ABSTRACT
Background
Whether consumption of sugar-sweetened beverages (SSBs) or artificially sweetened beverages (ASBs) is associated with the risk of breast cancer is of public health interest.
Objectives
We sought to evaluate associations between consumption of SSBs and ASBs and risks of total and subtype-specific breast cancer.
Methods
We followed 82,713 women from the Nurses’ Health Study (1980 to 2016) and 93,085 women from the Nurses’ Health Study II (1991 to 2017). Cumulatively averaged intakes of SSBs and ASBs from FFQs were tested for associations with incident breast cancer cases and subtypes using Cox regression models. We also evaluated the associations stratified by menopausal status, physical activity, BMI, and alcohol intake.
Results
We documented 11,379 breast cancer cases during 4,655,153 person-years of follow-up. Consumption of SSBs or ASBs was not associated with total breast cancer risk: pooled HRs comparing extreme categories (≥1/day compared with <1/month) were 1.03 (95% CI, 0.95–1.12) and 0.96 (95% CI, 0.91–1.02), respectively. We observed a suggestive interaction by BMI using pooled data (P-interaction = 0.08), where a modestly higher risk of breast cancer with each serving per day increment of SSBs was found in lean women (HR, 1.06; 95% CI, 1.01–1.11) but not among overweight or obese women (HR, 1.00; 95% CI, 0.95–1.06). Moreover, in the pooled, fully adjusted analysis, compared to infrequent consumers (<1/month), those who consumed ≥1 serving of ASBs per day had a lower risk of luminal A breast tumors (HR, 0.90; 95% CI, 0.80–1.01; P-trend = 0.02).
Conclusions
Although no significant associations were observed overall, consumption of SSBs was associated with a slightly higher risk of breast cancer among lean women. This finding could have occurred by chance and needs confirmation. Our findings also suggest no substantial increase in the risk of breast cancer with consumption of ASBs.
Keywords: breast cancer, epidemiology, diet, risk factors, tumor subtypes, sugar-sweetened beverages, prospective studies
See corresponding editorial on page 2511.
Introduction
Sugar-sweetened beverages (SSBs) are the single largest source of added sugar (39%) in the US diet (1), with 12% of the population consuming more than 3 servings per day. A typical 12-oz serving of soda contains 140 to 150 calories and 35.0 to 37.5 g of sugar. While heavy SSB intake has declined in the US population overall (2), certain groups (40- to 59-year-olds and non-Mexican Hispanic adults) have not decreased their consumption (3). According to the Global Burden of Disease Study 2016, SSBs were responsible for the second greatest increase in attributable deaths and disability adjusted life years between 1990 and 2016 (4).
Intake of SSBs increases weight gain (5) and is strongly linked to type 2 diabetes (independently of adiposity) (6), hypertension (7), coronary heart disease (8, 9), stroke (10), and mortality (11) in epidemiological studies. This is potentially important for breast cancer because this malignancy is 1 of the 13 obesity-related cancers (12). Independent from the obesity and adiposity pathways, SSBs also lead to activation of the insulin-signaling pathway, as well as elevation of markers of oxidative stress and inflammation, which jointly may raise the cancer risk (13), especially for breast cancer (14, 15), hepatocellular cancer (16), and diabetes-related carcinomas (including bladder, breast, colon-rectum, endometrium, liver, and pancreas cancers) (17). Artificially sweetened beverages (ASBs) are marketed as healthier and are often suggested as alternatives to SSBs, but much remains to be answered regarding their long-term health implications. ASB consumption, the main source of artificial sweeteners in the diet, has dramatically increased in the last 40 years in children and adults (18). In a recent meta-analysis, ASB consumption was linearly associated with risks of obesity and type 2 diabetes and nonlinearly associated with risks of hypertension and all-cause mortality (19); however, reverse causality cannot be ruled out as a driving factor in the observations. Some studies have also shown that some artificial sweeteners may negatively affect the gut microbiome and pathways associated with diabetes or obesity in both animals and humans (20, 21).
In contrast, very few studies have addressed the associations between SSB and ASB intakes and breast cancer incidence. The Continuous Update Project (22), a combined effort of the World Cancer Research Fund (WCRF) and the American Institute for Cancer Research (AICR), concluded that there is “limited–no conclusion” epidemiological evidence to support a link between sugary drinks and breast cancer. Yet this potential relationship raises increasing concerns due to its mechanistic plausibility. Only 5 prospective studies on sugary drinks and the breast cancer risk have been published showing contrasting results: 1 suggested an increased risk in premenopausal women [NutriNet-Santé prospective cohort (23), 693 cases], 2 suggested an increased risk in postmenopausal women [Melbourne Collaborative Cohort Study (24), 946 cases; Seguimiento Universidad de Navarra (25), 101 cases], and the other 2 observed no association [Framingham Offspring cohort (26), 124 cases; Canadian Study of Diet, Lifestyle, and Health (27), 870 cases]. Furthermore, only 2 studies (23, 24) have prospectively assessed the relationship between ASBs and breast cancer incidence, and results showed null associations. With regard to other cancer sites, in the Nurses’ Health Studies, greater diet soda consumption was linked to an increased risk of leukemia in both women and men (28). Contrarily, in an observational analysis within a randomized trial among patients with stage III colon cancer, ASB consumption was associated with significantly lower risks of cancer recurrence and death (29).
To address these knowledge gaps, we investigated the association between intakes of SSBs and ASBs and risks of total and subtype-specific breast cancer. We leveraged data from the original Nurses’ Health Study (NHS) and Nurses’ Health Study II (NHSII), 2 large, prospective US cohorts of young women with detailed and updated dietary intake assessments.
Methods
Subjects
The NHS is an ongoing study of 121,700 female nurses aged 30–55 years at enrollment in 1976, and the NHSII has followed 116,429 female nurses aged 25–42 years at recruitment in 1989. Every 2 years, participants have provided information on health-related factors and medical history.
Women were followed from baseline (1980 for the NHS and 1991 for the NHSII), when dietary information was first available, to 2016 in NHS and to 2017 in NHSII. We excluded women who died prior to baseline, had prevalent cancer, had missing data on SSB or ASB intake, or reported an implausible total energy intake (<600 or >3500 kcal/day), leaving 82,713 women from the NHS and 93,085 from the NHSII (Supplemental Figure 1). The study protocol was approved by the institutional review boards of the Brigham and Women's Hospital and Harvard T.H. Chan School of Public Health, and of participating state cancer registries as appropriate. The study procedures were in congruence with the ethical standards of the responsible institutional committees.
Dietary assessment
Diet was assessed with a validated FFQ administered in the NHS in 1980, 1984, 1986, and every 4 years thereafter and in the NHSII in 1991 and every 4 years thereafter. The numbers of FFQ food items have evolved: in the NHS, there were 61 items in 1980, 116 items in 1984 and 1986, and ≥130 items thereafter; in the NHSII, the FFQ consistently had ≥130 items. The FFQs included foods with a portion size, and participants were asked how often on average during the previous year they had consumed the foods specified. A standard portion size and 9 possible responses for the frequency of consumption, ranging from “never/almost never” to “6 or more times per day,” were given for each food item (available at https://www.nurseshealthstudy.org/participants/questionnaires).
Nutrient and energy intakes were calculated by multiplying the frequency of consumption of each unit of food and beverage recorded with the FFQs by its nutrient and energy contents and summing across all items, using the USDA database and complemented with information from the manufacturers (30, 31). Total SSBs were defined as caffeinated and noncaffeinated colas with sugar, other (noncola) carbonated beverages with sugar, and noncarbonated sweetened beverages (e.g., punch, lemonade, fruit drink, or sugared iced tea). We summed the consumption of these beverages as total SSBs. In addition, ASBs were defined as caffeinated, noncaffeinated, and noncarbonated low-calorie or diet beverages. Questions included the frequency of consumption over the past year for a standard 355 mL (12 oz) serving (1 glass/can/bottle) of each SSB or ASB.
The FFQ has been extensively validated in our cohorts by comparison with more detailed methods (31–33) and biomarkers of intakes (33). For example, in a comparison of the 1986 FFQ with multiple dietary records obtained in 1986, the mean correlation coefficients were 0.84 for SSBs and 0.36 for ASBs (32). Moreover, high- and low-energy beverages had low to moderate correlations with biomarkers (IL-6, C-reactive protein, tumor necrosis factor alpha receptor 2 (TNFaR2), adiponectin) in a recent study (34).
Case ascertainment
Invasive breast cancer cases were identified through self-report on the biennial questionnaires or through the National Death Index (35, 36). Then, authorization was requested from participants or the next of kin for access to medical records and pathology reports to confirm the diagnosis and gather tumor characteristic data, including disease stage and estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) statuses. For deceased cases, the next of kin was approached for this permission; deaths were reported by family members or the postal service or ascertained through a search of the National Death Index (35, 36).
Tissue microarrays, immunohistochemical analysis, and subtype classification
For approximately 70% of cases, breast cancer tissue samples were collected and tumor microarrays (TMA) were conducted to evaluate tumor characteristics by immunohistochemistry [details are described elsewhere (37–40)]. In brief, we collected archived formalin-fixed paraffin-embedded breast cancer blocks from participants with incident breast cancer diagnosed up through 2006. For molecular subtype classification, immunohistochemical staining information was available for the markers of ER, PR, HER2, cytokeratins 5/6 (CK 5/6), and epidermal growth factor receptor (EGFR) (41). Cases with TMAs were very similar to all eligible invasive cases in terms of demographics, breast cancer risk factors, and tumor characteristics.
For a subgroup of cases, we used definitions that correlated with gene expression profile classifications (42–47) for tumor molecular subtyping. In the NHS only, information on proliferative marker Ki-67 was available. For tumors missing Ki-67 expression data (NHSII tumors), the histologic grade was used. Hence, luminal A tumors were ER-positive and/or PR-positive, HER2 negative, and low in Ki-67 (or histologic grade 1 or 2). Luminal B tumors were either1) ER-positive and/or PR-positive and HER2 positive; or 2) ER-positive and/or PR-positive, HER2 negative, and high in Ki-67 (or histologic grade 3). HER2-enriched tumors were ER-negative, PR-negative, and HER2 positive. Basal-like tumors were ER-negative, PR-negative, HER2 negative, and CK 5/6 positive and/or EGFR positive. For assessing ER-positive compared with ER-negative tumors, the ER status was determined primarily from TMA slides and, if slides were unavailable, secondarily from pathology reports.
