Abstract
The availability of vaccines against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), provides hope towards mitigation of the coronavirus disease 2019 (COVID-19) pandemic. Vaccine safety and efficacy has not been established in individuals with chronic autoimmune diseases such as multiple sclerosis (MS). Anecdotal reports suggest that the vaccines may be associated with brain, spinal cord, peripheral nervous system, and cardiac inflammation. Based on the high morbidity and unpredictable course of COVID-19, and the need to achieve herd immunity, vaccination has been recommended for patients with MS. We report clinical and MRI features of seven individuals who received the Moderna (n = 3) or Pfizer (n = 4) SARS-CoV-2 mRNA vaccines. Within one to 21 days of either the first (n = 2) or second (n = 5) vaccine dose, these patients developed neurologic symptoms and MRI findings consistent with active CNS demyelination of the optic nerve, brain, and/or spinal cord. Symptoms included visual loss, dysmetria, gait instability, paresthesias, sphincter disturbance, and limb weakness. Age ranged from 24 to 64 (mean 39.1) years; five were woman (71.4%). The final diagnosis was exacerbation of known stable MS (n = 4, two were receiving disease-modifying therapy at the time of vaccination), new onset MS (n = 2), or new onset neuromyelitis optica (n = 1). All responded to corticosteroid (n = 7) or plasma exchange (n = 1) therapy, with five returning to baseline and two approaching baseline. Large prospective studies are required to further investigate any possible relationship between COVID-19 vaccines and acute CNS demyelination.
Keywords: COVID-19, SAR-CoV-2, Vaccination, Multiple sclerosis, Neuromyelitis optica, Demyelination, Inflammation
Introduction
In late 2019, an outbreak due to a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) led to the coronavirus disease 2019 (COVID-19) global pandemic and claimed many lives, especially in patients with comorbidities [1]. Early reports suggested that individuals with multiple sclerosis (MS) had an increased risk for a worse outcome after contracting COVID-19, based on certain disease characteristics and treatments [2–4] while other studies disputed these claims [5–7]. A recent study suggested no significant effects on MS relapse rates after COVID-19 infection [8]. Nonetheless, it is well recognized that in people without previous neurologic diseases, COVID-19 infection can cause a wide range of neurological complications affecting both the central and peripheral nervous system [9–17], including cases of new onset demyelinating disease or MS following COVID-19 infection [18–20].
In late 2020, the first vaccines for preventing SARS-CoV-2 infection became available with their mechanisms of action based on introducing the inactivated spike protein viral antigen to the host [21, 22]. While the COVID-19 vaccines have been shown to be remarkably safe and effective [23], there are rare reports of presumed post-vaccination neurologic conditions such as Bell’s palsy [24–26], Guillain–Barre syndrome (GBS) [22, 27, 28], transverse myelitis [15, 29–32], and the first manifestation of MS [33], or MS relapse [34, 35]. Cases of cardiac inflammation have also been reported after COVID-19 vaccination [36–42]. The pivotal clinical trials conducted on the safety and efficacy of the four major COVID-19 vaccines [Pfizer-BioNTech (BNT162b2), Moderna (mRNA-1273), Johnson & Johnson/Janssen (JNJ-78436735) and Oxford-AstraZeneca (ChAdOx1-S/nCoV-19, AZD1222)] excluded patients with major immunologic or infectious co-morbidities, including MS [30, 43–45]. Therefore, it is largely unknown to what extend COVID-19 vaccines are safe and effective in the MS population.
Vaccination has been generally recommended for patients with MS, based on the high morbidity associated with COVID-19, the unpredictable course of the disease, and the need for widespread immunization to achieve herd immunity, [46–52]. However, considering the paucity of safety data, some COVID vaccine hesitancy persists among patients with MS [53–55]. A recent report on COVID-19 vaccine safety in 555 MS patients suggested no increase in post-vaccination relapse rate [56].
Here we report the clinical and MRI features of seven individuals who received SARS-CoV-2 mRNA vaccines and, within a few weeks of either the first or second dose, developed new neurologic symptoms and MRI findings consistent with active CNS demyelination. This was either new onset demyelinating disease (MS, n = 2 or neuromyelitis optica-NMO, n = 1) or exacerbation of known MS (n = 4). All patients responded to corticosteroids (n = 7) or other (n = 1) immunotherapy, with five returning to baseline and two with partial recovery.
Case reports
The cases are summarized in Table 1. None of these individuals had any history of COVID-19 infection. Cerebrospinal fluid (CSF) analysis, performed in Cases 2, 4, and 5, is shown in Table 2. A narrative of each case is provided below. In the Albany, NY and Boston, MA areas, COVID-19 vaccination was made available to the public on December 14 and 15, 2020, respectively.
Table 1.
Subject’s characteristics
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | |
|---|---|---|---|---|---|---|---|
| Pre-vaccine data | |||||||
| Age (years) | 35 | 26 | 24 | 64 | 33 | 44 | 48 |
| Sex | Woman | Woman | Woman | Man | Man | Woman | Woman |
| Known demyelinating disease | Yes | No | Yes | No | No | Yes | Yes |
| Type of demyelinating disease | RRMS | - | RRMS | - | - | RRMS | CIS |
| MS disease duration (years) | 11 | - | 9 | - | - | 24 | 8 |
| Time since last clinical relapse | 7 years | - | 2.2 years | - | - | 14 years | 8 years |
| EDSS score at baseline | 1.0 | 0 | 0 | 0 | 0 | 0 | 1.0 |
| Disease-modifying therapy | natalizumab | none | fingolimod | none | none | none | none |
| Post-vaccine data* | |||||||
| New brain MRI enhancing lesions | 1 | 1 | 2 | 0 | 1 | 1 | - |
| New spinal cord enhancing lesions | 0 | 1 | - | Longitudinal/ patchy | 0 | 0 | - |
| New brain T2 lesions | 1 | 9 | 10 | 6 (confluent) | 7 | 1 | 3 |
| New spinal cord T2 lesions | 0 | > 2 | - | longitudinally extensive | 1 | 0 | 0 |
| New neurologic symptoms | right arm, dysmetria, balance/gait | blurred vision and pain OD | vision changes and pain OD | pain, paresthesias, urinary retention, constipation, balance/gait | blurred vision OS | ascending numbness, right sided weakness | pain OD, Lhermitte’s, balance/gait |
| Treatment for neurologic event (number of days) | IVMP (5) | IVMP (5) | IVMP (3) | IVMP (3), PLEX (5) | IVMP (3) | IVMP (3) | oral MP (3) |
| Neurologic outcome after acute treatments | back to baseline | back to baseline | back to baseline | partial recovery | back to baseline | back to baseline | close to baseline |
| EDSS score after treatment | 1.0 | 0 | 0 | 3.5 | 0 | 0 | 1.5 |
| Time of symptom onset post vaccination | 21 days after 2nd dose | 14 days after 2nd dose | 1 day after 2nd dose | 18 days after 1st dose | 1 day after 2nd dose | 6 days after 2nd dose | 15 days after 1st dose |
| COVID-19 vaccine type | Moderna (mRNA-1273) | Moderna (mRNA-1273) | Pfizer-BioNTech (BNT162b2) | Pfizer-BioNTech (BNT162b2) | Pfizer-BioNTech (BNT162b2) | Moderna (mRNA-1273) | Pfizer-BioNTech (BNT162b2) |
| Final diagnosis of neurologic event post-vaccine | MS exacerbation | New onset RRMS | MS exacerbation | New onset NMO | New onset RRMS | MS exacerbation | Conversion from CIS to RRMS |
MS disease duration time since first symptoms; EDSS expanded disability status scale, OD right eye, OS left eye, IVMP intravenous methylprednisolone, 1000 mg per day, Oral MP oral methylprednisolone, 1000 mg per day, PLEX plasma exchange, RRMS relapsing–remitting multiple sclerosis, NMO neuromyelitis optica, CIS clinically isolated demyelinating syndrome
*For cases 2, 4, and 5: MRI lesions presumed to be new, as no baseline MRI was available
Table 2.
