02, 0.26) p = 0.25 | CON = −0.01 CI:(−0.90, 0.88) p = 0.98 |
Interaction of pairwise risk and resilience phenotypes were tested in logistic regression analyses. Risk phenotypes are in rows and resilience phenotypes are in columns, and results of pairwise interactions are presented. CON values represent estimates of the contrast of the interaction term when Risk = 1 and Resilience = 1 versus Risk = 1 and Resilience = 0. CI, confidence interval.
4. Discussion
We undertook an innovative data-driven analysis to characterize brain-derived risk and resilience phenotypes for AUD, a leading cause of morbidity and mortality. A CCA identified multiple brain-behavior correlations associated with either risk or resilience, consistent with the diverse etiology of AUD. Brain phenotypes underlying risk versus resilience implicated largely distinct sets of brain networks, with frontoparietal networks associating with risk, and subcortical, limbic, and paralimbic networks relating to resilience. Furthermore, whereas the total number of risk or resilience phenotypes, as well as their interaction, predicted the probability of AUD, we also found evidence that specific resilience phenotypes may counteract specific risk phenotypes.
4.1. Risk phenotypes
We identified five orthogonal brain-behavior relationships related to AUD risk based on their association with individuals with AUD and, to an intermediate extent, their unaffected siblings, i.e., intermediate phenotypes. Notably, networks of executive control regions, including the dorsomedial and dorsolateral prefrontal cortex, precuneus, and inferior parietal cortex, were most strongly associated with AUD risk, suggesting a major role for brain regions outside of the mesolimbic dopamine system in AUD risk. The behaviors that most strongly correlated with these connections included diverse cognitive, affective, and personality measures.
Intriguingly, the first (strongest correlation) variate pair indicated that greater scores on certain measures of intelligence and cognition, such as matrix reasoning and working memory, were associated with risk for AUD. Executive function deficits in AUD primarily implicate impaired cognitive flexibility and inhibitory control (Day et al., 2015), but not intelligence (Bailey et al., 2020, Johnson et al., 2009, Maggs et al., 2008) (but see also (Müller et al., 2013)) or working memory (Hildebrandt et al., 2004, Tapert et al., 2004, van der Plas et al., 2009), except in reports that may be confounded by educational attainment (Rosoff et al., 2019), alcohol-related Korsakoff syndrome (Hildebrandt et al., 2004), and/or polysubstance use (Crone et al., 2006, Fernández-Serrano et al., 2010). We propose that Risk Phenotype #1 relates to a lack of cognitive flexibility. Specifically, whereas cognitive rigidity offers an advantage for tasks requiring a consistent application of established rules, the trade-off is an increased reliance on stimulus-directed behaviors (Cools and Robbins, 2004, Cools et al., 2007) and thus a vulnerability to addiction (Everitt and Robbins, 2016, McKim et al., 2016).
Consistent with prior evidence of poor behavioral control among individuals with AUD and their family members (Coskunpinar et al., 2013; DM et al., 2010), the strongest behavioral correlate for Risk Phenotype #2 was poor flanker task performance. Similarly, indices of delay discounting, a measure of impulsive choice, was also strongly correlated, as was anger/aggressive behavior, which similarly relates to inhibition deficits and impulsivity (Vigil-Colet and Codorniu-Raga, 2004). These relationships are consistent with findings that inhibitory control deficits precede substance use (Nigg et al., 2006, Tarter et al., 2004, Tarter et al., 2003), and represent a major risk pathway for addiction.
We further identified a risk phenotype (#3) consistent with a “deviance proneness” (Ohannessian and Hesselbrock, 2008, Sher, 1991) or externalizing pathway (Edwards et al., 2016, Hussong et al., 1998) of substance use/AUD, that was independent of the impulsivity phenotype, despite known relationships between impulsive and externalizing behaviors (Martel et al., 2017). Rather, the externalizing phenotype moderately correlated with somatic and internalizing symptoms, suggesting a common brain mechanism underlies these closely-intertwined behavioral syndromes as they relate to AUD.