Covariates
For both cohorts, information on lifestyle factors and medical history was obtained from biennial questionnaires. We included data on height, BMI at age 18, weight change since age 18, age at menarche, parity/age at first birth, breastfeeding, oral contraceptive use, family history of breast cancer in a first-degree relative, history of benign breast disease, age at menopause, postmenopausal hormone use, census-tract socioeconomic status, physical activity [metabolic equivalents (METs) in h/week], alcohol intake (g/day) and total energy intake (kcal/day). We additionally adjusted for a modified Alternate Healthy Eating Index (AHEI) score (48), without SSBs and alcohol. This score was calculated based on 9 foods and nutrients that are predictive of chronic disease risk, including fruit, vegetables, nuts and legumes, red and processed meat, whole grains, sodium, trans fat, long-chain omega-3 fatty acids, and other PUFAs. A higher score in the AHEI denoted better diet quality.
Statistical analysis
Data from the NHS and NHSII were pooled to increase statistical power. Participants contributed person-years from the date of return of the baseline FFQ (1980 in the NHS and 1991 in the NHSII) to the date of any cancer diagnosis except nonmelanoma skin cancer, death, or the end of follow-up (2016 in the NHS or 2017 in the NHSII for the main analysis and 2006 for the molecular subtype analysis), whichever happened first. The main outcome of the analysis was incident breast cancer (occurring in 1980–2016 in the NHS and in 1991–2017 in the NHSII) and secondary outcomes were the different breast cancer tumor subtypes.
We assessed the associations between categories of consumption of SSBs and ASBs and total and subtype-specific breast cancer using multivariable-adjusted time-varying Cox proportional hazards regression models, stratified by age (in months), 2-year time period at risk, and cohort (NHS or NHSII), to estimate HRs and 95% CIs. In order to reduce measurement error and within-person variation and to better represent long-term diet during follow-up, we performed the analysis using cumulative averages of dietary data that were created using repeated measures from the FFQs (calculated by taking the mean intake from all FFQs up to the beginning of a 2-year follow-up interval). We categorized SSB and ASB in groups based on the frequency of intake [1/month (reference), ≥1 to ≤4/month, >1 to <7/week, and ≥1/day], and linear trends were evaluated using the Wald test on a continuous variable representing median intakes of each category.
All variables with updated data throughout follow-up were entered into the models as time-varying covariates. Multivariable models were adjusted for race (self-reported), age at menarche, age at menopause, postmenopausal hormone use, oral contraceptive use history, parity and age at first birth, breastfeeding history, family history of breast cancer, history of benign breast disease, height, cumulatively updated alcohol intake, cumulatively updated total caloric intake, physical activity, BMI at age 18 years, a modified AHEI score (with SSBs and alcohol removed), and census-tract socioeconomic status. All models were mutually adjusted for SSB and ASB in categories. Because change in weight since age 18 may be an intermediate factor between SSBs or ASBs and breast cancer, we subsequently added this variable in a separate model.
To evaluate the latency between SSB or ASB intake and breast cancer, we performed latency analyses based on dietary data collected at different time points (49, 50). Briefly, in the cumulative average model, the mean SSB or ASB consumption from all FFQs up to the beginning of a follow-up interval was calculated; in the simple update model, the SSB or ASB consumption reported on the most recent FFQ before each follow-up interval was used; in the latency models, we used SSB or ASB consumptions reported at different latencies (i.e., 4–8, 8–12, 12–16, and 16–20 years) before a breast cancer diagnosis. For example, in the 8–12-year lag analysis, we examined the association of SSB consumption in 1980 with the risk of breast cancer between 1988 and 1992. Since the FFQs were collected every 4 years, the simple update model could be considered as a latency analysis of 0–4 years.
To test whether consumption of SSBs or ASBs and breast cancer risks differed by BMI (<25 compared with ≥25 kg/m2), physical activity [<21 compared with ≥21 METs-h/week (51)], or diet quality (below compared with above the AHEI median), we added interaction terms and used the Wald test for cross-product terms based on SSB or ASB intake (continuous variable) and the stratification variables.
Moreover, we used changes in beverage consumption updated every 4 years as a time-varying exposure to estimate the risk of breast cancer in the subsequent 4-year period. For instance, changes in total SSB consumption between 1986 and 1990 were used to evaluate the risk of breast cancer between 1990 and 1994, and so on. Participants were divided into 5 categories of change in beverage intakes: no change or relatively stable consumption (±0.14 serving/day or ±1.0 serving/week), increase or decrease in consumption ranging from 1.0 serving/week to 0.50 serving/day, and increase or decrease in consumption by >0.50 serving/day. To minimize the influence of outliers, changes in beverage consumption <0.5 and >99.5 percentiles were recoded into the value of the 0.5 and the 99.5 percentiles, respectively.
To examine differential associations of SSBs and ASBs with breast cancer risks by hormone receptor and molecular subtypes, we used the Lunn-McNeil approach to derive the P value for heterogeneity (52). All statistical tests were 2-sided with a P value of <0.05 and were performed using SAS version 9.4 (SAS Institute Inc.).
Results
During 4,655,153 person-years of follow-up, we documented 11,379 invasive breast cancer cases (NHS n = 7495; NHSII n = 3884). Characteristics of participants according to the frequency of SSB and ASB intakes are shown in Table 1. Women with higher intakes of SSBs tended to be younger, less physically active, and more likely to have lower AHEI scores. SSB consumption was also associated with a higher total energy intake and lower intakes of fruits, vegetables, coffee, and alcohol. Individuals with higher intakes of ASBs were more likely to have a greater BMI, be more physically active, consume more alcohol, and have higher AHEI scores. Frequent consumption of ASBs was also associated with a lower total energy intake and higher intakes of fruits, vegetables, and coffee.
TABLE 1.
Age and age-standardized baseline characteristics of women according to consumption of sugar-sweetened and artificially sweetened beverages at baseline1
| Nurses’ Health Study | Nurses’ Health Study II | |||||||
|---|---|---|---|---|---|---|---|---|
| <1/month | ≥1 to ≤4/month | >1 to <7/week | ≥1/day | <1/month | ≥1 to ≤4/month | >1 to <7/week | ≥1/day | |
| Sugar-sweetened beverages | ||||||||
| Participants, n | 31,702 | 18,424 | 20,326 | 12,261 | 31,171 | 22,613 | 24,137 | 15,164 |
| Age, years2 | 47.8 (7) | 46.9 (7.1) | 45.6 (7.1) | 44.5 (7.1) | 37.4 (4.5) | 36.6 (4.6) | 36.2 (4.7) | 35.8 (4.8) |
| Sugar-sweetened beverages, servings/day | 0 (0) | 0.1 (0) | 0.5 (0.2) | 1.9 (1.2) | 0 (0) | 0.1 (0) | 0.5 (0.2) | 2 (1.2) |
| Artificially sweetened beverages, servings/day | 0.7 (1.1) | 0.3 (0.7) | 0.3 (0.6) | 0.4 (0.8) | 1.5 (1.6) | 1.1 (1.4) | 0.7 (1.1) | 0.5 (1) |
| Self-reported African heritage, % | 0.9 | 1.3 | 1.8 | 3.1 | 0.8 | 1.1 | 2.0 | 3.0 |
| Age at menarche <12 years, % | 24.3 | 22.4 | 21.0 | 21.1 | 27.1 | 25.1 | 22.7 | 21.1 |
| BMI, kg/m2 | 24.4 (4.4) | 24.2 (4.3) | 24.5 (4.5) | 25 (5.1) | 24.8 (5.3) | 24.5 (5.2) | 24.1 (5.1) | 24.5 (5.8) |
| BMI at age 18 years, kg/m2 | 21.7 (3.1) | 21.3 (2.9) | 21.1 (2.8) | 21.1 (3.1) | 21.8 (3.5) | 21.3 (3.2) | 20.8 (3.1) | 20.7 (3.4) |
| Weight change since age 18 years, kg | 7 (10.6) | 7.9 (10) | 9 (10.3) | 10.5 (11.6) | 8 (11.8) | 8.7 (11.1) | 8.9 (10.7) | 10.1 (12) |
| Ever oral contraceptive use, % | 50.2 | 49.2 | 49.3 | 49.3 | 84.9 | 84.4 | 84.3 | 84.7 |
| Age at menopause (natural) | 49 (3.6) | 49 (3.8) | 49 (4.1) | 48.8 (4.7) | 37.1 (6.1) | 37.7 (5.2) | 39.1 (4.2) | 37.9 (4.8) |
| Parous, % | 91.8 | 92.8 | 93.3 | 92.5 | 71.4 | 74.5 | 77.7 | 75.3 |
| Parity, n | 3.1 (1.5) | 3.1 (1.5) | 3.2 (1.5) | 3.1 (1.5) | 2.1 (0.9) | 2.1 (0.9) | 2.1 (0.9) | 2.1 (0.9) |
| Breastfeeding, ≤6 months | 35.9 | 36.2 | 35.8 | 34.8 | 16.9 | 16.5 | 16.8 | 18.1 |
| Family history breast cancer, % | 6.1 | 6.2 | 6.1 | 6.1 | 6.1 | 5.9 | 5.8 | 6.1 |
| History of benign breast disease, % | 24.9 | 24.9 | 23.7 | 23.9 | 9.6 | 9.4 | 9.5 | 10.2 |
| Height, inches, continuous | 64.5 (2.4) | 64.5 (2.4) | 64.5 (2.4) | 64.5 (2.4) | 64.9 (2.6) | 64.9 (2.6) | 64.8 (2.6) | 64.8 (2.6) |
| Physical activity, METs-h/week | 13.2 (14) | 12 (13.3) | 11.4 (12.6) | 10.4 (11.9) | 23.3 (29.7) | 20.8 (26.5) | 19.2 (25.1) | 18.3 (26) |