Cerebrospinal fluid results
| CSF findings | Case 2 | Case 4 | Case 5 | Reference range (%/units) |
|---|---|---|---|---|
| WBC count | 19 | 1 | 4 | 0–5 /uL |
| Lymphocytes (%) | 91 | 78 | 50 | 0–100% |
| Monocytes (%) | 5 | 19 | 9 | 0–100% |
| Neutrophils (%) | 0 | 2 | 41 | 0% |
| Glucose | 94 | 89 | 88 | 50–75 mg/dL |
| Total protein | 19 | 39 | 51 | 5–55 mg/dL |
| Oligoclonal bands | 0 | 0 | > 5 | 0 |
| CSF/serum IgG index | 1.27 | 0.68 | 5.3 | < = 0.85 |
| RBC | 7 | None | 133 | 0–5/uL |
| Serum glucose | 131 | 157 | 130 | 70–110 mg/dL |
CSF was not obtained in Cases 1, 3, 6, and 7
WBC white blood Cells, CSF cerebrospinal fluid, IgG immunoglobulin G, RBC red blood cells
Case 1
A 35-year-old Caucasian woman was initially diagnosed with clinically isolated demyelinating syndrome (CIS) 11 years ago after she developed a left optic neuritis and brain MRI lesions. She was placed on weekly intramuscular interferon beta-1a. Despite starting disease-modifying therapy, she developed new brain lesions 10 months later, and she was diagnosed as converted to relapsing–remitting multiple sclerosis (RRMS). She preferred to remain on the same therapy. She was stable for 3 years and then developed a symptomatic partial myelitis associated with an acute enhancing MRI lesion in the cervical spinal cord. Her symptoms resolved after 3 days of high dose (1000 mg daily) intravenous methylprednisolone (IVMP). Her disease-modifying therapy was switched to natalizumab which led to a long period of disease stability at an Expanded Disability Status Scale (EDSS) score [57] of 1.0 with no evidence of clinical or MRI disease activity over the next 7 years. She received the first dose of the Moderna COVID-19 vaccine on January 5, 2021, and the second dose 28 days later. The second dose led to mild systemic side effects lasting only for 1 day. Twenty-one days after the second dose, she developed new neurologic symptoms; neurologic examination showed ataxia/dysmetria in the right upper extremity, and mild gait ataxia, with an EDSS score of 2.5. She received 2 days of 1000 mg IVMP per day based on the assumption that this was an MS relapse. MRI showed a new T2 hyperintense lesion in the right cerebellum that enhanced with gadolinium (Fig. 1). There were no other MRI changes compared to pre-pandemic imaging (Fig. 1). Serum JC virus and natalizumab neutralizing antibodies were negative. She then received 3 more days of high dose IVMP with rapid improvement of neurologic symptoms. Follow up MRI, 30 days after symptom onset, showed nearly complete resolution of the cerebellar lesion gadolinium enhancement (Fig. 1). During this entire episode she remained on monthly natalizumab and was able to work full time, having missed only 1 day of work during this period. Follow-up examination, 62 days after symptom onset, showed return of EDSS score back to the 1.0 baseline. She reported that all new neurologic symptoms had fully resolved.
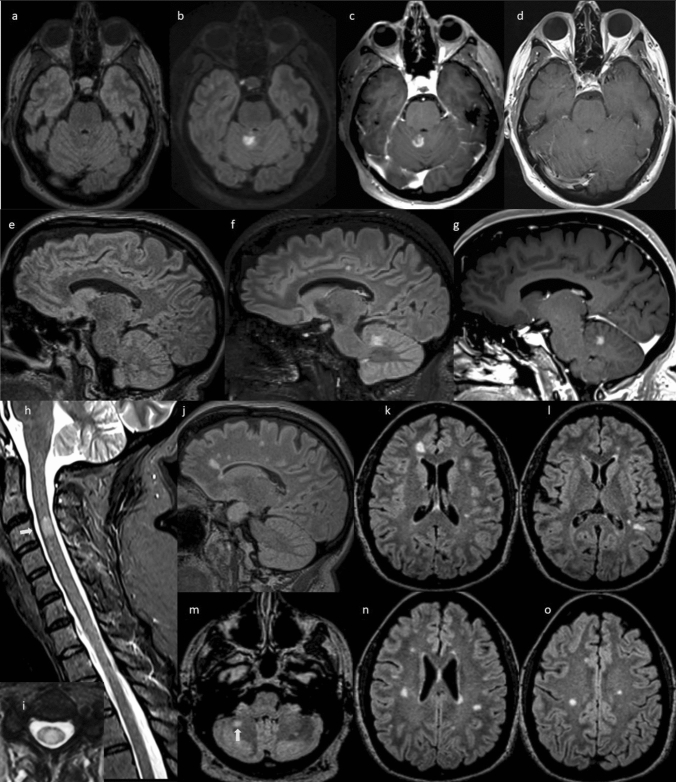
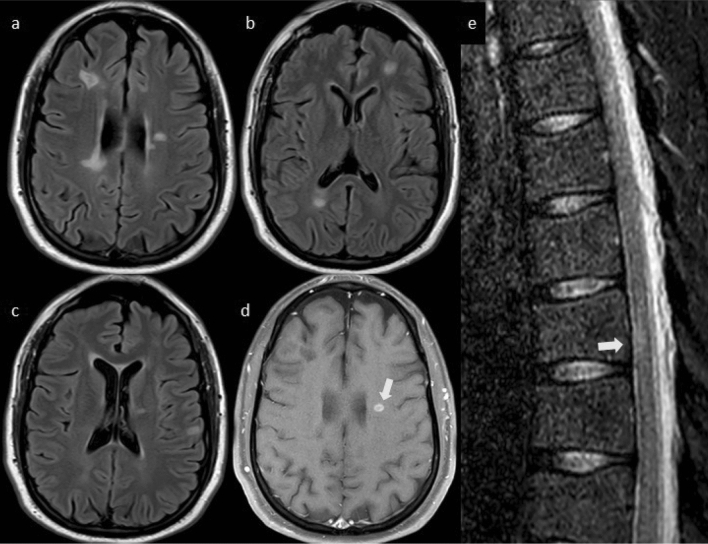
Fig. 1.