Risk Phenotype #4 was designated a social-emotional intelligence risk phenotype, as it related to better fMRI emotion task performance, measures of emotional health, and FC in brain regions important for social cognition such as superior temporal sulcus, temporoparietal junction, inferior frontal gyrus, and fusiform gyrus (Wolf et al., 2010). This risk phenotype may capture enhanced problem drinking among young people who are more socially engaged (Martins et al., 2017, Power et al., 2005). Indeed, social motives are a particularly strong risk factor for AUD for individuals with a positive family history of alcohol problems (Vaughan et al., 2009) and among younger individuals more generally (Corbin et al., 2011).
4.2. Resilience phenotypes
There is a paucity of published research on brain markers of resilience in AUD, much less a comprehensive analysis. In the current analysis, brain connections related to resilience were focused in the brain stem, cerebellum, medial prefrontal cortex, striatum, insula, and medial temporal lobe. These regions overlapped closely with regions involved in resilience to trauma and adversity (Bolsinger et al., 2018, Holz et al., 2020), indicating a potential shared neurobiology of resilience to heritable risk and resilience to stressors.
Resilience phenotype #2 correlated with fewer attention problems and, to a lesser extent, fewer internalizing symptoms, extending previous links between attention and resilience to psychopathology (Lee et al., 2019, Peng et al., 2012, Shi et al., 2018). Furthermore, treating symptoms of attention-deficit/hyperactivity disorder (ADHD) in childhood and adolescence lowers the risk for AUD associated with this developmental disorder (Mannuzza et al., 2008, Wilens, 2003). In fact, a link between attention ability in FH and resilience to college binge drinking has recently been demonstrated (Elton et al., 2021). This phenotype was associated with the greatest reductions in AUD risk both independently (Supplementary Table 4) and in interactions (Fig. 2). Taken together, these findings suggest that interventions targeting attention in individuals with familial risk for AUD, especially those with attention problems, may effectively promote resilience among these individuals.
This brain-behavior resilience pathway was associated with reduced antisocial personality and rule breaking. The brain results mirrored prior work identifying antisocial personality disorder effects on FC between the cerebellum and superior parietal regions (Tang et al., 2013). Antisocial personality is an important predictor of alcohol use problems above and beyond the risk conferred by FH (Hesselbrock and Hesselbrock, 1992). Antisocial behavior and conduct problems in childhood and adolescence are have both environmental (Basto-Pereira et al., 2016) and genetic (Rosenström et al., 2018) components. In fact, a genetic polymorphism and epigenetic regulation of the MAOA gene, for which high-activity variants protect against antisocial behavior (Kolla and Vinette, 2017), confers resilience against the increased risk for alcohol use problems among males with early life adversity (Nilsson et al., 2011, Bendre et al., 2018). Such mechanisms may represent important targets for promoting resilience to AUD among certain at-risk groups. Also, whereas Risk Phenotype #3 implicated increased rule breaking in AUD risk, Resilience Phenotype #3 strongly associated with reduced rule breaking and significantly moderated Risk Phenotype #3 (Fig. 2).
4.3. Combined effects of phenotypes
Similar to combining multiple risk genotypes in polygenic risk scores for AUD (Clarke et al., 2016, Taylor et al., 2016), our results suggest the potential utility of polyphenotypic scores. Previous work has demonstrated that prediction of AUD is improved when combining two independent risk phenotypes (Lange et al., 2010). We expand upon that work to demonstrate that risk phenotypes exhibit a dose-dependent relationship with AUD. In fact, a polyphenotypic brain risk score of 3+ using an upper quartile cut-off is associated with greater than 90% probability of having AUD (Fig. 2, Supplementary Table 3). In general, brain risk phenotypes provided more robust relationships than behavioral phenotypes, supporting the utility of neuroimaging markers in psychiatric illness (Aydin et al., 2019, Etkin, 2019). However, even a single risk behavior phenotype nearly tripled (OR = 2.8, Supplementary Table 3) the odds of having AUD, although effects of each independent risk phenotype varied (Supplementary Table 4). Resilience phenotypes also demonstrated dose-dependent (negative) relationships with AUD.
In this study, interaction analyses demonstrated that resilience phenotypes moderated the influence of risk phenotypes. These interactions are consistent with a “risk-protective” model in which resilience processes dampen effects of risk (Garmezy et al., 1984, Rutter, 1985); however, we also provide evidence that a greater number of protective phenotypes have a greater influence on reducing risk (Brook et al., 1990). The data further suggested that these interactions might be partly driven by specific risk-resilience phenotype pairs (Fig. 2), suggesting the potential utility of personalized intervention strategies dependent upon an individual’s profile of neurocognitive risk phenotypes.