| Alcohol consumption, g/day | 7.8 (11.6) | 5.8 (9.8) | 5.2 (9.2) | 5.3 (10.3) | 3.4 (6.5) | 3.2 (6) | 3 (5.8) | 2.5 (5.6) |
| Alternative Healthy Eating Index, score | 42.1 (9.4) | 39.8 (8.8) | 38.2 (8.6) | 36.9 (8.6) | 48.6 (9.9) | 44.5 (9.1) | 40.1 (8.4) | 35.6 (8.3) |
| Total energy intake, kcal/day | 1454 (462) | 1525 (472) | 1645 (483) | 1834 (534) | 1618 (501) | 1726 (508) | 1863 (528) | 2118 (562) |
| Fruit consumption, servings/day | 2.2 (1.6) | 2.1 (1.4) | 2.0 (1.4) | 2.0 (1.5) | 1.3 (1.0) | 1.2 (0.9) | 1.2 (0.9) | 1.0 (0.9) |
| Vegetable consumption, servings/day | 2.1 (1.3) | 1.9 (1.1) | 1.9 (1.1) | 1.9 (1.1) | 3.2 (2.1) | 3 (1.7) | 2.9 (1.7) | 2.7 (1.7) |
| Coffee consumption, servings/day | 2.5 (2.1) | 2.4 (2) | 2.2 (2) | 1.9 (1.9) | 1.8 (1.8) | 1.6 (1.7) | 1.4 (1.6) | 1.2 (1.5) |
| Artificially sweetened beverages | ||||||||
| Participants, n | 41,895 | 13,142 | 13,239 | 14,437 | 27,886 | 9,649 | 21,456 | 34,094 |
| Age, years2 | 46.8 (7.2) | 46.8 (7.1) | 46.7 (7.1) | 45.7 (7.1) | 36.5 (4.6) | 36.7 (4.7) | 36.8 (4.6) | 36.6 (4.7) |
| Sugar-sweetened beverages, servings/day | 0.5 (0.9) | 0.3 (0.6) | 0.3 (0.6) | 0.4 (0.8) | 0.9 (1.1) | 0.5 (0.8) | 0.3 (0.6) | 0.2 (0.5) |
| Artificially sweetened beverages, servings/day | 0 (0) | 0.1 (0) | 0.5 (0.2) | 1.8 (1.2) | 0 (0) | 0.1 (0) | 0.6 (0.2) | 2.4 (1.5) |
| Self-reported African heritage, % | 1.6 | 1.5 | 1.4 | 1.5 | 2.4 | 1.8 | 1.5 | 0.8 |
| Age at menarche <12 years, % | 20.5 | 23.8 | 24.3 | 25.5 | 20.3 | 23.9 | 25.4 | 27.6 |
| BMI, kg/m2 | 23.5 (4.1) | 24.8 (4.4) | 25.4 (4.6) | 26 (5) | 23.2 (4.8) | 24 (5.1) | 24.4 (4.9) | 25.7 (5.7) |
| BMI at age 18 years, kg/m2 | 20.9 (2.8) | 21.6 (3) | 21.9 (3) | 22.1 (3.3) | 20.4 (3) | 21.1 (3) | 21.3 (3.1) | 22 (3.6) |
| Weight change since age 18 years, kg | 7 (9.7) | 8.5 (10.4) | 9.5 (11) | 10.4 (12.1) | 7.7 (10.2) | 8 (10.9) | 8.4 (10.9) | 10 (12.6) |
| Ever oral contraceptive use, % | 48.4 | 50.6 | 51.0 | 51.3 | 82.2 | 82.9 | 85.4 | 86.6 |
| Age at menopause (natural) | 49 (3.7) | 49 (3.8) | 49.1 (3.9) | 48.8 (4.4) | 38.8 (4.2) | 36.8 (5.9) | 37.2 (5.8) | 37.7 (5.8) |
| Parous, % | 92.5 | 92.7 | 92.8 | 92.1 | 76.4 | 74.8 | 75.7 | 71.9 |
| Parity, n | 3.2 ± 1.5 | 3.1 ± 1.5 | 3.1 ± 1.5 | 3.1 ± 1.4 | 2.1 ± 0.9 | 2.1 ± 0.9 | 2.1 ± 0.9 | 2.1 ± 0.9 |
| Breastfeeding, ≤6 months | 34.9 | 36.6 | 37.3 | 36.0 | 15.5 | 15.6 | 16.7 | 19.0 |
| Family history breast cancer, % | 6.1 | 6.0 | 6.1 | 6.3 | 5.9 | 5.7 | 6.2 | 5.9 |
| History of benign breast disease, % | 25.0 | 23.7 | 24.0 | 23.8 | 10.2 | 9.3 | 9.6 | 9.2 |
| Height, inches, continuous | 64.5 (2.4) | 64.5 (2.4) | 64.6 (2.4) | 64.6 (2.4) | 64.8 (2.6) | 64.9 (2.6) | 64.9 (2.6) | 65 (2.6) |
| Physical activity, METs-h/week | 11.5 (12.9) | 12.5 (13.5) | 12.8 (13.6) | 12.4 (13.5) | 19.5 (26.4) | 20.6 (26) | 21.6 (27.8) | 21.5 (27.8) |
| Alcohol consumption, g/day | 6.4 (10.7) | 5.9 (10) | 6.5 (10) | 6.8 (11) | 2.7 (5.9) | 2.9 (5.5) | 3.3 (6) | 3.4 (6.3) |
| Alternative Healthy Eating Index, score | 39 (9.4) | 40.7 (9) | 40.9 (8.8) | 40.7 (9) | 41.2 (10.8) | 44.5 (10.6) | 44.5 (9.8) | 43.9 (9.7) |
| Total energy intake, kcal/day | 1607 (506) | 1530 (481) | 1533 (480) | 1550 (499) | 1861 (561) | 1759 (538) | 1742 (528) | 1770 (547) |
| Fruit consumption, servings/day | 2 (1.5) | 2.1 (1.4) | 2.2 (1.4) | 2.2 (1.5) | 1.2 (1) | 1.3 (1) | 1.3 (0.9) | 1.2 (0.9) |
| Vegetable consumption, servings/day | 1.9 (1.2) | 2 (1.2) | 2 (1.2) | 2.1 (1.3) | 2.8 (1.8) | 3 (1.8) | 3 (1.8) | 3.1 (1.9) |
| Coffee consumption, servings/day | 2.3 (2) | 2.3 (2) | 2.3 (2) | 2.2 (2) | 1.3 (1.6) | 1.6 (1.7) | 1.7 (1.7) | 1.6 (1.7) |
Values are means (SDs) for continuous variables and percentages (%) for categorical variables. Baseline was 1980 for the Nurses’ Health Study and 1991 for the Nurses’ Health Study II. All variables except age are standardized to the age distribution of the study population. MET, metabolic equivalent.
Value is not age adjusted.
In the NHS, a suggestive positive, though not statistically significant, association was observed in multivariable-adjusted model 1 (HR, 1.11; 95% CI, 1.00–1.22; P-trend = 0.06; Table 2). Multivariable model 1 and model 2 (which was further adjusted for the change in weight since age 18) showed similar results. Moreover, each serving per day increment in SSB was associated with a 4% higher risk of breast cancer (HR, 1.04; 95% CI, 0.99–1.09). There were no significant associations observed with SSB in the NHSII (HR per 1 serving/day increment 0.99; 95% CI, 0.94–1.05). The pooled HR comparing extreme categories was nonsignificant (1.03; 95% CI, 0.95–1.12; P-trend = 0.72). Furthermore, using pooled data, we additionally split the highest category of consumption into 2 categories (1/day and ≥2/day) but observed similar HRs in each category (data not shown). We also evaluated total breast cancer incidence according to type of SSB: cola and noncola beverages. In the model where the change in weight since age 18 was included, women who consumed ≥1 serving/day of cola beverages had a higher risk of breast cancer, although the association was not linear (HR, 1.10; 95% CI, 1.00–1.22; P-trend = 0.13; data not shown). Furthermore, we evaluated the latency between SSB consumption and breast cancer, and we did not observe a significant association in any lag (Supplemental Table 1). In an additional analysis, when we cross-classified by SSB and ASB intake (data not shown), a higher, nonsignificant risk of breast cancer was found for women with the highest intake of both SSBs and ASBs (≥1/day; HR, 1.09; 95% CI, 0.92–1.30) compared to the reference group (<1/month in ASB and SSB intake).
TABLE 2.
Risk of total breast cancer according to cumulative average intake of SSBs and ASBs in the NHS, NHSII, and pooled cohorts1
| <1/month | ≥1 to ≤4/month | >1 to <7/week | ≥1/day | P trend2 | Per 1 serving/day increase | ||
|---|---|---|---|---|---|---|---|
| Sugar-sweetened beverage category | |||||||
| NHS | Number of cases, n | 2141 | 1947 | 2864 | 543 | — | |
| Person years | 691,530 | 608,056 | 901,755 | 195,256 | — | ||
| Age-adjusted model | 1.00 | 1.01 (0.95–1.07) | 1.00 (0.94–1.06) | 1.04 (0.95–1.15) | 0.53 | 1.01 (0.97–1.06) | |
| Multivariable model 13 | 1.00 | 1.01 (0.94–1.07) | 1.01 (0.95–1.07) | 1.11 (1.00–1.22) | 0.06 | 1.05 (1.00–1.10) | |
| Multivariable model 24 | 1.00 | 1.00 (0.94–1.07) | 1.00 (0.94–1.06) | 1.09 (0.98–1.21) | 0.10 | 1.04 (0.99–1.09) | |
| NHS II | Number of cases, n | 928 | 984 | 1,507 | 465 | — | |
| Person years | 557,608 | 540,283 | 848,158 | 312,506 | — | ||
| Age-adjusted model | 1.00 | 1.10 (1.00–1.20) | 1.02 (0.94–1.11) | 0.96 (0.85–1.08) | 0.08 | 0.98 (0.93–1.03) | |
| Multivariable model 13 | 1.00 | 1.06 (0.97–1.17) | 0.96 (0.88–1.06) | 0.94 (0.83–1.08) | 0.14 | 1.00 (0.94–1.05) | |
| Multivariable model 24 | 1.00 | 1.06 (0.97–1.16) | 0.96 (0.87–1.05) | 0.93 (0.82–1.07) | 0.12 | 0.99 (0.94–1.05) | |
| Pooled5 | Number of cases, n | 3069 | 2931 | 4371 | 1008 | — | |
| Person years | 1,249,138 | 1,148,340 | 1,749,913 | 507,762 | — | ||
| Age-adjusted model | 1.00 | 1.03 (0.98–1.09) | 1.00 (0.96–1.05) | 1.00 (0.93–1.08) | 0.53 | 1.00 (0.96–1.03) | |
| Multivariable model 13 | 1.00 | 1.03 (0.98–1.08) | 1.00 (0.95–1.05) | 1.05 (0.97–1.14) | 0.49 | 1.03 (0.99–1.06) | |
| Multivariable model 24 | 1.00 | 1.02 (0.97–1.08) | 0.99 (0.94–1.04) | 1.03 (0.95–1.12) | 0.72 | 1.02 (0.99–1.06) | |
| Artificially sweetened beverage category | |||||||
| NHS | Number of cases, n | 2242 | 1123 | 2870 | 1260 | — | |
| Person years | 758,074 | 353,549 | 866,359 | 418,616 | — | ||
| Age-adjusted model | 1.00 | 1.02 (0.95–1.10) | 1.02 (0.96–1.08) | 1.01 (0.94–1.09) | 0.88 | 1.00 (0.97–1.04) | |
| Multivariable model 13 | 1.00 | 1.02 (0.95–1.10) | 1.01 (0.95–1.07) | 1.05 (0.98–1.13) | 0.22 | 1.02 (0.99–1.06) | |
| Multivariable model 24 | 1.00 | 1.00 (0.93–1.08) | 0.97 (0.92,1.03) | 0.99 (0.92–1.07) | 0.87 | 1.00 (0.97–1.04) | |
| NHS II | Number of cases, n | 935 | 408 | 1327 | 1214 | — | |
| Person years | 548,370 | 225,896 | 703,317 | 780,973 | — | ||
| Age-adjusted model | 1.00 | 1.02 (0.90–1.14) | 1.00 (0.92–1.09) | 0.88 (0.81–0.97) | <0.01 | 0.96 (0.93–0.98) | |
| Multivariable model 13 | 1.00 | 1.03 (0.91–1.16) | 0.99 (0.91–1.08) | 0.93 (0.84–1.01) | 0.04 | 0.98 (0.95–1.01) | |
| Multivariable model 24 | 1.00 | 1.02 (0.91–1.15) | 0.98 (0.89–1.07) | 0.91 (0.83–1.00) | 0.02 | 0.98 (0.95–1.00) | |
| Pooled5 | Number of cases, n | 3177 | 1531 | 4197 | 2474 | — | |
| Person Years | 1,306,444 | 579,445 | 1,569,676 | 1,199,588 | — | ||
| Age-adjusted model | 1.00 | 1.02 (0.96–1.09) | 1.01 (0.97–1.06) | 0.96 (0.91–1.01) | 0.01 | 0.98 (0.96–1.00) | |
| Multivariable model 13 | 1.00 | 1.02 (0.96–1.09) | 1.01 (0.96–1.06) | 1.00 (0.95–1.06) | 0.61 | 1.00 (0.98–1.02) | |
| Multivariable model 24 | 1.00 | 1.01 (0.95–1.07) | 0.98 (0.94–1.03) | 0.96 (0.91–1.02) | 0.08 | 0.99 (0.97–1.01) | |
Values are HRs with 95% CIs in parentheses. ASB, artificially sweetened beverage; MET, metabolic equivalent; NHS, Nurses’ Health Study; NHSII, Nurses’ Health Study II; SSB, sugar-sweetened beverage.