Serial 3 T MRI scans in case 1. Axial (a) and sagittal (e) T2 FLAIR MRI obtained 18 months prior to onset of new neurologic symptoms show two typical MS lesions in the corpus callosum, but no lesions in the cerebellum. Axial (b) and sagittal (f) T2 FLAIR and T1 post-gadolinium axial (c) and sagittal (g) MRI after COVID-19 vaccination and new neurologic symptom onset showing a new 1.5 × 1.4 × 1.9 cm T2 hyperintense lesion in the cerebellum with open-ring enhancement. Axial T1 post-contrast MRI (d) obtained 30 days after symptom onset, and after high dose intravenous methylprednisolone, shows nearly complete resolution of the gadolinium enhancement of the lesion. Her other MS lesions remained stable: sagittal STIR (h) and axial T2 (i) of the cervical spinal cord show a T2 hyperintense demyelinating lesion (white arrow). T2 FLAIR of the brain (j–o) show numerous typical MS lesions including one in the right cerebellum (white arrow)
Case 2
A 26-year-old white Hispanic woman presented with 2 days of new visual symptoms involving the right eye. Her symptoms began with mild blurring, which progressed over the next few days to worsening blurriness and pain with eye movement OD. Fourteen days prior to symptom onset, she had finished a full COVID-19 vaccination course. She received the first dose of the Moderna COVID-19 vaccine on December 27, 2020, and the second dose 29 days later without any significant immediate side effects after vaccination. She had a negative COVID-19 nasal PCR test on Jan 26, 2021, 1 day after the second vaccine dose. She had no significant past medical history. On examination, she had a relative afferent pupillary defect (RAPD), decreased visual acuity (20/50) and color desaturation OD. Visual field assessment showed a monocular central/inferior monocular deficit. MRI (Fig. 2) showed multiple T2 hyperintense periventricular, subcortical, posterior fossa, and spinal cord lesions with morphology suggesting MS. After gadolinium administration, two of the lesions enhanced (one each in the brain and spinal cord). Other inflammatory and infectious blood studies including antinuclear antibody, anti-neutrophil cytoplasmic antibody, and Lyme titer were unremarkable. Lumbar puncture performed 4 days after symptom onset (Table 2), showed an elevated IgG index (CSF/serum) at 1.27 (normal ≤ 0.85) and an elevated cell count. The CSF was otherwise normal on protein and glucose, without oligoclonal bands. She received 5 days of 1000 mg IVMP, 3 days after symptom onset, leading to gradual improvement of her symptoms. At follow-up visit, 14 days after symptom onset, she had returned to baseline and neurologic examination was normal. Given her clinical presentation and MRI findings, she was diagnosed with RRMS and started MS disease-modifying therapy. She showed a positive serum SARS-CoV-2 spike antibody, suggesting humoral immunity to the SARS-CoV-2 virus, when checked 8 months after completing COVID-19 vaccination (608.70 U/mL–negative < 0.80 U/mL).
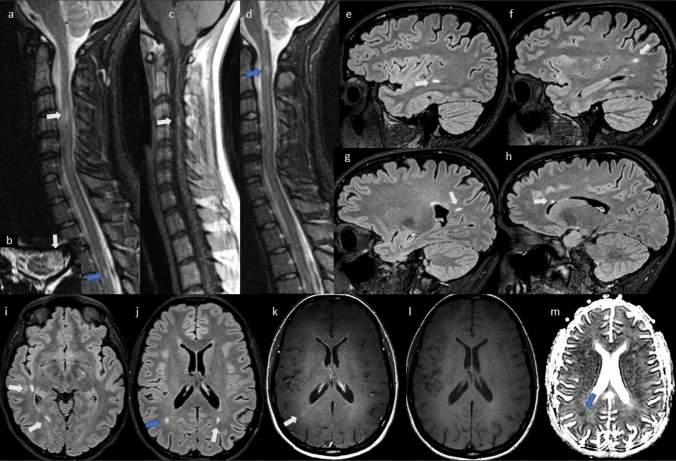
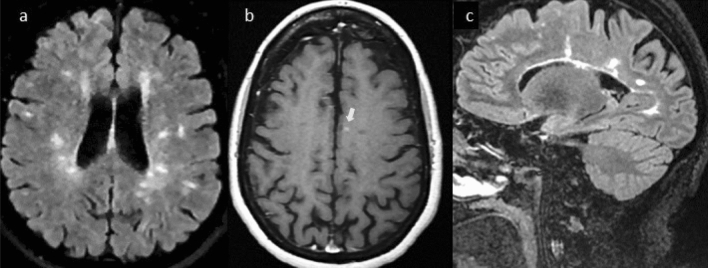
Fig. 2.
Serial 3 T MRI scans in case 2, obtained 2 days after symptom onset. Spinal cord sagittal STIR (a, d) and axial T2 (b) obtained 3 days after symptom onset, motion degraded, showing T2 hyperintense focal lesions suggestive of multiple sclerosis at C1-2 (d, blue arrow), C3-4 (a/b, white arrows), and T4-5 (a, blue arrow). After gadolinium administration, the spinal cord lesion at C4-5 enhanced on T1 images (c, white arrow). Brain sagittal (e–h) and axial (i, j) T2 FLAIR show multiple periventricular and subcortical lesions suggestive of multiple sclerosis (white arrows), with one of them likely active (j, blue arrow) because of enhancement on axial T1 post contrast (k, white arrow) compared to isointense appearance on T1 non contrast images (l). Consistent with the acuteness of the enhancing lesion, note the reduced signal (restricted diffusion) on the apparent diffusion coefficient map (m, blue arrow). No clear right optic nerve T2 abnormality or enhancement was detected (not shown)
Case 3
A 24-year-old Vietnamese woman presented with new onset left eye vision changes. She had a history of RRMS with symptom onset 9 years previously with sensory symptoms/paresthesias in the right face and upper extremity, followed by diplopia 5 years later. This second event led to an RRMS diagnosis. One year later, disease-modifying therapy with fingolimod was started. She had a stable disease course with no disability (EDSS 0), except for an episode of numbness in her left lower extremity with no new MRI lesions after 2 years of fingolimod treatment. This resolved after 3 days of IVMP. In the fourth year of fingolimod treatment, she had routine MRI that showed stable MS findings (Fig. 3). Sixty days later, she had received two doses of the Pfizer COVID-19 vaccine, the first dose on April 10, 2021, and the second dose 21 days later, without any systemic side effects. One day after the second dose, she developed new visual symptoms in the right eye with blurred vision and pain on eye movement. When examined 5 days after symptom onset, monocular decreased visual acuity (20/30), RAPD, and red desaturation were noted OD. There were no other neurologic symptoms or abnormalities on examination. MRI of the brain and orbit showed several new enhancing brain lesions without any optic MRI abnormalities (Fig. 3). She had a negative COVID-19 nasal PCR test on May 8, 2021, 7 days after completing vaccination. She was treated with 3 days of 1000 mg IVMP and her visual acuity improved to 20/25 OD. At follow-up, 11 days after discharge, she returned to baseline with a visual acuity of 20/20 bilaterally (EDSS 0). She showed a positive serum SARS-CoV- 2 spike antibody on May 20, 2021, (940.20 U/mL-negative < 0.80 U/mL) suggesting an adequate humoral immunity to the virus after vaccination.