4.4. Limitations
Although the inclusion of both affected (i.e., AUD) and unaffected (i.e., FH) siblings is a study strength, FH individuals generally drink an intermediate amount relative to CON and AUD, which obscures cause and effect relationships with alcohol use. Brain or behavioral alterations among FH may represent risk factors, alcohol toxicity effects (including in utero), protective factors, or compensatory changes. Additionally, the inclusion of different measures, including other known AUD risk phenotypes, such as sweet liking or alcohol sensitivity may have produced different canonical correlations. Similarly, this analysis focuses on behavior and personality phenotypes of AUD, but the inclusion of sensory, motor, and physiological and sleep quality measures that were excluded could have informed. In addition, the cross-sectional design does not allow for a clear test of the validity of the identified “risk” and “resilience” phenotypes. However, these hypothesis-generating results should be examined further in the context of a longitudinal study design. Furthermore, these risk and resilience phenotypes may play differing roles across the lifespan, and thus may not translate to older or younger populations. Finally, the groups sizes were relatively small, which may have resulted in less reliable findings.
4.5. Conclusions
This analysis revealed both intermediate phenotypes of AUD risk and adaptive neurocognitive mechanisms of resilience. These results have the potential to be extended to develop innovative brain-informed interventions for prevention or treatment of AUD. Furthermore, the combination of phenotypes could inform multidimensional typologies of risk within this heterogeneous disorder, leading to more effective, personalized treatment and early intervention strategies.
Acknowledgments
Acknowledgements
This work was supported by NIH awards K01AA026334 (AE) and P60AA011605 (CAB). The authors would like to thank Rhea Parikh and J. Hunter Allen for their assistance.
Declaration of Competing Interest
AE, JCG, and CAB declare no competing financial interests.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2021.102801.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Agrawal A., Lynskey M.T. Are there genetic influences on addiction: evidence from family, adoption and twin studies. Addiction. 2008;103(7):1069–1081. doi: 10.1111/j.1360-0443.2008.02213.x. [DOI] [PubMed] [Google Scholar]
- Anda R.F., Whitfield C.L., Felitti V.J., Chapman D., Edwards V.J., Dube S.R., Williamson D.F. Adverse childhood experiences, alcoholic parents, and later risk of alcoholism and depression. Psychiatr Serv. 2002;53(8):1001–1009. doi: 10.1176/appi.ps.53.8.1001. [DOI] [PubMed] [Google Scholar]
- Aydin O., Unal A.P., Arslan A. Development of neuroimaging-based biomarkers in psychiatry. Adv. Exp. Med. Biol. 2019;1192 doi: 10.1007/978-981-32-9721-0_9. [DOI] [PubMed] [Google Scholar]
- Bailey, A., Gerst, K., Finn, P., 2020. Intelligence moderates the relationship between delay discounting rate and problematic alcohol use. Psychology of addictive behaviors : journal of the Society of Psychologists in Addictive Behaviors 34. [DOI] [PMC free article] [PubMed]
- Basto-Pereira, M., Miranda, A., Ribeiro, S., Maia, A., 2016. Growing up with adversity: From juvenile justice involvement to criminal persistence and psychosocial problems in young adulthood. Child abuse & neglect 62. [DOI] [PubMed]
- Bendre M., Comasco E., Checknita D., Tiihonen J., Hodgins S., Nilsson K.W. Associations Between MAOA-uVNTR Genotype, Maltreatment, MAOA Methylation, and Alcohol Consumption in Young Adult Males. Alcohol. Clin. Exp. Res. 2018;42(3):508–519. doi: 10.1111/acer.13578. [DOI] [PubMed] [Google Scholar]
- Bolsinger, J., Seifritz, E., Kleim, B., Manoliu, A., 2018. Neuroimaging Correlates of Resilience to Traumatic Events-A Comprehensive Review. Frontiers in psychiatry 9. [DOI] [PMC free article] [PubMed]