P value for trend was calculated by assigning the median values to each quartile and modeling the median values as a continuous variable.
Multivariable model 1: stratified by age in months and calendar year, adjusted for SSB or ASB intake, race (non-Hispanic Caucasian, African, Asian, Hispanic Caucasian), age at menarche (<12, 12, 13, 14, >14 years), age at menopause (premenopausal, <45, 45–49, 50–52, ≥53 years), postmenopausal hormone use (never user; past user; current user: estrogen only for <5 years; current user: estrogen only for ≥5 years; current estrogen + progestin user for <5 years; current estrogen + progestin user for ≥5 years; current user of other types), oral contraceptive use history (never, ever), parity and age at first birth (nulliparous, 1 child before age 25, 1 child at ≥25 years of age, ≥2 children before age 25, ≥2 children ≥25 years of age), breastfeeding history (never, breastfed for ≤6 months, breastfed for >6 months), family history of breast cancer (yes or no), history of benign breast disease (yes or no), height (<1.60, 1.60–1.64, 1.65–1.69, 1.70–1.74, ≥1.75 meters), cumulatively updated alcohol intake (0, <5, 5–9, 10–14, ≥15 g/d), cumulatively updated total caloric intake (kcal/day, quintiles), physical activity (linear MET-h/week), BMI at age 18 years (<20.0, 20.0–21.9, 22.0–23.9, 24.0–26.9, ≥27.0 kg/m2), a modified Alternate Healthy Eating Index score (with SSBs and alcohol removed), and socioeconomic status.
Multivariable model 2 includes the variables from multivariable model 1 plus the change in weight since age 18 (lost ≥2 kg, lost 0–1 kg, gained 0–2 kg, gained 3–5 kg, gained 6–10 kg, gained 11–20 kg, gained 21–25 kg, gained >25 kg).
The pooled model was also stratified by cohort.
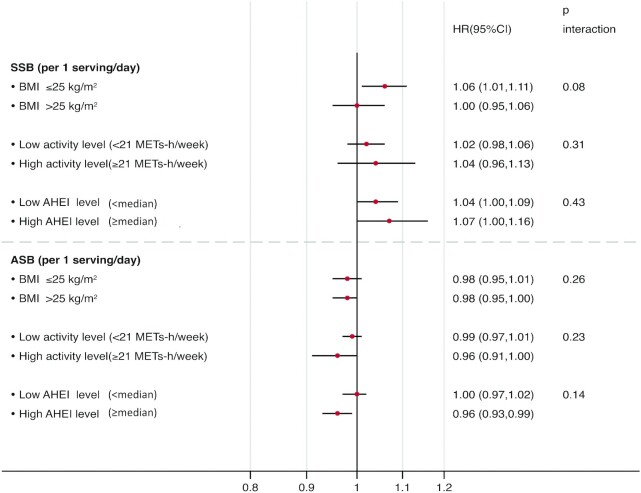
Figure 1 shows stratified analyses in the pooled data by BMI, physical activity, and diet quality. Although we did not observe a significant interaction by BMI (P-interaction = 0.08), a modest higher risk of breast cancer with each serving per day increment of SSBs was found in lean women (BMI < 25 kg/m2; HR, 1.06; 95% CI, 1.01–1.11), but not among overweight or obese women (BMI ≥ 25 kg/m2; HR, 1.00; 95% CI, 0.95–1.06). Although the interaction with physical activity (<21 METs-h/week compared with ≥21 METs-h/week) was not significant (P-interaction = 0.23), each serving per day of ASBs in women with a high activity level was associated with a 4% lower risk of breast cancer (HR, 0.96; 95% CI, 0.91–1.00).
FIGURE 1.
Total breast cancer according to SSB intake (servings/day) and ASB intake, stratified by BMI, physical activity, and diet quality (AHEI score), based on pooled data from both cohorts (NHS/NHSII). Data are stratified by age in months, cohort, and calendar year and are adjusted for SSB or ASB intake, race (non-Hispanic Caucasian, African, Asian, Hispanic Caucasian), age at menarche (<12, 12, 13, 14, >14 years), age at menopause (premenopausal, <45, 45–49, 50–52, ≥53 years), postmenopausal hormone use (never user; past user; current user: estrogen only for <5 years; current user: estrogen only for ≥5 years; current estrogen + progestin user for <5 years; current estrogen + progestin user for ≥5 years; current user of other types), oral contraceptive use history (never, ever), parity and age at first birth (nulliparous, 1 child before age 25, 1 child at ≥25 years of age, ≥2 children before age 25, ≥2 children ≥25 years of age), breastfeeding history (never, breastfed for ≤6 months, breastfed for >6 months), family history of breast cancer (yes or no), history of benign breast disease (yes or no), height (<1.60, 1.60–1.64, 1.65–1.69, 1.70–1.74, ≥1.75 meters), BMI at age 18 years (<20.0, 20.0–21.9, 22.0–23.9, 24.0–26.9, ≥27.0 kg/m2), physical activity (linear MET-h/week), diet quality using a modified AHEI score (with SSBs and alcohol removed), cumulatively updated alcohol intake (0, <5, 5–9, 10–14, ≥15 g/d), cumulatively updated total caloric intake (kcal/day, quintiles), change in weight since age 18 (lost ≥2 kg, lost 0–1 kg, gained 0–2 kg, gained 3–5 kg, gained 6–10 kg, gained 11–20 kg, gained 21–25 kg, gained >25 kg), and socioeconomic status. AHEI, Alternate Healthy Eating Index; ASB, artificially sweetened beverage; MET, metabolic equivalent; NHS, Nurses’ Health Study; NHSII, Nurses’ Health Study II; SSB, sugar-sweetened beverage.
While no association with ASB intake was observed in the NHS, ASB intake was inversely associated with the risk of breast cancer in the NHSII. Compared with women who consumed ASBs less than once per month, women who consumed ≥1 serving of ASBs per day had a 7% lower risk of breast cancer (HR, 0.93; 95% CI, 0.84–1.01; P-trend = 0.04). Additional adjustment for weight change since age 18 slightly strengthened the association (HR, 0.91; 95% CI, 0.83–1.00; P-trend = 0.02; Table 2).
Table 3 shows the breast cancer incidence by ER status and molecular phenotype, according to cumulative average intake categories of SSBs and ASBs using pooled data. SSB intake was not associated with an increased risk of any breast cancer subtype, and we did not observe heterogeneity by ER status or molecular phenotype. Nonetheless, the ASB intake was associated with a lower risk of luminal A breast tumors, with HRs across the categories of <1/month, ≥1 to ≤4/month, >1 to <7/week, and ≥1/day of 1.00 (reference), 1.05 (95% CI, 0.93–1.19), 0.95 (95% CI, 0.86–1.05), and 0.90 (95% CI, 0.80–1.01), respectively (P-trend = 0.02). Each serving per day increment in ASB intake was associated with a 6% lower risk of this cancer subtype (HR, 0.94; 95% CI, 0.90–0.98). Further adjustment for a mammographic exam in the last 2 years did not alter the results (data not shown). Given the between-studies heterogeneity for SSBs (P value = 0.02) using the Q-statistic, we presented the results stratified by cohort. As shown in Supplemental Table 2, a higher intake of SSBs was inversely associated with ER-positive and luminal A breast tumors in the NHSII, while there was a higher risk in the NHS for these tumors. Moreover, a higher intake of ASBs was inversely associated with ER-positive and luminal A breast tumors in the NHSII. Furthermore, we also tested whether the ASB intake and ER-positive or luminal A breast tumors differed by BMI (in kg/m2) in Supplemental Figure 2. Each serving per day increment in ASB intake was associated with 8% and 6% lower risks of luminal A and ER-positive breast cancer, respectively, among overweight/obese women [HR, 0.92 (95% CI, 0.88–0.96) and 0.94 (95% CI, 0.91–0.98), respectively] but not among lean women [HR, 0.97 (95% CI, 0.92–1.02) and 0.99 (95% CI, 0.95–1.03), respectively]; P-interactions of 0.10 and 0.01, respectively.