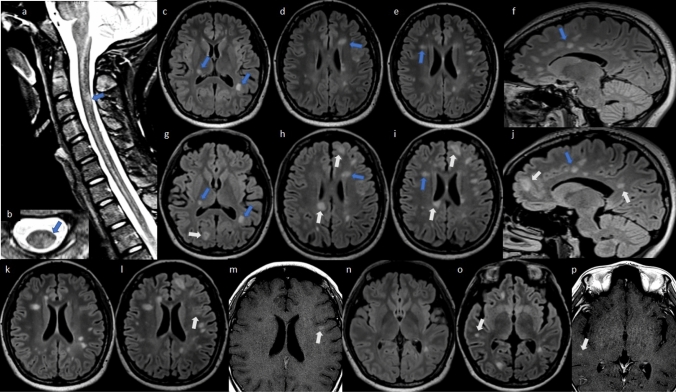
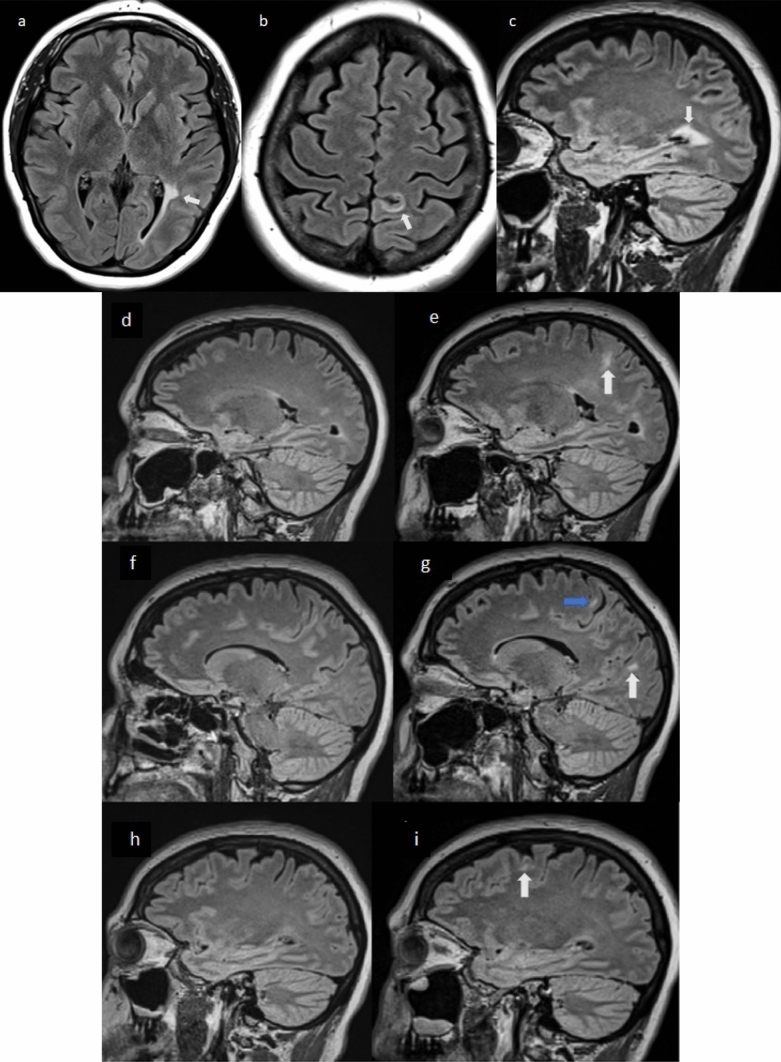
Fig. 3.
Serial 3 T MRI scans in case 3. Baseline scan: sagittal STIR (a) and axial T2 (b) of the cervical spinal cord and axial (c–e, k, n) and sagittal (f) T2 FLAIR MRI of the brain obtained 70 days prior to onset of new neurologic symptoms, showing multiple T2 hyperintense demyelinating lesions in the brain and one lesion in the spinal cord (blue arrows). Post-vaccination scan: axial (g–i, l, o) and sagittal (j) T2 FLAIR of the brain 6 days after new neurologic symptom onset and 7 days after COVID-19 vaccination, showing multiple new T2 hyperintense lesions (white arrows: new lesions, blue arrows: old lesions). T1 post-gadolinium axial images (m, p) show enhancement (white arrows) of two of the new FLAIR lesions. Spinal cord imaging was not obtained post vaccination due to the isolated visual symptoms
Case 4
A 64-year-old Caucasian man with no history of neurologic diseases, received the first dose of the Pfizer COVID-19 vaccine on April 2, 2021 without any immediate side effects post vaccination. Eighteen days after the first vaccine dose, he developed pain and paresthesia in his upper abdomen. Over the next 4 days, he developed right lower extremity numbness and weakness. His symptoms progressed to pain and numbness in the bilateral lower extremities, saddle anesthesia, sphincter dysfunction, and balance/gait difficulty. He required a cane to walk 200 feet. Brain and spinal cord MRI, 9 days after symptom onset, showed a minimally expansile central spinal cord edema-like T2 hyperintensity extending from the cervical spinal cord to the conus, with patchy areas of gadolinium enhancement, consistent with longitudinally extensive transverse myelitis (Fig. 4). Brain lesions were also demonstrated, suggesting demyelination (Fig. 4). Lumbar puncture, performed 9 days after symptom onset (Table 2), showed that CSF cells, protein, glucose, and IgG index were unremarkable, without oligoclonal bands. Neuromyelitis optica (NMO) antibody was strongly positive [serum titer > 1:100,000 (reference range < 1:5); CSF 1:128 (reference range < 1:2)]. He had positive SS-A/SS-B antibodies and underwent a salivary gland biopsy, which was consistent with Sjogren’s disease (focal lymphocytic sialadenitis and mild atrophic changes). He had negative COVID-19 nasal PCR tests on April 27, 2021 and May 1, 2021. The patient was treated with 3 days of 1 g IVMP with a partial response, followed by five sessions of plasma exchange. He showed improvement of his bowel/bladder symptoms and pain, with EDSS score improved to 3.5 from 6.0 when he was symptomatic. Residual symptoms included moderate sensory symptoms, mild right sided weakness, and bladder symptoms. The patient did not receive a second dose of the vaccine upon advice from his neurologist. Treatment with rituximab was initiated.
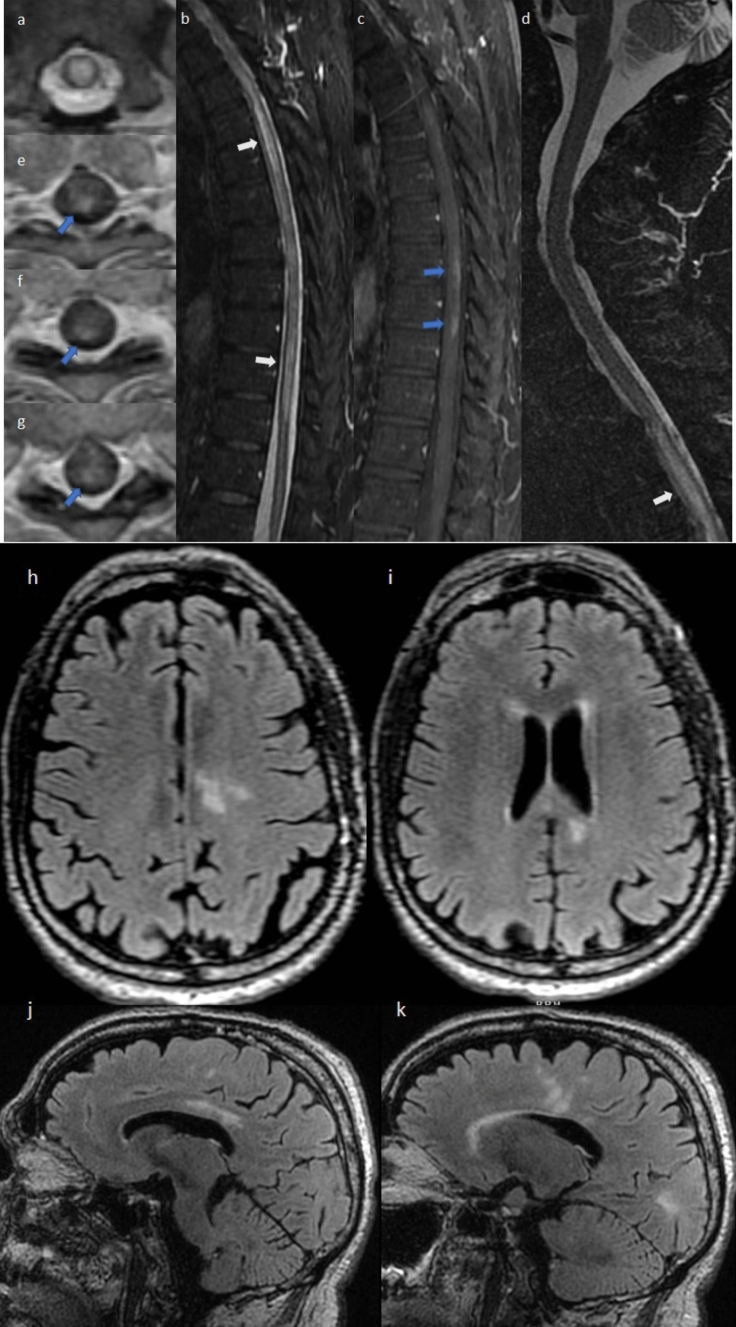
Fig. 4.