- Brook, J., Brook, D., Gordon, A., Whiteman, M., Cohen, P., 1990. The psychosocial etiology of adolescent drug use: a family interactional approach. Genetic, social, and general psychology monographs 116. [PubMed]
- Clarke T.-K., Smith A.H., Gelernter J., Kranzler H.R., Farrer L.A., Hall L.S., Fernandez-Pujals A.M., MacIntyre D.J., Smith B.H., Hocking L.J., Padmanabhan S., Hayward C., Thomson P.A., Porteous D.J., Deary I.J., McIntosh A.M. Polygenic risk for alcohol dependence associates with alcohol consumption, cognitive function and social deprivation in a population-based cohort. Addict. Biol. 2016;21(2):469–480. doi: 10.1111/adb.12245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools, R., Sheridan, M., Jacobs, E., D'Esposito, M., 2007. Impulsive personality predicts dopamine-dependent changes in frontostriatal activity during component processes of working memory. The Journal of neuroscience : the official journal of the Society for Neuroscience 27. [DOI] [PMC free article] [PubMed]
- Cools R., Robbins T.W. Chemistry of the adaptive mind. Philos. Transact. A Math. Phys. Eng. Sci. 2004;362(1825):2871–2888. doi: 10.1098/rsta.2004.1468. [DOI] [PubMed] [Google Scholar]
- Corbin, W., Iwamoto, D., Fromme, K., 2011. Broad social motives, alcohol use, and related problems: Mechanisms of risk from high school through college. Addictive behaviors 36. [DOI] [PMC free article] [PubMed]
- Ayca Coskunpinar, Allyson L. Dir, Melissa A. Cyders, 2013. Multidimensionality in impulsivity and alcohol use: a meta-analysis using the UPPS model of impulsivity. Alcoholism, clinical and experimental research 37. [DOI] [PMC free article] [PubMed]
- Crone, E.A., Wendelken, C., Donohue, S.E., Bunge, S.A., 2006. Neural evidence for dissociable components of task-switching. Cereb Cortex 16, 475-486. [DOI] [PubMed]
- Cservenka A., Casimo K., Fair D.A., Nagel B.J. Resting state functional connectivity of the nucleus accumbens in youth with a family history of alcoholism. Psychiatry Res. 2014;221(3):210–219. doi: 10.1016/j.pscychresns.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A., Fair D.A., Nagel B.J. Emotional processing and brain activity in youth at high risk for alcoholism. Alcohol. Clin. Exp. Res. 2014;38(7):1912–1923. doi: 10.1111/acer.12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A., Alarcón G., Jones S.A., Nagel B.J. Advances in human neuroconnectivity research: applications for understanding familial history risk for alcoholism. Alcohol Res. 2015:89–95. [PMC free article] [PubMed] [Google Scholar]
- Day, A., Kahler, C., Ahern, D., Clark, U., 2015. Executive Functioning in Alcohol Use Studies: A Brief Review of Findings and Challenges in Assessment. Current drug abuse reviews 8. [DOI] [PMC free article] [PubMed]
- Dick, D., Smith, G., Olausson, P., Mitchell, S., Leeman, R., O'Malley, S., Sher, K., 2010. Understanding the construct of impulsivity and its relationship to alcohol use disorders. Addiction biology 15. [DOI] [PMC free article] [PubMed]
- Doucet, G., Bassett, D., Yao, N., Glahn, D., Frangou, S., 2017. The Role of Intrinsic Brain Functional Connectivity in Vulnerability and Resilience to Bipolar Disorder. The American journal of psychiatry 174. [DOI] [PMC free article] [PubMed]
- Ducci, F., Goldman, D., 2008. Genetic approaches to addiction: genes and alcohol. Addiction (Abingdon, England) 103. [DOI] [PMC free article] [PubMed]
- Edwards, A., Gardner, C., Hickman, M., Kendler, K., 2016. A prospective longitudinal model predicting early adult alcohol problems: evidence for a robust externalizing pathway. Psychological medicine 46. [DOI] [PMC free article] [PubMed]
- Elton, A., Allen, J.H., Yorke, M., Khan, F., Lin, Q., Boettiger, C.A., 2021. High Trait Attention Promotes Resilience and Reduces Binge Drinking Among College Students With a Family History of Alcohol Use Disorder. Frontiers in psychiatry 12, 672863-672863. [DOI] [PMC free article] [PubMed]