TABLE 3.
Risk of breast cancer by estrogen receptor status and molecular subtypes according to cumulative average intake of SSBs and ASBs using pooled data from the Nurses' Health Studies (NHS and NHSII)1
| <1/month | ≥1 to ≤4/month | >1 to <7/week | ≥1/day | P trend2 | Per 1 serving/day increase | |
|---|---|---|---|---|---|---|
| Sugar-sweetened beverage category | ||||||
| By ER status | ||||||
| Estrogen receptor positive | ||||||
| Number of cases, n | 2019 | 1852 | 2832 | 599 | — | |
| Person years | 1,250,140 | 1,149,365 | 1,751,379 | 508,153 | — | |
| Multivariable-adjusted model 3 | 1.00 | 0.99 (0.92–1.05) | 0.99 (0.93–1.06) | 1.02 (0.92–1.13) | 0.68 | 1.03 (0.98–1.07) |
| Estrogen receptor negative | ||||||
| Number of cases, n | 453 | 428 | 635 | 177 | — | |
| Person years | 1,251,580 | 1,150,699 | 1,753,462 | 508,552 | — | |
| Multivariable-adjusted model 3 | 1.00 | 1.04 (0.91–1.19) | 0.99 (0.87–1.13) | 1.05 (0.86–1.28) | 0.76 | 0.97 (0.89–1.06) |
| P-heterogeneity by ER status4 = 0.99 | ||||||
| By molecular subtype 5 | ||||||
| Luminal A | ||||||
| Number of cases, n | 765 | 677 | 985 | 246 | — | |
| Person years | 942,382 | 818,719 | 1,192,135 | 384,338 | — | |
| Multivariable-adjusted model 3 | 1.00 | 1.00 (0.90–1.11) | 0.98 (0.88–1.08) | 1.03 (0.88–1.21) | 0.89 | 1.00 (0.93–1.08) |
| Luminal B | ||||||
| Number of cases, n | 311 | 287 | 461 | 88 | — | |
| Person years | 942,773 | 819,060 | 1,192,571 | 384,477 | — | |
| Multivariable-adjusted model 3 | 1.00 | 0.99 (0.84–1.17) | 1.03 (0.88–1.20) | 0.94 (0.72–1.22) | 0.66 | 0.99 (0.88–1.12) |
| HER2 | ||||||
| Number of cases, n | 62 | 66 | 101 | 21 | — | |
| Person years | 942,994 | 819,265 | 1,192,915 | 384,538 | — | |
| Multivariable-adjusted model 3 | 1.00 | 1.05 (0.73–1.50) | 0.97 (0.68–1.37) | 0.81 (0.47–1.40) | 0.38 | 0.92 (0.71–1.19) |
| Basal-like | ||||||
| Number of cases, n | 77 | 76 | 122 | 28 | — | |
| Person years | 942,980 | 819,259 | 1,192,889 | 384,529 | — | |
| Multivariable-adjusted model 3 | 1.00 | 1.14 (0.82–1.57) | 1.17 (0.86–1.60) | 0.87 (0.54–1.40) | 0.38 | 0.84 (0.67–1.05) |
| P-heterogeneity by molecular subtype4 = 0.65 | ||||||
| Artificially sweetened beverage category | ||||||
| By ER status | ||||||
| Estrogen receptor positive | ||||||
| Number of cases, n | 2014 | 1011 | 2715 | 1562 | — | |
| Person years | 1,307,507 | 579,966 | 1,571,087 | 1,200,478 | — | |
| Multivariable-adjusted model 3 | 1.00 | 1.03 (0.95–1.11) | 0.97 (0.91–1.03) | 0.97 (0.90–1.04) | 0.18 | 0.97 (0.94–1.00) |
| Estrogen receptor negative | ||||||
| Number of cases, n | 501 | 213 | 603 | 376 | — | |
| Person years | 1,308,930 | 580,719 | 1,573,107 | 1,201,537 | — | |
| Multivariable-adjusted model 3 | 1.00 | 0.95 (0.81–1.12) | 1.01 (0.89–1.15) | 0.98 (0.85–1.14) | 0.92 | 1.01 (0.97–1.07) |
| P-heterogeneity by ER status4 = 0.62 | ||||||
| By molecular subtype 5 | ||||||
| Luminal A | ||||||
| Number of cases, n | 790 | 381 | 939 | 563 | — | |
| Person years | 1,006,598 | 422,837 | 1,055,686 | 852,453 | — | |
| Multivariable-adjusted model 3 | 1.00 | 1.05 (0.93–1.19) | 0.95 (0.86–1.05) | 0.90 (0.80–1.01) | 0.02 | 0.94 (0.90–0.98) |
| Luminal B | ||||||
| Number of cases, n | 304 | 154 | 419 | 270 | — | |
| Person years | 1,007,001 | 423,043 | 1,056,133 | 852,706 | — | |
| Multivariable-adjusted model 3 | 1.00 | 1.04 (0.85–1.26) | 0.98 (0.84–1.14) | 1.12 (0.94–1.33) | 0.19 | 1.03 (0.97–1.10) |
| HER2 | ||||||
| Number of cases, n | 81 | 37 | 86 | 46 | — | |
| Person years | 1,007,221 | 423,157 | 1,056,440 | 852,895 | — | |
| Multivariable-adjusted model 3 | 1.00 | 1.04 (0.70–1.55) | 0.90 (0.66–1.25) | 0.85 (0.58–1.26) | 0.63 | 1.03 (0.90–1.18) |
| Basal-like | ||||||
| Number of cases, n | 89 | 31 | 109 | 74 | — | |
| Person years | 1,007,204 | 423,158 | 1,056,422 | 852,875 | — | |
| Multivariable-adjusted model 3 | 1.00 | 0.81 (0.53–1.22) | 1.11 (0.83–1.50) | 1.04 (0.74–1.46) | 0.79 | 0.97 (0.86–1.09) |
| P-heterogeneity by molecular subtype4 = 0.20 | ||||||
Values are HRs with 95% CIs in parentheses. Data on estrogen receptor status are from the NHS with follow-ups from 1980 to 2016 and the NHSII with follow-ups from 1991 to 2017) and data on molecular subtypes are from the NHS with follow-ups from 1980 to 2006 and the NHSII with follow-ups from 1991 to 2005. ASB, artificially sweetened beverage; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; MET, metabolic equivalent; NHS, Nurses’ Health Study; NHSII, Nurses’ Health Study II; SSB, sugar-sweetened beverage.
P values for trend were calculated by assigning the median values to each quartile and modeling the median values as a continuous variable.
Multivariable-adjusted model stratified by age in months and calendar year, adjusted for SSB or ASB intake, race (non-Hispanic Caucasian, African, Asian, Hispanic Caucasian), age at menarche (<12, 12, 13, 14, >14 years), age at menopause (premenopausal, <45, 45–49, 50–52, ≥53 years), postmenopausal hormone use (never user; past user; current user: estrogen only for <5 years; current user: estrogen only for ≥5 years; current estrogen + progestin user for <5 years; current estrogen + progestin user for ≥5 years; current user of other types), oral contraceptive use history (never, ever), parity and age at first birth (nulliparous, 1 child before age 25, 1 child at ≥25 years of age, ≥2 children before age 25, ≥2 children ≥25 years of age), breastfeeding history (never, breastfed for ≤6 months, breastfed for >6 months), family history of breast cancer (yes or no), history of benign breast disease (yes or no), height (<1.60, 1.60–1.64, 1.65–1.69, 1.70–1.74, ≥1.75 meters), cumulatively updated alcohol intake (0, <5, 5–9, 10–14, ≥15 g/d), cumulatively updated total caloric intake (kcal/day, quintiles), physical activity (linear MET-h/week), BMI at age 18 years (<20.0, 20.0–21.9, 22.0–23.9, 24.0–26.9, ≥27.0 kg/m2), a modified Alternate Healthy Eating Index score (with SSBs and alcohol removed), socioeconomic status, and change in weight since age 18 (lost ≥2 kg, lost 0–1 kg, gained 0–2 kg, gained 3–5 kg, gained 6–10 kg, gained 11–20 kg, gained 21–25 kg, gained >25 kg).
For testing heterogeneity by subtype, we used the Lunn-McNeil approach.
Due to smaller sample sizes in analyses, to ensure that models would run, covariate categorizations were simplified.
No interaction by menopausal status was observed for SSB or ASB intake; nonetheless, a suggestive, nonsignificantly higher risk of postmenopausal breast cancer was observed among women who consumed ≥1 serving of SSBs per day compared to women in the lowest category of consumption (HR, 1.07; 95% CI, 0.97–1.19; P-trend = 0.40; pooled data; Table 4). Furthermore, women who consumed ≥1serving/day of ASBs compared to those with a low consumption (<1 serving/month), had a 10% lower risk of premenopausal breast cancer (HR, 0.90; 95% CI, 0.80–1.01), although the test for a linear trend was not significant (P-trend = 0.20). We also tested whether the SSB or ASB intake and the risk of breast cancer differed by BMI in premenopausal or postmenopausal women in Supplemental Table 3, but no further associations were found.
TABLE 4.
Association between categories of cumulatively updated SSB and ASB intake and premenopausal or postmenopausal breast cancer risk using pooled data from the Nurses' Health Studies (NHS and NHSII)1
| <1/month | ≥1 to ≤4/month | >1 to <7/week | ≥1/day | P trend2 | Per 1 serving/day increase | |
|---|---|---|---|---|---|---|
| Categories of sugar-sweetened beverage category | ||||||
| Premenopausal breast cancer | ||||||
| Number of cases/person years | 671/421,460 | 596/389,514 | 906/578,796 | 330/236,214 | ||
| Multivariable-adjusted model3 | 1.00 | 0.94 (0.84–1.06) | 0.90 (0.81–1.01) | 0.96 (0.82–1.12) | 0.95 | 1.02 (0.95–1.08) |
| Postmenopausal breast cancer | ||||||
| Number of cases/person years | 2195/717,260 | 2160/662,476 | 3203/1,018,196 | 582/208,073 | — | |
| Multivariable-adjusted model3 | 1.00 | 1.05 (0.99–1.11) | 1.03 (0.97–1.09) | 1.07 (0.97–1.19) | 0.40 | 1.04 (0.99–1.09) |
| P-interaction by menopausal status = 0.42 | ||||||
| Categories of artificially sweetened beverage category | ||||||
| Premenopausal breast cancer | ||||||
| Number of cases/person years | 758/473,410 | 295/184,463 | 739/461,417 | 711/506,695 | — | |
| Multivariable-adjusted model3 | 1.00 | 0.97 (0.84–1.11) | 0.90 (0.81–1.00) | 0.90 (0.80–1.01) | 0.20 | 0.99 (0.96–1.03) |
| Postmenopausal breast cancer | ||||||
| Number of cases/person years | 2220/714,680 | 1151/348,395 | 3230/984,132 | 1539/558,798 | — | |
| Multivariable-adjusted model3 | 1.00 | 1.01 (0.94–1.09) | 0.99 (0.94–1.05) | 0.96 (0.90–1.03) | 0.12 | 0.98 (0.95–1.01) |
| P-interaction by menopausal status = 0.77 | ||||||
Values are HRs with 95% CIs in parentheses. ASB, artificially sweetened beverage; MET, metabolic equivalent; NHS, Nurses’ Health Study; NHSII, Nurses’ Health Study II; SSB, sugar-sweetened beverage.