3 T MRI scans in case 4. Cervical and thoracic spinal axial T2 (a) and sagittal STIR (b, d), obtained 8 and 9 days after neurologic symptom onset, show longitudinally extensive high signal abnormalities with cord swelling in the thoracic spinal cord (white arrows). Gadolinium-enhanced sagittal (c) and axial T1 (e–g) images show patchy posterior lesion enhancement at T1/2-T5 and T9-T10/11 (blue arrows). Brain axial (h, i) and sagittal (j, k) T2 FLAIR obtained 9 days after onset of new symptoms, showing multiple T2 white matter signal abnormalities in the corpus callosum with extension into the left frontal and parietal white matter, suggestive of demyelination. Brain imaging with gadolinium showed the lesions to be non-enhancing (not shown)
Case 5
A 33-year-old Caucasian man with no significant past medical history received the first dose of the Pfizer COVID-19 vaccine on December 18, 2020, with limited side effects (arm pain). The second dose was administered 20 days later. One day after the second vaccine dose, he developed unilateral painless blurring of vision. Examination showed a visual acuity of 20/50 OS. Brain MRI, 39 days after symptom onset, demonstrated multiple T2 hyperintense white matter lesions with a single gadolinium-enhancing lesion consistent with an active demyelinating process (Fig. 5). CSF studies, obtained 40 days after symptom onset (Table 2), showed more than five oligoclonal bands with elevated IgG index. He had a normal CSF cell count, protein, and glucose. NMO serum antibody was negative. He was treated with 3 days of 1000 mg IVMP followed by an oral prednisone taper. Seven days later, left eye visual acuity improved to 20/30 OS with poor low contrast visual acuity but no red color desaturation or RAPD. Thirty days later, visual symptoms returned to normal (acuity 20/20 OU), and he was started on disease modifying therapy.
Fig. 5.
3 T MRI in case 5. Brain: axial (a–c) T2 FLAIR obtained 39 days after new neurologic symptom onset, showing periventricular and juxtacortical T2 lesions typical of multiple sclerosis; note one of the lesions ring-enhances with gadolinium (d, white arrow). Spinal cord: thoracic sagittal STIR (e) shows a T2 hyperintense spinal cord lesion suggestive of multiple sclerosis (white arrow). No cervical spinal cord enhancing lesions or new cervical spinal cord T2 lesions were seen (not shown)
Case 6
A 44-year-old Caucasian woman had her first MS symptoms at age 20 when she developed optic neuritis. She was diagnosed with RRMS 2 years later but remained off disease-modifying therapy despite having active disease with several relapses. She started intramuscular interferon beta-1a at age 29 but this was poorly tolerated. One year later, she had an episode of myelitis and was place on glatiramer acetate for a few months and was again unable to tolerate injections. Her neurological complaints resolved, and she remained off disease-modifying therapy for the next 14 years with stable disease clinically (EDSS 0) and by MRI. She had a small gadolinium-enhancing lesion in the right pericallosal white matter reported on brain MRI 2 years prior to this presentation but without any new neurological symptoms, followed by stable subsequent imaging. She received the first dose of the Moderna COVID-19 vaccine on January 24, 2021, followed by a second dose 30 days later. She had a transient low-grade fever after the second dose, but no other systemic symptoms. Six days after the second vaccine dose, she developed new neurological symptoms including numbness that ascended from her feet to the middle of her waist without any bowel or bladder incontinence. On exam, she had an EDSS score of 1.5 with mild right deltoid and iliopsoas weakness, brisk deep tendon reflexes, and slightly diminished sensation to pinprick in the left back. MRI, 1 day after symptom onset, showed a new enhancing lesion in the brain (Fig. 6). She was treated with 3 days of 1000 mg IVMP and, 30 days later, the neurologic exam returned to normal (EDSS 0); she was started on disease-modifying therapy.
Fig. 6.
3 T MRI scans in case 6. Axial (a) and sagittal (c) T2 FLAIR of the brain obtained 1 day after new neurologic symptom onset showing stable burden of periventricular and juxtacortical T2 lesions when compared to previous imaging 2 years prior to symptom onset (not shown). There is a new 3-mm gadolinium enhancing lesion in the centrum semiovale, adjacent to the falx in the left cerebral hemisphere (b, white arrow). No new spinal cord lesions were noted by MRI during this episode (not shown)
Case 7
A 48-year-old Caucasian woman was diagnosed with a clinically isolated demyelinating syndrome (CIS) in the setting of sensory complaints 8 years previously. Her MS disease-modifying therapy history included a 9-month course of dimethyl fumarate which was poorly tolerated, followed by 6 years of glatiramer acetate. At her last neurologic visit (May 2019), she had clinical and MRI stability. Her baseline MS symptoms included sensory symptoms, occasional Lhermitte's symptoms, and decreased visual acuity OD (20/25); her EDSS score was stable at 1.0 for many years. When the pandemic started in early 2020, she decided to discontinue disease-modifying therapy. She received the first dose of the Pfizer COVID-19 vaccine on March 5, 2021, which was initially well tolerated. Fifteen days after the first vaccine dose, she developed a painful sensation behind her right eye, worsening with eye movement. She also noted increased Lhermitte’s phenomenon and new balance difficulty with occasional tripping. Brain MRI showed three new T2 hyperintense white matter lesions compared to prior imaging 2 years earlier (Fig. 7). Gadolinium was not administered with the new scan. All post-vaccination neurologic symptoms showed improvement after 3 days of high dose oral corticosteroids (1000 mg methylprednisolone daily) but she continued to have persistent mild symptoms. She received the second dose of the vaccine, 30 days after the first dose, without any new symptoms. At follow-up, 90 days after the initial event, EDSS score was 1.5, and the patient reported improvement but felt worse than her pre-vaccination baseline.
Fig. 7.
Serial 3 T MRI brain scans in case 7. Baseline (2 years prior to COVID-19 vaccination): T2 FLAIR of the brain (a–c) show three typical MS lesions (white arrows). Sagittal T2 FLAIR brain scans (d, f, h), also obtained at baseline, without any lesions. Post-vaccination: sagittal T2 FLAIR (e, g, i) obtained at the time of new neurologic symptoms following COVID-19 vaccination. Note three new lesions with typical multiple sclerosis morphology (white arrows). Also note that one of the lesions seen on image g (blue arrow), is an extension of the same lesion seen on image e
Discussion
We report seven cases of acute demyelinating disease of the CNS (optic nerve, brain, and/or spinal cord) following either the first (n = 2) or second (n = 5) dose of an mRNA COVID-19 vaccination (Moderna n = 3; Pfizer n = 4). All patients experienced new neurologic symptoms, occurring 1–21 (mean 13.7) days after vaccination, attributable to involvement of the optic nerve, brain, and/or spinal cord, including visual loss, dysmetria, gait instability, paresthesias, sphincter disturbance, and limb weakness. The individuals ranged in age from 24–64 (mean 39.1) years. Five were woman (71.4%). Four persons had previously diagnosed demyelinating disease, either RRMS (n = 3) or CIS (n = 1). Two of these four individuals were receiving disease-modifying therapy at the time of COVID-19 vaccination. The patients were stable in the years preceding COVID-19 vaccination. They all had no evidence of disease activity by clinical or MRI measures for at least 2 years. They were also free from MS clinical relapses for the previous 2.2 to 14 (mean 7.5) years. The other three patients in our case series had no previous history of demyelinating disease. The patients had low neurologic disability before vaccination with a normal neurologic examination (n = 5) or minimal abnormalities (EDSS = 1, n = 2). In the setting of new neurologic symptoms after vaccination, MRI with gadolinium was obtained in 6 patients, and showed enhancing lesions involving the brain and/or spinal cord. One patient (case 7) who did not receive gadolinium, had new T2 hyperintense lesions in the brain compared to MRI obtained 2 years before the vaccine. The new MRI lesions cannot be definitely linked to vaccination, as they could have appeared any time since the last scan. All patients returned to baseline (n = 5) or close to baseline (n = 2) after high dose IV steroids (n = 6) or IV steroids followed by plasma exchange (n = 1). The final diagnosis in these individuals was exacerbation of known MS (n = 4), new onset RRMS (n = 2), or new onset NMO (n = 1).