- Ersche K.D., Meng C., Ziauddeen H., Stochl J., Williams G.B., Bullmore E.T., Robbins T.W. Brain networks underlying vulnerability and resilience to drug addiction. PNAS. 2020;117(26):15253–15261. doi: 10.1073/pnas.2002509117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin, A., 2019. A Reckoning and Research Agenda for Neuroimaging in Psychiatry. The American journal of psychiatry 176. [DOI] [PubMed]
- Everitt, B., Robbins, T., 2016. Drug Addiction: Updating Actions to Habits to Compulsions Ten Years On. Annual review of psychology 67. [DOI] [PubMed]
- Fernández-Serrano M.J., Pérez-García M., Schmidt Río-Valle J., Verdejo-García A. Neuropsychological consequences of alcohol and drug abuse on different components of executive functions. J. Psychopharmacol. (Oxford, England) 2010;24(9):1317–1332. doi: 10.1177/0269881109349841. [DOI] [PubMed] [Google Scholar]
- Garavan H., Albaugh M. Connecting with resilience. Biol. Psychiatry. 2019;85(8):621–622. doi: 10.1016/j.biopsych.2019.02.004. [DOI] [PubMed] [Google Scholar]
- Garmezy, N., Masten, A., Tellegen, A., 1984. The study of stress and competence in children: a building block for developmental psychopathology. Child development 55. [PubMed]
- Herting M.M., Fair D., Nagel B.J. Altered fronto-cerebellar connectivity in alcohol-naive youth with a family history of alcoholism. Neuroimage. 2011;54:2582–2589. doi: 10.1016/j.neuroimage.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselbrock M.N., Hesselbrock V.M. Relationship of family history, antisocial personality disorder and personality traits in young men at risk for alcoholism. J. Stud. Alcohol. 1992:53. doi: 10.15288/jsa.1992.53.619. [DOI] [PubMed] [Google Scholar]
- Hildebrandt H., Brokate B., Eling P., Lanz M. Response shifting and inhibition, but not working memory, are impaired after long-term heavy alcohol consumption. Neuropsychology. 2004;18(2):203–211. doi: 10.1037/0894-4105.18.2.203. [DOI] [PubMed] [Google Scholar]
- Holz, N., Tost, H., Meyer-Lindenberg, A., 2020. Resilience and the brain: a key role for regulatory circuits linked to social stress and support. Molecular psychiatry 25. [DOI] [PubMed]
- https://www.humanconnectome.org/study/hcp-young-adult, 2017. HCP1200 July 2017 release of high-level rfMRI connectivity analyses.
- Hussong A., Curran P., Chassin L. Pathways of risk for accelerated heavy alcohol use among adolescent children of alcoholic parents. J. Abnormal Child Psychol. 1998:26. doi: 10.1023/a:1022699701996. [DOI] [PubMed] [Google Scholar]
- Johnson W., Hicks B.M., McGue M., Iacono W.G. How intelligence and education contribute to substance use: hints from the Minnesota twin family study. Intelligence. 2009;37 doi: 10.1016/j.intell.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler K.S., Aggen S.H., Prescott C.A., Crabbe J., Neale M.C. Evidence for multiple genetic factors underlying the DSM-IV criteria for alcohol dependence. Mol. Psychiatry. 2012;17:1306–1315. doi: 10.1038/mp.2011.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler K.S., Sundquist K., Ohlsson H., Palmér K., Maes H., Winkleby M.A., Sundquist J. Genetic and familial environmental influences on the risk for drug abuse: a national Swedish adoption study. Arch. Gen. Psychiatry. 2012;69(7):690–697. doi: 10.1001/archgenpsychiatry.2011.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Spoon J., Deater-Deckard K., Holmes C., Lee J., Chiu P., King-Casas B. Behavioral and neural inhibitory control moderates the effects of reward sensitivity on adolescent substance use. Neuropsychologia. 2016;91:318–326. doi: 10.1016/j.neuropsychologia.2016.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolla, N.J., Vinette, S.A., 2017. Monoamine Oxidase A in Antisocial Personality Disorder and Borderline Personality Disorder. Current behavioral neuroscience reports 4. [DOI] [PMC free article] [PubMed]