P value for trend was calculated by assigning the median values to each quartile and modeling the median values as a continuous variable.
Multivariable model stratified by age in months and calendar year, adjusted for SSB or ASB intake, race (non-Hispanic Caucasian, African, Asian, Hispanic Caucasian), age at menarche (<12, 12, 13, 14, >14 years), age at menopause (premenopausal, <45, 45–49, 50–52, ≥53 years), postmenopausal hormone use (never user; past user; current user: estrogen only for <5 years; current user: estrogen only for ≥5 years; current estrogen + progestin user for <5 years; current estrogen + progestin user for ≥5 years; current user of other types), oral contraceptive use history (never, ever), parity and age at first birth (nulliparous, 1 child before age 25, 1 child at ≥25 years of age, ≥2 children before age 25, ≥2 children ≥25 years of age), breastfeeding history (never, breastfed for ≤6 months, breastfed for >6 months), family history of breast cancer (yes or no), history of benign breast disease (yes or no), height (<1.60, 1.60–1.64, 1.65–1.69, 1.70–1.74, ≥1.75 meters), cumulatively updated alcohol intake (0, <5, 5–9, 10–14, ≥15 g/d), cumulatively updated total caloric intake (kcal/day, quintiles), physical activity (linear MET-h/week), BMI at age 18 years (<20.0, 20.0–21.9, 22.0–23.9, 24.0–26.9, ≥27.0 kg/m2), a modified Alternate Healthy Eating Index score (with SSBs and alcohol removed), socioeconomic status, and change in weight since age 18 (lost ≥2 kg, lost 0–1 kg, gained 0–2 kg, gained 3–5 kg, gained 6–10 kg, gained 11–20 kg, gained 21–25 kg, gained >25 kg).
Supplemental Table 4 presents the HRs for incidence of breast cancer according to updated 4-year changes in beverage consumption using pooled data from both cohorts. Participants who increased their ASB or SSB intake by >0.50 servings/day or by >0.07 to 0.50 servings/day had a risk of breast cancer in the subsequent 4 years similar to that of participants who maintained a stable intake.
Discussion
In these 2 large, prospective cohorts of US women, no significant associations of SSB or ASB intake with breast cancer were observed overall. However, we observed a positive association between intake of SSBs and the risk of breast cancer among lean women that was independent of weight changes and other established dietary and nondietary breast cancer risk factors. We also found a modest inverse association between ASB intake and the risk of luminal A breast tumors, particularly among obese women. Because this is the first evaluation of ASB intake and subtype-specific breast tumors, confirmation is needed. Given our study's observational design, we cannot completely exclude the possibility of residual confounding. Nonetheless, the observational findings persisted after adjusting for known and suspected predictors of breast cancer.
Although further investigation of this association is warranted, there are several potential mechanisms that may contribute to these observed results. Adiposity plays a key role in postmenopausal breast cancer, with higher concentration of adipocytokines, hyperinsulinemia, and insulin resistance leading to breast cancer cell growth (53). Nonetheless, a mechanism independent of adiposity is likely given that the positive association among lean women was evident after an adjustment for weight change. Indeed, whereas we observed no association with SSB intake among overweight/obese women, greater consumption of SSB among lean women was positively associated with the cancer risk, even after adjusting for weight changes. Aside from obesity, the mechanisms underlying a link between SSBs and breast cancer may stem from the high amounts of rapidly absorbable carbohydrates, such as any form of sugar or high-fructose corn syrup, which are the primary sweeteners used in SSBs and bring rapid and dramatic increases in blood glucose and insulin concentrations. Increased insulin concentrations may influence breast cancer risk either directly, by stimulating insulin receptors in breast tissue, or indirectly, by augmenting the bioactivity of insulin‐like growth factor I (IGF‐I), which stimulates cell proliferation and inhibits apoptosis (54). Moreover, there is evidence that both insulin and IGF‐I stimulate the synthesis of sex steroids, particularly androgens, and decrease the concentration of sex‐hormone‐binding globulin, which in turn produces an increased tissue concentration of estrogens, formed by local conversion of the androgens. Further, the caramel coloring used in SSBs [4-methylimidazole (55)] is high in advanced glycation end products, which may additionally increase insulin resistance and inflammation (56).
While the association between SSB intake and cardiometabolic health has been extensively studied (57), little is known about whether intake of these beverages impacts the risks of total and subtype-specific breast tumors. Inconsistent findings between consumption of SSBs and breast cancer in the epidemiologic literature may be due to differences in exposure definitions, which have included intakes of sugars, sweets, and desserts as individual foods or as part of an overall dietary pattern that included sweet foods and/or beverages (58). Consumption of dessert foods, sweet beverages, and added sugars was positively associated with breast cancer risks in a population-based case-control study in Long Island, New York (58). In the latter study, and analogous to our results, women with lower BMIs exhibited a stronger association between dessert consumption and breast cancer compared to those with higher BMIs. A recent study using data from the Nurses’ Health Studies (59) showed that higher adherence to a diabetes reduction risk score (where SSBs scored negative) was inversely associated with the risk of breast cancer, particularly among lean women and independent of weight changes. Hence, encouraging lifestyle modifications to lessen the risk of developing insulin resistance and hyperinsulinemia may be a promising primary prevention strategy for breast cancer. In fact, a study evaluating the association between adherence to the WCRF/AICR cancer prevention recommendations and the breast cancer incidence in African-American women found that adherence to the recommendation to limit intake of sugary beverages was protective against breast cancer, particularly ER-/PR-positive tumors (60).
Similar to our results, null findings were observed for an association between total consumption of sugary beverages and breast cancer in multivariable-adjusted models in the Framingham Offspring cohort (26) and in the Canadian Study of Diet, Lifestyle, and Health (27). A positive association between sugary drink consumption and the risk of breast cancer was found in the NutriNet-Santé cohort study, specifically among premenopausal women (61). In our results, we showed a suggestive, nonsignificantly higher risk of postmenopausal breast cancer for women consuming 1 or more servings of SSBs per day compared to those with infrequent consumption (<1/month). In contrast, researchers from the Melbourne Collaborative Cohort Study (24) and the Seguimiento Universidad de Navarra cohort study (25) observed an increased risk of postmenopausal breast cancer. Nonetheless, in a previous study of the European Prospective Investigation into Cancer and Nutrition (EPIC)-France cohort (62), rapidly absorbed carbohydrates were associated with the risk of postmenopausal breast cancer in women who were overweight and women with large waist circumferences.
In contrast to SSBs, which represent the largest source of added sugar in the American diet (1), ASBs contain few to no calories, which makes them a likely attractive substitute for SSBs. However, observational studies and intervention trials have shown inconclusive results regarding their health effects (63, 64). In the present study, null results were observed for ASB intake and the overall breast cancer risk, consistent with results from the NutriNet-Santé cohort study (23). As far as other tumor locations are concerned, a previous study in our cohorts found that ASBs were associated with higher risks for non-Hodgkin lymphoma (only in men), multiple myeloma (only in men), and leukemia (both men and women) (28). Although these results could be explained by biological mechanisms (i.e., the potential carcinogenicity of aspartame), they could also be attributable to chance, as in our case.
While emerging research shows mixed results (65), additional high-quality, long-term research is needed to understand the mechanisms of action of ASBs. Moreover, in order to advance in this field, it will be necessary to go beyond ASBs and take into account the different types of artificial sweeteners (e.g., aspartame or acesulfame K), as well as other food and beverage sources.
Strengths and limitations
The strengths of our study include a large number of study participants, long-term follow-up, extensive dietary and covariate information, and the availability of tissue information for the determination of molecular subtypes. Prospective and repeated assessments of SSB and ASB intakes via validated FFQs allowed us to capture long-term intakes, reflecting true changes and minimizing the extent of measurement error and recall bias. Breast cancer was examined independently due to its intricate and unique etiology, as represented by the influence of exposures across the life course, hormonal and nonhormonal exposures, and tumor molecular heterogeneity. Also, dietary associations may differ by tumor subtype. Thus, it is noteworthy that we had the unique opportunity to study the potential associations between SSBs and specific molecular breast cancer subtypes (luminal A, luminal B, HER2-enriched and basal-like), which were not examined in previous prospective studies.
However, several limitations need to be acknowledged. First, the use of dietary assessments in observational research has been a point of debate caused by self-reported intakes and measurement error (66), which would likely be nondifferential in relation to the breast cancer risk and thus tend to underestimate associations. However, assessments of SSB/ASB intakes may be less prone to measurement error, because consumption of these beverages are relatively easy to measure. Furthermore, our FFQs showed good correlations when extensively validated against diet records and biomarkers, and the use of repeated measures of diet and lifestyle in our analyses could further reduce random measurement errors and in turn represent long-term habits. Second, another limitation includes the possibility of residual confounding despite adjusting for many potential confounders in our analysis, although the similar socioeconomic backgrounds of participants helped to minimize this potential bias. Higher SSB intake could serve as a marker of a globally unhealthy diet, and incomplete adjustment for various factors could lead to an overestimation of the association between SSBs and breast cancer. Reverse causation cannot be totally ruled out; however, latency analyses showed similar associations as those seen in the main analyses. Moreover, we made multiple comparisons (SSBs and ASBs, premenopausal and postmenopausal subgroups, and subtypes of tumors) in this analysis and we cannot rule out the presence of a type I error as an explanation for our results. Finally, our study was conducted among a predominately non-Hispanic white population (Caucasians) of health professionals, which minimizes potential confounding by socioeconomic factors but may limit generalizability to populations with different underlying breast cancer risks.