Our case series adds to other recently published reports of the first manifestation of MS after the Pfizer COVID-19 vaccine [33], MS relapse 3 days after the Sputnik V COVID-19 vaccine [34], MS relapse after the Pfizer COVID-19 vaccine [35], and four cases of acute myelitis after the AstraZeneca COVID-19 vaccine [29, 30, 32].
Vaccines are key to preventing a range of infectious diseases; failing to get vaccinated increases the risk of viral infections which may, in turn, lead to worsening of MS [4, 58, 59]. Rare cases of vaccination-associated demyelination of the peripheral nervous system (PNS) or CNS have been reported; including Bell’s palsy, GBS, myelitis, and MS after vaccinations for influenza, hepatitis B, rabies, typhoid fever, smallpox, tetanus, polio, tuberculosis [60–63] and, most recently COVID-19 [24–35]. These anecdotal reports raised concerns regarding the safety of vaccination in patients with MS [50–52, 64–69]. A small study of yellow fever vaccination and MS found increased MS relapses after vaccination with the live attenuated vaccine [70]. However, several large studies including double-blind placebo-controlled studies on influenza vaccines as well as retrospective evaluations of numerous vaccines have failed to establish any causality between live or inactivated vaccination and an increased risk of new onset MS or exacerbation of known MS [46–52, 64–69, 71–76].
Infection by the SARS-CoV-2 virus may lead to an overactive immune system response, triggering both adaptive and innate immune activation, particularly in susceptible individuals [4, 77]. It is not clear whether COVID-19 vaccines may also induce an overactive immune response in a subset of recipients. While autoimmune sequelae have been anecdotally reported after COVID-19 vaccination, including thrombotic thrombocytopenia [78], myelitis [29–32], new onset MS [33], MS relapse [34, 35], and pericarditis/myocarditis [36–42], there is no clear consensus that COVID-19 vaccines increase these risks beyond the expected (normal) background rate of these occurrences. The rarity of diseases such as GBS after COVID-19 vaccination, argues against a causal relationship between the two [22, 27]. More recent studies have shown an increased risk of GBS in the 6 weeks after receiving the Johnson & Johnson COVID-19 vaccine, while no increased risk for GBS has been observed with the mRNA-based vaccines [79, 80]. This has led to a change in the prescribing information for the Johnson & Johnson vaccine by the U.S. Food and Drug Administration, which was preceded by a similar warning from European regulators for the AstraZeneca COVID-19 vaccine [79].
Our observations suggest that, in some individuals, COVID-19 vaccination may carry a short-term risk of CNS demyelination. This is in contrast with a recent study performed in Israel on 555 individuals with MS who received the Pfizer COVID-19 vaccine [56]. The authors reported a similar relapse rate after vaccination compared to the 3-year relapse rate prior to vaccination. This study may have been limited by the sample size, lack of a comparison group of unvaccinated MS patients from the pandemic time period, the ascertainment of relapses by telemedicine, and the absence of MRI scans. In our study, as well as in the only available case reports of MS activity after COVID-19 vaccination [33–35], MRI has been an integral tool to define the presence of new/active inflammatory demyelination, with gadolinium administered to all patients except one.
Our report is anecdotal and does not prove a cause-and-effect relationship between SARS-CoV-2 mRNA vaccines and active CNS demyelinating disease. We do not know the number of people with MS who were vaccinated against COVID-19 in the communities from which these cases were derived (Boston, MA and Albany, NY). We do not present any calculation of the rate of side effects in proportion to the total people with MS who were vaccinated, as these data are not readily available. A claim of causality has an inherent ascertainment bias, as we are only reporting adverse cases that came to our attention.
In conclusion, we present a case series of seven individuals with CNS demyelination following COVID-19 vaccination. Symptoms were transitory and responsive to corticosteroid therapy alone (n = 6) or corticosteroids followed by plasma exchange therapy (n = 1). Given the large number of patients with MS who have received COVID-19 vaccination, it is notable that there are only a few reports of individuals experiencing new CNS inflammatory disease activity after vaccination. Large prospective controlled studies/formal registries are required to establish a possible relationship between COVID-19 vaccines and acute CNS demyelination. At the present time, it would seem that the benefits of COVID-19 vaccination outweigh any potential risks to the MS population.
Acknowledgements
We are grateful to Dr. Howard L. Weiner for helpful comments.
Funding
None.
Declarations
Conflicts of interest
Dr. Bhattacharyya has received consulting fees from Alexion and research support from Alexion and UCB. Dr. Katz has served on speaker’s bureaus and advisory boards for Biogen, EMD-Serono, Genentech, Novartis, and Sanofi-Genzyme. Dr. Houtchens has received consulting fees from Biogen, EMD Serono Novartis, and Genentech, and research support from Biogen, Genentech, and Sanofi-Genzyme. Dr. Edwards has received fees for speaking and consulting from Biogen and EMD Serono and research grant support from Biogen, EMD Serono, Genentech, Novartis, and Sanofi-Genzyme. Dr. Bakshi has received consulting fees from EMD Serono and research support from BMS/Celgene and EMD Serono. The other authors have nothing to disclose.
Ethical approval
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Permission was obtained from all patients to be included in this report.