- Lange L.A., Kampov-Polevoy A.B., Garbutt J.C. Sweet liking and high novelty seeking: independent phenotypes associated with alcohol-related problems. Alcohol Alcohol. 2010;45(5):431–436. doi: 10.1093/alcalc/agq040. [DOI] [PubMed] [Google Scholar]
- Lee, D., Lee, S., Park, C., Kim, B., Lee, C., Cha, B., Seo, J., Choi, J., 2019. The Mediating Effect of Impulsivity on Resilience and Depressive Symptoms In Korean Conscripts. Psychiatry investigation 16. [DOI] [PMC free article] [PubMed]
- Luthar, S., Sawyer, J., Brown, P., 2006. Conceptual issues in studies of resilience: past, present, and future research. Annals of the New York Academy of Sciences 1094. [DOI] [PMC free article] [PubMed]
- Maggs J.L., Patrick M.E., Feinstein L. Childhood and adolescent predictors of alcohol use and problems in adolescence and adulthood in the National Child Development Study. Addiction (Abingdon, England) 2008;103(s1):7–22. doi: 10.1111/j.1360-0443.2008.02173.x. [DOI] [PubMed] [Google Scholar]
- Mannuzza, S., Klein, R.G., Truong, N.L., Moulton, J.L., 3rd, Roizen, E.R., Howell, K.H., Castellanos, F.X., 2008. Age of methylphenidate treatment initiation in children with ADHD and later substance abuse: prospective follow-up into adulthood. The American journal of psychiatry 165. [DOI] [PMC free article] [PubMed]
- Martel, M.M., Levinson, C.A., Lee, C.A., Smith, T.E., 2017, Impulsivity Symptoms as Core to the Developmental Externalizing Spectrum. Journal of abnormal child psychology 45. [DOI] [PMC free article] [PubMed]
- Martins J.G., de Paiva H.N., Paiva P.C.P., Ferreira R.C., Pordeus I.A., Zarzar P.M., Kawachi I., Ryabinin A.E. New evidence about the “dark side” of social cohesion in promoting binge drinking among adolescents. PLoS ONE. 2017;12(6) doi: 10.1371/journal.pone.0178652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martz, M., Cope, L., Hardee, J., Brislin, S., Weigard, A., Zucker, R., Heitzeg, M., 2019. Frontostriatal Resting State Functional Connectivity in Resilient and Non-Resilient Adolescents with a Family History of Alcohol Use Disorder. Journal of child and adolescent psychopharmacology 29. [DOI] [PMC free article] [PubMed]
- McKim, T., Bauer, D., Boettiger, C., 2016. Addiction History Associates with the Propensity to Form Habits. Journal of cognitive neuroscience 28. [DOI] [PMC free article] [PubMed]
- ME, M., LM, C., JE, H., SJ, B., A, W., RA, Z., MM, H., 2019. Frontostriatal Resting State Functional Connectivity in Resilient and Non-Resilient Adolescents with a Family History of Alcohol Use Disorder. Journal of child and adolescent psychopharmacology 29. [DOI] [PMC free article] [PubMed]
- Mitchell, S., 2011. The genetic basis of delay discounting and its genetic relationship to alcohol dependence. Behavioural processes 87. [DOI] [PMC free article] [PubMed]
- Morean M.E., Corbin W.R. Subjective response to alcohol: a critical review of the literature. Alcohol. Clin. Exp. Res. 2010;34:385–395. doi: 10.1111/j.1530-0277.2009.01103.x. [DOI] [PubMed] [Google Scholar]
- Müller M., Kowalewski R., Metzler S., Stettbacher A., Rössler W., Vetter S. Associations between IQ and alcohol consumption in a population of young males: a large database analysis. Social Psychiatry Psychiatric Epidemiol. 2013:48. doi: 10.1007/s00127-013-0666-2. [DOI] [PubMed] [Google Scholar]
- Nigg J.T., Wong M.M., Martel M.M., Jester J.M., Puttler L.I., Glass J.M., Adams K.M., Fitzgerald H.E., Zucker R.A. Poor response inhibition as a predictor of problem drinking and illicit drug use in adolescents at risk for alcoholism and other substance use disorders. J. Am. Acad. Child Adolesc. Psychiatry. 2006;45:468–475. doi: 10.1097/01.chi.0000199028.76452.a9. [DOI] [PubMed] [Google Scholar]
- Nilsson, K.W., Comasco, E., Åslund, C., Nordquist, N., Leppert, J., Oreland, L., 2011, MAOA genotype, family relations and sexual abuse in relation to adolescent alcohol consumption. Addiction biology 16. [DOI] [PubMed]