Conclusions
In these large prospective cohort studies of US women, SSB and ASB intakes were not significantly associated with the risk of breast cancer overall. However, among lean women, independently of weight changes, consumption of SSBs was modestly associated with an increased risk of breast cancer. Although a suggestive interaction between SSBs and BMI appears plausible and coherent, we cannot exclude the possibility of type I errors; therefore, our results must be prudently applied.
Ample evidence exists to discourage consumption of SSBs in place of healthy alternatives, in order to reduce the risk of chronic diseases and improve overall health and quality of life.
Supplementary Material
Acknowledgments
We thank the following state cancer registries for their help with providing information for confirming cancer self-reports in participants: AL, AR, AZ, CA, CO, CT, DE, FL, GA, IA, ID, IL, IN, KY, LA, MA, MD, ME, MI, NC, ND, NE, NH, NJ, NY, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, and WY. The authors assume full responsibility for analyses and interpretation of these data.
The authors’ responsibilities were as follows – AR-N, WCW, BAR, AHE: designed and conducted the research; AR-N, AHE, BAR: analyzed the data or performed the statistical analysis; AR-N: had primary responsibility for the final content; and all authors: wrote the paper and read and approved the final manuscript.
Notes
This study was supported by grants UM1 CA186107, U01 CA176726, P01 CA87969, and R01 CA50385 from the National Institutes of Health and the Breast Cancer Research Foundation. AR-N was supported by a fellowship from the Spanish Association Against Cancer Scientific Foundation (FC AECC).
Author disclosures: AR-N was supported by a fellowship from the Spanish Association Against Cancer Scientific Foundation (FC AECC).
The funding sources did not participate in the design or conduct of the study; collection, management, analysis or interpretation of the data; or preparation, review, or approval of the manuscript
Supplemental Figures 1 and 2 and Supplemental Tables 1–4 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: AICR, American Institute for Cancer research; AHEI, Alternate Healthy Eating Index; ASB, artificially sweetened beverage; CK 5/6, cytokeratins 5/6; EGFR, epidermal growth factor receptor; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; IGF-I, insulin-like growth factor I; MET, metabolic equivalent; NHS, Nurses’ Health Study; NHSII, Nurses’ Health Study II; PR, progesterone receptor; SSB, sugar-sweetened beverage; TMA, tumor microarrays; TNFaR2, tumor necrosis factor alpha receptor 2; WCRF, World Cancer Research Fund.
Contributor Information
Andrea Romanos-Nanclares, Department of Preventive Medicine and Public Health, School of Medicine, University of Navarra, Pamplona, Spain; Navarra Institute for Health Research (IdiSNA), Pamplona, Spain.
Laura C Collins, Department of Pathology, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA, USA.
Frank B Hu, Channing Division of Network Medicine, Department of Medicine, Brigham & Women's Hospital, and Harvard Medical School, Boston, MA, USA; Department of Nutrition, Harvard T. H. Chan School of Public Health, Boston, MA, USA; Department of Epidemiology, Harvard T. H. Chan School of Public Health, Boston, MA, USA.
Walter C Willett, Channing Division of Network Medicine, Department of Medicine, Brigham & Women's Hospital, and Harvard Medical School, Boston, MA, USA; Department of Nutrition, Harvard T. H. Chan School of Public Health, Boston, MA, USA; Department of Epidemiology, Harvard T. H. Chan School of Public Health, Boston, MA, USA.
Bernard A Rosner, Channing Division of Network Medicine, Department of Medicine, Brigham & Women's Hospital, and Harvard Medical School, Boston, MA, USA; Department of Biostatistics, Harvard T. H. Chan School of Public Health, Boston, MA, USA.
Estefania Toledo, Department of Preventive Medicine and Public Health, School of Medicine, University of Navarra, Pamplona, Spain; Navarra Institute for Health Research (IdiSNA), Pamplona, Spain; Centro de Investigacion Biomedica en Red Fisiopatologia de La Obesidad y La Nutrición (CIBEROBN), Institute of Health Carlos III, Madrid, Spain.
A Heather Eliassen, Channing Division of Network Medicine, Department of Medicine, Brigham & Women's Hospital, and Harvard Medical School, Boston, MA, USA; Department of Epidemiology, Harvard T. H. Chan School of Public Health, Boston, MA, USA.
References
- 1.Department of Health and Human Services . Department of Agriculture (US) 2015–2020 dietary guidelines for Americans. 8th ed, December 2015. [Google Scholar]
- 2.Liu J, Rehm CD, Onopa J, Mozaffarian D. Trends in diet quality among youth in the United States, 1999–2016. JAMA. 2020;323(12):1161–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vercammen KA, Moran AJ, Soto MJ, Kennedy-Shaffer L, Bleich SN. Decreasing trends in heavy sugar-sweetened beverage consumption in the United States, 2003 to 2016. J Acad Nutr Diet. 2020;120(12):1974–1985.e5. [DOI] [PubMed] [Google Scholar]
- 4.GBD 2016 Risk Factors Collaborators . Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet North Am Ed. 2017;390(10100):1345–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malik VS, Pan A, Willett WC, Hu FB. Sugar-sweetened beverages and weight gain in children and adults: A systematic review and meta-analysis. Am J Clin Nutr. 2013;98:1084–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imamura F, O'Connor L, Ye Z, Mursu J, Hayashino Y, Bhupathiraju SN, Forouhi NG. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: Systematic review, meta-analysis, and estimation of population attributable fraction. BMJ. 2015;351:h3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwingshackl L, Schwedhelm C, Hoffmann G, Knüppel S, Iqbal K, Andriolo V, Bechthold A, Schlesinger S, Boeing H. Food groups and risk of hypertension: A systematic review and dose-response meta-analysis of prospective studies. Adv Nutr. 2017;8(6):793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fung TT, Malik V, Rexrode KM, Manson JE, Willett WC, Hu FB. Sweetened beverage consumption and risk of coronary heart disease in women. Am J Clin Nutr. 2009;89(4):1037–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Koning L, Malik VS, Kellogg MD, Rimm EB, Willett WC, Hu FB. Sweetened beverage consumption, incident coronary heart disease, and biomarkers of risk in men. Circulation. 2012;125(14):1735–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernstein AM, De Koning L, Flint AJ, Rexrode KM, Willett WC. Soda consumption and the risk of stroke in men and women. Am J Clin Nutr. 2012;95(5):1190–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malik VS, Li Y, Pan A, De Koning L, Schernhammer E, Willett WC, Hu FB. Long-term consumption of sugar-sweetened and artificially sweetened beverages and risk of mortality in US adults. Circulation. 2019;139(18):2113–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. Body fatness and cancer–Viewpoint of the IARC Working Group. N Engl J Med. 2016;375(8):794–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klement RJ, Kämmerer U. Is there a role for carbohydrate restriction in the treatment and prevention of cancer?. Nutr Metab (Lond). 2011;8:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong J-Y, Qin L-Q. Dietary glycemic index, glycemic load, and risk of breast cancer: Meta-analysis of prospective cohort studies. Breast Cancer Res Treat. 2011;126(2):287–94. [DOI] [PubMed] [Google Scholar]
- 15.Schlesinger S, Chan DSM, Vingeliene S, Vieira AR, Abar L, Polemiti E, Stevens CAT, Greenwood DC, Aune D, Norat T. Carbohydrates, glycemic index, glycemic load, and breast cancer risk: A systematic review and dose-response meta-analysis of prospective studies. Nutr Rev. 2017;75(6):420–41. [DOI] [PubMed] [Google Scholar]
- 16.Laguna JC, Alegret M, Roglans N. Simple sugar intake and hepatocellular carcinoma: Epidemiological and mechanistic insight. Nutrients. 2014;6(12):5933–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi Y, Giovannucci E, Lee JE. Glycaemic index and glycaemic load in relation to risk of diabetes-related cancers: A meta-analysis. Br J Nutr. 2012;108(11):1934–47. [DOI] [PubMed] [Google Scholar]
- 18.Sylvetsky AC, Rother KI. Trends in the consumption of low-calorie sweeteners. Physiol Behav. 2016;164(Pt B):446–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qin P, Li Q, Zhao Y, Chen Q, Sun X, Liu Y, Li H, Wang T, Chen X, Zhou Qet al. Sugar and artificially sweetened beverages and risk of obesity, type 2 diabetes mellitus, hypertension, and all-cause mortality: A dose-response meta-analysis of prospective cohort studies. Eur J Epidemiol. 2020;35(7):655–71. [DOI] [PubMed] [Google Scholar]
- 20.Palmnäs MS, Cowan TE, Bomhof MR, Su J, Reimer RA, Vogel HJ, Hittel DS, Shearer J. Low-dose aspartame consumption differentially affects gut microbiota-host metabolic interactions in the diet-induced obese rat. PLoS One. 2014;9(10):e109841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suez J, Korem T, Zeevi D, Zilberman-Schapira G, Thaiss CA, Maza O, Israeli D, Zmora N, Gilad S, Weinberger Aet al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 2014;514(7521):181–6. [DOI] [PubMed] [Google Scholar]
- 22.World Cancer Research Fund, American Institute for Cancer Research . Continuous Update Project Report: Diet, nutrition, physical activity and breast cancer. London; 2017; Available from: wcrf.org/breast-cancer–2017 [Google Scholar]
- 23.Chazelas E, Srour B, Desmetz E, Kesse-Guyot E, Julia C, Deschamps V, Druesne-Pecollo N, Galan P, Hercberg S, Latino-Martel Pet al. Sugary drink consumption and risk of cancer: Results from NutriNet-Santé prospective cohort. BMJ. 2019;366:l2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hodge AM, Bassett JK, Milne RL, English DR, Giles GG. Consumption of sugar-sweetened and artificially sweetened soft drinks and risk of obesity-related cancers. Public Health Nutr. 2018;21(9):1618–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romanos-Nanclares A, Toledo E, Gardeazabal I, Jiménez-Moleón JJ, Martínez-González MA, Gea A. Sugar-sweetened beverage consumption and incidence of breast cancer: The Seguimiento Universidad de Navarra (SUN) project. Eur J Nutr. 2019;58:2875–86. [DOI] [PubMed] [Google Scholar]