References
- 1.Zhou P, Yang X-L, Wang X-G, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sormani MP, De Rossi N, Schiavetti I, et al. Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann Neurol. 2021;89:780–789. doi: 10.1002/ana.26028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng C, Kar I, Chen CK, et al. Multiple sclerosis disease-modifying therapy and the COVID-19 pandemic: implications on the risk of infection and future vaccination. CNS Drugs. 2020;34:879–896. doi: 10.1007/s40263-020-00756-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sadeghmousavi S, Rezaei N. COVID-19 and multiple sclerosis: predisposition and precautions in treatment. SN Compr Clin Med [Epub ahead of print] 2020 doi: 10.1007/s42399-020-00504-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersen KM, Mehta HB, Palamuttam N, et al. Association between chronic use of immunosuppresive drugs and clinical outcomes from coronavirus disease 2019 (COVID-19) hospitalization: a retrospective cohort study in a large US health system. Clin Infect Dis [Epub ahead of print] 2021 doi: 10.1093/cid/ciaa1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moreno-Torres I, Meca Lallana V, Costa-Frossard L, et al. Risk and outcomes of COVID-19 in patients with multiple sclerosis. Eur J Neurol [Epub ahead of print] 2021 doi: 10.1111/ene.14990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barzegar M, Mirmosayyeb O, Gajarzadeh M, et al. COVID-19 among patients with multiple sclerosis: a systematic review. Neurol Neuroimmunol Neuroinflamm. 2021;8:e1001. doi: 10.1212/NXI.0000000000001001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Etemadifar M, Sedaghat N, Aghababaee A, et al. COVID-19 and the risk of relapse in multiple sclerosis patients: a fight with no bystander effect? Mult Scler Relat Disord. 2021;51:102915. doi: 10.1016/j.msard.2021.102915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paterson RW, Brown RL, Benjamin L, et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020;143:3104–3120. doi: 10.1093/brain/awaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharifian-Dorche M, Huot P, Osherov M, et al. Neurological complications of coronavirus infection; a comparative review and lessons learned during the COVID-19 pandemic. J Neurol Sci. 2020;417:117085. doi: 10.1016/j.jns.2020.117085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gutiérrez-Ortiz C, Méndez-Guerrero A, Rodrigo-Rey S, et al. Miller Fisher syndrome and polyneuritis cranialis in COVID-19. Neurology. 2020;95:e601–e605. doi: 10.1212/WNL.0000000000009619. [DOI] [PubMed] [Google Scholar]
- 13.Toscano G, Palmerini F, Ravaglia S, et al. Guillain-Barré syndrome associated with SARS-CoV-2. N Engl J Med. 2020;382:2574–2576. doi: 10.1056/NEJMc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sedaghat Z, Karimi N. Guillain Barre syndrome associated with COVID-19 infection: a case report. J Clin Neurosci. 2020;76:233–235. doi: 10.1016/j.jocn.2020.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Román GC, Gracia F, Torres A, et al. Acute transverse myelitis (ATM): clinical review of 43 patients with COVID-19-associated ATM and 3 post-vaccination ATM serious adverse events with the ChAdOx1 nCoV-19 vaccine (AZD1222) Front Immunol. 2021;12:653786. doi: 10.3389/fimmu.2021.653786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Helms J, Kremer S, Merdji H, et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382:2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palao M, Fernández-Díaz E, Gracia-Gil J, et al. Multiple sclerosis following SARS-CoV-2 infection. Mult Scler Relat Disord. 2020;45:102377. doi: 10.1016/j.msard.2020.102377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore L, Ghannam M, Manousakis G. A first presentation of multiple sclerosis with concurrent COVID-19 infection. eNeurologicalSci. 2021;22:100299. doi: 10.1016/j.ensci.2020.100299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Domingues RB, Mendes-Correa MC, de Moura Leite FBV, et al. First case of SARS-COV-2 sequencing in cerebrospinal fluid of a patient with suspected demyelinating disease. J Neurol. 2020;267:3154–3156. doi: 10.1007/s00415-020-09996-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heinz FX, Stiasny K. Profiles of current COVID-19 vaccines. Wien Klin Wochenschr. 2021;133:271–283. doi: 10.1007/s00508-021-01835-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bourdette D, Killestein J. Quelling public fears about Guillain-Barre syndrome and COVID-19 vaccination. Neurology. 2021;96:1021–1022. doi: 10.1212/WNL.0000000000011882. [DOI] [PubMed] [Google Scholar]
- 23.Pormohammad A, Zarei M, Ghorbani S, et al. Efficacy and safety of COVID-19 vaccines: a systematic review and meta-analysis of randomized clinical trials. Vaccines (Basel) 2021;9:467. doi: 10.3390/vaccines9050467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colella G, Orlandi M, Cirillo N. Bell’s palsy following COVID-19 vaccination. J Neurol [Epub ahead of print] 2021 doi: 10.1007/s00415-021-10462-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ozonoff A, Nanishi E, Levy O. Bell’s palsy and SARS-CoV-2 vaccines. Lancet Infect Dis. 2021;21:450–452. doi: 10.1016/S1473-3099(21)00076-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cirillo N, Doan R. Bell’s palsy and SARS-CoV-2 vaccines-an unfolding story. Lancet Infect Dis. 2021;21:1210–1211. doi: 10.1016/S1473-3099(21)00273-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Márquez Loza AM, Holroyd KB, Johnson SA, et al. Guillain- Barré syndrome in the placebo and active arms of a COVID-19 vaccine clinical trial: temporal associations do not imply causality. Neurology [Epub ahead of print] 2021 doi: 10.1212/WNL.0000000000011881. [DOI] [PubMed] [Google Scholar]
- 28.Waheed S, Bayas A, Hindi F, et al. Neurological complications of COVID-19: Guillain-Barre syndrome following Pfizer COVID-19 vaccine. Cureus. 2021;13:e13426. doi: 10.7759/cureus.13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knoll MD, Wonodi C. Oxford-AstraZeneca COVID-19 vaccine efficacy. Lancet. 2021;397:72–74. doi: 10.1016/S0140-6736(20)32623-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goss AL, Samudralwar RD, Das RR, Nath A. ANA investigates: neurological complications of COVID-19 vaccines. Ann Neurol. 2021;89:856–857. doi: 10.1002/ana.26065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh Malhotra H, Gupta P, Prabhu V, et al. COVID-19 vaccination-associated myelitis. QJM [Epub ahead of print] 2021 doi: 10.1093/qjmed/hcab069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Havla J, Schultz Y, Zimmermann H, et al. First manifestation of multiple sclerosis after immunization with the Pfizer-BioNTech COVID-19 vaccine. J Neurol [Epub ahead of print] 2021 doi: 10.1007/s00415-021-10648-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Etemadifar M, Sigari AA, Sedaghat N, et al. Acute relapse and poor immunization following COVID-19 vaccination in a rituximab-treated multiple sclerosis patient. Hum Vaccin Immunother [Epub ahead of print] 2021 doi: 10.1080/21645515.2021.1928463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maniscalco GT, Manzo V, Di Battista ME, et al. Severe multiple sclerosis relapse after COVID-19 vaccination: a case report. Front Neurol. 2021;12:1398. doi: 10.3389/fneur.2021.721502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shay DK, Shimabukuro TT, DeStefano F. Myocarditis occurring after immunization with mRNA-based COVID-19 vaccines. JAMA Cardiol [Epub ahead of print] 2021 doi: 10.1001/jamacardio.2021.2821. [DOI] [PubMed] [Google Scholar]
- 37.Snapiri O, Rosenberg Danziger C, Shirman N, et al. Transient cardiac injury in adolescents receiving the BNT162b2 mRNA COVID-19 vaccine. Pediatr Infect Dis J [Epub ahead of print] 2021 doi: 10.1097/INF.0000000000003235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Albert E, Aurigemma G, Saucedo J, Gerson DS. Myocarditis following COVID-19 vaccination. Radiol Case Rep. 2021;16:2142–2145. doi: 10.1016/j.radcr.2021.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abu Mouch S, Roguin A, Hellou E, et al. Myocarditis following COVID-19 mRNA vaccination. Vaccine. 2021;39:3790–3793. doi: 10.1016/j.vaccine.2021.05.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.CDC (2020) COVID-19 Vaccination. In: centers for disease control and prevention. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/myocarditis.html. Accessed 26 Jun 2021