- Ohannessian C., Hesselbrock V. A comparison of three vulnerability models for the onset of substance use in a high-risk sample. J. Stud. Alcohol drugs. 2008:69. doi: 10.15288/jsad.2008.69.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi, K., Anderson, C., Bolger, E., Khan, A., McGreenery, C., Teicher, M., 2019. Susceptibility or Resilience to Maltreatment Can Be Explained by Specific Differences in Brain Network Architecture. Biological psychiatry 85. [DOI] [PMC free article] [PubMed]
- Oreland, L., Lagravinese, G., Toffoletto, S., Nilsson, K., Harro, J., Robert Cloninger, C., Comasco, E., 2018. Personality as an intermediate phenotype for genetic dissection of alcohol use disorder. Journal of neural transmission (Vienna, Austria: 1996) 125. [DOI] [PMC free article] [PubMed]
- Peng L., Zhang J., Li M., Li P., Zhang Y., Zuo X., Miao Y., Xu Y. Negative life events and mental health of Chinese medical students: the effect of resilience, personality and social support. Psychiatry Res. 2012;196:138–141. doi: 10.1016/j.psychres.2011.12.006. [DOI] [PubMed] [Google Scholar]
- Power T., Stewart C., Hughes S., Arbona C. Predicting patterns of adolescent alcohol use: a longitudinal study. J. Stud. Alcohol. 2005:66. doi: 10.15288/jsa.2005.66.74. [DOI] [PubMed] [Google Scholar]
- Roosa, M., 2000. Some thoughts about resilience versus positive development, main effects versus interactions, and the value of resilience. Child development 71. [DOI] [PubMed]
- Tom Rosenström, Fartein Ask Torvik, Eivind Ystrom, Nikolai Olavi Czajkowski, Nathan A Gillespie, Steven H Aggen, Robert F Krueger, Kenneth S Kendler, Ted Reichborn-Kjennerud, 2018, Prediction of alcohol use disorder using personality disorder traits: a twin study Addiction (Abingdon, England) 113. [DOI] [PMC free article] [PubMed]
- Rosoff D., Clarke T., Adams M., McIntosh A., Davey Smith G., Jung J., Lohoff F. Educational attainment impacts drinking behaviors and risk for alcohol dependence: results from a two-sample Mendelian randomization study with ∼780,000 participants. Mol. Psychiatry. 2019 doi: 10.1038/s41380-019-0535-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M. Resilience in the face of adversity. Protective factors and resistance to psychiatric disorder. Br. J. Psychiatry. 1985;147 doi: 10.1192/bjp.147.6.598. [DOI] [PubMed] [Google Scholar]
- SAMHSA, 2019. Results from the 2018 National Survey on Drug Use and Health. United States.
- Sher K.J. University of Chicago Press; 1991. Children of Alcoholics: A Critical Appraisal of Theory and Research. [Google Scholar]
- Sher, K., Grekin, E., Williams, N., 2005. The development of alcohol use disorders. Annual review of clinical psychology 1. [DOI] [PubMed]
- Shi, M., Liu, L., Sun, X., Wang, L., 2018. Associations between symptoms of attention-deficit/ hyperactivity disorder and life satisfaction in medical students: the mediating effect of resilience. BMC medical education 18. [DOI] [PMC free article] [PubMed]
- Smith S., Nichols T., Vidaurre D., Winkler A., Behrens T., Glasser M., Ugurbil K., Barch D., Van Essen D., Miller K. A positive-negative mode of population covariation links brain connectivity, demographics and behavior. Nature Neurosci. 2015;18 doi: 10.1038/nn.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spadoni, A.D., Simmons, A.N., Yang, T.T., Tapert, S.F., 2013. Family history of alcohol use disorders and neuromaturation: a functional connectivity study with adolescents. The American journal of drug and alcohol abuse 39. [DOI] [PMC free article] [PubMed]
- Spadoni A., Simmons A., Yang T., Tapert S. Family history of alcohol use disorders and neuromaturation: a functional connectivity study with adolescents. Am. J. Drug Alcohol Abuse. 2013:39. doi: 10.3109/00952990.2013.818680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, Y., Jiang, W., Liao, J., Wang, W., Aijing, A., 2013. Identifying individuals with antisocial personality disorder using resting-state FMRI. PloS one 8 (4). [DOI] [PMC free article] [PubMed]