- 26.Makarem N, Bandera EV, Nicholson JM, Parekh N. Consumption of sugars, sugary foods, and sugary beverages in relation to cancer risk: A systematic review of longitudinal studies. Annu Rev Nutr. 2018;38:17–39. [DOI] [PubMed] [Google Scholar]
- 27.Arthur RS, Kirsh VA, Mossavar-Rahmani Y, Xue X, Rohan TE. Sugar-containing beverages and their association with risk of breast, endometrial, ovarian and colorectal cancers among Canadian women. Cancer Epidemiol. 2021;70:101855. [DOI] [PubMed] [Google Scholar]
- 28.Schernhammer ES, Bertrand KA, Birmann BM, Sampson L, Willett WC, Feskanich D. Consumption of artificial sweetener- and sugar-containing soda and risk of lymphoma and leukemia in men and women. Am J Clin Nutr. 2012;96(6):1419–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guercio BJ, Zhang S, Niedzwiecki D, Li Y, Babic A, Morales-Oyarvide V, Saltz LB, Mayer RJ, Mowat RB, Whittom Ret al. Associations of artificially sweetened beverage intake with disease recurrence and mortality in stage III colon cancer: Results from CALGB 89803 (Alliance). PLoS One. 2018;13(7):e0199244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Willett WC, Sampson L, Browne ML, Stampfer MJ, Rosner B, Hennekens CH, Speizer FE. The use of a self-administered questionnaire to assess diet four years in the past. Am J Epidemiol. 1988;127(1):188–99. [DOI] [PubMed] [Google Scholar]
- 31.Yuan C, Spiegelman D, Rimm EB, Rosner BA, Stampfer MJ, Barnett JB, Chavarro JE, Subar AF, Sampson LK, Willett WC. Validity of a dietary questionnaire assessed by comparison with multiple weighed dietary records or 24-hour recalls. Am J Epidemiol. 2017;185(7):570–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salvini S, Hunter DJ, Sampson L, Stampfer MJ, Colditz GA, Rosner B, Willett WC. Food-based validation of a dietary questionnaire: The effects of week-to-week variation in food consumption. Int J Epidemiol. 1989;18(4):858–67. [DOI] [PubMed] [Google Scholar]
- 33.Willett W, Lenart E. Reproducibility and validity of food frequency questionnaires. In: Nutritional Epidemiology. 3rd ed New York: Oxford University Press; 2013. 96–141. [Google Scholar]
- 34.Tabung FK, Smith-Warner SA, Chavarro JE, Wu K, Fuchs CS, Hu FB, Chan AT, Willett WC, Giovannucci EL. Development and validation of an empirical dietary inflammatory index. J Nutr. 2016;146(8):1560–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the National Death Index and Equifax Nationwide Death Search. Am J Epidemiol. 1994;140(11):1016–9. [DOI] [PubMed] [Google Scholar]
- 36.Stampfer MJ, Willett WC, Speizer FE, Dysert DC, Lipnick R, Rosner B, Hennekens CH. Test of the National Death Index. Am J Epidemiol. 1984;119(5):837–9. [DOI] [PubMed] [Google Scholar]
- 37.Colditz GA, Rosner BA, Chen WY, Holmes MD, Hankinson SE. Risk factors for breast cancer according to estrogen and progesterone receptor status. J Natl Cancer Inst. 2004;96(3):218–28. [DOI] [PubMed] [Google Scholar]
- 38.Hirko KA, Willett WC, Hankinson SE, Rosner BA, Beck AH, Tamimi RM, Eliassen AH. Healthy dietary patterns and risk of breast cancer by molecular subtype. Breast Cancer Res Treat. 2016;155(3):579–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tamimi RM, Baer HJ, Marotti J, Galan M, Galaburda L, Fu Y, Deitz AC, Connolly JL, Schnitt SJ, Colditz GAet al. Comparison of molecular phenotypes of ductal carcinoma in situ and invasive breast cancer. Breast Cancer Res. 2008;10(4):R67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Collins LC, Marotti JD, Baer HJ, Tamimi RM. Comparison of estrogen receptor results from pathology reports with results from central laboratory testing. J Natl Cancer Inst. 2008;100(3):218–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang J, Zhang X, Beck AH, Collins LC, Chen WY, Tamimi RM, Hazra A, Brown M, Rosner B, Hankinson SE. Alcohol consumption and risk of breast cancer by tumor receptor expression. Horm Cancer. 2015;6(5-6):237–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, Hernandez-Boussard T, Livasy C, Cowan D, Dressler Let al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10(16):5367–74. [DOI] [PubMed] [Google Scholar]
- 43.Cheang MC, Voduc D, Bajdik C, Leung S, McKinney S, Chia SK, Perou CM, Nielsen TO. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Res. 2008;14(5):1368–76. [DOI] [PubMed] [Google Scholar]
- 44.Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, Watson M, Davies S, Bernard PS, Parker JSet al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009;101(10):736–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van de Rijn M, Perou CM, Tibshirani R, Haas P, Kallioniemi O, Kononen J, Torhorst J, Sauter G, Zuber M, Köchli ORet al. Expression of cytokeratins 17 and 5 identifies a group of breast carcinomas with poor clinical outcome. Am J Pathol. 2002;161(6):1991–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brenton JD, Carey LA, Ahmed A, Caldas C. Molecular classification and molecular forecasting of breast cancer: Ready for clinical application?. J Clin Oncol. 2005;23(29):7350–60. [DOI] [PubMed] [Google Scholar]
- 47.Abd El-Rehim DM, Pinder SE, Paish CE, Bell J, Blamey RW, Robertson JF, Nicholson RI, Ellis IO. Expression of luminal and basal cytokeratins in human breast carcinoma. J Pathol. 2004;203(2):661–71. [DOI] [PubMed] [Google Scholar]
- 48.McCullough ML, Feskanich D, Stampfer MJ, Giovannucci EL, Rimm EB, Hu FB, Spiegelman D, Hunter DJ, Colditz GA, Willett WC. Diet quality and major chronic disease risk in men and women: Moving toward improved dietary guidance. Am J Clin Nutr. 2002;76(6):1261–71. [DOI] [PubMed] [Google Scholar]
- 49.Lee JE, Willett WC, Fuchs CS, Smith-Warner SA, Wu K, Ma J, Giovannucci E. Folate intake and risk of colorectal cancer and adenoma: Modification by time. Am J Clin Nutr. 2011;93(4):817–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Willett W. Nutritional Epidemiology. 3rd ed New York: Oxford University Press; 2013. [Google Scholar]
- 51.Martínez ME, Giovannucci E, Spiegelman D, Hunter DJ, Willett WC, Colditz GA. Leisure-time physical activity, body size, and colon cancer in women. J Natl Cancer Inst. 1997;89(13):948–55. [DOI] [PubMed] [Google Scholar]
- 52.Lunn M, McNeil D. Applying Cox regression to competing risks. Biometrics. 1995;51(2):524–32. [PubMed] [Google Scholar]
- 53.Simone V, D'Avenia M, Argentiero A, Felici C, Rizzo FM, De Pergola G, Silvestris F. Obesity and breast cancer: Molecular interconnections and potential clinical applications. Oncologist. 2016;21(4):404–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaaks R, Lukanova A. Energy balance and cancer: The role of insulin and insulin-like growth factor-I. Proc Nutr Soc. 2001;60(1):91–106. [DOI] [PubMed] [Google Scholar]
- 55.Smith TJ, Wolfson JA, Jiao D, Crupain MJ, Rangan U, Sapkota A, Bleich SN, Nachman KE. Caramel color in soft drinks and exposure to 4-methylimidazole: A quantitative risk assessment. PLoS One. 2015;10(2):e0118138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leis-Keeling K. Comprehensive evaluation of soft drinks effects on health, and nutritional strategies to reverse damage. Nutr Perspect. 2010;33:15–23. [Google Scholar]
- 57.Malik VS, Hu FB. Sugar-sweetened beverages and cardiometabolic health: An update of the evidence. Nutrients. 2019;11(8):1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bradshaw PT, Sagiv SK, Kabat GC, Satia JA, Britton JA, Teitelbaum SL, Neugut AI, Gammon MD. Consumption of sweet foods and breast cancer risk: A case-control study of women on Long Island, New York. Cancer Causes Control. 2009;20(8):1509–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kang JH, Peng C, Rhee JJ, Farvid MS, Willett WC, Hu FB, Rosner BA, Tamimi R, Eliassen AH. Prospective study of a diabetes risk reduction diet and the risk of breast cancer. Am J Clin Nutr. 2020;112(6):1492–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nomura SJO, Dash C, Rosenberg L, Yu J, Palmer JR, Adams-Campbell LL. Adherence to diet, physical activity and body weight recommendations and breast cancer incidence in the Black Women's Health Study. Int J Cancer. 2016;139(12):2738–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chazelas E, Srour B, Desmetz E, Kesse-Guyot E, Julia C, Deschamps V, Druesne-Pecollo N, Galan P, Hercberg S, Latino-Martel P, et al. Sugary drink consumption and risk of cancer: Results from NutriNet-Santé prospective cohort. BMJ. 2019;366:l2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lajous M, Boutron-Ruault MC, Fabre A, Clavel-Chapelon F, Romieu I. Carbohydrate intake, glycemic index, glycemic load, and risk of postmenopausal breast cancer in a prospective study of French women. Am J Clin Nutr. 2008;87(5):1384–91. [DOI] [PubMed] [Google Scholar]
- 63.Fowler SP, Williams K, Resendez RG, Hunt KJ, Hazuda HP, Stern MP. Fueling the obesity epidemic? Artificially sweetened beverage use and long-term weight gain. Obesity. 2008;16(8):1894–900. [DOI] [PubMed] [Google Scholar]
- 64.Narain A, Kwok CS, Mamas MA. Soft drinks and sweetened beverages and the risk of cardiovascular disease and mortality: A systematic review and meta-analysis. Int J Clin Pract. 2016;70(10):791–805. [DOI] [PubMed] [Google Scholar]
- 65.Johnson RK, Lichtenstein AH, Anderson CAM, Carson JA, Després JP, Hu FB, Kris-Etherton PM, Otten JJ, Towfighi A, Wylie-Rosett J; American Heart Association Nutrition Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Quality of Care and Outcomes Research; and Stroke Council . Low-calorie sweetened beverages and cardiometabolic health: A science advisory from the American Heart Association. Circulation. 2018;138(9):e126–40. [DOI] [PubMed] [Google Scholar]
- 66.Satija A, Stampfer MJ, Rimm EB, Willett W, Hu FB. Perspective: Are large, simple trials the solution for nutrition research?. Adv Nutr. 2018;9(4):378–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.