- 41.PINHO AC (2021) Comirnaty and spikevax: possible link to very rare cases of myocarditis pericarditis. In: European medicines agency. https://www.ema.europa.eu/en/news/comirnaty-spikevax-possible-link-very-rare-cases-myocarditis-pericarditis. Accessed 12 Jul 2021
- 42.(2021) Clinical considerations: myocarditis after mRNA COVID-19 vaccines | CDC. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/myocarditis.html. Accessed 1 Jul 2021
- 43.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stephenson KE, Le Gars M, Sadoff J, et al. Immunogenicity of the Ad26.COV2.S vaccine for COVID-19. JAMA. 2021;325:1535–1544. doi: 10.1001/jama.2021.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Riva A, Barcella V, Benatti SV, et al. Vaccinations in patients with multiple sclerosis: a Delphi consensus statement. Mult Scler. 2021;27:347–359. doi: 10.1177/1352458520952310. [DOI] [PubMed] [Google Scholar]
- 47.Centonze D, Rocca MA, Gasperini C, et al. Disease-modifying therapies and SARS-CoV-2 vaccination in multiple sclerosis: an expert consensus. J Neurol [Epub ahead of print] 2021 doi: 10.1007/s00415-021-10545-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.COVID-19 vaccine guidance for people living with MS. In: national multiple sclerosis society. https://www.nationalmssociety.org/coronavirus-covid-19-information/multiple-sclerosis-and-coronavirus/covid-19-vaccine-guidance. Accessed 13 Jun 2021
- 49.Updated global COVID-19 advice for people with MS. In: EMSP - European multiple sclerosis platform. http://www.emsp.org/news-messages/coronavirus-disease-covid-19-and-multiple-sclerosis/. Accessed 13 Jun 2021
- 50.Zrzavy T, Kollaritsch H, Rommer PS, et al. Vaccination in multiple sclerosis: friend or foe? Front Immunol. 2019;10:1883. doi: 10.3389/fimmu.2019.01883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Williamson EML, Chahin S, Berger JR. Vaccines in multiple sclerosis. Curr Neurol Neurosci Rep. 2016;16:36. doi: 10.1007/s11910-016-0637-6. [DOI] [PubMed] [Google Scholar]
- 52.Frederiksen JL, Topsøe Mailand M. Vaccines and multiple sclerosis. Acta Neurol Scand. 2017;136(Suppl 201):49–51. doi: 10.1111/ane.12837. [DOI] [PubMed] [Google Scholar]
- 53.Ehde DM, Roberts MK, Herring TE, Alschuler KN. Willingness to obtain COVID-19 vaccination in adults with multiple sclerosis in the United States. Mult Scler Relat Disord. 2021;49:102788. doi: 10.1016/j.msard.2021.102788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xiang XM, Hollen C, Yang Q, et al. COVID-19 vaccination willingness among people with multiple sclerosis. Mult Scler J Exp Transl Clin. 2021;7:20552173211017160. doi: 10.1177/20552173211017159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Diem L, Friedli C, Chan A, et al. Vaccine hesitancy in patients with multiple sclerosis: preparing for the SARS-CoV-2 vaccination challenge. Neurol Neuroimmunol Neuroinflamm. 2021;8:e991. doi: 10.1212/NXI.0000000000000991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Achiron A, Dolev M, Menascu S, et al. COVID-19 vaccination in patients with multiple sclerosis: what we have learnt by February 2021. Mult Scler. 2021;27:864–870. doi: 10.1177/13524585211003476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 58.Steelman AJ. Infection as an environmental trigger of multiple sclerosis disease exacerbation. Front Immunol. 2015;6:520. doi: 10.3389/fimmu.2015.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Panitch HS. Influence of infection on exacerbations of multiple sclerosis. Ann Neurol. 1994;36(Suppl):S25–28. doi: 10.1002/ana.410360709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rowhani-Rahbar A, Klein NP, Lewis N, et al. Immunization and Bell’s palsy in children: a case-centered analysis. Am J Epidemiol. 2012;175:878–885. doi: 10.1093/aje/kws011. [DOI] [PubMed] [Google Scholar]
- 61.Wajih Ullah M, Qaseem A, Amray A. Post vaccination Guillain Barre syndrome: a case report. Cureus. 2018;10:e2511. doi: 10.7759/cureus.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bakshi R, Mazziotta JC. Acute transverse myelitis after influenza vaccination: magnetic resonance imaging findings. J Neuroimaging. 1996;6:248–250. doi: 10.1111/jon199664248. [DOI] [PubMed] [Google Scholar]
- 63.Miller H, Cendrowski W, Shapira K. Multiple sclerosis and vaccination. Br Med J. 1967;2:210–213. doi: 10.1136/bmj.2.5546.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gout O. Vaccinations and multiple sclerosis. Neurol Sci. 2001;22:151–154. doi: 10.1007/s100720170014. [DOI] [PubMed] [Google Scholar]
- 65.Marshall E. A shadow falls on hepatitis B vaccination effort. Science. 1998;281:630–631. doi: 10.1126/science.281.5377.630. [DOI] [PubMed] [Google Scholar]
- 66.Ascherio A, Zhang SM, Hernán MA, et al. Hepatitis B vaccination and the risk of multiple sclerosis. N Engl J Med. 2001;344:327–332. doi: 10.1056/NEJM200102013440502. [DOI] [PubMed] [Google Scholar]
- 67.Langer-Gould A, Qian L, Tartof SY, et al. Vaccines and the risk of multiple sclerosis and other central nervous system demyelinating diseases. JAMA Neurol. 2014;71:1506–1513. doi: 10.1001/jamaneurol.2014.2633. [DOI] [PubMed] [Google Scholar]
- 68.DeStefano F, Verstraeten T, Jackson LA, et al. Vaccinations and risk of central nervous system demyelinating diseases in adults. Arch Neurol. 2003;60:504–509. doi: 10.1001/archneur.60.4.504. [DOI] [PubMed] [Google Scholar]
- 69.Confavreux C, Suissa S, Saddier P, et al. Vaccinations and the risk of relapse in multiple sclerosis. Vaccines in multiple sclerosis study group. N Engl J Med. 2001;344:319–326. doi: 10.1056/NEJM200102013440501. [DOI] [PubMed] [Google Scholar]
- 70.Farez MF, Correale J. Yellow fever vaccination and increased relapse rate in travelers with multiple sclerosis. Arch Neurol. 2011;68:1267–1271. doi: 10.1001/archneurol.2011.131. [DOI] [PubMed] [Google Scholar]
- 71.Myers LW, Ellison GW, Lucia M, et al. Swine influenza virus vaccination in patients with multiple sclerosis. J Infect Dis. 1977;136(Suppl):S546–554. doi: 10.1093/infdis/136.supplement_3.s546. [DOI] [PubMed] [Google Scholar]
- 72.Miller AE, Morgante LA, Buchwald LY, et al. A multicenter, randomized, double-blind, placebo-controlled trial of influenza immunization in multiple sclerosis. Neurology. 1997;48:312–314. doi: 10.1212/wnl.48.2.312. [DOI] [PubMed] [Google Scholar]
- 73.Piyasirisilp S, Hemachudha T. Neurological adverse events associated with vaccination. Curr Opin Neurol. 2002;15:333–338. doi: 10.1097/00019052-200206000-00018. [DOI] [PubMed] [Google Scholar]
- 74.Mailand MT, Frederiksen JL. Vaccines and multiple sclerosis: a systematic review. J Neurol. 2017;264:1035–1050. doi: 10.1007/s00415-016-8263-4. [DOI] [PubMed] [Google Scholar]
- 75.Farez MF, Correale J. Immunizations and risk of multiple sclerosis: systematic review and meta-analysis. J Neurol. 2011;258:1197–1206. doi: 10.1007/s00415-011-5984-2. [DOI] [PubMed] [Google Scholar]
- 76.Huttner A, Eperon G, Lascano AM, et al. Risk of MS relapse after yellow fever vaccination: a self-controlled case series. Neurol Neuroimmunol Neuroinflamm. 2020;7:e726. doi: 10.1212/NXI.0000000000000726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Boziki MK, Mentis A-FA, Shumilina M, et al. COVID-19 immunopathology and the central nervous system: implication for multiple sclerosis and other autoimmune diseases with associated demyelination. Brain Sci. 2020;10:345. doi: 10.3390/brainsci10060345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wise J. Covid-19: rare immune response may cause clots after AstraZeneca vaccine, say researchers. BMJ. 2021;373:n954. doi: 10.1136/bmj.n954. [DOI] [PubMed] [Google Scholar]
- 79.U.S. puts new warning on J&J coronavirus vaccine for autoimmune disorder - Reuters. https://www.reuters.com/business/healthcare-pharmaceuticals/us-announce-new-warning-jj-coronavirus-vaccine-autoimmune-disorder-washington-2021-07-12/. Accessed 13 Jul 2021
- 80.CDC (2021) COVID-19 vaccination considerations for persons with underlying medical conditions. In: centers for disease control and prevention. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/underlying-conditions.html. Accessed 21 Jul 2021