- Tapert S., Schweinsburg A., Barlett V., Brown S., Frank L., Brown G., Meloy M. Blood oxygen level dependent response and spatial working memory in adolescents with alcohol use disorders. Alcoholism, Clin. Exp. Res. 2004;28 doi: 10.1097/01.alc.0000141812.81234.a6. [DOI] [PubMed] [Google Scholar]
- Tarter R.E., Kirisci L., Mezzich A., Cornelius J.R., Pajer K., Vanyukov M., Gardner W., Blackson T., Clark D. Neurobehavioral disinhibition in childhood predicts early age at onset of substance use disorder. Am. J. Psychiatry. 2003;160:1078–1085. doi: 10.1176/appi.ajp.160.6.1078. [DOI] [PubMed] [Google Scholar]
- Tarter R.E., Kirisci L., Habeych M., Reynolds M., Vanyukov M. Neurobehavior disinhibition in childhood predisposes boys to substance use disorder by young adulthood: direct and mediated etiologic pathways. Drug Alcohol Depend. 2004;73:121–132. doi: 10.1016/j.drugalcdep.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Taylor M., Simpkin A.J., Haycock P.C., Dudbridge F., Zuccolo L. Exploration of a polygenic risk score for alcohol consumption: a longitudinal analysis from the ALSPAC Cohort. PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0167360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya J.G., Elmore A.L., Wallace A.L., Langbehn D.R., Kramer J.R., Kuperman S., O’Leary D.S. Association between age and familial risk for alcoholism on functional connectivity in adolescence. J. Am. Acad. Child Adolescent Psychiatry. 2019;58 doi: 10.1016/j.jaac.2018.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya J., Elmore A., Wallace A., Langbehn D., Kramer J., Kuperman S., O'Leary D. Association between age and familial risk for alcoholism on functional connectivity in adolescence. J. Am. Acad. Child Adolesc. Psychiatry. 2019;58 doi: 10.1016/j.jaac.2018.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Plas E., Crone E., van den Wildenberg W., Tranel D., Bechara A. Executive control deficits in substance-dependent individuals: a comparison of alcohol, cocaine, and methamphetamine and of men and women. J. Clin. Experimental Neuropsychol. 2009;31 doi: 10.1080/13803390802484797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen D.C., Smith S.M., Barch D.M., Behrens T.E., Yacoub E., Ugurbil K. The WU-Minn Human Connectome Project: an overview. NeuroImage. 2013;80:62–79. doi: 10.1016/j.neuroimage.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan, E., Corbin, W., Fromme, K., 2009. Academic and social motives and drinking behavior. Psychology of addictive behaviors : journal of the Society of Psychologists in Addictive Behaviors 23. [DOI] [PMC free article] [PubMed]
- Vigil-Colet, A., Codorniu-Raga, M.J., 2004. Aggression and inhibition deficits, the role of functional and dysfunctional impulsivity. 37, 1431-1440.
- Wilens, T.E., 2003. Does the medicating ADHD increase or decrease the risk for later substance abuse? Revista brasileira de psiquiatria (Sao Paulo, Brazil) 25 (3). [DOI] [PubMed]
- Wills T., Ainette M., Stoolmiller M., Gibbons F., Shinar O. Good self-control as a buffering agent for adolescent substance use: an investigation in early adolescence with time-varying covariates. Psychol. Addictive Behav. 2008:22. doi: 10.1037/a0012965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A, Wills, Michael G Ainette, Mike Stoolmiller, Frederick X Gibbons, Ori Shinar, 2008. Good self-control as a buffering agent for adolescent substance use: an investigation in early adolescence with time-varying covariates. Psychology of addictive behaviors : journal of the Society of Psychologists in Addictive Behaviors 22. [DOI] [PMC free article] [PubMed]
- Wolf I., Dziobek I., Heekeren H. Neural correlates of social cognition in naturalistic settings: a model-free analysis approach. NeuroImage. 2010;49 doi: 10.1016/j.neuroimage.2009.08.060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.